Abstract
Banana (Musa spp.), a major cash and staple fruit crop in many parts of the world, is infected by Fusarium wilt, which contributes up to 100% yield loss and causes social consequences. Race 1 and race 2 of Panama wilt caused by Fusarium oxysporum f. sp. cubense (Foc) are prevalent worldwide and seriously affect many traditional varieties. The threat of Foc tropical race 4 (Foc TR4) is looming large in African counties. However, its incidence in India has been confined to Bihar (Katihar and Purnea), Uttar Pradesh (Faizabad), Madhya Pradesh (Burhanpur) and Gujarat (Surat). Management of Foc races by employing fungicides is often not a sustainable option as the disease spread is rapid and they negatively alter the biodiversity of beneficial ectophytes and endophytes. Besides, soil drenching with carbendazim/trifloxystrobin + tebuconazole is also not effective in suppressing the Fusarium wilt of banana. Improvement of resistance to Fusarium wilt in susceptible cultivars is being addressed through both conventional and advanced breeding approaches. However, engineering of banana endosphere with bacterial endophytes from resistant genotypes like Pisang lilly and YKM5 will induce the immune response against Foc, irrespective of races. The composition of the bacterial endomicrobiome in different banana cultivars is dominated by the phyla Proteobacteria, Bacteroidetes and Actinobacteria. The major bacterial endophytic genera antagonistic to Foc are Bacillus, Brevibacillus, Paenibacillus, Virgibacillus, Staphylococcus, Cellulomonas, Micrococcus, Corynebacterium, Kocuria spp., Paracoccus sp., Acinetobacter spp. Agrobacterium, Aneurinibacillus, Enterobacter, Klebsiella, Lysinibacillus, Micrococcus, Rhizobium, Sporolactobacillus, Pantoea, Pseudomonas, Serratia, Microbacterium, Rhodococcus, Stenotrophomonas, Pseudoxanthomonas, Luteimonas, Dokdonella, Rhodanobacter, Luteibacter, Steroidobacter, Nevskia, Aquicella, Rickettsiella, Legionella, Tatlockia and Streptomyces. These bacterial endophytes promote the growth of banana plantlets by solubilising phosphate, producing indole acetic acid and siderophores. Application of banana endophytes during the hardening phase of tissue-cultured clones serves as a shield against Foc. Hitherto, MAMP molecules of endophytes including flagellin, liposaccharides, peptidoglycans, elongation factor, cold shock proteins and hairpins induce microbe-associated molecular pattern (MAMP)-triggered immunity to suppress plant pathogens. The cascade of events associated with ISR and SAR is induced through MAPK and transcription factors including WRKY and MYC. Studies are underway to exploit the potential of antagonistic bacterial endophytes against Foc isolates and to develop an understanding of the MAMP-triggered immunity and metabolomics cross talk modulating resistance. This review explores the possibility of harnessing the potential bacterial endomicrobiome against Foc and developing nanoformulations with bacterial endophytes for increased efficacy against lethal pathogenic races of Foc infecting banana.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02833-5.
Keywords: Bacterial endophytes, Banana, Fusarium wilt, Foc, Plant immunity, Growth promotion
Introduction
Bananas and plantains are major fruit crops grown across the tropics and subtropics of the world due to their economic and nutritional significance. They are considered as the fourth most important staple food crop after cereals in many parts of Africa to meet human dietary and calorific requirements. There has been a consistent rise in the demand and trade for banana at the global level revealing the significance of banana as a major cash crop. Cultivated in 845 thousand hectares in the world, the crop has been an integral part of economic well-being of small and medium farm holders in many parts of Asia. Although bananas are affected by many biotic stresses, the incidence of Fusarium wilt race 4 (Fusarium oxysporum f. sp. cubense, both tropical and subtropical races) has emerged as the major threat in recent times. Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc TR4) pathogen is known to affect all cultivated varieties including the Cavendish cultivars, which were known to resist other races of Foc. Recently, the incidence of Foc TR4 has been reported in South-East Asia and small pockets of the Indian subcontinent. Specifically, in some parts of Bihar and Gujarat, suspected incidence indicates the potential threat of outbreak in the near future. Hence, there is an urgent need for managing and combating the spread of Fusarium wilt of banana. Since complete reliability on chemical protection is yet to become a reality for managing Fusarium wilt in banana, and taking into account the adverse effects of fungicidal formulations on the soil and environment, it is imperative to find alternative and sustainable approaches to manage this fungal pathogen to safeguard the global banana industry. Biocontrol measures involving selection of endophytes have been a part of integrated disease management in several agricultural and horticultural crops (Vinodkumar et al. 2015; Dheepa et al. 2016). Identification and exploitation of bacterial endophytes associated with resistance and susceptibility could be a possible and viable strategy to complement existing management strategies. This review attempts to provide an overview of bacterial endophytic associations in banana and their implications to control Fusarium wilt.
Significance of banana bacterial endophytes
Endophytes are known to mobilise nutrients to their host plants, modulate plant growth and induce resistance in banana. The major bacterial endomicrobiomes associated with growth promotion, nutrient mobilisation and induction of systemic resistance in banana cv. Grand Naine belong to the class Actinobacteria, α-Proteobacteria, γ-Proteobacteria and Firmicutes. Actinobacteria include Arthrobacter, Brevibacterium, Corynebacterium, Curtobacterium, Kocuria, Kytococcus, Micrococcus, Naumannella, Rothia, and Tessaracoccus. Endophytes pertaining to α-Proteobacteria and γ-Proteobacteria have been identified as Brevundimonas, Enterobacter, Klebsiella, Pseudomonas, Serratia and Sphingomonas. The class Firmicutes comprises of the genera Bacillus and Staphylococcus (Sekhar and Pious 2015). Endophytic bacterial communities have the genes for the synthesis of indole acetic acid (Zuniga et al. 2013), cytokinins (Bhore et al. 2010) and gibberellins (Shahzad et al. 2016) responsible for plant growth promotion. Besides, they produce various plant hormones and aid in growth promotion (Santoyo et al. 2016). The growth hormones play a key role in boosting plant growth and to withstand biotic as well as abiotic stresses. They also produce cell wall degrading enzymes (chitinase, β-1,3-glucanase), antimicrobial compounds, reduce endogenous stress-related ethylene (ET), trigger-induced systemic resistance (ISR), quench the quorum sensing molecules of phytopathogens, and compete for niche and resources (Compant et al. 2010; Glick 2014; Rolli et al. 2015; Tsurumaru et al. 2015; Miliute et al. 2016; Santoyo et al. 2016). The endophytic bacteria Klebsiella pneumoniae promoted plant growth of micropropagated ‘Prata Ana’ banana plantlets (Fernandes et al. 2013). Biopriming of micropropagated banana plantlets with endophytic B. subtilis promoted plant growth by increasing plant height, pseudostem girth, number of leaves and leaf area (Rajamanickam et al. 2018). Endophytic Bacillus spp., Streptomyces luteogriseus and Pseudomonas fluorescens increased the solubilisation efficiency of inorganic phosphate (Matsuoka et al. 2013). Biopriming of tissue-cultured banana plantlets with endophytic B. subtilis induced the expression of defence-related chemicals and enzymes, such as phenol, phenylalanine ammonia lyase, peroxidase and polyphenol oxidase, and pathogenesis-related proteins, such as chitinase and β-1,3-glucanase. This in turn modulates the plant immune system and enhances resistance in banana (Harish et al. 2009; Rajamanickam et al. 2018). The endophytic bacterium B. subtilis is effective against root knot nematode, burrowing nematode and root lesion nematode infecting banana (Jonathan and Umamaheswari 2006). Similarly, the endophytes from banana roots, viz. Pseudomonas spp., Bacillus spp., Streptomyces spp., were found in abundance. In Streptomyces sp., the strain designated as SA has the ability to colonise the inner part of root systems and articulate the biocontrol efficacy against banana nematode Meloidogyne javanica. Pseudomonas spp. exhibited greater root colonisation ability compared with Bacillus spp. and Streptomyces spp. (Su et al. 2017). Based on the literature survey, several classes of endophytes with diverse functions are known to exist in banana.
