Abstract
The need for highly effective vaccines that induce robust and long-lasting immunity has never been more apparent. However, for reasons that are still poorly understood, immune responses to vaccination are highly variable between different individuals and different populations. Furthermore, vaccine immunogenicity is frequently suboptimal in the very populations who are at most risk from infectious disease, including infants, the elderly, and those living in low-income and middle-income countries. Although many factors have the potential to influence vaccine immunogenicity and therefore vaccine effectiveness, increasing evidence from clinical studies and animal models now suggests that the composition and function of the gut microbiota are crucial factors modulating immune responses to vaccination. In this Review, we synthesize this evidence, discuss the immunological mechanisms that potentially mediate these effects and consider the potential of microbiota-targeted interventions to optimize vaccine effectiveness.
Subject terms: Immunization, Vaccines, Microbiome
This Review discusses evidence from clinical studies and animal models regarding the effects of the gut microbiota on modulating immune responses to vaccination as well as the immunological mechanisms that potentially mediate these effects.
Introduction
Global immunization programmes prevent an estimated 2–3 million deaths per year and markedly reduce disease morbidity1. Vaccines have led to the eradication or near eradication of diseases such as smallpox and polio, reduce disease severity when infection does occur, and can prevent the development of certain cancers, such as cervical cancer, by targeting the causative infectious agent (human papilloma virus). Vaccination also reduces antibiotic usage and therefore antibiotic resistance. For example, pneumococcal conjugate vaccines and oral rotavirus vaccines (ORVs) prevent an estimated 23.8 million and 13.6 million episodes of antibiotic-treated illness, respectively, among children under 5 years of age in low-income and middle-income countries (LMICs) each year2. In fact, vaccination programmes have been so successful that many people, especially those living in high-income countries (HICs) with low burdens of infectious disease, had become complacent to the threats posed by infectious disease. In 2020, the world was shaken from this complacency by the COVID-19 pandemic and the need to rapidly develop a vaccine against SARS-CoV-2 that is highly effective in populations across the globe. Furthermore, effective vaccines against many other serious infectious diseases, including malaria, tuberculosis and HIV-1, are still urgently required.
Vaccines mainly mediate protection by inducing B cells that produce antigen-specific antibodies, although T cells also contribute to the protection mediated by some vaccines, such as the Bacillus Calmette–Guérin (BCG) vaccine for tuberculosis, and are crucial to ensuring the induction of high-affinity antibodies and immune memory3. For reasons that are poorly understood, both B cell and T cell responses to vaccination are highly variable between individuals. For example, antibody titres induced by the inactivated seasonal influenza vaccine can vary ~100-fold between individuals, antibody responses to conjugated pneumococcal vaccines and the Haemophilus influenzae type b (Hib) vaccine vary up to 40-fold, and cytokine recall responses vary up to 10 log-fold in BCG-vaccinated infants4. As we discuss in more detail below, vaccine immunogenicity has frequently been reported to be impaired in individuals living in LMICs compared with those in HICs (Fig. 1). Compared with healthy adults, vaccine immunogenicity is also much poorer in infants5 and in the elderly6. These data suggest that specific host factors that vary between LMICs and HICs and at the extremes of life strongly influence the quality and durability of the immune response to vaccination and therefore vaccine effectiveness, although it should be noted that factors other than immunogenicity can also impact vaccine efficacy. Increasing evidence points to the gut microbiota — which is highly variable between individuals, over the course of life7,8 and between different populations around the world9 (Box 1) — as a crucial factor modulating immune responses to vaccination10. Here, we assess the evidence from clinical cohort studies, interventional studies and animal models that suggests that the gut microbiota plays an important role in modulating vaccine immunogenicity. Data from animal models are strongly supportive of the crucial role of gut microbiota in regulating immune responses to vaccination; however, further work is needed to better define the role of the gut microbiota in modulating vaccine immunogenicity in different human populations.
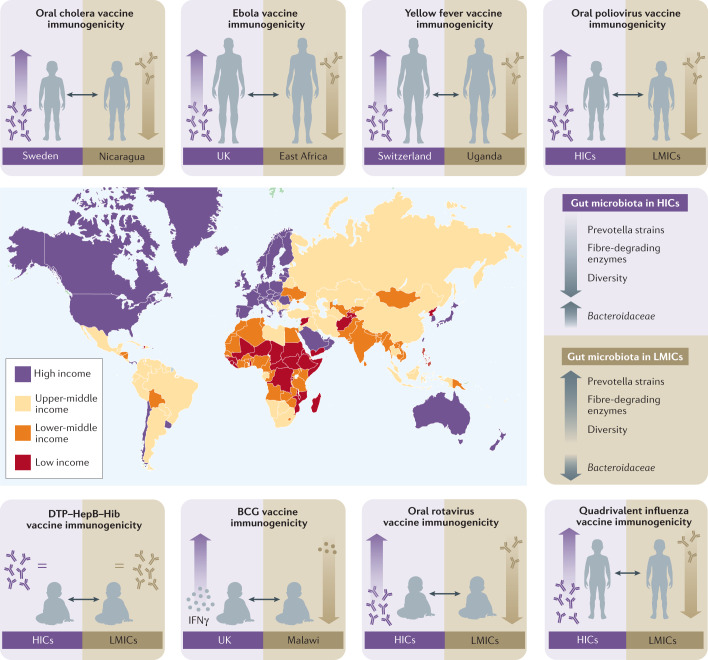
Fig. 1. Differences in the composition and functional capacity of the gut microbiota between low-income and high-income countries correlate with differences in vaccine immunogenicity.
Highlighted are example studies that have compared vaccine immunogenicity in individuals from low-income and middle-income countries (LMICs; red, orange and yellow) to those living in high-income countries (HICs; purple); see Table 1 for further details. The data for oral vaccines having reduced immunogenicity in LMICs are particularly convincing but further work is required to confirm whether responses to parenteral vaccines are impaired in LMICs as many of the reports so far are based on post hoc analyses of independent cohorts. Intriguingly, reported differences in vaccine immunogenicity correlate with differences in the composition and functional capacity of the gut microbiota between these populations. Classifications of income status are based on data from the World Bank, which within the broad category of LMICs, classifies countries as low income (red), lower-middle income (orange) and upper-middle income (yellow). BCG, Bacillus Calmette-Guérin; DTP–HepB–Hib, diphtheria, tetanus, pertussis–hepatitis B virus–Haemophilus influenzae type B; IFNγ, interferon-γ.
Box 1 Variation in the gut microbiota between populations.
Metagenomic studies comparing the composition of the microbiota in stool samples collected from ‘Westernized’ countries (involving urbanization, industrialization and the adoption of high-fat diets) with those from lower-income and less-industrialized countries have found substantial differences9. The gut microbiota of populations from industrialized countries tends to have a lower diversity102 and to be more heavily dominated by Bacteroidaceae. By comparison, populations living a traditional lifestyle (hunter–gatherer and traditional agriculturalist) across Africa, Asia and South America have an increased abundance of the genus Prevotella in their gut microbiota. Additionally, more taxonomically uncharacterized species have also been found to be present in the microbiota of non-Westernized populations, and certain families of bacteria, such as the Spirochaetaceae and Succinivibrionaceae, have been found to be prevalent in the Hadza hunter–gatherer people of Tanzania and other similar populations but are rare or undetected in the gut microbiota from Westernized countries103. In fact, a recent analysis of >9,000 adult metagenomes from 32 different countries identified >200 species-level ‘genome bins’ that were enriched among non-Westernized adult populations. The gut microbiota of children living in non-industrialized settings are also different to those from industrialized countries. For example, Enterobacteriaceae have been found to be significantly more abundant in children from the European Union than in those from a rural setting in Burkina Faso104. Many factors associated with urbanization also differentially affect birth delivery mode, nutrition and medication use, which in turn are known to differentially affect the gut microbiota of infants across urbanization gradients105. It is not just the composition of the gut microbiota that differs between these populations. The microbiota in more traditional populations has a greater encoded functional capacity for the utilization of complex plant carbohydrates as well as many other metabolic functions9,103. These differences in the gut microbiota between populations seem to be predominantly driven by diet and environment rather than genetics, as the microbiota of immigrants from less-industrialized countries who migrate to the USA undergo rapid changes, including a loss of diversity and replacement of Prevotella species with Bacteroidaceae, therefore becoming more similar to Westernized populations106.
