Abstract
Purpose:
While evidence indicates that Fusobacterium nucleatum may promote colorectal carcinogenesis through its suppressive effect on T-cell-mediated antitumor immunity, the specific T-cell subsets involved remain uncertain.
Experimental Design:
We measured F. nucleatum DNA within tumor tissue by quantitative PCR on 933 cases (including 128 F. nucleatum-positive cases) among 4,465 incident colorectal carcinoma cases in two prospective cohorts. Multiplex immunofluorescence combined with digital image analysis and machine learning algorithms for CD3, CD4, CD8, CD45RO (PTPRC isoform), and FOXP3 measured various T-cell subsets. We leveraged data on Bifidobacterium, microsatellite instability (MSI), tumor whole exome sequencing, and M1/M2-type tumor-associated macrophages [by CD68, CD86, IRF5, MAF, and MRC1 (CD206) multimarker assay]. Using the 4,465 cancer cases and inverse probability weighting method to control for selection bias due to tissue availability, multivariable-adjusted logistic regression analysis assessed the association between F. nucleatum and T-cell subsets.
Results:
The amount of F. nucleatum was inversely associated with tumor stromal CD3+ lymphocytes (multivariable odds ratio, 0.47, 95% confidence interval, 0.28–0.79, for F. nucleatum-high vs. negative category; Ptrend=0.0004) and specifically stromal CD3+CD4+CD45RO+ cells (corresponding multivariable odds ratio, 0.52, 95% confidence interval, 0.32–0.85; Ptrend=0.003). These relationships did not substantially differ by MSI status, neoantigen loads, or exome-wide tumor mutational burden. F. nucleatum was not significantly associated with tumor intraepithelial T cells or with M1 or M2 tumor-associated macrophages.
Conclusions:
The amount of tissue F. nucleatum is associated with lower densities of stromal memory helper T cells. Our findings provide evidence for the interactive pathogenic roles of microbiota and specific immune cells.
Keywords: colorectal neoplasms, immunology, microbiome, molecular pathological epidemiology, tumor microenvironment
Introduction
Accumulating evidence indicates the complex role of the microbiota and host immunity in carcinogenesis (1–4). As T cell-mediated adaptive immune response influences tumor development (5,6), immunotherapies targeting immune checkpoints that regulate T cell activity have emerged as promising treatment strategies for various types of malignancy, including colorectal cancer (7–9). In colorectal cancer, high densities of tumor-infiltrating immune cells, including CD3+ cells, CD8+ cells, and CD45RO+ cells have been associated with better survival (10–12). Recent advances in multiplex immunofluorescence technologies enable detailed evaluation of cellular phenotype as well as information on spatial distribution of each cell in relation to tumor epithelium and stroma, thereby providing deeper insights into the tumor immune microenvironment (13).
Fusobacterium nucleatum (F. nucleatum), Gram-negative anaerobic bacterium, is near ubiquitous in the oral cavity yet rarely found in other anatomic sites under healthy conditions (14,15). In disease states it has been identified in various extra-oral sites with studies demonstrating an enrichment of F. nucleatum in colorectal carcinoma tissue compared to adjacent normal tissue (16–18). Experimental studies have shown that F. nucleatum activates the WNT/CTNNB1 (beta-catenin) signaling pathway in colorectal cancer cells, promotes tumor growth (19,20), and inhibits T cell-mediated immune responses against colorectal cancer (20,21). Consistent with these findings, a higher amount of F. nucleatum DNA in colorectal cancer tissue has been associated with advanced disease stage, worse survival, and lower T cell density in tumor (18,22). However, the relationship between F. nucleatum and specific T cell subsets in the colorectal tumor microenvironment have not been well-defined.
Using a multiplex assay that allows detailed characterisation of T cells and their spatial localisation within tumor intraepithelial and stromal regions, this analysis represents among the first human studies to test the hypothesis that F. nucleatum DNA amount in tumor tissue might be inversely associated with specific T cell subset densities.
Methods
Study Population
We utilized data from two prospective cohort studies in the U.S., the Nurses’ Health Study (NHS, 121,701 women aged 30–55 years followed since 1976) and the Health Professionals Follow-up Study (HPFS, 51,529 men aged 40–75 years followed since 1986) (22–24). Study participants have been sent questionnaires biennially to update information on lifestyle factors and newly-diagnosed diseases including colorectal cancer. The follow-up rate has been more than 90% for each follow-up questionnaire cycle in both cohort studies. In both studies, the National Death Index was used to ascertain deaths of study participants and identify unreported lethal colorectal cancer cases. Study physicians, who were blinded to exposure data, reviewed medical records of identified colorectal cancer cases to confirm the disease diagnosis and to collect data on tumor size, tumor anatomical location, and disease stage based on the American Joint Committee on Cancer TNM classification. We included both colon and rectal carcinomas based on the colorectal continuum model (25). In the cohort studies, 4,465 incident colorectal carcinoma cases had been documented up to 2012. We attempted to collect formalin-fixed paraffin-embedded (FFPE) tumor tissue blocks from hospitals throughout the U.S. (where colorectal cancer patients had undergone surgical resection), resulting in 933 cases with sufficient tissue and T-cell data. A single pathologist (S.O.), blinded to other data, reviewed hematoxylin and eosin-stained tissue sections and recorded pathological features. Tumor differentiation was categorized into well/moderate vs. poor (> 50% vs. ≤ 50% gland formation, respectively).
The study was conducted in accordance with the U.S. Common Rule. All participants gave written informed consent for the study. This study was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health (Boston, MA), and those of participating registries as required.
Multiplex Immunofluorescence Analyses for T Cells and Macrophages in Tumor
We constructed tissue microarrays of colorectal cancer cases with sufficient tissue materials, including up to four tumor cores from each case in a tissue microarray block (26). As previously described (27,28), 4 μm sections from tissue microarray blocks were sequentially stained using the following antibodies/fluorescent dyes for T cell, in order: anti-CD3 antibody (clone F7.2.38; Dako; Agilent Technologies, Carpenteria, CA)/Opal-520, anti-FOXP3 (clone 206D, Biolegend, San Diego, CA, USA)/Opal-540, anti-CD45RO (one of PTPRC isoforms) (clone UCHL1, Dako)/Opal-650, anti-CD8 (clone C8/144B, Dako)/Opal-570, anti-CD4 (clone 4B12, Dako)/Opal-690, anti-KRT (keratin, pan-cytokeratins) (clone AE1/AE3, Dako, and clone C11, Cell signaling, Danvers, MA)/Opal-620 (Supplementary Figure S1) [with standardized protein nomenclature recommended by a panel of experts (29)].
Digital images of all tissue microarray cores were acquired at 200x magnification using the Vectra multispectral imaging platform (Vectra 3.0, Akoya Biosciences Hopkinton, MA). Images of each core underwent tissue segmentation to characterize regions of tumor epithelium and peritumoral stroma based on cytokeratin expression using supervised machine learning algorithms within Inform 2.4.1 (Akoya Biosciences). Following tissue segmentation, cell enumeration and segmentation was performed using the DAPI signal to aid in identification of nuclei (Supplementary Figure S1). We evaluated Comparison of T-cell subset densities between the initial and re-processed data demonstrated a high degree of concordance (Pearson’s r 0.48–0.99, Spearman’s r 0.77–0.99) between the density measurements. Each cell was further segmented into nuclear, cytoplasmic and membranous compartments. A separate supervised machine learning algorithm was used to identify T cells based upon a combination of cytomorphology and subcellular T cell marker expression patterns. We evaluated self-reproducibility and the reliability of tissue segmentation and T cell identification using supervised machine learning and confirmed a high degree of concordance (J.B.). We also evaluated pathologist-to-pathologist (J.B. and A.D.C.) concordance through their independent analyses starting from raw multispectral image data and confirmed moderate to high concordance. This single cell data was then used to calculate T cell subpopulation densities within separate regions. Aggregate tumor-level densities were then determined by calculating the average density for each subset across all regions from each tumor. T cell densities were initially classified into quartile categories (C1-C4). If more than 25% of all cases had zero density of a specific cell type, these zero-value cases were grouped together (C1 category), and the remaining (non-zero-value) cases were divided into tertile categories according to density (C2 to C4).
