ABSTRACT
Multiple species of obligate intracellular bacteria in the genus Chlamydia are important veterinary and/or human pathogens. These pathogens all share similar biphasic developmental cycles and transition between intracellular vegetative reticulate bodies and infectious elementary forms, but vary substantially in their host preferences and pathogenic potential. A lack of tools for genetic engineering of these organisms has long been an impediment to the study of their biology and pathogenesis. However, the refinement of approaches developed in C. trachomatis over the last 10 years, and adaptation of some of these approaches to other Chlamydia spp. in just the last few years, has opened exciting new possibilities for studying this ubiquitous group of important pathogens.
Keywords: Chlamydia, genetics, intracellular, pathogenesis
This is a review of contemporary molecular genetic tools that have been used to dissect the pathogenic properties of obligate intracellular bacteria in the genus Chlamydia.
INTRODUCTION
Bacteria in the phylum Chlamydiae are obligatory intracellular parasites that infect a wide range of phylogenetically diverse eukaryotic hosts. The most studied bacteria in this phylum include human and livestock pathogens in the genus Chlamydia, and recent advances in the genetic analysis of these bacteria are the focus of this review.
A total of three Chlamydia species (spp.), C. trachomatis, C. pneumoniae and C. psittaci, cause most human infections, but other less common zoonotic species including C. abortus are sometimes transmitted to humans from livestock with fatal consequences (Pichon et al. 2020). C. trachomatis strains have traditionally been separated into two biovars (Stephens and Kuo 1984), which we now know reflect an ancient and well-supported phylogenetic branch (Harris et al. 2012). Less invasive strains in the trachoma biovar infect and proliferate in mucosal epithelial cells, whereas more invasive strains in the lymphogranuloma venereum biovar infect a wider variety of cells (Moulder 1991). Trachoma biovar strains can be further subdivided into sexually transmitted urogenital strains that infect the lower genitourinary and gastrointestinal (GI) tracts and cause the sexually transmitted disease chlamydia, and fomite transmitted ocular strains that infect the conjunctival epithelium and cause the disease trachoma. Lymphogranuloma biovar strains cause the urogenital disease lymphogranuloma venereum (LGV) and are also sexually transmitted but, unlike the urogenital strains, can traverse mucosal membranes. Invasiveness of the LGV strains may also be linked to the ability to egress from the basolateral surface of epithelial cells and spread to sub-mucosal fibroblasts and macrophages (Davis and Wyrick 1997). Nonetheless, C. trachomatis disease groups are not silos, and horizontal gene exchange between ocular and urogenital (Harris et al. 2012) and LGV and urogenital (Borges et al. 2021) strains has been documented and may be behind the emergence of hybrid strains with intermediate characteristics (Somboonna et al. 2011). C. pneumoniae is spread by respiratory droplets (Kuo et al. 1995). C. pneumoniae is a common and endemic agent of community acquired pneumonia in humans, and epidemiological evidence has implicated this pathogen as an etiological agent or co-factor in a range of cardiovascular and neurological syndromes. C. psittaci is usually acquired by direct contact with birds (Chu, Yarrarapu and Durrani 2021), although human to human transmission has been observed (Broholm et al. 1977; Wallensten, Fredlund and Runehagen 2014), and elicits the disease psittacosis in humans. C. psittaci infection can elicit mild and self-limited symptoms, similar to those caused by C. pneumoniae, or rapidly progress to severe pneumonia with systemic complications, possibly when C. psittaci disseminates in macrophages (Knittler and Sachse 2015). Generally, less is known about the pathogenesis of veterinary Chlamydia spp., although it does appear that fecal-oral transmission is important in the spread of many of these pathogens (Rank and Yeruva 2014). However, the mouse pathogen C. muridarum, a close C. trachomatis relative, has been studied extensively in mice because this pathogen can reproduce many aspects of urogenital chlamydia in humans (R. P. Morrison and Caldwell 2002).
Genetic analysis in Chlamydia spp. has lagged behind other less common bacterial pathogens for multiple reasons. First, Chlamydiae cannot be cultivated axenically, and isolating clonal populations of these organisms via limiting dilution and/or plaque assays, a key step in most genetic approaches, is labor intensive. Second, Chlamydiae have a complex developmental cycle and these pathogens transition between infectious intracellular reticulate body (RB) and infectious elementary body (EB) forms (Moulder 1991). Vegetative, division-competent RBs are wall-less forms with a biologically active and non-condensed genome, while the infectious EBs have a tightly compacted nucleoid and a rigid cell wall. The genomes of EBs are also tightly packed with histones and these particles are encased by a disulfide cross-linked protein chlamydial outer membrane complex (COMC). Introduction of DNA into Gram-negative organisms usually requires physical contact between donors and recipients (conjugation/transduction) or delivery of DNA across the bacterial cytoplasmic and outer membranes (transformation). However, delivery of DNA into RBs requires traversing the host plasma membrane, inclusion and two bacterial membranes, and the COMC and histone-compacted genomes pose different challenges in EBs. Third, early whole genome sequencing (WGS) efforts focused on a few similar Chlamydia spp. (Stephens et al. 1998; Kalman et al. 1999; Read et al. 2003; Carlson et al. 2005). Observations that these organisms lacked many pathways found in other bacteria, shared many chlamydia-specific open reading frames of unknown function, and exhibited minimal sequence variation suggested that manipulation of highly conserved core Chlamydia genes could be difficult.
