Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) first emerged in December 2019 in Wuhan, China, and has since spread rapidly worldwide. As researchers seek to learn more about COVID-19, the disease it causes, this novel virus continues to infect and kill. Despite the socioeconomic impacts of SARS-CoV-2 infections and likelihood of future outbreaks of other pathogenic coronaviruses, options to prevent or treat coronavirus infections remain limited. In current clinical trials, potential coronavirus treatments focusing on killing the virus or on preventing infection using vaccines largely ignore the host immune response. The relatively small body of current research on the virus indicates pathological responses by the immune system as the leading cause for much of the morbidity and mortality caused by COVID-19. In this review, we investigated the host innate and adaptive immune responses against COVID-19, collated information on recent COVID-19 experimental data, and summarized the systemic immune responses to and histopathology of SARS-CoV-2 infection. Finally, we summarized the immune-related biomarkers to define patients with high-risk and worst-case outcomes, and identified the possible usefulness of inflammatory markers as potential immunotherapeutic targets. This review provides an overview of current knowledge on COVID-19 and the symptomatological differences between healthy, convalescent, and severe cohorts, while offering research directions for alternative immunoregulation therapeutic targets.
Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) and its associated pathology, COVID-19, have been of particular concern recently due to the worldwide disruption they have caused1. As the disease continues to spread, the development of prophylactic and therapeutic approaches is urgently required. The number of patients requiring intensive care is a critical point in this pandemic. A better understanding of the pathophysiology leading to disease progression is thus urgently needed, especially because the factors that drive severe lung pathology during infection with highly pathogenic human coronaviruses are poorly understood2.
The pathogen itself or an overwhelmed immune response can cause infected lesions3. Focusing on SARS-CoV-2, studies have observed that this virus can trigger immune responses that can be dysregulated in severe patients, leading to further injury to multiple organs4,5. An unbalanced immune response and exacerbated release of proinflammatory cytokines contribute to major complications of coronavirus infection, including acute respiratory distress syndrome (ARDS) and pulmonary fibrosis6,7. Yet the immune components that drive severe lung pathology during infection with highly pathogenic human coronaviruses are not clearly understood. Therefore, development of any potential treatments should focus not only on directly killing the virus or preventing infection with a vaccine, but also on managing immune and inflammatory responses8.
In this review, we investigated current knowledge on SARS-CoV-2 infection, focusing particularly on its immunologic features. We aimed to detail the mechanisms involving the host innate and adaptive immune responses against COVID-19 and to present information on recent COVID-19 experimental data. We found which immune components and secreted cytokines can induce systemic immune responses and histopathology in patients with COVID-19. Our findings elucidate the changes in immune mechanisms, the immune microenvironment, and immunopathogenesis upon SARS-CoV-2 infection. They provide a new research direction for developing alternative immunoregulation therapeutic targets and may assist in the discovery of potential immune-related hallmarks to define patient recovery and outcome.
Immune System Activation Under SARS-CoV-2 Infection
Humans have two types of immunities: innate (rapid and non-specific response) and adaptive (slow and specific response). In the innate immunity, myeloid cells, such as monocytes, macrophages, dendritic cells (DCs), and granulocytes, are critical in defending against foreign pathogens. Innate lymphocytes, such as natural killer (NK) cells and innate lymphoid cells (ILCs), also work in the innate immune response. In the adaptive immune system, B cells and T cells are crucial lymphocytes9. Immune response to SARS-CoV-2 involves both the innate and adaptive immunity2.
ACE2 Receptor-Mediated Inflammatory Response
At first contact with the respiratory mucosa, the SARS-CoV-2 virus infects cells via expression of the surface receptors angiotensin-converting enzyme 2 (ACE2) and type II transmembrane serine protease (TMPRSS2)10. Viral replication and release then occur, causing the host cell to undergo pyroptosis and release damage-associated molecular patterns (PAMPs), including viral nucleic acids and oligomers. The host’s initial innate immune function is the first line of defense against viral infection11,12. In the first days after infection, innate immune cells, including macrophages, DCs, neutrophils, and NK cells, initially activate by recognizing viral PAMPs via pattern-recognition receptors (PRRs)13.
Upon SARS-CoV-2 binding to the ACE2 receptor to enter the target cell, the renin–angiotensin system activates and angiotensin II (Ang II) level increase. Elevated Ang II levels are strongly associated with viral load and lung injury in patients with severe COVID-1914,15. In addition, studies have shown that the ACE2–Ang II axis can induce cytokine secretions, including interleukin 6 (IL-6), monocyte chemotactic protein 1 (MCP-1), vascular cell adhesion molecule 1 (VCAM-1), and selectin E, to induce macrophage infiltration, endothelial dysfunction, thrombin formation, and impaired fibrinolysis14,16–18. ACE2 appears to play two roles in COVID-19. It first acts as a receptor for SARS-CoV-2 to enter the host and then, due to the increase in Ang II expression from the ACE2–AngII axis, it later recruits additional macrophages to the site of infection14.
Host Inflammatory Response
The activated innate immune system generates various pro-inflammatory cytokines and chemokines, including IL-6, CXCL10 (IP-10), macrophage inflammatory protein 1α (MIP1α), MIP1β, and MCP-1. These chemokines then attract monocytes, macrophages, and T cells to the infection site, promoting further inflammation and establishing a pro-inflammatory feedback loop, including the recruitment of NK cells2. In addition, innate antigen-presenting cells (APCs), such as DCs and macrophages, will also be at the infection site to present viral antigens to virus-specific T cells. This leads to activation of the body’s adaptive immunity, which is mediated by virus-specific B (humoral immunities) and T cells (cellular immunities)8,19.
In the adaptive immunity, cell-mediated immunity and antibody production have critical roles in COVID-19 infection20. Decreased absolute numbers of T lymphocytes (CD4+ and CD8+ T cells) occur in both mild and severe cases, with a more notable decrease observed in severe cases. In fact, a decrease in CD8+ T and B cells and an increase in the CD4+/ CD8+ ratio may be independent predictors of poor treatment outcomes20. Decreased IFN-γ expression by CD4+ T cells is also more notable in severe cases than in moderate ones21. Overall, the low innate and adaptive antiviral defenses and high pro-inflammatory cues lead to multi-organ damage and contribute to COVID-1922.
Gender Differences in COVID-19
While men and women have similar susceptibility to SARS-CoV-2 infection, evidence suggests that men with COVID-19 tend to exhibit more severe morbidity and mortality than do women23,24. Public meta-analyses have also shown that the odds of requiring admission to an intensive treatment unit for COVID-19 is three times higher among men. Furthermore, the mortality rate among men is 2.4 times higher24, and the case fatality rate (CFR) for men was shown to be 1.7 times higher than that for women across 38 countries25. In SARS patients, sex was observed to have had a role in mortality24.
To further understand differences between sexes, studies have shown that biological sex has direct effects on the immune components of innate and adaptive immunity by modulating genetic variants26–32, transcription factors33, epigenetic modification34,35, sex hormones (including: estrogens36,37, progesterone38–42, and testosterone43, and microbiome variances44. Therefore, sex may to some extent determine the host immune response to COVID-19 infection as well as the disease course and clinical outcome44. Given that women are at 8-9 times higher risk of developing autoimmune disease (AD) compared to men, it is possible that upon COVID-19 infection, their increased immune function may enhance their anti-inflammatory regulation and antiviral defense45–47.
