Abstract
Data from observational studies have suggested an involvement of abnormal glycaemic regulation in the pathophysiology of psychiatric illness. This may be an attractive target for clinical intervention as glycaemia can be modulated by both lifestyle factors and pharmacological agents. However, observational studies are inherently confounded, and therefore, causal relationships cannot be reliably established. We employed genetic variants rigorously associated with three glycaemic traits (fasting glucose, fasting insulin, and glycated haemoglobin) as instrumental variables in a two-sample Mendelian randomisation analysis to investigate the causal effect of these measures on the risk for eight psychiatric disorders. A significant protective effect of a natural log transformed pmol/L increase in fasting insulin levels was observed for anorexia nervosa after the application of multiple testing correction (OR = 0.48 [95% CI: 0.33-0.71]—inverse-variance weighted estimate). There was no consistently strong evidence for a causal effect of glycaemic factors on the other seven psychiatric disorders considered. The relationship between fasting insulin and anorexia nervosa was supported by a suite of sensitivity analyses, with no statistical evidence of instrument heterogeneity or horizontal pleiotropy. Further investigation is required to explore the relationship between insulin levels and anorexia.
Subject terms: Genetics, Risk factors, Psychiatric disorders
Introduction
Psychiatric disorders are complex phenotypes aetiologically influenced by a range of environmental [1, 2] and genetic factors [3–7]. Currently, psychiatric disorders are treated with a combination of medication [8, 9] and psychotherapy approaches [10]. Often these interventions address the symptoms of the disease without targeting the underlying mechanisms of action, and thus, managing psychiatric disorders remains difficult for many patients [11, 12]. To address this, we need to better understand the risk factors and underlying pathophysiology of these conditions such that novel intervention strategies can be implemented.
There has been increasing interest in the relationship between glycaemic regulation and psychiatric illness. Dysglycaemia has well characterised systemic effects, however, its importance in the brain is often underappreciated. Insulin has been implicated in many neurological processes including synaptic plasticity and cognition [13–15], whilst neurons are dependent on glucose as their major energy source [16]. A disproportionately high burden of comorbid type 2 diabetes has been observed in several psychiatric disorders, including: schizophrenia (SZ) [17], bipolar disorder (BP) [17, 18], major depressive disorder (MDD) [17], autism spectrum disorder (ASD) [19], and Tourette’s syndrome (TS) [20]. In addition, glycaemic abnormalities have been observed through direct serum measurement, including an association of elevated glycated haemoglobin with attention deficit hyperactive disorder (ADHD) [21], insulin resistance with psychotic experiences [22], and elevated insulin sensitivity in anorexia [23]. Lifestyle factors and metabolic consequences of antipsychotic medication, such as weight gain [24, 25], likely contribute to these associations. However, data from treatment naïve, first episode psychosis patients provides evidence of glycaemic dysregulation in these disorders beyond what is directly attributable to medication effects and lifestyle [22, 26, 27]. This relationship is further supported by genetic studies. For instance, linkage disequilibrium score regression (LDSC) has demonstrated a negative genetic correlation between anorexia and both fasting inulin and glucose as indexed by common genomic variation [28]. Polygenic risk score for schizophrenia has also been associated with insulin resistance [29], whilst there is evidence of shared genome-wide association study (GWAS) association signals for schizophrenia and type 2 diabetes which display statistical colocalisation [30]. Given the importance of glycaemic regulation in the brain, and the direct significance of insulin signalling, these data suggest that dysglycaemia may be involved in the pathogenesis of psychiatric disorders. This could have implications for clinical monitoring and precision medicine as this system can be modulated through direct pharmacological intervention and lifestyle alterations.
The literature supporting the relationship between glycaemic traits and psychiatric disorders is largely composed of observational studies, preventing direct causal inferences. Randomised controlled trials (RCT) are viewed as an effective method to overcome this, however they are expensive and difficult to conduct with large sample sizes. An alternative method for inferring causal relationships between traits is Mendelian randomisation (MR), which is an analytical method to determine the causal effect of an exposure on an outcome by comparing the association of genetic instrumental variables (IV) with the outcome, relative to the IV effect on the exposure [31]. Genetic variants which are rigorously associated with the exposure — discovered through GWAS — are selected as IVs, which in turn serve as proxies for the exposure. Two-sample MR is particularly advantageous as only GWAS summary statistics are required for the exposure and outcome traits of interest. Mendel’s principle of independent assortment and random segregation ensure that these IVs will be randomized, and thus, their random distribution in the population emulates the random distribution of an exposure for individuals in an RCT [32, 33]. In the present study, we have applied this approach to probe the causal effects of glycaemic traits on the risk for psychiatric disorders and observed a significant protective effect of elevated fasting insulin levels on the risk of anorexia nervosa (AN).
Methods
Selection of genetic instrumental variables
IVs for MR are genetic variants associated with a particular effect size for a trait. There are three main assumptions which underlie the use of these IVs: [34–36]
IV1: the variant is rigorously associated with the exposure;
IV2: the variant is independent of all confounders of the exposure-outcome relationship (“exclusion-restriction assumption”); and
IV3: the variant is associated with the outcome only by acting through the exposure (independent conditional on the exposure and confounders).
IV1 is the only assumption which can be directly quantified [37]; thus, we implement models (described below) to evaluate evidence for violations of these core assumptions. Specifically, pleiotropy, wherein a variant is associated with multiple phenotypes, may invalidate an IV if said pleiotropy constitutes an alternate causal pathway between the variant and the outcome (horizontal pleiotropy) [32].
We chose three core glycaemic traits to use as exposures in this study for which well-powered GWAS data were available: fasting insulin, fasting glucose, and glycated haemoglobin (HbA1c). IVs were genome-wide significant SNPs (P < 5 × 10−8, such that IV1 is satisfied) from the largest GWAS available for each trait [38, 39]. Fasting insulin and fasting glucose data were obtained from the same meta-analysis of non-diabetic individuals of European ancestry (fasting insulin: N = 108,557, unit of effect = ln pmol/L; fasting glucose: N = 133,310, unit of effect = mmol/L). Fasting insulin GWAS data were originally obtained from serum samples. The fasting insulin GWAS provided summary statistics with and without covariation for body mass index (BMI). Both SNP effect sizes were considered as IVs due to the complex relationship between insulin and weight gain [40–42]. Fasting glucose data were obtained from either plasma or from whole blood and corrected to plasma levels [38]. IVs for HbA1c were obtained from the European subset of a GWAS meta-analysis (N = 123,665, unit of effect = % HbA1c). Genome-wide significant SNPs were further categorised in this study as those acting through glycaemic pathways and those acting through erythrocytic pathways via annotation with GWAS catalogue associations as described in Wheeler et al. [39]. We chose to utilise the full set of significant SNPs as IVs, as well as the subset of the lead SNPs specifically annotated as glycaemic, to reduce potential horizontal pleiotropy (gHbAlc). The F statistic was calculated using equation one where R2 is the variance in the outcome explained by each SNP (estimated using the squared sum of the get_r_from_pn() function), k is the number of viable IVs and N is the sample size (Table 1), demonstrating all IVs were sufficiently strong (F > 10).
| 1 |
Table 1.
