ABSTRACT
The mechanisms of vertebrate Hedgehog signaling are linked to the biology of the primary cilium, an antenna-like organelle that projects from the surface of most vertebrate cell types. Although the advantages of restricting signal transduction to cilia are often noted, the constraints imposed are less frequently considered, and yet they are central to how Hedgehog signaling operates in developing tissues. In this Review, we synthesize current understanding of Hedgehog signal transduction, ligand secretion and transport, and cilia dynamics to explore the temporal and spatial constraints imposed by the primary cilium on Hedgehog signaling in vivo.
KEY WORDS: Hedgehog, Cell cycle, Cilia, Signaling
Summary: This Review synthesizes current understanding of Hedgehog signal transduction, ligand secretion and transport, and cilia dynamics to explore the temporal and spatial constraints imposed by the primary cilium on Hedgehog signaling in vivo.
Introduction
The primary cilium is a microtubule-based organelle that acts as a cellular antenna. Concentrating membrane receptors and signaling components, the primary cilium projects into the signal-rich extracellular environment and transduces signals to influence cell fate (Anvarian et al., 2019). Defects in primary cilium structure and function can lead to a spectrum of developmental disorders, many of which are rooted in disrupted signaling (Reiter and Leroux, 2017). Of the signaling pathways associated with the primary cilium, vertebrate Hedgehog (Hh) signaling is the best characterized with respect to its relationship to the cilium (Bangs and Anderson, 2016). Hh signaling was originally identified for its patterning role in Drosophila (Nüsslein-Volhard and Wieschaus, 1980) and has important functions in the growth and morphogenesis of many vertebrate tissues, notably the nervous system, limb and skeleton (Chiang et al., 1996; Echelard et al., 1993; Riddle et al., 1993). Hh operates as a mitogen, driving proliferation and tissue growth, or as a morphogen, instructing cell fate, often through concentration gradients of secreted Hh ligands (Dessaud et al., 2008; Ericson et al., 1997; Wechsler-Reya and Scott, 1999). The biochemical mechanisms of vertebrate Hh signaling have been recently reviewed (Kong et al., 2019). Briefly, in the absence of Hh ligand, the Hh receptor patched 1 (Ptch1) inhibits the activator smoothened (Smo), keeping the pathway in its off state (Fig. 1A). Binding of one of the three vertebrate Hh ligands (Sonic hedgehog, Shh; Indian hedgehog, Ihh; or Desert hedgehog, Dhh) to Ptch1 releases the inhibition of Smo and allows downstream pathway activation mediated by the activator forms of Gli transcription factors.
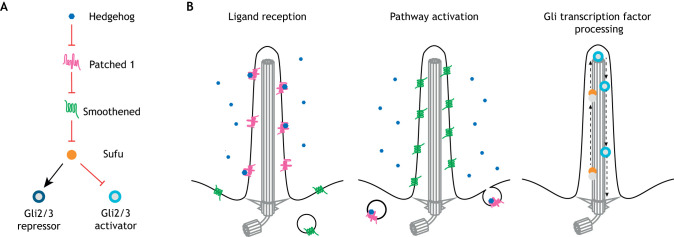
Fig. 1.
Vertebrate Hh signaling requires the primary cilium for multiple aspects of signal transduction. (A) Schematic depicting the Hh signaling pathway. (B) Three parts of Hh signal transduction are thought to occur in cilia: ligand reception, pathway activation (via patched 1 and smoothened) and Gli transcription factor processing.
In vertebrates, Hh signaling requires the primary cilium. A primary cilium consists of nine microtubule doublets that extend from the membrane-docked mature centriole (termed a ‘basal body’, once docked) to form an axoneme. The axoneme is surrounded by a ciliary membrane, which is a specialized and distinct compartment of the plasma membrane. Transport in and out of the cilium, which is necessary for ciliogenesis, maintenance and signaling, occurs bi-directionally along microtubules via the process of intraflagellar transport (Ishikawa and Marshall, 2017; Kozminski et al., 1993). The discovery that mutations in intraflagellar transport pathway genes cause defects in Hh signaling provided the first evidence for the role of primary cilia in Hh signaling (Huangfu et al., 2003).
Much subsequent work has identified three distinct aspects of vertebrate Hh signaling that occur in cilia (Fig. 1B). First, cilia may be important for initial signal reception, as the Ptch1 receptor concentrates in the ciliary membrane (Rohatgi et al., 2007). Second, cilia are important for pathway activation, as the repression of Smo by Ptch1 occurs in cilia, and Smo accumulates in the ciliary membrane when signaling is activated (Corbit et al., 2005; Rohatgi et al., 2007). Finally, cilia are required for transcription factor processing, as the Gli2 and Gli3 transcription factors travel via intraflagellar transport to the tips of cilia and are processed into their activator or repressor forms (Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005).
Although primary cilia have traditionally been studied in quiescent cells, recent advances have provided insight into the temporal dynamics and spatial localization of cilia in developing tissues. It has thus become clear that our understanding of the Hh pathway must expand to reflect the unique cell biology of this organelle. In this Review, we synthesize recent work about the mechanisms of Hh signal transduction, the dynamics of cilia in cycling cells, and the signal transport and reception of Hh ligand to highlight how reliance on a cilium imposes spatial and temporal constraints on Hh signaling and also creates opportunities for regulation. We also pose key unanswered questions in the interest of guiding future work in this area.
Constrained in time: Hedgehog signaling and cilia dynamics in proliferating cells
The dynamics of cilia assembly and disassembly during the cell cycle
Primary cilia have a close relationship to the cell cycle and are often associated with the quiescent cell cycle state. In many cell types, a cilium is assembled from a membrane-docked mature ‘mother’ centriole upon cell cycle exit into G0 (Fig. 2A). Accordingly, serum starvation, which results in cell cycle exit, is often used to induce ciliation in cell culture. Cell cycle re-entry from G0 is often accompanied by cilium disassembly (Pugacheva et al., 2007; Tucker et al., 1979), and several studies suggest that disassembly is required for re-entry. For example, depletion of the centrosome-associated proteins Tctex1 (Li et al., 2011; Saito et al., 2017), Cpap/Cenpj (Ding et al., 2019; Gabriel et al., 2016) or Nde1 (Kim et al., 2011), among others, causes cilia disassembly defects and a delay in S-phase entry. Excision of the ciliary tip, an event likely associated with disassembly, has also been shown to be required for G1 entry from quiescence (Phua et al., 2017). Timely cilium disassembly is particularly relevant in the developing brain, where delayed cell cycle entry impairs neural progenitor production and is a cause of microcephaly (Ding et al., 2019; Doobin et al., 2016; Gabriel et al., 2016; Li et al., 2011; Zhang et al., 2019).
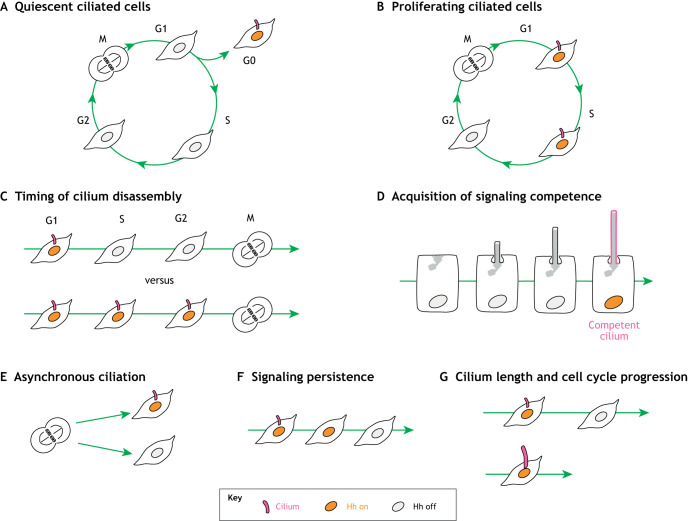
Fig. 2.
Cilia dynamics in proliferating cells can impact Hh signal transduction. (A) Some cells only ciliate upon cell cycle exit, restricting Hh signal transduction (orange nucleus) to the quiescent (G0) cell state. (B) Other cells ciliate during the cell cycle, allowing Hh signal transduction when cilia are present. In these proliferating cells, several aspects of cilia dynamics influence Hh signal transduction. (C) The timing of cilia assembly and disassembly determines when cells can transduce Hh signaling; persistence of cilia until G2 allows signaling to occur over more of the cell cycle. (D) There may be a refractory period during cilia assembly in which newly assembled cilia are not yet competent for signal transduction. (E) Asynchronous ciliation between sister cells can lead to differences in Hh signaling. (F) There is evidence that some aspects of Hh pathway activity can persist after cilium disassembly. (G) Cilium length is important for regulating cell cycle entry as well as Hh signaling. This figure is drawn assuming a constant source of ligand.
However, cilia are not found exclusively in quiescent cells. Proliferating cells in many developmental contexts and even some cancers possess cilia and require cilia and Hh signaling for their proliferation. Granule neuron precursors in the developing cerebellum are a well-studied example of a cell type that requires Hh pathway activity for proliferation, and loss of cilia in these cells leads to cell cycle arrest (Kenney and Rowitch, 2000; Spassky et al., 2008; Wechsler-Reya and Scott, 1999). Hh-dependent cancers, such as medulloblastoma and basal cell carcinoma, also require cilia for proliferation, unless mutations activate the pathway downstream of the essential cilium-mediated steps (Han et al., 2009; Wong et al., 2009). Thus, primary cilia can also promote rather than inhibit the cell cycle in contexts where they transduce proliferative signals.
In cycling cells, the cilium is only transiently present during the cell cycle. Cilia assemble in G1 and disassemble sometime before mitosis (Fig. 2B), although the exact timing is variable. Analysis of cells stimulated to enter the cell cycle by serum addition suggests that G1 and G2 are frequently points of cilium disassembly (Fig. 2C) (Pugacheva et al., 2007; Tucker et al., 1979). However, there is a need to study cilia disassembly in the context of normal cell cycles in culture and in vivo. Simultaneous imaging of cell cycle stage and cilia in mice found primary cilia on S/G2 cells in all tissues analyzed, suggesting that persistence of cilia past G1/S is common in vivo (Ford et al., 2018). Similarly, cilia in medulloblastoma cells persist through S phase (Ho et al., 2020), and cilia in dividing neural progenitors of the chick neural tube disassemble in late G2 (Spear and Erickson, 2012).
Although the timing of cilium disassembly is variable, the current understanding is that cilia must disassemble by mitosis. In general, mitotic cells lack cilia (Archer and Wheatley, 1971), and failure of cilium disassembly in chicken neural tube progenitors is correlated with absence of mitotic cells (Spear and Erickson, 2012). However, the requirement for cilium disassembly for successful completion of mitosis has not been rigorously examined, and cilium disassembly after entry into mitosis has been observed in PtK1 cells (Rieder et al., 1979).
