Abstract
Background Context:
Degenerative lumbar conditions are prevalent, disabling, and frequently managed with decompression and fusion. Black patients have lower spinal fusion rates than White patients.
Purpose:
Determine whether specific lumbar fusion procedure utilization differs by race/ethnicity and whether length of stay or inpatient complications differ by race/ethnicity after accounting for procedure performed.
Study Design:
Large database retrospective cohort study
Patient Sample:
Lumbar fusion recipients at least age 50 in the 2016 National Inpatient Sample with diagnoses of degenerative lumbar conditions.
Outcome Measures:
Type of fusion procedure used and inpatient safety measures including length of stay (LOS), prolonged LOS, inpatient medical and surgical complications, mortality, and cost.
Methods:
We examined the association between race/ethnicity and the safety measures above. Covariates included several patient and hospital factors. We used multiple linear or logistic regression to determine the association between race and fusion type [PLF, P/TLIF, ALIF, PLF + P/TLIF, and PLF + ALIF (anterior-posterior fusion)] and to determine whether race was associated independently with inpatient safety measures, after adjustment for patient and hospital factors.
Results:
Fusion method use did not differ among racial/ethnic groups, except for somewhat lower anterior-posterior fusion utilization in Black patients compared to White patients [crude OR: 0.81 (0.67–0.97)]. Inpatient safety measures differed by race/ethnicity for rates of prolonged LOS (Blacks 18.1%, Hispanics 14.5%, Whites 11.7%), medical complications (Blacks 9.9%, Hispanics 8.7%, Whites 7.7%), and surgical complications (Blacks 5.2%, Hispanics 6.9%, Whites 5.4%). Differences persisted after adjustment for procedure type as well as patient and hospital factors. Blacks and Hispanics had higher risk for prolonged LOS compared to Whites [adjusted OR Blacks 1.39 (95% CI 1.22–1.59); Hispanics 1.24 (95% CI 1.02–1.52)]. Blacks had higher risk for inpatient medical complications compared to Whites [adjusted OR 1.24 (95% CI 1.05–1.48)], and Hispanics had higher risk for inpatient surgical complications compared to Whites [adjusted OR 1.34 (95% CI 1.06–1.68)].
Conclusions:
Fusion method use was generally similar between racial/ethnic groups. Inpatient safety measures, adjusted for procedure type, patient and hospital factors, were worse for Blacks and Hispanics.
Level of Evidence:
Prognostic Level II
Keywords: Lumbar Spinal Fusion, National Inpatient Sample, Disparities, Race, Ethnicity
Introduction:
Degenerative lumbar conditions are prevalent, disabling, and often associated with loss of structural integrity, including spondylolisthesis, degenerative scoliosis, or instability1,2. If nonoperative treatment is unsuccessful, these conditions are frequently managed with surgical decompression and fusion3,4. Elective lumbar spinal fusion is an increasingly common procedure in the United States5,6. Several fusion methods are available, with varying complexity, invasiveness, and cost. The literature is inconclusive on whether any of these procedures results in better safety measures than the others7-9. Thus, treatment decisions are individualized and discretionary. While there has been a trend in the last 20 years toward more complex fusion with use of interbody techniques10-12, there are limited data on whether this trend has occurred across all patient populations.
Differences in spine surgical care by race/ethnicity have been reported. These include disparities in access to imaging13, surgical hospitalization rates14, postoperative morbidity and mortality15-20, and improvement in pain and function after surgery21,22. Furthermore, Black patients are less likely than White patients to receive spinal fusion surgery15,21,23. Prior work on procedure utilization rates has largely focused on surgical vs. nonoperative management. For patients committed to surgical treatment with lumbar fusion, it is unknown whether specific procedure utilization differs by race/ethnicity. Similarly, it remains unknown whether safety measures for a given fusion procedure differ between racial/ethnic groups.
Using the National Inpatient Sample (NIS), this study investigated two main questions in a population with degenerative lumbar spinal disease that received lumbar fusion. First, for patients undergoing lumbar fusion, does procedure selection differ by race/ethnicity? Second, do lumbar fusion inpatient safety measures differ by race/ethnicity after adjusting for procedure type? We hypothesized that Whites receive more expensive/complex procedures (e.g. anterior-posterior fusion) than Blacks or Hispanics and that inpatient safety measures are worse for Blacks and Hispanics than for Whites even after adjusting for the procedure performed.
Materials and Methods:
Data Source
This was a retrospective cohort study using hospital discharge information from the NIS. The NIS, developed for the Healthcare Cost and Utilization Project (HCUP), is the largest publicly available all payer inpatient healthcare database in the U.S. and approximates a 20% stratified sample of all discharges from U.S. hospitals to yield national estimates of inpatient stays24. The NIS conforms to the definition for a limited data set and patient data are deidentified. Our study received an exemption from our hospital institutional review board.
Study Population
We obtained hospital discharge information from the 2016 NIS for patients who met diagnostic, age, and procedure criteria. Patients were required to have at least one of the following degenerative lumbar conditions: lumbar spinal stenosis, spondylolisthesis, degenerative scoliosis, or lumbar instability. We constructed a list of acceptable International Classification of Diseases, Tenth Revision, Clinical Modification (ICD 10 CM) codes for each diagnosis (Appendix A). We required patient age to be at least 50 years to enhance the likelihood the underlying spine problem was degenerative in nature. Patients must have undergone lumbar fusion. The procedure categories were: posterolateral fusion (PLF), posterior or transforaminal lumbar interbody fusion (P/TLIF), anterior lumbar interbody fusion (ALIF, also includes direct and extreme lateral interbody fusions DLIF and XLIF), PLF + P/TLIF, and PLF + ALIF (“anterior-posterior fusion”). We constructed a list of acceptable International Classification of Diseases, Tenth Revision, Procedure Coding System (ICD 10 PCS) codes for each procedure category (Appendix B). The ICD-10-PCS coding system provided elaborate detail between procedures to allow us to confidently distinguish procedures from each other. We also consulted with a professional coding specialist to ensure our coding scheme assigned the correct codes to procedures.
Data Elements
The independent variable of interest was patient race. HCUP collects race and ethnicity (i.e. Hispanic or not) separately from data sources and HCUP coding incorporates race and ethnicity into a single data element for the NIS. Ethnicity takes precedence over race, so our categories included non-Hispanic Whites, non-Hispanic Blacks, Hispanics, other, and missing. The outcome for the first set of analyses was the fusion method performed. Outcomes for the second set of analyses were LOS, prolonged LOS, inpatient medical and surgical complications, mortality, and cost. Prolonged LOS was a binary variable for LOS longer than the 90th percentile for the fusion procedure performed. Inpatient medical complications included cardiovascular, pulmonary, and renal/urinary complications. Inpatient surgical complications included hemorrhagic, infectious/wound, neurologic, and thromboembolic complications. We constructed a list of acceptable ICD 10 CM codes for each complication category (Appendix C). We specified inpatient medical and surgical complications as binary variables that indicated whether a patient had at least one complication from the constructed lists. We calculated cost for each discharge by converting from total charges, using HCUP cost to charge ratios provided at the hospital level25. Additional data elements used for analysis included patient race, age, sex, Elixhauser Comorbidity Index (ECI, calculated using HCUP software tools26), zip code median household income (in quartiles as provided by NIS27), payer/insurance (private, Medicare, Medicaid, other), hospital bedsize (small, medium, and large28), hospital location/teaching status (rural, urban nonteaching, urban teaching29), U.S. hospital region, and hospital fusion volume. We created the hospital fusion volume variable by ranking hospitals by total fusion procedures performed and dividing them into tertiles. While the NIS does not provide the absolute value for hospital fusion volume, ranking hospital volume is appropriate since NIS approximates a random sample of all discharges30.
