Abstract
The NLRP3-IL-1β pathway plays an important role in adipose tissue (AT)-induced inflammation and the development of obesity-associated comorbidities. We aimed to determine the impact of NLRP3 on obesity and its associated metabolic alterations as well as its role in adipocyte inflammation and extracellular matrix (ECM) remodeling. Samples obtained from 98 subjects were used in a case−control study. The expression of different components of the inflammasome as well as their main effectors and inflammation- and ECM remodeling-related genes were analyzed. The impact of blocking NLRP3 using siRNA in lipopolysaccharide (LPS)-mediated inflammation and ECM remodeling signaling pathways was evaluated. We demonstrated that obesity (P < 0.01), obesity-associated T2D (P < 0.01) and NAFLD (P < 0.05) increased the expression of different components of the inflammasome as well as the expression and release of IL-1β and IL-18 in AT. We also found that obese patients with T2D exhibited increased (P < 0.05) hepatic gene expression levels of NLRP3, IL1B and IL18. We showed that NLRP3, but not NLRP1, is regulated by inflammation and hypoxia in visceral adipocytes. We revealed that the inhibition of NLRP3 in human visceral adipocytes significantly blocked (P < 0.01) LPS-induced inflammation by downregulating the mRNA levels of CCL2, IL1B, IL6, IL8, S100A8, S100A9, TLR4 and TNF as well as inhibiting (P < 0.01) the secretion of IL1-β into the culture medium. Furthermore, blocking NLRP3 attenuated (P < 0.01) the LPS-induced expression of important molecules involved in AT fibrosis (COL1A1, COL4A3, COL6A3 and MMP2). These novel findings provide evidence that blocking the expression of NLRP3 reduces AT inflammation with significant fibrosis attenuation.
Keywords: NLRP3, Inflammasone, Inflammation, Obesity, Type 2 diabetes, Nonalcoholic fatty liver disease
Subject terms: Inflammasome, Interleukins
Introduction
The worldwide obesity epidemic is emerging as a major global health challenge. Sustained obesity is a risk factor for an expanding number of chronic diseases, ranging from type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD) and cardiovascular diseases to renal, gastrointestinal and psychological disorders as well as different types of cancer.1–4 Obesity-associated chronic and unresolved inflammation is a well-recognized mechanism promoting adipose tissue (AT) dysfunction, favoring the development of its related comorbidities.5,6 Importantly, accumulating data have shown that in the obese state, the AT is infiltrated by a large number of immune cells that influence the metabolic status through the release of cytokines and other inflammatory mediators.7,8 Consequently, both immune and metabolic responses must be tightly regulated for a suitable cellular homeostasis.9 In this context, the AT emerges as an important organ connecting immunity and metabolism.10 Tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interleukin (IL)-1β and IL-6 have been extensively studied in the context of inflammation and metabolism, but specifically, the inflammasomes, as major mediators of innate immunity, constitute a significant link between the immune and metabolic systems.11–13
The inflammasomes are important intracellular multiprotein complexes that recognize a variety of danger signals, including pathogen-associated molecular patterns and host-derived danger signals (danger-associated molecular patterns, DAMPs).14 Different inflammasomes have been identified according to their main constituent: (i) the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) family consisting of the members NLRP1, NLRP2, NLRP3, NLRP6, NLRC4 and, potentially, NLRP12 and (ii) the PYHIN family that includes the AIM2 and IFI16 members.15 In the field of immunometabolism, the NLRP3 inflammasome is one of the most studied.16 The NLRP3 inflammasome assembles in response to a wide array of signals and subsequently acts as a high-molecular-weight platform for facilitating caspase-1 processing, promoting the release of IL-1β and IL-18. IL-1β is recognized as an important contributor to the development of T2D due to its potent inflammatory actions.17,18 In contrast, and unexpectedly since its levels are increased in obesity, IL-18 counteracts the development of diet-induced metabolic dysfunctions.19,20 However, the insulin-desensitizing effects of IL-1β have been proposed to override the IL-18 action since mice lacking both IL-1β and IL-18 exhibited an increased insulin sensitivity.21–23 Recently, IL-18 has been proposed to be activated in AT via the NLRP1 inflammasome,20 while NLRP3 is the main contributor to the increased levels of IL-1β in obesity. In this sense, the detrimental effect of the NLRP3-IL-1β pathway as a contributor of obesity-induced inflammation and insulin resistance22,24 together with the protective role of the NLRP1-IL-18 pathway ameliorating metabolic dysfunctions20,23 have been proposed as two antagonic mechanisms controlled by two cytokines from the same family, the IL-1 family.23
Chronic inflammation in visceral AT (VAT) is a cornerstone of immune activation in obesity leading to a dysfunctional AT characterized by impaired angiogenesis, local hypoxia, altered extracellular matrix (ECM) remodeling and finally, fibrosis.25 These metabolic alterations and the limited healthy expansion of the AT are associated with adverse metabolic consequences.26 A failure in the capacity for AT expansion together with impaired ECM remodeling has been proposed as a key factor linking positive energy balance and T2D development since ectopic lipid accumulation in nonadipocyte cells promotes lipotoxic insults, including insulin resistance.26 In this sense, the AT not only constitutes an important endocrine organ but also a mechanical support capable of responding to relevant signaling events regulating metabolic homeostasis.27 Compelling evidence suggests that the activation of the NLRP3-IL1β pathway has a central role in promoting obesity-associated comorbidities. However, the role of the NLRP3-IL1β pathway in regulating the crosstalk between inflammation and ECM remodeling in the context of obesity remains poorly understood. Therefore, we first tested whether obesity and its associated pathologies T2D and NAFLD influence the expression levels of different inflammasome components and their main effector molecules IL1B and IL18. The regulation of NLRP3 and NLRP1 by different inflammatory mediators and hypoxia was further analyzed in human visceral adipocytes. We also aimed to investigate the potential regulatory roles and mechanisms of NLRP3 in ECM remodeling and inflammation in human adipocytes by blocking its expression.
Results
Obesity and obesity-associated T2D upregulate the expression of different components of the inflammasome
The baseline characteristics of the subjects included in the study are summarized in Table 1. No differences in age between the groups were observed (P = 0.134). As expected, BMI, BF, the waist and hip circumference as well as the waist-to-hip ratio (WHR) were increased (P < 0.001) in both groups of patients with obesity compared to those of the lean volunteers. As expected, obese patients with T2D were more insulin-resistant than control and normoglycemic volunteers, exhibiting increased concentrations of glucose (P < 0.001), insulin (P < 0.001) and HOMA index (P < 0.01) as well as a lower QUICKI index (P < 0.001). Obesity was also associated with higher concentrations of leptin (P < 0.01), and patients with T2D exhibited increased circulating concentrations of triglycerides (P < 0.01) together with reduced levels of HDL-cholesterol (P < 0.01). Moreover, patients with obesity showed a proinflammatory profile exhibiting higher (P < 0.01) concentrations of the inflammatory markers CRP, fibrinogen, homocystein and vWF antigen. Obese patients with T2D showed a significant reduction (P < 0.01) in the AST/ALT ratio as well as increased levels of γ-GT (P < 0.05) (Table 1). No differences regarding the white blood cells were found between the groups.
Table 1.
