Abstract
Background
The impact of pulmonary hypertension (PH) on outcomes after surgical tricuspid valve replacement (TVR) and repair (TVr) is unclear. We sought to characterize PH in patients undergoing TVR/TVr, based on invasive hemodynamics and evaluate the effect of PH on mortality.
Methods
We identified 86 consecutive patients who underwent TVR/TVr with invasive hemodynamic measurements within 3 months before surgery. We used Kaplan-Meier survival and restricted mean survival time (RMST) analyses to quantify the effects of PH on survival.
Results
The mean age was 63 ± 13 years, 59% were female, 45% had TVR, 55% had TVr, 39.5% had isolated TVR/TVr, and 60.5% had TVR/TVr concomitant with other cardiac surgeries). Eighty-six percent of these patients had PH with a mean pulmonary artery pressure of 30 ± 10 mm Hg, pulmonary vascular resistance (PVR) of 2.5 (interquartile range: 1.5-3.9) Wood units (WU), pulmonary arterial compliance of 2.3 (1.6-3.6) mL/mm Hg, and pulmonary arterial elastance of 0.8 (0.6-1.2) mm Hg/mL. Cardiac output was mildly reduced at 4.0 ± 1.4 L/min, with elevated right-atrial pressure (14 ± 12 mm Hg) and pulmonary capillary wedge pressure (19 ± 7 mm Hg). Over a median follow-up of 6.3 years, 22% of patients died. Patients with PVR ≥ 2.5 WU had lower RMST over 5 years compared with patients with PVR < 2.5 WU.
Conclusion
PH is common in patients undergoing TVR/TVr, with combined pre- and postcapillary being the most common type. PVR ≥ 2.5 WU is associated with lower survival at 5-year follow-up.
Résumé
Contexte
On connaît mal les répercussions de l’hypertension pulmonaire (HP) chez les patients qui ont subi une intervention chirurgicale de remplacement de la valve tricuspide (RVT) ou de réparation de la valve tricuspide (rVT). Nous avons tenté de caractériser l’HP chez les patients ayant subi un RVT ou une rVT en fonction des paramètres de surveillance hémodynamique effractive et d’évaluer l’effet de l’HP sur la mortalité.
Méthodologie
Nous avons relevé 86 patients consécutifs ayant subi un RVT ou une rVT qui avaient fait l’objet de mesures hémodynamiques effractives dans les trois mois précédant l’intervention chirurgicale. Pour quantifier les effets de l’HP sur la survie, nous avons analysé la survie au moyen de la méthode de Kaplan-Meier et de la survie moyenne restreinte.
Résultats
Les patients avaient en moyenne 63 ± 13 ans; 59 % d’entre eux étaient des femmes; 45 % avaient subi un RVT et 55 %, une rVT; 39,5 % avaient subi seulement un RVT ou une rVT lors de l’intervention chirurgicale; 60,5 % avaient subi un RVT ou une rVT en même temps qu’une autre intervention cardiaque. Quatre-vingt-six pour cent de ces patients présentaient une HP avec une pression artérielle pulmonaire moyenne de 30 ± 10 mmHg, une résistance vasculaire pulmonaire (RVP) de 2,5 (intervalle interquartile : 1,5 à 3,9) unités de Wood (UW), une compliance artérielle pulmonaire de 2,3 (1,6 à 3,6) ml/mmHg et une élastance artérielle pulmonaire de 0,8 (0,6 à 1,2) mmHg/ml. On a observé une légère baisse du débit cardiaque à 4,0 ± 1,4 L/min, ainsi qu’une augmentation de la pression auriculaire droite (14 ± 12 mmHg) et de la pression artérielle pulmonaire d’occlusion (19 ± 7 mmHg). Sur une période médiane de suivi de 6,3 ans, 22 % des patients sont décédés. Le taux de survie moyenne restreinte à 5 ans était plus faible chez les patients présentant une RVP ≥ 2,5 UW que chez les patients présentant une RVP < 2,5 UW.
Conclusion
L’HP est fréquente chez les patients subissant un RVT ou une rVT, le type le plus courant étant l’HP mixte (pré-capillaire et post-capillaire). Une RVP ≥ 2,5 UW est associée à un taux de survie à 5 ans plus faible.
Moderate or severe tricuspid regurgitation (TR) affects more than 1 million patients in the United States. Pertinently, the presence of TR is associated with high morbidity not only in those with multivalvular disease or left-sided heart failure but also in those with severe, isolated TR.1 At present, the only class I indication for surgical tricuspid valve replacement (TVR) or repair (TVr) is severe TR in patients undergoing surgery for left-sided valve disease.2,3 Despite this, the number of TVR and TVr performed in the United States has more than doubled over a 10-year period (1998 to 2008), indicating a perceived clinical need for the procedure.4 Although there may be a mortality benefit in early treatment of TR in specific populations,1,5,6 nearly 85% of TVR/TVr are performed concomitant with other cardiac surgeries, and only 15% are isolated TVR/TVr.4 Importantly, the average mortality remains high after TVR/TVr and has not improved over time, which highlights the need for better patient selection.4
Severe pulmonary hypertension (PH) is currently a contraindication for TVR/TVr.2,3. This is because of the fear of worsening right-ventricular (RV) function from unfavourable preload and afterload conditions after TVR/TVr, in the presence of PH. The high RV afterload caused by PH can lead to RV dysfunction and failure. In addition, the sudden increase in RV preload after TVR/TVr (with the correction of the “pop-off” mechanism) can worsen RV function. However, the threshold of PH that is associated with increased mortality after TVR/TVr is unclear7, 8, 9, 10 because the current literature on the impact of PH on outcomes after TVR/TVr is limited by the lack of invasive hemodynamic measurements. Previous studies predominantly have used echocardiographic estimated RV systolic pressure for risk stratification. Thus, there remain no guidelines as to the objective invasive hemodynamic measures of PH that are associated with poor outcomes after TVR/TVr.
Therefore, in this article, we seek to characterize PH in patients undergoing TVR/TVr, based on invasive hemodynamics and to evaluate the effect of these invasive hemodynamic measures of PH on short-term and long-term mortality after TVR/TVr.
