Abstract
Youth at clinical high risk (CHR) are a unique population enriched for precursors of major psychiatric disorders, especially schizophrenia (SCZ). Recent neuroimaging findings point to abnormalities in the thalamus of patients with SCZ, including chronic and early course patients, as well as in CHR individuals relative to healthy comparison groups, thus suggesting that thalamic dysfunctions are present even before illness onset. Furthermore, modeling data indicate that alteration between excitatory and inhibitory control, as reflected by alteration in GABAergic and glutamatergic balance (i.e., GABA/Glu), may underlie thalamic deficits linked to the risk and development of psychosis. There is, however, a lack of in vivo evidence of GABA/Glu thalamic abnormalities in the CHR state. Magnetic resonance spectroscopic imaging (MRSI) 7 Tesla (7 T) provides enhanced resolution to quantify GABA and Glu levels in the thalamus of CHR individuals. In this study, we performed 7 T MRSI in 15 CHR and 20 healthy control (HC) participants. We found that GABA/Glu was significantly reduced in the right medial anterior and right medial posterior thalamus of CHR relative to HC groups. The GABA/Glu reduction was negatively correlated with general symptoms in the right medial anterior thalamus, as well as with disorganization symptoms in the right medial posterior thalamus. Altogether, these findings indicate that GABA/Glu abnormalities are present in the thalamus before the onset of full-blown psychosis and are associated with symptom severity, thus providing putative molecular and neuronal targets for early interventions in youth at CHR.
Subject terms: Predictive markers, Psychosis, Neuroscience, Risk factors
Introduction
Youth at clinical high risk (CHR) are a unique population enriched for precursors of major psychiatric disorders, including schizophrenia (SCZ), who also experience emergent cognitive impairments, social dysfunction, and sub-syndromal clinical symptoms. Identifying the neuronal and molecular mechanisms underlying these dysfunctions in CHR individuals may therefore contribute to unveil the neurodevelopmental vulnerabilities and abnormalities before full-blown psychopathology, which in turn could provide novel targets for early, more effective treatment interventions.
Previous neuroimaging work has shown that thalamic abnormalities are present in CHR individuals [1]. For instance, recent studies have reported lower thalamic volumes in CHR [2, 3] patients relative to healthy control (HC) subjects. Furthermore, decreased structural and functional connectivity between the thalamus and the cortex has been demonstrated in CHR compared to HC individuals [4–6], and these alterations were associated with CHR clinical symptoms and cognitive and sensory deficits [7].
The thalamus consists primarily of glutamatergic and GABAergic neurons. Glutamate as the primary excitatory and GABA as the primary inhibitory neurotransmitter are metabolically closely related to each other [8]. GABA is directly synthesized from glutamate using the enzyme glutamate decarboxylase, and the GABA is transaminated into amino acid glutamate in GABA degradation [9]. Synthesis, degradation, and transport are the three processes involved in the homeostasis of Glu and GABA, which in turn maintains the balance of excitation and inhibition, which is crucial for brain function [10]. Several studies have utilized magnetic resonance spectroscopy (MRS) to study the metabolic-related anomalies in the thalamus of CHR. Lower N-acetylaspartate (NAA) and glutamate levels have been reported in these MRS studies [11, 12]. In contrast, thalamic GABA concentration, and its relationship with glutamate (i.e., GABA/Glu ratio), has not been investigated in CHR. In vivo GABA measurement in the thalamus is challenging due to the relatively low concentration and the high degree of spectral overlap of GABA with other neurotransmitter resonances (e.g., creatine and amino acids). Nonetheless, MRS acquisition paradigms have been recently developed to better separate/suppress macromolecules from GABA, thus allowing a more accurate GABA measurement [13, 14]. Furthermore, employing 7 T scans enhances the sensitivity of the measurements of metabolic concentration by increasing the signal-to-noise ratio and spectral resolution, which in turn enables better separation of compounds, including GABA and glutamate, at a higher precision than at lower field strength [15–17]. Additionally, MRS imaging (MRSI), compared to localized MRS, allows one to position multiple well-defined regions of interest in specific brain structures, as well as to better evaluate GABA and glutamate concentration.
In this study, we employed 7 T MRSI to investigate the GABA–glutamate balance (i.e., GABA/Glu ratio) in the thalamus of CHR relative to HC subjects. To assess for specificity, differences in five other neurometabolite ratios (GABA/Cre, Glu/Cre, tNAA/Cre, tCho/Cre, and Gln/Cre) were examined in the CHR and HC groups. Furthermore, the association between GABA/Glu ratio and clinical assessments in CHR individuals were investigated.
