Abstract
Top‐tier evidence on the safety/tolerability of 80 medications in children/adolescents with mental disorders has recently been reviewed in this journal. To guide clinical practice, such data must be combined with evidence on efficacy and acceptability. Besides medications, psychosocial interventions and brain stimulation techniques are treatment options for children/adolescents with mental disorders. For this umbrella review, we systematically searched network meta‐analyses (NMAs) and meta‐analyses (MAs) of randomized controlled trials (RCTs) evaluating 48 medications, 20 psychosocial interventions, and four brain stimulation techniques in children/adolescents with 52 different mental disorders or groups of mental disorders, reporting on 20 different efficacy/acceptability outcomes. Co‐primary outcomes were disease‐specific symptom reduction and all‐cause discontinuation (“acceptability”). We included 14 NMAs and 90 MAs, reporting on 15 mental disorders or groups of mental disorders. Overall, 21 medications outperformed placebo regarding the co‐primary outcomes, and three psychosocial interventions did so (while seven outperformed waiting list/no treatment). Based on the meta‐analytic evidence, the most convincing efficacy profile emerged for amphetamines, methylphenidate and, to a smaller extent, behavioral therapy in attention‐deficit/hyperactivity disorder; aripiprazole, risperidone and several psychosocial interventions in autism; risperidone and behavioral interventions in disruptive behavior disorders; several antipsychotics in schizophrenia spectrum disorders; fluoxetine, the combination of fluoxetine and cognitive behavioral therapy (CBT), and interpersonal therapy in depression; aripiprazole in mania; fluoxetine and group CBT in anxiety disorders; fluoxetine/selective serotonin reuptake inhibitors, CBT, and behavioral therapy with exposure and response prevention in obsessive‐compulsive disorder; CBT in post‐traumatic stress disorder; imipramine and alarm behavioral intervention in enuresis; behavioral therapy in encopresis; and family therapy in anorexia nervosa. Results from this umbrella review of interventions for mental disorders in children/adolescents provide evidence‐based information for clinical decision making.
Keywords: Children, adolescents, pharmacotherapy, psychotherapies, psychosocial interventions, brain stimulation, ADHD, autism, disruptive behavior disorders, efficacy, acceptability
Many mental disorders have an onset with clinically relevant manifestations in childhood or adolescence, followed frequently by a chronic illness course into adulthood1, 2. Many disorders with an earlier onset are first diagnosed in adulthood, with a delay ranging for example from 6 to 8 years for mood disorders and from 9 to 23 years for anxiety disorders 3 . Due to their interference with attainment of biopsychosocial milestones, mental and neurodevelopmental disorders in children and adolescents are among the leading causes of global burden of disease and years lived with disability 4 . This situation makes the appropriate delivery of evidence‐based and effective treatments for youth with mental disorders a key priority in the public health field.
Pharmacological, psychosocial and brain stimulation options are available for the management of many mental disorders in children and adolescents. However, for several of them, what should be considered the first line treatment strategy – based on efficacy, effectiveness, acceptability and tolerability/safety – remains uncertain.
A number of randomized controlled trials (RCTs) have been conducted to assess the efficacy, acceptability and tolerability of medications across different disorders in children and adolescents. The results from many of these RCTs have been pooled in pairwise meta‐analyses (MAs) or network meta‐analyses (NMAs)5, 6, 7, 8. While most antidepressants outperform placebo to treat depression in adults 9 , most antidepressants have not been shown to be superior to placebo in children and adolescents with major depressive disorder7, 10. Similarly, yet to a lower extent, antidepressants may not be as effective in children and adolescents with anxiety disorders as in adults 11 .
On the other hand, RCTs comparing psychosocial interventions with waiting list or no intervention control groups generally show a large effect size in youth with depression 10 or anxiety 12 disorders. Yet, when compared with placebo/sham interventions, most significant findings favoring psychosocial interventions vs. placebo disappear10, 12. Effect sizes also vary according to design, blinding, patient selection (baseline severity) and choice of the control group 13 in trials assessing combination treatments, whose superiority to monotherapies has not been consistently confirmed within and across disorders in children/adolescents.
Differences in inclusion criteria, outcomes, and a variety of features defining quality across MAs and NMAs limit the clinical value and impact of such a rich, yet complex body of evidence. Umbrella reviews may overcome these problems to some degree by taking the totality of the evidence from existing MAs and NMAs into account, and filtering top‐tier meta‐analytic estimates according to pre‐established criteria. It is paramount to provide clinicians with structured and standardized summaries, translating the massive data into actionable clinical information.
To our knowledge, no umbrella review is available of the evidence from MAs and NMAs of RCTs on the efficacy and acceptability of pharmacological, psychosocial, and brain stimulation treatment options for the core symptoms and associated problems of the full range of mental disorders in children and adolescents. The present study aims to fill this gap, as previously done in this journal concerning the safety and tolerability of 80 pharmacological agents used for the management of child and adolescent mental disorders 14 .
We focused on disease‐specific symptom reduction and treatment response as efficacy measures, and on measures of acceptability that could be compared across the three different treatment modalities, namely all‐cause discontinuation and intolerability‐related discontinuation. Following this approach, this umbrella review intends to provide practitioners with an evidence‐based atlas of therapeutic tools to inform clinical decision making, where a balance needs to be struck between efficacy, acceptability/tolerability, and safety.
METHODS
Search, inclusion and exclusion criteria
This umbrella review followed an a priori protocol (available upon request). We conducted a systematic search in PubMed, PsycINFO, and Cochrane database up to January 9, 2021, using an exhaustive combination of key words (full search string available upon request). We also manually searched bibliographies of included meta‐analyses. Two independent authors conducted title/abstract screening, full‐text assessment, and data extraction into a pre‐defined excel spreadsheet. A third author triple‐checked extracted data, and resolved any conflict.
Included were: a) NMAs or MAs of RCTs, b) of a priori defined 48 psychotropic medications, 20 psychosocial interventions, and four brain stimulation interventions, c) in children and/or adolescents, d) with any of 52 a priori defined mental disorders, e) reporting on 20 a priori defined outcomes within a specific disorder. Exclusion criteria were: a) systematic reviews without meta‐analysis, b) pooling of studies other than RCTs, c) interventions for other than pre‐defined disorders/outcomes.
Whenever two NMAs or MAs reported on the same combination of disorder, intervention, comparison and outcome, we considered the comparison with more RCTs, the minimum being at least one direct comparison for NMAs.
Included disorders, interventions, and comparisons
Mental disorders of interest, as grouped in the ICD‐11 15 , were: a) neurodevelopmental disorders (autism spectrum disorder, attention‐deficit/hyperactivity disorder (ADHD), disorders of intellectual development, developmental speech or sound disorders, developmental learning disorders, developmental motor coordination disorders), b) schizophrenia and other primary psychotic disorders (schizophrenia, schizoaffective disorder, schizotypal disorder, acute and transient psychotic disorder), c) catatonia, d) mood disorders (bipolar and related disorders, depressive disorders), e) anxiety or fear‐related disorders (generalized anxiety disorder, panic disorder, agoraphobia, specific phobia, social anxiety disorder, separation anxiety disorder, selective mutism), f) obsessive‐compulsive and related disorders (obsessive‐compulsive disorder, body dysmorphic disorder, body‐focused repetitive disorders), g) movement disorders (Tourette's disorder, other tic disorder), h) disorders specifically associated with stress (post‐traumatic stress disorder (PTSD), complex PTSD, prolonged grief disorder, reactive attachment disorder, disinhibited social engagement disorder), i) dissociative disorders (dissociative neurological symptom disorder, dissociative amnesia, trance disorder, dissociative identity disorder), j) feeding and eating disorders (anorexia nervosa, bulimia nervosa, binge eating disorder, avoidant‐restrictive food intake disorder, pica, rumination‐regurgitation disorder), k) elimination disorders (enuresis, encopresis), l) disorders of bodily distress or bodily experience (bodily distress disorder, body integrity dysphoria), m) disorders due to substance use or addictive behaviors, n) impulse control disorders (pyromania, kleptomania, compulsive sexual behavior disorder, intermittent explosive disorder), o) disruptive behavior or dissocial disorders (oppositional defiant disorder, conduct disorder).
Interventions included medications, psychosocial interventions, and brain stimulation techniques.
Medications comprised antidepressants (bupropion, mirtazapine, nefazodone, vilazodone, desvenlafaxine, duloxetine, venlafaxine, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, clomipramine, desipramine, imipramine, nortriptyline, amitriptyline); antipsychotics (fluphenazine, haloperidol, molindone, trifluoperazine, amisulpride, aripiprazole, asenapine, clozapine, loxapine, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, thioridazine, ziprasidone); anti‐ADHD medications (amphetamines, atomoxetine, clonidine, guanfacine, methylphenidate, modafinil); mood stabilizers (carbamazepine, lamotrigine, lithium, oxcarbazepine, topiramate, valproate); and others (oxybutynin, desmopressin).
Psychosocial interventions included behavioral therapy, cognitive behavioral therapy (CBT), problem solving, dialectical behavioral therapy, family‐based therapy, interpersonal psychotherapy, mentalization based therapy, psychodynamic psychotherapy, supportive therapy, social skills training, acceptance and commitment therapy, mindfulness, eye movement desensitization and reprocessing, narrative exposure therapy, cognitive remediation therapy, cognitive training, parent‐child interaction therapy, play therapy, art therapy, and occupational therapy.
Brain stimulation interventions included transcranial magnetic stimulation, transcranial direct current stimulation, electroconvulsive therapy, and neurofeedback.
Comparators were labeled as active drug, active psychosocial intervention, treatment as usual (TAU)/low intensity psychosocial intervention, waiting list/no treatment, or placebo/sham.
Outcomes
Co‐primary outcomes were disease‐specific primary symptom reduction and all‐cause discontinuation (“acceptability”).
Secondary continuous outcomes were measures of aggressive behavior, anxiety (other than anxiety disorders), cognition (other than ADHD), depressive symptoms (other than depressive episode/disorder), irritability, suicidal ideation, global illness severity, functioning (as defined by authors), and quality of life.
Secondary categorical outcomes were study‐defined treatment response, remission, relapse, hospitalization, discontinuation due to inefficacy, discontinuation due to intolerability, suicide attempt, completed suicide, and death. When available, treatment estimates from clinicians, teachers, parents, and children/adolescents were considered separately.
Quality of evidence
The quality of MAs and NMAs was measured using A Measurement Tool for the Assessment of Multiple Systematic Reviews (AMSTAR‐PLUS)16, 17 to quantify both the methodological quality of MAs and NMAs with the first 11 items (AMSTAR) and of included RCTs with six additional items (AMSTAR‐Content).
Methodological quality was categorized into low (<4), medium (4‐7), and high (>7). Content quality was categorized into low (<4), medium (4‐6), and high (>6). The lowest score between methodological and content quality determined the overall MA or NMA quality.
Statistical analysis
We converted continuous non‐standardized outcomes, such as weighted mean differences, to standardized mean differences (SMDs), and binary outcomes to odds ratio (ORs) with Comprehensive Meta‐Analysis (CMA), Version 3 18 . We then calculated the mean SMD for the primary efficacy outcome across pharmacological, psychosocial, and brain stimulation interventions for each disorder against placebo/sham and waiting list/no intervention, as well as for active controlled monotherapy and combination treatment studies, prioritizing clinician rating, followed by teacher, parent, and then subject‐rated estimates. For treatment response, in case no data were available for the continuous primary efficacy outcome, we converted ORs to SMDs, using CMA.
Whenever data conversion was not possible, we kept the original effect sizes as reported. Whenever we included data from meta‐analyses that used fixed‐effects models, we recalculated the meta‐analysis using random‐effects models 19 . For consistent and easy comparison, we harmonized effect sizes as follows: SMD<0 favors intervention, OR/risk ratio (RR) <1 favors intervention for discontinuation, suicide or relapse, while OR/RR>1 favors intervention for response or remission.
RESULTS
Search results and literature coverage
The search process is described in Figure 1. Out of 5,231 initial hits, we assessed 910 MAs and NMAs at full text level. Of these, we excluded 806, with specific reasons (list available upon request). The list of all included MAs and NMAs is available in Table 1, also indicating the number of included RCTs and participants, as well as the methodological quality (AMSTAR score) together with the quality of included RCTs (AMSTAR‐Content median score).
Figure 1.
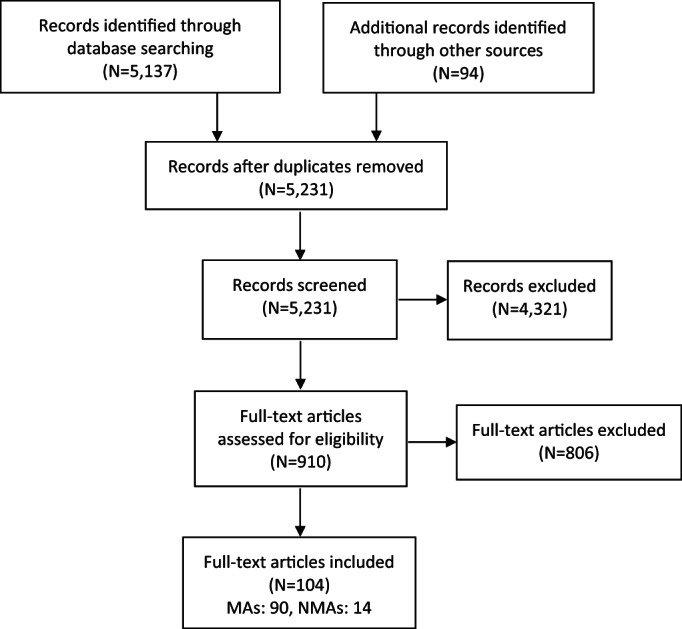
PRISMA flow chart, MAs – meta‐analyses, NMAs – network meta‐analyses
Table 1.
