Abstract
Background
Patients with ulcerative colitis (UC) are at elevated risk of cardiovascular disease vs the general population, despite a lower prevalence of traditional risk factors, including hyperlipidemia. Mechanistic studies in patients with rheumatoid arthritis and psoriasis suggest that tofacitinib restores serum lipids to preinflammation levels by reversing inflammation-induced cholesterol metabolism changes. We reviewed data on lipid levels and cardiovascular events, alongside recommendations for managing lipid levels during tofacitinib treatment in patients with UC, based on up-to-date expert guidelines.
Methods
Data were identified from a phase 3/open-label, long-term extension (OLE) tofacitinib UC clinical program (cutoff May 27, 2019). Literature was identified from PubMed (search terms “lipid,” “cholesterol,” “lipoprotein,” “cardiovascular,” “inflammation,” “atherosclerosis,” “tofacitinib,” “rheumatoid arthritis,” “psoriasis,” “inflammatory bowel disease,” “ulcerative colitis,” “hyperlipidemia,” and “guidelines”) and author knowledge. Data were available from 4 phase 3 clinical trials of 1124 patients with moderately to severely active UC who received ≥1 dose of tofacitinib 5 or 10 mg twice daily in induction (two identical trials), maintenance, and OLE studies (treatment duration ≤6.8 years; 2576.4 patient-years of drug exposure).
Results
In the OLE study, tofacitinib treatment was not associated with major changes from baseline in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol/high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol/high-density lipoprotein cholesterol, with lipid levels and ratios generally remaining stable over time. The major adverse cardiovascular events incidence rate was 0.26/100 patient-years (95% confidence interval, 0.11-0.54).
Conclusions
Lipid levels and ratios remained generally unchanged from baseline in the OLE study after tofacitinib treatment, and major adverse cardiovascular events were infrequent. Long-term studies are ongoing.
ClinicalTrials.gov identifiers
Keywords: clinical trials, lipids, tofacitinib
INTRODUCTION
Patients with inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), have an elevated risk of cardiovascular morbidity relative to the general population, despite a lower prevalence of classic cardiovascular risk factors (eg, high body mass index, diabetes, hyperlipidemia, and hypertension).1-4 A systematic review and meta-analysis reported a 19% increase in the risk of ischemic heart disease, and an 18% increase in the risk of cerebrovascular accidents in patients with IBD.4
Tofacitinib is an oral, small-molecule Janus kinase inhibitor for the treatment of UC.5 In clinical studies, tofacitinib treatment has been associated with generally reversible increases in serum lipids during the first few months of therapy,6-10 with maximum effects observed within 6 weeks.5 However, these changes have not been associated with an increased cardiovascular risk in previous individual studies in rheumatoid arthritis and psoriasis.6, 10 The incidence rate (IR) of major adverse cardiovascular events (MACE; defined as adjudicated cardiovascular mortality and nonfatal cardiovascular events, including myocardial infarction and cerebrovascular events) among 4827 patients with 8699 patient-years of exposure in rheumatoid arthritis clinical studies was low and stable over time (5 years) at 0.37/100 patient-years (95% confidence interval [CI], 0.26-0.52).6 In previous reports from a UC clinical trial program (cutoff December 2016), in which patients received tofacitinib for up to 4.4 years, the IRs of MACE remained low at 0.24/100 patient-years (95% CI, 0.07-0.62).11, 12 There is an ongoing postmarketing, open-label, endpoint-driven safety study (surveillance study A3921133; ClinicialTrials.gov identifier: NCT02092467) comparing tofacitinib 5 or 10 mg twice daily (BID) to tumor necrosis factor inhibitor therapy in patients with rheumatoid arthritis who are ≥50 years of age and have ≥1 cardiovascular risk factor.13
This article reviews available evidence on lipid levels and cardiovascular events during tofacitinib treatment in patients with UC, discusses these data in the context of available data in other indications, and provides recommendations based on up-to-date expert guidelines on the practical management of lipid levels in patients with UC receiving tofacitinib.
METHODS
Data on serum lipid levels and cardiovascular events during tofacitinib treatment were obtained from the phase 3/open-label, long-term extension (OLE) tofacitinib UC clinical program (cutoff May 27, 2019). Relevant literature was identified from searches of PubMed using search terms such as “lipid,” “cholesterol,” “lipoprotein,” “cardiovascular,” “inflammation,” “atherosclerosis,” “tofacitinib,” “rheumatoid arthritis,” “psoriasis,” “inflammatory bowel disease,” “ulcerative colitis,” “hyperlipidemia,” and “guidelines.” Further relevant information was identified from the reference lists of articles returned using these search terms, and from the authors’ experience and knowledge of the literature. These queries formed a conceptual framework to interpret lipid findings in studies of tofacitinib in UC. Guidance for lipid management is based on the most up-to-date expert guidelines.
EFFECTS OF TOFACITINIB ON SERUM LIPIDS IN UC CLINICAL TRIALS
The phase 3/OLE tofacitinib UC clinical program comprises 4 studies: 2 identical, 8-week induction studies of patients with moderately to severely active UC (OCTAVE Induction 1 [ClinicalTrials.gov identifier: NCT01465763] and OCTAVE Induction 2 [ClinicalTrials.gov identifier: NCT01458951]), a 52-week maintenance study that enrolled responders from OCTAVE Induction 1 and 2 (OCTAVE Sustain [ClinicalTrials.gov identifier: NCT01458574]), and an ongoing OLE study that enrolled patients who completed OCTAVE Induction 1 and 2 and were nonresponders or who completed or demonstrated treatment failure in OCTAVE Sustain (OCTAVE Open [ClinicalTrials.gov identifier: NCT01470612]). Details of these studies have been described previously and are summarized in Supplementary Table S1 and Supplementary Fig. S1.11, 12, 14, 15
The phase 3/OLE tofacitinib UC clinical program includes 1124 patients with moderately to severely active UC who received at least 1 dose of tofacitinib 5 or 10 mg BID in the phase 3 induction, maintenance, and OLE studies, with treatment duration up to 6.8 years and with 2576.4 patient-years of drug exposure. Patients had a mean age of 41.2 years, and the majority had a body mass index <25 kg/m2. Approximately 5% of patients in the cohort were current smokers and 31% were former smokers (Table 1).
TABLE 1.