In summary, although the details of cilia dynamics during the cell cycle remain to be fully characterized, it is clear that the cilium is a transient organelle that is assembled and disassembled during each cell cycle. An important prediction then is that the potential of a cell to transduce Hh signaling might also be transient with respect to the cell cycle. If so, what are the implications of primary cilium transience on Hh signal transduction and cellular decisions informed by those signals?
The implications of cilia dynamics on Hedgehog signaling
The dynamic nature of cilia suggests that Hh signal transduction might not be constant throughout the cell cycle. Accordingly, it was recently shown that Hh signal transduction during the cell cycle is correlated with cilium presence in medulloblastoma cells (Ho et al., 2020). This cell cycle dependence is reminiscent of other signaling pathways with peaks of transduction at specific cell cycle phases (Davidson et al., 2009; Kim et al., 2019), but is unique in that the presence or absence of an organelle is the cause of the signaling changes. Although Hh signal transduction is generally correlated with cilium presence, there may be refractory periods following cilium assembly or prior to disassembly during which cilia are not competent for signaling due to structural changes or altered signaling component localization (Fig. 2D). Tagged Smo localizes to pre-ciliary membranes early in ciliogenesis (Lu et al., 2015; Wu et al., 2018), arguing against a delay in localizing signaling components to newly assembled cilia, although further characterization of signaling competence over the lifetime of a cilium is needed. It is also unknown whether signaling output is proportional to the duration of ciliation during the cell cycle (Fig. 2C). Just as cells regulate their ciliation state to modulate Hh signaling at developmental transitions (Chang et al., 2019; Das and Storey, 2014), perhaps cells can also fine-tune the duration of cilium presence during the cell cycle to maintain the optimal level of Hh signaling, potentially explaining the variability between cell types.
As well as showing variability between cell types, cilium assembly and disassembly are asynchronous within the same population (Fig. 2E). For example, the daughter cell inheriting the oldest centriole in a mitotic division typically assembles a cilium first (Anderson and Stearns, 2009; Paridaen et al., 2013; Piotrowska-Nitsche and Caspary, 2012). Moreover, live imaging of cilium disassembly shows it is a highly asynchronous and heterogeneous process; cilia disassemble by a variety of mechanisms, including rapid deciliation and gradual resorption (Mirvis et al., 2019). It has also been suggested that cilia may assemble and disassemble more than once within a single cell cycle (Spalluto et al., 2013; Tucker et al., 1979), although this phenomenon has not been observed directly by imaging.
Periods of the cell cycle in which a cell lacks a cilium likely cause gaps in Hh signaling, with the length of the gap depending on the timing of cilium assembly and disassembly in a given cycle. Although the initiation of ciliogenesis has been well characterized in cells that have exited the cell cycle, the factors controlling cilium assembly in cycling cells are not well understood. In non-cycling cells, serum withdrawal stimulates Cp110 removal from the distal end of the mother centriole (Čajánek and Nigg, 2014; Goetz et al., 2012; Xu et al., 2016), inhibition of the cilia disassembly factor Aurora A (Inaba et al., 2016; Inoko et al., 2012; Kasahara et al., 2014, 2018), autophagy-mediated degradation of the ciliogenesis inhibitor Ofd1 (Tang et al., 2013) and microtubule stabilization, which promotes apical centrosome migration (Pitaval et al., 2017). Recent work has linked serum starvation with the Rab11-Rab8 cascade that traffics vesicles to the centriole to initiate ciliogenesis (Walia et al., 2019). How these mechanisms apply in cycling cells that form cilia without imposition of serum starvation is unknown. Overall, a potential consequence of this variability in cilium formation is that cells in the same population may not experience equivalent amounts of Hh signaling. Are there developmental consequences of this variability or are there buffering mechanisms to mitigate them?
We propose that cells have mechanisms to ‘remember’ signaling between ciliated phases (Fig. 2F) as a means of dealing with the intrinsic variability of cilium presence. Evidence for such mechanisms comes from the neural tube, where developmental decisions are made based on the amount of Hh that cells are exposed to. Here, progenitors cells integrate Hh signal concentration and duration over time to control differential gene expression rather than measuring Hh signaling at a single point (Dessaud et al., 2007). These cells must then not only integrate signals over time but also must do so as they are rapidly proliferating (Briscoe and Novitch, 2008; Kicheva et al., 2014). In support of this model, recent work has shown that Hh pathway activity from the previous cell cycle influences the decision to enter the cell cycle, revealing that cells can retain a memory of signaling between cell cycles (Ho et al., 2020). One aspect of this memory is persistence of cyclin D1 over times that span the ciliated signaling windows (Ho et al., 2020), although additional mechanisms likely exist. For example, it is possible that some part of the centrosome/cilium complex that persists between ciliated periods also contributes to memory of signaling. The ciliary remnant is a candidate for such a structure: it is a vesicle derived from the disassembled cilium that remains attached to the centriole, and it provides a memory of the ciliated state into the next cell cycle (Paridaen et al., 2013). In fact, Smo is present in the remnant following pathway stimulation, suggesting that Hh signaling components can be inherited in this way (Viol et al., 2020).
Cilium structure in the context of the cell cycle
Aspects of cilium structure can also influence signaling. The caliber of the primary cilium is thought to be similar in most cases, but cilium length can be highly variable between cell types. Importantly, changes in cilium length for a given cell type have consequences for both cell cycle progression and Hh signaling (Fig. 2G). Depletion of Nde1 or Cpap/Cenpj results in elongated cilia and a delay of cell cycle entry (Ding et al., 2019; Doobin et al., 2016; Gabriel et al., 2016; Kim et al., 2011). Mutations that affect cilium length are also often associated with defects in Hh signaling (Caspary et al., 2007; Huangfu and Anderson, 2005). Although aberrant cilium length is not always accompanied by altered Hh signaling (Bangs et al., 2015; Bonnafe et al., 2004; Gigante et al., 2020), the association with structural alterations is consistent with the observation that the correct concentration of signaling components in cilia is crucial for their signaling function (Mahjoub and Stearns, 2012). If cilium length is a tunable variable for a cell, is the optimum for Hh signal transduction the same as that for other functions associated with proliferation, or must Hh-responsive cycling cells make trade-offs that growth-arrested cells need not?
Another unexplored question is whether short-lived primary cilia on cycling cells (i.e. those present for only part of one cell cycle) are structurally different from long-lived cilia on differentiated cells, such as neurons or kidney epithelial cells. Is the structure of a cilium that must disassemble different from the structure of one that need not, and how might any differences affect Hh signaling? One hint comes from work on modifications to tubulin in the axoneme. In MDCK cells, prolonged starvation-induced cell cycle arrest causes the accumulation of tubulin glycylation modifications, which are associated with increased axoneme stability (Gadadhar et al., 2017). Although tubulin glycylation has not been studied in the context of Hh signaling, it is relevant that another tubulin modification, polyglutamylation, does influence Hh signaling. Reduced polyglutamylation impairs Gli3 transit to the cilium tip and consequently reduces Gli1 expression (He et al., 2018; Hong et al., 2018). Thus, it will be interesting to directly investigate how cilium age affects signaling capacity through structural changes. New advances in electron microscopy provide an exciting avenue to better understand variations in cilium structure between cell types and conditions (Kiesel et al., 2020; Sun et al., 2019).
We conclude that, unlike cells that are quiescent and have a long-lived primary cilium, cells in a proliferating tissue have dynamic primary cilia that are a source of variability in signal transduction capacity, both in a given cell through time and among cells in the same tissue. Although this variability is still largely uncharacterized, recent results have shown that considering Hh signaling in light of cilia dynamics can lead to insights into how the Hh pathway functions in developmental contexts. An exciting suggestion of these findings is that cells might control whether and when to elaborate a cilium, and to fine-tune the structure of that cilium to add additional layers of regulation onto Hh signal reception.
Constrained in space: Hedgehog signaling in ciliated epithelia
The location and positioning of cilia
The primary cilium is a small compartment compared with the size of a typical animal cell. Averaging about 5 μm in length, the primary cilium has an estimated volume that is about 5000 times smaller than the rest of the cell and a surface area about 500 times smaller (Nachury, 2014). Although this small size is thought to improve sensitivity by allowing a high local concentration of signaling components, a consequence is that cilium-based receptors are concentrated in only a small region of the cell membrane. If a cell is surrounded by uniform ligand concentration, as occurs in most cell culture experiments, the location of the solitary signaling organelle likely is of no consequence. But how does cilium position affect signaling in the complex milieu of the developing tissues of the embryo?
The location of the cilium on a cell is determined by the docking of the mature centriole to the plasma membrane. In epithelial cells, the initiation of ciliogenesis is marked by accumulation of ciliary vesicles at the end of this centriole as well as migration of the centriole toward the apical surface. Centriole migration to the apical surface requires actomyosin contractility and microtubule network remodeling (Hong et al., 2015; Pitaval et al., 2017). Actin is also cleared from the site of apical docking (Francis et al., 2011; Jewett et al., 2021), and a recent study (Insinna et al., 2019) identified a membrane channel connecting the ciliary vesicle to the plasma membrane during ciliogenesis that may guide the centriole to the apical membrane.
In epithelial cells, the cilium is almost exclusively an apical organelle, with rare exceptions, including some neuronal progenitors, exhibiting basolateral cilia (Lepanto et al., 2016; Wilsch-Brauninger et al., 2012). The apical location of cilia would seem to impose a spatial constraint on Hh signaling. Each cell in an epithelium has membrane contact with two compartments, apical and basal, separated by restrictive tight junctions and adherens junctions. Hh ligand may be present in either or both of these compartments, depending on the relative position of Hh-sending cells. However, the apical cilia project only into the apical compartment. So, how does the restricted location of the cilium affect the response of an epithelium to basal versus apical sources of Hh? To address this question, we examine current understanding of the spatial implications of Hh secretion, transport and reception, as well as examples of epithelial Hh signaling that provide insight into how signaling operates in vivo.
Ligand source and its relationship to cilium location in epithelia
In some epithelial signaling contexts, the spatial relationship between Hh ligand source and primary cilia is straightforward, with Hh in the same compartment as the primary cilia of the receiving cells. For example, Shh within the embryonic cerebrospinal fluid in mice has direct access to the apical primary cilia of radial glia, which project into the ventricle (Huang et al., 2010) (Fig. 3A). This choroid plexus-derived Shh is required for radial glia cell proliferation in the embryonic cerebellum prior to Shh production by the cerebellum itself (Huang et al., 2009, 2010). Shh and Ihh have also been detected in the apical fluid of the mouse epididymis, where they can access primary cilia of the epididymal epithelium (Girardet et al., 2020). Importantly, the apical presence of Hh ligand does not guarantee its access to primary cilia, as primary cilia only constitute a small proportion of the apical membrane. Thus, it is also relevant that there are many regulators of ligand transport and ligand-membrane interactions, including glypicans and co-receptors, that may influence how the Hh ligand interacts with the cilium (Capurro et al., 2008; Wierbowski et al., 2020).
