Abstract
This study investigated the effects of cultivar, fruit presence and tree age on whole-plant partitioning of dry matter and energy equivalents (i.e., glucose equivalents). Young trees of two cultivars characterized by different vigor (i.e., Arbequina, low vigor and Frantoio, high vigor) were either completely deflowered from 2014 to 2017 or never, providing two contrasting levels of cumulated reproductive growth over the following 4 years. Total vegetative dry matter growth over the 4 years was assessed by destructive samplings (whole tree). Plant growth was inversely correlated to reproductive efforts, with Arbequina producing more and growing less than Frantoio. Deflowered trees grew similarly across cultivars, although deflowered Arbequina grew statistically less than deflowered Frantoio by the fourth year, due to abundant flower production. Total reproductive (flowers + fruit) and vegetative biomass production were the same for all cultivars and treatments. Arbequina had a greater distribution of dry matter in directly productive structures (current and one-year-old shoots) and in leaves. This allows it to increase the number of current and following-year production sites, and to save in the resources invested in non-productive sinks (roots, trunk and branches), thus liberating resources for reproductive growth. Greater investments in leaves allow it to intercept more light and thus to increase assimilation. Increased assimilation and increased partitioning towards productive structures, and decreased competition by non-productive structures might contribute to explain the greater early bearing attitude of this cultivar.
Keywords: Dry matter partitioning, Harvest index, Harvest increment, Growth, Canopy to root ratio, Olea europaea
dry matter partitioning, harvest index, harvest increment, growth, canopy to root ratio, Olea europaea
1. Introduction
Knowledge on the relationship between vegetative and reproductive growth is of fundamental importance in fruit trees, where the presence or absence of fruit has effects on photosynthesis and on vegetative growth (Flore and Lakso 1989; Forshey and Elfving 1989; Jackson 1989; Wright 1989). This is primarily due to the functional relationship between production of photosynthates in the leaves (source), and carbohydrate partitioning to competing sinks, such as growing fruits and shoots (Egli et al., 1985; Wardlaw 1990; Marcelis, 1996).
Several eco-physiological models have been created to simulate the vegetative and reproductive development of plants (e.g. Grossman and DeJong, 1994; Allen et al., 2005). In these models, plant growth is the result of interacting physiological processes such as photosynthesis, respiration, carbon translocation and growth, which can all be identified at different levels of organization (i.e. cell, organ and plant) (Dixon, 1990). According to a mechanistic modelling approach, the plants grow as collections of semiautonomous but interacting organs that compete for resources (Grossman and DeJong, 1994). Dry matter partitioning is determined by the availability of resources, the conditional growth capacity and maintenance requirements of each organ, and the relative ability of each organ to compete for these resources (DeJong, 1999; Marcelis and Heuvelink, 1999). Carbohydrate partitioning within a tree is not a genetically programmed process, but a result of the unique combination of competing organs and their relative abilities to compete for limited amounts of carbohydrates (Lakso and Flore, 2003). Dry matter partitioning often responds to an established hierarchy among different sinks, where seeds and fruits dominate over vegetative parts (Wardlaw, 1990). In fact, fruits demand large quantities of photosynthates and the growth of branches and, especially, of the root system, decreases as the fruit load increases (Lakso and Flore, 2003).
The appropriate balance between vegetative and reproductive growth is a high priority research area in horticulture, as cropping efficiency gains are expected by reductions in the amount of vegetative growth required for reproductive growth (Elfving, 1988). In cultivated plants, the harvest index (HI) expresses the efficiency of dry matter partitioning as the ratio of biomass invested in the economically relevant product and the total biomass of the plant (Donald, 1962). The HI increases with tree age and depends on factors such as variety, rootstock, agro-ecological conditions and crop management (Fischer et al., 2012). In tree crops, the HI is often replaced by the harvest increment (H Incr), defined as the ratio between the increment in the harvested part and the total above-ground increment, over a period of one year or longer (Cannell, 1985). While a high H Incr is desirable, a sufficient development in vegetative organs is necessary to intercept radiation and to absorb water and nutrients.
Since, in fruit trees, reproductive and vegetative growth occur simultaneously for several months, there is a strong competition for the resources available between the reproductive and the vegetative organs of the plant (Forshey and Elfving, 1989; DeJong, 1999; Wünsche and Ferguson, 2005). This competition is well established in mature trees of several species (Stevenson and Shackel, 1998; Costes et al., 2000; Berman and DeJong, 2003), including olive (Monselise and Goldschmidt, 1982; Rallo and Suárez, 1989; Obeso, 2002; Connor and Fereres, 2005; Lavee, 2007; Dag et al., 2010; Castillo-Llanque and Rapoport, 2011), and it is even more striking in young fruit trees, where removing flowers and preventing fruit development results in dramatic increases in vegetative growth (Chandler and Heinicke, 1926; Verheij, 1972; Forshey and Elfving, 1989; Embree et al., 2007). Recently, it has been found that deflowering resulted in strong increments in vegetative growth also in young olive trees, and that this eliminated differences in vigor (i.e. vegetative growth) between plants of the cultivar Arbequina (low vigor) and those of the cultivar Frantoio (high vigor), suggesting that cultivar differences in vigor may be explained in terms of different dry matter partitioning towards fruit (Rosati et al., 2017, 2018a). This demonstrated that competition for resources plays a major role in determining tree growth in young olive trees, suggesting that tree growth is source limited (Rosati et al., 2018b).
There is little information on dry matter partitioning into tree components in adult olive trees, with some notable exceptions (e.g. Villalobos et al., 2006), and quantitative data on dry matter partitioning during the first years of growth are particularly scarce (Scariano et al., 2008; Di Vaio et al., 2012, 2013). Furthermore, there is virtually no information on how dry matter partitioning is affected by fruit presence and cultivar (Rosati et al., 2018b), or by tree age/size.
This study aimed at assessing whole-plant partitioning, in terms of both dry matter and energy equivalents (i.e. glucose equivalents), as a function of cultivar (low vigor vs. high vigor), fruit presence (fruiting vs. deflowered trees), and tree age.
2. Materials and methods
The study was carried out at the Department of Agricultural and Environmental Sciences of the University of Perugia (Latitude N 43° 6′ 10″, Longitude E 12° 23’ 39”). One-year-old plants, originated from rooted cuttings, were transplanted in 9.5-L pots in February 2014 and grown there until March 2016, then transplanted in 30-L pots where they were grown until the end of the experiment in February 2018. The trees were grown outdoors and were regularly fertigated using drip irrigation. A total of 24 Arbequina and 24 Frantoio trees were used. Half of the plants were deflowered every year while the others were allowed to fruit. Frantoio, however, had no flowers the first season and only Arbequina could be deflowered. In 2015, 2016, and 2017 instead, both cultivars bore inflorescences and could be deflowered. The two treatments were called fruiting (Fr) and non-fruiting (NF). In NF treatments, inflorescences were removed every year in May (white stage, Sanz-Cortés et al., 2002Sanz-Cortés et al., 2002), when flowers were not yet open. All inflorescences were then oven-dried at 80 °C until reaching constant weight, and then weighed to determine their dry mass per plant. For the fruiting plants inflorescence weight was estimated based on inflorescence number and the individual inflorescence dry weight (as measured from the deflowered plants).
In November of all years, fruits, when present, were harvested and oven-dried at 80 °C until reaching constant weight. Trunk cross-sectional area was calculated from the trunk diameter measured on the main stem, 5 cm above its insertion on the original cutting wood. The diameter was measured at the beginning of each year (2014, 2015, 2016, 2017, 2018), before the start of vegetative growth.
At the end of experiment (February 2018), three random plants per treatment were uprooted from the pots. Their roots were carefully washed, paying special attention to recovering all of them, including dead roots if present. Sample plants were separated into their main components: roots, trunk, branches and leaves. All parts were then oven-dried to constant weight at 80 °C. Before drying, a subsample of 30 leaves per plant was separated and its leaf area was determined using the SigmaScan Pro 5.0 for Windows analysis program (SPSS Science, Chicago, IL, USA). After area measurements, the subsamples were also dried and wheighed as for the other leaves. The total leaf area was estimated from the dry weight of all sample leaves and the area per unit weight as calculated from the subsamples.