Methods
Demographic and clinical characteristics
Study participants underwent a comprehensive screening assessment, including Structured Clinical Interview for DSM-5 Disorders, Structured Interview for Prodromal Syndromes (SIPS), and Childhood Brain Injury Interview. In CHR, clinical symptoms were assessed using the SIPS [18]. The SIPS is used to determine CHR status, including the presence of subthreshold psychotic symptoms. It contains the Scale of Prodromal Symptoms (SOPS), a scale that assesses the severity of high-risk symptoms and how they change over time. The SOPS has four subscales: Positive (SOPS_PS), Negative (SOPS_NS), Disorganization (SOPS_DS), and General (SOPS_GS). A group of clinical interviewers from the Western Psychiatric Institute with extensive experience in assessing prodromal individuals and healthy comparison subjects performed the interviews and scoring.
For the CHR group, a total of 22 participants were recruited. Of those, five individuals were found to be ineligible due to metal in their body (i.e., intrauterine device, braces), whereas two were excluded due to claustrophobia. For the HC group, a total of 21 participants were recruited, 1 of whom was excluded due to poor data quality. Therefore, data from 15 CHR (age: 20.1 ± 4 years and 7 females) and 20 HC (age: 21.6 ± 4.11 years and 11 females) participants were used in this study. Table 1 shows demographic and clinical variables for the CHR and HC groups.
Table 1.
Clinical and demographic variables of the subjects.
| Clinical measures | HC (N = 20, 11 females), mean ± STD | CHR (N = 15, 7 females), mean ± STD | p |
|---|---|---|---|
| Age (years) | 21.6 ± 4.11 | 20.1 ± 4 | 0.272 |
| IQ | 111.7 ± 11.7 | 102.5 ± 11.2 | 0.015 |
| SOPS_PS | N/A | 12.9 ± 6.7 | |
| SOPS_NS | N/A | 12.6 ± 6.7 | |
| SOPS_DS | N/A | 5.3 ± 2.8 | |
| SOPS_GS | N/A | 7 ± 3.9 |
The bolded p-value indicates a significant difference in IQ between CHR and HC.
N/A not applicable.
Recruitment
CHR and HC participants were recruited through online and physical advertisements in the community and referrals from other studies. CHR individuals were also enrolled through UPMC clinical settings, referrals from other clinicians, and outside sources. Our study was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent prior to completing study procedures and received financial compensation for participation in the study.
Eligibility
Eligibility criteria for each study participant included (1) ages 12–35 years; (2) speaking English fluently enough to participate in clinical assessments and study procedures; (3) able to travel to Western Psychiatric Hospital to participate in the assessments; (4) no lifetime history of diagnosed neurological disorder or head injury resulting in loss of consciousness for >1 min, (5) no pregnancy, (6) no history of alcohol or drug dependence within the past 12 months; and (7) ability to provide informed consent. CHR had to meet the following criteria: (1) meet one of the four possible prodromal states, as assessed by the SIPS: (I) Genetic Risk and Functional Deterioration State, (II) Attenuated Positive Symptom State, Currently Progressive, (III) Brief Intermittent Psychotic State, and (IV) Youth and Schizotypy; and (2) no diagnosis of a psychotic disorder. HC participants had to meet the following criteria: (1) no Axis-1 disorder diagnosis, (2) no high-risk syndrome diagnosis, and (3) no first-degree relatives with known psychotic disorder diagnosis.
1H spectroscopy and imaging
Data were acquired on a 7 T Siemens Magnetom scanner using an 8-channel multiple transmit system with a body gradient coil and an 8 × 2 transceiver array (2 rows, 8 coils/row, Resonance Research Inc.) A VHOS shim insert coil (with a 38-cm inner diameter) providing first–fourth-order shims and partial fifth-order shims (Resonance Research Inc.) was used for higher degree/order B0 shimming [19]. B1 shimming-based outer volume suppression with two B1+ distributions was used: a “homogeneous” distribution for excitation of the brain and a “ring” distribution to selectively suppress extra-cranial tissues, as described previously [20].
Planar MRSI data were acquired in a slice angulated along the thalamic plane using a slice selective J-refocused coherence transfer sequence [21] with echo time/repetition time = 34/1500 ms, matrix size = 24 × 24 over a field of view of 216 × 216 mm2, and slice thickness = 10 mm (nominal voxel size = 9 × 9 × 10 mm3). A hanning filter was applied in the spatial domain prior to Fourier transformation resulting in in-plane distribution approximating a circle having a full-width at half maximum diameter of ~16.9 mm. The integrated volume weighted by efficiency is identical to an unfiltered data set. Water suppression was achieved via a frequency selective inversion recovery preparation module and optimized semi-selective refocusing pulses [20]. An MP2RAGE sequence [22] with 1.0-mm isotropic resolution was used for anatomic identification.
The MRSI data was reconstructed using a Hanning filter along spatial axes. Gaussian broadening 4 Hz was used in the spectral domain along with a 100-Hz convolution difference. The matched scout images were used to weight and phase the data for coil recombination.
