Abstract
Objective
This study aimed to determine the possibility of subclinical myocardial dysfunction detected by strain echocardiography in the late period of children with Kawasaki disease.
Material and Methods
The study enrolled 30 patients with Kawasaki disease with a follow-up period of at least 12 months and 30 healthy age- and gender-matched children. During the follow-up period, standard echocardiography, pulsed and tissue Doppler, and strain echocardiography were recorded for both groups.
Results
The mean age at the time of the diagnosis was 2.6±2.3 years (2 months–11 years). The mean follow-up period after the diagnosis was 3.55±2.20 years. Conventional echocardiography, M mode, pulsed and tissue Doppler values, and myocard performance index did not reveal significant differences. Left ventricle strain and strain rate parameters obtained by apical four-, three-, and two-chamber views did not show statistical differences between patients and controls. There was a positive correlation between the duration of follow-up and global four- and three-chamber longitudinal strain and global longitudinal strain values (r=0.465, p=0.010; r=0.414, p=0.023; r=0.492, p=0.006, respectively), whereas global radial strain showed negative correlation (r=−0.517, p=0.003).
Conclusion
The analysis of systolic strain and strain rate did not detect a subclinical myocardial dysfunction in the long-term follow-up of Kawasaki disease. However, strain values showed variability with the follow-up periods, which indicates that Kawasaki disease might cause left ventricular dysfunction in the later phases. Therefore, a follow-up of children with a diagnosis of Kawasaki disease is of capital importance.
Keywords: Kawasaki disease, left ventricular function, speckle tracking imaging, strain
What is already known on this topic?
The involvement of the cardiovascular system is the determinant of prognosis in Kawasaki disease.
It has been reported that, even in patients without coronary artery involvement, the disease may cause cardiovascular morbidity and mortality in the late period.
Strain echocardiography is a new echocardiographic method that makes it possible to evaluate the regional function and deformation and the global function of the ventricles.
What this study adds on this topic?
The goal of our study was to determine the potential subclinical or asymptomatic myocardial dysfunction in long-term follow-up of patients with Kawasaki disease.
We aimed to show the efficiency of strain and strain rate in detecting subclinical myocardial dysfunction and their role in the follow-up of these patients.
Introduction
Kawasaki disease (KD) is a self-limited acute vasculitis of childhood that predominantly affects children <5 years of age (1). The involvement of the cardiovascular system is the determinant of prognosis in KD. Besides the coronary artery involvement, mitral or tricuspid regurgitation, valvulitis, or myocardial dysfunction owing to myocarditis may also occur during the acute phase. Ectasia or aneurysm of coronary arteries may be encountered in 15–25% of untreated patients (2). The treatment of intravenous immunoglobulin has reduced the incidence of coronary artery aneurysms from 25% to approximately 4% (1). New dilations may be detected in the coronary arteries years after the disease. It has also been reported that in patients even with no coronary artery involvement, the disease may cause cardiovascular morbidity and mortality in the late period (3).
There are studies in the literature analyzing the left ventricular myocardial changes in the acute phase of KD by various echocardiographic methods. There are limited data about the cardiac functions after the acute phase of the disease. Subclinical myocardial dysfunction cannot be detected by conventional echocardiography. However, we did not detect studies describing the long-term follow-up results of ventricular myocardial changes before the patients experience the common morbidities of adulthood, such as obesity, hypertension, and atherosclerosis.
Strain echocardiography is a new reliable echocardiographic method that makes it possible to evaluate the regional function and deformation and the global function of the ventricles. There are few studies about left ventricular (LV) myocardial deformation in the long term of KD. Therefore, the aim of our study was to determine the potential subclinical or asymptomatic myocardial dysfunction in long-term follow-up of patients with KD using two-dimensional (2D) strain echocardiography. In addition to conventional echocardiographic methods, we aimed to determine the possibility of subclinical myocardial dysfunction detected by strain echocardiography in the late period of children with KD.
Material and Methods
Study population
The study consisted of 30 patients diagnosed with KD at our department between 2005 and 2012 and 30 age- and gender-matched healthy children. All patients fulfilled the diagnostic criteria for KD. All patients had intravenous immunoglobulin (IVIG; 2 g/kg) and oral aspirin (80–100 mg/kg/day). Oral aspirin dose was reduced to 3–5 mg/kg/day 48 to 72 hours after the cessation of fever and completely stopped after the echocardiographic evaluation at the end of the eighth week, which revealed no persistence of coronary artery dilation. Medical records of the patients, including age at diagnosis, duration of the disease and follow-up, and therapy, were evaluated.
