Abstract

The review presents data on the synthesis as well as studies of biological activity of new derivatives of pyrimido[1,2-a]benzimidazoles published over the last decade. The bibliography of the review includes 136 sources.
Keywords: 2-aminobenzimidazole; pyrimido[1,2-a]benzimidazoles; polynitrogen-containing heteroarenes; heterocyclization; multicomponent reactions
Nitrogen-containing heterocyclic compounds are the basis of many natural and synthetic biologically active substances.1 More than two-thirds of the known drugs used in clinical practice contain heterocyclic and, above all, nitrogen-containing fragments within their structure. Over the past decades, the chemistry of aza-heterocycles has received considerable attention due to the wide spectrum of their biological activity and numerous therapeutic applications in medicine.
Of nitrogenous heterocycles, azoloazines containing fragments similar to the natural heterocycles purines and pyrimidines are currently of great practical importance. Thus, non-natural nucleosides abacavir, famciclovir, remdesivir are known, which are the products of structural modifications of all the components of the nucleotide exhibiting excellent indicators of antiviral action (Fig. 1).2–4
Figure 1.
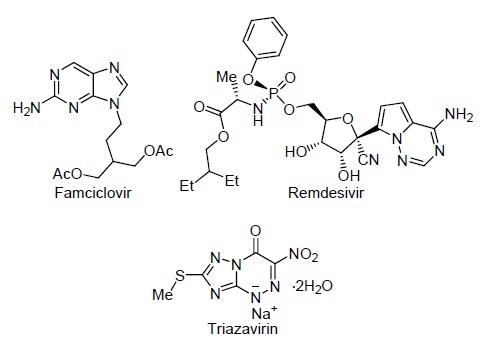
Azoloazine-based antiviral drugs.
In addition to the generally accepted nucleoside forms, the azoloazines themselves are relevant in the search for means of combating diseases on a global scale. Nitroazolo-[5,1-c][1,2,4]triazines and nitroazolo[1,5-a]pyrimidines, a new family of antiviral compounds has been found.5 The medication Triazavirin (2-methylsulfanyl-6-nitro[1,2,4]-triazolo[5,1-c][1,2,4]triazin-7-one sodium salt dihydrate) showed a broad spectrum of antiviral action and high efficacy. The medication protects against infection caused by influenza viruses,6–9 ARVI,10 tick-borne encephalitis. 11,12 Triazavirin has been shown to be effective in treating patients with moderate COVID-19.13,14 Antiviral medication 5-methyl-6-nitro-7-oxo-4,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine L-arginine salt monohydrate is at the stage of clinical trials.15,16
At the same time, this is not the only method of constructing the important heterocyclic structures with relevant biological activity. Thus, numerous compounds containing benzimidazole scaffold which are isosteres of the nitrogenous bases of nucleic acids are known.17 Some of the most important drugs containing the benzimidazole structural element are shown in Figure 2.
Figure 2.
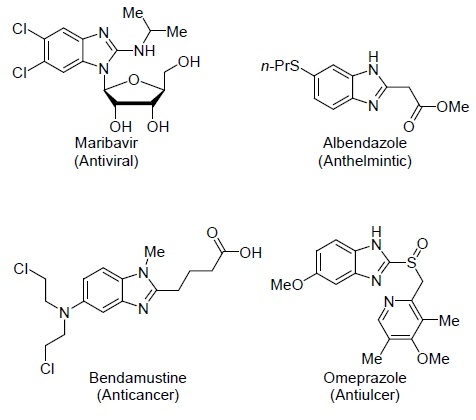
The drugs based on benzimidazole.
Currently, numerous compounds containing the benzimidazole moiety are known to exhibit various types of biological activity, including analgesic,18 antibacterial,19 anticancer,20,21 antifungal,22 antiHIV,23 anti-inflammatory,24 antimalarial,25 antimicrobial,26 antioxidant activity,27 as well as anti-tuberculosis28 and varied antiviral activities.29 Thus, the creation of pharmacologically sound benzimidazole derivatives is an important task that requires complex synthetic approaches.
Among the methods for the structural modification of benzimidazole scaffolds, the approach consisting in the creation of polycyclic condensed analogs with the participation of five- and six-membered structures is of particular interest and independent significance. Of the large number of polycyclic derivatives of benzimidazoles, pyrimido[1,2-a]benzimidazoles are of significant interest, having structural similarity both with benzimidazoles and with various azolo[1,5-a]pyrimidines which have also proven themselves as structures with relevant biological properties, including antiviral,5 antibacterial,30 antiseptic,31,32 anticancer,33 and anti-glycation34 effects. In addition, annulated benzimidazoles with a conjugated planar structure exhibit relevant photophysical properties and find application in optoelectronics as phosphors and fluorescent dyes in textile and polymer materials.35 Besides, pyrimido[1,2-a]benzimidazoles are of interest from the aspect of further modifications in the creation of macrocyclic derivatives, including, in particular, purinobenzimidazoles which are not described in the literature.
This review examines and discusses the data on the main methods of construction and the possibilities of practical application of pyrimido[1,2-a]benzimidazole derivatives published in the past 10 years. The increased interest in such heterocyclic systems is due to the promise of the emergence of unique properties (biological active, photophysical, structural, etc.) because of the practical significance of the benzimidazole and azolo[1,5-a]-pyrimidine scaffolds contained in pyrimido[1,2-a]-benzimidazoles.
To obtain the target heterocyclic systems of this type, two main synthetic strategies are currently actively used: the reaction of aminobenzimidazoles with bifunctional synthetic equivalents, and the construction of a pyrimidobenzimidazole structure by the method of multicomponent reactions.
The reaction of aminobenzimidazoles with bifunctional synthetic equivalents
One of the approaches to the construction of pyrimido-[1,2-a]benzimidazoles and related polycyclic derivatives is based on the annulation of substituted benzimidazoles with bifunctional synthetic equivalents, the nature of which determines the reaction conditions. The most common and widely used example of bifunctional synthetic equivalents are derivatives of unsaturated carbonyl compounds.
A team of authors from Egypt described the synthesis of pyrimidobenzimidazole derivative 3 containing a pyrazole substituent at position 2 (Scheme 1). It was demonstrated that the reaction of 2-aminobenzimidazole (1a) with the unsaturated ketone derivative 2 in EtOH under the conditions of basic catalysis leads to the target compound in good yield (71%).36 Another close illustration of the reaction of 2-aminobenzimidazoles with α,β-unsaturated carbonyl compounds 4 is the preparation of pyrimido[1,2-a]-benzimidazoles 5a–t using a highly active reusable catalyst based on heterogeneous layered double hydroxides on a polyacrylic support (PAA-g-LDH) (Scheme 1, Table 1).37 Carrying out the reaction without a solvent made it possible to obtain the final products 5a–t in more than 85% yields.

Scheme 1
Table 1.
The yields of pyrimido[1,2-a]benzimidazoles 5a–t
| Compound | R | R1 | Reaction time, min | Yield, % |
|---|---|---|---|---|
| 5a | 4-i-PrC6H4 | H | 20 | 92 |
| 5b | 3-MeOC6H4 | H | 22 | 89 |
| 5c | 4-ClC6H4 | H | 22 | 90 |
| 5d | 4-MeC6H4 | H | 28 | 91 |
| 5e | 4-FC6H4 | H | 24 | 90 |
| 5f | 3,4,5-(MeO)3C6H2 | H | 29 | 91 |
| 5g | 4-EtOC6H4 | H | 20 | 92 |
| 5h | 3-O2NC6H4 | H | 25 | 85 |
| 5i | 3-BrC6H4 | H | 29 | 89 |
| 5j | 3-MeC6H4 | 3-OMe | 28 | 91 |
| 5k | 2-MeC6H4 | 3-Me | 24 | 91 |
| 5l | 3-FC6H4 | 4-Me | 28 | 89 |
| 5m | 4-MeC6H4 | 4-Me | 23 | 90 |
| 5n | 2-FC6H4 | 4-Br | 29 | 91 |
| 5o | 4-i-PrC6H4 | 4-Me | 22 | 92 |
| 5p | 3,4,5-(MeO)3C6H2 | 4-Me | 25 | 89 |
| 5q | 4-BrC6H4 | 4-Me | 29 | 88 |
| 5r | 2-ClC6H4 | 4-F | 26 | 87 |
| 5s | 4-FC6H4 | 4-F | 25 | 89 |
| 5t | 3-MeC6H4 | 4-Br | 27 | 87 |
Later publications report reactions with ethyl 3-cinnamoyl-5-methyl-1-phenyl-1H-pyrazoles 6a,b and 8a–d in EtOH with AcOH as a catalyst.38,39 However, in contrast to the above approach, in these reactions, a pyrazole substituent is introduced at position 4 of the benzimidazo[1,2-a]pyrimidine system with the formation of products 7a,b and 9a–d (Scheme 1).
Photochemical condensation of 2-aminobenzimidazole (1a) and 2-(4-chlorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one (10) in the presence of KOH and DMF40 is demonstrated in Scheme 2. It was found that 312 nm is the most suitable wavelength for the reaction within 2 h resulting in 96% yield of product 11. These examples indicate the importance of selection of conditions in the construction of the pyrimidobenzimidazole molecular structure.

Scheme 2
In 2014, Gao et al. developed an approach to the synthesis of the tracer for positron emission tomography T808 (18) and the corresponding mesylate precursor T808P (19) which are necessary for the detection of Alzheimer's disease (Scheme 3). The developed method included interaction of 2-aminobenzimidazole (1a) with 4-ethoxybutan-2-one (12). Subsequent hydrolysis of the trichloromethyl group in compound 13 leads to the formation of 2-hydroxypyrimido[1,2-a]benzimidazole (14), bromodeoxygenation of which and further nucleophilic substitution with 2-(piperidin-4 yl)ethanol (16) leads to the formation of adduct 17. Treatment of the latter with N,N-diethyltrifluorosulfamine and methanesulfonyl chloride leads to reference standard 18 in 51% yield and mesylate 19 in 75% yield. A number of patent studies are devoted to the synthesis and elucidation of the properties of derivatives of compound 15 in relation to neurodegenerative diseases.42–44

Scheme 3
β-Enamine derivatives of ketones are important representatives of unsaturated carbonyl compounds for the construction of the pyrimidobenzimidazole system. Egyptian authors report the use of enaminonitriles 20 and 22 in regioselective synthesis of pyrimidobenzimidazole derivatives 21, 23. The use of pyridine as a solvent allows the target products to be obtained in good yields (Scheme 4).45
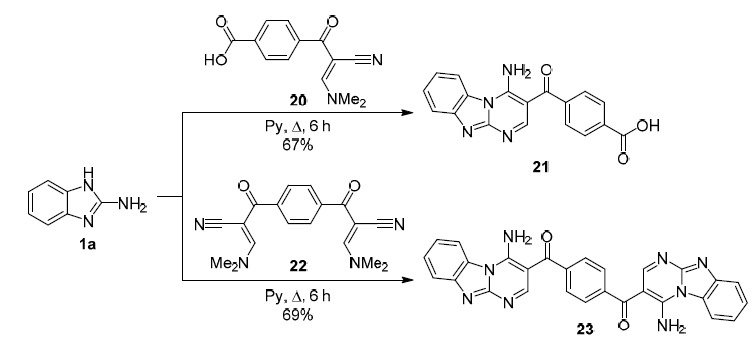
Scheme 4
Brazilian scientists described the cyclocondensation reaction of β-enamino diketone 24 and 2-aminobenzimidazole (1a) resulting in the formation of a glyoxylic derivative of pyrimido[1,2-a]benzimidazole 25.46 The reaction is characterized by a good yield of the product. The obtained glyoxylate 25 is converted into
pyrazinones and quinoxalinones 26a–e by the action of a number of 1,2-diamines (Scheme 5).

Scheme 5
Also presented are the results on the preparation of pyrimido[1,2-a]benzimidazoles 28, 30 containing thiophene and thieno[2,3-b]pyridinone fragments employing enaminones 27, 29 (Scheme 6).47,48 Interest in thiophenecontaining derivatives is justified by the wide representation of this important structural fragment in biologically active compounds such as vitamin H, xanthopappin A, etc.

Scheme 6
Another variation of the reaction of compound 1a with β-enamines was demonstrated by Egyptian scientists using pyridazine derivative 31 as an example. The process takes place upon heating under reflux in pyridine to form pyrimidobenzimidazole 32 containing the pyridazine fragment which is relevant in medicinal chemistry (drugs hydralazine, dihydralazine) (Scheme 7).49 The approaches presented in this work using enamino ketones indicate the great potential of these reagents as building blocks in the creation of pyrimidobenzimidazole scaffold.