Anthropometric and biochemical characteristics of subjects included in the study
| Lean | Obese NG | Obese T2D | |
|---|---|---|---|
| n (males, females) | 24 (6, 18) | 35 (6, 29) | 39 (10, 29) |
| Age (years) | 36 ± 3 | 40 ± 2 | 43 ± 2 |
| BMI (kg/m2) | 22.1 ± 3.0 | 42.5 ± 5.2** | 45.8 ± 7.4***,†† |
| Body fat (%) | 22.4 ± 1.6 | 52.4 ± 5.5*** | 52.5 ± 7.2*** |
| Waist circumference (cm) | 75 ± 10 | 119 ± 14** | 128 ± 13**,† |
| Hip circumference (cm) | 94 ± 6 | 128 ± 9** | 133 ± 12**,† |
| Waist-to-hip ratio | 0.80 ± 0.07 | 0.93 ± 0.09** | 0.96 ± 0.08** |
| SBP (mmHg) | 105 ± 6 | 121 ± 16** | 134 ± 15**,†† |
| DBP (mmHg) | 66 ± 7 | 75 ± 7** | 84 ± 8**,†† |
| Fasting glucose (mg/dL) | 85 ± 5 | 89 ± 6 | 129 ± 7**,†† |
| 2 h OGTT glucose (mg/dL) | – | 113 ± 6 | 205 ± 4††† |
| Fasting insulin (μU/mL) | 6.8 ± 2.0 | 16.7 ± 6.4 | 20.2 ± 1.7* |
| 2 h OGTT insulin (μU/mL) | – | 72.7 ± 8.6 | 140.0 ± 18.1††† |
| HOMA | 1.5 ± 0.8 | 3.9 ± 0.8 | 5.6 ± 4.0** |
| QUICKI | 0.371 ± 0.037 | 0.330 ± 0.038 | 0.306 ± 0.024***,† |
| Triglycerides (mg/dL) | 67 ± 7 | 95 ± 7 | 139 ± 12**,† |
| Cholesterol (mg/dL) | 176 ± 7 | 190 ± 7 | 196 ± 6 |
| LDL-cholesterol (mg/dL) | 103 ± 7 | 116 ± 6 | 118 ± 6 |
| HDL-cholesterol (mg/dL) | 64 ± 3 | 54 ± 2 | 48 ± 2** |
| Leptin (ng/mL) | 8.1 ± 1.3 | 56.1 ± 3.9*** | 48.2 ± 5.3** |
| Leukocytes (×106) | 7.35 ± 0.42 | 7.65 ± 0.55 | 7.56 ± 0.36 |
| CRP (mg/L) | 1.0 ± 0.2 | 9.3 ± 1.4** | 7.3 ± 1.1* |
| Fibrinogen (mg/dL) | 215 ± 18 | 395 ± 13** | 352 ± 16** |
| von Willebrand factor (%) | 56 ± 8 | 128 ± 12** | 131 ± 10** |
| Homocysteine (μmol/L) | 6.8 ± 0.4 | 9.1 ± 0.5* | 9.6 ± 0.5** |
| AST (U/L) | 13 ± 1 | 17 ± 2 | 15 ± 1 |
| ALT (U/L) | 10 ± 3 | 22 ± 3 | 24 ± 2 |
| AST/ALT | 1.74 ± 0.21 | 0.81 ± 0.05** | 0.71 ± 0.04** |
| ALP (U/L) | 93 ± 8 | 94 ± 5 | 93 ± 5 |
| γ-GT (U/L) | 11 ± 2 | 19 ± 2 | 29 ± 5* |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, CRP C-reactive protein, DBP diastolic blood pressure, γ-GT γ-glutamyltransferase, HOMA homeostatic model assessment, NG normoglycemic, OGTT oral glucose tolerance test, QUICKI quantitative insulin sensitivity check index, SBP systolic blood pressure, T2D type 2 diabetes
Data are presented as the mean ± SEM. CRP concentrations were logarithmically transformed for statistical analysis. Differences between groups were analyzed by one-way ANOVA followed by Tukey’s post hoc tests or by unpaired two-tailed Student’s t tests, where appropriate. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. lean. †P < 0.05, ††P < 0.01 and †††P < 0.01 vs. obese NG
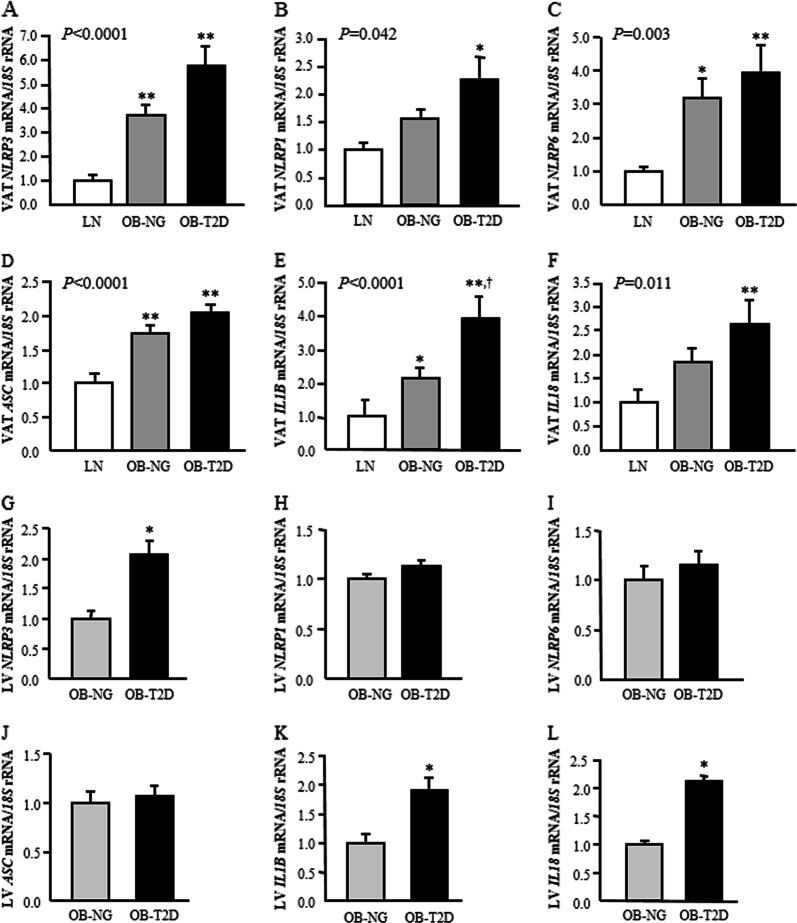
To verify whether obesity and obesity-associated T2D are involved in the activation of the inflammasome, the analysis of the expression levels of genes involved in the inflammasome pathway was performed in VAT. The results showed increased (P < 0.01) NLRP3, NLRP6 and ASC mRNA levels in VAT in obesity- and obesity-associated T2D. We also found increased gene expression levels of NLRP1 in patients with T2D compared with the levels in control (P < 0.01) and normoglycemic volunteers (P < 0.05) (Fig. 1a–d). In this sense, the mRNA expression of the inflammasome components in VAT correlated with BMI, BF and leptin (Supplemental Table 1). To corroborate which cell type preferentially contributed to the elevated levels of the inflammasome components previously observed, adipocytes and SVFCs were isolated from VAT samples obtained from patients with obesity. Although mRNA levels for all components were evident in SVFCs (P < 0.01), gene expression was also detected in mature adipocytes (Supplemental Fig. 1). The assembly of an active inflammasome leads to caspase-1 activation, triggering the maturation and release of the important proinflammatory mediators IL-1β and IL-18. The gene expression levels of CASP1 and IL1B were increased (P < 0.01) in both groups of obese patients, with IL1B levels being further upregulated (P < 0.05) in patients with T2D (Table 2, Fig. 1e). We also detected that IL18 levels were higher (P < 0.05) in patients with T2D (Fig. 1f). Notably, a significant association (P < 0.05) of the mRNA levels of these inflammation-related factors with NLRP3 and NLRP1 expression was also observed (Supplemental Table 1). Since the NLRP3 inflammasome also synergized with NOD2 for the synthesis of proinflammatory cytokines, we analyzed its mRNA in VAT, which was significantly upregulated (P < 0.01) in both groups of patients with obesity (Table 2). Remarkably, the gene expression levels of NLRP3 and NLRP1 were highly associated (P < 0.001) with the mRNA levels of NOD2 (Supplemental Table 1).
Fig. 1.
Gene expression levels in visceral adipose tissue and liver of the main inflammasome components in obesity and obesity-associated type 2 diabetes. Bar graphs show the mRNA levels of a, g NLRP3, b, h NLRP1, c, i NLRP6, d, j ASC, e, k IL1B and f, l IL18 in visceral adipose tissue and liver, respectively, from lean volunteers (LN), obese NG subjects (OB-NG) and obese patients with T2D (OB-T2D). Bars represent the mean ± SEM. Differences between groups were analyzed by one-way ANOVA followed by Tukey’s tests. *P < 0.05 and **P < 0.01 vs. lean subjects. †P < 0.05 vs. OB-NG
Table 2.