Network and pairwise meta‐analyses of randomized controlled trials (RCTs) of pharmacological, psychosocial and brain stimulation interventions in children and adolescents with mental disorders included in the umbrella review
| Source | Number of RCTs/ patients | Intervention | Controls | Outcomes | A | C | |
|---|---|---|---|---|---|---|---|
|
Anxiety disorders | |||||||
| Wang et al 79 | MA | 115/7,719 | AD | PBO | PE, REM | 10 | 4 |
| Dobson et al 11 | NMA | 22/2,623 | AD | PBO | RES, ACD, AED, S | 7 | 5 |
| Zhang et al 80 | MA | 7/358 | CB | WL/NT | PE | 9 | 2 |
| James et al 12 | MA | 87/5,964 | CB | PBO, WL/NT, TAU, PS | PE, REM, DEP, F, ACD | 11 | 3 |
| Zhou et al 78 | NMA | 101/6,625 | CB | PBO, WL/NT, TAU, PS | PE, QoL, ACD | 11 | 2 |
| Sigurvinsdóttir et al 81 | MA | 81/5,913 | CB | WL/NT, TAU, PS | REM | 10 | 1 |
| James et al 82 | MA | 41/1,955 | CB | TAU, PS | PE, REM | 11 | 1.5 |
|
Anorexia nervosa | |||||||
| Fisher et al 99 | MA | 21/1,407 | FB | TAU, PS | PE, ACD, REM | 10 | 1 |
| van den Berg et al 100 | MA | 15/1,279 | PS | TAU | PE | 9 | 2 |
| Zeeck et al 97 | NMA | 18/1,247 | FB, PSD‐O | PS | PE | 7 | 1 |
|
Social anxiety disorder | |||||||
| Yang et al 83 | MA | 17/1,134 | CB | PBO, WL/NT | PE, REM, DEP, QoL, ACD | 10 | 2 |
| Kreuze et al 84 | MA | 42/3,239 | CB | PBO, TAU, LIP | AG, F | 10 | 2.5 |
|
Attention‐deficit/hyperactivity disorder (ADHD) | |||||||
| Cortese et al 5 | NMA | 133/18,199 | AD, STIM, α2 | PBO, AD, STIM | PE, AED, GLO | 11 | 9 |
| Otasowie et al 22 | MA | 6/216 | AD | PBO | PE, GLO | 10 | 3 |
| Punja et al 23 | MA | 23/2,675 | STIM | PBO | PE, COG, GLO | 10 | 4 |
| Stuhec et al 34 | MA | 28/4,699 | AD | PBO | PE | 8 | 2 |
| Luan et al 21 | NMA | 73/15,025 | AD, STIM, α2 | PBO, PHARMA | PE, AED, ID | 7 | 4 |
| Catalá‐López et al 20 | NMA | 190/26,114 | AP, AD, STIM, α2, CB, CT, NF, COMB | PBO | RES, ACD, GLO | 10 | 4 |
| Schachter et al 36 | MA | 62/2,897 | STIM | PBO | AG | 9 | 1 |
| Schwartz et al 37 | MA | 25/3,928 | AD, STIM | PBO | AG, F, QoL, S | 7 | 5 |
| Coghill et al 38 | MA | 60/1,993 | STIM | PBO | COG | 8 | 2 |
| Storebø et al 39 | MA | 185/12,245 | STIM | PBO | QoL | 8 | 5 |
| Bangs et al 40 | MA | 32/7,248 | AD, STIM | PBO | S | 3 | 4 |
| Hirota et al 41 | MA | 12/2,276 | α2+ | PBO | PE, ACD, AED, ID | 6 | 3.5 |
| Storebø et al 42 | MA | 25/2,690 | SKILL, COMB | WL/NT | PE, COG, F | 11 | 2 |
| Sun et al 24 | MA | 8/423 | STIM | PBO | PE, ACD, AED | 11 | 2 |
| Battagliese et al 25 | MA | 24/1,690 | BT | MIX | PE, AG, COG, F | 7 | 1 |
| Faraone et al 26 | MA | 4/216 | STIM | STIM | AG | 2 | 3 |
| Van Doren et al 27 | MA | 10/506 | NF | PHARMA, PS | PE, RES, ACD | 8 | 2 |
| Cortese et al 28 | MA | 16/759 | CT | MIX | PE, COG | 11 | 1 |
| Daley et al 29 | MA | 32/2,077 | BT | MIX | PE, COG | 9 | 2 |
| Bikic et al 30 | MA | 12/1,054 | SKILL | MIX | PE, COG | 8 | 2 |
| Mulqueen et al 31 | MA | 8/399 | BT | MIX | PE | 6 | 1 |
| Cortese et al 32 | MA | 13/520 | NF | MIX | PE, COG | 9 | 1.5 |
| Bussalb et al 33 | MA | 16/706 | NF | MIX | PE | 4 | 2 |
| Faraone et al 35 | MA | 7/384 | STIM | PBO | AG | 2 | 2 |
|
Autism spectrum disorder | |||||||
| Maneeton et al 44 | MA | 3/408 | AP | PBO | PE, RES, GLO | 7 | 4 |
| Maneeton et al 52 | MA | 7/372 | AP | PBO | REL, RES | 7 | 3.5 |
| Zhou et al 53 | MA | 64/3,499 | STIM | PBO | PP | 9 | 3 |
| Murza et al 54 | MA | 16/837 | SKILL | WL/NT | F | 8 | 0.5 |
| Fletcher‐Watson et al 56 | MA | 22/695 | SKILL | WL/NT, TAU | F | 10 | 1 |
| Sturman et al 55 | MA | 4/113 | STIM | PBO | PE | 10 | 1 |
| Cohen et al 57 | MA | 15/995 | AP | PBO | RES | 5 | 1 |
| Hirota et al 58 | MA | 7/171 | MS | PBO | RES, AG, ACD, AED, ID | 6 | 4 |
| Fallah et al 43 | NMA | 8/878 | AP | PBO, AP | AG | 7 | 1 |
| D'Alò et al 59 | MA | 15/1,124 | AP | PBO | ACD, AED | 9 | 5 |
| Ospina et al 60 | MA | 69/2,585 | BT | WL/NT, PS | PE | 9 | 1 |
| Reichow et al 61 | MA | 5/196 | SKILL | WL/NT | PE | 10 | 1 |
| James et al 12 | MA | 87/5,964 | CB | WL/NT, TAU | ANX | 11 | 0.5 |
| Tachibana et al 62 | MA | 32/594 | PS | TAU | PE | 11 | 1 |
| Nevill et al 63 | MA | 19/1,205 | PCI | TAU/LIP, MIX | PE, COG | 5 | 1 |
| Yu et al 45 | MA | 14/555 | BT | TAU | PE, F | 9 | 0 |
| Oono et al 46 | MA | 17/919 | PCI | MIX | PE, F, GLO | 10 | 1 |
| Parsons et al 47 | MA | 21/925 | SKILL | MIX | PE | 9 | 1 |
| Kreslins et al 48 | MA | 10/470 | CB | MIX | ANX | 9 | 0 |
| Tarver et al 49 | MA | 9/521 | PCI | MIX | AG | 8 | 2 |
| Soares et al 50 | MA | 18/1,266 | SKILL | MIX | F | 8 | 2 |
| Postorino et al 51 | MA | 8/653 | PCI | MIX | IR | 8 | 1 |
|
Bipolar disorder, depressive episode | |||||||
| Maneeton et al 106 | MA | 3/251 | AP | PBO | PE, RES, REM, GLO, ACD, AED | 9 | 3 |
|
Bipolar disorder, manic episode | |||||||
| Meduri et al 107 | MA | 22/5,437 | AP | PBO | PE, RES, ACD, AED, ID | 10 | 5 |
| Liu et al 108 | MA | 46/2,666 | MS | PBO | RES | 7 | 6 |
| Jochim et al 109 | MA | 25/3,252 | MS, AP | PBO, MS | ACD | 10 | 4 |
|
Bulimia nervosa | |||||||
| Linardon et al 101 | MA | 79/NR | CB | PS | PE | 6 | 0 |
|
Depressive disorders | |||||||
| Zhou et al 10 | NMA | 71/9,510 | AD, PSD‐O, FB, CB, COMB | PBO, WL/NT, TAU/LIP, PHARMA, PS | PE, ACD, S | 11 | 5 |
| Cipriani et al 7 | NMA | 34/5,260 | AD | PBO, PHARMA | RES, AED | 11 | 5 |
| Spielmans & Gerwig 64 | MA | 8/1,756 | AD | PBO | QoL | 5 | 5 |
| Kato et al 65 | MA | 40/8,890 | AD | PBO | REL | 9 | 3 |
| Whittington et al 66 | MA | 2/376 | AD | PBO | REM | 9 | 2.5 |
| Watanabe et al 67 | MA | 27/1,744 | PSD‐O | WL/PBO | RES | 7 | 2 |
| Cox et al 68 | MA | 9/882 | AD, CB, COMB | PHARMA, PS | REM, S | 10 | 3 |
| Dubicka et al 69 | MA | 5/1,206 | COMB | PHARMA, PS | RES, F, S | 7 | 3 |
| Klein et al 70 | MA | 11/809 | CB | MIX | PE | 8 | 4 |
|
Disruptive behavior/dissocial/conduct disorders | |||||||
| Seida et al 92 | MA | 62/NR | AP | PBO | PE, AG, GLO | 9 | 3.5 |
| Loy et al 93 | MA | 10/896 | AP | PBO | PE, AG | 10 | 4 |
| Pringsheim et al 94 | MA | 18/1,195 | MS | PBO | AG | 10 | 2 |
| Ipser & Stein 95 | MA | 14/823 | PHARMA | PBO | AG, ACD, GLO, RES | 6 | 1.5 |
| Battagliese et al 25 | MA | 24/1,690 | CB | WL/NT, MIX | PE | 7 | 1.5 |
| McQuire et al 96 | MA | 14/912 | AP, MS | PBO | AG | 8 | 2 |
|
Developmental coordination disorder | |||||||
| Miyahara et al 116 | MA | 15/649 | SKILL | WL/NT | PE | 10 | 1 |
|
Eating disorders | |||||||
| Couturier et al 98 | MA | 6/369 | FB | PS | REM | 8 | 3 |
|
Encopresis | |||||||
| Freeman et al 114 | MA | 10/562 | COMB | TAU | PE, RES | 7 | 1 |
| Brazzelli et al 115 | MA | 21/1,371 | COMB | TAU | RES | 10 | 1 |
|
Enuresis | |||||||
| Caldwell et al 86 | MA | 74/5,983 | BT, COMB | PHARMA, PS, WL/NT | PE, RES | 11 | 1 |
| Caldwell et al 87 | MA | 64/4,071 | AD, COMB | PBO, PHARMA, PS | PE, RES | 11 | 1 |
| Caldwell et al 88 | MA | 16/1,643 | BT | PS, WL/NT | RES | 10 | 1 |
| Buckley et al 89 | MA | 27/1,803 | SKILL, COMB | TAU, PHARMA | REM | 10 | 1 |
| Deshpande et al 90 | MA | 40/2,440 | AD, COMB | PHARMA | RES, REL | 10 | 1 |
| Peng et al 91 | MA | 15/1,502 | PHARMA | PS | ACD | 9 | 4 |
| Song et al 85 | NMA | 18/1,649 | PHARMA, COMB | PHARMA, PS | RES, REL | 9 | 4 |
|
Obsessive‐compulsive disorder | |||||||
| Skapinakis et al 71 | NMA | 86/15,585 | AD, CB, COMB | PBO, WL/NT, PHARMA, PS | PE, ACD | 10 | 3 |
| Maneeton et al 72 | MA | 3/188 | AD | PBO | RES, GLO | 9 | 2 |
| McGuire et al 73 | MA | 20/1,296 | AD, CB | PBO, TAU/LIP, WL/NT | RES, REM | 8 | 1 |
| Locher et al 74 | MA | 36/6,778 | AD | PBO | AED | 10 | 4 |
| Geller 75 | MA | 12/1,044 | AD | PBO | GLO | 8 | 3 |
| Uhre et al 76 | MA | 12/791 | CB, AD | PBO, WL/NT, PS | REM, F, QoL | 9 | 1 |
| Johnco et al 77 | MA | 21/1,423 | CB, AD | PBO, WL/NT, TAU/LIP, PS | ACD | 6 | 1 |
|
Post‐traumatic stress disorder | |||||||
| Gillies et al 117 | MA | 14/758 | CB | WL/NT, TAU/LIP | PE, RES, ANX, DEP, ACD | 10 | 1 |
|
Schizophrenia spectrum disorders | |||||||
| Krause et al 102 | NMA | 28/3,003 | AP | PBO, PHARMA | PE, RES, ACD, ID | 11 | 3 |
| Arango et al 103 | NMA | 13/2,210 | AP | PBO, PHARMA | GLO, AED | 9 | 7 |
| Pagsberg et al 8 | NMA | 12/2,158 | AP | PBO, PHARMA | GLO | 8 | 3 |
| Sarkar & Grover 104 | MA | 15/995 | AP | PHARMA | PE | 5 | 1 |
| Kumar et al 105 | MA | 13/1,112 | AP | PHARMA | AED | 8 | 1 |
|
Tic disorder | |||||||
| Bloch et al 110 | MA | 9/477 | STIM, AD | PBO | PE | 4 | 1 |
| Yu et al 111 | MA | 15/1,070 | MS | PHARMA | RES | 7 | 3 |
|
Tourette's disorder | |||||||
| Hollis et al 112 | MA | 40/2,422 | AP, α2, STIM, BT | PBO, MIX | PE | 8 | 1 |
| Zheng et al 113 | MA | 6/528 | AP | PHARMA | PE | 10 | 2 |
MA – meta‐analysis, NMA – network meta‐analysis, A – AMSTAR, C – AMSTAR‐Content (median), AD – antidepressants, CB – cognitive‐based, FB – family‐based, PS – active psychosocial, PSD‐O – psychodynamic‐oriented, STIM – stimulants, α2 – α2‐agonists (+=augmentation with), AP – antipsychotics, CT – cognition‐targeted, NF – neurofeedback, COMB – combination of more than one treatment, SKILL – skills training, BT – behavioral treatment, MS – mood stabilizers, PCI – parent‐child interaction, PHARMA – mixed medications, PBO – placebo, WL – waiting list, NT – no treatment, TAU – treatment as usual, LIP – low‐intensity psychosocial intervention, MIX – mixed active/inactive control group, PE – primary efficacy outcome, REM – remission, REL – relapse, RES – response, S – suicidality, ACD – all‐cause discontinuation, AED – discontinuation due to adverse events, ID – discontinuation due to inefficacy, DEP – depressive symptoms, ANX – anxiety symptoms, AG – aggressivity, QoL – quality of life, GLO – global illness severity, COG – cognition, F – functioning, NR – not reported
We ultimately included 14 NMAs and 90 MAs, reporting on 15 disorders or groups of disorders. For ADHD, we included three NMAs5, 20, 21 and 21 MAs22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42; for autism, one NMA 43 and 21 MAs12, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63 (including one focusing on comorbid anxiety disorders and autism) 12 ; for depressive disorders, two NMA7, 10 and seven MAs64, 65, 66, 67, 68, 69, 70; for obsessive‐compulsive disorder, one NMA 71 and six MAs72, 73, 74, 75, 76, 77; for anxiety disorders, two NMAs11, 78 and five MAs12, 79, 80, 81, 82 (plus two MAs specific on social anxiety disorder83, 84); for enuresis, one NMA 85 and six MAs86, 87, 88, 89, 90, 91, for disruptive behavior/dissocial/conduct disorders, five MAs92, 93, 94, 95, 96 (plus one focusing on youth with comorbid ADHD) 25 ; for eating disorders, one NMA 97 and four MAs98, 99, 100, 101; for schizophrenia spectrum disorders, three NMAs8, 102, 103 and two MAs104, 105; for bipolar disorder, four MAs106, 107, 108, 109; for tic disorder, two MAs110, 111; for Tourette's disorder, two MAs112, 113; for encopresis, two MAs114, 115; for developmental coordination disorder, one MA 116 ; and for PTSD, one MA 117 .
Overall, 85.4% of a priori selected medications were covered for at least one of the two co‐primary outcomes, which was the case for 55% of the psychosocial interventions, and 25% of the brain stimulation interventions. Moreover, 70% of a priori selected outcomes were covered across monotherapy medication treatments (anti‐ADHD medications: 65%; antidepressants: 55%; antipsychotics: 40%; mood stabilizers: 25%), 80% across psychosocial interventions, and 20% across brain stimulation interventions.
Among monotherapy medication treatments with data on co‐primary outcomes, those most covered by the literature were atomoxetine (11 outcomes), methylphenidate (9 outcomes), amphetamines and risperidone (8 outcomes), aripiprazole, fluoxetine, guanfacine, lurasidone and quetiapine (7 outcomes), and asenapine, clonidine, olanzapine, paliperidone and sertraline (6 outcomes). Monotherapy psychosocial interventions most covered by the literature were CBT (12 outcomes), behavioral therapy (9 outcomes), parent‐child interaction therapy (7 outcomes), and CBT‐oriented, psychodynamic‐oriented and family‐based therapies (6 outcomes). Among brain stimulation interventions, neurofeedback was the only modality with data that could be included in this umbrella review (4 outcomes).
