Abstract
The purpose of this study was to assess the association between seawater temperature and Vibrio vulnificus cases in coastal regions of Korea. All V. vulnificus cases in coastal regions notified to the Korea Disease Control and Prevention Agency between 2003 and 2016 were included in this work. Data for seawater temperature on the south, west, and east coast during the study period were provided by the Korea Oceanographic Data Center of the National Institute of Fisheries Science. We used a generalized additive model and performed a negative binomial regression analysis. In total, 383 notified cases were analyzed (west coast: 196 cases, south coast: 162, and east coast: 25). The maximum seawater temperature was the most significant predictor of V. vulnificus cases on the south and east coasts (relative risk according to the 1 °C increase in seawater temperature (RR) = 1.35 (95% confidence interval (CI): 1.19–1.53) and 1.30 (95% CI: 1.06–1.59), respectively). However, the mean seawater temperature was the most significant predictor for the west coast (RR = 1.34 (95% CI: 1.20–1.51)). These results indicate that continuously monitoring seawater temperature increase in each coastal area is crucial to prevent V. vulnificus infections and protect high-risk groups, such as persons with liver disease.
Keywords: Vibrio vulnificus infection, seawater, temperature, generalized additive models, relative risk
1. Introduction
Vibrio vulnificus infection is an acute and fatal infectious disease caused by V. vulnificus. V. vulnificus is a halophilic Gram-negative bacterium that belongs to the Vibrio genus of the Vibrionaceae family. This pathogen can cause primary septicemia in high-risk populations, such as individuals with chronic liver disease, diabetes, immunodeficiency, iron storage impairment, and end-stage kidney disease, and it has a fatality rate of 50–60% [1,2]. Besides sepsis, potentially fatal wound infections can occur when wounds are exposed to V. vulnificus-contaminated warm seawater during recreational and leisure activities. Moreover, one can be infected with this bacterium through the consumption of uncooked or undercooked seafood [1]. The fatality rate of wound infections ranges from 15% [3] to 25% [4]. Three biotypes of V. vulnificus cause severe disease in human; biotype 1 is the most common and accounts for the entire illness, including primary sepsis associated with a fatality rate exceeding 50% [1]. V. vulnificus infection requires antibiotic treatment and surgical removal of necrotic tissue [1]. V. vulnificus infections are commonly observed in several coastal cities in Japan [5,6], Taiwan [7], and the United States [8,9,10,11]. Moreover, there is a clear seasonality, and cases peak during warm months when the water temperature increases.
V. vulnificus can be found in warm coastal regions worldwide and in estuarine environments where water temperatures range from 9 to 31 °C [1]. In an ecological study focusing on the eastern estuarine waters of North Carolina in the United States, V. vulnificus was isolated only at water temperatures between 15 and 27 °C. Furthermore, its presence was associated with water temperature, dissolved oxygen levels, and levels of other Vibrio species [12]. It is known that high water temperatures greatly promote V. vulnificus proliferation and its ability to infect humans. Therefore, water temperature is a reliable predictor of pathogenic V. vulnificus infection cases [4,13,14,15,16,17,18]. This bacterium is rarely isolated during winter, when the water temperature in the coastal regions of the Gulf of Mexico falls below 20 °C [4,13,14]. However, the bacterium becomes unculturable at low temperatures, leading to very few cases of infection. The lower limit of growth for V. vulnificus is known to be a seawater temperature of 13 °C, and below 13 °C, V. vulnificus cells enter the viable but nonculturable (VBNC) state [4]. In South Korea, a country with a high prevalence of V. vulnificus infection [17], several studies have assessed the seasonality [19] and geographic distribution of the bacterium [20] and developed mathematical models of the effects of global warming on seawater temperature [21]. However, the effect of water temperature on the occurrence of V. vulnificus infection has been poorly assessed quantitatively. Thus, this study was conducted to assess the risk of V. vulnificus infection depending on coastal water temperature.
2. Materials and Methods
2.1. V. vulnificus Cases in South Korea
In South Korea, V. vulnificus infection was declared as a notifiable infectious disease on August 1 2000, and population surveillance programs were implemented afterward. A total of 761 V. vulnificus infection cases were reported to the Korea Disease Control and Prevention Agency (KDCA) by National Infectious Disease Surveillance System (NIDSS) from 2003 to 2016. Epidemiological investigations were conducted in all 761 of these cases. The data for this study were derived from epidemiological investigations conducted during that period and complete epidemiological datasets. Among these cases, 383 were linked to the consumption of raw seafood or exposure to seawater in coastal cities, counties, and districts.
The present study included confirmed and suspected cases of V. vulnificus infection (hereinafter referred to as cases). Cases met the case definition criteria of nationally notifiable infectious diseases put forth by the KDCA in 2020. Confirmed cases included people with clinical symptoms of a V. vulnificus infection and a confirmatory infection diagnosis according to the examination criteria. Suspected cases included people with clinical symptoms indicative of V. vulnificus infection and epidemiological association (seafood consumption, seawater contact and underlying diseases, etc.) who tested negative for the pathogen.
