Abstract
N-type voltage-gated calcium channels (CaV2.2) are predominantly expressed at presynaptic terminals, and their function is regulated by auxiliary α2δ and β subunits. All four mammalian α2δ subunits enhance calcium currents through CaV1 and CaV2 channels, and this increase is attributed, in part, to increased CaV expression at the plasma membrane. In the present study we provide evidence that α2δ-1, like α2δ-2, is recycled to the plasma membrane through a Rab11a-dependent endosomal recycling pathway. Using a dominant-negative Rab11a mutant, Rab11a(S25N), we show that α2δ-1 increases plasma membrane CaV2.2 expression by increasing the rate and extent of net forward CaV2.2 trafficking in a Rab11a-dependent manner. Dominant-negative Rab11a also reduces the ability of α2δ-1 to increase CaV2.2 expression on the cell-surface of hippocampal neurites. In contrast, α2δ-3 does not enhance rapid forward CaV2.2 trafficking, regardless of whether Rab11a(S25N) is present. In addition, whole-cell CaV2.2 currents are reduced by co-expression of Rab11a(S25N) in the presence of α2δ-1, but not α2δ-3. Taken together these data suggest that α2δ subtypes participate in distinct trafficking pathways which in turn influence the localisation and function of CaV2.2.
Subject terms: Neuroscience, Cellular neuroscience
Introduction
The CaV1 and CaV2 voltage-gated calcium channels associate with auxiliary α2δ and ß subunits1. The α2δ subunits are glycosyl-phosphatidylinositol-anchored extracellular proteins2–4. There are four known mammalian α2δ isoforms (α2δ-1-4) which are thought to have similar functions and topological features, despite a surprising lack of sequence homology between them5. The α2δ subunits have long been known to enhance whole-cell currents through CaV channels6–9. However, to date there is limited evidence that α2δs strongly influence single-channel properties of CaV channels8. As such, α2δ-mediated enhancement of CaV currents has often been attributed primarily to increased channel trafficking and plasma membrane insertion8,10–13.
The development of functional exofacially-tagged CaV2 constructs10, has allowed us to define populations of plasma membrane-inserted CaV2 channels using imaging approaches. This method was previously used to demonstrate that heterologous expression of α2δ-1 increases cell-surface CaV2.2 expression, consistent with earlier models of α2δ-1 function10. However, subsequent studies on the proteolytic processing of α2δ have found that unprocessed α2δ-1 is unable to enhance macroscopic CaV currents despite increasing cell-surface CaV2.2 expression in cell lines14,15. These studies provide convincing evidence that the α2δ-mediated increase in CaV currents is dependent on an increase in cell-surface CaV2.2 expression, but also requires a molecular activation switch, triggered by the proteolytic cleavage of α2δ into α2 and δ. Presently, it is unclear whether α2δ isoforms differ significantly in their ability to enhance CaV currents, and it remains to be seen if α2δ subtypes have uniform trafficking mechanisms particularly with regard to their effect on CaV localisation.
In a previous study16, we found that the cell-surface localization of α2δ-2, following its heterologous expression, was increased by recycling from Rab11a-dependent endosomes back to the plasma membrane. Rab11a belongs to the Rab family of small GTPases which regulate a multitude of intracellular trafficking pathways, facilitating membrane targeting, cargo sorting and vesicle fusion events through recruitment of effectors and direct interactions with cargo proteins17. Rab11 proteins are particularly involved in trafficking through recycling endosomes18,19, and inhibition of Rab11a-dependent recycling can be achieved through expression of the dominant-negative mutant Rab11a(S25N), which is locked in an inactive GDP-bound conformation20. Cell-surface expression of α2δ-2 is reduced in the presence of Rab11a(S25N)16, and this occludes the effect of the α2δ ligand gabapentin, which itself reduces cell-surface expression of α2δ-1 and α2δ-211,16. These data provide a mechanistic explanation for the action of gabapentin, whereby α2δ bound to gabapentin is prevented from recycling to the plasma membrane via Rab11-positive endosomes, resulting in a loss of α2δ cell-surface expression16. Mutational studies have revealed that gabapentin acts by binding to a site including an RRR motif in the α2 domain, which is present in α2δ-1 and α2δ-221–24. However, since α2δ-3 is also widely distributed in the nervous system, if there were functional differences, for example between α2δ-1 and α2δ-3, this might influence the trafficking and localisation of associated CaV channels.
In the present study, we wished to determine to what extent the enhancement of plasma membrane CaV2.2 expression, a previously-demonstrated feature of α2δ-1 function, is conserved for α2δ-3. We then sought to elucidate further the mechanisms through which α2δs increase cell-surface CaV2.2, and to understand how this process influences the localization and function of CaV2.2 in cell lines and hippocampal neurons. We find that α2δ subunits have a differential effect on CaV2.2 trafficking; although all α2δ proteins tested increase CaV2.2 cell-surface expression to varying extents, only α2δ-1 and α2δ-2 rapidly increase net forward trafficking of CaV2.2; by contrast α2δ-3 does not influence this process, within a 45-min time-frame. Further examination showed that the enhanced forward trafficking of CaV2.2 is a Rab11a-dependent effect and that α2δ-1 and α2δ-2 participate in Rab11a-dependent recycling, whereas α2δ-3 does not.
Results
Rab11a-dependent recycling affects α2δ-1 but not α2δ-3 cell-surface expression
Trafficking through Rab11a-positive recycling endosomes enhances α2δ-2 membrane expression, a pathway that is inhibited by gabapentin16. The α2 domain of both α2δ-1 and α2δ-2 contains a triple RRR motif, the third R of which is required for gabapentin binding, and is absent from the α2δ-3 and α2δ-4 sequences12,25. Here, we considered the possibility that α2δ-1 and α2δ-2 enhance cell-surface CaV2.2 expression, at least in part, by facilitating the recycling of CaV2.2 through Rab11a-positive endosomes. Further to this, we speculated that α2δ-3 may traffic independently of this pathway.
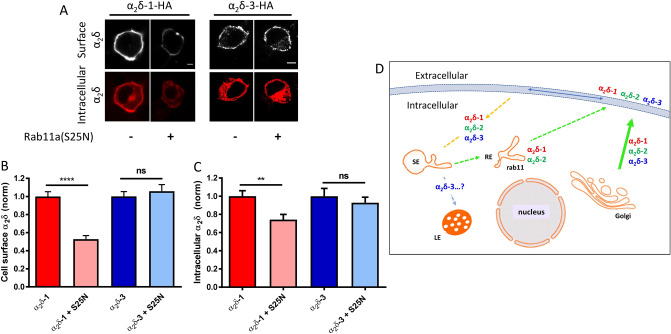
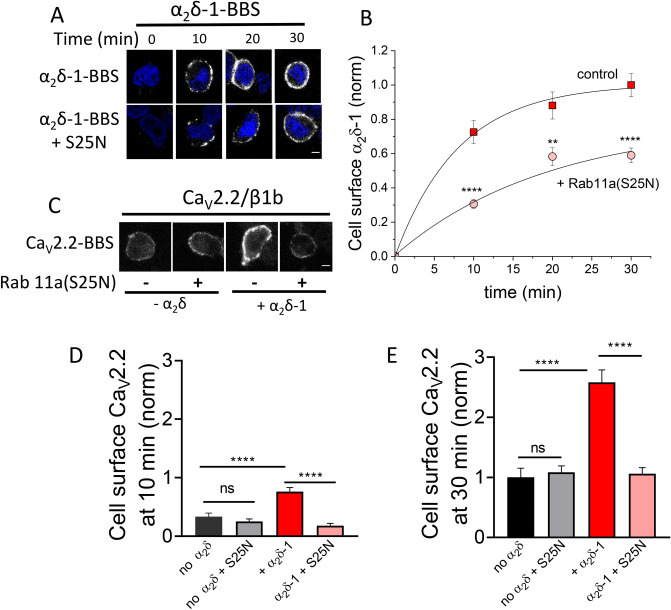
To test this hypothesis, we have used the dominant-negative Rab11a(S25N) mutant, which we found previously to inhibit cell-surface expression of α2δ-216, and thus a similar result was expected for α2δ-1. In our previous study16 we found no effect of co-expressing WT Rab11a on any aspects of α2δ trafficking or calcium channel function, and thus it was not included as an additional control in the present study. We compared cell-surface expression of HA-tagged α2δ-1 and α2δ-3, in the absence and presence of Rab11a(S25N) (Fig. 1A,B). Cell-surface α2δ-3 immunostaining is typically weaker than that for α2δ-1 (Fig. 1A), so we used antigen retrieval to maximise available HA signal. We found a reduction in cell-surface α2δ-1-HA staining of 47%, when co-expressed with Rab11a(S25N) (Fig. 1B). In contrast α2δ-3 showed no significant difference in cell-surface expression whether expressed with or without Rab11a(S25N) (Fig. 1B). These data provide evidence that α2δ-1, like α2δ-216, is regulated by Rab11a-dependent recycling and suggest that cell-surface expression of α2δ-3 is not promoted by trafficking through this endosomal recycling pathway (Fig. 1D). This result was supported by measurement of the intracellular α2δ levels which were strongly reduced by Rab11a(S25N) in the case of α2δ-1 but not α2δ-3 (Fig. 1A,C). This suggests that block of recycling endosome function may lead re-routing of α2δ-1 to degradation pathways (Fig. 1D), although this will require further experimentation.
