Abstract
Aims
Trimethylamine N‐oxide (TMAO) is a metabolite derived from the gut microbiota. Elevated TMAO levels are associated with a poor prognosis in patients with heart failure with reduced ejection fraction. However, the prognostic effect of elevated TMAO levels on heart failure with preserved ejection fraction (HFpEF) remains unclear.
Methods and results
We consecutively enrolled 146 patients who were hospitalized and discharged from Tottori University Hospital with the primary diagnosis of HFpEF (ejection fraction ≥ 50%). High TMAO levels were defined as those greater than the median value in the patients (20.37 μmol/L). Patients with high TMAO levels had a significantly higher prevalence of prior hospitalization for heart failure and severe renal dysfunction than those with low TMAO levels. They also had a significantly higher acylcarnitine to free carnitine ratio than those with low TMAO levels, which indicated abnormal fatty acid metabolism and relative carnitine deficiency. After adjustment for differences in the patients' background in multivariate analysis, high TMAO levels remained independently associated with a high incidence of the composite endpoints of death due to cardiac causes and hospitalization for heart failure (adjusted hazard ratio, 1.91; 95% confidence interval, 1.01 to 3.62; P < 0.05). There was a significant interaction between TMAO and nutritional status on the primary outcome, and the prognostic effect of TMAO was enhanced in patients with malnutrition.
Conclusions
Elevated TMAO levels at discharge are associated with an increased risk of post‐discharge cardiac events in patients with HFpEF, especially those with the complication of malnutrition.
Keywords: Carnitine, Gut microbiota, Malnutrition
Introduction
Changes in the gut microbiota and its metabolites are closely associated with the pathogenesis and prognosis of various diseases, including hypertension, 1 diabetes, 2 peripheral vascular disease, 3 and myocardial infarction. 4 Heart failure (HF) is the end‐stage manifestation of various types of cardiovascular disease. Recent evidence has also highlighted the potential importance of an altered gut microbiota and its metabolites on the pathophysiology and prognosis of HF. 5 , 6 , 7 , 8 , 9 Hypoperfusion and congestion of the intestine lead to a decrease in blood flow to intestinal epithelial cells, resulting in cell hypoxia, anaerobic metabolism, and overexpression of sodium/hydrogen exchanger 3. 9 , 10 This enhances sodium absorption, and hydrogen ions are exchanged into the gut lumen, decreasing the local pH of the gut and altering the composition of the intestinal microbiota. 9 , 10 , 11 An imbalance in the gut microbiota leads to an increase in harmful metabolites produced by gut bacteria. Trimethylamine N‐oxide (TMAO) is a molecular metabolite that is derived from the gut microbiota. 12 Choline, betaine, and l‐carnitine, which are included in red meat, eggs, fish, and dairy products, are converted to trimethylamine via certain gut microbial enzymes and then metabolized by the liver to TMAO. This promotes atherosclerosis and is associated with increased morbidity and mortality in coronary artery disease. 10 , 12 , 13 TMAO also directly affects the heart by inducing myocardial hypertrophy and fibrosis, 14 inflammation, 15 and mitochondrial dysfunction of the myocardium, 16 which may affect development and prognosis of HF. 9 The association between TMAO and prognosis has been investigated in patients with HF with reduced ejection fraction (HFrEF). 6 , 8 However, the association between TMAO and prognosis in patients with HF with preserved ejection fraction (HFpEF) has not been well established. 17 , 18 Therefore, this study aimed to examine the prognostic effect of elevated TMAO levels in patients with HFpEF.
Methods
Patients
The present study consecutively enrolled 146 patients who were hospitalized for acute decompensated HF and discharged from Tottori University Hospital with the primary diagnosis of HFpEF from January 2012 to December 2017.
Patients with HFpEF were defined as follows 19 , 20 : (i) patients with symptoms and signs of HF as defined by the Framingham criteria, together with pulmonary congestion on X‐rays or pulmonary hypertension evaluated by Doppler echocardiography as previously described; and (ii) patients with a left ventricular ejection fraction ≥ 50%. The following patients were excluded: those with surgically unrepaired severe valve disease or congenital disease; those with arrhythmia requiring pacemaker treatment (complete atrial ventricular block or sick sinus syndrome); and those with constrictive pericarditis, primary pulmonary hypertension, pulmonary embolism, or acute myocardial infarction. Patients under chronic dialysis and those with chronic diseases of the gastrointestinal tract were also excluded.