Region of interest (ROI) placement and voxel reconstruction
To provide a reproducible selection of ROIs across subjects, as well as to account for variations in metabolite ratios across the thalamic formation, spectroscopic data were processed using semi-automatic anatomically guided reconstructions, as described previously [23]. The centers of the ROI were defined from the anatomic images. First, the thalamic boundaries were manually outlined on the anatomic images, and a midline between the medial and lateral boundaries was automatically calculated. The midline was divided into four equidistant loci along its length. Six spectroscopic voxels were then reconstructed from the MRSI data: two voxels were positioned at the most posterior and most anterior midline locations, respectively, whereas the other four voxels were positioned at the levels of the intermediate midline positions, translating along a tangent at those levels, so as to provide two 9-mm voxels at each level. These coordinates were then used in the voxel-shifting reconstruction [23, 24] to gather data over the ROI.
Spectral analysis
Spectral data (in the range of 1.8–4.0 ppm) were quantified with LCModel [15] using 14 basis metabolite functions (NAA, N-acetylaspartylglutamate (NAAG), aspartate, lactate, creatine, γ-aminobutyric acid, glucose, glutamate, glutamine, glutathione, glycerophosphorylcholine (GPC), phosphorylcholine (PCh), myo-inositol, and taurine) and default macromolecule components. The basis functions were calculated from GAMMA simulations incorporating the effects of the pulse sequence [25]. To avoid quantification errors introduced by the partial volume effects and by the inhomogeneous distribution of radiofrequency fields, the metabolite concentration ratios, rather than their absolute concentrations, were measured. Six neurometabolite ratios: tNAA/Cre, GABA/Cre, Glu/Cre, tCho/Cre, Gln/Cre, and GABA/Glu, were computed, with Cre representing creatine, tNAA the total of NAA and NAAG, and tCho the total of major choline-containing compounds, GPC and PCh. Our main focus was on examining GABA/Glu as a readout of GABA–glutamate balance. The Cramer–Rao lower bound (CRLB) values for each ratio were utilized to filter out data of poor spectral quality. Spectra were excluded if CRLB > 7 for the major singlet resonances tNAA, Cre or tCho, or CRLB > 15 for GABA, Glu, or Gln. Based on that criteria, one CHR participant’s data were excluded (i.e., all six thalamic ROIs on both right and left sides were excluded). A representative spectrum of the thalamus is shown in Fig. 1.
Fig. 1. A representative MRSI spectrum of the right medial anterior thalamus (top), individual fits for the major compounds of interest, their Cramer–Rao lower bound values, and a residual (a difference between the measured and fitted spectra).
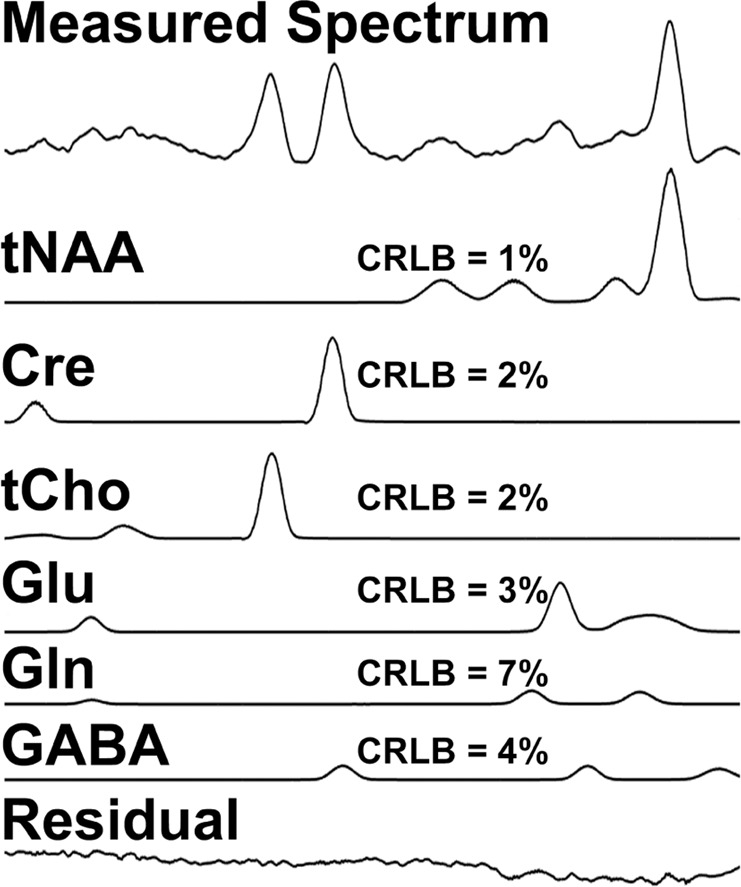
Gln glutamine; for other abbreviations, see text.
