This systematic review and meta-analysis evaluates stereotactic radiosurgery for brainstem metastasis in the context of prospective trials of stereotactic radiosurgery or molecular therapy for nonbrainstem brain metastases.
Key Points
Question
Is stereotactic radiosurgery (SRS) safe and effective for the treatment of brainstem metastasis (BSM), and how does this approach compare with SRS or targeted therapy for nonbrainstem brain metastasis (BM)?
Findings
This systematic review and meta-analysis of 32 studies comprising 1446 patients found associations with high local control (86%), high therapeutic ratio of symptom relief and tumor response (50%-60%) when compared with targeted therapy (17%-56%), rare significant toxic effects (2.4%), and rare death from BSM progression (2.7%). The neurologic death rate in patients with BSM who were treated with SRS was equivalent to that in patients with BM who were treated with SRS on prospective trials.
Meaning
Given the risks of acute morbidity or death from BSM growth in the context of the efficacy and safety of SRS for BSM, future trials of targeted therapy or immunotherapy for BM should consider including patients with BSM after treatment with SRS.
Abstract
Importance
Owing to the proximity to critical neurologic structures, treatment options for brainstem metastases (BSM) are limited, and BSM growth can cause acute morbidity or death. Stereotactic radiosurgery (SRS) is the only local therapy for BSM, but efficacy and safety of this approach are incompletely understood because patients with BSM are excluded from most clinical trials.
Objective
To perform a systematic review and comparative meta-analysis of SRS studies for BSM in the context of prospective trials of SRS or molecular therapy for nonbrainstem brain metastases (BM).
Data Sources
A comprehensive search of Pubmed/MEDLINE and Embase was performed on December 6, 2019.
Study Selection
English-language studies of SRS for BSM with at least 10 patients and reporting 1 or more outcomes of interest were included. Duplicate studies or studies with overlapping data sets were excluded. Studies were independently evaluated by 2 reviewers, and discrepancies were resolved by consensus. A total of 32 retrospective studies published between 1999 and 2019 were included in the analysis.
Data Extraction and Synthesis
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed to identify studies. Study quality was assessed using Methodological Index for Non-Randomized Studies criteria. Fixed and random-effects meta-analyses and meta-regressions were performed for the outcomes of interest.
Main Outcomes and Measures
Primary study outcomes included 1-year and 2-year local control and overall survival, objective response rate, symptom response rate, neurological death rate, and rate of grade 3 to 5 toxic effects as described in Common Terminology Criteria for Adverse Events, version 4.0.
Results
The 32 retrospective studies included in the analysis comprised 1446 patients with 1590 BSM that were treated with SRS (median [range] dose, 16 [11-39] Gy; median [range] fractions, 1 [1-13]). Local control at 1 year was 86% (95% CI, 83%-88%; I2 = 38%) in 1410 patients across 31 studies, objective response rate was 59% (95% CI, 47%-71%; I2 = 88%) in 642 patients across 17 studies, and symptom improvement was 55% (95% CI, 47%-63%; I2 = 41%) in 323 patients across 13 studies. Deaths from BSM progression after SRS were rare (19 of 703 [2.7%] deaths across 19 studies), and the neurologic death rate in patients with BSM (24%; 95% CI, 19%-31%; I2 = 62%) was equivalent to the neurologic death rate in patients with BM who were treated on prospective trials. The rate of treatment-related grade 3 to 5 toxic effects was 2.4% (95% CI, 1.5%-3.7%; I2 = 33%) in 1421 patients across 31 studies. These results compared favorably to trials of targeted or immunotherapy for BM, which had a wide objective response rate range from 17% to 56%.
Conclusions and Relevance
Results of this systematic review and meta-analysis show that SRS for BSM was associated with effectiveness and safety and was comparable to SRS for nonbrainstem BM, suggesting that patients with BSM should be eligible for clinical trials of SRS. In this analysis, patients treated with SRS for BSM rarely died from BSM progression and often experienced symptomatic improvement. Given the apparent safety and efficacy of SRS for BSM in the context of acute morbidity or death from BSM growth, consideration of SRS at the time of enrollment on emerging trials of targeted therapy for BM should be considered.
Introduction
Brainstem metastases (BSM) represent 4% to 7% of all secondary intracranial tumors1,2,3,4 but are not amenable to resection owing to a high risk of morbidity or mortality from damage to critical neurologic structures. Stereotactic radiosurgery (SRS) is an effective treatment for nonbrainstem brain metastases (BM) that does not cause the neurocognitive deficits of whole-brain radiotherapy (WBRT).5 Nearly all prospective trials of SRS for BM have excluded patients with BSM.5,6,7,8,9,10 Thus, reports of SRS for BSM are limited to small retrospective series,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43 and the efficacy and safety of SRS for BSM are incompletely understood. To address this unmet need, we performed a quantitative meta-analysis of SRS for BSM to estimate pooled measures of efficacy and safety in the context of prospective trials of SRS or molecular therapy for nonbrainstem BM.
Methods
A comprehensive search of English-language literature was performed in PubMed/MEDLINE and Embase with the search terms (srs OR stereotactic OR radiosurgery OR knife) AND (brainstem OR medulla OR pons OR pontine OR medullary) AND (metastasis/exp OR metastasis OR metastases/exp OR metastases OR metastatic) on December 6, 2019. Inclusion criteria were: (1) reports on SRS or stereotactic radiotherapy of BSM including (2) at least 1 outcome of local control (LC), 1-year or 2-year overall survival (OS), neurologic death, and/or toxic effect that were (3) written in English language and were (4) composed of at least 10 patients. Case reports were excluded, as were studies in which characteristics and outcomes of BSM could not be disaggregated from other tumor types, studies that focused on technical or other nonclinical aspects of SRS, and studies that contained duplicate reports of overlapping data sets. Study authors and institutions were examined to determine any possibility of data set overlap. No attempt was made to contact study authors to obtain missing data. Study quality was assessed using Methodological Index for Non-randomized Studies criteria44 (Supplement 1), and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines were followed.45 Criteria used within studies for local failure, partial response, symptom response, and neurologic death were collected (Supplement 1). Most commonly, Response Evaluation Criteria in Solid Tumours or similar criteria were used to determine local failure and objective response, and attribution of neurologic death was most commonly based on previously established and uniform criteria.46 Further details on the search and review process for non-BSM SRS trials and targeted/molecular therapy trials, as well as a qualitative assessment of study quality, are reported in eMethods in Supplement 2. Institutional review board approval was not required.
