Abstract
Purpose
Lipocalin 2 (LCN2) is an adipokine involved in many physiological functions, including bone metabolism. We previously demonstrated its implication in mouse models of mechanical unloading-induced osteoporosis and in a cohort of bed rest volunteers. We therefore aimed at studying its involvement in postmenopausal osteoporosis.
Methods
We measured serum LCN2 and correlated its levels to Dickkopf WNT Signaling Pathway Inhibitor 1 (DKK1), Tartrate Resistant Acid Phosphatase 5B (TRAcP5B), sclerostin, urinary N-terminal telopeptide of type I collagen (NTX), serum C-terminal telopeptide of type I collagen (CTX), parathyroid hormone and vitamin K by ELISA performed in a cohort of younger (50–65 years) and older (66–90 years) osteoporotic women in comparison to healthy subjects. A cohort of male healthy and osteoarthritic patients was also included. Sobel mediation analysis was used to test indirect associations among age, LCN2 and DKK1 or NTX.
Results
LCN2 levels were unchanged in osteoporotic and in osteoarthritis patients when compared to healthy subjects and did not correlate with BMD. However, serum LCN2 correlated with age in healthy women (R = 0.44; P = 0.003) and men (R = 0.5; P = 0.001) and serum concentrations of DKK1 (R = 0.47; P = 0.003) and urinary NTX (R = 0.34; P = 0.04). Sobel mediation analysis showed that LCN2 mediates an indirect relationship between age and DKK1 (P = 0.02), but not with NTX, in healthy subjects.
Conclusions
Taken together, the results suggest a hitherto unknown association between LCN2, DKK1 and age in healthy individuals, but not in postmenopausal osteoporotic women.
Abbreviations: BALP, bone-specific alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; CTX, C-terminal telopeptide of type I collagen; DKK1, Dickkopf WNT Signaling Pathway Inhibitor 1; IL, interleukin; LCN2, lipocalin 2; NfκB, nuclear factor kappa-B; NTX, N-terminal telopeptide of type I collagen; PTH, parathyroid hormone; RANKL, receptor activator of nuclear factor kappa-B; TNF, tumor necrosis factor; TRAcP5B, tartrate-resistant acid phosphatase 5B
Keywords: Osteoporosis, Osteoarthritis, Lipocalin-2, NGAL, DKK1, Wnt
1. Introduction
Lipocalin 2 (LCN2) is a small secreted glycoprotein, often defined as adipokine, which has been implicated in many physiological and pathological functions (Kjeldsen et al., 1993). Its role in the musculoskeletal system is not yet fully understood. LCN2 responds to reduced mechanical stimulation in both bone and muscle and is upregulated under mechanical unloading in mouse models as well as in humans (Capulli et al., 2009; Rucci et al., 2015; Gambara et al., 2017). Surprisingly, its absence is also a negative determinant of bone health, acting indirectly on osteoblasts through the modulation of energy metabolism (Capulli et al., 2018). Importantly, LCN2 overexpression stimulates the production of RANKL and IL-6, resulting in increased osteoclastogenesis and reduced osteoblast differentiation (Rucci et al., 2015). Moreover, high LCN2 levels have been shown to predict fracture-related hospitalization in elderly women (Lim et al., 2015). This knowledge, together with our experimental observations (Capulli et al., 2009; Rucci et al., 2015; Capulli et al., 2018), prompted us to investigate its possible implication in human bone pathology.
Postmenopausal osteoporosis is a generalized skeletal disease characterised by progressive bone loss, accompanied also by increased bone marrow adiposity, associated with a reduction of its osteogenic potential (Kawai et al., 2012). We compared two groups of osteoporotic female patients, one younger (age 50–65) and another older (age 66–90), with healthy age-matched women. Furthermore, since LCN2 was found to be increased in the synovial fluid of osteoarthritic knees (Gupta et al., 2007), we investigated if changes in circulating LCN2 could be detected in both osteoarthritic males and females compared to control subjects.
Our results show that LCN2 serum levels are unchanged in osteoporotic vs healthy women and did not differ between osteoarthritic and healthy subjects. However, we show that LCN2 is positively correlated with age in healthy women and men, but not in osteoporotic women. Moreover, in healthy women LCN2 serum levels correlate with PTH, vitamin K and urinary NTX. LCN2 also correlates with DKK1 in healthy women and, using the Sobel mediation analysis, we show that LCN2 mediates an indirect correlation between age and DKK1.
2. Materials and methods
2.1. Patient characteristics
The present study includes healthy, osteoporotic and osteoarthritic men and women arising from two separate cohorts of patients and healthy subjects.
The “Oslo Cohort” comprised healthy, osteoporotic (Table I), and osteoarthritic (Table II) men and women. All healthy participants had BMD T-score > −1. Bone densitometry was performed by DXA, using a Lunar Prodigy DF + 12649 densitometer with the Lunar iDXA encore software, version 12.30 (GE Medical Systems, Madison, USA). The osteoporotic patients had established osteoporosis according to the WHO's definition, with at least one fragility fracture (also see legend to Table I) and/or vertebral compression causing height loss >3.5 cm diagnosed by conventional X-ray examination, DXA-scans or a few self-reported. Also, fragility non-vertebral fractures (costa, wrist, femoral neck) were included. Reliable information regarding number of fractures per patient at the time of inclusion or total fractures in the group, is not available. Participants were recruited consecutively at Lovisenberg Diaconal Hospital outpatient clinic in Oslo (Norway), through advertisements in newspapers, or as patients selected for hip replacement, as described (Gautvik et al., 2020). Serum samples were collected in the morning from fasting individuals. The donors did not include persons with secondary diseases or taking medication known to affect bone metabolism. The study was approved by the Norwegian Regional Ethical Committee (REK no: 2010/2539) and conducted according to the Declaration of Helsinki. Verbal and written informed consents were administered to all participants in the study (Gautvik et al., 2020).
Table I.