Figure 1.
Dominant-negative Rab11a(S25N) reduces steady-state cell-surface expression of HA-tagged α2δ-1 but not α2δ-3. (A) Confocal images of cell-surface (top row) and intracellular (bottom row) HA staining in N2a cells expressing α2δ-1-HA (left) or α2δ-3-HA (right) in the absence or presence of Rab11a(S25N). Scale bars (5 μm) refer to either α2δ-1 or α2δ-3 expressing cells. Cell surface HA was detected in non-permeabilised cells and intracellular HA subsequently detected following permeabilization (see Methods). (B) Normalized mean cell-surface α2δ expression for α2δ-1 control (red), α2δ-1 + Rab11a(S25N) (pink), α2δ-3 control (blue), α2δ-3 + Rab11a(S25N) (light blue). (C) Normalized mean intracellular α2δ expression for α2δ-1 control (red), α2δ-1 + Rab11a(S25N) (pink), α2δ-3 control (blue), α2δ-3 + Rab11a(S25N) (light blue). For (B) and (C), mean fluorescence intensity per cell was normalized to mean α2δ control conditions for each experiment. Normalised data were then pooled from 3 separate experiments and plotted as mean ± SEM. Each cell was measured for cell-surface and intracellular HA expression: α2δ-1 control (n = 159 cells), α2δ-1 + Rab11a(S25N) (n = 161 cells), α2δ-3 control (n = 134 cells), α2δ-3 + Rab11a(S25N) (n = 135 cells). Statistical significance was determined using Student’s unpaired t test with Welch’s correction; **, P = 0.0022; ****, P < 0.0001. (D) Diagram of pathways for α2δ membrane expression and recycling, and Rab11 expression. SE = sorting endosome, RE = recycling endosome, LE = late endosome/lysosome. Dotted pathways represent hypothetical routes.
Cell-surface CaV2.2 expression is reduced by Rab11a(S25N) when α2δ-1 or α2δ-2 are present
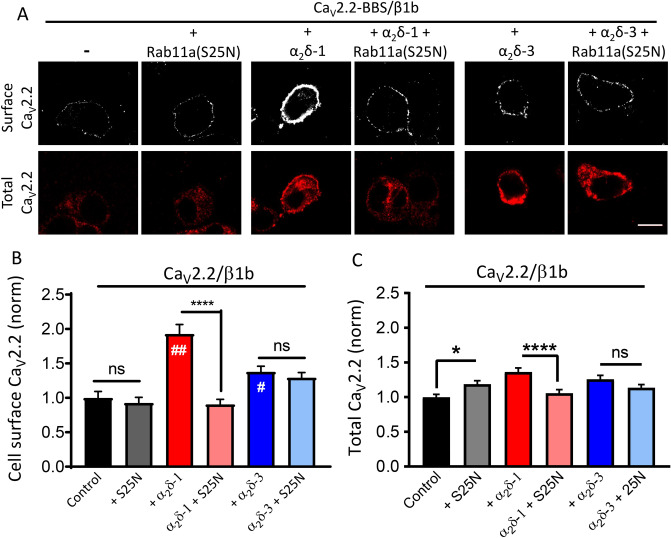
In our previous study16, we found that inhibition of α2δ-2 recycling through Rab11a-positive endosomes reduced whole-cell CaV2.2 currents. However, a direct effect on plasma membrane insertion of associated CaV channels was not examined. Here we compared the plasma membrane expression of exofacially-tagged CaV2.2, containing a bungarotoxin binding site (BBS) tag10, when co-expressed with ß1b, either alone or together with α2δ-1, or α2δ-3. We then examined the effect of Rab11a(S25N) (Fig. 2A). Plasma membrane-inserted CaV2.2 was increased by 92% and 38% when co-expressed with α2δ-1 and α2δ-3, respectively (Fig. 2B), in agreement with our previous study14. However, Rab11a(S25N) co-expression reduced the plasma membrane level of CaV2.2 by 53% when α2δ-1 was present, but had no effect in the presence of α2δ-3, or in the absence of α2δ (Fig. 2B). Total CaV2.2 levels were also reduced by Rab11a(S25N) in the presence of α2δ-1 but not in the absence of α2δ or presence of α2δ-3 (Fig. 2A,C). Following these findings, we also compared the effect of Rab11a(S25N) on plasma membrane CaV2.2 in the presence α2δ-2 (Fig. S1A,B). As expected, cell-surface CaV2.2 expressed with α2δ-2 and β1b was reduced by 35% by Rab11a(S25N) (Fig. S1B).
Figure 2.
Steady-state cell-surface CaV2.2 is reduced by Rab11a(S25N) when expressed with α2δ-1, but not α2δ-3. (A) Confocal images of cell-surface (top row, BBS, live-labelled with α-BTX) and total (bottom row, II-III loop Ab) CaV2.2-BBS expressed in N2a cells with β1b and either: no α2δ, α2δ-1, or α2δ-3, in the absence or presence of Rab11a(S25N). Scale bar applies to all images = 5 μm. (B) Mean cell-surface CaV2.2-BBS levels expressed in N2a cells with β1b and: no α2δ (black, n = 142), no α2δ + Rab11a(S25N) (grey, n = 145 cells), α2δ-1 (red, n = 148 cells), α2δ-1 + Rab11a(S25N) (pink, n = 140 cells), α2δ-3 (blue, n = 132 cells), α2δ-3 + Rab11a(S25N) (light blue, n = 144 cells), normalized to the control level in the absence of α2δ. Statistical significance was determined using one-way ANOVA (F = 17.72, P < 0.0001) and Sidak’s multiple comparison post hoc tests; ns, P = 0.986 for control, P = 0.975 for α2δ-3; ****, P < 0.0001; ##, P < 0.0001 vs no α2δ; #, P = 0.0213 vs no α2δ. (C) Total CaV2.2-BBS in cells shown in (B). Statistical significance was determined using one-way ANOVA (F = 6.752, P < 0.0001) and Sidak’s multiple comparison tests; ns, P = 0.284; *, P = 0.0302; ***, P < 0.0001. For (B) and (C), each cell was measured for cell-surface BBS expression in non-permeabilised cells using α-BTX-AF488, and for total CaV2.2 following permeabilization using the II-III loop Ab (see Methods). Fluorescence intensity per cell was normalized to mean CaV2.2/β1b control condition for each experiment. Normalized data were then pooled from 3 separate experiments and plotted ± SEM.
Together, these data suggest that α2δ-1 and α2δ-2 enhance cell-surface CaV2.2 expression, in part through Rab11a-dependent recycling to the plasma membrane, which CaV2.2 is unable to access independently of these α2δ subunits. In contrast, although α2δ-3 produced a 38% increase in CaV2.2 cell-surface expression (Fig. 2B), this was independent of a Rab11a-dependent recycling pathway, suggesting that α2δ-3 is only able to promote cell-surface CaV2.2 trafficking through another pathway, such as direct trafficking from the Golgi apparatus.
