Abstract
Background & Aims
Hepatic enzymes play a major role in the metabolic elimination of cortisol, and reduced rates of cortisol clearance have been consistently observed in patients with chronic liver disease. It is less clear whether there are concomitant abnormalities of adrenocortical function in patients with cirrhosis. In the present study, we sought to assess adrenocortical function in patients with cirrhosis using measures of free cortisol appearance and elimination rates that are independent of serum concentrations of cortisol binding proteins.
Methods
Post hoc analysis used computer-assisted numerical and modelling methods with serial total and free cortisol concentration data to obtain rates of free cortisol appearance and elimination. Rate parameters were obtained in 114 patients with chronic liver disease, including Child-Pugh (CP) ≤8 (n = 53) and CP >8 (n = 61).
Results
Maximal cortisol secretion rate (CSRmax) was significantly decreased (p = 0.01) in patients with cirrhosis with CP >8 (0.28 nM/s; 95% CI 0.24–0.34) compared with those with CP ≤8 (0.39 nM/s; 95% CI 0.33–0.46), and CSRmax was negatively correlated with CP score (r = −0.19, p = 0.01). Free cortisol elimination rate was significantly (p = 0.04) decreased in the CP >8 group (0.16 ± 0.20 min−1) compared with that in the CP ≤8 group (0.21 ± 0.21 min−1), and free cortisol elimination rates were negatively correlated with CP score (r = −0.23, p = 0.01). A significant correlation between CSRmax and free cortisol elimination rate (r = 0.88, p <0.001) was observed.
Conclusions
CSRmax and free cortisol elimination rates were significantly reduced according to severity of cirrhosis. In contrast to stimulated total cortisol concentrations, CSRmax estimates were independent of cortisol-binding protein concentrations. Results provide additional evidence of subnormal adrenocortical function in patients with cirrhosis.
Lay summary
We applied numerical analytic methods to characterise adrenocortical function in patients with varying stages of chronic liver disease. We found that patients with more severe cirrhosis have decreased rate of free cortisol elimination and decreased maximal cortisol secretion rate, which is a measure of adrenocortical function. In contrast to conventional measures of adrenocortical function, those obtained using numerical methods were not affected by variation in corticosteroid binding globulin and albumin concentrations. We conclude that patients with cirrhosis demonstrate measurable abnormalities of adrenocortical function, evidence of which supports aspects of the hepatoadrenal syndrome hypothesis.
Keywords: Liver disease, Computer-assisted numerical analysis, Hydrocortisone, Metabolic clearance rate, Adrenal insufficiency
Abbreviations: ACLF, acute-on-chronic liver failure; ACTH, adrenocorticotrophin; AI, adrenal insufficiency; CBG, corticosteroid-binding globulin; CIRCI, critical illness-related corticosteroid insufficiency; CP, Child-Pugh; CPR, cortisol production rate; CRP, C-reactive protein; CRT, corticosteroid replacement therapy; CSR, cortisol secretion rate; CSRbase, basal CSR (before ACTH stimulation); CSRmax, maximal CSR; HPA, hypothalamic–pituitary–adrenal; HSD, hydroxysteroid dehydrogenase; INR, international normalised ratio; MCR, metabolic clearance rate; MELD, model for end-stage liver disease; PAI, primary adrenal insufficiency; RAI, relative adrenal insufficiency; RCT, randomised clinical trial; SAI, secondary adrenal insufficiency; SCOTCH, Supplemental Corticosteroids in Cirrhotic Hypotensive Patients With Suspicion of Sepsis; STB, standardised beta
Graphical abstract
Highlights
-
•
Free cortisol appearance and elimination rates were obtained by computer-assisted numerical analysis.
-
•
Maximal cortisol secretion rates, a measure of adrenocortical function, were inversely related to severity of cirrhosis.
-
•
Variation in concentrations of cortisol binding proteins bias measures of adrenocortical function that rely on total cortisol.
-
•
Chronically reduced rate of free cortisol elimination in patients with cirrhosis may contribute to the pathophysiology of subnormal adrenocortical function.
-
•
Evidence of subnormal adrenocortical function supports the hepatoadrenal syndrome hypothesis.
Introduction
Enzymes expressed in the liver play a primary role in the metabolic elimination of cortisol, and cirrhosis has served as a clinical model for reduced rates of cortisol clearance.[1], [2], [3], [4] The experimental evidence for a concomitant association between severity of chronic liver disease and clinically relevant abnormalities of adrenocortical function is less clear and, thus, remains a hypothesis. One interpretation of the hepatoadrenal syndrome hypothesis is that chronic liver disease leads to associated, chronic alterations in regulatory and functional relationships of the hypothalamic–pituitary–adrenal (HPA) axis.2,5,6 A corollary hypothesis is that these alterations may in turn affect adrenocortical function among patients with chronic liver disease that reduce their ability to achieve or sustain the adaptive increase in cortisol secretion rate (CSR), which occurs as part of the normal physiological response to critical illness in otherwise euadrenal persons without cirrhosis.7
Many reports in the literature suggest a high prevalence of adrenal insufficiency (AI) in patients with chronic liver disease.[8], [9], [10] However, assessment of adrenocortical function in patients with cirrhosis is complicated by several factors, including lower serum concentrations of corticosteroid-binding globulin (CBG) and albumin,10,11 abnormalities of hepatic protein glycosylation that may influence CBG binding affinity,12 and decreased rates of cortisol clearance.2,5 The importance of high-affinity, reversible binding of cortisol to CBG as a determinant of total cortisol concentrations has been consistently observed in studies using a diversity of experimental methods.[13], [14], [15] It follows that application of cut scores for stimulated total cortisol concentrations developed in reference populations having normal distributions of CBG and albumin concentrations will lead to false-positive results when applied to patient populations having decreased levels of CBG and albumin,[16], [17], [18], [19] including cirrhosis and critical illness. Several studies suggest that measurements of stimulated serum -free or salivary cortisol concentrations provide a more accurate measure of adrenocortical functional in patients with cirrhosis when compared with those of total cortisol.[20], [21], [22] In the future, pending clinical standardisation and validation of cut scores,23 such tests may prove useful in the evaluation of adrenocortical function in patients with chronic liver disease.