Biodiversity of bacterial endophytes in banana—the key for induction of resistance against Foc isolates
Bacterial endophytes are highly diverse, and vary within plant species, varieties, plant parts as well as with phenological stages of crop growth. A phylogenetic tree constructed based on nucleotide sequence illustrates the endophytic diversity of the bacterial community in banana (Supplementary Table 1; Supplementary Fig. 1). Identification of bacterial endophytes from shoot tip culture of Grand Naine banana revealed the presence of Bacillus, Brevibacillus, Paenibacillus, Virgibacillus and Staphylococcus pertaining to Firmicutes. Actinobacteria include Cellulomonas, Micrococcus, Corynebacterium and Kocuria spp., α-Proteobacteria includes Paracoccus sp., and γ-Proteobacteria comprises Pseudomonas and Acinetobacter spp. (Thomas et al. 2008). In roots of Brazilian banana ‘Prata Ana’ belonging to Pome sub group (Musa AAB), about 150 g-positive and 51 g-negative bacteria have been isolated. Identification based on partial sequencing with 16S rDNA revealed that all the isolates belong to 15 different species represented in 10 genera including Agrobacterium, Aneurinibacillus, Bacillus, Enterobacter, Klebsiella, Lysinibacillus, Micrococcus, Paenibacillus, Rhizobium and Sporolactobacillus. In general, the predominant Bacillus species associated with banana are B. amyloliquefaciens, B. axarquiensis, B. cereus, B. flexus, B. megaterium, B. methylotrophicus, B. licheniformis, B. pumilus, B. safensis, B. subtilis, B. tequilensis and B. thuringiensis. The dominance of B. pumilus and B. subtilis is prominent among the identified species (Suzane et al. 2013).
Besides the Bacillus species, Agrobacterium tumefaciens has also been identified as an endophyte in banana revealing the marked diversity of endophytic Gram-negative and Gram-positive bacteria (Suzane et al. 2013; Bubici et al. 2019). More than 75% of endophytic bacterial isolates are Gram-negative from banana shoot tip cultures as against the relatively low representation of Gram-positive endophytic bacteria (Habiba et al. 2002; Thomas et al. 2008; Ganen et al. 2009; Suzane et al. 2013). The Gram-positive endophytic species isolated from banana roots are B. cereus, B. subtilis, B. megaterium and B. pumilus (Elvira-Recuenco and Vuurde 2000; Araujo et al. 2002). Similarly, the endophytic isolates identified from red banana are Bacillus spp. (47.05%), followed by Proteobacteria (41.17%) and Actinobacteria (11.76%). The γ-Proteobacteria subgroup isolated in banana cultivars comprise of Pantoea, Pseudomonas, Serratia and Klebsiella. The wilt resistant cultivar red banana has a unique group of α-Proteobacteria, viz. Rhizobium and Microbacterium of Actinobacteria. The endophytic Rhodococcus has been identified in the highly susceptible banana cv. Rasthali. The dominant endophytic bacterial genera in the tested banana cultivars are Bacillus (38.24%), followed by Pantoea (32.35%) in Rasthali, whereas Bacillus (36.97%) and Klebsiella (29.41%) were predominant in red banana (Karthik et al. 2017). Unravelling the composition and network relationship of endomicrobiome in roots of dwarf Cavendish banana of mother plant and suckers revealed a low diversity in the endosphere of banana roots. Among the endomicrobiome associated with banana roots of mother plant and suckers, Pseudomonas was identified as the predominant genus through co-occurrence network analysis. Besides, mother plants and suckers had a significant difference in the recruitment of endomicrobiome. There was a significant difference in the diversity of endomicrobiome during different phenological stages, thus emphasising the need to select appropriate stages to harvest the antifungal property of endophytes against Foc strains. Culturable bacteria in the root endosphere are dominated by the phyla Proteobacteria, Bacteroidetes and Actinobacteria. The major culturable bacterial genera isolated as endophytes were identified as Pseudomonas, Rhizobium, Streptomyces and Actinophytocola. The increased abundance of culturable bacteria was confirmed as different species of Pseudomonas (Gomez-Lama Cabanas et al. 2021).
Streptomyces griseorubiginosus were frequently isolated from the leaves and roots of healthy and wilt-affected banana plants. The diversity of the Streptomyces is high in wilted leaves compared with healthy leaves. Likewise, the diversity of actinomycete communities has also been observed between wilting and healthy roots. The population or the proportion of a particular strain is known to vary within the plant system depending on the host organ during the advent of disease incidence. The proportion of antagonistic S. griseorubiginosus against Foc was higher in healthy roots than in wilting roots. However, there was no difference in the population of antagonistic strains isolated from healthy and wilting leaves (Cao et al. 2004b). Thus, the diversity of banana endophytes varies depending on the varieties, resistant and susceptible cultivars, stages of crop growth and even between various plant parts of banana. Hence, knowledge on diversity of endomicrobiome in banana will help in understanding the distribution of beneficial endophytes throughout the crop growth period and in exploring the in planta resistance against Foc isolates (Fig. 1).
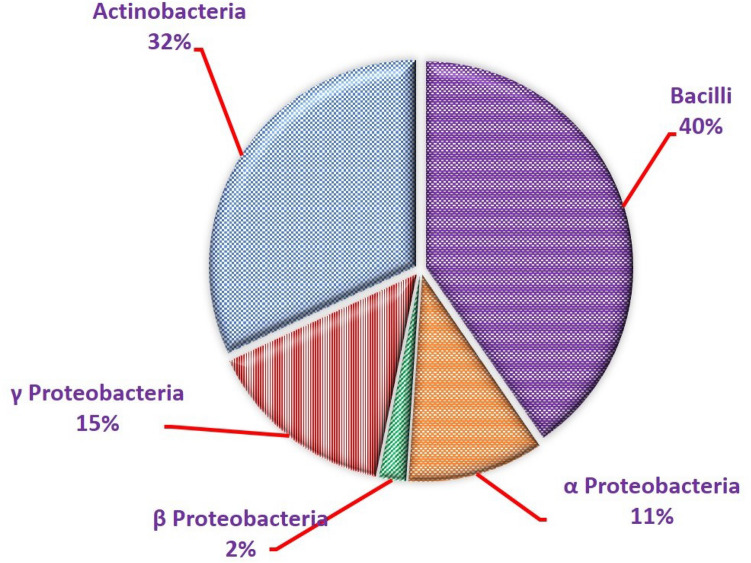
Fig. 1.
