Abstract
Background
Pelvic organ prolapse (POP) is the downward descent of the female pelvic organs into or through the vagina. The symptom that most strongly correlates with and is most specific for POP is a feeling of vaginal bulging. Stress urinary incontinence (SUI) is an involuntary loss of urine upon physical exertion or sneezing or coughing. Conservative (non-surgical) treatment options for both conditions include vaginal pessaries. We conducted a health technology assessment of vaginal pessaries for the treatment of POP and SUI, which included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding vaginal pessaries, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using ROBIS, the Cochrane Risk of Bias tool, and the Newcastle–Ottawa Scale and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search and conducted a cost–utility analysis with a 10-year horizon from a public payer perspective. We also analyzed the budget impact of publicly funding vaginal pessaries for individuals with pelvic organ prolapse and/or stress urinary incontinence in Ontario. We explored the underlying values, needs, and priorities of those who have lived experience with POP and/or SUI, as well as the preferences and perceptions of both patients and providers of vaginal pessaries.
Results
We included 15 studies in the clinical evidence review. Compared with no treatment for people with SUI, pessaries were associated with a significant improvement in some symptoms at 14 days follow-up (SUI subscore of Urinary Symptom Profile, mean difference −2.20; 95% CI −3.47 to −0.93; GRADE: Very low). Compared with pelvic floor muscle training (PFMT), pessaries were associated with no difference in improvement at 12 months follow-up for some symptoms (Urinary Distress Inventory subscale of the Pelvic Floor Distress Inventory, risk ratio = 0.86; 95% CI 0.64 to 1.16; GRADE: Low). For people with POP, pessaries were associated with a significant improvement in the Pelvic Organ Prolapse Distress Inventory score and in sexual function compared with PFMT plus feedback/electrical stimulation/lifestyle advice at 12- and 24-month follow ups (GRADE: Low). Pessary continuation rate at 12 months follow up was reported to be 60% (44/74 patients) (GRADE: Very low).
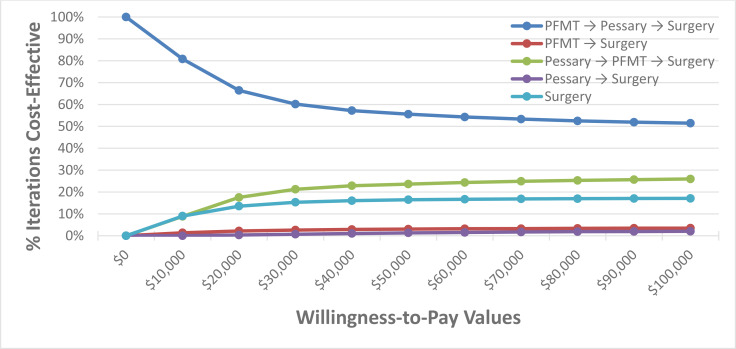
When evaluating various POP and SUI treatments in sequential order, pessaries were within the most cost-effective treatment sequence; therefore, it is likely to be a cost-effective intervention for treating POP and SUI. There was a high degree of certainty that pessaries were cost-effective in a population with POP, and a moderate degree of certainty in a population with SUI. When the treatment sequence of pessaries and surgery was compared with surgery alone, the pessaries treatment sequence dominates surgery in the cohort with POP, and in the cohort with SUI pessaries had an incremental cost-effectiveness ratio (ICER) of $1,033 per QALY gained. The annual budget impact of publicly funding vaginal pessaries in Ontario over the next 5 years ranges from $0.3 million in year 1 to $0.5 million in year 5 for POP, and $0.2 million in year 1 to $0.3 million in year 5 for SUI.
We included one study in our quantitative evidence review and spoke to 29 people in our direct patient engagement. The evidence indicated that patient preferences vary and that patients accept the risks of their chosen treatment option. The 24 people we spoke with who had direct experience with vaginal pessaries reported that their POP and/or SUI limited their social activities and restricted their activity levels, taking a huge emotional toll. Many were hesitant or even fearful of surgery due to side effects and perceived failure rate of the surgery. Most people reported that pessaries relieved most or all of their symptoms, allowing them to return to their normal daily activities. However, wait times for pessary fittings could be as long as 2 years, and out-of-pocket expenses could be a barrier for people without extended insurance.
Conclusions
For people with SUI, vaginal pessaries may improve symptoms compared with no treatment, but the evidence is very uncertain. Pessaries may result in little to no difference in longer-term improvement of SUI symptoms compared with PFMT. For people with POP, pessaries may improve some longer-term symptoms, as well as sexual function compared with PFMT. For people with symptomatic POP and SUI, vaginal pessaries may be a cost-effective intervention to be used within a stepped care model (a sequence of interventions followed after the current treatment proves ineffective). We estimate that publicly funding vaginal pessaries in Ontario would result in a total 5-year budget impact of $2.0 million for POP and $1.3 million for SUI. People with POP and/or SUI reported pessary use as being an effective treatment option to manage their symptoms.
Objective
This health technology assessment evaluates the effectiveness, safety, and cost-effectiveness of vaginal pessaries for people with pelvic organ prolapse or stress urinary incontinence. It also evaluates the budget impact of publicly funding vaginal pessaries and the experiences, preferences, and values of people with pelvic organ prolapse or stress urinary incontinence.
Background
Health Condition
Pelvic organ prolapse (POP) is the downward descent of the pelvic organs (i.e., vagina, uterus, bladder, and/or rectum) into or through the vagina.7 Prolapse of the anterior vaginal wall (cystocele) is the most common form of POP, detected twice as often as posterior vaginal prolapse (rectocele) and three times as often as apical prolapse (uterine and/or post-hysterectomy vaginal vault prolapse).7 In most cases of symptomatic POP, prolapse of multiple segments of the vagina are noted.7 Vaginal childbirth, advancing age, increasing body mass index, straining due to constipation and prior hysterectomy are the most consistent risk factors for POP.
The symptom that most strongly correlates with and is most specific for POP is a feeling of vaginal bulging or a vaginal bulge that can be seen or felt. People who develop symptoms may present with a single symptom such as feeling a vaginal bulge or pelvic pressure, or they may present with a combination of symptoms (some that are nonspecific for POP). A cross-sectional study of 237 people who were evaluated for POP found that 73% had concurrent urinary incontinence, 86% had urinary urgency/frequency, and 31% had fecal incontinence. Incidence of other voiding dysfunctions (abnormally slow and/or incomplete micturition, based on abnormally slow urine flow rates and or abnormally high post-void residuals) ranged from 34% to 62%.8 The severity of symptoms varies and is the driving factor in patient decisions to present at hospital.7 People with severe prolapse may develop erosions of the vagina or cervix, which can present with vaginal bleeding or spotting or more serious complication such as urinary tract infection, or obstruction of the ureter with hydronephrosis or renal failure. Symptoms may negatively affect body image, quality of life, and a person's ability to perform day to day activities.9
Stress urinary incontinence (SUI) is an involuntary loss of urine upon physical exertion, or with an increase in the intra-abdominal pressure upon sneezing or coughing.10 SUI represents 50% of cases of urinary incontinence worldwide and is more prevalent than other types of urinary incontinence in females under 55 years of age.11 The risk factors for SUI include obesity, menopause, number of pregnancies, delivering larger babies during childbirth, vaginal deliveries, use of medications that relax the urethral sphincter, presence of a lung disease causing chronic cough, and prior pelvic surgeries.10 SUI often remains unreported and undertreated. The underlying reasons for underreporting include the reluctance of patients to disclose their incontinence and urinary symptoms due to embarrassment, lack of knowledge about treatment options, fear of surgery, or belief that SUI is an inevitable part of aging.10 Stress urinary incontinence can have a devastating effect on people living with this condition and their families. People suffer the physical discomfort associated with incontinence as well as potential social isolation and psychological distress.12 Clinically, SUI is present alone or in association with pelvic organ prolapse (POP).10
Clinical Need and Target Population
In the United States, loss of vaginal or uterine support is seen in approximately 30% to 76% of people presenting for routine gynaecological care, with 3% to 6% of those experiencing descent beyond the vaginal opening.7 One population-based study in the United States found that about 3% of 1,961 adult females surveyed reported symptomatic vaginal bulging.13 Similarly published Canadian data were not identified.
While POP is often asymptomatic, the prevalence among parous people (people who bore children) is 50%.14 Among nulliparous people (who have not born children) aged 20 to 39 years old, the prevalence is 1.6%.15
About 50% of females with urinary incontinence report SUI as the primary or sole symptom of incontinence.16 The prevalence of SUI appears to increase with age initially, peaks around the fourth or fifth decade, and then decreases with increasing age.16
Current Treatment Options
First line treatment for POP and SUI includes lifestyle and behavioural interventions, pelvic floor muscle training (supervised by a trained professional, such as a nurse practitioner, nurse continence advisor, physician, or physiotherapist), and vaginal pessaries.9
Second line treatment involves surgical intervention (e.g., midurethral sling or pelvic reconstruction); however, these interventions may not be pursued as an option, particularly when the individual does not want surgery, wants to delay surgery, or is a poor candidate for surgery.9,12
Health Technology Under Review
Pessaries are devices inserted into the vagina to support its internal structure and can be divided into two main categories: those designed for the treatment of SUI and those used to treat POP. Pessaries can be further divided into support and space-filling, and have different shapes and sizes.9 Support pessaries are easier for patients because they tend to be simpler to remove and insert. Space-filling pessaries are primarily used to support severe POP, especially when the vagina drops after hysterectomy. Pessaries are designed to stay in place when pelvic support muscles are weak. In general, the ring-with-support (ring) pessary is the most commonly used support pessary. Space-filling pessaries are used with patients who are unable to retain a ring pessary. The Gellhorn pessary is the most commonly used space-filling pessary.
Most pessaries are made of medical-grade silicone.9 They are durable and able to withstand sterilization in an autoclave, and are resistant to absorption of vaginal discharge and odours.
For POP, the aim of pessaries is to decrease the frequency and severity of prolapse symptoms.9 The use of pessaries may also avoid or delay the need for surgery. For SUI, pessaries are designed to support the urethra and bladder wall, and possibly increase functional urethral length. The aim is to reduce or prevent leakage when intra-abdominal pressure increases.
Regulatory Information
Table 1 describes some pessaries with current active licenses according to Health Canada's Medical Devices Active License Listing (MDALL) database.17
Table 1:
Vaginal Pessaries With Active Licences from Health Canada17
| Device (Manufacturer) | License No. | Device Class | Approved Indication(s) |
|---|---|---|---|
| Coopersurgical Inc Vaginal Support Pessary (Cube Family) Silicone |
61777 | II | Uterine prolapse, stress urinary incontinence, incomplete cervix and complications such as rectocele, cystocele, or retro-displacement. |
| Coopersurgical Inc Donut Pessary vaginal support pessary |
63820 | III | Uterine prolapse, stress urinary incontinence, incomplete cervix and complications such as rectocele, cystocele, or retro-displacement. |
| MedGyn Products International Inc and MedGyn Products, Inc MedGyn Ring pessary with support |
99715 | II | These pessaries are used for non-surgical management of mild prolapse (first or second degree) with or without cystocele, rectocele, or urinary incontinence. |
Ontario Context
Many people with POP and/or SUI do not have a supplementary insurance plan to help with the cost of a pessary. The Ontario Schedule of Benefits includes a physician fee code for pessary fitting (G398, limited to once a year), but the pessary device is not publicly funded (i.e., the patient must pay out of pocket).18 An estimated 15% of people who are prescribed pessaries have coverage through private insurance (Jennifer Skelly, PhD, personal communication, December 2019). Other Canadian provinces also report coverage for the procedure but not the device.
A pessary costs approximately $50 to $150 and lasts approximately 5 to 10 years, depending on the type of pessary and how frequently it is removed (for cleaning, examination, etc.) (Jennifer Skelly, PhD, Personal Communication, December 2019). Expert consultation suggested that a Gellhorn pessary may need to be replaced every 1 to 2 years. The pessary may require more frequent replacement if it absorbs odours from vaginal discharge. Additionally, the size of the pessary may need to be reduced if the vaginal tissue atrophies (e.g., due to advancing age).
Surgery for POP and SUI is publicly funded in Ontario. However, there may be long wait times for a surgical consultation (average 101 days for POP and 136 days for SUI)19 and many people may not be good candidates for surgery owing to medical condition or frailty.
Pelvic floor muscle training is not publicly funded. Private physiotherapy coverage through supplemental insurance varies depending on an individual's plan and may only partially cover costs.
Expert Consultation
We engaged with experts in the specialty areas of nurse continence advisory (registered nurses who focus on first line methods of managing incontinence), gynecology, family medicine, pelvic reconstruction surgery, and physiotherapy to help inform our understanding of aspects of the health technology and our methodologies and to contextual the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD 42019142348), available at https://www.crd.york.ac.uk/PROSPERO.
Clinical Evidence
Research Question
What are the effectiveness and safety of vaginal pessaries for the treatment of people with pelvic organ prolapse and/or stress urinary incontinence?
Methods
Clinical Literature Search
We performed a clinical literature search on June 25, 2019, to retrieve studies published from January 1, 2000, until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS-EED). We used the EBSCOhost interface to search the Cumulative Index to Nursing & Allied Health Literature (CINAHL).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.20
We created database auto-alerts in MEDLINE, Embase, and CINAHL and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. The grey literature search was updated on December 2, 2019. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published between January 1, 2000, and June 25, 2019
Randomized controlled trials, systematic reviews, meta-analyses, and observational studies
Exclusion Criteria
Animal and in vitro studies
Nonsystematic reviews, narrative reviews, abstracts, editorials, letters, case reports, and commentaries
PARTICIPANTS
Adults (≥ 18 years) with symptomatic pelvic organ prolapse (POP) and/or stress urinary incontinence (SUI)
INTERVENTION
Included: vaginal pessaries
Excluded: weighted vaginal cones, electrical devices (i.e., devices that aim to improve pelvic floor muscle tone), pharmaceutical pessaries (used to deliver a drug through the skin of the vagina)
COMPARATOR
Control (i.e., no active treatment)
Conservative treatment (e.g., pelvic floor exercises)
Surgery (e.g., midurethral sling/pelvic reconstruction)
OUTCOME MEASURES
Quality of life
Improvement of symptoms
Prevention of worsening prolapse
Patient satisfaction
Complications
Delayed need for surgery; surgeries avoided
Sexual function
Pessary compliance
Urinary voiding/anorectal dysfunction (anal incontinence, defecatory dysfunction)
Lower urinary tract symptoms
Outcomes are reported for short- and long-term follow-up (e.g., ≤ 3 months and > 3 months)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence21 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items to collect information on the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, whether the study compared two or more groups)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, time points at which the outcomes were assessed)
Statistical Analysis
Due to clinical and methodological heterogeneity, meta-analysis was not appropriate. Instead, a narrative summary of results is provided.
Critical Appraisal of Evidence
We assessed risk of bias using the Cochrane Risk of Bias tool for randomized controlled trials (RCTs),22 ROBIS for systematic reviews,23 and the Newcastle–Ottawa Scale for observational studies24 (Appendix 2). When available, the risk of bias assessment from the included systematic reviews is reported.
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.25 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Clinical Literature Search
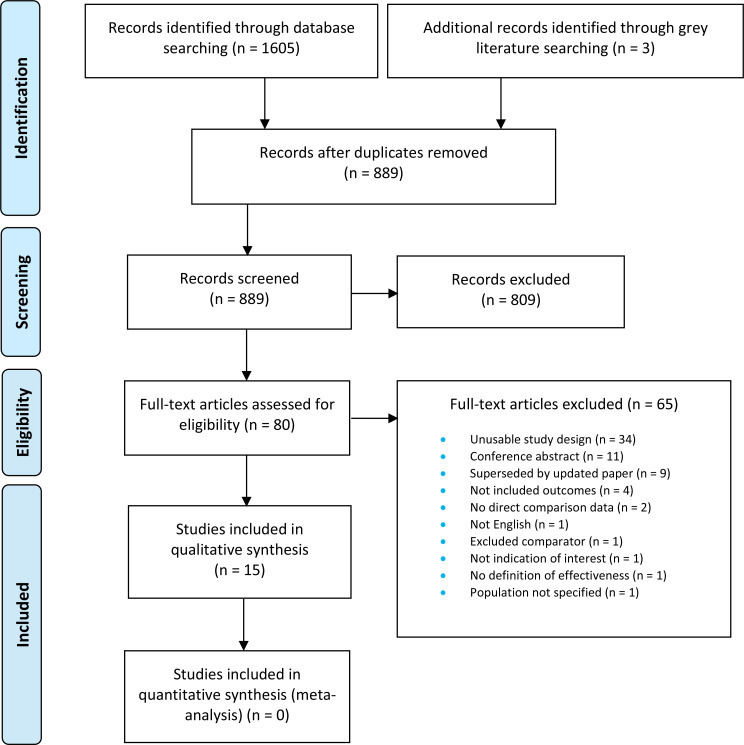
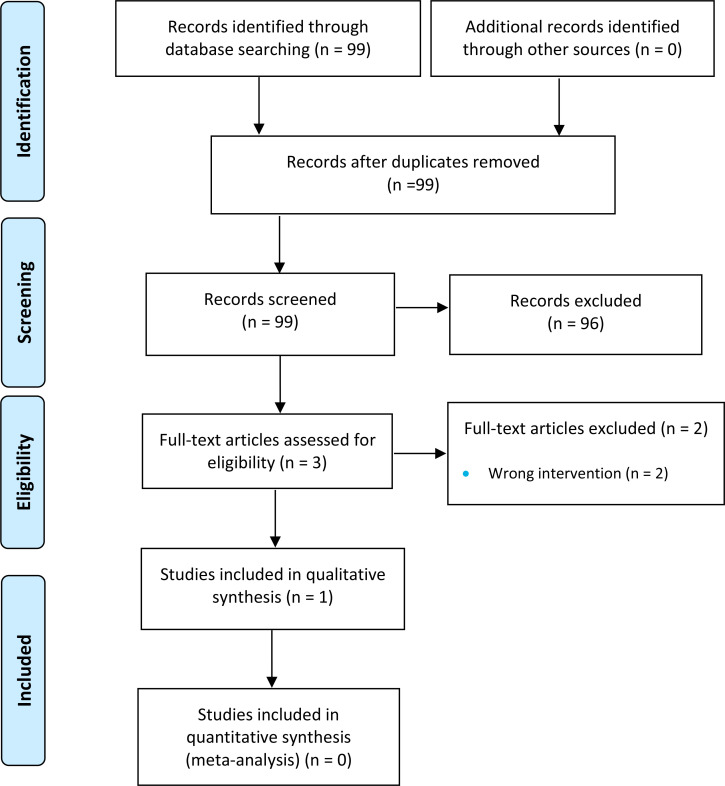
The literature search yielded 889 citations published between January 1, 2000, and June 25, 2019, after removing duplicates. We reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of 80 articles for further assessment. See Appendix 4 for a selected list of studies excluded after the full-text review. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.26
Stress Urinary Incontinence
One systematic review by Lipp et al12 was identified that assessed the effects of mechanical devices in the management of adult female urinary incontinence, particularly SUI. Characteristics of the systematic review are shown in Table A1, Appendix 2. We rated the risk of bias of the systematic review as low (Appendix 3).
The literature search in Lipp et al12 included four RCTs (published as five studies) (Table 2).27–31 We excluded one study30 from our analysis since it only reported data for an outcome (24-hour pad test) that is not included in our analysis. We did not identify any studies other than the Lipp et al review.
Table 2:
Characteristics of Studies Included in Systematic Review by Lipp et al12 for Stress Urinary Incontinence
| Author, Year, Country | Study Design and Methods | Participants | Inclusion/Exclusion Criteria | Intervention | Outcomes of Interest |
|---|---|---|---|---|---|
| Cornu et al,27 2012, France | Randomized parallel group trial | N = 55 SUI assessed by clinical examination (stress test) or mixed incontinence with predominant SUI |
Inclusion criteria: Age ≥ 18 y, postmenopausal or using contraception, no vaginal delivery in past 2 mo, no bladder/vaginal disease, no acute or recurrent urinary infection, no POP > stage 2 as per POPQ classification, no surgical intervention for SUI in the past 6 mo, no drug treatment for UI in last month, no PFMT underway. Exclusion criteria: not specified. |
Use of intravaginal device (75NC007) for up to 24 h per day for 14 d. Group 1: 75NC007 (n = 29) Group 2: wearing no mechanical device (n = 26) 75NC007: thermoplastic elastomer supplied in two sizes: medium and small. Inserted into vagina with or without an applicator. Automatically locates beneath urethra and bladder, removed by pulling string on cylindrical part of device. Device may be inserted by patient and must be discarded and replaced with a new device 24 h after insertion. |
Incontinence episode frequency according to bladder diaries. Urinary symptom profile score. 24-h pad weighing test. CONTILIFE questionnaire. |
| Nygaard et al,28 1995 United States |
Randomized controlled crossover trial | N = 18 SUI diagnosed by stress test |
Inclusion criteria: History of exercise incontinence; physical ability to perform 40 min exercise and positive stress test. Patients excluded if prolapse of uterus or vagina, stenotic vagina, or pelvic mass. Exclusion criteria: not specified. |
All participated in each of three separate standardized exercise sessions: Group 1. Wearing a Hodge pessarya with support (n = 18) Group 2. Wearing a Tampax super tampona (n = 18) Group 3. Wearing no mechanical device (n = 18) |
Pad weighing test. Patient self reported discomfort. |
| Richter et al,29 2010 United States |
Randomized parallel group trial (ATLAS trial) | N = 445 SUI only, SUI predominant, or mixed incontinence symptoms |
Inclusion criteria: ≥ 18 y of age, ambulatory and able to attend clinic, symptoms of SUI, SUI for at least 3 mo, adequately completed 7-d bladder diary for at least 5 d, with number of SUI episodes exceeding number of other types of incontinence, stable storage of oral/vaginal estrogen for past 8 wk if used, ability to complete bladder diary and questionnaires in English, stage 0, 1, or 2 prolapse as per POPQ classification. Exclusion criteria: urinary tract infection, pregnant or planning pregnancy, patient within 6 mo postpartum, severe atrophic vaginitis, patient strongly desires surgery within 12 mo, within 3 mo of failed surgery for SUI, current medication for incontinence, vaginal foreign body, currently using pessary or used one within last 2 months, neurologic conditions that may impact bladder symptoms. |
Randomized into one of three groups for an 8-wk treatment period. Group 1: intravaginal pessary (n = 149) Group 2: behavioural therapy (PFMT) (n = 146) Group 3: combined pessary and PFMT (n = 150) A physician- or nurse-fitted intravaginal pessary (continence ring or dish, type not specified). PFMT comprised of four visits at 2 weekly intervals that included instructions on PFMT and exercise, as well as skills for active use of muscles to prevent stress and urge incontinence. Participants given individualized prescriptions for daily PFMT and practice. After the 8-wk treatment period, participants in the combined group could continue in the trial while using only one of the therapies (PFMT alone or pessary alone). |
Subjective assessment by patients (“much better” or “very much better”). Validated questionnaire on symptom severity. Patient Satisfaction Survey (PSQ). Incontinence episodes over 7 d. |
| Kenton et al,31 2012 United States |
Randomized parallel group trial (ATLAS trial) | N = 295 SUI only, SUI predominant, or mixed incontinence symptoms |
Same as Richter et al29 | Pessary PFMT | UDI UIQ POPDI POPIQ CRADI CRAIQ QUID |
Abbreviations: CRADI, Colorectal Anal Distress Inventory; CRAIQ, Colorectal Anal Impact Questionnaire; PFMT, pelvic floor muscle training; POP, pelvic organ prolapse; POPDI, POP Distress Inventory; POPIQ, POP Impact Questionnaire; POPQ, POP Questionnaire; PSQ, patient satisfaction questionnaire; QUID, Questionnaire for Urinary Incontinence Diagnosis; SUI, stress urinary incontinence; UDI, Urogenital Distress Inventory; UI, urinary impact; UIQ, UI Questionnaire.
A Hodge pessary is a ring with a support placed at the neck of the cervix. It is plastic coated, with wires that allow it to be shaped for different anatomies. The Tampax super tampon (commercially available) is placed in the vagina. The tampon string was “hidden” as participants were blinded to treatments. Both devices were placed by the investigator.
IMPROVEMENT OF SYMPTOMS
Pessaries Compared With No Treatment
One randomized controlled trial (RCT) reported on improvement of symptoms for pessaries compared with no treatment27 and one RCT presented results for pessaries compared with pelvic floor muscle training (PFMT) (Table 3).29,31
Table 3:
Results of Randomized Controlled Trials Reporting Improvement of Symptoms in Patients With Stress Urinary Incontinence
| Comparison | Symptom | Results |
|---|---|---|
| Cornu et al,27 2012 (vaginal loop “75NC007” pessary versus no treatment)a | ||
| Pessary (n = 29) No treatment (n = 26) | Incontinence episode frequency (14-d follow-up) | Pessary, mean (SD): −31.7 (65.1) No treatment, mean (SD): −7.6 (24.5), MD = −24.10 (95% CI −49.60 to 1.40) |
| SUI subscore of USP (14-d follow-up) | Pessary: −2.4 (2.5) No treatment: −0.2 (2.3), MD = −2.20 (95% CI −3.47 to −0.93) |
|
| Dysuria subscore of USP (14-d follow-up) | Pessary: −0.2 (0.8) No treatment: 0.3 (0.8), MD = −0.50 (95% CI −0.92 to −0.08) |
|
| Richter et al,29 2010, and Kenton et al,31 2012 (dish/ring pessary versus PFMT, pessary alone versus dish/ring pessary + PFMT, dish/ring pessary + PFMT versus PFMT alone)a | ||
| Pessary (n = 149) PFMT (n = 146) |
Proportion reporting continence better on PGI-I (3 mo) | Pessary: 59/149 PFMT: 72/146, RR = 0.80 (95% CI 0.62 to 1.04) |
| Proportion reporting continence better on PGI-I (12 mo) | Pessary: 47/149 PFMT: 48/146, RR = 0.96 (95% CI 0.69 to 1.34) |
|
| No bothersome symptoms on UDI subscale of PFDI (3 mo) | Pessary: 49/149 PFMT: 71/146, RR = 0.68 (95% CI 0.51 to 0.90) |
|
| No bothersome symptoms on UDI subscale of PFDI (12 mo) | Pessary: 52/149 PFMT: 59/146, RR = 0.86 (95% CI 0.64 to 1.16) |
|
| > 75% reduction weekly incontinence episodes (3 mo) | Pessary: 69/149 PFMT: 68/146, RR = 0.99 (95% CI 0.78 to 1.27) |
|
| > 75% reduction weekly incontinence episodes (12 mo) | Pessary: 51/149 PFMT: 54/146, RR = 0.93 (95% CI 0.68 to 1.26) |
|
| Improved UIQ score (3 mo) | Pessary, mean (SD): −31.4 (50) PFMT, mean (SD): −32.1 (38.4), MD = 0.70 (95% CI −9.46 to 10.86) |
|
| Improved UDI score (3 mo) | Pessary, mean (SD): −33.9 (38.5) PFMT, mean (SD): −30.7 (33.4), MD = −3.20 (95% −11.42 to 5.02) |
|
| Improved QUID stress score (3 mo) | Pessary, mean (SD): −4.2 (6.2) PFMT, mean (SD): −4 (3.6), MD = −0.20 (95% CI −1.35 to 0.95) |
|
| Pessary (n = 149) Pessary + PFMT (n = 150) |
Improved PGI-I (3 mo) | Pessary: 59/149 Pessary + PFMT: 80/150, RR = 0.74 (95% CI 0.58 to 0.95) |
| Improved PGI-I (12 mo) | Pessary: 47/149 Pessary + PFMT: 49/150, RR = 0.97 (95% CI 0.69 to 1.34) |
|
| No bothersome symptoms on UDI subscale of PFDI (3 mo) | Pessary: 49/149 Pessary + PFMT: 66/150, RR = 0.75 (95% CI 0.56 to 1.00) |
|
| No bothersome symptoms on UDI subscale of PFDI (12 mo) | Pessary: 52/149 Pessary + PFMT: 49/150, RR = 1.07 (95% CI 0.78 to 1.47) |
|
| > 75% reduction weekly incontinence episodes (3 mo) | Pessary: 69/149 Pessary + PFMT: 80/150, RR = 0.87 (95% CI 0.69 to 1.34) |
|
| > 75% reduction weekly incontinence episodes (12 mo) | Pessary: 51/149 Pessary + PFMT: 52/150, RR = 0.99 (95% CI 0.72 to 1.35) |
|
| Pessary + PFMT (n = 150) PFMT (n = 146) |
Improved PGI-I (3 mo) | Pessary + PFMT: 80/150 PFMT: 72/146, RR = 1.08 (95% CI 0.87 to 1.35) |
| Improved PGI-I (12 mo) | Pessary + PFMT: 49/150 PFMT: 48/146, RR = 0.99 (95% CI 0.72 to 1.38) |
|
| No bothersome symptoms on UDI | Pessary + PFMT: 66/150 | |
| subscale of PFDI (3 mo) | PFMT: 71/146, RR = 0.90 (95% CI 0.71 to 1.16) | |
| No bothersome symptoms on UDI subscale of PFDI (12 mo) | Pessary + PFMT: 49/150 PFMT: 59/146, RR = 0.81 (95% CI 0.60 to 1.09) |
|
| 75% reduction weekly incontinence episodes (3 mo) | Pessary + PFMT: 80/150 PFMT: 68/146, RR = 1.15 (95% CI 0.91 to 1.44) |
|
| > 75% reduction weekly incontinence episodes (12 mo) | Pessary + PFMT: 52/150 PFMT: 54/146, RR = 0.94 (95% CI 0.69 to 1.27) |
|
Abbreviations: CI, confidence interval; MD, mean difference; PFMT, pelvic floor muscle training; PFDI, Pelvic Floor Distress Inventory; PGI-I, Patient Global Impression of Improvement; QUID, Questionnaire for Urinary Incontinence Diagnosis; RCT, randomized controlled trial; RR, risk ratio; SD, standard deviation; SUI, stress urinary incontinence; UDI, urinary distress inventory; UIQ, Urinary Impact Questionnaire; USP, Urinary Symptom Profile.
For pessary compared with no treatment, there was no significant difference in the number of incontinence episodes per week, over a follow-up period of 2 weeks (mean difference [MD] = −24.10, 95% confidence interval [CI] −49.60 to 1.40).27 However, people with pessaries had significantly improved subscores in the SUI category (MD = −2.20, 95% CI −3.47 to −0.93) as well as the dysuria category (MD = −0.50, 95% CI −0.92 to −0.08) of the Urinary Symptom Profile questionnaire (USP) compared with people who received no treatment.27 The USP is a standardized tool assessing urinary symptoms among people with stress, urge, frequency, or urinary obstructive symptoms for use in clinical practice to complement clinical measures and diagnosis.32
We rated the quality of the evidence as very low, downgrading for risk of bias and imprecision (Table A5, Appendix 3).
Pessaries Compared With Active Treatment
Richter et al29 and Kenton et al31 reported the improvement of symptoms in patients treated with: a) a pessary compared with PFMT, b) a pessary alone compared with a pessary plus PFMT, and c) a pessary plus PFMT compared with PFMT alone. Validated scales used to measure symptoms were the Patient Global Impression of Improvement (PGI-I) used to rate the response of a condition to a therapy,33 Urinary Distress Inventory (UDI),34 Pelvic Floor Distress Inventory (PFDI, which incorporates UDI as a sub-scale),35 Urinary Impact Questionnaire (UIQ),36 and the Questionnaire for Urinary Incontinence Diagnosis (QUID).37
Overall, people receiving pessaries were statistically significantly more likely to report bothersome symptoms compared with PFMT alone on the UDI subscale of the PFDI at 3 months follow-up. However, at 12 months follow-up, this difference narrowed and there was no significant difference between the treatment groups.
After 3 months, people receiving pessaries alone were statistically significantly less likely to report that their continence was much or very much better compared with pessary combined with PFMT PGI-I. However, by 12 months, the proportions were similar (47/149 compared with 49/150, respectively (Table 3).
There were no significant differences in any of the symptom outcomes for the comparison of people treated with a pessary plus PFMT versus PFMT alone.29
We rated the quality of the evidence as low, downgrading for risk of bias (Table A5, Appendix 3).
QUALITY OF LIFE
One RCT, by Cornu et al,27 reported no significant difference in quality of life (as measured with the CONTILIFE questionnaire38) between patients treated with a vaginal loop pessary compared with no treatment (Table 4).
Table 4:
Results of the Randomized Controlled Trial Reporting Quality of Life in People With Stress Urinary Incontinence Treated With a Vaginal Loop Pessary or No Treatmenta
| Comparison | Quality of Life | Results |
|---|---|---|
| Pessary (n = 22) No treatment (n = 24) |
CONTILIFE questionnaire (14 d) | Pessary, mean (SD): −12.7 (22.6) No treatment, mean (SD): −2.4 (11.3), MD = −10.30 (95% CI −20.77 to 0.17) |
We rated the quality of the evidence of this outcome for pessary compared with no treatment as very low, downgrading for risk of bias and imprecision (Table A5, Appendix 3).
PATIENT SATISFACTION
The RCT by Richter et al29 reported fewer people treated with pessaries alone were satisfied with treatment at 3-months follow-up compared with people who were treated with a pessary plus PFMT (Table 5). However, by 12 months, the proportions were similar. Satisfaction with treatment was determined using the validated Patient Satisfaction Questionnaire.29
Table 5:
Results of the Randomized Controlled Trial by Richter et al29 Reporting Satisfaction With Treatment in Patients With Stress Urinary Incontinence Treated With a Pessary or Pelvic Floor Muscle Therapya
| Comparison | Satisfaction | Results |
|---|---|---|
| Pessary (n = 146) PFMT (n = 149) |
Satisfaction with treatment (PSQ) (3 mo) | Pessary: 94/146 PFMT: 110/149, RR = 0.87 (95% CI 0.75 to 1.02) |
| Satisfaction with treatment (PSQ) (12 mo) | Pessary: 75/46 PFMT: 79/149, RR = 0.97 (95% CI 0.78 to 1.21) |
|
| Pessary (n = 146) Pessary + PFMT (n = 150) |
Satisfaction with treatment (PSQ) (3 mo) | Pessary: 94/146 Pessary + PFMT: 118/150, RR = 0.82 (95% CI 0.71 to 0.95) |
| Satisfaction with treatment (PSQ) (12 mo) | Pessary: 75/146 Pessary + PFMT: 81/150, RR = 0.95 (95% CI 0.77 to 1.18) |
|
| Pessary + PFMT (n = 150) PFMT (n = 146) |
Satisfaction with treatment (PSQ) (3 mo) | Pessary + PFMT: 118/150 PFMT: 110/146, RR = 0.82 (95% CI 0.54 to 1.23) |
| Satisfaction with treatment (PSQ) (12 mo) | Pessary + PFMT: 81/150 PFMT: 79/146, RR = 1.15 (95% CI 0.92 to 1.44) |
There were no significant differences in satisfaction between pessary compared with PFMT or pessary plus PFMT compared with PFMT alone reported at either 3 or 12 months follow-up by Richter et al29 (Table 5).
We rated the certainty of the evidence for this outcome for a) pessary compared with PFMT, b) pessary alone compared with pessary plus PFMT, and c) pessary plus PFMT compared with PFMT alone as low, downgrading for risk of bias (Table A5, Appendix 3).
COMPLICATIONS
One RCT, by Nygaard et al28 reported discomfort or pain experienced by people with SUI who received the Hodge pessary compared with no treatment, tampon compared with no treatment, or tampon compared with the Hodge pessary. Participants wore the tampon or Hodge pessary during three separate 40-minute standardized aerobics sessions. After each aerobics session, participants were asked if they experienced any discomfort or pain while exercising. There was no significant difference between the three groups in self-reported discomfort or pain (Table 6).
