Abstract
Introduction
Recently, nephronophthisis (NPH) has been considered a monogenic cause of end-stage renal disease (ESRD) in adults. However, adult-onset NPH is difficult to accurately diagnose and has not been reported in a cohort study. In this study, we assessed the genetic background and clinicopathologic features of adult NPH.
Methods
We investigated 18 sporadic adult patients who were suspected as having NPH by renal biopsy. We analyzed 69 genes that cause hereditary cystic kidney disease and compared clinicopathologic findings between patients with and without pathogenic mutations in NPH-causing genes.
Results
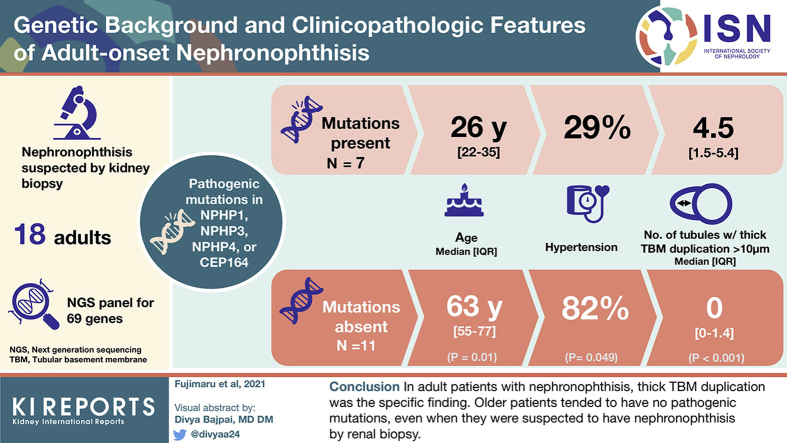
Seven of 18 patients had pathogenic NPH-causing mutations in NPHP1, NPHP3, NPHP4, or CEP164. Compared with patients without pathogenic mutations, those with pathogenic mutations were significantly younger but did not significantly differ in the classic NPH pathologic findings, such as tubular cysts. On the other hand, the number of tubules with thick tubular basement membrane (TBM) duplication, which was defined as >10-μm thickness, was significantly higher in patients with genetically proven adult NPH than in those without pathogenic mutations. α-Smooth muscle actin (α-SMA)-positive myofibroblasts were detected inside thick TBM duplication.
Conclusions
In adult patients with NPH, thick TBM duplication was the specific finding. Our analysis also suggested that older patients tended to have no pathogenic mutations, even when they were suspected to have NPH by renal biopsy. These findings could be the novel clinical clue for the diagnosis of NPH in adult patients.
Keywords: adult-onset kidney disease, chronic kidney disease, human genetics, nephronophthisis, renal cystic disease, renal pathology
Graphical abstract
NPH is an autosomal recessive kidney disease and is the most frequent genetic cause of pediatric ESRD.1, 2, 3 In principle, molecular genetic analysis is currently the only method for the accurate clinical diagnosis of NPH.3,4 To date, more than 25 different genes have been found to be associated with NPH.3 Because of the increasing number of NPH genes identified, Sanger sequencing has become more tedious and costly, and comprehensive mutation analysis using next-generation sequencing is now required.5 Recently, we have developed a target panel of genes that are related with inherited renal cystic diseases, including NPH.6 This system enables us to perform comprehensive mutation analysis of NPH. However, in the real clinical setting, genetic testing is not performed routinely because of the limited availability of sequencing platforms and the associated high cost.
Recently, advances in genetics have revealed the importance of adult-onset NPH. In 2018, a Dutch study on 5 international cohorts reported that 26 of 5606 patients (0.5%) with adult-onset ESRD showed homozygous NPHP1 deletions,7 suggesting that NPH is a relatively frequent monogenic cause of adult-onset ESRD. Although the importance of NPH in adult patients has been increasing, only a few case reports are available, and there had been no clinical cohort studies on adult NPH, probably because of the difficulty in the diagnosis.
In a real clinical setting around adult-onset NPH patients, a major clinical problem is the difficulty in an accurate diagnosis. In the Dutch study mentioned previously, only 3 (12%) patients were correctly diagnosed as NPH, and the other 88% were misdiagnosed as other kidney diseases or were defined as having chronic kidney disease with unknown etiology. Several factors make the diagnosis of NPH very difficult in adult patients. First, the extrarenal abnormalities in NPH are fewer in adult patients than in pediatric cases.7, 8, 9, 10, 11 In addition, the clinical and radiologic symptoms of NPH are unspecific compared with those in the more common causes of chronic kidney disease.9, 10, 11 Therefore, most cases of adult-onset NPH are suspected only after renal biopsy.11,12 However, although the renal histology exhibits a characteristic triad of corticomedullary cysts, TBM disruption, and tubulointerstitial nephropathy,1,13 it is not disease specific and is commonly seen in any chronic tubulointerstitial disorder.4,14 This absence of specific histologic findings makes the correct diagnosis of adult NPH more difficult.
