Abstract
Background & Aims:
The tumor microbiome of patients with pancreas ductal adenocarcinoma (PDAC) includes bacteria normally present in the upper gastrointestinal tract. If the predominant source of intratumoral bacteria in patients with PDAC is retrograde migration from the duodenum, duodenal fluid could be a representative biospecimen for determining microbiome profiles of patients with PDAC or at risk of developing PDAC.
Methods:
We performed a case-control study comparing bacterial and fungal (16S and 18S rRNA) profiles of secretin-stimulated duodenal fluid collections from 308 patients undergoing duodenal endoscopy including 134 normal pancreas controls, 98 patients with pancreatic cyst(s) and 74 patients with PDAC.
Results:
Alterations in duodenal fluid microbiomes with diminished alpha diversity were significantly associated with age >70 and proton pump inhibitor use. Patients with PDAC had significantly decreased duodenal microbial alpha diversity compared to age-matched controls with normal pancreata and those with pancreatic cyst(s). There was evidence of enrichment of Bifidobacterium genera in the duodenal fluid of patients with PDAC compared to controls and those with pancreatic cyst(s). There were also enrichment of duodenal fluid Fusobacteria and Rothia bacteria among patients with PDAC with short-term survival. Duodenal fluid microbiome profiles were not significantly different between controls and patients with pancreatic cyst(s).
Conclusion:
Patients with PDAC have alterations in their duodenal fluid microbiome profiles compared to patients with pancreatic cysts and those with normal pancreata.
Keywords: Pancreatic cancer, microbiome, mycobiome, duodenal fluid
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the third most common cause of cancer-related deaths in the United States. The overall prognosis of patients with PDAC remains poor but has been improving, (5-year survival currently at 9%).1, 2 Recent studies have found that gut dysbiosis contributes to the pathogenesis of various diseases including cancer.3–5 The systemic metabolic consequences of gut microbial dysbiosis may contribute to cancer development 6 and responses to chemotherapy and immunotherapy.7 For example, tumor gut microbiome profiles have been linked to outcome after a PDAC diagnosis.8 Most such studies have used stool for microbiome profiling, although several have identified disease associations with the oral microbiome.9, 10 For example, in a study of PLCO and CPSII cohorts, carriers of the pathogenic periodontal bacteria Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans had an increased risk of development of PDAC.9
Studies in mice show gut microbiota can translocate into the pancreas especially in the presence of pancreatic cancer and germ-free mice and antibiotic-treated mice have reduced pancreatic dysplasia and tumor growth.11 Consistent with these findings, a recent study found concordance between an individual’s pancreatic and duodenal tissue microbiomes.12 If alterations in the gut microbiome are important for pancreatic cancer risk and progression, microbial alterations in the duodenal fluid may be most relevant to identifying and mediating this risk. Although the duodenal and oral microbiomes are similar, there are also differences.13, 14 Proton pump inhibitor (PPI) use, which is known to cause gut dysbiosis15, 16 and has recently been associated with pancreatic cancer risk in some,17, 18 but not all studies,19 and with cholangitis risk,20 would be expected to particularly affect gastric and duodenal microbiomes.21 We tested the hypothesis that duodenal fluid may contain microbial alterations associated with PDAC.
MATERIAL AND METHODS
Study population and sample collection
Patient biospecimens and clinical information were obtained from 308 participants enrolled in the Cancer of the Pancreas Screening (CAPS) studies at Johns Hopkins University,22, 23 134 normal pancreas controls, 98 with pancreatic cyst(s), and 74 with PDAC. Normal pancreas controls and subjects with pancreas cysts underwent endoscopic ultrasound for another upper gastrointestinal indication and did not have another cancer diagnosis. Covariate information was obtained from the medical record. Regular PPI use was defined as maintenance daily or alternate day use. Regular PPI users were divided into non-regular or low-dose (over-the-counter) users and regular or prescription-strength users. Duodenal fluid samples were collected from the duodenal lumen over 5 minutes through the endoscopic channel after intravenous human synthetic secretin (kindly provided by ChiRhoClin, Inc. Burtonsville, MD) infusion (0.2 μg/kg over 1 minute, administered as part of a clinical trial to evaluate the diagnostic yield of these samples clinicaltrials.gov NCT02000089), and aliquoted and stored at −80°C. The duodenal fluid microbiomes collected pre- and post-secretin infusion from ten subjects were compared and found to be very similar (Figure S1). To assess potential microbial contamination associated with collecting duodenal fluid, we analyzed sterilized water aspirated from four sterilized echoendoscopes; no amplifiable microbial DNA was detected. Fresh-frozen pancreatic cancer tissue, obtained at surgical resection along with adjacent non-neoplastic pancreas and/or normal duodenal tissue, was available for microbiome analysis from twelve patients who had undergone endoscopic duodenal fluid collection. All authors had access to the study data and reviewed and approved the final manuscript. All study elements were approved by the Johns Hopkins Hospital institutional review board, and written informed consent was obtained from all patients. Subjects participated at enrollment by consenting to studies aimed at understanding risk factors and biomarkers of pancreatic cancer development and outcome.
Bacterial and fungal DNA PCR
Primers were used to amplify the 16S V3–V4 rRNA region,24 and 18S ITS1 rRNA region (Table S1).25 Bacterial and fungal DNA standards were used as positive controls (Femto, Zymo).
Bacterial and Fungal rRNA gene sequencing
See Supplemental Methods.
Statistical analysis
Propensity score matching was performed to match for age. Statistical analyses were performed using JMP® v.13.0 (SAS Institute, NC, USA). Patient characteristics between two groups were compared using Pearson’s chi-squared test for categorical variables and Student’s t-test for continuous measures. Linear discriminant analysis (LDA) effect size (LEfSe) was used to evaluate greatest differences in taxa between groups.26 P<0.05 was considered statistically significant. Multiple comparisons adjustments were made using the Benjamini-Hochberg method.
RESULTS
Duodenal fluid microbial profiles in normal pancreas controls
The phylum, class, order, family and genus structure of the duodenal fluid microbiota in normal pancreas controls is shown in Figure S2. The following phyla had >1% abundance: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, Patescibacteria and Epsilonbacteraeota.
We found regular PPI use was associated with an altered duodenal microbiome. PPI users had significantly lower alpha diversity than PPI non-users by observed OTUs (p=0.0006), Shannon Index (p=0.0004) and Faith PD (p=0.03, Figure 1A). PPI users had significant enrichment of Streptococcus by genus level analysis (Figure 1B) and by LEfSe analysis (Figure 1C). Alpha diversity was compared among the age-matched three groups based on daily dose of PPI: non-users vs. low-dose users vs. regular or high-dose users (Table S2). There was dose-dependent decrease in bacterial alpha diversity among the three groups (observed OTUs, p=0.001; Shannon Index, p=0.001)(Figure S3). We also found lower alpha diversity in those >70 years old and in current smokers (Figure S4). Reports have implicated FUT2 genotypes in gut microbiota composition,27 and resistance and/or susceptibility to certain infections,28 but LEfSe analysis identified few differences by secretor/non-secretor status (Figure S5).