We used standard echocardiographic imaging rather than tissue Doppler, strain, and strain rate (SR) at the acute and subacute phase of KD in these patients. Patients with a follow-up period of at least more than 12 months after the diagnosis were included in the study. Standard and tissue Doppler and strain and SR echocardiograms were performed after 3.55±2.20 years of follow-up of patients with KD.
The control group consisted of 30 age- and gender-matched children referred to the department of pediatric cardiology for evaluation of cardiac murmur. Children who had cardiac, rheumatic, endocrinologic, renal or other chronic diseases were excluded from the study.
All procedures performed involving human participants were in accordance with the ethical standards of the local ethics committee and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the ethics committee of Kocaeli University Research and Application Hospital (KAEK/2011/46). Written informed consent forms were obtained from the parents and/or relatives of all the patients in the study.
Echocardiographic examination
Transthoracic echocardiography recordings were obtained by a single operator from all the children. Echocardiography consisting of transthoracic 2D and Doppler scans was performed for all patients with Vivid 7 Dimension (GE Vingmed Ultrasound, Horten, Norway). A 2.5 MHz probe was used. We used midazolam (0.3 mg/kg intranasal) for sedation of the children as required.
Conventional 2D echocardiography
Lateral decubitus position; apical four-, three-, and two-chamber; and parasternal short-axis (papillary muscle level) views of the left ventricle were taken at end-expiratory apnea. From each view, three cardiac cycles were stored in cine-loop format for subsequent offline analysis by investigators blinded to patient data. The following parameters were measured according to the established criteria by the American Society of Echocardiography (4). M-modes of 2D images were obtained from the parasternal long-axis views. Interventricular septal wall thickness, LV posterior wall thickness, and LV internal diameters were measured in all children. Systolic functions of the left ventricle were assessed by using shortening fraction, ejection fraction, and LV myocardial performance index (Tei index).
Pulsed Doppler and tissue Doppler echocardiography
By positioning the sample volume at the tips of the mitral leaflets from apical four-chamber views, pulsed Doppler recording of transmitral flow velocities was made. The measurements included peak atrial filling wave (A), peak early LV filling wave (E), isovolumic relaxation time, and deceleration time. Tissue Doppler imaging was analyzed to obtain peak systolic, early diastolic (E’) and late diastolic (A’) annular velocities, and myocardial performance index (Tei index) from the lateral mitral annulus.
Two-dimensional speckle tracking echocardiography
Apical four-, three-, and two-chamber views were used to obtain longitudinal strain and SR of the left ventricle. Parasternal short-axis views at the level of the papillary muscle were utilized to obtain circumferential and radial strain and SR. Images of three cardiac cycles (60–90 frames/s) were uploaded to EchoPAC version 6.1.0, GE Vingmed Ultrasound USA software for analysis of speckle tracking–based myocardial mechanics. The software automatically split up the images into six distinct segments, which were defined and entitled by the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association (4). These segments were used to calculate longitudinal, radial, and circumferential strain values. Averages of the six segments in the short-axis and four-chamber views were taken to obtain global strain. Coronary artery dilations detected by echocardiography were assessed according to the criteria set by the Japanese Ministry of Health (5). Echocardiograms were recorded and interpreted by experienced cardiologists.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (Statistical Product and Service Solutions Company, Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation. Differences between two groups were analyzed using the independent samples t-test. To analyze the associations between continuous variables, Pearson’s correlation was used. A p-value <0.05 was considered as statistically significant.
Results
Clinical features
The patient group consisted of 18 (60%) males and 12 (40%) females. The male to female ratio was 1.5. The mean age at the time of the diagnosis was 2.6±2.3 years (2 months–11 years). Five of the subjects were <6 months old, 21 of them were 6 months to 5 years old, and four were >5 years old.
Mean admission time after the onset of disease was 8.6±3.5 days. A total of 20 (66.6%) patients were diagnosed as KD in the acute phase of the illness (first 10 days) and 10 (33.3%) in the subacute phase of the disease (after 10 days). Of the 30 patients, 25 (83%) were diagnosed with typical KD and five (17%) with incomplete KD. All patients had IVIG (2 g/kg) and oral aspirin (80–100 mg/kg/day). The starting day of the treatment was 8.6±3.5 (4–15) days. A repeating dose of IVIG was given to four patients because of persistent fever. Echocardiographic exams revealed pericardial effusion in six patients and mild mitral regurgitation in the acute phase. Transient coronary artery dilation was detected in three patients (10%), but no coronary artery aneurysms were observed. All three patients with coronary artery involvement were male, and they were all diagnosed as incomplete KD. We did not encounter the persistence of coronary artery dilation in their long-term follow-up.
