Abstract
STUDY QUESTION
What are the sperm and egg donor rejection rates after expanded carrier screening (ECS)?
SUMMARY ANSWER
Using an ECS panel looking at 46/47 genes, 17.6% of donors were rejected.
WHAT IS KNOWN ALREADY
The use of ECS is becoming commonplace in assisted reproductive technology, including testing of egg and sperm donors. Most national guidelines recommend rejection of donors if they are carriers of a genetic disease. If the use of ECS increases, there will be a decline in the number of donors available.
STUDY DESIGN, SIZE, DURATION
A review of the current preconception ECS panels available to donors was carried out through an online search. The genetic testing results of donors from Cryos International were analysed to determine how many were rejected on the basis of the ECS.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Data on gamete donors and their carrier status was provided by Cryos International, who screen donors using their own bespoke ECS panel. The ECS panels identified through the review were compared to the Cryos International panel and data.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 16 companies and 42 associated ECS panels were reviewed. There were a total of 2673 unique disorders covered by the panels examined, with a mean of 329 disorders screened. None of these disorders were common to all panels. Cryos International screen 46 disorders in males and 47 in females. From 883 candidate donors, 17.6% (155/883) were rejected based on their ECS result. Carriers of alpha-thalassaemia represented the largest proportion of those rejected (19.4%, 30/155), then spinal muscular atrophy (15.5%, 24/155) and cystic fibrosis (14.8%, 23/155).
LIMITATIONS, REASONS FOR CAUTION
Panel information was found on company websites and may not have been accurate.
WIDER IMPLICATIONS OF THE FINDINGS
This study highlights the need for consistent EU regulations and guidelines that allow genetic matching of gamete donors to their recipients, preventing the need to reject donors who are known carriers. A larger ECS panel would be most beneficial; however, this would not be viable without matching of donors and recipients.
STUDY FUNDING/COMPETING INTEREST(S)
No specific funding was obtained. J.C.H. is the founder of Global Women Connected, a platform to discuss women’s health issues and the Embryology and PGD Academy, who deliver education in clinical embryology. She has been paid to give a lecture by Cryos in 2019. A-B.S. is an employee of Cryos International.
TRIAL REGISTRATION NUMBER
N/A
Keywords: donor, egg donation, sperm donation, genetic testing, expanded carrier screening
Introduction
Carrier genetic testing was introduced in the 1970s to determine whether a person was a carrier of a genetic disorder to prevent the transmission of an autosomal recessive or X-linked disease (Kraft et al., 2019; Rowe and Wright, 2019). Testing was originally only used for specific ethnic groups where there is a higher frequency of autosomal recessive diseases, such as Tay–Sachs disease in the Ashkenazi Jewish populations (Harper et al., 2018).
In 2010, expanded carrier screening (ECS) was developed that offers a more cost-effective alternative to carrier genetic testing, increasing preconception and prenatal care and allowing the testing of a large number of genes (Srinivasan et al., 2010). The addition of genes to ECS panels is simple and relatively inexpensive, resulting in the production of commercial ECS panels that screen for hundreds of disorders (Chokoshvili et al., 2017; Chokoshvili et al., 2018). ECS is used for low-risk populations to identify carriers of single gene disorders that are not on the typical guidelines and is offered regardless of ancestry and ethnicity (Bajaj and Gross, 2014; Edwards et al., 2015; Dungan, 2018; Harper et al., 2018).
In 2011, it was suggested that targeted next-generation sequencing could be used within ECS, testing for a further 448 severe childhood-onset disorders (Bell et al., 2011; Grody et al., 2013). It was recommended by the European Society of Human Genetics that ECS panels should prioritize genes that are associated with severe childhood-onset disorders (Henneman et al., 2016). ECS is marketed to couples who are considering having children.
Data suggest that everyone will carry at least one pathogenic variant that is associated with severe recessive childhood diseases; therefore, the more genes and associated diseases that are tested through ECS, the more carriers will be found (Bell et al., 2011; Silver et al., 2016).
In 2010, the main genetic testing of sperm donors was for cystic fibrosis, as well as chromosome analyses and haemoglobin evaluations, with further testing for people of specific populations and ethnicities (Sims et al., 2010). There has been much debate on whether gamete donors should undergo ECS (Mertes et al., 2018; Pennings, 2020). Some countries exclude donors who are carriers so the more genes tested, the less donors would be available. One study found that of 143 donors tested, 41% of them were carriers of one or more conditions (Urbina et al., 2017).
Cryos International, founded in 1981, is the largest sperm bank in the world, supplying frozen donor sperm and eggs to over 100 countries (Cryos International, 2019a, b). They have used a bespoke ECS panel which screens males for 46 genetic disorders and females for 47.
