Summary
Biofilm formation in living organisms is associated to tissue and implant infections, and it has also been linked to the contribution of antibiotic resistance. Thus, understanding biofilm development and being able to mimic such processes is vital for the successful development of antibiofilm treatments and therapies. Several decades of research have contributed to building the foundation for developing in vitro and in vivo biofilm models. However, no such thing as an “all fit” in vitro or in vivo biofilm models is currently available. In this review, in addition to presenting an updated overview of biofilm formation, we critically revise recent approaches for the improvement of in vitro and in vivo biofilm models.
Subject areas: Microbiology, Microbiofilms
Graphical abstract
Microbiology; Microbiofilms
Introduction
Bacteria may seem simple entities when compared with eukaryotic organisms. However, their molecular machinery allows them to become rapidly adapted to a variety of environmental conditions. For instance, the ability of bacteria to modulate gene expression allows them to finely tune their growth rate depending on nutrient availability (Planson et al., 2020). Bacteria can also shift from an individual and free-floating (planktonic) state to a community-like condition where they improve their ability to survive in harsh environments. This self-organized arrangement, known as biofilm, consists of a three-dimensional (3D) microbial structure with cells enclosed within a self-produced extracellular matrix that may be attached to a substratum (Costerton et al., 1999). Bacteria may also autoaggregate or coaggregate to form biofilms in pure or mixed cultures, respectively, if no solid substrate is available for their attachment (Bjarnsholt et al., 2009; Alhede et al., 2011). Biofilm bacteria exhibit distinct metabolism and gene expression than their planktonic counterparts and present an altered phenotype with increased tolerance to host immune defense mechanisms and exogenously administered antimicrobial substances (Nickel et al., 1985). For instance, sessile bacteria are between 10 and 10,000 times more resistant to antimicrobial agents in comparison to bacteria in planktonic state (Olson et al., 2002a; Luppens et al., 2002; Mah and O'Toole, 2001; Nickel et al., 1985).
Biofilms have been present on earth for around 3.4 billion years (Hall-Stoodley et al., 2004), performing important roles in biogeochemical cycling processes (Paerl and Pinckney, 1996). Biofilms can colonize a great variety of abiotic and biotic surfaces (Yin et al., 2019; Hall-Stoodley et al., 2004). The ideal biotic environment for bacteria to thrive must include a reserve of nutrients, humidity, and an appropriate temperature. Thus, humans are a perfect source of biotic microenvironments for bacterial colonization and biofilm formation, which in most of the cases lead to infectious diseases. The first scientist to describe microbial aggregates in his own dental plaque was Anthony van Leeuwenhoek (1683), and approximately 180 years after, Louis Pasteur (1864) reported bacterial aggregates in wine (Høiby, 2014). Although the term biofilm had been previously used in microbiological and environmental reports, it was in 1985 that J.W. Costerton introduced this term to the field of medical microbiology (Høiby, 2017). Since then, thousands of reports trying to explain the nature, behavior, and potential methods of biofilm eradication have been published. Hence, one may question what has delayed a full understanding of biofilms after more than three centuries of study.
According to the National Institutes of Health, approximately 80% of microbial infections have a biofilm-related etiology (NIH, 2002). In general, biofilm microbial infections can be classified as:
-
(1)
Intrinsic to host tissues, and
-
(2)
Associated with indwelling medical devices (Sun et al., 2013).
The former are mainly chronic opportunistic infections that develop in different tissues and organs, including cystic fibrosis, osteomyelitis, conjunctivitis, vaginitis, urethritis, nonhealing wounds, native valve infectious endocarditis, some pediatric respiratory infections such as otitis media and rhinosinusitis, as well as diverse oral diseases, including caries, periodontitis, halitosis, and gingivitis (Filardo et al., 2019; Lebeaux et al., 2014; Hamilos, 2019). The second type of biofilm-associated infections is characterized by the development of biofilms on medical devices, such as intravascular and urinary catheters, pacemakers, heart valves, contact lenses, breast implants, endotracheal tubes, and orthopedic implants (Zimmerli and Sendi, 2017; Donlan, 2001; Lebeaux et al., 2014; Beloin et al., 2017).
The chronic nature of biofilm-related diseases and their high resistance to antimicrobial substances and antibiotics represent an economical burden for health care systems worldwide. In this comprehensive review, we have highlighted the importance of acknowledging the complexity of biofilm formation and development. This will allow the reader to consider a more conscious selection or creation of the appropriate models to better mimic the native conditions under which biofilms are formed. Such complexity can only be tackled using scientific interdisciplinarity. Thus, this review has been structured for welcoming scientists from different fields of expertise to the field of biofilms. Schematics and brief explanations of fundamental concepts are included in this work to help understand abstract concepts and mechanisms. In the first section, we offer the reader an overall picture of biofilm development by presenting a general overview of the cellular, molecular, mechanical, and physicochemical mechanisms and events involved. One of our goals is to discuss the wide variety of special considerations affecting biofilm formation and development, which are usually underestimated when selecting the experimental models for the study of biofilms and the evaluation of potential antibiofilm strategies. Moreover, we consider that once having a better understanding of such, the reader will become more critical when evaluating the previous and more recently developed in vitro and in vivo biofilm models (see Sections “in vitro models for biofilm assessment” and “in vivo models for biofilm assessment”). Finally, a brief discussion of the general advantages and limitations of biofilm models/studies is presented along with a road map that may serve as a guide for the selection of biofilm models.
Fundamental concepts on biofilm development
The outer and inner surfaces of the human body are ideal hosts for microbial colonization. However, depending on the site of entry, microbes face important environmental challenges that culminate in stress of these cells. Some of such stressors include cellular and humoral immune defense mechanisms, forces exerted by body fluids, and variability in the access to oxygen and nutrients. To withstand these challenges, bacteria modulate their genetic makeup (for review, see Beitelshees et al., 2018). Hence, biofilm formation arises as a microbial mechanism of defense to ensure survival of bacteria. There is not a single type of biofilm; rather than that, biofilms will depend on the type of microbes forming them, interactions with host immune effectors, and the physicochemical and mechanical properties of the microenvironment. This reflects the level of complexity for the study of biofilms and the development of effective antimicrobials for their eradication.
Biofilms are varied in microbial composition; they can be formed solely by single microorganisms (monospecies biofilms) (Cole et al., 2004; Tolker-Nielsen et al., 2000; Su et al., 2012), by a combination of two or more microbes (multispecies biofilms) from the same and/or different species and strains (Gibbons and Nygaard, 1970; Tolker-Nielsen et al., 2000; Price et al., 2020; Burmølle et al., 2006), or even by the integration of microbes from different taxonomic levels (interkingdom biofilms) (Tchekmedyian et al., 1986; El-Azizi et al., 2004; Adam et al., 2002). The term “polymicrobial biofilm” is often employed to refer to any combination of microorganisms into the biofilm, independently of their phylogeny. Both bacteria and fungi (yeast and filamentous) have the ability to produce biofilms (Costerton et al., 1999; Chandra et al., 2001; Mowat et al., 2009). Despite this high variability, common processes and mechanisms of biofilm development have been found to be shared between species. Hence, although biofilm morphology and physiology vary depending on the initial microbe(s) involved, the microenvironment and interactions occurring (or not) between the microorganisms and a potential surface, the complex series of events that take place during biofilm development are usually broadly grouped to allow a better understanding.
It is important to make clear that these are not, by any means, single events following a straight and unique line. A variety of different processes will occur and potentially overlap during biofilm development, where some of them may be exclusive for particular microorganisms and microenvironment conditions. Because of this complexity, and in order to provide an overview for interdisciplinary scientists, we have decided to present biofilm development by introducing such series of events following the traditional model of five main stages. Despite the use of clearly identified and labeled stages of biofilm formation and development, the actual processes occurring under native conditions are far more complex, dynamic, and varied.
Hence, in this review, we use this structure as an overall picture and provide a more detailed discussion in the following subsections to show some of the events and mechanisms involved, as well as the potential consequences of such, to highlight the multifactorial nature of biofilm formation and development. We consider that in order to develop relevant biofilm models, it is important to understand the molecular and cellular events that influence biofilm formation and heterogeneity at each stage, which as a consequence influence their susceptibility to potential antimicrobial strategies.
Since the current knowledge on biofilms has been mostly derived from in vitro studies and surface-attached biofilms, where Pseudomonas aeruginosa has served as a model microorganism over several years of research, we will mainly focus on surface-related biofilm development. As previously mentioned, and later discussed, biofilms of clinical relevance are also found to be not necessarily attached to a surface. Because of their significance, several research studies on this matter are also briefly discussed throughout this section.
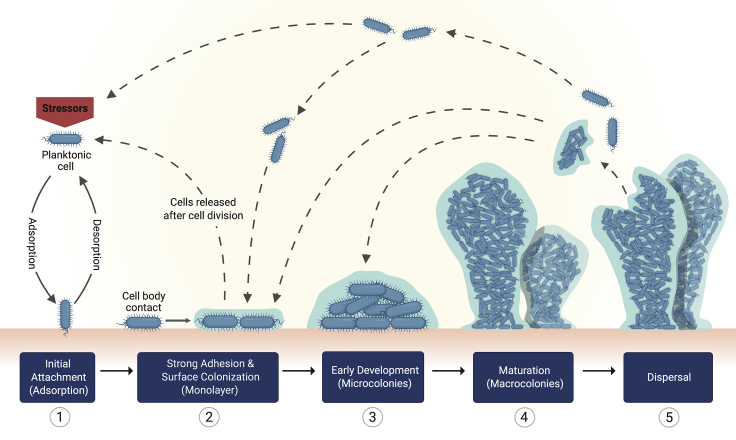
The main stages of bacterial biofilm formation may include the following: (1) adsorption, (2) adhesion, (3) formation of microcolonies, (4) maturation, and (5) dispersal (Figure 1). In general, these stages apply for both bacterial and yeast biofilms (Costerton et al., 1987; Stoodley et al., 2002; Chandra et al., 2001; Blankenship and Mitchell, 2006; Harding et al., 2009; O'Toole et al., 2000). Some authors have proposed to subdivide them to explain biofilm formation by filamentous fungi. Specifically, in this case, the formation of microcolonies considers the germling and/or formation of a monolayer, which leads to mycelial development, hyphal layering, and hyphal bundling (Harding et al., 2009).
Figure 1.
Schematic representation for single bacterial species biofilm formation on a solid surface
The schematic depicts the five main steps for the formation and spreading of biofilms.
Adsorption of bacterial cells to the surface: reversible attachment
Planktonic bacteria move toward a surface by the effect of physical and gravitational forces and by sensing changes in physicochemical properties (Xu et al., 1998; Kimkes and Heinemann, 2020). Biofilms can be formed onto abiotic or biotic surfaces, differing in some of the mechanisms for their anchorage (discussed later). Initially, bacterial cells become adsorbed to a substrate through nonspecific interactions in both abiotic and biotic surfaces (Bos et al., 1999) (Figure 2). These involve a series of attractive and repulsive physicochemical interactions between bacteria and the surface, where Lifshitz-van der Waals forces, electrostatic interactions, and Lewis acid-base hydrophobic forces are the first to participate (Ren et al., 2018; Bos et al., 1999). Steric forces given by bacterial structures, such as the polymeric brush layer in P. aeruginosa, P. putida, and Escherichia coli, also influence surface interactions (Berne et al., 2018). The net result between attractive and repulsive forces dictates the strength of bacterial adhesion, which is variable depending on the surface, microbial species, and surrounding medium (An and Friedman, 1998). Furthermore, most bacteria have extracellular projections of varied size, structure, and function generally known as bacterial appendages. Apart from influencing cell morphology, these surface-associated filamentous structures gear bacterial cells up to promote locomotion, survival, niche acquisition, and modulation of immune response in the host (Yang et al., 2016). Flagella and pili are two families of bacterial appendages that play main roles during the initial interactions with the target surface.
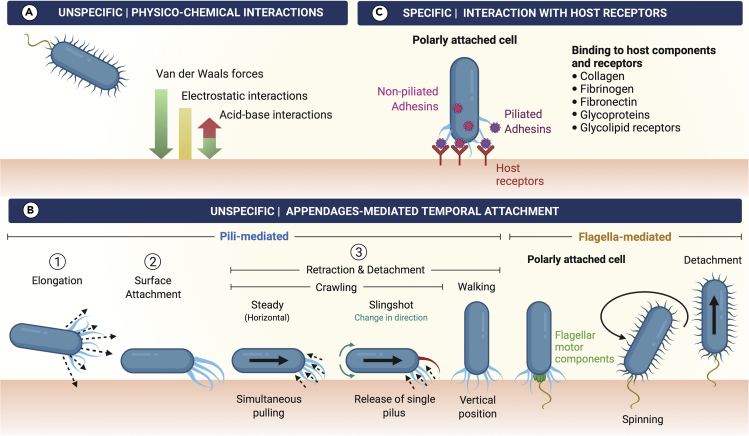
Figure 2.
Schematic representation for the main nonspecific and specific interactions between bacteria and surfaces
(A) Some physicochemical interactions include the attractive van der Waals forces; attractive or repulsive electrostatic interactions, which depend on the microenvironment conditions, where the presence of a conditioning film may contribute to reducing repulsion; and the attractive/repulsive acid-base interactions (Kimkes and Heinemann, 2020).
(B: Left side) Pili-mediated temporal attachment. Pili elongation allows attachment to the surface, whereas pili retraction may cause the bacterium to be tugged toward the surface, reach different directions, change from horizontal to vertical (and vice versa) orientations by using different types of motility, or it may be released back.
(B: Right side) Flagella-mediated temporal attachment may be caused because of their hydrophobic nature, as well as by some of the flagellar motor components. When flagella become anchored to the surface, the polarly attached cells spin around, often leading to detachment of bacteria.
(C) Specific temporal attachment may be mediated by binding of adhesins, expressed onto the bacterial surface or at the tip of certain pili appendages, to particular host receptors.
Flagella can either be found as long helical filament(s) located outside the cell, like in P. aeruginosa (O'Toole and Kolter, 1998) or, residing within the periplasmic space (Nakamura and Minamino, 2019) such as in spirochetes like Borrelia burgdorferi (Kumar et al., 2017; Sapi et al., 2012). These structures allow bacterial motion toward a gradient of nutrients (chemotaxis-directed motility) (Yang et al., 2016) and other types of motility to achieve surface migration. Pili, on the other hand, are hair-like structures varied in composition, which surround the bacterial cell body to serve as virulence factors during infection (Proft and Baker, 2008). Pili are proteinaceous polymers composed by “pilin” subunits and are also involved in a bacterial locomotion style known as twitching motility (O'Toole and Kolter, 1998; Yang et al., 2016). Pili can be found both in Gram-negative and Gram-positive bacteria (Proft and Baker, 2008; Dramsi et al., 2006; Abbot et al., 2007). Gram-negative bacteria have five different types of pili where two members of the chaperone-usher pili family, type I and P pili, have an adhesin protein assembled at the tip: FimH and PapG, respectively (Hospenthal et al., 2017; Fronzes et al., 2008; Dodson et al., 2001; Choudhury et al., 1999). The amino-terminal lectin domain of these adhesins serves for the specific interaction of bacteria with host proteins (Hospenthal et al., 2017). Recently, type IV pili, a well-characterized bacterial structure in Gram-negative bacteria (Craig et al., 2004; Craig et al., 2006; O'Toole and Kolter, 1998), has been reported to be also present in a diversity of Gram-positive bacteria having motility and adherence functions as well as a role in biofilm formation (Imam et al., 2011; Varga et al., 2006; Varga et al., 2008).
Apart from the initial nonspecific physicochemical interactions (Figure 2A) and once surpassing the repulsive forces between bacteria and the surface, an unspecific or specific appendages-mediated temporal attachment occurs. The first (Figure 2B) may be mediated by pili polymerization to favor pili extension and attachment, as well as pili depolymerization events, for retraction and detachment from the surface (Merz et al., 2000; O'Toole and Kolter, 1998; Skerker and Berg, 2001; Sun et al., 2000). These mechanisms allow surface exploration by bacteria in horizontal (crawling motility) (Conrad et al., 2011; Merz et al., 2000) and/or vertical positions (walking motility) (Conrad et al., 2011; Gibiansky et al., 2010). It has also been shown that bacteria can change their direction by the release of single pilus (slingshot) while crawling (Jin et al., 2011) or alter their orientation with respect to the surface as a consequence of pili retraction where the cell is reoriented from a horizontal to an upright position, being able to switch between both of them (Conrad et al., 2011; Sangermani et al., 2019).
Flagella are also involved in surface exploration through swimming motility (Conrad et al., 2011), and as these are hydrophobic structures, they tend to adhere to surfaces of the same nature (Friedlander et al., 2015). Moreover, flagellar motor components have been reported to be involved in both the initial attachment and strong attachment stages of biofilm development (Schniederberend et al., 2019; Toutain et al., 2007). Some bacterial cells have been found spinning in a polar position, which suggests attachment of flagella to the substrate (Conrad et al., 2011; Sauer et al., 2002; Toutain et al., 2007), and it generally precedes cell detachment (Conrad et al., 2011). Depending on the function that needs to be achieved, any or a combination of the previous processes is carried out by the bacterium. For instance, in smooth and dry inert materials, such as glass and quartz, an unspecific appendage-mediated temporal attachment would require type IV pili-mediated extension, attachment, and retraction mechanisms, as observed in P. aeruginosa (Piepenbrink and Sundberg, 2016; Skerker and Berg, 2001). In the case of abiotic surfaces, like implants and medical devices, the initial bacterial adherence takes place within the formed conditioning film, which may be used by the attached bacteria as a source of nutrients (for review, see Khatoon et al., 2018). Specifically, conditioning films are thin biopolymeric coatings formed at the interface of a material surface and the aqueous medium provided by body fluids and secretions (Donlan, 2002). They can be also formed onto living tissues such as tooth enamel surfaces in the oral cavity (Marsh and Bradshaw, 1995). The presence of conditioning films consequently modifies the physicochemical properties of the surface influencing bacterial adherence (Herrmann et al., 1988; Lorite et al., 2011; Wang et al., 1993). Other factors including roughness, porosity, pore topology, charge, and hydrophobicity/hydrophilicity of the surface also influence bacterial adherence (Feng et al., 2015; Fletcher, 1988; Fletcher and Loeb, 1979; Friedlander et al., 2013; Singh et al., 2011).
Regarding the specific temporal attachment at this stage, bacteria utilize piliated and nonpiliated bacterial adhesins to mediate binding to host components, including extracellular matrix (ECM) proteins, such as collagen, fibrinogen, and fibronectin, as well as glycoproteins and glycolipid receptors (Gries et al., 2020; Lee et al., 1994; Pouttu et al., 1999; Tielker et al., 2005). The initial specific binding is a reversible attachment mechanism employed by bacteria to sense the surface microenvironment. In order to achieve this, bacterial cells use the swimming and twitching motilities provided by flagella and pili to explore and sense the local microenvironment (Berne et al., 2018; Ellison et al., 2017; Merz et al., 2000), transmit signals, and modulate gene expression (Li et al., 2012). For instance, the flagellar rotary motor of Caulobacter crescentus has been reported to be involved in surface adherence, where it functions as a mechanosensitive device to promote surface adaptation and anchoring (Hug et al., 2017). Bacteria at this stage are found attached by the cell pole (Agladze et al., 2005; Li et al., 2012), which enables them to be easily detached by retraction and spinning along their axis (Gibiansky et al., 2010; Merz et al., 2000).
Bacterial interactions with surfaces allow them not only to sense the surface but also to become progressively adapted to it by successive attachment-retraction events (Lee et al., 2018; Li et al., 2012). Bacterial sensing through structural elements (mechanosensing) includes adherence via appendages (Hoffman et al., 2015; Hug et al., 2017), surface proteins (Siryaporn et al., 2014), piliated adhesins (Thomas et al., 2002), cell wall deformation (Otto and Silhavy, 2002), and stimuli-receptor interactions (Lower et al., 2010).
Bacterial adhesion to the surface: increased adhesion strength
The mechanical cues experienced during surface sensing cause molecular alterations at the biochemical level (mechanotransduction), which gradually increase the attachment strength of the loosely adhered bacterial cells (Hug et al., 2017) (Figure 3). The intracellular signaling driven by mechanotransduction contributes to the production/expression of more adhesins to reinforce adhesion (Hug et al., 2017; Li et al., 2012).
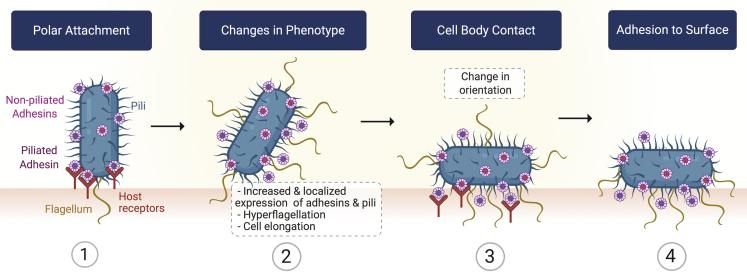
Figure 3.
Schematic representation for the functional steps involved in bacteria adhesion to a solid surface
Participation of bacterial appendages can be observed throughout the biofilm development stages. The interplay between pili and flagellum rotation has been reported to encourage the transition from a temporal bacterial attachment toward a stable, often called “irreversible,” state (Li et al., 2012). Particularly, some components of the bacteria flagellar motor contribute to reaching a stronger bacterial adhesion to the surface (Toutain et al., 2007). For recent reviews on bacteria-surface interactions, please refer to Berne et al. (2018) and Kimkes and Heinemann (2020). Furthermore, biofilm-forming bacteria experience changes in phenotype during biofilm development (Sauer et al., 2002; Southey-Pillig et al., 2005). For instance, it has been shown that upon surface sensing, some bacteria such as Vibrio parahaemolyticus switch from using a single polar flagellum to mixed flagellation by expressing multiple lateral flagella (Belas and Colwell, 1982). Proteus mirabilis bacteria, one of the main species responsible for catheter-associated urinary tract infections (Jacobsen et al., 2008), also show changes in phenotype after contact with solid surfaces such as an increased expression of lateral flagella and bacterial elongation (Fusco et al., 2017; Jones et al., 2004). Lateral flagella play important roles in cell adhesion, invasion, and biofilm formation as reported for Aeromonas species biofilms (Gavín et al., 2003; Gavín et al., 2002).
Moreover, together with the bacterial cell wall lipopolysaccharides (Chao and Zhang, 2011) and de novo production of proteins (Hinsa et al., 2003), flagella contribute to changes in the orientation of bacterial cells (Gu et al., 2016; Toutain et al., 2007) from a polar attachment to a flat position where the bacterial cell body directly contacts the surface enhancing adhesion strength. However, there are some bacterial species that do not require this change in orientation to reach a strong surface adhesion, as the case of C. crescentus which irreversibly attaches in a polar orientation by producing polarly localized adhesins (Li et al., 2012).
Consequences of bacterial adhesion to the surface
The first two stages of biofilm development serve bacteria to sense and evaluate the microenvironment of a potential target for colonization. Depending on the nature of the initial bacterial interactions with the surface, the subsequent mechanotransducive mechanisms determine whether planktonic bacteria will continue into the next stages of biofilm development or not (for review, see Stones and Krachler, 2016). If bacteria become adapted to the surface and microenvironmental conditions, other important alterations at the biochemical level take place. Some of these involve the activation of metabolic processes (Sauer and Camper, 2001), including protective metabolic pathways (Bhomkar et al., 2010; Sauer and Camper, 2001), regulation of two-component systems (Persat et al., 2015), and upregulation of virulence factors (Gode-Potratz et al., 2011; Persat et al., 2015) to evade and modulate host immune response, being bacterial adhesion a virulence factor per se (Busscher and van der Mei, 2012).
Implicit in the mechanisms required for surface recognition/interactions and acquired properties is the fact that these involve bacterial genetic expression of target genes for better responding to all the challenges and stressors that bacteria are subject to. Part of these events is mediated by the second messenger molecule bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP). This signaling molecule stimulates the biosynthesis of adhesins and extracellular polymeric substance (EPS) matrix components while reducing/inhibiting various forms of motility, contributing to the strong adhesion reached at this stage (for review, see Hengge, 2009). Moreover, at this point, bacteria not only become prepared to mount an adequate response for themselves or neighboring cells during the adsorption and adhesion stages but also has been reported that bacteria are able to “keep track” of these events retaining a multigenerational memory through the oscillations in the levels of the second messenger molecule cyclic adenosine 3′,5′-monophosphate (cAMP) and type IV pili activity (Lee et al., 2018). Finally, it has been recently reported that the biofilm forming environmental bacteria C. crescentus experience cell differentiation and speed up cell cycle progression after surface sensing (Snyder et al., 2020).
Cell growth and division: formation of microcolonies
Once a strong bacteria-to-surface adhesion has been achieved, attached bacteria grow and divide either by binary fission (Costerton et al., 1995; Read et al., 1989) or asymmetric division (Conrad et al., 2011; Laventie et al., 2019) (Figure 4). This implies cell proliferation and colonization of the surface, which leads to activation of second messengers, intercellular communication, and initial secretion of EPS matrix (Sauer and Camper, 2001). When the “monolayer biofilm” has been formed, any or a combination of three potential mechanisms may follow:
-
(1)
Additional planktonic bacteria are recruited from the bulk solution via agglutinins, such as Staphylococcus aureus surface protein G (Geoghegan et al., 2010) and the aggregative adherence fimbriae in E. coli (Nataro et al., 1992);
-
(2)
One of the planktonic bacteria that resulted from cell division detaches/moves away from the surface and potentially follows any of two pathways: land onto (or become attracted to) a recently formed layer of cells or stay free to initiate the colonization of other sites (Conrad et al., 2011; Laventie et al., 2019);
-
(3)
The initiation of biofilm formation occurs in the absence of a substrate, and the aggregated bacterial cells then land directly onto the monolayer biofilm or onto a noncolonized surface for further biofilm development (Kragh et al., 2016). The terms “flocks” and “clusters” are used to refer to autoaggregated (same species) or coaggregated (different species) bacteria in suspension. Hence, biofilm formation does not necessarily require a solid surface in order to succeed, and this is observed in infections with biofilm-related etiology, including cystic fibrosis (Bjarnsholt et al., 2009) and rhinosinusitis (Cryer et al., 2004).
