Abstract
Abnormalities in the complement system have been described in patients with schizophrenia, with those individuals having greater frequency of complement component 4A (C4A) alleles and higher C4A transcript levels in postmortem brain tissue. Importantly, abnormalities in C4A and other complement molecules have been associated with synaptic pruning abnormalities that occur during neurodevelopment. A few studies have investigated C4 levels in living patients with schizophrenia, but all of them did so using peripheral blood samples. No studies have examined C4 levels in cerebrospinal fluid (CSF), presumably a better biofluid choice given its intimate contact with the brain. Therefore, we report for the first time on C4 levels in CSF and plasma of patients with schizophrenia. In this study, we obtained CSF in 32 patients with schizophrenia spectrum disorders and 32 healthy volunteers and peripheral blood samples in 33 SSD and 31 healthy volunteers. C4 levels were measured using Abcam ELISA assays. Univariate analysis did not show a statistically significant difference in CSF C4 values between groups. However, a multivariable analysis showed a statistically significant increase in CSF C4 levels between groups after adjusting for sex and age. We also observed a high correlation between CSF C4 levels and age. By contrast, plasma C4 levels were not significantly different between groups. CSF and plasma C4 levels were not significantly correlated. Therefore, the use of CSF samples is critical and should be complementary to the use of peripheral blood samples to allow for a comprehensive understanding of complement C4 abnormalities in schizophrenia.
Subject terms: Biomarkers, Schizophrenia
Introduction
The complement system, besides assisting the immune system with critical tasks such as opsonization, chemotaxis/inflammation, and cell lysis [1], has other functions in the central nervous system such as promoting synaptic pruning [2] and facilitating neuronal migration during neurodevelopment [3]. Excessive synaptic pruning [4] and impaired neuronal migration [5] have been described in schizophrenia; therefore, it is possible that some of these findings may be the consequence of abnormalities in the complement system. The most compelling data supporting this hypothesis was reported by Sekar et al. [2]. They found: (1) a greater frequency of component 4A (C4A) alleles in patients with schizophrenia, (2) a 1.4-fold greater C4A mRNA in postmortem brain tissue of schizophrenia compared to individuals without schizophrenia, and (3) deposition of C4 in neuronal bodies, axons and dendrites in human postmortem brain tissue and cultured human cortical neurons. Similarly, Sellgren et al. demonstrated that the long form of the C4A gene (C4AL) was associated with increased deposition of C3 in neurons and increased synaptic engulfment by microglia [5].
Sex, age, and obesity seem to affect complement C4 levels. Kamitaki et al. [6] demonstrated that healthy men and women undergo age-dependent elevation in cerebrospinal fluid (CSF) C4 levels, but noted that this change starts occurring earlier in men (20s–30s years of age) compared to females (close to menopause). Gaya da Costa et al. [7] examined serum samples from 120 Caucasian individuals and found lower activity of the alternative pathway of the complement in females, although they did not find sex differences in C4 levels nor a correlation with age. In regards to obesity, Nilsson et al. reported increased levels of C3 and C4 in association with high BMI values and other risk factors for cardiovascular disease [8].
Few studies have investigated transcript and protein levels of complement molecules, particularly of C4, in patients with schizophrenia and findings have been inconsistent. Some authors have found differences between groups of ultrahigh risk or schizophrenia patients and healthy volunteers (HV) [9–11] whereas others did not find any differences [12, 13] Unfortunately, the study designs, the C4 assays and the patients selected for the studies were quite different between studies which could explain some of the inconsistent results. In the only longitudinal study reported in the literature, Mondelli et al. [14] found that baseline complement C4 serum levels predicted response to treatment in 25 FEP patients who were followed up for 1 year, with nonresponders having higher C4 levels at baseline compared to responders.
Notably, all prior studies used peripheral blood to obtain complement C4 levels and none used CSF, a biofluid in intimate contact with the brain and readily obtained by performing a lumbar puncture. Therefore, our aim is to report for the first time on complement C4 levels in CSF and plasma from patients with schizophrenia spectrum disorders (SSD) and a group of HV. Furthermore, we will investigate the correlation between CSF and plasma levels as well as exploratory correlations with demographic and clinical variables. We hypothesize that C4 levels will be increased in patients with SSD compared to HV and that CSF and plasma levels will be poorly correlated.