Statistical analysis
A three-way analysis of covariance (ANCOVA) with ROI position, brain hemisphere, and participant group as independent variables; neurometabolite ratios as the dependent variable; and age, intelligence quotient (IQ), and sex as covariates was conducted. Post hoc independent samples t tests were conducted with Bonferroni correction for multiple comparisons. Furthermore, we performed a Pearson correlation analysis between GABA/Glu and the subscales of the SOPS.
Results
Differences in neurometabolite ratios across ROIs
The ANCOVA analysis for GABA/Glu revealed significant differences between groups (F = 6.420, p = 0.012) and ROI positions (F = 2.539, p = 0.028). No significant interactions between Group × ROI Position (F = 0.779, p = 0.565), Group × Brain Hemisphere (F = 0.124, p = 0.725), ROI Position × Brain Hemisphere (F = 0.702, p = 0.622), and Group × ROI Position × Brain Hemisphere (F = 0.571, p = 0.722) were found. The ANCOVA revealed no significant effect of age (F = 1.275, p = 0.260), IQ (F = 0.392, p = 0.532), or sex (F = 0.936, p = 0.334) as covariates. Post hoc unpaired t test analyses revealed a significant GABA/Glu reduction (p = 0.0041, t = −3.135, df = 27) in CHR participants compared to HCs in the right medial anterior thalamus. This effect was significant even after Bonferroni correction (p = 0.0492, Fig. 2). A GABA/Glu reduction was also observed in the right medial posterior thalamus (p = 0.0148, t = −2.612, df = 26), although this effect did not survive Bonferroni correction (p = 0.1776, Fig. 2). Individual and average values of GABA/Glu in CHR and HC groups are shown in Fig. 3. Supplementary Table 1 shows the post hoc analysis for six ROIs in the right and left hemispheres. Previous work has established that tNAA/Cre can be utilized as a marker of gross neuronal injury in a variety of neurological disorders, such as epilepsy, traumatic brain injury and neurodegenerative diseases [26]. We therefore computed tNAA/Cre in the two ROIs that had significantly reduced GABA/Glu in CHR. We found no difference in the tNAA/Cre of the right medial anterior (p = 0.9464) and right medial posterior (p = 0.7514) thalamic nuclei of the CHR and HC groups.
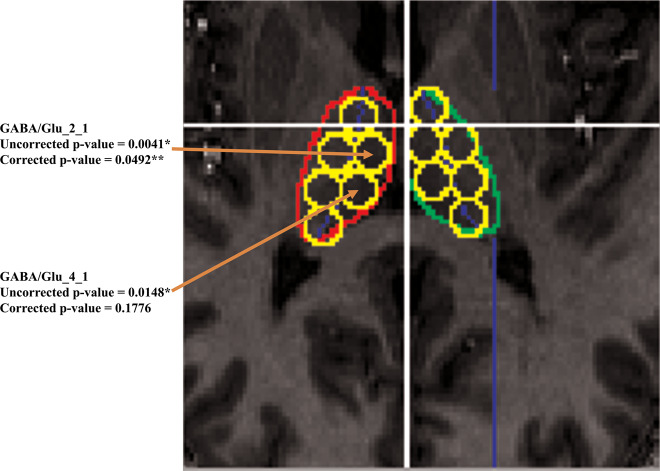
Fig. 2. Example figure displaying 6 ROIs in right and 6 ROIs in left thalamus and pointing out the ROIs with significant GABA/Glu differences between HC and CHR groups.
Significant differences between CHR and HC were found in the GABA/Glu ratio of the right medial anterior thalamus (GABA/Glu_2_1) and right medial posterior thalamus (GABA/Glu_4_1).
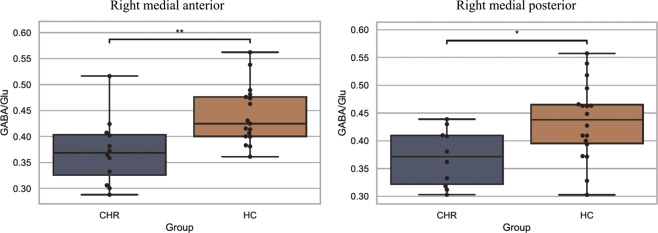
Fig. 3. Box plots displaying thalamic ROIs with significant reductions of GABA/Glu in CHR compared to HC.
The left panel displays a significant reduction in the GABA/Glu ratio of CHR compared to HC in the right medial anterior thalamus (p = 0.0041). The right panel displays a significant reduction in the GABA/Glu ratio of CHR compared to HC in the right medial posterior thalamus (p = 0.0148).
