Abstract
Acupuncture is an important alternative therapy in treating major depressive disorder (MDD), but its efficacy and safety are still not well assessed. This study is the first network meta-analysis exploring the effectiveness and safety of acupuncture, common pharmacological treatments or other non-medication therapies for MDD. Eight databases including PubMed, Embase, Allied and Complementary Medicine Database, Cochrane Library, Wan Fang Data, China National Knowledge Infrastructure, China Biology Medicine disc, and Chongqing VIP Database were searched up to Jan 17, 2021. Articles were screened and selected by two reviewers independently. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the certainty of the evidence. A total of 71 eligible studies were included. The network analysis results indicated that the combined interventions of electro-acupuncture (EA) with selective serotonin reuptake inhibitors (SSRIs) and manual acupuncture (MA) with SSRIs were more effective in improving depression symptoms compared with acupuncture alone, pharmacological interventions alone, or other inactive groups. Among all the regimens, EA with SSRIs was found to have the highest effect in improving depression symptoms of MDD. In addition, there were slight differences in the estimations of the various treatment durations. The combination of acupuncture and serotonin-norepinephrine reuptake inhibitors (SNRIs) was found to be more effective than SNRIs alone. In conclusion, acupuncture and its combinations could be safe and effective interventions for MDD patients. EA with SSRIs seems to be the most effective intervention among the assessed interventions. Well-designed and large-scale studies with long-term follow-up should be conducted in the future.
Subject terms: Depression, Therapeutics
Introduction
Major Depressive Disorder (MDD) is a serious mood disorder characterized as depressive mood and loss of interest. MDD affects up to 3.0% (2.4–3.8%) of the population worldwide1. In the United States (US), the 12-month prevalence of MDD is approximately 7%, and the rate in females could even be 1.5–3 folds higher than males at the early time of adolescence2. Diagnosis of MDD requires a period of major depressive episode which shows depressed mood, and loss of interest nearly every day for at least 2 weeks3. With the high recurrence of MDD (35 and 85% in the general population and specialized mental health care settings respectively after 15 years)4, uncontrolled and severe MDD causes continuously suicidal behaviors and creates extra medical and economic burdens5,6.
The second-generation antidepressants (SGAs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), etc., are considered and commonly applied as first-line treatment options for MDD7. However, side effects and non-response can occur commonly8. Patients treated with SNRIs complained of side effects such as sleep disturbances, sexual dysfunction, appetite changes, and headache9. Dizziness, fatigue, constipation, and dry mouth occur more frequently in patients using tricyclic antidepressants (TCAs)10. 30–50% of the patients show non-response to the treatment with antidepressants11. Due to these reasons, a variety of nonpharmacological approaches, including psychology consulting and complementary and alternative medicine (CAM), are adopted for the treatment of MDD. And acupuncture is one of the most commonly used nonpharmacological treatments. In the US, it is estimated that 0.6% of patients suffering from severe depression choose acupuncture12.
In recent decades, existing systematic reviews and meta-analyses suggested combination of acupuncture and SSRIs or SNRIs in treating MDD patients. As network meta-analysis (NMA) is a more efficient approach in evaluating and ranking multiple interventions, we conducted this study to assess the effectiveness and safety of different techniques of acupuncture in treating patients with MDD.
Methods
Search strategy for identification of studies
The systematic search was conducted in eight databases, PubMed, Embase, Allied and Complementary Medicine Database (AMED), Cochrane Library, Wan Fang Data, China National Knowledge Infrastructure (CNKI), China Biology Medicine disc (CBM, CBMdisc), and Chongqing VIP Database (CQVIP), from their inception to Jan 17, 2021. The following terms were used in the search strategies: (Acupuncture, Acupuncture Therapy, Electroacupuncture, Acupuncture, needling, electrostimulation, auriculoacupuncture, Electro-acupuncture, Electroacupuncture) and (depression, depressive disorder). The search strategies were adapted and specified for different databases. Details of the search strategies were listed in the Supplementary Method.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and its extension statement, the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (PRISMA-NMA), were regarded as the templates when reporting this systematic review and network meta-analyses13,14. This study was registered in PROSPERO, number CRD42019136229.
Study selection
Two reviewers (Z.C. Hu and L. Yao) independently evaluated studies for inclusion. Any disagreements were reviewed by the third reviewer (L.L.D. Zhong) and resolved by discussion among all reviewers. Studies that met the following criteria were included: (1) randomized control trials (RCTs) that adopted a double-blind, single-blind, or quasi-blind design; (2) patients met established diagnostic criteria of major depressive disorder, including the Diagnostic and Statistical Manual of Mental Disorders (DSM), the International Classification of Diseases (ICD) and the Chinese Classification of Mental Disorders (CCMD); (3) types of acupuncture were included: manual acupuncture (MA), electro-acupuncture (EA); (4) acupuncture alone or combined with antidepressant medications was compared with antidepressant medications, blank control, waitlist control, placebo control, or other non-medication therapies. Studies with the diagnosis of post-stroke depression, postpartum depression, depression during pregnancy, and depression due to the general medical condition were excluded.
Data abstraction
Two independent reviewers (Z.C. Hu and W.Y. Huang) extracted data from selected RCTs. Characteristics such as first author, titles of study, participants (gender, age, duration, sample sizes), study design (randomization, blinding), interventions, control interventions, outcome measures, results, and adverse events were recorded in a pre-made form. Pharmacological treatments evaluated were sorted by the five main antidepressants types: SSRIs, SNRIs, TCAs, monoamine oxidase inhibitors (MAOIs), noradrenaline and specific serotoninergic antidepressants (NASSAs). Acupuncture treatments were sorted by EA, MA, sham EA, and sham MA. Any disagreements were reviewed by the third reviewer (W.C. Lam) and resolved by discussion among all reviewers.
Outcomes
Hamilton Depression Rating Scale (HDRS, also abbreviated as HAMD) and Self-Rated Depression Scale (SDS) were defined as the primary efficiency outcome measures. Side Effect Rating Scale (SERS), Treatment Emergent Symptom Scale (TESS), and the number of adverse events or patients dropping out of the study due to any reason were defined as the primary safety outcome. Other assessment questionnaires measuring the depression level of MDD patients were collected at the same time.
Quality assessment
The identified trials were assessed independently by two reviewers (W.C. Lam and L. Yao). The risks of bias of the included RCTs were assessed using Revised Cochrane risk-of-bias tool for randomized trials (RoB 2)15. The appraisal of acupuncture procedure was based on the criteria of the Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA)16. Any disagreements were reviewed by the third reviewer (L.L.D. Zhong) and resolved by discussion among all reviewers.
Data synthesis and analysis
A network plot was constructed to illustrate all the relationships of the included interventions. Nodes represented the competing treatments, and edges represented the available direct comparisons between pairs of treatments. The size of the node and the width of the edges in the network plot were both weighted according to the number of studies involved in each direct comparison. The effects of multiple interventions were compared by estimating mean differences (MDs) on the change score between final and baseline scores on depression symptoms measured by the same scales. For studies that did not report the mean change from baseline, we calculated the mean change score in each intervention arm as the mean final score minus mean baseline score. For a trial that did not report the standard deviation (SD) of the change score, it was computed as , where SDB and SDF were the SDs of the baseline and final scores, and a moderate correlation coefficient of r = 0.5 between baseline and final irritability score was assumed. Since a higher score represents worse depression symptoms and the change score was defined as the final minus baseline score. A treatment was considered more efficacious than another treatment if the corresponding estimate of MD on the change score was negative and the 95% confidence interval (CI) did not include zero. The NMA was conducted based on the same scale to decrease potential heterogeneity and ensure the similarity of the outcomes data.
Bayesian NMAs with the package ‘gemtc’ V.0.8.1 of RStudio software (ver. 0.96.315; RStudio Inc, Boston, MA, USA) was performed to compare the effects of different prophylactic agents. The Markov Chains Monte Carlo sampler was used to generate samples. A total of 10 000 simulations for each chain was set as the ‘burn-in’ period. Posterior summaries were based on 100 000 subsequent simulations. Model convergence was assessed using the Brooks–Gelman–Rubin plots method. Global heterogeneity was assessed on the bias of the magnitude of heterogeneity variance parameter estimated from the NMA models using the mtc.anohe command of the ‘gemtc’ package. The normal likelihood used for the mean change score was continuous17. A random-effects network meta-analyses were performed for the NMA to account for the potential heterogeneity in the data. The comparative efficacies between the antimanic drugs were expressed using sham MA as reference.
A node splitting method was used to examine the inconsistency between direct and indirect comparisons when a loop connecting three arms exists18. The ranking probabilities for all treatments were also estimated, and a treatment hierarchy using the probability of being the best treatment was obtained19. This process was performed using the cumulative ranking curve (SUCRA). The SUCRA index ranged between 0 and 1, where the treatments with higher SUCRA values were considered to have better efficacy. Moreover, the subgroup analyses were conducted according to the different treatment duration to further explore the potential resource of heterogeneity. All outcomes from included studies were divided into three groups based on the duration of treatment, short-term as 1 ≤ x ≤ 4 weeks, mid-term as 4 < x ≤ 8 weeks, and long-term as x > 8 weeks.
Assessing certainty of the evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) were used to assess the certainty of the direct, indirect, and network estimates for all outcomes. The certainty of direct evidence of the randomized trials starts from high and can be rated down to be moderate, low and very low20. Certainty ratings of indirect estimates start at the lowest GRADE rating of the direct comparisons that contributed to the most-dominant first order loop, with a further rating down for intransitivity when present21,22. Ratings of the certainty of estimates for direct and indirect estimates to inform the rating of network estimates include risk of bias, inconsistency, indirectness, and publication bias, while imprecision was assessed at the network level. For the certainty of network estimates, we started with the estimate—direct or indirect—that dominates (contribution > 50%) the network estimate or use the higher of the direct and indirect estimates if they both contributed importantly to the network estimate. If incoherence is present, when both the direct and indirect evidence has the same certainty of evidence: we used the network estimate, but rate down the certainty of evidence; when the direct and indirect evidence does not have the same certainty of evidence: we used the higher certainty evidence instead of the network estimate. We used the MAGICapp platform to develop GRADE summary of finding tables for each outcome.
Results
Study identification
The flow diagram of literature selection was shown in Fig. 1 with reasons for exclusion at each stage. According to the prespecified selection criteria, 71 eligible studies and a total of 5856 individuals were assessed with eligibility and included in the review.
Figure 1.
Flowchart of literature selection on systematic reviews on acupuncture for treating major depressive disorder.
Characteristics of the included studies
The aggregated characteristics of the included RCTs were shown in Table 1. 16 studies3,24,31,34,37,49,53,56,60,65,75,76,83,86–88 met DSM (III revision: 1; IIIR revision: 1; IV revision: 10; V revision: 4), 45 studies26–29,32,33,35,36,38–42,44–46,48,50,53,54,57–59,61–63,66–70,74,77–82,84,85,88–90,92,93 met CCMD (3rd version: 44; 2R: 1), 14 studies25,30,40,43,47,51,52,55,64,71–73,80,91 met ICD (10th revision: 13; 9th revision: 1).
Table 1.
The aggregated characteristics of the included RCTs.