Proportions of bacterial endophytes in banana conferring resistance against Fusarium wilt of banana. Where 19—Bacilli are B. pumilus, B. thuringiensis, B. subtillis, B. cereus, B. amyloliquefaciens, B. flexus, B. megaterium, B. methylotrophicus, B. axarquienses, B. licheniformis, B. tequlensis, B. safensis, B. okhensis, Panibacillus sp., Staphylococcus pasteuri, Staphylococcus epidermidis, Sporalactobacillus sp. and Aneurinibacillus sp., Lysinibacillus sp.; 15—Actinobacteria are Micrococcus luteus, M. terreus, M. yunnanensis, M. endophyticus, Kytococcus sedentarius, Brevibacterium pityocampae, Arthrobacter chlorophenolicus, Curtobacterium oceanosedimentum, Corynebacterium lipophiloflavum, Kocuria rhizophila, Jatrophihabitans endophyticus, Tessaracoccus flavescens, Kocuria palustris, Rothia terrae and Naumannella halotolerans; 7—γ-Proteobacteria are Klebsiella pneumoniae, K. variicola, Pseudomonas aeruginosa, P. putida, Enterobacter cloacae, Serratia marcescens and S. nematodiphila; 5—α-Proteobacteria are Agrobacterium tumefaciens, Rhizobium sp., Sphingomonas mucosissima, S. panni and Brevundimonas vesicularis; 1—β-Proteobacteria is Pseudoacedovorax intermedius
Metagenomic approaches for exploring bacterial endophytes against Fusarium wilt
High-throughput sequencing technologies have been widely used to identify a large number of microbial communities in a sample, revealing the abundances of even rare microbial species. The pyrosequencing of enterobacterial 16S rRNA gene fragments from pseudostem of banana revealed the presence of abundant endosphere bacterial community with 14 genera, including Enterobacter (31.9%), Pantoea (14.0%), Raoultella (12.3%), Klebsiella (11.4%) and Serratia (11.3%) (Rossmann et al. 2019). The composition of enteric bacteria in the endosphere differed strongly from the rhizosphere soil. The genus Yersinia has been exclusively found in the banana endosphere. The dominant role of Enterics is due to the permanent nature and vegetative propagation of banana and the amendments of human as well as animal manure in traditional cultivation practices. Further, SSCP analysis of 16S rRNA/ITS genes amplified from DNA obtained from the endosphere confirmed the highest degree of heterogeneity of bacterial communities. The analysis showed that the identified endophytic communities are Flavobacteria, Sphingobacteria, α-Proteobacteria (Brevundimonas), β-Proteobacteria (Delftia, Herbaspirillum, Azoarcus, Acidovorax and Diaphorobacter), as well as γ-Proteobacteria (Buttiauxella, Pseudomonas and Serratia). With regard to antifungal activity against Fusarium wilt, screening of randomly selected 1152 bacterial species indicated that the highest proportion of bacterial strains with antagonistic properties has been noticed in the endosphere (9.4%), followed by the rhizosphere (6.5%) and soil (4.4%). These antagonistic isolates have been classified into five groups: (i) Burkholderia, (ii) Serratia, (iii) Pseudomonas, (iv) Bacillus 1 and (v) Bacillus 2 (Rossmann et al. 2019). Similarly, Zhai et al. (2016) reported that more than 99.08% of bacterial communities from the representative sequences of each operational taxonomic unit of banana root endophytes belong to Actinobacteria and others to Thermoleophilia (0.82%). Further analysis of the data set revealed that the most dominant family is Nocardioidaceae (56.37%), followed by Pseudonocardiaceae (14.36%), Nocardiaceae (9.77%), Microbacteriaceae (3.77%), Dietziaceae (2.67%), Dermabacteraceae (1.35%), Micrococcaceae (1.18%), Micromonosporaceae (1.01%), Mycobacteriaceae (0.91%), Actinosynnemataceae (0.85%), Corynebacteriaceae (0.70%), Kineosporiaceae (0.30%), Cellulomonadaceae (0.10%) and Promicromonosporaceae (0.10%). In the recent past, there are emerging threats to banana cultivation due to the outbreak of fungal pathogens. Since endomicrobiome are in abundance compared to rhizomicrobiome, exploitation of microbial diversity, balanced interaction between plant and pathogens are the key to prevent successful interactions between banana and pathogens. Hence, selection of endophytes based on the ecology is the only possibility for sustainable production of banana in the agricultural ecosystem. Thus, analysis of structure and function of banana-associated endomicrobiome, through metagenomic approaches, viz. NGS, pyrosequencing and phylochip-based metagenomics, will pave the way for identifying antagonists, not only of antifungal nature to Foc isolates, but would also be employed for mobilisation of nutrients, plant growth promotion and induction of systemic resistance (Supplementary Fig. 2).
Bacterial endophytes from non-host plants effective against Foc races infecting banana
Banana endophytes contribute in triggering resistance response against Foc races infecting banana. Apart from banana, the bacterial endophytes in weeds, medicinal plants and Capsicum frutescens could also serve as a repertoire to explore the antagonistic action against Foc isolates in banana (Ting et al. 2009; He et al. 2002). Furthermore, endophytic Burkholderia cenocepacia 869T2 isolated from Chrysopogon zizanioides roots were effective against Foc isolates. TC plantlets of banana cocultured with 869T2 reduced the incidence of Foc TR4 by up to 86% under field conditions (Ho et al. 2015). Thus, the bacterial endophytes of non-host origin also suppress the pathogenic Foc isolates by triggering the immune response in banana. Inoculation of endophytic Serratia marcescens ITBB B5-1 from Hevea brasiliensis into the banana plantlets produced more chitinase and glucanase, and reduced the severity of Foc R4 in greenhouse and field conditions by up to 79% and 70%, respectively (Tan et al. 2015). Diazotrophic endophytes, namely Burkholderia sp. and Herbaspirillum sp. isolated from pineapple roots and stems (Weber et al. 2007), and an Acremonium sp. from Kandelia candel (Liu and Lu 2013) also promote plant growth and help in nutrient mobilisation in banana.
Banana endophytes as plant growth promoters
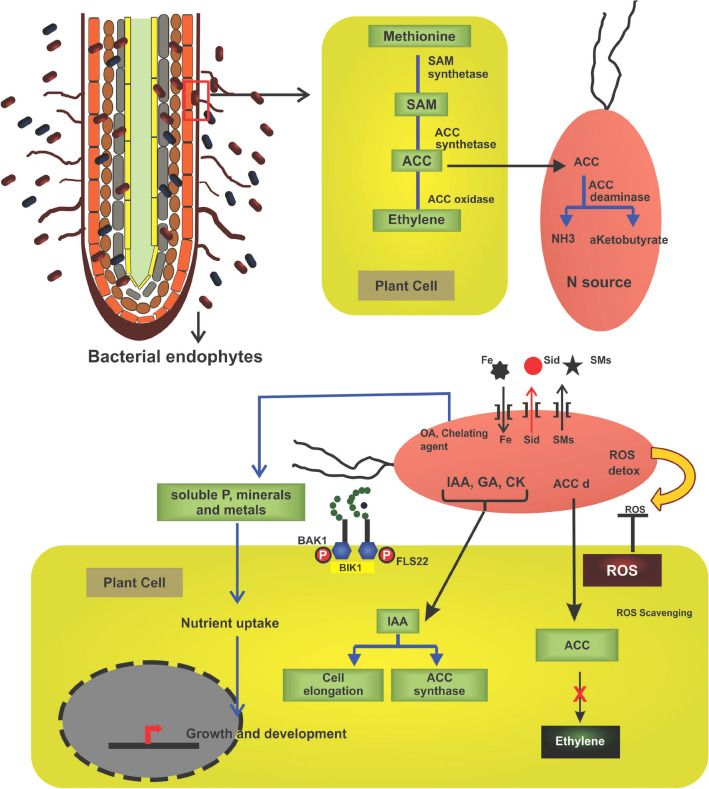
The microbes present inside the plant system alter the hormonal levels that enable the plant to biotic and abiotic stresses. In addition, it also promotes plant growth activities. The endophytes derived from the somatic embryogenic cultures of Musa acuminata AAA cv. Grand Naine were identified as Ralstonia sp. and Bacillus sp. The Ralstonia sp. produced indole acetic acid and siderophores and promoted plant growth. It also solubilised phosphate and produced ammonia (Jimtha et al. 2014). Banana endophytes with growth-promoting properties enhance plant growth and increase the innate immunity of banana against soil-borne pathogens (Adeline et al. 2007). The endophytic bacterial isolate UPM39B3 Serratia isolated from wild banana genotypes enhanced the growth in cultivated banana cultivars. It increased the total number of leaves, plant height, girth of pseudostem and root biomass. Biohardening the diseased plantlets with endophytic Serratia prior to planting of commercial banana cultivar improved growth of plantlets and protected them from Fusarium wilt (Adeline et al. 2007). The bacterial endophytes isolated from wild genotypes are amenable to artificial inoculation. The non-host-specific nature of these endophytes could be exploited to harvest its benefit in other commercial banana cultivars as well. Delivering these endophytes at the hardening phase of tissue-cultured clones could benefit early establishment of endophytes prior to planting in the field and thus providing a shield against some or the majority of soil-borne pathogens. Growth promotion of banana plantlets when subjected to endophytic inoculation has been attributed to production of plant growth regulators by endophytes (Porter et al. 1979). The endophytes trigger plant growth by producing auxins and cytokinins. Auxins stimulate cell division (Daly and Inman 1958) and may contribute to increased root biomass and formation of root hairs. Likewise, cytokinins induce root elongation (Daly and Inman 1958), and thereby increased root biomass. Besides, cytokinins are also known to enhance nutrient accumulation and transportation (Kiraly et al. 1967; Dekhuijzen and Overeem 1971; Sziraki et al. 1975; Vizarova 1979), and thus contribute to plant growth promotion (Fig. 2). Bacteria contain a lot of MAMP molecules that include flagellin, liposaccharides, peptidoglycans, elongation factor, cold shock proteins and hairpins that induce MAMP-triggered immunity and result in suppression of plant pathogens. They can be sensed by specific pattern recognition receptors in the plant, which in turn activates the plant defence mechanism in the plant.
Fig. 2.
