Abstract
Background & Aims
Coronavirus-19 disease (COVID-19) is associated with hepatocellular liver injury of uncertain significance. We aimed to determine whether development of significant liver injury during hospitalization is related to concomitant medications or processes common in COVID-19 (eg, ischemia, hyperinflammatory, or hypercoagulable states), and whether it can result in liver failure and death.
Methods
There were 834 consecutive patients hospitalized with COVID-19 who were included. Clinical, medication, and laboratory data were obtained at admission and throughout hospitalization using an identified database. Significant liver injury was defined as an aspartate aminotransferase (AST) level 5 or more times the upper limit of normal; ischemia was defined as vasopressor use for a minimum of 2 consecutive days; hyperinflammatory state was defined as high-sensitivity C-reactive protein value of 100 mg/L or more, and hypercoagulability was defined as D-dimer 5 mg/L or more at any time during hospitalization.
Results
A total of 105 (12.6%) patients developed significant liver injury. Compared with patients without significant liver injury, ischemia (odds ratio [OR], 4.3; range, 2.5–7.4; P < .0001) and tocilizumab use (OR, 3.6; range, 1.9–7.0; P = .0001) were independent predictors of significant liver injury. Although AST correlated closely with alanine aminotransferase (R = 0.89) throughout hospitalization, AST did not correlate with the international normalized ratio (R = 0.10) or with bilirubin level (R = 0.09). Death during hospitalization occurred in 136 (16.3%) patients. Multivariate logistic regression showed that significant liver injury was not associated with death (OR, 1.4; range, 0.8–2.6; P = .2), while ischemic (OR, 2.4; range, 1.4–4.0; P = .001), hypercoagulable (OR, 1.7; range, 1.1–2.6; P = .02), and hyperinflammatory (OR, 1.9; range, 1.2–3.1; P = .02) disease states were significant predictors of death.
Conclusions
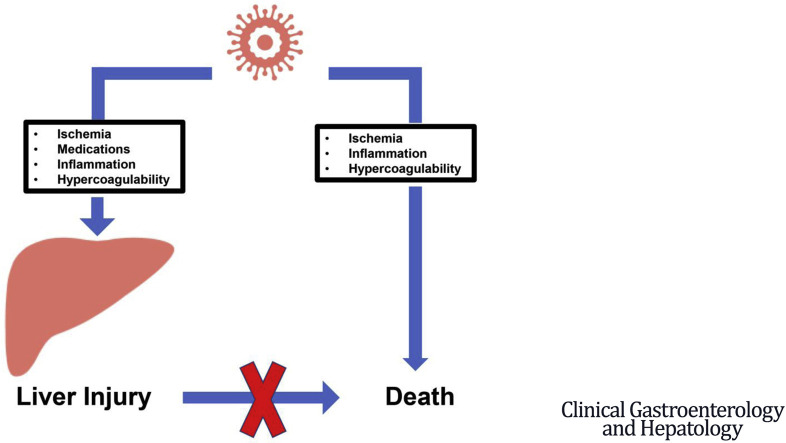
Liver test abnormalities known to be associated with COVID-19 are secondary to other insults, mostly ischemia or drug-induced liver injury, and do not lead to liver insufficiency or death.
Keywords: COVID-19, Liver Injury, Hepatitis
Abbreviations used in this paper: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; COVID-19, coronavirus-19 disease; hs-CRP, high-sensitivity C-reactive protein; ICU, intensive care unit; IL, interleukin; INR, international normalized ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TBIL, total bilirubin; ULN, upper limit of normal
Graphical abstract
What You Need to Know.
Background
Coronavirus-19 disease (COVID-19) is associated with abnormal aminotransferases, but it remains unclear if severe acute respiratory syndrome coronavirus 2 per se or concomitant factors such as ischemia, medications, and hyperinflammatory and hypercoagulable states are responsible for liver injury.
Findings
Significant liver injury (aspartate aminotransferase level >5× upper limit of normal) was uncommon in COVID-19 and was associated with other factors, mainly ischemia or drug-induced liver injury. Importantly, significant liver injury was not associated with death.
Implications for patient care
Concomitant factors play a major role in the pathogenesis of significant liver injury during COVID-19. This liver injury is not directly associated with liver insufficiency or death.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has tropism for cells that express angiotensin-converting enzyme 2 receptors. In the liver, these receptors appear to be expressed mostly in cholangiocytes and, to a much lesser degree, in hepatocytes.1 Liver histologic findings in patients who died from coronavirus-19 disease (COVID-19) are predominantly steatosis and mild lobular/portal inflammation.2 , 3 These changes are nonspecific and do not suggest cholestatic injury, as would be expected if SARS-CoV-2 led to cytopathic liver injury.
Liver test abnormalities have been noted since early reports of COVID-19 in China,4 with pooled prevalence rates of 15%5 and 19%,6 while in a large US series, comprising 5700 patients, abnormal admission aspartate aminotransferase (AST) levels were reported in 58% of patients, with only 2% having levels greater than 15 times the upper limit of normal (ULN).7 The most common abnormality consists of an increase in AST level, being consistently higher than the alanine aminotransferase (ALT) level, and with higher levels observed in patients with severe COVID-19.8, 9, 10, 11, 12 Alkaline phosphatase (AP) and serum total bilirubin (TBIL) levels were increased in less than 15% of patients at admission.9 Therefore, clinically, the injury was mostly hepatocellular.
Studies looking at peak liver test abnormalities during hospitalization have shown that up to 83% of patients develop some abnormality in AST levels but most abnormalities were minor and less than 20% of them exceeded 5 times the ULN.8, 9, 10, 11 , 13 , 14 Higher and continued AST or ALT abnormalities were observed mostly in patients with severe COVID-198, 9, 10, 11 , 13 , 14 and, therefore, peak AST/ALT levels have been shown to be an independent predictor of death.8 , 10 , 14 However, these rapid communications reported on peak AST/ALT levels, but did not correlate changes in aminotransferase levels occurring throughout hospitalization with concomitant events common in COVID-19 (such as ischemia, medications, and hyperinflammatory or hypercoagulable states) that could be associated with an increase in AST levels.
The objectives of the present study were to determine whether significant increases in AST level occurring during hospitalization in patients with COVID-19 are related directly to concomitant processes common in COVID-19 that could cause liver injury (thereby making direct liver injury less likely) and whether these significant increases in AST level are associated with liver insufficiency and/or associated independently with death.
Patients and Methods
Between April 1 and April 30, 2020, 899 consecutive adult patients admitted to Yale New Haven Hospital with COVID-19 were identified by query of the electronic health record. This time period was chosen because, by then, a specific treatment algorithm for COVID-19 had been established (Supplementary Figure 1). Sixty-five patients were excluded for various reasons (34 were admitted for reasons other than COVID-19 but had an incidental positive SARS-CoV-2 test, 15 had opted out, 11 had no liver tests, 3 had inconclusive SARS-CoV-2 results, and 2 were <18 years of age). Therefore, the study is based on a cohort of 834 patients admitted with COVID-19 who had at least 1 set of liver injury tests (AST, ALT, AP) and liver function tests (international normalized ratio [INR], TBIL, albumin). Patients were followed up until June 26, 2020, thereby providing a minimum of 8 weeks of observation. This study was deemed exempt from regulatory requirements applicable to human subject research.