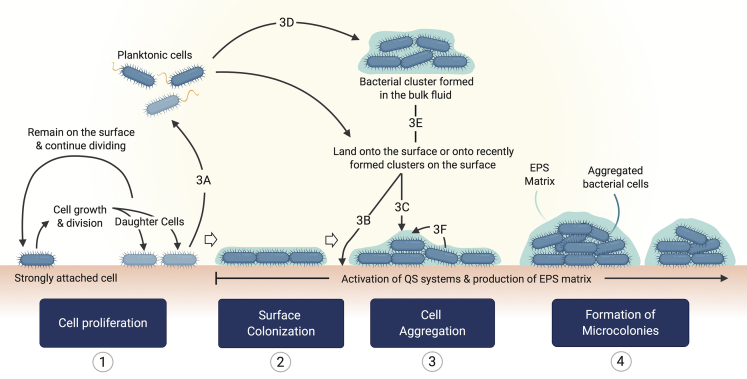
Figure 4.
Potential routes for the formation of microcolonies
Microcolony formation arises from the accumulation of cells in continuous growth and division and may be enhanced by the incorporation of planktonic cells from the bulk fluid or product of cell division and the integration of bacterial clusters. (1) Once bacteria become strongly attached, the colonization of the surface takes place by means of cell growth and division. One of the daughter cells may remain attached, and the other may be released from the surface (3A), where it becomes free to colonize other sites (2) by landing onto target surfaces (3B) or, it may become part of recently formed bacterial clusters either on the surface (3C) or the bulk fluid (3D). Bacterial clusters formed in the absence of a solid substrate may colonize new surfaces or land onto biofilms under development (3E). During cell proliferation and biofilm formation, QS signals and production of EPS matrix occur. As cell density increases, some of the bacteria slide along each other (3F) leading to the formation of small bacterial aggregates, which correspond to “immature” biofilms known as microcolonies (4).
Moreover, some reports suggest that cell aggregation mediated by either substrate attachment or occurring substrate free do not exclude each other, and both can occur simultaneously as shown for joint implant-associated infections (Stoodley et al., 2011; Stoodley et al., 2008). A number of agglutinins contribute to cell aggregation depending on the bacterial species, and although they are mainly proteinaceous, such as pili, flagella, M proteins, microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), and β-barrel proteins; some other molecules such as carbohydrates (for review, see Trunk et al., 2018) and even the extracellular DNA (eDNA) act as agglutinin (Das et al., 2010).
Cellular aggregation by any of the aforementioned mechanisms leads to the formation of bacterial clusters known as microcolonies. As microcolonies are being formed, the initial single layer of attached cells become multilayered by the accumulation of cells, causing an increase in thickness, which leads to the transition from a two-dimensional to a 3D arrangement (Su et al., 2012). In order to reach this spatial distribution, bacteria must communicate chemically with each other and express particular genes for the secretion of an EPS matrix, which will serve as a structural support while also allowing cellular communication for the stabilization of microcolonies and further biofilm network (Dominiak et al., 2011; Koo et al., 2010; Xiao and Koo, 2010).
Furthermore, a role in the formation of microcolonies has been attributed to type IV pili-mediated twitching motility (O'Toole and Kolter, 1998) and the poly-N-acetyl-glucosamine (PNAG) produced by some species, including S. epidermis (Gerke et al., 1998) and E. coli (Amini et al., 2009). At this stage, microcolonies may be able to move as a whole entity across the surface, as shown by P. aeruginosa (O'Toole and Kolter, 1998). The ability of small clusters to displace themselves onto a surface and fuse with each other to form microcolonies has been described by Haagensen et al. (2015) for Acinetobacter sp. strain C6. These representative examples invite us to be cautious and avoid labeling this, and the previous strong adhesion stages as “irreversible attachment” of bacterial cells as this may not be accurate.
Production of EPS matrix
Following adhesion, bacteria secrete an EPS matrix, which contributes in different ways to biofilm development, being particularly important for the formation of microcolonies where it serves as a structural support by filling the space between cells and covering them all over. Hence, the EPS matrix assists in the multilayer arrangement of bacterial cells, acting as an interphase with the surrounding microenvironment (Allison and Sutherland, 1987; Yang et al., 2011). Although the biofilm composition is variable depending on the microorganism(s) involved, biofilm maturation stage, and microenvironmental conditions, most biofilms are constituted by approximately 50%–90% EPS matrix (Frølund et al., 1996; Nielsen et al., 1997). The remaining percentage corresponds to the embedded microorganisms, which, although reduced compared with the EPS matrix, have a cell density that can be considered as high given that biofilm development usually starts from single or few cells and reaches cell numbers of a few thousands, as shown in studies of single-cell tracking (Drescher et al., 2016; Paula et al., 2020).
Molecular components of the EPS matrix
Chemical composition of the EPS matrix varies along with the biofilm development process (Chao and Zhang, 2012), and it is influenced by the composition of the environment, temperature, physical and mechanical stress, and cellular interactions (Flemming et al., 2016). Biofilm EPS matrix is mainly composed of water, which accounts for up to 97% of its content (Zhang et al., 1998). The molecular elements that confer the structural and functional properties of the EPS matrix are contained in the remaining 3%. Among these, polysaccharides usually represent the major component (Wingender et al., 2001), including exopolysaccharides (such as PNAG (Roux et al., 2015)), lipo-associated and wall-associated teichoic acids (Gross et al., 2001), and cellulose (Zogaj et al., 2001). Proteins are the second major EPS matrix component, although in some cases they have been reported to be present in larger proportion than polysaccharides (Jahn et al., 1999). Other EPS matrix components include eDNA (Whitchurch et al., 2002) and ions (Wang et al., 2019). Also, extracellular proteinaceous bacterial structures such as pili and flagella (van Schaik et al., 2005), together with eDNA (Jennings et al., 2015) and curli (amyloid structures) (Romero et al., 2010), serve as structural elements that contribute to stabilize and strengthen the EPS matrix.
EPS matrix functions
The EPS matrix fills in the space between the microbes forming the biofilm, facilitating cell-cell adhesion (Romero et al., 2010) and bringing cells in close proximity to allow intercellular interactions and horizontal gene transfer (Hausner and Wuertz, 1999). The rich and heterogeneous infrastructure of the EPS matrix provides the required elements to confer the architecture and mechanical stability of biofilms. Moreover, it shields and protects the inner bacteria from (1) the external microenvironment (Izano et al., 2007; Mulcahy et al., 2008), (2) the host immune response (discussed in the next sections), and (3) protozoan grazing (Matz et al., 2004).
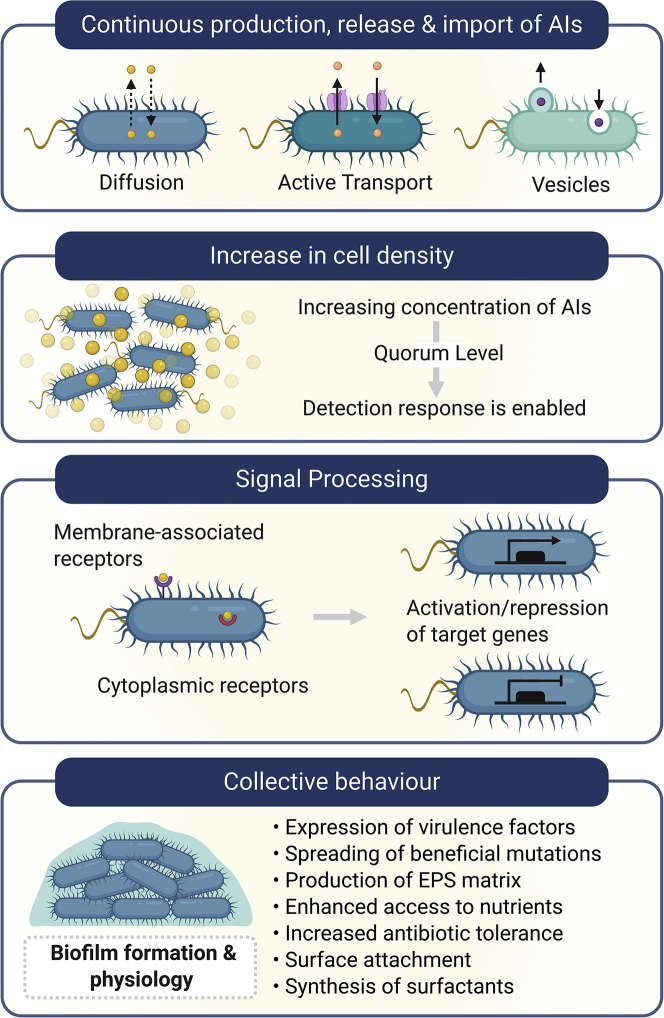
Cell-to-cell communication enhances biofilm development
The effective communication between bacterial cells at this stage is a key factor for the transition from a fairly simple aggregated state into the initial bacterial arrangements, which will further develop into a complex and well-structured community style of living: the biofilm. For this purpose, a cell-to-cell communication mechanism known as quorum sensing (QS) is utilized by bacteria to collectively adapt through the activation/repression of target genes (Fuqua et al., 1994). This communication is mediated by small diffusible or transported molecules, collectively known as autoinducers (AIs), which are continuously being produced by bacteria (Pearson et al., 1999). As cell density increases, AIs accumulate in the surroundings (Figure 5). Once this increase reaches a threshold concentration, often referred to as quorum level, response regulator proteins part of the QS systems become activated by binding to AI molecules, leading to the modulation of gene transcription (for review, see Haque et al., 2019). As a consequence of this intercellular communication mechanism, bacteria become synchronized to express particular QS phenotypic profiles. There are several QS systems that may be shared between different bacterial species or may be specific for certain species in particular (for review, see Haque et al., 2019 and Waters and Bassler, 2005). It has been widely reported that QS contributes to biofilm development, from the formation of microcolonies to biofilm maturation and dispersal (Boles and Horswill, 2008; Davies et al., 1998; de Kievit et al., 2001), mainly by influencing its architecture and promoting a series of beneficial phenotypic traits. Some of the biofilm-associated collective behaviors influenced by QS include the spread of beneficial mutations such as the regulation of EPS matrix production (Sakuragi and Kolter, 2007), modulation of nutrient utilization (An et al., 2014), expression of virulence factors (Erickson et al., 2002; O'Loughlin et al., 2013), synthesis of biosurfactants (Pearson et al., 1997), and regulation of antimicrobial resistance (Shih and Huang, 2002; Zhao et al., 2020).
Figure 5.
QS influences biofilm formation and physiology
AIs are continuously being produced by bacteria; they constantly move in and out of the cell by diffusion, active transport, or into vesicles, depending on their chemical nature. When the concentration of AIs reaches a threshold level as consequence of increased cell density, the detection response is enabled, and they bound to the corresponding membrane-associated or intracellular receptors, activating these response regulator proteins. As a consequence, signal processing leads to collective transcription of target genes to produce a communal behavior of the bacterial population. One of the potential responses is the expression of a series of beneficial traits involved in biofilm physiology.
Biofilm maturation
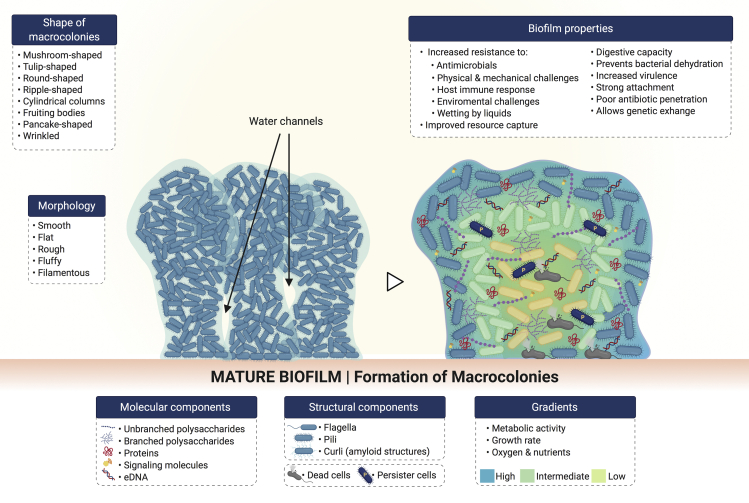
The regulation of biofilm EPS matrix production by second messenger molecules and QS systems at this stage contributes to reaching a stable biofilm structure (Colvin et al., 2012). After the formation of microcolonies, cell proliferation and aggregation of bacteria continues increasing along with the synthesis of EPS until reaching an optimal cell density, as regulated by QS. At this point, multiple layers form the microcolonies, and these acquire complex structural features through adhesive and disruptive processes (O'Toole et al., 2000). Here, bacterial microcolonies spread out forming larger aggregates that are referred to as “macrocolonies” or “towers” (Gupta et al., 2016; Ha and O'Toole, 2015), causing the biofilm to thicken (Figure 6). Biofilm thickness is variable depending on the species, substrate, time required for maturation, microenvironment conditions such as nutrient availability and shear flow, as well as the methods applied for its determination (Heydorn et al., 2000a; Heydorn et al., 2000b; Janakiraman et al., 2009; Jean et al., 2004; Meyer et al., 2011; Suarez et al., 2019). Most biofilms are found to be multispecies, where each microorganism influences the morphology and architecture of the resulting biofilm; which may be different from monospecies biofilms. For instance, the mean thickness of in vitro monospecies biofilms of P. aeruginosa and Klepsiella pneumoniae was found to be 29 and 100 μm, respectively; whereas a multispecies biofilm composed of both microorganisms reached a mean thickness of 400 μm (Murga et al., 1995). Furthermore, most of the time, a mature biofilm is characterized by sessile EPS matrix-enclosed macrocolonies that are separated by a well-defined network of channels.
Figure 6.
Morphology and architecture of mature biofilms
Molecular and structural components contribute to the 3D spatial organization and physiology of biofilms. Phenotypically different bacteria are found along the gradients observed in mature biofilms.
Biofilm morphology and architecture
Biofilms are highly heterogeneous in their 3D structure, morphology, and physiology (for review, see Evans et al., 2020 and Stewart and Franklin, 2008). Depending on their chemical and structural composition, their morphology may be smooth and flat, rough, fluffy, or filamentous (Flemming and Wingender, 2010). Pores, channels for gas and nutrient exchange, and dense areas of packed cells are the main components of the mature biofilm structure (Lawrence et al., 1991; Robinson et al., 1984; Stoodley et al., 1994). It has been reported that channels within the biofilm are formed by substances secreted by bacteria to degrade and remodel the biofilm EPS matrix. Some of them include nucleases (Seper et al., 2011) and detergent-like molecules (Periasamy et al., 2012). Moreover, specialized bacterial subpopulations known as “swimmers” have been reported as capable to swim at high speed within the biofilm leading to the formation of transient pores to increase nutrient flow (Houry et al., 2012). Biofilm morphology and structural components and arrangements contribute to the formation of mature biofilms in a variety of architectures, from flat and undifferentiated biofilms (Chung et al., 2014) to the classic tall mushroom-like macrocolonies found in the biofilm model microorganism P. aeruginosa (Klausen et al., 2003). For a recent review on biofilm architecture, please refer to the study by Kassinger and van Hoek (2020).
Gradients within the biofilm
Bacterial cells occupy different regions within the biofilm architecture, and as a consequence, their proximity to the external microenvironment, access to nutrients, interactions with the host immune defenses, and the forces they are subject to are variable. Hence, as the microenvironment and requirements are different for bacteria in different regions, the presence of gradients is observed within the biofilm (Figure 6). Some of them include gradients in terms of growth rate (Wentland et al., 1996), metabolic activity (Walters et al., 2003), oxygen concentration (de Beer et al., 1994; Walters et al., 2003), and pH (Hunter and Beveridge, 2005). For instance, bacterial cells in the inner biofilm regions show reduced metabolic activity (Walters et al., 2003), which provides them with the beneficial property of being resistant to conventional antibiotics (for review, see Høiby et al., 2010). Moreover, genetic diversity is found among biofilm-living bacteria, which is observed by the presence of bacterial subpopulations showing different phenotypes (Boles et al., 2004; Vlamakis et al., 2008). A cell type known as “persister cells,” was reported two decades ago in P. aeruginosa biofilms (Brooun et al., 2000; Spoering and Lewis, 2001). Persister cells were in the stationary phase of the cell life cycle, assuming a dormant phenotype where they neither grow nor divide, exhibiting a high antibiotic tolerance. The current thought is that such cells may be ubiquitous among bacterial species (Fisher et al., 2017), being able to “survive treatment by all known antimicrobials” (Lewis, 2007). Although persisters show a reduced metabolic activity, they have the ability of becoming active and restarting growth once the stressor has been removed (Roberts and Stewart, 2005). This represents a great advantage not only for antimicrobial resistance and infection chronicity but also further spread and colonization. Furthermore, a second type of dormant cells with increased resistance to antimicrobials has been reported for a wide range of bacteria, with Staphylococcus being one of the most studied microorganisms (for review, see Kahl et al., 2016). These cells are referred to as “small colony variants” because of their reduced size: one-tenth of the size of the colonies formed by their wild-type counterparts (Proctor et al., 2006). Small colony variants have been reported to be strong biofilm producers (Malone, 2015) in chronic and implant-related infections (Baddour and Christensen, 1987; Fauvart et al., 2011; Häußler, et al., 2003).
Living in a biofilm community: microbial interactions
Most biofilms are composed by more than one species living in close proximity. As consequence, social interactions arise among them in order to succeed and ensure biofilm formation as a strategy to withstand the effects of different stressors. Microbial interactions in multispecies and interkingdom biofilms have been widely reviewed (Krüger et al., 2019; Shirtliff et al., 2009; Wargo and Hogan, 2006). Here, we present a general classification of microbial interactions and their spatial arrangement within the biofilm rather than discussing particular examples of each one. Microbial interactions in mixed biofilms occur through competition (Fazli et al., 2009), cooperation (Castonguay et al., 2006; Paula et al., 2020), and chemical communication (Riedel et al., 2001), being not mutually exclusive. Taking into consideration that multispecies and interkingdom biofilms are found colonizing a wide variety of environments, it is inviting to assume that although antagonistic interactions occur to some degree during biofilm formation for certain species, synergistic cooperative interactions may predominate (for review, see Elias and Banin, 2012). As previously mentioned, both host microenvironment-bacteria and bacteria-bacteria interactions result in genetic modifications to adapt and respond as required. It has been reported for environmental (Gao et al., 2016) and health-related biofilms (Jakubovics, 2015) that intercellular interactions are responsible for the regulation of genetic expression in a coordinated fashion to modulate not only cell density, antimicrobial resistance, and virulence factors but also the spatial distribution of the species within the biofilm.
Microbial spatial distribution can be explained as three different modes:
-
(1)
Spatial segregation, where each microorganism produces its own microcolony because of competitive or weakly cooperative interactions resulting in formation of monospecies microcolonies (Kara et al., 2007);
-
(2)
Spatial intermixing, where both species are found coaggregated as result of strong cooperation, or one microorganism being exploited by the other (Kim et al., 2020); and
-
(3)
Spatial stratification, where the microorganisms are found forming different layers within the biofilm as a consequence of either strongly cooperative or strongly competitive interactions (Fazli et al., 2009; Kim et al., 2020). Liu et al. (2016) reviewed the influence of microbial interactions on spatial distribution in multispecies biofilms (Liu et al., 2016).
Moreover, the nature of microbial interactions is also dependant on the bacterial timing of colonization. For instance, in a model of dental biofilm, the interactions between Streptococcus mutans and Streptococcus sanguinis were competitive if one species colonized the surface before the other; whereas both species were able to coexist when there was a simultaneous colonization (Kreth et al., 2005).
Bacterial cells and clusters are dispersed from the biofilm
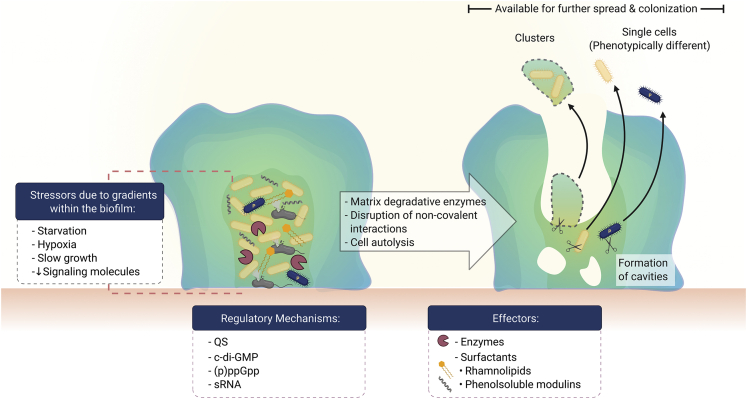
At some point after reaching maturity, biofilms suffer a partial structural loss that may occur by detachment and/or dispersion (Figure 7). Detachment involves four mechanisms that imply the release or loss of biofilm portions as a consequence of mechanical and shear stress (abrasion, erosion, and sloughing) as well as the impact of immune attack from the host (grazing) (for review, see Petrova and Sauer, 2016). Dispersion, on the other hand, implies more than being torn away from the biofilm by external stressors; it requires the sensing and processing of particular signals, which culminate in the expression of the corresponding physiological alterations (for review, see Rumbaugh and Sauer, 2020).
Figure 7.
Biofilm dispersal
Bacterial regulatory mechanisms drive biofilm disruption for the release of individual cells and bacterial clusters into the bulk fluid where they become available for further spread and colonization.
The inputs that prompt biofilm dispersal (Figure 7) may arise within the biofilm or may be induced in response to alterations in the microenvironment, such as variations in nitric oxide levels (Barraud et al., 2009), oxygen tension (Thormann et al., 2005), temperature (Kaplan and Fine, 2002), and changes in the availability of nutrients (Sauer et al., 2004). Furthermore, the gradients that were initially useful during biofilm differentiation to allow bacterial survival and optimization of available resources now become dispersion cues from within the biofilm. These gradients cause bacteria in the deepest zones to become exhausted because of the stress generated by starvation, hypoxia, scarce signaling molecules, and slow growth. As a consequence, these stressors cause the activation of regulatory mechanisms, including QS (Boles and Horswill, 2008), c-di-GMP (An et al., 2010; Barraud et al., 2009; Gjermansen et al., 2010), small regulatory RNAs (sRNAs) (Chua et al., 2014), cis-2-decenoic acid (Davies and Marques, 2009), and the stringent response mediators guanosine tetraphosphate and guanosine pentaphosphate (collectively known as (p)ppGpp) (Díaz-Salazar et al., 2017). Produced signals may culminate in events and self-produced bacterial substances that contribute to the remodeling process of the biofilm structure at this stage. Some of them include the production and release of enzymes to degrade the EPS matrix components (Boles and Horswill, 2008); disruption of noncovalent interactions by bacterial surfactants such as rhamnolipids (Boles et al., 2005) and phenol-soluble modulins (Wang et al., 2011); and cell death, which leads to the formation of cavities within the biofilm (Ma et al., 2009). The latter has been reported to be used by motile bacteria as points of escape from the biofilm and is often referred to as “central hollowing” or “seeding dispersal” (Ma et al., 2009; Purevdorj-Gage et al., 2005; Sauer et al., 2002). In general, individual cells or cell clusters may be released from a mature biofilm (Stoodley et al., 2001), and these become available for further spread and colonization (Figure 7).
The aforementioned mechanisms highlight the fact that biofilm dispersion is a well-regulated process affecting particular cells and regions within the biofilm. Furthermore, the dispersed cells have the advantage of having gone through changes in phenotype during biofilm development as well as further genetic modifications after the stress conditions generated by biofilm gradients. These modifications that once permitted them to express a more virulent and resistant phenotype are partially maintained; hence, dispersed cells are more virulent than their planktonic counterparts but less than those within the parent biofilm (Chua et al., 2014).
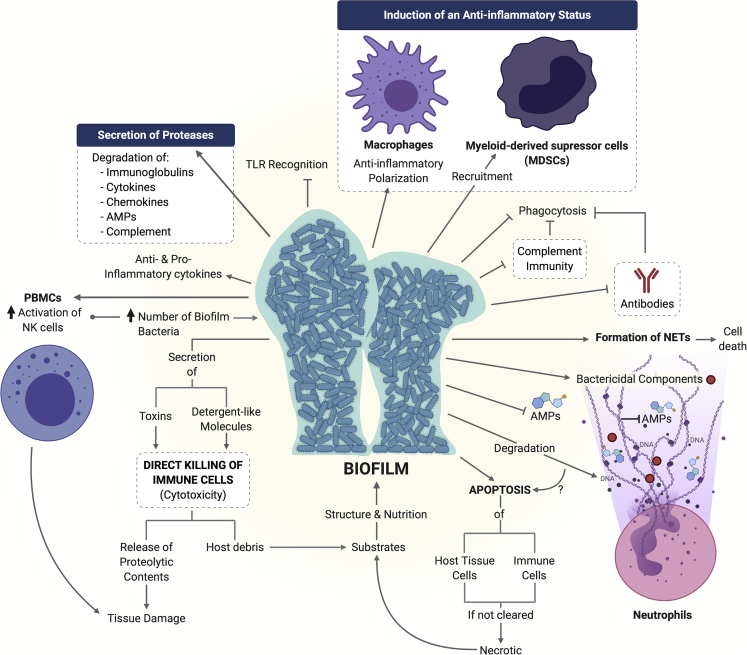
Biofilms modulate and evade immune responses from the host
As any other element recognized as foreign by the immune system, biofilm formation elicits the activation of different cells and mechanisms intended to clear it from the host. Although immune cells and mediators offer a wide variety of strategies to prevent pathogenic microbial invasion, biofilm-living microorganisms as well as biofilm molecular and structural components have the ability of modulating and evading immune attack (Figure 8). Some of the main mechanisms that contribute to this immune regulation include the following:
-
(1)
Activation and/or impaired activity of innate immune cells such as monocytes/macrophages (Kaya et al., 2020), neutrophils (Hong et al., 2009), and natural killer cells (Kaya et al., 2020);
-
(2)
Induction of an increased secretion of proinflammatory and anti-inflammatory cytokines (Kaya et al., 2020);
-
(3)
Modulation of recognition by toll-like receptors (TLRs) (Shang et al., 2019; Thurlow et al., 2011);
-
(4)
Shielding of epitopes recognized by immunoglobulins and impaired opsonization (Kristian et al., 2008; Langereis and Weiser, 2014);
-
(5)
Hindered complement activity (Kristian et al., 2008; Langereis and Weiser, 2014);
-
(6)
Impaired phagocytosis (Rose and Bermudez, 2014; Scherr et al., 2015; Thurlow et al., 2011);
-
(7)
Secretion of proteases that contribute to the degradation of cytokines (Fletcher et al., 1998; Mochizuki et al., 2014), chemokines (Mochizuki et al., 2014), complement proteins (Hong and Ghebrehiwet, 1992), and antimicrobial peptides (AMPs) (Maisetta et al., 2011);
-
(8)
Induction of an anti-inflammatory status by recruitment of myeloid-derived suppressor cells (Heim et al., 2014) and skewing of macrophage polarization toward an M2 anti-inflammatory status (Thurlow et al., 2011); and
-
(9)
Direct killing of immune cells by the secretion of toxins (Scherr et al., 2015) and detergent-like molecules (Jensen et al., 2007; Wang et al., 2007), causing the release of intracellular components of immune cells, which in turn may lead to host tissue damage and further cytotoxicity (Parks et al., 2009).