Suboptimal vaccine responses in LMICs
For reasons that are incompletely understood, vaccine immunogenicity has frequently been reported to be impaired in individuals from LMICs compared with those living in HICs. The evidence is particularly clear for orally administered vaccines but this also seems to be the case for some licensed and candidate parenteral vaccines.
Oral vaccine immunogenicity
Orally administered vaccines, including ORVs, oral poliovirus vaccine (OPV), and oral vaccines against cholera and typhoid, have consistently been found to be less immunogenic in individuals from LMICs (Table 1) who experience the greatest burden of enteric disease11. For example, average IgA titres in response to ORVs are fourfold lower in infants from LMICs than in those from HICs12. Poorer immunogenicity translates to poorer vaccine efficacy. A recent meta-analysis of all randomized controlled trials of the Rotarix and RotaTeq ORVs found that vaccine efficacy in HICs was 98% after 2 weeks and 94% after 12 months, compared with 66% and 44%, respectively, in LMICs13. Serum vibriocidal antibody titres, which are a correlate of protection in children immunized with oral cholera vaccine, are also significantly lower in children from LMICs14. In addition, close to 100% of children in HICs seroconvert following immunization with OPV compared with only ~70% in LMICs15. Several other oral vaccines, including those against Shigella spp., enterotoxigenic Escherichia coli and Campylobacter jejuni, are currently in development. Trials investigating a live-attenuated Shigella vaccine candidate have shown high levels of immunogenicity in adults in the USA but little or no immunogenicity in infants in Bangladesh16.
Table 1.
Immunogenicity of oral and parenteral vaccines in high-income countries compared with low and middle-income countries
| Vaccine | Populations compared | Differences observed in vaccine immunogenicity | Vaccine immunogenicity (HICs versus LMICs) | Head-to-head comparison? | Same vaccine schedule? | Ref. |
|---|---|---|---|---|---|---|
| ORV | HICs versus LMICs | IgA titres to ORV fourfold lower in infants from LMICs | + | No; review of multiple different studies | Yes | 12 |
| ORV | HICs versus LMICs | Meta-analysis of RCTs of ORV: vaccine efficacy in HICs 94% (after 12 months) compared with 44% in LMICs | + | No; meta-analysis | Yes | 13 |
| Oral cholera vaccine | Sweden versus Nicaragua | Mean IgA titres to CTB 1.6–1.9-fold lower in children in Nicaragua than in children in Sweden; vibriocidal antibody concentrations also much higher in children in Sweden | + | Yes | Yes | 14 |
| OPV | HICs versus LMICs | ~100% of individuals in HICs seroconvert following OPV compared with ~70% in LMICs | + | No; review of multiple different studies | No | 15 |
| Oral Shigella vaccine (candidate) | USA versus Bangladesh | High levels of immunogenicity in adults in the USA but little or no immunogenicity in infants in Bangladesh | + | No | No | 16 |
| DTP–HepB–Hib vaccine | HICs versus Indonesia | Similar levels of immunogenicity for infants in HICs and in Indonesia | = | No | Yes | 17 |
| Quadrivalent influenza vaccine | Europe/Mediterranean, Asia-Pacific and Central America | Higher efficacy in children from HICs (73.4%) with lowest efficacy in LMICs (30.3%) | + | Yes | Yes, but some vaccine strain differences in different regions | 18 |
| RTS,S (malaria vaccine) | Burkina Faso, Ghana, Gabon, Kenya, Tanzania, Malawi and Mozambique | Efficacy after 3 doses ranges from 40% to 77% at 11 different trial sites across 7 African countries | NA | Yes | Yes | 19 |
| YF-17D vaccine | Switzerland versus Uganda | Antigen-specific T cell and neutralizing antibody responses threefold and twofold lower, respectively, in vaccine recipients from Uganda compared with Switzerland | + | Yes | Yes | 20 |
| Ebola vaccine | UK versus Tanzania, Kenya and Uganda | 23% higher antibody titres in vaccine recipients in UK compared with 3 East African countries | + | No; post hoc analysis of data from three phase I trials | Yes | 21 |
| PCV7, PCV10 and PCV13 | HICs versus LMICs | Meta-analysis: higher mean antibody titres in Africa, Southeast Asia and the Western Pacific compared with Europe and the Americas | – | No; meta-analysis | Yes | 22 |
| BCG vaccine | UK versus Malawi | 3 months after BCG vaccination: 100% of infants in the UK had IFNγ response to PPD compared with 53% of infants in Malawi | + | Yes | Yes | 23 |
| HIV-1 vaccine | USA versus Kenya, Rwanda and South Africa | Significantly lower T cell responses in vaccine recipients in East Africa compared with South Africa or the USA | + | Yes | Yes | 24 |
+, increased immunogenicity in HICs; –, decreased immunogenicity in HICs; =, no difference in immunogenicity between HICs and LMICs; BCG, Bacillus Calmette–Guérin; CTB, cholera toxin B subunit; DTP–HepB–Hib, diphtheria, tetanus, pertussis–hepatitis B virus–Haemophilus influenzae type B; HICs, high-income countries; IFNγ, interferon-γ; LMICs, low-income and middle-income countries; NA, not applicable; OPV, oral poliovirus vaccine; ORV, oral rotavirus vaccine; PCV, pneumococcal conjugate vaccine; PPD, Mycobacterium tuberculosis purified protein derivative; RCT, randomized controlled trial; YF-17D, yellow fever 17D.
Antibody and T cell responses to parenteral vaccines
Some parenteral vaccines, such as the combined diphtheria, tetanus, pertussis–hepatitis B virus–Hib (DTP–HepB–Hib) vaccine, seem to have comparable levels of immunogenicity in low-income countries and HICs17. However, a growing number of studies suggest that responses to other licensed and candidate parenteral vaccines could be impaired in individuals from LMICs (Table 1). For example, a recent phase III trial to evaluate the efficacy of an inactivated quadrivalent influenza vaccine over five influenza seasons in children in three geographically diverse regions found that there was a higher level of vaccine efficacy in cohorts from countries with higher socioeconomic status18. The efficacy of the RTS,S malaria vaccine after three doses has been observed to vary from 40% to 77% at 11 trial sites across 7 African countries19, which suggests that vaccine immunogenicity may also vary at a more geographically localized level. B cell responses to the yellow fever 17D (YF-17D) vaccine have also been found to be substantially lower in immunized individuals from Uganda than in those from Switzerland20, and data from three phase I trials of an Ebola candidate vaccine carried out in the UK and three East African countries show that antibody concentrations measured 1 year after immunization were significantly higher in participants from the UK21. Interestingly, whereas most studies suggest that vaccine efficacy is lower in LMICs, a recent systematic review and meta-analysis of antibody responses to 7-valent, 10-valent or 13-valent pneumococcal conjugate vaccines (PCV7, PCV10 or PCV13) found that studies carried out in Africa, Southeast Asia and the Western Pacific reported higher antibody titres than studies carried out in Europe and the Americas22; a potential explanation for these data is not yet clear. Further, more carefully controlled studies are warranted to compare parenteral vaccine immunogenicity in HICs and LMICs as many of the studies outlined here have compared vaccine immunogenicity in independent cohorts or in post hoc analyses of the data.
Increasing evidence also suggests that vaccine-induced T cell immunity could be impaired in individuals in LMICs. Antigen-specific CD8+ T cell responses were markedly lower in individuals from Uganda receiving the YF-17D vaccine than in those from Switzerland20, although these differences could be due to differences in the viral load observed between populations. Furthermore, 3 months after BCG vaccination, 100% of infants in the UK had an interferon-γ (IFNγ) response to the Mycobacterium tuberculosis purified protein derivative, compared with only 53% of infants in Malawi23. T cell responses to a candidate adenovirus vector-based HIV-1 vaccine were lower in trial participants from Kenya than in participants from South Africa or the USA24. These data could have important implications for SARS-CoV-2 vaccines, for which T cell-mediated immunity may be important for protection25.