Figure 1. Duodenal fluid microbiomes of PPI users and non-users:
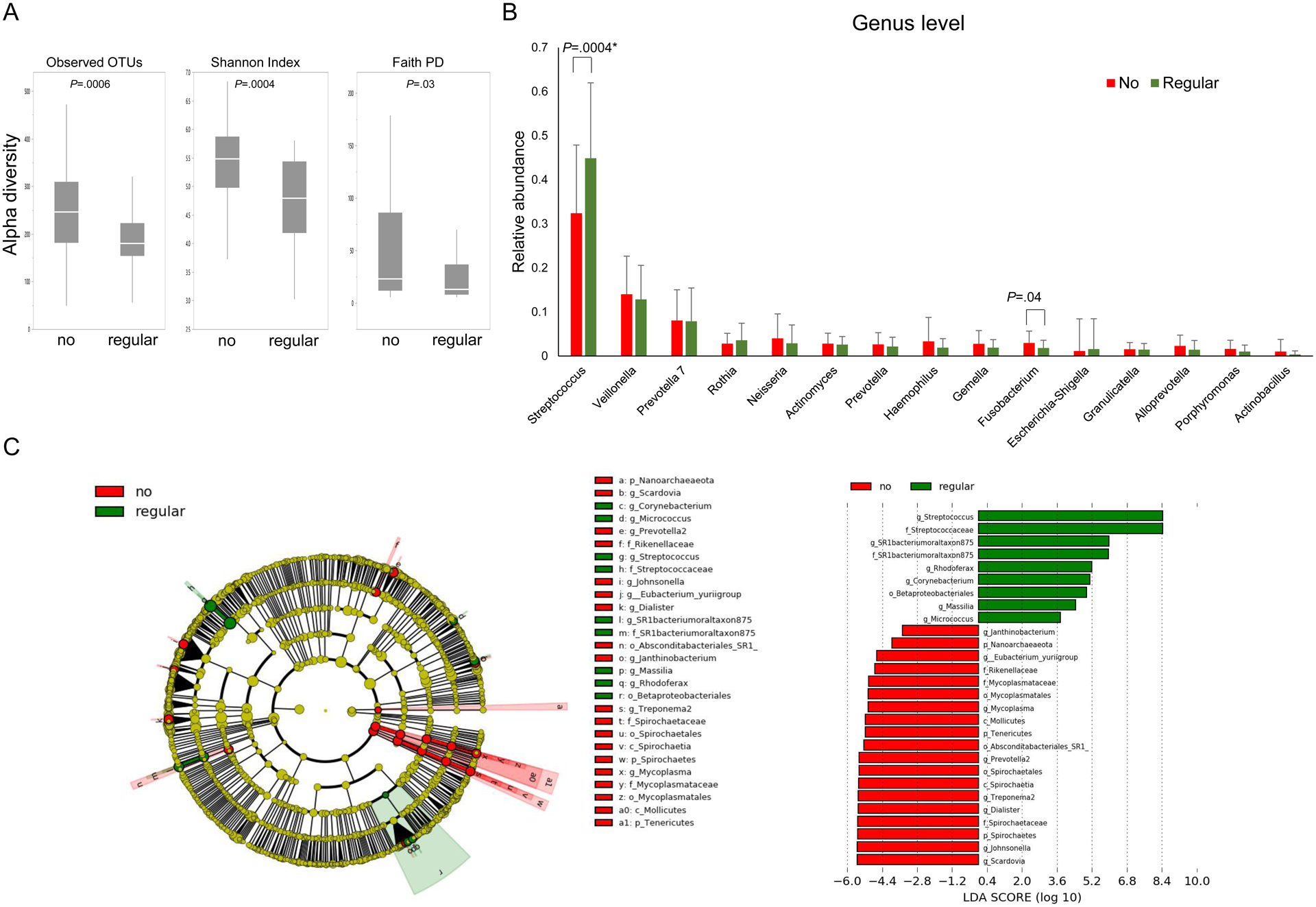
(A) The duodenal microbiomes of regular PPI users and non-users in normal pancreas controls were analyzed for alpha-diversity measures. (B) The relative abundance of genera between the two groups. (C) LDA score computed from features differentially abundant between regular-PPI users and non-users.
Duodenal fluid microbiomes of patients with PDAC vs. normal pancreas
Patients with PDAC were matched to controls by age and had similar demographic characteristics but were more likely to have diabetes, biliary obstruction, and to be regular PPI users than normal pancreas controls (Table 1). Duodenal fluid bacterial DNA levels were higher among those with PDAC compared to normal pancreas controls (p=0.04, Figure 2A). Alpha diversity measures (Figure 2B), including observed OTUs (p=0.005), Shannon index (p=0.03) and Faith’s PD (p=0.006) were significantly decreased in patients with PDAC compared to normal pancreas controls. When stratified by PPI status, the lower alpha diversity in PDAC vs. controls was confined to non-PPI users (Figure S5). Beta diversity measured by unweighted (p=0.001 using PERMANOVA, Figure 2C), but not weighted (p=0.071, Figure 2D), Unifrac principal coordinate analysis was significantly different in patients with PDAC compared to normal pancreas controls.
Table 1.
Patient Characteristics: : PDAC vs. normal pancreas
| Bacterial analysis | Fungal analysis | |||||
|---|---|---|---|---|---|---|
| Normal | PDAC | p value | Normal | PDAC | p value | |
| Cases | 63 | 63 | 43 | 43 | ||
| Age (years); mean (range) | 63.6 (41.6 – 79.5) | 65.3 (42.2 – 85.5) | 0.253 | 64.4 (50.4 – 79.5) | 66.3 (50.1 – 84.6) | 0.2624 |
| Sex | 0.073 | 0.2424 | ||||
| Male (%) | 30 (47.6%) | 40 (63.5%) | 23 (53.5%) | 28 (65.2%) | ||
| Female (%) | 33 (52.4%) | 23 (36.5%) | 20 (46.5%) | 18 (34.9%) | ||
| Race | 0.76 | 0.6724 | ||||
| White (%) | 57 (90.4%) | 57 (90.4%) | 39 (90.7%) | 40 (93.0%) | ||
| Non-white (%) | 5 (8.0%) | 4 (6.4%) | 4 (9.3%) | 2 (4.7%) | ||
| Missing (%) | 1 (1.6%) | 2 (3.2%) | 0 (0%) | 1 (2.3%) | ||
| Smoking status | 0.201 | 0.2483 | ||||
| Never smoker (%) | 44 (69.8%) | 36 (57.1%) | 30 (70.0%) | 23 (53.5%) | ||
| Former smoker (%) | 18 (28.6%) | 23 (36.5%) | 12 (27.9%) | 17 (39.5%) | ||
| Current smoker (%) | 1 (1.6%) | 4 (6.4%) | 1 (2.3%) | 3 (7.0%) | ||
| Alcohol consumption | 0.354 | 0.3535 | ||||
| Never or occasional (%) | 36 (57.1%) | 36 (57.1%) | 26 (60.5%) | 24 (55.8%) | ||
| Regular or heavy (%) | 27 (42.9%) | 25 (39.7%) | 17 (39.5%) | 17 (39.5%) | ||
| Missing (%) | 0 (0%) | 2 (3.2%) | 0 (0%) | 2 (4.7%) | ||
| Diabetes | <.0001 | 0.0281 | ||||
| Yes (%) | 5 (7.9%) | 22 (34.9%) | 6 (14.0%) | 13 (30.2%) | ||
| No (%) | 58 (92.1%) | 38 (60.3%) | 37 (86.0%) | 27 (62.8%) | ||
| Missing (%) | 0 (0%) | 3 (4.8%) | 0 (0%) | 3 (7.0%) | ||
| PPI use | 0.005 | 0.0162 | ||||
| Regular (%) | 15 (23.8%) | 34 (54.0%) | 7 (16.3%) | 17 (39.5%) | ||
| None (%) | 48 (76.2%) | 29 (46.0%) | 36 (83.7%) | 26 (60.5%) | ||
| Probiotics | 0.094 | 0.3145 | ||||
| Yes (%) | 1 (1.6%) | 5 (7.9%) | 0 (0%) | 1 (2.3%) | ||
| No (%) | 62 (98.4%) | 58 (92.1%) | 43 (100%) | 42 (97.7%) | ||
