Introduction
Globally, chronic kidney disease (CKD) represents an important non-communicable disease with significant morbidity and mortality. An estimated 10% of the world’s population had CKD in 2015 with approximately 1.2 million deaths in 2017 (1,2), and the burden is expected to rise at the rate of 6% per annum (3,4). By 2030, more than 70% of patients suffering from end-stage kidney disease (ESKD) worldwide will be in low and lower middle income countries of the world including African countries (5). Significant disparities in the burden of CKD exist worldwide, where economically disadvantaged communities, notably those on the African continent and those of the African diaspora, continue to bear a disproportionate burden of the disease (2,6). This disparity is fueled by a convergence of genetic and environmental risk factors (7,8). A recent meta-analysis showed an overall prevalence of CKD of 15.8% in the general population in Africa, with up to 4.6% of adults having moderate to severe kidney dysfunction (9). In Africa, more than 80% of the continental burden of CKD is in sub-Saharan Africa (SSA), with the highest prevalence in West Africa (8). The burden of CKD among African Americans, who share substantial genetic ancestry with West Africans (10), is similarly high; African Americans represent 13% of the USA population, but account for 35% of the patients on dialysis (11).
Genetic Susceptibility
It has long been noted that African Americans have an increased risk of progressive CKD and ESKD relative to their non-African counterparts, even after accounting for health disparities (12–14). A familial risk of chronic renal failure, often with different underlying etiologies, was noted in African American families (12,15). CKD has been observed to have higher incidence and faster progression rates in African Americans compared to Americans of European descent, consequently the risk of developing ESKD is 4-fold higher in African Americans (13,14,16–18). This is independent of socioeconomic status or the presence of traditional clinical risk factors.
These observations prompted a search for genetic risk for the development of CKD in people of African descent. Employing an admixture mapping approach to exploit the mixed African-European ancestry of African Americans, focal segmental glomerulosclerosis (FSGS) and HIV-associate nephropathy (collapsing nephropathy) was linked to a region on chromosome (chr) 22 and replicated for non-diabetic ESKD (19). A second group identified the same chr 22 association with non-diabetic ESKD, but notably, not with diabetic ESKD (20). Subsequently, Genovese and colleagues identified two alleles, termed G1 and G2 in the APOL1 gene, encoding apoliprotein L1, which were responsible for the chr 22 admixture signal (21). Carriage of any combination of the two APOL1 risk alleles accounts for about 70% of excess risk of development, progression and severity of CKD in the African American population (22).
APOL1 Gene and Protein
APOL1 functions as part of the innate immune system and is regulated by antiviral pathways; notably, it is potently upregulated by interferons (23,24). Of the 6 members of the APOL gene family, only APOL1 has acquired a secretory signal peptide permitting cellular export of the APOL1 protein into the blood stream (25). Intracellular APOL1 protein lacking a signal peptide is retained within the cell; it is this protein isoform that leads to kidney injury (26,27). Circulating APOL1 is largely produced by the liver and is a minor component of high-density lipid particles that are incorporated into trypanosome lytic factor (TFL), which, after ingestion by African trypanosomes, lyses the parasite (28,29). Unlike most common variants that tend to have small effect sizes, APOL1 G1 and G2 variants have large effect sizes, which is not atypical for mutations that have undergone balancing selection by lethal pathogens (i.e. sickle cell and malaria) (30). Among African primates, only the baboon, gorilla and humans retain a functional APOL1 gene and the gene is absent in all other mammals (31). In addition, one human completely lacks both copies of a functional APOL1 gene but shows no evidence of kidney disease, indicating that APOL1 protein is not essential for life or for kidney homeostasis (32).
APOL1 Protein and African Trypanosomiasis
Although APOL1 protein protects humans from infection by T.b. brucei, humans are susceptible to infection and disease by T.b. rhodensiense and T.b. gambiense, the pathogens causing acute and chronic human African trypansomiasis (HAT), respectively, affecting millions of Africans (33). T.b. rhodensiense and T.b. gambiense have evolved different mechanisms to escape APOL1 lysis. T.b. rhodesiense expresses a serum resistance associated (SRA) protein that binds and inactivates APOL1 protein, while T.b. gambiense resist lysis by a hydrophobic β-sheet of the T.b. gambiense-specific glycoprotein (TgsGP) (21,34–36). The APOL1 G2 variant, located within the serum resistant associated (SRA) binding site, restores partial ability of the APOL1 protein to lyse T.b. rhodesiense through its low affinity for SRA, conferring a selective advantage against acute trypanosomiasis (21,36,37). The G1 variant protein does not prevent infection by T.b. gambiense or T.b. rhodensiense, but the G1 allele is associated with asymptomatic T.b. gambiense infection and undetectable parasitemia in individuals from West Africa (38). The APOL1 G1 allele and, to a lesser extent the G2 allele, underwent a selective sweep in west Africa within the last 10 000 years and are prevalent in populations throughout sub-Saharan Africa and the African diaspora (21,39,40). These findings support the prevailing hypothesis that G1 and G2 variants have been subject to balance selecting in West Africa, with heterozygous advantage against trypanosomiasis and homozygous disadvantage in susceptibility to CKD.
Distribution Of APOL1 Variants
The APOL1 renal risk variants are found exclusively on African-derived chromosomes and are not present on European or Asian chromosomes (39,41). The G1 and G2 variants arose independently in separate events on two different haplotypes of chromosome 22 and have not undergone a recombination event; hence, G1 and G2 are not observed on the same haplotype (21). As shown in Figure 1, the highest prevalence of the APOL1 high-risk variants was found in West African populations notably in Ghana and Nigeria where a prevalence is as high as 40% among the Yoruba and Igbo people of southwestern Nigeria (39,42). East African populations appear to have lowest prevalence of the high-risk alleles in Africa (21,39,43). It is important to note that even within a country, the frequencies of the G1 and G2 variants may vary considerably among ethnic groups (39).
Figure 1 .