Instrumental variables selected for each glycaemic exposure.
| Exposure | Number of IVs | Variance explained (%) | F statistic | Sample size | Units |
|---|---|---|---|---|---|
| Fasting insulin | 14 | 0.64 | 40.66 | 108,557 | ln pmol/L |
| Fasting glucose | 32 | 3.31 | 130.27 | 133,310 | mmol/L |
| Fasting glycated haemoglobin (all) | 38 | 2.42 | 80.65 | 123,665 | % glycated haemoglobin |
| Fasting glycated haemoglobin (glycaemic) | 15 | 0.85 | 70.39 | 123,665 | % glycated haemoglobin |
The number of IVs, variance explained, F statistic, sample size and units are described for the glycaemic exposures. The F statistic was calculated from the number of IVs, variance explained and sample size as described previously [90]. The variance explained was only from the IVs utilised in this study [43].
Hereafter, we refer to four exposures, as opposed to three, as there are two IV sets used for HbA1c, all SNPs and SNPs annotated as glycaemic (gHbA1c). IVs for all four exposures (fasting insulin, fasting glucose, HbA1c, and gHbA1c) were clumped to remove variants in linkage disequilibrium (r2 < 0.001) upon importation into the TwoSampleMR (version 0.4.25) R package [43].
Outcome data
We selected eight psychiatric disorders for which GWAS data were available as the outcome traits in this study: AN, ADHD, ASD, BP, MDD, obsessive compulsive disorder (OCD), SZ, and TS. Outcome data were restricted to GWAS summary statistics from subjects of European ancestry in accordance with the exposure data, with the respective sample sizes as follows – AN: N = 72,515 [28], ADHD: N = 53,293 [European subset] [44], ASD: N = 46,351 [45], BP: N = 51,710 [46], MDD: N = 1,730,005 [23andMe cohorts were not included in public release of the summary statistics from the psychiatric genomics consortium] [47], OCD: N = 9725 [48], SZ: N = 105,318 [49], and TS: N = 14,307 [50]. The collection of individual demographic, clinical and genetic data was supervised by the respective institutional ethics review boards for each study after obtaining informed consent of participants. No further ethics approval was required for our analyses as we only accessed summary level meta-data. The number of genome-wide significant SNPs and SNP-based heritability for the outcome GWAS are detailed in Table 2. There was no sample overlap between the glycaemic exposures and the psychiatric outcomes to the best of our knowledge based on the contributing cohorts for each GWAS. However, this cannot be proven definitely, as we do not have access to the raw genotype/phenotype data of each of the studies. We also performed MR in the opposite direction using genome-wide significant variants for each of the above psychiatric traits as IVs. In this instance, we utilised a different GWAS of fasting insulin and FG as our outcome. The fasting insulin summary statistics produced by Scott et al. used as MR IVs followed up ~66,000 SNPs from previous GWAS. Whilst this is the largest sample size GWAS for this trait, the limited number of SNPs made it unsuitable to identify IV-outcome effects. Therefore, we utilised the smaller sample size GWAS summary statistics from Manning et al. (N = 33,823) with more SNPs available as a greater number of psychiatric IVs were encompassed by these summary statistics.
Table 2.
Characteristics of the psychiatric genome-wide association studies utilised as outcomes.
| Outcome | Cases/Controls | SNP heritability | GWAS hits |
|---|---|---|---|
| Attention deficit/ hyperactivity disorder | 19,099/34,194 | 0.22 | 12 |
| Anorexia nervosa | 16,992/55,525 | 0.11 | 8 |
| Autism spectrum disorder | 18,381/27,969 | 0.12 | 5 |
| Bipolar disorder | 20,352/31,358 | 0.17 | 19 |
| Major depressive disorder | 59,851/113,154 | 0.08 (0.10)a | 44 (5)b |
| Obsessive compulsive disorder | 2688/7037 | 0.28 | 0 |
| Schizophrenia | 40,675/64,643 | 0.23 | 145 |
| Tourette’s syndrome | 4819/9488 | 0.21 | 1 |
SNP heritability reported on the liability scale assuming the following population prevalence: attention deficit hyperactive disorder = 5%, anorexia = 0.9%, autism = 1.2%, bipolar = 0.5%, major depressive disorder = 15%, schizophrenia = 0.7%, obsessive compulsive disorder = 2.5%, Tourette’s syndrome = 0.8%. GWAS hits denotes the number of independent, genome-wide significant variants reported by the original study.
aThe reported heritability estimate by the MDD publication is given, with the liability scale SNP-heritability for the publicly available subset in parentheses.
bThe MDD GWAS study reports 44 genome-wide significant SNPs, however, only a subset of this cohort has publicly available summary stats, and this subset has 5 genome-wide significant SNPs.
Two sample Mendelian randomisation approach
First, we investigated the effect of fasting insulin, fasting glucose, HbA1c, and gHbA1c on the risk for each of the eight psychiatric disorders described above using an inverse-variance weighted effect model with multiplicative random effects (IVW) [51]. The positive strand was inferred where possible otherwise palindromic SNPs were removed [52]. We performed composite approaches and sensitivity analyses for exposure-outcome relationships which were significant after Bonferroni correction for the four exposures tested for eight outcomes [P < 1.56 × 10−3, α = 0.05/(8 × 4)]. The reverse MR analyses utilised the same IVW estimator approach. Beta estimates for nominally significant models with a binary exposure were converted to the liability scale assuming a population prevalence of 0.7% and 0.9% for SZ and AN, respectively [53].
The IVW model is limited such that even one invalid IV can bias the overall estimate. Therefore, for estimates with corrected significance we sought to overcome this limitation by using the outlier-robust MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) method [51, 54]. MR-PRESSO is underpinned by the residual sum of squares (RSS), which serves as a heterogeneity measure of ratio estimates. Specifically, an IVW estimate using the IVs is calculated in a leave-one out fashion; if the RSS is decreased significantly relative to a simulated Gaussian distribution of expected RSS, then that variant is excluded from the IVW model. Simulations have demonstrated that this methodology is best suited to instances when less than half of the IVs exhibit horizontal pleiotropy [54]. Three additional MR approaches were implemented to better account for potential invalid IVs: a weighted median estimate, a weighted mode estimate, and MR-Egger. The weighted median model takes the median of the ratio estimates (as opposed to the mean in the IVW model), such that upweighting (with second order weights [55]) is applied to ratio estimates with greater precision [36]. An advantage of this approach is that it is subject to the ‘majority valid’ assumption, whereby an unbiased causal estimate will still be obtained if less than 50% of the model weighting arises from invalid IVs. Mode-based estimators are subject to the related ‘plurality valid’ assumption [56]. Finally, an MR-Egger model was constructed [35]. This is an adaption of Egger regression wherein the exposure effect is regressed against the outcome with an intercept term added to represent the average pleiotropic effect. The I2 statistic for the IV-exposure effects was calculated to assess the relative strength of the no-measurement error assumption, and thus, the suitability of using an MR-Egger model. The conventional threshold of I2 > 0.9 was utilised to deem the IVs appropriate for MR-Egger [57]. We also estimated the effect of fasting insulin on anorexia using generalised summary-data-based Mendelian Randomisation (GSMR) [58]. GSMR is statistically similar to an IVW approach but is implemented using an R package from an independent research group, which serves as an important replication from a technical perspective.