Analysis of gene expression levels of inflammation- and extracellular matrix remodeling-related markers in VAT
| Gene | Lean | Obese NG | Obese T2D |
|---|---|---|---|
| IL1A | 1.00 ± 0.33 | 0.87 ± 0.11 | 1.50 ± 0.35 |
| IL6 | 1.00 ± 0.40 | 5.23 ± 2.41** | 6.46 ± 1.07***,† |
| IL8 | 1.00 ± 0.44 | 3.50 ± 1.56 | 3.67 ± 0.89 |
| IL10 | 1.00 ± 0.50 | 2.84 ± 0.44*** | 5.07 ± 0.92*** |
| IL32 | 1.00 ± 0.28 | 4.37 ± 0.58** | 5.12 ± 0.50** |
| TNF | 1.00 ± 0.29 | 1.25 ± 0.14* | 1.46 ± 0.28* |
| HIF1A | 1.00 ± 0.20 | 1.53 ± 0.16 | 1.89 ± 0.23* |
| NOD2 | 1.00 ± 0.34 | 2.97 ± 0.85** | 3.56 ± 0.49** |
| TLR4 | 1.00 ± 0.11 | 1.63 ± 0.13 | 1.81 ± 0.19* |
| FGF2 | 1.00 ± 0.18 | 0.63 ± 0.07 | 0.69 ± 0.07 |
| MMP2 | 1.00 ± 0.15 | 1.26 ± 0.28 | 1.72 ± 0.29 |
| MMP9 | 1.00 ± 0.44 | 2.64 ± 0.76* | 3.86 ± 0.75** |
| SPP1 | 1.00 ± 0.25 | 6.24 ± 0.45** | 4.13 ± 1.32* |
| TNC | 1.00 ± 0.20 | 7.97 ± 1.81** | 7.09 ± 1.30*** |
| TNMD | 1.00 ± 0.35 | 1.50 ± 0.19* | 2.00 ± 0.40* |
| TGFB | 1.00 ± 0.19 | 1.38 ± 0.12 | 2.36 ± 0.26**,†† |
| CASP1 | 1.00 ± 0.10 | 3.04 ± 0.48** | 2.53 ± 0.36** |
| CASP3 | 1.00 ± 0.21 | 1.02 ± 0.11 | 1.18 ± 0.18 |
| CASP7 | 1.00 ± 0.17 | 1.06 ± 0.10 | 1.38 ± 0.22 |
Analysis of mRNA levels in visceral adipose tissue (VAT) of LN, obese NG and obese T2D volunteers (LN: n = 10; OB-NG: n = 30; OB-IGT + T2D: n = 30)
CASP caspase, HIF1A hypoxia inducible factor 1 α, IL interleukin, MMP matrix metalloproteinase, NOD2 nucleotide binding oligomerization domain containing 2, SPP1 osteopontin, TGFB1 transforming growth factor-β, TLR4 toll-like receptor-4, TNC tenascin C, TNMD tenomodulin, TNF tumor necrosis factor-α
Data represent the mean ± SEM of the ratio between gene expression and 18S rRNA. Differences between groups were analyzed by one-way ANOVA followed by Tukey’s post hoc tests. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. lean. †P < 0.05 and ††P < 0.01 vs. obese NG
Given that the liver also constitutes a major metabolic organ responsible for energy utilization, we aimed to investigate the expression of NLRP3 and NLRP1 and their effectors in the liver. No changes were found between obese patients with or without T2D in the hepatic expression of NLRP1, but importantly, the gene expression levels of NLRP3 and its proinflammatory mediators IL1B and IL18 were significantly upregulated (P < 0.05) in the liver of obese patients with T2D (Fig. 1g–l). Moreover, an association between the hepatic mRNA levels of NLRP3 and BMI (P = 0.006) and the inflammatory marker CRP (P = 0.012) was detected. The gene expression levels of NLRP3 in the liver were also significantly correlated with IL1B (r = 0.72; P < 0.001) and NLRP1 (r = 0.35; P = 0.018) (Supplemental Table 2).
Interestingly, we also found a significant and positive association between the gene expression levels in VAT and liver of NLRP3 (r = 0.62; P < 0.001) and IL1B (r = 0.39; P = 0.020).
Impact of the inflammasome in obesity-associated NAFLD and NASH
Apart from T2D, one of the best-known hepatic derangements associated with obesity is NAFLD. NAFLD consists of a spectrum of liver disease from steatosis (fatty infiltration of the liver) to nonalcoholic steatohepatitis (NASH), which consists of steatosis mixed with inflammation, balloon degeneration, variable degrees of fibrosis and Mallory body formation.28 Thus, we aimed to investigate whether NAFLD and NASH are involved in the activation of the inflammasome.
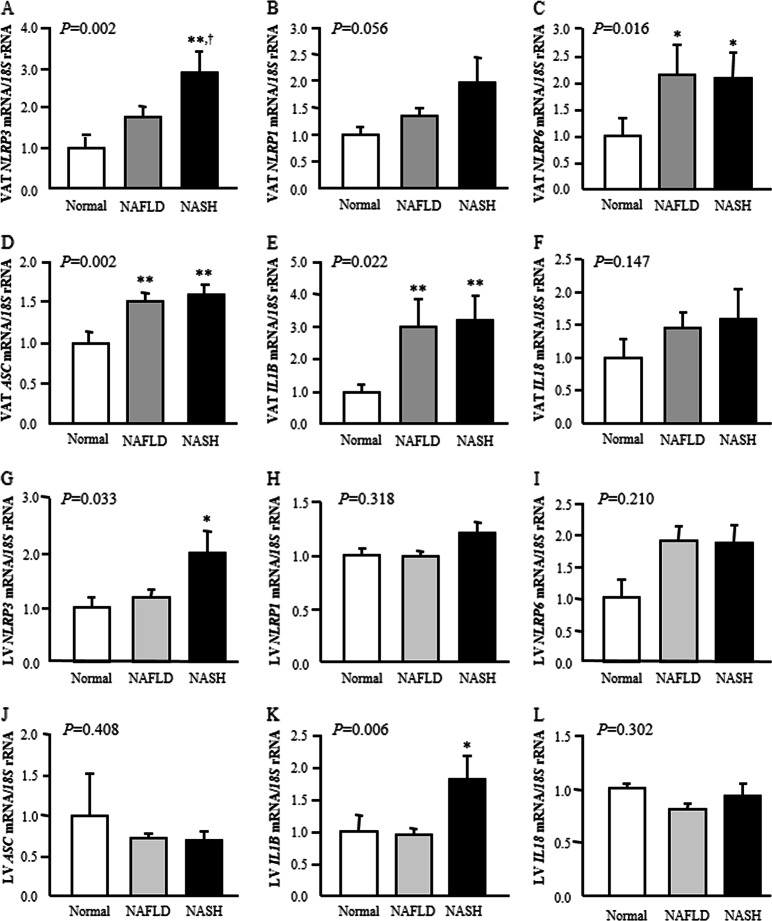
Obese subjects suffering from NASH exhibited higher mRNA levels of NLRP3 (P < 0.05) in VAT compared with levels in both volunteers with normal liver and subjects with NAFLD (Fig. 2a). We also found that the presence of NAFLD and NASH was associated with higher gene expression levels of NLRP6 (P < 0.05) and ASC (P < 0.01) as well as the effector IL1B (P < 0.01) in VAT (Fig. 2c–e). Although patients with liver disease exhibited increased mRNA levels of NLRP1 and IL18, the differences were not statistically significant (Fig. 2b, f). No differences were found between patients with or without NAFLD and NASH in the gene expression of NLRP1, NLRP6, ASC and IL18 in the liver, but notably, mRNA levels of NLRP3 (P < 0.05) and its proinflammatory mediator IL1B (P < 0.01) were significantly upregulated in the liver of obese patients with NASH (Fig. 2g–l). We also detected a positive association (r = 0.40; P = 0.007) between NLRP3 gene expression in the liver and circulating levels of ALT, a marker of liver injury (Supplemental Table 2).
Fig. 2.
Effect of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) on visceral adipose tissue and hepatic gene expression levels of the main inflammasome components. Bar graphs show the mRNA levels of a, g NLRP3, b, h NLRP1, c, i NLRP6, d, j ASC, e, k IL1B and f, l IL18 in visceral adipose tissue and liver according to hepatic function. Bars represent the mean ± SEM. Differences between groups were analyzed by one-way ANOVA followed by Tukey’s tests. *P < 0.05 and **P < 0.01 vs. normal liver. †P < 0.05 vs. NAFLD
Increased inflammation and ECM remodeling in human VAT and liver in obesity are related to NLRPs
Since NLRP3 and NLRP1 promote inflammation-related signaling, their association with important inflammatory mediators was analyzed in VAT and liver. Both groups of obese subjects exhibited higher VAT mRNA levels (P < 0.01) of the inflammatory mediators IL6, IL10, IL32 and TNF compared to levels in lean volunteers, with mRNA levels of IL6 also being upregulated (P < 0.05) in patients with T2D compared with NG subjects (Table 2). We found that HIF1A and TLR4 levels were increased in patients with T2D (Table 2). NLRP3 mRNA levels were significantly associated with the proinflammatory cytokines IL6, IL8, IL32, HIF1A and TNF, whereas NLRP1 expression was correlated with IL1A expression levels (Supplemental Table 1). NLRP3 also exhibited an association with circulating levels of fibrinogen (Supplemental Table 1). In this line, we found that hepatic mRNA expression of NLRP3 was significantly associated with IL6, IL8 and IL32 (Supplemental Table 2).
