Abstract
Irreversible pulmonary hypertension (PH) mainly results from vascular remodeling, in which the aberrant growth of pulmonary arterial smooth muscle cells (PASMCs) plays a significant role. Our previous work suggested that KLF5 and HIF1α are closely associated with the pathogenesis of hypoxic PH as they intervene in the growth of PASMCs. MicroRNAs (miRNAs) have been demonstrated to be involved in the control of cell proliferation and apoptosis. In the present study, we detected the expression of six miRNAs connected with KLF5 in hypoxia-exposed rat PH models and PASMCs and then further investigated the role of miR-320-3p in the abnormal proliferation of hypoxic PASMCs and in the progression and treatment outcomes of hypoxia-induced PH. The results indicated that miR-320-3p was downregulated in hypoxia-exposed rat PH models, hypoxia-induced PASMCs and chronic thromboembolic pulmonary hypertension (CTEPH) patients. Moreover, miR-320-3p directly regulated the expression of KLF5 and HIF1α. miR-320-3p mimics inhibited proliferation and migration and promoted apoptosis in hypoxic PASMCs. KLF5 and HIF1α reversed the above effects of miR-320-3p. In conclusion, miR-320-3p plays a certain role in the progression of hypoxic PH via KLF5 and HIF1α and might be a potent therapeutic tool for PH.
Keywords: Pulmonary hypertension, miRNA, KLF5, HIF1α, vascular remodeling
Introduction
Pulmonary hypertension (PH) is a chronic, progressive and life-threatening disease characterized by vasoconstriction and pulmonary-selective vascular remodeling. PH has been divided into five groups in the clinic, and hypoxia-induced PH is classified as part of the third group, “PH due to lung disease” [1,2]. Vascular remodeling underlying irreversible PH occurs in all three layers of distal pulmonary arteries, in which cells within the vessel wall present a proproliferative and antiapoptotic phenotype under hypoxic conditions. The aberrant biological behavior of pulmonary arterial smooth muscle cells (PASMCs) plays a crucial role. Previously approved therapies targeting vasoconstriction have had poor curative efficacy. Although some specific therapeutic methods targeting vascular remodeling, including molecular targeted therapeutics and natural products, have been explored in some studies, the current options for therapies to reverse vascular remodeling are limited [1,3,4]. Therefore, developing a more effective and selective therapeutic strategy targeting smooth muscle remodeling for PH is a promising research direction.
Many molecular mechanisms have been authenticated in vascular remodeling [4]. Kruppel-like factor 5 (KLF5), a zinc-finger transcription factor that is upregulated in PH, promotes PASMC proliferation and apoptosis resistance, and the induction of hypoxia relies on STAT3 activation. Moreover, small interfering RNA targeting KLF5 inhibited hypoxia-induced vascular remodeling and significantly decreased mean pulmonary arterial pressure (mPAP), RV/(LV+S) and pulmonary vascular resistance (PVR) in a rat PH model [5]. Our previous study revealed that KLF5 exerts biological effects on hypoxic PASMCs by upregulating and directly interacting with Hypoxia-inducible factor 1 subunit alpha (HIF1α) [6]. As a transcription factor, KLF5 can also regulate genes related to the cell cycle, angiogenesis, apoptosis and migration, such as cyclin D1, VEGFα, Survivin, and MMP9 [7-10]. KLF5 can also mediate WNT signaling, RAS signaling, NOTCH signaling, JNK signaling, and TGFβ signaling [11-15]. HIF1α upregulation under oxidative stress plays a significant role in the proliferation of PASMCs by targeting VEGFα rather than HIF2α [16].
MicroRNAs (miRNAs), which are 21-23 nucleotides in length, repress gene expression at the transcription level by targeting the 3’ untranslated region (3’UTR) of the genes. It is generally believed that at least 1/3 of human genes can be regulated by miRNA [17], and miRNA plays a pivotal role in cellular activities such as cell proliferation, migration, and apoptosis. miR-15a and miR-16-1 prolong the G0/G1 phases of the cell cycle and shorten the S phase to inhibit the cell growth of gastric adenocarcinoma by targeting YAP1 [18]. Ectopic expression of miR-224 promotes the proliferation, migration, and invasion of non-small-cell lung cancer cells [19]. miR-449a promotes apoptosis and inhibits the migration of liver cancer cells by targeting SOX4 [20]. Recent studies have demonstrated that miRNAs are related to the development and treatment response of PH [21,22]. To determine the conserved homologous miRNAs in humans and rats, we used bioinformatics software (TargetScan, miRwalk, miRanda) to identify miRNAs with seed regions that have direct binding sites for the 3’UTR of KLF5. Thus, miR-9a-5p, miR-145-5p, miR-153-3p, miR-320-3p, miR-375-3p and miR-495 were further explored. The expression profile, function, and potential therapeutic value of these miRNAs in pulmonary arterial hypertension (PAH) progression are still unclear.
This study revealed the expression profile of miR-320-3p in hypoxia-induced PASMCs and chronic thromboembolic pulmonary hypertension (CTEPH) patients (data from GSE56914). Further, this study showed that miR-320-3p attenuates proliferation and migration and enhances apoptosis in hypoxia-induced PASMCs via KLF5 and HIF1α. Thus, the present study provides a new therapy for PAH.
Materials and methods
Ethics statement
The rat experiments were approved by the Committee of Huazhong University of Science and Technology and Tongji Hospital and followed NIH guidelines (Guide for the Care and Use of Laboratory Animals).
Animal experiments and hemodynamic measurements
The rats (Sprague-Dawley, male, 6 weeks old) were randomly divided into normoxia and hypoxia groups (n=6 per group). Rats in the hypoxia group were put into a hypobaric chamber (10% O2 and 90% N2) for 8 h every day for 4 weeks, while rats in the normoxia group inhabited a normoxic environment (21% O2).
After hypoxic treatment for 4 weeks, the rats were anesthetized to measure right ventricular systolic pressure (RVSP) by inserting a polyvinyl catheter into the right ventricle. The signals were continuously recorded by Power Lab (AD Instruments, Australia).