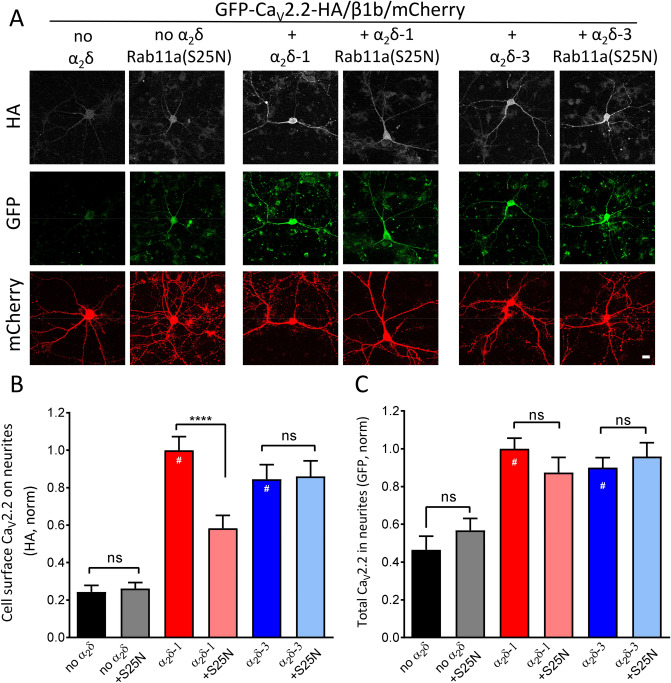
Rab11a(S25N) reduces CaV2.2 expression in neurites of cultured hippocampal neurons when co-expressed with α2δ-1 but not α2δ-3
We have previously demonstrated that CaVß subunits are able to increase plasma membrane expression of CaV2.2 in non-neuronal cells, even in the absence of α2δ, although there is generally an approximately twofold increase in the presence of α2δ-110,14 (see Fig. 2B). However, in primary hippocampal neuronal cultures, we have found the presence of α2δ to be critical for localisation of CaV2.2 to neurites14. Here, we examined whether CaV2.2 expression on the surface of hippocampal neurites is also influenced by Rab11a in an α2δ-dependent manner. To do this we used an exofacially HA-tagged and N-terminally GFP-tagged CaV2.2 construct26, which allowed us to quantify cell-surface and total CaV2.2 expression in non-permeabilised neurons. We expressed GFP-CaV2.2-HA plus β1b, with or without α2δ-1 or α2δ-3, and with or without Rab11a(S25N) (Fig. 3A). In accordance with our previous studies14, expression of CaV2.2 on the cell-surface of neurites (which had the appearance of dendrites, see Methods) was extremely low in the absence of α2δ (Fig. 3A,B), and we found no effect of co-expression of Rab11a(S25N) on this. We observed a ~ fourfold increase in cell-surface CaV2.2-HA expression with α2δ-1, and ~ 3.5-fold increase with α2δ-3, relative to control (Fig. 3B). Expression of GFP in the neurites, representing total CaV2.2, was also elevated by co-expression of both α2δ-1 and α2δ-3, by 2.1-fold and 1.9-fold, respectively (Fig. 3C). Consistent with our findings in non-neuronal cells, cell-surface CaV2.2-HA was reduced by co-expression of Rab11a(S25N) in the presence of α2δ-1 (by ~ 44%), but was not affected in the presence of α2δ-3 (Fig. 3B). However, we saw no change in total CaV2.2-GFP expression in the neurites with Rab11a(S25N), either in the absence or presence of α2δ-1 or α2δ-3 (Fig. 3C). Together these data suggest that Rab11a-dependent recycling enhances neurite CaV2.2 cell-surface expression in an α2δ-selective manner.
Figure 3.
Rab11a(S25N) reduces plasma membrane-inserted CaV2.2 in hippocampal neurites when expressed with α2δ-1 but not without α2δ or with α2δ-3. (A) Confocal images of rat hippocampal pyramidal neurons expressing GFP-CaV2.2-HA/β1b/mCherry control, + Rab11a(S25N), α2δ-1, α2δ-1 + Rab11a(S25N), α2δ-3 or α2δ-3 + Rab11a(S25N). Scale bar applies to all images = 20 μm. Top row, HA immunostaining in non-permeabilised neurons to measure cell surface CaV2.2; middle row, GFP to determine total CaV2.2 expression; bottom row, mCherry (transfection marker) expression. (B) Normalized mean cell-surface GFP-CaV2.2-HA, determined from HA staining in non-permeabilised neurons, in the processes of pyramidal rat neurons expressed with β1b/mCherry for control (black), Rab11a(S25N) (grey), α2δ-1 (red), α2δ-1 + Rab11a(S25N) (pink), α2δ-3 (blue) or α2δ-3 + Rab11a(S25N) (light blue). Statistical significance was determined using one-way ANOVA (F = 16.32, P < 0.0001) and Sidak’s multiple comparison post hoc tests; ns, P > 0.999 for both control vs S25N and α2δ-3 vs α2δ-3 + S25N; ****, P < 0.0001. Significance vs no α2δ control: #, P < 0.0001 for both α2δ-1 and α2δ-3. (C) Normalized mean total GFP-CaV2.2-HA (determined from GFP density) in the processes of pyramidal rat neurons expressed with β1b/mCherry for control (black), Rab11a(S25N) (grey), α2δ-1 (red), α2δ-1 + Rab11a(S25N) (pink), α2δ-3 (blue) or α2δ-3 + Rab11a(S25N) (light blue). Statistical significance was determined using one-way ANOVA (F = 8.74, P < 0.0001) and Sidak’s multiple comparison post hoc tests; ns, P = 0.925 for control vs S25N; P = 0.559 for α2δ-1 vs α2δ-1 + S25N; P = 0.974 for α2δ-3 vs α2δ-3 + S25N. Significance vs no α2δ control: #, P < 0.0001 for both α2δ-1 and α2δ-3. For (B) and (C), between 2–5 neuronal processes were measured and averaged per cell, 10–15 cells per condition per experiment, for a total of 3 transfections. Total cell numbers for each condition: control (n = 30 cells), Rab11a(S25N) (n = 19 cells), α2δ-1 (n = 51 cells), α2δ-1 + Rab11a(S25N) (n = 42 cells), α2δ-3 (n = 46) and α2δ-3 + Rab11a(S25N) (n = 40 cells). Normalized mean fluorescence intensity per neuron was pooled from three separate experiments and data are plotted ± SEM values.
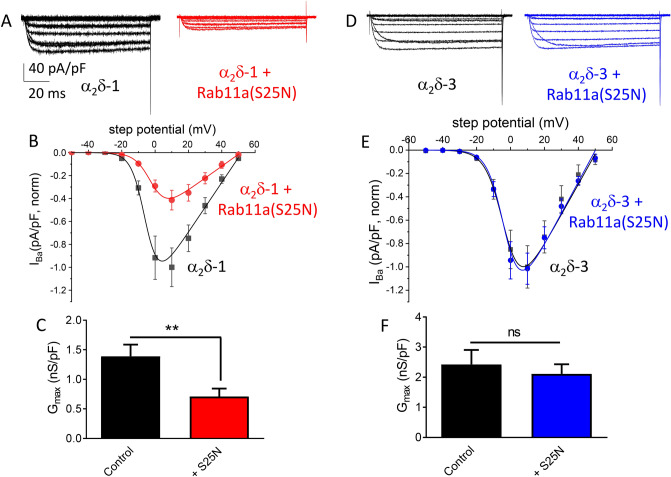
Rab11a(S25N) reduces whole-cell CaV2.2 currents when co-expressed with α2δ-1 but not α2δ-3
Here we investigated whether the reduction in cell-surface CaV2.2 expression by Rab11a(S25N) correlates to a change in whole-cell CaV currents and whether any change we observe is α2δ-dependent. We used patch-clamp recording to measure whole-cell Ba2+ currents in tsA201 cells expressing CaV2.2/β1b/α2δ, with or without Rab11a(S25N) (Fig. 4A). We found that the peak Ba2+ current density was reduced by 59% at + 10 mV, when α2δ-1 and Rab11a(S25N) were co-expressed, compared to α2δ-1 expressed alone (Fig. 4A,B). Corresponding maximum conductance (Gmax) values were reduced from 1.4 ± 0.2 to 0.7 ± 0.1 nS/pF by the presence of Rab11a(S25N) (Fig. 4C). We saw no significant changes to steady-state inactivation under the two conditions (Fig. S2A), supporting the view that current density changes occurred as a result of reduced plasma membrane CaV2.2 expression rather than changes to biophysical properties of the channels.