Quality of included evidence
Among 14 NMAs of RCTs, the median AMSTAR score was 9.5 (interquartile range, IQR: 7‐11), and the median AMSTAR‐Content score was 4 (IQR: 2.75‐5). The median overall quality score across all effect sizes was low in six NMAs (42.9%), moderate in six (42.9%), high in the remaining two (14.2%).
Among 90 MAs of RCTs, the median AMSTAR score was 9 (IQR: 7‐10) and the median AMSTAR‐Content score was 2 (IQR: 1‐3). The median overall quality score across all effect sizes was low in 71 MAs (78.9%), moderate in 19 (21.1%), and high in none.
Across NMAs and MAs of RCTs of medications, the median AMSTAR quality score was 10 (IQR: 7‐11), being low in 0.8%, moderate in 24.7%, and high in 74.4% of the NMAs/MAs, while the AMSTAR‐Content median quality score was 4 (IQR: 3‐5), being low in 30.1%, moderate in 58.6%, and high in 11.3%.
Across NMAs and MAs of RCTs of psychosocial interventions, the median AMSTAR quality score was 11 (IQR: 10‐12), being low in none of the NMAs/MAs, moderate in 8.2%, and high in 91.8%, while the median AMSTAR‐Content quality score was 2 (IQR: 1‐3), being low in 87.4%, moderate in 12.6%, and high in none.
Across brain stimulation interventions, the median AMSTAR quality score was 9 (IQR: 8‐10), being low in none of the NMAs/MAs, medium in 16.7%, and high in 83.3%, while the median AMSTAR‐Content quality score was 2 (IQR: 1‐4), being low in 66.7%, moderate in 33.3%, and high in none.
Efficacy, acceptability and tolerability of pharmacological, psychosocial, and brain stimulation interventions (Tables 2‐7)
ADHD
Results for ADHD are shown in Tables 2, 6 and 7. Amphetamines, methylphenidate, desipramine and modafinil had the largest effect size for the primary efficacy outcome.
Table 2.
Efficacy and effectiveness of pharmacological, psychosocial and brain stimulation interventions vs. inactive control in children/adolescents with neurodevelopmental and disruptive behavior/dissocial/conduct disorders
| Outcome | Intervention | Effect size (95% CI) | Control | Number of RCTs/patients | Q |
|---|---|---|---|---|---|
| Attention‐deficit/hyperactivity disorder (ADHD) | |||||
| Pharmacological interventions | |||||
| Efficacy (clinician‐rated) | Amphetamines | SMD=–1.02 (–1.19 to –0.85) | PBO/Sham | 46/9,926 | H |
| Methylphenidate | SMD=–0.78 (–0.93 to –0.62) | PBO/Sham | 46/9,926 | H | |
| Clonidine | SMD=–0.71 (–1.17 to –0.24) | PBO/Sham | 46/9,926 | H | |
| Guanfacine | SMD=–0.67 (–0.85 to –0.50) | PBO/Sham | 46/9,926 | H | |
| Modafinil | SMD=–0.62 (–0.84 to –0.41) | PBO/Sham | 46/9,926 | H | |
| Atomoxetine | SMD=–0.56 (–0.66 to –0.45) | PBO/Sham | 46/9,926 | H | |
| Efficacy (teacher‐rated) | Desipramine | SMD=–0.97 (–1.66 to –0.28) | PBO/Sham | 2/89 | L |
| Methylphenidate | SMD=–0.82 (–1.16 to –0.48) | PBO/Sham | 16/1,843 | H | |
| Modafinil | SMD=–0.76 (–1.15 to –0.37) | PBO/Sham | 16/1,843 | H | |
| Amphetamines | SMD=–0.55 (–0.83 to –0.27) | PBO/Sham | 5/745 | M | |
| Guanfacine | SMD=–0.63 (–1.62 to 0.35) | PBO/Sham | 16/1,843 | H | |
| Atomoxetine | SMD=–0.32 (–0.82 to 0.18) | PBO/Sham | 16/1,843 | H | |
| Efficacy (parent‐rated) | Desipramine | SMD=–1.42 (–1.99 to –0.85) | PBO/Sham | 2/99 | L |
| Amphetamines | SMD=–1.07 (–1.36 to –0.79) | PBO/Sham | 23/3,796 | H | |
| Methylphenidate | SMD=–0.84 (–0.95 to –0.72) | PBO/Sham | 23/3,796 | H | |
| Atomoxetine | SMD=–0.60 (–0.71 to –0.50) | PBO/Sham | 23/3,796 | H | |
| Modafinil | SMD=–0.46 (–0.61 to –0.31) | PBO/Sham | 23/3,796 | H | |
| Bupropion | SMD=–0.32 (–0.69 to 0.05) | PBO/Sham | 2/124 | L | |
| Guanfacine | SMD=–0.23 (–0.90 to 0.45) | PBO/Sham | 23/3,796 | H | |
| Efficacy (mixed‐rated) | Atomoxetine | SMD=–0.17 (–0.23 to –0.11) | PBO/Sham | 36/7,579 | M |
| Amphetamines | SMD=–0.18 (–0.28 to –0.09) | PBO/Sham | 36/7,579 | M | |
| Methylphenidate | SMD=–0.14 (–0.21 to –0.08) | PBO/Sham | 36/7,579 | M | |
| Guanfacine | SMD=–0.16 (–0.26 to –0.05) | PBO/Sham | 36/7,579 | M | |
| Clonidine | SMD=–0.10 (–0.23 to 0.03) | PBO/Sham | 36/7,579 | M | |
| Response | Desipramine | OR=36.76 (9.17‐214) | PBO/Sham | 113/19,398 | M |
| Amphetamines | OR=7.45 (5.1‐11.09) | PBO/Sham | 113/19,398 | M | |
| Modafinil | OR=5.51 (3.04‐10.32) | PBO/Sham | 113/19,398 | M | |
| Methylphenidate | OR=5.26 (4.09‐6.82) | PBO/Sham | 113/19,398 | M | |
| Clonidine | OR=3.96 (1.89‐8.41) | PBO/Sham | 113/19,398 | M | |
| Atomoxetine | OR=3.63 (2.81‐4.73) | PBO/Sham | 113/19,398 | M | |
| Guanfacine | OR=3.29 (2.27‐4.82) | PBO/Sham | 113/19,398 | M | |
| Aggressive behavior | Amphetamines | SMD=–1.15 (–1.38 to –0.93) | PBO/Sham | 3/84 | L |
| Methylphenidate | SMD=–0.26 (–1.10 to 0.68) | PBO/Sham | 2/181 | L | |
| Atomoxetine | RR=1.34 (0.91 to 1.97) | PBO/Sham | 15/2,067 | M | |
| Cognition: executive memory | Methylphenidate | SMD=–0.26 (–0.39 to –0.13) | PBO/Sham | 7/468 | L |
| Cognition: non‐executive memory | Methylphenidate | SMD=–0.60 (–0.79 to –0.41) | PBO/Sham | 8/635 | L |
| Cognition: reaction time | Methylphenidate | SMD=–0.21 (–0.30 to –0.12) | PBO/Sham | 21/1,095 | L |
| Cognition: response inhibition | Methylphenidate | SMD=–0.41 (–0.55 to –0.27) | PBO/Sham | 16/846 | L |
| Acceptability | Clonidine | OR=0.40 (0.20‐0.78) | PBO/Sham | 171/22,961 | M |
| Methylphenidate | OR=0.59 (0.46‐0.75) | PBO/Sham | 171/22,961 | M | |
| Aripiprazole | OR=0.61 (0.02‐25.34) | PBO/Sham | 171/22,961 | M | |
| Modafinil | OR=0.67 (0.37‐1.24) | PBO/Sham | 171/22,961 | M | |
| Desipramine | OR=0.70 (0.17‐2.89) | PBO/Sham | 171/22,961 | M | |
| Amphetamines | OR=0.78 (0.52‐1.18) | PBO/Sham | 171/22,961 | M | |
| Guanfacine | OR=0.79 (0.54‐1.14) | PBO/Sham | 171/22,961 | M | |
| Atomoxetine | OR=0.85 (0.68‐1.07) | PBO/Sham | 171/22,961 | M | |
| Bupropion | OR=1.54 (0.39‐6.76) | PBO/Sham | 171/22,961 | M | |
| Tolerability | Methylphenidate | OR=1.31 (0.79‐2.25) | PBO/Sham | 60/12,188 | M |
| Modafinil | OR=1.34 (0.57‐3.18) | PBO/Sham | 60/12,188 | M | |
| Amphetamines | OR=1.38 (0.64‐3.00) | PBO/Sham | 60/12,188 | M | |
| Clonidine | OR=2.32 (0.63‐8.94) | PBO/Sham | 58/NR | H | |
| Bupropion | OR=3.60 (0.34‐130) | PBO/Sham | 60/12,188 | M | |
| Atomoxetine | OR=1.48 (1.01‐2.18) | PBO/Sham | 60/12,188 | M | |
| Guanfacine | OR=3.39 (1.93‐6.3) | PBO/Sham | 60/12,188 | M | |
| Discontinuation due to inefficacy | Amphetamine | OR=0.11 (0.05‐0.20) | PBO/Sham | 45/9,087 | M |
| Clonidine | OR=0.29 (0.13‐0.56) | PBO/Sham | 45/9,087 | M | |
| Methylphenidate | OR=0.31 (0.18‐0.53) | PBO/Sham | 45/9,087 | M | |
| Guanfacine | OR=0.37 (0.26‐0.54) | PBO/Sham | 45/9,087 | M | |
| Atomoxetine | OR=0.47 (0.33‐0.67) | PBO/Sham | 45/9,087 | M | |
| Bupropion | OR=1.97 (0.19‐57.4) | PBO/Sham | 45/9,087 | M | |
| Functioning | Atomoxetine | SMD=–0.48 (–0.62 to –0.33) | PBO/Sham | 8/1,308 | M |
| Functioning: academic | Amphetamines | SMD=–0.56 (–0.73 to –0.39) | PBO/Sham | 8/826 | M |
| Global illness improvement | Amphetamines | OR=7.71 (5.52‐10.77) | PBO/Sham | 40/NR | H |
| Atomoxetine | OR=2.28 (1.38‐3.76) | PBO/Sham | 40/NR | H | |
| Guanfacine | OR=3.63 (2.36‐5.57) | PBO/Sham | 40/NR | H | |
| Methylphenidate | OR=5.57 (3.99‐7.79) | PBO/Sham | 40/NR | H | |
| Modafinil | OR=3.22 (1.91‐5.43) | PBO/Sham | 40/NR | H | |
| Clonidine | OR=2.78 (0.91‐8.53) | PBO/Sham | 40/NR | H | |
| Global illness severity | Amphetamines | SMD=–0.86 (–1.72 to –0.01) | PBO/Sham | 2/86 | M |
| Desipramine | OR=26.41 (7.41‐94.18) | PBO/Sham | 2/103 | L | |
| Quality of life | Methylphenidate | SMD=–0.61 (–0.80 to –0.42) | PBO/Sham | 3/514 | M |
| Atomoxetine | SMD=–0.39 (–0.50 to –0.28) | PBO/Sham | 16/2,361 | M | |
| Suicide attempt | Atomoxetine | RR=0.84 (0.03‐20.00) | PBO/Sham | 23/3,883 | L |
| Suicidal ideation | Atomoxetine | RR=1.67 (0.83‐3.36) | PBO/Sham | 15/2,517 | M |
| Pharmacological augmentation | |||||
| Efficacy | α2‐agonists + stimulants | SMD=–0.36 (–0.51 to –0.21) | PBO/Sham | 3/719 | M |
| Acceptability | α2‐agonists + stimulants | RR=0.74 (0.37‐1.48) | PBO/Sham | 3/726 | L |
| Tolerability | α2‐agonists + stimulants | RR=0.77 (0.05‐12.50) | PBO/Sham | 3/726 | L |
| Discontinuation due to inefficacy | α2‐agonists + stimulants | RR=0.49 (0.21‐1.13) | PBO/Sham | 3/726 | M |
| Psychosocial interventions | |||||
| Efficacy (mixed‐rated) | Social skills training | SMD=–0.39 (–0.63 to –0.15) | WL/NT | 15/2,857 | L |
| Efficacy (teacher‐rated) | Social skills training | SMD=–0.26 (–0.47 to –0.05) | WL/NT | 14/1,379 | M |
| Efficacy (parent‐rated) | Social skills training | SMD=–0.54 (–0.81 to –0.26) | WL/NT | 11/1,206 | L |
| Efficacy (clinician‐rated) | Social skills training | SMD=–3.15 (–9.88 to 3.57) | WL/NT | 2/107 | L |
| Response | Behavioral therapy | OR=2.97 (1.53‐5.88) | PBO/Sham | 113/19,398 | M |
| Cognitive training | OR=0.70 (0.12‐3.87) | PBO/Sham | 113/19,398 | M | |
| Acceptability | Behavioral therapy | OR=0.58 (0.33‐0.99) | PBO/Sham | 171/22,961 | M |
| Cognitive training | OR=1.32 (0.71‐2.52) | PBO/Sham | 171/22,961 | M | |
| Functioning: academic | Social skills training | SMD=–0.15 (–0.31 to 0.01) | WL/NT | 5/642 | M |
| Global illness severity | Behavioral therapy | OR=2.99 (1.21‐7.31) | PBO/Sham | 113/19,398 | M |
| Cognitive training | OR=0.39 (0.01‐5.80) | PBO/Sham | 113/19,398 | M | |
| Functioning: social skills (mixed‐rated) | Social skills training | SMD=–0.29 (–0.47 to –0.11) | WL/NT | 19/2,649 | L |
| Functioning: social skills (parent‐rated) | Social skills training + parental involvement | SMD=–0.43 (–0.70 to –0.15) | WL/NT | 4/337 | L |
| Social skills training | SMD=–0.19 (–0.32 to –0.06) | WL/NT | 15/1,609 | M | |
| Functioning: social skills (teacher‐rated) | Social skills training + parental involvement | SMD=–0.15 (–0.41 to 0.12) | WL/NT | 4/632 | M |
| Social skills training | SMD=–0.11 (–0.22 to 0.00) | WL/NT | 11/1,271 | M | |
| Functioning: emotional (mixed‐rated) | Social skills training | SMD=0.20 (–0.01 to 0.41) | WL/NT | 5/353 | L |
| Functioning: emotional (parent‐rated) | Social skills training | SMD=0.27 (–0.05 to 0.59) | WL/NT | 3/173 | L |
| Functioning: emotional (teacher‐rated) | Social skills training | SMD=0.02 (–0.68 to 0.72) | WL/NT | 2/129 | L |
| Brain stimulation interventions | |||||
| Response | Neurofeedback | OR=1.96 (0.52‐8.26) | PBO/Sham | 113/19,398 | M |
| Acceptability | Neurofeedback | OR=0.59 (0.31‐1.14) | PBO/Sham | 171/22,961 | M |
| Combined interventions | |||||
| Response | Methylphenidate + parent training | OR=55.63 (3.18‐29.52x10 2 ) | PBO/Sham | 113/19,398 | M |
| Methylphenidate + clonidine | OR=21.91 (5.52‐105.40) | PBO/Sham | 113/19,398 | M | |
| Atomoxetine + parent training | OR=2.48 (0.51‐11.79) | PBO/Sham | 113/19,398 | M | |
| Acceptability | Methylphenidate + clonidine | OR=0.32 (0.13‐0.77) | PBO/Sham | 171/22,961 | M |
| ADHD and disorders of intellectual development | |||||
| Efficacy | Methylphenidate | SMD=–0.88 (–1.14 to –0.61) | PBO/Sham | 8/424 | L |
| Acceptability | Methylphenidate | OR=1.68 (0.68‐4.14) | PBO/Sham | 4/215 | L |
| Tolerability | Methylphenidate | OR=4.82 (0.98‐23.63) | PBO/Sham | 4/215 | L |
| Autism spectrum disorder | |||||
| Pharmacological interventions | |||||