Although the conventional wisdom that chlamydiae would remain intractable to classical genetic approaches has been reconsidered, this pessimism was never universal and some approaches from the pre-genetics era remain relevant. For example, early studies confirmed that (1) these organisms were mutable (Rodolakis and Souriau 1983), (2) specific mutants could be enriched using antibiotics and other selections (Binet and Maurelli 2005), (3) identified telling evidence of recombination (Millman, Tavare and Dean 2001) and (4) interrogated the functions of some key proteins and pathways using chemical genetics (Huang, Lesser and Lory 2008). Separately, comparative genomics, surrogate genetic approaches in Escherichia coli and biochemical analysis played key roles in identifying many Chlamydia virulence factors and tropism determinants, including major outer membrane protein (Momp; Caldwell, Kromhout and Schachter 1981), tryptophan synthase (Shaw et al. 2000; Fehlner-Gardiner et al. 2002), inclusion membrane proteins (Incs; Rockey, Heinzen and Hackstadt 1995), translocated actin recruiting protein (Tarp; Clifton et al. 2004), polymorphic outer membrane proteins (Pmps; Henderson and Lam 2001), and outer membrane cysteine rich protein B (OmcB; Stephens et al. 2001), among others. Finally, approaches for complementation of natural phenotypic variants were developed that involved tasking the host cell with producing chlamydial factors or co-infecting with other chlamydia strains to deliver effector proteins (Nelson et al. 2007). We refer readers to other reviews that delve into the history of chlamydia genetics more deeply because some of these approaches remain the only viable approaches for testing specific hypotheses (Bastidas and Valdivia 2016; Brothwell et al. 2018).
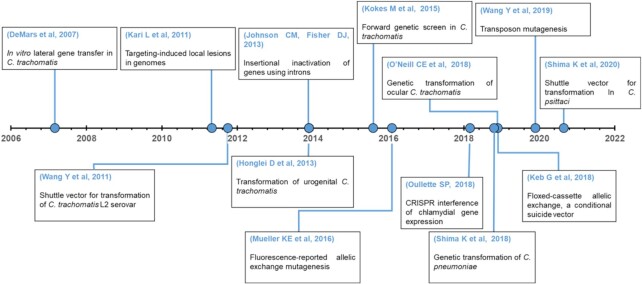
A few key advances played outsized roles in the development of contemporary chlamydial genetic approaches and Fig. 1 shows a non-exhaustive timeline of some key advances. First, Demars and colleagues demonstrated intra- and inter-species lateral gene (LGT) transfer of chlamydial genes in vitro by co-infecting single cells with isolates carrying different spontaneous antibiotic resistance alleles and selecting for recombinants with the corresponding antibiotics (Demars et al. 2007; DeMars and Weinfurter 2008). This provided the first evidence of natural competence and elucidated strategies for mapping genes to phenotypes that are still being harnessed and refined today (Suchland et al. 2009, 2019; Jeffrey et al. 2013). Second, the Caldwell group demonstrated that mutagens could be used to generate nonsense alleles of chlamydial genes inside and outside of the chlamydia plasticity zone (PZ), and that specific mutants could be identified in larger populations of mutants using a simple PCR- and endonuclease-based approach (Kari et al. 2011). Conversely, the Valdivia group showed that pools of heavily mutagenized strains could be screened for specific phenotypes, and then the mutations that elicited these phenotypes could be identified by WGS, LGT and genetic linkage analysis (Nguyen and Valdivia 2012). Collectively, these studies demonstrated that forward and reverse genetic approaches were feasible and identified surprisingly large numbers of genes whose products were partially or totally dispensable in cell culture. Finally, Wang and colleagues stably transformed a C. trachomatis LGV isolate with a shuttle vector constructed from this strain's endogenous plasmid and the origin of replication, β-lactamase (bla) and chloramphenicol transferase (cat) genes from an E. coli plasmid, using a calcium chloride transformation protocol (Wang et al. 2011). Their results confirmed earlier observations that DNA could be introduced into chlamydiae (Binet and Maurelli 2009) and that these organisms could recognize foreign promoters and transgenes (Tam, Davis and Wyrick 1994; Yu et al. 2006). Additionally, their observation that introducing a plasmid into a plasmid negative LGV isolate restored glycogen production confirmed the plasmids can play important roles in chlamydial physiology and the possibility of complementation from chlamydial shuttle vectors. These advances set the stage for the adaptation of molecular genetic tools from other bacteria and the development of new Chlamydia-specific approaches, which have rapidly increased the breadth of hypotheses that are testable in these pathogens.
Figure 1.
Timeline of key events in Chlamydial genetics.
Reverse genetic analysis of the chlamydial plasmid
The development of shuttle vectors from the C. trachomatis LGV plasmid permitted reverse genetic analysis of the roles of plasmid-encoded genes in chlamydial development and virulence, which were implied by earlier observations that naturally occurring plasmid-free isolates and artificially cured isolates behaved differently (Matsumoto et al. 1998; O'Connell et al. 2007). Transformation of naturally plasmid-deficient or plasmid-cured Chlamydia with engineered plasmids containing mutations or deletions in specific plasmid open reading frames (pORFs) identified roles for at least six of the eight plasmid encoded proteins (Pgp1–Pgp8) and a non-coding RNA in plasmid maintenance, virulence and/or gene regulation (Zhong 2017). Transformation of C. trachomatis with recombinant plasmids in which specific pORFs had been deleted (Song et al. 2013), or stop codons were introduced into specific pORFs (Gong et al. 2013), revealed plasmids that did not express Pgp 1, 2 and 6, or from which pORF8 was deleted, were not stable, indicating that these are plasmid maintenance factors. Another study that compared transcriptomes of strains transformed with plasmids lacking specific pORFs revealed that Pgp4 is a transcriptional regulator of Pgp3 expression from the plasmid and multiple chromosomal genes (Carlson et al. 2008; Song et al. 2013). These include genes that drive chlamydial glycogen synthesis and lytic exit (Yang et al. 2015). Pgp3 is a major virulence factor required for C. muridarum to colonize the mouse gut (Shao et al. 2018), for C. muridarum to elicit hydrosalpinx in female mouse upper genital tract (Liu, Huang, et al. 2014), and for C. trachomatis to persist in the female mouse upper genital tract (Liu, Huang, et al. 2014; Yang et al. 2020). The molecular details of how Pgp3 functions are less clear, but Pgp3 deficient strains are more sensitive to antimicrobial peptides and acid stress, which may explain these disease phenotypes (Hou et al. 2015; Yang et al. 2020). Pgp5 plays a more subtle role in C. muridarum virulence in the upper female mouse upper genital tract (Huang et al. 2015) and a role in negative regulation of plasmid genes (Liu, Chen, et al. 2014). Overall, reverse genetic analysis of pORFs revealed that the plasmid plays a central role in the pathogenesis of some Chlamydia spp. Notably, these roles mean that modification of chlamydial plasmids can elicit unintended phenotypes, and we agree with others that best practices necessitate the inclusion of empty shuttle vector controls in experiments (Rahnama and Fields 2018).