| Source | Study design | Population | Treatment | Outcome measures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of arm | Diagnosis criteria | No | Age | Intervention Group (type; duration; frequency; or drug name; dose; duration; frequency) | Comparator Group (type; duration; frequency; or drug name; dose; duration; frequency) | Third Group (type; duration; frequency; or drug name; dose; duration; frequency) | ||||||
| Ai et al.23 | 2018 | 2 | DSM-V | I: 50; C: 50 | I: 20.1 ± 3.6; C: 20.2 ± 3.5 | MA + paroxetine | MA: 1 time/ d, 6 w; paroxetine: 20 mg/time, 1 time/ d, 6 w | Paroxetine | 20 mg/time, 1 time/ d, 6 w | N/A | N/A | HAMD-17(0, 1, 2, 4, 6 w); response rates |
| Allen et al.24 | 2006 | 3 | DSM-IV | I: 53; C: 52; T: 52 | I: 23.3 ± 11.4; C:24.6 ± 12.8; T: 22.7 ± 14.0 | MA | 2 times/ w for first 4 w, 1 time/ w for another 4 w, 8 w | MA | 2 times/ w for first 4 w, 1 time/ w for another 4 w, 8 w | Wait-list | 8w | HAMD-17 (0, 4, 8, 12, 16 w), BDI (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 w); response rates (8, 16 w), remission rates (8, 16 w) |
| Chen et al.25 | 2014 | 2 | ICD-10 | I: 40; C: 33 | I: 36.2 ± 11.7; C: 35.0 ± 10.5 | MA + Paroxetine | MA: 3 times/ w, 6 w; Paroxetine: 1 time/ d, 10–20 mg/ time, 6 w | Paroxetine | 1 time/ d, 10–20 mg/ time, 6 w | N/A | N/A | HAMD-17(0, 1, 2, 4, 6w), SERS( 2, 4, 6w); response rates |
| Chen et al.26 | 2010 | 2 | CCMD-III | I: 33; C: 30 | I:65.30 ± 3.592; C: 65.10 ± 3.736 | MA | 5 time/ w, 6 w | Fluoxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A | HAMD(0, 2, 4, 6w); response rates |
| Chen et al.27 | 2011 | 2 | CCMD-III | I: 30; C: 30 | I: 43.9 ± 11.2; C: 49.0 ± 13.4 | EA + Fluoxetine | EA: 6 times/ w, 8 w; Fluoxetine: 1 time/ d, 20 mg/ time, 8 w | Fluoxetine | 1 time/ d, 20 mg/ time, 8 w | N/A | N/A | HAMD-17(0, 1, 4, 8 w); response rates |
| Dong et al.28 | 2017 | 2 | CCMD-III | I: 30; C: 30 | I: 15.6 ± 1.40; C: 14.9 ± 1.45 | MA + Psychotherapy | MA: 1 time/ d, 10 d one session, another session after 2d, 30 d; Psychotherapy: 1 times/ w, 4 w | Sertraline + Psychotherapy | Sertraline: 1 time/ d, 50 mg/ time, 30 d; Psychotherapy: : 1 times/ w, 4 w | N/A | N/A | HAMD-24(0, 10, 20, 30d) |
| Duan et al.29 | 2008 | 3 | CCMD-III | I: 25; C: 25; T: 25 | I: 50.12 ± 4.32; C: 49.72 ± 5.47; T: 48.93 ± 7.60 | EA | 6 time/ w, 6 w | Fluoxetine | 20 mg/ d, 6 w | EA + Fluoxetine | EA: 6 time w, 6 w; Fluoxetine: 20 mg/ d, 6 w | HAMD(0, 6w), TESS (2, 4, 6w); response rates |
| Feng et al.30 | 2015 | 3 | ICD-10 | I: 60; C: 60; T: 60 | I: 36.4 ± 9.9; C: 37.1 ± 9.8; T: 36.6 ± 10.0 | MA + SSRIs | MA: 3 times/ w, 6 w; Fluoxetine: 20–60 mg/ d; or Paroxetine: 20–60 mg/ d; or Citalopram: 20–60 mg/ d; or Sertraline: 50–200 mg/ d; or Fluvoxamine: 50–300 mg/d; 1–2 times/ d, 6w | SSRIs | Fluoxetine: 20–60 mg/ d; or Paroxetine: 20–60 mg/ d; or Citalopram: 20–60 mg/ d; or Sertraline: : 50–200 mg/ d; or Fluvoxamine: 50–300 mg/d; 1–2 times/ d, 6w | HC | HC | MADRS(0, 1, 2, 4, 6w); SERS(1, 2, 4, 6w) |
| Gallagher et al.31 | 2001 | 3 | DSM-IV | 38 | 18–45 | MA | 8 w | MA | 8 w | Wait-list | 8 w | HRSD-19(0, 8 w) |
| Gu et al.32 | 2015 | 2 | CCMD-III | I: 30; C: 30 | I: 61.83 ± 10.33; C: 63.53 ± 10.11 | MA | 1 time/ d, 30 d | Fluoxetine | 1 time/ d, 20 mg/ time, 30 d | N/A | N/A | HAMD-17(0, 30d) |
| Guo et al33 | 2019 | 2 | CCMD-3 | I: 22; C: 22 | I: 40.86 ± 9.84; C: 42.09 ± 10.71 years | MA + Fluoxetine | MA: 1 time/ d, 3w; Fluoxetine: 20 mg/ time,1 time/ d, 3 W | Fluoxetine | 20 mg/ time,1 time/ d, 3 W | N/A | N/A | HAMD-17, PHQ-9(0, 3w); response rate |
| Han et al34 | 2019 | 2 | DSM-V | I: 25; C: 25 | I: 37.0 (32. 0, 41. 5); C: 39.0 (35.0, 46.5) | EA | 3 times/ w, 6 w | MA | 3 times/ w, 6 w | N/A | N/A | HAMD, SDS(0,6w); response rate |
| Huang et al.35 | 2013 | 2 | CCMD-III | I: 30; C: 30 |
I: 49.25 ± 14.03; C: 50.78 ± 12.96 |
MA + Paroxetine | MA: 3 times/ w, 6 w; Paroxetine: 1 time/ d, 20–40 mg/ time, 6 w | Paroxetine | 1 time/ d, 20–40 mg/ time, 6 w | N/A | N/A | HAMD(0, 1, 6w), SDS(0, 1, 6w); Asberg(1, 6w); response rates |
| Jiang et al.36 | 2008 | 2 | CCMD-III | I: 34; C: 34 | I: 36.4 ± 11.7; C: 35.6 ± 13.1 | MA + Citalopram |
MA: 1 time/ 2 d, 40 d Citalopram: 20 mg/ d, 1 time/ d, 40 d |
Citalopram | 20 mg/ d, 1 time/ d, 40 d | N/A | N/A | HAMD-17(0, 1, 2, 4, 6w), CGI-SI(0, 2, 4, 6w); TESS; response rates |
| Li et al.37 | 2013 | 2 | DSM-IV | I: 62; C: 62 | I: 33.9 ± 12.0; C: 34.8 ± 12.4 | EA + Sertraline | EA: 6 times/ w, 8 w; Sertraline: 50–100 mg/ d, 8 w | Sertraline | 50–100 mg/d, 8 w | N/A | N/A | HAMD-17(0, 1, 2, 4, 8w) |
| Li et al.38 | 2004 | 3 | CCMD-III | I: 30; C: 30; T: 50 | I: 41.8 ± 14.6; C: 39.4 ± 13.4; T: 45.8 ± 14.5 | MA | 5 times/ w, 6 w | Fluoxetine | 1 time/ d, 20 mg/ time, 6 w | MA 2 | 5 times/ w, 6 w | HRSD, SDS(0, 6 w); response rates |
| Lin et al.39 | 2004 | 2 | CCMD-III | I: 29; C: 28 | I: 40.3 ± 11.5; C: 44.6 ± 12.7 | MA + Paroxetine |
MA: 1 time/ d, 5 time/ w, 6 w; Paroxetine: 1 time/ d, 10 mg/ time, 6 w |
Paroxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A |
HAMD-17(0, 1, 2, 4, 6w), HAMA(0, 1, 2, 4, 6w); CGI TESS; response rates |
| Li et al.40 | 2017 | 2 | ICD-10, CCMD-III | I: 30; C: 30 | I: 34 ± 8; C:35 ± 8 | MA + Paroxetine | MA: 6 time/ w, 8 w; Paroxetine: 10- 20 mg/ time, 1 time/ d, 8w | Paroxetine | 10- 20 mg/ time, 1 time/ d, 8w | N/A | N/A | HAMD-17(0,1,2,3,4,5,6,7,8w); response rates |
| Lin et al.41 | 2005 | 2 | CCMD-III | I: 30; C: 23 | I: 41.7 ± 12.1; C: 43.1 ± 11.5 | MA + Fluoxetine | MA: 5 time/ w, 6 w; Fluoxetine: 1 time/ d, 20 mg/ time, 6 w | Fluoxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A | HAMD-17(0, 1, 2, 4, 6w), HAMA(0, 1, 2, 4, 6w); CGI, TESS; response rates |
| Lin et al.42 | 2014 | 2 | CCMD-III | I: 61; C: 61 | NR | MA + Fluoxetine | 1 time/ d, 5 time/ w, 4 w | Fluoxetine | 1 time/ d, 20 mg/ time, 4 w | N/A | N/A | HAMD-17(0, 1, 2, 4w), SDS(0, 4w ); response rates |
| Liu et al.43 | 2018 | 2 | ICD-10 | I: 21; C: 21 | I: 44 ± 10; C:43 ± 9 | Fluoxetine + MA | MA: 1 time/ d for first 3d, then once every 3d, 8 w; Fluoxetine: 10 mg, 1 time/ d, 8w | Fluoxetine + Sham MA | Sham MA: 1 time/ d for first 3d, then once every 3d, 8 w; Fluoxetine: 10 mg, 1 time/ d, 8w | N/A | N/A | MADRS(0, 4, 8w), SDS(0, 4, 8w); response rates |
| Liu et al.44 | 2005 | 2 | CCMD-III | I: 21; C: 20 | I: 48.9 ± 12.0; C: 49.0 ± 13.4 | EA + SSRIs* | EA: 6 w; SSRIs: 6 w* | SSRIs* | SSRIs: 6 w* | N/A | N/A | HAMD-17(0, 1, 2, 4, 6w) |
| Liu et al.45 | 2014 | 3 | CCMD-III | I: 45; C: 45; T: 45 | I: 47.11 ± 8.32; C:47.58 ± 8.21; T: 48.11 ± 7.97 | MA + SSRIs | MA: 1 time/ 2 d, 4 w; Fluoxetine: 20 ~ 60 mg/ d; or Paroxetine: 20–60 mg/ d; or Citalopram: 20–10 mg/ d; or Sertraline: 50–200 mg/ d; 1time/ d, 4 w | SSRIs | Fluoxetine: 20 ~ 60 mg/ d; or Paroxetine: 20–60 mg/ d; or Citalopram: 20–10 mg/ d; or Sertraline: 50–200 mg/ d; 1time/ d, 4 w | HC | HC | HAMD-17(0, 1, 2, 4w), SERS(1, 2, 4w); response rates |
| Liu et al.46 | 2015 | 2 | CCMD-III | I: 45; C: 45; T: 45 | I: 36 ± 11; C: 37 ± 11; T: 36 ± 11 | MA + SSRIs | MA: 1 time/ 2 d, 4 w; SSRI: Fluoxetine: 20–60 mg/ d; or Paroxetine: 20–60 mg/ d; or Citalopram: 20–60 mg/ d; or Sertraline: 50–200 mg/ d; or Fluvoxamine: 50–300 mg/d; 1–2 times/ d, 4 w | SSRIs | Fluoxetine: 20 ~ 60 mg/ d; or Paroxetine: 20–60 mg/ d; or Citalopram: 20–60 mg/ d; or Sertraline: 50–200 mg/ d; or Fluvoxamine: 50–300 mg/d; 1-2times/ d, 4 w | HC | HC | HAMD-17(0, 1, 2, 4w) |
| Liu et al.47 | 2017 | 3 | ICD-10 | I: 47; C: 48; T: 45 |
I: 47.11 ± 9.10; C:47.27 ± 9.03; T:47.18 ± 9.21 |
MA + Venlafaxine | MA: 5 times/ w, 8w; Venlafaxine: 75–225 mg/ d, 8w | Venlafaxine | 75–225 mg/ d, 8w | HC | HC | HAMD-17(0, 8w) |
| Lu et al.48 | 2017 | 2 | CCMD-III | I: 30; C: 30 | I:52.7 ± 17.4; C:56.7 ± 16.3 | MA + Escitalopram |
EA: 3 times/ w, 2 w; Escitalopram :20 mg, 1 time/ d, 2w |
Escitalopram | 20 mg, 1 time/ d, 2w | N/A | N/A | HAMD(0, 1, 2w), |
| Luo et al.49 | 2003 | 3 | DSM-IV | I: 31; C: 32; T: 32 | I: 30 ± 11; C: 34 ± 13; T: 32 ± 12 | EA + Placebo | EA: 1 time/ d, 5 time/ w; Placebo: 1 time/ d, 20 mg/ time | Fluoxetine + Sham EA | Fluoxetine: 1 time/ d, 20 mg/ time; Sham EA: 1 time/ d, 5 time/w | Placebo + sham-EA | Placebo: 1 time/ d, 20 mg/ time; sham-EA: 1 time/ d, 5 time/ w | HAMD, SDS, CGI(0, 2, 4, 6w); Asberg(0, 6w) |
| Ma et al.50 | 2011 | 2 | CCMD-III | I: 31; C: 29 |
I: 51.1 ± 12.85; C:50.9 ± 11.29 |
MA | 5 time/ w, 6 w | Fluoxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A | HAMD-17(0, 2, 4, 6w), Asberg(2, 4, 6w); response rates |
| Ma et al.51 | 2011 | 2 | ICD-10 | I: 26; C: 29 |
I: 46.27 ± 13.13 C: 40.52 ± 14.21 |