| Efficacy: inappropriate speech (mixed‐rated) | Aripiprazole | SMD=–0.30 (–0.50 to –0.09) | PBO/Sham | 3/400 | L |
| Efficacy: stereotypic (mixed‐rated) | Aripiprazole | SMD=–0.32 (–0.53 to–0.12) | PBO/Sham | 3/400 | M |
| Methylphenidate | SMD=–0.18 (–0.46 to 0.11) | PBO/Sham | 5/127 | M | |
| Atomoxetine | SMD=–0.16 (–0.50 to 0.18) | PBO/Sham | 4/281 | L | |
| Efficacy: overall (teacher‐rated) | Methylphenidate | SMD=–0.53 (–1.26 to 0.19) | PBO/Sham | 2/37 | L |
| Efficacy: social interaction (parent‐rated) | Methylphenidate | SMD=–0.21 (–0.6 to 0.18) | PBO/Sham | 2/90 | L |
| Efficacy: social interaction (teacher‐rated) | Methylphenidate | SMD=–0.51 (–1.07 to 0.05) | PBO/Sham | 3/103 | L |
| Efficacy: stereotypic (parent‐rated) | Methylphenidate | SMD=–0.34 (–0.84 to 0.17) | PBO/Sham | 3/NR | L |
| Efficacy: social withdrawal (mixed‐rated) | Aripiprazole | SMD=–0.13 (–0.33 to 0.08) | PBO/Sham | 3/400 | M |
| Response | Risperidone | OR=2.57 (1.35‐4.86) | PBO/Sham | 3/241 | L |
| Aripiprazole | RR=2.08 (1.24‐3.46) | PBO/Sham | 3/400 | L | |
| Aggressive behavior | Risperidone | SMD=–0.29 (–0.48 to –0.11) | PBO/Sham | 8/878 | L |
| Aripiprazole | SMD=–0.24 (–0.40 to –0.08) | PBO/Sham | 8/878 | L | |
| Valproate | SMD=–0.18 (–0.71 to 0.35) | PBO/Sham | 2/57 | M | |
| Lurasidone | SMD=–0.05 (–0.27 to 0.18) | PBO/Sham | 8/878 | L | |
| Acceptability | Risperidone | RR=0.52 (0.32‐0.86) | PBO/sham | 6/379 | M |
| Antipsychotics | RR=0.61 (0.48‐0.78) | PBO/Sham | 15/1,124 | M | |
| Aripiprazole | RR=0.67 (0.49‐0.90) | PBO/Sham | 5/526 | M | |
| Haloperidol | RR=0.80 (0.24‐2.62) | PBO/Sham | 2/60 | M | |
| Mood stabilizers | RR=1.27 (0.53‐3.06) | PBO/Sham | 5/125 | M | |
| Tolerability | Risperidone | RR=0.71 (0.17‐2.92) | PBO/Sham | 5/339 | M |
| Antipsychotics | RR=0.99 (0.55‐1.79) | PBO/Sham | 12/1,010 | M | |
| Mood stabilizers | RR=1.13 (0.36‐3.53) | PBO/Sham | 4/112 | M | |
| Aripiprazole | RR=1.24 (0.57‐2.71) | PBO/Sham | 4/493 | M | |
| Discontinuation due to inefficacy | Mood stabilizers | RR=2.11 (0.36‐12.42) | PBO/Sham | 3/60 | M |
| Global illness severity | Aripiprazole | SMD=–0.54 (–0.77 to –0.32) | PBO/Sham | 3/400 | M |
| Risperidone | OR=10.5 (4.80‐22.60) | PBO/Sham | 6/446 | L | |
| Mood stabilizers | RR=1.55 (0.39‐6.21) | PBO/Sham | 3/77 | L | |
| Relapse | Risperidone | RR=0.30 (0.13‐0.68) | PBO/Sham | 2/56 | M |
| Psychosocial interventions | |||||
| Efficacy: emotion recognition (mixed‐rated) | Computer‐assisted interaction | SMD=–0.53 (–1.12 to 0.05) | WL/NT | 2/48 | L |
| Social skills training | SMD=–0.34 (–0.88 to 0.20) | WL/NT | 2/54 | L | |
| Efficacy: social competence (mixed‐rated) | Social skills training | SMD=–0.47 (–0.78 to –0.16) | WL/NT | 4/178 | L |
| Anxiety (subject‐rated) | Cognitive behavioral therapy | SMD=–0.61 (–1.54 to 0.33) | WL/NT | 5/181 | L |
| Anxiety (parent‐rated) | Cognitive behavioral therapy | SMD=–1.12 (–1.91 to –0.34) | WL/NT | 7/244 | L |
| Functioning: joint attention | Skills training‐joint attention | SMD=–0.66 (–0.93 to –0.40) | WL/NT | 9/417 | L |
| Disruptive behavior/dissocial/conduct disorders (with or without ADHD) | |||||
| Pharmacological interventions | |||||
| Efficacy (clinician‐rated) | Risperidone | SMD=–0.48 (–0.71 to –0.24) | PBO/Sham | 4/293 | L |
| Efficacy (parent‐rated) | Risperidone | SMD=–0.79 (–1.06 to –0.52) | PBO/Sham | 2/225 | M |
| Efficacy (mixed‐rated) | Risperidone | SMD=–0.32 (–0.49 to –0.16) | PBO/Sham | 4/590 | M |
| Response: aggressive behavior | Valproate | OR=15.6 (1.91‐128.1) | PBO/Sham | 2/47 | L |
| Lithium | RR=4.56 (1.97‐10.56) | PBO/Sham | 3/116 | L | |
| Aggressive behavior (clinician‐rated) | Mixed (risperidone, quetiapine) | SMD=–0.24 (–0.76 to 0.29) | PBO/Sham | 2/57 | L |
| Aggressive behavior (parent‐rated) | Risperidone | SMD=–0.72 (–0.99 to –0.46) | PBO/Sham | 3/238 | M |
| Aggressive behavior (mixed‐rated) | Risperidone | SMD=–0.60 (–0.89 to –0.31) | PBO/Sham | 2/188 | L |
| Mixed (risperidone, lithium, methylphenidate) | SMD=–1.93 (–3.88 to 0.02) | PBO/Sham | 4/172 | L | |
| Acceptability | Mixed (risperidone, lithium, methylphenidate) | RR= 0.97 (0.60‐1.55) | PBO/Sham | 8/631 | L |
| Global illness severity | Risperidone | SMD=–1.31 ( –1.88 to –0.74) | PBO/Sham | 2/58 | L |
| Mixed (risperidone, quetiapine) | SMD=–0.30 (–0.49 to –0.12) | PBO/Sham | 5/435 | M | |
| Mixed (carbamazepine, lithium, amphetamines) | RR= 2.39 (1.10‐5.21) | PBO/Sham | 4/136 | L | |
| Psychosocial interventions | |||||
| Efficacy (parent‐rated) | Parental + child behavioral interventions | SMD=–1.00 (–1.68 to –0.32) | WL/NT | 3/207 | L |
| Intellectual disabilities and disruptive behavior/dissocial disorders (with or without ADHD) | |||||
| Aggressive behavior (clinician‐rated) | Risperidone | SMD=–1.09 (–1.39 to –0.79) | PBO/Sham | 4/257 | L |
| Aripiprazole | SMD=–0.64 (–0.91 to –0.36) | PBO/Sham | 2/308 | L | |
| Valproate | SMD=–0.06 (–0.75 to 0.63) | PBO/Sham | 2/57 | L | |
| Aggressive behavior (mixed‐rated) | Risperidone | SMD=–0.70 (–1.01 to –0.39) | PBO/Sham | 3/266 | L |
| Developmental coordination disorders | |||||
| Efficacy | Skills training | SMD=–0.27 (–0.85 to 0.31) | WL/NT | 2/51 | L |
| Tic disorder | |||||
| Efficacy: tics (clinician‐rated) | Desipramine | SMD=–0.44 (–0.91 to 0.02) | PBO/Sham | 2/75 | L |
| Methylphenidate | SMD=–0.28 (–0.58 to 0.03) | PBO/Sham | 4/191 | L | |
| Tourette's disorder | |||||
| Efficacy (clinician‐rated) | Antipsychotics (haloperidol, pimozide, risperidone, ziprasidone) | SMD=–0.74 (–1.08 to –0.41) | PBO/Sham | 4/75 | L |
| Guanfacine | SMD=–0.73 (–1.26 to –0.20) | PBO/Sham | 2/58 | L | |
| Methylphenidate | SMD=–0.17 (–0.46 to 0.11) | PBO/Sham | 4/161 | L | |
RCTs – randomized controlled trials, SMD – standardized mean difference, OR – odds ratio, RR – risk ratio, PBO – placebo, WL – waiting list, NT – no treatment, NR – not reported, Q – quality (H – high, M – medium, L – low). Bold prints indicate significant values. SMDs<0 indicate that intervention is more effective than control. For discontinuation outcomes (acceptability, tolerability, inefficacy) and relapse, OR/RR<1 favors the intervention. For response and remission, OR/RR>1 favors the intervention.
Table 6.
Efficacy and effectiveness of pharmacological, psychosocial and brain stimulation interventions vs. active psychological intervention or drug condition in children/adolescents (only significant differences are reported)
| Outcome | Intervention | Effect size (95% CI) | Control | Number of RCTs/patients | Q |
|---|---|---|---|---|---|
| Anorexia nervosa | |||||
| Efficacy: weight gain | FT | SMD=–0.44 (–0.74 to –0.14) | Other than FT | 4/178 | L |
| Anxiety disorders | |||||
| Efficacy (mixed‐rated) | CBT‐Group | SMD=–0.44 (–0.82 to –0.06) | CBT‐Individual | 101/6,625 | L |
| Attention‐deficit/hyperactivity disorder (ADHD) | |||||
|
Efficacy (clinician‐rated) |
Amphetamines | SMD=–0.24 (–0.44 to –0.05) | Methylphenidate | 46/NR | H |
| Methylphenidate | SMD=–0.22 (–0.39 to –0.05) | Atomoxetine | 46/NR | H | |
| Efficacy (parent‐rated) | Methylphenidate | SMD=–1.07 (–1.74 to –0.40) | Bupropion | 23/NR | H |
| Methylphenidate | SMD=–0.23 (–0.37 to –0.10) | Atomoxetine | 23/NR | H | |
| Response | Methylphenidate | OR=1.44 (1.08‐1.92) | Atomoxetine | 113/19,398 | M |
| Aggressive behavior | Amphetamines | SMD=–0.35 (–0.56 to –0.13) | Methylphenidate | 2/132 | L |
| Acceptability | Methylphenidate | OR=0.68 (0.52‐0.91) | Atomoxetine | 171/22,961 | M |
| Tolerability | Methylphenidate | OR=0.39 (0.18‐0.83) | Guanfacine | 60/12,188 | M |
| Discontinuation due to inefficacy | Amphetamines | OR=0.23 (0.10‐0.44) | Atomoxetine | 45/9,087 | M |
| Global illness severity | Amphetamines | OR=3.39 (1.95‐5.88) | Atomoxetine | 40/NR | H |
| Efficacy: inattention (mixed‐rated) | Neurofeedback | SMD=0.44 (0.02 to 0.86) | Stimulants | 4/161 | L |
| Acceptability | Neurofeedback | OR=0.45 (0.21‐0.95) | COG TR | 171/22,961 | M |
| Response | BT+stimulants | OR=4.76 (2.50‐9.09) | BT | 113/19,398 | M |
| BT+stimulants | OR=4.58 (2.49‐8.75) | Stimulants | 113/19,398 | M | |
| Autism spectrum disorder | |||||
| Efficacy: stereotypic (clinician‐rated) | BT‐IT | SMD=–0.78 (–1.42 to –0.13) | BT‐CI | 2/40 | L |
| Efficacy: distal social behavior (clinician‐rated) | BT‐IT | SMD=–0.98 (–1.64 to –0.32) | BT‐CI | 2/40 | L |
| Bipolar disorder, manic episode | |||||
|
Efficacy (clinician‐rated) |
Risperidone | SMD=–1.01 (–1.29 to –0.74) | Valproate | 2/228 | M |
| Enuresis | |||||
| Acceptability | Desmopressin | OR=0.45 (0.29‐0.71) | BT‐Alarm | 15/1,502 | M |
| Efficacy | BT‐Alarm | SMD= –0.43 (–0.77 to –0.08) | Desmopressin | 4/285 | L |
| Relapse | BT‐Alarm | OR=0.15 (0.03‐0.53) | Desmopressin | 12/1,381 | M |
| Efficacy |
Desmopressin+ BT‐Alarm |
SMD= –0.58 (–0.89 to –0.26) | Desmopressin | 2/156 | L |
| Response | Desmopressin+anticholinergics | OR=2.80 (1.50‐5.40) | Desmopressin | 15/1,350 | M |
| Imipramine+oxybutynin | RR=1.47 (1.09‐2.00) | Imipramine | 2/101 | L | |
| Imipramine+oxybutynin | RR=1.46 (1.06‐2.01) | Oxybutynin | 2/100 | L | |
| Desmopressin+BT‐Alarm | RR=1.32 (1.08‐1.62) | Desmopressin | 5/359 | L | |
| Relapse | Oxybutynin+ imipramine | RR=0.50 (0.30‐0.81) | Oxybutynin | 2/81 | L |
| Oxybutynin+ imipramine | RR=0.48 (0.31‐0.74) | Imipramine | 2/85 | L | |
| Depressive disorders | |||||
|
Efficacy (clinician‐rated) |
Fluoxetine | SMD=–1.65 (–2.34 to –0.95) | Nortriptyline | 70/8,906 | M |
| Response | Fluoxetine | OR=3.02 (1.04‐7.22) | Nortriptyline | 34/5,260 | M |
| Tolerability | Paroxetine | OR=0.22 (0.08‐0.87) | Imipramine | 34/5,260 | M |
| Fluoxetine | OR=0.31 (0.13‐0.95) | Duloxetine | 34/5,260 | M | |
| Suicidal ideation | CBT | SMD=–0.27 (–0.51 to –0.03) | SSRIs | 2/268 | L |
| Remission | CBT+SSRI | OR=2.15 (1.15‐4.02) | CBT+PBO | 2/173 | M |
| Functioning | CBT+SSRI | SMD=–0.20 (–0.33 to –0.08) | Standalone AD | 4/850 | L |
| Schizophrenia spectrum disorders | |||||
|
Efficacy (clinician‐rated) |
Haloperidol | SMD=–1.35 (–2.16 to –0.55) | Fluphenazine | 28/3,003 | L |
| Clozapine | SMD=–0.86 (–1.54 to –0.17) | Olanzapine | 28/3,003 | L | |
| SGAs | SMD=–0.36 (–0.56 to –0.16) | FGAs | 4/243 | L | |
| Response | Risperidone | OR=5.53 (2.01‐15.18) | Haloperidol | 28/3,003 | L |
| Tic disorder | |||||
| Response | Topiramate | RR=1.10 (1.02‐1.18) | Haloperidol/tiapride | 14/1,017 | M |
| Topiramate | RR=1.09 (1.01‐1.19) | Haloperidol | 10/727 | L | |
RCTs – randomized controlled trials, SMD – standardized mean difference, OR – odds ratio, RR – risk ratio, PBO – placebo, Q – quality (H – high, M – medium, L – low), BT – behavioral therapy, BT‐IT– behavioral therapy imitative interaction, BT‐CI – behavioral therapy contingency interaction, CBT – cognitive behavioral therapy, FT – family therapy, COG TR ‐ cognitive training, AD – antidepressant, SSRI – selective serotonin reuptake inhibitor, SGAs – second‐generation antipsychotics, FGAs – first‐generation antipsychotics, NR – not reported. SMDs<0 indicate that intervention is more effective than control. For discontinuation outcomes (acceptability, tolerability, inefficacy) and relapse, OR/RR<1 favors the intervention. For response and remission, OR/RR>1 favors the intervention.
