Abstract
Sepsis induces significant immune dysregulation characterized by lymphocyte apoptosis and alterations in the cytokine milieu. Because cancer patients exhibit a ten-fold greater risk of developing sepsis compared to the general population, we aimed to understand how pre-existing malignancy alters sepsis-induced immune dysregulation. To address this question, we assessed the impact of tumor-specific CD8+ T cells on the immune response in a mouse model of cecal ligation and puncture (CLP)-induced sepsis. Tumor-bearing animals containing Thy1.1+ tumor-specific CD8+ T cells were subjected to CLP and groups of animals received anti-Thy1.1 mAb to deplete tumor-specific CD8+ T cells or isotype control. Results indicated that depleting tumor-specific T cells significantly improved mortality from sepsis. The presence of tumor-specific CD8+ T cells resulted in increased expression of the 2B4 coinhibitory receptor on and increased apoptosis of endogenous CD8+ T cells. Moreover, tumor-specific T cells were not reduced in number in the tumors during sepsis but did exhibit impaired IFN-γ production in the tumor, tumor draining lymph node, and spleen 24 hours after CLP. Our research provides novel insight into the mechanisms by which pre-existing malignancy contributes to increased mortality during sepsis.
Introduction
Cancer is a common comorbidity in patients with sepsis, with more than 20% of hospitalized sepsis patients in the United States also having cancer (1). Solid cancer is the most common underlying cause of death from sepsis (2); cancer is also responsible for higher mortality in patients with sepsis (1, 3). Mortality in sepsis patients with lung cancer is particularly high, at roughly 37% (1, 3). This is particularly concerning given that more than 7% of lung cancer patients develop sepsis within the first year of diagnosis (4). While many of these patients will recover from sepsis, they may experience prolonged organ and immunological dysfunction associated with higher risk for mortality (5).
One of the most striking immunological impairments during sepsis is the dysfunction of T cells. Sepsis induces many populations of T cells to undergo apoptosis, or to upregulate co-inhibitory markers and become exhausted (6, 7). Interestingly, recent studies suggest that T cell populations differ in their susceptibility to sepsis-induced dysfunction and apoptosis. For instance, skin resident memory CD8+ T cells are resistant to sepsis induced dysfunction compared to the memory cells in the circulation (8). In patients with Staphylococcus aureus infection, central memory CD45RA-CCR7+ T cells, but not naïve T cells, are significantly decreased (9). In a model of polymicrobial sepsis in mice, CD44hi CD11ahi memory CD8+ T cells are similarly depleted and have a higher rate of apoptosis compared to naïve T cells (10). In particular, T cells in the thymus and spleen are significantly reduced, while their levels are maintained in the liver and lungs (11).
A few studies have addressed the interaction of tumors and infections. For example, in 1920, Dr. Coley published a study showing that infection with Streptococcus led to tumor remission in over 25% of his sarcoma patients (12, 13). Recently, Roberts et al. found that intratumoral injections of attenuated Clostridium novyi improved tumor clearance in a rat model of glioma and in pet canines with spontaneously occurring solid tumors (14). One murine study using the cecal ligation and puncture (CLP) model of sepsis reported that sepsis leads to the expansion of regulatory T cells that suppress anti-tumor immunity (15). Animals that recovered from sepsis and that were inoculated with tumors experienced enhanced tumor growth compared to mice that were never septic (15). While these studies addressed how sepsis or bacterial infection impacted tumor-specific T cells, none have addressed the converse—how tumor-specific cells impact sepsis pathogenesis.
Here we report a clinically relevant model that allows us to assess the tumor-specific T cell response during sepsis. In this study, we demonstrate that there are tissue-specific alterations in the function of tumor-specific CD8+ T cells during sepsis. Moreover, we demonstrate that cancer sepsis mortality is linked to the presence of tumor-specific CD8+ T cells. Specifically, these cells modulate the apoptosis and phenotype of non-tumor specific CD8+ T cells that are important for the resolution of the septic insult. This study therefore provides insight into how anti-tumor immunity affects sepsis and may help lead to novel therapeutic strategies tailored for cancer patients with sepsis.
Materials and Methods
Mice
Eight to twelve-week-old C57BL/6J male mice were acquired from Jackson Laboratory. TCR transgenic mice possessing T cells specific for SIINFEKL/ Kb were purchased from Jackson Labs and crossed with Thy1.1+ animals (also Jackson Labs). B6.Cg-Thy1a/CyTg(TcraTcrb)8Rest/J transgenic mice possessing TCRs specific for the mouse homologue of human premelanosome protein (pMel, gp100) were also acquired from Jackson Laboratory and bred to Thy1.1+ mice at Emory University. All animals were housed and maintained in accordance with Emory University IACUC guidelines and studies were approved by Emory IACUC (Protocol: 201700361).
Murine cancer cell lines
LLC1 cells were acquired from ATCC (CRL-1642) and LLC-OVA was kindly provided by Dr. Gabrilovich at the Wistar Institute (16). LLC1 was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma) with 10% FBS, 1% P/S and 1% HEPES. LLCOVA was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma) with 10% FBS, 1% P/S, 1% HEPES and 0.5 mg/ml of G418. The ATCC guidelines for LLC1 culturing and cryopreservation were followed. B16F10 melanoma cells engineered to express hgp100 protein were kindly provided by Dr. Nicholas Restifo, NIH (17). B16-hgp100 cells were cultured in RPMI (RPMI, Sigma) with 10% FBS, 1% P/S, 1% HEPES, 1% L-glutamine, and 0.05 mM 2-mercaptoethanol. Guidelines for B16 gp100 culture and cryopreservation outlined by the Restifo lab were followed (18, 19).
Lung cancer and melanoma models
For the lung cancer model, two million OT-I T cells were adoptively transferred into naïve B6 mice via IV injection and the next day 100,000 LLC-OVA or 50,000 LLC cells were injected subcutaneously into the right flank. For the melanoma model, 1x106 gp100-specific CD8+ cells were adoptively transferred into naïve B6 mice via IV injection. The next day, 400,000 B16-hgp100 cells were injected subcutaneously into the right flank. Tumor volume was monitored using a caliper. After three weeks, cancer-bearing animals were randomized to the experimental groups.
Tumor-specific T cell depletion experiments
For anti-Thy1.1 depletion experiments, 250µg of anti-mouse Thy1.1 (BioXCell) or isotype control (BioXCell) were administered intraperitoneally one day prior to cecal ligation and puncture.
Cecal ligation and puncture
Cecal ligation and puncture (CLP) surgery was performed on the mice to induce polymicrobial sepsis. Mice were anesthetized using isoflurane and given pain relief medication (0.1 mg/kg buprenorphine, McKesson Medical) prior to the surgery. Ceca were ligated 50%-60% and punctured twice using 25 gauge needles (20). After surgery, mice received 1 mL saline subcutaneously and 4 doses of antibiotics (50 mg/kg ceftriaxone and 35 mg/kg metronidazole) at 0, 12, 24, and 36 hours following surgery. In some experiments, ceca were not ligated or punctured (sham surgery controls).