Cell culture and experiments
PASMCs isolated from four-week-old male SD rats were cultured according to our previous study method [23]. PASMCs were cultured in Dulbecco’s modified Eagle’s medium:F-12 (DMEM/F12) (Promotor, China) containing 10% certified FBS (BI, Israel). The cells were cultured at 37°C in a CO2 incubator with 21% O2, 5% CO2, and 74% N2 or in a three-gas incubator with 5% O2, 5% CO2, and 90% N2. The cells were transfected with mimics/control mimics at 50 nM or inhibitor/control inhibitor at 100 nM with Lipofectamine 3000 reagents (Invitrogen, USA). The miR-320-3p reagents were all purchased from RiboBio (Guangzhou, China). The KLF5 and HIF1α overexpression plasmids (with pcDNA3.1 as the vector backbone) were purchased from GeneChem Biotech (Shanghai, China).
Quantitative real-time PCR
Total RNA from pulmonary artery samples or PASMCs was extracted by using TRIzol reagent. For miRNA profile detection, the miDETECT A Track miRNA qRT-PCR Starter Kit and Bulge-Loop miRNA qRT-PCR Start Kit (RiboBio, Guangzhou, China) were used for specific reverse transcription and amplification. Specific reverse transcription primers for miRNAs (rno-miR-9a-5p, miR-145-5p, miR-153-3p, miR-320-3p, miR-375-3p, miR-495) and U6 RNA were obtained from RiboBio (Guangzhou, China). mRNA expression was quantified using a TB Green kit (Takara) on a CF-Connect system (Bio-Rad, USA). U6 and actin were used as internal controls.
Western blotting
PASMCs were lysed using ice-cold RIPA lysis buffer (Bioyear, Wuhan, China). A bicinchoninic acid (BCA) kit (Bioyear) was used to measure the concentrations of proteins following the manufacturer’s protocol. Proteins were electrophoresed by SDS-PAGE, transferred to PVDF membranes and incubated with different antibodies against KLF5, HIF1α, PCNA, survivin, MMP9, BAX, Bcl2, cleaved caspase-3, and actin. The protein bands were detected by an enhanced chemiluminescence detection system (Bio-Rad, Hercules, USA).
Luciferase reporter assay
For the wild-type reporter vector, fragments of the wild-type 3’UTRs of KLF5 and HIF1α were designed to contain the putative miR-320-3p binding site. In contrast, the mutant reporter vector was designed to retain point mutations in the seed regions of the miR-320-3p binding sites. The wild-type and mutant vectors were cloned into the GV272 vector expressing firefly luciferase protein by GeneChem Biotech (Shanghai, China). PASMCs were cotransfected with GV272-KLF5/HIF1α-WT or GV272-KLF5/HIF1-Mut, miR-320-3p or control mimics, and then the relative luciferase activities were quantified by the Dual-Luciferase Reporter Assay System (Promega, USA) after 48 h of transfection.
Cell counting kit-8 assay
The viability of PASMCs exposed to hypoxic conditions for 24 h was detected by the Cell Counting Kit-8 (CCK-8) assay. After incubating the cells with 10 μl CCK-8 reagent for 2 h, the absorbance of treated PASMCs in 96-well plates was measured at 450 nm.
EdU proliferation assay
The proliferation of PASMCs after hypoxic treatment for 24 h was determined by an EdU proliferation assay kit (RiboBio). The EdU activity was determined using inverted fluorescence microscopy (Nikon, Japan).
Cell migration assay
The migration of treated PASMCs was detected with a Transwell migration assay kit (Corning). The treated PASMCs were replanted into 24-well upper Transwell chambers containing DMEM/F12 with 5% FBS, while the lower chamber was filled with 15% FBS. After hypoxic exposure for 8 h, the migrated cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet for 15 min. Five random fields per chamber were observed using a light microscope to count the number of migrated cells.
Annexin V/PI assay
Cell apoptosis was detected using an annexin V/propidium iodide (PI) detection kit (RiboBio). Briefly, treated PASMCs digested with EDTA-free trypsin and the supernatant were collected into tubes. After centrifugation at 1000 rpm for 5 min with PBS, the treated PASMCs were incubated with Annexin V/PI reagents for 15 min and analyzed by flow cytometry (BD Bioscience, USA) within 1 h.
Data and statistical analysis
The data were analyzed using GraphPad Prism software. Student’s t-test was performed for comparisons between two groups. One-way ANOVA was performed for multiple groups. Then, Dunnett’s test was used to compare the control group with other groups, and the Sidak test was used to compare two certain groups. However, if the standard deviation (SD) was significantly different in multiple groups, Brown-Forsythe and Welch ANOVA followed by Dunnett’s T3 and Tamhane’s T2 tests was performed. Differences with a P value less than 0.05 were considered statistically significant.
Results
The expression profile of miR-320-3p in hypoxia-induced PH rat models and PASMCs exposed to hypoxic conditions
After hypoxic treatment for 4 weeks, the expression levels of the six miRNAs in the pulmonary artery of hypoxia-induced PH rat models were determined by qRT-PCR. The data showed that the expression of miR-320-3p and miR-153-3p was significantly decreased after hypoxic exposure (Figure 1A). The expression of the six miRNAs was detected in hypoxia-induced PASMCs, and the results showed that both miRNAs were downregulated (Figure 1B). The data from a Gene Expression Omnibus (GEO) dataset (GSE56914) also showed that miR-320-3p expression was decreased in a group of CTEPH patients at the plasma level (Figure 1C). Among the miRNAs, miR-320-3p had the lowest expression levels in vivo and vitro (Figure 1A-C). Then, qRT-PCR and western blotting were performed to further explore the hypoxia-induced dynamic changes in miR-320-3p, KLF5, and HIF1α expression at the indicated time points (0, 0.5, 1, 2, 4, 8, 16, and 24 h). The expression of miR-320-3p was upregulated before 1 h but later downregulated gradually. At 8 h of exposure to hypoxia, the miR-320-3p expression level was decreased by 62.6% compared with that at 0 h (Figure 1F). On the other hand, KLF5 and HIF1α expression were increased at 8 h, while miR-320-3p expression was significantly decreased (Figure 1D-F). The protein expression of KLF5 was significantly increased at 4 h and 8 h, while the expression of HIF1α was significantly increased at 8 h. (Figure 1G-I). The results suggest that miR-320-3p expression is downregulated in hypoxia-induced PH and PASMCs.