| Antibiotics | 0.171 | 0.5567 | ||||
| Yes (%) | 1 (1.6%) | 4 (6.3%) | 1 (2.3%) | 2 (4.6%) | ||
| No (%) | 62 (98.4%) | 59 (93.7%) | 42 (97.7%) | 41 (93.4%) | ||
| Biliary obstruction | 0 | 11 (17.5%) | 6E-04 | 0 | 8 (18.6%) | 0.0055 |
| Tumor location | ||||||
| head (%) | 42 (66.7%) | 29 (67.4%) | ||||
| body (%) | 15 (23.8%) | 12 (27.9%) | ||||
| tail (%) | 6 (9.5%) | 2 (4.7%) | ||||
| Tumor Stage | ||||||
| IA (%) | 2 (3.2%) | 0 (0%) | ||||
| IB (%) | 6 (9.5%) | 2 (4.7%) | ||||
| IIA (%) | 9 (14.3%) | 6 (13.9%) | ||||
| IIB (%) | 18 (28.6%) | 12 (27.9%) | ||||
| III (%) | 9 (14.3%) | 6 (13.9%) | ||||
| IV (%) | 19 30.1%) | 17 (39.5%) | ||||
Figure 2. Duodenal fluid bacterial microbiomes in patients with PDAC vs. Normal pancreas:
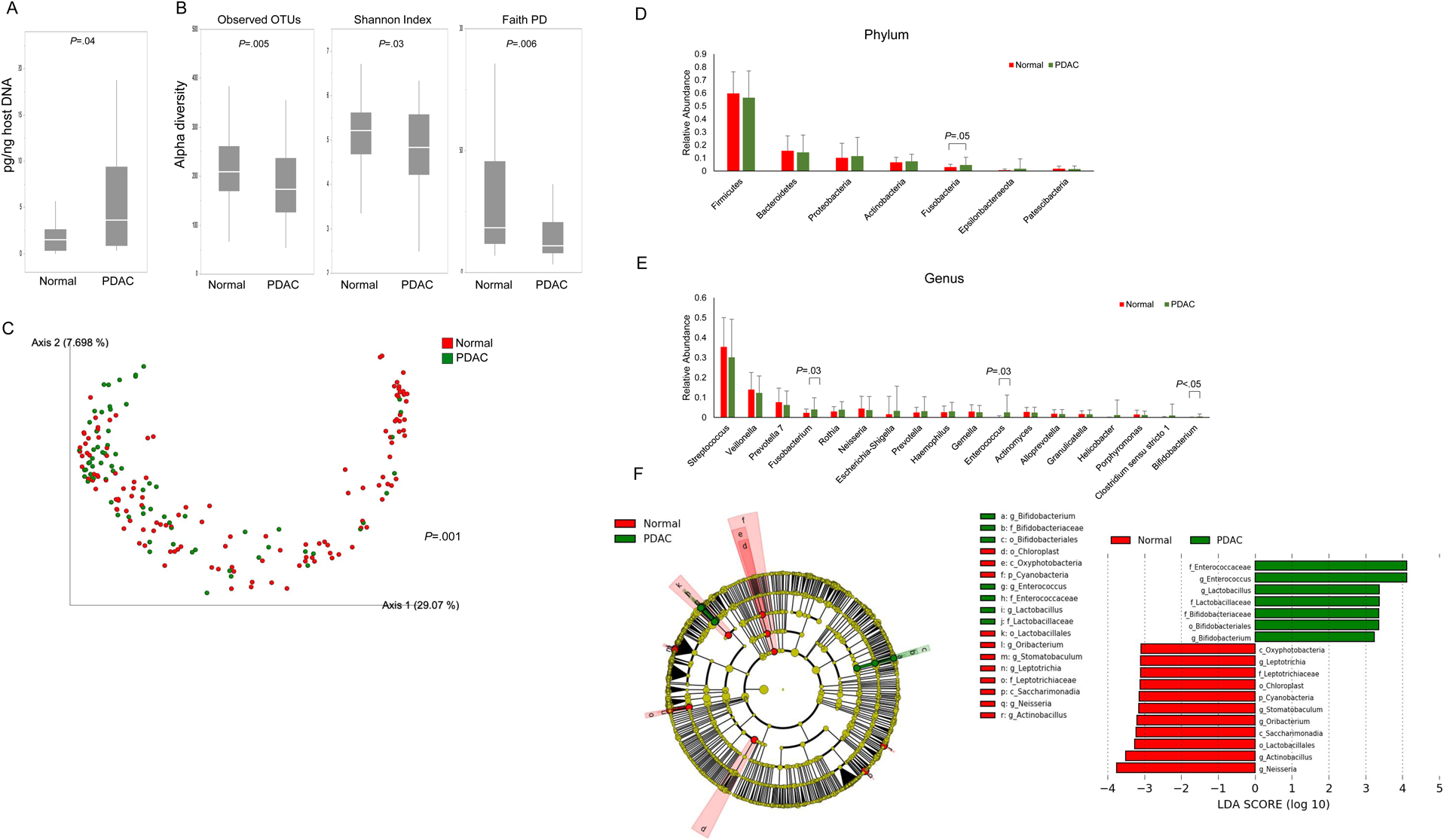
(A) Duodenal fluid bacterial concentrations. (B) The duodenal microbiomes of PDAC vs. normal pancreas controls were analyzed for alpha diversity measures. The beta diversity among these groups were compared by (C) Unweighted Unifrac plot. P values were determined by pairwise PERMANOVA. The relative abundance of (D) phylum and (E) genera between PDAC vs. normal pancreas controls. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score >3.0.
We next compared relative microbe counts and found Fusobacteria phylum abundance was higher in those with PDAC (P=0.05), though not significantly different after multiple comparison adjustment (Figure 2D–E). At the genus level, Fusobacterium, Enterococcus and Bifidobacterium were higher in patients with PDAC compared to normal pancreas controls (Fusobacterium; P=0.03, Enterococcus; P=0.03, Bifidobacterium; P<0.05); but not statistically significant so after multiple comparison adjustment. Stratified by PPI status, Fusobacterium was significantly more abundant in PDAC cases and Streptococcus in age-matched normal pancreas controls, among PPI users but not non-users, though these differences were no longer significant after multiple comparison correction (Figure S7).
LEfSe analysis (Figure 2F) found patients with PDAC had a predominance of Enterococcaceae, Lactobacillaceae and Bifidobacteriaceae compared to normal pancreas controls. Excluding nine patients that reported recent use of probiotics and/or antibiotics yielded similar results (Figure S8A–C), as did excluding cases and controls with diabetes (Figure S8D).
Duodenal fluid microbiomes of patients with PDAC vs. pancreatic cyst(s)
We compared duodenal bacterial microbiomes of patients with PDAC to age-matched patients with pancreatic cyst(s) (Table 2, Figure 3). Patients with PDAC had significantly reduced alpha diversity than those with pancreatic cyst(s) (Figure 3B). Beta diversity was also significantly different (unweighted Unifrac plot, p=0.010)(Figure 3C). Duodenal fluid samples from patients with PDAC trended towards higher levels of Escherichia-Shigella, Enterococcus, Clostridium sensu stricto 1 and Bifidobacterium DNA (Figure 3D–E). LEfSe analysis (Figure 3F) showed a similar predominance of Enterococcaceae, Lactobacillaceae and Bifidobacteriaceae at the family level among patients with PDAC, while those with pancreas cysts showed predominance of Porphyromonadaceae, Corynebacteriaceae and Leptotrichiaceae.
Table 2.