In exploratory analyses, we evaluated tumor-associated macrophage (TAM) densities and polarization using a separate multiplex immunofluorescence panel that included a pan-macrophage marker (CD68), two markers generally expressed in M1 TAMs (CD86, IRF5), two markers generally expressed in M2 TAMs [MAF, MRC1 (CD206)], a tumor cell marker [KRT, (keratin)], and DAPI, as previously described (30). We calculated an M1:M2 polarization index using the formula “(CD86 × IRF5) / (MRC1 × MAF)” based on the expression level of each protein. The TAMs within the highest 30% of the index were regarded as M1-like TAMs, while the macrophages within the lowest 30% were regarded as M2-like TAMs for these analyses, according to our previous study (30). We calculated each cell density (cell count per mm2) in tumor intraepithelial and stromal regions separately, and macrophage densities were classified into quartile categories (C1-C4).
DNA Analyses for Fusobacterium nucleatum and Bifidobacterium genus in Tumor
Genomic DNA was extracted from archival FFPE tissue sections of colorectal carcinoma using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). As previously described (18,31), we performed quantitative polymerase chain reaction (PCR) assays to measure the amounts of F. nucleatum and Bifidobacterium genus DNA in tumor tissue, using SLCO2A1 (for F. nucleatum) or a universal 16S primer set (for Bifidobacterium genus) as reference genes. Cases with any detectable F. nucleatum DNA (or Bifidobacterium genus) were categorized as low vs. high based on the median level of F. nucleatum (or Bifidobacterium genus), while cases without detectable F. nucleatum (or Bifidobacterium genus) were categorized as negative.
Evaluation of Tumor Molecular Characteristics
Tumor MSI status was analyzed using PCR of 10 microsatellite markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40,D18S55, D18S56, D18S67, and D18S487), and MSI-high was defined as presence of instability in ≥ 30% of the markers, as previously described (24,28,32). Using bisulfite-treated DNA, methylation status of eight CpG island methylator phenotype (CIMP)-specific promoters (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) and long interspersed nucleotide element-1 (LINE-1) was determined as previously described (24,28,32). CIMP-high was defined as ≥ 6 methylated promoters of eight promoters, and CIMP-low/negative as 0–5 methylated promoters as previously described (24,28,32). PCR and pyrosequencing were performed for KRAS (codons 12, 13, 61, and 146), BRAF (codon 600), and PIK3CA (exons 9 and 20), as previously described (23,24,28,33). Whole exome sequencing was performed using DNA from tumor and matched normal tissue pairs, as previously described (34). Exome-wide tumor mutational burden was defined as the number of nonsynonymous somatic mutations identified per megabase in the sequenced exome. Using a neoantigen prediction pipeline for somatic mutations, the neoantigen loads (i.e., the number of proteins that likely give rise to immunogenic peptides in the tumor microenvironment) was estimated by counting peptides that bind to personal human leukocyte antigen molecules with high affinity (< 500 nM). Using NetMHCpan (version 2.4) (35), we predicted the binding affinities of all possible 9- and 10-mer mutant peptides to the corresponding human leukocyte antigen alleles inferred by the POLYSOLVER algorithm), as previously described (28,34). Based on all colorectal cancers with available whole exome sequencing data, neoantigen loads and exome-wide tumor mutational burden were divided into quartiles (Q1 to Q4).
Statistical Analysis
All statistical analyses were conducted using SAS software (version 9.4, SAS Institute, Cary, NC), and all P values were two-sided. We used the two-sided α level of 0.005 as recommended (36). The current study started specific primary hypothesis testing that was an assessment of a statistical trend of the association of the amount of F. nucleatum DNA (negative, low, and high; as an ordinal predictor variable) with each T cell subset density (an ordinal outcome variable). All other analyses represented secondary analyses. In secondary analyses to assess the association between F. nucleatum DNA amount and each of categorical clinicopathological variables, the chi-square test was performed. To compare continuous variables, an analysis of variance assuming equal variances or Spearman correlation test was performed.
For our primary hypothesis testing, we conducted initial analyses to select T cell subset variables using Spearman’s correlation tests that assessed the correlations of F. nucleatum DNA amount with densities of T cell subsets. To control for selection bias due to tissue data availability, we used inverse probability weighting (IPW) method (24,37), which used 4,465 incident colorectal cancer cases including the 933 cases with tissue data. First, we constructed a multivariable logistic regression model that had covariates as predictors and tissue data availability as an outcome variable. Based on the fitted regression model using all of the 4,465 cases, we calculated the probability of tissue availability in each case with a set of covariates. Then, each of 933 tissue data-available cases was weighted by the inverse of the probability. Weights greater than the 95th percentile were truncated and set to the value of the 95th percentile to reduce outlier effects (37). We confirmed that results without weight truncation did not differ substantially from those with weight truncation (data not shown). The logistic regression analyses without IPW yielded similar results (Supplementary Table S1) to the IPW-adjusted model.
To control for confounding, multivariable IPW-adjusted logistic regression analyses initially included sex (female vs. male), age at diagnosis (continuous), year of diagnosis (continuous), family history of colorectal cancer in any first-degree relative (present vs. absent), tumor location (proximal colon vs. distal colon vs. rectum), MSI status (MSI-high vs. non-MSI-high), CIMP status (high vs. low/negative), LINE-1 methylation level (continuous), KRAS status (mutant vs. wild-type), BRAF status (mutant vs. wild-type), and PIK3CA status (mutant vs. wild-type). A backward elimination was conducted with a threshold P of 0.20 to select variables for the final models. Cases with missing data [family history of colorectal cancer in a first-degree relative (0.9%), tumor location (0.4%), MSI (2.9%), CIMP (7.0%), KRAS (2.9%), BRAF (2.0%), and PIK3CA mutation (8.5%)] were included in the majority category of a given categorical covariate to limit the degrees of freedom of the models. For the cases with missing data on LINE-1 methylation (2.7%), we assigned a separate indicator variable. We confirmed that excluding the cases with missing information in any of the covariates did not substantially alter results (data not shown). The proportional odds assumption was assessed using the ordinal logistic regression model using the ordinal categories of T cell subset density (negative and tertile or quartile; as an ordinal outcome variable). We observed evidence of violation of this assumption in CD3+CD4+ cells, while those assumption in the other subsets were generally satisfied (P > 0.07). Therefore, for CD3+CD4+ cells, we used binary variable dichotomized at the median value as the outcome variable in logistic regression analysis.
In secondary analyses, we assessed the statistical interaction between F. nucleatum status in colorectal cancer tissue (negative, low, and high) and MSI status (high vs. non-high), neoantigen loads [high (Q3–4) vs. low (Q1–2)], or exome-wide tumor mutational burden [high (Q3–4) vs. low (Q1–2)] in relation to T cell subset densities. We used the Wald test for the cross-product in multivariable-adjusted logistic regression models. We estimated the odds ratio for a unit increase in the three ordinal categories of the amount of F. nucleatum in strata of MSI status, neoantigen loads, or exome-wide tumor mutational burden using re-parameterization of the interaction term in a single regression model (32). For interaction analyses, the proportional odds assumption in an ordinal logistic regression model was violated in subpopulations defined by MSI status, neoantigen loads, and exome-wide tumor mutational burden, therefore we used binary outcome variables for all of the T cell measurements.