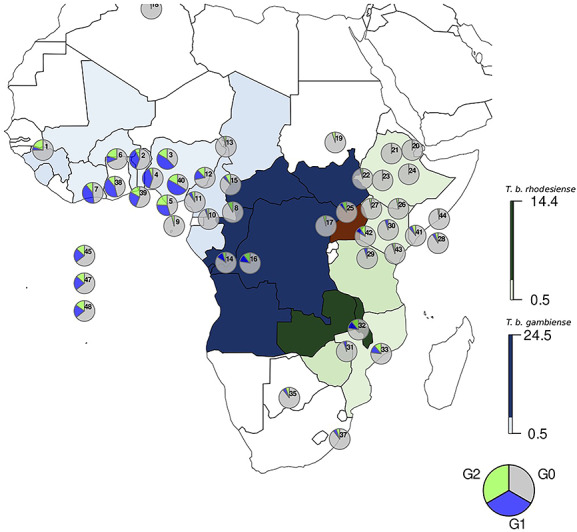
Distribution of human African trypansomiasis (T.b. gambiense and T.b. rhodesiense) and APOL1 G1 and G2 allele frequencies in Africa. The distribution of G1, G2 and GO frequencies are represented by pie charts and overlap the distribution of T.b. gambiense (blue), T.b. rhodesiense (green) and both (red) as presented in Franco et al. 2020 (97). Supplementary Material, Table S1 lists the haplotype frequencies and location of the sampled populations. For countries where T.b. gambiense and T.b. rhodesiense cases have not been reported recently, the frequency of infections has been set to 0.5%. These include Benin, Mali, Nigeria and Togo for T.b. gambiense, and Mozambique, Ethiopia and Kenya for T.b. rhodesiense (97).
APOL1 G1 and G2 risk variants are found throughout the African diaspora in individuals with recent African ancestry (40). During the 16th to 19th century, ~ 12 million Africans from West Africa were brought to enslavement in the Caribbean and the Americas during the trans-Atlantic slave trade, driving gene flow from Africans to Americans. As a result of this relocation, admixture with European populations and Native Americans caused a mixed genomic profile of the population now referred to as African American (44). Approximately 50% of African Americans carry at least one APOL1 risk allele and ~ 13% of African Americans carry two APOL1 risk alleles (45). In African Americans, the frequency for G1 is 20–42% and 13–15% for G2 (46). The high G1 and G2 frequencies from African individuals from the Atlantic coast of Africa are reflected in African Americans, in African and Hispanic Caribbeans and in South Americans, particularly in Brazil (47,48). Hispanics who migrated from the Caribbean to New York also carry the G1 and G2 variants, but at lower frequencies (41).
APOL1 Associations with CKD in the USA and SSA
In spite of the high frequencies of APOL1 G1 and G2 risk variants in the African population, there are limited data on APOL1-associated diseases in Africans residing on the African continent. Hence, most current knowledge about the role of APOL1 genetic variants on diseases are extrapolations from studies on Africans in the African diaspora, mainly African Americans in the USA.
Although most individuals with APOL1 high-risk genotypes do not develop disease, the lifetime risk of CKD for carriers is estimated to be approximately 20%. APOL1 is associated with a spectrum of progressive chronic kidney disease ranging in severity from arterionephrosclerosis to the most severe form of focal segmental glomerulosclerosis (FSGS), collapsing glomerulopathy, which is often fulminate, frequently irreversible and rapidly progressive (Fig. 2) (49). The APOL1 renal risk variants are most strongly associated with FSGS and HIV-associated nephropathy (HIVAN) with odds ratios (OR) of 17 and 29, respectively (46). Approximately 70% of African Americans with FSGS and HIVAN carry high-risk genotypes in contrast to 13% in the general US black population (46). Both conditions are characterized by podocyte effacement and detachment, which suggests APOL1-mediated injury to the kidney podocyte, a key component of the tripartite renal filtration barrier (50–52). African American patients with systemic lupus erythematosus (SLE) have a 5.4-fold greater odds of developing a collapsing glomerulopathy (53,54). In addition to untreated HIV infection, other viral infections, including cytomegalovirus and BK polyoma virus infection in recipients of kidney allografts from APOL1 high-risk donors, have been associated with de novo collapsing glomerulopathy (55). Treatment with therapeutic interferon-gamma has also been associated with collapsing nephropathy in those with APOL1 high-risk genotypes, which remits upon cessation of the drug (23). These findings suggest that ‘second hits’ that potently activate interferon pathways or high levels of exogenous interferon trigger collapsing glomerulopathy, likely by upregulating APOL1 levels in the kidney to a critical threshold (23).
Figure 2 .
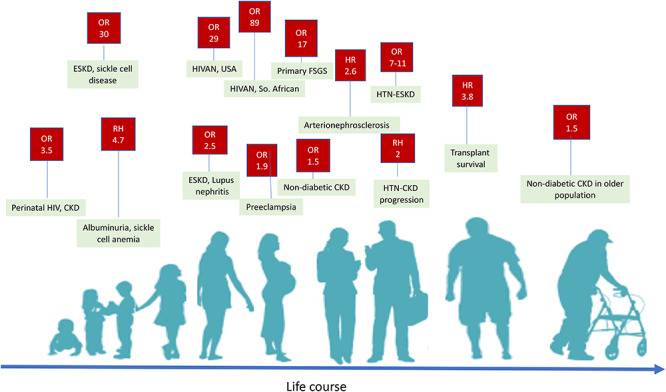
Shown are conditions and diseases and their point ORs and relative hazards (RHs) associated with APOL1 high-risk genotypes across the life course. Peak years of onset are from adolescence to middle age, after which risk of CKD decreases (46). Risk of preeclampsia is determined predominately by carriage of APOL1 high-risk genotypes of the fetus, although maternal genotype may also contribute to risk (88–90).