Figure 1.
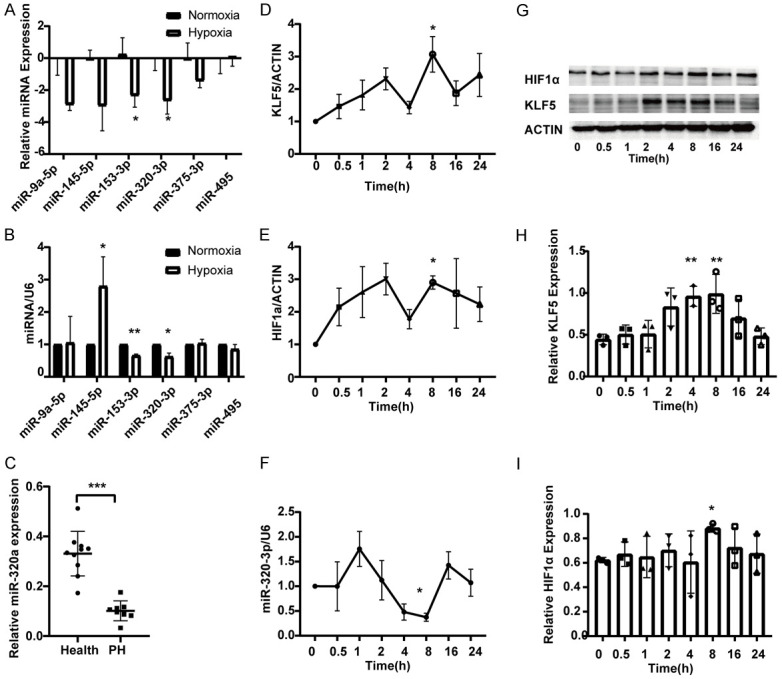
Downregulated miR-320-3p in hypoxic PH rat model and in hypoxic-exposed PASMC and dynamic changes in miR-320-3p, KLF5 and HIF1α expression in vitro. (A, B) The levels of miRNAs, including miR-9a-5p, miR-145-5p, miR-153-3p, miR-320-3p, miR-375-3p and miR-495 in rat pulmonary artery at the 4th week after hypoxia or hypoxic-exposed pulmonary artery smooth muscle cells (PASMCs) in vitro via real-time PCR. (C) GSE56914 from Gene Expression Omnibus (GEO) database was performed to analyze the expression of miR-320-3p in plasma of CTEPH patients (n=8). The expression levels of KLF5 (D), HIF1α (E) and miR-320-3p (F) in PASMC exposed to 5% O2 (hypoxia) at the indicated time points were determined by real-time PCR. (G-I) The protein expressions of KLF5 and HIF1α were analyzed with western blotting and the protein levels were evaluated. The levels of miR-135a-5p were normalized to that of U6 RNA. *P < 0.05, **P < 0.01, ***P < 0.005. (PAH, pulmonary arterial hypertension).
KLF5 and HIF1α are direct target genes of miR-320-3p
After transfection with control inhibitor/miR-320-3p inhibitor in normoxic conditions or control mimics/miR-320-3p mimics in hypoxic conditions, PASMCs were collected to determine the expression levels of KLF5 and HIF1α by qRT-PCR and western blotting. The data showed that overexpression of miR-320-3p significantly decreased the mRNA expression of KLF5 and HIF1α compared to that in the normal and control mimic groups (Figure 2A). The protein expression of KLF5 and HIF1α was highly upregulated in PASMCs after exposure to hypoxia but was suppressed by miR-320-3p mimics (Figure 2B). The dual-luciferase reporter assay showed that the luciferase activity of the GV272-KLF5-WT reporter in PASMCs was suppressed by miR-320-3p mimics while that of GV272-KLF5-Mut was not altered, indicating a trend similar to that for the reporter vector of HIF1α (Figure 2C, 2D). All the above results indicate that miR-320-3p could directly target KLF5 and HIF1α in hypoxia-induced PASMCs.
Figure 2.
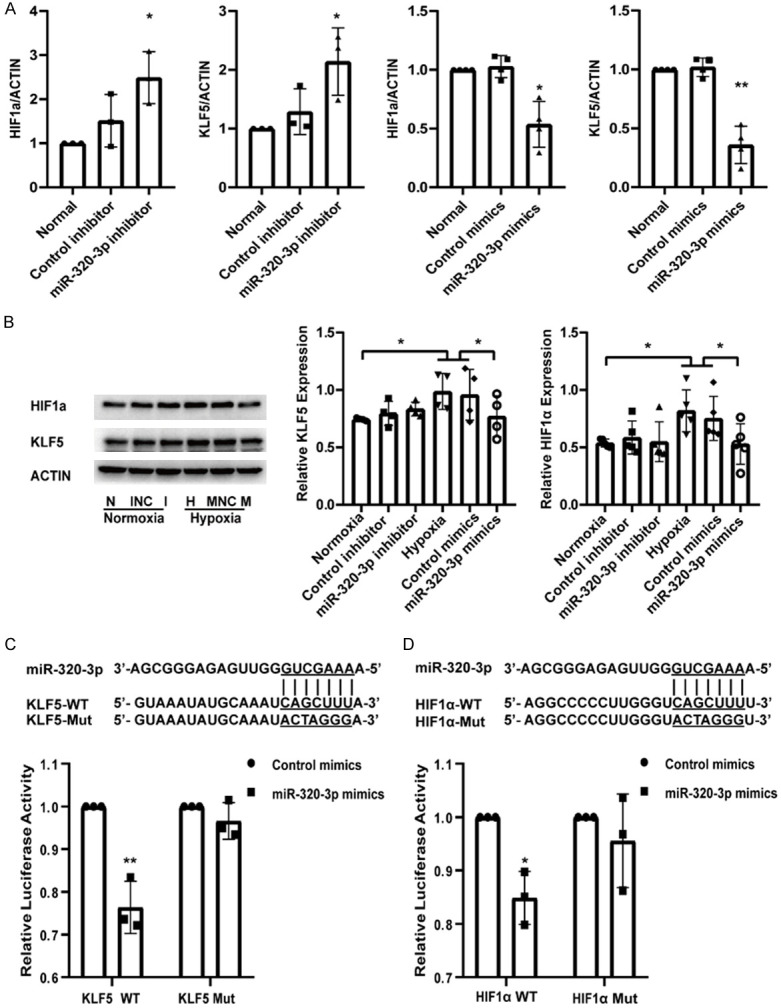
miR-320-3p reduced KLF5 and HIF1α expression and targeted the two genes directly. A. The mRNA expression of KLF5 and HIF1α in normal, control inhibitor/miR-320-3p inhibitor groups or control mimics/miR-320-3p mimics groups. B. PASMCs were transfected with control mimics or miR-320-3p mimics in normoxia or with control inhibitor or miR-320-3p inhibitor in hypoxia and KLF5 and HIF1α protein expression were analyzed. C, D. PASMCs were co-transfected with control mimics/miR-320-3p mimics and KLF5-WT/KLF5-Mut plasmid or HIF1α-WT/HIF1α-Mut plasmid for 48 h, and then the luciferase activity was detected. *P < 0.05, **P < 0.01. (WT, wild type; Mut, mutative type).