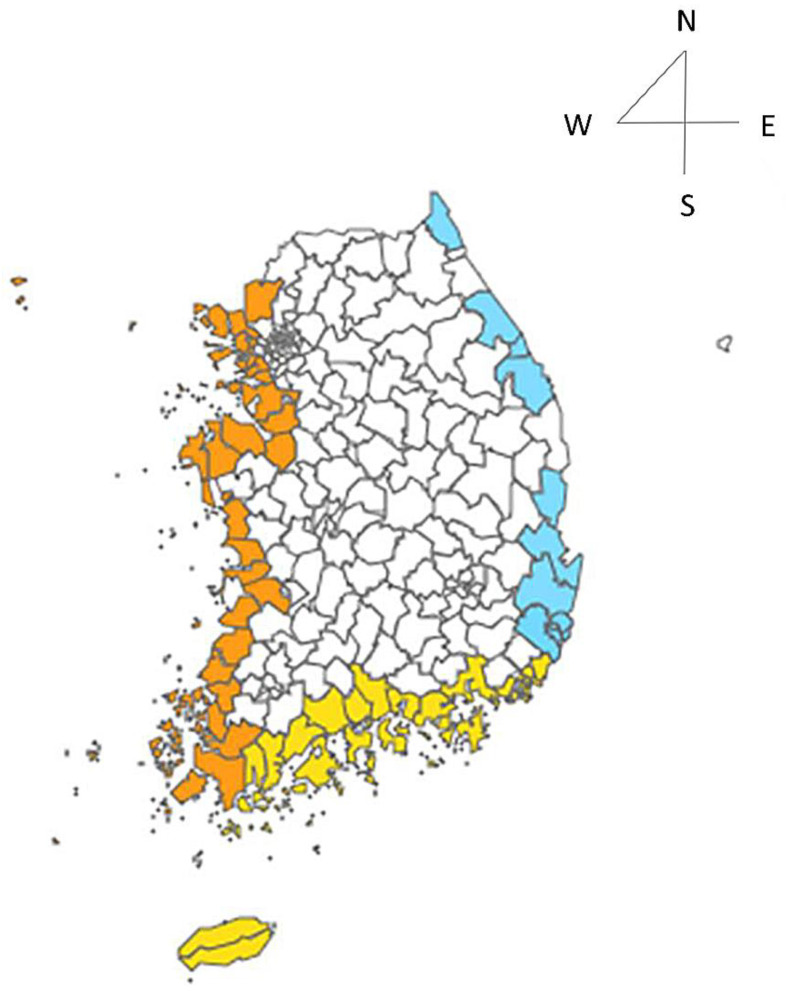
The subjects of the study were 383 cases residing in the inland or coastal areas of Korea during the study period. They were selected from the 761 cases for which epidemiological investigations were conducted. In the study, the selection criterion for the coastal area was where the 383 cases were infected in cities, counties, and districts adjacent to the sea. Figure 1 shows coastal regions where at least one case occurred. Since Korea is surrounded by three coasts to the west, south and east, Wando and Busan were used as markers to divide the coastal area into 3 sections. Those cities are the stationary coastal seawater temperature observation areas used by the Korea Oceanographic Data Center of the National Institute of Fisheries Science. The coast was divided into three coastal areas: the south coast and the west coast divided at Wando, and the south coast and the east coast divided at Busan.
Figure 1.
Coastal areas examined in the study.
2.2. Seawater Temperatures
To assess the association between seawater temperatures and V. vulnificus cases reported between 2003 and 2016, seawater temperature data were obtained from the Korea Oceanographic Data Center. Seawater temperatures were analyzed for each day and coastal region (south, west, and east coasts) to calculate the minimum, average, and maximum monthly temperatures.
Considering that it is more important to examine the duration of high-water temperatures than temperature alone, the number of days within a month on which the seawater temperature exceeded the expected temperature range (18–24 °C) was calculated. The seawater temperature indices were determined through a literature review. The mean temperature at which V. vulnificus can proliferate in July (21.2 °C), August (24.1 °C), and September (23.1 °C), as well as at the maximum number of identified cases and the maximum mean temperature in August (24.1 °C) were used in this study.
2.3. Statistical Analysis
We performed a descriptive analysis to examine the epidemiological characteristics of confirmed cases at city, county, and district levels. In addition, the absolute water temperature (minimum, mean, and maximum) and the number of days on which the water temperature exceeded the expected temperature range (18–24 °C) were calculated. The link between the number of monthly V. vulnificus cases per coastal region and the seawater temperature indices was investigated.
Pearson’s correlation analysis was performed to determine the linear relationship between the monthly number of V. vulnificus infections between 2003 and 2016 and each seawater temperature indicator. A generalized additive model (GAM) was applied to predict the nonlinear relationship between the two variables. The monthly number of cases in coastal regions (N) was the dependent variable and the seawater temperature indicators were the independent variables; a smoothing spline function was used. After each coastal model fit, the variable with the lowest Akaike information criterion (AIC) value among the water temperature indicators was selected as a predictor (Equation (1)).
| log[E(Yi)] = s (seawater temperature indicators, degrees of freedom: df) + time + seasonality + offset variable | (1) |
In this equation, E(Yi) refers to the monthly number of V. vulnificus cases in coastal regions, and a cubic spline smoothing function was used. The analysis was adjusted for the effector variables time trends (calendar time) and seasonality. The analysis was also adjusted for the population as an offset variable throughout the study period (2003–2016).
