Abstract
Purpose:
In this first-in-human, phase I, graft-versus-host disease (GVHD) prevention trial (NCT02891603) we combine pacritinib (PAC), a JAK2 inhibitor, with sirolimus to concurrently reduce T-cell costimulation via mTOR and IL-6 activity. We evaluate the safety of pacritinib when administered with sirolimus plus low-dose tacrolimus (PAC/SIR/TAC) after allogeneic hematopoietic cell transplantation (alloHCT).
Patients and Methods:
The preclinical efficacy and immune modulation of PAC/SIR were investigated in xenogeneic GVHD. Our phase I trial followed a 3+3 dose escalation design, including dose level 1 (PAC 100mg daily), level 2 (PAC 100mg twice daily), and level 3 (PAC 200mg twice daily). The primary endpoint was to identify the lowest biologically active and safe dose of PAC with SIR/TAC (n=12). Acute GVHD was scored through day +100. Allografts included 8/8 HLA-matched related or unrelated donor peripheral blood stem cells.
Results:
In mice, we show that dual JAK2/mTOR inhibition significantly reduces xenogeneic GVHD and increases peripheral Treg potency as well as Treg induction from conventional CD4+ T cells. PAC 100mg twice a day was identified as the minimum biologically active and safe dose for further study. JAK2/mTOR inhibition suppresses pathogenic Th1 and Th17 cells, spares Tregs and anti-leukemia effector cells, and exhibits preliminary activity in preventing GVHD. PAC/SIR/TAC preserves donor CMV immunity and permits timely engraftment without cytopenias.
Conclusions:
We demonstrate that PAC/SIR/TAC is safe and preliminarily limits acute GVHD, preserves donor CMV immunity, and permits timely engraftment. The efficacy of PAC/SIR/TAC will be tested in our ongoing phase II GVHD prevention trial.
Keywords: GVHD, JAK2, pacritinib, mTOR
Introduction
Nearly a decade has passed since JAK2 was identified as a therapeutic target to control human alloreactive T cells (1). Since that time, major gains in clinical translation have occurred. The first JAK inhibitor, ruxolitinib, was recently approved for use in steroid-refractory graft-versus-host disease (GVHD) (2). Distinct from selective JAK2 blockade (1), ruxolitinib is a dual JAK1/2 inhibitor that simultaneously targets a broad range of cytokines, inflammatory and otherwise (3–6). Others and we have shown that ruxolitinib indeed reduces pathogenic Th1 and Th17 cells in GVHD, but also limits the numbers and activity of beneficial NK cells, CD8+ cytotoxic T lymphocytes, and immunosuppressive regulatory T cells (Tregs) (3–6). While relapse of underlying hematologic malignancies does not appear to be problematic with ruxolitinib, cytomegalovirus (CMV) reactivation and cytopenias do pose a challenge when treating recipients of allogeneic hematopoietic cell transplantation (alloHCT) (2). Further, the durability of responses by ruxolitinib do wane over time and could be a result of Treg impairment (2).
We have shown that pacritinib (PAC), a selective JAK2 inhibitor, reduces alloreactivity mediated by murine or human T cells (6). Unlike ruxolitinib, pacritinib spares IL-2 signal transduction along with Tregs and their suppressive function (6). Conversely, pacritinib readily eliminates IL-6 activity and reduces pathogenic T effectors of GVHD (6). Pacritinib offers a degree of finesse in its selective immune suppression, lending its use to GVHD prevention. In this setting, pressure against pathogenic T cells is needed, yet key mediators of antiviral immunity, graft-versus-leukemia (GVL), and allotolerance must be left unperturbed. Our premise is that pacritinib is well positioned for GVHD prevention based on these unique immune effects.
To enhance the immune suppressive potency afforded by pacritinib, and leverage its beneficial effects toward Tregs, we combined pacritinib with our sirolimus (target range 5–14ng/ml) and low dose tacrolimus (3–7ng/ml) (SIR/TAC) GVHD prophylaxis regimen (7,8). Exposure to SIR and specifically limiting the dose of TAC (3–7ng/ml) assists in the reconstitution and function of Tregs across conditioning regimens and donor sources (7,9). The addition of JAK2 inhibition to mTOR blockade also parallels our prior study in mice where we demonstrated that concurrent suppression of T cell costimulation and cytokine activation prevents GVHD while maintaining GVL (10). In that past study, we used a bispecific inhibitor of JAK2 and Aurora kinase A, AJI-100 (10). Conceptually, PAC/SIR/TAC parallels that approach as CD28 signals through mTOR as well as Aurora kinase A (10). Thus, PAC/SIR/TAC provides concurrent inhibition of CD28-meditated T cell costimulation plus JAK2 cytokine activation.
In this phase I clinical trial, we demonstrate that PAC/SIR/TAC is safe and provides evidence of early activity in GVHD prevention. PAC/SIR/TAC leads to an immune phenotype that mirrors our prior observations in mice transplanted with CD4+ JAK2-deficient T cells (6). PAC/SIR/TAC promotes Tregs and substantially improves the ratio of Tregs to pathogenic effectors of GVHD. Importantly, PAC/SIR/TAC permits timely donor engraftment, avoids cytopenias, and is not associated with excess CMV reactivation. We provide supporting data in mice, demonstrating the long-term efficacy of concurrent JAK2/mTOR inhibition in xenogeneic GVHD as well as key benefits regarding Treg biology and function. Our ongoing phase II trial is testing the efficacy of PAC/SIR/TAC in GVHD prevention.
Materials and Methods
Included subjects:
Included were subjects ≥ age 18. HLA-A, -B, -C, and -DRB1 matched-related or unrelated donors were allowed. The graft source was mobilized peripheral blood stem cells (PBSC, 5–10 × 106 CD34+ cells/kg). Remission criteria included: acute leukemia (complete remission with < 5% marrow blasts, no peripheral blasts, and ANC > 1000/uL), myelodysplastic syndrome and chronic myeloid leukemia (< 5% marrow blasts), myeloproliferative neoplasms (< 5% marrow and peripheral blood blasts, and no JAK2 inhibitor therapy within 4 weeks preceding HCT date), and Hodgkin or non-Hodgkin lymphoma (complete or partial response). Adequate vital organ function (LVEF ≥ 50%; FEV, FVC and adjusted DLCO ≥ 50% of predicted values; transaminases < 2 times upper limit of normal values; creatinine clearance ≥ 50 cc/min) and adequate Karnofsky performance status (≥ 80%) were required. The following were exclusion criteria: uncontrolled infection, history of HIV or hepatitis B or C infection, HCT-CI > 4,(11) QTcF > 450ms, coagulation studies (PT, PTT, thrombin time) > 2 x upper limit of normal values, ≥ grade 3 cardiac or bleeding complications within the preceding 6 months, uncontrolled current myocardial infarction/angina, or class III-IV congestive heart failure. To reduce treatment heterogeneity, the following restrictions were enforced: Conditioning regimens could include only myeloablative PK-targeted IV busulfan/Fludarabine, or a reduced intensity Fludarabine/melphalan regimen (per program standard procedures). Any planned use of post-HCT cyclophosphamide, anti-thymocyte globulin, alemtuzumab, or bortezomib was prohibited. Finally, planned use of post-HCT maintenance (e.g., tyrosine kinase inhibitors, JAK1/2 inhibitors) was exclusionary to avoid drug interactions and confounding of toxicity assessment for pacritinib.