Patient Characteristics: PDAC vs. pancreatic cyst
| Bacterial analysis | Fungal analysis | |||||
|---|---|---|---|---|---|---|
| Pancreas cyst | PDAC | p value | Pancreas cyst | PDAC | p value | |
| Cases | 72 | 72 | 51 | 51 | ||
| Age (years); mean (range) | 65.8 (42.9 – 87.8) | 66.7 (42.2 – 85.5) | 0.5513 | 66.6 (49.0 – 87.3) | 67.4 (51.3 – 85.5) | 0.6339 |
| Sex | 0.0924 | 0.1627 | ||||
| Male (%) | 36 (50.0%) | 46 (63.9%) | 25 (49.0%) | 32 (62.8%) | ||
| Female (%) | 36 (50.0%) | 26 (36.1%) | 26 (51.0%) | 19 (37.2%) | ||
| Race | 0.5494 | 0.6721 | ||||
| White (%) | 65 (90.3%) | 65 (90.3%) | 47 (92.2%) | 46 (90.2%) | ||
| Non-white (%) | 5 (6.9%) | 5 (6.9%) | 3 (5.9%) | 3 (5.9%) | ||
| Missing (%) | 3 (3.4%) | 2 (2.8%) | 1 (1.9%) | 2 (3.9%) | ||
| Smoking status | 0.1958 | 0.8619 | ||||
| Never smoker (%) | 48 (66.6%) | 39 (54.2%) | 28 (54.9%) | 26 (51.0%) | ||
| Former smoker (%) | 22 (30.6%) | 29 (40.3%) | 21 (41.2%) | 22 (43.1%) | ||
| Current smoker (%) | 1 (1.4%) | 4 (5.6%) | 2 (3.9%) | 3 (5.9%) | ||
| Missing (%) | 1 (1.4%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Alcohol consumption | 0.3594 | 0.746 | ||||
| Never or occasional (%) | 44 (61.1%) | 42 (58.3%) | 32 (62.8%) | 29 (56.9%) | ||
| Regular or heavy (%) | 28 (38.9%) | 28 (38.9%) | 18 (35.3%) | 20 (39.2%) | ||
| Missing (%) | 0 (0%) | 2 (2.8%) | 1 (1.9%) | 2 (3.9%) | ||
| Diabetes | 0.0042 | 0.0641 | ||||
| Yes (%) | 12 (16.7%) | 26 (36.1%) | 10 (19.6%) | 16 (31.3%) | ||
| No (%) | 60 (83.3%) | 43 (59.7%) | 41 (80.4%) | 32 (62.8%) | ||
| Missing (%) | 0 (0%) | 3 (4.2%) | 0 (0%) | 3 (5.9%) | ||
| PPI use | 0.0001 | 0.0218 | ||||
| Regular (%) | 15 (20.8%) | 37 (51.4%) | 12 (23.5%) | 23 (45.1%) | ||
| None (%) | 57 (39.6%) | 35 (18.6%) | 39 (76.5%) | 28 (54.9%) | ||
| Probiotics | 0.4669 | 1 | ||||
| Yes (%) | 3 (2.1%) | 5 (6.9%) | 3 (5.9%) | 3 (5.9%) | ||
| No (%) | 69 (79.2%) | 67 (93.1%) | 48 (94.1%) | 48 (94.1%) | ||
| Antibiotics | 0.1721 | 0.1689 | ||||
| Yes (%) | 1 (1.4%) | 4 (5.6%) | 1 (1.9%) | 4 (7.8%) | ||
| No (%) | 71 (98.6%) | 68 (94.4%) | 50 (98.1%) | 47 (92.2%) | ||
| Cyst or Tumor location | ||||||
| head (%) | 30 (41.7%) | 49 (68.1%) | 18 (35.3%) | 34 | ||
| body (%) | 28 (38.9%) | 17 (23.6%) | 22 (43.1%) | 13 | ||
| tail (%) | 14 (19.4%) | 6 (8.3%) | 11 (21.6%) | 4 | ||
| Cyst size (cm); mean (range) | 1.24 (0.2–2.8) | 1.25 (0.2–8.0) | ||||
| Number of cysts; mean (range) | 2.41 (1–8) | 2.5 (1–8) | ||||
| Worrisome features | ||||||
| Yes (%) | 8 (11.1%) | 5 (9.8%) | ||||
| MPD dilation | 4 | 1 | ||||
| Mural nodule | 4 | 4 | ||||
| No (%) | 64 (88.9%) | 46 (90.2%) | ||||
| Stage | ||||||
| IA (%) | 2 (2.8%) | 2 (3.9%) | ||||
| IB (%) | 7 (9.7%) | 3 (5.9%) | ||||
| IIA (%) | 10 (13.9%) | 7 (13.7%) | ||||
| IIB (%) | 20 (27.8%) | 14 (27.5%) | ||||
| III (%) | 9 (12.5%) | 6 (11.8%) | ||||
| IV (%) | 24 (33.3%) | 19 (37.2%) | ||||
Figure 3. Duodenal fluid bacterial microbiomes in patients with PDAC vs pancreatic cyst(s):
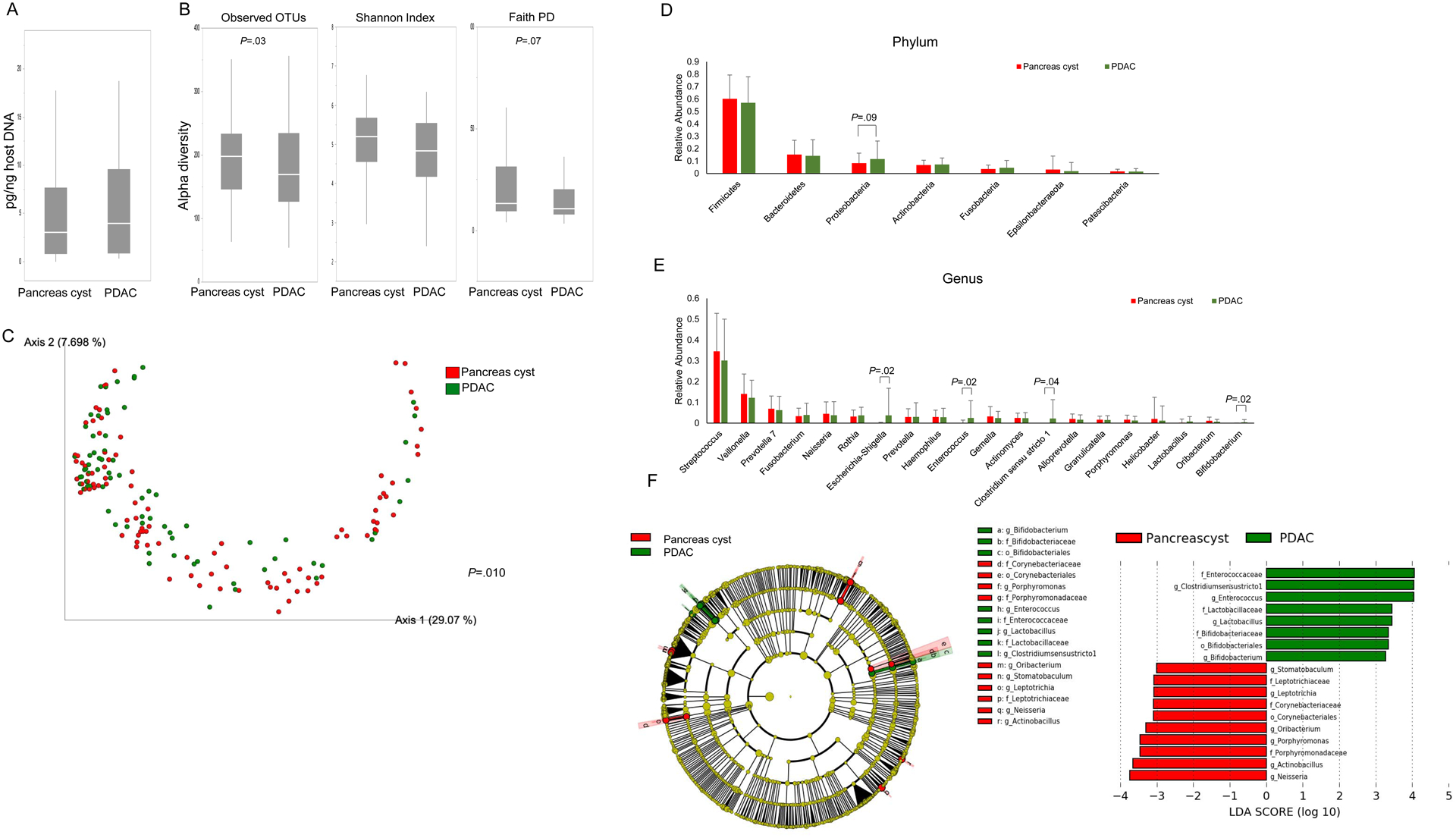
(A) Duodenal fluid bacterial concentrations PDAC subjects vs. age-matched subjects with a pancreas cyst. (B) The duodenal microbiomes of PDAC and pancreas cyst subjects were analyzed for alpha-diversity measures. Beta diversity compared by (C) unweighted, and weighted Unifrac plot. The relative abundance of phylum (D) and genera (E) between the two groups. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score >3.0.
Duodenal fluid microbiomes in patients with normal pancreas vs. pancreatic cyst(s)
Duodenal bacterial DNA levels in patients with pancreas cysts were not significantly increased compared to age-matched subjects with a normal pancreas (p=0.06, Figure S9A)(Table S3). Patients with pancreas cysts had lower alpha diversity than normal pancreas controls (Figure S8B). Beta diversity (unweighted Unifrac plot) was also significantly different (p=0.031)(Figure S9C–D). Phylum, genus and LEfSe comparisons did not identify differences between the normal pancreas and pancreatic cyst subjects (Figure S9E–G).