The aims of this study were to carry out a preconception ECS panel review and to analyse the effects of ECS testing on gamete donors from a large egg and sperm bank.
Materials and methods
ECS donor panel review
Data collection
An online search was performed using Google search engine (www.google.co.uk). The search was carried out on the 30th June 2020 in 1 day, using the same computer. Advanced Google search (https://www.google.co.uk/advanced_search) was used to create this query: all these words: ‘donor carrier screening’; any of these words: ‘expanded’, ‘universal’, ‘next-generation’, ‘sequencing’, ‘panel’, ‘genetic’, ‘testing’, ‘preconception’, ‘prenatal’, ‘reproductive’; language set to English. All pages and entries, including advertisements, that were produced using this search were investigated. Panels that were advertised for a specific ethnicity were excluded from the review.
Panel analysis
All of the websites and preconception panels identified were searched for the company country of origin, the number of genes and subsequent number of inherited disorders covered in the panel, cost of one test and whether the panels are recommended for the genetic testing of sperm and egg donors. If one company offered multiple panels, all were included in the analysis. When a list of diseases and genes were unavailable, the companies were contacted via email to request further information.
All genes and disorders covered by all panels were analysed and compared. Due to the large variation between the names of disorders and genes across the panels, a comprehensive search using the database Online Mendelian Inheritance in Man® (OMIM) was carried out. This search prevented duplication of disorders and genes that go by different names. The disorders were identified using their unique phenotype MIM numbers, and the name of the disorder represented in the table reflects the preferred title listed in OMIM.
The disorder inheritance patterns were also noted; however, there were some discrepancies when comparing the panels to OMIM, therefore, the OMIM inheritance patterns were used.
Cryos International donor data
Data were received from Cryos International regarding their sperm and egg donors who had undergone genetic testing using Cryos’ bespoke ECS panels ‘CGT 46 male’ and ‘CGT 47 female’. The data included the donor ID allocated when initially donated, sex, department where donation occurred, the result of ECS, and if they were a positive carrier, and which gene was affected.
The variables analysed included
The number of gamete donors accepted and rejected;
The carrier status of the rejected donors; and
The impact of the sex of the donor.
Panel review and donor data analysis
The panels used for gamete donors by Cryos International were compared with the panels identified in the review. The positive carriers found using Cryos International ECS panels were compared to all panels to determine whether any carriers would have been missed if other panels had been used in their place.
Results
ECS donor panel review
Companies offering ECS
Through the online search, a total of 18 pages and 196 results, including advertisements, were identified. From the 196 results, 17 companies were found to be offering ECS. One company, however, did not have their panel available even after contact, therefore was excluded from the review. This resulted in 16 companies and 42 associated panels being evaluated. These companies were
qGenomics
Genomic Diagnostics
Igenomix
Natera
Eurofins Genoma
Fulgent Genetics
VCGS
Centogene
Thermo Fisher
Virtus Diagnostics
Sema4
Integrated Genetics
Myriad Women’s Health
Invitae
GenPath Diagnostics
Progenity
The companies and panels were anonymized and summarized in Supplementary Table SI. Eight companies had more than one ECS panel on offer. Six companies and their nine associated panels did not mention their recommendation for gamete donors. Company 16 had one panel recommended for both sperm and egg donors (panel 16G) and one only for egg donors (panel 16E). All other panels are recommended for gamete donor screening. The most extensive panel was by company 16, panel 16A; with 1577 genes and more than 1600 disorders screened. It was found that 50% of the companies were based in the USA. The price was not available for all panels, but the price for screening between 3 and 553 genes ranged between $345 and $750.
Comprehensive review of the panels
A summary of all unique diseases and genes found across all 42 panels can be found in Supplementary Table SII. It includes the disease name preferred by OMIM, the gene associated, phenotype MIM number and the inheritance pattern for all panels. There were a total of 2673 unique disorders covered by combining all 42 panels, with a mean of 329 disorders screened.
