Abstract
Background
The purpose of this study is to update a previous critical review of adverse events in pregnant and postpartum populations.
Methods
The following databases were searched: PubMed, CINAHL, Index to Chiropractic Literature, Cochrane Database of Systematic Reviews/Cochrane Central Register of Controlled Trials and MEDLINE. We included all study design types as it was determined a priori that there would not be enough high-quality research on spinal manipulative therapy (SMT) in these populations to make any determinations. The Scottish Intercollegiate Guidelines Network (SIGN) and CARE (CAse REport) checklists were used for quality rating.
Results
This update found one case study that demonstrated a serious adverse event in the cervical spine following SMT and a handful of minor and transient adverse events in the low back following SMT.
Conclusions
There was limited evidence of adverse events following SMT in these populations. Although we are calling for improved reporting of such events in future studies, it may be that such injuries are rare.
Keywords: chiropractic, spinal manipulative therapy, manual therapy, pregnancy, postpartum, adverse events
Abstract
Contexte
La présente étude vise à mettre à jour les résultats d’un examen critique des effets défavorables des manipulations vertébrales chez la femme enceinte et la femme en postpartum.
Méthodologie
On a interrogé les bases de données suivantes : PubMed, CINAHL, Index to Chiropractic Literature, Cochrane Database of Systematic Reviews/ Cochrane Central Register of Controlled Trials et MEDLINE. On a retenu toutes les études parce qu’il avait été établi antérieurement que le nombre de recherches de bonne qualité sur les manipulations vertébrales (MV) chez la femme enceinte et la femme en postpartum était insuffisant pour trancher toute question. On s’est servi des listes de vérification Scottish Intercollegiate Guidelines Network (SIGN) et CARE (CAse REport) pour évaluer la qualité des études.
Résultats
Une étude de cas faisait état d’un grave effet indésirable à la colonne cervicale après des MV et d’une poignée d’effets indésirables mineurs et transitoires à la colonne lombaire.
Conclusions
Il existe peu de preuves que les MV ont des effets indésirables chez les populations à l’étude. Il faudrait plus de données. Mais il est permis d’affirmer que ces effets indésirables sont rares.
MOTS CLÉS: chiropratique, manipulations vertébrales, grossesse, postpartum, effets indésirable
Introduction
Musculoskeletal pain is a frequent complaint during pregnancy and the postpartum period. Low back pain (LBP), pelvic girdle pain (PGP), carpal tunnel syndrome, and mid-back pain are common complaints in these groups, with LBP being the most common complaint among pregnant women. The prevalence of low back pain during pregnancy has been reported as up to 90% of pregnant women1–6 and may continue into the postpartum period with up to 75% of women reporting symptoms six months following birth7–12 and approximately 8–20% still suffering from pregnancy-related pain two to three years after giving birth13. Both pregnant and postpartum women have described the back pain as moderate, severe or disabling1, 7 and interfering with life in general; interrupting activities of daily living, sleep and child rearing1, 8, 13, 14. Unfortunately, many primary health care providers consider pregnancy-related back pain to be a normal and unavoidable occurrence15–17 and patients often receive little or no treatment suggestions to manage their condition18,19. The etiology of pregnancy-related back pain is unknown.17, 20 It has been suggested that causation is multifactorial and some of the proposed mechanisms include, but are not limited to, maternal weight gain, biomechanical changes due to pregnancy17, 21, changes in abdominal musculature to accommodate the growing fetus22–24 and/ or increased circulating relaxin25 producing ligamentous laxity26. In general, women are more susceptible to increases in joint laxity than men.27, 28 It has been suggested that hormonal changes may be responsible for these differences.29–31 By the twelfth week of pregnancy production of the hormone relaxin is increased and “relaxes” the joints and ligaments for labour and delivery of the baby through the vaginal canal.32,33 This change in hormonal milieu does not dissipate upon delivery and it is suggested that women immediately postpartum may continue to experience hormone-mediated ligament laxity. It is important to note that this increase in ligament laxity is not targeted just at the pelvis34 thereby making these women more susceptible to various musculoskeletal injuries during this time.
Low back pain (LBP)35, neck pain36–38 and headaches39 are significant causes of pain and disability in the non-pregnant population. Approximately 80% of the population experience at least one episode of LBP in their lifetime35, 30–50% experience neck pain in a given year40 and approximately 50% of people will experience a headache within the last year41. One effective treatment option for patients experiencing any of these pains includes spinal manipulative therapy (SMT)42–47; whereby a localized force of high velocity and low amplitude (HVLA) is applied in the direction of the spinal segment. In the non-pregnant population, severe adverse events following SMT are rare48–53 with most events being reported in lower level of evidence studies such as case reports or case series54, 55. It is noteworthy that there are published case reports describing vertebral artery dissection and stroke following manipulation in the non-pregnant population.52 However, most cases of extracranial vertebral artery dissections are thought to occur spontaneously in individuals with other risk factors such as connective tissue disorders, migraine, hypertension or vessel abnormalities.52 At this time, the current evidence does not find excess risk for vertebral artery dissection from individuals seeking care from chiropractors compared to primary care.52, 56
Effective treatment options for pregnancy or postpartum related-back pain are not well known.57–59 There are few well designed randomized controlled trials60–62 (RCTs) investigating chiropractic care on pregnancy and postpartum-related spine pain, with most of the current evidence for this population being case studies. Although chiropractors report seeing pregnant and postpartum patients regularly59, 63, the lack of evidence for these two populations is surprising given the impact pain can have on a woman’s life during these time periods. Similarly, there is little information regarding the safety of treatment options, such as SMT, in these populations. Given the coagulability status64, 65 of these women and the plethora of hormonal and biomechanical changes that occur as a result of pregnancy and into the postpartum period, it is possible that some treatment options, such as SMT, may be contraindicated in these populations.
Our 2012 critical review of the literature identified four case reports50, 51, 66, 67 and one prospective observational cohort study68 reporting adverse events in seven individuals (five pregnant and two postpartum) following SMT69. Events ranged from minor pain following treatment, to fracture, stroke and epidural hematoma. This is an update of that previous paper and our aim is to systematically review the literature for any reported cases of iatrogenic injuries following SMT and other manual treatments.
Methods
Similar to our first review69, in this updated review we determined a priori that limiting our review to systematic reviews (SR) and RCTs would exclude valuable information regarding adverse events, so cohort and case reports were included. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) and was registered with PROSPERO (no. CRD42019048918).
Literature search parameters
A literature search strategy (Appendix 1) was developed in collaboration with a health sciences librarian (KM). The following items were considered in developing the strategy:
Participants/Population
Women who were either pregnant or postpartum (up to 6 weeks after birth) with spine and/or pelvic girdle musculoskeletal complaints.
Intervention
The interventions examined included spinal SMT and any other manual therapies performed by chiropractors, osteopaths and physiotherapists; as the latter two can deliver similar treatment plans to pregnant women, these terms were also included.57, 58, 70
Comparators
There were no restrictions for the comparison group which may include: active treatments (such as exercise), placebos/shams, usual obstetric care (UOBC) or no treatments.
Outcomes
The presence of adverse events/iatrogenic injuries.
Search strategy
The following databases were used in the search strategy: PubMed, CINAHL, Index to Chiropractic Literature, Cochrane Database of Systematic Reviews/Cochrane Central Register of Controlled Trials and MEDLINE. Search terms consisted of subject headings specific to each database (i.e. MeSH in MEDLINE) and free text words relevant to pregnancy, postpartum, low back pain, pelvic girdle pain, chiropractic, etc. Publications in the search were restricted to the English language and from the date of our last review (October 2011) until November 2018. An additional search strategy was employed when reviewing systematic reviews (SR). Similar to Hawk et al.71 and others46, two investigators (CAW and SW) searched each included SR for eligible studies not identified through the formal search. Any that were deemed potentially acceptable were added to the list of studies to be analyzed.
Screening
Titles were screened independently by two reviewers (SW and CAW). Disagreements on eligibility were resolved by discussion. The same two investigators reviewed the abstracts and articles. If there was disagreement between the reviewers, a third investigator also reviewed (KS) either the abstract or full-text article and the majority rating was used following a group discussion. Studies of unacceptable quality were excluded from the evidence tables.
Eligibility criteria
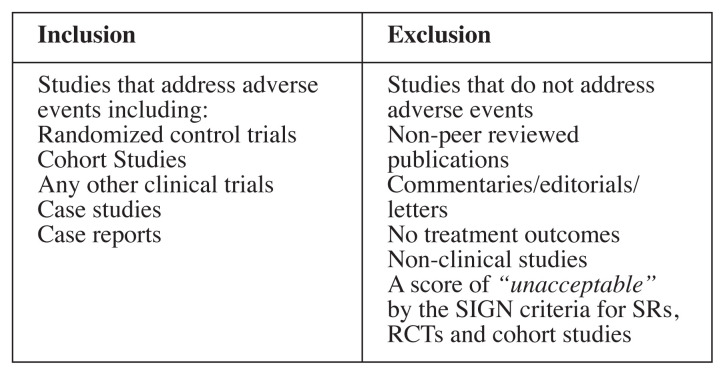
The eligibility criteria for articles in the search can be found in Figure 1.
Figure 1.