As exploratory analyses, we performed Spearman’s correlation tests that assessed the correlation between F. nucleatum and TAMs densities. We further assessed the association of Bifidobacterium genus with T cell subset densities.
Results
During the longitudinal follow-up of the two prospective cohort studies, we documented 4,465 incident colorectal cancer cases, including 933 cases with available data on F. nucleatum and T cells in tumor tissue. We used covariate data of the 4,465 cases to adjust for selection bias in the 933 cases in multivariable analyses to conduct our primary hypothesis testing. F. nucleatum DNA was detected using a quantitative PCR assay in 128 (14%) of the 933 cases. Table 1 shows clinical, pathological, and molecular features of colorectal cancer cases according to the amount of F. nucleatum DNA. Greater amounts of F. nucleatum DNA were associated with poor tumor differentiation, MSI-high status, CIMP-high status, BRAF mutation, higher tumor neoantigen loads, and higher exome-wide tumor mutational burden (P < 0.005; with the α level of 0.005) but not with KRAS or PIK3CA mutation.
Table 1.
Clinical, Pathological, and Molecular Characteristics of Colorectal Cancer Cases According to Fusobacterium nucleatum DNA Amount in Tumor Tissue
| F. nucleatum DNA in tumor tissue | |||||
|---|---|---|---|---|---|
| Characteristic* | All cases (N = 933) | Negative (N = 805) | Low (N = 64) | High (N = 64) | P value† |
| Sex | 0.61 | ||||
| Female (NHS) | 513 (55%) | 439 (55%) | 35 (55%) | 39 (61%) | |
| Male (HPFS) | 420 (45%) | 366 (45%) | 29 (45%) | 25 (39%) | |
| Mean age ± SD (years) | 69.1 ± 8.8 | 69.0 ± 8.9 | 70.6 ± 8.6 | 68.9 ± 8.0 | 0.37 |
| Year of diagnosis | 0.13 | ||||
| 1995 or before | 300 (32%) | 268 (33%) | 13 (20%) | 19 (30%) | |
| 1996–2000 | 307 (33%) | 264 (33%) | 20 (31%) | 23 (36%) | |
| 2001–2012 | 326 (35%) | 273 (34%) | 31 (48%) | 22 (34%) | |
| Family history of colorectal cancer in first-degree relative(s) | 0.69 | ||||
| Absent | 730 (79%) | 627 (78%) | 51 (81%) | 52 (83%) | |
| Present | 195 (21%) | 172 (22%) | 12 (19%) | 11 (17%) | |
| Tumor location | 0.084 | ||||
| Cecum | 166 (18%) | 135 (18%) | 16 (25%) | 15 (23%) | |
| Ascending to transverse colon | 300 (32%) | 253 (32%) | 23 (36%) | 24 (38%) | |
| Descending to sigmoid colon | 276 (30%) | 251 (30%) | 10 (16%) | 15 (23%) | |
| Rectum | 187 (20%) | 162 (20%) | 15 (23%) | 10 (16%) | |
| Tumor differentiation | < 0.0001 | ||||
| Well to moderate | 846 (91%) | 745 (93%) | 52 (83%) | 49 (77%) | |
| Poor | 86 (9.2%) | 60 (7.5%) | 11 (17%) | 15 (23%) | |
| AJCC disease stage | 0.090 | ||||
| I | 201 (23%) | 184 (25%) | 8 (14%) | 9 (15%) | |
| II | 284 (33%) | 235 (32%) | 22 (38%) | 27 (44%) | |
| III | 251 (29%) | 214 (29%) | 22 (38%) | 15 (25%) | |
| IV | 127 (15%) | 111 (15%) | 6 (10%) | 10 (16%) | |
| MSI status | < 0.0001 | ||||
| Non-MSI-high | 750 (83%) | 669 (86%) | 42 (69%) | 39 (61%) | |
| MSI-high | 156 (17%) | 112 (14%) | 19 (31%) | 25 (39%) | |
| CIMP status | < 0.0001 | ||||
| Low/negative | 708 (82%) | 627 (84%) | 46 (78%) | 35 (58%) | |
| High | 160 (18%) | 122 (16%) | 13 (22%) | 25 (42%) | |
| Mean LINE-1 methylation level ± SD (%) | 62.5 ± 9.6 | 62.2 ± 9.6 | 63.0 ± 9.3 | 64.8 ± 9.9 | 0.099 |
| KRAS mutation | 0.33 | ||||
| Wild-type | 536 (59%) | 466 (59%) | 30 (51%) | 40 (63%) | |
| Mutant | 370 (41%) | 318 (41%) | 29 (49%) | 23 (37%) | |
| BRAF mutation | 0.0009 | ||||
| Wild-type | 776 (85%) | 680 (86%) | 52 (85%) | 44 (69%) | |
| Mutant | 138 (15%) | 109 (14%) | 9 (15%) | 20 (31%) | |
| PIK3CA mutation | 0.95 | ||||
| Wild-type | 715 (84%) | 618 (84%) | 46 (82%) | 51 (84%) | |
| Mutant | 139 (16%) | 119 (16%) | 10 (18%) | 10 (16%) | |
| Neoantigen loads | 0.0002 | ||||
| Q1 (lowest) | 107 (25%) | 94 (26%) | 6 (19%) | 7 (23%) | |
| Q2 | 105 (25%) | 91 (25%) | 10 (31%) | 4 (13%) | |
| Q3 | 106 (25%) | 100 (28%) | 4 (13%) | 2 (6.7%) | |
| Q4 (highest) | 106 (25%) | 77 (21%) | 12 (37%) | 17 (57%) | |
| Exome wide tumor mutation burden | 0.001 | ||||
| Q1 (lowest) | 107 (25%) | 95 (26%) | 6 (19%) | 6 (20%) | |
| Q2 | 106 (25%) | 95 (26%) | 7 (22%) | 4 (13%) | |
| Q3 | 105 (25%) | 95 (26%) | 7 (22%) | 3 (10%) | |
| Q4 (highest) | 106 (25%) | 77 (21%) | 12 (38%) | 17 (57%) | |
| Median stromal CD3+ cell density (IQR) (cells/mm2) | 145 (22–493) | 162 (26–538) | 94 (6.8–302) | 71 (6.9–238) | 0.0002‡ |
| Median stromal CD3+CD4+ cell density (IQR) (cells/mm2) | 76 (3.8–361) | 84 (4.9–392) | 44 (0–207) | 37 (0–164) | 0.0007‡ |
| Median stromal CD3+CD8+ cell density (IQR) (cells/mm2) | 10 (0–54) | 10 (0–56) | 11 (0–56) | 9.3 (0–34) | 0.69‡ |
| Median stromal CD3+CD4+CD45RO+ cell density (IQR) (cells/mm2) | 57 (0–287) | 67 (2.9–329) | 33 (0–162) | 24 (0–134) | 0.0006‡ |
| Median stromal CD3+CD4+CD45RO− cell density (IQR) (cells/mm2) | 7.2 (0–48) | 7.7 (0–51) | 0 (0–38) | 4.2 (0–26) | 0.034‡ |
| Median stromal overall macrophage density (IQR) (cells/mm2) | 871 (489–1428) | 859 (479–1399) | 966 (579–1667) | 894 (609–1477) | 0.33‡ |
| Median stromal M1-like macrophage density (IQR) (cells/mm2) | 175 (65–398) | 170 (62–379) | 202 (68–548) | 192 (85–452) | 0.14‡ |
| Median stromal M2-like macrophage density (IQR) (cells/mm2) | 192 (69–429) | 192 (69–435) | 191 (69–435) | 196 (63–431) | 0.33‡ |
| Bifidobacterium genus DNA in tumor tissue | |||||
| Negative | 631 (71%) | 556 (72%) | 38 (64%) | 37 (62%) | 0.094 |
| Low | 128 (14%) | 104 (14%) | 9 (15%) | 15 (25%) | |
| High | 128 (14%) | 108 (14%) | 12 (20%) | 8 (13%) | |
Percentage indicates the proportion of patients with a specific clinical, pathologic, or molecular characteristic among all patients or in strata of F. nucleatum DNA amount.