Supplementary Figure 1.
Treatment algorithm for hospitalized patients with coronavirus-19 disease (COVID-19) at the time of the study. BH, Bridgeport Hospital; BMI, body mass index; BNP, brain natriuretic peptice; CBC, complete blood count; CMP, complete metabolic panel; CRP, C-reactive protein; CXR, chest x-ray; ECMO, extracorporeal membrane oxygenation; EKG, electrocardiogram; FDA, Food and Drug Administration; GH, Greenwich Hospital; HgbA1c, hemoglobin A1c; hs-CRP, high-specificity C-reactive protein; HTN, hypertension; ICU, intensive care unit; ID, Infectious Diseases; LMH, Lawrence and Memorial Hospital; MICU, Medical Intensive Care Unit; PCR, polymerase chain reaction; PT, prothrombin time; PTT, partial thromboplastin time; Q8H, every 8 hours; QTc, correct QT interval; RL, Radiologic Technologies; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAS, staf and associate specialist; WH, Westerly Hospital; YNHH, Yale New Haven Hospital; YNHHS, Yale New Haven Hospital System; YSM, Yale School of Medicine.
Data Collection
Relevant clinical data were pulled from each unique hospitalization instance to generate the database. Admission demographic information included age, sex, race, ethnicity, history of diabetes, hypertension, coronary artery disease, obesity, obstructive lung disease, congestive heart failure, and underlying liver disease. Comorbidities were determined based on International Classification of Diseases 9th revision and 10th revision codes. Relevant laboratory tests were collected at admission and throughout hospitalization. The administration and timing of relevant medications at any time during hospitalization, including vasopressors and potentially hepatotoxic drugs (eg, tocilizumab, antibiotics, lopinavir/ritonavir), were collected. Time of admission was subtracted from time-to-order to determine the time elapsed from hospital admission to each order instance. All admission data, medication data, laboratory values, and laboratory dates (initial, peak, and most recent) were verified manually by chart review (M.C., Z.T., C.R., N.D., and D.C.).
Definitions
Liver test abnormalities were defined based on the Yale New Haven Hospital laboratory reference range standards (Supplementary Table 1). Because an increase in AST levels is the most common abnormality in COVID-19 and based on American College of Gastroenterology guidelines,15 we defined significant liver injury as an AST level increased by 5 or more times the ULN at any time during hospitalization.
In addition to medications, conditions/disease states that occur commonly during the course of COVID-19 and that could cause liver injury were defined as follows: ischemic injury was defined as the use of vasopressors (norepinephrine, vasopressin, dopamine, and phenylephrine) for at least 2 consecutive days; a hyperinflammatory state was defined as any high-sensitivity C-reactive protein (hs-CRP) level of 100 mg/L or higher; and a hypercoagulable state was defined as any D-dimer level of 5 mg/L or higher.
Statistical Analysis
Descriptive statistics were summarized using medians and ranges for continuous variables and counts and proportions for categoric variables.
To investigate factors associated independently with significant liver injury, relevant medications or disease states associated significantly with an AST level greater than 5 times the ULN at any time during hospitalization on univariate analysis (by the Fisher exact test for categoric variables, by the Wilcoxon rank-sum test for continuous variables) then were analyzed using a multivariable logistic regression model (excluding variables that intercorrelated significantly). To investigate whether significant liver injury was an independent predictor of death, this and other medications/diseases states associated significantly with death on univariate analysis were analyzed using a multivariable logistic regression model (excluding variables that intercorrelated significantly). As sensitivity analyses, both models were adjusted further for potential confounders, specifically age, sex, race, body mass index (BMI), and comorbidities. A sensitivity analysis using an ALT level 5 or more times the ULN also was performed.
Within-patient correlations between changes from baseline in AST vs ALT, INR, AP, TBIL, CRP, and D-dimer throughout hospitalization were explored using bivariate longitudinal mixed-effects regression models. Because of the highly skewed nature of laboratory values, log-transformed laboratory values were used. For reference, a log AST of 4.0 equals an AST of 55 IU/mL. Changes from baseline in these log-transformed variables over the hospitalization period were plotted for several representative patients. Trends in log-transformed AST over time were examined between ischemia status and tocilizumab treatment status using mixed-effects models and the trajectory plots of log-transformed AST.
P values less than or equal to .05 were considered statistically significant. All analyses were conducted using SAS (version 9.4; Cary, NC) and R (version 3.6.2; Vienna, Austria).
Results
Characteristics of the 834 patients included in the study are shown in Table 1 . The median age was 68 years, 48% were male, the median BMI was 28.2, and 40% were obese. Only 51 patients (6.1%) had underlying liver disease (mostly without cirrhosis). The median length of hospital stay was 10 days (range, 0–83 d), with 8 patients still hospitalized at the end of the follow-up evaluation. Of the 834 patients, 273 (33%) were placed on steroids; 259 (31%) required admission to step-down units or intensive care units (ICUs); 133 (16%) received at least 1 dose of vasopressors, with 102 (12%) of them remaining on vasopressors for at least 2 days; and 108 (13%) were intubated. All 102 patients requiring vasopressors for at least 2 days were in step-down units/ICUs, 99 (97%) were on at least 1 antibiotic, 93 (91%) were intubated, and 77 (76%) were on steroids. As shown in Supplementary Table 2, 13 different classes of antibiotics were used, the most common being β-lactams, followed by vancomycin (mostly in combination with piperacillin/tazobactam). COVID-19–specific treatments included hydroxychloroquine in 725 (87%), tocilizumab in 502 (60%), and only 51 patients (6%) received remdesivir.
Table 1.