Demographics and Baseline Characteristics of Patients With Ulcerative Colitis in the Phase 3 Induction, Maintenance, and OLE Studies of Tofacitinib Who Received Tofacitinib 5 or 10 mg BID
| Tofacitinib (all) (N = 1124) |
|
|---|---|
| Age, y, mean (SD) | 41.2 (13.9) |
| Male, n (%) | 658 (58.5) |
| Race, n (%) White Black Asian Other Unspecified |
897 (79.8) 10 (0.9) 144 (12.8) 39 (3.5) 34 (3.0) |
| Body mass index (kg/m2), n (%)* <25 25 to <30 ≥30 |
665 (59.2) 303 (27.0) 155 (13.8) |
| Smoking history, n (%)† Current Never Stopped |
58 (5.2) 716 (63.7) 350 (31.1) |
| Disease duration, y, mean (SD) | 8.2 (7.0) |
| Extent of disease, n (%)†, ‡ Proctosigmoiditis Left-sided colitis Extensive/pancolitis Proctitis |
163 (14.5) 380 (33.9) 577 (51.5) 1 (0.1) |
| Total Mayo score at baseline, mean (SD)§ | 8.6 (2.0) |
| Oral corticosteroid use at baseline, n (%) | 505 (44.9) |
| Prior tumor necrosis factor inhibitor failure, n (%)† | 583 (51.9) |
| Treatment duration, d, median (range) | 686 (1 to 2494) |
*N = 1123.
†Based on data collected at the start of the phase 3 induction studies.
‡N = 1121.
§N = 1122.
Abbreviations: BID, twice daily; N, number of patients; n, number of patients within the given category; OLE, open-label, long-term extension; SD, standard deviation.
In healthy women and men without diabetes, the Reynolds Risk Score can be used to predict the risk of having a future heart attack, stroke, or major heart disease in the next 10 years, based on age, blood pressure, smoking status, history of heart attacks in parents (<60 years of age), total cholesterol levels, and high-sensitivity C-reactive protein levels (marker of inflammation).16, 17 A mean Reynolds Risk Score of 2.1 following 8 weeks of tofacitinib 10 mg BID induction therapy has previously been reported.12 At baseline in the tofacitinib UC clinical program, the majority of patients had a <5% risk of a cardiovascular event in the next 10 years based on their Reynolds Risk Score (Supplementary Table S2). Higher proportions of patients in the groups of males >45 years of age and females >55 years of age had markers of cardiovascular risk (Reynolds Risk Score ≥5%, body mass index ≥25 kg/m2, hypertension, diabetes, hyperlipidemia, and history as an ex-smoker) (Supplementary Table S2).
Serum lipid parameters were measured at baseline and at week 8 in the induction studies, up to week 61 for patients who received tofacitinib 5 or 10 mg BID or placebo in OCTAVE Sustain, and up to month 48 for patients who received tofacitinib 5 or 10 mg BID in the OLE study (Supplementary Table S3). After 8 weeks of tofacitinib 10 mg BID induction therapy, serum levels of total cholesterol (total-c), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) increased relative to baseline and remained elevated up to week 61 for patients on maintenance treatment using tofacitinib 5 or 10 mg BID.12 Differences for tofacitinib vs placebo for total-c, HDL-c, and LDL-c generally remained stable between weeks 17 and 61.12 Changes in total-c/HDL-c and LDL-c/HDL-c ratios were minimal during the induction and maintenance studies.12
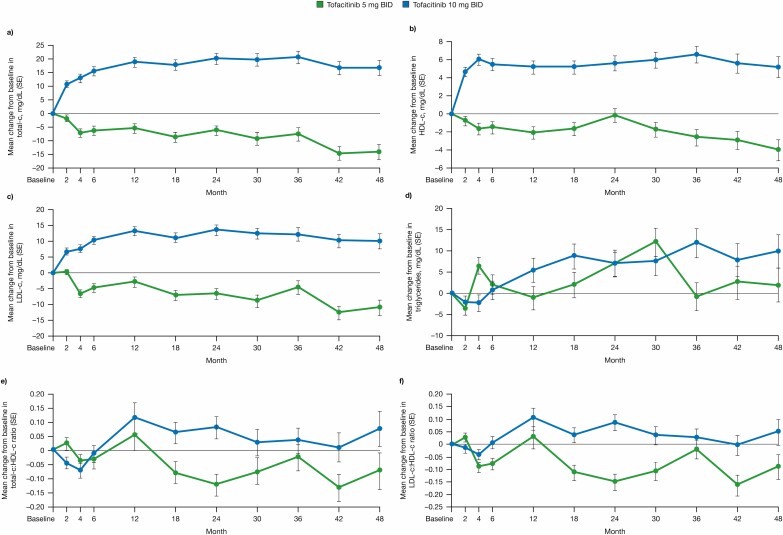
Patients from OCTAVE Sustain who entered the OLE study in remission received tofacitinib 5 mg BID; all other patients entering the OLE study (ie, patients with treatment failure and those who were not in remission) received tofacitinib 10 mg BID. The mean changes from the OLE study baseline in total-c, HDL-c, LDL-c, and triglycerides to month 48 of the OLE study are summarized in Fig. 1, with tofacitinib 5 or 10 mg BID not associated with major changes from baseline over time. Mean levels of LDL-c at month 48 were only slightly higher than the recommended target for patients with a low cardiovascular risk (<116 mg/dL).18 Lipid levels and ratios were generally stable over time in the OLE study.
FIGURE 1.
Mean change from baseline in lipid levels to month 48 in the OLE study. A, Total-c. B, HDL-c. C, LDL-c. D, Triglycerides. E, Ratio of total-c/HDL-c. F, Ratio of LDL-c/HDL-c. Error bars represent standard error. Abbreviations: BID, twice daily; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; OLE, open-label, long-term extension; total-c, total cholesterol; SE, standard error.
The mean percentage changes from the OLE study baseline at month 48 for total-c, HDL-c, LDL-c, and triglycerides are shown in Table 2, and the shift tables for total-c, HDL-c, LDL-c, and triglycerides in the OLE study are shown in Table 3.
TABLE 2.
Mean (SD) Percent Change from Baseline in Total-c, HDL-c, LDL-c, and Triglycerides Over Time in the OLE Study
| Visit (mo) |
Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | |||
|---|---|---|---|---|---|
| n | % (SD) | n | % (SD) | ||
| Total-c | 12 | 164 | –1.6 (14.1) | 480 | 11.4 (21.1) |
| 24 | 139 | –1.5 (15.6) | 399 | 12.2 (21.4) | |
| 36 | 121 | –2.4 (16.4) | 328 | 12.8 (23.2) | |
| 48 | 52 | –5.1 (18.2) | 213 | 10.4 (21.6) | |
| HDL-c | 12 | 164 | –2.0 (17.1) | 479 | 11.3 (27.4) |
| 24 | 139 | 1.1 (15.4) | 399 | 12.2 (27.2) | |
| 36 | 121 | –2.5 (14.6) | 328 | 13.6 (27.7) | |
| 48 | 53 | –5.1 (11.8) | 213 | 10.9 (28.7) | |
| LDL-c | 12 | 161 | –0.1 (19.9) | 475 | 14.4 (30.0) |
| 24 | 135 | –2.8 (23.9) | 397 | 14.9 (31.0) | |
| 36 | 119 | –1.3 (24.0) | 326 | 14.5 (33.3) | |
| 48 | 51 | –5.1 (26.2) | 213 | 12.4 (32.3) | |
| Triglycerides | 12 | 164 | 6.1 (38.5) | 479 | 10.6 (49.8) |
| 24 | 139 | 15.4 (52.8) | 399 | 13.8 (55.4) | |
| 36 | 120 | 12.2 (47.0) | 328 | 19.6 (65.8) | |
| 48 | 52 | 16.0 (43.2) | 213 | 18.3 (64.1) |
Abbreviations: BID, twice daily; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; n, number of patients with non-missing data; OLE, open-label, long-term extension; SD, standard deviation; total-c, total cholesterol.