Overdispersion (variance greater than the mean) was observed for the dependent variables (monthly number of V. vulnificus infection cases). Therefore, negative binomial regression was used to calculate the risk of infection depending on seawater temperature increase. The relative risk (RR) of V. vulnificus cases incidence per 1 °C increase in seawater temperature along with 95% confidence intervals (CIs) and the relationship between V. vulnificus cases and seawater temperature were analyzed. SAS software version 9.4 (SAS Institute, Cary, NC, USA) and MGCV package in R 3.1.4 software were used for the analysis. The level of significance was set at p < 0.05.
The data were publicly available and the institutional review board of Korea University granted exemption for this study (KUIRB-2018-0025-1).
3. Results
3.1. Research Subjects
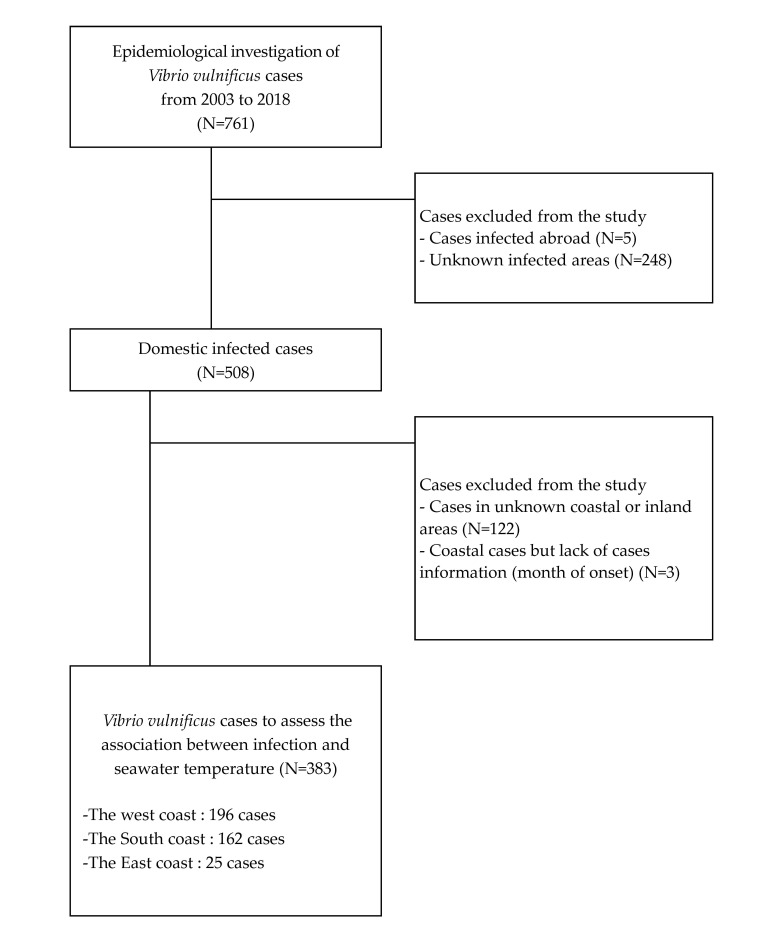
A total of 761 V. vulnificus cases were reported between 2003 and 2016. Five cases of infections that occurred abroad and 248 with unknown locations at which infection occurred were excluded. Of the 508 remaining cases, 117 registered in the inland area and 8 lacking necessary information (city, county, district, and month of onset) were excluded from the study. Thus, 383 cases in coastal cities, counties, and districts were included in the descriptive epidemiology study and for the analysis of the association between water temperature and V. vulnificus infections (Figure 2).
Figure 2.
The selection of the study subjects.
3.2. Epidemiological Characteristics of V. vulnificus Cases in Cities, Counties, and Districts Near the Coast
The general and epidemiological characteristics of V. vulnificus cases are shown in Table 1. Overall, 329 (85.9%) of the cases involved men, and the mean age of the cases was 58.2 ± 11.2 years. As for occupation, housewife and unemployed were the most common, with 157 (44.7%), and 340 (96.6%) had underlying diseases. According to the lifestyle survey of the cases, 257 (84.5%) had a history of drinking, and 128 (57.4%) had a history of smoking. On the west coast, the rate was the highest, with 196 cases (51.2%).
Table 1.
General characteristics of the V. vulnificus cases (N = 383).