Flow cytometry and intracellular cytokine staining
After tissue processing and homogenization, samples were washed with FACS buffer and resuspended in Fc block according to manufacturer instructions (TruStain FcX, BioLegend). After incubation on ice, extracellular antibodies were added and allowed to stain for 30 minutes. Samples were then washed and analyzed by flow cytometry. The following antibodies were used for extracellular staining: CD8 (clone 53–6.7, BioLegend), CD4 (clone GK1.5, Biolegend), CD28 (clone E18, BioLegend), CD44 (clone IM7, BioLegend), PD-1 (clone 29F1A12, BioLegend), TIGIT (clone IG9, BioLegend), Tim-3 (clone RMT3–23, BioLegend), 2B4 (clone eBio244F4, eBioscience), Thy1.1 (clone HIS51, Thermo Fisher Scientific), LIVE/DEAD Fixable Aqua Dead Cell Stain (Thermo Fisher Scientific), CD3 (clone 500A2, BD), CD45 (clone 30-F11, BD), CD160 (clone CNX46–3, BD).
For intracellular cytokine staining, splenocytes were harvested and stimulated with 30 ng/ml PMA and 400 ng/ml ionomycin in the presence of GolgiStop for 5 h at 37 °C. The following antibodies were used for cytokine staining: IFN-γ (clone XMG1.2), TNF (clone MP6-XT22), IL-2 (clone JES6–5H4) (all BD Pharmingen). CellEvent Caspase-3/7 Green Flow Cytometry Assay Kit (ThermoFisher) was used for Caspase 3/7 staining. Absolute cell counts were obtained using CountBright Absolute Counting Beads (ThermoFisher). BD LSRII and LSR Fortessa flow cytometers were used for collecting data. Analysis of flow cytometry data was performed using FlowJo and Cytobank.
Serum cytokine measurement
For serum cytokine detection, blood was obtained 24 hours post-surgery. Cytokine levels were measured using BD Cytometric Bead Array (CBA) Mouse Inflammation Kits and Bio-Plex Pro Mouse Cytokine Assays. Assays were done according to manufacturer’s protocols.
Statistics
2-tailed Student’s t test, Mann-Whitney non-parametric test, 1-way ANOVA, and log-rank tests were used. Outliers were identified and removed using Grubbs’ test. Data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism Software.
Results
LLC-OVA activates OT-I cells in vitro and in vivo
To establish a model to interrogate the impact of tumor-specific T cells on sepsis pathogenesis, Lewis lung carcinoma (LLC) cells (or saline) were injected into the flanks of mice, and three weeks later, sepsis was induced via cecal ligation and puncture (CLP). As we have previously shown, mice with pre-existing tumors exhibited significantly increased mortality following sepsis (Fig. 1A). In order to determine if the presence of tumor-specific T cells contributes to worsened sepsis morality in cancer animals, we developed an antigen-specific solid tumor model. OVA-expressing LLC cells (LLC-OVA) were used to generate tumors, and OVA-specific CD8+ OT-I T cells expressing the congenic marker Thy1.1 were used to track the tumor-specific CD8+ T cell response. First, to confirm OT-I cells recognize OVA antigen on LLC-OVA, LLC-OVA cells were co-cultured with OT-I cells in vitro. After four days of co-culture with LLC-OVA, OT-I cells exhibited increased expression of CD25 and CD69 compared to OT-I cells co-cultured with non-OVA-expressing LLC cells (Fig. 1B). This indicated that the OT-I response to LLC-OVA is specific to the OVA antigen rather than the LLC cells themselves. Next, OT-I cells were adoptively transferred into naïve animals and LLC-OVA cells were injected s.c. in the thigh (Fig. 1C). Three weeks after LLC-OVA implantation, virtually all the OT-I cells in cancer mice were CD44hi, suggesting that tumor specific OT-I cells are activated by LLC-OVA in the host (Fig. 1D). The frequency of OT-I cells in the tumor, tumor draining lymph node (TDLN, right inguinal lymph node), and spleen were assessed by flow cytometry (OT-I gating strategy is shown in Supplemental Figure 1). We found that OT-I cells constituted a larger proportion of CD8+ T cells in the TDLN and tumors compared to spleen (Fig. 1D). Moreover, OT-I cells found in tumors displayed a higher frequency of CD44+, PD-1+, 2B4+, and Tim-3+ cells as compared to OT-I cells in the spleen (Fig. 1E). These phenotypes are consistent with previous literature and indicate that OT-I cells develop an exhausted phenotype when continually exposed to tumor antigens. Overall, these results demonstrated that OT-I cells are specifically activated by LLC-OVA in our model.
Figure 1. LLC-OVA activates OT-I cells in vitro and in vivo.
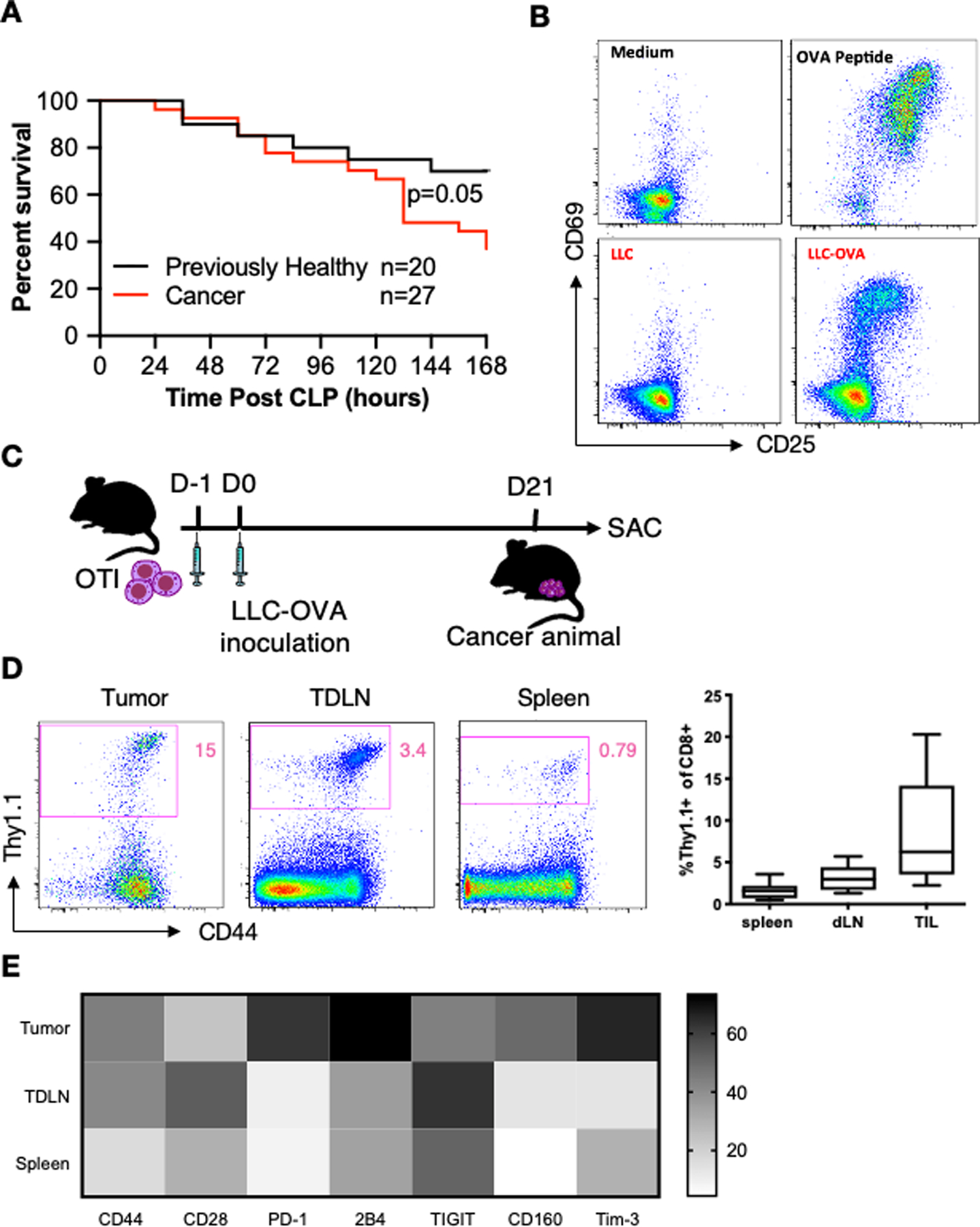
(A) Graph summarizing the survival difference between cancer and previously healthy mice following CLP. Cancer was induced in mice through inoculation with LLC cells in the right flank. Mice who did not receive LLC cells served as previously healthy controls. Three weeks later, all mice underwent CLP. Mice were then monitored for survival for 7 days. (B) OT-I splenocytes were co-cultured with medium, OVA peptide, LLC or LLC-OVA for four days. Representative flow cytometry plots show staining for the activation markers CD25 and CD69 after incubation for each condition. All cells were gated on CD3+CD8+Thy1.1+ cells (see Supplemental Figure 1 for gating strategy for tumor-specific cells). (C) Schematic showing the timeline for LLC-OVA cancer experiments. OT-I cells were adoptively transferred into animals. One day later, LLC-OVA was inoculated into the right thigh. Three weeks later, mice were euthanized, and OT-I cells were harvested from various tissues. (D) Representative flow cytometry plots (gated on live CD45+CD3+CD8+ cells) showing the percentage of OT-I cells of total CD8+ T cells in the tumor, TDLN, and spleen (left) and summary graph (right). (E) Heatmap showing the activation and exhaustion phenotype of OT-I cells from the tumor, TDLN, and spleen. The MFI of each surface marker was used to generate the heatmap.
