Abstract
Adolescent exposure to chronic stress, a risk factor for mood disorders in adulthood, sensitizes the neuroinflammatory response to a subsequent immune challenge. We previously showed that chronic adolescent stress (CAS) in rats led to distinct patterns of neuroimmune priming in adult male and female rats. However, sex differences in the neuroimmune consequences of CAS and their underlying mechanisms are not fully understood. Here we hypothesized that biological sex would dictate differential induction of inflammation-related transcriptomic pathways and immune cell involvement (microglia activation and leukocyte presence) in the hippocampus of male and female rats with a history of CAS. Adolescent rats underwent CAS (six restraint and six social defeat episodes during postnatal days 38–49), and behavioral assessments were conducted in adolescence and adulthood. Neuroimmune measures were obtained following vehicle or a systemic lipopolysaccharide (LPS) challenge in adulthood. CAS led to increased time in the corners of the open field in adolescence. In males, CAS also increased social avoidance. As adults, CAS rats displayed an exaggerated enrichment of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway and chemokine induction following LPS challenge, and increased number of perivascular CD45+ cells in the hippocampus. However, CAS females, but not males, showed exaggerated glucocorticoid receptor (GR) pathway enrichment and increased microglial complexity. These results provide further insight to the mechanisms by which peripheral immune events may influence neuroimmune responses differentially among males and females and further demonstrate the importance of adolescent stress in shaping adult responses.
Subject terms: Neuroimmunology, Stress and resilience, Emotion
Introduction
Appreciation has grown for the plausible role of neuroimmune sequalae to contribute to sex differences in neuropsychiatric diseases and stress-related disorders [1]. These sex differences emerge with the onset of puberty, thus highlighting adolescence as a possible critical period for diverging consequences of stress exposure among males and females. In humans, stress during adolescence is associated with a subsequent pro-inflammatory phenotype that may be linked to psychiatric illnesses [2, 3]. In addition, chronic stress plays an instrumental role by exaggerating immune reactivity in brain regions involved in regulating the stress response, including the hippocampus [4, 5]. Transcriptomic studies reveal excessive immune signaling in the hippocampus of depressed individuals [6] as well as rodents that have experienced chronic stress [7, 8]. Furthermore, some of these differences are sex-specific [9], mirroring the influence of sex on transcriptomic profiles of humans with depression [10]. There are also baseline sex differences in immune-related genes [11], suggesting a potential basis for sex differences in the stress-regulated neuroimmune transcriptome. Using a chronic adolescent stress (CAS) model in rats, here we sought to elucidate transcriptional pathways differentially impacted by CAS in male and female rats, with a focus on inflammatory and endocrine pathways known to be altered by chronic stress.
Using RNA-Seq, we assessed changes to nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and glucocorticoid receptor (GR) signaling pathways—primary mediators of the innate immune system and HPA axis, respectively [12],—to probe their potential involvement in CAS-induced neuroimmune priming [4]. Deficits in GR signaling, which are implicated in stress-related disorders including depression [13], allow excessive inflammatory signaling to occur [12]. In turn, exaggerated NFκB signaling has been linked to the pathophysiology of early life stress [14, 15], bipolar disorder [16], post-traumatic disorder [15, 17], and major depressive disorder [14, 16]. Furthermore, building on our previous work demonstrating differential peripheral immune sensitization in males and females [18], we additionally sought to assess the sex-specific influence of CAS on promoting immune-to-brain cellular traffic. The neuroimmune network hypothesis proposes that early life adversity strengthens immune-to-brain crosstalk via sensitizing immune cells of the brain as well as myeloid cells in the periphery to subsequent immune activation [19]. Both the brain’s resident immune cells, microglia [20, 21], as well as peripheral monocytes and macrophages of the spleen [22, 23] and bone marrow [24] have been reported to assume a hyper-inflammatory and primed phenotype following various forms of stressors. Although stress-induced infiltration of leukocytes to the brain has been reported to occur in female mice [25–27], and microglia from stressed adult male and female rats display distinct morphological profiles [28], currently it is not clear whether there are sex differences in the extent to which adolescent stress dysregulates immune cells in the brain. Therefore, we also aimed to determine whether CAS differentially impacts the number, composition, and activation of immune cells in the hippocampus of males and females using complementary approaches.
Methods and materials
Animals
Male and female Wistar rats from Charles Rivers (Durham, NC) were bred to generate litters. Rats were housed on a 14:10 reverse light/dark cycle with standard rat chow and water available ad libitum. Litters were culled to four male and four female pups on postnatal day (PND) 3, and rats were weaned on PND 21. One hundred and seventeen male and female Wistar rats were used for RNA-sequencing (n = 47 total; 5–6/group) and quantitative PCR studies (n = 70 additional; 6–8/group) at Emory University (see Table S1). A separate group of rats raised from timed-pregnant dams (Charles River, Durham, NC) at Virginia Commonwealth University was used for behavioral (n = 52 total; 12–14/group), immunohistochemistry, and flow cytometry experiments (n = 49 total; 6–7/group). Throughout all experiments, rats were housed in AAALAC (American Association for Accreditation of Laboratory Animal Care)-accredited facilities, and all studies were approved by the Institutional Animal Care and Use Committees at the respective universities.
Chronic adolescent stress (CAS)
CAS was performed as detailed previously [4, 18, 29–36]. On PND35, rats were randomly assigned to non-stressed (NS) or CAS groups. CAS rats were individually housed, and underwent CAS per experimental timeline in Fig. 1A. The mixed-modality chronic stress paradigm consisted of six random exposures to social defeat and restraint stress each, which took place across 12 days spanning mid-adolescence in the rat (PND38–49). For restraint stress, each Wistar rat was placed in a clear plexiglass restraint tube (Braintree Scientific, Braintree, MA) for 1 h. In the social defeat paradigm, each Wistar rat was placed in the home cage of a same-sex, adult Long Evans rat that is trained to demonstrate aggressive behavior toward the experimental rat. During the first 2 min of social defeat (habituation), the two rats were separated by a perforated, clear barrier that allows visual and olfactory cues. The barrier was subsequently removed, and rats were observed for 5 min of physical interaction or three pins, whichever came first, followed by an additional 25 min separated by the barrier. Upon completion of the stress paradigm (a total of 12 restraint/social defeat sessions), rats were allowed to mature into adulthood without further stressor exposure.