Scheme 7
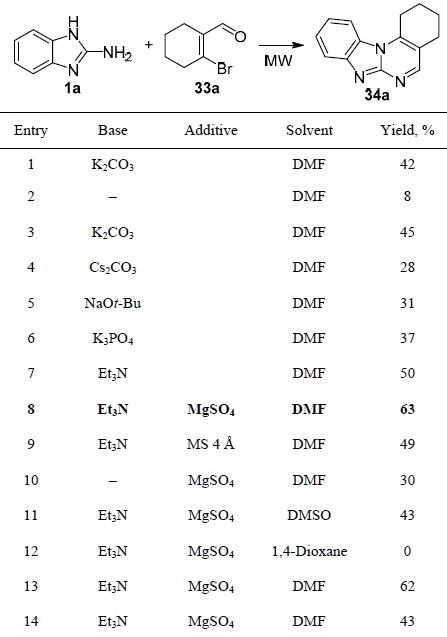
The use of α,β-unsaturated aldehydes to construct the pyrimidobenzimidazole scaffold was illustrated by Cho et al. The research team showed that β-bromo-α,β-unsaturated aldehydes 33a–j react with 2-aminobenzimidazole (1a) to form pyrimido[1,2-a]benzimidazoles 34a–j (Scheme 8).50 Optimization of the synthesis conditions (Table 2) showed that the use of DMF as a solvent under microwave irradiation in the presence of Et3N and MgSO4 is optimal. The developed approach represents a novel and efficient method for the synthesis of the hybrid structure of pyrimidobenzimidazoles from readily available β-bromo-α,β-unsaturated aldehydes.

Scheme 8
Table 2.
Optimization of conditions for the synthesis of pyrimido[1,2-a]benzimidazole 34a
Chromene aldehydes 35a–f were also successfully used in the synthesis of a number of condensed pentacyclic chromeno[3',2':5,6]pyrimido[1,2-a]benzimidazoles 36a–f (Scheme 9).51 According to the authors of the publication, the reaction was carried out under the conditions of sonochemical activation which resulted in product high yields (up to 88%) and also made it possible to significantly reduce the reaction time to 10 min. Due to the limited solubility of compounds 36a–f, for unambiguous determination of the structure by NMR, the methylation of compound 36a was carried out with the formation of N-methyl derivative 37a the structure of which was established on the basis of 2D NMR spectra. In addition, the reaction of derivatives 36a–f with thiocarboxylic acids was investigated and a mechanism for the formation of the products 38a–f was proposed. The author's interpretation of the formation of thiazolinones 38 involves the reaction of thioglycolate with the C-7 atom and the subsequent opening of the pyrimidine fragment. A significant advantage of the described approach is that in all reactions the chromone fragment of the molecule remains intact since the opening of the pyran ring is a limiting factor in many reactions with the participation of chromones.

Scheme 9
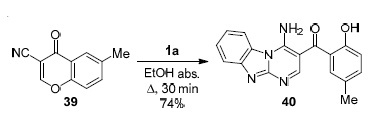
The instability of the chromone fragment is convincingly presented in a publication from 2016. Thus, the reaction of chromonecarbonitrile 39 with 2-aminobenzimidazole (1a) proceeds via the γ-opening of the pyrone ring followed by cycloaddition to the nitrile group and the formation of the pyrimidobenzimidazole structure 40. Condensation was successfully carried out by heating in EtOH under reflux for 30 min with a product yield of 74% (Scheme 10).52
Scheme 10
In addition, a method was developed for the construction of condensed derivatives of pyrimido[1,2-a]benzimidazoles 42a–r by cyclocondensation of 2-aminobenzimidazole (1a) with isoflavones 41a–r in MeOH in the presence of 3 equiv of MeONa (Scheme 11).53 This process is also accompanied by opening of the pyran ring followed by cyclocondensation. The use of the developed synthetic strategy makes it possible to access pyrimido[1,2-a]-benzimidazole derivatives 42a–r in high yields.

Scheme 11
Another example of the construction of the pyrimidobenzimidazole system by condensation of aminobenzimidazole with unsaturated carbonyl compounds was demonstrated in a study the authors of which synthesized a series of 4-arylpyrimido[1,2-a]benzimidazoles 44a–d by cyclization of methyl cinnamates 43a–d with 2-aminobenzimidazole (1a) in DMF in the presence of K2CO3 (Scheme 12).54 The research team points to the regioselectivity of the process and postulates the formation of only 4-substituted pyrimidobenzimidazoles.

Scheme 12
Unsaturated carbonyl compounds are not limited to derivatives containing an ethylene moiety. A nontrivial method for constructing the pyrimidobenzimidazole fragment was proposed by researchers from France. They developed a simple and convenient method for the regioselective synthesis of fluorinated pyrimido[1,2-a]-benzimidazole 46 by condensation of ethyl 4,4,4-trifluorobut-2-inoate (45) with 2-aminobenzimidazole (1а).
Pyrimido[1,2-a]benzimidazolone 46 thus obtained was subsequently used to obtain a number of new 2-amino- and 2-mercaptopyrimido[1,2-a]benzimidazoles 47, 48a,b (Scheme 13).55 In addition, compounds 47, 48 are relevant as participants in further functional transformations.

Scheme 13
Thus, due to the wide variety of components for condensation with the participation of 2-aminobenzimidazole, as well as the existence of effective catalytic systems, the creation of pyrimidobenzimidazoles based on α,β-unsaturated carbonyl compounds can be considered an effective approach.
derivatives are important substrates for the formation of a pyrimidine fragment in the structure of condensed pyrimidobenzimidazoles. Fang et al. developed an efficient, not using metal catalysts method for the synthesis of benzimidazo[1,2-a]quinazoline derivatives 51a–i, 52a–c involving the step of ipso substitution of halogen in arylcarbonyl compounds 49a–i, 50a–c (Scheme 14).56 Thus, derivatives of 2-fluoro-, 2-chloro-, 2-bromo-, and 2-nitro-substituted arylaldehydes 49a–i and ketones 50a–c are very reactive in this kind of transformations, which allows the process to be carried out with yields of target products of more than 63%. Detailed studies have been carried out to optimize the reaction conditions. It was found that the best conditions for the process is to carry out the reaction in DMF in the presence of K2CO3 at 135°C.

Scheme 14
A group of Indian researchers proposed a three-step synthesis of condensed acridines 55a–k containing a pyrimidobenzimidazole structural fragment.57 At the first step, the synthesis of cyclohexanone derivative 53 from (2-amino-5-chlorophenyl)(phenyl)methanone (52) and cyclohexane-1,3-dione under the conditions of catalysis by H2SO4 at 150°C for 6 h is carried out. The second step is the aldol-crotonic condensation of tetrahydroacridine 53 with aromatic aldehydes, the products of which, α,β-unsaturated acridones 54a–k, are reacted with 2-aminobenzimidazole (1a) under the conditions of catalysis by KOH. This synthesis route allowed the researchers to obtain 8-aryl-2-chloro-16-phenyl-6,7-dihydrobenzimidazo-[1',2':1,2]pyrimido[4,5-a]acridines 55a–k in 82–95% yields in the last step (Scheme 15).

Scheme 15
The use of carboxylic acid derivatives in the synthesis of pyrimidobenzimidazoles is illustrated.58,59 Gnanasekaran et al. have developed a method for the preparation of a series of benzimidazo[2,1-b]quinazolin-12(6H)-ones and pyrido-[2',3':4,5]pyrimido[1,2-a]benzimidazol-5(11H)-ones 57a–h and 57'a–h via the reaction of 2-aminobenzimidazoles 1a,b with 2-haloaroyl chlorides 56a–h (Scheme 16).58 Thus, the treatment of 2-aminobenzimidazoles 1a,b with acid chloride 56a–h in the presence of NaHCO3 in DMF at –10°С leads to acylation of the nitrogen atom of the imidazole ring. Upon subsequent heating of the reaction mixture to 75°C, an intramolecular SNAr process occurs accompanied by the formation of the pyrimidine ring. The yields of the reaction products are 76–98%. In addition, it was found that under the conditions of the acylation reaction of the obtained compounds, the formation of only 6-acetyl isomers 58a–e is observed.

Scheme 16
Galeterone derivatives containing the pyrimidobenzimidazole fragment were successfully synthesized by the reaction of 2-aminobenzimidazoles 1a–c with 16-dehydropregnenolone acetate (59). The target galeterone derivatives were obtained in good yields by heating under reflux in dry MeCN in the presence of p-TsOH (Scheme 17).60 Under these conditions, in addition to the predominant aromatic products 60a–c, 60'c, D-homoketones 61a–c, 61'c were obtained which were formed as autoxidation products. In addition, from the mixture of derivatives 60c, 60'c and 61c, 61'c, the regioisomers were isolated as individual products. Compound 60”a was obtained in 42% yield by lowering the reaction temperature and replacing aprotic MeCN with EtOH.

Scheme 17
An earlier publication also investigated the reaction of 2-aminobenzimidazole (1a) with 16-dehydropregnenolone acetate (59). It was found that the reaction under basic catalysis conditions also proceeds with the formation of two derivatives 60a and 61a (Scheme 18).61

Scheme 18
A demonstration of the use of 1,3-dicarbonyl compounds in the construction of the pyrimidobenzimidazole ring is presented in a publication from 2019. Researchers developed a simple and effective method for the synthesis of 2-polyfluoroalkylbenzimidazo[1,2-a]pyrimidine-4-carbaldehyde derivatives 63a,b with yields of up to 86% starting from 3-(polyfluoroacetyl)pyruvaldehyde dimethyl acetal 62a,b and 2-aminobenzimidazole (1a) (Scheme 19).62 As the authors of the study note, the introduction of fluorinated substituents into the pyrimidobenzimidazole molecule is an effective method for changing the physical and chemical properties, as well as the biological activity of this class of compounds. The choice of reaction conditions has a decisive influence on the direction of the reaction. Thus, carrying out the reaction in MeCN in the presence of 3 equiv of (EtO)3B is characterized by high regioselectivity (98%) in favor of 2-fluoroalkyl-substituted acetals of pyrimido[1,2-a]benzimidazole-4-carbaldehydes 63a,b. When trifluoroethanol is used without the presence of a Lewis acid, 4-fluoroalkyl derivatives 64a,b are formed.

Scheme 19
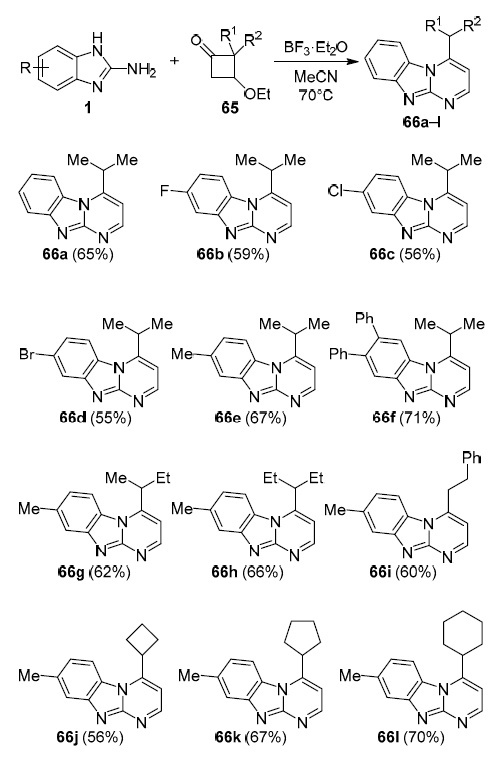
Kong et al. successfully synthesized a number of pyrimido[1,2-a]benzimidazole derivatives 66a–l via a chemoselective reaction of 2-aminobenzimidazoles 1 with 3-ethoxycyclobutanones 65 under the conditions of catalysis by Lewis acid BF3·Et2O. The researchers found that when using monohalogenated 2-aminobenzimidazoles 1, the formation of regioisomeric products is observed (similar to Scheme 19, according to 1H NMR spectroscopy), whereas in the case of unsubstituted and 5-methyl-aminobenzimidazoles, the process proceeds regioselectively. In addition, it was noted that the developed approach can be easily scaled without decrease of the yield of the target products (Scheme 20).63
Scheme 20
The above approach to the construction of the pyrimidobenzimidazole structure using cyclic ketones is not the only example of the use of this kind of carbonyl compounds. Thus, a group of Chinese researchers used squaraic acid chloride (67) in the synthesis of a series of alkyl 2-chloropyrimido[1,2-a]benzimidazole-3-carboxylates 68a–g (Scheme 21).64 Remarkably, the study revealed that the reaction proceeds in various alcohols with the formation of the corresponding alkyl derivatives of pyrimidobenzimidazole carboxylates 68a–g, while in aprotic solvents (MeCN, THF, and DMF) the reaction mixture underwent resinification and individual reaction products could not be isolated due to the extreme instability of the squaraic acid derivative and the resulting intermediates.