Clinical characteristics and serum bone turnover biomarkers of healthy and osteoporotic women analysed in this study – partially reported in Jemtland et al. (Jemtland et al., 2011).
| Healthy | Osteoporosisx | |
|---|---|---|
| Age (years) | 62.48 ± 7.77 (44) | 70.53 ± 7.74⁎⁎⁎ (29) |
| Weight (kg) | 70.23 ± 10.25 (44) | 62.99 ± 11.24⁎⁎ (29) |
| BMI (Kg/m2) | 25.09 ± 3.43 (44) | 23.3 ± 3.61⁎ (29) |
| L1-L4 T-score | 0.25 ± 0.86 (41) | −3.35 ± 0.78⁎⁎⁎ (24) |
| L1-L4 Z-score | 1.27 ± 0.89 (41) | −1.70 ± 0.66⁎⁎⁎ (24) |
| Skull BMD (g/cm2) | 2.38 ± 0.26 (38) | 1.82 ± 0.32⁎⁎⁎ (29) |
| Total body T-score | 0.90 ± 0.84 (38) | −2.09 ± 0.90⁎⁎⁎ (29) |
| Total body Z-score | 1.72 ± 0.84 (38) | −0.63 ± 1.20⁎⁎⁎ (29) |
| DKK1 (pmol/l) | 33.47 ± 10.79 (39) | 33.78 ± 13.06 (23) |
| Urine NTX(mM) | 49.97 ± 32.52 (34) | 77.00 ± 50.56⁎ (18) |
| TRAcP5B (U/l) | 3.13 ± 1.28 (39) | 3.60 ± 2.06 (23) |
| Sclerostin (ng/ml) | 0.91 ± 0.27 (39) | 0.68 ± 0.18⁎⁎⁎ (23) |
| Subjects with fracture (n) | 2 | 22 |
Data presented as Mean ± SD. Number of subjects in parentheses.
P < 0.05 vs healthy.
P < 0.001 vs healthy.
P = 0.0001 vs healthy.
Patients had at least one fragility fracture and otherwise fulfilled the WHO criteria for “established osteoporosis” with T-score < −2.5 in the spine and/or hip (Reppe et al).
Table II.
Clinical characteristics of healthy and osteoarthritic women and men analysed in this study – partially reported in Jemtland et al. (Jemtland et al., 2011).
| Healthy | Osteoarthritis | |
|---|---|---|
| Female subjects | ||
| Age (years) | 62.48 ± 7.77 (44) | 66.69 ± 10.5 (16) |
| Skull BMD (g/cm2) | 2.38 ± 0.26 (38) | 2.36 ± 0.26 (14) |
| Total body T-score | 0.90 ± 0.84 (38) | 0.62 ± 0.98 (13) |
| Total body Z-score | 1.72 ± 0.84 (38) | 1.22 ± 0.69⁎ (13) |
| Subjects with fracture (n) | 2 | 6 |
| Male subjects | ||
| Age (years) | 69.62 ± 6.01 (21) | 72.82 ± 8.08 (10) |
| Weight (kg) | 88.5 ± 10.37 (21) | 85.01 ± 18.46 (10) |
| BMI (Kg/m2) | 27.46 ± 2.63 (21) | 25.81 ± 4.48 (10) |
| Skull BMD (g/cm2) | 2.21 ± 0.26 (21) | 2.18 ± 0.22 (10) |
| Total body T-score | 1.25 ± 1.04 (21) | 0.89 ± 1.25 (10) |
| Total body Z-score | 1.28 ± 0.83 (21) | 1.29 ± 1.29 (10) |
| Subjects with fracture (n) | ? Not available | 1(?) |
Data presented as Mean ± SD. Number of subjects in parentheses.
P < 0.05 vs healthy.
We also employed the “L'Aquila healthy males cohort”, which includes healthy male volunteers (N = 15, age = 37.27 ± 11.09 years), who were enrolled for another study (manuscript in preparation) involving a bout of high-intensity physical exercise, which consisted in the “Gran Sasso d'Italia Vertical Run”, an on-foot race that took place at high altitude. For the purpose of this work, we exploited the sera harvested before the run, thus representing the basal levels of LCN2 of the partecipants, that were included in the correlations with age. Subjects were given a self-administered informed consent in English or Italian depending on their preferred language. The protocol was approved by Asl (Agenzia Sanitaria Locale) Ethical Committee Milan 1, Milan, Italy (protocol ID: MARC01).
2.2. Materials and detection methods
Human (cat#DLCN20) LCN2 ELISA kit was from R&D (Minneapolis, MN). TRAcP5B, DKK1, and Sclerostin ELISA kits were from TECO Medical AG (Sissach, Switzerland). Urinary NTX was measured using the Osteomark Urine NTx Kit (Wampole Laboratories, Inc., Princeton, NJ, USA). Analyses of BALP and Ca2+ were performed using photometric methods employing the Modular P equipment (Roche) and kits according to manufacturer's instructions. Serum PTH was measured as the intact hormone by the Immulite 2000 two-site chemiluminescent immunometric assay (Siemens, Deerfield, IL). Serum CTX was measured using the “β-CrossLaps/serum” kit by Roche. Serum levels of 25(OH) vitamin D were measured using radioimmunoassay from DiaSorin (Saluggia, Italy). Intact osteocalcin was measured using Luminoimmunanalyzis (BRAHMS, Hennigsdorf, Germany). Vitamin K was measured using the internal standard MK-4 and BHT as an antioxidant and analysis on a SPE-HPLC System with a HP 1100 liquid chromatograph (Agilent Technologies, Palo Alto, CA, USA) with a HP1100 fluorescence detector, emission: 265 nm excitation: 450 nm; vitamin K were electrochemically reduced (−0.75 V) on-line prior to (FLD) detection. Vitamin K's were separated on a 2.1 mm × 50 mm reversed phase column. A two-point calibration curve was made from analysis of a 3% albumin solution enriched with known phylloquinone concentrations. The recovery is >95%, and the method is linear from 0.05–4 μg/L and the limit of detection is 0.01 μg/L. Further technical details on these assays can be found in Reppe et al. (Reppe et al., 2010) and Jemtland et al. (Jemtland et al., 2011).
2.3. Statistical analysis
Correlation analyses were performed using Pearson's correlation test. When comparing two groups, we first performed D'Agostino-Pearson omnibus normality test. If at least one of the distributions was not Gaussian, we performed non-parametric Mann-Whitney test, otherwise we performed Student's t-test. Statistics was performed using Graphpad Prism v7.00 or Systat SigmaPlot v14.00 or Jamovi (v 1.6); a P value ≤0.05 was considered statistically significant.
To create the mediation model among variables, a linear regression analysis was performed to evaluate the relationship between the dependent variable and one (simple linear regression) explanatory variable. For these analyses, R and P values are reported in the text and in the figures. A test of mediation examines whether the effect (c′) of the independent variable (X) on the dependent variable (Y) occurs via a third intervening variable or mediator (M). In this work we tested whether the relationship between two selected variables (X and Y) was mediated by LCN2 (M). Finally, we used the Sobel, Aroian and Goodman tests to explore the significance of mediation effect (Chin et al., 2014; Torres-Costoso et al., 2015; Chan et al., 2014). The mediation models have been adjusted to age and BMI.
