Key Points
Question
What is the immunogenicity of COVID-19 messenger RNA (mRNA) vaccines in pregnant and lactating women?
Findings
In this cohort study involving 103 women who received a COVID-19 mRNA vaccine, 30 of whom were pregnant and 16 of whom were lactating, immunogenicity was demonstrated in all, and vaccine-elicited antibodies were found in infant cord blood and breast milk. Pregnant and nonpregnant vaccinated women developed cross-reactive immune responses against SARS-CoV-2 variants of concern.
Meaning
In a small convenience sample, COVID-19 mRNA vaccines were immunogenic in pregnant and lactating women and induced immune responses against SARS-CoV-2 variants.
Abstract
Importance
Pregnant women are at increased risk of morbidity and mortality from COVID-19 but have been excluded from the phase 3 COVID-19 vaccine trials. Data on vaccine safety and immunogenicity in these populations are therefore limited.
Objective
To evaluate the immunogenicity of COVID-19 messenger RNA (mRNA) vaccines in pregnant and lactating women, including against emerging SARS-CoV-2 variants of concern.
Design, Setting, and Participants
An exploratory, descriptive, prospective cohort study enrolled 103 women who received a COVID-19 vaccine from December 2020 through March 2021 and 28 women who had confirmed SARS-CoV-2 infection from April 2020 through March 2021 (the last follow-up date was March 26, 2021). This study enrolled 30 pregnant, 16 lactating, and 57 neither pregnant nor lactating women who received either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) COVID-19 vaccines and 22 pregnant and 6 nonpregnant unvaccinated women with SARS-CoV-2 infection.
Main Outcomes and Measures
SARS-CoV-2 receptor binding domain binding, neutralizing, and functional nonneutralizing antibody responses from pregnant, lactating, and nonpregnant women were assessed following vaccination. Spike-specific T-cell responses were evaluated using IFN-γ enzyme-linked immunospot and multiparameter intracellular cytokine–staining assays. Humoral and cellular immune responses were determined against the original SARS-CoV-2 USA-WA1/2020 strain as well as against the B.1.1.7 and B.1.351 variants.
Results
This study enrolled 103 women aged 18 to 45 years (66% non-Hispanic White) who received a COVID-19 mRNA vaccine. After the second vaccine dose, fever was reported in 4 pregnant women (14%; SD, 6%), 7 lactating women (44%; SD, 12%), and 27 nonpregnant women (52%; SD, 7%). Binding, neutralizing, and functional nonneutralizing antibody responses as well as CD4 and CD8 T-cell responses were present in pregnant, lactating, and nonpregnant women following vaccination. Binding and neutralizing antibodies were also observed in infant cord blood and breast milk. Binding and neutralizing antibody titers against the SARS-CoV-2 B.1.1.7 and B.1.351 variants of concern were reduced, but T-cell responses were preserved against viral variants.
Conclusion and Relevance
In this exploratory analysis of a convenience sample, receipt of a COVID-19 mRNA vaccine was immunogenic in pregnant women, and vaccine-elicited antibodies were transported to infant cord blood and breast milk. Pregnant and nonpregnant women who were vaccinated developed cross-reactive antibody responses and T-cell responses against SARS-CoV-2 variants of concern.
This study assesses the immunogenicity of the current COVID-19 mRNA vaccines in pregnant and lactating women against both the original SARS-CoV-2 USA-WA1/2020 strain as well as against the B.1.1.7 and B.1.351 variants of concern.
Introduction
Pregnant women with symptomatic COVID-19 have a higher risk of intensive care unit admission, mechanical ventilation, and death compared with other women in their reproductive years.1 Increases in preterm birth and stillbirth also have been observed in pregnancies complicated by COVID-19.2 Maternal-fetal virus transmission in utero is rare,2 and it appears that newborns receive passive immunity through antibody transfer via the placenta and from breast milk following natural infection.3,4 Vaccination during pregnancy has reduced maternal morbidity and mortality from influenza and neonatal morbidity from pertussis through passive immunity.5,6
The theoretical risks of COVID-19 vaccination in pregnancy and during lactation are limited, and the current vaccines have a favorable safety profile and high efficacy in nonpregnant individuals. The Centers for Disease Control and Prevention7 recommended that pregnant and lactating women have access to the available COVID-19 vaccines. In the month following Emergency Use Authorization of 2 COVID-19 messenger RNA (mRNA) vaccines in December 2020, 11 087 pregnant women received a COVID-19 vaccine in the United States.8 However, pregnant and lactating women were excluded from phase 3 vaccine efficacy trials9,10,11; thus, data on vaccine safety and immunogenicity in these populations remain limited.
New genetic variants have evolved from the initial SARS-CoV-2 sequence. The D614G variant is associated with enhanced infectivity,12 the B.1.1.7 variant is associated with greater transmissibility,13 and the B.1.351 variant appears to evade natural immunity from prior infection14,15 and partially escapes from neutralizing antibodies. The objective of this study was to assess the immunogenicity of the current COVID-19 mRNA vaccines in pregnant and lactating women against both the original SARS-CoV-2 USA-WA1/2020 strain as well as against the B.1.1.7 and B.1.351 variants of concern.
Methods
Study Population
The Beth Israel Deaconess Medical Center institutional review board approved this study and the parent biorepository study; participants provided written informed consent. We conducted an exploratory, descriptive cohort study of women 18 years or older who had received a COVID-19 vaccine from December 2020 through March 2021 or had had confirmed SARS-CoV-2 infection from April 2020 through March 2021 using samples collected in a larger hospital-wide, prospective data and tissue biorepository. The date of the last follow-up was March 26, 2021.
To recruit participants planning to be vaccinated, we screened clinic schedules; to recruit participants with confirmed infection, we also screened inpatient admissions. Participants also self-referred from flyers posted in the hospital. All participants provided blood, some provided infant cord blood at delivery, and some provided breast milk. Samples were collected close to each vaccine dose and 2 to 8 weeks after the second dose for the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine. The analysis presented herein includes pregnant, lactating, and nonpregnant women aged 18 to 45 years who were vaccinated or infected (Table 1). To further characterize the study population, participants were asked to provide their race and ethnicity based on specified categories for each; they could select multiple race categories. Participants also reported if they had fever symptoms following either vaccine dose.