To compare categorical data between subgroups classified by F. nucleatum DNA amount, the chi-square test was performed, unless otherwise noted. To compare continuous variables, an analysis of variance was performed.
To assess associations between F. nucleatum DNA amount (continuous) and densities of T cell subsets and macrophage (continuous), the Spearman’s correlation test was performed.
Abbreviations: AJCC, American Joint Committee on Cancer; CIMP, CpG island methylator phenotype; HPFS, Health Professionals Follow-up Study; IQR, interquartile range; LINE-1, long-interspersed nucleotide element-1; MSI, microsatellite instability; NHS, Nurses’ Health Study; SD, standard deviation.
Initial analyses using Spearman’s correlation test on each of the T cell subset densities in tumor intraepithelial and stromal regions revealed that the densities of CD3+ cells, CD3+CD4+ cells, and CD3+CD4+CD45RO+ cells in tumor stromal areas were inversely correlated with the amount of F. nucleatum (P < 0.005 with the α level of 0.005) (Table 1 and Figure 1A). In contrast, the amount of tissue F. nucleatum was not associated with intraepithelial densities of T cell subsets. Representative multiplex immunofluorescence and cell-phenotype images of F. nucleatum negative, low, and high cases are shown in Figure 1B.
Figure 1.
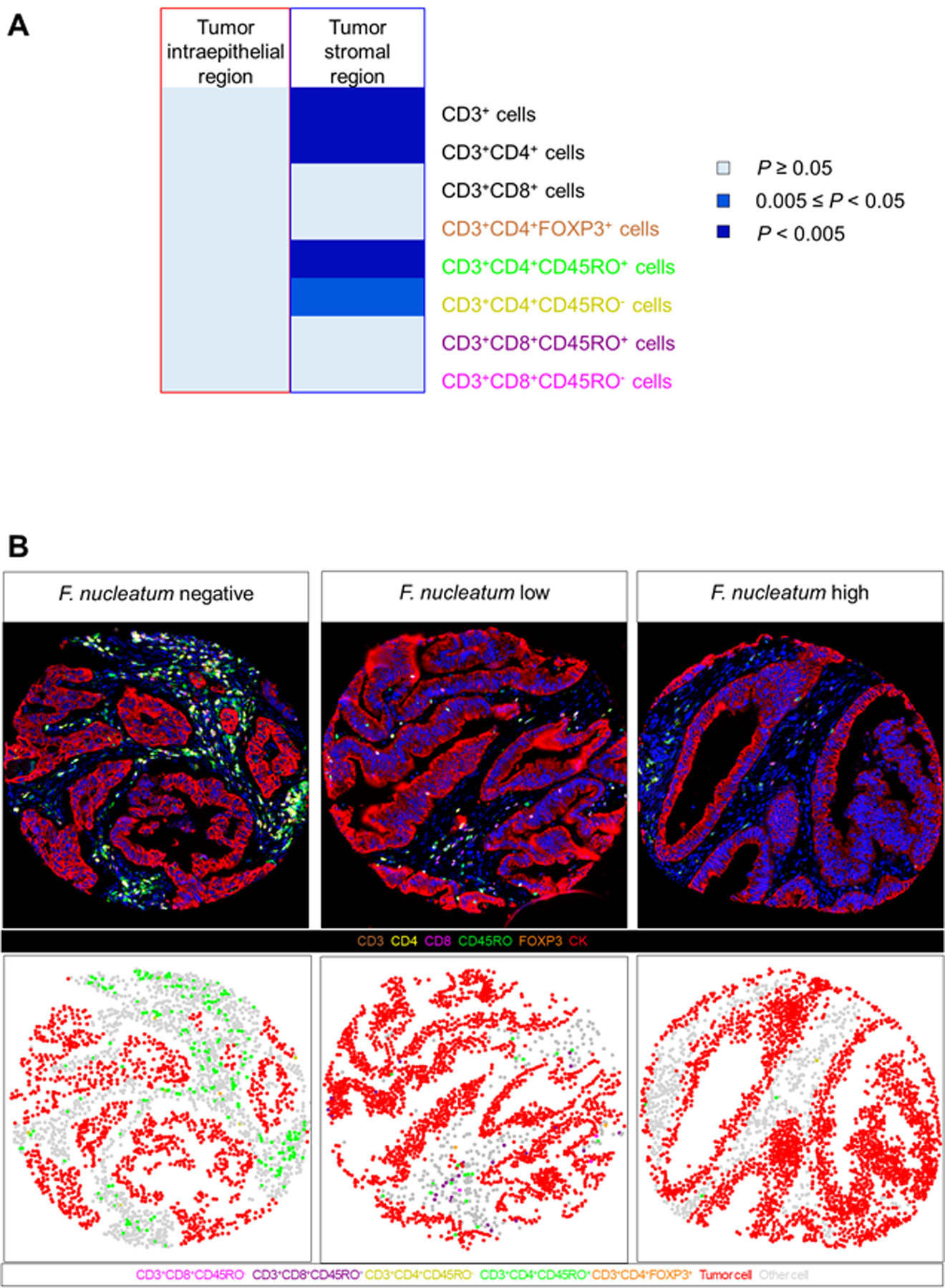
Relationships between Fusobacterium nucleatum and T cell subsets in the colorectal cancer microenvironment. (A) Correlation between Fusobacterium nucleatum DNA amount in tumor tissue and density of T cell subsets in tumor intraepithelial and stromal regions. (B) Multiplex Immunofluorescence and cell-phenotype images of representative Fusobacterium nucleatum negative, low, and high case.
In our primary hypothesis testing, we used a logistic regression analysis to assess the association of the amount of F. nucleatum DNA with the densities of T cell subsets which were selected by the initial analyses (Table 2, and Supplementary Tables S1 and S2). In the multivariable analyses, the amount of F. nucleatum DNA in colorectal cancer tissue was inversely associated with densities of stromal CD3+ cells (Ptrend = 0.0004) and CD3+CD4+CD45RO+ cells (Ptrend = 0.003) with the α level of 0.005. For a unit increase in four ordinal categories of stromal CD3+ cell density, multivariable odd ratios (ORs) were 0.51 [95% confidence interval (CI), 0.30–0.87] for F. nucleatum-low cases and 0.47 (95% CI, 0.28–0.79) for F. nucleatum-high cases, compared with F. nucleatum-negative cases. For a unit increase in four ordinal categories of stromal CD3+CD4+CD45RO+ cell density, multivariable ORs were 0.67 (95% CI, 0.41–1.10) for F. nucleatum-low cases and 0.52 (95% CI, 0.32–0.85) for F. nucleatum-high cases, compared with F. nucleatum-negative cases. The amount of F. nucleatum in colorectal cancer tissue was not significantly associated with the density of CD3+CD4+ cells or CD3+CD4+CD45RO− cells (with the α level of 0.005).
Table 2.