Scheme 21
Keto esters and their derivatives are another important synthon in the series of 1,3-dicarbonyl compounds for the construction of the pyrimidine ring. A method was developed for the synthesis of a wide range of pyrimido-[1,2-a]benzimidazol-4-one derivatives 70a–h by cyclocondensation of β-keto esters 69 with 2-aminobenzimidazole (1a) under the conditions of microwave activation.65 Of note is the high yields of products (74–94%) as well as a short reaction time (3 min). In addition, a number of derivatives 71a–h containing a methylenecoumarin fragment in their molecules were synthesized on the basis of the obtained compounds (Scheme 22).

Scheme 22
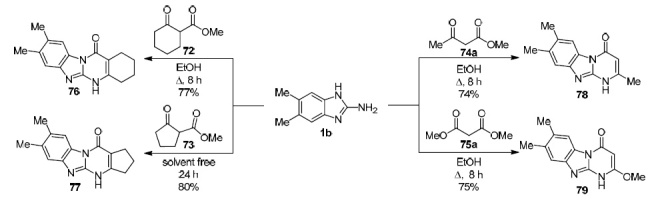
In addition to the above approach, a group of authors in 2015 published data on the condensation of various β-keto esters 72–75 with 2-amino-5,6-dimethylaminobenzimidazole (1b).66 The reactions were carried out by heating under reflux in EtOH, and in all cases good yields of the corresponding pyrimido[1,2-a]benzimidazoles 76–79 were attained (Scheme 23). The only drawback of this method is the long reaction time (8–24 h) in comparison with the conditions shown in Scheme 22. It should be noted that in both cases the formation of pyrimido[1,2-a]benzimidazol-4-ones was noted; however, the formation of pyrimido-[1,2-a]benzimidazol-2-ones can also be assumed.
Scheme 23
This is confirmed by a study on the synthesis of 1,4-dimethylpyrimido[1,2-a]benzimidazol-2(1H)-one (81), obtained by alkylation of pyrimidobenzimidazole 80 with dimethyl sulfate hydrate. In addition, the study provides detailed data on the properties of compound 81 revealed by X-ray structural analysis and quantum-chemical calculations (Scheme 24).67

Scheme 24
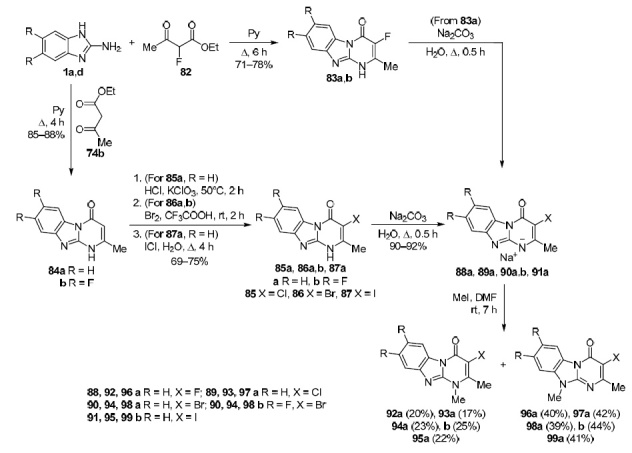
The use of ethyl acetoacetate derivatives 74b and 82 for the construction of the pyrimidobenzimidazole structure was demonstrated. Methods for the synthesis of halogenated 2-methylpyrimido[1,2-a]benzimidazol-4(10H)-ones 83a,b, 84a,b, 85a, 86a,b, 87a were developed, the alkylation of their sodium salts 88a, 89a, 90a,b, 91a was studied, and the structure of the regioisomeric methylation products 92a, 93a, 94a,b, 95a, 96a, 97a, 98a,b, 99a was established (Scheme 25).68 Regioisomeric pairs 92a/96a, 93a/97a, 94a/98a, 94b/98b, 95a/99a were resolved using column chromatography.
Scheme 25
An example of the use of dibasic acid esters described by a team of Italian researchers is the multistep synthesis of novel 10-substituted 2-(1-piperazinyl)pyrimido[1,2-a]-benzimidazol-4-ones 103a–o using malonic ester 75b.69 The synthetic strategy included the initial alkylation of 2-aminobenzimidazole (1a), condensation of the obtained products 100a–o with malonic ether 75b effected by EtONa, chlorodeoxygenation of the obtained pyrimidobenzimidazole-2,4-diones 101a–o, and nucleophilic ipso substitution of the halogen in compounds 102a–o by the piperazine moiety (Scheme 26).

Scheme 26
Derivatives of keto esters are also widely used in the synthesis of the pyrimidine moiety. A publication from 2011 presents the results of the synthesis of new 3-benzimidazolylpyrimidobenzimidazoles 106 under conditions of phase-transfer catalysis by benzyltriethylammonium chloride (BTEAC). Initially, the condensation of benzimidazole 1a with hydrazones of ethyl acetoacetate 104 was carried out, as a result of which pyrimidobenzimidazoles 105 were obtained. The subsequent reaction of azo derivatives 105 with carbene obtained in situ from 3-chloro-3-methylbut-1-yne in a basic medium completes the transformation (Scheme 27).70

Scheme 27
Derivatives of bifunctional compounds containing an active methylene group which are widely used in the synthesis of pyrimidobenzimidazole derivatives deserve special attention. A study of Polish researchers was devoted to the reaction of 2-diethoxyphosphoryl-3-methoxyacrylate (107) with aza-heterocycles, including 2-aminobenzimidazole (1a). It was shown that carrying out the reaction in xylene at 140°С for 30 h leads to the formation of the final pyrimidobenzimidazolone 108.71 The relatively mild conditions under which pyrimidinone 108 is formed, in comparison with other considered heterocycles, clearly reflect the higher nucleophilicity of the nitrogen atom of benzimidazole which favors intramolecular N-acylation (Scheme 28).

Scheme 28
The publication of Russian researchers is yet another demonstration of the use of α-alkoxymethylene derivatives of keto esters (by the example of compounds 109a–c). The authors report on the regiodirected synthesis of polyfluoroalkyl derivatives of benzimidazopyrimidines 110–113 a–c, as well as the route of the process depending on the fluoroalkyl substituent (Scheme 29).72
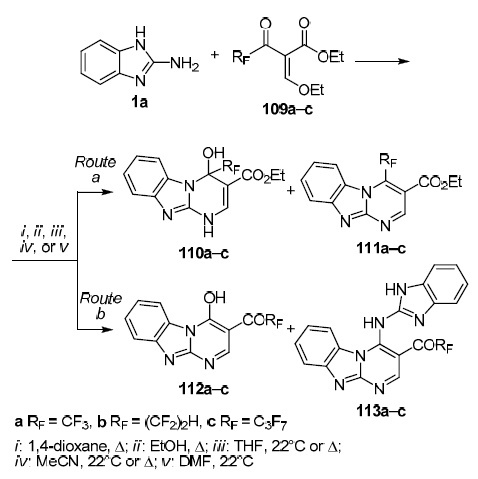
Scheme 29
It was found that the nature of the solvent used and the size of the fluoroalkyl moiety have a decisive influence on the ratio of the resulting compounds. Thus, for 2-ethoxymethylidene-4,4,4-trifluoroacetoacetate (109a), cyclization via route a is preferable in 1,4-dioxane, whereas in polar solvents this cyclization route becomes the only one. Tetrafluoroethyl-containing ester 109b shows such selectivity only in polar aprotic solvents, while in protondonating EtOH and weakly polar 1,4-dioxane, the formation of pairs of reaction products 110b/111b and 112b/113b in about equal total yields is observed which indicates that realization of heterocyclization routes a and b are equally probable. At the same time, the reaction of heptafluoropropyl-substituted ester 109c is characterized by a complete lack of selectivity since, regardless of the solvent used, cyclization proceeds along both routes (a and b).
A recent study reports the use of 3-ethoxy-2-methylsulfonylacrylonitrile (114) in the synthesis of novel aminomethylsulfonylpyrimidines. In particular, the reaction of compounds 1а and 114 in 1,4-dioxane in the presence of Et3N yielded 4-amino-3-(methylsulfonyl)pyrimido[1,2-a]-benzimidazole (115) (Scheme 30).73

Scheme 30
An example of the creation of nitro derivatives of pyrimidobenzimidazoles is the synthesis of 3-nitropyrimido-[1,2-a]benzimidazol-4-ones 117a–c and a detailed study of the alkylation of these derivatives described in a publication from 2017. Based on the studies carried out, it was found that alkylation proceeds at the N-1 and N-10 atoms with the formation of two series of regioisomeric products 118–121 a–c and 122–125 a–c, respectively (Scheme 31). In addition, the ratio of regioisomers was determined, which depends on the nature of the used alkyl iodide. In the case of alkylation with methyl, ethyl, or n-propyl iodide, the ratio of the products of 1- and 10-substitution is 1:0.7. In the reaction with isopropyl iodide, this ratio is 1:0.5.74

Scheme 31
Wong and colleagues presented a series of publications on the synthesis of pyrimidobenzimidazoles 127, 129, 131 via the reaction of 2-aminobenzimidazole (1а) with Baylis–Hillman acetates, alcohols, and amines 126, 128, 130, respectively.75–77 The methods presented by the authors assume varying conditions, catalysts, and reagents (Scheme 32).

Scheme 32
By changing the catalyst and solvent, a team of Indian researchers obtained pyrimidobenzimidazole derivatives 133a–k via the reaction of Morita–Baylis–Hillman acetates 132a–k and 2-aminobenzimidazole (1a) (Scheme 33).78

Scheme 33
Another approach for the construction of polycyclic derivatives of pyrimidobenzimidazoles is the synthesis of hexacyclic derivative 135 was demonstrated in a publication from 2014.79 Compound 135 was obtained by annulation of 2-aminobenzimidazole (1a) and benzothiazolopyrimidine 134 (Scheme 34). The authors of this paper paid much attention to the study of the mechanism and regioselectivity of the process.

Scheme 34
As mentioned earlier, reactions catalyzed by microwave irradiation are quite common and relevant in condensation processes that take place with the elimination of low molecular weight compounds (H2O, alcohols, etc.). Adhering to this approach, a method was developed for the synthesis of pyrimidobenzimidazolone derivatives 137a–g based on 2-aminobenzimidazole (1a) and phenylethanones 136 (Scheme 35).80

Scheme 35
To conclude, the methods for constructing a pyrimidobenzimidazole scaffold based on bifunctional synthetic equivalents have on the whole been developed in sufficient detail, although it cannot be said that they are completely exhausted.
Construction of pyrimidobenzimidazole structure by multicomponent reactions
Among the methods for constructing the azolopyrimidine fragment, multicomponent reactions (MCRs) occupy a special place. Due to its simplicity in application and wide possibilities for varying all components, MCRs open unconventional synthetic routes for the creation of new hard-to-access heterocyclic molecular structures. In the literature, examples of the use of MCR for the synthesis of pyrimidobenzimidazoles are represented by threecomponent reactions, the main components of which are derivatives of benzimidazoles and aldehydes. The third component is labile and is often an active CH component. This section of the review is systematized according to the variation of the third component of an MCR.
The development of new catalytic systems and determination of their efficiency in MCR was the goal of a large number of studies related to the development of MCR methodology.81–96 The use of new catalysts and their systems allows: simplifying the procedure for obtaining the target product, increasing the selectivity of the process, reducing its cost, and making it less environmentally harmful due to the use of nontoxic solvents. The latter condition is of particular importance for the use of the method in the pharmaceutical industry when the environmental friendliness of production and the purity of the resulting medicinal compound come to the fore as criteria for assessing the possibility of its technological application.
Numerous studies81–101 present the synthesis of pyrimidobenzimidazole derivatives 140 by the three-component reaction of 2-aminobenzimidazole (1а), aromatic aldehyde derivatives 138, and cyclohexane-1,3-diones 139 (Scheme 36). Considering the uniformity of the synthetic route used in the presented publications, special attention is paid to the development and study of various catalytic systems.