Figure 8.
Biofilm mechanisms of immune evasion
Some reported strategies utilized by different biofilms to overcome and modulate host innate and adaptive immune responses are shown.
Neutrophils are one of the innate immune system cells that play an active role during the initial stages of biofilm development. They are stimulated by biofilms to produce neutrophil extracellular traps (NETs) (Bhattacharya et al., 2018; Oveisi et al., 2019), one of the killing mechanisms of these immune cells. However, NETs are ineffective for biofilm clearance (Bhattacharya et al., 2018; Hong et al., 2009) as their main constituents, namely DNA, AMPs, and other microbicidal substances, are degraded by biofilm components (Bryzek et al., 2019; Sultan et al., 2019). Moreover, formation of NETs may lead to death of neutrophils (Desai et al., 2016). If cell death occurs (Bhattacharya et al., 2018), the released products may contribute to host tissue damage (Manzenreiter et al., 2012) and/or biofilm formation (Walker et al., 2005). Furthermore, biofilms can also induce programmed cell death (apoptosis) in immune cells as well as other host tissues (Rose and Bermudez, 2014; Schwarzer et al., 2012; Singh et al., 2019b; Tateda et al., 2003). Finally, debris from dead cells may serve as nutritional and structural substrates for further biofilm development (Parks et al., 2009; Walker et al., 2005), in the same manner as dead bacterial cells within the biofilm do (López et al., 2009).
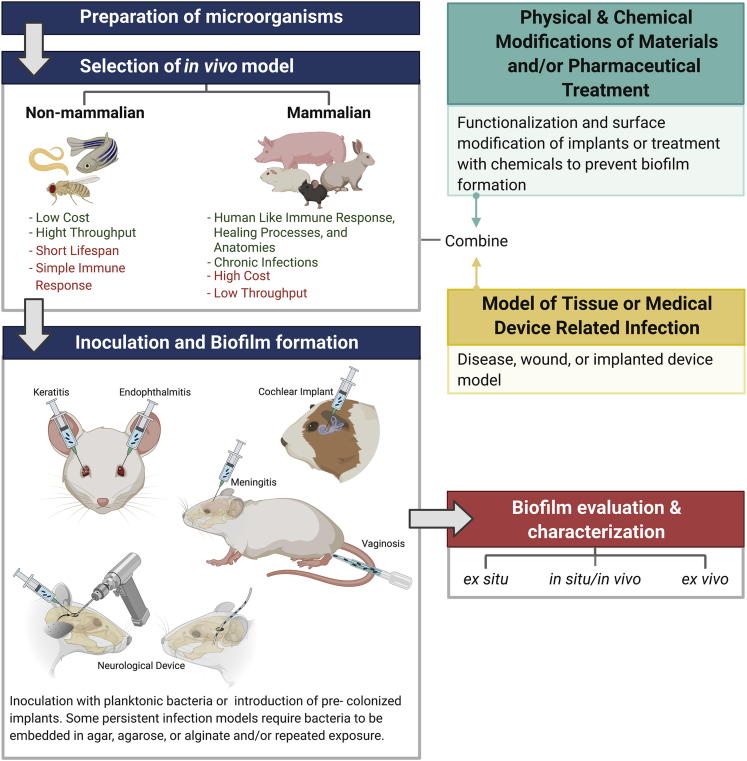
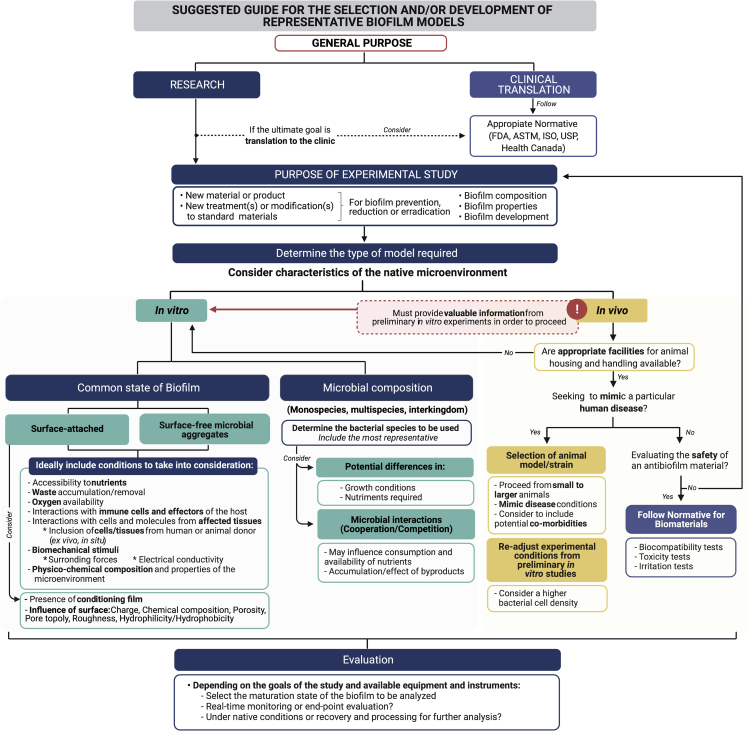
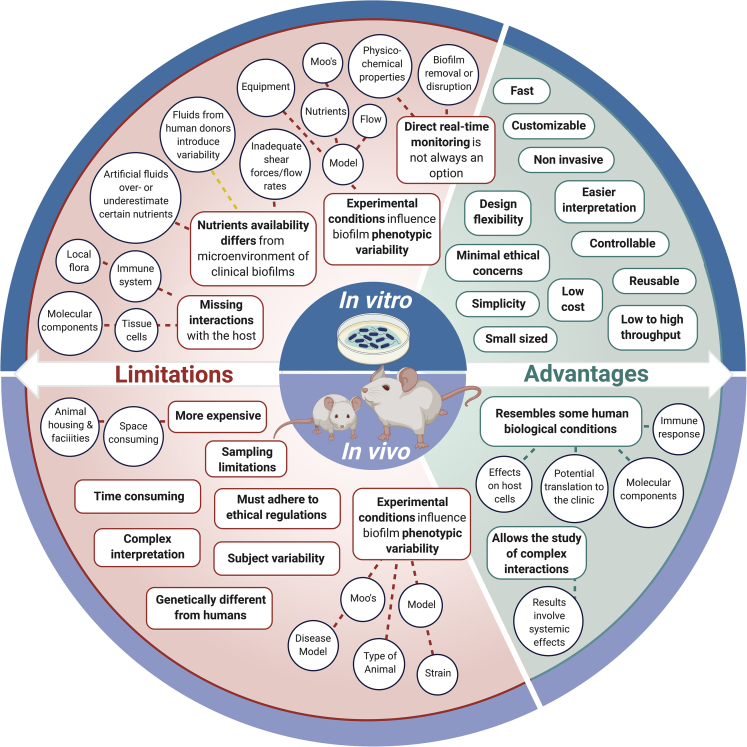
A meaningful part of the knowledge we currently have about biofilms, including their formation mechanisms, development stages, and the formulation of possible antibiotic or antibiofilm strategies, comes from biofilm models, which have been developed both in vitro or in vivo; each one with its own characteristics, uses, advantages, and drawbacks.
In vitro models for biofilm assessment
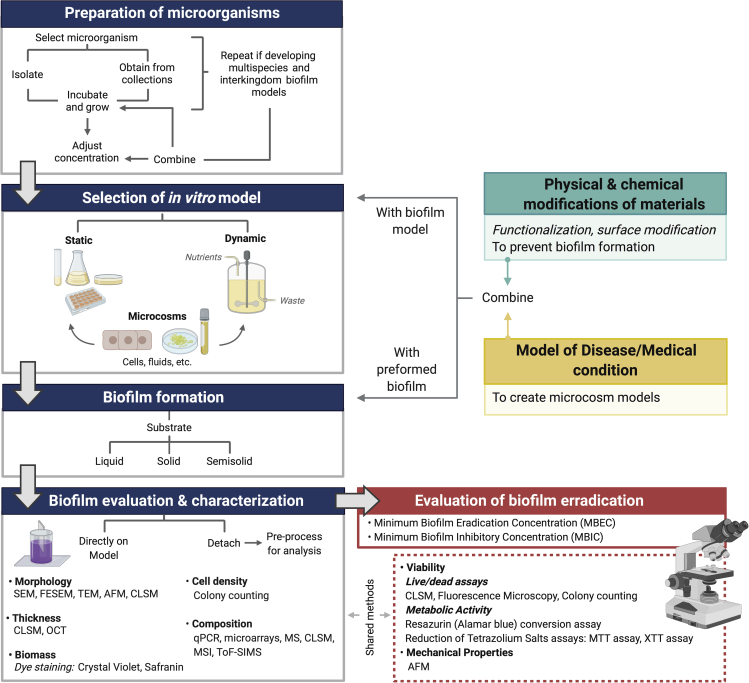
In vitro biofilm models (which do not use a living host) are used as the first step in testing new materials and methods meant to interact with biofilms. In vitro work is relatively simple, inexpensive, and high throughput compared with animal models, which are often used to further test promising candidates obtained from the use of in vitro models. The key difference between in vivo and in vitro models is the presence of the host immune system, but other important differences such as lower oxygen availability in many in vivo conditions are often unaddressed. In vitro biofilm models range from simple glass slides and Petri dishes to more elaborated systems that consider the physicochemical and mechanical properties required when trying to replicate biofilm formation in particular settings. More complex in vitro biofilm models may also consider cellular interactions with the host as well as the incorporation of monitoring systems for real-time assessment of biofilm formation. The variety of in vitro biofilm models can be classified into two main groups (Table 1) depending on the availability of nutrients over time:
-
(1)
Static or closed models: Nutrients and oxygen supply are limited to the initially provided conditions, or they are periodically renewed after removal of waste products (batch cultures);
-
(2)
Dynamic or open models: A continuous nutrient supply and waste removal over time are provided. Moreover, there is also the possibility to improve traditional in vitro biofilm models by translating them into microcosm models, either in static or dynamic setups, by including cells and/or substances that are typically found in the condition intended to be simulated. For instance, the incorporation of saliva for oral biofilm studies (Guggenheim et al., 2001) and supplementation of culture media with plasma and red blood cells to develop a chronic wound biofilm model (Sun et al., 2008) have been reported.
Table 1.
General characteristics of in vitro static and dynamic biofilm models
| Static |
Dynamic |
|
|---|---|---|
 |
 |
|
| Nutrient supply | Finite (may be replenished periodically) | Continuous |
| Flow | No | Yes, over the biofilm surface |
| Shear stress | Null to mild (if shaken) | Adjustable: Different hydrodynamic conditions can be adapted |
| Waste and planktonic cells | Accumulated | Removed as nutrients are replenished |
| Length of experiments | Short (days) | Long (from days to weeks) |
| Throughput screening | High: Different species/strains and conditions can be simultaneously evaluated | Low to medium |
| Technical complexity | Low | Medium to high |
| Complexity of setup procedures | None | Considerable, time consuming |
| Specialized equipment | Little (requires mostly common laboratory equipment) | Required (e.g., reactors or fermenting systems) |
| Cost per replicate | Low | Medium to high |
| Particularly useful for | Early stages of biofilm development Genetic screening |
Study of mature biofilms |
Currently used in vitro biofilm models
The currently used and well-established in vitro biofilm models have been extensively reviewed (Azeredo et al., 2017; Coenye and Nelis, 2010; Lebeaux et al., 2013). Biofilm formation in tubes and glass slides as models is not usually included because of their simplicity. However, it is important to acknowledge their significance and contribution during the initial biofilm experimental studies, which took place several decades ago. Particularly, the earliest reported custom-made device to analyze biofilm formation in vitro consisted of a lead and wood carrier suitable to hold glass slides, which was then submerged into seawater to study the initial stages of fouling (Zobell and Allen, 1935). Aside from this report, the development of more elaborate in vitro biofilm models really began in the 80s with the fabrication of the modified Robbins device (McCoy et al., 1981), the constant depth film fermenter (Peters and Wimpenny, 1988), the perfused biofilm fermenter (Gilbert et al., 1989), flow cell biofilm reactors (Caldwell and Lawrence, 1986; Pedersen, 1982), and the still widely used microtiter plate (Christensen et al., 1985). Several biofilm models and devices were proposed during the 90s, 2000s, and early 2010s; including the multiplaque artificial mouth (Sissons et al., 1991), drip flow reactor (Xu et al., 1998), Calgary biofilm device (CBD) (Ceri et al., 1999), colony biofilm model (Anderl et al., 2000), rotating disk reactor (Pitts et al., 2001), CDC biofilm reactor (Donlan et al., 2004), the Kadouri drip-fed biofilm assay (Merritt et al., 2005), the biofilm ring test (Chavant et al., 2007), microfluidic biochips (Lee et al., 2008; Richter et al., 2007), and the BioFlux device (Fluxion Biosciences) (Benoit et al., 2010). Although each of them offers different types of improvements for growing and studying biofilms, all of them present inherent disadvantages and limitations (for review, see Lebeaux et al., 2013). For instance, oral biofilms are widely studied because of their clinical relevance, as they are associated with several oral diseases, such as periodontitis (Socransky et al., 1998). The Zurich model was developed in 2001 for the study of oral multispecies biofilms under controlled static conditions (Guggenheim et al., 2001). Since then, because of its simplicity and despite its limitations, this model has allowed several methodological modifications to increase the complexity of biofilm microbial composition (Ammann et al., 2012), microbial dynamics (Thurnheer et al., 2016), and to include potential cellular and molecular interactions with the host (Belibasakis et al., 2015; Belibasakis et al., 2013).
Because of the fact that there is not an ideal biofilm model and since a reproducible model suitable for evaluating the efficacy of potential antimicrobials is essential, the use of standardized methods for specific biofilm devices and particular biofilm-producing bacteria has been proposed as an alternative. Specifically, the American Society for Testing and Materials (ASTM International) has implemented standardized test methods to provide the guidelines and specifications for the accurate and reproducible formation of biofilms and testing of antimicrobial substances. Four types of biofilm devices are associated to one or more ASTM standardized methods. The drip flow reactor and rotating disk reactor are used to evaluate biofilm formation in a continuous flow under low and medium shear stress, respectively, following their associated standard methods (ASTM E2647-20 and ASTM E2196-17) (ASTM, 2017a; ASTM, 2020). For evaluating disinfectant capacity, the CBD (ASTM E2799-17) and the CDC biofilm reactor (ASTM E2562-17, ASTM E3161-18, ASTM E2871-19) are utilized (ASTM, 2017c; ASTM, 2019; ASTM, 2017b; ASTM, 2018a). The colony biofilm model has also been recently adapted to develop a standard test method (ASTM E3180-18) to grow and quantify Bacillus subtilis biofilms (ASTM, 2018b). These standardized methods are mainly used for environmental biofilms, and there is still a need for standardized models, methods, and devices suitable for the evaluation of medical-related biofilms despite their potential associated limitations (Coenye et al., 2018; Malone et al., 2017b).
The majority of scientific reports regarding the study of biofilm development and evaluation of different antimicrobial substances and methodological approaches employ biofilm models that have been used for several decades, where most of the time only slight modifications or adaptations have been proposed. The two most popular in vitro biofilm models (by number of citations) are the microtiter plate and CBD, followed by flow cells. This prevalence may be explained by their simplicity and availability. Because of the great diversity of biofilm-related infections and biofilm formation methods and devices used; there is not a unique standard method for the experimental design of these studies. Instead, an open, flexible, and adaptable methodology (Figure 9) for studying biofilms allows scientists to explore and analyze them depending on the objectives being pursued.
Figure 9.
Schematic depiction for the overall steps involved in the current standard in vitro protocols of biofilm formation
Blue heading boxes show the general methodology followed in studies of biofilm development; same procedure is utilized when investigating the efficacy of biofilm eradication strategies (red box). Moreover, if prevention of biofilm formation is the goal of the study (green box) or if microcosm models are required (yellow box), functionalization/modification of materials and development of the appropriate disease models are respectively needed. After this, the materials under evaluation and the disease models may be combined with the chosen in vitro model for biofilm formation or with a preformed biofilm. Evaluation and characterization of the biofilm may be done directly on the model or detachment may be required for processing and analysis, depending on the chosen methods. Representative examples of the methods and techniques used for characterizing biofilms are shown; some of them are also used to evaluate the effect of antibiofilm strategies. SEM, scanning electron microscopy; FESEM, field emission SEM; TEM, transmission electron microscopy; AFM, atomic force microscopy; OCT, optical coherence tomography; qPCR, quantitative real-time PCR; MS, mass spectrometry; MSI, MS imaging; ToF-SIMS, time-of-flight secondary ion MS.
Recently developed (from 2015 to date) in vitro biofilm models
Because of the high volume of biofilm models reported as “new” or “novel” in the literature, we have selected the models discussed in the following subsections by having an inclusion criteria limited to studies published from 2015 to date, which include a unique or an innovative way of biofilm formation and/or offer an improved biofilm monitoring and evaluation strategy. Also, several research articles report “new biofilm models for particular diseases and conditions;” however, in many cases, the innovative feature of the study is the way in which the disease is being reproduced, not the biofilm development on that particular condition. Hence, papers where the biofilm is formed via traditional methods were excluded.
Static in vitro biofilm models
The main characteristics of the recently developed static in vitro biofilm models (Table 2) show a tendency to (1) utilize small sized or miniaturized devices; (2) offer a platform for real-time monitoring of biofilm development; (3) evaluate biofilm properties under native conditions (undisrupted); (4) allow the in situ evaluation of potential antimicrobial treatments; (5) improve reproducibility by forming biofilms with customized shapes and dimensions; (6) consider the maturation state of the biofilm; and (7) include particular elements to better resemble the microenvironment of biofilm formation for certain medical conditions.
Table 2.
Representative examples for recentain vitro biofilm models as of 2015
| Graphic depiction | Description | Application | Microorganism(s) evaluated | Advantages | Limitations |
|---|---|---|---|---|---|
| Recent static in vitro biofilm models | |||||
| 3D bioprinted biofilm construct (Ning et al., 2019) | |||||
 |
|
|
|
|
|
| Patterned SLIPS (“slippery” lubricant-infused porous surface) (Bruchmann et al., 2017) | |||||
 |
|
|
|
|
|
| The dissolvable bead (Dall et al., 2017) | |||||
 |
|
|
|
|
|
| Impedance-based multielectrode array (Goikoetxea et al., 2018) | |||||
 |
|
|
|
|
|
| Vertical capacitance aptamer-functionalized sensor (Song et al., 2019) | |||||
 |
|
|
|
|
|
| Gold mushroom-like nanoplasmonic biochip (Funari et al., 2018) | |||||
 |
|
|
|
|
|
| Biofilm rheometer plate (Grumbein et al., 2016) | |||||
 |
|
|
|
|
|
| Human plasma biofilm model (hpBIOM) (Besser et al., 2020; Besser et al., 2019) | |||||
 |
|
|
|
|
|
| Recent dynamic in vitro biofilm models | |||||
| Microcalorimetry flow system (Said et al., 2015) | |||||
 |
|
|
|
|
|
| Duckworth Biofilm Device (Duckworth et al., 2018) | |||||
 |
|
|
|
|
|
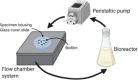
| Flow chamber system for medical implants (Rath et al., 2017) | |||||
 |
|
|
|
|
|
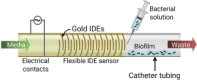
| Flexible impedimetric detection platform (Huiszoon et al., 2019) | |||||
 |
|
|
|
|
|
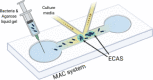
| Microfluidic agarose channel device (Jung et al., 2015) | |||||
 |
|
|
|
|
|
| Microfluidic wound model (Wright et al., 2015) | |||||
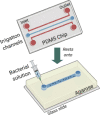 |
|
|
|
|
|
| Microfluidic artificial teeth device (Lam et al., 2016) | |||||
 |
|
|
|
|
|
The models listed in this table include some examples of how different multidisciplinary research groups have proposed to approach and overcome certain limitations of previous and currently used biofilm models. Because of specific goals being pursued, equipment and/or methodologies proposed are mostly particular of certain laboratories and may not be easily implementable in most research facilities. Despite this, they provide out-of-the-box strategies to study and analyze biofilms and may serve as inspiration for the development of more accurate biofilm models.
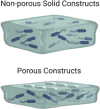
Similar to the greater antimicrobial resistance observed in biofilms as compared with planktonic bacteria, the structure and spatial arrangement of the biofilm influence this response, with a decreasing susceptibility to antimicrobials as biofilm thickness increases. For example, Ning et al. (2019) produced bioprinted bacterial biofilm constructs with thicknesses ranging from 0.25 to 4 mm using a double-crosslinked alginate bioink in a custom-made bioprinter. This technique offers the advantage of providing the user with a high degree of control over the production of constructs with predesigned shapes and dimensions. The authors printed solid and porous structures where the latter may facilitate oxygen diffusion, suggesting that these constructs may be suitable for the study of both aerobic and anaerobic bacterial biofilms depending on the design. This protocol allows the production of biofilm constructs robust enough to be manipulated for further analysis, including imaging and antimicrobial testing. The novelty of this model is that biofilm constructs can be allowed to mature for up to 28 days in culture, which is a great advantage considering that depending on the species and experimental conditions, it can take over 10 days for a biofilm to reach structural maturity (Stoodley et al., 2002). Hence, the antimicrobial efficacy of different substances can be evaluated at a particular time frame of biofilm formation for different bacterial species or strains. However, as previously mentioned, the biofilm properties are dependent on the interactions with the substrate which, in this case, would be limited to biofilms developed on soft surfaces with the additional inconvenience of other elements involved being unrepresented such as interactions with immune- and tissue-specific cells and molecules.
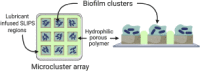
Bruchmann et al. (2017) also considered the importance of biofilm formation in 3D clusters to better resemble biofilm development under natural conditions. The authors addressed the need for reliable and controlled biofilm formation strategies to enable reproducibility in terms of shape and size of biofilms in order to establish proper comparisons. They took advantage of the biofilm-resistant properties of the so-called slippery lubricant-infused porous surface (SLIPS) (Epstein et al., 2012) and formed hydrophobic-hydrophilic patterns with predefined and equally sized geometries onto glass slides. As result, microcluster arrays where the biofilm can be grown into the hydrophilic areas are formed creating a set of multiple identical 3D biofilm clusters. However, after evaluating different shapes for a set of P. aeruginosa strains, it was found that the geometry of SLIPS as well as the size of the clusters and distance between them had an influence in biofilm formation and interactions between biofilm clusters. In order to properly apply this model for drug screening, the architectural conditions of the SLIPS for different types or strains of bacteria would require standardization. As most of the in vitro biofilm models for drug screening, this approach lacks the cellular and molecular interactions that have an important influence in driving biofilm formation and final properties.
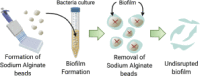
Another proposed strategy to model biofilm formation for further antimicrobial drug screening where the biofilm 3D structure is intended to be undisrupted is the dissolvable bead method (Dall et al., 2017). In this in vitro model, the biofilm is grown onto preformed sodium alginate beads by their incubation in culture broth. Once the biofilm has been established, the alginate beads are dissolved, and the biofilm structure remains intact. This protocol provides a rapid, simple, and cost-effective way of biofilm formation and drug screening. However, it results in a more homogeneous biofilm because of the uniform access to nutrients by bacterial cells, a situation that does not occur in natural biofilm formation where the bacteria in the deepest zones have limited access to nutrients and oxygen, originating a gradient of cells phenotypically different. Furthermore, as the size and shape of alginate beads is not exactly the same, it is possible that differences between biofilms in different beads arise because of the variability in the bacterial cell-alginate bead interactions. The same publication year, a different research group also reported the use of alginate beads as a platform for biofilm formation (Sønderholm et al., 2017). However, in this case, a less concentrated alginate solution was combined with a bacterial solution prior to the formation of beads. The resulting biofilms were observed as dense bacterial clusters mainly accumulated at the periphery of the beads but were also found within the beads.
The following three biofilm models have as a common objective the monitoring of biofilm development. There are many interesting reports about the employment of microsystems for the noninvasive characterization and sensing of bacterial biofilms (for review, see Subramanian et al., 2020), including microfluidic platforms and optical, mechanical, and electrochemical microsystems.
Biofilm structural and cellular components possess dielectric properties that can be exploited for their characterization by electrochemical systems (Subramanian et al., 2020). A representative model based on this class of biosensors is the biofilm impedance chamber reported by Goikoetxea et al. (2018). In this in vitro model, the microelectrode arrays (MEAs) served two functions: act as the substratum for biofilm formation and provide combined electrical recording and optical stimulation. Impedance was then recorded between working electrodes placed at the bottom of the MEA and an external platinum coil electrode. The MEA-based electrochemical impedance spectroscopy measurements allowed the characterization of structural differences of bacterial biofilms by detecting changes on the electrode surface at particular frequencies. According to the authors, changes in the low frequencies are related to the production of ECM and cell growth on the MEA surface, whereas those detected in the middle frequencies correspond to alterations in the supernatant that are associated to metabolites and planktonic cells. Although this model represents a fast, label-free, sensitive, and nondestructive method to characterize biofilms in terms of attachment and maturation, it requires important technical and theoretical considerations for appropriate data interpretation.
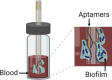
Song et al. (2019) developed an aptamer-functionalized sensor to analyze the biofilm formation in blood samples. In general, if bacteria were present in the sample, they would become attached to the sensor, affecting the electrical properties. The system was designed to allow the simultaneous measurement of capacitance, conductance, and impedance for the parallel monitoring of bacterial growth and biofilm formation. The sensor was vertically oriented in order to ensure that the alterations of electrical properties were being caused by bacteria and not by deposition of blood cells onto the sensor. An important advantage is that most of the materials and equipment required to replicate this system are part of most research laboratories. Also, apart from being a useful in vitro model for the evaluation of biofilm life cycle, this monitoring can also be done in the presence of antimicrobials to evaluate their efficacy. Moreover, the sensor can be removed from the system to allow further analysis of the formed biofilm. However, one implicit disadvantage is the potential influence of the aptamer-bacteria interactions on biofilm development.
