Abstract
OBJECTIVES:
To determine the extent to which newborns with neonatal abstinence syndrome (NAS) are concentrated in some hospitals as compared with newborns without NAS and whether care quality and safety differed among these hospitals. We hypothesized that newborns with NAS would be cared for in poorer-quality hospitals.
METHODS:
Secondary analysis of 3 2016 data sets: (1) the panel study of effects of changes in nursing on patient outcomes-US survey of hospital registered nurses regarding work conditions and safety, (2) inpatient discharge abstracts, and (3) the American Hospital Association annual survey. Newborns in 266 hospitals from the 4 states where the panel study of effects of changes in nursing on patient outcomes was conducted were included. We used Lorenz curves to determine if newborns with NAS were concentrated in different hospitals than newborns without NAS and whether care quality and safety differed among those hospitals. Quality and safety were assessed by staff nurses by using standard survey questions.
RESULTS:
Of the 659403 newborns in this study, 3130 were diagnosed with noniatrogenic NAS. We found that newborns with NAS were cared for in different hospitals compared with newborns without NAS (Gini coefficient 0.62, 95% confidence interval, 0.56–0.68) and that the hospitals in which they received care were rated as having poorer quality and safety (Gini coefficient 0.12, 95% confidence interval, 0.01–0.23).
CONCLUSIONS:
Newborns with NAS are cared for in poorer-quality hospitals than other newborns. Our findings are of concern because poorer-quality care is linked to patient outcomes. As stakeholders seek to address the opioid epidemic and improve outcomes of newborns with NAS, our findings suggest the importance of examining hospital factors.
Infants exposed to opioids in utero and who exhibit withdrawal signs after birth are diagnosed with neonatal abstinence syndrome (NAS). The number of newborns with NAS has increased fivefold in the past 10 years,1 with at least 1 newborn with NAS born every 15 minutes in the United States.2 In the most recent reports, researchers estimate that 32 128 newborns with NAS were born in 2016.3 Although this number represents <1% of all annual births, the care of newborns with NAS is more complicated, and their hospital stays are 5 times longer and 10 times more expensive than normal newborns.4 Additionally, they have higher rates of readmission within 30 days.5
Caring for newborns with NAS is challenging and time-consuming because of their complex physiologic and behavioral needs.6,7 NAS commonly affects the neurologic, respiratory, and gastrointestinal systems. Clinical signs vary for each infant and are dependent on several factors, including type and amount of opioid drug exposure, the drug’s half-life, and last maternal use. These medical needs require heightened monitoring, scoring the newborn’s withdrawal status, and dosing narcotics per local protocols for infants requiring treatment. The infants have higher caloric needs related to heightened energy expenditure and loss of calories from vomiting and diarrhea. Although they have higher caloric needs, these newborns have difficulty feeding.6
The approaches for soothing a newborn with NAS include decreased sound and light, tight swaddling, and being held. These high-touch, time-consuming interventions are difficult to achieve in many neonatal units. Newborns with NAS are uniquely vulnerable because of the confluence of their needs with the medical and social needs of their parents. Given their complex needs and poorer outcomes, it is important that the hospital environments in which newborns with NAS receive care meet their care needs.
There is evidence that other vulnerable groups are disproportionately cared for in hospitals with poorer resources.8 In another vulnerable newborn population, very low birth weight (VLBW) newborns, Black newborns are clustered by race in hospitals with poorer-quality care compared with newborns of other races and ethnicities.9 The opioid epidemic is known to be concentrated in certain geographic areas, especially rural communities, where access to health services may be limited.10 There is the potential that the geographic concentration of the opioid epidemic resulted in the clustering of newborns with NAS into hospitals with poorer-quality health services. Where newborns with NAS receive care, however, and the quality of care in those hospitals is not known. In this article, we define quality using nurse survey measures associated with the quality and safety of the units in which nurses work. Our purpose with this study was to determine the extent to which newborns with NAS are concentrated in some hospitals as compared with newborns without NAS and whether care quality and safety differed among those hospitals. We hypothesized that newborns with NAS would be cared for in poorer-quality hospitals.
METHODS
This is a retrospective cohort study involving the secondary analysis of 3 data sets: (1) the panel study of effects of changes in nursing on patient outcomes (RN4CAST) nurse surveys, (2) hospital discharge abstracts, and (3) the American Hospital Association annual survey. These 3 data sets were made available as part of a federally funded study (R01 NR004513 [principal investigator Linda Aiken]) and were merged on a unique hospital identifier.
Data Sources
RN4CAST is a survey of registered nurses (RNs) conducted in 2016 in 4 geographically diverse states that include ~25% of US births annually.11 The RN survey was designed to measure nursing organizational and workforce characteristics and hospital care quality. The survey sample was obtained from a 30% random sample of RNs from state licensure lists. Respondents identified the hospital at which they were employed. The response rate was 26%. A more detailed description of the RN4CAST sampling and recruitment approach has been described elsewhere.12
Newborn data from 2016 were derived from hospital discharge abstracts from the California Office of Statewide Health Planning and Development, the Healthcare Cost and Utilization Project State Inpatient Database for Florida, the New Jersey Department of Health and Senior Services, and the Pennsylvania Health Care Cost Containment Council. Newborn demographic characteristics, including sex, race, insurance status, and length of stay, were obtained from these data sources.