miR-320-3p mimics inhibited the proliferation and migration and promoted the apoptosis of PASMCs
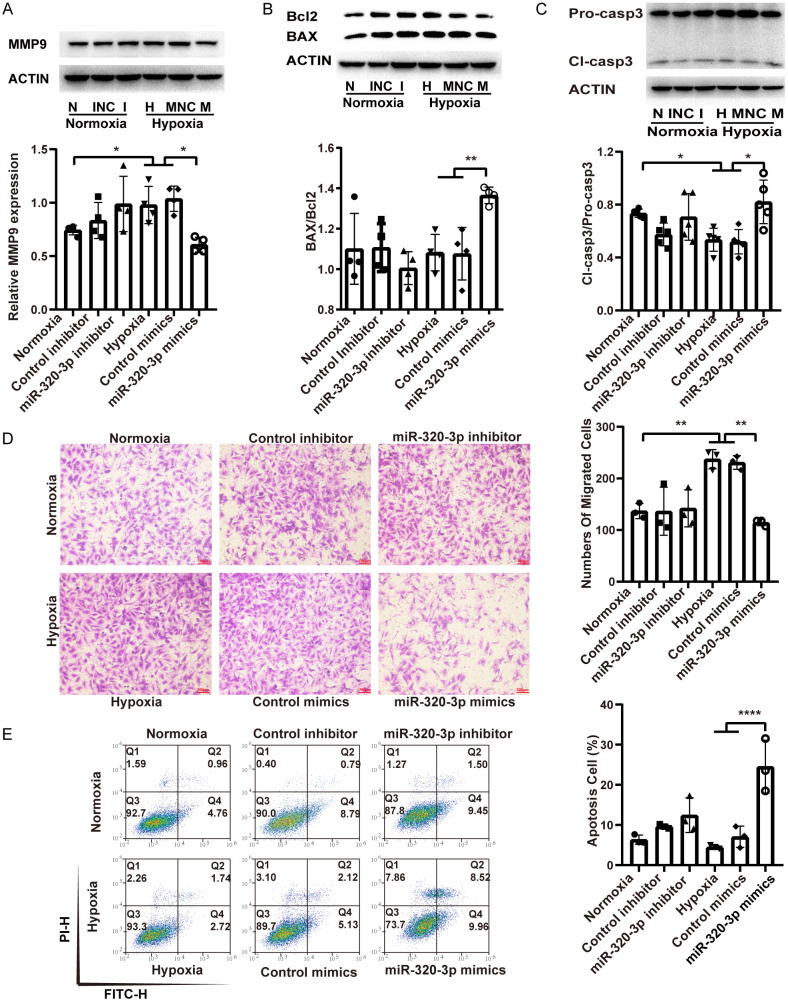
To explore the role of miR-320-3p in the proliferation, migration, and apoptosis of PASMCs, PASMCs were transfected with miR-320-3p mimics for 8 h under hypoxic (5% O2) exposure or with inhibitor under normoxic conditions. Western blot analysis revealed that miR-320-3p mimics attenuated the hypoxia-induced upregulation of the PCNA and survivin proteins (Figure 3A). The CCK-8 and EdU staining assays showed that miR-320-3p mimics attenuated the effects of hypoxia on the viability and proliferation of cells (Figure 3B-D). However, the miR-320-3p inhibitor did not affect PASMC proliferation but did affect the expression of survivin under normoxic conditions (Figure 3A-D). Western blot analysis showed that the upregulation of MMP9 expression induced by hypoxia was attenuated by miR-320-3p mimics (Figure 4A). The migration assay revealed that the increased number of migrated cells under hypoxic conditions was significantly suppressed by miR-320-3p mimics (Figure 4D). However, the miR-320-3p inhibitor did not significantly impact PASMC migration (Figure 4A, 4D). As shown in Figure 4B and 4C, miR-320-3p mimics reversed the hypoxia-induced decreases in the ratios of BAX/Bcl2 and cleaved caspase-3/procaspase-3 (Figure 4B, 4C). The Annexin V/PI assay results emphasized that miR-320-3p mimics enhanced cell apoptosis (Figure 4E). However, the miR-320-3p inhibitor had no evident effect on PASMC apoptosis (Figure 4B, 4C, 4E). The results above indicate that miR-320-3p plays a determinant role in hypoxia-induced PH.
Figure 3.