Clinical Symptoms
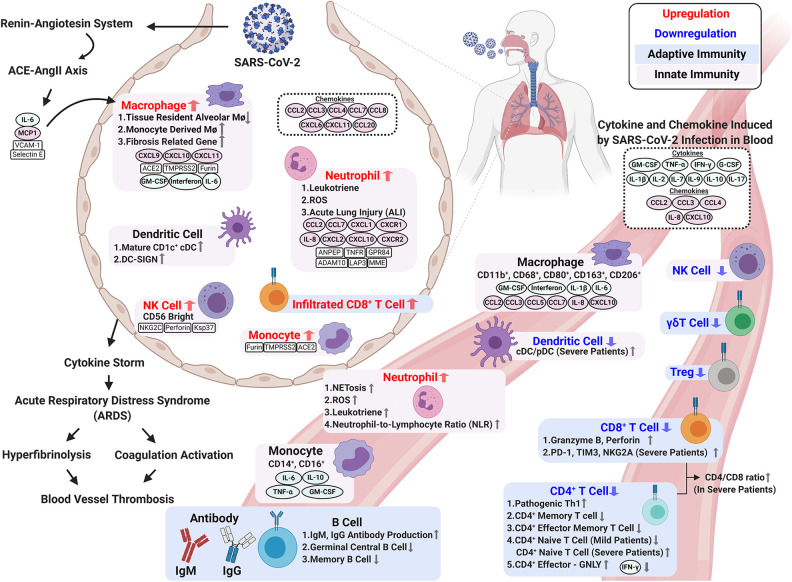
The abnormal and exaggerated cytokine storm that occurs in critically ill COVID-19 patients is a major factor of COVID-19 mortality. Development of a cytokine storm is also a sign of disease escalation in other viral diseases, such as SARS, Middle Eastern respiratory syndrome (MERS), and influenza48–50. In COVID-19, hyperactivity of the immune system stimulates elevated cytokines including IL-6, IL-8, IL-1β, IL-2, IL-4, IL-7, IL-10, IFN-γ, TNF-α, MCP-1, GM-CSF, CCL2, CCL3, CCL5, and CXCL10 (IP-10). This results in a cytokine storm and lung immunopathology symptoms, including acute lung injury (ALI), systemic inflammatory response syndrome, and ARDS51,52. Moreover, intravascular coagulation, blood vessel thrombosis, and hyper-fibrinolysis coexist in COVID-19-induced ARDS and become more severe as the disease progresses53–55. Changes in immune mechanisms, the immune microenvironment, and immunopathogenesis upon SARS-CoV-2 infection are described in the following sections and summarized in Fig. 1.
Figure 1.
Changes in immune mechanisms, the immune microenvironment, and immunopathogenesis upon SARS-CoV-2 infection. Immune response to SARS-CoV-2 infection involves innate and adaptive immunity. Activated innate immune cells trigger a strong immune response to secrete cytokines, which cause a cytokine storm and ARDS. Elevated circulating cytokines (e.g., IL-1β, IL-2, IL-7, IL-9, IL-10, IL-17, G-CSF, GM-CSF, IFN-γ, and TNF-α) are present in patients with severe COVID-19. BALF samples from COVID-19 patients contain accumulations of various immune cell-attracting chemokines (e.g., CCL2, CCL3, CCL4, CCL7, CCL8, CCL20, CXCL6, IL-8, CXCL10 (IP-10), and CXCL11). A cytokine storm during SARS-CoV-2 results in ARDS, which induces intravascular coagulation and hyperfibrinolysis and causes high thrombus burden in COVID-19 patients. In terms of adaptive immunity, SARS-CoV-2 infection significantly decreases total adaptive immunity lymphocytes and impairs their ability to defend against the virus. Upon infection, CD4+ T cells differentiate less frequently into Th1 cells, and this is associated with the decreased IFN-γ production for antiviral response. Severe COVID-19 patients exhibit the exhausted phenotype CD8+ T cell with high PD-1 and Tim-3 expression. Interestingly, compared to mild cases, severe COVID-19 patients have higher counts of activated CD8+ T cells in circulation to produce cytotoxic granzyme B and perforin. The humoral response is less affected by the virus. The increase in activated B cells gives greater antibody production and better protection to eliminate the virus. Figure created with BioRender.
Innate Responses
The innate immune system is the first line of defense against infection from various pathogens, including viruses. Cells composing the innate immune system include myeloid cells (monocytes, macrophages, DCs, and granulocytes), cytotoxic lymphocyte NK cells, and ILCs (Group 1, Group 2, and Group 3)12. These cells orchestrate the direct clearance and eradication of pathogens and contribute to the generation of long-lived adaptive immune responses56.
The innate immune response to coronaviruses can be induced upon recognizing pathogen associated molecular patterns (PAMPs), including SARS-CoV-2 positive-sense RNA genomes, viral RNA in endosomes, and viral protein PRRs such as TLR-513. This results in the excessive accumulation of monocytes, macrophages, and neutrophils in the lungs57,58. In the innate immunity, APCs such as DCs and macrophages can be used to process viral antigens and activate the host’s humoral and cellular immunities8,19. There are two major classes of MHCs involved in antigen presentation: MHC I and MHC II. SARS-CoV mainly depends on MHC I molecule presentation59; by contrast, SARS-CoV-2 could influence the antigen presentation of SARS-CoV-2 variants to MHC II with a single mutation60. The innate immune cell subset changes and cytokine secretions in SARS-CoV-2 infection are described in the following sections and summarized in the Table 1.
Table 1.
Representative Innate Immune Cell Subset Changes and Cytokine Secretions in SARS-CoV-2 Infection.
| Immune Cell Subset | Changes in Frequency | Changes in Phenotype | Reference |
|---|---|---|---|
| Macrophage | Highly proinflammatory macrophage microenvironment in the lungs in severe cases Increased monocyte-derived macrophages but depleted tissue-resident alveolar macrophages in lungs |
In the lungs, ACE2, TMPRSS2, and furin overexpression occur in macrophages. Inflammatory macrophage subsets show increased GM-CSF, IL-6, IFN, and IP-10. In the peripheral system, monocyte-derived macrophages express both M1 and M2 markers (CD11b+ CD14+CD16+ CD68+ CD80+ CD206+ CD163+) CD68+ CD169+ macrophages can be found in the lymph node subcapsular sinus and splenic marginal zone |
56,58,61–66 |
| Monocyte | Higher percentage of CD14+CD16+ inflammatory monocytes in the peripheral blood and may increase migration to the lungs Increased peripheral classical monocytes during recovery |
In the lungs, ACE2, TMPRSS2, and furin overexpression occur in monocytes. Unique populations of CD14+ CD16+ monocytes can be found in patients with COVID-19 Increased monocyte secretion cytokines (e.g., IL-6, TNFα, GM-CSF, and IL- 10) are present in the peripheral blood in severe cases |
20,56,58,61,62,64,67,68 |
| Neutrophil | Increased neutrophil-to-lymphocyte ratio (NLR), neutrophil-to-CD8+ T cell ratio (N8 R), and increased total circulating neutrophils in severe cases. All of these ratios are considered potential prognostic factors | SARS-CoV-2 can activate neutrophils and NETosis. Activated neutrophils release leukotrienes and ROS, thus triggering cytotoxicity such as ALI and cytokine storm Down-regulation of degranulation and activation pathways. COVID-19-infected cells express upregulation of neutrophil-attracting chemokine genes, including TNFR, IL-8, CXCR1, CXCR2, ADAM10, GPR84, MME, ANPEP, and LAP3 |
56,58,69–81 |
| Dendritic Cell (DC) | BALF of convalescent patients contains increased mature cDCs In the peripheral system, the total number of DCs is reduced with impaired activity, and with increased ratios of cDCs to pDCs in severe cases |
CD1c+ cDCs accumulated in the lungs and reduced blood DCs with impaired activity pDCs show impaired activity in the severe acute COVID-19 phase |
67,80,82,83 |
| Natural Killer (NK) Cell | Decreased total number in the peripheral blood during infection; increased infiltration of NK cells in the lungs | In severe cases, lung-infiltrated NK cells are activated with high expression of perforin, NKG2C, and Ksp37 Increased expression of the inhibitory receptor NKG2A could induce NK and NKT cell exhaustion |
12,20,56,84 |
Macrophages and Monocytes in COVID-19
Mononuclear phagocytes contribute substantially to innate and adaptive immunity by sensing and responding to microbial threats by producing inflammatory molecules that eliminate pathogens. Circulating monocytes are the largest type of leukocyte and can differentiate into tissue-resident macrophages and myeloid lineage DCs85. Resident subcapsular sinus macrophages and hilar lymph node macrophages are the majority of macrophages presenting viral antigens to activate specific T cells, which secrete cytotoxic perforin, granzyme B, and interferon-γ to kill infected cells. Thus, these macrophages play a protective role against viral infection by capturing viral particles86.