Data collection
Medical records were reviewed for demographics, medical history, co‐morbidities, laboratory data, echocardiograms, medications, and the clinical course. All measurements were taken at discharge when HF control was stable. Echocardiographic data were measured according to the recommendation of the American Society of Echocardiography. 21 , 22 Early diastolic mitral annular velocity was obtained from the septal site of the mitral leaflet. We collected prognostic information from electronic medical records for patients followed at our institution and conducted a telephone survey for patients followed at other hospitals every year.
Measurement of trimethylamine N‐oxide and serum l‐carnitine levels
Fasting serum and plasma blood samples were collected, and these samples were then immediately processed and frozen at −80°C until later analysis. TMAO levels were measured by ultra‐performance liquid chromatography‐quadrupole time‐of‐flight mass spectrometry (Agilent Technologies, CA, USA) as previously described. 23 High TMAO levels were defined as those greater than the median value of the patients (20.37 μmol/L). Serum l‐carnitine levels were measured by enzymatic cycling methods (carnitine assay kit; Kainos Laboratories Co., Tokyo, Japan) as previously described. 24 , 25
Geriatric Nutritional Risk Index
The Geriatric Nutritional Risk Index (GNRI) was recently postulated as a nutritional risk screening tool in patients with HFpEF. The GNRI was calculated from serum albumin levels and body mass index (BMI) as previously described. 19
Clinical outcomes
We evaluated the composite endpoints of death due to cardiac causes and first events of unplanned re‐hospitalization for HF during the follow‐up period. Death from a cardiac cause was defined as death due to HF and sudden death.
The investigation conformed to the principles outlined in the Declaration of Helsinki. The study was approved by the research ethics committee of Tottori University. Written informed consent was provided by each subject.
Statistical analysis
Continuous variables are expressed as median and interquartile range. Differences in continuous variables were compared using the Mann–Whitney U test. Categorical variables were compared using Fisher's exact test. The event‐free survival curve after discharge from hospital was estimated by the Kaplan–Meier method. Cox proportional hazards models were used to assess the effect of high TMAO levels on the primary outcome and its interaction with each subgroup. To adjust for differences in the patients' background, a propensity score indicating high TMAO levels was entered into the Cox hazard model. For each patient, a propensity score was calculated by logistic regression analysis, into which age, sex, and all baseline characteristics that were associated with high TMAO levels (P < 0.05) were entered: history of HF hospitalization, the levels of haemoglobin, blood urea nitrogen, estimated glomerular filtration rate, acylcarnitine, and free carnitine, and acylcarnitine/free carnitine ratio. A P value < 0.05 was considered statistically significant. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (Version 2.13.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients' characteristics
The median age of the cohort was 80 (73–85) years, and 53.6% of the patients were women. Underweight (BMI < 18 kg/m2) and obesity (BMI > 25 kg/m2), according to the definition in Japanese, 26 were found in 23.8% and 17.9% of the patients, respectively. Moderate or severe nutritional risk as assessed by the GNRI (<92) 19 was found in 55.6% of the patients. A total of 20% of the patients were in New York Heart Association functional class III. A history of hospitalization for HF was found in 21.2% of the patients. The prevalence of cardiac co‐morbidities, including coronary artery disease and atrial fibrillation, was 31.8% and 49.0%, respectively. Non‐cardiac co‐morbidities, including chronic obstructive pulmonary disease, diabetes mellitus, anaemia (haemoglobin levels of <13 g/dL in men and <12 g/dL in women), and severe renal dysfunction (chronic kidney disease stages 4–5; estimated glomerular filtration rate < 30 mL/min/1.73 m2), were found in 7.3%, 40.4%, 73.3%, and 27.1% of the patients, respectively.
Figure 1 shows the distribution of TMAO in the patients. The median value of TMAO was 20.37 (10.45–38.31) μmol/L. Patients with high TMAO levels had a significantly higher prevalence of prior hospitalization for HF and severe renal dysfunction than those with low TMAO levels (P < 0.05) (Table 1 ). Patients with high TMAO levels also had significantly lower haemoglobin levels and higher levels of blood urea nitrogen, free carnitine, and acylcarnitine, and the acylcarnitine to free carnitine ratio than those with low TMAO levels (P < 0.05). There were no significant differences in B‐type natriuretic peptide levels and other parameters between the two groups.
Figure 1.