Table 1. Characteristics of Participants (n = 103).
| No. (%) of womena | |||||
|---|---|---|---|---|---|
| Vaccinated | Unvaccinated, infected | ||||
| Nonpregnant, nonlactating (n = 57) | Pregnant (n = 30)b | Lactating (n = 16) | Nonpregnant (n = 6) | Pregnant (n = 22) | |
| Age, median (IQR), y | 30 (25-35) | 35 (32-36) | 34 (32-36) | 34 (33-38) | 31 (28-36) |
| Race, No. | 53 | 28 | 16 | 6 | 18 |
| White | 38 (72) | 24 (86) | 13 (81) | 3 (50) | 10 (56) |
| Black | 3 (6) | 0 | 0 | 2 (33) | 5 (28) |
| Asian | 6 (11) | 3 (11) | 3 (19) | 0 | 0 |
| Otherc | 6 (11) | 1 (4) | 0 | 1 (17) | 3 (17) |
| Ethnicity, No. | 51 | 28 | 15 | 6 | 19 |
| Hispanic or Latina | 7 (14) | 1 (4) | 1 (7) | 3 (50) | 4 (21) |
| Vaccine | |||||
| BNT162b2 (Pfizer-BioNTech) | 34 (60) | 11 (37) | 11 (69) | ||
| mRNA-1273 (Moderna) | 23 (40) | 19 (63) | 5 (31) | ||
| Prior SARS-CoV-2 infection | 1 (2) | 2 (7) | 1 (6) | 6 (100) | 22 (100) |
| Gestational age at first vaccine dose, wk | |||||
| <14 | 5 (17) | ||||
| 14 to <28 | 15 (50) | ||||
| ≥28 | 10 (33) | ||||
| Gestational age at symptom onset, wk | |||||
| <14 | 3 (14) | ||||
| 14 to <28 | 7 (32) | ||||
| ≥28 | 12 (55) | ||||
| Days from second vaccine dose to sample collection, median (IQR) | 21 (17-27) | 21 (14-36) | 26 (18-31) | ||
| Days from symptom onset to sample collection, median (IQR) | 12 (10-20) | 41 (15-140) | |||
| Fever within 48 h, No./total (%)d | |||||
| After first dose | 1/52 (2) | 0/30 | 0/16 | ||
| After second dose | 27/52 (52) | 4/29 (14) | 7/16 (44) | ||
Abbreviation: IQR, interquartile range.
Participants were chosen from the biorepository based on their age, pregnancy status, and sample availability at the time of the analysis.
Includes 3 participants who were vaccinated during pregnancy who also provided breast milk samples at delivery.
Other indicates multiracial, unknown or unobtainable, or free-text designations that included Latina, Salvadoran, and Spanish.
Temperature of 38 °C or higher.
Enzyme-Linked Immunosorbent Assay
SARS-CoV-2 spike receptor binding domain (RBD)–specific binding antibodies in serum and milk were assessed by enzyme-linked immunosorbent assay (ELISA) (Table 2). The 96-well plates were coated with 2 μg/mL of wild-type SARS-CoV-2, variant B.1.1.7 (containing mutation N501Y) (A.G. Schmidt),16 or B.1.351 (containing mutations K417N, E484K, N501Y) RBD protein in 1× Dulbecco phosphate-buffered saline (DPBS) and incubated at 4 °C overnight.
Table 2. Glossary of Immunologic Assays.
| Assay | What can it detect? | Immunologic interpretation |
|---|---|---|
| Humoral immune response | ||
| Receptor binding domain (RBD) IgG and IgA binding antibody | Quantifies IgG or IgA antibody that binds to SARS-CoV-2 spike RBD after vaccination or infection using ELISA |
|
| Pseudovirus neutralizing antibody (NT50) | Functional assay that measures serum antibodies that can neutralize viral particles (inhibit cell entry) after vaccination or infection |
|
| Systems serology | ||
| Antibody–dependent cellular phagocytosis (ADCP) | Functional assay that detects monocyte phagocytosis (clearance) of antibody-antigen (SARS-CoV-2 spike) immune complexes | Measures antibody-dependent antiviral cellular function (monocytes) |
| Antibody–dependent neutrophil phagocytosis (ADNP) | Functional assay that detects neutrophil phagocytosis (clearance) of antibody-antigen SARS-CoV-2 spike immune complexes | Measures antibody–dependent antiviral cellular function (neutrophils) |
| Antibody–dependent complement deposition (ADCD) | Functional assay that detects antibody-driven SARS-CoV-2 spike–specific complement activation | Measures antibody–dependent antiviral complement activity |
| Cellular immune response | ||
| IFN-γ enzyme-linked immunospot (ELISPOT) | Quantity of TH1 (IFN-γ) proinflammatory cellular response to SARS-CoV-2 spike peptides after vaccination or infection |
|
| Intracellular cytokine staining (ICS) | Quantity of TH1 (IFN-γ) response to SARS-CoV-2 spike peptides in CD4 and CD8 T-cell subsets after vaccination or infection; IFN-γ response by CD4, CD8, and central memory CD4 and CD8 T-cell subsets is assessed using flow cytometry gating |
|
Abbreviation: TH1, type 1 helper T cells.
After incubation, plates were washed once with wash buffer (0.05% Tween 20 in 1× DPBS) and blocked with 350 μL of casein block solution per well for 2 to 3 hours at room temperature. Following incubation, block solution was discarded and plates were blotted dry. Serial dilutions of heat-inactivated serum or breast milk diluted in Casein block were added to wells, and plates were incubated for 1 hour at room temperature, prior to 3 more washes and a 1-hour incubation with a 1:4000 dilution of anti–human IgG horseradish peroxidase (HRP) (Invitrogen, ThermoFisher Scientific) or a 1:1000 dilution of anti–human IgA HRP (Bethyl Laboratories Inc) at room temperature in the dark. Plates were washed 3 times, and 100 μL of SeraCare KPL TMB SureBlue Start solution was added to each well; plate development was halted by adding 100 μL of SeraCare KPL TMB Stop solution per well. The absorbance at 450 nm, with a reference at 650 nm, was recorded with a VersaMax microplate reader (Molecular Devices). For each sample, the ELISA end point titer was calculated using a 4-parameter logistic curve fit to calculate the reciprocal serum dilution that yields a corrected absorbance value (450 nm-650 nm) of 0.2. Interpolated end point titers were reported.
