Summary
Congenital anomalies are a worldwide health problem that places a burden on the family and society. Chromosome abnormalities are one of the leading causes for congenital anomalies in newborns. Despite the remarkable development in cytogenetic services in the past years, still there are limited data from Middle East countries. The current study aimed to evaluate the prevalence and patterns of chromosomal aberrations in newborns admitted to the neonatal intensive care unit (NICU) with major congenital anomalies at Medina province in the western region of Saudi Arabia. Out of 2,541 live births, 150 newborns were selected based on the presence of major birth defects. Demographic and clinical data were collected from hospital medical records and statistically analyzed. The prevalence of major congenital anomalies was 10.7/1,000 live births (95% CI: 9.076- 12.583). The most common congenital anomalies in descending order were congenital heart disease, musculoskeletal and chromosome abnormalities. The birth prevalence of chromosome abnormalities was 4.22/1,000 live births (95% CI: 3.211-5.441). The most common chromosome abnormality was Down syndrome-nondisjunction type (66%). Advanced parental age was strongly associated with chromosome aberrations (p < 0.001) while consanguinity was evident in cases with normal karyotype (p < 0.001). High birth prevalence of chromosome abnormalities in newborns with congenital anomalies in Al Madinah was evident and advanced parental age is a potential risk factor. A local registry system for congenital anomalies is highly recommended to provide proper health services to high risk families.
Keywords: congenital anomalies, chromosome abnormalities, birth prevalence, newborns, Down syndrome
1. Introduction
Congenital anomalies or Birth defects are defined as abnormalities of structure or function, including metabolic that are present at birth. Congenital anomalies in children vary from minor to major anomalies, and despite remarkable development in various treatment services, they remain an important cause of infant mortality and childhood disability (1,2).
From WHO reports, major congenital anomalies have been recorded in about three million newborns per year with a prevalence rate of 3% (1). On the other hand, there are some variations in the prevalence of congenital anomalies between different countries. Generally, in developed countries it ranged between 45-50/1,000 live births (3,4) while in the middle east and Africa it was between 20-30/1,000 live births (5-9). High numbers of deaths in low and middle income countries have been reported (3,10). Indeed underestimation in underdeveloped countries is mainly related to lack of local registry systems and records of actual cases.
There are several etiological factors of congenital anomalies like genetic, chromosomal, environmental and multifactorial. However; in many cases the cause is unknown (idiopathic). Chromosomal abnormalities have their impact on general health and wellbeing causing multiple problems including either mental retardation and/or physical disabilities. An underlying chromosome aberration is found to cause gross phenotypic anomalies in conjunction with mental retardation (11-13).
These facts encouraged governmental health authorities all over the world to establish proper cytogenetics diagnostic facilities in collaboration with well-trained genetic counseling services to provide information and increase awareness in the community to guide high risk families.
Although there are several perinatal studies on the prevalence of congenital anomalies in different regions of Saudi Arabia; up until now there are no available data on the birth prevalence of chromosomal abnormalities in newborns with congenital anomalies at Medina province in the western region of Saudi Arabia.
The present study aimed to assess the prevalence and patterns of chromosomal abnormalities in newborns with major congenital anomalies delivered at a tertiary care maternity hospital in Medina province in the western region of Saudi Arabia.
2. Materials and Methods
2.1. Materials
The current retrospective descriptive study was conducted at Al Madinah Maternity and Children Hospital (MMCH) in Al-Madinah Al-Munawarah, the capital of Medina province. MMCH is considered the main tertiary care hospital that provides integrated medical care to pregnant mothers at Al Madinah in the western region of Saudi Arabia.
The study started January 2019 and was conducted for a period of twelve months based on data extracted from hospital medical records concerning newborns aged from one to 28 days with major congenital anomalies and admitted to the neonatal intensive care unit (NICU). Newborns with minor congenital anomalies, inborn errors of metabolism, home delivered or referred from outside the hospital were excluded.
Out of 2,541 live births admitted to the NICU, 150 newborns had major congenital anomalies. Clinical assessment by a specialist and full investigations regarding Echo, abdominal ultrasound, CT brain, X-ray and referral for chromosomal analysis were done routinely to all cases and data were fed to hospital medical records.
Congenital anomalies were categorized according to body parts affected based on the International Statistical Classification of Diseases and Related Health Problems (ICD-10) classification (14). Chromosome aberrations were categorized according to the International System for Human Cytogenetic Nomenclature (ISCN) 2016 (15).