Scheme 36
A team of Iranian scientists describe the use of sulfamic acid as a reusable green catalyst under the conditions of heating in MeCN for the synthesis of derivatives 140 (Table 3). It was noted that the use of sulfamic acid as a catalyst provides certain advantages, including ease of use, ease of isolation, and good product yields, as well as the possibility of its reuse.81
Table 3.
Examples of NH2SO3H-catalyzed three-component reaction of compounds 1а, 138, and 139 to obtain compounds 140
| Entry | Ar | R | Reaction time, min | Yield, % |
|---|---|---|---|---|
| 1 | Ph | Me | 15 | 94 |
| 2 | 4-ClС6Н4 | Me | 15 | 90 |
| 3 | 4-BrС6Н4 | Me | 15 | 90 |
| 4 | 4-MeOС6Н4 | Me | 18 | 90 |
| 5 | 4-HOС6Н4 | Me | 20 | 90 |
| 6 | 4-O2NС6Н4 | Me | 18 | 95 |
For the synthesis of derivatives 140, catalytic systems were developed in the form of modified Fe3O4 nanoparticles containing:82–87
silica with terminal sulfo groups;82
L-proline fragments;83
chitosan structures;84
starch with n-butylsulfo group;85
titanium dioxide functionalized with sulfo groups;86
system Cu@Fe3O4.87
Among the advantages of these catalysts are high efficiency (yields of derivatives 140 of over 90%), reusability, and compliance with the principles of green chemistry. In addition, one of the main features of these catalytic systems is their magnetic activity, which makes it possible to separate the catalyst from the reaction mixture using an external magnet.
Another group of catalysts used for MCR shown in Scheme 36 are ionic liquids 141–145 (Fig. 3).88–92 Among the advantages of the considered catalysts are their cheapness, ease of preparation, and high stability. In addition, the research emphasizes the possibility of reuse of catalysts without losing their activity. In addition, it should be noted that the significant reduction in the reaction time and high yields of derivatives 140 make these catalysts attractive for many other studies in the field of synthesis of various nitrogenous heterocycles. While not proven, they are assumed to be of a general nature.
Figure 3.

Ionic liquids used in MCR synthesis of pyrimido-[1,2-a]benzimidazoles 140.
A series of studies was devoted to the use of acid catalysts in the synthesis of derivatives 140.93–96 Considered among the catalysts are polyvinylpyrrolidonium hydrogen sulfate,93 (CH2)4(DABCO–SO3H)2Cl4 nanoparticles, 94 PTSA,95 and AcOH.96 Among the advantages of using this series of catalysts, high yields of reaction products, ease of use, and environmental friendliness of the process are given.
The main tasks for optimizing the conditions for carrying out any synthetic process, along with increasing the yield of the target product, include the reduction of the reaction time. Microwave radiation is used to intensify many reactions in organic chemistry. From this point of view, the implementation of the process as shown in Scheme 36 under microwave irradiation conditions without solvent and catalysts,97 as well as under the conditions of catalysis by Sc(OTf)3 98 is of great synthetic importance.
In 2016, the synthesis of derivatives 140 using a deep eutectic solvent 146, which is a mixture of choline chloride and glycerol was described (Fig. 4).99 Along with high yields, short reaction times, and mild reaction conditions, the main advantage of the investigated solvent is its biodegradability.
Figure 4.

The structure of solvent 146.
The synthesis of a number of novel condensed tetracyclic thiopyrano[3,4:4,5]pyrimido[1,2-a]benzimidazol-4-ones 149a–m from 2-aminobenzimidazole (1а), aromatic aldehydes 147, and thio derivative 148 (as an active CH component) was investigated.100 The process was carried out under the conditions of heating the components in an AcOH medium at 50°C for 8–10 h (Scheme 37).

Scheme 37
Another interesting example was demonstrated in the work of Egyptian researchers by a method of obtaining poly(tetrahydrobenzimidazo[2,1-b]quinazolin-1-one) 152 by multicomponent condensation of 2-aminobenzimidazole (1a), polyaldehyde 150, and dimedone (151) in DMF catalyzed by ZnO nanoparticles under the conditions of microwave radiation (Scheme 38).101

Scheme 38
A innovative method for constructing benzimidazoquinazolinone structures 154a–h is presented in a publication from 2016.102 The difference between this approach and the above is that benzyl halide derivatives 153 which are oxidized with trimethylamine N-oxide are used instead of an aromatic aldehyde (Scheme 39).

Scheme 39
A large number of studies103–120 devoted to the synthesis of pyrimidobenzimidazoles 157 using MCR of keto esters and their various derivatives 155 as well as malononitrile (156) are presented in general in Scheme 40.

Scheme 40
A series of publications103–106 was devoted to the study of MCRs of malononitrile (156), 2-aminobenzimidazole (1а), and aromatic aldehydes 138. Alum103 was used as a condensation catalyst; at the same time PTSC was successfully used,104 and Mexican researchers carried out the synthesis by heating the components under reflux in H2O.105 In addition, the successful use of polyvinylpyrrolidonium perchlorate as a highly efficient catalyst in the preparation of 1,4-dihydropyrimido[1,2-a]benzimidazole-3-carbonitrile derivatives 157 (R1 = NH2, R2 = CN) is reported.106
The selectivity of the process of the MCR of 2-aminobenzimidazole (1a), aromatic aldehydes 158a–f, ethyl cyanoacetate (155a), and malononitrile (156) (Scheme 41) was studied.108,109 In the above studies, compounds exhibiting basic properties were investigated as condensation catalysts: NaOAc, Et3N, and MgO. It has been shown that their use provides high yields and product purity. In addition, it is noted that in this case the reaction proceeds selectively with the formation of products 161a,b and 162a–f. The regioselectivity of the process is explained by the formation of the Knoevenagel condensation products 159 and 160, the further reaction of which with aminobenzimidazole 1a is due to the increased electrophilicity of the methylene and ethoxycarbonyl fragments. As a result, the reaction products 161' and 162' are not preferred in the given process conditions.

Scheme 41
Another example of a MCR using malononitrile (156) and aromatic aldehydes 163a–l was described in 2018.110 It is noteworthy that the developed approach uses a nanostructured ionic liquid, a salt of imidazole and trinitromethane [HIMI]C(NO2)3 (Scheme 42). As noted by the authors, the use of this catalyst significantly reduces the reaction time, increases the yields of pyrimidobenzimidazoles 164a–l, and also allows the process to be carried out in accordance with the principles of green chemistry.
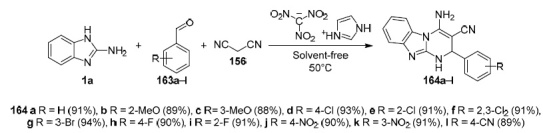
Scheme 42
Keto esters and diketones are important substrates for the construction of the pyrimidobenzimidazole scaffold.111–120 In these studies, the present synthetic strategy for the preparation of pyrimidobenzimidazoles corresponds to the approach shown in Scheme 36. The relevance of the research lies in demonstrating the broad capabilities of various catalysts and their systems in MCRs.
Thus, the synthesis of 1,4-dihydropyrimido[1,2-a]benzimidazole-3-carboxylates 169a–y using ionic liquids 167, 168 as catalysts and in the absence of solvents (Scheme 43) was presented.111,112 It was noted that when using these catalysts condensation proceeded smoothly with a wide range of substrates 165, 166, and the products were obtained in good or excellent yields (Table 4). In addition, the low cost of catalysts and the possibility of their reuse, as well as the ease of product isolation, are the main advantages of the developed methods.

Scheme 43
Table 4.
The yields of 1,4-dihydropyrimido- [1,2-a]benzimidazole-3-carboxylates 169a–y
| Compound | R | R1 | R2 | Yield, % |
|---|---|---|---|---|
| 169a | 4-FC6H4 | Et | Ph | 95 |
| 169b | 4-ClC6H4 | Et | Ph | 92 |
| 169c | 3-ClC6H4 | Et | Ph | 91 |
| 169d | Et | t-Bu | Me | 90 |
| 169e | Pr | t-Bu | Me | 92 |
| 169f | 2-Thienyl | t-Bu | Me | 89 |
| 169g | 4-O2NC6H4 | t-Bu | Me | 94 |
| 169h | 4-MeC6H4 | t-Bu | Me | 95 |
| 169i | 3-ClC6H4 | t-Bu | Me | 91 |
| 169j | 4-NCC6H4 | Et | Me | 93 |
| 169k | 4-MeOC6H4 | Et | Me | 92 |
| 169l | 1,3-Benzodioxol-5-yl | Et | Me | 89 |
| 169m | 4-EtOC6H4 | Et | Me | 93 |
| 169n | 2,5-Me2C6H3 | Et | Me | 91 |
| 169o | 2,4-F2C6H3 | Et | Me | 90 |
| 169p | 2-F-5-BrC6H3 | Et | Me | 89 |
| 169q | C6H11 | Et | Me | 92 |
| 169r | 2-F-4-BrC6H3 | Et | Me | 89 |
| 169s | 2-F-4-BrC6H3 | Me | Me | 91 |
| 169t | C6H11 | Me | Me | 90 |
| 169u | 2,4-F2C6H3 | Me | Me | 91 |
| 169v | 4-NCC6H4 | Me | Me | 92 |
| 169w | 1,3-Benzodioxol-5-yl | Me | Me | 88 |
| 169x | 2-F-5-BrC6H3 | Me | Me | 90 |
| 169y | 2,5-Me2C6H3 | Me | Me | 91 |
In a number of studies on the preparation of pyrimido-[1,2-a]benzimidazoles, the catalytic activity of some natural compounds, such as L-proline,113 thiamine hydrochloride,114 and citric acid was investigated.115 Along with all the advantages of organocatalysts, the described procedures are carried out in aqueous media, which expands the field of application of the MCR. At the same time, the efficiency of catalysts, a wide range of substrates used, and mild reaction conditions significantly expand the possibilities for the synthesis of relevant pyrimido[1,2-a]-benzimidazole derivatives.
A simple and effective method for the synthesis of pyrimido[1,2-a]benzimidazole derivatives 169m, 172a–m (Table 5) using N,N'-dichlorobis(2,4,6-trichlorophenyl)urea (CC-2) (171), aromatic aldehydes 170, and keto esters 74a,b was demonstrated by Indian researchers (Scheme 44).116 The main advantage of the method is that reagent 171 is converted during the reaction into insoluble 1,3-bis(2,4,6-trichlorophenyl)urea which can easily be separated by simple filtration and converted back to CC-2 by treatment with AcOH/Cl2/NaOH.
Table 5.
The yields of of 1,4-dihydropyrimido-[1,2-a]benzimidazole-3-carboxylates 169m, 172a–m
| Compound | R | R1 | Yield, % |
|---|---|---|---|
| 169m | 4-EtOC6H4 | Et | 78 |
| 172a | 4-MeOC6H4 | Et | 82 |
| 172b | 4-EtC6H4 | Et | 76 |
| 172c | 4-Me2CH | Et | 72 |
| 172d | 4-FC6H4 | Et | 68 |
| 172e | 4-O2NC6H4 | Et | 70 |
| 172f | 3-O2NC6H4 | Me | 68 |
| 172g | 4-НONC6H4 | Et | 71 |
| 172h | 4-MeOC6H4 | Et | 75 |
| 172i | 3-НOC6H4 | Et | 66 |
| 172j | 4-Me2NC6H4 | Et | 65 |
| 172k | 4-F3CC6H4 | Et | 58 |
| 172l | 3,4,5-(MeО)3C6H2 | Me | 55 |
| 172m | Indolyl* | Me | 55 |
*No information is provided in the publication as to which isomer of indole aldehyde was used.

Scheme 44
In 2014, Indian researchers studied the effect of zinc perchlorate (Zn(ClO4)2·6H2O) on the MCR of aldehydes 173, ethyl acetoacetate (74b), and 2-aminobenzimidazole (1a) in the synthesis of a number of pyrimido[1,2-a]-benzimidazole derivatives 172j, 174a–i (Scheme 45).117 The authors note high yields of the reaction products; however, in the case of using aldehydes containing an OH group in position 2 or 4 of the phenyl ring, a decrease in the yields of the target product is observed.

Scheme 45
Another catalytic system used in the synthesis of pyrimido[1,2-a]benzimidazoles is H3PO4 supported on Al2O3.118 Among the advantages of this catalyst are the possibility of its reuse without loss of efficiency, as well as carrying out the reaction without a solvent. In addition to the above approach, a team of authors from Iran reported the use of N,N,N',N'-tetrabromobenzene-1,3-disulfonamide (TBBDA) (175) and poly(N-bromo-N-ethylbenzene-1,3-disulfonamide) (PBBS) (176) as MCR catalysts (Fig. 5).119 These catalysts also allow solvent-free reactions.
Figure 5.