Inclusion and exclusion criteria
Evaluation of risk of bias
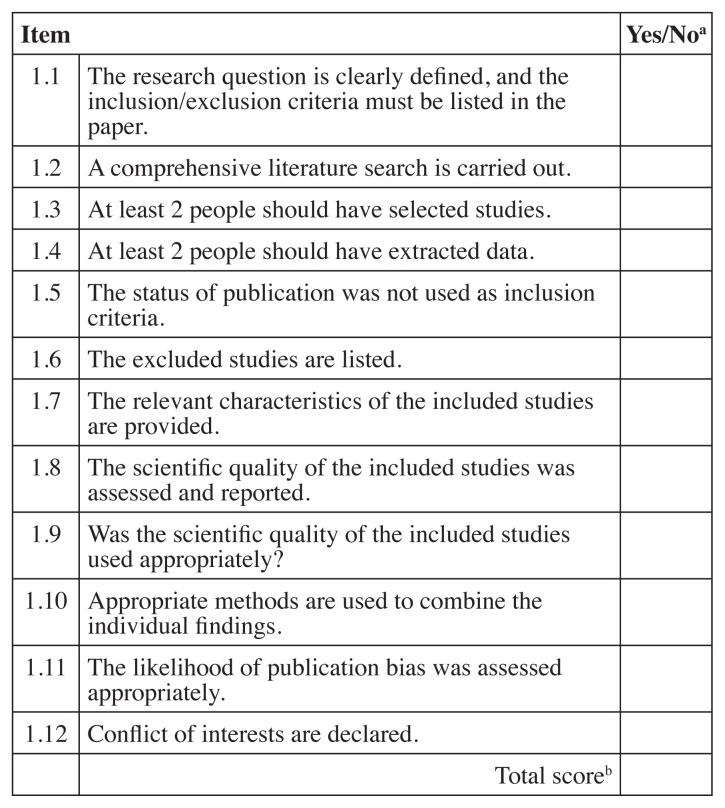
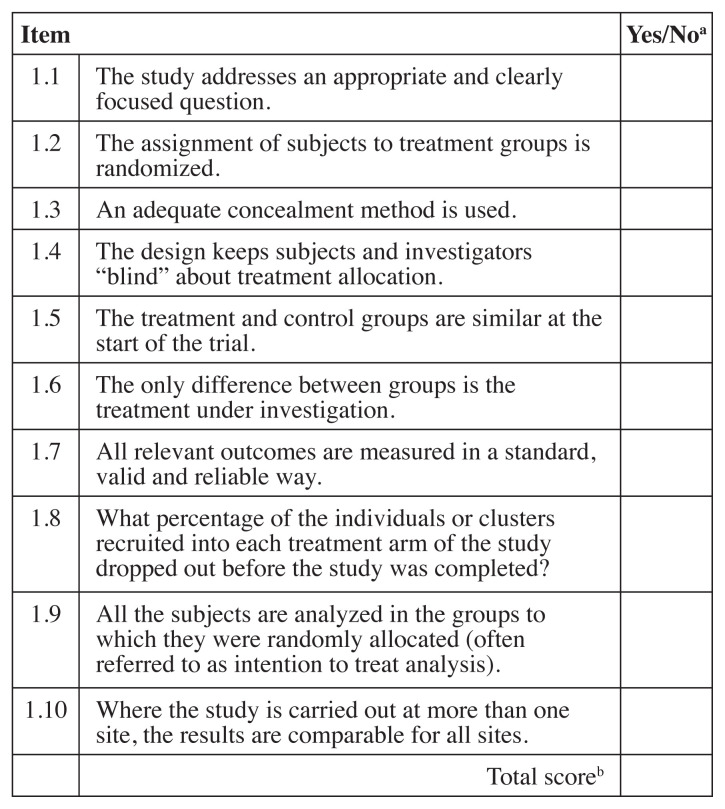
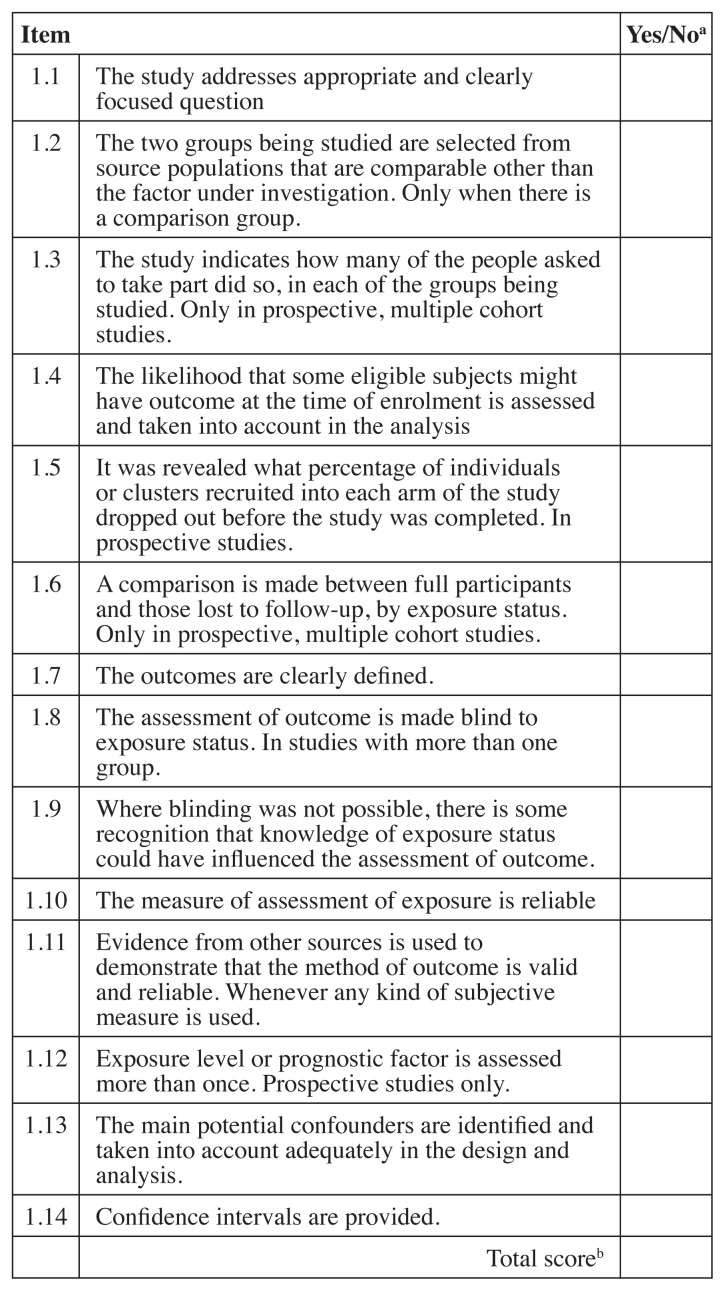
As previously performed by Hawk et al.71 and others57, 58 the Scottish Intercollegiate Guideline Network (SIGN) checklists were used to evaluate systematic reviews/meta-analyses72 (both abbreviated as “SR”) and cohort studies73 and a modified SIGN checklist was used to review RCTs71, 74. The modified SIGN RCT checklist combined information from the original checklist about concealment and blinding of the investigators, and it added three other items including patient blinding, sample size justification and if the required sample same size was reached (items 3, 4, 5 and 9). Unlike the original SIGN RCT checklist74, the modified one did not take into consideration dropouts or compare results from different sites71. Two of the original The SIGN checklists score each article as “high quality, low risk of bias”, “acceptable quality, moderate risk of bias”, “low quality, high risk of bias” or “unacceptable” quality. Any studies that were scored as “unacceptable” quality were removed from further analysis. Each level was defined by scoring the checklists and assigning a value of “1” for each “yes” response. Figures 2, 3 and 4 list the items in each checklist and explain the scoring system used to determine quality rating.
Figure 2.
SIGN checklist for systematic review72
SIGN – Scottish Intercollegiate Guideline Network
aRating: “Yes” = 1, “No” or unable to tell from the article = 0
bScoring: Sum of items - >9 high quality, low risk of bias; 6–9 acceptable quality, moderate risk of bias; <6 low quality, high risk of bias; if 1 and/or 3 are “no” Unacceptable quality (reject)
Figure 3.
Modified SIGN Randomized controlled trial checklist74
SIGN – Scottish Intercollegiate Guideline Network
aRating: “Yes” = 1, “No” or unable to tell from the article = 0
bScoring: Sum of items - 9–10 high quality, low risk of bias; 6–8 acceptable quality, moderate risk of bias; 3–5 low quality, high risk of bias; 0–2 or if item 1 and/or 3 are “no” unacceptable quality (reject)
Figure 4.
SIGN Cohort study checklist73
SIGN – Scottish Intercollegiate Guideline Network
aRating: “Yes” = 1, “No” or unable to tell from the article = 0
bScoring: Sum of items – 12–14 high quality, low risk of bias;
9–11 acceptable, moderate risk of bias; 6–8 low quality, high risk of bias; <6 unacceptable quality.
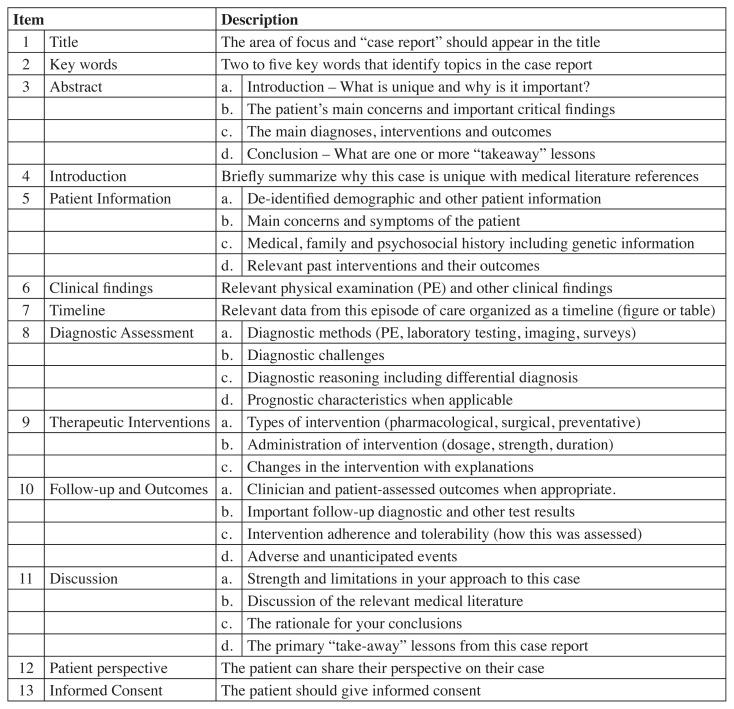
For case reports, the CARE (CAse REport) checklist for case reports was employed.75 The CARE checklist evaluates 13 main areas over 30 specific items (Figure 5). Although there is no scoring system for this checklist, we decided a priori that each item would be worth “1” and a high score would indicate a more robust case report. A consensus-based decision between reviewers on whether the internal validity of the case reports was acceptable for inclusion in the current review.
Figure 5.
CAse REport (CARE) Checklist75
Two investigators (CAW and SW) evaluated each article. If there was a disagreement between the two reviewers, a third investigator (KS) was asked to review. The majority rating was used after discussion among reviewers.
Data extraction
Variables for data extraction was determined a priori and completed by two investigators (CAW and SW) and the third author (KS) verified all of the data presented in the tables. All information extracted was entered into a Microsoft Word table.