Plant growth promotional activities of bacterial endophytes. The endophytic bacteria balance the hormones through biosynthesis of salicylic acid, jasmonic acid which are essential in development, metabolism, and stress responses. Where SAM S-adenosyl methionine; FLS2 flagellin sensitive 2; BIK BAK1 like; BAK1 brassinosteroid insensitive1-associated kinase1; IAA indole acetic acid; OA organic acid; ACC 1-aminocyclopropane carboxylic acid; GA gibberellic acid; ROS reactive oxygen species; Sid siderophore, SMs secondary metabolites; CK cytokinin
γ-Proteobacteria community as an endophytic indicator against Fusarium wilt
The diversity in the composition of γ-Proteobacteria community serves as a potential indicator of healthy banana. The composition of the γ-Proteobacterial microbiome in healthy banana Gros Michel plants was high in Pseudomonadales, Enterobacteriales, Xanthomonadales and Legionellales (Koberl et al. 2017). Among the Pseudomonadales, beneficial Pseudomonas and Stenotrophomonas are predominant in healthy banana plants. Besides, members of Pseudoalteromonadaceae are also responsible for the health of banana plants. The banana endophytes pertaining to Pseudomonadaceae are highly dominant from Nicaragua, but the dominance of Moraxellaceae has been observed in the banana grown in Costa Rica. Thus, depending upon the location and fertility status of the soil, there is a shift in the abundance of beneficial endophytes pertaining to γ-Proteobacteria in healthy plants compared to the infected banana plants. Stenotrophomonas, Pseudoxanthomonas, Luteimonas, Dokdonella, Rhodanobacter and Luteibacter pertaining to Xanthomonadales; Steroidobacter and Nevskia belonging to Sinobacteraceae; Aquicella and Rickettsiella belonging to Coxiellaceae; and Legionella and Tatlockia pertaining to Legionellaceae also decide the healthy nature of banana plants. Hitherto, the dominance of the enterobacterial community is more in Fusarium wilt-infected banana plantlets, which are well known for degradation activity. The major dominating genera in wilt-infected plants are Erwinia, followed by Enterobacter, Serratia and Citrobacter. Foc-infected plants were exclusively dominated by the families Thiotrichaceae, Alcanivoracaceae, Succinivibrionaceae and Chromatiaceae (Koberl et al. 2017). Based on the perusal of the literature, it is evident that a shift in the population of endophytes in healthy and wilt-infected banana plants varies depending on geographical locations, soil fertility status and stages of crop growth. Exploration of banana endophytes to trigger in planta resistance is only in the infancy stage. However, to explore the merits of the beneficial endophytes, a thorough understanding on the distribution of bacterial endomicrobiome in banana is highly important. Hence, metagenomic approaches of banana endomicrobiome will aid in engineering the banana endosphere with beneficial endophytes to trigger in planta resistance against Fusarium wilt of banana.
Induction of in planta resistance by bacterial endophytes against Foc races
The plant system harbours numerous microorganisms on its own (Marasco et al. 2012; Rashid et al. 2012). Association of endophytic microbiome decides the immune response of banana to biotic and abiotic stress. Among the endophytic microbiome, bacterial endophytes play a vital role in increasing resistance to Fusarium wilt of banana (Souza et al. 2014). Though the endophytes in plant system are considered as a subset of rhizosphere and root-associated bacterial population (Marquez-Santacruz et al. 2010), the functional role of keystone species enables it to provide resistance against pathogens. The increased ability of endophytes to survive inside the plant system and their efficient communication with plants is advantageous than rhizospheric bacteria (Ali et al. 2012; Coutinho et al. 2015). Biological control of Fusarium wilt of banana dates back to 1945 onwards (Thaysen and Butlin 1945). Diverse microbes of beneficial or neutral nature coexist inside the plant tissues as endophytes without causing any detrimental effect to the host (Hardoim et al. 2015). Beneficial endophytes promote plant health through mobilisation of nitrogen and phosphorus, synthesis of phytohormones and by triggering ISR (Compant et al. 2010). Apart from this, its endophytes also promote suppression of plant pathogens. Antagonistic bacterial endophytes have been employed as an effective complementary and sustainable alternative strategy in many crop species against systemic pathogenic fungal invasions. The complex endomicrobiomes in banana have been complemented with different microorganisms (Mia et al. 2010).
The coexistence of bacteria as endophytes in banana was published in the 1990s and during the last 2 decades, and increased research focus is being bestowed upon mining of endophytes to harness its beneficial aspects.
Bacterial endophytes associated with banana include Azospirillum amazonense, Azospirillum brasilense, Bacillus, Burkholderia cepacia, Burkholderia spp., Citrobacter spp., Enterobacter spp., Herbaspirillum spp., Klebsiella spp., Klebsiella variicola, Ochrobactrum, Pantoea, Serratia and Staphylococcus epidermidis (Rosenblueth et al. 2004; Thomas et al. 2008; Ting et al. 2008; Jie et al. 2009). Several bacterial endophytes have been attributed to enhance plant growth regulatory functions, especially in regulation of source–sink relationship and in enhancing resistance against biotic and abiotic stress in banana cultivation.
(Cao et al. 2004a, b; Jaizme-Vega et al. 2004; Jie et al. 2009). The endophytic P. aeruginosa isolate FJAT-346-PA colonised both roots and stems of banana and promoted plant growth. Besides, it also reduced Fusarium wilt of banana by up to 82–84% under field conditions (Yu et al. 2010). The endophytic S. griseorubiginosus has been isolated from both leaves and roots of diseased and healthy banana plants (Cao et al. 2004a, b). The isolates from the diseased plant were not found to differ in their identity from the isolates obtained from healthy banana plants. However, the isolates from S. griseorubiginosus were unable to suppress the pathogenic Foc isolate. The case was the reverse with the isolates collected from healthy banana plants, which were able to suppress the pathogenic Foc isolate R4 by up to 47%. Instead, antifungal activity of S. griseorubiginosus S96 was lost when the medium was amended with FeCl3, suggesting that siderophore production decides the antifungal nature and triggering of in planta resistance by the endophytes (Cao et al. 2005). Souza et al. (2014) stated that Bacillus spp. isolated from the endosphere of Musa cultivars from Brazil were antagonistic to Foc and Colletotrichum guaranicola. Among the diversified population of bacterial endophytes only.
Pseudomonas aeruginosa, Klebsiella variicola and Enterobacter cloacae showed antifungal activity against Foc. As several rhizomicrobiomes can colonise the endosphere, manipulation of banana rhizomicrobiome supplemented with organic amendments conferred resistance to Foc TR4 in China (Shen et al. 2015; Xue et al. 2015; Fu et al. 2016).
The banana plantlets produced through micropropagation are highly susceptible to Foc isolates. It is due to the absence of the microbiome that is naturally associated with banana plantlets. Hence, biohardening of banana plantlets with the effective bacterial endophytes during tissue culture and hardening process protects the banana seedling to maturity phase against Foc isolates. Coculturing of banana plants with Pseudomonas fluorescens Pf1, Bacillus subtilis EPB 10 and EPB 56 increased in planta resistance and improved the nutrient status of leaf, vegetative growth, bunch yield and fruit quality (Kavino et al. 2014; Kavino and Manoranjitham 2018). Combined application of endophytic bacteria like P. putida C4r4, Achromobactrum sp. Gcr1, Rhizobium sp. Lpr2, and B. flexus Tvpr1 and rhizospheric bacteria B. cereus Jrb1, P. putida Jrb2, Bacillus sp. Jrb6, and Jrb7 isolates from banana germplasm are effective in controlling Foc race. Besides, it also increased banana yield under field conditions (Thangavelu and Gopi 2015). Similarly, introduction of naturally occurring uncultivated endophytes into banana plantlets raised through tissue culture also suppressed Fusarium infection in banana plantations (Lian et al. 2009). Likewise, Belgrove et al. (2011) reported that biohardening of banana TC plants with endophytes suppressed banana wilt caused by Foc R4 by up to 67%, besides promoting plant growth (Supplementary Fig. 3). Biohardening with banana endophyte Sphingobacterium tabacisoli (UHEB) induced the resistance response against Foc in the plantlets of susceptible cv. Rasthali (Ajit Kumar et al. 2020).