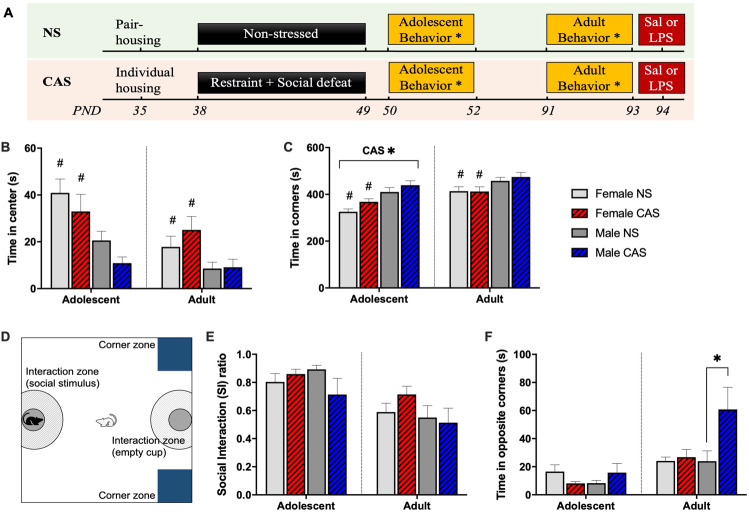
Fig. 1. Chronic adolescent stress (CAS) led to anxiety-like behavior and male-specific social avoidance.
A Female and male rats of NS or CAS background were behaviorally tested once in adolescence on postnatal day (PND)50, and again in adulthood on PND91. B Adolescent CAS rats displayed no change in time spent in the center, but (C) a significant increase in time spent in the corners (p < 0.05). D Social interaction arena setup is depicted. E CAS did not impact SI ratio in either sex when assessed in adolescence or adulthood. F CAS led to a significant increase in time spent in opposite corners in males (p < 0.05), but not in females. Data are presented as mean ± SEM. CAS*, main effect of CAS (p < 0.05); *, effect of CAS (p < 0.05) within males; # main effect of sex. CAS Chronic Adolescent Stress, NS Non-stressed, SI social interaction.
Behavioral assessments
Rats underwent open field and social interaction (SI) tests immediately following the end of the CAS paradigm (PND50–52), and again in adulthood (PND91–93) to assess anxiety-like and social avoidance behaviors as described in Supplementary. For open field test, the outcomes assessed were time spent in the center, and the corners of the open field. For SI test, the outcomes assessed were time spent in interaction zone of the cup containing a social stimulus and time spent in corners opposite from it.
Immune challenge in adulthood with lipopolysaccharide (LPS)
On PND94, 45 days after stress exposure concluded, all rats received a single intraperitoneal injection of either saline or LPS (L3880, Sigma Aldrich) (0.25 mg/kg; 750,000 Endotoxin Units). At 2 or 4 h after injection rats were anesthetized with Euthasol (150 mg/kg), and transcardially perfused with ice-cold PBS to prevent peripheral immune cells and inflammatory markers present in the blood from confounding outcomes measured in brain tissue (see experimental design in Fig. 1A).
RNA-Seq and quantitative PCR
Bulk RNA-Sequencing was performed by the Nonhuman Primate Genomics Core at Yerkes National Primate Research Center and analysis conducted as detailed in Supplementary. Briefly, the edgeR package was used for normalization and differential expression analysis [37]. Log2 expression of each gene was modeled as a function of sex, stress, LPS stimulus, and batch (date of sequencing). The Quasi-likelihood F Test was performed to test for differences in gene expression between these factors. The effect of CAS at baseline was assessed within each sex using the pairwise contrasts (1) Male-CAS-Saline (M.CAS.Sal) versus Male-NS-Saline (M.NS.Sal) and (2) Female-CAS-Saline (F.CAS.Sal) versus Female-NS-Saline (F.NS.Sal). Differentially expressed genes (nominal p < 0.05 and fold change > 1.2) from these pairwise comparisons were then used to perform GeneGo MetaCore (St. Joseph, MI, USA) pathway enrichment analyses in order to identify biological pathways differentially enriched at baseline (FDR < 0.05).
Moreover, pairwise contrasts of “LPS versus Saline” were conducted within each of (1) Female-NS, (2) Female-CAS, (3) Male-NS, and (4) Male-CAS groups to identify differentially expressed genes. To assess how a background of CAS impacts LPS-induced pathway enrichment compared to NS, Gene Set Enrichment Analysis (GSEA; Broad Institute) was conducted [38, 39]. To perform GSEA, we used the Molecular Signatures Database’s (MSigDB) Hallmark (H) collection, which includes 50 hallmark pathways representing well-defined biological processes, with the addition of the GR regulatory network (PID_REG_GR_PATHWAY) from MSigDB’s C2 curated collection: Pathway Interaction Database (NCI, NIH and Nature Publishing Group). Tables S2 and S3 list genes contained in GSEA pathways. qPCR was conducted as previously published [18] and described in Supplementary. Primer sequences are provided in Table S4.
Flow cytometry
To assess leukocyte infiltration to the hippocampus, flow cytometry was conducted as described in Supplementary and Fig S1.
Immunohistochemistry
Following saline perfusion, one hemisphere of the brain was post-fixed in 4% PFA for 48 h. Coronal sections (40 uM thickness) were stained with IBA-1 (Wako Chemicals USA Inc., Richmond, VA) to assess microglial count and morphology. Separately, sequential immunofluorescent staining with CD45 (Santa Cruz, Dallas, TX USA), RECA-1 (Abd Serptec; Hercules, CA, USA), and IBA-1 (Wako, Richmond, VA, USA) was performed to assess the perivascular presence of CD45+ leukocytes in the hippocampus as detailed in Supplementary.
Microglial count and morphology
The number of microglia in the hippocampus was estimated using unbiased stereology and microglial morphology was assessed using ImageJ (NIH) as described in Supplementary and Fig S2.
Statistical analysis
Analyses were conducted in SPSS 25.0, and detailed in Supplementary. Behavior, flow cytometry, stereology, qPCR, and perivascular CD45 data were analyzed via three-way ANOVAs modeling the main effect of sex, stress, and stimulus and their interactions.
Behavioral data from adolescence and adulthood were analyzed separately in order to assess the immediate and enduring impact of CAS. Significant interactions from ANOVA were followed up with Holm-Sidak post-hoc test. Microglial morphology data were analyzed via generalized estimating equation models to accommodate the clustered data structure (up to 24 cells analyzed per rat). Gene expression data are presented as mean fold change ± SEM. All other data are expressed as mean ± SEM. Significance threshold was set to α = 0.05 for all analyses.
Results
CAS leads to anxiety-like behavior in the open field in adolescence
In adolescence, CAS rats displayed no change in time spent in center (F(1,48) = 1.93, p = 0.171) (Fig. 1B) but a significant increase in time spent in the corners (F(1,48) = 4.73, p = 0.035) (Fig. 1C). The effect of CAS was no longer observed in adulthood (center: F(1,48) = 0.85, p = 0.363; corners: F(1,48) = 0.17, p = 0.679). Female rats spent more time in the center and less time in the corners of the open field compared to males in both adolescence (center: F(1,48) = 13.68, p < 0.001; corners: F(1,48) = 22, p < 0.001) and adulthood (center: F(1,48) = 8.88, p = 0.004; corners: F(1,48) = 8.33, p = 0.006).