Baseline Characteristics and Data on Admission Stratified by Absence or Presence of Significant Liver Injury (AST >5× ULN) During Hospitalization
| Characteristic |
Overall (834) |
Univariate |
||
|---|---|---|---|---|
| AST <5× ULN (N = 729) | AST >5× ULN (N = 105) | P value | ||
| Age, y | 68.0 (20.0–103.0) | 70.0 (20.0–98.0) | 60.0 (21.0–103.0) | <.001 |
| Sex | <.001 | |||
| Male, % | 47.6 | 45.3 | 63.8 | |
| Female, % | 52.4 | 54.7 | 36.2 | |
| Race, N (%) | <.001 | |||
| White | 459 (55.0) | 421 (57.7) | 38 (36.2) | |
| Black | 240 (28.8) | 207 (28.4) | 33 (31.4) | |
| Asian or Pacific Islander | 14 (1.7) | 12 (1.7) | 2 (1.9) | |
| Other | 121 (14.5) | 89 (12.2) | 32 (30.5) | |
| Ethnicity: Hispanic or Latino | 147 (17.6) | 111 (15.2) | 36 (34.3) | <.001 |
| Median BMI, kg/m2 (range) | 28.2 (14.6–73.9) | 28.1 (14.6–73.9) | 28.3 (17.0–56.8) | .2 |
| BMI >30 kg/m2 | 336 (40.3) | 290 (39.8) | 46 (43.8) | .4 |
| Comorbidities, N (%) | ||||
| Coronary artery disease | 97/716 (13.5) | 85/625 (13.6) | 12/91 (13.2) | .9 |
| Hypertension | 631 (75.6) | 562 (77.1) | 69 (65.7) | .01 |
| Congestive heart failure | 249 (29.8) | 233 (31.9) | 16 (15.2) | <.001 |
| Chronic lung disease | 277 (33.2) | 252 (34.6) | 25 (23.8) | .03 |
| Diabetes | 357 (42.8) | 322 (44.2) | 35 (33.3) | .04 |
| Pre-existing liver disease, N (%) | 51 (6.1) | 42 (5.7) | 9 (8.6) | .3 |
| No cirrhosis | 38 (4.5) | 30 (4.1) | 8 (7.6) | |
| Compensated cirrhosis | 10 (1.2) | 9 (1.2) | 1 (0.1) | |
| Decompensated cirrhosis | 3 (0.3) | 3 (0.4) | 0 | |
| Admission liver biochemistries | ||||
| AST, U/L | 40.0 (9.0–3054.0) | 37.0 (9.0–162.0) | 89.0 (16.0–3054.0) | <.001 |
| Alanine aminotransferase, U/L | 26.0 (4.0–904.0) | 24.0 (4.0–281.0) | 57.0 (9.0–904.0) | <.001 |
| Total bilirubin, mg/dL | 0.4 (0.1–5.1) | 0.4 (0.1–5.1) | 0.5 (0.1–4.0) | .03 |
| Alkaline phosphatase, U/L | 74.5 (22.0–838.0) | 74.0 (22.0–838.0) | 79.0 (32.0–417.0) | .4 |
| Albumin, mg/dL | 3.5 (1.7–5.4) | 3.6 (1.8–5.4) | 3.5 (1.7–4.7) | .09 |
| INR | 1.0 (0.8–9.9) | 1.0 (0.8–9.9) | 1.0 (0.8–5.4) | .1 |
| Inflammatory markers | ||||
| hs-CRP, mg/L (N = 829) | 76.9 (0.2–445.0) | 71.7 (0.2–445.0) | 114.2 (1.8–301.0) | <.001 |
| Ferritin, ng/dL (N = 820) | 531.5 (7.0–9365.0) | 480.0 (7.0–8903.0) | 1017.0 (19.0–9365.0) | <.001 |
| Fibrinogen, mg/dL (N = 812) | 509.5 (115.0–861.0) | 496.0 (115.0–861.0) | 596.0 (217.0–861.0) | <.001 |
| D-Dimer, mg/dL (N = 820) | 1.0 (0.2–35.2) | 1.0 (0.2–35.2) | 1.1 (0.2–33.9) | .7 |
| Cytokines | ||||
| IL6 pg/mL (N = 774) | 6.0 (0–945.0) | 6.0 (0–931.0) | 9.0 (4.0–945.0) | .03 |
| IL8 pg/mL (N = 782) | 4.0 (4.0–192.0) | 4.0 (4.0–21.0) | 4.0 (4.0–192.0) | .6 |
| IL1β pg/mL (N = 778) | 4.0 (4.0–213.0) | 4.0 (4.0–213.0) | 4.0 (4.0–132.0) | .8 |
| IFN-ϒ pg/mL (N = 779) | 4.0 (4.0–130.0) | 4.0 (4.0–130.0) | 4.0 (4.0–30.0) | .5 |
| TNF-α pg/mL (N = 781) | 4.0 (4.0–267.0) | 4.0 (4.0–267.0) | 4.0 (4.0–158.0) | .8 |
NOTE. Values are reported as median (ranges) unless otherwise specified.
AST, aspartate aminotransferase; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; IFN, interferon; IL, interleukin; INR, international normalized ratio; TNF, tumor necrosis factor; ULN, upper limit of normal.
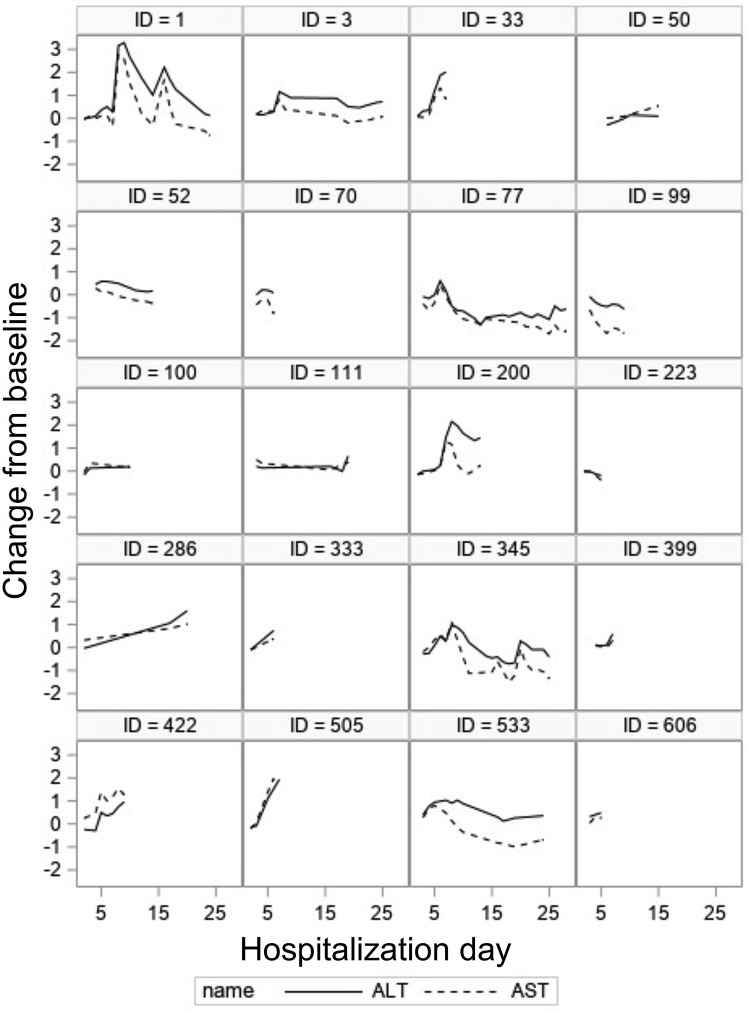
On admission, AST level was abnormal in 521 patients (62.5%), ALT level was abnormal in 281 patients (33.7%), 99 patients (11.9%) had an abnormal AP level, and 26 patients (3.1%) had an abnormal TBIL level (Supplementary Table 3). Consistent with other studies, AST level was higher than ALT level. In most patients, abnormalities were mild at 1 to 2 times the ULN. A median of 7 liver panel tests (range, 1–136 liver panel tests) per patient were available, with only 46 patients having a single set of tests. The median time to peak AST level for the entire cohort was 3 days (interquartile range, 1–6 d) after admission. AST level normalized in 222 patients (43%) in a median period of 4.4 days (range, 0–29.8 d). There was a strong correlation (R = 0.89) between changes in AST and changes in ALT levels over time (Figure 1 ). However, only minimal correlation was observed over time between changes in AST level and changes in TBIL level (R = 0.09), between AST and AP level (R = 0.15), or between AP and TBIL level (R = 0.24).