Expression of microbial genes for the induction of Foc resistance in banana
Plant growth-promoting rhizobacteria (PGPR) can influence plant health either directly or indirectly. PGPR can cause direct effect on plants through the production of antimicrobial compounds, which interact and suppress plant pathogens. Contrarily, PGPR contain enzymes and compounds that are indirectly involved in triggering the immune response of banana through induction of defence-related proteins. Incorporation of desirable traits for development of disease-resistant banana through transgenic approaches could be employed as a tool for the expression of microbial genes. The enzyme 1-aminocyclopropane 1-carboxylate deaminase from PGPR catalyses the breakage of ethylene precursor in plants and results in biotic/abiotic stress tolerance (Glick et al. 1998; Saravanakumar and Samiyappan 2007). The signal molecule ethylene helps in recognition of microbes and its level during host–pathogen interactions may affect the development of disease symptoms. Transgenic plants or endosphere microbes overexpress the genes for chitinases, β-1,3-glucanase, and other antimicrobial peptide (AMP) genes of microbial origin exhibit enhanced levels of resistance to pathogen infection or delay symptom expression. Manipulation of microbial consortia in banana endosphere can enhance immune response of banana to pathogens. Biohardening of banana plantlets with genetically modified endophytes for superior traits increases disease resistance and immune response of banana plantlets without altering the banana genome (Orozco-Mosqueda et al. 2018).
Some endophytes modulate the level of ethylene, which acts as a signal for the recognition of microbes (Vorholt 2012). Some PGPR can lower ethylene level by synthesising 1-aminocyclopropane-1-carboxylate (ACC) deaminase which cleaves the compound ACC, the immediate precursor of ethylene (Glick 2012; Santoyo et al. 2016). Liu et al. (2019) stated the possibility to improve resistance to banana Fusarium wilt using engineered endophytic microbes by expressing ACC deaminase on the cell walls. The endophytic bacterial isolates were selected based upon the production of IAA, ethylene, ACC deaminase activity, sensitivity to phytotoxin, fusaric acid (100 µg/ml—rated as tolerant) and plant growth promotion activities. The endophytes Kosakonia sp. (S1), Enterobacter sp. (E5) and Klebsiella sp. (Kb) engineered with ACC deaminase on cell walls effectively reduced Fusarium wilt disease index in banana by up to 63%, which confirmed the role of ACC deaminase to resist Fusarium infection in banana. Wilting symptoms in banana were not correlated with overgrowth of pathogen in the tissue, but might be due to the ethylene level induced by the pathogen and fusaric acid in accelerating the wilt symptoms. Thus, the in planta resistance in banana against Foc races is determined by the bacterial endophytes that produce more of ACC deaminase and the ability to tolerate phytotoxicity of fusaric acid.
Transgenic banana plants expressing chitinase (chit42) gene from Trichoderma viride enhance disease resistance against Fusarium wilt (Hu et al. 2013). Similarly, transgenic banana plants with endochitinase gene ThEn-42 from T. harzianum along with the grape stilbene synthase (StSy) gene increased tolerance to sigatoka disease (Vishnevetsky et al. 2011). Overexpression of the endochitinase gene in transgenic banana led to higher levels of chitinases and inhibited the growth of fungal pathogen by degrading chitin present in the fungal cell wall. The dominant endophytic banana colonisers in the pseudostem of transgenic banana expressing other host genes are Methylobacterium mesophilicum, Microbacterium phyllosphaerae, Methylobacterium adhaesivum, Bacillus and Paenibacillus, which might be also responsible for increasing the immune response of banana to Foc races (Nimusiima et al. 2015). Primary metabolites are required for normal cellular functions, whereas secondary metabolites secreted outside the cells are involved in plant–microbe interactions, which may either increase or decrease plant defence. Basu et al. (2017) reported that expression of cryptogein gene of fungal origin in the roots of Tylophora indica enhanced the biochemical basis of defence response to prevent infection of the pathogen. Bacteria with MAMP molecules include flagellin, liposaccharides, peptidoglycans, elongation factor, cold shock proteins and hairpins that induce MAMP-triggered immunity and result in suppression of plant pathogens (Fig. 3). They can be sensed by specific pattern recognition receptors in the plant, which activate the plant defence mechanism. Hence, cloning of defence genes and MAMP genes of microbial origin or plant origin to the endophytes will trigger immune response to Foc isolates.
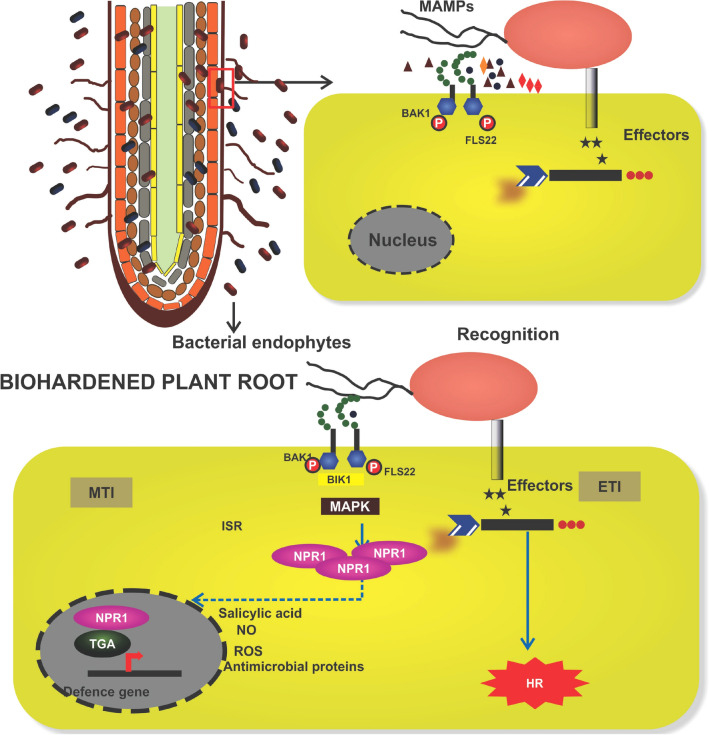
Fig. 3.
MAMP-triggered immunity by bacterial endophytes. The MAMP molecules from endophytic bacteria are recognized by the pattern recognition receptor (PRR) present in the plasma membrane. This results in calcium and hydrogen ion influx and K+ efflux occurs. This redox and pH changes in the cell leads to long distance transport of signal. The mitogen-activated protein kinase (MAPK) gene gets activated first and the activated MAPKKK phosphorylate to MPKK and MPK which leads into transcriptional reprogramming. The transcriptional factors like WRKY, ACS2/6, ERF 6/104, TGA, MYB/MYC gets activated to regulate the defence genes expression. This complex interaction results in production of pathogenesis-related genes, phytoalexin, callose deposition, ROS, defensin. Where, MAMP microbe-associated molecular pattern; FLS2 flagellin sensitive 2; BAK1 brassinosteroid insensitive1-associated kinase1; BIK BAK1 like; MTI MAMP-triggered immunity; TGA transcription factor; NPR1 non-pathogenesis-related 1; ETI effector-triggered immunity; NO nitric oxide; ROS reactive oxygen species
Novel delivery systems for bacterial endophytes
Bacterial biocontrol agents are applied in the form of talc-based, granular formations or liquid-based formulations for the management of diseases in banana (Harish et al. 2009; Rajamanickam et al. 2018). However, constraints pertaining to these formulations include reduced shelf life of the antagonists and adaptability of the formulation to cultivation practices like drip irrigation, necessitating a new strategy for efficient use of biocontrol agents. In this aspect, nanotechnology and its application in the field of plant pathology has to be explored. There are two different successful possibilities for the application of nanotechnology in the field of plant pathology. One is the nano-based delivery of bioagents and another could be the nanoscale conversion of antimicrobial compounds produced by bioagents. Nanotechnology has been applied successfully in the field of agriculture towards nano-based delivery of fertilisers and pesticides. These smart delivery systems enhance the efficiency of the product and reduce the required dosage (Duhan et al. 2017). Different carriers have been employed for nano delivery of drugs in the field of human medicine and agriculture. When a material is reduced to nano size, the property of the material is changed and its behaviour is entirely different. Nanoparticles have been reported to possess high surface to volume ratio, which in turn increases their reactivity and possible biochemical activity (Dubchak et al. 2010). For instance, 1 g of gold converted to nanoscale covers an area of 100 km2 (Buffat and Borel 1976). The same approach may be applied to the microbial secretome. The crude metabolites or antimicrobial compounds produced by the beneficial endophytic bacteria may be converted to nanoscale. This might increase the efficiency of the compounds and may impart them with hitherto unknown and unexplored properties.