The role of immune status before immunization
As well as showing substantial geographical variation, vaccine immunogenicity is also significantly lower in infants5 and in the elderly6. For example, influenza vaccine efficacy is estimated to be 70–90% in younger adults compared with 30–50% in those over 65 years of age6,26. Similarly, responses to pneumococcal polysaccharide and HepB vaccines are significantly poorer in the elderly6. Although an in-depth discussion of changes in immune status with ageing is outside the scope of this Review, an individual’s immune status before vaccination is increasingly recognized as being closely linked to how well they respond to vaccination. Immune status at baseline has been found to be predictive of responses to vaccines, including influenza, YF-17D, HepB and malaria vaccines27. Immune status is significantly altered in infancy and in later life28 and is also significantly different between individuals from low-income countries and HICs29. This provides a plausible rationale for the differences in responses to vaccination observed in these populations. Although a broad range of factors have been suggested to alter vaccine immunogenicity and efficacy (Fig. 2), including host factors such as genetics, diet and nutrition, maternal antibodies, prior exposure and other infections, and vaccine-related factors such as the degree of match between vaccine and circulating strains4, increasing evidence suggests that the gut microbiota is an important and targetable factor influencing the baseline immune status and the response to vaccination.
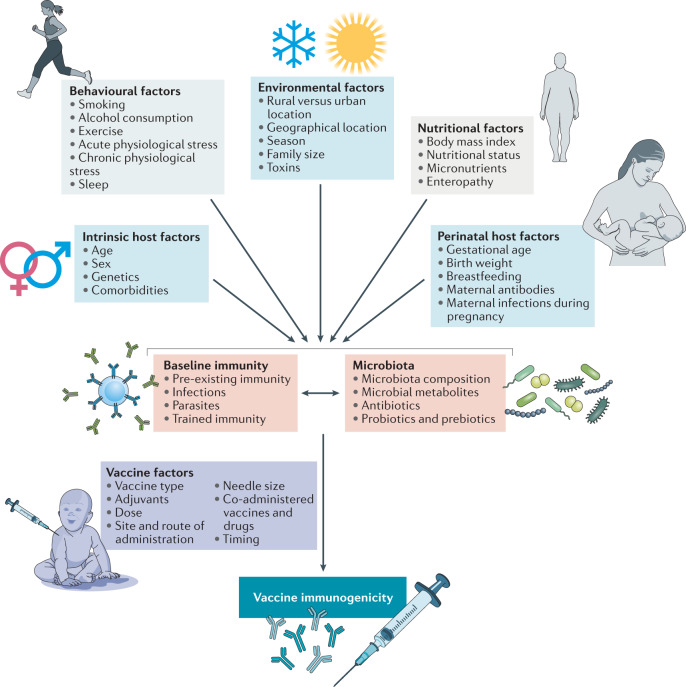
Fig. 2. Factors with the potential to influence vaccine immunogenicity and/or efficacy.
A range of intrinsic host factors (such as age, sex, genetics and comorbidities) and extrinsic factors (such as perinatal, nutritional, environmental and behavioural factors) have been suggested to influence vaccine immunogenicity and/or efficacy (reviewed in detail in ref.4). The influence of these factors on vaccine immunogenicity is likely mediated indirectly via the effects of these factors on baseline immunity and/or the composition of the microbiota. Vaccine immunogenicity is also, of course, dependent on vaccine-intrinsic factors such as the adjuvant used, and vaccine efficacy may be influenced by factors other than vaccine immunogenicity such as the degree of match between the vaccine and the strains circulating at the time.
Microbiota effects on vaccine responses
The composition of the gut microbiota is highly variable between individuals, particularly between Westernized and non-Westernized populations (Box 1). Furthermore, the diversity and stability of the microbiota are highly variable in infancy and decline with ageing, correlating with reduced vaccine immunogenicity (Fig. 3). Given the availability of a range of cost-effective and scalable interventions to modulate the gut microbiota30, including diet, prebiotics and probiotics, the gut microbiota is increasingly being considered an attractive target to enhance vaccine effectiveness in vulnerable populations. In the following sections, we assess the evidence from clinical cohort studies, interventional studies and animal models to indicate that the gut microbiota can modulate immune responses to vaccination.
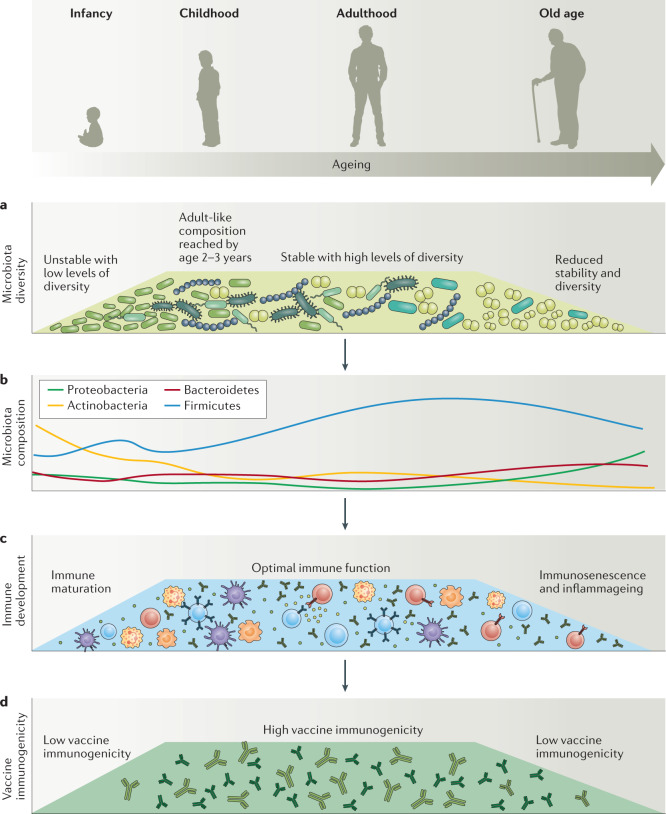
Fig. 3. Differences in the gut microbiota of infants and the elderly compared with that of young adults correlate with altered immune status and suboptimal vaccine immunogenicity.
a | The composition of the gut microbiota in early life is unstable and has low levels of diversity, with a small number of bacterial families tending to dominate. Over time, the diversity of the gut microbiota increases until an adult-like composition is reached between 2 and 3 years of age8. The adult gut microbiota is more complex than in infancy (higher levels of diversity) but is also more homogeneous between individuals and, in the absence of external perturbations (such as antibiotics), is generally quite stable. b | As people age, the diversity and stability of the gut microbiota decline7. There is also an increased relative abundance of the inflammation-associated Proteobacteria and a decrease in Actinobacteria. c | The composition of the gut microbiota can strongly influence immune function and the baseline status of the immune system at the time of vaccination. Baseline immune status has been shown to be predictive of responses to vaccination in several studies27. An in-depth discussion of other factors that influence changes in immune status with ageing is outside the scope of this Review. d | Compared with healthy adults, vaccine immunogenicity is poorer in infants5 and in the elderly6. Increasing data suggest causal links between these phenomena.
Correlative evidence from clinical cohort studies
Associations between the composition of the infant microbiota and responses to vaccination have been reported in several observational clinical cohort studies (Supplementary Table 1), including two studies (one in infants from Ghana31 and one in infants from Pakistan32) that found significant associations between the faecal microbiota and responses to ORVs. In the study in Ghana, the faecal microbiota of vaccine responders (n = 39) was more similar to that of age-matched infants from the Netherlands (n = 154) than to that of vaccine non-responders (n = 39); infants from the Netherlands were all assumed to be vaccine responders as ORV seroconversion rates are >90% in infants from Northern European countries. An increased relative abundance of Streptococcus bovis was significantly correlated with an enhanced response to vaccination, whereas the relative abundances of Bacteroides and Prevotella species were negatively correlated with vaccine responses. The study in infants from Pakistan was smaller (n = 10 per group) and found an increased relative abundance of Gram-negative bacteria, particularly Proteobacteria, in vaccine responders in infants from both Pakistan and the Netherlands. Interestingly, an increased ratio of Enterobacteriaceae to Bacteroides species in vaccine responders was also observed in the study in Ghana31, leading the authors to speculate that lipopolysaccharide (LPS) produced by Gram-negative Enterobacteriaceae could enhance vaccine responses.
Other studies in infants in India and Nicaragua did not find any significant associations between the faecal microbiota and responses to ORVs33,34. Interestingly, however, before correction for multiple statistical comparisons, the Nicaraguan study, which was likely to be significantly underpowered with only 25 infants seroconverting, did identify significant associations between the relative abundances of several genera in the faecal microbiota (including Enterobacteriaceae) and ORV seroconversion. The seroconversion rate in the Indian study was very low (31%) and the authors speculated that all infants might have harboured a microbiota that was inhibitory to rotavirus vaccine replication. A study of responses to OPV in 107 infants in China found that the relative abundance of Bifidobacteria in the infant faecal microbiota was correlated with increased poliovirus-specific IgA responses35. By contrast, another study in infants in India did not find any significant differences in the relative abundances of specific taxa between responders and non-responders to OPV36. However, in both studies, a higher diversity of the microbiota was associated with poorer responses to vaccination. The relative abundance of Bifidobacteria in early infancy has also been found to be significantly associated with CD4+ T cell responses and antibody responses to several parenteral vaccines assessed at 2 years of age37, which indicates that the gut microbiota might also modulate responses to non-orally administered vaccines.