Figure 1.
Correlation between aspartate aminotransferase (AST) (red) and alanine aminotransferase (ALT) (blue) throughout hospitalization in 20 randomly selected patients. The correlation in the overall population was strong (R = 0.89).
Factors associated with the development of significant liver injury during hospitalization
Significant liver injury was observed in only 25 patients (3%) at the time of admission, however, it developed during hospitalization in 105 patients (12.6%) (Supplementary Table 3). Patients with significant liver injury were younger, more likely to be male, black or Hispanic, and have higher aminotransferase levels at admission (Table 1).
As shown in Table 2 , patients with significant liver injury were sicker, as indicated by higher rates of ICU admission, intubation, and treatment with steroids, vasopressors, and tocilizumab. Patients with pre-existing liver disease were not more likely to develop significant liver injury. On multivariable analysis, an ischemic state and tocilizumab use were the only 2 independent predictors of significant liver injury (Table 2). The same 2 predictors were identified when using an ALT level 5 or more times the ULN as a marker of severe liver injury (Supplementary Table 4). Sensitivity analyses that further adjusted the multivariable model for potential confounders of age, sex, race, and BMI gave identical conclusions (data not shown).
Table 2.
Events/Disease States Occurring Throughout Hospitalization Associated With Significant Liver Injury (AST ≥5× ULN)
| Characteristic |
Overall (834) |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| AST <5× ULN (N = 729) | AST ≥5× ULN (N = 105) | P value | Odds ratio | 95% CI | P value | ||
| Length of stay, d | 10 (0–83) | 10 (0–77) | 13 (1–83) | <.001 | – | – | – |
| Hospitalization status, N (%) | .3 | – | – | – | |||
| Discharged | 690 (82.7) | 608 (83.4) | 82 (78.1) | ||||
| Still admitted | 8 (1.0) | 6 (0.8) | 2 (1.9) | ||||
| Deceased | 136 (16.3) | 115 (15.8) | 21 (20.0) | ||||
| Conditions during follow-up period, N (%) | |||||||
| Intensive care unit | 259 (31.1) | 196 (26.9) | 63 (60.0) | <.001 | – | – | – |
| Venous thromboembolism | 4 (0.5) | 4 (0.5) | 0 | 1.0 | – | – | – |
| Intubation | 108 (12.9) | 62 (8.5) | 46 (43.8) | <.001 | – | – | – |
| Medications, N (%) | |||||||
| Antibiotics | 543 (65.1) | 468 (64.2) | 75 (71.4) | .1 | – | – | – |
| Hydroxychloroquine | 725 (86.9) | 628 (86.1) | 97 (92.4) | .08 | – | – | – |
| Tocilizumab | 502 (60.2) | 410 (56.2) | 92 (87.6) | <.001 | 3.6 | 1.9–7.0 | .0001 |
| Remdesivir | 51 (6.1) | 40 (5.5) | 11 (10.5) | .05 | – | – | – |
| Steroid | 273 (32.7) | 224 (30.7) | 49 (46.7) | .001 | 0.7 | 0.4–1.2 | .2 |
| Statin | 343 (41.1) | 305 (41.8) | 38 (36.2) | .3 | – | – | – |
| NSAID | 369 (44.2) | 315 (43.2) | 54 (51.4) | .1 | – | – | – |
| Disease states | |||||||
| Ischemic | 102 (12.2) | 60 (8.2) | 92 (40.0) | <.001 | 4.3 | 2.5–7.4 | <.0001 |
| Hyperinflammatory | 484 (58.4) | 401 (55.4) | 83 (79.0) | <.001 | 1.5 | 0.9–2.6 | .1 |
| Hypercoagulable | 251 (30.6) | 197 (27.6) | 54 (51.4) | <.001 | 1.3 | 0.8–2.2 | .3 |
NOTE. Values are reported as median (ranges) unless otherwise specified. Ischemic state was defined as vasopressor use for at least 2 days; hyperinflammatory state was defined as any high-sensitivity C-reactive protein value ≥100 mg/L; hypercoagulable state was defined as any D-dimer level ≥5 mg/L. Because all patients on vasopressors were in the intensive care unit and most were intubated/on antibiotics, these variables were not entered into the multivariable model. Remdesivir was not included because only 51 patients received it.
AST, aspartate aminotransferase; NSAID, nonsteroidal anti-inflammatory drug; ULN, upper limit of normal.
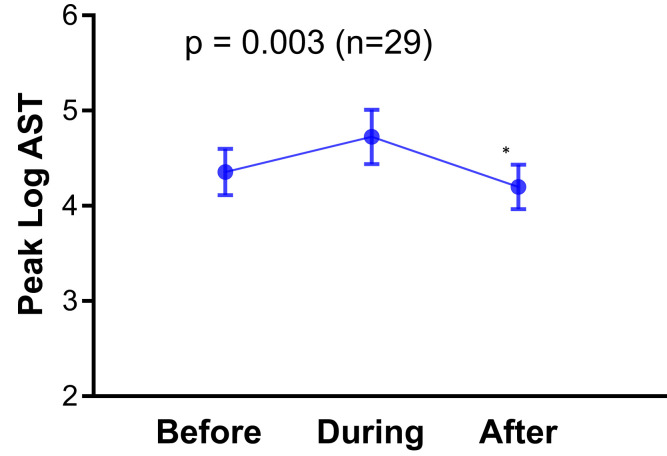
To emphasize the relationship between significant liver injury and ischemia, in 29 patients with AST values for 3 time periods (up to 2 days before, during, and up to 4 days after vasopressor discontinuation), the peak AST level was higher during vasopressor use (log 4.72), compared with before (log 4.35) or after vasopressors (log 4.19) (P = .003) (Supplementary Figure 2). Furthermore, in 41 patients with paired AST values before and during vasopressor use the peak AST level was higher during vasopressor use (log 4.79 vs log 4.47; P = .018), and in 76 patients with paired AST values during and after vasopressor use the peak AST level was higher during vasopressor use (log 4.7 vs log 4.32; P < .001).
Supplementary Figure 2.
Log aspartate aminotransferase (AST) values in 29 patients at 3 different time periods: up to 2 days before vasopressor use, during vasopressor use, and up to 4 days after vasopressor discontinuation.
To emphasize the relationship between AST level and tocilizumab use, in 426 patients with paired AST values (before and after tocilizumab), peak AST level was higher after tocilizumab, both in the 2-week period (log 4.45) and in the 4-week period (log 4.48) after its use compared with peak AST value before tocilizumab (log 3.91) (P < .001 for both comparisons).