Primary outcomes included were 1-year LC, objective response rate (ORR; defined as partial response or complete response), symptom response rate, 1-year and 2-year OS, neurologic death, and grade 3 to 5 toxic effects as described in Common Terminology Criteria for Adverse Events, version 4.0.47 Quantitative meta-analysis and meta-regression were performed in R software, version 3.6.2 (R Foundation for Statistical Computing). Fixed-effect and random-effect pooled binomial proportions were estimated using a random intercept logistic regression model via the metaprop function in the meta package, using a logit transformation and continuity correction of 0.5 and other default settings. Heterogeneity was assessed using I2 and τ, as estimated by maximum likelihood, and tested using the Wald test. The I2 cutoffs of 25%, 50%, and 75% were used to distinguish low, low-moderate, moderate-high, and high heterogeneity.48 When significant heterogeneity was present (I2 >50%), outlier studies were identified using Cook distance, and meta-analysis was performed with and without outlier studies. When any heterogeneity was present (I2 >0%), the results of the random-effects model were reported in the text (eMethods in Supplement 2). Meta-regression was undertaken using the metareg function with default settings and a linear random/mixed effects model with a moderator equal to the variable of interest. Publication bias was assessed using funnel plots and Egger regression test (eMethods in Supplement 2). Unless otherwise specified, all statistical tests were 2-tailed, and P ≤ .05 was considered significant. P values in the context of heterogeneity measures (I2 and τ) were based on Wald tests for heterogeneity, unless otherwise specified.
Results
Characteristics of Included Studies
We identified a total of 32 retrospective studies and no prospective studies published between 1999 and 2019 that reported outcomes of SRS for 1590 BSM in 1446 patients (eFigure 1 in Supplement 2 and Supplement 3). The characteristics and aggregated outcomes for the included studies are reported in Tables 1, 2, and 3, in which 31 studies reflect a mixed population of BSM in patients (n = 1409) from lung (n = 633 [44.9%]), breast (n = 286 [20.2%]), melanoma (n = 141 [10.0%]), and kidney or genitourinary (n = 106 [7.5%]) cancers. The median age of all included patients was 58 years (patient-level range, 22-92 years; study-level interquartile range [IQR], 56.7-60.3 years), median Karnofsky Performance Status score was 80 (patient-level range, 20-100; study-level IQR, 80-90), and median clinical follow-up was 8.5 months (study-level IQR, 5.7-11.0 months). Reported BSM were small (median volume, 0.40 cm3 [patient-level range, 0.0025-24.88 cm3; study-level IQR, 0.20-1.05 cm3]) among the total population (Table 2) and were treated with SRS using Gamma Knife (n = 1067 [73.8%]), linear accelerator (n = 248 [17.2%]), or Cyberknife (n = 131 [9%]) (Table 3). There was no significant evidence of publication bias (eFigure 2 in Supplement 2).
Table 1. Patient Characteristics.
| Characteristic | No. (%)a |
|---|---|
| Patients/BSM (n = 32 studies) | 1446/1590 |
| Median No. patients/BSM (range) | 36.5/40.5 (14-200/19-222) |
| Sex, median (range), % | |
| Male | 47.6 (41.7-61.3) |
| Female | 52.4 (38.7-58.3) |
| Median age (range), y | 58 (22-92) |
| Median KPS score (range) | 80 (20-100) |
| RPA (n = 15 studies; 715 patients) | |
| 1 | 93 (13.0) |
| 2 | 522 (73.0) |
| 3 | 100 (14.0) |
| Median GPA (range) (n = 3 studies; 256 patients) | 1.5 (0.0-4.0) |
| Median BSBM (range) (n = 3 studies; 115 patients) | 2 (0-3) |
| Median imaging follow-up (range), mo (n = 13 studies; 615 patients) | 7.6 (4.8-15.3) |
| Median clinical follow-up (range), mo (n = 27 studies; 1257 patients) | 8.5 (3.2-16.8) |
| Primary cancer site (n = 31 studies; 1409 patients) | |
| Lung | 633 (44.9) |
| Median NSCLC (range), % (n = 17 studies) | 90.0 (79.2-100) |
| Breast | 286 (20.2) |
| Melanoma | 141 (10.0) |
| Renal/genitourinary | 106 (7.5) |
| Gastrointestinal | 63 (4.5) |
| Other/unknown | 110 (7.8) |
Abbreviations: BSBM, Basic Score for Brain Metastases; BSM, brainstem metastases; GPA, graded prognostic assessment; KPS, Karnofsky Performance Status; NSCLC, non–small cell lung cancer; RPA, recursive partitioning analysis.
Unless specified, values in parentheses represent the full range reported across studies.
Table 2. Brainstem Metastases (BSM) and Treatment Characteristics.
| Characteristic | No. (%)a |
|---|---|
| BSM location (n = 30 studies; 1495 lesions) | |
| Pons | 934 (62.5) |
| Midbrain | 342 (22.9) |
| Medulla | 155 (10.4) |
| CPA or BS adjacent | 25 (1.7) |
| Solitary brain metastasis (n = 29 studies; 1335 patients) | 312 (23.4) |
| % With solitary BM, median (IQR) | 24.3 (18.3-34.2) |
| Patients with extracranial metastasis (n = 9 studies; 586 patients) | 370 (63.1) |
| % With extracranial metastasis, median (IQR) | 70.8 (56.0-78.6) |
| Symptomatic BSM (n = 22 studies; 1104 patients) | 546 (49.5) |
| % Symptomatic, median (IQR) | 46.8 (33.5-71.1) |
| Prior or combined WBRT (n = 30 studies; 1392 patients) | 609 (43.8) |
| % WBRT, median (IQR) | 43.8 (30.4-65.6) |
| WBRT + SBRT as boost (n = 28 studies; 1392 patients) | 67 (4.8) |
| Chemotherapy (n = 6 studies; 366 patients) | 198 (54.1) |
| Immunotherapy (n = 2 studies; 63 patients) | 12 (19.0) |
| BSM volume, median (range), cc (n = 31 studies; 1409 patients) | 0.40 (0.0025-24.88) |
Abbreviations: BM, brain metastases; BS, brainstem; cc, cubic centimeter (mL); CPA, cerebello-pontine angle; IQR, interquartile range; SBRT, stereotactic body radiation therapy; WBRT, whole-brain radiotherapy.