Similar results were obtained when an age-matched three-group (PDAC, pancreatic cyst, normal pancreas) analysis was performed (Figure S10). If these groups are stratified by PPI status (non-users and regular-users), bacterial alpha diversity were different among the three subgroups: Faith’s PD (p=0.036 in non-users, p=0.078 in regular-users, respectively)(Figure S11A,B). Across the six subgroups, bacterial alpha diversity was significantly decreased in both PPI users and non-users with PDAC compared to PPI non-users with normal pancreas: (Faith’s PD, Figure S11C). When stratified by smoking status, bacterial alpha diversity in non-smokers, (there were too few smokers), was significantly reduced in the PDAC group compared to age-matched controls: observed OTUs (p=0.025) and Faith’s PD (p=0.004)(Figure S12A). See also supplemental results.
Duodenal fluid mycobiomes
Duodenal fluid fungal DNA concentrations were lower than bacterial DNA concentrations in PDAC cases and controls (Figures 2A, S13). Fungal DNA levels were higher in patients with PDAC compared to normal pancreas controls and those with pancreas cyst(s) (p=0.01, p=0.03, Figure S9). The phylum, class, order, family and genus structure of the duodenal fluid fungal mycobiota of normal pancreas controls is shown in Figure S14. The duodenal fluid of PPI users was enriched with several of the normally less abundant luminal fungi (Figure S15). The duodenal mycobiomes of an age-matched set of FUT2 secretors and non-secretors with a normal pancreas did not show any significant differences (Figure S16).
One measure of fungal DNA alpha diversity, Faith’s PD, was significantly decreased in patients with PDAC compared to normal pancreas controls (p=0.02, Figure 4A). Beta diversity was also significantly different (Figure 4B, unweighted Unifrac; p=0.009, Figure 4C, weighted Unifrac plot; p=0.009). We next analyzed relative fungal microbial abundance by patient group (Figure 4D–E). At the phylum level, we found higher levels of Asomycota in patients with PDAC (P=0.02, non-significant after correction and higher levels of Nakaseomyces at the genus level (P=0.01, non-significant after correction) compared to those with a normal pancreas. LEfSe analysis (Figure 4F) revealed a predominance of Nakaseomyces and Skeletocutis at the genus level in patients with PDAC.
Figure 4. Duodenal fluid mycobiomes in patients with PDAC vs. those with normal Pancreata:
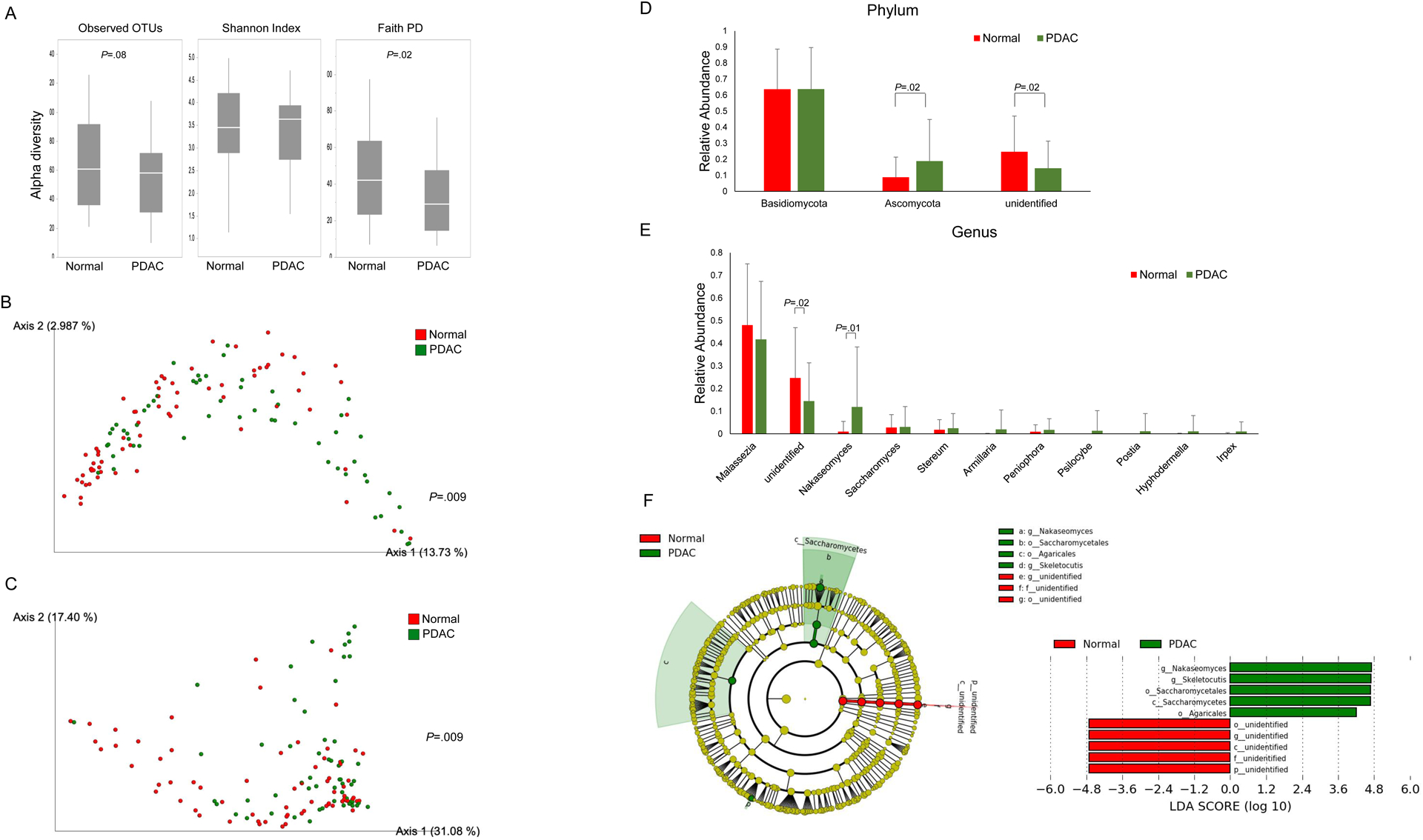
(A) Duodenal mycobiome alpha-diversity measures. The beta diversity compared by (B) Unweighted, and (C) Weighted Unifrac plot. Relative phylum (D) and genera (E) abundance between groups. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score >3.0.
Fewer fungal OTUs were observed in patients with PDAC compared to those with pancreatic cyst(s) (Figure S17A). Beta diversity was also significantly different (p=0.017)(Figure S17B–C). Patients with PDAC had significantly more Basidiomycota and more Nakaseomyces (non-significant after correction) and less Saccharomyces at the genus level (significant) than those with pancreatic cysts (Figure S10E, S17D–E). We also found a predominance of Saccharomyces in the pancreas cyst(s) group by LEfSe analysis (Figure S17F).
Among patients with a pancreas cyst compared to those with a normal pancreas we found similar fungal alpha diversity (Figure S18A) but significantly different beta diversity (unweighted, p=0.001)(Figure S18B–C). There were also significant differences in the relative fungal abundance in phyla and genera between the normal pancreas and pancreas cyst groups (Figure S18D–E). Patients with normal pancreata had higher levels of Basidiomycota at the phylum level and Malassezia at the genus level whereas Ascomycota and Saccharomyces were significantly enriched in those with pancreas cyst(s). Similar relative abundances were found by LEfSe analysis (Figure S18F).
Pancreas and duodenal tissue microbiome/mycobiomes
Profiles of pancreatic cancer tissue, adjacent non-cancer pancreatic tissue, normal duodenal tissue from patients who underwent pancreatic resection (Table S4) and their duodenal fluid microbiomes (Figure S19, S20) and mycobiomes (Figure S21, S22) showed broad similarities, including a preponderance of Proteobacteria, the most common phyla in the upper gastrointestinal tract, confirming previous reports.8, 11, 12, 29, 30 Overall, there were fewer bacterial genera in the pancreatic tumors than in duodenal fluid as well as cases where bacterial genera were detected in pancreatic cancer tissue but not in duodenal fluid.
