Abstract
Recent progress in biosensors have quantitively expanded current capabilities in exploratory research tools, diagnostics and therapeutics. This rapid pace in sensor development has been accentuated by vast improvements in data analysis methods in the form of machine learning and artificial intelligence that, together, promise fantastic opportunities in chronic sensing of biosignals to enable preventative screening, automated diagnosis, and tools for personalized treatment strategies. At the same time, the importance of widely accessible personal monitoring has become evident by recent events such as the COVID-19 pandemic. Progress in fully integrated and chronic sensing solutions is therefore increasingly important. Chronic operation, however, is not truly possible with tethered approaches or bulky, battery-powered systems that require frequent user interaction. A solution for this integration challenge is offered by wireless and battery-free platforms that enable continuous collection of biosignals. This review summarizes current approaches to realize such device architectures and discusses their building blocks. Specifically, power supplies, wireless communication methods and compatible sensing modalities in the context of most prevalent implementations in target organ systems. Additionally, we highlight examples of current embodiments that quantitively expand sensing capabilities because of their use of wireless and battery-free architectures.
1. Introduction
Rapid progress of highly integrated sensing technologies enabled by advances in electronics (Keyes, 1988; Popovici et al., 2013; Rasouli and Phee, 2010) materials (Joung, 2013; Khan et al., 2016; Lacour et al., 2005; L. Wang et al., 2019) and sensors (Bonato, 2005; K. et al., 2004; Ray et al., 2019) form the basis of symbiosis between biology and digital technologies. Sensors and sensing systems that enable continuous and precise collection of high-fidelity biological signals are key technologies to realize the next generation of digital medicine. Such systems require sophisticated hardware with substantial power requirements and consequently wired interfaces that tether subjects to a specific location, which complicates recovery, limits applicable use cases, and negatively impacts signal fidelity through induction of motion artifacts (Hamilton et al., 2000; Hartmut Gehring et al., 2002). Further, tethers limit operation during daily activity thereby lowering quality of life, especially in the case of diagnosis and therapy of chronic illnesses (Bade et al., 2018; Bize et al., 2007; Rejeski and Mihalko, 2001). The ability to monitor biosignals continuously, however, coupled with modern data analysis tools such as artificial intelligence (AI) (Chen and Asch, 2017; Ravì et al., 2017; Zhu et al., 2020), is predicted to yield significantly improved diagnostic and therapeutic capabilities (Obermeyer and Emanuel, 2016). To realize such advanced systems, there is a substantial need to increase seamless recording capabilities that operate autonomously and without user interaction to enable continuous data streams for diagnosis, fundamental research and therapeutics (Richard, 2015).
A direct requirement for chronic sensing is wireless operation (Dieffenderfer et al., 2015; Gaddam et al., 2008; Lynch and Loh, 2006; Patel et al., 2012; Zubiete et al., 2011). Current wireless sensing platforms in both wearable and implantable form factors rely largely on battery-powered designs to eliminate the need for tethered connections while providing capabilities in wearable sensing (Baig et al., 2017; Patel et al., 2012), neuroscience discovery (Farahmand et al., 2012), and organ specific biosignal recording (Kiourti et al., 2014; Ledet et al., 2012; Oliver et al., 2009). Although more suitable than their tethered counterparts, these systems fall short in yielding continuous, high-fidelity signals due to the bulk associated with batteries, which impacts system modulus and therefore conformality to the target organ system, resulting in reduced interface quality (Heikenfeld et al., 2018). Batteries inherently reduce functional lifetime and require subject interaction during charging events, which leads to loss of compliance, reduction in overall recording time and in the case of fundamental research studies, negatively impacts experimental outcomes (Q. Huang et al., 2016; Seneviratne et al., 2017; W. Xu et al., 2019).
A solution to this problem is offered by a new class of wireless and battery-free sensing systems in both wearable and implantable form factor with broad applications in medicine and research, encompassing a wide range of sensing modalities (Ferguson and Redish, 2011; Khan et al., 2020). These devices are characterized by their use of soft mechanics for conformal application to their target organ system and wireless, battery-free operation capabilities that reduce bulk while enabling continuous function without the need for user intervention. Beyond their use in human-centered applications (Ray et al., 2019), these systems have been introduced into animal-model research as investigative tools for development of novel sensing platforms (Burton et al., 2020; Hao Zhang et al., 2019) and closed-loop systems (Liu et al., 2020; Mickle et al., 2019), where physiological constraints prohibit the use of traditionally powered devices and enable insight into fundamental working principles of biological processes and underlying physiologies. Implementation of wireless, battery-free sensing systems require careful consideration of use-case, application, and sensing requirements to yield device form factors that allow for intimate integration with target organ systems to extract performance benefits over conventional approaches. Figure 1 summarizes key components that comprise these wireless and battery-free sensing systems, typical metrics for each component are provided in Supplementary Table 1 This review discusses key building blocks for these systems, namely wireless power transfer, data communication, current compatible sensing modalities and use cases for sending for these modalities in the context of their application in organ systems. It also highlights strategies for impactful combination of the components into wireless, battery-free sensors and sensor systems and provides a snapshot of current capabilities, while examining rationale behind design choices and highlighting current barriers and opportunities.
Figure 1.
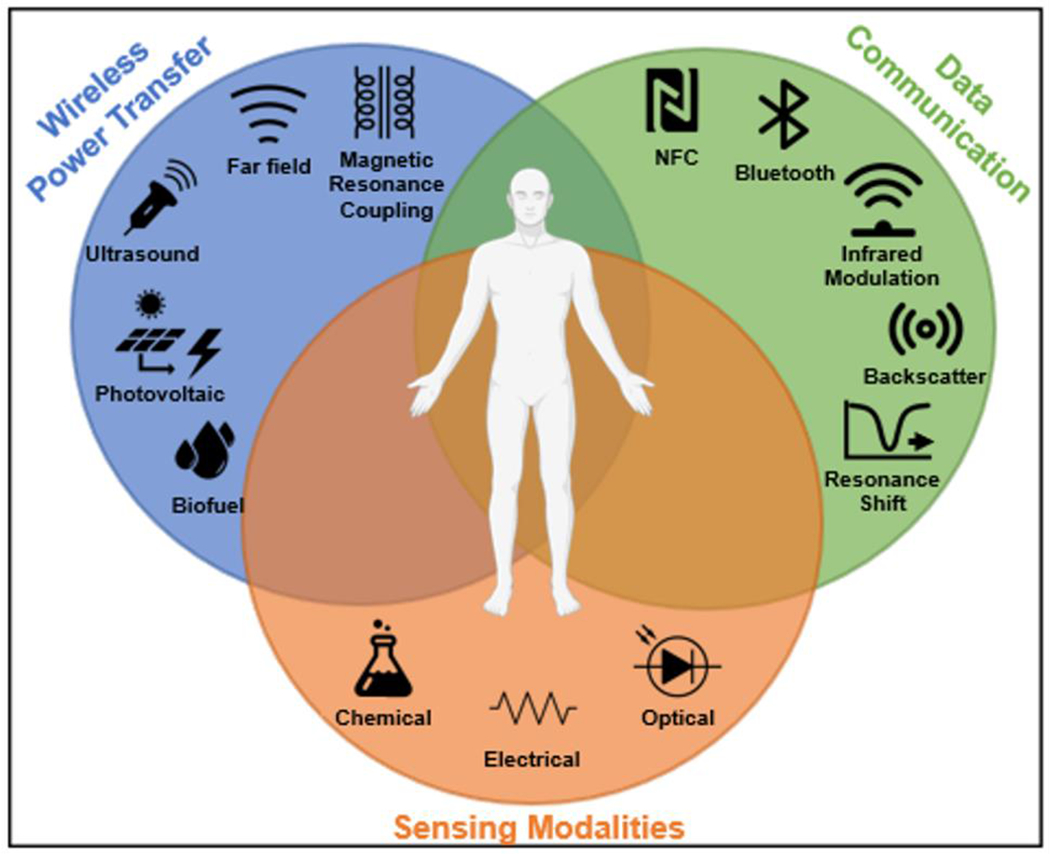
Key components of wireless, battery-free sensing systems.
2. Implementation of wireless, battery-free sensing systems
Key aspects in the creation of wireless, battery-free sensing include the strategy for power supply and technology for wireless communication. The availability of power often drives design choices of the wireless sensor system, dictates system capabilities, and is considered the bottleneck for most applications. In this section, we will explore currently available power casting and harvesting techniques, their operation principles, general advantages and challenges towards the use in the creation of fully integrated sensor systems. We will also identify links to wireless communication capabilities, options for sensing modalities and discuss practical combinations.
2.1. Power casting and harvesting techniques
Wireless power transfer (WPT) schemes lay the foundation for system-level integration of wireless, battery-free wearable and implantable sensing platforms and their capabilities largely determine system (communication bandwidth, sensing modality, operational range, form factor etc.) and sensor performance (resolution, sampling rate, accuracy, specificity, etc.) (Ahire and Gond, 2017; Hui et al., 2013; Radousky and Liang, 2012; Xie et al., 2013). Generally, wireless, battery-free sensing systems are powered by two categories of power sources (Amar et al., 2015): 1. externally directed energy that can be harvested by using radio frequency (RF) antennas (X. Tian et al., 2019), magnetic resonant coils (Bandodkar et al., 2019b; Lin et al., 2020), photovoltaic cells (S. Lee et al., 2018) or ultrasonic transducers (Hinchet et al., 2019; Seo et al., 2016) that we call power casting techniques in this review; 2. internal energy provided by the subject that can be converted to useful electrical power by piezo/tribo/thermoelectric power converters or bio-fuels (Bullen et al., 2006; Dagdeviren et al., 2017; S. Park et al., 2018; Settaluri et al., 2012; He Zhang et al., 2019) that we call energy harvesting techniques in this review. Operation mechanisms, representative device embodiments and implementations, that expand the use case over conventional tethered or battery-powered systems, are summarized in Figure 2 and Figure 3. In the following section, we will provide an overview of the notable progress of WPT methods and explore considerations when choosing a power supply strategy and highlight examples of their implementations.
Figure 2. External power sources for wireless, battery-free sensors.
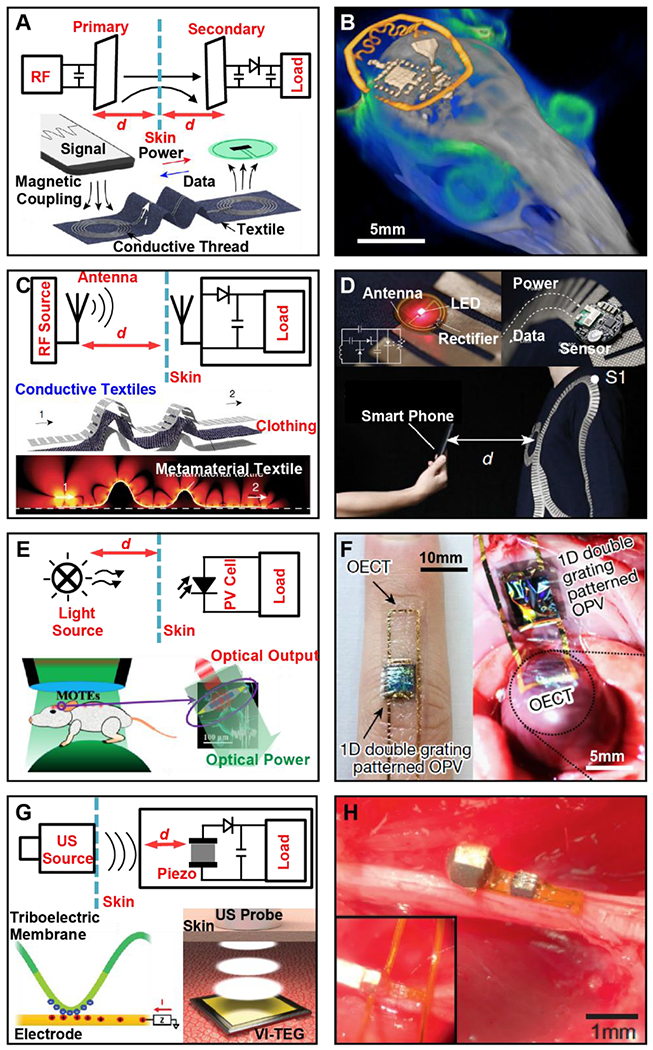
A, Operation mechanism of magnetic resonant coupling power transfer (upper half); Illustration of an E-textile-enabled near-field power transfer strategy to drive body sensor networks (lower half). B, 3D rendering of a micro-CT scan and MRI scan of a mouse implanted with a wireless photometric probe to monitor the Ca2+ dynamics in the brain. C, Operation mechanism of RF transmission-based power transfer (upper half); illustration and simulated electric field distribution of a wearable body area power transfer network enabled by metamaterial textile. D, Photographs of cardiac indicator (upper left) and temperature sensors (upper right) powered by the metamaterial textile, and demonstration of secure communication using a smartphone (lower image). E, Operation mechanism of photovoltaic-powered implantable sensors (upper half); Implementation strategy of a microscale optoelectronically transduced electrode (MOTE) with green light as input power source and red light as output for data communication. F, Photographs of sensors comprised of flexible organic photovoltaic (OPV) cells and organic electrochemical transistors (OECT) for wearable (left) and implantable (right) cardiac signal recording. G, Operation mechanism of ultrasonic power transfer with a piezoelectric crystal as implantable transducer (upper half); a vibrating and implantable triboelectric generator (VI-TEG) used to harvest ultrasonic energy (lower half). H, An ultrasonic-powered miniaturized device for wireless recording in the peripheral nervous system.
Figure 3. Energy harvesting power sources for wireless, battery-free sensors.
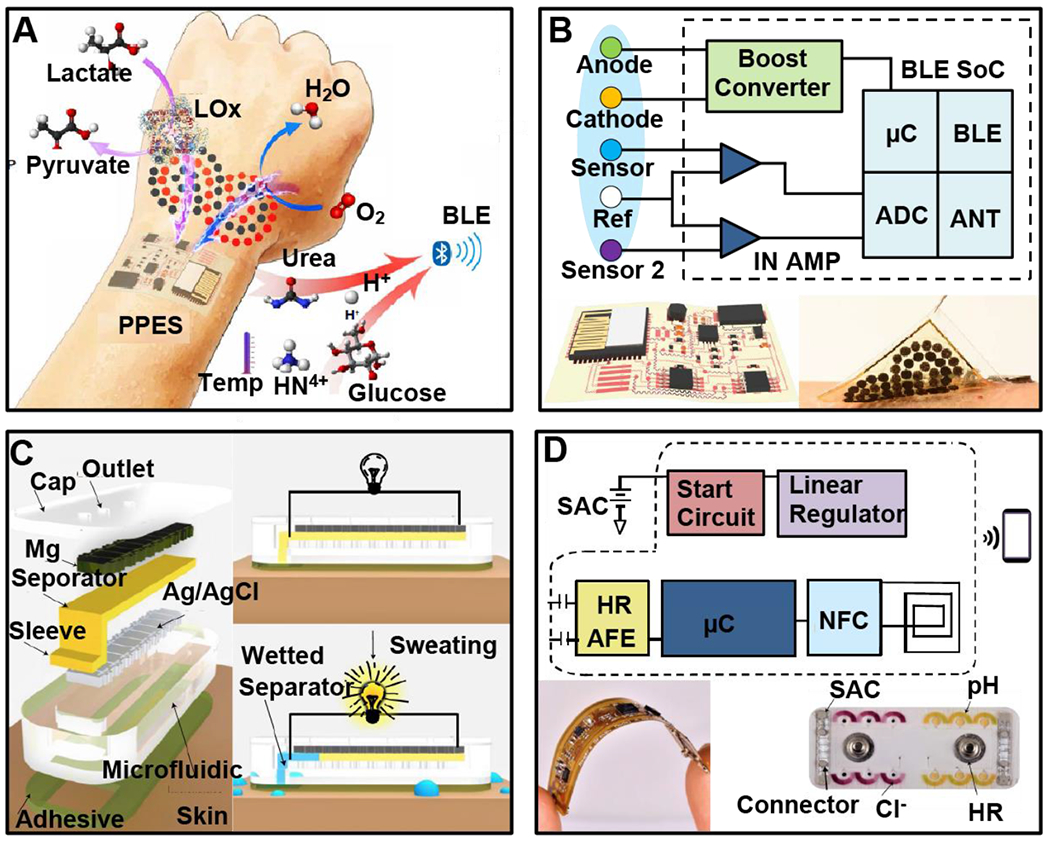
A, Operation mechanism and system construct of a soft electronic skin with multiplexed and wireless sensing functions powered by nanoengineered biofuel cells (BFCs). B, Block diagram of the system function (upper), schematic illustration (lower left) and photographs (lower right) of the BFC-powered wireless E-skins. C, Device structure and operation principle of sweat-activated batteries for driving epidermal electronic and microfluidic systems. D, Block diagram of the system function (upper), and photograph of the electronic (lower left) and microfluidic (lower right) modules.
2.1.1. Power casting techniques
Utilization of RF energy casting by an external source specifically designed to power wireless battery-free sensors in the nonradiative near-field regime (between 0 and λ from the transmitter) has demonstrated power transfer for stable operation over distances of up to 60 cm (Yu et al., 2019). Typically, frequencies in the range of 0.1 – 200 MHz (Barman et al., 2015; Ramrakhyani et al., 2011; Stoecklin et al., 2016) are utilized because of low tissue absorption (< 20 mW/kg) (Elder and Cahill, 1984; Gutruf and Rogers, 2018). Figure 2A (upper schematic) illustrates the operational principle of near-field magnetic resonant coupling (MRC) that relies on the impedance matched and tuned primary and secondary antennas with and without passive resonating elements that can be used to increase operating distance and harvesting efficiency (Imura and Hori, 2017). Here, the secondary coil antenna captures the magnetic field induced by the primary coil antenna that generates alternating current (AC) which is subsequently rectified to direct current (DC) and voltage regulated to drive the electronic circuits and sensing components (Barman et al., 2015; Hui et al., 2013). A power level of up to 500 mW can be obtained with MRC (Yu et al., 2019). This power transfer scheme is compatible with established radio-frequency identification (RFID) or near-field communication (NFC) (13.56 MHz) technologies that allows for simultaneous wireless communication through load modulation techniques (Lazaro et al., 2018), which enables sensor systems with small form factor and multimodal sensing capabilities (Chung et al., 2019; Han et al., 2018). The non-radiative nature of MRC confines its use to a distance less than the diameter of the transmitting coil antenna (Barman et al., 2015), limiting the utility of this powering scheme in applications such as body-wide sensor networks where sensor nodes are located throughout the body with distances up to a meter and various relative orientations (Cao et al., 2009). Recently, wearable near-field enabled clothing has been demonstrated by embroidering conductive thread on conventional textiles (Jiang et al., 2020; Lin et al., 2020) to form connected planar inductor patterns that function as relays between physically separated locations and enable wireless powering and data connectivity well-beyond the range of normal NFC (Figure 1A, lower schematic) (Lin et al., 2020), thereby highlighting a potential pathway to extend current limitations in power transfer for this technology.
In the context of implantable sensors, MRC is particular advantageous because of the relatively low specific absorption rate by tissues (Cecil et al., 2010; Elder and Cahill, 1984; Gutruf and Rogers, 2018) and high robustness against changes in dielectric environment resulting in stable device operation across various complex arenas such as those containing aquatic environments (Burton et al., 2020). In addition, MRC allows for a high level of design flexibility of the geometry of the receiving coil antenna, therefore the device layout can be adapted for complex anatomical structures (Samineni et al., 2017). In recent years, numerous wireless, battery-free implantable sensors have been demonstrated using MRC, that can operate in freely moving subjects in established test arenas for neuroscience discovery without addition of any externalized hardware to the subject. (Burton et al., 2020; Kang et al., 2016; Mickle et al., 2019; Hao Zhang et al., 2019) Figure 2B depicts a representative example where a subdermally implantable photometric device monitors the Ca2+ dynamics with cell specificity in the brains of freely-behaving mice (Burton et al., 2020).
The operation mechanism of far field RF radiation power casting is shown in Figure 2C (upper schematic), where radiative electromagnetic (EM) energy is transmitted into the surrounding space and reaches distances in the far field region (>> 2λ, where λ is 0.1 – 1m) (X. Huang et al., 2016; Merli et al., 2011), leading to an operational range (0.1 – 3 m) large enough to cover body area networks (Cao et al., 2009). However, the high frequency (up to 2.5 GHz (X. Tian et al., 2019)) RF energy used here shows relatively high tissue absorption (SAR ~ 0.1 - 6.15 W/kg) (Ho et al., 2015; X. Huang et al., 2016; Montgomery et al., 2015; Poon et al., 2010) resulting in heating effects and safety concerns, limiting their utility in implantable sensing devices. In addition, due to the directionality of EM wave propagation evoked by anisotropy of antennas, the power transfer efficiency strongly depends on spatial alignment of both the transmitting and receiving antennas (X. Huang et al., 2016; Park et al., 2015) and is prone to interference by surrounding dielectric materials with high electric susceptibility or conductive materials (Dobkin, 2012). As a result, hot spots and dead zones of EM energy density may appear, leading to concentrated tissue absorption or insufficient power transfer, respectively. A recent report of metamaterial textiles provides a viable solution to these transmission challenges where clothing is structured by patterns of conductive textiles that support surface-plasmon-like mode and confines the RF waves propagation close to the body surface (Figure 2C, lower schematic), yielding wearable multi-nodal sensor network with high energy efficiency wireless communication (Figure 2D, lower image) (X. Tian et al., 2019). Photovoltaic (PV) effect can be harnessed to convert light in the near infrared (NIR, e.g. 780 - 850 nm (Lu et al., 2018b; Moon et al., 2017)) and visible (~ 400 – 750 nm (Obaid and Lu, 2019)) regions into electrical power for wireless sensors, as depicted schematically in Figure 2E (upper schematic) (Jinno et al., 2017; S. Park et al., 2018). Energy can be either harvested from environmental light (S. Park et al., 2018) (e.g. sunlight, for wearable applications) or, more commonly, cast by dedicated light sources (J. Kim et al., 2020; S. Lee et al., 2018; Lim et al., 2020) (e.g. LED or laser, for implantable applications). Inorganic PV cells based on Si or III-V semiconductors have the advantages of good stability, mature technologies, as well as compatibility with standard microfabrication processes (Chen et al., 2018; Kim et al., 2012, 2010), the latter enabling ultraminiaturized devices that incorporate application specific integrated circuits (ASICs) for high-performance, low-power-consuming, on-board data processing, as well as monolithic integration of optical communication modules (Figure 2E, lower diagram) (S. Lee et al., 2018). The implementation of PV devices for deep-tissue implants is limited due to the absorption and scattering of light by biological tissues (Bevilacqua et al., 1999; Yaroslavsky et al., 2002). For example, with the incident light energy kept below the threshold of tissue damage (250 – 300 mW/mm2 for 20 minutes of continuous exposure, corresponding to a heating of 5 °C in the illuminated brain tissue (Podgorski and Ranganathan, 2016)), the operation depth is estimated to be less than ~ 3 mm even for ultra-low power ASIC microscale neural recorder that consumes only ~ 1 μW (S. Lee et al., 2018). Intrinsically flexible materials such as organic PV are suitable for wearable applications that undergo substantial mechanical manipulation and strain (Jinno et al., 2017). In a recent study, ultra-flexible organic PV cells were used to drive organic electrochemical transistors (OECT) attached on the finger or the surface of heart to record cardiac signals, highlighting the potential for self-powered wearable and implantable sensors (Figure 2F) (S. Park et al., 2018).