Even in adult patients, accurate diagnosis of NPH is important because management options, such as kidney transplantation and appropriate genetic counseling, are available.12 Therefore, knowledge on the specific clinical and histologic findings in adult NPH is highly required. Furthermore, the Dutch study only investigated complete NPHP1 deletion in adult NPH using genome wide association study data.7 Although complete NPHP1 deletion is responsible for 20% of pediatric NPH cases,2, 3, 4 more than 25 genes have been reported to cause NPH.3 Therefore, adult NPH cases may be caused by other kinds of mutations in different genes. However, because only a few case reports are available, the genetic background of adult patients with NPH remains unknown.
The present study aimed to assess the genetic background and clinicopathologic features of adult NPH wherein adult patients who were suspected to have NPH on renal biopsy were analyzed.
Methods
Patients
We investigated 18 adult patients who were suspected to have NPH by renal biopsy. After the pathologists in each institution suspected NPH based on the presence of tubular dilatation or TBM thickening and lamellation, the clinicians consulted us for genetic testing of NPH. All patients had no extrarenal findings, such as retinitis pigmentosa or liver function disorder, and no family history of autosomal dominant chronic kidney disease. Patients younger than 17 years were excluded. The patients were recruited at 16 institutions in Japan between 2015 and 2019. This study was approved by the research ethics committee of each institution.
The clinical data of the patients at the time of renal biopsy were collected from the medical records. The estimated glomerular filtration rate was calculated using the Japanese glomerular filtration rate equation.15 A liver or renal cyst was defined as the presence of at least 1 cyst detected by computed tomography or magnetic resonance imaging.
Genetic Analysis
Comprehensive genetic testing was performed using capture-based next-generation sequencing of 69 genes that cause 9 types of hereditary cystic kidney disease, including NPH, NPH-related ciliopathies (Joubert syndrome, Meckel syndrome, Senior-Løken syndrome, Bardet-Biedl syndrome, and skeletal ciliopathies), autosomal dominant polycystic kidney disease, autosomal recessive polycystic kidney disease, and autosomal dominant tubulointerstitial kidney disease6 (Supplementary Table S1). The detailed methods are described in Supplementary Methods, as well as in our previous reports.6,16
To detect large genomic rearrangements, such as gross deletions or duplications, copy number variation analysis was conducted using Copy Number Analysis for Targeted Resequencing (http://contra-cnv. sourceforge.net/).17 If homozygous entire deletion of NPHP1 was detected by copy number variation analysis, we performed polymerase chain reaction for exons 1, 10, and 20 of NPHP1. The primer sequences are shown in Supplementary Table S2.
Pathologic Assessment
For each patient, the tissue slides that were stained with hematoxylin-eosin, periodic acid-Schiff, and periodic acid–methenamine silver were digitized using the NanoZoomer HT Scan system (Hamamatsu Photonics, Hamamatsu, Japan). Whole standard glass slides were scanned at 40× magnification (0.23 μm/pixel).
We defined 3 types of pathologic findings that were classically known to be specific for NPH; these included tubular diverticulum, tubular floret, and tubular cyst (Supplementary Figure S1A–C).13,14 For tubular diverticulum, tubular lumens that extended through the long axis of the tubule were excluded. A tubular floret was defined as branching in at least 4 directions (Supplementary Figure S1A).14 A tubular cyst was defined as having >200 μm in diameter (Supplementary Figure S1C).14 Atrophic tubules, which were defined according to the Banff working classification,18 were excluded. In addition, we defined thick TBM duplication as thickness >10 μm. Tubules with >50% fibrosis within the thick TBM duplication were excluded.
A nephrologist and a pathologist blindly assessed the pathologic findings using the tissue slides with periodic acid–methenamine silver stain. First, they counted the number of the 3 types of tubules (i.e., tubular diverticulum, tubular floret, and tubular cyst) in the specimen of each patient. Thereafter, they assessed and calculated the number of tubules that had thick TBM duplication as follows: number of tubules with thick TBM duplication = (number of tubules with thick TBM duplication/total number of counted tubules) × 10. The cumulative number of the 3 types of counted tubules was noted.
Low-vacuum Scanning Electron Microscopic and Immunofluorescence Analysis
To investigate what the essence of thick TBM duplication was, we performed low-vacuum scanning electron microscopic (Hitachi, Tokyo, Japan) analysis and immunofluorescence (IF) analysis in the representative cases with or without pathogenic mutations. For low-vacuum scanning electron microscopic analysis, 5-μm formalin-fixed paraffin-embedded sections were stained with periodic acid–methenamine silver to evaluate the TBM as described previously.19,20 For IF analysis, monoclonal antibody against α-SMA (Merck KGaA, Darmstadt, Germany) for myofibroblast and Phaseolus vulgaris (Vector Laboratories, Inc., Burlingame, CA) for proximal tubules, respectively, were used in formalin-fixed paraffin-embedded sections. Streptavidin, Alexa Fluor 488 conjugate and goat antimouse IgG (H+L; Thermo Fisher Scientific Inc, Waltham, MA), and Alexa Fluor 568 (Thermo Fisher Scientific Inc) were used, and sections were counterstained with 4′,6-diamidino-2-phenylindole. IF images were captured using BZ-X800 (Keyence, Osaka, Japan).