CAS males display increased social avoidance
The SI test arena is shown in Fig. 1D. In adolescence, SI ratio was not impacted by sex (F(1,46) = 0.16, p = 0.69), CAS (F(1,46) = 0.78, p = 0.38), or their interaction (F(1,46) = 2.85, p = 0.098), and similar results were observed in adulthood (sex: F(1,46) = 2.1, p = 0.15; stress: F(1,46) = 0.29, p = 0.59, sex*stress: F(1,46) = 0.97, p = 0.33) (Fig. 1E). Time spent in opposite corners, a metric of social avoidance [40], was not impacted by sex (F(1,46) = 0.004, p = 0.95), CAS (F(1,46) = 0.01, p = 0.95), or their interaction (F(1,46) = 3.55, p = 0.066) in adolescence (Fig. 1F). However, in adulthood while sex did not significantly impact time spent in opposite corners (F(1,46) = 3.09, p = 0.085), CAS significantly increased time spent in opposite corners (F(1,46) = 4.19, p = 0.046) (Fig. 1F). Follow-up tests revealed that CAS led to increased time spent in opposite corners in males (t(46) = 2.81, p = 0.014), but not females (t(46) = 0.19, p = 0.851).
CAS sex-specifically alters baseline and LPS-induced enrichment of transcriptional pathways
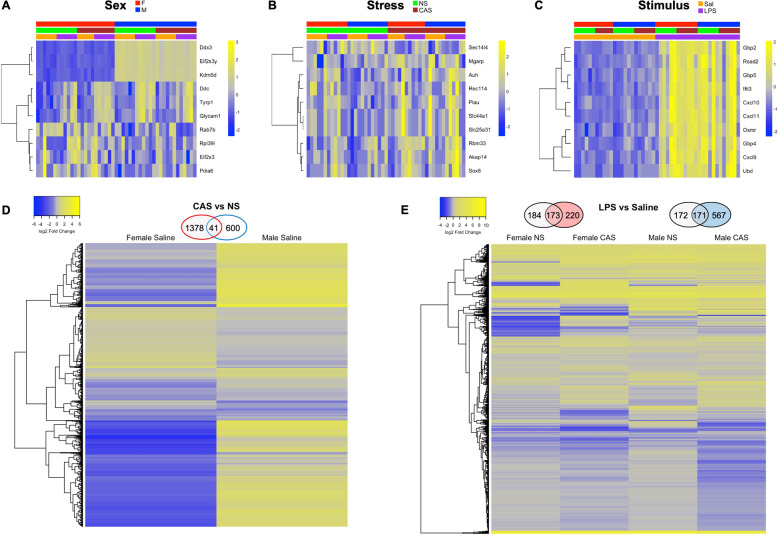
Differential expression analysis was conducted using edgeR by modeling the main effects of LPS, sex, and stress. Expression heatmaps of the top ten genes from each of the main effects are shown in Fig. 2A–C. Furthermore, pairwise comparisons assessed baseline (unstimulated) differences in gene expression between CAS rats and their NS litter mates, by sex (Fig. 2D). Female, CAS, and saline-injected rats (F.CAS.Sal) had 437 upregulated and 982 downregulated genes compared to Female, NS, saline rats (F.NS.Sal). Male, CAS, saline rats (M.CAS.Sal) had 559 upregulated and 82 downregulated genes compared to Male, NS, saline rats (M.NS.Sal). CAS-associated genes at baseline (Saline) were largely distinct between males and females with similar numbers of upregulated genes across males and females and greater number of downregulated genes in female rats. Genes differentially regulated by CAS in female rats enriched pathways related to signal transduction, histone deacetylases, and cytoskeleton remodeling. Genes differentially regulated by CAS in male rats were related to G protein-coupled receptor signaling, Notch signaling, and NMDA receptor trafficking. A complete list of genes from each contrast, along with the pathways they enrich, are shown in Table S5A–C.
Fig. 2. Chronic adolescent stress (CAS)-associated genes were largely distinct between females and males at baseline.
Female and male rats of NS or CAS background received a systemic injection of either saline or LPS in adulthood. Four hours later, hippocampal tissue was collected for bulk RNA-Seq. Differential expression analysis on the hippocampal transcripts was performed using the package edgeR in Bioconductor. A–C Expression of top ten DEGs are shown for the main factors of sex, stress, and LPS. D The impact of CAS on baseline (unstimulated) gene expression was suassessed within each sex using the contrasts “F.CAS.Sal versus F.NS.Sal” and “M.CAS.Sal versus M.NS.Sal.” The number of differentially expressed genes (DEG) from each paired contrast is displayed in Venn diagrams above the heatmaps. CAS-associated genes were largely distinct between males and females with a greater number of upregulated genes in female rats and similar numbers of downregulated genes across males and females. Subsequent pathway analyses showed that genes differentially regulated by CAS in female rats enriched pathways related to signal transduction, histone deacetylases, and cytoskeleton remodeling. Genes differentially regulated by CAS in male rats were related to G protein-coupled receptor signaling, Notch signaling, and NMDA receptor trafficking (Tables S5–6). E LPS-induced changes in gene expression were assessed within each of the four groups (Female-NS, Female-CAS, Male-NS, Male-CAS) using the contrast “LPS versus Sal.” Similar clusters of genes were differentially regulated by LPS across the four groups, with substantial DEG overlap between NS and CAS conditions within each sex. See GSEA pathway results in detail in Table 1, S7–10 and Fig S1. CAS Chronic Adolescent Stress, NS Non-stressed, Sal Saline.
LPS challenge led to differential expression of 357 genes in Female NS, 393 genes in Female CAS rats, with 173 genes common between the groups. In males, LPS challenge led to differential expression of 343 genes in NS, 738 genes in CAS rats, with 171 genes common between the groups (Fig. 2E). A complete list of genes from each contrast are shown in Table S6. To identify transcriptional pathways sensitized to immune activation by a background of CAS, we used Gene Set Enrichment Analysis (GSEA) [41]. In GSEA, we contrasted LPS-challenged and saline-treated rats within each of Female CAS, Female NS, Male CAS, and Male NS backgrounds. Acute immune challenge led to significant enrichment (nominal p < 0.05 and FDR q value < 0.1) of 21 pathways in female NS, 24 in female CAS, 23 in male NS, and 24 in male CAS (See Tables S7–10 for a list of pathways significantly enriched by LPS in each of the four groups). A qualitative comparison of the normalized enrichment scores (NES) of LPS-induced pathways across stressed and NS rats revealed that genes regulated by NFκB in response to Tumor Necrosis Factor (pathway name “TNFA_SIGNALING_VIA_NFκB”) was among the Top 10 pathways enriched to a greater extent in CAS compared to NS rats of either sex (Table 1). Pathways related to adipogenesis, myogenesis, apical junction, and MYC targets were also enriched to a greater extent in CAS rats of either sex. In CAS females, LPS challenge led to greater enrichment of the GR (“PID_REG_GR_PATHWAY”), early estrogen response, TGF-beta signaling, and p53 pathways compared to Female-NS rats. In CAS males, LPS challenge led to greater enrichment of interferon alpha and gamma response as well as metabolic pathways (glycolysis and xenobiotic metabolism) compared to NS males.