Statistical Analysis
We used SAS survey procedures (Version 9.4, SAS Institute, Cary, NC, USA) to account for survey stratum, survey cluster, and survey weight in all analyses. We calculated the distribution of fusion methods for each race/ethnicity. We used multiple logistic regression to determine whether race/ethnicity was associated with the use of each fusion method, compared to all other fusion methods combined. We examined the overall association of inpatient safety measures with race/ethnicity and stratified by procedure. To determine whether race was associated with inpatient safety measures, we performed multiple logistic regression analyses for prolonged LOS, inpatient medical complications, and inpatient surgical complications, and multiple linear regression analyses for log transformed LOS and cost. For all regression analyses, we used sequential models to examine the effects of additional covariate adjustments. In addition, we used the propensity score approach to create a 1:1 sample of Blacks and Whites matched on age, sex, Elixhauser Comorbidity Index, zip code median household income, payer/insurance, hospital bedsize, hospital location/teaching status, U.S. hospital region, and hospital fusion volume. The matched White controls were selected using the greedy nearest-neighbor matching techniques with exact match on age, sex, hospital bedsize, location/teaching status and region. Balance of covariates between propensity score matched groups was assessed using the standardized differences. The same process was repeated to create comparable groups of Hispanics and Whites. We used Wilcoxon rank sum tests to compare continuous outcomes (LOS, cost) and two sample chi square tests for categorical outcomes (type of fusion procedure, inpatient complications) in the matched samples. Statistical significance was set at two sided p ≤ 0.05.
Results:
Sample Description
The sample included 29,814 discharges, yielding a 2016 national estimate of 149,070 patients age ≥ 50 undergoing lumbar fusion (Table 1). Approximately 58.4% were female and 41.6% were male. Race/ethnicity was 79.5% White, 7.4% Black, 4.5% Hispanic, 3.3% other, and 5.2% missing (Appendix D shows cohort information by race/ethnicity). For payer/insurance, 58% had Medicare, 32.8% had private insurance, 3.7% had Medicaid, and 5.3% had other.
Table 1 –
National Estimates of Lumbar Spinal Fusion Admissions: Cohort Characteristics for Overall Population and Within Procedure
| Cohort Characteristics |
Overall (N = 149,070) |
PLF only1 (N = 49,950) |
P/TLIF only1 (N = 52,770) |
ALIF only1 (N = 8,790) |
PLF + P/TLIF1 (N = 25,995) |
Anterior- posterior fusion1 (N =11,565) |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| 50-59 | 38,105 (25.6%) | 10,605 (21.2%) | 14,510 (27.5%) | 2,545 (29%) | 7,030 (27%) | 3,415 (29.5%) |
| 60-69 | 57,415 (38.5%) | 18,065 (36.2%) | 20,765 (39.3%) | 3,555 (40.4%) | 10,470 (40.3%) | 4,560 (39.4%) |
| 70+ | 53,550 (35.9%) | 21,280 (42.6%) | 17,495 (33.2%) | 2,690 (30.6%) | 8,495 (32.7%) | 3,590 (31%) |
| Sex | ||||||
| Male | 61,950 (41.6%) | 21,260 (42.6%) | 21,455 (40.7%) | 3,585 (40.8%) | 10,885 (41.9%) | 4,765 (41.2%) |
| Female | 87,040 (58.4%) | 28,670 (57.4%) | 31,295 (59.3%) | 5,200 (59.2%) | 15,085 (58%) | 6,790 (58.7%) |
| Missing | 80 (0.1%) | <50 | <50 | <50 | <50 | <50 |
| Race/Ethnicity | ||||||
| White | 118,475 (79.5%) | 39,815 (79.7%) | 41,680 (79%) | 7,000 (79.6%) | 20,705 (79.6%) | 9,275 (80.2%) |
| Black | 11,065 (7.4%) | 3,785 (7.6%) | 4,020 (7.6%) | 630 (7.2%) | 1,920 (7.4%) | 710 (6.1%) |
| Hispanic | 6,780 (4.5%) | 2,205 (4.4%) | 2,465 (4.7%) | 410 (4.7%) | 1,080 (4.2%) | 620 (5.4%) |
| Other | 4,970 (3.3%) | 1,650 (3.3%) | 1,660 (3.1%) | 360 (4.1%) | 775 (3%) | 525 (4.5%) |
| Missing | 7,780 (5.2%) | 2,495 (5%) | 2,945 (5.6%) | 390 (4.4%) | 1,515 (5.8%) | 435 (3.8%) |
| Median household income for patient zip code | ||||||
| Q1: 0 - 25th percentile | 33,715 (22.6%) | 10,815 (21.7%) | 12,885 (24.4%) | 1,885 (21.4%) | 5,890 (22.7%) | 2,240 (19.4%) |
| Q2: 26th - 50th percentile | 37,560 (25.2%) | 12,355 (24.7%) | 13,550 (25.7%) | 1,960 (22.3%) | 6,865 (26.4%) | 2,830 (24.5%) |
| Q3: 51th - 75th percentile | 39,635 (26.6%) | 13,795 (27.6%) | 13,740 (26%) | 2,290 (26.1%) | 6,900 (26.5%) | 2,910 (25.2%) |
| Q4: 76th - 100th percentile | 35,660 (23.9%) | 12,180 (24.4%) | 11,735 (22.2%) | 2,475 (28.2%) | 5,915 (22.8%) | 3,355 (29%) |
| Missing | 2,500 (1.7%) | 805 (1.6%) | 860 (1.6%) | 180 (2%) | 425 (1.6%) | 230 (2%) |
| Payer/insurance | ||||||
| Private insurance | 48,955 (32.8%) | 14,225 (28.5%) | 18,050 (34.2%) | 3,005 (34.2%) | 9,395 (36.1%) | 4,280 (37%) |
| Medicare | 86,510 (58%) | 31,765 (63.6%) | 29,485 (55.9%) | 4,800 (54.6%) | 14,280 (54.9%) | 6,180 (53.4%) |
| Medicaid | 5,560 (3.7%) | 1,600 (3.2%) | 2,230 (4.2%) | 355 (4%) | 945 (3.6%) | 430 (3.7%) |