Another viable technology for WPT, although limited to implantable sensors, is ultrasonic (US) power casting (Figure 2G, upper schematic) (Basaeri et al., 2016) where an externalized ultrasonic transducer is placed in direct contact with the skin to generate US waves (wavelength ~ 0.1 – 1.5 mm (Nelson et al., 2009)) that propagate through tissues. Traditionally, a piezoelectric crystal is used to convert the mechanical vibrations into electrical energy to power the implant (Rosa and Yang, 2016; Seo et al., 2016). An alternative approach based on triboelectric effect has recently been proposed utilizing the US waves to induce micrometer-scale displacement of a polymer thin membrane to generate electrical energy through contact electrification (Figure 2G, lower half) (Hinchet et al., 2019). This technology is however only suitable for driving implants that consume < 100 μW due to its low efficiency (optimal efficiency ~ 0.03% at a distance of 5 mm) (Hinchet et al., 2019). US has the ability to penetrate deeper (> 10 cm (Kim et al., 2015)) into tissue compared to MRC, unless impeded by tissues with high absorption coefficient such as bone (Izadifar et al., 2017). Figure 2H presents an example of such a ultrasound-powered mm-scale sensing device that records electrophysiological signals such as electromyogram (EMG) and electroneurogram (ENG) in anesthetized rats (Seo et al., 2016).
However, a requirement of this technology is the need for direct skin contact for the power casting device with impedance matching agents. This limits its applicability in freely behaving subjects or wearable devices as motion may induce impedance changes at the skin interface. Other challenges of using US transduction to drive wireless sensors include sensitivity to misalignment (Denisov and Yeatman, 2010) as well as potential cavitation effects (Nelson et al., 2009).
2.1.1. Energy harvesting techniques
In recent years, biological energy sources have attracted increasing attention as means to realize self-powered sensing systems (Huang et al., 2019; J. Wang et al., 2019). A potentially suitable power source for wearable and implantable sensing application are biofuel cells (BFC) that convert the chemical energy stored in biofluids such as sweat (Gao et al., 2016) and saliva (Mink et al., 2014) (for wearable devices), blood (Chen et al., 2019) and gastric fluid (Nadeau et al., 2017) (for implantable or ingestible devices) to power by oxidization of substances such as glucose or lactate, producing power typically ranging from 0.2 to 100 μW/cm2 (Gonzalez-Solino and Lorenzo, 2018; Yu et al., 2020). Other technologies include piezo-/tribo-electric devices that generate a peak power of less than 1 mW from skeletal motion (Hwang et al., 2015) and a few tens of μW from visceral peristalsis (Dagdeviren et al., 2014; Yao et al., 2018) with an average power factor of > 10 lower than peak power (Song et al., 2020), which is currently insufficient to support continuous system-level functions of fully integrated sensing devices. Temperature difference between the human body and the environment can be utilized to generate electric power via thermoelectric conversion. Recent advances in scalable fabrication and soft mechanics design have enabled compliant thermoelectric generators with form factors tailored for wearable applications (Nan et al., 2018; Wen et al., 2020). Current embodiments feature a modest power density (Nan et al., 2018; Settaluri et al., 2012), < 30 μW/cm2, with typical skin-environment thermal gradient (~ 10 K), which, at sufficient sizes and good thermal coupling to the skin, can power devices with low energy requirements (Leonov et al., 2008).
Most recently, innovations in material engineering and device design have resulted in encouraging progress in perspiration-enabled electrochemical biofuel cells (BFCs) (Bandodkar et al., 2020; Talkhooncheh et al., 2020; Yu et al., 2020) for wearable sensing devices. Figure 3A illustrates the layered makeup of a flexible and self-contained electronic skin powered by nanoengineered BFCs that yield power density of ~ 3.5 mW/cm2, sufficient for driving a monolithically integrated system that contains multiplexed metabolic sensors, signal conditioning components, and a Bluetooth system-on-chip (BLE SoC) module (Figure 3B) (Yu et al., 2020). An alternative strategy to exploit perspiration for electrical power generation is displayed in Figure 3C where a thin-film Mg-Ag/AgCl primary battery is activated once the cellulose membrane separator absorbs sufficient sweat and creates electrolytic conditions to close the circuit (Bandodkar et al., 2020), yielding a single-use sweat activated cell (SAC) with a power density of ~ 1.5 mW/cm2 and stable operation for ~ 5 hours when driving a flexible wireless heart-rate sensor (Figure 3D). (Amar et al., 2015; Zebda et al., 2018)
In summary, power casting schemes such as MRC (Burton et al., 2020; Y. Zhang et al., 2019) and RF transmission (Sample et al., 2008; X. Tian et al., 2019) are advantageous for supporting system-level functionalities for wireless and battery-free sensing technologies due to their technological maturity, high and continuous power availability (Amar et al., 2015; Huang et al., 2019). Biological power sources that harvest mechanical, thermal and biochemical sources in general suffer from inconsistent nature of energy delivery that hinder integration of continuously operating digital systems (Li et al., 2020; Zebda et al., 2018). Encouraging developments in this field are biofuel-based systems that are powered by sweat that can sustain multimodal devices with digital communication with prevalent protocols (Bandodkar et al., 2020; Yu et al., 2020).
2.2. Wireless communication schemes
An integral component of wireless, battery-free sensors is the wireless communication that provides pathways for transmitting sensor signals and/or control commands. Wireless communication technologies that have been used for wearable and implantable sensing devices include simplex communication such as optical modulation (Burton et al., 2020; S. Lee et al., 2018; Zhang et al., 2019), ultrasonic (Seo et al., 2016) or RF backscatter (Vasisht et al., 2018), and passive resonance shifting (Boutry et al., 2019), as well as duplex communication such as near-field communication (NFC) (Bandodkar et al., 2019b; Chung et al., 2019; Krishnan et al., 2018; Yu et al., 2019; Zulqarnain et al., 2020) and Bluetooth Low Energy (BLE) (Mickle et al., 2019; Yu et al., 2020). Factors that determine the choice of communication methods include power budget, data rate, operation distance, hardware system complexity and form factor, as well as technological maturity. For example, infrared (IR) modulation can be used for data uplink of miniaturized subdermal sensors due to its small footprint (~0.5 mm2) and well-established protocols (Burton et al., 2020; Zhang et al., 2019). RF backscatter is the preferred choice for body networks of passive sensors due to its extremely low power consumption and relatively long operation distance (in the range of meters) (Jameel et al., 2019) however is not easily translated due to the lack of widely-accessible hardware. BLE features duplex communication with high data rate (up to 200 kbps) and established infrastructures (Tosi et al., 2017), thus is often used where high-speed data communication (uplink) and control command transfer (downlink) are required (Mickle et al., 2019), nonetheless the relatively high power consumption (> 5 mW) (Tosi et al., 2017) limits the choice of power sources. Detailed discussion of the wireless communication technologies commonly used for wearable and implantable devices is extensively covered by review articles focusing on the topic (Cao et al., 2009; Liang and Yuan, 2016; Zatout, 2012).
2.3. Sensing Modalities
Wireless, battery-free systems leverage sensing modalities which utilize favorable mechanical properties and resulting intimate integration with sensing targets that result in high fidelity data streams outperforming their tethered counterparts. Unlocking this fundamental advantage requires system engineering with limited power and footprint in mind, restricting the choice of sensing modalities based on operation location and application scenario. Given this dependency, it is most appropriate to review sensing modalities in conjunction with their respective target organ system and use case, outlined in section 3.
2.4. System architectures for wireless and battery-free sensors
In recent years, battery-free sensing systems have emerged as wearable or implantable tools to measure a wide range of biophysical, biochemical and physiological signals. We provide a comprehensive survey (Supplementary Information, Table S1) of the current research landscape which counts 49 papers of wireless, battery-free sensors and sensor systems. The dataset is compiled of work spanning the years of 2008 to 2021 and is comprised of sensors that provide digital data streams. Not included in this survey are passive sensors that rely on LC circuits (Niu et al., 2019), which are much simpler constructs that are very useful sensors for short time periods and simple applications, though have limited utility in chronic sensing applications. Figure 4 displays a visual compilation of the reported system architectures as a 3D scatter plot covering power source, communication method and sensing modality. Larger data points imply higher prevalence of corresponding system architectures and color indicates wearable or implantable system form factor.
Figure 4.
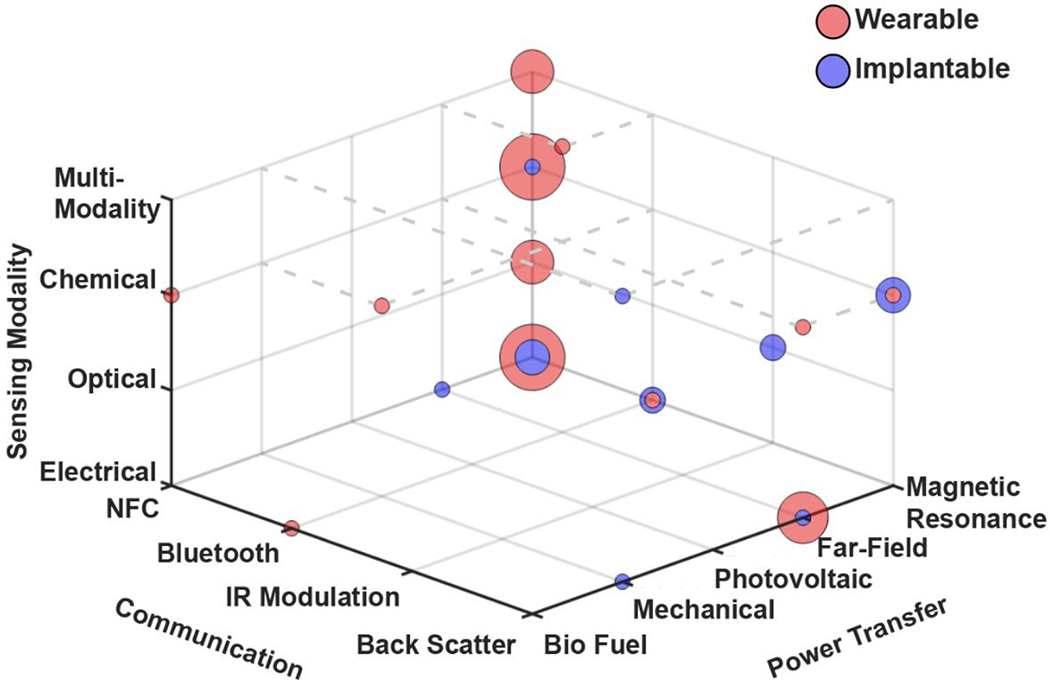
3D scatter plot summarizing recent work in wireless, battery-free sensing devices
The visualization reveals that near field powering as well as near field-based communication methods are the primary choice to enable single and multi-modal sensors. This is evident by statistical analysis which reveals that 70% of wearable sensors and 73% of implantable sensors utilize MRC. Data communication methods show a similar trend for wearable sensors of which 67% use NFC communication and 21% use backscatter. The exception, however, are implantable sensors which do not have a dominant communication technology of choice, here backscatter (18%) and NFC (15%) are the most popular, however a larger variety of communication methods is prevalent.
The popularity of near field power transfer and communication follows from a high compatibility with on-body applications (discussed in section 2.1) and also the availability of commercially available fully-integrated ICs that combine energy harvesting, communication and in some cases computation (Lazaro et al., 2018) in a compact package that can be seamlessly combined with existing sensing modalities (Bandodkar et al., 2019b). The miniaturized form factor also enables systems that feature high mechanical compliance, which is beneficial for sensing performance (Chung et al., 2019; Heo et al., 2018; Jeong et al., 2017; Jia et al., 2019). MRC is also often coupled with BT when higher data rates and longer communication distance are required (Mickle et al., 2019). The exception is implantable devices, where no dominant architecture has emerged. This is largely due to challenging electromagnetic conditions where communication methods suffer from attenuation in the surrounding tissues (Elder and Cahill, 1984).
3. Embodiments
Wireless, battery-free sensor systems require the combination of efficient energy harvesting and reliable data communication schemes that are tailored specifically towards the application to achieve best system performance. Often optimized solutions are application driven and engineered towards the sensing application and unique challenges arise from the locations of the sensing target and the broader application scenario. To discuss system architectures, sensor performance and unique advantages derived from wireless, battery-free operation, this section is organized by target organ.
3.1. Central and peripheral nervous system
Interfaces with the central and peripheral nervous system become increasingly important tools to treat and diagnose neurodegenerative diseases (Cagnan et al., 2019), control prosthetics (Krasoulis et al., 2017), supplement rehabilitation (Osuagwu et al., 2016) and provide tools for the neuroscience community to decipher the working principle of the brain (Seymour et al., 2017). These sensing and stimulation techniques, broadly defined as neuromodulation, currently use tethered and head mounted devices that are prone to infection (Biran et al., 2007), limit subject mobility (Armstrong et al., 2013), and restrict investigations to single subject study and 2D environments (Bin et al., 2017). Battery-powered systems suffer from limited operation times, bulky form factors, and weight that impacts subject mobility (Harrison et al., 2011). For exploratory research both tethered and battery-operated devices may impact animal behaviors and require operator interactions that impose experimental biases (Lu et al., 2018a). Wireless, battery-free operation of these neuromodulation systems allow for small, lightweight devices that intimately interface with the central and peripheral nervous system to enable high-fidelity recording in experimental paradigms that extend to 3D ethologically relevant environments, multisubject investigations, and flying subjects (Burton et al., 2020; Chestek et al., 2009; Harrison et al., 2011, 2009; Shon et al., 2017; Thomas et al., 2012). These demonstrations expand the toolset for exploratory neuroscience and enable capabilities that match or outperform their tethered or battery-powered counterparts without effecting subject behaviors.
3.1.1. Cell specific neuronal activity sensing
Recording activity of specific neuronal populations is an important tool to decipher the central and peripheral nervous system (Cui et al., 2013; Falkner et al., 2016; Gunaydin et al., 2014). This is commonly accomplished through direct electrical readout of cell activity in the electrode vicinity, which typically requires high channel count arrays to capture activity of neuronal ensembles to gain mechanistic insight (Legatt et al., 1980). To capture cell type specific activity, photometric approaches can use viral vectors to deliver genetically encoded calcium indicators (GECIs) to select neuronal populations to observe Ca2+ dynamics, a proxy for recording neuronal activity (Zhao et al., 2011). Common photometric approaches require tethers (Armstrong et al., 2013; Murayama et al., 2007) or battery-powered devices (Khiarak et al., 2018; Kim et al., 2013; Lu et al., 2018a) that have a large impact on subject behavior and mobility while limiting experimental paradigms (Dombeck et al., 2010; Lu et al., 2018a; Miyamoto and Murayama, 2016). Wireless battery-free sensor systems offer a unique opportunity to expand current sensing capabilities by shrinking system size to enable subdermal implantation. This has been demonstrated in recent work on wireless photometers as shown in Figure 5A (Burton et al., 2020). The device utilizes MRC power transfer methods (13.56 MHz, discussed in section 2.1) in a thin (1.2 mm), highly miniaturized (10.5 × 7 mm), and light-weight (45mg) device capable of harvesting >17 mW in a 25.5 cm × 25.5 cm cage, allowing for stable operation with a peak power consumption 10.37 mW (Burton et al., 2020). This low power consumption for optoelectronic sensing schemes is attributed to advanced digital power management of the analog to digital converters (ADC), comparators, and timers to minimize power consumption temporarily to 119 μW, yielding lower average power needs (Burton et al., 2020; Lu et al., 2018a). The injectable photometric sensor probes are comprised of a blue (468 nm) micro-scale inorganic light emitting diode (μ-LED) and a micro-scale inorganic photodetector (μ-IPD) laminated with a narrow-band optical filter to reject blue light and selectively pass fluorescence signal from the Ca2+ (523 nm), as shown in Figure 5A. A micro-controller (μC) enables precise timing of GECI excitation and digitally samples the photodetector to record fluorescence. IR data uplink is utilized to send sensor results to an external receiver as shown in Figure 5B. Low power data communication (~3 mW) is realized though IR communication (discussed in section 2.2) (Burton et al., 2020; Tsimbalo et al., 2015). In this application IR communication was chosen because of low component count and minimal footprint on the device, that enables a form factor for subdermal implantation. This photometric device can record the fluorescence of 12.5 μM Oregon Green 488 BAPTA-2, serving as a benchtop analog dye of GECIs, with the ability to detect calcium concentrations between 0.0625 and 32 μM (Figure 5C), covering the physiological ranges of intracellular calcium in mammalian neurons during rest and activation (Burton et al., 2020; Grienberger and Konnerth, 2012) and outperforms recently reported battery-powered devices with similar sensor architectures (Lu et al., 2018a). The device represents a significant advance over current photometric systems because of its fully implantable form factor that expands recording capabilities to multiple subjects in three dimensional experimental paradigms and underwater experiments, such as the Morris water maze, which is difficult or impossible to realize with current tethered and battery-powered systems (Burton et al., 2020). The nonmagnetic component choice further enables compatibility with noninvasive imaging modalities. The advantage gained by wireless, battery-free architecture also extends to future devices. Because of the use of scalable fabrication and off the shelf electronic components, function can be readily adapted to new fluorescent indicators sensitive to voltage, proteins, metal ions, and pH (Adam et al., 2019; T.-W. Chen et al., 2013; Greenwald et al., 2018; Martynov et al., 2018). Implementation of multimodality sensing systems is also possible, combining stimulation with sensing modalities to expand neuromodulation capabilities to create the next generation of exploratory tools to decipher the central and peripheral nervous systems.
Figure 5. Wireless battery-free neural interfaces.
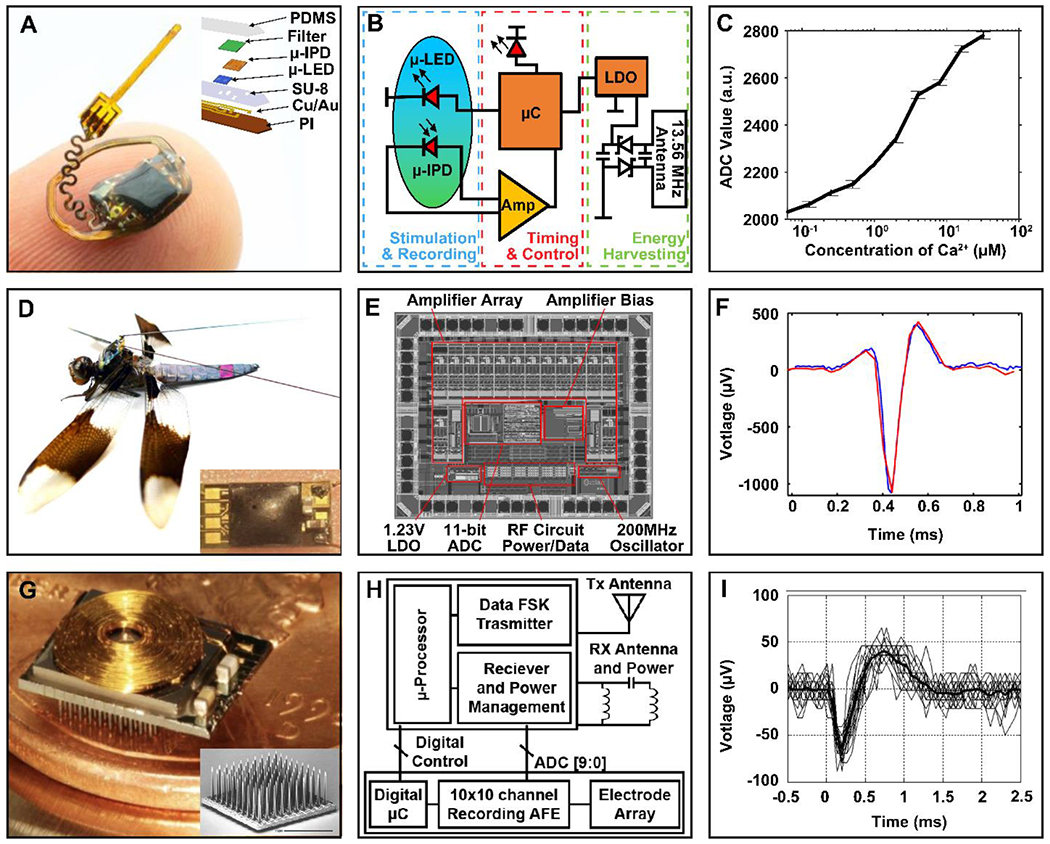
(A) Photograph of a wireless, battery-free photometry device balanced on a finger with an inset of an exploded view makeup of the photometric sensor probe. (B) Block diagram of the photometry device function. (C) Fluorescence recording with standard errors of titrated calcium solutions (0.0625 μM - 32 μM) and calcium dye (12.5 μM). (D) Photograph of a wireless neural recording system mounted on a dragonfly with an inset of a close-up view of the device. (E) Die layout of the 14-channel digital telemetry IC. (F) Wireless acquisition (red) of a reference neural spike (blue) in a dragonfly. (G) Photograph of a 100-channel wireless neural interface device with inset of a scanning electron micrograph of the 100-channel electrode. (H) Block diagram of the 100-Channel wireless neural recording system. (I) Wireless acquisition of multiple neural signals recorded from auditory cortex of cat.