Surfactin is an AMP produced by Bacillus species and has been reported with antifungal, antibacterial, antiviral activity against various human and plant pathogens. More recently, surfactin has been reported with anticancer activity against various types of cancer (Wang et al. 2009; Meena et al. 2016). It was also proposed that nanotechnology-assisted surfactin delivery for cancer treatment may be made possible. Similarly, delivery of surfactin through nanocarriers such as polymeric nanoparticles, nanofibres (Ahire et al. 2017), nanomicelles (Nozhat et al. 2012) and microemulsions (Kural and Gursoy 2011) have been previously reported. Similar applications can be developed to also manage plant pathogens; however, none of them have been applied in the field of plant pathology. Bacillus sp. produces a variety of AMPs that include iturin, surfactin, fengycin, bacilysin and bacillomycin with antifungal activity (Vinodkumar et al. 2017). Application of these AMPs individually or in combination through nano-based delivery systems to banana plants could possibly increase their potential. Apart from the secretomes of bacterial endophyte-mediated delivery, endophytic bacteria can be also delivered at standardised concentrations by coating onto the water-soluble nanofibres or nanopolymers. Further, exploring the delivery of plant growth-promoting endophytes, AMPs and other antimicrobial secretomes of antagonistic bacteria could open up a new era in the field of plant pathology to combat threatening diseases like Fusarium wilt. Combined application of bacteria with nanoparticles as carriers with reported antimicrobial activity may be attempted. For instance, Pickering emulsion that contained spores and crystals from Bacillus thuringiensis and iron oxide nanoparticles successfully increased the bioactivity of B. thuringiensis towards the management of lepidopteran pests of Brassica crops (Bashir et al. 2016). Antifungal activity of silver and zinc nanoparticles has been reported to be effective against Fusarium sp. (Madbouly et al. 2017; Dimkpa et al. 2013). The combined delivery of these nanoparticles with bacterial endophytes might suppress and inhibit the growth of Fusarium in banana.
Future outlook
Advances in the genomics era have opened avenues to map the endomicrobiome of banana to understand the existence and diversity of microbes including fungi, bacteria and Actinobacteria. The tritrophic interactions between endophytes, pathogens and banana plants can be understood better through transcriptome profiling. It helps in understanding the regulation of different defence genes through which potential endophytes can be screened and employed for the management of biotic and abiotic stress in banana. The effective endophytes can be delivered to the microbe’s free clones multiplied through tissue culture labs and the delivery mechanism can be optimised to induce MAMP-triggered immunity for confronting and managing the fungal, bacterial and viral pathogens infecting banana. Endophytes play a significant role in improving plant health. Plant mechanisms supporting the population dynamics of endophytes without any deleterious effect to the host system have to be investigated for the management of Foc races.
The contribution of endophytes towards plant growth, defence mechanisms against pathogens, correlation of the endomicrobiome role in tailoring root to shoot architecture and plant health are areas for further research. Selection of the effective isolate by considering various plant growth-promoting traits can elucidate a potential bacterial endophyte that could be used as both biocontrol agent and biofertiliser. This can enable microbiome engineering, breeding for crops that can host diverse beneficial endophytes and delivery of stage-specific endophytes to manage Foc races and to improve banana plant and soil health. Employing beneficial bacterial endophytes is an appropriate alternative for replacing chemical fertilisers and to overcome Foc races and abiotic stress. Intensive research into plant growth-promoting traits in delivering endophytes to the field and to the suitable ecological niche has to be carried out to harvest the benefits of the microbiome in the endosphere. Furthermore, metagenomic approaches would facilitate a faster and less labour-intensive method to screen for traits that could be readily detected in the genome of endophytic bacteria for effective management of Foc races infecting banana.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
SN and KS have conceptualized the review; SN, MV, SR and RS have written the manuscript. All authors have proofread and reviewed the manuscript.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Adeline SYT, Sariah M, Jugah K, Son R, Gurmit S. Endophytic microorganisms as potential growth promoters of banana. Biocontrol. 2007;53:541–553. [Google Scholar]
- Ahire JJ, Robertson DD, Van Reenen AJ, Dicks LMT. Surfactin-loaded polyvinyl alcohol (PVA) nanofibers alters adhesion of Listeria monocytogenes to polystyrene. Mater Sci Eng. 2017;77:27–33. doi: 10.1016/j.msec.2017.03.248. [DOI] [PubMed] [Google Scholar]
- Ajit Kumar S, Ashok B, Ananya B. Endophyte mediated activation of defense enzymes in banana plants pre-immunized with covert endophytes. Indian Phytopathol. 2020 doi: 10.1007/s42360-020-00245-8. [DOI] [Google Scholar]
- Ali S, Charles TC, Glick BR. Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane-1-carboxylate deaminase. J Appl Microbiol. 2012;113(5):1139–1144. doi: 10.1111/j.1365-2672.2012.05409.x. [DOI] [PubMed] [Google Scholar]
- Araujo WL, Marcon J, Maccheroni W, Jr, Van Elsas JD, Van Vuurde JWL, Azevedo JL. Diversity of endophytic bacterial populations and their interactions with Xylella fastidiosa in citrus plants. Appl Environ Microbiol. 2002;68:4906–4914. doi: 10.1128/AEM.68.10.4906-4914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir O, Claverie J, Lemoyne P, Vincent C. Controlled-release of Bacillus thurigiensis formulations encapsulated in light-resistant colloidosomal microcapsules for the management of lepidopteran pests of Brassica crops. Peer J. 2016;4:2524. doi: 10.7717/peerj.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Roychowdhury D, Joshi RK, Jha S. Effects of cryptogein gene on growth, phenotype and secondary metabolite accumulation in co-transformed roots and plants of Tylophora indica. Acta Physiol Plant. 2017;39:3. [Google Scholar]
- Belgrove A, Steinberg C, Viljoen A. Evaluation of nonpathogenic Fusarium oxysporum and Pseudomonas fluorescens for panama disease control. Plant Dis. 2011;95(8):951–959. doi: 10.1094/PDIS-06-10-0409. [DOI] [PubMed] [Google Scholar]
- Bhore SJ, Ravichantar N, Loh CY. Screening of endophytic bacteria isolated from leaves of Sambung nyawa (Gynura procumbens (Lour.) Merr.) for cytokinin-like compounds. Bioinformation. 2010;5(5):191. doi: 10.6026/97320630005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubici G, Kaushal M, Prigigallo MI, Gomez-Lama Cabanas C, Mercado-Blanco J. Biological control agents against Fusarium wilt of banana. Front Microbiol. 2019;10:616. doi: 10.3389/fmicb.2019.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffat P, Borel JP. Size effect on the melting temperature of gold particles. Phys Rev A. 1976;13(6):2287. [Google Scholar]
- Cao L, Qiu Z, Dai Q, Tan H, Lin Y, Zhou S. Isolation of endophytic actinomycetes from roots and leaves of banana (Musa acuminata) plants and their activities against Fusarium oxysporum f. sp. cubense. World J Microbiol Biotechnol. 2004;20:501–504. [Google Scholar]
- Cao L, Qiu Z, You J, Tan H, Zhou S. Isolation and characterization of endophytic Streptomycete antagonists of Fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol Lett. 2005;247(2):147–152. doi: 10.1016/j.femsle.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Compant S, Clement C, Sessitsch A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem. 2010;42:669–678. [Google Scholar]
- Coutinho BG, Licastro D, Mendonça-Previato L, Cámara M, Venturi V. Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol Plant Microbe Interact. 2015;28(1):10–21. doi: 10.1094/MPMI-07-14-0225-R. [DOI] [PubMed] [Google Scholar]
