Abstract
Metabolic pathways mediated by human gut bacteria have emerged as potential therapeutic targets because of their association with the pathophysiology of various human diseases. The anaerobic transformation of choline into trimethylamine (TMA) by gut microbiota is directly linked to type 2 diabetes, fatty liver disease, and cardiovascular diseases. Structural analogs of choline have been developed as competitive inhibitors of choline TMA-lyase (CutC), a key enzyme for the conversion of choline to TMA. However, weak to moderate CutC inhibitory profiles of the choline analogs limit their further advancement into clinical translation. In this study, we introduce a glycomimetic-based approach for the identification of CutC inhibitors with intestinal metabolic stability. Our workflow started with screening of a small library of glycomimetics for metabolic stability in the presence of human intestinal S9 fraction. Further screening using an in vitro CutC inhibitory assay identified a benzoxazole ligand (BO-I) as a CutC inhibitor with an IC50 value of 2.4 ± 0.3 μM. Kinetic analysis revealed that BO-I functions as a non-competitive inhibitor of CutC. Interestingly, BO-I reduced the production of TMA in whole cell assays of multiple bacterial strains as well as in complex biological environments. Therefore, structural optimization of BO-I holds promise for the development of efficient gut microbiota-targeted small molecules.
Metabolic pathways mediated by human gut bacteria have emerged as potential therapeutic targets because of their association with the pathophysiology of various human diseases.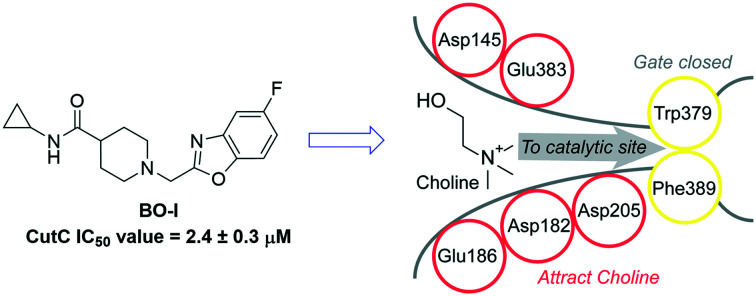
Introduction
The gut microbiota is a dynamic population of microorganisms residing in the human gastrointestinal tract that plays key roles in developing a balanced immune system, nutrient metabolism, and direct inhibition of pathogens.1–4 Additionally, gut microbiota mediates bidirectional communication between human gut and brain, known as the gut–brain axis (GBA).5 Clinical studies reveal direct interaction of gut flora with intestinal cells and the central nervous system through neuroendocrine and metabolic pathways.6 Recent studies have demonstrated the association between gut microbiota and various human diseases such as neurological disorders, chronic kidney disease (CKD), liver cirrhosis, and gastrointestinal disorders.7–11 A linkage between the levels of metabolites in human serum produced by gut bacteria and pathophysiology of various human diseases has been demonstrated.12,13
Anaerobic metabolism of choline by gut bacteria is an established disease-associated gut microbial activity.14 Trimethylamine (TMA) is produced by gut microbiota from different sources, including choline, glycine betaine, and carnitine.14 Choline, a source of carbon and energy for gut bacteria, is the major source for TMA production by gut microbiota which is further oxidized to trimethylamine N-oxide (TMAO) in the human body.15 Increased TMAO levels have been associated with elevated risk of atherosclerosis in vivo.16 Moreover, elevated TMAO levels have been linked with various diseases such as CKD, type 2 diabetes, and fatty liver disease.17–19 The metabolic pathway of choline by gut bacteria proceeds through the cleavage of C–N bonds in choline via choline TMA-lyase (CutC) to generate TMA and acetaldehyde (Fig. 1A).20 The produced TMA is further oxidized by hepatic flavin-dependent monooxygenase 3 (FMO3) to produce TMAO (Fig. 1A).21 Excretion of TMA and reduced FMO3 activity are detected in patients with inherited metabolic disorder trimethylaminuria.22–24 Therefore, targeting TMA-lyase (CutC) and FMO3 is identified as a potential therapeutic strategy to reduce the levels of TMAO production associated with CKD and atherosclerosis.25,26 In this context, using an antisense oligonucleotide to knockout FMO3 in mice reduced circulating TMAO levels and atherosclerosis.27In vivo FMO3 inhibition was confounded by metabolic interactions as revealed by decreased levels of hepatic lipids, plasma lipids, ketone bodies, glucose, and insulin.27 These results confirmed the regulatory function of FMO3 on glucose and lipid metabolism in a dose-dependent manner.27 Additionally, FMO3 knockdown results in stimulation of basal and liver X receptor (LXR)-stimulated macrophage reverse cholesterol transport which improves cholesterol balance in mice.28 However, the side effects associated with the inhibition of FMO3 such as hepatic inflammation and TMA accumulation have directed research efforts towards targeting CutC as a promising therapeutic target.27–29
Fig. 1. (A) Two-step metabolic pathway for choline by CutC and FMO3. (B) Chemical structures of CutC inhibitors.
The catalytic activity of CutC and its substrate specificity have been studied using homology modeling and mutagenesis experiments which triggered research to identify CutC inhibitors.30 Anaerobic choline metabolism mediated by CutC is activated by a S-adenosylmethionine (SAM) activating protein (CutD).30 A glycyl radical mechanism is proposed for CutC catalytic function and starts via hydrogen abstraction from a cysteine residue (Cys489) producing a thienyl radical intermediate that starts the interaction with choline.20
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)-based high-throughput screening identified compound 1 (Fig. 1B) as an inhibitor of TMA production in Clostridium hathewayi.31 A structural analog of choline, 3,3-dimethyl-1-butanol (2 in Fig. 1B), is a reported inhibitor of CutC activity that reduces the production of TMA by bacteria.25 Moreover, compound 2 decreased the levels of circulating TMA in mice models upon oral administration.25 Recent biochemical data revealed poor inhibition of CutC by compound 2in vitro, and thus, in vivo activity of 2 is attributed to interaction with alternative targets rather than direct binding to CutC.32 Iodomethylcholine is another example of choline analogs that has demonstrated the ability to reduce TMAO production in vivo.26 Furthermore, betaine aldehyde has demonstrated CutC in vitro inhibitory activity with a half maximal inhibitory concentration (IC50) value of 26 μM; however, poor reduction of the production of TMA was observed in whole cell assasys.32 Compound 3 (Fig. 1B) has recently been identified as a cyclic choline analog with improved CutC inhibitory activity (IC50 value of 2.9 μM).33 However, 3 featured a half maximal effective concentration (EC50) value of 60 μM in the inhibition of the conversion of choline to TMA in a human fecal suspension.33 Therefore, the development of small-molecule CutC inhibitors that maintain activity in a complex gut microbiota environment is of great importance to identify leads for further in vivo screening.