EA + Paroxetine | EA: 3 times/ w, 6w; Paroxetine: 10–20 mg/ d, 1times/ d, 6w | Paroxetine | 10–20 mg/ d, 1times/ d, 6w | N/A | N/A | HAMD-17, SERS(0, 1, 2, 4, 6w), CGI(0, 6 w) |
| Ma et al.52 | 2011 | 2 | ICD-10 |
I: 26; C: 29 |
I: 46.27 ± 13.13 C: 40.52 ± 14.21 |
EA + Paroxetine | EA: 3 times/ w, 6 w; Paroxetine: 10–20 mg/ d, 1 time/ d, 6w | Paroxetine | 10–20 mg/ d, 1 time/ d, 6w | N/A | N/A | HAMD-17, SDS(0, 1, 2, 4, 6w) |
| Ma et al53 | 2020 | 2 | CCMD-3/DSM-V | I: 30; C: 32 | 22–70 | MA | 3 times/ w, 8w | Sham MA | 3 times/ w, 8w | N/A | N/A | HAMD, SDS, TESS(0, 4, 8,12w) |
| Pei et al.54 | 2006 | 2 | CCMD-III | I: 62; C: 58 | I: 18–61; C:20–64 | MA | 5 time/ w, 6 w | Fluoxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A | HAMD(0, 2, 4, 6w); response rates |
| Qu et al.55 | 2013 | 3 | ICD-10 | I: 58; C: 54; T: 48 | I: 33.2 ± 9.0; C: 32.3 ± 9.6; T: 34.4 ± 10.8 | EA + Paroxetine | EA: 3 times/ w, 6 w; Paroxetine:10–20 mg/ d, 6 w | MA + Paroxetine |
MA: 3 times/ w, 6 w; Paroxetine:10–20 mg/ d, 6 w |
Paroxetine | Paroxetine:10–20 mg/ d, 6 w | HAMD-17 (0, 1, 2, 4, 6w, 10w follow-up), SDS (0, 1, 2, 4, 6w, 10w follow-up), CGI-S (0, 1, 2, 4, 6w, 10w follow-up); response rates |
| Roschke et al.56 | 2000 | 3 | DSM-III-R | I: 22; C: 24; T: 24 | I: 49 ± 13; C: 47 ± 9; T: 49 ± 11 | MA + Mianserin | MA: 3 times/ w, 4 w; Mianserin: 90–120 mg/ d, 4 w ; | Sham MA + Mianserin | Mianserin: 90–120 mg/ d, 4 w; Sham MA: 3 times/ w, 4 w | Mianserin | 90–120 mg/ d, 4 w | GAS, BRMS, CGI, Bf-S (twice/ w for 8 w); response rates |
| Shi et al.57 | 2015 | 3 | CCMD-III | I: 30; C: 30; T: 30 | I: 52.33 ± 9.93; C: 48.46 ± 8.44; T: 49.94 ± 9.41 | MA | 5 times/ w, 8 w | MA | 5 times/ w, 8 w | Fluoxetine | 1 time/ d, 20 mg/ time, 8 w | HAMD-17(0, 4, 8w); response rates |
| Song et al.58 | 2013 | 2 | CCMD-III | I: 30; C: 30 | I: 42.32 ± 12.47; C: 43.74 ± 12.52 | MA | 6 times/ w, 6 w | Fluoxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A | HAMD(0, 2, 4, 6w), Asberg (2, 4, 6w); response rates |
| Sun et al.59 | 2012 | 2 | CCMD-III | I: 20; C: 20 | I: 32.5 ± 10.3; C: 31.5 ± 11.4 | EA + Venlafaxine | EA: 5 times/ w, 2 w; Venlafaxine: 1 time/ d, 75–150 mg/ time, 2 w | Venlafaxine | 1 time/ d, 75–150 mg/ time, 2 w | N/A | N/A | HAMD(0, 1, 2w); TESS; response rates |
| Sun et al.60 | 2013 | 3 | DSM-IV | I: 25; C: 25; T: 25 |
I: 43.10 ± 13.86; C:42.56 ± 10.70; T:40.72 ± 12.80 |
EA | 5 times/ w, 6 w | EA | 5 times/ w, 6 w | Fluoxetine | 20 mg/ d, 6 w | HDRS-24 (0, 2, 4, 6 w) |
| Tang et al.61 | 2003 | 2 | CCMD-IIR | I: 32; C: 32 | I: 18–51; C: 19–56 | EA + Amitriptyline | EA: 1w-2w: 7 times/ w; 3w-6w: 3 times/ w; 6w; Amitriptyline: 1 time/ d, 50 mg/ time, 6 w | Amitriptyline | 2 times/ d, 25–75 mg/ time, 6 w | N/A | N/A | SDS(0, 3, 6w), SAS(0, 3, 6w); response rates |
| Tian et al.62 | 2008 | 2 | CCMD-III | I: 30; C: 30 | I: 35.1 ± 14.3; C: 34.8 ± 15.1 | EA + Clomipramine* |
EA: 5 times/ w, 6 w; Clomipramine: 1 time/d , 25–75 mg/ time, 6 w* |
Clomipramine* | 1 time/d , 25–250 mg/ time, 6w; | N/A | N/A | HAMD-17(0, 6 w); response rates |
| Wang et al.63 | 2018 | 2 | CCMD-III | I: 40; C: 40 | I: 34.2 ± 10.9; C: 33.4 ± 11.8 | MA + Fluvoxamine | 5 times/ w, 6w; Fluvoxamine: 100 ~ 150 mg/ d, 6 w | Fluvoxamine | 100 ~ 150 mg/ d, 6 w | N/A | N/A | HAMD-24(0, 2, 4, 6w), SERS(1, 2, 4, 6w); response rates |
| Wang et al.64 | 2014 | 3 | ICD-10 | I: 23; C: 32; T:17 | I: 47 ± 11; C: 45 ± 12; T: 48 ± 9 | EA + Paroxetine | EA: 1 time/ 2 d, 6 w; Paroxetine: 10–20 mg/ d, 1 time/ d, 6 w | MA + Paroxetine | MA: 3 times/w, 6 w; Paroxetine: 10–20 mg/ d, 1 time/ d, 6 w | Paroxetine | 10–20 mg/ d, 1 time/ d, 6 w | HAMD-17(0, 1, 2, 4, 6w), SERS(0, 2, 4, 6w), WHOQOL—BREF(0, 6w); response rates |
| Wang et al.65 | 2016 | 2 | DSM-IV | I: 32; C: 32 | I:41.3 ± 5.2;C:42.1 ± 4.7 | MA | 1 time/ d, 6 time/ w, 8 w | Mirtazapine | 1 time/ d, 15–45 mg/ time, 8 w | N/A | N/A | HAMD-24(0, 4, 8w); Asberg; response rates |
| Wang et al.66 | 2007 | 3 | CCMD-III | I: 35; C: 35 | I: 17–65; C: 19–63 | EA | 5 times/ w, 12 w | Sertraline | 25–75 mg/ d, 12 w | HC | HC | HAMD-17(0, 2, 4, 8, 12) |
| Wang et al.67 | 2007 | 2 | CCMD-III | I: 30; C: 30 | I: 18–68; C: 17–70 | EA* | 5 times/ w, 12 w* | Sertraline* | 25–7 5 mg/ d, 1 time/ d, 12 w* | N/A | N/A | HAMD-17(0, 2, 4, 8, 12) |
| Wang et al.68 | 2006 | 2 | CCMD-III | I: 38; C: 38 | I: 18–65; C: 19–63 | EA* | 5 times/ w, 24 w* | Sertraline* | 25–75 mg/d, 1 time/ d, 24 w* | N/A | N/A | HAMD-17(0, 6, 12, 24); response rates |
| Wang et al.69 | 2007 | 2 | CCMD-III | I: 50; C: 50 | I: 17–80; C: 19–73 | EA* | 5 time/ w, 12 w* | Sertraline* | 1time/ d, 25–75 mg/ time, 12 w* | N/A | N/A | HAMD-17(0, 2, 4, 8, 12w); response rates |
| Wang et al.70 | 2008 | 2 | CCMD-III | I: 50; C: 42 | I: 52.8 ± 14.1; C: 52.1 ± 15.4 | MA + SSRIs* | MA: 5 time/w, 4w; SSRIs* | SSRIs* | SSRIs* | N/A | N/A | HAMD-17, SDS(0, 1, 2, 4w ); response rates |
| Wang et al.71 | 2010 | 2 | ICD-10 |
I: 30; C: 30 |
I: 35.7 ± 11.1 C: 41.2 ± 9.0 |
EA + Fluoxetine | EA: 7 times/ w, 6 w; Fluoxetine: 20 mg/ d, 1 time/ d, 6 w | Fluoxetine | 20 mg/ d, 1 time/ d, 6 w | N/A | N/A | HAMD-17(0, 2, 4, 6w); response rates |
| Wang et al.72 | 2014 | 2 | ICD-9 | I: 47; C: 29 | NR | MA + SSRIs/ SNRIs | MA: 5 times/ w, 6 w; Fluoxetine: 20 mg, 1 time/ d; or Paroxetine: 20 mg, 1 time/d; r Duloxetine: 40 mg, 1 time/ d, 6w | SSRIs/ SNRIs | Fluoxetine: 20 mg, 1 time/ d; or Paroxetine: 20 mg, 1 time/d; r Duloxetine: 40 mg, 1 time/ d, 6w | N/A | N/A | HAMD-17 (0, 1, 2, 4, 6 w) |
| Wang et al.73 | 2017 | 2 | ICD-10 | I: 22; C: 24 | I: 44.5 ± 10.47; C: 43.78 ± 9.10 | MA + Fluoxetine | MA : 1 time/d for the first three days, 1 time/ 3 d for the reminder of the 8-w trial; Fluoxetine: 20 mg/ d | Sham MA + Fluoxetine | Sham MA : 1 time/d for the first three days, 1 time/ 3 d for the reminder of the 8-w trial; Fluoxetine: 20 mg/ d | N/A | N/A | MADRS, SDS(0, 8 w) |
| Wang et al74 | 2020 | 2 | CCMD-3 | I: 48; C: 48 | I: 34.19 ± 8.4; C: 32.71 ± 8.2 | Venlafaxine + MA* | MA: 3 times/ w, 12 w; Venlafaxine: 1st w:75 mg/d, 2ed w:150 mg/d, 3-6th w 225 mg/d, 12w* | Venlafaxine* | MA: 3 times/ w, 12 w; Venlafaxine: 1st w:75 mg/d, 2ed w:150 mg/d, 3-6th w 225 mg/d, 12w* | N/A | N/A | HAMD-17, SERS(0, 4, 8, 12 w); response rates |
| Wang et al75 | 2019 | 3 | DSM-V | I: 30; C: 30; T:30 | I: 32 ± 8; C: 32 ± 7;32 ± 8 | Venlafaxine + MA* | MA: 3 times/ w, 12 w; Venlafaxine:1st w:75 mg/d, 2ed w:150 mg/d, 3-6th w 225 mg/d, 12w* | Venlafaxine * | 1st w:75 mg/d, 2ed w:150 mg/d, 3-6th w 225 mg/d, 12w* | HC | HC | HAMD-17, BDI(0,2,8,12w), SERS(2,8,12w) |
| Wang et al.76 | 2013 | 2 | DSM-IV | I: 30; C: 30 | I: 48.1 ± 13.40; C: 47.10 ± 10.60 | EA | 3 times/ w, 24 w | Paroxetine | 20–60 mg/ d, 1 time/ d, 24 w | N/A | N/A | MMPI, MADRS, SDS, SAS(0, 24w) |
| Wen et al.77 | 2003 | 2 | CCMD-III | I: 31; C: 30 | I: 31.6 ± 13.6; C:32.7 ± 14.1 | EA + SSRIs | EA: 1 time/ d, 6 w; SSRI | SSRIs | SSRIs | N/A | N/A | HAMD(0, 2, 4, 6w); response rates |
| Wu et al.78 | 2010 | 2 | CCMD-III | I: 33; C: 33 | I: 68.52 ± 4.84; C: 69.64 ± 5.19 | EA + Citalopram | EA: 5 times/ w, 6w; Citalopram: 20–40 mg/ d, 6 w | Citalopram | 20–40 mg/ d, 6w | N/A | N/A | HAMD-17, TESS (0, 1, 2, 4, 6 w); response rates |
| Xu et al.79 | 2009 | 2 | CCMD-III | I: 21; C: 20 | I: 34; C: 31 | MA | 6 w | Fluoxetine | 20 mg/d, 6 w | N/A | N/A | HAMD-17(0, 6 w); response rates |
| Xu et al.80 | 2004 | 2 | CCMD-III、ICD-10 | I: 30; C: 30 | I: 42.5 ± 8.5; C:45.3 ± 9.2 | MA | 1 time/ d, 30d | Fluoxetine | 20–40 mg/ d, 1 time/ d, 30d | N/A | N/A | HAMD-24(0, 1, 2, 4w) |
| Xu et al.81 | 2011 | 3 | CCMD-III | I: 25; C: 30; T: 25 | I: 47.51 ± 8.21; C: 47.42 ± 8.89; T: 48.01 ± 8.14 | MA + SSRIs | MA: 3 times/ w, 6 w; Paroxetine, 20–60 mg, 1 time/ d, 6 w; or Sertraline: 50–200 mg, 1 time/ d, 6 w; or Fluoxetine: 20–80 mg, 1 time/ d, 6 w | SSRIs | Paroxetine,20–60 mg, 1 time/ d, 6 w; or Sertraline: 50–200 mg, 1 time/ d, 6 w; or Fluoxetine: 20–80 mg, 1 time/ d, 6 w | MA + Moxibustion + SSRIs | MA + Moxibustion: 3 times/ w, 6 w; Paroxetine,20–60 mg, 1 time/ d, 6 w; or Sertraline: 50–200 mg, 1 time/ d, 6 w; or Fluoxetine: 20–80 mg, 1 time/ d, 6 w | HAMD-17(0, 1, 2, 4, 6w); response rates |
| Yang et al.82 | 2012 | 3 | CCMD-III | I: 30; C: 30; T: 30 | I: 32.23 ± 13.98; C:33.40 ± 15.51; T:34.63 ± 12.71 | EA + CBT | 2 time/ w, 8 w; CBT: 1 time/w, 60-90 min/ time, 8 times | EA | 2 time/ w, 8 w | CBT | 1 time/w, 60-90 min/ time, 8 times | HAMD-17, CES-D(0.8w); response rates |
| Yi et al.83 | 2011 | 3 | DSM-IV | I: 14; C:14; T:14 | I: 37.0 ± 8.6; C:33.6 ± 8.4; T:35.5 ± 7.4 | MA + Fluoxetine | MA: 5 time/w, 30 d; Fluoxetine: 1time/ d, 20 mg/ time, 30 d | Fluoxetine | 1 time/ d, 20 mg/ time, 30 d | MA | 5 time/ w, 30 d | HAMD-17(0, 30d) |