Factors associated with death
A total of 136 patients (16.3%) died from COVID-19 during hospitalization. Compared with survivors, those who died had higher rates of ICU admission, intubation, management with steroids, vasopressors, and tocilizumab (Table 3 ). Patients who died also were more likely to have a hypercoagulable or hyperinflammatory state. Patients with pre-existing liver disease were not more likely to die. On multivariable analysis, vasopressor use (ischemia), a hyperinflammatory state, and a hypercoagulable state were independent predictors of death. Significant liver injury (AST ≥5× ULN) was not an independent predictor of death (Table 3). This finding was confirmed in sensitivity analyses that adjusted the multivariable model for baseline characteristics (age, sex, race, BMI, and comorbidities) (Supplementary Table 5) and in an analysis excluding 46 patients with a single admission value of AST (Supplementary Table 6). The lack of an association with death also was identified when using an ALT value 5 or more times the ULN as a marker of severe liver injury (Supplementary Table 7).
Table 3.
Events/Disease States Occurring During Hospitalization Associated With Death
| Characteristic |
Overall (834) |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| Alive (N = 698) | Dead (N = 136) | P value | Odds ratio | 95% CI | P value | ||
| Conditions during follow-up period, N (%) | – | – | – | ||||
| Intensive care unit | 259 (31.1) | 184 (26.4) | 75 (55.1) | <.001 | |||
| Venous thromboembolism | 4 (0.5) | 2 (0.3) | 2 (1.5) | .1 | |||
| Intubation | 108 (12.9) | 70 (10.0) | 38 (27.9) | <.001 | |||
| Medications, N (%) | |||||||
| Antibiotics | 543 (65.1) | 430 (61.6) | 113 (83.1) | <.001 | – | – | – |
| Hydroxychloroquine | 725 (86.9) | 617 (88.4) | 108 (79.4) | .004 | – | – | – |
| Tocilizumab | 502 (60.2) | 406 (58.2) | 96 (70.6) | .007 | 1.2 | 0.7–1.9 | .5 |
| Remdesivir | 51 (6.1) | 38 (5.4) | 13 (9.5) | .07 | – | – | – |
| Steroid | 273 (32.7) | 207 (29.6) | 66 (48.5) | <.001 | 1.5 | 1.0–2.3 | .06 |
| Statin | 343 (41.1) | 298 (42.7) | 45 (33.1) | .04 | – | – | – |
| NSAID | 369 (44.2) | 315 (45.1) | 54 (39.7) | .2 | – | – | – |
| Disease states, N (%) | |||||||
| AST ≥5× ULN | 105 (12.6) | 84 (12.0) | 21 (15.4) | .3 | 1.4 | 0.8–2.6 | .2 |
| Ischemic | 102 (12.2) | 69 (9.9) | 33 (24.3) | <.001 | 2.4 | 1.4–4.0 | .001 |
| Hyperinflammatory | 484 (58.4) | 383 (55.0) | 101 (76.0) | <.001 | 1.9 | 1.2–3.1 | .02 |
| Hypercoagulable | 251 (30.6) | 186 (27.0) | 65 (50.0) | <.001 | 1.7 | 1.1–2.6 | .02 |
NOTE. Values are reported as median (ranges) unless otherwise specified. Ischemic state = vasopressor use for at least 2 days; hyperinflammatory state = any high-sensitivity C-reactive protein value ≥100 mg/L; hypercoagulable state = any D-dimer ≥5 mg/L. Because all patients on vasopressors were in the intensive care unit and most were intubated and on antibiotics and/or hydroxychloroquine, these variables were not entered in the multivariable model.
AST, aspartate aminotransferase; CI, confidence interval; NSAID, nonsteroidal anti-inflammatory drug; ULN, upper limit of normal.
The AST level throughout hospitalization did not correlate with any of the following: hs-CRP (R = 0.04), D-dimer (R = 0.15), TBIL (R = 0.09) or, importantly, with the INR (R = 0.10) (Figure 2). Of the 105 patients with significant liver injury, there were only 8 patients (7.6%) in whom the peak AST level was associated with an INR greater than 1.5 as an indicator of liver failure (encephalopathy was not evaluable because all of these patients were sedated). Characteristics of these patients are shown in Supplementary Table 8.
Figure 2.
Correlation between aspartate aminotransferase (AST) (red) and international normalized ratio (INR) (blue) throughout hospitalization in 20 randomly selected patients. There was no clear correlation in the overall population (R = 0.10).
There was no significant difference in hospitalization status for those who were discharged, still admitted, or died at the time of follow-up evaluation (P = .3) between patients with and without significant liver injury during hospitalization (Table 2).
Discussion
The development of liver test abnormalities during hospitalization for COVID-19 were frequent. However, the pathophysiology of these abnormalities and their relationship to increased mortality, vis-à-vis concomitant confounders (ischemia, medications, and hyperinflammatory and/or hypercoagulable states), remains unclear. Abnormalities were predominantly hepatocellular and consistently showed AST values higher than ALT values. This is unlike most viral hepatitides (hepatitis A, B, or C) in which ALT value is higher than AST value. Conditions in which AST value is higher than ALT value are those that predominantly affect the hepatic centrilobular area such as alcoholic liver disease, drug-induced liver disease, and ischemic injury, although in these conditions AST levels are generally higher than those noted in patients with COVID-19.16 Death from hepatocellular liver injury is always associated with liver insufficiency, which in the acute setting is indicated by an increased INR and hepatic encephalopathy. Despite thousands of patients with COVID-19 reported in the literature, there have been only 2 reports of acute hepatitis that could be ascribed to SARS-CoV-2,17 , 18 only 1 of whom developed severe acute hepatitis with liver failure (with prolonged INR and encephalopathy) with some histologic evidence of viral hepatitis but without confirmatory viral inclusions and no liver polymerase chain reaction.18
Aminotransferase abnormalities during COVID-19 are commonly mild, with only 20% or so of patients developing increases in AST values of 5 or more times the ULN. The objectives of this study were to determine whether liver injury during hospitalization is more reflective of concomitant factors than direct liver injury by SARS-CoV-2, and whether this liver injury is related independently to death in patients with COVID-19.
Several studies have looked specifically at abnormalities in liver tests during hospitalization.8, 9, 10, 11 , 13 , 14 They were mostly rapid communications performed at the beginning of the pandemic that relied on deidentified data obtained from administrative databases without confirmation from medical record review and using admission data and/or peak abnormalities during hospitalization.8, 9, 10 Similar to our study, all of the studies showed that aminotransferase abnormalities were more marked in patients with severe COVID-19 (using different definitions of severity). Although a small study by Bloom et al13 could not find any factor that would predict significant liver injury, larger studies identified peak ferritin and peak interleukin (IL)6 levels8; age, male sex, fever, dyspnea, gastrointestinal symptoms, antibiotics, steroid, leukocyte, and platelet counts10; or preadmission AST and tocilizumab levels.9 Regarding factors associated with death, the study by Phipps et al8 showed that age, diabetes, intubation, and peak ALT levels were independent predictors of death, while the study by Lei et al10 showed that, compared with other liver tests, peak AST level was associated with the highest risk of death. However, variables were analyzed in these studies without accounting for intercorrelations among them.
Our study correlated pathophysiologically relevant disease states during COVID-19 that potentially could be associated with significant liver injury during hospitalization and established intercorrelations among predictive variables. We defined significant liver injury using an AST value more than 5 times the ULN and, although one can argue that the source of AST is the muscle, we showed a very close correlation between AST and ALT values and previous data showing a lack of correlation between AST and creatine kinase values, minimize this possibility for the majority of cases.