3. Results
3.1. LCN2 serum levels are unchanged in postmenopausal osteoporotic and in osteoarthritic patients
Our previous results demonstrated that serum LCN2 significantly increased in human volunteers subjected to bed rest-induced bone loss, while it appeared to be reduced in ovariectomized mice compared to control littermates (Rucci et al., 2015). To clarify the LCN2 involvement in postmenopausal osteoporotic patients, we analysed the serum levels in 44 healthy and 29 women with established postmenopausal osteoporosis with at least one fragility fracture (Table I). Clinical details of this cohort are further described in Jemtland et al. (Jemtland et al., 2011).
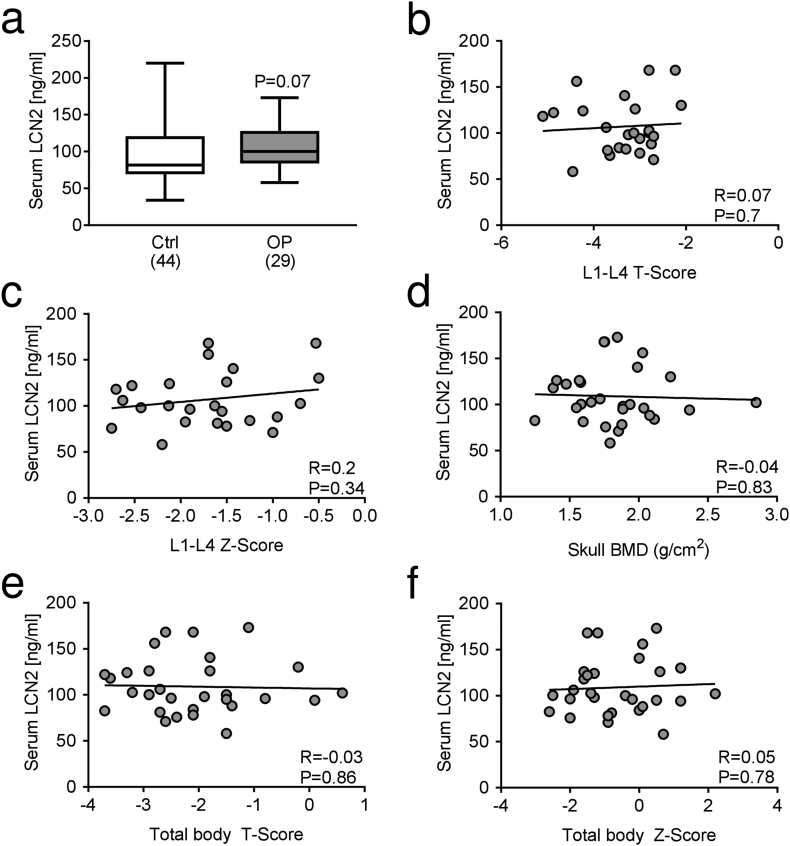
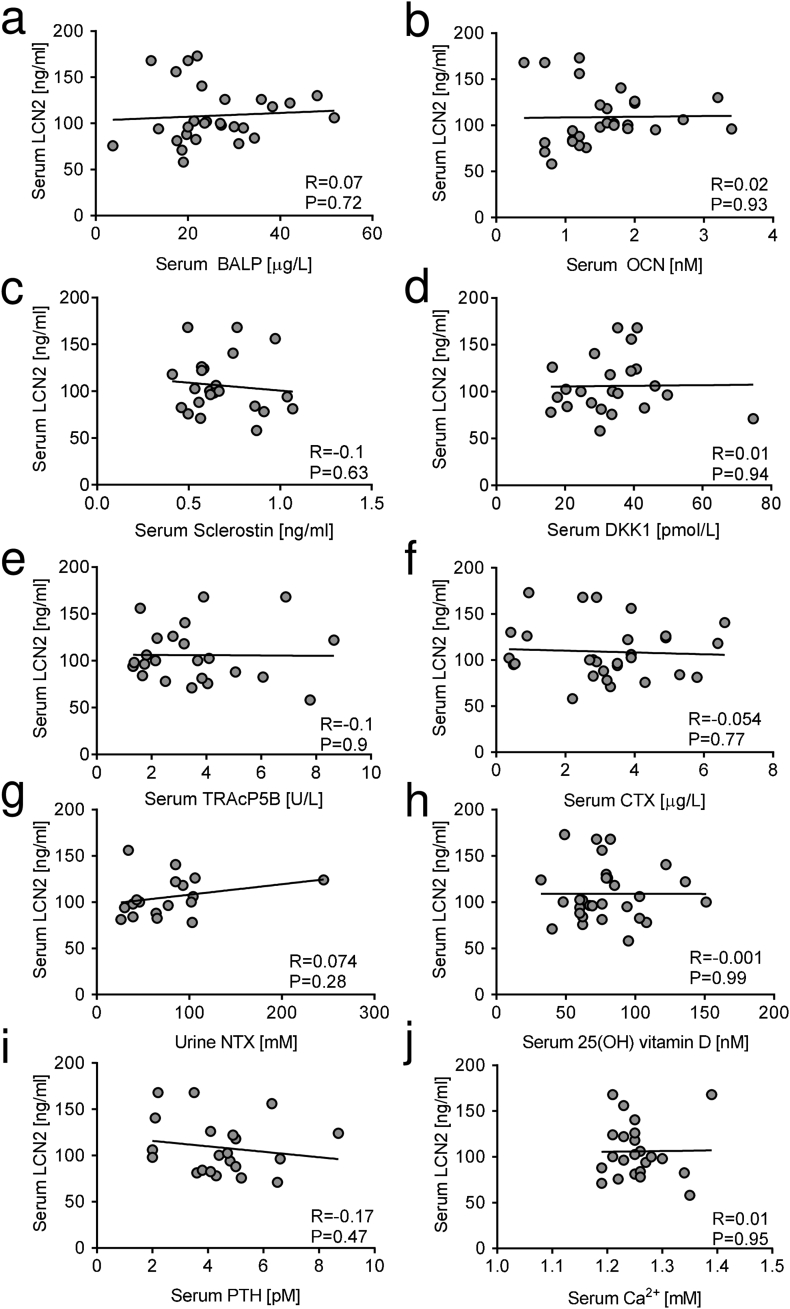
The analysis shows a trend of increase (P = 0.07) of serum LCN2 levels in osteoporotic versus healthy women (Fig. 1a). Moreover, in osteoporotic patients serum LCN2 did not correlate with L1-L4 BMD T- (Fig. 1b) and Z-scores (Fig. 1c), skull BMD (Fig. 1d), total body T- (Fig. 1e) and Z-score (Fig. 1f). Comparison between serum levels of LCN2 and serum or urinary bone turnover biomarkers in osteoporotic women showed no statistical correlations of LCN2 with the osteoblast serum biomarkers BALP (Fig. 2a), OCN (Fig. 2b), Sclerostin (Fig. 2c) and DKK1 (Fig. 2d). The osteoclast serum biomarker TRAcP5B (Fig. 2e), and the resorption biomarkers serum CTX (Fig. 2f) and urinary NTX (Fig. 2g) also did not correlate with LCN2 in osteoporotic women. No correlations were found between LCN2 and serum levels of 25(OH) vitamin D (Fig. 2h), PTH (Fig. 2i) and calcium (Fig. 2j). Finally, no significant correlation was observed between LCN2 serum levels and bone density scores (Suppl. Fig. 1) or serum/urine biomarkers (Suppl. Fig. 2) in healthy women either.
