Abstract
Dietary restriction (DR) decreases body weight, improves health, and extends lifespan. DR can be achieved by controlling how much and/or when food is provided, as well as by adjusting nutritional composition. Because these factors are often combined during DR, it is unclear which are necessary for beneficial effects. Several drugs have been utilized that target nutrient-sensing gene pathways, many of which change expression throughout the day, suggesting that the timing of drug administration is critical. Here, we discuss how dietary and pharmacological interventions promote a healthy lifespan by influencing energy intake and circadian rhythms.
Subject terms: Circadian rhythms and sleep, Ageing, Metabolism, Feeding behaviour
Circadian clocks link physiologic processes to environmental conditions and a mismatch between internal and external rhythms has negative effects on organismal health. In this review, the authors discuss the interactions between circadian clocks and dietary interventions targeted to promote healthy aging.
Introduction
Aging is a major risk factor for chronic diseases, including obesity, diabetes, cancer, cardiovascular disease, and neurodegenerative disorders1. Improvements in healthcare have increased life expectancy worldwide, but as the aged population increases, frailty and morbidity have become a public health burden. Through the Healthy Life Expectancy (HALE) indicator, the World Health Organization estimates that worldwide humans spend >10% of our lives suffering from age-related diseases. Aging research currently focuses on closing the gap between lifespan (living longer) and healthspan (remaining healthier for longer). While lifespan can be easily determined with survival curves, healthspan is more complex to quantify. Several biomarkers of healthspan are used in animal models2,3 and humans4, ranging from levels of metabolites (glucose, cholesterol, fatty acids), biological processes (inflammation, autophagy, senescence, blood pressure) to biological functions (behavior, cognition, cardiovascular performance, fitness, and frailty).
A major challenge is to understand, mechanistically, the progressive deregulation of metabolic function and to design interventions to delay the onset of age-related diseases. Research in animal models has revealed that the aging process can be targeted using genetic, nutritional, and pharmacological interventions5.
Dietary restriction widely improves lifespan and healthspan1, and some of its benefits could be mediated by the circadian system. Interactions between circadian clocks and aging-related pathways, such as the sirtuin6–8, insulin/IGF-19, and mTOR10 signaling pathways, support this hypothesis; yet, the mechanisms are not fully understood. Here, we review the literature from animal models and human trials assessing the effects of feeding paradigms and food quality on circadian rhythms, health, and lifespan. Finally, we discuss concepts of circadian medicine as an opportunity for tailoring antiaging interventions.
Circadian rhythms and aging
In mammals, the circadian system controls daily (~24 h) rhythms in behavior and physiology11. This evolutionarily conserved timekeeping mechanism allows organisms to synchronize internal processes with environmental timing cues, ensuring optimal organismal adaptation12.
The central clock, located in the hypothalamic suprachiasmatic nucleus (SCN), synchronizes peripheral clocks in tissues throughout the body via humoral and neural signals13. Peripheral clocks are cell-autonomous, and while synchronized by the central clock, do not require SCN inputs to generate rhythms. Rather, they are composed of transcriptional/translational negative feedback loops of transcriptional activators (CLOCK/BMAL1) and repressors (PER/CRY) driving their own oscillations14 and regulating the rhythmic expression of genes involved in key cellular functions15,16 (Fig. 1). Glucose, fatty acid, and cholesterol metabolic pathways are under circadian control, and the disruption of clock genes alters metabolism and worsens health status11. Moreover, components of nutrient-sensing pathways associated with aging exhibit tissue-specific oscillations due to a direct crosstalk with core clock genes15 (Figs. 1–2, see Caloric Restriction below).
Fig. 1. Crosstalk between molecular components of circadian clock, nutrient-sensing, and metabolic pathways.
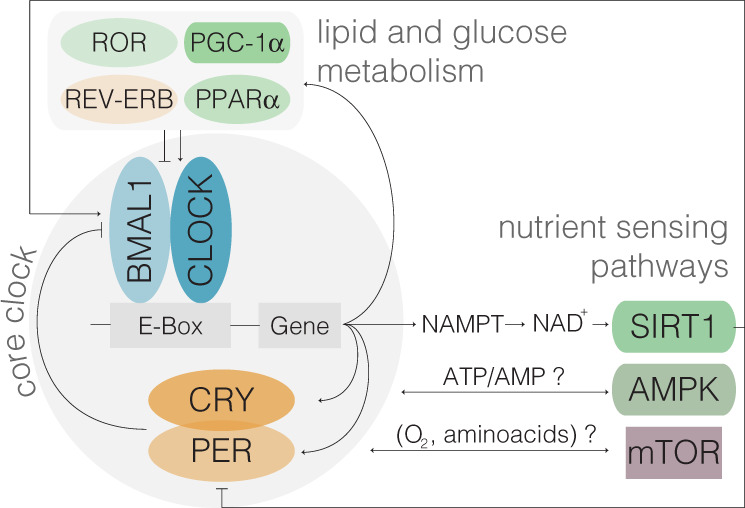
Core clock proteins CLOCK/BMAL1 (transcriptional activators) and PER/CRY (repressors) are engaged in an autoregulatory transcriptional/translational feedback loop leading to 24 h oscillations in gene expression, activity and protein levels. The molecular clock also regulates the rhythmic expression of genes involved in several cellular functions and nutrient-sensing pathways, which in turn feedback to the core clock machinery. CLOCK (Clock), BMAL1 (Arntl), PER (Period), CRY (Cryptochrome), SIRT1 (Sirtuin 1), AMPK (5’ AMP-activated protein kinase), PGC-1α (PPARγ co-activator 1a), mTOR (Mammalian target of rapamycin), ROR (RAR- Related Orphan Receptor), Rev-Erb (Nr1d1), and PPAR (Peroxisome Proliferator Activated Receptor).
Fig. 2. Aging-related pathways regulated by dietary interventions display circadian oscillations.
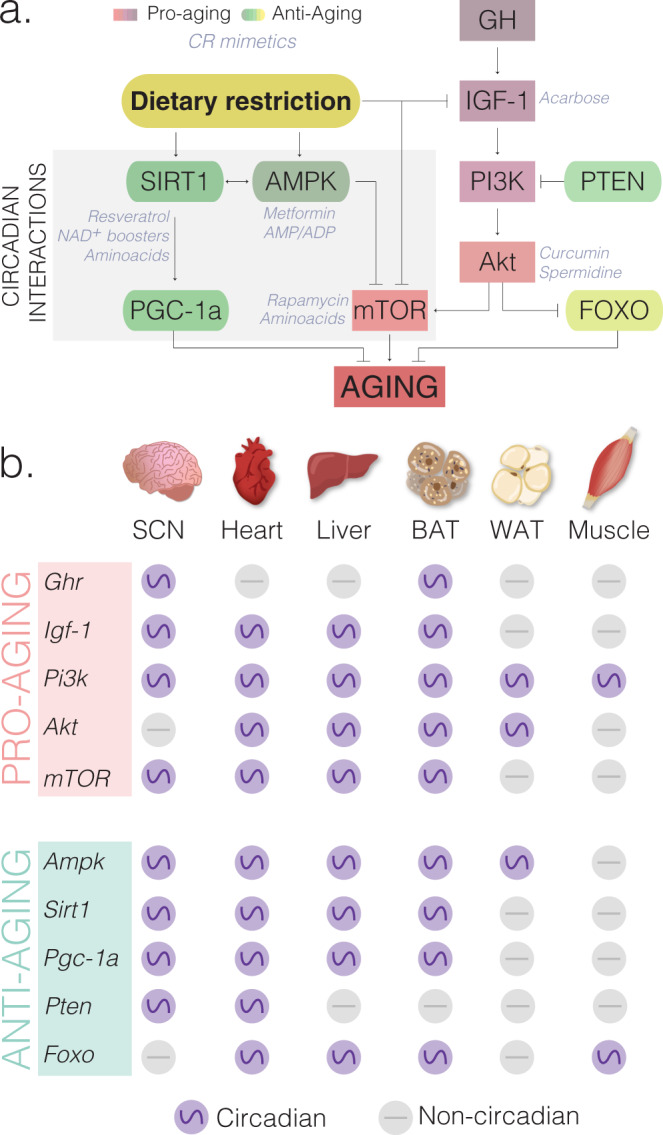
a Dietary interventions influence nutrient-sensing pathways50 by inducing antiaging (yellow-green gradient) and reducing proaging (pink-purple gradient) molecules. There are known direct interactions between nutrient-sensing pathways involve in aging (SIRT1, AMPK, NAMPT, mTOR) and core clock genes (CLOCK/BMAL and PER/CRY). Several compounds known as caloric restriction mimetics (CRM, shown as gray font color) target specific nutrient-sensing genes and mimic the health benefits of CR without reducing food intake. CRMs tested by ITP and other laboratories include acarbose, curcumin, spermidine, rapamycin, metformin, resveratrol, NAD+ boosters and amino acids (reviewed by ref. 172). CRMs (gray font color) are indicated next to or below their molecular targets. b Aging-related genes are expressed in a circadian manner (purple circles) in at least one tissue. Genes that are noncircadian in each tissue are represented as gray circles. RNA-seq and microarray containing circadian datasets for liver16 and other tissues extracted from CircaDB (http://circadb.hogeneschlab.org, Hogenesch laboratory). Proaging molecules: GH (growth hormone), GHR (growth hormone receptor), IGF-1 (insulin-like growth factor 1), PI3K (Phosphoinositide 3-kinase), AKT (also known as Protein kinase B), and mTOR (Mechanistic or Mammalian target of rapamycin). Antiaging molecules: SIRT1 (Sirtuin 1), AMPK (5’ AMP-activated protein kinase), PGC-1α (PPARγ co-activator 1a), PTEN (Phosphatase and tensin homolog), FOXO (Forkhead Box O). Core clock components CLOCK/BMAL1 (transcriptional activators) and PER/CRY (transcriptional repressors). See also Supplementary Table 1 for additional tissues and aging-related genes in mouse, baboon, and humans.