Methods
Patient population
We identified all consecutive adult patients ≥ 18 years of age who underwent TVR or TVr and also had right-heart catheterization (RHC) within 3 months before TVR/TVr at the University of Minnesota Medical Center, from October 1, 2000 to October 1, 2019. We included both patients who had isolated TVR/TVr and patients who had TVR/TVr concomitant with other cardiac surgeries. We excluded patients who had TVR/TVr at the time of left-ventricular assist device implantation or cardiac transplantation and those for whom the data could not be retrieved because of lack of electronic health record.
Covariates
The following baseline variables at the time of TVR/TVr were analyzed: age; sex; and comorbid conditions including hypertension, chronic kidney disease, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, and concomitant cardiac surgery. Comorbidities were identified using International Classification of Disease (ICD) codes.
Surgical TVR and TVr
The decision to perform TVR vs TVr was at the discretion of the treating surgeon. However, as a general institutional guideline, TVr was usually performed if there was isolated annular dilatation, typically when done in conjunction with mitral-valve procedures. However, if there was extreme annular dilatation (> 45 mm), TVR was favoured over TVr. For TVR, the St. Jude Epic bioprosthetic valve (Abbott Laboratories, Chicago, IL) was used, typically sizes 29-33. For TVr, a reduction annuloplasty was typically done using the Edwards MC 3 (Edrards Lifesciences, Irvine, CA) annuloplasty ring (sizes usually 30-32 mm).
Hemodynamic data
Patients underwent RHC in the University of Minnesota cardiac catheterization laboratory. Hemodynamics was obtained using a 7-French, balloon-tipped, flow-directed catheter placed either into the internal jugular vein or the common femoral vein. The following hemodynamic variables were recorded at the end of expiration: right-atrial pressure, RV systolic and end-diastolic pressures; systolic, diastolic, and mean pulmonary artery pressures (mPAP); and pulmonary capillary wedge pressure (PCWP). Cardiac output was determined as the mean of three measurements with the thermodilution method or indirect Fick method, based on total body oxygen consumption, as estimated via the formula of LaFarge and Miettinen.11.
PH was defined as mPAP > 20 mm Hg as per the 6th World Symposium on PH consensus statement.12 Isolated postcapillary PH was defined as mPAP > 20 mm Hg, PCWP > 15 mm Hg, and pulmonary vascular resistance (PVR) < 3 Wood units (WU).12 Combined pre- and postcapillary PH was defined as mPAP >20 mm Hg, PCWP > 15 mm Hg, and PVR ≥ 3 WU.12 Transpulmonary gradient (TPG) was calculated as the difference between mPAP and PCWP. The static and the dynamic afterload of the RV were estimated by calculating PVR and pulmonary arterial compliance (PAC), respectively. PVR was calculated in WU as the difference between mPAP and PCWP divided by the cardiac output. PAC (mL/mm Hg) was calculated as the ratio of stroke volume to the pulmonary artery pulse pressure, as previously described.13 Diastolic pulmonary artery gradient (DPG) was calculated as the difference between the diastolic PAP and PCWP. Total RV afterload was estimated by calculating pulmonary artery elastance (Ea). As previously described, in patients with normal PAP, Ea was defined as mPAP divided by stroke volume; however, in patients with PH, Ea was defined as systolic PAP divided by stroke volume.14 RV stroke work was calculated a: stroke volume x (mean PAP, mean right-atrial pressure) x 0.0136 gm/m per beat.
Echocardiography
In a subset of patients (n = 34) with RV-focused preoperative echocardiographic images, we measured RV fractional area change (RVFAC) to assess RV systolic function and RV basal diameter to evaluate RV enlargement.15 RVFAC was calculated as the difference between RV end-diastolic area and end systolic area over RV end-diastolic area.
Vital statistics
Vital statistics were obtained for all patients by chart review and the Minnesota Death Index. For each death, the date and cause of death was collected. In all patients who were not identified as deceased using the Minnesota Death Index, it was possible to establish vital status by chart review.
Statistical analysis
Categorical data were expressed as frequency and proportions, whereas continuous data were presented as mean ± standard deviation if normally distributed and as median (interquartile range [IQR]) if non-normally distributed. Histograms were created to assess for normality of distribution for all continuous variables. Patients were categorized into groups based on whether they had TVR or TVr and isolated TVR/TVr vs concomitant TVR/TVr. We used unpaired Student's t-tests for comparing normally distributed continuous variables and the Wilcoxon-Mann Whitney test for non-normally distributed continuous variables; χ2 or the Fisher exact test was performed to compare proportions for categorical variables, as appropriate. We used logistic regression to identify clinical determinants of PH (mPAP >20 mm Hg) in patients undergoing surgical TVR/TVr. To determine the impact of PH on mortality, Kaplan-Meier survival analyses were performed, with entry into the study defined as the date of TVR/TVr. The primary endpoint was all-cause mortality. Patients were censored if lost to follow-up or at study completion (October 2019). We used restricted mean survival time (RMST) analysis to estimate the effect of invasive hemodynamic measures of PH and RV function on survival in those with PH who underwent TVR/TVr.16, 17, 18 We categorized patients based on the median value of invasive hemodynamic measures and compared RMST over 5 years between groups. In the adjusted model for RMST, we adjusted for the type of tricuspid valve surgery as well as concomitant surgeries. All statistical analyses were performed with STATA Version 15 (StataCorp, College Station, TX) or GraphPad Prism Version 8.0 (SYSTAT, San Jose, CA). For all statistical analysis, a P value of < 0.05 was considered significant.