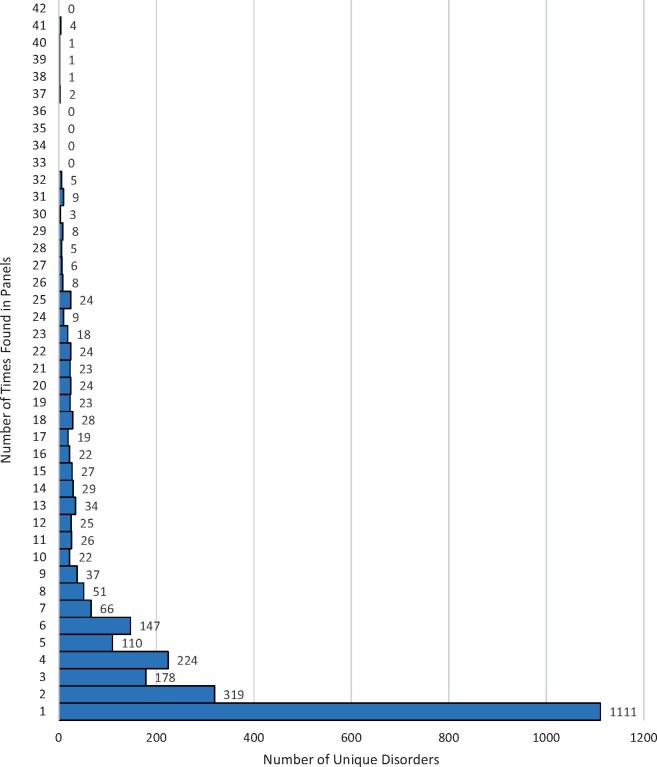
Figure 1 shows the number of times each of the 2673 unique disorders were found across the 42 panels. It was found that there were no diseases that were present in all 42 panels. Screening for cystic fibrosis (gene CFTR) and spinal muscular atrophy types 1, 2 and 3 (gene SMN1) were offered by 41 of the 42 panels (97.6%), screening for spinal muscular atrophy type 4 (gene SMN1) was offered by 40 panels (95.2%), screening for fragile X syndrome (gene FMR1) was offered by 39 panels (92.9%) and screening for sickle cell anaemia and beta-thalassaemia (gene HBB) was offered by 37 panels (88.1%).
Figure 1.
The number of times unique disorders were found across the panels. The 2673 unique disorders found according to the number of times they were used in the 42 different panels identified.
Four panels were the same: company 4, panel 4A; company 6, panel 6A; company 8, panel 8A; and company 10, panel 10A. All four panels only offered screening for three disorders: cystic fibrosis, fragile X syndrome and spinal muscular atrophy.
Figure 2 illustrates the inheritance pattern of the 2673 unique disorders found. There were 1711 disorders (64.0%) that were inherited in an autosomal recessive pattern. The next most common inheritance was autosomal dominant with 298 disorders (11.2%).
Figure 2.
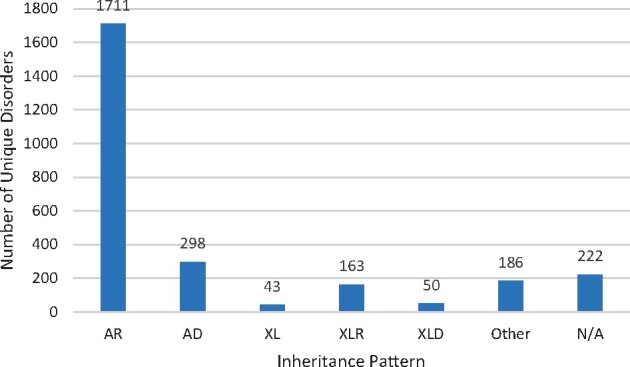
The number of unique disorders according to inheritance pattern. The 2673 unique disorders found according to their inheritance pattern as listed in OMIM. AR, autosomal recessive; AD, autosomal dominant; XL, X-linked; XLR, X-linked recessive; XLD, X-linked dominant. ‘Other’ refers to all other inheritance patterns and combinations as outlined in Supplementary Table SII. N/A is where the inheritance pattern was not available.
Cryos International donor data
ECS bespoke panels
The genes, related diseases tested for in panels CGT 46 and CGT 47, and the number of donors carrying each mutation are shown in Table I.
Table I.
