Abstract
Aims
Allograft rejection following heart transplantation (HTx) is a serious complication even in the era of modern immunosuppressive regimens and causes up to a third of early deaths after HTx. Allograft rejection is mediated by a cascade of immune mechanisms leading to acute cellular rejection (ACR) and/or antibody‐mediated rejection (AMR). The gold standard for monitoring allograft rejection is invasive endomyocardial biopsy that exposes patients to complications. Little is known about the potential of circulating miRNAs as biomarkers to detect cardiac allograft rejection. We here present a systematic analysis of circulating miRNAs as biomarkers and predictors for allograft rejection after HTx using next‐generation small RNA sequencing.
Methods and results
We used next‐generation small RNA sequencing to investigate circulating miRNAs among HTx recipients (10 healthy controls, 10 heart failure patients, 13 ACR, and 10 AMR). MiRNA profiling was performed at different time points before, during, and after resolution of the rejection episode. We found three miRNAs with significantly increased serum levels in patients with biopsy‐proven cardiac rejection when compared with patients without rejection: hsa‐miR‐139‐5p, hsa‐miR‐151a‐5p, and hsa‐miR‐186‐5p. We identified miRNAs that may serve as potential predictors for the subsequent development of ACR: hsa‐miR‐29c‐3p (ACR) and hsa‐miR‐486‐5p (AMR). Overall, hsa‐miR‐486‐5p was most strongly associated with acute rejection episodes.
Conclusions
Monitoring cardiac allograft rejection using circulating miRNAs might represent an alternative strategy to invasive endomyocardial biopsy.
Keywords: Heart transplantation, MiRNA, Allograft rejection, Biomarker
Introduction
Heart transplantation (HTx) is the only causative therapy for end‐stage heart failure (HF) patients. Over 5000 hearts are transplanted yearly. 1 Although the overall 1 year survival of 86% observed between 2009 and 2013 represents an improvement compared with previous eras, graft failure caused by rejection remains the leading cause of death beyond 30 days after transplantation. 1 The two major rejection mechanisms leading to allograft rejection are acute cellular rejection (ACR) and antibody‐mediated rejection (AMR). Endomyocardial biopsy is the gold standard for monitoring allograft rejection, but the need for frequent biopsies during the first year after HTx exposes patients to complications such as significant patient discomfort, valve injury, and the risk of potentially life‐threatening complications including cardiac perforation, tamponade, and arrhythmias. 2 , 3 Thus, there is a need for new methods for non‐invasive graft rejection monitoring.
Potential biomarkers of rejection derived by peripheral blood sampling have been studied. One commercially available bioassay to test for allograft rejection, Allomap (XDx, Brisbane, CA), is based on leucocyte gene expression profiling. 4 Other examples include human leucocyte antigen antibodies, anti‐endothelial cell antibodies, soluble CD30 from membrane glycoprotein, CXCR3‐binding chemokines, and IFN‐γ ELISPOT assay or flow cytometry, although these offer insufficient specificity and sensitivity to differentiate rejections from other immune responses. 5 Classic cardiac biomarkers such as troponin and brain natriuretic peptide have also been investigated but due to the non‐specificity for the immunological process have not been established as distinct markers of rejection. 6 , 7 , 8 Most recently, cell‐free DNA (cfDNA)‐based methods are being developed. 9 , 10 , 11 The concept of utilizing cfDNA as rejection biomarker is based on the release of genomic DNA during cell death, and initial studies show that circulating cfDNA levels correlate with severity of HF and that donor‐derived cell‐free DNA measurements show promise in being able to detect (or rule out) acute rejection. 12 , 13 , 14
MiRNAs represent a class of regulatory short single‐stranded non‐coding RNAs with tissue specificity that can be detected in the blood. 15 Although their regulatory mechanisms and specific targets are not completely understood, they are promising candidates for allograft rejection biomarkers. 16 Evidence suggests that urinary or serum miRNAs may be used for allograft rejection monitoring for kidney transplant rejection. 17 , 18 , 19 Plasma miRNA may be a marker for acute rejection in liver transplantation. 20 Further, profiles of miRNAs have been shown to be able to indicate the severity of HF. 21
Limited data on circulating miRNA in cardiac rejection are available from animal models, and a small number of human studies using quantitative polymerase chain reaction (qPCR)‐based profiling assays have been conducted. 22 , 23 , 24 , 25 However, these studies all used a biased approach focusing on preselected miRNAs with a known function relevant to cardiac allograft rejection, endothelial activation, injury, and vascular inflammation.
Here, we present the, to our knowledge, first study using next‐generation small RNA sequencing to investigate circulating miRNAs among HTx recipients and their potential for the prediction and detection of allograft rejection.
Material and methods
Study design and clinical data
We included a total of 43 patients, recruited at New York Presbyterian/Columbia University Medical Center, with serum samples ranging from January 2003 to October 2013. The cohort consisted of four groups: a group of 10 control patients with HF but no HTx (Ctr), a group of 10 patients following HTx but no allograft rejection (TX), a group of 13 patients following HTx who were diagnosed with ACR (all International Society for Heart and Lung Transplantation Grade 3A), and a group of 10 patients following HTx who presented with AMR. Serial samples were analysed from patients with ACR and AMR (ACR: mean of 3.8 per patient and AMR: mean of 3.5 samples per patient) at various time points before, during, and after rejection in order to detect dynamic changes in miRNA profiles related to rejection.
Sample collection
Blood was obtained from all patients and processed for miRNA isolation from serum. The samples were stored at −80°C until analysis. In the rejection cohort, samples from before and after the rejection episode were available for analysis, collected at the time of cardiac allograft implantation or, respectively, during the first follow‐up surveillance myocardial biopsy visit showing no evidence of rejection on histology. Rejection episodes were defined by endomyocardial biopsy, the gold standard for the detection of cardiac allograft rejection. The rejection type was determined according to the International Society for Heart and Lung Transplantation guidelines for HTx rejection by interpretation by two independent experienced cardiac transplant pathologists at the Columbia University Department of Pathology. The study was approved by the Institutional Review Board at Columbia University. The investigation conforms to the principles outlined in the Declaration of Helsinki.