Table 1.
Top ten gene sets enriched to a greater extent in Chronic Adolescent Stress (CAS) rats compared to Non-Stressed (NS) rats following an acute immune challenge with LPS in adulthood.
| Gene set | Female CAS | Female NS | ΔNES | ||
|---|---|---|---|---|---|
| NES | FDR | NES | FDR | (CAS - NS) | |
| ADIPOGENESISa | 1.52 | 0.021 | 0.83 | 1 | 0.69 |
| APICAL_JUNCTIONa | 1.55 | 0.018 | 0.93 | 0.672 | 0.62 |
| ESTROGEN_RESPONSE_EARLY | 1.34 | 0.1 | 0.87 | 0.758 | 0.47 |
| TGF_BETA_SIGNALING | 1.43 | 0.049 | 0.98 | 0.744 | 0.45 |
| MYOGENESIS* | 1.35 | 0.098 | 0.95 | 0.671 | 0.4 |
| PID_REG_GR_PATHWAY | 1.88 | 0.001 | 1.57 | 0.015 | 0.31 |
| UV_RESPONSE_UP | 1.65 | 0.009 | 1.37 | 0.079 | 0.28 |
| TNFA_SIGNALING_VIA_NFKBa | 2.71 | 0 | 2.48 | 0 | 0.23 |
| MYC_TARGETS_V2a | 1.51 | 0.024 | 1.29 | 0.142 | 0.22 |
| P53_PATHWAY | 1.86 | 0.001 | 1.72 | 0.003 | 0.14 |
| Gene set | Male CAS | Male NS | ΔNES | ||
|---|---|---|---|---|---|
| NES | FDR | NES | FDR | (CAS - NS) | |
| ADIPOGENESISa | 1.62 | 0.009 | 0.59 | 1 | 1.03 |
| MYOGENESISa | 1.83 | 0.001 | 0.95 | 0.709 | 0.88 |
| MYC_TARGETS_V2a | 1.58 | 0.013 | 0.93 | 0.727 | 0.65 |
| INTERFERON_GAMMA_RESPONSE | 3.6 | 0 | 3.12 | 0 | 0.48 |
| TNFA_SIGNALING_VIA_NFKBa | 2.87 | 0 | 2.45 | 0 | 0.42 |
| KRAS_SIGNALING_UP | 1.94 | 0.001 | 1.52 | 0.027 | 0.42 |
| GLYCOLYSIS | 1.45 | 0.035 | 1.07 | 0.505 | 0.38 |
| XENOBIOTIC_METABOLISM | 1.84 | 0.001 | 1.49 | 0.035 | 0.35 |
| APICAL_JUNCTIONa | 1.66 | 0.007 | 1.31 | 0.114 | 0.35 |
| INTERFERON_ALPHA_RESPONSE | 3.27 | 0 | 2.95 | 0 | 0.32 |
Using the Molecular Signatures Database’s Hallmark (H) gene set in Gene Set Enrichment Analysis (GSEA), differential enrichment of 50 “hallmark” pathways representing well-defined biological processes as well as a gene set for glucocorticoid receptor regulatory network from Pathway Interaction Database (NCI, NIH and Nature Publishing Group) was assessed between LPS-challenged and saline-treated rats of Female CAS, Female NS, Male CAS, and Male NS backgrounds. Of the significantly enriched gene sets, Top 10 pathways with the largest difference in NES (ΔNES) between CAS and NS groups are shown for each sex.
CAS chronic adolescent stress, NS non-stressed, NES normalized enrichment score, FDR false discovery rate.
aPathways whose enrichment is exaggerated by CAS in both males and females.
CAS exaggerates LPS-induced enrichment of the NFκB signaling pathway and expression of NFκB subunits and regulators
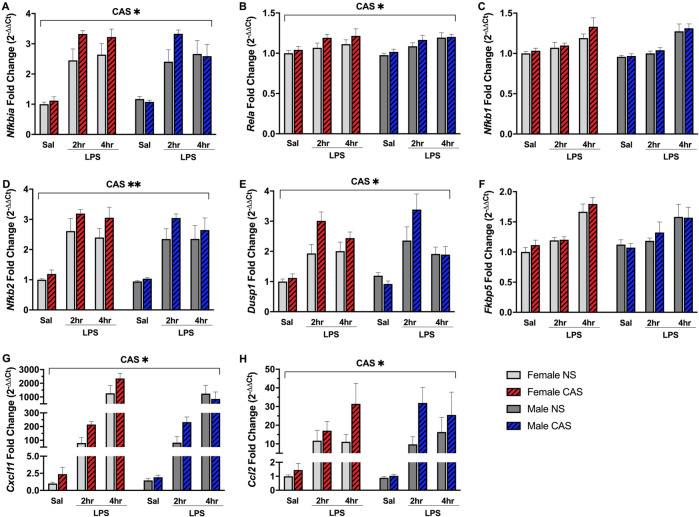
As indicated by the GSEA results in Table 1, the magnitude of TNFA_SIGNALING_VIA_NFκB pathway enrichment following LPS challenge revealed a greater cumulative increase in CAS male and female rats (NES of 2.87 and 2.71) compared to same-sex NS controls (NES of 2.45 and 2.48). Several members of the NFκB transcriptional complex including Nfkbia, Nfkb1, and Nfkb2 were among the “leading-edge” genes driving the enrichment of the TNFA_SIGNALING_VIA_NFκB pathway. Because prior exposure to stressors or stress hormones can alter the inflammatory response to an immune challenge [42], we assessed a time course of NFκB response to LPS via targeted qPCR, focusing on mRNA expression of canonical (Nfkbia, Nfkb1, Rela) and non-canonical (Nfkb1, Nfkb2) cascade subunits. LPS stimulus induced the mRNA expression of Nfkbia (F(2,105) = 103.84, p < 0.001), Rela (F(2,105) = 15.21, p < 0.001), Nfkb1 (F(2,105) = 38.66, p < 0.001), and Nfkb2 (F(2,105) = 120.24, p < 0.001) (Fig. 3A–D). There was no effect of sex on Nfkbia, Rela, Nfkb1, and Nfkb2 (p > 0.177). CAS rats displayed exaggerated hippocampal mRNA expression of Nfkbia (F(1,105) = 4.21, p = 0.043), Rela (F(1,105) = 5.26, p = 0.024), and Nfkb2 (F(1,105) = 8.19, p = 0.005) compared to NS rats. CAS did not impact the expression of Nfkb1 (F(1,105) = 2.72, p = 0.102).
Fig. 3. Chronic Adolescent Stress (CAS) exaggerated the expression of NFκB subunits and chemokines.