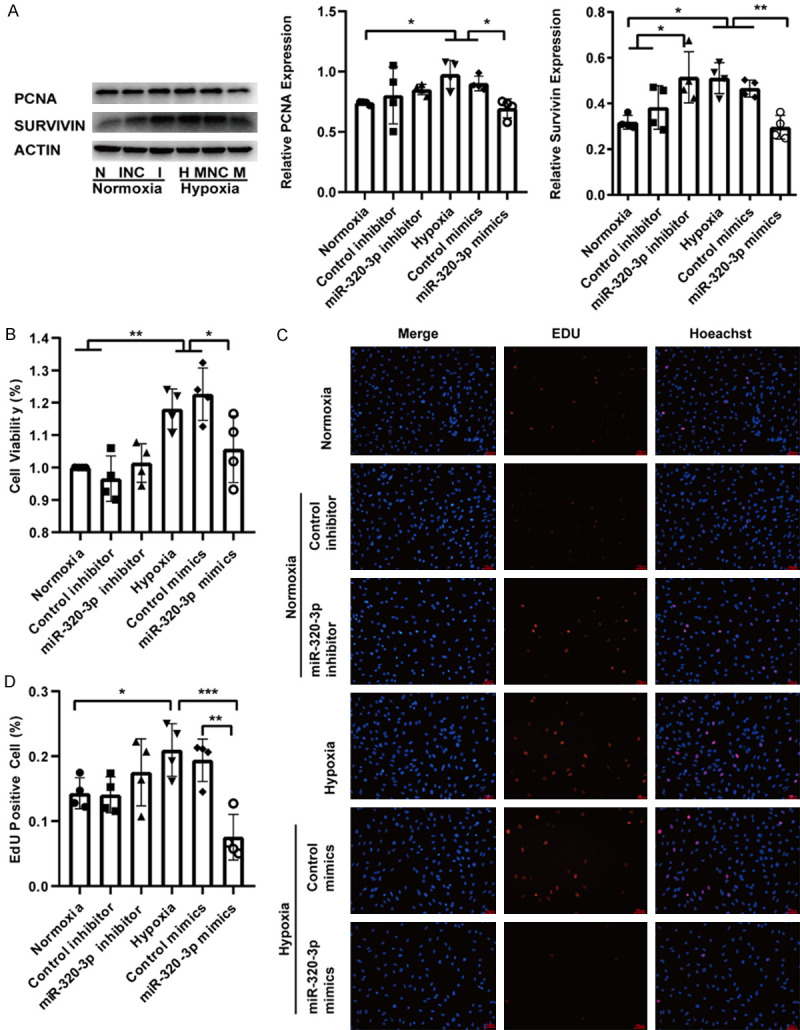
miR-320-3p inhibited the proliferation of PASMCs. A. The protein expression of PCNA and Survivin were analyzed with western blotting and the proteins expression were evaluated. B. The viability of PASMCs was determined by CCK-8 tests. C. The EDU proliferation assay was used to measure PASMCs proliferation ability. D. Quantitation of EDU positive cells. All images are presented at 400× magnification. The data statistics are shown as mean ± SD. CCK-8, cell-counting kit-8. *P < 0.05, **P < 0.01, ***P < 0.005.
Figure 4.
miR-320-3p inhibited migration and promoted apoptosis of PASMCs. A. The protein related to migration (MMP9) was detected by western blotting. B, C. The expression level of apoptosis proteins (Bcl2, BAX, pro-caspase3, cleaved-caspase3) and the ratio of BAX/Bcl2 and Cl-caspase3/Pro-caspase3 were calculated. D. The PASMC migration ability was measured by Transwell assay and the number of migrated cells was counted. E. The cell apoptosis of transfected cells was evaluated by Annexin V/PI assay and the percentage of apoptotic cells was analyzed. *P < 0.05, **P < 0.01, ***P < 0.005.
KLF5 and HIF1α reversed the effects of miR-320-3p mimics on PASMC proliferation, migration, and apoptosis
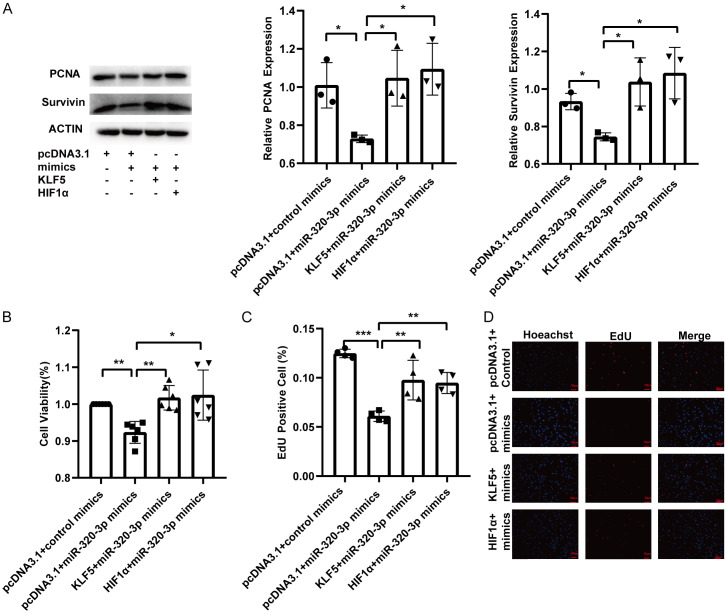
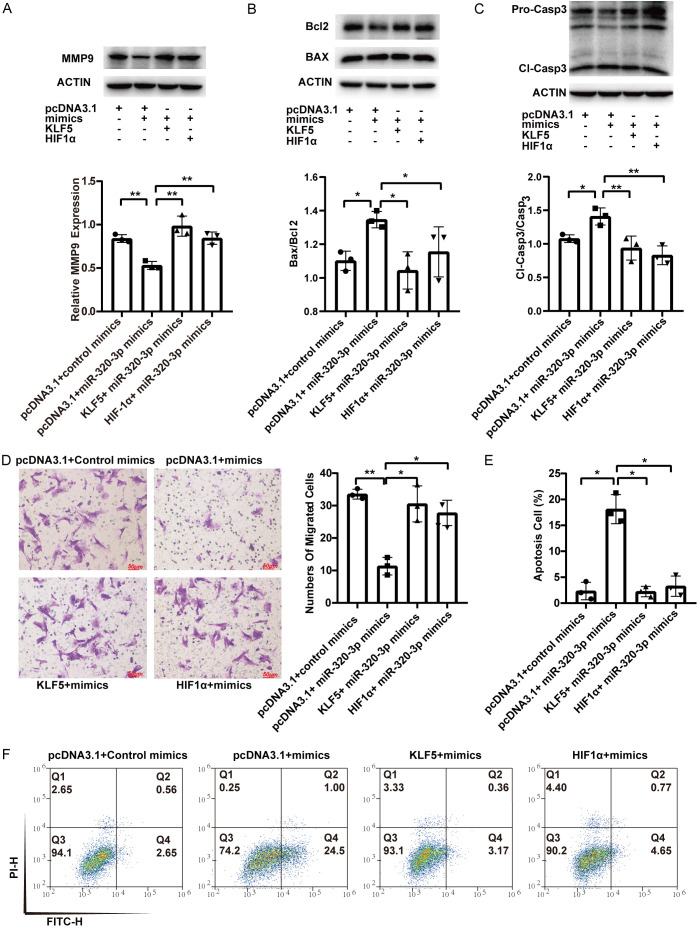
Rescue experiments showed that overexpression of KLF5 and HIF1α upregulated the expression of PCNA and survivin, which was suppressed by miR-320-3p mimics (Figure 5A). Overexpression of KLF5 and HIF1α reversed the suppressive effects of miR-320-3p on cell viability and proliferation (Figure 5B-D). The expression of MMP9, which was reduced by miR-320-3p mimics, was increased by KLF5 and HIF1α overexpression (Figure 6A). The decrease in the number of migrated cells induced by miR-320-3p mimics was reversed by the overexpression of KLF5 and HIF1α (Figure 6D). Overexpression of KLF5 and HIF1α prevented the increase in the ratio of BAX/Bcl2 and cleaved caspase-3/procaspase-3 induced by miR-320-3p (Figure 6B, 6C). The upregulation of apoptosis induced by miR-320-3p mimics was attenuated by the enforced expression of KLF5 and HIF1α (Figure 6E, 6F). These results show that KLF5 and HIF1α reverse the effects of miR-320-3p mimics in hypoxia-induced PASMCs.
Figure 5.
KLF5 and HIF1α restored the attenuated proliferation of PASMCs affected by miR-320-3p. PASMCs were transfected with control mimics or miR-320-3p mimics for 12 hr., and then transfected with pcDNA3.1, KLF5 plasmid or HIF1α plasmid. A. Proteins related to proliferation (PCNA and Survivin) were determined by western blotting. B. The PASMC viability was detected by CCK-8 tests. C, D. The PASMC proliferation ability was measured by EdU proliferation assay. *P < 0.05, **P < 0.01, ***P < 0.005.
Figure 6.
KLF5 and HIF1α reversed the attenuated migration and enhanced apoptosis of PASMCs affected by miR-320-3p. A. The expression level of MMP9. B, C. Proteins related to apoptosis (Bcl2, BAX, pro-Caspase3 and cleaved-Caspase3) were detected. D. Crystal violet staining of PASMC was presented at 200× magnification for the transwell assay and the number of migrated cells was calculated manually. E, F. The cell apoptosis of transfected cells was evaluated by Annexin V/PI assay and the percentage of apoptotic cells was analyzed. *P < 0.05, **P < 0.01, ***P < 0.005.
Discussion