3.1.2. Multimodal electrophysiological recording
Capturing electrical activity of neuronal populations is important for species where genetic modification is not available. Creating technology to expand animal model diversity is especially important to draw universal mechanistic and circuit level insight on the function of the nervous system (White, 2016). An example of this technology is recent work involving insects to capture spinal and motor neural activation to gain insight into communication pathways that control specific muscle groups, for example, experiments that track small subjects while monitoring prey drive or escape behaviors (Fotowat et al., 2011; Harrison et al., 2011; Olberg et al., 2000). Current methods of analyzing neural and muscular activity in small insects are heavy and too bulky to record effectively in freely flying insects (Harrison et al., 2011). Wireless and battery-free device architectures can significantly advance current capabilities. For application in insects, a high degree of miniaturization is required, which can be accomplished with the use of ASICs that combine both energy harvesting, communication, signal amplification, digitalization and processing to yield a very compact device form factor (6.8 mm × 4.6 mm) and low mass (38 mg), as shown in Figure 5D, compared to that of battery-powered versions of similar systems (13.0 mm × 9.5 mm, 790 mg) (Harrison et al., 2011; Thomas et al., 2012). Two light-weight gold wires (50 μm) form a dipole antenna that extends down the length of the dragonfly and provides up to 6 mW from far-field wireless power transfer (915 Mhz) from a central power casting system inside a flight arena for insects (discussed in section 2.1). The device consumes 1.23 mW of power, allowing for continuous operation up to ~1.91 m (Thomas et al., 2012). In this application far field energy harvesting is an advantageous technological choice because subjects are small and therefore do not have significant impact on electromagnetic environment and tissue absorption is less significant, resulting in long operational range capabilities. This system is capable of recording 10 extracellular neural and 4 EMG channels using hook shaped wires to establish an electrical interface with the spinal cord or muscle. Data is transmitted at 5 Mbps using back scatter communication (discussed in section 2.2) with energy consumption of 4 picojoules per bit (Liu et al., 2019), resulting in suitable performance to cover the bandwidth requiernments that are imposed by 14 channel recordings with sufficient sampling rate to capture neural activity spikes. Figure 5 E shows a microscopic image of the ASIC die (2.36mm × 1.88 mm, 250 μm thick) that includes the functionality described above. Figure 5F shows device performance over multiple channels of the device. The system is capable of recording at 11-bit resolution with a sampling rate of 250 Hz–10 kHz for spinal recording and 5 Hz–700 Hz for EMG recording, sufficient for comprehensive recordings of the central and peripheral nervous systems of insects. The demonstration of such a device highlights the benefits of system integration in an ASIC that contributes to the miniaturization and reduction in power consumption via the elimination of peripherals that are required for broadly applicable and programmable IC’s. The device also demonstrates that sensing capabilities can be expanded towards new species, increasing the animal model pool to insects. However, it should be noted that this is a highly optimized sensing solution that requires the subject to be very small in size and addition of sensing modalities will require a redesign of the ASIC, which is typically lengthy and costly.
3.1.3. High density 100 channel electrophysiological recording
High density neural recordings are important tools for deciphering the working principle of the brain and offer an avenue towards tailored therapeutics, such as seizure detection, and have been an active area of research for the last decade (Miccoli et al., 2019; Obien et al., 2015; Viventi et al., 2011). This form of neural interface is also translatable to human applications and devices of this type are actively translated towards diagnostic, therapeutic and brain machine interface applications. A popular sensing format of this device class are microelectrode array (MEA) that allow for high density recordings and enable investigation into neurological diseases, brain injuries, and effects of pharmaceuticals through precise spatial mapping of action potential propagation over time (Cheng et al., 2019; Ferrea et al., 2012; Ghane-Motlagh and Sawan, 2013). A typical bottleneck for these devices is the high power consumption that is required due to the higher channel count, thus limiting current technologies to tethered connections or large battery packs (Chestek et al., 2009) that often require bulky connectors and ports (Suner et al., 2005) to supply power to the device and allow transmission of high-fidelity recorded signals. Both of these approaches are acceptable for exploratory neuroscience with large animal models that are under constant supervision and care, however, are not a form factor that is likely to be adopted for the operation in patients with active lifestyles. Wireless, battery-free sensing approaches offer a path towards fully implanted systems via integration of both energy harvesting components, communication and low power analog frontend for MEA neural interfaces as shown in the bottom right of Figure 5G. Recently, devices that enable direct neural recording capability of up to 100 channels in an implantable wireless form factor (8 mm × 7.6 mm) (Cheng et al., 2012; Harrison et al., 2009, 2007; Sharma et al., 2011; Thurgood et al., 2009; Watkins et al., 2006) as shown in the center of Figure 5G, allow for chronic recording of neural signals with short distance telemetry. Integration of both energy harvesting and communication yields miniaturized form factors. Wireless power transfer using near field MRC (discussed in section 2.1) with a frequency of 2.64 MHz, reducing tissue absorption (Ziaie et al., 1997) and delivering up to 30 mW of power at distances of up to 2 cm from the primary antenna (Cheng et al., 2012; Harrison et al., 2008, 2007).
The 100 channel MEA is micro-machined silicon and features platinum electrodes, projecting 1.5 mm deep with an 80 μm electrode separation(Nordhausen et al., 1996) (in the inset of Figure 5G) and is used to penetrate the target area of the brain. The recorded signals are wirelessly transmitted using backscatter communication (discussed in section 2.2) at 433 Mhz with a data rate of 330 kb/s (Chestek et al., 2009) and a separate antenna dedicated to this frequency, see functional diagram Figure 5H. The higher frequency is utilized to support the high data rate requirement. Additionally, these devices can be programmed to select which channels are digitized and turn off select amplifiers that are not providing useful neural signals, thus lowering overall power consumption. These devices are capable of receiving commands to set voltage threshold for spike detection of local field potentials using comparators, reducing overall communication bandwidth and power consumption to transmit neurological signal wirelessly (Cheng et al., 2012; Harrison et al., 2008, 2007). The sensor can record neural potentials with 100 channels to measure local field potentials and detect activity spikes with potentials of 50-500 μV and has a discrete spike detection mode where only spike amplitude and time of the event are transmitted to realize low power operation (Cheng et al., 2012; Harrison et al., 2008, 2007). In-vivo experiment shows validation of device operation with 30 superimposed signals from the auditory cortex of a cat as shown in Figure 5I. Demonstration of such high channel count operation with wireless, battery-free device architectures highlight that systems operating with full subdermal implantation are possible in the near future for large animal models and humans, greatly expanding the potential of neural interfaces towards the realization of digital medicine and electroceutical intervention for chronic and neurodegenerative disease (Vesuna et al., 2020). Recent interest of commercial companies such as Neuralink, Amuza and Intan Technologies highlights the potential of this technological approach and forecasts impact on diagnostics, therapeutics and potentially performance enhancing applications (Grand et al., 2013; Musk, 2019; Petkos et al., 2019).
3.2. Cardiovascular system
Understanding and treating cardiovascular diseases often requires high-fidelity and chronic data acquisition for accurate diagnosis to delineate subtle changes in physiology that indicate onset or current status of disease (Hillebrand et al., 2013; Muller et al., 1989; Wilmot et al., 2012). Current practices in cardiac monitoring and diagnostics either require tethered connections in clinical settings or large wearable devices that are not suitable for long-term recordings (Hong et al., 2019; Walsh 3rd et al., 2014). Recent works in battery-free, wireless cardiac sensing devices have moved towards eliminating the need for tethered connections and battery-powered supplies. This provides solutions to currently cumbersome systems that yield improvements of signal fidelity (Alberto et al., 2020; Besnoff et al., 2013; Chung et al., 2019; Kim et al., 2017; Kim et al., 2016; H. Zhang et al., 2019) while broadening the scope of diagnostic and monitoring capabilities to detect chronic changes in physiology (Ray et al., 2019). In this section, we review sensing modalities used in cardiovascular applications, namely optical and electrical schemes, to measure oxygen saturation, biopotentials, and flow dynamics. We also explore both wearable and implantable systems implementing these functionalities and discuss how these devices advance the state of cardiac sensing capabilities.
3.2.1. Optical
Optical sensing approaches are useful tools in cardiovascular medicine and utilized in many applications such as photoplethysmography (PPG) (Allen, 2007) and pulse oximeters (Tremper, 1989), which use a narrowband light sources and photodetectors to probe target tissue with light and extract heart rate and blood oxygenation information via absorption variation induced by the oxygenation state of hemoglobin (Jubran, 2015, 1999; Liu et al., 2016). Conventional methods of pulse oximetry are unsuitable for long-term recording due to susceptibility to motion artifacts associated with increased mass of power supplies and electronics (DeMeulenaere, 2007; Sinex, 1999; Yan and Zhang, 2008). Changes of relative orientation of the light source and detector induced by the weight of the sensor system assembly and underlying vasculature causes net changes in signal, interrupting continuous acquisition (Barker, 2002; Hayes and Smith, 2001). Because of high power requirements (~10 mW) of optoelectronic components paired with a Nyquist frequency of 0.5 Hz with most systems operating closer to or above 5 Hz (Choi and Shin, 2017) (See SI Table 2), the lifetime of battery-powered devices is limited, and form factors are not sufficiently small for implantation or long-term, continuous use in wearable application scenarios. Wireless and battery-free devices have been demonstrated as viable solutions to these shortcomings.
An example of this device class is a highly miniaturized wearable pulse oximeter (Jeonghyun Kim et al., 2017) which utilizes MRC (discussed in section 2.1) and NFC (discussed in section 2.2) to provide indefinite and motion artifact-free blood oxygenation information. The device, shown in Figure 6A, uses a μC and a bare-die NFC IC to facilitate power management, device function, and data communication. NFC is chosen for this use case due to its technological maturity and availability in modern smartphones with sufficient power availability (up to 20 mW for devices of this size) (Lazaro et al., 2020).
Figure 6. Wireless battery-free oximetry sensors.
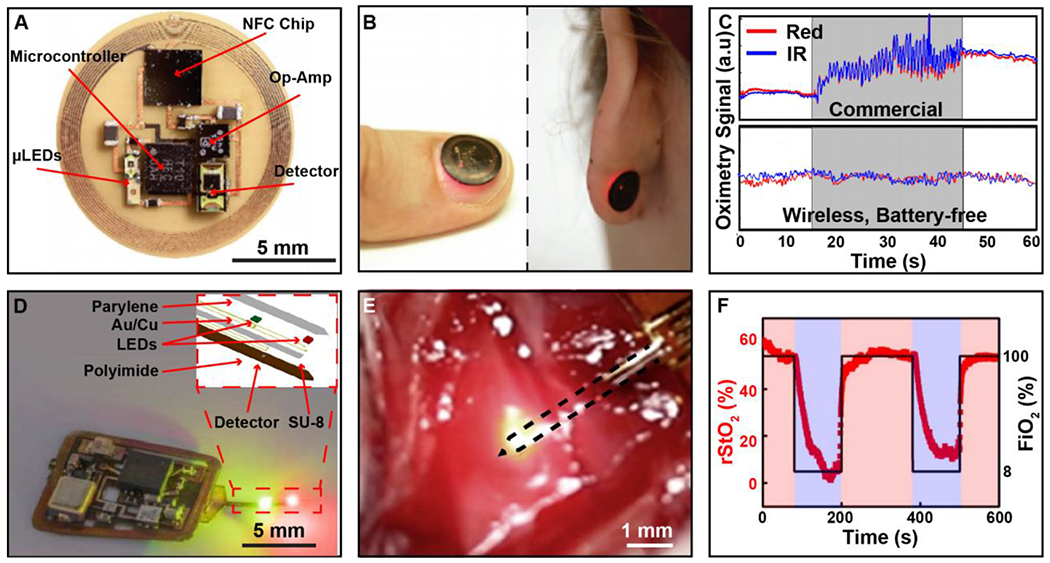
A) Photographic image of oximeter device with key electrical component labeled. B) Image of device during operation while applied to the fingernail and earlobe. C) Plot of red and infrared (IR) signal from both wireless, battery-free and commercially available devices during user motion. D) Image of wireless localized oximeter device during operation. Inset shows exploded view make up of injectable probe. E) Image of oximetry probe implanted in tissue region of the femoral artery. F) Plot of rStO2 readout in tissue region while anesthetized rat is exposed to FiO2 concentrations. of 100% (red) and 8% (purple).
A red and infrared LED are located 2 mm away from the photodetector in the same plane to achieve an optimized optical path through the tissue. The components are optically separated using a black, elastomeric encapsulation. This allows for a soft, intimate optical interface with the fingernail, epidermis and other body parts, a key aspect in increasing signal fidelity that is not possible with current battery-powered systems due to their inherent rigidity. Onboard amplification and digitalization render a compact device with ultra-small footprint and flexible mechanics that enables the use on a variety of body locations, increasing utility in comparison to tethered and battery-powered solutions. The diverse functionality of the system is shown in Figure 6B, where the device can be attached and function with high-fidelity on topologically complex regions such as the fingernail or earlobe. Figure 6C shows the enhanced fidelity of data collection during motion when compared to commercially available devices. In this experiment, devices undergo induced motion as indicated with grey shading. The inertia of commercial device experiences large motion artifacts, rendering the data acquired during that time unusable. The wireless and battery-free system, due to its favorable mechanics and miniaturization, can collect high-fidelity signals without any effect from motion, enabling continuous data collection without signal loss. SpO2 values collected from the wireless, battery-free system falls within a standard deviation of 1.5%, which is significantly improved compared to current state of the art systems that offer a 2-3% tolerance on SpO2 estimations. All currently demonstrated battery-free devices for this application operate using MRC and NFC, because of power availability needed for operation of the optoelectronics, small footprint to provide favorable mechanical properties, and robust data communication pathway coupled with widely available network infrastructure such as smart phones (Opperman and Hancke, 2011; Sidén et al., 2011). Current technology is still limited in operation range due to power requirements of the system and the use of MRC. With the improvement of power transfer techniques and development of low power methodologies for pulse oximetry, the functional range can be increased.
In addition to wearable systems, wireless and battery-free oximetry devices have been demonstrated for the use in localized implantable oxygenation measurements for use in animal model research, which allows for investigation into a variety of medically relevant parameters such as neural activity (Zhang et al., 2019) and tumor microenvironments (Hopf and Rollins, 2007). Devices such as those shown in Figure 6D provide the capability to gain valuable insights in a freely moving in vivo and ethnologically relevant environments, which would otherwise require the subject to be under anesthesia or immobilized, prohibiting longitudinal mechanistic and diagnostic insight (Reichelt et al., 2008; Theodor et al., 2014). This device features a near-field coupling coil for MRC power transfer and IR communication, a system architecture used in photometric recordings described in section 2.1 and 2.2. The device is designed to measure localized oxygenation (Zhang et al., 2019a). This detection method is enabled by microfabrication of the sensing probes with inorganic microscale LEDs and photodetectors that use an outline of less than 300 μm, enabling direct injection into the target organ for probing into the region of interest and minimizing tissue damage in comparison to full device implantation in the target region (sensor probe makeup shown in inset Figure 6D). Operation of the device is demonstrated in Figure 6E, where the oximetry probe has been placed into the tissue region proximal to the femoral artery of an anesthetized rat. The fidelity of measurement is detailed in Figure 6G. In this experiment, the local oxygen saturation near the femoral artery was measured while the subject was exposed to varying levels of oxygen. In this work the devices were also used in freely moving subjects to probe oxygenation levels in the rodent brain during exposure to hypoxic environments. The improved fidelity of wireless, battery-free systems allows for real-time data acquisition of local oxygenation plasticity of target organ, which is not possible with conventional devices. These new class of implantable oximetry devices highlight the possibility to enable investigation into cardiac and local oxygen perfusion dynamics in freely moving subjects, expanding possible experimental paradigms. The approach also has potential to be used as a therapeutic device to monitor surgery recovery and personalize pharmacological therapies.
3.2.1. Electrophysiological and Electromagnetic
Electrical recording of cardiovascular biosignals can be acquired using electrophysiological sensing that records small voltage signals that arise from the depolarization of cardiomyocytes, a technique often used in noninvasive devices with more recent developments implementing electromagnetic sensing schemes that can record blood flow dynamics in vivo for advanced diagnostics (Liebl et al., 2019; Parthasarathy et al., 2016). These signals require high-fidelity recording systems and continuous monitoring to extract diagnostic information, particularly for diseases which may present sporadically such as intermittent arrythmia. The most commonly used sensing system are electrocardiograms (ECGs) that extract heart rate and give insight into functional events, such as the QRS complex (Becker, 2006; Meek and Morris, 2002). In the case of noninvasive recording methods, impedance with the skin plays a critical role in extracting high-fidelity signals (Heikenfeld et al., 2018). Currently for most clinically relevant applications this is accomplished with hydrogel electrodes that offer low impedance (Shay et al., 2018) and reduce motion artifacts. This, however, results in a limited lifetime (McAdams, 2006; Searle and Kirkup, 2000) leading to implementation of bulky systems with interchangeable electrode schemes that are not suitable for continuous long-term recording, prohibiting their use for early onset detection of disease (Walsh 3rd et al., 2014).This issue is exacerbated in fragile patient populations such as neonates, as currently employed wet electrode systems utilize adhesives that result in skin injuries, leaving lifelong scars (Cartlidge et al., 1990; Lund, 2014). Wireless, battery-free sensing systems in combination with advanced electrode technology can overcome many of the challenges and have been widely investigated for use in electrophysiological monitoring (Besnoff et al., 2013; Chung et al., 2019; Jeong et al., 2019; Mandal et al., 2010; Zulqarnain et al., 2020). These devices feature miniaturized electronics with soft, conformal mechanics that enable the use of epidermal dry electrodes that offer low impedance to the skin without the use of adhesives (Chen et al., 2014; Chlaihawi et al., 2018; Li et al., 2018; L. Tian et al., 2019) enabling detection of discreet changes in cardiac potentials with operating conditions (200 Hz sampling and >15mW power consumption) on par with conventional wired methods (see SI Table 2). Figure 7A shows a demonstration of such a device that features a binodal dry electrode system, MRC and NFC communication (see section 2.1 and 2.2) with epidermal-like mechanics for the use in neonatal intensive care. MRC and NFC were selected to enable simultaneous power harvesting and data communication using a single antenna resulting in minimal peripheral component requirements that allow for soft mechanics required for high-fidelity operation. The operation scenario is defined, the NICU bed of the premature infant, which is a small operational space that can be equipped with a dedicated antenna system to power the device and receive data continuously without direct contact to the patient. To overcome typical data rate limitations of NFC, the device handles processing and analysis on the senor side, reducing bandwidth requirements. Other similar wearable system have been developed that enable operation at longer distances of 1 m, through the use of far field energy harvesting systems and Bluetooth communication protocols (Alberto et al., 2020; Besnoff et al., 2013). Figure 7B shows an infant patient with an attached ECG system to wirelessly record ECG signals. The system incorporates ultra-thin circuits that are structured with serpentine patterns to enable epidermal mechanics (Zhang et al., 2013). The contact relies solely on Van der Waals forces to minimize stresses at the epidermal interface and eliminate the need for adhesives. System mechanics and resulting sensing performance enable immediate and secondary benefits to health outcome such as increased child-parents bonding, which has been shown to be an important factor in neonatal development (Pineda et al., 2018). The use of nonmagnetic components allows for compatibility with common medical imaging techniques, such as MRI and X-ray, without significant image distortion, a favorable characteristic for long-term use in a hospital setting such as the NICU. Figure 7C shows heart rate data extracted from the device in comparison to the wired, gold standard data. The fidelity of this system matches the signal quality of the gold standard, with little variation in the QRS complex despite the use of a binodal system over conventional three electrode systems. Conceptually this class of device utilizes scalable monolithic fabrication, allowing for easy expansion of sensing modalities. The devices shown here are also capable of recording local skin temperature and photoplethysmographyasmorapgy (Chung et al., 2019).The successful implementation of systems that leverage wireless, battery-free system architectures to enable epidermal mechanics to conform to the epidermis highlights an important avenue towards systems that record chronically and also impact sensing modalities that rely on acquisition of electronic signals from other organs such as muscles and related applications in electromyography (Liu et al., 2018) and more recently in electroencephalography (see section 3.1.3).
Figure 7. Wireless battery-free sensors for applications in cardiovascular health assessment.
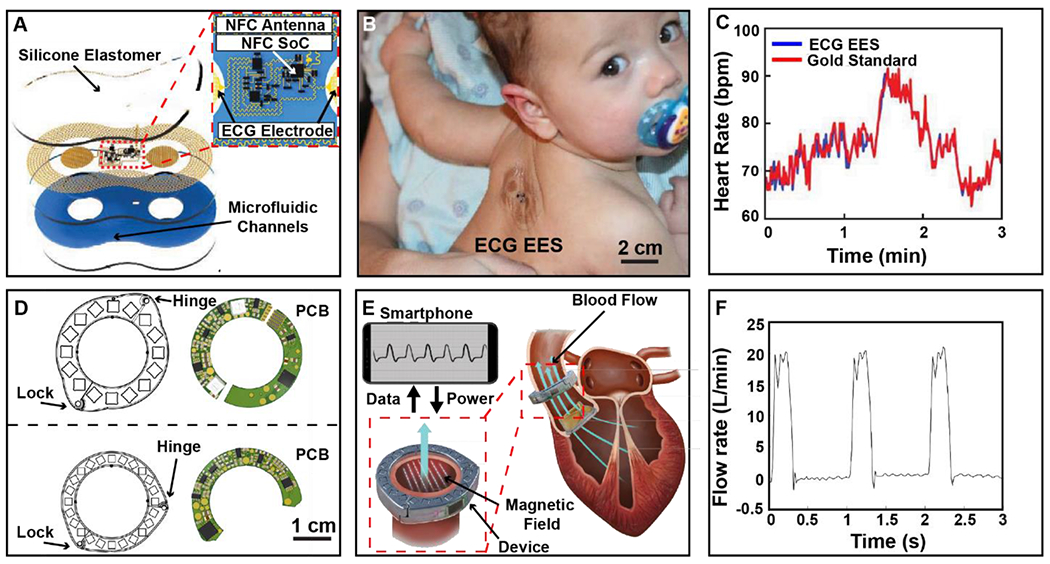
A) Schematic illustration of wireless, battery-free ECG system used for epidermal application on NICU patients. B) Photographic image of ECG device placed on the back of an infant. C) Graph of heart rate data collected using the ECG epidermal electronic system (EES) compared to the gold standard, wired system. D) Illustration of two devices with varying magnetic structures and their complementary printed circuit boards. E) Illustration of device function showing power and data transfer to the device with implementation on the aortic valve. F) Graph showing in vitro data collection at physiological conditions.