Results
Baseline characteristics
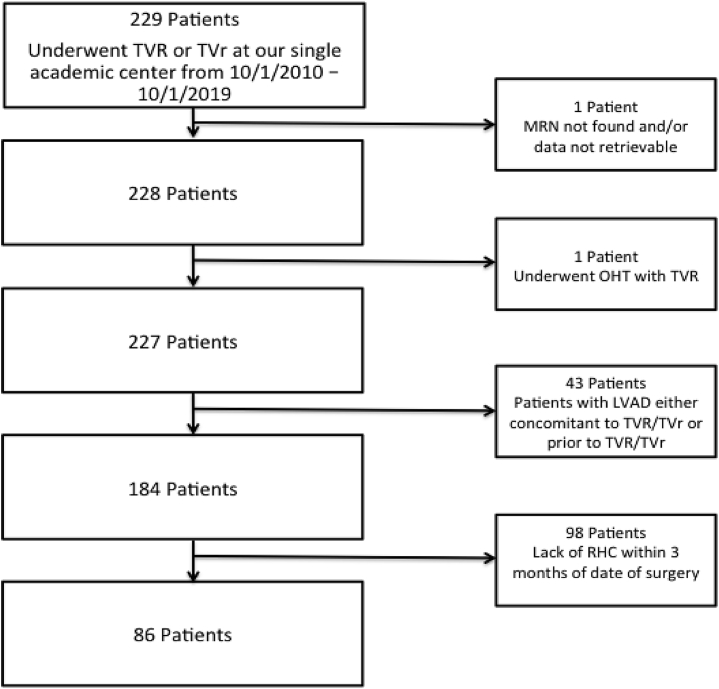
We identified 86 patients who met our inclusion and exclusion criteria (Fig. 1). The mean age was 63 ± 13 years, and there was a female predominance (59.3%) (Table 1). The majority of patients undergoing either TVR or TVr had multiple comorbid conditions: 88.3% had atrial fibrillation, 72% had systemic hypertension, 49% had chronic kidney disease, 42% had diabetes mellitus, and 22% had chronic obstructive lung disease (Table 1). The most common indication for tricuspid valve surgery was secondary TR (78%), followed by primary TR (19%) and tricuspid stenosis (3%) (Supplemental Table S1). Of the 86 patients, 39 underwent TVR (45%), and 47 underwent TVr (55%). Concomitant cardiac surgeries were performed in 60.5% of patients, whereas the remaining 39.5% of patients underwent isolated TVR/TVr. Mitral or aortic valve replacement was the most common concomitant cardiac surgery (44.2%), followed by coronary artery bypass grafting with concomitant mitral or aortic valve replacement (11.6%), congenital heart surgery (2.3%), coronary artery bypass grafting surgery (1.2%), and pulmonary valve replacement (1.2%).
Figure 1.
Study flow chart for patient selection. LVAD, left-ventricular assist device; MRN, medical record number; OHT, orthotopic heart transplant; RHC, right-heart catheterization.
Table 1.
Baseline clinical and hemodynamic characteristics in patients undergoing TVR/TVr
| Characteristics | All patients (N = 86) | TVR (N = 39) | TVr (N = 47) | P value | Isolated TVR/TVr (N = 34) | Concomitant TVR/TVr (N = 52) | P value |
|---|---|---|---|---|---|---|---|
| Age, years (n = 86) | 64 ± 13 | 63 ± 12 | 64 ± 14 | 0.82 | 66 ± 10 | 62 ± 15 | 0.16 |
| Female, % | 59.3% (51) | 69.3% (27) | 51.1% (24) | 0.08 | 65% (22) | 56% (29) | 0.41 |
| Comorbidities, % (n) | |||||||
| Hypertension | 72% (62) | 64.1% (25) | 78.7% (37) | 0.13 | 62% (21) | 79% (41) | 0.08 |
| Diabetes mellitus | 41.8% (36) | 56.4% (22) | 29.7% (14) | 0.01 | 50% (17) | 36.5% (19) | 0.22 |
| Chronic kidney disease | 48.8% (42) | 51.2% (20) | 46.8% (22) | 0.68 | 52.9% (18) | 46.2% (24) | 0.54 |
| Stage 1 (GFR > 90) | 10% (8) | 12% (4) | 8.5 % (4) | 7.1% (2) | 11.5% (6) | ||
| Stage II (GFR 60-89) | 41% (33) | 36.5% (12) | 44.7% (21) | 50% (14) | 36.5% (19) | ||
| Stage III (GFR 30-59) | 36% (29) | 36.5% (12) | 36.2% (17) | 35.7% (10) | 36.5% (19) | ||
| Stage IV (GFR 15-29) | 6.5% (5) | 6% (2) | 6.4% (3) | 3.6 % (1) | 7.7% (4) | ||
| Stage V (GFR < 15) | 6.5% (5) | 9% (3) | 4.3% (2) | 3.6% (1) | 7.7% (4) | ||
| COPD | 22.1% (19) | 25.6% (10) | 19.2% (9) | 0.47 | 32.4% (11) | 15.4% (8) | 0.06 |
| Atrial fibrillation | 88.3% (76) | 89.7% (35) | 87.2% (41) | 0.72 | 82.4% (28) | 92.3% (48) | 0.16 |
| MELD score (n = 33) | 12 ± 7 | 13 ± 7 | 12 ± 6 | 0.72 | 12 ± 8 | 12 ± 6 | 0.92 |
| Hemodynamics | |||||||
| Heart rate, bpm (n = 77) | 79 ± 16 | 76 ± 14 | 81 ± 17 | 0.11 | 76 ± 14 | 80 ± 17 | 0.36 |
| SBP, mm Hg (n = 73) | 119 ± 23 | 123 ± 26 | 116 ± 20 | 0.20 | 121 ± 26 | 118 ± 22 | 0.61 |
| DBP, mm Hg (n = 73) | 69 ± 11 | 69 ± 12 | 70 ± 11 | 0.85 | 69 ± 13 | 69 ± 11 | 0.97 |