Tumor-specific T cells worsen sepsis survival
Because cancer septic mice have a higher mortality compared to septic mice without cancer (21, 22), we next assessed whether tumor-specific T cells contributed to this increase in sepsis mortality. To do this, anti-Thy1.1 antibody (or isotype control) was administered to deplete OT-I cells three weeks following adoptive transfer of Thy1.1+ OT-I and LLC-OVA tumor induction. One day later, mice were subjected to cecal ligation and puncture (CLP) to induce sepsis (Fig. 2A). Flow cytometry confirmed that anti-Thy1.1 antibody treated animals have no detectable OT-I cells remaining (Fig. 2B). Strikingly, depletion of tumor-specific T cells improved 7-day survival compared to non-depleted isotype control-treated mice (Fig. 2C). These data suggested that tumor-specific T cells directly contributed to the increased mortality from sepsis in mice with cancer compared to previously healthy animals. To assess whether this effect was reproducible in a different cancer model, we repeated the survival experiment using a B16-hgp100 melanoma model. Mice underwent adoptive transfer of pMel Thy1.1+ CD8+ TCR tg T cells and cancer cell injection with B16-hgp100 cells. After 3 weeks, tumor-specific T-cells were depleted using an anti-Thy1.1 antibody and then 24 hours later the animals were subjected to CLP. Significantly increased survival was observed in the mice with tumor-specific T-cell depletion compared to non-depleted controls (Fig. 2D). These data show that the improved survival in cancer septic mice following tumor-specific T-cell depletion is not exclusive to lung cancer or to the OVA transgenic mouse model, and strengthen the conclusion that tumor-specific T cells contribute to sepsis mortality in these animals.
Figure 2. Tumor-specific T cells worsen sepsis survival.
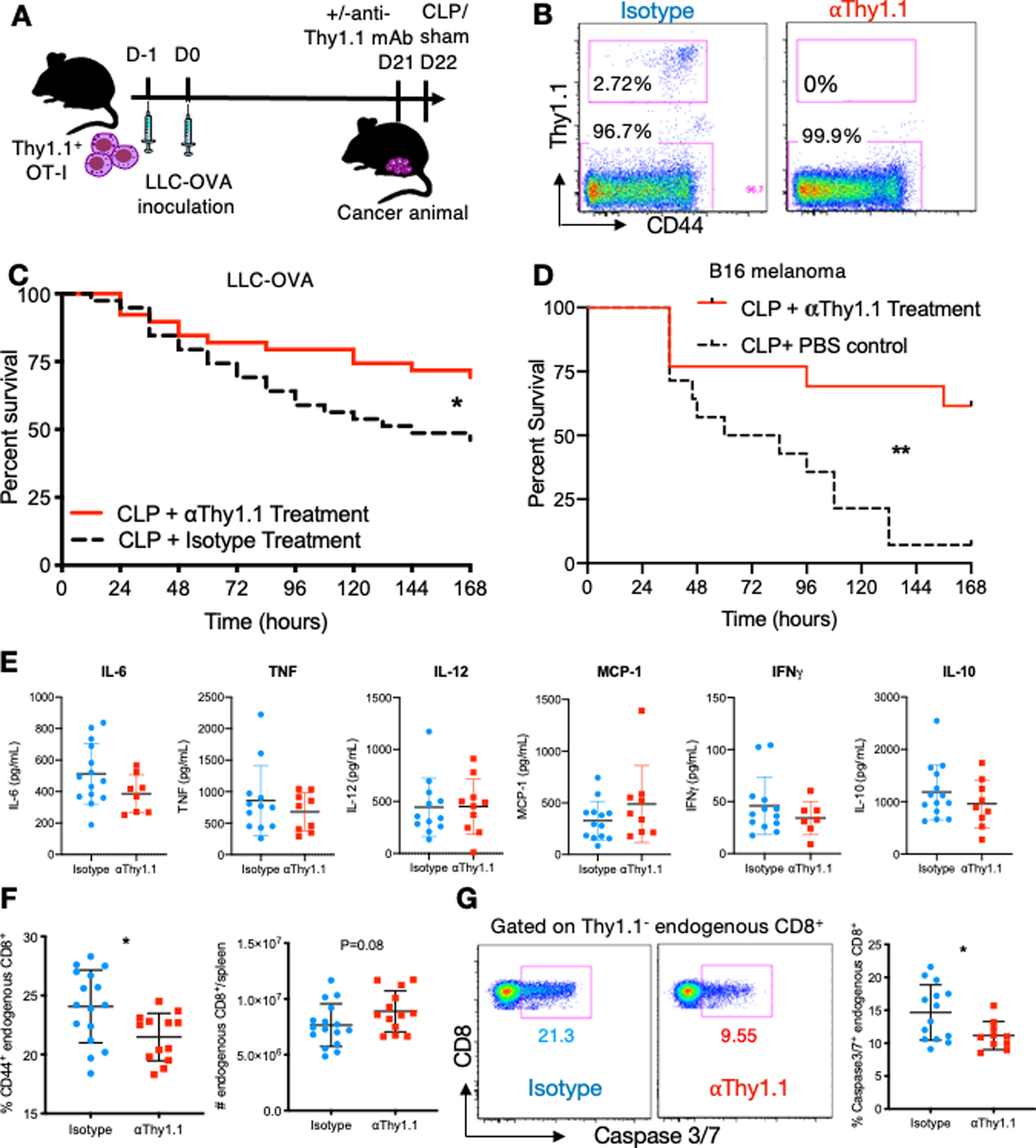
(A) Schematic of experimental design. OT-I cells were adoptively transferred into animals. One day later, LLC-OVA was inoculated into the right thigh. Three weeks later, mice were randomized to receive anti-Thy1.1 mAb or isotype control, and were subjected to CLP one day later. (B) Representative flow cytometry plots showing tumor-specific OT-I (Thy1.1+) and endogenous (Thy1.1-) CD8+ T cells following treatment with isotype control or anti-Thy1.1 depleting antibody. (C) Survival curve comparing the mortality of cancer septic mice that received treatment with isotype control or anti-Thy1.1 depletion antibody. Antibodies were administered one day prior to CLP and survival was monitored for 7 days. (D) CD8+ pMEL T cells were adoptively transferred into animals. One day later, B16 melanoma cells were inoculated into the right thigh. Three weeks later, mice were randomized to receive anti-Thy1.1 mAb or isotype control, and were subjected to CLP one day later. (E) Summary graphs showing serum cytokine concentrations at 24 hours following CLP in OT-I/LLC-OVA model. (F) Summary graphs showing the frequency of CD44+ endogenous CD8+ T cells and absolute number of endogenous CD8+ T cells at 48 hours following CLP in the spleen in OT-I/LLC-OVA model. (E) Representative flow cytometry plots and summary data showing Caspase 3/7 staining in the endogenous CD8+ T cell population for both isotype treated and anti-Thy1.1 treated mice in OT-I/LLC-OVA model. *p≤0.05, **p≤0.01.