Unless specified, values in parentheses represent the full range reported across studies.
Table 3. Radiation Therapy Modality, Dose, and Outcomes.
| Radiation therapy modality and dose | Median No. (range)a |
|---|---|
| Gamma Knife (n = 21 studies; 1067 patients) | |
| Prescribed or marginal dose, Gy | 16 (6-30) |
| Prescribed BED10, Gy | 41.6 (29.9-60.0) |
| Isodose, % | 50 (26-98) |
| Max dose, Gy (n = 7 studies) | 31.4 (23.5-33.0) |
| Max BED10, Gy | 134.4 (78.7-237.6) |
| Fractions | 1 (1-1) |
| Linear accelerator (n = 7 studies; 248 patients) | |
| Prescribed or marginal dose, Gy | 13.7 (11.1-39.0) |
| Prescribed BED10, Gy | 32.5 (23.6-50.7) |
| Isodose, % | 85 (73-90) |
| Max dose, Gy (n = 2 studies) | 14.5 (12.7-16.2) |
| Max BED10, Gy | 42.4 (27.9-49.5) |
| Fractions | 1 (1-13) |
| Cyberknife (n = 4 studies; 131 patients) | |
| Prescribed or marginal dose, Gy | 17.5 (16-24) |
| Prescribed BED10, Gy | 43.1 (28.8-50.4) |
| Isodose, % | 80 (75-80) |
| Max dose, Gy (n = 2 studies) | 23.3 (20.3-26.3) |
| Max BED10, Gy | 61.1 (40.0-95.5) |
| Fractions | 1.5 (1-5) |
| Outcomes | |
| Symptom improvement, No. (%) (n = 13 studies; 323 symptomatic patients) | 323 (63.0) |
| PR/CR, No. (%) (n = 17 studies; 642 patients evaluated) | 391 (60.9) |
| CR (n = 15 studies; 438 patients evaluated) | 67 (15.3) |
| PR (n = 15 studies; 438 patients evaluated) | 185 (42.2) |
| SD (n = 17 studies; 642 patients evaluated) | 175 (27.3) |
| 1-y/crude LC, median (IQR) (n = 31 studies; 1410 patients) | 87.5 (79.5-90.8) |
| 1-y OS, median (IQR) (n = 27 studies; 1254 patients) | 32.0 (30.9-40.0) |
| 2-y OS, median (IQR) (n = 22 studies; 959 patients) | 13.0 (9.3-16.0) |
| Cause of death, No. (%) | |
| Brainstem lesion(s) (n = 19 studies; 703 deaths) | 19 (2.7) |
| Distal brain lesion(s) (n = 17 studies; 643 deaths) | 120 (18.7) |
| % Death from distal brain lesion(s), median (IQR) | 23.1 (16.7-29.3) |
| Combined neurologic death (n = 17 studies; 643 deaths) | 139 (21.6) |
| Combined neurologic death, median (IQR) | 27.8 (18.9-32.1) |
| Death from systemic disease (n = 17 studies; 643 deaths) | 441 (68.6) |
| % Death from systemic disease, median (IQR) | 66.7 (45.5-79.1) |
| ARE/RN, No. (%) (n = 31 studies; 1386 patients) | 21 (1.5) |
| Symptomatic ARE (n = 30 studies; 1361 patients) | 15 (1.1) |
| Any symptomatic toxic effects, No. (%) (n = 30 studies, 1397 patients) | 78 (5.6) |
| Grade ≥3 toxic effect (n = 31 studies; 1421 patients) | 37 (2.6) |
| Grade 5 toxic effect (n = 31 studies; 1421 patients) | 4 (0.3) |
Abbreviations: ARE/RN, adverse radiation effect/radionecrosis; BED10, biological effective dose with α/β = 10; CR, complete response; IQR, interquartile range; LC, local control; OS, overall survival; PR, partial response; SD, stable disease.
Unless specified, values in parentheses represent the full range reported across studies.
Treatment Response and BSM Control
The 1-year LC was 86% (95% CI, 83%-88%; I2 = 38%; P = .12 for heterogeneity; Figure 1A11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43) in 1410 patients across 31 studies, the ORR was 59% (95% CI, 47%-71%; I2 = 88%; P < .001; Figure 1B12,15,17,18,19,20,21,23,26,28,29,31,36,37,38,40,41,42) in 642 patients across 17 studies (eFigure 3A in Supplement 2), and the rate of symptom improvement was 55% (95% CI, 47%-63%; I2 = 41%; P = .06; Figure 1C12,13,23,26,29,31,32,35,37,38,40,41,42) in 323 patients across 13 studies. Prior or concurrent WBRT (coefficient, −1.90; QM = 5.26; P = .02), or symptomatic BSM (coefficient, −2.43; QM = 5.11; P = .02) were associated with decreased ORR. Neither SRS dose nor SRS modality was predictive for local control (eFigure 4 in Supplement 2).
Figure 1. Forest Plots and Pooled Outcomes for Stereotactic Radiosurgery for Treatment of Brainstem Metastases.