Table 6:
Results of the Randomized Controlled Trial by Nygaard et al28 Reporting Complications in Patients With Stress Urinary Incontinence Treated With a Hodge Pessary or Tampon, or With No Treatmenta
| Comparison | Complications | Results |
|---|---|---|
| Hodge Pessary (n = 18) | Discomfort or pain | Hodge pessary: 4/18 |
| No treatment (n = 18) | No treatment: 0/18, RR = 9.00 (95% CI 0.52 to 155.86) | |
| Tampon (n = 18) | Discomfort or pain | Tampon: 2/18 |
| No treatment (n = 18) | No treatment: 0/18, RR = 5.00 (95% CI 0.26 to 97.37) | |
| Tampon (n = 18) | Discomfort or pain | Tampon: 2/18 |
| Hodge Pessary (n = 18) | Hodge pessary: 4/18, RR = 0.50 (95% CI 0.10 to 2.40) |
We rated the certainty of the evidence of this outcome for pessary alone compared with tampon alone or no treatment as low, downgrading due to risk of bias and imprecision (Table A5, Appendix 3).
DELAYED NEED FOR SURGERY
None of the included studies reported on this outcome.
PESSARY WITHDRAWAL
The RCT by Richter et al29 reported significantly more people treated with pessary alone (26%, 39/149) withdrew after 3-months follow-up due to failure, lack of efficacy, or dissatisfaction compared with PFMT alone (15%, 22/146) (Table 7). However, this difference was attenuated after 12 months with no significant difference between the groups.29
Table 7:
Results of the Randomized Controlled Trial by Richter et al29 Reporting Withdrawal Due to Failure, Lack of Efficacy, or Dissatisfaction in Patients With Stress Urinary Incontinence Treated With a Pessary or Pelvic Floor Muscle Therapya
| Comparison | Symptom | Results |
|---|---|---|
| Pessary (n = 149) PFMT (n = 146) |
Withdrawal (3 mo) | Pessary: 39/149 PFMT: 22/146, RR = 1.74 (95% CI 1.09 to 2.78) |
| Withdrawal (12 mo) | Pessary: 53/149 PFMT: 47/146, RR = 1.10 (95% CI 0.80 to 1.52) |
|
| Pessary (n = 149) Pessary + PFMT (n = 150) |
Withdrawal (3 mo) | Pessary: 39/149 Pessary + PFMT: 18/150, RR = 2.18 (95% CI 1.31 to 3.63) |
| Withdrawal (12 mo) | Pessary: 53/149 Pessary + PFMT: 39/150, RR = 1.37 (95% CI 0.97 to 1.93) |
|
| Pessary + PFMT (n = 150) PFMT (n = 146) |
Withdrawal (3 mo) | Pessary + PFMT: 18/150 PFMT: 22/146, RR = 0.80 (95% CI 0.45 to 1.42) |
| Withdrawal (12 mo) | Pessary + PFMT: 39/150 PFMT: 47/146, RR = 0.81 (95% CI 0.56 to 1.16) |
Similarly, significantly more people treated with pessary alone (26%, 39/149) withdrew after 3-months follow-up due to failure, lack of efficacy, or dissatisfaction compared with pessary combined with PFMT (12%, 18/150) (Table 7). However, by 12 months, the authors reported was no significant difference between the two treatment groups.12,29
We rated the certainty of the evidence of this outcome for a) pessary compared with PFMT, b) pessary alone compared with pessary plus PFMT, and c) pessary plus PFMT compared with PFMT alone as low, downgrading for risk of bias (Table A5, Appendix 3).
SEXUAL FUNCTION
None of the included studies reported on this outcome.
ANORECTAL OR URINARY VOIDING DYSFUNCTION
None of the included studies reported on this outcome.
LOWER URINARY TRACT SYMPTOMS
None of the included studies reported on this outcome.
Pelvic Organ Prolapse
Three systematic reviews were identified that assessed the use of pessaries for the treatment of POP.9,39,40 Characteristics of the systematic reviews are shown in Table A1, Appendix 2.
We assessed the risk of bias of the systematic reviews by the National Institute for Health and Care Excellence (NICE)39 and by Bugge et al9 as low (Appendix 3). The risk of bias of the systematic review by de Albuquerque Coelho et al40 was assessed as high due to the lack of an explicit statement about comparators or outcomes of interest or discussion regarding bias in primary studies. No meta-analysis was conducted in the systematic reviews.
The NICE systematic review39 included two RCTs41,42 and the systematic review by Bugge et al9 included one RCT.43 Four of the studies included in the systematic review by de Albuquerque Coelho et al40 were eligible for inclusion in our systematic review,43–46 including an RCT by Cundiff et al.43 The other three studies were observational in design.44–46
In addition to the primary studies identified from the systematic reviews, two additional studies were identified in our literature search.47,48 The characteristics of the eight included primary studies are shown in Table 8. Data extracted from the systematic reviews were checked against the primary studies.
Table 8:
Characteristics of Included Studies for Pelvic Organ Prolapse
| Author, Year, Country | Study Design and Methods | Participants | Inclusion/Exclusion Criteria | Intervention | Outcomes of Interest |
|---|---|---|---|---|---|
| Cheung et al,42 2016 Hong Kong (Included in NICE systematic review) |
Randomized parallel group trial | N = 276 Symptomatic POP |
Inclusion criteria: people with symptoms of prolapse, stage 1 to 3 POP, using POPQ system; no previous treatment received. Exclusion criteria: active complications arising from the prolapse; impaired mobility; cognitive impairment; language barrier. |
PFMT: standardized PFMT course offered by registered nurse specialist. Pessary: Standardized PFMT course plus fitting of a vaginal pessary. Estrogen cream was offered if there was a vaginal ulcer. All participants received PFMT (teaching session within 2 wk after first consultation, and three individual training sessions at 4, 8 and 16 wk). Advised to practice daily with at least two sets of 8–12 pre-set exercise repetitions per day, with 8–10 exercises per session at least 2×/wk. Both groups received a phone consultation 2 wk post sessions. If pessary slipped out, participant was offered a reassessment and replacement. If pessary fitting was unsuccessful, conservative management or surgery was discussed. |
Assessed at 6 and 12 mo follow-up: POPDI UDI CRADI POPIQ UIQ CRAIQ Adverse events De novo urinary symptoms Improvement of pre-existing urinary symptoms |
| Panman et al,41 2016 Netherlands (Included in NICE systematic review) |
Randomized parallel group trial | N = 162 POP stage 2, n = 120 POP stage 3, n = 42 |
Inclusion criteria: aged ≥ 55 y and registered in a participating primary care practice. Exclusion criteria: people who had undergone prolapse treatment in the previous year; people who are currently undergoing treatment for another urogynecological disorder; participants with a pelvic organ malignancy, impaired mobility, severe or terminal illness, or cognitive impairment; insufficient Dutch language comprehension. |
PFMT: exercises during face to face and at home (3–5×/wk, 2–3×/d). All participants started with the same exercise regimen, which was later tailored to the needs of each individual. For participants with an overactive pelvic floor, relaxation exercises were used rather than contraction. All participants were taught the “the knack” – how to contract their pelvic floor muscles before and during any increase in abdominal pressure. Data was collected on toilet habits and lifestyle (e.g., diet, smoking, body weight). Pessary: participants in whom the pessary fell out or who experienced discomfort within the first 2 wk were refitted and reviewed again after an additional 2 wk. Pessary fitting was regarded as unsuccessful if it was not successfully fitted after three attempts. In cases of vaginal discharge, irritation, or erosions, participants were advised not to wear the pessary for 2 wk. Topical estrogen was suggested in cases of discharge or ulceration due to vaginal atrophy. |
Assessed at 3 and 12 mo follow-up: PFDI POPDI CRADI UDI PFIQ PISQ PCS MCS Self reported change of symptoms Adverse events |
| Cundiff et al,43 2007 United States (Included in systematic review by de Albuquerque Coelho et al40and Bugge et al9) |
Randomized crossover trial | N = 134 Symptomatic POP of stage 3 or greater |
Inclusion criteria: people presenting with POP (stage 2 or greater) at six clinical practices who expressed interest in nonsurgical treatment. Exclusion criteria: pregnancy, prior pessary use, and vaginal narrowing or agglutination on exam that was felt to compromise pessary use. |
Ring pessary with support Gelhorn pessary | Assessed at 1, 6 and 12 wk follow-up: PFDI PFIQ POPQ |
| Abdool et al,45 2011 United Kingdom (Included in de Albuquerque Coelho et al40) |
Observational comparative retrospective | N = 554 Symptomatic POP |
Inclusion criteria: people recruited 2002–2007 who were in the pessary group and chose pessaries rather than surgery as a treatment option. Exclusion criteria: women fitted with pessaries solely for urinary incontinence or those who underwent concomitant urinary incontinence surgery. |
Vaginal pessary. When participants opted for pessary, the ring was the first choice. If the ring was not retained, then sexually active people were fitted with a cube pessary while people who were not sexually active were fitted with a Gellhorn or donut pessary. Concomitant vaginal estrogen was prescribed only if there was evidence of vaginal atrophy. Surgery | Assessed at 1 y follow-up: SPSQ |
| Mamik et al44 2013 United States (Included in de Albuquerque Coelho et al40) |
Observational comparative Prospective |
N = 100 Symptomatic POP ≥ stage 2 |
Inclusion criteria: aged ≥ 18 years, eligible for either surgery or pessary treatment, able to read and write in English, and able to give consent. | Pessary Surgery |
Assessed at 3 mo follow-up: Patient goals (categorized qualitatively by expert consensus) PGI-I PFDI PISQ BIS |
| Lone et al46 2015 United Kingdom (Included in de Albuquerque Coelho et al40) |
Observational comparative retrospective | N = 287 Symptomatic POP |
Inclusion criteria: people recruited 2009–2010 who were in the pessary group and chose pessaries rather than surgery as a treatment option. Exclusion criteria: people fitted with pessaries solely for urinary incontinence or who underwent concomitant urinary incontinence surgery. |
Vaginal pessary. When participants opted for pessary, the ring was the first choice. If ring not retained, Gellhorn or donut pessary fitted if patient not sexually active and a cube pessary if patient sexually active. Concomitant vaginal estrogen only prescribed if evidence of vaginal atrophy. Surgery |
Assessed at 1 y follow-up: ICIQ-VS ICIQ-UI |
| Coolen et al,47 2018 Netherlands |
Observational comparative prospective | N = 113 Symptomatic POP ≥ Stage 2 |
Inclusion criteria: symptomatic POP ≥ Stage 2. Exclusion criteria: previous surgery for correction of POP or urinary incontinence or previously treated with pessary, contraindication to surgical intervention, isolated rectocele without prolapse of any other compartment (due to possible insufficient support for a pessary). |
Shelf (Falk) or ring pessary (with or without central support)-follow-up visit 6 wk after fitting and every 3–4 mo for cleaning and vaginal inspection. Surgery. Correction of all compartments that required surgery. Technique chosen based on gynecologist's discretion. When SUI was diagnosed prior to surgery, the patient and surgeon decided whether to perform concomitant incontinence procedure or to first perform POP surgery only, with additional SUI surgery later. Additional interventions could include physiotherapy and incontinence surgery in pessary group and physiotherapy, incontinence surgery, or surgery for recurrent prolapse in surgery group. |
Assessed at 12 mo follow-up: UDI Complications |
| Sung et al,48 2016 United States |
Observational comparative prospective | N = 160 Symptomatic POP ≥ Stage 2 |
Inclusion criteria: aged ≥ 18 years; participants choosing any type of POP surgery or anticipating long-term pessary use. Exclusion criteria: no symptomatic, documented POP; no cognitive or language barriers; patients planning short-term pessary use. |
Pessary Surgery |
Assessed at 12 mo follow-up: Achievement of patient symptom or function goals PROMIS |
Abbreviations: BIS, body image scale; CRADI, Colorectal Anal Distress Inventory; CRAIQ, Colorectal Anal Impact Questionnaire; ICIQ-VS, International Consultation on Incontinence Questionnaire-Vaginal Symptoms; ICIQ-UI, International Consultation on Incontinence Questionnaire-Urinary Incontinence; MCS, mental component score; PCS, physical component score; PFDI, Pelvic Floor Distress Inventory; PFIQ, Pelvic Floor Impact Questionnaire; PFMT, pelvic floor muscle training; PGI-I, Patient Global Improvement Index; PISQ, Pelvic Incontinence Sexual Questionnaire; POP, pelvic organ prolapse; POPDI, POP Distress Inventory; POPIQ, POP Impact Questionnaire; POPQ, POP Quantification System; PROMIS, Patient Reported Outcomes Measurement System; SPSQ, Satisfaction With Performance Scaled Questionnaire; SUI, stress urinary incontinence; UDI, Urogenital Distress Inventory; UIQ, Urinary Impact Questionnaire.
IMPROVEMENT OF SYMPTOMS
Pessary Plus PFMT Compared With PFMT Alone
One RCT by Cheung et al42 reported a clinically important difference favouring pessary plus PFMT compared with PFMT alone on the POP Distress Inventory (POPDI) at 6 and 12 months in people with POP (Table 9).39 There was no significant difference observed between pessary plus PFMT and PFMT alone on the Urogenital Distress Inventory (UDI) or the Colorectal Anal Distress Inventory (CRADI) scores at 6 or 12 months.39 However, as noted by NICE,39 the mean difference (MD) between treatment groups was not estimable from the data presented by Cheung et al,42 because they reported the median and interquartile range for all symptom scores (Table 9). There was a significant difference favouring PFMT plus pessary over PFMT alone on the POP Impact Questionnaire (POPIQ) scores at 12 months, but not at 6 months for people with POP.39 Additionally, NICE reported no significant difference between PFMT plus pessary and PFMT alone on the UIQ or the Colorectal Anal Impact Questionnaire (CRAIQ) scores at 6 and 12 months in people with POP (Table 9).39
Table 9:
Results of Randomized Controlled Trials Reporting Improvement of Symptoms in People With Pelvic Organ Prolapse
| Comparisona | Symptom | Results |
|---|---|---|
| Cheung et al42 | ||
| Pessary + PFMT (n = 139) PFMT (n = 137) |
POPDI (6 mo) | Pessary + PFMT, median (IQR): 40.7 (11.3–100) PFMT, median (IQR): 54.8 (22.6–103.6), P = .02 |
| POPDI (12 mo) | Pessary + PFMT, median (IQR): 32.1 (12.5–78.6) PFMT, median (IQR): 49.4 (21.4–95.2), P = .04 |
|
| UDI (6 mo) | Pessary + PFMT, median (IQR): 42.8 (21.0–81.3) PFMT, median (IQR): 41.0 (19.8–80.7), P = .87 |
|
| UDI (12 mo) | Pessary + PFMT, median (IQR): 39.4 (16.9–74.7) PFMT, median (IQR): 37.5 (16.7–67.5), P = .57 |
|
| CRADI (6 mo) | Pessary + PFMT, median (IQR): 42.3 (12.1–86.9) PFMT median (IQR): 40.6 (15.5–83.0), P = .92 |
|
| CRADI (12 mo) | Pessary + PFMT, median (IQR): 32.1 (15.8–75.5) PFMT median (IQR): 32.1 (14.9–68.0), P = .80 |
|
| POPIQ (6 mo) | Pessary + PFMT, median (IQR): 5.6 (0–42.4) PFMT median (IQR): 8.3 (0–76.5), P = .22 |
|
| POPIQ (12 mo) | Pessary + PFMT, median (IQR): 0.3 (0–22.2) PFMT median (IQR): 8.9 (0–64.9), P = .02 |
|
| UIQ (6 mo) | Pessary + PFMT, median (IQR): 15.3 (1.6–48.6) PFMT median (IQR): 11.1 (0–56.9), P = .33 |
|
| UIQ (12 mo) | Pessary + PFMT, median (IQR): 13.3 (0–40.3) PFMT median (IQR): 9.7 (0–54.8), P = .71 |
|
| CRAIQ (6 mo) | Pessary + PFMT, median (IQR): 0 (0–5.6) PFMT median (IQR): 0 (0–8.5), P = .90 |
|
| CRAIQ (12 mo) | Pessary + PFMT, median (IQR): 0 (0–5.6) PFMT median (IQR): 0 (0 to 5.6), P = .77 |
|
| Panman et al41 | ||
| Pessary (n = 82) PFMT + feedback/electrical stimulation/lifestyle advice (n = 80) |
PFDI (3 mo) PFDI (12 mo) |
N= 112, MD = 0.50 (95% CI −8.79 to 9.79) N = 111, MD = 4.40 (95% CI −4.86 to 13.66) |
| PFDI (24 mo) | N = 138, MD = 6.90 (95% CI −1.31 to 15.11) | |
| CRADI (3 mo) | N = 113, MD = 2.00 (95% CI −1.83 to 5.83) | |
| CRADI (12 mo) | N = 114, MD = 1.10 (95% CI −2.67 to 4.87) | |
| CRADI (24 mo) | N = 141, MD = 2.10 (95% CI −1.27 to 5.47) | |
| UDI (3 mo) | N = 114, MD = −3.60 (95% CI −8.21 to 1.01) | |
| UDI (12 mo) | N = 115, MD = −0.50 (95% CI −5.05 to 4.05) | |
| UDI (24 mo) | N = 140, MD = −1.00 (95% CI −5.04 to 3.04) | |
| POPDI (3 mo) | N = 115, MD = 2.90 (95% CI −0.62 to 6.42) | |
| POPDI (12 mo) | N = 117, MD = 4.10 (95% CI 0.64 to 7.56) | |
| POPDI (24 mo) | N = 141, MD = 4.70 (95% CI 1.61 to 7.79) | |
Abbreviations: CRADI, Colorectal Anal Distress Inventory; CRAIQ, Colorectal Anal Impact Questionnaire; IQR, interquartile range; MD, mean difference; PFDI, Pelvic Floor Distress Inventory; PFMT, pelvic floor muscle training; POPDI, Pelvic Organ Prolapse Distress Inventory; POPIQ, Pelvic Organ Prolapse Impact Questionnaire; RCT, randomized controlled trial; UDI, Urogenital Distress Inventory; UIQ, Urinary Impact Questionnaire.
The median scores of the POPDI and POPIQ were also compared within the two groups. Median POPDI and POPIQ scores in the pessary plus PFMT group improved significantly at 6 and 12 months when compared at baseline (P = .05), whereas no significant difference was observed in the PFMT alone group. NICE rated the certainty of the evidence using GRADE of symptom outcomes for pessary plus PFMT compared with PFMT alone as very low, downgrading for risk of bias and imprecision (Table A6, Appendix 3).39
Pessary Compared With PFMT Plus Feedback/Electrical Stimulation/Lifestyle Advice
The RCT by Panman et al41 compared pessary alone (mostly ring pessaries) with PFMT plus feedback, electrical stimulation, and/or lifestyle advice. The POPDI measured at 12 and 24 months showed a significant difference favouring pessary use over PFMT plus feedback/electrical stimulation/lifestyle (Table 9).41
Interestingly, within the pessary alone group, the mean POPDI score improved by 4.2 points (24%) from baseline (17.4 ± standard deviation [SD] 13.5) to 3 months (13.2 ± 12.5), and the improvement remained until the end of the study at 24 months (12.9 ± 13.1). Within the PFMT plus feedback/electrical stimulation/lifestyle advice group, the mean POPDI score improved slightly from baseline (16.9 ± 13.0) to 3 months (15.6 ± 13.6), but worsened by 24 months (17.1 ± 15.9). A within-group statistical test was not reported by the authors.
NICE rated the GRADE of symptom outcomes for pessary compared with PFMT plus feedback/electrical stimulation/lifestyle advice as low, downgrading for risk of bias (Table A6, Appendix 3).39
One randomized crossover trial by Cundiff et al43 compared a ring pessary with support to a Gelhorn pessary in people with POP. The authors randomly assigned 134 people to use each pessary for 3 months. At the end of follow-up, they collected data for 94 ring and 99 Gelhorn pessary patients. They did not present primary data in tables, but displayed intra- and inter-group change in symptom scores in graphs.
The POPDI scales and subscales measured statistically and clinically significant improvements from baseline for both pessaries (P = .05).9 The POPIQ scales measured statistically significant improvement from baseline for both pessaries (P = .05), and a clinically significant improvement from baseline in POPIQ was found for the Gelhorn pessary.9 There were no significant differences in terms of improvement in POPDI (P = .99) or POPIQ (P = .29) in direct comparisons between the ring with support and the Gellhorn pessaries.43 The authors also reported no significant difference in UDI (P = .62), UIQ (P = .74), Colorectal Anal Distress Inventory (CRADI; P = .91), and Colorectal Anal Impact Questionnaire (CRAIQ; P = .29) scores between the two pessaries.43
We rated the GRADE of this outcome for ring with support pessary compared with Gelhorn pessary as very low, downgraded for risk of bias (Table A6, Appendix 3).
Pessary Compared With Surgery
Two prospective, observational, comparative studies reported on improvement of POP symptoms in people treated with a pessary compared with surgery.46,47
In a study by Coolen et al,47 the UDI prolapse domain score was the primary outcome assessed in women treated with pessaries compared with surgery. Significant differences were noted in baseline characteristics between the study groups. The pessary group was significantly older than the surgery group (P = .05) and had higher POP Quantification stages of the anterior (P = .01) and posterior (P = .02) pelvic compartments than the surgery group.
After 12 months of treatment, Coolen et al47 reported a significant difference in the median prolapse domain scores between patients who received pessaries compared with surgery (0 [10th to 90th percentile: 0 to 33] in the pessary group and 0 [10th to 90th percentile: 0 to 0] in the surgery group) (Table 10). This means that 10% of participants in the pessary group had a score of 33 or more in the genital prolapse symptom domain while all participants in the surgery group had a score of 0 in the same domain. This indicates that UDI prolapse symptoms were less severe in patients who underwent surgery compared with treatment with a pessary. Other domain scores were not significantly different.
Table 10:
Results of the Observational Study by Coolen et al47 Reporting Improvement of Symptoms in Patients With Pelvic Organ Prolapse Treated With a Pessary or Surgery
| Comparison | Symptom | Results |
|---|---|---|
| Pessary (n = 60) Surgery (n = 26) |
UDI prolapse | Pessary, median (10–90 percentile): 0.0 (0–33) Surgery, median (10–90 percentile): 0.0 (0–0), P < .01 |
| UDI overactive bladder | Pessary, median (10–90 percentile):0.0 (0–33) Surgery, median (10–90 percentile): 5.6 (0–56), P = .56 |
|
| UDI incontinence | Pessary, median (10–90 percentile): 16.7 (0–35) Surgery, median (10–90 percentile): 33.3 (0–50), P = .96 |
|
| UDI obstructive micturition | Pessary, median (10–90 percentile): 0.0 (0–35) Surgery, median (10–90 percentile): 0.0 (0–33), P = .39 |
|
| UDI pain/discomfort | Pessary, median (10–90 percentile): 0.0 (0–33) Surgery, median (10–90 percentile): 0.0 (0–33), P = .74 |
|
| UDI recurrent bladder infections (never) | Pessary: 24/60 (40%) Surgery: 12/26 (46%), P = .42 |
|
| IIQ physical | Pessary, median (10–90 percentile): 0.0 (0–33) Surgery, median (10–90 percentile): 0.0 (0–13), P = .07 |
|
| IIQ mobility | Pessary, median (10–90 percentile): 0.0 (0–33) Surgery, median (10–90 percentile): 0.0 (0–31), P = .71 |
|
| IIQ social | Pessary, median (10–90 percentile): 0.0 (0–11) Surgery, median (10–90 percentile): 0.0 (0–9), P = .86 |
|
| IIQ shame | Pessary, median (10–90 percentile): 0.0 (0–22) Surgery, median (10–90 percentile): 0.0 (0–17), P = .99 |
|
| IIQ emotional | Pessary, median (10–90 percentile): 0.0 (0–37) Surgery, median (10–90 percentile): 0.0 (0–11), P = .31 |
Abbreviations: IIQ, Incontinence Impact Questionnaire; UDI, Urinary Distress Inventory.
The second prospective, observational comparative study, by Lone et al,46 used the International Consultation on Incontinence Questionnaire–Vaginal Symptoms (ICIQ-VS) and –Urinary Incontinence (ICIQ-UI) to assess patients after 1 year of treatment for POP using a pessary (ring: 101/133, Gelhorn: 28/133, cube: 2/133, or donut: 2/133) or surgery (n = 154). Twelve patients discontinued use of their pessary within 6 months of insertion. Reasons for discontinuation included difficulty in retaining the pessary (n = 7), vaginal discomfort (n = 2), and vaginal discharge (n = 3).46
There were no significant differences between the pessary-treated and surgery patients at baseline, except for age.46 The mean age (±SD) of people who received a pessary was 67 ± 14.1 years, compared with 59 ± 11.9 years for people who chose surgery, P = .03.46 At the 1-year follow-up, 80 people in the pessary group (60%) and 103 people in the surgery group (67%) completed the ICIQ-VS questionnaire.46 There was significant improvement between baseline and follow-up in all vaginal and quality of life symptoms for people treated with a pessary or surgery (P < .05).46
The authors reported no significant differences in the change in reported vaginal, sex, or quality of life scores experienced by people treated with a pessary compared with surgery (P = .12, .25, and .36, respectively).46 The authors did not explicitly specify whether the “change in score” was the mean change in scores, and no standard deviation was reported.
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgrading for risk of bias (Table A6, Appendix 3).
QUALITY OF LIFE
Pessary Compared With PFMT Plus Feedback/Electrical Stimulation/Lifestyle Advice
One RCT, by Panman et al,41 showed no significant difference between pessary alone and PFMT plus feedback/electrical stimulation/lifestyle advice on Pelvic Floor Impact Questionnaire scores at 3, 12, and 24 months (Table 11).
Table 11:
Results of the Randomized Controlled Trial by Panman et al41 Reporting Quality of Life in Patients With Pelvic Organ Prolapse Treated With a Pessary or Pelvic Floor Muscle Therapya
| Comparison | Quality of Life | Results |
|---|---|---|
| Pessary (n = 82) | PFIQ (3 mo) | N = 106, MD = 1.30 (95% CI −6.25 to 8.85) |
| PFMT + feedback/electrical stimulation/ | PFIQ (12 mo) | N = 116, MD = −4.20 (95% CI −11.28 to 2.88) |
| lifestyle advice (n = 80) | PFIQ (24 mo) | N = 130, MD = 2.10 (95% CI −4.48 to 8.68) |
NICE rated the certainty of the evidence of this outcome for pessary compared with PFMT plus feedback/electrical stimulation/lifestyle advice as low, downgrading for risk of bias (Table A6, Appendix 3).39
Pessary Compared With Surgery
One observational study by Abdool et al45 assessed women treated with pessaries compared with surgery using the validated Sheffield POP quality of life questionnaire (SPS-Q) at the time of treatment and at a 1-year follow-up. The SPS-Q is a 13-item quality of life assessment tool that addresses the impact of POP on bladder, bowel, and sexual function.45
At baseline, there were no significant differences between the groups except for mean age, which was significantly higher in the pessary group compared with the surgery group (68.4 ± 13.08 vs. 60.4 ± 12.25, respectively). No P value was reported.45
Of 554 people treated for POP, 359 chose a pessary (ring: 296, Gelhorn: 50, cube: 8, and donut: 5) and 195 chose surgery.45 After 1 year of treatment, the SPS-Q was completed by 68% (n = 164) of people treated with a pessary and 55% (n = 107) of people who underwent surgery.45 When age was controlled as a potential confounder, there were no significant differences in any of the SPS-Q scores between patients who chose a pessary compared with those who opted for surgery at 1 year follow-up (all P values > .05).45 The authors did not explicitly specify whether the “change in score” was the mean change in scores and no standard deviation was reported. The authors reported a statistically significant improvement in prolapse, urinary, bowel, and sexual function symptoms from baseline to follow-up in both pessary users and the surgery group (P < .05).45
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgrading for risk of bias (Table A6, Appendix 3).
PATIENT SATISFACTION
Pessary Compared With Surgery
One prospective observational study, by Sung et al,48 reported patient goal attainment at 12 months follow-up after for people with POP who were treated with a pessary compared with surgery. Eighty people were enrolled in each treatment group. Goal attainment consisted of patient reported “symptom goals” (i.e., improvement in prolapse, urinary, bowel symptoms) and “function goals” (i.e., improvement in physical, social, emotional, and sexual function) at baseline and posttreatment.48
In terms of baseline characteristics, people who chose pessary over surgery were significantly older (64.2± 13.0 years [SD] vs. 59.0 ± 10.0 years [SD], respectively), P = .005.48 Additionally, people in the pessary group had a significantly higher median POPQ compared with those treated with surgery (stage 3 vs. 2, range 1–4, respectively; P = .04).
At follow-up, data were available for 64/80 (80%) people who were treated with pessaries and 72/80 (90%) who underwent surgery.48 In general, a higher proportion of people in the surgery arm reported successfully achieving some symptom and functional goals compared with people who chose a pessary (Table 12). However, the authors did not report comparative statistics between these two groups.
Table 12:
Results of the Observational Study by Sung et al48 Reporting Post-Treatment Goal Achievement in People With Pelvic Organ Prolapse Treated With Either a Pessary or Surgerya
| Patient Reported Goals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | All Symptom Goals | Prolapse | Urinary | Bowel | Pain/Discomfort | All Function Goals | Physical | Social | Emotional | Sexual |
| Surgery N = 72 |
43/59 (72.9%) |
24/25 (96.0%) |
27/39 (69.2%) |
5/8 (62.5%) |
23/25 (92.0%) |
31/43 (72.1%) |
26/31 (83.9%) |
4/6 (66.7%) |
4/5 (80.0%) |
10/16 (62.2%) |
| Pessary N = 64 |
42/60 (70.0%) |
23/29 (79.3%) |
23/36 (63.9%) |
5/7 (71.4%) |
20/25 (80%) |
27/44 (61.4%) |
22/35 (62.9%) |
4/5 (80.0%) |
6/7 (85.7%) |
3/9 (33.0%) |
Data represent participants who reported having baseline goals in each category that were subsequently achieved after treatment.
Another prospective observational study, by Mamik et al,44 assessed patient self-reported goal attainment among people with POP who chose surgery (n = 50) compared with those who chose a pessary (n = 50). Goals listed by the participants were categorized through qualitative consensus by five physicians as symptom (prolapse, urinary, bowel, pain), quality of life (physical activity, emotional, sex), avoidance (avoid surgery or make sure problem does not get worse), and body image goals.44 At 3 months follow-up, participants were asked if they met the goals they listed at the initial visit. They scored how well they met their goals on a scale of 0 to 10, with 0 representing not meeting the goal and 10 completely meeting the goal.44 The authors reported that there was no significant difference in baseline characteristics between the treatment groups.44 The distribution of the staging of POP for each group was not reported.
At 3 months follow-up, data were available for 65 people (30 in the pessary group and 35 in the surgery group) (Table 13).44 Overall, people who received surgery ranked their goal attainment higher than people who were treated with a pessary.
Table 13:
Results for the Observational Study by Mamik et al44 Reporting Goal Attainment in Patients With Pelvic Organ Prolapse Treated With a Pessary or Surgery
| Pessary (n = 30) Mean score (SD) |
Surgery (n = 35) Mean score (SD) |
P Value | |
|---|---|---|---|
| Goal 1 | 6.4 (3.0) | 8.6 (1.6) | < .001 |
| Goal 2 | 6.3 (2.6) | 8.6 (1.8) | .001 |
| Goal 3 | 6.3 (3.3) | 8.2 (2.2) | .03 |
Abbreviation: SD, standard deviation.
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgrading for risk of bias (Table A6, Appendix 3).
COMPLICATIONS
Pessary Plus PFMT Compared With PFMT Alone
One RCT42 reported no significant differences between pessary plus PFMT compared with PFMT alone on abnormal vaginal bleeding or “significant vaginal discharge” at 12 months follow-up (Table 14).39 Fifty-six of 132 patients (42.4%) failed to retain their pessary.42
Table 14:
Results for Randomized Controlled Trials Reporting Complications in Patients With Pelvic Organ Prolapse Treated With a Pessary or Pelvic Floor Muscle Training
| Comparisona | Complications | Results |
|---|---|---|
| Cheung et al42 | ||
| Pessary + PFMT (n = 132) PFMT (n = 128) |
Abnormal vaginal bleeding (12 mo) | Pessary + PFMT: 9/132 (6.8%) PFMT: 4/128 (3.1%), RR = 2.18 (95% CI 0.69–6.91) |
| Significant vaginal dischargeb (12 mo) | Pessary + PFMT: 6/132 (4.5%) PFMT: 2/128 (1.6%), RR = 2.91 (95% CI 0.60–14.15) |
|
| Failed to retain pessary | Pessary + PFMT: 56/132 (42.4%) PFMT: NA |
|
| Panman et al41 | ||
| Pessary (n = 82) PFMT + feedback/electrical stimulation/lifestyle advice (n = 80) |
Adverse events (24 mo): | Pessary: 21/35 (60%)
|
Abbreviations: CI, confidence interval; NA, not applicable; PFMT, pelvic floor muscle training; RCT, randomized controlled trial; RR, Risk Ratio; UI, urinary incontinence.
Significant defined as the discharge being unusual and bothersome.
NICE rated the certainty of the evidence for this outcome using GRADE for pessary plus PFMT compared with PFMT alone as very low, downgrading for risk of bias and imprecision (Table A6, Appendix 3).39
Pessary Compared With PFMT Plus Feedback/Electrical Stimulation/Lifestyle Advice
One RCT, by Panman et al41 showed a significant difference favouring PFMT plus feedback/electrical stimulation/lifestyle advice over pessary use on adverse events at 24 months in people with POP (Table 14).
NICE rated the certainty of the evidence using GRADE of symptom outcomes for pessary compared with PFMT plus feedback/electrical stimulation/lifestyle advice as very low, downgrading for risk of bias and imprecision (Table A6, Appendix 3).39
Pessary Compared With Surgery
One prospective observational study by Coolen et al47 reported complications in patients who chose pessary or surgery for the treatment of symptomatic POP. In the pessary group, the most common side effects were vaginal discharge (20%) and vaginal pain (14%)(Table 15).47 In the surgery group, side effects included bleeding during or after surgery, which required reoperation.
Table 15:
Results for the Observational Study by Coolen et al47 Reporting Complications in Patients With Pelvic Organ Prolapse Treated With a Pessary or Surgery
| Comparison | Results |
|---|---|
| Pessary (n = 74) Surgery (n = 39) |
Pessary group: n (%)
Surgery group: n (%) Complications during surgery: 2 (5)
Complications post-surgery: 13 (33)
|
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgrading for risk of bias (Table A6, Appendix 3).
DELAYED NEED FOR SURGERY
None of the included studies reported this outcome.
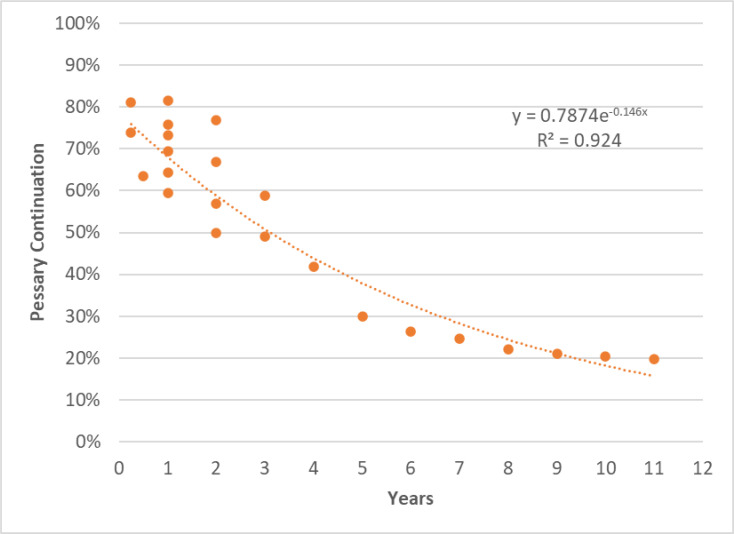
PESSARY COMPLIANCE
One prospective observational comparative study, by Coolen et al,47 reported a pessary continuation rate at 12 months follow-up of 60% (44/74 patients)(Table 16). The reasons for discontinuation were pessary expulsion, urinary incontinence, vaginal pain or discharge, no symptom reduction, or urinary retention.47
Table 16:
Results for the Observational Study by Coolen et al47 Reporting Pessary Compliance in Patients With Pelvic Organ Prolapse Treated With a Pessary
| Continuation rates (N = 74) n (%) | Reason for discontinuation (N = 30) n (%) |
|---|---|
| 4 Weeks: 60 (81) | Pessary expulsion: 7 (23) |
| 3 Months: 60 (81) | Urinary incontinence: 6 (20) |
| 6 Months: 47 (64) | Vaginal pain: 6 (20) |
| 1 Year: 44 (60) | Vaginal discharge: 5 (17) |
| No symptom reduction: 5 (17) | |
| Urinary retention: 1 (3) |
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgrading for risk of bias (Table A6, Appendix 3).
