Abstract
Orbitofrontal cortex (OFC) is thought to be involved in appropriate processing of rewarding stimuli, and abnormal OFC structure and function has been found in patients with substance use disorders. Atypical patterns of the H-sulcus in the OFC have been primarily identified with schizophrenia, but also with bipolar disorder, both of which are associated with comorbid substance use. Given the high rates of substance use within Axis I psychiatric disorders, it is reasonable to consider how frequencies of OFC patterns in populations with only substance use compare to controls. This information is crucial to disentangle whether atypical frequencies of H-sulcus sulcogyral patterns within psychopathology are associated with the psychiatric or substance use phenotype. Here, we present the first analysis of H-sulcus sulcogyral patterns in a population of adult black men with (n = 84) and without (n = 24) cocaine use disorder (CUD). We find that OFC sulcogyral patterns are not significantly different from the control group, indicating that OFC sulcogyral patterns are not disrupted in patients with CUD. As exploratory analyses, we describe OFC sulcogyral pattern subtypes in this cohort as well as an additional control group (n = 52), in order to add to the growing body of literature on OFC sulcogyral pattern characterization.
Keywords: OFC, Ventromedial prefrontal cortex, Gyrification, Sulcus
1. Introduction
Cocaine is a highly addictive and powerful stimulant, with use estimates of 2.5% in US individuals 12 and older (NIDA, 2015). Cocaine use and corresponding deaths related to cocaine use has been increasing in recent years (CBHSQ, 2016), highlighting substance use as a major public health concern (CDC, 2016). Many individuals with substance use disorders also have co-occurring psychiatric conditions, including generalized anxiety disorder (Fatseas et al., 2010), depression (Armstrong and Costello, 2002; CDC, 2015), attention deficit hyperactivity disorder (Tims et al., 2002), or conduct disorder (Zulauf et al., 2014). Lifetime crack cocaine use, specifically, has been previously associated with co-occurring post-traumatic stress disorder (Narvaez et al., 2014). While it is common for substance use and psychiatric disorders to co-occur, the influence of drug use on the manifestation of psychiatric symptoms is difficult to tease apart. Given the public health concern of cocaine use and its relationship with multiple psychiatric conditions, understanding neurobiological alterations that may predate and thus contribute to risk of substance use disorders would be prudent.
One morphological marker of interest is the H-shaped sulcal patterns within the orbitofrontal cortex (OFC). Although atypical patterns within the H-shaped sulcus have primarily been associated with schizophrenia (Chakirova et al., 2010; Isomura et al., 2017; Lavoie et al., 2014; Nakamura et al., 2007; Takayanagi et al., 2010) and more recently, bipolar disorder (Patti and Troiani, 2018), both of these patient populations typically have a history of substance abuse (Cerullo and Strakowski, 2007; Margolese et al., 2004). Thus, it has remained a lingering question whether substance abuse in these populations also contributes to the observed atypical brain morphology. One recent study assessed OFC sulcogyral morphology in cannabis users (Chye et al., 2017). We are not aware of other published studies assessing the frequency distributions of OFC H-sulcus patterns in patients with cocaine use disorder (CUD).
The characteristic sulcal and gyral folding within the OFC region develops in utero and is thought to remain stable throughout the life course (Armstrong et al., 1995). Although individual variability exists within the gyrification process, it is remarkable the degree in which similar patterns develop across individuals. These sulcogyral, H-shaped patterns were first characterized in macaques and humans (Chiavaras and Petrides, 2000). Within each hemisphere of the OFC, one of three H-shaped patterns are identified based on the continuity and intersection of the medial, lateral, and transverse orbital sulci, and were named based on the frequency by which they present within an adult human population that lacked psychiatric diagnoses (Chiavaras and Petrides, 2000). It should be noted that although there were originally three main pattern types, subsequent work has added a fourth (Chakirova et al., 2010; Nakamura et al., 2019; Nishikawa et al., 2016; Watanabe et al., 2014).
Previously, uncommon pattern types have been found to occur at increased frequencies within populations with psychopathology including schizophrenia, (Chakirova et al., 2010; Lavoie et al., 2014; Takayanagi et al., 2010) depression, (Whittle et al., 2014), and bipolar disorder (Patti and Troiani, 2018). Given the genetic, etiological, and behavioral overlap across multiple psychiatric conditions, it is reasonable to consider how atypical sulcogyral morphology may present as a transdiagnostic indicator for psychiatric dysfunction (Patti and Troiani, 2018).
Multi-morbidity within psychiatric conditions is common, as those with substance use disorders also frequently are diagnosed with psychiatric disorders (Rounsaville et al., 1998; Goldner et al., 2014). While OFC pattern distribution alone was not found to be associated with cannabis use in a previous study, increased frequencies of atypical patterns were informative of lifetime use of cannabis, as well as co-occurring depressive symptoms (Chye et al., 2017). Similarly, although atypical frequency distributions of OFC H-sulcus patterns were not identified in patients with Obsessive Compulsive Disorder, less common pattern type expression was associated with Obsessive Compulsive Disorder (OCD) illness severity (Delahoy et al., 2019).
Here, we were interested to expand on this work in substance use, by considering how sulcal patterns varied within a population of individuals with cocaine use, who were not previously diagnosed with an axis 1 psychiatric disorder. We sought to address if H-sulcus sulcogyral pattern frequencies differ between patients diagnosed with CUD compared to controls. In addition to describing and assessing frequency distributions of the four documented OFC sulcogyral pattern types (Type I, II, III, and IV, described below), we also include visual depictions of patient’s individual morphology, or pattern subtypes, following from the original paper by Chivaras and Petrides (2000). Subtypes exist because the characterization of an overarching pattern is merely based on the continuity or discontinuity of the medial orbital sulcus (MOS) and lateral orbital sulcus (LOS; Type I: discontinuous MOS and continuous LOS; Type II: continuous LOS and continuous MOS; Type III: discontinuous MOS and discontinuous LOS; Type IV: continuous MOS and discontinuous LOS). Thus, an individual hemisphere of a given brain can be characterized as one of four patterns. However, there is additional variability in other OFC sulci that are not considered in pattern characterization. For example, there is variability in the number of intermediate and posterior sulci in the OFC, as well as variability in length and shape of the transverse orbital sulcus. There is also variability in whether and which OFC sulci intersect, which is not considered when characterizing the H-sulcal pattern. Outside of the seminal paper on 50 human brains, no studies have described or assessed the distribution of pattern subtypes. Here, as exploratory analyses, we describe all of the pattern subtypes in the cocaine abuse disorder population and controls and include both visual and written descriptions of these differences between pattern subtypes. In addition, we assess whether the proportion of subtypes are significantly different than controls. For this analysis, in order to have a comparable number of controls, we include pattern type distribution data from our own database of pattern subtypes across control data previously published by our lab. Finally, the current analysis was completed within a sample of African American men. While we did not expect sulculgyral frequencies within the control sample to differ from those of other published control populations, this work emphasizes a need to include more racially diverse samples within research protocols, as the majority of sulcogyral tracing studies have had predominantly white and Asian samples.