Treatment program:
Phase I dose finding procedures followed a standard 3+3 dose escalation design and included dose level 1 (pacritinib 100mg daily), level 2 (100mg twice daily), and level 3 (200mg twice daily). Dose level 1 represented the lowest possible dose capable of inhibiting pSTAT3 in CD4+ T cells, based on prior data (12). Dose finding was governed by a composite of clinical safety (dose limiting toxicity, DLT events) and a key pharmacodynamic assessment (day +21 pSTAT3 in blood CD4 T cells after HCT, with a goal of achieving <35% CD4+, pSTAT3+ T cells (13,14)). Pacritinib was given at the full intended dose (per dose level) from day 0 onward, followed by treatment at 50% of original dose from day +70 through +83, then at 25% of original dose from day +84 through +100, and then stopped. Concurrent therapy with strong CYP3A4 inhibitors required 50% dose reduction in pacritinib. Specific drug interruption and discontinuation rules were based on QTcF prolongation, left ventricular systolic dysfunction, diarrhea, and any other ≥ grade 3 adverse events deemed at least possibly related to pacritinib therapy. Management of sirolimus and tacrolimus followed usual program standard operating procedures, and included guidelines for drug level targets (SIR 5–14 ng/ml, TAC 3–7 ng/ml) and management of drug interactions. Surveillance for signs, symptoms, and laboratory findings of thrombotic microangiopathy (TMA) was followed, and the protocol included guidance in how to manage and mitigate TMA if observed. The protocol did not mandate the duration of tacrolimus or sirolimus administration after HCT.
Outcome measures:
The phase I trial had a composite primary endpoint, which considered both safety and the biologic effect of pacritinib therapy, with the objective of identifying the lowest biologically active safe dose. Clinical DLT events included: left ventricular systolic dysfunction ≥ grade 3, myocardial infarction ≥ grade 3, atrial fibrillation or flutter ≥ grade 3, supraventricular tachycardia ≥ grade 3, gastrointestinal hemorrhage ≥ grade 3, intracranial hemorrhage ≥ grade 3, or any other ≥ grade 3 adverse events considered at least possibly related to pacritinib therapy. The DLT observation period was 100 days post-HCT. Success for the day +21 PD assessment was defined as CD4+ pSTAT3+ T cells <35%.
Secondary outcomes included engraftment (time to neutrophil (ANC > 500) and platelet (PLT > 20 without transfusion) recovery), donor chimerism, acute and chronic GVHD per consensus criteria (15,16), GVHD therapy requirements, TMA (17), hepatic veno-occlusive disease (VOD) (18), discontinuation of immune suppression, infectious complications including cytomegalovirus reactivation, and HCT outcomes including malignancy relapse, non-relapse morality, overall survival, and causes of death.
Detailed methods regarding flow cytometry, allogeneic mixed leukocyte reactions, Treg suppression assays, IACUC-approved xenogeneic GVHD mouse experiments are included as Supplemental Material.
Study Approval:
Written informed consent was obtained from eligible patients on our IRB-approved study, A Phase I/II GVHD Prevention Trial Combining Pacritinib With Sirolimus-Based Immune Suppression, at Moffitt Cancer Center (NCT02891603). This was done in accordance with the principles set forth within the Declaration of Helsinki. NSG mice (male or female, age 6- to 24-weeks-old) were purchased from The Jackson Laboratory and housed within American Association for Laboratory Animal Care–accredited Animal Resource Centers at Moffitt Cancer Center or the University of Minnesota. All mice were treated in adherence with the NIH Guide for the Care and Use of Laboratory Animals according to protocols approved by institutional animal care and use committees.
Statistical methods:
The phase I dose finding procedure followed a 3+3 design, however incorporated both the described clinical DLT events and the key pharmacodynamic measure. Dose escalation proceeded until a safe dose level was determined (i.e., ≤ 1/6 subjects experiencing a DLT event) where the key pharmacodynamic measure was met. Baseline characteristics and major outcome variables were summarized using descriptive statistics. Correlative and murine data are reported as mean values ± SEM. ANOVA was used for group comparisons, including a Dunnett’s test for correction of multiple comparisons with a control. For comparison of murine survival curves, a log-rank test was used. The statistical analysis was conducted using Prism software version 5.04 (GraphPad) for correlative and murine data. Statistical significance was defined by a 2-tailed P less than 0.05.
Results
Xenogeneic GVHD
Concurrent JAK2 or STAT3/mTOR blockade synergistically reduces xenogeneic GVHD mediated by human T cells
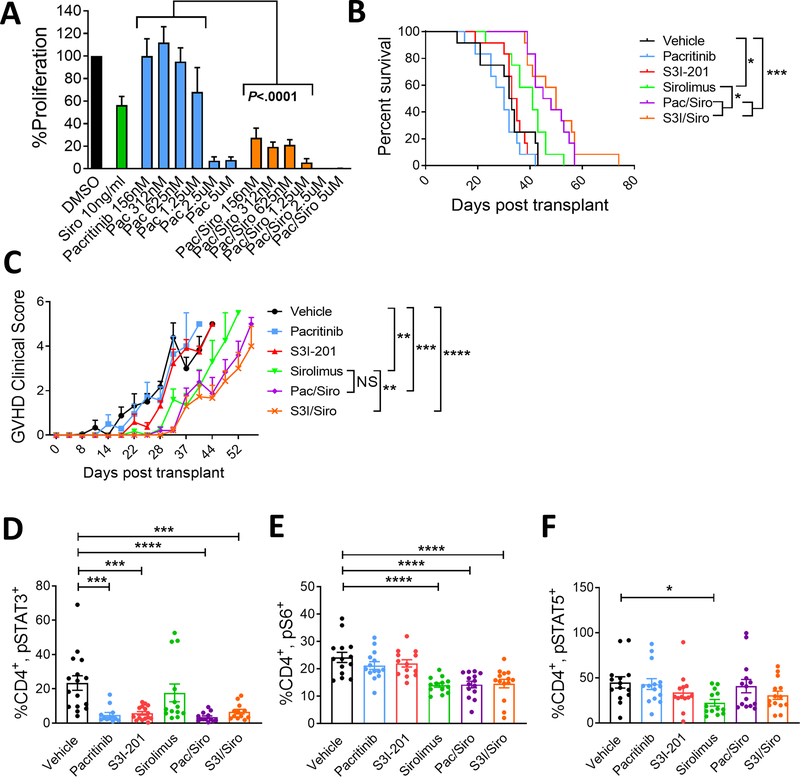
To test the effect of dual JAK2 or STAT3/mTOR inhibition on alloreactive human T cells, mixed leukocyte reactions (T cell to DC ratio 30:1) were incubated with varying doses of pacritinib alone or with a fixed, clinically relevant concentration of sirolimus (10ng/ml) (Figure 1A). Consistent with clinical experience (19), sirolimus alone moderately reduced T cell proliferation against allogeneic monocyte-derived dendritic cells (moDC) (Figure 1A). Pacritinib at 2.5μM and greater also inhibited alloreactive T effectors. We previously demonstrated that this minimum concentration of pacritinib (2.5μM) decreases the frequency of CD4+, pSTAT3+ T cells, permits CD4+ T cell STAT5 phosphorylation, and suppresses the proliferation of alloreactive T cells in vitro (6). Importantly, the combination of pacritinib plus sirolimus significantly suppressed T cell proliferation even at low nanomolar concentrations of the JAK2 inhibitor (Figure 1A).
Figure 1. Concurrent inhibition of JAK2 or STAT3 plus mTOR improves xenogeneic GVHD survival in NSG mice.