Attempts to transform chlamydiae with shuttle vectors derived from different strains and species also identified plasmid sub-regions that dictate plasmid host-range. For example, Song et al. (2014) were able to transform C. muridarum and a C. trachomatis trachoma isolate with shuttle vectors derived from their native plasmids, but not a similar vector derived from an LGV plasmid. Although there have been other reports that indicate restrictions to the transfer of plasmids between trachoma and LGV strains are more nuanced (O'Neill et al. 2018), there is strong evidence that a region in coding sequence 2 (CDS2) limits the host-range of some plasmids. For example, when a plasmid + C. muridarum nigg isolate was transformed with a C. trachomatis derived shuttle vector the transformants contained recombinant plasmids in which CDS2 from the native C. muridarum plasmid had replaced C. trachomatis CDS2 (Wang et al. 2014). Other unknown factors could prevent maintenance of foreign plasmids. For example, Shima showed that a shuttle vector constructed from the plasmid of a C. pneumoniae isolate could stably transform a range of human and veterinary C. pneumoniae strains and C. felis, but not C. trachomatis, C. muridarum, C. caviae, C. pecorum, or C. abortus isolates (Shima et al. 2018). Considering another report that C. psittaci can be stably transformed with a vector derived from its plasmid (Shima et al. 2020), is seems likely that a broad range of Chlamydia spp. can be transformed with vectors constructed from their corresponding plasmids, but the prospects for ‘universal’ shuttle vectors seem less promising.
Expanding the potential and applications of chlamydia shuttle vectors
Shuttle vectors have come a long way since their humble beginning less than 10 years ago. An early limitation was that the use of antibiotic resistance genes that conferred resistance to tetracyclines and β-lactams was limited by NIH guidelines and biosafety concerns. Some of these regulations have been now been clarified and this limitation has been further mitigated by validation of additional antibiotic resistance genes (chloramphenicol, spectinomycin, blasticidin, etc.; Ding et al. 2013; Xu et al. 2013; Lowden et al. 2015). Nonetheless, more antibiotic resistance markers are needed because some of the existing markers are less effective in specific Chlamydia spp., and specific applications, like complementation of antibiotic resistant mutants from shuttle vectors, require multiple resistance markers. For example, we have observed that many C. muridarum isolates are less sensitive to β-lactam antibiotics than C. trachomatis for unknown reasons (unpublished).
Many individual innovations have increased the breadth of what can be accomplished with shuttle vectors. One of the earliest was the addition of transgenes encoding various fluorescent proteins to facilitate live-cell imaging of these pathogens (Agaisse and Derre 2013; Wang et al. 2011). A wide array of fluorescent markers have now been validated, including for sophisticated approaches like split-GFP (Wang, Hybiske and Stephens 2018). Ectopic expression of proteins tagged with small epitopes, including FLAG, has facilitated the localization of chlamydial proteins when highly-specific antibodies are unavailable. In one notable application, Weber et al. (2015) systematically evaluated which computationally-predicted C. trachomatis Incs actually localized to the inclusion membrane. Another advance that substantially increased control of the timing and dosage of transgene expression was the introduction of tetracycline regulated control elements (Wickstrum et al. 2013); similar elements have now been incorporated into many vectors. Finally, expression of type III secreted chlamydial Inc proteins as fusions with ascorbate peroxidase, an enzyme that biotinylates interacting and proximal proteins, has been used by to identify networks of proteins that interact at the host–pathogen interface (Rucks et al. 2017; Dickinson et al. 2019). Although many of these approaches have only been validated in C. trachomatis LGV, it seems likely that they could be adapted for use in the rapidly growing list of successfully transformed Chamydia spp. Importantly, the above studies are only representative examples; we refer readers to another review that discusses advances in vector design in more detail (Bastidas and Valdivia 2016).
Irrespective of the availability of natural or experimentally generated chlamydial mutants, prior to development of stable transformation it was usually not possible to differentiate if mutations exerted their effects in cis or in trans. However, in the last few years successful complementation of phenotypes from shuttle vectors has become more common (Rahnama and Fields 2018). For example, expression of tryptophan biosynthesis genes from a shuttle vector in a C. trachomatis trachoma isolate confirmed that a functional trpBA operon was sufficient to explain the different abilities of C. trachomatis ocular and urogenital strains to be indole rescued in tryptophan limiting conditions (O'Neill et al. 2018). Among other notable examples, the phenotype of an interferon-sensitive C. trachomatis LGV point mutant was reversed by expression of the chlamydial protease Ptr from a shuttle vector (Panzetta et al. 2019).
Strategies for generating mutants and downstream forward and reverse genetic analyses
In the first report to describe the application of reverse genetics in Chlamydiae, Kari et al. (2011) used low doses of ethane-methyl sulfonate (EMS) to generate pools of C. trachomatis serovar D isolates that contained, on average, less than one mutagen-induced single nucleotide variant (SNV) per genome. These isolates were then expanded and screened for mutants that contained SNVs in genes of interest using a mismatch specific endonuclease and a technique initially developed in plant genetics called targeting-induced local lesions in genomes (TILLING) (McCallum et al. 2000). Their initial study identified an isogenic trpB null mutant that could not be indole rescued in tryptophan-replete conditions (Kari et al. 2011), and subsequent studies demonstrated that this approach is broadly applicable by isolating isogenic null mutants of the transcription factor ChxR (Yang et al. 2017), polymorphic protein D (Kari et al. 2014) and Pgp3 (Yang et al. 2020). Higher mutagen doses were used to systematically assess if nonsense mutants of C. muridarum plasticity zone genes were dispensable in cell culture and mice in another study, confirming that this approach works in other Chlamydia species (Rajaram et al. 2015). TILLING is labor intensive, but can theoretically be used to generate complete and/or partial loss of function alleles in any Chlamydia spp. that can be cultured in vitro without disrupting the plasmid. These factors make TILLING an attractive approach when targeted approaches for generating mutants are unavailable, would alter the native plasmid and/or complete ablation of gene function is lethal. In another mutagen-based reverse genetic approach, mutants were generated using higher mutagen doses so that the library isolates contained multiple SNVs, and then the genomes of the isolates were WGS in pools (Kokes et al. 2015). In addition to revealing that many C. trachomatis genes can tolerate missense mutations, this study identified 99 different nonsense mutations in 84 different genes. Collectively, the above observations indicated that chlamydial genomes were much more malleable than previously believed.