In February 2016 (mid-term of the experiment) three plants per treatment were also sampled and treated as above. In February 2014 (beginning of the experiment) six plants per cultivar (instead of three) were also sampled and handled as above. These six initial plants were sampled just before the start of the experiment and were in addition to the 24 plants per cultivar used in the experiment for subsequent measurements.
No trunk and branch biomass was lost during the experiment, since no pruning was carried out, and no damage occurred. Canopy to root ratio was calculated in 2016 and in 2018 as the ratio of total above-ground biomass/root biomass. The bearing branches biomass (previous-year + current-year shoots with leaves) to structural wood ratio (branches + trunk + roots) was calculated with data collected in 2018.
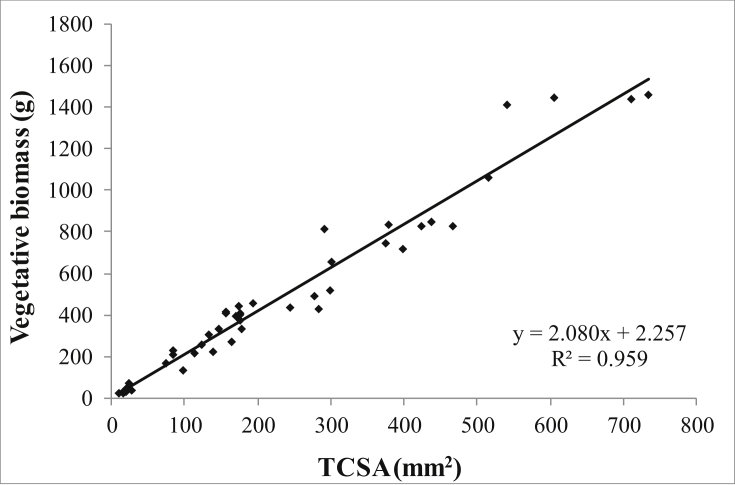
Since reproductive growth was measured on all plants (12 and 9 per treatment respectively in 2016 and 2018), whereas biomass was measured only on the six (2014) or three (2016 and 2018) plants per cultivar, in order to compare data for vegetative and reproductive growth on all 12 and 9 plants per treatment, vegetative growth for each plant we estimated based on allometry. Thus, all data available on total plant vegetative biomass were plotted against the respective trunk cross-sectional area (TCSA), calculated from trunk diameter. Since this relationship was not statistically different among the treatments, a single fit was used across all treatments (Figure 1). This was used to estimate total plant vegetative biomass from the TCSA of all trees at all sampling dates (February 2014, 2015, 2016, 2017 and 2018). This allowed to estimate the total vegetative growth for each plant as done for the individual three plants, but using the estimated initial and final biomass of each plant. The harvest increment (H Incr) for each year was calculated as the ratio between flowers + fruit biomass and total biomass increment (vegetative + reproductive).
Figure 1.
Relationships between total plant vegetative biomass, measured in February 2014 on six plants per cultivar and in 2016 and in 2018 on three plants per treatment in Frantoio and 6 plants in Arbequina, and the respective trunk cross-sectional area (TCSA). There was not significant difference between treatments so a single fit (P < 0.001) was used for all data.
The dry matter values of each component were converted to gram of glucose equivalents in order to compare the reproductive growth with the vegetative growth on an equal energy basis. To this aim, we used the conversion factors (i.e., the amount of dry matter produced per gram of glucose, g g−1) from Penning de Vries et al. (1974), and data from Mariscal et al. (2000) for the composition of vegetative parts, assuming 33.5% of oil in fruit dry matter, as measured in the fruits from both cultivars in 2017 (data not shown).
Net assimilation rate (NAR) over 2015 and 2017 was calculated as the total vegetative plus reproductive growth (in glucose equivalents) per unit final leaf area (measured in February 2016 and 2018). Data are presented as means ± SE. Treatment and cultivar effects were analyzed by two-way and one-way analysis of variance (ANOVA), and averages were compared using the Student–Newman–Keuls test (probability level <0.05). Differences between linear fits were analyzed by analysis of covariance ANCOVA.
3. Results
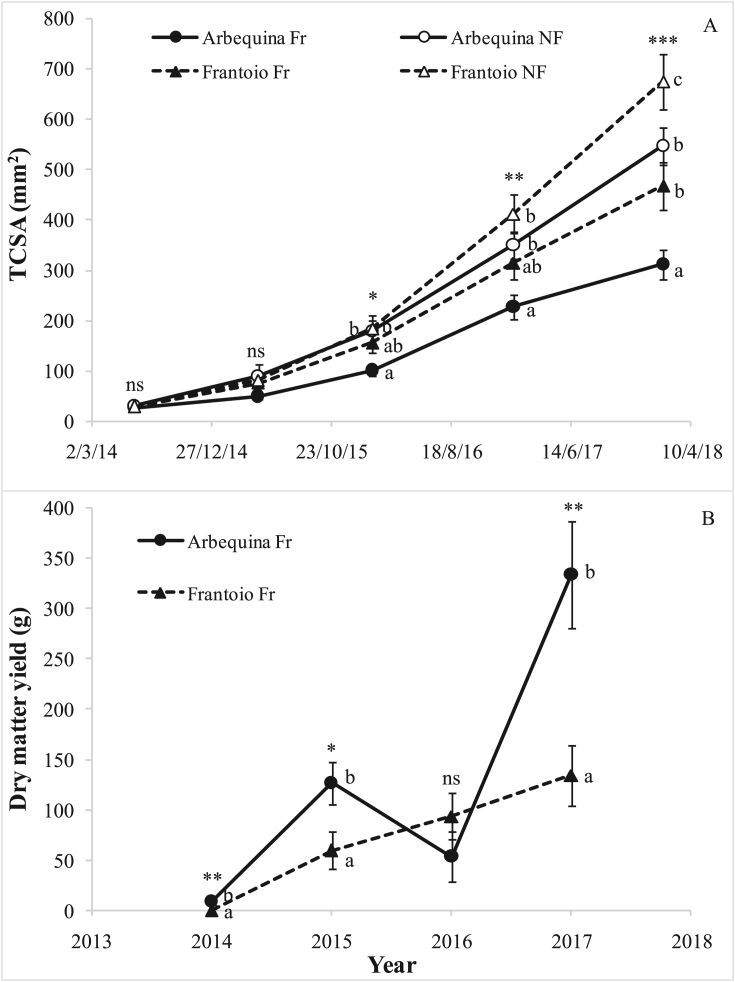
At the beginning of the experiment all the plants had a similar TCSA, while after one year, Arbequina Fr began to lag behind the other treatments, though the difference became statistically significant only in subsequent years (Figure 2A). At the end of the trial, the TCSA in Arbequina Fr was less than half that of Frantoio NF, but also Arbequina NF had an almost double TCSA, compared to Arbequina Fr. Frantoio NF differed significantly from Arbequina NF only at the end of the experiment. Arbequina began to bear fruit from the first year (2014) (Figure 2B). Frantoio produced fruit only from the second year, and its average yield was less than half that of Arbequina, both in 2015 and 2017, and overall.
Figure 2.