A) 5-day alloMLRs were treated with a range of pacritinib concentrations, sirolimus (10ng/ml), both, or DMSO control. Graph shows allogeneic DC-stimulated T cell proliferation (mean ± SEM) after 5 days of culture. n=4 experiments in triplicate. NSG mice received 25×106 human peripheral blood mononuclear cells on day 0. Mice received vehicle control, pacritinib (50mg/kg po BID), S3I-201 (2.5mg/kg ip daily), sirolimus (0.5mg/kg ip daily), pacritinib plus sirolimus, or S3I-201 plus sirolimus from day 0 until day +14. Mice were monitored for GVHD survival (B) and severity (C, mean ± SEM). Transplanted NSG mice were humanely euthanized at day +14 and human T cells were acquired from the spleens and phosphoproteins were analyzed by flow cytometry. Graph shows the frequency (mean ± SEM) of human D) CD4+, pSTAT3+ T cells, E) CD4+, pS6+ T cells, and F) CD4+, pSTAT5+ T cells. Pooled data from 3 independent experiments is shown, 12 mice per group. ANOVA (A, C, D, E, F) and Log-Rank test (B). *P<0.05, **P=0.01–0.001, ***P=0.001–0.0001, ****P<0.00001. NS=not significant
The efficacy of JAK2/mTOR inhibition was investigated in vivo using our established model of xenogeneic GVHD (10). Immunodeficient NSG mice were transplanted with 25×106 human peripheral blood mononuclear cells (PBMC) and then treated with vehicle, pacritinib, S3I-201 (S3I); an inhibitor of downstream STAT3, sirolimus, PAC/SIR, or S3I/SIR for 14 days. We previously demonstrated that pacritinib administered at 100mg/kg twice a day significantly suppressed human alloreactive T cells targeting skin grafts in vivo (6). As we were interested in testing the potential synergy of PAC/SIR, a lower but biologically active dose of pacritinib (50mg/kg) was used in these experiments. The combination of JAK2 or downstream STAT3 inhibition plus sirolimus significantly improved survival from xenogeneic GVHD in mice, compared to either inhibitor alone (Figure 1B). GVHD clinical scores were significantly reduced by sirolimus and the combination treatment groups, with S3I/SIR performing greater than sirolimus (Figure 1C).
STAT3 and S6 phosphorylation are biologic readouts for JAK2 and mTOR activation, respectively, in T cells (1,13,14). Mice treated with pacritinib, S3I-201, PAC/SIR, or S3I/SIR exhibited a significantly decreased frequency of CD4+, pSTAT3+ human T cells in the mouse spleens at day +14 (Figure 1D). Conversely, mice treated with the mTOR inhibitor, sirolimus, alone or in combination with pacritinib or S3I-201 demonstrated significantly less CD4+, pS6+ T cells (Figure 1E). Only the mice treated with concurrent mTOR blockade plus pacritinib or S3I-201 had a significant reduction in both the frequency of CD4+, pSTAT3+ and pS6+ T cells (Figure 1E). Thus, providing evidence that concurrent JAK2 or pSTAT3 plus mTOR inhibition successfully reduces relevant downstream signaling pathways in vivo. Further, these data confirm that a reduced dose of pacritinib at 50mg/kg exhibits on-target pathway inhibition of JAK2 (Figure 1D). IL-2/pSTAT5 responses were largely intact among mice in the control and treatment groups, though the frequency of CD4+, pSTAT5+ T cells was significantly reduced among mice treated with sirolimus (Figure 1F). The similar survival outcomes of PAC/SIR and S3i/SIR strengthen the hypothesis that targeting IL-6 receptor signal transduction, either at the level of JAK2 or downstream STAT3, plus mTOR inhibition significantly reduces the clinical impact of GVHD.
Targeting JAK2 or STAT3/mTOR suppresses Th1 and Th17 cells
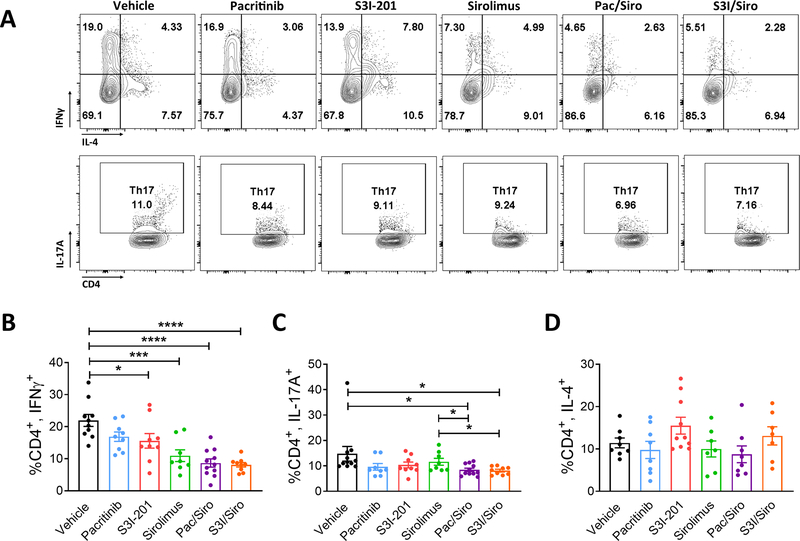
While NSG mice treated with S3I, sirolimus, PAC/SIR, or S3I/SIR exhibited a significant decrease in frequency of pathogenic Th1 cells in the spleen at day +14, the greatest reductions trended toward the dual pathway blockade groups (Figure 2A,B). However, when DC-allostimulated T cells were cultured with vehicle, pacritinib, S3I-201, sirolimus, PAC/SIR, or S3I/SIR in vitro, production of the inflammatory IFNγ, IL-13, and GM-CSF cytokines was only significantly suppressed with PAC/SIR or S3I/SIR (Supplemental Figure 1). Additionally, only PAC/SIR and S3I/SIR treated mice had a significant reduction in human Th17 cells (Figure 2A,C). The frequency of human Th2 cells in the recipient NSG spleens were similar across all experimental groups (Figure 2A,D).
Figure 2. Combined inhibition of JAK2 or STAT3 plus mTOR reduces pathogenic, human Th1 and Th17 cells in NSG mice.
A) Representative contour plots and bar graphs show the frequency (mean ± SEM) of B) CD4+, IFNγ+ Th1, C) CD4+, IL-17+ Th17, D) CD4+, IL-4+ Th2 cells from NSG spleens recovered at day +14, following transplantation with human PBMCs and then treated with vehicle, pacritinib, S3I-201, sirolimus, pacritinib plus sirolimus, or S3I-201 plus sirolimus for 2 weeks. Pooled data from 3 independent experiments is shown, up to 11 mice per group. ANOVA (B, C). *P<0.05, ***P=0.001–0.0001, ****P<0.00001.