In the current research demographic and clinical data regarding gender, consanguinity, family history of the presence of any inherited/genetic disorders and medical history of any congenital anomalies like central nervous system (CNS), congenital heart diseases (CHDs), gastrointestinal anomalies and limb anomalies were collected and statistically analyzed.
The study approval was obtained from the local ethical committee (IRB 533; H-03-M-048). The study protocol was in agreement with the Declaration of Helsinki guidelines 1975, as revised in 2013.
2.2. Statistical analysis
Prevalence per 1,000 live births was calculated and descriptive statistics were used to represent the qualitative data and the frequency of numerical and structural chromosome abnormalities. Comparison between variables was done by Chi square test or Fisher Exact test. Odds ratio (OR) at 95% confidence interval (95% CI) was used for assessment of association between chromosome anomalies and some demographic variables. P value was considered statistically significant at p ≤ 0.05.
3. Results
Out of 13,988 live births delivered at the gynecology and obstetrics department in maternity and children hospital; total admissions in NICU were 2,541 newborns, major congenital anomalies were detected in 150 newborn infants (3.7%) in which 74 (49.3%) were males and 76 (50.7%) were females. The overall birth prevalence rate was 1.07% (10.7/1,000 live births). The most common congenital anomalies were related to the circulatory system (congenital heart diseases), followed by musculoskeletal system and then suspected chromosome anomaly (Figure 1).
Figure 1.
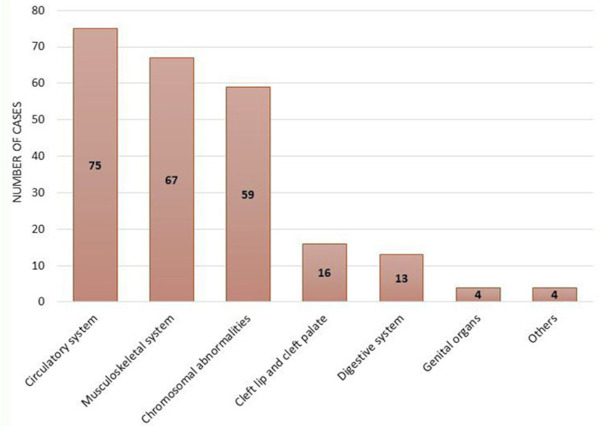
The frequency of newborns with birth defects according to body parts affected. The most frequent congenital anomalies in the studied cases were congenital heart diseases (75 cases) followed by musculoskeletal anomalies (67 cases), chromosome abnormalities (59 cases), cleft lip and palate (16 cases), gastrointestinal anomalies (13 cases), genital anomalies (4 cases), and others (4 cases). The frequency of congenital anomalies in the above figure exceeds the total number of studied newborns as one single infant may contain more than one anomaly.
Chromosome abnormalities were found in 59 newborn infants with congenital anomalies (39.3%) accounting for 2.3% of total NICU admissions. The prevalence rate of chromosomal abnormalities was 0.42% (4.22/1,000 live births).
Normal karyotype was found in 91 (60.7%), 43 newborns had normal male karyotype (46,XY) and 48 newborns had normal female karyotype (47.35% and 52.75%; respectively). On the other hand, abnormal karyotypes were found in 59 (39.3%) newborn infants. According to gender, males were more prone to chromosome abnormalities especially numerical abnormalities than females, however the difference was statistically non-significant (p = 0.527) (Table 1).
Table 1. Birth prevalence of chromosome abnormalities in newborns with congenital anomalies.
| Characteristic | Total | Male | Female | M:F ratio | p | per 1,000 live births | 95% CI |
|---|---|---|---|---|---|---|---|
| Newborns with congenital anomalies | 150 (100%) | 74 (94.3%) | 76 (50.7%) | 0.97:1 | - | 10.723 | 9.076-12.583 |
| Normal karyotype | 91 (60.7%) | 43 (47.35%) | 48 (52.75%) | 0.9:1 | 0.527 | 6.51 | 5.238-7.987 |
| Chromosomal abnormalities | 59 (39.3%) | 31 (52.5%) | 28 (47.45%) | 1.1:1 | 4.22 | 3.211-5.441 | |
| Numerical abnormalities | 47 (79.7%) | 27 (57.45%) | 20 (42.55%) | 1.35:1 | 0.135 | 3.36 | 2.469-4.468 |
| Structural abnormalities | 12 (20.3%) | 4 (33.3%) | 8 (66.7%) | 0.5:1 | 0.858 | 0.443-1.498 |
M:F ratio male to female ratio.