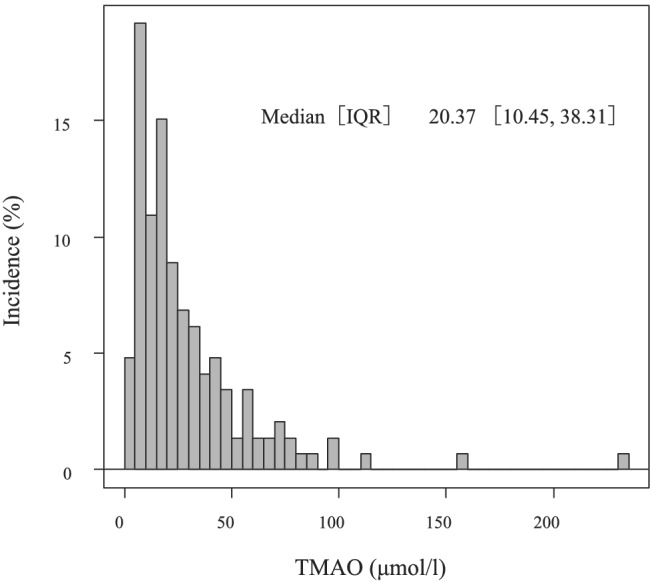
Distribution of TMAO levels in the patients. IQR, interquartile range; TMAO, trimethylamine N‐oxide.
Table 1.
Characteristics of patients with low and high trimethylamine N‐oxide levels
| Low TMAO (n = 73) | High TMAO (n = 73) | P value | |
|---|---|---|---|
| Age (years) | 80 [71–83] | 81 [75–86] | 0.10 |
| Female, n (%) | 40 (54.8) | 40 (54.8) | 1.00 |
| BMI (kg/m2) | 21.23 [19.03–23.44] | 21.03 [18.44–23.12] | 0.54 |
| GNRI | 91.44 [86.489–7.85] | 90.84 [85.97–95.30] | 0.45 |
| SBP (mmHg) | 122 [110–132] | 115 [107–130] | 0.14 |
| Prior HF hospitalization, n (%) | 10 (13.7) | 21 (28.8) | 0.04 |
| NYHA class I/II/III/IV, n | 0/57/16/0 | 0/60/13/0 | 0.68 |
| Co‐morbidity condition | |||
| Coronary artery disease, n (%) | 19 (26.0) | 27 (37.0) | 0.21 |
| Hypertension, n (%) | 60 (82.2) | 57 (78.1) | 0.68 |
| Dyslipidaemia, n (%) | 35 (48.6) | 30 (41.7) | 0.50 |
| Diabetes, n (%) | 26 (35.6) | 33 (45.2) | 0.31 |
| Atrial fibrillation, n (%) | 37 (50.7) | 36 (49.3) | 1.00 |
| COPD, n (%) | 4 (5.5) | 7 (9.6) | 0.53 |
| Anaemia, n (%) | 50 (68.5) | 57 (78.1) | 0.26 |
| CKD stage 4–5, n (%) | 8 (11.0) | 28 (38.4) | <0.01 |
| Medication | |||
| ACE‐I/ARB, n (%) | 62 (84.9) | 60 (82.2) | 0.82 |
| Beta‐blocker, n (%) | 57 (78.1) | 55 (75.3) | 0.85 |
| Loop diuretics, n (%) | 64 (87.7) | 69 (94.5) | 0.24 |
| Mineralocorticoid blocker, n (%) | 31 (42.5) | 27 (37.0) | 0.61 |
| Laboratory values | |||
| Haemoglobin (g/dL) | 11.50 [10.20–12.70] | 11.00 [9.60–11.90] | 0.03 |
| Sodium (mEq/L) | 139.00 [138.00–142.00] | 140.00 [138.00–142.00] | 0.60 |
| BUN (mg/dL) | 22.00 [17.00–26.70] | 37.30 [28.20–52.00] | <0.01 |
| eGFR (mL/min/1.73 m2) | 55.57 [42.82–73.10] | 32.49 [24.23–45.58] | <0.01 |
| CRP (mg/dL) (n = 145) | 0.16 [0.06–0.53] | 0.23 [0.08–0.54] | 0.44 |
| BNP (pg/mL) (n = 145) | 160.95 [72.28–301.48] | 164.00 [89.80–329.60] | 0.40 |
| TMAO (μmol/L) | 10.39 [6.92–15.58] | 38.34 [27.16–57.79] | <0.01 |
| FC (μmol/L) | 63.60 [52.40–71.70] | 68.80 [58.70–84.70] | 0.01 |
| AC (μmol/L) | 13.30 [11.50–16.30] | 21.00 [15.50–27.50] | <0.01 |
| AC to FC ratio | 0.23 [0.18–0.28] | 0.28 [0.20–0.37] | <0.01 |
| Echocardiography | |||
| LV mass index (g/m2) | 125.20 [108.10–148.10] | 120.50 [103.02–145.42] | 0.57 |
| RWT | 0.49 [0.42–0.56] | 0.47 [0.42–0.55] | 0.41 |
| LVEF (%) | 59.80 [55.00–66.00] | 59.10 [54.45–65.00] | 0.36 |
| E' (septal) (cm/s) (n = 140) | 4.20 [3.30–5.10] | 4.10 [3.20–5.20] | 0.81 |
| E/E' (septal) ratio (n = 140) | 17.98 [13.10–24.66] | 19.29 [13.29–23.03] | 0.97 |
| LA volume index (mL/m2) (n = 139) | 55.33 [41.26–71.47] | 58.54 [47.79–77.15] | 0.21 |
| TRPG (mmHg) | 26.00 [19.25–29.00] | 24.00 [20.00–32.00] | 0.74 |
