Key Points
Question
Does antimicrobial therapy in addition to usual care improve clinical outcomes in patients with idiopathic pulmonary fibrosis?
Findings
In this pragmatic randomized clinical trial that included 513 adults with idiopathic pulmonary fibrosis the addition of co-trimoxazole (trimethoprim-sulfamethoxazole) or doxycycline to usual care compared with usual care alone resulted in a rate of first nonelective respiratory hospitalization or death of 20.4 vs 18.4 events per 100 person-years, a difference that was not statistically significant.
Meaning
Among adults with idiopathic pulmonary fibrosis, addition of co-trimoxazole or doxycycline compared with usual care did not significantly improve the time to nonrespiratory hospitalization or death.
Abstract
Importance
Alteration in lung microbes is associated with disease progression in idiopathic pulmonary fibrosis.
Objective
To assess the effect of antimicrobial therapy on clinical outcomes.
Design, Setting, and Participants
Pragmatic, randomized, unblinded clinical trial conducted across 35 US sites. A total of 513 patients older than 40 years were randomized from August 2017 to June 2019 (final follow-up was January 2020).
Interventions
Patients were randomized in a 1:1 allocation ratio to receive antimicrobials (n = 254) or usual care alone (n = 259). Antimicrobials included co-trimoxazole (trimethoprim 160 mg/sulfamethoxazole 800 mg twice daily plus folic acid 5 mg daily, n = 128) or doxycycline (100 mg once daily if body weight <50 kg or 100 mg twice daily if ≥50 kg, n = 126). No placebo was administered in the usual care alone group.
Main Outcomes and Measures
The primary end point was time to first nonelective respiratory hospitalization or all-cause mortality.
Results
Among the 513 patients who were randomized (mean age, 71 years; 23.6% women), all (100%) were included in the analysis. The study was terminated for futility on December 18, 2019. After a mean follow-up time of 13.1 months (median, 12.7 months), a total of 108 primary end point events occurred: 52 events (20.4 events per 100 patient-years [95% CI, 14.8-25.9]) in the usual care plus antimicrobial therapy group and 56 events (18.4 events per 100 patient-years [95% CI, 13.2-23.6]) in the usual care group, with no significant difference between groups (adjusted HR, 1.04 [95% CI, 0.71-1.53; P = .83]. There was no statistically significant interaction between the effect of the prespecified antimicrobial agent (co-trimoxazole vs doxycycline) on the primary end point (adjusted HR, 1.15 [95% CI 0.68-1.95] in the co-trimoxazole group vs 0.82 [95% CI, 0.46-1.47] in the doxycycline group; P = .66). Serious adverse events occurring at 5% or greater among those treated with usual care plus antimicrobials vs usual care alone included respiratory events (16.5% vs 10.0%) and infections (2.8% vs 6.6%); adverse events of special interest included diarrhea (10.2% vs 3.1%) and rash (6.7% vs 0%).
Conclusions and Relevance
Among adults with idiopathic pulmonary fibrosis, the addition of co-trimoxazole or doxycycline to usual care, compared with usual care alone, did not significantly improve time to nonelective respiratory hospitalization or death. These findings do not support treatment with these antibiotics for the underlying disease.
Trial Registration
ClinicalTrials.gov Identifier: NCT02759120
This clinical trial investigates whether the addition of antimicrobial treatments co-trimoxazole or doxycycline to usual care improves outcomes among patients with idiopathic pulmonary fibrosis better than usual care alone.
Introduction
Lung dysbiosis, observed as increased bacterial load and/or loss of diversity, has been reported in patients with idiopathic pulmonary fibrosis (IPF)1,2 and is associated with disease progression and a local and systemic immune response,3,4 potentially contributing to acute exacerbations, hospitalizations, and decreased survival. Although initial randomized trial data suggested improved outcomes with co-trimoxazole (trimethoprim-sulfamethoxazole) therapy in patients with fibrosing interstitial lung diseases,5 a large placebo-controlled trial failed to document an improvement in clinical outcomes with this agent.6 Preliminary data suggested that doxycycline can improve outcomes7,8 and inhibit metalloproteinases in patients with IPF.7 Because antimicrobial therapy has been suggested to favorably alter the lung microbial community in other chronic disorders,9,10 this study was designed to address the hypothesis that an antimicrobial therapeutic strategy, with either co-trimoxazole or doxycycline, reduces the risk of nonelective respiratory hospitalization or death among patients with IPF.
Methods
This randomized clinical trial used an approach of 2 potentially effective antimicrobial therapies to simulate clinical practice. This trial was designed with knowledge of the EME-TIPAC (Efficacy and Mechanism Evaluation of Treating Idiopathic Pulmonary Fibrosis With the Addition of Co-trimoxazole) study6 with the expectation that this therapeutic strategy would better simulate clinical practice, facilitate enrollment, and provide robust results in a timely fashion. The study protocol is provided in Supplement 1; the statistical analysis plan in Supplement 2; and the investigator’s assessment of the PRECIS-2 criteria11,12 in eFigure 1 in Supplement 3. The study was approved by institutional review boards at each institution, and each patient provided written informed consent.
Participants
The detailed inclusion and exclusion criteria are enumerated in eTable 1 in Supplement 3. Exclusions were limited and designed to minimize the risk of antimicrobial therapy given the limited in-person follow-up visits.12 Key exclusions included allergies to both antimicrobial agents, allergy or intolerance to tetracycline and features that increase the risk of co-trimoxazole use, pregnancy, use of an investigational therapy, or concomitant use of immunosuppressive therapy. The National Heart, Lung, and Blood Institute required collection of race, ethnicity, and sex data from participating patients with data self-reported based on fixed categories (American Indian, Asian, Native Hawaiian or other Pacific Islander, Black or African American, White, or other) (Table 1).