Pseudovirus Neutralizing Antibody Assay
The SARS-CoV-2 pseudoviruses expressing a luciferase reporter gene were generated in a similar approach, described previously.3,17,18,19 In brief, the packaging construct psPAX2 (AIDS Resource and Reagent Program), luciferase reporter plasmid pLenti-CMV Puro-Luc (Addgene), and spike protein expressing pcDNA3.1-SARS-CoV-2 SΔCT were cotransfected into HEK293T cells with calcium phosphate. Pseudoviruses were also generated using spike plasmids harboring mutations found in the USA-WA1/2020 variant (mutation D614G), B.1.1.7 variant (Global Initiative on Sharing All Influenza Data [GISAID] accession number, EPI_ISL_601443), and B.1.351 variant (GISAID accession number, EPI_ISL_712096). The supernatants containing the pseudotype viruses were collected 48 hours after transfection; pseudotype viruses were purified by filtration with a 0.45-μm filter.
To determine the neutralization activity of human adult and infant cord blood serum and whole breast milk, HEK293T-hACE2 cells were seeded in 96-well tissue culture plates at a density of 1.75 × 104 cells per well overnight. Three-fold serial dilutions of heat-inactivated serum samples were prepared and mixed with 50 μL of pseudovirus. The mixture was incubated at 37 °C for 1 hour before adding to HEK293T-hACE2 cells. After 48 hours, cells were lysed in Steady-Glo Luciferase Assay (Promega Corp) according to the manufacturer’s instructions. SARS-CoV-2 neutralization titers (NT50) were defined as the sample dilution at which a 50% reduction in relative light units was observed relative to the average of the virus control wells.
Systems Serology
For the functional analysis of sera samples, bead-based assays were used to quantify antibody–dependent cellular phagocytosis (ADCP), antibody–dependent neutrophil phagocytosis (ADNP), and antibody–dependent complement deposition (ADCD), as previously described.19 Fluorescent streptavidin beads (ThermoFisher) were coupled to biotinylated SARS-CoV-2 Spike trimer (LakePharma) and incubated with diluted serum (ADCP and ADNP, 1:100; ADCD, 1:10). For ADCP, THP-1 cells (ATCC), derived from a human monocytic cell line, were added to the immune complexes and incubated for 16 hours at 37 °C. For ADNP, primary neutrophils were isolated using an ammonium chloride potassium lysis buffer from whole blood.
After a 1-hour incubation at 37 °C, neutrophils were stained with an anti-CD66b PacBlue detection antibody (Biolegend). For the ADCD assay, lyophilized guinea pig complement component 3b (C3b) (Sigma) was resuspended according to manufacturer’s instructions and diluted in a gelatin veronal buffer with calcium and magnesium (Boston BioProducts). After incubation, C3 was detected with fluorescein-conjugated goat IgG fraction to guinea pig complement C3 (MPbio). For ADCP, events were gated on bead-positive cells, whereas neutrophils were defined as CD66b positive followed by gating on bead-positive neutrophils for ADNP. ADCP and ADNP data were reported as the phagocytic score, calculated using the following formula: phagocytic score = {[percentage of bead-positive cells] × [geometric mean MFI (mean fluorescence index) for bead-positive cells]}/1000. ADCD was reported as the MFI of C3 deposition.
IFN-γ Enzyme-Linked Immunospot Assay
Enzyme-linked immunospot (ELISPOT) assay plates were coated with mouse anti–human IFN-γ monoclonal antibody (MabTech) at 1 μg per well and incubated overnight at 4 °C. Plates were washed with DPBS and blocked with R10 media (RPMI with 10% heat-inactivated fetal bovine serum [FBS] with 1% of 100× penicillin-streptomycin, 1 M of HEPES buffer, 100 mM of sodium pyruvate, 200 mM of L-glutamine, and 0.1% of 55 mM of 2-mercaptoethanol) for 2 to 4 hours at 37 °C. Peptides from wild-type, B.1.1.7, and B.1.351 variant spike (21st Century Biochemicals) were prepared and plated at a concentration of 2 μg per well, and 100 000 cells per well were added to the plate.
The peptides and cells were incubated for 15 to 20 hours at 37 °C. All steps following this incubation were performed at room temperature. The plates were washed with an ELISPOT wash buffer and incubated for 2 to 4 hours with 1 μg/mL of biotinylated mouse anti–human IFN-γ monoclonal antibody (MabTech). The plates were washed again and incubated for 2 to 3 hours with 1.33 μg/mL of conjugated goat antibiotin alkaline phosphatase (Rockland Inc). The final wash was followed by adding nitor-blue tetrazolium and 5-bromo-4-chloro-3-indolyphosphate p-toludine salt (NBT/BCIP chromogen) substrate solution for 7 minutes. The chromogen was discarded, and the plates were washed with water and dried in a dim place for 24 hours. Plates were scanned and counted on an immunospot analyzer (Cellular Technologies Ltd).
Intracellular Cytokine Staining Assay
Peripheral blood mononuclear cells were resuspended at a concentration of 106 cells in 100 μL of R10 media supplemented with a CD49d monoclonal antibody (1 μg/mL) and a CD28 monoclonal antibody (1 μg/mL). Each sample was assessed with mock (100 μL of R10 plus 0.5% dimethyl sulfoxide; background control), peptides (2 μg/mL), and/or 10 pg/mL of phorbol myristate acetate and 1 μg/mL of ionomycin (Sigma-Aldrich) (100 μL; positive control) and incubated at 37 °C for 1 hour. After incubation, 0.25 μL of GolgiStop (BD Bioscience), which contains monensin, and 0.25 μL of GolgiPlug (BD Bioscience), which contains brefeldin A, in 50 μL of R10 was added to each well and incubated at 37 °C for 8 hours and then held at 4 °C overnight.