AC, acylcarnitine; ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP; B‐type natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; E, early diastolic mitral inflow; E', early diastolic annular velocity; eGFR, estimated glomerular filtration rate; FC, free carnitine; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RWT, relative wall thickness; SBP, systolic blood pressure; TMAO, trimethylamine N‐oxide; TRPG, tricuspid regurgitation pressure gradient.
Data are median and interquartile range.
Trimethylamine N‐oxide and cardiac events
During the mean follow‐up of 872 ± 500 days, the composite endpoints of death due to cardiac causes and hospitalization for HF occurred in 20 (27.4%) patients in the low TMAO group and in 34 (46.6%) patients in the high TMAO group (unadjusted hazard ratio, 2.06; 95% confidence interval, 1.18 to 3.58; P = 0.01). We also confirmed that, when TMAO was divided into quartiles, the highest and second highest quartile groups had significantly poorer prognosis compared with the lowest quartile group (P < 0.05) (Supporting Information, Figure S1 ). Even after adjustment for differences in the patients' background using the propensity score, high TMAO levels were independently associated with a high incidence of primary outcomes (adjusted hazard ratio, 1.91; 95% confidence interval, 1.08 to 3.62; P < 0.05) (Table 2 and Figure 2 ). We also examined whether the effect of high TMAO levels on cardiac events was modified by age, sex, co‐morbidities, and values of B‐type natriuretic peptide and carnitine (Figure 3 ). We found a significant interaction between TMAO and nutritional status as assessed by the GNRI on the primary outcome (P < 0.01). High TMAO levels were associated with a worse prognosis in patients with low nutritional status compared with those with a preserved nutritional status.
Table 2.
Cox hazard analysis for clinical outcomes between the low and high trimethylamine N‐oxide groups
| Low TMAO no. of events/patients | High TMAO no. of events/patients | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) a | P value | |
|---|---|---|---|---|---|---|
| Primary composite outcomes | 20/73 | 34/73 | 2.06 (1.18–3.58) | 0.01 | 1.91 (1.01–3.62) | <0.05 |
| Cardiac cause death | 5/73 | 14/73 | 3.31 (1.19 9.21) | 0.02 | 2.03 (0.62–6.64) | 0.10 |
| HF hospitalization | 18/73 | 29/73 | 1.93 (1.07 3.49) | 0.03 | 1.96 (0.99–3.87) | 0.05 |
CI, confidence interval; HF, heart failure; HR, hazard ratio; TMAO, trimethylamine N‐oxide.
Adjusted for propensity scores for high TMAO levels (age, sex, prior hospitalization for HF, haemoglobin, blood urea nitrogen, estimated glomerular filtration rate, free carnitine, acylcarnitine, and the acylcarnitine to free carnitine ratio).
Figure 2.