TABLE 3.
Maximum Observed Concentrations of LDL-c, HDL-c, Total-c, and Triglycerides Up to Month 48 in the OLE Study According to OLE Study Baseline Levels
| OLE Study Treatment | Maximum Observed Concentrations of LDL-c (mg/dL), n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | <100 | 100 to <130 | 130 to <160 | 160 to <190 | ≥190 | |
| Tofacitinib 5 mg BID (n = 174) | <100 100 to <130 130 to <160 160 to <190 ≥190 |
18 (10.3) 1 (0.6) 0 (0.0) 0 (0.0) 0 (0.0) |
17 (9.8) 16 (9.2) 1 (0.6) 0 (0.0) 0 (0.0) |
6 (3.4) 24 (13.8) 15 (8.6) 1 (0.6) 0 (0.0) |
0 (0.0) 8 (4.6) 18 (10.3) 13 (7.5) 3 (1.7) |
0 (0.0) 2 (1.1) 3 (1.7) 12 (6.9) 16 (9.2) |
| Tofacitinib 10 mg BID (n = 752) | <100 100 to <130 130 to <160 160 to <190 ≥190 |
146 (19.4) 31 (4.1) 4 (0.5) 1 (0.1) 1 (0.1) |
104 (13.8) 73 (9.7) 12 (1.6) 2 (0.3) 0 (0.0) |
50 (6.6) 103 (13.7) 29 (3.9) 6 (0.8) 1 (0.1) |
11 (1.5) 38 (5.1) 36 (4.8) 19 (2.5) 3 (0.4) |
4 (0.5) 15 (2.0) 32 (4.3) 20 (2.7) 11 (1.5) |
| Maximum Observed Concentrations of HDL-c (mg/dL), n (%) | ||||||
| Baseline | <40 | 40 to <60 | ≥60 | |||
| Tofacitinib 5 mg BID (n = 175) |
<40 40 to <60 ≥60 |
2 (1.1) 0 (0.0) 0 (0.0) |
9 (5.1) 30 (17.1) 4 (2.3) |
1 (0.6) 32 (18.3) 97 (55.4) |
||
| Tofacitinib 10 mg BID (n = 753) | <40 40 to <60 ≥60 |
10 (1.3) 8 (1.1) 1 (0.1) |
42 (5.6) 146 (19.4) 30 (4.0) |
17 (2.3) 159 (21.1) 340 (45.2) |
||
| Maximum Observed Concentrations of Total-c (mg/dL), n (%) | ||||||
| Baseline | <200 | 200 to <240 | ≥240 | |||
| Tofacitinib 5 mg BID (n = 175) | <200 200 to <240 ≥240 |
33 (18.9) 5 (2.9) 0 (0.0) |
20 (11.4) 22 (12.6) 2 (1.1) |
6 (3.4) 29 (16.6) 58 (33.1) |
||
| Tofacitinib 10 mg BID (n = 753) | <200 200 to <240 ≥240 |
228 (30.3) 23 (3.1) 6 (0.8) |
145 (19.3) 65 (8.6) 10 (1.3) |
85 (11.3) 109 (14.5) 82 (10.9) |
||
| Maximum Observed Concentrations of Triglycerides (mg/dL), n (%) | ||||||
| Baseline | <150 | 150 to <200 | 200 to <500 | ≥500 | ||
| Tofacitinib 5 mg BID (n = 175) | <150 150 to <200 200 to <500 ≥500 |
77 (44.0) 2 (1.1) 1 (0.6) 0 (0.0) |
32 (18.3) 2 (1.1) 2 (1.1) 0 (0.0) |
19 (10.9) 19 (10.9) 16 (9.1) 0 (0.0) |
1 (0.6) 0 (0.0) 3 (1.7) 1 (0.6) |
|
| Tofacitinib 10 mg BID (n = 753) | <150 150 to <200 200 to <500 ≥500 |
460 (61.1) 21 (2.8) 8 (1.1) 0 (0.0) |
104 (13.8) 11 (1.5) 7 (0.9) 0 (0.0) |
71 (9.4) 29 (3.9) 36 (4.8) 0 (0.0) |
4 (0.5) 0 (0.0) 2 (0.3) 0 (0.0) |
Baseline defined as month 0 of the OLE study. % is the overall percentage of patients with the observation within a treatment group. The denominator is the total n for the treatment group. Abbreviations: BID, twice daily; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; OLE, open-label, long-term extension; total-c, total cholesterol.
The use of lipid-lowering agents was permitted in the tofacitinib UC clinical trials, based on the patients’ risk factors and local/national guidance. The dose of lipid-lowering agents, which included Anatomical Therapeutic Chemical 2 lipid-modifying agents (excluding fish oil preparations), was increased for 1.9% of patients, and 7.7% of patients added new lipid-lowering agents.
The findings on lipid parameters in the tofacitinib UC trials were generally similar to those of clinical studies of tofacitinib in patients with other inflammatory conditions6-8, 10 as well as studies of other Janus kinase inhibitors in rheumatoid arthritis and CD.19-21 For example, in a phase 2 study of patients with CD, treatment with the Janus kinase-1 selective inhibitor, filgotinib, was associated with increases in LDL-c and HDL-c levels of 12% and 11%, respectively, at week 20, with a corresponding change of 3% in the LDL-c/HDL-c ratio.20 It is important to note that across the other conditions studied in the tofacitinib clinical trials, ie, rheumatoid arthritis, psoriasis, and psoriatic arthritis, where patients have different clinical characteristics and demographics, the data also showed no significant change in the ratios of LDL-c/HDL-c and/or total-c/HDL-c,6, 7, 10 both of which are markers of increased cardiovascular risk.22
Implications of Lipid Elevations on Cardiovascular Safety in Patients Receiving Tofacitinib
In the phase 3/OLE tofacitinib UC clinical program, 7 adjudicated MACE (defined as death due to cardiovascular causes, or nonfatal myocardial infarction or stroke) were reported, with an IR of 0.26/100 patient-years (2654.66 patient-years of exposure; 95% CI, 0.11-0.54); the IR remained stable from December 2016 (IR, 0.24; 95% CI, 0.07-0.62).12 MACE were reported for 2 patients during induction (acute coronary syndrome in a patient who received a predominant dose of tofacitinib 5 mg BID [defined as average daily dose <15 mg], and fatal aortic dissection in a patient who received a predominant dose of tofacitinib 10 mg BID [defined as average daily dose ≥15 mg]). The fatal event of aortic dissection occurred in a male patient aged 39 years who had untreated baseline hyperlipidemia (LDL-c, 189 mg/dL; total-c, 308 mg/dL); this event was considered by the investigator to be unrelated to study treatment.