The American Hospital Association annual survey data from 2016 were used to measure hospital characteristics, and state urban or rural status was derived from core-based statistical area classification.
Samples
The nurse sample included staff RNs who worked in newborn nurseries or the NICU. The hospital sample included hospitals with at least 3 nurse respondents, to generate stable aggregate measures. The newborn sample included all newborns (Diagnosis Related Group 795, version 34) in their initial hospital stay. Newborns transferred in on the basis of admission source of an acute care inpatient facility were excluded. Newborns with NAS were identified by International Classification of Diseases, 10th Revision code P96.1. Newborns with iatrogenic NAS were excluded from the NAS subsample because they do not represent newborns who develop NAS because of maternal substance use in utero.13 Newborns with iatrogenic NAS were identified if they were born with a weight of <1500 g or diagnosed with a condition or procedure that likely warranted pharmacologic opioid treatment (eg, chronic lung disease, intraventricular hemorrhage, periventricular leukomalacia, necrotizing enterocolitis, spontaneous bowel perforation, or those requiring cardiac surgery or extracorporeal membrane oxygenation).14 Newborns with iatrogenic NAS were included in the newborns without NAS subsample.
Variables
Quality and Safety Measures
The RN4CAST nurse survey queried nurses regarding quality and safety on their nursing units with the following questions. The quality question asked, “In general, how would you describe the quality of nursing care delivered to patients in your work setting?” The responses were excellent, good, fair, and poor. The safety question asked, “Please give your current practice setting an overall grade on patient safety.” The responses were A (excellent), B (good), C (acceptable), D (poor), and F (failing). We calculated the average percentage of nurses who responded “good” or “excellent” to each question and then computed the mean of these 2 question averages. These questions have been used extensively internationally to measure quality of nursing care and patient safety.15,16 The predictive validity of the nurse-assessed quality rating has been established in a study of 396 hospitals in 4 US states.17 In that study, the nurse-assessed quality significantly predicted patient mortality, failure to rescue, patient satisfaction, and process-of-care composite scores for acute myocardial infarction, heart failure, pneumonia, and surgery.17 The patient safety grade question is part of the Agency for Healthcare Research and Quality safety culture survey.18
Newborn Measures
Newborn demographic characteristics, including sex, race, insurance status, state, length of stay, and discharge disposition, were derived from state discharge records. The hospital newborn volume was also derived from aggregated state discharge records and was categorized into 1 to <1000, 1000 to <2000, 2000 to <3000, and ≥3000.
Hospital Characteristics
Hospital characteristics, including bed size, teaching and technology status, state, and rurality, were derived from the American Hospital Association annual survey.
The number of licensed beds determined the size of the hospital (≤100 beds, 101–250 beds, ≥250 beds). The 3 teaching status categories were based on the ratio of medical residents or fellows to hospital beds (nonteaching, no medical trainees; minor teaching, 0–0.25 per bed; major teaching, ≥0.25 per bed). Technology status (high- or low-technology status) indicated whether a hospital performed major organ transplants or open-heart surgery. Hospital quality quartile was derived from RN4CAST nurse survey data and defined by the percentage of nurses rating quality and safety as good or excellent at the hospital level.
Analytic Procedure
We described the characteristics of newborns with and without NAS and the hospitals in which they received care using descriptive statistics including frequencies, t tests, medians, and SDs. We calculated an average quality and safety score derived from the RN4CAST nurse survey data as described above for each hospital and then classified hospitals into quartiles on the basis of this score. We described the characteristics of hospitals across the quality and safety quartiles. We then evaluated the distribution of newborns with and without NAS across the quartiles.
Overview of Lorenz Curves
We produced 2 Lorenz curves displaying concentration and care quality, respectively, for newborns with NAS relative to all newborns without NAS.9 Lorenz curves generate Gini coefficients, which range from 0.00 to 1.00. Contrary to typical hospital ranking schemes, a higher Gini coefficient indicates a greater magnitude of uneven distribution.
Utility of Lorenz Curves
We used Lorenz curves for both determining the distribution of newborns with NAS as compared with newborns without NAS across hospitals and across hospitals of differing quality of care. Lorenz curves are a method for examining unequal distribution in any system.9,19 The curve produces a Gini coefficient that measures the extent of unequal distribution in a defined subgroup. If the Gini coefficient is not statistically significant, distribution is considered to be equal. If it is statistically significant, then there is unequal distribution between groups.19,20 Previously, Lorenz curves have been used to examine the distribution of VLBW newborns across NICUs of varying quality by race and ethnicity.9 These curves are well suited to describe the distribution of newborns with NAS across hospitals with varying quality compared with newborns without NAS.