The next day, the cells were washed twice with DPBS, stained with aqua live-or-dead dye for 10 minutes, and then stained with predetermined titers of monoclonal antibodies (mAbs) against CD279 (clone EH12.1, BB700), CD4 (clone L200, BV711), CD27 (clone M-T271, BUV563), CD8 (clone SK1, BUV805), and CD45RA (clone 5H9, APC H7) for 30 minutes. Cells were then washed twice with a 2% FBS-DPBS buffer and incubated for 15 minutes with 200 μL of BD CytoFix/CytoPerm fixation/permeabilization solution. Cells were washed twice with 1× Perm Wash buffer (BD Biosciences Perm/Wash Buffer 10× in the CytoFix/CytoPerm Fixation/ Permeabilization kit diluted with MilliQ water and passed through 0.22-μm filter) and stained intracellularly with mAbs against Ki67 (clone B56, BB515), IL-21 (clone 3A3-N2.1, PE), CD69 (clone TP1.55.3, ECD), IL-10 (clone JES3-9D7, PE CY7), IL-13 (clone JES10-5A2, BV421), IL-4 (clone MP4-25D2, BV605), TNF (clone Mab11, BV650), IL-17 (clone N49-653, BV750), IFN-γ (clone B27; BUV395), IL-2 (clone MQ1-17H12, BUV737), IL-6 (clone MQ2-13A5, APC), and CD3 (clone SP34.2, Alexa 700) for 30 minutes. Cells were washed twice with 1× Perm Wash buffer and fixed with 250 μL of freshly prepared 1.5% formaldehyde. Fixed cells were transferred to a 96-well round bottom plate and analyzed by BD FACSymphony system.
Statistical Analysis
Descriptive statistics were calculated using SAS 9.4 (SAS Institute Inc) and GraphPad Prism 8.4.3 (GraphPad Software). Data are presented as median with interquartile range (IQR) or proportion with standard deviation (SD).
Results
Enrollment
The hospital-wide biorepository enrolled 103 women aged 18 to 45 years who received an mRNA COVID-19 vaccine and had serum available for analysis; an additional 4 individuals declined to participate. Among these 103 participants, 30 were pregnant; 16 were lactating; and 57 were neither pregnant nor lactating (Table 1). Samples were obtained a median of 21 days (IQR, 17-27 days) after the second vaccine dose from nonpregnant women, 21 days (IQR, 14-36 days) from pregnant women, and 26 days (IQR, 19-31 days) from lactating women. Nine pregnant women delivered during the study and contributed infant cord blood. Fifty-six participants (54%) received BNT162b2; 47 (46%) received mRNA-1273. Prior SARS-CoV-2 infection was diagnosed in 4 (4%) of the vaccinated participants. Among pregnant participants, 5 (17%) received their first vaccine dose in the first trimester, 15 (50%) in the second, and 10 (33%) in the third.
The hospital-wide biorepository also enrolled 70 women aged 18 to 45 years who were tested for SARS-CoV-2, including 60 pregnant women; an additional 76 individuals declined to participate. The analysis presented herein includes 22 pregnant and 6 nonpregnant unvaccinated women with SARS-CoV-2 infection as comparators who had serum available for analysis. These participants were more likely to self-identify as Black or Hispanic than were the vaccinated women (Table 1). Among women who had not been vaccinated but had been infected, the median time from symptom onset (or positive polymerase chain reaction [PCR] test result among those who were asymptomatic) to sample collection was 12 days (IQR, 10-20 days) for nonpregnant women and 41 days (IQR, 15-140 days) for pregnant women. Among participants infected but not vaccinated, 1 nonpregnant (17%) and 3 pregnant (14%) women experienced severe disease.
Reactogenicity
After the second dose, fever was reported in 27 nonpregnant (52%, SD; 7%), 4 pregnant (14%; SD, 6%), and 7 lactating (44%; SD, 12%) women (Table 1). Among nonpregnant women, 5 did not report whether they had a fever within 48 hours after the first or second dose; 1 pregnant woman did not report whether she had a fever after the second dose. No severe adverse events or pregnancy or neonatal complications were observed.
Serum Binding and Functional Antibody Responses
The median RBD–IgG binding antibody titers in nonpregnant (37 839), pregnant (27 601), and lactating (23 497) women after the second vaccine dose were higher than their baseline prevaccination titers (28) (Figure 1A). Among pregnant women, median binding antibody titer was 27 601 following vaccination and was 1321 after infection. The median binding antibody titer was 37 839 following vaccination and was 771 after infection in nonpregnant women. Similarly, the median pseudovirus NT50 in vaccinated nonpregnant (901), pregnant (910), and lactating individuals (783) were higher than the prevaccination titers (20) (Figure 1B). Among those who were not vaccinated but were infected, the median NT50 values were 148 among those who were pregnant and 193 among those who were not pregnant. Among vaccinated individuals, ADNP activity was quantified with median phagocytic score of 58 in nonpregnant, 27 in pregnant, and 12 in lactating individuals (Figure 1C). The median MFI for ADCD among vaccinated nonpregnant women was 376; pregnant women, 402; and lactating women, 333 (Figure 1D). The median phagocytic score for ADCP among vaccinated nonpregnant women was 277; for pregnant women, 282; and for lactating women, 249 (Figure 1E).
Figure 1. SARS-CoV-2 Binding and Functional Antibody Responses in Serum From Vaccinated and Unvaccinated, Infected Pregnant, Lactating, and Nonpregnant Women .