Forward genetic screens have long and storied history in microbiology and have now been applied to identify functions of several Chlamydia genes. Nguyen and Valdivia screened a library of chemically-mutagenized C. trachomatis LGV isolates for isolates with altered plaque morphologies. The phenotypes of two mutants were mapped to SNVs in the genes for the glycogen branching enzyme GlgB and the general secretion pathway protein GspE using LGT and genetic linkage analysis (Nguyen and Valdivia 2012). Screens of chemically-mutagenized libraries have now identified novel genes that mediate F-actin assembly around the inclusion (InaC; Kokes et al. 2015), interferon resistance and inclusion integrity (CpoS and Igs4; Sixt et al. 2017; Giebel et al. 2019), persistence (Ptr, Ctl0225; Muramatsu et al. 2016; Panzetta et al. 2019) and temperature sensitive alleles (Brothwell et al. 2016). Identifying which SNV is linked to a phenotype of interest has been the rate-limiting step in these screens, although this has been accomplished using LGT (Nguyen and Valdivia 2012), complementation (Panzetta et al. 2019) and genetic suppressor mapping (Giebel et al. 2019).
Transposon mutagenesis was only recently described in C. trachomatis and C. muridarum, but has already provided many insights (Fischer et al. 2017; LaBrie et al. 2019; Suchland et al. 2019; Wang et al. 2019). In the first detailed study, 105 unique transposon mutants were generated by transforming C. trachomatis LGV with a suicide vector encoding Himar1 transposase and the bla gene flanked by inverted repeats (LaBrie et al. 2019). A total of 54 transposon insertions were identified in genes that had not been inactivated previously. Some of the mutants had obvious phenotypes, but no phenotypes were detected in many mutants that had insertions in suspected chlamydial virulence factors. Interestingly, a mutant with an insertion in Ct339/ComEC, a component of the competence machinery in other bacteria, was unable to uptake DNA via LGT, supporting that this protein mediates competence in Chlamydia spp. A total of 33 stable transposon mutants were generated in C. muridarum using a similar approach, demonstrating that transposon mutagenesis is feasible in other transformable Chlamydia spp. (Wang et al. 2019). Most recently, Suchland et al. used transposon mutants to drive inter-species LGT crosses using the on-board antibiotic resistance genes in the transposons (Suchland et al. 2019). In comparison to intra-species LGT crosses, the regions of recombined DNA from these homeologous exchanges were more limited to regions proximal to the transposon-encoded resistance genes. This yielded a library of C. muridarum/C. trachomatis chimeras in which the individual isolates contained most of the genome from one parent and small regions of the chromosome from the other parent, which could be invaluable for mapping unique characteristics of these species to specific genes.
Three targeted approaches for gene inactivation in Chlamydia have now been described: insertion of mobile type II introns (TargeTron), Fluorescence-Reported Allelic Exchange Mutagenesis (FRAEM) and Crispr Interference (Crispri). In the first approach, DNA sequences in the substrate recognition region of TargeTron type II introns are altered to permit insertion of the intron into specific target sites. In the first application of this approach in Chlamydia, the intron and an onboard bla gene were introduced by transformation with a suicide vector, and a stable incA::GII(bla)mutant was selected with ampicillin (Johnson and Fisher 2013). Lowden et al. subsequently used a second intron encoding a spectinomycin resistance gene (aadA) to generate a C. trachomatis LGV incA::GII(aadA), rsbV1::GII(bla) double mutant (Lowden et al. 2015). TargeTron has been the most widely used approach for targeted gene disruption in Chlamydia so far, and has now been used to disrupt an extensive list of genes in C. trachomatis LGV that have been the subject of multiple recent reviews (Hooppaw and Fisher 2015; Bastidas and Valdivia 2016; Rahnama and Fields 2018). This approach has utility outside of C. trachomatis too; Filcek et al. used it to target genes in C. caviae (Filcek et al. 2019; Table 1). In contrast, the FRAEM approach developed by Mueller et al. uses a conditional suicide vector to force recombination between longer regions of homology on the chlamydial chromosome (Mueller, Wolf and Fields 2016). Fluorescent marker genes in and outside of recombination regions on the FRAEM vector permit visual differentiation of single and double cross-over events, and thus, this system can be used to generate insertions and deletions. A concern that FRAEM cassettes could elicit polar effects was addressed in another study that developed Floxed-cassette Allelic Exchange Mutagenesis (FLAEM; Keb, Hayman and Fields 2018). FLAEM uses a loxP flanked gfp-bla cassette so that the cassette can subsequently be removed by expressing Cre recombinase in trans. FRAEM/FLAEM have been used successfully to delete genes for type III secreted effectors that have not been inactivated by other targeted methods including Tarp and TmeE (Keb et al. 2018; Ghosh et al. 2020). The most recent addition to the Chlamydia reverse genetics toolbox has been the adaptation of Crispri to generate conditional knockouts (Ouellette 2018). In the original manuscript, Ouellette showed that co-expression of a catalytically inactive Cas9 variant from S. aureus and a guide RNA targeting the 5′ UTR of incA in C. trachomatis decreased the amount of IncA in RBs. After some fine tuning to address vector stability, the Ouellette group subsequently knocked down expression of the ClpP2X protease operon and showed that ClpP2 was essential for normal development of C. trachomatis LGV (Wood et al. 2020). Importantly, specificity of the interfering RNA was confirmed by complementing with a dCa9s-ClpP2 transcriptional fusion. This conditional knockdown approach could be especially useful for studying genes that cannot be irreversibly inactivated.
Table 1.