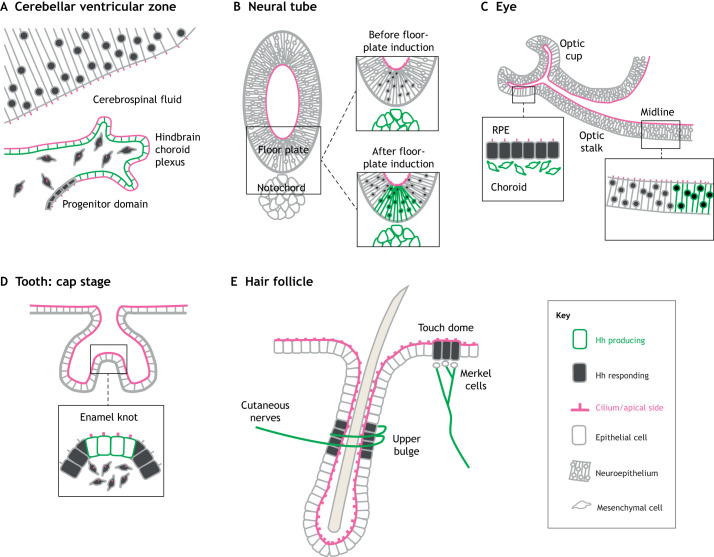
Fig. 3.
The relationship between ligand source and cilia in Hh-responsive epithelia in vivo. Cells secreting Hh ligand are outlined in green and cells responding to that Hh source are filled with gray. Magenta highlights the apical side and cilia. (A) In the developing mouse brain, Shh from the hindbrain choroid plexus epithelium signals to the nearby cerebellum via the apical cerebrospinal fluid, to the adjacent progenitor domain and to the underlying mesenchyme. (B) During neural tube development, Shh from the notochord signals from the basal side to the overlying ventral neural tube, inducing the floor plate. The floor plate then begins production of Shh, which signals to the rest of the neural tube. (C) During eye development, Shh from the midline signals to the adjacent neuroepithelium, inducing the optic stalk. In addition, Ihh from the choroid signals to the maturing retinal pigment epithelium (RPE) from the basal side of the RPE. (D) At the cap stage of tooth development, Shh from the enamel knot signals to both the adjacent epithelium and the underlying mesenchyme. (E) In the hair follicle, cutaneous nerves signal to cells of the upper bulge. Within the skin, nerves innervating Merkel cells provide a source of Shh for the developing touch dome.
In other tissues, the source of Hh is basal to the receiving cells, and the ligand is therefore separated from the primary cilium by the epithelial barrier. The classic example of this scenario is in the neural tube (Fig. 3B). Floor plate induction occurs when notochord-derived Shh signals to the most ventral neural tube cells. This notochord-derived signal is necessary and sufficient for floor plate induction (Matise et al., 1998; Patten and Placzek, 2002; Roelink et al., 1994; Tanabe et al., 1995). The apical cilia of the presumptive floor plate cells project into the lumen of the neural tube, but the notochord is on the basal side of the epithelium (Fig. 3B). Similarly, prior to maturation, cells in the retinal pigment epithelium (RPE) of the eye have apical cilia and respond to Ihh secreted by the developing choroid on the basal side of the epithelium (Dakubo et al., 2008; Lehmann et al., 2020; Portal et al., 2019) (Fig. 3C). Thus, there are well-defined examples of both apical and basal sources of Hh ligand involved in developmental signaling.
The spatial relationship between ligand source and primary cilia is not always so clear. For example, there are many examples of epithelial signaling centers that signal to other cells within the same epithelium. This process occurs in the neural tube, where floor-plate cells, after being induced by the notochord, secrete their own Shh (Dessaud et al., 2008) (Fig. 3B). This floor-plate-derived Shh is required to maintain the neural progenitor domains in the neural tube established by notochord-derived Shh (Yu et al., 2013). Similarly, midline floor-plate cells signal to optic stalk cells during early eye morphogenesis (Chiang et al., 1996; Ekker et al., 1995; Macdonald et al., 1995) (Fig. 3C). Moreover, in tooth development, Shh is expressed by cells in the epithelial bud tip that will become the enamel knot, and signals to cells of the surrounding epithelium and underlying mesenchyme (Hardcastle et al., 1998; Wang et al., 2005) (Fig. 3D). In these cases, cilia are apical, but whether the ligand is apical or basal is unclear and requires further understanding of its secretion and transport.
Evidence from Drosophila, which does not require cilia for Hh signaling, shows that there are both apical and basal routes of Hh release in epithelia. Newly synthesized Hh ligand is initially presented apically, and then endocytosed and trafficked for apical or basal secretion (Ayers et al., 2010; Callejo et al., 2011; D'Angelo et al., 2015). Secretion primarily occurs in the form of exosomes, although there is also evidence for soluble and lipoprotein-associated ligand (Gradilla et al., 2014; Panáková et al., 2005; Zeng et al., 2001). Basally secreted Hh is transported along cytonemes: long actin-based filopodial extensions present on the basal side of Drosophila epithelial cells that are required for signal transport and reception (Bischoff et al., 2013; Chen et al., 2017; González-Méndez et al., 2017). A key player in Hh secretion is Dispatched, a multipass transmembrane protein closely related to the Hh receptor Patched. Dispatched is required for the apical endocytosis of Hh as well as its basal secretion (Callejo et al., 2011; D'Angelo et al., 2015). Its mammalian homolog dispatched 1 has a conserved function in the basal secretion of Shh (Etheridge et al., 2010).
Comparatively less is known about the polarity of Hh secretion in vertebrates. One study showed that Shh secretion from MDCK cells is primarily basal and dispatched 1 dependent, although some apical Shh is present (Etheridge et al., 2010). Basal secretion is consistent with the observation that Hh-producing epithelia often signal to underlying ciliated mesenchymal tissues, as in the mammalian prostate and bladder (Peng and Joyner, 2015; Roberts et al., 2017), choroid plexus (Fig. 3A) (Huang et al., 2009), and tooth (Fig. 3D) (Hardcastle et al., 1998). Cytonemes also have a role in vertebrate Hh signaling (Hall et al., 2021; Sanders et al., 2013), and have been identified on the basal side of the developing optic stalk epithelium (Cardozo et al., 2014). Interestingly, in the neural tube, Shh accumulation has been observed both on the basal side of the neuroepithelium (Gritli-Linde et al., 2001) and in a ventral-dorsal gradient in the apical lumen (Chamberlain et al., 2008), although the relative contributions from the floor plate are unclear because notochord-derived Shh is found in both locations. Thus, in the case of epithelial cells signaling to cells within the same epithelium, there is strong evidence for a basal source of ligand, although apical ligand may also be present.
Shh can also be produced by nerve cells, which innervate many epithelial tissues and therefore provide an external source of ligand. However, the spatial relationship between Shh source and cilia in these contexts remains largely uncharacterized. In the hair follicle, Shh-producing sensory neurons wrap around the upper bulge region of the follicle, activating Hh signaling in those epithelial cells (Brownell et al., 2011; Lehman et al., 2009) (Fig. 3E). In development and maintenance of the touch dome, Shh-producing nerves innervate specialized epithelial sensory cells termed Merkel cells, which are located basally to Hh-responsive, apically ciliated basal keratinocytes (Elofsson et al., 1984; Ezratty et al., 2011; Peterson et al., 2015; Xiao et al., 2015, 2016) (Fig. 3E). In the adult brain, Hh-producing neurons project processes to the ventral subventricular zone, where Shh promotes self-renewal of apically ciliated neuronal progenitors (Garcia et al., 2010; Ihrie et al., 2011; Petrova and Joyner, 2014; Tong et al., 2014). It remains unexplored how and where Hh ligand is released from these neurons to activate signaling.
These complex spatial relationships raise the issue of whether Hh ligand is able to move between apical and basal compartments. Evidence in favor of this comes from the observation that Shh is localized within the neural tube lumen, even in the absence of Shh production by the floor plate, suggesting it is derived from the notochord (Chamberlain et al., 2008; Christ et al., 2016; Himmelstein et al., 2017). Transcytosis has been proposed as a potential mechanism for this movement, supported by putative visualization of notochord-derived Shh within the floor plate (Chamberlain et al., 2008). Another possible explanation for these results is that Shh moves between – rather than through – the floor-plate cells. Indeed, at the time of floor-plate induction, junctions between neural tube cells loosen, changing the restrictive nature of this barrier (Aaku-Saraste et al., 1996). Shh might therefore move between cells by diffusion, although recent work in cell culture (Li et al., 2018) and the adrenal gland (Mateska et al., 2020) suggests that membrane contact with ciliated receiving cells, perhaps by the extension of thin cytonemes through the space between cells (Sanders et al., 2013), is necessary for Shh transport.
Overall, these examples demonstrate that the source of Hh ligand in Hh-responsive epithelia may be apical or basal, or both. Although further work is needed to fully define the sources and transport route taken for the Hh source for each tissue, the diversity of signaling configurations described here suggests that epithelia are able to respond to both basal and apical sources of Shh; whether that is directly or through transport of the ligand, however, remains to be determined.
Hedgehog reception and its relationship to the primary cilium
The predominant model for Hh signaling in mammalian cells is that Hh ligand must bind to its receptor Ptch1 in the ciliary membrane to activate signaling. This model is based on two major lines of evidence. First, Ptch1 localizes to and concentrates in the ciliary membrane when the pathway is in the off state; when ligand is added, Shh localizes to cilia and causes Ptch1 exit from cilia (Rohatgi et al., 2007). Second, localization to the cilium compartment is required for Ptch1 and Smo to function in signaling. Removing the C-terminal tail of Ptch1 disrupts cilia localization while maintaining ligand binding (Kim et al., 2015). This non-ciliary Ptch1 cannot inactivate Smo, as evidenced by its failure to rescue pathway activation in Ptch1−/− mouse embryonic fibroblasts. Similarly, Smo that cannot localize to the cilium does not activate the pathway (Corbit et al., 2005). Biochemical experiments have also provided insights into the mechanisms of Smo regulation by Ptch1 in cilia. For example, recent work has shown that cholesterol is an endogenous ligand for Smo and provides evidence that Ptch1 regulates Smo by modulating its access to cholesterol (Byrne et al., 2016; Huang et al., 2016; Luchetti et al., 2016; Petrov et al., 2021). Analysis of the lipid composition of cilia has revealed that cilia exhibit lower cholesterol accessibility compared with the plasma membrane, which may be important for regulating Smo activity (Kinnebrew et al., 2019). Together, these experiments suggest that cilia concentrate signaling components in an environment optimized for signal transduction.