Ischemic liver injury results from a global decrease in blood flow to the liver associated with hypotension and therefore we considered vasopressor use as a surrogate for ischemic liver injury.19 Because most of the patients on vasopressors also were intubated, this also would add to the possibility of ischemic damage resulting from hypo-oxygenation. Initially, we considered any use of vasopressors (in 133 patients) during hospitalization but realized that some patients had received only 1 dose of vasopressors and therefore changed our definition to include 102 patients who had been on vasopressors for at least 2 consecutive days. The so-defined ischemic state was the most important predictor of significant liver injury in our study and a significant predictor of death (independent of AST value). Of note, results using the original definition of ischemia were not different (results not shown).
It also has been proposed that the liver injury could be from a hyperinflammatory state that is characterized by immune dysregulation and hypercytokinemia, resulting in cytokine storm syndrome.20 Although IL6 increases were only modest in our study and not at levels described in other cytokine storm syndromes such as in chimeric antigen T-cell therapy in which it exceeds 1000 pg/mL, we decided to incorporate a marker of hyperinflammation given the finding of high ferritin (another marker of inflammation) as a predictor of liver injury in the study by Phipps et al.8 However, because there was a significant correlation between ferritin, IL6, and hs-CRP values, we defined it solely as a hs-CRP level greater than 100 mg/L. Although hs-CRP levels did not correlate with AST levels throughout hospitalization and was not predictive of significant liver injury, the inflammatory state was predictive of death, as has been shown in other studies.13 , 21
Another potential cause of liver injury is a hypercoagulable state, which also has been associated with COVID-19. Studies have shown increased markers for endothelial cell and platelet activation in patients with severe disease, potentially resulting in microthrombi22, 23, 24 that could affect liver blood flow. We initially defined the hypercoagulable state as increased D-dimer and fibrinogen levels, however, because these tests also correlated significantly, we defined it solely as a D-dimer level of 5 mg/L or higher throughout hospitalization. Although the hypercoagulable state was not predictive of significant liver injury, it was associated with mortality.
To investigate drug-induced liver injury we looked at all medications used during hospitalization. Drugs commonly used in patients with COVID-19 known to cause drug-induced liver injury are tocilizumab,9 , 25 lopinavir/ritonavir,9 , 25 , 26 hydroxychloroquine,9 , 25 remdesivir,8 , 25 , 27 and antibiotics. Because most patients on pressors were on antibiotics and the classes of antibiotics varied, they were not incorporated in multivariate models. On univariate analysis both tocilizumab and remdesivir were significantly more common in patients with significant liver injury. Remarkably, and similar to the study by Hundt et al,9 we found tocilizumab use to be an independent predictor of significant liver injury, but not of death.
One of the main issues is the association between significant liver injury during hospitalization and death in patients with COVID-19. We found that significant liver injury was not an independent predictor of death, whereas disease states associated with COVID-19 (ischemic, hyperinflammatory, and hypercoagulable) were predictive. Furthermore, AST levels did not correlate with INR (Figure 2), indicating that abnormalities were not associated with liver insufficiency. In detailing the characteristics of only 8 patients with AST levels more than 15 times the ULN associated with an INR greater than 1.5, all had an abnormal serum creatinine concentration (range, 1.8–7.1), which suggests the liver injury was part of a multiorgan failure state.
In conclusion, although direct viral injury cannot be entirely excluded, our study indicates that concomitant factors, mainly ischemia and drug-induced liver injury, play a major role in the pathogenesis of significant liver injury during COVID-19. Perhaps more importantly, this liver injury is not associated directly with liver insufficiency or death.
Acknowledgments
CRediT Authorship Contributions
Michael Chew, MD (Conceptualization: Equal; Data curation: Lead; Formal analysis: Supporting; Investigation: Lead; Methodology: Lead; Project administration: Lead; Supervision: Supporting; Validation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Zeyu Tang (Conceptualization: Equal; Data curation: Lead; Investigation: Supporting; Methodology: Supporting; Project administration: Equal; Software: Lead; Validation: Lead; Writing – review & editing: Supporting)
Christopher Radcliffe (Conceptualization: Supporting; Data curation: Equal; Methodology: Equal; Validation: Equal; Writing – review & editing: Supporting)
Dennis Caruana (Conceptualization: Supporting; Data curation: Supporting; Methodology: Supporting; Validation: Equal; Writing – review & editing: Supporting)
Natty Doilicho (Conceptualization: Supporting; Data curation: Supporting; Methodology: Supporting; Validation: Supporting)
Maria M. Ciarleglio (Conceptualization: Supporting; Formal analysis: Lead; Methodology: Equal; Validation: Equal; Writing – review & editing: Supporting)
Yanhong Deng (Conceptualization: Equal; Formal analysis: Lead; Methodology: Equal; Validation: Lead; Writing – review & editing: Supporting)
Guadalupe Garcia-Tsao (Conceptualization: Lead; Data curation: Supporting; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Supervision: Lead; Validation: Equal; Visualization: Equal; Writing – original draft: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the Yale Liver Center National Institutes of Health grant P30 DK34989.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.05.022.
Supplementary Material
Supplementary Table 1.
Laboratory Reference Ranges
| Laboratory value | Reference range |
|---|---|
| Total bilirubin | ≤1.2 mg/dL |
| Alkaline phosphatase | ≤122 U/L |
| Aspartate aminotransferase | ≤33 U/L |
| Alanine aminotransferase | ≤34 U/L |
| Albumin | >3.5 g/dL |
| High-sensitivity C-reactive protein, mg/L | <1.0: lower relative cardiovascular risk 1.0–3.0: average relative cardiovascular risk 3.1–10.0: higher relative cardiovascular risk >10.0: persistent increase may be associated with infection and inflammation |
| Ferritin | ≤400 ng/dL |
| Fibrinogen | ≤464 mg/dL |
| D-dimer (mg/L) | ≤0.68 mg/L |
| IL6 | ≤5 pg/mL |
| IL8 | ≤5 pg/mL |
| IL1β | ≤36 pg/mL |
| Interferon-γ | ≤5 pg/mL |
| TNF-α | ≤22 pg/mL |
IL, interleukin; TNF, tumor necrosis factor.
Supplementary Table 2.
Antibiotics Used in 834 Patients Admitted With Coronavirus-19 Disease
| Antibiotic class | N (%) |
|---|---|
| β-lactam | 411 (49.3) |
| Vancomycin | 278 (33.3) |
| Piperacillin/tazobactam | 224 (27.5) |
| Tetracycline | 220 (26.4) |
| Metronidazole | 62 (7.4) |
| Macrolide | 49 (5.9) |
| Amoxicillin/clavulanate | 40 (4.9) |
| Sulfamethoxazole/trimethoprim | 35 (4.2) |
| Aminoglycoside | 14 (1.7) |
| Clindamycin | 5 (0.6) |
| Carbapenem | 9 (1.1) |
| Linezolid | 3 (0.4) |
| Daptomycin | 2 (0.2) |
Supplementary Table 3.