2. Methods
2.1. Participants
Structural images were obtained from a compilation of seven cohorts, all collected as part of research studies completed at the University of Pennsylvania School of Medicine. Details regarding recruitment and data collection have been previously published elsewhere (Childress et al., 2008; Wetherill et al., 2014; Young et al., 2014). All participants provided their written and informed consent to the Institutional Review Boards (IRBs) to University of Pennsylvania and all participating institutions. Briefly, the majority of the cocaine-using population in the Philadelphia area is African American, with the samples only including a handful of white participants. Thus, we restricted our sample and analyses to only include right-handed, African American men (see Table 1 for demographic information) with available neuroimaging data. CUD participant inclusion criteria specified that participants be treatment seeking, ages 18–55 years old, and met DSM-IV or DSM-III-R criteria for cocaine dependence. Smoking was the primary route of exposure, and participants reported using cocaine at least 8–30 days before screening. In addition, CUD participants were required to be available for a 7–10 day inpatient stay.
Table 1.
Demographic Characteristics of included participants.
| Participant Status CUD Patients (n = 84) | Control Group (n = 24) e | ||
|---|---|---|---|
|
| |||
| Characteristic | |||
| Mean (SD) | Mean (SD) | Group Comparison a T-test (p-value) | |
| Age | 45 (7) | 38 (6) | 4.9 (<0.01) |
| Missing b | 2 (2%) | – | |
| Education (years) | 13 (2) | 15 (2) | 4.3 (<0.01) |
| Missing | 24 (29%) | – | |
| Cocaine Use (years) | 18 (9) | – | |
| Missing | – | ||
| Alcohol Use (years) | 17 (12) | – | |
| Missing | 14(17%) | ||
| Cannabis Use | 40 (48) | – | |
| Missing | 24 (29) | ||
| N (%) | N (%) | Group Comparison a X2 test (p-value) |
|
| History of Physical/ Sexual Abuse c | 49 (58) | 10 (42) | 1.8 (0.18) |
| Missing | 2 (2) | – | |
| Cohort d | A: 18 (21) | B: 24 (100) | |
| C: 29 (29) | |||
| D: 11 (13) | |||
| E: 8 (10) | |||
| F: 12 (14) | |||
| G: 6 (7) | |||
Welch Two Sample T-test used to assess differences in the distribution of continuous covariates between CUD patients and control group subjects, X2 independence tests were used to evaluate associations between categorical covariates and CUD patients and control group subjects.
Participants with missing covariate information are presented as the number and percent missing.
Abuse history determined based on participant responses to Addiction Severity Index questions.
Seven cohorts were harmonized to create our sample indicated by letter A:G. Note that all control participants were ascertained from the same cohort, and that this cohort did not contain any CUD patients.
This does not include demographic information for the external control group. Demographic information within the external control group has been previously described (Patti and Troiani et al. 2018)
Note: All participants in our sample are African American Males.
Both CUD and control participants were recruited through advertisements in the Philadelphia metropolitan area. All participants underwent psychiatric and medical screening before enrollment. Exclusion criteria for both CUD and control groups included diagnosis of other psychiatric disorders (as determined by administration of Mini International Neuropsychiatric Interview (MINI). Additional exclusion criteria at the time of scanning included issues preventing structural magnetic resonance imaging (MRI) image acquisition (i.e. claustrophobia) or abnormalities detected on MRIs. We included a total of n = 108 participants from the University of Pennsylvania cohorts, participants with CUD (n = 84), and those identified as controls (n = 24).
2.1.1. Participant demographic and phenotype characterization
It should be noted that not all of the same demographic information was acquired from each of the cohorts, resulting in some information that is missing between them. Here, we report age, education, alcohol use, cannabis use, history of abuse, and years of cocaine use. The MINI (Sheehan et al., 1998) was used to assess cocaine dependence within the CUD patient sample, or the presence of other psychiatric disorders within the control population. To assess drug, alcohol use, and physical/sexual abuse history, study staff administered the Addiction Severity Index (McLellan et al., 1992).
2.2. Image acquisition
MRI scanning was conducted at one Siemens 3T scanner, located at the hospital of University of Pennsylvania, as described previously (Childress et al., 2008; Wetherill et al., 2014; Young et al., 2014). High-resolution, 5-minute anatomical images (T1-weighted 3D MPRAGE) were collected for each participant with the following parameters: 160 axial slices, 1 mm slice thickness, TR = 1620 ms, TE = 3.87 ms, matrix= 192 × 256, and flip angle 15° We excluded participants with excessive motion, as the additional noise within the OFC limited our ability to make accurate tracing classifications. This included the removal of 13 participants (CUD, n = 10) and (control, n = 3) from our final analysis.
2.3. Data analysis
2.3.1. Preprocessing
The anatomical images were normalized by first stripping non-brain tissue using FMRIB Software Library (FSL) Brain Extraction Tool (BET) (Smith et al., 2004), then aligned along the anterior commissure-posterior commissure plane to adjust for head tilt (using FMRIB Linear Image Registration Tool, FLIR (Jenkinson et al., 2002; Jenkinson and Smith, 2001) after registration to an MNI template, and resampled into 1 mm cubic voxels. The fractional intensity threshold in BET was set to 0.3. This sometimes resulted in residual skull or brainstem being left in the image but insured that we did not inadvertently remove portions of the brain surface.