The model of cilia as a specialized compartment for Hh reception projecting into a signal-rich environment is consistent with other modes of ciliary signaling. Motile cilia in the trachea contain bitter taste receptors that are thought to sense noxious chemicals in the airway and increase ciliary beating frequency (Shah et al., 2009). Olfactory GPCRs concentrate in olfactory cilia that project into the odorant-rich environment of the olfactory epithelium (Mykytyn and Askwith, 2017). It is interesting to note that, although most differentiated neurons have cilia localized to their cell bodies, olfactory and other sensory cilia are located at the tip of dendrites (Jenkins et al., 2009). This location of the olfactory cilium thus appears to be specialized specifically for its function. Concentration of Ptch1 in cilia may have a similar consequence for Hh signaling.
Other work, however, brings into question the requirement for Hh ligand binding to Ptch1 at cilia. Functional experiments have suggested that basal Shh is actually the relevant source of Shh during neural tube differentiation. Addition of the Shh-blocking antibody (5E1) to the apical lumen has no effect on Hh response in this context (Etheridge et al., 2010), and notochord grafting inside the apical lumen also has no effect on Hh target gene expression (Kahane and Kalcheim, 2020). Consistent with this model, there is evidence that, in vertebrates, as in Drosophila (González-Méndez et al., 2017), Hh receptors localize to the basal sides of cells. For example, the Hh co-receptor Cdon, which can negatively regulate the pathway by acting as a sink for ligand, is present on the basal side of the developing optic vesicle epithelium (Cardozo et al., 2014). Basal Ptch1 has also been described as a mediator of long-range Shh transport in embryonic stem cell cultures, although basal localization of Ptch1 has not been directly visualized (Etheridge et al., 2010).
There is also evidence that the interaction between Ptch1 and Smo can occur outside cilia. Reconstitution experiments have shown that Ptch1 can inhibit Smo activity outside of the specialized ciliary compartment (Myers et al., 2017). Examples of non-canonical, transcription-independent Shh signaling also provide evidence of these signaling molecules functioning outside of cilia. For example, Shh can regulate axon guidance by binding to Ptch1 and the co-receptor Boc at the growth cone, activating Smo to promote local cytoskeletal rearrangement and axon turning (Charron et al., 2003; Ferent et al., 2019a; Okada et al., 2006; Yam et al., 2009). The binding of Shh to Ptch1 occurs at a distance from the cell body where the cilium is located, and it has been shown that axon guidance in mouse commissural axons does not require cilia-localized Arl13b (Ferent et al., 2019b). Similarly, there is evidence that Shh-mediated chemotaxis in fibroblasts occurs independently of cilia (Bijlsma et al., 2012). Other work, however, shows that Arl13b in cilia is necessary for axon guidance in the chicken neural tube and chemotaxis in fibroblasts (Mariani et al., 2016; Toro-Tapia and Das, 2020), and so the issue of whether Ptch1 and Smo function outside of cilia remains unresolved.
Given the existing data, it is clear that the primary cilium is required to process and transduce Hh signals in mammalian cells, but we are not yet able to conclude whether it is also a necessary site of signal reception for Hh. Addressing this fundamental question of cilia biology will require directly testing whether Hh ligand must have access to the primary cilium to exert its signaling effect.
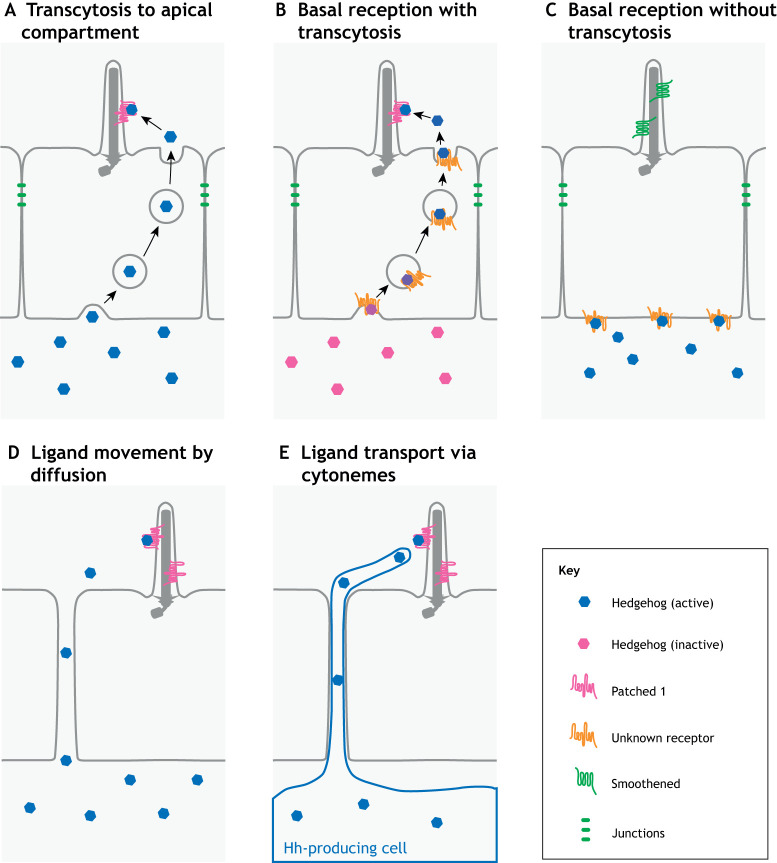
With our current understanding, we can propose a range of explanations to reconcile the apparent constraint of an apical antenna with the clear evidence for a basal source of ligand. One model is that Hh ligands undergo transcytosis to reach the apical cilium for reception. Hh ligands could be transcytosed alone or bound to a receptor (Fig. 4A). Although there is only limited direct evidence for transcytosis in the floor plate (Chamberlain et al., 2008), it is relevant to note that apico-basal trafficking of both Ptch and Hh does occur in Drosophila, which do not require cilia for Hh signaling. These apico-basal trafficking pathways might therefore be modified for signal reception in vertebrates (Callejo et al., 2011; González-Méndez et al., 2020). A variation of this model is that initial basal reception of ligand is a necessary step for signal transduction, perhaps mediating biochemical changes necessary for reception at cilia (Fig. 4B). This model is reminiscent of the finding that initial apical presentation of Hh ligand is necessary for proper secretion in Drosophila (D'Angelo et al., 2015). A second model is that the binding of Hh ligand to basal Ptch1 is sufficient to activate the pathway (Fig. 4C). This would require communication between the basal domain and the cilium, for which no mechanism currently exists. A third model is that, in cases where cells in an epithelium respond to a basal source of Hh ligand, the ligand can gain access to the apical compartment by moving between cells by diffusion (Fig. 4D) or even by cytoneme transport (Fig. 4E). Importantly, all of these models invoke the potential for developmental regulation of epithelial responsiveness to basal Shh ligand by modulating receptor localization, transcytosis or cell-cell junctions. We also note that some combination of these models is also possible; e.g. reception may occur at both ciliary and non-ciliary Ptch1, potentially explaining the observations that do not support a singular model.
Fig. 4.
Potential models for how epithelial cells respond to a basal source of Hh. (A) Active Hh ligand may undergo transcytosis to reach the apical compartment where it then binds to its receptor patched 1 (Ptch1) at the primary cilium. (B) Initial basal reception of inactive ligand may also be necessary prior to its reception at cilia. The basal receptor could be Ptch1 or another Hh-binding protein. (C) Another possibility is that Hh activates the pathway by binding to a receptor on the basal side. In this case, the Hh ligand itself would not reach the cilium but the pathway is still activated. (D,E) Alternatively, the epithelium may not restrict movement of Hh ligand between cells in tissues where signaling occurs from a basal source. In this model, Hh could move by diffusion (D) or transport (E) via filopodial extensions termed cytonemes. In these drawings, Hh is depicted as a soluble ligand but the models do not make that assumption.
Conclusions and future perspectives
This Review has explored the constraints imposed on the Hh pathway by the primary cilium. At first glance, the apical position and transient presence of the primary cilium would seem to impose limits on the time and place of ligand reception; this would presumably be balanced by the benefits of restricting Hh signaling to this organelle. Here, we have raised the possibility that these restrictions might also provide the potential for fine-tuned regulation of signaling in space and time. For example, regulation of ciliary dynamics or ligand localization would make it possible for some cells in a given context to be sensitive to a ligand while others nearby are not. In this regard, it has been shown that cilia submerged within a deep ciliary pit have different Hh signaling properties than fully exposed cilia, suggesting that changing the exposure of the cilium to the extracellular environment might be a regulatory mechanism to change signaling potential (Mazo et al., 2016). It is also interesting to consider that basal secretion of Hh ligand from ciliated epithelial cells into a different compartment from the apical primary cilia may provide an opportunity to prevent autocrine signaling activation of the epithelial cells themselves.
It is interesting to consider the implications of these constraints for Drosophila Hh signaling, which does not require the primary cilium. It has been proposed that some benefits of restricting signaling to a small, specialized compartment, such as the sensitivity to long-range signaling and the insulation from regulatory cholesterol sensing, are not necessary in Drosophila (Bangs and Anderson, 2016; Kinnebrew et al., 2019). Having identified the constraints imposed by the vertebrate primary cilium, we can further speculate that the opportunities for fine-tuning signaling that result from regulation of these constraints may be a vertebrate-specific evolutionary development. This advance might have occurred coincident with the establishment of a broader role for Hh signaling in dorsal-ventral patterning during neural development (Ren et al., 2020).
Our discussions here also highlight the need to develop new approaches for studying the Hh pathway in culture beyond serum-starved fibroblasts. Such G0 cells model neither the cilium dynamics that occur in cycling cells nor the separation of ligand and cilium that often occurs in an epithelium. New medulloblastoma cell lines that maintain Hh pathway dependence in culture have been a useful development (Zhao et al., 2015, 2017), but more options are needed, especially to extend these findings to different cell types. Similarly, taking advantage of relevant Hh-responsive epithelial cultures, such as embryonic stem cell-derived 3D neuroepithelial cysts that model the neural tube (Meinhardt et al., 2014; Ranga et al., 2016), will be important.
Extension of genetic screening efforts to these more relevant systems will also be important for identifying unique regulators of Hh pathway-dependent proliferation. Although many screens have interrogated the molecular players in Hh signaling (Breslow et al., 2018; Pusapati et al., 2018; Rack et al., 2014; Wheway et al., 2015), these screens have been carried out in quiescent cells. As a result, any specific regulators of Hh pathway-dependent proliferation, including those in the pathway itself, those that regulate the assembly and disassembly of cilia in cycling cells, or those that mediate signaling memory through non-ciliated phases, would have been missed. Developing a screening approach for specifically identifying regulators of Hh pathway-dependent proliferation will be an important advance, and may be useful for identifying new drug targets.
New tools are also needed to fully address the mechanisms underlying these constraints. First, our understanding of cilia dynamics would benefit significantly from tools that allow simultaneous long-term imaging of the cell cycle and cilia dynamics. Progress in this area has been made using a combination of a Fucci cell cycle sensor and a tagged Arl13b cilia marker (Ford et al., 2018). This type of imaging will be very important to further define and compare cilia dynamics in a variety of cell types. Arl13b overexpression has known effects on cilia structure, so it will be important to validate this method with parallel approaches (Larkins et al., 2011). Similarly, imaging efforts should focus on visualizing the Hh ligand and the subcellular localization of its receptors. Thus far, it has been difficult to visualize Hh gradients in tissues or to fully characterize Hh binding locations on the cilium or cell surface.