The endocrine system is also regulated in a circadian manner17. In humans, insulin, ghrelin, adiponectin, and cortisol are elevated in the morning/afternoon, while melatonin, thyroid stimulating hormone, prolactin, leptin, growth hormone (GH), and fibroblast growth factor 21 (FGF-21), are elevated in the evening/night17. These rhythmic hormones regulate feeding and sleep, and also synchronize endogenous clocks18. Hormonal rhythms may be central to coordinate internal clocks during aging, as timed-administration of insulin, corticosterone, and prolactin mimicking the rhythmic pattern seen in young rats, is sufficient to reverse insulin resistance and reduce body fat in aged rats19. Circadian clocks can regulate metabolic and endocrine rhythms independently of feeding and sleep20,21. For example, while nutrients account for circulating glucose during feeding events, the liver clock provides the glucose required during the fasting phase, leading to nearly constant levels of glucose throughout the day20. Interestingly, aspects of aging are reversed in parabiosis experiments, in which the circulatory systems of aged and young mice are connected22. Although possible, whether humoral rhythms from young mice contribute to aging reversal is unknown.
The systemic influence of circadian rhythms on tissue homeostasis, sleep regulation, and behavior is well established; with direct links to aging23,24. High-amplitude circadian rhythms correlate with wellbeing and increased lifespan in animal models regardless of food composition25–27, while circadian rhythms decrease in amplitude with normal aging and often exhibit a shift in phase23,28. Additionally, aged animals have defects in entrainment/synchronization to light/dark cycles8,29–31, which impairs the ability of the organism to predict and adapt to environmental changes.
A mismatch between internal clocks and daily changes in the environment is detrimental to survival. Mice with free-running periods of ~24 h live 20% longer than mice whose periods deviate significantly from 24h32. Conversely, mice with internal period of ~24 h reduce their lifespan when exposed to a short day of 4 h light/4 h dark as compared to a 24 h day (12 h light/12 h dark)33. Furthermore, rodents deficient in clock genes have shortened lifespans, including Clock−/− mice25, Bmal1−/− mice34, and 20-h-period golden hamsters carrying a tau mutation35. Remarkably, interventions that restore proper circadian rhythmicity improve longevity. For instance, transplantation of fetal SCN into aged animals increases rhythmicity and extends lifespan35–37. Conversely, genetic perturbation of circadian genes in peripheral tissues in rodents is associated with metabolic disorders20,38–41. Disruption of the clock through lifestyle (i.e., jet lag, shift work) is associated with decreased lifespan in mice42 as well as increased risk of cancer43, cardiovascular disease, and metabolic disruption in humans44. How aging perturbs the function of internal clocks remains an open question, but collectively these data suggest that understanding the circadian regulation of physiology and metabolism may provide novel insights for designing and implementing antiaging interventions.
Feeding paradigms in animal models
In the last two decades we have come to appreciate that, in addition to caloric content, timing of feeding and fasting periods between meals influence wellbeing45,46. Recent findings highlight the influence of dietary restriction (DR) on circadian clocks, yet the mechanisms linking circadian systems with metabolism, epigenetic signatures, and age-related diseases remain to be elucidated. Furthermore, the role of peripheral clocks in regulating metabolic homeostasis during aging is still unclear. Understanding the mechanistic links between circadian regulation during dietary interventions is an exciting avenue that may provide insights on how to achieve optimal health benefits throughout life. Here we discuss how health and lifespan are influenced by the most commonly used DR paradigms: caloric restriction (CR), time restriction (TR), and intermittent fasting (IF), as well as changes in dietary composition, in animal models (Fig. 3).
Fig. 3. Dietary interventions protect against chronic diseases and promote lifespan.
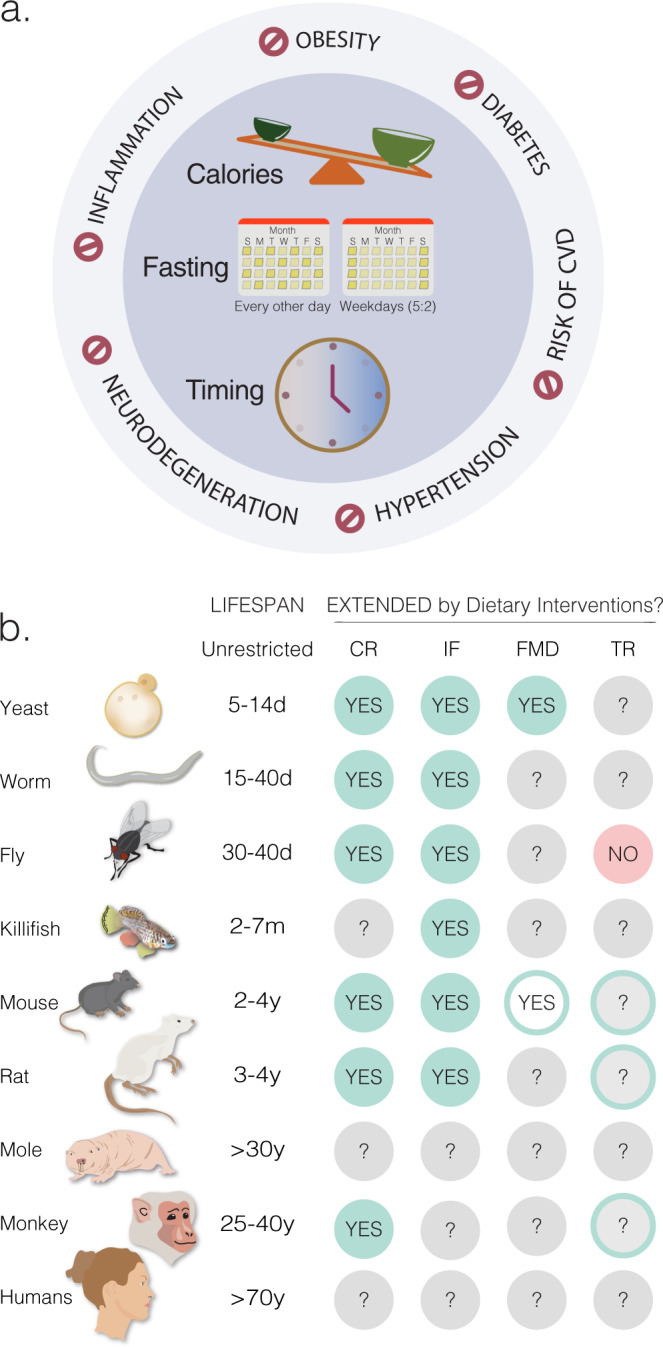
a Dietary restriction improves healthspan in several species by reducing the risk for obesity, diabetes, cardiovascular disease (CVD), hypertension, neurodegeneration, and inflammation. Although these benefits have been associated with a reduction in the number of calories consumed, extending fasting periods, and restricting the timing of food intake, the individual contribution of these factors remains unknown, since classical dietary restriction protocols often combines one or more of these parameters. b Dietary restriction extends lifespan in most model organisms used in aging research. Green circles labeled as “YES” represent dietary interventions that increase both maximum and median lifespan. Green circles with white centers labeled as “YES” represent the extension of median but not maximum lifespan. Red circles labeled as “NO” refer interventions that have no effect on median or maximum lifespan. Gray circles labeled as “?” indicate conditions that have not yet been tested. Although time-restricted feeding (TR) does not extend lifespan in flies204, caloric restriction (CR) protocols that promote longevity in monkeys, rats and mice also involve TR as well (shown as green outer circles with gray centers labeled with “?”). Dietary interventions: Caloric Restriction (CR)27,77,205,206. Intermittent Fasting (IF)92,207 includes either periodic fasting (PF, also known as every-other-day feeding EOD) or 5 days fasting/low calories followed by 2 days unrestricted intake (Weekdays 5:2). Fasting Mimicking Diet (FMD)100 with 5 days of a low-caloric diet every 3–4 months. Time-restricted feeding (TR)204, in which food is available ~8–12 h exclusively during the active period.
