It is believed that the adaptive immune system responds to nonself according to its specificity and memory properties, whereas the innate immune system lacks adaptive characteristics. The specificity of the adaptive immune response is based on irreversible changes in the DNA sequence by retroposons encoding site-specific recombinases, which generate unlimited numbers of receptors to allow B lymphocytes and T lymphocytes to specifically recognize antigens. Recent studies have shown that innate myeloid cells can engage in allogeneic recognition with specificity and respond to subsequent triggers more strongly than the primary response, a process revealing the immune memory property termed “trained immunity”.1,2 After encountering allogeneic splenocytes 1 or 4 weeks prior to the experiment, monocytes of lymphocyte-deficient Rag2-deficient mice cause a delayed-type hypersensitivity-like reaction, suggesting that this type of allogeneic antigen recognition is manifested in memory responses.3 Liu et al. showed that macrophages from mice primed with allogeneic cells readily recognized and rejected allogeneic cells.4 Importantly, Chu et al. recently demonstrated that allogeneic antigen-primed macrophages alone acutely rejected allogeneic skin grafts with specificity after adoptive transfer of these cells into MHC-matched immunodeficient mice.5 In the present study, we found that macrophages primed with the help of CD4+ T cells in the priming stage could reject allogeneic cells and skin grafts due to long-term memory properties (at least 4 months) in a mouse model in which immunodeficient mice received adoptive transfer of primed macrophages. These results revealed that primed macrophages gained long-term memory features to specifically mediate allograft rejection.
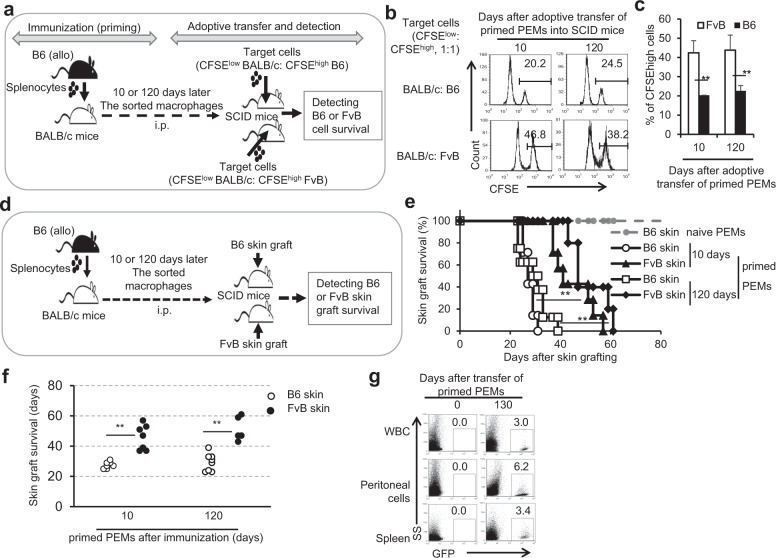
Macrophages can display memory as a result of bacterial infection,6,7 but this immune memory phenotype is short term and nonspecific in the studied models. Therefore, we tried to determine whether macrophages primed with the help of CD4+ T cells exhibited a specific memory trait in the rejection of allogeneic splenocytes over the long term. The results showed that primed B6 mice could efficiently eliminate primed donor BALB/c cells but not third-party allogeneic C3H splenocytes at various time points after immunization (Supplementary Fig. 1A, B). Similarly, we observed that B6 cell-immunized BALB/c mice specifically eliminated B6 splenocytes at 10 or 120 days after immunization without obvious elimination of third-party allogeneic FvB cells (Supplementary Fig. 1C, D). To further identify whether primed macrophages exhibit memory in the process of rejecting allogeneic splenocytes, we adoptively transferred the primed PEMs into MHC-matched SCID mice to test the clearance of allogeneic cells 120 days after BALB/c mice were immunized with B6 splenocytes (Fig. 1a). The results showed that SCID mice with transfer of primed, MHC-matched BALB/c PEMs isolated from BALB/c mice immunized with B6 splenocytes 120 days earlier could specifically reject priming donor B6 splenocytes similar to primed BALB/c PEMs isolated from BALB/c mice immunized with B6 splenocytes 10 days earlier (Fig. 1b, c). Thus, the ability of the primed macrophages to mediate the specific rejection of allogeneic cells could be maintained for at least 120 days after immunization in hosts.