MACE were also reported for 2 patients during maintenance (myocardial infarction in a patient who received a predominant dose of tofacitinib 5 mg BID, and hemorrhagic stroke in a patient who received a predominant dose of tofacitinib 10 mg BID), and 3 patients during the OLE study (acute myocardial infarction and cerebellar hemorrhage in 2 patients who received a predominant dose of tofacitinib 5 mg BID, and cerebrovascular accident in a patient who received a predominant dose of tofacitinib 10 mg BID). Both myocardial infarction events and the acute coronary syndrome event led to temporary tofacitinib discontinuation, and the hemorrhagic stroke, cerebrovascular accident, and cerebellar hemorrhage led to permanent tofacitinib discontinuation. Of the 7 patients with MACE, 5 had multiple cardiovascular risk factors at baseline.
The low incidence of MACE in the tofacitinib UC clinical studies was comparable with that reported in the clinical trials of tofacitinib in rheumatoid arthritis (MACE IR, 0.37; 95% CI, 0.26-0.52 in OLE studies [n = 4827; 8699 patient-years of exposure]),6 psoriasis (MACE IR, 0.37; 95% CI, 0.22-0.57; n = 3623; 5204 patient-years of exposure),10, 23 and psoriatic arthritis (MACE IR, 0.24; 95% CI, 0.05-0.70; n = 783; 1238 patient-years of exposure) programs.7 A retrospective cohort study of 459 patients with UC who had received treatment with tumor necrosis factor inhibitors revealed an IR for MACE of 2.37/100 patient-years of exposure; however, it should be noted that this result is based on data from a claims database, and so cannot be directly compared with the incidence derived from a clinical trial setting.24
A phase 3 trial of tofacitinib in psoriasis has also demonstrated reductions of circulating biomarkers of cardiovascular risk, including high-sensitivity C-reactive protein, for tofacitinib vs placebo.10 However, assessment of the risk of cardiovascular disease in relation to LDL-c elevation with tofacitinib treatment will require longer-term exposure and follow-up across indications.
CARDIOVASCULAR RISKS
Chronic Inflammation
Atherogenic dyslipidemia, characterized by increased serum concentrations of LDL-c, apolipoprotein B (the main structural protein component of LDL particles), and triglycerides, and low serum concentrations of HDL-c, is an established risk factor for cardiovascular disease.25, 26 Infiltration of the arterial wall by apolipoprotein B-containing lipoproteins is an initiating event in the pathogenesis of atherosclerotic plaques, and is closely linked to inflammation, which modulates both the atherogenic lipid profile and the endothelium.26, 27
Chronic systemic inflammation is associated with changes in atherogenic lipid metabolism that may impact the risk of atherosclerosis and cardiovascular disease.28 Levels of serum lipids and lipoproteins can change significantly during acute and chronic inflammation.29, 30 Previous studies in rheumatoid arthritis have reported lower lipid levels in patients with active inflammation vs those without.31-34 Despite this finding, the risk of cardiovascular disease is elevated in patients with rheumatoid arthritis, suggesting a complex interaction between systemic inflammation and atherogenic lipids.35 It has been demonstrated that systemic inflammation can influence the composition and function of LDL and HDL particles, and may therefore promote a more atherogenic profile.36, 37 The function of HDL particles is important for reverse cholesterol transport, an essential pathway for maintaining cholesterol homeostasis, which prevents atherosclerotic plaque formation and toxic intracellular levels of cholesterol. This pathway removes free cholesterol from tissues to the HDL particle for esterification by lecithin-cholesterol acyltransferase, to generate a mature HDL particle, which can be delivered either directly to the liver or via cholesterol ester transfer protein and LDL for fecal excretion. Dysfunction of HDL particles has been identified in patients with rheumatoid arthritis and psoriasis.37, 38 In rheumatoid arthritis, it has been suggested that increases in cholesterol ester catabolism without an increase in cholesterol esterification drive low levels of cholesterol in the setting of active inflammation.38 Consistent with findings in patients with rheumatoid arthritis, levels of serum LDL-c and total-c are lower in patients with active IBD than in healthy control patients, and correlate with the extent of systemic inflammation.39
Potential Mechanistic Effects of Tofacitinib on Lipids and Inflammation
The relationship between the effects of tofacitinib on serum lipids and its effects on inflammation through Janus kinase inhibition is unclear. Similar effects on lipids have been reported with tofacitinib in other patient populations with inflammatory conditions6, 40-42 and with other classes of anti-inflammatory agents,43-45 supporting a link between suppression of active inflammation and elevation of lipid levels.
Immunosuppressive agents used for the treatment of IBD have also been associated with changes in lipid parameters in patients with other inflammatory conditions. For example, meta-analyses have shown that, in patients with rheumatoid arthritis, use of tumor necrosis factor inhibitors is associated with significant elevations from baseline in total-c and HDL-c.43, 44 Similarly, in patients with ankylosing spondylitis, elevation of total-c and HDL-c in response to tumor necrosis factor inhibitor therapy correlated with a reduction in systemic inflammation measured by C-reactive protein.46 Furthermore, an inverse relationship was observed at week 8 between decreases in high-sensitivity C-reactive protein levels and increases in total-c, HDL-c, and LDL-c levels in patients with UC who received placebo or tofacitinib 10 mg BID in the OCTAVE Induction 1 and 2 studies.12 Physicians managing patients with IBD therefore need to be aware of the potential for therapy-induced elevation in lipid concentrations and need to implement risk-management strategies appropriate for the individual patient’s level of cardiovascular risk.
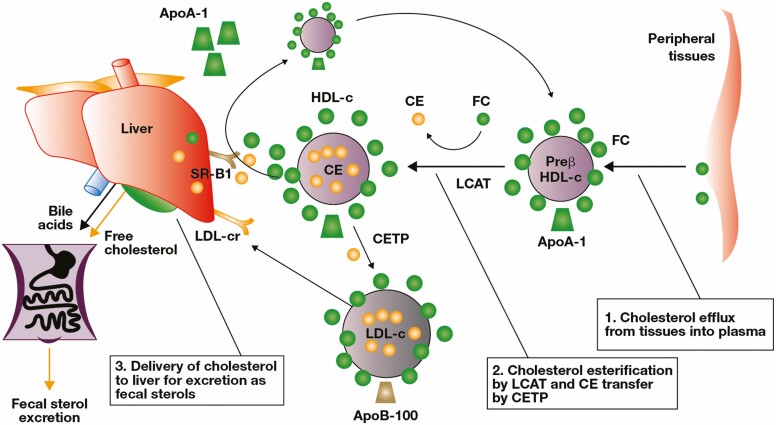
A phase 1 mechanism of action study of tofacitinib investigated the possible mechanisms through which tofacitinib might reverse the abnormal lipid profile associated with active inflammation in rheumatoid arthritis.38 In this study, it was demonstrated that the cholesterol ester fractional catabolic rate was higher in patients with rheumatoid arthritis compared with healthy volunteers, while the cholesterol ester production rate and rate of cholesterol efflux were similar. This suggests that lower levels of serum total-c, LDL-c, and HDL-c in rheumatoid arthritis are driven by increased cholesterol ester catabolism (Fig. 2). In addition, tofacitinib treatment was associated with a decrease in the cholesterol ester fractional catabolic rate and a concomitant significant increase in serum HDL-c towards levels found in healthy volunteers. Markers of anti-atherogenic HDL functions (antioxidant and efflux functions) also improved with tofacitinib treatment, including decreased serum amyloid A and increased activity and mass of lecithin-cholesterol acyltransferase, the enzyme that promotes the conversion of free cholesterol to esters for transport to the liver (Fig. 2). In line with these findings, data from a clinical study of patients with psoriasis have also demonstrated an increase in the activity of lecithin-cholesterol acyltransferase and paraoxonase-1, and a decrease in HDL-associated serum amyloid A following tofacitinib treatment.10 The implications of these beneficial effects of tofacitinib on anti-atherogenic HDL function for cardiovascular risk require further investigation.