Sensitivity and pleiotropy analyses
The key assumption of the MR-Egger model is referred to as Instrument Strength Independent of Direct Effect (InSIDE), which assumes that there is no significant correlation between direct IV effects on the outcome and genetic association of IVs with the exposure [35, 59]. In other words, the InSIDE assumption is violated if pleiotropic effects act through a confounder of the exposure-outcome association. We employed three primary methods to evaluate evidence for unbalanced pleiotropy: The intercept from the MR Egger model, Cochran’s Q, and the MR PRESSO global pleiotropy test. First, the Egger intercept was tested as to whether it was significantly different from zero, as a nonzero intercept may indicate unbalanced pleiotropy or violation of the InSIDE assumption, given that the intercept represents the mean pleiotropic effect [60]. Furthermore, as heterogeneity amongst the IV exposure-outcome ratio estimates could be caused by horizontal pleiotropy, we quantified this heterogeneity using Cochran’s Q statistic [43, 61, 62]. Finally, a global pleiotropy test was implemented via the MR-PRESSO framework, which utilised the expected and observed RSS [54]. A leave-one-out analysis was then performed to assess whether causal estimates are biased by a single IV, which may indicate the presence of outliers, and the sensitivity of the estimate to said outliers [43]. The MR Steiger directionality test utilizes the phenotypic variance explained by IV SNPs, comparing the instruments’ association with the exposure and outcome to determine if there is evidence that the assumed direction of causality is correct [63]. For binary traits, the trait population prevalence was used to calculate variance explained and convert to the liability scale using the lower and upper bounds of population prevalence estimates used by the GWAS for consistency (0.9% and 4% respectively for anorexia) [28, 64, 65]. To investigate the significance of BMI-associated SNPs [66] on the relationship between outcome and exposure, SNPs associated with both traits were removed and the IVW estimate recalculated. All MR analyses were performed using the TwoSampleMR v0.4.25 package [43] in R v3.6.1 [67], with the exception of the MR-PRESSO model which utilised the MRPRESSO package v1.0 and the GSMR estimate performed with the gsmr package v1.0.9.
Latent causal variable model to estimate the genetic causality proportion of fasting insulin on risk for anorexia nervosa
Fasting insulin and AN display significant genome-wide genomic correlation as indexed by LDSC [28]. This correlation may confound MR, and thus, we implemented a latent causal variable model (LCV) as an additional approach to investigate whether this correlation represents a causal relationship [68]. Briefly, the LCV method assumes that a latent variable mediates the genetic correlation between two traits and tests whether this latent variable displays stronger correlation with either of the traits. Using fourth moments of the bivariate effect size distributions of all SNPs in both GWAS datasets, and their LD structure, a posterior mean estimate of the genetic causality proportion (GCP) is derived which quantifies how much of the genomic architecture of one trait effects another. GCP values range from −1 to 1, with more positive values indicating greater partial genetic causality of trait one on two, and vice versa for more negative values. Full genetic causality is described as GCP = 1 or −1, which is rare in practice [68], with partial genetic causality occurring within these limits. A two-sided t test was used to assess whether the estimated GCP was significantly different from zero. The RunLCV.R and MomentFunctions.R scripts were leveraged to perform these analyses (https://github.com/lukejoconnor/LCV/tree/master/R). The Manning et al. fasting insulin GWAS was once more utilised for these analyses due to its more complete summary statistics and because it was the basis for the previously performed LDSC between anorexia and fasting insulin [28, 69]. Both summary statistics were cleaned and formatted in a standardised way (“munged”) prior to analysis with the LCV model [68, 70, 71].
Results
Evidence of a protective effect of fasting insulin on anorexia nervosa
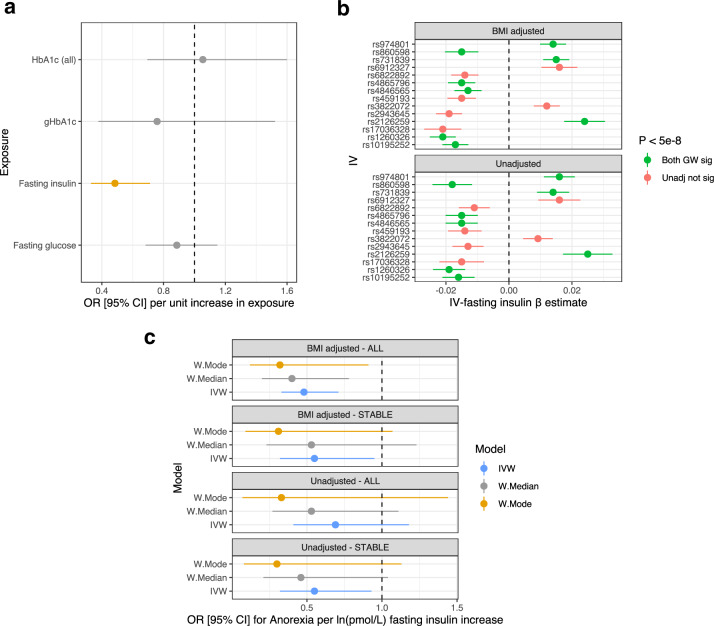
The selected glycaemic IVs explained ~3.33%, 0.64%, 2.42%, 0.85% of exposure variance of fasting glucose, fasting insulin, and glycated haemoglobin levels (HbA1c, gHbA1c), respectively. IVs were selected by clumping for LD to remove correlated variants and excluding palindromes for which the correct strand could not be inferred. An IVW model was used to estimate the casual effect of the four exposures on the eight psychiatric disorder outcomes. We revealed a significant protective effect of a unit increase [ln(pmol/L)] in fasting insulin levels on anorexia after the applying Bonferroni correction (OR = 0.48, [95% CI:0.33–0.71], P = 2.27 × 10−4) (Fig. 1a). It should be noted that this model utilised fasting insulin IVs from a GWAS adjusted for BMI, IVs unadjusted for BMI attenuated the causal estimate, although the point estimate was directionally consistent: OR = 0.69 [95% CI: 0.41–1.18], P = 0.178. The impact of covariation for BMI is discussed further in the subsequent section. In addition, the relationship between fasting insulin and MDD was nominally significant (uncorrected P < 0.05) [OR = 0.85, 95% CI: 0.74–0.97, P = 0.015], whilst all other causal estimates were not significant after utilising either the Bonferroni or less conservative Benjamini-Hochberg method for multiple testing correction. (Supplementary Table 1).