Variation in (A) trunk cross sectional area (TCSA) and (B) in fruit yield (dry matter), over the experimental period (2014–2018) in different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting). Each point is the average of the 9 trees that were non-destructively sampled until the end of the experiment. Bars denote standard errors. Different letters denote significant differences within each year. Averages were compared using the Student–Newman–Keuls test; probability level: ns not significant; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
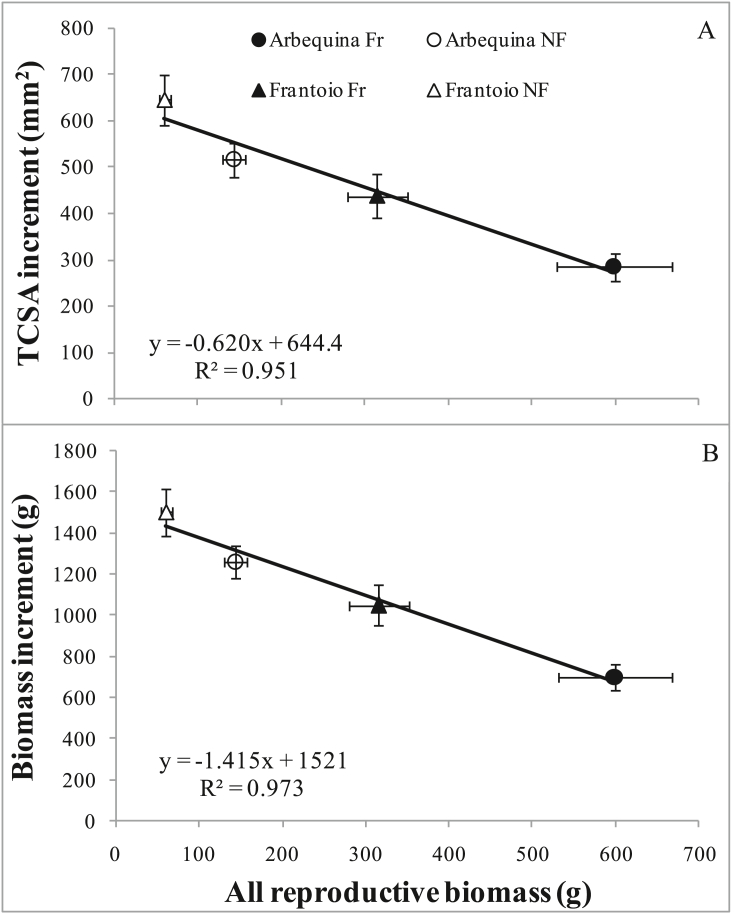
The increase in TCSA or in vegetative biomass over the 4 years was strongly (R2 > 0.95) correlated to the total production (fruit + flowers) over the same time period (Figure 3).
Figure 3.
Relationship between the total (over four years) increments in (A) trunk cross-sectional area (TCSA) and (B) vegetative biomass and total reproductive biomass (fruit + flowers), for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting). Each point is the average of 9 trees. Bars denote standard errors.
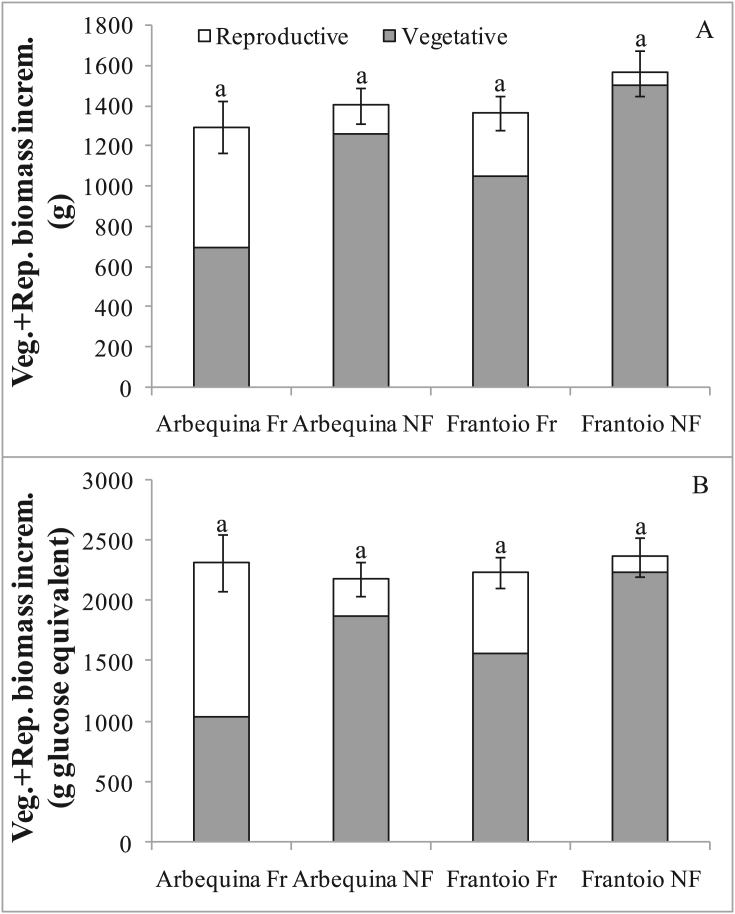
Despite significant differences in vegetative growth over the four years, the total (i.e. vegetative + reproductive) growth was similar and not statistically different between treatments, both in terms of dry matter (Figure 4A) and glucose equivalents (Figure 4B).
Figure 4.
(A) Vegetative and reproductive biomass increments over 4 years, for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting). (B) Vegetative and reproductive biomass increments as in (A) but expressed in glucose equivalents. Data are averages of 9 plants. Bars denote standard errors of the total (vegetative + reproductive) increments. Different letters denote significant (P < 0.05) differences in total (vegetative + reproductive) increments.
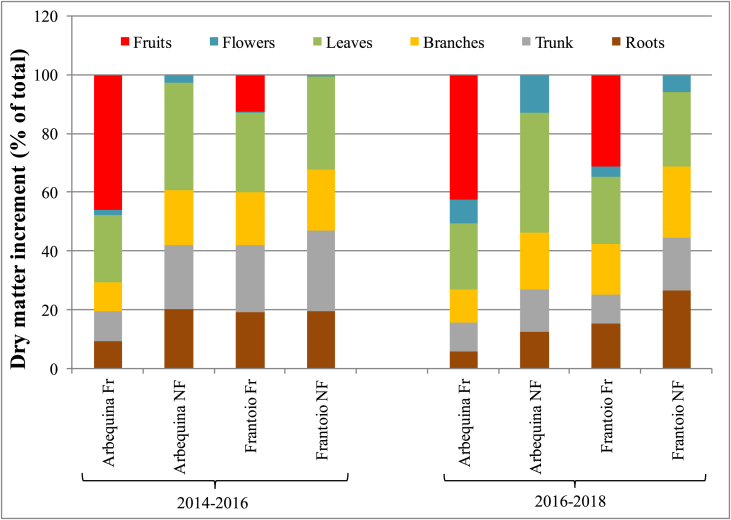
The relative biomass increments calculated over the total reproductive + vegetative biomass increments, for the 2014–2016 and the 2016–2018 periods, are shown in Figure 5. The relative investment in vegetative components obviously differed between fruiting and non-fruiting treatments, because of the fruit production: Fr treatments invested in fruits, thus reducing the relative investments in all other plant parts. For Arbequina Fr, the relative biomass increment in fruit (i.e. H Incr) was 46% of the whole biomass increment in the first period and 42% in the second period. For Frantoio, the numbers were 12% and 31% respectively.
Figure 5.
Partitioning of vegetative and reproductive dry matter increments in young olive trees for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting), in two periods: 2014–2016 and 2016–2018. Data from 6 plants per cultivar in 2014 and 3 plants per cultivar/fruiting treatment in 2016 and 2018.