Table 7.
Efficacy and effectiveness of pharmacological, psychosocial and brain stimulation interventions vs. mixed control conditions in children/adolescents (only significant differences are reported)
| Outcome | Intervention | Effect size (95% CI) | Control | Number of RCTs/patients | Q |
|---|---|---|---|---|---|
| Attention‐deficit/hyperactivity disorder (ADHD) | |||||
| Efficacy (mixed‐rated) | BI | SMD=–0.55 (–0.77 to –0.32) | WL/AC/LIP | 6/333 | L |
| Efficacy (probably blinded rater) | COG TR | SMD=–0.20 (–0.40 to –0.01) | Mixed | 11/566 | L |
| Efficacy (most proximal rater) | COG TR | SMD=–0.37 (–0.66 to –0.09) | Mixed | 14/727 | L |
| BT | SMD=–0.35 (–0.50 to –0.19) | Mixed | 19/1,430 | L | |
| Efficacy (teacher‐rated) | ST | SMD=–0.26 (–0.52 to –0.01) | Mixed | 6/615 | L |
| Efficacy (parent‐rated) | BT‐Parental | SMD=–0.65 (–1.05 to –0.25) | TAU/WL/LIP | 8/399 | L |
| ST | SMD=–0.56 (–0.74 to –0.38) | Mixed | 10/934 | L | |
| Aggressive behavior | BI | SMD=–0.40 (–0.71 to –0.10) | Mixed | 5/350 | L |
| Functioning: academic | ST | SMD=–0.33 (–0.51 to –0.14) | Mixed | 7/695 | L |
| BT | SMD=–0.28 (–0.59 to –0.06) | Mixed | 9/817 | L | |
| Efficacy (most proximal rater) | Neurofeedback | SMD=–0.35 (–0.59 to –0.11) | Mixed | 13/540 | M |
| Efficacy (parent‐rated) | Neurofeedback | SMD=–0.32 (p=0.013) | Mixed | 16/706 | L |
| Autism spectrum disorder | |||||
| Efficacy: socialization (mixed‐rated) | PCIT | SMD=–0.22 (–0.36 to –0.09) | Mixed | 13/846 | L |
| Efficacy: language (mixed‐rated) | PCIT | SMD=–0.16 (–0.31 to –0.02) | Mixed | 13/785 | L |
| Efficacy: language comprehension (parent‐rated) | PCIT | SMD=–0.29 (–0.56 to –0.01) | Mixed | 3/204 | L |
| Anxiety (clinician‐rated) | CBT | SMD=–1.05 (–1.65 to –0.45) | TAU/WL | 6/208 | L |
| Anxiety (parent‐rated) | CBT | SMD=–1.00 (–1.80 to –0.21) | TAU/WL | 7/283 | L |
| Aggressive behavior | PCIT | SMD = –0.67 (–0.85 to –0.49) | Mixed | 9/521 | L |
| Functioning: shared/joint attention | ST‐ToM | SMD=–0.55 (–0.99 to –0.11) | TAU/WL | 2/88 | L |
| PCIT | SMD=–0.41 (–0.68 to –0.14) | Mixed | 3/215 | L | |
| Functioning: social skills | SST‐Computer | SMD=–0.93 (–1.29 to –0.57) | TAU/WL | 5/138 | L |
| SST | SMD=–0.83 (–1.07 to –0.60) | TAU/WL | 18/1,266 | L | |
| SST‐Face to face | SMD=–0.81 (–1.08 to –0.53) | TAU/WL | 14/1,128 | L | |
| Functioning: parent synchrony | PCIT | SMD=–0.90 (–1.23 to –0.56) | Mixed | 3/244 | L |
| Global illness severity | PCIT | SMD=–0.30 (–0.52 to –0.08) | Mixed | 6/316 | L |
| Irritability | PCIT | SMD=–0.59 (–0.88 to –0.30) | Mixed | 8/653 | L |
| Depressive disorders | |||||
| Efficacy (mixed‐ rated) | CBT | SMD=–0.53 (–0.82 to –0.24) | Mixed | 11/809 | M |
| Oppositional defiant disorder (ODD) | |||||
| Efficacy (mixed‐rated) | BI | SMD=–0.79 (–0.93 to –0.64) | WL/AC | 17/NR | L |
| Tourette's disorder | |||||
| Efficacy (clinician‐rated) | BT | SMD=–0.64 (–0.99 to –0.29) | WL/LIP | 2/133 | L |
| Disruptive behavior/dissocial/conduct disorders (with or without ADHD) | |||||
|
Efficacy: ADHD symptoms (mixed‐ rated) |
BI | SMD=–0.34 (–0.64 to –0.05) | WL/AC | 11/518 | L |
| Efficacy: ADHD symptoms (parent‐rated) | BI | SMD=–0.68 (–0.91 to –0.44) | WL/AC | 5/322 | L |
| Efficacy: externalizing (mixed‐rated) | BI | SMD=–0.52 (–0.68 to –0.36) | WL/AC | 10/881 | L |
|
Efficacy: ODD symptoms (mixed‐ rated) |
BI | SMD=–0.88 (–1.24 to –0.51) | WL/AC | 10/335 | L |
| Efficacy: ODD symptoms (parent‐rated) | BI | SMD=–0.81 (–1.20 to –0.42) | WL/AC | 4/199 | L |
| Aggressive behavior | BI | SMD =–0.28 (–0.46 to –0.10) | WL/AC | 18/794 | L |
| Cognition: attention | BI | SMD=–0.38 (–0.52 to –0.23) | WL/AC | 15/588 | L |
| Functioning | BI | SMD=–0.39 (–0.52 to –0.26) | WL/AC | 22/1,027 | L |
RCTs – randomized controlled trials, SMD – standardized mean difference, WL – waiting list, AC – active control, TAU – treatment as usual, LIP – low intensity psychosocial intervention, Q – quality (H – high, M – medium, L – low), BT – behavioral therapy, CBT – cognitive behavioral therapy, COG TR – cognitive training, BI – combination of parental and child behavioral interventions, ST – skills training, PCIT – parent‐child interaction therapy, SST – social skills training, ST‐ToM – skills training: precursors of Theory of Mind, NR – not reported. SMDs<0 indicate that intervention is more effective than control.
Focusing on the two best interventions, amphetamines had the highest effect size based on the clinician‐rated primary efficacy outcome vs. placebo (large effect size), and were superior to placebo also regarding response (large effect size), aggressive behavior (large effect size), academic functioning (medium effect size), global illness severity (large effect size), and less discontinuation due to inefficacy (large effect size), without significant differences regarding all‐cause discontinuation (“acceptability”) or discontinuation due to intolerability (see Table 2).
Methylphenidate had medium to large effect sizes regarding the primary efficacy outcome vs. placebo across different raters, and was superior to placebo regarding other‐than‐attention cognition broadly (small to medium effect size), global illness improvement (large effect size), quality of life (medium effect size), acceptability (small effect size), and less discontinuation due to inefficacy (medium effect size), without significant differences concerning discontinuation due to intolerability. The efficacy of methylphenidate was also confirmed in youth with comorbid intellectual disability (see Table 2).
Clonidine, guanfacine and atomoxetine were also effective regarding the primary efficacy outcome, but with less consistent results across raters. Among psychosocial interventions, social skills training improved the primary efficacy outcome and functioning (small to medium effect size); however, the control group was waiting list/no treatment. Only behavioral therapy outperformed placebo for response (small effect size), impact on global illness severity (small effect size), and acceptability (small effect size). Neurofeedback did not show any significant efficacy outcome, nor any difference emerged on acceptability (see Table 2).
Alpha‐2 agonists were an effective augmentation strategy when added to stimulants vs. placebo (small effect size). Importantly, combined interventions, and specifically methylphenidate with parent training or with clonidine, and atomoxetine with parent training, showed large effect sizes regarding response vs. placebo (see Table 2). Additionally, behavioral therapy plus stimulants was superior both to behavioral therapy alone and to stimulants alone regarding response (large effect size), without any differences in acceptability (see Table 6).
In head‐to‐head comparisons, amphetamines outperformed methylphenidate, which outperformed bupropion (large effect sizes) and atomoxetine (small effect size) on the primary efficacy outcome. Amphetamines were superior to atomoxetine in reducing discontinuation due to inefficacy, and better than methylphenidate for aggressive behavior (small effect size), while methylphenidate was superior to atomoxetine regarding acceptability (medium effect size), and to guanfacine regarding less discontinuation due to intolerability (medium effect size). Stimulants were superior to neurofeedback regarding cognition, and neurofeedback outperformed cognitive training on acceptability (see Table 6).
Autism spectrum disorder
Results for autism spectrum disorder are shown in Tables 2, 5, 6 and 7.
Table 5.
Efficacy and effectiveness of pharmacological, psychosocial and brain stimulation interventions vs. treatment as usual (TAU) or low intensity psychosocial intervention (LIP) in children/adolescents (only significant differences are reported)
| Outcome | Intervention | Effect size (95% CI) | Control | Number of RCTs/patients | Q |
|---|---|---|---|---|---|
| Anxiety disorders | |||||
|
Efficacy (mixed‐ rated) |
CBT‐Group | SMD=–0.84 (–1.47 to –0.21) | TAU | 101/6,625 | L |
| Functioning | CBT | SMD=–1.06 (–1.57 to –0.55) | TAU/LIP/PBO/Sham | 5/467 | L |
| Remission |
CBT‐Individual+P |
OR=8.56 (3.10‐23.66) | TAU | 5/172 | L |
| Autism spectrum disorder | |||||
| Efficacy: overall (mixed‐rated) | PCIT | SMD=–0.22 (–0.41 to –0.03) | TAU/LIP | 6/420 | L |
| Efficacy: reciprocity (clinician‐rated) | Mixed psychosocial interventions | SMD=–0.53 (–0.78 to –0.29) | TAU | 8/380 | L |
| Cognition: developmental quotient | Mixed psychosocial interventions | SMD=–0.36 (–0.66 to –0.05) | TAU | 5/232 | L |
| Cognition | PCIT | SMD=–0.24 (–0.46 to –0.03) | TAU/LIP | 6/334 | L |
| Anxiety disorder remission | CBT | OR=11.25 (3.11‐40.79) | TAU | 4/142 | L |
| Depressive disorders | |||||
|
Efficacy (clinician ‐rated) |
IPT | SMD=–0.66 (–1.22 to –0.09) | TAU |
70/8,906 |
L |
| Encopresis | |||||
| Efficacy: soiling | BT+TAU | SMD=–0.35 (–0.63 to –0.07) | TAU | 4/209 | L |
| Response | BT+TAU | RR=1.78 (1.25‐2.55) | TAU | 4/216 | L |
| Obsessive‐compulsive disorder | |||||
| Response | BT‐ERP | RR=1.71 (1.29‐2.25) | TAU/LIP | 4/271 | L |
| Acceptability | BT‐ERP | RR=0.60 (0.39‐0.93) | TAU/LIP | 4/251 | L |
RCTs – randomized controlled trials, SMD – standardized mean difference, OR – odds ratio, RR – risk ratio, PBO – placebo, Q – quality (H – high, M – medium, L – low), BT – behavioral therapy, BT‐ERP – behavioral therapy with exposure and response prevention, CBT – cognitive behavioral therapy, IPT – interpersonal therapy, PCIT – parent‐child interaction therapy, P – parental involvement. SMDs<0 indicate that intervention is more effective than control. For discontinuation outcomes (acceptability, tolerability, inefficacy) and relapse, OR/RR<1 favors the intervention. For response and remission, OR/RR>1 favors the intervention.
Aripiprazole was superior to placebo regarding the primary efficacy outcome, as well as response, aggressive behavior, global illness severity, and acceptability (all small effect sizes). Risperidone showed the same profile, yet with a large effect size regarding response. Both aripiprazole and risperidone were not different from placebo concerning discontinuation due to intolerability (see Table 2).
Among psychosocial interventions, social skills training had a small to large effect size regarding the primary efficacy outcome and functioning, and CBT had a large effect concerning anxiety across different control groups (see Table 2). Parent‐child interaction therapy and other mixed psychosocial interventions had a small to medium effect size for the primary efficacy outcome vs. TAU, as well as a small effect regarding cognition. Parent‐child interaction therapy also improved aggression (medium effect size), irritability (medium effect size), and functioning (large effect size). Finally, behavioral therapy with an imitative component had a large effect size for the primary efficacy outcome against other active psychosocial interventions without the imitative component (see Tables 5, 6 and 7).
Depressive disorders
Results for depressive disorders are shown in Tables 3, 5, 6 and 7.
Table 3.