List of genes/operons analyzed using genetic techniques since 2017.
| Genetic technique | Organism | Gene/operon | Reference |
|---|---|---|---|
| Targetron | Chlamydia trachomatis L2 | ct105, ct228, ct622andct456 | Cossé et al. (2018), Faris et al. (2020), Pais et al. (2019) and Shaw et al. (2018) |
| Chlamydia cavie | incAandsinC | Filcek et al. (2019) | |
| FRAEM | Chlamydia trachomatis L2 | Tarp | Ghosh et al. (2020) |
| FLAEM | Chlamydia trachomatis L2 | tmeA | Keb et al. (2018) |
| TILLING | Chlamydia trachomatis A297 | pgp3 | Yang et al. (2020) |
| CRISPRi | Chlamydia trachomatis L2 | incAandclpP2X | Ouellette (2018) and Wood et al. (2020) |
| Complementation in trans | Chlamydia trachomatis A297 | trpRBA | O'Neill et al. (2018) |
| Chlamydia trachomatis L2 | ptr | Panzetta et al. (2019) | |
| Transposon mutagenesis | Chlamydia muridarum | glgB | Wang et al. (2019) |
| Chlamydia trachomatis D | ct339 | LaBrie et al. (2019) |
CONCLUSION AND FUTURE PERSPECTIVES
New genetic tools for Chlamydia spp. are now being reported regularly, and it is especially encouraging that many new approaches are extending the genetic toolbox beyond C. trachomatis. Although none of these tools are without their limitations, the toolbox is now deep and rich enough that many hypotheses can be tested by adaptable investigators. Nonetheless, we think that advances in a few areas discussed below could substantially broaden the utility of chlamydial genetic approaches.
Genetic screens in Chlamydia spp. have largely been limited to mutagen-derived libraries so far because the labor intensiveness and cost of generating large and rich mutant pools using targeted gene inactivation, gene knockdown and transposon mutagenesis remains prohibitive. One limitation of such screens is that mutagen-generated nonsense mutations in the 3′ end of genes may not lead to complete inactivation of gene function. Comprehensive libraries of ‘tagged’ mutants could permit interrogation with powerful approaches, such as TN-seq, which have been used to generate comprehensive lists of virulence factors and essential genes in other bacterial pathogens (Cain et al. 2020). Low transformation efficiency has often been identified as the key bottleneck in the generation of these sorts of libraries. However, at least two groups have demonstrated delivery of DNA into EBs using electroporation (Tam et al. 1994; Binet and Maurelli 2009). Highly efficient transformation protocols have also been described that employed shuttle vector-conjugated dendrimers (Gerard et al. 2013; Kannan et al. 2013), and dendrimers have been used to transform other intracellular pathogens (Oki et al. 2015). Thus, although development of the original transformation protocol was a landmark accomplishment, even modest improvements in transformation efficiency could have outsized effects.
Vector effects were noted by Wang et al. (2011) upon the introduction of the first shuttle vector and continue to be a major limitation. In essence, attempts to complement chromosomal mutations using transgenes expressed from shuttle vectors sometimes fail for unclear reasons and/or the transgenic plasmids have other unanticipated effects unrelated to the transgenes. Possible explanations include, but are not limited to, inappropriate timing and extent of gene expression and that manipulation of the plasmid could alter the expression of chromosomal and plasmid genes. In our hands, vector effects can be even more pronounced in animal models (unpublished). Isogenic strain pairs have been used to avoid vectors effects, but cannot differentiate if complementation occurs in cis or in trans. Better understanding of transcriptional regulation in Chlamydiae in general, fine tuning of promoters, development of alternate shuttle vectors compatible with native chlamydial plasmids, and/or continued improvement of inducible/repressible expression vectors could all help address these issues. However, the best long-term approach might be the development of techniques for expression of single copies of transgenes from neutral chromosomal sites and/or unmarked allelic repair.
Third, there has been a divergence between the numbers of new chlamydial mutants being reported and the numbers of new phenotypes. Some of this disparity may be explained by redundant and non-functional genes, but other mutants may not have yet been evaluated in their proper contexts. New models could facilitate searches for these missing phenotypes. For, example, multiple groups have pioneered the use of 3-dimensional organoid systems to more accurately model interactions between Chlamydia spp. and complex tissues (Kessler et al. 2019; Bishop et al. 2020; Dolat and Valdivia 2021). In another study, Filcek showed that a C. caviae sinC insertion mutant was severely attenuated in chick embryos (Filcek et al. 2019). This straightforward model is attractive because use of eggs is subject to less administrative regulation compared to adult animals. Finally, models of human cervical, fallopian tube and rectal chlamydia that utilize C. muridarum in mice are well-established (Murthy, Li and Ramsey 2018), but approaches for modeling sexual and vertical transmission of chlamydia are more limited (Mount, Bigazzi and Barron 1973; Rank et al. 2003). Pal, Tifrea and de la Maza (2019) recently developed a sexual transmission model that permits study of urethral responses to C. muridarum infection in male mice, sexual transmission from male to female mice and vertical transmission from mothers to pups. This model is compelling because C. muridarum encodes tropism determinants that play disproportionate roles in the gut and cervix (Morrison et al. 2018, 2020), but it is unknown if other genes evolved to facilitate sexual transmission, vertical transmission and/or pathogenesis in the male urethra.
In conclusion, it is an exciting time in chlamydia research, and hopefully increased genetic tractability of these pathogens will inspire new investigators to enter the field. Since tools now exist for the generation of mutants and complementation in all three species of Chlamydia that commonly infect humans, and an increasing array of veterinary pathogens, the field seems poised to make new insights that could have broad implications in microbial pathogenesis.
FUNDING
DE Nelson was supported by grant AI099278 from the National Institutes of Health. A Banerjee was supported by a graduate fellowship from the Cagientas Foundation.
Contributor Information
Arkaprabha Banerjee, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
David E Nelson, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Conflicts of interest
None declared.