| Mean RA pressure, mm Hg (n = 82) | 14 ± 12 | 14 ± 7 | 13 ± 6 | 0.32 | 13 ± 6 | 14 ± 6 | 0.58 |
| RVSP, mm Hg (n = 83) | 44 ± 14 | 40 ± 12 | 47 ± 15 | 0.01 | 40 ± 10 | 46 ± 16 | 0.03∗ |
| RVEDP, mm Hg (n = 81) | 9 ± 6 | 9 ± 6 | 10 ± 6 | 0.42 | 9 ± 6 | 9 ± 6 | 0.55 |
| SPAP, mm Hg (n = 86) | 45 ± 13 | 40 ± 11 | 48 ± 14 | < 0.01 | 40 ± 10 | 48 ± 15 | 0.01 |
| DPAP, mm Hg (n = 86) | 22 ± 8 | 20 ± 7 | 24 ± 8 | 0.02 | 20 ± 6 | 23 ± 8 | 0.11 |
| MPAP, mm Hg (n = 86) | 30 ± 10 | 27 ± 8 | 33 ± 10 | < 0.01 | 27 ± 7 | 32 ± 10 | 0.03 |
| PCWP, mm Hg (n = 83) | 19 ± 7 | 17 ± 7 | 21 ± 8 | 0.02 | 18 ± 7 | 20 ± 8 | 0.21 |
| PA saturation, % (n = 43) | 61 ± 10 | 63 ± 8 | 59 ± 12 | 0.20 | 62 ± 9 | 61 ± 10 | 0.69 |
| CO, L/min (n = 74) | 4.0 ± 1.4 | 3.9 ± 1.3 | 4.1 ± 1.5 | 0.59 | 4.1 ± 1.1 | 4.0 ± 1.5 | 0.68 |
| CI, L/min/m2 (n = 74) | 2.1 ± 0.6 | 2.1 ± 0.5 | 2.1 ± 0.6 | 0.50 | 2.2 ± 0.5 | 2.0 ± 0.6 | 0.30 |
| TPG, mm Hg (n = 82) | 11 ± 5 | 10 ± 4 | 12 ± 6 | 0.11 | 9 ± 4 | 12 ± 6 | 0.02 |
| DPG, mm Hg (n = 83) | 3 ± 4 | 2 ± 3 | 3 ± 4 | 0.80 | 2 ± 3 | 3 ± 4 | 0.30 |
| PVR, Wood units (n = 72) | 2.5 (1.5-3.9) | 2.2 (1.5-3.9) | 2.6 (1.4-3.9) | 0.48 | 2.0 (1.5-2.9) | 2.7 (1.8-4.2) | 0.07 |
| PAC, mL/mm Hg (n = 67) | 2.3 (1.6-3.6) | 2.5 (1.8-3.7) | 2.1 (1.3-3.0) | 0.07 | 2.6 (2.2-3.5) | 1.9 (1.3-3.6) | 0.05 |
| PA elastance, mm Hg/mL (n = 67) | 0.8 (0.6-1.2) | 0.7 (0.6-0.9) | 0.9 (0.6-1.4) | 0.07 | 0.7 (0.6-0.9) | 0.9 (0.6-1.4) | 0.04 |
bpm, beats per minute; CI, cardiac index; CO, cardiac output; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; DPG, diastolic pulmonary gradient; GFR, glomerular filtration rate; MELD, model for end-stage liver disease; MPAP, mean pulmonary artery pressure; PA, pulmonary artery; PAC, pulmonary artery compliance; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance, RA, right atrial; RVEDP, right-ventricular end-diastolic pressure; RVSP, right-ventricular systolic pressure; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; TPG, transpulmonary gradient; TVr, tricuspid valve repair; TVR, tricuspid valve replacement.
Statistically significant P value of < 0.05.
Hemodynamic characterization of PH in patients undergoing TVR/TVr
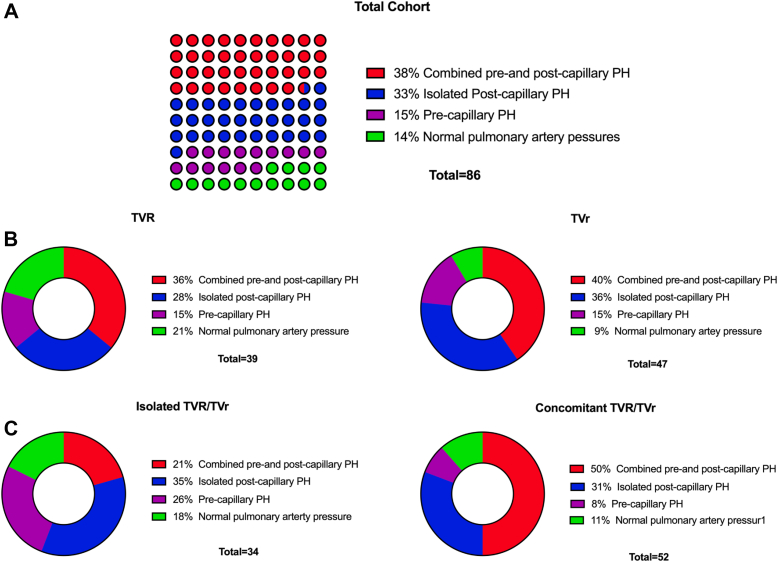
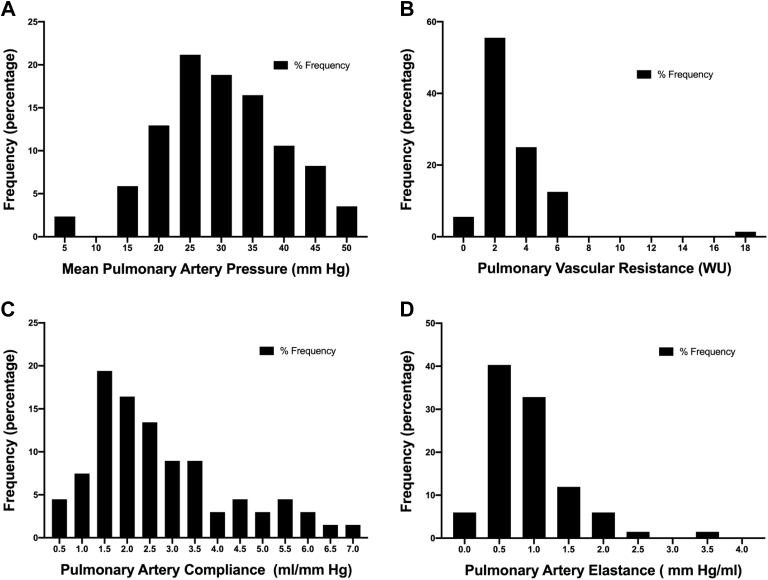
The median time interval between RHC and TVR/TVr was 22.5 (IQR: 7-51) days. On invasive hemodynamic characterization, the majority of patients (86%) who underwent TVR/TVr had PH. Combined pre- and postcapillary PH was the most common at 38%, after which, 33% of patients had isolated postcapillary PH; only 15% exhibited only precapillary PH, and the remainder 14% of our cohort did not have PH (Fig. 2A). The severity of PH was mild with an mPAP of 30 ± 10 mm Hg, PVR of 2.5 (IQR: 1.5-3.9) WU, PAC of 2.3 (1.6-3.6) mL/mm Hg, and Ea of 0.8 (0.6-1.2) mm Hg/mL (Table 1). Figure 3 displays the distribution of mPAP, PVR, PAC, and Ea in our cohort. Cardiac output and index were mildly reduced at 4.0 ± 1.4 L/min and 2.1 ± 0.6 L/min per m2, respectively. Biventricular filling pressures were elevated with a mean right-atrial pressure and a PCWP pressure of 14 ± 12 mm Hg and 19 ± 7 mm Hg, respectively.