Upon COVID-19 infection, circulating monocytes and resident macrophages participate in all stages of SARS-CoV-287. Human monocytes and macrophages seem to be a widespread target upon COVID-19 infection due to the expression of ACE2, TMPRSS2, and furin on both cells64. In vitro, both SARS-CoV and SARS-CoV-2 can equally infect type-I and -II pneumocytes as well as alveolar macrophages88,89. A highly proinflammatory macrophage microenvironment is present in the lungs of patients with severe COVID-1961, and autopsies on COVID-19 patients have revealed high infiltration of macrophages within the area of bronchopneumonia70,90. Moreover, with ACE2 and nucleoprotein (NP) antigen expression, CD68+ and CD169+ macrophages are highly infiltrating to the lymph node subcapsular sinus and splenic marginal zone of COVID-19 patients58. Furthermore, COVID-19 may selectively induce macrophages to produce IL-6, not TNF-α or IL-1β, to induce lymphocytopenia or lymphocyte necrosis91. Therefore, the depletion of tissue-resident alveolar macrophages and an abundance of inflammatory monocyte-derived macrophages are more associated with severe COVID-19 cases58,61,70,92.
Monocytes exhibit significant morphological and functional differences in patients with COVID-19, especially those requiring prolonged hospitalization and ICU admission68. The CD14+ CD16+ monocytes subset is found more in the peripheral blood of COVID-19 patients compared to healthy populations, and these larger-than-normal monocytes are easily detected on forward scatter in flow cytometry68. In severe COVID-19 cases, classical monocytes exhibit higher expressions of type I interferons (IFNs), TNF, and IL-1-driven inflammatory responses, but not with mild cases; this suggests that the exacerbating inflammatory cytokines may be critical in the progression to severe COVID-1922.
In addition to CD14+CD16+, larger-than-normal CD11b+, CD68+, CD80+, CD163+, CD206+ macrophages can also be found in the peripheral blood of patients COVID-19. Since CD80 is considered an M1 marker, while CD163 and CD206 are considered M2 markers, both M1 and M2 macrophages along with monocytes are present in the peripheral blood cells of patients with COVID-1968. Moreover, in moderate and severe COVID-19 cases, a relatively higher frequency of M1-like macrophages and higher concentration of chemokines, such as CXCL9, CXCL10 (IP-10), and CXCL11, can be found in the bronchoalveolar lavage fluid (BALF) compared to healthy individuals61,93.
Highlighting the effect of SARS-CoV-2 on macrophages, the cytokine storm in COVID-19 cases tends to fit the pattern of macrophage activation syndrome with lymphocytopenia. Immunohistochemical testing shows that infected macrophages produce high levels of IL-6 rather than TNF-α in COVID-19-infected spleen tissue and lymph nodes compared to healthy tissue. The SARS spike protein activates increased transcription of several cytokines in macrophages. These include granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, CXCL10 (IP-10), IL-1β, IL-8, and IFNs64. However, SARS-CoV-2 does not activate any IFN response, including that of IFN type I, II, and III, from infected monocyte-derived macrophages (MDMs)82.
BALF samples from COVID-19 patients show accumulations of monocyte-attractant chemokines in the lungs, including CCL2, CCL3, CCL4, CCL7, CCL8, CCL20, CXCL6, and CXCL1193,94. In addition, the activated blood-circulating macrophages further release attractant chemokines (such as CCL2, CCL3, CCL5, CCL7, and CXCL10 (IP-10), as well as of the soluble form of the α-chain of the IL-2 receptor) to locally accumulated mononuclear macrophages95. Many other elevated circulating cytokines are also present in patients with COVID-19, especially in those requiring ICU admission, thus triggering severe cytokine syndrome and exacerbating the severity of the disease58,85. These include IL-1β, IL-2, IL-7, IL-9, IL-10, IL-17, G-CSF, GM-CSF, IFN-γ, TNF-α, IL-8, CXCL10 (IP-10), MCP-1 (CCL2), MIP1A (CCL3), and MIP1B (CCL4), and many of these cytokines and chemokines are secreted from monocytes and macrophages93,96.
Finally, single-cell RNA sequencing (scRNA-seq) and bulk RNA-seq analysis of the heterogeneity of macrophages in patients’ BALF with moderate and severe COVID-19 reveals abundant MDMs in severe cases61. Twenty macrophage subclusters have been identified61, with one subset of COVID-19-associated macrophages exhibiting higher tissue repair and fibrosis-related genes, similar to that seen in liver cirrhosis65. This suggests that the pathogenicity of infiltrating macrophages could extend beyond the promotion of acute inflammation and is in line with the fibrotic complications observed in patients under mechanical ventilation97.
Natural Killer Cells
NK cells, an innate cytotoxic lymphocyte, provide innate immune defense against acute viral infection and cancer. Compared to healthy people, patients with moderate or severe COVID-19 have low peripheral NK cell and lower total lymphocyte numbers20,98–102. Yet compared mild cases, patients with severe COVID-19 have significantly lower total lymphocytes but no significant difference in NK cell numbers20.
The single-cell landscape of immune cells in the BALF of patients with COVID-19 exhibits increased NK cell numbers upon infection12,61,103. Flow cytometry reveals strong NK cell activation in the peripheral blood of patients with COVID-19, and single-cell RNA sequencing of BALF from COVID-19 patients shows two clusters of NK cells linked to disease status. Activated NK cell clusters show high levels of perforin, NKG2C, and Ksp37 expression, which is associated with disease severity, and the CD56bright NK cluster is present in severe hyperinflammation COVID-19 cases12.
For cytotoxic lymphocytes, such as NK cells and CD8+ T cells, NKG2A is an inhibitory receptor to recognize “self” MHC I on target cells104,105. As an inhibitory receptor, it induces NK cell and CD8+ T cell exhaustion in cancer and chronic viral infections106,107. In severe COVID-19 cases, increased NKG2A expression can induce NK and CD8+ T cell exhaustion and trigger cytotoxic lymphocytes to have impaired cytokine production56,84. Thus, the functional exhaustion of cytotoxic lymphocytes appears to be associated with SRAS-CoV-2 infection.