Evidence from interventional studies
Several observational and interventional studies have investigated whether antibiotic-driven perturbations of the gut microbiota lead to altered responses to vaccination. A study analysing a subset of infants enrolled in the Melbourne Infant Study: BCG for Allergy and Infection Reduction (MIS BAIR)38 found that seroprotection rates or antibody concentrations (assessed at 7 and 13 months of age) in response to routine infant immunizations (DTP, HepB, Hib, poliovirus and PCV13 vaccines) were not significantly different in infants exposed to antibiotics before vaccination (the majority of infants received antibiotics in the first week of life). However, the sample size of infants exposed to antibiotics in this study was modest (n = 29). A randomized controlled trial (RCT) investigating the effect of azithromycin on the immunogenicity of OPV in healthy infants found that azithromycin, administered once daily for 3 days before vaccination 11 days later, did not improve vaccine immunogenicity despite reducing biomarkers of environmental enteropathy and the prevalence of pathogenic intestinal bacteria39. Environmental enteropathy is a poorly defined state of intestinal inflammation that is often accompanied by bacterial overgrowth in the small intestine, which has been suggested to lead to impaired oral vaccine immunogenicity40.
Another RCT in adult volunteers (n = 21 per group) found that anti-rotavirus IgA titres were not significantly altered in participants randomized to receive a cocktail of broad-spectrum antibiotics 36 hours before vaccination with an ORV. However, there was an increased proportion of volunteers with a more than twofold increase in anti-rotavirus IgA titre by day 7 after vaccination compared with baseline (which the authors refer to as IgA boosting) in another group treated with vancomycin alone41. Interestingly, consistent with the study investigating the association between the composition of the faecal microbiota and responses to ORVs in infants in Ghana31, an increased ratio of Enterobacteriaceae to Bacteroides species in adult vaccine recipients in this RCT was associated with enhanced IgA boosting.
Recently, a systems vaccinology approach was used to comprehensively assess the impact of broad-spectrum antibiotics on innate and adaptive immune responses to influenza vaccination42. Broad-spectrum antibiotics (vancomycin, neomycin and metronidazole) were administered to healthy young adults (n = 11) before and after vaccination. Antibiotic treatment resulted in a 10,000-fold, although transient, reduction in gut bacterial load and a marked and long-lasting reduction in bacterial diversity. However, there was no significant impact on neutralizing or antigen-binding antibody responses induced by vaccination. To determine whether this was owing to pre-existing immunity, the experiment was repeated with individuals who had low baseline antibody titres against influenza virus. Interestingly, in these participants, antibiotic treatment markedly reduced H1N1-specific neutralization and IgG1 and IgA binding antibody titres. In addition, in both study groups, antibiotic treatment resulted in increased transcriptional signatures of inflammation, which were similarly increased in healthy elderly subjects immunized with seasonal influenza vaccine43, and divergent metabolic trajectories. There was a 1,000-fold reduction in serum levels of secondary bile acids after antibiotic treatment, which was highly correlated with transcriptional signatures of inflammation, suggesting a role for secondary bile acids in suppressing inflammation. Integrative modelling of the multi-omics datasets revealed significant associations between particular bacterial species and metabolic phenotypes. Taken together, these results suggest that antibiotic-driven changes to the gut microbiota can induce major changes in the metabolome, alter inflammatory responses and impair antibody responses to vaccination.
In summary, interventional studies carried out so far suggest that antibiotic-driven changes to the gut microbiota can influence responses to influenza vaccination and possibly to ORVs, although all of the studies are limited by a small sample size and by the fact that antibiotics were typically administered shortly before immunization. Interestingly, antibiotic treatment did not affect responses to the influenza vaccine in recipients with high levels of pre-existing immunity, which is consistent with the limited effect of antibiotics on responses to ORVs in individuals who had high levels of baseline seropositivity41. It is therefore possible that the microbiota has a more marked effect on primary responses to vaccination than on secondary immune responses. Another limitation is that several of the studies so far have assessed the impact of antibiotics on vaccine responses in adults. In mice (see next section), it has been shown that antibiotic exposure in infancy but not in adulthood leads to impaired responses to five parenteral vaccines licenced for infants44, which suggests that alterations to the microbiota in early life may have greater effects on responses to vaccination than alterations in adulthood. This in turn raises the question of what impact antibiotics could have on responses to primary vaccination in neonates and infants. An ongoing clinical study will assess antibody responses to routine infant immunizations in infants directly exposed to antibiotics in the neonatal period or indirectly to maternally administered antibiotics intrapartum in comparison to infants not exposed to antibiotics45.
Evidence from animal models
Data from animal models provide convincing evidence that the gut microbiota plays an important role in modulating immune responses to vaccination. For example, antibiotic-treated and germ-free mice had enhanced IgG and IgA responses to an orally administered mouse rotavirus strain intended to model responses to ORVs46. By contrast, germ-free mice and pups born to dams treated with a cocktail of antibiotics had impaired IgG responses following immunization with the model antigen ovalbumin, although the differences were modest and were only observed when pups were immunized at 7 days of age and not in those immunized later47. More convincingly, a study investigating the impact of the microbiota on antibody responses to the seasonal influenza vaccine found that antibiotic-treated, germ-free or Tlr5–/– adult mice (see section below for further discussion of the relevance of Toll-like receptor 5 (TLR5) signalling) had markedly impaired antibody responses compared with littermate control mice48. A similar effect was also observed in this study when mice were vaccinated with the inactivated poliovirus vaccine; however, antibody responses to several other adjuvanted vaccines (such as the Recombivax HepB vaccine) and live vaccines (such as the YF-17D vaccine) were not impaired in Tlr5–/– mice48. More recently, it was found that mice exposed to antibiotics as infants had significantly impaired antibody responses to five different live or adjuvanted licenced vaccines that are administered to infants worldwide44. These impaired responses could be rescued by faecal microbiota transplantation (FMT) from age-matched untreated mice. The potential for antibiotic-driven dysregulation of the microbiota to influence responses to vaccination has important implications given that, from 2000 to 2015, antibiotic consumption increased by 65% worldwide (from 21.1 billion to 34.8 billion defined daily doses), primarily driven by increased antibiotic usage in LMICs49. Interestingly, responses to the same live or adjuvanted vaccines were not impaired in adult mice treated with antibiotics. These data are consistent with the study discussed above48, which also indicated that the gut microbiota does not play an important role in regulating antibody responses to live or adjuvanted vaccines in adult mice. These data suggest that there is a potential ‘window of opportunity’ in early life, during which the microbiota may have a more marked influence on immune responses to vaccination. This is consistent with other studies that have highlighted a similar ‘window’ for the microbiota to imprint on the immune system in other contexts. For example, in mice, the intestinal microbiota induces a strong immune response at weaning that, if inhibited, can lead to a pathological imprinting that drives disease susceptibility in later life50.
Microbiota and lymphocyte responses
Aside from studies that have directly investigated the role of the microbiota in responses to vaccination, several other studies have highlighted important roles for the gut microbiota in modulating B cell and T cell responses that likely have important implications for the effects of the microbiota on vaccine immunogenicity. For example, two recent preclinical studies have shown that mucosal or systemic microbiota exposure shapes B cell repertoires, which has important implications for antigen-specific vaccine responses51,52. In the first study, adult germ-free mice immunized with Group A Streptococcus (GAS) had significantly reduced B-1 cell clonotypes and serum antibodies specific for an immunodominant cell-wall polysaccharide of GAS51. Colonizing germ-free mice with a conventional microbiota restored these responses. These data could have important implications for infant polysaccharide conjugate vaccines such as PCV13. Indeed, we have previously shown that responses to PCV13 are impaired in mice exposed to antibiotics as infants44 and in germ-free mice (D.J.L. and M.A.L., unpublished observation). The second study showed that sequential exposure to different microorganisms in the intestine of adult germ-free mice led to the attenuation of pre-existing IgA responses to microorganisms encountered previously52. This contrasted with sequential systemic microbial exposures, which led to a diverse IgG repertoire that could efficiently respond to these different encounters. These data may provide an explanation for poor responses to oral vaccines, particularly in settings where there is a high burden of environmental microorganisms in the gut.