Systematic Reviews (SRs)
Information extracted from SRs included: citation (first author and year of publication) and quality assessment, type of treatment/intervention, number of studies included, number of participants and type of studies included, results of that assessment and overall conclusions of the review.
Randomized Controlled Trials (RCTs)
Information extracted from RCTs included: study identification by citation (first author and year of publication) and quality assessment, patient population information, mean age and mean symptom duration, treatment/intervention, comparison group, dosage, adverse events reported and overall study conclusions.
Cohort studies
Information extracted from cohort studies included: study identification by citation (first author and year of publication) and quality assessment, patient population information, mean age and mean symptom duration, intervention, dosage, adverse events reported and overall study conclusions.
Case reports
Data extracted from the case reports included: study identification by citation (first author and year of publication), case presentation, treatment, and adverse events reported.
Results
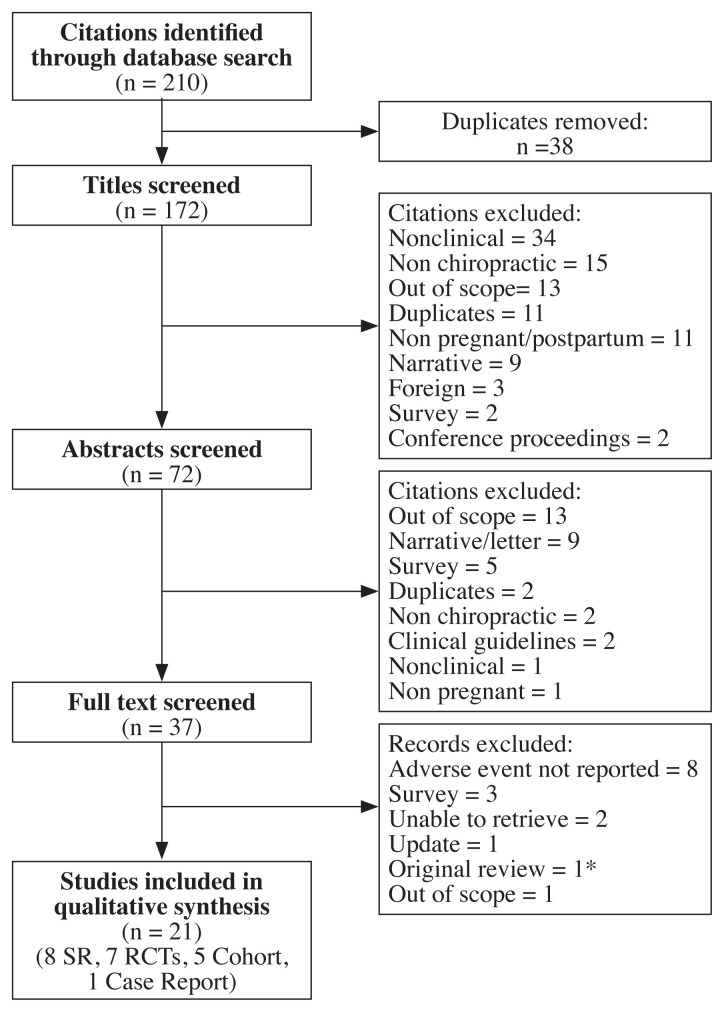
The initial database searches yielded 210 manuscripts (172 after duplicates removed). Of these, 21 were included in the review (8 SRs, 7 RCTs, 5 cohort, and 1 case study); see Figure 6 for the study flow diagram. Reasons for exclusion included: adverse events not reported, outside of the scope of the review, commentary/letter/ narrative review, no outcomes reported, not a clinical study, non-chiropractic, abstract/conference proceeding, non-English, and not reported in a peer-reviewed journal. Excluded studies are listed in Appendix 2.
Figure 6.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram.
*Stuber et al. (2012) not included in this analysis
Systematic reviews
Table 1 lists each item on the Risk of Bias assessment instrument of included SRs. Of the eight SRs included, four were of “high quality”18, 76–78, two were of “acceptable quality”70, 79, and two were of “unacceptable quality”80, 81 and removed from analysis. Overall, only a qualitative analysis could be completed because of the lack of homogeneity between the trials (specifically regarding SMT) and limited methodological quality, as well as variation between individual studies (i.e., gestational age, number of participants, types of intervention, duration and frequency of intervention, outcome measures, and condition diagnosis). Table 2 summarizes the included SRs. One of the SRs examined a variety of treatment options76 for the pregnant patient experiencing back pain, two examined osteopathic manipulative therapy (OMT)77, 78, one assessed complementary and alternative medicine (CAM)18 as a treatment option, one examined modalities70 and the final SR looked at physical therapy79 in general. The four “high quality” SRs recorded adverse events of which almost all were considered transient and minor.18, 76–78 In addition, one of the SRs stated that there were no issues related to any of the deliveries or neonates76 and another suggested that CAM, such as chiropractic, was a safe option compared to no treatment at all for pregnancy-related back pain.18 The two “acceptable quality” SRs70, 79 did not record specific adverse events for any intervention they examined.
Table 1.
Risk of bias assessment of included SRs with the SIGN checklist.
| First author and year published | Items on SIGN checklista | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 | #12 | Total | Qualityb | |
| Liddle, 201576 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | H |
| Franke, 201778 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | H |
| Ruffini, 201377 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | H |
| Hall, 201618 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 12 | H |
| Gutke, 201570 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | A |
| Sharma, 201479 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 8 | A |
| Majchrzyki, 201580 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | U |
| Posadaski, 201181 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | U |
SIGN = Scottish Intercollegiate Guideline Network
See Figure 2 for Quality assessment SIGN checklist itemsa and scoringb for SRs
Table 2.
Evidence table for SRs including treatment/intervention, quality rating, number and type of studies and overall study conclusions.
| Citation and quality* | Treatment/ intervention | Number and studies and participants and type of studies | Adverse events reported | Overall study conclusion |
|---|---|---|---|---|
|
| ||||
| Liddle 201576 High |
Multimodal | 34 studies (n=5,121): pertaining to: | The adverse event that were reported were considered transient and minor and mostly experienced by those who received acupuncture. | Overall, there is simply not good enough quality evidence to make confident decisions about treatments for these complaints. When reported, there were no lasting side effects on any of the studies. |
| LBP | LBP | LBP | ||
| 16 RCTs | Overall, there were no serious adverse events to mother or fetus to report. Exercise (Group or individual): Studies reported no adverse events as a result of the intervention Support devices: No adverse events reported Manual therapy: One trial reported no adverse events; 1 trial reported that adverse events were similar amongst the groups, but no further details were given; 1 did not report on adverse events; 1 trial reported post-treatment soreness but no adverse effects as a result of the treatment TENS: No adverse event to report Taping: No adverse event reported |
There is low quality evidence that exercise improves pain and disability for women with LBP. Exercise interventions (from five to 20 weeks duration) improved the level of LBP and disability than women who just received regular prenatal care. | ||
|
|
||||
| PGP | PGP | PGP | ||
| 6 RCTs | Overall, no long-lasting adverse effects were reported. Acupuncture: Data not provided on adverse events, but some Issues with needles (pain, bleeding, fainting). Exercise + Education: No adverse events reported Belts: Adverse effects not measured Craniosacral Therapy: some discomfort with belt, drowsiness and temporary increase in PGP |
In general, there is less evidence on treatment for pelvic pain. There is evidence from single studies that suggesting that acupuncture or craniosacral therapy improved PGP more than usual prenatal care. | ||
|
| ||||
| Both LBP and PGP | Both LBP & PGP | Both LBP & PGP | ||
| 12 RCTs | Overall, adverse events were minor and transient, when reported by subjects or investigators. There were no reported problems with any of the deliveries and neonates. Acupuncture: minor and transient adverse effects including bruising, local pain, nausea, weakness, heat or sweating Physiotherapy: some adverse effects, such as preterm uterine contractions, pre-eclampsia but unlikely to have been caused by physiotherapy |
There is moderate quality evidence that exercise results in less sick leave and fewer women reporting pain. Although the results are variable, exercise (eight to 12 weeks duration) reduced the number of women who reported back pain and land-based exercises reduced sick leave in 2 studies. However, 2 other studies suggested that sick leave was no better at preventing LBP or PGP than usual care. In addition, there is evidence from low quality studies that multimodal care (manual therapy, exercise and education) reduced pain and functional disability, but not sick leave. | ||
|
| ||||
| Franke 201778 High |
OMT | 8 RCTs* Pregnancy: 5 RCT Postpartum: 3 RCT *5 of 8 were grey literature |
Only 1 of the studies reported on adverse events and they suggested that they were minor in nature; occasionally patients reported they were tired following treatment. In personal communication, authors of 2 other studies, they reported no adverse event occurred. | Clinically relevant effects of OMT were found for reducing pain and improving functional status in pregnant and postpartum (3 months posttreatment) women experiencing LBP. |
|
| ||||
| Ruffini 201677 High |
OMT | 24 studies total but those pertaining to: | Overall, adverse events were not sufficiently described; only 3studies mentioned adverse events. Researchers suggested a more systematic reporting of adverse events in order to obtain solid and generalizable results. | OMT can be considered effective on pregnancy-related back pain. |
| Pregnancy | Pregnancy | |||
| 8 studies (n=914) 4 RCTs, 2 case controls, 1 observational study and 1 case-series |
Craniosacral Therapy: Minor events listed in the intervention group including increased PGP, elastic belt discomfort and drowsiness. Minor events listed in the control group including elastic belt discomfort and increases in PGP | |||
|
| ||||
| Labour and delivery | Labour and delivery | |||
| 4 studies (n=597): 1 RCT, 2 case-series and 1 observational study | Only reported adverse events in 2 studies and determined that OMT was well tolerated | |||
|
| ||||
| Hall 2016 High18 | CAM | 11 full text articles on 10 RCTs (n=1,198) |
Researchers stated that their findings are similar to others in that very few adverse events have been reported in the literature and suggest complementary manual therapies are a safe option compared to no treatment at all. | There is limited evidence to support the use of complementary manual therapies as an option for managing LBP and PGP during pregnancy. |
|
| ||||
| Gutke 201570 Acceptable |
Modalities | 34 RCTs; 8 CCTs; 3 long-term follow ups; 2 observational studies 4 observational retrospective studies; 1 experimental case study; 1 case series; and 3 pilot studies | No specific adverse events were recorded for any intervention (acupuncture, exercise, pelvic belt, physiotherapy, massage). | There was evidence for the positive effects of acupuncture and pelvic belts but weak for specific exercises. |
|
| ||||
| Sharma, 201479 Acceptable |
Physical therapy | 9 RCTs; 1 cohort; 3 CS | No specific adverse events were recorded for any interventions (exercise, pelvic/sacroiliac belt, muscle energy techniques, soft tissue mobilization, postural alignment). | These authors recommend a combination of specific stabilizing exercises, nonelastic sacroiliac belt in the high position and ergonomic educationas the most beneficial interventions in the management of sacroiliac dysfunction/PGP for pregnant individuals experiencing this pain. |
Scottish Intercollegiate Guideline Network (SIGN) Quality rating: >9=high quality, low risk of bias (H); 6–9=acceptable quality, moderate risk of bias (A); <6=low quality, high risk of bias (L) CAM = complementary alternative medicine; CCT = controlled clinical trials; CS = case series; LBP = low back pain; OMT = osteopathic manipulative therapy; PGP = pelvic girdle pain;
Randomized controlled trials
Table 3 lists each item on the Risk of Bias assessment instrument of included RCTs. Of the seven RCTs identified, six were of “acceptable quality”60, 82–86 and one was of “low quality”.87 Table 4 shows the data extraction of each RCT. There were five studies involving OMT83–87 as the intervention, one study examining SMT60, and one study which provided multimodal treatment82. Of the five studies that examined OMT, four were compared to sham or placebo ultrasound and/or usual obstetric care (UOBC)83–85, 87 and one did not have a comparison group86. All of the studies that applied an OMT protocol to pregnant women in the third trimester did not report any specific adverse events with respect to worsening their back pain and/or an increase in poor labour and delivery outcomes.83–85, 87 One study that examined the effects of OMT in women experiencing postpartum-related back pain did state that there were no serious adverse events reported, however occasionally participants did complain of being tired following the intervention.86
Table 3.