An interesting optical microsystem for studying biofilm formation in vitro was developed by Funari et al. (2018). The authors fabricated what they called a “nanomushroom-based localized surface plasmon resonance (LSPR) substrate” for the real-time monitoring of biofilm formation and drug screening. Briefly, it consisted the formation of mushroom-like structures (nanomushrooms [NMs]) with stems of silicon dioxide and gold caps. The glass support containing this NM substrate was fused to a polydimethylsiloxane support with preformed wells. The principle of this in vitro model is that the charge on the NM substrate will increase as the bacterial growth and EPS production do. As a consequence, the frequency of plasmonic resonances will increase as well, causing a decrease in the LSPR wavelength of the NM caps allowing a real-time monitoring of biofilm formation (Funari et al., 2018). This optical microsystem is suitable for both biofilm characterization and antimicrobial drug screening. Although this model offers important advantages, one of the main inconveniences is that it may not be easily reproduced in different research settings because of the requirement of specialized equipment for its fabrication. Moreover, design refinements would be required in order to reduce the scattering in the LSPR signal and decrease the local stress of cells to improve cell viability.
Furthermore, apart from characterizing biofilms by monitoring changes during their formation to study life cycle, the evaluation of rheological properties is fundamental to establish multidisciplinary studies for the development of innovative strategies to elucidate unanswered questions on biofilm fitness (Araújo et al., 2019; Hohne et al., 2009; Körstgens et al., 2001). Grumbein et al. (2016) designed and built a device suitable for the in situ evaluation of biofilm mechanical properties, namely rupture forces and tensile strengths. The device consisted of two polytetrafluoroethylene sample holder sections, which form a chamber that serves as a support for a solid nutrition layer (Singh et al., 2019a). Each end consists of a semicircular cavity that narrows into a straight connection area between both holder parts. Once the agar solidifies into the chamber forming a continuous layer, an incision is made on it at the contact zone of both holders. They are kept in close contact, and a certain volume of liquid bacterial culture is evenly spread over the agar layer. The device is incubated, and once the biofilm is formed over the sliced agar, this is placed in a system where a lateral stretching force is applied to the biofilm at the contact zone of the sample holders. A sensor detects the force, and this is then amplified and processed for data acquisition. Apart from allowing the measurement of native biofilm properties, the setup could be modified for the evaluation of multiple samples, treatments, or conditions at the same time. However, this device does not permit the evaluation of multiple biomechanical properties in the same sample. Also, it is restricted to the evaluation of biofilms formed on solid or semisolid substrates, excluding biofilms not physically attached to a surface. An important consideration is that a low force signal detection is obtained, which leads to the need of specialized users who can adequately select and apply the proper methods and filters to optimize the signal-to-noise ratio of measured data.
Finally, Besser et al. (2019) and (2020) developed a human plasma biofilm model (hpBIOM), which considers the influence of cellular and molecular components on nonhealing chronic wounds-associated biofilms (Besser et al., 2020; Besser et al., 2019). It consists of ellipsoid-shaped clot discs comprising human blood plasma preserves and buffy coats. For the fabrication of the hpBIOM, a bacterial solution with the pathogen of interest is prepared and combined with a plasma/buffy coat mixture. The resulting coagula-like discs with integrated pathogens are suitable for the evaluation of potential antimicrobial substances. The hpBIOM has noteworthy characteristics; first, the structural support for biofilm development is a human-based material present in the in vivo condition; second, it incorporates immune system components (white cells, platelets, and complement system); and last, it structurally resembles the chronic wound milieu. These qualities are finally translated into the development of a more realistic in vitro model where biofilm formation is influenced by its interaction with the cellular and molecular immunological components of the host. However, in order to better resemble the chronic wound microenvironment during biofilm formation, it should also consider the incorporation of “damaged” skin cells. Moreover, stability of the hpBIOM is dependent on the microbial pathogens being incorporated to the matrix. Furthermore, observed results are not generalizable, which is both an advantage and a disadvantage. It would be a drawback in the sense that immune competence would be donor specific, influencing biofilm formation and response to antimicrobial testing, requiring the elaboration of hpBIOMs for each patient. This last point emphasizes its advantage of offering personalized medicine in patients with nonhealing chronic wounds. In this case, bacteria could be isolated from the patient's wound, cultured, and included in an hpBIOM to test a range of antimicrobials and variable doses to find the best antibiofilm treatment for that particular patient. However, further improvements are required to avoid the problem of short time stability of the hpBIOM for certain pathogens as well as the consideration of design modifications to include dermal layers to the model. A research study by Crone et al. (2015) previously reported a similar end up product for the in vitro modeling of chronic wound biofilm infections. This consisted a semisolid gel where bacteria were entrapped between two layers of agar-enriched media casted in a plastic holder. With the aim to better mimic the wound microenvironment, they included chopped meat powder as damaged tissue protein in a culture media supplemented with 25% defibrinated and lysed horse blood and 25% bovine plasma. Nonetheless, these modifications did not influence biofilm growth in terms of number of cells and structure of the aggregates as compared with regular culture media, but it did have an impact on the biofilm response to the treatment evaluated being more susceptible to disruption under these conditions. A peculiar reported result was the obtention of four microcolonies within the gels that were visible to the naked eye. Hence, although the authors also presented an in vitro biofilm model for chronic wounds, which has a similar appearance to the hpBIOM, these are actually different approaches where the hpBIOM shows greater potential.
There is a substantial amount of research studies where the development of new biofilm models is considered an important factor to increase the potential of transitioning antimicrobial therapies and products to the clinic. However, most of the time, these studies consider the inclusion of slight modifications to previously reported models (Brown et al., 2019; Garuglieri et al., 2018; Pabst et al., 2016) pulling along inherent drawbacks of the original ones or, some other times, they focus on a particular characteristic that wants to be replicated or improved, leaving important conditions unaddressed (Pinck et al., 2017).
In general, although the new in vitro models presented in this section offer special advantages for the study of biofilms and evaluation of potential antimicrobials, they still face important drawbacks. These are found summarized in Table 2, where apart from the microbial species used, the different strains chosen as well as the initial and final cell density also show the high variability in the conditions reported when studying biofilms. Moreover, it is worth mentioning that the purpose of presenting the previously discussed models was not to propose them as “to use models” but to show how some of the limitations of previous models had been tackled by different research groups. Biofilm models cannot, and should not, be limited to a single formation/evaluation strategy because of the wide variety of microenvironments under which biofilms are formed. Ideally, models that better recapitulate native conditions of biofilm development would consider combining different strategies to improve biofilm formation and evaluation, depending on the objectives being pursued.
Dynamic in vitro biofilm models
Compared with most static models, dynamic models incorporate the effect of shear forces in different and varied ways, ranging from fluidics systems that push a mobile liquid phase through a solid phase container (e.g., the high-throughput Bioflux device; Benoit et al., 2010) or by simply placing glass beads inside the wells of a microtiter on a rotating platform (Konrat et al., 2016). While dynamic models offer the advantage of more closely replicating certain in vivo conditions, their disadvantages frequently come in the form of higher costs and difficulty of use. Clinically important conditions such as chronic wounds, where ∼80% of wounds contain biofilms (Malone et al., 2017a), demand dynamic conditions (such as those caused by the flow of fluids from the vasculature into a wound) to be considered in the development and evaluation of potential antimicrobials.
New dynamic models
Most of the recently published in vitro dynamic models involve slight modifications of previously developed models instead of a completely novel approach for biofilm development and/or evaluation. Although this implies carrying already identified limitations associated to that particular device or model, the adaptation of currently available technologies is also a convenient approach if it provides significant improvements (Table 2). For instance, Said et al. (2015) incorporated a bioreactor to a commercial calorimeter (2277 TAM Thermal Activity Monitor; TA Instruments) to allow the supply of bacterial culture flow from a bioreactor to the calorimeter, passing by a heat exchange system. This simple setup allowed the real-time in situ monitoring of biofilm development in the lumen of medical tubing materials without disruption. Although the heat flow signal implies the net result of different processes occurring in the system (Braissant et al., 2010), the authors were able to relate it with bacterial metabolic activity as an indicator of biofilm development, as previously reported (Wentzien et al., 1994). Because of the ability of the system to support a replenishing flow, the length of experiments is not limited by nutrient exhaustion as in batch conditions, and thus, longer experiments can be performed to investigate the entire life cycle of the biofilm. However, because of the high costs of the calorimeter equipment, this setup is limited to laboratories where the instrument is already available. Moreover, this is a large sized equipment that also limits its applicability. Combining this approach with miniaturization of the calorimeter (Lerchner et al., 2008) could be an alternative to overcome these limitations. Another proposed model that is based on the principle of flow cells is the Duckworth Biofilm Device (Duckworth et al., 2018). The Duckworth Biofilm Device is a custom-made 3D printed flow system device intended to replicate the biofilms found in wound infections by delivering a flow of nutritious media at the bottom of an agar disc that is topped with a semipermeable membrane upon which a biofilm is formed. This arrangement serves to model the air-liquid interface found in infected wounds, and the flow provided by the model mimics the exudate found in some chronic wounds. However, it does not allow regulation of the exudate level and requires a considerable amount of media.
Taking into account the importance of evaluating biofilm formation on medical implants, Rath et al. (2017) proposed a dental implant infection model that consists of a reusable flow chamber system where discs of different materials are tested on their capacity to allow biofilm formation. An interesting feature is that the experimental setup included the recirculation of nutritious media and bacteria through the chamber containing the material to be evaluated, mimicking physiological flow conditions of the oral cavity. Although this chamber system showed to be reproducible for monospecies biofilms, it may require further optimization if the characteristic multispecies biofilms found in the oral cavity want to be replicated. Moreover, real-time detection and monitoring of biofilm formation is of vital importance in clinical settings as it would allow the implementation of treatments that may prevent biofilm development if detected at early stages. A recently published study reported the potential of a flexible impedimetric detection platform to monitor biofilm development into the lumen of urinary catheters (Huiszoon et al., 2019). It consists of gold interdigitated electrodes, which are fitted into the lumen of the catheter to provide impedimetric readings that, according to the authors, can be associated to biofilm formation and growth. Moreover, apart from their utility as detectors, gold interdigitated electrodes were used to produce an electric field to evaluate their potential as a platform for treating biofilms in combination with lower dosages of antibiotics. Despite this may be a promising alternative for biofilm detection, monitoring, and treatment, it still faces some technical challenges that would need to be addressed before moving to a clinical setting.
As previously mentioned, there is a tendency to develop biofilm models in miniaturized devices. Here, we will present some recent representative examples that may find clinical application for biofilms of medical importance. For an extended revision on microsystems for biofilm development and characterization, the reader is referred to Subramanian et al. (2020).
Apart from growing onto a great diversity of surfaces, bacteria are able to colonize soft mucous tissues (Bjarnsholt et al., 2009) as well as the intracellular space (Anderson et al., 2003) where shear stress is barely present (Blake, 1973; Worlitzsch et al., 2002). In this sense, Jung et al. (2015) adapted a previously reported device (Choi et al., 2013) to develop a microfluidic agarose channel system that exposes bacterial colonies embedded in an agarose gel to a constant supply of fresh nutrients delivered at very low shear forces. The resulting bacterial colonies developed into embedded cell aggregative structures (ECASs), which were found to present several characteristics that resemble biofilms. For instance, ECAS produced EPS and became eventually burst to release planktonic cells, thus suggesting that this model replicates the full biofilm life cycle. Although gel-entrapped bacteria somehow resemble mucous environments, it was found that agarose concentration and fixing process influence the maturation and integrity of ECAS; which would require further improvement of experimental conditions. Wright et al. (2015) also utilized an agarose gel in a microfluidic wound model, which, in this case, served the function of allowing the diffusion of amino acids from the lateral injection ports of a PDMS chip placed on top of it, toward a central bacterial solution channel to resemble the concentration gradients of wounds. Notably, this arrangement provided an easy way of incorporating a chemotactic stimulus while allowing real-time monitoring of biofilm development via an observation port. Finally, a recently published Microfluidic Artificial Teeth Device offered the possibility of studying biofilm formation and development in 128 incubation chambers where different microenvironmental conditions, such as shear stress, nutrients, and oxygen levels, can be independently adjusted (Lam et al., 2016). Even though this device offered several advantages over other models, including the real-time monitoring and quantitative characterization of biofilm development, it demands specialized technical abilities for both device fabrication and operation, which limits its applicability.
It is noteworthy that all the dynamic models presented here have as a common limitation the fact that the interaction of biofilms with the cellular and molecular components of the host is not being considered or intended to be simulated. This is, generally speaking, the main issue found in most of the in vitro biofilm models that have been used for decades and are, unfortunately, still being developed in such a way most of the times. However, there are some published models that are exempted from this tendency. For instance, Millhouse et al. (2014) reported an in vitro static model where the coculture of epithelial cells with a multispecies biofilm was considered with the intention to simulate the conditions under which periodontal biofilms develop in vivo. Although in this study the influence of host cells was taken into account, biofilm formation was performed onto coverslips prior to the coculture setting, which ultimately limited the resemblance of this model to the development of periodontitis.
In vivo models for biofilm assessment
While in vitro biofilm models are powerful tools when it comes to reproducibly testing the effects of simple treatments and factors, they fail to account for the dynamic and complex nature of the interactions at play between the host (i.e., immune system) and bacteria that make up the biofilm as well as the potential for interaction with other bacteria. As such, the development of robust in vivo models is key in validating in vitro results and a necessary step in the testing of new treatments and devices on the path toward clinical translation.
The use of in vivo models to study pathogenic biofilms as well as the development of antibiofilm therapies is not new and has been a key and essential step in the development of our understanding of biofilm formation, treatment, and prevention. The earliest example of an in vivo model being used to study biofilms dates back to the 1940s (Scheman et al., 1941), where rabbits were used as a model for studying osteomyelitis. Since then, there has been a significant amount of research dedicated to the development and study of in vivo models. Nowadays, in vivo models of biofilm formation related to tissue infections, device-related infections, and systemic infections can be found (Lebeaux et al., 2013). Notable tissue infections that have been investigated include models for lung infections, chronic wounds, and ear, nose, and throat infections. Common device-based infections have focused on models for orthopedic implants, subcutaneous devices, and urinary tract catheters. While mammalian in vivo models employing mouse, rat, rabbit, and pigs are most prevalent because of the fact that they allow for the presentation of representative human pathology through similar anatomies, healing processes, and immune response, (Barré-Sinoussi and Montagutelli, 2015), other animals as well as several nonmammalian models have been developed. Although nonmammalian models eliminate some of the practical problems associated with the cost of raising, and housing small and large animals (Ziegler et al., 2016), their inability to present complex immune responses, narrow optimal growth temperature, and short life span have limited their applicability in studying certain infections/pathogens and make them unsuitable for the study of chronic infections. That being said, there are a number of situations in which these simple nonmammalian models (i.e., C. elegans (Diard et al., 2007), D. rerio (Neely et al., 2002), and D. melanogaster (de Bentzmann et al., 2012)) are ideal, such as high-throughput screening studies (Letamendia et al., 2012).
In the next sections, we will highlight some of the currently employed in vivo biofilm models, discuss recent advancements in the development of models that target tissue- and device-related infections that were previously not addressed and touch on the methods used to characterize the biofilm both in vivo and postmortem/ex vivo.
Currently used in vivo biofilm models
In vivo biofilm models have enabled researchers to amass a considerable amount of information regarding biofilm-related infections. Most of the currently employed and well-established in vivo biofilm models have been previously reviewed (Lebeaux et al., 2013). These models have varied in levels of complexity ranging from the simple injection of an inoculate into a catheter (Hagberg et al., 1983) to more complex orthopedic surgeries (Williams et al., 2012), and although there are models for almost all tissue-related infections as well as device-related infections, there is no standard model for each type of infection as the model chosen is highly dependent on the research question one is interested in addressing and may be influenced by the immune system of the host, the size and surface area of the device or implant, and environment in which they find themselves (Lebeaux et al., 2013). Further complicating the use, selection, and development of in vivo models is accessibility to the biofilm for characterization. Unlike in vitro models where the biofilm is typically grown on an easily accessible transparent substrate, most in vivo biofilms can be difficult to characterize and monitor in real time under in situ conditions because of the fact that they are typically internal. While surface wounds, dental caries, and biofilm-related eye infections are exceptions and can be monitored using techniques such as in vivo confocal laser scanning microscopy (CLSM) (Schlafer and Meyer, 2017) and optical coherence tomography (Dsouza et al., 2019; Lenton et al., 2012), typically the biofilm analysis of in vivo models must be carried out postmortem on ex vivo tissue. While there are few examples of in situ in vivo monitoring of biofilms using advanced imaging techniques, such as microcomputed tomography (Stadelmann et al., 2015) and micropositron emission tomography (Garrido et al., 2014); these techniques are highly specialized, expensive, and require trained technicians and as such are typically difficult to access. Some of the key steps in the design and study of in vivo biofilms are presented and summarized in Figure 10.
Figure 10.
Schematic depiction for the overall steps involved in the design of in vivo protocols of biofilm formation and characterization
Blue heading boxes show the general methodology followed in studies of in vivo biofilm development. The red heading box highlights the methods that can be used to characterize biofilm progression. If prevention of biofilm formation is the goal of the study (green box) or if disease or implanted medical device models are required (yellow box), functionalization/modification of materials and development of the appropriate disease models are respectively needed. After this, the materials under evaluation and the disease models may be combined to generate an appropriate in vivo model for biofilm formation. Evaluation and characterization of the biofilm can be done in situ using advanced in vivo techniques, ex situ through the analysis of swabs and excised tissue samples, and ex vivo (i.e., histological analysis of tissue at end point of study).
It is evident in examining the in vivo models developed to date, that there is not much standardization (Coenye et al., 2020). While it is understandable that researchers want to modify the models to better answer their research question, at the same time by using different animals (species and/or strain), modifying the route and/or dosage of inoculant, and varying the length of the experiment it becomes quite difficult to compare results from different studies. This lack of comparability can be a problem when trying to move devices and techniques toward translation in the clinic, considering there are many who question the validity of certain in vitro models because of the low efficiency of translation in the clinical setting (van der Worp et al., 2010; von Herrath and Nepom, 2005).
Currently, one can find established and well-characterized in vivo biofilm models for tissue-related infections, such as cystic fibrosis (Semaniakou et al., 2019), chronic obstructive pulmonary disease (Pang et al., 2008), bronchitis (Yanagihara et al., 1997), tract infections (Anderson et al., 2003), kidney stones (Satoh et al., 1984), pyelonephritis (Nickel et al., 1987), intestinal infections (Bohnhoff et al., 1964; Nell et al., 2010), gall bladder infections (Sukupolvi et al., 1997), chronic wounds (Dai et al., 2010; Simonetti et al., 2008; Akiyama et al., 1996; Nakagami et al., 2008; Gurjala et al., 2011; Davis et al., 2008; Mastropaolo et al., 2005), infective endocarditis (Dai et al., 2010; Durack et al., 1973; Veloso et al., 2011), chronic otitis media (Chaney et al., 2011; Dohar et al., 2005; Ehrlich et al., 2002; Eriksson et al., 2006; Swords et al., 2004), chronic rhinosinusitis (Abreu et al., 2012; Ha et al., 2007; Johansson et al., 1988), dental caries (Bainbridge et al., 2010; Catalán et al., 2011; Fitzgerald and Keyes, 1960), periodontitis (Bainbridge et al., 2010; Hasturk et al., 2007; Settem et al., 2012), and osteomyelitis (Scheman et al., 1941; Brady et al., 2006; Funao et al., 2012; Poeppl et al., 2011). While the development of many of these models followed closely with the introduction of new techniques for biofilm characterization and imaging, the introduction of bioluminescent bacterial strains and bioluminescence imaging had a great impact on the development of chronic wound models (Avci et al., 2018). Through the isolation and transfection of genes coding for light-emitting proteins in biofilm forming bacterial strains, in situ biofilm conditions can now be visualized and monitored. One of the more commonly employed biofilm-forming bioluminescent bacteria that has been employed is S. aureus, specifically strains Xen29, Xen36, Xen40, and ALC2906 (Pribaz et al., 2012).
Established models pertaining to device-related infections include vascular catheters (Fernández-Hidalgo et al., 2010; Chauhan et al., 2012; Rupp et al., 1999), urinary catheters (Davis et al., 1995; Olson et al., 1989; Guiton et al., 2010; Kurosaka et al., 2001; Fung et al., 2003; Allison et al., 2011; Cirioni et al., 2011; Haraoka et al., 1995), orthopedic implants (Mayberry-Carson et al., 1984; Sanzén and Linder, 1995; Del Pozo et al., 2009; Evans et al., 1993; Poelstra et al., 2000; Lucke et al., 2003; Prabhakara et al., 2011; Fitzgerald, 1983; Petty et al., 1985; Philipov et al., 1995; Williams et al., 2012), prosthetic joints (Schurman et al., 1978; Blomgren and Lindgren, 1981; Southwood et al., 1985; Belmatoug et al., 1996; Bernthal et al., 2010), endotracheal tubes (Berra et al., 2004; Fernández-Barat et al., 2012; Olson et al., 2002b), vascular grafts (Tollefson et al., 1987; Aboshady et al., 2012), tissue fillers (Arad et al., 2013; Jacombs et al., 2012), contact lenses (Sun et al., 2010), dental implants (Freire et al., 2011; Rimondini et al., 1997), as well as several other subcutaneous models (Ensing et al., 2005; Hansen et al., 2009; Illingworth et al., 1998; Nakamoto et al., 1994; Anguita-Alonso et al., 2007; Engelsman et al., 2010; Rediske et al., 2000).
Recently developed in vivo biofilm models
Many tissue-related infections and commonly used medical devices have well-established animal models that allow the study of biofilm-related disease progression and testing of new drugs, treatments, materials, and coatings. However, over the last decade, there has been a push to develop new models that address the shortcomings of available models or fill the holes where no model currently exists. In Table 3, we highlight several new in vivo biofilm models, which allow for the study of tissue- and device-related infections. Since in vivo biofilm models imply a more complex scenario because of all the potential interactions that may arise, and considering that differences in the species, sex, and strain of the animal chosen, as well as differences in age and weight influence the outcome of the model developed, we considered important to include these data as reference for comparison between the models presented. Moreover, the methods employed for analyzing these biofilm models and the main outcomes achieved with them are briefly summarized in the same table.
Table 3.
Recently developed in vivo biofilm models
| Description | Application | Microorganism(s) | Biofilm analysis | Outcome(s) |
|---|---|---|---|---|
| Recent in vivo biofilm models for tissue-related infection | ||||
| Endophthalmitis (Sadaka et al., 2014) | ||||
|
|
|
|
|
| Keratitis (Saraswathi and Beuerman, 2015) | ||||
|
|
|
|
|
| Keratitis (Ponce-Angulo et al., 2020) | ||||
|
|
|
|
|
| Vaginosis (Nash et al., 2016) | ||||
|
|
|
|
|
| Vaginosis (Hymes et al., 2013) | ||||
|
|
|
|
|
| Meningitis (Grumbein et al., 2016; Zhang et al., 2018) | ||||
|
|
|
|
|
| Chronic abscess infections (Pletzer et al., 2017) | ||||
|
|
|
|
|
| Recent in vivo biofilm models for device-related infection | ||||
| Cochlear implant (Cevizci et al., 2015) | ||||
|
|
|
|
|
| Neurological device (Glage et al., 2017) | ||||
|
|
|
|
|
| Neurological device (Snowden et al., 2012) | ||||
|
|
|
|
|
While several in vivo models to study biofilm adhesion and formation on contact lenses have been reported (Sun et al., 2010), until recently there was no accepted model in which to study the association of noncontact lens-related corneal biofilms with bacterial keratitis. In 2015, Saraswathi and Beuerman (2015) developed a mouse model in which they could study the role of P. aeruginosa biofilms in the progression of corneal keratitis. Using 7-8-week-old C57BL/6 mice, they were able to follow the progression of P. aeruginosa derived corneal biofilms from planktonic state to microcolony formation in a corneal injury model. The injury model was created by introducing four superficial abrasions of 1–2 mm into the corneal epithelium to which an aliquot of P. aeruginosa was added. By following the progression of the infection using slit lamp microscopy over a period of 7 days and imaging the biofilm formed on the enucleated eyes with a combination of CLSM, scanning electron microscopy, and transmission electron microscopy at days 1, 2, 3, 5, and 7, they were able to illustrate biofilm formation on the corneal surface in a standard infection, which suggests that mature biofilms are a common component of keratitis and could account for therapeutic difficulties when resistance to treatment is observed (Saraswathi and Beuerman, 2015). This study was followed by one from Ponce-Angulo et al. (2020) in which they examined biofilm formation in a mixed keratitis immunosuppressed murine model. The coinfection of S. aureus and F. falciforme was introduced through inoculation of a micropocket incision that was made in the limboscleral border of the cornea. Using optical and fluorescence microscopy techniques along with transmission electron microscopy of excised tissue, they were able to confirm and characterize the growth of a mixed bacterial and fungal biofilm (Ponce-Angulo et al., 2020).
Another biofilm-related tissue infection that afflicts the eye is endophthalmitis. In 2014, Sadaka et al. (2014) developed a mouse model in which biofilm-related anterior chamber infections characteristic of endophthalmitis could be studied. Interested in showing a link between the branched-chain amino acid responsive transcription regulator CodY and endophthalmitis, the study looked to investigate the impact of CodY deletion on S. aureus virulence within a murine anterior chamber model. Briefly, the anterior chamber of the right eye was inoculated with S. aureus using a 35G needle on a NanoFil syringe just anterior to the limbus without touching the iris while using the left eye as an untreated control. In order to assess the growth of the bacteria at 24 h after inoculation, some of the eyes were enucleated and homogenized to allow for quantitative plating, whereas others were fixed for histological analysis via light microscopy. In addition to illustrating S. aureus biofilm formation in the anterior chamber, the study revealed a link between CodY regulation and endophthalmitis repression while also showing that the addition of branched-chain amino acids to eye drops could possibly reduce the progression of bacterial-related endophthalmitis (Sadaka et al., 2014).