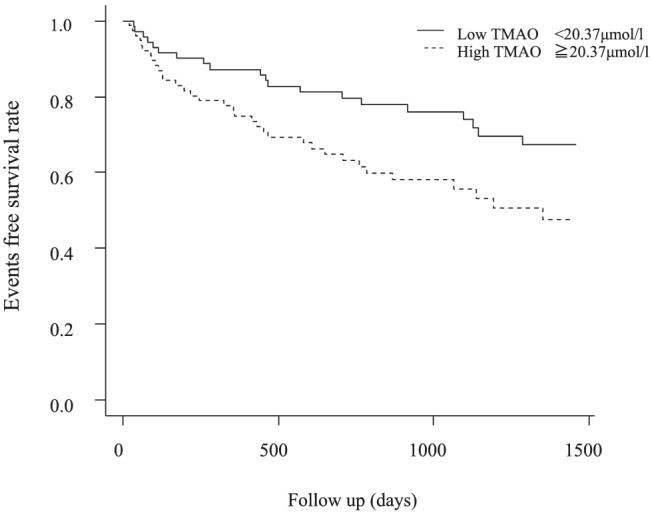
Kaplan–Meier curve for the composite endpoints of death due to cardiac causes and hospitalization for heart failure between the low and high TMAO groups, with adjustment for differences in the patients' background. TMAO, trimethylamine N‐oxide.
Figure 3.
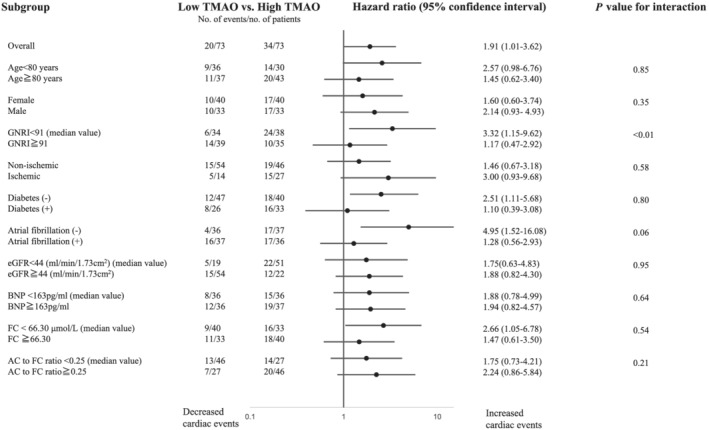
The association between high TMAO levels and the composite endpoints of death due to cardiac causes and hospitalization for heart failure in the subgroups. AC, acylcarnitine; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; FC, free carnitine; GNRI, Geriatric Nutritional Risk Index; TMAO, trimethylamine N‐oxide.
Discussion
The present study showed that elevated TMAO levels, which is a metabolite derived from the gut microbiota, were independently associated with an increase in composite endpoints of readmission for HF and cardiac death in patients with HFpEF. Furthermore, the prognostic effect of elevated TMAO levels was likely to be enhanced in patients with HFpEF and a low nutritional status.
Previous studies have reported that elevated TMAO levels are associated with a poor prognosis in patients with HFrEF. 5 , 6 , 7 , 8 In contrast, the prognostic effect of TMAO in HFpEF has not been well established. Schuett et al. reported that elevated TMAO levels predicted all‐cause mortality and cardiovascular mortality in patients with HFrEF, but not in those with HFpEF. 17 However, Salzano et al. showed that, similar to our report, elevated TMAO levels provided prognostic information in patients with HFpEF. 18 One of the potential reasons for the inconsistent results among studies could be partially explained by the fact that the HFpEF population is heterogeneous. 27 Because we found that the effect of TMAO on prognosis may interact with nutritional status, the prognostic effect of TMAO may depend on the phenotype of HFpEF. Furthermore, regional and racial differences have been reported to affect TMAO values and its effects on prognosis in HF. 28 , 29 In a previous study reported by Salzano et al., the median levels of TMAO in the enrolled patients with HFpEF were 6.6 (4.3–12.2) μmol/L, and the cut‐off value used to predict prognosis in HFpEF was the highest quartile of TMAO levels in patients without HF, which was 5 μmol/L or higher. 18 On the other hand, TMAO levels of the enrolled patients with HFpEF in our study were 20.4 (10.5–38.3) μmol/L. The TMAO values in our study were higher than those in the study by Salzano et al., which may be due to racial differences and the higher proportion of renal dysfunction in the enrolled patients. Yazaki et al. have reported that Japanese patients with HF have significantly higher TMAO levels than Caucasian patients with HF: 9.9 (5.2–22.8) μmol/L vs. 5.9 (3.6–10.8) μmol/L. 28 In addition, because TMAO is excreted by the kidneys, renal dysfunction increases the levels of TMAO. In a previous study examining TMAO in patients with chronic kidney disease, the median levels of TMAO ranged from 43 to 88 μmol/L. 30 The median levels of estimated glomerular filtration rate in our study were lower than those in the study by Salzano et al. 18 : estimated glomerular filtration rate, 44 (30–60) vs. 69 (50–85) mL/min/1.73 m2, which may also explain high levels of TMAO in the current study.