| Zhang et al.84 | 2007 | 2 | CCMD-III | I: 38; C: 42 | I: 41.57 ± 1.22; C: 39.82 ± 2.16 | EA | 1 time/ d, 6 w | Paroxetine | 1 time/ d, 20 mg/ time, 6 w | N/A | N/A | HAMD-17(0, 2, 4, 6w) |
| Zhang et al.85 | 2012 | 2 | CCMD-III | I: 20; C: 20 | I: 47.58 ± 9.45; C: 45.65 ± 10.45 | MA | 4 w | Flupentixol + Melitracen | Flupentixol: 0.5 mg/time + Melitracen: 10 mg/time; 1 time/ d, 4 w | N/A | N/A | HAMD-17(0, 4w); response rates |
| Zhang et al.86 | 2007 | 2 | DSM-III | I: 22; C: 20 | I: 36.6 ± 9.7; C: 37.1 ± 10.2 | EA + Paroxetine | EA: 6 times/ w, 6 w; Paroxetine: 10–40 mg/ d, 6 w | Paroxetine | 10–40 mg/ d, 6 w | N/A | N/A | HAMD-17(0, 1, 2, 4, 6 w), TESS (1, 2, 4, 6 w); response rates |
| Zhang et al.87 | 2009 | 2 | DSM-IV | I: 40; C: 40 | I: 36.2 ± 11.7; C: 35.5 ± 12.0 | MA + Fluoxetine (low dose) + placebo* | MA: 5 times/ w, 6 w; 10 mg fluoxetine + 1 placebo pill/ d in the first 2 w, followed by 10 mg fluoxetine + 2 placebo pills/d in the next 4 w* | Sham MA + Fluoxetine (normal dose)* | Sham MA: 5 times/ w, 6 w ; 20 mg/d in the first 2 w, followed by 30 mg fluoxetine/d in the next 4 w* | N/A | N/A | response rate, HRSD-17, HRSA (0, 2, 4, 6 w); SERS, acupuncture-specific side-effect checklist (2, 4, 6 w) |
| Zhao et al.88 | 2010 | 3 | CCMD-III、DSM-IV | I: 30; C: 30; T: 30 | I: 36.5 ± 14.7; C: 39.1 ± 12.3; T: 37.1 ± 12.3 | EA | 1 time/ d, 5 time/ w, 6w | EA | 1 time/ d, 5 times/ d, 6 w | Fluoxetine | 20 mg/ time, 1 time/ d, 6 w | HAMD-24(0, 2, 4, 6 w); response rates |
| Zhao et al.89 | 2010 | 2 | CCMD-III | I: 48; C: 45 | I: 40.9 ± 13.9; C: 41.5 ± 13.9 | EA + Paroxetine* | EA: 7 times/ w, 3 w; Paroxetine: 10–40 mg/ d, 3 w* | Paroxetine* | 10–40 mg/ d, 3 w* | N/A | N/A | HAMD(0, 3w); response rates |
| Zhao et al.90 | 2006 | 2 | CCMD-III | I: 38; C:38 | I: 18–65; C:19–63 | EA* | 5 time/ w, 12 w* | Sertraline* | 1 time/ d, 25–75 mg/ time, 12 w* | N/A | N/A | HAMD-17(0, 1, 2, 8); response rates |
| Zhao et al.91 | 2019 | 3 | ICD-10 | I: 161; C: 160; T:156 | I: 41.42 ± 12.53;C: 41.18 ± 12.00; T: 41.76 ± 12.85 | MA + SSRIs | MA: 3 times/ w, 6 w; Paroxetine, Fluoxetine, Sertraline, Fluvoxamine, Citalopram, or Escitalopram: 10–20 mg/d, 6 w | EA + SSRIs | EA: 3 times/ w, 6 w; Paroxetine, Fluoxetine, Sertraline, Fluvoxamine, Citalopram, or Escitalopram :10–20 mg/d, 6 w | SSRIs | Paroxetine, Fluoxetine, Sertraline, Fluvoxamine, Citalopram, or Escitalopram: 10–20 mg/d, 6 w | response rate, remission rate(6w); early onset rate(1w), HAMD-17(0,1,2,4,6,10w), SDS(0,1,2,4,6,10w), CGI(6w), SERS(2,4,6w) |
| Zheng et al.92 | 2012 | 2 | CCMD-III | I: 44; C: 54 | I: 47.11 ± 9.52; C: 48.07 ± 10.09 | MA | 1 time/ 2d, 3 times/ w, 6 w | SSRI | Paroxetine,20–60 mg, 1 time/ d, 6 w; or Sertraline: 50–200 mg, 1 time/ d, 6 w; or Fluoxetine: 20–80 mg, 1 time/ d, 6 w | N/A | N/A | HAMD-17(0, 1, 2, 4, 6w, f), SERS(0, 1, 2, 4, 6w); response rates |
| Zhu et al.93 | 2018 | 2 | CCMD-III | I: 33; C: 32 | I: 42.9 ± 5.0; C:42.1 ± 4.3 | MA + SSRIs* | MA: 5 time/ w, 6w; SSRIs, 6w* | SSRIs* | SSRIs, 6w* | N/A | N/A | HAMD-24,heart rate variability (0, 6w) |
NR not reported, N/A not available, I intervention group, C comparator group, T third group, HC healthy central group, d day, w week, SSRIs selective serotonin reuptake inhibitors, SERS/Asberg total scores of rating scale for side effects, PHQ-9 patient health questionnaire-9, HAMD/ HRSD/ HDRS the Hamilton Depression Rating Scale, CGI the clinical global impression, TESS Treatment Emergent Symptom Scale, MADRS Montgomery–Asberg Depression Rating Scale, SAS Self-Rating Anxiety Scale, SDS Self-Rating Depression Scale, MMPI Minnesota Multiphasic Personality Inventory, CES-D The Center for Epidemiologic Studies Depression Scale, BDI Beck Depression Inventory, WHOQOL-BREF World Health Organization Quality of Life Instruments(26 item), BRMS Bech- Rafaelsen Melancholia scale, Bf- S The ZERSSEN Mood Scale.
*Benzodiazepines (etc. Clonazepam, Estazolam, Zolpidem) was permitted.
The included studies were published between 2000 and 2020. 6823,25–30,32–55,57–93 of the RCTs originated in China, 2 of the RCTs24,31 originated in the United States, 156of the RCTs originated in German. 59 studies25–30,32–54,57–59,61–71,74,75,77–85,88–90,92,93 were published in Chinese, while 12 studies23,24,31,55,56,60,72,73,76,86,87,91, were in English. 50 RCTs23,25–27,32–37,39–44,48,50–54,58,59,61–63,65,67–80,84–87,89,90,92,93 were two-arm trials, and 2124,28–31,38,45–47,49,55–57,60,64,66,81–83,88,91 were three-arm trials. Treatment duration for acupuncture or related therapies ranged from 2 to 24 weeks.
Network meta-analysis
Change in depression scores
The network plot was presented in Fig. 2. Twelve interventions were involved: EA with SSRIs, MA with SSRIs, EA with SNRIs, MA with SNRIs, SNRIs, EA, MA, SSRIs, NASSAs, sham EA, sham MA, and sham EA with SSRIs. The two types of depression drugs, SSRIs and NASSAs, were included in this NMA. However, three therapies included the EA with SNRIs, MA with SNRIs, and SNRIs therapies were not able to form a connected loop with other interventions. Therefore, they were not be compared and analyzed in the main NMA.
Figure 2.

Network plot.
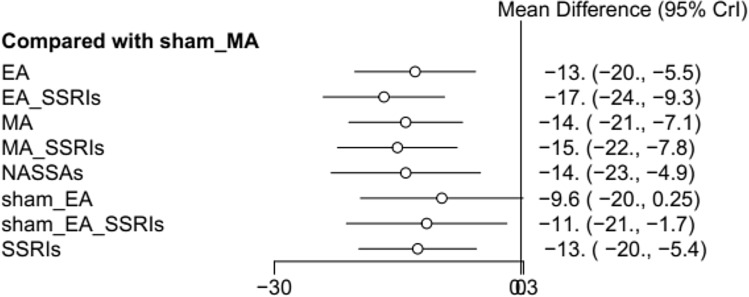
Fifty studies involving 3881 patients in main NMA reported changes in depression scores using the HAMD scale. Six three-arm-based studies and 44 two-arm-based studies were included. Among these studies, 19 studies (n = 19, 38.00%) were comparing MA plus SSRIs with SSRIs alone. And the rest were MA versus (vs) SSRIs (n = 11, 22.00%) and EA plus SSRIs vs SSRIs (n = 11, 22.00%), EA vs SSRIs (n = 10, 20.00%). The results of the NMA of different interventions were displayed in Table 2. For the combined interventions, the results of NMA indicated that EA with SSRIs was more effective in improving depression symptoms compared with MA, Sham EA, Sham MA, and SSRIs (MD: − 2.64, 95% CI: − 5.19 to − 0.10; MD: − 7.04, 95% CI: − 14.10 to − 0.03; MD: − 16.65, 95% CI: − 23.98 to − 9.34; MD: − 4.11, 95% CI: − 5.89 to − 2.33). And for MA with SSRIs, it seemed to be more effective as compared to SSRIs (MD: − 2.47, 95% CI: − 3.85 to − 1.11). For the acupuncture alone, MA was better than sham MA in reducing depression symptoms (MD: − 14.02, 95% CI: − 20.89, − 7.15). The EA could be more effective for relieving the depression symptoms compared with sham MA (MD: − 12.87, 95% CI: − 20.15 to − 5.56). Among all the interventions, EA with SSRIs seemed to achieve superior outcomes when compared to sham MA (MD: − 17.00, 95% CI: − 24.00 to − 9.30) (Fig. 3).
Table 2.
Results of network meta-analysis for all possible treatment effects.
| EA | − 3.78 (− 6.26, − 1.33) | − 1.14 (− 3.63, 1.32) | − 2.14 (− 4.37, 0.04) | − 1.11 (− 7.57, 5.27) | 3.26 (− 3.32, 9.84) | 1.43 (− 4.94, 7.8) | 12.87 (5.56, 20.15) | 0.33 (− 1.49, 2.12) |
| 3.78 (1.33, 6.26) | EA with SSRIs | 2.64 (0.1, 5.19) | 1.63 (− 0.52, 3.79) | 2.66 (− 3.81, 9.1) | 7.04 (0.03, 14.1) | 5.2 (− 1.63, 12.06) | 16.65 (9.34, 23.98) | 4.11 (2.33, 5.89) |
| 1.14 (− 1.32, 3.63) | − 2.64 (− 5.19, − 0.1) | MA | − 1.01 (− 3.24, 1.22) | 0.03 (− 5.9, 5.93) | 4.4 (− 2.63, 11.45) | 2.56 (− 4.27, 9.45) | 14.02 (7.15, 20.89) | 1.47 (− 0.37, 3.3) |
| 2.14 (− 0.04, 4.37) | − 1.63 (− 3.79, 0.52) | 1.01 (− 1.22, 3.24) | MA with SSRIs | 1.03 (− 5.3, 7.36) | 5.41 (− 1.52, 12.37) | 3.57 (− 3.15, 10.36) | 15.02 (7.8, 22.25) | 2.47 (1.11, 3.85) |
| 1.11 (− 5.27, 7.57) | − 2.66 (− 9.1, 3.81) | − 0.03 (− 5.93, 5.9) | − 1.03 (− 7.36, 5.3) | NASSAs | 4.38 (− 4.79, 13.59) | 2.54 (− 6.49, 11.63) | 14 (4.91, 23.07) | 1.44 (− 4.74, 7.64) |
| − 3.26 (− 9.84, 3.32) | − 7.04 (− 14.1, − 0.03) | − 4.4 (− 11.45, 2.63) | − 5.41 (− 12.37, 1.52) | − 4.38 (− 13.59, 4.79) | Sham EA | − 1.83 (− 8.51, 4.83) | 9.62 (− 0.26, 19.46) | − 2.94 (− 9.78, 3.9) |
| − 1.43 (− 7.8, 4.94) | − 5.2 (− 12.06, 1.63) | − 2.56 (− 9.45, 4.27) | − 3.57 (− 10.36, 3.15) | − 2.54 (− 11.63, 6.49) | 1.83 (− 4.83, 8.51) | Sham EA with SSRIs | 11.46 (1.74, 21.12) | − 1.09 (− 7.75, 5.53) |
| − 12.87 (− 20.15, − 5.56) | − 16.65 (− 23.98, − 9.34) | − 14.02 (− 20.89, − 7.15) | − 15.02 (− 22.25, − 7.8) | − 14 (− 23.07, − 4.91) | − 9.62 (− 19.46, 0.26) | − 11.46 (− 21.12, − 1.74) | Sham MA | − 12.54 (− 19.64, − 5.44) |
| − 0.33 (− 2.12, 1.49) | − 4.11 (− 5.89, − 2.33) | − 1.47 (− 3.3, 0.37) | − 2.47 (− 3.85, − 1.11) | − 1.44 (− 7.64, 4.74) | 2.94 (− 3.9, 9.78) | 1.09 (− 5.53, 7.75) | 12.54 (5.44, 19.64) | SSRIs |
The estimates of mean difference of treatments in the columns versus rows presented in the lower diagonal elements (while those of the row treatments vs. column treatments are presented in the upper diagonal elements). Significant results are in bold and underscored.