Neutrophils
The COVID-19 immunopathology can be exacerbated by neutrophil recruitment and related activity. Neutrophils are recruited by infected endothelial cells and form web-like chromatin structures known as neutrophil extracellular traps (NETs) as the forefront of innate immunity108. Patients with COVID-19 have elevated specific markers of NETs, including myeloperoxidase-DNA and citrullinated histone H3, proving that SARS-CoV-2 can activate NETosis109,110. In addition, compared with COVID-19 survivors, nonsurvivors have higher white blood cell (WBC) and neutrophil counts but lower lymphocyte and platelet counts111. The peripheral blood of patients with COVID-19 exhibits kinetic changes in WBCs, neutrophils, and monocytes, as well as in different lymphocyte subsets. Compared with mild cases, patients with severe COVID-19 show significant increases in total WBC counts at the time of onset (within 3 days) but not during the following period of disease progression70,71.
Upon viral infection, neutrophils are involved in early antiviral defense112. The initial pyroptotic of the infected endothelial airway can induce IL-1β overexpression and trigger neutrophils migration and T-cell activation76,113. The activated neutrophils further release leukotrienes and reactive oxygen species (ROS), thus triggering cytotoxicity such as ALI and a cytokine storm. Neutrophils may also cause endothelial injury, which can further promote viral systemic dissemination76–78,80. Through degranulation and lysis, neutrophils can be cytotoxic114 and can aggravate lung inflammation upon SARS-CoV-2 infection115,116. In addition, RNA-seq analysis of BALF samples shows that multiple neutrophil-attracting chemokine genes are upregulated in the COVID-19 infected lung microenvironment, and these include TNFR, IL-8, CXCR1, CXCR2, ADAM10, GPR84, MME, ANPEP, and LAP381.
An increased peripheral neutrophil-to-lymphocyte ratio (NLR) can be seen in severe SARS-CoV-2 cases and is likely associated with an unfavorable prognosis81,84,117. This correlates with low expression of type I and III IFNs and high expression of pro-inflammatory factors and chemokines22. Increased serum level of the NLR ratio and lower percentages of monocytes, eosinophils, and basophils have been associated with disease severity and death. Severe cases tend to also have higher NLR and increasing inflammatory cytokines, chemokines, and other markers (including C-reactive protein, ferritin, and D-dimers)58,72,75. Furthermore, four contributing variables, namely WBC count, neutrophil count, neutrophil-to-CD8+ T cell ratio (N8 R), and NLR, are considered potential prognostic factors of the disease70,71. NLR appears to be the most useful prognostic factor affecting the prognosis in severe cases117.
The COVID-19 immunopathology can be exacerbated by neutrophil recruitment and related activity. Excessive upregulation of the neutrophil-attracting chemokines, including CXCL1, CXCL2, IL-8, CXCL10 (IP-10), CCL2 and CCL7, can be found in COVID-19 BALF samples93,94. From an anatomopathological perspective, patients who die from COVID-19 show marked lung infiltration by neutrophils73. In severe cases, microscopic examination of the autopsy lung tissues shows an increased number of neutrophils and histiocytes (acute bronchopneumonia) in the airspaces71,90.
Dendritic Cells
Dendritic cells (DCs) are a diverse group of professional APCs that provide an important bridge linking the innate and adaptive immune responses. DCs played a crucial role in two previous human coronavirus outbreaks: SARS and MERS-CoV80,118. In both diseases, like COVID-19, clinical manifestation includes rapid and progressive acute pneumonia with altered multiorgan functions. However, compared to MERS-CoV, which infects monocyte-derived DCs (moDCs) and rapidly generated high levels of IFN-γ80,118, SARS-CoV-2 does not activate any IFN response from infected moDCs82.
DCs comprise several subsets, including plasmacytoid DCs (pDCs) and conventional DCs (cDCs)119. Upon COVID-19 infection, total peripheral DC populations are reduced and their activity is impaired80,83,120. Decreased CD123+ pDCs and CD141+ DCs and increased CD1c+ cDCs have been reported in the lungs and bronchoscopy infiltrates67,80. In addition, when severe acute infection occurs, the ratios of CD123+ pDCs to CD11c+ cDCs decrease and significant functional impairment occurs80,83. Since pDCs are critical for type I IFN secretion and the initial antiviral response, the reduction in pDCs could contribute to acute COVID-19 pathogenesis and have implications for treatment response83,120.
Moreover, aging causes a reduction in plasmacytoid DC numbers, as well as their pathogen-sensing functions via TLRs120,121. In addition, the DC-specific intracellular adhesion molecule-grabbing nonintegrin (DC-SIGN) is a C-type lectin receptor present on the surface of both macrophages and DCs to recognize viral PAMPs. In older adults, the increased DC-SIGN expression may be involved in the pathogenesis of more severe SARS-CoV-2 infection. The combination of SARS-CoV-2 and age-mediated DC dysfunctions may be central to the increased susceptibility of severe infection and poor outcomes in older patients120.
Adaptive Immunity
Lymphocytes, especially T cells and B cells, carry out the adaptive immune response in the human body. Although they can take days or even weeks to become established, differentiated lymphocytes have an important role in controlling and shaping the immune response by providing various immune-related functions and long-lasting protection. The T cells can either kill an infected cell (cytotoxic CD8+ T cells or CTLs) or balance the immune response (CD4+ helper T cells), while the B cells are related to antibody production, also known as the humoral immunity. Each of cell type is critical for eliminating SARS-CoV-2 infection122. Cytotoxic CD8+ T lymphocytes, CD4+ effector-GNLY (granulysin), and NK cells are also necessary for controlling viral infection, and the functional exhaustion of cytotoxic lymphocytes correlates with disease progression123–125.
Adaptive immunity, including cell-mediated and humoral immunity, is critical in regulating COVID-19 infection20. Patients with COVID-19 exhibit a decrease in total lymphocytes and the mean counts of the three main lymphocyte populations (T, B, and NK cells), and this is more pronounced in severe cases. In particular, T and NK cells decrease markedly below normal levels, while B cells remain within the lower normal range72. Severe cases are not only associated with decreased total lymphocyte numbers, but also with increased naive T helper cells as well as decreased memory T helper cells and memory subsets72. Effector T cells, such as IFN-γ secreting CD4+ T cells, also tend to be lower in severe cases than in moderate cases. In addition, dysregulation of lymphocytes, including T cell exhaustion, occurs in most cases. Among all the adaptive immune cells, CD8+ T, CD4+ T, and B cells (and the CD4+/CD8+ ratio) are significantly associated with inflammatory status and are indicated as independent predictors of poor treatment outcomes20,21. The adaptive immune cell subset changes and cytokine secretions in SARS-CoV-2 infection are described in the following sections and summarized in the Table 2.
Table 2.