Fig. 1. Effect of glycaemic traits on the risk of anorexia nervosa.
a Forest plot of the IVW estimates of the relationship between glycaemic exposures and anorexia nervosa. The estimates represent an odds ratio (OR) per unit increase in the exposure, with the error bars denoting the 95% confidence interval. The glycaemic exposures were as follows: fasting insulin, fasting glucose. glycated haemoglobin (HbA1c (all)), and a subset of glycaemic glycaeted haemoglobin lead SNPs. There was a significant protective effect of fasting insulin on anorexia nervosa after the application of multiple testing correction, and thus, that estimate is shaded orange. b Comparison of the IV-exposure association effect size for fasting insulin instrumental variables with, and without, phenotypic covariation for body mass index (BMI). The two panels plot the beta estimate of the 14 SNP-fasting insulin associations (error bars are 95% confidence interval) derived from the GWAS with or without adjustment for BMI. IV-estimates highlighted green were associated with fasting insulin at genome-wide significance (P < 5 × 10−8) irrespective of BMI adjustment (“both GW sig”), whilst red shaded SNP-exposure effects were only significant upon covariation for BMI. c Sensitivity analyses of BMI adjusted and unadjusted fasting insulin instrumental variables. We defined the instrumental variables for fasting insulin as follows: all IVs unadjusted for BMI, all IVs adjusted for BMI, IVs significant irrespective of BMI (stable IVs – estimates with and without BMI adjustment used). The forest plot denotes three MR estimators (IVW, weighted median, and weighted mode) using each of these IV subsets; each point represents the odds ratio for anorexia nervosa per natural log transformed pmol/L fasting insulin.
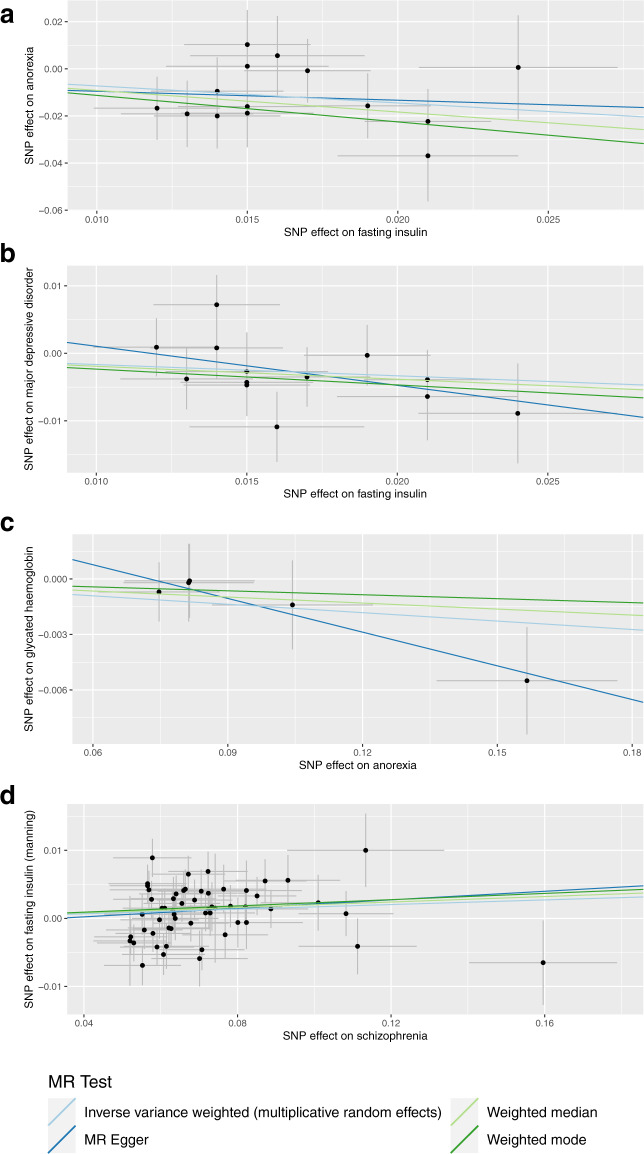
We subjected the causal estimate of fasting insulin on the risk for anorexia to a suite of sensitivity analyses to assess the rigor of our derived IVW estimate and evidence of violations of core MR assumptions (Fig. 2a, Supplementary Tables 3–6). No IVs were detected as outliers using the MR-PRESSO approach. Furthermore, the weighted median model supported the putative protective effect of elevated fasting insulin on risk for anorexia derived from the IVW (ORWeighted Median = 0.40, [95% CI:0.21–0.77], P = 6.3 × 10−3), as did the weighted mode estimator (ORWeighted Mode = 0.32 [95% CI: 0.13–0.84], P = 0.037). The MR-Egger model was not significant; however, the causal estimate was in the same direction of effect as the other two approaches, albeit with an extremely wide confidence interval (OREgger = 0.68, 95% CI: 0.05–9.10). It should be noted that the MR-Egger method typically has notably less power than other approaches, particularly when fewer IVs are used [35]. The causal estimate between fasting insulin and anorexia using both the MR-PRESSO and GSMR approaches was also practically identical to the IVW estimator, although the GSMR estimate displayed a larger standard error (ORGSMR = 0.48, [95% CI:0.30–0.79], P = 3.7 × 10−3). There was no compelling evidence for unbalanced pleiotropy amongst IVs utilised in this construct: heterogeneity between IV effects was not significant (Q = 8.24, df = 13, P = 0.827), the intercept of the MR egger regression did not significantly differ from zero (intercept = −5.7 × 10−3, P = 0.795), and the MR PRESSO test of global pleiotropy was also not significant. A leave-one out recalculation of the causal estimate did not indicate that a single IV or subset of IVs were unduly influencing the model.
Fig. 2. Sensitivity analyses of causal estimates.
The scatterplots represent the IV effects on the exposure and outcome variables (black point), with the confidence intervals for both estimates denoted by the horizontal and vertical lines, respectively. Each coloured slope is indicative of the causal effect of a unit increase in the exposure on the outcome, estimated by the method in the legend utilised to shade the trendline – that is, inverse-variance weighted effect with multiplicative random effects (light blue), weighted median (light green), weighted mode (dark green), and MR-Egger (dark blue). The four panels correspond to a different exposure-outcome pair: (a) fasting insulin → anorexia nervosa, (b) fasting insulin → major depressive disorder, (c) anorexia nervosa → HbA1c, and (d) schizophrenia → fasting insulin.
The impact of BMI adjustment on fasting insulin instrumental variables
We observed a more significant effect of fasting insulin on AN liability using fasting insulin IVs from a GWAS in which covariation for BMI was applied. Given the putative bidirectional relationship between anorexia and BMI [46], we sought to investigate evidence of BMI related confounding on our causal estimate. First, the variance explained by the insulin IVs adjusted for BMI was larger (0.69%) than the unadjusted IVs (0.52%), suggesting greater power to detect an effect using the BMI adjusted SNPs. We directly compared the effect size of the BMI adjusted IVs in the insulin GWAS to the same SNPs without BMI adjustment to identify stable fasting insulin IVs which reached genome-wide significance regardless of covariation for BMI (Fig. 1b). There were eight such IVs which were significantly associated with fasting insulin irrespective of BMI adjustment (Supplementary table 7). The causal estimate of fasting insulin on AN was recalculated using only these eight IVs, with both the BMI adjusted and unadjusted IV-insulin effect sizes yielding a significant protective impact of fasting insulin on liability to AN [IVW with multiplicative random effects estimate]—BMI adjusted stable IVs: OR = 0.55 [95% CI: 0.32–0.95], P = 0.03; BMI unadjusted stable IVs: OR = 0.55 [95% CI: 0.32–0.93], P = 0.027 (Fig. 1c). The change in IV-fasting insulin effect size upon covariation for BMI was relatively small in most instances, with no reversal of the direction of effect relative to the tested allele (Supplementary Table 7). Importantly, this stable subset of IVs remained sufficiently strong, as quantified by the F-statistic: FAdjusted BMI = 53.33, FUnadjusted = 38.09. These data support that the utilisation of BMI adjusted IVs does not constitute collider bias, although this possibility cannot be definitively excluded. Moreover, we identified five fasting insulin IVs which were also associated with BMI at genome wide significance (P < 5 × 10−8) and recalculated the IVW estimate with these instruments removed. The effect size observed in this reduced IVW model was not greatly attenuated (OR = 0.51 [95% CI: 0.32–0.83], P = 7.03 × 10−3, Supplementary Table 8), supporting that the relationship between insulin and anorexia was not unduly biased by horizontal pleiotropy through IV effects on BMI.