Autosomal chromosome abnormalities were more often detected in the enrolled infants than sex chromosome abnormalities (89.8%% and 10.2%; respectively). Moreover, numerical chromosome abnormalities were recorded in 47 (79.7%) cases while structural abnormalities were seen only in 12 (20.3%) newborns. The most common autosomal abnormality was non-disjunction Down syndrome; trisomy 21 (62.7%) while classic Turner syndrome (45, X) was the noticeable sex chromosome abnormality (3.4%) (Table 2 and Table 3).
Table 2. The birth prevalence according to type of chromosome abnormality.
| Type of chromosome abnormality | Number | Percentage | per 1,000 live births | 95% CI |
|---|---|---|---|---|
| Autosomal chromosome abnormalities | 53 | 90% | 3.789 | 2.837-4.956 |
| Down syndrome | 39 | 66% | 2.77 | 1.983-3.811 |
| Trisomy 21 | 37 | 63% | 2.61 | 1.862-3.646 |
| Translocation Down syndrome | 2 | 3% | 0.143 | 0.017-0.517 |
| Trisomy 18 | 5 | 9% | 0.357 | 0.116-0.834 |
| Trisomy 13 | 3 | 5% | 0.215 | 0.044-0.627 |
| Others | 6 | 10% | 0.429 | 0.157-0.934 |
| Sex chromosome abnormalities | 6 | 10% | 0.429 | 0.157-0.934 |
| Classic Turner syndrome | 2 | 3% | 0.143 | 0.017-0.517 |
| Others | 4 | 7% | 0.286 | 0.078-0.732 |
| Total cases of chromosome anomalies | 59 | 100% | 4.22 | 3.211-5.441 |
Table 3. The karyotype patterns detected in newborns with congenital anomalies.
| Chromosome abnormality | Number | Percentage |
|---|---|---|
| 47,XY,+21 | 24 | 40.7 |
| 47,XX,+21 | 13 | 22.03 |
| 47,XX,t(14;21)+21 | 1 | 1.7 |
| 47,XY,t(21;21)+21 | 1 | 1.7 |
| 47,XY,+18 | 3 | 5.1 |
| 47,XX,+18 | 2 | 3.4 |
| 47,XX,+13 | 3 | 5.1 |
| 45,X | 2 | 3.4 |
| 46,X,i(Xq) | 1 | 1.7 |
| 45,X/46,XX | 1 | 1.7 |
| 46,X,del(Xp)(p11) | 1 | 1.7 |
| 46,XX,t(X;3) | 1 | 1.7 |
| 46,XY,t(13;14) | 1 | 1.7 |
| 46,XY,inv(9) | 1 | 1.7 |
| 46,XX,del(13) | 1 | 1.7 |
| 46,XX,del(18q) | 1 | 1.7 |
| 46,XY,del(18q) | 1 | 1.7 |
| 46,XX,del(p5) | 1 | 1.7 |
| Total | 59 | 100 |
Chromosome abnormalities were more often noticed in full term infants (p = 0.050) and significantly associated with maternal age above 35 years and paternal age more than 40 years (p < 0.001). Despite seventy-nine newborn infants that were born to consanguineous parents (52.7%), chromosome abnormalities were more common in infants delivered from non-consanguineous parents than consanguineous parents (p < 0.001) (Table 4).
Table 4. The demographic characteristics of newborn infants included in the study.