Inverse Probability Weighting (IPW)-Adjusted Logistic Regression Analysis to Assess the Associations of Fusobacterium nucleatum (Predictor) with T Cell Density (Outcome)
| Univariable OR (95% CI)* | Multivariable OR (95% CI)*,† | |
|---|---|---|
| Model for stromal CD3+ cell density (as an ordinal outcome variable) | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.54 (0.33–0.91) | 0.51 (0.30–0.87) |
| High | 0.51 (0.30–0.84) | 0.47 (0.28–0.79) |
| Ptrend‡ | 0.001 | 0.0004 |
| Model for stromal CD3+CD4+ cell density (as a binary outcome variable) | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.86 (0.49–1.48) | 0.80 (0.46–1.39) |
| High | 0.52 (0.29–0.91) | 0.50 (0.28–0.89) |
| Ptrend§ | 0.023 | 0.015 |
| Model for stromal CD3+CD4+CD45RO+ cell density (as an ordinal outcome variable) | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.66 (0.40–1.09) | 0.67 (0.41–1.10) |
| High | 0.53 (0.33–0.85) | 0.52 (0.32–0.85) |
| Ptrend‡ | 0.003 | 0.003 |
| Model for stromal CD3+CD4+CD45RO− cell density (as an ordinal outcome variable) | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.73 (0.43–1.25) | 0.74 (0.43–1.28) |
| High | 0.75 (0.47–1.17) | 0.75 (0.47–1.17) |
| Ptrend‡ | 0.12 | 0.13 |
IPW was applied to reduce a bias due to the availability of tumor tissue after cancer diagnosis (see “Statistical Analysis” subsection for details).
The multivariable ordinal logistic regression model initially included age, sex, year of diagnosis, family history of colorectal cancer, tumor location, microsatellite instability, CpG island methylator phenotype, long-interspersed nucleotide element-1 methylation level, and KRAS, BRAF, and PIK3CA mutation status. A backward elimination with a threshold P of 0.20 was used to select variables for the final model. The variables which remained in the final models are shown in Supplementary Table S2.
Ptrend was calculated by the linear trend across the ordinal categories of F. nucleatum DNA amount (negative, low, and high, as an ordinal predictor variable) in the IPW-adjusted ordinal logistic regression model for the densities of T cells (4 ordinal categories, as an ordinal outcome variable).
To avoid violation of the proportional odds assumption, the density of CD3+CD4+ cell was dichotomized at the median value of subset. Ptrend was calculated by the linear trend across the ordinal categories of F. nucleatum DNA amount (negative, low, and high, as an ordinal predictor variable) in the IPW-adjusted logistic regression model for the density of CD3+CD4+ cell (binary categories, as an outcome variable).
Abbreviations: CI, confidence interval; IPW, inverse probability weighting; OR, odds ratio.
As secondary analyses, we examined the statistical interaction between F. nucleatum and MSI status in relation to densities of stromal CD3+ cells and CD3+CD4+CD45RO+ cells. We did not observe a significant interaction between F. nucleatum and MSI status (Table 3).
Table 3.
Inverse Probability Weighting (IPW)-Adjusted Logistic Regression Analysis to Assess the Associations of Fusobacterium nucleatum (Predictor) with T Cell Density (Outcome) in Strata of MSI Status
| N = 906 | Univariable OR (95% CI)* | Multivariable OR (95% CI)*,† |
|---|---|---|
| Model for stromal CD3+ cell density‡ (as a binary outcome variable) | ||
| Non-MSI-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.55 (0.27–1.11) | 0.56 (0.28–1.15) |
| High | 0.36 (0.16–0.79) | 0.36 (0.16–0.80) |
| MSI-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.60 (0.21–1.68) | 0.58 (0.20–1.66) |
| High | 0.52 (0.21–1.31) | 0.58 (0.23–1.46) |
| Pinteraction§ | 0.53 | 0.47 |
| Model for stromal CD3+CD4+CD45RO+ cell density‡ (as a binary outcome variable) | ||
| Non-MSI-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.56 (0.28–1.12) | 0.55 (0.28–1.11) |
| High | 0.43 (0.20–0.91) | 0.42 (0.20–0.89) |
| MSI-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.76 (0.27–2.14) | 0.72 (0.25–2.08) |
| High | 0.57 (0.23–1.45) | 0.62 (0.25–1.54) |
| Pinteraction§ | 0.52 | 0.46 |
IPW was applied to reduce a bias due to the availability of tumor tissue after cancer diagnosis (see “Statistical Analysis” subsection for details).
The multivariable logistic regression model initially included age, sex, year of diagnosis, family history of colorectal cancer, tumor location, CpG island methylator phenotype, long-interspersed nucleotide element-1 methylation level, and KRAS, BRAF, and PIK3CA mutation status. A backward elimination with a threshold P of 0.20 was used to select variables for the final model.
To avoid violation of the proportional odds assumption, the densities of T cells were dichotomized at the median value of each subset.
Pinteraction (two-sided) was calculated using the Wald test for the cross product of F. nucleatum DNA amount (negative, low, and high, as an ordinal predictor variable) and MSI status (high vs. non-high) in the IPW-adjusted logistic regression model.
Abbreviations: CI, confidence interval; IPW, inverse probability weighting; OR, odds ratio.
Additional secondary analyses were performed using a subset of cases with available neoantigen loads or exome-wide tumor mutational burden data as measured by whole exome sequencing. Evaluation of the statistical interaction between F. nucleatum and neoantigen loads (or exome-wide tumor mutational burden) in relation to densities of stromal CD3+ cells and CD3+CD4+CD45RO+ cells did not identify a significant interaction between F. nucleatum and neoantigen loads (Table 4) or exome-wide tumor mutational burden (Table 5).
Table 4.
Inverse Probability Weighting (IPW)-Adjusted Logistic Regression Analysis to Assess the Associations of Fusobacterium nucleatum (Predictor) with T Cell Density (Outcome) in Strata of Tumor Neoantigen Loads
| N = 424 | Univariable OR (95% CI)* | Multivariable OR (95% CI)*,† |
|---|---|---|
| Model for stromal CD3+ cell density‡ (as an outcome variable) | ||
| Neoantigen-low | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.86 (0.26–2.80) | 0.94 (0.28–3.13) |
| High | 0.50 (0.12–2.18) | 0.47 (0.10–2.23) |
| Neoantigen-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.49 (0.16–1.49) | 0.51 (0.17–1.55) |
| High | 0.71 (0.27–1.90) | 0.80 (0.29–2.25) |
| Pinteraction§ | 0.71 | 0.85 |
| Model for stromal CD3+CD4+CD45RO+ cell density‡ (as an outcome variable) | ||
| Neoantigen-low | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.84 (0.26–2.73) | 0.90 (0.27–2.98) |
| High | 0.49 (0.11–2.12) | 0.49 (0.11–2.13) |
| Neoantigen-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.51 (0.17–1.58) | 0.53 (0.18–1.60) |
| High | 0.74 (0.28–1.98) | 0.89 (0.32–2.50) |
| Pinteraction§ | 0.88 | 0.99 |
IPW was applied to reduce a bias due to the availability of tumor tissue after cancer diagnosis (see “Statistical Analysis” subsection for details).
The multivariable logistic regression model initially included age, sex, year of diagnosis, family history of colorectal cancer, tumor location, microsatellite instability, CpG island methylator phenotype, long-interspersed nucleotide element-1 methylation level, and KRAS, BRAF, and PIK3CA mutation status. A backward elimination with a threshold P of 0.20 was used to select variables for the final model.
To avoid violation of the proportional odds assumption, the densities of T cells were dichotomized at the median value of each subset.