Disorders and genes tested by Cryos International.a
| Disease | Genes tested | Number of Cryos donors identified as carriers |
|---|---|---|
| Abetalipoproteinemia | MTTP | 0 |
| Alpha-thalassaemia | HBA | 30 |
| Alport syndrome | COL4A3, COL4A4 | 1, 1 |
| Arthrogryposis | SLC35A3 | 0 |
| Bardet–Biedl syndrome | BBS1, BBS10, BBS2 | 2, 2, 0 |
| Beta-thalassaemia/sickle cell disease | HBB | 8 |
| Bloom syndrome | BLM | 1 |
| Canavan’s disease | ASPA | 5 |
| Carnitine palmitoyl transferase II deficiency | CPT2 | 1 |
| Carnitine transporter deficiency | SLC22A5 | 6 |
| Congenital amegakaryocytic thrombocytopenia | MPL | 4 |
| Congenital disorder of glycosylation Type 1a | PMM2 | 8 |
| Cystic fibrosis | CFTR | 23 |
| Dyskeratosis congenita | RTEL1 | 1 |
| Ehlers–Danlos type VIIC | ADAMTS2 | 0 |
| Familial dysautonomia | IKBKAP | 0 |
| Familial hyperinsulinism | ABCC8 | 0 |
| Fanconi anaemia type C | FANCC | 1 |
| b Fragile X syndrome | FMR1 | 2 |
| Galactosemia | GALT | 4 |
| Gaucher’s disease | GBA | 4 |
| Glycogen storage disease type 1a | G6PC | 2 |
| Joubert syndrome 2 | TMEM216 | 0 |
| Maple syrup urine disease type 1B | BCKDHB | 0 |
| Maple syrup urine disease type 3 | DLD | 0 |
| Mucolipidosis type IV | MCOLN1 | 1 |
| Multiple sulfatase deficiency | SUMF1 | 0 |
| Nemaline myopathy | NEB | 0 |
| Niemann–Pick type A | SMPD1 | 1 |
| Phosphoglycerate dehydrogenase deficiency | PHGDH | 1 |
| Polycystic kidney disease | PKHD1 | 4 |
| Retinitis pigmentosa | DHDDS | 1 |
| Smith–Lemli–Opitz syndrome | DHCR7 | 8 |
| Spinal muscular atrophy | SMN1 | 24 |
| Tay–Sachs disease | HEXA | 1 |
| Tyrosemia type 1 | FAH | 5 |
| Usher syndrome type IF | PCDH15 | 0 |
| Usher syndrome type III | CLRN1 | 1 |
| Walker–Warburg syndrome | FKTN | 0 |
| Wilson disease | ATP7B | 5 |
| Zellweger syndrome | PEX1, PEX2, PEX6 | 4, 0, 1 |
The disorders and genes tested for by Cryos International in the panel CGT 46 male and CGT 47 female and the donors who tested positive.
The additional gene tested for in CGT 47 female.
Candidate donors
Information about 883 candidate gamete donors were received from Cryos International. These were all the donors who had been tested using these panels. These data included the donor ID, sex, department donated at and carrier status. Of these candidate gamete donors, 84.6% (747/883) were male and 15.4% (136/883) were female.
Of the total 883 candidate donors, 82.5% (728/883) were found not to be a carrier for any of the genes tested. Of these donors, 84.9% (618/728) were male and 15.1% (110/728) were female. Table II summarizes the ECS results from testing the Cryos donors.
Table II.
The number of donors tested using Cryos International’s ECS panel and the number of donors who tested positive for one or more mutations.
| Number of donors (%) | |
|---|---|
| Total number of donors tested | 883 |
| Candidates with no mutations | 728 (82.4) |
| Candidates with mutations: | 155 (17.6) |
| 1 mutation | 149 |
| 2 mutations | 5 |
| 3 mutations | 0 |
| 4 mutations | 1 |
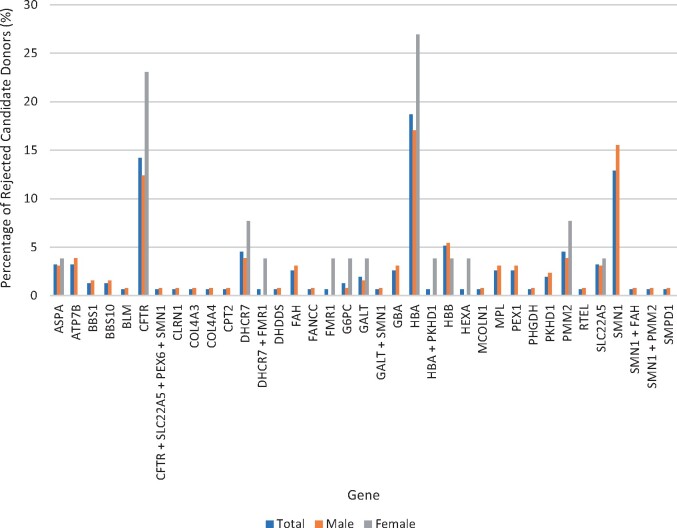
The percentage of total male and female rejected candidate donors according to the genes they were found to be carriers of can be seen in Fig. 3. For total rejected candidate donors, 19.4% (30/155) were carriers of HBA, 15.5% (24/155) of SMN1 and 14.8% (23/155) of CFTR. For male rejected candidate donors, 18.6% (24/129) were carriers of SMN1, 17.1% (22/129) carriers of HBA and 13.2% (17/129) of CFTR. For female rejected candidate donors, 30.8% (8/26) were carriers of HBA, 23.1% (6/26) of CFTR and 11.5% (3/26) of DHCR7.