Fig. 1.
LCN2 serum levels in osteoporotic patients and correlation with bone density scores. Measurements of LCN2 levels in sera of (a) osteoporotic (OP) patients compared to healthy subjects (Ctrl); number of subjects is indicated in parentheses. Correlation analyses run between serum LCN2 and L1-L4 vertebrae (b) T- and (c) Z-score, (d) skull BMD, and total body (e) T- and (f) Z-score. (a) Mann-Whitney test. (b-f) Pearson's correlation analysis. P and R values are shown in the graphs.
Fig. 2.
Correlation analysis between LCN2 serum levels and bone turnover biomarkers in osteoporotic patients. Correlation analysis carried out in osteoporotic patients, between serum LCN2 and serum (a) BALP, (b) OCN, (c) Sclerostin, (d) DKK1, (e) TRAcP5B, (f) CTX, (g) urinary NTX, (h) serum 25(OH) vitamin D, (i) PTH and (j) ionized calcium. Pearson's correlation analysis. P and R values are shown in the graphs.
Based on the evidence that LCN2 is increased in the synovial fluid of osteoarthritic knees (Gupta et al., 2007), we evaluated its serum levels in 16 women and 10 men affected by osteoarthritis, but with BMD within normal range. We found no differences compared to healthy subjects (Suppl. Fig. 3).
3.2. LCN2 serum levels increase with age in healthy subjects
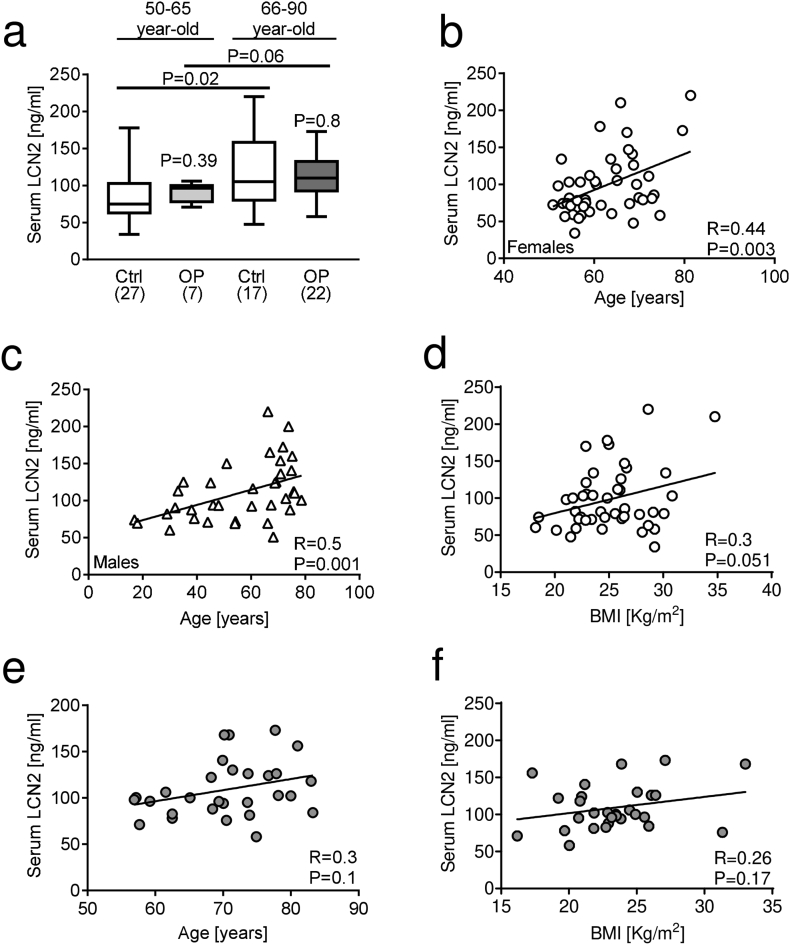
Stratification of patients according to age, i.e. 50–65 and 66–90 years old, to possibly discriminate between early-postmenopausal (50–65) versus late postmenopausal osteoporosis (66–90), also resulted in no significant differences in serum LCN2 levels between either age groups of patients and healthy women. However, a significant increase in LCN2 serum levels was detected in older vs younger healthy women, and a similar trend (P = 0.06) was found in older vs younger osteoporotic women (Fig. 3a).
Fig. 3.