- Daly JM, Inman RE. Changes in auxin levels in safflower hypocotyls infected with Puccinia carthami. Phytopathol. 1958;48:91–97. [Google Scholar]
- Dekhuijzen HM, Overeem JC. The role of cytokinins in clubroot formation. Physiol Plant Pathol. 1971;1(2):151–161. [Google Scholar]
- Dheepa R, Vinodkumar S, Renukadevi P, Nakkeeran S. Phenotypic and molecular characterization of chrysanthemum white rust pathogen Puccinia horiana (Henn) and the effect of liquid based formulation of Bacillus spp. for the management of chrysanthemum white rust under protected cultivation. Biol Control. 2016;103:172–186. [Google Scholar]
- Dimkpa CO, McLean JE, Britt DW, Anderson AJ. Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals. 2013;26(6):913–924. doi: 10.1007/s10534-013-9667-6. [DOI] [PubMed] [Google Scholar]
- Dubchak S, Ogar A, Mietelski JW, Turnau K. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span J Agric Res. 2010;1:103–108. [Google Scholar]
- Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S. Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvira-Recuenco M, Van Vuurde JWL. Natural incidence of endophytic bacteria in pea cultivars under field conditions. Can J Microbiol. 2000;46:1036–1041. doi: 10.1139/w00-098. [DOI] [PubMed] [Google Scholar]
- Fernandes TP, Nietsche S, Costa MR, Xavier AA, Pereira DFGS, Pereira MCTP. Potential use of endophytic bacteria to promote the plant growth of micropropagated banana cultivar ‘Prata Ana’. Afr J Biotechnol. 2013;12(31):4915–4919. [Google Scholar]
- Fu L, Ruan Y, Tao C, Li R, Shen Q. Continuous application of bioorganic fertilizer induced resilient culturable bacteria community associated with banana Fusarium wilt suppression. Sci Rep. 2016;6:27731. doi: 10.1038/srep27731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganen STS, Nietsche S, Pereira MCT, Reis ST, Xavier AA, Santos TM, Fernandes TP. Microbial contamination in explants of banana cultivars ‘Galil18’and Tropical. Acta Hort. 2009;829:341–344. [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012 doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol. 1998;190:52–57. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Gomez-Lama Cabanas C, Fernandez-Gonzalez AJ, Cardoni M, Valverde-Corredor A, Lopez-Cepero J, Fernandez-Lopez M, Mercado-Blanco J. The banana root endophytome: differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f. sp. cubense. J Fungi. 2021;7:194. doi: 10.3390/jof7030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habiba U, Reza S, Saha ML, Khan MR, Hadiuzzaman S. Endogenous bacterial contamination during in vitro culture of banana: identification and prevention. Plant Tissue Cult. 2002;12:117–124. [Google Scholar]
- Hardoim PR, Van Overbeek LS, Berg G, Pirttila AM, Compant S, Campisano A, Doring M, Sessitsch A. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 2015;79(3):293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish S, Kavino M, Kumar N, Balasubramanian P, Samiyappan R. Induction of defence-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Biol Control. 2009;51(1):16–25. [Google Scholar]
- He H, Cai X, Hong Y, Guan X, Hu F. Selection of endophytic antifungal bacteria from Capsicum. Chin J Biol Control. 2002;18:171–175. [Google Scholar]
- Ho YN, Chiang HM, Chao CP, Su CC, Hsu HF, Guo CT, Hsieh JL, Huang CC. In-planta biocontrol of soilborne Fusarium wilt of banana through a plant endophytic bacterium, Burkholderia cenocepacia 869T2. Plant Soil. 2015;387(1–2):295–306. [Google Scholar]
- Hu GH, Wei YR, Huang YH, Yi GJ. An efficient protocol for the production of chit42 transgenic Furenzhi banana (Musa spp. AA group) resistant to Fusarium oxysporum. Vitro Cell Dev Biol Plant. 2013 doi: 10.1007/s11627-013-9525-9. [DOI] [Google Scholar]
- Jaizme-Vega MC, Rodríguez-Romero AS, Guerra MSP. Potential use of rhizobacteria from the Bacillus genus to stimulate the plant growth micropropagated bananas. Fruits. 2004;59:83–90. [Google Scholar]
- Jie L, Zifeng W, Lixiang C, Hongming T, Patrik I, Zide J, Shining Z. Artificial inoculation of banana tissue culture plantlets with indigenous endophytes originally derived from native banana plants. Biocontrol. 2009;51:427–434. [Google Scholar]
- Jimtha JC, Smitha PV, Anisha C, Deepthi T, Meekha G, Radhakrishnan EK, Gayatri GP, Remakanthan A. Isolation of endophytic bacteria from embryogenic suspension culture of banana and assessment of their plant growth promoting properties. Plant Cell Tissue Organ Cult. 2014;118(1):57–66. [Google Scholar]
- Jonathan EI, Umamaheswari R. Biomanagement of nematodes infesting banana by bacterial endophytes (Bacillus subtilis) Indian J Nematol. 2006;36(2):213–216 . [Google Scholar]
- Karthik M, Pushpakanth P, Krishnamoorthy R, Senthilkumar M. Endophytic bacteria associated with banana cultivars and their inoculation effect on plant growth. J Hortic Sci Biotechnol. 2017;92(6):568–576. [Google Scholar]
- Kavino M, Manoranjitham SK. In vitro bacterization of banana (Musa spp.) with native endophytic and rhizospheric bacterial isolates: novel ways to combat Fusarium wilt. Eur J Plant Pathol. 2018;151(2):371–387. [Google Scholar]
- Kavino M, Manoranjitham SK, Balamohan TN, Kumar N, Karthiba L, Samiyappan R. Enhancement of growth and Panama wilt resistance in banana by in vitro co-culturing of banana plantlets with PGPR and endophytes. Int Symp Trop Subtrop Fruits. 2014;19:277–282. [Google Scholar]
- Kiraly Z, Elhammad ME, Pozsar BI. Increased cytokinin activity of rust-infected bean and broad bean leaves. Phytopathology. 1967;57(1):93. [Google Scholar]
- Koberl M, Dita M, Martinuz A, Staver C, Berg G. Members of γ-Proteobacteria as indicator species of healthy banana plants on Fusarium wilt-infested fields in Central America. Sci Rep. 2017;7:45318. doi: 10.1038/srep45318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kural FH, Gursoy RN. Formulation and characterization of surfactin-containing self-microemulsifying drug delivery systems (SF-SMEDDS) Hacet Univ J Fac Pharm. 2011;30:171–186. [Google Scholar]
- Lian J, Wang ZF, Cao LX, Tan HM, Inderbitzin P, Jiang ZD, Zhou SN. Artificial inoculation of banana tissue culture plantlets with indigenous endophytes originally derived from native banana plants. Biol Control. 2009;51:427–434. [Google Scholar]
- Liu Y, Lu Q. Inhibitory effects of Acremonium sp. on Fusarium wilt in bananas. Afr J Agric Res. 2013;8(48):6241–6249. [Google Scholar]
- Liu Y, Zhu A, Tan H, Cao L, Zhang R. Engineering banana endosphere microbiome to improve Fusarium wilt resistance in banana. Microbiome. 2019;7:74. doi: 10.1186/s40168-019-0690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Qiu Z, Dai X, Tan H, Lin Y, Zhou S. Isolation of endophytic actinomycetes from roots and leaves of banana (Musa Acuminata) plants and their activities against Fusarium oxysporum f. sp. cubense. World J Microbiol Biotechnol. 2004;20:501–504. [Google Scholar]
- Madbouly AK, Abdel-Aziz MS, Abdel-Wahhab MA. Biosynthesis of nanosilver using Chaetomium globosum and its application to control Fusarium wilt of tomato in the greenhouse. IET Nanobiotechnol. 2017;11(6):702–708. [Google Scholar]
- Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Abou-Hadid AF, El-Behairy UA, Sorlini C, Cherif A, Zocchi G, Daffonchio D. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE. 2012;7:48479. doi: 10.1371/journal.pone.0048479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Santacruz HA, Hernandez-Leon R, Orozco-Mosqueda MC, Velazquez-Sepulveda I, Santoyo G. Diversity of bacterial endophytes in roots of Mexican husk tomato plants (Physalis ixocarpa) and their detection in the rhizosphere. Gen Mol Res. 2010;9:2372–2380. doi: 10.4238/vol9-4gmr921. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Akiyama M, Kobayashi K, Yamaji K. Fe and P solubilization under limiting conditions by bacteria isolated from Carex kobomugi roots at the Hasaki coast. Curr Microbiol. 2013;66:314–321. doi: 10.1007/s00284-012-0276-3. [DOI] [PubMed] [Google Scholar]
- Meena KR, Dhiman R, Sharma A, Kanwar SS. Applications of lipopeptide (s) from a Bacillus sp: an overview. Res J Recent Sci. 2016;5:50–54. [Google Scholar]
- Mia MAB, Shamsuddin ZH, Mahmood M. Use of plant growth promoting bacteria in banana: a new insight for sustainable banana production. Int J Agr Biol. 2010;12:459–467. [Google Scholar]