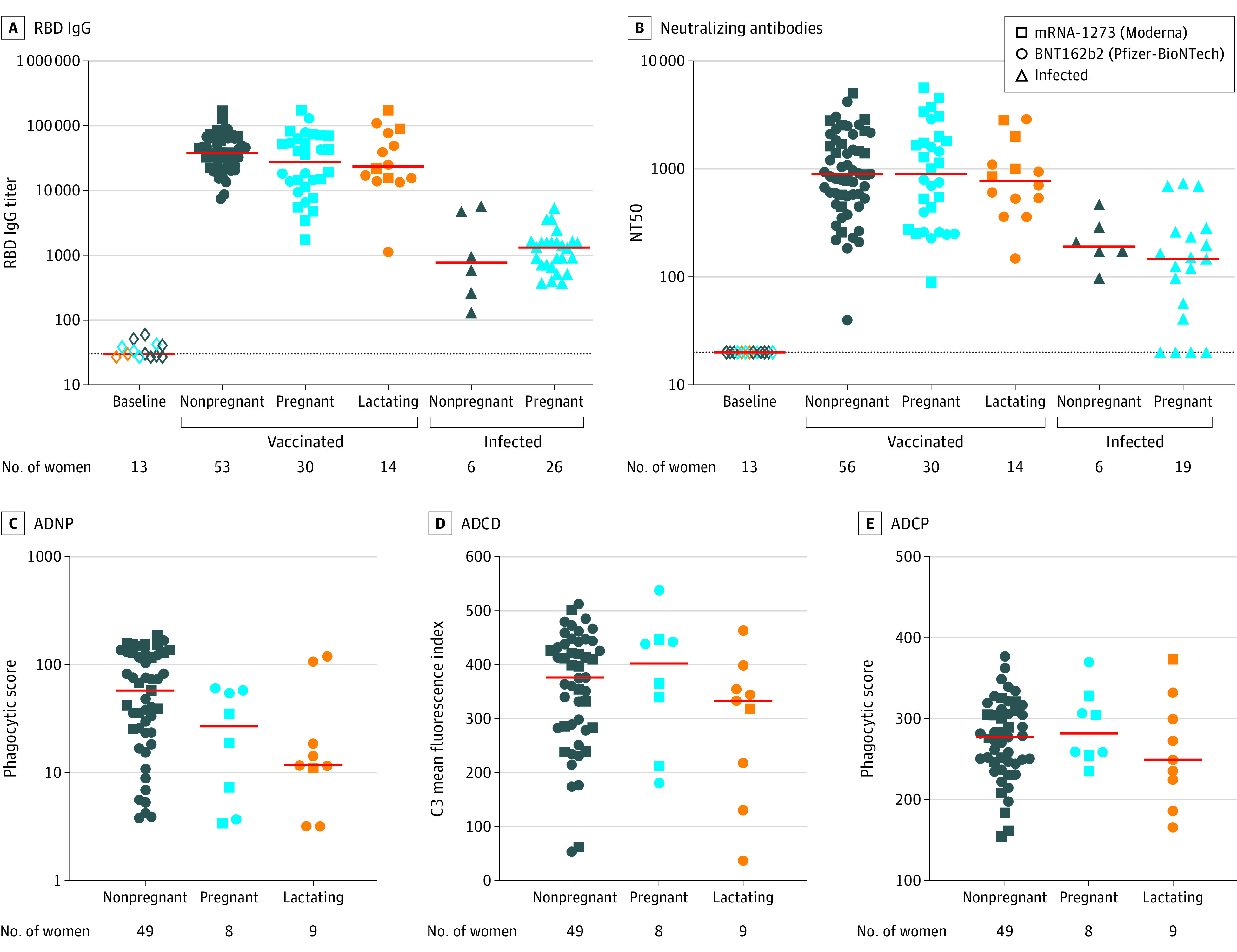
This figure presents serum binding and functional antibody responses following COVID-19 vaccination and SARS-CoV-2 infection among women 45 years or younger.
A and B, Each panel compares vaccine antibody responses at 2 through 8 weeks after the second dose to nonpregnant and pregnant women who were unvaccinated and infected. Thirteen women (7 nonpregnant, 4 pregnant, and 2 lactating) who had baseline samples collected within 7 days of their first vaccine dose were selected based on the earliest sample availability and were analyzed as a negative assay control.
C, D, and E, Systems serology was used to quantify spike-specific antibody–dependent neutrophil phagocytosis (ADNP), antibody–dependent complement deposition (ADCD), and antibody–dependent monocyte cellular phagocytosis (ADCP).
For an explanation of antibody binding, neutralizing, and systems serology assays see Table 2. The red bars indicate the median; the dotted lines in panels A and B, the limit of detection; C3, complement component 3; NT50, neutralizing antibody titer serum dilution.
Binding and Neutralizing Antibodies in Cord Blood
Nine paired maternal and infant cord blood samples were used to evaluate transplacental transfer of vaccine–elicited binding and neutralizing antibodies. Median maternal serum RBD IgG binding antibody titers at delivery were 14 953 compared with 19 873 in cord blood (Figure 2A). The median maternal NT50 at delivery was 1016 compared with 324 in cord blood (Figure 2B). In the unvaccinated infected maternal and infant dyads, the median RBD IgG binding antibody titers at delivery were 1342 in maternal sera and 635 in cord blood (Figure 2A), and the median NT50 was 151 in maternal sera compared with 164 in cord blood (Figure 2B).
Figure 2. SARS-CoV-2 Binding and Neutralizing Antibody Responses in Infant Cord Blood and Breast Milk From Women Following Vaccination or Infection.