So far, most work in this area has focused on the role of the microbiota in modulating vaccine-induced antibody responses. However, T helper cell responses are crucial for optimal B cell responses to vaccination3. Furthermore, T cell-mediated immunity is thought to play a crucial role in the protection induced by some licenced vaccines (such as the yellow fever and BCG vaccines) and candidate vaccines (such as for HIV-1)3. The importance of T cell-mediated immunity to SARS-CoV-2 is also increasingly recognized25 and induction of T cell immunity by a SARS-CoV-2 vaccine may be desirable for long-term protection. Although the ability of the microbiota to substantially modulate T cell responses has been reported in many other contexts53, including regulating responses to influenza virus infection54,55, relatively few studies have directly investigated the effects of the microbiota on T cell responses to vaccination. We have shown that early-life antibiotic exposure in mice leads to increased cytokine production by T cells during recall responses to antigens in a range of infant vaccines44 but it is not known whether this affects vaccine-mediated protective immunity.
Potential mechanisms
The mechanisms by which the microbiota might modulate immune responses to vaccination are currently incompletely understood. Several potential mechanisms have been proposed, including the natural adjuvant hypothesis, the modulation of B cell responses by microbial metabolites and microbiota-encoded epitopes that are cross-reactive with vaccine antigens. Here, we discuss the evidence for and against these potential mechanisms. Most likely, the microbiota can influence vaccine responses in multiple ways. Potential redundancies between the different pathways involved and a dependency on the specific composition of the microbiota in different contexts may explain why it has so far been challenging to fully decipher these mechanisms.
Innate sensing of the microbiota by pattern recognition receptors
One potential way in which the microbiota is likely to modulate vaccine responses is by providing natural adjuvants that enhance responses to vaccination (Fig. 4a). Adjuvants are pharmacological or immunological agents, such as aluminium salts (alum), that function to accelerate, prolong or enhance antigen-specific immune responses. Commonly used vaccine adjuvants directly or indirectly activate antigen-presenting cells, such as dendritic cells (DCs), via pattern recognition receptors (PRRs) such as TLRs or NOD-like receptors (NLRs), which also detect microbial molecules, including those produced by the microbiota56. For example, TLR5-mediated sensing of flagellin produced by the gut microbiota has been shown to be required for optimal antibody responses to the non-adjuvanted influenza vaccine48. Consistent with these data, the expression level of TLR5 on human peripheral blood mononuclear cells correlated with the magnitude of antibody titres in a haemagglutination inhibition assay57. However, in contrast to these data, another study did not find a strong dependency on TLR5 for antibody responses to influenza vaccine or PCV13 in young mice44. The reasons for these different results are not entirely clear but it is possible that differences in the composition of the microbiota play a role. Compared with non-littermate wild-type mice, which had a significantly different gut microbiota, Tlr5–/– mice did have substantially impaired antibody responses to PCV13. Conversely, compared with littermate wild-type mice, which had a similar gut microbiota, antibody responses to PCV13 were not impaired44.
Fig. 4. Potential mechanisms by which the microbiota could modulate vaccine immunogenicity and efficacy.
a | Immunomodulatory molecules produced by the microbiota, such as flagellin and peptidoglycan, have been shown in animal models to modulate vaccine responses by providing natural adjuvants that are sensed by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NOD2, expressed by antigen-presenting cells. Other immunomodulatory molecules, such as lipopolysaccharide, may also similarly modulate responses. PRRs expressed by T cells and B cells may also sense these molecules directly. b | Dendritic cells (DCs) have a crucial role in immune responses to vaccination by presenting vaccine antigens to T cells and secreting immunomodulatory cytokines. The microbiota regulates the production of type I interferons by plasmacytoid DCs (pDCs), which in turn instruct a specific metabolic and epigenomic state in conventional DCs (cDCs) that enhances T cell priming. c | Immunomodulatory metabolites produced by the microbiota, such as short-chain fatty acids (SCFAs), can enhance B cell metabolism to support the energy demands of antibody production and can increase the expression of genes involved in plasma cell differentiation and class switching, potentially altering responses to vaccination. d | Increasing data suggest that the microbiota can encode epitopes that are cross-reactive with pathogen-encoded or vaccine-encoded epitopes. The presence of cross-reactive B cells or T cells could potentially alter the responses to vaccination.
The activation of alternative PRR signalling pathways sensing microbial products other than flagellin might also provide similar adjuvant signals. Consistent with this possibility, the sensing of microbiota-produced peptidoglycan by NOD2 has been shown to be required for optimal responses to intranasal immunization with the model antigen human serum albumin and cholera toxin58. Furthermore, the influence of the microbiota on B-1 cell responses to GAS has been found to depend on MYD88, a key adaptor protein downstream of multiple TLRs51. Further work is needed to assess whether other PRRs can also mediate the influence of the microbiota on vaccine responses. For example, although it is well established that bacterial LPS (sensed via TLR4) can have an adjuvant effect on responses to vaccination56,59, it remains to be shown whether TLR4-mediated sensing of LPS produced by the gut microbiota can modulate vaccine immune responses. This may be further complicated by the fact that different bacterial taxa in the microbiota produce different types of LPS that have varying degrees of immunogenicity60.
The ability of the microbiota to provide natural vaccine adjuvants is likely also dependent on other factors, including the amount of specific immunomodulatory products that are produced and whether these products are confined to the gut or escape into the periphery. Blooms of specific pathobionts, such as members of the LPS-producing Enterobacteriaceae, which frequently overgrow when the gut is inflamed or following antibiotic exposure, may lead to increased levels of LPS in the gut and in the periphery61, thereby influencing responses to vaccines that are administered concurrently with these blooms. The ability of the microbiota to influence the responses to parenteral vaccines is also likely dependent on gut barrier integrity. When the gut epithelial barrier is compromised, for example owing to gut inflammation, malnutrition or antibiotic exposure, increased levels of antigens and immunomodulatory products produced by the gut microbiota are readily detected in the periphery, where they can modulate systemic immune responses62. Furthermore, metabolites produced by specific taxa in the gut microbiota can support gut barrier integrity by upregulating epithelial tight junction proteins63, which suggests that the abundance of specific taxa in the gut microbiota may alter the degree of gut ‘leakiness’ and therefore the amount of microbiota-produced natural adjuvants in the periphery.
Microbiota-mediated reprogramming of antigen-presenting cells
Antigen-presenting cells, such as DCs, play a crucial role in presenting vaccine antigens to T cells and controlling the magnitude, quality and durability of the ensuing immune response. PRRs control the key functions of these cells56 and increasing data indicate that the microbiota can potently modulate DC function, suggesting a potential way in which the microbiota could act as a natural adjuvant to vaccination (Fig. 4b). After intranasal immunization with inactive cholera toxin, TLR-mediated sensing of the microbiota by lung DCs led to the upregulation of the gut-homing receptors α4β7 integrin and CC-chemokine receptor 9 on IgA+ B cells. The migration of these cells from the lung to the gut resulted in protection against oral challenge with cholera toxin64. In germ-free mice receiving intranasal immunization with inactive cholera toxin, the levels of antigen-specific IgA in the gut were significantly reduced. Furthermore, depletion of the microbiota by broad-spectrum antibiotics has been shown to inhibit the TLR-dependent production of total IgA in the lungs of mice and humans in intensive care units, contributing to increased susceptibility to Pseudomonas aeruginosa infection65.
Recently, the microbiota has also been shown to regulate the constitutive production of type I interferon by plasmacytoid DCs66. Type I interferon production by plasmacytoid DCs induces a specific epigenomic and metabolic state in conventional DCs such that they more efficiently prime antigen-specific T cell responses. The microbiota may also have an adjuvant effect on vaccine responses through effects on other antigen-presenting cells. For example, DCs were found to be dispensable for the ability of microbiota-produced flagellin to enhance the antibody responses to the non-adjuvanted influenza vaccine48. Instead, these effects were dependent on macrophages, as macrophage-depleted mice failed to mount a detectable antibody response to the vaccine at 7 days after immunization. The microbiota has also been shown to modulate antigen presentation by intestinal epithelial cells67, which could have implications for immune responses to oral vaccines and, as discussed in more detail below, the microbiota can also exert direct effects on B cells and T cells.