Duodenal fluid microbiomes and survival of patients with PDAC
We compared bacterial profiles of short- vs. longer-term survivors stratified at the 75th percentile survival cut-off. At the genus level, after adjusting for multiple comparisons correction, duodenal samples from short-term survivors were significantly more enriched with Fusobacterium, Rothia, and Neisseria (Figure S23).
DISCUSSION
We find: (i) patients with PDAC had higher levels of amplifiable bacterial and fungal DNA in their duodenal fluid than controls with a normal pancreas even after controlling for age, smoking and PPI use; (ii) and that microbial diversity is decreased in patients with PDAC compared to controls with a normal pancreas. The duodenal fluid microbial profiles we observed in those with a normal pancreas are similar to that have been reported previously in healthy controls in duodenal biopsies.14, 31 The constituents of the duodenal microbiome may be particularly important if retrograde colonization of pancreatic tissues is important in PDAC pathogenesis. We also find that in our normal pancreas controls the duodenal fluid microbiomes of regular PPI users had numerous differences compared to non-users, similar to studies comparing stool microbiomes.15, 16 The duodenal fluid microbiome alterations associated with PPI use could be etiologically important given the potential association between PPI use and pancreatic cancer risk.17–19
The bacterial shifts with PPI use include an increase in increase in predominantly oral bacteria such as Streptococcus and Fusobacteria. We found Fusobacterium which has been associated with PDAC outcome32 is enriched in the duodenal fluid microbiomes of patients with PDAC compared to normal pancreas controls and is more abundant in PDAC patients with short survival. Periodontitis is associated with pancreas cancer and oral Fusobacterium has also been associated with several cancers included PDAC,33, 34 and has been detected in pancreatic cystic tumors35. Although we found higher levels of Fusobacterium in the duodenal fluid of patients with PDAC compared to normal pancreas controls, Fusobacterium was only occasionally detected in resected pancreas tissues, consistent with prior studies.8 Fusobacterium-containing tumors have been associated with a poor prognosis in patients with colorectal, esophageal cancer and PDAC.36–38
Bifidobacterium was also enriched in patients with PDAC compared to those with pancreatic cyst(s) and normal pancreas. Repopulation of the germ-free KC model with Bifidobacterium pseudolongum accelerated pancreatic oncogenesis and could be detected in the pancreas of treated mice.11 Del Castillo et al reported a higher prevalence of Bifidobacterium in pancreatic/duodenal tissue samples from PDAC subjects compared with controls.12 We also found higher levels of fungal DNA in patients with pancreatic cancer and higher levels of Ascomycota in duodenal fluid samples from patients with pancreatic cysts. A recent report by Aykut et al39 found PDAC tumors frequently contained Malassezia DNA, the most abundant genus in duodenal fluid, and found evidence for a pathogenic mechanism. Another emerging microbial pathogenic mechanism is the DNA damage signature in multiple cancer genomes including pancreas created by colibactin toxin produced by some E. coli strains.40, 41
Similar to our results, Ren et al reported decreased microbial alpha diversity in patients with PDAC.32 A recent study reported higher alpha diversity in the tumor microbiome of long-term survivors after a PDAC diagnosis.8 We also observed significant differences in beta diversity between patients with PDAC compared to normal pancreas controls.
Comparison of the microbial profiles of resected pancreatic cancer tissues with duodenal fluid revealed examples of concordance as well as discordance of bacterial and fungal genera consistent with the hypothesis that the main source of microbes within pancreatic tumors is the upper gastrointestinal tract with discordance possibly reflecting examples of hematogenous sources of bacteria.42
The limitations of this study include: (i) performance at a single academic medical center, (ii) confounding factors between cases and controls and (iii) pathophysiological consequences of having pancreatic cancer which can cause microbiome/mycobiome alterations. Larger prospective studies that include other geographic regions and populations are needed to confirm our findings and prospective collection of duodenal fluids prior to a pancreatic cancer diagnosis is needed to establish microbiome alterations associated with pancreatic cancer risk.
In conclusion, we find alterations in the duodenal fluid microbiome and mycobiome in patients with pancreatic cancer. Further studies are needed to determine if duodenal microbiome profiles could be used to better stratify the pancreatic cancer risk of patients under pancreatic surveillance.
Supplementary Material
Supplementary Figure Legends
Supplementary Figure 1 Relative abundance of duodenal bacteria in duodenal fluid (DJ) and post-secretin duodenal fluid (PDJ).
Supplementary Figure 2 Relative abundance of each taxa in normal pancreas group. The data shown were filtered by frequency higher than 1%. (A) phylum level (B) class level (C) order level (D) family level (E) genus level.
Supplemental Figure 3 Duodenal fluid microbiomes associated with PPI dose:
(A) The duodenal bacterial microbiomes of non-PPI regular users vs. low dose PPI users vs. regular or high dose PPI users in normal pancreas controls were analyzed for alpha diversity measures. (B) The fungal microbiomes of the three groups were analyzed for alpha diversity measures. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplementary Figure 4 The duodenal bacterial microbiomes of normal pancreas group was analyzed for alpha diversity measures, including Observed OTUs, Shannon Index and Faith PD. (A) Age (B) Alcohol (C) Smoking (D) DM (E) BMI.
Supplementary Figure 5 Duodenal fluid microbiomes among patients with normal pancreata stratified by FUT2 status: (A) The duodenal microbiome alpha-diversity measures. (B) Relative Genus abundance. (C) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 6 Duodenal fluid alpha diversity comparisons in PDAC vs normal pancreas controls stratified by PPI use.
Supplementary Figure 7 Genus level comparison of duodenal fluid microbiomes in PDAC vs. normal controls: (Top): PPI and non-PPI users. (Bottom) Streptococcus, Fusobacteria and Lactobacillus duodenal fluid abundance in PPI users: PDAC vs. normal controls.
Supplementary Figure 8 Duodenal fluid microbiomes: PDAC vs Normal Pancreas excluding patients who reported recent use of probiotics and antibiotics (A)–(C) and (D) excluding patients with diabetes. (A) Duodenal mycobiome alpha-diversity measures. (B) Relative Genus abundance. (C)–(D) Taxonomic cladogram from LEfSe analysis.
Supplementary Figure 9 Duodenal fluid bacterial microbiomes in patients with pancreas cyst(s) vs. Normal pancreas:
(A) Bacterial content was compared in normal pancreas group and pancreas cyst group who were matched with age. (B) The duodenal microbiomes of normal pancreas group and pancreas cyst group were analyzed for alpha-diversity measures, including Observed OTUs, Shannon Index and Faith PD. The beta diversity among these groups were tested by (C) Unweighted Unifrac plot and (D) Weighted Unifrac plot. P values were determined by pairwise PERMANOVA. (E) There were no significant differences in the relative abundance of phylum between two groups. The date showed are filtered by frequency higher than 1%. (F) There were no significant differences in the relative abundance of genus between two groups. The date showed are filtered by frequency higher than 1%. (G) Taxonomic cladogram from LEfSe analysis, showing taxonomic association from between pancreas cyst group and PDAC group.