APOL1 risk alleles are strongly associated (OR ~ 2–7) with end-stage kidney disease attributed to hypertension (OR 2–7), but not with diabetic ESKD (19–21,56,57). The predilection of African Americans with hypertension-attributed CKD to progress to ESKD likely results from APOL1-mediated arterionephrosclerosis and global glomerulosclerosis (58,59). APOL1 high-risk genotypes are associated with higher rates of progression to ESKD and steeper decline in estimated glomerular filtration rate (eGFR) in African Americans with established chronic kidney disease, regardless of diabetes status (60). The finding of a more rapid rate of progression in patients with CKD secondary to diabetes may be due to undetected APOL1-associated arterionephrosclerosis or FSGS in diabetic patients, since renal biopsies were not performed (60). Renal biopsies of patients with APOL1 high-risk genotypes and progressive or late-stage CKD may resolve the role of APOL1 in patients with diabetic nephropathy. APOL1 variants are also associated with earlier onset of proteinuria in both pediatric and adult sickle cell populations (61,62). In African Americans with CKD attributed to hypertension, those with APOL1 high-risk genotypes were 80% more likely to develop proteinuria and once proteinuria is established, it is the dominant risk factor for decline in eGFR regardless of APOL1 risk status (63). Similarly, in young to middle-aged adults with preserved kidney function, there is no significant difference in eGFR decline by APOL1 risk status; however, APOL1 high-risk status is associated with earlier onset of proteinuria, followed by a downward eGFR trajectory (64). It is important to note that among both those with preserved kidney function and in those with reduced kidney function, in the absence of proteinuria, eGFR slopes are similar between carriers of APOL1 high-risk and low-risk genotypes (64). These studies support accumulating evidence that the first manifestation of APOL1-mediated kidney injury is proteinuria resulting from podocyte injury.
Surprisingly few case–control studies and no longitudinal studies for APOL1 associations with kidney disease have been reported in sub-Saharan Africa (see Table 1). A small study of adults in the DRC comprising 83 controls and 79 cases with hypertension-attributed CKD reported that 12.7% of cases carried high-risk genotypes compared to only 2.4% of the controls (OR 7.7) (65). A recent study of 412 healthy children from the general population and 401 HIV-positive children living in Kinshasa, Democratic Republic of Congo (DRC), reported that children from the general population with APOL1 high-risk genotypes had lower eGFR (91 vs 97 mL/min/1.73m2); however, children with HIV had much higher prevalence of albuminuria and levels of albuminuria were higher with incomplete suppression of viral load (66). A large, cross-sectional study representing multiple ethnic groups from the Atlantic coastal region of Nigeria reported that over 70% of participants carried APOL1 high-risk genotypes; however, APOL1 high-risk genotypes were only associated with CKD in the setting of HIV infection (OR 2.5) (67). In the absence of HIV infection, there was no reported association between CKD and APOL1 high-risk genotypes (67). However, since this study only reported the associations for G1 or G2 alleles and not carriage of 2 risk alleles in the compound heterozygous state, effect sizes are likely underestimated. Kasembeli et al. reported that among HIV-infected adults living in South Africa, 79% of participants with biopsy confirmed HIVAN carried high-risk APOL1 genotypes compared to only 3.3% of the HIV-positive control group (OR 89) (68). In contrast, in African Americans, the OR for biopsy-confirmed HIVAN is 29 (46). The difference in effect sizes may represent differences in genetic background, circulating HIV strains or viral burden or other environmental influences. The striking association of APOL1 high-risk genotype and kidney disease in children and adults with HIV infection provides additional support that HIV infection is a strong ‘second hit’ promoting podocyte injury and glomerulosclerosis. Further sufficiently powered case–control studies for renal phenotypes and longitudinal cohort studies across different ethnic groups, risk groups and geographical regions of Africa are warranted to quantify APOL1 effect sizes and rates of progression to clinical endpoints. Performing these studies in multiple ethnic groups and geographical regions should identify genetic and environmental factors that attenuate or exacerbate APOL1 penetrance.
Table 1.
Case–control studies showing ORs for APOL1 high-risk genotypes associated with kidney disease in the Americas and sub-Saharan Africa for kidney disease
| Phenotype | Country | Setting | No. of cases | No. of controls | OR | Ref. |
|---|---|---|---|---|---|---|
| Non-diabetic ESKD | USA | Adults on dialysis | 1002 | 923 | 7.3 | (21) |
| ESKD | Brazil | Adults on dialysis | 106 | 106 | 10.95 | (48) |
| Stage 5 CKD | SA | Adults, mean eGFR 8 (4–12) | 70 | 58 | 0.85a | (98) |
| CKD | DRC | Adults, hypertensive CKD | 79 | 83 | 7.7 | (65) |
| CKD | Nigeria | Adults | 44 | 43 | 4.8 | (42) |
| FSGS | USA | Adults | 192 | 176 | 10.5 | (21) |
| FSGS | USA | Mostly adults | 217 | 383 | 17 | (46) |
| FSGS | SA | Adults | 22 | 108 | 2.1a | (68) |
| HIVAN | USA | Adults, HIV+ | 54 | 237 | 29 | (46) |
| HIVAN | SA | Adults, HIV+ | 78 | 108 | 89 | (68) |
| Albuminuria | USA | Young to middle-aged adults | 2.9 | 2.9 | (64) | |
| Albuminuria | DRC | Pediatric population | 2.1 | 40 | 412 | (66) |
| Albuminuria | DRC | Pediatric population, HIV+ | 22.0 | 72 | 329 | (66) |
aNot statistically significant. SA, South Africa; USA, United States of America; DRC, Democratic Republic of the Congo.
APOL1 and Kidney Transplantation
Studies have shown that donor APOL1 high-risk allele status significantly affects kidney allograft survival in the recipient, with shorter survival in kidneys from donors with two high-risk alleles compared to those with one or no risk allele (27). In contrast, the APOL1 risk status of the kidney recipient has no influence on allograft survival (69). APOL1 high-risk status may also have consequences for the kidney donor. Studies have shown that living kidney donors with high-risk genotypes have lower eGFR at donation and lower eGFR rebound following kidney donation, experience more hypertension than APOL1 low-risk kidney donors and may be at increased risk for ESKD post-donation (70,71). The APOL1 long-term Kidney Transplant Outcomes Network (APOLLO) study is designed to assess the effects of APOL1 renal risk variants on outcomes for living donors and for recipients of kidneys, following deceased and living kidney transplantation (72,73).