Immunomodulation by microbiota-derived metabolites
In addition to molecules sensed by PRRs, the gut microbiota also produces a large number of metabolites68 that have the potential to modulate immune responses (Fig. 4c). Amongst the best studied of these are short-chain fatty acids (SCFAs), such as acetate, butyrate and propionate, which are the main metabolic end products of bacterial fermentation in the colon. SCFAs have been shown to increase oxidative phosphorylation, glycolysis and fatty acid synthesis in B cells to support the energy demands of optimal homeostatic (non-pathogen-specific) antibody responses and antibody responses to Citrobacter rodentium infection69. This study also showed that SCFAs enhanced the expression of genes involved in plasma cell differentiation and class switching. However, a more recent study reported that SCFAs inhibit rather than enhance antibody responses to intragastrically administered ovalbumin as well as inhibiting autoantibody responses70. Given these conflicting reports, further work is needed to assess the impact of SCFAs on antibody responses to oral and parenteral vaccines.
Aside from SCFAs, the immunomodulatory properties of many other microbiota-derived metabolites, including secondary bile acids and tryptophan metabolites, are increasingly being uncovered. For example, antibiotic treatment has been shown to significantly reduce levels of secondary bile acids, the reduction of which correlated with enhanced inflammatory signatures in humans immunized with the influenza vaccine42. As mentioned earlier, microbiota-derived metabolites may also indirectly regulate immune responses to vaccination by enhancing gut barrier integrity71, thus potentially reducing the escape of microbial molecules that enhance parenteral vaccine responses. Further work is also needed to assess the immunomodulatory effects of these metabolites on other immune cell populations that regulate responses to vaccination such as T cells and DCs. SCFAs have potent effects on T cells in other contexts72 but whether SCFAs can regulate vaccine-induced T cell-mediated immunity remains to be investigated.
Microbiota-encoded cross-reactive antigens
Previous studies in humans have identified CD4+ memory T cells specific for pathogen-encoded antigens in individuals who were not previously infected with those pathogens73. A potential explanation is T cell receptor (TCR) cross-reactivity to environmental antigens, particularly antigens and epitopes encoded by the gut microbiota (Fig. 4d). Circulating and tissue-resident CD4+ T cells with reactivity to the intestinal microbiota are abundant in healthy individuals74 and bioinformatic predictions suggest that there is extensive sharing of the TCR epitope repertoire between the human proteome, the gut microbiota and pathogenic bacteria75 and therefore, presumably, with vaccine-encoded pathogen antigens. Increasing data suggest that the presence of these cross-reactive T cells (and, in some cases, cross-reactive B cells76) can modulate immune responses to pathogens by either dampening or enhancing the immunogenicity of pathogen-associated antigenic epitopes76,77. The presence of cross-reactive T cells at baseline has also been shown to positively correlate with immune responses to the influenza vaccine78. The origin of the cross-reactive T cells was not identified in these studies but other studies have identified T cells that cross-react with both influenza virus-derived peptides and epitopes encoded by taxa in the microbiota73. Recently, cross-reactivity between MHC class I-restricted tumour antigens and epitopes of a bacteriophage protein encoded in the genome of Enterococcus hirae has been reported. Mice colonized with E. hirae harbouring this bacteriophage had improved responses to immunotherapy79. Whether epitopes encoded by the gut microbiota stimulate B cells or T cells that cross-react with vaccine antigens and alter responses to immunization is currently unknown but this potential mechanism by which the gut microbiota could influence vaccine responses is worthy of further investigation.
Targeting the microbiota
Microbiota-targeted interventions, including prebiotics, probiotics, synbiotics, FMT and small-molecule drugs that inhibit specific microbial processes, are being extensively investigated in many contexts30,80. Whereas some of these, such as FMT, are unlikely to be feasible at a population scale or are at very early stages of development, such as microbiota-targeted drugs, others such as probiotics (with or without prebiotics or other dietary interventions) are potentially more attractive interventions to improve vaccine efficacy owing to their safety, cost effectiveness and scalability81. Probiotics are already proving to be effective interventions to prevent diseases such as necrotizing enterocolitis, acute diarrhoea and sepsis82–84. Furthermore, a recent preclinical study has shown that synbiotics can enhance responses to the oral cholera vaccine in mice colonized with the microbiota of non-responder infants85.
A recent systematic review86 reported that only ~50% of the 26 RCTs carried out so far have found a beneficial effect of probiotics on vaccine responses (Supplementary Table 2). However, these trials had several limitations, including small sample sizes (n <50 per group in many studies). Furthermore, differences in the probiotic strains investigated, including their purity and viability, and the timing, duration and dose of administration make these studies difficult to compare directly. Perhaps more importantly, none of these trials specifically recruited participants with a disrupted microbiota (for example, those exposed to antibiotics). Twelve of the 26 RCTs did not report whether or not participants were exposed to antibiotics; 9 specifically excluded these participants and, in those studies that included antibiotic-exposed participants, the sample size was very small. It is highly unlikely that administering probiotics to already well-colonized, healthy infants would have a significant effect on the immune response to vaccination. Well-powered RCTs that assess the beneficial effects of microbiota-targeted interventions in infants with a disrupted microbiota are therefore warranted.
Trials investigating the impact of microbiota-targeted interventions, such as prebiotics and probiotics, on vaccine responses in the elderly have mostly examined responses to the influenza vaccine. These studies found little to no improvement in vaccine responses in elderly individuals supplemented with probiotics87–90; however, the number of participants was small and these studies mostly used probiotics containing Lactobacillus strains, which are not commonly found in the adult gut. Further research is needed to identify adult-adapted strains of probiotics that might be more beneficial as interventions in the elderly. Some studies have suggested that probiotics can unexpectedly delay the re-establishment of a diverse microbiota following antibiotic exposure91, although normal recolonization may not be required for a beneficial effect. For example, administering Bifidobacterium bifidum-based probiotics to antibiotic-treated mice prevented colonization by pathobionts and suppressed inflammation without fully restoring a diverse gut microbiota92. Given the potential effects of gut barrier integrity and of the translocation of microbial products on responses to vaccination, microbiota-targeted interventions may not need to fully restore the gut microbiota to a ‘healthy’ state to be of benefit.
Conclusions and perspectives
The studies discussed above provide strong support for the idea that the gut microbiota modulates both B cell and T cell responses to vaccination, although much further work is required. A growing number of observational studies in infants have identified associations between specific bacterial phyla and families in the gut microbiota and immune responses to vaccination37; however, causal links remain to be proven. The identification of the exact species or strains of bacteria that mediate these effects is crucial to demonstrating these causal links and to elucidating the mechanisms involved. Cohort studies so far have been limited in this regard as they have used 16S rRNA sequencing, which lacks species-specific and strain-specific resolution, rather than shotgun metagenomics. Furthermore, interventional studies, for example, assessing the impact of probiotics or antibiotics on vaccine responses, have so far been significantly underpowered and have provided evidence for only relatively modest effects of the microbiota on vaccine responses41,42,86, although they do suggest a greater impact on antibody responses in humans with low levels of pre-existing immunity and major effects on the metabolome and innate immune responses42,93. Larger, better-powered trials are now needed to expand on these data, particularly trials that assess interventions targeted to the appropriate populations such as infants living in LMICs.
As mentioned above, a broad range of other factors have been suggested to alter vaccine immunogenicity and/or efficacy (Fig. 2), including intrinsic host factors (age, sex and genetics), nutritional factors (diet, body mass index, micronutrients and enteropathy), perinatal factors (breastfeeding, gestational age and intrapartum antibiotics), environmental factors (geographical location, rural versus urban location, family size and season) and other infections4. Most of these factors have also been shown to influence the composition of the gut microbiota and baseline immunity. Deconvoluting these highly complex interdependent relationships in human cohort studies is exceptionally challenging. These complexities necessitate studies that use multi-omic systems vaccinology approaches with appropriate statistical controls for potential confounding factors, similar to those applied recently to investigate the links between the gut microbiota and responses to the influenza vaccine42,93.