Analytic Approach for Lorenz Curves
To develop the concentration Lorenz curves, we ranked hospitals by proportion of newborns with NAS relative to newborns without NAS. The cumulative study population percentage of newborns with and without NAS was plotted on the x- and y-axes. The diagonal line indicates that each hospital’s fraction of newborns with NAS equals the population fraction of newborns without NAS. When the proportion of newborns with NAS in a hospital differed from the overall study population, the curve would not fall on the diagonal line. Deviations below the diagonal line indicate a hospital has more newborns with NAS than the study population. For the concentration analysis, the Lorenz curve always fell below the diagonal because the hospitals were ranked to maximize the difference in cumulative distributions. To generate the quality Lorenz curve, units were ranked by their average quality and safety score. If newborns with NAS were cared for in units with lower quality and safety ratings, the Lorenz curve would fall below the diagonal and the Gini would be positive; if newborns with NAS were cared for in units with higher quality and safety ratings, the curve would fall above the diagonal and the Gini would be negative. For both curves, the Gini coefficient and 95% confidence interval (CI) were generated by bootstrapping. In addition, we generated the Lorenz curves by state to examine whether the patterns were consistent or were driven by a particular state or states.
This study received exempt status from the University of Pennsylvania Institutional Review Board and is not considered human subjects research. All analyses were performed with Stata/IC version 15.1 (Stata Corp, College Station, TX).
RESULTS
The final newborn sample comprised 659 403 newborns in 266 hospitals. There were 3957 newborns diagnosed with NAS (0.6%). Twenty-one percent (n = 827) of newborns with NAS in the initial sample were classified as iatrogenic, so these newborns were reclassified as newborns without NAS. Therefore, 3130 (0.5%) newborns were in the NAS sample and 656 273 (99.5%) newborns did not have NAS. Our sample comprises more than half of all hospitals with newborns in 2016 in the 4 states (266 of 467 = 57%). The hospitals that were excluded for lack of nurse survey data had proportionately fewer newborns. Therefore, our final sample accounts for 79% of all newborns and 76% of newborns with NAS (after excluding iatrogenic NAS). Compared with newborns without NAS, more newborns with NAS were white and on Medicaid. Newborns with NAS had a longer length of stay (14 vs 2 days; Table 1). There was a mean of 11.77 (SD 18.39) and median of 6 newborns with NAS per hospital, whereas in 27 (10.15%) of hospitals, there were no newborns with NAS.
TABLE 1.
Characteristics of Newborns
| Characteristic | Newborns With NAS (n = 3130) | Newborns Without NAS (n = 656 273) | P |
|---|---|---|---|
| Race, n | 3025 | 633 070 | <.001 |
| White, n (%) | 2491 (82.35) | 254 695 (41.81) | — |
| Black, n (%) | 208 (6.88) | 78 221 (12.36) | — |
| Hispanic, n (%) | 236 (7.80) | 194 825 (30.77) | — |
| Asian American, n (%) | 13 (0.43) | 59 301 (9.37) | — |
| Other, n (%) | 77 (2.55) | 36 028 (5.69) | — |
| Insurance status, n | 3016 | 642 380 | <.001 |
| Medicaid or other, n (%) | 2652 (87.93) | 323 086 (50.30) | — |
| Private, n (%) | 364 (12.07) | 319 294 (49.70) | — |
| Sex, n | 3130 | 656129 | .338 |
| Female, n (%) | 1612 (51.50) | 332 290 (50.64) | — |
| Male, n (%) | 1518 (48.50) | 323 839 (49.36) | — |
| Length of stay, n | 3130 | 656 272 | — |
| Median ± SD | 14 ± 14.81 | 2 ± 8.52 | <.001 |
—, not applicable.
Hospitals in the sample were predominately large with high-technology status (Table 2). Of the 4 newborn volume levels, the largest fraction (32%) comprised hospitals with 1000 to <2000 newborns in 2016. All but 9 hospitals were classified as urban. Across quality quartiles from top to bottom, the proportion of nonteaching hospitals decreased from 33% to 18% (P ≤ .001) (Table 2). New Jersey and California had disproportionately more hospitals in the top quality quartile, whereas Florida had disproportionately fewer. Pennsylvania’s hospitals were equally distributed across the quality quartiles.
TABLE 2.