Concurrent JAK2 or STAT3/mTOR blockade maintains peripheral Tregs, but significantly improves Treg conversion from CD4+ Tconv
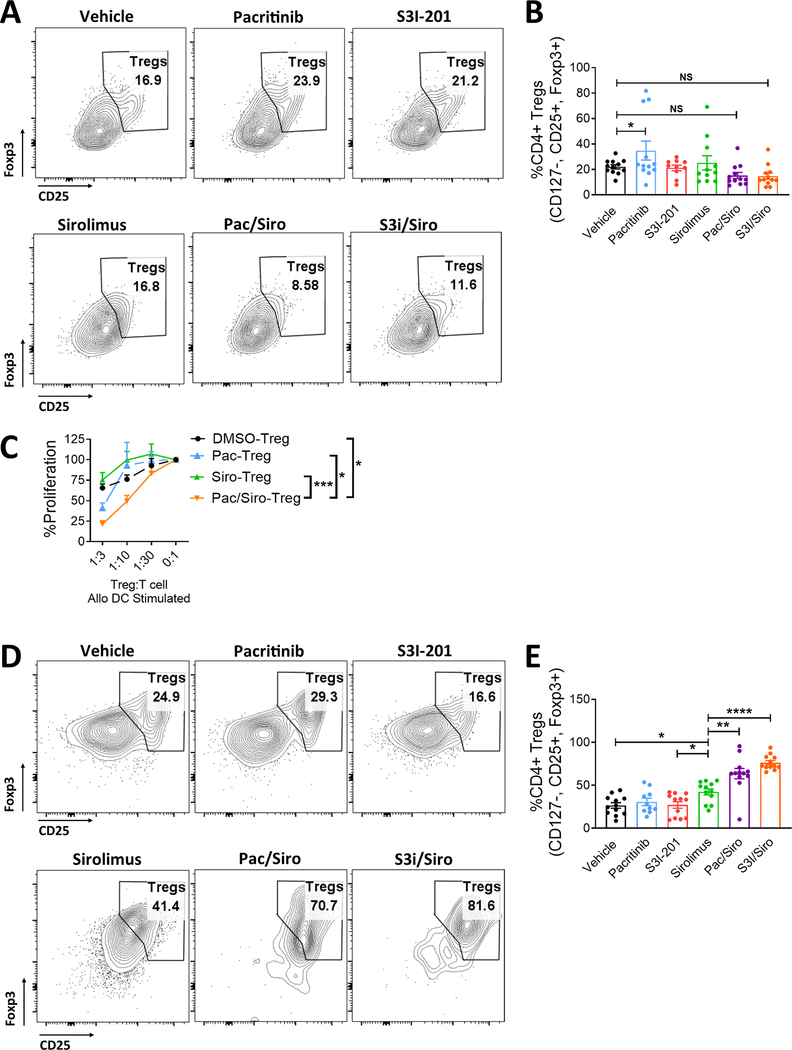
Similar to our previous data with single agent pacritinib (6), JAK2 inhibition alone significantly increased the amount of Tregs in the mouse spleen at day +14 (Figure 3A,B). However, this was not observed with the S3i-201, sirolimus, or either of the combination treatments (Figure 3A,B). Importantly, exposing peripheral Tregs to PAC/SIR in vitro significantly improved their suppressive potency against alloreactive T cells compared to vehicle or single agent controls (Figure 3C). This suggests that while the frequency of Tregs may be similar to controls when blocking JAK2/mTOR, the beneficial effects on Treg function may contribute toward the observed reduction in GVHD. Additionally, we have previously demonstrated that dual inhibition of T cell costimulation and IL-6 signal transduction substantially improved inducible Treg generation and potency, by way of concurrently targeting CD28 via aurora kinase A and IL-6 by JAK2 using a bispecific drug, AJI-100 (10). Further supporting that concept, we now show that dual inhibition of mTOR plus JAK2 or STAT3 leads to a significant gain in the frequency of Tregs when the mice are transplanted with Treg-depleted PBMCs at the outset (Figure 3D,E). Even when compared to sirolimus, an inhibitor with well-established Treg effects, the PAC/SIR and S3i/SIR combination produced significantly more human Tregs at day +14 in the mouse spleens (Figure 3D,E).
Figure 3. Concurrent JAK2 or STAT3/mTOR blockade maintains peripheral Tregs, but significantly improves Treg conversion from CD4+ Tconv.
Transplanted NSG mice treated with vehicle, pacritinib, S3I-201, sirolimus, pacritinib plus sirolimus, or S3I-201 plus sirolimus for 2 weeks were humanely euthanized at day +14. A) Representative contour plots and B) bar graph shows the frequency of human Tregs within the mouse spleens at day +14. Purified human peripheral Tregs were expanded with CD3/CD28 beads (Treg:bead ratio 1:1) for 5 days in the presence of vehicle, pacritinib 1.25μM, sirolimus 10ng/ml, or pacritinib plus sirolimus. Medium was supplemented with human recombinant IL-2 (20 IU/ml). C) The suppressive potency of vehicle-, pacritinib-, sirolimus-, or pacritinib plus sirolimus-treated Tregs was tested at different ratios of Treg to T cell responders in alloMLRs. No drugs were added to the suppression assay. Data are shown as mean ± SEM. N = 4 independent experiments. To investigate how dual JAK2 or STAT3 plus mTOR blockade impacts Treg induction in vivo, NSG mice were transplanted with Treg-depleted PBMCs. Mice received vehicle, pacritinib, S3I-201, sirolimus, pacritinib plus sirolimus, or S3I-201 plus sirolimus for 2 weeks as described. D) Representative contour plots and E) bar graph shows the frequency (mean ± SEM) of human Tregs recovered at day +14 for the mouse spleens. Pooled data from 3 independent experiments is shown, 12 mice per group. ANOVA (C, E). *P<0.05, **P=0.01–0.001, ***P=0.001–0.0001, ****P<0.00001.
Concurrent inhibition of JAK2 or STAT3/mTOR preserves donor anti-leukemia immunity
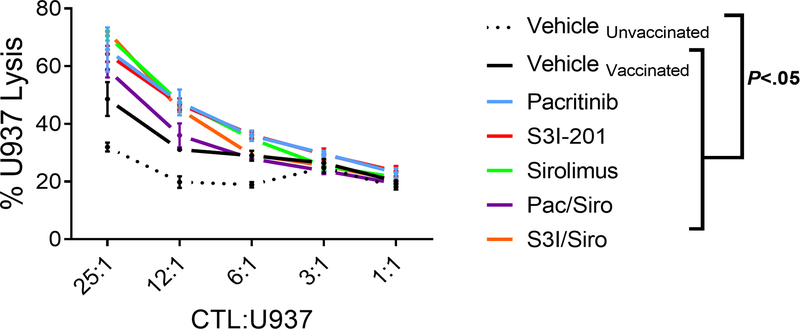
To test donor anti-leukemia immunity, human antitumor cytotoxic T lymphocytes were generated in vivo and then their specific killing of tumor targets was evaluated in vitro. NSG mice received human PBMCs and were inoculated with irradiated U937 cells on days 0 and +7. Mice were treated with vehicle, pacritinib, S3i-201, sirolimus, PAC/SIR, or S3i/SIR from day 0 to day +14. Human CD8+ T cells were isolated from the spleens of euthanized recipients during days +14 and cultured against fresh U937 target cells without further drug exposure in vitro. Human CD8+ cytotoxic T lymphocytes (CTL) from any of the U937-inoculated group exhibited significant killing of fresh U937 targets (Figure 4). This was consistent with our prior studies showing pacritinib and S3i-201 maintain GVL in transplanted mice (6,20).
Figure 4. Concurrent inhibition of JAK2 or STAT3/mTOR preserves donor anti-leukemia immunity.
NSG mice received 25×106 human peripheral blood mononuclear cells on day 0. An inoculum of 1×107 irradiated U937 cells were given to all mice, except the “unvaccinated” group, on days 0 and +7. Mice received vehicle control, pacritinib (50mg/kg po BID), S3I-201 (2.5mg/kg ip daily), sirolimus (0.5mg/kg ip daily), pacritinib plus sirolimus, or S3I-201 plus sirolimus from day 0 until day +14. Mice were humanely euthanized on day +14, the CD8+ T cells were purified, and anti-tumor killing of fresh U937 cells was determined in vitro. One representative experiment of 3 is shown using 3 mice per group. ANOVA.
Baseline patient characteristics of PAC/SIR/TAC phase I trial
The positive resultant data from our xenograft experiments served as proof-of-concept that dual JAK2/mTOR inhibition can reduce GVHD and spare anti-leukemia activity. As such, we investigated the safety and preliminary activity of PAC/SIR/TAC as GVHD prophylaxis following HLA-matched related or unrelated alloHCT. The primary aim of this phase I trial was to identify the lowest biologically active dose of pacritinib, defined as achieving <35% circulating CD4+, pSTAT3+ T cells at day +21, that is also safe when combined with sirolimus-based immune suppression (13). This pSTAT3 threshold was determined by our prior work showing that the risk for grade II-IV acute GVHD was increased among patients with >35% circulating CD4+, pSTAT3+ T cells at day +21 (13,14). A total of 12 evaluable patients were treated on this phase I trial (NCT02891603), with 6 patients each included in pacritinib dose levels 1 (100 mg/day) and 2 (100 mg twice a day). Pacritinib was administered orally from days 0 to day +70, then tapered to off on day +100 to mitigate the risk of JAK inhibitor withdrawal syndrome (21,22). Overall, the patients comprising the two dose level cohorts were similar in regard to age, gender, race, and ethnicity (Supplemental Table 1). Additionally, disease and pre-transplant response status, functional status, comorbidities, conditioning regimens, donor characteristics, and allograft source were comparable between the pacritinib dose level groups (Supplemental Table 1).