Figure 4.
Rab11a(S25N) reduces whole-cell CaV2.2 currents when expressed with α2δ-1, but not α2δ-3. (A) Example whole-cell current traces for CaV2.2 expressed in tsA-201 cells with: β1b/α2δ-1 (black) and β1b/α2δ-1 + Rab11a(S25N) (red). Scale bars (40 pA/pF and 20 ms) refer to all traces. (B) Mean IV plots for CaV2.2 with: β1b/α2δ-1 (black squares, n = 15) and β1b/α2δ-1 + Rab11a(S25N) (red circles, n = 14), fitted by a modified Boltzmann function. The potentials for 50% activation were -4.23 ± 0.76 mV, and -1.39 ± 1.26 mV, respectively. (C) Mean Gmax values obtained by fitting each individual trace in (B) to a modified Boltzmann function CaV2.2/β1b/α2δ-1 (black), CaV2.2/β1b/α2δ-1 + Rab11a(S25N) (red). Statistical significance determined by Student’s unpaired t-test; **, P = 0.0093. (D) Example whole-cell current traces for CaV2.2 with: β1b/α2δ-3 (black) and β1b/α2δ-3 + Rab11a(S25N) (blue). (E) Mean IV plots for CaV2.2 with: β1b/α2δ-3 (black squares, n = 15) and β1b/α2δ-3 + Rab11a(S25N) (blue circles, n = 14), fitted by a modified Boltzmann function. The potentials for 50% activation were -4.21 ± 1.59 mV and -3.93 ± 0.98 mV, respectively. (F) Mean Gmax values obtained by fitting each individual trace in (E) to a modified Boltzmann function CaV2.2/β1b/α2δ-3 (black), CaV2.2/β1b/α2δ-3 + Rab11a(S25N) (blue). Data are plotted as mean ± SEM. Statistical significance determined by Student’s unpaired t-test; ns, P = 0.5816.
Having established that Rab11a(S25N) reduced both α2δ-1-mediated CaV2.2 plasma membrane expression and CaV2.2 current enhancement to similar extents, it was necessary to determine whether Rab11a(S25N) would produce a reduction in α2δ-3-mediated CaV2.2 current enhancement. In contrast with the effect of α2δ-1, we found no difference in mean current density (Fig. 4D,E), Gmax (Fig. 4F) or steady-state inactivation (Fig. S2B) when Rab11a(S25N) was co-expressed with α2δ-3, compared to α2δ-3 alone. This result is consistent with our observation that cell-surface expression of CaV2.2 is unaffected by Rab11a-dependent recycling when expressed with α2δ-3.
Net forward trafficking of CaV2.2 is enhanced by α2δ-1 and α2δ-2, but not α2δ-3
Having provided evidence that α2δ-3 enhances steady-state plasma membrane CaV2.2 expression, albeit to a smaller degree than α2δ-1 (Fig. 2B), we considered two possible explanations for this effect: (1) α2δ subunits increase forward trafficking of CaV2.2 to the cell-surface, or (2) α2δ subunits reduce the rate of CaV2.2 endocytosis from the cell-surface. Previously, we found that the rate of CaV2.2 endocytosis was not altered by expression of α2δ-110,27. In the present study, we used an α-bungarotoxin (BTX) live-labelling approach to compare the rates of net forward trafficking of CaV2.2-BBS expressed with β1b either alone or together with α2δ-1, α2δ-2 or α2δ-3 (Fig. 5A). When compared to control conditions, we found that co-expression of α2δ-1 and α2δ-2 produced significantly higher insertion of CaV2.2 into the plasma membrane, at each time point tested, starting at 15 min (Fig. 5B). In contrast, the appearance of CaV2.2 on the cell-surface did not differ significantly between the control and α2δ-3 conditions up to 45 min, but there was a statistically significant increase of 23% at 60 min (Fig. 5B), which is consistent with our observation of its effect on steady-state CaV2.2 expression (Fig. 2B). Estimates for initial rates of net forward CaV2.2 trafficking were made using the slope value of a straight line between 0 and 15 min. While there was a 2.7-fold increase in initial rate of CaV2.2 appearance on the cell-surface with α2δ-1 co-expression compared to control (Fig. 5C), we found no significant difference in initial CaV2.2 trafficking rates for the α2δ-3 condition. Together these data support a role for α2δ-1 in enhancing rapid plasma membrane-insertion of CaV2.2 by increasing the rate of forward trafficking, whereas α2δ-3 had no clear effect on the initial rate of forward CaV2.2 trafficking (Fig. 5C).
Figure 5.
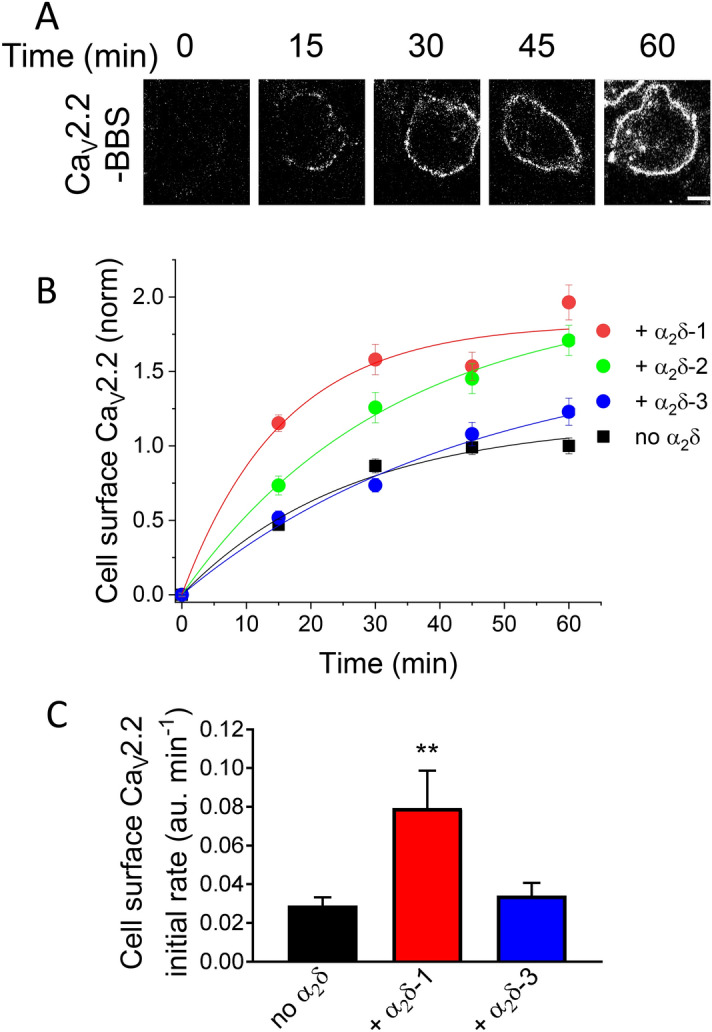
Net forward trafficking of CaV2.2-BBS is increased by α2δ-1 and α2δ-2, but not by α2δ-3. (A) Example images from forward trafficking assay for CaV2.2-BBS expressed in N2a cells with β1b and α2δ-1. Cells were live-labelled with α-BTX-AF488 for 0, 15, 30, 45, and 60 min, following pre-incubation with unlabelled α-BTX at 17 °C for 30 min. Scale bar = 5 μm. (B) Normalized mean cell-surface CaV2.2-BBS fluorescence in cells expressing CaV2.2-BBS and β1b alone (black) or with either α2δ-1 (red), α2δ-2 (green) or α2δ-3 (blue). An average of 30—60 cells were analysed per time point, for each condition in an individual experiment. The data were collected as paired experiments between CaV2.2/β1b (control) and CaV2.2/β1b + α2δ (n = 3 each), with a total of 9 for CaV2.2/β1b. Individual experiments were normalized to mean 60 min fluorescence of the paired CaV2.2/β1b condition, before being pooled together. Data were fitted with an exponential association equation; the time constant (τ) = 26.1 min (no α2δ), 15.6 min (+ α2δ-1), 32.4 min (+ α2δ-2) and 44.9 min (+ α2δ-3) and asymptotic normalized cell-surface CaV2.2 (amplitude) = 1.17 (no α2δ), 1.82 (+ α2δ-1), 2.0 (+ α2δ-2) and 1.63 (+ α2δ3). (C) Initial rate of net forward CaV2.2-BBS trafficking. The gradient of straight line between 0–15 min was obtained as an average for each individual experiment and summarised (in a.u. / min), for CaV2.2/β1b control (black, n = 9), CaV2.2/β1b + α2δ-1 (red, n = 3), and CaV2.2/β1b + α2δ-3 (blue, n = 3). Data are mean ± SEM. Statistical significance determined by one-way ANOVA (F = 9.333, P = 0.0036) and Dunnett’s multiple comparison test vs no α2δ, ** P = 0.0021.