Characteristics of Sample Hospitals Overall and by Quality Quartile (N = 266)
| Characteristic | Overall | Top Quality Quartile | Second Quality Quartile | Third Quality Quartile | Bottom Quality Quartile | P Among Quality Quartiles |
|---|---|---|---|---|---|---|
| Bed size, n | 266 | 66 | 67 | 66 | 67 | .526 |
| <100, n (%) | 8 (3.01) | 4 (50.00) | 1 (12.50) | 1 (12.50) | 2 (25.00) | — |
| 100–249, n (%) | 77 (28.95) | 18 (23.38) | 16 (20.78) | 20 (25.97) | 23 (29.87) | — |
| ≥250, n (%) | 181 (68.05) | 44 (24.31) | 50 (27.62) | 45 (24.86) | 42 (23.20) | — |
| Teaching status, n | 266 | 66 | 67 | 66 | 67 | <.001 |
| None, n (%) | 113 (42.48) | 37 (32.74) | 31 (27.43) | 25 (22.12) | 20 (17.70) | — |
| Minor, n (%) | 125 (46.99) | 18 (14.40) | 26 (20.80) | 36 (28.80) | 45 (36.00) | — |
| Major, n (%) | 28 (10.53) | 11 (39.29) | 10 (35.71) | 5 (17.86) | 2 (7.14) | — |
| Technology status, n | 262 | 65 | 65 | 66 | 66 | .305 |
| Low, n (%) | 97 (37.02) | 22 (22.86) | 19 (19.59) | 28 (28.87) | 28 (28.87) | — |
| High, n (%) | 165 (62.98) | 43 (26.06) | 46 (27.88) | 38 (23.03) | 38 (23.03) | — |
| State, n | 266 | 66 | 67 | 66 | 67 | .142 |
| California, n (%) | 119 (44.74) | 34 (28.57) | 31 (26.05) | 25 (21.01) | 29 (24.37) | — |
| Florida, n (%) | 64 (24.06) | 7 (10.94) | 15 (23.44) | 22 (34.38) | 20 (31.25) | — |
| New Jersey, n (%) | 34 (12.78) | 12 (35.29) | 10 (29.41) | 7 (20.59) | 5 (14.71) | — |
| Pennsylvania, n (%) | 49 (18.42) | 13 (26.53) | 11 (22.45) | 12 (24.49) | 13 (26.53) | — |
| Geographic status, n | 265 | 66 | 66 | 66 | 67 | .228 |
| Rural, n (%) | 9 (3.40) | 4 (50.00) | 1 (12.50) | 0 (0.00) | 3 (37.50) | — |
| Urban, n (%) | 256 (96.60) | 62 (24.22) | 64 (25.00) | 66 (25.00) | 64 (25.00) | — |
| Newborn volume, n | 265 | 66 | 67 | 66 | 67 | .051 |
| 1–<1000, n (%) | 47 (17.74) | 11 (21.57) | 8 (15.69) | 14 (27.45) | 18 (35.29) | — |
| 1000–<2000, n (%) | 85 (32.08) | 15 (21.13) | 13 (18.31) | 20 (28.17) | 23 (32.39) | — |
| 2000–<3000, n (%) | 65 (24.53) | 16 (25.00) | 18 (28.13) | 18 (28.13) | 12 (18.75) | — |
| ≥3000, n (%) | 68 (25.66) | 24 (30.00) | 28 (35.00) | 14 (17.50) | 14 (17.50) | — |
—, not applicable.
Statistically significant differences for newborns with NAS were noted for several hospital characteristics, but the percent differences were modest. Compared with newborns without NAS, newborns with NAS were disproportionately in hospitals in Pennsylvania. By contrast, California had proportionately few newborns with NAS relative to the overall distribution of newborns without NAS. Most newborns without NAS (55%) were born in the highest-volume hospitals. Fewer newborns with NAS were born in the highest-volume hospitals (41%) and proportionately more were born in midvolume (1000–<2000) hospitals (27%) than were newborns without NAS (16%).
We found that the newborns with NAS were cared for in different hospitals than other newborns (Fig 1). The hospital concentration index for newborns with NAS was 0.62 (95% CI, 0.56–0.68). When the index equals 0.00, there is a random distribution of newborns with NAS across all hospitals. When the index equals 1.00, newborns with NAS are the hospital’s newborn population. The hospital quality index for newborns with NAS when compared with newborns without NAS was 0.12 (95% CI, 0.01–0.23) (Fig 2). When the quality index equals 0.00, the quality of care is the same in hospitals regardless of the concentration of newborns with NAS. When the quality index equals 1.00, the hospitals in which newborns with NAS are concentrated are always of poorer quality. Compared with newborns with NAS, newborns without NAS were more often in top-quartile hospitals (27% vs 18%). Conversely, newborns with NAS, compared with newborns without NAS, were more often in bottom-quartile hospitals (23% vs 19%) (Table 3). The state-specific graphs revealed that the concentration curves were consistent across states (Supplemental Fig 3). The quality curves were consistent across all states except Florida, which showed no quality difference across hospitals in which newborns with NAS were concentrated as compared with other hospitals (Supplemental Fig 4).
FIGURE 1.