In vivo blood flow dynamics monitoring, is an important metric to asses cardiovascular health, however, sensing flow accurately is difficult because arterial specificity is needed, which cannot easily be achieved with noninvasive devices (Gradman, 2012; M. et al., 2018; Tarbell et al., 2014; Yoram and R., 2006). Diseases such as atherosclerosis (Texon et al., 1965), deep vein thrombosis (Fortuny et al., 2015), and aortic valve disease (Hope et al., 2011) are examples that feature disruptions in blood flow dynamics with serious impacts on health. Implementation of diagnostic and preventative chronic sensing solutions would dramatically increase patient outcomes. For example, patients with aortic valve disease have an increased risk of cardiovascular complications with higher rates of mortality and morbidity (Goland et al., 2007; Tjang et al., 2007) due to dysfunction of artificial heart valves and subsequent thrombosis or pannus formation (Ma et al., 2015). Early discovery of flow obstruction and mechanical failures associated with heart valves can prompt effective intervention, which is vital for improving outcomes for these patients (Abraham and Perl, 2017). Because patients undergo surgery as part of their treatment, there is an opportunity to implement sensing devices invasively without additional risk, wireless and battery-free devices are essential in this application scenario (Cong et al., 2009) because they do not require secondary surgery for replacement of batteries that can pose additional health risks to the patient (Jones et al., 2001). An example of such a device is an implant to monitor blood flow in vivo is shown in Figure 7D (Vennemann et al., 2020). The device is capable of detecting variation of flow rate through the ascending aorta. The device operates using both MRC and NFC as a means of power harvesting and data transfer. As with many implantable systems discussed in section 2.1 and 2.2, MRC is used in this case due to its ability to propagate deeper into the body, while providing enough power to sustain digital electronic device operation and NFC is used as communication method because of minimal data rate requirements and small footprint required for implementation. An overview of device function is demonstrated in Figure 7E. This system exploits faraday’s law and provides a constant magnetic field using passive magnets to produce a lateral magnetic field cross sectional to the artery. Electrodes embedded on the arterial walls detect the induced voltage by the flow of conductive liquid (blood), which is proportional to the fluid velocity. Results of this in vitro study are displayed in Figure 7F. Sensing fidelity enables the visualization of peak systolic flow and flow when the valve is closed. Implemented in vivo, this system can give insight into cardiac cycle variability and subtle, transient changes of flow, which can indicate the onset of heart valve failure. Recent advances in implantable cardiovascular devices benefit substantially from wireless power transfer and communication capabilities and enable sensing systems that collect information for peripheral blood flow (Yeshwant and Ghaffari, 2019) and changes in blood pressure (DeHennis and Wise, 2006; Murphy et al., 2013; Park et al., 2016) expanding current exploratory research tools and diagnostic capabilities substantially. Rapid development of these sensors suggests development of body networked systems in the near future that utilize wearable and implantable devices to provide a holistic insight into cardiac health. Challenges to enable these architectures are communication protocols with very little signal delay to resolve cardiac dynamics.
3.3. Endocrine and Metabolic System
A more recent application of wireless and battery-free sensor platforms has been their use in electrochemical investigation, specifically analysis of biomarkers of the endocrine and metabolic system in sweat and interstitial fluid (Bandodkar et al., 2019c; Bandodkar and Wang, 2014; Kassal et al., 2018; Scholten and Meng, 2018; Zhao et al., 2019). Monitoring of these biochemical markers involved in metabiotic diseases has vast relevance for many patient populations suffering from illnesses such as hypokalemia (Kardalas et al., 2018) and diabetes (Giesbertz and Daniel, 2016; Vashist, 2012). Gold standard methods of detection mostly rely on separated biomarker collection from the subject and subsequent, temporally shifted analysis in laboratory grade equipment, which has significant drawbacks due to sample degradation and significant limitations in sampling rate (Harker et al., 2006; LeGrys et al., 2007). A recent development for monitoring of diabetes, a pancreatic disease where the body fails to produce the amount of insulin to regulate sugar levels in the blood (Nathan et al, 1993), is the deployment of continuous glucose monitoring systems (GCMs) which represents an area of development with advances in device technology that enable patients with diabetes to monitor insulin levels continuously and ensure proper interventions (Rodbard, 2016). This is achieved via a insertion of a probe into the interstitial area of the body to continuously monitor glucose levels, and in some cases pump insulin in a closed-loop system (Bruen et al., 2017; Penfornis et al., 2011). Devices feature a bulky system that is clipped on a belt and features invasive methods of data collection that are unfavorable for many patients as they are generally painful and time consuming (Villena Gonzales et al., 2019). These devices represent the current state of the art in biosensing for endocrine and metabolic sensors. Progress in this area can affect the quality of life for a large patient population. For example, an estimated 415 million people living with diabetes worldwide (Chatterjee et al., 2017). Investigation into sweat-based extraction of biomarkers as the means for noninvasive monitoring of markers such as glucose, potassium and chloride (Baker and Wolfe, 2020; Heikenfeld, 2016; Heikenfeld et al., 2018; Moyer et al., 2012) is therefore a very active research area.
3.3.1. Wearable Endocrine and Metabolic Sensors
Wireless, battery-free sensing approaches offer opportunities to enable the realization of devices with conformal epidermal mechanics and minimally invasive implants that require little to no patient interaction with the ability to monitor multiple metabolites continuously to overcome current technological challenges. An example of such a device is presented in Figure 8A. This platform is comprised of two components, namely a one-time use microfluidic chip for on skin sweat collection, known as epifluidics (Choi et al., 2018; Koh et al., 2016), and biochemical analysis electronics for interfacing with passive sensors embedded in the epifluidic platform. The system relies on MRC and NFC (discussed in section 2.1 and 2.3) for this device architecture due to compatibility with smartphone systems, allowing for simultaneous power transfer, data communication, and in this case automated image collection to read out colorimetric sensing elements present in the epifluidic channels. The sensor architecture in this device is, unlike the highly integrated systems discussed in the previous sections, intentionally modular to facilitate reusability of the wireless, battery-free electronics and the one-time use epifluidics sensor that is worn on the skin to capture sweat. Figure 8B shows the system in use on the skin and highlights the diverse sensing modalities that make use of colorimetric analysis that require photographic imaging of the device to determine changes color and shading to extract values for pH and chloride monitoring, as well as automated calculations of sweat rate and sweat volume. The epifluidic system also includes biofuel-based sensors are able to detect sweat-based metabolites such lactate and glucose (Choi et al., 2018; Reeder et al., 2019). Figure 8C highlights data collected using the wearable device and is compared to the standard method of lactate detection, nuclear magnetic resonance spectroscopy (NMR) (Harker et al., 2006). In this example, the biofuel cell system continuously measures the change in lactate production in the sweat over time. This continuous collection enables insight into discrete changes in concentration over time which stands in stark contrast to the NMR analysis that only provides temporally averaged values with possible degradation of the sample between capture and analysis. Devices such as the epidermal device featured in Figure 8A–C represent a rapidly growing research area that harnesses sweat to analyze biomarkers for continuous sensing of multiple biomarkers including glucose (Bandodkar et al., 2019b; Hourlier-Fargette et al., 2020; G. Xu et al., 2019a), lactate (Bandodkar et al., 2019b; Hourlier-Fargette et al., 2020), chloride (Bandodkar et al., 2020, 2019b), sweat pH (Bandodkar et al., 2019b; Mazzaracchio et al., 2021; Song et al., 2020; G. Xu et al., 2019a) and other physiologically relevant biomarkers (Bandodkar et al., 2020, 2019a; Cheng et al., 2021; Hourlier-Fargette et al., 2020; Kim et al., 2018; S. B. Kim et al., 2020; Song et al., 2020; G. Xu et al., 2019a, 2019b). Sensing systems of this device class use primarily MRC and NFC due to it’s robust infrastructure, and recent work featured in section 2.1 has utilized RF and other internal power harvesting techniques to eliminate the need of a power casting source (G. Xu et al., 2019a) leading the way towards fully autonomous embodiments with broad impact in health diagnostics and preventative screening for individuals who are at risk for metabolic disease (Gao et al., 2016; Jia et al., 2013).
Figure 8. Devices for real-time metabolic.
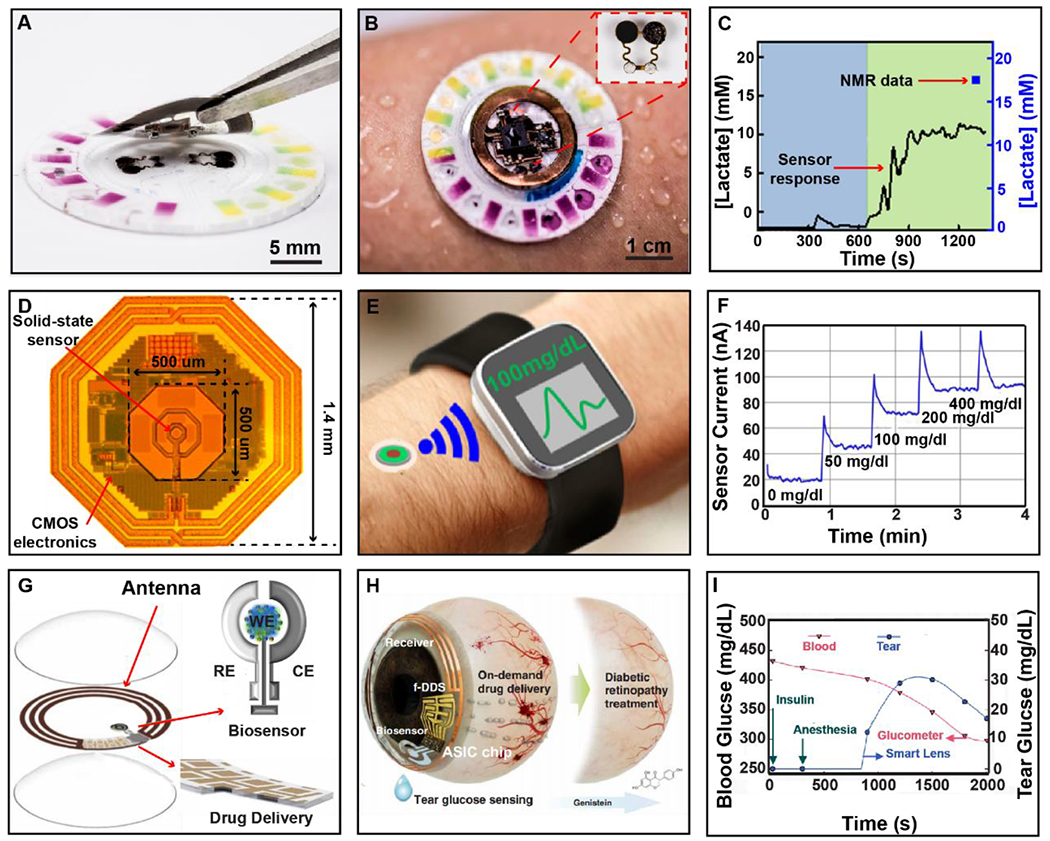
A) Photographic image showing two-part device structure with removable NFC electronics and single use epifluidic patch. B) Photographic Image of device on perspiring subject with inset showing biofuel cell for continuous metabolite sensing. C) Continuous lactate detection during exercise with comparison to gold-standard, single collection NMR analysis. D) Micrographic image of integrated wireless platform with key features labeled. E) Illustration of proposed device function. F) In Vitro response of GOx coated sensor when exposed to varying concentrations of Glucose solution. G) Layered makeup of device components for power transfer and data communication, glucose biosensor, and flexible drug delivery system integrated into a contact lens. H) Illustration of device function for closed-loop diabetic retinopathy treatment. I) In vivo response of smart lens compared to glucometer during treatment with insulin.
3.3.2. Implantable Endocrine and Metabolic Sensors
For the most accurate representation of physiologically relevant biochemical levels, it is beneficial to implement implantable systems, as they can collect biodata directly from the interstitial fluid for more accurate and real time information and do not suffer from epidermal interface erosion (Arora, 2013; Ming Li et al., 2007; Scholten and Meng, 2018). Implantable devices of this nature face harsher constrains on system design as form factor, power transfer, and sensing modality have to factor in patient comfort, system robustness, and invasiveness required for the implantation procedure. Current implantable systems for this application suffer from poor lifetime and biocompatibility (H. Lee et al., 2018) and generally require transdermal probes for operation (Corrie et al., 2015; Hage et al., 2010; Lucarelli et al., 2012), limiting patient function and increasing discomfort due to secondary procedures to recover sensors after use. Therefore, miniaturization of the implanted sensor architecture is often chosen as a design philosophy. A representative device this class of sensor system is shown in Figure 8D (Mujeeb-U-Rahman et al., 2019a). This system utilizes ultra-small CMOS-based wireless, battery-free glucose monitoring systems in a form factor ideal for minimally invasive implantation. For this device, a CMOS based system including sensing, power, and communication electronics serves as the platform of the solid-state sensor used to detect interstitial levels of glucose. This device functions using RF power transfer (see section 2.1) at a frequency of 900 MHz, which is suitable for subdermal implantation when combined with a powering and readout system in close proximity. Backscattering is used for communication because it can be implemented on one chip and enables low-power operation (5 mW) (Liu et al., 2019; Mujeeb-U-Rahman et al., 2019b). Additionally, the proximity to the biofluid results in a fast response time that is significantly improved over traditional transcutaneous devices (H. Lee et al., 2018). Figure 8E displays working principle, where the implant is powered by a wearable transmitter that casts power and communicates and functions as a gateway via Bluetooth to relay information for storage and analysis. Figure 8F shows the sensors response to varying concentrations of glucose in physiologically relevant ranges (Bruen et al., 2017). The device demonstrates significantly improved interstitial fluid lag when compared to other continuous glucose monitoring systems, with high sensitivity (40 mg/dl) during periods of hypoglycemia. This sensitivity is achieved with lifetimes of over 1 month of operation and high sampling rates of 1 kHz (See SI Table 2). Implantable systems of this form factor provide precise, effective means of delivering high-fidelity information without on-going risk of infection or interface failure that can avoid serious consequences for health outcomes (Ahmadi and Jullien, 2009; DeHennis et al., 2016; Kropff et al., 2017) Utilization of external, battery-free power sources enables long-term data collection from the system without the need for repeated insertion of probes or changing of batteries. More broadly, the approach of using an external wearable power source and readout electronics in combination with miniaturized implantable sensors has the potential to be highly impactful for chronic applications that require high fidelity signal acquisition over a long time. The access to interstitial fluid has the potential to expand sensing targets well beyond glucose monitoring towards fully integrated closed-loop disease management, which can have significant impact on diagnostic and therapeutic digital medicine capabilities.
3.3.3. Ocular Endocrine and Metabolic Sensors
The miniaturization achieved though implementation of wireless, battery-free strategies, coupled with progression of material science towards mechanically compliant systems has proliferated use towards unique target organ systems that are inaccessible or impractical targets with the current state of technology. One such example has been the development of smart contact lenses, which provide an avenue to sense intraocular pressure (G.-Z. Chen et al., 2014) and tear-based biomarkers (Ku et al., 2020; Liao et al., 2012; Zhang et al., 2011). This is significant because tear-based fluids contain a rich amount of biochemical information and can be accessed without invasive methods (Farandos et al., 2015). Recent attempts to use this sensing target with passive resonator designs which can be realized with few components, typically a capacitive or resistance-based sensor coupled with an antenna loop, however, chronic system stability of this class is usually poor (Chen et al., 2013; Kim et al., 2017). Recent innovations in this class of devices have expanded the functionality with active digital sensing components. The device featured in Figure 8G (Keum et al., 2020), shows such a system that incorporates a tear-based glucose sensing platform with flexible drug delivery that monitors tear glucose concentrations with active, on-demand pharmaceutical delivery. The device uses MRC power transfer at 443 MHz (see section 2.1) and low-power backscatter communication (see section 2.2). The system is fully integrated on a single die ASIC that houses an analog interface that can detect μA changes in current while simultaneously deploying drug delivery capabilities in a contact lens form factor (Shown in Figure 8H). This device implements real-time electrochemical detection of glucose concentration via using bioconjugated electrodes, specifically a platinum working electrode coated with Chitosan and polyvinyl alcohol hydrogels that contain glucose oxidase (GOx). Glucose is oxidized at the working electrode to produce a current representing the concentration of glucose. Sensing performance of 1μA/15mg dl−1 is superior to conventional tear-based analysis systems that do not allow for continuous monitoring(La Belle et al., 2016; Lee et al., 2019). Control of drug delivery is modulated through a voltage-controlled dissolution of a gold membrane, allowing for release of pharmaceuticals. Figure 8I shows system function during an in vivo study involving diabetic small animal models that are treated with insulin. The system shows good agreement with blood glucose levels considering lag inherent in glucose presentation in tear fluid, which has been previously studied (Aihara et al., 2018; Sen and Sarin, 1980). This and other demonstrations (Gulsen and Chauhan, 2004; Joohee Kim et al., 2017; Kownacka et al., 2018; Ku et al., 2020; La Belle et al., 2016; Liao et al., 2012; Zhang et al., 2011) show that tear fluid as a sensing target and the eye as a location for noninvasive drug delivery are an opportunity to advance diagnostic and therapeutic capabilities and substantially improve the quality of life for a large patient population. The location of these sensors requires the use of wireless and battery-free system architectures because of the need for low profile layout that occupies minimal space to avoid irritation and clear vision. The success of this class of devices will largely depend on sensor stability and technological maturity of power casting and integrated receiving systems that enable continuous operation in the eye (Chiou et al., 2017).
3.4. Other Physiological Systems
Wireless, battery-free sensing systems can expand capabilities to a broad range of target organ systems. In this section, we capture most impactful sensing systems outside of commonly targeted organ systems discussed in prior sections with demonstrations of devices that expand current sensing technologies. We discuss platforms that are made possible with implementation of this technology, and briefly describe approaches that go beyond the implementation of a sensor or a sensor system and demonstrate device which can operate in a closed loop.
3.4.1. Integumentary
The epidermis is the largest organ on the body and is susceptible to many injuries and diseases that are difficult to monitor with the current state of technology. Epidermal interfaces are traditionally plagued with interfacial challenges due to high impedance caused by high rates of epidermal turnover (Halprin, 1972). Wireless, battery-free sensing systems can provide a platform to address these challenges by enabling an intimate contact that results in lower impedance and lower motion artefacts, resulting in continuous recording capabilities such as the devices outlined in sections 3.1.1, 3.2.1, and 3.3.1. This high fidelity interface is not only limited to optical and electrical signals but can also be extended towards mechanical and thermal signatures (Kwak et al., 2020) that enable advanced applications in the detection of wound healing (Hattori et al., 2014), skin hydration (Krishnan et al., 2017), and blood perfusion (Webb et al., 2015). Figure 9A shows such a device used for thermal characterization of the skin in an ultra-small, conformal form factor. This system relies on MRC and NFC (see sections 2.1 and 2.2) to provide power to the thermal actuator/sensing unit while simultaneously utilizing thermography to capture changes in temperature to determine variations in thermal conductivity and heat capacity of the skin (Krishnan et al., 2018). Thermal actuation is a power intensive process, thus adequate power availability is essential for system function. For this use case, MRC is used as the power transfer method due to its high power-availability (~ 20 mW for this embodiment) (Lazaro et al., 2020). Communication is achieved with NFC, which is the preferred candidate for on skin applications (see section 2.2). The sensing principle relies on transfer of heat to the superficial epidermal layer using constant power thermal actuation and simultaneous monitoring of temperature to calculate the thermal conductivity of the underlying tissue. This scheme is only possible when thermal mass of the sensor/actuator combination is low and the thermal coupling to the target tissue is good, which can only be efficiently achieved with thin epidermal interfaces. At a low constant power (2 mW) the sensor/actuator combination can locally heat the underlying tissue below the threshold of sensation (Δ 6K) (Krishnan et al., 2018), providing sensor function with no user perception. The change in temperature, can then be used to compute thermal conductivity of the underlying tissue layers. This sensor configuration can achieve a resolution of 0.02 W m−1 K−1, which enables the insight into tissue health and properties such as hydration (Krishnan et al., 2017), wound healing (Hattori et al., 2014), and infection (Madhvapathy et al., 2018). Figure 9B shows a subject’s arm, in which the device was used to probe tissue properties of a class II burn injury. This system is able to detect the subtle changes in thermal conductivity evoked by the severity of burn damage to the underlying epidermis, corresponding results are shown in figure 9C, that clearly show distinction in thermal properties between the probed tissue locations. In the same work the authors also report on the capability to detect other conditions such as ischemia (Regas and Ehrlich, 1992) and flow in shunted arteries and more recent work has used the technique as monitors for shunt flow in hydrocephalous patients (Krishnan et al., 2020). The work also shows the capability to track skin hydration over several days, this is possible due to the dry epidermal interface that is stable despite epidermal turnover. These capabilities highlight the impact of this technology when coupled with wireless and battery-free devices to continuously assess skin health and highlight possible applications in chronic wound healing (Dargaville et al., 2013; Pasche et al., 2009), skin transplant health (Jain and Shakya, 2009) and skin irritation, conditions that effect a large patient populations.
Figure 9. Advance applications for wireless, battery-free systems.
