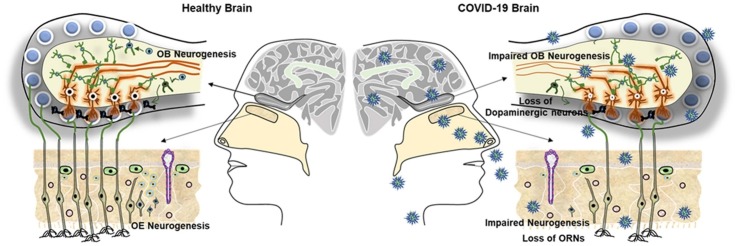
Graphical abstract
Abbreviations: ACE2, angiotensin converting enzyme 2; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; AOS, accessory olfactory system; COVID-19, coronavirus disease 2019; CT, computerized tomography; DAT-SPECT, Dopamine transporter single-photon emission computed tomography; DCX, Doublecortin; DDC, dopa decarboxylase; EPL, external plexiform layer; GABA, Gamma aminobutyric acid; GBC, globose basal cell; GCL, granule cell layer; GG, Grüneberg ganglion; GML, glomerular layer; GPCR, G Protein-Coupled Receptor; HBC, Horizontal basal cell; HD, Huntington’s disease; HIV, human immunodeficiency virus; IPL, internal plexiform layer; JE, Japanese encephalitis; MCL, mitral cell layer; MHV, mouse hepatitis virus; MOS, Main olfactory system; MRI, magnetic resonance imaging; NSC, neural stem cells; OB, olfactory bulb; OE, olfactory epithelium; ONL, olfactory nerve layer; OR, olfactory receptor; ORN, olfactory receptor neurons; OSN, olfactory sensory neuron; PD, Parkinson’s disease; PSA-NCAM, polysialylated-neural cell adhesion molecule; RMS, rostral migratory stream; RT-PCR, Real-time reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVZ, subventricular zone; TMPRSS2, transmembrane serine protease 2
Keywords: COVID-19, Anosmia, Olfactory dysfunction, Parkinson’s disease, Neurogenesis
Abstract
Anosmia, a neuropathogenic condition of loss of smell, has been recognized as a key pathogenic hallmark of the current pandemic SARS-CoV-2 infection responsible for COVID-19. While the anosmia resulting from olfactory bulb (OB) pathology is the prominent clinical characteristic of Parkinson's disease (PD), SARS-CoV-2 infection has been predicted as a potential risk factor for developing Parkinsonism-related symptoms in a significant portion of COVID-19 patients and survivors. SARS-CoV-2 infection appears to alter the dopamine system and induce the loss of dopaminergic neurons that have been known to be the cause of PD. However, the underlying biological basis of anosmia and the potential link between COVID-19 and PD remains obscure. Ample experimental studies in rodents suggest that the occurrence of neural stem cell (NSC) mediated neurogenesis in the olfactory epithelium (OE) and OB is important for olfaction. Though the occurrence of neurogenesis in the human forebrain has been a subject of debate, considerable experimental evidence strongly supports the incidence of neurogenesis in the human OB in adulthood. To note, various viral infections and neuropathogenic conditions including PD with olfactory dysfunctions have been characterized by impaired neurogenesis in OB and OE. Therefore, this article describes and examines the recent reports on SARS-CoV-2 mediated OB dysfunctions and defects in the dopaminergic system responsible for PD. Further, the article emphasizes that COVID-19 and PD associated anosmia could result from the regenerative failure in the replenishment of the dopaminergic neurons in OB and olfactory sensory neurons in OE.
1. Introduction
The coronavirus disease (COVID-19) pandemic continues due to the spread of new variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection worldwide (Kandasamy, 2020; Korber et al., 2020; Lai et al., 2020; Krishna Murthy et al., 2021; Selvaraj et al., 2021; Zheng, 2020). The typical symptoms of COVID-19 include anosmia and ageusia along with fever, dry cough and shortness of breath (Ali and Alharbi, 2020; Huang et al., 2020; Kandasamy, 2020a; Meng et al., 2020). The angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) expressing ciliated cells of the nasal mucosa are the primary targets of initial SARS-CoV-2 infection (Beghi et al., 2020; Sungnak et al., 2020). A higher level of viral load is found in the nasal cavity of both symptomatic and asymptomatic SARS-CoV-2 infected individuals (Gengler et al., 2020; Meng et al., 2020; Ra et al., 2021). SARS-CoV-2 spreads from nasal epithelium to different brain regions through the neural pathway of the olfactory bulb (OB) and induces cytokine storm responsible for neuroinflammation, thereby leading to various neurological deficits (DosSantos et al., 2020; Zhang et al., 2020). Anosmia, a clinical state of loss of smell, has been ascertained as a key symptom of COVID-19 regardless of the diagnostic criteria, sex and age (Aziz et al., 2021; Jain et al., 2020; Spinato et al., 2020). Anosmia can either occur as the only symptom in COVID-19 subjects or take place along with other clinical signs of COVID-19 (Lechien et al., 2020; Meng et al., 2020). While the duration of anosmia may be shorter in most COVID-19 cases, it may also be irreversible in subjects with severe OB dysfunction (Gori et al., 2020; Vavougios, 2020). At present, the underlying pathogenic basis for anosmia in patients with COVID-19 remains obscure. Several magnetic resonance imaging (MRI) and computerized tomography (CT) based brain imaging reports, and neuro clinical correlates have clearly indicated the dysfunction or atrophy of OB in subjects with COVID-19 (Chiu et al., 2020; Galougahi et al., 2020; Kandemirli et al., 2021). Defect in the dopamine system, OB pathology and loss of smell are the prominent clinical characteristics of Parkinson's disease (PD) (Balestrino and Schapira, 2020; Barresi et al., 2012; Haehner et al., 2011; Kouli et al., 2018). However, the underlying biological basis of anosmia and the potential link between COVID-19 and PD remains obscure.
Ample experimental studies in rodents suggest that the occurrence of neural stem cell (NSC) mediated neurogenesis in the olfactory epithelium (OE) and OB is important for the sense of smell (Brann and Firestein, 2014; Lledo and Valley, 2016), whereas impairment of olfactory neurogenesis due to various pathogenic stimuli including neuroinflammation and oxidative stress is associated with loss of smell (Boesveldt et al., 2017; Lazarini et al., 2014). Notably, the regulation of neurogenesis in OB is negatively altered during viral infections and various neuropathogenic conditions including PD (Kandasamy et al., 2015; Lazarini et al., 2014; Loseva et al., 2009; May et al., 2012). Though the occurrence of neurogenesis in the human OB is a controversial subject (Bergmann et al., 2012), there exists considerable experimental evidence that strongly supports the incidence of ongoing neurogenesis in the human OB during adulthood (Durante et al., 2020; Lazarini et al., 2014; Lledo and Valley, 2016; Pignatelli and Belluzzi, 2010; Winner et al., 2006). Therefore, this article attempts to survey the recent reports on SARS-CoV-2 mediated dysfunction in OB and defects in the dopaminergic system responsible for PD. Further, this article postulates a hypothesis that anosmia resulting from pathogenic events of COVID-19 and PD could be linked to dysregulation of adult neurogenesis in OB and OE.