Adrenocortical function has also been evaluated using higher-order research methodologies that are independent of or adjust for variation in CBG and albumin concentrations. These include (i) stable isotope dilution methodology6,24 and (ii) estimation of free cortisol appearance and elimination rates using numerical methods.25,26 Compared with controls, cortisol production rates (CPRs) obtained by stable isotope dilution have been reported to be normal27,28 or decreased2,6,29 in patients with cirrhosis. Free cortisol appearance and elimination rates obtained using numerical methods have been characterised in healthy controls,25,26 as well as in patients with critical illness,30,31 secondary AI (SAI),15 sepsis, and septic shock,7,30 but not, to our knowledge, in patients with chronic liver disease. A useful measure of adrenocortical function obtained using numerical methods is the maximal cortisol secretion rate (CSRmax), defined as the CSR that is obtained under conditions in which adrenocorticotrophin (ACTH) concentrations exceed the threshold for maximal adrenocortical stimulation.25,26 CSRmax is a key parameter defining the non-linear relationship between ACTH concentrations and CSR.7,15,25,26 When compared with that in healthy controls,25,26 CSRmax is significantly decreased in patients with primary AI (PAI) as well as (chronic) SAI.15,32,33
As evidence of subnormal adrenocortical function in patients with cirrhosis is important to the development of the hepatoadrenal syndrome hypothesis, we sought in the present investigation to further characterise adrenocortical function in patients with cirrhosis using methodologies that combine computer-assisted numerical and modelling analysis with experimental manipulation of CSR by ACTH stimulation. The question of whether adrenocortical function is ‘normal’ or ‘subnormal’ among patients with chronic liver disease is clinically relevant, as evidence of subnormal adrenocortical function could affect management decisions regarding corticosteroid replacement therapy (CRT) and clinical outcomes specific to patients with liver disease34,35 during episodes of acute illness commonly observed in patients with cirrhosis.23
Patients and methods
Patients
The study population included patients without sepsis (n = 95) and those with sepsis (n = 27). Laboratory methods, total and free cortisol assay characteristics, and ACTH1-24 stimulation protocol have been previously reported by Thévenot et al.11 The clinical study that generated data for this post hoc analysis was conducted in accordance with guidelines of the 1975 Declaration of Helsinki with institutional review committee approval,11 and informed consent was obtained from all participants. Of the original study participants, 7 patients with sepsis and 1 without sepsis were excluded from further analysis because the total cortisol increment above baseline during ACTH1-24 stimulation was less than 3.2% (see Supplementary data).
Estimation of CSRmax and free cortisol elimination rates
CSRmax and free cortisol elimination rates were obtained by computer-assisted numerical analysis using a dynamic 3-compartment model. Free cortisol appearance and elimination rates were obtained by an iterative least squares solution of 3 simultaneous, non-linear differential equations as previously described.7,15,25,26 Additional methodological details for obtaining free cortisol appearance and elimination rate parameters are described in the Supplementary data. Basal (unstimulated) CSR (CSRbase) was estimated as previously described.7
Statistical analysis
Descriptive data are reported as mean and SD. CSRmax was log-transformed to symmetrise the data distribution and reported by inverse transformation to geometric mean and asymmetric 95% CIs. To express the distribution of free cortisol elimination rate (α) in terms of free cortisol half-life, the half-life distribution is reported as median and IQR. Comparisons between the groups with mild and severe liver disease were done using a t test, where severity of liver disease was stratified by Child-Pugh (CP) score ≤8 (mild, n = 53) or >8 (severe, n = 61) as previously described.36 The group with severe cirrhosis included patients with (n = 20) and without (n = 41) concomitant sepsis. Comparisons between the CP groups were done using ANOVA with Fisher’s least significant difference method of post hoc pairwise comparisons. Correlations were evaluated using Pearson (r) correlation. To determine whether the effects of disease severity were driven by inclusion of patients with sepsis, CSRmax and free cortisol elimination rates and related correlations were analysed both with and without inclusion of patients with sepsis. Predictors of Δ-cortisol (increment between total cortisol concentrations at baseline and 60 min post-ACTH1-24) were analysed in the multivariate context using standardised beta (STB) analysis. STB is a unitless measure equal to the SD of the outcome variable (y, Δ-cortisol) multiplied by the multivariate regression coefficient (β) and divided by the SD of the predictor variable (x).15
Results
Participants
Baseline characteristics of the study population (n = 114) stratified by CP letter as well as sepsis are shown in Table 1. Results were similar to those previously reported by Thévenot et al.11 with significant differences between clinical groups for various measures including concentrations of CBG, albumin, and both total and free cortisol. The characteristics and cortisol concentration data for patients excluded from the analysis (n = 8) are reported in the Supplementary data.
Table 1.
Baseline characteristics of the 114 study patients according to severity of liver disease.