We interpreted the long-term cardiac findings of 30 patients with KD in our study, and a control group consisting of 30 healthy subjects was formed. The mean time of follow-up after the diagnosis was 3.55±2.20 years. We did not find a substantial difference in terms of the ECG findings of the two groups.
Conventional 2D echocardiography
There was no substantial difference between the left ventricle M mode values of both groups. Systolic dysfunction was not detected in any patient. Conventional echocardiography did not reveal a significant difference between the M mode and pulse wave tissue Doppler, E and A waves, E/A ratio, and deceleration values of both groups (Table 1).
Table 1.
Comparison of left ventricle M mode and pulsed wave Doppler variables between groups
| Variables | Patients (n=30) | Control (n=30) | p |
|---|---|---|---|
| LVDd (mm/m2) | 43.07±8.92 | 44.91±7.21 | 0.385 |
| LVIDs (mm/m2) | 27.21±5.32 | 28.72±4.70 | 0.249 |
| FS (%) | 36.03±3.39 | 36.21±3.83 | 0.848 |
| LVMI (g/m2) | 66.73±27.10 | 61.66±19.11 | 0.405 |
| E (m/sec) | 1.00±0.15 | 1.01±0.16 | 0.647 |
| A (m/sec) | 0.61±0.12 | 0.60±0.12 | 0.780 |
| E:A | 1.75±0.40 | 1.74±0.34 | 0.844 |
| DT (msec) | 118.80±24.30 | 118.50±22.1 | 0.969 |
| E’ (m/sec) | 0.18±0.03 | 0.18±0.03 | 0.671 |
| A’ (m/sec) | 0.11±0.17 | 0.09±0.13 | 0.608 |
| E’:A’ | 2.80±0.70 | 2.74±0.70 | 0.714 |
| S (m/sec) | 0.08±0.01 | 0.09±0.02 | 0.113 |
A, peak velocity of late transmitral flow; A’, peak velocity of diastolic mitral annular motion as determined by pulsed wave Doppler; DT, deceleration time; E, peak velocity of early diastolic transmitral flow; E’, peak velocity of early diastolic mitral annular motion as determined by pulsed wave Doppler; E:A, ratio of E to A; E':A', ratio of E' to A'; FS, fractional shortening; LVDd, left ventricular internal dimension at end-diastole; LVIDs, left ventricular internal dimension at end-systole; LVMI, left ventricular mass index, S: Systolic myocardial velocity.
Tissue Doppler echocardiography
Early diastolic wave, A’, E’/A’ ratio, systolic myocardial velocity, and myocardial performance index (Tei index) values obtained by tissue Doppler imaging did not show any significant differences between the groups (Table 1).
Two-dimensional speckle tracking echocardiography
Strain and SR parameters obtained by apical four-, three-, and two-chamber views were not statistically different between patients and controls (Table 2). Radial and circumferential peak systolic strain values also did not differ between the two groups. Moreover, detailed segmental analysis of strain was not different between the groups. We found no statistical differences between the patients and the control group in terms of global strain values, Tei index, and fractional shortening (Table 3).
Table 2.