2. Frequency of anosmia and olfactory dysfunction in COVID-19
The SARS-CoV-2 infection has been known to cause olfactory dysfunction in the majority of COVID-19 cases (Meng et al., 2020; Sedaghat et al., 2020; Sungnak et al., 2020). Several recent reports indicate that the neuroinvasion of SARS-CoV-2 initially indcuces neuroinflammation-mediated damage to the olfactory receptor neurons (ORNs) of the nasal neuroepithelium and spread to other brain regions via OB (Gori et al., 2020; Mahalaxmi et al., 2021). Hyposmia or anosmia has been reported to be highly prevalent among COVID-19 patients (Meng et al., 2020; Sedaghat et al., 2020; Speth et al., 2020). A retrospective study by Lechien JR et al. reported the occurrence of 85.6 % and 88 % of olfactory and gustatory dysfunctions respectively in patients with mild to moderate symptoms of COVID-19 (Lechien et al., 2020). A smartphone-app-based COVID-19 like symptom tracker study by Menni C et al. revealed a strong association between the loss of smell in 64.76 % of patients who confirmed positive for SARS-CoV-2 infection by real-time reverse transcription-polymerase chain reaction (RT-PCR) (Menni et al., 2020). A clinical report from China indicated 5.1 % of hospitalized COVID-19 patients experiencing loss of smell (Mao et al., 2020). Klopfenstein T et al. reported that 47 % of COVID-19 patients in Europe exhibited anosmia along with dysgeusia (Klopfenstein et al., 2020). Von Bartheld CS et al. reported higher incidences of olfactory impairments and taste disorders in COVID-19 cases in the Western population than in COVID-19 patients in East Asia, and the difference appears to be independent of age and disease severity (von Bartheld et al., 2020). Quantitative meta assessments of the olfactory defects upon SARS-CoV-2 infection reported some degree of loss of smell in nearly all COVID-19 patients worldwide (Moein et al., 2020; Mullol et al., 2020). There exist many reports that describe olfactory dysfunction varies from person to person irrespective of age. A Spanish multicenter cross-sectional study by Rojas-Lechuga MJ et al. indicated that 70.1 % of COVID-19 subjects confirmed by RT-PCR with a mean age of 46.5 ranging from 21 years to 89 years reported the occurrence of smell loss (Rojas-Lechuga et al., 2021). Gori A et al. indicated that COVID-19 causes severe complications in patients with advanced chronological age due to age-related defects of olfactory neuroepithelium and deterioration of OB homeostasis (Gori et al., 2020). An Italian multicenter‐study by Vaira LA et al. reported that 51.5 % of COVID-19 patients showed olfactory disorders regardless of the difference in gender and age (Vaira et al., 2020). In the meantime, Giacomelli A et al. reported 5.1 % of COVID-19 cases at the mean age group of 60 with OD in the Italian population (Giacomelli et al., 2020). A UK based cohort study by Tomlins, J. et al. reported 4% of SARS-CoV-2 positive subjects with anosmia in the median age group of 75 (Tomlins et al., 2020). A study by Bénézit F et al. indicated 20 % of hyposmia among COVID-19 patients in France who were tested positive for the presence of SARS-CoV-2 by RT-PCR (Bénézit et al., 2020). Luers JC et al. reported that 73.6 % of COVID-19 patients (mean age 38) confirmed by RT-PCR showed olfactory dysfunction (Luers et al., 2020). In a pilot multicenter case-control study from Spain, Beltrán‐Corbellini. A et al. reported anosmia in 45.2 % of COVID-19 patients (mean age 52.6) confirmed by RT-RCR (Beltrán-Corbellini et al., 2020). Notably, a case-control study by Mangal V et al. observed a significant prevalence of olfactory dysfunction in asymptomatic cases with SARS-CoV-2 infection (Mangal et al., 2021). An olfactory-action meter-based study by Bhattacharjee AS et al. revealed a significant reduction in the smell detection abilities of asymptomatic COVID-19 subjects compared to normal healthy individuals (Bhattacharjee et al., 2020).
Given the comorbid pathological nature of SARS-CoV-2 infection, neuropathogenesis seen in COVID-19 appears to be overlapped with the clinical characteristic of the sporadic form of various neurodegenerative disorders (Ferini-Strambi and Salsone, 2020; Kandasamy, 2020; Marshall, 2020; Rebholz et al., 2020). However, studies on abnormal cellular and molecular bases for the neurological deficits in COVID-19 are at the early stage. Anosmia resulting from OB pathology is the prominent clinical characteristic of many neurodegenerative disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS) (Hawkes, 2003; Rebholz et al., 2020). The degree of olfactory dysfunction in many neurodegenerative diseases varies and occurs along with other symptoms in the late stage of the disease (Doty, 2017; Rebholz et al., 2020). Unlike other neurological diseases, anosmia resulting from the pathogenic destruction of OB has been ascertained as the non-motor early clinical feature of PD (Doty, 2017, 2012). The SARS-CoV-2 infection has been predicted as a potential risk factor for developing Parkinsonism-related symptoms in a significant portion of COVID-19 patients and survivors (Brundin et al., 2020; Sulzer et al., 2020). SARS-CoV-2 infection appears to induce defects in the dopamine system, loss of dopaminergic neurons, and accelerates synucleinopathies in the brain which have been considered as the underlying causes of PD (Brundin et al., 2020; Li et al., 2020a,b; Merello et al., 2021; Sulzer et al., 2020; Yavarpour-Bali and Ghasemi-Kasman, 2020). Moreover, PD patients have been reported to be highly susceptible to SARS-CoV-2 infection, and COVID-19 related pathogenic changes appear to exacerbate the clinical features of PD (Brundin et al., 2020; Sulzer et al., 2020). Thus, a reciprocal link between COVID-19 and PD has become a topic of scientific focus.
3. Possible relationship between COVID-19 and Parkinson’s disease
While ageing and age-related diseases are the major risk factors of COVID‐19 infection, recent reports indicated the possibilities for the occurrence of PD upon SARS-CoV-2 infection (Sulzer et al., 2020). PD is one of the common late-onset neurodegenerative disorders mainly characterized by movement disorders including bradykinesia, tremor, imbalance and rigidity with postural instability (DeMaagd and Philip, 2015; Greenland and Barker, 2018; Kouli et al., 2018; Williams and Litvan, 2013). The movement disorders in PD arises mainly due to the loss of dopaminergic neurons in the substantia nigra leading to failure in the nigrostriatal pathway of the brain (Alexander, 2004; Sonne et al., 2020). The dysfunction of the dopaminergic system and degeneration of dopaminergic neurons in the OB contribute to olfactory defects in PD (Alexander, 2004; Paß et al., 2020). Besides, PD has been characterized by the presence of Lewy bodies and α-Synucleinopathy due to a dysfunctional proteostasis network in the brain (Beyer et al., 2009; Lehtonen et al., 2019). Several lines of evidence states that the impairment of the sense of smell in PD could also be due to Lewy bodies and aggregation of α-Synuclein mediated failure of the dopaminergic system in OB (Han et al., 2020; Rey et al., 2018; Sengoku et al., 2008). Many recent studies indicate that PD results from unknown complex interactions between the genetic and environmental factors, while mutations in some genes including alpha-Synuclein, Parkin, and Leucine-rich repeat kinase 2 (LRRK2) leads to genetic forms of PD (Nuytemans et al., 2010). There exists a considerable number of experimental proofs that viral infections can trigger the accumulation of Lewy bodies and α- Synuclein in the brain (Takahashi and Yamada, 1999; Tulisiak et al., 2019).
Previous case reports suggest that transient secondary parkinsonism can result from several viral infections including human immunodeficiency virus (HIV), Japanese encephalitis (JE) and coxsackie viruses (Jang et al., 2009a). Limphaibool N et al. reported that herpes simplex 1, Epstein-Barr, hepatitis C, and influenza A virus can also increase the chance of developing PD in the long run (Limphaibool et al., 2019). With reference to the alteration of the dopaminergic system upon viral infections, an earlier study by Jang H et al. showed that intranasally administered influenza virus leads to a selective decrease of dopaminergic neurons in the substantia nigra of the experimental mouse brain (Jang et al., 2009b). Similarly,the possibilities of SARS-CoV-2 infection mediated degeneration of dopaminergic neurons in the brain may not be excluded.
In 1985, Fishman et al. reported the selective affinity of coronavirus mouse hepatitis virus (MHV)-A59 to the basal ganglia, a key brain structure that controls the movement, and observed an abnormal posture, locomotion difficulties, neuronal loss, and gliosis in the substantia nigra of the experimental mouse brain (Fishman et al., 1985). Notably, recent data indicate the expression of ACE2 and dopa decarboxylase (DDC), the enzymes important for the synthesis of dopamine in the non-neuronal cells (Nataf, 2020). SARS-CoV infection has been reported to downregulate the expression of ACE2 that could in part play a role in the impairment of the production of dopamine (Khalefah and Khalifah, 2020; Kuba et al., 2005; Verdecchia et al., 2020). Moreover, SARS-CoV-2 infection appears to induce α-Synuclein aggregation and Lewy body-like pathology in the brain of COVID-19 cases (Brundin et al., 2020; Pavel et al., 2020). Chan CP et al. reported that SARS-CoV uses the endoplasmic reticulum as a site for the synthesis and processing of viral proteins which could afftect the endogenous protein clearance mechanisms (Chan et al., 2006). The impairment of proteostasis in the brains of subjects with COVID-19 could interfere with the clearance of α-Synuclein, thereby resulting in its abnormal aggregation (Pavel et al., 2020). A recently published neuroimaging study by Mikhal E Cohen and colleagues reported that a SARS-CoV-2 positive case diagnosed with olfactory defects developed sporadic Parkinsonism (Cohen et al., 2020). Another case report by Ingrid Faber and group reported that a neurologically normal COVID-19 positive survivor developed akinetic‐rigid parkinsonism due to defects in dopamine transport in the brain (Faber et al., 2020). Further, a dopamine transporter single-photon emission computed tomography (DAT-SPECT) based study by Méndez-Guerrero, A. et al. reported that a 58-year-old SARS-CoV-2 positive male patient showed hypokinetic rigid syndrome and bilateral decrease in presynaptic dopamine uptake in the brain (Méndez-Guerrero et al., 2020). Thus, it suggests that pathogenic events of COVID-19 are likely to induce a sporadic form of PD. In the physiological state, dopaminergic neurons in OB plays a prominent role in odour detection and discrimination (Wilson and Sullivan, 1995). For the routine funtion of OB in the normal brain, dopaminergic neurons need to be replenished through the NSC-derived ongoing adult neurogenesis (Morrison, 2016). Considering the fact, it can be expected that the impairment in neuronal replacement of dopaminergic neurons in OB may be responsible for the loss of smell in PD and COVID-19. Therefore, the dysregulation of the synthesis of dopamine, degeneration of dopaminergic neurons and impaired neuroregenerative potential of NSCs in the olfactory system resulting from SARS-CoV-2 infection may collectively contribute to the risk of developing the sporadic form of PD in COVID-19 patients and survivors.
