Abstract
Objectives
The WHO Access, Watch and Reserve (AWaRe) classification has been developed to support countries and hospitals in promoting rational use of antibiotics while improving access to these essential medicines. We aimed to describe patterns of worldwide antibiotic use according to the AWaRe classification in the adult inpatient population.
Methods
The Global Point Prevalence Survey on Antimicrobial Consumption and Resistance (Global-PPS) collects hospital antibiotic use data using a standardized PPS methodology. Global-PPS 2015, 2017 and 2018 data, collected by 664 hospitals in 69 countries, were categorized into AWaRe groups to calculate proportional AWaRe use, Access-to-Watch ratios and the most common indications for treatment with selected Watch antibiotics. Only prescriptions for systemic antibiotics on adult inpatient wards were analysed.
Results
Regional Access use ranged from 28.4% in West and Central Asia to 57.7% in Oceania, whereas Watch use was lowest in Oceania (41.3%) and highest in West and Central Asia (66.1%). Reserve use ranged from 0.03% in sub-Saharan Africa to 4.7% in Latin America. There were large differences in AWaRe prescribing at country level. Watch antibiotics were prescribed for a range of very different indications worldwide, both for therapeutic and prophylactic use.
Conclusions
We observed considerable variations in AWaRe prescribing and high use of Watch antibiotics, particularly in lower- and upper-middle-income countries, followed by high-income countries. The WHO AWaRe classification has an instrumental role to play in local and national stewardship activities to assess prescribing patterns and to inform and evaluate stewardship activities.
Introduction
Global antibiotic consumption in the healthcare sector increased by 65% between 2000 and 2015, highlighting the need for a multi-level and coordinated set of actions in global efforts to curb the threat of antimicrobial resistance (AMR).1 Optimizing antimicrobial use in hospital and community settings through antimicrobial stewardship (AMS) is considered a key element in the global AMR response.2 At the same time, many low- and middle-income countries (LMICs) are confronted with challenges such as poor quality of antibiotics, loosely regulated over-the-counter sales and limited access to essential antibiotics.3,4 WHO aimed to address this need to balance excess use of antibiotics with access restrictions, by classifying a total of 180 antibiotics available worldwide into three groups; Access, Watch and Reserve (AWaRe) and by integrating these groups in the Model List of Essential Medicines (EML).5 Antibiotics in the Access group are considered as first- or second-line agents in the empiric treatment of a number of common infectious syndromes and should therefore be widely available and affordable. The Watch group includes antibiotics that have a higher risk of selecting for resistance and that are used as first- or second-line options for a limited number of indications only. These are the antibiotics that need to be monitored and prioritized as targets for stewardship programmes. Finally, the Reserve group contains a set of last-resort antibiotics that need to be intensively monitored and should only be used under certain specific conditions in order to conserve their effectiveness. A fourth category of ‘Not recommended’ was added for fixed-dose combinations of broad-spectrum antibiotics for which use is not evidence-based.6 WHO proposes to use the AWaRe classification as a tool to support monitoring of antibiotic prescribing and inform AMS programmes and has introduced a new target, stating that by 2023 at least 60% of national antibiotic consumption should come from the Access group.6,7 Translating antimicrobial consumption data into AWaRe categories may provide useful insights into global prescribing patterns and will help to explore the potential of this newly developed classification as a stewardship tool.
Point prevalence surveys (PPSs) have shown their applicability as a cross-sectional audit of antimicrobial use in hospitals, thus addressing the need for a uniform approach for antimicrobial consumption surveillance.8–11 PPS data have been used to classify hospital antibiotic use in children; however, few studies have used this approach to investigate AWaRe patterns in the adult inpatient population.12 The aim of this paper is to describe and visualize therapeutic and prophylactic AWaRe antibiotic use patterns in adult inpatients using data collected in the Global Point Prevalence Survey on Antimicrobial Consumption and Resistance (Global-PPS) network and to investigate the main indications for which a selection of common Watch antibiotics were prescribed globally. The results reported here are not aimed at making comparisons between countries, considering the context-related factors that could influence antibiotic prescribing, such as local resistance patterns and availability of antibiotics.
Methods
Data collection
We analysed data on antimicrobial prescribing in hospitalized patients from the Global-PPS network. The Global-PPS was conducted in 2015, 2017 and 2018, using a standardized methodology for collecting cross-sectional data on antimicrobial use in hospitalized adults, children and neonates. For Belgium, 2017 ECDC-PPS data have been integrated into the Global-PPS tool and therefore were also included in the Belgian dataset.13 Patient-level data included patient characteristics, information on the prescribed antimicrobials and their indication, as well as a number of quality indicators such as guideline compliance. Survey data were entered and validated using a web application, developed by the University of Antwerp. Patient data were anonymized by means of a unique, non-identifiable survey number generated by the Global-PPS software. Details of the Global-PPS methodology have been described elsewhere.11
Data analysis
All Global-PPS data collected on adult wards in 2015, 2017 and 2018 were analysed. Data from paediatric hospitals and hospitals without adult patient-level data were excluded from the dataset. Only prescriptions for ‘antibacterials for systemic use’, classified as ATC J01, were included in the analysis. Antimycotics and antifungals (J02 and D01BA), TB drugs (J04A), antivirals (J05), intestinal anti-infectives (A07AA), antimalarials and other antiprotozoals (P01) were not analysed.
Antibiotics were labelled as ‘Access’, ‘Watch’, ‘Reserve’ or ‘Not recommended’ using the 2019 WHO AWaRe Classification Database (Table S1, available as Supplementary data at JAC Online). Antibiotics not included in the AWaRe classification were listed as ‘Unclassified’.
Proportional Access, Watch and Reserve use was assessed by calculating the number of prescriptions in each category relative to the total number of prescriptions and was stratified according to UN region and subregion, country and World Bank country classification.14,15 A unique prescription was defined as one antibiotic and one route of administration. The five UN regions were split into 11 subregions, based on the number of hospitals in the dataset. Subregions for Africa were Northern Africa and sub-Saharan Africa. The Americas region was broken down into Northern America and Latin America, and subregions for Europe were Northern, Eastern, Southern and Western Europe. For the Asian region, countries were grouped into two subregions: West and Central Asia (including Western and Central Asia) and East and South Asia (including Eastern, Southern and South-eastern Asia). We report the five most commonly prescribed antibiotics for therapeutic and prophylactic use, at ATC5 level, by region, and a green, yellow and red colour code is used to visualize Access, Watch and Reserve prescribing, respectively. To determine the relative use of Access and Watch antibiotics, Access-to-Watch ratios were calculated and presented in relation to the overall country median. Countries with fewer than three participating hospitals were excluded from country-level analyses. For a selection of Watch antibiotics (azithromycin, ciprofloxacin, ceftriaxone, vancomycin and meropenem), we report the most common indications for prescription. Similarly, the most common indications were assessed for Reserve antibiotics. Finally, we report the proportion of Reserve antibiotics prescribed empirically. Data were exported from the Global-PPS web application to a Microsoft Excel 2016 database (Microsoft Corporation, Redmond, WA, USA) and were analysed using Microsoft Excel and SPSS version 25 (IBM Corporation, Armonk, NY, USA).