FIGURE 2.
Reverse cholesterol transport pathway (reproduced with permission from Charles-Schoeman et al38). Abbreviations: ApoA-1, apolipoprotein A-1; ApoB-100, apolipoprotein B-100; CE, cholesterol ester; CETP, cholesterol ester transfer protein; FC, free cholesterol; HDL-c, high-density lipoprotein cholesterol; LCAT, lecithin-cholesterol acyltransferase; LDL-c, low-density lipoprotein cholesterol; LDL-cr, LDL-c receptor; SR-B1, scavenger receptor class B type 1.
MANAGEMENT OF LIPIDS IN PATIENTS RECEIVING TOFACITINIB
Long-term follow-up studies will further clarify whether lipid elevations observed during tofacitinib therapy increase the risk of cardiovascular mortality and morbidity. It is important for gastroenterologists to recognize the impact that tofacitinib has on inflammation and lipid levels, and to monitor lipids in individual patients based on clinical guidelines for hyperlipidemia if results are abnormal.
Clinical guidelines for reducing the risk of atherosclerotic cardiovascular disease18, 47 are based on risk categories determined by clinical factors (eg, existing cardiovascular disease, diabetes, chronic kidney disease, familial hyperlipidemia) and estimates of cardiovascular risk determined using risk calculation systems, such as the Framingham Score,48 Reynolds Risk Score,16 Risk Calculator,47 and Systematic Coronary Risk Estimation chart49 in Europe.18 These systems consider multiple interacting factors such as age, sex, smoking history, blood pressure, cholesterol, and sometimes, levels of high-sensitivity C-reactive protein.
Lipid Profiling for Monitoring Dyslipidemia
The European Society of Cardiology and European Atherosclerosis Society recommend that lipid profiling (LDL-c, HDL-c, triglycerides, and non-HDL-c or apolipoprotein B) should be conducted for all patients with cardiovascular disease or clinical conditions/family history associated with increased risk of cardiovascular disease, and that risk factor screening, including lipid profiling, should be considered for all asymptomatic male patients >40 years of age and female patients >50 years of age or who are postmenopausal.18 It is also recommended that risk assessment using a system such as Systematic Coronary Risk Estimation, which was developed to directly estimate total fatal cardiovascular risk in European clinical practice,49 should be conducted for all other individuals, because other factors, including inflammatory disorders, may modify cardiovascular risk.18 LDL-c is recommended as the primary lipid parameter for risk estimation and management, but HDL-c, non-HDL-c, and triglycerides should also be evaluated before treatment of dyslipidemia. Levels of LDL-c at which intervention is recommended are classified as a function of risk (Supplementary Table S4). The American College of Cardiology/American Heart Association Task Force 2018 report47 included the identification of patient groups who will benefit from statin therapy, and the age groups, risk factors, and LDL-c concentration ranges used to define these are summarized in Supplementary Table S5. These guideline recommendations highlight the importance of using risk assessment to identify patients who will benefit from intervention.
Interventions for Dyslipidemia
A treat-to-target approach based on a patient’s calculated cardiovascular risk is recommended in European guidelines.18 European Society of Cardiology/European Atherosclerosis Society guidelines recommend a primary objective of reducing LDL-c levels, with the treatment goal determined by risk category (see Supplementary Table S4 for full list of risk categories).18 American College of Cardiology/American Heart Association guidelines define intensity of statin therapy according to the required percentage LDL-c reduction (high intensity: ≥50%; moderate intensity: 30% to 49%; low intensity: <30%).47
Guideline recommendations on interventions to help patients achieve these goals include lifestyle modification of risk factors (heart-healthy diet, maintaining healthy body weight, regular physical activity, cessation of smoking, etc.) in conjunction with the use of lipid-lowering agents.18, 47 Patients at low or moderate risk, in whom lipid levels remain uncontrolled, and patients at high or very high risk of cardiovascular disease with LDL-c levels ≥70 mg/dL and ≥55 mg/dL, respectively, can receive a high-intensity HMG-CoA reductase inhibitor (statin) at the highest recommended or highest tolerable dose to reach the goal.18 For elderly patients, therapy may be started at a lower dose and titrated to achieve the target level, particularly when drug interactions are possible or renal function is significantly impaired.18 Additional treatment options for those patients who cannot tolerate high-dose statin therapy, or who do not achieve target lipid levels, include combination therapy with cholesterol absorption inhibitors and proprotein convertase subtilisin/kexin type 9 inhibitors.18 Investigating secondary causes of hyperlipidemia in patients should also be indicated,47 such as those due to metabolic causes, including hypothyroidism. Intensification of lipid-lowering therapy or referral to a specialist should be considered in those patients who do not respond to primary lipid-lowering therapy and lifestyle modification. Referral to specialist clinics may also be considered initially for those with documented cardiovascular disease or high risk at baseline.18
Lipid levels, adherence to statin therapy and lifestyle modification, and adverse events should be monitored regularly for patients who are receiving lipid-lowering agents.18, 47
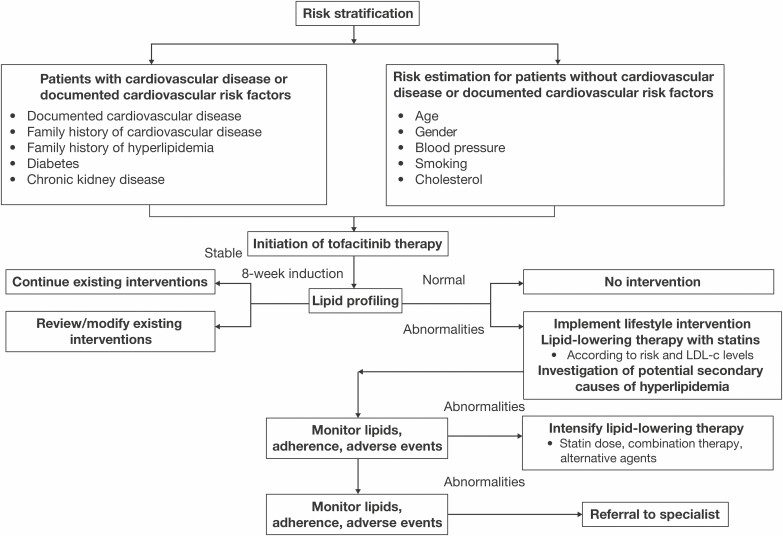
For patients initiating tofacitinib therapy, levels of lipid parameters should be monitored within approximately 4 to 8 weeks of initiating therapy, and the patient should be managed according to current clinical guidelines.5, 50 Following 8 weeks of tofacitinib induction therapy, lipid levels may be expected to have stabilized due to the effects of therapy on inflammation. Use of lipid panels before treatment initiation or during periods of acute flare may not be informative, due to the effects of acute inflammation in suppressing lipid metabolism leading to low lipid levels. However, for those patients with recognized cardiovascular risk factors, lipid profiles prior to treatment with tofacitinib should be monitored and managed as per applicable clinical guidelines. General recommendations for lipid management in patients with UC initiating tofacitinib therapy are summarized in Fig. 3.