4. The olfactory system and its cellular components and a revisit on neurogenesis in the olfactory bulb in adult humans
The olfactory system plays an important role in communicating the chemosensory input to the brain (Pinto, 2011; Sharma et al., 2019). In vertebrates, the olfactory system is comprised of the main olfactory system (MOS) and the accessory olfactory system (AOS) (Taniguchi et al., 2011). The MOS contains OE in the nasal cavity responsible for recognizing volatile compounds, while the AOS has the vomeronasal organ through which the non-volatile pheromones are identified (Mucignat-Caretta, 2010; Suárez et al., 2012; Vargas-Barroso et al., 2017). OE is composed of olfactory sensory neurons (OSNs), multipotent basal neuronal stem cells such as globose basal cells (GBCs) and horizontal basal cells (HBCs) (Carter et al., 2004). In addition, OE contains supporting cell populations like microvillar cells, sustentacular cells, and bowman’s gland lining cells (Choi and Goldstein, 2018; Purves et al., 2001; Schwob et al., 2017). The GBCs are mitotically active cells that give rise to sustentacular cells and sensory neurons in OE (Carter et al., 2004; Goldstein and Schwob, 1996; Leung et al., 2007). While HBCs generally remain quiescent in the physiological state, they get activated upon injury and microbial infection (Carter et al., 2004; Iwai et al., 2008; Joiner et al., 2015). In the nasal cavity, the olfactory receptor (OR) genes are expressed mainly in the sensory neurons of OE (Dang et al., 2018; Mombaerts, 2004; Niimura and Nei, 2006). ORs belong to G Protein-Coupled Receptor (GPCR) superfamily and are classified into two different groups, namely class I and class II, based on the phylogenetic difference in mammals (Fleischer et al., 2009; Spehr and Munger, 2009). While class I receptors mediate the signaling of water-soluble odorants, class II receptors are responsible for the perception of aerial odorants (Mezler et al., 2001; Spehr and Munger, 2009). In mammals, the majority of ORs are classified as class II receptors, and class I receptors are expressed to a lesser extent (Bozza et al., 2009; Ferrer et al., 2016). The odorant molecule dissolves in the mucus layer of OE and activates the ORNs (Izquierdo-Dominguez et al., 2020; Nagashima and Touhara, 2010). The bipolar ORNs extend axons through the cribriform plate and make the synaptic connection with the dendrites of neurons in the glomeruli of OB (Beites et al., 2009; Choi and Goldstein, 2018). In addition to OE, the other two regions of MOS include the Grüneberg ganglion (GG) and the septal organ (SO) which send projections to OB (Suárez et al., 2012). In turn, OB transfers the information to other brain regions through the olfactory cortex (Gire et al., 2013; Shepherd, 2010).
OB is a distinct projected tissue structure present in the forebrain of vertebrates that receive axonal input from OR expressing chemosensory neurons of OE and transfer the information to other brain regions including the cortex, amygdala, hypothalamus and hippocampus (Gire et al., 2013; Miyasaka et al., 2009; Price et al., 1991). In rodents and other mammals, OB is larger and located at the anterior portion of the brain (McGann, 2017). In humans and primates, OB is relatively small and located below the frontal lobe of the brain (McGann, 2017; Semendeferi et al., 1997). OB consists of multiple layers that include the olfactory nerve layer (ONL), glomerular layer (GML), external plexiform layer (EPL), mitral cell layer (MCL), internal plexiform layer (IPL) and granule cell layer (GCL) (Nagayama et al., 2014). The synaptic organization in different layers of OB is evident in rodent brains, whereas in humans, the circumferential organization of layers and medial-lateral symmetry are not well defined (Maresh et al., 2008). There are two types of neurons that project via the olfactory tract to higher areas of cortical centers, namely mitral cells located in MCL and tufted cells in EPL (Harvey and Heinbockel, 2018; Nagayama et al., 2014). The axons of ORNs synapse with the dendrites of mitral and tufted cells in OB (Kim et al., 2020; Nezlin et al., 2003; Vassar et al., 1994). The mitral cells project their axons from OB to other regions of the brain (Igarashi et al., 2012; Nagayama et al., 2010). The OB also contains dopaminergic neurons in the GML and periglomerular area which plays a prominent role in the detection, discrimination as well as modulation of odour (Paß et al., 2020; Pignatelli and Belluzzi, 2017). Dopaminergic neurons in OB appear to modulate the activity of olfactory sensory fibers, mitral cells and tufted cells together with with granule neurons of GCL (Berkowicz and Trombley, 2000; Cave and Baker, 2009; Pignatelli and Belluzzi, 2017). Notably, the granule neurons of GCL and dopaminergic neurons of GML in OB are constantly generated through the cellular regenerative process known as adult neurogenesis (Kandasamy et al., 2015; Lazarini et al., 2014).
In most vertebrates, neurogenesis in the olfactory system is crucial for daily life activities including food-seeking, pathfinding, social interactions and sexual behaviours (Feierstein, 2012). In OE, locally residing NSCs and progenitor cells such as HBCs and GBCs represent the cellular regenerative resource for the continual generation of new ORNs throughout adulthood (Brann and Firestein, 2014). Besides, rodent studies have established that NSCs in the subventricular zone (SVZ) generates immature neurons called neuroblasts which migrate through the rostral migratory stream (RMS) to OB where they differentiate and functionally integrate as gamma aminobutyric acid (GABA) ergic and dopaminergic interneurons (Kandasamy et al., 2015; Ming and Song, 2011; Winner et al., 2006). The occurrence of adult neurogenesis in OB responsible for olfaction has also been reported in many other mammalian species including primates (Kornack and Rakic, 2001). Unlike in rodents, an extra migratory stream containing immature neurons towards the ventromedial prefrontal cortex has been reported in forebrains of human infants (Sanai et al., 2011). Considering the fact, local progenitor cells residing in the human olfactory system has been speculated to be responsible for adult neurogenesis (Pagano et al., 2000). However, the occurrence of adult neurogenesis in the forebrain of humans has been a controversial subject (Berger et al., 2020; Sanai et al., 2011). There exists supportive evidence that neurogenesis in the SVZ takes place in the adult human brains but it is less active compared to the brains of adult rodents (Parolisi et al., 2018). The rate of turnover of new neurons in the human brain appears to be relatively slow (Bergmann et al., 2012; Spalding et al., 2013). A previous carbon dating analysis of the neuronal DNA indicated that the occurrence of neurogenesis is highly limited in the adult human OB (Ernst and Frisén, 2015; McGann, 2017). However, subsequent studies revealed strong evidence for the existence of ongoing neurogenesis in the human olfactory system in adulthood (Brann and Firestein, 2014; Curtis et al., 2012; Liu and Martin, 2003; Whitman and Greer, 2009). An immunofluorescence labelling study by Bédard and Parent, reported the expression of well-known markers of neurogenesis such as Doublecortin (DCX), NeuroD, polysialylated-neural cell adhesion molecule (PSA-NCAM) and TuJ1 in OB of the human brains (Bédard and Parent, 2004). Using a transcriptome analysis, Lötsch et al. validated the existence of neurogenic markers in OB of the adult human brain (Lötsch et al., 2014). Besides, it has been testified that transcriptional profiles of the basal progenitor populations of HBCs and GBCs present in the adult human OE are similar to that of rodent brains (Schwob et al., 2017). A recent single-cell RNA sequencing and immunohistochemical analysis by Durante et al. demonstrated the presence of NSCs and immature neurons in the human OB (Durante et al., 2020). Even though the morphology and cellular marker expression patterns of human and rodent OB may differ to some extent, both human OB and OE harbour NSCs, and immatureand mature newborn neurons (Lledo and Valley, 2016; Song et al., 2016; Whitman and Greer, 2009). Moreover, neurospheres derived from human olfactory mucosa biopsies appeared to have self-renewing capacity and differentiated into neurons and glial cells (Murrell et al., 2005). Taken together, the presence of NSCs and the occurrence of neurogenesis in the olfactory system of the human brain are increasingly evident.