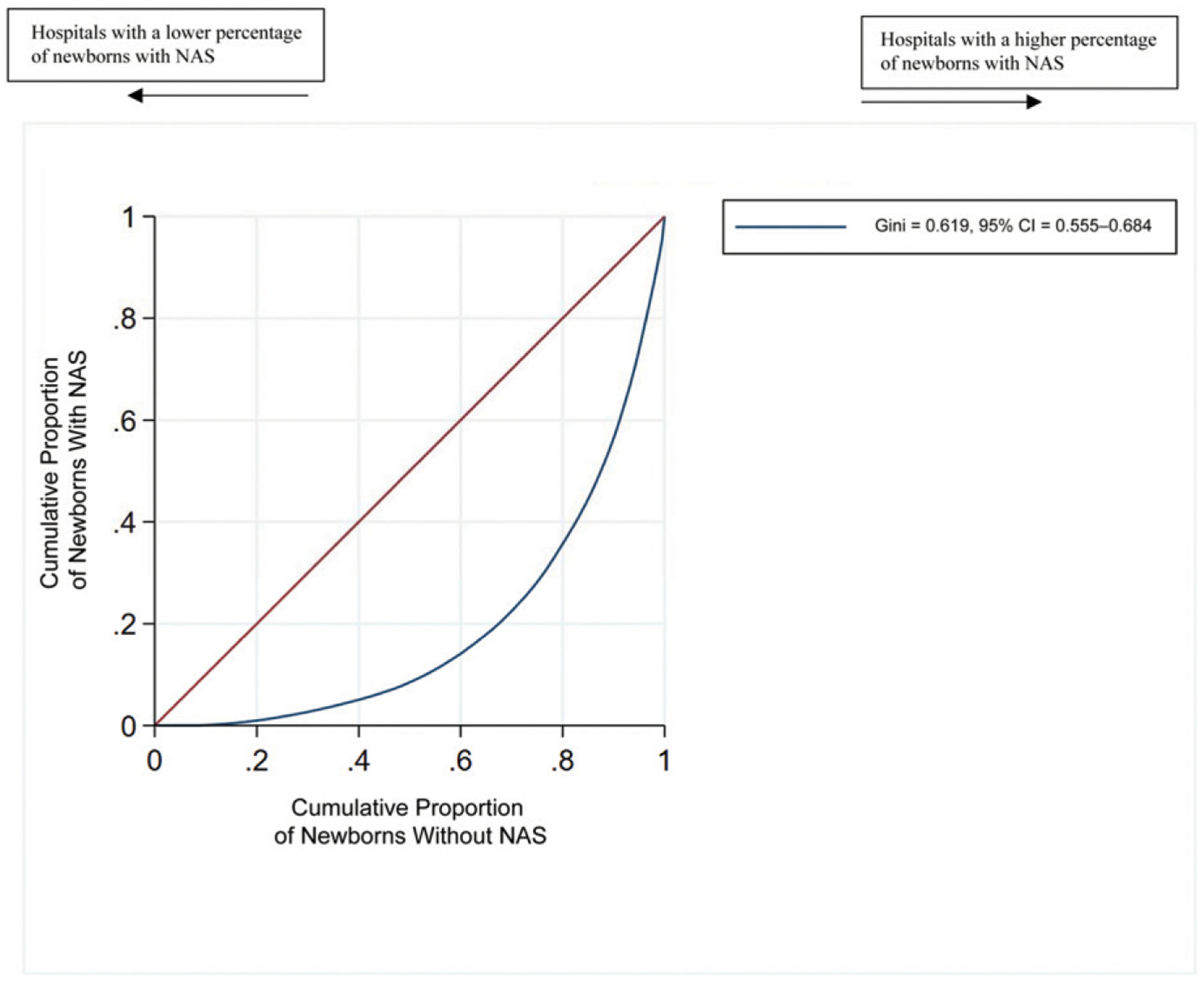
Concentration Lorenz curve for newborns with and without NAS (sample of 659 ÷≠× newborns in 266 hospitals). The curve below the diagonal indicates that newborns with NAS are concentrated in some hospitals as compared with newborns without NAS.
FIGURE 2.
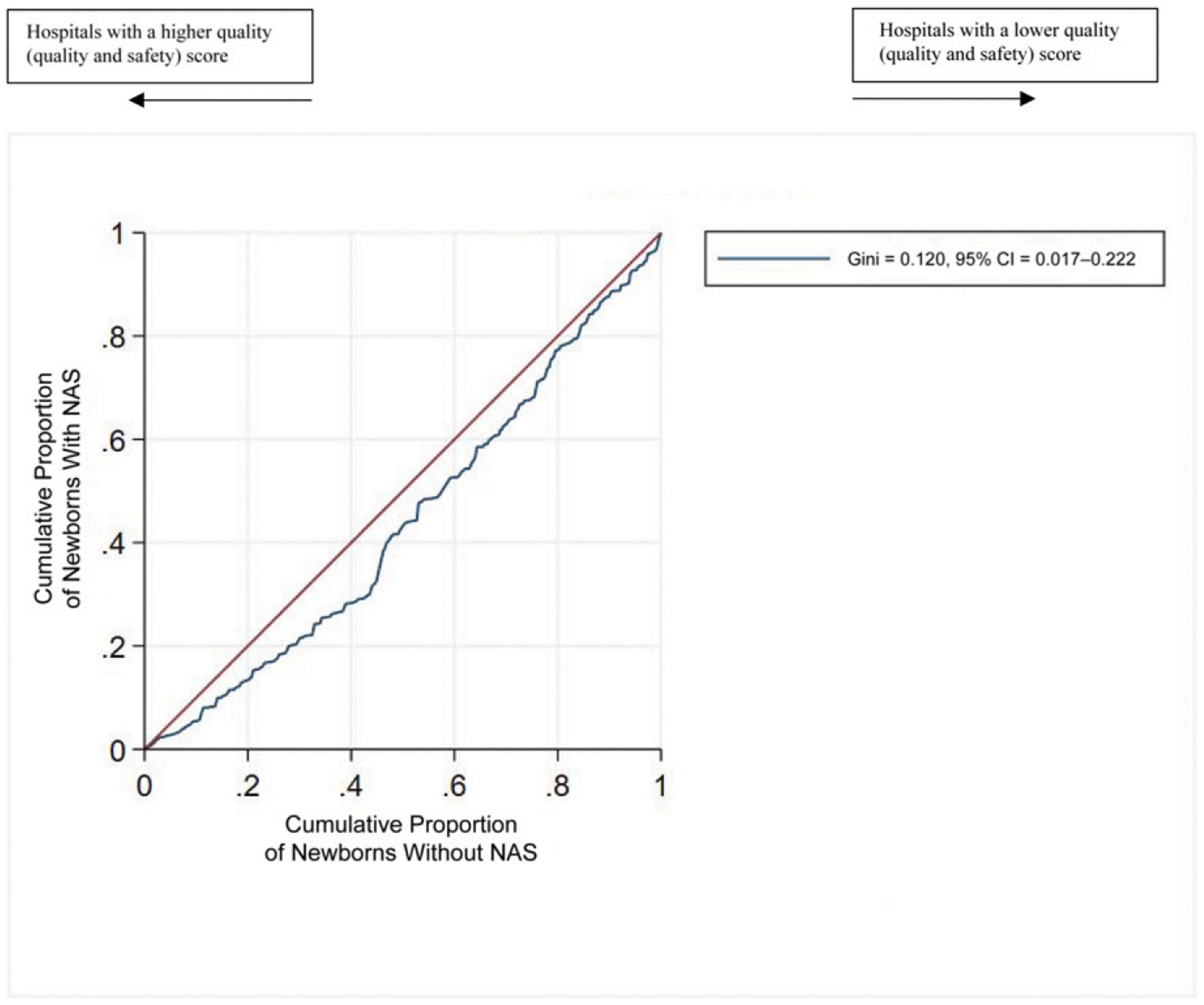
Quality Lorenz curve for newborns with and without NAS (sample of 659 ÷≠× newborns in 266 hospitals). The line below the diagonal indicates that newborns with NAS are cared for in hospitals with poorer quality and safety ratings than newborns without NAS.
TABLE 3.
Distribution of Newborns With and Without NAS by Hospital Characteristics
| Characteristic | Newborns With NAS (n = 3130) | Newborns Without NAS (n = 656 273) | P |
|---|---|---|---|
| Bed size, n | 3130 | 656 273 | .017 |
| <100, n (%) | 24 (0.77) | 4849 (0.74) | — |
| 100–249, n (%) | 601 (19.20) | 139 765 (21.30) | — |
| ≥250, n (%) | 2505 (80.03) | 511 659 (77.96) | — |
| Teaching status, n | 3130 | 656 273 | <.001 |
| None, n (%) | 1237 (39.52) | 254 972 (38.85) | — |
| Minor, n (%) | 1261 (40.29) | 303 484 (46.24) | — |
| Major, n (%) | 632 (20.19) | 97 817 (14.90) | — |