The structures of catalysts 175 and 176.
A method was developed for the synthesis of new adenosine receptor A2B antagonists. Among the synthesized structures, the most significant in terms of their activity were pyrimido[1,2-a]benzimidazole derivatives 178a–h which were synthesized via a MCR of aldehydes 177, keto esters 74b,c, and 2-aminobenzimidazole (1a).120 Optimization of the synthesis showed that that the synergistic use of chloroacetic acid (as a catalyst) and microwave irradiation led to a significant increase in the yields of compounds 178a–h (Scheme 46).

Scheme 46
Among other substrates used in MCRs, derivatives of terminal alkynes are of interest.121–123 Thus, the regioselective synthesis of pyrimido[1,2-a]benzimidazoles 181a–aa by the reaction of 2-aminobenzimidazoles 1, aldehydes 179, and alkynes 180 is described. The reaction is carried out in MeCN under reflux using a copper and silver salt system as a catalyst (Scheme 47).121

Scheme 47
A particular example of the use of alkyne derivatives in the synthesis of pyrimido[1,2-a]benzimidazoles is a work by Chinese researchers.124,125 The authors have developed an approach to the synthesis of disubstituted pyrimido-[1,2-a]benzimidazoles 184a–o using the MCR of benzimidazole 1a, aldehydes 182, and alkynecarboxylic acids 183 in the presence of a catalytic amount of CuI and K2CO3 (Scheme 48).

Scheme 48
In 2014, multicomponent condensation between acetophenones 184, aminobenzimidazole 1a, and aromatic aldehydes 185 was carried out by Hassaneen and Farghaly.126 The reaction was carried out in H2O in the presence of H-ferrierite zeolite for the short period of time of 8–15 min. The developed method made it possible to obtain a new series of pyrimido[1,2-a]benzimidazoles 186a–f in good yields (Scheme 49).

Scheme 49
Chinese researchers have demonstrated the possibility of using 1-benzyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4) as an effective catalyst in the threecomponent condensation of aminobenzimidazole 1а, 1,4-naphthoquinone (187), and aromatic aldehydes 188 for the synthesis of derivatives of pyrimido[1,2-a]benzimidazoles 189a–o (Scheme 50).127 When selecting the conditions, it was found that carrying out the reaction in the absence of a solvent at 100°C allows one to achieve better yields (Table 6).

Scheme 50
Table 6.
The yields of pyrimido[1,2-a]benzimidazole derivatives 89a–o
| Compound | R | Reaction time, min | Yield, % |
|---|---|---|---|
| 189a | 4-ClC6H4 | 11 | 83 |
| 189b | 3-FC6H4 | 11 | 83 |
| 189c | 3-HOC6H4 | 12 | 85 |
| 189d | 4-FC6H4 | 12 | 86 |
| 189e | 3-ClC6H4 | 11 | 86 |
| 189f | 3-ClC6H4 | 12 | 86 |
| 189g | 2,3-Cl2C6H3 | 12 | 85 |
| 189h | 4-BrC6H4 | 12 | 85 |
| 189i | 2-BrC6H4 | 12 | 84 |
| 189j | 2-ClC6H4 | 14 | 82 |
| 189k | 3,4-(MeO)2C6H3 | 11 | 86 |
| 189l | 2-MeOC6H4 | 12 | 84 |
| 189m | 3,4-Cl2C6H3 | 14 | 83 |
| 189n | 2,3-(MeO)2C6H3 | 13 | 85 |
| 189o | 2,4-Cl2C6H3 | 14 | 86 |
As mentioned earlier, microwave irradiation in most cases has a positive effect on the course of a MCR. Thus, in one of the studies, microwave irradiation was used for the three-component condensation of benzimidazole 1a, ketone derivatives 190, and aldehydes 191, 193 to obtain pyrimido-[1,2-a]benzimidazole derivatives (Scheme 51). The yields of compounds 192a–g ranged from 60 to 93%, the yields of compounds 194a–g – from 67 to 92%.128

Scheme 51
In another example of constructing the pyrimido[1,2-a]-benzimidazole structure under the MCR conditions, 2-aminobenzimidazole (1а), aromatic aldehydes 195, and (E)-N-methyl-1-(methylsulfanyl)-2-nitroethylenamine (196) were used as the reaction components.129,130 The process was carried out by melting the components in the presence of a novel ionic liquid based on imidazolium dication grafted onto a polyethylene glycol methacrylate support PEGMA-g-TEGBDIM,129 or by heating in EtOH under reflux in the presence of PTSA as a catalyst (Scheme 52).130

Scheme 52
A characteristic of the described approach lies in the possibility of accessing nitro derivatives of pyrimido-benzimidazoles 197a–v. It is known that nitroazolopyrimidines and their derivatives possess useful biological activity.33,131–135 In addition, the presence of such an important structural fragment as the nitro group seems to be promising from the point of view of further transformations, for example, in the creation of polycyclic purine-like structures.136
Biological activity of pyrimidobenzimidazole derivatives
Some examples of biologically active derivatives of pyrimidobenzimidazoles are presented below. Compounds 7a,b were tested for antimicrobial and antifungal activity in vitro against two fungal species, namely Aspergillus niger and Syncephalastrum racemosum, and four bacterial species: Gram-positive Staphylococcus aureus, Enterococcus faecalis and Gram-negative Klebsiella pneumoniae and Pseudomonas aeruginosa (Table 7). Compounds 7a,b did not show antifungal activity. The antimicrobial activity of these compounds was found to be lower than that of the reference drugs.
Table 7.
Antimicrobial activity of compounds 7a,b*
| Compound | Gram-positive bacteria | Gram-negative bacteria | ||
|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis | Klebsiella pneumoniae | Pseudomonas aeruginosa | |
| 7a | 17.3 ± 0.63 | NA** | 17.4 ± 0.58 | NA |
| 7b | 19.3 ± 1.2 | NA | 19.2 ± 0.58 | NA |
| Ampicillin | 23.8 ± 1.2 | 27.4 ± 0.72 | NA | NA |
| Ciprofloxacin | NA | NA | 25.3 ± 1.2 | 23.4 ± 0.63 |
*Activity is expressed as the diameter of the zone of inhibition (mean ± standard deviation), mm.
**NA – no activity.
Pyrimidobenzimidazoles 9a–d were tested for antibacterial activity against Gram-positive bacteria Bacillus subtilis and Staphylococcus aureus and Gramnegative bacteria Escherichia coli and Pseudomonas aeruginosa (Table 8).39
Table 8.
In vitro antibacterial activity of compounds 9a–d*
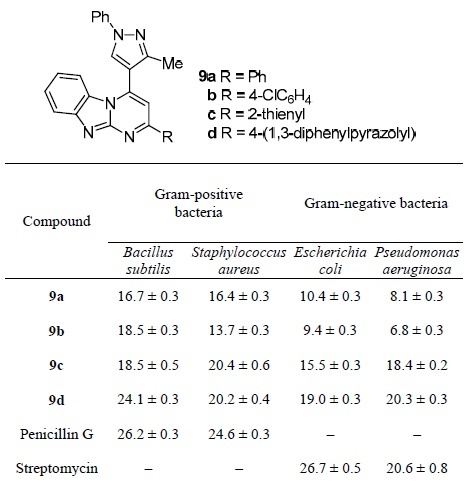
*Activity is expressed as the diameter of the zone of inhibition (mean ± standard deviation), mm.
As seen in Table 8, compound 9d showed the best activity against both Gram-positive and Gram-negative bacteria: its values of the zones of inhibition are close to those of the reference drugs.
The study of the biological activity of pyrimidobenzimidazole structures 21, 23 was presented in a study from 2017.45 In vitro antiproliferative activity of these compounds against the MCF-7 cell line was investigated. While compound 23 showed good activity, benzoic acid derivative 21 was found to be inactive (IC50 values 18.2 and 41 μg/ml, respectively). Antimicrobial activity of compounds 21 and 23 against Gram-positive bacteria Bacillus subtilis, Streptococcus pneumoniae and Gramnegative bacteria Pseudomonas aeruginos and Escherichia coli was investigated. Compared with the reference drug amphotericin B, compounds 21 and 23 exhibited moderate activity (Table 9).
Table 9.
Antimicrobial activity of compounds 21 and 23*
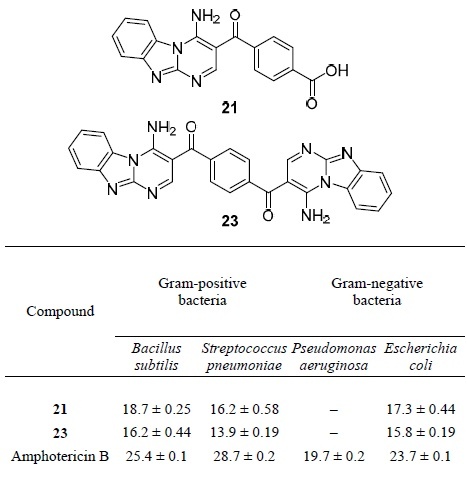
*Activity is expressed as the diameter of the zone of inhibition (mean ± standard deviation), mm.
In vitro studies of a wide range of pyrimido[1,2-a]-benzimidazoles 55a–k for antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Vibrio parahaemolyticus, and Proteus mirabilis were presented using chloramphenicol as a standard antibacterial agent with a broad spectrum of action. Based on the results of the in vitro studies, compounds 55a,c were selected as candidates for further biological tests (Table 10).57
Table 10.
In vitro antibacterial activity of compounds 55a,c

The synthesized galeterone derivatives containing a pyrimidobenzimidazole fragment were tested for antiproliferative activity. The values of the maximum inhibition of cell proliferation (GI50) of compounds 60a–c, 60'c, 61a–c, and 60”a lie in the middle micromolar range (Table 11).60
Table 11.
Antiproliferative activity of compounds 60, 60', 61, 61', and 60”a
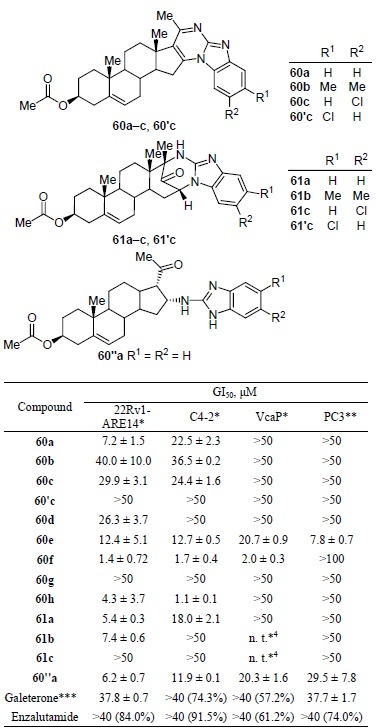
*Androgen receptor positive cell lines.
**Androgen receptor negative cell line.
***Data in parentheses represent viability in the presence of 40 μM concentration of compound.
*4 Not tested.
Pyrimidobenzimidazole derivatives 70, 71 a–h65 showed antibacterial activity with a MIC of 2 μg/ml against Grampositive bacteria and 1 μg/ml against Gram-negative bacteria. Compounds 70, 71 a–h showed antibacterial activity superior to ciprofloxacin against Enterococcus faecalis with a MIC of 0.2 to 0.8 μg/ml.
The antifungal activity of these compounds was evaluated in comparison with flucanozole (MIC 16 μg/ml for Candida albicans and 8 μg/ml for Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, Fusarium oxysporum, and Penicillium chrysogenum). Compounds 70f,g were highly active against Aspergillus fumigatus and Aspergillus flavus with a MIC of 0.2 μg/ml. Compounds 70b,d,e,g,h showed excellent activity against Fusarium oxysporum with a MIC of 0.2 μg/ml.
Cytotoxicity, expressed as percentage (%) of dead cells at a concentration of a test compound of 100 μg/ml, was assessed in comparison with 5-fluorouracil (88% dead cells) on Dalton's Lymphoma Ascites cells. Compounds 70b,d,f,g and 71b,d,e,g were highly active (>70% dead cells) against these cells.
Compounds 76–79 were tested for antimicrobial and antifungal activity.66 Compound 77 exhibited the broadest spectrum of antimicrobial activity. All compounds exhibited antifungal activity. The best indicators for compounds 76 and 77 were against Aspergillus flavus; for compound 79 – against Fusarium oxysporum, and for compound 78 – against Aspergillus ochraceus.
Pyrimidobenzimidazoles 129 were evaluated for pesticidal activity.75 Derivatives 129a–d were the most active against Plutella xylostella at a concentration of 100 mg/ml with a lethality of 70–90%. At a concentration of compounds 129a–d of 500 mg/ml, the lethality against Tetranychus cinnabarinus was 100% (Table 12).
Table 12.
Toxicity of compounds 129a–d against Tetranychus cinnabarinus and Plutella хylostella