Dominant-negative Rab11a reduces net forward trafficking of α2δ-1
Next, we compared net forward trafficking rates of α2δ-1-BBS in the presence or absence of Rab11a(S25N) (Fig. 6A). We found that net forward trafficking of α2δ-1-BBS was reduced by co-expression of Rab11a(S25N), with a 41% reduction in cell-surface α2δ-1-BBS after 30 min (Fig. 6B), consistent with the reduction in cell-surface α2δ-1-HA induced by Rab11a(S25N) under steady-state conditions (Fig. 1B). We then compared net forward trafficking of CaV2.2-BBS, when expressed with or without α2δ-1 and with or without Rab11a(S25N), at two time points, 10 and 30 min (Fig. 6C–E). As expected, α2δ-1 enhanced net-forward trafficking of CaV2.2, and Rab11a(S25N) abolished this increase at both time points. In sum, these data support the conclusion that α2δ-1 membrane expression is enhanced by forward-trafficking from Rab11a-positive recycling endosomes, and that CaV2.2 can traffic through this pathway when co-expressed with Rab11a-sensitive α2δ-1, but not with α2δ-3 (Fig. 1D).
Figure 6.
Rab11a(S25N) reduces net forward trafficking of α2δ-1 and CaV2.2/β1b/α2δ-1. (A) Confocal images of α2δ-1-BBS expressed in tsA-201 cells, alone (top row) or with Rab11a(S25N) (bottom row), at each time point of net forward trafficking assay. Cells were live-labelled with α-BTX-AF488 for 0, 10, 20, and 30 min. Nuclei staining with DAPI (blue). Scale bar = 5 μm. (B) Net forward α2δ-1-BBS trafficking, as in (A). Normalized mean cell-surface α2δ-1-BBS expressed either alone (red squares) or with Rab11a(S25N) (pink circles). Data were collected from 3 transfections with approximately 30 to 50 cells analysed per time point in each experiment. Individual experiments were normalized to the α2δ-1-BBS 30 min time point, before being pooled together. Data were fitted to a single exponential association, with a time constant, τ = 8.09 min for α2δ-1 control and 19.45 min for α2δ-1 + Rab11a(S25N). Statistical significance was determined using Student’s t test to compare mean fluorescence values between conditions at each time point, ****P < 0.0001; **, P = 0.0018. (C) Confocal images of cell-surface CaV2.2-BBS expressed in N2a cells with β1b either alone or with α2δ-1, in the absence or presence of Rab11a(S25N) after 30 min of net forward trafficking assay. Scale bar = 5 μm. (D,E) Normalized mean cell-surface CaV2.2-BBS expressed in N2a cells with β1b and: no α2δ (black), no α2δ + Rab11a(S25N) (grey), + α2δ-1 (red) and + α2δ-1 + Rab11a(S25N) (pink). Cells were live-labelled with α-BTX-AF488 for 10 min (D) or 30 min (E). Fluorescence intensity per cell was normalized to mean CaV2.2/β1b controls at 30 min before being pooled from 3 separate experiments and plotted as mean fluorescence ± SEM. For (D) no α2δ (black, n = 105 cells), no α2δ + Rab11a(S25N) (grey, n = 98 cells), + α2δ-1 (red, n = 123 cells) and + α2δ-1 + Rab11a(S25N) (pink, n = 112 cells). Statistical significances were determined: using one-way ANOVA (F = 23.12, P < 0.0001) and Sidak’s post hoc tests (**** P < 0.0001; ns, P = 0.672). For (E) no α2δ (black, n = 105 cells), no α2δ + Rab11a(S25N) (grey, n = 104 cells), + α2δ-1 (red, n = 132 cells) and + α2δ-1 + Rab11a(S25N) (pink, n = 118 cells). Statistical significances were determined: using one-way ANOVA (F = 27.24, P < 0.0001) and Sidak’s post hoc tests (**** P < 0.0001, ns, P = 0.975).
Discussion
In this study we have shown that α2δ subunits differentially enhance plasma membrane expression of CaV2.2 in both cell lines and primary neuronal cultures, and have implicated Rab11a-dependent recycling in this process. Rab11 proteins are particularly involved in trafficking through recycling endosomes18,19, and here we inhibited this route through expression of the dominant-negative mutant Rab11a(S25N)20. Our data indicate that α2δ-1 and α2δ-2 promote both the steady-state cell-surface CaV2.2 level and net forward trafficking of CaV2.2 through a Rab11a-dependent mechanism (Fig. 1D). Blockade of this pathway leads to a reduction in cell-surface CaV2.2 and α2δ-1 and may lead to increase in degradation, as judged by the reduction in their intracellular levels. However, α2δ-3 does not appear to participate in the Rab11a-dependent recycling pathway, and does not increase net forward CaV2.2 trafficking in the short-term (Fig. 5B,C). Despite this, α2δ-3 consistently increases plasma membrane-inserted CaV2.2 under steady-state conditions14; and the present study, although to a smaller extent than α2δ-1. Inhibition of Rab11a-dependent recycling also reduces whole-cell CaV2.2 currents, when expressed with α2δ-1, but has no effect with α2δ-3. Our results indicate that one of the main drivers of α2δ-1-mediated enhancement of CaV2.2 plasma membrane-insertion is recycling back to the plasma membrane via recycling endosomes. In primary hippocampal neurites, we find that CaV2.2 membrane expression is largely dependent on α2δ, and inhibition of Rab11a-dependent recycling also lowers cell-surface CaV2.2 in neurites in the presence of α2δ-1. The neurites examined here were of dendritic appearance, 100–150 μm from the soma, and in future it will be of interest to also examine trafficking in axons, because of the differential distribution of α2δ subunits in neurons28,29.
Earlier work from this laboratory has shown that α2δ-2 participates in recycling through Rab11a-positive recycling endosomes and that this pathway is interrupted by gabapentin16. The gabapentin-binding RRR motif is positioned just upstream of the VWA domain in both α2δ-1 and α2δ-2, and it has been hypothesised that gabapentinoids displace an endogenous ligand in the binding pocket that includes the RRR motif11,21,30. Previous studies using the α2δ-1(R217A) or α2δ-2(R282A) mutants, which show much lower affinity for binding gabapentin, found them to have lower ability to enhance CaV2 currents compared to their wild-type counterparts, as well as reduced endosomal recycling11,16,21. These findings are consistent with the hypothesis that binding of an endogenous ligand in the gabapentin binding site is important for association of α2δ-1 and α2δ-2 with endosomal sorting partners. As α2δ-3 lacks a gabapentin binding site, we hypothesised that it would not participate in Rab11a-dependent recycling and would thereby be unable to promote forward trafficking of CaV2.2 through this pathway, although the direct route via the Golgi apparatus would still be available16. In agreement with this, we find inhibition of Rab11a-dependent recycling did not influence α2δ-3 trafficking, unlike for α2δ-1 or α2δ-2. Presently it is unclear why α2δ-3 does not participate in Rab11a-dependent recycling, although α2δ-3 shares only 25.7% sequence homology with α2δ-1 (ClustalO), which may result in conformational differences that preclude an interaction with a Rab11a effector.