| Technology status, n | 3110 | 646 628 | .594 |
| Low, n (%) | 987 (31.74) | 202 346 (31.29) | — |
| High, n (%) | 2123 (68.26) | 444 282 (68.71) | — |
| State, n | 3130 | 656 273 | <.001 |
| California, n (%) | 570 (18.21) | 319 705 (48.72) | — |
| Florida, n (%) | 1053 (33.64) | 161 629 (24.63) | — |
| New Jersey, n (%) | 435 (13.90) | 85 474 (13.02) | — |
| Pennsylvania, n (%) | 1072 (34.25) | 89 465 (13.63) | — |
| Geographic status, n | 3124 | 652 025 | <.001 |
| Rural, n (%) | 60 (1.92) | 8576 (1.32) | — |
| Urban, n (%) | 3064 (98.08) | 643 449 (98.68) | — |
| Newborn volume, n | 3130 | 656 273 | <.001 |
| 1–<1000, n (%) | 269 (8.59) | 35 113 (5.35) | — |
| 1000–<2000, n (%) | 832 (26.58) | 104 942 (15.99) | — |
| 2000–<3000, n (%) | 719 (22.97) | 154 538 (23.55) | — |
| ≥3000, n (%) | 1310 (41.85) | 361 680 (55.11) | — |
| Quality quartile, n | 3130 | 656 273 | <.001 |
| Top quartile, n (%) | 571 (18.24) | 178 780 (27.24) | — |
| Second quartile, n (%) | 1061 (33.90) | 211 317 (32.20) | — |
| Third quartile, n (%) | 778 (24.86) | 138 787 (21.15) | — |
| Bottom quartile, n (%) | 720 (23.00) | 127 389 (19.41) | — |
—, not applicable.