Safety and dose limiting toxicities
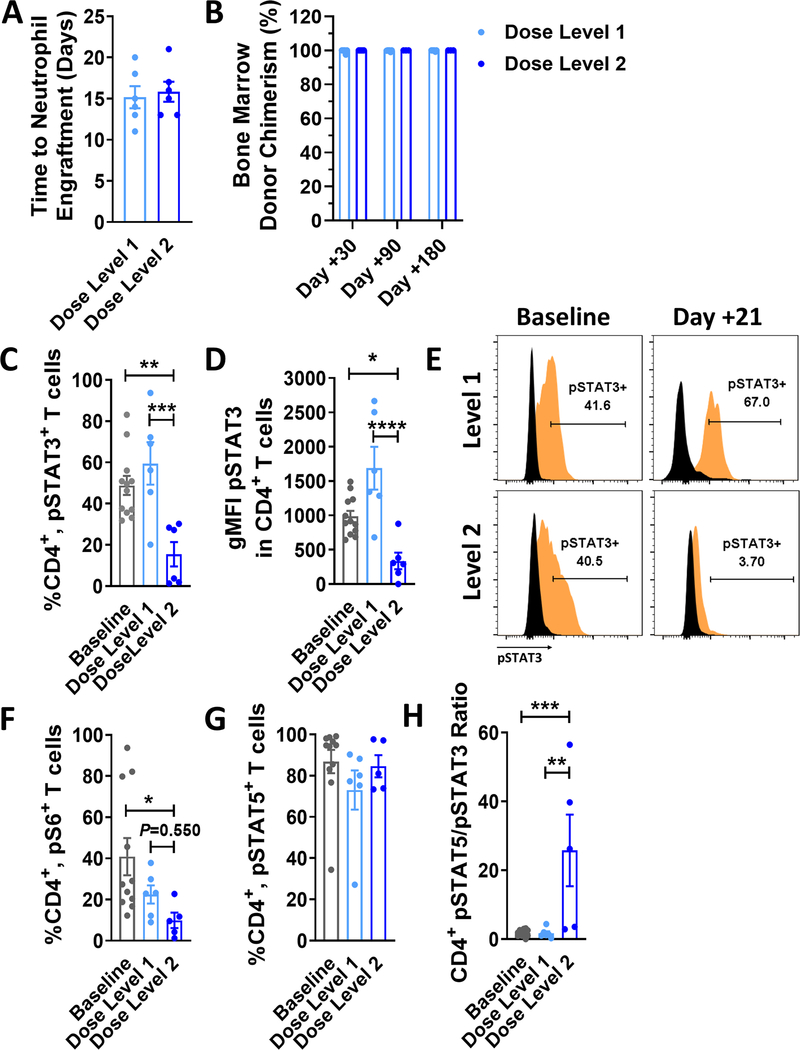
Median follow up time for surviving patients at time of this analysis was 18 months (range 3.7 – 29.6 months). A single dose limiting toxicity (DLT) was observed in dose level I only and consisted of angioedema possibly related to pacritinib. No patients treated on dose level 2 experienced any DLTs. Neither CMV reactivation nor disease was observed among patients treated at dose level 2, with only a single case of CMV reactivation among dose level 1 (Supplemental Table 2). Complete data on adverse events (AEs) including infectious complications are summarized in Supplemental Table 3. No patients treated with PAC/SIR/TAC developed VOD. Grade 1–2 TMA was observed among 2 dose level 2 subjects (one grade 1 resolved without intervention, and one grade 2 resolved after tacrolimus discontinuation). Additional safety data (QTcF, cardiac ejection fraction monitoring, and coagulation studies) are presented in Supplemental Figure 2. Two deaths occurred during the conduct of the study. One patient died of relapsed disease in dose level 1. In dose level 2, one patient died of steroid-refractory grade IV acute GVHD after prematurely discontinuing tacrolimus for non-TMA acute kidney injury and electively stopping pacritinib. Importantly, PAC/SIR/TAC allowed for timely neutrophil (median 15 days) and platelet (median 10 days) engraftment (Figure 5A, Supplemental Figure 3) and permitted full donor chimerism by day +30 (Figure 5A,B and Supplemental Table 4).
Figure 5. Engraftment, pharmacodynamics, and pharmacokinetics of PAC/SIR/TAC.
A) Graph shows the time of engraftment in days post allo-HCT (mean ± SEM) among patients treated on dose level 1 or dose level 2 of the phase I trial. B) Donor chimerism (mean ± SEM) within the recipient bone marrow is shown at 1, 3, and 6 months post allo-HCT. The C) frequency and D) geometric MFI (mean ± SEM) of CD4+, pSTAT3+ T cells at day +21 are shown. E) Representative histograms are shown for pSTAT3 gating on CD4+ T cells at baseline/pretransplant and day +21 (isotype = black, pSTAT3 = orange). A goal of the phase I trial was to identify a safe dose of pacritinib that achieved <35% CD4+, pSTAT3+ T cells at day +21. Graphs depict the frequency (mean ± SEM) of F) CD4+, pS6+ (downstream of mTOR) and G) CD4+, pSTAT5+ T cells. H) Graph shows the ratio (mean ± SEM) of pSTAT5+/pSTAT3+ CD4+ T cells in circulation at day +21 post allo-HCT. ANOVA (C, D, F, H). *P<.05, **P=.001–.01, ***P=.0001–.001, and ****P<.0001. Baseline data were acquired pretransplant between days −30 and −5.
Pharmacokinetics and pharmacodynamics
The frequency of CD4+, pSTAT3+ T cells at day +21 among patients treated at dose level 1 were not reduced compared to baseline values (Figure 5C). Importantly, PAC/SIR/TAC at dose level 2 significantly decreased the amount of CD4+, pSTAT3+ T cells (Figure 5C). This pharmacodynamic data, along with the favorable safety profile, identified pacritinib 100mg twice a day as the recommended phase II dose (RP2D). Additionally, dose level 2 of PAC/SIR/TAC significantly reduced the fluorescence intensity of pSTAT3 among circulating CD4+ T cells at day +21 (Figure 5D,E). Study drug adherence and pharmacokinetic studies for pacritinib 100mg daily and twice a day are detailed in Supplemental Tables 5 and 6. Among those cases that stopped pacritinib before full planned duration of study therapy, a withdrawal syndrome was not observed despite not tapering the drug first or using steroid therapy to address this possibility. Sirolimus suppresses mTOR signal transduction, and we confirmed that PAC/SIR/TAC at dose level 2 also significantly decreased downstream protein S6 phosphorylation in peripheral CD4+ T cells (Figure 5F). Given that pacritinib inhibits JAK2 and subsequent STAT3 phosphorylation, without impairing common gamma chain receptor cytokine signal transduction, we confirmed that CD4+ T cells from patients treated at dose level 2 exhibited intact pSTAT5 (Figure 5G). Further, dose level 2 of PAC/SIR/TAC significantly increased the ratio of pSTAT5 to pSTAT3 among CD4+ T cells (Figure 5H). A high CD4+ T cell pSTAT5/pSTAT3 ratio favors Tregs and opposes pathogenic T cell subsets, like Th1 and Th17 (13,14,23). Consistent with the central hypothesis of this interventional trial, dose level 2 of PAC/SIR/TAC concurrently inhibited T cell costimulation via mTOR blockade and cytokine activation by JAK2.