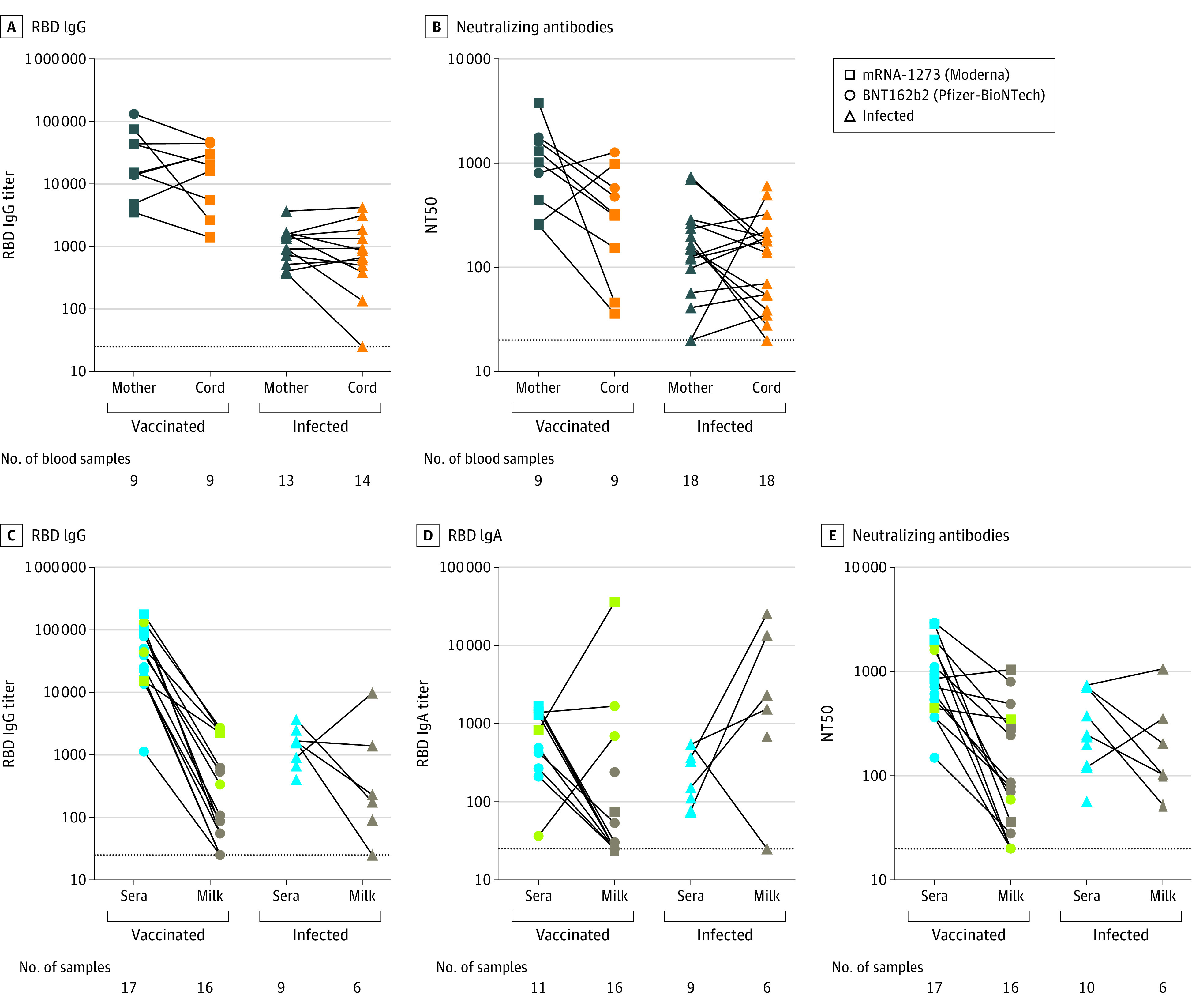
A and B, The paired sera samples from maternal blood and cord blood at delivery were used to measure transplacental transfers of the SARS-CoV-2 receptor binding domain (RBD) and binding neutralizing antibody levels after 2 doses of vaccines compared with levels in women who were not vaccinated but were infected with SARS-CoV-2.
C, D, and E, Paired sera samples and breast milk from lactating participants were used to assess IgG and IgA RBD binding antibody and neutralizing antibody levels and compare them between women who were vaccinated and women who were not vaccinated but were infected with SARS-CoV-2. Three participants (green data points) were vaccinated during pregnancy and provided breast milk in the immediate postpartum period. These 3 participants are included as vaccinated in the figure and are included in Table 1 with the pregnant group.
An explanation of binding and neutralizing assays can be found in Table 2. The red bars indicate the median and the dotted lines, the limit of detection.
Binding and Neutralizing Antibodies in Breast milk
RBD IgG and IgA binding antibodies and neutralizing antibodies were assessed in breast milk following vaccination and infection. The median serum IgG binding antibody titer was 25 055 after vaccination and 1593 following natural infection. Median breast milk IgG titer was 97 in vaccinated and 203 in infected individuals (Figure 2C). The median serum IgA–binding antibodies were 820 after vaccination and 152 after infection. The median breast milk IgA binding antibodies were 25 after vaccination and 1940 after infection (Figure 2D). The median NT50 in breast milk was 75 following vaccination and 153 following infection (Figure 2E).
T-Cell Responses in Peripheral Blood
Spike-specific T-cell responses were evaluated in 18 pregnant, 7 lactating, and 12 nonpregnant individuals with available peripheral blood mononuclear cells (PBMCs). The median ELISPOT responses in vaccinated pregnant individuals was 270 spot-forming cells (SFCs) per million PBMCs, 185 SFCs per million PBMCs in lactating, and 435 SFCs per million PBMCs in nonpregnant women. The percent of spike-specific IFN-γ production by CD4 T cells, CD4 central memory T cells, CD8 T cells, and CD8 central memory T cells were comparable in pregnant, lactating, and nonpregnant women (Figure 3).
Figure 3. SARS-CoV-2 Spike–Specific Cellular Immune Responses in Vaccinated Pregnant, Lactating, and Nonpregnant Women.