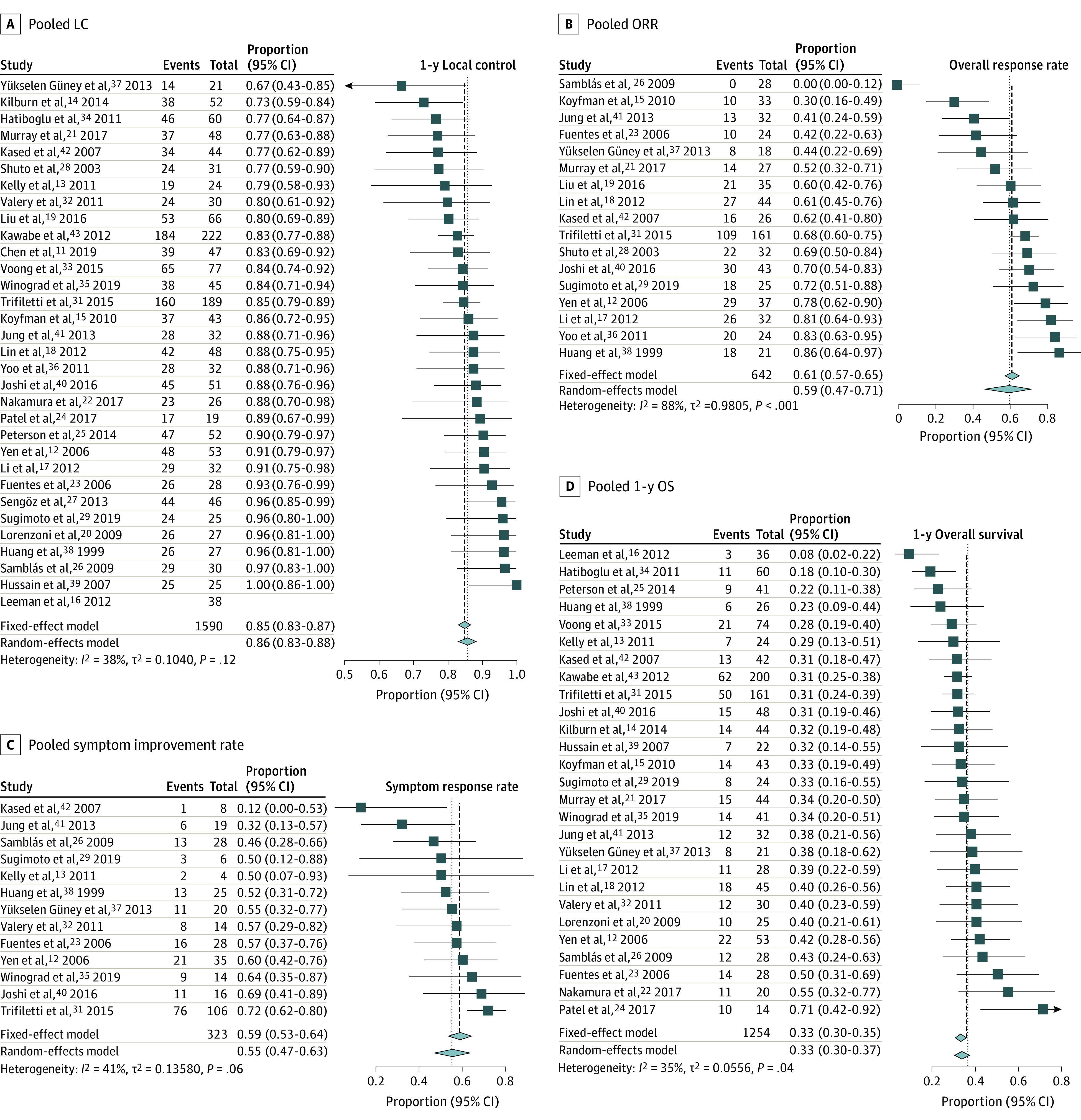
A, Pooled local control (LC) for studies reporting this end point. The I2 indicates low-to-moderate heterogeneity. B, Pooled objective response rate (ORR) for studies reporting this metric. The I2 indicates significant heterogeneity. Outlier analysis using Cook distance (eFigure 3 in Supplement 2) identified Samblás et al26 and Koyfman et al15 as outlier studies. Exclusion of these studies reduced heterogeneity but did not result in substantial change in the pooled ORR (65%; 95% CI, 58%-72%). C, Pooled symptom improvement rates based on narrative reports by authors of each study. The I2 indicates low-to-moderate heterogeneity. D, Pooled 1-year overall survival (OS).
Overall Survival, Death From BSM, and Neurologic Death
The 1-year OS was 33% (95% CI, 30%-37%; I2 = 35%; P = .04; Figure 1D12,13,14,15,16,17,18,20,21,22,23,24,25,26,29,31,32,33,34,35,37,38,39,40,41,42,43) in 1254 patients across 27 studies, and the 2-year OS was 13% (95% CI, 11%-16%; I2 = 30%; P = .10; eFigure 8 in Supplement 2) in 959 patients across 22 studies. Death attributed to BSM progression was rare (19 of 703 [2.7%] deaths across 19 studies). The rate of neurologic death was 24% (95% CI, 19%-31%; I2 = 62%; P < .001; Figure 2A12,13,14,16,18,19,22,23,26,32,34,36,38,39,40,41,42,43 and eFigure 3B in Supplement 2), accounting for 643 deaths across 17 studies. On meta-regression, symptomatic BSM (coefficient, 1.33; QM = 4.36; P = .04) was associated with greater rates of neurologic death (eTable 1 in Supplement 2).
Figure 2. Forest Plots and Pooled Rates of Toxic Effects and Neurologic Death for Stereotactic Radiosurgery of Brainstem Metastases.