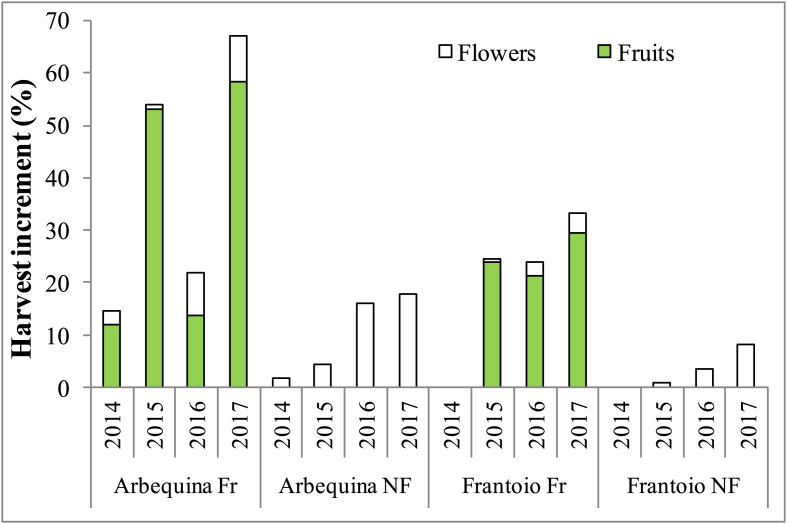
The H Incr was further analyzed for individual years (Figure 6). In the 2nd and 4th year of observation, the H Incr calculated only for the fruit (Fruit H Incr) was about 53% and 58% respectively, in Arbequina; these values were about double those achieved in Frantoio Fr. The H Incr calculated only for the flowers (Flower H Incr), tended to increase over time and with defruiting in both cultivars, reaching 16% and 18% in Arbequina NF in the 3rd and 4th year, respectively, while in Frantoio the highest value was only 8% in 2017. In the Fr treatments the flower H Incr reached 9% for Arbequina (in 2017) and 4% for Frantoio (in 2017); the values obtained with the Fr treatments were therefore around half of those obtained with defruiting.
Figure 6.
Harvest increment in young olive trees for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting), for every year during the experimental period (2014–2018). Data are from 9 plants. The denominator (i.e. total biomass increment) includes vegetative + reproductive (i.e. fruit + inflorescences) biomass increments.
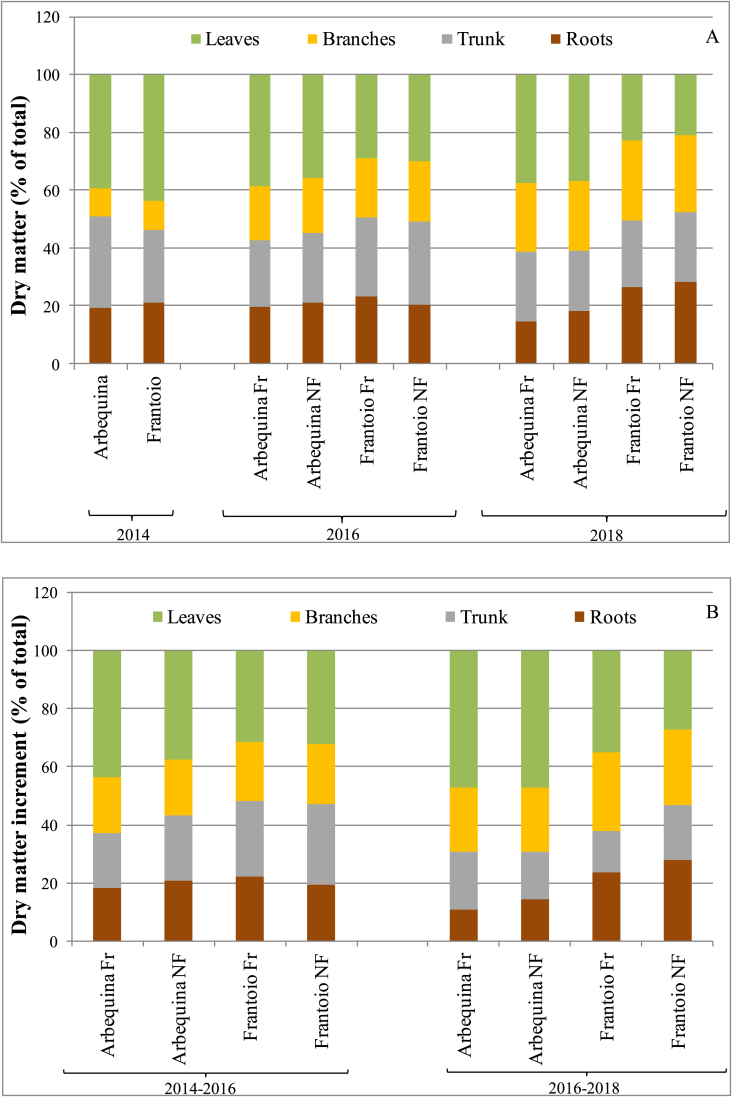
Given that fruiting reduced the partitioning towards all vegetative components (Figure 5), the question arises as to whether the proportions among vegetative components remain constant or whether they are also affected by fruiting. To analyze this, we calculated the percentage investments among vegetative components alone, in terms of both plant dry matter composition and partitioning of dry matter increments (Figure 7). Fruiting had no significant effects on the relative partitioning of vegetative dry matter into the different plant parts. We therefore carried out the ANOVA pooling data from the two fruiting treatments (Tables 1 and 2). At the beginning of the trial (2014) the dry biomass composition in the different parts of the plant was similar between cultivars with about 40% dry matter in leaves, 40% in trunk + branches and 20% in roots (Figure 7A). Nonetheless, Arbequina had slightly (+7%) but significantly more trunk mass and slightly (-5%) but significantly less leaf mass (Figure 7A and Table 1). Over the years, substantial differences began to emerge between the two cultivars with Arbequina maintaining a similar, and not significantly different across the years (Table 1), proportion of leaves, while in Frantoio relative leaf mass dropped significantly over time, down to 29.5% in 2016 (average between the two fruiting treatments) and to 22% in 2018, when Arbequina had 37% of leaf mass. Roots remained around 20% for all treatments until 2018, when they increased significantly to about 27% (i.e. average of the two treatments) in Frantoio, and decreased (but not significantly) to about 17% in Arbequina, making the difference in root composition significant between cultivars in 2018. In both cultivars there was also a marked and significant increase over time in the percentage of branches, which was however more pronounced in Frantoio. This increase was mostly to the detriment of leaves in Frantoio, of roots and trunk in Arbequina
Figure 7.
(A) Dry matter composition and (B) partitioning of vegetative dry matter increments of young olive trees for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting), in 2014, 2016, and 2018. Data from 6 plants per cultivar in 2014 and 3 plants per cultivar/fruiting treatment in 2016 and 2018. Statistics are reported in Tables 1 and 2.
Table 1.
Probability (P) values from ANOVA relative to data from Figure 6A and Student–Newman–Keuls test. For the Student–Newman–Keuls test, within the same plant part (i.e. same column), different letters between cultivars within the same year, or between years within the same cultivar, are significantly different (P < 0.05).
| Two-way ANOVA | Roots | Trunk | Branches | Leaves |
|---|---|---|---|---|
| Cultivar | <0.001 | 0.888 | 0.028 | <0.001 |
| Year | 0.534 | 0.008 | <0.001 | <0.001 |
| Cultivar x Year |
0.005 |
0.004 |
0.287 |
<0.001 |
| One-way ANOVA | ||||
| Arbequina (vs. 3 years) | 0.279 | 0.001 | <0.001 | 0.468 |
| Frantoio (vs. 3 years) | 0.006 | 0.229 | <0.001 | <0.001 |
| 2014 (Arb. vs. Fra.) | 0.442 | 0.003 | 0.828 | 0.026 |
| 2016 (Arb. vs. Fra.) | 0.522 | 0.106 | 0.161 | 0.014 |
| 2018 (Arb. vs. Fra.) | <0.001 | 0.504 | 0.030 | <0.001 |
| Student–Newman–Keuls test | |||||
|---|---|---|---|---|---|
| Year | Cultivar | Roots | Trunk | Branches | Leaves |
| 2014 | Arbequina | a | b | a | a |
| Frantoio | a | a | a | b | |
| 2016 | Arbequina | a | a | a | b |
| Frantoio | a | a | a | a | |
| 2018 |
Arbequina | a | a | a | b |
| Frantoio |
b |
a |
b |
a |
|
| Cultivar |
Year |
Roots |
Trunk |
Branches |
Leaves |
| Arbequina | 2014 | a | b | a | a |
| 2016 | a | a | b | a | |
| 2018 | a | a | c | a | |
| Frantoio | 2014 | a | a | a | c |
| 2016 | a | a | b | b | |
| 2018 | b | a | c | a | |
Table 2.