APOL1 and Other Disease Associations
The association of APOL1 risk alleles with cardiovascular disease (CVD) is conflicting; the earliest studies showed that the APOL1 high-risk genotype is associated with a range of CVD conditions including incident myocardial infarction, stroke, heart failure, cardiac revascularization and cardiovascular death (74,75). However, others have not observed statistically significant associations between APOL1 high-risk genotypes and increased risk of CVD in cohort studies of African Americans or in a two-stage meta-analysis of 23 305 individuals (76–78).
Preeclampsia is a common complication of pregnancy characterized by systemic hypertension, albuminuria and maternal endothelial dysfunction in pregnancy, which results from placentation defects with imbalance in angiogenic factors (79–81). The overall model-based incidence rate of preeclampsia is 4.6%, with the highest incidence in African countries (5.6%) (82). Preeclampsia accounts for 16% of maternal deaths; in sub-Saharan African, prevalence rates as high as 26% have been reported (83,84). Mothers of African descent in Africa and the Africa diaspora have higher rates of pregnancies complicated by preeclampsia (84). Globally, preeclampsia accounts for 900 000 infant deaths per year, with the largest burden being in sub-Saharan Africa (83). A role for APOL1 in preeclampsia is supported by several lines of evidence: 1) APOL1 mRNA and protein is highly expressed in the placenta (85); 2) circulating autoantibodies against APOL1 protein are present in the blood of women experiencing preeclampsia (86); and 3) dams of transgenic mice pups constitutively expressing reference or variant APOL1 develop a preeclampsia-like phenotype with smaller than expected litter sizes (87). Two recent studies found that carriage of APOL1 risk variants by the fetus, but not the mother, increases the odds of maternal preeclampsia by 50–90% (88,89). The first study reported a recessive inheritance in two independent cohorts of African American mother–baby pairs, while the second study reported associations for additive and recessive modes of inheritance (88,89). A third study from South Africa that had DNA from mothers, but not babies, reported that carriage of one APOL1 risk allele by the mother was associated with increased risk of preeclampsia (90), whereas two previous studies found no significant association with maternal carriage of 1 or 2 risk alleles for preeclampsia. This finding is not in conflict with the previous studies, since the mothers are obligate carriers of at least one APOL1 risk allele for fetuses carrying 2 risk alleles (88,91). Given that preeclampsia is a significant cause of infant and maternal mortality and may therefore affect reproductive fitness, it is interesting to speculate that modifying genes may attenuate APOL1 penetrance for preeclampsia in West African populations with high prevalence of APOL1 risk alleles.
Summary
APOL1 renal risk alleles have profound influence on a spectrum of kidney disease in individuals of recent African descent over the life course. APOL1 risk variants require ‘second hits’ (e.g. viral infection, auto-immune diseases, sickle cell anemia, glomerular hyperfiltration) for renal disease to manifest; upregulation of APOL1 by interferons is a potent second hit. However, beyond exposure to therapeutic interferon and certain viral infections, it is still unclear why most individuals with APOL1 high-risk status never develop kidney disease. Africa comprises 1000s of ethnolinguistic groups with extensive genetic diversity living on a continent undergoing epidemiological transition. The study of APOL1 associations in case–control studies and longitudinal studies in SSA may shed new light on genetic and environmental exposures that initiate CKD and/or modify CKD progression. In addition, knowledge of APOL1 prevalence and disease associations may inform public health policies and resource allocation. Recent advances in understanding the pathophysiological mechanisms of APOL1-associated CKD may lead to new therapeutic options (92,93). An APOL1 antisense drug targeting APOL1 has been shown to ameliorate proteinuria in animal models (94). If particular drug therapies (e.g. blood pressure lowering drugs that block the renin angiotensin aldosterone system) are proven effective in clinical trials, knowledge of at-risk populations susceptible to APOL1-related kidney disease may justify screening for APOL1 or for biomarkers of APOL1-mediated renal injury (95). APOL1 risk variants provide fertile soil for the development of severe glomerulopathies and progressive kidney disease and warrant further study in sub-Saharan Africa and in the African diaspora (96).
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with federal funds from the Frederick National Laboratory for Cancer Research, under Contract HHSN261200800001E. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), Frederick National Laboratory, Center for Cancer Research (C.A.W.). M.A.C. and J.-T.B. are funded by the South African National Research Foundation (NRF). The content of this publication does not necessarily reflect the views or policies of the NRF or Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Conflict of Interest statement. No conflicts of interest to report by any author.
Contributor Information
Aminu Abba Yusuf, Department of Haematology, Bayero University Kano and Aminu Kano Teaching Hospital, Kano, Nigeria.
Melanie A Govender, Faculty of Health Sciences, Sydney Brenner Institute for Molecular Bioscience, University of the Witwatersrand, Johannesburg, South Africa.
Jean-Tristan Brandenburg, Faculty of Health Sciences, Sydney Brenner Institute for Molecular Bioscience, University of the Witwatersrand, Johannesburg, South Africa.
Cheryl A Winkler, Molecular Genetic Epidemiology Section, Basic Research Laboratory, Frederick National Laboratory for Cancer Research, NCI, Frederick, MD 21701, USA.
Contributions
The initial draft was written by all authors.
References
- 1. Kassebaum, N.J., Aurora, M., Barber, R.M., Brown, J., Carter, A. and Casey, D.C. (2016) Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388, 1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia-Garcia, G. and Jha, V. (2015) World Kidney Day 2015: CKD in disadvantaged populations. Am. J. Kidney Dis., 65, 349–353. [DOI] [PubMed] [Google Scholar]
- 3. Collaboration, G.B.D.C.K.D (2020) Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 395, 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mortality, G.B.D. and Causes of Death, C (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, 385, 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanifer, J.W., Jing, B., Tolan, S., Helmke, N., Mukerjee, R., Naicker, S. and Patel, U. (2014) The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob. Health, 2, e174–e181. [DOI] [PubMed] [Google Scholar]
- 6. Pugsley, D., Norris, K.C., Garcia-Garcia, G. and Agodoa, L. (2009) Global approaches for understanding the disproportionate burden of chronic kidney disease. Ethn. Dis., 19, S1-1–S1-2. [PubMed] [Google Scholar]
- 7. Labuschagne, I. and Nel, J. (2017) Chronic kidney disease is still a major health challenge in Africa [internet]. https://theconversation.com/chronic-kidney-disease-is-still-a-major-health-challenge-in-africa-73977.