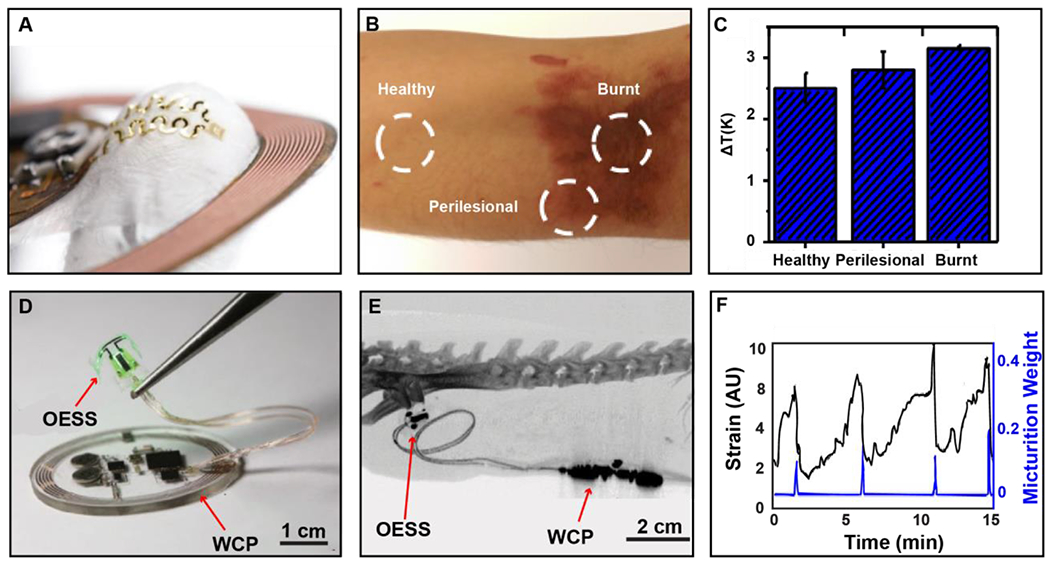
A) Photographic image of device showing flexibility of epidermal actuator/sensing combination. B) Photographic Image illustrating testing conditions of subject’s epidermis. C) Thermal characteristics based on health status of epidermis corresponding to panel B. D) Photographic image of closed-loop system showing the wireless control and power module (WCP) and optoelectronic stimulation and sensing module (OESS) featuring a strain gauge andμ-ILED for optogenetic stimulation. E) CT image showing device 1-month post-implantation with sensor and stimulation interface secured circumferentially around the bladder and subdermally implanted wireless battery-free system. F) Graph displaying recorded date during closed-loop operation of the system with continuous strain gauge data (black) and micturition events measured by a balance (blue).
3.4.3. Urinary
The ability to deliver power continuously to power digital electronics naturally suggests the usage of on-board computing on the device itself. The sensor systems that we review in the previous sections, however, only do this in a limited capacity to, for example, to reduce bandwidth requirement for communication links (section 2.2). An approach that is only rarely realized are closed loop systems that couple sensing modalities with actuation or modulation to realize autonomous systems that automatically treat chronic illness, aid in management of disabilities and offer advanced insight into fundamental mechanisms of human and animal physiology (Parastarfeizabadi and Kouzani, 2017; Renard, 2002; Scholten and Meng, 2018; Sun and Morrell, 2014). Current trends in electronic medicine have been focused on development and understanding treatment strategies using stimulation of peripheral nervous system paired with targeted biosensors to refine stimulus delivery (Cameron, 2004; Famm et al., 2013). One organ system that is rarely targeted due to complex anatomy, is the urinary system, specifically targeting bladder dysfunction through closed-loop modulation (de Groat and Tai, 2015; Siegel et al., 2000). Wired systems for this application have been utilized to treat and examine voiding dysfunctions through electrical stimulation, however current systems are highly invasive and require a clinical setting with limited chronic operation appeal (J. et al., 2006; Park et al., 2018). Wireless and battery-free systems provide a favorable alternative due to their robust long-term data collection and transmission capabilities with on-board processors to handle data analysis coupled with the ability of system miniaturization that enables full implantation (Zhong et al., 2020). One such system is shown in Figure 9F this fully integrated, closed-loop system features a wireless control and power module and an optoelectrical stimulation and sensing platform. This system is utilized in small animal model research and uses optogenetic stimulation of the sacral nerve to regulate bladder activity while simultaneously recording strain on the bladder. MRC is used for continuous power deliver to the rodent subjects as discussed in section 2.1 and has been successfully deployed in devices discussed in sections 3.1, 3.2 and 3.3. BLE is used for data communication to adjust and monitor closed loop parameters. A circumferential strain gauge attached to the bladder (as shown in Figure 9E) collects information on extension, which is an indicator of fill level. The strain gauge is made from a carbon black doped elastomer to provide flexible mechanics to enable full implantation and overall soft mechanics of the system. Stimulation is carried out with μ -ILED nerve cuff that is comprised of low-modulus silicone to provide soft mechanics in an optically clear platform that seamlessly interfaces with peripheral nerves. Information from the strain gauge is translated to bladder size and computed to determine if micturition events are consistent, hyper-, or hypoactive. If there is variation in the micturition events when correlated to strain gauge values, optogenetic stimulation via μ-ILED is used to void the bladder in a closed loop fashion to supplement natural function. Figure 9F shows strain gauge data with correlated micturition events as detected by the subject’s closed loop implant and stimulation events that are computed by the system to return to regular bladder activity.
Demonstrations of closed loop regulation capability highlight advanced computational capabilities that can be accomplished with wireless, battery-free devices and showcase a promising avenue for the realization of systems that automatically treat disease or supplement body function without clinical intervention.
4. Conclusion
Wireless, battery-free sensing systems can provide a platform that can be fundamentally advantageous for biosensing applications through the intimate integration opportunities enabled by a highly miniaturized form factor, soft mechanics, low weight and uninterrupted operation. Wireless power supply or power harvesting coupled with suitable data communication strategies can yield highly capable systems in wearable and implantable format. As demonstrated by most recent advances highlighted in this review, this device class quantitively expands current capabilities over conventional sensing approaches and rapid rate of development suggests increasingly capable bioelectronic systems in the near future. Specifically, improvements to wireless, battery-free power transfer and power harvesting provides opportunities to increase both operational range and the inclusion of power-hungry sensing modalities. This potentially enables the development of sensing platforms that can expand use cases of existing technologies towards sensing modalities not yet realized in wireless and battery-free format. Examples that would expand current sensing capabilities include impedance spectroscopy, continuous blood pressure assessment, and on device continuous analysis requiring multiple streams of high-fidelity data and AI analysis approaches which require more capable computing on the device. Conceptually however, the idea of continuously monitored health with biosensors is only in its infancy. With the expansion and proliferation of wireless and battery free systems, a standardized infrastructure will be needed to support both the continuous powering of these systems and means of data aggregation compliant with current HIPAA requirements. The increased data stream by continuously operating systems and enhanced fidelity enabled by soft mechanics enables advanced disease assessment and prediction if analysis methods can be created that need little human supervision. AI and machine learning offer these attributes if reliable models can be created that take multimodal data streams into account. Assuming increased efficiency of power harvesting and power casting techniques, closed-loop system functionality beyond the boundaries of research paradigms can enable advanced diagnostics and support organ systems. Sensor platforms, as the ones reviewed here, form the basis of progression towards autonomous and integrated healthcare solutions that have the potential to drastically improve the current standard of care and health outcomes.
Supplementary Material
Acknowledgements
We acknowledge support from the department of Biomedical Engineering at the University of Arizona. P Gutruf and T Stuart acknowledge support from the Flinn Foundation Translational Bioscience Seed Grants Pilot Program, P Gutruf and A Burton acknowledge support from the National Institutes of Health (1R01NS112535-01).
References
- Abraham WT, Perl L, 2017. Implantable Hemodynamic Monitoring for Heart Failure Patients. J. Am. Coll. Cardiol. 70, 389–398. 10.1016/j.jacc.2017.05.052 [DOI] [PubMed] [Google Scholar]
- Adam Y, Kim JJ, Lou S, Zhao Y, Xie ME, Brinks D, Wu H, Mostajo-Radji MA, Kheifets S, Parot V, Chettih S, Williams KJ, Gmeiner B, Farhi SL, Madisen L, Buchanan EK, Kinsella I, Zhou D, Paninski L, Harvey CD, Zeng H, Arlotta P, Campbell RE, Cohen AE, 2019. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569, 413–417. 10.1038/s41586-019-1166-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahire DB, Gond VJ, 2017. Wireless power transfer system for biomedical application: A review, in: 2017 International Conference on Trends in Electronics and Informatics (ICEI). pp. 135–140. 10.1109/ICOEI.2017.8300903 [DOI] [Google Scholar]
- Ahmadi MM, Jullien GA, 2009. A Wireless-Implantable Microsystem for Continuous Blood Glucose Monitoring. IEEE Trans. Biomed. Circuits Syst. 3, 169–180. 10.1109/TBCAS.2009.2016844 [DOI] [PubMed] [Google Scholar]
- Aihara M, Kubota N, Kadowaki T, 2018. Study of the Correlation between Tear Glucose Concentrations and Blood Glucose Concentrations. Diabetes 67, 944–P. 10.2337/db18-944-P [DOI] [Google Scholar]
- Alberto J, Leal C, Fernandes C, Lopes PA, Paisana H, de Almeida AT, Tavakoli M, 2020. Fully Untethered Battery-free Biomonitoring Electronic Tattoo with Wireless Energy Harvesting. Sci. Rep. 10, 5539. 10.1038/s41598-020-62097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, 2007. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 28, R1. [DOI] [PubMed] [Google Scholar]
- Amar A, Kouki A, Cao H, 2015. Power approaches for implantable medical devices. sensors 15, 28889–28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Krook-Magnuson E, Oijala M, Soltesz I, 2013. Closed-loop optogenetic intervention in mice. Nat. Protoc. 8, 1475–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora N, 2013. Recent advances in biosensors technology: a review. Octa J. Biosci. 1. [Google Scholar]
- Bade BC, Brooks MC, Nietert SB, Ulmer A, Thomas DD, Nietert PJ, Scott JB, Silvestri GA, 2018. Assessing the Correlation Between Physical Activity and Quality of Life in Advanced Lung Cancer. Integr. Cancer Ther. 17, 73–79. 10.1177/1534735416684016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig MM, GholamHosseini H, Moqeem AA, Mirza F, Lindén M, 2017. A Systematic Review of Wearable Patient Monitoring Systems – Current Challenges and Opportunities for Clinical Adoption. J. Med. Syst. 41, 115. 10.1007/s10916-017-0760-1 [DOI] [PubMed] [Google Scholar]
- Baker LB, Wolfe AS, 2020. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 120, 719–752. 10.1007/s00421-020-04323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandodkar AJ, Choi J, Lee SP, Jeang WJ, Agyare P, Gutruf P, Wang S, Sponenburg RA, Reeder JT, Schon S, Ray TR, Chen S, Mehta S, Ruiz S, Rogers JA, 2019a. Soft, Skin-Interfaced Microfluidic Systems with Passive Galvanic Stopwatches for Precise Chronometric Sampling of Sweat. Adv. Mater. 31, 1902109. 10.1002/adma.201902109 [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Gutruf P, Choi J, Lee K, Sekine Y, Reeder JT, Jeang WJ, Aranyosi AJ, Lee SP, Model JB, others, 2019b. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294. 10.1126/sciadv.aav3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandodkar AJ, Jeang WJ, Ghaffari R, Rogers JA, 2019c. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 12, 1–22. 10.1146/annurev-anchem-061318-114910 [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Lee SP, Huang I, Li W, Wang S, Su C-J, Jeang WJ, Hang T, Mehta S, Nyberg N, Gutruf P, Choi J, Koo J, Reeder JT, Tseng R, Ghaffari R, Rogers JA, 2020. Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems. Nat. Electron. 10.1038/s41928-020-0443-7 [DOI] [Google Scholar]
- Bandodkar AJ, Wang J, 2014. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371. 10.1016/J.TIBTECH.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Barker SJ, 2002. “Motion-resistant” pulse oximetry: a comparison of new and old models. Anesth. Analg. 95, 967–972. [DOI] [PubMed] [Google Scholar]
- Barman S. Das, Reza AW, Kumar N, Karim ME, Munir AB, 2015. Wireless powering by magnetic resonant coupling: Recent trends in wireless power transfer system and its applications. Renew. Sustain. energy Rev. 51, 1525–1552. [Google Scholar]
- Basaeri H, Christensen DB, Roundy S, 2016. A review of acoustic power transfer for bio-medical implants. Smart Mater. Struct. 25, 123001. [Google Scholar]
- Becker DE, 2006. Fundamentals of electrocardiography interpretation. Anesth. Prog. 53, 53–64. https://doi.org/10.2344/0003-3006(2006)53[53:FOEI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnoff JS, Deyle T, Harrison RR, Reynolds MS, 2013. Battery-free multichannel digital ECG biotelemetry using UHF RFID techniques, in: 2013 IEEE International Conference on RFID (RFID). pp. 16–22. 10.1109/RFID.2013.6548130 [DOI] [Google Scholar]
- Bevilacqua F, Piguet D, Marquet P, Gross JD, Tromberg BJ, Depeursinge C, 1999. In vivo local determination of tissue optical properties: applications to human brain. Appl. Opt. 38, 4939–4950. [DOI] [PubMed] [Google Scholar]
- Bin N-R, Song H, Wu C, Lau M, Sugita S, Eubanks JH, Zhang L, 2017. Continuous Monitoring via Tethered Electroencephalography of Spontaneous Recurrent Seizures in Mice. Front. Behav. Neurosci. 11, 172. 10.3389/fnbeh.2017.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA, 2007. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. Part A 82A, 169–178. 10.1002/jbm.a.31138 [DOI] [PubMed] [Google Scholar]
- Bize R, Johnson JA, Plotnikoff RC, 2007. Physical activity level and health-related quality of life in the general adult population: A systematic review. Prev. Med. (Baltim). 45, 401–415. 10.1016/j.ypmed.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Bonato P, 2005. Advances in wearable technology and applications in physical medicine and rehabilitation. J. Neuroeng. Rehabil. 2, 2. 10.1186/1743-0003-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry CM, Beker L, Kaizawa Y, Vassos C, Tran H, Hinckley AC, Pfattner R, Niu S, Li J, Claverie J, others, 2019. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47. [DOI] [PubMed] [Google Scholar]
- Bruen D, Delaney C, Florea L, Diamond D, 2017. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors (Basel). 17, 1866. 10.3390/s17081866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen RA, Arnot TC, Lakeman JB, Walsh FC, 2006. Biofuel cells and their development. Biosens. Bioelectron. 10.1016/j.bios.2006.01.030 [DOI] [PubMed] [Google Scholar]
- Burton A, Obaid SN, Vázquez-Guardado A, Schmit MB, Stuart T, Cai L, Chen Z, Kandela I, Haney CR, Waters EA, Cai H, Rogers JA, Lu L, Gutruf P, 2020. Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl. Acad. Sci. 201920073. 10.1073/pnas.1920073117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnan H, Denison T, McIntyre C, Brown P, 2019. Emerging technologies for improved deep brain stimulation. Nat. Biotechnol. 37, 1024–1033. 10.1038/s41587-019-0244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron T, 2004. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J. Neurosurg. Spine 100, 254–267. 10.3171/spi.2004.100.3.0254 [DOI] [PubMed] [Google Scholar]
- Cao H, Leung V, Chow C, Chan H, 2009. Enabling technologies for wireless body area networks: A survey and outlook. IEEE Commun. Mag. 47, 84–93. [Google Scholar]
- Cartlidge PHT, Fox PE, Rutter N, 1990. The scars of newborn intensive care. Early Hum. Dev. 21, 1–10. 10.1016/0378-3782(90)90105-R [DOI] [PubMed] [Google Scholar]
- Cecil S, Schmid G, Lamedschwandner K, Morak J, Schreier G, Oberleitner A, Bammer M, 2010. Numerical Assessment of Specific Absorption Rate in the Human Body Caused by NFC Devices, in: 2010 Second International Workshop on Near Field Communication. pp. 65–70. 10.1109/NFC.2010.14 [DOI] [Google Scholar]
- Chatterjee S, Khunti K, Davies MJ, 2017. Type 2 diabetes. Lancet 389, 2239–2251. 10.1016/S0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- Chen G-Z, Chan I-S, Lam DCC, 2013. Capacitive contact lens sensor for continuous non-invasive intraocular pressure monitoring. Sensors Actuators A Phys. 203, 112–118. 10.1016/j.sna.2013.08.029 [DOI] [Google Scholar]
- Chen G-Z, Chan I-S, Leung LKK, Lam DCC, 2014. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med. Eng. Phys. 36, 1134–1139. 10.1016/j.medengphy.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Chen H, Bai Z, Dai X, Zeng X, Cano ZP, Xie X, Zhao M, Li M, Wang H, Chen Z, others, 2019. In Situ Engineering of Intracellular Hemoglobin for Implantable High-Performance Biofuel Cells. Angew. Chemie 131, 6735–6740. [DOI] [PubMed] [Google Scholar]
- Chen H, Xiang S, Li W, Liu H, Zhu L, Yang S, 2018. Inorganic Perovskite Solar Cells: A Rapidly Growing Field. Sol. RRL. [Google Scholar]
- Chen JH, Asch SM, 2017. Machine Learning and Prediction in Medicine - Beyond the Peak of Inflated Expectations. N. Engl. J. Med. 376, 2507–2509. 10.1056/NEJMp1702071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Op de Beeck M, Vanderheyden L, Carrette E, Mihajlović V, Vanstreels K, Grundlehner B, Gadeyne S, Boon P, Van Hoof C, 2014. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors (Basel). 14, 23758–23780. 10.3390/s141223758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Li X, Xu G, Lu Y, Low SS, Liu G, Zhu L, Li C, Liu Q, 2021. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron. 172, 112782. 10.1016/j.bios.2020.112782 [DOI] [PubMed] [Google Scholar]
- Cheng K, Zou X, Cheong JH, Xue R, Chen Z, Yao L, Cha H, Cheng SJ, Li P, Liu L, Andia L, Ho CK, Cheng M, Duan Z, Rajkumar R, Zheng Y, Goh WL, Guo Y, Dawe G, Park W, Je M, 2012. 100-Channel wireless neural recording system with 54-Mb/s data link and 40%-efficiency power link, in: 2012 IEEE Asian Solid State Circuits Conference (A-SSCC). pp. 185–188. [Google Scholar]
- Cheng M-Y, Damalerio RB, Chen W, Rajkumar R, Dawe GS, 2019. Ultracompact Multielectrode Array for Neurological Monitoring. Sensors (Basel). 19, 2286. 10.3390/s19102286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestek CA, Gilja V, Nuyujukian P, Kier RJ, Solzbacher F, Ryu SI, Harrison RR, Shenoy KV, 2009. HermesC: Low-Power Wireless Neural Recording System for Freely Moving Primates. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 330–338. 10.1109/TNSRE.2009.2023293 [DOI] [PubMed] [Google Scholar]
- Chiou J-C, Hsu S-H, Huang Y-C, Yeh G-T, Liou W-T, Kuei C-K, 2017. A Wirelessly Powered Smart Contact Lens with Reconfigurable Wide Range and Tunable Sensitivity Sensor Readout Circuitry. Sensors . 10.3390/s17010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlaihawi AA, Narakathu BB, Emamian S, Bazuin BJ, Atashbar MZ, 2018. Development of printed and flexible dry ECG electrodes. Sens. Bio-Sensing Res. 20, 9–15. 10.1016/j.sbsr.2018.05.001 [DOI] [Google Scholar]
- Choi A, Shin H, 2017. Photoplethysmography sampling frequency: pilot assessment of how low can we go to analyze pulse rate variability with reliability? Physiol. Meas. 38, 586–600. 10.1088/1361-6579/aa5efa [DOI] [PubMed] [Google Scholar]
- Choi J, Ghaffari R, Baker LB, Rogers JA, 2018. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 4, eaar3921. 10.1126/sciadv.aar3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HU, Kim BH, Lee JY, Lee J, Xie Z, Ibler EM, Lee K, Banks A, Jeong JY, Kim J, others, 2019. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science (80-. ). 363, eaau0780. 10.1126/science.aau0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong P, Chaimanonart N, Ko WH, Young DJ, 2009. A Wireless and Batteryless 10-Bit Implantable Blood Pressure Sensing Microsystem With Adaptive RF Powering for Real-Time Laboratory Mice Monitoring. IEEE J. Solid-State Circuits 44, 3631–3644. 10.1109/JSSC.2009.2035551 [DOI] [Google Scholar]
- Corrie SR, Coffey JW, Islam J, Markey KA, Kendall MAF, 2015. Blood, sweat, and tears: developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 140, 4350–4364. 10.1039/c5an00464k [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM, 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242. 10.1038/nature11846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdeviren C, Javid F, Joe P, von Erlach T, Bensel T, Wei Z, Saxton S, Cleveland C, Booth L, McDonnell S, others, 2017. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 1, 807. [DOI] [PubMed] [Google Scholar]
- Dagdeviren C, Yang BD, Su Y, Tran PL, Joe P, Anderson E, Xia J, Doraiswamy V, Dehdashti B, Feng X, Lu B, Poston R, Khalpey Z, Ghaffari R, Huang Y, Slepian MJ, Rogers JA, 2014. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. U. S. A. 111, 1927–1932. 10.1073/pnas.1317233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargaville TR, Farrugia BL, Broadbent JA, Pace S, Upton Z, Voelcker NH, 2013. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 41, 30–42. 10.1016/j.bios.2012.09.029 [DOI] [PubMed] [Google Scholar]