EA electroacupuncture, MA manual acupuncture, SSRIs selective serotonin reuptake inhibitors, NASSAs noradrenaline and specific serotoninergic antidepressants, TCAs tricyclic antidepressants.
Figure 3.
Forest plot compared with sham MA.
Table 3 presented the mean values of SUCRA, the hierarchy of eleven treatments on outcomes. According to SUCRA, EA plus SSRIs had the highest probability on improving depression symptoms with probabilities of 0.9518. The next was MA with SSRIs (0.784). The probability of MA was very close to NASSAs, and the mean values of SUCRA were 0.6421 and 0. 6162 respectively. And the probability of EA was 0.4648. The lowest was sham MA group with probabilities of 0.0052.
Table 3.
Ranking probability of different interventions.
| Rank | Treatments, SUCRA | Different treatments durations | |
|---|---|---|---|
| Short-term (1 ≤ x ≤ 4 weeks) | Mid-term (4 < x ≤ 8 weeks) | ||
| 1 | EA with SSRIs, 0.9518 | EA with SSRIs, 0.9104 | EA with SSRIs, 0.9737 |
| 2 | MA with SSRIs, 0.784 | MA with SSRIs, 0.8589 | MA with SSRIs, 0.8147 |
| 3 | MA, 0.6421 | EA, 0.4939 | MA, 0.6329 |
| 4 | NASSAs, 0.6162 | MA, 0.463 | NASSAs, 0.607 |
| 5 | EA, 0.4648 | NASSAs, 0.4592 | SSRIs, 0.4576 |
| 6 | SSRIs, 0.3961 | Sham EA with SSRIs, 0.3579 | EA, 0.4339 |
| 7 | Sham EA with SSRIs, 0.3912 | SSRIs, 0.2722 | Sham EA with SSRIs, 0.3599 |
| 8 | Sham EA, 0.2486 | Sham EA, 0.1846 | Sham EA, 0.2177 |
| 9 | Sham MA, 0.0052 | Sham MA, 0.0027 |
The separated NMA results of acupuncture with SNRIs showed that MA plus SNRIs had the highest probability on improving depression symptoms with probabilities of 0.8994, followed by EA plus SNRIs (0.3956) and SNRIs (0.205).
Inconsistency between direct and indirect comparisons
Assessment of inconsistency between direct and indirect comparisons using a node-splitting model showed that there were no inconsistencies among most studies (P > 0.05). The details of results were listed in Table 4.
Table 4.
Node-splitting analysis of inconsistency.
| Comparison | Direct RoM 95%CI | Indirect RoM 95%CI | Network RoM 95%CI | P-value |
|---|---|---|---|---|
| EA with SSRIs vs EA | − 5.2 (− 11.00, 0.94) | − 3.5 (− 6.30, − 0.75) | − 3.8 (− 6.20, − 1.30) | 0.614 |
| MA vs EA | 1.8 (− 4.80, 8.50) | − 1.6 (− 4.30, 1.00) | − 1.2 (− 3.60, 1.30) | 0.339 |
| MA with SSRIs vs EA | 8.3 (3.90, 13.00) | − 3.9 (− 5.70, − 2.00) | − 2.2 (− 4.40, 0.06) | 0.000 |
| SSRIs vs EA | − 0.47 (− 2.00, 1.00) | − 3.6 (− 2.10, 9.20) | 0.33(− 1.5, 2.10) | 0.176 |
| MA with SSRIs vs EA with SSRIs | 2.2 (− 2.30, 6.80) | 1.40 (− 1.10, 4.00) | 1.6 (− 0.52, 3.80) | 0.760 |
| MA with SSRIs vs MA | − 3.7 (− 9.90, 2.50) | − 0.57 (− 3.00, 1.90) | − 1.00 (− 3.20, 1.20) | 0.351 |
| SSRIs vs MA | 1.8 (− 0.10, 3.80) | − 1.8 (− 8.70, 5.20) | 1.5 (− 0.37, 3.30) | 0.322 |
RoM ratio of mean.
Subgroup analysis
The change in depression scores at the short-term (1 ≤ x ≤ 4 weeks) was reported among 40 studies, 41 studies reporting the change in depression scores at the mid-term (4 < x ≤ 8 weeks), six studies reporting the change in depression scores at the long-term (x > 8 weeks). The data of different interventions were analyzed according to the different treatment duration. For the short-term, there were eight different interventions. The treatment of EA with SSRIs had the largest probability of being the top rank intervention (0.9014), followed by MA with SSRIs (0.8589), EA (0.4939), MA (0.4630), and NASSAs (0.4592). For the mid-term, the highest probability on improving depression symptoms was EA with SSRIs similarly, with the probability of 0.9737. MA with SSRIs, MA, and NASSAs followed closely with probabilities of 0.8147, 0.6329, and 0.6070, respectively. For the long-term, six studies with four treatments (EA, SSRIs, MA with SNRIs, and SNRIs) were included. However, their network was disconnected.
Fourteen studies34,35,38,42,43,49,52,53,55,61,70,73,76,91 reported the change scores using the SDS. Besides, 3 studies30,43,73 used the Montgomery-Asberg Depression Rating Scale (MADRS). The corresponding network analysis failed to be conducted due to the limited number of studies.
Adverse events
Twenty-four reported the presence of adverse events24,25,28,36,39,41,43,50,53,55,58,59,61,62,74–76,78,80,84,85,87,89,91. Among the acupuncture groups and control groups, the main comparable adverse reactions found were needle-related pain (6 cases)24,76 and skin erythema of acupoints (2 cases)28,87. These symptoms were slight and persisted for less than 2 days. One of the included studies reported that MA with SSRIs and EA with SSRIs groups had significantly fewer side effects as compared with the SSRIs group91. One serious adverse event was reported requiring hospitalization due to abnormal behaviors and confusion of mind in the MA with SSRIs group91. Due to a limited number of studies that reported the same adverse outcome, it was not analyzed using NMA.
Quality of evidence
Figure 4 and Table 5 presented the assessment results of the risks of bias. Most RCTs had a low risk of bias in selection of the reported result (n = 70, 99%) and missing outcome data (n = 49, 69%). However, a high proportion had concerns of bias in reporting measurement of the outcome (n = 68, 96%), randomization process (n = 63, 89%), and deviations from the intended interventions (n = 55, 77%). Regarding reports of interventions specified to acupuncture, STRICTA showed that majority of the RCTs reported details of needling (n = 71, 100%), details of other interventions administered to the acupuncture group (n = 47, 66%), instructions to practitioners, and information and explanations to patients (n = 40, 56%), and precise description of the control or comparator (n = 68, 96%). However, many RCTs did not report the descriptions of participating acupuncturists (n = 58, 82%), nor rationale for the control or comparator (n = 46, 65%). The details of the appraisal of acupuncture procedure based on STRICTA were presented in Table 6.
Figure 4.
Risk of bias summery for 66 included studies.
Table 5.
Risk of bias assessment for 66 included studies.
| Source | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall | |
|---|---|---|---|---|---|---|---|
| Ai et al.23 | 2018 | S | L | L | S | L | S |
| Allen et al.24 | 2006 | S | L | L | L | L | S |
| Chen et al.25 | 2014 | S | L | L | S | L | S |
| Chen et al.26 | 2010 | S | S | L | S | L | S |
| Chen et al.27 | 2011 | S | S | S | S | L | S |
| Dong et al.28 | 2017 | S | S | L | S | L | S |
| Duan et al.29 | 2008 | S | S | L | S | L | S |
| Feng et al.30 | 2015 | S | S | S | S | L | S |
| Gallagher et al.31 | 2001 | S | L | H | S | L | H |
| Gu et al.32 | 2015 | L | S | S | S | L | S |
| Guo et al33 | 2019 | S | S | L | S | L | S |
| Han et al34 | 2019 | L | S | L | S | L | S |
| Huang et al.35 | 2013 | S | S | L | S | L | S |
| Jiang et al.36 | 2008 | S | S | L | S | L | S |
| Li et al.37 | 2013 | S | S | S | S | L | S |
| Li et al.38 | 2004 | S | S | L | S | L | S |
| Lin et al.39 | 2004 | S | S | L | S | L | S |
| Li et al.40 | 2017 | S | S | S | S | L | S |
| Lin et al.41 | 2005 | S | S | L | S | L | S |
| Lin et al.42 | 2014 | S | S | S | S | L | S |
| Liu et al.43 | 2018 | S | S | S | S | L | S |
| Liu et al.44 | 2005 | S | S | L | S | L | S |
| Liu et al.45 | 2014 | S | S | L | S | L | S |
| Liu et al.46 | 2015 | S | S | L | S | L | S |
| Liu et al.47 | 2017 | S | S | S | S | L | S |
| Lu et al.48 | 2017 | S | S | S | S | L | S |
| Luo et al.49 | 2003 | S | L | S | S | L | S |
| Ma et al.50 | 2011 | L | L | L | S | L | S |
| Ma et al.51 | 2011 | S | L | L | S | L | S |
| Ma et al.52 | 2011 | S | S | L | S | L | S |
| Ma et al53 | 2020 | S | S | L | S | L | S |
| Pei et al.54 | 2006 | S | S | L | S | L | S |
| Qu et al.55 | 2013 | L | L | L | S | L | S |
| Roschke et al.56 | 2000 | S | L | S | S | L | S |
| Shi et al.57 | 2015 | L | L | L | S | L | S |
| Song et al.58 | 2013 | S | L | L | S | L | S |
| Sun et al.59 | 2012 | S | S | L | S | L | S |
| Sun et al.60 | 2013 | L | L | L | S | L | S |
| Tang et al.61 | 2003 | S | S | L | S | L | S |
| Tian et al.62 | 2008 | S | S | L | S | L | S |
| Wang et al.63 | 2018 | S | S | S | S | L | S |
| Wang et al.64 | 2014 | S | S | L | S | L | S |
| Wang et al.65 | 2016 | S | L | L | S | L | S |
| Wang et al.66 | 2007 | S | S | S | S | L | S |
| Wang et al.67 | 2007 | S | S | S | S | L | S |
| Wang et al.68 | 2006 | S | S | L | S | L | S |
| Wang et al.69 | 2007 | S | S | S | S | L | S |
| Wang et al.70 | 2008 | S | S | L | S | L | S |
| Wang et al.71 | 2010 | S | S | L | S | L | S |
| Wang et al.72 | 2014 | S | S | L | S | L | S |
| Wang et al.73 | 2017 | S | L | L | L | L | S |
| Wang et al74 | 2020 | S | S | L | S | L | S |
| Wang et al75 | 2019 | S | S | L | S | L | S |
| Wang et al.76 | 2013 | S | L | L | S | L | S |
| Wen et al.77 | 2003 | S | S | L | S | L | S |
| Wu et al.78 | 2010 | S | S | L | S | L | S |
| Xu et al.79 | 2009 | S | S | S | S | L | S |
| Xu et al.80 | 2004 | S | S | L | S | L | S |
| Xu et al.81 | 2011 | S | S | L | S | L | S |
| Yang et al.82 | 2012 | S | S | S | S | L | S |
| Yi et al.83 | 2011 | S | S | L | S | L | S |
| Zhang et al.84 | 2007 | S | S | S | S | L | S |
| Zhang et al.85 | 2012 | S | S | L | S | L | S |
| Zhang et al.86 | 2007 | S | S | H | S | S | H |
| Zhang et al.87 | 2009 | L | L | L | L | L | L |
| Zhao et al.88 | 2010 | S | S | L | S | L | S |
| Zhao et al.89 | 2010 | S | S | S | S | L | S |
| Zhao et al.90 | 2006 | S | S | S | S | L | S |
| Zhao et al.91 | 2019 | L | S | L | S | L | S |
| Zheng et al.92 | 2012 | S | S | L | S | L | S |
| Zhu et al.93 | 2018 | S | S | L | S | L | S |
L low risk, S some concerns, H high risk.
Table 6.