| Characteristics | Classification | N | (%) |
|---|---|---|---|
| Sex | |||
| Male | 329 | (85.9) | |
| Female | 54 | (14.1) | |
| Age (years) | |||
| ≤39 | 13 | (3.4) | |
| 40–64 | 269 | (70.2) | |
| ≥65 | 101 | (26.4) | |
| Occupation (N = 351) | |||
| Fishery and fishery product related workers | 35 | (10.0) | |
| Agriculture | 65 | (18.5) | |
| White-collar workers, professional | 18 | (5.1) | |
| Service, distribution industry, self-employed | 32 | (9.1) | |
| Blue-collar workers * | 44 | (12.6) | |
| Housewife, unemployed | 157 | (44.7) | |
| Drinking (N = 304) | |||
| Yes | 257 | (84.5) | |
| No | 7 | (2.3) | |
| Unknown | 40 | (13.2) | |
| Smoking (N = 223) | |||
| Yes | 128 | (57.4) | |
| No | 20 | (9.0) | |
| Unknown | 75 | (33.6) | |
| Underlying diseases (N = 352) | |||
| Yes | 340 | (96.6) | |
| No | 8 | (2.3) | |
| Unknown | 4 | (1.1) | |
| V. vulnificus cases by year | |||
| 2003 | 42 | (11.0) | |
| 2004 | 29 | (7.6) | |
| 2005 | 30 | (7.8) | |
| 2006 | 44 | (11.5) | |
| 2007 | 26 | (6.8) | |
| 2008 | 16 | (4.2) | |
| 2009 | 8 | (2.1) | |
| 2010 | 37 | (9.7) | |
| 2011 | 25 | (6.5) | |
| 2012 | 31 | (8.1) | |
| 2013 | 25 | (6.5) | |
| 2014 | 33 | (8.6) | |
| 2015 | 15 | (3.9) | |
| 2016 | 22 | (5.7) | |
| Coastal area | |||
| West coast | 196 | (51.2) | |
| South coast | 162 | (42.3) | |
| East coast | 25 | (6.5) | |
| Route of infection | |||
| Seafood consumption | 336 | (87.7) | |
| Seawater exposure | 22 | (5.7) | |
| Seafood consumption and seawater exposure | 24 | (6.3) | |
| Unknown | 1 | (0.3) |
* Blue-collar workers: construction workers, civil engineers, architects, etc.
3.3. Annual Indicators Related to Water Temperature and Seawater Temperature
Table 2 summarizes the minimum, mean, and maximum seawater temperatures during the study period.
Table 2.
Seawater temperatures and indicators by year from 2003 to 2016.
| Year | Min. Seawater Temperature (°C) |
Mean Seawater Temperature (°C) |
Max. Seawater Temperature (°C) |
|---|---|---|---|
| 2003 | 9.9 | 15.1 | 20.1 |
| 2004 | 10.3 | 15.5 | 20.6 |
| 2005 | 9.7 | 15.3 | 21.0 |
| 2006 | 9.6 | 15.1 | 20.2 |
| 2007 | 10.8 | 15.8 | 20.5 |
| 2008 | 7.6 | 15.7 | 20.9 |
| 2009 | 11.1 | 16.0 | 20.3 |
| 2010 | 9.0 | 15.0 | 20.1 |
| 2011 | 9.1 | 14.9 | 20.0 |
| 2012 | 10.8 | 15.4 | 20.1 |
| 2013 | 11.1 | 15.7 | 19.6 |
| 2014 | 12.0 | 16.0 | 19.8 |
| 2015 | 11.4 | 15.7 | 19.4 |
| 2016 | 12.1 | 16.2 | 19.5 |
| Mean | 10.3 | 15.5 | 20.2 |
The mean seawater temperature in Korea between 2003 and 2016 was 15.5 °C. The minimum seawater temperature was the highest in 2016 (12.1 °C), and the maximum seawater temperature was the highest in 2005 (21.0 °C).
3.4. Monthly Coastal V. vulnificus Cases in Cities, Counties, and Districts and Seawater Temperature
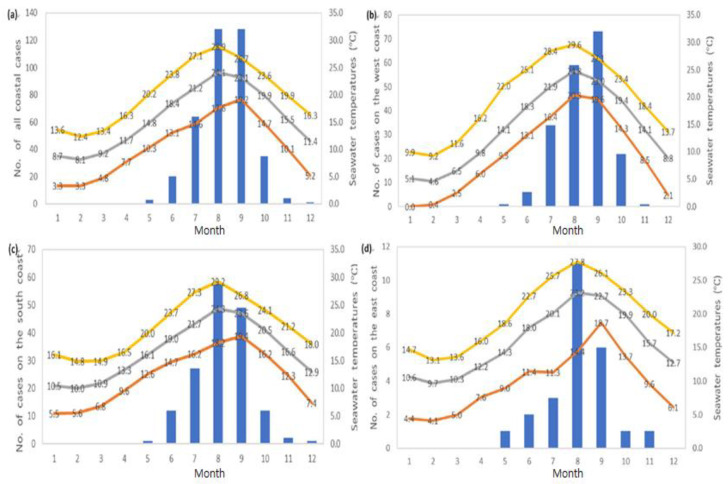
V. vulnificus cases mostly occurred between July and September of each year (Figure 3). Throughout the 14 years covered by our analysis, the total number of monthly cases was highest in August and September (128 cases per month), followed by July (64 cases). The mean and maximum monthly seawater temperatures of the three Korean coasts were highest in August (Figure 3a). The numbers of V. vulnificus infections on the south and east coasts in August were 58 and 11, respectively. However, the number of V. vulnificus infections on the west coast was the highest in September, with 73 cases, and the average water temperature was 23.0 °C (Figure 3b). The highest temperatures were 29.2 and 27.8 °C in August on the south and east coast, respectively (Figure 3c,d).
Figure 3.