The ongoing adult neurogenesis in the forebrain has been linked to the maintenance and rewiring of the neural circuit in the olfactory system (Pignatelli and Belluzzi, 2010). Notably, adult neurogenesis in OB is important for odour-based learning, odour discrimination, and mating behaviours (Lledo and Valley, 2016; Pignatelli and Belluzzi, 2010). Moreover, a number of neurodegenerative diseases including PD have been characterized by impaired neurogenesis in OB which is accountable for deficits in olfactory neuroplasticity, leading to anosmia (Höglinger et al., 2004; Marxreiter et al., 2013; Winner et al., 2006; Winner and Winkler, 2015). Besides, many viruses including rhinovirus, Epstein bar virus, and coronaviruses are known to induce dysfunction of OB due to inflammation (Bonzano et al., 2020; Lechien et al., 2020; Suzuki et al., 2007; van Riel et al., 2015). While neuroinflammation has been known to suppress the proliferation of NSCs and adult neurogenesis (Fan and Pang, 2017; Kandasamy et al., 2020, 2015, 2011, 2010), the possibilities of SARS-CoV-2 infection-mediated defect in neurogenesis responsible for anosmia in the olfactory system may not be excluded.
5. Impaired neurogenesis in the olfactory system as a potential cause of anosmia in COVID-19 and PD
In general, upper respiratory tract infection has been known to be associated with OB dysfunction (Welge-Lüssen and Wolfensberger, 2006). However, the occurrence of anosmia resulting from OB pathology has been reported in the majority of COVID-19 cases regardless of the onset of respiratory dysfunctions (Whitcroft and Hummel, 2020). Thus, the World Health Organization (WHO) has recognized anosmia as a distinct early symptom of SARS-CoV-2 infection. Similarly, anosmia has been well recognized as a pre-clinical symptom of PD (Fullard et al., 2017). To note, the sensory neurons that express the odorant receptors responsible for the sense of smell are continuously replenished throughout life unlike the other nerve cells in mammalian brains (Brann and Firestein, 2014). In the olfactory system, these sensory neurons of OE project excitatory synapses with inhibitory interneurons in GCL of OB through the generation of axonal binding in GML(Imamura et al., 2020; Lledo et al., 2008). Notably, ORNs in OE are known to be positive for the markers of immature neurons such as DCX during their generation in adulthood (Saaltink et al., 2012). While the inhibitory interneurons play a major role in the synaptic input of sensory neurons (Arnson and Strowbridge, 2017; Lledo et al., 2004), adult neurogenesis contributes to the generation of GABAergic inhibitory interneurons in GCL of OB (Lledo and Valley, 2016; Pallotto and Deprez, 2014). Moreover, the axonal processing of sensory neurons in glomeruli appears to be important for the olfactory discrimination (Malnic et al., 2010), in which, continual generation ofdopaminergic neurons from NSCs and neural progenitors appears to be highly important (Kandasamy et al., 2015; Lazarini et al., 2014; Pignatelli et al., 2009) Thus, the regulation of olfactory neurogenesis provides a cellular mechanism for the sense of smell in physiological conditions (Gheusi and Lledo, 2014).
To note, reduction of dopamine in the adult brain appears to alter the number of mitotic cells in the forebrain of PD patients and the animal models of PD (Höglinger et al., 2004; Winner et al., 2006). O'Sullivan et al. reported the negative correlation between disease duration and the number of Musashi-positive NSCs of SVZ in the postmortem brain samples of non-dementia PD victims (O’Sullivan et al., 2011). Moreover, Ziabreva I et al. have also validated the reduced number of Musashi-positive NSCs and progenitor cells of SVZ in the postmortem brain with Lewy body pathology (Ziabreva et al., 2007). Moreover, many experimental models of PD have been characterized by impaired neurogenesis in OB (May et al., 2012; Winner and Winkler, 2015). Notably, neuroinflammation appears to impair adult neurogenesis in various neurodegenerative conditions including PD (Kandasamy et al., 2020, 2011; Taupin, 2008). While reduction of dopamine synthesis has been known to cease the neurogenic capacity of the adult brain, blockade of neuroinflammation appears to enhance adult neurogenesis, thereby compensating the replenishment of the dopaminergic neurons in OB and restores olfactory sensory processing in PD (Lazarini et al., 2014).
Previously, the extent of epithelial destruction and load of SARS-CoV-2 in OB has been correlated to the varying severity of olfactory dysfuntion responsible for anosmia (Akerlund et al., 1995; Imam et al., 2020; Yamagishi et al., 1988). Recently, it has been speculated that the loss of smell noticed in COVID-19 arises because of defects in the supporting cells of OE and vascular pericytes of OB, that could induce a change in the function of ORNs (Butowt and von Bartheld, 2020; Solomon, 2021; Vaira et al., 2020). A case study testified that obstruction in the olfactory cleft caused by inflammation may be a possible reason for anosmia in COVID-19 (Eliezer et al., 2020), while RNA sequencing and immunostaining studies revealed that ORNs and OE are positive for ACE2 and TMPRSS2 (Bilinska et al., 2020; Brann et al., 2020). However, Naeini AS et al. did not find any significant mucosal changes or olfactory cleft abnormality in the CT imaging in COVID-19 patients with anosmia and thereby highlighted further experiments are required to elucidate the aetiology of olfactory loss in COVID-19 (Naeini et al., 2020). Gane SB et al. hypothesized that anosmia in COVID-19 patients could occur due to the destruction of the ORNs in OE (Gane et al., 2020). The whole RNA sequencing data of olfactory mucosa in humans showed that the expression of SARS-CoV-2 entry related genes are prominent in most neuronal cells of the olfactory system including NSCs and progenitors (Brann et al., 2020). Considering the aforementioned facts, the regenerative aspect of the human olfactory system responsible for the production of new neurons that involves the sense of smell needs to be revisited in COVID-19 conditions. Notably, many neurological deficits noticed in COVID-19 have been related to neuroinflammation due to the prominent cytokine storm (Kandasamy, 2021). Thus, neuroinflammation has been recognized as a key determinant for the abnormalities notcied in the dopaminergic system in substantia nigra and OB responsible for anosmia in PD (Höglinger et al., 2004; Taupin, 2008). The proliferative and differentiation potentials of NSCs are known to be drastically impaired by neuroinflammation in PD. Therefore, it can be hypothesized that the pathogenesis of COVID-19 associated neuroinflammation could be an underlying basis for the defect in the dopaminergic system leading to the development of sporadic PD. Eventually, the pathogenic events of PD and COVID-19 might be in part overlapped by impaired NSC-mediated neurogenesis in OB and OE leading to anosmia.
6. Conclusion
The human OE is highly sensitive to environmental changes and susceptible to the chemosensory deprivation and inflammation. OB plays an intermediate role between OE and higher brain areas in processing the odour information. The regulation of NSC-mediated neurogenesis in OB and OE is important for olfaction. While the regulation of neurogenesis in OB and OE is negatively influenced by neuroinflammation, olfactory impairments resulting from neuroinflammation represent a pathogenic interlink between COVID-19 and PD. Considering the fact, SARS-CoV-2 infection in the nasal route may be associated with a reduction in the NSC population of GBCs and HBCs which are involved in renewing the ORNs in OE. Besides, the SARS-CoV-2-mediated neuroinflammation in OB would also be associated with the impairment of neurogenesis at the level of defects in proliferation and dopaminergic differentiation of NSCs in the proximity of GML similar to the pathogenesis seen in PD. Thus, failure in the replenishment of degenerating ORNs in OE and dopaminergic neurons in OB in subjects with COVID-19 and PD might be the underlying basis of anosmia. To defend and overcome this obstacle, the delivery of proneurogenic drugs and therapeutic measures against dopamine alterations needs to be considered to manage COVID-19. Also, further experiments are indispensable to analyze neurogenesis and the overlapping mechanisms with other neurological diseases in the brains of COVID-19 patients.
Author contributions
MK conceived the present idea, hypothesis and generated the illustration. HSR, SR and RKR further developed the hypothesis and performed the literature search and made the initial draft. HSR, SR, RKR and MK contributed to the revision of article, made critical comments and suggestions. All authors discussed the content and contributed to the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
M.K. has been supported by the Faculty Recharge Programme, University Grants Commission (UGC-FRP), New Delhi, India. M.K. received a research grant (SERB-EEQ/2016/000639) and an Early Career Research Award (SERB-ECR/2016/000741) from the Science and Engineering Research Board (SERB), government of India. The authors acknowledge, RUSA (Rashtriya Uchchatar Shiksha Abhiyan) 2, Biological Sciences, Bharathidasan University for the financial support, UGC-SAP, DST-FIST for the infrastructure of the Department of Animal Science, Bharathidasan University. R.K.R. was supported as JRF from the project grant-ECR/2016/000741, SERB.