Efficacy and effectiveness of pharmacological, psychosocial and brain stimulation interventions vs. inactive control in children/adolescents with schizophrenia spectrum, depressive, and bipolar disorders
| Outcome | Intervention | Effect size (95% CI) | Control | Number of RCTs/patients | Q |
|---|---|---|---|---|---|
| Schizophrenia spectrum disorders | |||||
| Efficacy (clinician‐rated) | Olanzapine | SMD=–0.74 (–1.05 to –0.44) | PBO/Sham | 28/3,003 | L |
| Risperidone | SMD=–0.62 (–0.89 to –0.34) | PBO/Sham | 28/3,003 | L | |
| Lurasidone | SMD=–0.48 (–0.71 to –0.25) | PBO/Sham | 28/3,003 | M | |
| Aripiprazole | SMD=–0.43 (–0.63 to –0.24) | PBO/Sham | 28/3,003 | M | |
| Quetiapine | SMD=–0.42 (–0.65 to –0.19) | PBO/Sham | 28/3,003 | M | |
| Paliperidone | SMD=–0.42 (–0.66 to –0.18) | PBO/Sham | 28/3,003 | L | |
| Asenapine | SMD=–0.38 (–0.66 to –0.11) | PBO/Sham | 28/3,003 | M | |
| Ziprasidone | SMD=–0.14 (–0.40 to 0.11) | PBO/Sham | 28/3,003 | L | |
| Response | Risperidone | OR=3.46 (1.92‐6.23) | PBO/Sham | 28/3,003 | L |
| Olanzapine | OR=2.64 (1.07‐4.18) | PBO/Sham | 28/3,003 | L | |
| Lurasidone | OR=2.56 (1.45‐4.48) | PBO/Sham | 28/3,003 | M | |
| Paliperidone | OR=2.12 (1.07‐4.18) | PBO/Sham | 28/3,003 | L | |
| Quetiapine | OR=1.86 (1.03‐3.32) | PBO/Sham | 28/3,003 | M | |
| Asenapine | OR=1.73 (0.96‐3.10) | PBO/Sham | 28/3,003 | M | |
| Global illness severity | Olanzapine | SMD=–0.6 (–1.18 to –0.02) | PBO/Sham | 13/2,210 | M |
| Risperidone | SMD=–0.50 (–0.73 to –0.27) | PBO/Sham | 12/2,158 | L | |
| Paliperidone | SMD=–0.44 (–0.67 to –0.22) | PBO/Sham | 12/2,158 | L | |
| Lurasidone | SMD=–0.41 (–0.77 to –0.05) | PBO/Sham | 13/2,210 | M | |
| Quetiapine | SMD=–0.41 (–0.77 to –0.05) | PBO/Sham | 13/2,210 | M | |
| Ziprasidone | SMD=–0.40 (–0.68 to –0.12) | PBO/Sham | 13/2,210 | M | |
| Aripiprazole | SMD=–0.35 (–0.59 to –0.11) | PBO/Sham | 13/2,210 | M | |
| Asenapine | SMD=–0.29 (–0.53 to –0.06) | PBO/Sham | 13/2,210 | M | |
| Acceptability | Paliperidone | OR=0.26 (0.08‐0.80) | PBO/Sham | 28/3,003 | L |
| Risperidone | OR=0.31 (0.14‐0.72) | PBO/Sham | 28/3,003 | L | |
| Olanzapine | OR=0.36 (0.15‐0.85) | PBO/Sham | 28/3,003 | L | |
| Lurasidone | OR=0.53 (0.18‐1.55) | PBO/Sham | 28/3,003 | M | |
| Ziprasidone | OR=0.59 (0.22‐1.58) | PBO/Sham | 28/3,003 | L | |
| Quetiapine | OR=0.63 (0.27‐1.43) | PBO/Sham | 28/3,003 | M | |
| Asenapine | OR=0.91 (0.33‐2.56) | PBO/Sham | 28/3,003 | M | |
| Aripiprazole | OR=1.48 (0.60‐3.67) | PBO/Sham | 28/3,003 | M | |
| Tolerability | Lurasidone | OR=0.45 (0.16‐1.22) | PBO/Sham | 13/2,210 | M |
| Ziprasidone | OR=0.99 (0.45‐2.30) | PBO/Sham | 13/2,210 | M | |
| Risperidone | OR=2.38 (0.57‐13.56) | PBO/Sham | 13/2,210 | M | |
| Aripiprazole | OR=2.54 (0.70‐14.48) | PBO/Sham | 13/2,210 | M | |
| Asenapine | OR=2.67 (0.82‐12.47) | PBO/Sham | 13/2,210 | M | |
| Quetiapine | OR=3.29 (0.92‐16.75) | PBO/Sham | 13/2,210 | M | |
| Olanzapine | OR=7.76 (1.23‐87.44) | PBO/Sham | 13/2,210 | M | |
| Paliperidone | OR=23.12 (2.38‐778.70) | PBO/Sham | 13/2,210 | M | |
| Discontinuation due to inefficacy | Paliperidone | OR=0.10 (0.04‐0.28) | PBO/Sham | 28/3,003 | L |
| Olanzapine | OR=0.14 (0.06‐0.31) | PBO/Sham | 28/3,003 | L | |
| Risperidone | OR=0.17 (0.07‐0.42) | PBO/Sham | 28/3,003 | L | |
| Ziprasidone | OR=0.41 (0.20‐0.84) | PBO/Sham | 28/3,003 | L | |
| Lurasidone | OR=0.39 (0.09‐1.77) | PBO/Sham | 28/3,003 | M | |
| Asenapine | OR=0.63 (0.23‐1.73) | PBO/Sham | 28/3,003 | M | |
| Depressive disorders | |||||
| Pharmacological interventions | |||||
| Efficacy (clinician‐rated) | Fluoxetine | SMD=–0.51 (–0.84 to –0.18) | PBO/Sham | 70/8,906 | M |
| Desipramine | SMD=–0.43 (–1.26 to 0.39) | PBO/Sham | 70/8,906 | M | |
| Duloxetine | SMD = –0.22 (–0.85 to 0.42) | PBO/Sham | 70/8,906 | M | |
| Venlafaxine | SMD = –0.25 (–0.87 to 0.36) | PBO/Sham | 70/8,906 | M | |
| Mirtazapine | SMD = –0.23 (–0.97 to 0.51) | PBO/Sham | 70/8,906 | M | |
| Citalopram | SMD=–0.18 (–0.89 to 0.55) | PBO/Sham | 70/8,906 | M | |
| Escitalopram | SMD=–0.17 (–0.88 to 0.54) | PBO/Sham | 70/8,906 | M | |
| Paroxetine | SMD=–0.16 (–0.67 to 0.35) | PBO/Sham | 70/8,906 | M | |
| Nefazodone | SMD=–0.14 (–0.85 to 0.57) | PBO/Sham | 70/8,906 | M | |
| Desvenlafaxine | SMD=–0.12 (–0.79 to 0.54) | PBO/Sham | 70/8,906 | M | |
| Sertraline | SMD=–0.11 (–0.71 to 0.49) | PBO/Sham | 70/8,906 | M | |
| Imipramine | SMD=–0.03 (–0.75 to 0.68) | PBO/Sham | 70/8,906 | M | |
| Vilazodone | SMD=–0.09 (–1.09 to 0.90) | PBO/Sham | 70/8,906 | M | |
| Amitriptyline | SMD=0.08 (–1.11 to 1.27) | PBO/Sham | 70/8,906 | M | |
| Nortriptyline | SMD= 1.14 (0.46‐1.81) | PBO/Sham | 70/8,906 | M | |
| Response | Nefazodone | OR=2.1 (1.06‐4.89) | PBO/Sham | 34/5,260 | M |
| Duloxetine | OR=1.74 (1.12‐2.84) | PBO/Sham | 34/5,260 | M | |
| Fluoxetine | OR=1.70 (1.25‐2.39) | PBO/Sham | 34/5,260 | M | |
| Desipramine | OR=1.59 (0.67‐4.84) | PBO/Sham | 34/5,260 | M | |
| Escitalopram | OR=1.53 (0.96‐2.58) | PBO/Sham | 34/5,260 | M | |
| Sertraline | OR=1.44 (0.79‐2.97) | PBO/Sham | 34/5,260 | M | |
| Paroxetine | OR=1.3 (0.89‐1.99) | PBO/Sham | 34/5,260 | M | |
| Venlafaxine | OR=1.16 (0.72‐2.03) | PBO/Sham | 34/5,260 | M | |
| Citalopram | OR=1.02 (0.62‐1.82) | PBO/Sham | 34/5,260 | M | |
| Imipramine | OR=0.83 (0.48‐1.54) | PBO/Sham | 34/5,260 | M | |
| Nortriptyline | OR=0.57 (0.24‐1.64) | PBO/Sham | 34/5,260 | M | |
| Amitriptyline | OR=0.22 (0.05‐2.78) | PBO/Sham | 34/5,260 | M | |
| Acceptability | Nefazodone | OR=0.49 (0.21‐1.39) | PBO/Sham | 66/9,075 | M |
| Vilazodone | OR=0.59 (0.27‐1.54) | PBO/Sham | 66/9,075 | M | |
| Nortriptyline | OR=0.76 (0.28‐3.41) | PBO/Sham | 66/9,075 | M | |
| Fluoxetine | OR=0.78 (0.56‐1.15) | PBO/Sham | 66/9,075 | M | |
| Mirtazapine | OR=0.83 (0.40‐2.08) | PBO/Sham | 66/9,075 | M | |
| Desvenlafaxine | OR=0.85 (0.47‐1.74) | PBO/Sham | 66/9,075 | M | |
| Citalopram | OR=0.96 (0.52‐1.97) | PBO/Sham | 66/9,075 | M | |
| Duloxetine | OR=1.04 (0.62‐1.96) | PBO/Sham | 66/9,075 | M | |
| Venlafaxine | OR=1.12 (0.53‐2.70) | PBO/Sham | 66/9,075 | M | |
| Amitriptyline | OR=1.16 (0.29‐12.13) | PBO/Sham | 66/9,075 | M | |
| Paroxetine | OR=1.3 (0.81‐2.27) | PBO/Sham | 66/9,075 | M | |
| Escitalopram | OR=1.4 (0.77‐2.86) | PBO/Sham | 66/9,075 | M | |
| Sertraline | OR=162 (0.83‐3.22) | PBO/Sham | 66/9,075 | M | |
| Desipramine | OR=2.21 (0.88‐7.67) | PBO/Sham | 66/9,075 | M | |
| Imipramine | OR=2.51 (1.26‐6.25) | PBO/Sham | 66/9,075 | M | |
| Tolerability | Amitriptyline | OR=0.10 (0.02‐32.16) | PBO/Sham | 34/5,260 | M |
| Fluoxetine | OR=1.03 (0.5‐2.7) | PBO/Sham | 34/5,260 | M | |
| Citalopram | OR=1.13 (0.45‐3.66) | PBO/Sham | 34/5,260 | M | |
| Nefazodone | OR=1.29 (0.3‐21.89) | PBO/Sham | 34/5,260 | M | |
| Mirtazapine | OR=1.36 (0.41‐10.99) | PBO/Sham | 34/5,260 | M | |
| Paroxetine | OR=1.59 (0.77‐3.95) | PBO/Sham | 34/5,260 | M | |
| Escitalopram | OR=1.64 (0.46‐13.49) | PBO/Sham | 34/5,260 | M | |
| Desipramine | OR=2.85 (0.83‐21.8) | PBO/Sham | 34/5,260 | M | |
| Sertraline | OR=2.94 (0.94‐17.19) | PBO/Sham | 34/5,260 | M | |
| Duloxetine | OR=2.80 (1.20‐9.42) | PBO/Sham | 34/5,260 | M | |
| Venlafaxine | OR=3.19 (1.01‐18.7) | PBO/Sham | 34/5,260 | M | |
| Imipramine | OR=5.49 (1.96‐20.86) | PBO/Sham | 34/5,260 | M | |
| Quality of life | Mixed (fluoxetine, paroxetine, sertraline) | SMD=–0.11 (–0.26 to 0.03) | PBO/Sham | 3/765 | M |
| Relapse | SSRIs | OR=0.34 (0.18‐0.64) | PBO/Sham | 3/164 | L |
| Remission | Fluoxetine | RR=1.82 (1.25‐2.63) | PBO/Sham | 2/315 | M |
| Sertraline | RR=1.09 (0.72‐1.61) | PBO/Sham | 2/376 | M | |
| Suicide attempt/ideation | Nefazodone | OR=0.29 (0.06‐6.31) | PBO/Sham | 34/NR | M |
| Mirtazapine | OR=0.53 (0.10‐40.83) | PBO/Sham | 34/NR | M | |
| Imipramine | OR=0.59 (0.19‐3.07) | PBO/Sham | 34/NR | M | |
| Desvenlafaxine | OR=0.74 (0.41‐1.49) | PBO/Sham | 34/NR | M | |
| Escitalopram | OR=0.94 (0.44‐2.55) | PBO/Sham | 34/NR | M | |
| Duloxetine | OR=0.93 (0.55‐1.71) | PBO/Sham | 34/NR | M | |
| Fluoxetine | OR=1.11 (0.74‐1.75) | PBO/Sham | 34/NR | M | |
| Paroxetine | OR=1.71 (0.81‐5.05) | PBO/Sham | 34/NR | M | |
| Citalopram | OR=1.18 (0.46‐4.43) | PBO/Sham | 34/NR | M | |
| Vilazodone | OR=1.96 (0.45‐100.00) | PBO/Sham | 34/NR | M | |
| Sertraline | OR=2.22 (0.75‐12.5) | PBO/Sham | 34/NR | M | |
| Venlafaxine | OR=8.33 (1.92‐NC) | PBO/Sham | 34/NR | M | |
| Psychosocial interventions | |||||
| Efficacy (clinician‐rated) | IPT | SMD=–1.37 (–2.04 to –0.7) | WL/NT | 70/8,906 | L |
| PSOLV | SMD=–1.26 (–2.48 to –0.03) | WL/NT | 70/8,906 | L | |
| FT | SMD=–1.03 (–1.66 to –0.4) | WL/NT | 70/8,906 | L | |
| CBT | SMD=–0.94 (–1.40 to –0.48) | WL/NT | 70/8,906 | L | |
| IPT | SMD=–0.70 (–1.29 to –0.12) | PBO/Sham | 70/8,906 | L | |
| FT | SMD=–0.36 (–0.95 to 0.24) | PBO/Sham | 70/8,906 | L | |
| CBT | SMD=–0.27 (–0.72 to 0.18) | PBO/Sham | 70/8,906 | L | |
| PSD‐O | SMD=0.08 (–0.67 to 0.84) | PBO/Sham | 70/8,906 | L | |
| Response | PSD‐O | RR=1.68 (1.08‐2.63) | WL/PBO/Sham | 2/83 | L |
| Acceptability | IPT | OR=0.53 (0.20‐1.15) | PBO/Sham | 66/9,075 | M |
| IPT | OR=0.65 (0.19‐1.62) | WL/NT | 66/9,075 | M | |
| CBT | OR=0.65 (0.32‐1.16) | PBO/Sham | 66/9,075 | M | |
| PSOLV | OR=0.77 (0.01‐4.40) | WL/NT | 66/9,075 | M | |
| CBT | OR=0.77 (0.34‐1.48) | WL/NT | 66/9,075 | M | |
| FT | OR=0.84 (0.35‐1.72) | PBO/Sham | 66/9,075 | M | |
| PSD‐O | OR=0.96 (0.37‐1.93) | PBO/Sham | 66/9,075 | M | |
| BT | OR=1.27 (0.19‐4.32) | PBO/Sham | 66/9,075 | M | |
| Suicide attempt/ideation | IPT | OR=0.64 (0.04‐2.59) | PBO/Sham | 34/NR | M |
| CBT | OR=11.31 (0.01‐46.11) | PBO/Sham | 34/NR | M | |
| PSD‐O | OR=8.64 (0.01‐40.05) | PBO/Sham | 34/NR | M | |
| Combination interventions | |||||
| Efficacy (clinician‐rated) |
Fluoxetine+CBT |
SMD=–0.73 (–1.39 to –0.07) | PBO/Sham | 70/8,906 | M |
| Acceptability |
Fluoxetine+CBT |
OR=0.75 (0.39‐1.65) | PBO/Sham | 66/9,075 | M |
| Suicide attempt/ideation |
Fluoxetine+CBT |
OR=0.88 (0.41‐2.35) | PBO/Sham | 34/NR | M |
| Bipolar disorder, depressive episode | |||||
| Efficacy (clinician‐rated) | Quetiapine | SMD=–0.10 (–0.32 to 0.13) | PBO/Sham | 2/224 | M |
| Response | Quetiapine | RR=1.1 (0.89‐1.35) | PBO/Sham | 3/250 | L |
| Acceptability | Quetiapine | RR=0.73 (0.36‐1.49) | PBO/Sham | 2/225 | L |
| Global illness severity | Quetiapine | SMD=–0.20 (–0.46 to –0.06) | PBO/Sham | 2/224 | M |
| Remission | Quetiapine | RR=1.23 (0.90‐1.68) | PBO/Sham | 3/250 | L |
| Tolerability | Quetiapine | RR=0.31 (0.11‐1.01) | PBO/Sham | 2/225 | L |
| Bipolar disorder, manic episode | |||||
|
Efficacy (clinician‐rated) |
Aripiprazole | SMD=–1.08 (–1.32 to –0.85) | PBO/Sham | 2/339 | M |
| Response | Mixed (mood stabilizers and antipsychotics) | OR=2.24 (z=8.12, p<0.001) | PBO/Sham | 9/1,362 | M |
| Aripiprazole | RR=1.86 (1.43‐2.43) | PBO/Sham | 2/332 | M | |
| SGAs | z=10.34, p<0.001 | PBO/Sham | 6/1,190 | H | |
| Mood stabilizers | z=2.06, p=0.04 | PBO/Sham | 2/172 | M | |
| Acceptability | Aripiprazole | RR=0.80 (0.51‐1.27) | PBO/Sham | 2/339 | M |
| Valproate | OR=1.77 (0.83‐3.78) | PBO/Sham | 2/179 | M | |
| Tolerability | Aripiprazole | RR=5.19 (0.92‐29.25) | PBO/Sham | 2/339 | M |
| Discontinuation due to inefficacy | Aripiprazole | RR=0.27 (0.09‐0.82) | PBO/Sham | 2/339 | M |
RCTs – randomized controlled trials, SMD – standardized mean difference, OR – odds ratio, RR – risk ratio, PBO – placebo, WL – waiting list, NT – no treatment, NR – not reported, NC – not calculable, Q – quality (H – high, M – medium, L – low), BT – behavioral therapy, CBT – cognitive behavioral therapy, FT – family therapy, IPT – interpersonal therapy, PSD‐O – psychodynamic‐oriented, PSOLV – problem solving, SSRIs – selective serotonin reuptake inhibitors, SGAs – second‐generation antipsychotics. Bold prints indicate significant values. SMDs<0 indicate that intervention is more effective than control. For discontinuation outcomes (acceptability, tolerability, inefficacy) and relapse, OR/RR<1 favors the intervention. For response and remission, OR/RR>1 favors the intervention.