Number of V. vulnificus cases and seawater temperatures by month from 2003 to 2016. (a) Total cases of all coasts, (b) west coast, (c) south coast, and (d) east coast. Blue bar represents the monthly number of cases, yellow line: maximum seawater temperature, gray line: average seawater temperature, red line: minimum seawater temperature.
3.5. Assessment of the Correlation between Seawater Temperature and V. vulnificus Cases
3.5.1. Correlation Analysis
Pearson’s correlation analysis revealed a positive relationship between seawater temperature and V. vulnificus cases and statistically significant correlation coefficients ranging from 0.667 to 0.693 (Table 3).
Table 3.
Correlation between seawater temperature indicators and V. vulnificus cases.
| Cases | Mean Seawater Temperature (°C) | Maximum Seawater Temperature (°C) | Minimum Seawater Temperature (°C) | |
|---|---|---|---|---|
| Cases | 1 | |||
| Mean seawater temperature (°C) | 0.693 * | 1 | ||
| Maximum seawater temperature (°C) | 0.686 * | 0.981 * | 1 | |
| Minimum seawater temperature (°C) | 0.667 * | 0.959 * | 0.914 * | 1 |
* Significance at p < 0.001.
3.5.2. Results of General Additive Model Analysis
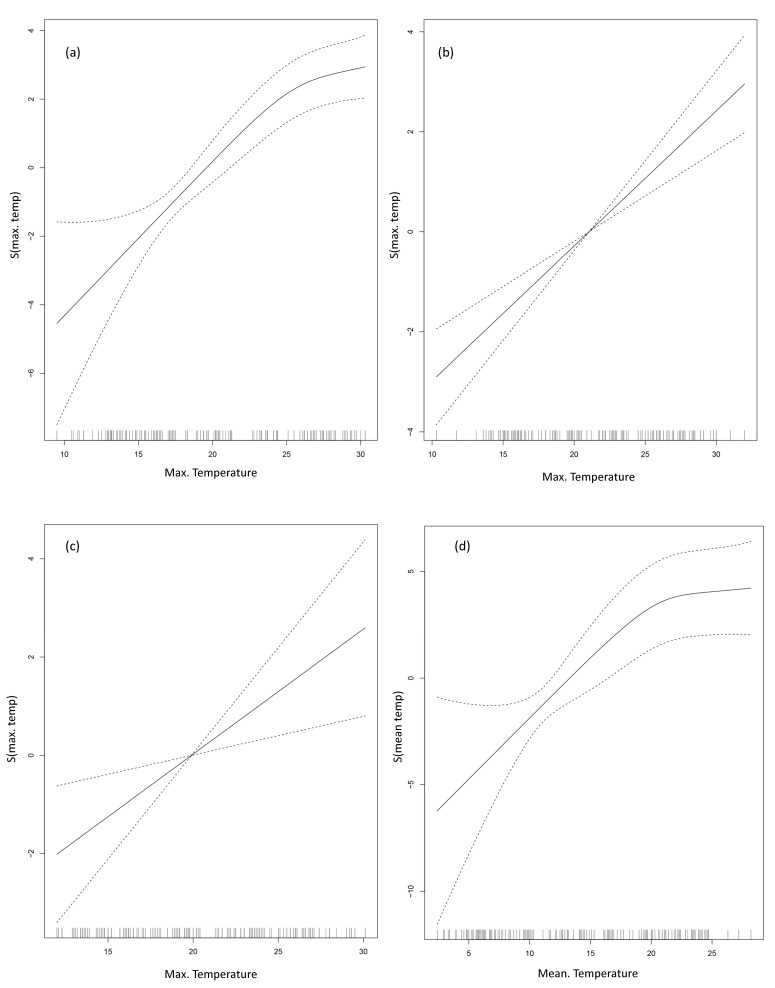
GAM analysis of all coastal, southern coastal, and eastern coastal data showed that the maximum monthly seawater temperature had the lowest AIC value. However, the mean seawater temperature had the lowest AIC value on the west coast (Figure 4).
Figure 4.
Association between seawater temperature and the incidence of V. vulnificus cases on each coast (general additive model analysis). The central solid line represents central estimates, and the dashed line represents 95% confidence intervals. (a) All coasts, (b) south coast, (c) east coast, and (d) west coast.
The adjusted-R2 of the water temperature for the incidence of all V. vulnificus infection cases was 70%. The adjusted-R2 of water temperature for the occurrence of cases was the highest for the west coast at 61%, followed by the south and east coasts at 49% and 22%, respectively.
Table 4 shows the risk of V. vulnificus infection cases for every 1 °C increase in seawater temperature on each coast, calculated by selecting the variable with the lowest AIC obtained with a GAM model.
Table 4.
Relative risk of V. vulnificus cases.
| Coast | Seawater Temperature Index | Relative Risk * | 95% Confidence Interval |
p |
|---|---|---|---|---|
| All coasts | Maximum seawater temperature | 1.37 | 1.24–1.52 | 0.001 |
| South coast | Maximum seawater temperature | 1.35 | 1.19–1.53 | 0.001 |
| East coast | Maximum seawater temperature | 1.30 | 1.06–1.59 | 0.011 |
| West coast | Mean seawater temperature | 1.34 | 1.20–1.51 | 0.001 |
* Adjusted for seasonality and time trends.