Another approach to untangle these interdependent relationships is the use of well-designed preclinical studies, which can control for these factors by tightly regulating the environment and diet, by age-matching and sex-matching, and using littermates. One approach that we are using to establish causal relationships between specific bacterial taxa and vaccine responses is to monocolonize germ-free mice with specific strains of bacteria from the microbiota that have been associated with altered vaccine responses in human cohorts and then assess the immune responses to the same vaccines in these mice. A similar approach has been previously used to identify immunomodulatory taxa in the gut microbiota in other contexts94. These experiments may identify novel probiotic strains that have a disproportionately beneficial impact on vaccine responses and could also be used to screen for more personalized probiotics depending on an individual’s microbiota85. This is important, as one cannot assume that a probiotic that is beneficial in infants in HICs will have the same effect in infants in LMICs, where the composition of the microbiota is likely to be substantially different (Box 1). Vaccine responses are also frequently suboptimal in other vulnerable groups such as the elderly6 or those with obesity95. Both ageing and obesity are associated with a dysregulation of the gut microbiota. Given that elderly individuals and individuals with obesity are at higher risk from COVID-19, interventions that can boost vaccine effectiveness (including SARS-CoV-2 vaccines) in these populations, even modestly, may be highly desirable96.
We have outlined several potential immunological mechanisms by which the microbiota could influence immune responses to vaccination. However, given the sometimes contradictory data in different studies, most of the proposed mechanisms remain as plausible hypotheses rather than established facts. Further work is now needed to more fully elucidate these mechanisms in different contexts. These efforts may be complicated by redundancies between different pathways and potential dependencies between the relative importance of certain mechanisms and the composition of the gut microbiota in specific individuals or populations. A better understanding of how the microbiota regulates vaccine responses in different populations and in different contexts may also inform the use of more tailored population-specific adjuvants to enhance responses to vaccination. Much of the research in this field has so far focused on the gut microbiota and mainly its bacterial component. The microbiota at other sites may also play crucial roles depending on the route of vaccination. For example, NOD2-mediated sensing of the microbiota is required for optimal responses to intranasal immunization with a model antigen58. Furthermore, the composition of the nasal microbiota has been associated with IgA responses to a live attenuated influenza vaccine97. The skin microbiota could also influence immune responses to intradermal vaccines98, which may be increasingly important given the interest in using microneedle arrays for vaccine delivery99. Further work is also needed to investigate the potential of the mycobiome and virome to modulate vaccine responses given our increasing understanding of their contributions to modulating immune responses in general79,100. For example, recent work has suggested that enteric viruses have a greater impact than the bacterial microbiota on responses to OPV36. Finally, it is increasingly recognized that vaccines not only induce specific immunity but can also have potent non-specific effects on immune responses to unrelated infections101. Whether or not the gut microbiota can influence the non-specific effects of vaccines is, to our knowledge, almost completely unexplored. Given the increasing appreciation of the potential importance of these effects, investigations in this area should be prioritized.
Supplementary information
Acknowledgements
This work was supported by grants from the Australian National Health and Medical Research Council (APP1156415 and APP1098429 to D.J.L.) and by an EMBL Australia group leader award (to D.J.L.). Work in B.P.’s laboratory is supported by NIH grants U19AI090023 (to B.P.), U19AI057266 (to R. Ahmed and B.P.), the Sean Parker Cancer Institute, the Soffer endowment, and the Violetta Horton endowment. The authors thank other members of the Lynn laboratory and S. Wesselingh for helpful comments on the manuscript. The authors regret that they were unable to cite all of the relevant literature in this Review owing to space limitations.
Glossary
- Parenteral vaccines
Vaccines administered non-orally by injection into the body.
- Prebiotics
Substrates that are selectively used by the host microbiota, conferring a health benefit.
- Probiotics
Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.
- Secondary bile acids
Metabolites (such as deoxycholic acid and lithocholic acid) that are produced by the metabolism of primary bile acids synthesized in the liver (such as cholic acid and chenodeoxycholic acid) by the gut microbiota.
- Adjuvanted vaccines
Vaccines containing an adjuvant, a substance that enhances the response of the immune system to vaccine antigens.
- Faecal microbiota transplantation
(FMT). The administration of a solution of faecal matter from a donor to a recipient to transfer the donor’s microbiota to the recipient.
- B-1 cell
A specialized innate-like B cell that predominates in fetal and neonatal life.
- Haemagglutination inhibition assay
An assay that uses the ability of some viruses to haemagglutinate (bind) red blood cells in order to titrate the antibody response to viral infection or vaccination.
- Synbiotics
A mixture of live microorganisms (probiotics) and substrates selectively used by host microorganisms (prebiotics) that together confer a health benefit on the host.
Author contributions
All authors contributed to the writing, editing and preparation of this Review.
Competing interests
D.J.L. receives funding from GlaxoSmithKline as part of a collaborative research agreement in this area. B.P. serves on the External Immunology Network of GlaxoSmithKline and is on the scientific advisory board of Medicago. S.C.B. and M.A.L. declare no competing interests.
Footnotes
Peer review information
Nature Reviews Immunology thanks D. Kasper, G. Stefanetti and P. Zimmermann for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41577-021-00554-7.
References
- 1.Andre FE, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008;86:140–146. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewnard JA, Lo NC, Arinaminpathy N, Frost I, Laxminarayan R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. 2020;581:94–99. doi: 10.1038/s41586-020-2238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegrist, C. A. Vaccine immunology. in Vaccines (eds Plotkin, S. A., Orenstein, W. A. & Offit, P. A.) 17–36 (Elsevier Inc, 2008).
- 4.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019;32:e00084–18. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PrabhuDas M, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat. Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 6.Ciabattini A, et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin. Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 7.DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28:180–189. doi: 10.1016/j.chom.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Backhed F, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Pasolli E, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176:649–662. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynn DJ, Pulendran B. The potential of the microbiota to influence vaccine responses. J. Leukoc. Biol. 2017;103:225–231. doi: 10.1189/jlb.5MR0617-216R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker EP, et al. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018;13:97–118. doi: 10.2217/fmb-2017-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel M, et al. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J. Infect. Dis. 2013;208:284–294. doi: 10.1093/infdis/jit166. [DOI] [PubMed] [Google Scholar]
- 13.Clark A, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect. Dis. 2019;19:717–727. doi: 10.1016/S1473-3099(19)30126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallander HO, et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002;21:138–145. doi: 10.1016/S0264-410X(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 15.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev. Infect. Dis. 1991;13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 16.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat. Rev. Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rusmil K, et al. The immunogenicity, safety, and consistency of an Indonesia combined DTP-HB-Hib vaccine in expanded program on immunization schedule. BMC Pediatr. 2015;15:219. doi: 10.1186/s12887-015-0525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dbaibo G, et al. Quadrivalent influenza vaccine prevents illness and reduces healthcare utilization across diverse geographic regions during five influenza seasons: a randomized clinical trial. Pediatr. Infect. Dis. J. 2020;39:e1–e10. doi: 10.1097/INF.0000000000002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rts SCTP. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11:e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muyanja E, et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J. Clin. Invest. 2014;124:3147–3158. doi: 10.1172/JCI75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasin C, et al. Dynamics of the humoral immune response to a prime-boost Ebola vaccine: quantification and sources of variation. J. Virol. 2019;93:e00579–19. doi: 10.1128/JVI.00579-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe YJ, Blatt DB, Lee HJ, Choi EH. Associations between geographic region and immune response variations to pneumococcal conjugate vaccines in clinical trials: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;92:261–268. doi: 10.1016/j.ijid.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Lalor MK, et al. Population differences in immune responses to Bacille Calmette-Guerin vaccination in infancy. J. Infect. Dis. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden LR, et al. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann. Intern. Med. 2016;164:313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox RJ, Brokstad KA. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat. Rev. Immunol. 2020;20:581–582. doi: 10.1038/s41577-020-00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 27.Tsang JS, et al. Improving vaccine-induced immunity: can baseline predict outcome? Trends Immunol. 2020;41:457–465. doi: 10.1016/j.it.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill DL, et al. Immune system development varies according to age, location, and anemia in African children. Sci. Transl Med. 2020;12:eaaw9522. doi: 10.1126/scitranslmed.aaw9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wargo JA. Modulating gut microbes. Science. 2020;369:1302–1303. doi: 10.1126/science.abc3965. [DOI] [PubMed] [Google Scholar]
- 31.Harris VC, et al. The infant gut microbiome correlates significantly with rotavirus vaccine response in rural Ghana. J. Infect. Dis. 2017;215:34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris V, et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2018;9:93–101. doi: 10.1080/19490976.2017.1376162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker EPK, et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine. 2018;36:264–272. doi: 10.1016/j.vaccine.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fix J, et al. Association between gut microbiome composition and rotavirus vaccine response among Nicaraguan infants. Am. J. Trop. Med. Hyg. 2020;102:213–219. doi: 10.4269/ajtmh.19-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao T, et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines. 2020;5:47. doi: 10.1038/s41541-020-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Praharaj I, et al. Influence of nonpolio enteroviruses and the bacterial gut microbiota on oral poliovirus vaccine response: a study from South India. J. Infect. Dis. 2019;219:1178–1186. doi: 10.1093/infdis/jiy568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huda MN, et al. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics. 2019;143:e20181489. doi: 10.1542/peds.2018-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann P, et al. Biological sex influences antibody responses to routine vaccinations in the first year of life. Acta. Paediatr. 2020;109:147–157. doi: 10.1111/apa.14932. [DOI] [PubMed] [Google Scholar]
- 39.Grassly NC, et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double-blind randomised placebo-controlled trial in seronegative Indian infants. Lancet Infect. Dis. 2016;16:905–914. doi: 10.1016/S1473-3099(16)30023-8. [DOI] [PubMed] [Google Scholar]
- 40.Gilmartin AA, Petri WA., Jr. Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015;370:20140143. doi: 10.1098/rstb.2014.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris VC, et al. Effect of antibiotic-mediated microbiome modulation on rotavirus vaccine immunogenicity: a human, randomized-control proof-of-concept trial. Cell Host Microbe. 2018;24:197–207. doi: 10.1016/j.chom.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Hagan T, et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178:1313–1328. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakaya HI, et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015;43:1186–1198. doi: 10.1016/j.immuni.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynn MA, et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23:653–660. doi: 10.1016/j.chom.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 45.The Australian New Zealand Clinical Trials Registry (ANZCTR). A clinical study to determine whether antibiotic-driven dysbiosis is associated with impaired vaccine responses in infants. ANZCTRhttps://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373071&isReview=true (2017).