Table 1. Baseline Characteristics of Patients.
| Parameter | No. (%) of patients | |
|---|---|---|
| Usual care + antimicrobial therapy (n = 254) | Usual care (n = 259) | |
| Sex | ||
| Women | 60 (23.6) | 51 (19.7) |
| Men | 194 (76.4) | 208 (80.3) |
| Age, median (IQR), y | 71.0 (66.9-76.3) | 72.1 (67.7-76.1) |
| Race, No.a | 251 | 257 |
| White | 231 (89.9) | 244 (94.9) |
| Asian | 9 (3.6) | 5 (1.9) |
| African American or Black | 7 (2.8) | 4 (1.6) |
| Another race | 4 (1.6) | 3 (1.2) |
| American Indian | 0 | 1 (0.3) |
| Smoking status | ||
| Current | 1 (0.4) | 2 (0.8) |
| Formerb | 150 (59.1) | 175 (67.6) |
| Comorbid conditions, No./total (%)c | ||
| Gastroesophageal reflux | 156/251 (62.2) | 149/256 (58.2) |
| Hyperlipidemia | 144/252 (57.1) | 154/257 (59.9) |
| Hypertension | 127/252 (50.4) | 129/258 (50.0) |
| Coronary artery disease | 69 (27.2) | 79 (30.5) |
| Diabetes | 49 (19.3) | 39 (15.1) |
| Emphysema or chronic bronchitis | 25 (9.8) | 28 (10.8) |
| Myocardial infarction | 20/251 (8.0) | 32 (12.4) |
| Atrial fibrillation | 21/252 (8.3) | 31/258 (12.0) |
| Valvular heart disease | 7/252 (2.8) | 13/256 (5.1) |
| Congestive heart failure | 8 (3.1) | 12/258 (4.7) |
| Stroke | 9 (3.5) | 10/258 (3.9) |
| Moderate or severe kidney disease | 9/253 (3.6) | 8 (3.1) |
| Connective tissue disease | 2/249 (0.8) | 1/258 (0.4) |
| Prior medications, No.d | 253 | 259 |
| Proton pump inhibitors | 158 (62.5) | 158 (61.0) |
| Prednisone (>1 mo) | 43 (17.0) | 53 (20.5) |
| Azathioprine | 1 (0.4) | 39 (1.22) |
| H2 blocker | 49 (19.4) | 42 (16.2) |
| N-acetylcysteine | 14 (5.5) | 13 (5.0) |
| IPF-specific medication prior to randomization, No. | 253 | 259 |
| Pirfenidone | 158 (62.5) | 160 (61.8) |
| Nintedanib | 101 (39.9) | 97 (37.5) |
Abbreviations: H2, histamine 2; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range
Self-reported from fixed categories.
Former smoker was defined as stopped smoking 1 or more month prior to screening.
Self-reported.
Medication at the time of enrollment and any prior usage.
Randomization
Patients were randomized to the 2 strategies in a 1:1 allocation ratio using a computer-generated algorithm. Prior to randomization, eligible patients and their physician were offered a preference between the co-trimoxazole or doxycycline stratum; the protocol advised co-trimoxazole if possible (Figure 1).12 Once patients were randomized their follow-up schedule varied based on their assigned treatment group (Supplement 1).12
Figure 1. Recruitment, Randomization, and Patient Flow.
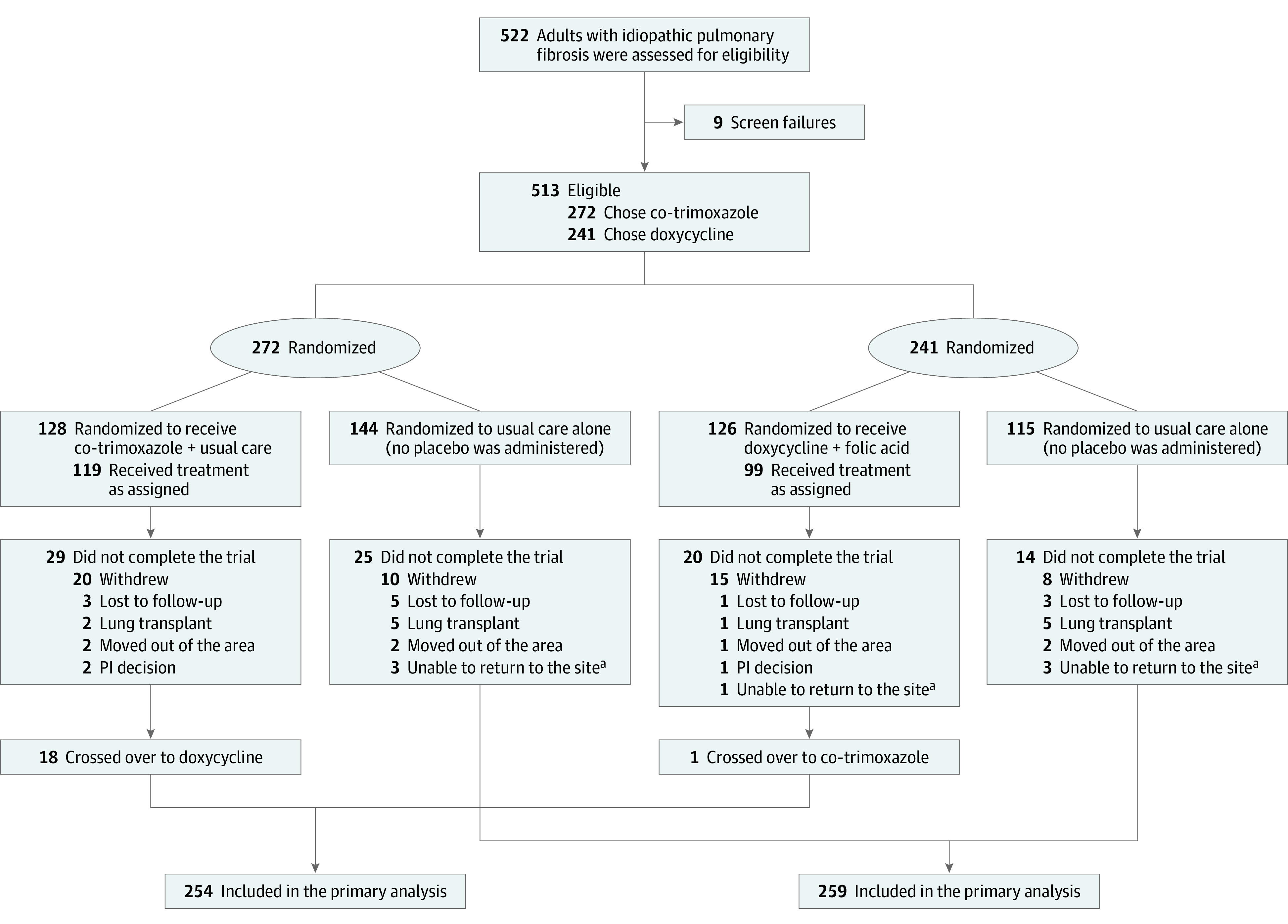
Recruitment of 513 patients with idiopathic pulmonary fibrosis who were randomized to 2 therapeutic strategies in a 1:1 allocation ratio. Prior to randomization eligible patients and their physician were offered a preference for the co-trimoxazole (trimethoprim-sulfamethoxazole) or doxycycline stratum; the protocol specified co-trimoxazole in the absence of a selection.12
aUnable to return to the treatment site in a timely fashion because of either the COVID-19 shutdown or scheduling difficulties.