Ethics
The need to obtain ethical approval was dependent on local policies. This was arranged by hospitals individually, if needed, and a document stating the Global-PPS data privacy principles was available to inform these applications.
Results
General results
Between January 2015 and December 2018, 721 hospitals from 73 countries participated in at least one Global-PPS survey period. Data from 55 paediatric hospitals and two hospitals with data quality issues were excluded. The final dataset included 80 671 patients on at least one systemic antibiotic (106 105 prescriptions) admitted to adult wards in 664 hospitals throughout 69 countries. A list of participating countries with the respective number of hospitals, participations, patients and prescriptions is included in Table S2. Of all participating hospitals, 352 (53.0%) were from high-income countries, 144 (21.7%) from upper-middle-income countries, 141 (21.2%) from lower-middle-income countries and 27 (4.1%) from low-income countries. Tertiary hospitals represented the majority of all institutions (267; 40.2%), followed by secondary hospitals (243; 36.6%), primary care centres (76; 11.4%), institutions for specialized care (50; 7.5%) and infectious diseases hospitals (28; 4.2%). Overall, pneumonia was the most common reason for prescription of antibiotics on adult wards (19.2%), followed by skin and soft tissue infections (9.8%) and intra-abdominal infections (7.0%).
Proportional AWaRe use by region
West and Central Asian hospitals had the highest percentage of Watch antibiotics (66.1%) and the lowest Access percentage (28.4%) (Figure 1). The highest Access percentage (57.7%) and the lowest Watch percentage (41.3%) were observed in Oceania. Within Europe, Access percentages ranged from 30.2% in Eastern Europe to 55.2% in Northern European hospitals. The overall percentage of Reserve use was 2.0%, ranging from 0.03% in sub-Saharan Africa to 4.7% in Latin America. The highest percentage of not-recommended antibiotics was seen in Northern Africa (2.3%). Up to 64.9% of all not-recommended antibiotic prescriptions worldwide were for cefoperazone/β-lactamase inhibitor.
Figure 1.
Proportional antibiotic use in adult inpatients according to the AWaRe classification, by UN region. C, countries; H, hospitals; P, prescriptions.
Proportional AWaRe use by income level
Stratification by World Bank country classification (Figure 2) showed that low-income countries had the highest Access percentage (62.8%), the lowest Watch percentage (36.0%) and no Reserve prescriptions on adult wards. Upper-middle- and lower-middle-income countries showed similar patterns of Access and Watch prescribing. Access antibiotics accounted for 33.0% and 33.5% of prescriptions in upper-middle- and lower-middle-income countries, respectively. Watch percentages were high, with up to 62.2% in upper-middle-income countries, 63.4% in lower-middle-income countries and 53.1% in high-income countries. Reserve prescribing was highest in upper-middle-income countries (3.0%), followed by high-income countries (1.9%) and lower-middle-income countries (1.4%).
Figure 2.
Proportional antibiotic use in adult inpatients according to the AWaRe classification, by income level. C, countries; H, hospitals; P, prescriptions.
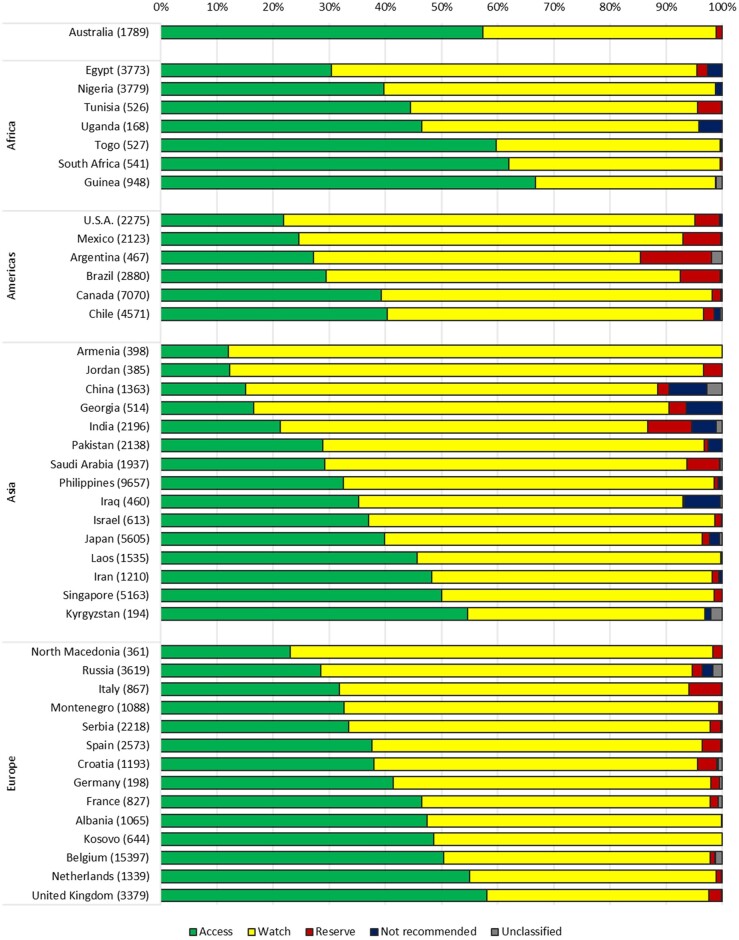
Proportional AWaRe use by country
The highest Access percentages at country level were observed in sub-Saharan countries such as Guinea (66.7%), South Africa (61.9%) and Togo (59.8%) (Figure 3). Access prescribing was lowest in Armenia (12.1%), Jordan (12.2%) and China (15.1%). The percentage of Watch prescribing was high in Armenia (87.9%) and Jordan (84.4%), whereas Guinea (32.1%), South Africa (37.7%) and the UK (39.5%) reported the lowest Watch percentages. Reserve prescribing was highest in Argentina (12.6%), India (7.8%) and Brazil (7.1%). For a number of participating countries, such as Nigeria, Guinea, Togo, Laos, Kosovo, Kyrgyzstan and Armenia, no Reserve prescriptions were reported.