Materials and methods
Subjects
Inclusion and exclusion criteria
Patients were included in the study if they (1) fulfilled DSM-IV criteria for schizophrenia, schizophreniform disorder, schizoaffective disorder, or Psychosis Not Otherwise Specified; (2) were 15–59 years old, and (3) were willing and capable of providing informed consent. Subjects were excluded from participation if they (1) were prescribed an anticoagulant; (2) had a history of an organic brain disorder, (3) had a clinically significant thrombocytopenia or coagulopathy based on screening blood tests that prevented them for having a lumbar puncture, or (4) had a diagnosis of a substance-induced psychotic disorder. HV were recruited from the community and were excluded if they had an Axis I diagnosis or if they had a first-degree relative with a known or suspected Axis I disorder, based on family history questionnaire. They had the same inclusion/exclusion criteria except for the criterium related to diagnoses.
Recruitment and consent
Patients were recruited from the inpatient and outpatient departments at The Zucker Hillside Hospital, a psychiatric hospital member of Northwell Health in Glen Oaks, NY and the New York Presbyterian Hospital, Westchester Division. HV were recruited from the general population via word of mouth, newspaper and internet advertisements, and poster flyers. All subjects provided written informed consent to a protocol approved by the Institutional Review Board of the Northwell Health System and the Weill Cornell Medical College. After providing informed consent, subjects provided urine for a urine toxicology test and underwent a physical exam for height, weight and waist circumference and baseline ratings. Psychopathology ratings were administered using the Brief Psychiatric Rating Scale (BPRS) and the schedule for assessment of negative symptoms. A neuropsychological assessment was administered using the MATRICS Consensus Cognitive Battery (MCCB) battery.
CSF and blood collection
Subjects underwent a lumbar puncture, performed using a standard technique with a 25 gauge, Whitacre point spinal needle after subcutaneous lidocaine was applied. The procedure was conducted with patient sitting up and all procedures took place at 2 pm. 15–25 cc of CSF were obtained from each subject. The first 2–3 mls obtained after collecting the fluid were sent for routine testing (cell count, proteins, glucose, and VDRL). CSF samples with macroscopic blood as consequence of traumatic procedures were not included in these analyses. A blood draw to obtain 10 cc of peripheral blood was also performed prior to the lumbar puncture procedure. Both CSF and blood samples were centrifuged to remove cells in the case of CSF samples or to obtain plasma in the case of blood. Both sample types were flash frozen with liquid nitrogen and stored at −80 °C at the biorepository of the Feinstein Institute for Medical Research. CSF and blood samples were sent to the core laboratory CTSC Weill Cornell Medicine, where complement component C4 concentrations were determined using CSF (ab108825) and plasma (ab108824) ELISA kits from Abcam.
Statistical analysis
Baseline characteristics were compared between patients and HV using t tests for normally distributed continuous variables or Wilcoxon rank sum tests for continuous variables not normally distributed. C4 levels were considered normally distributed after checking their distributions in patients and HV. A multivariable linear regression model was built using CSF C4 levels as the dependent variable with subject type (SSD vs. HV), sex and age as the covariates. Sex and age were selected a priori as they are commonly included in models to adjust for potential sex and age effects. The model was tested for confounding factors and interactions. The correlation between C4 levels in CSF and plasma was calculated using the pairwise Pearson correlation coefficient. In the overall sample, C4 values were correlated with other variables of interest such as age, body mass index (BMI) and neurocognitive domain scores. Within the SSD group, we examined the correlation between CSF C4 levels and duration of illness, total BPRS score, number of hospitalizations, and antipsychotic dose. To convert antipsychotic dosages to a chlorpromazine dose, we utilized the package chlorpromazineR version 0.1.2 [15] within R version 3.6.3 [16]. Within this package, we employed the widely accepted chlorpromazine conversion key found in the manuscript by Leucht et al. [17].