Caloric restriction
CR extends lifespan and improves health in several organisms1. CR reduces oxidative stress, increases insulin sensitivity, modulates neuroendocrine responses, impacts central nervous system function47, reduces necroptosis48, and delays the onset of neoplasia49. Overall, CR ameliorates several hallmarks of aging, including deregulated nutrient-sensing pathways, mitochondrial dysfunction, impaired autophagy, stem cell senescence, DNA damage, and inflammation1.
CR promotes longevity by targeting highly conserved pathways across species50. These include inhibition of IGF-1 and mTOR as well as the activation of SIRT1, NAMPT, AMPK, FGF-21, and PGC-1α signaling pathways (Fig. 2a). These molecules exhibit tissue-specific circadian oscillations (Fig. 2b and Supplementary Table 1), and some of them feedback to molecular components of the clock14,15,23 (Fig. 1). For instance, CLOCK-BMAL1 rhythmically activate NAMPT, the rate-limiting enzyme in NAD+ biosynthesis, leading to circadian oscillations of NAD+ levels7. As a NAD+-dependent deacetylase, SIRT1 directly binds to CLOCK-BMAL1 and rhythmically deacetylates and promotes degradation of PER2, contributing to the maintenance of robust circadian rhythms7,51. Furthermore, AMPK, an energy sensor of AMP/ATP ratios, destabilizes the circadian transcriptional repressor CRY152.
Inhibition of the insulin/IGF-1 signaling pathway increases lifespan from C. elegans53 to mice54, and polymorphisms in this pathway have been found in centenarians55. Intriguingly, IGF-1 levels decline with both normal and premature aging, perhaps as a defensive response to energy scarcity56. Igf-1 expression oscillates in mouse tissues, including the liver, where most IGF-1 is produced57. Additionally, IGF-1 rhythms in the liver and blood are abolished in mice lacking core clock genes (Cry1/2−/− mice)9. Downstream targets of IGF-1 also display tissue-specific oscillations, suggesting that IGF-1 signaling throughout the body is under circadian control57. IGF-1 may be one of signals that synchronizes peripheral clocks to feeding time58; however, circadian regulation of the IGF-1 pathway throughout the lifespan has been largely unexplored.
mTOR plays a critical role in energy metabolism, and regulates circadian period in flies59 and mice60. It has been implicated in the entrainment of the circadian clock in the mouse brain61 and the activity of the mTOR complex oscillates in several brain regions and in peripheral tissues60. CR restores the rhythmic activity of mTOR that is lost under ad lib feeding in arrhythmic mutant mice62, perhaps through self-imposed feeding/fasting cycles associated with CR protocols63. Moreover, mTOR inhibition upon fasting is driven by induction of the core clock protein PER264. These studies indicate that both endogenous clocks and CR regulate the rhythmic activity of mTOR.
A genome-wide circadian analysis of liver65 and epithelial stem cells66 showed that CR rescues the age-related decline in circadian metabolic pathways in mice. These findings suggest that the aging process has minimal effect on core clock machinery, and instead promotes tissue-specific reprogramming of the transcriptome. Interestingly, BMAL1-deficient mice not only have reduced lifespan but also exhibit premature aging phenotypes such as sarcopenia, cataracts, less subcutaneous fat (aged skin), reduction in organ size, and impaired hair growth34,67. Surprisingly, CR decreases survival in Bmal1−/− mice, while failing to reduce IGF-1 and insulin levels68 or to improve circadian rhythmicity of clock genes69. It is unclear whether this is a specific effect of BMAL1, or whether CR also has detrimental effects in other clock mutant mice.
Tissue-specific responses to CR add an extra layer of complexity in understanding the relative contributions of nutrient-sensing pathways to longevity. For example, activation of SIRT1 has been proposed to mediate the effects of CR on lifespan. Yet, although CR increases SIRT1 in most tissues, it decreases it in liver70. Moreover, while brain-specific expression of SIRT1 is sufficient to extend lifespan in mice71, whole-body overexpression of SIRT1 has no such beneficial effect72. Experimental disruption of mTOR signaling in mice also has tissue-specific effects: in adipose tissue mTOR inhibition is beneficial, but in muscle it is deleterious73. A recent study showed that tissue-specific nutritional memory impairs the full benefits of CR if applied late in life74. Switching 24-month-old female mice from ad lib to CR slightly increases lifespan as compared to ad lib controls, but does not reach the lifespan extension observed in the life-long CR cohort74. Genome-wide comparisons revealed that while the liver was mostly reprogrammed by CR, ~13% of the genes in the white adipose tissue involved in lipogenesis and inflammation, were unresponsive to late-onset CR74. Therefore, many questions remain about the beneficial effects of CR in different tissues, in which tissue-specific circadian rhythms may play a significant role (Fig. 2 and Supplementary Table 1).
Two independent studies demonstrated that CR improves metabolic fitness and healthspan in rhesus monkeys, but reported a discrepancy on longevity, with either no effect75 or improvement76 with CR. Survival results were reconciled by considering differences in feeding behavior, age of treatment, genetic background, and other variables, and concluded that CR improves longevity in non-human primates77. The timing of food access partly explains the discrepancy in survival: CR had no effect on survival in the study that allowed the control group to eat ad lib, but only during the daytime, as food was removed every night77. This study was later replicated in mice, in which animals eating a single meal ad lib with variable periods of fasting lived longer than mice with 24 h food access49. It remains unknown whether these mechanisms also apply to humans.
A major confounding aspect of most CR regimens is that they change both the amount of food eaten and the temporal pattern of food intake23. CR protocols are often constrained by human schedules, with food provided in the morning when nocturnal rodents would not normally eat. With limited food access, rodents tend to eat their allotment as soon as the food is available, thereby self-imposing daily cycles of feeding and fasting63. Because food intake synchronizes metabolic function and hormone production throughout the body, the timing of food intake is critical. An intriguing study found no differences in lifespan in calorically restricted female mice with access to a single meal during the daytime, a single meal at night, or 6 meals at night78, suggesting that long-term effects of CR could be independent of feeding time. However, an important consideration is that CR mice eat shortly after food is made available;63 which allows the animal to remain close to its normal nocturnal behavior (driven by the light/dark cycle) by extending the active phase for <2 h. Alternatively, perhaps day-fed and night-fed groups extend lifespan through different mechanisms, as revealed by changes body temperature rhythms with opposite 24 h profiles78. Further experiments are required to elucidate, which possibility accounts for these findings. Altogether, despite many studies using CR, the contribution of the timing of food access by itself has become a new area of exploration to unravel the antiaging mechanisms induced by CR.
Time restriction
Environmental signals, including food, can act as powerful non-photic clock entraining agents79. When food access is restricted to a few hours in the daytime (when nocturnal animals normally eat only sparingly), rodents increase their activity anticipating the time of food availability80,81. Such unnatural TR paradigms also shift the phase of circadian rhythms by many hours in peripheral clocks such as the liver, without a significant effect on the SCN80–83. This internal desynchrony in the organism, with central and peripheral clocks out of phase, has been associated with metabolic disorder. However, a combination of TR with either CR or highly palatable food results in a stronger stimulus capable of resetting both central and peripheral clocks84. In traditional TR protocols, a large shift in the core transcriptional clock in the liver is accompanied by complex changes in phase and amplitude of clock-controlled genes83. Thus, it is clear that feeding/fasting cycles are powerful cues for peripheral circadian clocks, with both CR and TR reprograming the circadian profiles of the transcriptome and metabolome27,65,66,81,83,85–88.
Mice consume 75–85% of their calories at night (active phase), and high-fat diet (HFD) dramatically disrupts this feeding pattern by increasing ~50% of intake during the rest phase89. Such diet-induced disruption in feeding behavior contributes to the development of obesity and diabetes in mice given HFD. Indeed, eating exclusively at the wrong time of the day increases weight gain and susceptibility to obesity, diabetes, and cardiovascular disease, while also negatively impacting learning and memory85–87,90. Conversely, restricting food intake to the active phase protects against HFD-induced obesity, hepatic steatosis, hyperinsulinemia, and inflammation85,90. While HFD dampens circadian rhythms of metabolic genes89, limiting food during the night-time restores the proper oscillations and prevents metabolic syndrome in WT85,87 and arrhythmic clock mutant mice91. Despite the short-term metabolic benefits, the impact of TR on longevity remains to be determined. Because TR is difficult to maintain long term, automated feeders may prove valuable in overcoming methodological limitations63.