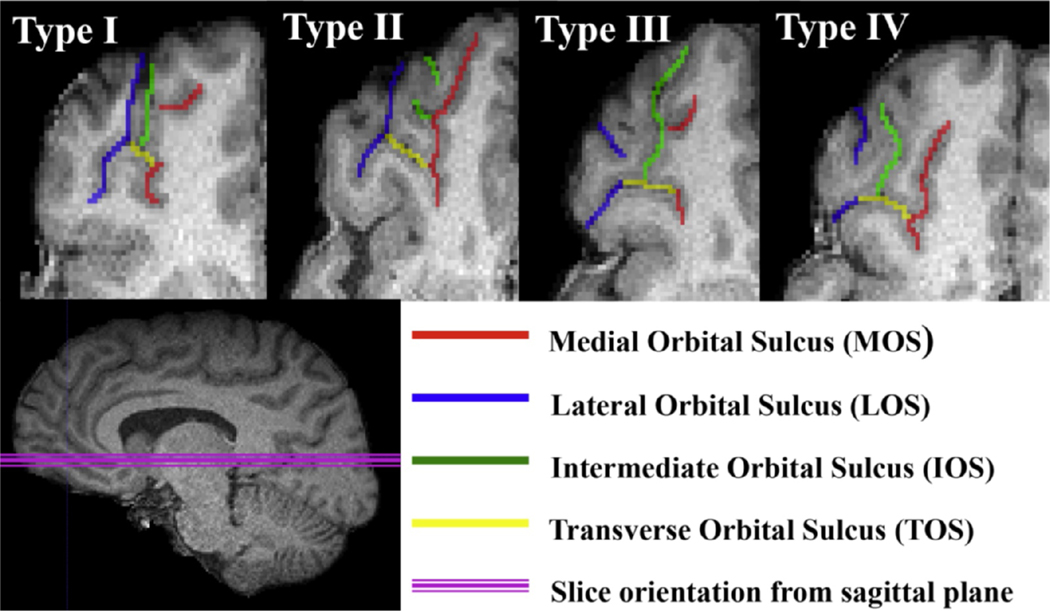
OFC sulcolgyral pattern types were classified based off manual tracings from normalized images using the software ITK-SNAP (Yushkevich et al., 2006). Pattern type in each hemisphere was determined based on the continuity of the medial (MOS) and lateral (LOS) sulcus and categorized into four main types (Chakirova et al., 2010; Chiavaras and Petrides, 2000). Type I is identified by a discontinuous MOS and continuous LOS, Type II has a continuous MOS and LOS, Type III discontinuous MOS and LOS, and Type IV has a continuous MOS and a discontinuous LOS (see Fig. 1). In addition to classifying the main sulcogyral pattern types, the original paper by Chiavaras and Petrides also identified that variability exists within each of the main pattern types, by identifying six pattern subtypes within each main pattern type. As we identified novel subtypes within each of the main pattern types, we classified and recorded these as an extension to those originally described by Chiavaras and Petrides.
Fig. 1. Depictions of each pattern type.
Axial slices from the right hemisphere T1 image of example subjects depicting each pattern type, with tracing overlaid to illustrate each sulcus. Type I pattern is distinguished by its discontinuous Medial Orbital Sulcus (MOS) and continuous Lateral Orbital Sulcus (LOS). Type II is defined by a continuous MOS and LOS. Type III is distinguished by a discontinuous MOS and discontinuous LOS. Type IV is defined by a continuous MOS and discontinuous LOS. Red line indicates MOS. Blue line indicates LOS. Yellow line indicates Transverse Orbital Sulcus (TOS). Green line indicates Intermediate Orbital Sulcus (IOS). Pink line indicates orientation of the orbitofrontal cortex from the sagittal plane.
Each participant’s bilateral H-sucal patterns were independently traced, characterized, and subtyped by two tracers (M.P. and S.W.) who were both unaware of CUD patient/control group status. Inter-rater reliability was assessed in a random subset (n = 10) participants from both CUD and control cohorts. Inter-rater reliability between M.P and V.T was reliable (κ = 0.83 (95% CI, 0.50 to 1.15).
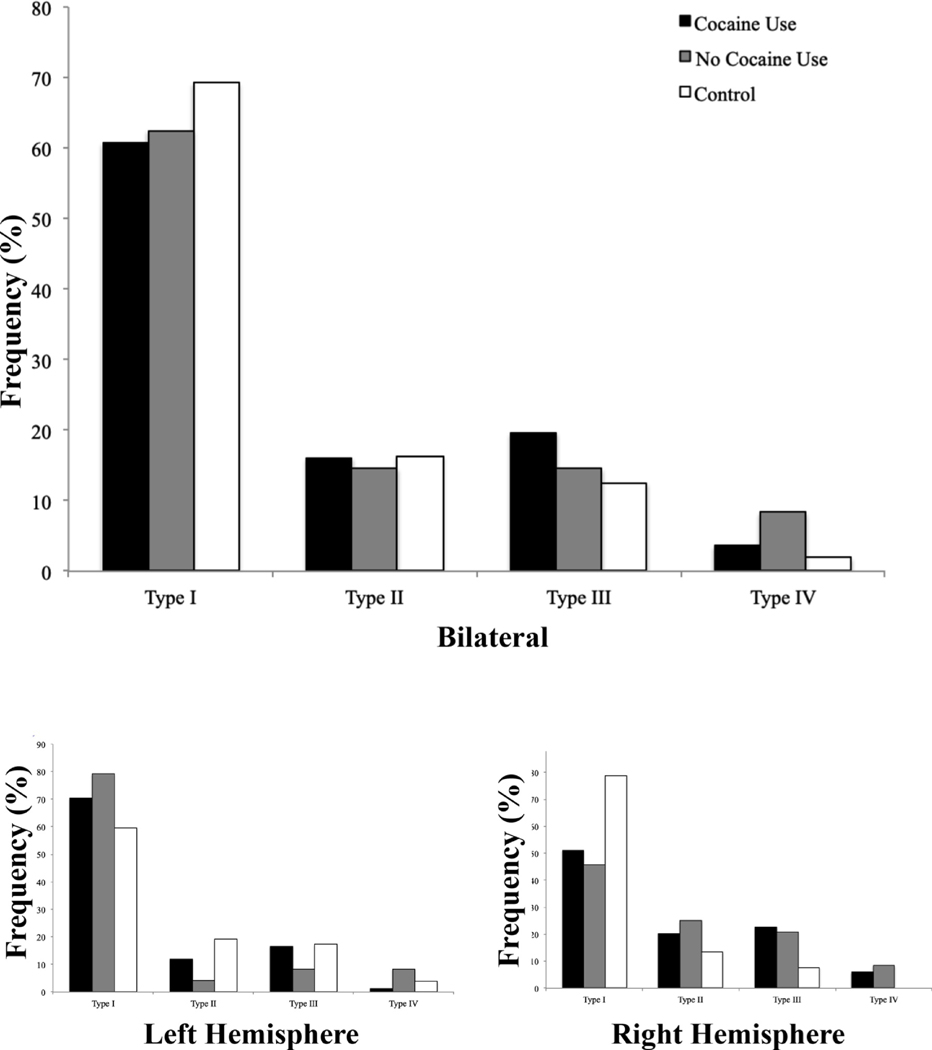
Fig. 2. Frequency distributions for pattern types.