FIGURE 3.
Algorithm for management of lipids in patients receiving tofacitinib. Risk assessment, stratification of patient, and monitoring of lipids should be based on clinical practice guidelines for hyperlipidemia (European Society of Cardiology, European Atherosclerosis Society, American College of Cardiology, American Heart Association). Abbreviations: LDL-c, low-density lipoprotein cholesterol.
Evidence for Effects of Statin Therapy in Patients With IBD
Although there are no randomized clinical trials examining the impact of LDL-lowering with statin therapy in patients with IBD, LDL-lowering in the general population (irrespective of comorbidities) has been associated with improved cardiovascular outcomes. Atorvastatin 10 mg once daily concomitant with tofacitinib 10 mg BID has been investigated in a 12-week, placebo-controlled, phase 2 study in patients with rheumatoid arthritis.51 The study demonstrated rapid and significant reductions in LDL-c, triglycerides, and apolipoprotein B with administration of atorvastatin at week 6 for 6 weeks, supporting the use of statin therapy as a feasible and well-tolerated approach to reducing global cardiovascular risk in patients with rheumatoid arthritis.51 However, further studies are needed to determine how lipid-lowering therapy should be used when stopping or interrupting tofacitinib treatment. In addition, it remains unclear what impact the lowering of lipid levels with statins has on cardiovascular risk over time in patients with IBD. The antiatherogenic effect of statins is also mediated through nonlipid mechanisms, including direct effects in the vascular wall that have an impact on endothelial function, vascular inflammation, smooth muscle cells, and plaque stability.52, 53 Endothelial dysfunction, which occurs in the early, subclinical phase of atherosclerosis, has been identified in patients with UC regardless of traditional cardiovascular risk factors.54, 55 During active disease in patients with IBD, damage to the intestinal vascular endothelial cells is more prominent.56 This raises the question of whether cardiovascular risk in patients with IBD can be modified by treatment to reduce disease activity, and whether there are benefits of statin therapy in IBD beyond lipid-lowering.
In patients with rheumatoid arthritis who received atorvastatin vs placebo, a numerical improvement in rheumatoid arthritis disease activity endpoints (American College of Rheumatology responses) was reported in addition to the effects on lipids.51 A similar anti-inflammatory effect of statin therapy (on the rheumatoid arthritis endpoints Disease Activity Score in 28 joints, swollen joint counts, C-reactive protein, and erythrocyte sedimentation rate) was described in a placebo-controlled study of patients receiving disease-modifying antirheumatic drugs.57 It is established that statins have pleiotropic anti-inflammatory properties in addition to lipid-lowering effects,58 and have an impact on immunity and mucosal inflammation in the gastrointestinal tract.58 Studies in animal models indicat that statins may modify disease activity in IBD through the beneficial effects of decreasing both inflammation and fibrosis; 58-61 however, there are currently limited data from clinical studies to support these effects in patients.
A small, open-label study of 10 patients with CD demonstrated that atorvastatin, added to standard anti-inflammatory therapy, reduced markers of systemic and mucosal inflammation (eg, C-reactive protein and fecal calprotectin) and numerically improved Crohn’s Disease Activity Index scores.62 A large retrospective study of a U.S. administrative claims database, which included 1986 statin-exposed patients with IBD, showed that statin exposure was associated with a reduction in the rate of first prescription for oral corticosteroids (courses ≥14 days) among the patients with UC (n = 1132; hazard ratio, 0.75; 95% CI, 0.62-0.91).63 A trend towards reduced use of tumor necrosis factor inhibitors and rates of abdominal surgery or hospitalization in patients with UC was also reported.63 In addition, protective effects of statin exposure against the onset of IBD, including UC, and against the development of colorectal cancer among patients with IBD have been reported and may also support an anti-inflammatory effect of statins.64-67 In contrast, a small, placebo-controlled study of atorvastatin showed no benefit of statin therapy vs placebo in patients with UC,68 indicating a need for larger, randomized studies to investigate potential effects on inflammation and disease activity.
In the phase 3/OLE tofacitinib UC clinical program, 71 (6.3%) patients were receiving lipid-lowering agents at baseline and received concomitant treatment with tofacitinib. Further studies are required to elucidate the effects of statin therapy in conjunction with tofacitinib on disease activity and cardiovascular risk in patients with UC.
CONCLUSIONS
Patients with IBD have an increased risk of cardiovascular disease relative to the general population, despite low prevalence of established cardiovascular risk factors and lower levels of serum cholesterol. Systemic inflammation may contribute to a pro-atherogenic lipid profile through effects on the composition and function of LDL-c and HDL-c particles.
Elevations in LDL-c, HDL-c, total-c, and triglycerides have been reported in clinical trials of tofacitinib in patients with UC. However, ratios of LDL-c/HDL-c and total-c/HDL-c, which have been identified as strong predictors of cardiovascular risk in the general population, were generally unaffected by tofacitinib treatment, and levels of LDL-c were only slightly higher than the recommended target for patients with a low cardiovascular risk. In addition, MACE were reported at low frequency in the phase 3/OLE tofacitinib UC clinical program, and a low proportion of patients increased their dose of, or added, a new lipid-lowering agent.
Mechanistic studies in other inflammatory conditions have shown that control of systemic inflammation with tofacitinib is associated with a reversal of increased cholesterol ester metabolism, as well as improvement of markers of anti-atherogenic HDL function. The clinical significance of lipid elevations induced by tofacitinib and also observed with other anti-inflammatory agents is not yet fully determined. Longer-term studies are required to further evaluate the risk for cardiovascular disease and the impact of stopping or interrupting therapy on lipid profiles.