| Other | 7,930 (5.3%) | 2,300 (4.6%) | 2,980 (5.6%) | 630 (7.2%) | 1,355 (5.2%) | 665 (5.8%) |
| Missing | 115 (0.1%) | 60 (0.1%) | <50 | <50 | <50 | <50 |
| Elixhauser Comorbidity Index | ||||||
| 0-1 | 55,245 (37.1%) | 17,145 (34.3%) | 21,000 (39.8%) | 3,375 (38.4%) | 9,645 (37.1%) | 4,080 (35.3%) |
| 2-3 | 64,920 (43.5%) | 22,360 (44.8%) | 22,430 (42.5%) | 3,685 (41.9%) | 11,275 (43.4%) | 5,170 (44.7%) |
| 4+ | 28,905 (19.4%) | 10,445 (20.9%) | 9,340 (17.7%) | 1,730 (19.7%) | 5,075 (19.5%) | 2,315 (20%) |
| Hospital Characteristics | ||||||
| Bedsize2 | ||||||
| Small | 33,070 (22.2%) | 11,000 (22%) | 9,805 (18.6%) | 1,765 (20.1%) | 6,820 (26.2%) | 3,680 (31.8%) |
| Medium | 39,645 (26.6%) | 12,110 (24.2%) | 15,735 (29.8%) | 2,560 (29.1%) | 6,485 (24.9%) | 2,755 (23.8%) |
| Large | 76,355 (51.2%) | 26,840 (53.7%) | 27,230 (51.6%) | 4,465 (50.8%) | 12,690 (48.8%) | 5,130 (44.4%) |
| Location/teaching status | ||||||
| Rural | 5,235 (3.5%) | 1,540 (3.1%) | 1,925 (3.6%) | 295 (3.4%) | 1,085 (4.2%) | 390 (3.4%) |
| Urban nonteaching | 39,895 (26.8%) | 12,155 (24.3%) | 14,700 (27.9%) | 2,355 (26.8%) | 7,390 (28.4%) | 3,295 (28.5%) |
| Urban teaching | 103,940 (69.7%) | 36,255 (72.6%) | 36,145 (68.5%) | 6,140 (69.9%) | 17,520 (67.4%) | 7,880 (68.1%) |
| Hospital Division3 | ||||||
| New England | 6,025 (4%) | 2,650 (5.3%) | 2,335 (4.4%) | 185 (2.1%) | 555 (2.1%) | 300 (2.6%) |
| Middle Atlantic | 16,510 (11.1%) | 7,110 (14.2%) | 5,170 (9.8%) | 885 (10.1%) | 2,360 (9.1%) | 985 (8.5%) |
| East North Central | 25,440 (17.1%) | 9,850 (19.7%) | 8,790 (16.7%) | 880 (10%) | 4,840 (18.6%) | 1,080 (9.3%) |
| West North Central | 10,365 (7%) | 3,630 (7.3%) | 3,065 (5.8%) | 345 (3.9%) | 2,460 (9.5%) | 865 (7.5%) |
| South Atlantic | 32,740 (22%) | 10,520 (21.1%) | 12,260 (23.2%) | 2,375 (27%) | 5,395 (20.8%) | 2,190 (18.9%) |
| East South Central | 10,075 (6.8%) | 3,165 (6.3%) | 4,040 (7.7%) | 485 (5.5%) | 1,775 (6.8%) | 610 (5.3%) |
| West South Central | 17,020 (11.4%) | 5,090 (10.2%) | 5,775 (10.9%) | 1,005 (11.4%) | 3,065 (11.8%) | 2,085 (18%) |
| Mountain | 12,685 (8.5%) | 2,885 (5.8%) | 5,385 (10.2%) | 840 (9.6%) | 2,835 (10.9%) | 740 (6.4%) |
| Pacific | 18,210 (12.2%) | 5,050 (10.1%) | 5,950 (11.3%) | 1,790 (20.4%) | 2,710 (10.4%) | 2,710 (23.4%) |
| Hospital Fusion Procedure Volume4 | ||||||
| Lowest | 7,825 (5.2%) | 2,815 (5.6%) | 3,070 (5.8%) | 535 (6.1%) | 965 (3.7%) | 440 (3.8%) |
| Middle | 32,750 (22%) | 10,480 (21%) | 13,575 (25.7%) | 1,930 (22%) | 4,805 (18.5%) | 1,960 (16.9%) |
| Highest | 108,495 (72.8%) | 36,655 (73.4%) | 36,125 (68.5%) | 6,325 (72%) | 20,225 (77.8%) | 9,165 (79.2%) |
PLF indicates posterolateral fusion, P/TLIF indicates posterior or transforaminal lumbar interbody fusion, ALIF indicates anterior lumbar interbody fusion (also includes extreme lateral interbody fusion, XLIF, and direct lateral interbody fusion, DLIF), and anterior-posterior fusion indicates a combined PLF + ALIF procedure.
Bedsize categories are based on number of hospital beds and are specific to the hospital location (Northeast, Midwest, Southern, or Western) and teaching status (rural, urban nonteaching, or urban teaching).
The NIS hospital divisions are the nine U.S. Census Bureau Divisions.
Hospital Fusion Procedure Volume: Hospitals are ranked from lowest to highest total fusion procedure volume and divided into tertiles. Relative ranking of hospitals is feasible given NIS sampling methodology (random sampling of discharges from all hospitals).
There were demographic differences between racial/ethnic groups (Appendix D). Blacks and Hispanics were younger than Whites (percentages in the lowest age category, 50-59 years, were 34.5% and 34.1% for Blacks and Hispanics, and 24.3% for Whites). Blacks and Hispanics lived in zip codes with lower median household incomes than Whites (percentage in Q1 were 44.1% and 32.2% for Blacks and Hispanics and 20.8% for Whites). Higher percentages of Blacks and Hispanics compared to Whites had Medicaid as primary payer/insurance (8.1% and 7.5% vs. 3% for Whites). Blacks had more comorbidities than Hispanics and Whites (percentages with ECI ≤ 1 were 28.4% for Blacks, 36.7% for Hispanics, 37.5% for Whites; percentages with ECI ≥ 4 were 25.3% for Blacks, 19% for Hispanics, 19.2% for Whites). Hispanics and Blacks received more surgery at lower volume centers than Whites (percentages in the lowest tertile were 10.5% for Hispanics, 6.8% for Blacks, 4.7% for Whites and in the highest tertile were 60.8% for Hispanics, 69.4% for Blacks, 73.9% for Whites). There were regional differences regarding where racial/ethnic groups received surgery with Blacks receiving most in the South Atlantic division (38.9%) and Hispanics receiving most in the West South Central (28.9%) and Pacific divisions (23.2%).