Figure 2.
Characterization of pulmonary hypertension by invasive hemodynamic measurements in patients undergoing TVR or TVr. (A) Total cohort, (B) TVR vs TVr, and (C) isolated TVR/TVr vs concomitant TVR/TVr. PH, pulmonary hypertension; TVr, tricuspid valve repair, TVR, tricuspid valve replacement.
Figure 3.
Distribution of pulmonary vascular hemodynamic parameters in patients undergoing TVR or TVr. (A) Mean pulmonary artery pressures, (B) pulmonary vascular resistance, (C) pulmonary artery compliance, and (D) pulmonary artery effective elastance. TVr, tricuspid valve repair; TVR, tricuspid valve replacement; WU, Wood units.
On comparing invasive hemodynamics between those who underwent TVR vs TVr, patients who underwent TVr more often had PH, especially combined pre- and postcapillary PH and isolated postcapillary PH (Fig. 2B), with higher PA pressures (mPAP: 33 ± 10 vs 27 ± 8 mm Hg, P < 0.01), and PCWP (21 ± 8 vs 17 ± 7 mm Hg, P = 0.02), and a trend to a lower PAC (2.1 [1.3-3.0] vs 2.5 [1.8-3.7] mL/mm Hg, P = 0.07) and a higher Ea (0.9 [0.6-1.4] vs 0.7 [0.6-0.9] mm Hg/mL, P = 0.07), compared with patients who underwent TVR (Table 1).
On comparing invasive hemodynamics between those who underwent isolated TVR/TVr vs concomitant TVR/TVr, as expected, patients who underwent concomitant TVR/TVr more often had combined pre- and postcapillary and isolated postcapillary PH and less often had precapillary PH (Fig. 2C). Patients who underwent concomitant TVR/TVr had higher PA pressures (mPAP: 32 ± 10 vs 27 ± 7 mm Hg, P = 0.03), higher TPG (12 ± 6 vs 9 ± 4, P = 0.02), higher Ea (0.9 [0.6-1.4] vs 0.7 [0.6-0.9] mm Hg/mL, P = 0.04), higher PVR (2.7 [1.8-4.2] vs 2.0 [1.5-2.9] WU, P = 0.07), albeit not statistically significant (2.7 [1.8-4.2] vs 2.0 [1.5-2.9] WU, P = 0.07) and lower PAC (1.9 [1.3-3.6] vs 2.6 [2.2-3.5], P = 0.05) compared with patients who underwent isolated TVR/TVr (Table 1).
Effect of pulmonary vascular hemodynamics on outcomes after TVR/TVr
There were 19 deaths (22.4%) during a median follow up of 6.3 years. Of the total 19 deaths, 7 were cardiac related (37%), 9 were noncardiac related (47%), and cause of death was unclear in 3 patients (16%). For the total study cohort, the 30-day survival was 98%, 1-year survival was 95%, 3-year survival was 91%, and 5-year survival was 87%. The 30-day, 1-year survival, 3-year survival, and 5-year survival in patients with no PH was 100%/100%/100%/80%, isolated postcapillary PH was 100%/96%/96%/91%, combined pre- and postcapillary PH was 94%/91%/88%/88%, and prcapillary PH was 100%/100%/82%/82%.
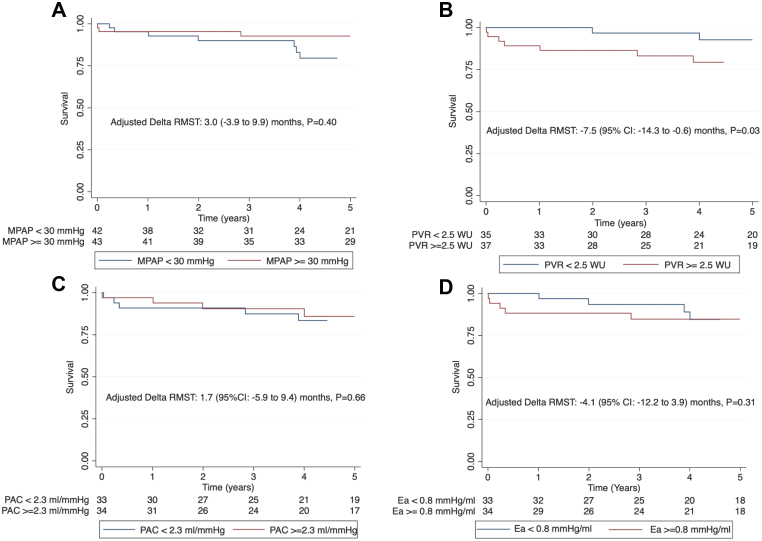
On comparison of RMST based on various hemodynamic parameters, patients with PVR ≥ 2.5 WU had lower RMST over 5 years compared with those with PVR < 2.5 WU (survival time 51 vs 58 months, unadjusted ΔRMST: –7.3 (95% confidence interval [CI] ,–14.1 to –0.6 months, P = 0.034, and adjusted ΔRMST: –7.5 [–14.3 to –0.6] months, P = 0.032) (Table 2, Fig. 4). There was no difference in RMST over 5 years when patients were categorized by the median values of mPAP, PAC, and Ea (Table 2, Fig. 4).
Table 2.