Results
Subjects
Thirty-two SSD patients and 32 HV were included in the CSF analysis. The mean age of subjects was 38.5 years (SD = 11.6), 70.3% were male (n = 45), and 43.8% were black (n = 28). SSD patients were younger compared to HV (mean age = 36.1 years, SD = 12.4 vs. 41.0, SD = 10.3, p = 0.09). Four patients and none of the HV had a urine toxicology test positive for THC. SSD patients had been hospitalized a median of 5 times in their lifetime and all of them were on antipsychotic treatment. Twelve (44.4%) of SSD patients were on antidepressants, 4 (14.8%) were on mood stabilizers and eight (29.6%) were on benzodiazepines. Expectedly, SSD patients had higher BMI (29.2 vs. 25.9, p = 0.03) and a lower MCCB composite score (25.9 vs. 45.4, p < 0.0001) compared to HV (Table 1). Another partially overlapping group of 64 subjects were included in the plasma analysis (33 SSD and 31 HV) (Supplementary Table 2). Twenty-four HV and 23 SSD patients provided data for both CSF and plasma analysis.
Table 1.
Baseline characteristics of subjects—cerebrospinal fluid analysis.
| Overall sample (N = 64) |
SSD (n = 32) |
Healthy volunteers (n = 32) |
p value | |
|---|---|---|---|---|
| Age, mean (SD) | 38.5 (11.6) | 36.1 (12.4) | 41.0 (10.3) | 0.09 |
| Male sex, n (%) | 45 (70.3) | 25 (78.1) | 20 (62.5) | 0.17 |
| Non-hispanic ethnicity, n (%) [sample size] | 30 (85.7) [35] | 11 (78.6) [14] | 19 (90.5) [21] | 0.3 |
| Race | 0.56 | |||
| Black, n (%) | 28 (43.8) | 15 (46.9) | 13 (40.6) | |
| White, n (%) | 21 (31.3) | 8 (25.0) | 13 (40.6) | |
| Other, n (%) | 15 (22.4) | 9 (25.7) | 6 (18.8) | |
| Diagnosis | NA | |||
| Schizophrenia, n (%) | NA | 19 (59.4) | NA | |
| Schizoaffective disorder, n (%) | NA | 10 (31.3) | NA | |
| Psychosis NOS, n (%) | NA | 3 (9.4) | NA | |
| BPRS score, mean (SD) | NA | 29.8 (8.6) [29] | NA | NA |
| Number of hospitalizations, median (IQR) | 5 (2–8.5) [20] | NA | NA | |
| Length of illness, median years (IQR) | NA | 14.0 (3.1–25.7) [21] | NA | NA |
| Medications | ||||
| Antipsychotics, n (%) | NA | 27 (100) [27] | NA | NA |
| Antidepressants, n (%) | NA | 12 (44.4) [27] | NA | NA |
| Mood stabilizers, n (%) | NA | 4 (14.8) [27] | NA | NA |
| Benzodiazepines, n (%) | NA | 8 (29.6) [27] | NA | NA |
| THC + in Utox, n (%) | 4 (6.5) [62] | 4 (13.3) [30] | 0 [30] | 0.03 |
| BMI, mean (SD) | 27.5 (5.7) [57] | 29.2 (7.1) [28] | 25.9 (3.3) [29] | 0.03 |
| MCCB composite score, mean (SD) | 35.6 (16.2) [60] | 25.9 (15.2) [30] | 45.4 (10.4) [30] | <0.0001 |
NOS not otherwise specified, BPRS Brief Psychiatric Rating Scale, THC tetrahydrocannabinol, Utox urine toxicology test, BMI body mass index, MCCB Matrics Consensus Cognitive Battery.
[ ]: number of subjects if different from group sample size.
CSF analysis
Mean C4 levels were 4278.5 ng/ml (SD = 2284.1) in the SSD group and 3641.1 ng/ml (SD = 1673.9) in HV (t = −1.2675, p = 0.21). The multivariable linear regression analysis showed that being a SSD patient was associated with a significant increase in 919.4 ng/ml in CSF C4 levels compared to HV (p = 0.035) after adjusting for age (p < 0.0001) and sex (p = 0.02) (n = 64, R2: 0.37, p < 0.0001). The same multivariable analysis showed that being a male was associated with an increase in 1050.0 ng/ml in CSF C4 levels (p = 0.02) and that for each increase in 1 year of age CSF C4 value levels increase 90.6 ng/ml (p < 0.0001) (Table 2). An interaction between subject type (SSD vs. HV) and age was tested by introducing an interaction term in the model but it was not statistically significant (subject type × age: p value = 0.37). In addition, we computed semi-partial correlation coefficients and found that age explains the largest part of the C4 CSF variance (sr2 = 0.29) whereas subject type (SSD vs. HV) and sex explain a similar amount of the variance (sr2 = 0.07 and 0.08 respectively).