National Lumbar Fusion Utilization
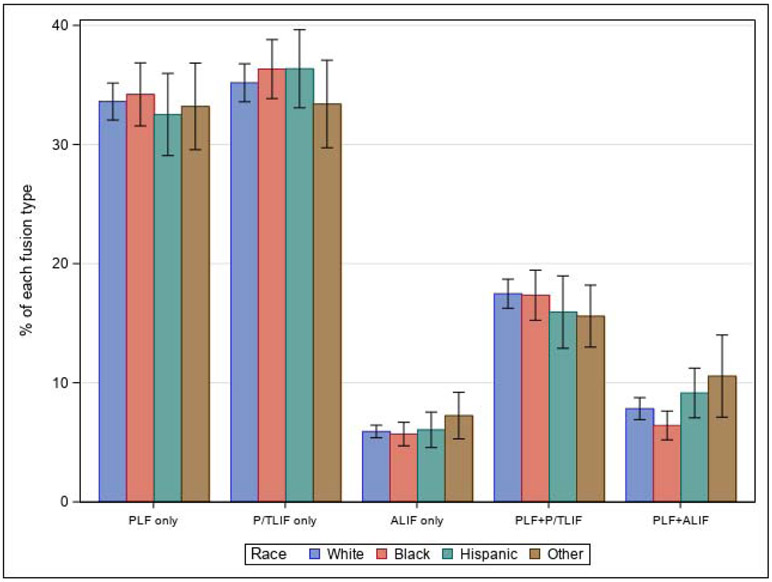
National estimates for utilization of each fusion method were 49,950 for PLF only (33.5%), 52,770 for P/TLIF only (35.4%), 8,790 for ALIF only (5.9%), 25,995 for PLF + P/TLIF (17.4%), and 11,565 for anterior-posterior fusion (7.8%) (Table 1). We observed regional variation in procedure use (e.g. the Pacific division performed 12.2% of the nation’s fusion procedures but performed 23.4% of all anterior-posterior fusions). Proportional use for each procedure was similar across racial/ethnic groups (Figure 1). Multiple logistic regression for anterior-posterior fusion vs. all other procedures demonstrated decreased odds of anterior-posterior fusion use in Blacks compared to Whites [Table 2, OR: 0.81 (0.67–0.97)]. This effect was attenuated after adjustment for hospital factors [Table 2 Model 3, adjusted OR: 0.84 (0.69–1.02)]. Adjustment for SES factors prior to hospital factors attenuated the effect to a lesser degree [adjusted OR: 0.81 (0.67–0.97)], indicating greater contribution from hospital factors. Regression analyses for all other procedures demonstrated similar odds ratios between racial/ethnic groups (Appendix E for all procedures, Appendix F for entire model). In the propensity score sample, where Blacks and Whites were matched on all patient and hospital factors, we observed further attenuation of the decreased odds of anterior-posterior fusion use in Blacks compared to Whites [Table 3, OR: 0.89 (0.70–1.13)].
Figure 1:
National fusion method utilization in 2016 as a proportion of all fusion procedures within racial/ethnic groups. Within each race/ethnicity, different procedures were used at similar proportions. The use of anterior-posterior fusion in Blacks was statistically significantly lower than Whites. PLF indicates posterolateral fusion, P/TLIF indicates posterior or transforaminal lumbar interbody fusion, ALIF indicates anterior lumbar interbody fusion (also includes extreme lateral interbody fusion, XLIF, and direct lateral interbody fusion, DLIF), and PLF + ALIF indicates anterior-posterior fusion.
Table 2 –
Odds Ratios of Anterior-Posterior Fusion vs. All Other Fusion Procedures by Race/Ethnicity
| Anterior-Posterior Fusion1 (OR, 95% CI) |
|
|---|---|
| Model 1: Race alone | |
| White | Reference |
| Black | 0.81 (0.67–0.97) |
| Hispanic | 1.19 (0.92–1.52) |
| Other | 1.39 (0.97–2) |
| Model 2: Model 1 + age, sex, comorbidities2 | |
| White | Reference |
| Black | 0.76 (0.64–0.92) |
| Hispanic | 1.15 (0.89–1.48) |
| Other | 1.39 (0.97–1.99) |
| Model 3: Model 2 + hospital factors3 | |
| White | Reference |
| Black | 0.84 (0.69–1.02) |
| Hispanic | 0.96 (0.76–1.22) |
| Other | 1.15 (0.83–1.59) |
| Model 4: Model 3 + SES factors4 | |
| White | Reference |
| Black | 0.87 (0.71–1.06) |
| Hispanic | 0.99 (0.78–1.25) |
| Other | 1.13 (0.82–1.55) |
Odds ratios were calculated for the anterior-posterior fusion procedure vs. all other fusion methods. Anterior-posterior fusion indicates a combined PLF + ALIF procedure.
Model 2 adjusted for comorbidities using the Elixhauser comorbidity index.
The hospital factors included in regression model 3 were hospital bedsize, location/teaching status, U.S. division, and fusion procedure volume.
The socioeconomic factors included in regression model 4 were payer/insurance and median household income for the patient’s zip code. White was used as the reference race category.
Table 3 –
Procedure Utilization and Inpatient Safety Measures for Propensity Score Matched Populations: White vs. Black and White vs. Hispanic
| White (n=2158) | Black (n=2158) | Odd Ratio or Difference [95% CI] |
p value1 | |
|---|---|---|---|---|
| Fusion Utilization | 0.45 | |||
| PLF2 | 724 (33.5%) | 734 (34%) | 1.02 [0.90, 1.16] | |
| P/TLIF only2 | 797 (36.9%) | 791 (36.7%) | 0.99 [0.87, 1.12] | |
| ALIF only2 | 140 (6.5%) | 121 (5.6%) | 0.86 [0.67, 1.10] | |
| PLF + P/TLIF2 | 342 (15.8%) | 373 (17.3%) | 1.11 [0.94, 1.30] | |
| Anterior-posterior fusion2 | 155 (7.2%) | 139 (6.4%) | 0.89 [0.70, 1.13] | |
| LOS (days)* | 3 (2–5) | 3 (3–5) | 0 [0, 0] | <0.0001 |
| Cost (US dollars)*3 | 27526 (19665–39617) | 29121 (21150–40756) | 1643 [870, 2383] | 0.004 |
| Prolonged LOS4 | 305 (14.13%) | 390 (18.07%) | 1.34 [1.14, 1.58] | 0.0004 |
| Inpatient Medical Complications | 169 (7.83%) | 214 (9.92%) | 1.30 [1.05, 1.60] | 0.016 |
| Inpatient Surgical Complications | 109 (5.05%) | 114 (5.28%) | 1.05 [0.80, 1.37] | 0.73 |
| Mortality | 3 (0.14%) | 5 (0.23%) | 1.67 [0.40, 6.99] | 0.51 |
| White (n=1310) | Hispanic (n=1310) | Odd Ratio or Difference [95% CI] |
p value1 | |
| Fusion Utilization | 0.38 | |||
| PLF2 | 420 (32.1%) | 428 (32.7%) | 1.03 [0.87, 1.21] | |
| P/TLIF only2 | 502 (38.3%) | 476 (36.3%) | 0.92 [0.78, 1.08] | |
| ALIF only2 | 91 (6.9%) | 80 (6.1%) | 0.87 [0.64, 1.19] | |
| PLF + P/TLIF2 | 177 (13.5%) | 209 (16%) | 1.22 [0.98, 1.51] | |
| Anterior-posterior fusion2 | 120 (9.2%) | 117 (8.9%) | 0.97 [0.74, 1.27] | |
| LOS (days)* | 3 (2–4) | 3 (2–5) | 0 [0, 0] | 0.002 |
| Cost (US dollars)*3 | 29677 (20606–42699) | 31340 (22221–45054) | 1698 [649, 2711] | 0.005 |
| Prolonged LOS4 | 156 (11.91%) | 191 (14.58%) | 1.26 [1.01, 1.58] | 0.044 |
| Inpatient Medical Complications | 104 (7.94%) | 115 (8.78%) | 1.12 [0.85, 1.47] | 0.44 |
| Inpatient Surgical Complications | 77 (5.88%) | 92 (7.02%) | 1.21 [0.88, 1.65] | 0.23 |
| Mortality | 1 (0.08%) | 2 (0.15%) | 2.00 [0.18, 22.13] | 0.62 |
Data reported as median (Q1–Q3)
Wilcoxon signed-rank test was performed for LOS and cost; Chi-squared test was performed for prolonged LOS, inpatient medical complications, and inpatient surgical complications; Fisher’s exact test was performed for mortality.