Statistical Analysis
All statistical analyses were performed using JMP version 15 (SAS Institute, Cary, NC). Nonnormally distributed variables were expressed as the median with interquartile range. The Mann-Whitney U test was used to compare the medians of continuous variables, such as age, and the chi-square test was used to compare the percentages of categoric variables, such as sex, between patients with pathogenic mutations and those without pathogenic mutations. A P value < 0.05 was considered statistically significant.
Results
Patient Characteristics
The patient characteristics are presented in Table 1. The median age at renal biopsy was 52 years; 6 patients (33%) were men, and 11 patients (61%) had hypertension. For the renal manifestations, 14 patients (78%) had proteinuria. The median estimated glomerular filtration rate was 18.4 ml/min per 1.73 m2. Chronic kidney disease was stage 3 in 3 patients (17%), stage 4 in 8 patients (44%), and stage 5 in 7 patients (39%). No patient received dialysis. Nine patients had at least 1 renal cyst identified on imaging, such as computed tomography or magnetic resonance imaging.
Table 1.
Characteristics of patients at the time of renal biopsy
| Characteristics | N = 18 |
|---|---|
| Age, years | 52 (25.8–74.5) |
| Male | 6 (33) |
| Hypertension | 11 (61) |
| Proteinuria | 14 (78) |
| Serum Cr, mg/dl | 2.4 (1.60–3.66) |
| eGFR, ml/min per 1.73 m2 | 18.4 (10.4–29.0) |
| CKD stage | |
| 3 | 3 (17) |
| 4 | 8 (44) |
| 5 | 7 (39) |
| Liver cyst | 2 (11) |
| Renal cyst | 9 (50) |
CKD, chronic kidney disease; Cr, creatine; eGFR, estimated glomerular filtration rate.
Values are reported as medians (25th–75th percentile) or numbers (%).
Panel-based Genetic Diagnosis of NPH
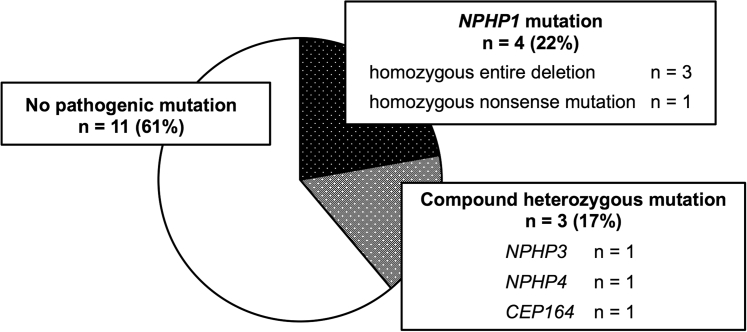
A summary of the results of our comprehensive genetic testing is presented in Figure 1. Of the 7 patients (39%) who had pathogenic mutations related with NPH; 4 patients had a mutation in NPHP1; and the other 3 patients had compound heterozygous mutations in NPHP3, NPHP4, and CEP164, respectively. Notably, to the best of our knowledge, the patient with the CEP164 mutation was the first case in an adult. Of the patients with an NPHP1 mutation, 3 patients (patient numbers 883, 896, and 1207) were detected to have homozygous entire deletion of NPHP1 by copy number variation analysis. The regions that were expected to be identified by copy number variation analysis are shown in Supplementary Table S3. In all 3 patients, no polymerase chain reaction products were detected in exons 1, 10, and 20 of NPHP1; this confirmed complete gene deletion of NPHP1. The other patient (patient number 1107) had homozygous nonsense mutation in NPHP1 (Supplementary Table S4). The details of the mutations in the patients with compound heterozygous mutations in NPHP3, NPHP4, and CEP164 are shown in Supplementary Table S4.
Figure 1.
Disease-causing mutations in adults with suspected nephronophthisis (NPH). Of the 7 patients (39%) who had pathogenic mutations related with NPH, 4 patients had a mutation in NPHP1, and the other 3 patients had compound heterozygous mutations in NPHP3, NPHP4, and CEP164, respectively.