Intervention
The antimicrobial therapy strategy consisted of trimethoprim 160 mg–sulfamethoxazole 800 mg twice a day plus folic acid 5 mg daily or with doxycycline with a weight-based dosing (100 mg once daily if actual body weight was <50 kg or 100 mg twice daily if ≥50 kg). The addition of folate to co-trimoxazole minimized the risk of leukopenia associated with folic acid metabolism inhibition by trimethoprim.13 After randomization, patients assigned to the antimicrobial groups were provided prescription drug vouchers to defray the study drug cost; no placebo agent was provided. Patients were expected to continue on the assigned therapy until the end of the study.
End Points
The primary end point of this study was time to first nonelective, respiratory hospitalization or all-cause mortality. Although forced vital capacity (FVC) has been used as a primary end point in several pivotal trials, the use of clinically relevant parameters has been suggested as a potential advance that is particularly relevant in the setting that clinical trials have been conducted in the postantifibrotic treatment era.14 Ascertainment of these events included the impression of the investigative clinician (site investigator) and central adjudication group (clinical end point committee) to confirm the clinical cause of a hospitalization or a mortality event.15 Although the site investigators and participants were not blinded to treatment allocation, the central adjudication group was blinded both to treatment allocation and to the site investigator’s classification of the potential event. The initial central adjudication was provided by a single blinded central committee member. If this assessment agreed with the site investigator’s assessment with respect to elective vs nonelective and respiratory vs nonrespiratory primary cause, the final event classification was achieved. If not, the event was reviewed by a phase 2 adjudication committee that was also blinded to treatment allocation, and a final consensus classification was achieved. The same process was used to confirm the occurrence of deaths and their dates, based on review of submitted medical records.
There were 13 prespecified secondary end points (eTable 2 in Supplement 3). Due to the COVID-19 pandemic, not all events were able to be adjudicated because some site personnel were pulled for clinical duty, limiting their ability to obtain final clinical data. Due to the timing of the study, none of the patients were affected by clinically identified SARS-CoV-2 infection. The best available classification, used for primary analyses, was the decision of the clinical end point committee, if available, or the clinical site interpretation, if not. Targeted adverse events of special interest such as arrhythmia, diarrhea, hyperkalemia, rash, and vomiting were assessed. To provide simplicity, patients had minimal in-person study-specific visits with data collection coinciding with clinical visits.12
Sample Size
The planned sample size of 500 patients was expected to provide adequate power to test the primary hypothesis under a range of event rates. Based on IPFnet data, the primary end point event rates were anticipated to vary depending on the gender, age, and lung physiology (GAP) index scores of the enrolled patients.16 The statistical power for designs enrolling 500 patients with usual care group events rates varying from 24% to 36% and 12-month treatment effects varying from 30% to 35% are shown in eTable 3 in Supplement 3. The proposed design was expected to provide adequate power under most scenarios. One planned interim analysis for efficacy was scheduled to occur after 300 patients had 12 months of follow-up.
Statistical Analysis
Baseline characteristics were summarized using mean (SDs) or median (interquartile range [IQR]) for continuous variables and frequency in each category for nominal variables. The primary analysis of the primary end point was based on the as-randomized populations. Patients receiving lung transplants during the course of follow-up were censored for all end points at the time of the transplant. The primary comparison between the 2 randomized groups was based on a time-to-event analysis using a multivariable adjusted Cox proportional hazards regression model. The Cox model included treatment group, age, sex, baseline diffusing capacity of lung for carbon monoxide (Dlco), baseline FVC, use of N-acetylcysteine at enrollment, use of nintedanib or pirfenidone at enrollment, and preference of antimicrobial agent prior to randomization. Multiple imputation was performed on age, sex, baseline Dlco, baseline FVC, N-acetylcysteine use at enrollment, use of standard of care medications at enrollment, and preference of antimicrobial agent prior to randomization, using PROC MI, assuming that the data are missing at random with an arbitrary missing pattern; that is, the full conditional specification method was used to generate the 20 imputed data sets. The final inferential results, including the interaction P values, were generated by averaging, using PROC MIANALYZE, the inferential results across the 20 imputed data sets. Hazard ratios (HRs) and 95% CIs were used to summarize the differences between treatment groups. Kaplan-Meier estimates were used to display event rates by treatment group. Participants who were event-free (ie, patients without any respiratory hospitalization or death event at the time of analysis) were censored at their last visit or lung transplant. To assess the interaction of randomized treatment and prespecified antimicrobial agent a variable was added to the model. Departures from the Cox model assumptions were assessed by the examination of the martingale residuals. A post hoc analysis was applied using robust standard errors to account for a possible clustering effect at the enrolling site level. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. The significance threshold was 2-sided at .05. All analyses were performed using SAS version 9.4 software (SAS Institute Inc).
Results
The data and safety monitoring board (DSMB) met on December 18, 2019, to review the first planned efficacy analysis for CleanUP-IPF and unanimously voted to terminate the study early for futility. The determination was based on the low likelihood of the study demonstrating a statistically significant positive effect of the intervention. Neither the protocol nor the DSMB statistical analysis plan contained a formal strategy for a futility analysis. Although no clear harm signal was seen, the DSMB noted a higher rate of the primary outcome, mortality, and serious adverse events in the intervention group that did not reach statistical significance (eAppendix Supplement 4).
Participants
A total of 513 patients (mean age, 71 years; 21.6% women) at 35 US clinical centers were randomized from August 2017 to June 2019 to receive usual care plus antimicrobials (128, co-trimoxazole; 126 doxycycline) or to usual care alone (259) and were followed up through January 2020 (Figure 1). Of the participants, 336 (65.5%) were older men with moderate to severe IPF with a high frequency of comorbid conditions, particularly cardiovascular diseases, diabetes, and gastroesophageal reflux disease (Table 1 and Table 2). The 2 treatment groups were well matched. Of the 512 study patients with available data, 466 (91%) were taking concurrent antifibrotic medications. Missing information for key baseline variables was no more than 6% and was more than 2% in only 2 baseline variables. Twelve patients were lost to follow-up.