- de Groat WC, Tai C, 2015. Impact of Bioelectronic Medicine on the Neural Regulation of Pelvic Visceral Function. Bioelectron. Med. 2015, 25–36. [PMC free article] [PubMed] [Google Scholar]
- DeHennis A, Getzlaff S, Grice D, Mailand M, 2016. An NFC-Enabled CMOS IC for a Wireless Fully Implantable Glucose Sensor. IEEE J. Biomed. Heal. Informatics 20, 18–28. 10.1109/JBHI.2015.2475236 [DOI] [PubMed] [Google Scholar]
- DeHennis AD, Wise KD, 2006. A fully integrated multisite pressure sensor for wireless arterial flow characterization. J. Microelectromechanical Syst. 15, 678–685. 10.1109/JMEMS.2006.876668 [DOI] [Google Scholar]
- DeMeulenaere S, 2007. Pulse Oximetry: Uses and Limitations. J. Nurse Pract. 3, 312–317. 10.1016/j.nurpra.2007.02.021 [DOI] [Google Scholar]
- Denisov A, Yeatman E, 2010. Ultrasonic vs. inductive power delivery for miniature biomedical implants, in: 2010 International Conference on Body Sensor Networks. pp. 84–89. [Google Scholar]
- Dieffenderfer JP, Goodell H, Bent B, Beppler E, Jayakumar R, Yokus M, Jur JS, Bozkurt A, Peden D, 2015. Wearable wireless sensors for chronic respiratory disease monitoring, in: 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN). pp. 1–6. 10.1109/BSN.2015.7299411 [DOI] [Google Scholar]
- Dobkin DM, 2012. The rf in RFID: uhf RFID in practice. Newnes. [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW, 2010. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440. 10.1038/nn.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JA, Cahill DF, 1984. Biological effects of radiofrequency radiation. Health Effects Research Laboratory, Office of Research and Development, US~…. [Google Scholar]
- Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D, 2016. Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci. 19, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M, 2013. A jump-start for electroceuticals. Nature 496, 159–161. 10.1038/496159a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmand S, Vahedian H, Eslami MA, Sodagar AM, 2012. Wearable, battery-powered, wireless, programmable 8-channel neural stimulator, in: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. pp. 6120–6123. 10.1109/EMBC.2012.6347390 [DOI] [PubMed] [Google Scholar]
- Farandos NM, Yetisen AK, Monteiro MJ, Lowe CR, Yun SH, 2015. Contact Lens Sensors in Ocular Diagnostics. Adv. Healthc. Mater. 4, 792–810. 10.1002/adhm.201400504 [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Redish AD, 2011. Wireless communication with implanted medical devices using the conductive properties of the body. Expert Rev. Med. Devices 8, 427–433. 10.1586/erd.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrea E, Maccione A, Medrihan L, Nieus T, Ghezzi D, Baldelli P, Benfenati F, Berdondini L, 2012. Large-scale, high-resolution electrophysiological imaging of field potentials in brain slices with microelectronic multielectrode arrays. Front. Neural Circuits 6, 80. 10.3389/fncir.2012.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuny G, Herrero J, Puigjaner D, Olivé C, Marimon F, Garcia-Bennett J, Rodríguez D, 2015. Effect of anticoagulant treatment in deep vein thrombosis: A patient-specific computational fluid dynamics study. J. Biomech. 48, 2047–2053. 10.1016/j.jbiomech.2015.03.026 [DOI] [PubMed] [Google Scholar]
- Fotowat H, Harrison RR, Gabbiani F, 2011. Multiplexing of Motor Information in the Discharge of a Collision Detecting Neuron during Escape Behaviors. Neuron 69, 147–158. 10.1016/j.neuron.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddam A, Mukhopadhyay SC, Gupta G. Sen, Guesgen H, 2008. Wireless Sensors Networks based monitoring: Review, challenges and implementation issues, in: 2008 3rd International Conference on Sensing Technology. pp. 533–538. 10.1109/ICSENST.2008.4757163 [DOI] [Google Scholar]
- Gao W, Emaminejad S, Nyein HYY, Challa S, Chen KV, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D, Lien DH, Brooks GA, Davis RW, Javey A, 2016. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509--+. 10.1038/nature16521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghane-Motlagh B, Sawan M, 2013. A review of Microelectrode Array technologies: Design and implementation challenges, in: 2013 2nd International Conference on Advances in Biomedical Engineering. pp. 38–41. 10.1109/ICABME.2013.6648841 [DOI] [Google Scholar]
- Giesbertz P, Daniel H, 2016. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care 19. [DOI] [PubMed] [Google Scholar]
- Goland S, Czer LSC, De Robertis MA, Mirocha J, Kass RM, Fontana GP, Chang W, Trento A, 2007. Risk Factors Associated With Reoperation and Mortality in 252 Patients After Aortic Valve Replacement for Congenitally Bicuspid Aortic Valve Disease. Ann. Thorac. Surg. 83, 931–937. 10.1016/j.athoracsur.2006.10.047 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Solino C, Lorenzo DM, 2018. Enzymatic Fuel Cells: Towards Self-Powered Implantable and Wearable Diagnostics. Biosensors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradman AH, 2012. Strategies for combination therapy in hypertension. Curr. Opin. Nephrol. Hypertens. 21. [DOI] [PubMed] [Google Scholar]
- Grand L, Ftomov S, Timofeev I, 2013. Long-term synchronized electrophysiological and behavioral wireless monitoring of freely moving animals. J. Neurosci. Methods 212, 237–241. 10.1016/j.jneumeth.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald EC, Mehta S, Zhang J, 2018. Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem. Rev. 118, 11707–11794. 10.1021/acs.chemrev.8b00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A, 2012. Imaging calcium in neurons. Neuron 73, 862–885. 10.1016/j.neuron.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Gulsen D, Chauhan A, 2004. Ophthalmic Drug Delivery through Contact Lenses. Invest. Ophthalmol. Vis. Sci. 45, 2342–2347. 10.1167/iovs.03-0959 [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K, 2014. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutruf P, Rogers JA, 2018. Implantable, wireless device platforms for neuroscience research. Curr. Opin. Neurobiol. 50, 42–49. [DOI] [PubMed] [Google Scholar]
- Hage C, Mellbin L, Rydén L, Wernerman J, 2010. Glucose monitoring by means of an intravenous microdialysis catheter technique. Diabetes Technol. Ther. 12, 291–295. 10.1089/dia.2009.0150 [DOI] [PubMed] [Google Scholar]
- Halprin KM, 1972. Epidermal “Turnover Time”—A Re-Examination. Br. J. Dermatol. 86, 14–19. [DOI] [PubMed] [Google Scholar]
- Hamilton PS, Curley MG, Aimi RM, Sae-Hau C, 2000. Comparison of methods for adaptive removal of motion artifact, in: Computers in Cardiology 2000. Vol.27 (Cat. 00CH37163). pp. 383–386. 10.1109/CIC.2000.898537 [DOI] [Google Scholar]
- Han S, Kim J, Won SM, Ma Y, Kang D, Xie Z, Lee K-T, Chung HU, Banks A, Min S, Heo SY, Davies CR, Lee JW, Lee C-H, Kim BH, Li K, Zhou Y, Wei C, Feng X, Huang Y, Rogers JA, 2018. Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 10. 10.1126/scitranslmed.aan4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker M, Coulson H, Fairweather I, Taylor D, Daykin CA, 2006. Study of metabolite composition of eccrine sweat from healthy male and female human subjects by 1H NMR spectroscopy. Metabolomics 2, 105–112. 10.1007/s11306-006-0024-4 [DOI] [Google Scholar]
- Harrison RR, Fotowat H, Chan R, Kier RJ, Olberg R, Leonardo A, Gabbiani F, 2011. Wireless Neural/EMG Telemetry Systems for Small Freely Moving Animals. IEEE Trans. Biomed. Circuits Syst. 5, 103–111. 10.1109/TBCAS.2011.2131140 [DOI] [PubMed] [Google Scholar]
- Harrison RR, Kier RJ, Chestek CA, Gilja V, Nuyujukian P, Ryu S, Greger B, Solzbacher F, Shenoy KV, 2009. Wireless Neural Recording With Single Low-Power Integrated Circuit. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 322–329. 10.1109/TNSRE.2009.2023298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RR, Kier RJ, Kim S, Rieth L, Warren DJ, Ledbetter NM, Clark GA, Solzbacher F, Chestek CA, Gilja V, 2008. A wireless neural interface for chronic recording, in: 2008 IEEE Biomedical Circuits and Systems Conference. IEEE, pp. 125–128. [Google Scholar]
- Harrison RR, Watkins PT, Kier RJ, Lovejoy RO, Black DJ, Greger B, Solzbacher F, 2007. A Low-Power Integrated Circuit for a Wireless 100-Electrode Neural Recording System. IEEE J. Solid-State Circuits 42, 123–133. [Google Scholar]
- Hartmut Gehring MD, ME HM, Schmucker P, 2002. The effects of motion artifact and low perfusion on the performance of a new generation of pulse oximeters in volunteers undergoing hypoxemia. Respir. Care 47, 48–60. [PubMed] [Google Scholar]
- Hattori Y, Falgout L, Lee W, Jung S-Y, Poon E, Lee JW, Na I, Geisler A, Sadhwani D, Zhang Y, Su Y, Wang X, Liu Z, Xia J, Cheng H, Webb RC, Bonifas AP, Won P, Jeong J-W, Jang K-I, Song YM, Nardone B, Nodzenski M, Fan JA, Huang Y, West DP, Paller AS, Alam M, Yeo W-H, Rogers JA, 2014. Multifunctional Skin-Like Electronics for Quantitative, Clinical Monitoring of Cutaneous Wound Healing. Adv. Healthc. Mater. 3, 1597–1607. 10.1002/adhm.201400073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Smith PR, 2001. A new method for pulse oximetry possessing inherent insensitivity to artifact. IEEE Trans. Biomed. Eng. 48, 452–461. 10.1109/10.915711 [DOI] [PubMed] [Google Scholar]
- Heikenfeld J, 2016. Non-invasive Analyte Access and Sensing through Eccrine Sweat: Challenges and Outlook circa 2016. Electroanalysis 28, 1242–1249. 10.1002/elan.201600018 [DOI] [Google Scholar]
- Heikenfeld J, Jajack A, Rogers J, Gutruf P, Tian L, Pan T, Li R, Khine M, Kim J, Wang J, Kim J, 2018. Wearable sensors: modalities, challenges, and prospects. Lab Chip 18, 217–248. 10.1039/C7LC00914C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SY, Kim J, Gutruf P, Banks A, Wei P, Pielak R, Balooch G, Shi Y, Araki H, Rollo D, Gaede C, Patel M, Kwak JW, Peña-Alcántara AE, Lee K-T, Yun Y, Robinson JK, Xu S, Rogers JA, 2018. Wireless, battery-free, flexible, miniaturized dosimeters monitor exposure to solar radiation and to light for phototherapy. Sci. Transl. Med. 10. 10.1126/scitranslmed.aau1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, Rosendaal FR, Dekkers OM, 2013. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose–response meta-regression. EP Eur. 15, 742–749. 10.1093/europace/eus341 [DOI] [PubMed] [Google Scholar]
- Hinchet R, Yoon H-J, Ryu H, Kim M-K, Choi E-K, Kim D-S, Kim S-W, 2019. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science (80-. ). 365, 491–494. 10.1126/science.aan3997 [DOI] [PubMed] [Google Scholar]
- Ho JS, Tanabe Y, Iyer SM, Christensen AJ, Grosenick L, Deisseroth K, Delp SL, Poon ASY, 2015. Self-Tracking Energy Transfer for Neural Stimulation in Untethered Mice. Phys. Rev. Appl. 4, 24001. 10.1103/PhysRevApplied.4.024001 [DOI] [Google Scholar]
- Hong YJ, Jeong H, Cho KW, Lu N, Kim D-H, 2019. Wearable and Implantable Devices for Cardiovascular Healthcare: from Monitoring to Therapy Based on Flexible and Stretchable Electronics. Adv. Funct. Mater. 29, 1808247. 10.1002/adfm.201808247 [DOI] [Google Scholar]
- Hope MD, Hope TA, Crook SES, Ordovas KG, Urbania TH, Alley MT, Higgins CB, 2011. 4D Flow CMR in Assessment of Valve-Related Ascending Aortic Disease. JACC Cardiovasc. Imaging 4, 781 LP – 787. 10.1016/j.jcmg.2011.05.004 [DOI] [PubMed] [Google Scholar]
- Hopf HW, Rollins MD, 2007. Wounds: An Overview of the Role of Oxygen. Antioxid. Redox Signal. 9, 1183–1192. 10.1089/ars.2007.1641 [DOI] [PubMed] [Google Scholar]
- Hourlier-Fargette A, Schon S, Xue Y, Avila R, Li W, Gao Y, Liu C, Kim SB, Raj MS, Fields KB, Parsons BV, Lee K, Lee JY, Chung HU, Lee SP, Johnson M, Bandodkar AJ, Gutruf P, Model JB, Aranyosi AJ, Choi J, Ray TR, Ghaffari R, Huang Y, Rogers JA, 2020. Skin-interfaced soft microfluidic systems with modular and reusable electronics for in situ capacitive sensing of sweat loss, rate and conductivity. Lab Chip. 10.1039/D0LC00705F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Mei Y, Wang W, Zhang Q, 2016. Battery-free sensing platform for wearable devices: The synergy between two feet, in: IEEE INFOCOM 2016 - The 35th Annual IEEE International Conference on Computer Communications. pp. 1–9. 10.1109/INFOCOM.2016.7524543 [DOI] [Google Scholar]
- Huang X, Liu Y, Kong GW, Seo JH, Ma Y, Jang K-I, Fan JA, Mao S, Chen Q, Li D, others, 2016. Epidermal radio frequency electronics for wireless power transfer. Microsystems Nanoeng. 2, 16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wang L, Wang H, Zhang B, Wang X, Stening RYZ, Sheng X, Yin L, 2019. Materials Strategies and Device Architectures of Emerging Power Supply Devices for Implantable Bioelectronics. Small 0, 1902827. 10.1002/smll.201902827 [DOI] [PubMed] [Google Scholar]
- Hui SYR, Zhong W, Lee CK, 2013. A critical review of recent progress in mid-range wireless power transfer. IEEE Trans. Power Electron. 29, 4500–4511. [Google Scholar]
- Hwang G-T, Kim Y, Lee J-H, Oh S, Jeong CK, Park DY, Ryu J, Kwon H, Lee S-G, Joung B, others, 2015. Self-powered deep brain stimulation via a flexible PIMNT energy harvester. Energy Environ. Sci. 8, 2677–2684. [Google Scholar]
- Imura T, Hori Y, 2017. Unified Theory of Electromagnetic Induction and Magnetic Resonant Coupling. Electr. Eng. Japan 199, 58–80. 10.1002/eej.22953 [DOI] [Google Scholar]
- Izadifar Z, Babyn P, Chapman D, 2017. Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge. Ultrasound Med. Biol. 43, 1085–1104. 10.1016/j.ultrasmedbio.2017.01.023 [DOI] [PubMed] [Google Scholar]
- J. WB, W. BJ, J. GK, M. GW, 2006. Closed Loop Electrical Control of Urinary Continence. J. Urol. 175, 1559–1563. 10.1016/S0022-5347(05)00657-9 [DOI] [PubMed] [Google Scholar]
- Jain M, Shakya M, 2009. Study of temperature variation in human peripheral region during wound healing process due to plastic surgery. Appl. Math. Sci. 3, 2651–2662. [Google Scholar]
- Jameel F, Duan R, Chang Z, Liljemark A, Ristaniemi T, Jantti R, 2019. Applications of Backscatter Communications for Healthcare Networks. IEEE Netw. 33, 50–57. 10.1109/MNET.001.1900109 [DOI] [Google Scholar]
- Jeong H, Wang L, Ha T, Mitbander R, Yang X, Dai Z, Qiao S, Shen L, Sun N, Lu N, 2019. Modular and Reconfigurable Wireless E-Tattoos for Personalized Sensing. Adv. Mater. Technol. 4, 1900117. 10.1002/admt.201900117 [DOI] [Google Scholar]
- Jeong YR, Kim J, Xie Z, Xue Y, Won SM, Lee G, Jin SW, Hong SY, Feng X, Huang Y, Rogers JA, Ha JS, 2017. A skin-attachable, stretchable integrated system based on liquid GaInSn for wireless human motion monitoring with multi-site sensing capabilities. NPG Asia Mater. 9, e443–e443. 10.1038/am.2017.189 [DOI] [Google Scholar]
- Jia W, Bandodkar AJ, Valdés-Ramírez G, Windmiller JR, Yang Z, Ramírez J, Chan G, Wang J, 2013. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 85, 6553–6560. 10.1021/ac401573r [DOI] [PubMed] [Google Scholar]
- Jia W, Jiang H, Yang X, Wang W, Wang Z, Wang JJ, Wu Y, 2019. Passive Implantable Wireless Intracranial Pressure Monitoring Based on Near Field Communication, in: 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS). pp. 1–4. 10.1109/BIOCAS.2019.8919064 [DOI] [Google Scholar]
- Jiang Y, Pan K, Leng T, Hu Z, 2020. Smart Textile Integrated Wireless Powered Near Field Communication Body Temperature and Sweat Sensing System. IEEE J. Electromagn. RF Microwaves Med. Biol. 4, 164–170. 10.1109/JERM.2019.2929676 [DOI] [Google Scholar]
- Jinno H, Fukuda K, Xu X, Park S, Suzuki Y, Koizumi M, Yokota T, Osaka I, Takimiya K, Someya T, 2017. Stretchable and waterproof elastomer-coated organic photovoltaics for washable electronic textile applications. Nat. Energy 2, 780–785. [Google Scholar]
- Jones JM, O’Kane H, Gladstone DJ, Sarsam MAI, Campalani G, MacGowan SW, Cleland J, Cran GW, 2001. Repeat heart valve surgery: Risk factors for operative mortality. J. Thorac. Cardiovasc. Surg. 122, 913–918. 10.1067/mtc.2001.116470 [DOI] [PubMed] [Google Scholar]
- Joung Y-H, 2013. Development of implantable medical devices: from an engineering perspective. Int. Neurourol. J. 17, 98–106. 10.5213/inj.2013.17.3.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubran A, 2015. Pulse oximetry. Crit. Care 19, 272. 10.1186/s13054-015-0984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubran A, 1999. Pulse oximetry. Crit. Care 3, R11–R17. 10.1186/cc341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- K. A,E, J. G,L, D. M, O. K, 2004. A review of low-power wireless sensor microsystems for biomedical capsule diagnosis. Microelectron. Int. 21, 8–19. 10.1108/13565360410549675 [DOI] [Google Scholar]
- Kang S-K, Murphy RKJ, Hwang S-W, Lee SM, Harburg DV, Krueger NA, Shin J, Gamble P, Cheng H, Yu S, others, 2016. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71. 10.1038/nature16492 [DOI] [PubMed] [Google Scholar]
- Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, Vryonidou A, 2018. Hypokalemia: a clinical update. Endocr. Connect. 7, R135–R146. 10.1530/EC-18-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassal P, Steinberg MD, Steinberg IM, 2018. Wireless chemical sensors and biosensors: A review. Sensors Actuators B Chem. 266, 228–245. 10.1016/j.snb.2018.03.074 [DOI] [Google Scholar]
- Keum DH, Kim S-K, Koo J, Lee G-H, Jeon C, Mok JW, Mun BH, Lee KJ, Kamrani E, Joo C-K, Shin S, Sim J-Y, Myung D, Yun SH, Bao Z, Hahn SK, 2020. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 6, eaba3252. 10.1126/sciadv.aba3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes RW, 1988. Miniaturization of electronics and its limits. IBM J. Res. Dev. 32, 84–88. 10.1147/rd.321.0024 [DOI] [Google Scholar]
- Khan SR, Pavuluri SK, Cummins G, Desmulliez MPY, 2020. Wireless Power Transfer Techniques for Implantable Medical Devices: A Review. Sensors (Basel). 20, 3487. 10.3390/s20123487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Y, Ostfeld AE, Lochner CM, Pierre A, Arias AC, 2016. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 28, 4373–4395. 10.1002/adma.201504366 [DOI] [PubMed] [Google Scholar]
- Khiarak MN, Martel S, Koninck Y. De, Gosselin B, 2018. Wireless Optoelectronic Fiber Photometry Headstage for Deep Brain Structures Monitoring, in: 2018 IEEE Life Sciences Conference (LSC). pp. 9–12. 10.1109/LSC.2018.8572276 [DOI] [Google Scholar]
- Kim A, Ochoa M, Rahimi R, Ziaie B, 2015. New and emerging energy sources for implantable wireless microdevices. IEEE Access 3, 89–98. [Google Scholar]
- Kim D-H, Lu N, Huang Y, Rogers JA, 2012. Materials for stretchable electronics in bioinspired and biointegrated devices. MRS Bull. 37, 226–235. 10.1557/mrs.2012.36 [DOI] [Google Scholar]
- Kim Jeonghyun, Gutruf P, Chiarelli A, Yun Heo S, Cho K, Xie Z, Banks A, Han S, Jang K-I, Woo Lee J, Lee K-T, Feng X, Huang ying-sheng, Fabiani M, Gratton G, Paik U,A. Rogers J, 2017. Oximetry: Miniaturized Battery-Free Wireless Systems for Wearable Pulse Oximetry (Adv. Funct. Mater. 1/2017). Adv. Funct. Mater. 27. 10.1002/adfm.201770007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Joohee, Kim M, Lee M-S, Kim K, Ji S, Kim Y-T, Park J, Na K, Bae K-H, Kyun Kim H, Bien F, Young Lee C, Park J-U, 2017. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997. 10.1038/ncomms14997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Seo J, Jung D, Lee T, Ju H, Han J, Kim N, Jeong J, Cho S, Seol JH, Lee J, 2020. Active photonic wireless power transfer into live tissues. Proc. Natl. Acad. Sci. 117, 16856 LP – 16863. 10.1073/pnas.2002201117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Jeonghyun, Salvatore GA, Araki H, Chiarelli AM, Xie Z, Banks A, Sheng X, Liu Y, Lee JW, Jang K-I, Heo SY, Cho K, Luo H, Zimmerman B, Kim Joonhee, Yan L, Feng X, Xu S, Fabiani M, Gratton G, Huang Y, Paik U, Rogers JA, 2016. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2, e1600418–e1600418. 10.1126/sciadv.1600418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R-H, Kim D-H, Xiao J, Kim BH, Park S-I, Panilaitis B, Ghaffari R, Yao J, Li M, Liu Z, Malyarchuk V, Kim DG, Le A-P, Nuzzo RG, Kaplan DL, Omenetto FG, Huang Y, Kang Z, Rogers JA, 2010. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater. 9, 929–937. 10.1038/NMAT2879 [DOI] [PubMed] [Google Scholar]