Risk of bias assessment of included RCTs.
| First author and year published | Items on Modified SIGN checklista | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | Qualityb | |
| Gausel, 2017 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 6 | A |
| Schwerla, 201586 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 8 | A |
| Hensel, 201687 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 6 | A |
| Peterson, 201260 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | A |
| Licciardone, 201083 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 6 | A |
| Licciardone, 201384 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | A |
| Hensel, 201685 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 5 | L |
RCTs = randomized controlled trials; SIGN = Scottish Intercollegiate Guideline Network
See Figure 3 for Quality assessment SIGN checklist itemsa and scoringb for randomized controlled trials
Table 4.
Evidence table for RCTs including quality rating, patient information, intervention and comparison group, dosage, adverse events reported and study conclusions.
| Citation and quality* | Patient population, mean age, mean symptom duration | Intervention | Comparison group(s) | Dosage | Adverse event reported | Conclusion |
|---|---|---|---|---|---|---|
| Gausel 201782 Acceptable |
N=56, pregnant women, less than 29 wks, with 1-sided PGP Age (mean yrs): TG: 28.9 CG: 29.9 GA (mean wks): 23.1 Onset: Prior to 18–29 wks |
TG: SMT, mobs, STT, exercises and advice chosen by the chiropractor | CG: UOBC | TG: Number of treatments individualized by the chiropractor | Reported: At follow-up appts, women were asked to recall any negative reactions. No serious or long-lasting adverse events were reported. Although adverse events following SMT during pregnancy are rare, treatments should not be performed over a long period of time unless there is a positive response. Future studies should track possible adverse events throughout the study. |
There were no statistically significant differences between the treatment group and control group with respect to sick leave, pain, disability or general health status. |
| Schwerla 201586 Acceptable |
n=80, postpartum women with nonspecific LBP or PGP; at least 3mo and 5/10 on VAS Age (Mean wk): TG=33.9 CG=33.3 GA: TG= postpartum CG= postpartum Onset: Within the past 3 to 15 mo Duration: TG: 9.8 mo CG: 9.7 mo |
TG: OMT could include direct and indirect visceral and cranial techniques | CG: No tx but told they were put on a wait list to be scheduled 2 mo later | 8 wks 4 txs 40–60 min |
Reported: No serious adverse events were recorded during the study period. Occasionally, participants complained of being tired following the intervention. |
OMT applied 4 times to postpartum women led to clinically relevant positive changes in pain intensity and functional disability. |
| Licciardone 201083 Acceptable |
n=146, pregnant women, third trimester with or without LBP Age (Mean yrs) TG=23.8 CG1=23.7 CG2=23.8 GA: Enrolled 28–30 wks Onset: Not stated. Duration: Not stated. |
TG: UOBC + OMT: Standardized OMT protocol during 3rd trimester | CG1: UOBC + SUT CG2: UOBC |
Up to 7 treatment in conjunction with OB appointments at 30, 32, 34, 36, 37, 38 and 39 wks gestation 30 min |
No specific adverse events reported. But the authors stated that the study demonstrated important clinical benefits without any appreciable harms in back-specific functioning when OMT is provided as complementary therapy in the third trimester. | OMT does halt or lessen back pain during the third trimester of pregnancy; however the possibility of minimally important harms cannot be ruled out. |
| Hensel 201687 Acceptable |
n=400, pregnant women, 3rd trimester Age (Mean yrs): TG=24.0 CG1=24.1 CG2=24.7 GA: Enrolled at 30 wks Onset: Not stated Duration: Not stated |
TG: OMT= Usual care + standardized OMT protocol | CG1: PUT CG2: UOBC |
OMT and PUT groups provided 7 visits within 24 hours of OB visit 20 min over 9 wks |
No specific adverse events reported.The authors did state that the OMT protocol did not increase the risk of precipitous labour, conversion to cesarean delivery or meconium-stained amniotic fluid Although the OMT group experienced longer labour, there was no increased incidence of complications during delivery including perineal laceration, episiotomy or need for forceps or vacuum |
Those who received OMT protocol in addition to usual care had a slower rate of deterioration of their pain and back-specific functioning during the third trimester. The OMT protocol appears to be a safe and effective way to manage back pain and function during pregnancy. |
| Hensel 201685 Low |
n=400, pregnant women, 3rd trimester Age (Mean yrs): TG=24.1 CG1=24.1 CG2=24.8 |
TG: OMT= Usual care + standardized OMT protocol | CG1: PUT CG2: UOBC |
OMT and PUT groups provided 7 visits within 24 hours of OB visit 20 min over 9 wks |
No specific adverse events reported. When using high-risk status and labour and delivery outcomes as an index for safety, no greater risk in the OMT group was found. | The OMT protocol applied in the third trimester of pregnancy, is a safe intervention with respect to labour and delivery outcomes. |
| Peterson 201260 Acceptable |
n = 57, pregnant women with LBP and/or PGP reproducible by palpation Age: TG1= 31.1 TG2=29.7 CG= 28.7 GA: TG1= 25.7 TG2= 27.0 CG=23.7 Onset: TG1=16.1 TG2=13.9 CG=11.6 Duration: During pregnancy |
TG1: SMT= HVLA for L/S and SI JT; blocks used to adjust Sacro Occiptial Technique Category II pelvis; activator to adjust pelvis TG 2: NET= chiropractic mind-body technique; combines desensitization procedures with 5 element Chinese medicine + chiropractic adjustment |
CG: Individualized home exercises + Information | All TGs: Paralleled prenatal care schedule; 1x/mo until 28 wks; 2x/mo until 36 wks; 1x/wk thereafter CG: 5 x/wk 15 min |
Reported: Participants were asked at each assessment if they experienced any adverse events as a result of the intervention. No adverse events were reported but the study participants in any group. However, 6% of SMT and exercise and 18% of NET participants produced soreness |
All 3 interventions appear to provide clinically meaningful improvements in function and pain intensity. |
| Licciardone 201384 Acceptable |
N= 144, pregnant women in 3rd trimester with or without LBP Age: TG: 23.8 CG1: 23.7 CG2: 23.8 GA: enrolled between 28–30 wks Onset: not stated Duration: not stated |
TG: OMT + UOBC | CG1: SUT + UOBC CG2: UOBC |
Up to 7 treatment in conjunction with OB appointments at 30, 32, 34, 36, 37, 38 and 39 wks gestation 30 min |
No adverse events specifically reported. The authors did state that there was no SS between study groups in the rates of development of high-risk obstetric conditions or delivery prior to wk 39 | OMT has medium to large treatment effects in preventing progressive back-specific dysfunction during the 3rd trimester. |
Modified Scottish Intercollegiate Guideline Network (SIGN) Quality RCT rating: 9–10 high quality, low risk of bias; 6–8 acceptable quality, moderate risk of bias; 3–5 low quality, high risk of bias; 0–2 or if item 1 and/or 3 are “no unacceptable quality (reject)
CG – control group; GA – gestational age; HVLA – high velocity low amplitude; LBP – low back pain; L/S – lumbar spine; min – minute; mo – month; mobs – mobilization; NET = neuroemotional technique; OB – obstetrician; OMT – osteopathic manipulative therapy; PUT – placebo ultrasound therapy; SI JT – sacroiliac joint; SMT – spinal manipulative therapy; SS = statistial significance; STT – soft tissue therapy; SUT – sham ultrasound therapy; TG – treatment group; tx – treatment; txs – treatments; UOBC – usual obstetric care; wk – week; wks – weeks; x/ – times per; yrs – years
Two RCTs included SMT in their study design; one compared a multimodal approach including SMT to UOBC82 and the other compared SMT and exercise to neuroemotional technique (NET) and a control group consisting of individual home exercises and information. Both of these studies asked patients to recall any negative reactions to treatment at the follow up visit. Both studies did not have any serious adverse or long-lasting events to report. However, the study involving SMT and exercise compared with NET did state that 6% and 18% of participants experienced soreness, respectively.