Recent evidence suggests that during progressive obesity, AT fibrosis restricts adipocyte expansion, promoting the development of different comorbidities. In this sense, we detected a marked increase in the amount of total fibrosis in the VAT from obese volunteers with (P < 0.01) and without (P < 0.05) T2D compared to that of lean subjects (Supplemental Fig. 2). Thus, we evaluated the association of NLRP3 and NLRP1 with other important factors regulating ECM remodeling in VAT. Obese subjects showed increased (P < 0.05) mRNA expression levels of the ECM remodeling genes MMP9, SPP1, TNC, TNMD and TGFB in VAT, with the latter also being upregulated (P < 0.01) in patients with T2D (Table 2). No differences were observed in the gene expression levels of FGF2 and MMP2. We found a positive correlation of NLRP3 gene expression levels with MMP2, MMP9, TNC and TGFB (Supplemental Table 1). The expression levels of NLRP3 in the liver were also associated with the expression of MMP2, MMP9 and TGFB (Supplemental Table 2).
NLRP3, but not NLRP1, is regulated by inflammation and hypoxia in visceral adipocytes
Since NLRP inflammasomes can sense a wide array of DAMPs, human visceral adipocytes were stimulated with a panel of inflammation-related factors. Hypoxia has been proposed as a risk factor for chronic inflammation in obesity29 and is involved in the regulation of NLRP3 activation in adipocytes.30 Therefore, adipocytes were also cultured under hypoxic conditions, which were mimicked by the divalent transition-metal ion cobalt.
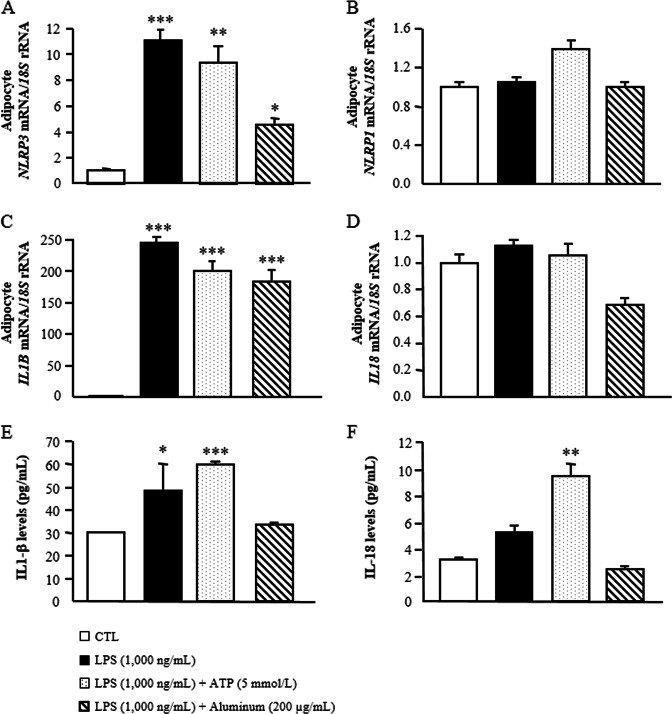
The expression of NLRP3 and NLRP1 as well as the expression and secretion of its main targets IL-1β and IL-18 were analyzed. We first primed human adipocytes with LPS, and the second insults for inflammasome activation were provided by a treatment with ATP or with aluminum. As shown in Fig. 3a, b, LPS alone as well as LPS combined with ATP (P < 0.01) or aluminum (P < 0.05) significantly increased the mRNA levels of NLRP3 in human adipocytes without changes in NLRP1 expression. After the treatments, we also detected a strong increase (P < 0.0001) in the expression of IL1B, but no differences were found in the mRNA levels of IL18 (Fig. 3c, d). In this sense, treatment with LPS followed by ATP promoted an increase (P < 0.001) in IL-1β secretion into the culture medium (Fig. 3e). An increase in the release of IL-18 was detected after the combined stimulus with LPS and ATP (Fig. 3f). Furthermore, the gene expression levels of NLRP3 were strongly induced (P < 0.01) by TNF-α in visceral adipocytes (Supplemental Fig. 3a). Similarly, hypoxic conditions upregulated (P < 0.05) NLRP3 expression (Supplemental Fig. 3c). No differences were detected in the expression of NLRP1 after the different treatments. IL-4 and IL-13, described as anti-inflammatory cytokines, had no effect on the regulation of both NLRP3 and NLRP1 (Supplemental Fig. 3e–h).
Fig. 3.
LPS alone or combined with ATP or aluminum promotes the expression and secretion of IL-1β by priming the expression of the NLRP3 inflammasome in human visceral adipocytes. Gene expression levels of a NLRP3, b NLRP1, c IL1B and d IL18 as well as released levels of e IL-1β and f IL-18 in cultured human visceral adipocytes incubated with LPS (1000 ng/mL) for 24 h as well as with LPS (1000 ng/mL) for 3 h in the presence or absence of ATP (5 mmol/L) and aluminum (200 μg/mL) for another 24 h. Gene expression levels in unstimulated cells were assumed to be 1. Values are the mean ± SEM (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Dunnett’s tests. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. unstimulated cells
NLRP3 silencing ameliorated LPS-induced inflammation and fibrosis in human visceral adipocytes
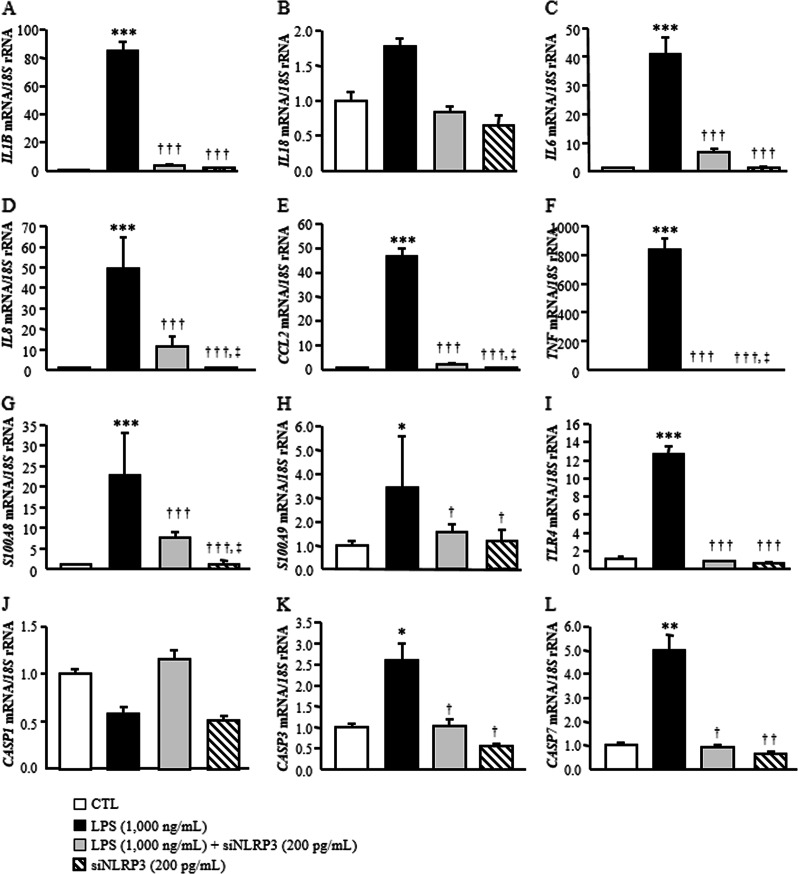
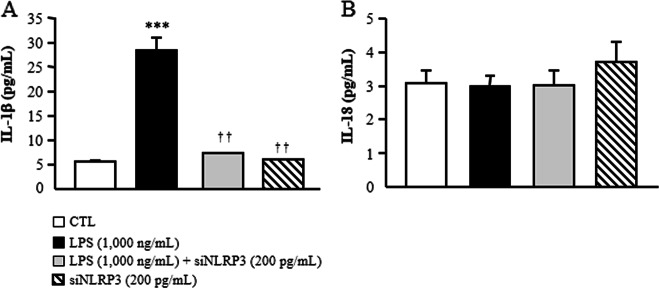
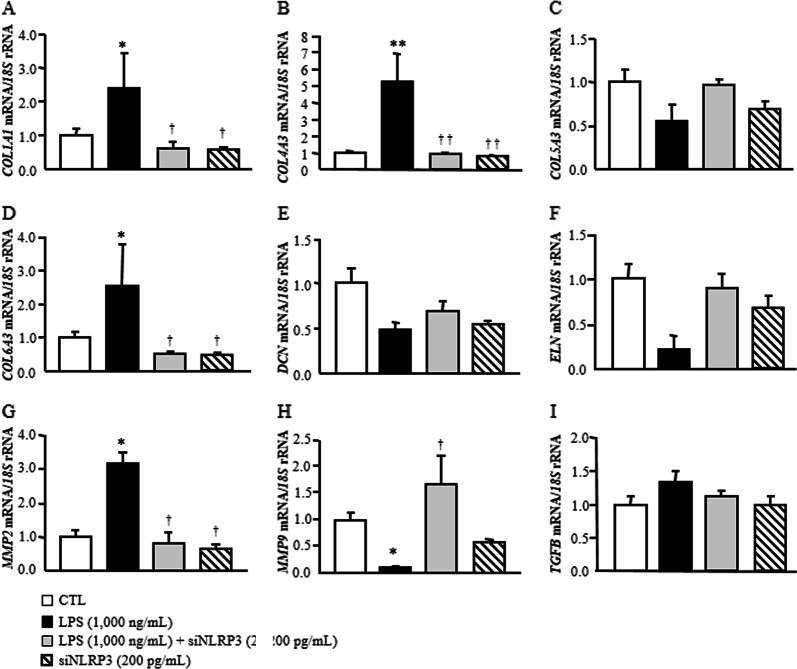
Based on the relevance of NLRP3 in visceral obesity-associated inflammation, we reduced the constitutive expression levels of NLRP3 in human visceral adipocytes using a specific siRNA. As shown in Fig. 4, LPS significantly upregulated (P < 0.001) the expression of inflammation-related genes (CCL2, IL1B, IL6, IL8, S100A8, S100A9, TLR4 and TNF) compared to that of the control group, whereas the inhibition of NLRP3 significantly blocked the LPS-induced effect by downregulating (P < 0.001) the gene expression levels of all the analyzed molecules. In line with our previous results, no significant differences were found regarding the expression levels of IL18. To gain further insight into the effect of the inhibition of NLRP3 on the inflammatory response, we measured the secretion levels of IL-1β and IL-18 into the culture medium after siNLRP3 treatment. Although no effects were found for IL-18, LPS promoted an increase (P < 0.01) in IL1-β secretion into the medium, and this effect was reduced (P < 0.01) by the inhibition of NLRP3 (Fig. 5). Recent evidence suggests that during progressive obesity, the development of AT fibrosis restricts adipocyte expansion. We found that the downregulation of NLRP3 attenuated the LPS-induced expression of COL1A1, COL4A3, COL6A3 and MMP2. Surprisingly, LPS promoted a downregulation (P < 0.01) of MMP9 and ELN mRNA that increased after siNLRP3 treatment (Fig. 6). No differences were observed for COL5A3, TGF and DCN, a protein highly involved in collagen fibril assembly.
Fig. 4.
Knockdown of NLRP3 ameliorated LPS-induced inflammation in human visceral adipocytes. Gene expression levels of inflammatory factors in human visceral adipocytes incubated in the presence or absence of LPS (1000 ng/mL) for 3 h, followed by transfection with or without 100 pmol/L NLRP3 siRNA/2 × 105 cells/well for another 24 h. Gene expression levels in scrambled siRNA cells (CTL) were assumed to be 1. Values are the mean ± SEM (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Tukey’s tests. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. scrambled siRNA. †P < 0.05, ††P < 0.01 and †††P < 0.001 vs. adipocytes stimulated with LPS. ‡P < 0.05 vs. adipocytes stimulated with LPS and siNLRP3
Fig. 5.
Secreted levels of IL-1β and IL-18 after NLRP3 siRNA treatment in human visceral adipocytes. a IL-1β and b IL-18 concentrations in the culture media of human visceral adipocytes incubated in the presence or absence of LPS (1000 ng/mL) for 3 h, followed by transfection with or without 100 pmol/L NLRP3 siRNA/2 × 105 cells/well for another 24 h. Values are the mean ± SEM (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Tukey’s tests. ***P < 0.001 vs. unstimulated cells. ††P < 0.01 vs. adipocytes stimulated with LPS