Like many of the other in vivo models, we have touched on the models that have been recently developed are rather simple in design. Vaginosis is another tissue-related infection for which there was up until recently no animal model in which to study biofilms. In 2013, Hymes et al. (2013) developed a murine vaginosis model to demonstrate that eDNA secreted from G. vaginalis biofilms is critical for the structural integrity of newly forming and established biofilms. Vaginal biofilms were induced through intravaginal infections through inoculation with G. vaginalis. The progression of the infection was assessed by quantitative culture of vaginal swabs. During the initial inoculation, the G. vaginalis was either added in combination with DNase or a gelatin control. The results from this study showed that intravaginal treatment with DNase inhibits de novo biofilm formation while also liberating G. vaginalis from existing biofilms without killing, a process that decreased biofilm density and possibly enhance the effect of antimicrobials (Hymes et al., 2013). While this finding is interesting, there was actually no direct analysis of biofilm formation in this model, something that was improved on by Nash et al. (2016), who were looking to develop a similar diabetic murine model in which to study vaginal infections caused by C. glabrata, C. albicans, and whether the presence of C. glabrata enhances the pathogenesis of C. albicans. Again, intravaginal infections were induced through inoculation with either C. glabrata or cocultures with C. albicans. Colonization was assessed by quantitative culture of vaginal swabs and visualization of biofilm infection, and inflammatory immunopathogenic response was accomplished through CLSM. Overall, they were able to determine that C. glabrata neither does elicit the same immunopathology as C. albicans during vaginal colonization nor does its presence enhance C. albicans pathogenesis (Nash et al., 2016).
The first animal model of biofilm-mediated meningitis was reported by (Zhang et al., 2018) to investigate the role of S. suis-derived biofilms in meningitis. The infection was introduced via injection of S. suis inoculum through the intracranial subarachnoidal route of infection, which is located 3.5 mm rostral from the bregma. Colonization was assessed by quantitative culture and light microscopy of histopathological samples after sacrifice. This model can be not only used to study the mechanism of bacterial meningitis or the efficacy of new drugs but also showed that disruption of the blood-brain or blood-cerebrospinal fluid barrier by S. suis are important steps in the development of meningitis (Zhang et al., 2018).
Recently, Pletzer et al. (2017) developed a simple murine abscess model for studying subcutaneous chronic Gram-negative infections. Unlike previous chronic infection models, this model is not technically challenging and can be easily tracked without sacrificing the animals. Subcutaneous injection of the Gram-negative bacteria results in the formation of a visible abscess whose progression can be monitored visually and sized using calipers. Furthermore, inoculation with luminescent bacteria allows for visualization of disease progression using noninvasive in vivo imaging system. Extraction of the abscess followed by homogenization and plating allows for quantitative analysis of colony-forming units. Furthermore, dissemination of the infection can be determined by postmortem analysis of the surrounding organs.
In addition to these tissue-related infection models, a few new biofilm models pertaining to device-related infections have been recently established. These include a model for biofilm formation on cochlear implants (Cevizci et al., 2015) and a couple of neurological devices such as central nervous system catheters (Snowden et al., 2012) and surgical screws (Glage et al., 2017). In 2015, Cevizci et al. (2015) developed a guinea pig model in which to study biofilm-mediated infection of implanted cochlear devices and to test a QS inhibitor. The model was generated by instilling S. pneumoniae into the middle ear of the guinea pigs through the tympanic membrane, while small pieces of cochlear implant were implanted under the skin in the retroauricular area after being soaked in a pneumococcal solution. Using scanning electron microscopy analysis, they were able to visualize biofilm formation on the explanted device pieces and confirm that their novel QS inhibitor could prevent biofilm formation on the implants (Craig et al., 2004). As for the neurological devices, Glage et al. (2017) developed a rat model to test whether intraoperative infection of intracranial screws with S. aureus would lead to biofilm formation and inflammatory reaction, whereas Snowden et al. (2012) developed a mouse model to characterize the nature of the inflammatory response during biofilm growth within a central nervous system catheter.
The more we learn about the underlying complexity of biofilm formation in living organisms, the more evident is the need to further advance in developing in vitro models that more closely mimic the in vivo setting where biofilms form and mature. By now, we expect the reader to agree with us that the “one-fit-all” approach is certainly unrealistic and not practical. Further, when one is presented with the choice of selecting or developing an in vitro biofilm; the first step should be assuming that no model will ever be able to fully recapitulate how bacteria will be challenged in vivo. Next, deciding on what critical factor(s) must be incorporated in an in vitro biofilm model (Figure 11) are equally important as acknowledging the intrinsic limitation of that specific model. Thus, for example, not including the immune response and cellular interactions for most of the current in vitro biofilm models have impacted on how we understand biofilm formation and bacterial colonization in living organisms.
Figure 11.
Suggested decision-making system for selecting a biofilm model
Although recent progress in the field has considered incorporating the 3D feature component of biofilms (Ning et al., 2019), most studies have focused on using flat surfaces as main target. This rather oversimplistic conception is a consequence of not considering the multifactorial, and interdependent, mechanisms involved in biofilm formation (“fundamental concepts on biofilm development” section). Thus, a large proportion of currently available in vitro models focus on surfaces (including mechanical deformation and geometry), and availability of nutrients (in vitro models for biofilm assessment section;Table 2). When looking at the inherent polymicrobial nature of biofilms formed in living organisms; most of the currently available in vitro models are limited to a single bacteria strain (Table 2). Modeling biofilm formation in a single-species fashion has also intrinsic limitations, particularly when testing materials/therapies designed for preventing, eradicating, and even sensing biofilms. Such limitations, however, go beyond of what can be recapitulated in the laboratory, as the polymicrobial flora of biofilms, despite of having common components, is truly a fingerprint of each individual, including microorganisms that cannot be cultured in the laboratory. Further limitations for in vitro models have more to do with variability between the same model used in different laboratories and include using (1) bacteria of the same species but different strains, (2) different media composition and nutritional content, (3) growth time of the biofilm, and (4) chosen method for evaluating biofilm formation and maturity (including real-time monitoring), to name some factors.
In vivo models (in vivo models for biofilm assessment section) have also their own limitations. For starters, the immune system in animals, particularly in small rodents, is not fully comparable to that from humans. Apart from being expensive, assessing biofilm in vivo needs of special setup for preventing potential infection of other colonies within the facilities. Further, most studies are short term and carried out in small sample sizes. To test the susceptibility of biomedical devices to bacterial infection using nonclinical bacterial isolates, most tests rely on using relatively large bacterial densities (Table 3). Moreover, most of the in vivo models, including those regulatorily approved, use healthy animals that are even further apart from what is seen in patients who need to have implants or interventional procedures. A representative example of this was recently reported by part of our team when assessing bacterial biofilm prevention (P. aeruginosa) in skin repair. In that study, diabetic mice showed impaired wound healing and were more prone to bacterial infection, when compared with their nondiabetic pairs (Lazurko et al., 2020). Another important factor to consider as a drawback for in vivo models of biofilms rely on the variability of methodologies for evaluating and quantifying biofilm formation (Table 3). As a final note, one must also acknowledge the ethical boundaries for developing in vivo models that more closely recapitulate human diseases. Thus, for example, in the case of diabetic foot ulcers, despite the relevance of incorporating the pressure factor in the lower limb wounds; the currently available animal models for that disease aim for assessing biofilms that do not compromise the animal mobility. In Figure 12, we have schematically summarized some of what we consider limitations and advantages of in vitro and in vivo biofilm models. This list is by no means exhaustive but aims to highlight some of the factors revised in the present review.
Figure 12.
Schematic representation for the limitations and advantages of in vitro and in vivo biofilm models
Next generation of integrative biofilm models
The last 50 years of research in the biofilm and infectious diseases field have brought us closer to developing more functional models of bacterial infection in living organisms. Despite the limitations present in both in vitro and in vivo models of biofilms, the advantages of the models we have currently available would be a microbiologist's dream in the 1950s. Biofilms can be modeled in vitro at a relatively low cost, with a somewhat decent array of customizable alternatives, including high-throughput capabilities and other options (Table 2; Figure 12). Despite the current limitations, in vivo biofilm models allow for more mechanistically accurate assessment of their development and progress within a complex organism. In Figures 9 and 10, we included a road map on how in vitro and in vivo biofilm studies are normally carried out. Moreover, we proposed a schematic in Figure 11 to offer a simplified guide for scientists on where to start and what is important to consider when there is the need to choose or develop more representative biofilm models for a given condition or an experimental setting.
Ex vivo models could be a good compromise for providing a more biologically relevant in vitro bacterial colonization. The cornea is considered an immune-privileged organ, meaning triggering an immune response to the corneal structure is relatively tricky. Very recently, rabbit and porcine corneas have been proposed as an alternative for developing “in vitro” models that allow the evaluation of potential antimicrobials in fungal and bacterial keratitis (Okurowska et al., 2020; Pinnock et al., 2017). However, when working in perfused tissues, such as the heart, modelling endocarditis needs to encompass the physiological temperature, volume, flow, and shear conditions (Lauten et al., 2020) with the innate immune response. The rapid progress in microfluidics and organ-on-a-chip, in what is called “body-on-a-chip” (Dehne and Marx, 2020), could open a new door for seemingly integrating endogenous immune response and high-throughput capabilities in the study of biofilms. Such technologies could be, for example, coupled with the fabrication of 3D bioprinted structures to more closely mimic the complex structure present in living organisms.
Further refinement of biofilm models will need to integrate advanced computing tools to pinpoint critical features in biological systems that need to be more finely replicated for in vitro models. Humanized animal models could be the next frontier for developing more precise in vivo models for biofilms, but the ethical issues must be carefully considered. A more viable avenue that could be explored for in vivo studies are animal models with comorbidities, such as diabetes, cardiovascular diseases, or obesity, that could more closely mimic underlying medical conditions that human patients would present.
It is difficult to predict what the next generation of advanced models in biofilms would look like. Nevertheless, the recent advances in biofilm models discussed in this review illustrate how crossdisciplinarity is contributing to accelerating the development of more physiologically accurate biofilm models.
Acknowledgments
This work was made possible by funding from the Natural Sciences and Engineering Research Council of Canada RGPIN-2015-0632 to E.I.A. and Canadian Institutes of Health Research to E.I.A., E.J.S., T.-F.M., and M.G. M.G holds the Canada Research Chair in Biomaterials and Stem Cells in Ophthalmology. E.I.A. also thanks the New Frontiers in Research Fund-Exploration Stream and to the Government of Ontario for an Early Career Research Award.
Author contributions
The article was written through contributions from all authors. All authors have given approval to the final version of the article. Figures in this work were created using the BioRender.com platform.
Declaration of interests
The authors have no conflicts of interest to declare.
References
- Abbot E.L., Smith W.D., Siou G.P.S., Chiriboga C., Smith R.J., Wilson J.A., Hirst B.H., Kehoe M.A. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell Microbiol. 2007;9:1822–1833. doi: 10.1111/j.1462-5822.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Aboshady I., Raad I., Shah A.S., Vela D., Dvorak T., Safi H.J., Buja L.M., Khalil K.G. A pilot study of a triple antimicrobial-bonded Dacron graft for the prevention of aortic graft infection. J. Vasc. Surg. 2012;56:794–801. doi: 10.1016/j.jvs.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Abreu N.A., Nagalingam N.A., Song Y., Roediger F.C., Pletcher S.D., Goldberg A.N., Lynch S.V. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012;4:151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam B., Baillie G.S., Douglas L.J. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 2002;51:344–349. doi: 10.1099/0022-1317-51-4-344. [DOI] [PubMed] [Google Scholar]
- Agladze K., Wang X., Romeo T. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J. Bacteriol. 2005;187:8237–8246. doi: 10.1128/JB.187.24.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Kanzaki H., Tada J., Arata J. Staphylococcus aureus infection on cut wounds in the mouse skin: experimental staphylococcal botryomycosis. J. Dermatol. Sci. 1996;11:234–238. doi: 10.1016/0923-1811(95)00448-3. [DOI] [PubMed] [Google Scholar]
- Alhede M., Kragh K.N., Qvortrup K., Allesen-Holm M., Van Gennip M., Christensen L.D., Jensen P.Ø., Nielsen A.K., Parsek M., Wozniak D., Molin S., Tolker-Nielsen T., Høiby N., Givskov M., Bjarnsholt T. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One. 2011;6:e27943. doi: 10.1371/journal.pone.0027943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D.G., Sutherland I.W. The role of exopolysaccharides in adhesion of freshwater bacteria. Microbiology. 1987;133:1319–1327. [Google Scholar]
- Allison K.R., Brynildsen M.P., Collins J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S., Goodarzi H., Tavazoie S. Genetic dissection of an exogenously induced biofilm in laboratory and clinical isolates of E. coli. PLoS Pathog. 2009;5:e1000432. doi: 10.1371/journal.ppat.1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann T.W., Gmür R., Thurnheer T. Advancement of the 10-species subgingival Zurich Biofilm model by examining different nutritional conditions and defining the structure of the in vitrobiofilms. BMC Microbiol. 2012;12:227. doi: 10.1186/1471-2180-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J.H., Goo E., Kim H., Seo Y.-S., Hwang I. Bacterial quorum sensing and metabolic slowing in a cooperative population. Proc. Natl. Acad. Sci. U S A. 2014;111:14912. doi: 10.1073/pnas.1412431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S., Wu J., En, Zhang L.-H. Modulation of Pseudomonas aeruginosa biofilm dispersal by a cyclic-di-GMP phosphodiesterase with a putative hypoxia-sensing domain. Appl. Environ. Microbiol. 2010;76:8160. doi: 10.1128/AEM.01233-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y.H., Friedman R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Anderl J.N., Franklin M.J., Stewart P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000;44:1818–1824. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G.G., Palermo J.J., Schilling J.D., Roth R., Heuser J., Hultgren S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- Anguita-Alonso P., Giacometti A., Cirioni O., Ghiselli R., Orlando F., Saba V., Scalise G., Sevo M., Tuzova M., Patel R., Balaban N. RNAIII-inhibiting-peptide-loaded polymethylmethacrylate prevents in vivo Staphylococcus aureus biofilm formation. Antimicrob. Agents Chemother. 2007;51:2594–2596. doi: 10.1128/AAC.00580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad E., Navon-Venezia S., Gur E., Kuzmenko B., Glick R., Frenkiel-Krispin D., Kramer E., Carmeli Y., Barnea Y. Novel rat model of methicillin-resistant Staphylococcus aureus-infected silicone breast implants: a study of biofilm pathogenesis. Plast. Reconstr. Surg. 2013;131:205–214. doi: 10.1097/PRS.0b013e3182778590. [DOI] [PubMed] [Google Scholar]
- Araújo G.R.D.S., Viana N.B., Gómez F., Pontes B., Frases S. The mechanical properties of microbial surfaces and biofilms. Cell Surf. 2019;5:100028. doi: 10.1016/j.tcsw.2019.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTM . ASTM International; 2017. ASTM E2196-17, Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown with Medium Shear and Continuous Flow Using Rotating Disk Reactor. [Google Scholar]
- ASTM . ASTM International; 2017. ASTM E2562-17, Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown with High Shear and Continuous Flow Using CDC Biofilm Reactor. [Google Scholar]
- ASTM . ASTM International; 2017. ASTM E2799-17, Standard Test Method for Testing Disinfectant Efficacy against Pseudomonas aeruginosa Biofilm Using the MBEC Assay. [Google Scholar]
- ASTM . ASTM International; 2018. ASTM E3161-18, Standard Practice for Preparing a Pseudomonas aeruginosa or Staphylococcus aureus Biofilm Using the CDC Biofilm Reactor. [Google Scholar]
- ASTM . ASTM International; 2018. ASTM E3180-18, Standard Test Method for Quantification of a Bacillus Subtilis Biofilm Comprised of Vegetative Cells and Spores Grown Using the Colony Biofilm Model. [Google Scholar]
- ASTM . ASTM International; 2019. ASTM E2871-19, Standard Test Method for Determining Disinfectant Efficacy against Biofilm Grown in the CDC Biofilm Reactor Using the Single Tube Method. [Google Scholar]
- ASTM . ASTM International; 2020. ASTM E2647-20, Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown Using Drip Flow Biofilm Reactor with Low Shear and Continuous Flow. [Google Scholar]
- Avci P., Karimi M., Sadasivam M., Antunes-Melo W.C., Carrasco E., Hamblin M.R. In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence. 2018;9:28–63. doi: 10.1080/21505594.2017.1371897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeredo J., Azevedo N.F., Briandet R., Cerca N., Coenye T., Costa A.R., Desvaux M., Di Bonaventura G., Hébraud M., Jaglic Z. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017;43:313–351. doi: 10.1080/1040841X.2016.1208146. [DOI] [PubMed] [Google Scholar]
- Baddour L.M., Christensen G.D. Prosthetic valve endocarditis due to small-colony staphylococcal variants. Rev. Infect. Dis. 1987;9:1168–1174. doi: 10.1093/clinids/9.6.1168. [DOI] [PubMed] [Google Scholar]
- Bainbridge B., Verma R.K., Eastman C., Yehia B., Rivera M., Moffatt C., Bhattacharyya I., Lamont R.J., Kesavalu L. Role of Porphyromonas Gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect. Immun. 2010;78:4560–4569. doi: 10.1128/IAI.00703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N., Schleheck D., Klebensberger J., Webb J.S., Hassett D.J., Rice S.A., Kjelleberg S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009;191:7333. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Montagutelli X. Animal Models are essential to biological research: issues and perspectives. Future Sci. OA. 2015;1:Fso63. doi: 10.4155/fso.15.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitelshees M., Hill A., Jones C.H., Pfeifer B.A. Phenotypic variation during biofilm formation: implications for anti-biofilm therapeutic design. Materials (Basel) 2018;11:1086. doi: 10.3390/ma11071086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas M.R., Colwell R.R. Scanning electron microscope observation of the swarming phenomenon of Vibrio parahaemolyticus. J. Bacteriol. 1982;150:956. doi: 10.1128/jb.150.2.956-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belibasakis G.N., Kast J.I., Thurnheer T., Akdis C.A., Bostanci N. The expression of gingival epithelial junctions in response to subgingival biofilms. Virulence. 2015;6:704–709. doi: 10.1080/21505594.2015.1081731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belibasakis G.N., Thurnheer T., Bostanci N. Interleukin-8 responses of multi-layer gingival epithelia to subgingival biofilms: role of the “red complex” species. PLoS One. 2013;8:e81581. doi: 10.1371/journal.pone.0081581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmatoug N., Crémieux A.C., Bleton R., Volk A., Saleh-Mghir A., Grossin M., Garry L., Carbon C. A new model of experimental prosthetic joint infection due to methicillin-resistant Staphylococcus aureus: a microbiologic, histopathologic, and magnetic resonance imaging characterization. J. Infect. Dis. 1996;174:414–417. doi: 10.1093/infdis/174.2.414. [DOI] [PubMed] [Google Scholar]
- Beloin C., Fernández-Hidalgo N., Lebeaux D. Understanding biofilm formation in intravascular device-related infections. Intensive Care Med. 2017;43:443–446. doi: 10.1007/s00134-016-4480-7. [DOI] [PubMed] [Google Scholar]
- Benoit M.R., Conant C.G., Ionescu-Zanetti C., Schwartz M., Matin A. New device for high-throughput viability screening of flow biofilms. Appl. Environ. Microbiol. 2010;76:4136–4142. doi: 10.1128/AEM.03065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne C., Ellison C.K., Ducret A., Brun Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018;16:616–627. doi: 10.1038/s41579-018-0057-5. [DOI] [PubMed] [Google Scholar]
- Bernthal N.M., Stavrakis A.I., Billi F., Cho J.S., Kremen T.J., Simon S.I., Cheung A.L., Finerman G.A., Lieberman J.R., Adams J.S., Miller L.S. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010;5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra L., De Marchi L., Yu Z.X., Laquerriere P., Baccarelli A., Kolobow T. Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs, and endotracheal tube. Anesthesiology. 2004;100:1446–1456. doi: 10.1097/00000542-200406000-00017. [DOI] [PubMed] [Google Scholar]
- Besser M., Dietrich M., Weber L., Rembe J.D., Stuermer E.K. Efficacy of antiseptics in a novel 3-dimensional human plasma biofilm model (hpBIOM) Sci. Rep. 2020;10:4792. doi: 10.1038/s41598-020-61728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser M., Terberger J., Weber L., Ghebremedhin B., Naumova E.A., Arnold W.H., Stuermer E.K. Impact of probiotics on pathogen survival in an innovative human plasma biofilm model (hpBIOM) J. Transl. Med. 2019;17:243. doi: 10.1186/s12967-019-1990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Berends E.T.M., Chan R., Schwab E., Roy S., Sen C.K., Torres V.J., Wozniak D.J. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc. Natl. Acad. Sci. U S A. 2018;115:7416. doi: 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhomkar P., Materi W., Semenchenko V., Wishart D.S. Transcriptional response of E. coli upon FimH-mediated fimbrial adhesion. Gene Regul. Syst. Biol. 2010;4:GRSB.S4525. doi: 10.4137/grsb.s4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T., Jensen P.Ø., Fiandaca M.J., Pedersen J., Hansen C.R., Andersen C.B., Pressler T., Givskov M., Høiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- Blake J. A note on mucus shear rates. Respir. Physiol. 1973;17:394–399. doi: 10.1016/0034-5687(73)90012-1. [DOI] [PubMed] [Google Scholar]
- Blankenship J.R., Mitchell A.P. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Blomgren G., Lindgren U. Late hematogenous infection in total joint replacement: studies of gentamicin and bone cement in the rabbit. Clin. Orthop. Relat. Res. 1981;155:244–248. [PubMed] [Google Scholar]
- Bohnhoff M., Miller C.P., Martin W.R. Resistance of the mouse's intestinal tract to experimental salmonella infection. Ii. Factors responsible for its loss following streptomycin treatment. J. Exp. Med. 1964;120:817–828. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles B.R., Horswill A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles B.R., Thoendel M., Singh P.K. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl. Acad. Sci. U S A. 2004;101:16630. doi: 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles B.R., Thoendel M., Singh P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005;57:1210–1223. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- Bos R., Van Der Mei H.C., Busscher H.J. Physico-chemistry of initial microbial adhesive interactions – its mechanisms and methods for study. FEMS Microbiol. Rev. 1999;23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Brady R.A., Leid J.G., Camper A.K., Costerton J.W., Shirtliff M.E. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect. Immun. 2006;74:3415–3426. doi: 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O., Wirz D., Göpfert B., Daniels A.U. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 2010;303:1–8. doi: 10.1111/j.1574-6968.2009.01819.x. [DOI] [PubMed] [Google Scholar]
- Brooun A., Liu S., Lewis K. A dose-response study of antibiotic resistance inPseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2000;44:640. doi: 10.1128/aac.44.3.640-646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.L., Johnston W., Delaney C., Rajendran R., Butcher J., Khan S., Bradshaw D., Ramage G., Culshaw S. Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells. Sci. Rep. 2019;9:15779. doi: 10.1038/s41598-019-52115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchmann J., Pini I., Gill T.S., Schwartz T., Levkin P.A. Patterned SLIPS for the formation of arrays of biofilm microclusters with defined geometries. Adv. Healthc. Mater. 2017;6:1601082. doi: 10.1002/adhm.201601082. [DOI] [PubMed] [Google Scholar]
- Bryzek D., Ciaston I., Dobosz E., Gasiorek A., Makarska A., Sarna M., Eick S., Puklo M., Lech M., Potempa B. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019;15:e1007773. doi: 10.1371/journal.ppat.1007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmølle M., Webb J.S., Rao D., Hansen L.H., Sørensen S.J., Kjelleberg S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl. Environ. Microbiol. 2006;72:3916–3923. doi: 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher H.J., van der Mei H.C. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012;8:e1002440. doi: 10.1371/journal.ppat.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D.E., Lawrence J.R. Growth kinetics ofPseudomonas fluorescens microcolonies within the hydrodynamic boundary layers of surface microenvironments. Microb. Ecol. 1986;12:299–312. doi: 10.1007/BF02011173. [DOI] [PubMed] [Google Scholar]