REFERENCES
- Agaisse H, Derre I. A C. trachomatis cloning vector and the generation of C. trachomatis strains expressing fluorescent proteins under the control of a C. trachomatis promoter. PLoS One. 2013;8:e57090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas RJ, Valdivia RH. Emancipating Chlamydia: advances in the genetic manipulation of a recalcitrant intracellular pathogen. Microbiol Mol Biol Rev. 2016;80:411–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet R, Maurelli AT. Frequency of spontaneous mutations that confer antibiotic resistance in Chlamydia spp. Antimicrob Agents Chemother. 2005;49:2865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet R, Maurelli AT. Transformation and isolation of allelic exchange mutants of Chlamydia psittaci using recombinant DNA introduced by electroporation. Proc Natl Acad Sci. 2009;106:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop RC, Boretto M, Rutkowski MRet al. . Murine endometrial organoids to model chlamydia infection. Front Cell Infect Microbiol. 2020;10:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges V, Isidro J, Correia Cet al. . Transcontinental dissemination of the L2b/D-Da recombinant Chlamydia trachomatis Lymphogranuloma venereum (LGV) strain: need of broad multi-country molecular surveillance. Clin Infect Dis. 2021. DOI: 10.1093/cid/ciab067. [DOI] [PubMed] [Google Scholar]
- Broholm KA, Bottiger M, Jernelius Het al. . Ornithosis as a nosocomial infection. Scand J Infect Dis. 1977;9:263–7. [DOI] [PubMed] [Google Scholar]
- Brothwell JA, Muramatsu MK, Toh Eet al. . Interrogating genes that mediate Chlamydia trachomatis survival in cell culture using conditional mutants and recombination. J Bacteriol. 2016;198:2131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothwell JA, Muramatsu MK, Zhong Get al. . Advances and obstacles in the genetic dissection of Chlamydial virulence. Curr Top Microbiol Immunol. 2018;412:133–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain AK, Barquist L, Goodman ALet al. . A decade of advances in transposon-insertion sequencing. Nat Rev Genet. 2020;21:526–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JH, Porcella SF, McClarty Get al. . Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73:6407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JH, Whitmire WM, Crane DDet al. . The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;76:2273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Yarrarapu SNS, Durrani MI. Psittacosis. In StatPearls. Treasure Island (FL), 2021. [Google Scholar]
- Clifton DR, Fields KA, Grieshaber SSet al. . A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci. 2004;101:10166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossé MM, Barta ML, Fisher DJet al. . The Loss of Expression of a Single Type 3 Effector (CT622) Strongly Reduces Chlamydia trachomatis Infectivity and Growth. Front Cell Infect Microbiol. 2018;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CH, Wyrick PB. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect Immun. 1997;65:2914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars R, Weinfurter J, Guex Eet al. . Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis. J Bacteriol. 2007;189:991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R, Weinfurter J. Interstrain gene transfer in Chlamydia trachomatis in vitro: mechanism and significance. J Bacteriol. 2008;190:1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson MS, Anderson LN, Webb-Robertson BMet al. . Proximity-dependent proteomics of the Chlamydia trachomatis inclusion membrane reveals functional interactions with endoplasmic reticulum exit sites. PLoS Pathog. 2019;15:e1007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Gong S, Tian Yet al. . Transformation of sexually transmitted infection-causing serovars of Chlamydia trachomatis using Blasticidin for selection. PLoS One. 2013;8:e80534. DOI: 10.1371/journal.pone.0080534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolat L, Valdivia RH. An endometrial organoid model of Chlamydia-epithelial and immune cell interactions. J Cell Sci. 134, 2021. DOI: 10.1242/jcs.252403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris R, McCullough A, Andersen SEet al. . The Chlamydia trachomatis secreted effector TmeA hijacks the N-WASP-ARP2/3 actin remodeling axis to facilitate cellular invasion. PLoS Pathog. 2020;16:e1008878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlner-Gardiner C, Roshick C, Carlson JHet al. . Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J Biol Chem. 2002;277:26893–903. [DOI] [PubMed] [Google Scholar]
- Filcek K, Vielfort K, Muraleedharan Set al. . Insertional mutagenesis in the zoonotic pathogen Chlamydia caviae. PLoS One. 2019;14:e0224324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Harrison KS, Ramirez Yet al. . Chlamydia trachomatis-containing vacuole serves as deubiquitination platform to stabilize Mcl-1 and to interfere with host defense. Elife. 2017;6. DOI: 10.7554/eLife.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard HC, Mishra MK, Mao Get al. . Dendrimer-enabled DNA delivery and transformation of Chlamydia pneumoniae. Nanomed Nanotechnol Biol Med. 2013;9:996–1008. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ruelke EA, Ferrell JCet al. . Fluorescence-reported allelic exchange mutagenesis-mediated gene deletion indicates a requirement for Chlamydia trachomatis tarp during in vivo infectivity and reveals a specific role for the C terminus during cellular invasion. Infect Immun. 2020;88. DOI: 10.1128/IAI.00841-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel AM, Hu S, Rajaram Ket al. . Genetic screen in Chlamydia muridarum reveals role for an interferon-induced host cell death program in antimicrobial inclusion rupture. mBio. 2019;10. DOI: 10.1128/mBio.00385-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Yang Z, Lei Let al. . Characterization of Chlamydia trachomatis plasmid-encoded open reading frames. J Bacteriol. 2013;195:3819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SR, Clarke IN, Seth-Smith HMet al. . Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet. 2012;44:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Lam AC. Polymorphic proteins of Chlamydia spp.–autotransporters beyond the Proteobacteria. Trends Microbiol. 2001;9:573–8. [DOI] [PubMed] [Google Scholar]
- Hooppaw AJ, Fisher DJ. A coming of age story: Chlamydia in the post-genetic era. Infect Immun. 2016;84:612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Dong X, Yang Zet al. . Chlamydial plasmid-encoded virulence factor Pgp3 neutralizes the antichlamydial activity of human cathelicidin LL-37. Infect Immun. 2015;83:4701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lesser CF, Lory S. The essential role of the CopN protein in Chlamydia pneumoniae intracellular growth. Nature. 2008;456:112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhang Q, Yang Zet al. . Plasmid-encoded Pgp5 is a significant contributor to Chlamydia muridarum induction of hydrosalpinx. PLoS One. 2015;10:e0124840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey BM, Suchland RJ, Eriksen SGet al. . Genomic and phenotypic characterization of in vitro-generated Chlamydia trachomatis recombinants. BMC Microbiol. 2013;13:142. DOI: 10.1186/1471-2180-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Fisher DJ. Site-specific, insertional inactivation of incA in Chlamydia trachomatis using a group II intron. PLoS One. 2013;8:e83989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman S, Mitchell W, Marathe Ret al. . Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat Genet. 1999;21:385–9. [DOI] [PubMed] [Google Scholar]