After adjusting for time and seasonality, the maximum seawater temperature was the most reliable predictor of V. vulnificus risk on the south and east coasts. The relative risks per 1 °C increase in seawater temperature on the south and east coast were 1.35 (95% CI: 1.19–1.53) and 1.30 (95% CI: 1.06–1.59), respectively. The mean seawater temperature was the most reliable predictor on the west coast and showed a relative risk of 1.34 (95% CI: 1.20–1.51).
4. Discussion
The purpose of this study was to assess the association between domestic seawater temperature and V. vulnificus cases. V. vulnificus cases were most frequently observed in August and September. In August, the mean and maximum seawater temperatures were highest at 24.1 and 28.9 °C, respectively. A positive correlation was observed between each seawater temperature indicator and the number of infection cases. After adjusting for time trend and seasonality, the risk of V. vulnificus infection was significantly higher when seawater temperatures were high.
Several V. vulnificus cases associated with the consumption of contaminated seafood or exposure to contaminated seawater have been reported in coastal regions worldwide. A total of 4754 cases of Vibrio infections were reported by the Centers for Disease Control’s Cholera and Other Vibrio Illness Surveillance (COVIS) system in the United States between 1997 and 2006. Of these, 1210 (25%) cases had developed non-foodborne Vibrio infections after being in contact with seawater, and 72% of the V. vulnificus infection cases involved residents of Gulf Coast states [10]. In Japan, 94 cases of V. vulnificus infection occurred between 1999 and 2000, and 50 (53%) occurred in Kyushu. Interestingly, 43 cases occurred in the coastal regions of the Ariake and Yatsushiro seas, which have several tidal flats [6]. In Taiwan, most of the reported cases (>90%) from 1995 to 2000 involved residents of the southern region of the country [7].
In the present study, V. vulnificus cases were frequently observed between July and September in Korea. The number of cases was highest on the west coast, followed by the south and east coasts. This study also shows that the risk of V. vulnificus cases increases with increasing seawater temperatures, consistent with findings from previous studies [21,22,23,24,25,26,27,28,29].
The first V. vulnificus case in our study was observed in May, when the maximum seawater temperature at the coast was 20.2 °C. In contrast, the maximum seawater temperature from January to April, when cases were not reported, was 12.4–16.3 °C. The cases of V. vulnificus infection from 1988 to 2010 reported to the COVIS system in the United States had a distinct seasonal distribution. Infections rarely occurred when the seawater temperature was below 13 °C [30]. It is known that seawater temperature plays a central role in the growth of this bacterium and that infections are rarely reported in winter when the seawater temperature is lower than 15 °C [27].
In this study, positive correlations (r = 0.414–0.805; p < 0.001) were observed between the number of V. vulnificus infection cases in coastal regions and the nine water temperature indicators. The adjusted-R2 of water temperature for the incidence of total V. vulnificus infections was 70%. A previous study reported a linear increase in the presence of V. vulnificus at seawater temperatures between 11 and 30 °C [24]. In our model, after adjusting for time trend and seasonality, the RR of V. vulnificus infection on the south, west, and east coasts according to the 1 °C increase in seawater temperature were 1.35 (95% CI: 1.19–1.53), 1.34 (95% CI: 1.20–1.51), and 1.30 (95% CI: 1.06–1.59), respectively. The estimated exposure–response relationship between Vibrio infections and the threshold of 16 °C demonstrated an RR of 1.14 (95% CI: 1.02–1.27) for a two-week lag. The estimated risk was in line with the findings of previous studies [26,29]. For each 1 °C increase in seawater temperature above 20 °C, the probability of the V. vulnificus cases increased tenfold [26].
The maximum seawater temperature was the most sensitive index to predict the number of cases on the south and east coasts, while the mean seawater temperature was the most reliable one on the west coast in this study. The highest incidence was observed in August on the south and east coasts when the maximum and mean seawater temperatures were the highest. However, on the west coast, which had the highest infection prevalence among the three coasts, the highest incidence was registered in September, although the seawater temperature was highest in August. Given that the same surveillance and reporting system is used nationwide, delayed reporting cannot explain this discrepancy. However, it has been reported that the abundance of V. vulnificus is dependent on the salinity and turbidity of seawater as well as the water temperature [24]. In addition, the increase in the number of V. vulnificus cases during hot seasons is not only due to the increased number of bacteria but also to increased exposure to seawater, since more people gather at beaches for leisure activities such as swimming, surfing, and fishing. It has been reported that the amount of V. vulnificus in shellfish (oysters) increases when seawater temperature is high, posing greater health risks to people who eat raw oysters [31]. Further studies are needed to explain the discrepancy between high temperatures and the number of cases on the Korean west coast. One of possible explanation for this discrepancy is the characteristics of west seawater. On the west coast, the seawater temperature between June and September is the highest compared to that of the other two coasts, and the turbidity of the seawater is much higher, which may contribute to the growth of bacteria and contamination of the seafood.
The primary limitation of this study is that it was an ecological study that assessed the relationship between seawater temperatures and V. vulnificus infection cases in coastal regions. Second, marine environmental factors, such as salinity, turbidity, dissolved oxygen, and suspended particulate matter, which may affect V. vulnificus infections, were not considered in this study. Lastly, our work did not consider the number of beach visitors, and behavioral patterns, which may affect the risk of infection in the population during the summer.