SEXUAL FUNCTION
Pessary Compared With PFMT Plus Feedback/Electrical Stimulation/Lifestyle Advice
One RCT, by Panman et al,41 showed a significant difference favouring pessary over PFMT plus feedback/electrical stimulation/lifestyle advice on Pelvic Incontinence Sexual Questionnaire scores at 3, 12, and 24 months (Table 17).39
Table 17:
Results for the Randomized Controlled Trial by Panman et al41 Reporting Sexual Function in Patients With Pelvic Organ Prolapse Treated With a Pessary or Pelvic Floor Muscle Therapya
| Comparison | Sexual Function | Results |
|---|---|---|
| Pessary (n = 82) | PISQ (3 mo) | n = 44, MD = 2.70 (95% CI 0.87–4.53) |
| PFMT + feedback/electrical | PISQ (12 mo) | n = 48, MD = 2.60 (95% CI 0.88–4.32) |
| stimulation/lifestyle advice (n = 80) | PISQ (24 mo) | n = 130, MD = 1.30 (95% CI 0.25–2.35) |
NICE rated the certainty of the evidence using GRADE of symptom outcomes for pessary compared with PFMT plus feedback/electrical stimulation/lifestyle advice as low, downgrading for risk of bias (Table A6, Appendix 3).39
Pessary Compared With Surgery
One prospective observational study, by Coolen et al,47 reported no significant difference in sexual function after 12 months of treatment with a pessary or with surgery in people with POP (P = .21) (Table 18). The authors did not provide details about the sexual function questionnaire used for their study.
Table 18:
Results for Observational Study by Coolen et al47 Reporting Sexual Function in People With Pelvic Organ Prolapse Treated With a Pessary or Surgery
| Comparison | Sexual Function | Resultsa |
|---|---|---|
| Pessary (n = 60) Surgery (n = 26) |
Sexual function questionnaire | Pessary: 35/53 (68%) Surgery: 21/27 (82%), P = .21 |
Numbers reported by study authors. The cohort given the sexual function questionnaire may not match the cohort chosen for the comparison.
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgrading for risk of bias (Table A6, Appendix 3).
ANORECTAL OR URINARY VOIDING DYSFUNCTION
Pessary Plus PFMT Compared With PFMT Alone
One RCT by Cheung et al42 showed no significant difference between pessary plus PFMT compared with PFMT alone for de novo urinary voiding difficulty in women with POP (Table 19). However, a significant difference was observed for improvement of urinary voiding difficulty favouring pessary plus PFMT over PFMT alone in women with POP (Table 19).
Table 19:
Results for Randomized Controlled Trial by Cheung et al42 Reporting Anorectal or Urinary Voiding Dysfunction in People With Pelvic Organ Prolapse Treated With a Pessary or Pelvic Floor Muscle Therapya
| Complications | Results |
|---|---|
| De novo urinary voiding difficulty | Pessary + PFMT: 10/92 (10.9%) PFMT: 8/97 (8.2%), P = .54; RR = 1.74 (95% CI: 0.71–4.24) |
| Improvement of urinary voiding difficulty | Pessary + PFMT: 25/40 (62.5%) PFMT: 11/31 (35.5%), P = .02; RR = 5.51 (95% CI: 3.01–10.10) |
We rated the certainty of the evidence of this outcome for pessary plus PFMT compared with PFMT alone as very low, downgraded for risk of bias (Table A6, Appendix 3).
Pessary Compared With Surgery
A prospective, observational, comparative study by Coolen et al47 reported no significant differences in the various Defecatory Distress Inventory domain scores at 12 months follow-up as reported by people with POP who underwent treatment with a pessary or surgery (Table 20).
Table 20:
Results for Observational Study by Coolen et al47 Reporting Anorectal or Urinary Voiding Dysfunction in Patients With Pelvic Organ Prolapse Treated With a Pessary or Surgery
| Comparison | Symptom | Results |
|---|---|---|
| Pessary (n = 60) Surgery (n = 26) |
DDI constipation | Pessary: median (10–90 percentile) = 0.0 (0–17) Surgery: median (10–90 percentile) = 0.0 (0–55), P = .69 |
| DDI obstructive defecation | Pessary: median (10–90 percentile) = 0.0 (0–18) Surgery: median (10–90 percentile) = 0.0 (0–33), P = .21 |
|
| DDI pain/discomfort | Pessary: median (10–90 percentile) = 0.0 (0–33) Surgery: median (10–90 percentile) = 0.0 (0–22), P = .25 |
|
| DDI incontinence | Pessary: median (10–90 percentile) = 0.0 (0–33) Surgery: median (10–90 percentile) = 0.0 (0–0), P = .20 |
|
| DDI incontinence flatus | Pessary: median (10–90 percentile) = 0.0 (0–33) Surgery: median (10–90 percentile): 33.3 (0–67), P = .18 |
Abbreviation: DDI, Defecatory Distress Inventory.
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgraded for risk of bias (Table A6, Appendix 3).
LOWER URINARY TRACT SYMPTOMS
Pessary Plus PFMT Compared With PFMT Alone
One RCT, by Cheung et al,42 reported significantly more women developing de novo SUI after receiving a pessary plus PFMT compared with women undergoing PFMT alone (Table 21). However, there was no significant difference in the development of de novo urge UI in women of either treatment group.42
Table 21:
Results for Randomized Controlled Trial by Cheung et al42 Reporting Lower Urinary Tract Symptoms in People With Pelvic Organ Prolapse Treated With Pessary or Pelvic Floor Muscle Therapya
| Complications | Results |
|---|---|
| De novo SUI | Pessary + PFMT: 24/50 (48.0%) PFMT: 13/58 (22.4%), P = .01; RR = 2.14 (95% CI: 1.22–3.75) |
| De novo urge UI | Pessary + PFMT: 17/73 (23.3%) PFMT: 19/84 (22.6%), P = .85; RR = 1.03 (95% CI: 0.58–1.83) |
We rated the certainty of the evidence of this outcome for pessary plus PFMT compared with PFMT alone as very low, downgraded for risk of bias (Table A6, Appendix 3).
One prospective, observational comparative study, by Lone et al,46 used the ICIQ-UI to assess people after 1 year of treatment for POP who chose a pessary (ring: 101/133, Gelhorn 28/133, cube 2/133, and donut 2/133) or surgery (n = 154). Twelve discontinued use of the pessary within 6 months of insertion. Reasons for discontinuation were difficulty in retaining the pessary (n = 7), vaginal discomfort (n = 2), and vaginal discharge (n = 3).46
There were no significant differences between the pessary-treated and surgery patients at baseline, except for age.46 The mean (±SD) age of people who received a pessary was 67 ± 14.1 years compared with 59 ± 11.9 years for people who chose surgery, P = .03.46
The authors noted that, prior to the intervention, 33% of all participants in the study (N = 287) documented symptoms of SUI, 21% of urgency urinary incontinence, and 46% had mixed urinary incontinence.46 Within each study group, significant improvement was noted in the overall ICIQ-UI score at 1 year (P < .05).
The authors reported no significant difference in the change in ICIQ-UI scores experienced by people treated with a pessary compared with surgery (P = .14) at 1 year.46 It is unclear whether the change in ICIQ-UI score reported by the authors was the mean change in scores as this was not explicitly specified and no standard deviation was reported.
We rated the certainty of the evidence of this outcome for pessary compared with surgery as very low, downgraded for risk of bias (Table A6, Appendix 3).
Ongoing Studies
We are aware of the following ongoing studies that have potential relevance to this review. We could not locate information that any of these studies has been published at the time of writing of this assessment.
Study evaluating the efficacy and safety of Yoni. Fit in women with stress urinary incontinence (ClinicalTrials.gov Identifier: NCT03978741)
A clinical study to assess the safety of a disposable intra-vaginal device for stress urinary incontinence (ClinicalTrials.gov Identifier: NCT02160314)
The impact of pessaries in older women's quality of life: a systematic review. PROSPERO 2018 CRD42018103206. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018103206
A systematic review of the use of pessaries for the management of pelvic organ prolapse in women. PROSPERO 2016 CRD42016046793. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016046793
Discussion
Strengths and Limitations
STRESS URINARY INCONTINENCE
Overall, the quality of the evidence related to pessaries for the treatment of SUI was rated as low to very low, downgraded due to risk of bias.
For the treatment of SUI, pessaries were compared with no treatment,27,28 PFMT,29 pessary plus PFMT,29 and a tampon.28 Additionally, one study compared pessary plus PFMT to PFMT alone.29 Follow-up periods ranged from less than 1 day to 12 months. The device reported in the study by Cornu et al27 is not available in Canada.
Significant Improvement of Symptoms Within Pessary Trial Arms
Richter et al29 did not report within-group differences for each of the treatment arms; however, the authors noted that, after 12 months of therapy, one-third of all participants and over one-half of participants still using the assigned treatment reported improved symptom outcomes and satisfaction. The authors concluded that their 1-year data support the consideration of pessaries as a reasonable alternative for people wishing to avoid or defer surgery for SUI and who are not interested in or able to adhere to behavioural therapy (e.g., PFMT).29
Kenton et al31 similarly noted that both the pessary and PFMT groups had clinically meaningful within-group improvement on each of the symptom measures (UDI, UIQ, and QUID); however, the score improvement did not differ significantly between the two treatment arms. Given this, the authors concluded that both PFMT and pessaries have a clinically important role in the treatment of SUI.31 Patients may see symptom improvement with either treatment modality, and individual patient characteristics and preferences can inform decisions among nonsurgical treatment options.31
Challenges in Conducting Randomized Controlled Trials With Pessaries
Conducting RCTs involving interventions such as pessaries for SUI is difficult, with particular obstacles to blinding and defining an appropriate placebo arm.12 Patient blinding may not be feasible, and provider blinding would not be possible.12 In the study by Nygaard et al,28 people allocated to no treatment had a ring pessary inserted into the vagina and then immediately removed. Thirteen of the 18 participants correctly identified that no pessary was in place.28
The systematic review by Lipp et al12 stated that some trial reports were unclear regarding randomization and methods of blinding participants and outcome assessors. The studies generally had small sample sizes,27,28 except for the study by Richter et al (N = 446).29 The addition of behavioural therapy (i.e., PFMT) to the use of a pessary led to small but statistically significant improvements in patient satisfaction, withdrawal, and perceived improvements at 3 months; however, the differences in these effects were attenuated after 12 months, at which point the study authors found no significant differences between the groups.12 Lipp et al12 recommended interpreting these results with caution as participants receiving behavioural therapy had four clinic visits, whereas most people in the pessary-only group had one clinic visit. Higher levels of clinician contact could impact on participant perceptions of satisfaction and improvement. The authors further suggested that, to definitively establish whether combining PFMT with a pessary provides patient benefits, clinician contact must be controlled for and a more robust range of outcome measures should be provided.
PELVIC ORGAN PROLAPSE
The NICE systematic review for symptomatic POP consisted of two RCTs.41,42 In one, pessaries were compared with PFMT plus biofeedback/electrical stimulation/lifestyle advice.41 The other compared pessaries plus PFMT with PFMT alone.42 Follow-up assessments ranged from 3 to 12 months. No RCTs were identified that assessed the effectiveness of pessaries compared with surgery in people with POP. However, five observational studies were identified.44,45,47–49 and the overall quality of evidence was rated as very low, downgraded due to risk of bias.
Recommendations for Use of Pessaries to Treat POP by NICE and Choosing Wisely
The NICE review concluded that there was very low quality evidence for the use of pessaries and that adverse effects are more common with pessary use plus PFMT compared with PFMT alone.39 Despite their assessment of the evidence, the NICE committee found that pessary use remains an important alternative to surgical intervention for people at all stages of POP, including advanced prolapse, and recommended that pessaries should be considered as an option for treatment of prolapse symptoms.39 The NICE committee noted that ongoing pessary care may be difficult for people with physical or cognitive impairment and that it is therefore important for some patients who have an impairment to have an appointment in a pessary clinic every 6 months.39
Similar to NICE, Choosing Wisely, along with the American Urogynecologic Society, recommends the use of pessaries for the treatment of POP.50 The recommendation states, “Nonsurgical treatment options for pelvic organ prolapse include pessaries, which are removable devices that are placed into the vagina to support the prolapsed organs (i.e., uterus, vagina, bladder and/or rectum). A pessary trial can be offered to almost all females with pelvic organ prolapse. Exceptions include people with an active vaginal infection and those who would be noncompliant with follow-up.”
Significant Improvement of Symptoms Within Pessary Trial Arms
Panman et al41 showed no significant difference in the change of pelvic floor symptoms measured by the Pelvic Floor Distress Inventory during the 24-month period in which pessary and PFMT were compared. However, people in the pessary group did show a significantly greater reduction in prolapse-specific symptoms (POPDI) (e.g., vaginal bulging, heaviness or pressure in pelvic area, and vaginal splinting for micturition or bowel movement) compared with people in the PFMT group. The POPDI score decreased by almost 26% between baseline and 24 months in the pessary group (indicating less distress related to prolapse symptoms), whereas the score increased slightly for people in the PFMT group. The authors stated that this suggests that people with typical prolapse symptoms benefit from pessary treatment more than from PFMT, which is plausible given that pessaries redress prolapse directly.41 Panman et al41 also found that more people in the pessary group reported one or more complications compared with people undergoing PFMT. However, a majority of the people in the pessary group continued pessary treatment despite the complications.
Challenges in Conducting Randomized Controlled Trials With Pessaries
Similar to the limitations in studies investigating pessary treatment for SUI, trials involving interventions such as pessaries for POP are difficult to design and implement, especially in terms of blinding of the patients and investigators.
One study, by Coolen et al,47 was originally designed as a RCT, but changed to a prospective observational study when a large majority of potential participants expressed a very strong preference for one treatment option over the other. Previous studies also showed strong patient preference for one or the other of two interventions.51 It is possible that physicians counselled patients differently depending on individual characteristics such as age, comorbidity, sexual activity, POP stage, and symptoms. The authors suggested that the heterogeneity between the treatment groups may be a reflection of normal daily practice, as the average age of participants in the pessary group was higher than in the surgery group, a cohort characteristic similar to earlier studies.44,45,47–49 Lamers et al51 also found that the likelihood of preferring pessary treatment over surgery increases with patient age.
Conclusions
Stress Urinary Incontinence
Compared with no treatment, pessaries:
May improve symptoms, but the evidence is very uncertain
May have little to no effect on quality of life or complications, but the evidence is very uncertain
Compared with PFMT alone, pessaries may result in:
Less improvement in short-term symptoms
Little to no difference in longer-term improvement of symptoms or patient satisfaction
Compared with PFMT alone, pessaries combined with PFMT may result in:
Little to no difference in improvement of symptoms or patient satisfaction
Pelvic Organ Prolapse
Compared with PFMT combined with feedback/electrical stimulation/lifestyle advice, pessaries:
May improve some longer-term symptoms and sexual function
May not improve quality of life
Compared with surgery, pessaries:
May have little to no effect on improvement of symptoms, quality of life, or satisfaction, but the evidence is very uncertain
Economic Evidence
Research Question
What is the cost-effectiveness of vaginal pessaries for the treatment of people with pelvic organ prolapse and/or stress urinary incontinence?
Methods
Economic Literature Search
We performed an economic literature search on June 26, 2019, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE, Embase, and CINAHL and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. The grey literature search was updated on December 2, 2019. See the Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published between January 1, 2000, and June 26, 2019
Cost-benefit analyses, cost-utility analyses, cost-effectiveness analyses, or cost-minimization analyses
Exclusion Criteria
Narrative reviews, letters/editorials, case reports, commentaries, abstracts, posters, unpublished studies, cost analyses
PARTICIPANTS/POPULATION
Adults (≥ 18 years) with symptomatic pelvic organ prolapse (POP) and/or stress urinary incontinence (SUI)
INTERVENTION
Vaginal pessaries
COMPARATOR
Control (i.e., no active treatment)
Conservative treatment (e.g., pelvic floor muscle exercises)
Surgery (e.g., midurethral sling/pelvic reconstruction)
OUTCOME MEASURES
Costs
Health outcomes (e.g., quality-adjusted life-years)
Incremental costs
Incremental effectiveness
Incremental cost-effectiveness ratios
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, incremental cost-effectiveness ratios)
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.52 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
The economic literature search yielded 98 citations published from database inception until June 26, 2019, after removing duplicates. We excluded a total of 87 articles based on information in the title and abstract. We then obtained the full texts of 11 potentially relevant articles for further assessment and excluded six; see Appendix 5 for a list of studies excluded after full-text review. Five studies met our inclusion criteria. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al, 2009.26
Overview of Included Economic Studies
Table 22 provides a summary of the five included studies.
Table 22:
Results of Economic Literature Review—Summary
| Author, Year, Country of Publication | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Intervention(s) and Comparator(s) | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness (ICER, USD/QALY) | ||||
| Pelvic Organ Prolapse | ||||||
| Hullfish et al, 201153 United States |
Cost–utility analysis Decision analytic model (Markov) Perspective not specified 1-y time horizon No discounting |
Post-hysterectomy females with at least stage III apical prolapse of the vagina Mean age: 65 y |
Expectant management Pessary VRS Traditional ASC surgery Robot-assisted ASC surgery |
Total QALYs: Pessary = 0.867 VRS = 0.95 |
2007 USD Pessary = $10,000 VRS = $15,000 |
VRS vs. pessary = $60,240 Pessaries and VRS dominate all other interventions |
| Panman et al, 201641 Netherlands |
Cost–utility analysis Randomized controlled trial Perspective not specified 2-y follow-up No discounting |
162 women (82 pessary, 80 PFMT) At least 55 y of age, with symptomatic prolapse Mean age (y):
|
Pessary PFMT |
Total QALYs:a Pessary = −0.024 PFMT = −0.065 |
2015 USD Direct medial costs:
|
Bootstrapped = −$27,439 (95% CI: −91,974 to 74,695) |
| Stress Urinary Incontinence | ||||||
| Richardson et al, 201454 United States |
Cost–utility analysis Decision analytic model (decision tree) Third-party payer perspective 1-y time horizon No discounting |
Women with uncomplicated symptomatic SUI Mean age 50 y |
Pessary PFMT MUS |
QALYs not provided | 2012 USD Costs not provided |
MUS vs. PFMT = $32,132 |
| Simpson et al, 201955 Canada |
Cost–utility analysis Decision analytic model (decision tree) Health system perspective 1-y time horizon No discounting |
Healthy adult women at least 18 y of age, with predominant symptoms of SUI Mean age not specified |
Pessary PFMT Uresta Impressa |
Total QALYs: Pessary = 0.8580 PFMT = 0.8941 Uresta = 0.8818 Impressa = 0.8863 |
2017 USD Pessary = $55 PFMT = $609 Uresta = $304 Impressa = $347 |
Uresta vs. Pessary = $43,785 Impressa vs. Uresta = $43,970 PFMT vs Impressa = $44,098 |
| Von Bargen et al, 201556 United States |
Cost–utility analysis Decision analytic model (Markov) Societal perspective Lifetime time horizon 3% discount rate |
Healthy women with SUI Mean age: 45 y |
Conservative management Pessary PFMT PFMT with electrical stimulation MUS |
Total QALYs: Pessary = 18.98 MUS = 18.94 |
2012 USD Pessary = $11,411 MUS = $17,779 |
Pessary dominates. In CEAC, pessary is the most cost-effective below $50,000/QALY, while at $60,000/QALY and above MUS is the most cost-effective |
Abbreviations: ASC, abdominal sacrocolpopexy; CEAC, cost-effectiveness acceptability curve; MUS, midurethral sling; ICER, incremental cost-effectiveness ratio; PFMT, pelvic floor muscle training; QALY, quality adjusted life years; SUI, stress urinary incontinence; VRS, vaginal reconstructive surgery.
We caution any interpretation in the authors published results as they likely derived their incremental QALYs as the utility change.
PELVIC ORGAN PROLAPSE
Hullfish et al53 conducted a cost–utility analysis (Markov model) of pessaries compared with expectant management, vaginal reconstructive surgery (VRS), traditional abdominal sacrocolpopexy (ASC) surgery, and robotic assisted ASC surgery, in a population of females with stage III or higher apical prolapse of the vagina. For their inputs, the authors took transition probabilities from their literature review and estimated utilities. Costs were either estimated by the authors or were taken from a nationwide hospital discharge database. The analysis was run over a 1-year time horizon. The authors did not explicitly state the perspective, but their analysis indicates a US payer perspective. Discounting was not used given the short time horizon. The incremental cost-effectiveness ratios (ICERs) were not provided, but a cost-effectiveness acceptability curve was; it indicated that two options were considered potentially cost-effective: pessary use and VRS (where other options were dominated by having fewer quality-adjusted life years [QALYs] gained and greater costs). Pessary use was the optimal strategy below a willingness-to-pay (WTP) threshold of $5,600 USD, while VRS was the optimal strategy above it. However, there was a large degree of uncertainty as the probability of pessaries or VRS being cost-effective plateaued at approximately 45% and 65%, respectively. The authors conducted one-way sensitivity analyses and found the model's results changed when values were changed for the following five variables “probability of POP complication, probability of surgery following pessary, utility of pessary use, probability of late complications for VRS, and the proportional cost estimate for robotic-assisted ASC as a percentage of the median total hospitalization charge for patients with ASC.”53
Panman et al41 carried out a cost–utility analysis alongside a randomized control trial, evaluating both pessaries and pelvic floor muscle training (PFMT). The trial collected data on 162 women from 20 clinical practices in the Netherlands. The people recruited were female, at least 55 years of age, who had symptoms of prolapse, but no prior treatment for prolapse. Follow-up occurred at 3, 12, and 24 months after either a successful pessary fitting or the start of PFMT. There was no discounting of outcomes, as trial data was used in place of an economic model. The authors stated that there was a loss in QALYs from baseline in both the pessary and PFMT trial arms, where the loss was slightly lower in the pessary arm. Caution is warranted in interpreting the results as, while the authors refer to QALY loss, we assume they meant loss in utility, because a negative QALY implies that the health state is worse than death. After bootstrapping, the authors reported a negative ICER of −$27,439 per QALY (95% CI: −$91,974 to $74,695), indicating an additional saving of $27,439 USD per QALY lost. A cost-effectiveness plane indicated that 95% of simulations were in the southeast quadrant (i.e., greater effect and lower cost for pessary group), while the remaining 5% were in the southwest quadrant (i.e., less effect and lower cost for pessary group). The authors did not perform sensitivity analyses.
STRESS URINARY INCONTINENCE
Richardson et al54 designed a cost–utility analysis (decision tree) of pessaries compared with PFMT and surgery (i.e., mid-urethral sling), in a population with uncomplicated, symptomatic stress urinary incontinence. Direct costs from Medicare reimbursement were used to provide cost estimates, and effectiveness and utility data were taken from the published literature. Discounting was not used because the analysis was run over a 1-year time horizon. The results of the analysis are from a US third-party payer perspective. The study found that surgery was the preferred strategy, with an ICER of $32,132 USD per QALY gained compared with PFMT. Despite the low cost of a pessary, it was always less effective than treatment alternatives, leading the authors to conclude that it was never the preferred scenario. Sensitivity analyses showed that the results were sensitive to several variables on clinical effectiveness and costs. Specifically, the most cost-effective treatment would change if the subjective cure rate was greater than 40.5% for PFMT (reference case = 33%) and 43.5% for a pessary (reference case = 32%).
Simpson et al55 undertook a cost–utility analysis (decision tree) of pessaries compared with PFMT, Uresta (Resilia Inc), a self-fitting intravaginal incontinence device, and Impressa (Kimberly-Clark Worldwide, Inc), a disposable tampon device, using a Canadian health system perspective. The simulated population were healthy adult females with predominant symptoms of SUI who had failed initial treatment with Kegel exercises. Model inputs were taken from the literature, with a preference for randomized studies. The best available literature was used where randomized studies were unavailable. Costs were taken from the subject companies (i.e., Resilia and Kimberly-Clark) and publicly available data. The authors indicated they used only utility data that had been validated in the literature. They used a 1-year time horizon to reflect the follow-up data available in the literature because most costs were incurred within the first year of treatment; therefore, they did not use discounting. The model took a health system perspective to account for both public and private payers (e.g., self-pay and personal insurance). Pelvic floor muscle training was favoured as the most cost-effective nonsurgical treatment option, followed closely by Impressa and Uresta. The ICER for pessary vs. Uresta was $1,156 USD per QALY gained. Despite pessaries having the lowest cost, the authors noted that it performed poorly, with both the lowest cure rate and the lowest cost-effectiveness and therefore they felt pessaries shouldn't be considered as a first-line treatment for SUI. Sensitivity analyses indicated the results were sensitive to parameter changes, specifically changes in the probability of success with PFMT.
Von Bargen et al56 conducted a cost–utility analysis (Markov model) of pessaries compared with PFMT, PFMT with electrical stimulation, conservative (non-surgical) management, and surgery (i.e., mid-urethral sling). The theoretical cohort included healthy women with SUI who required treatment. Treatment success and utility values were derived from the medical literature, except in cases where data was lacking, in which case expert opinion was used. Costs were estimated from Medicare reimbursement codes and the cost of lost productivity was not included. The model used a societal perspective, and modeled the cohort over a lifetime horizon using yearly cycles and a 3% discount rate. Based on the mean reference case results, pessaries dominate surgery. However, in the cost-effectiveness acceptability curve for all interventions, and at a WTP threshold of $60,000 USD per QALY gained, surgery overtakes pessaries in having the highest percentage of iterations being cost-effective. The percentage of cost-effective iterations for both the pessary and surgery group remained below 70% across various WTP thresholds. Although multivariable sensitivity analyses were mentioned, both multivariable and one-way sensitivity analyses are not presented.
Applicability of the Included Studies
Appendix 6 provides the results of the quality appraisal checklist for economic evaluations applied to the included studies. All studies identified were deemed partially applicable to the research question. Studies varied in the comparators they evaluated, with only two including all interventions of interest.54,56 Only one study55 took a Canadian health system perspective, while three were primarily based on data from the United States,53,54,56 and one from the Netherlands.41 The time horizon was limited to 1-year in most studies,53–55 with the exception of one model using a lifetime horizon,56 and one randomized controlled trial (RCT) with 2-years of data.41 Although Simpson et al55 took a Canadian health system perspective, it did not include surgery in its analysis and took a 1-year time horizon, thus making it unsuitable to evaluating long-term adherence of conservative interventions. Additionally, Simpson et al55 had other minor limitations (e.g., the model did not incorporate pessary fitting and cleaning costs). Therefore, the study was deemed only partially applicable to the research question.
Discussion
The economic evidence review identified five studies, two in a population with POP,41,53 and three in a population with SUI.54–56 In the two POP studies, different comparators were used in each study. One study41 found that pessary use was the optimal strategy when compared with PFMT, while the other study53 found that pessary use was optimal only below a WTP threshold of $5,600 USD per QALY gained when compared with surgery. In the three studies on SUI, the comparators also differed by whether they included surgery or other mechanical devices (e.g. Uresta and Impressa). In the two studies including surgery,54,56 surgery was the preferred strategy, although one56 found it overtook pessaries only after a WTP threshold of $60,000 USD per QALY gained. In the study looking at nonsurgical treatment options,55 PFMT was favoured as the most cost-effective, followed closely by Impressa and Uresta. Overall, there appeared to be no consistent trend across all five studies. Since these studies were only partially applicable to the research question, we decided to conduct a primary economic evaluation.
There were methodological differences across studies that may have led to inconsistent results. In the POP population, the RCT by Panman et al41 evaluated only non-surgical interventions. Furthermore, it stated that QALYs decreased from baseline regardless of the intervention used, but the authors likely found a utility decrease instead of a QALY decrease. Hullfish et al53 did not find a similar waning utility for non-surgical interventions in their model and it is uncertain whether surgical interventions would also have a similar waning utility over time. Future modeling efforts in a population with POP may need to incorporate diminishing utility values for non-surgical interventions.
There was only one study that included both Impressa and Uresta in the analysis, and the authors found that their clinical efficacy estimates were limited. The only literature Simpson et al55 found on the efficacy of Impressa was from a 28-day trial, so they used disposable tampon data to estimate long-term efficacy. The authors found two studies on Uresta, with one coming directly from the manufacturer. Based on expert opinion, Simpson et al55 reported that the cure rate appeared inflated, so they developed a new estimate based on a urogynecologist's opinion. Compared with other common interventions in POP and SUI (i.e., pessary, PFMT, and surgery), publications on Uresta and Impressa are fewer and of lower methodological quality, resulting in a large degree of uncertainty.
To accurately measure the true cost-effectiveness of conservative therapies (i.e., pessaries and PFMT), a time horizon beyond the typically reported 1–2 years may be required to account for the continued attrition or discontinuation rates. In their RCT, Panman et al41 reported a 25% attrition rate (n = 12) among those successfully fitted with a pessary. In addition to pessary attrition rates, individuals must be successfully fitted with a pessary at the start of treatment. Previously reported success rates vary between 41% and 86%.41 This data shows that pessary fitting failed in a sizeable population of symptomatic people, indicating that the treatment may not be suitable for all symptomatic cases of prolapse. Using data from the ATLAS trial29, Richardson et al54 found after 1 year, 45% and 57% of participants were still using their pessaries and performing PFMT, respectively, suggesting that low adherence to conservative therapies is also present in populations with SUI. In addition to the low adherence to PFMT exercises, Von Bargen et al56 reported that the long-term success of PFMT declines over time (previous studies have shown a 5% to 10% decrease in muscle strength per week after completion of pelvic floor exercises). Given the chronic nature of POP and SUI and the reported high discontinuation rate of conservative therapies, models with short time horizons may overestimate the benefits of these interventions.
All studies found in this economic evidence review evaluated comparative effectiveness to determine the most cost-effective intervention; however, as the local treatment pathways recommend non-invasive conservative therapies before surgery, an economic evaluation using treatment sequencing may be most appropriate in informing cost-effectiveness.57,58
Conclusions
The economic literature review identified five economic evaluations comparing pessaries with various other interventions for people with POP or SUI. Overall, results were mixed. In the two studies found for a population with POP, both pessaries and surgery were found to be cost-effective. Of the three studies examining a population with SUI, none found that pessaries were the intervention most likely to be cost-effective. Two studies found surgery was most likely to be cost-effective, while PFMT was favoured in the third.
Primary Economic Evaluation
The published economic evaluations identified in the economic literature review did not adequately answer our research questions; therefore, we conducted a primary economic evaluation. One key limitation was that all identified studies used head-to-head comparisons between interventions. When both conservative and surgical interventions are compared in this manner, it does not represent local guidelines, which advise a stepped-care approach to treating the conditions.57,58 We created our own model because the results were inconsistent, with differing interventions evaluated, perspectives, methodologies, and conclusions. All but two studies identified incorporated a 1-year time horizon, which fails to account for discontinuation rates of pessaries over time. Further, the study that took a lifetime horizon used a constant discontinuation rate, which may overestimate the effectiveness of pessaries. Owing to these limitations, we conducted a primary economic evaluation.
Research Questions
From the perspective of the Ontario Ministry of Health:
-
1.
What is the cost-effectiveness of various treatment strategies, including vaginal pessaries, PFMT, and surgery, for the treatment of people with symptomatic pelvic organ prolapse (POP)?
-
2.
What is the cost-effectiveness of various treatment strategies, including vaginal pessaries, PFMT, and surgery, for the treatment of people with symptomatic stress urinary incontinence (SUI)?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.59
Analysis
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis adhered to the Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines60 when appropriate and represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions.
We conducted a cost–utility analysis to determine the costs and health outcomes (i.e., quality-adjusted life-years [QALYs]) associated with each treatment strategy. We chose this type of analysis because utility inputs are available and a generic outcome measure such as the QALY allows decision-makers to make comparisons across different conditions and interventions. The outcomes reported are total costs and total QALYs for each treatment, and incremental cost per QALY gained compared with the next most effective strategy. For this analysis, incremental costs and QALYs are key outcomes considered by decision-makers, while total costs and QALYs of treatment options are informative measures for decision-makers.
Target Population
We evaluated two distinct populations using two economic models. The first model was specific to females (≥ 18 years) with symptomatic SUI, where SUI is the involuntary loss of urine from effort, physical exertion, or with an increase in the intra-abdominal pressure upon sneezing or coughing. The second model was specific to females with symptomatic POP, where POP results in the downward descent of the pelvic organs (i.e., vagina, uterus, bladder, and/or rectum) into or through the vagina. As individuals can present with both conditions, model inputs derived from the literature will be assigned to either POP or SUI based on the underlying primary treatment and the condition it's targeting.
The model's population demographics were based on weighted averages from published studies used in the clinical review (Appendix 7, Tables A8 and A9). The modeled POP and SUI populations were on average 66 and 50 years old, respectively.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health.
Interventions
According to local guidelines from the Canadian Urological Association and the Society of Obstetricians and Gynaecologists of Canada, conservative first line treatments for POP and SUI include supervised pelvic floor muscle training and vaginal pessaries, while second line treatments typically involve surgical interventions (e.g., midurethral sling, pelvic floor reconstruction).57,58 In the Ontario context, public funding is limited or absent for PFMT and pessaries. For pessaries, only the fitting is publicly funded, not the device itself. Supervised PFMT led by a physiotherapist primarily relies on private insurance coverage. Public coverage of physiotherapist-led PFMT is specific to individuals who have a referral and are under 19 or over 65 years of age, have had an overnight hospital stay requiring physiotherapy, or are a recipient of the Ontario Disability Support Program (ODSP). It should be noted that in addition to physiotherapists, other professions (i.e. nurse practitioners, midwives, physicians) could receive training on PFMT and advise patients on how to initiate PFMT. Despite variation in the availability of public funding for PFMT, we include it in our reference case analysis as it is a recommended first line (i.e., before surgery) treatment under Canadian guidelines.57,58
As local guidelines support the use of non-invasive conservative therapies before surgery, we evaluated the cost-effectiveness of treatment sequences instead of performing a head-to-head comparison.57,58 Each sequence consists of multiple interventions used after the former treatment is unsuccessful or discontinued. See Table 23 for a summary of the sequences evaluated in each of our reference case models. All interventions within the most cost-effective treatment sequence will be considered cost-effective when used in the indicated sequence. Therefore, pessaries will be considered cost-effective if the most cost-effective treatment sequence contains pessaries.
Table 23:
Treatment Sequences Evaluated in the Primary Economic Model
| Treatment Sequences | Patient Population | Outcomes |
|---|---|---|
| Pessary → PFMT → Surgery | ||
| PFMT → Pessary → Surgery | ||
| Pessary → Surgery | People with pelvic organ prolapse | Costs, QALYs, ICER |
| PFMT → Surgery | ||
| Surgery | ||
| Pessary → PFMT → Surgery | ||
| PFMT → Pessary → Surgery | ||
| Pessary → Surgery PFMT → Surgery | People with stress urinary incontinence | Costs, QALYs, ICER |
| Surgery | ||
Abbreviations: ICER, incremental cost-effectiveness ratio; PFMT, pelvic floor muscle training; QALY, quality-adjusted life year.