*Concentration of compounds 129a–d 500 mg/ml.
**Concentration of compounds 129a–d 100 mg/ml.
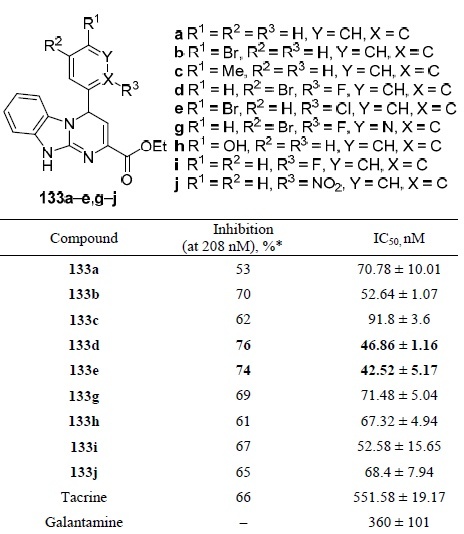
For structures 133a–e,g–j, an in vitro study of inhibition of acetylcholinesterase was carried out. The results in comparison with known inhibitors are presented (Table 13).78 Compounds 133d,e showed the best result among other compounds 133 and significantly higher inhibitory efficacy at lower IC50 values in comparison with drugs tacrine and galantamine.
Table 13.
The profile of acetylcholinesterase inhibition in vitro by compounds 133a–e,g–j
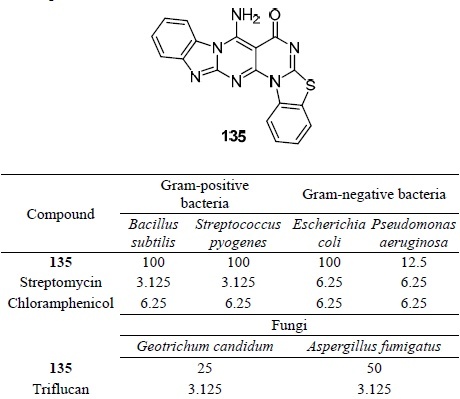
Compound 135 was studied against Gram-positive, Gramnegative bacteria, as well as against the fungi Geotrichum candidum and Aspergillus fumigatus79 (Table 14). Compound 135 exhibited both antimicrobial and antifungal activity, but showed MIC values much higher than those of the reference drugs.
Table 14.
Antimicrobial and antifungal activity (MIC, μg/ml) of compound 135
Pyrimidobenzimidazole derivatives 137a–g were evaluated in vitro for antioxidant activity80 (Table 15). The evaluation was carried out in two directions: a) interaction with DPPH (1,1-diphenyl-2-picrylhydrazyl) and b) inhibition of sodium linoleate peroxidation by 2,2'-azobis-(2-amidinopropane) dihydrochloride (AAPH) and soy lipoxygenase (LOX). Such well-known antioxidant agents as nordihydroguaiaretic acid (NDGA), ionol (BHT), and trolox were selected as objects of comparison. According to the results of the study, the most effective compound with antioxidant activity against peroxidation was compound 137a, while derivatives 137d,e showed moderate activity.
Table 15.
Antioxidant activity of compounds 137a–g assessed by the interaction with DPPH and in vitro inhibition of sodium linoleate peroxidation by APPH and LOX, and the lipophilicity index (C logP)

The affinity for human adenosine receptors A1, A2A, A2B, and A3, expressed in transfected HeLa (hA2A) and HEK-293 (hA2B) cells for compounds 178a–h was studied in vitro (Table 16).120 Along with tricyclic compounds, monocyclic 3,4-dihydropyridin-2(1H)-ones, bicyclic pyrrolopyrimidinones, and furopyrimidinediones were also considered. Pyrimidobenzimidazoles, in particular compounds 178a–d,g,h, showed the best affinity for the hA2B receptor (Ki ≤ 25 nM). The selective A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) and the selective A2A receptor antagonist ZM241385 were used as reference drugs.
Table 16.
Affinity of pyrimidobenzimidazoles 178a–h for human adenosine receptors
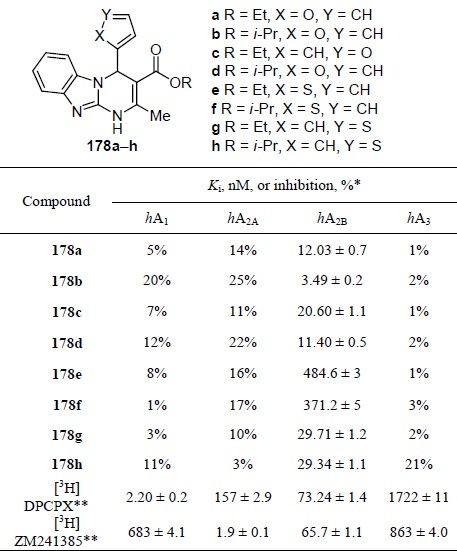
*Data are expressed as Ki or percent inhibition of specific binding at 1 μM concentration of compound (n = 2) for those compounds that did not completely displace radioligand binding.
** [3H]DPCPX and [3H]ZM241385 were used as radioligands in binding assays.
A team of scientists from India synthesized pyrimidobenzimidazole derivatives 192, 194 a–g and studied in vitro their antimicrobial, anti-tuberculosis (Table 17), and antimalarial activity (Table 18).128 Most of the compounds tested for antimicrobial activity showed excellent potential in relation to Salmonella typhi, Streptococcus pneumoniae, Bacillus subtilis, and Clostridium tetani compared to the antibiotic ampicillin. Compounds 192c,g and 194f,g (MIC 100 μg/ml) exhibited activity greater than ampicillin (MIC 250 μg/ml) and equal to ciprofloxacin (MIC 100 μg/ml).
Table 17.
In vitro anti-tuberculosis activity of compounds 192, 194 a–g against Mycobacterium tuberculosis H37Rv at a concentration of 250 μg/ml