Figure 3.
Proportional antibiotic use in adult inpatients according to the AWaRe classification, by country. Number of prescriptions is shown in parentheses. Countries with fewer than three participating hospitals are not included in the representation.
Most commonly prescribed antibiotics by region
Ceftriaxone was the most commonly used antibiotic for therapeutic use on adult wards worldwide, ranging from 2.5% of therapeutic prescriptions in Northern Europe to 24.8% in Eastern Europe (Figure 4). Piperacillin/tazobactam was the number one antibiotic in Northern Europe (15.9%) and Northern America (17.4%), whereas amoxicillin/clavulanic acid was widely used in Western Europe (27.0%). In Northern America the top five antibiotics for therapeutic use consisted entirely of Watch antibiotics. Results for surgical prophylaxis (Figure 5) showed more Access prescribing for almost all regions compared with therapeutic prescribing. Cefazolin was the number one antibiotic for surgical prophylaxis in Oceania (68.1%), Northern America (67.1%), Western Europe (63.3%) and Latin America (34.7%). Ceftriaxone was widely used as prophylaxis for surgery in Eastern Europe (34.4%), Southern Europe (24.8%), West and Central Asia (23.6%) and Northern Africa (19.7%). In sub-Saharan Africa up to 23.6% of prescriptions for surgical prophylaxis were for metronidazole, followed by ceftriaxone (23.2%).
Figure 4.
Top five most commonly prescribed antibiotics for therapeutic use in adult inpatients, by UN region. C, countries; H, hospitals; P, prescriptions; inhib, inhibitor. Access antibiotics are coloured green and Watch antibiotics are coloured yellow.
Figure 5.
Top five most commonly prescribed antibiotics for surgical prophylaxis in adult inpatients, by UN region. C, countries; H, hospitals; P, prescriptions; inhib, inhibitor. Access antibiotics are coloured green and Watch antibiotics are coloured yellow.
Access-to-Watch ratio by country
The median Access-to-Watch ratio was 0.7 (IQR 0.5–0.9) (Figure 6). In 9 out of 43 countries included in the analyses, the Access-to-Watch ratio was higher than 1, meaning that in these countries the proportion of Access antibiotics prescribed on adult wards was higher than the Watch proportion. The majority of countries, however, had an Access-to-Watch ratio lower than 1. The lowest ratio (0.1) was observed in Armenia and Jordan, whereas Guinea had the highest Access-to-Watch ratio (2.1), followed by South Africa (1.6), Togo (1.5) and the UK (1.5).
Figure 6.
(a) Country-level Access-to-Watch ratio for adult inpatients clustered by UN region. The dotted line marks the overall country median (0.7). Countries with fewer than three participating hospitals are not included in the representation. (b) Country-level Access-to-Watch ratio for adult inpatients clustered by income level. The dotted line marks the overall country median (0.7). Countries with fewer than three participating hospitals are not included in the representation.
Most common indications for selected Watch antibiotics
Accounting for 13.3% (11.0% in Oceania to 19.7% in Africa) of all included prescriptions, and prescribed for a wide range of indications, ceftriaxone was the most commonly used antibiotic on adult wards worldwide (Table S3). Surgical prophylaxis represented up to 24.2% (1.1% in Northern America to 35.2% in Africa) of worldwide ceftriaxone use. Overall, 20.0% of ceftriaxone prescriptions were for treatment of pneumonia, ranging from 10.0% in Africa to 35.1% in Northern America. Ciprofloxacin constituted up to 6.2% (2.7% in Oceania to 8.2% in Europe) of overall antibiotic use. The most common reason for prescription of ciprofloxacin was urinary tract infection (21.6%; 6.6% in Africa to 36.8% in Northern America), the majority of which (13.6% of all ciprofloxacin prescriptions) were for infection of the lower urinary tract. Skin and soft tissue infections (12.3%) were another important indication for ciprofloxacin use, with up to 18.1% of all ciprofloxacin prescriptions in Africa being prescribed for this reason. Meropenem represented 4.3% of all antibiotic prescriptions (1.9% in Africa to 6.9% in Latin America). The main indications for meropenem treatment were pneumonia (27.9%; 13.0% in Oceania to 29.8% in Latin America), intra-abdominal infection (11.7%; 5.1% in Africa to 15.0% in Europe) and sepsis (7.8%; 3.7% in Oceania to 15.2% in Africa). Parenteral vancomycin accounted for 3.7% (1.1% in Africa to 8.1% in Northern America) of all prescriptions in the dataset. Up to 15.7% of prescriptions were for pneumonia, ranging from 11.4% in Africa and Europe to 19.8% in Latin America. Skin and soft tissue infections and bone and joint infections constituted 15.4% (13.4% in Europe to 17.1% in Northern America) and 9.7% (2.6% in Africa to 21.8% in Oceania) of prescriptions, respectively. Azithromycin, finally, accounted for 2.0% (0.9% in Latin America to 3.7% in Northern America) of overall antibiotic use. Up to 54.5% of azithromycin prescriptions were for treating pneumonia, ranging from 26.1% in Europe to 69.7% in Asia.
Worldwide use of Reserve antibiotics
Analyses of the 2118 Reserve prescriptions in the dataset showed that linezolid was the most commonly used Reserve antibiotic worldwide (29.9% of all Reserve prescriptions; 19.1% in East and South Asia to 62.1% in Northern Africa). Overall, colistin accounted for 27.0% of Reserve use (8.7% in Northern America to 50.5% in West and Central Asia). In Northern America, daptomycin represented up to 30.1% of all Reserve prescriptions. Reserve antibiotics were mainly used to treat pneumonia (26.2%), skin and soft tissue infections (12.9%) and intra-abdominal infections (10.5%). Empirical prescribing of Reserve antibiotics ranged from 34.3% in high-income countries to 41.4% and 53.0% in upper-middle-income and lower-middle-income countries, respectively.