Fig. 6.
Effect of blocking NLRP3 on the LPS-induced expression of extracellular matrix remodeling genes in human visceral adipocytes. Gene expression levels of fibrosis-related factors in human visceral adipocytes incubated in the presence or absence of LPS (1000 ng/mL) for 3 h, followed by transfection with or without 100 pmol/L NLRP3 siRNA/2 × 105 cells/well for another 24 h. Gene expression levels in scrambled siRNA cells (CTL) were assumed to be 1. Values are the mean ± SEM (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Tukey’s tests. *P < 0.05 and **P < 0.01 vs. scrambled siRNA. †P < 0.05 and ††P < 0.01 vs. adipocytes stimulated with LPS
To explore the role of IL-1β in the regulation of inflammation- and fibrosis-related genes, we also reduced the constitutive expression levels of IL1B in human visceral adipocytes using a specific siRNA (Supplemental Fig. 4). In line with our previous results, we observed that LPS increased (P < 0.001) the mRNA expression of IL8, CCL2 and TNF, whereas the inhibition of IL1B downregulated (P < 0.01) their gene expression levels (Supplemental Fig. 4a–c). However, no differences in the expression of the ECM molecules COL1A1, COL4A3 and COL6A3 after both LPS treatment and the inhibition of IL1B were detected (Supplemental Fig. 4d–f).
Discussion
A huge body of knowledge supports the link between the inflammasome pathway and its central role in immune sensing as well as its key function in the development of different chronic inflammatory diseases.11,13,31,32 The most important finding of our investigation is that blocking the expression of NLRP3 reduces AT inflammation with a significant attenuation of fibrosis, most likely by the decrease in the production of IL-1β. We also demonstrated that obesity, obesity-associated T2D and NAFLD are involved in the activation of different components of the inflammasome as well as its main effector IL-1β in VAT. Consistently, we found that NLRP3, IL1B and IL18 gene expression levels were significantly increased in the liver from obese patients with T2D. Additionally, we further revealed that NLRP3, but not NLRP1, is regulated by inflammation-related factors and hypoxia in visceral adipocytes.
The relationship between the expression of the NLRP3 inflammasome components and IL-1β in AT with the development of insulin resistance and severity of T2D in individuals with obesity has been elegantly demonstrated by Vandanmagsar et al.24 Reportedly, mice lacking Nlrp3 exhibited decreased weight gain and fat mass as well as improved glucose homeostasis and insulin sensitivity.21,24,33 These observations are quite similar in humans, since obesity and T2D are associated with increased expression of the NLRP3 inflammasome subunit in AT and liver.24 Similarly, we found that obese patients with and without T2D exhibited increased levels of different inflammasome components, including NLRP3, NLRP1, NLRP6 and ASC, in the VAT. The positive association found between NLRP3 and NLRP1 with anthropometric parameters as well as with circulating leptin concentrations strengthens their functional role in obesity and its associated comorbidities. Notably, the metabolic unhealthy phenotype has been associated with an upregulation of NLRP3 in VAT as well as in its infiltrated macrophages.34 Furthermore, a significant reduction in NLRP3 and IL1B gene expression levels in AT from patients with obesity and T2D was detected 1 year after weight loss achieved by calorie restriction and exercise.24 However, no differences after laparoscopic adjustable gastric banding surgery were found.35 Although the involvement of the NLRP1 inflammasome in metabolic processes has been scarcely evaluated, an increase in AT in the obese state and a dysregulated glucose metabolism in Nlrp1-deficient mice have been demonstrated.20 NLRP3 has also been directly associated with pancreatic β-cell dysfunction during T2D by the activation of IL-1β, which is able to inhibit insulin signaling.36 Glyburide, an oral antihyperglycemic agent, has been described to inhibit NLRP3-mediated IL-1β release in monocytes, supporting the therapeutic potential of NLRP3 in T2D.37 The increased levels of CASP1, IL1B and IL18 detected in our study in both groups of patients with obesity indicate the activation of the inflammasome pathway. It must be highlighted that CASP1 mRNA needs to be translated before activating IL-1β. However, the combined increase in the expression of all NLRP inflammasome components and targets suggests that VAT from patients with obesity is primed to release the proinflammatory factors IL-1β and IL-18.38 In addition, the NLRP3 inflammasome participates in the development of NAFLD pathogenesis by promoting inflammation.39 In the liver from mice with hepatosteatosis, NLRP3 expression levels were elevated,40 while Nlrp3-knockout mice were protected against the development of diet-induced steatohepatitis.24 In this regard, we found increased NLRP3, IL1B and IL18 expression levels in the liver of patients with T2D, together with higher expression of NLRP3 and IL1B in the VAT from patients suffering from NAFLD. Furthermore, we found a negative association between NLRP3 and ALT, a reliable and sensitive marker of chronic liver disease that correlates with the severity of NAFLD.41
AT is composed of different cellular types, and the identification of the source responsible for adipose inflammasome component expression is not completely elucidated. We found that although all the analyzed inflammasome components were detected in mature adipocytes, their expression was more evident in the SVF, with macrophages being the primary component of the SVF during obesity.42 In this line, the expression of NLRP3 in macrophages has been shown to promote high-fat diet-induced insulin resistance in mice.43 Increased gene and protein expression of NLRP3, IL-1β and IL-18 in myeloid cells isolated from subjects with T2D has also been revealed.44 However, microarray studies have identified that the expression of inflammasome-related genes in adipocytes, but not in SVFCs, correlated with adiposity phenotypes.45 Thus, a common activation in both adipocytes and macrophages has been proposed with an early activation of adipocyte inflammasomes.