Cohort Studies
Table 5 lists each item on the Risk of Bias assessment instrument of included cohort studies. Of the cohort studies included two were of “acceptable quality”88, 89, one was of “low quality”90 and two were considered “unacceptable quality”91,92. The two “unacceptable quality” studies were removed. Table 6 shows the data extraction of each cohort study. In the first “acceptable quality” cohort study, it was determined that following a high velocity thrust technique (HVTT) for a maximum of two attempts per symptomatic side, 80% of participants reported an improvement of 50% or more within the first 24 to 72 hours following the intervention.88 In this cohort study, no subject was determined to have greater disability or pain after the intervention.88 The second “acceptable quality” cohort study examined chiropractic treatment (unspecified method or frequency, left up to the treating clinician) on pregnant women with LBP and/or PGP at one, three, six and 12 months following the start of treatment.89 A large proportion of women undergoing chiropractic treatment reported clinically relevant improvements in their symptoms at all time points. Eighty-five percent of the participants were “very happy” or “happy” with their treatment and the authors reported that no adverse events had occurred.89 The final cohort study of “low quality” determined the effects of a 20 to 30 minute OMT treatment on women who delivered within 48 hours. Although their preliminary results suggested that OMT is efficacious for postpartum pain management, 18.6% of participants experienced a slight increase in tenderness and sharpness immediately following their treatment.90
Table 5.
Risk of bias assessment of included cohort studies.
| First author and published year | Items on SIGN checklist | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total | Qualityb | |
| Al-Sayegh, 201088 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8 | A |
| Peterson, 201489 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | A |
| Hastings, 201690 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 7 | L |
| Skarica, 201892 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | U |
| Haavik, 201691 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | U |
SIGN, Scottish Intercollegiate Guideline Network
See Figure 4 for Quality assessment SIGN checklist itemsa and scoringb for cohort studies
Table 6.
Evidence table for cohort studies including quality rating, patient information, intervention, dosage, adverse events reported and study conclusions.
| Citation and quality* | Patient population, mean age, mean symptom duration | Intervention | Dosage | Adverse event reported | Overall study conclusion |
|---|---|---|---|---|---|
| Al-Sayegh 201088 Acceptable |
n=69, postpartum women with LBP and/or PGP Age (Mean yrs): All: 31 TG1: 30 TG2: 34 GA: Postpartum Onset: Anytime during pregnancy or postpartum Duration: All: 28.9 wks TG1: 28.8 wks TG2: 29.9 wks |
All subjects HVTT + forward rocking G1: HVTT success G2: HVTT non-success |
2 attempts at each visit | Reported: In no case was a subject determined to have greater disability or pain after the intervention. | (80%) is enough to reassure the The pretest probability of success clinician about the decision to use HVTT lumbopelvic region in postpartum women experiencing LBP and/or PGP |
| Peterson 201489 Acceptable |
n=143, pregnant women with LBP, PGP or both Age (mean yrs): 32.96 GA (mean wks): 26.21 |
Chiropractic treatment (unspecified) | Was left to the discretion of the treating clinician | Reported: No adverse events were reported and 85% of patients were happy or very happy with their chiropractic treatment. | A large proportion of patients with LBP or PGP undergoing chiropractic treatment reported clinically relevant improvements in their symptoms at all time points up to 1 yr. |
| Hastings 201690 Low |
n= 75–80 pts approached, women who delivered within 48 hrs Age: not reported GA: postpartum |
OMT – based on somatic dysfunction; | Was left to the discretion of the treating clinician; 20–30 min; most commonly used myofascial release, balanced ligamentous tension and facilitated positional release | Reported: Slight increase in tenderness and sharpness immediately following OMT, although not SS, is consistent with what is already reported in the literature. It is believed to result from minor and temporary tissue irritation | Most postpartum patients undergoing chiropractic treatment reported clinically relevant improvements at all time points. |
Note: Skarica (2018)92 and Haavik (2016)91 were deemed unacceptable and removed from the data extraction table.
Scottish Intercollegiate Guideline Network (SIGN) Quality rating: 12–14 high quality, low risk of bias; 9–11 acceptable, moderate risk of bias; 6–8 low quality, high risk of bias; <6 unacceptable quality G = group; GA = gestational age; hrs = hours; HVTT = high velocity thrust technique; LBP = low back pain; min = minute; OMT = osteopathic manipulative therapy; PGP = pelvic girdle pain; pts = patients;
SS = statistically significant; TG = target group; wks = weeks; yr = year
Case studies
Table 7 lists each item on the CARE checklist.75 Only one case study93 reported a serious adverse event following SMT on the cervical spine in a 16 week pregnant woman (Table 8). Immediately following a cervical SMT treatment the patient experienced right-sided anterior neck pain and developed ipsilateral Horner’s syndrome as a result of a dissection of the right internal carotid artery. Four days following the treatment, the patient miscarried. The patient was admitted to the ICU and treated accordingly. One year later, the Horner’s symptoms still persisted.
Table 7.
CARE Case studies
| Morton, 201293 | ||
|---|---|---|
| 1. | Title | 1 |
| 2. | Key Words | 1 |
| 3. | Abstract | |
| a. | Introduction | 1 |
| b. | Patient’s main concerns and important clinical findings. | 1 |
| c. | The main diagnoses, intervention and outcomes. | 1 |
| d. | Conclusion – what are the “take away” lessons? | 1 |
| 4 | Introduction | 1 |
| 5 | Patient information | |
| a. | De-identified demographic and other patient information. | 1 |
| b. | Main concerns of the symptoms of the patient. | 1 |
| c. | Medical, family and psychosocial history including genetic information. | 1 |
| d. | Relevant past interventions and their outcomes. | 0 |
| 6. | Clinical findings | 0 |
| 7. | Timeline | 0 |
| 8. | Diagnostic Assessment | |
| a. | Diagnostic methods (PE, laboratory testing, imaging, surveys) | 0 |
| b. | Diagnostic challenges | N/A |
| c. | Diagnostic reasoning including differential diagnosis | N/A |
| d. | Prognostic characteristics when applicable | N/A |
| 9. | Therapeutic Intervention | |
| a. | Types of intervention (pharmacologic, surgical, preventive) | 0 |
| b. | Administration of intervention (dosage, strength, duration) | 0 |
| c. | Changes in the intervention with explanations | N/A |
| 10. | Follow up and Outcomes | |
| a. | Clinician and patient-assessed outcomes when appropriate. | 0 |
| b. | Important follow-up diagnostic and other test results. | 1 |
| c. | Intervention adherence and tolerability (how was this assessed). | N/A |
| d. | Adverse and unanticipated events. | 1 |
| 11 | Discussion | |
| a. | Strengths and limitations in your approach to the case. | 0 |
| b. | Discussion of the relevant medical literature. | 1 |
| c. | The rationale for your conclusion. | 1 |
| d. | Primary “take-away” lessons from this case report. | 1 |
| 12 | Patient perspective | 0 |
| 13. | Informed consent | 1 |
| Total | 16 | |
| Adverse events Reported | Yes |
Table 8.
Evidence tables for case studies including citation, case presentation and treatment and reported adverse events.
| Citation | Case Presentation | Treatment and Adverse Event reported |
|---|---|---|
| Morton, 2012 | A 31-yr old woman presented to the chiropractor at 16-wks GA with occipital HA. She has a 17-yr previous history of monthly, intermittent, bilateral occipital muscle tension HA that are unchanged with pregnancy. In addition, she had a history of migraine characterized by unilateral frontal HA, the last episode which had been 6-wks earlier. Patient was diagnosed with SLE 12 yrs earlier, complicated by renal involvement treated with azathioprine and prednisone, hypertension managed with labetalol and episodes of DVT and PE. She was heterozygous for prothrombin gene mutation but did not have lupus anticoagulant or anticardiolipin antibody. | Immediately following chiropractic treatment (not specified butbased on description, SMT was suggested), the subject reported severe right-sided anterior neck pain and developed ipsilateral Horner’s syndrome. MRI revealed dissection of the right internal carotid artery. It extended 5 cm distal to the carotid bulb to the horizontal intrapetrous segment. SLE flared up. 4 days after the onset of neurological symptoms, intrauterine fetal demise occurred. Tx: reported to ICU and treated with intravenous heparin and subsequently low-molecular weight heparin. Patient was placed on warfarin for 6 months. A follow-up MRI revealed a focal false aneurysm on the right internal carotid artery. One year later, Horner’s syndrome persists. |
cm – centimetre; DVT – deep vein thrombosis GA – gestational age; HA – headache; ICU – intensive care unit; MRI – magnetic resonance imaging; PE – pulmonary embolism; SLE – systemic lupus erythematosus; SMT – spinal manipulative therapy; Tx - treatment; yrs – years;
Discussion
This systematic review provides an update of the literature regarding SMT during pregnancy and the postpartum period, as well as a review of any adverse events associated with the reported studies. With the exception of one case study, all studies reported only minor and transient events. The case study demonstrated an adverse event following cervical spinal manipulation. When added to the results of our 2012 review (four events following cervical SMT and three events following lumbar SMT) adverse events following SMT in these populations still appear to be scarce.
One important revelation in this review is the lack of adverse events being reported, which was also highlighted in a few of the studies included in this review.77, 82 Tracking of adverse events was not common practice in higher quality studies, such as RCTs, until the CONSORT guidelines94, 95 were developed and changed over the years to encourage researchers to do so. Unfortunately, the reporting of adverse events is a missing component of research papers. In the current paper, the fact that very few adverse events were reported, does not mean that others did not happen. There has to be a greater effort made by researchers to report not only adverse events associated with studies but also to clearly state that no adverse events occurred when that is the case. Future research should not only focus on reporting the presence or absence of adverse events,76 but also determining the adverse events that occur at each of the different pain locations experienced by pregnant and postpartum patients. Recently, there has been a greater emphasis on delineating the various pain locations (lumbar spine LBP versus PGP versus combined pain) experienced by pregnant and postpartum patients.57,58,76,96,97 Robust trials on the effectiveness of SMT for cervical and thoracic spine in these populations are required to help inform decisions regarding care. By utilizing all of this information, future studies can be designed and ultimately determine possible prevention and effective management strategies for these populations.
Chiropractors are well versed in treating pregnant and postpartum patients.59 However, the evidence with respect to safe and effective treatment options, including SMT, in these patients is limited. Two recent SRs regarding pregnancy58 and postpartum-related back pain57 have suggested that SMT should be considered as a possible modality to treat these two populations. Although the strength for SMT in these two SRs was inconclusive, it has been suggested that a trial of care may be warranted to see if it produces symptomatic relief for patients.4, 57, 58 Determining conclusive evidence in these populations may be difficult simply because of the rarity of these events.93 In one RCT examining the effects of a multimodal program including SMT on LBP the authors suggest that although adverse events during pregnancy are rare, treatments should not be performed unless there is a positive response within a trial of care period.82 Unfortunately, there is even less evidence with respect to the safety and suggested treatment strategies for neck pain during the pregnant and postpartum period.