Table 2. Baseline Patient History and Quality of Life.
| Parameters | No. (%) of patients | |
|---|---|---|
| Usual care + antimicrobial therapy (n = 254) | Usual care (n = 259) | |
| FVC, No. | 247 | 255 |
| Median (IQR), L | 2.68 (2.14-3.23) | 2.73 (2.20-3.32) |
| Percent predicted, No. | 237 | 254 |
| Median (IQR) | 68.9 (56.7-81.8) | 71.17 (57.4-83.8) |
| FEV1, L, No. | 247 | 255 |
| Median (IQR) | 2.15 (1.76-2.64) | 2.23 (1.76-2.67) |
| Percent predicted, No. | 237 | 254 |
| Median (IQR) | 78.0 (64.1-92.2) | 77.2 (65.7-88.8) |
| FEV1:FVC, No. | 242 | 258 |
| Median (IQR) | 73.8 (72.3-75.1) | 73.5 (72.2-74.9) |
| FEV1:FVC <70%, No./total (%) | 13/242 (5.0) | 12/258 (5.0) |
| Dlco, No.a | 240 | 252 |
| Median (IQR), mL·min−1·mm Hg−1 | 10.74 (8.27-14.42) | 11.56 (8.74-14.73) |
| % Predicted, No. | 233 | 251 |
| Median (IQR) | 39.50 (29.32-48.61) | 38.37 (30.97-49.01) |
| GAP stage, No.b | 233 | 251 |
| I | 60 (25.8) | 54 (21.5) |
| II | 124 (53.2) | 133 (53.0) |
| III | 49 (21.0) | 64 (25.5) |
| Patient-reported parameters | ||
| Shortness of breath, No.c | 251 | 257 |
| Median (IQR) | 28.0 (11.0-51.0) | 30.0 (14.0-48.0) |
| EQ-5D-5Ld | ||
| Index, No. | 250 | 255 |
| Median (IQR) | 0.81 (0.73-1.00) | 0.81 (0.71-1.00) |
| Visual analog scale, No.e | 218 | 212 |
| Median (IQR) | 70.5 (54.0-88.0) | 75.00 (60.0-85.0) |
| ICECAP-O, No.f | 253 | 258 |
| Median (IQR) | 0.38 (0.18-0.52) | 0.40 (0.19-0.54) |
| Short Form Health Survey | ||
| 12-Item mental component, No.g | 252 | 258 |
| Median (IQR) | 54.8 (46.3-60.2) | 54.8 (48.9-59.9) |
| 12-item physical component, No.g | 251 | 258 |
| Median (IQR) | 40.4 (35.5-48.0) | 39.3 (34.2-46.5) |
| 6-item descriptive system, No.h | 249 | 258 |
| Median (IQR) | 0.72 (0.63-0.82) | 0.72 (0.66-0.80) |
| Fatigue severity scale, No.i | 252 | 253 |
| Median (IQR) | 3.89 (2.67-4.89) | 4.00 (2.67-5.22) |
| Leicester cough scale, No.j | 249 | 257 |
| Median (IQR) | 17.8 (14.4-19.8) | 18.3 (15.2-20.1) |
Abbreviations: Dlco, diffusing capacity of lung for carbon monoxide; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; IQR, interquartile range.
Corrected for hemoglobin by the coordinating center using the Cotes equation.17
The gender, age, physiology (GAP) index is a multidimensional scoring system that is associated with mortality in patients with IPF (higher scores indicate poorer prognosis).18
Score based on the University of California, San Diego, shortness of breath questionnaire (range, 0-120; higher values indicate worse shortness of breath).19
The 5-level EuroQol 5-dimension questionnaire (EQ-5D) quality-of-life score (range, −0.59 to 1; higher score indicates better health utility).20
Range, 0 to 100 (<70 are associated with frailty; higher scores indicate better health).20
ICEpop CAPability measure for Older people (ICECAP-O) summary score (range, 0-1; higher score indicates better well-being).21
Higher scoresindicate a better quality of life (range, 0-100).22
The 6-item descriptive system (SF6D) uses 6 of the SF-36 dimensions in economic evaluation studies (range, 0.29-1; higher score indicates better health).22
A 9-item scale with each item scored from 1 to 7 (higher score is worse).23
A cough-related quality-of-life score (range, 3-2; higher values represent better quality of life.)24
Study Conduct
The study recruited ahead-of-scheduled projections (eFigure 2 in Supplement 3), with a median follow-up of 12.7 months. Fifty-three patients (10%) withdrew consent (Figure 1); 49.5% of study patients were followed up for more than 12 months. One patient crossed over from usual care to usual care plus antimicrobial therapy. Within the antimicrobial therapy group, 18 patients switched from co-trimoxazole to doxycycline and 1 from doxycycline to co-trimoxazole therapy during the course of the study.
Adherence to study medication during the course of the study for the 7 days prior to assessment was 78.3% among the 201 patients who reported ) at 1 month, 66.2% at 6 months among 163 patients who reported, and 47.2% at 12 months among 163 patients who reported). There was a statistically significant difference in the withdrawal rates, with more patients withdrawing from the usual care plus antimicrobial therapy: 49 of 254 (19.3%) vs 39 of 259 (15.1%; P = .05) (eFigure 3 in Supplement 3).
Primary End Point
The time to event end point data, as defined by the clinical end point committee or clinical site, is presented in eFigure 4 and eTable 4 in Supplement 3, and its components, are enumerated in eTable 5 in Supplement 3. The total number of events were quantitatively similar by the committee, the clinical site, or the best available data. For adjudication of respiratory related nonelective hospitalizations the site determination result vs the clinical end point committee result were found to strongly agree with a κ of 0.78 (95% CI, 0.68-0.88) and a low and balanced disagreement rates of 10.3% when the site said yes and the clinical end point committee said no and 11.4% when the clinical end point committee said no and the site said yes.
When the database locked, a total of 108 primary end point events occurred: 52 events (20.4 events per 100 patient-years [95% CI, 14.8-25.9]) in the usual care plus antimicrobial therapy group and 56 events (18.4 events per 100 patient-years [95% CI, 13.2-23.6]) in the usual care group. The mean follow-up time was 13.1 months. There was no significant effect of antimicrobial therapy after multivariable adjustment (HR, 1.04 [95% CI, 0.71-1.53]; Figure 2, Table 3; and eTable 6 in Supplement 3) for time to the composite primary end point. A post hoc analysis accounting for the clustering of patients within the enrolling clinical site yielded a similar result (eTable 6 in Supplement 3). An examination of the martingale residuals did not reveal any departures from the assumed model. There was no significant interaction between the effect of the prespecified antimicrobial agent on the primary end point (adjusted HR for co-trimoxazole, 1.15 [95% CI, 0.68-1.95] vs doxycycline, 0.82 [95% CI, 0.46-1.47]; P = .66) (eFigure 5 in Supplement 3). The overall number of events rose by each GAP stage (eTable 7 in Supplement 3).
Figure 2. Time to the Primary Composite End Point and Its Components.