Supplemental Figure 10 Duodenal fluid microbiomes in patients with PDAC vs. pancreatic cyst(s) vs. normal pancreas:
(A) The duodenal bacterial microbiomes of PDAC vs. pancreatic cyst(s) vs. normal pancreas controls were analyzed for alpha diversity measures. (B, C) The relative abundance of bacterial genera among the three groups. (D) The duodenal fungal microbiomes of the three groups were analyzed for alpha diversity measures. (E) The relative abundance of fungal phylum among the three groups. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplementary Figure 11 Duodenal fluid microbiomes in patients with PDAC vs. pancreatic cyst(s) vs. normal pancreas stratified by PPI status:
The duodenal bacterial microbiomes of PDAC vs. pancreatic cyst(s) vs. normal pancreas controls were analyzed for alpha diversity measures in (A) non PPI users and (B) PPI regular users, and (C) when the three disease groups were stratified by PPI status. The duodenal fungal microbiomes of the three disease groups were analyzed for alpha diversity measures in (D) non PPI users and (E) PPI regular users, and (F) stratified by PPI status. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplemental Figure 12 Duodenal fluid mycobiomes in non-smoker patients with PDAC vs. pancreatic cyst(s) vs. normal pancreas:
The duodenal (A) bacterial and (B) fungal microbiomes of PDAC vs. pancreatic cyst(s) vs. normal pancreas controls of non-smokers were analyzed for alpha diversity measures. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplementary Figure 13 Duodenal fluid Fungal DNA abundance Fungal DNA abundance in patients with PDAC vs. a normal pancreas (left), patients with PDAC vs. pancreatic cyst(s) (middle), and patients with pancreatic cyst(s) vs. a normal pancreas (right panel).
Supplementary Figure 14 Duodenal fluid Fungal DNA in patients with normal pancreata The relative abundance of duodenal fungal DNA by (A) phylum, (B) class, (C) order, (D) family and (E) genus in patients with normal pancreata.
Supplementary Figure 15 Duodenal fluid mycobiomes among PPI users vs. non-users: (A) The duodenal mycobiome alpha-diversity measures. (B) Relative Genus abundance. (C) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 16 Duodenal fluid mycobiomes among patients with normal pancreata stratified by FUT2 status: (A) The duodenal mycobiome alpha-diversity measures. (B) Relative Genus abundance. (C) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 17 Duodenal fluid mycobiomes in patients with PDAC vs Pancreas cyst(s): (A) Duodenal mycobiome alpha-diversity measures. Beta diversity determined by (B) Unweighted and (C) Weighted Unifrac plots. The relative phylum (D) and genera (E) abundance between groups. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score >3.0.
Supplementary Figure 18 Duodenal fluid mycobiomes among patients with normal pancreas vs. pancreatic cyst (A) The duodenal mycobiome alpha-diversity measures, (B) beta diversity measures by Unweighted Unifrac plot and (C) Weighted Unifrac plot. P values were determined by pairwise PERMANOVA. (D) Differences in the relative phylum abundance between groups. (E) Differences in the relative genus abundance between groups. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 19 (A) Relative abundance of bacterial DNA in pancreatic tumors and duodenal fluid. (B–D) correlations of specific bacteria between duodenal fluid and pancreatic cancer tissue. The Y axis indicates the abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 20 Relative abundance of bacteria in matched pancreatic cancer tissue, duodenal fluid, normal pancreas and normal duodenum in selected patients. Normal=normal pancreas, PSJ=duodenal fluid. The Y axis is the abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 21 Relative abundance of fungal DNA by genus in pancreatic cancer tissue, normal pancreas, duodenal tissue and duodenal fluid. The Y axis is the abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 22 Relative abundance of fungal DNA in matched pancreatic cancer tissue, duodenal fluid, normal pancreas and normal duodenum. Normal=normal pancreas, PSJ=duodenal fluid. Y axis: abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 23 Top left: Alpha diversity measures in patients with PDAC stratified by tumor stage; early (Stage I and II vs. Stage III and IV). Top right: Bacterial genera in patients with PDAC stratified by tumor stage. Bottom left: Bacterial genera in patients with PDAC stratified by tumor location. Bottom right: Bacterial genera in patients with PDAC stratified by patient outcome (short vs. longer-term survivors).
Background:
Microbes within pancreas cancers are thought to arise predominantly from retrograde migration from the duodenum.
Findings:
Patients with pancreatic cancer have alterations in their duodenal fluid bacteria, even when other factors such as proton-pump inhibitor use are accounted for. Several duodenal bacteria among patients with pancreatic cancer with short-term survival.
Implications for patient care:
This study raises the possibility that factors that influence duodenal fluid bacterial profiles such as the use of proton-pump inhibitors could impact pancreatic cancer risk and survival after a pancreatic cancer diagnosis.
Grant Support:
This work was supported by NIH grants (CA210170 and CA62924), Susan Wojcicki and Dennis Troper, and by a Stand Up To Cancer-Lustgarten Foundation Pancreatic Cancer Interception Translational Cancer Research Grant (Grant Number: SU2C-AACR-DT25-17). Stand Up To Cancer is a program of the Entertainment Industry Foundation. SU2C research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. MG is the Sol Goldman Professor of Pancreatic Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any personal or financial conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Blackford AL, Canto MI, Klein AP, et al. Recent trends in the incidence and survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results analysis. J Natl Cancer Inst 2020;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 4.Garrett WS. Cancer and the microbiota. Science 2015;348:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier JB, Young VB, Skufca J, et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer. 2020;6:192–204. doi: 10.1016/j.trecan.2020.01.004. Epub 2020 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AM, Manghi P, Asnicar F, et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med 2019;25:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CL, O’Toole PW, Shanahan F. The Gut Microbiota in Causation, Detection, and Treatment of Cancer. Am J Gastroenterol 2019;114:1036–1042. [DOI] [PubMed] [Google Scholar]
- 8.Riquelme E, Zhang Y, Zhang L, et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019;178:795–806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 2018;67:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582–8. doi: 10.1136/gutjnl-2011-300784. Epub 2011 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pushalkar S, Hundeyin M, Daley D, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov 2018;8:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Castillo E, Meier R, Chung M, et al. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol Biomarkers Prev 2019;28:370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Guryn K, Leone V, Chang EB. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe. 2019;26:314–324. doi: 10.1016/j.chom.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasapolli R, Schutte K, Schulz C, et al. Analysis of Transcriptionally Active Bacteria Throughout the Gastrointestinal Tract of Healthy Individuals. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 15.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. doi: 10.1136/gutjnl-2015-310861. Epub 2015 Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–8. doi: 10.1136/gutjnl-2015-310376. Epub 2015 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusselaers N, Sadr-Azodi O, Engstrand L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J Gastroenterol 2019;6:019–01652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearns MD, Boursi B, Yang YX. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiol. 2017;46:80–84.: 10.1016/j.canep.2016.12.006. Epub 2017 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JK, Merchant SA, Schneider JL, et al. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am J Gastroenterol. 2020;115:706–715. doi: 10.14309/ajg.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 20.Min YW, Kang D, Shin JY, et al. Use of proton pump inhibitors and the risk of cholangitis: a nationwide cohort study. Aliment Pharmacol Ther. 2019;50:760–768. doi: 10.1111/apt.15466. Epub 2019 Aug 25. [DOI] [PubMed] [Google Scholar]
- 21.Mailhe M, Ricaboni D, Vitton V, et al. Repertoire of the gut microbiota from stomach to colon using culturomics and next-generation sequencing. BMC Microbiol. 2018;18:157. doi: 10.1186/s12866-018-1304-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Canto MI, Almario JA, Schulick RD, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018;155:740–751.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe T, Blackford AL, Tamura K, et al. Deleterious Germline Mutations Are a Risk Factor for Neoplastic Progression Among High-Risk Individuals Undergoing Pancreatic Surveillance. J Clin Oncol 2019;37:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013;41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellemain E, Carlsen T, Brochmann C, et al. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol 2010;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolde R, Franzosa EA, Rahnavard G, et al. Host genetic variation and its microbiome interactions within the Human Microbiome Project. Genome Med 2018;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Pendu J, Ruvoën-Clouet N, Kindberg E, et al. Mendelian resistance to human norovirus infections. Semin Immunol. 2006;18:375–86. doi: 10.1016/j.smim.2006.07.009. Epub 2006 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei QX, Huang CL, Luo SZ, et al. Characterization of the duodenal bacterial microbiota in patients with pancreatic head cancer vs. healthy controls. Pancreatology 2018;18:438–445. [DOI] [PubMed] [Google Scholar]
- 32.Ren Z, Jiang J, Xie H, et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017;8:95176–95191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis-cancer association. Ann Epidemiol 2003;13:312–6. [DOI] [PubMed] [Google Scholar]
- 34.Michaud DS, Joshipura K, Giovannucci E, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 2007;99:171–5. [DOI] [PubMed] [Google Scholar]
- 35.Gaiser RA, Halimi A, Alkharaan H, et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut. 2019;68:2186–2194. doi: 10.1136/gutjnl-2018-317458. Epub 2019 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamura K, Izumi D, Kandimalla R, et al. Intratumoral Fusobacterium nucleatum levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin Cancer Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015;6:7209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aykut B, Pushalkar S, Chen R, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019;574:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dziubańska-Kusibab PJ, Berger H, Battistini F, et al. Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat Med 2020;1:020–0908. [DOI] [PubMed] [Google Scholar]
- 41.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580:269–273. doi: 10.1038/s41586-020-2080-8. Epub 2020 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummins J, Tangney M. Bacteria and tumours: causative agents or opportunistic inhabitants? Infect Agent Cancer. 2013;8:11. doi: 10.1186/1750-9378-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure Legends
Supplementary Figure 1 Relative abundance of duodenal bacteria in duodenal fluid (DJ) and post-secretin duodenal fluid (PDJ).