To refine the scope of this analysis, we narrowed the specific interventions being evaluated. We did not distinguish between different types of PFMT and pessaries. Therefore, for PFMT, costs did not vary by whether electrical stimulation or other treatment-assistive methods were used. In addition, there may be some limitations to our approach (e.g., potential differences in discontinuation rates61) of grouping all pessary types, but our analysis looks at pessaries as a class, and we explore cost variations in sensitivity analyses.
We also excluded several treatments. Expectant management (i.e., watchful waiting) was not included as an intervention as the probability of success has not been defined in the literature, but it is assumed to be less effective than conservative treatments.56 To note, expectant management was indirectly modeled as an untreated state when all treatments were exhausted. Both Uresta and Impressa were excluded from the treatment sequences due to their limited published data and lack of long-term adherence data. Finally, simultaneous treatment use (e.g., supervised PFMT and pessary together) and its potential synergistic effects were excluded given a lack of published long-term adherence data.
Discounting and Time Horizon
As per CADTH guidelines, after the model's first year, an annual discount rate of 1.5% was applied to costs and QALYs.60 We also explored discount rates of 0%, 3%, and 5% in our sensitivity analyses.
A 10-year time horizon was used in the reference case as it is the maximum available published long-term follow-up for both pessaries and PFMT.62,63 Scenario analyses include a time horizon of 2 years and lifetime horizon. A lifetime horizon was not selected as the reference case given the uncertainty of predicting PFMT and pessary discontinuation data beyond the published literature. Furthermore, a short time horizon of 1 year, which was commonly used in previous cost-effectiveness analyses, was not selected in the reference case as it may bias results in favour of conservative therapies by not fully accounting for their high discontinuation rates over time.
Main Assumptions
The model's main assumptions were as follows:
Due to a lack of data, all individuals, regardless of their condition's severity, are assumed to have an equal probability of being successfully treated with an intervention
Mortality does not differ between the POP/SUI cohort and the general population
Pessary fitting and cleanings were costed based on available physician billing codes (other health care professionals also provide these services)
80% of pessary users go to a physician to have their pessary removed, cleaned, and reinserted; the remaining self manage their pessary
Those individuals who are successfully fitted with a pessary are fitted at their first physician fitting session
Due to a lack of data, pessary discontinuation rates do not differ by pessary type
In treating SUI and POP, pessaries are effective as long as individuals continue to adhere to their usage
There is a 6-month waiting period from the initial treatments onset before being able to attempt a new treatment within a treatment sequence
Individual treatment effectiveness is not impacted by treatment sequence
A portion of the patient population do not want, and will not undergo, surgery despite being eligible
Model Structure
We developed a Markov microsimulation model to estimate the long-term clinical and economic outcomes of different treatment sequences. A Markov microsimulation allows the use of trackers to follow individual patients and calculate their length of stay in each state. The cycle length was 6 months, which provides a minimum period before an individual can attempt the next intervention in the treatment sequence. This time horizon provides sufficient time to capture both the index visit and four to six sessions of PFMT. The model was built using TreeAge Pro 2019.64
The model included seven states:
Pessary: individuals being treated with a pessary. This state accounts for the probability of a successful fitting, pessary discontinuation over time, maintenance visits to a physician for removal/cleaning, and replacement of a pessary every 5 years
PFMT: individuals being treated with supervised PFMT. This state accounts for PFMT discontinuation over time
Surgery: individuals receiving either urethropexy or reconstruction (depending on the target population). This state accounts for those eligible and willing to undergo surgery
Surgically repaired: individuals who underwent surgery and it was successful
Reoperation: individuals who underwent surgery and it was unsuccessful, or who had a successful surgery but whose symptoms later returned requiring a reoperation. This state accounts for those eligible and unwilling to undergo a reoperation
Untreated: individuals living with SUI or POP who exhausted all conservative therapies and either had an unsuccessful surgery/reoperation or were not eligible or were unwilling to have a surgery/reoperation
Death: An absorbing state accounting for the general mortality rate in the cohort over time
Depending on the treatment sequence, individuals typically started with conservative treatments and exhausted them before moving to surgery. While using a conservative treatment, individuals either adhered to the treatment or discontinued. Those who discontinued were assigned the total treatment costs (as though they adhered within a given cycle), but their utility gain represented someone in the “Untreated” state. After the cycle length of 6 months, individuals could attempt the next treatment in their sequence. People whose next treatment in their sequence was surgery were tested as to whether they were eligible and willing to have surgery (this is not explicitly shown in Figure 3). Those who did not receive surgery moved to the “Untreated” state, while those who did receive surgery either no longer had symptomatic POP/SUI, or the surgery was unsuccessful and required a reoperation. Additionally, those who had a successful surgery and were non-symptomatic were at risk of becoming symptomatic again over time, requiring a reoperation. We limited the model to only one reoperation, at which point individuals will have exhausted available treatments. Either their condition is surgically repaired, or they live with untreated POP/SUI. Throughout this model, individuals were at risk of moving to the “Dead” state, an absorbing state which was based on Canadian age- and sex-specific life tables.
Figure 3: Model Structure.
aBecause each distinct treatment sequence results in an altered model structure, the presented model is simplified such that directional transitions between interventions are not explicitly shown, but are indicated by dotted lines. When a treatment's clinical effectiveness wanes or the individual discontinues pessary use or PFMT, they transition to the following treatment sequence on the following cycle. In the cycle before the transition, they are assigned the same utility value as those in the Untreated state.
The model structure was unique for our analysis. Costs and QALYs were accumulated as transitional events dependent on whether an event occurred (e.g., adherence to conservative therapy, surgery eligibility, and success). Given this structure, it was assumed that those who underwent PFMT or pessary use but by the end of the cycle did not adhere or maintain the clinical benefit, would not receive any benefit from the treatment for that cycle.
Figure 3 presents both the treatment sequences and the simplified diagram of the model structure, which will be used as two distinct models for the POP and SUI populations.
In our economic evaluation, we considered the financial impact of adverse events indirectly. For surgery, we accounted for reoperations, which would include adverse events that are severe or expensive to treat (e.g., bladder or vaginal perforation). Other adverse events that have a negligible impact on health effects or resources were excluded from the analysis (e.g., hematoma, urinary tract infection, vaginal discharge, vaginal erosion, or vaginal bleeding). However, these adverse events may be accounted for indirectly in adherence rates to conservative therapies.
Clinical Outcomes and Utility Parameters
The inputs used in the primary economic evaluation pulled from comparative and non-comparative studies. This differs from the clinical evidence review, which used comparative literature only. Comparative literature represents the gold standard in evaluating whether a treatment is effective and the degree to which it is effective, when compared against the standard treatment. Non-comparative literature was included in our analysis because the model is evaluating treatment sequencing, or stepped care, where individuals start with one intervention and progress to others after the current treatment proves ineffective. This reflects clinical practice, where patients can pursue different conservative therapies of their choice or move directly to surgery. Therefore, the model doesn't seek to find the most cost-effective intervention, which would require head-to-head comparative evidence, but instead identifies the most cost-effective treatment strategy using mixed comparative and non-comparative evidence.
HEALTH STATE AND EVENT OCCURRENCES
Table 24 summarizes the list of transition probabilities for both the POP and SUI models. Where possible, inputs were derived from the published literature that informed the clinical review and other relevant published literature, and in their absence expert opinion was used to inform the inputs. If applicable, all probabilities are adjusted to account for the model's biannual cycle length.
Table 24:
Probabilities Used in the Economic Model
| Model Parameters | Probability (%) | Standard Error (+/-) | Reference |
|---|---|---|---|
| Pessary | |||
| Self manage pessary | 20.00 | 0.025 | Assumptiona,b |
| Successfully fitted | |||
| POP | 77.94 | 0.030 | Pooled rate41,43,65–73 |
| SUI | 77.29 | 0.026 | Pooled rate67–69,71,72,74 |
| Discontinuation rate | See reference | — | Appendix 7, Figure A1 |
| Pelvic floor muscle training | |||
| Discontinuation rate | See reference | — | Appendix 7, Figure A2 |
| Surgery—SUI | |||
| Eligible and want surgery | 62.00 | 0.031 | Brazzeli et al, 201975 |
| Surgery success | 83.3 | 0.005 | Ford et al, 201776 |
| Reoperation—SUI | |||
| Eligible and want reoperation | 75.00 | — | Kilonzo et al, 200477 |
| Reoperation success | 79.20 | 0.028 | Van der Doelen et al, 201578 |
| Surgically repaired—SUI | |||
| Symptoms return (biannual) | |||
| Year 1–5 | 0.95 | 0.002 | Ford et al, 201776 |
| Year 5+ | 0.55 | 0.002 | Ford et al, 201776 |
| Surgery—POP | |||
| Eligible and want surgery | 55.02 | 0.031 | Patnam et al, 201979 |
| Surgery success | 94.35 | 0.010 | Brubaker et al, 201080 |
| Reoperation—POP | |||
| Eligible and want reoperation | 80.00 | — | Assumptiona |
| Reoperation success | 85.00 | 0.012 | Assumptiona |
| Surgically repaired—POP | |||
| Symptoms return (biannual) | |||
| Year 1–2 | 0.76 | — | Jacklin & Duckett, 201381 |
| Year 2+ | 0.19 | — | Jacklin & Duckett, 201381 |
Abbreviations: POP, pelvic organ prolapse; SUI, stress urinary incontinence.
Email communication, Aisling Clancy, MD, January 7, 2020.
Email communication, Jennifer Skelly, PhD, December 31, 2019.
Although Table 24 lists PFMT and pessary discontinuation rates as probabilities used in the economic model, the actual discontinuation rates over time are presented in Appendix 7, Figures A1 and A2. Given its physical nature, the discontinuation rate for pessaries was defined as individuals who no longer used the device. Because it is an exercise, the discontinuation rate for PFMT was based on the individual's subjective satisfaction with their prolapse/continence status. For pessaries, it was assumed that those who continued using the device were maintaining the benefits of the device. The data used to derive Figures A1 and A2 are presented in Appendix 7, Tables A10 and A11. The data was collected through a literature search on pessary and PFMT's discontinuation rates. Those studies with longitudinal discontinuation rates were plotted by year, and an exponential curve was fitted to estimate the discontinuation rate over time.
A weighted average was used when pooling successful pessary fitting rates. (We used the rates reported in individual studies, which may have used different standards and timeframes to determine whether a fitting was successful.) In studies where there was high overlap between the POP and SUI populations, the rate was included in both weighted averages. Overall, there was a marginal difference between the probabilities of having a successful fitting in the POP (77.94%) and SUI (77.29%) populations.
Inputs for patient preferences towards surgery were included to account for the subpopulation of people who are unwilling to undergo surgery despite being eligible. This distinct population must rely on conservative therapies to address their health condition. Evidence for surgical preference towards surgery was pulled from an observational study and a discrete choice experiment.
As stated previously, model accounted for only one reoperation following an unsuccessful surgery. The probability of a reoperation's success was assumed to be the same whether it was conducted after a failed initial surgery or after a successful surgery in which symptoms eventually returned.
MORTALITY
The model assumed the SUI and POP populations were at the same risk of mortality as the general population; therefore, we used age- and sex-specific mortality rates from the Ontario general population.82
HEALTH STATE UTILITIES
Utilities represent a person's preference for certain health outcomes, such as being continent. These are often measured on a scale of 0 (death) to 1 (full health). Utilities can be derived from several sources, including common questionnaires such as the SF-6D and the EQ-5D. In a population with POP, the minimally important difference (MID) in utilities has been estimated at 0.026 for the SF-6D and 0.025 for the EQ-5D.83
Utility data are summarized in Table 25 and were derived from the published literature using the EQ-5D. Utility values for pre- and post-treatment in both POP and SUI were used in the model.
Table 25:
Utilities Used in the Economic Model
| Model State | Utility | Standard Error (+/-) | Reference |
|---|---|---|---|
| Untreated POP | 0.810 | 0.004 | Harvie et al, 201983 |
| Treated POP | 0.868 | 0.006 | Harvie et al, 201983 |
| Untreated SUI | 0.810 | 0.022 | Harvie et al, 201484 |
| Treated SUI | 0.850 | 0.048 | Haywood et al, 200885 |
Abbreviations: POP, pelvic organ prolapse; SUI, stress urinary incontinence.
Cost Parameters
We included relevant costs that individuals incurred during conservative treatment and both before and after surgery. The costs consisted of professional fees, hospitalization costs, and device costs. To simplify the analysis, diagnostic testing was not included. Urodynamics, although sometimes ordered prior to surgery, have limited accessibility across the province, and the evidence supporting its use is debated. Previous publications have found that it is not cost-effective and does not improve surgical outcomes.86
All costs were reported in 2019 Canadian dollars. We obtained cost inputs from standard Ontario sources and published literature. The fees for professional visits, procedures and consultations were obtained from the Ontario Schedule of Benefits for Physician Services.18 Hospitalization costs were obtained from the Ontario Case Costing Initiative.87
Due to the complex nature of our analysis, we made several costing assumptions to simplify the analysis. First, we conservatively assumed that each year 80% of patients visit a physician to have their pessary removed, cleaned and reinserted (the remaining 20% would self-manage their pessary cleaning). Second, in our reference case analysis, we assumed that PFMT would not be administered with biofeedback or muscle stimulation given its higher cost and inconclusive incremental efficacy within RCTs and systematic reviews.88 Third, we assumed that need for follow-up sessions for PFMT would be low enough that the entire treatment costs of PFMT could be accounted for in the initial 6-month cycle. Finally, we assumed that post-operative outpatient visits for follow-up care or catheter removal represented a marginal cost compared with the total surgery cost, so they were excluded from the analysis.
Table 26 presents the costs of conservative treatments. The yearly maintenance costs in Table 26 are from pessary-users' visits to a physician to have their pessary removed and cleaned. Maintenance costs in the first year were lower than in following years, given the earlier initial fitting. The cost of a pessary was set at $50 for the reference case, the lowest cost in the range given by Simpson et al.55 The cost for PFMT was also derived from the same authors, modified to exclude the cost of the vaginal probe and muscle stimulation unit.55 This cost reflects PFMT sessions without biofeedback or muscle stimulation.
Table 26:
Costs of Conservative Treatments
| Cost Variable | Mean Cost ($ CAD) | Reference |
|---|---|---|
| Pessary | ||
| Pessary Device | 50.00 | Simpson et al, 201955 |
| Initial pessary fitting | ||
| Assessment | 47.45 | SoB A205 |
| Fitting | 61.30 | SoB G398 |
| Yearly maintenance (year 1)a | 100.15 | SoB A203, A204 |
| Yearly maintenance (year 2+)b | 147.60 | SoB A203, A204 |
| Pessary replacement (every 5 years) | ||
| Device | 55.50 | Simpson et al, 201955 |
| Fitting | 61.30 | SoB G398 |
| Pelvic Floor Muscle Training | ||
| Physiotherapist-led trainingc | 545 | Simpson et al, 201955 |
Abbreviation: SoB, physician schedule of benefits.
Three maintenance visits were assumed given the earlier implantation, with the first billed under A203, while the subsequent two visits were billed under A204.
Four maintenance visits were assumed, with the first two billed under A203 and the final two under A204.
SoB A205: consultation (obstetrics and gynecology)SoB G398: medical management of prolapse—initial pessary fitting or re-fitting as requiredSoB A203: specific assessment (obstetrics and gynecology)SoB A204: partial assessment (obstetrics and gynecology)
Tables 27 and 28 present the itemized surgical costs for SUI and POP. The surgical procedures outlined assume surgery for SUI was conducted as day surgery while for POP was done in an inpatient setting. This assumption reflects the typical local pathway as seen through surgical volumes by setting. The surgical codes used in Table 28 for POP were selected after consultation with clinical experts on the most commonly billed surgical codes for the target population. However, we acknowledge this is a simplification of complex clinical practice where various surgical codes are used depending on surgical technique and the location of the prolapse.
Table 27:
Cost of Stress Urinary Incontinence Surgical Interventions
| Cost Variable | Mean Cost ($ CAD) | Standard Error | Costing Sourcea |
|---|---|---|---|
| Surgery – Stress Urinary Incontinence | |||
| Pre-surgery consultation | 214.10 | SoB A935, A206 | |
| Surgeryb,c | |||
| Surgical | 381.60 | — | SoB S815 |
| Assistant | 120.40 | — | SoB S815 |
| Anesthesia | 150.10 | — | SoB S815 |
| Hospitalization (day surgery) | 2,236.00 | 19.51 | OCCId |
| Post-surgery consultation | 47.45 | — | SoB A203 |
| Reoperationb,e | |||
| Surgical | 489.70 | — | SoB S546 |
| Assistant | 168.56 | — | SoB S546 |
| Anesthesia | 225.15 | — | SoB S546 |
| Hospitalization (day surgery) | 1,775.00 | 106.38 | OCCI |
Abbreviations: OCCI, Ontario case costing initiative; SoB, physician schedule of benefits.
SoB A935, special surgical consultation (obstetrics and gynaecology)SoB A206, repeat consultation (obstetrics and gynaecology)SoB S815, repair—tension-free vaginal tape mid-urethral sling, by any method/approachSoB A203, specific assessment (obstetrics and gynecology)SoB S546, repeat procedure for failed retropubic or vaginal surgery for stress incontinence
Basic and time units were multiplied by either the anaesthesiologist unit fee ($15.01) or the assistant fee ($12.04) to calculate total cost.
The procedure time was estimated to be 1 hour. Assistant and Anesthesia costs were derived from six base units plus four time units.
Using Canadian Classification of Intervention codes 1.PL.74.CR-XX-N, 1.PL.74.AL-XX-N, 1.PL.74.AF-XX-N, 1.PL.74.LA-XX-N, 1.PL.74.DA-XX-N (fixation, bladder neck).
The procedure time was estimated at 1.5 hours. Assistant costs were derived from six base units and eight time units. Anesthesia costs were derived from seven base units and eight time units.
Table 28:
Cost of Pelvic Organ Prolapse Surgical Interventions
| Cost Variable | Mean Cost ($ CAD) | Standard Error | Costing Sourcea |
|---|---|---|---|
| Surgery—Pelvic Organ Prolapse | |||
| Pre-surgery consultation | 214.10 | SoB A935, A206 | |
| Surgeryb,c | |||
| Surgical | 616.60 | — | SoB S758 |
| Assistant | 168.56 | — | SoB S758 |
| Anesthesia | 210.14 | — | SoB S758 |
| Hospitalization (inpatient) | 6,245.00 | 56.22 | OCCId |
| Post-surgery—surgeon consultation | 47.45 | — | SoB A203 |
| Reoperationb,e | |||
| Surgical | 453.70 | — | SoB S812 |
| Assistant | 168.56 | — | SoB S812 |
| Anesthesia | 225.15 | — | SoB S812 |
| Hospitalization (inpatient) | 5,930.00 | 215.80 | OCCI |
Abbreviations: OCCI, Ontario case costing initiative; SoB, physician schedule of benefits.
SoB A935: special surgical consultation (obstetrics and gynaecology)SoB A206: repeat consultation (obstetrics and gynaecology)SoB S758: hysterectomy with anterior and posterior vaginal repair and including enterocoele and/or vault prolapse repair when renderedSoB A203: specific assessment (obstetrics and gynecology)SoB S812: post hysterectomy vault prolapse—repeat—repair by vaginal approach, may include enterocoele and/or anterior and posterior repair
Basic and time units were multiplied by either the anaesthesiologist unit fee ($15.01) or the assistant fee ($12.04) to calculate total cost.
The procedure time was estimated at 1.5 hours. Both Assistant and Anesthesia costs were derived from six base units and eight time units.
Using Canadian Classification of Intervention codes 1.RM.89.^^ (excision total, uterus and surrounding structures).
The procedure time was estimated at 1.5 hours. Assistant costs were derived from six base units and eight time units.
Anesthesia costs were derived from seven base units and eight time units.
Analysis
For the reference case analysis, we performed a probabilistic analysis to determine the mean incremental cost and mean incremental QALYs, and we calculated the incremental cost-effectiveness ratio (ICER) for all treatment sequences. Since more than two treatment sequences were being compared, we calculated the ICER using sequential analysis.60 Sequential analysis compares all treatment sequences and ranks them by increasing cost. Incremental costs and QALYs for each treatment sequence are calculated by comparing it with the next most costly treatment sequence. If a treatment sequence is dominated (i.e., more costly and less effective than at least one other sequence) or is subject to extended dominance, it is removed from the analysis and all remaining interventions are recalculated until only undominated treatment sequences remain. Extended dominance occurs when a given intervention is ruled out because its ICER is higher than the ICER of a more effective intervention.
We performed the probabilistic sensitivity analysis using a Monte Carlo simulation with 10,000 outer loops to capture parameter uncertainty, and 1,000 inner loops to capture patient variability (i.e., cohort age). We examined parameter uncertainty by specifying distributions around each estimate. The distributions used include gamma distributions for cost inputs, beta distributions for probability and utility inputs, and normal distributions for simulating age. In addition to the reference case results described, we present the impact of uncertainty and variability through a cost-effectiveness acceptability curve.
SCENARIO ANALYSES
As described in Table 29, we conducted several scenario analyses testing not only different input parameters, but also some of the assumptions required to estimate Ontario-specific costs. For each scenario, we recalculated the mean incremental costs and QALYs for each treatment, along with the ICER. All scenarios were performed probabilistically.
Table 29:
Variables Altered in Scenario Analyses
| Parameter | Reference Case | Scenario Analyses |
|---|---|---|
| Time horizon | 10 years | 2 years and lifetime horizon |
| Discount rate | 1.5% | 0%, 3%, 5% |
| Pessary device replacement | 5 years | 3 and 10 years |
| Pessary cost | $50.00 | $35.00, $91.08 |
| Patient preference for surgery | Include | Exclude |
| Pelvic floor muscle training as comparator | Include | Exclude |
Time Horizon
We varied the time horizon based on previously published studies. A time horizon of 2 years represents the most frequent follow-up period evaluated in observational studies on pessaries.61,63,73,89 A lifetime horizon was evaluated to fully account for the waning effectiveness seen in individual treatments. This time horizon has been used in a previous cost–utility analysis, as identified in the economic evidence review.56
Discounting
To evaluate the impact of uncertainty in the discount rate, we incorporated discount rates of 0% and 3% per year, along with an additional analysis at 5%, which corresponds to discount rates recommended in previous CADTH guidelines.60
Pessary device
Pessaries are durable and can typically be reused for multiple years. Based on expert opinion, we varied the pessary replacement time between 3 and 10 years (email communication, Aisling Clancy, MD, January 7, 2020; Jennifer Skelly, PhD, December 31, 2019).
Given pricing variation, pessary device costs were taken from different sources to estimate a minimum and maximum expected cost. The minimum cost was estimated based on a 30% reduction from the reference case price, while the maximum cost was estimated using costs from online distributors (Appendix 7, Table A12).
Other Scenarios
We performed a scenario analysis that excluded patient preferences towards surgery and reoperation. In this analysis, all patients who were eligible for surgery (90%) received it. Early discontinuation rates and the rate of successful pessary fitting were assumed to adequately reflect patient preferences for pessaries and PFMT.
Given PFMT's limited public funding, another scenario removed it as a comparator. This scenario aligns with a previous Canadian cost-effectiveness analysis by Simpson et al,55 who stated that PFMT can be prohibitively expensive to those without private insurance. Given PFMT's limited public funding, common practice in Ontario is to proceed to a pessary without PFMT.
Results
Reference Case Analysis
Table 30 presents the results of the sequential reference case analysis for the cohort with POP. As “PFMT → Pessary → Surgery” dominates all other treatment sequences (i.e., lower cost and higher effectiveness), we use this sequence as the comparator. “Surgery” had both the highest mean cost ($4,407) and the lowest mean QALYs gained (7.31) when compared with the other treatment sequences. The average cost of “Surgery” was noticeably lower than the costs shown in Table 28 due to the inclusion of individuals who were ineligible or unwilling to undergo surgery, and therefore did not accrue any surgical costs. The overall results indicate that, compared with surgery, as the number of interventions in a strategy increases, the costs decrease and the QALY gain increases.
Table 30:
Reference Case Analysis Results-Pelvic Organ Prolapse
| Strategy | Average Total Cost, $ CAD (95% CrI) | Incremental Cost, $ CADa (95% CrI) | Average Total Effect, QALY (95% CrI) | Incremental Effect, QALYb (95% CrI) | ICER |
|---|---|---|---|---|---|
| PFMT → Pessary → Surgery | 1,619.83 (1,437–1,812) | – | 7.504 (7.369–7.637) | – | – |
| Pessary → PFMT → Surgery | 1,918.36 (1,734–2,110) | 298.52 (90–508) | 7.494 (7.358–7.626) | −0.010 (−0.107 to 0.087) | Dominatedc |
| PFMT → Surgery | 2,165.14 (1,897–2,438) | 545.30 (320–773) | 7.483 (7.348–7.613) | −0.021 (−0.053 to 0.009) | Dominatedc |
| Pessary → Surgery | 3,858.68 (3,426–4,294) | 2,238.85 (1,858–2,622) | 7.395 (7.269–7.519) | −0.109 (−0.204 to −0.014) | Dominatedc |
| Surgery | 4,407.99 (3,846–4,970) | 2,788.16 (2,290–3,290) | 7.310 (7.191–7.430) | −0.194 (−0.308 to −0.082) | Dominatedc |
Abbreviations: CrI, credible interval; ICER, incremental cost-effectiveness ratio; PFMT, pelvic floor muscle training; QALY, quality-adjusted life-years.
Incremental cost = average cost (strategy B) – average cost (strategy A).
Incremental effect = average effect (strategy B) – average effect (strategy A).
A health care intervention is considered dominated when it is less effective and more costly than PFMT → Pessary → Surgery.
The cost-effectiveness acceptability curve for POP is presented in Figure 4 and shows the probability of all treatment sequences being cost-effective across a range of willingness-to-pay values. At a willingness-to-pay value of $50,000 per QALY, “PFMT → Pessary → Surgery” has the highest probability of being cost-effective (61.8%), followed by “Pessary → PFMT → Surgery” (37.1%). All other interventions are highly unlikely to be cost-effective, with approximately <1% of iterations being cost-effective across willingness-to-pay values.
Figure 4: Cost-Effectiveness Acceptability Curve-Pelvic Organ Prolapse.
The reference case sequential analysis results for SUI are presented in Table 31. As “PFMT → Pessary → Surgery” dominates all other treatment sequences (i.e., lower cost and higher effectiveness), we use this sequence as the comparator. “Pessary → Surgery” had the highest mean cost, but a higher QALY gain than the treatment sequence with the second highest mean cost (“Surgery”). As the number of interventions in a strategy increased, so too did the QALY gain increase (an outcome similar to what we found with POP). Where it differed from POP was that, as the number of interventions in a strategy increased, the costs did not decrease accordingly, as seen in “PFMT → Surgery” having a lower cost than “Pessary → PFMT → Surgery”.
Table 31:
Reference Case Analysis Results—Stress Urinary Incontinence
| Strategy | Average Total Cost, $ CAD (95% CrI) | Incremental Cost, $ CADa (95% CrI) | Average Total Effects, QALY (95% CrI) | Incremental Effect, QALYb (95% CrI) | ICER |
|---|---|---|---|---|---|
| PFMT → Pessary → Surgery | 1,211.70 (1,120–1,308) | – | 7.712 (6.831–8.389) | – | – |
| PFMT → Surgery | 1,404.15 (1,278–1,532) | 192.45 (84–296) | 7.697 (6.854–8.342) | −0.015 (−0.057; 0.029) | Dominatedc |
| Pessary → PFMT → Surgery | 1,525.42 (1,435–1,619) | 313.72 (208–418) | 7.705 (6.850–8.368) | −0.007 (−0.072; 0.057) | Dominatedc |
| Surgery | 2,274.27 (2,012–2,527) | 1,062.56 (824–1,291) | 7.577 (7.038–8.025) | −0.135 (−0.458; 0.244) | Dominatedc |
| Pessary → Surgery | 2,334.09 (2,122–2,535) | 1,122.39 (934–1,301) | 7.635 (6.957–8.168) | −0.077 (−0.271; 0.141) | Dominatedc |
Abbreviations: CrI, credible interval; ICER, incremental cost-effectiveness ratio; PFMT, pelvic floor muscle training; QALY, quality-adjusted life-years.
Incremental cost = average cost (strategy B) – average cost (strategy A).
Incremental effect = average effect (strategy B) – average effect (strategy A).
A health care intervention is considered dominated when it is less effective and more costly than PFMT → Pessary → Surgery.
Figure 5 shows the cost-effectiveness acceptability curve for SUI. “PFMT → Pessary → Surgery” has the highest probability of being cost-effective across all willingness-to-pay values. At a willingness-to-pay of $50,000 per QALY, “PFMT → Pessary → Surgery,” “Pessary → PFMT → Surgery,” and “Surgery” had probabilities of 55.5%, 23.6%, and 16.4%, respectively.
Figure 5: Model Structure—Stress Urinary Incontinence.
Scenario Analysis
Tables 32 and 33 present the results of all scenario analyses in the POP and SUI cohorts, respectively. The methods for each scenario analysis are previously described in Table 29.
Table 32:
Scenario Analysis Results—Pelvic Organ Prolapse
| Strategy | Cost ($ CAD) | Effect | ICER ($ CAD) | CE Probability (%)a |
|---|---|---|---|---|
| Time horizon | ||||
| 2 years | ||||
| Pessary → PFMT → Surgery | 559.66 | 1.680 | — | 6.4 |
| PFMT → Pessary → Surgery | 628.86 | 1.689 | 7,804.61 | 93.5 |
| Lifetime | ||||
| PFMT → Pessary → Surgery | 3,020.57 | 16.883 | — | 25.9 |
| Pessary → PFMT → Surgery | 3,254.65 | 16.895 | 19,431.07 | 30.8 |
| Discount rate | ||||
| 0% | ||||
| PFMT → Pessary → Surgery | 1,704.91 | 8.037 | — | 40.8 |
| 3% | ||||
| PFMT → Pessary → Surgery | 1,530.19 | 7.038 | — | 42.7 |
| 5% | ||||
| PFMT → Pessary → Surgery | 1,433.51 | 6.481 | — | 43.8 |
| Pessary device replacement | ||||
| 3 years | ||||
| PFMT → Pessary → Surgery | 1,617.35 | 7.510 | — | 42.0 |
| 10 years | ||||
| PFMT → Pessary → Surgery | 1,609.71 | 7.510 | — | 42.0 |
| Pessary cost | ||||
| $35 pessary | ||||
| PFMT → Pessary → Surgery | 1,612.60 | 7.510 | — | 42.0 |
| $112 pessary | ||||
| PFMT → Pessary → Surgery | 1,638.02 | 7.510 | — | 41.7 |
| Exclude patient preferences | ||||
| PFMT → Pessary → Surgery | 2,206.90 | 7.522 | — | 57.4 |
| Exclude PFMT as comparator | ||||
| Pessary → Surgery | 3,858.69 | 7.395 | — | 98.5 |
Abbreviations: CE, cost-effective; ICER, incremental cost-effectiveness ratio; PFMT, pelvic floor muscle training.
Based on a probabilistic sensitivity analysis using a cost-effectiveness acceptability curve at a willingness-to-pay of $50,000 per QALY gained.
Table 33:
Scenario Analysis Results–Stress Urinary Incontinence
| Strategy | Cost ($ CAD) | Effect | ICER ($ CAD) | CE Probability (%)a |
|---|---|---|---|---|
| Time horizon | ||||
| 2 years | ||||
| Pessary → PFMT → Surgery | 530.95 | 1.665 | – | 21.8 |
| PFMT → Pessary → Surgery | 597.06 | 1.672 | 10,452.79 | 72.9 |
| Lifetime | ||||
| PFMT → Surgery | 2,165.28 | 22.320 | – | 20.2 |
| Pessary → PFMT → Surgery | 2,597.81 | 22.345 | 17,416.25 | 23.3 |
| Discount rate | ||||
| 0% | ||||
| PFMT → Pessary → Surgery | 1,260.29 | 8.273 | – | 37.4 |
| 3% | ||||
| PFMT → Pessary → Surgery | 1,151.75 | 7.226 | – | 38.7 |
| 5% | ||||
| PFMT → Pessary → Surgery | 1,091.76 | 6.643 | – | 39.9 |
| Pessary device replacement | ||||
| 3 years | ||||
| PFMT → Pessary → Surgery | 1,207.93 | 7.721 | – | 38.3 |
| 10 years | ||||
| PFMT → Pessary → Surgery | 1,199.87 | 7.721 | – | 38.2 |
| Pessary cost | ||||
| $35 pessary | ||||
| PFMT → Pessary → Surgery | 1,202.94 | 7.721 | – | 38.3 |
| $112 pessary | ||||
| PFMT → Pessary → Surgery | 1,229.52 | 7.721 | – | 38.5 |
| Exclude patient preferences | ||||
| PFMT → Pessary → Surgery | 1,446.76 | 7.723 | 55.2 | |
| Exclude PFMT as comparator | ||||
| Surgery | 2,274.27 | 7.577 | – | 23.2 |
| Pessary → Surgery | 2,334.09 | 7.635 | 1,033.03 | 76.8 |
Abbreviations: CE, cost-effective; ICER, incremental cost-effectiveness ratio; PFMT, pelvic floor muscle training.
Based on a probabilistic sensitivity analysis using a cost-effectiveness acceptability curve at a willingness-to-pay of $50,000 per QALY gained.
We found that changes to the pessary device replacement frequency and the cost of the pessary device itself had little impact on the ICER to the reference case.
When the time horizon was altered to 2 years, both “PFMT → Pessary → Surgery” and “Pessary → PFMT → Surgery” were neither dominated nor subject to extended dominance. Notably, the probability of “PFMT → Pessary → Surgery” being cost-effective at $50,000 per QALY gained increased in the 2-year time horizon. This likely occurred because the use of conservative treatments delay individuals from receiving an expensive surgery, and in some cases the delay pushed the need for surgery beyond the 2-year time horizon. When the time horizon was extended beyond 2 years, the ICERs increased and the probability of “PFMT → Pessary → Surgery” being cost-effective at $50,000 per QALY gained decreased from 93.5% to 55.8% at 10 years (reference case), and to 25.9% at a lifetime horizon.
The discount rate was run at 0%, 3%, and 5% across both cohorts. Compared with the reference case, there was no change to the most cost-effective treatment sequence. As the discount rate increased, costs and QALY gain decreased, while the probability that “PFMT → Pessary → Surgery” was cost-effective increased slightly.
In one scenario, we excluded patient preferences towards receiving surgery. We assumed that 90% of the cohort would be eligible and all would receive surgery. Although the most cost-effective treatment remained unchanged, the probability of “PFMT → Pessary → Surgery” being cost-effective at $50,000 per QALY decreased. This shift likely occurs because the treatment sequence “Surgery” has a higher initial success rate compared with conservative therapies, resulting in the potential for individuals to spend less time being untreated and have a higher QALY gain.
The final scenario excluded PFMT as a comparator. In this scenario, the treatment sequence “Pessary → Surgery” dominates “Surgery” in the POP cohort and had an ICER of $1,033 per QALY gained in the SUI cohort.
Discussion
The results of the reference case analysis indicate that pessaries are likely to be cost-effective in a stepped care model for individuals with POP or SUI. In both cohorts, the treatment sequence “PFMT → Pessary → Surgery” was the most cost-effective and dominates all other treatment sequences. In the SUI cohort, treatment strategies that included PFMT had both the lowest costs and some of the highest gains in QALYs. This trend of PFMT driving improved QALY gains and decreased costs is less pronounced in POP, but strategies that included PFMT had better outcomes. This finding is likely heavily influenced by long-term PFMT effectiveness data used to model discontinuation, which had fewer long-term studies relative to the pessary literature. As shown in Figures A1 and A2, although pessaries have a lower discontinuation rate initially at 32% versus 43% at 1 year, PFMT has a slower discontinuation rate as time progresses, resulting in 33% of users finding PFMT still effective after 10 years, compared with only 18% of pessary users. Despite the higher initial discontinuation rate in PFMT, even when a 2-year time horizon is used, treatment sequences beginning with PFMT have a higher QALY gain. This may be due to both the slower discontinuation rate and the proportion of initial pessary users who cannot have their pessaries fitted.