It will also be necessary to develop new tools for controlling cilia dynamics. Although ciliation inhibitors exist, we have few tools to manipulate cilia once they are formed or to control in which phases of the cell cycle cells are ciliated. HDAC6 and Aurora A inhibitors that prevent cilia disassembly have been useful, but their independent effects on cell cycle progression limit their use (Pugacheva et al., 2007). Recent work shows that small molecule inhibition of the heterotrimeric ciliary kinesin 2 motor KIF3A/KIF3B/KAP is a promising alternative approach for acutely inhibiting anterograde intraflagellar transport to the cilia tip, leading to cilia loss in fibroblasts within 8 h (Engelke et al., 2019). Although this hours-long time scale required for cilia loss is too long for cell cycle experiments, the developments in this area are exciting. Similarly, modulating cilium location on the cell would be important to address spatial constraints, and results using micropatterned substrates suggest some avenues for further work in making this possible (Pitaval et al., 2010).
This Review has focused on the Hh pathway because of its significance in development and its well-defined relationship to cilia. However, Hh signaling is not the only signaling pathway transduced in cilia. GPCR, PDGFα, IGF1, Wnt and Notch signaling pathways all have some requirement for cilia (Anvarian et al., 2019). The implications of cilium position and dynamics on Hh signaling thus likely also have similar but unexplored implications on these signaling pathways. For example, Notch receptors localize to cilia in the developing skin, and cilia are required for Notch activity in the epidermis (Ezratty et al., 2011). Given that Notch signaling requires direct contact between membrane-bound ligand and receptor, cilium placement may determine cell interactions. Exploring this and other implications of cilia biology on the many functions of these signaling pathways will be an important area of future research and will allow us to better understand this organelle and its extensive influence on development.
Acknowledgements
We apologize to those whose work we were not able to cover due to space constraints. We thank Ljiljana Milenkovic for comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors’ research was supported by a National Research Service Award (1F31GM129950 to E.K.H.) and a National Institutes of Health grant (1R35GM130286 to T.S.). Deposited in PMC for release after 12 months.
References
- Aaku-Saraste, E., Hellwig, A. and Huttner, W. B. (1996). Loss of Occludin and functional tight junctions, but not ZO-1, during neural tube closure—Remodeling of the neuroepithelium prior to neurogenesis. Dev. Biol. 180, 664-679. 10.1006/dbio.1996.0336 [DOI] [PubMed] [Google Scholar]
- Anderson, C. T. and Stearns, T. (2009). Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr. Biol. 19, 1498-1502. 10.1016/j.cub.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B. and Christensen, S. T. (2019). Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199-219. 10.1038/s41581-019-0116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer, F. L. and Wheatley, D. N. (1971). Cilia in cell-cultured fibroblasts. II. Incidence in mitotic and post-mitotic BHK 21-C13 fibroblasts. J. Anat. 109, 277-292. [PMC free article] [PubMed] [Google Scholar]
- Ayers, K. L., Gallet, A., Staccini-Lavenant, L. and Thérond, P. P. (2010). The long-range activity of Hedgehog Is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev. Cell 18, 605-620. 10.1016/j.devcel.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Bangs, F. and Anderson, K. V. (2016). Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb. Perspect. Biol. 9, a028175. 10.1101/cshperspect.a028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs, F. K., Schrode, N., Hadjantonakis, A.-K. and Anderson, K. V. (2015). Lineage specificity of primary cilia in the mouse embryo. Nat. Cell Biol. 17, 113-122. 10.1038/ncb3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma, M. F., Damhofer, H. and Roelink, H. (2012). Hedgehog-stimulated chemotaxis is mediated by Smoothened located outside the primary cilium. Sci. Signal. 5, ra60-ra60. 10.1126/scisignal.2002798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, M., Gradilla, A.-C., Seijo, I., Andrés, G., Rodríguez-Navas, C., González-Méndez, L. and Guerrero, I. (2013). Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat. Cell Biol. 15, 1269-1281. 10.1038/ncb2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnafe, E., Touka, M., AitLounis, A., Baas, D., Barras, E., Ucla, C., Moreau, A., Flamant, F., Dubruille, R., Couble, P.et al. (2004). The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol. Cell. Biol. 24, 4417-4427. 10.1128/MCB.24.10.4417-4427.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow, D. K., Hoogendoorn, S., Kopp, A. R., Morgens, D. W., Vu, B. K., Kennedy, M. C., Han, K., Li, A., Hess, G. T., Bassik, M. C.et al. (2018). A CRISPR-based screen for Hedgehog signaling provides insights into ciliary function and ciliopathies. Nat. Genet. 50, 460-471. 10.1038/s41588-018-0054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe, J. and Novitch, B. G. (2008). Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos. Trans. R. Soc. B Biol. Sci. 363, 57-70. 10.1098/rstb.2006.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell, I., Guevara, E., Bai, C. B., Loomis, C. A. and Joyner, A. L. (2011). Nerve-derived Sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552-565. 10.1016/j.stem.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, E. F. X., Sircar, R., Miller, P. S., Hedger, G., Luchetti, G., Nachtergaele, S., Tully, M. D., Mydock-McGrane, L., Covey, D. F., Rambo, R. P.et al. (2016). Structural basis of Smoothened regulation by its extracellular domains. Nature 535, 517-522. 10.1038/nature18934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čajánek, L. and Nigg, E. A. (2014). Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole. Proc. Natl. Acad. Sci. USA 111, E2841-E2850. 10.1073/pnas.1401777111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo, A., Bilioni, A., Mollica, E., Gorfinkiel, N., Andrés, G., Ibáñez, C., Torroja, C., Doglio, L., Sierra, J. and Guerrero, I. (2011). Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. USA 108, 12591-12598. 10.1073/pnas.1106881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro, M. I., Xu, P., Shi, W., Li, F., Jia, A. and Filmus, J. (2008). Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev. Cell 14, 700-711. 10.1016/j.devcel.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Cardozo, M. J., Sánchez-Arrones, L., Sandonis, Á., Sánchez-Camacho, C., Gestri, G., Wilson, S. W., Guerrero, I. and Bovolenta, P. (2014). Cdon acts as a Hedgehog decoy receptor during proximal-distal patterning of the optic vesicle. Nat. Commun. 5, 4272. 10.1038/ncomms5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary, T., Larkins, C. E. and Anderson, K. V. (2007). The graded response to Sonic hedgehog depends on cilia architecture. Dev. Cell 12, 767-778. 10.1016/j.devcel.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Chamberlain, C. E., Jeong, J., Guo, C., Allen, B. L. and McMahon, A. P. (2008). Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development 135, 1097-1106. 10.1242/dev.013086 [DOI] [PubMed] [Google Scholar]
- Chang, C.-H., Zanini, M., Shirvani, H., Cheng, J.-S., Yu, H., Feng, C.-H., Mercier, A. L., Hung, S.-Y., Forget, A., Wang, C.-H.et al. (2019). Atoh1 controls primary cilia formation to allow for SHH-triggered granule neuron progenitor proliferation. Dev. Cell 48, 184-199.e5. 10.1016/j.devcel.2018.12.017 [DOI] [PubMed] [Google Scholar]
- Charron, F., Stein, E., Jeong, J., McMahon, A. P. and Tessier-Lavigne, M. (2003). The morphogen Sonic hedgehog is an axonal chemoattractant that collaborates with Netrin-1 in midline axon guidance. Cell 113, 11-23. 10.1016/S0092-8674(03)00199-5 [DOI] [PubMed] [Google Scholar]
- Chen, W., Huang, H., Hatori, R. and Kornberg, T. B. (2017). Essential basal cytonemes take up Hedgehog in the Drosophila wing imaginal disc. Development 144, 3134-3144. 10.1242/dev.149856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C., Litingtung, Y., Lee, E., Young, K. E., Corden, J. L., Westphal, H. and Beachy, P. A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407-413. 10.1038/383407a0 [DOI] [PubMed] [Google Scholar]
- Christ, A., Herzog, K. and Willnow, T. E. (2016). LRP2, an auxiliary receptor that controls Sonic hedgehog signaling in development and disease. Dev. Dyn. 245, 569-579. 10.1002/dvdy.24394 [DOI] [PubMed] [Google Scholar]
- Corbit, K. C., Aanstad, P., Singla, V., Norman, A. R., Stainier, D. Y. R. and Reiter, J. F. (2005). Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018-1021. 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- Dakubo, G. D., Mazerolle, C., Furimsky, M., Yu, C., St-Jacques, B., McMahon, A. P. and Wallace, V. A. (2008). Indian hedgehog signaling from endothelial cells is required for sclera and retinal pigment epithelium development in the mouse eye. Dev. Biol. 320, 242-255. 10.1016/j.ydbio.2008.05.528 [DOI] [PubMed] [Google Scholar]
- D'Angelo, G., Matusek, T., Pizette, S. and Thérond, P. P. (2015). Endocytosis of Hedgehog through dispatched regulates long-range signaling. Dev. Cell 32, 290-303. 10.1016/j.devcel.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Das, R. M. and Storey, K. G. (2014). Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 343, 200-204. 10.1126/science.1247521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, G., Shen, J., Huang, Y.-L., Su, Y., Karaulanov, E., Bartscherer, K., Hassler, C., Stannek, P., Boutros, M. and Niehrs, C. (2009). Cell cycle control of Wnt receptor activation. Dev. Cell 17, 788-799. 10.1016/j.devcel.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Dessaud, E., Yang, L. L., Hill, K., Cox, B., Ulloa, F., Ribeiro, A., Mynett, A., Novitch, B. G. and Briscoe, J. (2007). Interpretation of the Sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717-720. 10.1038/nature06347 [DOI] [PubMed] [Google Scholar]
- Dessaud, E., McMahon, A. P. and Briscoe, J. (2008). Pattern formation in the vertebrate neural tube: a Sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489-2503. 10.1242/dev.009324 [DOI] [PubMed] [Google Scholar]
- Ding, W., Wu, Q., Sun, L., Pan, N. C. and Wang, X. (2019). Cenpj regulates cilia disassembly and neurogenesis in the developing mouse cortex. J. Neurosci. 39, 1994-2010. 10.1523/JNEUROSCI.1849-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobin, D. J., Kemal, S., Dantas, T. J. and Vallee, R. B. (2016). Severe NDE1-mediated microcephaly results from neural progenitor cell cycle arrests at multiple specific stages. Nat. Commun. 7. 10.1038/ncomms12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard, Y., Epstein, D. J., St-Jacques, B., Shen, L., Mohler, J., McMahon, J. A. and McMahon, A. P. (1993). Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417-1430. 10.1016/0092-8674(93)90627-3 [DOI] [PubMed] [Google Scholar]