The significance of this study is that it is the first to estimate the risk of V. vulnificus incidence depending on the increase in seawater temperature in a quantitative manner using national incidence data of over 14 years. Our work also assessed the risk of infection in each of the coastal regions of Korea. Unexpectedly, our findings show that temperature predictors differ between coastal areas and that the sea temperature and the peak case incidence do not coincide on the west coast.
Considering population by coast, the incidence of V. vulnificus cases appears different, so it is necessary to further investigate whether liver diseases (cirrhosis, hepatitis, liver cancer, etc.), which are the representative risk diseases for V. vulnificus infection, differ by coast.
5. Conclusions
This study included 383 registered cases of V. vulnificus infection between 2003 and 2016 in coastal regions of Korea. We calculated the risk of V. vulnificus infection for every 1 °C increase in seawater temperature on the three coasts. The most reliable infection predictor based on seawater temperature indicators differs between coastal areas. On the west coast, the highest seawater temperature and the peak incidence of cases do not coincide, suggesting that factors other than seawater temperature are also relevant to predict infections. Therefore, it is necessary to consider people’s water activity and eating habits of raw seafood in hot seasons as well as seawater temperature to prevent V. vulnificus infections in coastal areas.
Acknowledgments
We would like to thank the Korea Oceanographic Data Center of National Institute of Fisheries Science for providing data on water temperature in the coastal waters of Korea.
Author Contributions
Conceptualization, J.K. and B.C.C.; Methodology, J.K. and B.C.C.; Software, J.K.; Validation, J.K. and B.C.C.; Formal analysis, J.K.; Investigation: J.K.; Resources, J.K.; Data curation: J.K.; Writing—original draft preparation, J.K.; Writing—review and editing, B.C.C.; Visualization, J.K.; Supervision, B.C.C.; Project administration, B.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The institutional review board (IRB) of Korea University granted exemption for this study (IRB exemption number: KUIRB-2018-0025-1).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used this study were extracted from publically available data set. The number of V. vulnificus cases has been available from URL: http://www.kdca.go.kr/npt/biz/npp/nppMain.do (accessed on 3 August 2020) [Korean]. The seawater temperature has been available from URL: http://www.nifs.go.kr/kodc/eng/index.kodc (accessed on 3 August 2020).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horseman M.A., Surani S.A. Comprehensive review of Vibrio vulnificus: An important cause of severe sepsis and skin and soft-tissue infection. Int. J. Infect. Dis. 2011;15:e157–e166. doi: 10.1016/j.ijid.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Oliver J.D. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol. Infect. 2005;133:383–391. doi: 10.1017/S0950268805003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bross M.H., Soch K., Morales R., Mitchell R.B. Vibrio vulnificus infection: Diagnosis and treatment. Am. Fam. Physician. 2007;76:539–544. [PubMed] [Google Scholar]
- 4.Oliver J.D. The biology of Vibrio vulnificus. Microbiol. Spectr. 2015;3 doi: 10.1128/microbiolspec.VE-0001-2014. [DOI] [PubMed] [Google Scholar]
- 5.Inoue Y., Miyasaka J., Ono T., Ihn H. The growth of Vibrio vulnificus and the habitat of infected patients in Kumamoto. Biosci. Trends. 2007;1:134–139. [PubMed] [Google Scholar]
- 6.Inoue Y., Ono T., Matsui T., Miyasaka J., Kinoshita Y., Ihn H. Epidemiological survey of Vibrio vulnificus infection in Japan between 1999 and 2003. J. Dermatol. 2008;35:129–239. doi: 10.1111/j.1346-8138.2008.00432.x. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh P.R., Lin C.Y., Tang H.J., Lee H.C., Liu J.W., Liu Y.C., Chuang Y.C. Vibrio vulnificus in Taiwan. Emerg. Infect. Dis. 2004;10:1363–1368. doi: 10.3201/eid1008.040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hlady W.G., Klontz K.C. The epidemiology of Vibrio infections in Florida, 1981–1993. J. Infect. Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 9.Klontz K.C., Lieb S., Schreiber M., Janowski H.T., Baldy L.M., Gunn R.A. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981–1987. Ann. Intern. Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 10.Dechet A.M., Yu P.A., Koram N., Painter J. Nonfoodborne Vibrio infections: An important cause of morbidity and mortality in the United States, 1997–2006. Clin. Infect. Dis. 2008;46:970–976. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- 11.Leng F., Lin S.L., Wu W., Zhang J.C., Song J.Q., Zhong M. Epidemiology, pathogenetic mechanism, clinical characteristics, and treatment of Vibrio vulnificus infection: A case report and literature review. Eur. J. Clin. Microbiol. 2019;38:1999–2004. doi: 10.1007/s10096-019-03629-5. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer C.S., Hite M.F., Oliver J.D. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl. Environ. Microbiol. 