We continue to support the suggestions from our previous review:69 (1) that contraindications to SMT are evident during a careful history and physical exam; (2) clinicians treating these two populations should consider prothrombotic and joint laxity risk factors when determining their treatment plan and attempt to minimize the risk of potentially dangerous and neurological complications; and (3) pregnant and postpartum women at higher risk for complications, such as those in a post-thrombotic state or possible joint laxity, should be treated with additional caution. These patients should be counselled with respect to the risks of SMT and educated as to the signs and symptoms of possible neurovascular complications.69 In addition, we believe that future studies should include the presence or absence adverse events. Reporting this information will help to inform stakeholders of the actual possible adverse events that may occur in these populations.
Strengths and limitations
A key strength of this review is that a thorough search of the literature was conducted by a health science librarian, multiple electronic databases were searched, and we employed a number of broad search terms. Another strength for this review is that we expanded our search to include all forms of literature including SRs and meta-analyses, RCTs, cohort and case studies. In general, the information garnered in this paper should provide practicing chiropractors, chiropractic educators, chiropractic patients and other allied health professionals a reasonable and evidence-based rationale to the safety of SMT in these two populations.
There are a few limitations associated with this review. The first is the number of studies available and the hierarchy of available evidence. Similar to our 2012 review, the majority of the papers identifying serious adverse events were case studies, and they are considered lower levels of evidence because of their high risk of bias. The second limitation is the reporting of adverse events, or lack thereof in clinical trials. In most of the papers included in this review there was no mention of whether or not an adverse event occurred following treatment. Similar to the limitations of our previous review, we suggest that given the lower levels of evidence and the lack of reporting of adverse events, the possibility of risk to pregnant and postpartum undergoing SMT cannot be measure or stated definitively. In addition, it cannot be determined if any such risk level is higher or lower than in non-pregnant or postpartum populations. There is a need to execute more robust high-quality studies, such as the SafetyNET active surveillance reporting system,98, 99 to rigorously track adverse events and potentially develop mitigation strategies in these populations. The third limitation is the time frame since the current search was completed. Although it has been two years since the last search, similar to what we found between the original study and the current one, we do not anticipate any major changes with respect to the reporting of adverse events. However, a future update will be completed in a more expedient manner. The final limitation is the restriction of our postpartum timeline of six weeks. The hormonal changes that occur with pregnancy do not automatically revert back to a pre-pregnancy state with birth of a child. Therefore, we maybe limiting the number of studies that could have been retrieved and the adverse events associated with them. Extending the postpartum timeline should be considered for a future update.
Conclusions
High quality studies, such as RCTs, regarding SMT for pregnancy- and postpartum-related spinal pain are lacking. This update of our previous review found one case study93 that demonstrated a serious adverse event following SMT in the cervical spine and a handful of minor and transient adverse events in the low back18, 60, 76, 77, 86. Although we are calling for improved reporting of such events in all papers going forward, it appears these events are rare. Future research should focus on the proper reporting of all adverse events while assessing efficacy of appropriate treatment options for these populations.
Supplementary Information
Appendix 1. Search strategy terms
| MEDLINE | |
|---|---|
| 1. | MH “Long Term Adverse Effects” |
| 2. | adverse event* |
| 3. | adverse reaction* |
| 4. | adverse effect* |
| 5. | side effect* |
| 6. | TI harm* or AB harm* |
| 7. | (increas* n2 pain*) or (incident* n2 pain*) |
| 8. | hematoma* |
| 9. | sprain* or strain* |
| 10. | (disc n2 herniat*) or (disk* n2 herniat*) |
| 11. | (disc n2 bulg*) or (disk* n2 bulg*) |
| 12. | thrombophil* or thrombosis* or hypercoag* |
| 13. | dissection* |
| 14. | stroke* |
| 15. | fractur* |
| 16. | MH Chiropractic |
| 17. | MH Manipulation, Spinal |
| 18. | MH Musculoskeletal Manipulations |
| 19. | MH Manipulation, Chiropractic |
| 20. | chiroprac* |
| 21. | spinal* n2 manip* |
| 22. | spinal* n2 adjust* |
| 23. | musculoskeletal n2 manip* |
| 24. | musculoskeletal* n2 adjust* |
| 25. | manual n2 therap* |
| 26. | manual* n2 adjust* |
| 27. | hvla |
| 28. | high velocity low amplitude* or high-velocity low-amplitude* or high velocity thrust* or high-velocity thrust* |
| 29. | audibl* n2 releas* |
| 30. | subluxat* |
| 31. | MH Pregnancy |
| 32. | MH Pregnant Women |
| 33. | MH Pregnancy Outcome |
| 34. | MH Pregnancy Complications |
| 35. | MH Prenatal Care |
| 36. | MH Postpartum Period |
| 37. | MH Parturition |
| 38. | pregnan* |
| 39. | childbirth* |
| 40. | antenatal* OR ante natal* OR ante-natal* |
| 41. | prenatal* OR pre natal* OR pre-natal* |
| 42. | postnatal* OR post natal* OR post-natal* |
| 43. | postpartum* OR post partum* OR post-partum* |
| 44. | perinatal* or peri natal* or peri-natal* |
| 45. | peirpartum* or peri-partum* |
| 46. | 1–15/ OR |
| 47. | 16–30/ OR |
| 48. | 31–45/ OR |
| 49. | 46 AND 47 AND 48 |
| 50. | LIMIT English |
| 51. | LIMIT January 1 2010–Nov 1 2018 |
Footnotes
The authors have no disclaimers, competing interests, or sources of support or funding to report in the preparation of this manuscript.
References
- 1.Malmqvist S, Kjaermann I, Andersen K, Økland I, Brønnick K, Larsen JP. Prevalence of low back and pelvic pain during pregnancy in a Norwegian population. J Manipulative Physiol Ther. 2012;35(4):272–278. doi: 10.1016/j.jmpt.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs FM, Garcia E, Royuela A, González L, Abraira V. Prevalence and factors associated with low back pain and pelvic girdle pain during pregnancy: a multicenter study conducted in the Spanish National Health Service. Spine. 2012;37(17):1516–1533. doi: 10.1097/BRS.0b013e31824dcb74. [DOI] [PubMed] [Google Scholar]
- 3.Vermani E, Mittal R, Weeks A. Pelvic girdle pain and low back pain in pregnancy: a review. Pain Pract. 2010;10(1):60–71. doi: 10.1111/j.1533-2500.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Vleeming A, Albert H, Ostgaard H, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning M. Low back and pelvic girdle pain of pregnancy: Recommendations for diagnosis and clinical management. J Clin Chiropr Pediatr. 2010;11(2):775–779. [Google Scholar]
- 6.Mogren I, Pohjanen A. Low back pain and pelvic pain during pregnancy. Spine. 2005;30(8):983–991. doi: 10.1097/01.brs.0000158957.42198.8e. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton DB, MacLennan AH, Kristiansson P. The prevalence of recalled low back pain during and after pregnancy: a South Australian population survey. Aust N Z J Obstetr Gyn. 2002;42(5):482–485. doi: 10.1111/j.0004-8666.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 8.Schytt A, Lindmark G, Waldenstrom U. Physical symptoms after childbirth: prevalence and associations with self-rated health. BJOG. 2005;112(2):210–217. doi: 10.1111/j.1471-0528.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Bastiaenen C, Bie R, Vlaeyen J, Goossens M, Leffers P, Wolters P, et al. Long-term effectiveness and costs of a brief self-management intervention in women with pregnancy-related low back pain after delivery. BMC Pregnancy Childbirth. 2008;8(19) doi: 10.1186/1471-2393-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mogren IM. Physical activity and persistent low back pain and pelvic pain post partum. BMC Public Health. 2008;8:417. doi: 10.1186/1471-2458-8-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindal E, Hauksson A, Arnardottir S, Hallgrimsson J. Low back pain, smoking and employment during pregnancy and after delivery - a 3-month follow-up study. J Obstet Gynaecol. 2000;20(3):263–266. doi: 10.1080/01443610050009575. [DOI] [PubMed] [Google Scholar]
- 12.Turgut F, Turgut M, Cetinsahin M. A prospective study of persistent back pain after pregnancy. Eur J Obstetr Gynec Reprod Biol. 1998;80:45–48. doi: 10.1016/s0301-2115(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 13.Gutke A, Lundberg M, Ostgaard H, Oberg B. Impact of postpartum lumbopelvic pain on disability, pain intensity, health-related quality of life, activity level, kinesiophobia, and depressive symptoms. Eur Spine J. 2011;20(3):440–448. doi: 10.1007/s00586-010-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuge B, Laerum E, Kirkesola G, Vøllestad N. The efficacy of a treatment program focusing on specific stabilizing exercises for pelvic girdle pain after pregnancy: a randomized controlled trial. Spine. 2004;29(4):351–359. doi: 10.1097/01.brs.0000090827.16926.1d. [DOI] [PubMed] [Google Scholar]
- 15.Pierce H, Homer CS, Dahlen HG, King J. Pregnancyrelated lumbopelvic pain: listening to Australian women. Nurs Res Pract. 2012;2012:387428. doi: 10.1155/2012/387428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari N, Hasson S, Naghdi S, Keyhani S, Jalaie S. Low back pain duirng pregnancy in Iranian women. Physiother Theory Pract. 2010;26:40–48. doi: 10.3109/09593980802664968. [DOI] [PubMed] [Google Scholar]