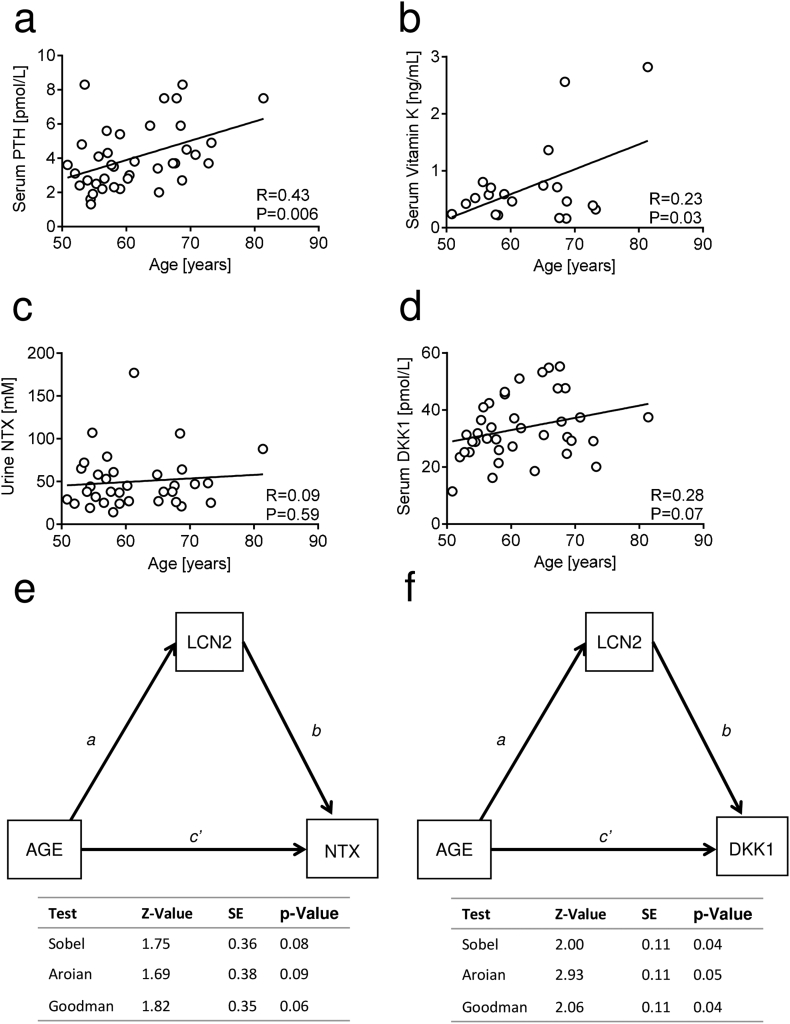
Correlation analysis between LCN2 serum levels and age, weight, and BMI. (a) LCN2 serum levels analysed in healthy and osteoporotic subjects stratified by age (50–65 years vs 66–90 years). Correlation analysis between LCN2 and age in healthy (b) women and (c) men. (d) Correlation analysis between LCN2 serum levels and BMI in healthy women. Correlation analysis between LCN2 serum levels and (e) age an(f) BMI in osteoporotic women. (a) Mann-Whitney test or Student's t-test, (b-f) Pearson's correlation test. P and R values are shown in the graphs.
Consistently, Pearson correlation analysis showed that LCN2 correlated positively with age in healthy females (Fig. 3b) and males (Fig. 3c). Considering that elderly people are prone to lose bone mass due to reduced mobility and/or physical activity, this result is in agreement with our previous data (Rucci et al., 2015) showing the positive correlation between LCN2 and duration of bed rest used as a model of disuse/inactivation osteoporosis. A trend of positive correlation (P = 0.051) was observed between BMI and LCN2 in healthy women (Fig. 3d). However, in osteoporotic women, LCN2 did not show any significant correlation with age (Fig. 3e) or BMI (Fig. 3f).
3.3. Correlation between LCN2 and bone turnover biomarkers in healthy women
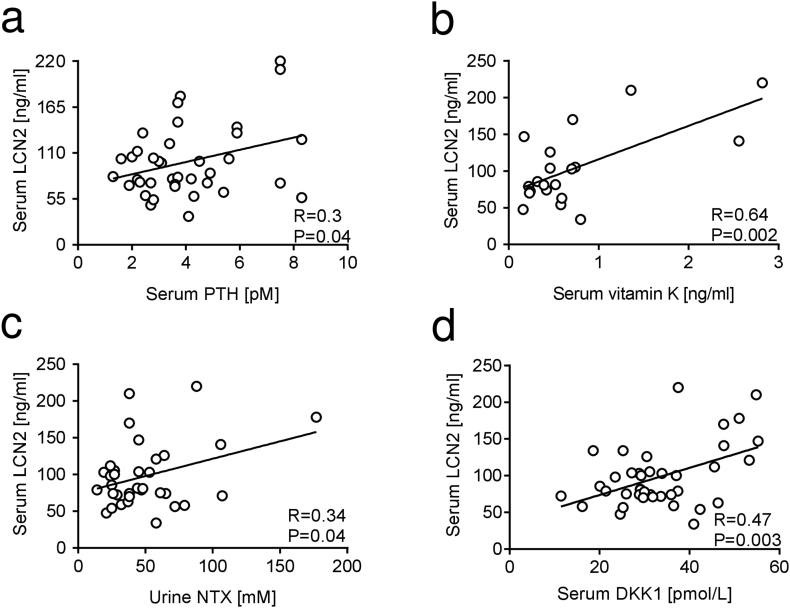
We next evaluated whether there was a correlation between LCN2 and bone turnover biomarkers in healthy women. Among them we also included vitamin K, since recent studies indicate this molecule as a potential therapeutic agent for bone fractures, due to its role as a coenzyme of the carboxylation of osteocalcin and matrix Gla protein (Fusaro et al., 2020; Fusaro et al., 2017). Intriguingly, LCN2 was directly correlated with serum levels of PTH (Fig. 4a), vitamin K (Fig. 4b), urinary NTX (Fig. 4c) and serum DKK1 (Fig. 4d) in healthy women. These relationships were confirmed by regression analyses adjusted for age (Suppl. Fig. 4a-d) and BMI values (Suppl. Fig. 4e-h).
Fig. 4.
Correlation analysis between LCN2 serum levels and bone turnover biomarkers in healthy women. Correlation analyses between serum LCN2 and serum (a) PTH, (b) vitamin K, (c) urinary NTX and (d) serum DKK1 in healthy women. Pearson's correlation test. P and R values are swown in the graphs.
We then aimed at understanding whether these correlations were reliable or confounded by age. Therefore, we performed correlation analyses between age and these variables. PTH showed significant correlation with age (Fig. 5a), and so did vitamin K (Fig. 5b), thus prompting us to conclude that the correlation observed between these factors and LCN2 are likely due to their linear correlation with age, rather than a real interaction with LCN2. Notably, NTX (Fig. 5c) and DKK1 (Fig. 5d) were not correlated with age. To assess whether the interaction observed in our model could be associative, we used three different statistical tests including Sobel, Aroian and Goodman tests to explore the significance of the mediation effects between the different variables. Notably, we could not find a significant indirect relationship between age and NTX when using LCN2 as predictor (Fig. 5e). We next built a mediation model between age and serum DKK1, using serum LCN2 as mediator, finding a statistically significant result (Fig. 5f), thus suggesting that LCN2 could be a mediator relating DKK1 serum levels to ageing. Similar results were observed when we adjusted the mediation analysis for the BMI value (Suppl. Fig. 5).