Pattern frequencies plotted for each pattern Type across all hemispheres (top panel) and for the left and right hemispheres (bottom panel). Black bar indicates cocaine use patient group, gray bar indicates no cocaine use group, and white bar indicates independent control group. Frequencies of pattern type for each group add up to a total frequency of 1. Please note that within the same participant, pattern types may differ between the left and right hemisphere.
2.3.2. Statistical analysis
Statistical analyses were performed using RStudio 1.2.1335 (RStudio Team, 2015). Chi-square tests were used to evaluate the associations between categorical variables between those with and without CUD. For small cells with less than 5 samples, Fisher’s exact tests were employed instead to adjust p-value. First, we assessed if demographic differences (i.e. age, education, and history of abuse) differed between those with and without CUD. For our main hypothesis, we compared differences in the frequency distributions within OFC sulcogyral pattern types in the left and right hemispheres of participants in the CUD group relative to those participants in the control group using Chi-Square statistics. We then compared differences in OFC pattern type distributions between left and right hemispheres between CUD patients and controls. P-values <0.05 are considered as significant.
Given the relatively small sample size of our available control population (n = 24) we also include additional independent controls (n = 52) from a sample that has been previously published from our group (Patti and Troiani, 2018) for subtype descriptions and as a reference for overall pattern distributions in Table 2.
Table 2.
Distribution of pattern types between CUD patients, controls, and independent controls.
| CUD Patients (n = 84) | Control Group (n = 24) | Independent Control Group a (n = 52) | All Group Comparison | |
|---|---|---|---|---|
| Pattern Type | N (%) | N (%) | N (%) | X2 (p-value)c |
| Total | ||||
| Type I | 102 (61) | 30 (63) | 72 (69) | 2.42 (0.49) |
| Type II | 27 (16) | 7 (15) | 17 (16) | |
| Type III | 33 (20) | 7 (15) | 13 (13) | |
| Type IV | 6 (4) | 4 (8) | 2 (2) | |
| Right Hemisphere | ||||
| Type I | 59 (70) | 19 (79) | 31 (60) | 5.61 (0.13) |
| Type II | 10 (12) | 1 (4) | 10 (19) | |
| Type III | 14 (17) | 2 (8) | 9 (17) | |
| Type IV | 1 (1) | 2 (8) | 2 (4) | |
| Left Hemisphere | ||||
| Type I | 43 (51) | 11 (46) | 41 (79) | 0.50 (0.92) |
| Type II | 17 (20) | 6 (25) | 7 (14) | |
| Type III | 19 (23) | 5 (21) | 4 (8) | |
| Type IV | 5 (6) | 2 (8) | 0 (-) |
For reference and comparison we included the number and distribution of pattern types from an independent control sample identified from a previously published paper (Patti and Troiani 2018).
Comparing CUD patients n = 84, to control group n = 24, and independent control group n = 52.
3. Results
Before completing analyses related to our hypotheses, we assessed any demographic differences between the CUD patients and both sets of controls groups. This was completed in order to confirm that characteristics of cocaine use disorder were present in the CUD group, as well as to establish any demographic differences that may influence interpretation. Patients who use cocaine were significantly older than those in the control group (Welch Two Sample T-test statistic 4.9, p-value <0.01; Table 1). Individuals in the control group also had greater educational attainment relative to the cases (Welch Two Sample T-test statistic 4.3, p-value <0.01; Table 1). H-sulcus patterns are thought to be laid down early in life and to not change with advancing age, so the age difference between CUD patients and controls is unlikely to influence results. Further, it is established that substance abuse disorders can limit educational attainment (Elliott and Lowman, 2015; Martin et al., 2015).
CUD patients had an average of 18 years of cocaine use, as well as 17 years of alcohol use, and a self-reported history of cannabis use. Thus, these CUD patients had a primary addiction of cocaine use disorder but were polysubstance users. Abuse history was also self-reported by CUD patents and controls, and was not consistently different, with 49% of CUD patients having a prior history of abuse and 42% of controls.
We next assessed whether the distribution of pattern types was different between cocaine users and non-users. We find that Type I patterns were the most frequent, followed by Type III patterns, Type II patterns, and Type IV patterns overall (Χ2 =2.42, p-value = 0.49; Table 2) and in the left and right hemispheres, individually (Χ2 =0.50, p-value = 0.92; Χ2 =5.61, p-value = 0.13; Table 2). Because the sample of controls is relatively small, we also include a comparison with an existing dataset from a previous publication from our lab. These analyses indicate that, compared to controls, the distribution of OFC sulcogyral patterns is not significantly different in CUD patients with a history of cocaine use.
As described in the Introduction, there is additional variation that exists in the OFC sulci and gyri that is not captured by H-sulcus pattern typing procedure, which only relies on the continuity of the medial orbital sulcus and/or lateral orbital sulcus. Following from the seminal paper by Chiavaras and Petrides (2000), as exploratory analyses, we characterize the subtype patterns of all CUD patients and controls. We have included descriptions and frequencies that each pattern subtype was observed in this data set. Rather than limiting our descriptions to those that we feel may be more important, we have chosen to expand on our characterizations to include all relevant pattern subtypes for the purpose of future discovery.