PH is a chronic, progressive vascular disease that leads to right heart failure in severe cases; thus, effective therapeutic strategies are urgently needed based on the currently limited treatment methods. Our present study aimed to explore a novel therapy for PH. Our research found that miR-320-3p was downregulated in vitro and in vivo in hypoxia-induced PASMCs as well as in CTEPH patients compared to controls. miR-320-3p downregulated the expression of KLF5 and HIF1α by directly targeting their 3’UTRs. Overexpression of miR-320-3p repressed the proliferation and migration and enhanced the apoptosis of PASMCs, and the effects were rescued by KLF5 and HIF1α overexpression. The miR-320-3p inhibitor did not affect cell proliferation, apoptosis or migration but did affect survivin expression under normoxic conditions. The present study demonstrates that the miR-320-3p/KLF5/HIF1α signaling pathway is involved in the progression of PAH and that miR-320-3p could be a new therapeutic strategy for PH.
Several recent studies discovered a series of miRNAs contributing to the regulation of pulmonary arterial vascular remodeling [21]. miR-204 significantly reduced the mPAP and vascular wall thickness in the monocrotaline (MCT)-PAH model [24]. The hypoxia-induced increases in RVSP and vascular wall thickness were significantly reduced in miR-143-/- mice [25]. miR-140-5p, miR-125a-5p, and miR-340-5p altered vascular remodeling by affecting the proliferation and apoptosis of PASMCs [26-29]. In the present study, enhanced expression of miR-320-3p inhibited cell proliferation and migration and promoted apoptosis in hypoxic PASMCs. It is well known that PAH and cancer have analogous hypoxic microenvironments; the function of miR-320-3p in PASMCs was identical to that found in studies in the cancer field. Rno-miR-320-3p has the same sequence as hsa-miR-320a. The plasma level of miR-320a was downregulated in a group of colorectal cancer patients [30]. miR-320a inhibited cell proliferation and migration and was downregulated in malignant pleural mesothelioma by targeting PDL1. Moreover, overexpression of p53 upregulated miR-320a [31]. In a hepatocellular carcinoma study, miR-320a exerted a tumor suppressor function by inhibiting ERK1/2 activation by targeting PBX3 [32]. Additionally, as a tumor suppressor gene, miR-320-3p inhibited cell proliferation in prostate cancer, ovarian cancer, cholangiocarcinoma, and gastric cancer [33-38]. Similarly, we found that miR-320-3p inhibited PASMC proliferation under hypoxic conditions.
Many studies have shown that KLF5 plays a crucial role in cell proliferation, apoptosis and migration based on multiple molecular mechanisms. At the transcriptional level, KLF5 directly targets genes related to proliferation, apoptosis and migration by binding to their GC-rich promoter regions. At the translational level, KLF5 directly interacts with p53 and PARP-1 [9,39]. KLF5 and p53 can bind to the promoters of HIF1α and survivin [9,40]. A recent study demonstrated that KLF5 was a target of miR-320-3p as well [37]. Our study explained that KLF5 and HIF1α were direct targets of miR-320-3p and reversed the effects caused by overexpression of miR-320-3p in hypoxia-treated PASMCs. Our recent study demonstrated that KLF5 mediates vascular remodeling by directly regulating HIF1α in hypoxic pulmonary tissue [6]. This result suggests that the miR-320-3p/KLF5/HIF1α signaling pathway might be involved in PH.