Since Rab11a is a membrane-associated cytoplasmic protein, and α2δ-1 is extracellular and therefore present in the lumen of recycling endosomes, any functional interaction between the two will involve other proteins. There are several studies that have examined the interaction of α2δs with other proteins; for review see31. For example, we have demonstrated an interaction between α2δ-1 and Low-Density Lipoprotein (LDL) Receptor-related Protein-1 (LRP1), a multifunctional receptor which mediates cargo trafficking. LRP1 and its chaperone Rap, promote α2δ-1 maturation and trafficking to the cell-surface, and also enhance CaV2.2 currents32. Since LRP1 has multiple intracellular and extracellular protein ligands, and contains several cytoplasmic adaptor protein binding motifs33, it may play a role in α2δ-1 trafficking via recycling endosomes.
In another study, neurexin-1α enhanced CaV2.1 currents when expressed with α2δ-1 but had no effect in the presence of α2δ-334, although no selective and specific interaction with neurexin-1α could be demonstrated, in contrast to a previous study35. α2δ-1 has also been reported to interact with thrombospondins (TSPs) 1–4, which are extracellular matrix proteins36. More recently, another study37 showed a weak interaction between α2δ-1 and TSP4, but was unable to demonstrate any interaction between cell-surface expressed α2δ-1 and TSP4, concluding that α2δ-1 and TSP4 may only interact intracellularly at high concentrations. A further study showed a low affinity interaction between α2δ-1 and TSP4, but not other TSPs38. However, any interaction of α2δ subunits with TSPs is unlikely to be involved in their Rab11-dependent recycling.
A number of studies have also linked Rab11a function to growth cone development39–41. Since we have demonstrated distinct trafficking pathways for α2δ-1 and α2δ-2 compared to α2δ-3 in relation to Rab11a-dependent recycling, it is possible that other demonstrated subtype-specific functions of α2δ proteins28,42 may also involve distinct trafficking pathways.
Materials and methods
Molecular biology
The following cDNAs were used: CaV2.2 (rabbit, D14157), CaV2.2-HA and CaV2.2-BBS10, GFP-CaV2.2-HA26, β1b (rat, X61394)43, α2δ-1 (rat, M86621)44, α2δ-1-HA12,45, α2δ-1-BBS, in which the BBS tag from α2δ-216 was inserted into α2δ-1 in the same position as the HA tag, α2δ-3 (AJ010949), α2δ-3-HA14. mCherry46 or CD847 were included as a transfection markers where stated. Human Rab11a (AF000231) containing the S25N mutation16,48, was obtained from From Prof. T. Hébert, McGill University.
Antibodies and other materials
Antibodies (Abs) used were: anti-HA Ab (rat monoclonal, Roche), anti-CaV2.2 II-III loop Ab (rabbit polyclonal)49. The following secondary Abs (raised in goat) were used: anti-rat or anti-rabbit IgG-Alexa Fluor (AF) 488 or 594 (ThermoFisher), biotin-labelled anti-rat IgG (Sigma). Other reagents used were BTX-AF488 (Invitrogen) and streptavidin-AF633 (Invitrogen).
Cell lines: culture and transfection
tsA-201 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen) supplemented with 10% Foetal Bovine Serum, 50 U/ml penicillin/streptomycin and 1% GlutaMAX (Invitrogen). Cells were maintained in tissue culture flasks at 37 °C in a humidified atmosphere of 5% CO2 and grown up to 70–90% confluence prior to transfection or further passage. Neuro2a (N2a) cells were cultured in 50% DMEM (with high glucose and L-glutamine) and 50% Opti-MEM (with L-glutamine), supplemented with 50 U/ml penicillin/streptomycin, 5% FBS, and 1% GlutaMAX (Life Technologies). The N2a cells were cultured to 80% confluence in a 5% CO2 incubator at 37 °C and passaged every 3 to 4 d.
For transient expression, N2a or tsA-201 cells were transfected using either PolyJet (SignaGen Laboratories) or Fugene 6 (Promega) in a 3:1 ratio with cDNA mix, according to the manufacturers’ instructions. A typical cDNA mix was 2 μg total DNA per 35 mm culture dish at a molar ratio of 3:2:2 of CaV2.2: β1b: α2δ. In experiments using Rab11a(S25N) the cDNA mix was 3:2:2:1 for CaV2.2: β1b: α2δ: Rab11a(S25N). In conditions lacking a given construct, the DNA mix was supplemented with the appropriate empty vector to have the same total DNA. For fluorescence imaging experiments in either tsA-201 or N2a cells, all cDNAs were in a pcDNA3, pRK5 or pCMV vector. In electrophysiological experiments using tsA-201 cells, all cDNAs were in a pMT2 vector, with the exception of Rab11a(S25N) (pCMV).
Hippocampal neuronal culture and transfection
Hippocampal neurons were isolated from the hippocampus of P0 rats, which were killed under a UK Home Office Schedule 1 procedure. The cerebrum was cut into two, and the hippocampi were extracted from each hemisphere in ice cold HBSS with 10 mM HEPES. The hippocampi were then cut into small segments and digested gently in Papain solution (7 U/ml Papain, 0.2 mg/ml L-Cysteine, 0.2 mg/ml Bovine Serum Albumin (BSA), 5 mg/ml glucose, 10 mM HEPES in HBSS with 30 Kunitz/ml DNase) in a shaking water bath for 40 min at 37 °C. The cells were washed twice with the growth medium (Neurobasal, 2% B27 supplement, 1 unit/ml penicillin, 1 μg/ml streptomycin, 1% GlutaMAX, 0.1% β-mercaptoethanol), and triturated gently. The cells were plated onto 18 mm circular coverslips that are coated with poly-L-lysine and laminin at 750 cells/μl, 100 μl per coverslip. The entire growth medium was changed after 2 h of plating, and half of the medium was then changed every 3–4 d.
Hippocampal neurons were transfected after 7 d in culture, using cDNAs cloned into pCAGGS50. 2 h prior to transfection, the growth medium was replaced with a mixture of 50% conditioned, and 50% fresh growth medium. The transfection process was as follows: 4 μg cDNA was mixed in a 50 μl total with Opti-MEM (Thermo Fisher), 2 μl Lipofectamine 2000 (Thermo Fisher) was mixed in a 50 μl total volume of Opti-MEM in separate tubes by pipetting; mixing was done by gentle pipetting. DNA and transfection reagent mixes were then combined and incubated for 5 min at room temperature before being added dropwise to cultures. Hippocampal neurons were cultured for a further 7 d after transfection before fixation and immunostaining.
Immunocytochemistry
Cells were plated onto either 20 mm square coverslips, or glass-bottomed dishes (MatTek Corporation) which were coated with poly-L-lysine prior to transfection, and cultured in a 5% CO2 incubator at 37 °C. After 36–48 h expression, cells were fixed with ice cold 4% paraformaldehyde (PFA), 4% sucrose in PBS, pH 7.4 at room temperature for 5 min. Where antigen retrieval was used, fixed samples were incubated in pH 6 citrate buffer at 98˚C for 10 min10. For labelling the HA epitope on the cell-surface in non-permeabilised conditions, cells were incubated with primary Ab with 2% BSA and 10% goat serum in PBS at room temperature for 1 h for cell lines or overnight at 4 °C for neurons (Fig. 3). Using the same protocol, no detectable permeabilization by PFA fixation in hippocampal neurons was observed in a previous study51. When permeabilization was required, cells were incubated with 0.2% Triton X-100 in PBS for 5 min10. The secondary Ab was added with 2.5% BSA and 10% goat serum in PBS and incubated for 1 h at room temperature. Cell nuclei were stained with 0.5 µM 4′,6′-diamidino-2-phenylindole (DAPI) in PBS for 10 min. The coverslips were mounted onto glass slides using Vectashield mounting medium (Vector Laboratories).