References
- Akerlund A., Bende M., Murphy C. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol. 1995;115:88–92. doi: 10.3109/00016489509133353. [DOI] [PubMed] [Google Scholar]
- Alexander G.E. Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004;6:259–280. doi: 10.31887/DCNS.2004.6.3/galexander. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I., Alharbi O.M.L. COVID-19: disease, management, treatment, and social impact. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnson H.A., Strowbridge B.W. Spatial structure of synchronized inhibition in the olfactory bulb. J. Neurosci. 2017;37:10468–10480. doi: 10.1523/JNEUROSCI.1004-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Goyal H., Haghbin H., Lee-Smith W.M., Gajendran M., Perisetti A. The association of “Loss of smell” to COVID-19: a systematic review and meta-analysis. Am. J. Med. Sci. 2021;361:216–225. doi: 10.1016/j.amjms.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrino R., Schapira A.H.V. Parkinson disease. Eur. J. Neurol. 2020;27:27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- Barresi M., Ciurleo R., Giacoppo S., Foti Cuzzola V., Celi D., Bramanti P., Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012;323:16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Bédard A., Parent A. Evidence of newly generated neurons in the human olfactory bulb. Dev. Brain Res. 2004;151:159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Beghi E., Feigin V., Caso V., Santalucia P., Logroscino G. COVID-19 infection and neurological complications: present findings and future predictions. Neuroepidemiology. 2020:1–6. doi: 10.1159/000508991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites C.L., Kawauchi S., Calof A.L. Olfactory neuron patterning and specification. Dev. Neurobiol. 2009;7:145–156. doi: 10.1016/b978-008045046-9.01046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Corbellini Á., Chico-García J.L., Martínez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J., Gómez-López A., Monreal E., Parra-Díaz P., Cortés-Cuevas J.L., Galán J.C., Fragola-Arnau C., Porta-Etessam J., Masjuan J., Alonso-Cánovas A. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur. J. Neurol. 2020;27:1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénézit F., Le Turnier P., Declerck C., Paillé C., Revest M., Dubée V., Tattevin P. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020;20:1014–1015. doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T., Lee H., Thuret S. Neurogenesis right under your nose. Nat. Neurosci. 2020;23:297–298. doi: 10.1038/s41593-020-0596-8. [DOI] [PubMed] [Google Scholar]
- Bergmann O., Liebl J., Bernard S., Alkass K., Yeung M.S.Y., Steier P., Kutschera W., Johnson L., Landén M., Druid H., Spalding K.L., Frisén J. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Berkowicz D.A., Trombley P.Q. Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 2000;855:90–99. doi: 10.1016/s0006-8993(99)02342-2. [DOI] [PubMed] [Google Scholar]
- Beyer K., Domingo-Sàbat M., Ariza A. Molecular pathology of Lewy body diseases. Int. J. Mol. Sci. 2009;10:724–745. doi: 10.3390/ijms10030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A.S., Joshi S.V., Naik S., Sangle S., Abraham N.M. Quantitative assessment of olfactory dysfunction accurately detects asymptomatic COVID-19 carriers. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesveldt S., Postma E.M., Boak D., Welge-Luessen A., Schöpf V., Mainland J.D., Martens J., Ngai J., Duffy V.B. Anosmia—a clinical review. Chem. Senses. 2017;42:513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano C., Borroni D., Lancia A., Bonzano E. Doxycycline: From Ocular Rosacea to COVID-19 Anosmia. New Insight Into the Coronavirus Outbreak. Front. Med. (Lausanne) 2020:7. doi: 10.3389/fmed.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T., Vassalli A., Fuss S., Zhang J.-J., Weiland B., Pacifico R., Feinstein P., Mombaerts P. Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron. 2009;61:220–233. doi: 10.1016/j.neuron.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann J.H., Firestein S.J. A lifetime of neurogenesis in the olfactory system. Front. Neurosci. 2014:8. doi: 10.3389/fnins.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.-J., Fletcher R.B., Das D., Street K., de Bezieux H.R., Choi Y.-G., Risso D., Dudoit S., Purdom E., Mill J., Hachem R.A., Matsunami H., Logan D.W., Goldstein B.J., Grubb M.S., Ngai J., Datta S.R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020:6. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P., Nath A., Beckham J.D. Is COVID-19 a Perfect Storm for Parkinson’s Disease? Trends Neurosci. 2020;43:931–933. doi: 10.1016/j.tins.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., von Bartheld C.S. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2020 doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.A., MacDonald J.L., Roskams A.J. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J. Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave J.W., Baker H. Dopamine systems in the forebrain. Adv. Exp. Med. Biol. 2009;651:15–35. doi: 10.1007/978-1-4419-0322-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.-P., Siu K.-L., Chin K.-T., Yuen K.-Y., Zheng B., Jin D.-Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80:9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu A., Fischbein N., Wintermark M., Zaharchuk G., Yun P.T., Zeineh M. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2020:1–2. doi: 10.1007/s00234-020-02554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi R., Goldstein B.J. Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018;3:35–42. doi: 10.1002/lio2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.E., Eichel R., Steiner-Birmanns B., Janah A., Ioshpa M., Bar-Shalom R., Paul J.J., Gaber H., Skrahina V., Bornstein N.M., Yahalom G. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19:804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.A., Low V.F., Faull R.L.M. Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Dev. Neurobiol. 2012;72:990–1005. doi: 10.1002/dneu.22028. [DOI] [PubMed] [Google Scholar]
- Dang P., Fisher S.A., Stefanik D.J., Kim J., Raper J.A. Coordination of olfactory receptor choice with guidance receptor expression and function in olfactory sensory neurons. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaagd G., Philip A. 2015. Parkinson’s Disease and Its Management; pp. 504–532. P T 40. [PMC free article] [PubMed] [Google Scholar]
- DosSantos M.F., Devalle S., Aran V., Capra D., Roque N.R., Coelho-Aguiar J., de M., Spohr T.C.Lde Se, Subilhaga J.G., Pereira C.M., D’Andrea Meira I., Niemeyer Soares Filho P., Moura-Neto V. Neuromechanisms of SARS-CoV-2: a review. Front. Neuroanat. 2020:14. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R.L. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 2012;46:527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R.L. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16:478–488. doi: 10.1016/S1474-4422(17)30123-0. [DOI] [PubMed] [Google Scholar]
- Durante M.A., Kurtenbach Stefan, Sargi Z.B., Harbour J.W., Choi R., Kurtenbach Sarah, Goss G.M., Matsunami H., Goldstein B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020;23:323–326. doi: 10.1038/s41593-020-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliezer M., Hautefort C., Hamel A.-L., Verillaud B., Herman P., Houdart E., Eloit C. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020;146:674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- Ernst A., Frisén J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015:13. doi: 10.1371/journal.pbio.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber I., Brandão P.R.P., Menegatti F., de Carvalho Bispo D.D., Maluf F.B., Cardoso F. Coronavirus disease 2019 and parkinsonism: a non-post-encephalitic case. Mov. Disord. 2020;35:1721–1722. doi: 10.1002/mds.28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L.-W., Pang Y. Dysregulation of neurogenesis by neuroinflammation: key differences in neurodevelopmental and neurological disorders. Neural Regen. Res. 2017;12:366–371. doi: 10.4103/1673-5374.202926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein C.E. Linking adult olfactory neurogenesis to social behavior. Front. Neurosci. 2012;6:173. doi: 10.3389/fnins.2012.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferini-Strambi L., Salsone M. COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? J. Neurol. 2020:1–11. doi: 10.1007/s00415-020-10070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Garcia-Esparcia P., Carmona M., Carro E., Aronica E., Kovacs G.G., Grison A., Gustincich S. Olfactory receptors in non-chemosensory organs: the nervous system in health and disease. Front. Aging Neurosci. 2016:8. doi: 10.3389/fnagi.2016.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P., Gass J., Swoveland P., Lavi E., Highkin M., Weiss Infection of the basal ganglia by a murine coronavirus. Science. 1985;229:877–879. doi: 10.1126/science.2992088. [DOI] [PubMed] [Google Scholar]