Fig. 5.
Correlation and mediation analysis among LCN2, PTH, vitamin K, NTX, DKK1 and age. Correlation analyses between age and serum (a) PTH, (b) vitamin K, (c) NTX and (d) serum DKK1. (e,f) Mediation models built to elucidate whether there is a relationship between age and (e) urine NTX or (f) serum DKK1 mediated by LCN2. All relevant parameters, including Z, standard error (SE) and P value are included in the graph and adjusted to age. (a-d) Pearson's correlation. R and P values are included in the graphs.
Taken together these results demonstrate that the LCN2 serum level increases with age and correlates with urinary NTX and DKK1. Moreover, LCN2 appears to mediate an indirect relationship between age and DKK1.
4. Discussion
The main finding of this study is a clear correlation between serum levels of LCN2 and age. Furthermore, serum LCN2 correlates with urinary NTX and with serum levels of DKK1 in healthy women and is possibly also indirectly involved in the relationship between DKK1 and ageing. A few earlier reports have already indicated the correlation between serum LCN2 and age but having different study designs. A first study found that serum LCN2 was related to age in patients undergoing elective percutaneous coronary intervention (Bachorzewska-Gajewska et al., 2006), while Bennet et al. observed, in a healthy paediatric population cohort, that urine LCN2 was significantly higher in the 10–<15 year and 15–<18 year age groups compared to 3–<5 years age group (Bennett et al., 2015). Consistent with our results, a similar correlation was observed in the sera of 30 volunteers, stratified in three groups of age: 18–22, 38–42 and 58–62 years, and the authors suggested a possible role for LCN2 in cell senescence (Bahmani et al., 2014). Our work, although confirmatory, allows to demonstrate this correlation in a larger cohort of healthy individuals stratified non only for age but also for gender.
LCN2 is a potential biomarker of inflammatory and metabolic conditions (Abella et al., 2015). In bone, its overexpression impairs the differentiation of bone-forming cells (osteoblasts) and increases their production of IL-6 and RANKL, eventually leading to increased osteoclastogenesis (Rucci et al., 2015). However, the role of LCN2 in bone is quite complex, with reports from another group showing that this protein inhibits in vitro RANKL-mediated osteoclastogenesis (Kim et al., 2015), and its absence promotes proliferation and differentiation of osteoclast precursors (Kim et al., 2016). On the other hand, Costa and colleagues (Costa et al., 2013) showed that Lcn2-transgenic mice, with high circulating levels of Lcn2, have increased levels of IL6 and osteoclasts. Moreover, LCN2 is a known IL-17, TNF-α, NfĸB and IL-1β-responsive gene (Veeriah et al., 2016; Zhao et al., 2014; Sommer et al., 2009), confirming its tight link with inflammation. Indeed, this association between inflammation and LCN2 may explain the correlation between this factor and ageing. In fact, a novel concept, termed “senoinflammation” (Chung et al., 2019) states that ageing is characterised by a chronic inflammatory state. Inflammatory cytokines involved in this aspect include IL-6, IL-1β and TNF-α, and LCN2 may be part of this chronic inflammatory signature correlated to ageing.
Another recent cross-sectional study by Liu and colleagues (Liu et al., 2018) carried out on 1012 outpatients, investigated the relationship between LCN2 and bone turnover biomarkers in healthy women. In this report, LCN2 was found to be positively correlated with serum CTX. Liu and colleagues also did not find any difference in LCN2 serum levels between women with or without osteoporotic fractures. The latter aspect is in line with our results, although our data was collected from a lower number of subjects. In fact, we did not find any evidence of an association between LCN2 serum levels neither with postmenopausal osteoporosis nor with osteoarthritis, two medical conditions where inflammatory processes are involved (Mundy, 2007; López-Otín et al., 2013).
Another prospective study by Lim et al. (Lim et al., 2015), carried out on 1009 osteoporosis patients over 70 years of age, showed that there was 30% multivariable-adjusted increase in the risk of any osteoporotic fracture per standard deviation increase in LCN2 serum levels. Although this may not seem in line with our work, the low power, and the difference in the composition of the cohorts may have been significant determinants of the discrepancies between Lim's and our report. Furthermore, Lim also found a positive correlation between LCN2 and age in osteoporotic women, which we were not able to detect.
Osteoarthritis involves one or a few of the major joints where cartilage is destroyed with secondary inflammatory responses also extending to the adjacent bone and it has been reported that LCN2 is increased in osteoarthritic synovial fluid. However, our study mitigates the relevance to use LCN2 as an osteoarthritis serum biomarker.
During ageing, people tend to show reduced mobility, eventually leading to a predisposition to gender-independent disuse osteoporosis. Moreover, one of the hallmarks of ageing is cell senescence (López-Otín et al., 2013), a phenomenon through which, following telomere attrition or replicative stress, cells stop their cell cycle permanently and change their phenotype, expressing specific markers such as β-galactosidase and inflammatory cytokines (Kuilman et al., 2010). This, together with the chronic/degenerative diseases which are also associated with ageing, increases the LCN2-activating transcription factor NfĸB (Poynter and Daynes, 1998; Tilstra et al., 2011), possibly contributing to our observations. Notably, Bahmani and colleagues suggested that, after senescence-inducing treatments, LCN2 expression is increased in HEK-293 cells (Bahmani et al., 2015) and in mesenchymal stem cells (Bahmani et al., 2014) in vitro, and that its overexpression can reduce the effects of cellular senescence. In their report, they also show a direct correlation between human ageing and serum levels of LCN2, consistent with our findings (Bahmani et al., 2014).