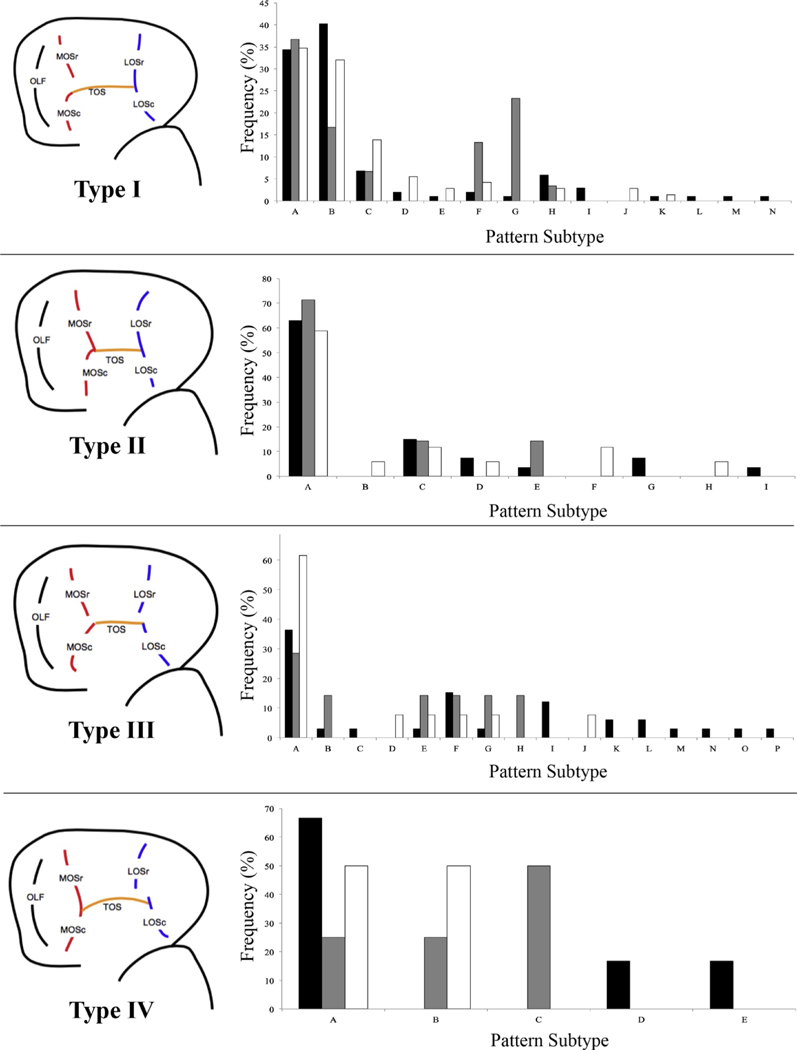
It is important to note that Chiavaras and Petrides characterized 50 human brains and described the most common 6 subtypes for each overall pattern; including Type I patterns A-F, Type II patterns A-F, and Type III patterns A-F. In the cases that an individual’s pattern was obviously different and could not be characterized as one of the previously described subtypes, we added a subtype and articulated how it was different from existing subtypes (see Fig. 3, Supplementary Methods, and Supplementary Figure S1). It is also important to note that Type IV was not originally identified in the sample by Chiavaras and Petrides, so Type IV subtypes are described for the first time here.
Fig. 3. Sulcogyral pattern subtype variation for all patients and controls.
Pattern Type cartoon exemplar on left for each of four panels, depicting a canonical pattern for each Type. Individual subtypes are labeled with letters, with the first 6 letters (A-F) matching the original pattern subtypes identified by Chiavaras and Petrides for pattern Types I, II, and III. Note that in this original descriptive paper, pattern Type IV (and corresponding subtypes) were not identified and therefore not included. Any new subtypes that we identified for pattern Types I, II, and III were labeled with the next alphabetical letter. Please note that not all previously identified pattern subtypes were identified in the sample described here, as reflected in the frequency graphs. See supplementary figures and descriptions for complete description of methods and individual cartoon examplars and descriptions of each pattern subtype.
For Type I patterns, we observed the same six subtypes described in Chiavaras and Petrides (2000), as well as eight additional subtypes. Additional subtypes were included mainly to account for variability in how the medial orbital sulcus extended in the rostral caudal direction, as well as how the MOS connected to the TOS. For example, none of the subtypes described in Chiavaras and Petrides (2000) included a rostral MOS that extended caudally beyond the plane at which the TOS appears (Supplementary Figure S1, Type I.7). Further, we added a subtype in which neither the rostral or caudal MOS intersected with the TOS (Supplementary Figure S1, Type I.10) and another in which the intermediate sulcus intersected the TOS, but neither the rostral or caudal MOS (Supplementary Figure S1, Type I.11). We describe each subtype completely in Supplementary Methods, including specific features that differentiate subtypes that appear similar.
For Type II pattern subtypes, we observed the originally described 6 subtypes, as well as 3 additional subtypes. Additional subtypes accounted for an absent TOS (Type II.7, a broken TOS (Type II.8) and a TOS that did not intersect the LOS (Type II.9).
For Type III pattern subtypes, we observed the original 6 subtypes, as well as 10 additional subtypes. Subtypes included individuals who had an absent caudal portion of the LOS (Type III.7), an intermediate sulcus that intersects with the TOS and has a caudal LOS that intersects with the TOS and extends rostrally (Type III.8), amongst others. As with the creation of additional Type I and Type II pattern subtypes, we followed the convention of Chiavaras and Petrides (2000), naming new subytypes if they differed in a novel way, while still remaining consistent with the previous pattern subtype conventions. For example, Chiavaras and Petrides (2000) differentiated pattern subtypes if the caudal portion of the MOS or LOS extended rostrally beyond the plane at which that sulcal segment intersected the TOS and whether or not the intermediate sulcus intersected portions of the sulcal group, even though intermediate sulcus continuity and connectivity is not considered as part of overall pattern subtyping. All of our novel subtypes are consistent with this previously described convention.