Representative Adaptive Immune Cell Subset Changes and Cytokine Secretions in SARS-CoV-2 Infection.
| Immune Cell Subset | Changes in Frequency | Changes in Phenotype | Reference | |
|---|---|---|---|---|
| CD4+/CD8+ T Cell Ratio | Increased ratio indicates poor efficacy after treatment | 20,21,126 | ||
| CD8+ T Cell | Mild | Decreased total number in the peripheral blood. Increased CD8+ T cells infiltrate the lungs with clonal expansion. In mild cases, multi-cytokine production of CD8+ T cells is higher compared to CD4+ T cells |
Upon SARS-CoV-2 infection, the circulating peripheral CD8+ T cells are activated and produce granzyme B and perforin. In convalescent patients, CD8+ T cells recognize the viral spike protein, M protein, and least eight SARS-CoV-2 ORFs |
8,21,61,68,88,102,127–134 |
| Severe | Further decreased total number | Circulating peripheral CD8+ T cells are activated and produce high amounts of granzyme B and perforin, yet may exhibit increased expression of exhaustion markers, including PD-1, Tim-3, and NKG2A | 20,21,61,127–129 | |
| CD4+ T Cell | Mild | Decreased total number. Decreased Treg, memory, and effector memory cells SARS-CoV-2 infection can prime CD4+ T lymphocytes to differentiate into Th17 and TFH. |
Decreased conventional Th1 and IFNγ secretion Increased pathogenic Th1 cells, which can secrete IL-6 and GM-CSF to induce CD14+ CD16+ monocyte activation Decreased CD4+ naïve, CD4+ memory, and effector memory subsets Increased CD4+ effector-GNLY subsets |
21,56,102,122,124,129–131,135–141 |
| Severe | Decreased in the most severe cases and increased percentage of over-activated with reduced IFNγ production Increased percentage in the naive subset but decreased in the memory and effector memory subsets. Numbers increase with recovery |
Increased CD4+ naïve subsets Decreased CD4+ memory, and effector memory subsets CD4+ T lymphocytes differentiate into pathogenic Th1 cells. Increased CD4+ effector-GNLY subsets |
20,72,124,128,135,142 | |
| Others T cell | Decreased numbers of total circulating γδT cells but increased frequency of CD4+ γδT cells | 143–145 | ||
| B cell | Mild | The humoral response is less affected by the virus. Most convalescent COVID-19 individuals have neutralizing antibodies B cells in geminal center are largely absent during the acute phase |
Within 1 week of illness onset, antibodies are detectable in approximately 40% of patients. Fifteen days after infection, the detectable antibody count rapidly increases to 100%, followed by IgM (94.3%) and IgG (79.8%) Specific antibodies against the RBD of the spike protein, SARS-CoV-2 NP, and the main protease Increased active state but decreased memory B cell subsets |
20,72,124,127,131,135,146–155 |
| Severe | Proportion of B cells is significantly higher than in moderate cases | |||
T Cells
Serious lymphopenia occurs when the absolute numbers of total T cells, CD4+ T cells, and CD8+ T cells are below the lower limit of normal. It can happen in patients with COVID-19, with more profound reductions observed in severe cases than in moderate cases21,66,102,131,156. Lymphopenia is correlated with p53 signaling overactivation69 accompanied by atrophy of the lymph nodes and spleen137. CD26 and CD147 inducing activation-induced cell death may also be responsible for lymphopenia88. Moreover, the more profound the lymphopenia, the worse the prognosis50,51.
Although with a lower T cell total count, the pervasiveness of highly cytotoxic effector T cell subsets (e.g., CD4+ effector-GNLY, CD8+ effector-GNLY, and NKT CD160) is related to convalescence in cases of moderate COVID-19124. Present in COVID-19 convalescent individuals are virus-specific CD4 and CD8 T cells that can recognize multiple regions of the SARS-CoV-2 N protein and S protein epitopes as well as antibodies against the viral receptor-binding domain (RBD)149,157. In addition, patients with long-lasting memory T cells toward SARS are most likely to generate SARS-CoV-2-specific T cell immunity and recover from SAR-CoV-2 infection. Pre-existing T cells preferentially boost N-specific T cells, whereas individuals with no history of SARS tend to have NSP7-, NSP13-, and ORF1-specific T cells157.
Regarding the CD4+/CD8+ T cell ratio, some studies have reported no significant difference in the CD4+/CD8+ T cell ratio, indicating that both CD4+ and CD8+ T cells are depleted during SARS-CoV-2 infection20,131, while multivariate analysis results have suggested that the CD4/CD8 ratio is significantly higher in severe cases than in moderate cases, with a higher ratio indicating a worse inflammatory status and poorer efficacy posttreatment20,126.
Finally, the professional APCs process viral antigens to activate virus-specific T cells and cytotoxic T lymphocytes (CTLs), by major histocompatibility complexes (MHC; or human leukocyte antigen, HLA, in humans)8,19. Disease in patients infected with SARS-CoV with HLA-B*46:01 (MHC I molecules) genotypes is more severe compared to those with different genotypes, although this has not been clinically validated for SARS-CoV-28,9,158–161.
CD8+ T Cell Response in COVID-19
Analysis of lymphocyte subsets associates COVID-19 patients with lymphopenia. Although with lymphopenia, infiltrated lymphocytes can still be observed in the lungs infected with SARS-CoV-2, and scRNA-seq analysis of BALF from COVID-19 patients exhibits an increase in CD8 T cell infiltrate with proliferation and clonal expansion61. SARS-CoV-2-specific CD8+ T cell are present in approximately 70% of convalescent patients, and these cells can recognize the viral spike protein, M protein, and at least eight SARS-CoV-2 ORFs130.
There appears to be heterogeneity in the CD8+ T cell immune response between patients. Some studies have reported that upon SARS-CoV-2 infection, the circulating peripheral CD8+ T cells are activated and produce high amounts of granzyme B and perforin to increase their cytotoxic response. In addition, these CD8+ cells appear to be clonally expanded in the lungs of mild COVID-19 diseases133. In mild cases, patients have more multi-cytokine production CD8+ T cells than CD4+ T cell132. Other studies have reported that CD8+ T cells in severe COVID-19 patients become exhausted due to the increased expression of exhaustion markers, including PD-1, Tim-3, TIGIT, HLA-DR, CD38, CD25, and NKG2A128,129.
CD4+ T Cell Response in COVID-19
Upon infection, activated CD4+ T cells, also known as T helper (Th) cells, produce pro-inflammatory cytokines (IFN-γ), and antiviral cytokines, such as granzyme B and TNFκ129,162. Compared with healthy people, patients with COVID-19 exhibit a decrease in total CD4+ T cell numbers and IFN-γ production, with even lower IFN-γ levels occurring in severe cases than in moderate cases21. In mild cases, patients have lower proportions of the naive, memory and effector memory CD4+ T cell subsets. In severe cases on the other hand, patients have increased naïve helper CD4+ T cell, yet memory T cell subsets (CD3+CD4+CD45RO+) are decreasing72,124,130. In contrast to memory or naïve-state cells, the proportion of CD4+ effector-GNLY (granulysin) is elevated in patients with COVID-19 and convalescence patients124.
There are five types of CD4+: T helper (Th)1, Th2, and Th17; regulatory T cells (Treg), and follicular helper T cells (TFH)138. SARS-CoV-2 infection can prime CD4+ T lymphocytes to differentiate into pathogenic Th1 cells (nonconventional Th1), Th17, and TFH 21,135,139,141,163. Normally, upon infection, activated Th1 effector T cells produce pro-inflammatory cytokines (IFN-γ) and antiviral cytokines, such as granzyme B, TNF-α, TNF-β, and TNF-κ129,162 to stimulate innate and T cell immune responses. In COVID-19 patients, conventional Th1 effector cells show a decrease in the total amount140,141 and lower IFN-γ production21. SARS-CoV-2 infection can also prime CD4+ T lymphocytes to differentiate into pathogenic Th1 cells and generate IL-6 and GM-CSF to promote activation of CD14+ CD16+ monocytes139. Furthermore, increased T cells (TFH) can help B cells to produce antibodies and drive CD8+ T cells’ cytotoxic response activation to kill virus-infected cells138. In addition, the count of Treg cells, which downregulate the induction and proliferation of effector T cells, is significantly lower in COVID-19 patients21,137, and this manifests as a lack of functional immunosuppression. Notably, a high NLR, reflecting a worsening of the inflammatory process, has been closely related with poor prognosis, disease severity, and death164.