We also found that in this context of AT-associated inflammation, patients with obesity included in the study exhibited increased circulating and expression levels of inflammation and ECM remodeling-related mediators in VAT, with their strong association with NLRP3 expression underlining the involvement of this molecule in AT inflammation. Different endogenous (homocysteine, free fatty acids, ceramides, reactive oxygen species, uric acid, calcium or cholesterol crystals) and exogenous (LPS, aluminum) danger signals trigger the activation of the inflammasomes.15 In this study, we detected that NLRP3, and hence IL-1β expression and release, is primed by exogenous (LPS or aluminum or TNF-α) and endogenous (ATP or TNF-α) factors that promote inflammation as well as by hypoxia in human visceral adipocytes without exerting any effect on the expression of NLRP1. Reportedly, NLRP3 expression levels were decreased in epididymal AT in adipocyte-specific Hif1a-knockout mice, confirming the role of hypoxia in regulating the activation of NLRP3.30 This finding demonstrates the main implication of NLRP3 in mediating inflammatory effects in VAT rather than NLRP1.
Given that the expanded AT with infiltrated macrophages is an important site of origin of inflammation in obesity,46 we investigated whether the blocking of NLRP3 by siRNA is capable of regulating obesity-associated AT fibrosis. First, we confirmed that NLRP3 gene silencing resulted in the downregulation of IL1B expression and release as well as that of other key inflammatory markers, including IL6, IL8, CCL2 and TNF. No differences were found in the expression and released levels of IL18, pointing to the NLRP1 inflammasome as the main inflammasome inducing IL-18 production in fat.20 In line with previous results,24,47 the reduction of NLRP3 expression did not downregulate the expression of CASP1 in visceral adipocytes, suggesting that NLRP3 is dispensable for LPS-induced caspase-1 activation and evidencing the potential implication of other specific inflammasomes, including NLRP6, in mediating AT-specific effects as well as the role of independent-inflammasome signaling pathways in promoting inflammation. In contrast, inhibition of CASP3 and CASP7 expression was observed after siNLRP3 treatment. Caspases 3 and 7 are known as apoptotic effector caspases, and following BAX/BAK activation, both act as an upstream signal to induce NLRP3-dependent IL-1β activation.48 TLR4 signaling, a well-known mediator of obesity-induced inflammation, has been described to induce NLRP3 inflammasome activity. Previous research has suggested a molecular crosstalk between the TLR signaling cascade and the NLRP3 inflammasome inducing a synergistic effect.49 In this sense, we found that the increased expression of TLR4 after LPS treatment was reversed after the inhibition of NLRP3, confirming the connection between these membrane and cytosolic danger signal receptors.
The central feature of fibrosis is the increased production and deposition of ECM.25 Consistent with the role of inflammation in prompting fibrosis, we found that the inhibition of NLRP3 in adipocytes from obese patients promoted a reduction in the expression of important molecules involved in ECM deposition and fibrosis, including different collagens (COL1A1, COL4A3, and COL6A3) and MMP2, an important protease known to regulate the turnover of the ECM. Interestingly, activation of the NLRP3-inflammasome pathway has been closely associated with enhanced collagen deposition and increased expression of connective tissue growth factor in the liver,50 as well as with pulmonary fibrosis.51 In this line, pancreatic Langerhans islets,52 kidneys53 and livers54 from Nlrp3-deficient mice have been shown to be protected from fibrosis. The activation of the NLRP3 inflammasome pathway has been associated with IL-1R/MyD88 signaling in the development of fibrosis both in lung and liver in experimental animal models.55 Interestingly, a selective NLRP3 chemical inhibitor attenuated liver fibrosis, suggesting that the reduced activity of the NLRP3 inflammasome might have beneficial effects in the treatment of liver injury.56 Thus, blocking the upstream factors in the proinflammatory signaling pathways may open new therapeutic opportunities.
AT is a heterogeneous and plastic tissue composed of mature adipocytes and other cells including preadipocytes, fibroblasts, immune cells (macrophages, lymphocytes, mast cells, eosinophils, neutrophils and foam cells) and vascular endothelial cells that interplay among them, contributing to the main functions of AT. The complex interaction between adipocytes and immune cells in the obese state leads to the development of inflammation and subsequent AT dysfunction. However, the exact cell type (adipocytes or AT macrophages (ATMs)) responsible for inflammasome activation in VAT remains to be clarified. Some studies have reported that the inflammasome components were highly expressed in the ATMs and colocalized only with ATM markers.24 However, other studies have demonstrated the expression of the inflammasome components in both mouse and human primary adipocytes.30,45,57 Moreover, the depletion of macrophages did not modify inflammasome activation in AT.21 Since our main aim was to reveal the central role of adipocytes in the regulation of the inflammasome in VAT, a limitation of our study is the exclusion of ATMs in the in vitro analysis. To further clarify the exact cellular type responsible for the activation of inflammasomes in VAT, the analysis of the regulation of the inflammasome system in adipocytes and ATMs at the same time will help to compare the contributions of both cell types. Another limitation of our study is that although we confirmed the downregulation of the mRNA levels of NLRP3 and IL1B after the respective siRNA treatments, the analysis of protein knockdown by western blot could confirm the efficacy of the siRNA treatments at the protein level.
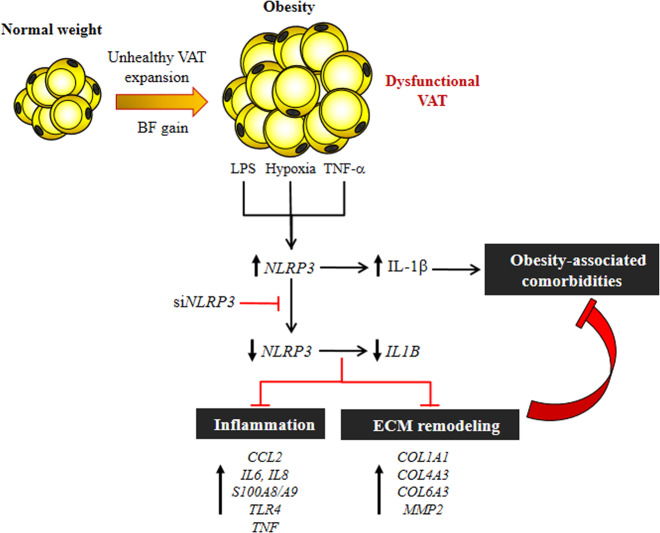
The inflammasomes are required to control the main elements of inflammation and tissue repair.14,58 Taken together, these novel findings provide robust support for the proposal that the blockade of NLRP3 activation, a key upstream protein in the inflammatory pathway in AT, can improve inflammation and fibrosis regulating the development of obesity-associated comorbidities, including T2D or NAFLD. A better characterization of the involvement of the NLRP3 inflammasome pathway in AT fibrosis opens up interesting new therapeutic targets for the reduction of collagen deposition and fibrosis in AT, providing a useful framework within which to understand the pathogenesis of obesity-linked disorders (Fig. 7).
Fig. 7.