Despite PFMT being a key driver of cost-effectiveness between treatment sequences, pessaries were shown to be an effective treatment. When PFMT was excluded, “Pessary → Surgery” dominates “Surgery” in POP (i.e., lower mean cost and higher mean QALY gain) and had an ICER of $1,033 per QALY gained in the SUI cohort. At a willingness to pay of $50,000 per QALY, the percentage of iterations found cost-effective was 98.5% in POP and 76.8% in SUI. When determining the cost-effectiveness of publicly funding pessaries, this scenario closely resembles the current landscape of public funding in Ontario, as PFMT has very limited public funding. Furthermore, this scenario removes a potentially biased estimate in PFMT discontinuation, which has inconsistent long-term outcome measurement in the published literature (see Strengths and Limitations, below, for further details).
Model Considerations
Despite modelling SUI and POP through two different economic models, many inputs used came from sources with populations that had symptoms of both SUI and POP. For instance, in one study with 10 years of data on pessary discontinuation rates, the population of pessary users was almost entirely (95.2%) individuals with concomitant POP and SUI.63 This high degree of symptom overlap is also apparent in corrective surgeries. Surgical procedures repairing bladder prolapse often uncover existing mechanical dysfunction of the urethra that, after prolapse repair, begins to manifest SUI, resulting in both prolapse and incontinence being repaired together. Overall, the model's results in both populations were similar, leading us to expect that pessaries will also be cost-effective in a population with both symptoms.
Without public funding, out-of-pocket costs for conservative therapies provide a barrier to accessing care. Specifically, PFMT costs may be deemed prohibitive as they are estimated at $545 for a complete series of physiotherapist-led trainings (four office visits). As the pessary device is also not publicly funded, individuals do not have a publicly funded conservative intervention. Therefore, they must pay out of pocket for a conservative therapy or pursue publicly funded surgery, if eligible. However, a large number of people with POP and SUI are elderly and some may not be surgical candidates due to comorbidities. In addition, others may have a preference to avoid surgery due to various factors such as media attention regarding vaginal mesh used in surgery and its associated possibility of persistent pelvic pain (email communications, Aisling Clancy, MD, January 2020; Sinéad Dufour, MD, December 2019; and Jennifer Skelly, PhD, December 2019). Our model accounted for eligibility and patient preferences towards interventions within each treatment sequence (Table 24). For PFMT and pessaries, we assumed patient preferences were accounted for by the high initial discontinuation rate. One area not explored was the likelihood of individuals whose preference was to go straight to surgery and avoid pessary use. This likely represents a small proportion of patients, as surgical wait times (from deciding with the surgeon to proceed with the surgery) in Ontario for SUI and POP are 97 and 116 days, respectively.90 Therefore, those individuals who desire surgery and decline conservative therapies would need to be comfortable with remaining untreated for at least 3 months before their surgery date.
The treatment sequences presented in our model assumed all treatments followed a stepped care approach, starting with conservative therapies, where applicable, and ending with surgery. However, as seen in Preferences and Values Evidence, below, some individuals may use pessaries after surgery. We accounted for this population, but assumed the number is minimal as clinical guidelines across POP and SUI recommend conservative therapies prior to surgery57,58 and because pessary fittings following a failed surgery are often more difficult due to scar tissue impeding the pessary's overall fit (email communications, Jennifer Skelly, PhD, December 2020; Aisling Clancy, MD, January 2020).
In our model, the untreated state represented treatment exhaustion in POP and SUI. We did not account for the potential costs of untreated individuals given a lack of available evidence to inform costing. However, it should be noted that from a societal perspective, incontinence products and other expenses may be regularly purchased to manage an individual's SUI.
Treatment discontinuation rates for conservative therapies are a key driver of an intervention's cost-effectiveness. Not explored in our analysis were other patient-level factors impacting discontinuation rates. An expert indicated that long-term adherence to pessaries may be lower in younger people who are sexually active, and patient satisfaction may be correlated with severity of the underlying prolapse or urinary incontinence (email communication, Aisling Clancy, MD, January 2020). Successful pessary fitting may also be impacted by many unique factors, such as whether vaginal estrogen cream was used prior to fitting, the experience level of the health professional fitting the pessary and the pessaries available to them, whether the patient is on hormone replacement therapy, or whether they had a prior vaginal surgery (i.e., prolapse repair or hysterectomy).
Cost-Effectiveness Literature on Pessaries
We identified one other Canadian study that evaluated both pessaries and PFMT in a population with symptomatic SUI. It conducted a head-to-head comparison over a 1-year time horizon and found that, when compared with pessaries, PFMT had an ICER of $15,346 per QALY gained. Given the short time horizon, it did not account for the discontinuation rate of conservative interventions. The study also only accounted for the cost of the pessary device, not the initial fitting and ongoing annual cleanings that were included in our model. Although the authors concluded that pessaries were not the preferred option when compared with PFMT, they also reported that pessaries have clinical utility for SUI in those with fiscal constraints or medical conditions that prevent them from participating in PFMT. Our analysis sought to evaluate the most cost-effective treatment sequence, so it did not directly compare pessaries with PFMT. However, as described earlier, PFMT was a key driver in the cost-effectiveness of the treatment sequences.
Strengths and Limitations
Our primary economic evaluation had several strengths. It is the first known analysis estimating the cost-effectiveness of treatment sequences for POP and SUI, accounting for the complexities of treating these chronic conditions through a stepped care approach. This methodology is advantageous over a direct head-to-head comparison, as comparing the cost-effectiveness of pessaries to surgery fails to account for the fact that pessaries are a conservative treatment often advised before progressing to surgery, and it is not intended to replace it. Another strength was the costing methodology used. Our analysis was applicable to the local setting through the use of Ontario-specific costs wherever possible. We also conducted detailed costing of our interventions. For pessaries, in addition to the cost of the devices, we also included the pessary fitting and regular cleaning costs and accounted for the subpopulation who self-manage their pessary maintenance. Finally, our time horizon was 10 years, which is useful when evaluating conservative treatments such as pessaries as they have a large discontinuation rate over time. To inform the pessary discontinuation rate, adherence data was pulled from the literature, pooled, and fitted to an exponential distribution used to predict discontinuation rates beyond what is reported in the published literature.
To model POP and SUI and their various interventions, the model required simplifying assumptions to answer the research question while limiting model complexity. For example, a single surgical success rate and cost was chosen despite the fact that there are numerous surgical techniques and procedures for POP, with various success rates. The model also limited the number of resurgeries to one due to a lack of evidence on success rate of sequential surgeries and the complexity of modelling the rates of additional resurgeries. Pessary refittings were also limited to one fitting, and the model assumed a pessary user did not switch pessary types before their existing pessary needed replacement. Any additional cost of fittings was assumed to occur in only a small percentage of patients, and thus have a marginal impact on the total cost. Finally, the model assumed that complications for pessaries that are rare or minor will typically be treated through temporary removal of the pessary, and treatments used for other complications, such as urinary tract infections, would have a marginal impact on the total cost.
Our analysis also has limitations. As identified in the clinical evidence review, many studies were deemed low quality based on GRADE score and, given the lower inherent GRADE score of the additional observational studies included in this economic analysis, we recommend interpreting the results with caution. We used utility values to represent untreated and successfully treated individuals. A utility difference was not used as the utility values for SUI, were taken from two different sources. When plotted on a distribution, there is a possibility of cross-over in the simulated utility values for untreated and treated, resulting in some iterations potentially having an untreated utility value higher than treated. This would result in a conservative underestimation of the overall QALY gain in the SUI population. Another limitation is the potential for input used to determine an individual's eligibility and preference for reoperation to be accounted for already in the probability that an individual requires a reoperation after their symptoms return post-surgery. This limitation may cause a minor underestimation of the QALY gain from surgery. Despite the potential overlap, both estimates were included, as eligibility and patient preferences towards reoperation are necessary inputs in the subpopulation of individuals who fail their initial surgery.
Another limitation is that evidence used to calculate the PFMT discontinuation rate was poor and lacked a standard definition across published studies identifying long-term PFMT success. To address this concern, scenario analyses excluding PFMT were conducted. One final limitation was the uncertainty in using predicted pessary and PFMT discontinuation rates beyond what is published in the literature to inform the lifetime horizon analysis. Given the uncertainty when using prediction, the reference case used a 10-year time horizon to reflect the data available in the literature, and a lifetime horizon was run as an exploratory scenario analysis.
Conclusions
Our economic analysis found that pessaries was within the most cost-effective treatment sequence; therefore, it's likely to be a cost-effective intervention for treating POP and SUI. In our reference case, there was a high degree of certainty that pessaries were cost-effective in a population with POP, and a moderate degree of certainty that pessaries were cost-effective in a population with SUI. When the treatment sequence of pessaries and surgery was compared with surgery alone, the pessaries treatment sequence dominates surgery in the cohort with POP, and in the cohort with SUI had an ICER of $1,033 per QALY gained.
Budget Impact Analysis
Research Question
-
1.
From the perspective of the Ontario Ministry of Health, what is the potential 5-year budget impact in Ontario of publicly funding vaginal pessaries for pelvic organ prolapse?
-
2.
From the perspective of the Ontario Ministry of Health, what is the potential 5-year budget impact in Ontario of publicly funding vaginal pessaries for stress urinary incontinence?
Methods
As reflected in the research question, the treatment sequencing approach that was conducted in the primary economic evaluation was not used in the budget impact analysis. In the primary economic evaluation, treatment sequencing was used to reflect the stepped care approach to treating pelvic organ prolapse (POP) and stress urinary incontinence (SUI) with multiple interventions. As conservative treatments are recommended prior to progressing to a more invasive intervention such as surgery, evaluating the cost-effectiveness of a pessary compared with surgery provides little insight into the most cost-effective way to treat POP and SUI. The primary economic evaluation concluded that the most cost-effective treatment sequences included pessaries. Since we have shown pessaries are likely to be a cost-effective intervention within a stepped care model for SUI and POP, this budget impact analysis focuses on estimating the additional cost required to publicly fund pessaries in the target populations. We did not estimate the budget impact of PFMT as it was outside the scope of this HTA.
Analytic Framework
We estimated the budget impact of publicly funding pessaries using the cost difference between two scenarios: the current scenario, which is the current clinical practice of not funding the pessary device, and the new scenario, which is the anticipated clinical practice of funding the pessary device. The model schematic is shown in Figure 6.
Figure 6: Schematic Model of Budget Impact.
aFitting and maintenance are covered under the current scenario, but the pessary device is not.
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions.
Key Assumptions
All assumptions used in the primary economic evaluation apply to the budget impact analysis
Publicly funding the pessary device increases the demand for pessaries and thus the use of physician services for pessary fittings and cleanings increases proportionately
Existing pessary users fitted prior to the first year of the budget impact are not costed
After pessary discontinuation, there are no additional costs
Patients who have undergone surgery do not switch to a pessary
Target Population
The target population was people with symptomatic POP or SUI who are treated with a pessary. Table 34 shows a breakdown of the calculation of the target population. First, the prevalence of POP (2.9%) and SUI (1.95%) were calculated using the incidence of POP and SUI among the Ontario adult female population. Using the combined prevalence numbers, the individual proportions were calculated. This value was later used to subcategorize the number of pessary fittings for people with POP and with SUI. The final target population counts used in the analysis are presented in Table 35.
Table 34:
Target Population Calculation
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Ontario adult female populationa | 5,929,863 | 6,018,768 | 6,099,538 | 6,173,292 | 6,246,523 |
| Prevalence of SUIb | 115,632 | 117,366 | 118,941 | 120,379 | 121,807 |
| Prevalence of POPc | 171,966 | 174,544 | 176,887 | 179,025 | 181,149 |
| Total prevalence of SUI and POP | 287,598 | 291,910 | 295,828 | 299,405 | 302,956 |
| Percent of total prevalence from POPd | 59.79% | 59.79% | 59.79% | 59.79% | 59.79% |
| Ontario first pessary fittings: | |||||
| Physician onlye | 5,837 | 5,953 | 6,187 | 6,420 | 6,654 |
| All healthcare professionalsf | 7,144 | 7,424 | 7,705 | 7,985 | 8,265 |
| Population expansion due to pessary public fundingg | 7,858 | 8,166 | 8,475 | 8,784 | 9,092 |
| Target population for budget impact analysish | |||||
| SUI | 3,159 | 3,283 | 3,407 | 3,532 | 3,656 |
| POP | 4,699 | 4,883 | 5,068 | 5,252 | 5,436 |
Abbreviations: POP, pelvic organ prolapse; SUI, stress urinary incontinence.
Ontario Ministry of Finance female population projections.91
Assuming a constant urinary incontinence prevalence of 3.9%, of which 50% consist of individuals with stress urinary incontinence.92
Assuming a constant pelvic organ prolapse prevalence of 2.9%.13
Disregarding potential crossover between populations.
Data provided by Ontario IntelliHealth. The number of unique patients being billed under Schedule of Benefits code G398 (medical management of prolapse—initial pessary fitting or re-fitting as required).
Assuming 20% of all pessary fittings are conducted by non-physicians (email communication, Aisling Clancy, MD, January 2020; Jennifer Skelly, PhD, December 2019).
Assuming a flat 10% increase to all pessary fittings across 5 years (email communication, Aisling Clancy, MD, January 2020; Risa Bordman, MD, December 2019).
Derived using the previously calculated 59.79% for pelvic organ prolapse, and 40.21% for stress urinary incontinence.
Table 35:
Target Population
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Pelvic Organ Prolapse | |||||
| Current scenario | 4,271 | 4,439 | 4,607 | 4,775 | 4,942 |
| New scenario | 4,699 | 4,883 | 5,068 | 5,252 | 5,436 |
| Stress Urinary Incontinence | |||||
| Current scenario | 2,872 | 2,985 | 3,098 | 3,210 | 3,323 |
| New scenario | 3,159 | 3,283 | 3,407 | 3,532 | 3,656 |
The annual number of pessary fittings was obtained from local Ontario IntelliHealth data, specifically using physician medical services data (i.e., the Ontario Health Insurance Plan [OHIP]). Specifically, we obtained data on people with a new pessary fitting, defined as individuals who had no previous record of an earlier pessary fitting. Of all pessary fittings, the percentage of individuals with POP or SUI who had their first fitting was constant over the last four years (2014–2017) at 51%. As data on pessary fittings was available only up to 2017, linear regression was used to estimate fittings in future years. The percentage of the total fittings that were first fittings was then estimated using the same historical percentage (i.e., 51%). As the administrative data used to estimate pessary fittings was specific to physician medical services, we used expert opinion to estimate that an additional 20% of fittings would come from other healthcare providers, such as nurses in pessary clinics (email communication, Aisling Clancy, MD, January 2020; Jennifer Skelly, PhD, December 2019). Finally, we assumed a 10% market expansion to the target population, as those unable to afford a pessary would no longer have a cost barrier to pessary use (email communication, Aisling Clancy, MD, January 2020; Risa Bordman, MD, December 2019).
In our scenario analyses we examined the target population under different assumptions (see Appendix 8 Table A13). The first scenario looked at the budget impact in the population who received a pessary fitting by physicians alone. The second assumed the expansion of pessary fittings would increase by 20% over the five years, representing individuals who previously could not afford a pessary without public funding.
Resources and Costs
For each cohort, we obtained the mean cost per patient from our primary economic evaluation's probabilistic analysis, specifically in a new treatment sequence not previously shown in the primary economic evaluation that evaluates pessaries alone. We used annual undiscounted costs for 5 years from the reference case analysis of the primary economic evaluation. These included resource use and costs related to medical devices and physician services. We provided a detailed description of these costs in the Primary Economic Evaluation, above. All costs are reported in 2019 Canadian dollars. The costs per patient for both the reference case and scenario analyses can be found in Appendix 8, Table A14. The costs varied slightly between the POP and SUI cohorts due to differential mortality and pessary fitting success rates. Furthermore, a noticeable increase in costs at year 5 between the current and new scenarios reflects the additional cost of a pessary replacement (we assume one pessary replacement every 5 years). As the budget impact analysis pulls costs from the companion cost-effectiveness analysis, the yearly costs account for pessary discontinuation over time, which is reflected in the decreasing annual cost per patient over the 5 years.
In our scenario analyses, we varied the cost of a pessary device (see Appendix 8, Table A14). Compared with the reference case, where a pessary costs $55.50, the scenarios used both a higher ($91.08) and lower cost ($35.00) for the devices. The details of these costs are previously described in the Primary Economic Evaluation, above.
Analysis
This analysis evaluated the incremental cost of funding pessary devices and their associated increase in volume compared with the current practice of only funding physician services related to pessaries. To account for projected physician services over a 5-year period, the budget impact analysis was conducted with a companion cost-effectiveness analysis (using undiscounted costs). This companion cost-effectiveness analysis was for pessaries alone, a new treatment sequence from what was previously examined that allowed us to estimate the yearly costs of pessaries.
Results
Reference Case
The results of the reference case analysis are presented in Table 36. The estimated budget impact of publicly funding pessaries ranged from $0.3 million in the first year to $0.5 million in fifth year for POP, and $0.2 million in the first year and $0.3 million in the fifth year for SUI. This equates to a 5-year total of $2.0 million and $1.3 million for POP and SUI, respectively.
Table 36:
Budget Impact Analysis Results
| Budget Impact ($ Millions)a | |||||||
|---|---|---|---|---|---|---|---|
| Population | Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| POP | Current | 0.717 | 1.070 | 1.388 | 1.676 | 1.938 | 6.788 |
| New | 1.022 | 1.419 | 1.779 | 2.105 | 2.492 | 8.816 | |
| Budget impact | 0.305 | 0.350 | 0.391 | 0.429 | 0.553 | 2.028 | |
| SUI | Current | 0.483 | 0.721 | 0.937 | 1.135 | 1.316 | 4.591 |
| New | 0.688 | 0.957 | 1.201 | 1.424 | 1.691 | 5.962 | |
| Budget impact | 0.206 | 0.236 | 0.264 | 0.290 | 0.376 | 1.371 | |
Abbreviations: POP, pelvic organ prolapse; SUI, stress urinary incontinence.
In 2019 Canadian dollars. Some budget impact numbers may not add up due to rounding.
Scenario Analyses
Tables 37 and 38 present the results from the scenario analyses on POP and SUI. If we assume there will be no expansion of pessary usage after public funding, the total 5-year budget would decrease to $1.4 and $0.9 million for POP and SUI, respectively. However, if we assume an expansion of 20%, hypothetically representing individuals who previously could not afford a pessary without public funding, the 5-year budget impact would rise to $2.8 and $1.9 million for POP and SUI, respectively. The scenario analyses also look at varying the mean cost of a pessary from $55.00 to a high of $91.08 and a low of $35.00. If the mean pessary cost was purchased at $91.08, the 5-year budget impact would rise to $3.1 and $2.1 million for POP and SUI, respectively. If the mean cost was reduced to $35.00, the 5-year budget impact would decrease to $1.6 and $1.0 million for POP and SUI, respectively. Another scenario looked at funding pessaries through the Ontario Assistive Devices Program, which would cover 75% of the device cost. In that scenario, the 5-year budget impact would decrease to $1.7 and $1.1 million for POP and SUI, respectively. Finally, the last scenario assumed device funding would be given to those without private insurance covering a pessary. It's estimated that 63% of the Ontario population have private insurance for prescription drugs, and it was assumed that same coverage would be applicable to medical devices such as a pessary.93 If funding was exclusive to those without private insurance, the 5-year budget impact would decrease to $1.3 and $0.7 million for POP and SUI, respectively.
Table 37:
Budget Impact Analysis Results—Pelvic Organ Prolapse
| Budget Impact ($ Millions)a | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Reference Case | ||||||
| Current scenario | 0.717 | 1.070 | 1.388 | 1.676 | 1.938 | 6.788 |
| New scenario | 1.022 | 1.419 | 1.779 | 2.105 | 2.492 | 8.816 |
| Budget impact | 0.305 | 0.350 | 0.391 | 0.429 | 0.553 | 2.028 |
| No Expansion | ||||||
| New scenario | 0.929 | 1.303 | 1.651 | 1.976 | 2.372 | 8.231 |
| Budget impact | 0.212 | 0.234 | 0.264 | 0.300 | 0.433 | 1.443 |
| 20% Expansion | ||||||
| New scenario | 1.115 | 1.548 | 1.940 | 2.296 | 2.718 | 9.618 |
| Budget impact | 0.398 | 0.479 | 0.552 | 0.620 | 0.780 | 2.829 |
| Low Pessary Cost | ||||||
| New scenario | 0.952 | 1.347 | 1.703 | 2.026 | 2.384 | 8.411 |
| Budget impact | 0.235 | 0.277 | 0.315 | 0.350 | 0.445 | 1.623 |
| High Pessary Cost | ||||||
| New scenario | 1.214 | 1.619 | 1.986 | 2.319 | 2.787 | 9.925 |
| Budget impact | 0.497 | 0.549 | 0.598 | 0.643 | 0.849 | 3.136 |
| 75% Pessary Device Funding | ||||||
| New scenario | 0.964 | 1.359 | 1.716 | 2.040 | 2.403 | 8.482 |
| Budget impact | 0.247 | 0.289 | 0.328 | 0.364 | 0.465 | 1.694 |
| Fund Privately Uninsured | ||||||
| New scenario | 0.875 | 1.266 | 1.634 | 1.978 | 2.334 | 8.087 |
| Budget impact | 0.158 | 0.197 | 0.246 | 0.302 | 0.395 | 1.299 |
In 2019 Canadian dollars. Some budget impact numbers may not add up due to rounding.
Table 38:
Budget Impact Analysis Results–Stress Urinary Incontinence
| Budget Impact ($ Millions)a | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Reference Case | ||||||
| Current scenario | 0.483 | 0.721 | 0.937 | 1.135 | 1.316 | 4.591 |
| New scenario | 0.688 | 0.957 | 1.201 | 1.424 | 1.691 | 5.962 |
| Budget impact | 0.206 | 0.236 | 0.264 | 0.290 | 0.376 | 1.371 |
| No Expansion | ||||||
| New scenario | 0.626 | 0.878 | 1.115 | 1.337 | 1.611 | 5.568 |
| Budget impact | 0.143 | 0.158 | 0.178 | 0.203 | 0.296 | 0.977 |
| 20% Expansion | ||||||
| New scenario | 0.751 | 1.044 | 1.310 | 1.554 | 1.845 | 6.504 |
| Budget impact | 0.269 | 0.323 | 0.373 | 0.419 | 0.530 | 1.913 |
| Low Pessary Cost | ||||||
| New scenario | 0.641 | 0.908 | 1.150 | 1.371 | 1.618 | 5.688 |
| Budget impact | 0.159 | 0.187 | 0.213 | 0.237 | 0.303 | 1.098 |
| High Pessary Cost | ||||||
| New scenario | 0.818 | 1.091 | 1.341 | 1.569 | 1.892 | 6.711 |
| Budget impact | 0.335 | 0.371 | 0.404 | 0.435 | 0.577 | 2.121 |
| 75% Pessary Device Funding | ||||||
| New scenario | 0.649 | 0.916 | 1.159 | 1.381 | 1.631 | 5.736 |
| Budget impact | 0.167 | 0.195 | 0.222 | 0.246 | 0.316 | 1.146 |
| Fund Privately Uninsured | ||||||
| New scenario | 0.588 | 0.852 | 1.089 | 1.305 | 1.523 | 5.357 |
| Budget impact | 0.106 | 0.131 | 0.152 | 0.170 | 0.208 | 0.766 |
In 2019 Canadian dollars. Some budget impact numbers may not add up due to rounding.
Discussion
This analysis built off the primary economic evaluation, which identified pessaries as a cost-effective intervention when used within a sequence of interventions treating POP and SUI. The budget impact analysis did not look at the full cost of a treatment sequence, but focussed on the cost of pessaries alone to estimate the additional cost expected if pessary devices are publicly funded. This includes the total cost of the pessary devices, as well as the additional costs in maintenance services, which are already covered in Ontario, due to the expected increase in pessary use.
In the reference case analysis, the total 5-year budget impact for POP and SUI were $2.0 and $1.3 million, respectively. Compared with current funding, which reimburses only pessary fittings and cleanings, the new funding scenario, which covers a pessary device at an average cost of $50 per device, is estimated to increase the pessary-related budget by roughly 30%. As local sources for pessary prices vary, our scenario analyses examined how the budget would be impacted if provincially funded pessaries were purchased at both higher and lower costs. If pessary funding were to be implemented, bulk purchasing strategies for both hospital and non-hospital settings present an opportunity to further reduce the estimated budget impact. Alternatively, cost sharing mechanisms such as co-payments through the Assistive Devices Program (which provides 75% coverage for medical devices) could be employed to improve access and control the costs required for public funding. Our analysis estimated, based on clinical expert consultation, that if pessary devices were publicly funded, current demand for pessaries would rise by 20%. This increase represents individuals who currently forgo purchasing a pessary due to the cost. The average age of people with POP approximates retirement age, and these people who rely on pension income may have more difficulty absorbing the costs of a pessary compared with working-age individuals. Funding strategies, including co-payments and subsidies for subpopulations who cannot afford a pessary, are alternative strategies that may improve access and equity while managing costs.
In one scenario analysis, we assumed that pessary device funding would be provided for those who lack coverage through private insurance. Applying this stricter eligibility criteria led to a large drop in the 5-year budget impact. However, there is a large degree of uncertainty in this estimate, as the number of Ontarians with private insurance for prescription drugs may not be a good indicator of coverage for medical devices such as the pessary. A further source of uncertainty is that the percentage of the population with private drug insurance varies significantly by age group; over 75% of those 25–64 years old, but less than 40% of those 65 years of age or older, have coverage.93 This difference is important as the average age of pessary users in the primary economic evaluation was 62 years for POP and 50 years for SUI. Given the older mean age of pessary users, it's possible the percentage of pessary users with private insurance is lower than the 63% estimate used in our analysis.
Publicly funding pessaries may lead to cost savings in several distinct areas not explored in this analysis. First in individuals with SUI, pessary use may decrease the uptake of medical supplies. Specifically, it may reduce the number of purchased incontinence supplies such as diapers, maxi pads, and existing medications that are not publicly funded. This could reduce societal costs or the need for local grants used for urinary incontinence (e.g., the Ontario Disability Support Program Income Support). If urinary incontinence is improved significantly with pessary use, it may also impact an individual's economic productivity by reducing rates of absenteeism or presenteeism. Second, pessary funding could delay or remove the need for costly surgery in a percentage of the target population. This concept is highlighted in the primary economic evaluation through its use of treatment sequences (stepped care approach), where conservative treatments are offered before surgery. This potential reduction of the need for surgery through pessary use was not explored in our analysis due to data limitations on the number of surgeries conducted in our target population. Finally, a 5-year budget impact would likely underestimate the long-term budget impact, as some surgical costs would be incurred at a later date.
The demand for pessaries was estimated using long-term historical data on pessary fittings; however, several factors may impact the accuracy of these historical projections. An aging population may lead to an increase in the number of people with SUI and POP requiring pessaries beyond what is predicted using historical data. Pessary use may also be impacted by aversion to vaginal mesh in surgeries, especially with its past media attention. If surgery rates stay below expectations due to these sensitivities, the use of alternative methods such as pessaries may exceed our estimates, increasing costs to the system.
Strengths and Limitations
Our analysis had several strengths. We used a companion primary economic evaluation, which allowed us to account for factors such as the long-term discontinuation rate. This approach results in a budget impact estimate that accurately predicts long-term pessary-related costs. An additional strength was the use of scenario analyses to explore uncertainty around both the target population and the cost of a pessary. This analysis was also strengthened by local administrative data, which provide an accurate historical trend of the number of physician pessary fittings.
Our analysis also had several limitations. Due to a lack of published Ontario data, there is uncertainty around the population that live with POP and/or SUI; especially the number of individuals who have both conditions. Given this unknown degree of overlap, the population breakdown by POP and SUI used in the budget impact analysis may differ from the true population makeup. However, the combined budget impacts of POP and SUI are based on local administrative data, which should reflect the overall population of pessary users.
Conclusions
Our budget impact analysis indicates that publicly funding pessaries for pelvic organ prolapse may result in extra spending of $0.3 million to $0.5 million annually for the next 5 years, with a 5-year total additional cost of $2.0 million. Funding pessaries for stress urinary incontinence would result in extra spending of $0.2 million to $0.3 million annually for the next 5 years, with a 5-year additional cost of $1.3 million. If pessaries are funded in both populations, the total 5-year budget impact would be about $3.3 million.
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of those who have lived experience with pelvic organ prolapse (POP) or stress urinary incontinence (SUI), as well as the preferences and perceptions of both patients and providers of vaginal pessaries.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).94–96 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are often inadequately explored in the published literature, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
For this analysis, we examined the preferences and values of people with POP or SUI who sought treatment with vaginal pessaries in two ways:
A review by Ontario Health of the quantitative evidence on patient and provider preferences and values
Direct engagement by Ontario Health with people with these conditions through interviews
Quantitative Evidence
Research Question
What is the relative preference of patients for pessary use in the treatment of POP and/or SUI compared with standard of care?
Methods
LITERATURE SEARCH
We performed a targeted literature search for quantitative evidence of preferences and values on July 4, 2019, to retrieve studies published from January 1, 2000 until the search date. We used the OVID interface to search MEDLINE. The search was based on the population and intervention of the clinical search strategy with a methodological filter applied to limit retrieval to quantitative evidence of preferences and values (modified from Selva et al97). See Appendix 1 for our literature search strategies, including all search terms.
ELIGIBILITY CRITERIA
The population and intervention components are the same as the clinical review, but the outcomes will be patient and/or provider preferences. No specific comparator is required.
Inclusion and exclusion criteria will be the same as the clinical review; however, included studies report patient or provider preferences toward the use of pessaries for the treatment of POP or SUI using quantitative methods.
LITERATURE SCREENING
A single reviewer conducted an initial screening of titles and abstracts using Covidence21 and then obtained the full text of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion.
DATA EXTRACTION
We extracted relevant data on study characteristics using a data form to collect information about the following:
Source (e.g., citation information, contact details, study type)
Methods (e.g., study design, study duration, participant recruitment)
Outcomes (e.g., outcomes measured, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], time points at which the outcomes were assessed)
STATISTICAL ANALYSIS
Results are summarized narratively. No additional statistical analyses were conducted beyond those reported in the primary studies.
CRITICAL APPRAISAL OF EVIDENCE
We did not undertake a formal critical appraisal of the included studies.
Results
LITERATURE SEARCH
The literature search of the quantitative evidence of preferences and values yielded 99 citations published between January 1, 2000 and July 4, 2019, after removing duplicates. We reviewed titles and abstracts to identify potentially relevant articles. We identified one study that met our inclusion criteria. Figure 7 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the literature search for quantitative evidence of preferences and values.
Figure 7: PRISMA Flow Diagram—Quantitative Evidence of Preferences and Values Search Strategy.
Source: Adapted from Moher et al.26
CHARACTERISTICS OF INCLUDED STUDIES
Thys et al98 used a structured interview to assess the willingness of patients with symptomatic POP to alter treatment preference (interviewers presented scenarios of increasing likelihood of complications or side-effects) by determining the conditions at which their treatment preference changes.
The authors began by interviewing three groups of 25 people with POP reflecting three treatments examined: 1) people who were untreated, 2) people who underwent prolapse surgery, and 3) people who were treated with a pessary. During the interview, all participants were informed about the benefits and complications of surgery and pessary treatment98 and were asked whether they preferred a treatment and, if so, which treatment they preferred.
At baseline, participants were older and symptoms existed longer in the pessary group compared with the other two groups.98 In the surgery group, 48% had used a pessary before undergoing surgery, and most of them received a pessary in expectation of their surgery date.
After extensive information was provided to participants about both treatment options, it was observed that people in the no-treatment group had a slightly higher preference for prolapse surgery compared with pessary use (48% vs. 36%) (Table 39).98 In the surgery group, 92% would opt for surgery in the event that they had to decide again. In the pessary group, 86% would still opt for pessaries.98
Table 39:
Observational Study by Thys et al98 Reporting Patient Preference in Patients With Pelvic Organ Prolapse
| Group | |||
|---|---|---|---|
| No Treatment (n = 25) | Surgery (n = 25) | Pessary (n = 25) | |
| Age, years (range) | 60 (48–79) | 56 (35–84) | 73 (45–84) |
| Physical therapy | 9 (36%) | 5 (20%) | 5 (20%) |
| History of pessary | 0 (0%) | 12 (48%) | 25 (100%) |
| History of surgery | 0 (0%) | 25 (100%) | 0 (0%) |
| Duration of complaints, months (range) | 7 (2–30) | 24 (4–100) | 36 (3–120) |
| Treatment preference | |||
| Pessary | 9 (36%) | 2 (8%) | 21 (84%) |
| Prolapse surgery | 12 (48%) | 23 (92%) | 4 (16%) |
| No preferable treatment | 4 (16%) | 0 (0%) | 0 (0%) |
In the surgery group, there were two patients who expressed a preference for pessaries. Of these, one had bladder retention after surgery, and the other experienced a recurrent prolapse and had a pessary inserted.98 In the pessary group, four patients (16%) expressed a preference for surgery. The primary factors for their preference to switch were mild pessary-related symptoms that had lasted for several years and the wish to receive a definite solution.98 These four patients considered themselves too old to undergo prolapse surgery.98
Participants were then presented with fictional scenarios regarding both treatment options.98 Participants who indicated that they preferred pessary use over prolapse surgery were presented with a scenario where there was a 5% risk of vaginal irritation. Participants who continued to prefer pessary use were presented with the same scenario, but the risk of vaginal irritation was increased in 5% increments until they switched their preference to prolapse surgery (the point of trade-off).98 The same exercise was performed for two additional risks—placement problems and incomplete symptom relief.
Participants who indicated a preference for prolapse surgery over pessary use were presented with a scenario where there was a 5% risk of SUI. Participants who continued to prefer prolapse surgery were presented with the same scenario, but the risk of SUI was increased in 5% increments until they switched their preference to pessary use (the point of trade-off).98 The same exercise was performed for risk of prolapse recurrence.
Table 40 shows the trade-offs concerning the advantages and disadvantages of both treatment options.98 Treated patients have a lower tolerance for the disadvantages of the alternative treatment option, and a higher tolerance of the disadvantages associated with their own treatment.98
Table 40:
Observational Study by Thys et al98 Reporting Patient Preference Trade-Offs in Patients With Pelvic Organ Prolapse
| Group | |||
|---|---|---|---|
| No Treatment (n = 25)a | Surgery (n = 25)a | Pessary (n = 25)a | |
| Surgery | |||
| Stress urinary incontinence | 22% | 62% | 6% |
| Recurrence of prolapse | 43% | 84% | 7% |
| Pessary | |||
| Vaginal irritation | 32% | 10% | 72% |
| Placing problems | 32% | 9% | 60% |
| Incomplete treatment | 17% | 7% | 79% |
Data are median percentages.
In the treatment-naïve group, patients switched preference from surgery to a pessary at a median risk of SUI of 22% and of recurrent prolapse of 43%. Patients switched preference from pessary to surgery at a median risk of vaginal irritation of 32%, placing problems of 32% and of incomplete symptom relief of 17%.98
Thys et al98 also investigated the factors determining whether patients prefer a certain treatment. According to the authors, more than half of the participants (N = 75) rated the ability to preserve the uterus as not important and that almost all rated the permanent solution that surgery provided to be important or very important (exact values unavailable because data were presented graphically).
Limitations to the study by Thys et al98 include:
There was no validated questionnaire available to measure treatment preference. Instead, the authors based their questionnaire on literature and expert opinion98
By focussing on individual complications, the fictional scenarios may not accurately represent the full range of advantages and disadvantages that may affect a patient's final treatment decision98
Participants were referred to the authors by a general practitioner. This selection method may have a bias towards patients who prefer surgery or in whom treatment with a pessary failed98
Conclusions
Thys et al98 concluded that when realistic assumptions about advantages and disadvantages of a specific treatment are made, most people with POP consider the disadvantages of the treatment option they pursued to be acceptable.