Carbohydrates are typical substrates of various metabolizing enzymes expressed by human gut microbiota.34–36 Moreover, carbohydrate-based prebiotics have demonstrated an ability to modulate the gut microbiome based on their chemical composition, monosaccharide profile, and glycosidic linkage.37 Therefore, optimization of the structure of carbohydrate-based therapeutics holds promise for further advancement in personalized microbiome modulation with precision (PMMP).37 Glycomimetics are structurally altered analogs of carbohydrates that are able to mimic their interaction with target proteins.38 Given the structural complexity and metabolic instability of carbohydrates, glycomimetics have emerged as promising scaffolds to identify leads as potential therapeutics for various diseases.38–42 However, glycomimetic-based approaches haven't been explored for targeting bacterial TMA-lyase (CutC). Herein, we report a benzoxazole-based ligand capable of reducing TMA levels in whole cell assays, which was identified from screening a glycomimetic library for in vitro CutC inhibitory activity.
Results and discussion
Aiming to discover glycomimetic ligands which can inhibit TMA production by CutC, we identified a small library of glycomimetic ligands and their structural analogs (627 compounds) from the Enamine glycomimetic library.43 The library represents a set of synthesized small molecules with molecular weights in the range of 140 to 450 Dalton that can mimic the interaction of carbohydrates with their target proteins. Members of this library are enriched with hydrogen bond acceptors and donors as well as O, N-containing aliphatic rings.43
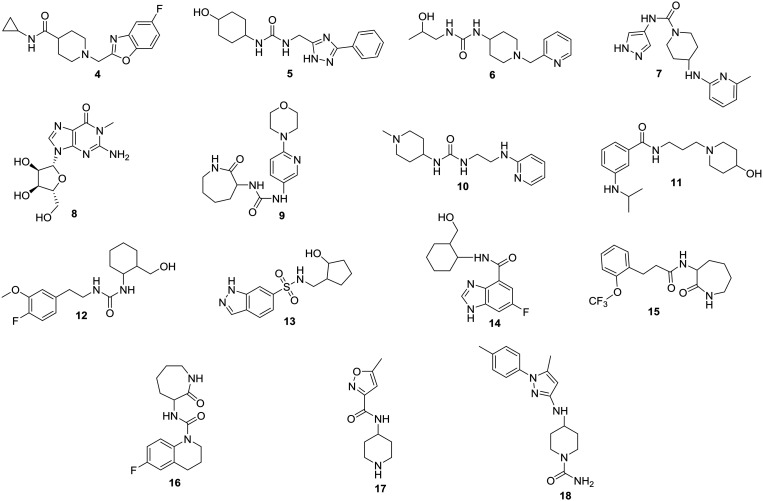
The metabolism of drugs by gut microbiota greatly affects their therapeutic efficiency.44,45 Additionally, intestinal metabolism is a critical factor in controlling the bioavailability and pharmacokinetic profiles of therapeutic agents.46,47 Thus, identification of small molecules with demonstrated metabolic stability in the complex human gastrointestinal system would be fundamental in advancing gut microbiota-targeted small molecules for further screening in animal models and eventually to clinical translation. As shown in Fig. 2, we subjected the glycomimetic library (627 compounds) to in vitro metabolic stability screening using mixed gender human intestinal S9 fraction. A final concentration of 33 μM of the tested compounds was used in the metabolic assay to reflect the predicted concentration of drugs in the gastrointestinal tract upon oral administration.48 Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to determine the percentage of intact compounds remaining upon incubation for 2 hours with intestinal S9 fraction. The in vitro metabolic screening identified compounds 4–18 (Fig. 3) with more than 90% remaining parent compound at the end of the incubation time (Table S1, ESI†). According to the workflow we developed (Fig. 2), compounds with in vitro metabolic stability were screened for the ability to reduce CutC-mediated production of TMA. Further screening of compounds 4–18 using CutC in vitro inhibitory assay identified compound 4 (BO-I, Fig. 3) as a novel CutC inhibitor.
Fig. 2. A schematic illustration of the workflow used in this study to identify CutC inhibitors with in vitro intestinal metabolic stability.
Fig. 3. Chemical structures of compounds 4–18.
The ability of compounds 4–18 to inhibit anaerobic choline metabolism by the cut gene cluster-containing human gut isolate Escherichia coli (E. coli) MS 200-1 was evaluated in a multi-dose screening format. The outcome of the screening is shown in Table 1 in comparison to compound 3 as a positive control. The investigated compounds were added to the bacterial culture as well as d9-choline as a substrate for CutC. Levels of d9-TMA generated by CutC were determined using liquid chromatography-mass spectrometry (LC-MS). Compound 4 (BO-I) featured an EC50 value of 5.4 ± 1.1 μM in the whole cell assay while the positive control (compound 3) displayed an EC50 value of 11 ± 1.5 μM. It is noteworthy to mention that the addition of BO-I to the bacterial culture had no impact on the uptake of choline by the bacteria at various tested concentrations (Fig. S1, ESI†). This finding highlights that the potential interaction of BO-I with CutC is likely to mediate reduced TMA production in the whole cell assay. Compounds 5 and 6 exhibited weak to moderate ability to reduce TMA production in whole cell assays with EC50 values of 79 ± 5.8 and 87 ± 6.9 μM, respectively. Additionally, compounds 7 and 8 featured a weak inhibitory effect on TMA production with EC50 values >100 μM. Compounds 9–18 had a minimal impact on the production of TMA in whole cell assays (Table 1).
Evaluation of the activity of compounds 3–18 in whole cell assays and in vitro CutC assay.