- Castonguay M.-H., Van Der Schaaf S., Koester W., Krooneman J., Van Der Meer W., Harmsen H., Landini P. Biofilm formation by Escherichia coli is stimulated by synergistic interactions and co-adhesion mechanisms with adherence-proficient bacteria. Res. Microbiol. 2006;157:471–478. doi: 10.1016/j.resmic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Catalán M.A., Scott-Anne K., Klein M.I., Koo H., Bowen W.H., Melvin J.E. Elevated incidence of dental caries in a mouse model of cystic fibrosis. PLoS One. 2011;6:e16549. doi: 10.1371/journal.pone.0016549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceri H., Olson M.E., Stremick C., Read R.R., Morck D., Buret A. The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevizci R., Düzlü M., Dündar Y., Noyanalpan N., Sultan N., Tutar H., Bayazit Y.A. Preliminary results of a novel quorum sensing inhibitor against pneumococcal infection and biofilm formation with special interest to otitis media and cochlear implantation. Eur. Arch. Otorhinolaryngol. 2015;272:1389–1393. doi: 10.1007/s00405-014-2942-5. [DOI] [PubMed] [Google Scholar]
- Chandra J., Kuhn D.M., Mukherjee P.K., Hoyer L.L., Mccormick T., Ghannoum M.A. biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney E.J., Nguyen C.T., Boppart S.A. Novel method for non-invasive induction of a middle-ear biofilm in the rat. Vaccine. 2011;29:1628–1633. doi: 10.1016/j.vaccine.2010.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y., Zhang T. Probing roles of lipopolysaccharide, type 1 fimbria, and colanic acid in the attachment of Escherichia coli strains on inert surfaces. Langmuir. 2011;27:11545–11553. doi: 10.1021/la202534p. [DOI] [PubMed] [Google Scholar]
- Chao Y., Zhang T. Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: from initial attachment to mature biofilm. Anal Bioanal. Chem. 2012;404:1465–1475. doi: 10.1007/s00216-012-6225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A., Lebeaux D., Decante B., Kriegel I., Escande M.C., Ghigo J.M., Beloin C. A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections. PLoS One. 2012;7:e37281. doi: 10.1371/journal.pone.0037281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavant P., Gaillard-Martinie B., Talon R., Hébraud M., Bernardi T. A new device for rapid evaluation of biofilm formation potential by bacteria. J. Microbiol. Methods. 2007;68:605–612. doi: 10.1016/j.mimet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Choi J., Jung Y.-G., Kim J., Kim S., Jung Y., Na H., Kwon S. Rapid antibiotic susceptibility testing by tracking single cell growth in a microfluidic agarose channel system. Lab Chip. 2013;13:280–287. doi: 10.1039/c2lc41055a. [DOI] [PubMed] [Google Scholar]
- Choudhury D., Thompson A., Stojanoff V., Langermann S., Pinkner J., Hultgren S.J., Knight S.D. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science. 1999;285:1061–1066. doi: 10.1126/science.285.5430.1061. [DOI] [PubMed] [Google Scholar]
- Christensen G.D., Simpson W.A., Younger J.J., Baddour L.M., Barrett F.F., Melton D.M., Beachey E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua S.L., Liu Y., Yam J.K.H., Chen Y., Vejborg R.M., Tan B.G.C., Kjelleberg S., Tolker-Nielsen T., Givskov M., Yang L. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 2014;5:4462. doi: 10.1038/ncomms5462. [DOI] [PubMed] [Google Scholar]
- Chung M.-C., Dean S., Marakasova E.S., Nwabueze A.O., Van Hoek M.L. Chitinases are negative regulators of Francisella novicida biofilms. PLoS One. 2014;9:e93119. doi: 10.1371/journal.pone.0093119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirioni O., Ghiselli R., Silvestri C., Minardi D., Gabrielli E., Orlando F., Rimini M., Brescini L., Muzzonigro G., Guerrieri M. Effect of the combination of clarithromycin and amikacin on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. J. Antimicrob. Chemother. 2011;66:1318–1323. doi: 10.1093/jac/dkr107. [DOI] [PubMed] [Google Scholar]
- Coenye T., Goeres D., Van Bambeke F., Bjarnsholt T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin. Microbiol. Infect. 2018;24:570–572. doi: 10.1016/j.cmi.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Coenye T., Kjellerup B., Stoodley P., Bjarnsholt T. The future of biofilm research - report on the '2019 Biofilm Bash. Biofilm. 2020;2:100012. doi: 10.1016/j.bioflm.2019.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T., Nelis H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods. 2010;83:89–105. doi: 10.1016/j.mimet.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Cole S.P., Harwood J., Lee R., She R., Guiney D.G. Characterization of monospecies biofilm formation by Helicobacter pylori. J. Bacteriol. 2004;186:3124–3132. doi: 10.1128/JB.186.10.3124-3132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin K.M., Irie Y., Tart C.S., Urbano R., Whitney J.C., Ryder C., Howell P.L., Wozniak D.J., Parsek M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012;14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad J.C., Gibiansky M.L., Jin F., Gordon V.D., Motto D.A., Mathewson M.A., Stopka W.G., Zelasko D.C., Shrout J.D., Wong G.C.L. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys. J. 2011;100:1608–1616. doi: 10.1016/j.bpj.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J.W., Cheng K.J., Geesey G.G., Ladd T.I., Nickel J.C., Dasgupta M., Marrie T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Lewandowski Z., Caldwell D.E., Korber D.R., Lappin-Scott H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Craig L., Pique M.E., Tainer J.A. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2004;2:363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- Craig L., Volkmann N., Arvai A.S., Pique M.E., Yeager M., Egelman Edward H., Tainer J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell. 2006;23:651–662. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Crone S., Garde C., Bjarnsholt T., Alhede M. A novel in vitro wound biofilm model used to evaluate low-frequency ultrasonic-assisted wound debridement. J. Wound Care. 2015;24:66–69, 72. doi: 10.12968/jowc.2015.24.2.64. [DOI] [PubMed] [Google Scholar]
- Cryer J., Schipor I., Perloff J.R., Palmer J.N. Evidence of bacterial biofilms in human chronic sinusitis. ORL. 2004;66:155–158. doi: 10.1159/000079994. [DOI] [PubMed] [Google Scholar]
- Dai T., Tegos G.P., Zhiyentayev T., Mylonakis E., Hamblin M.R. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg. Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall G.F., Tsang S.T.J., Gwynne P.J., Wilkinson A.J., Simpson A., Breusch S.J.B., Gallagher M.P. The dissolvable bead: a novel in vitro biofilm model for evaluating antimicrobial resistance. J. Microbiol. Methods. 2017;142:46–51. doi: 10.1016/j.mimet.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Das T., Sharma P.K., Busscher H.J., Van Der Mei H.C., Krom B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010;76:3405. doi: 10.1128/AEM.03119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.G., Marques C.N.H. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009;191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.H., Costerton J.W., Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- Davis C.P., Shirtliff M.E., Scimeca J.M., Hoskins S.L., Warren M.M. In vivo reduction of bacterial populations in the urinary tract of catheterized sheep by iontophoresis. J. Urol. 1995;154:1948–1953. [PubMed] [Google Scholar]
- Davis S.C., Ricotti C., Cazzaniga A., Welsh E., Eaglstein W.H., Mertz P.M. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008;16:23–29. doi: 10.1111/j.1524-475X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- de Beer D., Stoodley P., Roe F., Lewandowski Z. Effects of Biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994;43:1131–1138. doi: 10.1002/bit.260431118. [DOI] [PubMed] [Google Scholar]
- de Bentzmann S., Giraud C., Bernard C.S., Calderon V., Ewald F., Plésiat P., Nguyen C., Grunwald D., Attree I., Jeannot K. Unique biofilm signature, drug susceptibility and decreased virulence in Drosophila through the Pseudomonas aeruginosa two-component system PprAB. PLoS Pathog. 2012;8:e1003052. doi: 10.1371/journal.ppat.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kievit T.R., Gillis R., Marx S., Brown C., Iglewski B.H. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 2001;67:1865. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne E.-M., Marx U. Chapter 13 - human body-on-a-chip systems. In: Hoeng J., Bovard D., Peitsch M.C., editors. Organ-on-a-chip. Academic Press; 2020. pp. 429–439. [Google Scholar]
- Del Pozo J.L., Rouse M.S., Euba G., Kang C.I., Mandrekar J.N., Steckelberg J.M., Patel R. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob. Agents Chemother. 2009;53:4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai J., Mulay S.R., Nakazawa D., Anders H.-J. Matters of life and death. How neutrophils die or survive along NET release and is “NETosis” = necroptosis? Cell. Mol. Life Sci. 2016;73:2211–2219. doi: 10.1007/s00018-016-2195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diard M., Baeriswyl S., Clermont O., Gouriou S., Picard B., Taddei F., Denamur E., Matic I. Caenorhabditis elegans as a simple model to study phenotypic and genetic virulence determinants of extraintestinal pathogenic Escherichia coli. Microbes Infect. 2007;9:214–223. doi: 10.1016/j.micinf.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Díaz-Salazar C., Calero P., Espinosa-Portero R., Jiménez-Fernández A., Wirebrand L., Velasco-Domínguez M.G., López-Sánchez A., Shingler V., Govantes F. The stringent response promotes biofilm dispersal in Pseudomonas putida. Sci. Rep. 2017;7:18055. doi: 10.1038/s41598-017-18518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson K.W., Pinkner J.S., Rose T., Magnusson G., Hultgren S.J., Waksman G. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell. 2001;105:733–743. doi: 10.1016/s0092-8674(01)00388-9. [DOI] [PubMed] [Google Scholar]
- Dohar J.E., Hebda P.A., Veeh R., Awad M., Costerton J.W., Hayes J., Ehrlich G.D. Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope. 2005;115:1469–1472. doi: 10.1097/01.mlg.0000172036.82897.d4. [DOI] [PubMed] [Google Scholar]
- Dominiak D.M., Nielsen J.L., Nielsen P.H. Extracellular DNA is abundant and important for microcolony strength in mixed microbial biofilms. Environ. Microbiol. 2011;13:710–721. doi: 10.1111/j.1462-2920.2010.02375.x. [DOI] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms and device-associated infections. Emerging Infect. Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms: microbial life on surfaces. Emerging Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R.M., Piede J.A., Heyes C.D., Sanii L., Murga R., Edmonds P., El-Sayed I., El-Sayed M.A. Model system for growing and quantifying Streptococcus pneumoniae biofilms in situ and in real time. Appl. Environ. Microbiol. 2004;70:4980–4988. doi: 10.1128/AEM.70.8.4980-4988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramsi S., Caliot E., Bonne I., Guadagnini S., Prévost M.-C., Kojadinovic M., Lalioui L., Poyart C., Trieu-Cuot P. Assembly and role of pili in group B streptococci. Mol. Microbiol. 2006;60:1401–1413. doi: 10.1111/j.1365-2958.2006.05190.x. [DOI] [PubMed] [Google Scholar]
- Drescher K., Dunkel J., Nadell C.D., Van Teeffelen S., Grnja I., Wingreen N.S., Stone H.A., Bassler B.L. Architectural transitions in Vibrio cholerae biofilms at single-cell resolution. Proc. Natl. Acad. Sci. U S A. 2016;113:E2066. doi: 10.1073/pnas.1601702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dsouza R., Spillman D.R., Jr., Barkalifa R., Monroy G.L., Chaney E.J., White K.C., Boppart S.A. In vivo detection of endotracheal tube biofilms in intubated critical care patients using catheter-based optical coherence tomography. J. Biophotonics. 2019;12:e201800307. doi: 10.1002/jbio.201800307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth P.F., Rowlands R.S., Barbour M.E., Maddocks S.E. A novel flow-system to establish experimental biofilms for modelling chronic wound infection and testing the efficacy of wound dressings. Microbiol. Res. 2018;215:141–147. doi: 10.1016/j.micres.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Durack D.T., Beeson P.B., Petersdorf R.G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br. J. Exp. Pathol. 1973;54:142–151. [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G.D., Veeh R., Wang X., Costerton J.W., Hayes J.D., Hu F.Z., Daigle B.J., Ehrlich M.D., Post J.C. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287:1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- El-Azizi M.A., Starks S.E., Khardori N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms∗. J. Appl. Microbiol. 2004;96:1067–1073. doi: 10.1111/j.1365-2672.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- Elias S., Banin E. Multi-species biofilms: living with friendly neighbors. FEMS Microbiol. Rev. 2012;36:990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- Ellison C.K., Kan J., Dillard R.S., Kysela D.T., Ducret A., Berne C., Hampton C.M., Ke Z., Wright E.R., Biais N. Obstruction of pilus retraction stimulates bacterial surface sensing. Science. 2017;358:535. doi: 10.1126/science.aan5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsman A.F., Van Dam G.M., Van Der Mei H.C., Busscher H.J., Ploeg R.J. In vivo evaluation of bacterial infection involving morphologically different surgical meshes. Ann. Surg. 2010;251:133–137. doi: 10.1097/SLA.0b013e3181b61d9a. [DOI] [PubMed] [Google Scholar]
- Ensing G.T., Roeder B.L., Nelson J.L., Van Horn J.R., Van Der Mei H.C., Busscher H.J., Pitt W.G. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J. Appl. Microbiol. 2005;99:443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A.K., Wong T.-S., Belisle R.A., Boggs E.M., Aizenberg J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. U S A. 2012;109:13182–13187. doi: 10.1073/pnas.1201973109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson D.L., Endersby R., Kirkham A., Stuber K., Vollman D.D., Rabin H.R., Mitchell I., Storey D.G. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 2002;70:1783. doi: 10.1128/IAI.70.4.1783-1790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P.O., Li J., Ny T., Hellström S. Spontaneous development of otitis media in plasminogen-deficient mice. Int. J. Med. Microbiol. 2006;296:501–509. doi: 10.1016/j.ijmm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Evans C.R., Kempes C.P., Price-Whelan A., Dietrich L.E.P. Metabolic heterogeneity and cross-feeding in bacterial multicellular systems. Trends Microbiol. 2020;28:732–743. doi: 10.1016/j.tim.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R.P., Nelson C.L., Harrison B.H. The effect of wound environment on the incidence of acute osteomyelitis. Clin. Orthop. Relat. Res. 1993;286:289–297. [PubMed] [Google Scholar]
- Fauvart M., De Groote V.N., Michiels J. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011;60:699–709. doi: 10.1099/jmm.0.030932-0. [DOI] [PubMed] [Google Scholar]
- Fazli M., Bjarnsholt T., Kirketerp-Møller K., Jørgensen B., Andersen A.S., Krogfelt K.A., Givskov M., Tolker-Nielsen T. Nonrandom Distribution of Pseudomonas aeruginosa and Staphylococcus aureus in Chronic Wounds. J. Clin. Microbiol. 2009;47:4084. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Cheng Y., Wang S.-Y., Borca-Tasciuc D.A., Worobo R.W., Moraru C.I. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: how small is small enough? npj Biofilms Microbiomes. 2015;1:15022. doi: 10.1038/npjbiofilms.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Barat L., Li Bassi G., Ferrer M., Bosch A., Calvo M., Vila J., Gabarrús A., Martínez-Olondris P., Rigol M., Esperatti M. Direct analysis of bacterial viability in endotracheal tube biofilm from a pig model of methicillin-resistant Staphylococcus aureus pneumonia following antimicrobial therapy. FEMS Immunol. Med. Microbiol. 2012;65:309–317. doi: 10.1111/j.1574-695X.2012.00961.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Hidalgo N., Gavaldà J., Almirante B., Martín M.T., Onrubia P.L., Gomis X., Pahissa A. Evaluation of linezolid, vancomycin, gentamicin and ciprofloxacin in a rabbit model of antibiotic-lock technique for Staphylococcus aureus catheter-related infection. J. Antimicrob. Chemother. 2010;65:525–530. doi: 10.1093/jac/dkp499. [DOI] [PubMed] [Google Scholar]
- Filardo S., Di Pietro M., Tranquilli G., Sessa R. Biofilm in genital ecosystem: a potential risk factor for Chlamydia trachomatis infection. Can. J. Infect. Dis. Med. Microbiol. 2019;2019:1672109. doi: 10.1155/2019/1672109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A., Gollan B., Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R.H., JR. Experimental osteomyelitis: description of a canine model and the role of depot administration of antibiotics in the prevention and treatment of sepsis. J. Bone Jt. Surg. Am. 1983;65:371–380. [PubMed] [Google Scholar]
- Fitzgerald R.J., Keyes P.H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J. Am. Dent Assoc. 1960;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Nair S., Poole S., Henderson B., Wilson M. Cytokine degradation by biofilms of porphyromonas gingivalis. Curr. Microbiol. 1998;36:216–219. doi: 10.1007/s002849900297. [DOI] [PubMed] [Google Scholar]
- Fletcher M. Attachment of Pseudomonas fluorescens to glass and influence of electrolytes on bacterium-substratum separation distance. J. Bacteriol. 1988;170:2027–2030. doi: 10.1128/jb.170.5.2027-2030.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M., Loeb G.I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microbiol. 1979;37:67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire M.O., Sedghizadeh P.P., Schaudinn C., Gorur A., Downey J.S., Choi J.H., Chen W., Kook J.K., Chen C., Goodman S.D. Development of an animal model for Aggregatibacter actinomycetemcomitans biofilm-mediated oral osteolytic infection: a preliminary study. J. Periodontol. 2011;82:778–789. doi: 10.1902/jop.2010.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander R.S., Vlamakis H., Kim P., Khan M., Kolter R., Aizenberg J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. U S AS. 2013;110:5624. doi: 10.1073/pnas.1219662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander R.S., Vogel N., Aizenberg J. Role of flagella in adhesion of Escherichia coli to abiotic surfaces. Langmuir. 2015;31:6137–6144. doi: 10.1021/acs.langmuir.5b00815. [DOI] [PubMed] [Google Scholar]
- Frølund B., Palmgren R., Keiding K., Nielsen P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996;30:1749–1758. [Google Scholar]
- Fronzes R., Remaut H., Waksman G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 2008;27:2271–2280. doi: 10.1038/emboj.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funao H., Ishii K., Nagai S., Sasaki A., Hoshikawa T., Aizawa M., Okada Y., Chiba K., Koyasu S., Toyama Y. Establishment of a real-time, quantitative, and reproducible mouse model of Staphylococcus osteomyelitis using bioluminescence imaging. Infect. Immun. 2012;80:733–741. doi: 10.1128/IAI.06166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funari R., Bhalla N., Chu K.-Y., Söderström B., Shen A.Q. Nanoplasmonics for real-time and label-free monitoring of microbial biofilm formation. ACS Sensors. 2018;3:1499–1509. doi: 10.1021/acssensors.8b00287. [DOI] [PubMed] [Google Scholar]
- Fung L.C., Mittelman M.W., Thorner P.S., Khoury A.E. A novel rabbit model for the evaluation of biomaterial associated urinary tract infection. Can. J. Urol. 2003;10:2007–2012. [PubMed] [Google Scholar]
- Fuqua W.C., Winans S.C., Greenberg E.P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A., Coretti L., Savio V., Buommino E., Lembo F., Donnarumma G. biofilm formation and immunomodulatory activity of Proteus mirabilis clinically isolated strains. Int. J. Mol. Sci. 2017;18:414. doi: 10.3390/ijms18020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Zheng H., Ren Y., Lou R., Wu F., Yu W., Liu X., Ma X. A crucial role for spatial distribution in bacterial quorum sensing. Sci. Rep. 2016;6:34695. doi: 10.1038/srep34695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido V., Collantes M., Barberán M., Peñuelas I., Arbizu J., Amorena B., Grilló M.J. In vivo monitoring of Staphylococcus aureus biofilm infections and antimicrobial therapy by [18F]fluoro-deoxyglucose-MicroPET in a mouse model. Antimicrob. Agents Chemother. 2014;58:6660–6667. doi: 10.1128/AAC.03138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garuglieri E., Meroni E., Cattò C., Villa F., Cappitelli F., Erba D. Effects of sub-lethal concentrations of silver nanoparticles on a simulated intestinal prokaryotic-eukaryotic interface. Front. Microbiol. 2018;8:2698. doi: 10.3389/fmicb.2017.02698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavín R., Merino S., Altarriba M., Canals R., Shaw J.G., Tomás J.M. Lateral flagella are required for increased cell adherence, invasion and biofilm formation by Aeromonas spp. FEMS Microbiol. Lett. 2003;224:77–83. doi: 10.1016/S0378-1097(03)00418-X. [DOI] [PubMed] [Google Scholar]
- Gavín R., Rabaan A.A., Merino S., Tomás J.M., Gryllos I., Shaw J.G. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol. Microbiol. 2002;43:383–397. doi: 10.1046/j.1365-2958.2002.02750.x. [DOI] [PubMed] [Google Scholar]
- Geoghegan J.A., Corrigan R.M., Gruszka D.T., Speziale P., Gara J.P., Potts J.R., Foster T.J. Role of surface protein SasG in biofilm formation by Staphylococcus aureus</em>. J. Bacteriol. 2010;192:5663. doi: 10.1128/JB.00628-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke C., Kraft A., Süssmuth R., Schweitzer O., Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- Gibbons R.J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch. Oral Biol. 1970;15:1397–IN39. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibiansky M.L., Conrad J.C., Jin F., Gordon V.D., Motto D.A., Mathewson M.A., Stopka W.G., Zelasko D.C., Shrout J.D., Wong G.C.L. Bacteria use type IV pili to walk upright and detach from surfaces. Science. 2010;330:197. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- Gilbert P., Allison D.G., Evans D.J., Handley P.S., Brown M.R. Growth rate control of adherent bacterial populations. Appl. Environ. Microbiol. 1989;55:1308–1311. doi: 10.1128/aem.55.5.1308-1311.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjermansen M., Nilsson M., Yang L., Tolker-Nielsen T. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: genetic elements and molecular mechanisms. Mol. Microbiol. 2010;75:815–826. doi: 10.1111/j.1365-2958.2009.06793.x. [DOI] [PubMed] [Google Scholar]
- Glage S., Paret S., Winkel A., Stiesch M., Bleich A., Krauss J.K., Schwabe K. A new model for biofilm formation and inflammatory tissue reaction: intraoperative infection of a cranial implant with Staphylococcus aureus in rats. Acta Neurochir. 2017;159:1747–1756. doi: 10.1007/s00701-017-3244-7. [DOI] [PubMed] [Google Scholar]
- Gode-Potratz C.J., Kustusch R.J., Breheny P.J., Weiss D.S., Mccarter L.L. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goikoetxea E., Routkevitch D., De Weerdt A., Green J.J., Steenackers H., Braeken D. Impedimetric fingerprinting and structural analysis of isogenic E. coli biofilms using multielectrode arrays. Sens. Actuators B Chem. 2018;263:319–326. [Google Scholar]
- Gries C.M., Biddle T., Bose J.L., Kielian T., Lo D.D. Staphylococcus aureus fibronectin binding protein A mediates biofilm development and infection. Infect. Immun. 2020;88 doi: 10.1128/IAI.00859-19. e00859–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Cramton S.E., Götz F., Peschel A. Key role of teichoic acid net charge inStaphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001;69:3423. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbein S., Werb M., Opitz M., Lieleg O. Elongational rheology of bacterial biofilms in situ. J. Rheol. 2016;60:1085–1094. [Google Scholar]
- Gu H., Chen A., Song X., Brasch M.E., Henderson J.H., Ren D. How Escherichia coli lands and forms cell clusters on a surface: a new role of surface topography. Sci. Rep. 2016;6:29516. doi: 10.1038/srep29516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B., Giertsen E., Schüpbach P., Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J. Dental Res. 2001;80:363–370. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- Guiton P.S., Hung C.S., Hancock L.E., Caparon M.G., Hultgren S.J. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 2010;78:4166–4175. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Sarkar S., Das B., Bhattacharjee S., Tribedi P. Biofilm, pathogenesis and prevention—a journey to break the wall: a review. Arch. Microbiol. 2016;198:1–15. doi: 10.1007/s00203-015-1148-6. [DOI] [PubMed] [Google Scholar]
- Gurjala A.N., Geringer M.R., Seth A.K., Hong S.J., Smeltzer M.S., Galiano R.D., Leung K.P., Mustoe T.A. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen. 2011;19:400–410. doi: 10.1111/j.1524-475X.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- Ha D.-G., O'Toole G.A. c-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa review. Microbiol. Spectr. 2015;3 doi: 10.1128/microbiolspec.MB-0003-2014-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha K.R., Psaltis A.J., Tan L., Wormald P.J. A sheep model for the study of biofilms in rhinosinusitis. Am. J. Rhinol. 2007;21:339–345. doi: 10.2500/ajr.2007.21.3032. [DOI] [PubMed] [Google Scholar]
- Haagensen J.A.J., Hansen S.K., Christensen B.B., Pamp S.J., Molin S. Development of spatial distribution patterns by biofilm cells. Appl. Environ. Microbiol. 2015;81:6120. doi: 10.1128/AEM.01614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hamilos D.L. Biofilm formations in pediatric respiratory tract infection. Curr. Infect. Dis. Rep. 2019;21:6. doi: 10.1007/s11908-019-0658-9. [DOI] [PubMed] [Google Scholar]
- Hansen L.K., Brown M., Johnson D., Palme D.F., II, Love C., Darouiche R. In vivo model of human pathogen infection and demonstration of efficacy by an antimicrobial pouch for pacing devices. Pacing Clin. Electrophysiol. 2009;32:898–907. doi: 10.1111/j.1540-8159.2009.02406.x. [DOI] [PubMed] [Google Scholar]
- Haque S., Yadav D.K., Bisht S.C., Yadav N., Singh V., Dubey K.K., Jawed A., Wahid M., Dar S.A. Quorum sensing pathways in Gram-positive and -negative bacteria: potential of their interruption in abating drug resistance. J. Chemother. 2019;31:161–187. doi: 10.1080/1120009X.2019.1599175. [DOI] [PubMed] [Google Scholar]
- Haraoka M., Matsumoto T., Takahashi K., Kubo S., Tanaka M., Kumazawa J. Effect of prednisolone on ascending renal infection due to biofilm disease and lower urinary tract obstruction in rats. Urol. Res. 1995;22:383–387. doi: 10.1007/BF00296880. [DOI] [PubMed] [Google Scholar]
- Harding M.W., Marques L.L.R., Howard R.J., Olson M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009;17:475–480. doi: 10.1016/j.tim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Hasturk H., Kantarci A., Goguet-Surmenian E., Blackwood A., Andry C., Serhan C.N., Van Dyke T.E. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Hausner M., Wuertz S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 1999;65:3710. doi: 10.1128/aem.65.8.3710-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häußler S., Ziegler I., Löttel A., Götz F.V., Rohde M., Wehmhöhner D., Saravanamuthu S., Tümmler B., Steinmetz I. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 2003;52:295–301. doi: 10.1099/jmm.0.05069-0. [DOI] [PubMed] [Google Scholar]
- Heim C.E., Vidlak D., Scherr T.D., Kozel J.A., Holzapfel M., Muirhead D.E., Kielian T. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J. Immunol. 2014;192:3778. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P.E., Pittet D., Auckenthaler R., Lew P.D., Schumacher-Perdreau F., Peters G., Waldvogel F.A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Heydorn A., Ersbøll B.K., Hentzer M., Parsek M.R., Givskov M., Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology. 2000;146:2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- Heydorn A., Nielsen A.T., Hentzer M., Sternberg C., Givskov M., Ersbøll B.K., Molin S. Quantification of biofilm structures by the novel computer program comstat. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Hinsa S.M., Espinosa-Urgel M., Ramos J.L., O'toole G.A. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003;49:905–918. doi: 10.1046/j.1365-2958.2003.03615.x. [DOI] [PubMed] [Google Scholar]