| Demographic data | Normal karyotype (no.91) |
Abnormal karyotype (no.59) |
OR (95% CI) | p | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Sex: | ||||||
| Males | 43 | 47.3 | 31 | 52.5 | 1.235 (0.641-2.382) | 0.527 |
| Females | 48 | 52.7 | 28 | 47.5 | 0.809 (0.420-1.560) | |
| Gestational age (weeks) | ||||||
| Preterm (< 37) | 3 | 3.3 | 7 | 11.9 | 3.949 (0.978-15.937) | 0.050* |
| Full term (37-42) | 88 | 96.7 | 52 | 88.1 | 0.253 (0.063-1.022) | |
| Post term (> 42) | 0 | 0 | 0 | 0 | - | |
| Maternal age (years) | ||||||
| ≤ 35 | 68 | 74.7 | 19 | 32.2 | 0.161 (0.078-0.331) | < 0.001* |
| > 35 | 23 | 25.3 | 40 | 67.8 | 6.224 (3.023-12.820) | |
| Paternal age (years) | ||||||
| ≤ 40 | 70 | 76.9 | 12 | 20.3 | 0.077 (0.034-0.170) | < 0.001* |
| > 40 | 21 | 23.1 | 47 | 79.7 | 13.056 (5.867-29.05) | |
| Consanguinity | ||||||
| Present | 71 | 78.02 | 8 | 13.56 | 0.044 (0.018-0.108) | < 0.001* |
| Absent | 20 | 21.97 | 51 | 86.44 | 22.63 (9.244-55.407) | |
| Family history of genetic disease | ||||||
| Positive | 5 | 5.5 | 2 | 3.4 | 0.603 (0.113-3.218) | 0.705 |
| Negative | 86 | 94.5 | 57 | 96.6 | 1.657 (0.311-8.835) | |
*Statistically significant at p ≤ 0.05.
4. Discussion
Congenital anomalies are one of the leading causes of neonatal deaths and childhood disabilities creating a high burden on the family and community. In Saudi Arabia, prematurity and its complications, together with congenital anomalies, account for 85.5% of all causes of neonatal mortality (16,17).
One of the main causes of congenital anomalies is chromosome aberrations whether inherited or de novo. There is a paucity of chromosomal studies in the western region of Saudi Arabia especially the Medina province. The current study was directed to estimate the frequency of chromosome anomalies among newborns with major congenital anomalies delivered at the main tertiary care hospital in the country to which many pregnant mothers are admitted.
In the present study birth prevalence rate of congenital anomalies was 1.07% (10.7/1,000 live births), which was consistent with other national and regional studies from Al Ahsa at Saudi Arabia (1.14%) (18), Kuwait (1.25%) (19), Oman (1.2%) (20), Iran (1.12%) (21), Morocco (1.02%) (22), Pakistan (1.14%) (23), and Northeast India (1.2%) (8). However, birth prevalence rates of congenital anomalies were higher in other reports than that in the present study (4,9,24). In comparison to other Saudi studies, the rate was higher in Riyadh (3.9%) (25), Hofof (2.27%) (26), and Jeddah (2.8%) (27). Sallout et al. found a relatively high prevalence of congenital anomalies in the KFMC (King Fahd Medical City) population in Riyadh, more specifically 46.5 cases per 1,000 live births that may be related to a high consanguinity rate (7).
In the current study, the consanguinity rate was 52.7% in newborns with congenital anomalies and was strongly associated with congenital anomalies in newborns with normal karyotype. On the other hand, chromosomal abnormalities were more common in infants delivered from non-consanguineous parents than consanguineous parents (p < 0.001). The overall consanguinity rate is high in middle east countries. In the Kingdome of Saudi Arabia it was estimated to be 57.7 % as it is part of the customs and traditions of society (28). Close association between consanguineous mating as a risk factor and birth defects especially congenital heart diseases have been previously reported (29,30).
In addition to consanguinity, other factors may cause discrepancy between different studies like study design, inclusion and exclusion criteria and environmental exposure to teratogens.
The relatively low birth prevalence rate of congenital anomalies in our study could be related to the fact that it included only live births from Medina delivered in the assigned hospital with exclusion of referred or home delivered cases. Also enrolled newborns were admitted to NICU with major congenital anomalies with exclusion of cases with minor congenital anomalies or inborn errors of metabolism.
In the current study birth prevalence of chromosome abnormalities was 4.22/1,000 live births. In addition, numerical chromosome abnormalities were more prominent than structural chromosome abnormalities and Down syndrome (trisomy 21) was the most common recorded anomaly. In comparison with other studies, birth prevalence of chromosome abnormalities among newborns in Medina was within international rates, however it was relevantly higher compared to national studies (25,31). Table 5 summarizes the comparison between our study and some national and international studies.