Table 2.
Predictors of CSF C4 levels—multivariable linear regression analysis.
| Coefficient (B) | SE | 95% CI | t | p value | |
|---|---|---|---|---|---|
| Subject type (SSD vs. HV) | 919.4 | 427.3 | 64.8–1774.0 | 2.15 | 0.035 |
| Sex (male vs. female) | 1050.0 | 457.8 | 134.3–1965.8 | 2.29 | 0.025 |
| Age (years) | 90.6 | 18.3 | 53.9–127.2 | 4.94 | <0.001 |
CSF cerebrospinal fluid, SSD schizophrenia spectrum disorder patients, HV healthy volunteers.
N = 64, F = 11.68, p < 0.0001, R2 = 0.37.
Correlations with age, illness variables, and psychopathology
C4 levels in CSF were significantly correlated with age (r = 0.49, p < 0.0001) (Fig. 1) and this was observed in both SSD patients (r = 0.54, p = 0.0015) (Supplementary Fig. 1) and HV (r = 0.55, p = 0.0010) (Supplementary Fig. 2). On the other hand, within the SSD group, there were no significant correlations between CSF C4 levels and duration of illness (r = 0.33, p = 0.17), total BPRS score (r = 0.09, p = 0.66), number of hospitalizations (spearman rho = 0.39, p = 0.10) or antipsychotic dose expressed as chlorpromazine equivalents (spearman rho = 0.20, p = 0.29). Similarly, there were no significant correlations with BMI or MCCB composite or domain scores.
Fig. 1. Correlation between CSF C4 levels and age.
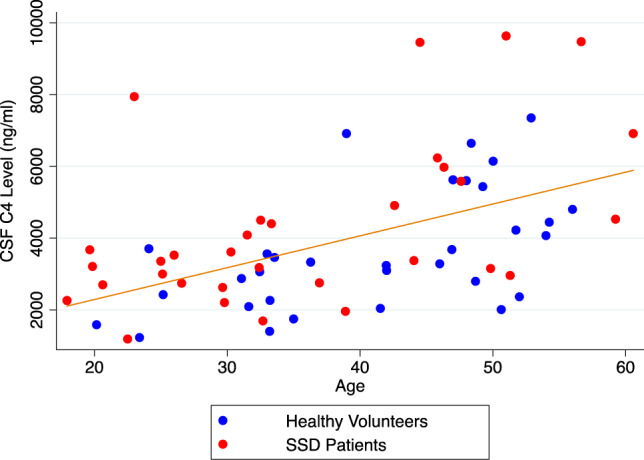
This figure shows a statistically signficant positive correlation between CSF C4 levels and age (Pearson’s correlation coefficient: 0.49, p < 0.0001, n = 64). Red dots depict schizophrenia spectrum disorder patients and blue dots depict healthy volunteers.
Plasma analysis
Mean C4 levels were 398.9 µg/ml (SD = 145.1) in the SSD group and 388.1 µg/ml (SD = 145.1) in HV (N = 64, t = −0.30, p = 0.77). The multivariable linear regression analysis showed that an SSD diagnosis was not significantly associated with plasma C4 levels (p = 0.25) after adjusting by sex and age (n = 64, R2: 0.19, p = 0.005) (Supplementary Table 2). In addition, plasma C4 levels were not correlated with age in the overall group (r = 0.1, p = 0.42). There were no correlations between plasma C4 levels and BMI or cognitive domains.
Correlation between CSF and plasma levels
C4 levels between CSF and plasma in all subjects were not significantly correlated (n = 47, r = −0.11, p = 0.45) (Fig. 2). Similarly, this correlation remained nonsignificant when looking separately at the SSD group (n = 23, r = 0.03, p = 0.90) and the HV group (n = 24, r = −0.29, p = 0.16).