Discussion
Overall AWaRe prescribing
Using 2015, 2017 and 2018 hospital PPS data, we assessed worldwide antibiotic prescribing patterns for adult inpatients based on the WHO AWaRe classification. To the best of our knowledge, this is the first analysis of hospital AWaRe prescribing in adults on a global level. Results show considerable differences in proportional use of Access, Watch and Reserve antibiotics between regions and countries. Similar levels of variation in AWaRe consumption were reported in other global studies.7,12,16 The overall use of Watch antibiotics was high and stratification by World Bank classification showed that hospitals in lower-middle- and upper-middle-income countries contributed substantially to the proportion of Watch antibiotics. This high use of broad-spectrum Watch antibiotics in middle-income settings may be attributed to a combination of improved access to these antibiotics in emerging economies, rising levels of AMR and increased incidence of infectious diseases due to urbanization.1,17–19 A global, longitudinal analysis of national sales data has shown that, while both Access and Watch consumption increased between 2000 and 2015, the increase in Watch antibiotics was more pronounced, especially in LMICs. As a result, the proportion of countries in which Access antibiotics constituted at least 60% of total consumption, the target set by WHO, decreased substantially between 2000 and 2015.7 High levels of Watch use could also be observed in Northern America, where the top five most commonly used antibiotics for therapeutic use consisted entirely of Watch antibiotics.
In contrast, hospitals from countries such as South Africa, Guinea and Togo had high Access-to-Watch ratios. Similar patterns have been observed in wholesales data from countries such as Burkina Faso and Burundi.20 It seems, therefore, that despite overall rising antibiotic consumption levels, access to antibiotics has not improved uniformly throughout LMICs and that certain hospitals in resource-poor settings are still faced with a need for effective antibiotics to treat drug-resistant infections.21–24
The use of Reserve antibiotics was particularly high in Latin American countries and in India, which may reflect the burden of increasingly resistant pathogens in these regions.19,25 No Reserve prescriptions were reported in hospitals from countries such as Laos, Guinea and Nigeria, which again may be attributed to availability issues for last-resort antibiotics in these settings. Worldwide, a large proportion of Reserve antibiotics were prescribed on an empirical basis. In lower-middle-income countries, up to 53.0% of all Reserve prescriptions were empirical, which likely indicates a lack of diagnostic capacity.
Indications for Watch antibiotics
Although considerable interregional differences were observed, a large proportion of prescriptions for key Watch antibiotics were issued for indications other than those for which they were included in the EML.5 Ceftriaxone was the most commonly prescribed Watch antibiotic globally and commonly used for surgical prophylaxis in some regions. These findings are worrisome, as the use of third-generation cephalosporins has been identified as a possible driver of ESBL-producing pathogens.26,27 The high number of ciprofloxacin prescriptions for indications such as skin and soft tissue infections and pneumonia is of particular concern in TB-endemic countries as fluoroquinolones are essential antibiotics in treatment regimens for MDR TB.28 A high burden of MRSA could explain the common use of vancomycin in Latin and Northern American hospitals.29,30 However, further investigation into the appropriateness of vancomycin use is needed, as it has been identified as a risk factor for nosocomial VRE infections, along with concerns of toxicity and maintaining effective concentrations.31,32 Azithromycin was commonly used for treatment and prophylaxis of respiratory infections. However, widespread use of azithromycin will result in reduced susceptibility of Streptococcus pneumoniae to macrolides, but also emerging resistance in typhoidal Salmonella strains.33,34
Limitations
The current study has important limitations, many of which are associated with the use of PPS data.10–12,35 First, the Global-PPS relies on voluntary participation and therefore the results reported here cannot be extrapolated to all hospitals within the same country or region. In terms of representativeness at country level, results need to be interpreted with caution, as some countries are clearly underrepresented. In our analyses, a total of three participating institutions per country was considered a hospital network and all countries with fewer than three participating hospitals were excluded from country-level analyses. Nevertheless, we caution against comparison of AWaRe use between countries and emphasize that the purpose of this study is not to benchmark participating countries. At regional level, results are likely biased towards countries with a large and active network, such as Belgium in Western Europe (15 397/17 953 prescriptions) or Russia in Eastern Europe (3619/4056 prescriptions). Second, participation from low-income countries was low. Only three low-income countries with more than two participating hospitals were included, all of which were located in sub-Saharan Africa. Third, these results are not corrected for factors that may have an impact on antibiotic prescribing: institutional characteristics, patient case mix, seasonality or number of times the hospital participated in the PPS. Fourth, although encoding of the indication for treatment was guided by online quality checks, local teams were responsible for attribution of the appropriate code to the identified diagnosis. Fifth, the selection of adult patients for this dataset was based on the type of ward to which they were admitted. As some hospitals did not have dedicated paediatric wards, a limited percentage, estimated at 2.8% of all included patients on adult wards, were younger than 18 years. Finally, factors such as local resistance data, infectious disease prevalence or availability of antibiotics are not considered here, but would be valuable in interpreting these findings.
Conclusions
The WHO AWaRe classification was used to describe antibiotic prescribing patterns in adult inpatients worldwide. Designed as a global stewardship tool, it provides local AMS teams and policymakers with a comprehensive method to monitor antibiotic use, set targets and design interventions, especially in high-burden settings where antibiotic consumption data are scarce. We observed large heterogeneity in AWaRe prescribing at country, regional and income levels. The data collected in the Global-PPS also allowed us to zoom in on the indications of some essential Watch antibiotics. Further research on local levels, integrating contextual information, could usefully explore some of the drivers behind the prescribing patterns reported here.