Table 5. Comparison between the birth prevalence of chromosome abnormalities in the current study and other national and international studies.
| Study region | Total Cases | Congenital anomalies | Chromosome abnormalities | per 1,000 live births | Ref. |
|---|---|---|---|---|---|
| Current study | 13,988 | 150 | 59 | 4.22 | - |
| Riyadh, Saudi Arabia | 28,376 | 296 | 79 | 2.78 | (25) |
| Jedda, Saudi Arabia | 98 | - | 78 | 2.8 | (31) |
| Aljahraa, Kuwait | 7,739 | 97 | 17 | 2.18 | (19) |
| Assiut, Upper Egypt | 5,000 | 103 | 28 | 5.6 | (9) |
| Tokyo, Japan | 14,835 | - | 93 | 6.27 | (32) |
| Goa | 8,551 | 166 | 40 | 4.7 | (33) |
| Denmark | 77,520 | 2,089 | 291 | 3.75 | (34) |
| Wales, England | 466,475 | 20,374 | 1,269 | 2.73 | (35) |
Using a literature review, there was no sufficient data regarding the prevalence of chromosome abnormalities in newborns in the western region of Saudi Arabia especially Medina province. Variations between different studies could be related to criteria of selection of cases and methods used for chromosome analysis. One of the influencing factors is the availability of advanced prenatal diagnostic techniques and the extent to which the society is aware of their importance, especially in light of religious regulations.
Another factor we have to take into account is the frequency of certain types of suspected chromosome anomaly in the studied population like, for example, most of the cases involved here were Down syndrome (66%), which directs towards numerical aberrations and aneuploidy as the most common abnormality in the studied cases.
Parents' age is also an important factor that must be taken into account. Maternal age above 35 years and paternal age above 40 years were highly associated with chromosome abnormalities in newborns with congenital anomalies enrolled in the present study while their association was non-significant in newborns with normal karyotypes. These findings match previous studies (36,37). It is well established that advanced maternal age is a risk factor for non-disjunction during chromosome segregation and aneuploidy. However, the exact mechanism is not yet well understood. In addition, some studies found an association between advanced paternal age (above 40 years) and impaired male reproduction in which chromosome aneuploidy and impaired chromatin integrity have been detected in male sperm. These changes could explain the association between advanced paternal age and some genetic disorders and chromosomal abnormalities like Trisomy 21 especially when it is taken along with advanced maternal age (38,39). Among most cases advanced paternal age is accompanied with advanced age of the mother making the association between paternal age and aneuploidy difficult to be assessed. Hence more precise studies are needed to find the exact role of paternal age.
Like other studies this study is not without limitations. Indeed, although our study was the first one that established the prevalence of chromosome abnormalities in newborns with birth defects, a retrospective design was preferred to suit the nature of this research and the information available. In addition; chromosomal abnormalities were diagnosed by standard karyotype methods, but some submicroscopic chromosome aberrations need more advanced techniques like chromosomal microarrays, which were not accessible.
In conclusion, birth prevalence of chromosome abnormalities in Medina province in the western region of Saudi Arabia was 4.22/1,000 live births. Congenital heart diseases were the most frequent congenital anomalies seen in newborn infants while Down syndrome was the most frequent chromosome anomaly. Consanguinity is a respectable risk factor for congenital anomalies in affected newborns with normal karyotypes whereas advanced maternal and paternal ages were evident risk factors for chromosomal abnormalities. We were trying to shed light on chromosome abnormalities as a cause of congenital anomalies in newborns. This study also makes it clear that there is a need to establish an updated national registry system for congenital anomalies and trends over time. In addition, cytogenetic examination should be afforded to newborns with congenital anomalies for proper diagnosis and genetic counseling for high risk families in Al Madinah in the west of Saudi Arabia.
Acknowledgements
Authors are grateful to the department of neonatal intensive care unit and department of statistics at the Madinah Maternity and Children Hospital (MMCH) in Al-Madinah Al-Munawarah for their supply of information and great cooperation to fulfil this work.
Funding:
None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. World Health Organization. Congenital anomalies. https://www.who.int/news-room/fact-sheets/detail/congenital-anomalies (accessed December 6, 2019).
- 2. Agha M M, Williams JI, Marrett L, To T, Dodds L. Determinants of survival in children with congenital abnormalities: A long-term population-based cohort study. Birth Defects Res A Clin Mol Teratol. 2006; 76:46-54. [DOI] [PubMed] [Google Scholar]
- 3. Christianson A, Howson C, Modell B. Global report on birth defects: the hidden toll of dying and disabled children. https://www.marchofdimes.org/materials/global-report-on-birth-defects-the-hidden-toll-of--d2unzZI5_VWOaLZnw6iHcx7hbpMWtWzTuIOU3DabcVY.pdf (accessed December 6, 2019).