The positive correlation between DKK1 and LCN2, and between NTX and LCN2 in healthy women is also a de novo finding and links LCN2 to these important negative determinants of bone mass, the former impairing bone formation, which is also a possible therapeutic target for osteoporosis (Roux, 2010), and the latter being a biomarker of bone resorption. We used a statistical mediation analysis to test a possible association between LCN2, age and DKK1.While no direct correlation was present between DKK1 and age per se, we found a significant association when considering LCN2 as a predictor. Albeit the finding does not prove an association between DKK1 and LCN2, it is suggestive of a causal relationship between the 3 variables that, however, needs to be confirmed in further studies, especially considering that DKK1 reduces bone accrual, and therefore could represent a determinant of low bone mass (Butler et al., 2011; Florio et al., 2016). Indeed, in another study from our group (manuscript in preparation) we investigated the role of LCN2 following acute high-intensity exercise in a healthy male cohort, finding a significant upregulation of serum LCN2 and DKK1, which also directly correlated, thus supporting the present results. If LCN2 is an upstream mediator/cofactor of DKK1, it would make for a good therapeutic target, since its removal or inhibition do not cause detectable side effects in mice (Rucci et al., 2015; Capulli et al., 2018).
Although the presented data do not rule out a possible role for LCN2 in human bone health, they show that in postmenopausal osteoporosis, LCN2 does not seem to have a significant functional meaning. However, LCN2 correlates with NTX and DKK1 in healthy women and men, indicating possible associative functions in bone biology which are worthy of further studies.
In this study, the number of participants is a limitation, especially for the osteoporosis group. Some of the analyses are thus underpowered and need to be validated in larger cohorts. However, from the present results we conclude that serum LCN2 is not increased in osteoporotic or osteoarthritic patients, but correlates with age, NTX and DKK1 in healthy subjects. The statistical models used also indicate a possible association between LCN2, DKK1 and ageing.
Transparency document
Transparency document
CRediT authorship contribution statement
Antonio Maurizi: Formal analysis, Investigation, Writing – review & editing, Visualization. Marco Ponzetti: Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Kaare M. Gautvik: Investigation, Resources, Writing – review & editing. Sjur Reppe: Investigation, Resources, Writing – review & editing. Anna Teti: Conceptualization, Methodology, Resources, Writing – review & editing, Funding acquisition. Nadia Rucci: Conceptualization, Methodology, Formal analysis, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was funded by the European Commission grant program “Collaborative Project – Large-scale integrating project” – Call identifier: FP7-HEALTH.2012.2.1.1-1-C Proposal No. 602300 - Acronym: SYBIL to A.T. and Agenzia Spaziale Italiana (ASI) N.2019-11-U.0 to A.T. and N.R.
Footnotes
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2021.101059.
Appendix A. Supplementary data
Supplementary figures
References
- Abella V., Scotece M., Conde J., Gómez R., Lois A., Pino J., Gómez-Reino J.J., Lago F., Mobasheri A., Gualillo O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers. 2015;20:565–571. doi: 10.3109/1354750X.2015.1123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachorzewska-Gajewska H., Malyszko J., Sitniewska E., Malyszko J.S., Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am. J. Nephrol. 2006;26:287–292. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- Bahmani B., Roudkenar M.H.abib., Halabian R., Jahanian-Najafabadi A., Amiri F., Jalili M.A.l. Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress. Cell Stress Chaperones. 2014;19:685–693. doi: 10.1007/s12192-014-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani B, Amiri F, Mohammadi Roushandeh A, Bahadori M, Dehghan Harati M, Habibi Roudkenar M (2015) The LCN2-engineered HEK-293 cells show senescence under stressful condition. Iran J Basic Med Sci 18:459–464. doi:10.22038/ijbms.2015.4407. [PMC free article] [PubMed]
- Bennett M.R., Nehus E., Haffner C., Ma Q., Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr. Nephrol. 2015;30:677–685. doi: 10.1007/s00467-014-2989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.S., Murray D.W., Hurson C.J., Obrien J., Doran P.P., Obyrne J.M. The role of Dkk1 in bone mass regulation: correlating serum Dkk1 expression with bone mineral density. J. Orthop. Res. 2011;29:414–418. doi: 10.1002/jor.21260. [DOI] [PubMed] [Google Scholar]
- Capulli M, Rufo A, Teti A, Rucci N (2009) Global transcriptome analysis in mouse calvarial osteoblasts highlights sets of genes regulated by modeled microgravity and identifies a “mechanoresponsive osteoblast gene signature.” J. Cell. Biochem. 107:240–252. doi: 10.1002/jcb.22120. [DOI] [PubMed]
- Capulli M., Ponzetti M., Maurizi A., Gemini-Piperni S., Berger T., Mak T.W., Teti A., Rucci N. A complex role for lipocalin 2 in bone metabolism: global ablation in mice induces osteopenia caused by an altered energy metabolism. J. Bone Miner. Res. 2018;33:1141–1153. doi: 10.1002/jbmr.3406. [DOI] [PubMed] [Google Scholar]
- Chan M.Y., Frost S.A., Center J.R., Eisman J.A., Nguyen T.V. Relationship between body mass index and fracture risk is mediated by bone mineral density. J. Bone Miner. Res. 2014;29:2327–2335. doi: 10.1002/jbmr.2288. [DOI] [PubMed] [Google Scholar]
- Chin K.Y., Ima-Nirwana S., Mohamed I.N., Hanapi Johari M., Ahmad F., Mohamed Ramli E.S., Wan Ngah W.Z. Insulin-like growth factor-1 is a mediator of age-related decline of bone health status in men. Aging Male. 2014;17:102–106. doi: 10.3109/13685538.2014.896895. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, Seo AY, Chung JH, Jung YS, Im E, Lee J, Kim ND, Choi YJ, Im DS, Yu BP (2019) Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis. 10:367–382. doi:10.14336/AD.2018.0324. [DOI] [PMC free article] [PubMed]
- Costa D., Lazzarini E., Canciani B., Giuliani A., Spanò R., Marozzi K., Manescu A., Cancedda R., Tavella S. Altered bone development and turnover in transgenic mice over-expressing lipocalin-2 in bone. J. Cell. Physiol. 2013;228:2210–2221. doi: 10.1002/jcp.24391. [DOI] [PubMed] [Google Scholar]