The Type IV pattern, consisting of a continuous MOS and discontinuous LOS, was not described in the first paper to identify OFC sulcogyral patterns in the H-sulcus. This pattern has been observed in most subsequent studies, however, and is either discarded from analysis or grouped with Type III patterns, since the Type III and Type IV patterns have a discontinuous LOS in common. We observed 5 different subtypes of Type IV patterns, including a ‘canonical’ Type IV pattern with a continuous MOS and discontinuous LOS, where the LOS extends caudally from the TOS (Type IV.1). The other observed Type IV pattern subtypes were differentiated by a caudal LOS that extended rostrally from the TOS with a rostral LOS that extended caudally beyond the plane that the caudal LOS intersected the TOS (Type IV.2), an absent caudal LOS (Type IV.3), and a rostral LOS that intersects an intermediate sulcus, but not the TOS (Type IV.4). The final subtype (Type IV.5) is differentiated from Type IV.2 in that the rostral LOS extends in the rostral-caudal axis along a more medial plane relative to the caudal portion.
It is not surprising that additional pattern subtypes exist beyond those described by Chiavaras and Petrides (2000). The original OFC H-sulcal patterns were described in a group of Caucasian persons with no known psychiatric diagnoses. Since this seminal paper, there has been consistent and overwhelming evidence that schizophrenia and some related brain disorders (bipolar disorder, autism) are associated with atypical distributions of these primary pattern frequencies. Thus, it is unsurprising that we observe additional patterns subtypes, given the (1) larger number of hemispheres in the current study and (2) the limited diversity of the original sample. The patients describe here do not have diagnoses that are consistent with those previously found to have atypical frequency distributions of the OFC H-sulcus (i.e. schizophrenia). However, it is possible that patients with brain disorders, including substance abuse disorders, have greater variability in the sulci that comprise the H-sulcus, even though the frequency distributions of the general H-sulcal pattern remains consistent with controls. See Table 3 for the total number of documented subtypes for each pattern, as well as the number and percent of subtypes identified for each group.
Table 3.
Number and percentages of subtypes for cases and controls for all subtypes.
| Cocaine Use (n = 84) | No Cocaine Use (n = 24) | Independent Control Group (n = 52) | All Controls b (n = 76) | ||
|---|---|---|---|---|---|
| Pattern Type | Documented Subtypes | Subtypes Identified (%) | Subtypes Identified (%) | Subtypes Identified (%) | Subtypes Identified (%) |
| Type I | 14 | 13 (92.9) | 6 (42.9) | 9 (64.3) | 10 (71.4) |
| Type II | 9 | 6 (66.7) | 3 (33.3) | 6 (66.7) | 6 (66.7) |
| Type III | 16 | 13 (81.3) | 6 (37.5) | 6 (37.5) | 8 (50.0) |
| Type IV | 5 | 3 (60) | 3(60) | 2 (40) | 3 (60) |
| Total a | 44 | 35 (79.5) | 18 (40.9) | 23 (52.3) | 27 (61.4) |
This considers the number of subtypes identified within cases and controls respectively, over the total number of previously documented subtypes. Note that the denominator is includes documented subtypes identified within this sample.
Includes all subtypes identified between both sets of control groups.
Although we are underpowered to assess for statistical differences in subtypes between the groups, we observe that overall, CUD patients may have a broader distribution of the pattern subtypes. That is, while the majority of both CUD patients and controls have the most common subtypes (Subtypes TypeX.1–2), Overall, CUD patients may have greater variability for the number of ways in which Type I and Type III patterns manifest, with ~93% and ~81% of all Type I and III subtypes, respectively, observed for CUD patients. This can be contrasted with only 38–64% of subtypes for Type I and III observed in the control group and independent control groups described here. In combining all identified subtypes for both the control group and independent control group, we still find that there are less subtypes identified within this control population ~61% overall, in comparison to the CUD group ~80%. This variability is particularly noticeable in comparing the more numerous subtypes identified in the CUD group for Type I ~93% and Type III ~81% in comparison to the overall control group for Type I ~71% and Type III 50%. While we do not identify differences in the overall distribution of sulcogyral pattern frequencies between the CUD group and controls, we do see a larger variation of sulcogyral pattern subtypes within the CUD group relative to controls; indicating future studies should include pattern subtype information so that data on subtype prevalence and distribution can be curated and relevance to diagnostic status evaluated.
4. Discussion
We do not find differences in the frequency distributions of OFC sulcogyral patterns in patients with CUD relative to controls. That is, CUD patients show the expected frequency distribution, with Type I patterns appearing most frequently and no increase in frequency of Type II, III, or IV patterns, relative to controls. Previous work has found increased frequency of Type II and III patterns in patients with schizophrenia (Chakirova et al., 2010; Isomura et al., 2017; Lavoie et al., 2014; Nakamura et al., 2007; Takayanagi et al., 2010) bipolar disorder (Patti and Troiani, 2018), and autism (Watanabe et al., 2014), but not OCD (Delahoy et al., 2019), ADHD (Patti and Troiani, 2018), or cannabis use disorder (Chye et al., 2017). However, please note that in OCD, increased symptom severity, as measured by the Y-BOCS was associated with increased prevalence of Type II and Type III patterns, despite no overall difference in pattern frequencies in OCD relative to controls. In addition, authors found that patients who had Type III pattern in their right hemisphere tended to use more cannabis in their lifetime, with lifetime use associated with greater depression symptoms. Although gambling use disorder is not a substance use disorder, it is considered an addiction disorder. A recent study showed that individuals with gambling disorder had increased Type II patterns when collapsing data across hemispheres (Li et al., 2019) but did not find an association with symptom severity.
A key finding in the current study is that patients with decades of drug use demonstrate the same OFC sulcogyral pattern frequencies as those without drug use. This indicates that previous results seen in schizophrenia and bipolar disorder are unlikely to be due to comorbid drug use in patients and strengthens the assumption that these OFC patterns are laid down early in life and do not change throughout the lifetime.