2003;69:3526–3531. doi: 10.1128/AEM.69.6.3526-3531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly M.T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 1982;44:820–824. doi: 10.1128/AEM.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strom M.S., Paranjpye R.N. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes. Infect. 2000;2:177–188. doi: 10.1016/S1286-4579(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 15.Froelich B.A., Noble R.T. Vibrio bacteria in raw oysters: Managing risks to human health. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150209. doi: 10.1098/rstb.2015.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heng S.P., Letchumanan V., Deng C.Y., Ab Mutalib N.S., Khan T.M., Chuah L.H., Chan K.G., Goh B.H., Pusparajah P., Lee L.H. Vibrio vulnificus: An environmental and clinical burden. Front. Microbiol. 2017;8:997. doi: 10.3389/fmicb.2017.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang S.J., Jung S.I., Peck K.R. Historical and clinical perspective of Vibrio vulnificus infections in Korea. Infect. Chemother. 2020;52:245–251. doi: 10.3947/ic.2020.52.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson C.N., Bowers J.C., Griffitt K.J., Molina V., Clostio R.W., Pei S.F., Laws E., Paranjpye R.N., Strom M.S., Chen A., et al. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States) Appl. Environ. Microbiol. 2012;78:7249–7257. doi: 10.1128/AEM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di D.Y.W., Lee A., Jang J., Han D., Hur H.G. Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Appl. Environ. Microbiol. 2017;83:e02680-16. doi: 10.1128/AEM.02680-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok J.S., Ryu A., Kwon J.Y., Kim B., Park K. Distribution of Vibrio species isolated from bivalves and bivalve culture environments along the Gyeongnam coast in Korea: Virulence and antimicrobial resistance of Vibrio parahaemolyticus isolates. Food Control. 2019;106:106697. doi: 10.1016/j.foodcont.2019.06.023. [DOI] [Google Scholar]
- 21.Chu C., Do Y., Kim Y., Saito Y., Lee S.D., Park H., Lee J.K. Mathematical modeling of Vibrio vulnificus infection in Korea and the influence of global warming. Osong Public Health Res. Perspect. 2011;2:51–58. doi: 10.1016/j.phrp.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomenchok L.E., Gidley M.L., Mena K.D., Ferguson A.C., Solo-Gabriele H.M. Children’s abrasions in recreational beach areas and a review of possible wound infections. Int. J. Environ. Res. Public Health. 2020;17:4060. doi: 10.3390/ijerph17114060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Cabanyero C., Sanjuan E., Fouz B., Pajuelo D., Vallejos-Vidal E., Reyes-Lopez F.E., Amaro C. The effect of the environmental temperature on the adaptation to host in the zoonotic pathogen Vibrio vulnificus. Front. Microbiol. 2020;11:489. doi: 10.3389/fmicb.2020.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs J.M., Rhodes M., Brown C.W., Hood R.R., Leight A., Long W., Wood R. Modeling and forecasting the distribution of Vibrio vulnificus in Chesapeake Bay. J. Appl. Microbiol. 2014;117:1312–1327. doi: 10.1111/jam.12624. [DOI] [PubMed] [Google Scholar]
- 25.Mahmud Z.H., Neogi S.B., Kassu A., Mai Huong B.T., Jahid I.K., Islam M.S., Ota F. Occurrence, seasonality and genetic diversity of Vibrio vulnificus in coastal seaweeds and water along the Kii Channel, Japan. FEMS Microbiol. Ecol. 2008;64:209–218. doi: 10.1111/j.1574-6941.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 26.Boer S.I., Heinemeyer E.A., Luden K., Erler R., Gerdts G., Janssen F., Brennholt N. Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microb. Ecol. 2013;65:1052–1067. doi: 10.1007/s00248-013-0221-4. [DOI] [PubMed] [Google Scholar]
- 27.Nowakowska J., Oliver J.D. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol. Ecol. 2013;84:213–222. doi: 10.1111/1574-6941.12052. [DOI] [PubMed] [Google Scholar]
- 28.Jeong H.S., Kim J.Y., Jeon S.M., Park M.S., Kim S.H. Genotypic characterization of Vibrio vulnificus clinical isolates in Korea. Osong Public Health Res. Perspect. 2011;2:8–14. doi: 10.1016/j.phrp.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza J.C., Trinanes J., Lohr W., Sudre B., Lofdahl M., Martinez-Urtaza J., Nichols G.L., Rocklov J. Environmental suitability of Vibrio infections in a warming climate: An early warning system. Environ. Health Persp. 2017;125:107004. doi: 10.1289/EHP2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker-Austin C., Oliver J.D. Vibrio vulnificus: New insights into a deadly opportunistic pathogen. Environ. Microbiol. 2018;20:423–430. doi: 10.1111/1462-2920.13955. [DOI] [PubMed] [Google Scholar]
- 31.Murphy S.K., Oliver J.D. Effects of temperature abuse on survival of Vibrio vulnificus in oysters. Appl. Environ. Microbiol. 1992;58:2771–2775. doi: 10.1128/AEM.58.9.2771-2775.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used this study were extracted from publically available data set. The number of V. vulnificus cases has been available from URL: http://www.kdca.go.kr/npt/biz/npp/nppMain.do (accessed on 3 August 2020) [Korean]. The seawater temperature has been available from URL: http://www.nifs.go.kr/kodc/eng/index.kodc (accessed on 3 August 2020).