As per the approved label, patients commencing tofacitinib therapy should have lipid levels monitored after approximately 4 to 8 weeks, and up-to-date expert guidelines for hyperlipidemia management should be followed. Physicians should follow general clinical guidance on stratification of patients according to cardiovascular risk prior to initiating any therapy, to identify those patients who may benefit from counseling and regular lipid monitoring, and to identify those requiring lipid-lowering intervention during tofacitinib therapy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients, investigators, and trial teams who were involved in the tofacitinib clinical trial programs. James Clark (Pfizer Inc, Cambridge, MA) provided critical review and discussion of the manuscript content. Statistical support was provided by Hyejin Jo (Syneos Health, Washington, DC). Medical writing support, under the guidance of the authors, was provided by Karl Baumforth, PhD, and Kirsteen Munn, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc (New York, NY) in accordance with Good Publication Practice (GPP3) guidelines.
Author contributions: All authors have contributed to the manuscript’s conception and design, critically reviewed it, approved the final version for submission, take public responsibility for the content, and agree to be accountable for all aspects of the work. All authors had full access to all the data in the manuscript and had final responsibility for the decision to submit for publication.
Supported by: These studies (NCT01465763, NCT01458951, NCT01458574, NCT01470612) were sponsored by Pfizer Inc.
Conflicts of interest: BES has served as a consultant for 4D Pharma, AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Capella Biosciences, Celltrion Healthcare, EnGene, Ferring Pharmaceuticals, Gilead, Lyndra, Oppilan Pharma, Otsuka, Palatin Technologies, Progenity, Protagonist Therapeutics, Rheos Medicines, Seres Therapeutics, Sienna Biopharmaceuticals, Takeda, Theravance Pharmaceuticals, and Vivelix Pharmaceuticals; has served on advisory boards for Celgene, Eli Lilly, Janssen, MedImmune, Millennium Pharmaceuticals, and Pfizer Inc; and has received research funding from Janssen and Takeda. JFC has served as a consultant or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Ferring Pharmaceuticals, Genentech, Johnson & Johnson, MedImmune, Merck & Co., Pfizer Inc, Protagonist Therapeutics, Second Genome, Seres Therapeutics, Shire, Takeda, Theradiag, and Tigenix; has been a speaker for AbbVie, Ferring Pharmaceuticals, and Takeda; has been part of the speakers’ bureau for Amgen; has stock options with Genfit and Intestinal Biotech Development; and has received research grants from AbbVie, Janssen, and Takeda. CH has served as a consultant for AbbVie, Ferring Pharmaceuticals, Genentech, Janssen, Pfizer Inc, Salix, and Takeda. MF has received grants, consulting fees, and/or honoraria for delivering lectures from Abbott, Akcea/Ionis, Amarin, Amgen, AstraZeneca, Daiichi Sankyo, Eli Lilly, Kowa, Merck & Co., Mylan, Pfizer Inc, Roche, Sanofi/Regeneron, and Servier. AA has served as a consultant and/or advisory board member for AbbVie, Allergan, Amgen, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Gilead, Hospira, Janssen, MSD, Mundipharma, Mylan, Pfizer Inc, Samsung Bioepis, Sandoz, Sofar, and Takeda; has received lecture and/or speaker bureau fees from AbbVie, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb, Chiesi, Ferring Pharmaceuticals, Gilead, Hospira, Janssen, Mitsubishi Tanabe, MSD, Mundipharma, Nikkiso, Otsuka, Pfizer Inc, Samsung Bioepis, Sandoz, Takeda, Tigenix, and Zambon; and has received research grants from MSD, Pfizer Inc, and Takeda. DQ, GSF, KK, LS, and CS are employees and stockholders of Pfizer Inc. PRT has served as a consultant, speaker, and/or advisory board member for Amarin, Amgen, Boehringer Ingelheim, Janssen, Novo Nordisk, Pfizer Inc, and Sanofi/Regeneron.
Data-sharing statement: Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (a) for indications that have been approved in the US and/or EU or (b) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
REFERENCES
- 1. Baena-Díez JM, Garcia-Gil M, Comas-Cufí M, et al. . Association between chronic immune-mediated inflammatory diseases and cardiovascular risk. Heart. 2018;104:119–126. [DOI] [PubMed] [Google Scholar]
- 2. Kirchgesner J, Beaugerie L, Carrat F, et al. ; BERENICE study group . Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. 2018;67:1261–1268. [DOI] [PubMed] [Google Scholar]
- 3. Rungoe C, Nyboe Andersen N, Jess T. Inflammatory bowel disease and risk of coronary heart disease. Trends Cardiovasc Med. 2015;25:699–704. [DOI] [PubMed] [Google Scholar]
- 4. Singh S, Singh H, Loftus EV Jr, et al. . Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382–3 93.e1. [DOI] [PubMed] [Google Scholar]
- 5. U.S. Food and Drug Administration. XELJANZ (tofacitinib): highlights for prescribing information. 2019. http://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed August 11, 2020.
- 6. Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, et al. . Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016;46:261–271. [DOI] [PubMed] [Google Scholar]
- 7. Gladman DD, Charles-Schoeman C, McInnes IB, et al. . Changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis: a pooled analysis across phase III and long-term extension studies. Arthritis Care Res (Hoboken). 2019;71:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mease P, Hall S, FitzGerald O, et al. . Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Ghosh S, Panés J, et al. ; Study A3921063 Investigators . Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 10. Wolk R, Armstrong EJ, Hansen PR, et al. . Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J Clin Lipidol. 2017;11:1243–1256. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Panés J, D’Haens GR, et al. . Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17:1541–1550. [DOI] [PubMed] [Google Scholar]
- 12. Sands BE, Taub PR, Armuzzi A, et al. . Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:123–132.e3. [DOI] [PubMed] [Google Scholar]
- 13. ClinicalTrials.gov. Safety study of tofacitinib versus tumor necrosis factor (TNF) inhibitor in subjects with rheumatoid arthritis. 2017. https://clinicaltrials.gov/ct2/show/NCT02092467. Accessed August 11, 2020.