Clinical Characteristics of Adult NPH Patients
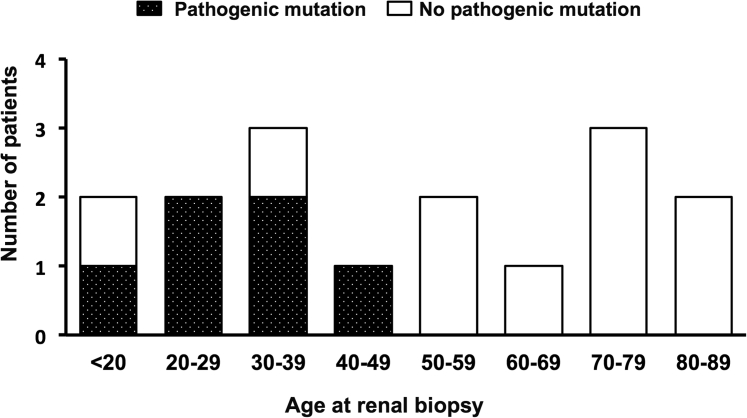
As shown in Table 2, the patients with pathogenic mutations were significantly younger compared with those who had no pathogenic mutations (median age, 26 vs. 63 years; P = 0.01). Interestingly, no patient who was >50 years old at the time of renal biopsy had pathogenic mutations (Figure 2). In addition, compared with patients who had no pathogenic mutations, there was a significantly higher proportion of men for those with pathogenic mutations (71% vs. 9%, P = 0.01) and a significantly lower incidence of hypertension (29% vs. 82%, P = 0.049) but a similar estimated glomerular filtration rate, level of proteinuria, and number of renal or liver cysts (at least 1 for each).
Table 2.
Phenotypic characterization of the patients according to the presence of pathogenic mutation
| Phenotype | Pathogenic mutation n = 7 | No pathogenic mutation n = 11 | P value |
|---|---|---|---|
| Clinical findings at renal biopsy | |||
| Age, years | 26 (22–35) | 63 (55–77) | 0.01 |
| Male | 5 (71) | 1 (9) | 0.01 |
| Hypertension | 2 (29) | 9 (82) | 0.049 |
| Proteinuria | 5 (71) | 9 (82) | 1.00 |
| eGFR, ml/min per 1.73 m2 | 28.8 (5.2–39.5) | 15.2 (10.4–25.9) | 0.53 |
| Liver cyst | 1 (14) | 1 (9) | 1.00 |
| Renal cyst | 3 (43) | 6 (55) | 1.00 |
| Pathologic findings | |||
| Tubule with thick TBM duplication, /10 counted tubulesa |
4.5 (1.5–5.4) | 0 (0–0.4) | <0.001 |
| Tubular diverticulum | 8 (5–9) | 5.5 (5–7) | 0.22 |
| Tubular floretb | 1 (0.5–2.5) | 2.5 (1–3.5) | 0.12 |
| Cystc | 0 (0–0) | 1 (0–1.5) | 0.15 |
| Total counted tubulesd | 9 (6–11.5) | 9.5 (8.5–11) | 0.56 |
eGFR, estimated glomerular filtration rate; TBM, tubular basement membrane.
Values are reported as median (25th–75th percentile) or numbers (%).
Number of tubules with thick TBM duplication divided by the total number of counted tubules and then multiplied by 10.
Branching in at least 4 directions.
Diameter >200 μm.
Sum of the number of tubular diverticula, tubular florets, and cysts.
Figure 2.
The age distribution of patients according to the presence of pathogenic mutations. No pathogenic mutations in the known genes are detected in patients >50 years old.
Pathologic Findings Specific for Adult-onset NPH
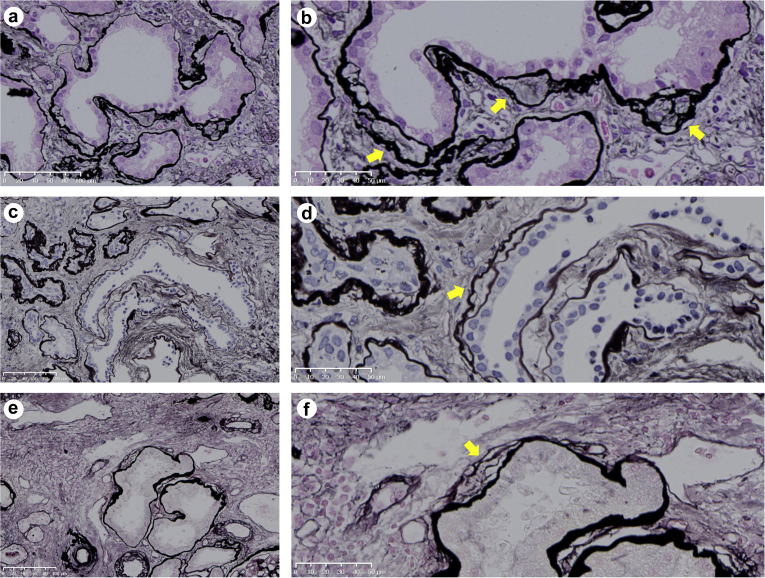
The 3 pathologic findings that are known to be specific for NPH were not significantly different between the adult patients with NPH and those who did not have a mutation causing NPH (Table 2). Therefore, we further searched for novel pathologic findings that were specific to adult NPH cases. We focused on TBM duplication, which was reported to be another specific finding in NPH.13,14 Although TBM duplication was reported in noninherited renal diseases,14 we noticed that the TBMs were quite thick and reduplicated in genetically proven adult NPH cases. Therefore, we focused on the thickness of TBM duplication; we defined thick TBM duplication as >10 μm (Figure 3). Interestingly, the number of tubules with thick TBM duplication was significantly higher in genetically proven adult NPH than in those who did not have pathogenic mutation (Table 2).