Acute and Chronic GVHD
Overall grade II-IV acute GVHD occurred in 2 subjects in dose level 1 (both GI involvement treated with beclomethasone and budesonide with no systemic prednisone or IV equivalent), and 1 subject in dose level 2 (steroid-refractory skin and GI involvement starting day +20 post-alloHCT after early discontinuation of tacrolimus for non-TMA acute kidney injury at day +13). Additional stage 1–2 skin (overall grade I) acute GVHD was observed in 1 subject in dose level 1 (resolved after topical steroid only), and 3 subjects in dose level 2 (resolved with topical agents only in 2 cases, and resolved after topical agents and 0.5mg/kg/day prednisone in 1 case). NIH chronic GVHD was uncommon, with 2 subjects affected by overall score 1 (mild) chronic GVHD in dose level 1, and 1 subject affected by overall score 1 (mild) chronic GVHD in dose level 2. None of these required systemic immune suppressive therapy for chronic GVHD. Full acute and chronic GVHD staging tables are presented in Supplemental Tables 7 and 8. With current follow up time, 4 total subjects have successfully stopped tacrolimus after intentional taper (median 8.8 months post-HCT, range 5.6–10.4), and 2 subjects stopped sirolimus (at 14.7 and 8.7 months post-HCT, respectively) following successful taper. Separately, 4 subjects stopped tacrolimus for other reasons (suspected allergy n=1, relapse n=1, TMA n=1, acute kidney injury n=1), and 1 patient stopped sirolimus (relapse).
Combined JAK2/mTOR inhibition polarizes Tregs over pathogenic Th1/Th17 cells
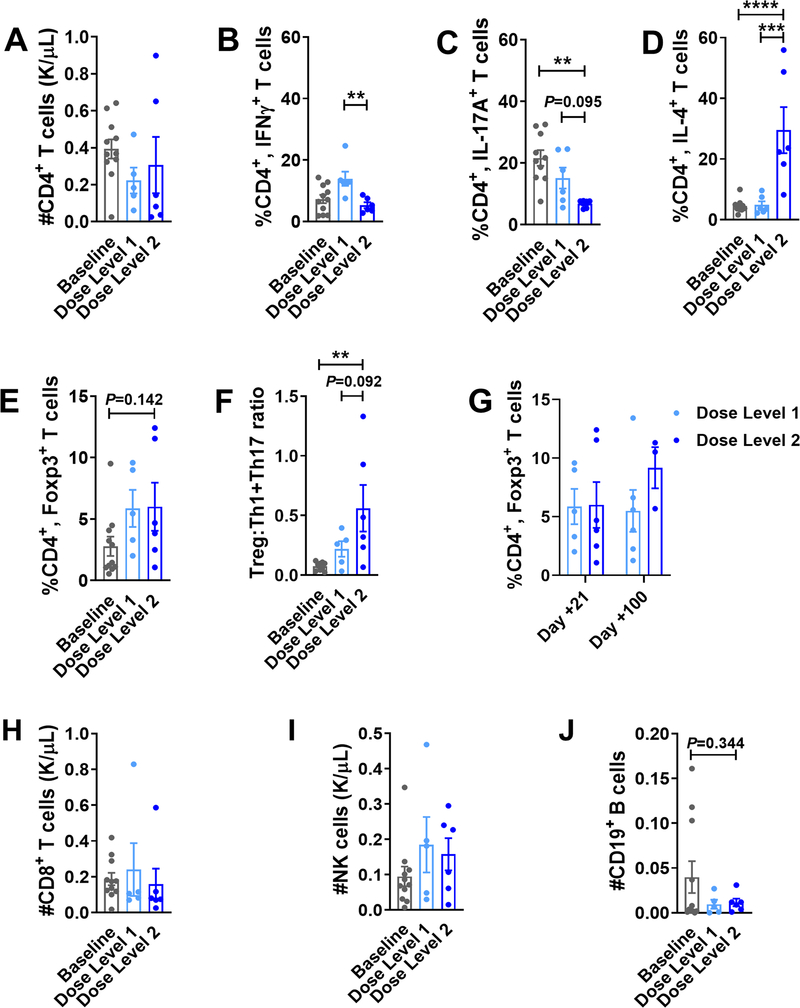
PAC/SIR/TAC at dose level 2 did not impair the reconstitution of CD4+ T cells, but as observed in our xenograft experiments it did significantly reduce the frequency of pathogenic Th1 and Th17 cells at day +21, compared to baseline values or subtherapeutic doses of pacritinib in dose level 1 (Figure 6A–C). Like mice transplanted with allogeneic, CD4+ JAK2-deficient T cells (6), PAC/SIR/TAC dose level 2 resulted in a significant increase in the frequency of Th2 cells (Figure 6D). Further, the percentage of CD4+ Tregs was maintained among patients treated at both dose levels of PAC/SIR/TAC (Figure 6E). Thus, the ratio of immune suppressive Tregs to pathogenic Th1 and Th17 cells was significantly increased among those treated with PAC/SIR/TAC dose level 2, compared to baseline controls (Figure 6F). While the frequency of Tregs at day +21 is similar among patients receiving dose level 1 or 2 of PAC/SIR/TAC, there was trend toward greater Tregs later at day +100 among dose level 2 (Figure 6G).
Figure 6. PAC/SIR/TAC favors Tregs over pathogenic Th1 and Th17 cells.
Bar graphs show the A) total number (mean ± SEM) of CD4+ T cells at day +21 and frequency (mean ± SEM) of B) CD4+, IFNγ+ Th1, C) CD4+, IL-17+ Th17, D) CD4+, IL-4+ Th2, and E) CD4+, CD127neg, CD25+, Foxp3+ Treg cells at day +21 among patients treated on dose level 1 or dose level 2 of the phase I trial. F) Graph depicts the ratio (mean ± SEM) of Treg to Th1 and Th17s at day +21. G) Graph shows Treg reconstitution at day +21 versus day +100 after allo-HCT. Bar graphs show the absolute numbers (mean ± SEM) of (H) CD8+ T cells, (I) NK cells, and (J) B cells at day +21 among patients treated on dose level 1 or dose level 2 of the phase I trial. ANOVA (B-F, J). **P=.001–.01, ***P=.0001–.001, and ****P<.0001. Baseline data were acquired pretransplant between days −30 and −5.
PAC/SIR/TAC maintains the reconstitution of effector T and NK cells
Importantly, dose level 2 of PAC/SIR/TAC preserves the number of circulating CD8+ T cells and NK cells at day +21, compared to baseline and dose level 1 (Figure 6H, I). PAC/SIR permitted NK cell cytolytic function against K562 target cells in vitro, albeit reduced compared to vehicle or ruxolitinib (JAK1/2 inhibitor) controls (Supplemental Figure 4A). However, PAC/SIR fully maintained NK cell proliferation in response to JAK1-dependent IL-2 and IL-15 (Supplemental Figure 4B), while ruxolitinib significantly curtailed NK cell expansion in vitro as previously observed (5,6). The absolute number of peripheral B cells at day +21 from patients treated at PAC/SIR/TAC dose level 2 was not significantly reduced compared to baseline or dose level 1 values (Figure 6J). Thus, while dose level 2 of PAC/SIR/TAC decreased the amount of pathogenic Th1 and Th17s, important effectors needed for GVL and antiviral immunity remained intact after alloHCT. Further, we investigated the impact of JAK2/mTOR inhibition on human T cell responses against recall antigens from infectious pathogens in vitro, including CMV, EBV, Influenza, and tetanus (CEFT) (Supplemental Figure 5A–D). CD4+ T cell proliferation, IFNγ production, and degranulation remained intact when stimulated by CEFT peptides and exposed to PAC/SIR. Conversely, ruxolitinib significantly reduced CD4+ T cell responses to recall antigens compared to PAC/SIR (Supplemental Figure 5A, C). While ruxolitinib and PAC/SIR significantly reduced CD8+ T cell proliferation when stimulated with CEFT, IFNγ production and degranulation remained intact compared to vehicle control (Supplemental Figure 5B, D).