Female and male rats of NS or CAS background received a systemic injection of either saline or LPS in adulthood. Two and four hours later, mRNA expression of NFκB pathway members Nfkbia, Rela, Nfkb1, and Nfkb2 (A–D), GR targets Dusp1 and Fkbp5 (E–F), and the chemokines Ccl2 and Cxcl11 (G–H) were assessed via quantitative PCR. NFκB pathway: CAS rats displayed exaggerated hippocampal mRNA expression of (A) Nfkbia, (B) Rela, and (D) Nfkb2 compared to NS rats (all p < 0.05). CAS did not significantly impact the expression of (C) Nfkb1. GR pathway: CAS potentiated the expression of (E) Dusp1 (p < 0.05). CAS did not alter the expression of (F) Fkbp5. Chemokines: CAS exaggerated the expression of (G) Ccl2 and (H) Cxcl11 (all p < 0.05). Data are mean fold change ± SEM. Main effect of CAS is denoted by CAS* (p < 0.05) or CAS** (p < 0.01). CAS Chronic Adolescent Stress, NS Non-stressed, LPS lipopolysaccharide, Sal Saline.
CAS potentiates GR signaling in the hippocampus of female rats
Per Table 1, the magnitude of the GR regulatory network pathway (PID_REG_GR_PATHWAY) enrichment following LPS challenge was greater in CAS females (NES = 1.88) compared to that of NS females (NES = 1.57) (Table 1). However, the GR regulatory network was not enriched to a greater degree in CAS males (NES = 0.84) compared to NS males (NES = 1.56). We examined expression of several GR-inducible genes via qPCR to assess post-LPS time course of CAS-mediated changes in GR transcriptional activity. These included the anti-inflammatory gene Dusp1 (encoding dual specificity phosphatase 1 which inhibits the MAPK signaling pathway [43]), and GR regulator gene Fkbp5 (FK506-binding protein 5) which is implicated in major depressive disorder [44]. Additional GR-inducible genes were Tsc22d (glucocorticoid-induced leucine zipper), Sgk1 (Serum/Glucocorticoid Regulated Kinase 1), and Nr3c1 (GR). LPS increased the expression of Dusp1 (F(2,105) = 59.32, p < 0.001), Fkbp5 (F(2,105) = 17.09, p < 0.001) (Fig. 3E, F), as well as Tsc22d3 (F(2,105) = 15.98, p < 0.001), Sgk1 (F(2,105) = 36.4, p < 0.001), and Nr3c1 (F(2,105) = 6.83, p = 0.002). There was no effect of sex on Dusp1, Fkbp5, Tsc22d3, Sgk1, or Nr3c1 (p > 0.62). CAS increased expression of Dusp1 (F(1,105) = 4.17, p = 0.044), but not Fkbp5 or Tsc22d3, Sgk1, or Nr3c1 (p > 0.266).
CAS potentiates chemokine signaling and increases perivascular myeloid cell presence in the hippocampus
Several of the leading-edge genes driving the exaggerated enrichment of the HALLMARK_TNFA_SIGNALING_VIA_NFκB pathway in CAS rats were genes related to chemokine signaling (e.g., Cxcl11, Ccl4, Ccl2, Cxcl10, Ccrl2) which play a key role in recruiting peripheral immune cells to sites of injury or inflammation. Therefore we measured the mRNA expression of Ccl2 and Cxcl11—two chemokines which have been implicated in stress-related pathology [4, 45, 46]. LPS strongly induced mRNA expression of Ccl2 (F(2,105) = 83.02, p < 0.001) and Cxcl11 (F(2,105) = 237.22, p < 0.001) (Fig. 3G, H). There was no effect of sex on expression of either chemokine (p > 0.657). CAS exaggerated the hippocampal mRNA expression of both Ccl2 (F(1,105) = 7.04, p = 0.009) (Fig. 3G) and Cxcl11 (F(1,105) = 4.2, p = 0.043) (Fig. 3H).
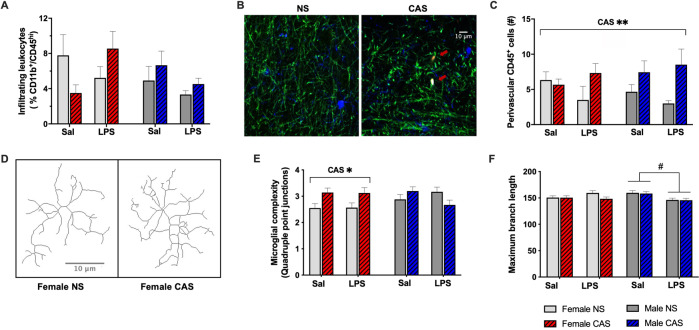
The extent of leukocyte infiltration was measured via flow cytometry and expressed as percent of CD11b+/CD45high myeloid cells, which include monocytes and neutrophils, relative to CD11b+ cells (gating shown in Fig S1). The proportion of infiltrating leukocytes was not impacted by sex (F(1,40) = 1.78, p = 0.189), CAS (F(1,40) = 0.22, p = 0.64), or LPS (F(1,40) = 0.09, p = 0.771) (Fig. 4A). Immunohistochemical experiments revealed that CAS increased the number of CD45+ cells in the perivascular area (F(1,36) = 8.52, p = 0.006) (Fig. 4B, C). There was no main effect of sex (F(1,36) = 8.52, p = 0.006) or stimulus (F(1,36) = 8.52, p = 0.006) on the number of perivascular CD45+ cells.
Fig. 4. CAS increased perivascular CD45 presence and sex-specifically alters microglial morphology in the hippocampus.
Female and male rats of NS or CAS background received a systemic injection of either saline or LPS in adulthood on PND94. Two hours later, leukocyte infiltration, perivascular CD45 immunoreactivity, and microglial morphology were assessed in the hippocampus. A The percent of CD11b+/CD45high infiltrating myeloid cells was not impacted by sex, stress, or LPS stimulus. B Representative CD45 staining (red, indicated by arrow) in close proximity to blood vessels (green) and distinct from microglia (blue) in the hippocampus of NS and CAS rats. C CAS increased CD45 immunoreactivity in the hippocampus (p < 0.01). D Representative microglia skeletons are shown to illustrate differential microglial complexity between females NS and CAS groups. E CAS increased microglial complexity within females (p < 0.05), but not males. F LPS decreased maximum branch length of microglia in males (p < 0.05), but not females. Data are presented as mean ± SEM. **CAS, main effect of CAS (p < 0.01); *CAS, effect of CAS (p < 0.05) within females; #, LPS effect within males. CAS Chronic Adolescent Stress, NS Non-stressed, LPS lipopolysaccharide, Sal Saline.