Table 18.
In vitro antimalarial activity of compounds 192, 194 a–g against Plasmodium falciparum
| Compound | IC50, μg/ml |
|---|---|
| 192a | 0.051 |
| 192b | 0.030 |
| 192c | 1.84 |
| 192d | 1.52 |
| 192e | 1.19 |
| 192f | 1,75 |
| 192g | 0.079 |
| 194a | 1.45 |
| 194b | 0.041 |
| 194c | 1.50 |
| 194d | 1.45 |
| 194e | 0.054 |
| 194f | 0.83 |
| 194g | 0.092 |
| Chloroquine | 0.020 |
| Quinine | 0.268 |
According to in vitro antifungal screening data, compound 194b exhibited activity against Candida albicans (MIC 100 μg/l), equivalent to nystatin, while compound 192f turned out to be also highly active (MIC 250 μg/ml), surpassing the known drug griseofulvin (MIC 500 μg/ml). The activity of compounds 192b,d,e and 194b,e,f against Trichophyton rubrum was equal to nystatin and griseofulvin (MIC 500 μg/ml).
When screening for anti-tuberculosis activity, it was found that compounds 192b and 194b,e have the highest efficacy with an inhibition rate of 91, 94, and 90%, respectively. The rest of the compounds showed weak inhibition of the growth of Mycobacterium tuberculosis.
Compounds 192a,g and 194b,e,g showed the best activity against the Plasmodium falciparum strain with IC50 values in the range of 0.030–0.092 μg/ml (Table 18). The authors identify compounds 192b and 194b,e as promising antimicrobial, anti-tuberculosis, and antimalarial agents.
This review systematizes the results on the creation of pyrimidobenzimidazole structures over the past 10 years. The obtained compounds demonstrate a wide spectrum of biological activity, comparable to the activity of existing drugs on the market. Thus, pyrimidobenzimidazoles are promising objects in the search for means of combating diseases on a global scale and deserve the most serious attention in further studies of their structural modifications.
Acknowledgments
The reported study was funded the RFBR, project No. 19-33-90161.
Footnotes
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(4), 383–409
Contributor Information
Victor V. Fedotov, Email: victor0493@mail.ru
Vladimir L. Rusinov, Email: nitro@ios.uran.ru
References
- 1.Joule, J.; Mills, K. Khimiya Geterotsiklicheskikh Soedinenii (Chemistry of Heterocyclic Compounds [Russian translation]); Yurovskaya M. А., Ed.; Moscow: Mir, 2004.
- 2.Andersen PI, Ianevski A, Lysvand H, Vitkauskiene A, Oksenych V, Bjørås M, Telling K, Lutsar I, Dumpis U, Irie Y, Tenson T, Kantele A, Kainov DE. Int. J. Infect. Dis. 2020;93:268. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Álvarez DM, Castillo E, Duarte LF, Arriagada J, Corrales N, Farías MA, Henriquez A, Agurto-Muñoz C, González PA. Front. Microbiol. 2020;11:139. doi: 10.3389/fmicb.2020.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Bujuq, N. Synthesis2020, 3735.
- 5.Rusinov, V. L.; Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Bull., Int. Ed.2018, 67, 573. [Izv. Akad. Nauk, Ser. Khim.2018, 573.]
- 6.Karpenko I, Deev S, Kiselev O, Charushin V, Rusinov V, Ulomsky E, Deeva E, Yanvarev D, Ivanov A, Smirnova O, Kochetkov S, Chupakhin O, Kukhanova M. Antimicrob. Agents Chemother. 2010;54:2017. doi: 10.1128/AAC.01186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiselev, О. I.; Deeva, E. G.; Melnikova, T. I.; Kozeletskaya, K. N.; Kiselev, A. S.; Rusinov, V. L.; Charushin, V. N.; Chupakhin, O. N. Voprosy virusologii2012, 57(6), 9. [PubMed]
- 8.Sologub TV, Tokin II, Midikari AS, Tsvetkov VV. Infektsionnye bolezni. 2017;15(3):25. doi: 10.20953/1729-9225-2017-3-25-32. [DOI] [Google Scholar]
- 9.Tikhonova, E. P.; Kuz'mina, T. Yu.; Andronova, N. V.; Tyushevskaya, O. A.; Elistratova, T. A.; Kuz'min, A. E. Kazanskii meditsinskii zhurnal2018, 99, 215.
- 10.Tokin, I. I.; Zubkova, T. G.; Drozdova, Yu. V.; Lioznov, D. A. Infektsionnye bolezni2019, 17(4), 13.
- 11.Loginova, S. Ya.; Borisevich, S. V.; Rusinov, V. L.; Ulomsky, E. N.; Charushin, V. N.; Chupakhin, O. N.; Sorokin, P. V. Antibiotiki i khimoterapiya2015, 60(5–6), 8. [PubMed]
- 12.Tikhonova, E. P.; Kuz'mina, T. Yu.; Anisimova, A. A.; Kalinina, Yu. S. Eksperimental’naya i klinicheskaya farmokolgiya2018, 81(9), 21.
- 13.Sabitov, A. U.; Belousov, V. V.; Edin, A. S.; Oleinichenko, E. V.; Gladunova, E. P.; Tikhonova, E. P.; Kuz'mina, T. Yu.; Kalinina, Yu. S.; Sorokin, P. V. Antibiotiki i khimoterapiya2020, 65(7–8), 27.
- 14.Wu X, Yu K, Wang Y, Xu W, Ma H, Hou Y, Li Y, Cai B, Zhu L, Zhang M, Hu X, Gao J, Wang Y, Qin H, Wang W, Zhao M, Wu X, Zhang Y, Li L, Li K, Du Z, Mol BWJ, Yang B. Engineering. 2020;6:1185. doi: 10.1016/j.eng.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeva, E. G.; Shevchik, Yu. I.; Shaldzhan, A. A.; Zagorodnikova, K. A.; Tumashov, A. A.; Baklykov, A. V.; Kotovskaya, S. K.; Chupakhin, O. N.; Charushin, V. N.; Rusinov, V. L.; Kopchuk, D. S. Razrabotka i registratsiya lekarstvennykh sredstv2018, (3), 172.
- 16.Slepukhin, P. A.; Voinkov, E. K.; Ulomsky, E. N.; Savateev, K. V.; Kopchuk, D. S.; Zyryanov, G. V.; Fedotov, V. V.; Charushin, V. N.; Chupakhin, O. N.; Rusinov, V. L. Chem. Heterocycl. Compd.2019, 55, 989. [Khim. Geterotsikl. Soedin.2019, 55, 989.]
- 17.Begunov, R. S.; Ryzvanovich, G. A. Russ. Chem. Rev.2013, 82, 77. [Usp. Khim.2013, 82, 77.]
- 18.Achar KCS, Hosamani KM, Seetharamareddy HR. Eur. J. Med. Chem. 2010;45:2048. doi: 10.1016/j.ejmech.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Podunavac-Kuzmanović SO, Cvetković DD. Chem. Ind. Chem. Eng. Q. 2011;17:33. doi: 10.2298/CICEQ100405050P. [DOI] [Google Scholar]
- 20.Andrzejewska M, Yépez-Mulia L, Cedillo-Rivera R, Tapia A, Vilpo L, Vilpo J, Kazimierczuk Z. Eur. J. Med. Chem. 2002;37:973. doi: 10.1016/S0223-5234(02)01421-6. [DOI] [PubMed] [Google Scholar]
- 21.LaBarbera DV, Skibo EB. Bioorg. Med. Chem. 2005;13:387. doi: 10.1016/j.bmc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Agh-Atabay N, Dulger B, Gucin F. Eur. J. Med. Chem. 2003;38:875. doi: 10.1016/S0223-5234(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 23.Sharma D, Narasimhan B, Kumar P, Judge V, Narang R, De Clercq E, Balzarini J. J. Enzyme Inhib. Med. Chem. 2009;24:1161. doi: 10.1080/14756360802694427. [DOI] [PubMed] [Google Scholar]
- 24.Sondhi SM, Rani R, Singh J, Roy P, Agrawal SK, Saxena AK. Bioorg. Med. Chem. Lett. 2010;20:2306. doi: 10.1016/j.bmcl.2010.01.147. [DOI] [PubMed] [Google Scholar]
- 25.Ndakala AJ, Gessner RK, Gitari PW, October N, White KL, Hudson A, Fakorede F, Shackleford DM, Kaiser M, Yeates C, Charman SA, Chibale K. J. Med. Chem. 2011;54:4581. doi: 10.1021/jm200227r. [DOI] [PubMed] [Google Scholar]
- 26.Ansari KF, Lal C. Eur. J. Med. Chem. 2009;44:4028. doi: 10.1016/j.ejmech.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Kerimov I, Ayhan-Kilcigil G, Can-Eke B, Altanlar N, İscan M. J. Enzyme Inhib. Med. Chem. 2009;22:696. doi: 10.1080/14756360701228558. [DOI] [PubMed] [Google Scholar]
- 28.Pieroni M, Tipparaju SK, Lun S, Song Y, Sturm AW, Bishai WR, Kozikowski AP. ChemMedChem. 2011;6:334. doi: 10.1002/cmdc.201000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou S, Marousek GI. Antimicrob. Agents Chemother. 2006;50:3470. doi: 10.1128/AAC.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oukoloff K, Lucero B, Francisco KR, Brunden KR, Ballatore C. Eur. J. Med. Chem. 2019;165:332. doi: 10.1016/j.ejmech.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savateev, K. V.; Fedotov, V. V.; Ulomsky, E. N.; Rusinov, V. L. Chem. Heterocycl. Compd.2018, 54, 197. [Khim. Geterotsikl. Soedinen.2018, 54, 197.]
- 32.Savateev, K. V.; Ulomsky, E. N.; Fedotov, V. V.; Rusinov, V. L.; Sivak, K. V.; Lyubishin, M. M.; Kuzmich, N. N.; Aleksandrov, A. G. Russ. J. Bioorg. Chem.2017, 43, 421. [Bioorgan. Khim.2017, 43, 402.]
- 33.Zhang N, Ayral-Kaloustian S, Nguyen T, Afragola J, Hernandez R, Lucas J, Gibbsons J, Beyer C. J. Med. Chem. 2007;50:319. doi: 10.1021/jm060717i. [DOI] [PubMed] [Google Scholar]
- 34.Savateev K, Fedotov V, Butorin I, Eltsov O, Slepukhin P, Ulomsky E, Rusinov V, Litvinov R, Babkov D, Khokhlacheva E, Radaev P, Vassiliev P, Spasov A. Eur. J. Med. Chem. 2020;185:111808. doi: 10.1016/j.ejmech.2019.111808. [DOI] [PubMed] [Google Scholar]
- 35.Manna SK, Das T, Samanta S. ChemistrySelect. 2019;4:8781. doi: 10.1002/slct.201901941. [DOI] [Google Scholar]
- 36.Ali KA, Ragab EA, Abdelghafar HS, Farag AM. Res. Chem. Intermed. 2015;42:3553. doi: 10.1007/s11164-015-2231-y. [DOI] [Google Scholar]
- 37.Veeranarayana Reddy, M.; Chandra Sekhar Reddy, G.; Thi Kim Lien, N.; Kim, D. W.; Jeong, Y. T. Tetrahedron Lett.2009, 73, 1317.
- 38.Abbas IM, Abdallah MA, Gomha SM, Kazem MSH. J. Heterocycl. Chem. 2017;54:3447. doi: 10.1002/jhet.2968. [DOI] [Google Scholar]
- 39.El-Hashash MAE-A, Gomha SM, El-Arab EE. Chem. Pharm. Bull. 2017;65:90. doi: 10.1248/cpb.c16-00759. [DOI] [PubMed] [Google Scholar]
- 40.Devipriya, D.; Roopan, S. M. J. Photochem. Photobiol., B2019, 190, 42. [DOI] [PubMed]
- 41.Gao M, Wang M, Zheng Q-H. Bioorg. Med. Chem. Lett. 2014;24:254. doi: 10.1016/j.bmcl.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Marik, J.; Lyssikatos, J. P.; Williams, S. US Patent 2016250358.
- 43.Cashion, D. K.; Chen, G.; Gangadharmath, U. B.; Kasi, D.; Kolb, H. C.; Liu, C.; Sinha, A.; Szardenings, A. K.; Walsh, J. C.; Wang, E.; Yu, C.; Zhang, W. CN Patent 102985411.
- 44.Berlin, M.; Crew, A. P.; Dong, H.; Flanagan, J. J.; Ishchenko, A. US Patent 2018125821.
- 45.Farag AM, Fahim AM. J. Mol. Struct. 2018;1179:304. doi: 10.1016/j.molstruc.2018.11.008. [DOI] [Google Scholar]
- 46.Campos PT, Rodrigues LV, Belladona AL, Bender CR, Bitencurt JS, Rosa FA, Back DF, Bonacorso HG, Zanatta N, Frizzo CP, Martins MAP, Beilstein J. Org. Chem. 2017;13:257. doi: 10.3762/bjoc.13.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mabkhot Y, Alatibi F, El-Sayed N, Kheder N, Al-Showiman S. Molecules. 2016;21:1036. doi: 10.3390/molecules21081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mabkhot, Y. N.; Aladdi, S. S.; Al-Showiman, S. S.; Al-Majid, A. M. A.; Barakat, A.; Ghabbour, H. A.; Shaaban, M. R. J. Chem.2015, 382381.
- 49.Ibrahim H, Behbehani H. Molecules. 2014;19:2637. doi: 10.3390/molecules19022637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho, S. L.; Dao, P. D. Q.; Cho, C. S. Synlett2017, 1811.
- 51.Drosos NM, Kakoulidou C, Raftopoulou M, Stephanidou-Stephanatou J, Tsoleridis CA, Hatzidimitriou AG. Tetrahedron Lett. 2017;73:1. doi: 10.1016/j.tet.2016.11.022. [DOI] [Google Scholar]
- 52.Ibrahim MA, El-Gohary NM. J. Heterocycl. Chem. 2016;53:859. doi: 10.1002/jhet.2355. [DOI] [Google Scholar]
- 53.Zhang Z-T, Qiu L, Xue D, Wu J, Xu F-F. J. Comb. Chem. 2010;12:225. doi: 10.1021/cc900064q. [DOI] [PubMed] [Google Scholar]
- 54.Deng X-Q, Quan L-N, Song M-X, Wei C-X, Quan Z-S. Eur. J. Med. Chem. 2011;46:2955. doi: 10.1016/j.ejmech.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 55.Abarbri, M.; Jismy, B.; Akssira, M.; Knez, D.; Guillaumet, G.; Gobec, S. New J. Chem.2019, 43, 9961.
- 56.Fang S, Niu X, Yang B, Li Y, Si X, Feng L, Ma C. ACS Comb. Sci. 2014;16:328. doi: 10.1021/co500001u. [DOI] [PubMed] [Google Scholar]
- 57.Palaniraja J, Kumar SS, Ramki S, Arunachalam P, Roopan SM. J. Mol. Liq. 2017;230:534. doi: 10.1016/j.molliq.2017.01.010. [DOI] [Google Scholar]