Figure 3.
Thick tubular basement membrane duplication. Thick tubular basement membrane duplication was defined as thickness of >10 μm (yellow arrows). (a and b) Patient number 883, periodic acid–methenamine silver (PAM) stain, 20× and 40× magnification, respectively. (c and d) Patient number 478, PAM stain, 20× and 40× magnification, respectively. (e and f) Patient number 896, PAM stain, 20× and 40× magnification, respectively. All slides were scanned on the NanoZoomer NDP system with 40× resolution (0.23 μm/pixel) (Hama-matsu Photonics, Hamamatsu-City, Japan).
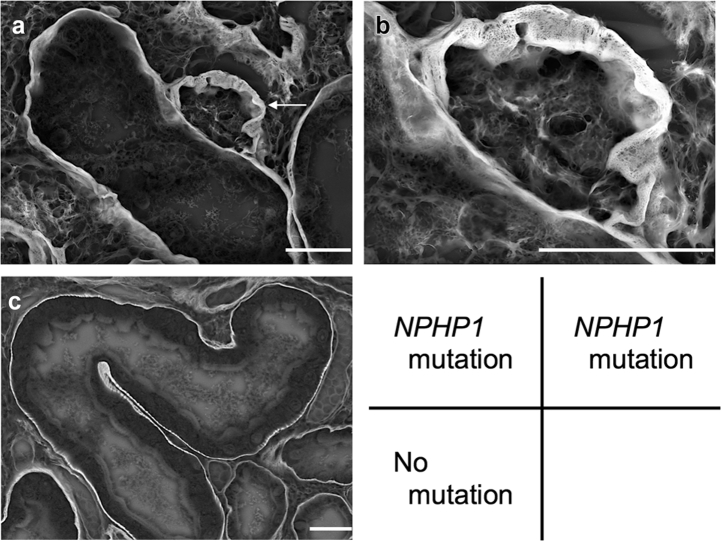
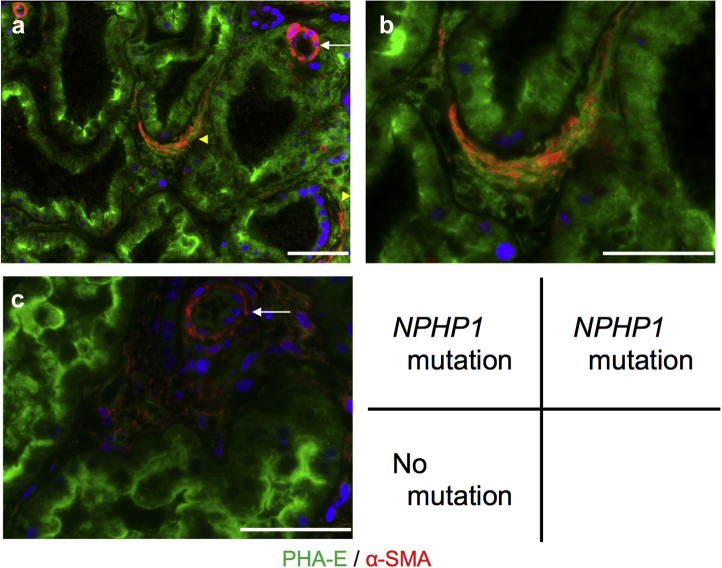
For investigating the ultrastructure and components of thick TBM duplication, we observed renal tissues of representative cases using low-vacuum scanning electron microscopy and IF. In low-vacuum scanning electron microscopic observations, thick TBM duplications were detected in the patient with homozygous entire deletion of NPHP1 (patient number 896) (Figure 4a and 4b). Furthermore, from immunofluorescence results, α-SMA–positive myofibroblasts were detected inside thick TBM duplication, in addition to vascular smooth muscle cells (Figure 5a and b). On the other hand, in the patient who had no pathogenic mutations related with NPH (patient number 669), thick TBM duplications were not detected in low-vacuum scanning electron microscopic analysis (Figure 4c). Additionally, α-SMA–positive myofibroblasts were detected only in vascular smooth muscle cells by IF observation (Figure 5c).
Figure 4.
Low-vacuum scanning electron microscopic imaging of the patient with pathogenic mutation in NPHP1 (patient number 896) and without pathogenic mutations (patient number 669). Low-vacuum scanning electron microscope images for periodic acid–methenamine silver using formalin-fixed paraffin-embedded samples (5-μm section). (a and b) Imaging of the patient with pathogenic mutation in NPHP1; b presents higher magnification images of a. The white arrow shows thick tubular basement membrane duplication. (c) imaging of the patient without pathogenic mutations. White bars = 20 μm.
Figure 5.