Figure 3.
Genes of rejected candidate donors. The genes of the rejected candidate donors who were found to be carriers through ECS. Percentage of total rejected candidate donors (blue), percentage of male rejected candidate donors (orange) and percentage of female rejected candidate donors (grey) corresponding to the gene they screened positive. ASPA, Canavan’s disease; ATP7B, Wilson disease; BBS1/BBS10, Bardet–Biedl syndrome; BLM, Bloom syndrome; CFTR, cystic fibrosis; CLRN1, Usher syndrome type III; COL4A3/COL4A4, Alport syndrome; CPT2, Carnitine palmitoyl transferase II deficiency; DHCR7, Smith–Lemli–Opitz syndrome; DHDDS, retinitis pigmentosa; FAH, Tyrosemia type 1; FANCC, Fanconi anaemia type C; FMR1, Fragile X syndrome; G6PC, glycogen storage disease type 1a; GALT, galactosaemia; GBA, Gaucher’s disease; HBA, alpha-thalassaemia; HBB, beta-thalassaemia/sickle cell disease; HEXA, Tay–Sachs disease; MCOLN1, mucolipidosis type IV; MPL, congenital amegakaryocytic thrombocytopenia; PEX1/PEX6, Zellweger syndrome; PHGDH, phosphoglycerate dehydrogenase deficiency; PKHD1, polycystic kidney disease; PMM2, congenital disorder of glycosylation type 1a; RTEL, dyskeratosis congenita; SLC22A5, carnitine transporter deficiency; SMN1, spinal muscular atrophy; SMPD1, Niemann–Pick type A.
Panel review and donor data analysis
A tabulated comparison of the 16 companies and 42 associated panels, identified by the ECS donor panel review, and the Cryos International bespoke panels, ‘CGT 46 male’ and ‘CGT 47 female’, can be found in Supplementary Table SIII. It was found that there were no diseases present in all panels, as found in the initial review. There were seven panels from six companies that would detect all diseases present in the Cryos International bespoke panels (Table III).
Table III.
The companies and associated panels that cover all the disorders and genes tested in the Cryos International panels and the number of genes the panels screen for.
| Company | Panel | Number of genes tested |
|---|---|---|
| 3 | 3E | 274 |
| 5 | 5A | 283 |
| 6 | 6D | >280 |
| 7 | 7A | 332 |
| 9 | 9C | 301 |
| 13 | 13C | 401 |
| 13 | 13D | 410 |
Discussion
The aims of this study were to carry out a preconception ECS panel review and to analyse the effects of ECS testing on gamete donors from a large egg and sperm bank.
ECS donor panel review
From the review, 16 companies offering 42 ECS panels were identified. There was a large diversity in the number genes being screened, from as little as 3 to 1577. Overall, there were 2673 unique diseases screened across all panels, none of which were present in all panels (Fig. 1). It was surprising that not all panels tested for pathogenic variants in CFTR (cystic fibrosis) or SMN1 (spinal muscular atrophy) that are the main diseases recommended by organizations, such as the American College of Medical Genetics (ACMG) and the American College of Obstetricians and Gynaecologists (ACOG) (Grody et al., 2013; Edwards et al., 2015; ACOG, 2017). A review that looked at the overall ECS panels not specific to gamete donors also found that there was a substantial difference in the number and types of diseases found across panels. They concluded that there needs to be more similarities between the panels, which could come to fruition by using consistent criteria (Chokoshvili et al., 2018).
Cryos International donor data
From the Cryos donors, 17.6% were rejected based on their carrier status. Carriers of alpha-thalassaemia (HBA) represented 19.4% of rejected donors and cystic fibrosis (CFTR) carriers represented 14.8% of rejected donors. In the USA, cystic fibrosis occurs in 1 in 2500–3500 Caucasian newborns (Genetics Home Reference, 2020).
The bespoke panels that Cryos International use allow the testing of a large number of diseases despite the potential for more donors being rejected. According to the World Health Organisation (WHO), the most common recessive or X-linked genetic diseases are alpha- and beta-thalassaemia, sickle cell anaemia, haemophilia, cystic fibrosis, Tay–Sachs disease and fragile X syndrome (WHO, 2020). The Cryos International panels screen for all except haemophilia, displaying that the panels offered are extremely comprehensive. There are only 11 of the 42 review panels that contain all of the most common recessive or X-linked diseases according to WHO (2020): 7A, 11A, 12A, 13C, 13D, 15A, 15C, 16A, 16B, 16G and 16I.