Whether high TMAO levels are a cause or result of poor prognosis of HF is controversial, 9 but, in the present study, we confirmed that high TMAO was associated with poor prognosis independent of established prognostic indicators such as previous hospitalization for HF, renal dysfunction, and anaemia. In addition, even if B‐type natriuretic peptide and nutritional indicators, BMI and GNRI, were included in the propensity score, high TMAO still remained to be associated with poor prognosis (P < 0.05) (data not shown). These results at least suggest that TMAO is not simply a marker for sicker and more debilitated patients. Several recent studies have suggested that TMAO itself may worsen HF as follows. TMAO directly affects the heart by inducing myocardial hypertrophy and fibrosis, 14 inflammation of endothelial cells of blood vessels, 15 and mitochondrial dysfunction of the myocardium. 16 TMAO was also found to contribute to interstitial fibrosis and dysfunction in the kidney, 31 which may promote sodium and water retention and increase the risk of worsening HF. 9 This finding is partially supported by our finding that high TMAO levels were associated with renal dysfunction. Because TMAO is excreted by the kidneys, renal failure increases TMAO and further worsens renal function, creating a vicious cycle. In our study, high TMAO levels were also associated with elevated levels of free carnitine and acylcarnitine and a high acylcarnitine to free carnitine ratio. Carnitine is metabolized by some gut microbial taxa to form TMAO, 12 but the association between carnitine metabolism and TMAO levels has not been well investigated in patients with HF. Carnitine plays an important role in energy metabolism in the cardiac and skeletal muscles through transporting fatty acids into the mitochondria. 24 Incomplete fatty acid metabolism results in the accumulation of highly toxic acyl CoA. 32 , 33 Free carnitine is consumed and reduced owing to detoxification of acyl CoA, and its metabolite, acylcarnitine, increases. 33 In addition, persistent local inflammation may result in leakage of carnitine from the myocardium into the bloodstream through myocardial cell membranes damage. 33 , 34 The levels of C‐reactive protein, a non‐specific marker of inflammation, were not different between the low and high TMAO groups in our study. However, it has been reported that TMAO induces local inflammation in the myocardium by increasing tumour necrosis factor‐α levels in a mouse model. 35 Therefore, even though free carnitine levels in the blood are high, carnitine in myocardial cells may be reduced due to myocardial cell membranes damage in patients with HFpEF with high TMAO. This may increase acylcarnitine but decrease free carnitine in the myocardium, resulting in an elevated acylcarnitine to free carnitine ratio that reflects relative carnitine deficiency. Our and other recent studies showed that an elevated acylcarnitine to free carnitine ratio was associated with a poorer prognosis in patients with HFpEF than in those with HFrEF. 24 , 36 The prognostic effect of TMAO may be partially explained by abnormal fatty acid metabolism and relative carnitine deficiency. The prognostic effect of TMAO was modified by nutritional status, with low body weight and low albumin levels, as assessed by the GNRI. The detailed mechanisms of our findings require further studies. However, the close relationship between gut microbiota and the nutritional status may contribute to the pathogenesis of HF. This is because chronic inflammation derived from intestinal microorganisms is involved in the pathogenesis of wasting and cachexia in patients with HF. 37
There are several limitations to this study. Although the HFA‐PEFF score has recently been proposed as a diagnostic criterion of HFpEF, 38 this study could not evaluate left ventricular filling pressure according to this diagnostic criterion. Thus, we may not be able to completely exclude HFpEF‐likely HF. Measurement of TMAO levels was only made at one time point. Changes in TMAO during the course of acute decompensated HF and recompensation are unknown. Thus, further studies are necessary to assess the best time point for the measurement of TMAO to predict outcome in HFpEF. In addition to gut bacteria, dietary habits may affect TMAO levels. 13 However, because our study included patients who were in the hospital and ate a therapeutic diet, dietary effects, even if present, were likely to be small. Racial and regional differences may affect TMAO values. 29 Furthermore, the sample size of this study was small. A large‐scale survey that includes a variety of races and regions is necessary in the future.
In conclusion, elevated TMAO levels at discharge are associated with an increased risk of post‐discharge cardiac events in patients with HFpEF, especially with the complication of malnutrition. Further investigations are necessary to assess interventions for the gut microbiota in patients with HFpEF.