CAS increases microglial complexity in the female hippocampus
To assess the impact of CAS on the resident immune cells in the brain, we examined microglial morphology and total number in the hippocampus. There was a significant sex*stress interaction on microglial morphological complexity, as indicated by the number of quadruple points created by intersecting branches (Wald Χ2 = 6.28, df = 1, p = 0.012). Specifically, CAS increased morphological complexity in females (mean difference = 0.67, SE = 0.22, p = 0.003), but not males (mean difference = −0.22, SE = 0.27, p = 0.4) (Fig. 4D, E). In addition, a sex*stimulus interaction was observed for maximum branch length (Wald Χ2 = 5.63, df = 1, p = 0.018). Specifically, LPS led to a decrease in maximum branch length within males (mean difference = −8.84, SE = 3.82, p = 0.021) but not within females (mean difference = 2.92, SE = 3.24, p = 0.368) (Fig. 4F). The estimated number of microglia in the hippocampus was not impacted by sex (F(1,40) = 2.78, p = 0.103), stress (F(1,40) = 0.74, p = 0.396), or LPS (F(1,40) = 2.16, p = 0.15).
Discussion
Together, the data presented here indicate that chronic exposure to adolescent stress leads to persistent behavioral and immunophenotypes that manifest differentially in males and females. Previously, CAS has been shown to lead to female-specific depressive-like behavior [31] immediately following CAS which persisted into adulthood. Herein, we demonstrated a complementary anxiety-like phenotype in adolescence as indicated by increased time spent in the corners of the open field. We also found that CAS led to social avoidance in males as indicated by increased time spent in opposite corners from a social stimulus rat during SI [47]. Given that the social stimulus used here was a novel rat of the defeater strain (Long Evans), it is possible that CAS-induced social avoidance in males is context-dependent with regards to the phenotypic characteristics of the defeaters, similar to reduced SI in defeated mice that is specifically induced by a stimulus of the aggressor strain [48]. While SI ratio was not significantly impacted, sex-specific and/or CAS-related changes in social behavior may be investigated via alternative measures of SI, including reduced social approach and increased social vigilance which have been demonstrated following social defeat in female and male mice [49, 50].
Several next generation sequencing studies have investigated sex differences in stress-induced changes in the rodent brain [9, 29, 51], but to our knowledge, this is the first study to report long-lasting immune-inducible changes primed by adolescent stress. At baseline, CAS led to largely distinct sets of DEGs in the male and female rat hippocampus, a result consistent with prior transcriptomic studies in depression [10] as well as acute and chronic stress [29, 51, 52]. Following LPS, hippocampal immune transcripts were inducible to a greater extent in CAS rats compared to their NS litter mates. These transcriptomic changes were epitomized by an exaggerated enrichment of the NFκB signaling pathway in both males and females. Our results therefore extend similar findings from male mice subjected to chronic restraint stress [53] and are consistent with data from chronically stressed humans showing upregulated transcripts with response elements for NFκB [54]. In addition, we found that CAS exaggerated induction of several key members of the NFκB transcriptional complex, consistent with previously reported effects of stress on NFκB expression and activity in rodents [55–58] and humans [14, 59].
While CAS-driven sensitization of NFκB induction was present in both sexes, it is nonetheless possible that stress-induced NFκB activity exerts different effects on behavioral outcomes in males and females. Indeed, whereas in stressed male rodents NFκB has been shown to be necessary for changes in synaptic strength [60] and neurogenesis [61] underlying behavioral susceptibility, in female mice subjected to chronic unpredictable stress NFκB protected against ovarian hormone-related depressive-like behavior [62]. Interestingly, CAS females exhibited enhanced GR signaling. Although the effects of GR are commonly conceptualized as anti-inflammatory [63], there are several contexts in which GR has been documented to enhance pro-inflammatory processes [20, 64–66]. In contrast, CAS males displayed lower GR pathway induction compared to NS males, which may be consistent with reports of diminished expression of transcripts bearing the GR response element in chronically stressed humans [67–69] and rodents [70].
Here we found that although CAS markedly potentiated chemokine induction following LPS challenge, there was no evidence of peripheral myeloid cell infiltration into the brain parenchyma in rats due to sex, stress, or LPS. Thus, peripheral myeloid cell infiltration could not have contributed to CAS-driven priming of inflammatory genes reported here. Notably, while there is mixed evidence of monocyte trafficking to the mouse brain following social defeat or LPS injection [21, 24, 71, 72], leukocyte infiltration in rats has only been documented following severe inflammatory insults such as ischemia [73], experimental allergic encephalomyelitis [74], traumatic brain injury [75], and glioma [76]. However, in the absence of overt parenchymal infiltration, it is possible that subtly activated peripheral immune cells become associated with blood vessels in the brain. Indeed, under normal circumstances circulating monocytes are known to patrol the brain’s vasculature, and give rise to perivascular macrophages upon receiving pro-inflammatory signals [77]. Here we found that CAS increased CD45+ cells in the perivascular area. Consistent with the flow cytometry results, obtained using hippocampal tissue after removal of blood vessels, very few CD45+ cells were seen in the parenchyma, with the vast majority associated with the endothelium. Consistent with previous reports at similar time points [77], LPS did not increase perivascular CD45 presence.
We also assessed the impact of CAS on microglial morphology to probe if microglia may be involved in CAS-related inflammatory gene expression priming [78–80]. Similar to another index of microglial hypercomplexity identified by Sholl analysis following chronic stress model in rats [81], here we found that CAS led to a more complex microglial morphology—as measured by the number of quadruple points, indicating intersecting branches of a ramified cell—in female rats only. Consistently, microglia have been demonstrated to be critical in neuroinflammatory and behavioral profiles seen in other models of chronic stress [82]. Additional work will be necessary to determine the functional implications of increased ramification of adult microglia following adolescent stress in females. A potential limitation of the current study is that estrous cycle was not controlled for at the time of tissue collection. Indeed, stress-related neuroinflammatory outcomes such as brain concentrations of the cytokine IL-1 have been shown to differ by estrous stage, with considerable upregulation during diestrus, proestrus, and estrus [83]. Previous studies from our group using the CAS paradigm have shown that plasma values of 17β-estradiol and progesterone were not different at baseline between CAS and NS females in adolescence and in adulthood [4, 31]. While terminal uterine weights, a proxy to estrous cycle stage [84], indicated that CAS females had lower uterine weights compared to NS females, uterine weights did not correlate with experimental endpoints collected on the same day, including microglial morphology, suggesting that estrous cycle stage was not a primary driver of observed differences in CAS females. Another limitation was that the single housing was not only a part of the intended stressor during CAS, but that it extended beyond the specific adolescent stress period. This design was selected because we’ve previously shown that individual housing alone does not produce equivalent effects to CAS [31] and retaining the individual housing condition eliminates the potential effects of pair-housing-induced noise in the data including social buffering effects (which may also be sex-specific) [85, 86], or heightened stress and potential injury particularly in males due to in-cage fighting. However, because the individual housing condition extended beyond the end of the adolescent stress period, it does represent a continuous stressor for CAS rats. While the current paradigm offers relevance to real-world scenarios whereby chronically stressed individuals are more likely to withdraw from SIs following their stressful experiences [87, 88], it does not allow complete isolation of the individual housing effects to the adolescent period. Consistent with our assessment of sustained individual housing versus CAS-effects [31], we’ve recently shown that the impact of chronic stressor exposure during adolescence in mice is evident on behavioral and neural outcomes in adulthood even when all groups are exposed to individual housing following the initial adolescent stress period [89].