In experiments probing for both cell-surface and intracellular HA epitopes (Fig. 1), cells were fixed and blocked as described above, then immunostained for 1 h with rat anti-HA Ab (1:500), followed by 1 h incubation with biotin-labelled anti-rat IgG (1:1000). Following this, cells were permeabilised with 0.2% Triton-X100 for 5 min and re-probed with rat anti-HA Ab for 1 h. Cells were then incubated with anti-rat-AF594 Ab (1:500) and streptavidin-AF633 (1:500), to visualise intracellular and extracellular HA epitopes, respectively. In experiments probing for cell surface BBS tag in CaV2.2 (Fig. 2, Fig. S1), cells were live-labelled with BTX-AF488 (10 µg/ml, Invitrogen) for 30 min at 17 °C. Cells were then washed, fixed, permeabilised and immunostained, using a rabbit Ab against the II-III loop to detect total CaV2.2, followed by secondary anti-rabbit-AF594 Ab.
Net forward trafficking assay
Transfected cells were plated onto 22 × 22 mm glass coverslips coated with poly-L-lysine, and cultured in a 5% CO2 incubator at 37 °C. N2a cells were used for CaV2.2-HA experiments and tsA-201 cells for α2δ-1 experiments, where higher expression levels were required to observe live labelling. After 40 h expression, cells were washed twice with Krebs–Ringer-HEPES (KRH) buffer and incubated with 10 μg/ml unlabelled BTX (Life technologies) for 30 min at 17 °C. The unbound BTX was washed off with KRH, and the cells were then incubated with BTX-AF488 (10 µg/ml) in KRH at 37 °C. To terminate the reaction, cells were washed twice with cold KRH and then fixed with 4% PFA, 4% sucrose in PBS at specified times for the kinetic assay. After fixation, cells were permeabilised and intracellular expression markers and/or nuclei were labelled as described above. The coverslips were then mounted onto glass slides using Vectashield mounting medium.
Confocal microscopy and analysis
All images were acquired using an LSM 780 scanning confocal microscope (Zeiss), equipped with a Plan-Apochromat 63x/1.4 or 40x/1.3 DICII oil immersion objective lens, in 16-bit mode. For experiments in which both AF594 and AF633 were used, sequential scanning was performed with 633 nm and 594 nm laser lines, with emission filters 638–747 nm or 597–695 nm, with beam splitters (MBS) 488/543/633 and MBS 458/514/594, respectively. For each experiment, the laser power, gain and acquisition settings were kept constant between images that were used for quantification, although laser power and gain settings were adjusted between experiments depending on expression and staining level of the samples. Where possible, the region of interest was determined by identifying cells with expression of a transfection marker or intracellular staining of the protein of interest (e.g. GFP, HA), without selecting for the cell-surface immunostaining, to avoid bias. In experiments where an appropriate intracellular expression marker was absent, nuclear staining with DAPI was used to identify viable cells (having an intact nucleus); cell-surface measurements were made for all viable cells per field of view. In addition, a 2 × 2 or 3 × 3 tile scan was performed to further remove the bias in selecting cells with high expression. For cell-surface expression analysis, images were taken usually with 1 μm optical section. Confocal images were imported and analysed in ImageJ (National Institutes of Health). The plasma membrane fluorescence was quantified using the freehand brush tool with a selection width of 0.66 μm and tracing the membrane region manually. If any cells show signs of intracellular staining for the extracellular epitope, because of damage-induced permeabilization, they are excluded from analysis. Total or intracellular fluorescence was quantified using the freehand selection tool, omitting the signal intensity from the nuclei. The background fluorescence in each channel was measured and subtracted from mean cell-surface or intracellular fluorescence measurements in image analysis.
For analysis of neurite expression in hippocampal neurons, an average of 10–15 cells were selected per condition for an individual experiment. Neurons were selected based on expression of free mCherry as a transfection marker. Between 2 and 5 neurites (with the appearance of dendrites) were measured per mCherry-positive cell to reduce over-representation of highly branched neurons. The freehand brush tool was used to manually trace neurites of lengths between 25 and 60 µm, and width of 2 µm, beginning at a distance of 100 µm from the cell soma. Neurites were selected and traced using mCherry to avoid bias, measurements were then taken in channels for expression of HA and/or GFP tags on expressed CaV2.2 constructs. Background fluorescence measurements were taken for each condition and subtracted from mean fluorescence values during image analysis. Mean neurite signal intensity for each channel was calculated for neurons of a given condition and normalized to the stated controls. Normalized data were then pooled between experiments with a minimum of three separate experiments.
In cell-surface expression and forward trafficking experiments, where relevant, all cells chosen for analysis contained intracellular CaV2.2 expression. For the forward trafficking assays, the membrane fluorescent intensities were fitted to the single exponential association. All experiments were repeated n = 3–5 times, and approximately 30–50 cells were analysed for each experiment. All data are presented as pooled cells, except for the trafficking initial rates, which are averages of separate experiments.
Electrophysiology
tsA-201 cells were transfected with cDNA mix containing CaV2.2, α2δ-1, β1b, and CD8 at a ratio of 3:2:2:0.8 using a Fugene transfection protocol. After 40 h expression, cells were replated in cell culture medium at 1 in 3 or 1 in 5 dilution depending on their confluency. Transfected cells were identified by CD8 expression, detected with CD8 Dynabeads (Life Technologies). Whole-cell currents were recorded in voltage-clamp mode in the following solutions; intracellular solution (mM): 140 Cs-aspartate, 5 EGTA, 2 MgCl2, 0.1 CaCl2, 2 K2ATP, 20 HEPES, pH 7.2, 310 mOsm; extracellular solution (mM): 1 BaCl2, 3 KCl, 1 NaHCO3, 1 MgCl2, 10 HEPES, 4 D-glucose, 160 tetraethylammonium bromide, pH 7.4, 320 mOsm. The borosilicate glass electrode resistance was between 1.5 and 4 MΩ. Cell capacitance and series resistance were compensated to 60–70%. Whole-cell currents were recorded on Axopatch-200B amplifier using pClamp 9 or 10 (Molecular Devices). The cells were held at − 90 mV, and 50 ms pulses were applied in + 10 mV steps between − 50 mV and + 50 mV. To correct for the leak current, P/8 leak subtraction protocol was applied. Recordings were made at 20 kHz sampling frequency and filtered at 5 kHz (lowpass 4-pole Bessel filter) in the amplifier. The digital low-pass 8-pole Bessel filter with 1 kHz 3 dB cut-off was applied in Clampfit 10.7 (Molecular Devices) before the current amplitudes were determined. Average peak currents were taken between 8 and 13 ms after the test potentials were applied and normalized to the cell capacitance to obtain current density. IV relationships were fit by a modified Boltzmann equation as follows: I = Gmax*(V-Vrev)/(1 + exp(-(V-V50, act)/k)), where I is the current density (in pA/pF), Gmax is the maximum conductance (in nS/pF), Vrev is the apparent reversal potential (mV), V50, act is the midpoint voltage for current activation (mV), and k is the slope factor.
Statistical analysis
The number of samples (n) in most of the experiments in this study is the total number of individual cells analysed, which are pooled from a minimum of three separate experiments unless stated otherwise. Data from individual experiments were normalized to their control conditions prior to being pooled to account for inter-experimental variance. Mean values and standard error of mean (SEM) were calculated for normalized, pooled data. The only exception is for the experiments determining the rates of CaV2.2 trafficking, where the mean and SEM values were determined from the mean of repeated experiments. Statistical analysis was performed using Student’s t test or one-way ANOVA with appropriate post-hoc test as stated, in GraphPad Prism 7.
Ethical approval
The use of neonatal mice for primary hippocampal cultures was carried out according to methods covered by UK Home Office Schedule I procedures. Ethical approval for Schedule I licence 1285 was obtained from the local UCL Animal Welfare Ethical Review Body (AWERB) and followed ARRIVE guidelines and regulations.
Supplementary information
Acknowledgements
The authors thank Wendy S Pratt and Kanchan Chaggar for technical support, and Dr. Laurent Ferron and Kjara Pilch for hippocampal cultures. We thank Drs. Karen Page and Manuela Nieto-Rostro for commenting on the manuscript.
Author contributions
J.O.M. performed all experimental work, and analysed data. A.C.D. analysed data. J.O.M. and A.C.D. conceived the study, co-wrote the manuscript and prepared the Figures.