- Kannan RM, Gerard HC, Mishra MKet al. . Dendrimer-enabled transformation of Chlamydia trachomatis. Microb Pathog. 2013;65:29–35. [DOI] [PubMed] [Google Scholar]
- Kari L, Goheen MM, Randall LBet al. . Generation of targeted Chlamydia trachomatis null mutants. Proc Natl Acad Sci. 2011;108:7189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari L, Southern TR, Downey CJet al. . Chlamydia trachomatis polymorphic membrane protein D is a virulence factor involved in early host-cell interactions. Infect Immun. 2014;82:2756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keb G, Hayman R, Fields KA. Floxed-cassette allelic exchange mutagenesis enables markerless gene deletion in Chlamydia trachomatis and can reverse cassette-induced polar effects. J Bacteriol. 2018;200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Hoffmann K, Fritsche Ket al. . Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nat Commun. 2019;10:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. 2015;73:1–15. [DOI] [PubMed] [Google Scholar]
- Kokes M, Dunn JD, Granek JAet al. . Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe. 2015;17:716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Jackson LA, Campbell LAet al. . Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995;8:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBrie SD, Dimond ZE, Harrison KSet al. . Transposon mutagenesis in Chlamydia trachomatis identifies CT339 as a ComEC homolog important for DNA uptake and lateral gene transfer. mBio. 2019;10. DOI: 10.1128/mBio.01343-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen C, Gong Set al. . Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol. 2014;196:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang Y, Yang Zet al. . Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun. 2014;82:5327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowden NM, Yeruva L, Johnson CMet al. . Use of aminoglycoside 3' adenyltransferase as a selection marker for Chlamydia trachomatis intron-mutagenesis and in vivo intron stability. BMC Res Notes. 2015;8:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Izutsu H, Miyashita Net al. . Plaque formation by and plaque cloning of Chlamydia trachomatis biovar trachoma. J Clin Microbiol. 1998;36:3013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum CM, Comai L, Greene EAet al. . Targeted screening for induced mutations. Nat Biotechnol. 2000;18:455–7. [DOI] [PubMed] [Google Scholar]
- Millman KL, Tavare S, Dean D. Recombination in the ompA gene but not the omcB gene of Chlamydia contributes to serovar-specific differences in tissue tropism, immune surveillance, and persistence of the organism. J Bacteriol. 2001;183:5997–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Giebel AM, Toh ECet al. . Chlamydia muridarum genital and gastrointestinal infection tropism is mediated by distinct chromosomal factors. Infect Immun, 2018;86:e00141–18. DOI: 10.1128/IAI.00141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Giebel AM, Toh Eet al. . A genital infection-attenuated Chlamydia muridarum mutant infects the gastrointestinal tract and protects against genital tract challenge. mBio. 2020;11. DOI: 10.1128/mBio.02770-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–90. Available at: https://www.ncbi.nlm.nih.gov/pubmed/2030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount DT, Bigazzi PE, Barron AL. Experimental genital infection of male guinea pigs with the agent of guinea pig inclusion conjunctivitis and transmission to females. Infect Immun. 1973;8:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KE, Wolf K, Fields KA. Gene deletion by fluorescence-reported allelic exchange mutagenesis in Chlamydia trachomatis. mBio. 2016;7:e01817–01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu MK, Brothwell JA, Stein BDet al. . Beyond tryptophan synthase: identification of genes that contribute to Chlamydia trachomatis survival during gamma interferon-induced persistence and reactivation. Infect Immun. 2016;84:2791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK, Li W, Ramsey KH. Immunopathogenesis of Chlamydial infections. Curr Top Microbiol Immunol. 2018;412:183–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Taylor LD, Shannon JGet al. . Phenotypic rescue of Chlamydia trachomatis growth in IFN-gamma treated mouse cells by irradiated Chlamydia muridarum. Cell Microbiol. 2007;9:2289–98. [DOI] [PubMed] [Google Scholar]
- Nguyen BD, Valdivia RH. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc Natl Acad Sci. 2012;109:1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell CM, Ingalls RR, Andrews CW Jr.et al. . Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;179:4027–34. [DOI] [PubMed] [Google Scholar]
- O'Neill CE, Skilton RJ, Pearson SAet al. . Genetic transformation of a C. trachomatis ocular isolate with the functional tryptophan synthase operon confers an indole-rescuable phenotype. Front Cell Infect Microbiol. 2018;8:434. DOI: 10.3389/fcimb.2018.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki AT, Seidman D, Lancina MG 3rdet al. . Dendrimer-enabled transformation of Anaplasma phagocytophilum. Microb Infect. 2015;17:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette SP. Feasibility of a conditional knockout system for Chlamydia based on CRISPR interference. Front Cell Infect Microbiol. 2018;8:59. DOI: 10.3389/fcimb.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais SV, Key CE, Borges Vet al. . CteG is a Chlamydia trachomatis effector protein that associates with the Golgi complex of infected host cells. Sci Rep. 2019;9:6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Tifrea DF, de la Maza LM. Characterization of the horizontal and vertical sexual transmission of Chlamydia genital infections in a new mouse model. Infect Immun. 2019;87. DOI: 10.1128/IAI.00834-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzetta ME, Lujan AL, Bastidas RJet al. . Ptr/CTL0175 is required for the efficient recovery of Chlamydia trachomatis from stress induced by gamma-interferon. Front Microbiol. 2019;10:756. DOI: 10.3389/fmicb.2019.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon N, Guindre L, Laroucau Ket al. . Chlamydia abortus in pregnant woman with acute respiratory distress Syndrome. Emerg Infect Dis. 2020;26:628–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnama M, Fields KA. Transformation of Chlamydia: current approaches and impact on our understanding of chlamydial infection biology. Microb Infect. 2018;20:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram K, Giebel AM, Toh Eet al. . Mutational analysis of the Chlamydia muridarum plasticity zone. Infect Immun. 2015;83:2870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Bowlin AK, Reed RLet al. . Characterization of Chlamydial genital infection resulting from sexual transmission from male to female guinea pigs and determination of infectious dose. Infect Immun. 2003;71:6148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Yeruva L. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun. 2014;82:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read TD, Myers GS, Brunham RCet al. . Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 2003;31:2134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DD, Heinzen RA, Hackstadt T. Cloning and characterization of a Chlamydia psittaci gene coding for a protein localized in the inclusion membrane of infected cells. Mol Microbiol. 1995;15:617–26. [DOI] [PubMed] [Google Scholar]