- 17.Sipko T, Grygier D, Barczyk K, Eliasz G. The occurrence of strain symptoms in the lumbosacral region and pelvis during pregnancy and after childbirth. J Manip Physiol Ther. 2010;33(5):370–377. doi: 10.1016/j.jmpt.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Hall H, Cramer H, Sundberg T, Ward L, Adams J, Moore C, et al. The effectiveness of complementary manual therapies for pregnancy-related back and pelvic pain: a systematic review with meta-analysis. Medicine. 2016;95(38) doi: 10.1097/MD.0000000000004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skaggs C, George J, Nelson D, Gross G, Prather H, Thompson P. Back and pelvic pain in an underserved United States pregnant population: a preliminary descriptive survey. J Manip Physiol Ther. 2007;30(2):130–134. doi: 10.1016/j.jmpt.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Lisi AJ. Chiropractic spinal manipulation for low back pain of pregnancy: a retrospective case series. J Midwif Women Health. 2006;51(1):e7–e10. doi: 10.1016/j.jmwh.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Pennick VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2007;2:CD001139. doi: 10.1002/14651858.CD001139.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Fast A, Weiss L, Ducommun EJ, Medina E, Butler JG. Low-back pain in pregnancy. Abdominal muscles, sit-up performance, and back pain. Spine. 1990;15(1):28–30. doi: 10.1097/00007632-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Gilleard W, Brown J. Structure and function of the abdominal muscles in primgravid subjects during pregnancy and the immediate postbirth period. Phys Ther. 1996;76(7):750–762. doi: 10.1093/ptj/76.7.750. [DOI] [PubMed] [Google Scholar]
- 24.Weis CA, Triano JJ, Barrett J, Campbell MD, Croy M, Roeder J. Ultrasound assessment of abdominal muscle thickness in postpartum vs nulliparous women. J Manip Physiol Ther. 2015;38(5):352–357. doi: 10.1016/j.jmpt.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansson P, Savardsudd K, von Schoultz B. Back pain during pregnancy. A prospective study. Spine. 1996;21(6):702–709. doi: 10.1097/00007632-199603150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Mens J, Vleeming A, Stoeckart R, Stam H, Snijders C. Understanding peripartum pelvic pain. Implications of a patient survey. Spine. 1996;21:1363–1370. doi: 10.1097/00007632-199606010-00017. [DOI] [PubMed] [Google Scholar]
- 27.Beighton P, Solomon L, Soskolne C. Articular mobility in an African population. Ann Rheumatol Dis. 1973;32:413418. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter C, Wilkinson J. Persistent joint laxity and congenital dislocation. J Bone Joint Surg (Br) 1964;(46B):40–25. [PubMed] [Google Scholar]
- 29.Bjorklund K, Bergstrom S, Nordström M, Ulmsten U. Symphyseal distention in relation to serum relaxin levels and pelvic pain in pregnancy. Acta Obstet Gynecol Scand. 2000;70:269–275. doi: 10.1080/j.1600-0412.2000.079004269.x. [DOI] [PubMed] [Google Scholar]
- 30.Marnach M, Ramin K, Ramsey P, Song S, Stensland J, An K. Characterization of the relationship between joing laxity and maternal hormones in pregnancy. Obstet Gynecol. 2003;101:331–335. doi: 10.1016/s0029-7844(02)02447-x. [DOI] [PubMed] [Google Scholar]
- 31.Calguneri M, Bird H, Wright A. Changes in joint laxity occurring during pregnancy. Ann Rheumatol Dis. 1982;41:126–128. doi: 10.1136/ard.41.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey M, Johnston S, Davies G. Mid-trimester serum relaxin concentrations and post-partum pelic floor dysfunction. Acta Obstet Gynecol Scand. 2008;87(12):1315–1321. doi: 10.1080/00016340802460321. [DOI] [PubMed] [Google Scholar]
- 33.Hansen A, Jensen D, Larsen E, Wilken-Jensen C, Petersen K. Relaxin is not realted to symptom-giving pelvic relaxation in pregnant women. Acta Obstet Gynecol Scand. 1996;75:245–249. doi: 10.3109/00016349609047095. [DOI] [PubMed] [Google Scholar]
- 34.Mens J, Pool-Goudzwaard A, Stam H. Mobility of the pelvic joints in pregnancy-related lumbopelvic pian: a systematic review. Obstet Gynecol Surv. 2009;64:200208. doi: 10.1097/OGX.0b013e3181950f1b. [DOI] [PubMed] [Google Scholar]
- 35.Stuber KJ, Smith DL. Chiropractic treatment of pregnancy-related low back pain: a systematic review of the evidence. J Manipulative Physiol Ther. 2008;31(6):447–454. doi: 10.1016/j.jmpt.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Haldeman S, Carroll L, Cassidy J. Findings from the Bone and Joint Decade 2000 to 2010 Task Force on Neck Pain and its Associated Disorders. J Occupational Environ Med. 2010;52:424–427. doi: 10.1097/JOM.0b013e3181d44f3b. [DOI] [PubMed] [Google Scholar]
- 37.Natvig B, Ihlebaek C, Grotle M, Brage S, Bruugsgaard D. Neck pain is often a part of widespread pain and is associated with reduced functioning. Spine. 2010;35:E1285–E1289. doi: 10.1097/BRS.0b013e3181e38e73. [DOI] [PubMed] [Google Scholar]
- 38.Bussieres AE, Stewart G, Al Zoubi F, Decina P, Descarreaux M, Hayden J. The treatment of whiplash and neck pain associated disorders: Canadian Chiropractic Guideline Initiative clinical practice guideline. J Manip Physiol Ther. 2016;39:523–604. doi: 10.1016/j.jmpt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Coulter I, Hurwitz E, Adams A. Patients using chiropractors in North America: who are they, and why are they in chiropractic care? Spine. 2002;27:291–296. doi: 10.1097/00007632-200202010-00018. [DOI] [PubMed] [Google Scholar]
- 40.Carbonell JL, Varela L, Velazco A, Cabezas E, Fernández C, Sánchez C. Oral methotrexate and vaginal misoprostol for early abortion. Contraception. 1998;57(2):83–88. doi: 10.1016/s0010-7824(98)00004-3. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Headache disorders. Geneva, Switzerland: World Health Organization; 2016. [Available from: http://www.who.int/news-room/fact-sheets/detail/headache-disorders. [Google Scholar]
- 42.Bishop P, Quon J, Fisher C, Dvorak M. The chiropractic hospital-based interventions reserach outcomes (CHIRO) study: a randomized controlled trial on the effectivenss of clinical preactic guidelines in the medical and chiropractic management of pateints with acute mechanical low back pain. Spine J. 2010;10:1055–1064. doi: 10.1016/j.spinee.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 43.McMorland G, Suter E. Chiropractic managment of mechanical neck and low back pain: a retrospective, outcome-based analysis. J Manip Physiol Ther. 2000;23:307–311. [PubMed] [Google Scholar]
- 44.Shekelle P, Coulter I. Cervical spine manipulation: summary report of a systematic review of the literature and multidisciplinary expert panel. J Spinal Dis. 1997;10:223226. [PubMed] [Google Scholar]
- 45.Nelson C. Principles of effective headache management. Topics Clin Chiropr. 1998;5:55–61. [Google Scholar]
- 46.Bryans R, Descarreaux M, Duranleau M, Marcoux M, Potter B, Ruegg R, et al. Evidenced-based guidelines for the chiropractic treatment of adults with headaches. J Manip Physiol Ther. 2011;34:274–289. doi: 10.1016/j.jmpt.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Khorsan R, Hawk C, Lisi AJ, Kizhakkeveettil A. Manipulative therapy for pregnancy and related conditions: a systematic review. Obstet Gynecol Surv. 2009;64(6):416–427. doi: 10.1097/OGX.0b013e31819f9ddf. [DOI] [PubMed] [Google Scholar]
- 48.Chung CLR, Cote P, Stern PJ, L’Esperance G. The association between cervical spine manipulation and carotid dissection: a systematic reivew of the literature. J Manip Physiol Ther. 2015;38(9):672–676. doi: 10.1016/j.jmpt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Hurwitz E, Carragee E, van der Velde G, Carroll L, Nordin M, Guzman J, et al. Treatment of neck pain: noninvasive interventions. J Manip Physiol Ther. 2009;32(2):S141–S175. doi: 10.1016/j.jmpt.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Heiner J. Cervical epidural hematoma after chiropractic spinal manipulation. The Am J Emerg Med. 2009;27(8):1023.e1–2. doi: 10.1016/j.ajem.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz A, Lutterbey G, von Engelhardt L, von Falkenhausen M, Stoffel M. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manip Physiol Ther. 2005;28(8):633–636. doi: 10.1016/j.jmpt.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Cassidy J, Boyle E, Cote P, Hogg-Johnson S, Silver F, Bondy S. Risk of vertebrobasilar stroke and chiropractic care: results of a population-based case-control and case-crossover study. J Manip Physiol Ther. 2009;32(2):S201–S208. doi: 10.1016/j.jmpt.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a risk assessement. J Manip Physiol Ther. 2004;27(3):197–210. doi: 10.1016/j.jmpt.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 54.Hurwitz E, Randhawa K, Torres P, Yu H, Verville L, Hartvigsen J, et al. The global spine care initiative: a systematic review of individual and communitybased burden of spinal disorders in rural populations in low- and middle-income communities. Eur Spine J. 2017;27(Supplement 6):802–815. doi: 10.1007/s00586-017-5393-z. [DOI] [PubMed] [Google Scholar]
- 55.Carroll LJ, Hurwitz EL, Cote P, Hogg-Johnson S, Carragee E, Nordin M, et al. Research Priorities and Methodological Implications. The Bone and Joint Decade 2000 –2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33:S214–S220. doi: 10.1097/BRS.0b013e318164462c. [DOI] [PubMed] [Google Scholar]
- 56.Boyle E, Cote P, Grier AR, Cassidy JD. Examining vertebrobasilar artery stroke in two Canadian provinces. Spine. 2008;33:S170–S175. doi: 10.1097/BRS.0b013e31816454e0. [DOI] [PubMed] [Google Scholar]
- 57.Weis CA, Pohlman KA, Draper C, da Silva-Oolup S, Stuber K, Hawk C. Chiropactic care of adults with postpartum-related low back, pelvic girdle and combination pain: a systematic review. J Manip Physiol Ther. 2020;43(7):732–743. doi: 10.1016/j.jmpt.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Weis CA, Pohlman KA, Draper C, da Silva-Oolup S, Stuber K, Hawk C. Chiropractic care for adults with pregnancy-related low back, pelvic girdle or combination pain: a systematic review. J Manip Physiol Ther. 2020;43(7):714–731. doi: 10.1016/j.jmpt.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Yuen T, Wells K, Benoit S, Yohanathan S, Capelletti L, Stuber K. Therapeutic interventions employed by Greater Toronto Area chiropractors on pregnant patients: results of a cross-sectional online survey. J Can Chiropr Assoc. 2013;57(2):132–142. [PMC free article] [PubMed] [Google Scholar]