- 8. Abd ElHafeez, S., Bolignano, D., D'Arrigo, G., Dounousi, E., Tripepi, G. and Zoccali, C. (2018) Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ Open, 8, e015069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaze, A.D., Ilori, T., Jaar, B.G. and Echouffo-Tcheugui, J.B. (2018) Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol., 19, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zakharia, F., Basu, A., Absher, D., Assimes, T.L., Go, A.S., Hlatky, M.A., Iribarren, C., Knowles, J.W., Li, J., Narasimhan, B. et al. (2009) Characterizing the admixed African ancestry of African Americans. Genome Biol., 10, R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saran, R., Robinson, B., Abbott, K.C., Agodoa, L.Y.C., Abertus, P., Ayanian, J., Balkrishnan, R., Bragg-Gresham, J., Cao, J. et al. (2016) US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis., 69, A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferguson, R., Grim, C.E. and Opgenorth, T.J. (1988) A familial risk of chronic renal failure among blacks on dialysis? J. Clin. Epidemiol., 41, 1189–1196. [DOI] [PubMed] [Google Scholar]
- 13. Saran, R., Robinson, B., Abbott, K.C., Bragg-Gresham, J., Chen, X., Gipson, D., Gu, H., Hirth, R.A., Hutton, D., Jin, Y. et al. (2020) US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis., 75, A6–A7. [DOI] [PubMed] [Google Scholar]
- 14. McClellan, W., Tuttle, E. and Issa, A. (1988) Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am. J. Kidney Dis., 12, 285–290. [DOI] [PubMed] [Google Scholar]
- 15. Freedman, B.I., Spray, B.J., Tuttle, A.B. and Buckalew, V.M., Jr. (1993) The familial risk of end-stage renal disease in African Americans. Am. J. Kidney Dis., 21, 387–393. [DOI] [PubMed] [Google Scholar]
- 16. Kiberd, B.A. and Clase, C.M. (2002) Cumulative risk for developing end-stage renal disease in the US population. J. Am. Soc. Nephrol., 13, 1635–1644. [DOI] [PubMed] [Google Scholar]
- 17. Lipworth, L., Mumma, M.T., Cavanaugh, K.L., Edwards, T.L., Ikizler, T.A., Tarone, R.E., McLaughlin, J.K. and Blot, W.J. (2012) Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS One, 7, e48407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cowie, C.C., Port, F.K., Wolfe, R.A., Savage, P.J., Moll, P.P. and Hawthorne, V.M. (1989) Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N. Engl. J. Med., 321, 1074–1079. [DOI] [PubMed] [Google Scholar]
- 19. Kopp, J.B., Smith, M.W., Nelson, G.W., Johnson, R.C., Freedman, B.I., Bowden, D.W., Oleksyk, T., McKenzie, L.M., Kajiyama, H., Ahuja, T.S. et al. (2008) MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat. Genet., 40, 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kao, W.H., Klag, M.J., Meoni, L.A., Reich, D., Berthier-Schaad, Y., Li, M., Coresh, J., Patterson, N., Tandon, A., Powe, N.R. et al. (2008) MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat. Genet., 40, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Genovese, G., Friedman, D.J., Ross, M.D., Lecordier, L., Uzureau, P., Freedman, B.I., Bowden, D.W., Langefeld, C.D., Oleksyk, T.K., Uscinski Knob, A.L. et al. (2010) Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science, 329, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freedman, B.I. (2013) APOL1 and nephropathy progression in populations of African ancestry. Semin. Nephrol., 33, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nichols, B., Jog, P., Lee, J.H., Blackler, D., Wilmot, M., D'Agati, V., Markowitz, G., Kopp, J.B., Alper, S.L., Pollak, M.R. et al. (2015) Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int., 87, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma, L., Divers, J. and Freedman, B.I. (2019) Mechanisms of injury in APOL1-associated kidney disease. Transplantation, 103, 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Page, N.M., Butlin, D.J., Lomthaisong, K. and Lowry, P.J. (2001) The human apolipoprotein L gene cluster: identification, classification, and sites of distribution. Genomics, 74, 71–78. [DOI] [PubMed] [Google Scholar]
- 26. Ma, L., Shelness, G.S., Snipes, J.A., Murea, M., Antinozzi, P.A., Cheng, D., Saleem, M.A., Satchell, S.C., Banas, B., Mathieson, P.W. et al. (2015) Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J. Am. Soc. Nephrol., 26, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeves-Daniel, A.M., DePalma, J.A., Bleyer, A.J., Rocco, M.V., Murea, M., Adams, P.L., Langefeld, C.D., Bowden, D.W., Hicks, P.J., Stratta, R.J. et al. (2011) The APOL1 gene and allograft survival after kidney transplantation. Am. J. Transplant., 11, 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shukha, K., Mueller, J.L., Chung, R.T., Curry, M.P., Friedman, D.J., Pollak, M.R. and Berg, A.H. (2017) Most ApoL1 is secreted by the liver. J. Am. Soc. Nephrol., 28, 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanhamme, L., Paturiaux-Hanocq, F., Poelvoorde, P., Nolan, D.P., Lins, L., Van Den Abbeele, J., Pays, A., Tebabi, P., Van Xong, H., Jacquet, A. et al. (2003) Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature, 422, 83–87. [DOI] [PubMed] [Google Scholar]