PLF indicates posterolateral fusion, P/TLIF indicates posterior or transforaminal lumbar interbody fusion, ALIF indicates anterior lumbar interbody fusion (also includes extreme lateral interbody fusion, XLIF, and direct lateral interbody fusion, DLIF), and anterior-posterior fusion indicates a combined PLF + ALIF procedure.
Total admission cost: Total charges for each hospital discharge were converted to total cost using hospital-specific cost-to-charge ratios, which are based on hospital accounting reports collected by the Centers for Medicare & Medicaid Services.
Prolonged LOS is defined as a hospital length of stay greater than the 90th percentile for the fusion procedure performed.
Inpatient Safety Measures
Table 4 shows unadjusted inpatient safety measures by race/ethnicity (Appendix G further stratifies by procedure). Mean LOS was longer for Blacks [4.4 days (95% CI 4.2–4.5)] and Hispanics [4.1 days (3.8–4.3)] compared to Whites [3.7 days (3.6–3.8)]. Rates of prolonged LOS were higher for Blacks [18.1% (95% CI 16.2–19.9)] and Hispanics [14.5% (12.1–17)] compared to Whites [11.7% (11–12.4)]. Rates of inpatient medical complication were higher in Blacks [9.9% (95% CI 8.6–11.2)] and Hispanics [8.7% (7–10.4)] compared to Whites [7.7% (7.3–8.1)]. Rates of inpatient surgical complication were higher in Hispanics [6.9% (95% CI 5.5–8.3)] compared to Whites [5.4% (5–5.7)]. Mortality was approximately 0.1% across all racial/ethnic groups. Mean total cost was higher for Hispanics [$37,088 (95% CI 34,908–39,267)] and Blacks [$34,699 (33,139–36,259)] than for Whites [$33,583 (32,622–34,544)].
Table 4 –
Unadjusted Inpatient Safety Measures for Lumbar Spinal Fusion by Race/Ethnicity
| Safety Measures | White | Black | Hispanic | Other |
|---|---|---|---|---|
| Average LOS (days) | 3.73 (3.64 - 3.81) | 4.35 (4.17 - 4.54) | 4.07 (3.84 - 4.3) | 4.14 (3.87 - 4.4) |
| Prolonged LOS1 (%) | 11.7 (11 - 12.4) | 18.1 (16.2 - 19.9) | 14.5 (12.1 - 17) | 15.4 (12.9 - 17.9) |
| Inpatient Medical Complication2 (%) | 7.7 (7.3 - 8.1) | 9.9 (8.6 - 11.2) | 8.7 (7 - 10.4) | 8 (6.3 - 9.8) |
| Inpatient Surgical Complication3 (%) | 5.4 (5 - 5.7) | 5.2 (4.3 - 6.2) | 6.9 (5.5 - 8.3) | 5.5 (4.1 - 7) |
| Mortality (%) | 0.1 (0.1 - 0.2) | 0.2 (0 - 0.4) | 0.1 (0 - 0.3) | 0.1 (0 - 0.3) |
| Total Admission Cost4 (US dollars) | 33,583 (32,622–34,544) | 34,699 (33,139–36,259) | 37,088 (34,908–39,267) | 38,434 (36,142–40,725) |
Prolonged LOS is defined as a hospital length of stay greater than the 90th percentile for the fusion procedure performed.
Inpatient medical complications included cardiovascular, pulmonary, or renal/urinary complications. We constructed a list of ICD-10-CM codes for each complication type (see Appendix C). To be a case, the patient’s hospital discharge needed to contain at least one complication code.
Inpatient surgical complications included hemorrhagic, infectious/wound, neurologic, and thromboembolic complications. We constructed a list of ICD-10-CM codes for each complication type (see Appendix C). To be a case, the patient’s hospital discharge needed to contain at least one complication code.
Total cost: Total charges for each hospital discharge were converted to total cost using hospital-specific cost-to-charge ratios, which are based on hospital accounting reports collected by the Centers for Medicare & Medicaid Services.
Table 5 shows multiple logistic regression analyses for prolonged LOS, inpatient medical complications, and inpatient surgical complications (Appendix H shows the entire model and additional outcomes LOS and cost). Many differences in safety measures between racial/ethnic groups persisted after adjustment for patient and hospital factors, including LOS [Appendix H Model 5 adjusted OR Blacks 1.12 (95% CI 1.09–1.16); Hispanics 1.06 (1.01–1.11)], prolonged LOS [Table 5 Model 5 adjusted OR Blacks 1.39 (95% CI 1.22–1.59); Hispanics 1.24 (1.02–1.52)], inpatient medical complications [Table 5 Model 5 adjusted OR Blacks 1.24 (95% CI 1.05–1.48)], inpatient surgical complications [Table 5 Model 5 adjusted OR Hispanics 1.34 (95% CI 1.06–1.68)], and cost [Appendix H Model 5 adjusted OR Blacks 1.05 (95% CI 1.02–1.08)]. We observed similar results in the propensity score samples, where racial/ethnic groups were matched on all available patient and hospital factors (Table 3).