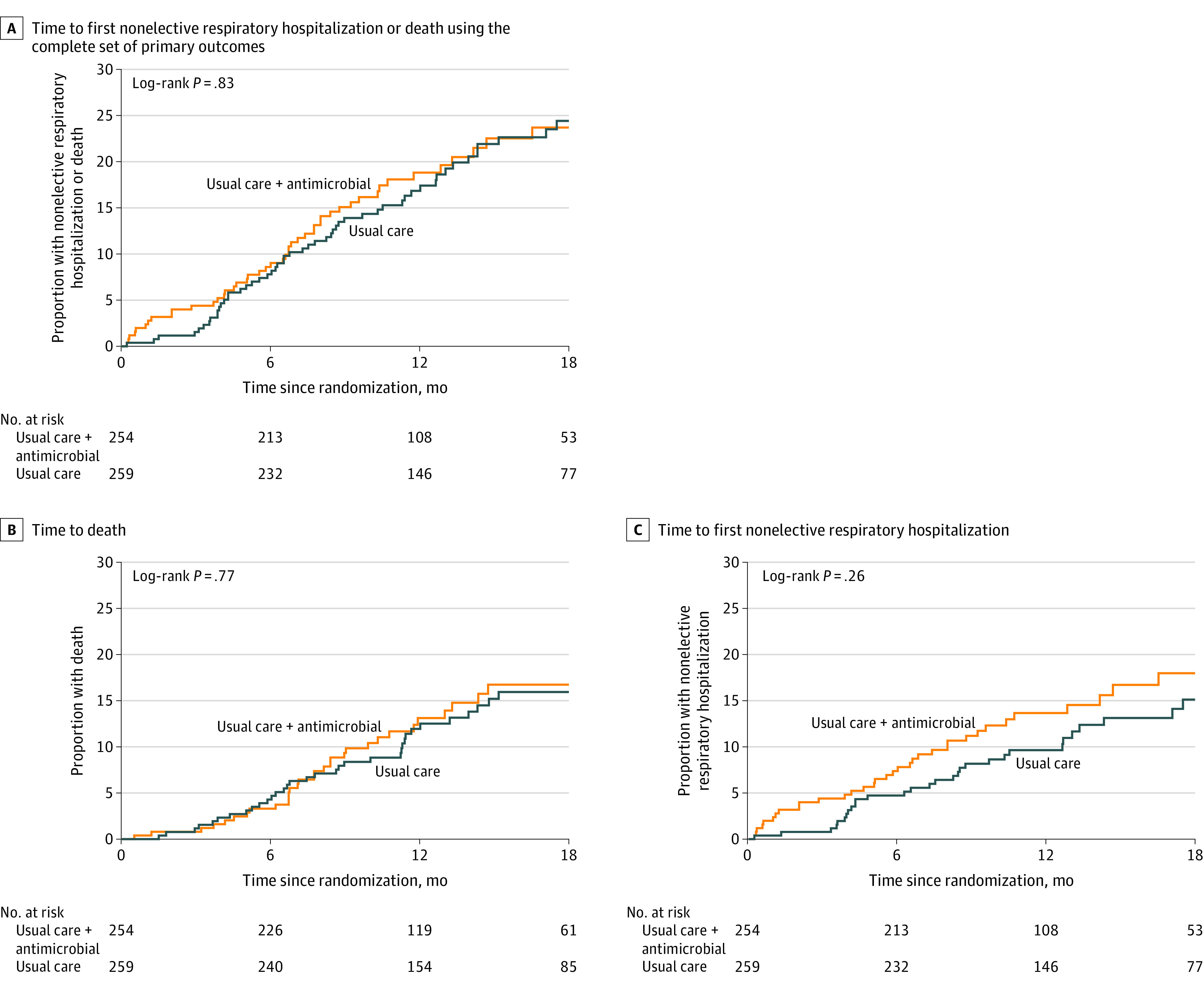
There were no statistically significant differences between the antimicrobial therapy plus usual care group and usual care–only group based on the log-rank test for this end point nor for the components.
Table 3. Primary and Secondary End Pointsa.
| Event | Usual care + antimicrobial therapy (n = 254) | Usual care (n = 259) | Difference (95% CI) | Hazard ratio (95% CI)b | ||
|---|---|---|---|---|---|---|
| Summary statistics | Events per 100 patient-years | Summary statistics | Events per 100 patient-years | |||
| Primary end point, No. (%) | ||||||
| Death or first nonelective, respiratory hospitalization | 52 (20.5) | 20.4 (14.8 to 25.9) | 56 (21.6) | 18.4 (13.2 to 23.6) | 2.0 (−5.7 to 9.6)c | Adjusted, 1.04 (0.71 to 1.53) Unadjusted, 1.11 (0.70 to 1.78) |
| Secondary end point, No. (%) | ||||||
| Death from any cause | 37 (14.6) | 13.5 (9.5 to 18.7) | 37 (14.3) | 11.5 (8.1 to 15.9) | 2.0 (−4.1 to 8.1)c | 1.11 (0.70 to 1.78) |
| Nonelective hospitalization | ||||||
| Respiratory | 36 (14.2) | 14.1 (9.9 to 19.5) | 31 (12.0) | 10.2 (6.9 to 14.5) | 3.9 (−2.1 to 10.0)c | 1.34 (0.82 to 2.17) |
| All-cause | 54 (21.3) | 22.6 (17.0 to 29.5) | 47 (18.1) | 15.8 (11.6 to 21.1) | 6.7 (−0.9 to 14.3)c | 1.36 (0.91 to 2.01) |
| Count of nonelective hospitalizations, per 100 y | 44 (17.3) | 16.1 (11.2 to 21.0) | 36 (13.9) | 11.2 (7.2 to 15.3) | 4.9 (−1.5 to 11.3)c | RR, 1.29 (0.82 to 2.02) |
| Respiratory | ||||||
| 0 | 218 (85.8) | 228 (88.0) | ||||
| 1 | 30 (11.8) | 26 (10.0) | ||||
| 2 | 4 (1.6) | 5 (1.9) | ||||
| 3 | 2 (0.8) | 0 | ||||
| All-cause per 100 y | 72 (28.3) | 26.4 (20.0 to 32.7) | 56 (21.6) | 17.5 (12.4 to 22.6) | 8.9 (0.8 to 17.0)c | RR, 1.44 (1.00 to 2.07) |
| 0 | 200 (78.7) | 212 (81.9) | ||||
| 1 | 39 (15.4) | 40 (15.4) | ||||
| 2 | 12 (4.7) | 6 (2.3) | ||||
| 3 | 3 (1.2) | 0 | ||||
| 4 | 0 (0.0) | 1 (0.4) | ||||
| Change from randomization to 12 mo | ||||||
| FVC, median (IQR), L | −0.10 (−0.28 to 0.03) | −0.14 (−0.29 to 0.03) | 0.04 (−0.02 to 0.10)b | |||
| Dlco, median (IQR), mL·min −1·mm Hg | −0.71 (−2.10 to 0.72) | −0.83 (−2.18 to 0.24) | 0.38 (−0.10 to 0.86)b | |||
| Respiratory infections, No. (%) | 34 (12.2) | 12.4 (8.1 to 16.8) | 49 (18.9) | 15.3 (10.5 to 20.1) | −2.8 (−9.3 to 3.6)c | RR, 0.71 (0.46 to 1.11) |
| 0 | 223 (87.8) | 216 (83.4) | ||||
| 1 | 28 (11.0) | 37 (14.3) | ||||
| 2 | 3 (1.2) | 6 (2.3) | ||||
| Change from randomization to 12 mo, median (IQR) | ||||||
| Shortness of breath | 5.00 (−2.00 to 15.00) | 4.00 (−2.00 to 12.00) | 0.59 (−4.62 to 3.43)b | |||
| Fatigue severity scale | 0.22 (−0.78 to 1.11) | 0.22 (−0.56 to 1.00) | 0.10 (−0.26 to 0.46)b | |||
| Leicester cough questionnaire | −0.30 (−2.32 to 0.86) | −0.27 (−2.09 to 0.88) | −0.01 (−0.82 to 0.80)b | |||
| EQ-5D score | 0.00 (−0.14 to 0.04) | 0.00 (−0.12 to 0.00) | 0.01 (−0.04 to 0.05)b | |||
| 12-Item Short Form Health Survey | ||||||
| Mental score | −1.05 (−5.84 to 2.79) | −1.29 (−6.03 to 2.90) | 0.46 (−1.45 to 2.37)b | |||
| Physical score | −1.49 (−6.39 to 3.63) | −1.96 (−5.62 to 1.88) | 0.46 (−1.18 to 2.10)b | |||
| SF6D score | 0.00 (−0.08 to 0.06) | 0.00 (−0.10 to 0.05) | 0.02 (−0.01 to 0.04)b | |||
| ICECAP-O | 0.00 (−0.10 to 0.15) | 0.00 (−0.09 to 0.12) | 0.01 (−0.03 to 0.05)b | |||
Abbreviations: Dlco, diffusing capacity of lung for carbon monoxide; Dlco, diffusing capacity of lung for carbon monoxide; EQ-5D, 5-level EuroQol 5-dimension questionnaire; FVC, forced vital capacity; ICECAP-O, CEpop CAPability measure for Older people; IQR, interquartile range; RR, risk ratio; SF6D, Short Form Health survey.
For a definition of measures and score ranges, see the Table 1 footnotes.
Difference in events per 100 person-years for antimicrobial therapy plus usual care vs usual care: point estimate (95% CI).