- 30. Raychaudhuri, S. (2011) Mapping rare and common causal alleles for complex human diseases. Cell, 147, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith, E.E. and Malik, H.S. (2009) The apolipoprotein L family of programmed cell death and immunity genes rapidly evolved in primates at discrete sites of host-pathogen interactions. Genome Res., 19, 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnstone, D.B., Shegokar, V., Nihalani, D., Rathore, Y.S., Mallik, L., Ashish, Zare, V., Ikizler, H.O., Powar, R. and Holzman, L.B. (2012) APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PLoS One, 7, e51546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kasembeli, A.N., Duarte, R., Ramsay, M. and Naicker, S. (2015) African origins and chronic kidney disease susceptibility in the human immunodeficiency virus era. World J. Nephrol., 4, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stephens, N.A. and Hajduk, S.L. (2011) Endosomal localization of the serum resistance-associated protein in African trypanosomes confers human infectivity. Eukaryot. Cell, 10, 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berberof, M., Perez-Morga, D. and Pays, E. (2001) A receptor-like flagellar pocket glycoprotein specific to Trypanosoma brucei gambiense. Mol. Biochem. Parasitol., 113, 127–138. [DOI] [PubMed] [Google Scholar]
- 36. De Greef, C., Imberechts, H., Matthyssens, G., Van Meirvenne, N. and Hamers, R. (1989) A gene expressed only in serum-resistant variants of Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol., 36, 169–176. [DOI] [PubMed] [Google Scholar]
- 37. O'Toole, J.F., Bruggeman, L.A., Madhavan, S. and Sedor, J.R. (2017) The cell biology of APOL1. Semin. Nephrol., 37, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper, A., Ilboudo, H., Alibu, V.P., Ravel, S., Enyaru, J., Weir, W., Noyes, H., Capewell, P., Camara, M., Milet, J. et al. (2017) APOL1 renal risk variants have contrasting resistance and susceptibility associations with African trypanosomiasis. elife. doi: 10.7554/eLife.25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Limou, S., Nelson, G.W., Kopp, J.B. and Winkler, C.A. (2014) APOL1 kidney risk alleles: population genetics and disease associations. Adv. Chronic Kidney Dis., 21, 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadkarni, G.N., Gignoux, C.R., Sorokin, E.P., Daya, M., Rahman, R., Barnes, K.C., Wassel, C.L. and Kenny, E.E. (2018) Worldwide frequencies of APOL1 renal risk variants. N. Engl. J. Med., 379, 2571–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosset, S., Tzur, S., Behar, D.M., Wasser, W.G. and Skorecki, K. (2011) The population genetics of chronic kidney disease: insights from the MYH9-APOL1 locus. Nat. Rev. Nephrol., 7, 313–326. [DOI] [PubMed] [Google Scholar]
- 42. Ulasi, I.I., Tzur, S., Wasser, W.G., Shemer, R., Kruzel, E., Feigin, E., Ijoma, C.K., Onodugo, O.D., Okoye, J.U., Arodiwe, E.B. et al. (2013) High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron. Clin. Pract., 123, 123–128. [DOI] [PubMed] [Google Scholar]
- 43. Behar, D.M., Kedem, E., Rosset, S., Haileselassie, Y., Tzur, S., Kra-Oz, Z., Wasser, W.G., Shenhar, Y., Shahar, E., Hassoun, G. et al. (2011) Absence of APOL1 risk variants protects against HIV-associated nephropathy in the Ethiopian population. Am. J. Nephrol., 34, 452–459. [DOI] [PubMed] [Google Scholar]
- 44. Salas, A., Carracedo, A., Richards, M. and Macaulay, V. (2005) Charting the ancestry of African Americans. Am. J. Hum. Genet., 77, 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gutiérrez, O.M., Limou, S., Lin, F., Peralta, C.A., Kramer, H.J., Carr, J.J., Bibbins-Domingo, K., Winkler, C.A., Lewis, C.E. and Kopp, J.B. (2018) APOL1 nephropathy risk variants, subclinical atherosclerosis and left ventricular mass in middle-aged black adults. Kidney Int., 93, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kopp, J.B., Nelson, G.W., Sampath, K., Johnson, R.C., Genovese, G., An, P., Friedman, D., Briggs, W., Dart, R., Korbet, S. et al. (2011) APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol., 22, 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tishkoff, S.A., Reed, F.A., Friedlaender, F.R., Ehret, C., Ranciaro, A., Froment, A., Hirbo, J.B., Awomoyi, A.A., Bodo, J.M., Doumbo, O. et al. (2009) The genetic structure and history of Africans and African Americans. Science, 324, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riella, C., Siemens, T.A., Wang, M., Campos, R.P., Moraes, T.P., Riella, L.V., Friedman, D.J., Riella, M.C. and Pollak, M.R. (2019) APOL1-associated kidney disease in Brazil. Kidney Int. Rep., 4, 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friedman, D.J. and Pollak, M.R. (2016) Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol. Metab., 27, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beckerman, P., Bi-Karchin, J., Park, A.S., Qiu, C., Dummer, P.D., Soomro, I., Boustany-Kari, C.M., Pullen, S.S., Miner, J.H., Hu, C.A. et al. (2017) Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat. Med., 23: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heymann, J., Winkler, C.A., Hoek, M., Susztak, K. and Kopp, J.B. (2017) Therapeutics for APOL1 nephropathies: putting out the fire in the podocyte. Nephrol. Dial. Transplant., 32, i65–i70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kopp, J.B., Winkler, C.A., Zhao, X., Radeva, M.K., Gassman, J.J., D'Agati, V.D., Nast, C.C., Wei, C., Reiser, J., Guay-Woodford, L.M. et al. (2015) Clinical features and histology of Apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J. Am. Soc. Nephrol., 26, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larsen, C.P., Beggs, M.L., Saeed, M. and Walker, P.D. (2013) Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J. Am. Soc. Nephrol., 24, 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hiraki, L.T. (2020) APOL1 gene—implications for systemic lupus erythematosus. J. Rheumatol., 47, 1155–1157. [DOI] [PubMed] [Google Scholar]