- 60.Peterson C, Haas M, Gregory W. A pilot randomized controlled trial comparing the efficacy of exercise, spinal manipulation, and neuro emotional technique for the treatment of pregnancy-related low back pain. Chiropr Man Therap. 2012;20(1):18. doi: 10.1186/2045-709X-20-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George J, Skaggs C, Thompson P, Nelson D, JAG, Gross G. A randomized controlled trial comparing a multimodal intervention and standard obstetrics care for low back and pelvic pain in pregnancy. Am J Obstet Gynecol. 2013;208(4):e1–e7. doi: 10.1016/j.ajog.2012.10.869. [DOI] [PubMed] [Google Scholar]
- 62.Kamel DM, Raoof NAA, Tantawy SA. Efficacy of lumbar mobilization on postpartum low back pain in Egyptian females: a randomized control trial. J Back Musculoskelet Rehabil. 2016;29(1):55–63. doi: 10.3233/BMR-150598. [DOI] [PubMed] [Google Scholar]
- 63.Sadr S, Pourkiani-Allah-Abad N, Stuber K. The treatment experience of patients with low back pain during pregnancy and their chiropractors: a qualitative study. Chiropr Man Therap. 2012;20(1):32–39. doi: 10.1186/2045-709X-20-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drife J. Thromboembolism: reducing maternal death and disability during pregnancy. Br Med J. 2003;67(1):177–190. [Google Scholar]
- 65.Stella C, Sibai B. Thrombophilia and adverse maternalperinatal outcome. Clin Obstet Gynecol. 2006;49:850–860. doi: 10.1097/01.grf.0000211954.66959.e1. [DOI] [PubMed] [Google Scholar]
- 66.Ng KP, Doube A. Stroke after neck manipulation in the post partum period. N Z Med J. 114(1143):498. [PubMed] [Google Scholar]
- 67.Parkin PJ, Wallis WE, Wilson JL. Vertebral artery occlusion following manipulation of the neck. N Z Med J. 1978;88:441–443. [PubMed] [Google Scholar]
- 68.Murphy DR, Hurwitz EL, McGovern EE. Outcome of pregnancy-related lumbopelvic pain treated according to a diagnosis-based decision rule: a prospective observational cohort study. J Manipulative Physiol Ther. 2009;32(8):616–624. doi: 10.1016/j.jmpt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Stuber KJ, Wynd S, Weis CA. Adverse events from spinal manipulation in the pregnant and postpartum periods: a critical review of the literature. Chiropr Man Therap. 2012;20:8. doi: 10.1186/2045-709X-20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gutke A, Betten C, Degerskar K, Pousette S, Olsen MF. Treatments for pregnancy-related lumbopelvic pain: a systematic review of physiotherapy modalities. Acta Obstet Gynecol Scand. 2015;94(11):1156–1167. doi: 10.1111/aogs.12681. [DOI] [PubMed] [Google Scholar]
- 71.Hawk C, Minkalis A, Khorsan R, Daniels C, Homack D, Gliedt J, et al. Systematic review of nondrug, nonsurgical treatment of shoulder conditions. J Manip Physiol Ther. 2017;40(5):293–319. doi: 10.1016/j.jmpt.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Scottish Intercollegiate Guideline Network (SIGN) Methodology checklist 1: systematic reviews and metaanalyses [Internet] Edinburgh: SIGN; 2015. [cited 2018 Sep 17]. Available from: https://www.sign.ac.uk/what-we-do/methodology/checklists/ [Google Scholar]
- 73.Scottish Intercollegiate Guideline (SIGN) Methodology checklist 3: cohort studies [Internet] Edinburgh: SIGN; 2015. [cited 2018 Sep 17]. Available from: https://www.sign.ac.uk/what-we-do/methodology/checklists/ [Google Scholar]
- 74.Scottish Intercollegiate Guideline (SIGN) Methodology checklist 2: controlled trials [Internet] Edinburgh: SIGN; 2015. [cited 2018 Sep 17]. Available from: https://www.sign.ac.uk/what-we-do/methodology/checklists/ [Google Scholar]
- 75.Gagnier J, Kienle G, Altman D, Moher D, Sox H, Riley D. The CARE guidelines: Consensus-based clinica case reporting guideline development. Glob Adv Health Med. 2013;2(5):38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liddle Sarah D, Pennick V. Interventions for preventing and treating low-back and pelvic pain during pregnancy. Cochrane Database Syst Rev. 2015;(9) doi: 10.1002/14651858.CD001139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruffini N, D’Alessandro G, Cardinali L, Frondaroli F, Cerritelli F. Osteopathic manipulative treatment in gynecology and obstetrics: a systematic review. Compl Ther Med. 2016;26:72–78. doi: 10.1016/j.ctim.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Franke H, Franke J-D, Belz S, Fryer G. Osteopathic manipulative treatment for low back and pelvic girdle pain during and after pregnancy: a systematic review and metaanalysis. J Bodywork Mov Ther. 2017;21(4):752–762. doi: 10.1016/j.jbmt.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 79.Sharma A, Sharma S, Steiner LA, Brudvig TJ. Identification and effectiveness of physical therapy interventions for sacroiliac joint dysfunction in pregnant and nonpregnant adults: a systematic review. J Women Health Phys Ther. 2014;38(3):110–117. [Google Scholar]
- 80.Majchrzycki M, Wolski H, Seremak-Mrozikiewicz A, Lipiec J, Marszałek S, Mrozikiewicz PM, et al. Application of osteopathic manipulative technique in the treatment of back pain during pregnancy. Ginekologia Polska. 2015;86(3):224–228. doi: 10.17772/gp/2066. [DOI] [PubMed] [Google Scholar]
- 81.Posadzki P, Ernst E. Spinal manipulation: an update of a systematic review of systematic reviews. N Z Med J. 2011;124(1340):55–71. [PubMed] [Google Scholar]
- 82.Gausel A, Kjaermann I, Malmqvist S, Andersen K, Dalen I, Larsen J, et al. Chiropractic management of dominating one-sided pelvic girdle pain in pregnant women; a randomized controlled trial. BMC Pregnancy Childbirth. 2017;17(1):331. doi: 10.1186/s12884-017-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Licciardone J, Buchanan S, Hensel K, King H, Fulda K, Stoll S. Osteopathic manipulative treatment of back pain and related symptoms during pregnancy: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(1):43:e1e8. doi: 10.1016/j.ajog.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Licciardone J, Aryal S. Prevention of progressive backspecific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113(10):728–736. doi: 10.7556/jaoa.2013.043. [DOI] [PubMed] [Google Scholar]
- 85.Hensel KL, Roane BM, Chaphekar AV, Smith-Barbaro P. PROMOTE Study: safety of osteopathic manipulative treatment during the third trimester by labor and delivery outcomes. J Am Osteopath Assoc. 2016;116(11):698–703. doi: 10.7556/jaoa.2016.140. [DOI] [PubMed] [Google Scholar]
- 86.Schwerla F, Rother K, Rother D, Ruetz M, Resch K-L. Osteopathic manipulative therapy in women with postpartum low back pain and disability: a pragmatic randomized controlled trial. J Am Osteopath Assoc. 2015;115(7):416–425. doi: 10.7556/jaoa.2015.087. [DOI] [PubMed] [Google Scholar]
- 87.Hensel K, Carnes M, Stoll S. Pregnancy research on osteopathic manipulation optimizing treatment effects: the PROMOTE study protocol. J Am Osteopath Assoc. 2016;116(11):716–724. doi: 10.7556/jaoa.2016.142. [DOI] [PubMed] [Google Scholar]
- 88.Al-Sayegh N, George S, Boninger M, Rogers J, Whitney S, Delitto A. Spinal mobilization of postpartum low back and pelvic girdle pain: an evidence-based clinical rule for predicting responders and nonresponders. PM R. 2010;2(11):995–1005. doi: 10.1016/j.pmrj.2010.07.481. [DOI] [PubMed] [Google Scholar]
- 89.Peterson C, Muhlemann D, Humphreys B. Outcomes of pregnant patients with low back pain undergoing chiropractic treatment: a prospective cohort study with short term, medium term and 1 year follow-up. Chiropr Man Therap. 2014;22(1):15. doi: 10.1186/2045-709X-22-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hastings V, McCallister AM, Curtis S, Valant R, Yao S. Efficacy of osteopathic manipulative treatment for management of postpartum pain. J Am Osteopath Assoc. 2016;116(8):502–509. doi: 10.7556/jaoa.2016.103. [DOI] [PubMed] [Google Scholar]
- 91.Haavik H, Murphy B, Kruger J. Effect of spinal manipulation on pelvic floor functional changes in pregnant and nonpregnant women: a preliminary study. J Manip Physiol Ther. 2016;39(5):339–347. doi: 10.1016/j.jmpt.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 92.Skarica B. Effectiveness of manual treatment on pregnancy symptoms: sefulness of manual treatment in treating pregnancy symptoms. Med Arch. 2018;72(2):131135. doi: 10.5455/medarh.2018.72.131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morton A. Internal carotid artery dissection following chiropractic treatment in a pregnant woman with Systemic Lupus Erythematosus. Chiropr Man Ther. 2012;20(38) doi: 10.1186/2045-709X-20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ioannidis JPA, Evans SJW, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT Statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 95.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Intl J Surg. 2010;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Tavares P, Barrett J, Hogg-Johnson S, Ho S, Corso M, Batley S, et al. Prevalence of low back pain, pelvic girdle pain, and combination pain in a postpartum Ontario population. J Obstet Gynaecol Canada. 2020;42(4):473480. doi: 10.1016/j.jogc.2019.08.030. [DOI] [PubMed] [Google Scholar]
- 97.Weis C, Barrett J, Tavares P, Draper C, Ngo K, Leung J, et al. Prevalence of low back pain, pelvic girdle pain, and combination pain in a pregnant Ontario population. J Obstet Gynaecol Canada. 2018;40(8):1038–1043. doi: 10.1016/j.jogc.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 98.Vohra S, Kawchuk G, Boon H, Caulfield T, Pohlman KA. SafetyNET: An interdisciplinary team supporting a safety culture for a spinal manipulation therapy. Eur J Integrat Med. 2014;6(4):473–477. [Google Scholar]
- 99.Pohlman KA, Funabashi M, Ndetan H, Hogg-Johnson S, Bodnar P, Kawchuk G. Assessing adverse events after chiropractic care at a chiropractic teaching clinic: an active surveillance pilot study. J Manip Physiol Ther. 2020;43(9):845–854. doi: 10.1016/j.jmpt.2020.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.