Table 5 –
Odds Ratios for Inpatient Safety Measures by Race/Ethnicity Obtained From Multiple Logistic Regression Analyses
| Prolonged LOS1 (OR, 95% CI) |
Inpatient
Medical Complications2 (OR, 95% CI) |
Inpatient
Surgical Complications3 (OR, 95% CI) |
|
|---|---|---|---|
| Model 1: Race alone | |||
| White | Reference | Reference | Reference |
| Black | 1.66 (1.47–1.89) | 1.32 (1.14–1.54) | 0.97 (0.8–1.18) |
| Hispanic | 1.28 (1.05–1.57) | 1.14 (0.92–1.42) | 1.31 (1.05–1.64) |
| Other | 1.37 (1.13–1.67) | 1.05 (0.82–1.33) | 1.03 (0.78–1.37) |
| Model 2: Model 1 + fusion procedure | |||
| White | Reference | Reference | Reference |
| Black | 1.66 (1.47–1.89) | 1.33 (1.14–1.55) | 0.97 (0.81–1.18) |
| Hispanic | 1.28 (1.05–1.57) | 1.14 (0.91–1.42) | 1.32 (1.06–1.65) |
| Other | 1.38 (1.13–1.67) | 1.04 (0.81–1.32) | 1.03 (0.78–1.37) |
| Model 3: Model 2 + age, sex, comorbidities | |||
| White | Reference | Reference | Reference |
| Black | 1.6 (1.4–1.83) | 1.27 (1.08–1.49) | 0.97 (0.8–1.18) |
| Hispanic | 1.33 (1.09–1.62) | 1.21 (0.97–1.5) | 1.37 (1.09–1.71) |
| Other | 1.53 (1.25–1.86) | 1.18 (0.92–1.51) | 1.08 (0.82–1.43) |
| Model 4: Model 3 + hospital factors4 | |||
| White | Reference | Reference | Reference |
| Black | 1.45 (1.27–1.66) | 1.26 (1.07–1.48) | 0.97 (0.8–1.17) |
| Hispanic | 1.28 (1.05–1.57) | 1.11 (0.89–1.38) | 1.34 (1.06–1.68) |
| Other | 1.54 (1.27–1.86) | 1.19 (0.94–1.51) | 1.07 (0.81–1.41) |
| Model 5: Model 4 + SES factors5 | |||
| White | Reference | Reference | Reference |
| Black | 1.39 (1.22–1.59) | 1.24 (1.05–1.48) | 0.97 (0.8–1.17) |
| Hispanic | 1.24 (1.02–1.52) | 1.1 (0.88–1.38) | 1.34 (1.06–1.68) |
| Other | 1.5 (1.24–1.81) | 1.19 (0.93–1.5) | 1.06 (0.81–1.41) |
Prolonged LOS is defined as a hospital length of stay greater than the 90th percentile for the fusion procedure performed.
Inpatient medical complications included cardiovascular, pulmonary, or renal/urinary complications. We constructed a list of ICD-10-CM codes for each complication type (see Appendix C). To be a case, the patient’s hospital discharge needed to contain at least one complication code.
Inpatient surgical complications included hemorrhagic, infectious/wound, neurologic, and thromboembolic complications. We constructed a list of ICD-10-CM codes for each complication type (see Appendix C). To be a case, the patient’s hospital discharge needed to contain at least one complication code.
Regression model 4 corrects for the following hospital factors: hospital bedsize, location/teaching status, U.S. division, and fusion procedure volume.
Regression model 5 corrects for the following socioeconomic factors: payer/insurance and median household income for the patient’s zip code. White race is the reference group.
Total cost: Total charges for each hospital discharge were converted to total cost using hospital-specific cost-to-charge ratios, which are based on hospital accounting reports collected by the Centers for Medicare & Medicaid Services.
Regression analyses demonstrated procedure effects on safety measures (Appendix H, Model 5). anterior-posterior fusion, compared to PLF, had higher odds of inpatient medical complication [adjusted OR 1.39 (95% CI 1.16–1.67)] and LOS [adjusted OR 1.15 (95% CI 1.1–1.21)]. P/TLIF only, compared to PLF, had lower odds of inpatient medical complication [adjusted OR 0.89 (95% CI 0.79–1)], inpatient surgical complication [adjusted OR 0.67 (95% CI 0.59–0.76)], and LOS [adjusted OR 0.90 (95% CI 0.88–0.92)]. PLF was associated with the lowest total cost and anterior-posterior fusion was associated with the highest total cost [ratio for anterior-posterior fusion vs. PLF: 1.86 (1.78–1.95)].
Discussion:
We found that lumbar fusion method use did not differ across racial/ethnic groups, with one exception: Blacks underwent anterior-posterior fusion less frequently than Whites and this effect was partially explained by hospital factors. We also found in adjusted analyses that Blacks and Hispanics had longer LOS and higher rates of prolonged LOS, Blacks had 24% higher odds of inpatient medical complication than Whites, and Hispanics had 34% higher odds of inpatient surgical complication than Whites.
Prior investigations have documented differences in spinal surgery rates/hospitalizations by race/ethnicity.14,15,22,23 Since the NIS sample consists of people who had hospitalizations but does not include the entire population of patients with the underlying conditions, we were not able to calculate rates of each fusion method using the NIS data. Our study focused on whether—among those already receiving lumbar fusion—the distribution of fusion methods differed by race.
Racial/ethnic differences in spine surgery outcomes have been reported for LOS17,31, infection32, complications or complex disposition16-18,20,33,34, mortality15,17,18, and functional improvement21. A meta analysis to characterize the effect of race/ethnicity on spine surgery outcomes found few studies and could not draw firm conclusions due to insufficient evidence22. More complex fusion methods, such as anterior-posterior fusion, have been associated with greater complications and costs10,11,35-37. Our analyses mirror these findings. However, we found patients receiving P/TLIF had shorter LOS and fewer inpatient complications compared to patients receiving PLF. This may reflect different overall health statuses between these populations (i.e. fusion patients with more comorbidites and higher surgical risk may receive the less intensive PLF).
To our knowledge, no prior study has investigated the association of race/ethnicity with utilization of particular spinal fusion procedures among patients undergoing spinal fusion. Patients with Medicaid or without insurance have been observed to be more likely to receive PLF and patients with private insurance or Medicare were more likely to receive interbody fusion35. Interbody fusion procedures are preferentially used in fee for service settings compared to salaried health systems38. We observed few differences in procedure use across racial/ethnic groups. Blacks were 19% less likely than Whites to receive anterior-posterior fusion; our analyses showed this was partly explained by hospital factors. Prior research suggests a potential overutilization of complex fusion, with more invasive procedures like the anterior-posterior fusion associated with worse outcomes10,35. Thus, lower anterior-posterior fusion use in Blacks may paradoxically reduce their exposure to risk. It would be appropriate to term this pattern a difference but not a disparity39.
To our knowledge, this is also the first work to investigate racial/ethnic differences in spinal fusion safety measures after accounting for procedure performed. Even after adjusting or matching for procedure, patient and hospital factors, certain disparities in safety measures persisted, including LOS and complications.
Inpatient safety measures may have been worse in Blacks and Hispanics for several reasons. Higher risk for inpatient medical complication for Black patients may reflect worse underlying health status, since we observed more comorbidities for Black patients compared to White patients. Higher risk for inpatient surgical complication for Hispanic patients may reflect differences in surgical care received or healthcare facilities accessed, since we observed regional differences in fusion utilization and a higher percentage of Hispanic patients receiving care from low volume centers. While we adjusted for relevant covariates, there may be residual confounding from unmeasured environmental differences, health differences undetected by ECI, socioeconomic factors not fully captured by insurance or zip code median income, and differences in care not captured by our hospital variables. Blacks and Hispanics may face barriers to accessing surgical care and present with more advanced disease. Disposition issues (e.g. placement in rehabilitation center) may disproportionately delay discharge for Blacks and Hispanics and prolong LOS34. It is also possible that Blacks and Hispanics receive suboptimal healthcare. Previous work has discussed healthcare segregation (i.e. limited access leading to uneven racial distribution across hospitals) as a potential source for disparities, in which hospitals serving communities of color cannot provide the same care given by hospitals serving mostly White populations22,23,40,41. In addition to health system issues, providers may have implicit biases and treat patients differently13,42-44.