National guidelines and donor carrier status
Within Europe, there is a discrepancy and a large variation between the guidelines and regulations in place regarding the use of donors who are carriers, with some recommending rejecting donors who are carriers or who have a family history. For example, in the UK, there is no law regarding this; however, there are guidelines set by the Association of Clinical Embryologists, the Association of Biomedical Andrologists, the British Fertility Society and the British Andrology Society. They state that the potential donor should not be a carrier of an autosomal recessive disease prevalent in their ethnic background; however, a carrier donor may be used under exceptional circumstances, such as for known donors (Clarke et al., 2019). In Denmark, it is the interpretation of the law that donors must be rejected if they are carriers of a recessive disease (Retsinformation, 2015a, b). Germany recommendations, 2006, state that men who are known to be autosomal recessive disease carriers are excluded from sperm donation. Egg donation is banned in Germany (Arbeitskreises für Donogene Insemination, 2006). Italy’s law states that carriers of genetic disorders are rejected from donation (Boggio, 2017).
Many commercial panels that are available to sperm and egg donors screen for a high number of diseases; but paired with current guidelines and legislations, many donors would be rejected. As we have shown in this study, even panels such as that used by Cryos International, which screen donors using a smaller panel, reject a relatively high proportion of potential donors.
A study that looked at the attitudes of gamete donors and recipients towards ECS found that most recipients would not reject a donor based solely on the family medical history (Amor et al., 2018). A similar study that looked into the perspectives of potential recipients of gamete donation regarding the carrier status of donors found that they were not very comfortable with using a donor who was a carrier for a severe condition (Jackson et al., 2017). The study did not mention matching, therefore, would the outcomes have been different if they were aware?
The need for the matching of gamete donors to their recipients
Presently, there are 25 532 Mendelian diseases listed on OMIM, compared to 23 621 in 2016 (Vas-de-Macedo and Harper, 2017; OMIM, 2020). This illustrates that the number of known Mendelian diseases is increasing; therefore, more genes will be available to screen. As the number of diseases screened for increases, more candidate donors will be found to be carriers and be rejected. This will result in decreasing numbers of gamete donors until the point where there are none. Increasing the number of genes screened has resulted in a higher proportion of carriers; screening 10 genes resulting in 8.6% carriers, Cryos International screening for 47 genes resulting in 17.6% carriers, and screening 200 genes resulting in 56% carriers (Boada, 2017; Fabiani et al., 2020).
There is an argument that it is best not to screen more genes, as the donors would be rejected, and the risk to the offspring is low. When rejecting gamete donors based on carrier status, using smaller panels would allow more donors to become available; however, this would not allow intended parents to have reproductive autonomy and potential knowledge of their own carrier status. In our opinion, the recipient’s reproductive autonomy implies that they should be able to choose a donor who has agreed to genetic matching, if they themselves are a known carrier. Deciding what to do with the knowledge of known carriership in either the donor or the recipient should be free of choice within the frame of informed decision-making as the risk of an affected child is still very low. On the other hand, if they have gone so far in the process and know they (recipient and donor) are a known carrier of the same recessive disease, then we are no longer talking low risk, hence due to the welfare of the child they should be constraints of either to choose another donor or go forward with PGD-M.
One of the main shortcomings of matching donors and recipients would be the increased cost of treatment for the recipients as they would also have to undergo ECS as well as genetic counselling; with increasing panel sizes increasing expense. This poses economical and ethical issues, meaning that the ECS of recipients would not be able to be mandatory. There is the possibility that donors will not be interested in donating if ECS is mandatory (De Wert et al., 2020; Pennings, 2020). This poses safety issues of donors as well as the donor’s children, such as an increased burden of knowledge that they may not have wanted (Pennings, 2020). Counselling, however, should be offered prior to ECS of donors, which would allow the donor to make an informed decision as to whether they want to carry on with the donation process.
Donors may not want to know the results of the ECS and this should be an option. However, if any donor who is a carrier is rejected, these donors would be aware that they were carrying one of the conditions tested for. This would not be an issue if the donor is selected after genetic matching
A situation may arise that individuals requiring gamete donation are aware they are carriers of a gene that donors are not tested for. In this case, the donors would need to be screened again for that gene, which would mean the donors may have to provide more samples to be tested. In the circumstance that recipients are tested with a different panel than the donors, different results would be obtained; therefore, matching would not be possible unless screening was reperformed. This highlights the need for a consistent panel if matching was to occur. Or that donor DNA is stored for future use on request.