The models were adjusted for the following factors: age, sex, baseline Dlco; baseline FVC, use of N-acetylcysteine at enrollment, use of nintedanib or pirfenidone at enrollment, and choice of antimicrobial agent prior to randomization.
Secondary End Points
In covariate adjusted analyses, there was no statistically significant difference in the time to death between the antimicrobial treatment and usual care (13.5 vs 11.5 events per 100 patient-years; HR, 1.11 [95% CI; 0.70-1.78]) or to the time to the first nonelective respiratory hospitalization (14.1 vs 10.2 hospitalizations per 100 patient-years; HR, 1.34 [95% CI, 0.82-2.17]) (Figure 2, Table 3, and eTable 6 in Supplement 3). Similarly, neither antimicrobial treatment added to usual care had a significant effect on the time to death (interaction P value = .28). Co-trimoxazole had an HR of 1.48 (95% CI, 0.78-2.81) and doxycycline, HR of 0.74 (95% CI, 0.37-1.49) on the time to the first nonelective respiratory hospitalization with an HR of 1.40 (95% CI, 0.72-2.75) for co-trimoxazole and an HR of 1.10 (95% CI, 0.53-2.26) for doxycycline (interaction P value = .79).
The time to the first nonelective all-cause hospitalization did not differ in statistical significance between antimicrobial therapy (22.6 hospitalizations per 100-patient years) and usual care (15.8 hospitalizations per 100 patient-years; HR, 1.36 [95% CI, 0.91- 2.01]) nor in the total number of nonelective all-cause hospitalizations (Table 3).
The total number of nonelective all-cause hospitalizations showed slightly higher rates in the usual care plus antimicrobial groups than in usual care alone group (26.4 vs 17.5 hospitalizations per 100 patient-years; rate ratio; 1.44 [95% CI, 1.00-2.07]). There were no statistically significant differences between usual care plus antimicrobial therapy vs usual care alone in the change between randomization and 12 months in FVC, Dlco, or patient-reported outcome parameters (Table 3, eTable 8, and eFigure 6 in Supplement 3).
Adverse Events
Numerically more respiratory serious adverse events in patients treated with usual care plus antimicrobial therapy were observed, whereas fewer infections were observed (Table 4). There were expected differences in prespecified adverse events of special interest. These included more episodes of diarrhea, vomiting, rash, arrhythmias, and hyperkalemia in the antimicrobial therapy group. Similar patterns of serious adverse events and prespecified events of special interest were observed for the co-trimoxazole and doxycycline cohorts (eTable 9 in Supplement 3).
Table 4. Patients With Serious Adverse Events and Adverse Events of Special Interest.
| Event | No. (%) of patients | |
|---|---|---|
| Usual care + antimicrobial therapy (n = 254) | Usual care (n = 259) | |
| Serious adverse events | ||
| Patients with ≥ 1 eventsa | 71 (28.0) | 65 (25.1) |
| Respiratory, thoracic and mediastinal | 42 (16.5) | 26 (10.0) |
| Idiopathic pulmonary fibrosis | 16 (6.3) | 5 (1.9) |
| Respiratory failure | 5 (2.0) | 4 (1.5) |
| Dyspnea | 5 (2.0) | 2 (0.8) |
| Cardiovascular | 11 (4.3) | 11 (4.2) |
| Infections | 7 (2.8) | 17 (6.6) |
| Pneumonia | 5 (2.0) | 7 (2.7) |
| Nervous system | 8 (3.1) | 3 (1.2) |
| Gastrointestinal | 3 (1.2) | 4 (1.5) |
| Metabolism and nutrition | 2 (0.8) | 4 (1.5) |
| Adverse events of special interestb | ||
| Diarrhea | 26 (10.2) | 8 (3.1) |
| Rash | 17 (6.7) | 0 |
| Vomiting | 12 (4.7) | 2 (0.8) |
| Hyperkalemia | 10 (3.9) | 2 (0.8) |
| Arrhythmia | 2 (0.8) | 5 (1.9) |
A serious adverse event was one that resulted in death or was life-threatening, required hospitalization or prolonged hospitalization, resulted in significant disability, or resulted in congenital anomaly.
An adverse event of special interest reflects one thought to be a potentially antibiotic-associated adverse event. The list of 5 adverse events of special interest was prepopulated.