Appraisal of acupuncture procedure based on STRICTA.
| Source | 1a | 1b | 1c | 2a | 2b | 2c | 2d | 2e | 2f | 2g | 3a | 3b | 4a | 4b | 5 | 6a | 6b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ai et al.23 | 2018 | TCM | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Allen et al.24 | 2006 | TCM | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Chen et al.25 | 2014 | TCM | Y | Y | N | N | Y | N | Y | Y | N | Y | Y | Y | Y | N | Y | Y |
| Chen et al.26 | 2010 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | Y |
| Chen et al.27 | 2011 | TCM | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | N | N | N | Y |
| Dong et al.28 | 2017 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y |
| Duan et al.29 | 2008 | TCM | Y | Y | N | N | N | N | Y | N | N | Y | Y | N | Y | N | N | Y |
| Feng et al.30 | 2015 | TCM | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Gallagher et al.31 | 2001 | TCM | Y | Y | N | Y | N | N | Y | N | N | Y | N | N | N | N | Y | Y |
| Gu et al.32 | 2015 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y |
| Guo et al33 | 2019 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y |
| Han et al34 | 2019 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | Y | Y |
| Huang et al.35 | 2013 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Jiang et al.36 | 2008 | TCM | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y |
| Li et al.37 | 2013 | TCM | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y |
| Li et al.38 | 2004 | TCM | Y | Y | N | N | Y | N | Y | Y | N | Y | Y | N | N | N | N | Y |
| Lin et al.39 | 2004 | TCM | Y | Y | N | N | Y | N | N | N | N | Y | Y | Y | N | N | N | Y |
| Li et al.40 | 2017 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | Y |
| Lin et al.41 | 2005 | TCM | Y | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | N | N | Y |
| Lin et al.42 | 2014 | TCM | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | N | N | N | Y |
| Liu et al.43 | 2018 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Liu et al.44 | 2005 | TCM | Y | Y | N | N | N | Y | Y | N | N | Y | N | Y | N | N | Y | Y |
| Liu et al.45 | 2014 | TCM | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Liu et al.46 | 2015 | TCM | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | Y |
| Liu et al.47 | 2017 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Lu et al.48 | 2017 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | Y |
| Luo et al.49 | 2003 | TCM | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y | Y | Y |
| Ma et al.50 | 2011 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y |
| Ma et al.51 | 2011 | TCM | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Ma et al.52 | 2011 | TCM | Y | Y | N | N | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y |
| Ma et al53 | 2020 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Pei et al.54 | 2006 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | N |
| Qu et al.55 | 2013 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Roschke et al.56 | 2000 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Shi et al.57 | 2015 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y |
| Song et al.58 | 2013 | TCM | Y | Y | N | N | Y | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y |
| Sun et al.59 | 2012 | TCM | Y | Y | N | N | N | N | Y | Y | N | Y | Y | Y | N | N | Y | Y |
| Sun et al.60 | 2013 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y | Y |
| Tang et al.61 | 2003 | TCM | Y | Y | N | N | N | N | Y | Y | N | Y | Y | Y | N | N | N | Y |
| Tian et al.62 | 2008 | TCM | Y | N | N | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y |
| Wang et al.63 | 2018 | TCM | Y | Y | N | N | N | N | Y | Y | N | Y | Y | Y | N | Y | N | Y |
| Wang et al.64 | 2014 | TCM | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Wang et al.65 | 2016 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | N | Y |
| Wang et al.66 | 2007 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | Y |
| Wang et al.67 | 2007 | TCM | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y |
| Wang et al.68 | 2006 | TCM | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y |
| Wang et al.69 | 2007 | TCM | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | N | N | N | N | Y |
| Wang et al.70 | 2008 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | Y |
| Wang et al.71 | 2010 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y |
| Wang et al.72 | 2014 | TCM | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Wang et al.73 | 2017 | TCM | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Wang et al74 | 2020 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Wang et al75 | 2019 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y |
| Wang et al.76 | 2013 | TCM | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y |
| Wen et al.77 | 2003 | TCM | Y | N | N | N | N | Y | Y | Y | N | Y | Y | N | N | N | N | N |
| Wu et al.78 | 2010 | TCM | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y |
| Xu et al.79 | 2009 | TCM | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | N | N | N | N | Y |
| Xu et al.80 | 2004 | TCM | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | Y | N | N | N | Y |
| Xu et al.81 | 2011 | TCM | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | N | N | Y |
| Yang et al.82 | 2012 | TCM | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y |
| Yi et al.83 | 2011 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Zhang et al.84 | 2007 | TCM | Y | Y | N | N | N | N | Y | Y | N | Y | Y | Y | Y | N | Y | N |
| Zhang et al.85 | 2012 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | N | N | N | Y |
| Zhang et al.86 | 2007 | TCM | Y | Y | N | Y | N | N | Y | N | N | Y | Y | Y | N | N | Y | Y |
| Zhang et al.87 | 2009 | TCM | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Zhao et al.88 | 2010 | TCM | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Zhao et al.89 | 2010 | TCM | Y | Y | N | N | Y | Y | Y | Y | N | Y | Y | Y | N | N | N | Y |
| Zhao et al.90 | 2006 | TCM | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | N | N | N | N | Y |
| Zhao et al.91 | 2019 | TCM | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Zheng et al.92 | 2012 | TCM | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
| Zhu et al.93 | 2018 | TCM | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y |
(1a) Style of acupuncture; (1b) Reasoning for treatment provided; (1c) Extent to which treatment was varied; (2a) Number of needle insertions per subject per session; (2b) Names of points used; (2c) Depth of insertion; (2d) Response sought; (2e) Needle stimulation; (2f) Needle retention time; (2g) Needle type; (3a) Number of treatment sessions; (3b) Frequency and duration of treatment sessions; (4a) Details of other interventions administered to the acupuncture group; (4b) Setting and context of treatment, including instructions to practitioners, and information and explanations to patients; (5) Description of participating acupuncturists; (6a) Rationale for the control or comparator in the context of the research question, with sources that justify this choice; (6b) Precise description of the control or comparator; Y: Reported; N: not available.
Summary of findings GRADE
The summary of quality of evidence of change in depression scores between comparisons was presented in Table 7. Because of high risk of bias, imprecise confidence interval, and inconsistency, almost all comparisons for the reduction of depression proved low quality evidence except for the comparison of EA with SSRIs vs EA (moderate quality evidence), which indicated that most comparisons might result in little or no difference in reducing depression scores.
Table 7.
Summary of findings’ table of comparisons in change in depression scores.
| Comparison | Direct estimates 95%CI | Certainty of evidence | Indirect estimates 95%CI | Certainty of evidence | Network estimates 95%CI | Certainty of evidence |
|---|---|---|---|---|---|---|
| EA with SSRIs vs EA | − 5.2 (− 11.00, 0.94) | Low a,b | − 3.5 (− 6.30, − 0.75) | Moderate a | − 3.8 (− 6.20, − 1.30) | Moderate a |
| MA vs EA | 1.8 (− 4.80, 8.50) | Low a,d | − 1.6 (− 4.30, 1.00) | Low a,b | − 1.14 (− 3.63, 1.32) | Very low a,b,c,d |
| MA with SSRIs vs EA | 8.3 (3.90, 13.00) | Low a,d | − 3.9 (− 5.70, − 2.00) | Low a,b | − 2.14 (− 4.37, 0.04) | Very low a,b,d,e |
| SSRIs vs EA | − 0.47 (− 2.00, 1.00) | Low a,b | − 3.6 (− 2.10, 9.20) | Low a,b | 0.33 (− 1.49, 2.12) | Low a,b |
| MA with SSRIs vs EA with SSRIs | 2.2 (− 2.30, 6.80) | Low a,b | 1.40 (− 1.10, 4.00) | Low a,b | 1.63 (− 0.52, 3.79) | Low a,b |
| MA with SSRIs vs MA | − 3.7 (− 9.90, 2.50) | Low a,b | − 0.57 (− 3.00, 1.90) | Low a,b | − 1.01 (− 3.24, 1.22) | Low a,b |
| SSRIs vs MA | 1.8 (− 0.10, 3.80) | Low a,b | − 1.8 (− 8.70, 5.20) | Low a,b | 1.47 (− 0.37, 3.3) | Low a,b |
aDowngrading for risk of bias; bdowngrading for imprecision (wide confidence interval); cdowngrading for incoherence; ddowngrading for inconsistency; edowngrading for intransitivity.
Discussion
Main results
To our knowledge, this study is the first NMA that explored the efficiency of different techniques of acupuncture comparing with common pharmacological treatments or other non-medication therapies for MDD. Comparing with the most updated meta-analyses focused on the effect of acupuncture on MDD94,95, NMA allows ranking of all different treatment options through the quantitative comparison of interventions from a comprehensive collection of literature. The pooled results showed that the combined interventions (EA with SSRIs, and MA with SSRIs) obtained a better efficacy for improving depression symptoms compared to acupuncture, pharmacological interventions alone, or other inactive groups. Even the studies observing SNRIs and SNRIs combined with EA or MA were not analyzed in main NMA, add-on therapies were more effective than pharmacological interventions alone. Among all the regimens, EA with SSRIs had the highest probability on improving depression symptoms, while the estimation of MA with SSRIs was very close to EA with SSRIs. Besides, for different treatment durations, there were slight differences. For the short-term (1 ≤ x ≤ 4 weeks) and mid-term (4 < x ≤ 8 weeks), both EA with SSRIs and MA with SSRIs achieved better efficacy. However, EA was more effective than MA for the short-term, while the situation reversed for the mid-term.
Based on the comparison of adverse effects among the groups from all included studies, acupuncture alone and its combinations were proved to be relatively safe therapies for MDD patients. Although one case91 of serious adverse effect was reported, no direct association between the intervention and the case was justified.
Considerable experimental and clinical evidence suggest that MDD is a neuro-endocrine-immune system disorder, and more novel mechanisms are explored basing on new genetic, epigenetic and optogenetic tools96. The exact mechanism why EA with SSRIs shows the best treatment efficiency for MDD patients is still not fully understood. According to early animal electrophysiological and immunohistochemical studies, EA can modify the activities of serotonergic neurons in the dorsal raphe (DR) and raphe magnus (RMg), activate serotonin- and catecholamine-containing neurons in the RMg and locus coeruleus97,98. In the clinical study, EA can restore the normal concentration of glial cell-derived neurotrophic factor (GDNF) in the serum of MDD patients which having similar effect to fluoxetine60. Furthermore, EA combined with SSRIs can increase serum 5-HT more rapidly, reduce pro-inflammatory cytokines secreted by TH1 cells, and increase anti-inflammatory cytokines secreted by TH2 cells99. Further studies are required to answer whether these observations are based on the simple add-on effects, or due to more complex vivo interaction pathways.
Implications for practice
The comparisons among various treatment approaches provided updated evidence for practitioners in the areas of CAM and integrative medicine and decision-makers in deriving public health policies. The results in the subgroup analysis indicated that acupuncture with common pharmacological treatments or acupuncture alone could be more effective for MDD even in a short treatment cycle. Under the synthesis of data, we suggest that acupuncture with common pharmacological treatments could be considered as better therapeutic approaches.
Nowadays, with the development of the registration system of acupuncturists and the increasing popularity of acupuncture services worldwide100, acupuncture could be a practical option for MDD patients. In the current clinical practice guideline developed by the American College of Physicians Clinical Guidelines Committee, acupuncture has been studied as a potential monotherapy and combination therapy with antidepressants on treating patients with MDD101. However, the citation of acupuncture articles is limited in the guideline. Although acupuncture trials are largely conducted and published on Chinese databases, the evidence from Chinese databases is largely skipped in the guideline. In this NMA, clinical trial data in recent years from Chinese databases was included. Results of this study provided significance evidence-based data by systematically estimating the clinical effect and safety of acupuncture and its combinations.
Limitation
This study had several limitations: (i) although various outcome measures were collected, only HAMD was included in NMA because of insufficient data from the other scales; (ii) included studies were mainly carried out in Chinese populations; (iii) incomplete reporting of trial details might have affected the reliability of results; (iv) only 9 types of interventions were analyzed for the main network analysis. We intended to involve more non-medication therapies. However, after systematic searching, we only found one study which explored the effect of cognitive-behavior therapy for MDD. Given the limited study data, it was not included in the NMA. Therefore, more studies focusing on non-medication interventions would be needed.
Authors of the RCTs included in this review could have improved their publications by reporting the details of randomization process and measurement of the outcomes, increasing the data transparency through demonstrating the design and every afford involved in the clinical study. Moreover, be specific to acupuncture-related trials, authors are encouraged to report the qualification or years in acupuncture practice for acupuncturists participated in the trials, and to provide justification for the choice of the control or comparator in the context of the research question.
Indeed, the lack of long-term follow-up studies made it difficult to achieve more profound research significance. Patients with MDD often suffer from longer disease cycles and high recurrence rates. We need more evidence to prove that acupuncture not only could show improvements on the depression rating scales, but also more benefits such as drug truncation, low recurrence rate, shorter treatment cycle.