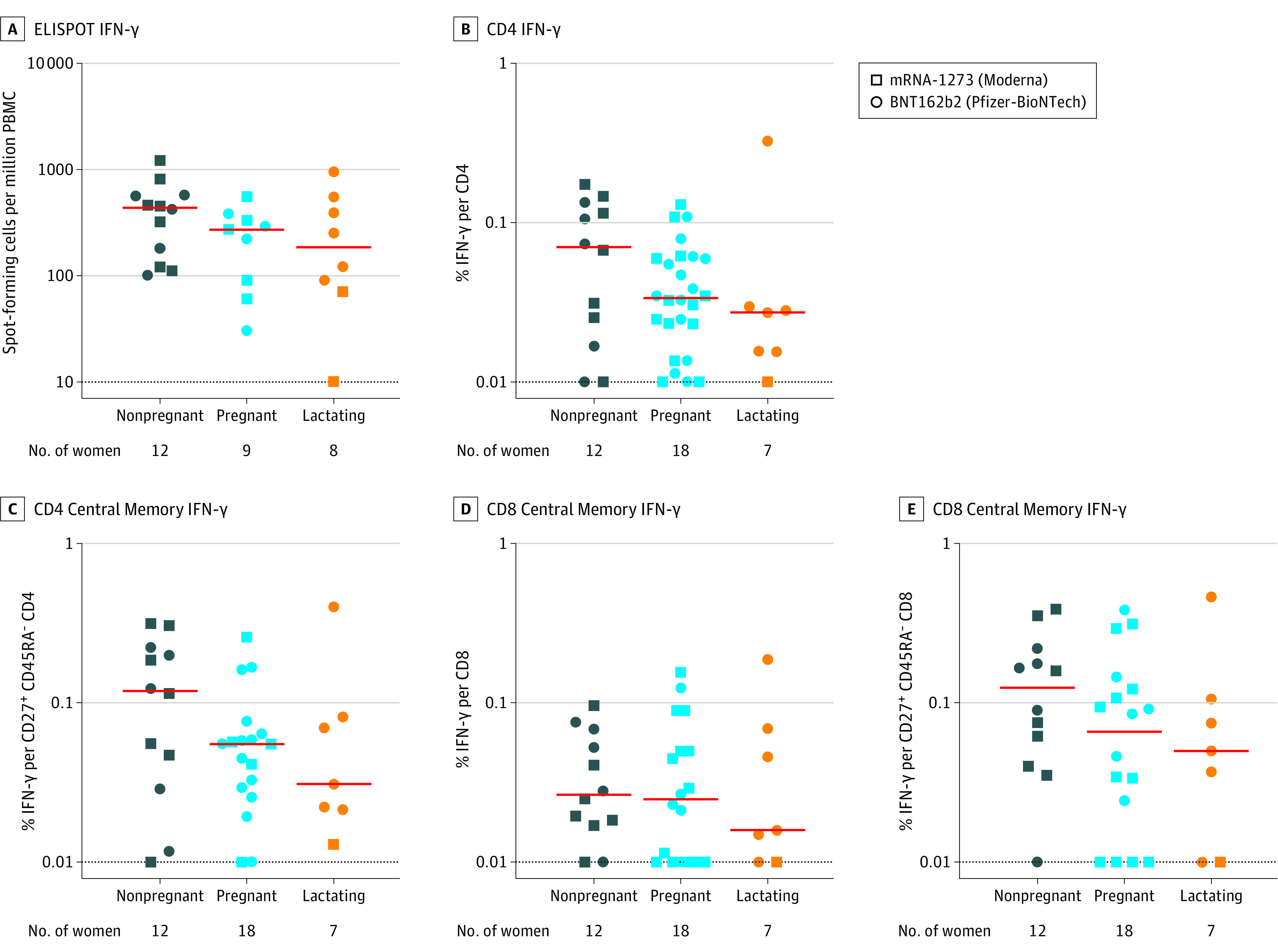
Peripheral blood mononuclear cells (PBMCs) following 2 doses of vaccines were stimulated with SARS-CoV-2 USA-WA1/2020 spike peptides. The T-cell responses were measured using IFN-γ enzyme-linked immunospot (ELISPOT) assays and multiparameter intracellular cytokine staining assays to assess IFN-γ total CD4 T cells, CD45RA− CD27+ central memory CD4 T cells, total CD8 T cells, and CD45RA− CD27+ central memory CD8 T cells.
The red bars indicate the median and the dotted lines, the limit of detection. See Table 2 for an explanation of ELISPOT and ICS assays.
Humoral and Cellular Immune Responses to SARS-CoV-2 Variants of Concern
Serum RBD IgG binding antibodies and neutralizing antibodies to the B.1.1.7 and B.1.351 variants of concern were evaluated.20 Binding antibody responses were comparable against wildtype USA-WA1/2020 and B.1.1.7 RBD proteins in nonpregnant, pregnant, and lactating women and in infant cord samples but were lower for the B.1.351 RBD protein (Figure 4A). The median neutralizing antibody titer in nonpregnant, pregnant, and lactating women was lower by 3.5-fold for the B.1.1.7 variant and 6-fold lower for the B.1.351 variant than for the USA-WA1/2020 variant (Figure 4B).
Figure 4. Vaccine-Elicited Humoral and Cellular Immune Responses Against SARS-CoV-2 Variants of Concern.

Serum receptor binding domain (RBD) IgG binding antibody titers and neutralizing antibody titers (NT50) were compared with SARS-CoV-2 wild-type USA-WA1/2020 and variants of concern B.1.1.7 and B.1.351 following 2 doses of vaccines, as well as in cord blood and in breast milk. Peripheral blood mononuclear cells (PBMCs) were stimulated with SARS-CoV-2 wild-type USA-WA1/2020, B.1.1.7, and B.1.351 spike peptides. IFN-γ T-cell responses were measured using enzyme-linked immunospot (ELISPOT) assays and multiparameter intracellular cytokine staining assays gated on total CD4 T cells, CD45RA−CD27+ central memory CD4 T cells, total CD8 T cells, and CD45RA− CD27+ central memory CD8 T cells.
The red bars indicate the median and the dotted lines, the limit of detection. See Table 2 for an explanation of antibody binding and neutralizing assays and ELISPOT and ICS assays.
Spike-specific T-cell responses were also compared with the wildtype USA-WA1/2020, B.1.1.7, and B.1.351 peptides by ELISPOT and ICS assays following vaccination. There were no differences in ELISPOT responses , CD4 T-cell responses, CD4 central memory T-cell responses, CD8 T-cell responses, or CD8 central memory T-cell responses across these variants.
Discussion
COVID-19 mRNA vaccines were immunogenic, as quantified by both humoral and cellular immune responses, in pregnant, lactating, and nonpregnant, nonlactating women. Following the second dose of the mRNA vaccines, 13% of pregnant women and 47% of nonpregnant women reported fever. These findings need to be confirmed using the national v-safe Centers for Disease Control and Prevention registry.21 Moreover, similar to prior studies,22 this study validates that vaccination elicits higher antibody responses than does infection.