The detailed protocols on RNA isolation, sRNA library preparation, and bioinformatic methods can be found in Supporting Information, Data S1 .
In brief, total RNA was isolated from 425 μL serum using commercially available kits; 8.5 μL RNA was used for sRNAseq cDNA library preparation. The cDNA library preparation for the tissue samples was performed according to a previously published protocol. 26
Bioinformatics analysis of RNA sequencing
Details on the methodological considerations of the bioinformatical analysis can be found in the Supporting Information, Data S1 . In brief, we analysed the FASTQ output files from the HiSeq 2500 next‐generation sequencing platform using a pipeline inspired by methods presented by Farazi et al. 27 and Brown et al. 28 The principal variations of these analysis guidelines were the use of Cutadapt 29 for trimming and demultiplexing, Bowtie 30 for simple alignments, and miRdeep2 31 , 32 , 33 , 34 , 35 , 36 , 37 for the quantification of miRNA levels. For the differential abundance analysis, we used DESeq2. 38 , 39 We performed this analysis on each possible pair of case–control groups, the control group always being the group ‘transplant without rejection (TX)’. Case patients were patients who had an allograft rejection episode at any point of the longitudinal study. The differential abundance analysis returned the list of miRNAs with the average count, a log2 fold change (logFC) for the case–control comparison, a P‐value from Wald test, and an adjusted P‐value using the Benjamini–Hochberg method, 40 reporting the false discovery rate. MiRNAs with an false discovery rate ≤0.05 were considered statistically significant.
Identification of biomarker miRNAs and prediction of abundance levels
We evaluated how predictive the significant miRNAs were by defining abundance level thresholds when considering a unique miRNA or linear combinations of features using logistic regression with least absolute shrinkage and selection operator (L1 regularization) when using multiple miRNAs. We built receiver operating characteristic curves, computed the area under the curve (AUC), and evaluated these metrics on the whole training set with cross‐validation, as well as by using a leave‐one‐out (LOO) strategy. Details on this approach can be found in Supporting Information, Data S1 . This approach enabled us to evaluate how generalizable the normalized levels of significant miRNAs can be to classify a new patient.
Results
Patient cohort
Twenty‐three patients were HTx recipients: of these, the majority were male (80% for AMR; 69% for ACR). Mean age was 48 ± 10 years in the AMR cohort and 51 ± 14 years for the ACR group. The aetiology of end‐stage HF leading to transplant was a non‐ischaemic cardiomyopathy in two‐thirds of cases, after coronary artery disease had been ruled out (Table 1 ).
TABLE 1.
Patient demographics
| AMR (n = 10) | ACR (n = 13) | No rejection—TX (n = 10) | No transplant—Ctr (n = 10) | P | |
|---|---|---|---|---|---|
| Male, n (%) | 8 (80) | 9 (69) | 8 (80) | 5 (50) | 0.42 |
| Age (years) | 48 ± 10 | 51 ± 14 | 46 ± 23 | 60 ± 19 | 0.17 |
| Aetiology, n (%) | 0.24 | ||||
| ICM | 3 (30) | 3 (23) | 5 (50) | 1 (10) | |
| NICM | 7 (70) | 10 (67) | 5 (50) | 9 (90) | |
| Medications | |||||
| Antiarrhythmics | 5 (50) | 3 (23) | 0 | 2 (20) | 0.22 |
| Inotropes/pressors | 4 (40) | 7 (54) | 0 | 0 | 0.68 |
| Steroids | 2 (20) | 11 (85) | 6 (60) | 0 | 0.003 |
ACR, acute cellular rejection; AMR, antibody‐mediated rejection; ICM, ischaemic cardiomyopathy; NICM, non‐ischaemic cardiomyopathy.
MiRNAs as stable markers of rejection
We assessed for miRNAs that are significant at all time for a given type of rejection (AMR, ACR, or ACR + AMR). For ACR, hsa‐miR‐615‐3p, hsa‐miR‐186‐5p, hsa‐miR‐139‐5p, and hsa‐miR‐151a‐5p were significantly different before, during, and after ACR (all increased) (Figure 1 ). For AMR, eight miRNAs were significant across all time points: hsa‐miR‐3615, hsa‐miR‐361‐5p, hsa‐miR‐186‐5p, hsa‐miR‐139‐5p, hsa‐miR‐151a‐5p, hsa‐miR‐150‐5p, hsa‐miR‐127‐3p, which were increased, and hsa‐miR‐182‐5p, which was decreased (Figure 2 ). Finally, the analysis combining both types of rejection (AMR + ACR) highlighted eight miRNAs significant at all time points: hsa‐miR‐186‐5p, hsa‐miR‐3615, hsa‐miR‐18a‐5p, hsa‐miR‐139‐5p, hsa‐miR‐151a‐5p, hsa‐miR‐150‐5p, hsa‐miR‐127‐3p, which were increased, and hsa‐miR‐486‐5p, which was decreased (Supporting Information, Data S2 and Table S9 ).
Figure 1.