| Characteristic | Child-Pugh A (n = 34) | Child-Pugh B (n = 29) | Child-Pugh C (n = 31) | Child-Pugh >8 and sepsis (n = 20) | p value∗ |
|---|---|---|---|---|---|
| Age (year) | 59.1 ± 7.9 | 61.7 ± 11.2 | 55.5 ± 9.4 | 58.0 ± 10.8 | 0.10 |
| Sex, n (%) | 0.77 | ||||
| Male | 25 (73.5) | 20 (69) | 19 (61.3) | 14 (70) | |
| Female | 9 (26.5) | 9 (31) | 12 (38.7) | 6 (30) | |
| BMI (kg/m2) | 27.0 ± 4.8 | 26.7 ± 5.4 | 24.9 ± 5.2 | 25.1 ± 5.6 | 0.28 |
| CBG (mg/L) | 45.5 ± 12.6 | 37.7 ± 10.4 | 26.6 ± 8.66 | 29.5 ± 14.4 | <0.001 |
| Albumin (g/dl) | 3.7 ± 0.5 | 2.7 ± 0.5 | 2.4 ± 0.5 | 2.7 ± 0.7 | <0.001 |
| Baseline total cortisol (μg/dl) | 15.5 ± 7.4 | 12.0 ± 4.9 | 11.7 ± 7.6 | 17.2 ± 7.3 | 0.01 |
| Baseline free cortisol (μg/dl) | 1.5 ± 0.9 | 1.5 ± 1.0 | 2.0 ± 1.4 | 3.5 ± 2.2 | <0.001 |
| MELD score | 10.0 ± 3.3 | 14.9 ± 4.1 | 23.9 ± 6.3 | 24.1 ± 6.9 | <0.001 |
| Total bilirubin (mg/dl) | 1.1 ± 0.5 | 3.3 ± 4.7 | 9.4 ± 9.1 | 8.2 ± 5.8 | <0.001 |
| INR | 1.2 ± 0.2 | 1.6 ± 0.3 | 2.5 ± 1.0 | 2.4 ± 0.8 | <0.001 |
| Prothrombin (s) | 72 ± 6.5 | 64.7 ± 7.6 | 59.8 ±8.7 | 60.0 ± 8.4 | <0.001 |
| Creatinine (mg/dl) | 0.9 ± 0.2 | 0.9 ± 0.4 | 0.9 ± 0.4 | 1.1 ± 0.6`` | 0.34 |
| CRP (mg/L) | 6.6 ± 7.7 | 17.0 ± 16.4 | 23.2 ± 22.9 | 55.6 ± 31.2 | <0.001 |
| Total cholesterol (mg/dl) | 65.6 ± 15.4 | 50.2 ± 15.4 | 46.3 ± 23.2 | 34.7 ± 15.4 | <0.001 |
| HDL (mg/dl) | 15.4 ± 7.7 | 15.4 ± 7.7 | 7.7 ± 3.9 | 7.7 ± 3.9 | <0.001 |
Data are presented as mean ± SD. CBG, corticosteroid-binding globulin; CRP, C-reactive protein; INR, international normalised ratio; MELD, model for end-stage liver disease. ∗By analysis of variance.
Role of cortisol-binding protein concentrations on measures of adrenocortical function
The dependence of stimulated total cortisol concentrations on CBG concentration was confirmed by our finding of a significant positive correlation between CBG and stimulated cortisol levels in both the groups with mild (r = 0.58, p <0.001, for 30 min and r = 0.44, p = 0.001, for 60 min post-ACTH1-24) and severe (r = 0.46, p = 0.003, for 30 min and r = 0.51, p <0.001, for 60 min post-ACTH1-24) cirrhosis. Albumin concentrations were also significantly correlated with stimulated cortisol concentrations in patients with mild cirrhosis (r = 0.43, p = 0.001, for 30 min and r = 0.42, p = 0.002, for 60 min post-ACTH1-24), By contrast, CSR estimates were independent of serum-binding protein concentrations, as confirmed by lack of significant correlations between CBG levels and CSRmax in both the groups with mild and severe cirrhosis. Similarly, no significant correlations between albumin concentration and CSRmax were observed in either of the groups with mild or severe cirrhosis.
Maximal cortisol secretion rate according to severity of cirrhosis
As shown in Fig. 1A, there was a trend toward significance (p = 0.06) for higher CSRmax in the group with mild liver disease (0.39 nM/s; 95% CI 0.33–0.46) compared with the group with severe liver disease (0.31 nM/s; 95% CI 0.26–0.37). When patients with sepsis were excluded from the analysis, CSRmax was significantly decreased (p = 0.01) in the group with severe liver disease (0.28 nM/s; 95% CI 0.24–0.34). ANOVA also demonstrated significant differences between CP groups (p = 0.02). As shown in Fig. 1B, CSRmax was significantly decreased in CP B (0.29 nM/s; 95% CI 0.24–0.35) and C (0.29 nM/s; 95% CI 0.23–0.36) compared with that in CP A (0.44 nM/s; 95% CI 0.35–0.55) (p <0.01 for both comparisons). CSRmax did not differ significantly between patients with severe cirrhosis and with or without concomitant sepsis. As well, CSRmax in patients with concomitant sepsis was not significantly different compared with CP groups A–C (Fig. 1B).
Fig. 1.
CSRmax by severity of liver disease.
(A) CSRmax (nmol/L/s) for patients grouped according to Child-Pugh score ≤8 or >8. (B) CSRmax (nmol/L/s) by CP letter (A–C) as well as the group having sepsis and CP score >8. Data presented are geometric mean and 95% confidence interval. §p = 0.06 (t test). ∗p <0.01 compared with CP A (ANOVA with Fisher's least significant difference method for pairwise comparisons). CP, Child-Pugh; CSRmax, maximal cortisol secretion rate.
We also observed a significant negative correlation between CP score and CSRmax (r = −0.19, p = 0.01). As shown in Table 2, this correlation was not driven by patients with sepsis, as the negative correlation between disease severity (CP) and CSRmax remained significant when patients with sepsis were excluded.
Table 2.
Correlations between CSRmax and markers of liver disease severity.
| Correlation with CSRmax (r) | p value | |
|---|---|---|
| All patients (n = 114) | ||
| Child-Pugh score | −0.19 | 0.01 |
| MELD | −0.16 | 0.10 |
| HDL | 0.14 | 0.04 |
| Total cholesterol | 0.12 | 0.20 |
| CRP | 0.33 | <0.01 |
| Creatinine | 0.11 | 0.26 |
| Free cortisol half-life | −0.88 | <0.01 |
| Patients without sepsis (n = 94) | ||
| Child-Pugh score | −0.29 | <0.01 |
| MELD | −0.14 | 0.17 |
| HDL | 0.23 | 0.03 |
| Total cholesterol | 0.20 | 0.05 |
| CRP | 0.10 | 0.33 |
| Creatinine | 0.16 | 0.13 |
| Free cortisol half-life | −0.88 | <0.01 |
CRP, C-reactive protein; CSRmax, maximal cortisol secretion rate; MELD, model for end-stage liver disease.