As such, we next sought to identify potential mechanisms underlying this effect. First, the cytokine milieu of isotype vs. anti-Thy1.1-treated mice was assessed. No significant differences in the concentration of serum IL-6, TNF, IL-12, MCP-1, IFN-γ or IL-10 between isotype and anti-Thy1.1 treated mice at 24 hours were observed (Fig. 2E). Next, we examined the impact of tumor-specific T cell depletion on the endogenous Thy1.1- CD8+ T cell population, which would be expected to participate in the sepsis response, two days following CLP. Results indicated that the frequency of activated CD44+ endogenous CD8+ T cells was significantly reduced by anti-Thy1.1 treatment (Fig. 2F), suggesting that the presence of tumor-specific T cells contributes to the activation of the endogenous CD8+ T cell population. Moreover, mice treated with anti-Thy1.1 exhibited a trend towards higher absolute numbers of endogenous CD8+ T cells compared to isotype-treated mice (Fig. 2F). Based on these differences, we hypothesized that tumor-specific T cells were causing endogenous CD8+ T cells to undergo apoptosis during sepsis. Indeed, results indicated that anti-Thy1.1 treatment significantly reduced the frequency of caspase 3/7 apoptotic cells among endogenous CD8+ T cells in septic animals (Fig. 2G). In sum, these results imply that tumor-specific T cells may contribute to sepsis pathogenesis and mortality through the induction of apoptosis in endogenous CD8+ T cells.
Depletion of tumor antigen-specific CD8+ T cells results in reduced expression of 2B4 on endogenous CD8+ T cells during sepsis
Based on our findings that the presence of tumor-specific CD8+ T cells increases the apoptosis of endogenous T cell populations, we sought to investigate changes in endogenous T cell phenotype that may underlie increased apoptosis. As such, expression of the coinhibitory receptors 2B4, PD-1, TIM-3, LAG-3, and TIGIT on the surface of Thy1.1-negative CD4+ and CD8+ T cells were compared between tumor-bearing septic mice and those that had been depleted of Thy1.1+ tumor-antigen-specific T cells as above. As in Fig 2, naïve B6 recipients of Thy1.1+ OT-I T cells were inoculated with LLC-OVA and allowed to develop tumors for 3 weeks. On day 21, mice were injected with anti-Thy1.1 depleting antibody or isotype control, underwent CLP 24h later, and were sacrificed 24h after that. Analysis of Thy1.1- CD4+ (Fig. 3A) and CD8+ (Fig. 3B) T cell populations in the spleen revealed no differences in the expression of PD-1, TIM-3, LAG-3, or TIGIT between the depleted vs. non-depleted groups. In contrast, expression of 2B4 on endogenous CD8+ T cells was significantly reduced following Thy1.1+ tumor-specific CD8+ T cell depletion.
Figure 3. Deletion of tumor-specific T cells results in decreased 2B4 expression on endogenous CD8+ T cells during sepsis.
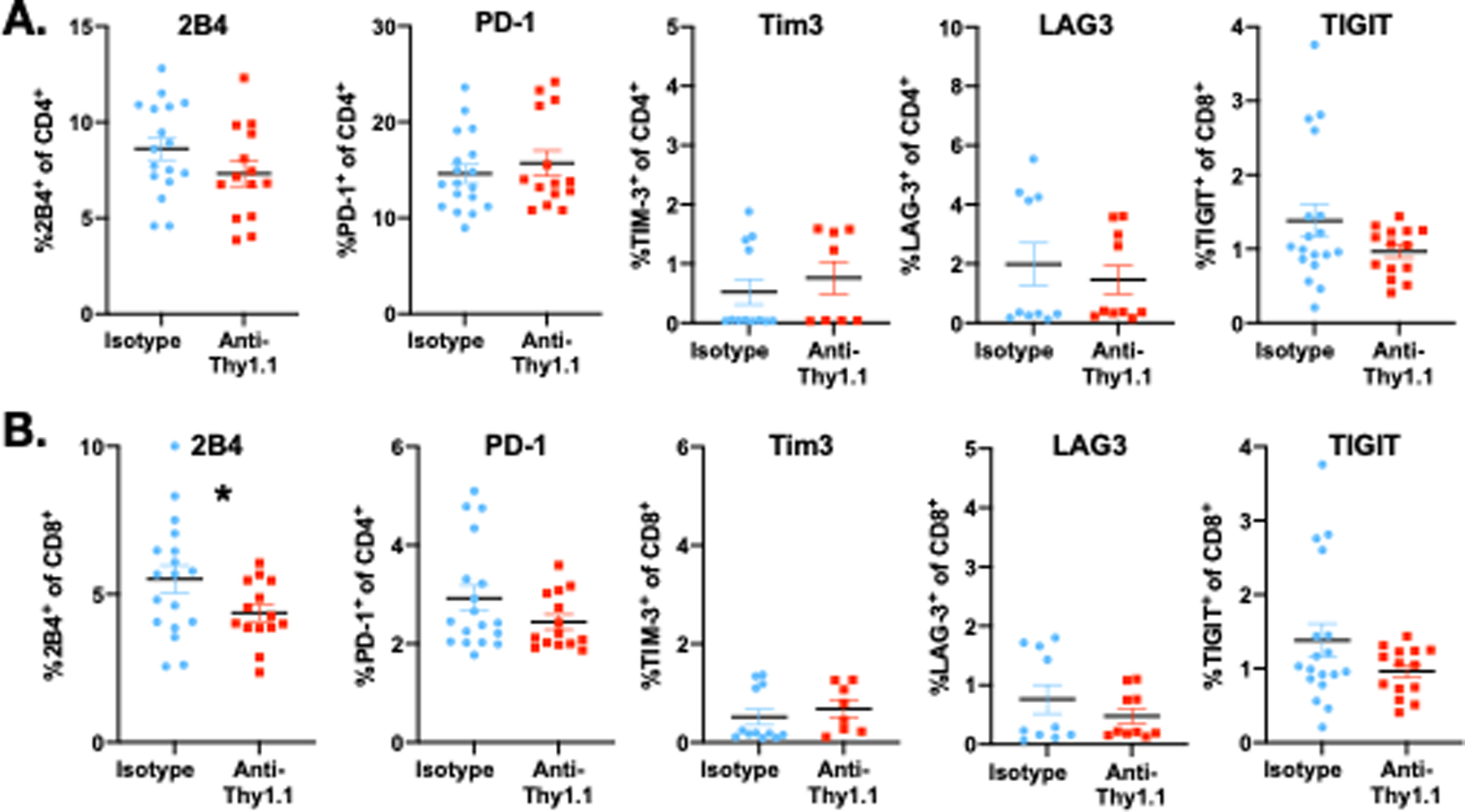
OT-I cells were adoptively transferred into animals. One day later, LLC-OVA was inoculated into the right thigh. Three weeks later, mice were randomized to receive αThy1.1 mAb or isotype control, and were subjected to CLP one day later. At 24h, mice were sacrificed and expression of coinhibitory molecules were examined on splenic CD4+ and CD8+ T cells. A, Expression of 2B4, PD-1, Tim3, LAG-3, and TIGIT on splenic CD4+ T cells isolated from 24h septic animals. B, Expression of 2B4, PD-1, Tim3, LAG-3, and TIGIT on splenic CD8+ T cells isolated from 24h septic animals. *p≤0.05