- 14. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 15. Lichtenstein GR, Loftus EV Jr, Bloom S, et al. . Tofacitinib, an oral Janus kinase inhibitor, in the treatment of ulcerative colitis: open-label, long-term extension study [abstract]. Am J Gastroenterol. 2017;112(Suppl 1):714. [Google Scholar]
- 16. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM, Paynter NP, Rifai N, et al. . C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–22 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 19. Genovese MC, Smolen JS, Weinblatt ME, et al. . Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68:2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vermeire S, Schreiber S, Petryka R, et al. . Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. [DOI] [PubMed] [Google Scholar]
- 21. Taylor PC, Kremer JM, Emery P, et al. . Lipid profile and effect of statin treatment in pooled phase II and phase III baricitinib studies. Ann Rheum Dis. 2018;77:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millán J, Pintó X, Muñoz A, et al. . Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 23. Wu JJ, Strober BE, Hansen PR, et al. . Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J Am Acad Dermatol. 2016;75:897–905. [DOI] [PubMed] [Google Scholar]
- 24. Lewis JD, Scott FI, Brensinger CM, et al. . Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-α-directed therapy for inflammatory bowel disease. Am J Gastroenterol. 2018;113:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. 2010;45:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linton MF, Yancey PG, Davies SS, et al. . The role of lipids and lipoproteins in atherosclerosis. In: De Groot LJ, Chrousos G, Dungan K, et al. , eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000. [Google Scholar]
- 27. Skeoch S, Bruce IN. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol. 2015;11:390–400. [DOI] [PubMed] [Google Scholar]
- 28. Feingold KR, Grunfeld C. The effect of inflammation and infection on lipids and lipoproteins. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2015. [Google Scholar]
- 29. Hudgins LC, Parker TS, Levine DM, et al. . A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J Lipid Res. 2003;44:1489–1498. [DOI] [PubMed] [Google Scholar]
- 30. Johnsson H, Panarelli M, Cameron A, et al. . Analysis and modelling of cholesterol and high-density lipoprotein cholesterol changes across the range of C-reactive protein levels in clinical practice as an aid to better understanding of inflammation-lipid interactions. Ann Rheum Dis. 2014;73:1495–1499. [DOI] [PubMed] [Google Scholar]
- 31. Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–469. [DOI] [PubMed] [Google Scholar]
- 32. Boers M, Nurmohamed MT, Doelman CJ, et al. . Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lazarevic MB, Vitic J, Mladenovic V, et al. . Dyslipoproteinemia in the course of active rheumatoid arthritis. Semin Arthritis Rheum. 1992;22:172–178. [DOI] [PubMed] [Google Scholar]
- 34. Steiner G, Urowitz MB. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin Arthritis Rheum. 2009;38:372–381. [DOI] [PubMed] [Google Scholar]
- 35. Myasoedova E, Crowson CS, Kremers HM, et al. . Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de la Llera Moya M, McGillicuddy FC, Hinkle CC, et al. . Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222:390–39 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta NN, Li R, Krishnamoorthy P, et al. . Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charles-Schoeman C, Fleischmann R, Davignon J, et al. . Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67:616–6 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romanato G, Scarpa M, Angriman I, et al. . Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment Pharmacol Ther. 2009;29:298–307. [DOI] [PubMed] [Google Scholar]
- 40. Papp KA, Menter MA, Abe M, et al. . Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two, randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949–9 61. [DOI] [PubMed] [Google Scholar]
- 41. Bachelez H, van de Kerkhof PC, Strohal R, et al. . Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–5 61. [DOI] [PubMed] [Google Scholar]
- 42. Bissonnette R, Iversen L, Sofen H, et al. . Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2015;172:1395–1406. [DOI] [PubMed] [Google Scholar]
- 43. Daien CI, Duny Y, Barnetche T, et al. . Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–86 8. [DOI] [PubMed] [Google Scholar]
- 44. van Sijl AM, Peters MJ, Knol DL, et al. . The effect of TNF-alpha blocking therapy on lipid levels in rheumatoid arthritis: a meta-analysis. Semin Arthritis Rheum. 2011;41:393–400. [DOI] [PubMed] [Google Scholar]
- 45. Gabay C, McInnes IB, Kavanaugh A, et al. . Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806–18 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heslinga SC, Peters MJ, Ter Wee MM, et al. . Reduction of inflammation drives lipid changes in ankylosing spondylitis. J Rheumatol. 2015;42:1842–184 5. [DOI] [PubMed] [Google Scholar]
- 47. Grundy SM, Stone NJ, Bailey AL, et al. . 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–e 350. [DOI] [PubMed] [Google Scholar]
- 48. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. . General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–7 53. [DOI] [PubMed] [Google Scholar]
- 49. Conroy RM, Pyörälä K, Fitzgerald AP, et al. . Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 50. European Medicines Agency. XELJANZ (tofacitinib): summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf. Accessed August 11, 2020.
- 51. McInnes IB, Kim HY, Lee SH, et al. . Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis. 2014;73:124–131. [DOI] [PubMed] [Google Scholar]
- 52. Antonopoulos AS, Margaritis M, Lee R, et al. . Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18:1519–15 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–6 57. [DOI] [PubMed] [Google Scholar]
- 54. Aloi M, Tromba L, Di Nardo G, et al. . Premature subclinical atherosclerosis in pediatric inflammatory bowel disease. J Pediatr. 2012;161:589–5 94.e1. [DOI] [PubMed] [Google Scholar]
- 55. Principi M, Mastrolonardo M, Scicchitano P, et al. . Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis. 2013;7:e427–e4 33. [DOI] [PubMed] [Google Scholar]
- 56. Caliskan Z, Keles N, Gokturk HS, et al. . Is activation in inflammatory bowel diseases associated with further impairment of coronary microcirculation? Int J Cardiol. 2016;223:176–1 81. [DOI] [PubMed] [Google Scholar]
- 57. McCarey DW, McInnes IB, Madhok R, et al. . Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–2021. [DOI] [PubMed] [Google Scholar]
- 58. Côté-Daigneault J, Mehandru S, Ungaro R, et al. . Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:724–732. [DOI] [PubMed] [Google Scholar]
- 59. Abe Y, Murano M, Murano N, et al. . Simvastatin attenuates intestinal fibrosis independent of the anti-inflammatory effect by promoting fibroblast/myofibroblast apoptosis in the regeneration/healing process from TNBS-induced colitis. Dig Dis Sci. 2012;57:335–344. [DOI] [PubMed] [Google Scholar]
- 60. Ikeda M, Takeshima F, Isomoto H, et al. . Simvastatin attenuates trinitrobenzene sulfonic acid-induced colitis, but not oxazalone-induced colitis. Dig Dis Sci. 2008;53:1869–18 75. [DOI] [PubMed] [Google Scholar]
- 61. Lei A, Yang Q, Li X, et al. . Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates murine colitis. Immunology. 2016;149:432–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grip O, Janciauskiene S, Bredberg A. Use of atorvastatin as an anti-inflammatory treatment in Crohn’s disease. Br J Pharmacol. 2008;155:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crockett SD, Hansen RA, Stürmer T, et al. . Statins are associated with reduced use of steroids in inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. 2012;18:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ananthakrishnan AN, Cagan A, Cai T, et al. . Statin use is associated with reduced risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:973–97 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Poynter JN, Gruber SB, Higgins PD, et al. . Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–21 92. [DOI] [PubMed] [Google Scholar]
- 66. Samadder NJ, Mukherjee B, Huang SC, et al. . Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011;117:1640–164 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ungaro R, Chang HL, Côté-Daigneault J, et al. . Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol. 2016;111:1416–14 23. [DOI] [PubMed] [Google Scholar]
- 68. Dhamija P, Hota D, Kochhar R, et al. . Randomized clinical trial: atorvastatin versus placebo in patients with acute exacerbation of mild to moderate ulcerative colitis. Indian J Gastroenterol. 2014;33:151–15 6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.