Discussion
Among adults with IPF, adding co-trimoxazole or doxycycline to usual care did not significantly improve time to nonelective respiratory hospitalization or death compared with usual care alone. These findings do not support treatment with these antibiotics for the underlying disease.
Neither EME-TIPAC nor this study supported a significant effect of either co-trimoxazole on the primary end point or on numerous secondary end points. Although both studies used different designs, they had a very similar composite of clinical outcomes that was thought to be clinically relevant14 and more reflective of the anticipated mechanism of action of antimicrobial therapy25 in contrast to the more traditional longitudinal change in FVC.26 As expected this study’s patient population was rapidly enrolled, was physiologically less severe, experienced greater comorbidity, and exhibited lower adherence to the study therapy. Despite these differences, the withdrawal rate was identical and the primary end point for co-trimoxazole therapy was similar between the 2 studies.
Increased gastrointestinal adverse events, particularly those treated with doxycycline, were identified. Rash and hyperkalemia were more frequently seen with co-trimoxazole therapy. These adverse events are reflective of those reported in other settings,27 were not serious in nature, and were similar to reactions to co-trimoxazole, noted in the EME-TIPAC study.6 Nevertheless, there was a numerical imbalance in respiratory serious adverse events but not infections with antimicrobial therapy. The latter observation suggests a possible disconnect between IPF progression and pneumonia, with the former potentially being less likely to be associated with bacterial infection.
This study extends the EME-TIPAC findings by suggesting that a broader antimicrobial therapy strategy was not effective in improving clinical outcomes. Several investigations have demonstrated an altered lung microbial community in patients with IPF that correlates closely with clinical outcomes1,2 and is associated with augmented local and systemic immune responses.3,4 Robust murine data support the association of the lung microbial community on increased fibroproliferative response28,29; however, antimicrobial therapy or lack of microbial populations in germ-free mice has ameliorated this association.28,29 The findings may reflect the complex nature of the lung microbial community in chronic lung disease and the approach to define causality by altering dysbiosis with antimicrobial therapy,30 including significant interpatient and intrapatient heterogeneity in the microbiome31 and its associated host response.3 The immunomodulatory effect of antimicrobial therapy may be more relevant than direct effect on the microbes,32 particularly as there appears to be a core resistome in chronic lung diseases.33 The results of this study, in conjunction with those of EME-TIPAC investigators, do not support the use of co-trimoxazole to modify disease progression in patients with IPF but do not negate the potential effects of other agents whose preclinical34 or preliminary clinical work suggest an antifibrotic34 and/or clinical effect.35
Because this study did not measure the number and type of lung microbes nor the direct effect of antibiotics on these microbes, this study could not exclude that antimicrobials may work in select subsets of patients with IPF experiencing increased dysbiosis. Other approaches for manipulation of the microbiome from fecal transplant, phage therapy, or selective immunization, among others, may prove successful. This study collected blood for DNA and RNA, along with buccal and fecal swabs for future analyses, including candidate genetic association studies and exploratory genome and transcriptome-wide studies.
Limitations
This study has several limitations. First, there was lack of placebo control and less intrusive adherence monitoring that may have limited the ability to robustly define treatment effect as was suggested in a preliminary study.5 Second, there was not a bronchoalveolar lavage or other identification of lung dysbiosis for patient selection. Third, the IPF diagnosis was established by the site investigator rather than by investigators in a centralized location, so it is possible that enrolled patients did not strictly meet current diagnostic criteria. Fourth, it is possible that the differential in smoking rates observed between treatment groups influenced levels of lung dysbiosis potentially influencing the lack of observed treatment effect.
Conclusions
Among adults with idiopathic pulmonary fibrosis, the addition of co-trimoxazole or doxycycline to usual care, compared with usual care alone, did not significantly improve time to nonelective respiratory hospitalization or death. These findings do not support treatment with these antibiotics for the underlying disease.
Study Protocol
Statistical Analysis Plan
eTable 1. Detailed inclusion and exclusion criteria
eTable 2. Pre-specified secondary endpoints
eTable 3. Statistical power assuming a sample size of 500 randomized patients
eTable 4. Primary endpoint and its components as a function of Clinical Endpoint Committee (CEC) Adjudication or Clinical Site determination
eTable 5. Total events and event rates for the primary endpoint and its components as a function of the Clinical Event Adjudication Committee, clinical site and best available determination
eTable 6. Multivariable modelling using best available data and multiple imputation for the primary composite and its components
eTable 7. Total events and event rates for the primary endpoint by GAP score as a function of the Clinical Event Adjudication Committee, Site Investigator, and Best Available determination
eTable 8. Multivariable modelling using multiple imputations for the change from baseline to 12 months in secondary endpoints
eTable 9. Serious adverse events and adverse events of special interest by pre randomization cohort
eFigure 1. PRECIS-2 Diagram
eFigure 2. Enrollment (Actual vs. Projection)
eFigure 3. Time to withdrawal from study
eFigure 4. Time to first non-elective respiratory hospitalization or death using the Clinical Endpoint Committee adjudicated (Panel A) and Site Investigator (Panel B) determined outcomes
eFigure 5. Time to first non-elective respiratory hospitalization or death for the Pre Randomization Co-trimoxazole (Panel A) or Doxycycline (Panel B) Cohorts
eFigure 6. Boxplots of FVC at each study visit
eAppendix. DSMB Communications
Nonauthor Collaborators. CleanUP-IPF Investigators of the Pulmonary Trials Cooperative
Data Sharing Statement
References
- 1.Han MK, Zhou Y, Murray S, et al. ; COMET Investigators . Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2(7):548-556. doi: 10.1016/S2213-2600(14)70069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molyneaux PL, Cox MJ, Willis-Owen SAG, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190(8):906-913. doi: 10.1164/rccm.201403-0541OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, Ma SF, Espindola MS, et al. ; COMET-IPF Investigators . Microbes are associated with host innate immune response in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196(2):208-219. doi: 10.1164/rccm.201607-1525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molyneaux PL, Willis-Owen SAG, Cox MJ, et al. Host-microbial interactions in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;195(12):1640-1650. doi: 10.1164/rccm.201607-1408OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shulgina L, Cahn AP, Chilvers ER, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013;68(2):155-162. doi: 10.1136/thoraxjnl-2012-202403 [DOI] [PubMed] [Google Scholar]