Pinteraction (two-sided) was calculated using the Wald test for the cross product of F. nucleatum DNA amount (negative, low, and high, as an ordinal predictor variable) and neoantigen loads [high (Q3–4) vs. low (Q1–2)] in the IPW-adjusted logistic regression model.
Abbreviations: CI, confidence interval; IPW, inverse probability weighting; OR, odds ratio.
Table 5.
Inverse Probability Weighting (IPW)-Adjusted Logistic Regression Analysis to Assess the Associations of Fusobacterium nucleatum (Predictor) with T Cell Density (Outcome) in Strata of Exome-wide Tumor Mutational Burden
| N = 424 | Univariable OR (95% CI)* | Multivariable OR (95% CI)*,† |
|---|---|---|
| Model for stromal CD3+ cell density‡ (as an outcome variable) | ||
| Exome-wide tumor mutational burden-low | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 1.08 (0.32–3.64) | 1.07 (0.30–3.76) |
| High | 0.48 (0.11–2.07) | 0.43 (0.09–2.09) |
| Exome-wide tumor mutational burden-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.42 (0.15–1.18) | 0.47 (0.17–1.33) |
| High | 0.68 (0.26–1.80) | 0.75 (0.28–2.06) |
| Pinteraction§ | 0.68 | 0.77 |
| Model for stromal CD3+CD4+CD45RO+ cell density‡ (as an outcome variable) | ||
| Exome-wide tumor mutational burden-low | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 1.15 (0.34–3.87) | 1.15 (0.34–3.97) |
| High | 0.51 (0.12–2.20) | 0.50 (0.12–2.19) |
| Exome-wide tumor mutational burden-high | ||
| Amount of F. nucleatum DNA | ||
| Negative | 1 (referent) | 1 (referent) |
| Low | 0.41 (0.14–1.14) | 0.43 (0.15–1.22) |
| High | 0.65 (0.25–1.71) | 0.78 (0.29–2.15) |
| Pinteraction§ | 0.71 | 0.82 |
IPW was applied to reduce a bias due to the availability of tumor tissue after cancer diagnosis (see “Statistical Analysis” subsection for details).
The multivariable logistic regression model initially included age, sex, year of diagnosis, family history of colorectal cancer, tumor location, microsatellite instability, CpG island methylator phenotype, long-interspersed nucleotide element-1 methylation level, and KRAS, BRAF, and PIK3CA mutation status. A backward elimination with a threshold P of 0.20 was used to select variables for the final model.
To avoid violation of the proportional odds assumption, the densities of T cells were dichotomized at the median value of each subset.
Pinteraction (two-sided) was calculated using the Wald test for the cross product of F. nucleatum DNA amount (negative, low, and high, as an ordinal predictor variable) and exome-wide tumor mutational burden [high (Q3–4) vs. low (Q1–2)] in the IPW-adjusted logistic regression model.
Abbreviations: CI, confidence interval; IPW, inverse probability weighting; OR, odds ratio.
In exploratory analyses, we did not observe a statistically significant association between F. nucleatum DNA amount and any TAM subset in tumor intraepithelial or stromal regions (P > 0.01; with the α level of 0.005) (Table 1 and Supplementary Table S3).
We further examined the association of Bifidobacterium genus with T cell subset densities and did not observe a significant association between Bifidobacterium genus and any T cell subset in intraepithelial or stromal regions (P > 0.2) (Supplementary Table S4).
Discussion
As colorectal cancer is a group of heterogenous tumors influenced by the microbiota and immune system, we conducted this study utilizing a molecular pathological epidemiology database (38) based on two the prospective cohort studies, to examine the relations between F. nucleatum and T cell infiltrates while controlling for confounders and selection bias. By employing a quantitative, multiplexed immunofluorescence assay. we found an inverse association of F. nucleatum with tumor stromal CD3+ T cells, particularly stromal CD3+CD4+CD45RO+ memory helper T cells that was independent of MSI status, neoantigen loads, and exome-wide tumor mutational burden. To our knowledge, our analysis is the first human population study to show the relationship between F. nucleatum amount and memory helper T cells in tumor stroma. Our findings not only aid the understanding of F. nucleatum associated immunosuppression but also underscore the importance of in-situ localization of specific T cell subsets in the colorectal cancer microenvironment.
The tumor immune microenvironment comprises transformed neoplastic cells, infiltrating immune cells, other stromal cells, and extracellular matrices. Recent advances in digital pathology have revealed the importance of both the characterization and localization of immune cells (10). Multiplex immunofluorescence allows detailed phenotyping of immune cells, which improves understanding of tumor-immune interactions (13). Mature T cells, expressing CD3, are largely comprised of CD8+ T cells and CD4+ helper T cells. Within these two major classes, naïve and memory T cells can be distinguished based on expression of CD45RO, and regulatory T cells can be identified by expression of the FOXP3 transcription factor (39). While T cells play a major role in the adaptive immune response against cancer, specific T cell subsets have divergent functions. Tumor-infiltrating lymphocytes, specifically CD8+ T cells, represent the cytotoxic arms of adaptive immune response and have been associated with better survival, while CD4+ T cells (mostly in the helper T cell lineage) appear to enhance anti-tumor activity of cytotoxic T cells (40,41). Tumor stromal lymphocytes, which are generally more abundant than intraepithelial ones, have also been associated with favorable prognosis in colorectal cancer (42); however, different cell types such as naïve, memory, and regulatory T cells may have different implications. Our intriguing findings suggest the potential interplay between F. nucleatum and memory helper T cells in tumor stroma.
There is a growing body of evidence on the influential role of microbiota on cancer immunosurveillance and the modulation of immunotherapy responsiveness (43–46). F. nucleatum has emerged as a potentially oncogenic microorganism, which may drive inflammation-related carcinogenesis and recruit myeloid-derived suppressor cells (20). Experimental studies also have shown that F. nucleatum may inhibit T cell- and natural killer cell-mediated immune response against colorectal cancer through the immune cell receptor TIGIT (21). In accordance with these experimental lines of evidence, our findings support the immunosuppressive role of F. nucleatum in the human colorectal cancer microenvironment. Interestingly, despite the ability of F. nucleatum to adhere and invade into tumor epithelial cells (19,47), the densities of T cells within tumor epithelial regions were not correlated with F. nucleatum levels. Given that invasive F. nucleatum distribution appears highly heterogenous and focal in the colorectal cancer tissues in terms of gross tumor center versus invasive margin, as well as microscopic tumor epithelia versus stroma (48), F. nucleatum may interact with stromal components including myeloid-derived suppressor cells, which inhibit T cell proliferation and induce T cell apoptosis (20). The amount of F. nucleatum in colorectal cancer has been associated with proximal tumor location, high-level MSI status, lower T cell infiltrates, and worse prognosis (18,49–51). Evidence also suggests that F. nucleatum may exert differential immunosuppressive or modulatory effects according to tumor MSI status (52). A better understanding of the interaction between F. nucleatum and specific immune cell phenotypes could have considerable implications on the development of therapeutic strategies that help overcome scarcity of T cell infiltration and increase the fraction of patients responding to immunotherapy.
We acknowledge limitations of this study. First, considering the cross-sectional nature of our study, we cannot exclude the possibility of reverse causation. Although it is possible that T cells may contribute in the elimination of F. nucleatum, our specific hypothesis was based on several lines of experimental evidence indicating that F. nucleatum suppresses the adaptive immune responses against colorectal cancer (21). Second, since our study was driven by a specific hypothesis, we focused on F. nucleatum in relation to T cells. Accumulating evidence suggests that various species of microbiota are involved in tumor development and anti-tumor immune response (1,2). Although we determined that there was no significant association between Bifidobacterium genus and T cell densities as an exploratory analysis, more comprehensive bacterial analyses such as metagenomic sequencing would help further characterize the relationship between tumor microbiota and the antitumor immune response. Third, we used the quantitative PCR assay for F. nucleatum in FFPE tissue specimens. Histopathology procedures and storage conditions may have influenced the detection rates and quantification. Nonetheless, our previous validation study using the quantitative PCR assay showed both a good concordance in detection of F. nucleatum in paired FFPE and frozen tissue specimens as well as high linearity and reproducibility of F. nucleatum measurements in FFPE tissue specimens (18).