Direct Patient Engagement
Methods
ENGAGEMENT PLAN
The engagement plan for this health technology assessment focused on consultation with patients to examine the experiences of people with pelvic organ prolapse (POP and/or stress urinary incontinence (SUI), including their experience with using pessaries.
We engaged people via phone interviews. We conducted qualitative interviews, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people affected by POP and/or SUI.
Our main task in interviewing was to understand what people told us and to gain an understanding of the story behind their experiences.105 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that supported our choice of an interview methodology.
PARTICIPANT OUTREACH
We used an approach called purposive sampling,106–109 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of health clinics, pelvic organ prolapse support groups, clinical specialists, and allied health professionals to spread the word about this engagement activity and to contact patients, families, and caregivers with experience with POP and/or SUI.
INCLUSION CRITERIA
We sought to speak with people and their caregivers who have been actively managing POP and/or SUI. These people were not required to have had direct experience with pessaries to participate.
We sought broad geographic, cultural, and socioeconomic representation to explore possible equity issues in accessing treatment for POP and/or SUI.
EXCLUSION CRITERIA
We did not set specific exclusion criteria.
PARTICIPANTS
We engaged with a total of 29 people, 28 by telephone, and one through a written submission over email. All participants had lived experience of pelvic organ prolapse with treatment for their condition. sixteen of the 29 participants were diagnosed with POP and the remaining 13 with POP and SUI.
We spoke to 24 people who had direct experience with pessaries. Duration of use varied from 2 weeks to 15 years. We also spoke to people who had experience using other forms of treatment, such as surgery and pelvic floor physiotherapy. Some used a pessary in conjunction with pelvic floor physiotherapy, while others used a pessary only after surgery.
Gaining an understanding of the day-to-day functioning of people with POP and/or SUI and their experiences with available treatments, including pessaries, helped us assess the potential value of pessaries from the perspective of people living with POP and/or SUI.
APPROACH
At the beginning of the interview, we explained the role of Ontario Health, the purpose of the health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a printed letter of information (Appendix 9). We then obtained participants' consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 20 to 90 minutes. The interviews were loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.110 Questions focused on the impact of pelvic organ prolapse on patients' and families' quality of life, their experiences with treatment options, and their perceptions of the benefits or limitations of using a pessary to manage their condition. See Appendix 10 for our interview guide.
DATA EXTRACTION AND ANALYSIS
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consisted of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.99,100 We used the qualitative data analysis software program NVivo101 to identify and interpret patterns in interview data. The patterns we identified allowed us to highlight the impact of POP and SUI and treatments on the people we interviewed.
Results
LIVED EXPERIENCE OF PELVIC ORGAN PROLAPSE AND STRESS URINARY INCONTINENCE
Participants reported that POP and SUI had a significant impact on their quality of life. They emphasized the constant struggle of managing the condition. The most commonly reported symptom was a bulge that protruded from the vagina. Other symptoms included pain, discomfort, pelvic pressure, backpain, incontinence, and difficulty voiding.
My uterus became visible on the outside…. It looked almost like a golf ball starting to protrude.
You feel horrible pressure and discomfort when you're peeing and you have something rubbing against your legs as you're walking.
Every time I went near a bathroom, I had to pee, every time. And it was like a rush, I could hardly get my pants down, I was peeing my pants or I'd drip. [If] anybody turned water on, I had to go to the bathroom right away. Things like that.
I had trouble with my bowel movements. I couldn't always even start the bowel movement normally, by sitting down on the toilet
When I felt like my bladder wasn't emptying completely…. I would always have to completely bend over to empty my bladder.
DIAGNOSIS OF PELVIC ORGAN PROLAPSE
People we spoke with reported a variety of experiences with the process of getting diagnosed with POP. Some reported a positive streamlined experience where they were seen, diagnosed, and provided treatment to management their prolapse. Others reported negative experiences, with the most common complaint being the long wait times to see a specialist.
They referred me to the [specialist], and told me it was a two-year wait to see her.
They said, it's going to be 18 months…And it really, really bothers me. Even now, and I think of how I was and how I was told that.
Referrals to specialists weren't always made in a timely manner and support from primary care providers was sometimes lacking.
After three years of talking to the doctor, he finally sent me to some specialists
He didn't examine anything at all. And he said basically, ‘Well you should just cut it out'…. It was a devastating thing to hear and I went back to work numb.
[It took] quite a number of years before they really figured out what was going on.
Other issues include misdiagnoses, conflicting information from physicians, and the condition not being taken seriously.
The doctor…examined me lying down, so he never saw it. He just kind of shook his head [and said], ‘there's nothing there.’ He sent me to one gynaecologist and they said there was nothing wrong. When I kept complaining… he sent me to another gynaecologist, and he's the one who said, ‘do you mind if I examine you standing up?'… And he said, ‘can you cough?’ and then it came out and he goes ‘oh, well this is what it is.'
My diagnosis process and consultation with doctors was complicated. I saw like five different doctors.
It was minimized…. I felt minimized, that this is not a big deal. It was a big deal to me, because it affected so much of my life.
There's all these women suffering in silence, suffering in silence because nobody talks about this. It's one of the last taboos…. A lot of women are forced to rely on obstetricians/gynaecologists who, to be quite honest, a lot of them, their information about prolapse is not excellent. Urogynecologists are the prolapse experts.
Most patients had no previous knowledge of POP and found the diagnosis unexpected. Many expressed frustration and anger due to the lack of awareness and information on POP, which they attributed to the stigmatizing nature of the condition.
Prolapse, it's so foreign, it's so alien, nobody talks about it. I had no idea what to do, I had no idea what was going on, nobody seems to know anything and I had to figure all this stuff out for myself. It was very frustrating.
Angry is what I felt that this information is not more public, that it's not more accessible, that you have to be so ashamed of the condition in the first place that you don't even bring it up to your doctor.
Well, I wasn't educated with pelvic prolapse. I had no idea about this, which kind of annoys me that I didn't know anything about [it]. I wish people were made aware…that this is something that can happen. I had no idea what it was. Not a clue. I didn't have any idea of what was starting to happen and that upsets me because I would have looked into it sooner.
DAY TO DAY IMPACT OF PELVIC ORGAN PROLAPSE AND STRESS URINARY INCONTINENCE
POP and SUI often restrict a person's activity level. Activities impacted range from simple tasks such as sitting, standing, and walking to more strenuous tasks such as bending, exercising, and heavy lifting. People with POP and SUI often have to adjust their daily routines or activities to cope with the symptoms.
Social impact
A majority of people we interviewed reported that POP and SUI symptoms limited their social activities. They described several social factors that were impacted, including their employment, relationships, and hobbies. Many drastically limited their socializing due to the fear of their prolapse getting worse or the symptoms being unmanageable.
Socializing, I was scared to do, I was scared to do anything except lie down, when I first discovered it.
I sing Wednesday nights and I couldn't stand for the two hours. I had to sit on a chair to sing, because I couldn't stand the whole time. I'd start and then I'd have to sit. So that was embarrassing.
And I'm good if I'm around home but then I'm always concerned when I'm out if I don't know where the bathroom is, so you know it's just this whole big thing.
Several of those interviewed reported being unable to exercise due to discomfort or the fear of making their prolapse worse. Those who also had SUI, limited their exercise due to the fear of having leaks.
I've completely stopped exercising because I'm terrified of making it worse… I'm terrified of doing anything. For a long time, I was afraid to even take my son out for walks, because I was worried that would make everything worse.
I used to walk an hour at a time and I had to cut my walk to about 10 minutes, because the prolapse–it would get worse on the walk. Like, just with gravity.
It was still affecting my activities of daily living. I'm a very physical person and I dance. I had to change from my tapping class to jazz, because I couldn't tap. And tap is my joy. I love tapping.
Several participants spoke of how simple day-to-day tasks became difficult, especially household chores and errands, due to their POP symptoms.
I'm limited in the daily tasks I can do. I can't pick up baskets of laundry anymore. I can't change the sheets on the bed, because I have to lift the corners of the mattress and I can't do that.
I couldn't walk around, couldn't rake the yard, couldn't lift anything heavier than a milk jug.
Participants spoke of how the limitations on certain activities and changes in lifestyle negatively impacted their relationships.
It's changed the way that I can interact with my son. I'll never be able to run around on the playground with him, or do anything like that, because it will damage my prolapse further. So it has completely changed that relationship.
I stopped doing certain things. Like even to go for a lunch with my friends at work. I knew I could only walk around the building across from us. Like I couldn't go for my normal walks. Yeah, it changed…. It changed everything.
[My husband] was very frustrated and he would get angry with me, and then I'd get defensive or quiet, and then we seemed to [drift] apart. For sure we didn't talk as much. He would go out [alone] more…then, of course, you get angry at yourself, and then you're frustrated, and then you're mad at them and it's not their fault. It's a whole series of emotions.
People with POP also experienced impacts on sexual relationships. They reported that they avoided sexual intercourse due to the fear of making their prolapse worse or feeling unattractive.
In terms of the relationship with my husband, in the beginning, until I had seen the specialist, I was afraid to have sex, because I didn't know what that would do to the prolapse.
I was afraid to have–my husband was afraid to touch me, like for sex. We didn't know what was going…. So we didn't….
Well, it certainly put a damper on our sex life because…you don't feel sexy or attractive when you've got things falling out of your vagina. You just don't. And you just feel like, it just makes you feel like you're worn out. I'm worn out, I'm a tired old worn out person.
In addition to the mental health impacts that affect sexual relationships, some people with POP reported that sexual intercourse was uncomfortable, sometimes painful.
Sex was not something that–it was very painful and I never knew why it was painful.
Sex was nonexistent. It just didn't happen. You feel so bad about yourself and so much pain and misery.
I also found that intercourse was very uncomfortable and still is. And to the point where I—my husband and I have not had intercourse in probably 2 years.
Work impact
Most people we interviewed were retired. The few who were employed reported that POP and SUI impacted their work life in many ways. They reflected on the difficulties of working in an office environment due to the constant sitting and the repercussions they faced due to the limitations imposed by their symptoms.
I'm back at work now and we have a system where we have laptops we can take home and I can't carry a laptop back and forth every day, because that impacts on my prolapse. The way I sit in the office all day isn't great. At least when I was home on [maternity] leave, I could lie down…. But now I'm in the office 8 hours a day, sitting, with gravity pulling everything down the entire time.
I had to sit more. I had to lay on the floor at work to do my [pelvic floor] exercises, to keep everything at bay.
I did miss some work due to the back pain with the prolapse initially. Now I'm on an attendance awareness program, which is totally humiliating because I've never had any issues with attendance before.
Emotional impact
Participants reported that complications from their condition had a huge emotional toll and impacted their mental health. The symptoms of POP and SUI limit their quality of life, primarily due to the discomfort and activity restrictions. Many people reported experiencing anxiety, fear, and depression due to the emotional burden of their prolapse and/or SUI.
I have to be constantly paying attention to my posture and making sure that my breathing is correct, so that I'm not making it worse. It's at the back of my mind all day, every day.
I've had issues with anxiety and depression for a long time and it made me deeply depressed because I felt like my body had betrayed me and I felt crippled. Literally. And the worst part is nobody can see it. I look perfectly healthy.
PELVIC ORGAN PROLAPSE AND STRESS URINARY INCONTINENCE TREATMENT OPTIONS
The people we interviewed reported seeking out different treatment options. The most commonly reported options were pelvic floor physiotherapy, pessaries, and surgery. Other, less common, options included laser treatments, Botox, and pessary-like alternatives. Participants reported varying results from these therapies, but pessaries provided the most relief.
I've never had a problem with discomfort while it [the pessary] was in there. I mean it just–it just solved the problem perfectly.
A majority of people who received pelvic floor physiotherapy had positive experiences and felt that it helped their symptoms. Most people reported positive results using pelvic floor physiotherapy along with a pessary.
It's like things got stretched out and then they got unstretched and the physiotherapy…that helped me tighten things up.
I've been doing [pelvic floor physiotherapy] for a few months now and I have been seeing improvements. [My therapist] downgraded my level of stress incontinence one degree.
Many people who sought physiotherapy for POP and/or SUI reported that cost was a barrier.
They're pretty expensive. I think they might have been like $95.00 a session. They were pretty pricey. Between me and my husband's insurance, it paid for half maybe per visit…. I might have gone eight times. So it wasn't a huge financial burden, but yeah, for somebody who didn't have insurance it could add up in a hurry.
It was quite expensive. I think my insurance didn't pay for all of it.
Surgery
Many participants expressed hesitancy and fear with the surgical treatment option. Some reported knowing of people (family or others) who had negative repercussions from a surgery that failed.
I didn't have to have surgery, which was a huge relief, having seen what my mother went through.
I've heard horror stories from people who have had surgery.
I don't want to have surgery yet, because I'm not sure that I'm done having a family. In any case, the surgeries have a huge failure rate. My mom has had two surgeries already and they've both failed. So, frankly, I don't think surgery is an option.
Others reported that they feared surgery because of its invasive nature. Surgery was not the preferred option and, if needed, they would like to delay it as long as possible.
In general, I'd like to avoid surgery. I think most people would probably like to avoid having a surgery. And certainly I don't like the idea of having a mesh inserted permanently. It just doesn't
make sense to me to have some foreign object in my body that may or may not cause me other types of pain.
I just felt that [removing an organ] was very invasive for the condition I had. While my condition was uncomfortable, it was not life-threatening. So the surgical option seemed extreme to me.
Some of the participants who had surgery reported that the surgery failed or even brought on another prolapse.
I had a super-cervical hysterectomy with concurrent tethering of vaginal to ligaments at back using mesh….I had erosion after 3 years of messy bloody discharge…. [The specialist] tried unsuccessfully to remove mesh.
I had the surgery but it just didn't seem to really help. It seemed [to work in] the beginning, but after a month or so, it [came] back. And I had a lot of pain after the surgery.
Well, the first thing I did was, I had surgery. And the surgery, the procedure, didn't hold on the operating table. They had to redo it and within two weeks it had let go again and I had the prolapse back.
Shortly after the surgery, the bulge returned. So [the surgeon] corrected one problem, but then I ended up with more of a rectal type of prolapse, which he says wasn't there when he did the surgery.
Pessary
A majority of people we interviewed reported that pessaries alleviated most if not all of their POP and SUI symptoms, resulting in an improvement of their overall quality of life. Patients reported that they were able to get back to functioning normally, performing basic day to day tasks, and even engaging in strenuous activities. Pessary use among people we interviewed ranged from 2 weeks to 15 years. Ten have been using a pessary for more than 5 years. Pessaries may need to be changed due to wear and tear or changes in the size or shape of the prolapse. Only two patients reported discontinuing the use of a pessary; one because their condition improved and the other because the pessary felt uncomfortable.
Pessaries need to be removed and cleaned on a regular basis to prevent infection. This can be done through self management at home, or by visiting a pessary clinic or health professional. Most patients reported that it was their decision to self manage or rely on a professional. A majority of people reported also using an estrogen cream or pill along with the pessary.
People we spoke to who self managed their pessary removal and cleaning reported that the process is fairly simple. Additionally, they went for an annual follow-up exam to ensure there was no erosion or infection.
It's easy to do. If you've ever used a diaphragm, it's no more difficult than that—or probably less. It's easy to do if you're mobile enough to be able to reach.
You fold it like a taco and in it goes. It's super easy.
Some of the participants said that there is a learning curve in the removal and placement of the pessary.
I didn't understand that if it slipped, I could push it back up. So I'd be walking around with a pessary that was slipping, that was like starting to fall out, and I did nothing to fix it. I would just be walking around with this really uncomfortable feeling of the pessary slipping and not knowing, I didn't know how to fix it…. I didn't really have a complete understanding of how to deal with a pessary, how to manage a pessary.
People we spoke to who had their pessary managed by a clinician reported going for check ups every 2 to 3 months. The pessary would be cleaned and the clinician would check to ensure that there was no erosion or infection. Patients may choose this option if they are unable to reach the pessary or feel more comfortable having the procedure managed by a health care provider. A minority of patients expressed frustration with the short follow-up periods.
I can't reach inside. I can't do anything myself. It has to be done by someone at the hospital.
I could do it at home, but I always said no, I do not want to do that. I want to come here and I want you guys to check me. I just feel better about it. I'm not comfortable doing it myself.
It's always been a nuisance having to have an appointment every 3 months and always having to make sure that I've got the pills to put in. It's just another chore that you have to do.
Some participants reported experiencing side effects from their pessary, including bleeding, erosion, infections, pain, extrusion. These were often addressed by removing the pessary for a short period to allow for healing. A few participants also reported experiencing the side effects due to being fitted with an incorrect pessary or they needed the size or type of pessary to be changed over time.
The second Ontario doctor I saw tried me with a cube pessary. It was excruciating. It was horrible! Fortunately, I didn't leave the hospital with it, so I turned it back in and said this isn't going to work.
Just a bad odour with some discharge that I had, but it was only for a short period of time. Once I phoned them and told them about the discharge that had a foul odour, they took me in and removed the pessary, cleaned it and kind of cleaned me with a long Q-tip, cleaned me inside. And I was without it for about six weeks.
One of them [pessary] had to be changed…The way it was sitting in that was causing pain in my back.
Social benefits
People who used a pessary consistently reported the pessary's ability to provide support for their prolapsed organ(s) and control their SUI, enabling them to go back to their normal day-to-day lives without interruptions.
Well, I'm able to go on with my life the way I was before, I can go for walks and there's nothing rubbing my leg. Everything is comfortable in there; I can pee normally. I just got back to the way that I used to be.
I'm able to walk around without feeling like I have to hold on to my crotch all the time to support myself.
I can go back to doing those activities…. I [can] get my pool membership again. So my physical well being…keeping mobile, trying to keep fit, it's…a huge benefit for because I had pretty much stopped most of those things.
It helped tremendously…it was much different, especially when bending and lifting planters and things at home and just heavy garbage, things like that,…because I felt back to normal.
The pessary has made an enormous difference in my life. It's incredible. I couldn't walk half a block without having to sit down and pull my muscles in just to relieve the pressure…. The quality of life is just tremendously different.
Emotional benefits
Pessaries reduced the emotional toll associated with POP and SUI. Most reported that using a pessary gave them a sense of normalcy and confidence, it allowed them to stop constantly thinking about their prolapse.
I don't spend every single second worrying that things are going to fall out, because I know that there's something stopping them from falling out. So just mentally, the impact is just huge. It's such a relief to have that worry gone
I feel great. I don't feel like a victim. I don't feel like something's wrong with me. I just feel fine. I feel normal.
The confidence it gave me in the sense that…I don't have to feel like an old lady…I'm still very vital. This was probably the single thing that made me feel like I'm young again.
With the pessary, …I can bend over, I can dig in my garden. Without it, I can't. It literally is the difference between whether or not I can function and not be completely depressed.
Medical benefits
People we interviewed who had used a pessary consistently reported that it was able to support their prolapsed organ(s) and control their SUI without the need for surgery. In a few cases, the grade of the prolapse reduced, leading to the need for a smaller sized pessary. In one case, the pessary allowed the pelvic floor muscles to tighten so that it was no longer needed at all. Additionally, people we spoke with reported that their pessaries reduced the incontinence they were experiencing.
The other thing is just, I don't feel a bulge.
If I don't wear the pessary, that vaginal wall comes right down and it just feels awful.
I didn't feel that pressure anymore…. If I take it out and try to walk around without it, immediately I can feel the difference.
Well, it's positive, because I don't have to be sitting on a lump.
Access barriers
Wait times to see a specialist were consistently reported as a barrier to diagnosis and treatment, with reported wait times of 2 months to 2 years.
A few people reported that their physician (primary care or specialist) would not prescribe them a pessary. Some sought another physician or used a pessary-like alternative.
My urogynecologist actually would not give me a pessary, because she said there was no point for me. My pelvic floor physiotherapist strongly disagreed. She recommended that I purchase–it's not a real pessary. It's a stress incontinence device, but that can be used as a pessary. So I've been using this makeshift pessary instead, because my urogynecologist refused [to issue a prescription].
To the first doctor, I said, ‘I talked to a friend and she has a pessary.’ He goes, ‘Oh those are for old ladies. You don't want one of those.'
I asked about a pessary. She [my gynecologist] said that…it wouldn't be a good fit for me. I wasn't happy with that decision [so] I went back to my family doctor and I said, I think I need to see a urogynecologist, not just a gynecologist. And at that time, she referred me to a urogynecologist–who I'm seeing now–who is much more proactive not to do surgery so fast, to try other methods.
The variety and number of pessaries that specialists had on hand for fittings varied. Some specialists have a limited selection, while others have an abundance of different sizes and types.
My own experience is that regular OB/GYN's…have very limited selection of pessaries. They always want to give a ring pessary when there's all different kinds of pessaries out there, and some are better for different types of prolapse.
Geographic barriers
Some participants who live outside major cities noted that there are geographic barriers to seeing a specialist for the initial diagnosis, as well as for follow-up appointments if self-management isn't an option.
It's hard, it's very difficult to find a urogynecologist, particularly in this area. It does require that I either go to Hamilton or Toronto for treatment and it—the place that I had been referred to for a better pessary fitting—is actually in Toronto…or in Barrie.
Sault Ste. Marie, there's no urogynecologist here. I have to go to Toronto or London.
Financial barriers
A few people we interviewed reported cost as a barrier when paying for a pessary out of pocket, with patients reporting costs of between $50 and $200. Most patients reported having private insurance that covered a majority of the cost, but they acknowledged that cost could be a barrier in the absence of insurance.
I'm on a very tight budget as it is. I live in a one-bedroom apartment…. My cost of living is going up all the time. I'm afraid my income isn't meeting all these increases and everything. And so something like that [a pessary] would have quite an impact.
Being on maternity leave and knowing that there's going to be an out-of-pocket cost definitely delayed my [decision] to book the appointment. Things might have been a little bit easier, especially as a new mom you're dealing with a lot, it didn't help that I had to deal with a prolapse.
It wouldn't greatly impact my financial situation, but it is still something that I wouldn't just go out and just buy quickly. I definitely would need to budget for it and take the time to make an informed decision to make sure that that was what I wanted to invest my money in.
At this point, if I had to pay for it, it would be an issue.
Discussion
The extensive outreach for this health technology assessment yielded engagement with 29 patients diagnosed with POP and/or SUI. People we spoke with discussed the pain and pressure of a prolapse and having a bulge protruding from their vagina as their main symptoms. They shared the burden of their condition and its disruption to their daily life, mental health, employment, and relationships. Each had experienced one or more of the main treatment options available: pessaries, surgery, and pelvic floor physiotherapy.
Patients who accessed pessaries reported a tremendous improvement in their quality of life. The pessary allowed them to continue menial activities such as sitting, standing, and walking without discomfort. It also allowed them to resume more strenuous activities like exercise and housework requiring bending and lifting. Additionally, many commented on the benefits of being able to avoid or delay surgery because of the pessary. Reported side effects from pessary use included bleeding, infection, pain, and extrusion. These were often resolved with temporary removal of the pessary or change in pessary type.
A few participants reported that the cost of a pessary would create financial stress for them. Most patients used their private health insurance to offset the cost of the pessary or reflected that they were financially stable enough that the cost did not have a negative impact on them. The limited impact of cost on the people we interviewed could be an indication that our outreach to pelvic floor physiotherapists across Ontario resulted in the recruitment of more financially stable patients. Applicability of our results is also limited by the fact that most participants resided in large urban areas. Those people we spoke to who resided in other areas reported geographic barriers to accessing a specialist for their prolapse and attending follow-up appointments to manage their pessary. Another significant barrier to access experienced by many of the participants was the long wait time to see a specialist. Reported wait times were as high as 2 years. There were also reports of misdiagnosis, conflicting information, and refusal to prescribe a pessary.
Conclusion
People with pelvic organ prolapse and/or stress urinary incontinence reported pessary use as being an effective treatment option to manage their symptoms. Several people spoke about the positive impact pessary use had on their social, emotional, and physical well being.
Most participants reported that their pessary was covered by their private insurance, but some participants said that cost was or could be a barrier. Common access barriers included specialist wait times, and conflicting information from physicians. Additional barriers included refusal to prescribe, misdiagnosis, and geographic barriers.
Thys et al97 concluded that patient preferences are a personal choice, with patients accepting the risks of their chosen treatment option. The authors' results also showed a higher preference for surgery. The direct patient engagement results were in line with treatment options being a personal choice, but indicated that people preferred more conservative options before considering surgery.
Conclusions of the Health Technology Assessment
For people with stress urinary incontinence, compared with no treatment, pessaries may improve some symptoms, but the evidence is very uncertain. They may have little to no effect on quality of life and on complications, but the evidence is very uncertain. Compared with PFMT alone, pessaries may result in less improvement in short-term symptoms and little to no difference in longer-term improvement of symptoms or in patient satisfaction. Compared with PFMT alone, pessaries plus PFMT may result in little to no difference in improvement of symptoms or patient satisfaction.
For people with pelvic organ prolapse, compared with PFMT combined with feedback/electrical stimulation/lifestyle advice, pessaries may improve some longer-term symptoms and sexual function, but may not improve quality of life. Compared with surgery, pessaries have little to no effect on improvement of symptoms, quality of life, or patient satisfaction, but the evidence is very uncertain.
The economic literature review identified five economic evaluations comparing pessaries with various other interventions to treat people with POP or SUI. Results were mixed. In two studies found for a population with POP, both pessaries and surgery were found to be cost-effective. Of the three studies found for a population with SUI, none found pessaries were most likely to be cost-effective.
Our economic analysis found a high degree of certainty that pessaries are cost-effective for treating POP and a moderate degree of certainty that pessaries are cost-effective for SUI. Our budget impact analysis indicates that publicly funding pessaries for POP may result in a 5-year total additional cost of $2.0 million. Funding pessaries for SUI may result in a 5-year additional cost of $1.3 million. If pessaries are funded in both populations, the total 5-year budget impact would be about $3.3 million.
People with pelvic organ prolapse reported that pessary use is an effective treatment option to manage their symptoms. Several people spoke about the positive impact pessary use had on their social, emotional, and physical well being. Cost could be a barrier, especially for people whose pessary will not be covered through private insurance. Common access barriers include specialist wait times and conflicting information from physicians. Additional barriers included refusal to prescribe, misdiagnosis, and geographic barriers.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologist was Kristen McMartin, the medical librarian was Corinne Holubowich, the health economist was Sean Tiggelaar, and the patient and public partnership analyst was Jigna Mistry.
The medical editor was Tim Maguire. Others involved in the development and production of this report were Jamie Turnbull, Claude Soulodre, Susan Harrison, Saleemeh Abdolzahraei, Elisabeth Smitko, Sarah McDowell, Vivian Ng, David Wells, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We would like to thank the following individuals for lending their expertise to the development of this report:
Risa Bordman, University of Toronto
Aisling Clancy, University of Ottawa
Sinéad Dufour, ptHealth, Hamilton
Nelly Faghani, Pelvic Health Solutions, Toronto
Marie-Andrée Harvey, Queen's University, Kingston
Muhammad Mamdani, Unity Health Toronto, Toronto
Jennifer Skelly, McMaster University, Hamilton
Kirsten Smith, University of Toronto
Cathy Vakil, Queen's University, Kingston
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Abbreviations
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CI
Confidence interval
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- ICIQ
International Consultation on Incontinence Questionnaire
- MD
Mean difference
- NICE
National Institute for Health and Care Excellence
- PFMT
Pelvic floor muscle training
- POP
Pelvic organ prolapse
- POPDI
POP Distress Inventory
- POPIQ
POP Impact Questionnaire
- QALY
Quality-adjusted life-year
- QUID
Questionnaire for Urinary Incontinence Diagnosis
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SUI
Stress urinary incontinence
- UDI
Urogenital Distress Inventory
- UIQ
Urinary Impact Questionnaire
- WTP
Willingness to pay
Glossary
- Adverse event
An adverse event is any unexpected problem that happens during or as a result of treatment, regardless of the cause or severity.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cost–benefit analysis
A cost–benefit analysis is a type of economic evaluation that expresses the effects of a health care intervention in terms of a monetary value so that these effects can be compared with costs. Results can be reported either as a ratio of costs to benefits or as a simple sum that represents the net benefit (or net loss) of one intervention over another. The monetary valuation of the different intervention effects is based on either prices that are revealed by markets or an individual or societal willingness-to-pay value.
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness acceptability curve
In economic evaluations, a cost-effectiveness acceptability curve is a graphical representation of the results of a probabilistic sensitivity analysis. It illustrates the probability of health care interventions being cost-effective over a range of willingness-to-pay values. Willingness-to-pay values are plotted on the horizontal axis of the graph, and the probability of the intervention of interest and its comparator(s) being cost-effective at corresponding willingness-to-pay values is plotted on the vertical axis.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost–utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Decision tree
A decision tree is a type of economic model used to assess the costs and benefits of two or more alternative health care interventions. Each intervention may be associated with different outcomes, which are represented by distinct branches in the tree. Each outcome may have a different probability of occurring and may lead to different costs and benefits.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Health Quality Ontario use an annual discount rate of 1.5% for both future costs and future benefits.
- Dominant
A health care intervention is considered dominant when it is more effective and less costly than its comparator(s).
- EuroQol–Five Dimensions (EQ-5D)
The EQ-5D is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The EQ-5D questionnaire consists of five questions relating to different domains of quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. For each domain, there are three response options: no problems, some problems, or severe problems. A newer instrument, the EQ-5D-5L, includes five response options for each domain. A scoring table is used to convert EQ-5D scores to utility values.
- Health-related quality of life
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Health state
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Microsimulation model
In economic evaluations, a microsimulation model (e.g., an individual-level or patient-level model) is used to simulate the health outcomes for a heterogeneous group of patients (e.g., patients of different ages or with different sets of risk factors) after receiving a particular health care intervention. The health outcomes and health events of each patient are modelled, and the outcomes of several patients are combined to estimate the average costs and benefits accrued by a group of patients. In contrast, a cohort model follows a homogeneous cohort of patients (e.g., patients of the same age or with the same set of risk factors) through the model and estimates the proportion of the cohort who will experience specific health events.
- Ministry of Health perspective
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Monte Carlo simulation
Monte Carlo simulation is an economic modelling method that derives parameter values from distributions rather than fixed values. The model is run several times, and in each iteration, parameter values are drawn from specified distributions. This method is used in microsimulation models and probabilistic sensitivity analysis.
- One-way sensitivity analysis
A one-way sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying one model input (i.e., a parameter) at a time between its minimum and maximum values to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Pelvic floor muscle training
Exercises designed to strengthen the muscles of the pelvic floor (the muscles under the uterus, bladder, and large intestine). It is performed by tightening and relaxing the muscles that control urine flow and is commonly used in the non-surgical management of prolapse.
- Probabilistic sensitivity analysis (PSA)
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Short-Form–Six Dimensions (SF-6D)
The SF-6D is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The classification system consists of six attributes (physical functioning, role limitations, social functioning, pain, mental health, and vitality), each associated with four to six levels, thus producing a total of 18,000 possible unique health states. A scoring table is used to convert SF-6D scores to health state values.
- Societal perspective
The perspective adopted in an economic evaluation determines the types of costs and health benefits to include. The societal perspective reflects the broader economy and is the aggregation of all perspectives (e.g., health care payer and patient perspectives). It considers the full effect of a health condition on society, including all costs (regardless of who pays) and all benefits (regardless of who benefits).