Free cortisol elimination rate in relation to severity of cirrhosis
As shown in Fig. 2A, free cortisol elimination rate constant (α) was significantly (p = 0.04) reduced in the group with severe cirrhosis (0.16 ± 0.2 min−1) than in the group with mild cirrhosis (0.21 ± 0.21 min−1). As α is inversely related to free cortisol half-life, these elimination constants correspond to median free cortisol half-lives of 8.2 min (IQR 3.6–18.9) in the group with severe cirrhosis and 5.0 min (IQR 2.2–11.1) in the group with mild cirrhosis. Free cortisol elimination rate remained significantly decreased (p = 0.03) in severe vs. mild liver disease when patients with sepsis were excluded from the analysis (data not shown). Free cortisol elimination rates by CP score (Fig. 2B) were significantly different by ANOVA (p = 0.04), with free cortisol elimination rates significantly reduced in CP groups B and C than in CP group A (p = 0.03 for both comparisons). The free cortisol elimination rate in the group with sepsis was not significantly different from that in the CP groups A–C (Fig. 2B). As shown in Table 3, we also observed a significant, negative correlation between CP score and free cortisol elimination rate (r = −0.23, p = 0.01). This correlation remained significant when patients with sepsis were excluded from the analysis (Table 3). Although albumin concentration and prothrombin time were significantly correlated with free cortisol half-life, correlations with several other measures of severity of cirrhosis, including model for end-stage liver disease score, were not significant (Table 3).
Fig. 2.
Free cortisol elimination rate constant by severity of liver disease.
Free cortisol elimination rate constant (α, min-1) by (A) CP ≤8 or >8 and (B) CP classification A–C and sepsis. Data presented are mean and SEM. §p = 0.04 CP ≤8 vs. >8 (t test). ∗p = 0.03 compared with CP A (ANOVA with Fisher's least significant method for pairwise comparisons). CP, Child-Pugh.
Table 3.
Correlations between free cortisol elimination rate (α) and markers of liver disease severity.
| Correlation with free cortisol elimination rate constant α (r) | p value | |
|---|---|---|
| All patients (n = 114) | ||
| Child-Pugh score | −0.23 | 0.01 |
| MELD | −0.15 | 0.12 |
| Albumin | 0.22 | 0.02 |
| INR | −0.13 | 0.18 |
| Prothrombin | 0.25 | 0.01 |
| Total bilirubin | −0.12 | 0.09 |
| Creatinine | 0.10 | 0.30 |
| Patients without sepsis (n = 94) | ||
| Child-Pugh score | −0.21 | 0.04 |
| MELD | −0.08 | 0.44 |
| Albumin | 0.22 | 0.03 |
| INR | −0.05 | 0.61 |
| Prothrombin | 0.24 | 0.02 |
| Total bilirubin | −0.09 | 0.38 |
| Creatinine | −0.11 | 0.28 |
INR, international normalised ratio; MELD, model for end-stage liver disease.
Relationship between free cortisol elimination rate and CSRmax
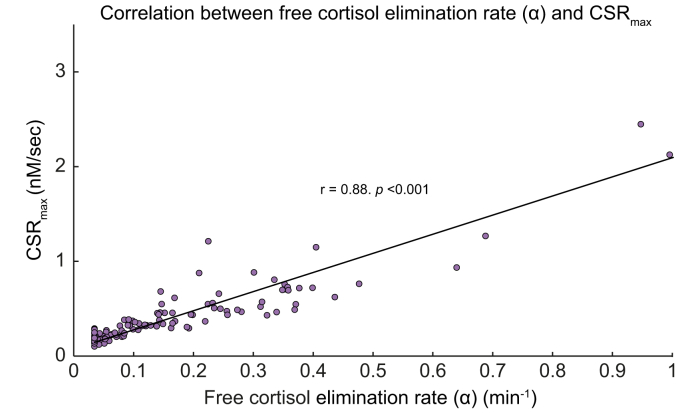
We examined several potential factors contributing to decreased CSRmax in patients with more severe cirrhosis. As shown in Table 2, there was a significant positive correlation between HDL concentration and CSRmax in patients with and without concomitant sepsis. We also observed a significant correlation between C-reactive protein (CRP) concentration and CSRmax. However, this association appeared to be driven by the inclusion of patients with sepsis, as the correlation between CRP and CSRmax was no longer significant when patients with sepsis were excluded from the analysis (Table 2). We also observed a significant positive correlation between free cortisol elimination rate constant (α) and CSRmax (r = 0.88, p <0.01), shown in Fig. 3. This correlation was not driven by inclusion of septic patients, as it remained significant and similar when septic patients with cirrhosis were excluded from the analysis (Table 2).
Fig. 3.
Correlation of CSRmax (nmol/L/s) on free cortisol elimination rate (α, min−1) was significant (r = 0.88, p <0.001).
Individual patients shown as open circles and regression trend line as solid line. Included in regression but not shown are 2 outliers having free cortisol elimination rate >1.0. CSRmax, maximal cortisol secretion rate.
Predictors of Δ-cortisol
In several studies, including the randomised clinical trial (RCT) of CRT vs. placebo in patients with cirrhosis and with sepsis and septic shock reported by Arabi et al.,34 a Δ-cortisol (ACTH-stimulated increment in total cortisol concentration above baseline) <248 nmol/L was associated with haemodynamic response to CRT. We performed a multivariate analysis to determine which predictor variables influence Δ-cortisol. As shown in STB analysis (Table 4), several predictor variables were significantly correlated with Δ-cortisol in our study population. For example, an STB of −0.55 means that a 1 SD increase in baseline CSR (as percent of maximum) results in a 0.55 SD decrease in Δ-cortisol and a 1 SD increase in CBG concentration results in a corresponding increase of 0.19 SD in Δ-cortisol.
Table 4.