Psychiatric disorders as the exposure phenotype
Bidirectional relationships were investigated using a reverse MR approach, whereby the psychiatric disorders were the exposures and the glycaemic traits acting as outcomes. There were five disorders with at least three IVs which overlapped the outcome GWAS (ADHD, AN, BP, MDD, and SZ), and thus, these phenotypes were considered as exposures. We demonstrated weak evidence of a positive causal effect of genetic liability to SZ on fasting insulin (β = 0.017 [95% CI: 0.004–0.03], P = 0.016) and a negative relationship between AN and HbA1c (β = −0.015 [95% CI: −0.03 to −0.002], P = 0.023), although these estimates did not survive multiple testing correction. Causal estimates using binary exposures are difficult to intuitively interpret given a unit increase represents a 2.72-fold multiplicative increase in the odds of the disorder [72]. Previously, it has been suggested that these estimates could be converted to the liability scale to represent the change in the outcome per standard deviation increase in liability to the disorder [53]. The converted IVW results assumed a 0.7% and 0.9% population prevalence of SZ and AN, respectively: β = 0.048 (SZ → fasting insulin), β = −0.041 (AN → HbA1c). We caution that binary exposures must be carefully interpreted in MR analyses, and thus, these models may be more appropriately considered as a test of the null hypothesis rather than a direct estimation of effect size, as discussed elsewhere [72]. The IVs selected for these two psychiatric exposures explained 0.26% (F = 37.73) and 2.29% (F = 43.37) of the phenotypic variance for AN and schizophrenia, respectively. There were no significant estimates between any of the remaining psychiatric exposures and glycaemic traits as outcomes (Supplementary Table 2). The IVW estimates for all exposure-outcome pairs, in both MR directions, are detailed in Table 3.
Table 3.
Causal relationships between glycaemic traits and psychiatric disorders estimated via two-sample Mendelian randomisation using an inverse-variance weighted effect model with multiplicative random effects.
| Trait one | Trait two | Glycaemic → psychiatric (beta)a | Psychiatric → glycaemic (beta)b |
|---|---|---|---|
| ADHD | Fasting insulin | 0.21 (0.34) | −0.0004 (0.02) |
| ADHD | Fasting glucose | 0.20 (0.12) | −0.008 (0.02) |
| ADHD | HbA1c (all) | 0.34 (0.20) | −0.02 (0.01) |
| ADHD | HbA1c (glycaemic) | 0.45 (0.37) | N/A |
| AN | Fasting insulin | −0.72 (0.20)** | −0.0005 (0.02) |
| AN | Fasting glucose | −0.12 (0.13) | −0.003 (0.03) |
| AN | HbA1c (all) | 0.05 (0.21) | −0.02 (0.01)* |
| AN | HbA1c (glycaemic) | −0.28 (0.36) | N/A |
| ASD | Fasting insulin | −0.06 (0.30) | N/A |
| ASD | Fasting glucose | −0.14 (0.12) | N/A |
| ASD | HbA1c (all) | −0.16 (0.15) | N/A |
| ASD | HbA1c (glycaemic) | −0.38 (0.26) | N/A |
| BP | Fasting insulin | −0.20 (0.52) | 0.003 (0.01) |
| BP | Fasting glucose | −0.15 (0.18) | −0.009 (0.01) |
| BP | HbA1c (all) | −0.10 (0.15) | 0.012 (0.01) |
| BP | HbA1c (glycaemic) | −0.51 (0.46) | N/A |
| MDD | Fasting insulin | −0.17 (0.07)* | −0.01 (0.02) |
| MDD | Fasting glucose | 0.02 (0.04) | 0.02 (0.02) |
| MDD | HbA1c (all) | 0.09 (0.05) | 0.01 (0.02) |
| MDD | HbA1c (glycaemic) | −0.11 (0.13) | N/A |
| OCD | Fasting insulin | 0.14 (0.65) | N/A |
| OCD | Fasting glucose | −0.19 (0.23) | N/A |
| OCD | HbA1c (all) | 0.40 (0.43) | N/A |
| OCD | HbA1c (glycaemic) | 0.38 (0.54) | N/A |
| SZ | Fasting insulin | 0.19 (0.29) | 0.02 (0.01)* |
| SZ | Fasting glucose | −0.13 (0.10) | 0.008 (0.01) |
| SZ | HbA1c (all) | 0.01 (0.14) | −0.0005 (0.004) |
| SZ | HbA1c (glycaemic) | −0.36 (0.26) | N/A |
| TS | Fasting insulin | 0.42 (0.42) | N/A |
| TS | Fasting glucose | 0.15 (0.19) | N/A |
| TS | HbA1c (all) | 0.16 (0.33) | N/A |
| TS | HbA1c (glycaemic) | 0.98 (0.56) | N/A |
Mendelian randomisation (IVW estimator with multiplicative random effects) was performed in both directions (subject to the availability of IVs), that is, glycaemic traits as the exposure (glycaemic → psychiatric), along with psychiatric disorders as the exposure. The glycaemic traits were as follows: fasting insulin (BMI adjusted, BMI unadjusted estimates available in Supplementary Tables 1 and 2), fasting glucose, glycaeted haemoglobin (all IVs = HbA1c (all), IVs annotated as glycaemic = HbA1c (glycaemic). Bolded beta estimates are statistically significant. N/A represents analyses where less than three overlapping IVs were available.
The psychiatric traits were: ADHD attention deficit hyperactivity disorder, AN anorexia nervosa, ASD autism spectrum disorder, BP bipolar disorder, MDD major depressive disorder, OCD obsessive compulsive disorder, SZ schizophrenia, TS Tourette’s syndrome.
aIVW beta estimates (standard error) of the effect of glycaemic on the risk of psychiatric disorders represent the log odds of the disorder per unit increase of the exposure. The unit of effects were as follows: fasting insulin = natural log transformed pmol/L, fasting glucose = mmol/L, HbA1c = % HbA1c.
bIVW beta estimates (standard error) using the psychiatric disorders as exposures represent the effect on glycaemic traits per 2.72-fold multiplicative increase in the odds of the psychiatric disorder, however, we treat this primarily as a test of the null hypothesis.
*P < 0.05, **P < 0.01.
We applied a subset of these sensitivity analyses (MR Egger, Weighted Median, and Weighted Mode estimator) to the three remaining IVW estimates which did not survive multiple testing correction (uncorrected P < 0.05) to evaluate the consistency of the causal estimate (Fig. 2b–d). All three exposure-outcome pairs had consistent point estimate effect directions, that is, a protective effect of fasting insulin on the risk of MDD (negative beta), a positive causal estimate between SZ and fasting insulin (positive beta), and a negative estimate between AN and HbA1c (negative beta). However, these models were only statistically significant in the case of the weighted median estimator of the schizophrenia to fasting insulin construct (Supplementary Table 3), with the confidence interval overlapping the null in most instances. As a result, we classified these trait pairs as having relatively weak evidence of causation in comparison to the fasting insulin to anorexia model.