The next step for this research would be to develop an ECS panel for use in matching donors and recipients that is consistent across clinics and beneficial for all parties involved. One way this could be done is by creating criteria for the inclusion of diseases that also encompasses all those recommended by the different organizations and societies. The panel should be targeted towards conditions and pathogenic variants that can be tested accurately and precisely and the clinical implications of which would be well understood, as well as preventing unnecessary burden for both donors and recipients (Dondorp et al., 2014; Stevens et al., 2017). The panel should be focussed on severe childhood-onset diseases in which their quality of life would be affected (Stevens et al., 2017; Kraft et al., 2019).
Conclusion
This study highlights the need for consistent EU regulations and guidelines that allows genetic matching of gamete donors to their recipients, preventing the need to reject donors who are known carriers. A larger ECS panel would be most beneficial; however, this would not be viable without matching of donors and recipients. This will ensure more equal genetic possibilities for people in need of fertility care no matter whether it is with the use of their own gametes or people in need of donor gametes.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article were provided by Cryos International by permission. Data will be shared on request to the corresponding author with permission of Cryos International.
Authors’ roles
J.C.H.: Primary supervisor, conception and design, interpretation of data for the work, drafting and revision of the article and final approval of the version to be published. A-B.S.: Conception and design, acquisition of data for the work, revision of the article and final approval of the version to be published. M.R.P.: Conception and design, analysis and interpretation of data for the work, drafting and revision of the article and final approval of the version to be published.
Funding
No specific funding was obtained.
Conflict of interest
J.C.H. is the founder of Global Women Connected, a platform to discuss women’s health issues and the Embryology and PGD Academy, who deliver education in clinical embryology. She has been paid to give a lecture by Cryos in 2019. A-B.S. is an employee of Cryos International.
Supplementary Material
References
- American College of Obstetricians and Gynecologists (ACOG). Carrier Screening for Genetic Conditions. 2017. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/03/carrier-screening-for-genetic-conditions (16 August 2020, date last accessed).
- Amor DJ, Kerr A, Somanathan N, McEwan A, Tome M, Hodgson J, Lewis S.. Attitudes of sperm, egg and embryo donors and recipients towards genetic information and screening of donors. Reprod Health 2018;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeitskreises für Donogene Insemination. Richtlinien des Arbeitskreises für Donogene Insemination zur Qualitätssicherung der Behandlung mit Spendersamen in Deutschland. 2006. https://www.donogene-insemination.de/content/uploads/2019/08/Richtl_Druckfassung.pdf (27 July 2020, date last accessed).
- Bajaj K, Gross SJ.. Carrier screening: past, present, and future. J Clin Med 2014;3:1033–1042. [Google Scholar]
- Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Ganusova EE, Mudge J, Langley RJ, Zhang L, Lee CC, Schilkey FD. et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med 2011;3:65ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada M. Genetic matching between recipients and oocyte donors. Curr Trends Clin Embryol 2017;4:52. [Google Scholar]
- Boggio A. The legalisation of gamete donation in Italy. Eur J Health Law 2017;24:85–104. doi: 10.1163/15718093-12341409. [DOI] [PubMed] [Google Scholar]
- Chokoshvili D, Vears DF, Borry P.. Growing complexity of (expanded) carrier screening: direct-to-consumer, physician-mediated, and clinic-based offers. Best Pract Res Clin Obstet Gynaecol 2017;44:57–67. [DOI] [PubMed] [Google Scholar]
- Chokoshvili D, Vears DF, Borry P.. Expanded carrier screening for monogenic disorders: Where are we now? Prenat Diagn 2018;38:59–66. [DOI] [PubMed] [Google Scholar]
- Clarke H, Harrison S, Perez MJ, Kirkman-Brown J.. UK guidelines for the medical and laboratory procurement and use of sperm, oocyte and embryo donors (2019). Hum Fertil (Camb) 2019;6:1–13. [DOI] [PubMed] [Google Scholar]
- Cryos International. A dream come true. 2019a. https://dk.cryosinternational.com/about-us (19 March 2020, date last accessed).
- Cryos International. Cryos International. 2019b. https://dk.cryosinternational.com/(19 March 2020, date last accessed).
- De Wert G, van der Hout S, Goddijn M, Vassena R, Frith L, Vermeulen N, Eichenlaub-Ritter U, on behalf of the ESHRE Ethics Committee. The Ethics of Preconception Expanded Carrier Screening in Applicants of Medically Assisted Reproduction. 2020. (Unpublished).