STB for Δ-cortisol vs. predictors.
| Predictor variable | STB | p value∗ |
|---|---|---|
| Baseline CSR (% CSRmax) | −0.55 | <0.001 |
| Albumin concentration | 0.31 | <0.001 |
| CBG concentration | 0.19 | 0.01 |
| Free cortisol half-life | 0.19 | 0.03 |
Δ-cortisol is defined as the total cortisol concentration 60 min post-ACTH1-24 (250 μg) minus baseline total cortisol concentration (at time zero). ACTH, adrenocorticotrophin; CBG, corticosteroid-binding globulin; CSR, cortisol secretion rate; CSRmax, maximal CSR; STB, standardised beta. ∗By Wald's test.
Discussion
Our finding that ACTH-stimulated total cortisol concentrations were significantly and positively correlated with CBG in both mild and severe cirrhosis groups highlight the potential for error in using stimulated total cortisol concentrations to assess adrenocortical function. This is especially a concern in patient populations such as chronic liver disease where variability of CBG and albumin concentrations is increased.8,11,19,21 By contrast, CSRmax was independent of CBG and albumin concentrations. As CSRmax is a particularly useful measure of adrenocortical function,7,15,25,26 our principal finding of decreased CSRmax in patients with more severe cirrhosis supports the hepatoadrenal syndrome hypothesis. These observations suggest that chronic liver disease is indeed associated with abnormalities of adrenocortical function that are not simply a result of variability in the distributions of CBG and albumin concentrations and their cognate effects on stimulated total cortisol concentration.1,2,4,37,38
We also observed that the rate of free cortisol elimination was reduced in patients with more severe degrees of chronic liver disease, which is consistent with previous reports of reduced cortisol metabolic clearance rate (MCR) and longer cortisol half-life in patients with cirrhosis.1,5,29 The mechanism of impaired metabolic elimination of free cortisol in patients with cirrhosis appears to be related to decreased activity of hepatic A-ring reductases (5-α- and 5-β-reductase)2,3,39 as well as decreased hepatic blood flow.5 A similar mechanism involving reduced expression and activity of 5-α-reductase and 5-β-reductase, as well as decreased 11-β-hydroxysteroid dehydrogenase 2 (11-HSD-2) activity, has been linked to reduced MCRs of cortisol in patients without cirrhosis but with critical illness and sepsis.24 In that study, measures of A-ring reductase activities were inversely related to serum levels of total bile acids,24 suggesting a putative mechanism or indicator of impaired metabolic elimination of cortisol that may be applicable as well to patients with chronic liver disease. We did not measure serum bile acid concentrations in the present study, but bile acid concentrations were reported to be increased according to severity of chronic liver disease in previous studies.40,41
Considering the mechanism for decreased CSRmax in chronic liver disease, in both animal and human studies,4,37,42 it was indicated that conditions associated with decreased rates of cortisol clearance can result in a secondary compensatory reduction in CSR. The reduction in baseline rates of cortisol production in patients with cirrhosis is viewed as adaptive, as under conditions where rates of free cortisol elimination are diminished, commensurately lower rates of cortisol secretion are needed to achieve comparable free cortisol concentrations.[1], [2], [3],38 Our observation of a significant positive correlation between free cortisol elimination rate constant and CSRmax (Fig. 3) is also consistent with studies in critically ill patients with cirrhosis, suggesting that reduced rates of cortisol clearance may have a causal role in the adaptive reduction in cortisol secretory capacity.24 Hypothyroidism is another example of HPA axis adaptation to chronically decreased rates of cortisol clearance in which adrenocortical hypofunction has been well documented.43 For example, CRT is recommended during treatment of myxoedema coma, as thyroid hormone replacement therapy in this setting results in rapid normalisation of cortisol MCR, whereas the compensatory increase in cortisol secretion may lag.44
Other potential mechanisms for adrenocortical function abnormalities in patients with cirrhosis have been proposed in the literature.45 These include decreased HDL concentrations, potentially impacting delivery of the steroidogenic precursor cholesterol to adrenocortical cells.23,46,47 Our finding of a positive correlation between HDL concentrations and CSRmax (Table 2) is consistent with previous reports suggesting this possibility.23,46,47 There is also evidence that cytokines and other mediators of inflammation may affect ACTH receptor expression as well as the dose–response relationship between ACTH concentration and CSR.8,24 A similar shift in the dose–response relationship between ACTH concentration and CSR has been observed as well in patients with SAI.15 In the present study, our finding that the association between CRP and CSRmax was driven by higher CRP concentrations in the group with sepsis is consistent with other reports of increased CSR in sepsis.7,24
In the present study, we observed CSRmax of 0.28 nM/s (95% CI 0.24–0.34) in patients with severe cirrhosis (sepsis excluded), which is intermediate to levels previously reported in healthy controls (0.44 nM/s) and patients with SAI (0.17 nM/s).15,25 Although we did not measure ACTH concentrations in the current study, significant reductions in plasma ACTH concentrations in more severe stages of cirrhosis have been previously reported48 and ACTH concentrations were not elevated in patients with acutely decompensated cirrhosis.23 These considerations, including normal or low ACTH concentrations, modest rather than marked decrease in CSRmax, and proposed pathogenesis involving reduced cortisol clearance, favour classification of adrenocortical function abnormalities associated with cirrhosis as a form of SAI rather than PAI.15,32,33
In considering the potential clinical implications of adrenocortical function abnormalities and treatment effects of CRT in patients with cirrhosis, it is useful to distinguish between alterations in adrenocortical function that develop during chronic liver failure in patients who are clinically stable and those that develop acutely as a result of concomitant illness such as sepsis, organ failure, and acute-on-chronic liver failure (ACLF). For purposes of discussion, we use the term baseline adrenocortical function to refer to integrated functionality of the HPA axis during periods of clinical stability and absent concurrent critical illness.