Other T Cells
Patients with COVID-19 have lower total circulating γδT cell counts and higher frequency of CD4+ γδT cells. This is notable because the CD4+ subset of γδT cells produce Th2 cytokines, which can then induce TMPRSS2 expression (the SARS-CoV-2 spike protein priming protease). Th2 immune responses, while able to increase TMPRSS2 expression, also decrease ACE2 expression143–145
B Cell and Humoral Response
B cells are critical adaptive immune cells for antibody production and the humoral immune response122. Upon COVID-19 infection, T lymphocyte responses are impaired by the virus, while B cell activities are not as affected as T cells135. In severe cases, the proportion of B cells is significantly higher than in moderate cases (20% vs. 10.8%)72,131,135. In addition, patients with COVID-19 exhibit higher proportions of activating B subsets and decreased memory B cells compared with healthy individuals124. Moreover, most convalescent patients with COVID-19 have detectable cellular immune responses and neutralizing antibodies135,149.
Most of them also display antibody responses between 6 and 21 days after infection150–152. Within 1 week of illness onset, antibodies are detectable in approximately 40% patients. Fifteen days after infection, the detectable antibody number rapidly increases to 100%, followed by IgM (94.3%), and IgG (79.8%). A strong positive correlation can also be observed between the Ab titer and clinical severity 15 days post infection148. In addition, SARS-CoV-2-specific antibodies against the RBD of the spike protein149, SARS-CoV-2 NP, and the main protease135.
The longevity of the antibody response remains unknown, yet most patients show high levels of SARS-CoV-2-specific memory B cells at 6-8 months post infection, suggesting that patients start to develop long-term protective immunity154. In the case of SARS or MERS-CoV infection, specific antibodies begin to wane at 12 to 52 weeks following infection and homologous re-infection can occur153. Serology testing is relatively ineffective for early detection because the detectability of virus-specific antibodies in the first 7 days from onset is below 40%148.
Moreover, the germinal center (GC) is a specialized area within the lymph nodes, where B cells can receive help from follicular T helper (TFH) cells to activate and become memory B cells or long-lived plasma cells. SARS infection can defeat GC responses, thus affecting B cell activity. As for COVID-19, GCs are also largely absent during the acute phase, and this absence is accompanied with a lack of BCL6-expressing B cells or TFH cells155.
Conclusions
This review investigated the various changes to immune mechanisms, the immune microenvironment, and COVID-19 immunopathogenesis. The COVID-19 pandemic is a current issue affecting people worldwide, and there is an urgent need to develop fundamental therapeutic interventions. Controlling the inflammatory response may be as important as targeting the virus. Thus, a better understanding of the immune processes and mechanisms is crucial for potential COVID-19 therapeutic drug development. Future research should consider focusing on identifying inflammatory biomarkers as potential immunotherapeutic targets and investigating the association between immune dysfunction and disease severity in SARS-Co-V-2 patients. The findings would be invaluable for vaccine development and evaluation.
Footnotes
Authors Note: Chih-Hung Ye and Wen-Lin Hsu contributed equally to this article.
Author Contributions: Conceptualization, S.-H. Yu, W.-L. Hsu, C.-H. Ye.; writing—original draft preparation, S.-H. Yu, W.-L. Hsu, C.-H. Ye.; writing—review and editing, S.-H. Yu, W.-L. Hsu, C.-H. Ye. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Young Scholar Fellowship Program by Ministry of Science and Technology (MOST) in Taiwan, under Grant MOST 109-2636-B-002-012.
ORCID iD: Shu-Han Yu  https://orcid.org/0000-0002-0351-6999
https://orcid.org/0000-0002-0351-6999
References
- 1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10(7):514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robba C, Battaglini D, Pelosi P, Rocco PRM. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 2020;14(9):865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasarmidi E, Tsitoura E, Spandidos DA, Tzanakis N, Antoniou KM. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review). Exp Ther Med. 2020;20(3):2557–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370:m3001. [DOI] [PubMed] [Google Scholar]
- 8. Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chowdhury MA, Hossain N, Kashem MA, Shahid MA, Alam A. Immune response in COVID-19: A review. J Infect Public Health. 2020;13(11):1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larenas-Linnemann D, Rodríguez-Pérez N, Arias-Cruz A, Blandón-Vijil MV, Del Río-Navarro BE, Estrada-Cardona A, Gereda JE, Luna-Pech JA, Navarrete-Rodríguez EM, Onuma-Takane E, Pozo-Beltrán CF, et al. Enhancing innate immunity against virus in times of COVID-19: trying to untangle facts from fictions. World Allergy Organ J. 2020;13(11):100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, Hertwig L, Strunz B, Lentini A, Reinius B, Brownlie D, Cuapio A, et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol. 2020;5(50):eabd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golonka RM, Saha P, Yeoh BS, Chattopadhyay S, Gewirtz AT, Joe B, Vijay-Kumar M. Harnessing innate immunity to eliminate SARS-CoV-2 and ameliorate COVID-19 disease. Physiol Genomics. 2020;52(5):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open. 2020;4(2):e138–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, He J, Zhong X, Gan H, Xia Y. CX3CL1/CX3CR1 axis contributes to angiotensin ii-induced vascular smooth muscle cell proliferation and inflammatory cytokine production. Inflammation. 2018;41(3):824–834. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Yang J, Yu X, Cheng S, Gan H, Xia Y. Angiotensin II-induced early and late inflammatory responses through NOXs and MAPK pathways. Inflammation. 2017;40(1):154–165. [DOI] [PubMed] [Google Scholar]
- 18. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, Song S, Ma Z, Mo P, Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carrel L, Brown CJ. When the Lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Philos Trans R Soc Lond B Biol Sci. 2017;372(1733):20160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, Pienkowski C, Chaumeil J, Mejia JE, Guery JC. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19):eaap8855. [DOI] [PubMed] [Google Scholar]
- 29. Fink AL, Engle K, Ursin RL, Tang WY, Klein SL. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A. 2018;115(49):12477–12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, Zachary JF, Huber SA, Blankenhorn EP, Teuscher C. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23(9):1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krementsov DN, Case LK, Dienz O, Raza A, Fang Q, Ather JL, Poynter ME, Boyson JE, Bunn JY, Teuscher C. Genetic variation in chromosome Y regulates susceptibility to influenza A virus infection. Proc Natl Acad Sci U S A. 2017;114(13):3491–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ. 2011;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. [DOI] [PubMed] [Google Scholar]
- 34. Marquez EJ, Chung CH, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, Mellert DJ, Kuchel GA, Banchereau J, Ucar D. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11(1):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Golden LC, Itoh Y, Itoh N, Iyengar S, Coit P, Salama Y, Arnold AP, Sawalha AH, Voskuhl RR. Parent-of-origin differences in DNA methylation of X chromosome genes in T lymphocytes. Proc Natl Acad Sci U S A. 2019;116(52):26779–26787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 37. Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am J Physiol Lung Cell Mol Physiol. 2016;310(5):L415–L425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10(5):1097–1107. [DOI] [PubMed] [Google Scholar]