| Compound | EC50 against E. coli MS 200-1 (μM) | IC50 against CutC (μM) |
|---|---|---|
| 3 | 11 ± 1.5 | 3.9 + 0.2 |
| 4 | 5.4 ± 1.1 | 2.4 ± 0.3 |
| 5 | 79 ± 5.8 | 49 + 5.4 |
| 6 | 87 ± 6.9 | 73 + 5.1 |
| 7 | 143 + 21 | 85 + 7.2 |
| 8 | 175 + 32 | 110 + 8.4 |
| 9 | 298 + 45 | 167 + 9.5 |
| 10 | 354 + 31 | 199 + 11.5 |
| 11 | 376 + 55 | 210 + 20.8 |
| 12 | 409 + 59 | 212 + 17.9 |
| 13 | 488 + 63 | 301 + 20.5 |
| 14 | >1000 | >500 |
| 15 | >1000 | >500 |
| 16 | >1000 | >500 |
| 17 | >1000 | >500 |
| 18 | >1000 | >500 |
In order to confirm the potential interference of BO-I with the catalytic activity of CutC, in vitro CutC screening assay was performed. Wild-type CutC from Desulfovibrio desulfuricans strain G20 was expressed and purified as described previously.30 Coupling the activity of CutC to the reduction of acetaldehyde by NADH-dependent yeast alcohol dehydrogenase (YADH) is an established tool for screening in vitro CutC inhibitory activity.32,33 Using this method to evaluate the CutC inhibitory activity of BO-I in a dose-dependent assay demonstrated an IC50 value of 2.4 ± 0.3 μM for BO-I (Table 1 and Fig. 4). The positive control 3 exhibits an IC50 value of 3.9 + 0.2 μM. Incubation of different concentrations of BO-I (5, 25, and 50 μM) with alcohol dehydrogenase had a negligible impact on its activity as revealed by alcohol dehydrogenase colorimetric assay (Fig. S2. ESI†). Additionally, evaluation of the ability of BO-I to bind both CutC and CutD was performed using surface plasmon resonance (SPR) screening. Flowing a solution of BO-I over immobilized CutC revealed dose-dependent binding with a Kd value of 4.05 ± 0.23 μM (Fig. S3. ESI†). Additionally, minimal binding affinity of BO-I to CutD was detected from SPR screening (Fig. S4. ESI†). Collectively, these findings illustrate that the interaction of BO-I with CutC is the driving force for reduced TMA production in whole cell assays. CutC inhibitory activities (expressed as IC50 values) for compounds 5–18 are displayed in Table 1 and are in consistence with their weak to moderate activity in whole cell assays.
Fig. 4. Dose–response curve of BO-I in CutC in vitro inhibition assay. Error bars represent the standard deviation (n = 3).
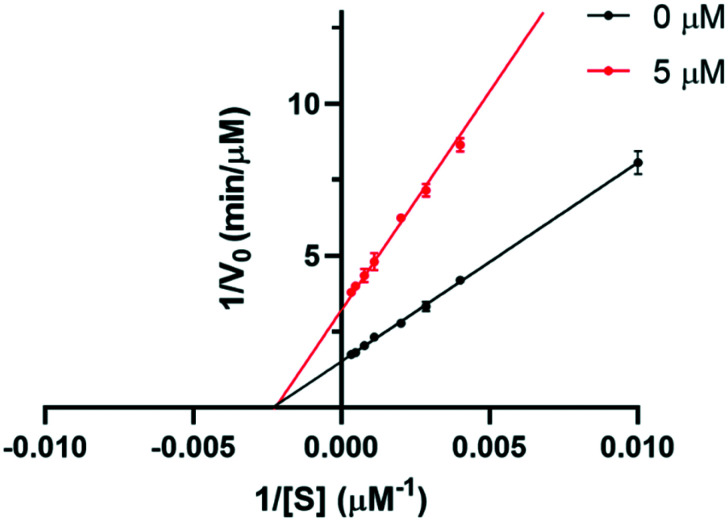
We next performed a kinetic study for the CutC activity in the presence of BO-I in order to gain further insight into its mode of action. The enzymatic reaction mediated by CutC was carried out in the presence and absence of BO-I at different concentrations of the CutC substrate, choline. The Michaelis–Menten plot of the reaction velocity against choline concentration (Fig. S5. ESI†) demonstrated that Vmax (maximum rate for the enzymatic reaction) was remarkably reduced in the presence of BO-I (5 μM). However, the addition of BO-I (5 μM) to the enzymatic reaction had no impact on the Michaelis constant (KM) of ∼420 μM. A Lineweaver–Burk plot (double reciprocal plot) for the kinetic study (Fig. 5) further reveals unchanged KM as well as reduced Vmax as a function of added BO-I (5 μM). Such trends are in consistence with activity profiles of non-competitive inhibitors of enzyme activities according to the Michaelis–Menten equation.49 Thus, we propose that the non-competitive inhibition of CutC by BO-I is the key to its ability to reduce TMA production in whole cell assays.
Fig. 5. Lineweaver–Burk double reciprocal plot of reaction velocity (1/V0) versus substrate concentration (1/[S]) in the absence and presence of BO-I (5 μM). Error bars represent the standard deviation (n = 3).
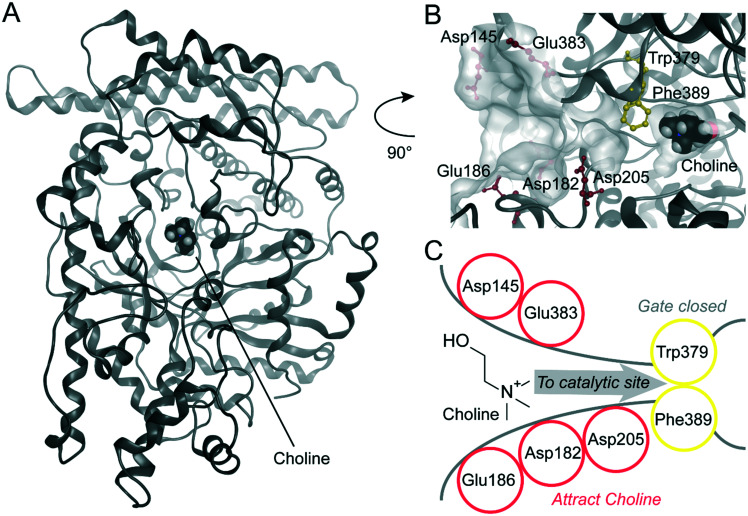
In order to rationalize the inhibitory activity of BO-I against CutC from D. desulfuricans and K. pneumoniae, we performed extensive molecular modelling for the protein–inhibitor complexes. For the two glycyl radical enzymes (GREs) benzylsuccinate synthase (BSS)50 and CutC from Klebsiella pneumoniae,51 a large-scale clam shell-like closure upon choline binding was postulated. A potential mechanism of the inhibition of BO-I might be the interference with this movement. For CutC from K. pneumoniae, the open choline-free and closed choline-bound states of the enzyme were solved by X-ray crystallography (PDB-IDs: 5A0Z, 5A0U).51 The large-scale opening movement opens a funnel to the buried catalytic site accommodating choline (Fig. S7, ESI†). The crystal structure of CutC from D. desulfuricans strain G20 by Bodea et al. in the closed state with bound choline shows Trp379 and Phe389 as gating residues for the entrance to the catalytic site (Fig. 6, PDB-ID: 5FAU).52 We suggest that the movement of Phe389/671 (D. desulfuricans/K. pneumoniae) is restricted in the closed state by Leu700/982 based on the X-ray structures (PDB-IDs: 5FAU, 5A0U)51,52 (Fig. S7, ESI†). In the open state X-ray structure of CutC from K. pneumoniae (PDB-ID: 5A0Z),51 an open channel is described by Kalnins et al., which is at a similar position to our suggested substrate entry funnel (Fig. S7, ESI†). The X-ray structure of the open state clearly shows that Phe is in an outward rotated position and we suggest that this leads to the funnel opening (Fig. S7, ESI†). We did not consider the open state of CutC towards inhibitor binding since the Leu-loop and other loops in the vicinity to the choline binding site are not resolved in the X-ray structure (PDB-ID: 5A0Z)51 (Fig. S7, ESI†). However, we surmise that CutC recognizes positively charged substrate choline through a network of residues with negatively charged side chains (D. desulfuricans: Asp145, Asp182, Glu186, Asp205, and Glu383) at the entry of this funnel (Fig. 6). Ionic long-range interactions represent a common mechanism for substrate