- Dondorp W, De Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, Barri P, Diedrich K, Eichenlaub-Ritter U, Tüttelmann F. et al. ESHRE Task Force on Ethics and Law 21: genetic screening of gamete donors: ethical issues. Hum Reprod 2014;29:1353–1359. [DOI] [PubMed] [Google Scholar]
- Dungan J. Expanded carrier screening: what the reproductive endocrinologist needs to know. Fertil Steril 2018;109:183–189. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Feldman G, Goldberg J,, Gregg AR, Norton ME, Rose NC, Schneider A, Stoll K, Wapner R, Watson MS.. Expanded carrier screening in reproductive medicine-points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol 2015;125:653–662. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Caroselli S, Girardi L, Patassini C, Poli M, Simon C, Capalbo A. High detection rates in both IVF and general population confirm the clinical utility of Expanded Carrier Screening for the management of reproductive genetic risk. 2020. (Unpublished). [DOI] [PubMed]
- Genetics Home Reference. Cystic fibrosis. 2020. https://ghr.nlm.nih.gov/condition/cystic-fibrosis#sourcesforpage (16 August 2020, date last accessed).
- Grody WW, Thompson BH, Gregg AR, Bean LH, Monaghan KG, Schneider A, Lebo RV.. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med 2013;15:482–483. [DOI] [PubMed] [Google Scholar]
- Harper JC, Aittomäki K, Borry P, Cornel MC, De Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I. et al. Recent developments in genetics and medically-assisted reproduction: from research to clinical applications. Eur J Hum Genet 2018;26:12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman L, Borry P, Chokoshvilli D, Cornel MC, van El CG, Forzano F, Hall A, Howard HC, Janssens S, Kayserili H. et al. Responsible implementation of expanded carrier screening. Eur J Hum Genet 2016;24:e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E, Edwards J, Besser A, Isley LJ.. Recipients’ perspectives regarding expanded carrier screening of gamete donors. Fertil Steril 2017;108:E265–E266. [Google Scholar]
- Kraft SA, Duenas D, Wilfond BS, Goddard KAB.. The evolving landscape of expanded carrier screening: challenges and opportunities. Genet Med 2019;21:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertes H, Lindheim SR, Pennings G.. Ethical quandaries around expanded carrier screening in third-party reproduction. Fertil Steril 2018;109:190–194. [DOI] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man (OMIM). OMIM Entry Statistics. 2020. https://omim.org/statistics/entry (18 August 2020, date last accessed).
- Pennings G. Expanded carrier screening should not be mandatory for gamete donors. Hum Reprod 2020;35:1256–1261. [DOI] [PubMed] [Google Scholar]
- Retsinformation. Vejledning om kvalitet og sikkerhed ved donation og testning af væv og celler. 2015a. https://www.retsinformation.dk/eli/retsinfo/2015/9356 (17 July 2020, date last accessed).
- Retsinformation. Vejledning om sundhedspersoners og vævscentres virksomhed og forpligtelser i forbindelse med assisteret reproduction. 2015b. https://www.retsinformation.dk/eli/retsinfo/2015/9351 (17 July 2020, date last accessed).
- Riigi Teataja. Criteria for the selection of cell, tissue, and organ donors, list of precluding circumstances for the donation of cells, tissues, or organs, list of mandatory laboratory studies established for a donor, and the conditions and procedure for carrying out these studies. 2015. https://www.riigiteataja.ee/en/eli/502112016002/consolide (5 August 2020, date last accessed).
- Rowe CA, Wright CF.. Expanded universal carrier screening and its implementation within a publicly funded healthcare service. J Community Genet 2019;11:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AJ, Larson JL, Silver MJ, Lim RM, Borroto C, Spurrier B, Morriss A, Silver LM.. Carrier screening is a deficient strategy for determining sperm donor eligibility and reducing risk of disease in recipient children. Genet Test Mol Biomarkers 2016;20:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims CA, Callum P, Ray M, Iger J, Falk RE.. Genetic testing of sperm donors: survey of current practices. Fertil Steril 2010;94:126–129. [DOI] [PubMed] [Google Scholar]
- Srinivasan BS, Evans EA, Flannick J, Patterson AS, Chang CC, Pham T, Young S, Kaushal A, Lee J, Jacobson JL. et al. A universal carrier test for the long tail of Mendelian disease. Reprod Biomed Online 2010;21:537–551. [DOI] [PubMed] [Google Scholar]
- Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J.. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol 2017;130:279–284. [DOI] [PubMed] [Google Scholar]
- Urbina MT, Benjamin I, Medina R, Jiménez R, Trías L, Lerner J.. Expanded carrier screening in gamete donors of Venezuela. JBRA Assist Reprod 2017;21:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas-de-Macedo C, Harper JC.. A closer look at expanded carrier screening from a PGD perspective. Hum Reprod 2017;32:1951–1956. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO). Genes and human diseases: Monogenic diseases. 2020. https://www.who.int/genomics/public/geneticdiseases/en/index2.html#Haemophilia (11 September 2020, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Cryos International by permission. Data will be shared on request to the corresponding author with permission of Cryos International.