Obesity-associated AT dysfunction favors the development of different comorbidities, including T2D and NAFLD. Danger signals, including endogenous (TNF-α) and exogenous (LPS) inflammatory factors as well as hypoxia, are able to induce the expression of the cytosolic innate immune signaling receptor NLRP3 and subsequently the activation of IL-1β. NLRP3 silencing using siRNA inhibits the expression and release of IL-β as well as important factors promoting inflammation (CCL2, IL6, IL8, S100A8, S100A9 and TNF) and fibrosis (COL1A1, COL4A3, COL6A3 and MMP2) in human visceral adipocytes. In this sense, direct targeting of NLRP3 could offer potential efficacy in the treatment of obesity-associated comorbidities
Material and methods
Patient selection
Blood and tissue samples from 98 subjects (22 males and 76 females) recruited from healthy volunteers and patients attending the Departments of Endocrinology & Nutrition and Surgery at the Clínica Universidad de Navarra were used. Volunteers underwent a body composition analysis and comorbidity evaluation by a multidisciplinary consultation team. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters, and body fat (BF) was estimated by air-displacement-plethysmography (Bod-Pod®, Life Measurements, Concord, CA). Obese patients were further subclassified into two groups (normoglycemia or T2D) following the criteria of the Expert Committee on the Diagnosis and Classification of Diabetes.59 The diagnosis of NAFLD and NASH was established by the anatomo-pathological analysis of liver biopsies obtained during bariatric surgery applying the criteria of Kleiner and Brunt by an expert pathologist masked to all the results of the assays.28 In all patients, ultrasonography was able to correctly identify steatosis compared with the histological analysis.
AT samples were collected from patients undergoing either Nissen fundoplication (for hiatal hernia repair in lean volunteers) or Roux-en-Y gastric bypass (RYGB) (for morbid obesity treatment in obese subjects) at the Clínica Universidad de Navarra. Both interventions were carried out via a laparoscopic approach. In addition, an intraoperative liver biopsy was performed in the obese patients during bariatric surgery to establish a histological diagnosis of the hepatic state as well as for gene expression studies. This procedure is not clinically justified in lean subjects. Tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C for subsequent analyses. The study was approved from an ethical and scientific standpoint by the Research Ethics Committee of the University of Navarra (protocol 2017.126), and written informed consent was obtained from the participants.
Analytical procedures
Plasma samples were obtained by venipuncture after an overnight fast. Glucose was analyzed by an automated analyzer (Hitachi Modular P800, Roche, Basel, Switzerland). Insulin was measured by means of an enzyme-amplified chemiluminescence assay (IMMULITE®, Diagnostic Products Corp., Los Angeles, CA) with intra- and interassay coefficients of variation of 4.2% and 5.7%, respectively. Insulin resistance and sensitivity were calculated using the HOMA and QUICKI indices, respectively.60 Total cholesterol, high-density lipoprotein (HDL-cholesterol) and low-density lipoprotein (LDL-cholesterol) levels were determined as previously described.61 Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and γ-glutamyltransferase (γ-GT) were measured by enzymatic tests in an automated analyzer (Roche/Hitachi Modular P800). The AST/ALT ratio was calculated as an indirect indicator of fatty liver disease.62 High-sensitivity CRP, fibrinogen, homocysteine and von Willebrand factor antigen (vWF) concentrations were determined as previously reported.61 Leptin was measured by a double-antibody RIA method (Linco Research, Inc., St. Charles, MO); intra- and interassay coefficients of variation were 5.0% and 4.5%, respectively.63 White blood cell count was measured using an automated cell counter (Beckman Coulter, Inc., Fullerton, CA).
RNA extraction and real-time PCR
RNA extraction was performed by homogenization with an Ultra-Turrax® T25 basic (IKA® Werke Gmbh, Staugen, Germany) using QIAzol® Reagent (Qiagen, Hilden, Germany) for AT and adipocytes and TRIzol® Reagent (Invitrogen, Carlsbad, CA) for liver biopsies. RNA purification was performed using the RNeasy Mini Lipid Kit (Qiagen) in AT and adipocytes and the RNeasy Mini kit (Qiagen) in liver biopsies. All samples were treated with DNase I (RNase Free DNase set, Qiagen). For first strand cDNA synthesis, constant amounts of 3 μg of total RNA were reverse transcribed in a 60 μL final volume using random hexamers (Roche) as primers and 300 units of M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA).
The transcript levels for apoptosis-associated speck-like protein containing a CARD (ASC); caspase (CASP)-1 CASP3, and CASP7; collagen (COL)-1A1, COL4A3, COL5A3, and COL6A3; decorin (DCN); elastin (ELN); fibroblast growth factor (FGF)-2; hypoxia inducible factor 1 α (HIF1A); interleukin (IL)-4, IL1B, IL4, IL6, IL8, and IL18; matrix metalloproteinase (MMP)-2 and MMP9; monocyte chemoattractant protein-1 (CCL2); nucleotide-binding oligomerization domain, leucine rich repeat and pyrin (NLRP)-1, NLRP-3, and NLRP-6; nucleotide binding oligomerization domain containing 2 (NOD2); osteopontin (SPP1); S100 calcium-binding protein A (S100A)-8 and S100A9; transforming growth factor-β (TGFB1); toll-like receptor-4 (TLR4); tenascin C (TNC); tumor necrosis factor-α (TNF); and tenomodulin (TNMD) were quantified by real-time PCR (7300 Real Time PCR System, Applied Biosystems, Foster City, CA) as previously described. Primers and probes (Supplemental Table 3) were designed using the software Primer Express 2.0 (Applied Biosystems) and purchased from Genosys (Sigma-Aldrich, Madrid, Spain). Primers or TaqMan® probes encompassing fragments of the areas from the extremes of two exons were designed to ensure the detection of the corresponding transcript, avoiding genomic DNA amplification. The cDNA was amplified under the following conditions: 95 °C for 10 min, followed by 45 cycles of 15 s at 95 °C and 1 min at 59 °C, using the TaqMan® Universal PCR Master Mix (Applied Biosystems). The primer and probe concentrations for gene amplification were 300 and 200 nmol/L, respectively. The endogenous control gene 18 S rRNA (Applied Biosystems) was the loading control for real-time PCR experiments, and relative quantification was calculated using the ΔΔCt formula. Relative mRNA expression was expressed as fold expression over the calibrator sample (the average of the gene expression corresponding to the lean group) as previously described.64 All samples were run in triplicate, and the average values were calculated.
Fibrosis characterization
Sections of formalin-fixed paraffin-embedded VAT (6 µm) were dewaxed with xylene and rehydrated with decreasing concentrations of ethanol. Fibrosis was localized by Sirius Red staining (Sigma). Images of five fields per section from each sample were obtained at ×200 magnification. Total fibrosis quantification was expressed as the ratio of fibrous tissue area stained with Sirius Red to the total tissue surface using ImageJ analysis software, as previously described.65
Adipocyte culture
Human stromovascular fraction cells (SVFCs) were isolated from visceral AT of obese NG subjects (BMI: 41.9 ± 3.3 kg/m2; BF, 56.0 ± 2.0%; glucose: 98 ± 5 mg/dL, glucose 2 h after an oral glucose tolerance test: 114 ± 8 mg/dL) as previously described.66 SVFCs were seeded at 2 × 105 cells/well and grown in adipocyte medium [DMEM/F-12 [1:1], 17.5 mol/L glucose, 16 μmol/L biotin, 18 μmol/L panthotenate, 100 μmol/L ascorbate and antibiotic-antimycotic] supplemented with 10% newborn calf serum (NCS). After 4 days, the medium was changed to adipocyte medium supplemented with 3% NCS, 0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX), 0.1 μmol/L dexamethasone, 1 μmol/L BRL49653 and 10 μg/mL insulin. After a 3-day induction period, cells were fed every 2 days with the same medium but without IBMX and BRL49653 supplementation for the remaining 7 days of adipocyte differentiation. Adipocyte differentiation efficacy was determined by examining the time course gene expression profiles of peroxisome proliferator-activated receptor γ (PPARG), a key transcriptional factor known to be involved in adipocyte differentiation as well as the mRNA levels of adiponectin (ADIPOQ), a marker of mature adipocytes. As shown in Supplemental Fig. 5, the gene expression levels of PPARG (P < 0.01) and ADIPOQ (P < 0.00001) significantly increased during adipocyte differentiation.
Differentiated human visceral adipocytes were serum-starved for 24 h and then incubated in the presence of LPS (1000 ng/mL) for 3 h, followed by the addition of the inflammasome triggers ATP (5 mmol/L) (Sigma) and aluminum (200 μg/mL) (Sigma) for another 24 h. In another set of experiments, cells were serum-starved for 24 h and then treated with increasing concentrations of TNF-α (1, 10 and 100 ng/mL) (Sigma), IL-4 (10, 100 and 1,000 ng/mL) (R&D systems, Minneapolis, MN), IL-13 (10, 100 and 1,000 ng/mL) (R&D systems), and CoCl2 (100 and 200 nmol/L) for 24 h.