Probability (P) values from ANOVA relative to data from Figure 6B and Student–Newman–Keuls test. For the Student–Newman–Keuls test, within the same plant part (i.e. same column), different letters between cultivars within the same year, or between years within the same cultivar, are significantly different (P < 0.05).
| Two-way ANOVA | Roots | Trunk | Branches | Leaves |
|---|---|---|---|---|
| Cultivar | <0.001 | 0.339 | 0.007 | <0.001 |
| Two-year period | 0.545 | 0.015 | <0.001 | 0.184 |
| Cultivar x Two-year period |
0.001 |
0.127 |
0.117 |
0.125 |
| One-way ANOVA | ||||
| Arbequina (vs. 2-year period) | 0.027 | 0.305 | 0.079 | 0.053 |
| Frantoio (vs. 2-year period) | 0.021 | 0.034 | <0.001 | 0.890 |
| 2014–2016 (Arb. vs. Fra.) | 0.641 | 0.055 | 0.253 | 0.009 |
| 2016–2018 (Arb. vs. Fra.) | <0.001 | 0.716 | 0.018 | <0.001 |
| Student–Newman–Keuls test | |||||
|---|---|---|---|---|---|
| Two-year period | Cultivar | Roots | Trunk | Branches | Leaves |
| 2014–2016 | Arbequina | a | a | a | b |
| Frantoio | a | a | a | a | |
| 2016–2018 |
Arbequina | a | a | a | b |
| Frantoio |
b |
a |
b |
a |
|
| Cultivar |
Two-year period |
Roots |
Trunk |
Branches |
Leaves |
| Arbequina | 2014–2016 | b | a | a | a |
| 2016–2018 | a | a | a | a | |
| Frantoio | 2014–2016 | a | b | a | a |
| 2016–2018 | b | a | b | a | |
All vegetative parts increased their biomass over time for all treatments and cultivars (Figure 7B). The percentage of the total biomass increment invested in the different plant parts (Figure 7B, Table 2) reflected the trends found for plant dry matter composition in Figure 7A (increased branch mass, decreased root mass in Arbequina and decreased leaf mass in Frantoio), although the changes were less pronounced because the increments were calculated over two year intervals, while the plant composition in Figure 7A reflects the cumulative effect over the tree life, up to the time of sampling.
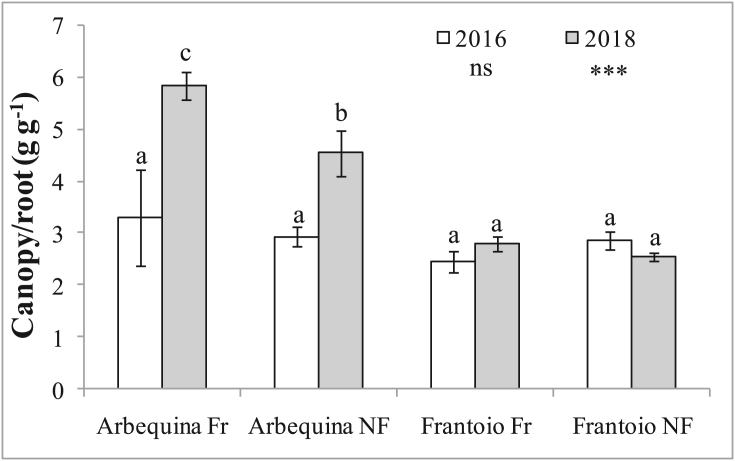
Canopy to root ratio was similar for all treatment in 2016 while in 2018 Arbequina had values almost double those of Frantoio, and Arbequina Fr had a significantly higher ratio than Arbequina NF (Figure 8).
Figure 8.
Canopy to root ratio of young olive trees for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting), calculated in 2016 and 2018. Data are averages of 3 plants. Bars denote standard errors. Averages within each year were compared using the Student–Newman–Keuls test; the probability level (ns = not significant; ∗∗∗ = P < 0.001), is indicated in the legend below the respective year. Different letters denote significant differences within each year.
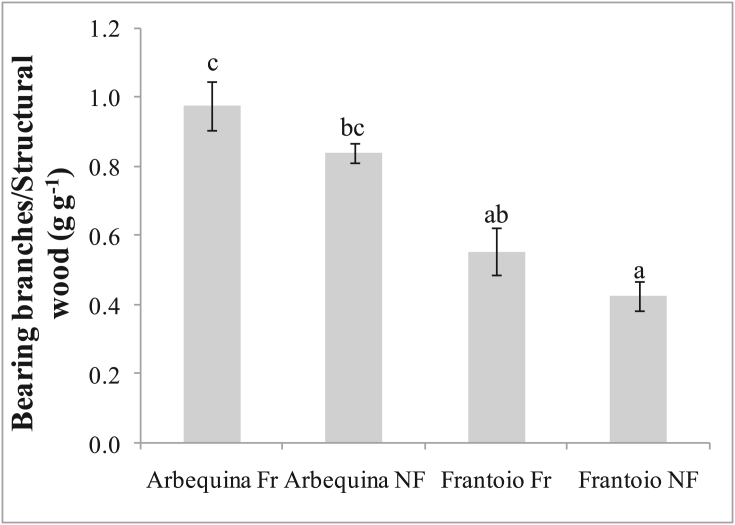
The bearing branches to structural wood biomass ratio, i.e. previous-year + current-year shoots with leaves to structural wood (branches + trunk + roots) ratio, in Arbequina was almost double that of Frantoio (Figure 9). Fruiting treatments consistently (i.e. for both cultivars) tended to have higher values than NF treatments, though the difference within cultivars were not statistically significant.
Figure 9.
Bearing branches (previous-year + current-year shoots with leaves) to structural wood (branches + trunk + roots) biomass ratio in young olive trees for different olive cultivars (Arbequina and Frantoio) and treatments (Fr = fruiting, NF = non-fruiting), calculated at the end of the experiment (2018). Data are averages of 3 plants. Bars denote standard errors. Averages were compared using the Student–Newman–Keuls test. Different letters denote significant (P < 0.05) differences.
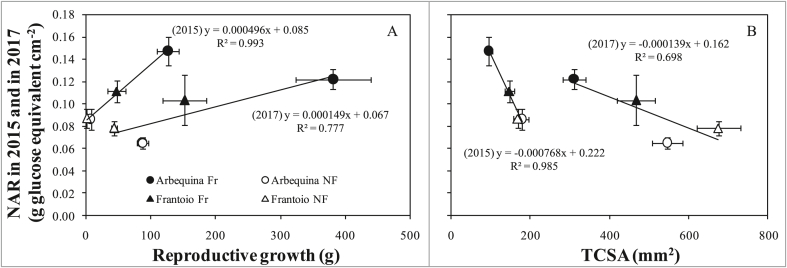
The net assimilation rate (NAR) increased with reproductive growth (Figure 10A) and decreased with TCSA (Figure 10B). In both cases the slopes were sharper in 2015 than in 2017 and, for each treatment combination, NAR was slightly lower in 2017, compared to 2015. However, ANCOVA gave significant differences between years only for Figure 10A, while fir Figure 10B the probability for the difference between years was P = 0.19.
Figure 10.
Relationship between net assimilation rate (NAR), calculated as total vegetative and reproductive growth (glucose equivalent year−1) per unit of final leaf area, and (A) reproductive growth, or (B) final trunk corss-sectional area (TCSA) in 2015 and in 2017. Each point is the average of 9 trees. Bars denote standard errors. Solid lines are fits across treatments within each year.
4. Discussion
Increments in TCSA (and thus for whole-tree biomass) were lower for fruiting than non-fruiting treatments (Figure 2A and Figure 3), in line with previous findings in olive trees (Fernández et al., 2015; Rosati et al., 2018a). The TCSA of Arbequina NF was comparable to that of Frantoio NF up to the first three years of observation, confirming that the low vigor generally observed in Arbequina is due to its early and abundant fruiting (up to 159 g of fruit + flower dry matter per tree in the first and second year after transplanting Rosati et al., 2018a; 2018b). In the fourth year, however, the TCSA in Frantoio NF was significantly greater than that of Arbequina NF (Figure 2A). Increments in TCSA (Figure 3) and in vegetative biomass were also greater for Frantoio NF compared to Arbequina NF (Figures 3 and 4). The greater vegetative growth of Frantoio NF was related to flower production (Figure 3). Arbequina NF, precisely because it was defruited every year, produced considerable quantities of floral biomass (Figures 3, 5, and 6), much higher than Frantoio, and the plant growth (TCSA and therefore biomass) was closely related to the reproductive effort in flowers + fruits (Figure 3).