Fig. 1.
Primed macrophages rejected allogeneic donor splenocytes and skin grafts with memory. a The flowchart for the donor and third-party splenocyte rejection assays in SCID mice with adoptive transfer of primed macrophages is shown. BALB/c mice were immunized with B6 splenocytes. By 10 or 120 days after immunization, the sorted B6 splenocyte-priming BALB/c PEMs and CFSE-labeled target splenocytes (BALB/c cells: B6/FvB cells = 1:1) were mixed at a 4:1 ratio and intraperitoneally injected into SCID mice. The survival of CFSE-labeled cells (b) and the total percentages of CFSEhigh cells among total CFSE-labeled cells (c) in SCID mice were determined 24 h later by flow cytometry. d The experimental procedure for investigation of donor and third-party skin graft rejection in SCID mice with adoptive transfer of primed macrophages is shown. BALB/c mice were immunized with B6 splenocytes. By 10 or 120 days after immunization, the sorted naive or primed BALB/c PEMs were adoptively transferred into MHC-matched SCID mice by i.v. injection, followed by transplantation of allogeneic priming donor B6 or third-party allogeneic FvB skin grafts. e The survival of B6 and FvB skin grafts in the SCID mice is shown. f The number of days of survival of allogeneic skin grafts in the SCID mice are summarized. g The percentages of transferred BALB/c splenocyte-primed B6-GFP+ macrophages were detected in the peripheral blood, peritoneal cavity, and spleen in syngeneic Rag2 KO mice 130 days after adoptive transfer. **P < 0.01 compared with the indicated groups
Do primed macrophages have the ability via memory to reject skin tissue allografts? The primed PEMs were sorted from naive or B6 splenocyte-immunized BALB/c mice and adoptively transferred into MHC-matched SCID mice. These SCID mice were subsequently grafted with either B6 or third-party FvB skin tissues, as shown in Fig. 1d. SCID mice that received naive PEMs received allogeneic B6 skin grafts and did not show evidence of rejection for more than 100 days. However, SCID mice that received B6 cell-primed PEMs efficiently rejected priming donor B6 skin grafts within 40 days, while they rejected third-party FvB skin grafts in a significantly delayed manner, regardless of whether the PEMs were isolated from BALB/c mice immunized with B6 splenocytes 10 days or 120 days ago (P < 0.01, Fig. 1e, f). Therefore, primed macrophages could somehow specifically reject allogeneic skin grafts, as reported previously.5 Importantly, the primed PEMs isolated from BALB/c mice that were immunized with B6 splenocytes 120 days ago could also efficiently reject priming donor B6 skin grafts after their adoptive transfer into MHC-matched SCID mice similar to primed PEMs isolated from BALB/c mice that were immunized with B6 splenocytes 10 days ago. These data indicated that the ability of the primed macrophages to specifically reject allogeneic skin grafts could be maintained for at least 120 days in mice after immunization.
After observing the memory properties of the primed macrophages, we wanted to determine whether these primed macrophages could live for a long period of time in vivo. We thus detected the presence of adoptively transferred primed macrophages in Rag2 KO mice (B6 background, H-2b) that received primed syngeneic GFP B6 PEMs (H-2b) prior to allogenic BALB/c skin grafting. As shown in Fig. 1g, a specific percentage of donor GFP-PEMs (~3–6%) could be detected in the peripheral blood, peritoneal cavity and spleens of Rag2 KO mice 130 days after adoptive transfer of the primed syngeneic GFP B6 PEMs into Rag2 KO recipient mice. These results indicated that primed macrophages could survive in mice for a long period of time.