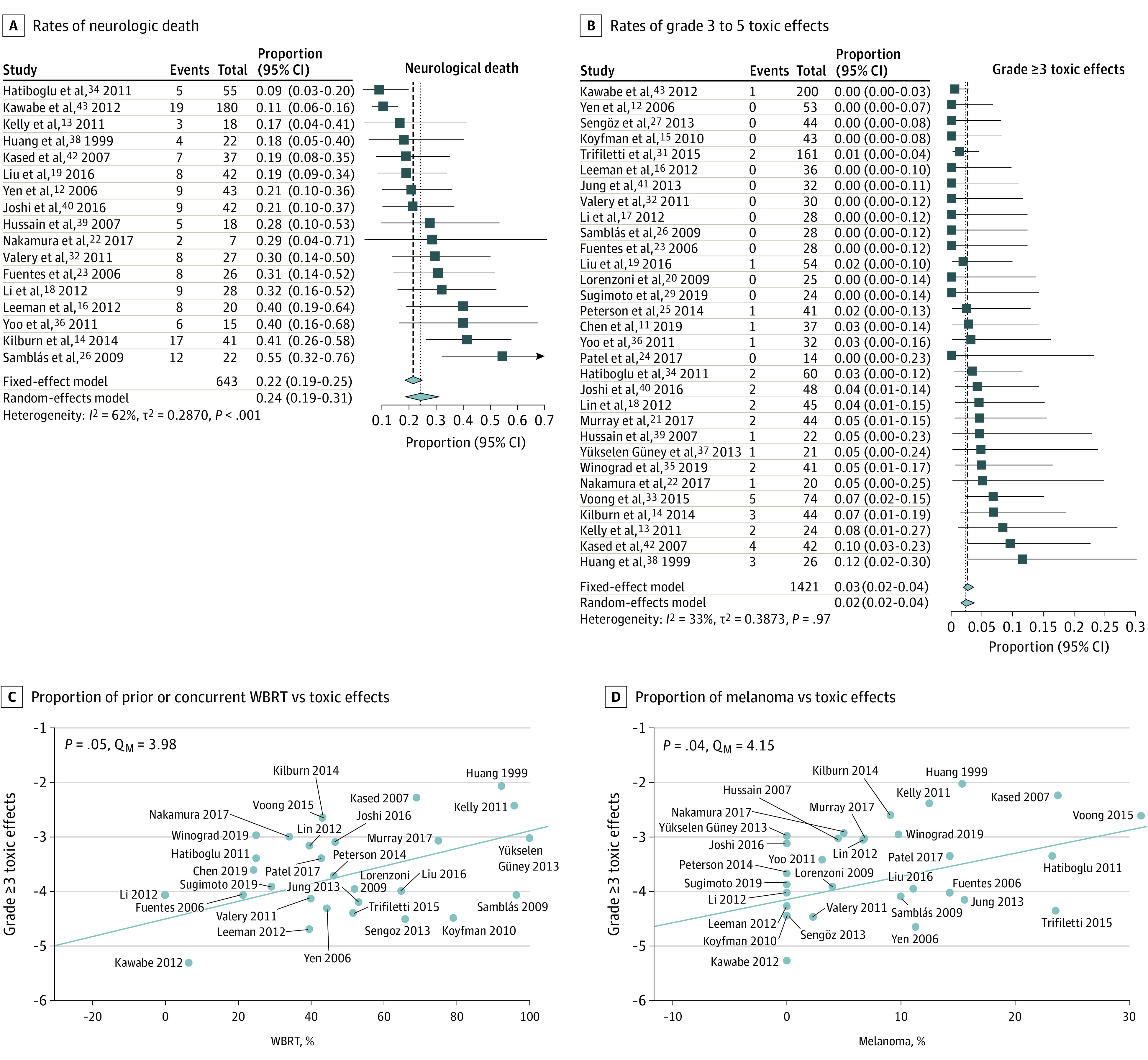
A, Forest plots and pooled estimates of rates of neurologic death. The I2 indicates moderate-to-high heterogeneity. Outlier analysis (eFigure 3 in Supplement 2) using Cook distance identified Samblás et al26 and Kawabe et al43 as outlier studies. Exclusion of these studies reduced heterogeneity but did not alter the pooled measure (25%; 95% CI, 20%-30%). B, Forest plots and pooled estimates of the rates of grade 3 to 5 toxic effects. C, Meta-regression of the proportion of prior or concurrent whole-brain radiotherapy (WBRT) vs rate of grade 3 to 5 toxic effects on a study-level basis is shown and indicates a statistically significant positive association between the rate of WBRT and rate of significant toxic effects. D, Meta-regression of proportion of melanoma vs rate of grade 3 to 5 toxic effects on a study-level basis is shown and indicates a greater proportion of patients with melanoma to be positively associated with greater grade 3 to 5 toxic effects within studies. For C and D, values on the y axes represent the logit transformed treatment effect based on a random-effects model. P values were obtained from a univariate linear random/mixed effects model with moderator equal to the variable of interest.
Treatment-Related Toxic Effects
A total of 78 (5.6%) patients experienced symptomatic toxic effects of any grade after SRS for BSM, and the rate of grade 3 to 5 toxic effects was 2.4% (95% CI, 1.5%-3.7%; I2 = 33%; P = .97; Figure 2B11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,29,31,32,33,34,35,36,37,38,39,40,41,42,43) in 1421 patients across 31 studies. The rate of adverse radiation effect/radionecrosis49 was 1.5% (21 of 1386 patients across 31 studies), and the rate of symptomatic radiation effect was 1.1% (15 of 1361 patients across 30 studies). A full accounting of all reported symptomatic and asymptomatic treatment complications (90 of 1421 [6.2%] patients; Supplement 4) revealed a substantial proportion occurred in patients with melanoma BSM (7 of 21 [33.3%] patients with known histological results vs 141 of 1409 [10.0%] in the overall cohort; Fisher exact test, P = .003) or renal cell carcinoma (RCC; 3 of 21 [14.3%] patients with known histological results vs 106 of 1409 [7.5%] in the overall cohort; Fisher exact test, P = .20), both of which are prone to hemorrhage.50 On univariate meta-regression (Figure 2C and D; eTable 1 in Supplement 2), melanoma BSM (coefficient, 4.26; QM = 4.15; P = .04) and WBRT (coefficient, 1.62; QM = 3.98; P = .046) were associated with higher grade 3 to 5 toxic effects, and these effects remained independently prognostic when considered together in multivariable meta-regression (QM = 8.37; P = .02). The median size of BSM in patients who developed toxic effects from SRS was 1.7 cm3 (n = 27; IQR, 0.87-2.22 cm3; range, 0.1-5.58 cm3) compared with 0.4 cm3 in the overall cohort.