recognition in enzymes.53 We suggest the interference with the substrate recognition of CutC as a second potential inhibition mechanism of BO-I. Hence, we probed the surfaces of CutC from D. desulfuricans and K. pneumoniae for plausible binding sites by docking BO-I in the vicinity to the bound choline. Our docking experiments suggest only two plausible binding conformations to CutC from D. desulfuricans (pose 1, Fig. 7A and pose 2, Fig. 7B) and one similar conformation to CutC from K. pneumoniae (Fig. S8, ESI†). All other docking poses of BO-I were regarded implausible according to our protocol detailed in the Experimental section. In both modelled binding conformations of BO-I to CutC from D. desulfuricans, the positively charged piperidine moiety of BO-I mimics choline and shows a charge interaction with Asp205 (Fig. 7A and B). According to modelled pose 1, the cyclopropyl moiety and the benzoxazole moiety in pose 2 of BO-I show hydrophobic contacts in a buried hydrophobic subpocket formed by Tyr189, Trp195, Ala210, and Val211. In putative pose 1, BO-I shows additional hydrophobic contacts via its benzoxazole moiety with Phe202, Phe388, and Leu709 (Fig. 7). In our model, we observed hydrophobic contacts between the cyclopropyl moiety of BO-I and Trp477, Tyr471, and Ile493 as well as between the fluorobenzoxazole moiety and Phe484, Tyr670, and Leu991 of CutC from K. pneumoniae (Fig. S8, ESI†). BO-I also establishes a salt bridge to Asp487, which is at a similar position to Asp205 in D. desulfuricans. Interestingly, our surmised binding pocket does not significantly change its steric shape between the open and closed states of CutC of K. pneumoniae. The root mean square deviation of the heavy atoms of the binding pocket residues is minimal with 1.0 Å (Fig. S8, ESI†). Additionally, a binding mode hypothesis for BO-I to the open state was obtained by docking to the suggested binding site in the open state structure of CutC (PDB-ID: 5A0Z).51 BO-I binding hypotheses to the closed and the open states closely resemble each other (Fig. S8, ESI†). This suggests that BO-I can bind to the entry channel of CutC in both open and closed states and hamper the choline binding, as well as the product leaving the active site. Furthermore, our modelled mode of inhibition of CutC by BO-I does not indicate interference with the transition of CutC between the open and closed states. In order to identify possible essential features for binding in the CutC substrate entry funnel causing the blockade by BO-I, we performed molecular dynamics (MD) simulations starting from the two plausible poses of BO-I as shown in Fig. 7A and B. Protein–inhibitor interaction dynamics were monitored by dynamic 3D pharmacophore analysis (dynophores, Fig. 7C and D).54 MD simulations starting from BO-I in pose 1 show a high frequency of ionic interaction with Asp205 (85%) and hydrophobic contacts for the cyclopropyl and benzoxazole moieties (87% and 88%, respectively, Table S2, ESI†). The benzoxazole moiety shows sporadic aromatic interactions (12%, aromatic interactions of benzene or isoxazole rings, Fig. S1†) with Phe202 and Phe383. In the MD simulations of pose 2, ionic interactions to Asp205 are frequent (64%, Table S2, ESI†) and the benzoxazole hydrophobic contacts are stable (97%). We observed that the cyclopropyl moiety in pose 2 moves from the less favourable solvent exposed position into ‘flipped pose 2’. In flipped pose 2, cyclopropyl is accommodated by a lipophilic cleft consisting of residues Phe202, Phe388, and Leu709 (Fig. 7B and D). Hence, we suggest a 3D pharmacophore model for the CutC inhibition of BO-I comprising two hydrophobic contact areas surrounding a positive ionizable feature representing the piperidine moiety (Fig. 7E). BO-I can interact with all the chemical features in our suggested 3D pharmacophore model in pose 1 or in ‘flipped’ pose 2′ (Fig. 7A and B). We surmise that the piperidine moiety of BO-I disrupts the long-range ionic interactions in the putative substrate entry funnel assumed to be necessary for choline recognition. The putatively hampered choline recognition likely leads to decelerated access to the catalytic site and decreased conversion to TMA.
Fig. 6. Suggested substrate recognition mechanism for CutC. (A) X-ray structure of CutC co-crystallized with choline in its central catalytic site (PDB-ID: 5FAU50). (B) Suggested substrate recognition funnel towards the catalytic site with annotations of key residues and transparent surfaces. Protein backbone is dark grey; suggested substrate entry funnel residues (red) and catalytic site gate residues (yellow) are highlighted. (C) Schematic 2D representation of the suggested substrate recognition mode of CutC. Negatively charged residues (red) putatively recognize choline and lead it to the catalytic site gate (yellow), which is closed in the X-ray structure.
Fig. 7. Binding hypotheses for BO-I to CutC. In the docking poses 1 (A) and 2 (B), BO-I putatively blocks the choline (grey ball model) substrate entry channel. BO-I moves during MD simulations from pose 2 to ‘flipped’ conformation (B; black arrow). Suggested molecular interaction dynamics of CutC and BO-I in pose 1 (C) and 2/‘flipped’ 2 (D) are shown as representative dynamic pharmacophores (yellow: lipophilic contacts; purple: cationic interaction). A simple suggested 2D pharmacophore model was derived for pose 1 and ‘flipped’ pose 2 (E). BO-I is represented as a ball and stick model and heavy atoms are color coded: grey shades – carbon, green – fluorine, red – oxygen, and dark blue – nitrogen. The lipophilic contact pharmacophore feature is colored yellow and the cationic interaction is purple.