Supplementary Material
Acknowledgements
We thank the members of the Global-PPS network:
Andi Koraqi, University Hospital Center ‘Mother Theresa’, Tirana, Albania; Iris Hoxha, University of Medicine Tirana, Tirana, Albania; Silva Tafaj, University Hospital ‘Shefqet Ndroqi’, Tirana, Albania; Wanda Cornistein, Hospital Argerich, Buenos Aires, Argentina; Rodolfo Quiros, Hospital Universitario Austral, Buenos Aires, Argentina; Martin Hojman, Clinica de los Virreyes—Hospital Bernardino Rivadavia, Buenos Aires, Argentina; Lilit Ghazaryan, Scientific Center of Drug and Medical Technology Expertise, Ministry of Health, Yerevan, Armenia; Kylie Horne, Monash Health, Melbourne, Australia; Kelly Cairns, The Alfred Hospital, Melbourne, Australia; Fiona Doukas, Concord Repatriation General Hospital, Sydney, Australia; Thomas Gottlieb, Concord Hospital Sydney, Australia; Jameela Al Salman, Salmaniya medical complex, Manama, Bahrain; Erica Sermijn, Algemeen Stedelijk Ziekenhuis, Aalst, Belgium; Katia Verhamme, Onze-Lieve-Vrouwziekenhuis, Aalst, Belgium; Christiane Brands, ZNA, Antwerp, Belgium; Bruno Van Herendael, GZA hospitals, Antwerp, Belgium; Lorenzo Filippin, Centre Hospitalier EpiCURA, Baudour, Belgium; Wouter Vandewal, AZ Sint-Lucas, Brugge, Belgium; Deborah Konopnicki, Saint-Pierre University Hospital, Brussels, Belgium; Evelyne Maillart, CHU Brugmann, Brussels, Belgium; Liliana Teixeira Lopes, CHU Brugmann, Brussels, Belgium; Pauline Papin, Clinique Saint-Jean, Brussels, Belgium; Ilse Smits, AZ Monica, Deurne, Belgium; Hilde Jansens, University Hospital Antwerp, Edegem, Belgium; Sofie Bartholomeus, Sint-Dimpna Ziekenhuis, Geel, Belgium; Anne-Marie Van den Abeele, St-Lucas Hospital, Ghent, Belgium; Sophia Steyaert, General Hospital Maria Middelares, Ghent, Belgium; Anne Piette, AZ Alma, Eeklo, Belgium; Franky Buyle, Ghent University hospital, Ghent, Belgium; Reinoud Cartuyvels, Jessa Hospital, Hasselt, Belgium; Stijn Jonckheere, Jan Yperman, Ieper, Belgium; Ingrid Wybo, Universitair Ziekenhuis Brussel, Jette, Belgium; Lorenz Vanneste, General Hospital Groeninge, Kortrijk, Belgium; Delphine Mathieu, Centre Hospitalier Universitaire Tivoli, La Louvière, Belgium; Eric Firre, Centre Hospitalier Regional de Liège, Liege, Belgium; Veerle Westelinck, AZ Sint-Maarten, Mechelen, Belgium; Philippe Gadisseux, Centre Hospitalier Mouscron, Mouscron, Belgium; Thierry Dugernier, Clinique Saint Pierre, Ottignies, Belgium; Kristof Bafort, Mariaziekenhuis, Pelt, Belgium; Viviane Gonissen, Hospital of Bois de l’Abbaye, Seraing, Belgium; Vanessa Vanderper, AZ Nikolaas, Sint-Niklaas, Belgium; Patrick Gabriels, Sint-Trudo ziekenhuis, Sint-Truiden, Belgium; Frank Weekers, AZ Turnhout, Turnhout, Belgium; Philippe Michel, CHR Verviers, Verviers, Belgium; Ann Van Liedekerke, AZ Sint-Elisabeth, Zottegem, Belgium; Michiel Costers, AZ Sint-Elisabeth, Zottegem, Belgium; and other members of the Belgian Point Prevalence Survey Study Group; Boudewijn Catry, Katrien Latour and Eline Vandael, Sciensano, Brussels, for the collection and integration of ECDC-PPS data for the year 2017; Amela Dedeic-Ljubovic, University Clinical Center of Sarajevo, Sarajevo, Bosnia and Herzegovina; Ana C. Gales, Universidade Federal de São Paulo, São Paulo, Brazil; Ana Paula Matos Porto, University of São Paulo, São Paulo, Brazil; Silvia Figueiredo Costa, Hospital das Clínicas FMUSP, São Paulo, Brazil; and other members of The Brazilian Global-PPS working group; Emma Keuleyan, Medical Institute—Ministry of the Interior, Sofia, Bulgaria; Apollinaire Beidi, MSF OCG, Maroua, Cameroon; Youssouph Cissohko, MSF OCG, Maroua, Cameroon; Habsatou Blakwe, MSF OCG, Maroua, Cameroon; Ngassa Batchaya Basile, HRM, Maroua, Cameroon; Greg J. German, Health PEI, Charlottetown, Prince Edward Island, Canada; Sarah Lutes, Health PEI, Charlottetown, Prince Edward Island, Canada; Jennifer Boswell, Health PEI, Charlottetown, Prince Edward Island, Canada; Dominik Mertz, Hamilton Health Sciences, Hamilton, Canada; Tuyen Nguyen, CISSSLAV, Laval, Canada; Timothy MacLaggan, Horizon Health Network, Moncton, Canada; Daniel Landry, CHUDGLD, Vitalité Health Network, Moncton, Canada; Anita Ang, Centre Hospitalier de l’Université de Montreal, Montreal, Canada; Daniel J. G. Thirion, McGill University Health Center, Montreal, Canada; Charles Frenette, McGill University Health Center, Montreal, Canada; Yannick Émond, Hôpital Maisonneuve-Rosemont, Montreal, Canada; Jacqueline Roberts, Perth and Smiths Falls District Hospital, Perth—Smiths Falls, Canada; Sandra Chang, Richmond Hospital, Richmond, Canada; Justin Kosar, Saskatchewan Health Authority, Saskatoon, Canada; Louis Valiquette, CIUSSS de l’Estrie—CHUS, Sherbrooke, Canada; Ginette Dutrisac, Health Sciences North, Sudbury, Canada; Kevin Afra, Fraser Health, Surrey, Canada; Allison McGeer, Sinai Health System—Mount Sinai Hospital, Toronto, Canada; Marie Carrier, Centre hospitalier affilié universitaire de Trois-Rivières—Hôpital Ste-Croix, CIUSSS Mauricie-et-Centre-du-Québec, Trois-Rivières, Canada; Jennifer Grant, Vancouver Coastal Health, Vancouver, Canada; Jaime Labarca, Department of Infectious Diseases, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile; Camila Carvajal, Department of Infectious Diseases, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile; HongYi Lin, The Nanjing Drum Tower Hospital, Nanjing, China; Qiang Wang, The First Affiliated Hospital of Xi’an Medical University, Xi’an, China; Jing Yang, The Third Central Hospital of Tianjin, Tianjin, China; Wenjie Yang, Tianjin First Central Hospital, Tianjin, China; Jorge A. Cortes, Universidad Nacional de Colombia, Bogotá, Colombia; Juan Villalobos-Vindas, Hospital México, San José, Costa Rica; Carlos Ramírez-Valverde, Hospital México, San José, Costa Rica; Jasminka Horvatic, Clinical Hospital Centre Zagreb, Zagreb, Croatia; Irina Pristas, University Hospital for Infectious Diseases, Zagreb, Croatia; Niki Paphitou, Nicosia