- Ekker, S. C., Ungar, A. R., Greenstein, P., von Kessler, D. P., Porter, J. A., Moon, R. T. and Beachy, P. A. (1995). Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol. 5, 944-955. 10.1016/S0960-9822(95)00185-0 [DOI] [PubMed] [Google Scholar]
- Elofsson, R., Andersson, A., Falck, B. and Sjöborg, S. (1984). The ciliated human keratinocyte. J. Ultrasructure Res. 87, 212-220. 10.1016/S0022-5320(84)80061-1 [DOI] [PubMed] [Google Scholar]
- Engelke, M. F., Waas, B., Kearns, S. E., Suber, A., Boss, A., Allen, B. L. and Verhey, K. J. (2019). Acute inhibition of heterotrimeric kinesin-2 function reveals mechanisms of intraflagellar transport in mammalian cilia. Curr. Biol. 29, 1137-1148.e4. 10.1016/j.cub.2019.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, J., Rashbass, P., Schedl, A., Brenner-Morton, S., Kawakami, A., Van Heyningen, V., Jessell, T. M. and Briscoe, J. (1997). Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90, 169-180. 10.1016/S0092-8674(00)80323-2 [DOI] [PubMed] [Google Scholar]
- Etheridge, L. A., Crawford, T. Q., Zhang, S. and Roelink, H. (2010). Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development 137, 133-140. 10.1242/dev.043547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty, E. J., Stokes, N., Chai, S., Shah, A. S., Williams, S. E. and Fuchs, E. (2011). A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145, 1129-1141. 10.1016/j.cell.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferent, J., Giguère, F., Jolicoeur, C., Morin, S., Michaud, J.-F., Makihara, S., Yam, P. T., Cayouette, M. and Charron, F. (2019a). Boc acts via numb as a Shh-dependent endocytic platform for Ptch1 internalization and Shh-mediated axon guidance. Neuron 102, 1157-1171.e5. 10.1016/j.neuron.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Ferent, J., Constable, S., Gigante, E. D., Yam, P. T., Mariani, L. E., Legué, E., Liem, K. F., Caspary, T. and Charron, F. (2019b). The ciliary protein Arl13b functions outside of the primary cilium in Shh-mediated axon guidance. Cell Rep. 29, 3356-3366.e3. 10.1016/j.celrep.2019.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, M. J., Yeyati, P. L., Mali, G. R., Keighren, M. A., Waddell, S. H., Mjoseng, H. K., Douglas, A. T., Hall, E. A., Sakaue-Sawano, A., Miyawaki, A.et al. (2018). A cell/cilia cycle biosensor for single-cell kinetics reveals persistence of cilia after G1/S transition Is a general property in cells and mice. Dev. Cell 47, 509-523.e5. 10.1016/j.devcel.2018.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, S. S., Sfakianos, J., Lo, B. and Mellman, I. (2011). A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J. Cell Biol. 193, 219-233. 10.1083/jcb.201009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, E., Wason, A., Ramani, A., Gooi, L. M., Keller, P., Pozniakovsky, A., Poser, I., Noack, F., Telugu, N. S., Calegari, F.et al. (2016). CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J. 35, 803-819. 10.15252/embj.201593679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadadhar, S., Dadi, H., Bodakuntla, S., Schnitzler, A., Bièche, I., Rusconi, F. and Janke, C. (2017). Tubulin glycylation controls primary cilia length. J. Cell Biol. 216, 2701-2713. 10.1083/jcb.201612050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, A. D. R., Petrova, R., Eng, L. and Joyner, A. L. (2010). Sonic Hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J. Neurosci. 30, 13597-13608. 10.1523/JNEUROSCI.0830-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante, E. D., Taylor, M. R., Ivanova, A. A., Kahn, R. A. and Caspary, T. (2020). ARL13B regulates Sonic hedgehog signaling from outside primary cilia. eLife 9, 711671. 10.7554/eLife.50434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardet, L., Bernet, A., Calvo, E., Soulet, D., Joly-Beauparlant, C., Droit, A., Cyr, D. G. and Belleannée, C. (2020). Hedgehog signaling pathway regulates gene expression profile of epididymal principal cells through the primary cilium. FASEB J. 34, 7593-7609. 10.1096/fj.202000328R [DOI] [PubMed] [Google Scholar]
- Goetz, S. C., Liem, K. F. and Anderson, K. V. (2012). The spinocerebellar ataxia-associated gene tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell 151, 847-858. 10.1016/j.cell.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Méndez, L., Seijo-Barandiarán, I. and Guerrero, I. (2017). Cytoneme-mediated cell-cell contacts for Hedgehog reception. eLife 6, 1-24. 10.7554/eLife.24045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Méndez, L., Gradilla, A.-C., Sánchez-Hernández, D., González, E., Aguirre-Tamaral, A., Jiménez-Jiménez, C., Guerra, M., Aguilar, G., Andrés, G., Falcón Pérez, J. M.et al. (2020). Polarized sorting of Patched enables cytoneme-mediated Hedgehog reception in the Drosophila wing disc. EMBO J. 39, e103629. 10.15252/embj.2019103629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilla, A.-C., González, E., Seijo, I., Andrés, G., Bischoff, M., González-Mendez, L., Sánchez, V., Callejo, A., Ibáñez, C., Guerra, M.et al. (2014). Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat. Commun. 5, 416. 10.1038/ncomms6649 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde, A., Lewis, P., McMahon, A. P. and Linde, A. (2001). The whereabouts of a morphogen: Direct evidence for short- and graded long-range activity of Hedgehog signaling peptides. Dev. Biol. 236, 364-386. 10.1006/dbio.2001.0336 [DOI] [PubMed] [Google Scholar]
- Hall, E. T., Dillard, M. E., Stewart, D. P., Zhang, Y., Wagner, B., Levine, R. M., Pruett-Miller, S. M., Sykes, A., Temirov, J., Cheney, R. E.et al. (2021). Cytoneme delivery of Sonic hedgehog from ligand-producing cells requires Myosin 10 and a dispatched-BOC/CDON co-receptor complex. eLife 10, e61432. 10.7554/eLife.61432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y.-G., Kim, H. J., Dlugosz, A. A., Ellison, D. W., Gilbertson, R. J. and Alvarez-Buylla, A. (2009). Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 15, 1062-1065. 10.1038/nm.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle, Z., Mo, R., Hui, C. C. and Sharpe, P. T. (1998). The Shh signalling pathway in tooth development: Defects in Gli2 and Gli3 mutants. Development 125, 2803-2811. [DOI] [PubMed] [Google Scholar]
- Haycraft, C. J., Banizs, B., Aydin-Son, Y., Zhang, Q., Michaud, E. J. and Yoder, B. K. (2005). Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, 0480-0488. 10.1371/journal.pgen.0010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, K., Ma, X., Xu, T., Li, Y., Hodge, A., Zhang, Q., Torline, J., Huang, Y., Zhao, J., Ling, K.et al. (2018). Axoneme polyglutamylation regulated by Joubert syndrome protein ARL13B controls ciliary targeting of signaling molecules. Nat. Commun. 9, 3310. 10.1038/s41467-018-05867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelstein, D. S., Cajigas, I., Bi, C., Clark, B. S., Van Der Voort, G. and Kohtz, J. D. (2017). SHH E176/E177-Zn 2+ conformation is required for signaling at endogenous sites. Dev. Biol. 424, 221-235. 10.1016/j.ydbio.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, E. K., Tsai, A. E. and Stearns, T. (2020). Transient primary cilia mediate robust Hedgehog pathway-dependent cell cycle control. Curr. Biol. 30, 2829-2835.e5. 10.1016/j.cub.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, H., Kiim, J. and Kim, J. (2015). Myosin heavy chain 10 (MYH10) is required for centriole migration during the biogenesis of primary cilia. Biochem. Biophys. Res. Commun. 461, 180-185. 10.1016/j.bbrc.2015.04.028 [DOI] [PubMed] [Google Scholar]
- Hong, S.-R., Wang, C.-L., Huang, Y.-S., Chang, Y.-C., Chang, Y.-C., Pusapati, G. V., Lin, C.-Y., Hsu, N., Cheng, H.-C., Chiang, Y.-C.et al. (2018). Spatiotemporal manipulation of ciliary glutamylation reveals its roles in intraciliary trafficking and Hedgehog signaling. Nat. Commun. 9, 1732. 10.1038/s41467-018-03952-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Ketova, T., Fleming, J. T., Wang, H., Dey, S. K., Litingtung, Y. and Chiang, C. (2009). Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development 136, 2535-2543. 10.1242/dev.033795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Liu, J., Ketova, T., Fleming, J. T., Grover, V. K., Cooper, M. K., Litingtung, Y. and Chiang, C. (2010). Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc. Natl. Acad. Sci. USA 107, 8422-8427. 10.1073/pnas.0911838107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, P., Nedelcu, D., Watanabe, M., Jao, C., Kim, Y., Liu, J. and Salic, A. (2016). Cellular cholesterol directly activates Smoothened in Hedgehog signaling. Cell 166, 1-12. 10.1016/j.cell.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu, D. and Anderson, K. V. (2005). Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325-11330. 10.1073/pnas.0505328102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu, D., Liu, A., Rakeman, A. S., Murcia, N. S., Niswander, L. and Anderson, K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83-87. 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Ihrie, R. A., Shah, J. K., Harwell, C. C., Levine, J. H., Guinto, C. D., Lezameta, M., Kriegstein, A. R. and Alvarez-Buylla, A. (2011). Persistent Sonic Hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron 71, 250-262. 10.1016/j.neuron.2011.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba, H., Goto, H., Kasahara, K., Kumamoto, K., Yonemura, S., Inoko, A., Yamano, S., Wanibuchi, H., He, D., Goshima, N.et al. (2016). Ndel1 suppresses ciliogenesis in proliferating cells by regulating the trichoplein-Aurora A pathway. J. Cell Biol. 212, 409-423. 10.1083/jcb.201507046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko, A., Matsuyama, M., Goto, H., Ohmuro-Matsuyama, Y., Hayashi, Y., Enomoto, M., Ibi, M., Urano, T., Yonemura, S., Kiyono, T.et al. (2012). Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J. Cell Biol. 197, 391-405. 10.1083/jcb.201106101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna, C., Lu, Q., Teixeira, I., Harned, A., Semler, E. M., Stauffer, J., Magidson, V., Tiwari, A., Kenworthy, A. K., Narayan, K.et al. (2019). Investigation of F-BAR domain PACSIN proteins uncovers membrane tubulation function in cilia assembly and transport. Nat. Commun. 10, 1-17. 10.1038/s41467-018-07882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, H. and Marshall, W. F. (2017). Intraflagellar transport and ciliary dynamics. Cold Spring Harb. Perspect. Biol. 9, 1-14. 10.1101/cshperspect.a021998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, P. M., Mcewen, D. P. and Martens, J. R. (2009). Olfactory cilia: linking sensory cilia function and human disease. Chem. Senses 34, 451-464. 