- Fleischer J., Breer H., Strotmann J. Mammalian olfactory receptors. Front. Cell. Neurosci. 2009:3. doi: 10.3389/neuro.03.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard M.E., Morley J.F., Duda J.E. Olfactory dysfunction as an early biomarker in parkinson’s disease. Neurosci. Bull. 2017;33:515–525. doi: 10.1007/s12264-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galougahi M.K., Ghorbani J., Bakhshayeshkaram M., Naeini A.S., Haseli S. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-Induced anosmia: the first report. Acad. Radiol. 2020;27:892–893. doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58:299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- Gengler I., Wang J.C., Speth M.M., Sedaghat A.R. Sinonasal pathophysiology of SARS‐CoV‐2 and COVID‐19: a systematic review of the current evidence. Laryngoscope Investig. Otolaryngol. 2020;5:354. doi: 10.1002/lio2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G., Lledo P.-M. Adult neurogenesis in the olfactory system shapes odor memory and perception. Prog. Brain Res. 2014;208:157–175. doi: 10.1016/B978-0-444-63350-7.00006-1. [DOI] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire D.H., Restrepo D., Sejnowski T.J., Greer C., De Carlos J.A., Lopez-Mascaraque L. Temporal Processing in the Olfactory System: Can We See a Smell? Neuron. 2013;78:416–432. doi: 10.1016/j.neuron.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B.J., Schwob J.E. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J. Neurosci. 1996;16:4005–4016. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori A., Leone F., Loffredo L., Cinicola B.L., Brindisi G., De Castro G., Spalice A., Duse M., Zicari A.M. COVID-19-Related anosmia: the olfactory pathway hypothesis and early intervention. Front. Neurol. 2020:11. doi: 10.3389/fneur.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland J.C., Barker R.A. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects. Stoker T.B., Greenland J.C., editors. Codon Publications; Brisbane (AU): 2018. The differential diagnosis of Parkinson’s disease. [Google Scholar]
- Haehner A., Hummel T., Reichmann H. Olfactory loss in parkinson’s disease. Parkinsons Dis. 2011;2011 doi: 10.4061/2011/450939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Zheng W., Wang X., Chen Z. Proteostasis of α-Synuclein and its role in the pathogenesis of parkinson’s disease. Front. Cell. Neurosci. 2020:14. doi: 10.3389/fncel.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J.D., Heinbockel T. Neuromodulation of synaptic transmission in the main olfactory bulb. Int. J. Environ. Res. Public Health. 2018:15. doi: 10.3390/ijerph15102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Mov. Disord. 2003;18:364–372. doi: 10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- Höglinger G.U., Rizk P., Muriel M.P., Duyckaerts C., Oertel W.H., Caille I., Hirsch E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K.M., Ieki N., An M., Yamaguchi Y., Nagayama S., Kobayakawa K., Kobayakawa R., Tanifuji M., Sakano H., Chen W.R., Mori K. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 2012;32:7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S.A., Lao W.P., Reddy P., Nguyen S.A., Schlosser R.J. Is SARS-CoV-2 (COVID-19) postviral olfactory dysfunction (PVOD) different from other PVOD? World J. Otorhinolaryngol. Head Neck Surg. 2020;6:S26–S32. doi: 10.1016/j.wjorl.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F., Ito A., LaFever B.J. Subpopulations of projection neurons in the olfactory bulb. Front. Neural Circuits. 2020:14. doi: 10.3389/fncir.2020.561822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai N., Zhou Z., Roop D.R., Behringer R.R. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Dominguez A., Rojas-Lechuga M., Mullol J., Alobid I. Olfactory dysfunction in the COVID-19 outbreak. J. Investig. Allergol. Clin. Immunol. 2020:30. doi: 10.18176/jiaci.0567. [DOI] [PubMed] [Google Scholar]
- Jain A., Kaur J., Rai A.K., Pandey A.K. Anosmia: a clinical Indicator of COVID-19 reinfection. Ear Nose Throat J. 2020 doi: 10.1177/0145561320978169. 145561320978169. [DOI] [PubMed] [Google Scholar]
- Jang H., Boltz D.A., Webster R.G., Smeyne R.J. Viral parkinsonism. Biochim. Biophys. Acta. 2009;1792:714–721. doi: 10.1016/j.bbadis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H., Boltz D., Sturm-Ramirez K., Shepherd K.R., Jiao Y., Webster R., Smeyne R.J. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. U S A. 2009;106(Aug. (33)):14063–14068. doi: 10.1073/pnas.0900096106. Epub 2009 Aug 10. PMID: 19667183; PMCID: PMC2729020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner A.M., Green W.W., McIntyre J.C., Allen B.L., Schwob J.E., Martens J.R. Primary cilia on horizontal basal cells regulate regeneration of the olfactory epithelium. J. Neurosci. 2015;35:13761–13772. doi: 10.1523/JNEUROSCI.1708-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M. Perspectives for the use of therapeutic Botulinum toxin as a multifaceted candidate drug to attenuate COVID-19. Med Drug Discov. 2020;6 doi: 10.1016/j.medidd.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2021;394:561–567. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M., Couillard-Despres S., Raber K.A., Stephan M., Lehner B., Winner B., Kohl Z., Rivera F.J., Nguyen H.P., Riess O., Bogdahn U., Winkler J., von Hörsten S., Aigner L. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J. Neuropathol. Exp. Neurol. 2010;69:717–728. doi: 10.1097/NEN.0b013e3181e4f733. [DOI] [PubMed] [Google Scholar]
- Kandasamy M., Reilmann R., Winkler J., Bogdahn U., Aigner L. Transforming growth factor-beta signaling in the neural stem cell niche: a therapeutic target for huntington’s disease. Neurol. Res. Int. 2011;2011 doi: 10.1155/2011/124256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M., Rosskopf M., Wagner K., Klein B., Couillard-Despres S., Reitsamer H.A., Stephan M., Nguyen H.P., Riess O., Bogdahn U., Winkler J., Hörsten Svon, Aigner L. Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of huntington’s disease is accompanied by striatal invasion of neuroblasts. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M., Anusuyadevi M., Aigner K.M., Unger M.S., Kniewallner K.M., de Sousa D.M.B., Altendorfer B., Mrowetz H., Bogdahn U., Aigner L. TGF-β signaling: a therapeutic target to reinstate regenerative plasticity in vascular dementia? Aging Dis. 2020;11:828–850. doi: 10.14336/AD.2020.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2021;28:28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalefah M.M., Khalifah A.M. Determining the relationship between SARS-CoV-2 infection, dopamine, and COVID-19 complications. J Taibah Univ Med Sci. 2020;15:550–553. doi: 10.1016/j.jtumed.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Choe J., Moon C. Distinct developmental features of olfactory bulb interneurons. Mol. Cells. 2020;43:215–221. doi: 10.14348/molcells.2020.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.-Y., Lepiller Q., Gendrin V., Zayet S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020;50:436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack D.R., Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouli A., Torsney K.M., Kuan W.-L. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects. Stoker T.B., Greenland J.C., editors. Codon Publications; Brisbane (AU): 2018. Parkinson’s disease: etiology, neuropathology, and pathogenesis. [PubMed] [Google Scholar]
- Krishna Murthy P., Sivashanmugam K., Kandasamy M., Subbiah R., Ravikumar V. Repurposing of histone deacetylase inhibitors: a promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci. 2021;266 doi: 10.1016/j.lfs.2020.118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F., Gabellec M.-M., Moigneu C., de Chaumont F., Olivo-Marin J.-C., Lledo P.-M. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J. Neurosci. 2014;34:14430–14442. doi: 10.1523/JNEUROSCI.5366-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen Š., Sonninen T.-M., Wojciechowski S., Goldsteins G., Koistinaho J. Dysfunction of cellular proteostasis in parkinson’s disease. Front. Neurosci. 2019:13. doi: 10.3389/fnins.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.T., Coulombe P.A., Reed R.R. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Li J., Long X., Zhu C., Wang H., Wang T., Lin Z., Li Jinghong, Xiong N. Olfactory dysfunction in recovered coronavirus disease 2019 (COVID-19) patients. Mov. Disord. 2020;35:1100–1101. doi: 10.1002/mds.28172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.-S., Chan L.-L., Chao Y.-X., Tan E.-K. Parkinson’s disease following COVID-19: causal link or chance occurrence? J. Transl. Med. 2020;18:493. doi: 10.1186/s12967-020-02670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limphaibool N., Iwanowski P., Holstad M.J.V., Kobylarek D., Kozubski W. Infectious etiologies of parkinsonism: pathomechanisms and clinical implications. Front. Neurol. 2019:10. doi: 10.3389/fneur.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Martin L.J. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. J. Comp. Neurol. 2003;459:368–391. doi: 10.1002/cne.10664. [DOI] [PubMed] [Google Scholar]
- Lledo P.-M., Valley M. Adult olfactory bulb neurogenesis. Cold Spring Harb. Perspect. Biol. 2016:8. doi: 10.1101/cshperspect.a018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P.-M., Saghatelyan A., Lemasson M. Inhibitory interneurons in the olfactory bulb: from development to function. Neuroscientist. 2004;10:292–303. doi: 10.1177/1073858404263460. [DOI] [PubMed] [Google Scholar]