Rodent studies examining sex differences in stress-related neuroimmune outcomes have found exaggerated pro-inflammatory responses more frequently in males than females [4, 58, 90]. Our results hereby confirm and extend the occurrence of stress-induced immune priming in female rodents. Although the experiments herein utilized LPS to induce immune activation, it would be reasonable to expect that non-pathogenic sources of inflammation, including psychological stressors, would similarly engage the NFκB pathway [91, 92] in the periphery and possibly in the brain. Furthermore, in females an estrogen response pathway was potentiated by CAS following LPS challenge, which could contribute to sex differences presented in the current study, as we’ve previously reported estrogen receptor-mediated transcriptional changes in CAS females [29]. In males, CAS enriched pathways involved in glycolysis and metabolism, which warrants future investigation into the role of stress in altered energy metabolism in the brain [89]. Taken together, our results provide a novel framework within which mechanisms mediating sex differences in chronic stress-related neuroimmune changes can be further explored.
Funding and disclosure
This work was supported by the National Institutes of Health National Institute of Nursing Research (NR014886; to GNN). The Yerkes NHP Genomics Core is supported in part by ORIP/OD P51OD011132. Services and products in support of the research project were generated by the VCU Massey Cancer Center Flow Cytometry Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. Microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059. SAR was supported by training grant T32- GM008602. MMH was supported by training grant K12GM093857. The authors declare no competing interests.
Supplementary information
Differential Gene Expression as Function of LPS
Gene Set Enrichment with LPS in CAS Females
Gene Set Enrichment with LPS in CAS Males
Acknowledgements
The authors would like to thank Julie S. Farnsworth for assistance with performing flow cytometry experiments.
Author contributions
MB conceived of, collected, and analyzed behavioral, microglial, flow cytometry, and qPCR data, conducted pathway analysis, and wrote the paper. DM collected and analyzed Iba-1 and CD45 staining data. MMH assisted with collection of Iba-1 and CD45 staining data, and contributed to interpretation of behavioral data. MGD, JCS, and GKT analyzed RNA-Seq data. SDB and JLD assisted with flow cytometry experimental design and data collection. SAR and SDK assisted with behavioral data collection, and contributed to experimental design. ZQ assisted with statistical analysis. MGT advised project design, and assisted with data interpretation. GNN conceived of project, acquired funding, advised project design and data interpretation, and revised the paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-00970-2.
References
- 1.Bekhbat M, Neigh GN. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain Behav Immun. 2018;67:1–12. doi: 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich KB, Chen E, Yu T, Miller GE, Brody GH. Exposure to Parental Depression in Adolescence and Risk for Metabolic Syndrome in Adulthood. Child Dev. 2017;90:1272–85. doi: 10.1111/cdev.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson-Leary J, Eacret D, Chen R, Takano H, Nicholas B, Bhatnagar S. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl Psychiatry. 2017;7:e1160. doi: 10.1038/tp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan GJ, Vallender EJ, Garrett MR, Challagundla L, Overholser JC, Jurjus G, et al. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:177–86. doi: 10.1016/j.pnpbp.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stankiewicz AM, Goscik J, Majewska A, Swiergiel AH, Juszczak GR. The Effect of Acute and Chronic Social Stress on the Hippocampal Transcriptome in Mice. PLoS ONE. 2015;10:e0142195. doi: 10.1371/journal.pone.0142195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XH, Chen JX, Yue GX, Liu YY, Zhao X, Guo XL, et al. Gene expression profile of the hippocampus of rats subjected to chronic immobilization stress. PLoS ONE. 2013;8:e57621. doi: 10.1371/journal.pone.0057621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35:16362–76. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–11. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trabzuni D, Ramasamy A, Imran S, Walker R, Smith C, Weale ME, et al. Widespread sex differences in gene expression and splicing in the adult human brain. Nat Commun. 2013;4:2771. doi: 10.1038/ncomms3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekhbat M, Rowson SA, Neigh GN. Checks and balances: the glucocorticoid receptor and NFkB in good times and bad. Front Neuroendocrinol. 2017;46:15–31. doi: 10.1016/j.yfrne.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–25. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 15.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–7. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 16.Miklowitz DJ, Portnoff LC, Armstrong CC, Keenan-Miller D, Breen EC, Muscatell KA, et al. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 2016;241:315–22. doi: 10.1016/j.psychres.2016.04.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guardado P, Olivera A, Rusch HL, Roy M, Martin C, Lejbman N, et al. Altered gene expression of the innate immune, neuroendocrine, and nuclear factor-kappa B (NF-kappaB) systems is associated with posttraumatic stress disorder in military personnel. J Anxiety Disord. 2016;38:9–20. doi: 10.1016/j.janxdis.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Bekhbat M, Howell PA, Rowson SA, Kelly SD, Tansey MG, Neigh GN. Chronic adolescent stress sex-specifically alters central and peripheral neuro-immune reactivity in rats. Brain Behav Immun. 2019;76:248–57. doi: 10.1016/j.bbi.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nusslock R, Miller GE. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: a Neuroimmune Network Hypothesis. Biol Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799–805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 23.McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry. 2016;79:803–13. doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, et al. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS ONE. 2013;8:e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, et al. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88:1890–7. doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- 27.Yin W, Gallagher NR, Sawicki CM, McKim DB, Godbout JP, Sheridan JF. Repeated social defeat in female mice induces anxiety-like behavior associated with enhanced myelopoiesis and increased monocyte accumulation in the brain. Brain Behav Immun. 2019;78:131–42. doi: 10.1016/j.bbi.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowson SA, Bekhbat M, Kelly SD, Binder EB, Hyer MM, Shaw G, et al. Chronic adolescent stress sex-specifically alters the hippocampal transcriptome in adulthood. Neuropsychopharmacology. 2019;44:1207–15. doi: 10.1038/s41386-019-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–8. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrell CS, Burgado J, Kelly SD, Johnson ZP, Neigh GN. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology. 2015;62:252–64. doi: 10.1016/j.psyneuen.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrell CS, Hardy E, Boss-Williams K, Weiss JM, Neigh GN. Sex and lineage interact to predict behavioral effects of chronic adolescent stress in rats. Behav Brain Res. 2013;248:57–61. doi: 10.1016/j.bbr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowson SA, Harrell CS, Bekhbat M, Gangavelli A, Wu MJ, Kelly SD, et al. Neuroinflammation and Behavior in HIV-1 Transgenic Rats Exposed to Chronic Adolescent Stress. Front Psychiatry. 2016;7:102. doi: 10.3389/fpsyt.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowson SA, Foster SL, Weinshenker D, Neigh GN. Locomotor sensitization to cocaine in adolescent and adult female Wistar rats. Behav Brain Res. 2018;349:158–62. doi: 10.1016/j.bbr.2018.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 40.Henriques-Alves AM, Queiroz CM. Ethological Evaluation of the Effects of Social Defeat Stress in Mice: beyond the Social Interaction Ratio. Front Behav Neurosci. 2015;9:364. doi: 10.3389/fnbeh.2015.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Cairns MJ. Gene set enrichment analysis of RNA-Seq data: integrating differential expression and splicing. BMC Bioinforma. 