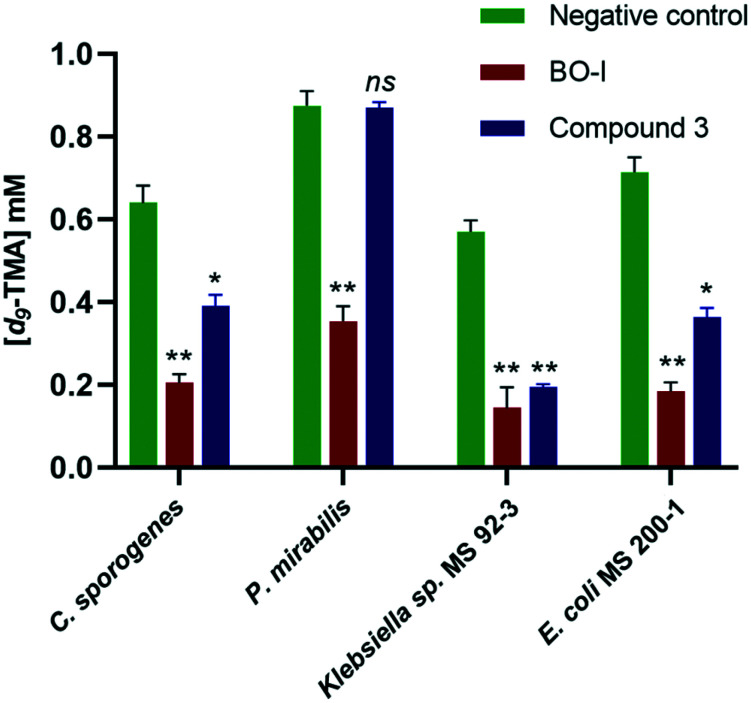
CutC inhibitors have demonstrated considerable variation in their ability to reduce TMA production in different bacterial strains.32,33 Thus, we evaluated the ability of BO-I to inhibit TMA production in whole cell assays using different bacterial strains, namely Clostridium sporogenes, Proteus mirabilis, Klebsiella sp. MS 92-3, and E. coli MS 200-1. The effect of incubation of BO-I (10 μM) on TMA production in the tested bacterial strains is displayed in Fig. 8. Remarkably, BO-I was able to significantly reduce the TMA levels in the bacterial culture of all the screened strains revealing its efficacy in various biological environments. In comparison to compound 3, BO-I was more efficient in reducing TMA production in Clostridium sporogenes, Proteus mirabilis, Klebsiella sp. MS 92-3, and E. coli MS 200-1 at 10 μM (Fig. 8). It is noteworthy to mention that BO-I (25 μM) didn't impact the growth kinetics of all the screened bacterial strains (Fig. S6. ESI†). Moreover, we tested the effectiveness of BO-I in impeding the transformation of choline into TMA in a human fecal suspension. Interestingly, BO-I featured an EC50 value of 38 ± 5.1 μM in the inhibition of TMA production. On the other hand, compound 3 as a positive control displayed an EC50 value of 83.9 ± 7.4 μM. The superior efficacy of BO-I in comparison to 3 renders BO-I as a promising lead for further optimization aiming at the development of potent CutC inhibitors.
Fig. 8. Levels of d9-TMA in Clostridium sporogenes, Proteus mirabilis, Klebsiella sp. MS 92-3, and E. coli MS 200-1 in the absence and presence of BO-I and compound 3 (10 μM). Error bars represent the standard deviation (n = 3). (*p < 0.05, **p < 0.005 relative to negative control, ns indicates non-significant difference).
Conclusions
In conclusion, we screened a focused library of glycomimetics and their structural analogs for intestinal metabolic stability followed by in vitro CutC inhibitory activity. Our screening strategy identified BO-I as a non-competitive inhibitor of CutC with an IC50 value of 2.4 ± 0.3 μM. The discovered inhibitor reduced the levels of TMA production in whole cell assays using different bacterial strains. Importantly, BO-I shows satisfactory inhibition of TMA production in the human fecal suspension (EC50 value of 38 ± 5.1 μM) showcasing its suitability for in vivo screening. Molecular modeling suggests a negatively charged entrance channel for choline, which is possibly blocked by BO-I leading to a reduced conversion of choline. Optimization of the lead identified in this study would remarkably advance research efforts aiming at the clinical translation of gut microbiota-targeted small molecules by offering tools for inhibiting TMA production.
Experimental
Screening for intestinal metabolic stability
Mixed gender human intestinal S9 fraction was purchased from Sekisui XenoTech, LLC. The reaction buffer was potassium phosphate buffer (100 mM) at pH 7.4. The assay was performed in 96-well plates with a final reaction volume of 220 μL consisting of: 83 μL reaction buffer, 85 μL of S9 fraction (4 mg mL−1), 2 μL from tested compound stock, and 50 μL from cofactor stock (NADPH, 1 mg mL−1) in the presence of MgCl2 (6 mM) and EDTA (1 mM). Tested compounds were screened in triplicate at a final concentration of 33 μM. Negative control for the experiment was performed by replacing the cofactor solution with the reaction buffer. The samples were incubated for 2 h at 37 °C before reaction termination. The samples were then centrifuged at 2500 rpm. 25 μL of the supernatant was diluted with water (350 μL) and acetonitrile (100 μL). Finally, the samples were transferred to 96-well plates for LC-MS/MS analysis.
Mass spectrometry was performed using an API 3000™ LC/MS/MS system, an enhanced high performance triple quadrupole mass spectrometer. An Atlantis dC18 column (Waters Chromatography), 2.0 mm × 100 mm, was used as a stationary phase. The mobile phase was a gradient of water and acetonitrile containing 0.1% formic acid with a flow rate of 0.25 mL min−1.
Whole cell assay for the assessment of TMA production
The assay was performed as previously described.32,33 Briefly, the bacterial strain employed in the whole cell assay was streaked onto an agar plate in an anaerobic chamber and incubated at 37 °C overnight. Choline chloride (1 mM) was added to a single colony of bacteria inoculated into 5 mL of brain heart infusion (BHI) medium. Subsequently, this culture is inoculated into a fresh medium supplemented with 1 mM choline chloride-(trimethyl-d9).
The tested compounds were dissolved in potassium phosphate buffer (100 mM) at pH 7.4 and added to 96-well plates (Corning), then the previously prepared bacterial culture was added to the assay plates. The final volume of the mixture was 220 μL. The assay plates were sealed and incubated at 37 °C for 4 h in an anaerobic chamber. Following the incubation time, each sample was diluted 4000-fold using a mixture of acetonitrile and 100 mM ammonium formate (95 : 5). The diluted samples were then analysed using LC-MS/MS for d9-choline and d9-TMA. Standard curves using various concentrations of d9-choline and d9-TMA (0.05–4 mM) were prepared. The d9-TMA production level was determined by comparing the peak integration to the standard curves. We next evaluated the dose–response profile of the tested compounds and determined the respective EC50 values. The dose–response curve was analyzed by nonlinear regression using GraphPad Prism 8.0.2 (GraphPad Software, Inc., La Jolla, CA, USA). Each data point from this assay represents the average of three independent measurements. Error bars represent the standard deviation.