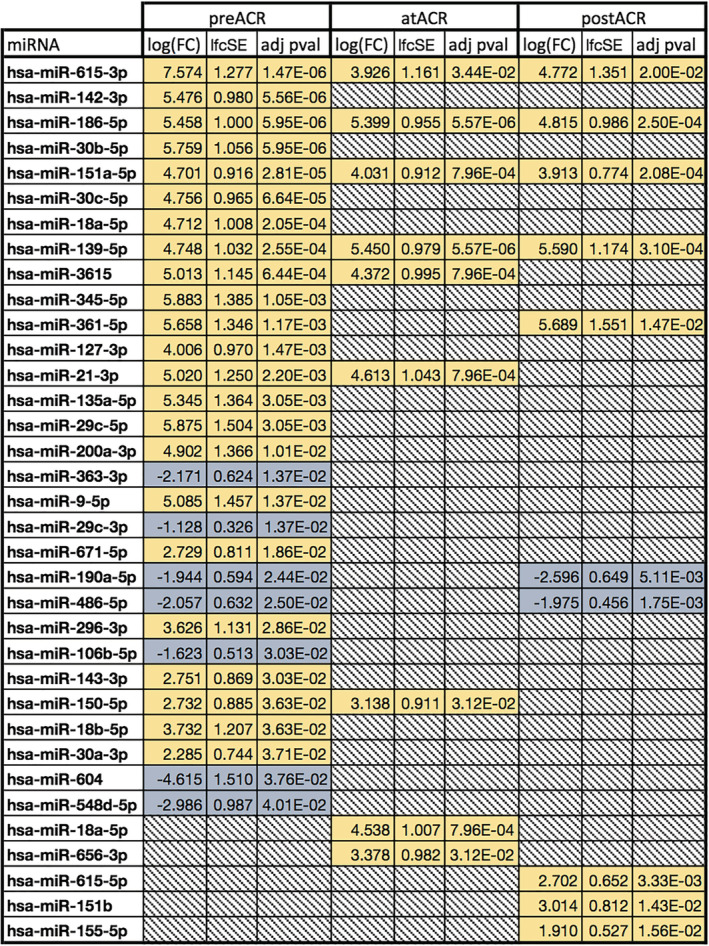
Significant miRNA abundance changes before, during, and after acute cellular rejection (ACR). Down‐regulated in blue, up‐regulated in yellow, and greyed out where non‐significant (P ≥ 0.05).
Figure 2.
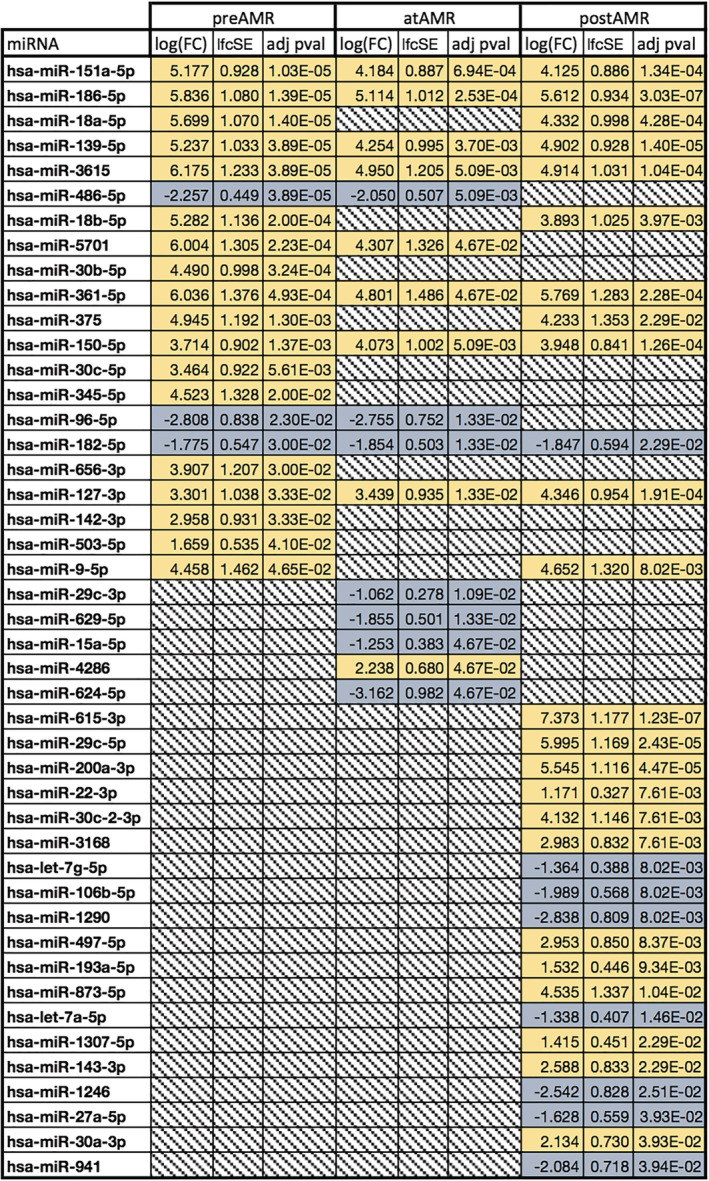
Significant miRNA abundance changes before, during, and after antibody‐mediated rejection (AMR). Down‐regulated in blue, up‐regulated in yellow, and greyed out where non‐significant (P ≥ 0.05).
Three miRNAs were consistently increased across all time points (P < 0.005) for all types of rejection: hsa‐miR‐139‐5p with an average log fold change (logFC) of 5.26 ± 0.37 in ACR and 4.80 ± 0.41 in AMR, hsa‐miR‐151a‐5p with 4.22 ± 0.35 in ACR and 4.50 ± 0.48 in AMR, and hsa‐miR‐186‐5p with an average logFC of 5.22 ± 0.29 in ACR and 5.52 ± 0.30 in AMR, all compared with transplant with no rejection subjects. These miRNAs with a consistently higher abundance in patients who have developed cardiac allograft rejections in their post‐transplant course might be an expression of an immunological predisposition to develop significant rejection episodes.
Rejection treatment‐sensitive miRNAs
Next, we assessed for miRNAs that would have a significant change in abundance both before and during the rejection [compared with controls (TX group)], but with no significant change after rejection treatment. For ACR, we identified four miRNAs that appeared to return to a level similar as the non‐rejection group (TX): hsa‐miR‐21‐3p, hsa‐miR‐3615, hsa‐miR‐18a‐5p, and hsa‐miR‐150‐5p. For AMR, three miRNAs present a similar pattern: hsa‐miR‐5701, hsa‐miR‐486‐5p, and hsa‐miR‐96‐5p. Elevated levels of these miRNAs before the histologically confirmed rejection episode is diagnosed could be indicative of preclinical rejection processes and therefore early markers of rejection.