- Kim SB, Koo J, Yoon J, Hourlier-Fargette A, Lee B, Chen S, Jo S, Choi J, Oh YS, Lee G, Won SM, Aranyosi AJ, Lee SP, Model JB, Braun PV, Ghaffari R, Park C, Rogers JA, 2020. Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat. Lab Chip 20, 84–92. 10.1039/C9LC01045A [DOI] [PubMed] [Google Scholar]
- Kim SB, Lee K, Raj MS, Lee B, Reeder JT, Koo J, Hourlier-Fargette A, Bandodkar AJ, Won SM, Sekine Y, Choi J, Zhang Y, Yoon J, Kim BH, Yun Y, Lee S, Shin J, Kim J, Ghaffari R, Rogers JA, 2018. Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition. Small 14, 1802876. 10.1002/smll.201802876 [DOI] [PubMed] [Google Scholar]
- Kim T, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim R-H, Lu C, Lee SD, Song I-S, Shin G, Al-Hasani R, Kim S, Tan MP, Huang Y, Omenetto FG, Rogers JA, Bruchas MR, 2013. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216. 10.1126/science.1232437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiourti A, Psathas KA, Nikita KS, 2014. Implantable and ingestible medical devices with wireless telemetry functionalities: A review of current status and challenges. Bioelectromagnetics 35, 1–15. 10.1002/bem.21813 [DOI] [PubMed] [Google Scholar]
- Koh A, Kang D, Xue Y, Lee S, Pielak RM, Kim J, Hwang T, Min S, Banks A, Bastien P, Manco MC, Wang L, Ammann KR, Jang K-I, Won P, Han S, Ghaffari R, Paik U, Slepian MJ, Balooch G, Huang Y, Rogers JA, 2016. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 LP–366ra165. 10.1126/scitranslmed.aaf2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kownacka AE, Vegelyte D, Joosse M, Anton N, Toebes BJ, Lauko J, Buzzacchera I, Lipinska K, Wilson DA, Geelhoed-Duijvestijn N, Wilson CJ, 2018. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecules 19, 4504–4511. 10.1021/acs.biomac.8b01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasoulis A, Kyranou I, Erden MS, Nazarpour K, Vijayakumar S, 2017. Improved prosthetic hand control with concurrent use of myoelectric and inertial measurements. J. Neuroeng. Rehabil. 14, 71. 10.1186/s12984-017-0284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Shi Y, Webb RC, Ma Y, Bastien P, Crawford KE, Wang A, Feng X, Manco M, Kurniawan J, Tir E, Huang Y, Balooch G, Pielak RM, Rogers JA, 2017. Multimodal epidermal devices for hydration monitoring. Microsystems &Amp; Nanoeng. 3, 17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SR, Arafa HM, Kwon K, Deng Y, Su C-J, Reeder JT, Freudman J, Stankiewicz I, Chen H-M, Loza R, Mims Marcus, Mims Mitchell, Lee K, Abecassis Z, Banks A, Ostojich D, Patel M, Wang H, Börekçi K, Rosenow J, Tate M, Huang Y, Alden T, Potts MB, Ayer AB, Rogers JA, 2020. Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. npj Digit. Med. 3, 29. 10.1038/s41746-020-0239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SR, Su C-J, Xie Z, Patel M, Madhvapathy SR, Xu Y, Freudman J, Ng B, Heo SY, Wang H, Ray TR, Leshock J, Stankiewicz I, Feng X, Huang Y, Gutruf P, Rogers JA, 2018. Wireless, Battery-Free Epidermal Electronics for Continuous, Quantitative, Multimodal Thermal Characterization of Skin. Small 14, 1803192. 10.1002/smll.201803192 [DOI] [PubMed] [Google Scholar]
- Kropff J, Choudhary P, Neupane S, Barnard K, Bain SC, Kapitza C, Forst T, Link M, Dehennis A, DeVries JH, 2017. Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial. Diabetes Care 40, 63 LP – 68. 10.2337/dc16-1525 [DOI] [PubMed] [Google Scholar]
- Ku M, Kim J, Won J-E, Kang W, Park Y-G, Park J, Lee J-H, Cheon J, Lee HH, Park J-U, 2020. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 6, eabb2891. 10.1126/sciadv.abb2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JW, Han M, Xie Z, Chung HU, Lee JY, Avila R, Yohay J, Chen X, Liang C, Patel M, Jung I, Kim J, Namkoong M, Kwon K, Guo X, Ogle C, Grande D, Ryu D, Kim DH, Madhvapathy S, Liu C, Yang DS, Park Y, Caldwell R, Banks A, Xu S, Huang Y, Fatone S, Rogers JA, 2020. Wireless sensors for continuous, multimodal measurements at the skin interface with lower limb prostheses. Sci. Transl. Med 12, eabc4327. 10.1126/scitranslmed.abc4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Belle JT, Adams A, Lin C-E, Engelschall E, Pratt B, Cook CB, 2016. Self-monitoring of tear glucose: the development of a tear based glucose sensor as an alternative to self-monitoring of blood glucose. Chem. Commun. 52, 9197–9204. 10.1039/C6CC03609K [DOI] [PubMed] [Google Scholar]
- Lacour SP, Jones J, Wagner S, Li T, Suo Z, 2005. Stretchable Interconnects for Elastic Electronic Surfaces. Proc. IEEE 93, 1459–1467. 10.1109/JPROC.2005.851502 [DOI] [Google Scholar]
- Lazaro A, Boada M, Villarino R, Girbau D, 2020. Study on the Reading of Energy-Harvested Implanted NFC Tags Using Mobile Phones. IEEE Access 8, 2200–2221. 10.1109/ACCESS.2019.2962570 [DOI] [Google Scholar]
- Lazaro A, Villarino R, Girbau D, 2018. A survey of NFC sensors based on energy harvesting for IoT applications. Sensors 18, 3746. 10.3390/s18113746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledet EH, D’Lima D, Westerhoff P, Szivek JA, Wachs RA, Bergmann G, 2012. Implantable Sensor Technology: From Research to Clinical Practice. JAAOS - J. Am. Acad. Orthop. Surg. 20. [DOI] [PubMed] [Google Scholar]
- Lee H, Hong YJ, Baik S, Hyeon T, Kim D-H, 2018. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 7, 1701150. 10.1002/adhm.201701150 [DOI] [PubMed] [Google Scholar]
- Lee S, Cortese AJ, Gandhi AP, Agger ER, McEuen PL, Molnar AC, 2018. A 250μm × 57μm Microscale Opto-electronically Transduced Electrodes (MOTEs) for Neural Recording. IEEE Trans. Biomed. Circuits Syst. 12, 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Cho YC, Bin Choy Y, 2019. Noninvasive Self-diagnostic Device for Tear Collection and Glucose Measurement. Sci. Rep. 9, 4747. 10.1038/s41598-019-41066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legatt AD, Arezzo J, Vaughan HG, 1980. Averaged multiple unit activity as an estimate of phasic changes in local neuronal activity: effects of volume-conducted potentials. J. Neurosci. Methods 2, 203–217. 10.1016/0165-0270(80)90061-8 [DOI] [PubMed] [Google Scholar]
- LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ, 2007. Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J. Pediatr. 151, 85–89. [DOI] [PubMed] [Google Scholar]
- Leonov V, Gyselinckx B, Hoof CV, Torfs T, Yazicioglu RF, Vullers RJM, Fiorini P, 2008. Wearable self-powered wireless devices with thermoelectric energy scavengers, in: 2nd European Conference & Exhibition on Integration Issues of Miniaturized Systems - MOMS, MOEMS, ICS and Electronic Components. pp. 1–8. [Google Scholar]
- Li J, Song E, Chiang C-H, Yu KJ, Koo J, Du H, Zhong Yishan, Hill M, Wang C, Zhang J, Chen Y, Tian L, Zhong Yiding, Fang G, Viventi J, Rogers JA, 2018. Conductively coupled flexible silicon electronic systems for chronic neural electrophysiology. Proc. Natl. Acad. Sci. 115, E9542 LP–E9549. 10.1073/pnas.1813187115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Zhe, Zheng Q, Wang ZL, Li Zhou, others, 2020. Nanogenerator-Based Self-Powered Sensors for Wearable and Implantable Electronics. Research 2020, 8710686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Yuan YJ, 2016. Wearable Medical Monitoring Systems Based on Wireless Networks: A Review. IEEE Sens. J. 16, 8186–8199. 10.1109/JSEN.2016.2597312 [DOI] [Google Scholar]
- Liao Y, Yao H, Lingley A, Parviz B, Otis BP, 2012. A 3-$\mu\hbox{W}$ CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid-State Circuits 47, 335–344. 10.1109/JSSC.2011.2170633 [DOI] [Google Scholar]
- Liebl M, Gleich B, Eberbeck D, Radon P, Rahmer J, Trahms L, Wiekhorst F, 2019. Noninvasive monitoring of blood flow using a single magnetic microsphere. Sci. Rep. 9, 5014. 10.1038/s41598-019-41416-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Moon E, Barrow M, Nason SR, Patel PR, Patil PG, Oh S, Lee I, Kim H-S, Sylvester D, Blaauw D, Chestek CA, Phillips J, Jang T, 2020. 26.9 A 0.19×0.17mm2 Wireless Neural Recording IC for Motor Prediction with Near-Infrared-Based Power and Data Telemetry, in: 2020 IEEE International Solid-State Circuits Conference - (ISSCC). pp. 416–418. 10.1109/ISSCC19947.2020.9063005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Kim H-J, Achavananthadith S, Kurt SA, Tan SCC, Yao H, Tee BCK, Lee JKW, Ho JS, 2020. Wireless battery-free body sensor networks using near-field-enabled clothing. Nat. Commun. 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Z, Li X, Zhu L, Xu G, Chen Z, Cheng C, Lu Y, Liu Q, 2020. Wireless, battery-free and wearable device for electrically controlled drug delivery: sodium salicylate released from bilayer polypyrrole by near-field communication on smartphone. Biomed. Microdevices 22, 53. 10.1007/s10544-020-00511-6 [DOI] [PubMed] [Google Scholar]
- Liu J, Yan BP-Y, Dai W-X, Ding X-R, Zhang Y-T, Zhao N, 2016. Multi-wavelength photoplethysmography method for skin arterial pulse extraction. Biomed. Opt. Express 7, 4313–4326. 10.1364/BOE.7.004313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Huang K, Zhou X, Durrani S, 2019. Next generation backscatter communication: systems, techniques, and applications. EURASIP J. Wirel. Commun. Netw. 2019, 69. 10.1186/s13638-019-1391-7 [DOI] [Google Scholar]
- Liu Y, Tian L, Raj MS, Cotton M, Ma Y, Ma S, McGrane B, Pendharkar AV, Dahaleh N, Olson L, Luan H, Block O, Suleski B, Zhou Y, Jayaraman C, Koski T, Aranyosi AJ, Wright JA, Jayaraman A, Huang Y, Ghaffari R, Kliot M, Rogers JA, 2018. Intraoperative monitoring of neuromuscular function with soft, skin-mounted wireless devices. npj Digit. Med. 1, 19. 10.1038/s41746-018-0023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Gutruf P, Xia L, Bhatti DL, Wang X, Vazquez-Guardado A, Ning X, Shen X, Sang T, Ma R, Pakeltis G, Sobczak G, Zhang H, Seo D-O, Xue M, Yin L, Chanda D, Sheng X, Bruchas MR, Rogers JA, 2018a. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl. Acad. Sci. U. S. A. 115, E1374–E1383. 10.1073/pnas.1718721115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Yang Z, Meacham K, Cvetkovic C, Corbin EA, Vázquez-Guardado A, Xue M, Yin L, Boroumand J, Pakeltis G, Sang T, Yu KJ, Chanda D, Bashir R, Gereau IV RW, Sheng X, Rogers JA, 2018b. Biodegradable Monocrystalline Silicon Photovoltaic Microcells as Power Supplies for Transient Biomedical Implants. Adv. Energy Mater. 8, 1703035. 10.1002/aenm.201703035 [DOI] [Google Scholar]
- Lucarelli F, Ricci F, Caprio F, Valgimigli F, Scuffi C, Moscone D, Palleschi G, 2012. GlucoMen Day continuous glucose monitoring system: a screening for enzymatic and electrochemical interferents. J. Diabetes Sci. Technol. 6, 1172–1181. 10.1177/193229681200600522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, 2014. Medical Adhesives in the NICU. Newborn Infant Nurs. Rev. 14, 160–165. 10.1053/j.nainr.2014.10.001 [DOI] [Google Scholar]
- Lynch JP, Loh KJ, 2006. A summary review of wireless sensors and sensor networks for structural health monitoring. Shock Vib. Dig. 38, 91+. [Google Scholar]
- M. RF, G. HM, J. WJ, J.H. de K.P., J.M. WJ, R.M. JM, A. BN, A.W. RA 2018. Energetics of Blood Flow in Cardiovascular Disease. Circulation 137, 2393–2407. 10.1161/CIRCULATIONAHA.117.033359 [DOI] [PubMed] [Google Scholar]
- Ma W-G, Hou B, Abdurusul A, Gong D-X, Tang Y, Chang Q, Xu J-P, Sun H-S, 2015. Dysfunction of mechanical heart valve prosthesis: experience with surgical management in 48 patients. J. Thorac. Dis. 7, 2321–2329. 10.3978/j.issn.2072-1439.2015.12.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhvapathy SR, Ma Y, Patel M, Krishnan S, Wei C, Li Y, Xu S, Feng X, Huang Y, Rogers JA, 2018. Epidermal Electronic Systems for Measuring the Thermal Properties of Human Skin at Depths of up to Several Millimeters. Adv. Funct. Mater. 28, 1802083. 10.1002/adfm.201802083 [DOI] [Google Scholar]
- Mandal S, Turicchia L, Sarpeshkar R, 2010. A Low-Power, Battery-Free Tag for Body Sensor Networks. IEEE Pervasive Comput. 9, 71–77. 10.1109/MPRV.2010.1 [DOI] [Google Scholar]
- Martynov VI, Pakhomov AA, Deyev IE, Petrenko AG, 2018. Genetically encoded fluorescent indicators for live cell pH imaging. Biochim. Biophys. Acta - Gen. Subj. 1862, 2924–2939. 10.1016/j.bbagen.2018.09.013 [DOI] [PubMed] [Google Scholar]
- Mazzaracchio V, Fiore L, Nappi S, Marrocco G, Arduini F, 2021. Medium-distance affordable, flexible and wireless epidermal sensor for pH monitoring in sweat. Talanta 222, 121502. 10.1016/j.talanta.2020.121502 [DOI] [PubMed] [Google Scholar]
- McAdams E, 2006. Bioelectrodes. Encycl. Med. Devices Instrum., Major Reference Works. https://doi.org/doi: 10.1002/0471732877.emd013 [DOI] [Google Scholar]
- Meek S, Morris F, 2002. ABC of clinical electrocardiography.Introduction. I-Leads, rate, rhythm, and cardiac axis. BMJ 324, 415–418. 10.1136/bmj.324.7334.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merli F, Bolomey L, Zurcher J-F, Corradini G, Meurville E, Skrivervik AK, 2011. Design, realization and measurements of a miniature antenna for implantable wireless communication systems. IEEE Trans. Antennas Propag. 59, 3544–3555. [Google Scholar]
- Miccoli B, Lopez CM, Goikoetxea E, Putzeys J, Sekeri M, Krylychkina O, Chang S-W, Firrincieli A, Andrei A, Reumers V, Braeken D, 2019. High-Density Electrical Recording and Impedance Imaging With a Multi-Modal CMOS Multi-Electrode Array Chip. Front. Neurosci. 13, 641. 10.3389/fnins.2019.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickle AD, Won SM, Noh KN, Yoon J, Meacham KW, Xue Y, McIlvried LA, Copits BA, Samineni VK, Crawford KE, Kim DH, Srivastava P, Kim BH, Min S, Shiuan Y, Yun Y, Payne MA, Zhang J, Jang H, Li Y, Lai HH, Huang Y, Park S-I, Gereau RW, Rogers JA, 2019. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365. 10.1038/s41586-018-0823-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Li C, Dong H, Cao X, T Luong JH, Zhang X, 2007. Implantable electrochemical sensors for biomedical and clinical applications: progress, problems, and future possibilities. Curr. Med. Chem. 14, 937–951. [PubMed] [Google Scholar]
- Mink JE, Qaisi RM, Logan BE, Hussain MM, 2014. Energy harvesting from organic liquids in micro-sized microbial fuel cells. NPG Asia Mater. 6, e89–e89. 10.1038/am.2014.1 [DOI] [Google Scholar]
- Miyamoto D, Murayama M, 2016. The fiber-optic imaging and manipulation of neural activity during animal behavior. Neurosci. Res. 103, 1–9. 10.1016/j.neures.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon ASY, 2015. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E, Blaauw D, Phillips JD, 2017. Subcutaneous Photovoltaic Infrared Energy Harvesting for Bio-Implantable Devices. IEEE Trans. Electron Devices 64, 2432–2437. 10.1109/TED.2017.2681694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer J, Wilson D, Finkelshtein I, Wong B, Potts R, 2012. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 14, 398–402. 10.1089/dia.2011.0262 [DOI] [PubMed] [Google Scholar]
- Mujeeb-U-Rahman M, Honarvar Nazari M, Sencan M, 2019a. A novel semiconductor based wireless electrochemical sensing platform for chronic disease management. Biosens. Bioelectron. 124–125, 66–74. 10.1016/j.bios.2018.09.077 [DOI] [PubMed] [Google Scholar]
- Mujeeb-U-Rahman M, Nazari MH, Sencan M, Antwerp W. Van, 2019b. A Novel Needle-Injectable Millimeter scale Wireless Electrochemical Glucose Sensing Platform for Artificial Pancreas Applications. Sci. Rep. 9, 17421. 10.1038/s41598-019-53680-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JE, Tofler GH, Stone PH, 1989. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79, 733–743. 10.1161/01.CIR.79.4.733 [DOI] [PubMed] [Google Scholar]
- Murayama M, Pérez-Garci E, Lüscher H-R, Larkum ME, 2007. Fiberoptic System for Recording Dendritic Calcium Signals in Layer 5 Neocortical Pyramidal Cells in Freely Moving Rats. J. Neurophysiol. 98, 1791–1805. 10.1152/jn.00082.2007 [DOI] [PubMed] [Google Scholar]
- Murphy OH, Bahmanyar MR, Borghi A, McLeod CN, Navaratnarajah M, Yacoub MH, Toumazou C, 2013. Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor. Biomed. Microdevices 15, 737–749. 10.1007/s10544-013-9759-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musk E, 2019. An integrated brain-machine interface platform with thousands of channels. bioRxiv 703801. 10.1101/703801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau P, El-Damak D, Glettig D, Kong YL, Mo S, Cleveland C, Booth L, Roxhed N, Langer R, Chandrakasan AP, Traverso G, 2017. Prolonged energy harvesting for ingestible devices. Nat. Biomed. Eng. 1, 22. 10.1038/s41551-016-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan K, Kang SD, Li K, Yu KJ, Zhu F, Wang J, Dunn AC, Zhou C, Xie Z, Agne MT, Wang H, Luan H, Zhang Y, Huang Y, Snyder GJ, Rogers JA, 2018. Compliant and stretchable thermoelectric coils for energy harvesting in miniature flexible devices. Sci. Adv. 4, eaau5849. 10.1126/sciadv.aau5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TR, Fowlkes JB, Abramowicz JS, Church CC, 2009. Ultrasound biosafety considerations for the practicing sonographer and sonologist. J. ultrasound Med. 28, 139–150. [DOI] [PubMed] [Google Scholar]
- Niu S, Matsuhisa N, Beker L, Li J, Wang S, Wang J, Jiang Y, Yan X, Yun Y, Burnett W, Poon ASY, Tok JB-H, Chen X, Bao Z, 2019. A wireless body area sensor network based on stretchable passive tags. Nat. Electron. 2, 361–368. 10.1038/s41928-019-0286-2 [DOI] [Google Scholar]
- Nordhausen CT, Maynard EM, Normann RA, 1996. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 726, 129–140. 10.1016/0006-8993(96)00321-6 [DOI] [PubMed] [Google Scholar]
- Obaid S, Lu L, 2019. Highly Efficient Microscale Gallium Arsenide Solar Cell Arrays as Optogenetic Power Options. IEEE Photonics J. 11, 1–8. [Google Scholar]
- Obermeyer Z, Emanuel EJ, 2016. Predicting the Future - Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 375, 1216–1219. 10.1056/NEJMp1606181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obien MEJ, Deligkaris K, Bullmann T, Bakkum DJ, Frey U, 2015. Revealing neuronal function through microelectrode array recordings. Front. Neurosci. 8, 423. 10.3389/fnins.2014.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olberg RM, Worthington AH, Venator KR, 2000. Prey pursuit and interception in dragonflies. J. Comp. Physiol. A 186, 155–162. 10.1007/s003590050015 [DOI] [PubMed] [Google Scholar]
- Oliver NS, Toumazou C, Cass AEG, Johnston DG, 2009. Glucose sensors: a review of current and emerging technology. Diabet. Med. 26, 197–210. 10.1111/j.1464-5491.2008.02642.x [DOI] [PubMed] [Google Scholar]
- Opperman CA, Hancke GP, 2011. Using NFC-enabled phones for remote data acquisition and digital control, in: IEEE Africon ‘ 11. pp. 1–6. 10.1109/AFRCON.2011.6072147 [DOI] [Google Scholar]
- Osuagwu BCA, Wallace L, Fraser M, Vuckovic A, 2016. Rehabilitation of hand in subacute tetraplegic patients based on brain computer interface and functional electrical stimulation: a randomised pilot study. J. Neural Eng. 13, 65002. 10.1088/1741-2560/13/6/065002 [DOI] [PubMed] [Google Scholar]
- Parastarfeizabadi M, Kouzani AZ, 2017. Advances in closed-loop deep brain stimulation devices. J. Neuroeng. Rehabil. 14, 79. 10.1186/s12984-017-0295-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Lee J-W, Kang M, Cho K, Cho BH, Lee K-S, 2018. Detecting Bladder Biomarkers for Closed-Loop Neuromodulation: A Technological Review. Int. Neurourol. J. 22, 228–236. 10.5213/inj.1836246.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Il, Shin G, Banks A, McCall JG, Siuda ER, Schmidt MJ, Chung HU, Noh KN, Mun JG-H, Rhodes J, Bruchas MR, Rogers JA, 2015. Ultraminiaturized photovoltaic and radio frequency powered optoelectronic systems for wireless optogenetics. J. Neural Eng. 12, 56002. 10.1088/1741-2560/12/5/056002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim J-K, Patil SJ, Park J-K, Park S, Lee D-W, 2016. A Wireless Pressure Sensor Integrated with a Biodegradable Polymer Stent for Biomedical Applications. Sensors (Basel). 16, 809. 10.3390/s16060809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Heo SW, Lee W, Inoue D, Jiang Z, Yu K, Jinno H, Hashizume D, Sekino M, Yokota T, others, 2018. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 561, 516. 10.1038/s41586-018-0536-x [DOI] [PubMed] [Google Scholar]
- Parthasarathy AB, Gannon K, Baker WB, Kavuri V, Mullen MT, Detre JA, Yodh AG, 2016. Functional monitoring of blood flow dynamics in brain with photon correlation techniques, in: Proc.SPIE. 10.1117/12.2225250 [DOI] [Google Scholar]