CutC in vitro inhibitory assay
The assay was performed as previously described.32,33 CutC and CutD were expressed and purified using previously reported procedures.32,33 In an anaerobic chamber, both CutD (40 μM) and Na dithionite (150 μM) were incubated for 15 min. A mixture of S-(5′adenosyl)-l-methionine (SAM, 200 μM) and CutC (20 μM) were added to the incubated mixture. The final reaction volume was 50 μL in polypropylene Eppendorf tubes. The reaction buffer was potassium phosphate buffer at pH 8.0 (50 mM) supplemented with 50 mM KCl. The reaction sample was incubated for 1 h followed by 20-fold dilution using the reaction buffer. To 50 μL of the diluted sample, a master mix of NADH (200 μM) and yeast alcohol dehydrogenase (YADH, 0.4 μM) was added. Subsequently, the mixture (180 μL) was added to 96-well plates (Corning), followed by the tested compound (10 μL) and choline (10 μL, 200 μM).
UV-vis absorbance was then measured at 340 nm every 20 s for 10 min starting from the complete addition of all reaction components. A negative control in which the reaction buffer (10 μL) replaces the tested compound (10 μL) was performed and subtracted from the measurements of the tested compounds. The initial rate of each tested concentration was normalized to the negative control. The dose–response curve was analyzed by nonlinear regression using GraphPad Prism 8.0.2 (GraphPad Software, Inc., La Jolla, CA, USA) by plotting the normalized CutC activity against the tested concentration. Each data point from this assay represents the average of three independent measurements. Error bars represent the standard deviation.
Alcohol dehydrogenase colorimetric assay
The assay was performed using an alcohol dehydrogenase assay kit from abcam (ab102533) according to the protocol provided by the manufacturer.
SPR screening
The screening was performed using a BIAcore S200. The proteins (CutC and CutD) were immobilized (20 μg ml−1) on a CM5 sensor chip (Biacore, GE Healthcare) using an amine coupling kit.
A stock solution of BO-I was prepared in DMSO at 1 mM final concentration. The samples were then serially diluted while maintaining the final DMSO concentration at 5% in all tested concentrations. The tested dilutions in potassium phosphate buffer (100 mM) at pH 7.4 containing 5% DMSO and 0.05% Tween20 were allowed to flow through both ligand-captured flow cells and reference flow cells at the same rate (30 μl min−1) and contact time (120 s). Solvent correction was included to avoid the impact of DMSO on the surface plasmon effect during binding analysis. Additional washing of the flow system using 50% DMSO in potassium phosphate buffer was then performed following each run. Kinetic analysis was performed at 25 °C.
Evaluation of TMA production in a human fecal suspension
Fecal matter from a single human donor was purchased from Lee Biosolutions, Inc. A suspension of the fecal matter was prepared by diluting it 1 : 10 (weight per volume) using a sterile anaerobic phosphate buffer solution. Following centrifugation of the diluted sample at 1000 rpm for 10 min at 4 °C, the supernatant was further suspended in a BHI medium 1 : 50 (v/v) supplemented with d9-choline (1 mM).
Each tested concentration (10 μL) was added to a 96-well plate (Corning), followed by the addition of the prepared human fecal suspension (200 μL). The assay plates were incubated overnight at 37 °C. Following the incubation period, further dilution of the samples was performed (4000 folds) using a mixture of acetonitrile and 100 mM ammonium formate (95 : 5). The diluted samples were then analysed using LC-MS/MS for d9-choline and d9-TMA as described above. All experiments were performed in triplicate.
Molecular docking
Molecular docking of BO-I to CutC was performed using GOLD (v.5.2; Genetic Optimisation for Ligand Docking, The Cambridge Crystallographic Data Centre, UK)55 in order to obtain binding hypotheses. A 3D starting geometry for BO-I was built in MOE (v2019.0102; Molecular Operating Environment, Chemical Computing Group ULC, Montreal, Canada). X-ray coordinates of CutC from D. desulfuricans (PDB-ID: 5FAU52) and K. pneumoniae (PDB-ID: 5A0U,51 chain H) in the closed state and open state from K. pneumoniae (PDB-ID: 5A0Z,51 chain B) were used as protein conformation and underwent structural preparation in MOE. The protein structure and ligand were protonated at pH 7.4 in MOE.56 The choline nitrogen served as the binding site sphere center. The binding site sphere radius was 25 Å since there was no initial binding site hypothesis. The genetic algorithm (GA) performed 50 runs at a search efficiency of 100% and the ‘diverse solution’ option was enabled. The ASP scoring function57 was used including subsequent rescoring with ChemScore.58 The resulting 50 diverse binding hypotheses of BO-I were energy minimized in the presence of CutC using LigandScout (v4.4; Inte:ligand, Vienna, Austria)59–61 and the MMFF94 force field.62 Ligand orientations were evaluated with respect to their geometry and interactions with CutC using pharmacophore analysis. Poses that placed the positively charged piperidine too close to basic residues and cis amide bonds were discarded. At least three interactions to the protein including the ionic interaction of piperidine were mandatory. The settings to dock BO-I to the suggested substrate funnel of the open state CutC differed in the following aspects. The binding site sphere center was set as the carboxylate carbon of Asp478 and the binding site radius was 10 Å. GA only performed 20 runs due to the smaller binding sphere.
Molecular dynamics simulations
Both CutC–BO-I complexes were prepared using Maestro (v11.7; Schrödinger, New York, USA). Hydrogen bonds were optimized in each system. The complexes were solved in the TIP4P water model63 within a cubic box with a 12 Å padding distance to the protein surface and neutralized with sodium ions. Osmotic pressure was adjusted with 0.15 M sodium chloride to achieve isosmotic conditions. Molecular dynamics (MD) simulations were carried out with Desmond (v5.5)64 on Nvidia GeForce water-cooled RTX 2080 Ti graphics cards (NVIDIA Corporation, Santa Clara, USA) in five replicates for each system. Both systems were relaxed and equilibrated according to the default protocol. MD runs were performed under a normal pressure of 1.01325 bar and a temperature of 300 K for 100 ns generating 2000 protein–ligand conformations per replicate.