Fasting
Fasting is the most extreme example of dietary restriction. Chronic intermittent fasting (IF) involves alternating 24 h of minimal food intake with 24 h of unrestricted intake, or cycles of periodic fasting (PF) for several days46. In rodents, IF extends lifespan, protects against obesity, cardiovascular disease, hypertension, diabetes, and neurodegenerative diseases92. IF improves overall health in aged animals by inducing autophagy93 and thermoregulation94 while enhancing glucose metabolism95 and promoting neuronal resistance to injury independently of weight loss and caloric intake96. Importantly, the effects of IF upon body weight and lifespan depend on genotype, strain, and age of treatment97. GH/IGF-1 signaling seems to be a key mediator of the effects of IF, since mice lacking GH receptor fail to respond to IF98. Interestingly, IF alters the phase of circadian genes in the liver99. However, the long-term effects of IF on central and peripheral clocks are unknown.
Fasting periods between meals extend lifespan in mice, regardless of caloric intake and food composition49. When provided one single meal per day (MF) matching ad lib intake, mice have variable fasting intervals. However, even with food starting during the rest phase (3 h before lights off), MF mice live longer than mice with 24 h food access. Within the MF group, it would be interesting to determine whether longer fasting periods correlate with increased survival. Given individual differences in food intake (both amount and feeding pattern), recording the unrestricted ad lib feeding pattern as a baseline for each mouse would be useful for determining to what extent each imposed feeding protocol (CR, TR, or IF) also affects other feeding parameters. Thus, further studies are required to disentangle the contributions of the amount and the timing of food intake on survival.
A fasting-mimicking diet (FMD) has been developed to confer similar benefits of prolonged fasting, without the burden of several days of starvation. FMD is a low-cal/low-protein diet, with plant base ingredients. In mice, FMD enhances cognitive function, reduces adiposity, ameliorates bone loss, reduces the incidence of neoplasia, and boosts the immune system, but does not prolong maximum lifespan100,101. FMD restores insulin secretion from pancreatic islets from Type 1 diabetic (T1D) patients and reverses both T1D and T2D phenotypes in mouse models. Also, it decreases circulating levels of IGF-1, insulin, and glucose, while increasing plasma concentrations of IGF-binding protein 1 and ketone bodies and increases the number of progenitor stem cells102. Therefore, this approach of mimicking fasting without actually fasting effectively prolongs healthspan. It would be interesting to explore whether FMD, given for a few days every 2–3 months, enhances healthspan by resetting internal clocks or restoring metabolic rhythms.
Changing food composition
Dietary restriction is extremely difficult to sustain long term, and compliance often decreases over time. As an alternative, several groups have explored reducing specific dietary components, rather than decreases in overall food intake. Two examples of modifications in dietary composition are ketogenic diets (KD) and protein restrictions (PR).
Ketogenic diets (KD) are low-carb, high-fat diets that recapitulate certain metabolic aspects of dietary restriction, such as reliance on fatty acid metabolism and production of ketone bodies as an energy source. In mice, KD improves healthspan, slows down age-associated neurological decline, and increases median, but not maximum lifespan103,104. Interestingly, KD reshapes liver and gut clocks105 increasing chromatin recruitment of BMAL1 and promoting higher amplitude rhythms in the liver, along with increasing rhythmicity in serum ketone bodies and PPARα target genes in the gut. Unlike HFD, which is rich in both in fat and carbohydrates, KD does not change feeding patterns, as mice continue eating mainly at night-time. The maintenance of rhythmic behavior with KD likely enhances tissue-specific rhythms and contributes to improving overall health status.
Limiting proteins or certain amino acids in the diet can also reduce the incidence of age-associated diseases and simultaneously increase lifespan106. Protein restriction increases a fasting-induced hepatokine, FGF-21, in rodents and humans107. In mice, overexpression of FGF-21 has been reported to extend lifespan by inhibiting GH/IGF-1 signaling pathways in the liver108. These FGF-21 overexpressing mice not only have elevated, but also highly rhythmic, levels of circulating FGF-21 that modulate circadian behavior109. In addition to PR, methionine restriction (MR) extends health and lifespan in mouse models of normal110 and accelerated aging111 as a consequence of reduced inflammation and increased autophagy and DNA stability. In contrast with these restrictive diets, which are difficult to implement long term, glycine supplementation mimics MR and promotes lifespan in rodents112. Glycine has also been implicated in regulating aspects of circadian biology. Glycine supplementation promotes sleep and hypothermia via NMDA receptors in the SCN in rats113 and it is able to synchronize SCN rhythms in vitro114. The mechanisms by which glycine acts on circadian clocks and how these cues are transmitted throughout the body are not yet fully understood. Taken together, these findings suggest that limiting specific dietary components is another viable alternative to improve metabolic function without constant CR. Further studies are required to establish whether restricting or supplementing at a specific optimal time of day could expand their benefits on healthspan and longevity.
Implications for human health
Caloric restriction and fasting-mimicking diets in humans
Long-term CR also improves several markers of health in humans, both in obese and non-obese individuals115,116. These include decreased body weight, metabolic rate, and oxidative damage117, lower incidences of cardiovascular disease118 and cancer;119 and decreased insulin-Akt-FOXO signaling pathway activity120. A new multicenter trial called the CALERIE, is currently assessing different aspects of human physiology as a consequence of long-term CR in over 200 individuals121,122. Even in clinical trials, long-term compliance with CR is low, and often only a partial goal of food restriction is achieved. However, highly motivated individuals, such as those in the long-term CR with Optimal Nutrition (CRONies) study, as well as observational studies of centenarians in Okinawa, Japan who have had CR for most of their lives115,118, provide evidence that CR can have benefits in humans.
Several short-term clinical trials have shown that alternate-day fasting can deliver benefits similar to CR in terms of weight loss and cardiometabolic health, including reduction in body weight and improved lipid profiles, lower blood pressure, and increased insulin sensitivity123–126. Fasting also appears to have anti-cancer properties in humans127, and it increases susceptibility of cancers to certain chemotherapeutics128. A recent clinical trial tested the feasibility of incorporating short-term fasting (72 h fasting around the time when the therapeutic agent is administered) into platinum-based chemotherapy. Similar to what was observed in mice, short-term fasting prior to chemotherapy administration appeared to protect against toxicity129. Additionally, fasting during chemotherapy was well-tolerated and reduced hematological toxicity in HER2-negative stage II/III breast cancer patients130.Nevertheless, implementing alternate-day fasting or similar interventions can still be extremely difficult.
Other diets have also been tested in humans with sleep abnormalities. A ketogenic diet131 can normalize sleep patterns in association with a loss in body mass132–134. In healthy, non-obese, normal sleepers, a ketogenic diet increases slow-wave sleep and decreases REM sleep compared to a high-carbohydrate, low-fat diet135. Similar to methionine restriction in animal models, low methionine levels found in vegan and vegetarian diets also confer metabolic benefits by increasing circulating levels of FGF-21 in humans136.
Time restriction
With increasingly sedentary lifestyles and 24/7 access to food and artificial light, the burden of chronic diseases has also grown. The detrimental effects of poor eating behaviors are not only consequences of unhealthy diet (high fat, high sugar, highly processed foods), but also the timing of when the food is consumed. More than half of the individuals in diet studies regularly eat over a period of 15 h or longer each day, fasting only while they sleep137,138. Interestingly, the timing of food intake relative to the natural increase in melatonin (which marks the beginning of night for each individual) is significantly associated with body fat percentage and body mass index139. Nonlean individuals consume most of their calories 1.1 h closer to melatonin onset than lean individuals, suggesting that the timing of food intake is also a key aspect of metabolism in humans.
In humans, as in mice, the timing of calorie consumption impacts metabolic status and body weight maintenance, even during a short-term feeding regimen. For example, in a 20-week dietary intervention in 420 obese and overweight individuals, the timing of the main meal (i.e., lunch for the Spanish population) was predictive of weight-loss success independent from total 24 h caloric intake140. Body weight and optimal metabolic activity also appear to depend on the time of day of the eating window. Implementing a time-restricted feeding schedule decreases body fat, fasting glucose and insulin levels, insulin resistance, hyperlipidemia, and inflammation in humans141–143. Remarkably, there are further benefits if the food is consumed earlier in the day. When a 6 h feeding period, with dinner before 3 PM was compared to a control schedule (12 h feeding period) for 5 weeks in a randomized crossover trial of prediabetic men, insulin sensitivity, blood pressure, and oxidative stress levels were improved during the early time-restricted feeding regimen without any effect on body weight144. Other studies have suggested that the additional benefits are not simply because early TR also increases the fasting period; instead, there appears to be an optimal time for food consumption. In fact, restricting food intake to the late afternoon or evening (after 4 PM; late TRF) either has no effect or worsens postprandial glucose levels, glucose tolerance, blood pressure, and lipid levels141,143,145. Furthermore, early dinner improved glucose tolerance especially for subjects carrying a diabetes risk allele MTNR1B (melatonin receptor)146, supporting links between circadian melatonin and glucose control in humans.