- 6.Wilson AM, Clark AB, Cahn T, et al. ; EME-TIPAC team . Effect of co-trimoxazole (trimethoprim-sulfamethoxazole) vs placebo on death, lung transplant, or hospital admission in patients with moderate and severe idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA. 2020;324(22):2282-2291. doi: 10.1001/jama.2020.22960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra A, Bhattacharya P, Paul S, Paul R, Swarnakar S. An alternative therapy for idiopathic pulmonary fibrosis by doxycycline through matrix metalloproteinase inhibition. Lung India. 2011;28(3):174-179. doi: 10.4103/0970-2113.83972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya P, Nag S, Bardhan S, et al. The role of long-term doxycycline in patients of idiopathic pulmonaryfibrosis: the results of an open prospective trial. Lung India. 2009;26(3):81-85. doi: 10.4103/0970-2113.53231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non–cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251-1259. doi: 10.1001/jama.2013.1937 [DOI] [PubMed] [Google Scholar]
- 10.Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non–cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309(12):1260-1267. doi: 10.1001/jama.2013.2290 [DOI] [PubMed] [Google Scholar]
- 11.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 12.Anstrom KJ, Noth I, Flaherty KR, et al. ; CleanUP-IPF Study Team . Design and rationale of a multi-center, pragmatic, open-label randomized trial of antimicrobial therapy—the study of clinical efficacy of antimicrobial therapy strategy using pragmatic design in Idiopathic Pulmonary Fibrosis (CleanUP-IPF) clinical trial. Respir Res. 2020;21(1):68. doi: 10.1186/s12931-020-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183(16):1851-1858. doi: 10.1503/cmaj.111152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson KF, Kass DJ. Clinical trials in idiopathic pulmonary fibrosis in the “posttreatment era.” JAMA. 2018;319(22):2275-2276. doi: 10.1001/jama.2018.6225 [DOI] [PubMed] [Google Scholar]
- 15.Andrade J, Schwarz M, Collard HR, et al. ; IPFnet Investigators . The Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet): diagnostic and adjudication processes. Chest. 2015;148(4):1034-1042. doi: 10.1378/chest.14-2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collard HR, Brown KK, Martinez FJ, Raghu G, Roberts RS, Anstrom KJ. Study design implications of death and hospitalization as end points in idiopathic pulmonary fibrosis. Chest. 2014;146(5):1256-1262. doi: 10.1378/chest.14-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi: 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 18.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi: 10.7326/0003-4819-156-10-201205150-00004 [DOI] [PubMed] [Google Scholar]
- 19.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619-624. doi: 10.1378/chest.113.3.619 [DOI] [PubMed] [Google Scholar]
- 20.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 21.Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people: preferences or capabilities? Soc Sci Med. 2006;62(8):1891-1901. doi: 10.1016/j.socscimed.2005.08.023 [DOI] [PubMed] [Google Scholar]
- 22.QualityMetric Health Outcomes Scoring Software 5.0 User’s Guide. QualityMetric Inc; 2016. [Google Scholar]
- 23.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121-1123. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 24.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339-343. doi: 10.1136/thorax.58.4.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collard HR, Bradford WZ, Cottin V, et al. A new era in idiopathic pulmonary fibrosis: considerations for future clinical trials. Eur Respir J. 2015;46(1):243-249. doi: 10.1183/09031936.00200614 [DOI] [PubMed] [Google Scholar]
- 26.Paterniti MO, Bi Y, Rekić D, Wang Y, Karimi-Shah BA, Chowdhury BA. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2017;14(9):1395-1402. doi: 10.1513/AnnalsATS.201606-458OC [DOI] [PubMed] [Google Scholar]
- 27.Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005;27(9):1329-1342. doi: 10.1016/j.clinthera.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 28.O’Dwyer DN, Ashley SL, Gurczynski SJ, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(9):1127-1138. doi: 10.1164/rccm.201809-1650OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knippenberg S, Ueberberg B, Maus R, et al. Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin. Thorax. 2015;70(7):636-646. doi: 10.1136/thoraxjnl-2014-206420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gosens R, Hiemstra PS, Adcock IM, et al. Host-microbe cross-talk in the lung microenvironment: implications for understanding and treating chronic lung disease. Eur Respir J. 2020;56(2):1902320. doi: 10.1183/13993003.02320-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson RP, Huffnagle GB, Flaherty KR, et al. Radiographic honeycombing and altered lung microbiota in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(12):1544-1547. doi: 10.1164/rccm.201903-0680LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal LN, Clemente JC, Wu BG, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax. 2017;72(1):13-22. doi: 10.1136/thoraxjnl-2016-208599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsheh MY, Haldar K, Bafadhel M, et al. Resistome analyses of sputum from COPD and healthy subjects reveals bacterial load-related prevalence of target genes. Thorax. 2020;75(1):8-16. doi: 10.1136/thoraxjnl-2019-213485 [DOI] [PubMed] [Google Scholar]
- 34.Krempaska K, Barnowski S, Gavini J, et al. Azithromycin has enhanced effects on lung fibroblasts from idiopathic pulmonary fibrosis (IPF) patients compared to controls. Respir Res. 2020;21(1):25. doi: 10.1186/s12931-020-1275-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macaluso C, Maritano Furcada J, Alzaher O, et al. The potential impact of azithromycin in idiopathic pulmonary fibrosis. Eur Respir J. 2019;53(2):1800628. doi: 10.1183/13993003.00628-2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eTable 1. Detailed inclusion and exclusion criteria
eTable 2. Pre-specified secondary endpoints
eTable 3. Statistical power assuming a sample size of 500 randomized patients
eTable 4. Primary endpoint and its components as a function of Clinical Endpoint Committee (CEC) Adjudication or Clinical Site determination
eTable 5. Total events and event rates for the primary endpoint and its components as a function of the Clinical Event Adjudication Committee, clinical site and best available determination
eTable 6. Multivariable modelling using best available data and multiple imputation for the primary composite and its components
eTable 7. Total events and event rates for the primary endpoint by GAP score as a function of the Clinical Event Adjudication Committee, Site Investigator, and Best Available determination
eTable 8. Multivariable modelling using multiple imputations for the change from baseline to 12 months in secondary endpoints
eTable 9. Serious adverse events and adverse events of special interest by pre randomization cohort
eFigure 1. PRECIS-2 Diagram
eFigure 2. Enrollment (Actual vs. Projection)
eFigure 3. Time to withdrawal from study
eFigure 4. Time to first non-elective respiratory hospitalization or death using the Clinical Endpoint Committee adjudicated (Panel A) and Site Investigator (Panel B) determined outcomes
eFigure 5. Time to first non-elective respiratory hospitalization or death for the Pre Randomization Co-trimoxazole (Panel A) or Doxycycline (Panel B) Cohorts
eFigure 6. Boxplots of FVC at each study visit
eAppendix. DSMB Communications
Nonauthor Collaborators. CleanUP-IPF Investigators of the Pulmonary Trials Cooperative
Data Sharing Statement