CD4+ T cell Aurora kinase A activation as a GVHD resistance mechanism
Others and we have demonstrated that CD4+ T cell Aurora kinase A is relevant to CD28 signal transduction and its primary signaling element, phosphorylated histone 3 serine 10 (pHser10), is highly expressed by alloreactive CD4+ T cells despite therapeutic doses of sirolimus (20,24,25). While we show that dual JAK2/mTOR inhibition effectively suppresses pSTAT3 and pS6 in CD4+ T cells, respectively, pH3ser10 is left largely unperturbed early after alloHCT (Supplemental Figure 6A). Interestingly, the frequency of CD4+, pH3ser10+ T cells are similar among baseline and dose level 2 treated patients (Supplemental Figure 6A). While we observed a significant decrease is the amount of CD4+, pH3ser10+ T cells between dose levels 1 and 2 of PAC/SIR/TAC, the percentage of Aurora kinase activated T cells was still over 50% (Supplemental Figure 6A). This suggests that sufficient JAK2 and mTOR inhibition may limit the overall activation of the CD4+ T cells but does little to counter aberrant Aurora kinase A activity. Similarly, NSG mice transplanted with human PBMCs still demonstrated high frequencies of CD4+, pH3ser10+ T cells among groups of animals treated with PAC/SIR or S3i/SIR, compared to controls (Supplemental Figure 6B). This identifies the potential for unchecked T cell costimulation via Aurora kinase A as a mechanism for GVHD resistance despite potent inhibition of JAK2 and mTOR.
Discussion
This phase 1 clinical trial is the first investigation to demonstrate that combining a selective JAK2 inhibitor, pacritinib, with sirolimus-based GVHD prophylaxis is safe. Importantly, PAC/SIR/TAC exhibits preliminary activity in GVHD prevention, without post-transplant cytopenias, CMV reactivation, or relapse. In part, pacritinib selectively inhibits pathogenic IL-6, without impairing common gamma chain receptor cytokines, like IL-2 and IL-15, which are critical for immune tolerance, antiviral immunity, and GVL (6). This is distinct from JAK1/2 inhibitors, where ruxolitinib exposure was linked to cytopenias and high rates of CMV reactivation among patients treated for steroid-refractory GVHD (2). Others and we have shown that ruxolitinib broadly impairs the signal transduction of IL-2 and IL-15, reducing the numbers and function of human NK cells, cytotoxic T lymphocytes, and Tregs (3–6). Here we demonstrate that the immune reconstitution of NK cells, effector T cells, and Tregs are unperturbed by pacritinib. Further, the combination of pacritinib and sirolimus significantly increases peripheral Treg suppressive potency.
The decision to combine pacritinib with SIR/TAC is supported by extensive translational investigations (1,6–10). We have refined the use of SIR/TAC in GVHD prevention and developed a strategy to achieve a low incidence of VOD or TMA (7–9). Of the two patients that developed TMA with PAC/SIR/TAC, the severity was mild with no major adverse consequences. In both cases of TMA, the patients improved with either no intervention or discontinuation of tacrolimus. Further, SIR/TAC enhances the immune reconstitution of Tregs and limits severe mucositis compared to tacrolimus plus methotrexate (TAC/MTX) (7–9,26). This is important as our published data in mice show that JAK2-deficient T cells favor a Treg phenotype over pathogenic Th17 cells (6). Our use of SIR/TAC includes a proportionally higher amount of sirolimus to tacrolimus, and that approach has demonstrated efficacy in reducing acute and chronic GVHD compared to TAC/MTX in a randomized controlled trial (7). While not a tacrolimus-free regimen, the limited exposure to tacrolimus in our SIR/TAC (target range 3–7ng/ml) compared to standard TAC/MTX (10–15ng/ml) further assists in Treg accumulation after alloHCT (7). Although CTN 0402, a multicenter randomized comparison of SIR/TAC to TAC/MTX as GVHD prophylaxis, resulted in equivalence with regard to the incidence of chronic GVHD, TAC/SIR was associated with a beneficial increase in the ratio of Tregs to conventional T cells after alloHCT (26,27).
The rationale to combine pacritinib with SIR/TAC was also based on extensive preclinical data showing concurrent inhibition of T cell costimulation via CD28 and cytokine activation from IL-6 synergistically reduced xenogeneic GVHD in mice (10). PAC/SIR/TAC represents our first clinical iteration of this approach, merging mTOR blockade with JAK2 inhibition. The data from this phase I trial provides proof-of-concept that dual JAK2/mTOR inhibition is safe and shows preliminary activity in preventing GVHD. Interestingly, aberrant Aurora kinase A activity appears to be possible route for resistance to PAC/SIR/TAC, primarily by bypassing mTOR inhibition (10,24,28). Aurora kinase A and mTOR share a common signal transduction pathway, mediated by CD28 activation (10,24,28). We have previously shown that dual JAK2/Aurora kinase A inhibition is synergistic using a novel bispecific inhibitor (10). Thus, clinical studies adding small molecule inhibitors of Aurora kinase A and JAK2 may overcome any potential resistance to mTOR blockade and warrant consideration.
There are important distinctions that require discussion to appreciate the nuanced implications of JAK1/2 versus JAK2 inhibition on the immune system post alloHCT. Starting with ruxolitinib, its broad inhibition of common gamma chain receptor cytokines, IL-6, and the p40 cytokines, IL-12 and IL-23, provide very potent suppression over alloreactive T cells (3–6,29). While this successfully achieves initial responses in GVHD therapy, the unbridled activity of ruxolitinib against beneficial Tregs, NK cells, and anti-tumor effectors poses a challenge toward durable allotolerance, antiviral immunity, and disease control. So far, these concerns derived from preclinical studies have primarily emerged as an increased risk for CMV reactivation (2). Importantly, waning complete responses by day 56 of ruxolitinib therapy may be secondary to the cumulative loss of Tregs deprived of IL-2 (2). We previously demonstrated pacritinib alone offers partial protection from alloreactive T cells, compared to ruxolitinib (6). However, the combination of PAC/SIR has enhanced suppression over human T cells in xenogeneic GVHD models and in vitro. We show the combination of PAC/SIR successfully inhibits mTOR and IL-6 signal transduction, reduces pathogenic Th1 and Th17 cells, maintains IL-2 activity in T cells, and enhances peripheral Treg function as well as Treg induction from Tconv. Further, PAC/SIR does not impair donor T cell antileukemia activity.
In conclusion, we demonstrate that PAC/SIR/TAC is safe in GVHD prevention and have identified pacritinib 100mg twice a day as the recommended phase II dose. Our phase II GVHD prevention trial of PAC/SIR/TAC is underway and actively accruing patients. This pending investigation is dedicated to studying the efficacy of PAC/SIR/TAC in GVHD prophylaxis after HLA-matched related or unrelated donor allografts for hematologic malignancies. The phase II experience will more completely address efficacy overall in GVHD prevention and provide an opportunity to examine the impact of individual factors (patient-level differences in variables associated with GVHD risk, impact of serial sirolimus and tacrolimus levels) on GVHD outcomes. Based on the successful completion of the PAC/SIR/TAC phase I and II trial, we look forward to future investigations aimed at eliminating tacrolimus from the combination of PAC/SIR, as we demonstrated in mice. We anticipate that uncoupling PAC/SIR from the calcineurin-inhibitor will permit unconstrained allotolerance by optimizing Treg proportions and function.