Immunofluorescence imaging of the patient with pathogenic mutations in NPHP1 (patient number 896) and without pathogenic mutations (patient number 669). (a and b) Imaging of the patient with pathogenic mutation in NPHP1. Alpha-smooth muscle action (α-SMA) (red) positive myofibroblasts were detected inside thick tubular basement membrane duplication (yellow arrowhead), in addition to vascular smooth muscle cells in the artery (white arrow); b presents higher magnification images of a. (c) Imaging of the patient without pathogenic mutations. α-SMA (red) was detected only in vascular smooth muscle cells (white arrow). Phaseolus vulgaris (PHA-E) (green) was used to identify proximal tubules. Nuclear counterstain with DAPI (blue). White bars = 100 μm.
In general, IgA nephropathy, diabetic nephropathy, and tubulointerstitial nephritis are known to have tubular interstitial lesions. Therefore, we assessed for the presence of tubules with thick TBM duplication in these noninherited renal diseases (Supplementary Table S5). Among the 9 patients, the diagnoses by renal biopsy were IgA nephropathy in 4 patients, diabetic nephropathy in 3 patients, and tubulointerstitial nephritis in 2 patients. Although most patients had >30% of tubulointerstitial fibrosis, we could not find any tubule that had thick TBM duplication in these samples. Therefore, thick TBM duplication could be specific for NPH.
Discussion
To the best of our knowledge, this was the first cohort study to investigate the clinicopathologic findings and genetic background of adult patients with NPH. Through genetic analysis of 18 adult patients who were suspected to have NPH by renal biopsy, we found pathogenic mutations related with NPH in 7 patients. Compared with patients who had no pathogenic mutations, adult patients who had genetically proven NPH were significantly younger and had a significantly higher proportion of men, a significantly lower incidence of hypertension, and a significantly higher number of tubules with thick TBM duplication.
In this study, the pathogenic mutations in the 7 patients with NPH were in the genes NPHP1, NPHP3, NPHP4, and CEP164. In a genome-wide association study on adult-onset ESRD patients, Snoek et al.7 analyzed only homozygous NPHP1 full gene deletions using generated genomic data. However, based on our results, 4 of 7 genetically confirmed NPH cases had pathogenic mutations other than homozygous NPHP1 full gene deletions. Moreover, mutations in CEP164 were reported to cause Senior-Løken syndrome or Joubert syndrome.21,22 In this study, patient number 930, who was a 26-year-old man, had compound heterozygous mutations in CEP164. To the best of our knowledge, this was the first reported case of adult NPH with a CEP164 mutation. These findings strongly indicated that comprehensive genetic testing may be useful for adult patients suspected to have NPH.
In our study, we detected α-SMA–positive cells in the thick TBM duplication. α-SMA is commonly used as a marker for myofibroblasts and the resulting fibrosis.23 Interestingly, through the study of primary cilia in cultured epithelial cells, primary cilia undergo a dynamic biphasic change during epithelial-myofibroblast transition as well as fibroblast-to-myofibroblast transition induced by transforming growth factor-β.24 Furthermore, the study of inhibition of ciliogenesis demonstrated that deficiency of primary cilia induces epithelial-to-mesenchymal transition.25 Therefore, because it is well-known that products of genes causing NPH are localized at segments associated with primary cilia, it could be possible that abnormal primary cilia in NPH patients result in increased α-SMA–positive myofibroblasts and fibrosis, resulting in thick TBM duplication.
In this study, adult patients with genetically proven NPH were significantly younger compared with those who had no pathogenic mutations at the time of renal biopsy. Snoek et al.7 analyzed 5606 patients with adult-onset ESRD and revealed that 26 (0.5%) of the patients had homozygous NPHP1 full gene deletions. Of the patients who were >50 years old at the time of the initiation of renal replacement therapy, only 2 had homozygous NPHP1 full gene deletions. Similar to the report by Snoek et al., our study showed that no patient who was >50 years old at the time of renal biopsy had pathogenic NPH mutations. These findings suggested that older patients tended to have no pathogenic mutations, even if they were suspected to have NPH by renal biopsy.
In this study, the incidence of hypertension was significantly higher in patients without pathogenic mutations than in those who had pathogenic mutations. Hypertensive nephrosclerosis is a disorder that is usually associated with disease chronicity. The renal pathologic features of arteriolar nephrosclerosis are characterized by the involvement of arteries, arterioles, glomeruli, and the tubulointerstitium26; the presence of chronic tubular and interstitial lesions in the form of tubular atrophy and interstitial fibrosis26,27; and lamellated TBMs in atrophic tubules.28 In this study, the patients who had no pathogenic mutation tended to have a relatively high number of tubular cysts. In aging kidneys, tubular diverticulum is often observed and is a probable source of renal cysts.29, 30, 31 Therefore, in patients without pathogenic mutations, tubular disorders can be caused by secondary factors, such as hypertension and aging. Moreover, we found that patients with pathogenic mutations included a relatively high proportion of men. However, in the Dutch study about adult-onset ESRD, 12 of 26 patients who had homozygous NPHP1 deletion were men,7 demonstrating no sex difference. Further study will be required to confirm this observation.