- Miliute I, Buzaite O, Gelvonauskiene D, Sasnauskas A, Stanys V, Baniulis D. Plant growth promoting, and antagonistic properties of endophytic bacteria isolated from domestic apple. Zemdirbyste. 2016;103:77–82. [Google Scholar]
- Nimusiima J, Koberl M, Tumuhairwe JB, Kubiriba J. Transgenic banana plants expressing Xanthomonas wilt resistance genes revealed a stable non-target bacterial colonization structure. Sci Rep. 2015;5:18078. doi: 10.1038/srep18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozhat Z, Asadi A, Zahri S. Properties of surfactin C-15 nanopeptide and its cytotoxic effect on human cervix cancer (HeLa) cell line. J Nanomater. 2012 doi: 10.1155/2012/526580. [DOI] [Google Scholar]
- Orozco-Mosqueda MDC, Rocha Granados C, Glick BR, Santoyo G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol Res. 2018 doi: 10.1016/j.micres.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Porter JK, Bacon CW, Robbins JD. Lysergic acid amide derivatives from Balansia epichloe and Balansia claviceps (Clavicipitaceae) J Nat Prod. 1979;42(3):309–314. [Google Scholar]
- Rajamanickam S, Karthikeyan G, Kavino M, Manoranjitham SK. Biohardening of micropropagated banana using endophytic bacteria to induce plant growth promotion and restrain rhizome rot disease caused by Pectobacterium carotovorum subsp. carotovorum. Sci Hortic. 2018;231:179–187. [Google Scholar]
- Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol. 2012;61:217–224. [Google Scholar]
- Rolli E, Marasco R, Vigani G, Ettoumi B, Mapelli F, Deangelis ML. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol. 2015;17:316–331. doi: 10.1111/1462-2920.12439. [DOI] [PubMed] [Google Scholar]
- Rosenblueth M, Martinez L, Silva J, Martinez-Romero E. Klebsiella variicola, a novel species with clinical and plant-associated isolates. Syst Appl Microbiol. 2004;19:827–837. doi: 10.1078/0723-2020-00261. [DOI] [PubMed] [Google Scholar]
- Rossmann B, Muller H, Smalla K, Mpiira S, Tumuhairwe JB, Staver C, Berga G. Banana associated microbial communities in Uganda are highly diverse but dominated by Enterobacteriaceae. Appl Environ Microbiol. 2019;78(14):4933–5494. doi: 10.1128/AEM.00772-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, del Carmen O-M, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Saravanakumar D, Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol. 2007;102:1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- Sekhar AC, Pious T. Isolation and identification of shoot-tip associated endophytic bacteria from banana cv. Grand Naine and testing for antagonistic activity against Fusarium oxysporum f. sp. cubense. Am J Plant Sci. 2005;6:943–954. [Google Scholar]
- Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, Kang SM, Yun BW, Lee IJ. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol Biochem. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Shen Z, Ruan Y, Xue C, Zhong S, Li R, Shen Q. Soils naturally suppressive to banana Fusarium wilt disease harbour unique bacterial communities. Plant Soil. 2015;393:21–33. [Google Scholar]
- Souza A, Cruz JC, Sousa NR, Procopio ARL, Silva GF. Endophytic bacteria from banana cultivars and their antifungal activity. Genet Mol Res. 2014;13(4):8661–8670. doi: 10.4238/2014.October.27.6. [DOI] [PubMed] [Google Scholar]
- Su L, Shen Z, Ruan Y, Tao C, Chao Y, Li R, Shen Q. Isolation of antagonistic endophytes from banana roots against Meloidogyne javanica and their effects on soil nematode community. Front Microbiol. 2017;8:2070. doi: 10.3389/fmicb.2017.02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzane A, Souza SA, Xavier AA, Costa MR, Cardoso A, Pereira MC, Nietsche S. Endophytic bacterial diversity in banana ‘Prata Ana’ (Musa spp.) roots. Genetics Mol Biol. 2013;36(2):252–264. doi: 10.1590/S1415-47572013000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziraki I, Balazs E, Kiraly Z. Increased levels of cytokinin and indoleacetic acid in peach leaves infected with Taphrina deformans. Physiol Plant Pathol. 1975;5(1):45–50. [Google Scholar]
- Tan D, Fu L, Han B, Sun X, Zheng P, Zhang J. Identification of an endophytic antifungal bacterial strain isolated from the rubber tree and its application in the biological control of banana Fusarium wilt. PLoS ONE. 2015;10:0131974. doi: 10.1371/journal.pone.0131974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavelu R, Gopi M. Field suppression of Fusarium wilt disease in banana by the combined application of native endophytic and rhizospheric bacterial isolates possessing multiple functions. Phytopathol Mediterr. 2015;54(2):241–252. [Google Scholar]
- Thaysen AC, Butlin KR. Inhibition of the development of Fusarium oxysporum cubense by a growth substance produced by Meredith’s actinomycetes. Nature. 1945;156(3974):781. [Google Scholar]
- Thomas P, Swarna GK, Roy PK, Patil P. Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv. Grand Naine. Plant Cell Tissue Organ Cult. 2008;93:55–63. [Google Scholar]
- Ting ASY, Meon S, Kadir J, Radu S, Singh G. Endophytic microorganisms as potential growth promoters of banana. Biocontrol. 2008;53:541–553. [Google Scholar]
- Ting ASY, Meon S, Kadir J, Radu S, Singh G. Induced host resistance by non-pathogenic Fusarium endophyte as a potential mechanism in Fusarium wilt management of banana. Pest Technol. 2009;3(1):67–72. [Google Scholar]
- Tsurumaru H, Okubo T, Okazaki K, Hashimoto M, Kakizaki K, Hanzawa E, Takahashi H, Asanome N, Tanaka F, Sekiyama Y, Ikeda S, Minamisawa K. Metagenomic analysis of the bacterial community associated with the taproot of sugar beet. Microbes Environ. 2015;30:63–69. doi: 10.1264/jsme2.ME14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinodkumar S, Nakkeeran S, Renukadevi P, Malathi VG. Biocontrol potentials of antimicrobial peptide producing Bacillus species: multifaceted antagonists for the management of stem rot of carnation caused by Sclerotinia sclerotiorum. Front Microbiol. 2017;8:446. doi: 10.3389/fmicb.2017.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinodkumar S, Rajeshkumar P, Nakkeeran S, Eraivan AAK. Developmental biology and infection cycle of Sclerotinia sclerotiorum causing stem rot of carnation in India. Afr J Microbiol Res. 2015;9:2328–2336. [Google Scholar]
- Vishnevetsky J, White TLJ, Palmateer AJ, Flaishman M, Cohen Y, Elad Y, Velcheva M, Hanania U, Sahar N, Dgani O, Perl A. Improved tolerance toward fungal diseases in transgenic Cavendish banana (Musa spp. AAA group) cv. Grand Nain Transgenic Res. 2011;20:61–72. doi: 10.1007/s11248-010-9392-7. [DOI] [PubMed] [Google Scholar]
- Vizarova G. Changes in the level of endogenous cytokinins of barley during the development of powdery mildew. J Phytopathol. 1979;95(4):329–341. [Google Scholar]
- Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012;10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- Wang CL, Ng TB, Cao XH, Jiang Y, Liu ZK, Wen TY, Liu F. CLP induces apoptosis in human leukemia K562 cells through Ca2+ regulating extracellular-related protein kinase ERK activation. Cancer Lett. 2009;276(2):221–227. doi: 10.1016/j.canlet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Weber OB, Muniz CR, Vitor AO, Freire FC, Oliveira VM. Interaction of endophytic diazotrophic bacteria and Fusarium oxysporum f. sp. cubense on plantlets of banana ‘Maça’. Plant Soil. 2007;298:47–56. [Google Scholar]
- Xue C, Ryan Penton C, Shen Z, Zhang R, Huang Q, Li R, Ruan Y, Shen Q. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci Rep. 2015;5:11124. doi: 10.1038/srep11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Xiao R, Liu B, Lin N, Chen L. Endophytic colonization of biocontrol bacterium FJAT-346-PA and its efficiency against banana Fusarium wilt. Acta Phytophylacica Sinica. 2010;37(6):493–498. [Google Scholar]
- Zhai Y, Wang W, Tan H, Cao L. A new approach to analyzing endophytic Actinobacterial population in the roots of banana plants (Musa sp., AAA) J Biochem Mol Biol Res. 2016;2(3):180–184. [Google Scholar]
- Zuniga A, Poupin MJ, Donoso R, Ledger T, Guiliani N, Gutierrez RA, Gonzalez B. Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol Plant Microbe Interact. 2013;26:546–553. doi: 10.1094/MPMI-10-12-0241-R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.