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: Jun 25, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database, CINAHL
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <May 2019>, EBM Reviews-Cochrane Database of Systematic Reviews <2005 to June 19, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 25>, Ovid MEDLINE(R) ALL <1946 to June 24, 2019>
Search Strategy:
————————————————————————–
-
1
exp Pelvic Organ Prolapse/ (31559)
-
2
Uterine Prolapse/ (7694)
-
3
((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) adj3 prolaps*).ti,ab,kf. (26747)
-
4
(vagin* adj2 bulg*).ti,ab,kf. (566)
-
5
Rectocele/ (3313)
-
6
Cystocele/ (3432)
-
7
(cystoc?ele* or rectoc?ele* or proctoc?ele* or sigmoidoc?ele* or urethroc?ele* or cystourethroc?ele*).ti,ab,kf. (6571)
-
8
Urinary Incontinence/ (44318)
-
9
Urinary Incontinence, Stress/ (21761)
-
10
(((urinar* or urge* or stress or bladder*) adj3 (incontinen* or continen*)) or SUI).ti,ab,kf. (89105)
-
11
Urinary Bladder, Overactive/ (7528)
-
12
((bladder or urinar*) adj2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)).ti,ab,kf. (43419)
-
13
or/1–12 (168949)
-
14
Pessaries/ (3975)
-
15
pessar*.ti,ab,kf. (4495)
-
16
((pubovagin* or vagin* or intravagin* or bladder*) adj3 (ring* or plug* or prosthes*)).ti,ab,kf. (4598)
-
17
((continen* or incontinen*) adj3 (device* or ring* or dish*)).ti,ab,kf. (668)
-
18
((urethra* or intraurethra*) adj3 (insert or inserts or plug* or prosthes*)).ti,ab,kf. (279)
-
19
((vagin* or bladder* or urethra*) adj2 support* adj2 mechan*).ti,ab,kf. (14)
-
20
(shaatz* or gellhorn* or risser* or tandem cube* or (hodge adj2 (knob* or support*)) or (ring adj2 support*) or gehrung* or donut or inflatoball* or incostress*).ti,ab,kf. (3006)
-
21
or/14–20 (14000)
-
22
13 and 21 (2852)
-
23
exp Animals/ not Humans/ (17114820)
-
24
22 not 23 (1985)
-
25
Case Reports/ or Congresses.pt. (2027106)
-
26
24 not 25 (1831)
-
27
limit 26 to english language [Limit not valid in CDSR; records were retained] (1526)
-
28
limit 27 to yr=“2000-Current” (1203)
-
29
28 use medall,cctr,coch,clhta,cleed (636)
-
30
exp pelvic organ prolapse/ (31559)
-
31
((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) adj3 prolaps*).tw,kw. (27004)
-
32
(vagin* adj2 bulg*).tw,kw. (575)
-
33
rectocele/ (3313)
-
34
exp cystocele/ (3435)
-
35
(cystoc?ele* or rectoc?ele* or proctoc?ele* or sigmoidoc?ele* or urethroc?ele* or cystourethroc?ele*).tw,kw. (6881)
-
36
urine incontinence/ (43193)
-
37
stress incontinence/ (32909)
-
38
(((urinar* or urge* or stress or bladder*) adj3 (incontinen* or continen*)) or SUI).tw,kw. (90945)
-
39
overactive bladder/ (19899)
-
40
((bladder or urinar*) adj2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)).tw,kw. (44661)
-
41
or/30–40 (172999)
-
42
exp vagina pessary/ (2396)
-
43
pessar*.tw,kw,dv. (4585)
-
44
((pubovagin* or vagin* or intravagin* or bladder*) adj3 (ring* or plug* or prosthes*)).tw,kw,dv. (4710)
-
45
((continen* or incontinen*) adj3 (device* or ring* or dish*)).tw,kw,dv. (711)
-
46
((urethra* or intraurethra*) adj3 (insert or inserts or plug* or prosthes*)).tw,kw,dv. (299)
-
47
((vagin* or bladder* or urethra*) adj2 support* adj2 mechan*).tw,kw,dv. (14)
-
48
(shaatz* or gellhorn* or risser* or tandem cube* or (hodge adj2 (knob* or support*)) or (ring adj2 support*) or gehrung* or donut or inflatoball* or incostress*).tw,kw,dv. (3038)
-
49
or/42–48 (13631)
-
50
41 and 49 (2760)
-
51
(exp animal/ or nonhuman/) not exp human/ (10325814)
-
52
50 not 51 (2730)
-
53
Case Report/ or conference abstract.pt. (7599423)
-
54
52 not 53 (1871)
-
55
limit 54 to english language [Limit not valid in CDSR; records were retained] (1559)
-
56
limit 55 to yr=“2000-Current” (1314)
-
57
56 use emez (703)
-
58
29 or 57 (1339)
-
59
58 use medall (526)
-
60
58 use emez (703)
-
61
58 use coch (6)
-
62
58 use cctr (101)
-
63
58 use clhta (2)
-
64
58 use cleed (1)
-
65
remove duplicates from 58 (809)
| CINAHL | ||
|---|---|---|
| # | Query | Results |
| S1 | (MH “Pelvic Organ Prolapse+”) | 2,648 |
| S2 | ((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) N3 prolaps*) | 3,039 |
| S3 | vagin* N2 bulg* | 48 |
| S4 | cystoc#ele* or rectoc#ele* or proctoc#ele* or sigmoidoc#ele* or urethroc#ele* or cystourethroc#ele* | 386 |
| S5 | (MH “Urinary Incontinence”) | 7,977 |
| S6 | (MH “Stress Incontinence”) | 2,614 |
| S7 | (((urinar* or urge* or stress or bladder*) N3 (incontinen* or continen*)) or SUI) | 13,355 |
| S8 | (MH “Overactive Bladder”) | 1,638 |
| S9 | ((bladder or urinar*) N2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)) | 3,808 |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 | 18,083 |
| S11 | (MH “Pessaries”) | 399 |
| S12 | pessar* | 552 |
| S13 | ((pubovagin* or vagin* or intravagin* or bladder*) N3 (ring* or plug* or prosthes*)) | 386 |
| S14 | ((continen* or incontinen*) N3 (device* or ring* or dish*)) | 83 |
| S15 | ((urethra* or intraurethra*) N3 (insert or inserts or plug* or prosthes*)) | 12 |
| S16 | ((vagin* or bladder* or urethra*) N2 support* N2 mechan*) | 0 |
| S17 | (shaatz* or gellhorn* or risser* or tandem cube* or (hodge N2 (knob* or support*)) or (ring N2 support*) or gehrung* or donut or inflatoball* or incostress*) | 482 |
| S18 | S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 1,474 |
| S19 | S10 AND S18 | 343 |
| S20 | PT Case Study or Proceedings | 353,274 |
| S21 | S19 NOT S20 | 293 |
| S22 | S19 NOT S20 Limiters – Published Date: 20000101–20191231 | 271 |
| S23 | S21 NOT S25 Limiters – Published Date: 20000101–20191231; English Language | 266 |
Economic Evidence Search
Economic Literature Search
Search date: June 26, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database, CINAHL
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <May 2019>, EBM Reviews-Cochrane Database of Systematic Reviews <2005 to June 19, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 25>, Ovid MEDLINE(R) ALL <1946 to June 25, 2019>
Search Strategy:
————————————————————————–
-
1
exp Pelvic Organ Prolapse/ (31559)
-
2
Uterine Prolapse/ (7694)
-
3
((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) adj3 prolaps*).ti,ab,kf. (26752)
-
4
(vagin* adj2 bulg*).ti,ab,kf. (566)
-
5
Rectocele/ (3313)
-
6
Cystocele/ (3432)
-
7
(cystoc?ele* or rectoc?ele* or proctoc?ele* or sigmoidoc?ele* or urethroc?ele* or cystourethroc?ele*).ti,ab,kf. (6571)
-
8
Urinary Incontinence/ (44324)
-
9
Urinary Incontinence, Stress/ (21763)
-
10
(((urinar* or urge* or stress or bladder*) adj3 (incontinen* or continen*)) or SUI).ti,ab,kf. (89122)
-
11
Urinary Bladder, Overactive/ (7529)
-
12
((bladder or urinar*) adj2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)).ti,ab,kf. (43425)
-
13
or/1–12 (168974)
-
14
Pessaries/ (3975)
-
15
pessar*.ti,ab,kf. (4496)
-
16
((pubovagin* or vagin* or intravagin* or bladder*) adj3 (ring* or plug* or prosthes*)).ti,ab,kf. (4602)
-
17
((continen* or incontinen*) adj3 (device* or ring* or dish*)).ti,ab,kf. (668)
-
18
((urethra* or intraurethra*) adj3 (insert or inserts or plug* or prosthes*)).ti,ab,kf. (279)
-
19
((vagin* or bladder* or urethra*) adj2 support* adj2 mechan*).ti,ab,kf. (14)
-
20
(shaatz* or gellhorn* or risser* or tandem cube* or (hodge adj2 (knob* or support*)) or (ring adj2 support*) or gehrung* or donut or inflatoball* or incostress*).ti,ab,kf. (3007)
-
21
or/14–20 (14006)
-
22
13 and 21 (2852)
-
23
economics/ (252505)
-
24
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (824729)
-
25
economics.fs. (420778)
-
26
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (879160)
-
27
exp “costs and cost analysis”/ (576480)
-
28
(cost or costs or costing or costly).ti. (261995)
-
29
cost effective*.ti,ab,kf. (322608)
-
30
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab,kf. (211721)
-
31
models, economic/ (12640)
-
32
markov chains/ or monte carlo method/ (80029)
-
33
(decision adj1 (tree* or analy* or model*)).ti,ab,kf. (41865)
-
34
(markov or markow or monte carlo).ti,ab,kf. (127871)
-
35
quality-adjusted life years/ (39345)
-
36
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (72482)
-
37
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (118214)
-
38
or/23–37 (2527204)
-
39
22 and 38 (186)
-
40
39 use medall,cctr,coch,clhta (60)
-
41
22 use cleed (1)
-
42
or/40–41 (61)
-
43
exp Animals/ not Humans/ (17115638)
-
44
42 not 43 (61)
-
45
Case Reports/ (2027690)
-
46
44 not 45 (60)
-
47
limit 46 to english language [Limit not valid in CDSR; records were retained] (52)
-
48
exp pelvic organ prolapse/ (31559)
-
49
((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) adj3 prolaps*).tw,kw. (27008)
-
50
(vagin* adj2 bulg*).tw,kw. (575)
-
51
rectocele/ (3313)
-
52
exp cystocele/ (3435)
-
53
(cystoc?ele* or rectoc?ele* or proctoc?ele* or sigmoidoc?ele* or urethroc?ele* or cystourethroc?ele*).tw,kw. (6881)
-
54
urine incontinence/ (43193)
-
55
stress incontinence/ (32911)
-
56
(((urinar* or urge* or stress or bladder*) adj3 (incontinen* or continen*)) or SUI).tw,kw. (90960)
-
57
overactive bladder/ (19900)
-
58
((bladder or urinar*) adj2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)).tw,kw. (44668)
-
59
or/48–58 (173021)
-
60
exp vagina pessary/ (2396)
-
61
pessar*.tw,kw,dv. (4586)
-
62
((pubovagin* or vagin* or intravagin* or bladder*) adj3 (ring* or plug* or prosthes*)).tw,kw,dv. (4713)
-
63
((continen* or incontinen*) adj3 (device* or ring* or dish*)).tw,kw,dv. (711)
-
64
((urethra* or intraurethra*) adj3 (insert or inserts or plug* or prosthes*)).tw,kw,dv. (299)
-
65
((vagin* or bladder* or urethra*) adj2 support* adj2 mechan*).tw,kw,dv. (14)
-
66
(shaatz* or gellhorn* or risser* or tandem cube* or (hodge adj2 (knob* or support*)) or (ring adj2 support*) or gehrung* or donut or inflatoball* or incostress*).tw,kw,dv. (3039)
-
67
or/60–66 (13636)
-
68
59 and 67 (2760)
-
69
Economics/ (252505)
-
70
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (128174)
-
71
Economic Aspect/ or exp Economic Evaluation/ (451666)
-
72
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw. (904804)
-
73
exp “Cost”/ (576480)
-
74
(cost or costs or costing or costly).ti. (261995)
-
75
cost effective*.tw,kw. (334890)
-
76
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab,kw. (222668)
-
77
Monte Carlo Method/ (63746)
-
78
(decision adj1 (tree* or analy* or model*)).tw,kw. (45667)
-
79
(markov or markow or monte carlo).tw,kw. (132928)
-
80
Quality-Adjusted Life Years/ (39345)
-
81
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (76314)
-
82
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw. (138857)
-
83
or/69–82 (2165838)
-
84
68 and 83 (182)
-
85
84 use emez (94)
-
86
(exp animal/ or nonhuman/) not exp human/ (10326632)
-
87
85 not 86 (94)
-
88
Case Report/ (4296746)
-
89
87 not 88 (93)
-
90
limit 89 to english language [Limit not valid in CDSR; records were retained] (86)
-
91
47 or 90 (138)
-
92
91 use medall (36)
-
93
91 use emez (86)
-
94
91 use coch (1)
-
95
91 use cctr (13)
-
96
91 use clhta (1)
-
97
91 use cleed (1)
-
98
remove duplicates from 91 (93)
| CINAHL | ||
|---|---|---|
| # | Query | Results |
| S1 | (MH “Pelvic Organ Prolapse+”) | 2,648 |
| S2 | ((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) N3 prolaps*) | 3,039 |
| S3 | vagin* N2 bulg* | 48 |
| S4 | cystoc#ele* or rectoc#ele* or proctoc#ele* or sigmoidoc#ele* or urethroc#ele* or cystourethroc#ele* | 386 |
| S5 | (MH “Urinary Incontinence”) | 7,979 |
| S6 | (MH “Stress Incontinence”) | 2,614 |
| S7 | (((urinar* or urge* or stress or bladder*) N3 (incontinen* or continen*)) or SUI) | 13,356 |
| S8 | (MH “Overactive Bladder”) | 1,639 |
| S9 | ((bladder or urinar*) N2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)) | 3,810 |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 | 18,086 |
| S11 | (MH “Pessaries”) | 399 |
| S12 | pessar* | 552 |
| S13 | ((pubovagin* or vagin* or intravagin* or bladder*) N3 (ring* or plug* or prosthes*)) | 386 |
| S14 | ((continen* or incontinen*) N3 (device* or ring* or dish*)) | 83 |
| S15 | ((urethra* or intraurethra*) N3 (insert or inserts or plug* or prosthes*)) | 12 |
| S16 | ((vagin* or bladder* or urethra*) N2 support* N2 mechan*) | 0 |
| S17 | (shaatz* or gellhorn* or risser* or tandem cube* or (hodge N2 (knob* or support*)) or (ring N2 support*) or gehrung* or donut or inflatoball* or incostress*) | 482 |
| S18 | S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 1,474 |
| S19 | S10 AND S18 | 343 |
| S20 | PT Case Study or Proceedings | 353,278 |
| S21 | S19 NOT S20 | 293 |
| S22 | (MH “Economics”) | 12,679 |
| S23 | (MH “Economic Aspects of Illness”) | 8,387 |
| S24 | (MH “Economic Value of Life”) | 582 |
| S25 | MH “Economics, Dental” | 121 |
| S26 | MH “Economics, Pharmaceutical” | 2,013 |
| S27 | MW “ec” | 163,978 |
| S28 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*) | 260,782 |
| S29 | (MH “Costs and Cost Analysis+”) | 103,625 |
| S30 | TI cost* | 48,009 |
| S31 | (cost effective*) | 35,956 |
| S32 | AB (cost* N2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)) | 27,427 |
| S33 | (decision N1 (tree* or analy* or model*)) | 7,080 |
| S34 | (markov or markow or monte carlo) | 5,012 |
| S35 | (MH “Quality-Adjusted Life Years”) | 3,942 |
| S36 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs) | 9,712 |
| S37 | ((adjusted N1 (quality or life)) or (willing* N2 pay) or sensitivity analys?s) | 15,491 |
| S38 | S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 | 352,290 |
| S39 | S21 AND S38 | 14 |
| S40 | S21 AND S38 Limiters – English Language | 14 |
Quantitative Evidence of Preferences and Values Search
Search date: July 4, 2019
Databases searched: Ovid MEDLINE
Search filter used: Quantitative preference evidence filter, modified from Selva et al.97
Database: Ovid MEDLINE(R) ALL <1946 to July 03, 2019>
Search Strategy:
————————————————————————–
-
1
exp Pelvic Organ Prolapse/ (11556)
-
2
Uterine Prolapse/ (5476)
-
3
((pelvi* or vagin* or uter* or urethra* or genital* or vault* or cervi* or bladder* or apical*) adj3 prolaps*).ti,ab,kf. (9735)
-
4
(vagin* adj2 bulg*).ti,ab,kf. (149)
-
5
Rectocele/ (672)
-
6
Cystocele/ (513)
-
7
(cystoc?ele* or rectoc?ele* or proctoc?ele* or sigmoidoc?ele* or urethroc?ele* or cystourethroc?ele*).ti,ab,kf. (2264)
-
8
Urinary Incontinence/ (21479)
-
9
Urinary Incontinence, Stress/ (10954)
-
10
(((urinar* or urge* or stress or bladder*) adj3 (incontinen* or continen*)) or SUI).ti,ab,kf. (33342)
-
11
Urinary Bladder, Overactive/ (4227)
-
12
((bladder or urinar*) adj2 (hyper* or overactiv* or over activ* or leak* or instab* or stab* or unstab* or urge* or frequen*)).ti,ab,kf. (15045)
-
13
or/1–12 (66535)
-
14
Pessaries/ (1417)
-
15
pessar*.ti,ab,kf. (1662)
-
16
((pubovagin* or vagin* or intravagin* or bladder*) adj3 (ring* or plug* or prosthes*)).ti,ab,kf. (1702)
-
17
((continen* or incontinen*) adj3 (device* or ring* or dish*)).ti,ab,kf. (230)
-
18
((urethra* or intraurethra*) adj3 (insert or inserts or plug* or prosthes*)).ti,ab,kf. (131)
-
19
((vagin* or bladder* or urethra*) adj2 support* adj2 mechan*).ti,ab,kf. (7)
-
20
(shaatz* or gellhorn* or risser* or tandem cube* or (hodge adj2 (knob* or support*)) or (ring adj2 support*) or gehrung* or donut or inflatoball* or incostress*).ti,ab,kf. (1256)
-
21
or/14–20 (5457)
-
22
13 and 21 (978)
-
23
exp Animals/ not Humans/ (4595265)
-
24
22 not 23 (967)
-
25
Case Reports/ or Congresses.pt. (2029411)
-
26
24 not 25 (813)
-
27
Attitude to Health/ (81785)
-
28
Health Knowledge, Attitudes, Practice/ (103804)
-
29
Patient Participation/ (24068)
-
30
Patient Preference/ (7307)
-
31
Attitude of Health Personnel/ (116099)
-
32
*Professional-Patient Relations/ (11174)
-
33
*Physician-Patient Relations/ (34261)
-
34
Choice Behavior/ (31026)
-
35
(choice or choices or value* or valuation*).ti. (190676)
-
36
(preference* or expectation* or attitude* or acceptab* or knowledge or point of view).ti,ab. (1120922)
-
37
((patient*1 or user*1 or men or women or personal or provider* or practitioner* or professional*1 or (health* adj2 worker*) or clinician* or physician* or doctor* or gynecologist* or gynaecologist* or nurse practitioner* or physiotherapist* or physio therapist* or physical therapist*) adj2 (participation or perspective* or perception* or misperception* or perceiv* or view* or understand* or misunderstand* or value*1)).ti,ab. (113339)
-
38
health perception*.ti,ab. (2543)
-
39
*Decision Making/ (39416)
-
40
(patient*1 or user*1 or men or women or personal or provider* or practitioner* or professional*1 or (health* adj2 worker*) or clinician* or physician* or doctor* or gynecologist* or gynaecologist* or nurse practitioner* or physiotherapist* or physio therapist* or physical therapist*).ti. (2312102)
-
41
39 and 40 (7234)
-
42
(decision* and mak*).ti. (26645)
-
43
(decision mak* or decisions mak*).ti,ab. (127618)
-
44
42 or 43 (129087)
-
45
(patient*1 or user*1 or men or women or personal or provider* or practitioner* or professional*1 or (health* adj2 worker*) or clinician* or physician* or doctor* or gynecologist* or gynaecologist* or nurse practitioner* or physiotherapist* or physio therapist* or physical therapist*).ti,ab. (7640228)
-
46
44 and 45 (80301)
-
47
(discrete choice* or decision board* or decision analy* or decision-support or decision tool* or decision aid* or latent class* or decision* conflict* or decision* regret*).ti,ab. (30736)
-
48
Decision Support Techniques/ (18957)
-
49
(health and utilit*).ti. (1362)
-
50
(gamble* or prospect theory or health utilit* or utility value* or utility score* or utility estimate* or health state or feeling thermometer* or best-worst scaling or time trade-off or TTO or probability trade-off).ti,ab. (12212)
-
51
(preference based or preference score* or preference elicitation or multiattribute or multi attribute).ti,ab. (2561)
-
52
or/27–38,41,46–51 (1686518)
-
53
26 and 52 (125)
-
54
limit 53 to yr=“2000-Current” (105)
-
55
limit 54 to english language (99)
Grey Literature Search
Performed on:
June 27 – July 3, 2019. Updated November 28 – December 2, 2019
Websites searched:
HTA Database Canadian Repository, Alberta Health Evidence Reviews, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Health Technology Assessment Database, Epistemonikos, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Council of Australian Governments Health Technologies, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, Health Technology Wales, Oregon Health Authority Health Evidence Review Commission, Veterans Affairs Health Services Research and Development, Italian National Agency for Regional Health Services (AGENAS), Australian Safety and Efficacy Register of New Interventional Procedures-Surgical (ASERNIP-S), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Ministry of Health Malaysia Health Technology Assessment Section, Swedish Agency for Health Technology Assessment and Assessment of Social Services, ClinicalTrials.gov, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used:
pessary, pessaries, vaginal ring, vaginal prostheses, vaginal support, incontinence device, pelvic organ prolapse, stress urinary incontinence, incontinence
Results from clinical search: (included in PRISMA): 3
Results from economic search: (included in PRISMA): 2
Ongoing HTAs (PROSPERO/EUnetHTA): 9
Ongoing clinical trials (ClinicalTrials.gov): 14
Appendix 2: Characteristics of Systematic Reviews
Table A1:
Characteristics of Included Systematic Reviews
| Author, Year | Objective | Literature Search Dates | Inclusion/Exclusion Criteria | Comparator | Outcomes |
|---|---|---|---|---|---|
| Stress Urinary Incontinence | |||||
| Lipp et al,12 2014 | To assess the effects of mechanical devices in the management of adult female urinary incontinence, particularly SUI. | January 1966 to August 2014 |
RCTs Inclusion criteria: mechanical devices to control urinary leakage, implanted by insertion into the vagina, within the urethra, or applied to the external surface of the urethra. The intervention focused on for the purposes of our systematic review was intravaginal devices (pessaries). Exclusion criteria: vaginal cones, electrical devices that aim to improve the function of the pelvic floor musculature, pads, catheters, and other collecting devices. |
Mechanical devices to control urinary leakage versus no treatment One mechanical device versus another mechanical device Mechanical device versus other treatments, for example, conservative therapies such as PFMT |
Patient symptoms Tolerability of device and side-effects Quality of life |
| Pelvic Organ Prolapse | |||||
| National Institute for Health and Care Excellence (NICE),39 2019 | To broadly assess the effects of conservative management options for POP. | January 1966 to June 2017 |
RCTs Inclusion criteria: women ≥ 18 years of age with POP who may be eligible for treatment with a pessary. |
Pessary versus no pessary Pessary versus PFMT |
Improvement in symptoms (self reported or through questionnaires) Patient satisfaction Quality of life Sexual function Adverse events |
| Bugge et al9 2013 | To determine effectiveness of pessaries for the treatment of POP. | January 1966 to March 2012 |
RCTs Inclusion criteria: women with symptomatic POP. |
Pessary versus control, waiting list, or no active treatment Pessary versus another treatment (lifestyle interventions, estrogen treatment, or physical interventions such as PFMT or surgery) Pessary plus another treatment versus the other treatment alone Pessary plus another treatment versus pessary alone One type of pessary versus a different type of pessary |
Perceived improvement in symptoms of POP Satisfaction with treatment Quality of life Complications |
| de Albuquerque Coelho et al,40 2016 | To assess the impact of pessary use on the quality of life of women with POP and to determine satisfaction rates and rationales for discontinuation. | January 1966 to May 2015 | Inclusion criteria: RCTs or observational studies; women with POP who were treated with a pessary. | No comparator stated | Quality of life Satisfaction Reason for discontinuation |
Abbreviations: PFMT, pelvic floor muscle training; POP, pelvic organ prolapse; RCT, randomized controlled trial; SUI, stress urinary incontinence.
Appendix 3: Critical Appraisal of Clinical Evidence
Table A2:
Risk of Biasa Among Systematic Reviews (ROBIS Tool)
| Phase 2 | Phase 3 | ||||
|---|---|---|---|---|---|
| Author, Year Indication | Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in the Review |
| Lipp et al,12 2014 | Low | Low | Low | Low | Low |
| Stress urinary incontinence | |||||
| National Institute for Health and Care Excellence,39 2019 | Low | Low | Low | Low | Low |
| Pelvic organ prolapse | |||||
| de Albuquerque Coelho et al,40 2016 | Highb | Low | Low | Highc | Highb,c |
| Pelvic organ prolapse | |||||
| Bugge et al,9 2013 | Low | Low | Low | Low | Low |
| Pelvic organ prolapse | |||||
Abbreviation: ROBIS, Risk of Bias in Systematic Reviews.
Possible risk of bias levels: low, high, unclear.
Potential bias due to no comparator (if any) or outcome stated.
Potential bias due to no discussion regarding bias in primary studies.
Table A3:
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Reporting | Baseline Comparability | Timing of Outcome Assessment | Other |
|---|---|---|---|---|---|---|---|---|
| Stress Urinary Incontinence | ||||||||
| Cornu et al,27 2012 | Low | Low | Unclear | Highc | Low | Unclear | Low | NR |
| Nygaard et al,28 1995 | Low | Low | Low | Low | Low | Low | Low | Ring inserted in vagina and immediately removed when participant is allocated to no treatment. 13/18 people correctly identified no mechanical device was in place. |
| Richter et al,29 2010 | Low | Low | Unclear | Low | Low | Low | Low | PFMT patients had more clinic visits than pessary-only patients. Higher levels of clinician contact could impact on patient perceptions of satisfaction and improvement. |
| Pelvic Organ Prolapse | ||||||||
| Cundiff et al,43 2007 | Unclear | Low | Unclear | Highd | Highe | NR | NR | Appropriateness of crossover design: unclear. Randomised treatment order: low. Risk of carry over effects: unclear. |
| Panman et al,41 2016 | Low | Low | Highf | Highg | Low | NR | NR | PFMT adherence: unclear. |
| Cheung et al,42 2016 | Low | Low | Highh | Highg | Low | NR | NR | PFMT adherence: unclear. |
Abbreviations: NR, not reported; PFMT, pelvic floor muscle training.
Possible risk of bias levels: low, high, and unclear.
Using the Cochrane Risk of Bias Tool, as reported in the systematic reviews by Lipp et al,12 Bugge et al,9 and the National Institute for Health and Care Excellence.39
Dropouts:14/55 (25%), intent to treat analysis performed.
Dropouts:49/134 (37%). Only 85 patients completed the study.
No formally stated primary outcome. Not all outcomes described in the methods were reported in the results. No report on vaginal discharge or bleeding.
Not blinded.
Greater than 10% patient dropout rate.
Assessor only blinded.
Table A4:
Risk of Biasa Among Observational Studies for the Comparison of Pessaries and Surgery
| Author, Year | Representativeness of Exposed Cohort | Selection of Nonexposed Cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest was Not Present at Start of Study | Comparability of Cohorts on Basis of Design or Analysis | Assessment of Outcome | Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | Score (out of 9) |
|---|---|---|---|---|---|---|---|---|---|
| Coolen et al,47 2018 | Yes | Yes | Yes | Yes | Nob | Noc | Yes | Nod | 5/9 |
| Sung et al,48 2016 | Yes | Yes | Yes | Yes | Noe | Nof | Yes | Nog | 5/9 |
| Mamik et al,44 2013 | Yes | Yes | Yes | Yes | Noe | Nof | Yes | Noh | 5/9 |
| Lone et al,49 2015 | Yes | Yes | Yes | Yes | Noe | Nof | Yes | Noi | 5/9 |
| Abdool et al,45 2011 | Yes | Yes | Yes | Yes | Noe | Nof | Yes | Noj | 5/9 |
Risk of bias assessed using Newcastle-Ottawa Quality Assessment Scale. A “Yes” in each category scores one point, except for the comparability of cohorts, which counts for a maximum of two points. The maximum total score is nine.
Patients chose intervention based on preference. Study started out as a randomized controlled trial, then became an observational study because a large number of patients had a strong preference for a particular treatment option. Patients who chose pessary were significantly older than patients who chose surgery.
Neither patients nor investigators were blinded.
Unclear accounting about patient dropouts at 1-year follow-up.
Patients chose intervention based on preference. Patients who chose pessary were significantly older than patients who chose surgery.
No discussion regarding patient or investigator blinding.
Median follow-up was 383 days (range 171–534) for surgery group and 223 days (range 11–446) for pessary group. The authors reported follow-up time in the pessary group was shorter due to failure to follow and overall pessary discontinuance.
One hundred participants recruited in total. Sixty-five people received treatment and gave follow-up data at 3 months (30 in the pessary group and 35 in the surgery group).
At 1 year, the questionnaire was completed by 80 participants (60%) in the pessary group and 103 (67%) in the surgery group.
At 1 year, the questionnaire was completed by 164 participants (68%) in the pessary group and 107 (55%) in the surgery group.
Table A5:
GRADE Evidence Profile for Comparison of Pessaries and No Treatment or Pelvic Floor Muscle Training or Tampons for People With Stress Urinary Incontinence
| Number of Studies, Design Author, Year | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Improvement of Symptoms—Pessary versus No Treatment | |||||||
| 1 RCT | Very serious limitations (−2)a | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| Cornu et al,27 2012 | limitations | limitations | (−1)b | ||||
| Improvement of Symptoms—Pessary versus PFMT or Pessary versus Pessary + PFMT or Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations (−2)c | No serious | No serious | No serious | Undetected | NA | ⊕ Low |
| Richter et al,29 2010 | limitations | limitations | limitations | ||||
| Quality of Life—Pessary versus No Treatment | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| Cornu et al,27 2012 | (−2)a | limitations | limitations | (−1)b | |||
| Patient Satisfaction—Pessary versus PFMT or Pessary versus Pessary + PFMT or Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations (−2)c | No serious | No serious | No serious | Undetected | NA | ⊕ Low |
| Richter et al,29 2010 | limitations | limitations | limitations | ||||
| Complications—Pessary versus No Treatment or Tampon versus No Treatment or Tampon versus Pessary | |||||||
| 1 RCT | Serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Low |
| Nygaard et al,28 1995 | (−1)d | limitations | limitations | (−1)d | |||
| Delayed Need for Surgery | |||||||
| 0 studies | — | — | — | — | — | — | — |
| Sexual Function | |||||||
| 0 studies | — | — | — | — | — | — | — |
| Compliance—Pessary versus PFMT or Pessary versus Pessary + PFMT or Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Low |
| Richter et al,29 2010 | (−2)b | limitations | limitations | limitations | |||
| Anorectal/Urinary Voiding Dysfunction | |||||||
| 0 studies | — | — | — | — | — | — | — |
| Lower Urinary Tract Symptoms | |||||||
| 0 studies | — | — | — | — | — | — | — |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable; PFMT, pelvic floor muscle training; RCT, randomized controlled trial.
Cornu et al reported 14/55 dropouts (25%) (although intent to treat analysis was performed). Blinding not stated. Baseline comparability unclear risk (e.g., significant difference in hysterectomy and USP questionnaire). The authors accounted for these differences by using change from baseline within groups.12
Wide confidence intervals.
Blinding was not described for participants or care providers. However, the authors stated that “outcome assessors were blinded to treatment group assignment.” PFMT patients had more clinic visits than pessary-only patients. Higher levels of clinician contact could impact on patient's perceptions of satisfaction and improvement.
Table A6:
GRADE Evidence Profile for Comparison of Vaginal Pessaries and PFMT or Another Pessary or Surgery for People With Pelvic Organ Prolapse
| Number of Studies, Design,a Author, Year | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Improvement of Symptoms – Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| Cheung et al,42 2016 | (−2)b | limitations | limitations | (−1)c | |||
| Improvement of Symptoms – Pessary versus PFMT + Feedback/Electrical Stimulation/Lifestyle Advice | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕⊕ Low |
| Panman et al,41 2016 | (−2)b | limitations | limitations | limitations | |||
| Improvement of Symptoms – Pessary versus Surgery | |||||||
| 2 Observational studies | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Coolen et al,47 2018 | (−1)d | limitations | limitations | limitations | |||
| Lone et al,49 2011 | |||||||
| Improvement of Symptoms – Ring Pessary versus Gelhorn Pessary | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Cundiff et al,43 2007 | (−2)e | limitations | limitations | limitations | |||
| Quality of Life – Pessary versus PFMT + Feedback/Electrical Stimulation/Lifestyle Advice | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕⊕ Low |
| Panman et al,41 2016 | (−2)b | limitations | limitations | limitations | |||
| Quality of Life – Pessary versus Surgery | |||||||
| 1 Observational study | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Abdool et al,45 2011 | (−1)d | limitations | limitations | limitations | |||
| Patient Satisfaction – Pessary versus Surgery | |||||||
| 2 Observational studies | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Sung et al,48 2016 | (−1)d | limitations | limitations | limitations | |||
| Mamik et al,44 2013 | |||||||
| Complications – Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| (−2)b | limitations | limitations | (−1)f | ||||
| Cheung et al,42 2016 | |||||||
| Complications – Pessary versus PFMT + Feedback/Electrical Stimulation/Lifestyle Advice | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| Panman et al,41 2016 | (−2)b | limitations | limitations | (−1)g | |||
| Delayed Need for Surgery | |||||||
| 0 studies | — | — | — | — | — | — | — |
| Sexual Function – Pessary versus PFMT + Feedback/Electrical Stimulation/Lifestyle Advice | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕⊕ Low |
| Panman et al,41 2016 | (−2)b | limitations | limitations | limitations | |||
| Sexual Function – Pessary versus Surgery | |||||||
| 1 Observational study | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Coolen et al,47 2018 | (−1)d | limitations | limitations | limitations | |||
| Pessary Compliance – Pessary versus Surgery | |||||||
| 1 Observational study | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Coolen et al,47 2018 | (−1)d | limitations | limitations | limitations | |||
| Anorectal/Urinary Voiding Dysfunction – Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| Cheung et al,42 2016 | (−2)b | limitations | limitations | (−1)c | |||
| Anorectal/Urinary Voiding Dysfunction – Pessary versus Surgery | |||||||
| 1 Observational study | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Coolen et al,47 2018 | (−1)d | limitations | limitations | limitations | |||
| Lower Urinary Tract Symptoms – Pessary + PFMT versus PFMT | |||||||
| 1 RCT | Very serious limitations | No serious | No serious | Serious limitations | Undetected | NA | ⊕ Very low |
| Cheung et al,42 2016 | (−2)b | limitations | limitations | (−1)c | |||
| Lower Urinary Tract Symptoms – Pessary versus Surgery | |||||||
| 1 observational study | Serious limitations | No serious | No serious | No serious | Undetected | NA | ⊕ Very low |
| Lone et al,49 2011 | (−1)d | limitations | limitations | limitations | |||
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable; PFMT, pelvic floor muscle training; RCT, randomized controlled trial.
Observational studies start at “Low” in GRADE.
High risk of bias due to incomplete blinding (assessor blinded only) and incomplete outcome data (>10% dropout rate). PFMT adherence unclear.
Precision is uncertain; no 95% CI reported.
Risk of bias due to comparability issues at baseline, high number of patients did not complete follow-up, blinding not addressed.
High dropout rate; only 85 of the 134 patients completed the study. No formally stated primary outcome. Not all outcomes described in the methods were reported in the results.
Precision is uncertain due to wide CI.
Issue regarding minimally important difference.
Appendix 4: Selected Excluded Studies—Clinical Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Miceli A, Dueñas-Diez JL. Effectiveness of ring pessaries versus vaginal hysterectomy for advanced pelvic organ prolapse. A cohort study. Int Urogynecol J. 2019 Dec;30(12):2161–9. | No definition of effectiveness. |
| Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008 Mar 18;148(6):459–73. | Same pessary studies as in 2014 Cochrane systematic review. Focussed on all nonsurgical treatments. |
| Scarabelot K, Pereira F, Ghizzo L, Willing J, Virtuoso J. Use of pessary in the treatment of pelvic floor dysfunctions: a systematic review. Physiotherapy Quarterly. 2018;26(1):1–8. | Population condition not specified. |
| Lovatsis D, Best C, Diamond P. Short-term Uresta efficacy (SURE) study: a randomized controlled trial of the Uresta continence device. Int Urogynecol J. 2017 Jan;28(1):147–150. | Outcome was pad test. No outcomes of interest. |
| Alnaif, B, Drutz HP. Bacterial vaginosis increases in pessary users. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(4):219–22. | Outcome was change in vaginal flora. No outcomes of interest. |
| Medina Lucena H, Williams K, Tincello DG, Lipp A, Shaw C. Evaluation of the IncoStress device for urinary incontinence: a feasibility study and pilot randomised controlled trial. Int Urogynecol J. 2019 Aug;30(8):1365–1369. | No direct comparison between intervention and comparator. |
| Thyssen H, Bidmead J, Lose G, Moller Bek K, Dwyer P, Cardozo L. A new intravaginal device for stress incontinence in women. BJU Int. 2001;88(9):889–92. | Outcome was pad test. No outcomes of interest. |
| Balk EM, Rofeberg VN, Adam GP, Kimmel HJ, Trikalinos TA, Jeppson PC. Pharmacologic and nonpharmacologic treatments for urinary incontinence in women: a systematic review and network meta-analysis of clinical outcomes. Ann Intern Med. 2019 Apr 2;170(7):465–79. | Pessaries combined in “behavioural therapy” group which also included bladder training, cones, education, heat therapy, weight loss, yoga, and spheres. No outcomes of interest. |
| Nager CW, Richter HE, Nygaard I, Paraiso MF, Wu JM, Kenton K, Atnip SD, Spino C, Pelvic Floor Disorders Network (PFDN). Incontinence pessaries: size, POPQ measures, and successful fitting. Int Urogynecol J Pelvic Floor Dysfunct. 2009 Sep;20(9):1023–8. | No outcome of interest. |
Appendix 5: Selected Excluded Studies—Economic Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Zhuo Y, Solak S, Jones K, Harmanli O. Optimizing treatment for pelvic organ prolapse using dynamic programming. Female Pelvic Med Reconstr Surg. 2015;1:S122-S3. | Poster with no full-text article. |
| Patterson D, Morse A, Flynn M. What is the best treatment for stage 2 cystocele? Female Pelvic Med Reconstr Surg. 2011;1:S142. | Poster with no full-text article. |
| Garbens A, Simpson AN, Baxter NN, Coyte PC, McDermott C, Hancock-Howard R. A cost–utility analysis of non-surgical treatments for the management of stress urinary incontinence in adult women. Int Urogynecol J. 2017;28 (1 Supplement 1):S136-S7. | Poster with no full-text article. |
| Betschart C, Cervigni M, Contreras Ortiz O, Doumouchtsis SK, Koyama M, Medina C, et al. Management of apical compartment prolapse (uterine and vault prolapse): a FIGO working group report. Neurourol Urodyn. 2017;36(2):507–13. | Clinical guideline. |
| Medical Advisory Secretariat. Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8(3):1–52. | Budget impact analysis only. |
| Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence (structured abstract). Health Technol Assess Database. 2016(4). | Pessaries not evaluated. |
Appendix 6: Results of Applicability Checklists for Studies Included in the Economic Literature Review
Table A7:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of Vaginal Pessaries
| Author, Year, Country of Publication | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where there is material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| Hullfish et al, 201153 United States |
Yes, but narrow population based on prolapse staging | Partially, does not include PFMT | No (United States) | No | Yes | NA, 1-y time horizon | Yes, but utilities were estimated by the authors | No | Partially applicable |
| Panman et al, 201641 Netherlands |
Yes (POP) | Partially, does not include surgery | No (Netherlands) | NA, an RCT was conducted, not an economic model | Yes | No, RCT data | Yes | No | Partially applicable |
| Richardson et al, 201454 United States |
Yes (SUI) | Yes | No (United States) | Yes, third-party payer perspective | Yes | NA, 1-y time horizon | Yes | No | Partially applicable |
| Simpson et al, 201955 Canada |
Yes (SUI) | Partially, does not include surgery | Yes (Canada – not province specific) | Yes, health system perspective | No, pessary fitting costs were not modelled | NA, 1-y time horizon | Yes | No | Partially applicable |
| Von Bargen et al, 201556 United States |
Yes (SUI) | Yes | No (United States) | Yes, societal perspective | Yes | Yes, 3% discount rate | Yes | Yes | Partially applicable |
Abbreviations: PFMT, pelvic floor muscle training; POP, pelvic organ prolapse; RCT, randomized controlled trial; SUI, stress urinary incontinence.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Appendix 7: Additional Primary Economic Evaluation Data
Table A8:
Population Demographics of Simulated Cohort—Pelvic Organ Prolapse
| Reference | Sample Size | Mean Age | Standard Deviation |
|---|---|---|---|
| Abdool et al, 201145 | 554 | 63.22 | 12.79 |
| Cheung et al, 201642 | 276 | 62.60 | 9.65 |
| Coolen et al, 201847 | 113 | 61.27 | 1.56 |
| Cundiff et al, 200743 | 134 | 60.92 | 14.39 |
| Lone et al, 201546 | 287 | 62.34 | 12.82 |
| Mamik et al, 201344 | 100 | 61.75 | 9.35 |
| Panman et al, 201641 | 162 | 65.25 | 6.91 |
| Sung et al, 201648 | 160 | 61.60 | 11.80 |
| Weighted average | 62.64 | 10.90 |
Table A9:
Population Demographics of Simulated Cohort—Stress Urinary Incontinence
Figure A1: Pessary Use Continuation Rate.