Endogenous and tumor-specific T cells numbers are equally lost in the circulation during sepsis
Based on our findings that the presence of tumor-specific T cells increases the apoptosis of other T cell populations, we further investigated the phenotypes and functionality of these tumor-specific CD8+ T cells during sepsis. Since sepsis induces T cell apoptosis and reduces the number of circulating T cells but has less impact on tissue resident T cell populations (8, 11, 23), we hypothesized that sepsis would result in a greater reduction of T cells in the circulation compared to the TDLN or tumor site. To address this question, we adoptively transferred OT-I cells into mice one day prior to injection with LLC-OVA. Three weeks later, mice underwent CLP or sham surgery before sacrifice at 24 hours; tumors, tumor draining lymph node (TDLN), and spleen were collected for T cell analysis (Fig. 4A). Consistent with our hypothesis, we found that the number of OT-I cells in the tumor was not different between mice receiving CLP and sham surgery (Fig. 4B. In contrast, the number of endogenous CD8+ T cells in the tumor decreased two-fold after CLP (Fig. 4C). In the TDLN, OT-I and non-OT-I CD8+ T cells were reduced over 4-fold (Fig. 4B, C). The number of both OT-I and non-OT-I CD8+ T cells were reduced ~1.5 fold in the spleen following CLP (Fig. 4C, D). These data indicate that while sepsis results in a loss of both tumor-specific and endogenous T cells in the TDLN and spleen, sepsis has no effect on the numbers of tumor-specific CD8+ T cells within the tumor.
Figure 4. Tumor-specific T cells are lost in the circulation during sepsis, but remain unchanged in tumor tissue.
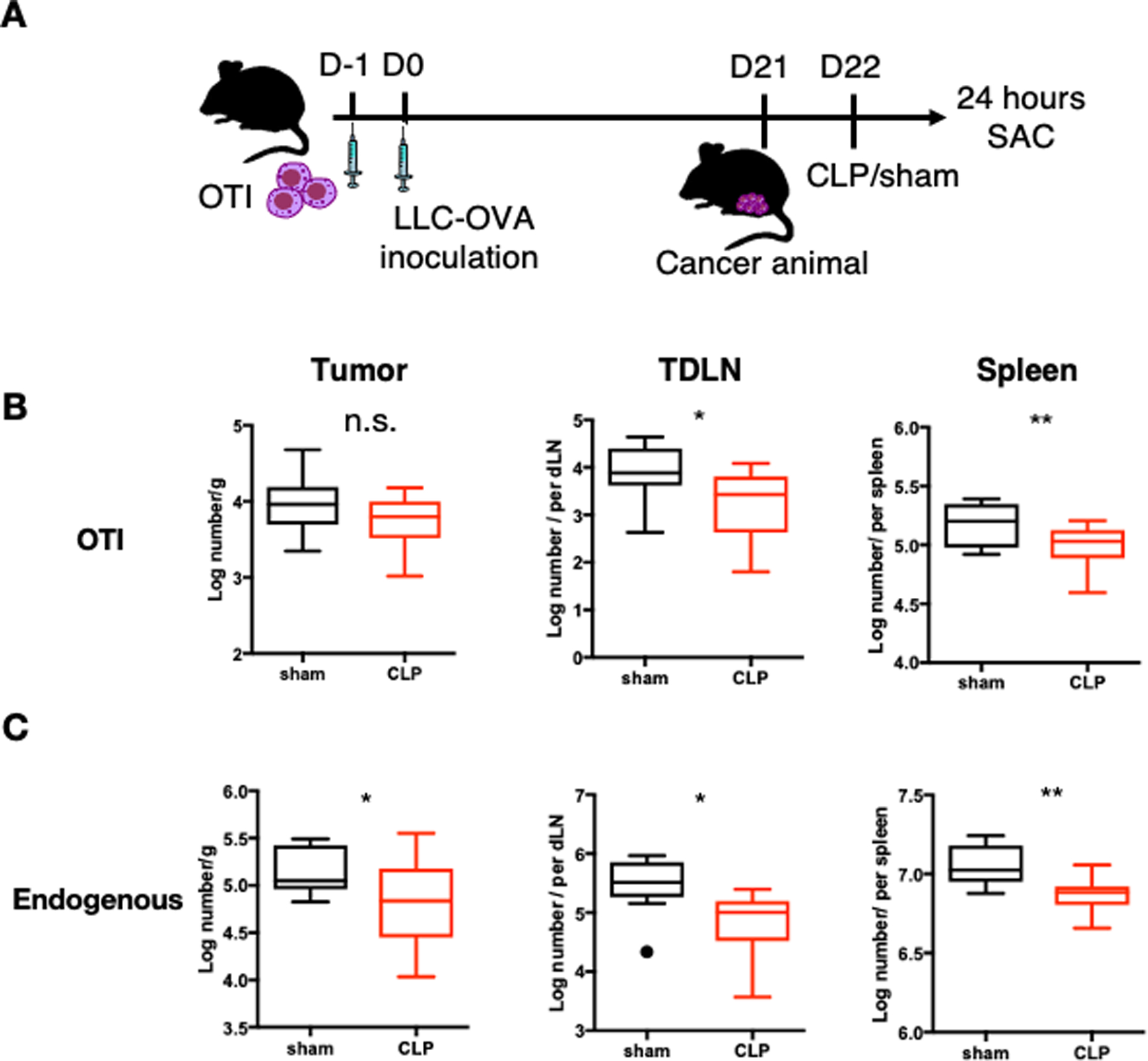
(A) Schematic showing the experimental design for Figure 3. LLC-OVA cancer animals were generated as previously described. CLP surgery was performed to induce polymicrobial sepsis and sham surgery was performed as a control on mice. 24 hours later, animals were sacrificed. (B) Summary graphs showing the absolute number of tumor-specific OT-I cells (gated on CD3+CD8+Thy1.1+ cells) in tumor, TDLN, and spleen. (C) Summary graphs showing the absolute number of endogenous CD8+ T cells (gated on CD3+CD8+Thy1.1- cells) in tumor, TDLN, and spleen. *p≤0.05, **p≤0.01, ***p≤0.001
Tumor-specific T cells undergo greater exhaustion in the circulation compared to the tumor microenvironment during sepsis
Systemic T cell exhaustion is considered a hallmark of sepsis-induced immune dysfunction and is characterized by the expression of multiple co-inhibitory receptors and decreased functionality. In our model, OT-I cells in the tumor microenvironment express high levels of co-inhibitory molecules prior to the induction of sepsis (Fig. 1E). To determine whether tumor-specific T cells become more exhausted during sepsis, we assessed the phenotype of OT-I T cells in cancer septic mice 24 hours after CLP. Tumor infiltrating OT-I T cells did not upregulate any co-inhibitory markers following CLP, although the MFI of CD28 decreased after sepsis (Fig. 5A). In the TDLN, OT-I T cells exhibited increased expression of PD-1 and TIGIT but no change in their CD28 MFI following CLP (Fig. 5B). OT-I cells in the spleen had a reduced CD28 MFI in addition to elevated 2B4 and TIGIT expression following CLP (Fig. 5C). These results are consistent with previous findings that sepsis-induced T cell dysfunction preferentially affects circulating lymphocytes (8, 11, 23).