The current study has notable strengths, including the use of a molecular pathological epidemiology database derived from two U.S.-based large prospective cohort studies. Because no experimental model could recapitulate the complexity of human tumor immune microenvironment, which can be modified by various factors including genetic and epigenetic alterations, lifestyle and environmental exposures, the microbiota, and host factors (53–55), the importance of the integrated data analyses on microbial features, tumor molecular characteristics, clinicopathological findings, and immunological profiling cannot be overemphasized. Second, our analyses within the prospective cohort studies enabled us to use the IPW method and covariate data of all 4,465 incident colorectal cancer cases to control for selection bias due to tissue data availability. Third, we utilized multiplexed immunofluorescence assays to identify and quantify specific subsets of T cells and macrophages in archival tumor tissue. In contrast to commonly used immunohistochemistry, our assays enabled us to deeply subclassify immune cells and discover the link between F. nucleatum and memory helper T cells. Fourth, unlike common studies based on cases drawn from few hospitals, our study subjects were derived from over a hundred of hospitals located throughout the U.S., which increases the generalizability of our findings. Nevertheless, our findings need to be validated in independent studies.
In conclusion, this cross-sectional study utilizing the two U.S.-wide prospective cohort studies has shown an inverse association of F. nucleatum DNA in colorectal carcinoma tissue with tumor stromal densities of CD3+ cell and CD3+CD4+CD45RO+ cells. These findings provide a compelling rationale for further investigations into the interplay of the microbiota and T lymphocytes in colorectal carcinoma, potentially leading to novel strategies for cancer prevention and therapy.
Supplementary Material
Translational Relevance.
Our multiplex immunofluorescence assay combined with digital image analyses and machine learning enabled us to robustly quantify T-cell subsets in 933 colorectal cancer cases within a large database of 4,465 incident colorectal cancers in two prospective U.S.-wide cohort studies. We found an inverse association of Fusobacterium nucleatum DNA amount with tumor stromal density of CD3+CD4+CD45RO+ helper memory T cells. Fewer memory T cells may contribute to a lack of immune attack on developing tumors. Our unique human population-based evidence for microbial-immune interactions supports possible interventions targeting the microbiota and/or immunity for cancer prevention and therapy.
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. This work was supported by U.S. National Institutes of Health (NIH) grants (P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; U01 CA167552 to W.C. Willett and L.A. Mucci; P50 CA127003 to C.S.F.; R01 CA118553 to C.S.F.; R01 CA169141 to C.S.F.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C.; R35 CA197735 to S.O.; R01 CA151993 to S.O.; K07 CA190673 to R.N.; K07 CA188126 to X.Z.; R01 CA225655 to J.K.L.; R01 CA248857 to S.O., U.P., and A.I.P.; P50 CA101942 (to G.J.F.); by Cancer Research UK’s Grand Challenge Award (UK C10674/A27140 to K.N., W.S.G., M.G., C.H., and S.O.); by Nodal Award (2016-02) from the Dana-Farber Harvard Cancer Center (to S.O. and G.J.F.); by a Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (SU2C-AACR-DT22-17 to C.S.F. and M.G.), and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance and SU2C. Stand Up to Cancer is a division of the Entertainment Industry Foundation. The indicated SU2C research grant is administered by the American Association for Cancer Research, the scientific partner of SU2C. J.B. was supported by a grant from the Australia Awards-Endeavour Scholarships and Fellowships Program. K.H. was supported by fellowship grants from the Uehara Memorial Foundation and the Mitsukoshi Health and Welfare Foundation. K.A. was supported by a grant from Overseas Research Fellowship (JP2018-60083) from Japan Society for the Promotion of Science. K.F. was supported by a fellowship grant from the Uehara Memorial Foundation. S.A.V. was supported by Finnish Cultural Foundation and Orion Research Foundation. J.A.M. research is supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, P fund and the George Stone Family Foundation. M.G. is supported by an ASCO Conquer Cancer Foundation Career Development Award. A.T.C. is a Stuart and Suzanne Steele MGH Research Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest: J.A.M. has received institutional research funding from Boston Biomedical, has served as an advisor/consultant to Ignyta and COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. A.T.C. previously served as a consultant for Bayer Healthcare and Pfizer Inc.. R.N. is currently employed by Pfizer Inc.; she contributed to this study before she became an employee of Pfizer Inc.. M.G. receives research funding from Bristol-Myers Squibb and Merck. C.S.F. previously served as a consultant for Agios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, Gilead Sciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, Merrimack Pharmaceuticals, Pfizer Inc, Sanofi, Taiho, and Unum Therapeutics; C.S.F. also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. G.J.F. reports grants from National Cancer Institute during the conduct of the study; personal fees from Roche, personal fees from Bristol-Myers Squibb, personal fees from Xios, personal fees from Orogimed, personal fees from Triursus, personal fees from iTeos, personal fees from NextPoint, personal fees from IgM, personal fees from Jubilant, personal fees from GV20, and personal fees from Trillium outside the submitted work. In addition, G.J.F. has a patent for CD274 (PD-L1) / PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Roche, a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Merck MSD, a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Bristol-Myers-Squibb, a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Merck KGA, a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Astra-Zeneca, a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Dako, a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Mayo Clinic, and a patent for CD274 (PD-L1)/PDCD1 (PD-1) pathway issued, licensed, and with royalties paid from Novartis. G.J.F. has equity in Nextpoint, Triursus, Xios, iTeos, IgM, GV20, and Geode. This study was not funded by any of these commercial entities. The other authors declare that they have no conflicts of interest.
Abbreviations:
- AJCC
American Joint Committee on Cancer
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- FFPE
formalin-fixed paraffin-embedded
- HPFS
Health Professionals Follow-up Study
- IPW
inverse probability weighting
- LINE-1
long-interspersed nucleotide element-1
- MSI
microsatellite instability
- NHS
Nurses’ Health Study
- OR
odds ratio
- PCR
polymerase chain reaction
- SD
standard deviation
- TAM
tumor-associated macrophage
Footnotes
Use of Standardized Official Symbols: We use HUGO (Human Genome Organisation)-approved official symbols (or root symbols) for genes and gene products, including BRAF, CACNA1G, CD3, CD4, CD8, CD68, CD86, CD274, CDH1, CDKN2A, CRABP1, CTNNB1, FOXP3, IGF2, IRF5, KRAS, KRT, MAF, MLH1, MRC1, NEUROG1, PDCD1, PIK3CA, PTPRC, RUNX3, SOCS1, TIGIT, and WNT; all of which are described at www.genenames.org. Gene symbols are italicized whereas symbols for gene products are not italicized.