In a laboratory setting participants were given three meals (breakfast, lunch, dinner) at 5 h intervals, beginning either 0.5 (early) or 5.5 (late) hours after waking. Interestingly, plasma glucose rhythms were delayed by over 5 hours and average glucose concentrations decreased following the late feeding schedule. Moreover, Per2 mRNA rhythms in adipose tissues were delayed by 1 h after 6 days of late feeding, indicating that human molecular clocks may be regulated by feeding time and could influence plasma glucose147. Similarly, another study showed that skipping breakfast influences the circadian rhythms of clock genes and their targets and is correlated with increased postprandial glycemic response in both healthy individuals and individuals with diabetes148. A recent randomized crossover trial elegantly showed that healthy patients exhibit lower glucose levels, reduced hunger, and increased diet-induced thermogenesis (amount of energy used for digestion and absorption of nutrients) when eating high caloric breakfasts as compared to high caloric dinners149. Taken together, these studies suggest that a restricted window of food intake early in the day is the most beneficial.
Consequences of misalignment of the circadian system
Genetic polymorphisms make some people more likely to behave as an early bird (early chronotype) or a night owl (late chronotype). A late chronotype may be detrimental in our scheduled society, as these individuals typically experience a mild form of circadian misalignment150. This link between chronotype and misalignment was also suggested in a study of 163 prediabetic or type 2 diabetic individuals’ eating and sleeping patterns. This study found that, after adjusting for age, sex, BMI, and statin use, a late chronotype was associated with higher inflammation in diabetic individuals151.
A forced misalignment of these circadian rhythms with the environment, as happens in jet lag and shift work, is detrimental to health152, with increased risk of type 2 diabetes153 and cancer154,155. This has been shown in the laboratory, when, during an 8-day gradual misalignment protocol between behavioral cycles (fasting/feeding and sleep/wake cycles) and endogenous circadian cycles, 10 adults showed decreased leptin, increased glucose despite increased insulin, increased mean arterial pressure, and reduced sleep efficiency156. This 8-day misalignment was also sufficient for 30% of the subjects to exhibit postprandial glucose responses in the range typical of a prediabetic state.
In another study, 14 healthy lean young men were exposed to a more drastic 12-h-shift misalignment protocol, after which insulin sensitivity tests and skeletal muscle biopsies were performed. After 3.5 days of circadian misalignment, these individuals showed normal hepatic insulin sensitivity but muscle insulin resistance due to reduced mitochondrial function and nonoxidative glucose disposal in the muscle157. Molecular analysis of muscle biopsies revealed that the molecular circadian clock was not aligned to the inverted behavioral cycle, possibly because it would take longer than 3.5 days to entrain to the 12 h shift. Nonetheless, fatty acid metabolism and PPAR signaling were drasticallly increased from 7 AM to 7 PM in the misaligned condition, revealing the human PPAR pathway as a key player in disturbed energy metabolism with circadian misalignment157. Although this protocol was extreme, it showed that glucose tolerance in humans can be altered even after a short-term circadian misalignment in a laboratory setting158.
Sleep restriction
In addition to the circadian aspects of sleep/wake cycles, the total amount of time spent sleeping also appears to have an impact on metabolism and health. More than 30% of adult men and women report fewer than 6 h of sleep per night, well below the National Sleep Foundation’s recommendations159. Patients with sleep disorders have altered glucose homeostasis and a higher risk for developing obesity. In healthy individuals, sleep deprivation is inversely correlated with body weight, suggesting that sleep deprivation plays a key role in regulating energy balance160. Total or partial sleep deprivation results in increased sympathetic nervous system activity, increased evening cortisol, and increased daytime GH levels161,162. After sleep restriction, leptin levels are lower and ghrelin levels are higher163,164. Indeed, a meta-analysis of 11 studies showed that after partial sleep deprivation energy intake was increased by ~385 kcal per subject165. Since there was no significant change in total energy expenditure or resting metabolic rate, sleep deprivation led to a positive energy balance, which in the long term may contribute to weight gain in these individuals. Importantly, sleep restriction not only increases energy intake but also extends the eating window and also increases the preference for frequent energy-rich snacks over regular meals166. Such altered patterns of food consumption likely contribute to the detrimental metabolic effects of sleep deprivation.
Circadian medicine: optimizing when to treat
The National Institute of Aging Intervention Testing Program (ITP) is a multicenter initiative assessing treatments with the potential to extend lifespan and delay disease onset and dysfunction in mice167. Successful treatments include FDA-approved drugs to treat cancer and diabetes, such as rapamycin (mTOR inhibitor)168,169 and metformin (AMPK activator)170,171. These drugs are classified as caloric restriction mimetics (CRMs) since they target similar molecular pathways and confer the health benefits of CR without actually limiting food intake (Fig. 2) (thoroughly reviewed in ref. 172). Genome-wide studies with circadian sampling in rodents, non-human primates, and human tissues have highlighted that the targets of >80% of the FDA-approved drugs exhibit daily rhythms57,173. Thus, dosage timing may help to optimize benefits of antiaging drugs, especially when acting on oscillating or moving targets (Figs. 2, 4).
Fig. 4. Model of how Circadian Medicine can be used as an optimized intervention to improve circadian rhythms and potentially promote lifespan.
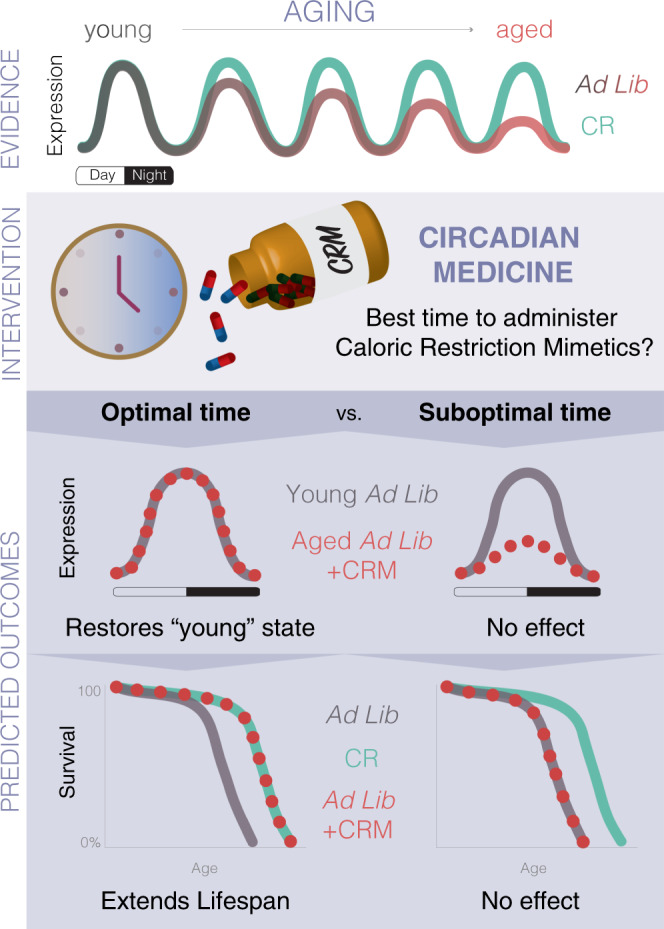
The top panel reflects the current evidence that, (1) aging-related pathways oscillate throughout the day; (2) circadian rhythms decline with age and restoring rhythms improves healthspan; and (3) CR, the most robust lifespan-extending intervention, remarkably protects against the age-dependent dampening of circadian rhythms. Circadian medicine introduces a time-of-day concept for administration of drugs. Considering most aging-related genes are circadian, perhaps there is an optimal time for interventions such as CR mimetics (CRMs). The hypothesis behind this model is that there is an optimal time to administer antiaging drugs, which can restore the proper rhythms targets, and consequently boost survival. If there were an optimal time, we would expect robust circadian rhythms even in an aged individual resembling a young state, potentially leading to lifespan extension. On the contrary, a suboptimal time of administration would not be effective or would require a higher dose to reach similar beneficial effect. Tailoring the treatment for each drug as to how often and what time of the day is still required, as it depends on pharmacokinetic properties, tissue-specific pathways, potential sex-differences, and other factors.