- Hoffman M.D., Zucker L.I., Brown P.J.B., Kysela D.T., Brun Y.V., Jacobson S.C. Timescales and frequencies of reversible and irreversible adhesion events of single bacterial cells. Anal. Chem. 2015;87:12032–12039. doi: 10.1021/acs.analchem.5b02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohne D.N., Younger J.G., Solomon M.J. Flexible microfluidic device for mechanical property characterization of soft viscoelastic solids such as bacterial biofilms. Langmuir. 2009;25:7743–7751. doi: 10.1021/la803413x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014;70:205–211. doi: 10.1111/2049-632X.12165. [DOI] [PubMed] [Google Scholar]
- Høiby N. A short history of microbial biofilms and biofilm infections. APMIS. 2017;125:272–275. doi: 10.1111/apm.12686. [DOI] [PubMed] [Google Scholar]
- Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Hong W., Juneau R.A., Pang B., Swords W.E. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the Chinchilla model for otitis media. J. Innate Immun. 2009;1:215–224. doi: 10.1159/000205937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Ghebrehiwet B. Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin. Immunol. Immunopathol. 1992;62:133–138. doi: 10.1016/0090-1229(92)90065-v. [DOI] [PubMed] [Google Scholar]
- Hospenthal M.K., Costa T.R.D., Waksman G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 2017;15:365–379. doi: 10.1038/nrmicro.2017.40. [DOI] [PubMed] [Google Scholar]
- Houry A., Gohar M., Deschamps J., Tischenko E., Aymerich S., Gruss A., Briandet R. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. U S A. 2012;109:13088. doi: 10.1073/pnas.1200791109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug I., Deshpande S., Sprecher K.S., Pfohl T., Jenal U. Second messenger–mediated tactile response by a bacterial rotary motor. Science. 2017;358:531. doi: 10.1126/science.aan5353. [DOI] [PubMed] [Google Scholar]
- Huiszoon R.C., Subramanian S., Ramiah Rajasekaran P., Beardslee L.A., Bentley W.E., Ghodssi R. Flexible platform for in situ impedimetric detection and bioelectric effect treatment of Escherichia coli biofilms. IEEE Trans. Biomed. Eng. 2019;66:1337–1345. doi: 10.1109/TBME.2018.2872896. [DOI] [PubMed] [Google Scholar]
- Hunter R.C., Beveridge T.J. Application of a pH-sensitive fluoroprobe (C-SNARF-4) for pH microenvironment analysis in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2005;71:2501. doi: 10.1128/AEM.71.5.2501-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymes S.R., Randis T.M., Sun T.Y., Ratner A.J. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J. Infect. Dis. 2013;207:1491–1497. doi: 10.1093/infdis/jit047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth B.L., Tweden K., Schroeder R.F., Cameron J.D. In vivo efficacy of silver-coated (Silzone) infection-resistant polyester fabric against a biofilm-producing bacteria, Staphylococcus epidermidis. J. Heart Valve Dis. 1998;7:524–530. [PubMed] [Google Scholar]
- Imam S., Chen Z., Roos D.S., Pohlschröder M. Identification of surprisingly diverse type IV pili, across a broad range of gram-positive bacteria. PLoS One. 2011;6:e28919. doi: 10.1371/journal.pone.0028919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izano E.A., Sadovskaya I., Vinogradov E., Mulks M.H., Velliyagounder K., Ragunath C., Kher W.B., Ramasubbu N., Jabbouri S., Perry M.B. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb. Pathog. 2007;43:1–9. doi: 10.1016/j.micpath.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen S.M., Stickler D.J., Mobley H.L.T., Shirtliff M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis</em>. Clin. Microbiol. Rev. 2008;21:26. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacombs A., Allan J., Hu H., Valente P.M., Wessels W.L., Deva A.K., Vickery K. Prevention of biofilm-induced capsular contracture with antibiotic-impregnated mesh in a porcine model. Aesthet. Surg. J. 2012;32:886–891. doi: 10.1177/1090820X12455429. [DOI] [PubMed] [Google Scholar]
- Jahn A., Griebe T., Nielsen P.H. Composition of pseudomonas putida biofilms: accumulation of protein in the biofilm matrix. Biofouling. 1999;14:49–57. [Google Scholar]
- Jakubovics N.S. Intermicrobial interactions as a driver for community composition and stratification of oral biofilms. J. Mol. Biol. 2015;427:3662–3675. doi: 10.1016/j.jmb.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Janakiraman V., Englert D., Jayaraman A., Baskaran H. Modeling growth and quorum sensing in biofilms grown in microfluidic chambers. Ann. Biomed. Eng. 2009;37:1206–1216. doi: 10.1007/s10439-009-9671-8. [DOI] [PubMed] [Google Scholar]
- Jean J.-S., Tsao C.-W., Chung M.-C. Comparative endoscopic and sem analyses and imaging for biofilm growth on porous quartz sand. Biogeochemistry. 2004;70:427–445. [Google Scholar]
- Jennings L.K., Storek K.M., Ledvina H.E., Coulon C., Marmont L.S., Sadovskaya I., Secor P.R., Tseng B.S., Scian M., Filloux A. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. U S A. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.Ø., Bjarnsholt T., Phipps R., Rasmussen T.B., Calum H., Christoffersen L., Moser C., Williams P., Pressler T., Givskov M. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–1338. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- Jin F., Conrad J.C., Gibiansky M.L., Wong G.C.L. Bacteria use type-IV pili to slingshot on surfaces. Proc. Natl. Acad. Sci. U S A. 2011;108:12617. doi: 10.1073/pnas.1105073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P., Kumlien J., Carlsöö B., Drettner B., Nord C.E. Experimental acute sinusitis in rabbits. A bacteriological and histological study. Acta Otolaryngol. 1988;105:357–366. doi: 10.3109/00016488809097019. [DOI] [PubMed] [Google Scholar]
- Jones B.V., Young R., Mahenthiralingam E., Stickler D.J. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect. Immun. 2004;72:3941. doi: 10.1128/IAI.72.7.3941-3950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.-G., Choi J., Kim S.-K., Lee J.-H., Kwon S. Embedded biofilm, a new biofilm model based on the embedded growth of bacteria. Appl. Environ. Microbiol. 2015;81:211. doi: 10.1128/AEM.02311-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl B.C., Becker K., Löffler B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin. Microbiol. Rev. 2016;29:401–427. doi: 10.1128/CMR.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.B., Fine D.H. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl. Environ. Microbiol. 2002;68:4943. doi: 10.1128/AEM.68.10.4943-4950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara D., Luppens S.B.I., Van Marle J., Özok R., Ten Cate J.M. Microstructural differences between single-species and dual-species biofilms of Streptococcus mutans and Veillonella parvula, before and after exposure to chlorhexidine. FEMS Microbiol. Lett. 2007;271:90–97. doi: 10.1111/j.1574-6968.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- Kassinger S.J., van Hoek M.L. Biofilm architecture: an emerging synthetic biology target. Synth. Syst. Biotechnol. 2020;5:1–10. doi: 10.1016/j.synbio.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya E., Grassi L., Benedetti A., Maisetta G., Pileggi C., Di Luca M., Batoni G., Esin S. In vitro interaction of Pseudomonas aeruginosa biofilms with human peripheral blood mononuclear cells. Front. Cell. Infect. Microbiol. 2020;10:187. doi: 10.3389/fcimb.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon Z., Mctiernan C.D., Suuronen E.J., Mah T.-F., Alarcon E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e01067. e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Barraza J.P., Arthur R.A., Hara A., Lewis K., Liu Y., Scisci E.L., Hajishengallis E., Whiteley M., Koo H. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. U S A. 2020;117:12375–12386. doi: 10.1073/pnas.1919099117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimkes T.E.P., Heinemann M. How Bacteria recognise and respond to surface contact. FEMS Microbiol. Rev. 2020;44:106–122. doi: 10.1093/femsre/fuz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen M., Aaes-Jørgensen A., Molin S., Tolker-Nielsen T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 2003;50:61–68. doi: 10.1046/j.1365-2958.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- Konrat K., Schwebke I., Laue M., Dittmann C., Levin K., Andrich R., Arvand M., Schaudinn C. The bead assay for biofilms: a quick, easy and robust method for testing disinfectants. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157663. e0157663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H., Xiao J., Klein M.I., Jeon J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010;192:3024. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körstgens V., Flemming H.C., Wingender J., Borchard W. Uniaxial compression measurement device for investigation of the mechanical stability of biofilms. J. Microbiol. Methods. 2001;46:9–17. doi: 10.1016/s0167-7012(01)00248-2. [DOI] [PubMed] [Google Scholar]
- Kragh K.N., Hutchison J.B., Melaugh G., Rodesney C., Roberts A.E.L., Irie Y., Jensen P.Ø., Diggle S.P., Allen R.J., Gordon V. Role of multicellular aggregates in biofilm formation. mBio. 2016;7 doi: 10.1128/mBio.00237-16. e00237–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J., Merritt J., Shi W., Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian S.A., Birkenstock T.A., Sauder U., Mack D., Götz F., Landmann R. biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J. Infect. Dis. 2008;197:1028–1035. doi: 10.1086/528992. [DOI] [PubMed] [Google Scholar]
- Krüger W., Vielreicher S., Kapitan M., Jacobsen I.D., Niemiec M.J. Fungal-bacterial interactions in health and disease. Pathogens. 2019;8:70. doi: 10.3390/pathogens8020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Miller K., Charon N.W., Legleiter J. Periplasmic flagella in Borrelia burgdoferi function to maintain cellular integrity upon external stress. PLoS One. 2017;12:e0184648. doi: 10.1371/journal.pone.0184648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka Y., Ishida Y., Yamamura E., Takase H., Otani T., Kumon H. A non-surgical rat model of foreign body-associated urinary tract infection with Pseudomonas aeruginosa. Microbiol. Immunol. 2001;45:9–15. doi: 10.1111/j.1348-0421.2001.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Lam R.H.W., Cui X., Guo W., Thorsen T. High-throughput dental biofilm growth analysis for multiparametric microenvironmental biochemical conditions using microfluidics. Lab Chip. 2016;16:1652–1662. doi: 10.1039/c6lc00072j. [DOI] [PubMed] [Google Scholar]
- Langereis J.D., Weiser J.N. Shielding of a lipooligosaccharide IgM epitope allows evasion of neutrophil-mediated killing of an invasive strain of nontypeable Haemophilus influenzae. mBio. 2014;5 doi: 10.1128/mBio.01478-14. e01478–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauten A., Martinović M., Kursawe L., Kikhney J., Affeld K., Kertzscher U., Falk V., Moter A. Bacterial biofilms in infective endocarditis: an in vitro model to investigate emerging technologies of antimicrobial cardiovascular device coatings. Clin. Res. Cardiol. 2020;110:323–331. doi: 10.1007/s00392-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laventie B.-J., Sangermani M., Estermann F., Manfredi P., Planes R., Hug I., Jaeger T., Meunier E., Broz P., Jenal U. A surface-induced asymmetric program promotes tissue colonization by Pseudomonas aeruginosa. Cell Host Microbe. 2019;25:140–152.e6. doi: 10.1016/j.chom.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Lawrence J.R., Korber D.R., Hoyle B.D., Costerton J.W., Caldwell D.E. Optical sectioning of microbial biofilms. J. Bacteriol. 1991;173:6558. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazurko C., Khatoon Z., Goel K., Sedlakova V., Eren Cimenci C., Ahumada M., Zhang L., Mah T.-F., Franco W., Suuronen E.J. Multifunctional nano and collagen-based therapeutic materials for skin repair. ACS Biomat. Sci. Eng. 2020;6:1124–1134. doi: 10.1021/acsbiomaterials.9b01281. [DOI] [PubMed] [Google Scholar]
- Lebeaux D., Chauhan A., Rendueles O., Beloin C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens (Basel) 2013;2:288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaux D., Ghigo J.-M., Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.K., De Anda J., Baker A.E., Bennett R.R., Luo Y., Lee E.Y., Keefe J.A., Helali J.S., Ma J., Zhao K. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc. Natl. Acad. Sci. U S A. 2018;115:4471–4476. doi: 10.1073/pnas.1720071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Kaplan J.B., Lee W.Y. Microfluidic devices for studying growth and detachment of Staphylococcus epidermidis biofilms. Biomed. Microdevices. 2008;10:489–498. doi: 10.1007/s10544-007-9157-0. [DOI] [PubMed] [Google Scholar]
- Lee K.K., Sheth H.B., Wong W.Y., Sherburne R., Paranchych W., Hodges R.S., Lingwood C.A., Krivan H., Irvin R.T. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Lenton P., Rudney J., Chen R., Fok A., Aparicio C., Jones R.S. Imaging in vivo secondary caries and ex vivo dental biofilms using cross-polarization optical coherence tomography. Dent Mater. 2012;28:792–800. doi: 10.1016/j.dental.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner J., Wolf A., Buchholz F., Mertens F., Neu T.R., Harms H., Maskow T. Miniaturized calorimetry — a new method for real-time biofilm activity analysis. J. Microbiol. Methods. 2008;74:74–81. doi: 10.1016/j.mimet.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Letamendia A., Quevedo C., Ibarbia I., Virto J.M., Holgado O., Diez M., Izpisua Belmonte J.C., Callol-Massot C. Development and validation of an automated high-throughput system for zebrafish in vivo screenings. PLoS One. 2012;7:e36690. doi: 10.1371/journal.pone.0036690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- Li G., Brown P.J.B., Tang J.X., Xu J., Quardokus E.M., Fuqua C., Brun Y.V. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol. Microbiol. 2012;83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Røder H.L., Madsen J.S., Bjarnsholt T., Sørensen S.J., Burmølle M. Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization. Front. Microbiol. 2016;7:1366. doi: 10.3389/fmicb.2016.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López D., Vlamakis H., Losick R., Kolter R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol. Microbiol. 2009;74:609–618. doi: 10.1111/j.1365-2958.2009.06882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorite G.S., Rodrigues C.M., De Souza A.A., Kranz C., Mizaikoff B., Cotta M.A. The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J. Colloid Interf. Sci. 2011;359:289–295. doi: 10.1016/j.jcis.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Lower S.K., Yongsunthon R., Casillas-Ituarte N.N., Taylor E.S., Dibartola A.C., Lower B.H., Beveridge T.J., Buck A.W., Fowler V.G., JR. A tactile response in Staphylococcus aureus. Biophys. J. 2010;99:2803–2811. doi: 10.1016/j.bpj.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke M., Schmidmaier G., Sadoni S., Wildemann B., Schiller R., Haas N.P., Raschke M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521–531. doi: 10.1016/s8756-3282(03)00050-4. [DOI] [PubMed] [Google Scholar]
- Luppens S.B.I., Reij M.W., Van Der Heijden R.W.L., Rombouts F.M., AbEE T. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 2002;68:4194. doi: 10.1128/AEM.68.9.4194-4200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Conover M., Lu H., Parsek M.R., Bayles K., Wozniak D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5:e1000354. doi: 10.1371/journal.ppat.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah T.-F.C., O'Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Maisetta G., Brancatisano F.L., Esin S., Campa M., Batoni G. Gingipains produced by Porphyromonas gingivalis ATCC49417 degrade human-β-defensin 3 and affect peptide's antibacterial activity in vitro. Peptides. 2011;32:1073–1077. doi: 10.1016/j.peptides.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Malone J.G. Role of small colony variants in persistence of Pseudomonas aeruginosa infections in cystic fibrosis lungs. Infect. Drug Resist. 2015;8:237–247. doi: 10.2147/IDR.S68214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M., Bjarnsholt T., Mcbain A.J., James G.A., Stoodley P., Leaper D., Tachi M., Schultz G., Swanson T., Wolcott R.D. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J. Wound Care. 2017;26:20–25. doi: 10.12968/jowc.2017.26.1.20. [DOI] [PubMed] [Google Scholar]
- Malone M., Goeres D.M., Gosbell I., Vickery K., Jensen S., Stoodley P. Approaches to biofilm-associated infections: the need for standardized and relevant biofilm methods for clinical applications. Expert Rev. Anti Infect. Ther. 2017;15:147–156. doi: 10.1080/14787210.2017.1262257. [DOI] [PubMed] [Google Scholar]
- Manzenreiter R., Kienberger F., Marcos V., Schilcher K., Krautgartner W.D., Obermayer A., Huml M., Stoiber W., Hector A., Griese M. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cystic Fibrosis. 2012;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Marsh P.D., Bradshaw D.J. Dental plaque as a biofilm. J. Ind. Microbiol. 1995;15:169–175. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- Mastropaolo M.D., Evans N.P., Byrnes M.K., Stevens A.M., Robertson J.L., Melville S.B. Synergy in polymicrobial infections in a mouse model of type 2 diabetes. Infect. Immun. 2005;73:6055–6063. doi: 10.1128/IAI.73.9.6055-6063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz C., Bergfeld T., Rice S.A., Kjelleberg S. Microcolonies, quorum sensing and cytotoxicity determine the survival of Pseudomonas aeruginosa biofilms exposed to Protozoan grazing. Environ. Microbiol. 2004;6:218–226. doi: 10.1111/j.1462-2920.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Mayberry-Carson K.J., Tober-Meyer B., Smith J.K., Lambe D.W., Jr., Costerton J.W. Bacterial adherence and glycocalyx formation in osteomyelitis experimentally induced with Staphylococcus aureus. Infect. Immun. 1984;43:825–833. doi: 10.1128/iai.43.3.825-833.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy W.F., Bryers J.D., Robbins J., Costerton J.W. Observations of fouling biofilm formation. Can. J. Microbiol. 1981;27:910–917. doi: 10.1139/m81-143. [DOI] [PubMed] [Google Scholar]
- Merritt J.H., Kadouri D.E., O'toole G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005;1:Unit 1B.1. doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz A.J., So M., Sheetz M.P. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- Meyer M.T., Roy V., Bentley W.E., Ghodssi R. Development and validation of a microfluidic reactor for biofilm monitoring via optical methods. J. Micromech. Microeng. 2011;21:054023. [Google Scholar]
- Millhouse E., Jose A., Sherry L., Lappin D.F., Patel N., Middleton A.M., Pratten J., Culshaw S., Ramage G. Development of an in vitroperiodontal biofilm model for assessing antimicrobial and host modulatory effects of bioactive molecules. BMC Oral Health. 2014;14:80. doi: 10.1186/1472-6831-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki Y., Suzuki T., Oka N., Zhang Y., Hayashi Y., Hayashi N., Gotoh N., Ohashi Y. Pseudomonas aeruginosa MucD protease mediates keratitis by inhibiting neutrophil recruitment and promoting bacterial survival. Invest. Ophthalmol. Vis. Sci. 2014;55:240–246. doi: 10.1167/iovs.13-13151. [DOI] [PubMed] [Google Scholar]
- Mowat E., Williams C., Jones B., Mcchlery S., Ramage G. The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm? Med. Mycol. 2009;47:S120–S126. doi: 10.1080/13693780802238834. [DOI] [PubMed] [Google Scholar]
- Mulcahy H., Charron-Mazenod L., Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga R., Stewart P.S., Daly D. Quantitative analysis of biofilm thickness variability. Biotechnol. Bioeng. 1995;45:503–510. doi: 10.1002/bit.260450607. [DOI] [PubMed] [Google Scholar]
- Nakagami G., Sanada H., Sugama J., Morohoshi T., Ikeda T., Ohta Y. Detection of Pseudomonas aeruginosa quorum sensing signals in an infected ischemic wound: an experimental study in rats. Wound Repair Regen. 2008;16:30–36. doi: 10.1111/j.1524-475X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto D.A., Rosenfield M.L., Haaga J.R., Merritt K., Sachs P.B., Hutton M.C., Graham R.C., Rowland D.Y. Young Investigator Award. In vivo treatment of infected prosthetic graft material with urokinase: an Animal Model. J. Vasc. Interv. Radiol. 1994;5:549–552. doi: 10.1016/s1051-0443(94)71552-9. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Minamino T. Flagella-driven motility of bacteria. Biomolecules. 2019;9:279. doi: 10.3390/biom9070279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash E.E., Peters B.M., Lilly E.A., Noverr M.C., Fidel P.L., Jr. A murine model of Candida glabrata vaginitis shows No evidence of an inflammatory immunopathogenic response. PLoS One. 2016;11:e0147969. doi: 10.1371/journal.pone.0147969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J.P., Deng Y., Maneval D.R., German A.L., Martin W.C., Levine M.M. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 1992;60:2297–2304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M.N., Pfeifer J.D., Caparon M. Streptococcus-zebrafish model of bacterial pathogenesis. Infect. Immun. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell S., Suerbaum S., Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat. Rev. Microbiol. 2010;8:564–577. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- Nickel J.C., Olson M., Mclean R.J., Grant S.K., Costerton J.W. An ecological study of infected urinary stone genesis in an animal model. Br. J. Urol. 1987;59:21–30. doi: 10.1111/j.1464-410x.1987.tb04573.x. [DOI] [PubMed] [Google Scholar]
- Nickel J.C., Ruseska I., Wright J.B., Costerton J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P.H., Jahn A., Palmgren R. Conceptual model for production and composition of exopolymers in biofilms. Water Sci. Technol. 1997;36:11–19. [Google Scholar]
- NIH N.I.O.H. Research on microbial biofilms. 2002. https://grants.nih.gov/grants/guide/pa-files/PA-03-047.html
- Ning E., Turnbull G., Clarke J., Picard F., Riches P., Vendrell M., Graham D., Wark A.W., Faulds K., Shu W. 3D bioprinting of mature bacterial biofilms for antimicrobial resistance drug testing. Biofabrication. 2019;11:045018. doi: 10.1088/1758-5090/ab37a0. [DOI] [PubMed] [Google Scholar]
- O'Toole G., Kaplan H.B., Kolter R. biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- O'Toole G.A., Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Okurowska K., Roy S., Thokala P., Partridge L., Garg P., Macneil S., Monk P.N., Karunakaran E. Establishing a porcine ex vivo cornea model for studying drug treatments against bacterial keratitis. J. Vis. Exp. 2020;159:e61156. doi: 10.3791/61156. [DOI] [PubMed] [Google Scholar]
- O’Loughlin C.T., Miller L.C., Siryaporn A., Drescher K., Semmelhack M.F., Bassler B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. U S A. 2013;110:17981. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M.E., Ceri H., Morck D.W., Buret A.G., Read R.R. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- Olson M.E., Harmon B.G., Kollef M.H. Silver-coated endotracheal tubes associated with reduced bacterial burden in the lungs of mechanically ventilated dogs. Chest. 2002;121:863–870. doi: 10.1378/chest.121.3.863. [DOI] [PubMed] [Google Scholar]
- Olson M.E., Nickel J.C., Khoury A.E., Morck D.W., Cleeland R., Costerton J.W. Amdinocillin treatment of catheter-associated bacteriuria in rabbits. J. Infect. Dis. 1989;159:1065–1072. doi: 10.1093/infdis/159.6.1065. [DOI] [PubMed] [Google Scholar]
- Otto K., Silhavy T.J. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. U S A. 2002;99:2287. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisi M., Shifman H., Fine N., Sun C., Glogauer N., Senadheera D., Glogauer M. Novel assay to characterize neutrophil responses to oral biofilms. Infect. Immun. 2019;87 doi: 10.1128/IAI.00790-18. e00790–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst B., Pitts B., Lauchnor E., Stewart P.S. Gel-entrapped Staphylococcus aureus bacteria as models of biofilm infection exhibit growth in dense aggregates, oxygen limitation, antibiotic tolerance, and heterogeneous gene expression. Antimicrob. Agents Chemother. 2016;60:6294–6301. doi: 10.1128/AAC.01336-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H.W., Pinckney J.L. A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb. Ecol. 1996;31:225–247. doi: 10.1007/BF00171569. [DOI] [PubMed] [Google Scholar]
- Pang B., Hong W., West-Barnette S.L., Kock N.D., Swords W.E. Diminished icam-1 expression and impaired pulmonary clearance of nontypeable Haemophilus influenzae in A mouse model of chronic obstructive pulmonary disease/emphysema. Infect. Immun. 2008;76:4959–4967. doi: 10.1128/IAI.00664-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks Q.M., Young R.L., Poch K.R., Malcolm K.C., Vasil M.L., Nick J.A. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J. Med. Microbiol. 2009;58:492–502. doi: 10.1099/jmm.0.005728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula A.J., Hwang G., Koo H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 2020;11:1354. doi: 10.1038/s41467-020-15165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.P., Pesci E.C., Iglewski B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.P., Van Delden C., Iglewski B.H. Active Efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K. Method for studying microbial biofilms in flowing-water systems. Appl. Environ. Microbiol. 1982;43:6. doi: 10.1128/aem.43.1.6-13.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S., Joo H.-S., Duong A.C., Bach T.-H.L., Tan V.Y., Chatterjee S.S., Cheung G.Y.C., Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. U S A. 2012;109:1281. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persat A., Inclan Y.F., Engel J.N., Stone H.A., Gitai Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U S A. 2015;112:7563–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A.C., Wimpenny J.W.T. A constant-depth laboratory model film fermentor. Biotechnol. Bioeng. 1988;32:263–270. doi: 10.1002/bit.260320302. [DOI] [PubMed] [Google Scholar]
- Petrova O.E., Sauer K. Escaping the biofilm in more than one way: desorption, detachment or dispersion. Curr. Opin. Microbiol. 2016;30:67–78. doi: 10.1016/j.mib.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty W., Spanier S., Shuster J.J., Silverthorne C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J. Bone Jt. Surg. Am. 1985;67:1236–1244. [PubMed] [Google Scholar]
- Philipov J.P., Pascalev M.D., Aminkov B.Y., Grosev C.D. Changes in serum carboxyterminal telopeptide of type I collagen in an experimental model of canine osteomyelitis. Calcif. Tissue Int. 1995;57:152–154. doi: 10.1007/BF00298436. [DOI] [PubMed] [Google Scholar]
- Piepenbrink K.H., Sundberg E.J. Motility and adhesion through type IV pili in Gram-positive bacteria. Biochem. Soc. Trans. 2016;44:1659–1666. doi: 10.1042/BST20160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinck S., Etienne M., Dossot M., Jorand F.P.A. A rapid and simple protocol to prepare a living biocomposite that mimics electroactive biofilms. Bioelectrochemistry. 2017;118:131–138. doi: 10.1016/j.bioelechem.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Pinnock A., Shivshetty N., Roy S., Rimmer S., Douglas I., Macneil S., Garg P. Ex vivo rabbit and human corneas as models for bacterial and fungal keratitis. Graefe's Archive Clin. Exp. Ophthalmol. 2017;255:333–342. doi: 10.1007/s00417-016-3546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts B., Willse A., Mcfeters G.A., Hamilton M.A., Zelver N., Stewart P.S. A repeatable laboratory method for testing the efficacy of biocides against toilet bowl biofilms. J. Appl. Microbiol. 2001;91:110–117. doi: 10.1046/j.1365-2672.2001.01342.x. [DOI] [PubMed] [Google Scholar]
- Planson A.-G., Sauveplane V., Dervyn E., Jules M. Bacterial growth physiology and RNA metabolism. Biochim. Biophys. Acta. 2020;1863:194502. doi: 10.1016/j.bbagrm.2020.194502. [DOI] [PubMed] [Google Scholar]
- Pletzer D., Mansour S.C., Wuerth K., Rahanjam N., Hancock R.E.W. New mouse model for chronic infections by gram-negative bacteria enabling the study of anti-infective efficacy and host-microbe interactions. mBio. 2017;8 doi: 10.1128/mBio.00140-17. e00140–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelstra K.A., Barekzi N.A., Grainger D.W., Gristina A.G., Schuler T.C. A novel spinal implant infection model in rabbits. Spine (Phila Pa 1976) 2000;25:406–410. doi: 10.1097/00007632-200002150-00003. [DOI] [PubMed] [Google Scholar]