ANCOVA results for other neurometabolites
In addition to tNAA/Cre, to examine how specific was the reduction in GABA/Glu in CHR, we performed three-way ANCOVA for Glu/Cre, GABA/Cre, and tCho/Cre and Gln/Cre. No significant differences between CHR and HC were observed for any of those measurements except for GABA/Cre (Supplementary Table 2). However, post hoc analyses failed to show any difference in thalamic GABA/Cre between the CHR and HC groups (Supplementary Table 3).
Correlations between neurometabolite ratios and clinical measures
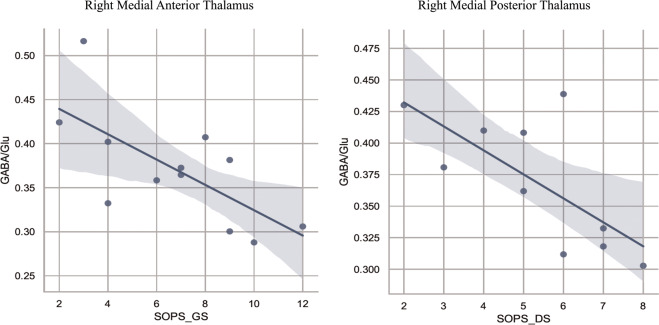
In CHR individuals, we conducted correlation analyses between GABA/Glu concentration in the right medial anterior and right medial posterior thalamic ROIs and the subscales of the SOPS. GABA/Glu deficits in the right medial anterior thalamus (p = 0.013, r = −0.692) was negatively related to the general symptom scores (SOPS_GS, Fig. 4, left). Furthermore, the reduction of GABA/Glu in the right medial posterior thalamus (p = 0.024, r = −0.701) was negatively correlated with the disorganization symptoms (SOPS_DS, Fig. 4, right). No other significant correlations were established between GABA/Glu in these two thalamic ROIs and clinical symptoms in CHR individuals (Supplementary Table 4).
Fig. 4. Correlations between thalamic GABA/Glu and SOPS subscales in CHR individuals.
The left panel shows GABA/Glu in the right medial anterior thalamus has a significant correlation with SOPS_GS (p = 0.013, r = −0.692) and the right panel displays a significant correlation between GABA/Glu in the right medial posterior thalamus and SOPS_DS (p = 0.024, r = −0.701).
Discussion
In the present study, we found that GABA/Glu within the thalamus was significantly decreased in CHR relative to HC subjects and that this decrease was localized to the medial thalamus. Furthermore, reduced GABA/Glu in the right medial anterior thalamus was inversely related to the SOPS_GS, whereas decreased GABA/Glu in the right medial posterior thalamus was negatively correlated with the SOPS_DS in CHR individuals.
To the best of our knowledge, this is the first study employing 7 T MRSI to assess GABA/Glu in the thalamus of CHR individuals. We focused on GABA/Glu based on increasing evidence that an imbalance between excitation and inhibition within the thalamo-cortical system play a critical role in the development and full manifestation of psychosis [27]. Glutamate is the main excitatory neurotransmitter, whereas GABA is the main inhibitory neurotransmitter in the brain. As such, a relative change in the two metabolites, rather than their absolute values, may best capture this imbalance. Consistent with this prediction, we established a significant reduction in GABA/Glu in the thalamus of CHR relative to HC. Also, we did not find any significant difference between groups when looking as GABA or glutamate separately. A reduction in thalamic glutamate was reported in a few recent studies in CHR individuals relative to HC [28–31]. Possible explanations for the discrepancy between these and our findings are the scanner resolution (3 vs. 7 T) and the measurement of glutamate (absolute vs. normalized relative to Cre concentration). By exploiting the higher resolution afforded by 7 T, we also measured for the first time GABA in the thalamus of CHR individuals. We found a reduction in GABA levels, which, however, did not reach significance. This suggests that GABA may be altered in prodromal individuals, but GABA/Glu represents the most sensitive measure to capture thalamic abnormalities in these individuals. We also found no NAA/Cre reduction in CHR relative to HC. Given that NAA/Cre is often utilized as a marker of neuronal damage in neurodegenerative disorders, this negative finding indicates that the reduction in GABA/Glu is not related to neuronal loss [26] but rather reflects neurodevelopment abnormalities in CHR. Furthermore, because no alterations in any other neurometabolite ratios were established, GABA/Glu may be specifically impaired in CHR youth.
It should be noted that, in our study we used Cre as an internal reference to quantify neurometabolite levels. The 3.0 ppm “creatine” resonance is actually the sum of Cre and PCre and has been used extensively as a normalization to energetic mass [32]. Thus small changes in metabolites solely due to differential inclusion of cerebrospinal fluid are factored out. Of note, previous studies found Cre abnormalities in brain cortices (e.g., anterior cingulate cortex, parieto-occipital cortex, and prefrontal cortex) in SCZ patients [33, 34]. We acknowledge that Cre can be altered by pathology; however, large changes in energetics or glial proliferation (increased Cre) typically reflect more severe pathology. Further, a reduction in selective in Cre would have increased all metabolite ratios to Cre, which was not observed. In our study, our main finding was GABA/Glu ratio abnormality in CHR, which is unlikely to be affected by creatine. We did not find any other neurometabolite differences (except GABA/Cre) between groups, which by itself might guide us to no differences in Cre level, otherwise we should find differences in more neurometabolites ratio between CHR and HC.