When patients with cirrhosis become acutely ill, multiple and complex mechanisms affecting the HPA axis evidently occur, as summarised in the concepts of critical illness-associated corticosteroid insufficiency (CIRCI).45,49 These HPA axis adaptations to critical illness are likely applicable to both patients without and with cirrhosis, but they are overlaid on different backgrounds of baseline adrenocortical function that may result in differential risk for cortisol deficiency and differential treatment effects of CRT. Our finding of reduced CSRmax provides additional evidence of subnormal baseline adrenocortical function in patients with chronic liver disease. These observations support the clinically relevant but as yet unproven proposition of the hepatoadrenal syndrome hypothesis that patients with cirrhosis may be at greater risk for relative cortisol deficiency and experience different treatment effects of CRT than may patients without cirrhosis in comparable, defined clinical settings of critical illness.
Arabi et al.34 conducted an RCT of CRT in patients with end-stage liver disease and concurrent sepsis or septic shock, which demonstrated significant, early benefit of CRT on important clinical outcomes, including (i) shock reversal, (ii) norepinephrine infusion rate, and (iii) intra-abdominal pressure. However, the study was considered a ‘negative clinical trial’ insofar as it was discontinued prematurely owing to lack of benefit of CRT on the primary study outcome (28-day mortality),34 which was driven largely by recurrence of shock after cessation of CRT. These considerations, in conjunction with the observation that Δ-cortisol <248 nmol/L was a significant and robust predictor of haemodynamic response to CRT, raise the possibility that a common factor, intrinsic adrenal hypofunction, was responsible for both the early, significant benefit of CRT and the later recurrence of shock after cessation of CRT.34 Arabi et al.34 reached a similar conclusion, stating ‘it is possible that relative adrenal insufficiency is inherent in patients with cirrhosis and not a temporary sepsis-related phenomenon’. An RCT assessing the treatment effects of CRT in hospitalised patients with hypotension and cirrhosis is currently in progress (Supplemental Corticosteroids in Cirrhotic Hypotensive Patients With Suspicion of Sepsis [SCOTCH], NCT 02602210), and such data are urgently needed to better understand the role of CRT in patients with cirrhosis during concomitant critical illness.
Interestingly, the cut score for Δ-cortisol (increment of total cortisol above baseline 60 min after stimulation with ACTH1-24 0.25 mg) <248 nmol/L was developed in non-cirrhotic patients with septic shock on the basis of its ability to predict treatment effect of CRT on 28-day mortality.50 In hospitalised patients with cirrhosis, relative adrenal insufficiency (RAI), defined by Δ-cortisol <248 nmol/L, is commonly observed, and among patients with ACLF, RAI was a significant and independent predictor of 90-day mortality.23 However, in studies of patients without cirrhosis but with septic shock, the use of the Δ-cortisol parameter for prediction of treatment effect of CRT is no longer recommended precisely because of difficulty in reproducing the finding in follow-on studies.51 As shown in our STB analysis (Table 4), Δ-cortisol, as it applies to the study population with cirrhosis, is a ‘composite’ measure influenced by several discrete factors. The multivariate interaction between the various factors that contribute to Δ-cortisol and the heterogeneity of their distributions in different study populations may account for the observed variation in the ability of the Δ-cortisol parameter to predict treatment effects of CRT across different clinical trials.52
There are several limitations to our study, including absence of a control group and post hoc analytic study design.11 The lack of a control group necessitated primary comparisons between more and less severe stages of chronic liver disease; historical data were used for indirect comparisons between populations with cirrhosis and control and SAI. As the analysis of cortisol appearance and elimination rates was post hoc, the results should be confirmed in follow-up investigations that include control participants and a cortisol sampling schedule prospectively designed for numerical and modelling analysis. An additional limitation is that the numerical method does not distinguish adrenal vs. extra-adrenal contributions to cortisol appearance rate.53 For example, in stable isotope dilution studies, hepatic 11-HSD1-mediated reduction of cortisone was identified as a source of extra-adrenal cortisol.54 Therefore, we cannot exclude the possibility that our finding of subnormal levels of CSRmax in patients with cirrhosis was, in part, attributable to reduced hepatic 11-HSD1 activity.
The exclusion of a significant proportion (7/27, 26%) of patients with concomitant sepsis from our analysis was a potential source of selection bias. If the excluded patients were the sickest, as suggested by significantly higher baseline total cortisol concentrations (see Supplementary data), this selection bias may have contributed to underestimation of severity of adrenocortical hypofunction in patients with cirrhosis and concomitant sepsis and limited generalisability of our data in the group with sepsis.
In summary, we conclude that (i) CSRmax is significantly reduced in patients having more severe cirrhosis, (ii) free cortisol elimination rate is significantly decreased in patients with more severe cirrhosis, (iii) CSRmax obtained using numerical methods provides a measure of adrenocortical function that is independent of variation in cortisol-binding protein concentrations, whereas both stimulated total cortisol and Δ-cortisol were affected by variation in CBG and albumin concentrations, and (iv) Δ-cortisol, historically applied in the definition of RAI, is a composite endpoint influenced by several identifiable and measurable prediction variables, potentially limiting its specificity as a measure of adrenocortical hypofunction. Our results suggest that the pre-test probability of subnormal baseline adrenocortical function is higher in patients with cirrhosis and higher still in patients with more severe manifestations of chronic liver disease. It remains to be determined, preferably on the basis of well-powered RCT studies like those recently completed for patients without cirrhosis but with septic shock,35,51 whether CRT has a useful role in the management of patients with cirrhosis during episodes of acute clinical decompensation and critical illness.
Financial support
This study is the result of work supported with resources and the use of facilities at the New Mexico VA Healthcare System facility in Albuquerque, NM, USA.
Authors’ contributions
Study concept: CML, RID, QRQ, FKU. Experiments: TT, SB, VDM. Numerical and modelling analysis: CML, RID, QRQ, FKU. Writing of article: CML, TT, SB, VDM, QRQ, FKU, RID
Data availability statement
De-identified data from this analysis may be made available by contacting the first author (CML) or the corresponding author (RID).