Comparison With Published Prospective Trials of SRS for BM
A literature search of clinical trials of SRS for BM yielded 5 prospective randomized trials5,6,8,9,10 and 1 prospective observational trial7 (eTable 2 and eFigure 1B in Supplement 2). Among these studies, 3 explicitly excluded BSM,6,9,10 2 did not specify inclusion or exclusion of BSM,5,8 and 1 explicitly included BSM but did not independently report outcomes for patients with BSM.7 Compared with BSM studies, prospective BM trials had a higher proportion of controlled extracranial disease, had a higher proportion of solitary brain metastasis, and excluded patients with poor performance status (Table 2 and Supplement 4). Nevertheless, the pooled rates of neurologic death between BSM studies and prospective BM trials were equivalent (BM: 22%; 95% CI, 14%-34%; I2 = 91%; P < .01 vs BSM: 24%; 95% CI, 19%-31%; Q = 0.11; between-group P = .74; eFigure 5 in Supplement 2). Moreover, LC (86% vs 81%) and rates of grade 3 to 5 toxic effects (2.4% vs 5.1%) among BSM studies compared favorably with prospective BM trials despite the lower SRS doses used to treat BSM (eFigure 6 in Supplement 2).
Comparison With Published Prospective Trials of Targeted or Immunotherapy for BM
A literature search of clinical trials reporting intracranial activity of targeted or immunotherapy for lung, breast, melanoma, and RCC BM yielded 43 prospective trials reporting intracranial ORR for 2255 patients (Supplement 5). Intracranial ORR was 43% (95% CI, 36%-50%; eFigure 7 in Supplement 2) in 1863 patients across 33 nonnegative studies but ranged from 17% (95% CI, 13%-21%) and 18% (95% CI, 8%-34%) in patients with unselected non–small cell lung cancer (NSCLC) and RCC treated with checkpoint inhibitors, respectively; 36% (95% CI, 25%-49%) and 45% (95% CI, 31%-60%) in patients with ERBB2-positive breast cancer and BRAF-mutant melanoma, respectively; and 56% (95% CI, 49%-63%) and 53% (95% CI, 35%-70%) for patients with ALK-mutated or EGFR-mutated NSCLC, respectively. Among trials with comparative chemotherapy arms, the intracranial ORR was 16.9% (95% CI, 10.3%-26.5%) in 83 patients across 3 studies.
Discussion
In this systematic review and comparative meta-analysis, we identified 1446 patients with 1590 BSM treated with SRS. Results revealed an 86% LC rate, a 2.7% death rate from BSM, a favorable therapeutic ratio (50%-60%), and a low risk of severe toxic effects (2.4%).
Results in the Context of Prior Work
Published studies of SRS for BSM primarily consist of small, retrospective, single-institution reports. Our search of the literature also identified 1 large, multi-institutional, retrospective analysis of 547 patients with BSM,30 which was excluded from the quantitative meta-analysis owing to overlap with other single-institution studies. The present results are consistent with this excluded study, which reported 1-year LC of 82% and a grade 3 to 5 toxic effect rate of 7.4%.
Despite the promising advances in molecular therapy for BM, a mere 8.3% of patients with metastatic cancer are eligible for genome-targeted therapy.51 Although 44% of patients with metastatic cancer are eligible for immunotherapy,52 the low intracranial response rate among patients with unselected NSCLC and RCC suggests SRS should be used to prevent acute morbidity or death from unchecked BSM growth. The brainstem contains life-sustaining neurologic structures but comprises just 2% of brain.53 Thus, timely, efficacious, and safe BSM treatment, which can be accomplished with single-fraction SRS, can be an indispensable bridge to clinical trial enrollment for patients with metastatic cancer. Unfortunately, a search of ClinicalTrials.gov in December 2020 revealed only 2 of 54 active phase 1 to 3 trials investigating SRS for brain metastases included patients with BSM (Supplement 6).
Key Findings
This study supports several moderate quality findings.54 Single-session SRS to a margin dose of 15 to 18 Gy (median, 16 Gy) for BSM smaller than 1 cm3 was associated with high LC, ORR, and symptomatic improvement, and a low risk of toxic effects or death from BSM. Dose reduction or fractionation of SRS could be considered for patients with BSM 1 cm3 or greater, or prior or concurrent WBRT.30,42
Strengths and Limitations
A strength of this study is that these findings are supported by the large sample size and number of studies, the low-to-moderate heterogeneity across studies (median I2 = 35% [range, 30%-62%]), and the corroboration of the findings by a partially independent report.30 Nevertheless, this study has several limitations. First, as a study-level meta-analysis of retrospective data, we were unable to fully identify or account for patient-level confounders and competing risks. Second, only 2 studies of SRS for BSM reported the prevalence of concurrent molecular/immune therapy,11,38 which limited our ability to investigate the efficacy or safety of SRS in combination with systemic therapy for BSM. The qualitative comparison with drug trials was limited to assessment of objective response owing to limited reporting of other outcomes specific to the central nervous system. Third, the studies included were subject to biases inherent to retrospective research, which may have particularly influenced recording of symptom improvement. Thus, the level of evidence we present is moderate.
Conclusions
Results of this systematic review and meta-analysis demonstrate that SRS for BSM has been associated with effectiveness, safety, and improved symptoms. Additionally, BSM size, prior WBRT, and histology results may be associated with treatment-related toxic effects. Regardless, patients with BSM are unlikely to experience severe toxic effects after SRS and have similar rates of neurologic death compared with patients with BM treated with SRS on prospective trials. Future trials incorporating SRS should consider inclusion of patients with BSM.
eTable. MINORS Criteria for Included Studies
eMethods
eFigure 1. PRISMA Flowcharts
eFigure 2. Funnel Plots for Publication Bias
eFigure 3. Heterogeneity After Excluding Outlier BSM Studies
eFigure 4. Outcomes Grouped by Radiation Modality
eFigure 5. Pooled Neurologic Death Rate of BSM Studies and BM Trials
eFigure 6. Pooled Outcomes for BM SRS Trials
eFigure 7. Forest Plot of Intracranial Response Rates for Central-Nervous System Penetrant Targeted and Immunotherapies
eFigure 8. Two-year overall survival for BSM studies
eTable 1. Univariate Meta-Regression P values of Study Level Characteristics in Relation to Outcomes of Interest
eTable 2. Characteristics of Published Trials of SRS for Non-Brainstem Intracranial Metastases
eReferences.