Fig. 2. Correlation between CSF and plasma C4 levels.
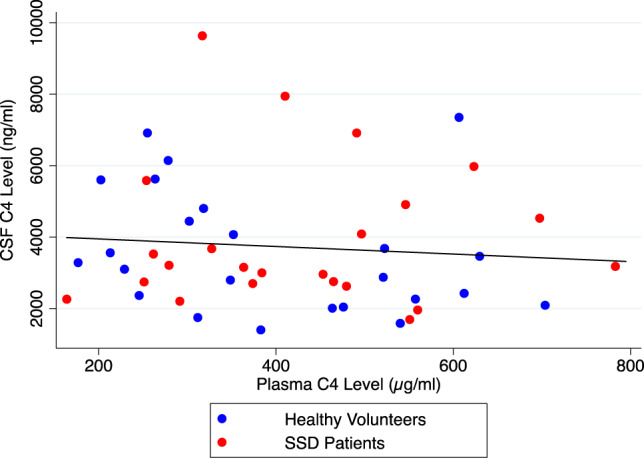
This figure shows a lack of correlation between CSF and plasma C4 levels (Pearson’s correlation coefficient: −0.11, p = 0.45, n = 47). Red dots depict schizophrenia spectrum disorder patients and blue dots depict healthy volunteers.
Discussion
To our knowledge, this is the first study that examined C4 levels in the CSF of patients with schizophrenia. Despite the fact that we did not find a statistically significant difference in CSF C4 levels between groups on a t test, we did find a statistically significant increase in CSF C4 levels between the groups after adjusting for sex and age. These findings are in contrast with results from plasma in which we did not observe significant differences in C4 levels between groups. Importantly, we were able to investigate the correlation between CSF and plasma levels and we observed that they were poorly correlated, which has implications for future studies and the interpretation of plasma results.
Given that there has not been another study investigating C4 levels in CSF in schizophrenia, we cannot directly compare our findings to other studies. However, our findings provide further support to the findings by Sekar et al. [2] and Kaminaki et al. [6] by showing C4 abnormalities in CSF of patients with schizophrenia. Unfortunately, we did not have genetic data to allow us to compare haplotype findings with those from Sekar et al. [2] but we hypothesize that schizophrenia patients with a higher number of copy number variants of the C4A gene would demonstrate a higher amount of C4 protein in CSF, as already demonstrated by Kaminaki et al. [6] but with HV. An important development would be to determine the relationship between C4A allele frequency and patient outcomes. Are those with higher frequency of C4A alleles be less responsive to treatment, more impaired cognitively, or less able to function in society?
A critical finding of our study is the very poor correlation between CSF and plasma levels of C4. Since we are the first group to report CSF C4 levels we cannot compare this finding to other groups. Therefore, a replication of this finding is crucial. Furthermore, even though measuring complement molecules in CSF may provide a more accurate picture of brain complement abnormalities, it is possible that measuring complement molecules in peripheral blood may better capture systemic complement abnormalities. Thus, we consider the use of CSF and blood as complimentary approaches in the study of complement abnormalities in schizophrenia.
Our finding that CSF C4 levels were highly positively correlated with age in both SSD patients and HV suggests that this correlation is independent from illness factors (e.g., duration of illness or cumulative antipsychotic exposure) but instead associated with aging. In this regard, Gaya da Costa et al. [7] examined serum samples of healthy individuals and found that the activities of the classical and the alternative pathway of the complement were higher in elderly subjects. Interestingly, they found a significant positive correlation between C5, C8, and C9 and age but did not find a significant correlation between serum C4 values and age. This is in agreement with our plasma results in which we did not find a significant correlation between plasma C4 levels and age. Furthermore, and in agreement with our findings, Kamitaki et al. [6] found that CSF C4 levels increased with age but noted that males had higher levels compared to females particularly in the reproductive years. Although not totally clear, this increase in C4 levels with age is likely multifactorial and partially the result of metabolic changes associated with age or as a compensatory mechanism for the impaired clearance of pathogens and cells due to a less active cellular immunity associated with aging [7].