Reduced thalamic GABA/Glu in the medial thalamus may be implicated in some of the functional and structural thalamo-cortical dysconnectivity, observed in psychotic patients and CHR individuals [3, 35–37]. Resting-state thalamo-cortical dysconnectivity has been found in both chronic and early course patients with psychotic disorders [37, 38]. Thalamo-cortical functional hypoconnectivity, specifically implicating the medial thalamus, has also been identified in CHR youth, with individuals converting to a psychotic disorder having the most prominent impairment [5]. Furthermore, structural imaging studies have established reduced thalamo-cortical white matter connectivity in SCZ and psychotic bipolar disorder [39], as well as in both first episode psychosis (FEP) and CHR individuals compared to HCs [4], and recent meta-analyses reported an association between SCZ and lower gray matter estimates in the medial thalamus [40, 41]. In previous work, we found a decrease in sleep spindles, electroencephalogram oscillations generated within the thalamus, in both SCZ and FEP patients relative to HC groups [35, 42–44]. In one of these studies, we found that reduced mediodorsal thalamic volumes were associated with decreased spindle density in patients with SCZ compared to HC [35]. Here we focused on investigating the potential molecular abnormalities in the thalamic nuclei of high-risk individuals. One intriguing hypothesis is that the reduced GABA/Glu reported in this study may be coupled with the functional and structural thalamic alterations reported by previous studies from our and other research groups. Additionally, given that it was observed in CHR participants, a reduction in GABA/Glu may represent an early thalamic abnormality that precedes and contributes to the thalamo-cortical dysconnectivity observed along with the development and full manifestation of psychotic disorders.
Another main finding of this study was the association between reduction in thalamic GABA/Glu and SOPS-GS and SOPS-DS clinical scores in CHR individuals. Previous work has reported an association between thalamic abnormalities and clinical symptoms in psychosis spectrum patients. For instance, one study with SCZ spectrum patients did find that greater Positive and Negative Syndrome Scale general symptom severity was negatively correlated with thalamo-cortical hypoconnectivity [36]. Furthermore, a brain-wide meta-analysis of first-episode and chronic stages of SCZ found that a reduction in thalamo-cortical functional connectivity was associated with the general symptom severity of these patients [45]. Here we reported that the reduced GABA/Glu was associated with worsening general symptoms in CHR individuals. We also found that GABA/Glu abnormalities were associated with an increase in the SOPS subscale related to disorganization symptoms. Altogether, these findings provide a putative molecular and neural mechanism underlying some of the clinical symptoms reported by psychosis spectrum patients. They also indicate that these associations are present before the illness onset, thus providing a window of opportunity for early clinical interventions.
Limitations and future directions
This study had a number of limitations and unanswered questions, which should be addressed in future work. First, our CHR sample size was relatively small. It would therefore be important to replicate the present findings in larger groups of CHR individuals to confirm whether the reduced thalamic GABA/Glu is a reliable biomarker of at-risk mental state. Nonetheless, by exploiting the enhanced resolution provided by 7 T MRSI, here we were able to characterize for the first time GABAergic and glutamatergic abnormalities in the medial thalamic nuclei of CHR youth.
Second, future studies are needed to establish whether the GABA/Glu thalamic reduction reported in this study can be utilized to predict and/or track the trajectory of illness in at-risk individuals. For example, if GABA/Glu deficits are particularly prominent in CHR transitioning to psychosis, this measure may represent a putative predictive biomarker of psychosis. Alternatively, reduced GABA/Glu may represent a measure of the overall impairment in CHR individuals that affect their social functioning and prognosis. In the present study, we established that reduced GABA/Glu thalamic activity was associated with worsening general and disorganized symptoms, both of which have been associated with functional outcomes in CHR and psychotic patients [46, 47].
Third, based on converging neuroimaging [48–50], neurophysiological [51], and postmortem [52, 53] evidence indicating thalamic dysfunction and excitatory/inhibitory imbalance in psychosis spectrum and CHR individuals, in this study, we focused on the thalamus and GABA/Glu neurometabolite. Building on the present findings, future studies should investigate whether GABA/Glu abnormalities are present in other brain regions, especially the prefrontal cortex, which is known to be heavily interconnected with the thalamus. This will help to establish the extent of GABAergic and glutamatergic abnormalities in CHR individuals, thus potentially revealing novel treatment targets for at-risk mental state.