Predictors and biomarkers
For this section, we assessed how the miRNAs identified previously can be predictive at the individual level, as some miRNAs might have significant abundance changes but without sufficient predictive power across all samples. For this, we selected an AUC cut‐off of 0.9.
We termed miRNAs that correctly classify ‘rejection’ patients before the rejection episode has occurred as ‘predictors’, and miRNAs than correctly classify patients at the time of the rejection as ‘biomarkers’.
Single predictors of rejection
We evaluated the ability of measured changes in miRNA levels to predict a rejection at the first measurement after transplantation, before any histologically diagnosed rejection is noted. This evaluation represents the ability of a miRNA to correctly classify patients between rejection (ACR and/or AMR) and no rejection (TX). Our first evaluation involved using the normalized abundance counts and compute specificity and sensitivity of a model only relying on these to perform the classification. As ACR predictor (elevated level before an ACR episode is diagnosed by biopsy), the miRNAs with abundance changes who performed the best with this method were hsa‐miR‐29c‐3p (AUC = 0.938), hsa‐miR‐486‐5p (AUC = 0.938), and hsa‐miR‐615‐3p (AUC = 0.913). The only miRNA with a LOO AUC above 0.9 showing sufficient generalizability was hsa‐miR‐29c‐3p (LOO AUC = 0.925) (Supporting Information, Figure S5A ).
As AMR predictor, we observed only two predictors with AUC > 90%: hsa‐miR‐486‐5p (AUC = 0.986) and hsa‐miR‐182‐5p (AUC = 0.914). Only hsa‐miR‐486‐5p presented a satisfying LOO AUC of 0.986 (Supporting Information, Figure S6A ). Combining the pre‐AMR and pre‐ACR groups in the differential analysis against the TX control, only hsa‐miR‐486‐5p emerged as a consistent predictor when combining the rejection types for both AUC (0.96) and LOO AUC (0.927) (Supporting Information, Figure S7A ). In this context, this miRNA could be interpreted as a general predictor for allograft rejection irrespective of the type of rejection, ACR or AMR.
Single biomarkers of rejection
We also studied the miRNAs with significant abundance change at the time of rejection (i.e. at ACR and at AMR) and evaluated their classification power between the ACR, AMR, and TX control patients.
Out of the nine miRNAs with abundance change at the diagnosis time point of ACR, no miRNA presented an AUC above 0.9, with the best biomarker being hsa‐miR‐615‐3p (AUC = 0.755 and LOO AUC = 0.755) (Supporting Information, Figure S5B ).
For AMR, three potential biomarkers of allograft rejection emerged: hsa‐miR‐29c‐3p (AUC = 0.967), hsa‐miR‐486‐5p (AUC = 0.944), and hsa‐miR‐629‐5p (AUC = 0.9). Only hsa‐miR‐29c‐3p presented acceptable generalizability with a LOO AUC of 0.944 (Supporting Information, Figure S6B ).
Combining both groups, only hsa‐miR‐486‐5p appears to be a satisfactory biomarker, with AUC 0.895 and LOO AUC of 0.9 (Supporting Information, Figure S7B).
Post‐treatment receiver operating characteristic curves for AMR, ACR, and AMR + ACR groups are in Supporting Information, Figures S5C , S6C , and S7C .
Combining biomarkers of acute cellular rejection and antibody‐mediated rejection
We also evaluated the LOO AUC by using all significant miRNAs identified for each case group as previously in order to assess how generalizable a combination of these biomarkers would be to classify new patients. For this, pre‐ACR markers showed a LOO AUC of 0.913, and pre‐AMR presented a high LOO AUC of 0.929. At rejection, significant miRNAs in ACR performed only with a LOO AUC of 0.636, while AMR biomarkers scored a LOO AUC of 0.956 (Figure 3 ). Finally, combining abundance levels between AMR and ACR groups, the significant miRNAs scored a LOO AUC of 0.64 before rejection and 0.65 at rejection (Supporting Information, Figure S8A and S8B ). The observation that combining miRNAs of both ACR and AMR leads to a poorer classification result suggests that different miRNAs are relevant for cellular vs. antibody‐mediated graft rejection processes.
Figure 3.
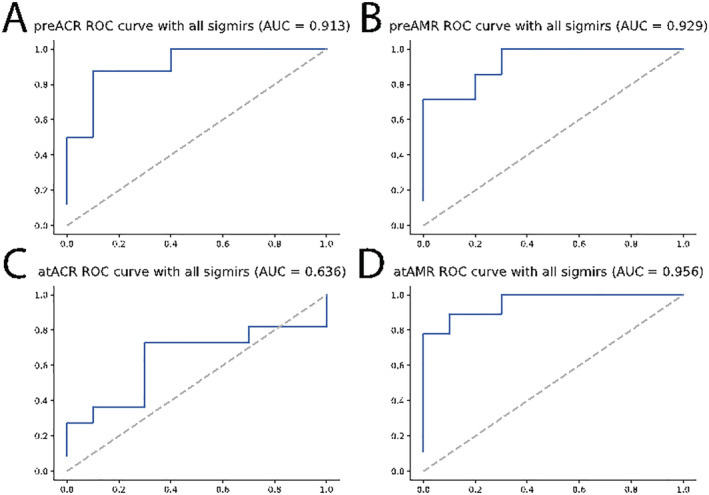
Receiver operating characteristic (ROC) curves from logistic regression with least absolute shrinkage and selection operator penalty evaluated with leave‐one‐out testing for predicting rejection (A) before acute cellular rejection (pre‐ACR) and (B) before antibody‐mediated rejection (pre‐AMR) and identifying rejection (C) at ACR and (D) at AMR. AUC, area under the curve.