Fluoxetine was the only pharmacological intervention that was superior to placebo on the primary efficacy outcome (medium effect size), as well as on response and remission (both small effect size). Nortriptyline worsened the primary efficacy outcome (large effect size), imipramine increased all‐cause drop‐out (small effect size), and imipramine, venlafaxine and duloxetine increased discontinuation due to intolerability (small to medium effect size). Venlafaxine increased suicidality (large effect size) (see Table 3).
Among psychosocial interventions, a large effect size on the primary efficacy outcome was apparent for interpersonal therapy, problem‐solving therapy, family therapy, and CBT vs. waiting list/no treatment. However, these results were not confirmed vs. placebo or vs. TAU, except for interpersonal therapy, that remained superior when compared to placebo and TAU (medium effect size) (see Tables 3 and 5).
CBT was also superior to mixed interventions regarding the primary efficacy outcome (medium effect size), and to selective serotonin reuptake inhibitors (SSRIs) regarding suicidality (small effect size) (see Tables 3 and 6). Psychodynamically‐oriented psychotherapy had a small effect size advantage regarding response, but no significant effect on the primary efficacy outcome vs. placebo (see Table 3).
As a combination treatment, CBT plus fluoxetine had a medium effect size advantage regarding the primary efficacy outcome vs. placebo (see Table 3), and CBT plus SSRI was superior concerning remission vs. CBT monotherapy, and functioning vs. antidepressant monotherapy (small effect size) (see Table 6).
Enuresis
Results for enuresis are shown in Tables 4 and 6.
Table 4.
Efficacy and effectiveness of pharmacological, psychosocial and brain stimulation interventions vs. inactive control in children/adolescents with anxiety, obsessive‐compulsive, stress‐related, and mixed disorders
| Outcome | Intervention | Effect size (95% CI) | Control | Number of RCTs/patients | Q |
|---|---|---|---|---|---|
| Anxiety disorders | |||||
| Pharmacological interventions | |||||
|
Efficacy (clinician‐rated) |
Paroxetine | SMD=–0.43 (–0.75 to –0.10) | PBO/Sham | 14/2,502 | M |
| Fluvoxamine | SMD=–0.36 (–0.61 to –0.10) | PBO/Sham | 14/2,502 | M | |
| Imipramine | SMD=–0.27 (–0.92 to 0.39) | PBO/Sham | 14/2,502 | M | |
| Guanfacine | SMD=–0.13 (–0.39 to 0.12) | PBO/Sham | 14/2,502 | M | |
| Fluoxetine | SMD=–0.11 (–0.33 to 0.12) | PBO/Sham | 14/2,502 | M | |
| Atomoxetine | SMD=–0.11 (–0.38 to 0.16) | PBO/Sham | 14/2,502 | M | |
| Duloxetine | SMD=–0.09 (–0.27 to 0.09) | PBO/Sham | 14/2,502 | M | |
| Sertraline | SMD=–0.08 (–0.25 to 0.09) | PBO/Sham | 14/2,502 | M | |
| Venlafaxine | SMD=–0.06 (–0.22 to 0.04) | PBO/Sham | 14/2,502 | M | |
| Efficacy (subject‐rated) | Fluoxetine | SMD=–0.51 (–0.85 to –0.18) | PBO/Sham | 2/154 | M |
| SNRIs | SMD=–2.14 (–9.75 to 5.48) | PBO/Sham | 3/622 | M | |
| Venlafaxine | SMD=–1.71 (–3.93 to 0.51) | PBO/Sham | 2/443 | M | |
| SSRIs | SMD=–0.42 (–0.96 to 0.12) | PBO/Sham | 4/197 | M | |
| Atomoxetine | SMD=–0.29 (–0.51 to 0.08) | PBO/Sham | 2/331 | M | |
| TCAs | SMD= 0.36 (–0.27 to 0.99) | PBO/Sham | 2/41 | M | |
| Efficacy (parent‐rated) | SSRIs | SMD=–0.82 (–1.38 to –0.27) | PBO/Sham | 2/96 | L |
| Response | Fluvoxamine | OR=8.17 (1.35‐49.40) | PBO/Sham | 19/2,656 | M |
| Sertraline | OR=6.05 (2.23‐49.40) | PBO/Sham | 19/2,656 | M | |
| Fluoxetine | OR=4.06 (1.49‐18.17) | PBO/Sham | 19/2,656 | M | |
| Guanfacine | OR=5.47 (0.74‐49.40) | PBO/Sham | 19/2,656 | M | |
| Atomoxetine | OR=4.06 (0.67‐24.53) | PBO/Sham | 19/2,656 | M | |
| Paroxetine | OR=3.67 (0.67‐20.09) | PBO/Sham | 19/2,656 | M | |
| Imipramine | OR=3.00 (0.61‐14.88) | PBO/Sham | 19/2,656 | M | |
| Venlafaxine | OR=2.46 (0.90‐6.69) | PBO/Sham | 19/2,656 | M | |
| Duloxetine | OR=2.01 (0.37‐11.02) | PBO/Sham | 19/2,656 | M | |
| Clomipramine | OR=1.22 (0.22‐6.69) | PBO/Sham | 19/2,656 | M | |
| Acceptability | Clomipramine | OR=0.55 (0.02‐7.39) | PBO/Sham | 20/2,679 | M |
| Paroxetine | OR=0.61 (0.12‐3.32) | PBO/Sham | 20/2,679 | M | |
| Fluvoxamine | OR=0.67 (0.11‐4.06) | PBO/Sham | 20/2,679 | M | |
| Sertraline | OR=0.67 (0.14‐2.72) | PBO/Sham | 20/2,679 | M | |
| Guanfacine | OR=0.67 (0.10‐4.95) | PBO/Sham | 20/2,679 | M | |
| Atomoxetine | OR=0.82 (0.15‐4.95) | PBO/Sham | 20/2,679 | M | |
| Duloxetine | OR=1.00 (0.18‐5.47) | PBO/Sham | 20/2,679 | M | |
| Venlafaxine | OR=1.11 (0.33‐3.67) | PBO/Sham | 20/2,679 | M | |
| Fluoxetine | OR=1.65 (0.50‐6.69) | PBO/Sham | 20/2,679 | M | |
| Imipramine | OR=2.01 (0.37‐9.97) | PBO/Sham | 20/2,679 | M | |
| Remission | Fluoxetine | RR=2.52 (1.19‐5.32) | PBO/Sham | 2/95 | L |
|
Suicide attempt/ ideation |
Sertraline | LogOR=–19.8 (–61.7 to 0.7) | PBO/Sham | 9/1,648 | M |
| Duloxetine | LogOR=0.2 (–2.5 to 2.8) | PBO/Sham | 9/1,648 | M | |
| Venlafaxine | LogOR=1.4 (–1.4 to 5.24) | PBO/Sham | 9/1,648 | M | |
| Atomoxetine | LogOR=6.6 (–31.6 to 22.7) | PBO/Sham | 9/1,648 | M | |
| Guanfacine | LogOR=16.1 (–1.0 to 58.3) | PBO/Sham | 9/1,648 | M | |
| Imipramine | LogOR=17.3 (–0.1 to 54.8) | PBO/Sham | 9/1,648 | M | |
| Paroxetine | LogOR=20.0 (1.7 to 60.47) | PBO/Sham | 9/1,648 | M | |
| Tolerability | Venlafaxine | LogOR=–0.8 (–3.8 to 2.1) | PBO/Sham | 15/2,516 | M |
| Atomoxetine | LogOR=0.0 (–5.3 to 5.3) | PBO/Sham | 15/2,516 | M | |
| Duloxetine | LogOR=0.2 (–3.9 to 4.3) | PBO/Sham | 15/2,516 | M | |
| Sertraline | LogOR=1.7 (–2.8 to 6.6) | PBO/Sham | 15/2,516 | M | |
| Paroxetine | LogOR=1.7 (–2.5 to 6.0) | PBO/Sham | 15/2,516 | M | |
| Fluovoxamine | LogOR=2.1 (–2.4 to 7.0) | PBO/Sham | 15/2,516 | M | |
| Fluoxetine | LogOR=2.5 (–1.8 to 7.9) | PBO/Sham | 15/2,516 | M | |
| Imipramine | LogOR=16.6 (–37.5 to 83.7) | PBO/Sham | 15/2,516 | M | |
| Guanfacine | LogOR=29.2 (2.2‐94.3) | PBO/Sham | 15/2,516 | M | |
| Psychosocial interventions | |||||
|
Efficacy (clinician ‐rated) |
CBT/BT | SMD=–0.85 (–1.12 to –0.57) | WL/NT | 7/358 | L |
| Efficacy (subject‐rated) | CBT‐Child only | SMD=–1.04 (–1.41 to –0.67) | WL/NT | 24/1,239 | L |
| CBT‐Group | SMD=–0.91 (–1.22 to –0.60) | WL/NT | 27/1,268 | L | |
| CBT | SMD=–0.67 (–0.88 to –0.47) | WL/NT | 45/2,831 | L | |
| CBT‐Child+P | SMD=–0.45 (–0.67 to –0.23) | WL/NT | 20/1,285 | L | |
| CBT‐Individual | SMD=–0.39 (–0.64 to –0.15) | WL/NT | 21/1,203 | L | |
| CBT | SMD=–0.31 (–0.51 to –0.11) | PBO/Sham | 15/978 | L | |
| CBT‐Parent only | SMD=0.04 (–0.38 to 0.46) | WL/NT | 5/307 | L | |
| Efficacy (parent‐rated) | CBT‐Group | SMD=–0.92 (–1.21 to –0.62) | WL/NT | 21/1,279 | L |
| CBT‐Child only | SMD=–0.87 (–1.21 to –0.53) | WL/NT | 13/734 | L | |
| CBT | SMD=–0.70 (–0.90 to –0.51) | WL/NT | 35/2137 | L | |
| CBT‐Child+P | SMD=–0.69 (–0.98 to –0.39) | WL/NT | 17/1,031 | L | |
| CBT‐Individual | SMD=–0.43 (–0.65 to –0.21) | WL/NT | 17/858 | L | |
| CBT‐Parent only | SMD=–0.37 (–0.77 to 0.04) | WL/NT | 5/372 | L | |
| CBT | SMD=–0.25 (–0.61 to 0.11) | PBO/Sham | 8/638 | L | |
| Efficacy (mixed‐rated) | BT‐Group | SMD=–1.43 (–2.36 to –0.51) | WL/NT | 101/6,625 | L |
| CBT‐Group | SMD=–1.43 (–1.76 to –1.09) | WL/NT | 101/6,625 | L | |
| BT‐Individual+P | SMD=–1.09 (–1.93 to –0.25) | WL/NT | 101/6,625 | L | |
| CBT‐Group+P | SMD=–0.99 (–1.31 to –0.68) | WL/NT | 101/6,625 | L | |
| CBT‐Individual | SMD=–0.99 (–1.30 to –0.68) | WL/NT | 101/6,625 | L | |
| CBT‐Individual+P | SMD=–0.84 (–1.16 to –0.53) | WL/NT | 101/6,625 | L | |
| CBT‐Group | SMD=–0.76 (–1.16 to –0.36) | PBO/Sham | 101/6,625 | L | |
| CBT‐Parent only | SMD=–0.70 (–1.22 to –0.19) | WL/NT | 101/6,625 | L | |
| CBT‐Internet | SMD=–0.61 (–1.02 to –0.20) | WL/NT | 101/6,625 | L | |
| BT‐Individual+Group | SMD=–0.73 (–1.59 to 0.13) | WL/NT | 101/6,625 | L | |
| CBT‐Individual+Group | SMD=–0.64 (–1.69 to 0.41) | WL/NT | 101/6,625 | L | |
| BT‐Individual+P | SMD=–0.42 (–1.29 to 0.44) | PBO/Sham | 101/6,625 | L | |
| CBT‐Group+P | SMD=–0.33 (–0.78 to 0.13) | PBO/Sham | 101/6,625 | L | |
| CBT‐Individual | SMD=–0.32 (–0.72 to 0.07) | PBO/Sham | 101/6,625 | L | |
| CBT‐Individual+P | SMD=–0.18 (–0.61 to 0.25) | PBO/Sham | 101/6,625 | L | |
| BT‐Individual+Group | SMD=–0.06 (–0.94 to 0.82) | PBO/Sham | 101/6,625 | L | |
| CBT‐Internet | SMD=0.06 (–0.48 to 0.60) | PBO/Sham | 101/6,625 | L | |
| Acceptability | CBT‐Individual+Group | OR=0.26 (0.05‐5.73) | WL/NT | 101/6,625 | L |
| BT‐Individual+P | OR=0.64 (0.22‐2.72) | WL/NT | 101/6,625 | L | |
| BT‐Individual+P | OR=0.81 (0.19‐2.27) | PBO/Sham | 101/6,625 | L | |
| CBT‐Group+P | OR=0.90 (0.46‐1.60) | PBO/Sham | 101/6,625 | L | |
| CBT‐Group | OR=0.85 (0.46‐1.44) | PBO/Sham | 101/6,625 | L | |
| BT | OR=0.90 (0.32‐3.95) | WL/NT | 101/6,625 | M | |
| CBT‐Individual | OR=0.92 (0.52‐1.52) | PBO/Sham | 101/6,625 | L | |
| CBT‐Group | OR=0.93 (0.57‐1.63) | WL/NT | 101/6,625 | L | |
| CBT | OR=1.09 (0.85‐1.41) | WL/NT | 45/3,158 | L | |
| CBT‐Group+P | OR=0.99 (0.67‐1.55) | WL/NT | 101/6,625 | M | |
| CBT | OR=1.00 (0.68‐1.49) | PBO/Sham | 12/797 | L | |
| CBT‐Internet | OR=1.02 (0.42‐2.08) | PBO/Sham | 101/6,625 | L | |
| CBT‐Individual | OR=1.02 (0.67‐1.67) | WL/NT | 101/6,625 | L | |
| CBT‐Internet | OR=1.05 (0.59‐2.05) | WL/NT | 101/6,625 | L | |
| CBT‐Individual+P | OR=1.11 (0.60‐1.90) | PBO/Sham | 101/6,625 | L | |
| BT‐Individual+Group | OR=1.13 (0.28‐3.19) | PBO/Sham | 101/6,625 | L | |
| BT‐Group | OR=1.21 (0.27‐22.51) | WL/NT | 101/6,625 | L | |
| CBT‐Individual+P | OR=1.23 (0.80‐2.02) | WL/NT | 101/6,625 | L | |
| CBT‐Parent only | OR=1.43 (0.75‐3.15) | WL/NT | 101/6,625 | L | |
| Depressive symptoms | CBT | SMD=–0.34 (–0.51 to –0.17) | WL/NT | 17/1,157 | L |
| CBT | SMD=–0.18 (–0.45 to 0.09) | PBO/Sham | 10/613 | L | |
| Functioning | CBT | SMD=–1.03 (–1.38 to –0.68) | WL/NT | 11/557 | L |
| Quality of life | CBT‐Parent only | SMD=–1.87 (–3.04 to –0.71) | WL/NT | 101/6,625 | L |
| CBT‐Individual | SMD=–1.13 (–1.82 to –0.45) | PBO/Sham | 101/6,625 | L | |
| CBT‐Individual | SMD=–1.01 (–1.55 to –0.48) | WL/NT | 101/6,625 | L | |
| CBT‐Internet | SMD=–0.86 (–1.57 to –0.15) | PBO/Sham | 101/6,625 | L | |
| CBT‐Group | SMD=–0.85 (–1.45 to –0.26) | PBO/Sham | 101/6,625 | L | |
| CBT‐Individual+P | SMD=–0.80 (–1.33 to –0.27) | WL/NT | 101/6,625 | L | |
| CBT‐Group+P | SMD=–0.75 (–1.34 to –0.17) | WL/NT | 101/6,625 | L | |
| CBT‐Group | SMD=–0.73 (–1.34 to –0.11) | WL/NT | 101/6,625 | L | |
| CBT‐Internet | SMD=–0.73 (–1.14 to –0.33) | PBO/Sham | 101/6,625 | L | |
| BT‐Individual+Group | SMD=–0.79 (–1.68 to 0.09) | WL/NT | 101/6,625 | L | |
| BT‐Individual+Group | SMD=–0.67 (–1.56 to 0.21) | WL/NT | 101/6,625 | L | |
| CBT‐Individual+Group | SMD=–0.55 (–1.78 to 0.69) | WL/NT | 101/6,625 | L | |
| Remission | CBT‐Child only | OR=10.42 (5.84‐7.60) | WL/NT | 19/1,184 | M |
| CBT‐Group | OR=6.25 (4.45‐8.78) | WL/NT | 25/1,532 | M | |