Figure 5. T cells in the spleen and tumor draining lymph node have increased co-inhibitory receptor expression in cancer sepsis.
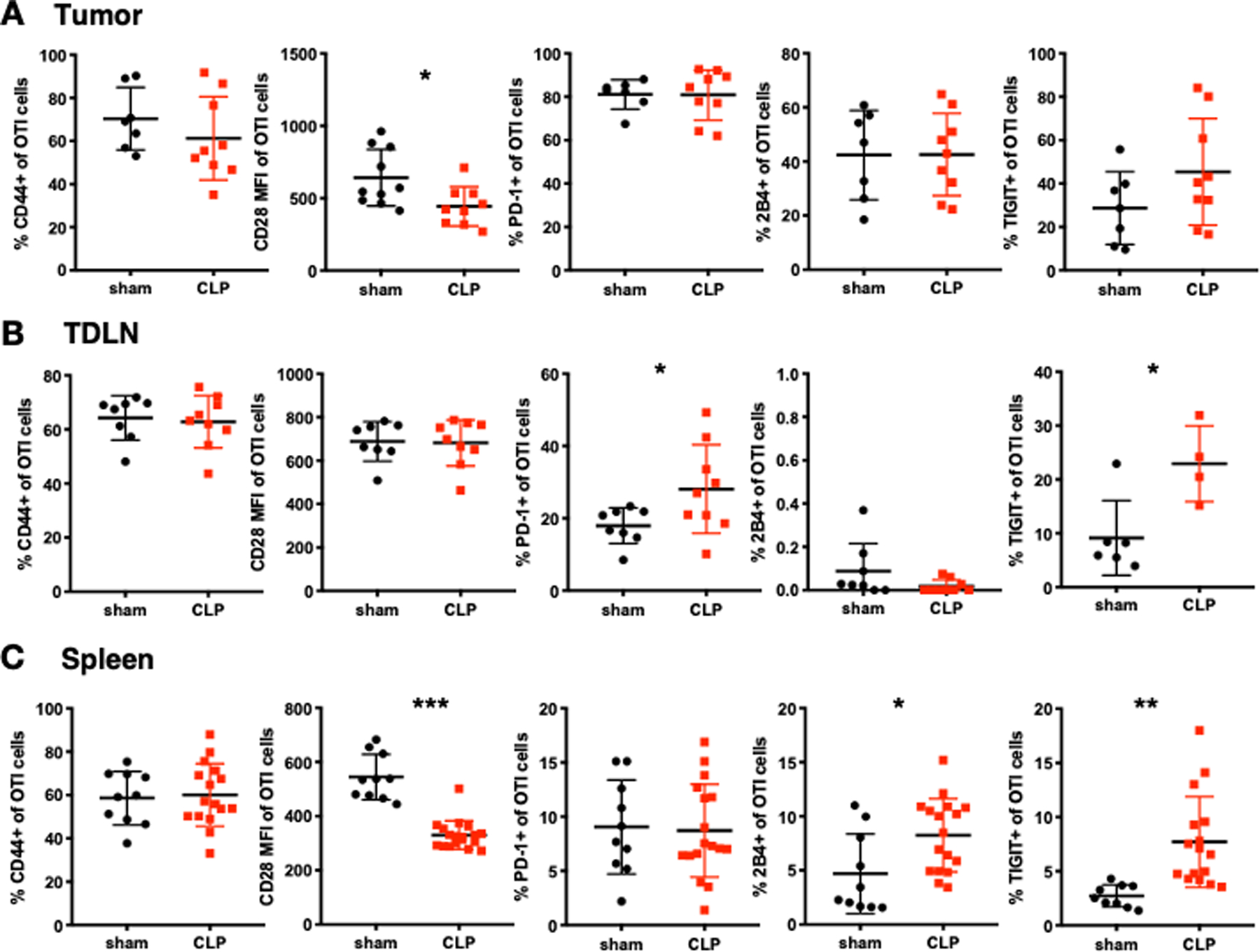
LLC-OVA cancer animals were sacrificed at 24 hours post CLP. The phenotype of OT-I cells (gated on CD3+CD8+Thy1.1+ cells) in sham and CLP mice were determined. (A) Summary graphs showing the frequency and MFI of activation (CD44, CD28) and exhaustion (PD-1, 2B4, TIGIT) markers on OT-I cells in the tumor. (B) Summary graphs showing the frequency and MFI of activation (CD44, CD28) and exhaustion (PD-1, 2B4, TIGIT) markers on OT-I cells in the tumor draining lymph node (TDLN) at 24 hours post CLP. (C) Summary graphs showing the frequency and MFI of activation (CD44, CD28) and exhaustion (PD-1, 2B4, TIGIT) markers on OT-I cells in the spleen at 24 hours post CLP. *p≤0.05, **p≤0.01, ***p≤0.001.
Tumor-specific T cells have impaired IFN-γ production and increased TNF production during sepsis
T cell exhaustion leads to impairments in the production of inflammatory cytokines such as IFN-γ and TNF. As the secretion of these cytokines are impacted by sepsis and are important for anti-tumor immunity, we assessed the impact of sepsis on the production of these cytokines. Twenty-four hours after CLP-induced sepsis, we found that the frequency of IFN-γ secreting OT-I cells was reduced in the tumor, TDLN, and spleen (Fig. 6A). In contrast, TNF secretion varied across tissues, with the frequency of TNF-secreting OT-I cells declining in the tumor while increasing in the TDLN and remaining unchanged in the spleen (Fig. 6B). Overall, these data suggest that tumor-specific T cells have impaired IFN-γ cytokine secretion during sepsis across all compartments. However, TNF production by tumor-specific T cells is enhanced in the TDLN compared to the circulation.
Figure 6. Sepsis impairs IFN-γ secretion by tumor-specific T cells in multiple organs.