Conclusion
Acupuncture and its combinations could be safe and effective interventions for MDD patients. What’s more, EA with SSRIs seems to be the most effective intervention among the assessed interventions. Well-designed and large-scale studies with long-term follow-up should be conducted in the future.
Supplementary Information
Acknowledgements
We would like to thank the funder for the support of the project " Efficacy and Safety of Acupuncture for Major Depression Disorder” (No.: FRG II/16-17/094, the Faculty Research Grant, Hong Kong Baptist University).
Abbreviations
- MDD
Major depressive disorder
- EA
Electro-acupuncture
- SSRIs
Selective serotonin reuptake inhibitors
- MA
Manual acupuncture
- SNRIs
Serotonin-norepinephrine reuptake inhibitors
- TCAs
Tricyclic antidepressants
- US
United States
- SGA
Second generation antidepressant
- CAM
Complementary and alternative medicine
- NMA
Network meta-analysis
- AMED
Allied and complementary medicine database
- CNKI
China national knowledge infrastructure
- CBM, CBMdisc
China biology medicine disc
- CQVIP
Chongqing VIP database
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PRISMA-NMA
PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions
- RCTs
Randomized control trials
- DSM
The diagnostic and statistical manual of mental disorders
- ICD
The international classification of diseases
- CCMD
The Chinese classification of mental disorders
- HDRS (also abbreviated as HAMD)
Hamilton Depression Rating Scale
- SDS
The Self-Rated Depression Scale
- SERS
Side Effect Rating Scale
- TESS
Treatment Emergent Symptom Scale
- STRICTA
The revised standards for reporting interventions in clinical trials of acupuncture
- GRADE
The grading of recommendations assessment, development and evaluation
- MDs
Mean differences
- SD
Standard deviation
- CI
Confidence interval
- SUCRA
The cumulative ranking curve
- MAOIs
Monoamine oxidase inhibitors
- NASSAs
Noradrenaline and specific serotoninergic antidepressants
- MADRS
The Montgomery-Asberg Depression Rating Scale
Author contributions
L.D. Zhong and Z.X. Bian were responsible for the conception and design of this study. Z.C. Hu and L. Yao performed the search and evaluated studies for inclusion. Z.C. Hu and W.Y. Huang extracted data from selected RCTs. W.C. Lam and L. Yao assessed the quality of selected RCTs. H.J. Li and L. Yao performed statistical analysis. L. Yao performed the GRADE assessment. Z.C. Hu, W.C. Lam and H.J. Li drafted the paper. All authors critically revised and approved the final paper. Z.C. Hu, W.C. Lam and H.J. Li contributed equally to this study.
Funding
The study was supported by the Faculty Research Grant, Hong Kong Baptist University entitled “Efficacy and Safety of Acupuncture for Major Depression Disorder”. (Ref. No.: FRG II/16-17/094). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hu Zhichao, Lam Wai Ching and Li Huijuan.
Contributor Information
Bian Zhaoxiang, Email: bzxiang@hkbu.edu.hk.
Zhong L. D. Linda, Email: ldzhong@hkbu.edu.hk
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88263-y.
References
- 1.Ferrari, A. J. et al. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med.43, 471–481. 10.1017/s0033291712001511 (2013). [DOI] [PubMed]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013).
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-4-TR). (American Psychiatric Association, 2000).
- 4.Hardeveld F, Spijker J, De Graaf R, Nolen WA, Beekman AT. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr. Scand. 2010;122:184–191. doi: 10.1111/j.1600-0447.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari AJ, et al. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg, P. E., Fournier, A. A., Sisitsky, T., Pike, C. T. & Kessler, R. C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry76, 155–162. 10.4088/JCP.14m09298 (2015). [DOI] [PubMed]
- 7.McIntyre, R. S., Suppes, T., Tandon, R. & Ostacher, M. Florida best practice psychotherapeutic medication guidelines for adults with major depressive disorder. J. Clin. Psychiatry78, 703–713. 10.4088/JCP.16cs10885 (2017). [DOI] [PubMed]
- 8.Gartlehner, G. et al. Nonpharmacological versus pharmacological treatments for adult patients with major depressive disorder, agency for healthcare research and quality (US) (2015). [PubMed]
- 9.Ferguson JM. SSRI antidepressant medications: Adverse effects and tolerability. Prim. Care. Companion. J. Clin. Psychiatry. 2001;3:22–27. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swedish Council on Health Technology Assessment. Treatment of Depression: A Systematic Review. Report No 166/2. PMID: 28876724 (SBU, 2004). [PubMed]
- 11.Bschor T, et al. Impact of citalopram on the HPA system. A study of the combined DEX/CRH test in 30 unipolar depressed patients. J. Psychiatr. Res. 2012;46:111–117. doi: 10.1016/j.jpsychires.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am. J. Psychiatry. 2001;158:289–294. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- 13.Hutton B, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/m14-2385. [DOI] [PubMed] [Google Scholar]
- 14.Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann. Intern. Med.151, 264–269. 10.7326/0003-4819-151-4-200908180-00135 (2009). [PMC free article] [PubMed]
- 15.Sterne JAC, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.MacPherson H, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA, extending the CONSORT statement. PLoS Med. 2010;7:e1000261. doi: 10.1371/journal.pmed.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias, S., Sutton, A. J., Ades, A. E. & Welton, N. J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Mak.33, 607–617. 10.1177/0272989x12458724 (2013). [DOI] [PMC free article] [PubMed]
- 18.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 19.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puhan, M.A. et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ349, g5630. 10.1136/bmj.g5630 (2014). [DOI] [PubMed]
- 22.Brignardello-Petersen R, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018;93:36–44. doi: 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ai C, et al. Therapeutic observation of cranial suture acupuncture in treating depression. J. Acupunct. Tuina Sci. 2018;16:161–166. doi: 10.1007/s11726-018-1043-1. [DOI] [Google Scholar]
- 24.Allen JJ, et al. Acupuncture for depression: A randomized controlled trial. J. Clin. Psychiatry. 2006;67:1665–1673. doi: 10.4088/jcp.v67n1101. [DOI] [PubMed] [Google Scholar]
- 25.Chen HD, et al. Effects of acupuncture in patients with mild or moderate depression treated with paroxetine hydrochloride. Chin. J. Inform. Tradition. Chin. Med. 2014;21:35–37. doi: 10.3969/j.issn.1005-5304.2014.08.010. [DOI] [Google Scholar]
- 26.Chen, J., Zhang, J. & Pei, Y. The study on curative effect of acupuncture therapy with the Shu-points of five Zangs and Ge-shu point on elderly depression. Int. J. Tradition. Chin. Med.32, 339–340. 10.3760/cma.j.issn.1673-4246.2010.04.032 (2010).
- 27.Chen L, Wang XJ, Wang LL. Clinical observation on combination of Tongdu Diaoshen acupuncture and Fluoxetine in treating 30 cases of depression. Jiangsu J. Tradition. Chin. Med. 2011;43:57–58. [Google Scholar]
- 28.Dong, Y., Huang, W., Zhang, Y. et al. The clinical efficacy observation of GV-regulating and brain-activating acupuncture combined with psychological ttherapy in the treatment of youngsters’ depression. Guiding J. Tradition. Chin. Med. Pharm.23, 67–68. 10.13862/j.cnki.cn43-1446/r.2017.02.01 (2017).
- 29.Duan DM, Tu Y, Chen LP. Assessment of effectiveness of electroacupuncture and Fluoxetine for treatment of depression with physical symptoms. Chin. Acupunct. Moxibustion. 2008;28:167–170. [PubMed] [Google Scholar]
- 30.Feng H, Liu Y, Yu ZH, et al. Synergism of liver-soothing and heart-nourishing acupuncture therapy on the treatment of depressive disorder by SSRIs and its influence on inflammatory chemokines. Chin. Gen. Pract. 2015;18:722–3726. doi: 10.3969/j.issn.1007-9572.2015.30.019. [DOI] [Google Scholar]
- 31.Gallagher SM, Allen JJ, Hitt SK, Schnyer RN, Manber R. Six-month depression relapse rates among women treated with acupuncture. Complement. Ther. Med. 2001;9:216–218. doi: 10.1054/ctim.2001.0470. [DOI] [PubMed] [Google Scholar]
- 32.Wei, G., Yaran, Z., Xiaoxian, J., Shizhe, D. & Yang, K. Clinical observation on tonifying kidney and spleen acupuncture method in treating 30 cases of mild depression. J. Tradition. Chin. Med.56, 1585–1587. 10.13288/j.11-2166/r.2015.18.015 (2015).
- 33.Guo Y, et al. Clinical observation on the treatment of depression with scalp acupuncture. J. Changchun Univ. Tradition. Chin. Med. 2019;35:289–291. [Google Scholar]
- 34.Han D, Zhang HL, Wang XL, Sun HS. Comparative analysis of clinical efficacy of electro-acupuncture and simple acupuncture in the treatment of first-episode mild-to-moderate depression. J. Tradition. Chin. Med. 2019;60:1307–1307. [Google Scholar]
- 35.Huang Y, et al. Anti-depressive effect of acupuncture on selective serotonin reuptake inhibitors. Chin. J. Integr. Tradition. West. Med. 2013;330:1341–1344. doi: 10.7661/CJIM.2013.10.1341. [DOI] [PubMed] [Google Scholar]
- 36.Jiang XY, Ren K, Zhao XW. Combination of acupuncture and Citalopram in treating 34 cases of depression. J. Chin. Acupunct. Moxibustion. 2008;24:18–19. [Google Scholar]
- 37.Li, G. et al. Analysis of electroacupuncture on treatment of depression and efficacy on cognitive function. Guangdong Med. J.34, 391–392. 10.13820/j.cnki.gdyx.2013.03.001 (2013).
- 38.Li GP, Du YH, Yan H, Zhang XJ, Huang LF. Clinical effect of Tiaoshen Shugan acupuncture on depression. Tianjin J. Tradition. Chin. Med. 2004;21:382–385. [Google Scholar]
- 39.Lin H, Li GQ, Zheng BZ. Clinical study on treatment of depression with combined acupuncture and antidepressant. J. Clin. Acupunct. Moxibustion. 2004;20:17–19. [Google Scholar]
- 40.Li, X. N., Gao, S., Wu, L., Mei, J. L. & Li, N. Clinical observation of acupuncture plus paroxetine for depression due to liver-qi stagnation. Shanghai J. Acupunct. Moxibustion 36, 138–141. 10.13460/j.issn.1005-0957.2017.02.0138 (2017).
- 41.Lin, H., Li, G. Q., Zhou, Z. B. & Liu, J. X. Observation on therapeutic effect of combination of acupuncture with drug on depression. Chin. Acupunct. Moxibustion25, 27–29. 10.13703/j.0255-2930.2005.01.012 (2005). [PubMed]
- 42.Lin H, Yu ZF, Kang FH. Th1/Th2 balance on depression patients by acupuncture treatment combined with antidepressant. J. Clin. Acupunct. Moxibustion. 2014;30:1–3. [Google Scholar]
- 43.Liu, J., Wang, A. A., Nie, G. N., Wang, X. Y. & Huang, J. Acupuncture for female depression: a randomized controlled trial. Chin. Acupunct. Moxibustion38, 375–378. 10.13703/j.0255-2930.2018.04.009 (2018). [DOI] [PubMed]
- 44.Liu LY, Wang LL, Lu M, Li D. Combination of electroacupuncture and SSRI drugs on effect of HAMD scale in patients of depression. Sichuan J. Tradition. Chin. Med. 2005;23:96–98. [Google Scholar]
- 45.Yi, L. et al. Clinical randomized study of acupuncture enhancing clinical effects of SSRIs antidepressants treating depression and regulating TH1/TH2 balance. Chin. Arch. Tradition. Chin. Med.32, 1927–1929. 10.13193/j.issn.1673-7717.2014.08.040 (2014).
- 46.Liu, Y., Feng, H., Mao, H. J. et al. Impact on serum 5-HT and TH1/TH2 in patients of depressive disorder at acute stage treated with acupuncture and western medication. Chin. Acupunct. Moxibustion35, 539–543. 10.13703/j.0255-2930.2015.06.003 (2015). [PubMed]
- 47.Liu Y, et al. Effect of acupuncture of soothing-liver and nourishing-heart method combined with venlafaxine on residual symptoms and serum monoamine neurotransmitter of depressive disorder patients. Chin. J. Gen. Pract. 2017;15:1378–1381. [Google Scholar]
- 48.Lu, M. J. & Zhang, J. B. Clinical observation on 60 patients of depression complicated with gastrointestinal symptoms treated with needling tender points on back section of DU meridian combined with western medicine. J. Tradition. Chin. Med.58, 2028–2031. 10.13288/j.11-2166/r.2017.23.012 (2017).