Dynamic 3D pharmacophores (dynophores)
CutC–BO-I interaction patterns occurring in the MD simulations were tracked using dynamic 3D pharmacophores (dynophores).54 Dynophores move the pharmacophore concept modeling from the statics to the dynamics.65 A dynophore model encompasses all protein–ligand interactions occurring during an MD simulation in point density clouds and allows conclusions on spatial distribution and statistics of interactions. MD simulation data were prepared in VMD (v1.9.3)66 for dynophore analysis. All five complete simulation replicas of one system were concatenated after the structural alignment to the binding site coordinates of the X-ray structure of CutC (PDB-ID: 5FAU52). The dynophore app is implemented in the LigandScout framework.59–61
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Electronic supplementary information (ESI) available: Experimental procedures, and supplementary figures. See DOI: 10.1039/d0md00218f
Notes and references
- Thursby E. Juge N. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I. Gibson G. Heinken A. Scott K. Swann J. Thiele I. Tuohy K. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P. D. Gut. 2018;67:1716. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala S. M. Talukdar R. Subramanyam C. Vuyyuru H. Sasikala M. Nageshwar Reddy D. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J. F. O'Riordan K. J. Cowan C. S. M. Sandhu K. V. Bastiaanssen T. F. S. Boehme M. Codagnone M. G. Cussotto S. Fulling C. Golubeva A. V. Guzzetta K. E. Jaggar M. Long-Smith C. M. Lyte J. M. Martin J. A. Molinero-Perez A. Moloney G. Morelli E. Morillas E. O'Connor R. Cruz-Pereira J. S. Peterson V. L. Rea K. Ritz N. L. Sherwin E. Spichak S. Teichman E. M. van de Wouw M. Ventura-Silva A. P. Wallace-Fitzsimons S. E. Hyland N. Clarke G. Dinan T. G. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Carabotti M. Scirocco A. Maselli M. A. Severi C. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Shreiner A. B. Kao J. Y. Young V. B. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J. A. Mazmanian S. K. Genome Med. 2018;10:98. doi: 10.1186/s13073-018-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H. Cani P. D. Mayer E. A. Gut. 2016;65:2035. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- Wang X.-Q. Zhang A.-H. Miao J.-H. Sun H. Yan G.-L. Wu F.-F. Wang X.-J. RSC Adv. 2018;8:42380–42389. doi: 10.1039/C8RA08094A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J. F. O'Riordan K. J. Sandhu K. Peterson V. Dinan T. G. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- Sharon G. Garg N. Debelius J. Knight R. Dorrestein P. C. Mazmanian S. K. Cell Metab. 2014;20:719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postler T. S. Ghosh S. Cell Metab. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel S. H. Warrier M. Annu. Rev. Nutr. 2017;37:157–181. doi: 10.1146/annurev-nutr-071816-064732. [DOI] [PubMed] [Google Scholar]
- Marcus A. B. Gianfranco F. Allan M. E. Curr. Drug Metab. 2005;6:227–240. doi: 10.2174/1389200054021807. [DOI] [PubMed] [Google Scholar]
- Wang Z. Klipfell E. Bennett B. J. Koeth R. Levison B. S. Dugar B. Feldstein A. E. Britt E. B. Fu X. Chung Y. M. Wu Y. Schauer P. Smith J. D. Allayee H. Tang W. H. DiDonato J. A. Lusis A. J. Hazen S. L. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J. Ling A. V. Manthena P. V. Gearing M. E. Graham M. J. Crooke R. M. Croce K. J. Esquejo R. M. Clish C. B. Vicent D. Biddinger S. B. Nat. Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas M.-E. Barton R. H. Toye A. Cloarec O. Blancher C. Rothwell A. Fearnside J. Tatoud R. Blanc V. Lindon J. C. Mitchell S. C. Holmes E. McCarthy M. I. Scott J. Gauguier D. Nicholson J. K. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12511. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H. Wang Z. Kennedy D. J. Wu Y. Buffa J. A. Agatisa-Boyle B. Li X. S. Levison B. S. Hazen S. L. Circ. Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciun S. Balskus E. P. Proc. Natl. Acad. Sci. U. S. A. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger S. K. Williams D. E. Pharmacol. Ther. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger J. Clark S. Massick S. Bechtel M. J. Clin. Aesthet. Dermatol. 2013;6:45–48. [PMC free article] [PubMed] [Google Scholar]
- Dolphin C. T. Janmohamed A. Smith R. L. Shephard E. A. Phillips I. R. Nat. Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- Christodoulou J. J. Paediatr. Child Health. 2012;48:153–155. doi: 10.1111/j.1440-1754.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- Wang Z. Roberts A. B. Buffa J. A. Levison B. S. Zhu W. Org E. Gu X. Huang Y. Zamanian-Daryoush M. Culley M. K. DiDonato A. J. Fu X. Hazen J. E. Krajcik D. DiDonato J. A. Lusis A. J. Hazen S. L. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B. Gu X. Buffa J. A. Hurd A. G. Wang Z. Zhu W. Gupta N. Skye S. M. Cody D. B. Levison B. S. Barrington W. T. Russell M. W. Reed J. M. Duzan A. Lang J. M. Fu X. Li L. Myers A. J. Rachakonda S. DiDonato J. A. Brown J. M. Gogonea V. Lusis A. J. Garcia-Garcia J. C. Hazen S. L. Nat. Med. 2018;24:1407–1417. doi: 10.1038/s41591-018-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. M. Wang Z. Lee R. Meng Y. Che N. Charugundla S. Qi H. Wu J. Pan C. Brown J. M. Vallim T. Bennett B. J. Graham M. Hazen S. L. Lusis A. J. J. Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier M. Shih D. M. Burrows A. C. Ferguson D. Gromovsky A. D. Brown A. L. Marshall S. McDaniel A. Schugar R. C. Wang Z. Sacks J. Rong X. Vallim T. A. Chou J. Ivanova P. T. Myers D. S. Brown H. A. Lee R. G. Crooke R. M. Graham M. J. Liu X. Parini P. Tontonoz P. Lusis A. J. Hazen S. L. Temel R. E. Brown J. M. Cell Rep. 2015;10:326–338. doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman J. R. Camp K. Fakharzadeh S. S. Fennessey P. V. Hines R. N. Mamer O. A. Mitchell S. C. Nguyen G. P. Schlenk D. Smith R. L. Tjoa S. S. Williams D. E. Yannicelli S. Curr. Drug Metab. 2003;4:151–170. doi: 10.2174/1389200033489505. [DOI] [PubMed] [Google Scholar]