- 39. Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016;12(9):e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hall OJ, Nachbagauer R, Vermillion MS, Fink AL, Phuong V, Krammer F, Klein SL. Progesterone-based contraceptives reduce adaptive immune responses and protection against sequential influenza a virus infections. J Virol. 2017;91(8):e02160–e02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vom Steeg LG, Klein SL. Sex steroids mediate bidirectional interactions between hosts and microbes. Horm Behav. 2017;88:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vom Steeg LG, Klein SL. Sex and sex steroids impact influenza pathogenesis across the life course. Semin Immunopathol. 2019;41(2):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gadi N, Wu SC, Spihlman AP, Moulton VR. What’s sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front Immunol. 2020;11:2147–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. 2018;9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun X, Wang T, Cai D, Hu Z, Chen J, Liao H, Zhi L, Wei H, Zhang Z, Qiu Y, Wang J, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharmacol Sci. 2018;22(15):4956–4961. [DOI] [PubMed] [Google Scholar]
- 50. Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. [DOI] [PubMed] [Google Scholar]
- 56. O’Connell P, Aldhamen YA. Systemic innate and adaptive immune responses to SARS-CoV-2 as it relates to other coronaviruses. Hum Vaccin Immunother. 2020;16(12):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu J, Wu P, Gao F, Qi J, Kawana-Tachikawa A, Xie J, Vavricka CJ, Iwamoto A, Li T, Gao GF. Novel immunodominant peptide presentation strategy: a featured HLA-A*2402-restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J Virol. 2010;84(22):11849–11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Sousa E, Ligeiro D, Lérias JR, Zhang C, Agrati C, Osman M, El-Kafrawy SA, Azhar EI, Ippolito G, Wang FS, Zumla A, et al. Mortality in COVID-19 disease patients: Correlating the association of major histocompatibility complex (MHC) with severe acute respiratory syndrome 2 (SARS-CoV-2) variants. Int J Infect Dis. 2020;98:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. [DOI] [PubMed] [Google Scholar]
- 62. Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, Liu X, Xie L, Li J, Ye J, Dong L, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bonnardel J, Guilliams M. Developmental control of macrophage function. Curr Opin Immunol. 2018;50:64–74. [DOI] [PubMed] [Google Scholar]
- 64. Booz GW, Altara R, Eid AH, Wehbe Z, Fares S, Zaraket H, Habeichi NJ, Zouein FA. Macrophage responses associated with COVID-19: A pharmacological perspective. Eur J Pharmacol. 2020;887:173547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, Portman JR, Matchett KP, Brice M, Marwick JA, Taylor RS, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, Qiang M, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020;189(3):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sanchez-Cerrillo I, Landete P, Aldave B, Sanchez-Alonso S, Sanchez-Azofra A, Marcos-Jimenez A, Avalos E, Alcaraz-Serna A, de Los Santos I, Mateu-Albero T, Esparcia L, et al. Differential redistribution of activated monocyte and dendritic cell subsets to the lung associates with severity of COVID-19. Preprint. medRxiv. 2020.05.13.20100925.
- 68. Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, Qian H, Dai T, Zhang T, Lai Y, Wang J, et al. COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2020;109(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, Wu H, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paces J, Strizova Z, Smrz D, Cerny J. COVID-19 and the immune system. Physiol Res. 2020;69(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Birra D, Benucci M, Landolfi L, Merchionda A, Loi G, Amato P, Licata G, Quartuccio L, Triggiani M, Moscato P. COVID 19: a clue from innate immunity. Immunol Res. 2020;68(3):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, Whitmore A, Heise MT, Baric RS. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5):e01753–e01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, Feng J, Jia Q, Song Q, Zhu B, Wang J. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and igg level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Dassler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217(6):e20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Soy M, Keser G, Atagunduz P, Tabak F, Atagunduz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jakovac H. COVID-19: is the ACE2 just a foe? Am J Physiol Lung Cell Mol Physiol. 2020;318(5): L1025–L1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Campana P, Parisi V, Leosco D, Bencivenga D, Della Ragione F, Borriello A. Dendritic cells and SARS-CoV-2 infection: still an unclarified connection. Cells. 2020;9(9):2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Didangelos A. COVID-19 hyperinflammation: what about neutrophils? mSphere. 2020;5(3):e00367–e00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang D, Chu H, Hou Y, Chai Y, Shuai H, Lee AC, Zhang X, Wang Y, Hu B, Huang X, Yuen TT, et al. Attenuated interferon and proinflammatory response in sars-cov-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J Infect Dis. 2020;222(5):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhou R, To KK, Wong YC, Liu L, Zhou B, Li X, Huang H, Mo Y, Luk TY, Lau TT, Yeung P, et al. Acute SARS-CoV-2 infection impairs dendritic cell and t cell responses. Immunity. 2020;53(4):864–877.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F. Role of monocytes/macrophages in Covid-19 pathogenesis: implications for therapy. Infect Drug Resist. 2020;13:2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lang PA, Recher M, Honke N, Scheu S, Borkens S, Gailus N, Krings C, Meryk A, Kulawik A, Cervantes-Barragan L, Van Rooijen N, et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology. 2010;52(1):25–32. [DOI] [PubMed] [Google Scholar]
- 87. Martinez FO, Combes TW, Orsenigo F, Gordon S. Monocyte activation in systemic Covid-19 infection: assay and rationale. EBioMedicine. 2020;59:102964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Garcia LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, Shuai H, Yang D, Hu B, Huang X, Zhang X, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71(6):1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front Immunol. 2020;11:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Park MD. Macrophages: a trojan horse in COVID-19? Nat Rev Immunol. 2020;20(6):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, Walzer T, Francois B, Seve P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pelaia C, Tinello C, Vatrella A, De Sarro G, Pelaia G. Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther Adv Respir Dis. 2020;14:1753466620933508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cabrera-Benitez NE, Laffey JG, Parotto M, Spieth PM, Villar J, Zhang H, Slutsky AS. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121(1):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, Mencarini J, Caporale R, Peruzzi B, Antonelli A, Trotta M, et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130(9):4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jiang Y, Wei X, Guan J, Qin S, Wang Z, Lu H, Qian J, Wu L, Chen Y, Chen Y, Lin X. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020;218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Demaria O, Carvelli J, Batista L, Thibult ML, Morel A, Andre P, Morel Y, Vely F, Vivier E. Identification of druggable inhibitory immune checkpoints on natural killer cells in COVID-19. Cell Mol Immunol. 2020;17(9):995–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thurmann L, Kurth F, Volker MT, Kazmierski J, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–979. [DOI] [PubMed] [Google Scholar]
- 104. Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagné F, et al. Human NK cell education by inhibitory receptors for MHC Class I. Immunity. 2006;25(2):331–342. [DOI] [PubMed] [Google Scholar]
- 105. Rapaport AS, Schriewer J, Gilfillan S, Hembrador E, Crump R, Plougastel BF, Wang Y, Le Friec G, Gao J, Cella M, Pircher H, et al. The inhibitory receptor NKG2A sustains virus-specific CD8+ T Cells in response to a lethal poxvirus infection. Immunity. 2015;43(6):1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, Sun R, Tian Z. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144(2):392–401. [DOI] [PubMed] [Google Scholar]