Unadjusted and adjusted difference in restricted mean survival time after TVR/TVr, based on pulmonary vascular hemodynamics
| Characteristics | Survival time, months | Unadjusted ΔRMST, months (95% CI) | P value | Adjusted ΔRMST, months (95% CI)† | P value |
|---|---|---|---|---|---|
| Mean PA pressure (≥ 30 mm Hg vs < 30 mm Hg) | 57 vs 54 | 2.8 (–3.4 to 8.9) | 0.38 | 3.0 (–3.9 to 9.9) | 0.40 |
| PVR (≥ 2.5 WU vs < 2.5 WU) | 51 vs 58 | –7.3 (–14.1 to –0.6) | 0.034∗ | –7.5 (–14.3 to –0.6) | 0.032∗ |
| PAC (≥ 2.3 mL/mm Hg vs < 2.3 mL/mm Hg) | 55 vs 53 | 1.6 (–6.1 to 9.3) | 0.68 | 1.7 (–5.9 to 9.4) | 0.66 |
| Ea (≥ 0.8 mm Hg/mL vs < 0.8 mm Hg/mL) | 52 vs 56 | –3.9 (–11.4 to 3.6) | 0.31 | –4.1 (–12.2 to 3.9) | 0.31 |
CI, confidence interval; Δ, delta; Ea, pulmonary artery effective elastance; PA, pulmonary artery; PAC, pulmonary artery compliance; PVR, pulmonary vascular resistance; RMST, restricted mean survival time; TVr, tricuspid valve repair; TVR, tricuspid valve replacement; WU, Wood units.
Statistically significant P value of <0.05.
Adjusted for type of tricuspid valve surgery (replacement vs repair), and isolated TVR/TVr vs concomitant TVR.
Figure 4.
Survival in patients undergoing TVR or TVr categorized by median values of invasive hemodynamic measures of pulmonary hypertension. (A) Mean pulmonary artery pressure, (B) pulmonary vascular resistance, (C) pulmonary artery compliance, and (D) pulmonary artery elastance. Adjusted restricted mean survival time (RMST) was 7.5 months lower over 5 years in patients with PVR ≥ 2.5 WU than those with PVR < 2.5 WU. Ea, pulmonary artery elastance; mPAP, mean pulmonary artery pressure; PAC, pulmonary artery compliance; PVR, pulmonary vascular resistance; TVr, tricuspid valve repair; TVR, tricuspid valve replacement; WU, Wood units.
On sensitivity analysis, we compared RMST based on whether patients had concomitant mitral or aortic valve surgery (n = 49) or not (n = 37). PVR > 2.5 WU was associated with lower RMST in patients who underwent TVR/TVr with concomitant mitral or aortic valve surgery. We saw a similar trend in patients who had TVR/TVr without concomitant mitral or aortic valve surgery, but this was not statistically significant (Supplemental Table S2).
RV function and PH
A subset of patients (n = 33) had preoperative RVFAC measurements. The mean RVFAC was 31 ± 12%. There was no difference in RVFAC between those with and without PH (31 ± 13 vs 29 ± 8 %, P = 0.77). Similarly, 34 of the 86 patients had preoperative RV basal diameter measurements. The mean RV basal diameter was 5.3 ± 1.0 cm, and there was no difference between those with and without PH (5.3 ± 1.1 vs 5.2 ± 0.3 cm, P = 0.83). There was no association between invasive hemodynamic measures of RV function including right atrial pressure, RV end diastolic pressure, cardiac index, and RV stroke work and postoperative survival after surgical TVR or TVr (Supplemental Fig. S1)
Discussion
Our study focuses on hemodynamic parameters in all patients who underwent TVR/TVr to identify the role of invasive hemodynamic measures of PH for risk stratification. The main findings are as follows: The majority of patients (86%) who underwent surgical TVR/TVr had PH, with combined pre- and postcapillary PH being the most common type; the severity of PH in patients who had surgical TVR/TVr was mild; and patients with PVR ≥ 2.5 WU lived, on average, 7.5 fewer months in 5-year follow-up than those with PVR < 2.5 WU after undergoing TVR/TVr.
The prevalence of TR is increasing with concomitant atrial fibrillation, as well as with intracardiac devices, and the number of tricuspid valve interventions has more than doubled over a 10-year period.19, 20, 21 Previous studies showed that moderate or greater TR is associated with poor prognosis.22 Yet, an extremely large number of these patients with moderate or severe TR are treated conservatively with medical management because of lack of clinical trials, and thus lack of guidelines establishing objective criteria for surgery, and based on studies that indicate high mortality (5% to 50%) with TVR.23,24 Very few studies have been able to elucidate objective clinical, hemodynamic, or imaging-guided parameters to risk stratify patients with TR. A single-centre echocardiography-based study comprising 86 patients showed that severe symptoms at time of surgery, New York Heart Association (NYHA) functional class III, and elevated PAPs unfavourably affected the long-term results.24 Topilsky and colleagues showed that the only echocardiographic parameter that independently predicted poor outcomes is the RV index of myocardial performance (RIMP), which is a global estimate of both systolic and diastolic function of the RV measured using Doppler echocardiography.25 However, RIMP is not done routinely. These studies suggest that earlier detection, patient selection, and subsequent intervention for TR might reduce adverse outcomes.