For practical reasons, most drugs tested in mice are supplemented in the diet or drinking water, which leads to voluntary night-time administration174. However, this may not be the best time to administer drugs whose targets have circadian rhythms of expression. Because numerous aspects of human physiology are under circadian control, there are windows of opportunity for interventions by simply administering drugs when their targets are at the right expression level to restore (Fig. 4). This is the basis of a concept known as Circadian Medicine, or Chronotherapy173 (Fig. 4). Circadian medicine as a model for antiaging interventions is based on current evidence that: (1) circadian rhythms decline with age8,27–31,65,66,175, (2) disruption of circadian rhythms leads to metabolic disorders20,38–40,43,44,90 and shortens lifespan25,32–37,42, while restoration of circadian rhythms promotes health19,26,91 and longevity35–37,175, and (3) aging-related pathways oscillate throughout the day6–10,51,57. Therefore, a plausible hypothesis is that there is an optimal time to administer drugs, which can restore the proper rhythms of targets, and ultimately result in lifespan extension (Fig. 4), while a suboptimal time would not have any benefit. This would require further tailoring treatments for each drug as to how often and what time of day it is needed, as it depends on pharmacokinetic properties, tissue-specific pathways, and potential sex-differences. Thus, moving forward, it will be necessary to develop new tools for administering interventions at specific times while controlling for frequency and amounts given. For example, it would be useful to develop an automated computer-controlled device to control access to drugs administered in the water or food, such as a gate alternating access between treated and nontreated water. For food-supplemented drugs, a separate compartment could be designed to deliver drugs as pills independently of the food dispenser. In addition, other promising approaches, such as mini-pumps, could be adapted for drug delivery.
There are many examples of treatments for aging-related diseases that are more effective when given at specific times of day, including cancer176–178, T2D179, and hypertension180. Aspirin extends lifespan in male mice181 and is used as a secondary prevention for cardiovascular disease (CVD) in humans182. The efficacy and outcome of aspirin treatment highly depends on dosing timing183–188. In humans, a randomized crossover trial showed that aspirin reduces blood clotting more effectively when taken at bedtime rather than in the morning186. Conversely, increased hemorrhage, risk of CVD and all-cause mortality was found in a placebo-controlled trial with healthy older adults (65+) receiving aspirin during breakfast187,188.
Polyamines are an interesting success story highlighting how a pharmacological intervention could mediate crosstalk between circadian clocks, metabolic pathways, and lifespan. Polyamines (e.g., putrescine, spermidine and spermine) are involved in cell growth, survival, and proliferation189. Despite an association of increased levels with cancer, both spermidine supplementation and high-polyamine diets increase lifespan in rodents189. Interestingly, polyamines show circadian oscillations and also influence circadian periodicity by regulating PER2/CRY1 interactions175. Remarkably, polyamine oscillations exhibit an age-dependent lengthening of period that can be reversed by dietary supplementation of polyamines in drinking water175.
Other potential drugs for delaying age-related diseases are those that target endogenous clocks. Screens to identify small molecules that modulate the clock have indeed proven useful at identifying clock-enhancing drugs190,191. One such molecule, a natural flavonoid compound called nobiletin (NOB) was found to mitigate body weight gain without altering food intake, stimulate energy expenditure and circadian activity, enhance glucose and insulin tolerance, diminish lipid content, and improve mitochondrial respiration in mice26,192. This study provided clear evidence that maintenance of a robust circadian organization within the organism protects against metabolic disruption.
Methods to infer individual internal timing
Internal circadian time varies among individuals, as it is influenced by many factors, including work schedules, feeding regimens, genetic predisposition, age, sex, environmental light levels, and seasons. Current efforts are dedicated for leveraging individual patient’s circadian clocks to personalize healthcare. Several algorithms have been developed to identify reliable markers of internal timing based on blood and brain transcriptomic datasets193–200. The first method developed to infer internal timing, called Molecular Timetable, is composed of ~100 time-indicating-genes identified from mouse liver microarray datasets193. Identifying circadian targets in humans has been challenging, since genome-wide datasets for most tissues rarely include time of day during which samples were collected. Some algorithms have been developed to this end: CYCLOPS that reveals human transcriptional rhythms in health and disease198; BodyTime, a simple assay to determine the internal timing of an individual from a single blood sample taken at any time during the day199; and more recently TimeSignature, a machine learning-based algorithm designed to accurately predict internal timing from blood samples (±2 h) using ~40 genes as predictor markers200. All of these provide promising tools for translational studies and individualized circadian medicine (Fig. 4).
In addition to individual variation in rhythms, the circadian system is highly amenable to resetting signals, including environmental changes (light/dark cycle, food availability), behavior (sleep, exercise, feeding), endogenous metabolites, and hormonal status. Moreover, pharmacological interventions to extend longevity in mice often exhibit sexual dimorphism201–203 and depend at what age the treatment starts112, and thus are important factors to consider in addition to the timing of antiaging therapies. Incorporating these variables in assessing individual internal timing pushes the need for developing novel computational tools.
Final remarks
As the human population ages, the increased risk of chronic diseases has become a public health burden worldwide. Additionally, ~39% of the world adult population is overweight due to unrestricted access to food and sedentary lifestyle, increasing the incidence of cardiovascular disease, obesity, diabetes, and stroke. Dietary interventions, including regulation of the amount and timing of food intake and length of fasting periods, have become attractive methods for mitigating age-related physical decline and chronic diseases. Although the specific mechanisms are far from being fully understood, a periodic break in energy intake appears to improve multiple risk factors and, in some cases, reverse disease progression in mice and humans. Going forward, it will be important to elucidate to what degree the effects of caloric restriction regimes are due to calories, fasting, and feeding time. In addition, pharmacological interventions targeting pathways improved by DR have become promising alternatives to restricted diets. Understanding how metabolic pathways change throughout the day may provide insights into when and how often treatments should be applied in order to minimize drug resistance and side effects (Fig. 4). Additionally, systematic studies are required to determine at what age treatment can be applied to provide maximum benefits. Integrating tissue-specific circadian oscillations in these pathways could also prove critical for pinpointing the optimal time to administer interventions in order to promote healthy aging.
Supplementary information
Acknowledgements
We thank Dr. Kimberly Cox for helpful manuscript editing and Fernando Augusto for the art production of figures. We apologize for the omission of relevant work owing to space constraints. This work was supported by the Howard Hughes Medical Institute, NIH grant R01 AG045795 (J.S.T. and C.B.G.), NIH/NIGMS grant R35 GM127122 (C.B.G.), and NIH/NIGMS 1K99GM132557 (F.R.-F.). J.S.T. is an Investigator and F.R.F. is an Associate in the Howard Hughes Medical Institute.
Author contributions
The review was written by V.A.R. and F.R.-F. and edited by J.S.T. and C.B.G.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Roman Kondratov, Frank Scheer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22922-6.