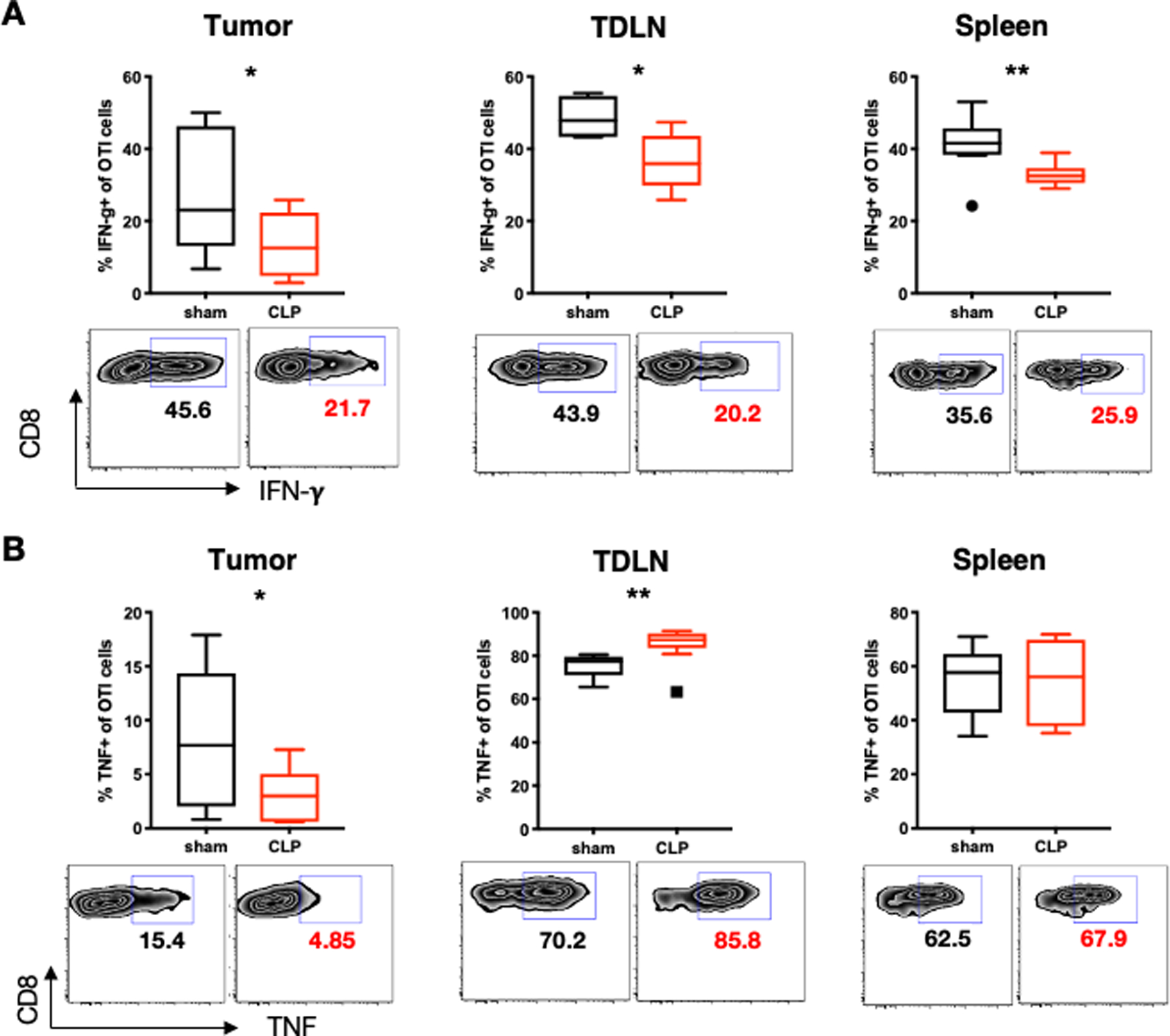
LLC-OVA cancer was induced 3 weeks prior to CLP as previously described. Animals were sacrificed at 24 hours post CLP. Splenocytes were collected and stimulated with PMA and Ionomycin for 4 hours before cytokine staining. (A) Representative flow plots and summary graphs showing the percentage of IFN-γ+ tumor-specific OT-I cells (gated on CD8+Thy1.1+) in the tumor, TDLN, and spleen. (B) Representative flow cytometry plots and summary graphs showing the percentage of TNF+ tumor-specific OT-I cells (gated on CD8+Thy1.1+) in the tumor, TDLN, and spleen. *p≤0.05, **p≤0.01.
Discussion
In this study, we used an antigen-specific cancer system to investigate how anti-tumor immunity shapes sepsis pathophysiology. We primarily assessed the phenotype of CD8+ T cells at 24 hours following CLP or sham surgery because the immune abnormalities that occur during sepsis occur rapidly, including changes in T cell phenotype and effector function that are present within 24 hours (24–27). We found that tumor-specific T cells contribute to sepsis mortality, potentially by contributing to the apoptosis of endogenous CD8+ cells. Tumor-specific CD8+ T cells in the tumor did not display a sepsis-induced increase in exhaustion markers as did tumor-specific CD8+ T cells in the TDLN/spleen and non-tumor-specific CD8+ T cells. Moreover, while cytokine secretion by tumor-specific CD8+ T cells was impaired early after CLP, at later time points tumor-specific CD8+ T cells in the tumor tissue exhibited increased cytokine secretion. Overall, these data support the conclusion that activated, tumor-specific CD8+ T cells are not subject to sepsis-induced immunosuppression, but instead their preserved function comes at a cost of increased attrition of non-tumor-specific CD8+ T cell populations during sepsis.
The finding that tumor-specific T cells contributed to the apoptosis of endogenous CD8+ T cells is significant because lymphocyte apoptosis is a well-known aspect of sepsis immune dysregulation and mortality and preventing apoptosis can attenuate death from sepsis (28). This was demonstrated in seminal studies from Hotchkiss’s laboratory, which showed that mice containing Bcl2-tg lymphocytes that cannot undergo apoptosis are resistant to death from sepsis (28). Moreover, adoptive transfer of even 106 Bcl2tg T cells into WT hosts (which contain 108 total T cells) also prevented death from sepsis (28). These data provide proof-of-principle that even small changes in the number of apoptotic T cells can impact mortality during sepsis.
Our study examined the phenotypes of endogenous T cell populations in cancer animals in which tumor antigen-specific T cells were present or had been depleted. Interestingly, the only significant difference in the expression of these inhibitory molecules in either the CD4+ or CD8+ T cell compartment was a decrease in the expression of 2B4 on CD8+ cells T cells following depletion of tumor-specific CD8+ T cells. These results are consistent with our recently published results showing that anti-2B4, but not anti-PD-1, was effective at improving sepsis mortality in cancer septic animals (29). Moreover, 2B4 expression was recently shown by Menner et al. to be a marker of susceptibility to apoptosis in the continued presence of antigen (30). Mechanistically, the increased propensity of 2B4+ cells to undergo apoptosis was driven by their loss of expression of the transcription factor Id3. Of note, retroviral expression of Id3 specifically increased the persistence of 2B4+ CD8+ T cells by decreasing their susceptibility to Fas/Fas ligand-mediated cell death (30). Thus, our data suggest a model in which tumor antigen-specific CD8+ T cells drive expression of 2B4 on endogenous CD8+ T cells during sepsis, and this may render them at increased risk of undergoing apoptosis. Future investigation will be aimed at rigorously testing the model and hypotheses raised by the data presented in this manuscript.
Our finding that OT-I T cells within the tumor were retained is consistent with previous studies showing that tissue resident T cells in lung and skin are less impaired during sepsis relative to circulating cells or those in secondary lymphoid organs (8, 11, 23, 31). Of note, in our study, although the OT-I cell count was preserved during sepsis in the tumor, non-OT-I cells at this site were decreased. These results are consistent with published data showing that sepsis results in the preferential depletion of a subset of memory-phenotype CD8+ T cells that remain “unactivated” (i.e., fail to upregulate activation markers) by apoptosis (32). These data therefore suggest that TCR-activation of OT-I cells at the site of the tumor may provide survival signals to these cells. The mechanisms behind the preserved survival of OT-I T cells in the tumor microenvironment during sepsis warrant further investigation.