The detection of binding and neutralizing antibodies in infant cord blood suggests efficient transplacental transfer of maternal antibodies. As with the recommendation for diphtheria and tetanus toxoids and acellular pertussis vaccination in pregnancy to protect vulnerable newborns against pertussis, maternal COVID-19 vaccination in pregnancy may confer similar benefits for newborns who may be ineligible for vaccination. Vaccination also elicited binding and neutralizing antibodies in breast milk, although IgA responses were low in breast milk, with the exception of early breast milk from participants receiving a vaccine during pregnancy. Differential breast milk IgG- and IgA-antibody production specific to respiratory pathogens has been described in the setting of maternal infection and vaccination,23 and future work should focus on delineating the timing of vaccination that optimizes delivery of breast milk antibodies to neonates. Other studies have similarly reported spike–specific binding antibodies in breast milk following vaccination.24 The results of this study complement these studies by demonstrating neutralizing antibodies in both cord blood and breast milk, suggesting the possibility that newborns may be protected by maternal vaccination.
Consistent with recent reports,15,25,26 reduced serum neutralizing antibody titers were evident against the B.1.1.7 variant that was originally identified in the UK and particularly against the B.1.351 variant that was originally identified in South Africa. Both vaccinated pregnant women and infant cord blood showed reductions in neutralizing antibody titers against these variants. In contrast, minimal reductions were observed against these variants for nonneutralizing antibody binding and for CD4 and CD8 T-cell responses in both pregnant and nonpregnant women following vaccination. These data suggest that there may be greater cross-reactivity for functional nonneutralizing antibodies and cellular immune responses than for neutralizing antibodies against SARS-CoV-2 variants of concern. The mechanistic roles of these different immune responses in protecting against COVID-19 infection and disease remain to be determined, but data from nonhuman primates suggest that both humoral and cellular immune responses may contribute to protection.17
Limitations
This study has several limitations. First, the study size is small, and thus conclusions about vaccine safety and tolerability could not be made. Second, the correlates of immunogenicity and protection against COVID-19 infection and disease have not yet been determined. Third, as a cohort study rather than a randomized clinical trial, any differences in the findings among the groups cannot be assumed to be causal. Fourth, given the reliance on a convenience sample of women who were willing to be vaccinated, the generalizability of the findings may be limited. Fifth, immune responses were evaluated at a short interval after vaccination; thus, conclusions regarding durability cannot be drawn from these results.
Conclusions
In this exploratory analysis of a convenience sample, receipt of a COVID-19 messenger RNA vaccine was immunogenic in pregnant women, and vaccine-elicited antibodies were transported to infant cord blood and breast milk. Pregnant and nonpregnant women who were vaccinated developed cross-reactive antibody responses and T-cell responses against SARS-CoV-2 variants of concern.
References
- 1.Zambrano LD, Ellington S, Strid P, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641-1647. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ. Pregnancy, postpartum care, and COVID-19 vaccination in 2021. JAMA. 2021;325(11):1099-1100. doi: 10.1001/jama.2021.1683 [DOI] [PubMed] [Google Scholar]
- 3.Edlow AG, Li JZ, Collier AY, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3(12):e2030455. doi: 10.1001/jamanetworkopen.2020.30455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pace RM, Williams JE, Jarvinen KM, et al. COVID-19 and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. MedRxiv. Preprint posted online September 18, 2020. doi: 10.1101/2020.09.16.20196071 [DOI]
- 5.Madhi SA, Cutland CL, Kuwanda L, et al. ; Maternal Flu Trial (Matflu) Team . Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918-931. doi: 10.1056/NEJMoa1401480 [DOI] [PubMed] [Google Scholar]
- 6.Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis. 2017;64(1):9-14. doi: 10.1093/cid/ciw633 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . Information about COVID-19 vaccines for People who are pregnant or breastfeeding. Accessed March 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 8.Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. doi: 10.15585/mmwr.mm7008e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021. doi: 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korber B, Fischer WM, Gnanakaran S, et al. ; Sheffield COVID-19 Genomics Group . Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812-827.e19. doi: 10.1016/j.cell.2020.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp SA, Datir RP, Collier DA, et al. Recurrent emergence and transmission of a SARS-CoV-2 spike deletion ∆H69/V70. bioRxiv. Preprint posted online December 21, 2020. 10.1101/2020.12.14.422555 [DOI]
- 14.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27(4):622-625. doi: 10.1038/s41591-021-01285-x [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Werner AP, Koch M, et al. serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384(15):1468-1470. doi: 10.1056/NEJMc2102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman J, Bals J, St Denis K, et al. Naive human B cells can neutralize SARS-CoV-2 through recognition of its receptor binding domain. bioRxiv. Preprint posted online February 10, 2021. doi: 10.1101/2021.02.02.429458 [DOI]
- 17.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630-634. doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369(6505):812-817. doi: 10.1126/science.abc4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806-811. doi: 10.1126/science.abc6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. SARS-CoV-2 variant of classifications and definitions. Accessed March 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html
- 21.Centers for Disease Prevention and Control . v-safe: after vaccination health checker. Accessed March 8, 2021. https://vsafe.cdc.gov/en/
- 22.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616-622. doi: 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atyeo C, Alter G. The multifaceted roles of breast milk antibodies. Cell. 2021;184(6):1486-1499. doi: 10.1016/j.cell.2021.02.031 [DOI] [PubMed] [Google Scholar]
- 24.Baird JK, Jensen SM, Urba WJ, Fox BA, Baird JR. SARS-CoV-2 antibodies detected in human breast milk post-vaccination. MedRxiv. Preprint posted online March 2, 2021. doi: 10.1101/2021.02.23.21252328 [DOI] [PMC free article] [PubMed]
- 25.Muik A, Wallisch A-K, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371(6534):1152-1153. doi: 10.1126/science.abg6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384(15):1466-1468. doi: 10.1056/NEJMc2102017 [DOI] [PMC free article] [PubMed] [Google Scholar]