- Rodolakis A, Souriau A. Response of ewes to temperature-sensitive mutants of Chlamydia psittaci (var ovis) obtained by NTG mutagenesis. Ann Rech Vet. 1983;14:155–61. Available at: https://www.ncbi.nlm.nih.gov/pubmed/6614792. [PubMed] [Google Scholar]
- Rucks EA, Olson MG, Jorgenson LMet al. . Development of a proximity labeling system to map the Chlamydia trachomatis inclusion membrane. Front Cell Infect Microbiol. 2017;7:40. DOI: 10.3389/fcimb.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Zhang T, Melero Jet al. . The genital tract virulence factor pGP3 is essential for Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun. 2018;86. DOI: 10.1128/IAI.00429-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Christiansen G, Roepstorff Pet al. . Genetic differences in the Chlamydia trachomatis tryptophan synthase alpha-subunit can explain variations in serovar pathogenesis. Microb Infect. 2000;2:581–92. [DOI] [PubMed] [Google Scholar]
- Shaw JH, Key CE, Snider TAet al. . Genetic Inactivation of Chlamydia trachomatis Inclusion Membrane Protein CT228 Alters MYPT1 Recruitment, Extrusion Production, and Longevity of Infection. Front Cell Infect Microbiol. 2018;8:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Wanker M, Skilton RJet al. . The gnetic transformation of Chlamydia pneumoniae. mSphere. 2018;3. DOI: 10.1128/mSphere.00412-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Weber MM, Schnee Cet al. . Development of a plasmid shuttle vector system for genetic manipulation of Chlamydia psittaci. mSphere. 2020;5. DOI: 10.1128/mSphere.00787-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt BS, Bastidas RJ, Finethy Ret al. . The Chlamydia trachomatis inclusion membrane protein CpoS counteracts STING-mediated cellular surveillance and suicide programs. Cell Host Microbe. 2017;21:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somboonna N, Wan R, Ojcius DMet al. . Hypervirulent Chlamydia trachomatis clinical strain is a recombinant between lymphogranuloma venereum (L(2)) and D lineages. mBio. 2011;2:e00045–00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Carlson JH, Whitmire WMet al. . Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect Immun. 2013;81:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Carlson JH, Zhou Bet al. . Plasmid-mediated transformation tropism of chlamydial biovars. Pathog Dis. 2014;70:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Kalman S, Lammel Cet al. . Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–9. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Koshiyama K, Lewis Eet al. . Heparin-binding outer membrane protein of chlamydiae. Mol Microbiol. 2001;40:691–9. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Kuo CC. Chlamydia trachomatis species-specific epitope detected on mouse biovar outer membrane protein. Infect Immun. 1984;45:790–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchland RJ, Carrell SJ, Wang Yet al. . Chromosomal recombination targets in Chlamydia interspecies lateral gene transfer. J Bacteriol. 2019;201. DOI: 10.1128/JB.00365-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchland RJ, Sandoz KM, Jeffrey BMet al. . Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob Agents Chemother. 2009;53:4604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam JE, Davis CH, Wyrick PB. Expression of recombinant DNA introduced into Chlamydia trachomatis by electroporation. Can J Microbiol. 1994;40:583–91. [DOI] [PubMed] [Google Scholar]
- Wallensten A, Fredlund H, Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January-February 2013. Eurosurveillance. 2014;19. DOI: 10.2807/1560-7917.es2014.19.42.20937. [DOI] [PubMed] [Google Scholar]
- Wang X, Hybiske K, Stephens RS. Direct visualization of the expression and localization of chlamydial effector proteins within infected host cells. Pathog Dis. 2018;76. DOI: 10.1093/femspd/fty011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cutcliffe LT, Skilton RJet al. . The genetic basis of plasmid tropism between Chlamydia trachomatis and Chlamydia muridarum. Pathog Dis. 2014;72:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kahane S, Cutcliffe LTet al. . Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011;7:e1002258. DOI: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, LaBrie SD, Carrell SJet al. . Development of transposon mutagenesis for Chlamydia muridarum. J Bacteriol. 2019;201. DOI: 10.1128/JB.00366-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MM, Bauler LD, Lam Jet al. . Expression and localization of predicted inclusion membrane proteins in Chlamydia trachomatis. Infect Immun. 2015;83:4710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrum J, Sammons LR, Restivo KNet al. . Conditional gene expression in Chlamydia trachomatis using the tet system. PLoS One. 2013;8:e76743. DOI: 10.1371/journal.pone.0076743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NA, Blocker AM, Seleem MAet al. . The ClpX and ClpP2 Orthologs of Chlamydia trachomatis perform discrete and essential functions in organism growth and development. mBio. 2020;11. DOI: 10.1128/mBio.02016-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Battaglia L, Bao Xet al. . Chloramphenicol acetyltransferase as a selection marker for chlamydial transformation. BMC Research Notes. 2013;6:377. DOI: 10.1186/1756-0500-6-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kari L, Lei Let al. . Chlamydia trachomatis plasmid gene protein 3 is essential for the establishment of persistent infection and associated immunopathology. mBio. 2020;11. DOI: 10.1128/mBio.01902-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kari L, Sturdevant GLet al. . Chlamydia trachomatis ChxR is a transcriptional regulator of virulence factors that function in in vivo host-pathogen interactions. Pathog Dis. 2017;75. DOI: 10.1093/femspd/ftx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Starr T, Song Let al. . Chlamydial lytic exit from host cells is plasmid regulated. MBio. 2015;6:e01648–01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Di Russo EG, Rounds MAet al. . Mutational analysis of the promoter recognized by Chlamydia and Escherichia coli sigma(28) RNA polymerase. J Bacteriol. 2006;188:5524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol. 2017;25:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]