- 49.Luo HC, Ureil H, Shen YC, et al. Comparative study of electroacupuncture and fluoxetine for treatment of depression. Chin. J. Psychiatry. 2003;36:215–219. [Google Scholar]
- 50.Ma, Q., Zhou, D. A. & Wang, L. P. Clinical curative effect and factor analysis of depression treated by acupuncture. Chin. Acupunct. Moxibustion31, 875–878. 10.13703/j.0255-2930.2011.10.006 (2011). [PubMed]
- 51.Ma XH, Zhang M, Zhang WY, et al. The effect evaluation of electroacupuncture improving therapeutic effects and decreasing the side-effects of Paroxetine for mild or moderate depression patients. Chin. J. Tradition. Chin. Med. Pharam. 2011;26:2876–2879. [Google Scholar]
- 52.Ma XH, Li WD, Xu K. Effect of electroacupuncture on therapeutic effects and onset time of paroxetine for mild or moderate depression patients. Chin. J. Behav. Med. Brain Sci. 2011;20:4–6. doi: 10.3760/cma.j.issn.1674-6554.2011.01.002. [DOI] [Google Scholar]
- 53.Ma J, et al. Efficacy and safety of acupuncture treatment on depression by unblocking Du Meridian and relieving depression: A randomized controlled trial. Liaoning J. Tradition. Chin. Med. 2020;47:180–182. [Google Scholar]
- 54.Pei Y, Zhang J, Chen J, Qian J. Clinical observation of acupuncture therapy with the Shu-points of five Zangs on treating patients with depression. Chin. J. Inf. Tradition. Chin. Med. 2006;13:62. [Google Scholar]
- 55.Qu SS, et al. A 6-week randomized controlled trial with 4-week follow-up of acupuncture combined with paroxetine in patients with major depressive disorder. J. Psychiatr. Res. 2013;47:726–732. doi: 10.1016/j.jpsychires.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Roschke, J. et al. The benefit from whole body acupuncture in major depression. J. Affect. Disord. 57, 73–81. 10.1016/s0165-0327(99)00061-0 (2000). [DOI] [PubMed]
- 57.Shi Y, Wang ZX, Zhou ZY, Lu CJ. Clinical study on needling method of purging liver and tonifying lungs in treating Taiyin cases with depression. J. Nanjing Univ. Tradition. Chin. Med. 2015;31:118–121. [Google Scholar]
- 58.Song SC, Lu Z, Wang LC, Chen H. Clinical observation on Xingnao Diaoshen acupuncture in treating patients with depression. Chin. J. Integr. Med. Cardiov. Cerebrovasc. Dis. 2013;11:556–557. [Google Scholar]
- 59.Sun, R. Z. et al. Control study of the effect of electroacupuncture plus venlafaxine in the treatment of depression. J. Neurosci. Ment. Health12, 593–594. 10.3969/j.issn.1009-6574.2012.06.017 (2012).
- 60.Sun, H. et al. Effects of electroacupuncture on depression and the production of glial cell line-derived neurotrophic factor compared with fluoxetine: a randomized controlled pilot study. J. Altern. Complement. Med.19, 733–739. 10.1089/acm.2011.0637 (2013). [DOI] [PMC free article] [PubMed]
- 61.Tang HM, Li HS, Feng B. Efficacy on combination of electroacupuncture and low dose Amitriptyline on treatment of patients with depression. Acta Chin. Med. Pharmacol. 2003;31:5–6. [Google Scholar]
- 62.Tian CH, Lou YM, Guo YM, Fan XQ. Combination of electroacupuncture with Clomipramine in the treatment of patients with depression, a clinical observation. Pract. J. Cardiac. Cereb. Pneum. Vasc. Dis. 2008;16:36. [Google Scholar]
- 63.Wang JM, Wang GQ, Zhou HP, Zhang Y. The clinical efficacy of acupuncture and fluvoxamine in the treatment of depression. J. Xuzhou Med. Univ. 2018;38:737–739. [Google Scholar]
- 64.Wang, S. H. et al. Study on alleviating side effect of paroxetine and improving quality of life using acupuncture in treatment of mild or moderate depression. Chin. J. Behav. Med. Brain Sci.23, 211–214. 10.3760/cma.j.issn.1674-6554.2014.03.006 (2014).
- 65.Wang, Q. S., Ji, X. D., Zhu, W. X. & Yu, H. Y. Effect of acupuncture on the serum brain-derived neurotrophic factor level in depressive patients with hyperactivity of fire due to Yin deficiency. J. Cap. Med. Univ.37, 176–180, 10.3969/j.issn.1006-7795.2016.02.014 (2016).
- 66.Wang XF, Zhao ZG, Ni AH. Electroacupuncture with the Shu-points of Wan Gu and Tai Chong point in the treatment of patients with depression, 35 cases. Shanxi J. Tradition. Chin. Med. 2007;28:723–725. [Google Scholar]
- 67.Wang XF, Zhang XP, Zhao ZG. Effect of electroacupuncture on treatment of depression and its effect on ACTH and cortisol level. J. Sichuan Tradition. Chin. Med. 2007;25:102–103. [Google Scholar]
- 68.Wang XF, Liu JP, Zhang XP, Zhao ZG. Electroacupuncture with the Shu-points of Wan Gu and Tai Chong point and Sertraline in the treatment of patients with depression: a controlled observation. J. Sichuan Tradition. Chin. Med. 2006;24:95–96. [Google Scholar]
- 69.Wang, X. F., Ali, T. L. K. & Zhao, Z. G. Effect of electroacupuncture with the Shu-points of Wan Gu and Tai Chong point on regulating ACTH and cortisol level. Liaoning J. Tradition. Chin. Med.34, 1145–1146. 10.13192/j.ljtcm.2007.08.125.wangxf.022 (2007).
- 70.Wang X, Wang L, Qiao H, Li J. Clinical observation on combination of acupuncture and antidepressants in treating 50 cases with depression. Jiangsu J. Tradition. Chin. Med. 2008;40:74–75. [Google Scholar]
- 71.Wang, Y., Shushanic, Song, M. & Tu, Y. Characteristics of electroacupuncture in anti-depression treatment. J. Beijing Univ. Tradition. Chin. Med.33, 210–213 (2010)
- 72.Wang, T., Wang, L., Tao, W. & Chen, L. Acupuncture combined with an antidepressant for patients with depression in hospital: A pragmatic randomised controlled trial. Acupunct. Med.32, 308–312. 10.1136/acupmed-2013-010469 (2014). [DOI] [PubMed]
- 73.Wang, Z. et al. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res.84, 18–26. 10.1016/j.jpsychires.2016.09.014 (2017). [DOI] [PMC free article] [PubMed]
- 74.Wang PR, et al. Therapeutic effect of acupuncture combined with venlafaxine hydrochloride on depression and its effects on 5-HT and hs-CRP. Chin. J. Tradit. Med. Sci. Technol. 2020;27:167–182. [Google Scholar]
- 75.Wang PR, et al. Therapeutic effect of the combined treatment with acupuncture and venlafaxine hydrochloride on depression based on diffusion tensor imaging technology. Chin. Acupunct. Moxibustion. 2019;39:571–575. doi: 10.13703/j.0255-2930.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Wang WD, et al. Effects of electro-acupuncture on personality traits in depression: A randomized controlled study. Chin. J. Integr. Med. 2013;19:777–782. doi: 10.1007/s11655-013-1594-4. [DOI] [PubMed] [Google Scholar]
- 77.Wen NY, Wang W, Du WD. Complementary therapeutic effect of electroacupuncture on refractory depression. Mod. J. Integr. Tradition. Chin. West. Med. 2003;12:1250. [Google Scholar]
- 78.Wu YH, Meng QM, Tang SY, Luo XF. Augment effect of electric acupuncture in the treatment of senile depression. J. Clin. Psychiatry. 2010;20:276–277. [Google Scholar]
- 79.Wang FM, Wang Q, Liu LX. Clinical observation on acupuncture in treating patients with depression. J. Clin. Acupunct. Moxibustion. 2009;25:27–28. [Google Scholar]
- 80.Xu, H. et al. Effects of acupuncture on the hypothalamus-pituitary-adrenal axis in the patient of depression. Chin. Acupunct. Moxibustion24, 78–80. 10.13703/j.0255-2930.2004.02.002 (2004).
- 81.Xu, L., Jiang, J. F. & Wang, L. L. Clinical observation on combination of electroacupuncture and SSRIs in treating patients with depression. J. Zhejiang Chin. Med. Univ.35,459–460, 10.16466/j .issn1005-5509.2011.03.019 (2011).
- 82.Yang, X. Q. et al. Therapeutic effect of early intervention with electro-acupuncture and cognitive behavioral therapy on mild depression of 30 cases. J. Tradition. Chin. Med.53, 936–938,968. 10.13288/j.11-2166/r.2012.11.015 (2012).
- 83.Yi Y, et al. Correlation between the liver meridian and the frontal lobe in depression by needling at Taichong (LV3): a resting-state fMRI study. Chin. J. Integr. Tradi West. Med. 2011;31:1044–1050. [PubMed] [Google Scholar]
- 84.Zhang CP, Huang YG, Chen ZX. Electroacupuncture and Paroxetine in the treatment of patients with depression, a controlled clinical study. J. Pract. Med. 2007;23:2949–2950. [Google Scholar]
- 85.Zhang KK, Liu L, Han XY. The treatment of depression with liver-qi stagnation type by acupuncturing middle line of forehead and liver-shu point. J. Clin. Acupunct. Moxibustion. 2012;28:23–25. doi: 10.1016/S1003-5257(13)60023-9. [DOI] [Google Scholar]
- 86.Zhang, G. J. et al. Clinical observation on treatment of depression by electro-acupuncture combined with Paroxetine. Chin. J. Integr. Med.13, 228–230. 10.1007/s11655-007-0228-0 (2007). [DOI] [PubMed]
- 87.Zhang, W. J., Yang, X. B. & Zhong, B. L. Combination of acupuncture and fluoxetine for depression: a randomized, double-blind, sham-controlled trial. J. Altern. Complement. Med.15, 837–844. 10.1089/acm.2008.0607 (2009). [DOI] [PubMed]
- 88.Zhao H, et al. Effects of electroacupuncture on depressive disorder recovery and serum cytokines. Chin. J. Rehabil. Theory Pract. 2010;16:774–777. [Google Scholar]
- 89.Zhao RJ, Bayahemaiti K. Influence of electro-acupuncture on P300 in patients with depression. J. Clin. Psychiatry. 2010;20:123–125. [Google Scholar]
- 90.Zhao ZG, Wang XF, Guo DZ. Clinical observation on electroacupuncture with the Shu-points of Wan Gu and Tai Chong point on 38 cases of depression. Jiangsu J. Tradition. Chin. Med. 2006;27:62. [Google Scholar]
- 91.Zhao B.C. et al. Manual or electroacupuncture as an add-on therapy to SSRIs for depression. J. Psychiatr. Res.114, 23–33. 10.1016/j.jpsychires.2019.04.005 (2019). [DOI] [PubMed]
- 92.Zheng, M., Lin, Y. H., Zhang, J. B., Wang, L. L. & Qiao, H. F. Clinical observation of acupuncture combined with antidepressants for 44 cases of depression. J. Tradition. Chin. Med.53, 927–929, 932. 10.13288/j.11-2166/r.2012.11.013 (2012).
- 93.Zhu, W. X., Wang, J., Wang, Q. S., Ji, X. D. & Yuan, G. Z. Effect of acupuncture on the autonomic nervous system in patients with major depressive disorder. Lishizhen Med. Mater. Med. Res.29, 381–383. 10.3969/j.issn.1008-0805.2018.02.044 (2018).
- 94.Armour M, et al. Acupuncture for depression: A systematic review and meta-analysis. J. Clin. Med. 2019;8:1140. doi: 10.3390/jcm8081140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye, J., Cheung, W. M. & Tsang, H. W. H. The neuroscience of nonpharmacological traditional Chinese therapy (NTCT) for major depressive disorder: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med.2019, 2183403. 10.1155/2019/2183403 (2019). [DOI] [PMC free article] [PubMed]
- 96.Ménard, C., Hodes G.E. & Russo S. J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience321, 138–162. 10.1016/j.neuroscience.2015.05.053 (2016). [DOI] [PMC free article] [PubMed]
- 97.Kwon YB, et al. Different frequencies of electroacupuncture modified the cellular activity of serotonergic neurons in brainstem. Am. J. Chin. Med. 2020;28:435–441. doi: 10.1142/S0192415X00000519. [DOI] [PubMed] [Google Scholar]
- 98.Lee, H. J. et al. Electroacupuncture reduces stress-induced expression of c-fos in the brain of the rat. Am. J. Chin. Med.32, 795–806. 10.1142/S0192415X04002405 (2004). [DOI] [PubMed]
- 99.Liu, Y. et al. Effect of soothing-liver and nourishing-heart acupuncture on early selective serotonin reuptake inhibitor treatment onset for depressive disorder and related indicators of neuroimmunology: A randomized controlled clinical trial. J. Tradition. Chin. Med.35, 507–513. 10.1016/s0254-6272(15)30132-1 (2015). [DOI] [PubMed]
- 100.Lam W.C., Lyu A.P., Bian Z.X. ICD-11: Impact on traditional Chinese medicine and world healthcare systems. Pharmaceut. Med.33, 373–377. 10.1007/s40290-019-00295-y (2019). [DOI] [PubMed]
- 101.Qaseem, A., Barry, M. J. & Kansagara, D. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med.164, 350–359. 10.7326/m15-2570 (2016). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.