- Lledo P.-M., Merkle F.T., Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loseva E., Yuan T.-F., Karnup S. Neurogliogenesis in the mature olfactory system: a possible protective role against infection and toxic dust. Brain Res. Rev. 2009;59:374–387. doi: 10.1016/j.brainresrev.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J., Schaeffeler E., Mittelbronn M., Winter S., Gudziol V., Schwarzacher S.W., Hummel T., Doehring A., Schwab M., Ultsch A. Functional genomics suggest neurogenesis in the adult human olfactory bulb. Brain Struct. Funct. 2014;219:1991–2000. doi: 10.1007/s00429-013-0618-3. [DOI] [PubMed] [Google Scholar]
- Luers J.C., Rokohl A.C., Loreck N., Wawer Matos P.A., Augustin M., Dewald F., Klein F., Lehmann C., Heindl L.M. Olfactory and gustatory dysfunction in coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2262–2264. doi: 10.1093/cid/ciaa525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalaxmi I., Kaavya J., Devi S.M., Balachandar V. COVID-19 and olfactory dysfunction: a possible associative approach towards neurodegenerative diseases. J. Cell. Physiol. 2021;236:763–770. doi: 10.1002/jcp.29937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B., Gonzalez-Kristeller D.C., Gutiyama L.M. In: The Neurobiology of Olfaction, Frontiers in Neuroscience. Menini A., editor. CRC Press/Taylor; Francis, Boca Raton (FL): 2010. Odorant receptors. [Google Scholar]
- Mangal V., Murari T., Vashisht R., Iqbal S.M., Meghana K., Gujrathi S., Ambade V., Tilak T., Aggarwal V., Manrai M., Verma V., Srinath R., Goel N., Yadav N.K., Menon A. Olfactory dysfunction among asymptomatic patients with SARS CoV2 infection: a case-control study. Indian J. Otolaryngol. Head Neck Surg. 2021:1–6. doi: 10.1007/s12070-021-02366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresh A., Rodriguez Gil D., Whitman M.C., Greer C.A. Principles of glomerular organization in the human olfactory bulb – implications for odor processing. PLoS One. 2008;3:e2640. doi: 10.1371/journal.pone.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. How COVID-19 can damage the brain. Nature. 2020;585:342–343. doi: 10.1038/d41586-020-02599-5. [DOI] [PubMed] [Google Scholar]
- Marxreiter F., Regensburger M., Winkler J. Adult neurogenesis in Parkinson’s disease. Cell. Mol. Life Sci. 2013;70:459–473. doi: 10.1007/s00018-012-1062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V.E.L., Nuber S., Marxreiter F., Riess O., Winner B., Winkler J. Impaired olfactory bulb neurogenesis depends on the presence of human wild-type alpha-synuclein. Neuroscience. 2012;222:343–355. doi: 10.1016/j.neuroscience.2012.07.001. [DOI] [PubMed] [Google Scholar]
- McGann J.P. Poor human olfaction is a nineteenth century myth. Science. 2017:356. doi: 10.1126/science.aam7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Guerrero A., Laespada-García M.I., Gómez-Grande A., Ruiz-Ortiz M., Blanco-Palmero V.A., Azcarate-Diaz F.J., Rábano-Suárez P., Álvarez-Torres E., de Fuenmayor-Fernández de la Hoz C.P., Vega Pérez D., Rodríguez-Montalbán R., Pérez-Rivilla A., Sayas Catalán J., Ramos-González A., González de la Aleja J. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–e2118. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am. J. Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S., Visconti A., Hysi P., Bowyer R.C.E., Mangino M., Falchi M., Wolf J., Ourselin S., Chan A.T., Steves C.J., Spector T.D. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merello M., Bhatia K.P., Obeso J.A. SARS-CoV-2 and the risk of Parkinson’s disease: facts and fantasy. Lancet Neurol. 2021;20:94–95. doi: 10.1016/S1474-4422(20)30442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezler M., Fleischer J., Breer H. Characteristic features and ligand specificity of the two olfactory receptor classes from Xenopus laevis. J. Exp. Biol. 2001;204:2987–2997. doi: 10.1242/jeb.204.17.2987. [DOI] [PubMed] [Google Scholar]
- Ming G., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka N., Morimoto K., Tsubokawa T., Higashijima S., Okamoto H., Yoshihara Y. From the olfactory bulb to higher brain centers: genetic visualization of secondary olfactory pathways in zebrafish. J. Neurosci. 2009;29:4756–4767. doi: 10.1523/JNEUROSCI.0118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Curr. Opin. Neurobiol. 2004;14:31–36. doi: 10.1016/j.conb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Morrison B.E. Discovery of nigral dopaminergic neurogenesis in adult mice. Neural Regen. Res. 2016;11:878–881. doi: 10.4103/1673-5374.184449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucignat-Caretta C. The rodent accessory olfactory system. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2010;196:767–777. doi: 10.1007/s00359-010-0555-z. [DOI] [PubMed] [Google Scholar]
- Mullol J., Alobid I., Mariño-Sánchez F., Izquierdo-Domínguez A., Marin C., Klimek L., Wang D.-Y., Liu Z. The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr. Allergy Asthma Rep. 2020:20. doi: 10.1007/s11882-020-00961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W., Féron F., Wetzig A., Cameron N., Splatt K., Bellette B., Bianco J., Perry C., Lee G., Mackay-Sim A. Multipotent stem cells from adult olfactory mucosa. Dev. Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- Naeini A.S., Karimi-Galougahi M., Raad N., Ghorbani J., Taraghi A., Haseli S., Mehrparvar G., Bakhshayeshkaram M. Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am. J. Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima A., Touhara K. Enzymatic conversion of odorants in nasal mucus affects olfactory glomerular activation patterns and odor perception. J. Neurosci. 2010;30:16391–16398. doi: 10.1523/JNEUROSCI.2527-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S., Enerva A., Fletcher M.L., Masurkar A.V., Igarashi K.M., Mori K., Chen W.R. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front. Neural Circuits. 2010:4. doi: 10.3389/fncir.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S., Homma R., Imamura F. Neuronal organization of olfactory bulb circuits. Front. Neural Circuits. 2014:8. doi: 10.3389/fncir.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID‐19. J. Med. Virol. 2020 doi: 10.1002/jmv.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlin L.P., Heermann S., Schild D., Rössler W. Organization of glomeruli in the main olfactory bulb of Xenopus laevis tadpoles. J. Comp. Neurol. 2003;464:257–268. doi: 10.1002/cne.10709. [DOI] [PubMed] [Google Scholar]
- Niimura Y., Nei M. Evolutionary dynamics of olfactory and other chemosensory receptor genes in vertebrates. J. Hum. Genet. 2006;51:505–517. doi: 10.1007/s10038-006-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuytemans K., Theuns J., Cruts M., Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum. Mutat. 2010;31:763–780. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan S.S., Johnson M., Williams D.R., Revesz T., Holton J.L., Lees A.J., Perry E.K. The effect of drug treatment on neurogenesis in Parkinson’s disease. Mov. Disord. 2011;26:45–50. doi: 10.1002/mds.23340. [DOI] [PubMed] [Google Scholar]
- Pagano S.F., Impagnatiello F., Girelli M., Cova L., Grioni E., Onofri M., Cavallaro M., Etteri S., Vitello F., Giombini S., Solero C.L., Parati E.A. Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells. 2000;18:295–300. doi: 10.1634/stemcells.18-4-295. [DOI] [PubMed] [Google Scholar]
- Pallotto M., Deprez F. Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front. Cell. Neurosci. 2014:8. doi: 10.3389/fncel.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolisi R., Cozzi B., Bonfanti L. Humans and dolphins: decline and fall of adult neurogenesis. Front. Neurosci. 2018:12. doi: 10.3389/fnins.2018.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paß T., Aßfalg M., Tolve M., Blaess S., Rothermel M., Wiesner R.J., Ricke K.M. The impact of mitochondrial dysfunction on dopaminergic neurons in the olfactory bulb and odor detection. Mol. Neurobiol. 2020;57:3646–3657. doi: 10.1007/s12035-020-01947-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel A., Murray D.K., Stoessl A.J. COVID-19 and selective vulnerability to Parkinson’s disease. Lancet Neurol. 2020;19:719. doi: 10.1016/S1474-4422(20)30269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli A., Belluzzi O. In: The Neurobiology of Olfaction, Frontiers in Neuroscience. Menini A., editor. CRC Press/Taylor; Francis, Boca Raton (FL): 2010. Neurogenesis in the adult olfactory bulb. [Google Scholar]
- Pignatelli A., Belluzzi O. Dopaminergic neurones in the main olfactory bulb: an overview from an electrophysiological perspective. Front. Neuroanat. 2017:11. doi: 10.3389/fnana.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli A., Ackman J.B., Vigetti D., Beltrami A.P., Zucchini S., Belluzzi O. A potential reservoir of immature dopaminergic replacement neurons in the adult mammalian olfactory bulb. Pflugers Arch. 2009;457:899–915. doi: 10.1007/s00424-008-0535-0. [DOI] [PubMed] [Google Scholar]