Fruit production in Arbequina Fr was also much higher than in Frantoio Fr (Figure 2B), except for the year 2016. The strong negative correlations found between reproductive biomass (flowers + fruits) production and increments in TCSA (R2 = 0.95) or in biomass (R2 = 0.97) (Figure 3) confirm that the cost of reproduction explains the different growth of young olive trees among various fruiting treatments and cultivars, as described in our previous work (Rosati et al., 2017, 2018a, 2018b). In fact, the increases in vegetative + reproductive biomass over the four years, expressed both in grams of dry matter and in grams of glucose equivalent, were not significantly different between the two cultivars and treatments (Figure 4), confirming what was previously observed with two-year-old plants (Rosati et al., 2018b). The meaning and implications of these findings were discussed in detail in the cited previous work and are not further discussed here.
Substantial differences were found in the distribution of dry matter, especially at the level of the biomass invested in leaves: while in Arbequina leaf dry matter was close to 40% at all sampling periods, in Frantoio it dropped significantly from about 40 to 30% in 2016 and close to 20% in 2018 (Figure 7A). Similar values were observed in 3-year-old olive trees (cv. Nocellara del Belice), in which the leaves represented almost 30% (Scariano et al., 2008), but no trend over the years was reported. This value is very similar to what we found in Frantoio, in 2016, when plants were the same age (3 years old). In 1-year-old trees (cv. Leccino), Di Vaio et al. (2012) reported 28% for both leaves and roots. These values are respectively lower (leaves) and higher (roots) than we found for one-year-old trees in 2014. This could be due to varietal differences or different experimental conditions. Di Vaio et al. (2013) reported the dry matter composition of two-year-old olive trees (Leccino, high vigor and Racioppella, low vigor) under different irrigation levels. Although the data did not report the percentage composition of the different plant components, we calculated it from the absolute values reported. In well irrigated Leccino trees, leaves represented about 25% of total dry matter in the first year, decreasing to about 16% in the second year. For less irrigated plants, the respective values were 22% and 14%. Similar results were obtained for the cv. Racioppella. Root biomass increased, from the first to the second year, from 16% to 28% in well-irrigated Leccino but increased less, from 30 to 34%, in Racioppella. The effect of different irrigation levels on dry matter partitioning is well documented in olive (Xiloyannis et al., 1999; Dichio et al., 2002; Bacelar et al., 2007; Di Vaio et al., 2012, 2013). Cultivar effect is also documented (Tognetti et al., 2002; Di Vaio et al., 2013). The effects of tree age and the trends in the variation of dry mater partitioning over the years has not been documented as well. The present results and their comparison with available literature (i.e. recalculated data from Di Vaio et al., 2013) indicate that there appears to be a general trend in decreasing leaf and increasing root relative biomass in most cultivars, except in Arbequina where we found that the leaf biomass fraction remained constant, at least from one to four years of age. Additionally, our data show that the branch biomass fraction increases over time, but this can be at the expense of leaves (Frantoio) or other plant parts (Arbequina). Perhaps the most striking difference between Arbequina and the other cultivars is its ability to maintain greater leaf biomass fractions (thus greater leaf area, relative to tree size) compared to other cultivars, confirming our previous results (Rosati et al., 2018c). Greater relative investments in leaves are advantageous in young trees, allowing them to intercept more light and therefore to increase the vegetative and productive growth potential (Rosati et al., 2018c).
In young plants of some fruit species, the H Incr can reach 75–80% (Cannell, 1985; Fischer et al., 2012). Dwarfing rootstocks are particularly efficient in diverting dry matter into fruits rather than into wood, with significant increases in HI observed in some fruit species such as apple, pear and cherry (Atkinson and Else, 2003). In Arbequina olive trees, Villalobos et al. (2006) reported average H Incr values around 50%, in line with our results on the same cultivar (Figures 5 and 6). In this work we also calculated the harvest increment referred only to flowers, which allows to better evaluate the reproductive effort in young olive plants, including the cost of flowering. The biomass invested in flowers in relation to the total increase in biomass of the plant was considerable in Arbequina already in the 3rd-4th year, with fruiting plants reaching 8–9%, and defruited plants reaching 16–18% (Figure 6). These higher percentages were most likely due to the fact that removing the fruit sink released the competition between vegetative and reproductive growth, resulting in greater vegetative growth, as clearly suggested by Figure 3, and greater flower induction and differentiation the following years. Famiani et al. (2019) observed that in 14-year-old Frantoio trees, the cost of flowering represented on average almost 17% of the total inflorescence + fruit biomass. Considering an H Incr of about 50% in adult plants (Villalobos et al., 2006), this value corresponds to about 8.5 % of the total biomass increment, thus nearly identical to the value found here for fruiting plants in this experiment. Considering the short period of development of the inflorescences (i.e. about one month) this value implies that during inflorescence development trees make a considerable effort in flower formation, with biomass allocation rates similar to those invested later in fruit growth (Famiani et al., 2019).
Canopy to root ratio increased considerably in 2018, compared to 2016, in Arbequina, compared to Frantoio Fr (Figure 8), confirming that this ratio varies between genotypes (Di Vaio et al., 2012, 2013). In addition, the present data also suggest that in Arbequina this ratio is higher in fruiting plants, compared to NF plants. This agrees with observations that roots are weaker sinks than other plant parts, particularly fruit, thus when more fruit is present (as in Arbequina in this study), root growth is reduced to a greater extent (Lakso and Flore, 2003).
The bearing branches to structural wood biomass ratio was close to 1 gg-1 in Arbequina Fr (Figure 9). That is, for every gram of dry matter invested in non-productive structures (branches + trunk + roots), there was one gram invested in productive structures (i.e. previous-year + current-year shoots with leaves). Arbequina's values were, on average between treatments, almost double Frantoio's values, in this experiment with young trees. Higher values of this ratio are considered advantageous in terms of productivity, allowing the plant to spend proportionally fewer resources in non-productive structures (Rosati et al., 2013, 2018c). In fact, trunk and branches are not directly productive structures of the plant (i.e. they do not photosynthesize), even though they are indispensable since they allow the leaves to be distributed in space, maximizing light interception and bringing water and nutrients from the soil to the leaves. Similarly, the root system is not directly productive, but it is necessary both to absorb and transport water and nutrients to the leaves, and to anchor and support the plants. How does Arbequina achieve greater partitioning into reproductive structures? It has been shown that, compared to other cultivars such as Frantoio, Arbequina has both thinner woody structures (trunk, branches and shoots), saving wood biomass per unit leaf biomass, and greater branching, implying that more main branches are supported by the same trunk, and more secondary branches are supported by each main branch and so on, up to the shoots, which are the production sites (Rosati et al., 2013, 2018c). Therefore, in Arbequina more leaves and production sites are supported with the same wood or, in other words, less wood is needed to support the same leaves and production sites. This finding is supported also by the present data showing greater proportional investments in leaves, than in trunk, branches and roots (Figure 7) and by the higher bearing branches to structural wood biomass ratio (Figure 9). These savings in non-productive structures reduce the size of the vegetative sink, thus reducing competition for resources between vegetative and reproductive growth. This might contribute to explain Arbequina's greater attitude towards earlier and higher fruit production.
Aside from the cultivar differences, the bearing branches to structural wood ratio tended to be higher for the Fr treatments, compared to the NF treatments, consistently for both cultivars, although the differences were not statistically significant in the present experiment. Further work might confirm this trend, which agrees with our previous results (Rosati et al., 2018c) showing that fruiting modifies canopy architecture, favoring greater leaf area per unit of total biomass, by producing shorter shoots with a higher leaf area/biomass ratio, thus allowing to better support the growing fruit.