- 4. Irvine B, Luo W, León JA. Congenital anomalies in Canada 2013: a perinatal health surveillance report by the Public Health Agency of Canada's Canadian Perinatal Surveillance System. Health Promot Chronic Dis Prev Can. 2015; 35:21-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muga R, Mumah SCJ, Juma PA. Congenital malformations among newborns in Kenya. African Journal of Food, Agriculture, Nutrition and Development. 2009; 9:814-819. [Google Scholar]
- 6. Ndibazza J, Lule S, Nampijja M, Mpairwe H, Oduru G, Kiggundu M, Akello M, Muhangi L, Elliott AM. A description of congenital anomalies among infants in Entebbe, Uganda. Birth Defects Res A Clin Mol Teratol. 2011; 91:857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sallout BI, Al Hoshan MS, Attyyaa RA, Al Suleimat AA. Antenatal diagnosis, prevalence and outcome of major congenital anomalies in Saudi Arabia: a hospital-based study. Ann Saudi Med. 2008; 28:272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baruah J, Kusre G, Bora R. Pattern of gross congenital malformations in a tertiary referral hospital in Northeast India. Indian J Pediatr. 2015; 82:917-922. [DOI] [PubMed] [Google Scholar]
- 9. Mohammed RM, Shawky AA, Soliman M, Ahmed M. Chromosomal study in newborn infants with congenital anomalies in Assiut University hospital: Cross-sectional study. Egyptian Journal of Medical Human Genetics. 2011; 12:79-90. [Google Scholar]
- 10. Toobaie A, Yousef Y, Balvardi S, St-Louis E, Baird R, Guadagno E, Poenaru D. Incidence and prevalence of congenital anomalies in low- and middle-income countries: A systematic review. J Pediatr Surg. 2019; 54:1089-1093. [DOI] [PubMed] [Google Scholar]
- 11. Polipalli SK, Karra VK, Jindal A, Puppala M, Singh P, Rawat K, Kapoor S. Cytogenetic analysis for suspected chromosomal abnormalities; A five years experience. J Clin Diagn Res. 2016; 10:GC01-GC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wellesley D, Dolk H, Boyd PA, et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet. 2012; 20:521-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson T, Howell S, Davis S, Wilson R, Janusz J, Boada R, Pyle L, Tartaglia N. Current survey of early childhood intervention services in infants and young children with sex chromosome aneuploidies. Am J Med Genet C Semin Med Genet. 2020; 184:414-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. Congenital malformation, deformation, and chromosomal abnormalities. Chapter XVII. In: International Statistical Classification of Diseases and Related Health Problems. 10th Revision (ICD-10). Fifth edition. (WHO, eds.). WHO Press, Geneva, Switzerland, 2016; pp.707-757. [Google Scholar]
- 15. McGowan-Jordan J, Simons A, Schmid M. ISCN: an international system for human cytogenomic nomenclature (2016). Basel; New York: Karger. 2016. [Google Scholar]
- 16. Mesleh RA, Kurdi AM, Sabagh TO, Algwiser AA. Changing trends in perinatal deaths at the Armed Forces Hospital, Riyadh, Saudi Arabia. J Obstet Gynaecol. 2001; 21:49-55. [DOI] [PubMed] [Google Scholar]
- 17. Al-Gazali L, Hamamy H, Al-Arrayad S. Genetic disorders in the Arab world. BMJ. 2006; 333:831-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Bu Ali WH, Balaha MH, Al Moghannum MS, Hashim I. Risk factors and birth prevalence of birth defects and inborn errors of metabolism in Al Ahsa, Saudi Arabia. Pan Afr Med J. 2011; 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madi SA, Al-Naggar RL, Al-Awadi SA, Bastaki LA. Profile of major congenital malformations in neonates in Al-Jahra region of Kuwait. East Mediterr Health J. 2005; 11:700-706. [PubMed] [Google Scholar]
- 20. Sawardekar KP. Profile of major congenital malformations at Nizwa Hospital, Oman: 10-year review. J Paediatr Child Health. 2005; 41:323-330. [DOI] [PubMed] [Google Scholar]