Supplementary Figure 2 Relative abundance of each taxa in normal pancreas group. The data shown were filtered by frequency higher than 1%. (A) phylum level (B) class level (C) order level (D) family level (E) genus level.
Supplemental Figure 3 Duodenal fluid microbiomes associated with PPI dose:
(A) The duodenal bacterial microbiomes of non-PPI regular users vs. low dose PPI users vs. regular or high dose PPI users in normal pancreas controls were analyzed for alpha diversity measures. (B) The fungal microbiomes of the three groups were analyzed for alpha diversity measures. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplementary Figure 4 The duodenal bacterial microbiomes of normal pancreas group was analyzed for alpha diversity measures, including Observed OTUs, Shannon Index and Faith PD. (A) Age (B) Alcohol (C) Smoking (D) DM (E) BMI.
Supplementary Figure 5 Duodenal fluid microbiomes among patients with normal pancreata stratified by FUT2 status: (A) The duodenal microbiome alpha-diversity measures. (B) Relative Genus abundance. (C) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 6 Duodenal fluid alpha diversity comparisons in PDAC vs normal pancreas controls stratified by PPI use.
Supplementary Figure 7 Genus level comparison of duodenal fluid microbiomes in PDAC vs. normal controls: (Top): PPI and non-PPI users. (Bottom) Streptococcus, Fusobacteria and Lactobacillus duodenal fluid abundance in PPI users: PDAC vs. normal controls.
Supplementary Figure 8 Duodenal fluid microbiomes: PDAC vs Normal Pancreas excluding patients who reported recent use of probiotics and antibiotics (A)–(C) and (D) excluding patients with diabetes. (A) Duodenal mycobiome alpha-diversity measures. (B) Relative Genus abundance. (C)–(D) Taxonomic cladogram from LEfSe analysis.
Supplementary Figure 9 Duodenal fluid bacterial microbiomes in patients with pancreas cyst(s) vs. Normal pancreas:
(A) Bacterial content was compared in normal pancreas group and pancreas cyst group who were matched with age. (B) The duodenal microbiomes of normal pancreas group and pancreas cyst group were analyzed for alpha-diversity measures, including Observed OTUs, Shannon Index and Faith PD. The beta diversity among these groups were tested by (C) Unweighted Unifrac plot and (D) Weighted Unifrac plot. P values were determined by pairwise PERMANOVA. (E) There were no significant differences in the relative abundance of phylum between two groups. The date showed are filtered by frequency higher than 1%. (F) There were no significant differences in the relative abundance of genus between two groups. The date showed are filtered by frequency higher than 1%. (G) Taxonomic cladogram from LEfSe analysis, showing taxonomic association from between pancreas cyst group and PDAC group.
Supplemental Figure 10 Duodenal fluid microbiomes in patients with PDAC vs. pancreatic cyst(s) vs. normal pancreas:
(A) The duodenal bacterial microbiomes of PDAC vs. pancreatic cyst(s) vs. normal pancreas controls were analyzed for alpha diversity measures. (B, C) The relative abundance of bacterial genera among the three groups. (D) The duodenal fungal microbiomes of the three groups were analyzed for alpha diversity measures. (E) The relative abundance of fungal phylum among the three groups. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplementary Figure 11 Duodenal fluid microbiomes in patients with PDAC vs. pancreatic cyst(s) vs. normal pancreas stratified by PPI status:
The duodenal bacterial microbiomes of PDAC vs. pancreatic cyst(s) vs. normal pancreas controls were analyzed for alpha diversity measures in (A) non PPI users and (B) PPI regular users, and (C) when the three disease groups were stratified by PPI status. The duodenal fungal microbiomes of the three disease groups were analyzed for alpha diversity measures in (D) non PPI users and (E) PPI regular users, and (F) stratified by PPI status. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplemental Figure 12 Duodenal fluid mycobiomes in non-smoker patients with PDAC vs. pancreatic cyst(s) vs. normal pancreas:
The duodenal (A) bacterial and (B) fungal microbiomes of PDAC vs. pancreatic cyst(s) vs. normal pancreas controls of non-smokers were analyzed for alpha diversity measures. * the p value was considered significant after Benjamini-Hochberg adjustment.
Supplementary Figure 13 Duodenal fluid Fungal DNA abundance Fungal DNA abundance in patients with PDAC vs. a normal pancreas (left), patients with PDAC vs. pancreatic cyst(s) (middle), and patients with pancreatic cyst(s) vs. a normal pancreas (right panel).
Supplementary Figure 14 Duodenal fluid Fungal DNA in patients with normal pancreata The relative abundance of duodenal fungal DNA by (A) phylum, (B) class, (C) order, (D) family and (E) genus in patients with normal pancreata.
Supplementary Figure 15 Duodenal fluid mycobiomes among PPI users vs. non-users: (A) The duodenal mycobiome alpha-diversity measures. (B) Relative Genus abundance. (C) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 16 Duodenal fluid mycobiomes among patients with normal pancreata stratified by FUT2 status: (A) The duodenal mycobiome alpha-diversity measures. (B) Relative Genus abundance. (C) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 17 Duodenal fluid mycobiomes in patients with PDAC vs Pancreas cyst(s): (A) Duodenal mycobiome alpha-diversity measures. Beta diversity determined by (B) Unweighted and (C) Weighted Unifrac plots. The relative phylum (D) and genera (E) abundance between groups. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score >3.0.
Supplementary Figure 18 Duodenal fluid mycobiomes among patients with normal pancreas vs. pancreatic cyst (A) The duodenal mycobiome alpha-diversity measures, (B) beta diversity measures by Unweighted Unifrac plot and (C) Weighted Unifrac plot. P values were determined by pairwise PERMANOVA. (D) Differences in the relative phylum abundance between groups. (E) Differences in the relative genus abundance between groups. (F) Taxonomic cladogram from LEfSe analysis. The criteria for feature selection is log LDA score > 3.0.
Supplementary Figure 19 (A) Relative abundance of bacterial DNA in pancreatic tumors and duodenal fluid. (B–D) correlations of specific bacteria between duodenal fluid and pancreatic cancer tissue. The Y axis indicates the abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 20 Relative abundance of bacteria in matched pancreatic cancer tissue, duodenal fluid, normal pancreas and normal duodenum in selected patients. Normal=normal pancreas, PSJ=duodenal fluid. The Y axis is the abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 21 Relative abundance of fungal DNA by genus in pancreatic cancer tissue, normal pancreas, duodenal tissue and duodenal fluid. The Y axis is the abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 22 Relative abundance of fungal DNA in matched pancreatic cancer tissue, duodenal fluid, normal pancreas and normal duodenum. Normal=normal pancreas, PSJ=duodenal fluid. Y axis: abundance of each bacterial DNA relative to total bacterial DNA.
Supplementary Figure 23 Top left: Alpha diversity measures in patients with PDAC stratified by tumor stage; early (Stage I and II vs. Stage III and IV). Top right: Bacterial genera in patients with PDAC stratified by tumor stage. Bottom left: Bacterial genera in patients with PDAC stratified by tumor location. Bottom right: Bacterial genera in patients with PDAC stratified by patient outcome (short vs. longer-term survivors).