The present study also has some limitations. First, we focused on revealing the mechanism of miR-320-3p in hypoxic PASMCs. Thus, PAH animal models should be implemented to further study the role of miR-320-3p in the pathophysiology of PAH. Second, since miRNAs target multiple genes, the miR-320-3p network that regulates the progression of PAH may be further explored. Third, the profile of miR-320-3p in CTEPH (the fourth PH type) plasma is based on the GEO database. Thus, the miR-320-3p plasma profile in hypoxic PAH (the third PH type) should be investigated further.
In conclusion, our findings demonstrate that miR-320-3p is downregulated in PH and that miR-320-3p attenuates hypoxia-induced proliferation and migration and enhances cell apoptosis via KLF5 and HIF1α. Our present study demonstrates that miR-320-3p exerts a novel, prominent and evident role in regulating the biological effect of hypoxia in PASMCs.
Acknowledgements
The present study was supported by the National Key Research and Development Programs of China (No. 2016YFC13045004, 2016-YFC0903602) and the National Natural Science Foundation of China (No. 81670048, and 81973987).
Disclosure of conflict of interest
None.
References
- 1.Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhrin P, Wang D, Mocan A, Waltenberger B, Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR, Tzvetkov NT, Horbańczuk J, Atanasov AG. Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: natural products inhibiting proliferation. Biotechnol Adv. 2018;36:1608–1621. doi: 10.1016/j.biotechadv.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Uhrin P, Mocan A, Waltenberger B, Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR, Tzvetkov NT, Horbańczuk J, Atanasov AG. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: molecular targets and pathways. Biotechnol Adv. 2018;36:1586–1607. doi: 10.1016/j.biotechadv.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Courboulin A, Tremblay VL, Barrier M, Meloche J, Jacob MH, Chapolard M, Bisserier M, Paulin R, Lambert C, Provencher S, Bonnet S. Krüppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir Res. 2011;12:128. doi: 10.1186/1465-9921-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, He Y, Xu Y, Huang X, Liu J, Xie M, Liu X. KLF5 mediates vascular remodeling via HIF-1α in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;310:L299–310. doi: 10.1152/ajplung.00189.2015. [DOI] [PubMed] [Google Scholar]
- 7.Wan H, Luo F, Wert SE, Zhang L, Xu Y, Ikegami M, Maeda Y, Bell SM, Whitsett JA. Kruppel-like factor 5 is required for perinatal lung morphogenesis and function. Development. 2008;135:2563–2572. doi: 10.1242/dev.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinoda Y, Ogata N, Higashikawa A, Manabe I, Shindo T, Yamada T, Kugimiya F, Ikeda T, Kawamura N, Kawasaki Y, Tsushima K, Takeda N, Nagai R, Hoshi K, Nakamura K, Chung UI, Kawaguchi H. Kruppel-like factor 5 causes cartilage degradation through transactivation of matrix metalloproteinase 9. J Biol Chem. 2008;283:24682–24689. doi: 10.1074/jbc.M709857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu N, Gu L, Findley HW, Chen C, Dong JT, Yang L, Zhou M. KLF5 Interacts with p53 in regulating Survivin expression in acute lymphoblastic leukemia. J Biol Chem. 2006;281:14711–14718. doi: 10.1074/jbc.M513810200. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Benjamin MS, Sun XD, Otto KB, Guo P, Dong XY, Bao YD, Zhou ZM, Cheng XH, Simons JW, Dong JT. KLF5 promotes cell proliferation and tumorigenesis through gene regulation in the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006;118:1346–55. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 11.Nakaya T, Ogawa S, Manabe I, Tanaka M, Sanada M, Sato T, Taketo MM, Nakao K, Clevers H, Fukayama M, Kuroda M, Nagai R. KLF5 regulates the integrity and oncogenicity of intestinal stem cells. Cancer Res. 2014;74:2882–2891. doi: 10.1158/0008-5472.CAN-13-2574. [DOI] [PubMed] [Google Scholar]
- 12.Nandan MO, Ghaleb AM, McConnell BB, Patel NV, Robine S, Yang VW. Kruppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations. Molecular Cancer. 2010;9:14. doi: 10.1186/1476-4598-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YZ, Nakagawa H, Tetreault MP, Billig J, Victor N, Goyal A, Sepulveda AR, Katz JP. Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer. Cancer Res. 2011;71:6475–6484. doi: 10.1158/0008-5472.CAN-11-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarapore RS, Yang YZ, Katz JP. Restoring KLF5 in esophageal squamous cell cancer cells activates the JNK pathway leading to apoptosis and reduced cell survival. Neoplasia. 2013;15:472–80. doi: 10.1593/neo.122126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Zhang B, Xiang L, Xia S, Kucuk O, Deng X, Boise LH, Dong JT. TGF-β causes docetaxel resistance in prostate cancer via the induction of Bcl-2 by acetylated KLF5 and protein stabilization. Theranostics. 2020;10:7656–7670. doi: 10.7150/thno.44567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad A, Ahmad S, Malcolm KC, Miller SM, Hendry-Hofer T, Schaack JB, White CW. Differential regulation of pulmonary vascular cell growth by hypoxia-inducible transcription factor-1α and hypoxia-inducible transcription factor-2α. Am J Respir Cell Mol Biol. 2013;49:78–85. doi: 10.1165/rcmb.2012-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 18.Kang W, Tong JHM, Lung RWM, Dong YJ, Zhao JH, Liang QY, Zhang L, Pan Y, Yang WQ, Pang JCS, Cheng ASL, Yu J, To KF. Targeting of YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor function in gastric adenocarcinoma. Mol Cancer. 