Comparison of left ventricle longitudinal strain
| View | Segment | S (%) SR (1/S) | Patients (n=30) | Control (n=30) | p |
|---|---|---|---|---|---|
| Apical four-chamber (Septal) | Apical | S | −20.62±5.71 | −21.53±5.94 | 0.550 |
| SR | −1.43±0.39 | −1.43±0.36 | 0.989 | ||
| Mid | S | −21.81±2.85 | −21.88±2.52 | 0.919 | |
| SR | −1.40±0.22 | −1.36±0.19 | 0.521 | ||
| Basal | S | −20.13±3.14 | −20.34±3.40 | 0.803 | |
| SR | −1.36±0.19 | −1.38±0.26 | 0.700 | ||
| Apical four-chamber (Lateral) | Apical | S | −17.47±7.64 | −18.70±6.83 | 0.516 |
| SR | −1.29±0.46 | −1.41±0.48 | 0.321 | ||
| Mid | S | −17.89±6.16 | −17.33±4.80 | 0.696 | |
| SR | −1.46±0.45 | −1.47±0.52 | 0.890 | ||
| Basal | S | −18.71±6.61 | −17.15±5.05 | 0.308 | |
| SR | −1.82±0.69 | −1.80±0.75 | 0.933 | ||
| Apical two-chamber (Inferior) | Apical | S | −22.17±4.92 | −22.94±3.84 | 0.503 |
| SR | −1.56±0.37 | −1.57±0.39 | 0.860 | ||
| Mid | S | −24.41±2.79 | −23.52±3.36 | 0.273 | |
| SR | −1.57±0.20 | −1.57±0.27 | 0.996 | ||
| Basal | S | −24.12±3.44 | −22.99±3.87 | 0.236 | |
| SR | −1.62±0.30 | −1.63±0.29 | 0.965 | ||
| Apical two-chamber (Anterior) | Apical | S | −17.80±6.36 | −19.16±5.76 | 0.389 |
| SR | −1.26±0.39 | −1.37±0.51 | 0.367 | ||
| Mid | S | −19.56±5.57 | −19.74±5.06 | 0.896 | |
| SR | −1.33±0.39 | −1.38±0.40 | 0.614 | ||
| Basal | S | −22.30±6.39 | −21.55±6.42 | 0.652 | |
| SR | −1.84±0.50 | −1.75±0.51 | 0.469 | ||
| Apical three-chamber (Posterior) | Apical | S | −19.23±5.76 | −20.03±6.21 | 0.605 |
| SR | −1.42±0.39 | −1.45±0.39 | 0.775 | ||
| Mid | S | −14.84±6.76 | −16.45±4.93 | 0.296 | |
| SR | −1.21±0.35 | −1.27±0.39 | 0.509 | ||
| Basal | S | −12.26±7.92 | −12.96±8.76 | 0.736 | |
| SR | −1.53±0.58 | −1.60±0.49 | 0.601 | ||
| Apical three-chamber (Anterior septal) | Apical | S | −21.18±5.49 | −21.81±6.33 | 0.681 |
| SR | −1.37±0.35 | −1.52±0.37 | 0.128 | ||
| Mid | S | −21.76±4.73 | −22.43±5.22 | 0.608 | |
| SR | −1.38±0.35 | −1.39±0.29 | 0.940 | ||
| Basal | S | −21.16±4.95 | −21.75±4.80 | 0.641 | |
| SR | −1.47±0.40 | −1.47±0.34 | 0.976 |
S, strain; SR, strain rate.
Table 3.
Comparison of global strain values, TEI index, EF, and FS
| Values | Patients (n=30) | Control (n=30) | p |
|---|---|---|---|
| Global four-chamber LS | −19.47±3.56 | −19.08±3.11 | 0.658 |
| Global two-chamber LS | −21.86±3.23 | −22.03±2.81 | 0.826 |
| Global three-chamber LS | −18.36±3.73 | −19.25±3.88 | 0.368 |
| Global RS | 48.70±18.30 | 44.35±20.50 | 0.499 |
| Global CS | −15.79±3.40 | −16.64±5.30 | 0.463 |
| Global LS | −19.91±2.74 | −20.12±2.66 | 0.747 |
| Tei index | 0.34±0.06 | 0.36±0.10 | 0.459 |
| EF | 66.33±4.42 | 66.65±4.76 | 0.791 |
| Shortening fraction | 36.03±3.32 | 36.21±3.83 | 0.848 |
CS, circumferential strain; EF, ejection fraction; FS, fractional shortening; LS, longitudinal strain; RS, radial strain.
There was a positive correlation between follow-up period and global four- and three-chamber longitudinal strain and global longitudinal strain values (r=0.465, p=0.010; r=0.414, p=0.023; r=0.492, p=0.006, respectively) (Figure 1), whereas global radial strain showed negative correlation (r=−0.517, p=0.003) (Figure 2). The study indicated a negative correlation between the risk factor of lower hemoglobin levels related to the cardiovascular system involvement in KD and the four-chamber global longitudinal strain (r=−0.444, p=0.014). There was also a positive correlation between the erythrocyte sedimentation rate and the four-chamber global longitudinal strain (r=0.383, p=0.037).
Figure 1.