PH commonly occurs in patients undergoing TVR/TVr,7, 8, 9, 10 which we confirm in our cohort. The prevalence of PH in our cohort was 86%. Despite the common occurrence of PH in patients undergoing TVR/TVr, there is lack of invasive hemodynamic characterization of PH. Previous studies describe PH in patients undergoing TVR/TVr, predominantly based on estimated systolic PAP from echocardiograms,7,8,10 However, echocardiography can significantly underestimate or overestimate PAPs.26 In addition, echocardiography lacks the ability to measure PCWP and cardiac output and thereby assess PVR, PAC, and Ea. Our study is unique, as we provide detailed hemodynamic characterization of PH in patients undergoing TVR/TVr. The high prevalence of PH in our cohort, especially combined pre- and postcapillary PH, is likely because of TVR/TVr being more often performed as a concomitant procedure to either mitral or aortic valve surgery. The majority of patients in our analysis underwent TVR/TVr in conjunction with mitral valve or aortic valve surgeries (56%), which is in alignment with class I guidelines. Mitral and aortic valve disease often causes PH caused by elevated PCWP.27,28 Interestingly, in our cohort, even in patients who underwent isolated TVR/TVr, isolated postcapillary and combined pre-and postcapillary PH was more common than precapillary PH. We suspect that this is predominantly because of residual PH from left-sided valvular heart disease as the majority of patients who underwent isolated TVR/TVr in our cohort had previous mitral or aortic valve replacement (18 of 34 patients). Indeed, residual PH after aortic or mitral valve replacement is common and is associated with poor prognosis.29,30
The severity of PH in patients undergoing TVR/TVr in our cohort was mild, with an mPAP of 30 ± 10 mm Hg and a median PVR of 2.5 (IQR: 1.5-3.9) WU. This mild severity of PH in our cohort was likely caused by selection bias and was a confounder and yielded a mean survival time that is higher than reported by other papers.31Patients undergoing TVR/TVr concomitant with other cardiac surgeries in our cohort had higher PAPs compared with those undergoing isolated TVR/TVr. Consistent with this, patients undergoing TVr had more severe PH when compared with patients undergoing TVR, as TVr is more often performed concomitantly with other cardiac surgeries. Age was the only clinical determinant of PH in our cohort. Although mPAP was higher in patients undergoing TVr (vs TVR) and in those undergoing TVR/TVr concomitantly with other cardiac surgeries (vs isolated TVR/TVr), the number of patients having mPAP >20 mm Hg was not different between these groups.
De Meester et al. reported invasive hemodynamics in 65 patients undergoing isolated TVR/TVr.9 There are several key differences between our study and the study from De Meester and colleagues. First, the prevalence of PH was higher in our cohort compared with the study by De Meester et al. (77% vs 51% of patients had PH, defined as invasively measured mPAP ≥ 25 mm Hg). Second, patients in our cohort had relatively higher mPAP compared with the patients in the report by De Meester et al. (30 ± 10 mm Hg vs 24 ± 9 mm Hg). The difference in the prevalence and the severity of PH between the 2 studies may be related to varied study cohorts. Our study includes both patients who had isolated TVR/TVr as well as those who had TVR/TVr concomitantly with other cardiac surgeries. In contrast, De Meester et al. studied only patients undergoing isolated TVR/TVr. Nationally, of all patients who underwent TVR/TVr, isolated procedures are a minority (38% isolated TVR and 7% isolated TVr). Therefore, our data are more comparable with national trends.4 Finally, De Meester et al. did not provide detailed pulmonary vascular hemodynamic characterization, which our study does.
At present, TVR/TVr is not recommended in patients with severe PH.2,3 However, there is no clear consensus on the threshold criteria for the severity of PH that is prohibitive of TVR/TVr. Multiple previous studies have associated the presence of PH with increased mortality after TVR/TVr, but they were predominantly based on estimated RV systolic pressure from the echocardiogram,7,8,10 which we postulate may not be as accurate as hemodynamic characterization. De Meester et al. showed that elevated invasive mPAP is associated with poor outcomes after TVR/TVr in patients who are younger than 59 years of age.9 Every 1 mm Hg increase in mPAP is associated with a 9% increase in mortality. Our study adds to this known literature and shows that even a mild elevation in PVR ≥ 2.5 is associated with reduced survival time. In our analysis, patients with PVR ≥ 2.5 WU, on average, lived 7.5 fewer months than those with PVR <2.5 WU when adjusted for type of tricuspid valve surgery and concomitant surgery. Our data suggest that patients should undergo TVR/TVr before they develop precapillary PH and their PVR increases owing to the potential survival benefit. Furthermore, in our data, only PVR was associated with poor outcomes and not the PAP. Taken together, these data suggest that invasive hemodynamic data is essential to risk stratify patients undergoing tricuspid valve surgery.
Our findings must be validated in larger, prospective, multicentre studies before translation into clinical practice. In addition, studies elucidating the role of RV function in relation to PH in TR are essential to risk stratify patients further. When we analyzed our data, we did not see any association between invasive hemodynamic measures of RV function and postoperative survival after surgical TVR or TVr (Supplemental Fig. S1). A better understanding of the effect of PH on outcomes in patients undergoing transcatheter TVR or TVr, which are becoming increasingly common,32 is also necessary in the future.
Study limitations
This is a single-centre observational study. Our sample size was small owing to single-centre design and lack of invasive hemodynamic data for nearly one-half our initially identified patient cohort. However, there was no significant difference in the survival characteristics between those with and without invasive hemodynamic data (Supplemental Fig. S2). We studied both patients who had TVr as well as TVR, but there was relative homogeneity with no significant difference in clinical characteristics between those who underwent TVR vs TVr (Table 1). Our study did not include preoperative echocardiographic data on RV function in all patients because of the lack of RV-focused images. Likewise, patients in our cohort did not undergo echocardiography as well as right-heart catheterization postoperatively in a systematic manner to assess residual TR and PH, and its effect on outcomes. Although we adjusted for concomitant vs isolated TVR/TVr and replacement vs repair, because of the small number of events in our cohort, we were unable to adjust for other covariates to assess the independent effect of PH on postoperative mortality. Furthermore, owing to the observational nature of our study, we cannot prove causality between PH and postoperative mortality. Finally, our study included only a small number of patients who had isolated TVR or TVr, but our data are more comparable with national trends. Despite these limitations, we consider that the observations from this detailed hemodynamic characterization of PH may help in risk stratifying patients who undergo TVR/TVr.
Conclusions
PH is common in patients undergoing TVR or TVr. Combined pre- and postcapillary PH is the most common type of PH, followed by isolated postcapillary PH. Precapillary PH is uncommon. Even a mild increase in PVR (> 2.5 WU) is associated with reduced survival time over 5 years compared with those who have PVR < 2.5 WU.