Assessment of the direction of causal effect between fasting insulin and anorexia nervosa
The MR models implemented in this study assume that the IVs impact the exposure, which in turn affects the outcome—however, in practice it is feasible that the orientation of the causal pathway is incorrect and that the outcome influences the exposure through the genetic instruments. To address this, we performed a Steiger directionality test. This method examined whether the phenotypic variance in the outcome explained by the IVs is less than that of the exposure to test the assumed causal direction. We calculated the variance in anorexia risk explained by the IVs and converted to the liability scale using the upper (4%) and lower (0.9%) bound of estimated population prevalence for anorexia [28]. The Steiger directionality test supported the hypothesis that the effect of fasting insulin on risk for anorexia is the correct causal direction as the variance explained by the IVs was lower for anorexia than fasting insulin (P = 1.35 × 10−27, P = 1.36 × 10−27 respectively for the upper and lower bound anorexia prevalence estimates) (Supplementary Table 9). Furthermore, the reverse IVW estimator did not indicate evidence of an effect from AN to fasting insulin (P = 0.986), although there was weak evidence of a negative relationship between anorexia liability and HbA1c, as described in the previous sections. Given the genetic correlation between fasting insulin and anorexia, we used a latent causal variable model to determine the proportion of trait one (fasting insulin) that genetically causes trait two (anorexia), which was quantified as the mean posterior estimate of the GCP. The sign of the mean posterior GCP estimate suggests that fasting insulin is partially genetically causal for anorexia; however, this was not significantly different from zero, likely due to the large standard error ( = 0.39, SE = 0.33, P = 0.26]. Whilst the LCV GCP estimate was not significant, there was strong evidence that the causal direction was not anorexia to insulin [H0: GCP= −1, P = 3.6 × 10−104], in accordance with the Steiger directionality test results (Supplementary Table 10).
Discussion
Glycaemic regulation is involved with many physiological processes, however, its role in neurological function has motivated investigation of this system in psychiatric disorders. Using a MR approach which leverages genetic IVs as proxies for three glycaemic traits, we uncovered evidence of a protective effect of elevated fasting insulin on the risk for anorexia nervosa. No strong evidence of a causal effect of any of the glycaemic traits was found testing seven other psychiatric phenotypes, although there was relatively weaker support for a protective effect of fasting insulin on depression. Notably, we did not replicate a previous study which demonstrated a risk increasing effect of fasting insulin on schizophrenia, however, we utilised a larger schizophrenia GWAS and different IVs [73]. Although previous analysis has shown a relationship between first episode psychosis and glycaemic dysregulation, and elevated rates of dysglycaemia in psychiatry, this was not supported by our MR model. Our inability to detect this relationship may be limited by the strength of the IV used, or the observed effects from previous analysis may be due to variables with shared genetic liability which influence glycaemic homoeostasis, such as inflammation or BMI. Interestingly, this study demonstrated there was weak evidence of a causal effect in the opposite direction, whereby genetic liability to schizophrenia was associated with increased fasting insulin, supporting previous data which demonstrated an association between schizophrenia PRS and insulin resistance [29]. We prefer to treat these binary exposure estimates as a test of the null hypothesis as the interpretation of effect sizes from binary exposures are not intuitive and may be subject to unrealistic assumptions related to the homogeneity of their effects [72]. The future availability of more data with the power to explain a larger portion of the variance in the exposures and outcomes, could yet yield more evidence of a causal relationship between dysglycaemia and other psychiatric disorders. The negative relationship between elevated insulin and anorexia risk derived in this study supports the negative genetic correlation observed between the two GWAS studies by LDSC [28]. However, it should be noted that genetic correlation between traits may confound MR estimates, which cannot be definitively ruled out in this study as our LCV estimate of partial genetic causality was not significantly different than zero. The association between a natural log transformed pmol/L increase in fasting insulin and odds of anorexia yielded an odds ratio of 0.48 [95% CI: 0.33–0.71]. To contextualize this unit of effect, we considered fasting insulin values from a large cohort of 10.5–11 year old female normal weight European participants [74]. We estimate that a unit increase from the 10th percentile of this cohort (with a fasting insulin concentration of 30.97 pmol/L) would correspond to ~84.19 pmol/L, which is roughly equivalent to the 90th percentile of the cohort (~86.91). This estimate derived from the IVW model was supported by sensitivity analyses which did not indicate any statistical evidence of unbalanced pleiotropy which would confound the IVs we selected.
The role of insulin signalling and glucose metabolism in the brain, and its interplay with the periphery, is complex, necessitating further research to specifically understand how fasting insulin could exert a protective effect on anorexia. The relationship between circulating insulin and weight gain may contribute to this protective effect given peripheral insulin and insulin therapy in the context of diabetes is associated with weight gain [42, 75]. There are a number of mechanisms by which this is proposed to be mediated, including the stimulatory effect of insulin on fatty acid storage and cell growth and a reduction in glycosuria [76–78]. Furthermore, MR analyses have supported a positive relationship between insulin and weight gain [79, 80]. Given the nature of the clinical presentation of anorexia, the effect of insulin on hunger and satiety is particularly pertinent. Increased insulin levels in the body results in higher levels of hunger and an increased pleasantness associated with sweet taste [81]. This corresponds to data from anorexia cohorts which report that individuals with anorexia have a reduced appreciation of sweet tastes [82]. In contrast, insulin is postulated to have an anorexigenic effect in the brain [83, 84], partly through its inhibition of the orexigenic agouti-related peptide and neuropeptide Y neurons [85, 86]. This may contradict the putative risk-decreasing effect of insulin on anorexia we observed in our study, however, there is evidence of a significant sexual dimorphism in this phenomenon. An example of this has been demonstrated using intranasal insulin administration, in which hunger was decreased only in male participants, conversely, there were positive cognitive enhancing effects seen only in women [87], although this was a small study (N = 32) that warrants replication in a larger cohort. This sexual dimorphism in the effect of insulin signalling is further supported by rodent data [88]. As anorexia is significantly more prevalent in females [89] it is possible that the sexual dimorphic effect of insulin on hunger signalling is contributing to this discrepancy in prevalence. However, the data supporting the central nervous system impact of insulin in humans are derived from studies with small sample sizes, and thus, further work is needed to resolve the relationship between insulin signalling and satiety. Moreover, many insights into the neurological consequences of insulin signalling arise from rodent models and caution must be exercised when directly extrapolating physiology from these models to humans.