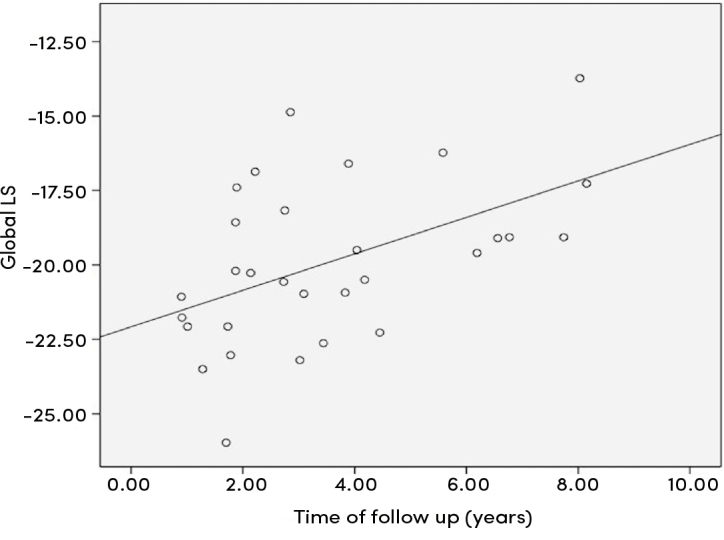
Correlation analysis between global longitudinal strain and follow-up time
Figure 2.
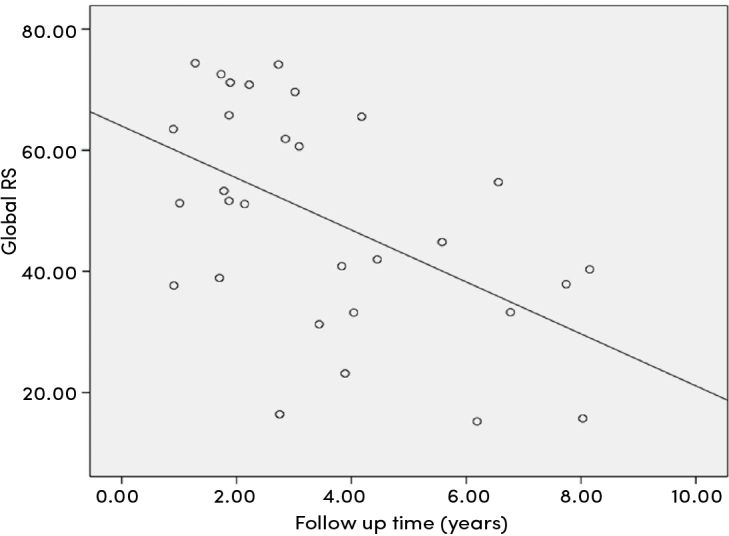
Correlation analysis between global radial strain and follow-up time
Discussion
Cardiovascular system involvement is the major factor affecting mortality and morbidity during the acute and long-term follow-up of patients with KD. Coronary artery lesions may lead to sudden death and ischemic heart disease in the long term (6). Patients who had been diagnosed as KD regardless of having coronary artery lesions bear the risks of obesity, dyslipidemia, and hypertension. As adults, these patients will present with cardiovascular complications such as endothelial dysfunction, atherosclerosis, and ischemic heart disease (7).
During the acute phase of the disease, myocarditis may give rise to ventricular dysfunction, which is attributed to myocardial edema and inflammation that lead to diastolic dysfunction. Ajami et al. (8) used the Tei index for estimating global myocardial dysfunction in patients with KD. The Tei index increased before IVIG treatment and decreased significantly after it. They also observed a decreased ejection fraction (EF) and fractional shortening (FS) during the acute phase in 35.7% of patients.
Our study showed that LV functions did not differ between children with a history of KD and healthy children in the late period of follow-up. We also detected normal Tei index during follow-up and no significant difference compared with the control group. These results suggest that cardiac functions of children with KD are not affected in the long term.
Because the number of patients with coronary artery involvement was quite low (there were only three patients with coronary artery dilation and none of the patients had coronary artery aneurysms), statistical analysis could not be performed between the two groups because of the small sample size. Larger series are needed to evaluate the outcome of cases with coronary artery lesions.
Speckle tracking imaging via echocardiography is a new and useful technique to evaluate global and regional myocardial deformation (9). Few studies have evaluated LV longitudinal systolic strain in children with KD during acute and convalescent phases.
A vast majority of patients with KD exhibit normal conventional echocardiographic metrics of cardiac function, such as EF and FS (10). Despite normal LV systolic function by routine echocardiographic measurements, patients with KD may have reduced longitudinal LV myocardial strain and SR, which may be more sensitive indicators of myocardial inflammation. This may provide supportive criteria to avoid delayed diagnosis of KD. Three recent studies have demonstrated lower (i.e., less negative) mean global longitudinal strain in cohorts of patients with acute-phase KD relative to controls (9–11).