General Hospital, Nicosia, Cyprus; Maija-Liisa Rummukainen, Central Finland Health Care District, Jyväskylä, Finland; Antoine Froissart, CHI de Créteil, Créteil, France; Philippe Vanhems, Service d’Hygiène Hospitalière, Epidémiologie, Infectiovigilance et Prévention, Hôpital Edouard Herriot, Hospices Civils de Lyon, Lyon, France; Karaman Pagava, Tbilisi State Medical University, Tbilisi, Georgia; Irma Korinteli, Tbilisi State Medical University, Tbilisi, Georgia; Tobias Brandt, AMEOS Klinikum, Bernburg, Germany; Johannes Gaertner, Klinikum Nuernberg, Nuernberg, Germany; Anthony Enimil, Komfo Anokye Teaching Hospital, Kumasi, Ghana; Emmanuel Roilides, Hippokration General Hospital, Thessaloniki, Greece; Edit Hajdú, University of Szeged, Szeged, Hungary; Sharmila Sengupta, Medanta The Medicity, Gurugram, Haryana, India; Sanjeev Singh, Amrita Institute of Medical Sciences, Kochi, India; Priyanka Patil, Breach Candy Hospital Trust, Mumbai, India; Aruna Poojary, Breach Candy Hospital Trust, Mumbai, India; and other members of The Global-PPS India working group; Jafar Soltani, Department of Pediatrics, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran; Gholamreza Pouladfar, Professor Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; Zahra Jafarpour, Professor Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; Cyrus Alinia, Urmia University of Medical Sciences, Urmia, Iran; Hadi Ameen, for the Iraqi hospitals, University of Antwerp, Antwerp, Belgium; David Fitzgerald, National Maternity Hospital, Dublin, Ireland; Mical Paul, Rambam Health Care Campus, Haifa, Israel; Yasmin Maor, Wolfson Medical Center—Tel Aviv University, Holon, Israel; Jacob Strahilevitz, Hadassah-Hebrew University, Jerusalem, Israel; Michal Chowers, Meir Medical Center, Kfar Saba, Israel; Elizabeth Temkin, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Arnoldo Luca, ASU FC, Udine, Italy; Noriomi Ishibashi, Saitama Medical University, Saitama, Japan; Yoshiaki Gu, Tohoku University Hospital, Sendai, Japan; and other members of The Japanese Global-PPS study group; Feras Darwish Elhajji, Faculty of Pharmacy, Applied Science Private University, Amman, Jordan; Aizhan Karabukayeva, University Medical Center, Nursultan, Kazakhstan; Denis Raka, University of Prishtina ‘Hasan Prishtina’, Medical Faculty, Prishtina, Kosovo; Baktygul Kambaralieva, Tokmok Territorial Hospital, Tokmok, Kyrgyzstan; Lelde Zarakauska, Pauls Stradiņš Clinical University Hospital, Riga, Latvia; Peter Zarb, Mater Dei Hospital, Msida, Malta; Blanca Estela Hernandez Chena, Hospital General Regional No. 6, Ciudad Madero, Mexico; Esteban Gonzalez-Diaz, Hospital Civil de Guadalajara Fray Antonio Alcalde, Guadalajara, Mexico; JuanCarlos Corona-Meléndez, Hospital Angeles Del Carmen, Guadalajara, Mexico; Darwin Stalin Torres Erazo, Hospital Regional de Alta Especialidad de la Peninsula de Yucatan, Merida, Mexico; Suria Elizabeth Loza-Jalil, Hospital de Especialidades Centro Medico Nacional Siglo XXI IMSS, Mexico City, Mexico; Julio Molina, Hospital Cardiologia IMSS Mty, Monterrey, Mexico; Jose Antonio Candelas, Hospital Angeles Torreon, Torreon, Mexico; Gordana Mijovic, Institute of Public Health, Podgorica, Montenegro; Natasa Duborija-Kovacevic, Medical Faculty of the University of Montenegro, Podgorica, Montenegro; Eefje Jong, Meander Medical Center, Amersfoort, The Netherlands; Jan Kluytmans, Department of Infection Control, Amphia Hospital, Breda, The Netherlands; Erika van Elzakker, Haga Hospital, The Hague, The Netherlands; Valentijn Schweitzer, University Medical Center, Utrecht, The Netherlands; Nicola Davies, Waitemata District Health Board, Auckland, New Zealand; Kenneth Iregbu, National Hospital, Abuja, Nigeria; Philip Nwajiobi-Princewill, National Hospital, Abuja, Nigeria; Ifeyinwa Nwafia, University of Nigeria Teaching Hospital, Ituku Ozalla, Enugu, Nigeria; Temitayo Fasuyi, University College Hospital, Ibadan, Nigeria; Aaron Aboderin, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria; Charles John Elikwu, Babcock University Teaching Hospital, Ilishan, Nigeria; Abayomi Fadeyi, University of Ilorin Teaching Hospital, Ilorin, Nigeria; Olafoyekemi Ola-Bello, State Specialist Hospital—Lagos University Teaching Hospital, Lagos, Nigeria; Oyinlola Oduyebo, Lagos University Teaching Hospital, Lagos, Nigeria; Akin Nelson Adedosu, Federal medical center, Owo, Nigeria; Agantem Ekuma, University of Uyo, Uyo, Nigeria; Erjona Shaqiri, Institute of public health, Skopje, North Macedonia; Zikria Saleem, Faculty of Pharmacy, University of Lahore, Lahore, Pakistan; Mari Rose De Los Reyes, Department of Health, Research Institute for Tropical Medicine, Muntinlupa City, The Philippines; and other members of the Philippines Antimicrobial Point Prevalence Survey Team; Luis Tavares, Centro Hospitalar Entre Douro e Vouga, Santa Maria da Feira, Portugal; Nam Joong Kim, Seoul National University College of Medicine, Seoul, Republic of Korea; Svetlana Rachina, Sechenov First Moscow State Medical University, Moscow, Russian Federation; Alwaleed R. Alharthi, King Abdallah Hospital, Bishah, Saudi Arabia; Mushira Enani, King Fahad Medical City, Riyadh, Saudi Arabia; Osama Faried, Prince Mutaeb Bin Abdulaziz hospital, Sakaka, Aljouf, Saudi Arabia; Mohamed Mirghani, Infection Control Directorate, Tabuk, Saudi Arabia; Biljana Carevic, Clinical center of Serbia, Belgrade, Serbia; Lili Radulovic, Zvezdara University Medical Center, Belgrade, Serbia; Gorana Dragovac, Institute of Public Health of Vojvodina, Medical Faculty of University of Novi Sad, Novi Sad, Serbia; Sock Hoon Tan, Tan Tock Seng Hospital, Singapore; Hui Lin Tay, Tan Tock Seng Hospital, Singapore; Jantjie Taljaard, Tygerberg Hospital, Cape Town, South Africa; Vindana Chibabhai, National Health Laboratory Service—Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa; Jennifer Joiner, George Regional Hospital, Western Cape, South Africa; Juan Jose