Note: Data from Table A10.
Figure A2: Pelvic Floor Muscle Training Continued Effectiveness.
Note: Data from Table A11.
Table A10:
Pessary Continuation by Study and Time
| Model Parameters | Number Continued | Percentage Continued |
|---|---|---|
| Hsieh et al, 201989 (N = 65) | ||
| 1 year | 53 | 81.5% |
| 2 year | 50 | 76.9% |
| Sarma et al, 200963 (N = 167) | ||
| Year 0 | 167 | 100.0% |
| Year 1 | 116 | 69.5% |
| Year 2 | 95 | 56.9% |
| Year 3 | 82 | 49.1% |
| Year 4 | 70 | 41.9% |
| Year 5 | 50 | 29.9% |
| Year 6 | 44 | 26.3% |
| Year 7 | 41 | 24.6% |
| Year 8 | 37 | 22.2% |
| Year 9 | 35 | 21.0% |
| Year 10 | 34 | 20.4% |
| Year 11 | 33 | 19.8% |
| Wolff et al, 201661 (N = 311) | ||
| Year 1 | 236 | 76.0% |
| Year 2 | 208 | 67.0% |
| Year 3 | 183 | 59.0% |
| Richter et al, 201029 (N = 149) | ||
| Year 0.25 | 94 | 63.1% |
| Year 1 | 75 | 50.3% |
| Nguyen et al, 200573 (N = 82) | ||
| Year 1 | 60 | 73.2% |
| Year 2 | 41 | 50.0% |
| Coolen et al, 201847 (N = 74) | ||
| Year 0.25 | 60 | 81.1% |
| Year 0.5 | 47 | 63.5% |
| Year 1 | 44 | 59.5% |
Table A11:
Pelvic Floor Muscle Training Continued Effectiveness by Study and Time
| Model Parameters | Number Continued | Percentage Continued |
|---|---|---|
| Cammu et al, 200062 (N = 45) | ||
| Year 1 | 24 | 53.3% |
| Year 5 | 19 | 42.2% |
| Year 10 | 16 | 35.6% |
| Richter et al, 201029 (N = 146) | ||
| Year 0.25 | 110.0 | 75.3% |
| Year 1 | 79.0 | 54.1% |
| Alewijnse et al, 2003102 (N = 129) | ||
| Year 1 | 83.0 | 64.4% |
| Fitz et al, 2019103 (N = 34) | ||
| Year 0.25 | 21.0 | 61.8% |
| Hagen et al, 2009104 (N = 19) | ||
| Year 0.5 | 12.0 | 63.0% |
| Hagen et al, 2014105 (N = 187) | ||
| Year 0.5 | 98.0 | 52.4% |
| Year 1 | 83.0 | 44.4% |
Table A12:
Online Canadian Medical Supplier Pessary Costs
| Manufacturer, Description | Cost ($ CAD) | Pessary Type |
|---|---|---|
| Milex MXKPDO05 – No Returns – MILEX Donut Pessary, 3 1/4” (82.6mm)., KIT | 120.57 | Donut |
| Milex MXKPCON02 – No Returns – MILEX Incontinence Ring 2 1/4″” O.D., KIT | 120.57 | Ring |
| Milex MXKPER04 – No Returns – MILEX All Silicone Flexible Ring Pessary #4, Without Support, 2 3/4” O.D., KIT | 120.57 | Ring |
| Milex MXKPERK4 – No Returns – MILEX All Silicone Flexible Ring Pessary with Knob without Support, 2.75” O.D | 120.57 | Ring |
| Milex MXKPGE3 – No Returns – MILEX Gelhorn Pessary 3”, ea | 120.57 | Gellhorn |
| Milex MXKPEHK00 – No Returns – MILEX Hodge Pessary with Knob #0: 2–3/4” × 1–3/4”, EACH | 115.23 | Hodge |
| Milex MXKPEC02 – MILEX Cube Pessary Size 2: 1 3/8” 35mm, ea | 120.57 | Cube |
| Milex MXKPECH07 – No Returns – MILEX Cube Pessary with Drainage Holes, size 7: 2 1/4”, EA | 99.00 | Cube |
| Milex MXKPRS00 – Milex Ring Pessary w/ Support Folding 1 3/4”, Size 0 – *No Returns*, KIT | 120.57 | Ring |
| Bioteque CU33D#2 – “Cube” Flexible Silicone Pessary, with Drain, 33mm., EA | 72.14 | Cube |
| Bioteque D2.00 – “Donut” Pessary 3Degree Support (D2.00), EACH | 72.14 | Donut |
| Bioteque DSH2 – Dish Pessary Without Support. (Flexible) 60mm (BIO-DSH2 #2)., EA | 72.14 | Dish |
| Bioteque GD2 – Gellhorn Pessary 2.00” With Drains (G2.00D), EACH | 72.14 | Gellhorn |
| Bioteque GHS2 – Gehrung Pessary with Support 60mm (GH60S#2), EA | 72.14 | Gehrung |
| Bioteque GS3 – Short Stem Gellhorn Pessary 2.25 #3 With Drains (GS2.25S#3), EACH | 72.14 | Gellhorn |
| Bioteque HDS3 – Hodge Pessary With Support, 80mm. (HD80S#3), EACH | 72.14 | Hodge |
| Bioteque M3 – Marland Pessary Without Support, 2.5” #3 (M2.50#3), EACH | 72.14 | Marland |
| Bioteque OVS8 – Oval Pessary with Support 3.75” #8 (O3.75S#8)., EACH | 72.14 | Oval |
| Bioteque R1 – Ring Pessary #1 without Support 2” (R2.00#1)., EACH | 72.14 | Ring |
| Bioteque RS2 – Ring Pessary #2 with Support 2.25” (R2.25S#2)., EA | 72.14 | Ring |
| Bioteque RKS3 – Ring Pessary with Knob with Support 2.50” (RK2.50S#3), EA | 72.14 | Ring |
| Bioteque RK4 – Ring Pessary with Knob #4 without Support, 2.75” O.D. 2” I.D.(RK2.75#4)., EA | 72.14 | Ring |
| Bioteque SH6 – Shaatz Pessary 3.00” (BIO-SH6#6), ea | 72.14 | Shaatz |
| Bioteque GS2 – Short Stem Gellhorn Pessary 2.00 #2 With Drains (BIO-GS2 #2), EA | 72.14 | Gellhorn |
| Bioteque RKS1 – Ring Pessary with Knob with Support 2.00” (BIO-RKS1 #1), EA | 72.14 | Ring |
| Milex MXKPER02 – No Returns – MILEX All Silicone Flexible Ring Pessary #2, Without Support, 2 1/4” O.D., KIT | 120.57 | Ring |
| Bioteque CU5 – “Cube” Flexible Silicone Pessary without Drain, 45mm.(CU5 #5)., EACH | 72.14 | Cube |
| Bioteque D1 – “Donut” Pessary 3Degree Support, 2.25” (Size #1), EACH | 80.16 | Donut |
| Milex MXKPCONDS06 – No Returns – MILEX Incontinence Dish folding w/ support 85mm, ea | 120.57 | Dish |
| Milex MXPGSK00 – Gehrung Pessary with Knob/Folding Size 0: 1 1/16 in. – No Returns, EA | 96.86 | Gehrung |
| Milex MXPRSK01 – No Returns – MILEX Ring Pessary with Support/Knob, Size 1: 2 inch, EA | 120.57 | Ring |
Source: CanMedDirect. https://www.canmeddirect.ca/catalogsearch/result/index/?cat=5&q=pessary
Appendix 8: Additional Budget Impact Analysis Data
Table A13:
Target Population for Scenario Analyses
| Cohort Size | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Reference case, health care professional fitting and 10% expansion | |||||
| Pelvic organ prolapse | 4,699 | 4,883 | 5,068 | 5,252 | 5,436 |
| Stress urinary incontinence | 3,159 | 3,283 | 3,407 | 3,532 | 3,656 |
| Physician fitting, no expansion | |||||
| Pelvic organ prolapse | 4,271 | 4,439 | 4,607 | 4,775 | 4,942 |
| Stress urinary incontinence | 2,872 | 2,985 | 3,098 | 3,210 | 3,323 |
| Health care professional fitting, 20% expansion | |||||
| Pelvic organ prolapse | 5,126 | 5,327 | 5,528 | 5,729 | 5,931 |
| Stress urinary incontinence | 3,447 | 3,582 | 3,717 | 3,853 | 3,988 |
Table A14:
Annual Costs by Cohort
| Cost ($) | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Reference case | |||||
| Pelvic organ prolapse | |||||
| Current | 167.77 | 76.05 | 64.91 | 55.35 | 47.14 |
| New | 217.50 | 76.05 | 64.91 | 55.35 | 66.09 |
| Stress urinary incontinence | |||||
| Current | 168.00 | 76.37 | 65.72 | 56.55 | 48.63 |
| New | 217.91 | 76.37 | 65.72 | 56.55 | 68.24 |
| Low pessary cost | |||||
| Pelvic organ prolapse | 202.59 | 76.04 | 64.91 | 55.35 | 60.41 |
| Stress urinary incontinence | 202.93 | 76.37 | 65.73 | 56.55 | 62.35 |
| High pessary cost | |||||
| Pelvic organ prolapse | 258.36 | 76.05 | 64.91 | 55.35 | 81.66 |
| Stress urinary incontinence | 258.91 | 76.37 | 65.73 | 56.55 | 84.34 |
Appendix 9: Letter of Information
Appendix 10: Interview Guide
I would like your permission to have an audio recording of this conversation so I can use your direct quotes and other information from this conversation to make a case for the decision makers. Your name or any other identifiers will not be placed in the report or the presentation and your privacy and your confidentiality will be protected. So do I have your permission to audio record this conversation?
Explain HTA
Pessary impact on different age groups
History
Before starting, comfort
Diagnosed with prolapse or stress urinary incontinence?
To start off can you tell me a little bit more information about your journey of having prolapse/SUI?
When were you diagnosed?
Were there any other health conditions that were diagnosed as well?
Symptoms?
Timeline?
Lived-Experience
How does your condition impact your life? (Day-to-day routine, work/school, activities, socializing)
What is the impact of POP/SUI and its progression on quality of life?
What is the impact of your condition on your quality of life? (Loss of independence?)
Impact on loved-ones/caregivers, sexual activity etc?
Therapies
What therapies have you used and their impact?
Cost of therapies (example, physio, pessaries?) – was it covered by insurance vs out of pocket?
Do you use any incontinence products such as pads?
Is accessibility to therapies/treatments an issue (are you able to take advantage of all potential therapies?)
Pessaries
How much information were you given about pessaries?
How was the decision-making process?
Fitting process – how many tries until perfect fit? (discomfort)
Pessary management – self management vs physician follow-up, how long have you had one? How many changes?
What are the results, impacts, change in quality of life (if applicable)?
Did you experience any potential side effects?
A Note About Terminology
As a government agency, Ontario Health can play an active role in ensuring that people of all identities and expressions recognize themselves in what they read and hear from us. We recognize that gender identities are individual and that many people who are candidates for vaginal pessaries may not identify as women. Thus, in this health technology assessment, we use gender-inclusive pronouns and terms as much as possible. However, when citing published literature that uses the terms “woman,” “women,” or “female,” we also use these terms for consistency with these cited studies.
Author contributions
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologist was Kristen McMartin, the medical librarian was Corinne Holubowich, the health economist was Sean Tiggelaar, and the patient and public partnership analyst was Jigna Mistry.
Key Messages
What Is This Health Technology Assessment About?
Pelvic organ prolapse (POP) refers to a condition in which the muscles and surrounding tissue supporting the vagina, uterus, bladder, and rectum stretch or weaken, allowing the organs to shift downwards. This can lead to a visible bulge and discomfort. Stress urinary incontinence (SUI) refers to the involuntary loss of urine during physical exertion, sneezing, or coughing. SUI can be made more likely by excess weight, menopause, pregnancy, chronic cough, pelvic surgery, or muscle-relaxing medication.
Initial treatment for POP and SUI typically includes lifestyle changes, exercises (known as pelvic floor muscle training), and devices that can be inserted into the vagina to assist with holding the organs in place. These devices are known as vaginal pessaries. Surgery is an option for some people who do not see improvement from these treatments.
This health technology assessment looked at how safe, effective, and cost-effective vaginal pessaries are for the treatment of POP and SUI. It also looked at the budget impact of publicly funding vaginal pessaries and at the experiences, preferences, and values of people with POP or SUI.
What Did This Health Technology Assessment Find?
Pessaries may improve some symptoms and sexual function compared with pelvic floor muscle training for POP. In the treatment of SUI, pessaries may have little to no difference in longer-term improvement of some symptoms compared with pelvic floor muscle training.
Compared with treatment regimens that do not include pessaries, treatment regimens that include vaginal pessaries may be cost-effective. We estimate that publicly funding vaginal pessaries for people with POP and/or SUI in Ontario over the next 5 years would cost about $0.3 million to $0.5 million for POP, and $0.2 million to $0.3 million for SUI.
People with POP or SUI with whom we spoke reported that they felt pessary use was an effective treatment option to manage their symptoms. However, conflicting information from health care providers and long wait times to see a specialist were common barriers to treatment.
Contributor Information
Ontario Health (Quality):
Kristen McMartin, Corinne Holubowich, Sean Tiggelaar, and Jigna Mistry
About Us
Ontario Health is an agency of the Government of Ontario. Our mandate is to connect and coordinate our province's health care system in ways that have not been done before to help ensure that Ontarians receive the best possible care. We work to support better health outcomes, patient experiences, provider experiences and value for money spent.
For more information about Ontario Health, visit ontariohealth.ca.
References
- (1).Zhuo Y, Solak S, Jones K, Harmanli O. Female Pelvic Medicine and Reconstructive Surgery. Conference: Optimizing treatment for pelvic organ prolapse using dynamic programming. 36th Annual Scientific Meeting of the American Urogynecologic Society; September/October Seattle (WA): Lippincott Williams and Wilkins; August 2015. p. S122–S3.
- (2).Patterson D, Morse A, Flynn M. What is the best treatment for stage 2 cystocele? Female Pelvic Medicine and Reconstructive Surgery Conference: 32nd Annual Scientific Meeting of the American Urogynecologic Society; September-October Providence (RI): Lippincott Williams and Wilkins; August 2011. p. S142.
- (3).Garbens A, Simpson AN, Baxter NN, Coyte PC, McDermott C, Hancock-Howard R. A cost-utility analysis of non-surgical treatments for the management of stress urinary incontinence in adult women. Int Urogynecol J. 2017;28 (1 Supplement 1):S136–S7. [Google Scholar]
- (4).Betschart C, Cervigni M, Contreras Ortiz O, Doumouchtsis SK, Koyama M, Medina C, et al. Management of apical compartment prolapse (uterine and vault prolapse): a FIGO Working Group report. Neurourol Urodyn. 2017;36(2):507–13. [DOI] [PubMed] [Google Scholar]
- (5).Medical Advisory Secretariat. Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis. Ontario Health Technol Assess Ser. 2008;8(3):1–52. [PMC free article] [PubMed] [Google Scholar]
- (6).Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence (structured abstract). Health Technol Assess Database. 2016(4):1. [DOI] [PubMed] [Google Scholar]
- (7).Barber MD. Pelvic organ prolapse. BMJ. 2016;354:i3853. [DOI] [PubMed] [Google Scholar]
- (8).Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185(6):1332–7. [DOI] [PubMed] [Google Scholar]
- (9).Bugge C, Adams EJ, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013(2):CD004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Al-Shaikh G, Syed S, Osman S, Bogis A, Al-Badr A. Pessary use in stress urinary incontinence: a review of advantages, complications, patient satisfaction, and quality of life. Int J Womens Health. 2018;10:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Frequently asked questions [Internet]. Canadian Continence Foundation; 2019. [updated 2019; cited 2019 May 13]. Available from: http://www.canadiancontinence.ca/EN/frequently-asked-questions.php [Google Scholar]
- (12).Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database Syst Rev. 2014(12):CD001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bordman R, Telner D, Jackson B, Little D. Step-by-step approach to managing pelvic organ prolapse: information for physicians. Can Fam Physician. 2007;53(3):485–7. [PMC free article] [PubMed] [Google Scholar]
- (15).Kow N, Goldman HB, Ridgeway B. Management options for women with uterine prolapse interested in uterine preservation. Curr Urol Rep. 2013;14(5):395–402. [DOI] [PubMed] [Google Scholar]
- (16).Reynolds WS, Dmochowski RR, Penson DF. Epidemiology of stress urinary incontinence in women. Curr Urol Rep. 2011;12(5):370–6. [DOI] [PubMed] [Google Scholar]
- (17).Health Canada. Medical devices active licence listing (MDALL) – your reference tool for licensed medical devices in Canada [Internet]. Ottawa: Government of Canada; 2019. [updated 2019 February 14; cited 2019 August 7]. Available from: https://health-products.canada.ca/mdall-limh/index-eng.jsp [Google Scholar]
- (18).Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act. Toronto (ON): Queen's Printer for Ontario; 2015. p. 746. [Google Scholar]
- (19).Ontario Health (Quality). System performance: time to patient's first surgical appointment [Internet]. Toronto (ON): Queen's Printer for Ontario. c2020. Available from: https://www.hqontario.ca/System-Performance/Wait-Times-for-Surgeries-and-Procedures/Wait-Times-for-Other-Surgeries-and-Procedures/Time-to-Patients-First-Surgical-Appointment [Google Scholar]
- (20).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (21).Covidence systematic review software [Internet]. Melbourne, Australia: Veritas Health Innovation; 2019. Available from: www.covidence.org [Google Scholar]
- (22).Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. 2016. In: Cochrane Methods [Internet]. Cochrane Database of Systematic Reviews. Available from: 10.1002/14651858.CD201601 [DOI]
- (23).Whiting P, Savović J, Higgins J, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute; 2019. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- (25).Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): GRADE Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [Internet]. [Google Scholar]
- (26).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cornu JN, Mouly S, Amarenco G, Jacquetin B, Ciofu C, Haab F, et al. 75NC007 device for noninvasive stress urinary incontinence management in women: a randomized controlled trial. Int Urogynecol J. 2012;23(12):1727–34. [DOI] [PubMed] [Google Scholar]
- (28).Nygaard I. Prevention of exercise incontinence with mechanical devices. J Reprod Med. 1995;40(2):89–94. [PubMed] [Google Scholar]
- (29).Richter HE, Burgio KL, Brubaker L, Nygaard IE, Ye W, Weidner A, et al. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Thyssen H, Bidmead J, Lose G, Moller Bek K, Dwyer P, Cardozo L. A new intravaginal device for stress incontinence in women. BJU Int. 2001;88(9):889–92. [DOI] [PubMed] [Google Scholar]
- (31).Kenton K, Barber M, Wang L, Hsu Y, Rahn D, Whitcomb E, et al. Pelvic floor symptoms improve similarly after pessary and behavioral treatment for stress incontinence. Female Pelvic Med Reconstr Surg. 2012;18(2):118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Haab F, Richard F, Amarenco G, Coloby P, Arnould B, Benmedjahed K, et al. Comprehensive evaluation of bladder and urethral dysfunction symptoms: development and psychometric validation of the Urinary Symptom Profile (USP) Questionnaire. Urology. 2008;71(4):646–56. [DOI] [PubMed] [Google Scholar]
- (33).Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189(1):98–101. [DOI] [PubMed] [Google Scholar]
- (34).Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory: Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131–9. [DOI] [PubMed] [Google Scholar]
- (35).Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388–95. [DOI] [PubMed] [Google Scholar]
- (36).Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory: Continence Program in Women (CPW) Research Group. Qual Life Res. 1994;3(5):291–306. [DOI] [PubMed] [Google Scholar]
- (37).Bradley CS, Rahn DD, Nygaard IE, Barber MD, Nager CW, Kenton KS, et al. The questionnaire for urinary incontinence diagnosis (QUID): validity and responsiveness to change in women undergoing non-surgical therapies for treatment of stress predominant urinary incontinence. Neurourol Urodyn. 2010;29(5):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Amarenco G, Arnould B, Carita P, Haab F, Labat JJ, Richard F. European psychometric validation of the CONTILIFE: a quality of life questionnaire for urinary incontinence. Eur Urol. 2003;43(4):391–404. [DOI] [PubMed] [Google Scholar]
- (39).National Institute for Health Care and Excellence. Urinary incontinence and pelvic organ prolapse in women: mangement. Evidence reviews for lifestyle and conservative mangement options for pelvic organ prolapse. NICE guideline NG123. April 2019. [PubMed]
- (40).de Albuquerque Coelho SC, de Castro EB, Juliato CR. Female pelvic organ prolapse using pessaries: systematic review. Int Urogynecol J. 2016;27(12):1797–803. [DOI] [PubMed] [Google Scholar]
- (41).Panman CM, Wiegersma M, Kollen BJ, Berger MY, Lisman-van Leeuwen Y, Vermeulen KM, et al. Effectiveness and cost-effectiveness of pessary treatment compared with pelvic floor muscle training in older women with pelvic organ prolapse: 2-year follow-up of a randomized controlled trial in primary care. Menopause. 2016;23(12):1307–18. [DOI] [PubMed] [Google Scholar]
- (42).Cheung RY, Lee JH, Lee LL, Chung TK, Chan SS. Vaginal pessary in women with symptomatic pelvic organ prolapse: a randomized controlled trial. Obstet Gynecol. 2016;128(1):73–80. [DOI] [PubMed] [Google Scholar]
- (43).Cundiff GW, Amundsen CL, Bent AE, Coates KW, Schaffer JI, Strohbehn K, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196(4):405.e1–8. [DOI] [PubMed] [Google Scholar]
- (44).Mamik MM, Rogers RG, Qualls CR, Komesu YM. Goal attainment after treatment in patients with symptomatic pelvic organ prolapse. Am J Obstet Gynecol. 2013;209(5):488.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Abdool Z, Thakar R, Sultan AH, Oliver RS. Prospective evaluation of outcome of vaginal pessaries versus surgery in women with symptomatic pelvic organ prolapse. Int Urogynecol J. 2011;22(3):273–8. [DOI] [PubMed] [Google Scholar]
- (46).Lone F, Thakar R, Sultan AH. One-year prospective comparison of vaginal pessaries and surgery for pelvic organ prolapse using the validated ICIQ-VS and ICIQ-UI (SF) questionnaires. Int Urogynecol J. 2015;26(9):1305–12. [DOI] [PubMed] [Google Scholar]
- (47).Coolen AWM, Troost S, Mol BWJ, Roovers J, Bongers MY. Primary treatment of pelvic organ prolapse: pessary use versus prolapse surgery. Int Urogynecol J. 2018;29(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lone F, Thakar R, Sultan AH, Karamalis G. A 5-year prospective study of vaginal pessary use for pelvic organ prolapse. Int J Gynaecol Obstet. 2011;114(1):56–9. [DOI] [PubMed] [Google Scholar]
- (50).Choosing Wisely. Don't exclude pessaries as a treatment option for pelvic organ prolapse [Internet]. Philadelphia (PA): ABIM Foundation; 2015. May 15 [cited 2020 Feb 18]. Available from: http://www.choosingwisely.org/clinician-lists/augs-pessaries-for-pelvic-organ-prolapse [Google Scholar]
- (51).Lamers BH, Broekman BM, Milani AL. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. Int Urogynecol J. 2011;22(6):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).National Institute for Health and Care Excellence. Process and methods guides. Appendix I: quality appraisal checklist–economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (53).Hullfish KL, Trowbridge ER, Stukenborg GJ. Treatment strategies for pelvic organ prolapse: a cost-effectiveness analysis. Int Urogynecol J. 2011;22(5):507–15. [DOI] [PubMed] [Google Scholar]
- (54).Richardson ML, Sokol ER. A cost-effectiveness analysis of conservative versus surgical management for the initial treatment of stress urinary incontinence. Am J Obstet Gynecol. 2014;211(5):565.e1–6. [DOI] [PubMed] [Google Scholar]
- (55).Simpson AN, Garbens A, Dossa F, Coyte PC, Baxter NN, McDermott CD. A cost-utility analysis of nonsurgical treatments for stress urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2019;25(1):49–55. [DOI] [PubMed] [Google Scholar]
- (56).Von Bargen E, Patterson D. Cost utility of the treatment of stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2015;21(3):150–3. [DOI] [PubMed] [Google Scholar]
- (57).Bettez M, Tu le M, Carlson K, Corcos J, Gajewski J, Jolivet M, et al. 2012. Update: guidelines for adult urinary incontinence collaborative consensus document for the Canadian Urological Association. Can Urol Assoc J. 2012;6(5):354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Robert M, Ross S. No. 186-conservative management of urinary incontinence. J Obstet Gynaecol Can. 2018;40(2):e119–e25. [DOI] [PubMed] [Google Scholar]
- (59).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (60).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada [Internet]. Ottawa (ON): The Agency; 2017. [cited 2018 Jan]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (61).Wolff B, Williams K, Winkler A, Lind L, Shalom D. Pessary types and discontinuation rates in patients with advanced pelvic organ prolapse. Int Urogynecol J. 2017;28(7):993–7. [DOI] [PubMed] [Google Scholar]
- (62).Cammu H, Van Nylen M, Amy JJ. A 10-year follow-up after Kegel pelvic floor muscle exercises for genuine stress incontinence. BJU Int. 2000;85(6):655–8. [DOI] [PubMed] [Google Scholar]
- (63).Sarma S, Ying T, Moore KH. Long-term vaginal ring pessary use: discontinuation rates and adverse events. BJOG. 2009;116(13):1715–21. [DOI] [PubMed] [Google Scholar]
- (64).TreeAge Software. TreeAge Pro 2019. R2 ed. Williamstown (MA): TreeAge Software; 2019. [Google Scholar]
- (65).Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–50. [DOI] [PubMed] [Google Scholar]
- (66).Ding J, Chen C, Song XC, Zhang L, Deng M, Zhu L. Successful use of ring pessary with support for advanced pelvic organ prolapse. Int Urogynecol J. 2015;26(10):1517–23. [DOI] [PubMed] [Google Scholar]
- (67).Fernando RJ, Sultan AH, Thakar R, Jeyanthan K. Management of the neglected vaginal ring pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):117–9. [DOI] [PubMed] [Google Scholar]
- (68).Hanson LA, Schulz JA, Flood CG, Cooley B, Tam F. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):155–9. [DOI] [PubMed] [Google Scholar]
- (69).Maito JM, Quam ZA, Craig E, Danner KA, Rogers RG. Predictors of successful pessary fitting and continued use in a nurse-midwifery pessary clinic. J Midwifery Womens Health. 2006;51(2):78–84. [DOI] [PubMed] [Google Scholar]
- (70).Manchana T. Ring pessary for all pelvic organ prolapse. Arch Gynecol Obstet. 2011;284(2):391–5. [DOI] [PubMed] [Google Scholar]
- (71).Mao M, Ai F, Zhang Y, Kang J, Liang S, Xu T, et al. Predictors for unsuccessful pessary fitting in women with symptomatic pelvic organ prolapse: a prospective study. BJOG. 2018;125(11):1434–40. [DOI] [PubMed] [Google Scholar]
- (72).Markle D, Skoczylas L, Goldsmith C, Noblett K. Patient characteristics associated with a successful pessary fitting. Female Pelvic Med Reconstr Surg. 2011;17(5):249–52. [DOI] [PubMed] [Google Scholar]
- (73).Nguyen JN, Jones CR. Pessary treatment of pelvic relaxation: factors affecting successful fitting and continued use. J Wound Ostomy Continence Nurs. 2005;32(4):255–61; quiz 62–3. [DOI] [PubMed] [Google Scholar]
- (74).Nager CW, Richter HE, Nygaard I, Paraiso MF, Wu JM, Kenton K, et al. Incontinence pessaries: size, POPQ measures, and successful fitting. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(9):1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Brazzelli M, Javanbakht M, Imamura M, Hudson J, Moloney E, Becker F, et al. Surgical treatments for women with stress urinary incontinence: the ESTER systematic review and economic evaluation. Health Technol Assess. 2019;23(14):1–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Ford AA, Rogerson L, Cody JD, Aluko P, Ogah JA. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Kilonzo M, Vale L, Stearns SC, Grant A, Cody J, Glazener CM, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20(4):455–63. [DOI] [PubMed] [Google Scholar]
- (78).van der Doelen MJ, Withagen MI, Vierhout ME, Heesakkers JP. Results of primary versus recurrent surgery to treat stress urinary incontinence in women. Int Urogynecol J. 2015;26(7):997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Patnam R, Sripad AA, Dengler E, Geller EJ, Wu JM. Moving on: how many women opt for surgery after pessary use for prolapse? Female Pelvic Med Reconstr Surg. 2019 Apr 18. [DOI] [PubMed]
- (80).Brubaker L, Maher C, Jacquetin B, Rajamaheswari N, von Theobald P, Norton P. Surgery for pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2010;16(1):9–19. [DOI] [PubMed] [Google Scholar]
- (81).Jacklin P, Duckett J. A decision-analytic Markov model to compare the cost-utility of anterior repair augmented with synthetic mesh compared with non-mesh repair in women with surgically treated prolapse. BJOG. 2013;120(2):217–23. [DOI] [PubMed] [Google Scholar]
- (82).Statistics Canada. Life tables, Canada, provinces and territories 1980/1982 to 2014/2016 [Internet]. Ottawa (ON): Statistics Canada. c2018. [updated 2018 June 28; cited 2018 October 17]. Available from: https://www.statcan.gc.ca/eng/reference/refcentre/index?MM=1 [Google Scholar]
- (83).Harvie HS, Honeycutt AA, Neuwahl SJ, Barber MD, Richter HE, Visco AG, et al. Responsiveness and minimally important difference of SF-6D and EQ-5D utility scores for the treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2019;220(3):265 e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Harvie HS, Shea JA, Andy UU, Propert K, Schwartz JS, Arya LA. Validity of utility measures for women with urge, stress, and mixed urinary incontinence. Am J Obstet Gynecol. 2014;210(1):85 e1–6. [DOI] [PubMed] [Google Scholar]
- (85).Haywood KL, Garratt AM, Lall R, Smith JF, Lamb SE. EuroQol EQ-5D and condition-specific measures of health outcome in women with urinary incontinence: reliability, validity and responsiveness. Qual Life Res. 2008;17(3):475–83. [DOI] [PubMed] [Google Scholar]
- (86).Hickling DR, Steele SS. The role of preoperative urodynamics in stress urinary incontinence surgery. Can Urol Assoc J. 2017;11(6 Suppl 2):S113–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Ministry of Health and Long-Term Care. Health data branch web portal: Ontario case costing [Internet]. Toronto (ON): Queen's Printer for Ontario. 2017. [cited 201X Mon XX]. Available from: https://hsim.health.gov.on.ca/HDBPortal [Google Scholar]
- (88).Bo K, Hilde G. Does it work in the long term? A systematic review on pelvic floor muscle training for female stress urinary incontinence. Neurourol Urodyn. 2013;32(3):215–23. [DOI] [PubMed] [Google Scholar]
- (89).Hsieh MF, Tsai HW, Liou WS, Lo CC, Lin ZH, An YF, et al. Long-term compliance of vaginal pessaries: does stress urinary incontinence matter? Medicine (Baltimore). 2019;98(14):e15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Ontario Health (Quality). Time from decision to having surgery or procedure [Internet]. Toronto (ON): Queen's Printer for Ontario; c2019. [updated Dec 2019. Available from: https://www.hqontario.ca/System-Performance/Wait-Times-for-Surgeries-and-Procedures/Wait-Times-for-Other-Surgeries-and-Procedures/Time-from-Decision-to-Having-Surgery-or-Procedure [Google Scholar]
- (91).Ontario Ministry of Finance. Ontario population projections, 2018–2046 [Internet]. Ottawa (ON): Queen's Printer for Ontario; c2010. [updated 2019 October 1; cited 2019 December 6]. Available from: https://www.fin.gov.on.ca/en/economy/demographics/projections/table7.html [Google Scholar]
- (92).Cameron Institute. The impact of incontinence in Canada: a briefing document for policy-makers [Internet]. Peterborough (ON): The Canadian Continence Foundation; 2014. Dec. Available from: https://www.canadiancontinence.ca/pdfs/en-impact-of-incontinence-in-canada-2014.pdf [Google Scholar]
- (93).Dinh T, Sutherland G. Understanding the gap: a pan-Canadian analysis of prescription drug insurance coverage [Internet]. Ottawa (ON): The Conference Board of Canada; 2017. Dec. Available from: https://www.conferenceboard.ca/temp/df2656a3-4ac9-445e-9a15-27070cc8fa7c/9326_Understanding-the-Gap__RPT.pdf [Google Scholar]
- (94).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (95).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (96).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (97).Selva A, Sola I, Zhang Y, Pardo-Hernandez H, Haynes RB, Martinez Garcia L, et al. Development and use of a content search strategy for retrieving studies on patients' views and preferences. Health Qual Life Outcomes. 2017;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Thys SD, Roovers JP, Geomini PM, Bongers MY. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74(1):6–12. [DOI] [PubMed] [Google Scholar]
- (99).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (100).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (101).NVivo qualitative data analysis software [Internet]. Doncaster, Victoria (Australia): QSR International Pty Ltd. Available from: https://www.qsrinternational.com/nvivo/home [Google Scholar]
- (102).Alewijnse D, Metsemakers JF, Mesters IE, van den Borne B. Effectiveness of pelvic floor muscle exercise therapy supplemented with a health education program to promote long-term adherence among women with urinary incontinence. Neurourol Urodyn. 2003;22(4):284–95. [DOI] [PubMed] [Google Scholar]
- (103).Fitz FF, Gimenez MM, de Azevedo Ferreira L, Matias MMP, Bortolini MAT, Castro RA. Pelvic floor muscle training for female stress urinary incontinence: a randomised control trial comparing home and outpatient training. Int Urogynecol J. 2019. [DOI] [PubMed] [Google Scholar]
- (104).Hagen S, Stark D, Glazener C, Sinclair L, Ramsay I. A randomized controlled trial of pelvic floor muscle training for stages I and II pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(1):45–51. [DOI] [PubMed] [Google Scholar]
- (105).Hagen S, Stark D, Glazener C, Dickson S, Barry S, Elders A, et al. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383(9919):796–806. [DOI] [PubMed] [Google Scholar]