We did not detect a significant difference one year after the onset of the disease between the patient and control groups in terms of strain values. Our data is supported by the fact that there are certain variations in the normalization of deformation by the convalescent phase of KD. Similarly, Xu et al. (11) demonstrated that at 6–8 weeks after the onset of KD, all LV strains had recovered to normal. Moreover, Frank et al. (9) studied 41 patients with KD and measured myocardial strain and SR by velocity vector imaging from pretreatment and convalescent echocardiograms. Their patients showed a significant range of myocardial strain on the acute-phase echocardiography. Those patients with initially lower strain improved significantly by the convalescent echo, whereas those with higher strain showed much less change or even a lower strain value at the later time point. The results were similar when using either longitudinal or circumferential strain. However, they also emphasized that strain may be affected by interstitial inflammation/edema, whereas SR may not be affected unless actual myocyte injury occurs. This fact is supported by Yu et al. (12), who identified depressed strain but not SR in the patients compared with controls during the acute phase, whereas McCandless et al. (10) found both strain and SR to be depressed in acute KD. As we do not have the echocardiographic values of patients in the acute phase of the disease, our study has no power to compare the changes between the acute phase of the disease and long-term course or the variations in the acute phase in terms of strain and SRs. On the other hand, compared with the studies aforementioned, our study is unique as all of the previous studies concentrated on the acute phase of the disease. In contrast, we investigated changes in the long-term follow-up period.
Although we did not detect myocardial dysfunction during our follow-up, we cannot conclude that these patients will not encounter any complications owing to KD. In our study, none of the patients had coronary artery aneurysms. Coronary artery dilation, which was detected in three patients, fully regressed. We know that even coronary artery aneurysms regress to normal internal lumen. The prognosis for adults who recovered from KD without coronary aneurysms is suggested to be good, but longitudinal studies have not been performed to test this hypothesis. Patients with either transient coronary artery dilation or no coronary artery luminal changes as noted on echocardiography after acute KD in childhood are currently recommended to require no pharmacological therapy beyond 6–8 weeks after the acute episode, no invasive cardiologic testing, and ongoing cardiovascular risk factor assessment and counseling at 3- to 5-year intervals. However, in case-control cross-sectional studies of a small number of patients, subtle ongoing abnormalities have been variously and sometimes inconsistently reported. Relative to controls, these include decreases in high-density lipoprotein, increases in markers of inflammation, the presence of varying degrees of endothelial dysfunction in both the coronary and systemic arteries, decreases in arterial compliance and distensibility, decreases in myocardial blood flow reserve, increases in carotid intima-media thickness (IMT) and myocardial fibrosis, and alterations in the coronary artery microvasculature (13–16).
Follow-up evaluations should be made during childhood, and it seems rational to continue this periodic re-assessment into adulthood. There are other options that can be useful in follow-up of patients with KD. Coronary artery calcium score by computed tomography may be useful as arterial calcification is a feature of the vascular lesions of KD. Late myointimal thickening of the coronary arterial wall can be detected by intravascular ultrasound. Non-invasive assessments of endothelial cell functions such as brachial artery flow-mediated dilation and structural assessments such as carotid IMT remain research tools at this time with uncertain prognostic significance (17).
Our study, which evaluated late-term cardiac functions, showed no significant differences between patient and control groups in terms of strain and SR values. Regional myocardial functions of our asymptomatic cases were found to be normal. Because the involvement of distal parts of the coronary arteries may occur in the acute phase of the disease, and conventional methods could not detect this, it was suggested that 2D speckle tracking echocardiography (STE) could show subclinical changes. However, no finding that reflects ischemia on cardiac segments was discovered. This circumstance indicates that the regional myocardial functions in the long term are normal. Thus, by taking into consideration the study conducted by Yu et al. (12), our study supports the idea that impairment of strain values in the acute phase occurs secondary to myocarditis.
Dedeoğlu et al. (18) reported that LV strain was impaired in the apical and basal segments of patients with antecedent KD during midterm follow-up by using STE. In this study, the median follow-up period was similar to our study. Seven patients had persistent coronary artery involvement. Although there was no significant difference in strain values between patients with KD with and without coronary aneurysms in left LV segments, impairment of basal and apical segments of the left ventricle may be due to cardiovascular sequelae in these patients.