References
- 1.Rajpoot M, Sharma AK, Sharma A, Gupta GK. Understanding the microbiome: Emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin Cancer Biol 2018;52(Pt 1):1–8 doi 10.1016/j.semcancer.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Inamura K Gut microbiota contributes towards immunomodulation against cancer: New frontiers in precision cancer therapeutics. Semin Cancer Biol 2020. doi 10.1016/j.semcancer.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Chen B, Du G, Guo J, Zhang Y. Bugs, drugs, and cancer: can the microbiome be a potential therapeutic target for cancer management? Drug Discov Today 2019;24(4):1000–9 doi 10.1016/j.drudis.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 4.El Bairi K, Jabi R, Trapani D, Boutallaka H, Ouled Amar Bencheikh B, Bouziane M, et al. Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut. Expert Rev Clin Pharmacol 2020;13(4):403–21 doi 10.1080/17512433.2020.1758063. [DOI] [PubMed] [Google Scholar]
- 5.Kamal Y, Schmit SL, Frost HR, Amos CI. The tumor microenvironment of colorectal cancer metastases: opportunities in cancer immunotherapy. Immunotherapy 2020;12(14):1083–100 doi 10.2217/imt-2020-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kather JN, Halama N. Harnessing the innate immune system and local immunological microenvironment to treat colorectal cancer. Br J Cancer 2019;120(9):871–82 doi 10.1038/s41416-019-0441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359(6382):1350–5 doi 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372(26):2509–20 doi 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciardiello D, Vitiello PP, Cardone C, Martini G, Troiani T, Martinelli E, et al. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat Rev 2019;76:22–32 doi 10.1016/j.ctrv.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391(10135):2128–39 doi 10.1016/s0140-6736(18)30789-x. [DOI] [PubMed] [Google Scholar]
- 11.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010;222(4):350–66 doi 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marisa L, Svrcek M, Collura A, Becht E, Cervera P, Wanherdrick K, et al. The Balance Between Cytotoxic T-cell Lymphocytes and Immune Checkpoint Expression in the Prognosis of Colon Tumors. J Natl Cancer Inst 2018;110(1) doi 10.1093/jnci/djx136. [DOI] [PubMed] [Google Scholar]
- 13.Gartrell RD, Marks DK, Hart TD, Li G, Davari DR, Wu A, et al. Quantitative Analysis of Immune Infiltrates in Primary Melanoma. Cancer Immunol Res 2018;6(4):481–93 doi 10.1158/2326-6066.Cir-17-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan CA, Garrett WS. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol 2019;17(3):156–66 doi 10.1038/s41579-018-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo K, Zhang Y, Xv C, Ji J, Lou G, Guo X, et al. Fusobacterium nucleatum, the communication with colorectal cancer. Biomed Pharmacother 2019;116:108988 doi 10.1016/j.biopha.2019.108988. [DOI] [PubMed] [Google Scholar]
- 16.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012;22(2):292–8 doi 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22(2):299–306 doi 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol 2015;1(5):653–61 doi 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14(2):195–206 doi 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14(2):207–15 doi 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015;42(2):344–55 doi 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta RS, Nishihara R, Cao Y, Song M, Mima K, Qian ZR, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol 2017;3(7):921–7 doi 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 2012;367(17):1596–606 doi 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haruki K, Kosumi K, Hamada T, Twombly TS, Väyrynen JP, Kim SA, et al. Association of autophagy status with amount of Fusobacterium nucleatum in colorectal cancer. J Pathol 2020;250(4):397–408 doi 10.1002/path.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61(6):847–54 doi 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 2007;356(21):2131–42 doi 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 27.Fujiyoshi K, Väyrynen JP, Borowsky J, Papke DJ Jr., Arima K, Haruki K, et al. Tumour budding, poorly differentiated clusters, and T-cell response in colorectal cancer. EBioMedicine 2020;57:102860 doi 10.1016/j.ebiom.2020.102860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau MC, Borowsky J, Väyrynen JP, Haruki K, Zhao M, Costa AD, et al. Tumor-Immune Partitioning and Clustering (TIPC) algorithm reveals distinct signatures of tumor-immune cell interactions within the tumor microenvironment. bioRxiv 2020:2020.05.29.111542 doi 10.1101/2020.05.29.111542. [DOI] [Google Scholar]
- 29.Fujiyoshi K, Bruford EA, Mroz P, Sims CL, O’Leary TJ, Lo AWI, et al. Opinion: Standardizing gene product nomenclature-a call to action. Proc Natl Acad Sci U S A 2021;118(3) doi 10.1073/pnas.2025207118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Väyrynen JP, Haruki K, Lau MC, Väyrynen SA, Zhong R, Dias Costa A, et al. The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol Res 2020. doi 10.1158/2326-6066.Cir-20-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosumi K, Hamada T, Koh H, Borowsky J, Bullman S, Twombly TS, et al. The Amount of Bifidobacterium Genus in Colorectal Carcinoma Tissue in Relation to Tumor Characteristics and Clinical Outcome. Am J Pathol 2018;188(12):2839–52 doi 10.1016/j.ajpath.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosho K, Irahara N, Shima K, Kure S, Kirkner GJ, Schernhammer ES, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One 2008;3(11):e3698 doi 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imamura Y, Lochhead P, Yamauchi M, Kuchiba A, Qian ZR, Liao X, et al. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature review. Mol Cancer 2014;13:135 doi 10.1186/1476-4598-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep 2016;15(4):857–65 doi 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen M, Andreatta M. NetMHCpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med 2016;8(1):33 doi 10.1186/s13073-016-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers EJ, Berk R, et al. Redefine statistical significance. Nat Hum Behav 2018;2(1):6–10 doi 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Nevo D, Nishihara R, Cao Y, Song M, Twombly TS, et al. Utility of inverse probability weighting in molecular pathological epidemiology. Eur J Epidemiol 2018;33(4):381–92 doi 10.1007/s10654-017-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol 2020. doi 10.1038/s41571-020-00445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel) 2016;8(3) doi 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bou Nasser Eddine F, Ramia E, Tosi G, Forlani G, Accolla RS. Tumor Immunology meets…Immunology: Modified cancer cells as professional APC for priming naive tumor-specific CD4+ T cells. Oncoimmunology 2017;6(11):e1356149 doi 10.1080/2162402x.2017.1356149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aspeslagh S, Morel D, Soria JC, Postel-Vinay S. Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol 2018;29(4):812–24 doi 10.1093/annonc/mdy050. [DOI] [PubMed] [Google Scholar]
- 42.Haruki K, Kosumi K, Li P, Arima K, Vayrynen JP, Lau MC, et al. An integrated analysis of lymphocytic reaction, tumour molecular characteristics and patient survival in colorectal cancer. Br J Cancer 2020;122(9):1367–77 doi 10.1038/s41416-020-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sears CL, Pardoll DM. The intestinal microbiome influences checkpoint blockade. Nat Med 2018;24(3):254–5 doi 10.1038/nm.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018;33(4):570–80 doi 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol 2017;18(8):843–50 doi 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 46.Murphy CL, OʼToole PW, Shanahan F. The Gut Microbiota in Causation, Detection, and Treatment of Cancer. Am J Gastroenterol 2019;114(7):1036–42 doi 10.14309/ajg.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 47.Abed J, Emgard JE, Zamir G, Faroja M, Almogy G, Grenov A, et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016;20(2):215–25 doi 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017;358(6369):1443–8 doi 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016;65(12):1973–80 doi 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, Masugi Y, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol 2016;7(11):e200 doi 10.1038/ctg.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol 2016;22(2):557–66 doi 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in Colorectal Cancer Relates to Immune Response Differentially by Tumor Microsatellite Instability Status. Cancer Immunol Res 2018;6(11):1327–36 doi 10.1158/2326-6066.Cir-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011;60(3):397–411 doi 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogino S, Nowak JA, Hamada T, Milner DA, Jr., Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol 2019;14:83–103 doi 10.1146/annurev-pathmechdis-012418-012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang ST, Cui WQ, Pan D, Jiang M, Chang B, Sang LX. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J Gastroenterol 2020;26(6):562–97 doi 10.3748/wjg.v26.i6.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