Conflict of interest
Y. Kinugasa received a grant from Novartis Pharma KK during the term of the study. Dr K. Yamamoto received lecturer's fees from Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., Mitsubishi Tanabe Pharma Co., Ltd., and Boehringer Ingelheim Co., Ltd. Dr K. Yamamoto also received research grants from St. Jude Medical Japan Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., Boston Scientific Co., Ltd., Johnson & Johnson, Biotronik Japan Inc., Japan Lifeline Co., Ltd., Astellas, Teijin Pharma Ltd., Mitsubishi Tanabe Pharma Co., Ltd., Fukuda Denshi, Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Public Health Research Foundation, Nihon Kohden Co., Ltd., and Novartis.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Nos 19K08582 and 20K08403).
Supporting information
Figure S1. Kaplan–Meier curve for the composite endpoints of death due to cardiac causes and hospitalization for HF according to the quartile values of TMAO.
Acknowledgement
We thank Ellen Knapp, PhD, from Edanz Group (https://en‐author‐services.edanzgroup.com/ac) for editing a draft of this manuscript.
Kinugasa, Y. , Nakamura, K. , Kamitani, H. , Hirai, M. , Yanagihara, K. , Kato, M. , and Yamamoto, K. (2021) Trimethylamine N‐oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction. ESC Heart Failure, 8: 2103–2110. 10.1002/ehf2.13290
References
- 1. Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, Xi X, Zhou X, Fan H. The gut microbial metabolite trimethylamine N‐oxide and hypertension risk: a systematic review and dose‐response meta‐analysis. Adv Nutr 2020; 11: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WH, Wang Z, Li XS, Fan Y, Li DS, Wu Y, Hazen SL. Increased trimethylamine N‐oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem 2017; 63: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WH. Trimethylamine N‐oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc 2016; 5: e004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan Y, Sheng Z, Zhou P, Liu C, Zhao H, Song L, Li J, Zhou J, Chen Y, Wang L, Qian H, Sun Z, Qiao S, Xu B, Gao R, Yan H. Plasma trimethylamine N‐oxide as a novel biomarker for plaque rupture in patients with ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv 2019; 12: e007281. [DOI] [PubMed] [Google Scholar]
- 5. Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjorndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015; 277: 717–726. [DOI] [PubMed] [Google Scholar]
- 6. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe‐generated metabolite trimethylamine‐N‐oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014; 64: 1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N‐oxide and prognosis in acute heart failure. Heart 2016; 102: 841–848. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T, Yazaki Y, Voors AA, Jones DJL, Chan DCS, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M, Ng LL. Association with outcomes and response to treatment of trimethylamine N‐oxide in heart failure: results from BIOSTAT‐CHF. Eur J Heart Fail 2019; 21: 877–886. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res 2021; 228: 109–125. [DOI] [PubMed] [Google Scholar]
- 10. Polsinelli VB, Marteau L, Shah SJ. The role of splanchnic congestion and the intestinal microenvironment in the pathogenesis of advanced heart failure. Curr Opin Support Palliat Care 2019; 13: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Failure 2016; 4: 220–227. [DOI] [PubMed] [Google Scholar]
- 12. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non‐meat protein on trimethylamine N‐oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019; 40: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M. Gut microbe‐derived metabolite trimethylamine N‐oxide induces cardiac hypertrophy and fibrosis. Lab Invest 2019; 99: 346–357. [DOI] [PubMed] [Google Scholar]
- 15. Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y. Trimethylamine N‐oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS‐TXNIP‐NLRP3 inflammasome. Biochem Biophys Res Commun 2016; 481: 63–70. [DOI] [PubMed] [Google Scholar]
- 16. Makrecka‐Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, Liepinsh E, Dambrova M. Trimethylamine N‐oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett 2017; 267: 32–38. [DOI] [PubMed] [Google Scholar]
- 17. Schuett K, Kleber ME, Scharnagl H, Lorkowski S, Marz W, Niessner A, Marx N, Meinitzer A. Trimethylamine‐N‐oxide and heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2017; 70: 3202–3204. [DOI] [PubMed] [Google Scholar]
- 18. Salzano A, Israr MZ, Yazaki Y, Heaney LM, Kanagala P, Singh A, Arnold JR, Gulsin GS, Squire IB, McCann GP, Ng LL, Suzuki T. Combined use of trimethylamine N‐oxide with BNP for risk stratification in heart failure with preserved ejection fraction: findings from the DIAMONDHFpEF study. Eur J Prev Cardiol 2020; 27: 2159–2162. [DOI] [PubMed] [Google Scholar]