Lin et al. (19) reported that patients with KD with coronary artery dilation had lower global strain values according to the control group. Two-dimensional and three-dimensional (3D) strain echocardiography was performed, and the duration of the follow-up time was more than 7 years. Left ventricle strains were normal in children without coronary artery dilations in the long term, similar to our study.
In our study, a positive correlation was determined between the duration of follow-up and global longitudinal strain, global four chamber longitudinal strain, and global three-chamber longitudinal strain values. In contrast, the duration of follow-up and global radial strain values showed a negative correlation. Initially, this result was attributed to the judgment that strain values in children might vary with increasing age. However, with the help of a thorough analysis of the literature, it was noticed that Sato et al. (20) had reported that peak systolic strain values in childhood did not vary with age or heart rate. Left ventricular strain may be influenced by age, gender, heart rate, blood pressure, and body surface area. Dallaire et al. (21) reported statistically significant associations between body size and strain values in children and also recommended using strain values normalized to body surface area. There are recent studies that have demonstrated maturational changes in LV deformation indices with somatic growth. Lorch et al. (22) found that LV longitudinal strain did not change significantly with age. Furthermore, longitudinal SR changed with age, highest in infancy then decreased until the age of 10. Klitsie et al. (23) also reported no linear relation between age and most global peak strain parameters. Kaku et al. (24) demonstrated that after 2–3 years of age, the normal range of deformation did not vary significantly according to those in young adults by using 3D echocardiography. According to these studies, there are still arguments on the standardization of normal strain echocardiographic values in children. Although data quality has improved in the recent studies, available pediatric nomograms for LV strain and SR are heterogeneous and therefore limited. In our study, although LV strain values evaluated by 2D STE did not reveal any difference between patients and controls, increased values of longitudinal strain were observed by increasing follow-up period. An increase in longitudinal strain value, which is expressed as a negative mathematical value, implies less shortening of LV myocardial fibers. Therefore, this result considers subclinical impairment of myocardial functions with increased duration of follow-up. These findings support the results of studies in adults who had KD in their childhood. The negative correlation between duration of follow-up and radial strain, which is expressed as a positive mathematical value, has also been interpreted similarly. In our study, the finding of normal cardiac functions in the long term may be related to the timing of diagnosis and treatment of the disease.
It was reported in children with a diagnosis of KD that they had an increased risk of atherosclerosis and cardiovascular morbidity in adulthood even if they had no coronary involvement initially. Normal strain values were detected in all patients in this study. However, this result does not denote that these children are not under risk for cardiovascular diseases in adulthood.
In contrast, variation in the strain values by the duration of follow-up, which is at least one year in our study, gives rise to the thought that LV systolic functions are impaired independently of coronary artery involvement in KD. Therefore, the follow-up of children with a diagnosis of KD is of capital importance. Although the echocardiographic examinations are found to be normal in late childhood and even in adulthood, follow-up should be sustained, and, in symptomatic patients, further examinations should be planned in recognition of coronary artery disease.
Study Limitations
We do not have the echocardiographic values of patients in the acute phase of the disease. Our study has no power to compare the changes between the acute phase of the disease and long-term course or the variations in the acute phase in terms of strain and SRs.
There were only three patients with coronary artery dilation and none of the patients had coronary artery aneurysms. Because of the small sample size, the statistical analysis could not be performed between the two groups.
Conclusion
Although there was no difference between groups by strain parameters, this study is one of the very few studies which investigates the late-term findings of KD by 2D strain echocardiography. Although we were not able to detect myocardial dysfunction by echocardiographic methods in patients with KD, strain values showed variability with the follow-up period, which indicates that KD might cause LV dysfunction in the later phases. Therefore, it appears that they should be kept under control for possible cardiovascular complications. More detailed results could be obtained by studies including bigger populations and longer follow-up periods.
Acknowledgement
We would like to thank all our children and their parents for their collaboration and patience.
Footnotes
Ethical Committee Approval: Ethics committee approval was received for this study from the ethics committee of Kocaeli University Research and Application Hospital (KAEK / 2011/46).