Our study had limitations. While we found compelling evidence for the role of tumor-specific T cells in causing immune impairment and mortality during sepsis, our study relied on only two types of cancer cell lines and the use of monoclonal TCR transgenic T cells. As such, we targeted only one antigen in each model from a pool of hundreds of antigens present on cancer cells. While OT-I and pMEL served as tumor antigen-specific cells in our model, it is also possible that some endogenous CD8+ T cells may have recognized other antigens on the tumors in these experiments. As human cancer patients have a wide variety of cancer types with thousands of antigens, future studies should confirm our results in other cancer models using polyclonal populations of tumor antigen-specific cells. In addition, in this study we did not elucidate the role of tumor-specific CD4+ T cells in cancer sepsis, or the effect on mortality beyond 7 days post-CLP due to animal welfare/ IACUC regulations. Moreover, the impact of cancer treatments such as immune checkpoint blockade and chemotherapy on the influence of tumor-specific T cells in sepsis pathogenesis is similarly unknown. Future work should determine whether tumor-specific T cells have a similar impact in the presence of these therapies.
Supplementary Material
Key points:
Tumor-specific CD8+ T cells worsen mortality in a mouse model of polymicrobial sepsis
This effect is linked to increased apoptosis of non-tumor-specific CD8+ T cells
This is linked to increased expression of 2B4 on non-tumor-specific CD8+ T cells
Acknowledgements
The authors would like to acknowledge Dr. Chrystal Paulos, Emory University, for helpful advice regarding the pMEL melanoma model.
This work was supported by GM104323, AI073707, and AI149724.
References
- 1.Hensley MK, Donnelly JP, Carlton EF, and Prescott HC. 2019. Epidemiology and Outcomes of Cancer-Related Versus Non-Cancer-Related Sepsis Hospitalizations. Crit Care Med 47: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, Anderson DJ, Warren DK, Dantes RB, Epstein L, Klompas M, C. Centers for Disease, and P. Prevention Prevention Epicenters. 2019. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw Open 2: e187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper AJ, Keller SP, Chan C, Glotzbecker BE, Klompas M, Baron RM, and Rhee C. 2020. Improvements in Sepsis-associated Mortality in Hospitalized Patients with Cancer versus Those without Cancer. A 12-Year Analysis Using Clinical Data. Ann Am Thorac Soc 17: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Te Marvelde L, Whitfield A, Shepheard J, Read C, Milne RL, and Whitfield K. 2020. Epidemiology of sepsis in cancer patients in Victoria, Australia: a population-based study using linked data. Australian and New Zealand journal of public health 44: 53–58. [DOI] [PubMed] [Google Scholar]
- 5.Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, Salluh JI, and Soares M. 2012. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care 27: 301–307. [DOI] [PubMed] [Google Scholar]
- 6.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, and Hotchkiss RS. 2011. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306: 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Monneret G, and Payen D. 2013. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue HH, Harty JT, Griffith TS, and Badovinac VP. 2017. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog 13: e1006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardura MI, Banchereau R, Mejias A, Di Pucchio T, Glaser C, Allantaz F, Pascual V, Banchereau J, Chaussabel D, and Ramilo O. 2009. Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS One 4: e5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serbanescu MA, Ramonell KM, Hadley A, Margoles LM, Mittal R, Lyons JD, Liang Z, Coopersmith CM, Ford ML, and McConnell KW. 2016. Attrition of memory CD8 T cells during sepsis requires LFA-1. J Leukoc Biol 100: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Yang WL, Matsuo S, and Wang P. 2015. Differential alterations of tissue T-cell subsets after sepsis. Immunol Lett 168: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy EF 2006. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 26: 154–158. [PMC free article] [PubMed] [Google Scholar]
- 13.DeWeerdt S 2013. Bacteriology: a caring culture. Nature 504: S4–5. [DOI] [PubMed] [Google Scholar]
- 14.Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, Helgason T, Szvalb AD, Bird JE, Roy-Chowdhuri S, Zhang HH, Qiao Y, Karim B, McDaniel J, Elpiner A, Sahora A, Lachowicz J, Phillips B, Turner A, Klein MK, Post G, Diaz LA Jr., Riggins GJ, Papadopoulos N, Kinzler KW, Vogelstein B, Bettegowda C, Huso DL, Varterasian M, Saha S, and Zhou S. 2014. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med 6: 249ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavassani KA, Carson W. F. t., Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, Hogaboam CM, and Kunkel SL. 2010. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood 115: 4403–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, and Gabrilovich D. 2011. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 121: 4015–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanada KI, Yu Z, Chappell GR, Park AS, and Restifo NP. 2019. An effective mouse model for adoptive cancer immunotherapy targeting neoantigens. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overwijk WW, and Restifo NP. 2001. B16 as a mouse model for human melanoma. Curr Protoc Immunol Chapter 20: Unit 20 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, and Restifo NP. 2003. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 198: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker CC, Chaudry IH, Gaines HO, and Baue AE. 1983. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94: 331–335. [PubMed] [Google Scholar]
- 21.Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM, Dominguez JA, Perrone EE, Dunne WM, Hotchkiss RS, Buchman TG, Linehan DC, and Coopersmith CM. 2010. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit Care Med 38: 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons JD, Mittal R, Fay KT, Chen CW, Liang Z, Margoles LM, Burd EM, Farris AB, Ford ML, and Coopersmith CM. 2016. Murine Lung Cancer Increases CD4+ T Cell Apoptosis and Decreases Gut Proliferative Capacity in Sepsis. PLoS One 11: e0149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danahy DB, Jensen IJ, Griffith TS, and Badovinac VP. 2019. Cutting Edge: Polymicrobial Sepsis Has the Capacity to Reinvigorate Tumor-Infiltrating CD8 T Cells and Prolong Host Survival. J Immunol 202: 2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, and Green JM. 2012. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care 16: R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O’Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, and Moldawer LL. 2011. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol 186: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martignoni A, Tschop J, Goetzman HS, Choi LG, Reid MD, Johannigman JA, Lentsch AB, and Caldwell CC. 2008. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock 29: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CW, Mittal R, Klingensmith NJ, Burd EM, Terhorst C, Martin GS, Coopersmith CM, and Ford ML. 2017. Cutting Edge: 2B4-Mediated Coinhibition of CD4(+) T Cells Underlies Mortality in Experimental Sepsis. J Immunol 199: 1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, and Karl IE. 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol 162: 4148–4156. [PubMed] [Google Scholar]
- 29.Chen CW, Xue M, Zhang W, Xie J, Coopersmith CM, and Ford ML. 2019. 2B4 but not PD-1 blockade improves mortality in septic animals with preexisting malignancy. JCI insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menner AJ, Rauch KS, Aichele P, Pircher H, Schachtrup C, and Schachtrup K. 2015. Id3 Controls Cell Death of 2B4+ Virus-Specific CD8+ T Cells in Chronic Viral Infection. J Immunol 195: 2103–2114. [DOI] [PubMed] [Google Scholar]
- 31.Danahy DB, Kurup SP, Winborn CS, Jensen IJ, Harty JT, Griffith TS, and Badovinac VP. 2019. Sepsis-Induced State of Immunoparalysis Is Defined by Diminished CD8 T Cell-Mediated Antitumor Immunity. J Immunol 203: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serbanescu MA, Ramonell KM, Hadley A, Margoles LM, Mittal R, Lyons JD, Liang Z, Coopersmith CM, Ford ML, and McConnell KW. 2016. Attrition of memory CD8 T cells during sepsis requires LFA-1. J Leukoc Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