DISCUSSION
Given the physiologic and social vulnerability of newborns with NAS, we were interested in whether these newborns were cared for in the same hospitals as other newborns and, if not, how the quality of care differed from that of hospitals caring for other newborns. We found that newborns with NAS were concentrated in hospitals of poorer quality. These findings are concerning because we assert that these newborns are particularly sensitive to the quality of care.
Our results indicate that newborns with NAS are concentrated in some hospitals as compared with newborns without NAS (Gini coefficient = 0.62; 95% CI, 0.56–0.68). Where newborns with NAS are born, and therefore receive care, is difficult to change. The hospitals in which newborns with NAS were concentrated had a poorer quality rating (Gini coefficient 0.12; 95% CI, 0.01–0.23). Almost one-quarter of newborns with NAS were cared for in hospitals with the bottom quartile of care quality, compared with only 19% of newborns without NAS. Quality of care is modifiable. Our evaluation of state-specific Lorenz curves revealed that Florida showed no difference in quality where newborns with NAS were concentrated, indicating, for unknown reasons, that this result does not apply to Florida. Future research could be used to explore how the birth hospital in Florida does not result in poorer-quality settings for newborns with NAS.
Although we are not aware of similar studies among newborns with NAS, Lorenz curves and Gini coefficients have been used to address questions on unequal distribution in various populations, often in public health.21–23 Horbar et al9 examined racial segregation and care inequality for VLBW infants. In a sample 743 NICUs with VLBW infants across the United States, they found that Black, Asian American, and Hispanic infants were cared for in different NICUs than white infants (Gini coefficient = 0.50, 95% CI, 0.46–0.53), termed “segregation,” and that Black infants received care in poorer-quality NICUs compared with white infants (Gini coefficient = 0.07, 95% CI, 0.02–0.13). Their measure of NICU quality was derived from a composite Baby–Measure of Neonatal Intensive Care Outcomes Research score, which would not be suitable for our query because it is only available in the NICU. Compared with their results, our Gini coefficients for concentration (0.62 compared with 0.50) and quality (0.12 compared with 0.07) were slightly worse, meaning newborns with NAS, as compared with newborns without NAS, are more often cared for in poorer-quality settings than are Black VLBW infants relative to white VLBW infants. Our study and the study by Horbar et al9 reveal that vulnerable patient populations (newborns with NAS and Black VLBW newborns) become more disadvantaged by site of care, in this case, birth location.
Higher nurse-reported quality and safety has also been linked with increased patient satisfaction and improved outcomes.15,24 As a result, we speculate that newborns with NAS would have better outcomes in hospitals with higher nurse-reported quality and safety. The potential for improved outcomes based on birth hospital was demonstrated in a different population in a study of racial segregation and care quality for Black mothers giving birth in New York City. Howell et al25 found that if Black mothers delivered in the same hospitals as white women, they would experience fewer episodes of severe maternal morbidity, equivalent to a 47.7% reduction in the Black maternal severe morbidity rate. Birth location may not be amenable to change, but the quality and safety of care can be improved through hospital quality improvement efforts and achieving high standards of nursing excellence.26
In future work, researchers should examine the association of quality of care and outcomes of newborns with NAS. Research exploring modifiable organizational characteristics of the hospitals in which newborns with NAS are cared for is needed. An additional area for exploration is the impact of nursing care and nursing organizational factors on outcomes of newborns with NAS given the distinctive nursing care needs of newborns with NAS. We acknowledge that racial subgroups within NAS deserve similar research attention. To our knowledge, however, evidence about potential racial disparities in the NAS population has not been reported.
Our sample includes a state (PA) with a large rural population; thus, we have some rural hospitals included in our study group. However, this number does not reflect the disproportionate impact that the opioid epidemic has on rural communities.27 We do believe that the poorer-quality trends that we observed here for newborns with NAS would likely be starker if we had more rural hospitals because rural hospitals tend to have fewer nursing resources.10 Our sample includes 76% of newborns with NAS born in the 4 states. Hospitals not included in this sample were more likely to be small, rural, and low-technology hospitals with low birth volumes; therefore, our results may not be generalizable to such hospitals. The measures used to derive hospital quality may not necessarily reflect the specific quality of newborn care because these are general measures. We considered quality and safety ratings of good or excellent to be satisfactory. We believe, however, that it is reasonable to desire excellent quality and safety for all newborns. Our conservative approach to measuring quality offers a more attainable target for hospitals, and yet we still observed notable disparities.
We found that newborns with NAS are concentrated in hospitals with poorer quality than all other newborns. As stakeholders seek to address the opioid epidemic and improve outcomes of newborns with NAS, our findings suggest the importance of examining hospital factors as they relate to outcomes of newborns with NAS.
Supplementary Material
ACKNOWLEDGMENT:
The authors wish to acknowledge Morgan Peele for her contributions to data management and analysis.
FUNDING:
Dr Clark’s postdoctoral fellowship and Ms French’s predoctoral fellowship are supported by funding from the National Institute of Nursing Research (T32NR007104). Funding for Panel Study of Effects of Changes in Nursing on Patient Outcomes was provided by the National Institute of Nursing Research (R01NR014855, principal investigator Linda Aiken). The current study was also supported by funding from the Leonard Davis Institute for Health Economics titled “Disparities in Neonatal Abstinence Syndrome Infant Outcomes and Related Nursing System Factors.” The funders did not participate in the work.