Discussion
In this study, we used next‐generation sequencing to identify miRNAs associated with cardiac allograft rejection. MiRNAs have ideal features for being potential biomarkers 41 : they can be easily extracted from blood, have a high tissue specificity, are highly stable as ribonucleic acid, and can be relatively easily and cost‐efficiently quantified even in a multi‐marker approach with modern sequencing technologies. Our analysis focused on a set of patients presenting at various time points following HTx who suffered from cardiac allograft rejection. This longitudinal analysis is a major strength of this analysis. This allowed the characterization of miRNA patterns of patients following HTx with and without allograft rejection.
We studied both types of rejection: ACR and AMR. We identified three miRNAs with significant abundance changes for both types of rejection and at all time points: hsa‐miR‐139‐5p, hsa‐miR‐151a‐5p, and hsa‐miR‐186‐5p. These miRNAs, showing a consistently higher abundance in transplant patients who develop an allograft rejection episode, might be an expression of an immunological predisposition to develop significant rejection episodes. Little is known about these specific miRNAs, but miR‐139‐5p has been linked to cardiac apoptotic pathways 42 and interestingly in this context of rejection processes to T lymphocyte immune mechanisms. 43 miR‐151 has been linked to myocardial damage 44 and similarly apoptotic processes. 45 A larger body of research on miR‐186 is available, with strong mechanistic evidence for its role in autophagy. 46 , 47
An important clinical scenario in which miRNA‐based biomarkers can be of great value is the detection of an allograft rejection before that rejection episode becomes histologically apparent or clinically relevant. Such a pre‐rejection diagnosis with high diagnostic power would allow a pre‐emptive adjustment of the immunosuppressive regimen. We were able to perform an analysis on samples taken from our patients before a histologically confirmed rejection episode developed and could identify several miRNAs that might qualify as predictors of rejection: for the prediction of allograft rejection, we demonstrated with two different methods that hsa‐miR‐29c‐3p can identify patients who will have ACR from baseline controls, whereas hsa‐miR‐486‐5p can classify patients who will have AMR before a rejection, diagnosed by conventional methods, develops.
Attempting to identify potential miRNA biomarkers of rejection, we applied the previously mentioned stringent criteria to candidates for both types of cardiac rejection (ACR and AMR); however, classification by abundance and logistic regression with LOO cross‐validation found no miRNAs in common with an AUC above 0.9 for ACR. Only hsa‐miR‐29c‐3p seemed to be a satisfactory biomarker for AMR. This particular miRNA might be an interesting candidate for the development of a miRNA‐based biomarker test to detect AMR. MiR‐29c has mostly been described in the oncological literature, but recently, this miRNA has been found to be part of a miRNA signature associated with lung transplant allograft dysfunction 48 as well as kidney transplant surveillance. 49
Several studies have been published investigating various modalities of non‐invasive allograft rejection surveillance using DNA, RNA, and protein‐based approaches. The initial studies designed based on the concept of a ‘liquid biopsy’ to assess for allograft rejection were the CARGO and CARGO‐II studies, from which AlloMap was marketed. 50 AlloMap analyses a leucocyte gene abundance profile to make predictions on rejection events. In other, small studies, a murine model of HTx showed that miR‐182 levels increased dramatically during rejection of an HTx. 23 In a pilot study on 10 patients using qPCR‐based profiling assays, six miRNAs (miR‐326, miR‐142‐3p, miR‐101, miR‐144, miR‐27a, and miR‐424) were found to be significantly increased during rejection compared with before rejection. 25 The largest study analysing circulating miRNAs as potential biomarkers of acute allograft rejection to date has been conducted by Van Huyen et al. 24 on 113 HTx recipients, which focused on 14 miRNAs relevant to cardiac allograft rejection, endothelial activation, injury, and vascular inflammation and also used qPCR to screen miRNAs in both endomyocardial biopsies and blood samples. In this study, Van Huyen et al. identified miR‐10a, miR‐31, miR‐92a, and miR‐155 in circulation to be correlated with myocardial tissue miRNA levels during rejection. Although statistically well powered, this study had a limited screening power as the miRNAs were preselected.
A study that uses circulating miRNA for allograft surveillance, similar to the work at hand, has recently been published: limiting their focus on ACR, Dewi et al. identified seven miRNAs that were significantly elevated in ACR compared with quiescent cardiac allografts and propose two miRNAs, miR‐142‐3p and miR‐101‐3p, as a potential diagnostic tool for the detection of rejection. 51
Our study has several limitations. The work is limited by a relatively small cohort, and we therefore noted disparities between patients within the groups, which suggests that larger case and control groups would be more representative of the variations in miRNA levels, which may result from related medical treatments, co‐morbidities, and genetic variability.
Further, the bioinformatics treatment of the raw sequencing data showed that only 10% of the total raw reads were mapped to human miRNA references due to quality control and spiked‐in synthetic RNAs that accounted for almost 44% of the reads that passed the Illumina quality control. Furthermore, our findings do not overlap with previously reported miRNAs during cardiac rejection. Of note, most prior studies in the field do not apply an unbiased approach but utilize preselected miRNAs based on the literature. However, the study at hand is a pilot study, and as outlined previously, generalizability is limited given the small sample size, as well as relatively low percentage of raw reads mapped to human miRNA, given the stringent quality control criteria applied. Furthermore, the non‐ischaemic cardiomyopathy subgroup within the HF cohort subsumes possibly different aetiologies of HF, which might have an effect on miRNA profiles. However, the exact aetiology of the cardiomyopathy could not definitively be determined and was labelled as non‐ischaemic after coronary artery disease had been ruled out.
In conclusion, the present study represents the to our knowledge first systematic approach using deep‐sequencing RNA analysis for the detection of circulating miRNA species that qualify as potential biomarkers for the prediction and detection of various types of cardiac allograft rejection.
Conflict of interest
None declared.