- 55. Chang, J.H., Husain, S.A., Santoriello, D., Stokes, M.B., Miles, C.D., Foster, K.W., Li, Y., Dale, L.A., Crew, R.J., Cohen, D.J. et al. (2019) Donor’s APOL1 risk genotype and ‘second hits’ associated with de novo collapsing glomerulopathy in deceased donor kidney transplant recipients: a report of 5 cases. Am. J. Kidney Dis., 73, 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lipkowitz, M.S., Freedman, B.I., Langefeld, C.D., Comeau, M.E., Bowden, D.W., Kao, W.H., Astor, B.C., Bottinger, E.P., Iyengar, S.K., Klotman, P.E. et al. (2013) Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int., 83, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Freedman, B.I., Kopp, J.B., Langefeld, C.D., Genovese, G., Friedman, D.J., Nelson, G.W., Winkler, C.A., Bowden, D.W. and Pollak, M.R. (2010) The Apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J. Am. Soc. Nephrol., 21, 1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kopp, J.B. (2013) Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr. Opin. Nephrol. Hypertens., 22, 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Freedman, B.I. and Murea, M. (2012) Target organ damage in African American hypertension: role of APOL1. Curr. Hypertens. Rep., 14, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parsa, A., Kao, W.H., Xie, D., Astor, B.C., Li, M., Hsu, C.Y., Feldman, H.I., Parekh, R.S., Kusek, J.W., Greene, T.H. et al. (2013) APOL1 risk variants, race, and progression of chronic kidney disease. N. Engl. J. Med., 369, 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zahr, R.S., Rampersaud, E., Kang, G., Weiss, M.J., Wu, G., Davis, R.L., Hankins, J.S., Estepp, J.H. and Lebensburger, J. (2019) Children with sickle cell anemia and APOL1 genetic variants develop albuminuria early in life. Haematologica, 104, e385–e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ashley-Koch, A.E., Okocha, E.C., Garrett, M.E., Soldano, K., De Castro, L.M., Jonassaint, J.C., Orringer, E.P., Eckman, J.R. and Telen, M.J. (2011) MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br. J. Haematol., 155, 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen, T.K., Tin, A., Peralta, C.A., Appel, L.J., Choi, M.J., Lipkowitz, M.S., Winkler, C.A. and Estrella, M.M. (2017) APOL1 risk variants, incident proteinuria, and subsequent eGFR decline in blacks with hypertension-attributed CKD. Clin. J. Am. Soc. Nephrol., 12, 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peralta, C.A., Bibbins-Domingo, K., Vittinghoff, E., Lin, F., Fornage, M., Kopp, J.B. and Winkler, C.A. (2016) APOL1 genotype and race differences in incident albuminuria and renal function decline. J. Am. Soc. Nephrol., 27, 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sumaili, E.K., Shemer, R., Kruzel-Davila, E., Cohen, E.P., Mutantu, P.N., Bukabau, J.B., Makulo, J.R.R., Mokoli, V.M., Luse, J.L., Pakasa, N.M. et al. (2019) G1 is the major APOL1 risk allele for hypertension-attributed nephropathy in Central Africa. Clin. Kidney J., 12, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ekulu, P.M., Nkoy, A.B., Betukumesu, D.K., Aloni, M.N., Makulo, J.R.R., Sumaili, E.K., Mafuta, E.M., Elmonem, M.A., Arcolino, F.O., Kitetele, F.N. et al. (2019) APOL1 risk genotypes are associated with early kidney damage in children in sub-Saharan Africa. Kidney Int. Rep., 4, 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ekrikpo, U.E., Kengne, A.P., Akpan, E.E., Effa, E.E., Bello, A.K., Ekott, J.U., George, C., Salako, B.L. and Okpechi, I.G. (2018) Prevalence and correlates of chronic kidney disease (CKD) among ART-naive HIV patients in the Niger-Delta region of Nigeria. Medicine (Baltimore), 97, e0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kasembeli, A.N., Duarte, R., Ramsay, M., Mosiane, P., Dickens, C., Dix-Peek, T., Limou, S., Sezgin, E., Nelson, G.W., Fogo, A.B. et al. (2015) APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J. Am. Soc. Nephrol., 26, 2882–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee, B.T., Kumar, V., Williams, T.A., Abdi, R., Bernhardy, A., Dyer, C., Conte, S., Genovese, G., Ross, M.D., Friedman, D.J. et al. (2012) The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am. J. Transplant., 12, 1924–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zwang, N.A., Shetty, A., Sustento-Reodica, N., Gordon, E.J., Leventhal, J., Gallon, L. and Friedewald, J.J. (2016) APOL1-associated end-stage renal disease in a living kidney transplant donor. Am. J. Transplant., 23:429–438. [DOI] [PubMed] [Google Scholar]
- 71. Doshi, M.D., Ortigosa-Goggins, M., Garg, A.X., Li, L., Poggio, E.D., Winkler, C.A. and Kopp, J.B. (2018) APOL1 genotype and renal function of black living donors. J. Am. Soc. Nephrol., 29, 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Freedman, B.I. and Moxey-Mims, M. (2018) The APOL1 long-term kidney transplantation outcomes network—APOLLO. Clin. J. Am. Soc. Nephrol., 13, 940–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Freedman, B.I., Moxey-Mims, M.M., Alexander, A.A., Astor, B.C., Birdwell, K.A., Bowden, D.W., Bowen, G., Bromberg, J., Craven, T.E., Dadhania, D.M. et al. (2020) APOL1 long-term kidney transplantation outcomes network (APOLLO): design and rationale. Kidney Int. Rep., 5, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mukamal, K.J., Tremaglio, J., Friedman, D.J., Ix, J.H., Kuller, L.H., Tracy, R.P. and Pollak, M.R. (2016) APOL1 genotype, kidney and cardiovascular disease, and death in older adults. Arterioscler. Thromb. Vasc. Biol., 36, 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ito, K., Bick, A.G., Flannick, J., Friedman, D.J., Genovese, G., Parfenov, M.G., Depalma, S.R., Gupta, N., Gabriel, S.B., Taylor, H.A., Jr. et al. (2014) Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ. Res., 114, 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grams, M.E., Surapaneni, A., Ballew, S.H., Appel, L.J., Boerwinkle, E., Boulware, L.E., Chen, T.K., Coresh, J., Cushman, M., Divers, J. et al. (2019) APOL1 kidney risk variants and cardiovascular disease: an individual participant data meta-analysis. J. Am. Soc. Nephrol., 