10.1093/chemse/bjp020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett, C. E., Soh, A. W. J., Lin, C. H., Lu, Q., Lencer, E., Westlake, C. J., Pearson, C. G. and Prekeris, R. (2021). RAB19 directs cortical remodeling and membrane growth for primary ciliogenesis. Dev. Cell 56, 325-340.e8. 10.1016/j.devcel.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane, N. and Kalcheim, C. (2020). Neural tube development depends on notochord-derived Sonic hedgehog released into the sclerotome. Development 147, dev183996. 10.1242/dev.183996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara, K., Kawakami, Y., Kiyono, T., Yonemura, S., Kawamura, Y., Era, S., Matsuzaki, F., Goshima, N. and Inagaki, M. (2014). Ubiquitin-proteasome system controls ciliogenesis at the initial step of axoneme extension. Nat. Commun. 5, 5081. 10.1038/ncomms6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara, K., Aoki, H., Kiyono, T., Wang, S., Kagiwada, H., Yuge, M., Tanaka, T., Nishimura, Y., Mizoguchi, A., Goshima, N.et al. (2018). EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat. Commun. 9, 758. 10.1038/s41467-018-03117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney, A. M. and Rowitch, D. H. (2000). Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 20, 9055-9067. 10.1128/MCB.20.23.9055-9067.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicheva, A., Bollenbach, T., Ribeiro, A., Valle, H. P., Lovell-Badge, R., Episkopou, V. and Briscoe, J. (2014). Coordination of progenitor specification and growth in mouse and chick spinal cord. Science 345, 1254927. 10.1126/science.1254927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel, P., Alvarez Viar, G., Tsoy, N., Maraspini, R., Gorilak, P., Varga, V., Honigmann, A. and Pigino, G. (2020). The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat. Struct. Mol. Biol. 27, 1115-1124. 10.1038/s41594-020-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Zaghloul, N. A., Bubenshchikova, E., Oh, E. C., Rankin, S., Katsanis, N., Obara, T. and Tsiokas, L. (2011). Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 13, 351-360. 10.1038/ncb2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., Hsia, E. Y. C., Brigui, A., Plessis, A., Beachy, P. A. and Zheng, X. (2015). The role of ciliary trafficking in Hedgehog receptor signaling. Sci. Signal. 8, ra55. 10.1126/scisignal.aaa5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W., Cho, Y. S., Wang, X., Park, O., Ma, X., Kim, H., Gan, W., Jho, E.-H., Cha, B., Jeung, Y.-J.et al. (2019). Hippo signaling is intrinsically regulated during cell cycle progression by APC/CCdh1. Proc. Natl. Acad. Sci. USA 116, 9423-9432. 10.1073/pnas.1821370116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew, M., Iverson, E. J., Patel, B. B., Pusapati, G. V., Kong, J. H., Johnson, K. A., Luchetti, G., Eckert, K. M., McDonald, J. G., Covey, D. F.et al. (2019). Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. eLife 8, e50051. 10.7554/eLife.50051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, J. H., Siebold, C. and Rohatgi, R. (2019). Biochemical mechanisms of vertebrate Hedgehog signaling. Development 146, dev166892. 10.1242/dev.166892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K. G., Johnson, K. A., Forscher, P. and Rosenbaum, J. L. (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519-5523. 10.1073/pnas.90.12.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins, C. E., Aviles, G. D. G., East, M. P., Kahn, R. A. and Caspary, T. (2011). Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol. Biol. Cell 22, 4694-4703. 10.1091/mbc.e10-12-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman, J. M., Laag, E., Michaud, E. J. and Yoder, B. K. (2009). An essential role for dermal primary cilia in hair follicle morphogenesis. J. Invest. Dermatol. 129, 438-448. 10.1038/jid.2008.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, G. L., Hanke-Gogokhia, C., Hu, Y., Bareja, R., Salfati, Z., Ginsberg, M., Nolan, D. J., Mendez-Huergo, S. P., Dalotto-Moreno, T., Wojcinski, A.et al. (2020). Single-cell profiling reveals an endothelium-mediated immunomodulatory pathway in the eye choroid. J. Exp. Med. 217, e20190730. 10.1084/jem.20190730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepanto, P., Davison, C., Casanova, G., Badano, J. L. and Zolessi, F. R. (2016). Characterization of primary cilia during the differentiation of retinal ganglion cells in the zebrafish. Neural Dev 11, 10. 10.1186/s13064-016-0064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, A., Saito, M., Chuang, J.-Z., Tseng, Y.-Y., Dedesma, C., Tomizawa, K., Kaitsuka, T. and Sung, C.-H. (2011). Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat. Cell Biol. 13, 402-411. 10.1038/ncb2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P., Markson, J. S., Wang, S., Chen, S., Vachharajani, V. and Elowitz, M. B. (2018). Morphogen gradient reconstitution reveals Hedgehog pathway design principles. Science 360, 543-548. 10.1126/science.aao0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A., Wang, B. and Niswander, L. (2005). Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103-3111. 10.1242/dev.01894 [DOI] [PubMed] [Google Scholar]
- Lu, Q., Insinna, C., Ott, C., Stauffer, J., Pintado, P. A., Rahajeng, J., Baxa, U., Walia, V., Cuenca, A., Hwang, Y.-S.et al. (2015). Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat. Cell Biol. 17, 228-240. 10.1038/ncb3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti, G., Sircar, R., Kong, J. H., Nachtergaele, S., Sagner, A., Byrne, E. F. X., Covey, D. F., Siebold, C. and Rohatgi, R. (2016). Cholesterol activates the G-protein coupled receptor smoothened to promote hedgehog signaling. eLife 5, e20304. 10.7554/eLife.20304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, R., Barth, K. A., Xu, Q., Holder, N., Mikkola, I. and Wilson, S. W. (1995). Midline signalling is required for Pax gene regulation and patterning of the eyes. Development 121, 3267-3278. [DOI] [PubMed] [Google Scholar]
- Mahjoub, M. R. and Stearns, T. (2012). Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Curr. Biol. 22, 1628-1634. 10.1016/j.cub.2012.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, L. E., Bijlsma, M. F., Ivanova, A. A., Suciu, S. K., Kahn, R. A. and Caspary, T. (2016). Arl13b regulates Shh signaling from both inside and outside the cilium. Mol. Biol. Cell 27, 3780-3790. 10.1091/mbc.e16-03-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateska, I., Nanda, K., Dye, N. A., Alexaki, V. I. and Eaton, S. (2020). Range of SHH signaling in adrenal gland is limited by membrane contact to cells with primary cilia. J. Cell Biol. 219, e201910087. 10.1083/jcb.201910087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise, M. P., Epstein, D. J., Park, H. L., Platt, K. A. and Joyner, A. L. (1998). Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125, 2759-2770. [DOI] [PubMed] [Google Scholar]
- Mazo, G., Soplop, N., Wang, W.-J., Uryu, K. and Tsou, M.-F. B. (2016). Spatial control of primary ciliogenesis by subdistal appendages alters sensation-associated properties of cilia. Dev. Cell 39, 424-437. 10.1016/j.devcel.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt, A., Eberle, D., Tazaki, A., Ranga, A., Niesche, M., Wilsch-Bräuninger, M., Stec, A., Schackert, G., Lutolf, M. and Tanaka, E. M. (2014). 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep. 3, 987-999. 10.1016/j.stemcr.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirvis, M., Siemers, K. A., Nelson, W. J. and Stearns, T. P. (2019). Primary cilium loss in mammalian cells occurs predominantly by whole-cilium shedding. PLoS Biol. 17, e3000381. 10.1371/journal.pbio.3000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, B. R., Neahring, L., Zhang, Y., Roberts, K. J., Beachy, P. A., Briscoe, J. and Chuang, P.-T. (2017). Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc. Natl. Acad. Sci. USA114, E11141-E11150. 10.1073/pnas.1717891115 [DOI] [PMC free article] [PubMed]
- Mykytyn, K. and Askwith, C. (2017). G-protein-coupled receptor signaling in cilia. Cold Spring Harb. Perspect. Biol. 9, a028183. 10.1101/cshperspect.a028183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury, M. V. (2014). How do cilia organize signalling cascades? Philos. Trans. R. Soc. B Biol. Sci. 369, 20130465. 10.1098/rstb.2013.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard, C. and Wieschaus, E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801. 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- Okada, A., Charron, F., Morin, S., Shin, D. S., Wong, K., Fabre, P. J., Tessier-Lavigne, M. and McConnell, S. K. (2006). Boc is a receptor for Sonic hedgehog in the guidance of commissural axons. Nature 444, 369-373. 10.1038/nature05246 [DOI] [PubMed] [Google Scholar]
- Panáková, D., Sprong, H., Marois, E., Thiele, C. and Eaton, S. (2005). Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58-65. 10.1038/nature03504 [DOI] [PubMed] [Google Scholar]
- Paridaen, J. T. M. L., Wilsch-Bräuninger, M. and Huttner, W. B. (2013). Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 155, 333-344. 10.1016/j.cell.2013.08.060 [DOI] [PubMed] [Google Scholar]
- Patten, I. and Placzek, M. (2002). Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr. Biol. 12, 47-52. 10.1016/S0960-9822(01)00631-5 [DOI] [PubMed] [Google Scholar]