This study had several limitations. First, the sample size of our study was small. Nevertheless, only a few adult cases of suspected NPH by renal biopsy have been reported,7, 8, 9,11 and no cohort studies on adult NPH cases have been available. Considering that there were only 26 patients with homozygous NPHP1 deletions even in the study using nationwide data from 5 countries,7 the number of patients in our study could be reasonable. Therefore, although we collected samples from all over Japan, this number of study patients was inevitable. Furthermore, to the best of our knowledge, this was the first study to investigate the clinicopathologic findings and genetic background of adult patients who were suspected to have NPH. We expect an increase in the number of cases in the future. Second, in our study, 2 of 7 patients with mutations in the genes related with nephronophthisis had only novel missense mutations. Therefore, we could not exclude the possibility that these missense mutations were not disease-causing mutations. Third, we could not analyze other genes that had been identified very recently as rare causal genes for NPH-related ciliopathies, such as C8orf37,32 KIAA0586,33 and MAPKBP1.34 However, the phenotypes of the patients with mutations in these genes were Bardet-Biedl syndrome, Joubert syndrome, or NPH with extrarenal findings. Therefore, considering that all patients in this study do not exhibit any extrarenal findings, it is unlikely that our 11 patients without identified pathogenic mutations have mutations in these genes.
In conclusion, our analysis showed that older patients tended to have no pathogenic mutations, even if they were suspected to have NPH by renal biopsy. On pathology, the number of tubules with thick TBM duplication could be an effective measure to diagnose NPH in adult patients. In addition, comprehensive genetic testing with a panel system could be useful for adult patients suspected to have NPH.
Disclosure
TM reports personal fees from honoraria for lectures and personal fees from employment outside the submitted work. JH has received a research grant from Otsuka Pharmaceutical Co. All the other authors declared no competing interests.
Acknowledgments
Supported in part by the Grants-in-Aid for Scientific Research (25221306, 16H05314, 19H01049, and 19H03672), the Challenging Exploratory Research (15K15327, 16K15467, and 18K19534), the Research Activity Start-up (17H06657, 20K22926), the Young Scientists (19K17733), and the Early-Career Scientists (19K17730) from the Japan Society of the Promotion of Science; the Health Labor Science Research Grant from the Ministry of Health Labor and Welfare, AMED under Grant Number JP18ek0109304; grants from the Yukiko Ishibashi Foundation; the Salt Science Research Foundation (1925, 2030); and low-vacuum scanning electron microscope device support from the LVSEM Study Group of Renal Biopsy and Hitachi High-Technologies Corporation in Japan Grant Number 007.
Footnotes
Supplementary Methods.
Supplementary References.
Table S1. Disease categories and the targeted genes included in this panel
Table S2. Primer sequences of NPHP1
Table S3. Detected copy number variants in NPHP1
Table S4. Mutations in the genes related with nephronophthisis
Table S5. Clinical characteristics of the pathologic control group
Figure S1. Representative pathologic findings.
Supplementary Material
Supplementary Methods.
Supplementary References.
Table S1. Disease categories and the targeted genes included in this panel
Table S2. Primer sequences of NPHP1
Table S3. Detected copy number variants in NPHP1
Table S4. Mutations in the genes related with nephronophthisis
Table S5. Clinical characteristics of the pathologic control group
Figure S1. Representative pathologic findings.
References
- 1.Hildebrandt F., Attanasio M., Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava S., Molinari E., Raman S. Many genes-one disease? Genetics of nephronophthisis (NPHP) and NPHP-associated disorders. Front Pediatr. 2017;5:287. doi: 10.3389/fped.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo F., Tao Y.-H. Nephronophthisis: A review of genotype-phenotype correlation. Nephrology (Carlton) 2018;23:904–911. doi: 10.1111/nep.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf M.T.F. Nephronophthisis and related syndromes. Curr Opin Pediatr. 2015;27:201–211. doi: 10.1097/MOP.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbritter J., Porath J.D., Diaz K.A. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet. 2013;132:865–884. doi: 10.1007/s00439-013-1297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimaru T., Mori T., Sekine A. Kidney enlargement and multiple liver cyst formation implicate mutations in PKD1/2 in adult sporadic polycystic kidney disease. Clin Genet. 2018;94:125–131. doi: 10.1111/cge.13249. [DOI] [PubMed] [Google Scholar]