- 21. Mashhadi Abdolahi H, Kargar Maher MH, Afsharnia F, Dastgiri S. Prevalence of congenital anomalies: a community-based study in the northwest of Iran. ISRN Pediatr. 2014; 2014:920940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elghanmi A, Razine R, Jou M, Berrada R. Congenital malformations among newborns in Morocco: A retrospective study. Pediatr Rep. 2020; 12:7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perveen F, Tyyab S. Frequency and pattern of distribution of congenital anomalies in the newborn and associated maternal risk factors. J Coll Physicians Surg Pak. 2007; 17:340-343. [PubMed] [Google Scholar]
- 24. Sachdeva S, Nanda S, Bhalla K, Sachdeva R. Gross congenital malformation at birth in a government hospital. Indian J Public Health. 2014; 58:54-56. [DOI] [PubMed] [Google Scholar]
- 25. Kurdi AM, Majeed-Saidan MA, Al Rakaf MS, AlHashem AM, Botto LD, Baaqeel HS, Ammari AN. Congenital anomalies and associated risk factors in a Saudi population: a cohort study from pregnancy to age 2 years. BMJ Open. 2019; 9:e026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Refat MY, Al-Moghanem M, McDonald P, Reyes L. Major birth defects at King Fahd Hofuf Hospital: Prevalence, risk factors and outcome. Ann Saudi Med. 1995; 15:339-343. [DOI] [PubMed] [Google Scholar]
- 27. Fida NM, Al-Aama J, Nichols W, Nichols W, Alqahtani M. A prospective study of congenital malformations among live born neonates at a University Hospital in Western Saudi Arabia. Saudi Med J. 2007; 28:1367-1373. [PubMed] [Google Scholar]
- 28. el-Hazmi MA, al-Swailem AR, Warsy AS, al-Swailem AM, Sulaimani R, al-Meshari AA. Consanguinity among the Saudi Arabian population. J Med Genet. 1995; 32:623-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Mouzan MI, Al Salloum AA, Al Herbish AS, Qurachi MM, Al Omar AA. Consanguinity and major genetic disorders in Saudi children: a community-based cross-sectional study. Ann Saudi Med. 2008; 28:169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Majeed-Saidan MA, Ammari AN, AlHashem AM, Al Rakaf MS, Shoukri MM, Garne E, Kurdi AM. Effect of consanguinity on birth defects in Saudi women: results from a nested case-control study. Birth Defects Res A Clin Mol Teratol. 2015; 103:100-104. [DOI] [PubMed] [Google Scholar]
- 31. Al-Qahtani MH. Chromosomal abnormalities in Saudi children of Jeddah city, Saudi Med J. Journal of King Abdulaziz University - Medical Sciences. 2008; 15:3-25. [Google Scholar]
- 32. Maeda T, Ohno M, Matsunobu A, Yoshihara K, Yabe N. A cytogenetic survey of 14,835 consecutive liveborns. Jinrui Idengaku Zasshi. 1991; 36:117-129. [DOI] [PubMed] [Google Scholar]
- 33. Vaz N, Shyama SK. Numerical chromosomal abnormalities in the malformed newborns of Goa. Int J Hum Genet. 2005; 5:237-240. [Google Scholar]
- 34. Garne E, Hansen AV, Birkelund AS, Andersen AM. Major congenital anomalies in a Danish region. Dan Med J. 2014; 61:A4825. [PubMed] [Google Scholar]
- 35. Iechyd Cyhoeddus Cymru Public Health Wales. Congenital anomaly register and information service for Wales- Annual review 2012 including data 1998 - 2011. http://www.caris.wales.nhs.uk/data-tables-annual-reviews.html (accessed January 13, 2021).
- 36. Loane M, Morris JK, Addor MC, et al. Twenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screening. Eur J Hum Genet. 2013; 21:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goetzinger KR, Shanks AL, Odibo AO, Macones GA, Cahill AG. Advanced maternal age and the risk of major congenital anomalies. Am J Perinatol. 2017; 34:217-222. [DOI] [PubMed] [Google Scholar]
- 38. Kaarouch I, Bouamoud N, Madkour A, Louanjli N, Saadani B, Assou S, Aboulmaouahib S, Amzazi S, Copin H, Benkhalifa M, Sefrioui O. Paternal age: Negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev. 2018; 85:271-280. [DOI] [PubMed] [Google Scholar]
- 39. Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, Romundstad LB, Söderström- Anttila V, Bergh C. The effect of paternal factors on perinatal and paediatric outcomes: A systematic review and meta-analysis. Hum Reprod Update. 2018; 24:320-389. [DOI] [PubMed] [Google Scholar]