Importantly, since race is a social construct rather than biological45, worse outcomes for Black and Hispanic patients do not suggest that race is a biological risk factor, but rather a marker for a range of social, structural, and care process factors that affect outcome. The explanations listed above (differences in environment, health status, SES, healthcare access and utilization, and interpersonal differential treatment) are all manifestations of structural racism46. Historical, cultural, and structural factors influence the relationship between the healthcare system and communities of color, contributing to distrust or unequitable treatment47-49.
Advantages of this study include a large, nationally representative sample that permitted us to analyze racial/ethnic subgroups of sufficient size to detect meaningful differences. The percentage of missing race data was low (5.2%) and characteristics between populations containing and missing race data were similar, so formal imputation was not performed.
Important study limitations include intrinsic drawbacks from retrospective review of administrative data, such as potentially inaccurate/incomplete documentation and lack of important clinical or radiologic details. Prior work suggests ICD-10 inpatient complication coding for spinal trauma may be less sensitive than prospective review.50 However, this would only bias our findings if this effect differed between racial groups. This misclassification is likely non-differential (similar across racial groups) and would bias our findings conservatively, toward the null. Since procedures are tied to reimbursement, ICD-10 procedure codes should be accurate. We constructed comprehensive ICD-10-CM lists of spine procedures and inpatient complications to mitigate potential inaccuracies. Some database variables were less precise (e.g. median income by zip code and payer serving as SES markers). Additionally, the NIS race categories were constructed to be mutually exclusive, which does not fully capture the diversity of racial identities (e.g. multiracial groups, Black Hispanics, Hispanics who also identify as White, etc.). We also acknowledge vast heterogeneity within the racial categories. Our focus was to determine whether disparities existed between Blacks and/or Hispanics and Whites, which also left a very heterogeneous “other” race category. We were unable to perform provider level analysis, which would help account for differences in training or preferences/comfort with specific procedures. Lastly, our analysis was limited to inpatient safety measures, so we could not follow the postoperative course past discharge when more complications would be expected.
Conclusions:
Fusion method use was generally similar between racial/ethnic groups, except for reduced anterior-posterior fusion use in Black patients compared to White patients. Even though the more invasive anterior-posterior fusion procedure, which is associated with worse outcomes, was utilized less for Black patients, Blacks and Hispanics had worse inpatient safety measures (LOS, prolonged LOS, medical and surgical complications). Our observation of worse safety measures for Black and Hispanic recipients of lumbar fusion procedures provides further impetus to understand the sources of these disparities and to implement changes to address them. Further research should emphasize contributions from structural racism46. While changing the most upstream social determinants is needed to fundamentally influence health outcomes51, areas for intervention in healthcare could include financial barriers, language barriers, workforce diversity, and institutional racism. Initiatives could include centers of excellence, quality metrics focused on tracking and reducing disparities, and insurance incentives to enhance access. Our data underscore the urgency of these research and policy goals to reduce disparities in outcome.
Supplementary Material
Highlights:
For lumbar fusion patients, procedure use similar for Blacks, Hispanics, and Whites
Anterior-posterior fusion use is lower for Black patients compared to White patients
Black and Hispanic patients have longer LOS than White patients after lumbar fusion
Blacks have higher odds for inpatient medical complication compared to Whites
Hispanics have higher odds for inpatient surgical complication compared to Whites
Acknowledgements:
We thank Robin Talbot (Partners Healthcare RCO) for her technical assistance with ICD spinal fusion coding.
Funding disclosure statement: This study was funded by the National Institutes of Health (NIH T32AR055885, P30AR072577, and K24AR057827) and the Scholars in Medicine Office at Harvard Medical School. The funding sources did not play a role in this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008. February 21;358(8):818–25. [DOI] [PubMed] [Google Scholar]
- 2.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016. January 4;352:h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eismont FJ, Norton RP, Hirsch BP. Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg. 2014. April;22(4):203–13. [DOI] [PubMed] [Google Scholar]
- 4.Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008. March;90(3):656–71. [PubMed] [Google Scholar]
- 5.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005. June 15;30( 12): 1441–5; discussion 1446-1447. [DOI] [PubMed] [Google Scholar]
- 6.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine. 2019. March 1;44(5):369–76. [DOI] [PubMed] [Google Scholar]
- 7.Matz PG, Meagher RJ, Lamer T, Tontz WL, Annaswamy TM, Cassidy RC, et al. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J Off J North Am Spine Soc. 2016. March; 16(3): 439–48. [DOI] [PubMed] [Google Scholar]
- 8.Abdu WA, Lurie JD, Spratt KF, Tosteson ANA, Zhao W, Tosteson TD, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine. 2009. October 1;34(21):2351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdu WA, Sacks OA, Tosteson ANA, Zhao W, Tosteson TD, Morgan TS, et al. Long-Term Results of Surgery Compared With Nonoperative Treatment for Lumbar Degenerative Spondylolisthesis in the Spine Patient Outcomes Research Trial (SPORT). Spine. 2018. January;43(23): 1619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010. April 7;303(13): 1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thirukumaran CP, Raudenbush B, Li Y, Molinari R, Rubery P, Mesfin A. National Trends in the Surgical Management of Adult Lumbar Isthmic Spondylolisthesis: 1998 to 2011. Spine. 2016. March;41(6):490–501. [DOI] [PubMed] [Google Scholar]
- 12.Saifi C, Cazzulino A, Laratta J, Save AV, Shillingford JN, Louie PK, et al. Utilization and Economic Impact of Posterolateral Fusion and Posterior/Transforaminal Lumbar Interbody Fusion Surgeries in the United States. Glob Spine J. 2019. April;9(2): 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey TS, Garrett JM. The relation of race to outcomes and the use of health care services for acute low back pain. Spine. 2003. February 15;28(4):390–4. [DOI] [PubMed] [Google Scholar]
- 14.Skolasky RL, Maggard AM, Thorpe RJ, Wegener ST, Riley LH. United States hospital admissions for lumbar spinal stenosis: racial and ethnic differences, 2000 through 2009. Spine. 2013. December 15;38(26):2272–8. [DOI] [PubMed] [Google Scholar]
- 15.Alosh H, Riley LH, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009. August 15;34(18):1956–62. [DOI] [PubMed] [Google Scholar]