- Peng, Y.-C. and Joyner, A. L. (2015). Hedgehog signaling in prostate epithelial-mesenchymal growth regulation. Dev. Biol. 400, 94-104. 10.1016/j.ydbio.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, S. C., Eberl, M., Vagnozzi, A. N., Belkadi, A., Veniaminova, N. A., Verhaegen, M. E., Bichakjian, C. K., Ward, N. L., Dlugosz, A. A. and Wong, S. Y. (2015). Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400-412. 10.1016/j.stem.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov, K., de Almeida Magalhaes, T. and Salic, A. (2021). Mechanism and ultrasensitivity in Hedgehog signaling revealed by Patched1 disease mutations. Proc. Natl. Acad. Sci. USA 118, e2006800118. 10.1073/pnas.2006800118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova, R. and Joyner, A. L. (2014). Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 141, 3445-3457. 10.1242/dev.083691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua, S. C., Chiba, S., Suzuki, M., Su, E., Roberson, E. C., Pusapati, G. V., Setou, M., Rohatgi, R., Reiter, J. F., Ikegami, K.et al. (2017). Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264-279.e15. 10.1016/j.cell.2016.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska-Nitsche, K. and Caspary, T. (2012). Live imaging of individual cell divisions in mouse neuroepithelium shows asymmetry in cilium formation and Sonic hedgehog response. Cilia 1, 6. 10.1186/2046-2530-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaval, A., Tseng, Q., Bornens, M. and Théry, M. (2010). Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J. Cell Biol. 191, 303-312. 10.1083/jcb.201004003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaval, A., Senger, F., Letort, G., Gidrol, X., Guyon, L., Sillibourne, J. and Théry, M. (2017). Microtubule stabilization drives 3D centrosome migration to initiate primary ciliogenesis. J. Cell Biol. 216, 3713-3728. 10.1083/jcb.201610039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portal, C., Rompolas, P., Lwigale, P. and Iomini, C. (2019). Primary cilia deficiency in neural crest cells models anterior segment dysgenesis in mouse. eLife 8, 1-25. 10.7554/eLife.52423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva, E. N., Jablonski, S. A., Hartman, T. R., Henske, E. P. and Golemis, E. A. (2007). HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351-1363. 10.1016/j.cell.2007.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusapati, G. V., Kong, J. H., Patel, B. B., Krishnan, A., Sagner, A., Kinnebrew, M., Briscoe, J., Aravind, L. and Rohatgi, R. (2018). CRISPR screens uncover genes that regulate target cell sensitivity to the morphogen Sonic Hedgehog. Dev. Cell 44, 113-129.e8. 10.1016/j.devcel.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack, P. G., Ni, J., Payumo, A. Y., Nguyen, V., Crapster, J. A., Hovestadt, V., Kool, M., Jones, D. T. W., Mich, J. K., Firestone, A. J.et al. (2014). Arhgap36-dependent activation of Gli transcription factors. Proc. Natl. Acad. Sci. USA 111, 11061-11066. 10.1073/pnas.1322362111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga, A., Girgin, M., Meinhardt, A., Eberle, D., Caiazzo, M., Tanaka, E. M. and Lutolf, M. P. (2016). Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl. Acad. Sci. USA 113, 201603529. 10.1073/pnas.1603529113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter, J. F. and Leroux, M. R. (2017). Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533-547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Q., Zhong, Y., Huang, X., Leung, B., Xing, C., Wang, H., Hu, G., Wang, Y., Shimeld, S. M. and Li, G. (2020). Step-wise evolution of neural patterning by Hedgehog signalling in chordates. Nat. Ecol. Evol. 4, 1247-1255. 10.1038/s41559-020-1248-9 [DOI] [PubMed] [Google Scholar]
- Riddle, R. D., Johnson, R. L., Laufer, E. and Tabin, C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401-1416. 10.1016/0092-8674(93)90626-2 [DOI] [PubMed] [Google Scholar]
- Rieder, C. L., Jensen, C. G. and Jensen, L. C. W. (1979). The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J. Ultrasructure Res. 68, 173-185. 10.1016/S0022-5320(79)90152-7 [DOI] [PubMed] [Google Scholar]
- Roberts, K. J., Kershner, A. M. and Beachy, P. A. (2017). The stromal niche for epithelial stem cells: a template for regeneration and a brake on malignancy. Cancer Cell 32, 404-410. 10.1016/j.ccell.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink, H., Augsburger, A., Heemskerk, J., Korzh, V., Norlin, S., Ruiz i Altaba, A., Tanabe, Y., Placzek, M., Edlund, T., Jessell, T. M.et al. (1994). Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 76, 761-775. 10.1016/0092-8674(94)90514-2 [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Milenkovic, L. and Scott, M. P. (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372-376. 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Saito, M., Otsu, W., Hsu, K.-S., Chuang, J.-Z., Yanagisawa, T., Shieh, V., Kaitsuka, T., Wei, F.-Y., Tomizawa, K. and Sung, C.-W. (2017). Tctex 1 controls ciliary resorption by regulating branched actin polymerization and endocytosis. EMBO Rep. 18, 1460-1472. 10.15252/embr.201744204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, T. A., Llagostera, E. and Barna, M. (2013). Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497, 628-632. 10.1038/nature12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, A. S., Ben-Shahar, Y., Moninger, T. O., Kline, J. N. and Welsh, M. J. (2009). Motile cilia of human airway epithelia are chemosensory. Science 325, 1131-1134. 10.1126/science.1173869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalluto, C., Wilson, D. I. and Hearn, T. (2013). Evidence for reciliation of RPE1 cells in late G1 phase, and ciliary localisation of cyclin B1. FEBS Open Bio. 3, 334-340. 10.1016/j.fob.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky, N., Han, Y.-G., Aguilar, A., Strehl, L., Besse, L., Laclef, C., Romaguera Ros, M., Garcia-Verdugo, J. M. and Alvarez-Buylla, A. (2008). Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 317, 246-259. 10.1016/j.ydbio.2008.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear, P. C. and Erickson, C. A. (2012). Apical movement during interkinetic nuclear migration is a two-step process. Dev. Biol. 370, 33-41. 10.1016/j.ydbio.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S., Fisher, R. L., Bowser, S. S., Pentecost, B. T. and Sui, H. (2019). Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. USA 116, 9370-9379. 10.1073/pnas.1821064116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe, Y., Roelink, H. and Jessell, T. M. (1995). Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation. Curr. Biol. 5, 651-658. 10.1016/S0960-9822(95)00130-8 [DOI] [PubMed] [Google Scholar]
- Tang, Z., Lin, M. G., Stowe, T. R., Chen, S., Zhu, M., Stearns, T., Franco, B. and Zhong, Q. (2013). Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 502, 254-257. 10.1038/nature12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, C. K., Han, Y.-G., Shah, J. K., Obernier, K., Guinto, C. D. and Alvarez-Buylla, A. (2014). Primary cilia are required in a unique subpopulation of neural progenitors. Proc. Natl. Acad. Sci. USA 111, 12438-12443. 10.1073/pnas.1321425111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro-Tapia, G. and Das, R. M. (2020). Primary cilium remodeling mediates a cell signaling switch in differentiating neurons. Sci. Adv. 6, eabb0601. 10.1126/sciadv.abb0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, R. W., Pardee, A. B. and Fujiwara, K. (1979). Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell 17, 527-535. 10.1016/0092-8674(79)90261-7 [DOI] [PubMed] [Google Scholar]
- Viol, L., Hata, S., Pastor-peidro, A., Neuner, A., Murke, F., Wuchter, P., Ho, A. D. and Giebel, B. (2020). Nek2 kinase displaces distal appendages from the mother centriole prior to mitosis. J. Cell Biol. 219, e201907136. 10.1083/jcb.201907136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia, V., Cuenca, A., Vetter, M., Insinna, C., Perera, S., Lu, Q., Ritt, D. A., Semler, E., Specht, S., Stauffer, J.et al. (2019). Akt Regulates a Rab11-Effector Switch Required for Ciliogenesis. Dev. Cell 50, 229-246.e7. 10.1016/j.devcel.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.-P., Åberg, T., James, M. J., Levanon, D., Groner, Y. and Thesleff, I. (2005). Runx2(Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J. Dent. Res. 84, 138-143. 10.1177/154405910508400206 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya, R. J. and Scott, M. P. (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron 22, 103-114. 10.1016/S0896-6273(00)80682-0 [DOI] [PubMed] [Google Scholar]
- Wheway, G., Schmidts, M., Mans, D. A., Szymanska, K., Nguyen, T.-M. T., Racher, H., Phelps, I. G., Toedt, G., Kennedy, J., Wunderlich, K. A.et al. (2015). An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell Biol. 17, 1074-1087. 10.1038/ncb3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierbowski, B. M., Petrov, K., Aravena, L., Gu, G., Xu, Y. and Salic, A. (2020). Hedgehog pathway activation requires coreceptor-catalyzed, lipid-dependent relay of the Sonic Hedgehog ligand. Dev. Cell 55, 450-467.e8. 10.1016/j.devcel.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsch-Brauninger, M., Peters, J., Paridaen, J. T. M. L. and Huttner, W. B. (2012). Basolateral rather than apical primary cilia on neuroepithelial cells committed to delamination. Development 139, 95-105. 10.1242/dev.069294 [DOI] [PubMed] [Google Scholar]
- Wong, S. Y., Seol, A. D., So, P.-L., Ermilov, A. N., Bichakjian, C. K., Epstein, E. H., Dlugosz, A. A. and Reiter, J. F. (2009). Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 15, 1055-1061. 10.1038/nm.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C.-T., Chen, H.-Y. and Tang, T. K. (2018). Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat. Cell Biol. 20, 175-185. 10.1038/s41556-017-0018-7 [DOI] [PubMed] [Google Scholar]
- Xiao, Y., Thoresen, D. T., Williams, J. S., Wang, C., Perna, J., Petrova, R. and Brownell, I. (2015). Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. Proc. Natl. Acad. Sci. USA 112, 7195-7200. 10.1073/pnas.1504177112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y., Thoresen, D. T., Miao, L., Williams, J. S., Wang, C., Atit, R. P., Wong, S. Y. and Brownell, I. (2016). A cascade of Wnt, Eda, and Shh signaling is essential for touch dome merkel cell development. PLoS Genet. 12, e1006150. 10.1371/journal.pgen.1006150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., Zhang, Y., Wei, Q., Huang, Y., Hu, J. and Ling, K. (2016). Phosphatidylinositol phosphate kinase PIPKIγ and phosphatase INPP5E coordinate initiation of ciliogenesis. Nat. Commun. 7, 10777. 10.1038/ncomms10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam, P. T., Langlois, S. D., Morin, S. and Charron, F. (2009). Sonic Hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 62, 349-362. 10.1016/j.neuron.2009.03.022 [DOI] [PubMed] [Google Scholar]
- Yu, K., McGlynn, S. and Matise, M. P. (2013). Floor plate-derived Sonic hedgehog regulates glial and ependymal cell fates in the developing spinal cord. Development 140, 1594-1604. 10.1242/dev.090845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X., Goetz, J. A., Suber, L. M., Scott, W. J., Schreiner, C. M. and Robbins, D. J. (2001). A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716-720. 10.1038/35079648 [DOI] [PubMed] [Google Scholar]
- Zhang, W., Yang, S.-L., Yang, M., Herrlinger, S., Shao, Q., Collar, J. L., Fierro, E., Shi, Y., Liu, A., Lu, H.et al. (2019). Modeling microcephaly with cerebral organoids reveals a WDR62–CEP170–KIF2A pathway promoting cilium disassembly in neural progenitors. Nat. Commun. 10, 2612. 10.1038/s41467-019-10497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Ponomaryov, T., Ornell, K. J., Zhou, P., Dabral, S. K., Pak, E., Li, W., Atwood, S. X., Whitson, R. J., Chang, A. L. S.et al. (2015). RAS/MAPK activation drives resistance to Smo inhibition, metastasis, and tumor evolution in Shh pathway-dependent tumors. Cancer Res. 75, 3623-3635. 10.1158/0008-5472.CAN-14-2999-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Pak, E., Ornell, K. J., Pazyra-Murphy, M. F., MacKenzie, E. L., Chadwick, E. J., Ponomaryov, T., Kelleher, J. F. and Segal, R. A. (2017). A transposon screen identifies loss of primary cilia as a mechanism of resistance to SMO inhibitors. Cancer Discov. 7, 1436-1449. 10.1158/2159-8290.CD-17-0281 [DOI] [PubMed] [Google Scholar]