Conflict of interest
The authors have no conflicts of interest to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100277.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Englert E.J.r.., Brown H., Wallach S., Simons E.L. Metabolism of free and conjugated 17-hydroxycorticosteroids in subjects with liver disease. J Clin Endocrinol Metab. 1957;17:1395–1406. doi: 10.1210/jcem-17-12-1395. [DOI] [PubMed] [Google Scholar]
- 2.Peterson R.E. Adrenocortical steroid metabolism and adrenal cortical function in liver disease. J Clin Invest. 1960;39:320–331. doi: 10.1172/JCI104043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson R.E., Wyngaarden J.B. The physiological disposition and metabolic fate of hydrocortisone in man. Ann N Y Acad Sci. 1955;61:297–305. doi: 10.1111/j.1749-6632.1955.tb42479.x. [DOI] [PubMed] [Google Scholar]
- 4.Urquhart J., Yates F.E., Herbst A.L. Hepatic regulation of adrenal cortical function. Endocrinology. 1959;64:816–830. doi: 10.1210/endo-64-5-816. [DOI] [PubMed] [Google Scholar]
- 5.Kawai S., Ichikawa Y., Homma M. Differences in metabolic properties among cortisol, prednisolone, and dexamethasone in liver and renal diseases: accelerated metabolism of dexamethasone in renal failure. J Clin Endocrinol Metab. 1985;60:848–854. doi: 10.1210/jcem-60-5-848. [DOI] [PubMed] [Google Scholar]
- 6.Cope C.L., Black E. The production rate of cortisol in man. Br Med J. 1958;1:1020–1024. doi: 10.1136/bmj.1.5078.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorin R.I., Qualls C.R., Torpy D.J., Schrader R.M., Urban F.K., III Reversible increase in maximal cortisol secretion rate in septic shock. Crit Care Med. 2015;43:549–556. doi: 10.1097/CCM.0000000000000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fede G., Spadaro L., Tomaselli T., Privitera G., Germani G., Tsochatzis E. Adrenocortical dysfunction in liver disease: a systematic review. Hepatology. 2012;55:1282–1291. doi: 10.1002/hep.25573. [DOI] [PubMed] [Google Scholar]
- 9.Karagiannis A.K., Nakouti T., Pipili C., Cholongitas E. Adrenal insufficiency in patients with decompensated cirrhosis. World J Hepatol. 2015;7:1112–1124. doi: 10.4254/wjh.v7.i8.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marik P.E., Gayowski T., Starzl T.E. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit Care Med. 2005;33:1254–1259. doi: 10.1097/01.ccm.0000164541.12106.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thevenot T., Borot S., Remy-Martin A., Sapin R., Cervoni J.P., Richou C. Assessment of adrenal function in cirrhotic patients using concentration of serum-free and salivary cortisol. Liver Int. 2011;31:425–433. doi: 10.1111/j.1478-3231.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 12.Simard M., Underhill C., Hammond G.L. Functional implications of corticosteroid-binding globulin N-glycosylation. J Mol Endocrinol. 2018;60:71–84. doi: 10.1530/JME-17-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton J.L., Hayward C., Direk N., Lewis J.G., Hammond G.L., Hill L.A. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. Plos Genet. 2014;10 doi: 10.1371/journal.pgen.1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bright G.M., Darmaun D. Corticosteroid-binding globulin modulates cortisol concentration responses to a given production rate. J Clin Endocrinol Metab. 1995;80:764–769. doi: 10.1210/jcem.80.3.7883828. [DOI] [PubMed] [Google Scholar]
- 15.Dorin R.I., Qiao Z., Bouchonville M., Qualls C.R., Schrader R.M., Urban F.K., III Characterization of cortisol secretion rate in secondary adrenal insufficiency. J Endocr Soc. 2017;1:945–956. doi: 10.1210/js.2017-00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arafah B.M. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–3745. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 17.Galbois A., Rudler M., Massard J., Fulla Y., Bennani A., Bonnefont-Rousselot D. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010;52:839–845. doi: 10.1016/j.jhep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Hamrahian A.H., Oseni T.S., Arafah B.M. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 19.Thevenot T., Borot S., Remy-Martin A., Sapin R., Penfornis A., Di Martino V. Assessing adrenal function in cirrhotic patients: is there a reliable test? Gastroenterol Clin Biol. 2009;33:584–588. doi: 10.1016/j.gcb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Fede G., Spadaro L., Tomaselli T., Privitera G., Piro S., Rabuazzo A.M. Assessment of adrenocortical reserve in stable patients with cirrhosis. J Hepatol. 2011;54:243–250. doi: 10.1016/j.jhep.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Fede G., Spadaro L., Tomaselli T., Privitera G., Scicali R., Vasianopoulou P. Comparison of total cortisol, free cortisol, and surrogate markers of free cortisol in diagnosis of adrenal insufficiency in patients with stable cirrhosis. Clin Gastroenterol Hepatol. 2014;12:504–512. doi: 10.1016/j.cgh.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Thevenot T., Dorin R., Monnet E., Qualls C.R., Sapin R., Grandclement E. High serum levels of free cortisol indicate severity of cirrhosis in hemodynamically stable patients. J Gastroenterol Hepatol. 2012;27:1596–1601. doi: 10.1111/j.1440-1746.2012.07188.x. [DOI] [PubMed] [Google Scholar]
- 23.Piano S., Favaretto E., Tonon M., Antonelli G., Brocca A., Sticca A. Including relative adrenal insufficiency in definition and classification of acute-on-chronic liver failure. Clin Gastroenterol Hepatol. 2020;18 doi: 10.1016/j.cgh.2019.09.035. 1188–96.e3. [DOI] [PubMed] [Google Scholar]
- 24.Boonen E., Vervenne H., Meersseman P., Andrew R., Mortier L., Declercq P.E. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–1488. doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorin R.I., Qiao Z., Qualls C.R., Urban F.K., III Estimation of maximal cortisol secretion rate in healthy humans. J Clin Endocrinol Metab. 2012;97:1285–1293. doi: 10.1210/jc.2011-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keenan D.M., Roelfsema F., Veldhuis J.D. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab. 2004;287:E652–E661. doi: 10.1152/ajpendo.00167.2004. [DOI] [PubMed] [Google Scholar]