Admission Liver Tests by Degree of Abnormality in 834 Patients Admitted With Coronavirus-19 Disease
| Variables | Total (N = 834) |
|---|---|
| AST, N (%) | |
| Normal | 313 (37.5) |
| 1–2× ULN | 347 (41.6) |
| 2–5× ULN | 149 (17.9) |
| ≥5× ULN | 25 (3.0) |
| AST >5× ULN anytime | 105 (12.6) |
| ALT, N (%) | |
| Normal | 553 (66.3) |
| 1–2× ULN | 185 (22.2) |
| 2–5× ULN | 83 (9.9) |
| ≥5× ULN | 13 (1.6) |
| ALT ≥5× ULN anytime | 93 (11.1) |
| Total bilirubin, N (%) | |
| Normal | 808 (96.9) |
| 1–2× ULN | 22 (2.6) |
| 2–5× ULN | 4 (0.5) |
| ≥5× ULN | 0 |
| Alkaline phosphatase, N (%) | |
| Normal | 735 (88.1) |
| 1–2× ULN | 84 (10.1) |
| 2–5× ULN | 14 (1.7) |
| ≥5× ULN | 1 (0.1) |
| Albumin, mg/dL, N = 831 | 3.5 (1.7–5.4) |
| INR (N = 825) | 1.0 (0.8–9.9) |
| Normal, N (%) | 721 (87.4) |
| 1–2× ULN, N (%) | 90 (10.9) |
| 2–5× ULN, N (%) | 7 (0.8) |
| ≥5× ULN, N (%) | 7 (0.8) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; ULN, upper limit of normal.
Supplementary Table 4.
Multivariate Model for Death Using AST Excluding 46 Patients Without Follow-Up Evaluation
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.1 | 1.0–1.1 | <.0001 |
| Sex | 1.6 | 1.0–2.6 | .05 |
| Race | 0.8 | 0.4–1.4 | .7 |
| BMI | 1.0 | 0.9–1.0 | .8 |
| Hypertension | 0.9 | 0.5–1.8 | .8 |
| Congestive heart failure | 0.9 | 0.5–1.6 | .8 |
| Chronic lung disease | 0.6 | 0.4–1.1 | .1 |
| Diabetes | 1.0 | 0.6–1.6 | 1.0 |
| Pre-existing liver disease | 0.8 | 0.3–2.1 | .6 |
| Vasopressor | 5.1 | 2.8–9.4 | <.0001 |
| Tocilizumab use | 1.5 | 0.8–2.7 | .1 |
| Hyperinflammatory | 1.5 | 0.9–2.7 | .1 |
| Hypercoagulable | 1.5 | 0.9–2.5 | .1 |
| ALT >5× ULN | 1.2 | 0.5–2.6 | .6 |
| Steroids | 1.9 | 1.1–3.2 | .01 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; OR, odds ratio; ULN, upper limit of normal.
Supplementary Table 5.
Multivariate Analysis for Predictors of ALT Level >5× ULN
| Characteristic | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Tocilizumab | 3.8 | 1.9–7.7 | .0002 |
| Ischemia | 3.2 | 1.8–5.6 | <.0001 |
| Hyperinflammatory | 1.4 | 0.8–2.4 | .2 |
| Hypercoagulable | 1.0 | 0.6–1.7 | .9 |
| Steroids | 1.0 | 0.6–1.7 | .9 |
ALT, alanine aminotransferase; ULN, upper limit of normal.
Supplementary Table 6.
Baseline Characteristics Associated With Death Using AST Value
| Characteristic | Overall (834) | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| Alive (N = 698) | Dead (N = 136) | P value | Odds ratio | 95% CI | P value | ||
| Age, y | 68 (20–103) | 66 (20–98) | 82 (29–103) | <.001 | 1.1 | 1.0–1.1 | <.0001 |
| Sex | .2 | 1.5 | 0.9–2.3 | .1 | |||
| Male, % | 47.6 | 46.5 | 52.9 | ||||
| Female, % | 52.4 | 53.4 | 47.1 | ||||
| Race, N (%) | .04 | 0.8 | 0.4–1.3 | .6 | |||
| White | 459 (55) | 53.3 | 63.9 | ||||
| Black | 240 (28.8) | 30.1 | 22.1 | ||||
| Asian or Pacific Islander | 14 (1.7) | 1.7 | 1.5 | ||||
| Other | 121 (14.5) | 14.9 | 12.5 | ||||
| Median BMI, kg/m2 (range) | 28.2 (14.6–73.9) | 28.4 (14.6–73.9) | 26.1 (15–64.8) | <.001 | 1 | 0.9–1.0 | .9 |
| Comorbidities, N (%) | |||||||
| Coronary artery disease | 97 (13.5) | 80 (13.5) | 17 (13.9) | .9 | – | – | – |
| Hypertension | 631 (75.6) | 519 (74.4) | 112 (82.3) | .05 | 0.8 | 0.4–1.6 | .6 |
| Congestive heart failure | 249 (29.8) | 201 (28.8) | 48 (35.3) | .1 | 0.9 | 0.5–1.5 | .7 |
| Chronic lung disease | 277 (33.2) | 237 (33.9) | 40 (29.4) | .3 | 0.6 | 0.4–1.1 | .1 |
| Diabetes | 357 (42.8) | 293 (42.0) | 64 (47.1) | .3 | 1.1 | 0.7–1.8 | .6 |
| Pre-existing liver disease, N (%) | 51 (6.1) | 45 (6.4) | 6 (4.4) | .4 | 0.9 | 0.3–2.5 | .8 |
| Compensated cirrhosis | 10 (1.2) | 8 (1.1) | 2 (1.5) | .5 | |||
| Decompensated cirrhosis | 3 (0.3) | 2 (0.3) | 1 (0.7) | .4 | |||
| Tocilizumab | 502 (60.2) | 406 (58.2) | 96 (70.6) | .007 | 1.2 | 0.7–2.0 | .6 |
| Steroid | 273 (32.7) | 207 (29.6) | 66 (48.5) | <.001 | 1.8 | 1.1–2.9 | .02 |
| Ischemia | 102 (12.2) | 69 (9.9) | 33 (24.3) | <.001 | 5 | 2.8–9.3 | <.0001 |
| Hyperinflammatory | 484 (58.4) | 383 (55.0) | 101 (76.0) | <.001 | 1.8 | 1.1–3.0 | .02 |
| Hypercoagulable | 251 (30.6) | 186 (27.0) | 65 (50.0) | <.001 | 1.4 | 0.9–2.3 | .7 |
| AST, >5× ULN | 105 (12.6) | 84 (12.0) | 21 (15.4) | .3 | 0.9 | 0.4–1.7 | .7 |
AST, aspartate aminotransferase; BMI, body mass index; ULN, upper limit of normal.
Supplementary Table 7.