Limited data are available in regards to the impact of antipsychotic medications on C4 levels. We could not find in the literature a study comparing C4 levels pre- and post-antipsychotic treatment. In a naturalistic longitudinal study, Mondelli et al. [14] investigated baseline serum levels of C4 as a predictor of response in first episode psychosis at a 1-year follow-up. However, follow-up C4 levels were either not measured or not reported, not allowing us to determine what impact antipsychotic treatment had on baseline levels. Indirect ways to determine the impact of treatment on C4 levels include comparing baseline C4 levels in antipsychotic-free vs. antipsychotic-treated patients. In that regard, Idonije et al. [18] compared C4 levels and did not find any different between antipsychotic-naive schizophrenia patients compared to medicated schizophrenia patients. Conversely, Maes et al. [19] found higher C4 plasma levels in antipsychotic-free schizophrenia patients compared to healthy controls but he did not see a difference between medicated schizophrenia patients and healthy controls. In our study, since all of our patients were on antipsychotic treatment and given that our study had a cross-sectional design, we were not able to directly determine the impact of antipsychotic treatment in our findings. However, we investigated the correlation between antipsychotic dose (chlorpromazine equivalents) and CSF C4 levels but this correlation was not significant. Therefore, new studies comparing C4 levels pre and post-antipsychotic treatment are required.
Strengths of our work include being the first group to report on C4 levels in CSF, the analysis of concomitant peripheral blood samples collected from the same subjects, and the incorporation of sex, age, and other relevant variables in our analysis. On the other hand, our study was limited due to the relatively small sample size, as usually observed in single site CSF studies. Furthermore, the HV group had older subjects and a higher proportion of females compared to SSD patients which, along with a relatively small sample size, might have decreased our power to detect differences between groups and increased the possibility of type 1 error. However, after adjusting for the potential effects of sex and age in CSF C4 levels in a multivariable analysis, we were able to observe a statistically significant difference between SSD patients and HV. Another limitation includes that the Abcam ELISA kits we used for the study were not designed to measure C4 fractions such as C4A or C4B. This is important since it was the greater expression of C4A alleles that was associated with schizophrenia diagnosis [2]. Similarly, the CSF and blood Abcam kits used for the analysis have different assay sensitivities which makes comparisons between CSF and blood results more difficult to interpret. On the other hand, CSF assays require higher assay sensitivities given the overall lower amount of substrates/molecules in CSF compared to peripheral blood. In addition, we were limited by our lack of genetic data, which would have helped us determine the impact of C4 schizophrenia risk variants in our CSF and blood data. We also lacked smoking data; however, we were able to look at the correlation between BMI and CSF C4 levels but this correlation was not statistically significant. Similarly, the vast majority of our subjects had negative urine toxicology tests, so we were not able to look at the impact of substance use in the outcomes of our study.
Future studies should look to replicate this CSF and plasma findings in an independent sample. In addition, investigators should complement their studies by measuring C4 subfractions (C4A, C4B), C3, C1q, inflammatory markers, and oxidative stress markers. Unfortunately, the number of groups conducting CSF research in schizophrenia is very small for us to be able to conduct an independent replication of this findings. Therefore, the creation of a consortium with the inclusion of several academic institutions is paramount to ensure a larger sample size while keeping a consistent CSF processing protocol across sites. The Alzheimer’s Disease Neuroimaging Initiative [20], which collected CSF samples across different sites would be an example to follow.
Funding and disclosure
This study was supported in parts by a NARSAD Young Investigator Grant (PI: JAG) from the Brain & Behavior Research Foundation and a K23MH100264 from the National Institute of Mental Health (PI: JAG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Brain & Behavior Research Foundation. JAG participated in an Alkermes advisory board. TL, EAB, and CM have nothing to disclose. AKM is a consultant to Genomind Inc, InformedDNA, and Janssen.
Supplementary information
Acknowledgements
Thanks to Juan Cordero and Yuan-Shan Zhu at the CTSC Weill Cornell Medicine.
Author contributions
JAG collected all data, performed statistical analyses, and wrote the paper. EAB and CM assisted with data collection, data cleaning, and reviewed critically the paper. TL and AKM assisted with study design, data interpretation, and revised the paper critically for important intellectual content.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00867-6).