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.hosspeds.org/cgi/doi/10.1542/hpeds.2020-003863
REFERENCES
- 1.Reddy UM, Davis JM, Ren Z, Greene MF; Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes Workshop Invited Speakers. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017;130(1):10–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jilani SM, Frey MT, Pepin D, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome - six states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(1):6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramphul K, Mejias SG, Joynauth J. An update on the burden of neonatal abstinence syndrome in the United States. Hosp Pediatr. 2020;10(2):181–184 [DOI] [PubMed] [Google Scholar]
- 4.Milliren CE, Gupta M, Graham DA, Melvin P, Jorina M, Ozonoff A. Hospital variation in neonatal abstinence syndrome incidence, treatment modalities, resource use, and costs across pediatric hospitals in the United States, 2013 to 2016. Hosp Pediatr. 2018;8(1):15–20 [DOI] [PubMed] [Google Scholar]
- 5.Patrick SW, Burke JF, Biel TJ, Auger KA, Goyal NK, Cooper WO. Risk of hospital readmission among infants with neonatal abstinence syndrome. Hosp Pediatr. 2015;5(10):513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 [Google Scholar]
- 7.Stover MW, Davis JM. Opioids in pregnancy and neonatal abstinence syndrome. Semin Perinatol. 2015;39(7):561–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks-Carthon JM, Kutney-Lee A, Sloane DM, Cimiotti JP, Aiken LH. Quality of care and patient satisfaction in hospitals with high concentrations of black patients. J Nurs Scholarsh. 2011;43(3):301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horbar JD, Edwards EM, Greenberg LT, et al. Racial segregation and inequality in the neonatal intensive care unit for very low-birth-weight and very preterm infants. JAMA Pediatr. 2019;173(5):455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JG, Plover CM, McChesney MC, Lake ET. Isolated, small, and large hospitals have fewer nursing resources than urban hospitals: implications for rural health policy. Public Health Nurs. 2019;36(4):469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark RRS, Lake ET. Association of clinical nursing work environment with quality and safety in maternity care in the United States. MCN Am J Matern Child Nurs. 2020;45(5):265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasater KB, Jarrín OF, Aiken LH, McHugh MD, Sloane DM, Smith HL. A methodology for studying organizational performance: a multistate survey of front-line providers. Med Care. 2019; 57(9):742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940 [DOI] [PubMed] [Google Scholar]
- 14.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004–2011. J Perinatol. 2014;34(11):867–872 [DOI] [PubMed] [Google Scholar]
- 15.Aiken LH, Sermeus W, Van den Heede K, et al. Patient safety, satisfaction, and quality of hospital care: cross sectional surveys of nurses and patients in 12 countries in Europe and the United States. BMJ. 2012;344:e1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lake ET, Hallowell SG, Kutney-Lee A, et al. Higher quality of care and patient safety associated with better NICU work environments. J Nurs Care Qual. 2016; 31(1):24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHugh MD, Stimpfel AW. Nurse reported quality of care: a measure of hospital quality. Res Nurs Health. 2012; 35(6):566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorra J, Famolaro T, Dyer MN, Nelson D, Khanna K. Hospital Survey on Patient Safety Culture: 2008 Comparative Database Report. Rockville, MD: Agency for Healthcare Research and Quality; 2008 [Google Scholar]
- 19.Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci USA. 2016;113(47):13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell CR, Firebaugh G. Is immigrant neighborhood inequality less pronounced in suburban areas? Soc Sci Res. 2016;57:161–176 [DOI] [PubMed] [Google Scholar]
- 21.Kerani RP, Handcock MS, Handsfield HH, Holmes KK. Comparative geographic concentrations of 4 sexually transmitted infections. Am J Public Health. 2005; 95(2):324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono T, Matsuda Y, Sasaki K, et al. Comparative analysis of cesarean section rates using Robson Ten-Group Classification System and Lorenz curve in the main institutions in Japan. J Obstet Gynaecol Res. 2016;42(10): 1279–1285 [DOI] [PubMed] [Google Scholar]
- 23.Pabayo R, Chiavegatto Filho AD, Lebrão ML, Kawachi I. Income inequality and mortality: results from a longitudinal study of older residents of São Paulo, Brazil. Am J Public Health. 2013;103(9): e43–e49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiken LH, Clarke SP, Sloane DM, Lake ET, Cheney T. Effects of hospital care environment on patient mortality and nurse outcomes. J Nurs Adm. 2008;38(5): 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kutney-Lee A, Stimpfel AW, Sloane DM, Cimiotti JP, Quinn LW, Aiken LH. Changes in patient and nurse outcomes associated with magnet hospital recognition. Med Care. 2015;53(6): 550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigg KK, Monnat SM, Chavez MN. Opioid-related mortality in rural America: geographic heterogeneity and intervention strategies. Int J Drug Policy. 2018;57:119–129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