- 19. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711. [DOI] [PubMed] [Google Scholar]
- 20. Nagai T, Yoshikawa T, Saito Y, Takeishi Y, Yamamoto K, Ogawa H, Anzai T, Investigators J. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction—a report from the Japanese Heart Failure Syndrome With Preserved Ejection Fraction (JASPER) registry. Circ J 2018; 82: 1534–1545. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 22. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 23. Heaney LM, Jones DJ, Mbasu RJ, Ng LL, Suzuki T. High mass accuracy assay for trimethylamine N‐oxide using stable‐isotope dilution with liquid chromatography coupled to orthogonal acceleration time of flight mass spectrometry with multiple reaction monitoring. Anal Bioanal Chem 2016; 408: 797–804. [DOI] [PubMed] [Google Scholar]
- 24. Kinugasa Y, Sugihara S, Yanagihara K, Miyagi M, Matsubara K, Kato M, Yamamoto K. Carnitine insufficiency is associated with adverse outcomes in patients with heart failure with preserved ejection fraction. J Aging Res Clin Pract 2016; inpress. 10.14283/jarcp.2016.116 [DOI] [Google Scholar]
- 25. Takahashi M, Ueda S, Misaki H, Sugiyama N, Matsumoto K, Matsuo N, Murao S. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem 1994; 40: 817–821. [PubMed] [Google Scholar]
- 26. Yamamoto K, Tsuchihashi‐Makaya M, Kinugasa Y, Iida Y, Kamiya K, Kihara Y, Kono Y, Sato Y, Suzuki N, Takeuchi H, Higo T, Miyazawa Y, Miyajima I, Yamashina A, Yoshita K, Washida K, Kuzuya M, Takahashi T, Nakaya Y, Hasebe N, Tsutsui H, Japanese Heart Failure Society ECWC . Japanese Heart Failure Society 2018 scientific statement on nutritional assessment and management in heart failure patients. Circ J 2020; 84: 1408–1444. [DOI] [PubMed] [Google Scholar]
- 27. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015; 17: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yazaki Y, Aizawa K, Israr MZ, Negishi K, Salzano A, Saitoh Y, Kimura N, Kono K, Heaney L, Cassambai S, Bernieh D, Lai F, Imai Y, Kario K, Nagai R, Ng LL, Suzuki T. Ethnic differences in association of outcomes with trimethylamine N‐oxide in acute heart failure patients. ESC Heart Failure 2020; 7: 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yazaki Y, Salzano A, Nelson CP, Voors AA, Anker SD, Cleland JG, Lang CC, Metra M, Samani NJ, Ng LL, Suzuki T. Geographical location affects the levels and association of trimethylamine N‐oxide with heart failure mortality in BIOSTAT‐CHF: a post‐hoc analysis. Eur J Heart Fail 2019; 21: 1291–1294. [DOI] [PubMed] [Google Scholar]
- 30. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe‐generated metabolite trimethylamine‐N‐oxide as cardiovascular risk biomarker: a systematic review and dose‐response meta‐analysis. Eur Heart J 2017; 38: 2948–2956. [DOI] [PubMed] [Google Scholar]
- 31. Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa‐Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota‐dependent trimethylamine N‐oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015; 116: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long‐chain fatty acid β‐oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African‐American women. J Nutr 2009; 139: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JR, Alberta H. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One 2015; 10: e0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Regitz V, Shug AL, Fleck E. Defective myocardial carnitine metabolism in congestive heart failure secondary to dilated cardiomyopathy and to coronary, hypertensive and valvular heart diseases. Am J Cardiol 1990; 65: 755–760. [DOI] [PubMed] [Google Scholar]
- 35. Zhang H, Meng J, Trimethylamine YH. N‐oxide supplementation abolishes the cardioprotective effects of voluntary exercise in mice fed a western diet. Front Physiol 2017; 8: 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoshihisa A, Watanabe S, Yokokawa T, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Takeishi Y. Associations between acylcarnitine to free carnitine ratio and adverse prognosis in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail 2017; 4: 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber‐Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole‐Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007; 50: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 38. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020; 22: 391–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier curve for the composite endpoints of death due to cardiac causes and hospitalization for HF according to the quartile values of TMAO.