- Craciun S. Marks J. A. Balskus E. P. ACS Chem. Biol. 2014;9:1408–1413. doi: 10.1021/cb500113p. [DOI] [PubMed] [Google Scholar]
- Winter M. Bretschneider T. Thamm S. Kleiner C. Grabowski D. Chandler S. Ries R. Kley J. T. Fowler D. Barlett C. Binetti R. Broadwater J. Luippold A. H. Bischoff D. Buttner F. H. SLAS Discovery. 2019;24:766–777. doi: 10.1177/2472555219838216. [DOI] [PubMed] [Google Scholar]
- Orman M. Bodea S. Funk M. A. Campo A. M.-D. Bollenbach M. Drennan C. L. Balskus E. P. J. Am. Chem. Soc. 2019;141:33–37. doi: 10.1021/jacs.8b04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollenbach M. Ortega M. Orman M. Drennan C. L. Balskus E. P. ACS Med. Chem. Lett. 2020 doi: 10.1021/acsmedchemlett.0c00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassard C. Lacroix C. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:453–460. doi: 10.1097/MCO.0b013e3283619e63. [DOI] [PubMed] [Google Scholar]
- Owolabi I. O. Dat-arun P. Takahashi Yupanqui C. Wichienchot S. J. Funct. Foods. 2020;66:103787. doi: 10.1016/j.jff.2020.103787. [DOI] [Google Scholar]
- Flint H. J. Scott K. P. Duncan S. H. Louis P. Forano E. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.-L. Cheung P. C.-K. J. Agric. Food Chem. 2019;67:12335–12340. doi: 10.1021/acs.jafc.9b04811. [DOI] [PubMed] [Google Scholar]
- Compain P. Molecules. 2018;23:1658. doi: 10.3390/molecules23071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alonzo D. Guaragna A. Palumbo G. Curr. Med. Chem. 2009;16:473–505. doi: 10.2174/092986709787315540. [DOI] [PubMed] [Google Scholar]
- Pratesi D. Matassini C. Goti A. Angeli A. Carta F. Supuran C. T. Spanevello R. Cardona F. ACS Med. Chem. Lett. 2020;11:727–731. doi: 10.1021/acsmedchemlett.9b00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaine T. Collins P. MacKinnon A. Sharma G. Stegmayr J. Rajput V. K. Mandal S. Cumpstey I. Larumbe A. Salameh B. A. Kahl-Knutsson B. van Hattum H. van Scherpenzeel M. Pieters R. J. Sethi T. Schambye H. Oredsson S. Leffler H. Blanchard H. Nilsson U. J. ChemBioChem. 2016;17:1759–1770. doi: 10.1002/cbic.201600285. [DOI] [PubMed] [Google Scholar]
- Magnani J. L. Ernst B. Discov. Med. 2009;8:247–252. [PubMed] [Google Scholar]
- https://enamine.net/hit-finding/focused-libraries/glycomimetic-library, Accessed on May 28th 2020
- Enright E. F. Gahan C. G. M. Joyce S. A. Griffin B. T. Yale J. Biol. Med. 2016;89:375–382. [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Zimmermann-Kogadeeva M. Wegmann R. Goodman A. L. Nature. 2019;570:462–467. doi: 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. B. Labissiere G. Curr. Drug Metab. 2007;8:694–699. doi: 10.2174/138920007782109788. [DOI] [PubMed] [Google Scholar]
- George C. F. Clin. Pharmacokinet. 1981;6:259–274. doi: 10.2165/00003088-198106040-00002. [DOI] [PubMed] [Google Scholar]
- Maier L. Pruteanu M. Kuhn M. Zeller G. Telzerow A. Anderson E. E. Brochado A. R. Fernandez K. C. Dose H. Mori H. Patil K. R. Bork P. Typas A. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. Perspect. Sci. 2015;4:3–9. doi: 10.1016/j.pisc.2014.12.002. [DOI] [Google Scholar]
- Funk M. A. Judd E. T. Marsh N. G. Elliot S. J. Drennan C. L. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10161–10166. doi: 10.1073/pnas.1405983111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins G. Kuka J. Grinberga S. Makrecka-Kuka M. Liepinsh E. Dambrova M. Tars K. J. Biol. Chem. 2015;290:21732–21740. doi: 10.1074/jbc.M115.670471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodea S. Funk M. A. Balskus E. P. Drennan C. L. Cell Chem. Biol. 2016;23:1206–1216. doi: 10.1016/j.chembiol.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B. Nicholls A. Science. 1995;268:1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- Bock A. Bermudez M. Krebs F. Matera C. Chirinda B. Sydow D. Dallanoce C. Holzgrabe U. De Amici M. Lohse M. J. Wolber G. Mohr K. J. Biol. Chem. 2016;291:16375–16389. doi: 10.1074/jbc.M116.735431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. Willett P. Glen R. C. Leach A. R. Taylor R. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- Labute P. Proteins. 2009;75:187–205. doi: 10.1002/prot.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooij W. T. Verdonk M. L. Proteins. 2005;61:272–287. doi: 10.1002/prot.20588. [DOI] [PubMed] [Google Scholar]
- Baxter C. A. Murray C. W. Clark D. E. Westhead D. R. Eldridge M. D. Proteins. 1998;33:367–382. doi: 10.1002/(SICI)1097-0134(19981115)33:3<367::AID-PROT6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Seidel T. Ibis G. Bendix F. Wolber G. Drug Discovery Today: Technol. 2010;7:e221–e228. doi: 10.1016/j.ddtec.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Wolber G. Langer T. J. Chem. Inf. Model. 2005;45:160–169. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]
- Wolber G. and Sippl W., in The Practice of Medicinal Chemistry, 4th edn, ed. C. G. Wermuth and D. Rognan, Elsevier Ltd, Philadelphia, PA, USA, 2015, pp. 489–507 [Google Scholar]
- Halgren T. A. Nachbar R. B. J. Comput. Chem. 1996;17:587–615. [Google Scholar]
- Abascal J. L. Vega C. J. Chem. Phys. 2005;123:234505. doi: 10.1063/1.2121687. [DOI] [PubMed] [Google Scholar]
- Bowers K., Chow E., Xu H., Dror R., Eastwood M., Gregersen B., Klepeis J., Kolossvary I., Moraes M., Sacerdoti F., Salmon J., Shan Y. and Shaw D., SC '06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, 2006, p. 43, 10.1109/SC.2006.54 [DOI] [Google Scholar]
- Schaller D. Šribar D. Noonan T. Deng L. Nguyen T. N. Pach S. Machalz D. Bermudez M. Wolber G. WIREs Comput. Mol. Sci. 2020;10:e1468. [Google Scholar]
- Humphrey W. Dalke A. Schulten K. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.