Funding
This study was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (R01HL114813) and the Else Kröner‐Fresenius Foundation (Else Kröner‐Fresenius‐Stiftung) (to P.C.S.).
Open Access funding enabled and organized by Projekt DEAL
Supporting information
Figure S1. (A) Pre ACR vs TX. (B) At ACR vs. TX. (C) Post ACR vs. TX.
Figure S2. (A) Pre AMR vs. TX. (B) At AMR vs. TX. (C) Post AMR vs. TX.
Figure S3. (A) Pre AMR + ACR vs. TX. (B) At AMR + ACR vs. TX. (C) Post AMR + ACR vs. TX.
Figure S4. (A) ACR patterns. (B) AMR patterns. (C) AMR + ACR patterns.
Figure S5. (A) preACR. (B) at ACR. (C) post ACR.
Figure S6. (A) Pre AMR. (B) At AMR. (C) Post AMR.
Figure S7. (A) pre ACR + AMR. (B) at ACR + AMR. (C) Post AMR + ACR.
Figure S8. (A) ROC curve, all sigmirs AMR + ACR before rejection. (B) ROC curve, all sigmirs AMR + ACR before rejection.
Figure S9. Significant miRNA abundances.
Table S1. 21 multiplexing adapters used for the miRNA sequencing.
Table S2. Adapter Id of each group across the five sequencing pools.
Table S3. Details of the number of reads in the source files, with number of reads discarded by Illumina quality control and the net count of reads.
Table S4. Adapter trimming before demultiplexing.
Table S5. Demultiplexing process, discarding reads that could not be assigned with certainty to a group.
Table S6. Quality control process removing reads shorter than 17 bps, reads with a Phred score lower than 10.
Table S7. Reads mapped to the hg38 human genome reference allowing for 1 mismatch.
Table S8. Reads mapped to the miRNA human reference allowing for 2 mismatches.
Table S9. Significantly differentially expressed miRNAs before, during and after both rejection types. Down regulated in blue, up‐regulated in yellow. Greyed out where non‐significant (P > = 0.05).
Data S1. Supplemental methods.
Data S2. Supplemental results.
Kennel, P. J. , Yahi, A. , Naka, Y. , Mancini, D. M. , Marboe, C. C. , Max, K. , Akat, K. , Tuschl, T. , Vasilescu, E.‐R. M. , Zorn, E. , Tatonetti, N. P. , and Schulze, P. C. (2021) Longitudinal profiling of circulating miRNA during cardiac allograft rejection: a proof‐of‐concept study. ESC Heart Failure, 8: 1840–1849. 10.1002/ehf2.13238
References
- 1. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: thirty‐second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34: 1244–1254. [DOI] [PubMed] [Google Scholar]
- 2. From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc Elsevier 2011; 86: 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saraiva F, Matos V, Gonçalves L, Antunes M, Providência LA. Complications of endomyocardial biopsy in heart transplant patients: a retrospective study of 2117 consecutive procedures. Transplant Proc 2011; 43: 1908–1912. [DOI] [PubMed] [Google Scholar]
- 4. Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Elashoff B, Baron H, Yee J, Valantine HA. Gene‐expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med Massachussetts Medical Society 2010; 362: 1890–1900. [DOI] [PubMed] [Google Scholar]
- 5. Heidt S, San Segundo D, Shankar S, Mittal S, Muthusamy ASR, Friend PJ, Fuggle SV, Wood KJ. Peripheral blood sampling for the detection of allograft rejection: biomarker identification and validation. Transplantation 2011; 92: 1–9. [DOI] [PubMed] [Google Scholar]
- 6. Patel PC, Hill DA, Ayers CR, Lavingia B, Kaiser P, Dyer AK, Barnes AP, Thibodeau JT, Mishkin JD, Mammen PPA, Markham DW, Stastny P, Ring WS, De Lemos JA, Drazner MH. High‐sensitivity cardiac troponin I assay to screen for acute rejection in patients with heart transplant. Circ Heart Fail Lippincott Williams and Wilkins 2014; 7: 463–469. [DOI] [PubMed] [Google Scholar]
- 7. Bader FM, Rogers RK, Kfoury AG, Gilbert EM, Horne BD, Stehlik J, Renlund DG. Time‐dependent changes in B‐type natriuretic peptide after heart transplantation: correlation with allograft rejection and function. Congest Heart Fail John Wiley & Sons, Ltd 2009; 15: 63–67. [DOI] [PubMed] [Google Scholar]
- 8. Martinez‐Dolz L, Almenar L, Moro J, Agüero J, Hervas I, Rueda J, Rivera M, Arnau MA, Mateo A, Salvador A. Prognostic value of brain natriuretic peptide in heart transplant patients. J Heart Lung Transplant 2007; 26: 986–991. [DOI] [PubMed] [Google Scholar]
- 9. De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, Neff NF, Okamoto J, Bernstein D, Weisshaar D, Quake SR, Khush KK. Circulating cell‐free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med American Association for the Advancement of Science 2014; 6: 241ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grskovic M, Hiller DJ, Eubank LA, Sninsky JJ, Christopherson C, Collins JP, Thompson K, Song M, Wang YS, Ross D, Nelles MJ, Yee JP, Wilber JC, Crespo‐Leiro MG, Scott SL, Woodward RN. Validation of a clinical‐grade assay to measure donor‐derived cell‐free DNA in solid organ transplant recipients. J Mol Diagnostics Elsevier BV 2016; 18: 890–902. [DOI] [PubMed] [Google Scholar]
- 11. Richmond ME, Kindel SJ, Schroder JN, Deshpande SR, Bichell DP, Wigger MA, Knecht KR, Pahl E, Gaglianello NA, Simpson PM, Mahle WT, Mitchell AT, Zangwill SD, Mitchell ME. Increase in donor fraction cell‐free DNA correlates with cellular and antibody mediated rejection (ACR/AMR) in adult and pediatric heart transplant recipients: DNA based transplant rejection test (DTRT)—a prospective blinded multicenter NIH/NHLBI funded clinical study. J Hear Lung Transplant Elsevier BV 2019; 38: S50. [Google Scholar]