- Poeppl W., Tobudic S., Lingscheid T., Plasenzotti R., Kozakowski N., Lagler H., Georgopoulos A., Burgmann H. Daptomycin, fosfomycin, or both for treatment of methicillin-resistant Staphylococcus aureus osteomyelitis in an experimental rat model. Antimicrob. Agents Chemother. 2011;55:4999–5003. doi: 10.1128/AAC.00584-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Angulo D.G., Bautista-Hernández L.A., Calvillo-Medina R.P., Castro-Tecorral F.I., Aparicio-Ozores G., López-Villegas E.O., Ribas-Aparicio R.M., Bautista-De Lucio V.M. Microscopic characterization of biofilm in mixed keratitis in a novel murine model. Microb. Pathog. 2020;140:103953. doi: 10.1016/j.micpath.2019.103953. [DOI] [PubMed] [Google Scholar]
- Pouttu R., Puustinen T., Virkola R., Hacker J., Klemm P., Korhonen T.K. Amino acid residue Ala-62 in the FimH fimbrial adhesin is critical for the adhesiveness of meningitis-associated Escherichia coli to collagens. Mol. Microbiol. 1999;31:1747–1757. doi: 10.1046/j.1365-2958.1999.01311.x. [DOI] [PubMed] [Google Scholar]
- Prabhakara R., Harro J.M., Leid J.G., Keegan A.D., Prior M.L., Shirtliff M.E. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 2011;79:5010–5018. doi: 10.1128/IAI.05571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribaz J.R., Bernthal N.M., Billi F., Cho J.S., Ramos R.I., Guo Y., Cheung A.L., Francis K.P., Miller L.S. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J. Orthop. Res. 2012;30:335–340. doi: 10.1002/jor.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price B.L., Morley R., Bowling F.L., Lovering A.M., Dobson C.B. Susceptibility of monomicrobial or polymicrobial biofilms derived from infected diabetic foot ulcers to topical or systemic antibiotics in vitro. PLoS One. 2020;15:e0228704. doi: 10.1371/journal.pone.0228704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R.A., Von Eiff C., Kahl B.C., Becker K., Mcnamara P., Herrmann M., Peters G. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- Proft T., Baker E.N. Pili in Gram-negative and Gram-positive bacteria — structure, assembly and their role in disease. Cell. Mol. Life Sci. 2008;66:613. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purevdorj-Gage B., Costerton W.J., StoodleY P. Phenotypic differentiation and seeding dispersal in non-mucoid and mucoid Pseudomonas aeruginosa biofilms. Microbiology. 2005;151:1569–1576. doi: 10.1099/mic.0.27536-0. [DOI] [PubMed] [Google Scholar]
- Rath H., Stumpp S.N., Stiesch M. Development of a flow chamber system for the reproducible in vitro analysis of biofilm formation on implant materials. PLoS One. 2017;12:e0172095. doi: 10.1371/journal.pone.0172095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read R.R., Eberwein P., Dasgupta M.K., Grant S.K., Lam K., Nickel J.C., Costerton J.W. Peritonitis in peritoneal dialysis: bacterial colonization by biofilm spread along the catheter surface. Kidney Int. 1989;35:614–621. doi: 10.1038/ki.1989.30. [DOI] [PubMed] [Google Scholar]
- Rediske A.M., Roeder B.L., Nelson J.L., Robison R.L., Schaalje G.B., Robison R.A., Pitt W.G. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob. Agents Chemother. 2000;44:771–772. doi: 10.1128/aac.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Wang C., Chen Z., Allan E., Van Der Mei H.C., Busscher H.J. Emergent heterogeneous microenvironments in biofilms: substratum surface heterogeneity and bacterial adhesion force-sensing. FEMS Microbiol. Rev. 2018;42:259–272. doi: 10.1093/femsre/fuy001. [DOI] [PubMed] [Google Scholar]
- Richter L., Stepper C., Mak A., Reinthaler A., Heer R., Kast M., Brückl H., Ertl P. Development of a microfluidic biochip for online monitoring of fungal biofilm dynamics. Lab Chip. 2007;7:1723–1731. doi: 10.1039/b708236c. [DOI] [PubMed] [Google Scholar]
- Riedel K., Hentzer M., Geisenberger O., Huber B., Steidle A., Wu H., Høiby N., Givskov M., Molin S., Eberl L. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001;147:3249–3262. doi: 10.1099/00221287-147-12-3249. [DOI] [PubMed] [Google Scholar]
- Rimondini L., Farè S., Brambilla E., Felloni A., Consonni C., Brossa F., Carrassi A. The effect of surface roughness on early in vivo plaque colonization on titanium. J. Periodontol. 1997;68:556–562. doi: 10.1902/jop.1997.68.6.556. [DOI] [PubMed] [Google Scholar]
- Roberts M.E., Stewart P.S. Modelling protection from antimicrobial agents in biofilms through the formation of persister cells. Microbiology. 2005;151:75–80. doi: 10.1099/mic.0.27385-0. [DOI] [PubMed] [Google Scholar]
- Robinson R.W., Akin D.E., Nordstedt R.A., Thomas M.V., Aldrich H.C. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl. Environ. Microbiol. 1984;48:127–136. doi: 10.1128/aem.48.1.127-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D., Aguilar C., Losick R., Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U S A. 2010;107:2230. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.J., Bermudez L.E. Mycobacterium avium biofilm attenuates mononuclear phagocyte function by triggering hyperstimulation and apoptosis during early infection. Infect. Immun. 2014;82:405. doi: 10.1128/IAI.00820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux D., Cywes-Bentley C., Zhang Y.-F., Pons S., Konkol M., Kearns D.B., Little D.J., Howell P.L., Skurnik D., Pier G.B. Identification of poly-N-acetylglucosamine as a major polysaccharide component of the Bacillus subtilis biofilm matrix. J. Biol. Chem. 2015;290:19261–19272. doi: 10.1074/jbc.M115.648709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh K.P., Sauer K. Biofilm dispersion. Nat. Rev. Microbiol. 2020;18:571–586. doi: 10.1038/s41579-020-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp M.E., Ulphani J.S., Fey P.D., Mack D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 1999;67:2656–2659. doi: 10.1128/iai.67.5.2656-2659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaka A., Palmer K., Suzuki T., Gilmore M.S. In vitro and in vivo models of Staphylococcus aureus endophthalmitis implicate specific nutrients in ocular infection. PLoS One. 2014;9:e110872. doi: 10.1371/journal.pone.0110872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said J., Walker M., Parsons D., Stapleton P., Beezer A.E., Gaisford S. Development of a flow system for studying biofilm formation on medical devices with microcalorimetry. Methods. 2015;76:35–40. doi: 10.1016/j.ymeth.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Sakuragi Y., Kolter R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa</em>. J. Bacteriol. 2007;189:5383. doi: 10.1128/JB.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangermani M., Hug I., Sauter N., Pfohl T., Jenal U. Tad pili play a dynamic role in Caulobacter crescentus surface colonization. mBio. 2019;10 doi: 10.1128/mBio.01237-19. e01237–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzén L., Linder L. Infection adjacent to titanium and bone cement implants: an experimental study in rabbits. Biomaterials. 1995;16:1273–1277. doi: 10.1016/0142-9612(95)98136-3. [DOI] [PubMed] [Google Scholar]
- Sapi E., Bastian S.L., Mpoy C.M., Scott S., Rattelle A., Pabbati N., Poruri A., Burugu D., Theophilus P.A.S., Pham T.V. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS One. 2012;7:e48277. doi: 10.1371/journal.pone.0048277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswathi P., Beuerman R.W. Corneal biofilms: from planktonic to microcolony formation in an experimental keratitis infection with Pseudomonas aeruginosa. Ocul. Surf. 2015;13:331–345. doi: 10.1016/j.jtos.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Satoh M., Munakata K., Kitoh K., Takeuchi H., Yoshida O. A newly designed model for infection-induced bladder stone formation in the rat. J. Urol. 1984;132:1247–1249. doi: 10.1016/s0022-5347(17)50115-9. [DOI] [PubMed] [Google Scholar]
- Sauer K., Camper A.K. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 2001;183:6579. doi: 10.1128/JB.183.22.6579-6589.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Camper A.K., Ehrlich G.D., Costerton J.W., Davies D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002;184:1140. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Cullen M.C., Rickard A.H., Zeef L.A.H., Davies D.G., Gilbert P. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 2004;186:7312. doi: 10.1128/JB.186.21.7312-7326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheman L., Janota M., Lewin P. The production of experimental osteomyelitis: preliminary report. J. Am. Med. Assoc. 1941;117:1525–1529. [Google Scholar]
- Scherr T.D., Hanke M.L., Huang O., James D.B.A., Horswill A.R., Bayles K.W., Fey P.D., Torres V.J., Kielian T. Staphylococcus aureus Biofilms Induce Macrophage Dysfunction through Leukocidin AB and Alpha-Toxin. mBio. 2015;6 doi: 10.1128/mBio.01021-15. e01021–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlafer S., Meyer R.L. Confocal microscopy imaging of the biofilm matrix. J. Microbiol. Methods. 2017;138:50–59. doi: 10.1016/j.mimet.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Schniederberend M., Williams J.F., Shine E., Shen C., Jain R., Emonet T., Kazmierczak B.I. Modulation of flagellar rotation in surface-attached bacteria: a pathway for rapid surface-sensing after flagellar attachment. PLoS Pathog. 2019;15:e1008149. doi: 10.1371/journal.ppat.1008149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurman D.J., Trindade C., Hirshman H.P., Moser K., Kajiyama G., Stevens P. Antibiotic-acrylic bone cement composites. Studies of gentamicin and Palacos. J. Bone Jt. Surg. Am. 1978;60:978–984. [PubMed] [Google Scholar]
- Schwarzer C., Fu Z., Patanwala M., Hum L., Lopez-Guzman M., Illek B., Kong W., Lynch S.V., Machen T.E. Pseudomonas aeruginosa biofilm-associated homoserine lactone C12 rapidly activates apoptosis in airway epithelia. Cell Microbiol. 2012;14:698–709. doi: 10.1111/j.1462-5822.2012.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaniakou A., Croll R.P., Chappe V. Animal models in the pathophysiology of cystic fibrosis. Front. Pharmacol. 2019;9:1475. doi: 10.3389/fphar.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seper A., Fengler V.H.I., Roier S., Wolinski H., Kohlwein S.D., Bishop A.L., Camilli A., Reidl J., Schild S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011;82:1015–1037. doi: 10.1111/j.1365-2958.2011.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settem R.P., El-Hassan A.T., Honma K., Stafford G.P., Sharma A. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect. Immun. 2012;80:2436–2443. doi: 10.1128/IAI.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L., Deng D., Buskermolen J.K., Roffel S., Janus M.M., Krom B.P., Crielaard W., Gibbs S. Commensal and pathogenic biofilms alter toll-like receptor signaling in reconstructed human gingiva. Front. Cell. Infect. Microbiol. 2019;9:282. doi: 10.3389/fcimb.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P.-C., Huang C.-T. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 2002;49:309–314. doi: 10.1093/jac/49.2.309. [DOI] [PubMed] [Google Scholar]
- Shirtliff M.E., Peters B.M., Jabra-Rizk M.A. Cross-Kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009;299:1–8. doi: 10.1111/j.1574-6968.2009.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti O., Cirioni O., Ghiselli R., Goteri G., Scalise A., Orlando F., Silvestri C., Riva A., Saba V., Madanahally K.D. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008;52:2205–2211. doi: 10.1128/AAC.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.V., Vyas V., Patil R., Sharma V., Scopelliti P.E., Bongiorno G., Podestà A., Lenardi C., Gade W.N., Milani P. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS One. 2011;6:e25029. doi: 10.1371/journal.pone.0025029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.K., Yadav V.K., Kalia M., Sharma D., Pandey D., Agarwal V. Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxo-dodecanoyl)-l-homoserine lactone triggers mitochondrial dysfunction and apoptosis in neutrophils through calcium signaling. Med. Microbiol. Immunol. 2019;208:855–868. doi: 10.1007/s00430-019-00631-8. [DOI] [PubMed] [Google Scholar]
- Singh P.K., Yadav V.K., Kalia M., Sharma D., Pandey D., Agarwal V. Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxo-dodecanoyl)-l-homoserine lactone triggers mitochondrial dysfunction and apoptosis in neutrophils through calcium signaling. Med. Microbiol. Immunol. 2019;208:855–868. doi: 10.1007/s00430-019-00631-8. [DOI] [PubMed] [Google Scholar]
- Siryaporn A., Kuchma S.L., O’toole G.A., Gitai Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc. Natl. Acad. Sci. U S A. 2014;111:16860. doi: 10.1073/pnas.1415712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons C.H., Cutress T.W., Hoffman M.P., Wakefield J.S.J. A multi-station dental plaque microcosm (artificial mouth) for the study of plaque growth, metabolism, pH, and mineralization. J. Dental Res. 1991;70:1409–1416. doi: 10.1177/00220345910700110301. [DOI] [PubMed] [Google Scholar]
- Skerker J.M., Berg H.C. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. U S A. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J.N., Beaver M., Smeltzer M.S., Kielian T. Biofilm-infected intracerebroventricular shunts elicit inflammation within the central nervous system. Infect. Immun. 2012;80:3206–3214. doi: 10.1128/IAI.00645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R.A., Ellison C.K., Severin G.B., Whitfield G.B., Waters C.M., Brun Y.V. Surface sensing stimulates cellular differentiation in Caulobacter crescentus</em>. Proc. Natl. Acad. Sci.U S A. 2020;117:17984. doi: 10.1073/pnas.1920291117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Sønderholm M., Kragh K.N., Koren K., Jakobsen T.H., Darch S.E., Alhede M., Jensen P.Ø., Whiteley M., Kühl M., Bjarnsholt T. Pseudomonas aeruginosa Aggregate Formation in an Alginate Bead Model System Exhibits In Vivo-Like Characteristics. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.00113-17. e00113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Lee S.M., Park I.H., Yong D., Lee K.S., Shin J.S., Yoo K.H. Vertical capacitance aptasensors for real-time monitoring of bacterial growth and antibiotic susceptibility in blood. Biosens. Bioelectron. 2019;143:111623. doi: 10.1016/j.bios.2019.111623. [DOI] [PubMed] [Google Scholar]
- Southey-Pillig C.J., Davies D.G., Sauer K. Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2005;187:8114. doi: 10.1128/JB.187.23.8114-8126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood R.T., Rice J.L., Mcdonald P.J., Hakendorf P.H., Rozenbilds M.A. Infection in experimental hip arthroplasties. J. Bone Jt. Surg. Br. 1985;67:229–231. doi: 10.1302/0301-620X.67B2.3980532. [DOI] [PubMed] [Google Scholar]
- Spoering A.L., Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001;183:6746. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann V.A., Potapova I., Camenisch K., Nehrbass D., Richards R.G., Moriarty T.F. In vivo MicroCT monitoring of osteomyelitis in a rat model. Biomed. Res. Int. 2015;2015:587857. doi: 10.1155/2015/587857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P.S., Franklin M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- Stones D.H., Krachler A.M. Against the tide: the role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 2016;44:1571–1580. doi: 10.1042/BST20160186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P., Conti S.F., Demeo P.J., Nistico L., Melton-Kreft R., Johnson S., Darabi A., Ehrlich G.D., Costerton J.W., Kathju S. Characterization of a mixed MRSA/MRSE biofilm in an explanted total ankle arthroplasty. FEMS Immunol. Med. Microbiol. 2011;62:66–74. doi: 10.1111/j.1574-695X.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- Stoodley P., Debeer D., Lewandowski Z. Liquid flow in biofilm systems. Appl. Environ. Microbiol. 1994;60:2711–2716. doi: 10.1128/aem.60.8.2711-2716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P., Nistico L., Johnson S., Lasko L.-A., Baratz M., Gahlot V., Ehrlich G.D., Kathju S. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J. Bone Jt. Surg. Am. 2008;90:1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P., Sauer K., Davies D.G., Costerton J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Stoodley P., Wilson S., Hall-Stoodley L., Boyle J.D., Lappin-Scott H.M., Costerton J.W. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl. Environ. Microbiol. 2001;67:5608. doi: 10.1128/AEM.67.12.5608-5613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P.-T., Liao C.-T., Roan J.-R., Wang S.-H., Chiou A., Syu W., Jr. Bacterial colony from two-dimensional division to three-dimensional development. PLoS One. 2012;7:e48098. doi: 10.1371/journal.pone.0048098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C., Piculell M., Modin O., Langenheder S., Persson F., Hermansson M. Thickness determines microbial community structure and function in nitrifying biofilms via deterministic assembly. Scientific Rep. 2019;9:5110. doi: 10.1038/s41598-019-41542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S., Huiszoon R.C., Chu S., Bentley W.E., Ghodssi R. Microsystems for biofilm characterization and sensing – a review. Biofilm. 2020;2:100015. doi: 10.1016/j.bioflm.2019.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Edelstein A., Rhen M., Normark S.J., Pfeifer J.D. Development of a murine model of chronic Salmonella infection. Infect. Immun. 1997;65:838–842. doi: 10.1128/iai.65.2.838-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A.R., Hoppenbrouwers T., Lemmens-Den Toom N.A., Snijders S.V., Van Neck J.W., Verbon A., De Maat M.P.M., Van Wamel W.J.B. During the early stages of Staphylococcus aureus biofilm formation, induced neutrophil extracellular traps are degraded by autologous thermonuclease. Infect. Immun. 2019;87 doi: 10.1128/IAI.00605-19. e00605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Qu F., Ling Y., Mao P., Xia P., Chen H., Zhou D. Biofilm-associated infections: antibiotic resistance and novel therapeutic strategies. Future Microbiol. 2013;8:877–886. doi: 10.2217/fmb.13.58. [DOI] [PubMed] [Google Scholar]
- Sun H., Zusman D.R., Shi W. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 2000;10:1143–1146. doi: 10.1016/s0960-9822(00)00705-3. [DOI] [PubMed] [Google Scholar]
- Sun Y., Chandra J., Mukherjee P., Szczotka-Flynn L., Ghannoum M.A., Pearlman E. A murine model of contact lens-associated fusarium keratitis. Invest. Ophthalmol. Vis. Sci. 2010;51:1511–1516. doi: 10.1167/iovs.09-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Dowd S.E., Smith E., Rhoads D.D., Wolcott R.D. In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen. 2008;16:805–813. doi: 10.1111/j.1524-475X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- Swords W.E., Moore M.L., Godzicki L., Bukofzer G., Mitten M.J., Voncannon J. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect. Immun. 2004;72:106–113. doi: 10.1128/IAI.72.1.106-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda K., Ishii Y., Horikawa M., Matsumoto T., Miyairi S., Pechere J.C., Standiford T.J., Ishiguro M., Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-Oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 2003;71:5785. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchekmedyian N.S., Newman K., Moody M.R., Costerton J.W., Aisner J., Schimpff S.C., Reed W.P. Case report: special studies of the hickman catheter of a patient with recurrent bacteremia and candidemia. Am. J. Med. Sci. 1986;291:419–424. doi: 10.1097/00000441-198606000-00009. [DOI] [PubMed] [Google Scholar]
- Thomas W.E., Trintchina E., Forero M., Vogel V., Sokurenko E.V. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- Thormann K.M., Saville R.M., Shukla S., Spormann A.M. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 2005;187:1014. doi: 10.1128/JB.187.3.1014-1021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow L.R., Hanke M.L., Fritz T., Angle A., Aldrich A., Williams S.H., Engebretsen I.L., Bayles K.W., Horswill A.R., Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011;186:6585. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnheer T., Bostanci N., Belibasakis G.N. Microbial dynamics during conversion from supragingival to subgingival biofilms in an in vitro model. Mol. Oral Microbiol. 2016;31:125–135. doi: 10.1111/omi.12108. [DOI] [PubMed] [Google Scholar]
- Tielker D., Hacker S., Loris R., Strathmann M., Wingender J., Wilhelm S., Rosenau F., Jaeger K.E. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151:1313–1323. doi: 10.1099/mic.0.27701-0. [DOI] [PubMed] [Google Scholar]
- Tolker-Nielsen T., Brinch U.C., Ragas P.C., Andersen J.B., Jacobsen C.S., Molin S. Development and dynamics of Pseudomonas biofilms. J. Bacteriol. 2000;182:6482–6489. doi: 10.1128/jb.182.22.6482-6489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson D.F., Bandyk D.F., Kaebnick H.W., Seabrook G.R., Towne J.B. Surface biofilm disruption. Enhanced recovery of microorganisms from vascular prostheses. Arch. Surg. 1987;122:38–43. doi: 10.1001/archsurg.1987.01400130044006. [DOI] [PubMed] [Google Scholar]
- Toutain C.M., Caizza N.C., Zegans M.E., O'toole G.A. Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Res. Microbiol. 2007;158:471–477. doi: 10.1016/j.resmic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Trunk T., Khalil H.S., Leo J.C. Bacterial autoaggregation. AIMS Microbiol. 2018;4:140–164. doi: 10.3934/microbiol.2018.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O'collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik E.J., Giltner C.L., Audette G.F., Keizer D.W., Bautista D.L., Slupsky C.M., Sykes B.D., Irvin R.T. DNA binding: a novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol. 2005;187:1455. doi: 10.1128/JB.187.4.1455-1464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J.J., Nguyen V., O'brien D.K., Rodgers K., Walker R.A., Melville S.B. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other Clostridia. Mol. Microbiol. 2006;62:680–694. doi: 10.1111/j.1365-2958.2006.05414.x. [DOI] [PubMed] [Google Scholar]
- Varga J.J., Therit B., Melville S.B. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 2008;76:4944–4951. doi: 10.1128/IAI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso T.R., Amiguet M., Rousson V., Giddey M., Vouillamoz J., Moreillon P., Entenza J.M. Induction of experimental endocarditis by continuous low-grade bacteremia mimicking spontaneous bacteremia in humans. Infect. Immun. 2011;79:2006–2011. doi: 10.1128/IAI.01208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamakis H., Aguilar C., Losick R., Kolter R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008;22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrath M.G., Nepom G.T. Lost in translation: barriers to implementing clinical immunotherapeutics for autoimmunity. J. Exp. Med. 2005;202:1159–1162. doi: 10.1084/jem.20051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T.S., Tomlin K.L., Worthen G.S., Poch K.R., Lieber J.G., Saavedra M.T., Fessler M.B., Malcolm K.C., Vasil M.L., Nick J.A. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect. Immun. 2005;73:3693. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M.C., Roe F., Bugnicourt A., Franklin M.J., Stewart P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003;47:317. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I.W., Anderson J.M., Marchant R.E. Staphylococcus epidermidis adhesion to hydrophobic biomedical polymer is mediated by platelets. J. Infect. Dis. 1993;167:329–336. doi: 10.1093/infdis/167.2.329. [DOI] [PubMed] [Google Scholar]
- Wang R., Braughton K.R., Kretschmer D., Bach T.-H.L., Queck S.Y., Li M., Kennedy A.D., Dorward D.W., Klebanoff S.J., Peschel A. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wang R., Khan B.A., Cheung G.Y.C., Bach T.-H.L., Jameson-Lee M., Kong K.-F., Queck S.Y., Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Flint S., Palmer J. Magnesium and calcium ions: roles in bacterial cell attachment and biofilm structure maturation. Biofouling. 2019;35:959–974. doi: 10.1080/08927014.2019.1674811. [DOI] [PubMed] [Google Scholar]
- Wargo M.J., Hogan D.A. Fungal—bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 2006;9:359–364. doi: 10.1016/j.mib.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Waters C.M., Bassler B.L. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Wentland E.J., Stewart P.S., Huang C.T., Mcfeters G.A. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 1996;12:316–321. doi: 10.1021/bp9600243. [DOI] [PubMed] [Google Scholar]
- Wentzien S., Sand W., Albertsen A., Steudel R. Thiosulfate and tetrathionate degradation as well as biofilm generation by Thiobacillus intermedius and Thiobacillus versutus studied by microcalorimetry, HPLC, and ion-pair chromatography. Arch. Microbiol. 1994;161:116–125. [Google Scholar]
- Whitchurch C.B., Tolker-Nielsen T., Ragas P.C., Mattick J.S. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Williams D.L., Haymond B.S., Woodbury K.L., Beck J.P., Moore D.E., Epperson R.T., Bloebaum R.D. Experimental model of biofilm implant-related osteomyelitis to test combination biomaterials using biofilms as initial inocula. J. Biomed. Mater. Res. A. 2012;100:1888–1900. doi: 10.1002/jbm.a.34123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender J., Strathmann M., Rode A., Leis A., Flemming H.-C. [25] Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. In: Doyle R.J., editor. Methods in Enzymology. Academic Press; 2001. pp. 302–314. [DOI] [PubMed] [Google Scholar]
- Worlitzsch D., Tarran R., Ulrich M., Schwab U., Cekici A., Meyer K.C., Birrer P., Bellon G., Berger J., Weiss T. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E., Neethirajan S., Weng X. Microfluidic wound model for studying the behaviors of Pseudomonas aeruginosa in polymicrobial biofilms. Biotechnol. Bioeng. 2015;112:2351–2359. doi: 10.1002/bit.25651. [DOI] [PubMed] [Google Scholar]
- Xiao J., Koo H. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J. Appl. Microbiol. 2010;108:2103–2113. doi: 10.1111/j.1365-2672.2009.04616.x. [DOI] [PubMed] [Google Scholar]
- Xu K.D., Stewart P.S., Xia F., Huang C.-T., Mcfeters G.A. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 1998;64:4035–4039. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Tomono K., Sawai T., Hirakata Y., Kadota J., Koga H., Tashiro T., Kohno S. Effect of clarithromycin on lymphocytes in chronic respiratory Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 1997;155:337–342. doi: 10.1164/ajrccm.155.1.9001333. [DOI] [PubMed] [Google Scholar]
- Yang D.C., Blair K.M., Salama N.R. Staying in shape: the impact of cell shape on bacterial survival in diverse environments. Microbiol. Mol. Biol. Rev. 2016;80:187–203. doi: 10.1128/MMBR.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Hu Y., Liu Y., Zhang J., Ulstrup J., Molin S. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ. Microbiol. 2011;13:1705–1717. doi: 10.1111/j.1462-2920.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- Yin W., Wang Y., Liu L., He J. Biofilms: the microbial "protective clothing" in extreme environments. Int. J. Mol. Sci. 2019;20:3423. doi: 10.3390/ijms20143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bishop P.L., Kupferle M.J. Measurement of polysaccharides and proteins in biofilm extracellular polymers. Water Sci. Technol. 1998;37:345–348. [Google Scholar]
- Zhang S., Gao X., Xiao G., Lu C., Yao H., Fan H., Wu Z. Intracranial subarachnoidal route of infection for investigating roles of Streptococcus suis biofilms in meningitis in a mouse infection model. J. Vis. Exp. 2018;137:e57658. doi: 10.3791/57658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yu Z., Ding T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms. 2020;8:425. doi: 10.3390/microorganisms8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A., Gonzalez L., Blikslager A. Large animal models: the key to translational discovery in digestive disease research. Cell. Mol. Gastroenterol. Hepatol. 2016;2:716–724. doi: 10.1016/j.jcmgh.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125:353–364. doi: 10.1111/apm.12687. [DOI] [PubMed] [Google Scholar]
- Zobell C.E., Allen E.C. The significance of marine bacteria in the fouling of submerged surfaces. J. Bacteriol. 1935;29:239–251. doi: 10.1128/jb.29.3.239-251.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogaj X., Nimtz M., Rohde M., Bokranz W., Römling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]