There are a number of future directions which arise from these data. Only Europeans were used in this analysis warranting its extension to trans-ethnic cohorts. As the negative relationship between insulin and anorexia is also evidenced using a genomic correlation approach, there is a need to investigate shared genes and biological pathways which may explain this association. This would be particularly valuable to interpret the causal estimate we uncovered, as individuals who develop anorexia may be genetically predisposed to have altered glycaemic homoeostasis. Furthermore, a well-powered, sex stratified GWAS of anorexia could be utilised to formally test whether the impact of insulin on anorexia risk displays sexual dimorphism, with sexual dimorphisms likely also evident in glycaemic traits themselves. As diagnosis of anorexia is highly skewed towards females, it will likely be a continued challenge to genotype larger male cohorts which approach the sample sizes available for female participants. There are also a number of other heritable psychiatric phenotypes for which the effect of glycaemic traits could be tested using MR, including measures like neuroticism and anxiety. It is also important to consider the inherent limitations of MR in light of our data. We did not uncover any statistical evidence of unbalanced pleiotropy amongst the fasting insulin IVs; however, this cannot be definitively proven and future replication in larger studies is paramount. Moreover, whilst the IVs selected for insulin were appropriately strong as quantified by an F-statistic, they still only explain a fraction of the phenotypic variance in fasting insulin. Despite these caveats and other methodological challenges associated with causal inference using IVs, we believe the fasting insulin—anorexia model to be reliable. In conclusion, we uncovered evidence of a protective effect of fasting insulin on the risk of anorexia nervosa, with further work now required to further understand the biological mechanisms underpinning this relationship.
Funding and disclosure
MJC is supported by a National health and medical research council (NHMRC) Senior Research Fellowship (1121474) and an NHMRC project grant (1147644), URL: https://www.nhmrc.gov.au/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no competing financial interests.
Supplementary information
Author contributions
WRR designed the study with input from DMA, MPG, and MJC. DMA and WRR performed the analyses. DMA, WRR, and MJC wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and the final manuscript. MPG and MJC supervised the project.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Danielle M. Adams, William R. Reay
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00847-w).
References
- 1.McGrath JJ, Saha S, Lim CCW, Aguilar-Gaxiola S, Alonso J, Andrade LH, et al. Trauma and psychotic experiences: transnational data from the World Mental Health Survey. Br J Psychiatry. 2017;211:373–80.. doi: 10.1192/bjp.bp.117.205955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468:203–12.. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 3.Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address pmhe, cross-disorder group of the psychiatric genomics c. genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469–-82.e11. doi: 10.1016/j.cell.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polderman TJC, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47:702–9. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- 5.Duncan LE, Ostacher M, Ballon J. How genome-wide association studies (GWAS) made traditional candidate gene studies obsolete. Neuropsychopharmacology. 2019;44:1518–23.. doi: 10.1038/s41386-019-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestadt G, Grados M, Samuels JF. Genetics of obsessive-compulsive disorder. Psychiatr Clin North Am. 2010;33:141–58.. doi: 10.1016/j.psc.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–51.. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson DC. Managing weight gain and metabolic issues in patients treated with atypical antipsychotics. J Clin Psychiatry. 2008;69:e04. doi: 10.4088/jcp.0208e04. [DOI] [PubMed] [Google Scholar]
- 9.Lake J, Turner MS. Urgent need for improved mental health care and a more collaborative model of care. Perm J. 2017;21:17–024.. doi: 10.7812/TPP/17-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cogn Ther Res. 2012;36:427–40.. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216–29.. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 13.Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–35.. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 14.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–36.. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:21. doi: 10.1155/2012/384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–97.. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15:166–74.. doi: 10.1002/wps.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, et al. Prevalence and predictors of type 2 diabetes mellitus in people with bipolar disorder: a systematic review and meta-analysis. J Clin Psychiatry. 2015;76:1490–9. doi: 10.4088/JCP.14r09635. [DOI] [PubMed] [Google Scholar]
- 19.Chen MH, Lan WH, Hsu JW, Huang KL, Su TP, Li CT, et al. Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care. 2016;39:788–93.. doi: 10.2337/dc15-1807. [DOI] [PubMed] [Google Scholar]
- 20.Brander G, Isomura K, Chang Z, Kuja-Halkola R, Almqvist C, Larsson H, et al. Association of tourette syndrome and chronic tic disorder with metabolic and cardiovascular disorders. JAMA Neurol. 2019;76:454–61. doi: 10.1001/jamaneurol.2018.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindblad F, Eickhoff M, Forslund AH, Isaksson J, Gustafsson J. Fasting blood glucose and HbA1c in children with ADHD. Psychiatry Res. 2015;226:515–6. doi: 10.1016/j.psychres.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Perry BI, Upthegrove R, Thompson A, Marwaha S, Zammit S, Singh SP, et al. Dysglycaemia, inflammation and psychosis: findings from the UK ALSPAC birth cohort. Schizophr Bull. 2019;45:330–8. doi: 10.1093/schbul/sby040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dostalova I, Smitka K, Papezova H, Kvasnickova H, Nedvidkova J. Increased insulin sensitivity in patients with anorexia nervosa: the role of adipocytokines. Physiol Res. 2007;56:587–94. doi: 10.33549/physiolres.931089. [DOI] [PubMed] [Google Scholar]
- 24.Schimmelmann BG, Schmidt SJ, Carbon M, Correll CU. Treatment of adolescents with early-onset schizophrenia spectrum disorders: in search of a rational, evidence-informed approach. Curr Opin Psychiatry. 2013;26:219–30.. doi: 10.1097/YCO.0b013e32835dcc2a. [DOI] [PubMed] [Google Scholar]
- 25.Correll CU, Detraux J, De Lepeleire J, De, Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14:119–36.. doi: 10.1002/wps.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:261–9. doi: 10.1001/jamapsychiatry.2016.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry BI, McIntosh G, Weich S, Singh S, Rees K. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry. 2016;3:1049–58.. doi: 10.1016/S2215-0366(16)30262-0. [DOI] [PubMed] [Google Scholar]
- 28.Watson H, Yilmaz Z, Thornton L, Hübel C, Coleman J, Gaspar H, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51:1207–14. [DOI] [PMC free article] [PubMed]
- 29.Tomasik J, Lago SG, Vázquez-Bourgon J, Papiol S, Suárez-Pinilla P, Crespo-Facorro B, et al. Association of insulin resistance with schizophrenia polygenic risk score and response to antipsychotic treatmentinsulin resistance and schizophrenia polygenic risk score and response to antipsychotic treatmentletters. JAMA Psychiatry. 2019;76:864–7. doi: 10.1001/jamapsychiatry.2019.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackinger S, Prins B, Mamakou V, Zengini E, Marouli E, Brčić L, et al. Evidence for genetic contribution to the increased risk of type 2 diabetes in schizophrenia. Transl Psychiatry. 2018;8:252. doi: 10.1038/s41398-018-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasooly D, Patel CJ. Conducting a reproducible mendelian randomization analysis using the r analytic statistical environment. Curr Protoc Hum Genet. 2019;101:e82. doi: 10.1002/cphg.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey, Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63.. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 33.Teumer A. Common methods for performing mendelian randomization. Front Cardiovasc Med. 2018;5:51. doi: 10.3389/fcvm.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiol (Camb, Mass) 2006;17:260–7. doi: 10.1097/01.ede.0000215160.88317.cb. [DOI] [PubMed] [Google Scholar]