eTable. Study Level Data for Included Studies
eTable. Catalogue of All Reported Complications of Stereotactic Radiosurgery for Brainstem Metastases
eTable. Prospective Trials Reporting Intracranial Response Rates of Targeted and Immuno-Therapy for Brain Metastases
eTable. Currently Active Phase I-III Trials Involving Stereotactic Radiosurgery for Brain Metastases Identified on Clinicaltrials.gov
References
- 1.Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia: a prospective autopsy study. Cancer. 1963;16:781-787. doi: [DOI] [PubMed] [Google Scholar]
- 2.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14. doi: 10.1007/s11060-004-8093-6 [DOI] [PubMed] [Google Scholar]
- 3.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45(7):741-744. doi: 10.1001/archneur.1988.00520310047016 [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: epidemiology. Handb Clin Neurol. 2018;149:27-42. doi: 10.1016/B978-0-12-811161-1.00002-5 [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044. doi: 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 6.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387-395. doi: 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 8.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491. doi: 10.1001/jama.295.21.2483 [DOI] [PubMed] [Google Scholar]
- 9.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401-409. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672. doi: 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Grimm J, Baker BR, et al. Fractionated stereotactic radiosurgery for brainstem metastasis and brainstem tolerance. Int J Radiat Oncol. 2019;105(1)(suppl):E72-E73. doi: 10.1016/j.ijrobp.2019.06.2328 [DOI] [Google Scholar]
- 12.Yen CP, Sheehan J, Patterson G, Steiner L. Gamma knife surgery for metastatic brainstem tumors. J Neurosurg. 2006;105(2):213-219. doi: 10.3171/jns.2006.105.2.213 [DOI] [PubMed] [Google Scholar]
- 13.Kelly PJ, Lin YB, Yu AYC, et al. Linear accelerator-based stereotactic radiosurgery for brainstem metastases: the Dana-Farber/Brigham and Women’s Cancer Center experience. J Neurooncol. 2011;104(2):553-557. doi: 10.1007/s11060-010-0514-0 [DOI] [PubMed] [Google Scholar]
- 14.Kilburn JM, Ellis TL, Lovato JF, et al. Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: is there a volume threshold for toxicity? J Neurooncol. 2014;117(1):167-174. doi: 10.1007/s11060-014-1373-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyfman SA, Tendulkar RD, Chao ST, et al. Stereotactic radiosurgery for single brainstem metastases: the Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2010;78(2):409-414. doi: 10.1016/j.ijrobp.2009.07.1750 [DOI] [PubMed] [Google Scholar]
- 16.Leeman JE, Clump DA, Wegner RE, Heron DE, Burton SA, Mintz AH. Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol. 2012;7:107. doi: 10.1186/1748-717X-7-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Xu D, Zhang Z, et al. Gamma Knife surgery for brainstem metastases. J Neurosurg. 2012;117(suppl):13-16. doi: 10.3171/2012.7.GKS121020 [DOI] [PubMed] [Google Scholar]
- 18.Lin CS, Selch MT, Lee SP, et al. Accelerator-based stereotactic radiosurgery for brainstem metastases. Neurosurgery. 2012;70(4):953-958. doi: 10.1227/NEU.0b013e31823c40fe [DOI] [PubMed] [Google Scholar]
- 19.Liu SH, Murovic J, Wallach J, et al. CyberKnife radiosurgery for brainstem metastases: management and outcomes and a review of the literature. J Clin Neurosci. 2016;25:105-110. doi: 10.1016/j.jocn.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Lorenzoni JG, Devriendt D, Massager N, et al. Brain stem metastases treated with radiosurgery: prognostic factors of survival and life expectancy estimation. Surg Neurol. 2009;71(2):188-195. doi: 10.1016/j.surneu.2008.01.029 [DOI] [PubMed] [Google Scholar]
- 21.Murray L, Menard C, Zadeh G, et al. Radiosurgery for brainstem metastases with and without whole brain radiotherapy: clinical series and literature review. J Radiat Oncol. 2017;6(1):21-30. doi: 10.1007/s13566-016-0281-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura M, Nishimura H, Mayahara H, et al. Investigation of the efficacy and safety of CyberKnife hypofractionated stereotactic radiotherapy for brainstem metastases using a new evaluation criterion: ‘symptomatic control’. J Radiat Res. 2017;58(6):834-839. doi: 10.1093/jrr/rrx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuentes S, Delsanti C, Metellus P, Peragut JC, Grisoli F, Regis J. Brainstem metastases: management using gamma knife radiosurgery. Neurosurgery. 2006;58(1):37-42. doi: 10.1227/01.NEU.0000190655.95669.5C [DOI] [PubMed] [Google Scholar]
- 24.Patel A, Mohammadi H, Dong T, et al. Brainstem metastases treated with Gamma Knife stereotactic radiosurgery: the Indiana University Health experience. CNS Oncol. 2018;7(1):15-23. doi: 10.2217/cns-2017-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson HE, Larson EW, Fairbanks RK, et al. Gamma knife treatment of brainstem metastases. Int J Mol Sci. 2014;15(6):9748-9761. doi: 10.3390/ijms15069748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samblás JM, Sallabanda K, Bustos JC, et al. Radiosurgery and whole brain therapy in the treatment of brainstem metastases. Clin Transl Oncol. 2009;11(10):677-680. doi: 10.1007/s12094-009-0423-x [DOI] [PubMed] [Google Scholar]
- 27.Sengöz M, Kabalay IA, Tezcanlı E, Peker S, Pamir N. Treatment of brainstem metastases with gamma-knife radiosurgery. J Neurooncol. 2013;113(1):33-38. doi: 10.1007/s11060-013-1086-6 [DOI] [PubMed] [Google Scholar]
- 28.Shuto T, Fujino H, Asada H, Inomori S, Nagano H. Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien). 2003;145(9):755-760. doi: 10.1007/s00701-003-0034-1 [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto T, Matsuda R, Tamamoto T, et al. Linac-based fractionated stereotactic radiotherapy with a micro-multileaf collimator for brainstem metastasis. World Neurosurg. 2019;132:e680-e686. doi: 10.1016/j.wneu.2019.08.049 [DOI] [PubMed] [Google Scholar]