Caston, Hospital Universitario Reina Sofía, Córdoba, Spain; María Núñez-Núñez, Hospital Universitario Clínico San Cecilio, Granada, Spain; Francisco Javier Martínez-Marcos, Hospital Juan Ramón Jiménez, Huelva, Spain; Guillermo Ojeda-Burgos, Hospital Universitario Virgen de la Victoria, Málaga, Spain; Maria Dolores Menendez, Hospital Monte Naranco, Oviedo, Spain; Pilar Retamar, Hospital Universitario Virgen Macarena, Sevilla, Spain; Juan E. Corzo, Hospital Universitario de Valme, Sevilla, Spain; Pinyo Rattanaumpawan, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; Mounerou Salou, Ministry of Health, Lomé, Togo; Basma Mnif, Habib Bourguiba University hospital, Sfax, Tunisia; Ahsen Oncul, Sisli Hamidiye Etfal Education and Research Hospital, Istanbul, Turkey; Peter Ahabwe Babigumira, China Uganda Friendship Hospital Naguru, Kampala, Uganda; James Olweny, Sustainable Development Systems Africa, Kampala, Uganda; Emily Marshall, University Hospitals Bristol NHS Foundation Trust, Bristol, UK; Ann McCorry, Southern Health and Social Care Trust, Craigavon, UK; Mamoon Aldeyab, Department of Pharmacy, School of Applied Sciences, University of Huddersfield, Huddersfield, UK; Priya Khanna, London North West University Healthcare NHS Trust, London, UK; Cairine Gormley, Western Health and Social Care Trust, Londonderry, UK; Sara Maloney, Doctors Hospital of Augusta, Augusta, GA, USA; Mandelin Cooper, Presbyterian St. Luke’s Medical Center, Denver, CO, USA; Laura Blackburn, HCA Houston Healthcare Clear Lake, Houston, TX, USA; Mallory Gessner-Wharton, HCA Houston Healthcare Kingwood, Houston, TX, USA; Lam Vu, West Houston Medical Center, Houston, TX, USA; Nickie Greer, Centennial Medical Center, Nashville, TN, USA; Gerard Gawrys, Methodist Hospital—HCA, San Antonio, TX, USA; Lisha Kronmann, Sentara Obici Hospital, Suffolk, VA, USA; Edgar Rios, HCA/HealthTrust, Sugar Land, TX, USA; Melissa Hudson, Colleton Medical Center, Walterboro, SC, USA; David A. Lindholm, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, OH, USA.
Funding
The Global Point Prevalence Survey is coordinated by the University of Antwerp, Belgium and sponsored through an unrestricted grant given to them annually by bioMérieux. The Global-PPS is also funded by a personal Methusalem grant to Herman Goossens from the Flemish government.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
Contributor Information
the Global-PPS network:
Andi Koraqi, Iris Hoxha, Silva Tafaj, Wanda Cornistein, Rodolfo Quiros, Martin Hojman, Lilit Ghazaryan, Kylie Horne, Kelly Cairns, Fiona Doukas, Thomas Gottlieb, Erica Sermijn, Katia Verhamme, Christiane Brands, Bruno Van Herendael, Lorenzo Filippin, Wouter Vandewal, Deborah Konopnicki, Evelyne Maillart, Liliana Teixeira Lopes, Pauline Papin, Ilse Smits, Hilde Jansens, Sofie Bartholomeus, Anne-Marie Van den Abeele, Sophia Steyaert, Anne Piette, Franky Buyle, Reinoud Cartuyvels, Stijn Jonckheere, Ingrid Wybo, Lorenz Vanneste, Delphine Mathieu, Eric Firre, Veerle Westelinck, Philippe Gadisseux, Thierry Dugernier, Kristof Bafort, Viviane Gonissen, Vanessa Vanderper, Patrick Gabriels, Frank Weekers, Philippe Michel, Ann Van Liedekerke, Michiel Costers, Boudewijn Catry, Amela Dedeic-Ljubovic, Ana C Gales, Ana Paula Matos Porto, Silvia Figueiredo Costa, Emma Keuleyan, Apollinaire Beidi, Youssouph Cissohko, Habsatou Blakwe, Ngassa Batchaya Basile, Greg J German, Sarah Lutes, Jennifer Boswell, Dominik Mertz, Tuyen Nguyen, Timothy MacLaggan, Daniel Landry, Anita Ang, Daniel J G Thirion, Charles Frenette, Yannick Émond, Jacqueline Roberts, Sandra Chang, Justin Kosar, Louis Valiquette, Ginette Dutrisac, Kevin Afra, Allison McGeer, Marie Carrier, Jennifer Grant, Jaime Labarca, Camila Carvajal, HongYi Lin, Qiang Wang, Jing Yang, Wenjie Yang, Jorge A Cortes, Juan Villalobos-Vindas, Carlos Ramírez-Valverde, Jasminka Horvatic, Irina Pristas, Niki Paphitou, Maija-Liisa Rummukainen, Antoine Froissart, Philippe Vanhems, Karaman Pagava, Irma Korinteli, Tobias Brandt, Johannes Gaertner, Anthony Enimil, Emmanuel Roilides, Edit Hajdú, Sharmila Sengupta, Sanjeev Singh, Priyanka Patil, Aruna Poojary, Jafar Soltani, Gholamreza Pouladfar, Zahra Jafarpour, Cyrus Alinia, Hadi Ameen, David Fitzgerald, Mical Paul, Yasmin Maor, Jacob Strahilevitz, Michal Chowers, Elizabeth Temkin, Arnoldo Luca, Noriomi Ishibashi, Yoshiaki Gu, Feras Darwish Elhajji, Aizhan Karabukayeva, Denis Raka, Baktygul Kambaralieva, Lelde Zarakauska, Peter Zarb, Blanca Estela Hernandez Chena, Esteban Gonzalez-Diaz, JuanCarlos Corona-Meléndez, Darwin Stalin Torres Erazo, Suria Elizabeth Loza-Jalil, Julio Molina, Jose Antonio Candelas, Gordana Mijovic, Natasa Duborija-Kovacevic, Eefje Jong, Jan Kluytmans, Erika van Elzakker, Valentijn Schweitzer, Nicola Davies, Kenneth Iregbu, Philip Nwajiobi-Princewill, Ifeyinwa Nwafia, Temitayo Fasuyi, Aaron Aboderin, Charles John Elikwu, Abayomi Fadeyi, Olafoyekemi Ola-Bello, Oyinlola Oduyebo, Akin Nelson Adedosu, Agantem Ekuma, Erjona Shaqiri, Zikria Saleem, Mari Rose De Los Reyes, Luis Tavares, Nam Joong Kim, Svetlana Rachina, Alwaleed R Alharthi, Mushira Enani, Osama Faried, Mohamed Mirghani, Biljana Carevic, Lili Radulovic, Gorana Dragovac, Sock Hoon Tan, Jantjie Taljaard, Vindana Chibabhai, Jennifer Joiner, Juan Jose Caston, María Núñez-Núñez, Francisco Javier Martínez-Marcos, Guillermo Ojeda-Burgos, Maria Dolores Menendez, Pilar Retamar, Juan E Corzo, Pinyo Rattanaumpawan, Mounerou Salou, Basma Mnif, Ahsen Oncul, Peter Ahabwe Babigumira, James Olweny, Emily Marshall, Ann McCorry, Mamoon Aldeyab, Priya Khanna, Cairine Gormley, Sara Maloney, Mandelin Cooper, Laura Blackburn, Mallory Gessner-Wharton, Lam Vu, Nickie Greer, Gerard Gawrys, Lisha Kronmann, Edgar Rios, Melissa Hudson, and David A Lindholm
References
- 1. Klein EY, Van Boeckel TP, Martinez EM. et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA 2018; 115: E3463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/.