NAR increased with reproductive growth for both periods considered (Figure 10A), implying improved leaf photosynthetic properties in the fruiting trees compared to NF ones or, alternatively, downregulation of photosynthesis in deflowered trees, in line with previous findings (DeJong, 1986), including in olive (Rosati et al., 2018b). These authors reported a detailed discussion on this subject, concluding that increasing vegetative growth and leaf area at decreasing reproductive efforts probably increased water stress limitation to photosynthesis in the potted plants.
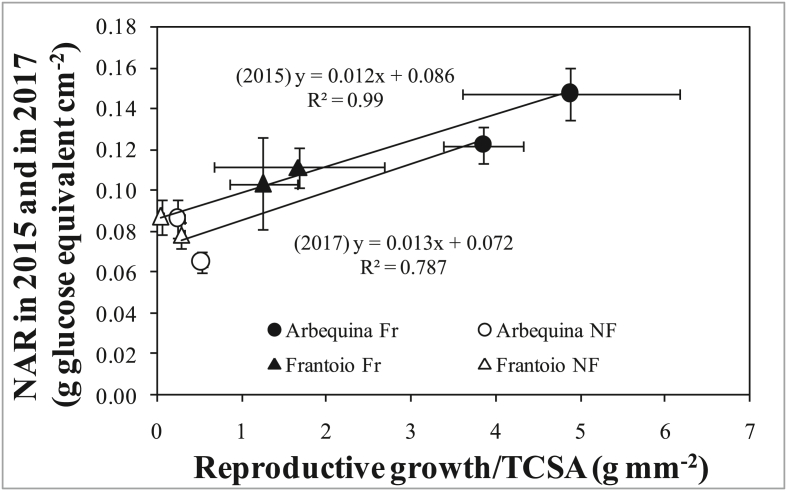
The regressions in Figure 10A were significantly different for the two years (2015 and 2017) with lower slope and lower NAR values within treatments for 2017, when trees were older and thus larger than in 2015. This could appear to suggest that the difference in NAR between years could be related to increased self-shading with increasing tree size/age (Matsuda et al., 2011). However, when NAR was plotted against TCSA (i.e. a good proxy of tree size), the regressions remained very distinct between years (although not significantly different, P = 0.19, Figure 10B), suggesting that tree size and self-shading were not able to explain differences among years. We therefore plotted NAR against the relative reproductive effort (reproductive growth relative to TCSA) and found almost identical and not significantly different regressions for the two years (Figure 11). This suggest that the main driving variable affecting NAR is the plant's relative reproductive effort (i.e. expressed relative to the plant size). This is sensible because the same amount of fruit will have different physiological impacts on trees of different size, representing a smaller relative biomass/energy effort in larger plants. Therefore, plants of different size/age cannot be compared in absolute terms but must be compared expressing their reproductive effort relative to their size. Expressing the reproductive effort in relative terms in Figure 11, the regressions remained slightly, although not significantly (P = 0.12) different, with lower NAR values, at equal relative reproductive effort, for 2017. Although not significant this trend is in line with increased self-shading at increasing plant age/size (2017 vs. 2015). In older/bigger (e.g. full size trees) trees, this effect can be more pronounced, reducing NAR more dramatically at increasing tree size (Matsuda et al., 2011).
Figure 11.
Relationship between net assimilation rate (NAR), calculated as total vegetative and reproductive growth (glucose equivalent) per unit of final leaf area, and relative reproductive growth in 2015 and in 2017. Each point is the average of 9 trees. Bars denote standard errors. Solid lines are fits across treatments within each year.
5. Conclusions
TCSA increments in defruited Arbequina plants (Arbequina NF) were comparable, up to the third year, to that of the Frantoio NF. In the fourth year, Arbequina NF plants were somewhat smaller than those of Frantoio NF, but they produced considerably more inflorescence mass over the years. When flower mass was considered as part of the reproductive effort, tree biomass growth over the four years was perfectly explained by the reproductive effort: vegetative biomass increments decreased linearly (R2 = 0.97) with increasing reproductive efforts, and total vegetative + reproductive biomass did not differ between either cultivars or fruiting treatments. This supports the hypothesis that differences in vigor between cultivars and fruit loads can be explained in terms of different dry matter partitioning among the various organs, with early bearing cultivars growing less. However, what makes Arbequina bear fruit earlier and more abundantly than Frantoio is not known. The results show that, compared to Frantoio, Arbequina has a greater distribution of dry matter in productive structures (current and one-year-old shoots) and in leaves. This allows it to increase the number of current and following-year production sites, and to save in the resources invested in non-productive sinks (roots, trunk and branches), thus liberating resources for reproductive growth. Arbequina maintains greater investments in leaf mass over the years, and this should allow it to intercept more light and thus to increase assimilation. Increased assimilation and increased partitioning towards productive structures, and decreased competition by non-productive structures might contribute to explaining the greater early bearing attitude of this cultivar.
Declarations
Author contribution statement
Andrea Paoletti; Franco Famiani: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Adolfo Rosati: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Allen M.T., Prusinkiewicz P., DeJong T.M. Using L-systems for modeling source–sink interactions, architecture and physiology of growing trees: the L-PEACH model. New Phytol. 2005;166:869–880. doi: 10.1111/j.1469-8137.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- Atkinson C.J., Else M.A. Enhancing harvest index in temperate fruit tree crops through the use of dwarfing rootstocks. In: Bekele F., End M.J., Eskes A.B., editors. Proceedings of International Workshop on cocoa Breeding for Improved Production Systems. 19-21 October 2003, Accra, Ghana. 2003. pp. 118–131. [Google Scholar]
- Bacelar E.A., Moutinho-Pereira J.M., Gonçalves B.C., Ferreira H.F., Correira C.M. Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2007;60:183–192. [Google Scholar]
- Berman M.E., DeJong T.M. Seasonal patterns of vegetative growth and competition with reproductive sink in peach. J. Hortic. Sci. Biotechnol. 2003;78:303–309. [Google Scholar]
- Cannell M.G.R. Dry matter partitioning in tree crops. In: Cannell M.G.R., Jackson J.E., editors. Attributes of Trees as Crop Plants. Inst. Terrestrial Ecology, Midlothian, Great Britain. 1985. pp. 160–193. [Google Scholar]
- Castillo-Llanque F., Rapoport H.F. Relationship between reproductive behavior and new shoot development in 5-year-old branches of olive trees (Olea europaea L.) Trees. 2011;25:823–832. [Google Scholar]
- Chandler W.H., Heinicke A.J. The effect of fruiting on the growth of Oldenburg apple trees. Proc. Am. Soc. Hortic. Sci. 1926;23:36–46. [Google Scholar]
- Connor D.J., Fereres E. The physiology of adaptation and yield expression in olive. Hort. Rev. 2005;31:155–229. [Google Scholar]
- Costes E., Fournier D., Salles J.C. Changes in primary and secondary growth as influenced by crop load in ‘Fantasme’ apricot trees. J. Hort. Sci. Biotechnol. 2000;75:510–519. [Google Scholar]
- Dag A., Bustan A., Avni A., Tzipori I., Lavee S., Riov J. Timing of fruit removal affects concurrent vegetative growth and subsequent return bloom and yield in olive (Olea europaea L.) Sci. Hortic. 2010;123:469–472. [Google Scholar]
- DeJong T.M. Fruit effects on photosynthesis in Prunus persica. Physiol. Plant. 1986;66:149–153. [Google Scholar]
- DeJong T.M. Developmental and environmental control of dry-matter partitioning in peach. Hortscience. 1999;34:1037–1040. [Google Scholar]
- Dichio B., Romano M., Nuzzo V., Xiloyannis C. Soil water availability and relationship between canopy and roots in young olive trees (cv Coratina) Acta Hortic. 2002;586:255–258. [Google Scholar]