This is the first OFC sulcogyral pattern study that includes a majority of African American males. Previous work has focused on primarily Caucasian and Asian populations, based primarily on the geographical location and sampling of laboratories that have expertise in this type of characterization. Because the overall patterns in the OFC have also been observed in non-human primates, it is expected that these patterns and consistency of frequency distributions would be observed across all races. However, it is interesting and important to increase our knowledge of pattern types in more diverse samples. Further, this lack of differences in frequency distribution in an African American sample suggests that atypical OFC sulcogyral patterns will also confer risk for schizophrenia in African American individuals.
We describe additional subtypes for Pattern Types I, II, and III and also describe pattern subtypes observed for Type IV for the first time. Although we do not observe pattern frequency differences in patients with CUD, our exploratory descriptions of pattern subtypes and their distributions across patient and control populations suggest an interesting new avenue for understanding sulcogyral variability within and between subtypes.
Evidence for atypical sulcogyral patterns in the OFC has predominantly been found in schizophrenia. As patients with other psychiatric diagnoses are found to have altered OFC sulcogyral patterns, one might wonder whether this diminishes the utility of this metric as a specific disease biomarker. We suggest that there is great potential in further understanding the impact of atypical sulcogyral patterns as a transdiagnostic marker, as they may reflect shared vulnerabilities that are present in multiple psychiatric illnesses. Further, sulcogyral patterns may be a brain marker that reflects a shared genetic risk factor that is common across multiple psychiatric and neurodevelopmental disorders (Anttila et al., 2018).
This study is not without limitations. It should be noted that inclusion criteria for all CUD participants was that individuals must be treatment seeking (Childress et al., 2008; Wetherill et al., 2014; Young et al., 2014). This indicates how participants with CUD included in our study may represent a different subset of individuals with CUD generally. Future work should characterize sulcogyral patterns within psychiatric patients where substance use history is well characterized. Given how the OFC is implicated in reward-based, and goal directed behavior (Kringelbach, 2005), we speculate that individuals who seek treatment for substance use disorders may be more similar to controls than initially hypothesized, given they are motivated to stop their drug use. Thus, individuals who seek treatment for substance use disorders are fundamentally different from those individuals who continue to use substances, yet do not seek treatment (Kessler et al., 2001). This could be why we were unable to detect differences in the frequency distributions of OFC sulcogyral patterns in patients with CUD relative to controls. Future studies should attempt to overcome these issues of selection bias, by investigating the frequencies of OFC sulcogyral patterns in more generalizable populations of individuals with substance use who did not seek treatment. An additional limitation is that our results were not corrected for multiple comparisons. We encourage future projects to obtain larger samples, such that the power necessary to perform rigorous statistical testing with multiple comparison corrections can be computed. This leads to a potential concern that our sample was obtained through harmonization of several smaller cohorts. While slightly different information was obtained through each cohort specific protocol, all participants were scanned using the same scanner. It is thus unlikely that differences in cohort influenced individual sulcogyral pattern classification. Finally, given the exploratory nature of our final analysis on pattern subtyping, we were unable to provide statistical analyses to further investigate differences between CUD patients and control groups. We encourage future studies to consider subtyping analyses, in addition to traditional pattern classification.
Supplementary Material
Acknowledgments
We are grateful for the individuals who agreed to participate in the cohort studies at the University of Pennsylvania School of Medicine, for without their participation this work would not be possible. This work was supported by a National Institute of Health Grant to V.T. [R01 DA044015]. Brain imaging re-analyzed in the current manuscript was acquired via support of NIDA Grants [R01 DA010241; R01 DA025906; P50-DA-12756; R21/R33 DA026114; Commonwealth of Pennsylvania (CURE); U54 DA039002; R01 DA039215]. P.R. is supported by T32 DA028874.
Abbreviations:
- CUD
Cocaine use disorder
- IOS
Intermediate orbital sulcus
- LOS
Lateral orbital sulcus
- MINI
Mini international neuropsychiatric interview
- MOS
Medial Orbital Sulcus
- OCD
Obsessive Compulsive Disorder
- OFC
Orbitofrontal Cortex
- TOS
Transverse Orbital Sulcus
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2020.111174.
Declaration of Competing Interest
All authors declare that there are no conflicts of interest.
References
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Murray R, 2018. Analysis of shared heritability in common disorders of the brain. Science 360 (6395). 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K, 1995. The ontogeny of human gyrification. Cereb. Cortex 5 (1), 56–63. 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Armstrong TD, Costello EJ, 2002. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J. Consult. Clin. Psychol. 70 (6), 1224–1239. 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- Center for Behavrioral Health Statistics and Quality (CBHSQ), 2015. National survey on drug use and health: detailed tables. Substance Abuse and Mental Health Services Administration. Rockville, MD: 2016. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), Center for Behavioral Health Statistics and Quality, 2015. Behavioral Health Trends in the United States: Results from the 2014. National Survey on Drug Use and Health. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2016. National center for health statistics. Underlying cause of death 1999–2015 on CDC WONDER online database, released december. In: Data are from the Multiple Cause of Death Files, 1999–2015 as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions Through the Vital Statistics Cooperative Program. [Google Scholar]