References
- 1.Woo JJ, Pouget JG, Zai CC, Kennedy JL. The complement system in schizophrenia: where are we now and what’s next? Mol Psychiatry. 2020;25:114–30. doi: 10.1038/s41380-019-0479-0. [DOI] [PubMed] [Google Scholar]
- 2.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N,, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelik A, Sapir T, Haffner-Krausz R, Olender T, Woodruff TM, Reiner O. Developmental activities of the complement pathway in migrating neurons. Nat Commun. 2017;8:15096. doi: 10.1038/ncomms15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muraki K, Tanigaki K. Neuronal migration abnormalities and its possible implications for schizophrenia. Front Neurosci. 2015;9:74. doi: 10.3389/fnins.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–85. doi: 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamitaki N, Sekar A, Handsaker RE, de Rivera H, Tooley K, Morris DL,, et al. Complement genes contribute sex-biased vulnerability in diverse disorders. Nature. 2020;582:577–81. doi: 10.1038/s41586-020-2277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaya da Costa M, Poppelaars F, van Kooten C, Mollnes TE, Tedesco F, Würzner R, et al. Age and sex-associated changes of complement activity and complement levels in a healthy caucasian population. Front Immunol. 2018;9:2664. doi: 10.3389/fimmu.2018.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson B, Hamad OA, Ahlström H, Kullberg J, Johansson L, Lindhagen L, et al. C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors. Eur J Clin Investig. 2014;44:587–96. doi: 10.1111/eci.12275. [DOI] [PubMed] [Google Scholar]
- 9.Laskaris L, Zalesky A, Weickert CS, Di Biase MA, Chana G, Baune BT,, et al. Investigation of peripheral complement factors across stages of psychosis. Schizophr Res. 2019;204:30–7. doi: 10.1016/j.schres.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Zhou FC, Lee JWY, Zhang QH, Sun ZL, Bo Q, He XX, et al. Higher serum C-reactive protein levels in catatonic patients: a comparison to non-catatonic patients and healthy controls. Schizophr Bull. 2020.10.1093/schbul/sbaa041. pii: sbaa041. [DOI] [PMC free article] [PubMed]
- 11.Melbourne JK, Rosen C, Feiner B, Sharma RP. C4A mRNA expression in PBMCs predicts the presence and severity of delusions in schizophrenia and bipolar disorder with psychosis. Schizophr Res. 2018;197:321–7. doi: 10.1016/j.schres.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopczynska M, Zelek W, Touchard S, Gaughran F, Di Forti M, Mondelli V, et al. Complement system biomarkers in first episode psychosis. Schizophr Res. 2019;204:16–22. doi: 10.1016/j.schres.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohoe G, Holland J, Mothersill D, McCarthy-Jones S, Cosgrove D, Harold D, et al. Genetically predicted complement component 4A expression: effects on memory function and middle temporal lobe activation. Psychol Med. 2018;48:1608–15. doi: 10.1017/S0033291717002987. [DOI] [PubMed] [Google Scholar]
- 14.Mondelli V, Di Forti M, Morgan BP, Murray RM, Pariante CM, Dazzan P. Baseline high levels of complement component 4 predict worse clinical outcome at 1-year follow-up in first-episode psychosis. Brain Behav Immun. 2020;S0889-1591:31082–7. doi: 10.1016/j.bbi.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Brown E, Shah P, Kim J. Using chlorpromazineR to calculate chlorpromazine-equivalent doses. 2019. https://cran.r-project.org/web/packages/chlorpromazineR/vignettes/walkthrough.html. Accesed 8 July 2020.
- 16.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 17.Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42:S90–4. doi: 10.1093/schbul/sbv167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idonije OB, Akinlade KS, Ihenyen O, Arinola OG. Complement factors in newly diagnosed Nigerian schizoprenic patients and those on antipsychotic therapy. Niger J Physiol Sci. 2012;27:19–21. [PubMed] [Google Scholar]
- 19.Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/s0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
- 20.Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. Swedish BioFINDER study group; Alzheimer’s disease neuroimaging initiative. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14:1470–81. doi: 10.1016/j.jalz.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