Recent studies have shown that innate immune cells exhibit stronger and more rapid inflammatory responses when challenged with subsequent triggers, a process revealing the immune memory property termed “trained immunity”.1 The trained immune response is mainly orchestrated by cellular metabolism reprogramming and epigenetic reprogramming, such as DNA methylation or transcription of long noncoding RNAs.8,9 Foster et al. reported that after TLR-mediated triggering, macrophages are capable of undergoing a chromatin modification program called LPS tolerance, which has features similar to those of memory, to adapt to repeated exposure.10 Our current study demonstrated that macrophages primed with allogeneic antigens in immunocompetent mice reject allogeneic priming donor splenocytes or skin grafts directly due to their specificity and memory properties.
Our recent studies showed that primed macrophages but not unprimed macrophages in immunodeficient mice could proliferate significantly upon re-encountering subsequent allogeneic skin grafts.5 In this study, we found that primed macrophages could survive in secondary recipients for at least 4 months and can display memory properties for specific rejection of priming donor allogeneic cells or skin grafts for at least 120 days. The long-term presence of primed macrophages offers an essential fundamental basis for the long-term memory features of primed macrophages. It is true that even when macrophages were primed with alloantigen almost 4 months earlier, the specific rejection capacity of the primed macrophages for priming donor splenocytes or skin grafts was not much different from that of macrophages primed for a short time (10–14 days). These results demonstrated for the first time that primed macrophages acquire memory characteristics to specifically reject allogeneic grafts in the long term. Thus, after activation in immunocompetent mice and with the help of CD4+ T cells in the priming phase, macrophages were endowed with an adaptive immunity feature to recall the primary immune response. The primed macrophages survived for a long time, which met the essential requirement for long-term memory features. The relevant molecular mechanisms are not clear and need to be investigated in the future. This study greatly challenges our traditional opinion about the innate immunity of macrophages and provides new insights and concepts for us to gain a deeper understanding of macrophage biological characteristics. Moreover, the clarification of primed macrophage-mediated allograft rejection will significantly aid in the reconsideration of the role of effector cells in allograft rejection and the complex process of allograft rejection, which would finally allow us to explore novel strategies for overcoming organ graft rejection. Recognizing the specificity and long-term memory characteristics of the primed macrophage-mediated immune response would significantly expand our understanding of macrophage biology far beyond that of innate immunity.
Supplementary information
Acknowledgements
The authors thank Dr. Zhanfeng Liang for his kind review of the manuscript and Qing Meng for her expert technical assistance. This work was supported by grants from the National Key R&D Program of China (2017YFA0105002, 2017YFA0104402, Y.Z.), the Chinese Government 13th Five-Year Plan (2017ZX10201101), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16030301, Y.Z.), the National Natural Science Foundation for General and Key Programs (C81530049, C81130055, C31470860, U1738111, Y.Z.), and the Fundamental Research Funds for the Central Universities (2018-JYBZZ-XJSJJ004, Z.C.).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Zhulang Chu, Chang Feng, Chenming Sun
Supplementary information
The online version of this article (10.1038/s41423-020-00521-7) contains supplementary material.
References
- 1.Netea MG, et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberbarnscheidt MH, Lakkis FG. Innate allorecognition. Immunol. Rev. 2014;258:145–149. doi: 10.1111/imr.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG. An innate response to allogeneic nonself mediated by monocytes. J. Immunol. 2009;183:7810–7816. doi: 10.4049/jimmunol.0902194. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Xiao X, Demirci G, Madsen J, Li XC. Innate NK cells and macrophages recognize and reject allogeneic nonself in vivo via different mechanisms. J. Immunol. 2012;188:2703–2711. doi: 10.4049/jimmunol.1102997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu Z, et al. Primed macrophages directly and specifically reject allografts. Cell Mol. Immunol. 2020;17:237–246. doi: 10.1038/s41423-019-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weavers H, Evans IR, Martin P, Wood W. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell. 2016;165:1658–1671. doi: 10.1016/j.cell.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing Z, et al. Innate immune memory of tissue-resident macrophages and trained innate immunity: re-vamping vaccine concept and strategies. J. Leukoc. Biol. 2020 doi: 10.1002/JLB.4MR0220-446R. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SC, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arts RJ, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24:807–819. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.