2015;14:52. doi: 10.1186/s12943-015-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui R, Meng W, Sun HL, Kim T, Ye ZQ, Fassan M, Jeon YJ, Li B, Vicentini C, Peng Y, Lee TJ, Luo ZH, Liu L, Xu D, Tili E, Jin V, Middleton J, Chakravarti A, Lautenschlaeger T, Croce CM. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci U S A. 2015;112:E4288–E4297. doi: 10.1073/pnas.1502068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandbothe M, Buurman R, Reich N, Greiwe L, Vajen B, Gurlevik E, Schaffer V, Eilers M, Kuhnel F, Vaquero A, Longerich T, Roessler S, Schirmacher P, Manns MP, Illig T, Schlegelberger B, Skawran B. The microRNA-449 family inhibits TGF-beta-mediated liver cancer cell migration by targeting SOX4. J Hepatol. 2017;66:1012–1021. doi: 10.1016/j.jhep.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Courboulin A, Ranchoux B, Cohen-Kaminsky S, Perros F, Bonnet S. MicroRNA networks in pulmonary arterial hypertension: share mechanisms with cancer? Curr Opin Oncol. 2016;28:72–82. doi: 10.1097/CCO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 22.Negi V, Chan SY. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight. 2017;2:e91327. doi: 10.1172/jci.insight.91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao X, He Y, Li X, Xu Y, Liu X. The IRE1α-XBP1 pathway function in hypoxia-induced pulmonary vascular remodeling, is upregulated by quercetin, inhibits apoptosis and partially reverses the effect of quercetin in PASMCs. Am J Transl Res. 2019;11:641–654. [PMC free article] [PubMed] [Google Scholar]
- 24.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Blanco FJ, Stevens H, Lu RF, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117:870–883. doi: 10.1161/CIRCRESAHA.115.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, Francis SE, Rowlands DJ, Lawrie A. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu TT, Zhang WF, Yin YL, Liu YH, Song P, Xu J, Zhang MX, Li P. MicroRNA-140-5p targeting tumor necrosis factor-α prevents pulmonary arterial hypertension. J Cell Physiol. 2019;234:9535–9550. doi: 10.1002/jcp.27642. [DOI] [PubMed] [Google Scholar]
- 28.Cai Z, Li J, Zhuang Q, Zhang X, Yuan A, Shen L, Kang K, Qu B, Tang Y, Pu J, Gou D, Shen J. MiR-125a-5p ameliorates monocrotaline-induced pulmonary arterial hypertension by targeting the TGF-β1 and IL-6/STAT3 signaling pathways. Exp Mol Med. 2018;50:45. doi: 10.1038/s12276-018-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou M, Zhang C, Chen J, Zhao S, Cui S, Tu J. Overexpression of microRNA-340-5p inhibits pulmonary arterial hypertension induced by APE by downregulating IL-1β and IL-6. Mol Ther Nucleic Acids. 2020;21:542–554. doi: 10.1016/j.omtn.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Fang ZX, Tang J, Bai YY, Lin HY, You HY, Jin HW, Lin LQ, You P, Li J, Dai Z, Liang XM, Su YH, Hu Q, Wang F, Zhang ZY. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J Exp Clin Cancer Res. 2015;34:86. doi: 10.1186/s13046-015-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa C, Indovina P, Mattioli E, Forte IM, Iannuzzi CA, Luzzi L, Bellan C, De Summa S, Bucci E, Di Marzo D, De Feo M, Mutti L, Pentimalli F, Giordano A. P53-regulated miR-320a targets PDL1 and is downregulated in malignant mesothelioma. Cell Death Dis. 2020;11:748. doi: 10.1038/s41419-020-02940-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZC, Li X, Sun W, Yue SQ, Yang JY, Li JJ, Ma B, Wang JL, Yang XS, Pu M, Ruan B, Zhao G, Huang QK, Wang L, Tao KS, Dou KF. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Sato S, Katsushima K, Shinjo K, Hatanaka A, Ohka F, Suzuki S, Naiki-Ito A, Soga N, Takahashi S, Kondo Y. Histone deacetylase inhibition in prostate cancer triggers miR-320-mediated Suppression of the androgen receptor. Cancer Res. 2016;76:4192–4204. doi: 10.1158/0008-5472.CAN-15-3339. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Duan P, Wang J, Lu X, Cheng J. miR-320 inhibited ovarian cancer oncogenicity via targeting TWIST1 expression. Am J Transl Res. 2017;9:3705–3713. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Yang J, Xiang YY, Pi J, Bian J. Overexpression of Hsa-miR-320 is associated with invasion and metastasis of ovarian cancer. J Cell Biochem. 2017;118:3654–3661. doi: 10.1002/jcb.26009. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H, Jiang X, Zhou X, Dong X, Xie K, Yang C, Jiang H, Sun X, Lu J. Neuropilin-1 regulated by miR-320 contributes to the growth and metastasis of cholangiocarcinoma cells. Liver Int. 2018;38:125–135. doi: 10.1111/liv.13495. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Xu Q, Shang J, Lu L, Chen G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol. 2019;234:17876–17885. doi: 10.1002/jcp.28418. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Gao S, Zhao Z, Liang G, Kong J, Feng X. MicroRNA-320d regulates tumor growth and invasion by promoting FoxM1 and predicts poor outcome in gastric cardiac adenocarcinoma. Cell Biosci. 2020;10:80. doi: 10.1186/s13578-020-00439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki T, Nishi T, Nagino T, Sasaki K, Aizawa K, Kada N, Sawaki D, Munemasa Y, Matsumura T, Muto S, Sata M, Miyagawa K, Horikoshi M, Nagai R. Functional interaction between the transcription factor Kruppel-like factor 5 and poly(ADP-ribose) polymerase-1 in cardiovascular apoptosis. J Biol Chem. 2007;282:9895–9901. doi: 10.1074/jbc.M608098200. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, Yun CC. Regulation of Hypoxia-inducible Factor 1 alpha (HIF-1 alpha) by Lysophosphatidic Acid Is Dependent on Interplay between p53 and Kruppel-like Factor 5. J Biol Chem. 2013;288:25244–25253. doi: 10.1074/jbc.M113.489708. [DOI] [PMC free article] [PubMed] [Google Scholar]