- 58.Gnanasekaran KK, Muddala NP, Bunce RA. Tetrahedron Lett. 2015;56:7180. doi: 10.1016/j.tetlet.2015.11.041. [DOI] [Google Scholar]
- 59.Yong, J. S.; Hyun, J. S.; Min, K. D.; Kwan, L. B.; Ill, L. H.; Hyun, L. J.; Hyun, M. S.; Sun, Y. E. KR Patent 102027962.
- 60.Jorda R, Řezníčková E, Kiełczewska U, Maj J, Morzycki JW, Siergiejczyk L, Bazgier V, Berka K, Rárová L, Wojtkielewicz A. Eur. J. Med. Chem. 2019;179:483. doi: 10.1016/j.ejmech.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 61.Wojtkielewicz A, Uścinowicz P, Siergiejczyk L, Kiełczewska U, Ratkiewicz A, Morzycki JW. Steroids. 2017;117:71. doi: 10.1016/j.steroids.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Belyaev DV, Chizhov DL, Kodess MI, Ezhikova MA, Rusinov GL, Charushin VN. Mendeleev Commun. 2019;29:249. doi: 10.1016/j.mencom.2019.05.002. [DOI] [Google Scholar]
- 63.Kong W, Zhou Y, Song Q. Adv. Synth. Catal. 2018;360:1943. doi: 10.1002/adsc.201701641. [DOI] [Google Scholar]
- 64.Liu Y, Xia G, Luo C, Sun J, Ye B, Yuan Y, Wang H. Tetrahedron Lett. 2015;56:5071. doi: 10.1016/j.tetlet.2015.07.050. [DOI] [Google Scholar]
- 65.Puttaraju, K. B.; Shivashankar, K.; Chandra; Mahendra, M.; Rasal, V. P.; Venkata Vivek, P. N.; Rai, K.; Chanu, M. B. Eur. J. Med. Chem.2013, 69, 316. [DOI] [PubMed]
- 66.Kouadri, Y.; Ouahrani, M. R.; Missaoui, B. E.; Chebrouk, F.; Gherraf, N. Asian J. Chem.2015, 27, 3675.
- 67.El Bakri Y, Anouar EH, Ramli Y, Essassi EM, Mague JT. J. Mol. Struct. 2018;1152:154. doi: 10.1016/j.molstruc.2017.09.074. [DOI] [Google Scholar]
- 68.Ulomskiy, E. N.; El'tsov, O. S.; Borisov, S. S.; Savateev, K. V.; Voinkov, E. K.; Fedotov, V. V.; Rusinov, V. L. Chem. Heterocycl. Compd.2014, 50, 1005. [Khim. Geterotsikl. Soedin.2014, 1090.]
- 69.Di Braccio M, Grossi G, Signorello MG, Leoncini G, Cichero E, Fossa P, Alfei S, Damonte G. Eur. J. Med. Chem. 2013;62:564. doi: 10.1016/j.ejmech.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 70.Sharma P, Kumar A, Sharma M, Singh J, Bandyopadhyay P, Sathe M, Kaushik MP. J. Enzyme Inhib. Med. Chem. 2011;27:294. doi: 10.3109/14756366.2011.587814. [DOI] [PubMed] [Google Scholar]
- 71.Modranka J, Janecki T. Tetrahedron. 2011;67:9595. doi: 10.1016/j.tet.2011.09.139. [DOI] [Google Scholar]
- 72.Goryaeva, M. V.; Burgart, Y. V.; Saloutin, V. I.; Chupakhin, O. N. Chem. Heterocycl. Compd.2012, 48, 372. [Khim. Geterotsikl. Soedin.2012, 395.]
- 73.Solomyannii, R. N.; Pil'o, S. G.; Slivchuk, S. R.; Prokopenko, V. M.; Rusanov, E. B.; Brovarets, V. S. Rus. J. Gen. Chem.2017, 87, 407. [Zh. Obshch Khim.2017, 87, 398.]
- 74.Fedotov, V. V.; Ulomskiy, E. N.; Gorbunov, E. B.; Eltsov, O. S.; Voinkov, E. K.; Savateev, K. V.; Drokin, R. A.; Kotovskaya, S. K.; Rusinov, V. L. Chem. Heterocycl. Compd.2017, 53, 582. [Khim. Geterotsikl. Soedin.2017, 53, 582.]
- 75.Ren C, Wang Y, Wang D, Chen Y, Liu L. Sci. China Chem. 2010;53:1492. doi: 10.1007/s11426-010-4033-9. [DOI] [Google Scholar]
- 76.Wang Y, Shafiq Z, Liu L, Wang D, Chen Y-J. J. Heterocycl. Compd. 2010;47:373. [Google Scholar]
- 77.Wang Y, Liu L, Wang D, Chen Y-J. Can. J. Chem. 2012;90:85. doi: 10.1139/v11-095. [DOI] [Google Scholar]
- 78.Koti Reddy E, Chandran R, Sajith AM, Dileep KV, Sadasivan C, Anwar S. RSC Adv. 2016;6:77431. doi: 10.1039/C6RA12507G. [DOI] [Google Scholar]
- 79.Bondock S. Res. Chem. Intermed. 2014;41:5451. doi: 10.1007/s11164-014-1672-z. [DOI] [Google Scholar]
- 80.Neochoritis CG, Zarganes-Tzitzikas T, Tsoleridis CA, Stephanidou-Stephanatou J, Kontogiorgis CA, Hadjipavlou-Litina DJ, Choli-Papadopoulou T. Eur. J. Med. Chem. 2011;46:297. doi: 10.1016/j.ejmech.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 81.Heravi MM, Derikvand F, Ranjbar L. Synth. Commun. 2010;40:677. doi: 10.1080/00397910903009489. [DOI] [Google Scholar]
- 82.Dam B, Pal AK, Gupta A. Synth. Commun. 2016;46:275. doi: 10.1080/00397911.2015.1135955. [DOI] [Google Scholar]
- 83.Fekri LZ, Nikpassand M, Khakshoor SN. J. Organomet. Chem. 2019;894:18. doi: 10.1016/j.jorganchem.2019.05.004. [DOI] [Google Scholar]
- 84.Maleki A, Aghaei M, Ghamari N. Chem. Lett. 2015;44:259. doi: 10.1246/cl.141074. [DOI] [Google Scholar]
- 85.Shamsi-Sani M, Shirini F, Mohammadi-Zeydi M. J. Nanosci. Nanotechnol. 2019;19:4503. doi: 10.1166/jnn.2019.16489. [DOI] [PubMed] [Google Scholar]
- 86.Tabrizian E, Amoozadeh A. RSC Adv. 2016;6:96606. doi: 10.1039/C6RA21048A. [DOI] [Google Scholar]
- 87.Jiang L, Druzhinin Z. RSC Adv. 2019;9:15061. doi: 10.1039/C9RA01509D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dehghan M, Davoodnia A, Bozorgmehr MR, Bamoharram FF. Org. Prep. Proced. Int. 2017;49:236. doi: 10.1080/00304948.2017.1320905. [DOI] [Google Scholar]
- 89.Lu, H.; Shen, J.; Shen, Z. CN Patent 107312008.
- 90.Seyyedi N, Shirini F, Langarudi MSN, Jashnani S. J. Iran. Chem. Soc. 2017;14:1859. doi: 10.1007/s13738-017-1125-x. [DOI] [Google Scholar]
- 91.Shirini F, Langarudi MSN, Daneshvar N, Mashhadinezhad M, Nabinia N. J. Mol. Liq. 2017;243:302. doi: 10.1016/j.molliq.2017.07.080. [DOI] [Google Scholar]
- 92.Shirini F, Mazloumi M, Seddighi M. J. Nanosci. Nanotechnol. 2018;18:1194. doi: 10.1166/jnn.2018.13954. [DOI] [PubMed] [Google Scholar]
- 93.Goli-Jolodar O, Shirini F, Seddighi M. RSC Adv. 2016;6:44794. doi: 10.1039/C6RA08486A. [DOI] [Google Scholar]
- 94.Goli-Jolodar O, Shirini F. J. Iran. Chem. Soc. 2017;14:2275. doi: 10.1007/s13738-017-1164-3. [DOI] [Google Scholar]
- 95.Mousavi MR, Maghsoodlou MT. Monatsh. Chem. 2014;145:1967. doi: 10.1007/s00706-014-1273-y. [DOI] [Google Scholar]
- 96.Mousavi MR, Maghsoodlou MT, Hazeri N, Habibi-Khorassani SM. J. Iran. Chem. Soc. 2015;12:1419. doi: 10.1007/s13738-015-0609-9. [DOI] [Google Scholar]
- 97.Maloo P, Roy TK, Sawant DM, Pardasani RT, Salunkhe MM. RSC Adv. 2016;6:41897. doi: 10.1039/C6RA05322J. [DOI] [Google Scholar]
- 98.Gajaganti S, Kumari S, Kumar D, Allam BK, Srivastava V, Singh S. J. Heterocycl. Chem. 2018;11:2578. doi: 10.1002/jhet.3314. [DOI] [Google Scholar]
- 99.Mahire VN, Patel VE, Mahulikar PP. Res. Chem. Intermed. 2016;43:1847. doi: 10.1007/s11164-016-2734-1. [DOI] [Google Scholar]
- 100.Shen S, Zhang H, Yang W, Yu C, Yao C. Chin. J. Chem. 2011;29:1727. doi: 10.1002/cjoc.201180308. [DOI] [Google Scholar]
- 101.Diab, H.; Abdelhamid, I.; Elwahy, A. Synlett2018, 1627.
- 102.Beerappa M, Shivashankar K. Synth. Commun. 2016;46:421. doi: 10.1080/00397911.2016.1140784. [DOI] [Google Scholar]
- 103.Karimi AR, Bayat F. Lett. Org. Chem. 2011;8:631. doi: 10.2174/157017811799304368. [DOI] [Google Scholar]
- 104.Reddy, M. V.; Oh, J.; Jeong, Y. T. C. R. Chim.2014, 17, 484.
- 105.Risley, V. A.; Henry, S.; Kosyrikhina, M. V.; Manzanares, M. R.; Payan, I.; Downer, C. D.; Hellmann, C. C.; Slambrouck, S. V.; Frolova, L. V. Chem. Heterocycl. Compd.2014, 50, 185. [Khim. Geterotsikl. Soedin.2014, 209.]
- 106.Abedini M, Shirini F, Mousapour M, Goli Jolodar O. Res. Chem. Intermed. 2016;42:6221. doi: 10.1007/s11164-016-2456-4. [DOI] [Google Scholar]
- 107.Arya K, Tomar R. Res. Chem. Intermed. 2013;41:3389. doi: 10.1007/s11164-013-1441-4. [DOI] [Google Scholar]
- 108.Sheibani H, Hassani F. J. Heterocycl. Compd. 2011;48:915. doi: 10.1002/jhet.640. [DOI] [Google Scholar]
- 109.Sheibani, H.; Babaie, M. Russ. Chem. Bull., Int. Ed.2013, 62, 2202. [Izv. Akad. Nauk, Ser. Khim.2013, 2202.]
- 110.Yarie M, Zolfigol MA, Baghery S, Khoshnood A, Alonso DA, Kalhor M, Bayat Y, Asgari A. J. Iran. Chem. Soc. 2018;15:2259. doi: 10.1007/s13738-018-1415-y. [DOI] [Google Scholar]
- 111.Reddy MV, Reddy AVS, Jeong YT. Res. Chem. Intermed. 2015;42:4893. doi: 10.1007/s11164-015-2328-3. [DOI] [Google Scholar]
- 112.Tran PH, Thi Bui T-P, Bach Lam X-Q, Thi Nguyen X-T. RSC Adv. 2018;8:36392. doi: 10.1039/C8RA07256F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalita SJ, Chandra Deka D, Mecadon H. RSC Adv. 2016;6:91320. doi: 10.1039/C6RA21376F. [DOI] [Google Scholar]
- 114.Liu J, Lei M, Hu L. Green Chem. 2012;14:840. doi: 10.1039/c2gc16499j. [DOI] [Google Scholar]
- 115.Warekar PP, Patil PT, Patil KT, Jamale DK, Kolekar GB, Anbhule PV. Synth. Commun. 2016;46:2022. doi: 10.1080/00397911.2016.1244273. [DOI] [Google Scholar]
- 116.Rao GBD, Acharya BN, Verma SK, Kaushik MP. Tetrahedron Lett. 2011;52:809. doi: 10.1016/j.tetlet.2010.12.039. [DOI] [Google Scholar]
- 117.Kaur N, Kaur K, Raj T, Kaur G, Singh A, Aree T, Park S-J, Kim T-J, Singh N, Jang DO. Tetrahedron. 2015;71:332. doi: 10.1016/j.tet.2014.11.039. [DOI] [Google Scholar]
- 118.Shaterian HR, Fahimi N, Azizi K. Res. Chem. Intermed. 2013;40:1879. doi: 10.1007/s11164-013-1087-2. [DOI] [Google Scholar]
- 119.Ghorbani-Vaghei R, Toghraei-Semiromi Z, Karimi-Nami R, Salimi Z. Helv. Chim. Acta. 2014;97:979. doi: 10.1002/hlca.201300361. [DOI] [Google Scholar]
- 120.El Maatougui A, Azuaje J, González-Gómez M, Miguez G, Crespo A, Carbajales C, Escalante L, García-Mera X, Gutiérrez-de-Terán H, Sotelo E. J. Med. Chem. 2016;59:1967. doi: 10.1021/acs.jmedchem.5b01586. [DOI] [PubMed] [Google Scholar]
- 121.Kumar A, Kumar M, Maurya S, Khanna RS. J. Org. Chem. 2014;79:6905. doi: 10.1021/jo5007762. [DOI] [PubMed] [Google Scholar]
- 122.Rawat M, Rawat DS. Tetrahedron Lett. 2018;59:2341. doi: 10.1016/j.tetlet.2018.05.005. [DOI] [Google Scholar]
- 123.Shinde, V. V.; Jeong, Y. T. New J. Chem.2015, 39, 4977.
- 124.Wu J, Luo H, Wang T, Sun H, Zhang Q, Chai Y. Tetrahedron. 2019;75:1052. doi: 10.1016/j.tet.2019.01.009. [DOI] [Google Scholar]
- 125.Сhai, Y.; Luo, H.; Wu, J.; Zhang, Q. CN Patent 108250202.
- 126.Hassaneen HME, Farghaly TA. J. Heterocycl. Chem. 2015;52:1154. doi: 10.1002/jhet.2152. [DOI] [Google Scholar]
- 127.Li Y-L, Cai G, Liu X-J, Wang K, Du B-X. J. Chem. Res. 2013;37:201. doi: 10.3184/174751913X13637768014150. [DOI] [Google Scholar]
- 128.Prasad, P.; Kalola, A. G.; Patel, M. P. New J. Chem.2018, 42, 12666.
- 129.Reddy MV, Byeon KR, Park SH, Kim DW. Tetrahedron. 2017;73:5289. doi: 10.1016/j.tet.2017.07.025. [DOI] [Google Scholar]
- 130.Jadhav AM, Kim YI, Lim KT, Jeong YT. Tetrahedron Lett. 2018;59:554. doi: 10.1016/j.tetlet.2018.01.008. [DOI] [Google Scholar]
- 131.Spasov AA, Babkov DA, Sysoeva VA, Litvinov RA, Shamshina DD, Ulomsky EN, Savateev KV, Fedotov VV, Slepukhin PA, Chupakhin ON, Charushin VN, Rusinov VL. Arch. Pharm. 2017;350:1700226. doi: 10.1002/ardp.201700226. [DOI] [PubMed] [Google Scholar]
- 132.Chupakhin, O. N.; Charushin, V. N.; Rusinov, V. L.; Ulomskii, E. N.; Kotovskaya, S. K.; Kiselev, O. I.; Deeva, E. G.; Savateev, K. V.; Borisov, S. S. 2013 RU Patent 2529487.
- 133.Savateev, K. V.; Ulomsky, E. N.; Borisov, S. S.; Voinkov, E. K.; Fedotov, V. V.; Rusinov, V. L. Chem. Heterocycl. Compd.2014, 50, 880. [Khim. Geterotsikl. Soedin., 953.]
- 134.Zhao, L.; Christov, P. P.; Kozekov, I. D.; Pence, M. G.; Pallan, P. S.; Rizzo, C. J.; Egli, M.; Guengerich, F. P. Angew. Chem., Int. Ed.2012, 51, 5466. [DOI] [PMC free article] [PubMed]
- 135.Combs, D.; Langevine, C. M.; Qiu, Y.; Zusi, F. C. WO Patent 2005011609.
- 136.Fedotov, V. V.; Ulomsky, E. N.; Savateev, K. V.; Mukhin, E. M.; Gazizov, D. A.; Gorbunov, E. B.; Rusinov, V. L. Synthesis2020, 3622.