- 12. Salzano A, Israr MZ, Garcia DF, Middleton L, D'Assante R, Marra AM, Arcopinto M, Yazaki Y, Bernieh D, Cassambai S, Page K, Rengo G, Bossone E, Cittadini A, Shaw JA, Suzuki T. Circulating cell‐free DNA levels are associated with adverse outcomes in heart failure: testing liquid biopsy in heart failure. Eur J Prev Cardiol 2020; 2047487320912375. 10.1177/2047487320912375 [DOI] [PubMed] [Google Scholar]
- 13. Richmond ME, Zangwill SD, Kindel SJ, Deshpande SR, Schroder JN, Bichell DP, Knecht KR, Mahle WT, Wigger MA, Gaglianello NA, Pahl E, Simpson PM, Dasgupta M, North PE, Hidestrand M, Tomita‐Mitchell A, Mitchell ME. Donor fraction cell‐free DNA and rejection in adult and pediatric heart transplantation. J Hear Lung Transplant Elsevier USA 2020; 39: 454–463. [DOI] [PubMed] [Google Scholar]
- 14. Khush KK, Patel J, Pinney S, Kao A, Alharethi R, DePasquale E, Ewald G, Berman P, Kanwar M, Hiller D, Yee JP. Noninvasive detection of graft injury after heart transplant using donor‐derived cell‐free DNA: a prospective multicenter study. Am J Transplant 2019; 19: 2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin Chem American Association for Clinical Chemistry Inc 2015: 56–63. [DOI] [PubMed] [Google Scholar]
- 16. Mas VR, Dumur CI, Scian MJ, Gehrau RC, Maluf DG. MicroRNAs as biomarkers in solid organ transplantation. Am J Transplant. NIH Public Access 2013: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorenzen JM, Volkmann I, Fiedler J, Schmidt M, Scheffner I, Haller H, Gwinner W, Thum T. Urinary miR‐210 as a mediator of acute T‐cell mediated rejection in renal allograft recipients. Am J Transplant 2011; 11: 2221–2227. [DOI] [PubMed] [Google Scholar]
- 18. Betts G, Shankar S, Sherston S, Friend P, Wood KJ. Examination of serum miRNA levels in kidney transplant recipients with acute rejection. Transplantation 2014; 97: e28–e30. [DOI] [PubMed] [Google Scholar]
- 19. Wilflingseder J, Regele H, Perco P, Kainz A, Soleiman A, Mühlbacher F, Mayer B, Oberbauer R. MiRNA profiling discriminates types of rejection and injury in human renal allografts. Transplantation Lippincott Williams and Wilkins 2013; 95: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu J, Wang Z, Tan CJ, Liao BY, Zhang X, Xu M, Dai Z, Qiu SJ, Huang XW, Sun J, Sun QM, He YF, Song K, Pan Q, Wu Y, Fan J, Zhou J. Plasma microRNA, a potential biomarker for acute rejection after liver transplantation. Transplantation 2013; 95: 991–999. [DOI] [PubMed] [Google Scholar]
- 21. Akat KM, Moore‐McGriff D, Morozov P, Brown M, Gogakos T, Da Rosa JC, Mihailovic A, Sauer M, Ji R, Ramarathnam A, Totary‐Jain H, Williams Z, Tuschl T, Schulze PC. Comparative RNA‐sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc Natl Acad Sci U S A National Academy of Sciences 2014; 111: 11151–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kennel PJ, Schulze PC. Novel biomarker approaches for managing patients with cardiac transplantation. Curr Heart Fail Rep Current Science Inc 2015: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei L, Wang M, Qu X, Mah A, Xiong X, Harris AGC, Phillips LK, Martinez OM, Krams SM. Differential expression of microRNAs during allograft rejection. Am J Transplant 2012; 12: 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Huyen JPD, Tible M, Gay A, Guillemain R, Aubert O, Varnous S, Iserin F, Rouvier P, François A, Vernerey D, Loyer X, Leprince P, Empana JP, Bruneval P, Loupy A, Jouven X. MicroRNAs as non‐invasive biomarkers of heart transplant rejection. Eur Heart J Oxford University Press 2014; 35: 3194–3202. [DOI] [PubMed] [Google Scholar]
- 25. Sukma Dewi I, Torngren K, Gidlöf O, Kornhall B, Öhman J. Altered serum miRNA profiles during acute rejection after heart transplantation: potential for non‐invasive allograft surveillance. J Heart Lung Transplant 2013; 32: 463–466. [DOI] [PubMed] [Google Scholar]
- 26. Hafner M, Renwick N, Farazi TA, Mihailović A, Pena JTG, Tuschl T. Barcoded cDNA library preparation for small RNA profiling by next‐generation sequencing. Methods 2012; 58: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farazi TA, Brown M, Morozov P, ten Hoeve JJ, Ben‐Dov IZ, Hovestadt V, Hafner M, Renwick N, Mihailović A, Wessels LFA, Tuschl T. Bioinformatic analysis of barcoded cDNA libraries for small RNA profiling by next‐generation sequencing. Methods Academic Press 2012; 58: 171–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown M, Suryawanshi H, Hafner M, Farazi TA, Tuschl T. Mammalian miRNA curation through next‐generation sequencing. Front Genet Frontiers 2013; 4: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin M. Cutadapt removes adapter sequences from high‐throughput sequencing reads. EMBnet Journal EMBnet Stichting 2011; 17: 10. [Google Scholar]
- 30. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory‐efficient alignment of short DNA sequences to the human genome. Genome Biol BioMed Central 2009; 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedländer MR, Chen W, Adamidi C, Maaskola J, Einspanier R, Knespel S, Rajewsky N. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol 2008; 26: 407–415. [DOI] [PubMed] [Google Scholar]