2013;14:S16. doi: 10.1186/1471-2105-14-S5-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly KA, Michalovicz LT, Miller JV, Castranova V, Miller DB, O’Callaghan JP. Prior exposure to corticosterone markedly enhances and prolongs the neuroinflammatory response to systemic challenge with LPS. PLoS ONE. 2018;13:e0190546. doi: 10.1371/journal.pone.0190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah S, King EM, Chandrasekhar A, Newton R. Roles for the mitogen-activated protein kinase (MAPK) phosphatase, DUSP1, in feedback control of inflammatory gene expression and repression by dexamethasone. J Biol Chem. 2014;289:13667–79. doi: 10.1074/jbc.M113.540799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–25. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 45.Girotti M, Donegan JJ, Morilak DA. Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain. Psychoneuroendocrinology. 2011;36:1164–74. doi: 10.1016/j.psyneuen.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, et al. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;68:1–8. doi: 10.1016/j.pnpbp.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barat P, Meiffred MC, Brossaud J, Fuchs D, Corcuff JB, Thibault H, et al. Inflammatory, endocrine and metabolic correlates of fatigue in obese children. Psychoneuroendocrinology. 2016;74:158–63. doi: 10.1016/j.psyneuen.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Ayash S, Schmitt U, Muller MB. Chronic social defeat-induced social avoidance as a proxy of stress resilience in mice involves conditioned learning. J Psychiatr Res. 2020;120:64–71. doi: 10.1016/j.jpsychires.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl Acad Sci USA. 2020;117:26406–13. doi: 10.1073/pnas.2011890117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman EL, Covington HE, 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF, et al. Fighting Females: neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biol Psychiatry. 2019;86:657–68. doi: 10.1016/j.biopsych.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marrocco J, Petty GH, Rios MB, Gray JD, Kogan JF, Waters EM, et al. A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun. 2017;8:808. doi: 10.1038/s41467-017-01014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCann KE, Sinkiewicz DM, Rosenhauer AM, Beach LQ, Huhman KL. Transcriptomic Analysis Reveals Sex-Dependent Expression Patterns in the Basolateral Amygdala of Dominant and Subordinate Animals After Acute Social Conflict. Mol Neurobiol. 2019;56:3768–79. doi: 10.1007/s12035-018-1339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2014;19:1171–8. doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–72. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z. The characteristic long-term upregulation of hippocampal NF-kappaB complex in PTSD-like behavioral stress response is normalized by high-dose corticosterone and pyrrolidine dithiocarbamate administered immediately after exposure. Neuropsychopharmacology. 2011;36:2286–302. doi: 10.1038/npp.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, et al. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–63. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 57.Madrigal JLM, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor κB-mediated mechanisms. J Neurochemistry. 2001;76:532–38. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 58.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–20. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagabhushan M, Mathews HL, Witek-Janusek L. Aberrant nuclear expression of AP-1 and NFkappaB in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav Immun. 2001;15:78–84. doi: 10.1006/brbi.2000.0589. [DOI] [PubMed] [Google Scholar]
- 60.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–21. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–74. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, et al. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–80. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Bosscher K, Schmitz ML, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–9. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–45. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Barrientos RM, Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, et al. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol Aging. 2015;36:1483–95. doi: 10.1016/j.neurobiolaging.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 2014;41:191–9. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224. doi: 10.1186/s12974-016-0672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32:11706–15. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–92. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 74.Mensah-Brown EP, Shahin A, Al Shamisi M, Lukic ML. Early influx of macrophages determines susceptibility to experimental allergic encephalomyelitis in Dark Agouti (DA) rats. J Neuroimmunol. 2011;232:68–74. doi: 10.1016/j.jneuroim.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Truettner JS, Bramlett HM, Dietrich WD. Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J Cereb Blood Flow Metab. 2017;37:2952–62. doi: 10.1177/0271678X16680003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Badie B, Schartner JM, Paul J, Bartley BA, Vorpahl J, Preston JK. Dexamethasone-induced abolition of the inflammatory response in an experimental glioma model: a flow cytometry study. J Neurosurg. 2000;93:634–9. doi: 10.3171/jns.2000.93.4.0634. [DOI] [PubMed] [Google Scholar]
- 77.Audoy-Remus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallieres L. Rod-Shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci. 2008;28:10187–99. doi: 10.1523/JNEUROSCI.3510-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karperien AL, Jelinek HF. Fractal, multifractal, and lacunarity analysis of microglia in tissue engineering. Front Bioeng Biotechnol. 2015;3:51. doi: 10.3389/fbioe.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morrison H, Young K, Qureshi M, Rowe RK, Lifshitz J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci Rep. 2017;7:13211. doi: 10.1038/s41598-017-13581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013;10:4. doi: 10.1186/1742-2094-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb cortex (N. Y, NY: 1991) 2013;23:1784–97. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- 82.McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23:1421–31. doi: 10.1038/mp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arakawa K, Arakawa H, Hueston CM, Deak T. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology. 2014;100:162–77. doi: 10.1159/000368606. [DOI] [PubMed] [Google Scholar]
- 84.Harrell CS, Burgado J, Kelly SD, Neigh GN. Ovarian steroids influence cerebral glucose transporter expression in a region- and isoform-specific pattern. J Neuroendocrinol. 2014;26:217–25. doi: 10.1111/jne.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez CI, Magcalas CM, Barto D, Fink BC, Rice JP, Bird CW, et al. Effects of sex and housing on social, spatial, and motor behavior in adult rats exposed to moderate levels of alcohol during prenatal development. Behav Brain Res. 2016;313:233–43. doi: 10.1016/j.bbr.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du Preez A, Onorato D, Eiben I, Musaelyan K, Egeland M, Zunszain PA, et al. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav Immun. 2021;91:24–47. doi: 10.1016/j.bbi.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 88.Girard JM, Cohn JF, Mahoor MH, Mavadati SM, Hammal Z, Rosenwald DP. Nonverbal Social Withdrawal in Depression: evidence from manual and automatic analysis. Image Vis Comput. 2014;32:641–47. doi: 10.1016/j.imavis.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaw GA, Hyer MM, Targett I, Council KR, Dyer SK, Turkson S, et al. Traumatic stress history interacts with sex and chronic peripheral inflammation to alter mitochondrial function of synaptosomes. Brain Behav Immun. 2020;88:203–19. doi: 10.1016/j.bbi.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hudson SP, Jacobson-Pick S, Anisman H. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience. 2014;277:239–49. doi: 10.1016/j.neuroscience.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75:970–81. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differential Gene Expression as Function of LPS
Gene Set Enrichment with LPS in CAS Females
Gene Set Enrichment with LPS in CAS Males