- 46.Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J. Infect. Dis. 2014;210:171–182. doi: 10.1093/infdis/jiu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamousé-Smith ES, Tzeng A, Starnbach MN. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS ONE. 2011;6:e27662. doi: 10.1371/journal.pone.0027662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh JZ, et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein EY, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl Acad. Sci. USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al Nabhani Z, et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity. 2019;50:1276–1288. doi: 10.1016/j.immuni.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 51.New JS, et al. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity. 2020;53:172–186. doi: 10.1016/j.immuni.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature. 2020;584:274–278. doi: 10.1038/s41586-020-2564-6. [DOI] [PubMed] [Google Scholar]
- 53.Kageyama T, Matsuo T, Kurakake R, Sano T. Relationship between T cells and microbiota in health and disease. Prog. Mol. Biol. Transl Sci. 2020;171:95–129. doi: 10.1016/bs.pmbts.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Abt MC, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichinohe T, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georg P, Sander LE. Innate sensors that regulate vaccine responses. Curr. Opin. Immunol. 2019;59:31–41. doi: 10.1016/j.coi.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Nakaya HI, et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D, et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 2016;22:524–530. doi: 10.1038/nm.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vatanen T, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh R, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019;10:89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruane D, et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J. Exp. Med. 2016;213:53–73. doi: 10.1084/jem.20150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robak OH, et al. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. J. Clin. Invest. 2018;128:3535–3545. doi: 10.1172/JCI97065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaupp L, et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell. 2020;181:1080–1096. doi: 10.1016/j.cell.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 67.Koyama M, et al. MHC Class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity. 2019;51:885–898. doi: 10.1016/j.immuni.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bittinger K, et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol. 2020;5:838–847. doi: 10.1038/s41564-020-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez HN, et al. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020;11:60. doi: 10.1038/s41467-019-13603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garrett WS. Immune recognition of microbial metabolites. Nat. Rev. Immunol. 2020;20:91–92. doi: 10.1038/s41577-019-0252-2. [DOI] [PubMed] [Google Scholar]
- 73.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hegazy AN, et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology. 2017;153:1320–1337. doi: 10.1053/j.gastro.2017.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bremel RD, Homan EJ. Extensive T-cell epitope repertoire sharing among human proteome, gastrointestinal microbiome, and pathogenic bacteria: implications for the definition of self. Front. Immunol. 2015;6:538. doi: 10.3389/fimmu.2015.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams WB, et al. HIV-1 VACCINES. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349:aab1253. doi: 10.1126/science.aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carrasco Pro S, et al. Microbiota epitope similarity either dampens or enhances the immunogenicity of disease-associated antigenic epitopes. PLoS ONE. 2018;13:e0196551. doi: 10.1371/journal.pone.0196551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkinson TM, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 79.Fluckiger A, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369:936–942. doi: 10.1126/science.aax0701. [DOI] [PubMed] [Google Scholar]
- 80.Cully M. Microbiome therapeutics go small molecule. Nat. Rev. Drug Discov. 2019;18:569–572. doi: 10.1038/d41573-019-00122-8. [DOI] [PubMed] [Google Scholar]
- 81.Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 82.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid. Based Child. Health. 2014;9:584–671. doi: 10.1002/ebch.1976. [DOI] [PubMed] [Google Scholar]
- 83.Szajewska H, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children – a 2019 update. Aliment. Pharmacol. Ther. 2019;49:1376–1384. doi: 10.1111/apt.15267. [DOI] [PubMed] [Google Scholar]
- 84.Panigrahi P, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407–412. doi: 10.1038/nature23480. [DOI] [PubMed] [Google Scholar]
- 85.Di Luccia B, et al. Combined prebiotic and microbial intervention improves oral cholera vaccination responses in a mouse model of childhood undernutrition. Cell Host Microbe. 2020;27:899–908. doi: 10.1016/j.chom.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmermann P, Curtis N. The influence of probiotics on vaccine responses – a systematic review. Vaccine. 2018;36:207–213. doi: 10.1016/j.vaccine.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 87.Maruyama M, et al. The effects of non-viable Lactobacillus on immune function in the elderly: a randomised, double-blind, placebo-controlled study. Int. J. Food. Sci. Nutr. 2016;67:67–73. doi: 10.3109/09637486.2015.1126564. [DOI] [PubMed] [Google Scholar]
- 88.Akatsu H, et al. Lactobacillus in jelly enhances the effect of influenza vaccination in elderly individuals. J. Am. Geriatr. Soc. 2013;61:1828–1830. doi: 10.1111/jgs.12474. [DOI] [PubMed] [Google Scholar]
- 89.Bunout D, et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. JPEN J. Parenter. Enteral Nutr. 2004;28:348–354. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
- 90.Boge T, et al. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 91.Kotler E, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–1423. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 92.Ojima MN, et al. Bifidobacterium bifidum suppresses gut inflammation caused by repeated antibiotic disturbance without recovering gut microbiome diversity in mice. Front. Microbiol. 2020;11:1349–1349. doi: 10.3389/fmicb.2020.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulendran B. Immunology taught by vaccines. Science. 2019;366:1074–1075. doi: 10.1126/science.aau6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geva-Zatorsky N, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168:928–943. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422–4429. doi: 10.1016/j.vaccine.2015.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ledford H. How obesity could create problems for a COVID vaccine. Nature. 2020;586:488–489. doi: 10.1038/d41586-020-02946-6. [DOI] [PubMed] [Google Scholar]
- 97.Salk HM, et al. Taxa of the nasal microbiome are associated with influenza-specific IgA response to live attenuated influenza vaccine. PLoS ONE. 2016;11:e0162803. doi: 10.1371/journal.pone.0162803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen YE, Fischbach MA, Belkaid Y. Skin microbiota-host interactions. Nature. 2018;553:427–436. doi: 10.1038/nature25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim E, et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeung F, et al. Altered immunity of laboratory mice in the natural environment is associated with fungal colonization. Cell Host Microbe. 2020;27:809–822. doi: 10.1016/j.chom.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aaby P, et al. The non-specific and sex-differential effects of vaccines. Nat. Rev. Immunol. 2020;20:464–470. doi: 10.1038/s41577-020-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Segata N. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr. Biol. 2015;25:R611–R613. doi: 10.1016/j.cub.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 103.Smits SA, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science. 2017;357:802–806. doi: 10.1126/science.aan4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brushett S, Sinha T, Reijneveld SA, de Kroon MLA, Zhernakova A. The effects of urbanization on the infant gut microbiota and health outcomes. Front. Pediatr. 2020;8:408. doi: 10.3389/fped.2020.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vangay P, et al. US immigration westernizes the human gut microbiome. Cell. 2018;175:962–972. doi: 10.1016/j.cell.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.