- 30.Trifiletti DM, Lee CC, Kano H, et al. Stereotactic radiosurgery for brainstem metastases: an international cooperative study to define response and toxicity. Int J Radiat Oncol Biol Phys. 2016;96(2):280-288. doi: 10.1016/j.ijrobp.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifiletti DM, Lee CC, Winardi W, et al. Brainstem metastases treated with stereotactic radiosurgery: safety, efficacy, and dose response. J Neurooncol. 2015;125(2):385-392. doi: 10.1007/s11060-015-1927-6 [DOI] [PubMed] [Google Scholar]
- 32.Valery CA, Boskos C, Boisserie G, et al. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phys. 2011;80(2):362-368. doi: 10.1016/j.ijrobp.2010.02.028 [DOI] [PubMed] [Google Scholar]
- 33.Voong KR, Farnia B, Wang Q, et al. Gamma knife stereotactic radiosurgery in the treatment of brainstem metastases: the MD Anderson experience. Neurooncol Pract. 2015;2(1):40-47. doi: 10.1093/nop/npu032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatiboglu MA, Chang EL, Suki D, Sawaya R, Wildrick DM, Weinberg JS. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011;69(4):796-806. doi: 10.1227/NEU.0b013e31821d31de [DOI] [PubMed] [Google Scholar]
- 35.Winograd E, Rivers CI, Fenstermaker R, Fabiano A, Plunkett R, Prasad D. The case for radiosurgery for brainstem metastases. J Neurooncol. 2019;143(3):585-595. doi: 10.1007/s11060-019-03195-y [DOI] [PubMed] [Google Scholar]
- 36.Yoo TW, Park ES, Kwon DH, Kim CJ. Gamma knife radiosurgery for brainstem metastasis. J Korean Neurosurg Soc. 2011;50(4):299-303. doi: 10.3340/jkns.2011.50.4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yükselen Güney Y, Işik Özşeker N, Altinişik Inan G, et al. Cyberknife radiosurgery and fractionated stereotactic radiotherapy for brainstem or adjacent-to-brainstem metastases. Turkiye Klin J Med Sci. 2013;33(6):1354-1359. doi: 10.5336/medsci.2012-32233 [DOI] [Google Scholar]
- 38.Huang CF, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91(4):563-568. doi: 10.3171/jns.1999.91.4.0563 [DOI] [PubMed] [Google Scholar]
- 39.Hussain A, Brown PD, Stafford SL, Pollock BE. Stereotactic radiosurgery for brainstem metastases: survival, tumor control, and patient outcomes. Int J Radiat Oncol Biol Phys. 2007;67(2):521-524. doi: 10.1016/j.ijrobp.2006.08.081 [DOI] [PubMed] [Google Scholar]
- 40.Joshi R, Johnson MD, Maitz A, Marvin KS, Olson RE, Grills IS. Utility of graded prognostic assessment in evaluation of patients with brainstem metastases treated with radiosurgery. Clin Neurol Neurosurg. 2016;147:30-33. doi: 10.1016/j.clineuro.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 41.Jung EW, Rakowski JT, Delly F, et al. Gamma Knife radiosurgery in the management of brainstem metastases. Clin Neurol Neurosurg. 2013;115(10):2023-2028. doi: 10.1016/j.clineuro.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 42.Kased N, Huang K, Nakamura JL, et al. Gamma knife radiosurgery for brainstem metastases: the UCSF experience. J Neurooncol. 2008;86(2):195-205. doi: 10.1007/s11060-007-9458-4 [DOI] [PubMed] [Google Scholar]
- 43.Kawabe T, Yamamoto M, Sato Y, et al. Gamma Knife surgery for patients with brainstem metastases. J Neurosurg. 2012;117(suppl):23-30. doi: 10.3171/2012.7.GKS12977 [DOI] [PubMed] [Google Scholar]
- 44.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 45.Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 46.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500. doi: 10.1056/NEJM199002223220802 [DOI] [PubMed] [Google Scholar]
- 47.Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. National Cancer Institute. Accessed April 7, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40.
- 48.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sneed PK, Mendez J, Vemer-van den Hoek JGM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373-386. doi: 10.3171/2014.10.JNS141610 [DOI] [PubMed] [Google Scholar]
- 50.Liew DN, Kano H, Kondziolka D, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases: clinical article. J Neurosurg. 2011;114(3):769-779. doi: 10.3171/2010.5.JNS1014 [DOI] [PubMed] [Google Scholar]
- 51.Marquart J, Chen EY, Prasad V. Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol. 2018;4(8):1093-1098. doi: 10.1001/jamaoncol.2018.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erbagci H, Keser M, Kervancioglu S, Kizilkan N. Estimation of the brain stem volume by stereological method on magnetic resonance imaging. Surg Radiol Anat. 2012;34(9):819-824. doi: 10.1007/s00276-012-0966-3 [DOI] [PubMed] [Google Scholar]
- 54.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. MINORS Criteria for Included Studies
eMethods
eFigure 1. PRISMA Flowcharts
eFigure 2. Funnel Plots for Publication Bias
eFigure 3. Heterogeneity After Excluding Outlier BSM Studies
eFigure 4. Outcomes Grouped by Radiation Modality
eFigure 5. Pooled Neurologic Death Rate of BSM Studies and BM Trials
eFigure 6. Pooled Outcomes for BM SRS Trials
eFigure 7. Forest Plot of Intracranial Response Rates for Central-Nervous System Penetrant Targeted and Immunotherapies
eFigure 8. Two-year overall survival for BSM studies
eTable 1. Univariate Meta-Regression P values of Study Level Characteristics in Relation to Outcomes of Interest
eTable 2. Characteristics of Published Trials of SRS for Non-Brainstem Intracranial Metastases
eReferences.
eTable. Study Level Data for Included Studies
eTable. Catalogue of All Reported Complications of Stereotactic Radiosurgery for Brainstem Metastases
eTable. Prospective Trials Reporting Intracranial Response Rates of Targeted and Immuno-Therapy for Brain Metastases
eTable. Currently Active Phase I-III Trials Involving Stereotactic Radiosurgery for Brain Metastases Identified on Clinicaltrials.gov