Informed Consent: Written informed consent forms were obtained from the parents and/or relatives of all the patients in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Ö.K., K.B.; Design – Ö.K.; Supervision – Ö.K.; Data Collection and/or Processing – Ö.K., M.D., E.Z.B.; Analysis and/or Interpretation – Ö.K., M.D., E.Z.B.; Literature Review – Ö.K., T.T.; Writing – Ö.K., K.B.; Critical Review – K.B.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from American Heart Association. Circulation. 2017;135:927–99. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6. [PubMed] [Google Scholar]
- 3.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–11. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerqueira MD, Weissman NJ, Dilsizian V, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 5.Research Committee on Kawasaki Disease Report of Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. Tokyo, Japan: Japanese Ministry of Health and Welfare; 1984. [Google Scholar]
- 6.Kato H, Sugimura T, Agati T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up story of 594 patients. Circulation. 1996;94:1379–85. doi: 10.1161/01.CIR.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 7.Senzaki H. Long-Term Outcome of Kawasaki Disease. Circulation. 2008;118:2763–72. doi: 10.1161/CIRCULATIONAHA.107.749515. [DOI] [PubMed] [Google Scholar]
- 8.Ajami G, Borzouee M, Amoogzar H, et al. Evaluation of myocardial function using the Tei index in patients with Kawasaki disease. Cardiol Young. 2010;20:44–8. doi: 10.1017/S1047951109991910. [DOI] [PubMed] [Google Scholar]
- 9.Frank B, Davidson J, Tong S, et al. Myocardial Strain and Strain Rate in Kawasaki Disease: Range, Recovery, and Relationship to Systemic Inflammation/Coronary Artery Dilation. J Clin Exp Cardiolog. 2016;7:432. doi: 10.4172/2155-9880.1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCandless RT, Minich LL, Wilkinson SE, et al. Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging. 2013;14:1061–8. doi: 10.1093/ehjci/jet041. [DOI] [PubMed] [Google Scholar]
- 11.Xu QQ, Ding YY, Lv HT, et al. Evaluation of left ventricular systolic strain in children with Kawasaki disease. Pediatr Cardiol. 2014;35:1191–7. doi: 10.1007/s00246-014-0915-5. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Choi H, Kim Y, et al. Analyses of left ventricular myocardial deformation by speckle-tracking imaging during the acute phase of Kawasaki Disease. Pediatr Cardiol. 2010;31:807–12. doi: 10.1007/s00246-010-9708-7. [DOI] [PubMed] [Google Scholar]
- 13.McCrindle BW. Kawasaki disease: a childhood disease with important consequences into adulthood. Circulation. 2009;120:6–8. doi: 10.1161/CIRCULATIONAHA.109.874800. [DOI] [PubMed] [Google Scholar]
- 14.Yutani C, Okano K, Kamiya T, et al. Histopathological study on right endomyocardial biopsy of Kawasaki disease. Br Heart J. 1980;43:589–92. doi: 10.1136/hrt.43.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonesaka S, Takahashi T, Matubara T, et al. Histopathological study on Kawasaki disease with special reference to the relation between the myocardial sequelae and regional wall motion abnormalities of the left ventricle. Jpn Circ J. 1992;56:352–8. doi: 10.1253/jcj.56.352. [DOI] [PubMed] [Google Scholar]
- 16.Haneda N, Mori C. Histopathologic and coronary angiographic assessment of effectiveness of aspirin or aspirin-and-gamma globulin in Kawasaki disease. Acta Paediatr Jpn. 1993;35:294–7. doi: 10.1111/j.1442-200X.1993.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: Myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–20. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedeoğlu R, Barut K, Oztunc F, et al. Evaluation of myocardial deformation in patients with Kawasaki disease using speckle-tracking echocardiography during mid-term follow-up. Cardiol Young. 2017;27:1377–85. doi: 10.1017/S1047951117000580. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Zheng J, Chen W, et al. Assessing left ventricular systolic function in children with a history of Kawasaki disease. BMC Cardiovasc Disord. 2020;20:131. doi: 10.1186/s12872-020-01409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato Y, Maruyama A, Ichihashi K. Myocardial strain of the left ventricle in normal children. J Cardiol. 2012;60:145–9. doi: 10.1016/j.jjcc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Dallaire F, Slorach C, Bradley T, et al. Pediatric reference values and z score equations for left ventricular systolic strain measured by two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2016;29:786–93. doi: 10.1016/j.echo.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimentional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr. 2008;21:1207–15. doi: 10.1016/j.echo.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Klitsie LM, Roest AAW, Van Der Hulst AE, et al. Assessment of intraventricular time differences in healthy children using two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2013;26:629–39. doi: 10.1016/j.echo.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Kaku K, Takeuchi M, Tsang W, et al. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:55–64. doi: 10.1016/j.echo.2013.10.002. [DOI] [PubMed] [Google Scholar]