- Pinto J.M. Olfaction. Proc. Am. Thorac. Soc. 2011;8:46–52. doi: 10.1513/pats.201005-035RN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Slotnick B.M., Revial M.F. Olfactory projections to the hypothalamus. J. Comp. Neurol. 1991;306:447–461. doi: 10.1002/cne.903060309. [DOI] [PubMed] [Google Scholar]
- Purves D., Augustine G.J., Fitzpatrick D., Katz L.C., LaMantia A.-S., McNamara J.O., Williams S.M. The olfactory epithelium and olfactory receptor neurons. Neuroscience. 2001 2nd edition. [Google Scholar]
- Ra S.H., Lim J.S., Kim G., Kim M.J., Jung J., Kim S.-H. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76:61–63. doi: 10.1136/thoraxjnl-2020-215042. [DOI] [PubMed] [Google Scholar]
- Rebholz H., Braun R.J., Ladage D., Knoll W., Kleber C., Hassel A.W. Loss of olfactory function—early Indicator for Covid-19, other viral infections and neurodegenerative disorders. Front. Neurol. 2020:11. doi: 10.3389/fneur.2020.569333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey N.L., Wesson D.W., Brundin P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018;109:226–248. doi: 10.1016/j.nbd.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Lechuga M.J., Izquierdo-Domínguez A., Chiesa-Estomba C., Calvo-Henríquez C., Villarreal I.M., Cuesta-Chasco G., Bernal-Sprekelsen M., Mullol J., Alobid I. Chemosensory dysfunction in COVID-19 out-patients. Eur. Arch. Otorhinolaryngol. 2021;278:695–702. doi: 10.1007/s00405-020-06266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaltink D.-J., Håvik B., Verissimo C.S., Lucassen P.J., Vreugdenhil E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: implications for neurogenesis. J. Comp. Neurol. 2012;520:2805–2823. doi: 10.1002/cne.23144. [DOI] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R.A., Mirzadeh Z., Tsai H.-H., Wong M., Gupta N., Berger M.S., Huang E., Garcia-Verdugo J.-M., Rowitch D.H., Alvarez-Buylla A. Corridors of migrating neurons in human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob J.E., Jang W., Holbrook E.H., Lin B., Herrick D.B., Peterson J.N., Coleman J.H. Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J. Comp. Neurol. 2017;525:1034–1054. doi: 10.1002/cne.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat A.R., Gengler I., Speth M.M. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol. Head. Neck Surg. 2020;163:12–15. doi: 10.1177/0194599820926464. [DOI] [PubMed] [Google Scholar]
- Selvaraj K., Ravichandran S., Krishnan S., Radhakrishnan R.K., Manickam N., Kandasamy M. Testicular atrophy and hypothalamic pathology in COVID-19: possibility of the incidence of male infertility and HPG Axis abnormalities. Reprod. Sci. 2021 doi: 10.1007/s43032-020-00441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K., Damasio H., Frank R., Van Hoesen G.W. The evolution of the frontal lobes: a volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. J. Hum. Evol. 1997;32:375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Sengoku R., Saito Y., Ikemura M., Hatsuta H., Sakiyama Y., Kanemaru K., Arai T., Sawabe M., Tanaka N., Mochizuki H., Inoue K., Murayama S. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J. Neuropathol. Exp. Neurol. 2008;67:1072–1083. doi: 10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- Sharma A., Kumar R., Aier I., Semwal R., Tyagi P., Varadwaj P. Sense of smell: structural, functional, mechanistic advancements and challenges in human olfactory research. Curr. Neuropharmacol. 2019;17:891–911. doi: 10.2174/1570159X17666181206095626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G.M. In: The Neurobiology of Olfaction, Frontiers in Neuroscience. Menini A., editor. CRC Press/Taylor; Francis, Boca Raton (FL): 2010. New perspectives on olfactory processing and human smell. [Google Scholar]
- Solomon T. Neurological infection with SARS-CoV-2 — the story so far. Nat. Rev. Neurol. 2021:1–2. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Olsen R.H.J., Sun J., Ming G., Song H. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb. Perspect. Biol. 2016:8. doi: 10.1101/cshperspect.a018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne J., Reddy V., Beato M.R. StatPearls Publishing; Treasure Island (FL): 2020. Neuroanatomy, Substantia Nigra, in: StatPearls. [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A., Possnert G., Mash D.C., Druid H., Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M., Munger S.D. Olfactory receptors: GPCRs and beyond. J. Neurochem. 2009;109:1570–1583. doi: 10.1111/j.1471-4159.2009.06085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth M.M., Singer-Cornelius T., Oberle M., Gengler I., Brockmeier S.J., Sedaghat A.R. Mood, Anxiety and Olfactory Dysfunction in COVID-19: Evidence of Central Nervous System Involvement? Laryngoscope. 2020;130:2520–2525. doi: 10.1002/lary.28964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C., Boscolo-Rizzo P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez R., García-González D., de Castro F. Mutual influences between the main olfactory and vomeronasal systems in development and evolution. Front. Neuroanat. 2012:6. doi: 10.3389/fnana.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Antonini A., Leta V., Nordvig A., Smeyne R.J., Goldman J.E., Al-Dalahmah O., Zecca L., Sette A., Bubacco L., Meucci O., Moro E., Harms A.S., Xu Y., Fahn S., Ray Chaudhuri K. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Parkinsons Dis. 2020:6. doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Saito K., Min W.-P., Vladau C., Toida K., Itoh H., Murakami S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Yamada T. Viral etiology for Parkinson's disease--a possible role of influenza A virus infection. Jpn. J. Infect. Dis. 1999;52(June (3)):89–98. PMID: 10507986. [PubMed] [Google Scholar]
- Taniguchi Kazumi, Saito S., Taniguchi Kazuyuki. Phylogenic outline of the olfactory system in vertebrates. J. Vet. Med. Sci. 2011;73:139–147. doi: 10.1292/jvms.10-0316. [DOI] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int. J. Med. Sci. 2008;5:127–132. doi: 10.7150/ijms.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins J., Hamilton F., Gunning S., Sheehy C., Moran E., MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J. Infect. 2020;81:e59–e61. doi: 10.1016/j.jinf.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulisiak C.T., Mercado G., Peelaerts W., Brundin L., Brundin P. Can infections trigger alpha-synucleinopathies? Prog. Mol. Biol. Transl. Sci. 2019;168:299–322. doi: 10.1016/bs.pmbts.2019.06.002. Epub 2019 Jul 3. PMID: 31699323; PMCID: PMC6857718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Fois A.G., Piombino P., Riu G.D. Potential pathogenesis of ageusia and anosmia in COVID‐19 patients. Int. Forum Allergy Rhinol. 2020;10:1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system: the olfactory nerve: a shortcut for viruses into the CNS. J. Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Vargas-Barroso V., Peña-Ortega F., Larriva-Sahd J.A. Olfaction and pheromones: uncanonical sensory influences and bulbar interactions. Front. Neuroanat. 2017:11. doi: 10.3389/fnana.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Chao S.K., Sitcheran R., Nun˜ez J.M., Vosshall L.B., Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vavougios G.D. Potentially irreversible olfactory and gustatory impairments in COVID-19: indolent vs. Fulminant SARS-CoV-2 neuroinfection. Brain Behav. Immun. 2020;87:107–108. doi: 10.1016/j.bbi.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld C.S., Hagen M.M., Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem. Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lüssen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323:2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- Whitman M.C., Greer C.A. Adult neurogenesis and the olfactory system. Prog. Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Litvan I. Parkinsonian syndromes. Continuum Minneap. Minn (Minneap Minn) 2013;19:1189–1212. doi: 10.1212/01.CON.0000436152.24038.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.A., Sullivan R.M. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J. Neurosci. 1995;15:5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B., Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B., Geyer M., Couillard-Despres S., Aigner R., Bogdahn U., Aigner L., Kuhn G., Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp. Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Hasegawa S., Nakano Y. Examination and classification of human olfactory mucosa in patients with clinical olfactory disturbances. Arch. Otorhinolaryngol. 1988;245:316–320. doi: 10.1007/BF00464640. [DOI] [PubMed] [Google Scholar]
- Yavarpour-Bali H., Ghasemi-Kasman M. Update on neurological manifestations of COVID-19. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.-Z., Chu H., Han S., Shuai H., Deng J., Hu Y., Gong H., Lee A.C.-Y., Zou Z., Yau T., Wu W., Hung I.F.-N., Chan J.F.-W., Yuen K.-Y., Huang J.-D. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–931. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziabreva I., Ballard C., Johnson M., Larsen J.P., McKeith I., Perry R., Aarsland D., Perry E. Loss of Musashi1 in Lewy body dementia associated with cholinergic deficit. Neuropathol. Appl. Neurobiol. 2007;33:586–590. doi: 10.1111/j.1365-2990.2007.00848.x. [DOI] [PubMed] [Google Scholar]