References
- 1.Lopez-Otin C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Richardson A, et al. Measures of healthspan as indices of aging in mice-—A recommendation. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:427–430. doi: 10.1093/gerona/glv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellantuono I, et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat. Protoc. 2020;15:540–574. doi: 10.1038/s41596-019-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deelen J, et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019;10:3346. doi: 10.1038/s41467-019-11311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilai N, Cuervo AM, Austad S. Aging as a biological target for prevention and therapy. JAMA. 2018;320:1321–1322. doi: 10.1001/jama.2018.9562. [DOI] [PubMed] [Google Scholar]
- 6.Nakahata Y, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey KM, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhari A, Gupta R, Patel S, Velingkaar N, Kondratov R. Cryptochromes regulate IGF-1 production and signaling through control of JAK2-dependent STAT5B phosphorylation. Mol. Biol. Cell. 2017;28:834–842. doi: 10.1091/mbc.e16-08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khapre RV, et al. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany NY) 2014;6:48–57. doi: 10.18632/aging.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rijo-Ferreira F, Takahashi JS, Figueiredo LM. Circadian rhythms in parasites. PLoS Pathog. 2017;13:e1006590. doi: 10.1371/journal.ppat.1006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014;10:466–475. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Challet E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019;15:393–405. doi: 10.1038/s41574-019-0210-x. [DOI] [PubMed] [Google Scholar]
- 19.Cincotta AH, et al. Circadian neuroendocrine role in age-related changes in body fat stores and insulin sensitivity of the male Sprague-Dawley rat. Chronobiol. Int. 1993;10:244–258. doi: 10.1080/07420529309059707. [DOI] [PubMed] [Google Scholar]
- 20.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc. Natl Acad. Sci. USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatfield D, Schibler U. Circadian glucose homeostasis requires compensatory interference between brain and liver clocks. Proc. Natl Acad. Sci. USA. 2008;105:14753–14754. doi: 10.1073/pnas.0807861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 23.Froy O. Circadian aspects of energy metabolism and aging. Ageing Res. Rev. 2013;12:931–940. doi: 10.1016/j.arr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Hood S, Amir S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 2017;127:437–446. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2:936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B, et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016;23:610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katewa SD, et al. Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell Metab. 2016;23:143–154. doi: 10.1016/j.cmet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki S, et al. Effects of aging on central and peripheral mammalian clocks. Proc. Natl Acad. Sci. USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpcell.1997.273.6.C1957. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci. Lett. 1998;258:167–170. doi: 10.1016/S0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]
- 31.Sellix MT, et al. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J. Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libert S, Bonkowski MS, Pointer K, Pletcher SD, Guarente L. Deviation of innate circadian period from 24 h reduces longevity in mice. Aging Cell. 2012;11:794–800. doi: 10.1111/j.1474-9726.2012.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park N, Cheon S, Son GH, Cho S, Kim K. Chronic circadian disturbance by a shortened light-dark cycle increases mortality. Neurobiol. Aging. 2012;33:1122 e1111–1122 e1122. doi: 10.1016/j.neurobiolaging.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 36.Hurd MW, Zimmer KA, Lehman MN, Ralph MR. Circadian locomotor rhythms in aged hamsters following suprachiasmatic transplant. Am. J. Physiol. 1995;269:R958–R968. doi: 10.1152/ajpregu.1995.269.5.R958. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Satinoff E. Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats. Am. J. Physiol. 1998;275:R1735–R1744. doi: 10.1152/ajpcell.1998.275.2.C358. [DOI] [PubMed] [Google Scholar]
- 38.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paschos GK, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson AJ, et al. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl Acad. Sci. USA. 2016;113:E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362:770–775. doi: 10.1126/science.aau2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speakman JR, Mitchell SE. Caloric restriction. Mol. Asp. Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Deepa SS, Unnikrishnan A, Matyi S, Hadad N, Richardson A. Necroptosis increases with age and is reduced by dietary restriction. Aging Cell. 2018;17:e12770. doi: 10.1111/acel.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell SJ, et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 2019;29:221–228 e223. doi: 10.1016/j.cmet.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asher G, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 52.Lamia KA, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 54.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl Acad. Sci. USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh PP, Demmitt BA, Nath RD, Brunet A. The genetics of aging: a vertebrate perspective. Cell. 2019;177:200–220. doi: 10.1016/j.cell.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crosby P, et al. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell. 2019;177:896–909 e820. doi: 10.1016/j.cell.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng X, Sehgal A. AKT and TOR signaling set the pace of the circadian pacemaker. Curr. Biol. 2010;20:1203–1208. doi: 10.1016/j.cub.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramanathan C, et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018;14:e1007369. doi: 10.1371/journal.pgen.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao R, Li A, Cho HY, Lee B, Obrietan K. Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock. J. Neurosci. 2010;30:6302–6314. doi: 10.1523/JNEUROSCI.5482-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tulsian R, Velingkaar N, Kondratov R. Caloric restriction effects on liver mTOR signaling are time-of-day dependent. Aging (Albany NY) 2018;10:1640–1648. doi: 10.18632/aging.101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 2017;26:267–277 e262. doi: 10.1016/j.cmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu R, et al. The circadian protein period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 Complex. Cell Metab. 2019;29:653–667 e656. doi: 10.1016/j.cmet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Sato S, et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170:664–677 e611. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solanas G, et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell. 2017;170:678–692 e620. doi: 10.1016/j.cell.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 67.Kondratov RV. A role of the circadian system and circadian proteins in aging. Ageing Res. Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2016;30:1634–1642. doi: 10.1096/fj.15-282475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel SA, Velingkaar N, Makwana K, Chaudhari A, Kondratov R. Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci. Rep. 2016;6:25970. doi: 10.1038/srep25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen D, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satoh A, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herranz D, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hahn O, et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab. 2019;1:1059–1073. doi: 10.1038/s42255-019-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mattison JA, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson W, Halberg F. Meal-timing, circadian rhythms and life span of mice. J. Nutr. 1986;116:2244–2253. doi: 10.1093/jn/116.11.2244. [DOI] [PubMed] [Google Scholar]
- 79.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 2011;104:535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Izumo M, et al. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. elife. 2014;3:e04617. doi: 10.7554/eLife.04617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 82.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vollmers C, et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl Acad. Sci. USA. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mendoza J, Clesse D, Pevet P, Challet E. Food-reward signalling in the suprachiasmatic clock. J. Neurochem. 2010;112:1489–1499. doi: 10.1111/j.1471-4159.2010.06570.x. [DOI] [PubMed] [Google Scholar]
- 85.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sherman H, et al. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 87.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eckel-Mahan KL, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaix, A., Lin, T., Le, H. D., Chang, M. W. & Panda, S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab.29, 303–319 (2018). [DOI] [PMC free article] [PubMed]
- 92.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Lopez N, et al. System-wide benefits of intermeal fasting by autophagy. Cell Metab. 2017;26:856–871 e855. doi: 10.1016/j.cmet.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Talan MI, Ingram DK. Effect of intermittent feeding on thermoregulatory abilities of young and aged C57BL/6J mice. Arch. Gerontol. Geriatr. 1985;4:251–259. doi: 10.1016/0167-4943(85)90007-X. [DOI] [PubMed] [Google Scholar]
- 95.Varady KA. Impact of intermittent fasting on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:300–302. doi: 10.1097/MCO.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 96.Anson RM, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl Acad. Sci. USA. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech. Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-Q. [DOI] [PubMed] [Google Scholar]
- 98.Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell. 2009;8:756–760. doi: 10.1111/j.1474-9726.2009.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirao A, et al. Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1045–G1053. doi: 10.1152/ajpgi.00330.2010. [DOI] [PubMed] [Google Scholar]
- 100.Brandhorst S, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei M, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017;9:eaai8700. doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng CW, et al. Fasting-mimicking diet promotes Ngn3-driven beta-cell regeneration to reverse diabetes. Cell. 2017;168:775–788 e712. doi: 10.1016/j.cell.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts MN, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2018;27:1156. doi: 10.1016/j.cmet.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newman JC, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26:547–557 e548. doi: 10.1016/j.cmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tognini P, et al. Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab. 2017;26:523–538 e525. doi: 10.1016/j.cmet.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Solon-Biet SM, et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 2015;11:1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bookout AL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller RA, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barcena C, et al. Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Rep. 2018;24:2392–2403. doi: 10.1016/j.celrep.2018.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller RA, et al. Glycine supplementation extends lifespan of male and female mice. Aging Cell. 2019;18:e12953. doi: 10.1111/acel.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawai N, et al. The sleep-promoting and hypothermic effects of glycine are mediated by NMDA receptors in the suprachiasmatic nucleus. Neuropsychopharmacology. 2015;40:1405–1416. doi: 10.1038/npp.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mordel J, et al. Activation of glycine receptor phase-shifts the circadian rhythm in neuronal activity in the mouse suprachiasmatic nucleus. J. Physiol. 2011;589:2287–2300. doi: 10.1113/jphysiol.2010.204693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7:173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- 116.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Redman LM, et al. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018;27:805–815 e804. doi: 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl Acad. Sci. USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diaz-Ruiz, A. et al. Benefits of caloric restriction in longevity and chemical-induced tumorigenesis are transmitted independent of NQO1. J. Gerontol. A Biol. Sci. Med. Sci. 74, 155–162 (2018). [DOI] [PMC free article] [PubMed]