- Pasche S, Angeloni S, Ischer R, Liley M, Luprano J, Voirin G, 2009. Wearable Biosensors for Monitoring Wound Healing. Adv. Sci. Technol. 57, 80–87. 10.4028/www.scientific.net/AST.57.80 [DOI] [Google Scholar]
- Patel S, Park H, Bonato P, Chan L, Rodgers M, 2012. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 9, 21. 10.1186/1743-0003-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfornis A, Personeni E, Borot S, 2011. Evolution of Devices in Diabetes Management. Diabetes Technol. Ther. 13, S-93–S-102. 10.1089/dia.2011.0058 [DOI] [PubMed] [Google Scholar]
- Petkos K, Koutsoftidis S, Guiho T, Degenaar P, Jackson A, Greenwald SE, Brown P, Denison T, Drakakis EM, 2019. A high-performance 8 nV/√Hz 8-channel wearable and wireless system for real-time monitoring of bioelectrical signals. J. Neuroeng. Rehabil. 16, 156. 10.1186/s12984-019-0629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Bender J, Hall B, Shabosky L, Annecca A, Smith J, 2018. Parent participation in the neonatal intensive care unit: Predictors and relationships to neurobehavior and developmental outcomes. Early Hum. Dev. 117, 32–38. 10.1016/j.earlhumdev.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski K, Ranganathan G, 2016. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon ASY, O’Driscoll S, Meng TH, 2010. Optimal frequency for wireless power transmission into dispersive tissue. IEEE Trans. Antennas Propag. 58, 1739–1750. [Google Scholar]
- Popovici E, Magno M, Marinkovic S, 2013. Power management techniques for Wireless Sensor Networks: A review, in: 5th IEEE International Workshop on Advances in Sensors and Interfaces IWASI. pp. 194–198. 10.1109/IWASI.2013.6576090 [DOI] [Google Scholar]
- Radousky HB, Liang H, 2012. Energy harvesting: an integrated view of materials, devices and applications. Nanotechnology 23, 502001. 10.1088/0957-4484/23/50/502001 [DOI] [PubMed] [Google Scholar]
- Ramrakhyani AK, Mirabbasi S, Chiao M, 2011. Design and optimization of resonance-based efficient wireless power delivery systems for biomedical implants. IEEE Trans. Biomed. Circuits Syst. 5, 48–63. 10.1109/TBCAS.2010.2072782 [DOI] [PubMed] [Google Scholar]
- Rasouli M, Phee LSJ, 2010. Energy sources and their development for application in medical devices. Expert Rev. Med. Devices 7, 693–709. 10.1586/erd.10.20 [DOI] [PubMed] [Google Scholar]
- Ravì D, Wong C, Deligianni F, Berthelot M, Andreu-Perez J, Lo B, Yang G, 2017. Deep Learning for Health Informatics. IEEE J. Biomed. Heal. Informatics 21, 4–21. 10.1109/JBHI.2016.2636665 [DOI] [PubMed] [Google Scholar]
- Ray TR, Choi J, Bandodkar AJ, Krishnan S, Gutruf P, Tian L, Ghaffari R, Rogers JA, 2019. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 10.1021/acs.chemrev.8b00573 [DOI] [PubMed] [Google Scholar]
- Reeder JT, Choi J, Xue Y, Gutruf P, Hanson J, Liu M, Ray T, Bandodkar AJ, Avila R, Xia W, Krishnan S, Xu S, Barnes K, Pahnke M, Ghaffari R, Huang Y, Rogers JA, 2019. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 5, eaau6356. 10.1126/sciadv.aau6356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regas FC, EHRLICH HP, 1992. Elucidating the Vascular Response to Burns with a New Rat Model. J. Trauma Acute Care Surg. 32. [DOI] [PubMed] [Google Scholar]
- Reichelt S, Fiala J, Werber A, Forster K, Heilmann C, Klemm R, Zappe H, 2008. Development of an Implantable Pulse Oximeter. IEEE Trans. Biomed. Eng. 55, 581–588. 10.1109/TBME.2007.902242 [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Mihalko SL, 2001. Physical Activity and Quality of Life in Older Adults. Journals Gerontol. Ser. A 56, 23–35. 10.1093/gerona/56.suppl_2.23 [DOI] [PubMed] [Google Scholar]
- Renard E, 2002. Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Curr. Opin. Pharmacol. 2, 708–716. 10.1016/S1471-4892(02)00216-3 [DOI] [PubMed] [Google Scholar]
- Richard B, 2015. Wearable sensors bring new benefits to continuous medical monitoring, real time physical activity assessment, baby monitoring and industrial applications. Sens. Rev. 35, 141–145. 10.1108/SR-10-2014-722 [DOI] [Google Scholar]
- Rodbard D, 2016. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol. Ther. 18, S2-3–S2-13. 10.1089/dia.2015.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa BMG, Yang GZ, 2016. Active implantable sensor powered by ultrasounds with application in the monitoring of physiological parameters for soft tissues, in: 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN). pp. 318–323. 10.1109/BSN.2016.7516281 [DOI] [Google Scholar]
- Samineni VK, Yoon J, Crawford KE, Jeong YR, McKenzie KC, Shin G, Xie Z, Sundaram SS, Li Y, Yang MY, others, 2017. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 158, 2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample AP, Yeager DJ, Powledge PS, Mamishev AV, Smith JR, 2008. Design of an RFID-Based Battery-Free Programmable Sensing Platform. IEEE Trans. Instrum. Meas. 57, 2608–2615. 10.1109/TIM.2008.925019 [DOI] [Google Scholar]
- Scholten K, Meng E, 2018. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 544, 319–334. 10.1016/j.ijpharm.2018.02.022 [DOI] [PubMed] [Google Scholar]
- Searle A, Kirkup L, 2000. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 21, 271–283. 10.1088/0967-3334/21/2/307 [DOI] [PubMed] [Google Scholar]
- Sen DK, Sarin GS, 1980. Tear glucose levels in normal people and in diabetic patients. Br. J. Ophthalmol. 64, 693–695. 10.1136/bjo.64.9.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne S, Hu Y, Nguyen T, Lan G, Khalifa S, Thilakarathna K, Hassan M, Seneviratne A, 2017. A Survey of Wearable Devices and Challenges. IEEE Commun. Surv. Tutorials 19, 2573–2620. 10.1109/COMST.2017.2731979 [DOI] [Google Scholar]
- Seo D, Neely RM, Shen K, Singhal U, Alon E, Rabaey JM, Carmena JM, Maharbiz MM, 2016. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91, 529–539. 10.1016/j.neuron.2016.06.034 [DOI] [PubMed] [Google Scholar]
- Settaluri KT, Lo H, Ram RJ, 2012. Thin thermoelectric generator system for body energy harvesting. J. Electron. Mater. 41, 984–988. [Google Scholar]
- Seymour JP, Wu F, Wise KD, Yoon E, 2017. State-of-the-art MEMS and microsystem tools for brain research. Microsystems Nanoeng. 3, 16066. 10.1038/micronano.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Rieth L, Tathireddy P, Harrison R, Oppermann H, Klein M, Töpper M, Jung E, Normann R, Clark G, Solzbacher F, 2011. Evaluation of the packaging and encapsulation reliability in fully integrated, fully wireless 100 channel Utah Slant Electrode Array (USEA): Implications for long term functionality, in: 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference. pp. 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay T, Velev OD, Dickey MD, 2018. Soft electrodes combining hydrogel and liquid metal. Soft Matter 14, 3296–3303. 10.1039/c8sm00337h [DOI] [PubMed] [Google Scholar]
- Shon A, Chu J-U, Jung J, Kim H, Youn I, 2017. An Implantable Wireless Neural Interface System for Simultaneous Recording and Stimulation of Peripheral Nerve with a Single Cuff Electrode. Sensors (Basel). 18. 10.3390/s18010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidén J, Skerved V, Gao J, Forsström S, Nilsson H-E, Kanter T, Gulliksson M, 2011. Home care with nfc sensors and a smart phone, in: Proceedings of the 4th International Symposium on Applied Sciences in Biomedical and Communication Technologies. pp. 1–5. [Google Scholar]
- Siegel SW, Catanzaro F, Dijkema HE, Elhilali MM, Fowler CJ, Gajewski JB, Hassouna MM, Janknegt RA, Jonas U, van Kerrebroeck PEV, Lycklama a Nijeholt AAB, Oleson KA, Schmidt RA, 2000. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology 56, 87–91. 10.1016/S0090-4295(00)00597-5 [DOI] [PubMed] [Google Scholar]
- Sinex JE, 1999. Pulse oximetry: principles and limitations. Am. J. Emerg. Med. 17, 59–67. [DOI] [PubMed] [Google Scholar]
- Song Y, Min J, Yu Y, Wang H, Yang Y, Zhang H, Gao W, 2020. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 6, eaay9842. 10.1126/sciadv.aay9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklin S, Yousaf A, Volk T, Reindl L, 2016. Efficient Wireless Powering of Biomedical Sensor Systems for Multichannel Brain Implants. IEEE Trans. Instrum. Meas. 65, 754–764. 10.1109/TIM.2015.2482278 [DOI] [Google Scholar]
- Sun FT, Morrell MJ, 2014. Closed-loop Neurostimulation: The Clinical Experience. Neurotherapeutics 11, 553–563. 10.1007/s13311-014-0280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP, 2005. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 524–541. 10.1109/TNSRE.2005.857687 [DOI] [PubMed] [Google Scholar]
- Talkhooncheh AH, Yu Y, Agarwal A, Kuo WW-T, Chen K-C, Wang M, Hoskuldsdottir G, Gao W, Emami A, 2020. A Biofuel-Cell-Based Energy Harvester With 86% Peak Efficiency and 0.25-V Minimum Input Voltage Using Source-Adaptive MPPT. IEEE J. Solid-State Circuits 1. 10.1109/JSSC.2020.3035491 [DOI] [Google Scholar]
- Tarbell JM, Shi Z-D, Dunn J, Jo H, 2014. Fluid Mechanics, Arterial Disease, and Gene Expression. Annu. Rev. Fluid Mech. 46, 591–614. 10.1146/annurev-fluid-010313-141309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texon M, Imparato AM, Helpern M, 1965. The Role of Vascular Dynamics in the Development of Atherosclerosis. JAMA 194, 1226–1230. 10.1001/jama.1965.03090240060016 [DOI] [PubMed] [Google Scholar]
- The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus, 1993. . N. Engl. J. Med. 329, 977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- Theodor M, Ruh D, Subramanian S, Förster K, Heilmann C, Beyersdorf F, Plachta D, Manoli Y, Zappe H, Seifert A, 2014. Implantable pulse oximetry on subcutaneous tissue, in: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. pp. 2089–2092. 10.1109/EMBC.2014.6944028 [DOI] [PubMed] [Google Scholar]
- Thomas SJ, Harrison RR, Leonardo A, Reynolds MS, 2012. A Battery-Free Multichannel Digital Neural/EMG Telemetry System for Flying Insects. IEEE Trans. Biomed. Circuits Syst. 6, 424–436. [DOI] [PubMed] [Google Scholar]
- Thurgood BK, Warren DJ, Ledbetter NM, Clark GA, Harrison RR, 2009. A Wireless Integrated Circuit for 100-Channel Charge-Balanced Neural Stimulation. IEEE Trans. Biomed. Circuits Syst. 3, 405–414. [DOI] [PubMed] [Google Scholar]
- Tian L, Zimmerman B, Akhtar A, Yu KJ, Moore M, Wu J, Larsen RJ, Lee JW, Li J, Liu Y, Metzger B, Qu S, Guo X, Mathewson KE, Fan JA, Cornman J, Fatina M, Xie Z, Ma Y, Zhang J, Zhang Y, Dolcos F, Fabiani M, Gratton G, Bretl T, Hargrove LJ, Braun PV, Huang Y, Rogers JA, 2019. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 10.1038/s41551-019-0347-x [DOI] [PubMed] [Google Scholar]
- Tian X, Lee PM, Tan YJ, Wu TLY, Yao H, Zhang M, Li Z, Ng KA, Tee BCK, Ho JS, 2019. Wireless body sensor networks based on metamaterial textiles. Nat. Electron. 2, 243–251. 10.1038/s41928-019-0257-7 [DOI] [Google Scholar]
- Tjang YS, van Hees Y, Körfer R, Grobbee DE, van der Heijden GJMG, 2007. Predictors of mortality after aortic valve replacement. Eur. J. cardio-thoracic Surg. 32, 469–474. [DOI] [PubMed] [Google Scholar]
- Tosi J, Taffoni F, Santacatterina M, Sannino R, Formica D, 2017. Performance Evaluation of Bluetooth Low Energy: A Systematic Review. Sensors (Basel). 17, 2898. 10.3390/s17122898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremper KK, 1989. Pulse oximetry. Chest. [DOI] [PubMed] [Google Scholar]
- Tsimbalo E, Fafoutis X, Mellios E, Haghighi M, Tan B, Hilton G, Piechocki R, Craddock I, 2015. Mitigating packet loss in connectionless Bluetooth Low Energy, in: 2015 IEEE 2nd World Forum on Internet of Things (WF-IoT). pp. 291–296. [Google Scholar]
- Vashist SK, 2012. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 750, 16–27. 10.1016/j.aca.2012.03.043 [DOI] [PubMed] [Google Scholar]
- Vasisht D, Zhang G, Abari O, Lu H-M, Flanz J, Katabi D, 2018. In-body backscatter communication and localization, in: Proceedings of the 2018 Conference of the ACM Special Interest Group on Data Communication. pp. 132–146. [Google Scholar]
- Vennemann B, Obrist D, Rösgen T, 2020. A smartphone-enabled wireless and batteryless implantable blood flow sensor for remote monitoring of prosthetic heart valve function. PLoS One 15, e0227372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna S, Kauvar IV, Richman E, Gore F, Oskotsky T, Sava-Segal C, Luo L, Malenka RC, Henderson JM, Nuyujukian P, Parvizi J, Deisseroth K, 2020. Deep posteromedial cortical rhythm in dissociation. Nature 586, 87–94. 10.1038/s41586-020-2731-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena Gonzales W, Mobashsher AT, Abbosh A, 2019. The Progress of Glucose Monitoring-A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors (Basel). 19, 800. 10.3390/s19040800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B, 2011. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605. 10.1038/nn.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JA 3rd, Topol EJ, Steinhubl SR, 2014. Novel wireless devices for cardiac monitoring. Circulation 130, 573–581. 10.1161/CIRCULATIONAHA.114.009024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, He T, Lee C, 2019. Development of neural interfaces and energy harvesters towards self-powered implantable systems for healthcare monitoring and rehabilitation purposes. Nano Energy 104039. [Google Scholar]
- Wang L, Lou Z, Jiang K, Shen G, 2019. Bio-Multifunctional Smart Wearable Sensors for Medical Devices. Adv. Intell. Syst. 1, 1900040. 10.1002/aisy.201900040 [DOI] [Google Scholar]
- Watkins PT, Kier RJ, Lovejoy RO, Black DJ, Harrison RR, 2006. Signal amplification, detection and transmission in a wireless 100-electrode neural recording system, in: 2006 IEEE International Symposium on Circuits and Systems. pp. 4 pp. – 2196. 10.1109/ISCAS.2006.1693054 [DOI] [Google Scholar]
- Webb RC, Ma Y, Krishnan S, Li Y, Yoon S, Guo X, Feng X, Shi Y, Seidel M, Cho NH, Kurniawan J, Ahad J, Sheth N, Kim J, Taylor VI JG, Darlington T, Chang K, Huang W, Ayers J, Gruebele A, Pielak RM, Slepian MJ, Huang Y, Gorbach AM, Rogers JA, 2015. Epidermal devices for noninvasive, precise, and continuous mapping of macrovascular and microvascular blood flow. Sci. Adv. 1, e1500701. 10.1126/sciadv.1500701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D-L, Deng H-T, Liu X, Li G-K, Zhang X-R, Zhang X-S, 2020. Wearable multi-sensing double-chain thermoelectric generator. Microsystems Nanoeng. 6, 68. 10.1038/s41378-020-0179-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BH, 2016. What genetic model organisms offer the study of behavior and neural circuits. J. Neurogenet. 30, 54–61. 10.1080/01677063.2016.1177049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJH, 2012. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 55, 2895–2905. 10.1007/s00125-012-2677-z [DOI] [PubMed] [Google Scholar]
- Xie L, Shi Y, Hou YT, Lou A, 2013. Wireless power transfer and applications to sensor networks. IEEE Wirel. Commun. 20, 140–145. 10.1109/MWC.2013.6590061 [DOI] [Google Scholar]
- Xu G, Cheng C, Liu Z, Yuan W, Wu X, Lu Y, Low SS, Liu J, Zhu L, Ji D, Li S, Chen Z, Wang L, Yang Q, Cui Z, Liu Q, 2019a. Battery-Free and Wireless Epidermal Electrochemical System with All-Printed Stretchable Electrode Array for Multiplexed In Situ Sweat Analysis. Adv. Mater. Technol. 4, 1800658. 10.1002/admt.201800658 [DOI] [Google Scholar]
- Xu G, Cheng C, Yuan W, Liu Z, Zhu L, Li X, Lu Y, Chen Z, Liu Jinglong, Cui Z, Liu Jingjing, Men H, Liu Q, 2019b. Smartphone-based battery-free and flexible electrochemical patch for calcium and chloride ions detections in biofluids. Sensors Actuators B Chem. 297, 126743. 10.1016/j.snb.2019.126743 [DOI] [Google Scholar]
- Xu W, Feng X, Wang J, Luo C, Li J, Ming Z, 2019. Energy Harvesting-Based Smart Transportation Mode Detection System via Attention-Based LSTM. IEEE Access 7, 66423–66434. 10.1109/ACCESS.2019.2918555 [DOI] [Google Scholar]
- Yan Y, Zhang Y, 2008. An Efficient Motion-Resistant Method for Wearable Pulse Oximeter. IEEE Trans. Inf. Technol. Biomed. 12, 399–405. 10.1109/TITB.2007.902173 [DOI] [PubMed] [Google Scholar]
- Yao G, Kang L, Li J, Long Y, Wei H, Ferreira CA, Jeffery JJ, Lin Y, Cai W, Wang X, 2018. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 9, 5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky AN, Schulze PC, Yaroslavsky IV, Schober R, Ulrich F, Schwarzmaier HJ, 2002. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 47, 2059. [DOI] [PubMed] [Google Scholar]
- Yeshwant K, Ghaffari R, 2019. A biodegradable wireless blood-flow sensor. Nat. Biomed. Eng. 3, 7–8. 10.1038/s41551-018-0345-4 [DOI] [PubMed] [Google Scholar]
- Yoram R, R. EE, 2006. Cardiology Is Flow. Circulation 113, 2679–2682. 10.1161/CIRCULATIONAHA.106.632687 [DOI] [PubMed] [Google Scholar]
- Yu X, Xie Z, Yu Y, Lee J, Vazquez-Guardado A, Luan H, Ruban J, Ning X, Akhtar A, Li D, others, 2019. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479. [DOI] [PubMed] [Google Scholar]
- Yu Y, Nassar J, Xu C, Min J, Yang Y, Dai A, Doshi R, Huang A, Song Y, Gehlhar R, others, 2020. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 5. 10.1126/scirobotics.aaz7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatout Y, 2012. Using wireless technologies for healthcare monitoring at home: A survey, in: 2012 IEEE 14th International Conference on E-Health Networking, Applications and Services (Healthcom). pp. 383–386. 10.1109/HealthCom.2012.6379443 [DOI] [Google Scholar]
- Zebda A, Alcaraz J-P, Vadgama P, Shleev S, Minteer SD, Boucher F, Cinquin P, Martin DK, 2018. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 124, 57–72. 10.1016/j.bioelechem.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Zhang Hao, Gutruf P, Meacham K, Montana MC, Zhao X, Chiarelli AM, Vázquez-Guardado A, Norris A, Lu L, Guo Q, others, 2019. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 5, eaaw0873. 10.1126/sciadv.aaw0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang He, Zhang J, Hu Z, Quan L, Shi L, Chen J, Xuan W, Zhang Z, Dong S, Luo J, 2019. Waist-wearable wireless respiration sensor based on triboelectric effect. Nano Energy 59, 75–83. [Google Scholar]
- Zhang J, Hodge W, Hutnick C, Wang X, 2011. Noninvasive diagnostic devices for diabetes through measuring tear glucose. J. Diabetes Sci. Technol. 5, 166–172. 10.1177/193229681100500123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mickle AD, Gutruf P, McIlvried LA, Guo H, Wu Y, Golden JP, Xue Y, Grajales-Reyes JG, Wang X, others, 2019. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu S, Fu H, Lee J, Su J, Hwang K-C, Rogers JA, Huang Y, 2013. Buckling in serpentine microstructures and applications in elastomer-supported ultra-stretchable electronics with high areal coverage. Soft Matter 9, 8062–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Guo H, Li J, Bandodkar AJ, Rogers JA, 2019. Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids. Trends Chem. 1, 559–571. 10.1016/j.trechm.2019.07.001 [DOI] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang Y-F, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, Campbell RE, 2011. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891. 10.1126/science.1208592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Qian B, Zhu Y, Ren Z, Deng J, Liu J, Bai Q, Zhang X, 2020. Development of an Implantable Wireless and Batteryless Bladder Pressure Monitor System for Lower Urinary Tract Dysfunction. IEEE J. Transl. Eng. Heal. Med. 8, 1–7. 10.1109/JTEHM.2019.2943170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, He T, Lee C, 2020. Technologies toward next generation human machine interfaces: From machine learning enhanced tactile sensing to neuromorphic sensory systems. Appl. Phys. Rev. 7, 31305. 10.1063/5.0016485 [DOI] [Google Scholar]
- Ziaie B, Nardin MD, Coghlan AR, Najafi K, 1997. A single-channel implantable microstimulator for functional neuromuscular stimulation. IEEE Trans. Biomed. Eng. 44, 909–920. 10.1109/10.634643 [DOI] [PubMed] [Google Scholar]
- Zubiete ED, Luque LF, Rodríguez AVM, Gonzalez IG, 2011. Review of wireless sensors networks in health applications, in: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. pp. 1789–1793. 10.1109/IEMBS.2011.6090510 [DOI] [PubMed] [Google Scholar]
- Zulqarnain M, Stanzione S, Rathinavel G, Smout S, Willegems M, Myny K, Cantatore E, 2020. A flexible ECG patch compatible with NFC RF communication. npj Flex. Electron. 4, 1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