- 107. André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, Rossi B, et al. Anti-NKG2A mAb Is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731–1743.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. [DOI] [PubMed] [Google Scholar]
- 109. Arcanjo A, Logullo J, Menezes CC, Giangiarulo TC, Dos Reis MC, de Castro GM, da Silva Fontes Y, Todeschini AR, Freire-de-Lima L, Decoté-Ricardo D, Ferreira-Pereira A, et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci Rep. 2020;10(1):19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhao Y, Nie HX, Hu K, Wu XJ, Zhang YT, Wang MM, Wang T, Zheng ZS, Li XC, Zeng SL. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect Dis Poverty. 2020;9(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Camp JV, Jonsson CB. A role for neutrophils in viral respiratory disease. Front Immunol. 2017;8:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Parisi V, Leosco D. Precision medicine in COVID-19: IL-1beta a potential target. JACC Basic Transl Sci. 2020;5(5):543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Haick AK, Rzepka JP, Brandon E, Balemba OB, Miura TA. Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology. J Gen Virol. 2014;95(Pt 3):578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154(1):197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhu B, Zhang R, Li C, Jiang L, Xiang M, Ye Z, Kita H, Melnick AM, Dent AL, Sun J. BCL6 modulates tissue neutrophil survival and exacerbates pulmonary inflammation following influenza virus infection. Proc Natl Acad Sci U S A. 2019;116(24):11888–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P, Lu J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23(8):101400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7(7):543–555. [DOI] [PubMed] [Google Scholar]
- 120. Borges RC, Hohmann MS, Borghi SM. Dendritic cells in COVID-19 immunopathogenesis: insights for a possible role in determining disease outcome. Int Rev Immunol. 2021;40(1–2):108–125. [DOI] [PubMed] [Google Scholar]
- 121. Garbe K, Bratke K, Wagner S, Virchow JC, Lommatzsch M. Plasmacytoid dendritic cells and their Toll-like receptor 9 expression selectively decrease with age. Hum Immunol. 2012;73(5):493–497. [DOI] [PubMed] [Google Scholar]
- 122. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walters P. Molecular Biology of the Cell, 4th ed. New York: Garland Science; 2002. [Google Scholar]
- 123. Zhang C, Wang XM, Li SR, Twelkmeyer T, Wang WH, Zhang SY, Wang SF, Chen JZ, Jin X, Wu YZ, Chen XW, et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat Commun. 2019;10(1):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang JY, Wang XM, Xing X, Xu Z, Zhang C, Song JW, Fan X, Xia P, Fu JL, Wang SY, Xu RN, et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol. 2020;21(9):1107–1118. [DOI] [PubMed] [Google Scholar]
- 125. Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pallotto C, Suardi LR, Esperti S, Tarquini R, Grifoni E, Meini S, Valoriani A, Di Martino S, Cei F, Sisti E, Piani F, et al. Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID-19): a retrospective multicentre study. Infect Dis (Lond). 2020;52(9):675–677. [DOI] [PubMed] [Google Scholar]
- 127. Yang X, Dai T, Zhou X, Qian H, Guo R, Lei L, Zhang X, Zhang D, Shi L, Cheng Y, Hu J, et al. Analysis of adaptive immune cell populations and phenotypes in the patients infected by SARS-CoV-2. medRxiv. 2020.03.23.20040675. [Google Scholar]
- 128. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17(5):541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, et al. Targets of t cell responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ganji A, Farahani I, Khansarinejad B, Ghazavi A, Mosayebi G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol Dis. 2020;83:102437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, Dejnirattisai W, Rostron T, Supasa P, Liu C, Lopez-Camacho C, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yang X, Dai T, Zhou X, Qian H, Guo R, Lei L, Zhang X, Zhang D, Shi L, Cheng Y, Hu J, et al. Naturally activated adaptive immunity in COVID-19 patients. J Cell Mol Med. 2020;24(21):12457–12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, Wei P, Ge J, Gou M, Li X, Sun L, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52(6):971–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of cytokines including interleukin-6 in covid-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1(1):20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Nat Sci Rev. 2020;7(6):998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schultheiss C, Paschold L, Simnica D, Mohme M, Willscher E, von Wenserski L, Scholz R, Wieters I, Dahlke C, Tolosa E, Sedding DG, et al. Next-generation sequencing of t and b cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity. 2020;53(2):442–455.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sallusto F. Heterogeneity of human CD4(+) T cells against microbes. Annu Rev Immunol. 2016;34:317–334. [DOI] [PubMed] [Google Scholar]
- 142. Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, Jia X, Wu M, Shi B, Xu S, Chen J, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. [DOI] [PubMed] [Google Scholar]
- 143. Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ gammadelta T cells in the absence of ITK results in elevated Ige production. Blood. 2009;114(3):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lei L, Qian H, Yang X, Zhang X, Zhang D, Dai T, Guo R, Shi L, Cheng Y, Zhang B, Zhou X, et al. The phenotypic changes of gammadelta T cells in COVID-19 patients. J Cell Mol Med. 2020;24(19):11603–11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL, Rios CL, Pruesse E, Nolin JD, Plender EG, Wechsler ME, et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11(1):5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mahal BA, Alshalalfa M, Kensler KH, Chowdhury-Paulino I, Kantoff P, Mucci LA, Schaeffer EM, Spratt D, Yamoah K, Nguyen PL, Rebbeck TR. Racial differences in genomic profiling of prostate cancer. N Engl J Med. 2020;383(11):1083–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, He L, Chen Y, Wu J, Shi Z, Zhou Y, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus Entry. J Virol. 2020;94(5):e02015–e02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang F, Gan R, Zhen Z, Hu X, Li X, Zhou F, Liu Y, Chen C, Xie S, Zhang B, Wu X, et al. Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals. Signal Transduct Target Ther. 2020;5(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. [DOI] [PubMed] [Google Scholar]
- 151. Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF, Wang DQ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. [DOI] [PubMed] [Google Scholar]
- 153. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101(8):791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, Piechocka-Trocha A, Lefteri K, Osborn M, Bals J, Bartsch YC, et al. Loss of Bcl-6-expressing t follicular helper cells and germinal centers in COVID-19. Cell. 2020;183(1):143–157 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Liu Z, Long W, Tu M, Chen S, Huang Y, Wang S, Zhou W, Chen D, Zhou L, Wang M, Wu M, et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect. 2020;81(2):318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, Chng MHY, Lin M, Tan N, Linster M, Chia WN, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. [DOI] [PubMed] [Google Scholar]
- 158. Ceraolo C, Giorgi FM. Genomic variance of the 2019-nCoV coronavirus. J Med Virol. 2020;92(5):522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A, Thompson RF. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94(13):e00510–e00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lin M, Tseng HK, Trejaut JA, Lee HL, Loo JH, Chu CC, Chen PJ, Su YW, Lim KH, Tsai ZU, Lin RY, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Li J, Guo M, Tian X, Wang X, Yang X, Wu P, Liu C, Xiao Z, Qu Y, Yin Y, Wang C, et al. Virus-host interactome and proteomic survey reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. Med (N Y). 2021;2(1):99–112.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Becattini S, Latorre D, Mele F, Foglierini M, De Gregorio C, Cassotta A, Fernandez B, Kelderman S, Schumacher TN, Corti D, Lanzavecchia A, et al. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science. 2015;347(6220):400–406. [DOI] [PubMed] [Google Scholar]
- 164. Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]