30, 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Langefeld, C.D., Divers, J., Pajewski, N.M., Hawfield, A.T., Reboussin, D.M., Bild, D.E., Kaysen, G.A., Kimmel, P.L., Raj, D.S., Ricardo, A.C. et al. (2015) Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int., 87, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen, T.K., Appel, L.J., Grams, M.E., Tin, A., Choi, M.J., Lipkowitz, M.S., Winkler, C.A. and Estrella, M.M. (2017) APOL1 risk variants and cardiovascular disease: results from the AASK (African American Study of Kidney Disease and Hypertension). Arterioscler. Thromb. Vasc. Biol., 37:1765–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Phipps, E., Prasanna, D., Brima, W. and Jim, B. (2016) Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin. J. Am. Soc. Nephrol., 11, 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hypertension in pregnancy. (2013) Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol., 122, 1122–1131. [DOI] [PubMed] [Google Scholar]
- 81. Levine, R.J., Maynard, S.E., Qian, C., Lim, K.H., England, L.J., Yu, K.F., Schisterman, E.F., Thadhani, R., Sachs, B.P., Epstein, F.H. et al. (2004) Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med., 350, 672–683. [DOI] [PubMed] [Google Scholar]
- 82. Abalos, E., Cuesta, C., Grosso, A.L., Chou, D. and Say, L. (2013) Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol., 170, 1–7. [DOI] [PubMed] [Google Scholar]
- 83. Khan, K.S., Wojdyla, D., Say, L., Gulmezoglu, A.M. and Van Look, P.F. (2006) WHO analysis of causes of maternal death: a systematic review. Lancet, 367, 1066–1074. [DOI] [PubMed] [Google Scholar]
- 84. Nakimuli, A., Chazara, O., Byamugisha, J., Elliott, A.M., Kaleebu, P., Mirembe, F. and Moffett, A. (2014) Pregnancy, parturition and preeclampsia in women of African ancestry. Am. J. Obstet. Gynecol., 210(510–520), e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wen, Q., Liu, L.Y., Yang, T., Alev, C., Wu, S., Stevenson, D.K., Sheng, G., Butte, A.J. and Ling, X.B. (2013) Peptidomic identification of serum peptides diagnosing preeclampsia. PLoS One, 8, e65571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Elliott, S.E., Parchim, N.F., Liu, C., Xia, Y., Kellems, R.E., Soffici, A.R. and Daugherty, P.S. (2014) Characterization of antibody specificities associated with preeclampsia. Hypertension, 63, 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bruggeman, L.A., Wu, Z., Luo, L., Madhavan, S.M., Konieczkowski, M., Drawz, P.E., Thomas, D.B., Barisoni, L., Sedor, J.R. and O'Toole, J.F. (2016) APOL1-G0 or APOL1-G2 transgenic models develop preeclampsia but not kidney disease. J. Am. Soc. Nephrol., 27, 3600–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reidy, K.J., Hjorten, R.C., Simpson, C.L., Rosenberg, A.Z., Rosenblum, S.D., Kovesdy, C.P., Tylavsky, F.A., Myrie, J., Ruiz, B.L., Haque, S. et al. (2018) Fetal—not maternal—APOL1 genotype associated with risk for preeclampsia in those with African ancestry. Am. J. Hum. Genet., 103, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miller, A.K., Azhibekov, T., O'Toole, J.F., Sedor, J.R., Williams, S.M., Redline, R.W. and Bruggeman, L.A. (2020) Association of preeclampsia with infant APOL1 genotype in African Americans. BMC Med. Genet., 21, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thakoordeen-Reddy, S., Winkler, C., Moodley, J., David, V., Binns-Roemer, E., Ramsuran, V. and Naicker, T. (2020) Maternal variants within the Apolipoprotein L1 gene are associated with preeclampsia in a South African cohort of African ancestry. Eur. J. Obstet. Gynecol. Reprod. Biol., 246, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hong, X., Rosenberg, A.Z., Zhang, B., Binns-Roemer, E., David, V., Lv, Y., Hjorten, R.C., Reidy, K.J., Chen, T.K., Wang, G. et al. (2020) Joint associations of maternal-fetal APOL1 genotypes and maternal country of origin with preeclampsia risk. Am. J. Kidney Dis. doi: 10.1053/j.ajkd.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Friedman, D.J. and Pollak, M.R. (2020) APOL1 nephropathy: from genetics to clinical applications. Clin. J. Am. Soc. Nephrol. doi: 10.2215/CJN.15161219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Friedman, D.J. and Pollak, M.R. (2020) APOL1 and kidney disease: from genetics to biology. Annu. Rev. Physiol., 82, 323–342. [DOI] [PubMed] [Google Scholar]
- 94. Aghajan, M., Booten, S.L., Althage, M., Hart, C.E., Ericsson, A., Maxvall, I., Ochaba, J., Menschik-Lundin, A., Hartleib, J., Kuntz, S. et al. (2019) Antisense oligonucleotide treatment ameliorates IFN-gamma-induced proteinuria in APOL1-transgenic mice. JCI Insight. doi: 10.1172/jci.insight.126124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aliyu, M.H., Wudil, U.J., Ingles, D.J., Shepherd, B.E., Gong, W., Musa, B.M., Muhammad, H., Sani, M.U., Abdu, A., Nalado, A.M. et al. (2019) Optimal management of HIV-positive adults at risk for kidney disease in Nigeria (Renal Risk Reduction ‘R3’ Trial): protocol and study design. Trials, 20, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kopp, J.B., Heymann, J. and Winkler, C.A. (2017) APOL1 renal risk variants: fertile soil for HIV-associated nephropathy. Semin. Nephrol., 37, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Franco, J.R., Simarro, P.P., Diarra, A. and Jannin, J.G. (2014) Epidemiology of human African trypanosomiasis. Clin. Epidemiol., 6, 257–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nqebelele, N.U., Dickens, C., Dix-Peek, T., Duarte, R. and Naicker, S. (2019) Low prevalence of Apolipoprotein L1 gene variants in black South Africans with hypertension-attributed chronic kidney disease. Clin. Nephrol., 91, 40–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