- 27.McCann V.J., Fulton T.T. Cortisol metabolism in chronic liver disease. J Clin Endocrinol Metab. 1975;40:1038–1044. doi: 10.1210/jcem-40-6-1038. [DOI] [PubMed] [Google Scholar]
- 28.Zumoff B., Bradlow H.L., Gallagher T.F., Hellman L. Cortisol metabolism in cirrhosis. J Clin Invest. 1967;46:1735–1743. doi: 10.1172/JCI105664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown H., Willardson D.G., Samuels L.T., Tyler F.H. 17-Hydroxycorticosteroid metabolism in liver disease. J Clin Invest. 1954;33:1524–1532. doi: 10.1172/JCI103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonen E., Meersseman P., Vervenne H., Meyfroidt G., Guiza F., Wouters P.J. Reduced nocturnal ACTH-driven cortisol secretion during critical illness. Am J Physiol Endocrinol Metab. 2014;306:E883–E892. doi: 10.1152/ajpendo.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbison B., Keenan D.M., Roelfsema F., Evans J., Phillips K., Rogers C.A. Dynamic pituitary–adrenal interactions in the critically ill after cardiac surgery. J Clin Endocrinol Metab. 2020;105:1327–1342. doi: 10.1210/clinem/dgz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bornstein S.R., Allolio B., Arlt W., Barthel A., Don-Wauchope A., Hammer G.D. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorin R.I., Qualls C.R., Crapo L.M. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 34.Arabi Y.M., Aljumah A., Dabbagh O., Tamim H.M., Rishu A.H., Al-Abdulkareem A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971–1977. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatesh B., Finfer S., Cohen J., Rajbhandari D., Arabi Y., Bellomo R. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 36.Bosch J., Thabut D., Albillos A., Carbonell N., Spicak J., Massard J. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology. 2008;47:1604–1614. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- 37.Yates F.E., Urquhart J., Herbst A.L. Impairment of the enzymatic inactivation of adrenal cortical hormones following passive venous congestion of the liver. Am J Physiol. 1958;194:65–71. doi: 10.1152/ajplegacy.1958.194.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Dorin R.I., Urban F.K., III, Qualls C.R. Letter to the Editor: prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J Clin Endocrinol Metab. 2021;106:e393–e394. doi: 10.1210/clinem/dgaa709. [DOI] [PubMed] [Google Scholar]
- 39.Peterson R.E. Metabolism of adrenocorticosteroids in man. Ann N Y Acad Sci. 1959;82:846–853. doi: 10.1111/j.1749-6632.1960.tb44966.x. [DOI] [PubMed] [Google Scholar]
- 40.Islam S., Poupon R.E., Barbare J.C., Chretien Y., Darnis F., Poupon R. Fasting serum bile acid level in cirrhosis. a semi-quantitative index of hepatic function. J Hepatol. 1985;1:609–617. doi: 10.1016/s0168-8278(85)80004-0. [DOI] [PubMed] [Google Scholar]
- 41.de Caestecker J.S., Jazrawi R.P., Nisbett J.A., Joseph A.E., Maxwell J.D., Northfield T.C. Direct assessment of the mechanism for a raised serum bile acid level in chronic liver disease. Eur J Gastroenterol Hepatol. 1995;7:955–961. doi: 10.1097/00042737-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Christy N.P., Wallace E.Z., Gordon W.E., Jailer J.W. On the rate of hydrocortisone clearance from plasma in pregnant women and in patients with Laennec's cirrhosis. J Clin Invest. 1959;38:299–305. doi: 10.1172/JCI103802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon G.G., Southren A.L. Thyroid-hormone effects on steroid-hormone metabolism. Bull N Y Acad Med. 1977;53:241–259. [PMC free article] [PubMed] [Google Scholar]
- 44.Jonklaas J., Bianco A.C., Bauer A.J., Burman K.D., Cappola A.R., Celi F.S. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annane D., Pastores S.M., Arlt W., Balk R.A., Beishuizen A., Briegel J. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a multispecialty task force of the society of critical care medicine (SCCM) and the European society of intensive care medicine (ESICM) Intens Care Med. 2017;43:1781–1792. doi: 10.1007/s00134-017-4914-x. [DOI] [PubMed] [Google Scholar]
- 46.Marik P.E. Adrenal-exhaustion syndrome in patients with liver disease. Intens Care Med. 2006;32:275–280. doi: 10.1007/s00134-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 47.Cicognani C., Malavolti M., Morselli-Labate A.M., Zamboni L., Sama C., Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157:792–796. [PubMed] [Google Scholar]
- 48.Zhang J., Yu H.-W., Li Y.-K., Wang K.-F., Jia L., Meng Q.-H. Reduced cortisol in the absence of bacterial infection in patients with hepatitis B virus cirrhosis. Genet Mol Res. 2015;14:7957–7963. doi: 10.4238/2015.July.17.3. [DOI] [PubMed] [Google Scholar]
- 49.Bornstein S.R. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360:2328–2329. doi: 10.1056/NEJMra0804635. [DOI] [PubMed] [Google Scholar]
- 50.Annane D., Sebille V., Charpentier C., Bollaert P.E., Francois B., Korach J.M. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 51.Annane D., Renault A., Brun-Buisson C., Megarbane B., Quenot J.P., Siami S. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 52.Annane D., Pastores S.M., Rochwerg B., Arlt W., Balk R.A., Beishuizen A. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intens Care Med. 2017;43:1751–1763. doi: 10.1007/s00134-017-4919-5. [DOI] [PubMed] [Google Scholar]
- 53.Basu R., Basu A., Grudzien M., Jung P., Jacobson P., Johnson M. Liver is the site of splanchnic cortisol production in obese nondiabetic humans. Diabetes. 2009;58:39–45. doi: 10.2337/db08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stimson R.H., Andrew R., McAvoy N.C., Tripathi D., Hayes P.C., Walker B.R. Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes. 2011;60:720–725. doi: 10.2337/db10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data from this analysis may be made available by contacting the first author (CML) or the corresponding author (RID).