- 3. Howard P, Pulcini C, Hara GL. et al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother 2015; 70: 1245–55. [DOI] [PubMed] [Google Scholar]
- 4. Cox JA, Vlieghe E, Mendelson M. et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–8. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO Model List of Essential Medicines, 21st List, 2019, 2019. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06.
- 6. Sharland M, Gandra S, Huttner B. et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis 2019; 19: 1278–80. [DOI] [PubMed] [Google Scholar]
- 7. Klein EY, Milkowska-Shibata M, Tseng KK. et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis 2021; 21: 107–15. [DOI] [PubMed] [Google Scholar]
- 8. Zarb P, Goossens H.. European Surveillance of Antimicrobial Consumption (ESAC): value of a point-prevalence survey of antimicrobial use across Europe. Drugs 2011; 71: 745–55. [DOI] [PubMed] [Google Scholar]
- 9. Ansari F, Erntell M, Goossens H. et al. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 2009; 49: 1496–504. [DOI] [PubMed] [Google Scholar]
- 10. Versporten A, Sharland M, Bielicki J. et al. The Antibiotic Resistance and Prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J 2013; 32: 242–53. [DOI] [PubMed] [Google Scholar]
- 11. Versporten A, Zarb P, Caniaux I. et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Heal 2018; 6: e619–29. [DOI] [PubMed] [Google Scholar]
- 12. Hsia Y, Lee BR, Versporten A. et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Heal 2019; 7: e861–71. [DOI] [PubMed] [Google Scholar]
- 13. Vandael E, Latour K, Goossens H. et al. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: results of the Global-PPS and ECDC-PPS 2017. Antimicrob Resist Infect Control 2020; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UN Statistics Division. Standard country or area codes for statistical use (M49). 2019. https://unstats.un.org/unsd/methodology/m49/overview/.
- 15.The World Bank. World Bank List of Economies (June 2019). 2019. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 16. Hsia Y, Sharland M, Jackson C. et al. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis 2019; 19: 67–75. [DOI] [PubMed] [Google Scholar]
- 17. Wi T, Lahra MM, Ndowa F. et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14: e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crump JA, Heyderman RS.. A perspective on invasive Salmonella disease in Africa. Clin Infect Dis 2015; 61: S235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gandra S, Mojica N, Klein EY. et al. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008–2014. Int J Infect Dis 2016; 50: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. WHO Report on Surveillance of Antibiotic Consumption: 2016-2018 Early implementation. 2018. https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf.
- 21. Laxminarayan R, Matsoso P, Pant S. et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016; 387: 168–75. [DOI] [PubMed] [Google Scholar]
- 22. Knowles R, Sharland M, Hsia Y. et al. Measuring antibiotic availability and use in 20 low-and middle-income countries. Bull World Health Organ 2020; 98: 177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendelson M, Røttingen JA, Gopinathan U. et al. Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 2016; 387: 188–98. [DOI] [PubMed] [Google Scholar]
- 24. Pulcini C, Beovic B, Béraud G. et al. Ensuring universal access to old antibiotics: a critical but neglected priority. Clin Microbiol Infect 2017; 23: 590–2. [DOI] [PubMed] [Google Scholar]
- 25. Karlowsky JA, Hoban DJ, Hackel MA. et al. Resistance among Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013–2015. Brazilian J Infect Dis 2017; 21: 343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paterson DL, Bonomo RA.. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 2005; 18: 657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larramendy S, Gaultier A, Fournier J-P. et al. Local characteristics associated with higher prevalence of ESBL-producing Escherichia coli in community-acquired urinary tract infections: an observational, cross-sectional study. J Antimicrob Chemother 2021; 76: 789–95. [DOI] [PubMed] [Google Scholar]
- 28. Jabeen K, Shakoor S, Hasan R.. Fluoroquinolone-resistant tuberculosis: implications in settings with weak healthcare systems. Int J Infect Dis 2015; 32: 118–23. [DOI] [PubMed] [Google Scholar]
- 29. Arias CA, Reyes J, Carvajal LP. et al. A prospective cohort multicenter study of molecular epidemiology and phylogenomics of Staphylococcus aureus bacteremia in nine Latin American countries. Antimicrob Agents Chemother 2017; 61: e00816-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jernigan JA, Hatfield KM, Wolford H. et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012–2017. N Engl J Med 2020; 382: 1309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fridkin SK, Edwards JR, Courval JM. et al. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 U.S. adult intensive care units. Ann Intern Med 2001; 135: 175–83. [DOI] [PubMed] [Google Scholar]
- 32. Remschmidt C, Behnke M, Kola A. et al. The effect of antibiotic use on prevalence of nosocomial vancomycin-resistant enterococci- an ecologic study. Antimicrob Resist Infect Control 2017; 6: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lubell Y, Turner P, Ashley EA. et al. Susceptibility of bacterial isolates from community-acquired infections in sub-Saharan Africa and Asia to macrolide antibiotics. Trop Med Int Heal 2011; 16: 1192–205. [DOI] [PubMed] [Google Scholar]
- 34. Crump JA, Sjölund-Karlsson M, Gordon MA. et al. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 2015; 28: 901–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al-Taani GM, Scott M, Farren D. et al. Longitudinal point prevalence survey of antibacterial use in Northern Ireland using the European Surveillance of Antimicrobial Consumption (ESAC) PPS and Global-PPS tool. Epidemiol Infect 2018; 146: 985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.