Adipocyte transfection with siRNA
Differentiated human visceral adipocytes were serum-starved for 24 h, and then two pairs of small interfering RNAs (siRNAs) for blocking NLRP3 expression (s128674 and s128675, Ambion, ThermoFisher Scientific, Waltham, MA) as well as another two pairs of siRNAs for downregulating IL1B expression (s7270 and s7171, Ambion, ThermoFisher Scientific) were annealed and transfected into adipocytes (100 pmol/L siRNA/2 × 105 cells/well) using 40 nmol of Lipofectamine 2000 (Invitrogen).61 The siRNA sequences were as follows: siNLRP3 s128674 sense AGGCUGAUCUGUUGCCGUATT, antisense UACGGCAACAGAUCAGCCUTG; siNLRP3 s128675 sense AGCUCACUCCUCUACUUGATT, antisense UCAAGUAGAGGAGUGAGCUCT; siIL1B s7270 sense GCACCUCUCAAGCAGAAAATT, antisense UUUUCUGCUUGAGAGGUGCTG; and siIL1B s7271 sense CGAUGCACCUGUACGAUCATT and antisense UGAUCGUACAGGUGCAUCGTG. A scrambled siRNA was used as a negative control. Treatment with NLRP3-s128675 resulted in a 48% average knockdown of NLRP3 mRNA, whereas no decreased expression was found after transfection with siNLRP3-s128674 (Supplemental Fig. 6a). Treatment with IL1B-s7270 and IL1B-s7271 decreased the expression of IL1B by 51% and 24%, respectively (Supplemental Fig. 6b). In this sense, NLRP3-siRNA s128675 and IL1B-s7270 were selected for NLRP3 and IL1B knockdown studies, respectively. Adipocytes were cultured in the presence or absence of LPS (1000 ng/mL) for 3 h followed by NLRP3-s128675 or IL1B-s7270 transfection for another 24 h.
Detection of inflammatory factors in adipocyte culture medium
The adipocyte-conditioned medium (ACM) was collected from differentiated adipocytes after treatment with different stimuli, centrifuged at 200 × g for 10 min and stored at −80 °C. In order to assess the concentrations of IL-1β and IL-18 in the ACM, commercially available ELISA kits (RayBiotech, Inc., Norcross, GA) were used according to the manufacturer’s instructions. The intra- and interassay coefficients of variation were <10% and <12%, respectively, for all analyzed molecules.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Differences in the proportion of subjects within groups regarding sex were assessed using the Chi-square test. Due to their non-normal distribution, C-reactive protein concentrations and gene expression levels were logarithmically transformed. The normal distribution of the other variables was adequate for the use of parametric tests. Differences between groups were assessed by one-way ANOVA followed by Tukey’s or Dunnett’s post hoc tests and two-tailed unpaired Student’s t tests as appropriate. Pearson’s correlation coefficients (r) were used to analyze the association between variables. The calculations were performed using the SPSS/Windows version 15.0 statistical package (SPSS, Chicago, IL). A P value < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
The authors gratefully acknowledge the valuable collaboration of all the members of the Multidisciplinary Obesity Team, Clínica Universidad de Navarra, Pamplona, Spain. This work was supported by Plan Estatal I+D+I from the Spanish Instituto de Salud Carlos III–Subdirección General de Evaluación y Fomento de la investigación–FEDER (grants number PI16/01217, PI17/02183 and PI17/02188), by the Gobierno de Navarra (10/2018) and by CIBEROBN, ISCIII, Spain.
Author contributions
X.U. collected and analyzed data, wrote the first draft of the manuscript, contributed to discussion, and reviewed the manuscript. J.G.-A., A.R. and S.B. collected and analyzed the data, contributed to the discussion, and reviewed the manuscript. V.V., C.S., and J.S. enrolled patients, collected data, contributed to discussion, and reviewed the manuscript. B.R. and R.M. collected data, contributed to discussion, and reviewed the manuscript. G.F. designed the study, wrote the first draft of the manuscript, contributed to discussion, and reviewed the manuscript. V.C. designed the study, collected and analyzed data, wrote the first draft of the manuscript, contributed to discussion, and reviewed the manuscript. V.C. and G.F. are guarantors for the contents of the article and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare no competing interests.
Ethics statement
The study was approved, from an ethical and scientific standpoint, by the Clínica Universidad de Navarra’s Ethical Committee responsible for research, and written informed consent of participants was obtained.
Contributor Information
Gema Frühbeck, Email: gfruhbeck@unav.es.
Victoria Catalán, Email: vcatalan@unav.es.
Supplementary information
The online version of this article (10.1038/s41423-019-0296-z) contains supplementary material.
References
- 1.GBDO Collaborators, et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James WPT. Obesity: a global public health challenge. Clin. Chem. 2018;64:24–29. doi: 10.1373/clinchem.2017.273052. [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Wollam J, Olefsky JM. An integrated view of immunometabolism. Cell. 2018;172:22–40. doi: 10.1016/j.cell.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Wu H. T cells in adipose tissue: critical players in immunometabolism. Front. Immunol. 2018;9:2509. doi: 10.3389/fimmu.2018.02509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab. 2017;25:11–26. doi: 10.1016/j.cmet.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 12.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 13.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 16.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat. Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 20.Murphy AJ, et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 2016;23:155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Netea MG, Joosten LA. The NLRP1-IL18 connection: a stab in the back of obesity-induced inflammation. Cell Metab. 2016;23:6–7. doi: 10.1016/j.cmet.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome-an allostatic perspective. Biochim. Biophys. Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin. Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 29.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int. J. Obes. (Lond.) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SY, et al. Adipocyte-derived lysophosphatidylcholine activates adipocyte and adipose tissue macrophage nod-like receptor protein 3 inflammasomes mediating homocysteine-induced insulin resistance. EBioMedicine. 2018;31:202–216. doi: 10.1016/j.ebiom.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahechu P, et al. NLRP3 inflammasome: a possible link between obesity-associated low-grade chronic inflammation and colorectal cancer development. Front. Immunol. 2018;9:2918. doi: 10.3389/fimmu.2018.02918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangan MSJ, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:688. doi: 10.1038/nrd.2018.149. [DOI] [PubMed] [Google Scholar]
- 33.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 34.Esser N, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487–2497. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- 35.Moschen AR, et al. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011;17:840–845. doi: 10.2119/molmed.2010.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jourdan T, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamkanfi M, et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kursawe R, et al. A role of the inflammasome in the low storage capacity of the abdominal subcutaneous adipose tissue in obese adolescents. Diabetes. 2016;65:610–618. doi: 10.2337/db15-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mridha AR, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017;66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csak T, et al. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Public Policy Committee of the American Association for the Study of Liver D. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HM, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin Z, et al. Transcriptome analysis of human adipocytes implicates the NOD-like receptor pathway in obesity-induced adipose inflammation. Mol. Cell Endocrinol. 2014;394:80–87. doi: 10.1016/j.mce.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 2017;127:74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuoka M, et al. TNF-alpha induces caspase-1 activation independently of simultaneously induced NLRP3 in 3T3-L1 cells. J. Cell Physiol. 2016;231:2761–2767. doi: 10.1002/jcp.25385. [DOI] [PubMed] [Google Scholar]
- 48.Vince JE, et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1beta activation. Cell Rep. 2018;25:2339–2353.e2334. doi: 10.1016/j.celrep.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 49.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wree A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasse P, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 52.Youm YH, et al. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–4045. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakker PJ, et al. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int. 2014;85:1112–1122. doi: 10.1038/ki.2013.503. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe A, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1248–G1257. doi: 10.1152/ajpgi.90223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert, S. et al. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep.36, 1–11 (2016). [DOI] [PMC free article] [PubMed]
- 56.Qu J, Yuan Z, Wang G, Wang X, Li K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int. Immunopharmacol. 2019;70:147–155. doi: 10.1016/j.intimp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Sun S, Xia S, Ji Y, Kersten S, Qi L. The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue. Diabetes. 2012;61:1471–1478. doi: 10.2337/db11-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 59.American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 60.Gómez-Ambrosi J, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37:2813–2821. doi: 10.2337/dc14-0937. [DOI] [PubMed] [Google Scholar]
- 61.Catalán V, et al. Increased interleukin-32 levels in obesity promote adipose tissue inflammation and extracellular matrix remodeling: effect of weight loss. Diabetes. 2016;65:3636–3648. doi: 10.2337/db16-0287. [DOI] [PubMed] [Google Scholar]
- 62.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 63.Muruzabal FJ, Frühbeck G, Gómez-Ambrosi J, Archanco M, Burrell MA. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002;128:149–152. doi: 10.1016/s0016-6480(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 64.Catalán V, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm. Metab. Res. 2007;39:495–500. doi: 10.1055/s-2007-982502. [DOI] [PubMed] [Google Scholar]
- 65.Divoux A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodríguez A, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int. J. Obes. (Lond.) 2009;33:541–552. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.