Fourth, in this work acquisition parameters were optimized to investigate GABA/Glu thalamic abnormalities in CHR relative to HC groups. However, it is also possible that other thalamic abnormalities were present in CHR individuals (e.g., decreased cortico-thalamic excitatory neurotransmission to the thalamus) and that altered GABA/Glu may be part of a process to achieve homeostasis. Combining MRSI with other neuroimaging modalities, such as functional MRI to characterize thalamo-cortical functional connectivity, might contribute to characterize these alterations.
Funding and disclosure
This study was funded by a National Institute of Mental Health BRAINS R01 MH113827 awarded to FF. The authors report no competing financial interests.
Supplementary information
Author contributions
GMQ and HPH contributed to the acquisition, analysis and interpretation of data, drafting and revising the work, and approving the final version to be published. AM and VEY contributed to the analysis and interpretation of data, drafting and revising the work, and approving the final version to be published. FF contributed to the conception and design of the work, the analysis and interpretation of data, drafting and revising the work, and approving the final version to be published.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gonzalo M. Quiñones, Ahmad Mayeli.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00920-4).
References
- 1.Lunsford-Avery JR, Orr JM, Gupta T, Pelletier-Baldelli A, Dean DJ, Watts AKS, et al. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151:148–53.. doi: 10.1016/j.schres.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper D, Barker V, Radua J, Fusar-Poli P, Lawrie SM. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res. 2014;221:69–77. doi: 10.1016/j.pscychresns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Harrisberger F, Buechler R, Smieskova R, Lenz C, Walter A, Egloff L, et al. Alterations in the hippocampus and thalamus in individuals at high risk for psychosis. NPJ Schizophr. 2016;2:1–6. doi: 10.1038/npjschz.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho KIK, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun J-Y, et al. Altered thalamo-cortical white matter connectivity: probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull. 2016;42:723–31.. doi: 10.1093/schbul/sbv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72:882–91. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao H, Chén OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9:1–9. doi: 10.1038/s41467-018-06350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steullet P. Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr Res. 2019. 10.1016/j.schres.2019.05.027. [DOI] [PubMed]
- 8.Cai H-L, Zhu R-H, Zhang X-H, Hu L, Yang W, Ye H-S. Elevated plasma γ-aminobutyrate/glutamate ratio and responses to risperidone antipsychotic treatment in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1273–8.. doi: 10.1016/j.pnpbp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Deutch AY, Roth RH. Pharmacology and biochemistry of synaptic transmission: classical transmitters. In: Byrne JH, Heidelberger R, Waxham MN, editors. From molecules to networks. An introduction to cellular and molecular neuroscience. Elsevier; 2014. p. 245–78.
- 10.Waagepetersen HS, Sonnerwald U, Schousboe A. Glutamine, glutamate and GABA: metabolic aspects. In: Lajtha A, Oja SS, Schousboe A, Saransaari P, editors. Handbook of neurochemistry and molecular neurobiology. Springer; 2007. p. 1–21.
- 11.Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia—a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Stone J, Broome M, Valli I, Mechelli A, McLean M, et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry. 2011;68:881–90. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- 13.Pan J, Duckrow R, Spencer D, Avdievich N, Hetherington H. Selective homonuclear polarization transfer for spectroscopic imaging of GABA at 7T. Magn Reson Med. 2013;69:310–16.. doi: 10.1002/mrm.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cudalbu C, Mlynárik V, Gruetter R. Handling macromolecule signals in the quantification of the neurochemical profile. J Alzheimers Dis. 2012;31:S101–15.. doi: 10.3233/JAD-2012-120100. [DOI] [PubMed] [Google Scholar]
- 15.Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–85.. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RA, et al. Comparison of single voxel brain MRS AT 3 T and 7 T using 32-channel head coils. Magn Reson Imaging. 2015;33:1013–8.. doi: 10.1016/j.mri.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreychenko A, Boer VO, Arteaga de Castro CS, Luijten PR, Klomp DW. Efficient spectral editing at 7 T: GABA detection with MEGA‐sLASER. Magn Reson Med. 2012;68:1018–25.. doi: 10.1002/mrm.24131. [DOI] [PubMed] [Google Scholar]
- 18.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–15.. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 19.Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med. 2012;68:1007–17.. doi: 10.1002/mrm.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med. 2010;63:9–19. doi: 10.1002/mrm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan J, Avdievich N, Hetherington H. J‐refocused coherence transfer spectroscopic imaging at 7 T in human brain. Magn Reson Med. 2010;64:1237–46.. doi: 10.1002/mrm.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49:1271–81.. doi: 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Hetherington H, Kuzniecky R, Vives K, Devinsky O, Pacia S, Luciano D, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007;69:2256–65.. doi: 10.1212/01.wnl.0000286945.21270.6d. [DOI] [PubMed] [Google Scholar]