Funding Sources
K.W.P. is funded by NIH K08 HL140100, the Cardiovascular Medical Research and Education Fund, a Lillehei Heart Institution Cardiovascular Seed Grant, and the United Therapeutics Jenesis Award. S.L.A. is funded by Canada Foundation for Innovation (229252 and 33012), a Tier 1 Canada Research Chair in Mitochondrial Dynamics and Translational Medicine (950-229252), the Queens Cardiopulmonary Unit (QCPU), and a grant from the William J. Henderson Foundation. S.Z.P. is funded by NIH T32 HL144472, a University of Minnesota Clinical and Translational Science award (NIH UL1 TR0029494) and a University of Minnesota Medical School Academic Investment Educational Program Grant. T.T. is funded by the Cardiovascular Medical Research and Education Fund.
Disclosures
K.W.P. served on an advisory board for Actelion. T.T. has served on an advisory board for Actelion, Gilead, and Altavant. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was approved by the University of Minnesota Institutional Review Board. The research reported has adhered to the relevant ethical guidelines.
See page 496 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca and at https://doi.org/10.1016/j.cjco.2020.12.008.
Supplementary Material
References
- 1.Park S., Suri R.M., Enriquez-Sarano M. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Img. 2014;7:1185–1194. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 3.Sorajja P., Erwin J.P., O’Gara P.T. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hallak A., Berzingi C.O., Alkhouli M., Hijazi M., Aljohani S., Alqahtani F. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuge O., Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg. 2006;132:1258–1261. doi: 10.1016/j.jtcvs.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 6.De Meester P., De Cock D., Van De Bruaene A. Additional tricuspid annuloplasty in mitral valve surgery results in better clinical outcome. Heart. 2015;101:720–726. doi: 10.1136/heartjnl-2014-306801. [DOI] [PubMed] [Google Scholar]
- 7.Civelek A., Ak K., Akgün S., Isbir S.C., Arsan S. Tricuspid valve replacement: an analysis of risk factors and outcomes. Thorac Cardiovasc Surg. 2008;56:456–460. doi: 10.1055/s-2008-1038730. [DOI] [PubMed] [Google Scholar]
- 8.Buzzatti N., Iaci G., Taramasso M. Long-term outcomes of tricuspid valve replacement after previous left-side heart surgery. Eur J Cardiothorac Surg. 2014;46:713–719. doi: 10.1093/ejcts/ezt638. discussion 9. [DOI] [PubMed] [Google Scholar]
- 9.De Meester P., Van De Bruaene A., Voigt J.U., Herijgers P., Budts W. Outcome and determinants of prognosis in patients undergoing isolated tricuspid valve surgery: retrospective single center analysis. Int J Cardiol. 2014;175:333–339. doi: 10.1016/j.ijcard.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Färber G., Tkebuchava S., Dawson R.S. Minimally invasive, isolated tricuspid valve redo surgery: a safety and outcome analysis. Thorac Cardiovasc Surg. 2018;66:564–571. doi: 10.1055/s-0038-1627452. [DOI] [PubMed] [Google Scholar]
- 11.LaFarge C.G., Miettinen O.S. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahapatra S., Nishimura R.A., Sorajja P., Cha S., McGoon M.D. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 14.Tampakakis E., Shah S.J., Borlaug B.A. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail. 2018;11:1–9. doi: 10.1161/CIRCHEARTFAILURE.117.004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Royston P., Parmar M.K.B. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregson J., Sharples L., Stone G.W., Burman C.-F., Öhrn F., Pocock S. Nonproportional hazards for time-to-event outcomes in clinical trials. JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2102–2112. doi: 10.1016/j.jacc.2019.08.1034. [DOI] [PubMed] [Google Scholar]
- 18.Kim D.H., Uno H., Wei L.J. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2:1179–1180. doi: 10.1001/jamacardio.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paniagua D., Aldrich H.R., Lieberman E.H., Lamas G.A., Agatston A.S. Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemakers leads. Am J Cardiol. 1998;82:1130–1132. doi: 10.1016/s0002-9149(98)00567-0. [DOI] [PubMed] [Google Scholar]
- 20.Vassileva C.M., Shabosky J., Boley T., Markwell S., Hazelrigg S. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–1049. doi: 10.1016/j.jtcvs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Fender E.A., Zack C.J., Nishimura R.A. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart. 2018;104:798–806. doi: 10.1136/heartjnl-2017-311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nath J., Foster E., Heidenreich P.A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Do Q.B., Pellerin M., Carrier M. Isolated tricuspid valve replacement: long-term results. Arch Mal Coeur Vaiss. 2000;93:1119–1124. [PubMed] [Google Scholar]
- 24.Litwiński P., Kołsut P., Sitko T. Results and factors associated with adverse outcome after tricuspid valve replacement. Kardiologia Polska. 2018;76:731–739. doi: 10.5603/KP.a2018.0026. [DOI] [PubMed] [Google Scholar]
- 25.Topilsky Y., Khanna A.D., Oh J., Nishimura R.A. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 26.Rich J.D., Shah S.J., Swamy R.S., Kamp A., Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan C.J., Wenaweser P., Ceylan O. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: insights from the new proposed pulmonary hypertension classification. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002358. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro P.A., al Zaibag M., Abdullah M. Pulmonary artery pressure and pulmonary vascular resistance before and after mitral balloon valvotomy in 100 patients with severe mitral valve stenosis. Am Heart J. 1993;125:1110–1114. doi: 10.1016/0002-8703(93)90121-o. [DOI] [PubMed] [Google Scholar]
- 29.Bermejo J., Yotti R., Garcia-Orta R. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J. 2018;39:1255–1264. doi: 10.1093/eurheartj/ehx700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeder M.T., Weber L., Buser M. Pulmonary Hypertension in aortic and mitral valve disease. Front Cardiovasc Med. 2018;5:40. doi: 10.3389/fcvm.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong W.K., Chen S.W., Chou A.H. Late outcomes of valve repair versus replacement in isolated and concomitant tricuspid valve surgery: a nationwide cohort study. J Am Heart Associ. 2020;9 doi: 10.1161/JAHA.119.015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asmarats L., Puri R., Latib A., Navia J.L., Rodes-Cabau J. Transcatheter tricuspid valve interventions: landscape, challenges, and future directions. J Am Coll Cardiol. 2018;71:2935–2956. doi: 10.1016/j.jacc.2018.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.