- Cerullo MA, Strakowski SM, 2007. The prevalence and significance of substance use disorders in bipolar type I and II disorder. Subst. Abuse Treat. Prev. Policy 2, 29. 10.1186/1747-597x-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakirova G, Welch KA, Moorhead TW, Stanfield AC, Hall J, Skehel P, McIntosh AM, 2010. Orbitofrontal morphology in people at high risk of developing schizophrenia. Eur. Psychiatry 25 (6), 366–372. 10.1016/j.eurpsy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M, 2000. Orbitofrontal sulci of the human and macaque monkey brain. J. Comp. Neurol. 422 (1), 35–54. [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, O’Brien CP, 2008. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE 3 (1), e1506. 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chye Y, Solowij N, Ganella EP, Suo C, Yucel M, Batalla A, Lorenzetti V, 2017. Role of orbitofrontal sulcogyral pattern on lifetime cannabis use and depressive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 79 (Pt B), 392–400. 10.1016/j.pnpbp.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Delahoy R, Bartholomeusz CF, Pemberton H, Alonso P, Pujol J, Cardoner N, Harrison BJ, 2019. An examination of orbitofrontal sulcogyral morphology in obsessive-compulsive disorder. Psychiatry Res. Neuroimag. 286, 18–23. 10.1016/j.pscychresns.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Elliott M, Lowman J, 2015. Education, income and alcohol misuse: a stress process model. Soc. Psychiatry Psychiatr. Epidemiol. 50 (1), 19–26. 10.1007/s00127-014-0867-3. [DOI] [PubMed] [Google Scholar]
- Fatseas M, Denis C, Lavie E, Auriacombe M, 2010. Relationship between anxiety disorders and opiate dependence–a systematic review of the literature: implications for diagnosis and treatment. J. Subst. Abuse Treat. 38 (3), 220–230. 10.1016/j.jsat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Goldner EM, Lusted A, Roerecke M, Rehm J, Fischer B, 2014. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict. Behav. 39 (3), 520–531. 10.1097/00005053-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Isomura S, Hashimoto R, Nakamura M, Hirano Y, Yamashita F, Jimbo S, Onitsuka T, 2017. Altered sulcogyral patterns of orbitofrontal cortex in a large cohort of patients with schizophrenia. NPJ Schizophr. 3, 3. 10.1038/s41537-016-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2), 825–841. 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S, 2001. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5 (2), 143–156. 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Berglund PA, Caraveo-Anduaga JJ, DeWit DJ, Greenfield SF, Vega WA, 2001. Patterns and predictors of treatment seeking after onset of a substance use disorder. Arch. Gen. Psychiatry 58 (11), 1065–1071. 10.1001/archpsyc.58.11.1065. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6 (9), 691–702. 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Bartholomeuz CF, Nelson B, Lin A, McGorry PD, Velakoulis D, Wood SJ, 2014. Sulcogyral pattern and sulcal count of the orbitofrontal cortex in individuals at ultra high risk for psychosis. Schizophr. Res. 154 (1–3), 93–99. 10.1016/j.schres.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Z, Boileau I, Dreher JC, Gelskov S, Genauck A, Sescousse G, 2019. Altered orbitofrontal sulcogyral patterns in gambling disorder: a multicenter study. Transl Psychiatry 9 (1), 186. 10.1038/s41398-019-0520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K, 2004. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr. Res. 67 (2–3), 157–166. 10.1016/s0920-9964(02)00523-6. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Conger RD, Sitnick SL, Masarik AS, Forbes EE, Shaw DS, 2015. Reducing risk for substance use by economically disadvantaged young men: positive family environments and pathways to educational attainment. Child. Dev. 86 (6), 1719–1737. 10.1111/cdev.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M, 1992. The fifth edition of the addiction severity index. J. Subst. Abuse Treat. 9 (3), 199–213. 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, Shenton ME, 2007. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain 130 (Pt 3), 693–707. 10.1093/brain/awm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Takahashi T, Takayanagi Y, Sasabayashi D, Katagiri N, Sakuma A, Suzuki M, 2019. Surface morphology of the orbitofrontal cortex in individuals at risk of psychosis: a multicenter study. Eur. Arch. Psychiatry Clin. Neurosci. 269 (4), 397–406. 10.1007/s00406-018-0890-6. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA). (2015). Drug factors: nationwide trends. https://www.drugabuse.gov/publications/drugfacts/nationwide-trends.
- Narvaez JC, Jansen K, Pinheiro RT, Kapczinski F, Silva RA, Pechansky F, Magalhaes PV, 2014. Psychiatric and substance-use comorbidities associated with lifetime crack cocaine use in young adults in the general population. Compr. Psychiatry 55 (6), 1369–1376. 10.1016/j.comppsych.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Takahashi T, Takayanagi Y, Furuichi A, Kido M, Nakamura M, Suzuki M, 2016. Orbitofrontal sulcogyral pattern and olfactory sulcus depth in the schizophrenia spectrum. Eur. Arch. Psychiatry Clin. Neurosci. 266 (1), 15–23. 10.1007/s00406-015-0587-z. [DOI] [PubMed] [Google Scholar]
- Patti MA, Troiani V, 2018. Orbitofrontal sulcogyral morphology is a transdiagnostic indicator of brain dysfunction. Neuroimage Clin. 17, 910–917. 10.1016/j.nicl.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Kranzler HR, Ball S, Tennen H, Poling J, Triffleman E, 1998. Personality disorders in substance abusers: relation to substance use. J. Nerv. Mental Dis. 186 (2), 87–95. 10.1097/00005053-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Team RStudio, 2015. RStudio: Integrated Development for R. RStudio, Inc., Boston, MA: URL. http://www.rstudio.com/. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 (Suppl 20), 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1), S208–S219. 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Masuda N, Mozue Y, Nakamura K, Suzuki M, 2010. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophr. Res. 121 (1–3), 55–65. 10.1016/j.schres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Tims FM, Dennis ML, Hamilton N, B JB, Diamond G, Funk R, Brantley LB, 2002. Characteristics and problems of 600 adolescent cannabis abusers in outpatient treatment. Addiction 97 (Suppl 1), 46–57. 10.1046/j.1360-0443.97.s01.7.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakamura M, Ohno T, Itahashi T, Tanaka E, Ohta H, Hashimoto R, 2014. Altered orbitofrontal sulcogyral patterns in adult males with high-functioning autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 9 (4), 520–528. 10.1093/scan/nst016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, Franklin TR, 2014. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacol. (Berl) 231 (7), 1397–1407. 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Bartholomeusz C, Yucel M, Dennison M, Vijayakumar N, Allen NB, 2014. Orbitofrontal sulcogyral patterns are related to temperamental risk for psychopathology. Soc. Cogn. Affect. Neurosci. 9 (2), 232–239. 10.1093/scan/nss126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Franklin TR, Roberts DC, Jagannathan K, Suh JJ, Wetherill RR, Childress AR, 2014. Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J. Neurosci. 34 (14), 5038–5043. 10.1523/jneurosci.4977-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G, 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31 (3), 1116–1128. 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zulauf CA, Sprich SE, Safren SA, Wilens TE, 2014. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr. Psychiatry Rep. 16 (3), 436. 10.1007/s11920-013-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.