- 24.Twieg D, Meyerhoff D, Hubesch B, Roth K, Sappey‐Marinier D, Boska MD, et al. Phosphorus‐31 magnetic resonance spectroscopy in humans by spectroscopic imaging: localized spectroscopy and metabolite imaging. Magn Reson Med. 1989;12:291–305. doi: 10.1002/mrm.1910120302. [DOI] [PubMed] [Google Scholar]
- 25.Smith S, Levante T, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson Ser A. 1994;106:75–105. [Google Scholar]
- 26.Öz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658–79.. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray JD, Anticevic A. Toward understanding thalamocortical dysfunction in schizophrenia through computational models of neural circuit dynamics. Schizophr Res. 2017;180:70–7. doi: 10.1016/j.schres.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenneberg C, Glenthoj BY, Hjorthoj C, Buchardt Zingenberg FJ, Glenthoj LB, Rostrup E, et al. Cerebral glutamate and GABA levels in high-risk of psychosis states: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Res. 2020;215:38–48. doi: 10.1016/j.schres.2019.10.050. [DOI] [PubMed] [Google Scholar]
- 29.Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–9. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Valli I, Stone J, Mechelli A, Bhattacharyya S, Raffin M, Allen P, et al. Altered medial temporal activation related to local glutamate levels in subjects with prodromal signs of psychosis. Biol Psychiatry. 2011;69:97–9. doi: 10.1016/j.biopsych.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM, et al. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology. 2014;39:2891–9. doi: 10.1038/npp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connett RJ. Analysis of metabolic control: new insights using scaled creatine kinase model. Am J Physiol Regul Integr Comp Physiol. 1988;254:R949–59.. doi: 10.1152/ajpregu.1988.254.6.R949. [DOI] [PubMed] [Google Scholar]
- 33.Öngür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–8. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibbo PG, Bernier D, Hanstock CC, Seres P, Lakusta B, Purdon SE. 3‐T proton magnetic spectroscopy in unmedicated first episode psychosis: a focus on creatine. Magn Reson Med. 2013;69:613–20.. doi: 10.1002/mrm.24291. [DOI] [PubMed] [Google Scholar]
- 35.Buchmann A, Dentico D, Peterson MJ, Riedner BA, Sarasso S, Massimini M, et al. Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage. 2014;102:540–47.. doi: 10.1016/j.neuroimage.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND. Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry. 2018;83:509–17.. doi: 10.1016/j.biopsych.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016–25.. doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheffield JM, Huang AS, Rogers BP, Giraldo-Chica M, Landman BA, Blackford JU, et al. Thalamocortical anatomical connectivity in schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2020;46:1062–71. [DOI] [PMC free article] [PubMed]
- 40.Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–56.. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92.. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 43.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–48.. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaskie RE, Graziano B, Ferrarelli F. Topographic deficits in sleep spindle density and duration point to frontal thalamo-cortical dysfunctions in first-episode psychosis. J Psychiatr Res. 2019;113:39–44. doi: 10.1016/j.jpsychires.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Wang Q, Zhang J, Rolls ET, Yang W, Palaniyappan L, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–48. doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corcoran C, Kimhy D, Parrilla-Escobar M, Cressman V, Stanford A, Thompson J, et al. The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2011;41:251. doi: 10.1017/S0033291710000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130:290–9.. doi: 10.1111/acps.12289. [DOI] [PubMed] [Google Scholar]
- 48.Reddy-Thootkur M, Kraguljac NV, Lahti AC. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders–a systematic review of magnetic resonance spectroscopy studies. Schizophr Res. 2020. 10.1016/j.schres.2020.02.001. [DOI] [PMC free article] [PubMed]
- 49.Takahashi T, Tsugawa S, Nakajima S, Plitman E, Chakravarty MM, Masuda F, et al. Thalamic and striato-pallidal volumes in schizophrenia patients and individuals at risk for psychosis: a multi-atlas segmentation study. Schizophr Res. 2020. 10.1016/j.schres.2020.04.016. [DOI] [PubMed]
- 50.Bojesen KB, Broberg BV, Fagerlund B, Jessen K, Thomas MB, Sigvard A, et al. Associations between cognitive function and levels of glutamatergic metabolites and GABA in antipsychotic-naïve patients with schizophrenia or psychosis. Biol Psychiatry. 2020. 10.1016/j.biopsych.2020.06.027. [DOI] [PMC free article] [PubMed]
- 51.Vukadinovic Z, Rosenzweig I. Abnormalities in thalamic neurophysiology in schizophrenia: could psychosis be a result of potassium channel dysfunction? Neurosci Biobehav Rev. 2012;36:960–8.. doi: 10.1016/j.neubiorev.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Seeman P, Guan H-C, Van, Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–5.. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 53.Danos P, Baumann B, Krämer A, Bernstein H-G, Stauch R, Krell D, et al. Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res. 2003;60:141–55.. doi: 10.1016/s0920-9964(02)00307-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.