- 32. Miga KH, Newton Y, Jain M, Altemose N, Willard HF, Kent EJ. Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res Cold Spring Harbor Laboratory Press 2014; 24: 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kozomara A, Griffiths‐Jones S. miRBase: integrating microRNA annotation and deep‐sequencing data. Nucleic Acids Res 2011; 39: D152–D157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kozomara A, Griffiths‐Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 2014; 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Griffiths‐Jones S. The microRNA registry. Nucleic Acids Res 2004; 32: 109D–1111D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Griffiths‐Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 2006; 34: D140–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Griffiths‐Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2007; 36: D154–D158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol BioMed Central Ltd. 2014; 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JYH, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. GBC 2004; 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57: 289–300. [Google Scholar]
- 41. Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM, Voinea SC. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cell MDPI AG 2020; 9: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DÍaz I, Calderón‐Sánchez E, Del Toro R, Ávila‐Médina J, De Rojas‐De Pedro ES, Domínguez‐Rodríguez A, Rosado JA, Hmadcha A, Ordóñez A, Smani T. miR‐125a, miR‐139 and miR‐324 contribute to urocortin protection against myocardial ischemia‐reperfusion injury. Sci Rep Nature Publishing Group 2017; 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hou T, Liao J, Zhang C, Sun C, Li X, Wang G. Elevated expression of miR‐146, miR‐139 and miR‐340 involved in regulating Th1/Th2 balance with acute exposure of fine particulate matter in mice. Int Immunopharmacol Elsevier B.V. 2018; 54: 68–77. [DOI] [PubMed] [Google Scholar]
- 44. Horváth M, Horváthová V, Hájek P, Štěchovský C, Honěk J, Šenolt L, Veselka J. MicroRNA‐331 and microRNA‐151‐3p as biomarkers in patients with ST‐segment elevation myocardial infarction. Sci Rep Nature Research 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen F, Ye X, Jiang H, Zhu G, Miao S. MicroRNA‐151 attenuates apoptosis of endothelial cells induced by oxidized low‐density lipoprotein by targeting interleukin‐17A (IL‐17A). J Cardiovasc Transl Res Springer 2020; 25: 1–9. [DOI] [PubMed] [Google Scholar]
- 46. Ouyang M, Lu J, Ding Q, Qin T, Peng C, Guo Q. Knockdown of long non‐coding RNA PVT1 protects human AC16 cardiomyocytes from hypoxia/reoxygenation‐induced apoptosis and autophagy by regulating miR‐186/Beclin‐1 axis. Gene Elsevier BV 2020; 754. [DOI] [PubMed] [Google Scholar]
- 47. Xu H, Li J, Zhao Y, Liu D. TNFα‐induced downregulation of microRNA‐186 contributes to apoptosis in rat primary cardiomyocytes. Immunobiology Elsevier GmbH 2017; 222: 778–784. [DOI] [PubMed] [Google Scholar]
- 48. Palleschi A, Gaudioso G, Edefonti V, Musso V, Terrasi A, Ambrogi F, Franzi S, Rosso L, Tarsia P, Morlacchi LC, Ferrero S, Nosotti M, Vaira V. Bronchoalveolar lavage‐microRNAs are potential novel biomarkers of outcome after lung transplantation. Transplant Direct Wolters Kluwer Health 2020; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borštnar Š, Večerić‐Haler Ž, Boštjančič E, Tkalec ŽP, Kovač D, Lindič J, Kojc N. Uromodulin and microRNAs in kidney transplantation—association with kidney graft function. Int J Mol Sci MDPI AG 2020; 21: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deng MC. The AlloMap™ genomic biomarker story: 10 years after. Clin Transplant Blackwell Publishing Ltd 2017; 31. [DOI] [PubMed] [Google Scholar]
- 51. Sukma Dewi I, Hollander Z, Lam KK, McManus JW, Tebbutt SJ, Ng RT, Keown PA, McMaster RW, McManus BM, Gidlöf O, Öhman J. Association of serum miR‐142‐3p and miR‐101‐3p levels with acute cellular rejection after heart transplantation. PLoS One Public Library of Science 2017; 12: e0170842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) Pre ACR vs TX. (B) At ACR vs. TX. (C) Post ACR vs. TX.
Figure S2. (A) Pre AMR vs. TX. (B) At AMR vs. TX. (C) Post AMR vs. TX.
Figure S3. (A) Pre AMR + ACR vs. TX. (B) At AMR + ACR vs. TX. (C) Post AMR + ACR vs. TX.
Figure S4. (A) ACR patterns. (B) AMR patterns. (C) AMR + ACR patterns.
Figure S5. (A) preACR. (B) at ACR. (C) post ACR.
Figure S6. (A) Pre AMR. (B) At AMR. (C) Post AMR.
Figure S7. (A) pre ACR + AMR. (B) at ACR + AMR. (C) Post AMR + ACR.
Figure S8. (A) ROC curve, all sigmirs AMR + ACR before rejection. (B) ROC curve, all sigmirs AMR + ACR before rejection.
Figure S9. Significant miRNA abundances.
Table S1. 21 multiplexing adapters used for the miRNA sequencing.
Table S2. Adapter Id of each group across the five sequencing pools.
Table S3. Details of the number of reads in the source files, with number of reads discarded by Illumina quality control and the net count of reads.
Table S4. Adapter trimming before demultiplexing.
Table S5. Demultiplexing process, discarding reads that could not be assigned with certainty to a group.
Table S6. Quality control process removing reads shorter than 17 bps, reads with a Phred score lower than 10.
Table S7. Reads mapped to the hg38 human genome reference allowing for 1 mismatch.
Table S8. Reads mapped to the miRNA human reference allowing for 2 mismatches.
Table S9. Significantly differentially expressed miRNAs before, during and after both rejection types. Down regulated in blue, up‐regulated in yellow. Greyed out where non‐significant (P > = 0.05).
Data S1. Supplemental methods.
Data S2. Supplemental results.