Supplementary Material
Translational Relevance:
GVHD remains an important cause of morbidity and mortality after alloHCT. Distinct from broadly acting GVHD prophylaxis, JAK2 inhibition suppresses alloreactive T cells, while sparing Tregs and graft-versus-leukemia (GVL). IL-6 activity via JAK2 and phosphorylated STAT3 in CD4+ T cells is associated with acute GVHD onset. T cell costimulation by CD28 and mTOR activation is also implicated in GVHD pathogenesis. We demonstrate the safety and preliminary efficacy of combined JAK2/mTOR inhibition in a phase I trial of PAC/SIR/TAC. Importantly, we show PAC/SIR/TAC effectively blocks pathogenic IL-6 and CD28 signal transduction and reduces critical T effectors of GVHD. Extensive correlative studies and data from mice show combined JAK2/mTOR blockade mechanistically increases the ratio of STAT5 to STAT3 in CD4+ T cells and promotes the induction of potent Tregs. The favorable safety profile and its impact on immune reconstitution after alloHCT make PAC/SIR/TAC a promising strategy to prevent GVHD.
Acknowledgments:
The Flow Cytometry, USF Comparative Medicine and Vivarium, Analytic Microscopy, Biostatistics, and Tissue Cores at Moffitt/USF were utilized in completing this work. The core facilities are supported partially by the Moffitt Cancer Center Support Grant, P30-CA076292. The University of Minnesota Research Animal Resource and Flow Cytometry Resource were also used to complete this work. Funding: This work was supported by R01 HL133823 (to B.C.B.) and R50 CA211447 (to H.R.L.).
Funding: This work was supported by R01 HL133823 (to B.C.B.) and R50 CA211447 (to H.R.L.).
COI statement: B.C.B. holds a patent (WO2015120436A2) related to CD4+ T cell pSTAT3 as a marker and therapeutic target of acute GVHD. N.J.L., H.R.L., and S.M.S. hold a patent (US7960434B2) related to the composition and use of S3I-201. B.C.B. additionally holds a provisional patent (WO2017058950A1) related to the use of JAK inhibitors for rejection and GVHD prevention. Neither the inventors nor their institutions have received payment related to claims described in the patent. All other authors have no competing financial interests to declare.
REFERENCES:
- 1.Betts BC, Abdel-Wahab O, Curran SA, St Angelo ET, Koppikar P, Heller G, et al. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood 2011;118(19):5330–9 doi 10.1182/blood-2011-06-363408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med 2020;382(19):1800–10 doi 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 3.Parampalli Yajnanarayana S, Stubig T, Cornez I, Alchalby H, Schonberg K, Rudolph J, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol 2015;169(6):824–33 doi 10.1111/bjh.13373. [DOI] [PubMed] [Google Scholar]
- 4.Schonberg K, Rudolph J, Vonnahme M, Parampalli Yajnanarayana S, Cornez I, Hejazi M, et al. JAK Inhibition Impairs NK Cell Function in Myeloproliferative Neoplasms. Cancer Res 2015;75(11):2187–99 doi 10.1158/0008-5472.CAN-14-3198. [DOI] [PubMed] [Google Scholar]
- 5.Curran SA, Shyer JA, St Angelo ET, Talbot LR, Sharma S, Chung DJ, et al. Human Dendritic Cells Mitigate NK-Cell Dysfunction Mediated by Nonselective JAK1/2 Blockade. Cancer Immunol Res 2017;5(1):52–60 doi 10.1158/2326-6066.CIR-16-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts BC, Bastian D, Iamsawat S, Nguyen H, Heinrichs JL, Wu Y, et al. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci U S A 2018;115(7):1582–7 doi 10.1073/pnas.1712452115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pidala J, Kim J, Jim H, Kharfan-Dabaja MA, Nishihori T, Fernandez HF, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica 2012;97(12):1882–9 doi 10.3324/haematol.2012.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pidala J, Kim J, Alsina M, Ayala E, Betts BC, Fernandez HF, et al. Prolonged sirolimus administration after allogeneic hematopoietic cell transplantation is associated with decreased risk for moderate-severe chronic graft-versus-host disease. Haematologica 2015;100(7):970–7 doi 10.3324/haematol.2015.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khimani F, Kim J, Chen L, Dean E, Rizk V, Betts B, et al. Predictors of overall survival among patients treated with sirolimus/tacrolimus vs methotrexate/tacrolimus for GvHD prevention. Bone Marrow Transplant 2017;52(7):1003–9 doi 10.1038/bmt.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betts BC, Veerapathran A, Pidala J, Yang H, Horna P, Walton K, et al. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Sci Transl Med 2017;9(372) doi 10.1126/scitranslmed.aai8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106(8):2912–9 doi 2005–05-2004 [pii] 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younes A, Romaguera J, Fanale M, McLaughlin P, Hagemeister F, Copeland A, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol 2012;30(33):4161–7 doi 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betts BC, Pidala J, Kim J, Mishra A, Nishihori T, Perez L, et al. IL-2 promotes early Treg reconstitution after allogeneic hematopoietic cell transplantation. Haematologica 2017;102(5):948–57 doi 10.3324/haematol.2016.153072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts BC, Sagatys EM, Veerapathran A, Lloyd MC, Beato F, Lawrence HR, et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol 2015;97(4):807–19 doi 10.1189/jlb.5A1114-532RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–8. [PubMed] [Google Scholar]
- 16.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401 e1 doi 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11(8):571–5 doi S1083879105003642 [pii] 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 18.McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984;4(1):116–22. [DOI] [PubMed] [Google Scholar]
- 19.Johnston L, Florek M, Armstrong R, McCune JS, Arai S, Brown J, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone marrow transplantation 2012;47(4):581–8 doi 10.1038/bmt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts BC, Veerapathran A, Beato F, Lawrence HR, Sebti SM, Pidala JA, et al. CD4+ T-Cell STAT3 Activity Is Increased before Acute Graft-Versus-Host Disease Onset and Its Pharmacologic Inhibition Selectively Controls Alloresponses. Blood 2014;124(21):3819- doi 10.1182/blood.V124.21.3819.3819. [DOI] [Google Scholar]
- 21.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc 2011;86(12):1188–91 doi 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palandri F, Palumbo GA, Elli EM, Polverelli N, Benevolo G, Martino B, et al. Ruxolitinib discontinuation syndrome: incidence, risk factors, and management in 251 patients with myelofibrosis. Blood Cancer J 2021;11(1):4 doi 10.1038/s41408-020-00392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betts BC, Veerapathran A, Pidala J, Yu XZ, Anasetti C. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. J Leukoc Biol 2014;95(2):205–13 doi 10.1189/jlb.0313154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furlan SN, Watkins B, Tkachev V, Flynn R, Cooley S, Ramakrishnan S, et al. Transcriptome analysis of GVHD reveals aurora kinase A as a targetable pathway for disease prevention. Sci Transl Med 2015;7(315):315ra191 doi 10.1126/scitranslmed.aad3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehx G, Somja J, Warnatz HJ, Ritacco C, Hannon M, Delens L, et al. Xenogeneic Graft-Versus-Host Disease in Humanized NSG and NSG-HLA-A2/HHD Mice. Front Immunol 2018;9:1943 doi 10.3389/fimmu.2018.01943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014;124(8):1372–7 doi 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gooptu M, Kim HT, Howard A, Choi SW, Soiffer RJ, Antin JH, et al. Effect of Sirolimus on Immune Reconstitution Following Myeloablative Allogeneic Stem Cell Transplantation: An Ancillary Analysis of a Randomized Controlled Trial Comparing Tacrolimus/Sirolimus and Tacrolimus/Methotrexate (Blood and Marrow Transplant Clinical Trials Network/BMT CTN 0402). Biol Blood Marrow Transplant 2019;25(11):2143–51 doi 10.1016/j.bbmt.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol 2007;8(1):64–73 doi 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 29.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 2014;123(24):3832–42 doi 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.