- 16.Lad SP, Bagley JH, Kenney KT, Ugiliweneza B, Kong M, Bagley CA, et al. Racial disparities in outcomes of spinal surgery for lumbar stenosis. Spine. 2013. May 15;38(11): 927–35. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld AJ, Zhang D, Walley KC, Bono CM, Harris MB. The influence of race and hospital environment on the care of patients with cervical spine fractures. Spine J Off J North Am Spine Soc. 2016; 16(5):602–7. [DOI] [PubMed] [Google Scholar]
- 18.Skolasky RL, Thorpe RJ, Wegener ST, Riley LH. Complications and mortality in cervical spine surgery: racial differences. Spine. 2014. August 15;39(18): 1506–12. [DOI] [PubMed] [Google Scholar]
- 19.Patil CG, Patil TS, Lad SP, Boakye M. Complications and outcomes after spinal cord tumor resection in the United States from 1993 to 2002. Spinal Cord. 2008. May;46(5):375–9. [DOI] [PubMed] [Google Scholar]
- 20.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009. July 1;302(1):58–66. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld AJ, Lurie JD, Zhao W, Bono CM. The effect of race on outcomes of surgical or nonsurgical treatment of patients in the Spine Patient Outcomes Research Trial (SPORT). Spine. 2012. August 1;37(17):1505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenfeld AJ, Sieg RN, Li G, Bader JO, Belmont PJ, Bono CM. Outcomes after spine surgery among racial/ethnic minorities: a meta-analysis of the literature. Spine J Off J North Am Spine Soc. 2011. May;11(5):381–8. [DOI] [PubMed] [Google Scholar]
- 23.Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly Medicare beneficiaries: 1989-2000. Med Care. 2005. April;43(4): 320–9. [DOI] [PubMed] [Google Scholar]
- 24.HCUP Databases [Internet]. Healthcare Cost and Utilization Project (HCUP). 2019. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp
- 25.Cost-to-Charge Ratio Files [Internet]. Healthcare Cost and Utilization Project (HCUP).2009. [cited 2019 Dec 8]. Available from: https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp
- 26.Elixhauser Comorbidity Software for ICD-10-CM (beta version) [Internet]. Healthcare Cost and Utilization Project (HCUP). 2018. [cited 2019 Dec 9]. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp
- 27.NIS Description of Data Elements: ZIPINC_QRTL Median household income for patient’s ZIP Code (based on current year) [Internet]. Healthcare Cost and Utilization Project (HCUP). [cited 2020 Aug 4]. Available from: https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp
- 28.NIS Description of Data Elements: HOSP_BEDDSIZE - Bedsize of hospital [Internet]. Healthcare Cost and Utilization Project (HCUP). [cited 2019 Aug 4]. Available from: https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp
- 29.NIS Description of Data Elements: HOSP_LOCTEACH - Location/teaching status of hospital [Internet]. Healthcare Cost and Utilization Project (HCUP). [cited 2020 Aug 4]. Available from: https://www.hcup-us.ahrq.gov/db/vars/hosp_locteach/nisnote.jsp [Google Scholar]
- 30.Houchens R, Ross D, Elixhauser A, Jiang J. Nationwide Inpatient Sample (NIS) Redesign Final Report [Internet]. Agency for Healthcare Research and Quality; 2014. April. (HCUP Methods Series; ). Report No.: 2014–04. Available from: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. [Google Scholar]
- 31.Walid MS, Hyer L, Ajjan M, Barth ACM, Robinson JS. Prevalence of opioid dependence in spine surgery patients and correlation with length of stay. J Opioid Manag. 2007. June;3(3):127–8, 130–2. [DOI] [PubMed] [Google Scholar]
- 32.Friedman ND, Sexton DJ, Connelly SM, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol. 2007. September;28(9): 1060–5. [DOI] [PubMed] [Google Scholar]
- 33.Patil CG, Lad SP, Santarelli J, Boakye M. National inpatient complications and outcomes after surgery for spinal metastasis from 1993-2002. Cancer. 2007. August 1; 110(3):625–30. [DOI] [PubMed] [Google Scholar]
- 34.Kalanithi PS, Patil CG, Boakye M. National Complication Rates and Disposition After Posterior Lumbar Fusion for Acquired Spondylolisthesis. Spine. 2009. August;34(18): 1963–9. [DOI] [PubMed] [Google Scholar]
- 35.Norton RP, Bianco K, Klifto C, Errico TJ, Bendo JA. Degenerative Spondylolisthesis: An Analysis of the Nationwide Inpatient Sample Database. Spine. 2015. August 1;40(15): 1219–27. [DOI] [PubMed] [Google Scholar]
- 36.Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J Off J North Am Spine Soc. 2014. September 1;14(9):2019–27. [DOI] [PubMed] [Google Scholar]
- 37.Schoenfeld AJ, Harris MB, Liu H, Birkmeyer JD. Variations in Medicare payments for episodes of spine surgery. Spine J Off J North Am Spine Soc. 2014. December 1;14(12):2793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenfeld AJ, Makanji H, Jiang W, Koehlmoos T, Bono CM, Haider AH. Is There Variation in Procedural Utilization for Lumbar Spine Disorders Between a Fee-for-Service and Salaried Healthcare System? Clin Orthop. 2017. December;475(12):2838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathore SS, Krumholz HM. Differences, disparities, and biases: clarifying racial variations in health care use. Ann Intern Med. 2004. October 19;141(8):635–8. [DOI] [PubMed] [Google Scholar]
- 40.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003. October 2;349(14): 1350–9. [DOI] [PubMed] [Google Scholar]
- 41.Jancuska JM, Hutzler L, Protopsaltis TS, Bendo JA, Bosco J. Utilization of Lumbar Spinal Fusion in New York State: Trends and Disparities. Spine. 2016. October 1;41(19):1508–14. [DOI] [PubMed] [Google Scholar]
- 42.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017. January;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, et al. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am J Public Health. 2015. December;105(12):e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor BA, Casas-Ganem J, Vaccaro AR, Hilibrand AS, Hanscom BS, Albert TJ. Differences in the work-up and treatment of conditions associated with low back pain by patient gender and ethnic background. Spine. 2005. February 1;30(3):359–64. [DOI] [PubMed] [Google Scholar]
- 45.Roberts D Fatal Invention: How Science, Politics, and Big Business Re-create Race in the Twenty-First Century. New York: The New Press; 2012. [Google Scholar]
- 46.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet Lond Engl. 2017. August;389(10077): 1453–63. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care [Internet]. Smedley BD, Stith AY, Nelson AR, editors. Washington (DC: ): National Academies Press (US); 2003. [cited 2019 Aug 2]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK220358/ [PubMed] [Google Scholar]
- 48.Eberly Lauren A, Richterman Aaron, Beckett Anne G., Wispelwey Bram, Marsh Regan H., Cleveland Manchanda Emily C., et al. Identification of Racial Inequities in Access to Specialized Inpatient Heart Failure Care at an Academic Medical Center. Circ Heart Fail. 2019. November 1;12(11):e006214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz JN. Patient preferences and health disparities. JAMA. 2001. September 26;286(12): 1506–9. [DOI] [PubMed] [Google Scholar]
- 50.Street JT, Thorogood NP, Cheung A, Noonan VK, Chen J, Fisher CG, et al. Use of the Spine Adverse Events Severity System (SAVES) in patients with traumatic spinal cord injury. A comparison with institutional ICD-10 coding for the identification of acute care adverse events. Spinal Cord. 2013. June;51(6):472–6. [DOI] [PubMed] [Google Scholar]
- 51.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.