- 7.Snoek R., van Setten J., Keating B.J. NPHP1 (nephrocystin-1) gene deletions cause adult-onset ESRD. J Am Soc Nephrol. 2018;29:1772–1779. doi: 10.1681/ASN.2017111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haghighi A., Savaj S., Haghighi-Kakhki H. Identification of an NPHP1 deletion causing adult form of nephronophthisis. Ir J Med Sci. 2016;185:589–595. doi: 10.1007/s11845-015-1312-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoefele J., Nayir A., Chaki M. Pseudodominant inheritance of nephronophthisis caused by a homozygous NPHP1 deletion. Pediatr Nephrol. 2011;26:967–971. doi: 10.1007/s00467-011-1761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Chen F., Wang J. Two novel homozygous mutations in NPHP1 lead to late onset end-stage renal disease: a case report of an adult nephronophthisis in a Chinese intermarriage family. BMC Nephrol. 2019;20:173. doi: 10.1186/s12882-019-1372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollée G., Fakhouri F., Karras A. Nephronophthisis related to homozygous NPHP1 gene deletion as a cause of chronic renal failure in adults. Nephrol Dial Transplant. 2006;21:2660–2663. doi: 10.1093/ndt/gfl348. [DOI] [PubMed] [Google Scholar]
- 12.Hudson R., Patel C., Hawley C.M. Adult-diagnosed nonsyndromic nephronophthisis in Australian families caused by biallelic NPHP4 variants. Am J Kidney Dis. 2020;76:282–287. doi: 10.1053/j.ajkd.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Waldherr R., Lennert T., Weber H.P. The nephronophthisis complex. A clinicopathologic study in children. Virchows Arch A Pathol Anat Histol. 1982;394:235–254. doi: 10.1007/BF00430668. [DOI] [PubMed] [Google Scholar]
- 14.Larsen C.P., Bonsib S.M., Beggs M.L. Fluorescence in situ hybridization for the diagnosis of NPHP1 deletion-related nephronophthisis on renal biopsy. Hum Pathol. 2018;81:71–77. doi: 10.1016/j.humpath.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Mori T., Hosomichi K., Chiga M. Comprehensive genetic testing approach for major inherited kidney diseases, using next-generation sequencing with a custom panel. Clin Exp Nephrol. 2017;21:63–75. doi: 10.1007/s10157-016-1252-1. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Lupat R., Amarasinghe K.C. CONTRA: copy number analysis for targeted resequencing. Bioinformatics. 2012;28:1307–1313. doi: 10.1093/bioinformatics/bts146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roufosse C., Simmonds N., Clahsen-van Groningen M. 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaga S., Kato M., Hirashima S. Rapid three-dimensional analysis of renal biopsy sections by low vacuum scanning electron microscopy. Arch Histol Cytol. 2010;73:113–125. doi: 10.1679/aohc.73.113. [DOI] [PubMed] [Google Scholar]
- 20.Okada S., Inaga S., Kawaba Y. A novel approach to the histological diagnosis of pediatric nephrotic syndrome by low vacuum scanning electron microscopy. Biomed Res. 2014;35:227–236. doi: 10.2220/biomedres.35.227. [DOI] [PubMed] [Google Scholar]
- 21.Chaki M., Airik R., Ghosh A.K. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150:533–548. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilboux T., Doherty D.A., Glass I.A. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med. 2017;19:875–882. doi: 10.1038/gim.2016.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gewin L., Zent R., Pozzi A. Progression of chronic kidney disease: too much cellular talk causes damage. Kidney Int. 2017;91:552–560. doi: 10.1016/j.kint.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozycki M., Lodyga M., Lam J. The fate of the primary cilium during myofibroblast transition. Mol Biol Cell. 2014;25:643–657. doi: 10.1091/mbc.E13-07-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han S.J., Jung J.K., Im S.-S. Deficiency of primary cilia in kidney epithelial cells induces epithelial to mesenchymal transition. Biochem Biophys Res Commun. 2018;496:450–454. doi: 10.1016/j.bbrc.2018.01.079. [DOI] [PubMed] [Google Scholar]
- 26.Freedman B.I., Cohen A.H. Hypertension-attributed nephropathy: what’s in a name? Nat Rev Nephrol. 2016;12:27–36. doi: 10.1038/nrneph.2015.172. [DOI] [PubMed] [Google Scholar]
- 27.Meyrier A. Nephrosclerosis: update on a centenarian. Nephrol Dial Transplant. 2015;30:1833–1841. doi: 10.1093/ndt/gfu366. [DOI] [PubMed] [Google Scholar]
- 28.Lusco M.A., Fogo A.B., Najafian B. AJKD atlas of renal pathology: tubular atrophy. Am J Kidney Dis. 2016;67:e33–e34. doi: 10.1053/j.ajkd.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Kanasaki K., Kitada M., Koya D. Pathophysiology of the aging kidney and therapeutic interventions. Hypertens Res. 2012;35:1121–1128. doi: 10.1038/hr.2012.159. [DOI] [PubMed] [Google Scholar]
- 30.Denic A., Glassock R.J., Rule A.D. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darmady E.M., Offer J., Woodhouse M.A. The parameters of the ageing kidney. J Pathol. 1973;109:195–207. doi: 10.1002/path.1711090304. [DOI] [PubMed] [Google Scholar]
- 32.Heon E., Kim G., Qin S. Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21) Hum Mol Genet. 2016;25:2283–2294. doi: 10.1093/hmg/ddw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachmann-Gagescu R., Phelps I.G., Dempsey J.C. KIAA0586 is mutated in Joubert syndrome. Hum Mutat. 2015;36:831–835. doi: 10.1002/humu.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macia M.S., Halbritter J., Delous M. Mutations in MAPKBP1 cause juvenile or late-onset cilia-independent nephronophthisis. Am J Hum Genet. 2017;100:323–333. doi: 10.1016/j.ajhg.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.