Funding
This research was funded, in part, by the Wellcome Investigator award to ACD (098360/Z/12/Z)]. For the purpose of Open Access, the corresponding author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. JOM was supported in part by the UCL Grand Challenges PhD program.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41598-021-89820-1.
References
- 1.Dolphin AC. Voltage-gated calcium channels: their discovery, function and importance as drug targets. Brain Neurosci. Adv. 2018;2:239821281879480. doi: 10.1177/2398212818794805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of a1 and partial amino acid sequence of a phosphorylation site of an independent b subunit. Proc. Natl. Acad. Sci. U.S.A. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jay SD, et al. Structural characterization of the dihydropyridine-sensitive calcium channel a2-subunit and the associated d peptides. J. Biol. Chem. 1991;266:3287–3293. doi: 10.1016/S0021-9258(18)49986-3. [DOI] [PubMed] [Google Scholar]
- 4.Davies A, et al. The a2d subunits of voltage-gated calcium channels form GPI-anchored proteins, a post-translational modification essential for function. Proc. Natl. Acad. Sci. USA. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol. 2016;594:5369–5390. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J. Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel a2d subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/S0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 8.Wakamori M, Mikala G, Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J. Physiol.-Lond. 1999;517:659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barclay J, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassidy JS, Ferron L, Kadurin I, Pratt WS, Dolphin AC. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary alpha2delta-1 subunits. Proc. Natl. Acad. Sci. USA. 2014;111:8979–8984. doi: 10.1073/pnas.1403731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrich J, et al. Pharmacological disruption of calcium channel trafficking by the a2d ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies A, et al. The calcium channel a2d–2 subunit partitions with CaV2.1 in lipid rafts in cerebellum: implications for localization and function. J. Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canti C, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadurin I, et al. Proteolytic maturation of α2δ represents a checkpoint for activation and neuronal trafficking of latent calcium channels. Elife. 2016;5:e1143. doi: 10.7554/eLife.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferron L, Kadurin I, Dolphin AC. Proteolytic maturation of alpha2delta controls the probability of synaptic vesicular release. Elife. 2018;7:e37507. doi: 10.7554/eLife.37507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. 2010;30:12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Marlin MC. Rab family of GTPases. Methods Mol. Biol. 2015;1298:1–15. doi: 10.1007/978-1-4939-2569-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren M, et al. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. U S A. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisfeld AJ, Kawakami E, Watanabe T, Neumann G, Kawaoka Y. RAB11A is essential for transport of the influenza virus genome to the plasma membrane. J. Virol. 2011;85:6117–6126. doi: 10.1128/JVI.00378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field MJ, et al. Identification of the a2d–1 subunit of voltage-dependent calcium channels as a novel molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. USA. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee NS, et al. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the a2d subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 23.Lotarski SM, et al. Anxiolytic-like activity of pregabalin in the Vogel conflict test in alpha2delta-1 (R217A) and alpha2delta-2 (R279A) mouse mutants. J. Pharmacol. Exp. Ther. 2011;338:615–621. doi: 10.1124/jpet.111.180976. [DOI] [PubMed] [Google Scholar]
- 24.Lotarski S, et al. Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in alphadelta (R217A) and alphadelta (R279A) mouse mutants. Epilepsy Res. 2014;108:833–842. doi: 10.1016/j.eplepsyres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Wang MH, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit a2d for gabapentin binding. Biochem. J. 1999;342:313–320. doi: 10.1042/bj3420313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macabuag N, Dolphin AC. Alternative splicing in CaV2.2 regulates neuronal trafficking via adaptor protein complex-1 adaptor protein binding motifs. J. Neurosci. 2015;35:14636–14652. doi: 10.1523/JNEUROSCI.3034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahimene S, et al. The a2d-like protein cachd1 increases N-type calcium currents and cell surface expression and competes with a2d–1. Cell Rep. 2018;25:1610–1621. doi: 10.1016/j.celrep.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler S, et al. Presynaptic alpha2delta-2 calcium channel subunits regulate postsynaptic GABAA receptor abundance and axonal wiring. J. Neurosci. 2019;39:2581–2605. doi: 10.1523/JNEUROSCI.2234-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bikbaev A, et al. Auxiliary alpha2delta1 and alpha2delta3 subunits of calcium channels drive excitatory and inhibitory neuronal network development. J. Neurosci. 2020;40:4824–4841. doi: 10.1523/JNEUROSCI.1707-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JP, Dissanayake VU, Briggs AR, Milic MR, Gee NS. Isolation of the [3H]gabapentin-binding protein/alpha 2 delta Ca2+ channel subunit from porcine brain: development of a radioligand binding assay for alpha 2 delta subunits using [3H]leucine. Anal. Biochem. 1998;255:236–243. doi: 10.1006/abio.1997.2447. [DOI] [PubMed] [Google Scholar]
- 31.Dolphin AC. Voltage-gated calcium channel alpha 2delta subunits: an assessment of proposed novel roles. F1000Res. 2018;7:1830. doi: 10.12688/f1000research.16104.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadurin I, Rothwell SW, Lana B, Nieto-Rostro M, Dolphin AC. LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary alpha2delta-1 subunit. Sci. Rep. 2017;7:43802. doi: 10.1038/srep43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brockhaus J, et al. alpha-neurexins together with alpha2delta-1 auxiliary subunits regulate Ca(2+) influx through Cav2.1 channels. J. Neurosci. 2018;38:8277–8294. doi: 10.1523/JNEUROSCI.0511-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong X-J, et al. Retrograde synaptic inhibition is mediated by α-neurexin binding to the α2δ subunits of N-type calcium channels. Neuron. 2017;95:1–15. doi: 10.1016/j.neuron.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eroglu C, et al. Gabapentin receptor alpha2delta-1 Is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lana B, et al. Thrombospondin-4 reduces binding affinity of [3H]-gabapentin to calcium-channel α2δ-1-subunit but does not interact with α2δ-1 on the cell-surface when co-expressed. Sci. Rep. 2016;6:24531. doi: 10.1038/srep24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Awaad E, et al. Direct, gabapentin-insensitive interaction of a soluble form of the calcium channel subunit alpha2delta-1 with thrombospondin-4. Sci. Rep. 2019;9:16272. doi: 10.1038/s41598-019-52655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Bergeijk P, Adrian M, Hoogenraad CC, Kapitein LC. Optogenetic control of organelle transport and positioning. Nature. 2015;518:111–114. doi: 10.1038/nature14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Homma Y, Fukuda M. Rabin8 regulates neurite outgrowth in both GEF activity-dependent and -independent manners. Mol. Biol. Cell. 2016;27:2107–2118. doi: 10.1091/mbc.E16-02-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldenring JR. Recycling endosomes. Curr. Opin. Cell Biol. 2015;35:117–122. doi: 10.1016/j.ceb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha(2)delta-3 is required for synaptic morphogenesis independent of its Ca(2+)-channel functions. Nat. Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pragnell M, Sakamoto J, Jay SD, Campbell KP. Cloning and tissue-specific expression of the brain calcium channel b-subunit. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-K. [DOI] [PubMed] [Google Scholar]
- 44.Kim H-L, Kim H, Lee P, King RG, Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel a2 subunit. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadurin I, et al. Calcium currents are enhanced by alpha2delta-1 lacking its membrane anchor. J. Biol. Chem. 2012;1287:33554–33566. doi: 10.1074/jbc.M112.378554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 47.Rougier JS, et al. Molecular determinants of voltage-gated sodium channel regulation by the Nedd4/Nedd4-like proteins. Am. J. Physiol. Cell Physiol. 2005;288:C692–701. doi: 10.1152/ajpcell.00460.2004. [DOI] [PubMed] [Google Scholar]
- 48.Dupre DJ, et al. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J. Biol. Chem. 2006;281:34561–34573. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- 49.Raghib A, et al. Dominant-negative synthesis suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs. J. Neurosci. 2001;21:8495–8504. doi: 10.1523/JNEUROSCI.21-21-08495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- 51.Meyer JO, et al. Disruption of the key Ca2+ binding site in the selectivity filter of neuronal voltage-gated calcium channels inhibits channel trafficking. Cell Rep. 2019;29:22–33. doi: 10.1016/j.celrep.2019.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.