- Florio M., Gunasekaran K., Stolina M., Li X., Liu L., Tipton B., Salimi-Moosavi H., Asuncion F.J., Li C., Sun B., Tan H.L., Zhang L., Han C.Y., Case R., Duguay A.N., Grisanti M., Stevens J., Pretorius J.K., Pacheco E., Jones H., Chen Q., Soriano B.D., Wen J., Heron B., Jacobsen F.W., Brisan E., Richards W.G., Ke H.Z., Ominsky M.S. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat. Commun. 2016;7:11505. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro M., Mereu M.C., Aghi A., Iervasi G., Gallieni M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 2017;14:200–206. doi: 10.11138/ccmbm/2017.14.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro M., Cianciolo G., Brandi M.L., Ferrari S., Nickolas T.L., Tripepi G., Plebani M., Zaninotto M., Iervasi G., la Manna G., Gallieni M., Vettor R., Aghi A., Gasperoni L., Giannini S., Sella S., Cheung A.M. Vitamin K and osteoporosis. Nutrients. 2020 doi: 10.11138/ccmbm/2017.14.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambara G., Salanova M., Ciciliot S., Furlan S., Gutsmann M., Schiffl G., Ungethuem U., Volpe P., Gunga H.C., Blottner D. Microgravity-induced transcriptome adaptation in mouse paraspinal longissimus dorsi muscle highlights insulin resistance-linked genes. Front. Physiol. 2017;8:279. doi: 10.3389/fphys.2017.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautvik K.M., Günther C.C., Prijatelj V., Medina-Gomez C., Shevroja E., Rad L.H., Yazdani M., Lindalen E., Valland H., Gautvik V.T., Olstad O.K., Holden M., Rivadeneira F., Utheim T.P., Reppe S. Distinct subsets of noncoding RNAs are strongly associated with BMD and fracture, studied in weight-bearing and non–weight-bearing human bone. J. Bone Miner. Res. 2020;35:1065–1076. doi: 10.1002/jbmr.3974. [DOI] [PubMed] [Google Scholar]
- Gupta K., Shukla M., Cowland J.B., Malemud C.J., Haqqi T.M. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–3335. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- Jemtland R., Holden M., Reppe S., Olstad O.K., Reinholt F.P., Gautvik V.T., Refvem H., Frigessi A., Houston B., Gautvik K.M. Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype. J. Bone Miner. Res. 2011;26:1793–1801. doi: 10.1002/jbmr.396. [DOI] [PubMed] [Google Scholar]
- Kawai M., de Paula F.J.A., Rosen C.J. New insights into osteoporosis: the bone-fat connection. J. Intern. Med. 2012;272:317–329. doi: 10.1111/j.1365-2796.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Yoon H.J., Yoon K.A., Gwon M.R., Jin Seong S., Suk K., Kim S.Y., Yoon Y.R. Lipocalin-2 inhibits osteoclast formation by suppressing the proliferation and differentiation of osteoclast lineage cells. Exp. Cell Res. 2015;334:301–309. doi: 10.1016/j.yexcr.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Ohk B, Kang WY, Seong SJ, Suk K, Lim M-S, Kim S-Y, Yoon Y-R (2016) Deficiency of lipocalin-2 promotes proliferation and differentiation of osteoclast precursors via regulation of c-fms expression and nuclear factor-kappa B activation. J Bone Metab 23:8. doi:10.11005/jbm.2016.23.1.8. [DOI] [PMC free article] [PubMed]
- Kjeldsen L., Johnsen A.H., Sengelov H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993;268:10425–10432. doi: 10.1016/s0021-9258(18)82217-7. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev. 24:2463–2479. doi:10.1101%2Fgad.1971610. [DOI] [PMC free article] [PubMed]
- Lim W.H., Wong G., Lim E.M., Byrnes E., Zhu K., Devine A., Pavlos N.J., Prince R.L., Lewis J.R. Circulating lipocalin 2 levels predict fracture-related hospitalizations in elderly women: a prospective cohort study. J. Bone Miner. Res. 2015;30:2078–2085. doi: 10.1002/jbmr.2546. [DOI] [PubMed] [Google Scholar]
- Liu D.M., Zhao H.Y., Zhao L., Zhang M.J., Liu T.T., Tao B., Sun L.H., Liu J.M. The relationship among serum lipocalin 2, bone turnover markers, and bone mineral density in outpatient women. Endocrine. 2018;59:304–310. doi: 10.1007/s12020-017-1504-1. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. doi:10.1016%2Fj.cell.2013.05.039. [DOI] [PMC free article] [PubMed]
- Mundy G.R. Osteoporosis and inflammation. Nutr. Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Poynter M.E., Daynes R.A. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. J. Biol. Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- Reppe S., Refvem H., Gautvik V.T., Olstad O.K., Høvring P.I., Reinholt F.P., Holden M., Frigessi A., Jemtland R., Gautvik K.M. Eight genes are highly associated with BMD variation in postmenopausal Caucasian women. Bone. 2010;46:604–612. doi: 10.1016/j.bone.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Roux S. New treatment targets in osteoporosis. Jt. Bone Spine. 2010;77:222–228. doi: 10.1016/j.jbspin.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Rucci N., Capulli M., Piperni S.G., Cappariello A., Lau P., Frings-Meuthen P., Heer M., Teti A. Lipocalin 2: a new mechanoresponding gene regulating bone homeostasis. J. Bone Miner. Res. 2015;30:357–368. doi: 10.1002/jbmr.2341. [DOI] [PubMed] [Google Scholar]
- Sommer G., Weise S., Kralisch S., Lossner U., Bluher M., Stumvoll M., Fasshauer M. Lipocalin-2 is induced by interleukin-1 β in murine adipocytes in vitro. J. Cell. Biochem. 2009;106:103–108. doi: 10.1002/jcb.21980. [DOI] [PubMed] [Google Scholar]
- Tilstra J.S., Clauson C.L., Niedernhofer L.J., Robbins P.D. NF-κB in aging and disease. Aging Dis. 2011;2:449–465. [PMC free article] [PubMed] [Google Scholar]
- Torres-Costoso A., Gracia-Marco L., Sánchez-López M., García-Prieto J.C., García-Hermoso A., Díez-Fernández A., Martínez-Vizcaíno V. Lean mass as a total mediator of the influence of muscular fitness on bone health in schoolchildren: a mediation analysis. J. Sports Sci. 2015;33:817–830. doi: 10.1080/02640414.2014.964750. [DOI] [PubMed] [Google Scholar]
- Veeriah V., Zanniti A., Paone R., Chatterjee S., Rucci N., Teti A., Capulli M. Interleukin-1β, lipocalin 2 and nitric oxide synthase 2 are mechano-responsive mediators of mouse and human endothelial cell-osteoblast crosstalk. Sci. Rep. 2016;6:29880. doi: 10.1038/srep29880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Elks C.M., Stephens J.M. The induction of lipocalin-2 protein expression in vivo and in vitro. J. Biol. Chem. 2014;289:5960–5969. doi: 10.1074/jbc.M113.532234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Supplementary figures