- Di Vaio C., Marra F.P., Scaglione G., La Mantia M., Caruso T. The effect of different vigour olive clones on growth dry matter partitioning and gas exchange under water deficit. Sci. Hortic. 2012;134:72–78. [Google Scholar]
- Di Vaio C., Marallo N., Marino G., Caruso T. Effect of water stress on dry matter accumulation and partitioning in pot-grown olive trees (cv Leccino and Racioppella) Sci. Hortic. 2013;164:172–177. [Google Scholar]
- Dixon R.K. Physiological processes and tree growth. In: Dixon R.K., Meldahl R.S., Ruark G.A., Warren W.G., editors. Forest Growth: Process Modelling of forest Growth Responses to Environmental Stress. Timber Press; Portland: 1990. pp. 21–32. [Google Scholar]
- Donald C.M. In search of yield. J. Aust. Inst. Agric. Sci. 1962;28:171–178. [Google Scholar]
- Egli D.B., Guffy R.D., Leggett J.E. Partitioning of assimilate between vegetative and reproductive growth in soybean. Agron. J. 1985;77:917–922. [Google Scholar]
- Embree C.G., Myra M.T., Nichols D.S., Wright A.H. Effect of blossom density and crop load on growth, fruit quality, and return bloom in ‘Honeycrisp’ apple. Hortscience. 2007;42:1622–1625. [Google Scholar]
- Elfving D.C. Economic effects of excessive vegetative growth in deciduous fruit trees. Hortscience. 1988;23:461–463. [Google Scholar]
- Famiani F., Farinelli D., Gardi T., Rosati A. The cost of flowering in olive (Olea europaea L.) Sci. Hortic. 2019;252:268–273. [Google Scholar]
- Fernández F.J., Ladux J.L., Searles P.S. Dynamics of shoot and fruit growth following fruit thinning in olive trees: same season and subsequent season responses. Sci. Hortic. 2015;192:320–330. [Google Scholar]
- Fischer G., Almanza-Merchán P.J., Ramírez F. Source-sink relationships in fruit species: a review. Rev. Colomb. Cienc. Hortíc. 2012;6:238–253. [Google Scholar]
- Flore J.A., Lakso A.N. Environmental and physiological regulation of photosynthesis in fruit crops. Hortic. Rev. 1989;11:111–157. [Google Scholar]
- Forshey C.G., Elfving D.C. The relationship between vegetative growth and fruiting in apple trees. Hortic. Rev. 1989;11:229–287. [Google Scholar]
- Grossman Y.L., DeJong T.M. PEACH: a simulation model of reproductive and vegetative growth in peach trees. Tree Physiol. 1994;14:329–345. doi: 10.1093/treephys/14.4.329. [DOI] [PubMed] [Google Scholar]
- Jackson J.E. The manipulation of fruiting. In: Wright C.J., editor. Manipulation of Fruiting. Butterworths; London: 1989. pp. 3–12. [Google Scholar]
- Lakso A.N., Flore J.A. Carbohydrate partitioning and plant growth. In: Baugher T.A., Singh S., editors. Concise Encyclopedia of Temperate Tree Fruit. Food Products Press; New York: 2003. pp. 21–30. [Google Scholar]
- Lavee S. Biennial bearing in olive (Olea europaea) Ann. Ser. His. Nat. 2007;17:101–112. [Google Scholar]
- Marcelis L.F.M. Sink strength as a determinant of dry matter partitioning in the whole plant. J. Exp. Bot. 1996;47:1281–1291. doi: 10.1093/jxb/47.Special_Issue.1281. [DOI] [PubMed] [Google Scholar]
- Marcelis L.F.M., Heuvelink E. Modelling fruit set, fruit growth and dry matter partitioning. Acta Hortic. 1999;499:39–49. [Google Scholar]
- Mariscal M.J., Orgaz F., Villalobos F.J. Radiation-use efficiency and dry matter partitioning of a young olive (Olea europaea) orchard. Tree Physiol. 2000;20:65–72. doi: 10.1093/treephys/20.1.65. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Suzuki K., Nakano A., Higashide T., Takaichi M. Responses of leaf photosynthesis and plant growth to altered source–sink balance in a Japanese and a Dutch tomato cultivar. Sci. Hortic. 2011;127:520–527. [Google Scholar]
- Monselise S., Goldschmidt E.E. Alternate bearing in fruit trees. Hort. Rev. 1982;4:128–173. [Google Scholar]
- Obeso J.R. The cost of reproduction in plants. New Phytol. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Penning de Vries F.W.T., Brunsting A.H.M., van Laar H.H. Products, requirements and efficiency of biosynthesis: a quantitative approach. J. Theor. Biol. 1974;45:339–377. doi: 10.1016/0022-5193(74)90119-2. [DOI] [PubMed] [Google Scholar]
- Rallo L., Suárez M.P. Seasonal distribution of dry matter within the olive fruitbearing limb. Adv. Hort. Sci. 1989;3:55–59. [Google Scholar]
- Rosati A., Paoletti A., Caporali S., Perri E. The role of tree architecture in super high density olive orchards. Sci. Hortic. 2013;161:24–29. [Google Scholar]
- Rosati A., Paoletti A., Pannelli G., Famiani F. Growth is inversely correlated with yield efficiency across cultivars in young olive (Olea europaea L.) trees. Hortscience. 2017;52:1525–1529. [Google Scholar]
- Rosati A., Paoletti A., Al Hariri R., Morelli A., Famiani F. Partitioning of dry matter into fruit explains cultivar differences in vigor in young olive (Olea europaea L.) trees. Hortscience. 2018;53:491–495. [Google Scholar]
- Rosati A., Paoletti A., Al Hariri R., Morelli A., Famiani F. Resource investments in reproductive growth proportionately limit investments in whole-tree vegetative growth in young olive trees with varying crop loads. Tree Physiol. 2018;38:1267–1277. doi: 10.1093/treephys/tpy011. [DOI] [PubMed] [Google Scholar]
- Rosati A., Paoletti A., Al Hariri R., Famiani F. Fruit production and branching density affect shoot and whole-tree wood to leaf biomass ratio in olive. Tree Physiol. 2018;38:1278–1285. doi: 10.1093/treephys/tpy009. [DOI] [PubMed] [Google Scholar]
- Sanz-Cortés F., Martínez-Calvo J., Badenes M.L., Bleiholder H., Hack H., Llácer G., Meier U. Phenological growth stages of olive trees (Olea europaea) Ann. Appl. Biol. 2002;140:151–157. [Google Scholar]
- Scariano L., Lo Bianco R., Di Marco L., Policarpo M. Dynamics of dry matter partitioning in young ‘Nocellara del Belice’ olive trees. Acta Hortic. 2008;791:397–401. [Google Scholar]
- Stevenson M.T., Shackel K.A. Alternate bearing in pistachio as a masting phenomenon: construction cost of reproduction versus vegetative growth and storage. J. Am. Soc. Hortic. Sci. 1998;123:1069–1075. [Google Scholar]
- Tognetti R., Costagli G., Minnocci A., Gucci R. Stomatal behaviour and water use efficiency in two cultivars of Olea europaea L. Agr. Med. 2002;132:90–97. [Google Scholar]
- Verheij E.W.M. Competition in apple as influenced by Alar sprays, fruiting, pruning and tree spacing. Meded. Landbouwhog. Wageningen. 1972;72–4:l–54. [Google Scholar]
- Villalobos F.J., Testi L., Hidalgo J., Pastor M., Orgaz F. Modelling potential growth and yield of olive (Olea europaea L.) canopies. Eur. J. Agron. 2006;24:296–303. [Google Scholar]
- Wardlaw I.F. The control of carbon partitioning in plants. New Phytol. 1990;116:341–381. doi: 10.1111/j.1469-8137.1990.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Wright C.J. Interactions between vegetative and reproductive growth. In: Wright C.J., editor. Manipulation of Fruiting. Butterworths; London: 1989. pp. 15–27. [Google Scholar]
- Wünsche J.N., Ferguson I.B. Crop load interactions in apple. Hortic. Rev. 2005;31:231–290. [Google Scholar]
- Xiloyannis C., Dichio B., Nuzzo V., Celano G. Defense strategies of olive against water stress. Acta Hortic. 1999;474:423–426. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.