- 120.Mercken EM, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin CK, et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern. Med. 2016;176:743–752. doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Das SK, et al. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am. J. Clin. Nutr. 2017;105:913–927. doi: 10.3945/ajcn.116.137232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—a narrative review of human and animal evidence. Behav. Sci. (Basel) 2017;7:4. doi: 10.3390/bs7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schubel R, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am. J. Clin. Nutr. 2018;108:933–945. doi: 10.1093/ajcn/nqy196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sundfor TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018;28:698–706. doi: 10.1016/j.numecd.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 126.Sundfor TM, Tonstad S, Svendsen M. Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. Eur. J. Clin. Nutr. 2019;73:1006–1014. doi: 10.1038/s41430-018-0370-0. [DOI] [PubMed] [Google Scholar]
- 127.Safdie FM, et al. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee C, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dorff TB, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360. doi: 10.1186/s12885-016-2370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Groot S, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. doi: 10.1186/s12885-015-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zuccoli G, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr. Metab. (Lond.) 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Willi SM, Oexmann MJ, Wright NM, Collop NA, Key LL., Jr. The effects of a high-protein, low-fat, ketogenic diet on adolescents with morbid obesity: body composition, blood chemistries, and sleep abnormalities. Pediatrics. 1998;101:61–67. doi: 10.1542/peds.101.1.61. [DOI] [PubMed] [Google Scholar]
- 133.Hallbook T, Lundgren J, Rosen I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia. 2007;48:59–65. doi: 10.1111/j.1528-1167.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 134.Husain AM, Yancy WS, Jr., Carwile ST, Miller PP, Westman EC. Diet therapy for narcolepsy. Neurology. 2004;62:2300–2302. doi: 10.1212/WNL.62.12.2300. [DOI] [PubMed] [Google Scholar]
- 135.Afaghi A, O’Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosci. 2008;11:146–154. doi: 10.1179/147683008X301540. [DOI] [PubMed] [Google Scholar]
- 136.Castano-Martinez T, et al. Methionine restriction prevents onset of type 2 diabetes in NZO mice. FASEB J. 2019;33:7092–7102. doi: 10.1096/fj.201900150R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta NJ, Kumar V, Panda S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE. 2017;12:e0172852. doi: 10.1371/journal.pone.0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McHill AW, et al. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017;106:1213–1219. doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garaulet M, et al. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. (Lond.) 2013;37:604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stote KS, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moro T, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016;14:290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tinsley GM, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 2017;17:200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 144.Sutton EF, et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–1221 e1213. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bandin C, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int. J. Obes. (Lond.) 2015;39:828–833. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 146.Lopez-Minguez J, Saxena R, Bandin C, Scheer FA, Garaulet M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin. Nutr. 2018;37:1133–1140. doi: 10.1016/j.clnu.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wehrens SMT, et al. Meal timing regulates the human circadian system. Curr. Biol. 2017;27:1768–1775 e1763. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jakubowicz D, et al. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: a randomized clinical trial. Diabetes Care. 2017;40:1573–1579. doi: 10.2337/dc16-2753. [DOI] [PubMed] [Google Scholar]
- 149.Richter J, et al. Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals. J. Clin. Endocrinol. Metab. 2020;105:dgz311. doi: 10.1210/clinem/dgaa353. [DOI] [PubMed] [Google Scholar]
- 150.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 151.Nimitphong H, et al. More evening preference is positively associated with systemic inflammation in prediabetes and type 2 diabetes patients. Sci. Rep. 2018;8:15882. doi: 10.1038/s41598-018-34045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Knutsson A. Health disorders of shift workers. Occup. Med. (Lond.) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 153.Vetter C, et al. Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care. 2018;41:762–769. doi: 10.2337/dc17-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Straif K, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 155.Jakubowicz D, et al. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58:912–919. doi: 10.1007/s00125-015-3524-9. [DOI] [PubMed] [Google Scholar]
- 156.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wefers, J. et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl Acad. Sci. USA115, 7789–7794 (2018). [DOI] [PMC free article] [PubMed]
- 158.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl Acad. Sci. USA. 2015;112:E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hirshkowitz M, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep. Health. 2015;1:233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat. Rev. Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brandenberger G, Gronfier C, Chapotot F, Simon C, Piquard F. Effect of sleep deprivation on overall 24 h growth-hormone secretion. Lancet. 2000;356:1408. doi: 10.1016/S0140-6736(00)02847-6. [DOI] [PubMed] [Google Scholar]
- 162.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. N. Y Acad. Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 165.Al Khatib HK, Harding SV, Darzi J, Pot GK. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2017;71:614–624. doi: 10.1038/ejcn.2016.201. [DOI] [PubMed] [Google Scholar]
- 166.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015;6:648–659. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miller RA, et al. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 168.Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 169.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Martin-Montalvo A, et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 2019;29:592–610. doi: 10.1016/j.cmet.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 173.Ruben MD, et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci. Transl. Med. 2018;10:eaat8806. doi: 10.1126/scitranslmed.aat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Refinetti R, Nelson DE, Menaker M. Social stimuli fail to act as entraining agents of circadian rhythms in the golden hamster. J. Comp. Physiol. A. 1992;170:181–187. doi: 10.1007/BF00196900. [DOI] [PubMed] [Google Scholar]
- 175.Zwighaft Z, et al. Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab. 2015;22:874–885. doi: 10.1016/j.cmet.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 176.Levi F, et al. Chemotherapy of advanced ovarian cancer with 4′-O-tetrahydropyranyl doxorubicin and cisplatin: a randomized phase II trial with an evaluation of circadian timing and dose-intensity. J. Clin. Oncol. 1990;8:705–714. doi: 10.1200/JCO.1990.8.4.705. [DOI] [PubMed] [Google Scholar]
- 177.Hrushesky WJ. Circadian timing of cancer chemotherapy. Science. 1985;228:73–75. doi: 10.1126/science.3883493. [DOI] [PubMed] [Google Scholar]
- 178.Hori K, Zhang QH, Li HC, Saito S, Sato Y. Timing of cancer chemotherapy based on circadian variations in tumor tissue blood flow. Int. J. Cancer. 1996;65:360–364. doi: 10.1002/(SICI)1097-0215(19960126)65:3<360::AID-IJC14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 179.Ferrannini E. Sodium-glucose transporter-2 inhibition as an antidiabetic therapy. Nephrol. Dial. Transpl. 2010;25:2041–2043. doi: 10.1093/ndt/gfq249. [DOI] [PubMed] [Google Scholar]
- 180.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am. J. Hypertens. 2008;21:948–954. doi: 10.1038/ajh.2008.216. [DOI] [PubMed] [Google Scholar]
- 181.Strong R, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Patrono, C. & Baigent, C. Role of aspirin in primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 16, 675–686 (2019). [DOI] [PubMed]
- 183.Tofler GH, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N. Engl. J. Med. 1987;316:1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 184.Ohdo S, Ogawa N, Song JG. Chronopharmacological study of acetylsalicylic acid in mice. Eur. J. Pharmacol. 1995;293:151–157. doi: 10.1016/0926-6917(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 185.Cohen M, Adams PC, McBride R, Blanke H, Fuster VV. Prospective comparison of patient characteristics and outcome of non-prior aspirin users versus aspirin users with unstable angina or non-Q-wave myocardial infarction treated with combination antithrombotic therapy. J. Thromb. Thrombolysis. 1997;4:275–280. doi: 10.1023/A:1008855220129. [DOI] [PubMed] [Google Scholar]
- 186.Bonten TN, et al. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension. 2015;65:743–750. doi: 10.1161/HYPERTENSIONAHA.114.04980. [DOI] [PubMed] [Google Scholar]
- 187.McNeil JJ, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 2018;379:1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McNeil JJ, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3:716–732. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen Z, Yoo SH, Takahashi JS. Development and therapeutic potential of small-molecule modulators of circadian systems. Annu. Rev. Pharmacol. Toxicol. 2018;58:231–252. doi: 10.1146/annurev-pharmtox-010617-052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cell Mol. Life Sci. 2013;70:2985–2998. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nohara K, et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019;10:3923. doi: 10.1038/s41467-019-11926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ueda HR, et al. Molecular-timetable methods for detection of body time and rhythm disorders from single-time-point genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2004;101:11227–11232. doi: 10.1073/pnas.0401882101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kasukawa T, et al. Human blood metabolite timetable indicates internal body time. Proc. Natl Acad. Sci. USA. 2012;109:15036–15041. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hughey JJ, Hastie T, Butte AJ. ZeitZeiger: supervised learning for high-dimensional data from an oscillatory system. Nucleic Acids Res. 2016;44:e80. doi: 10.1093/nar/gkw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Agostinelli F, Ceglia N, Shahbaba B, Sassone-Corsi P, Baldi P. What time is it? Deep learning approaches for circadian rhythms. Bioinformatics. 2016;32:3051. doi: 10.1093/bioinformatics/btw504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Laing EE, et al. Blood transcriptome based biomarkers for human circadian phase. elife. 2017;6:e20214. doi: 10.7554/eLife.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc. Natl Acad. Sci. USA. 2017;114:5312–5317. doi: 10.1073/pnas.1619320114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wittenbrink N, et al. High-accuracy determination of internal circadian time from a single blood sample. J. Clin. Invest. 2018;128:3826–3839. doi: 10.1172/JCI120874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Braun R, et al. Universal method for robust detection of circadian state from gene expression. Proc. Natl Acad. Sci. USA. 2018;115:E9247–E9256. doi: 10.1073/pnas.1800314115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Harrison DE, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Strong R, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16:1256–1266. doi: 10.1111/acel.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Villanueva JE, et al. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat. Commun. 2019;10:2700. doi: 10.1038/s41467-019-10563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 206.Terzibasi E, et al. Effects of dietary restriction on mortality and age-related phenotypes in the short-lived fish Nothobranchius furzeri. Aging Cell. 2009;8:88–99. doi: 10.1111/j.1474-9726.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 207.Catterson JH, et al. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol. 2018;28:1714–1724 e1714. doi: 10.1016/j.cub.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