Multivariate Model for Death Using ALT
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.1 | 1.0–1.1 | <.0001 |
| Sex | 1.5 | 0.9–2.3 | .1 |
| Race | 0.7 | 0.4–1.3 | .6 |
| BMI | 1.0 | 0.9–1.0 | .9 |
| Hypertension | 0.8 | 0.4–1.6 | .6 |
| Congestive heart failure | 0.9 | 0.5–1.5 | .7 |
| Chronic lung disease | 0.6 | 0.4–1.1 | .1 |
| Diabetes | 1.1 | 0.7–1.7 | .7 |
| Pre-existing liver disease | 0.9 | 0.3–2.4 | .8 |
| Vasopressor requirement | 5.2 | 2.9–9.5 | <.0001 |
| Tocilizumab use | 1.1 | 0.7–2.0 | .5 |
| Any inflammatory | 1.8 | 1.1–3.1 | .02 |
| Any hypercoagulable | 1.4 | 0.8–2.3 | .14 |
| Peak ALT value | 1.1 | 0.5–2.2 | .8 |
| Steroids | 1.7 | 1.0–2.9 | .02 |
ALT, alanine aminotransferase; BMI, body mass index; OR, odds ratio.
Supplementary Table 8.
Characteristics of 8 of 105 (7.6%) Patients Who Developed Liver Insufficiency During COVID-19
| ID | Peak AST | Peak ALT | Peak TBIL | Peak AP | Peak INR | sCr | Comments |
|---|---|---|---|---|---|---|---|
| 1 | 3663 | 2157 | 2.6 | 174 | 3.73 | 6.0 | 66-year-old with severe COVID-19 requiring intubation COVID Tx: hydroxychloroquine, tocilizumab Antibiotics: ceftriaxone, piperacillin/tazobactam, vancomycin Vasopressors: norepinephrine Complications: perforated diverticulitis and death |
| 2 | 3054 | 904 | 0.7 | 357 | 4.67 | 1.8 | 42-year-old with severe COVID-19 requiring intubation COVID-Tx: hydroxychloroquine, tocilizumab Antibiotics: ceftriaxone, doxycycline Vasopressors: norepinephrine, epinephrine Complications: cardiac arrest with shock and death |
| 3 | 5283 | 2724 | 9.2 | 211 | 2.56 | 1.8 | 29-year-old with severe COVID requiring intubation COVID-Tx: hydroxychloroquine, tocilizumab, remdesivir, steroids Antibiotics: ceftriaxone, doxycycline Vasopressors: norepinephrine, phenylephrine, vasopressin Complications: neuroleptic malignant syndrome with fever 108°F and death |
| 4 | 9441 | 3141 | 16.8 | 523 | 1.95 | 3.1 | 64-year-old with history of HIV presented with severe COVID requiring intubation COVID-Tx: hydroxychloroquine, tocilizumab, remdesivir, steroids Antibiotics: piperacillin/tazobactam, ciprofloxacin, daptomycin Vasopressors: norepinephrine, vasopressin, phenylephrine Complications: multiple infections and renal failure requiring CVVH and death |
| 5 | 1825 | 1691 | 2.7 | 77 | 3.18 | 6.9 | 74-year-old with severe COVID requiring intubation COVID-Tx: hydroxychloroquine, tocilizumab, steroids Antibiotics: piperacillin/tazobactam, vancomycin Vasopressors: norepinephrine, phenylephrine Complications: NSTEMI requiring heparin drip and hemorrhagic shock resulting in death |
| 6 | 1107 | 452 | 3.9 | 339 | 2.08 | 3.8 | 63-year-old severe COVID requiring intubation COVID-Tx: tocilizumab, steroids Antibiotics: piperacillin/tazobactam Vasopressors: norepinephrine, phenylephrine, vasopressin Complications: renal failure requiring CVVH and malignant hyperthermia with Tmax 108°F and death |
| 7 | 4221 | 1447 | 1.8 | 209 | 2.3 | 7.1 | 55-year-old severe COVID requiring intubation COVID-Tx: hydroxychloroquine, tocilizumab, steroids Antibiotics: ceftriaxone, ciprofloxacin, ertapenem Vasopressors: norepinephrine, epinephrine, phenylephrine, vasopressin Complications: acute kidney injury requiring dialysis and death |
| 8 | 8921 | 5106 | 1 | 228 | 1.89 | 3.2 | 89-year-old severe COVID requiring NRB COVID-Tx: Hydroxychloroquine Antibiotics: piperacillin/tazobactam Vasopressors: none but hypotensive and increasing lactate and transitioned to comfort measures only Complications: bacterial pneumonia and multi-organ failure and death |
NOTE. Of the 105 patients with severe liver injury, there were only 8 patients (7.6%) in whom peak AST level was associated with an INR greater than 1.5 (encephalopathy was not evaluable because all patients were sedated).
AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; COVID-19, coronavirus-19 disease; CVVH, continuous veno-venous hemofiltration; HIV, human immunodeficiency virus; INR, international normalized ratio; NRB, non-rebreather; NSTEMI, non-ST elevation myocardial infarction; sCr, serum creatinine; TBIL, total bilirubin; Tmax, maximal temperature; Tx, treatment.
References
- 1.Pirola C.J., Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagana S.M., Kudose S., Iuga A.C. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonzogni A., Previtali G., Seghezzi M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan S., Lim J.K., Altayar O. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020;159:739–758. doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao R., Qiu Y., He J.S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipps M.M., Barraza L.H., LaSota E.D. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hundt M.A., Deng Y., Ciarleglio M.M. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei F., Liu Y.M., Zhou F. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponziani F.R., Del Zompo F., Nesci A. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2 positive patients. Aliment Pharmacol Ther. 2020;52:1060–1068. doi: 10.1111/apt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Z., Chen L., Li J. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom P.P., Meyerowitz E.A., Reinus Z. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. 2021;73:890–900. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 14.Huang H., Chen S., Li H. The association between markers of liver injury and clinical outcomes in patients with COVID-19 in Wuhan. Aliment Pharmacol Ther. 2020;52:1051–1059. doi: 10.1111/apt.15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwo P.Y., Cohen S.M., Lim J.K. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 16.Kasarala G., Tillmann H.L. Standard liver tests. Clin Liver Dis (Hoboken) 2016;8:13–18. doi: 10.1002/cld.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wander P., Epstein M., Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115:941–942. doi: 10.14309/ajg.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melquist S., Estepp K., Aleksandrovich Y. COVID-19 presenting as fulminant hepatic failure: a case report. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strassburg C.P. Gastrointestinal disorders of the critically ill. Shock liver. Best Pract Res Clin Gastroenterol. 2003;17:369–381. doi: 10.1016/s1521-6918(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.Y.C., Hoiland R.L., Stukas S. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;56 doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manson J.J., Crooks C., Naja M. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goshua G., Pine A.B., Meizlish M.L. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConnell M., Kawaguchi N., Kondo R. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. Journal of Hepatology. 2021 doi: 10.1016/j.jhep.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhania N., Bansal S., Nimmatoori D.P. Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020;20:393–403. doi: 10.1007/s40256-020-00431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong G.L., Wong V.W., Thompson A. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5:776–787. doi: 10.1016/S2468-1253(20)30190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong P., Xu J., Yang D. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grein J., Myers R.P., Brainard D. Compassionate use of remdesivir in Covid-19. Reply. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]