- 35.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowden J, Davey, Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrecque J, Swanson SA. Understanding the assumptions underlying instrumental variable analyses: a brief review of falsification strategies and related tools. Curr Epidemiol Rep. 2018;5:214–20.. doi: 10.1007/s40471-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Ja Luan, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheeler E, Leong A, Liu C-T, Hivert M-F, Strawbridge RJ, Podmore C, et al. Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome-wide meta-analysis. PLoS Med. 2017;14:e1002383. doi: 10.1371/journal.pmed.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, Arriola L, et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes. 2014;63:4378–87.. doi: 10.2337/db14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Cantero J, Martin-Rodriguez JL, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL. Insulin resistance in lean and overweight non-diabetic Caucasian adults: Study of its relationship with liver triglyceride content, waist circumference and BMI. PLOS ONE. 2018;13:e0192663. doi: 10.1371/journal.pone.0192663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pennings N, Jaber J, Ahiawodzi P. Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation. Diabetes/Metab Res Rev. 2018;34:e2986. doi: 10.1002/dmrr.2986. [DOI] [PubMed] [Google Scholar]
- 43.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44.. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.OCD IOCDFGCI-Ga, (OCGAS CGAS. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23:1181–8. doi: 10.1038/mp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50:381–9. doi: 10.1038/s41588-018-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, et al. Interrogating the genetic determinants of tourette’s syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry. 2019;176:217–27.. doi: 10.1176/appi.ajp.2018.18070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65.. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717–26.. doi: 10.1093/ije/dyx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrne EM, Zhu Z, Qi T, Skene NG, Bryois J, Pardinas AF, et al. Conditional GWAS analysis identifies putative disorder-specific SNPs for psychiatric disorders. bioRxiv. 2019:592899. [DOI] [PMC free article] [PubMed]
- 54.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2018;48:728–42.. doi: 10.1093/ije/dyy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98.. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74.. doi: 10.1093/ije/dyw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Z, Zheng Z, Zhang F, Wu Y, Trzaskowski M, Maier R, et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun. 2018;9:224. doi: 10.1038/s41467-017-02317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slob EAW, Burgess S A comparison of robust mendelian randomization methods using summary data. bioRxiv. 2019:577940. [DOI] [PMC free article] [PubMed]
- 60.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89.. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–66.. [PubMed] [Google Scholar]
- 62.Bowden J, Hemani G, Davey, Smith G. Invited commentary: detecting individual and global horizontal pleiotropy in mendelian randomization—a job for the humble heterogeneity statistic? Am J Epidemiol. 2018;187:2681–5. doi: 10.1093/aje/kwy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007081–e. doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SH, Wray NR. Novel genetic analysis for case-control genome-wide association studies: quantification of power and genomic prediction accuracy. PLoS One. 2013;8:e71494–e. doi: 10.1371/journal.pone.0071494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SH, Goddard ME, Wray NR, Visscher PM. A better coefficient of determination for genetic profile analysis. Genet Epidemiol. 2012;36:214–24.. doi: 10.1002/gepi.21614. [DOI] [PubMed] [Google Scholar]
- 66.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166–74.. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Team R A language and environment for statistical computing. Computing. 2006;1.
- 68.O’Connor LJ, Price AL. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat Genet. 2018;50:1728–34.. doi: 10.1038/s41588-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manning AK, Hivert M-F, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44:659–69.. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. Analysis of shared heritability in common disorders of the brain. Science (New York, NY). 2018;360:eeap8757. [DOI] [PMC free article] [PubMed]
- 71.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33:947–52.. doi: 10.1007/s10654-018-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Z, Chen P, Chen J, Xu Y, Wang Q, Li X, et al. Glucose and Insulin-Related Traits, Type 2 Diabetes and Risk of Schizophrenia: A Mendelian Randomization Study. EBioMedicine. 2018;34:182–8. doi: 10.1016/j.ebiom.2018.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peplies J, Jiménez-Pavón D, Savva SC, Buck C, Günther K, Fraterman A, et al. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in pre-pubertal normal weight European children from the IDEFICS cohort. Int J Obes. 2014;38:S39–S47. doi: 10.1038/ijo.2014.134. [DOI] [PubMed] [Google Scholar]
- 75.Brown A, Guess N, Dornhorst A, Taheri S, Frost G. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655–68.. doi: 10.1111/dom.13009. [DOI] [PubMed] [Google Scholar]
- 76.Packianathan IC, Fuller NJ, Peterson DB, Wright A, Coward WA, Finer N. Use of a reference four-component model to define the effects of insulin treatment on body composition in type 2 diabetes: the ‘Darwin study’. Diabetologia. 2005;48:222–9. doi: 10.1007/s00125-004-1642-x. [DOI] [PubMed] [Google Scholar]
- 77.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with Type II diabetes mellitus. Diabetologia. 1999;42:406–12.. doi: 10.1007/s001250051172. [DOI] [PubMed] [Google Scholar]
- 78.Shank ML, Del Prato S, DeFronzo RA. Bedtime insulin/daytime glipizide. Effective therapy for sulfonylurea failures in NIDDM. Diabetes. 1995;44:165–72.. doi: 10.2337/diab.44.2.165. [DOI] [PubMed] [Google Scholar]
- 79.Xu L, Borges MC, Hemani G, Lawlor DA. The role of glycaemic and lipid risk factors in mediating the effect of BMI on coronary heart disease: a two-step, two-sample Mendelian randomisation study. Diabetologia. 2017;60:2210–20.. doi: 10.1007/s00125-017-4396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Astley CM, Todd JN, Salem RM, Vedantam S, Ebbeling CB, Huang PL, et al. Genetic evidence that carbohydrate-stimulated insulin secretion leads to obesity. Clin Chem. 2018;64:192–200. doi: 10.1373/clinchem.2017.280727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodin J. Insulin levels, hunger, and food intake: an example of feedback loops in body weight regulation. Health Psychol. 1985;4:1–24. doi: 10.1037//0278-6133.4.1.1. [DOI] [PubMed] [Google Scholar]
- 82.Kinnaird E, Stewart C, Tchanturia K. Taste sensitivity in anorexia nervosa: a systematic review. Int J Eat Disord. 2018;51:771–84.. doi: 10.1002/eat.22886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kullmann S, Heni M, Veit R, Scheffler K, Machann J, Häring H-U, et al. Intranasal insulin enhances brain functional connectivity mediating the relationship between adiposity and subjective feeling of hunger. Sci Rep. 2017;7:1627. doi: 10.1038/s41598-017-01907-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metabol. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Loh K, Zhang L, Brandon A, Wang Q, Begg D, Qi Y, et al. Insulin controls food intake and energy balance via NPY neurons. Mol Metab. 2017;6:574–84.. doi: 10.1016/j.molmet.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaga T, Inui A, Okita M, Asakawa A, Ueno N, Kasuga M, et al. Modest overexpression of neuropeptide Y in the brain leads to obesity after high-sucrose feeding. Diabetes. 2001;50:1206–10.. doi: 10.2337/diabetes.50.5.1206. [DOI] [PubMed] [Google Scholar]
- 87.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–44.. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 88.Clegg DJ, Riedy CA, Smith KAB, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 89.Klump KL. Puberty as a critical risk period for eating disorders: a review of human and animal studies. Horm Behav. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shu X, Wu L, Khankari NK, Shu X-O, Wang TJ, Michailidou K, et al. Associations of obesity and circulating insulin and glucose with breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol. 2018;48:795–806. doi: 10.1093/ije/dyy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.