| CBT‐Remote | OR=6.14 (2.97‐12.71) | WL/NT | 10/591 | L | |
| CBT | OR=5.45 (3.90‐7.60) | WL/NT | 39/2,697 | L | |
| CBT‐Individual | OR=4.53 (2.55‐8.03) | WL/NT | 17/1,165 | L | |
| CBT‐Individual+P | OR=4.08 (2.72‐6.11) | WL/NT | 19/1,142 | M | |
| CBT‐Child only | OR=3.58 (1.92‐6.65) | PBO/Sham | 7/509 | L | |
| CBT‐Group | OR=3.10 (1.14‐8.45) | PBO/Sham | 5/353 | L | |
| CBT‐Parent only | OR=2.83 (1.12‐7.16) | WL/NT | 4/371 | L | |
| CBT | OR=2.28 (1.33‐3.89) | PBO/Sham | 10/822 | L | |
| CBT‐Individual | OR=2.04 (1.06‐3.91) | PBO/Sham | 5/469 | L | |
| CBT‐Individual+P | OR=1.12 (0.65‐1.92) | PBO/Sham | 4/313 | L | |
| Social anxiety disorder | |||||
| Efficacy (subject‐rated) | CBT | SMD=–1.59 (–2.33 to –0.86) | WL/NT | 11/603 | L |
| BT | SMD=–1.22 (–2.06 to –0.38) | WL/NT/PBO/Sham | 4/169 | L | |
| CBT | SMD=–1.19 (–1.72 to –0.67) | WL/NT/PBO/Sham | 14/872 | L | |
| CBT‐Group | SMD=–1.19 (–1.93 to –0.45) | WL/NT/PBO/Sham | 11/670 | L | |
| CBT/BT | SMD=–1.13 (–1.59 to –0.68) | WL/NT/PBO/Sham | 17/1,016 | L | |
| CBT+P | SMD=–1.13 (–1.59 to –0.67) | WL/NT/PBO/Sham | 17/983 | L | |
| CBT‐Individual | SMD=–1.10 (–1.91 to –0.29) | WL/NT/PBO/Sham | 3/127 | L | |
| CBT‐Individual+Group | SMD=–0.80 (–1.19 to –0.41) | WL/NT/PBO/Sham | 3/115 | L | |
| CBT‐Child only | SMD=–0.75 (–1.24 to –0.26) | WL/NT/PBO/Sham | 2/70 | L | |
| CBT‐Internet | SMD=–0.52 (–1.01 to –0.03) | WL/NT/PBO/Sham | 2/143 | L | |
| Acceptability | CBT | RR=1.00 (0.72‐1.41) | WL/NT/PBO/Sham | 16/1,052 | M |
| Depressive symptoms | CBT/BT | SMD=–0.39 (–0.63 to –0.16) | WL/NT/PBO/Sham | 8/299 | L |
| Quality of life | CBT/BT | SMD=–0.79 (–1.17 to –0.41) | WL/NT/PBO/Sham | 9/552 | L |
| Remission | CBT/BT | RR=8.99 (5.27‐15.33) | WL/NT/PBO/Sham | 13/832 | L |
| Obsessive‐compulsive disorder | |||||
| Pharmacological interventions | |||||
|
Efficacy (clinician‐rated) |
Sertraline | SMD=–0.24 (–0.46 to –0.03) | PBO/Sham | 17/991 | L |
| Fluoxetine | SMD=–0.24 (–0.47 to –0.01) | PBO/Sham | 17/991 | L | |
| Clomipramine | SMD=–0.31 (–0.64 to 0.02) | PBO/Sham | 17/991 | L | |
| Fluvoxamine | SMD=–0.21 (–0.49 to 0.06) | PBO/Sham | 17/991 | L | |
| Response | Fluoxetine | RR=1.49 (1.15‐1.96) | PBO/Sham | 2/146 | L |
| SSRI/TCAs | RR=1.80 (1.43‐2.26) | PBO/Sham | 7/692 | L | |
| Acceptability | Fluoxetine | MOR=0.74 (0.25‐1.68) | PBO/Sham | 18/1,143 | L |
| Fluvoxamine | MOR=0.79 (0.24‐2.07) | PBO/Sham | 18/1,143 | L | |
| Sertraline | MOR=0.89 (0.32‐2.07) | PBO/Sham | 18/1,143 | L | |
| Paroxetine | MOR=1.12 (0.37‐3.42) | PBO/Sham | 18/1,143 | L | |
| Clomipramine | MOR=3.06 (0.54‐21.69) | PBO/Sham | 18/1,143 | L | |
| Tolerability | SSRIs | RR=3.59 (1.89‐6.84) | PBO/Sham | 7/807 | L |
| Global illness severity | Fluoxetine | SMD=–0.52 (–0.86 to –0.18) | PBO/Sham | 2/146 | L |
| SSRIs | SMD=–0.42 (–0.61 to –0.23) | PBO/Sham | 5/556 | M | |
| Remission | SSRIs | RR=2.06 (1.03‐4.13) | PBO/Sham | 3/302 | L |
| Pharmacological augmentation (in SSRI‐refractory cases) | |||||
| Response | Risperidone | OR=6.35 (1.48‐27.3) | PBO/Sham | 3/72 | M |
| Quetiapine | OR=2.33 (0.88‐6.20) | PBO/Sham | 3/102 | M | |
| Olanzapine | OR=2.74 (0.34‐21.9) | PBO/Sham | 2/70 | L | |
| Psychosocial interventions | |||||
|
Efficacy (clinician‐rated) |
CBT | SMD=–0.78 (–1.05 to –0.51) | WL/NT | 17/991 | L |
| BT | SMD=–0.72 (–1.20 to –0.24) | WL/NT | 17/991 | L | |
| CBT | SMD=–0.23 (–0.56 to 0.11) | PBO/Sham | 17/991 | L | |
| Response | CBT/BT‐ERP | RR=3.93 (2.52‐6.14) | WL/NT/PBO/Sham | 6/236 | L |
| Acceptability | CBT | MOR=0.49 (0.09‐2.40) | PBO/Sham | 18/1,143 | L |
| BT‐ERP | RR=0.80 (0.35‐1.84) | PBO/WL | 6/301 | L | |
| CBT | MOR=0.86 (0.23‐3.24) | PBO/Sham | 18/1,143 | L | |
| CBT | MOR=0.94 (0.21‐4.79) | WL/NT | 18/1,143 | L | |
| BT | MOR=14.28 (0.87‐785.20) | WL/NT | 18/1,143 | L | |
| Functioning (subject‐rated) | CBT | SMD=–1.15 (–2.11 to –0.19) | WL/NT | 3/194 | L |
| Functioning (parent‐rated) | CBT | SMD=–0.95 (–1.61 to –0.28) | WL/NT | 3/194 | L |
| CBT | SMD=–0.31 (–0.63 to 0.01) | PBO/Sham | 2/183 | L | |
| Remission | CBT | RR=2.33 (1.33‐4.00) | WL/NT | 4/271 | L |
| CBT | RR=1.59 (1.28‐1.96) | PBO/Sham | 3/153 | L | |
| Quality of life | CBT | SMD=–0.39 (–0.77 to –0.02) | WL/PBO/Sham | 2/223 | L |
| Combined interventions | |||||
| Efficacy | CBT+sertraline | SMD=–0.58 (–0.91 to –0.25) | PBO/Sham | 17/991 | L |
| Acceptability | CBT+sertraline | MOR=0.54 (0.08‐3.15) | PBO/Sham | 18/1,143 | L |
| Post‐traumatic stress disorder | |||||
| Efficacy | CBT | SMD=–1.34 (–1.79 to –0.89) | WL/NT | 3/98 | L |
| EMDR | SMD=–0.61 (–1.96 to 0.74) | WL/NT | 2/65 | L | |
| NET | SMD=–0.57 (–1.23 to 0.09) | WL/NT | 2/79 | L | |
| Response | CBT | OR=8.64 (2.01‐37.14) | WL/NT | 2/49 | L |
| NET | OR=3.82 (0.67‐21.8) | WL/NT | 2/78 | L | |
| Acceptability | NET | OR=5.13 (0.56‐47.28) | WL/NT | 2/83 | L |
| Anxiety symptoms | NET | SMD=–0.66 (–1.33 to 0.01) | WL/NT | 2/59 | L |
| Depressive symptoms | CBT | SMD=–0.8 (–1.47 to –0.131) | WL/NT | 3/98 | L |
| Enuresis | |||||
| Pharmacological interventions | |||||
| Efficacy | Imipramine | SMD=–0.46 (–0.67 to –0.24) | PBO/Sham | 4/347 | M |
| Response | Amitriptyline | RR=1.22 (1.02‐1.45) | PBO/Sham | 2/98 | L |
| Imipramine | RR=1.35 (1.11‐1.64) | PBO/Sham | 12/831 | L | |
|
Psychosocial interventions | |||||
| Efficacy | BT‐Alarm | SMD=–1.30 (–2.16 to –0.44) | WL/NT | 4/127 | L |
| Response | BT‐Alarm | RR=7.23 (1.40‐37.77) | WL/NT | 18/827 | L |
| BT‐Alarm | RR=1.59 (1.16‐2.17) | PBO/Sham | 2/181 | L | |
| BT‐Reward | RR=1.22 (1.03‐1.45) | WL/NT | 2/325 | L | |
RCTs – randomized controlled trials, SMD – standardized mean difference, OR – odds ratio, MOR – median odds ratio, RR – risk ratio, PBO – placebo, WL – waiting list, NT – no treatment, Q – quality (H – high, M – medium, L – low), BT – behavioral therapy, BT‐ERP – behavioral therapy with exposure and response prevention, CBT – cognitive behavioral therapy, EMDR – eye movement desensitization and reprocessing, NET – narrative exposure therapy, P – parental involvement, SSRIs – selective serotonin reuptake inhibitors, SNRIs – serotonin‐norepinephrine reuptake inhibitors, TCAs – tricyclic antidepressants. Bold prints indicate significant values. SMDs<0 indicate that intervention is more effective than control. For discontinuation outcomes (acceptability, tolerability, inefficacy) and relapse, OR/RR<1 favors the intervention. For response and remission, OR/RR>1 favors the intervention.
Among pharmacological interventions, imipramine outperformed placebo regarding the primary efficacy outcome and response (small effect size), and amitriptyline was superior to placebo with respect to response (small effect size) (see Table 4).
Behavioral therapy with alarm outperformed waiting list on the primary efficacy outcome (small effect size) and response (large effect size), and maintained a small effect size regarding response vs. placebo (see Table 4).
No clear superior treatment emerged in monotherapy head‐to‐head comparisons. Combination of desmopressin plus behavioral therapy with alarm was superior to desmopressin alone regarding the primary efficacy outcome (medium effect size) and response (small effect size), while combination of oxybutynin plus imipramine was superior to either imipramine or oxybutynin monotherapy (small effect size) (see Table 6).
Obsessive‐compulsive disorder
Results for obsessive‐compulsive disorder are shown in Tables 4 and 5.
Fluoxetine was the pharmacological intervention with the broadest efficacy, including primary efficacy outcome, response, and global illness severity vs. placebo (small effect sizes). SSRIs as a class also improved response, remission and global illness severity, yet had a higher discontinuation rate due to intolerability than placebo (see Table 4).
Among monotherapy psychosocial interventions, CBT was superior to waiting list regarding the primary efficacy outcome (medium effect size), response (small effect size), remission (small effect size), quality of life (small effect size) and functioning (large effect size), and also to placebo concerning remission (small effect size) (see Table 4). Behavioral therapy with exposure and response prevention outperformed TAU for both response and acceptability (small effect size) (see Table 5).
As a combination treatment, CBT and sertraline outperformed placebo (medium effect size) (see Table 4). No significant differences emerged in head‐to‐head comparisons.
Anxiety disorders
Results for anxiety disorders are shown in Tables 4, 5 and 6.
SSRIs (fluoxetine, fluvoxamine, paroxetine) outperformed placebo regarding the primary efficacy outcome, and response (small to medium effect). Fluoxetine also outperformed placebo with respect to remission (small effect size) (see Table 4). Sertraline reduced suicidality compared with placebo, but paroxetine increased it.
CBT was superior to waiting list in different formats (i.e., individual, Internet, group) regarding the primary efficacy outcome (small to large effect size), depressive symptoms (small effect size), remission (small to large effect size) and quality of life (large effect size). CBT was also superior to placebo with respect to quality of life (large effect size) and to TAU regarding the primary efficacy outcome, remission and functioning (large effect size). Group CBT was superior to individual CBT in head‐to‐head comparisons (small effect size) (see Tables 4, 5 and 6).
No meta‐analysis compared pharmacological vs. psychosocial interventions or combined treatment strategies.
Disruptive behavior/dissocial/conduct disorders
Results for disruptive behavior/dissocial/conduct disorders are shown in Tables 2 and 7.
Among pharmacological interventions, risperidone outperformed placebo across different raters regarding the primary efficacy outcome (medium effect size), aggressive behavior (medium effect size, also in people with intellectual disability), and global illness severity (medium effect size). Aggressive behavior was also improved by lithium and valproate (see Table 2).
Among psychosocial interventions, a combination of parental and child behavioral interventions had a large effect size vs. waiting list concerning the primary efficacy outcome, and a medium effect size vs. a mixed control group (see Tables 2 and 7).
Eating disorders
Results for eating disorders are shown in Table 6.
No meta‐analysis on pharmacological intervention met the inclusion criteria of this umbrella review. Among psychosocial interventions, family therapy outperformed other interventions in anorexia nervosa regarding the primary efficacy outcome (body weight, small effect size).
Schizophrenia spectrum disorders
Results for schizophrenia spectrum disorders are shown in Tables 3 and 6.
For schizophrenia, only pharmacological interventions were covered. All investigated antipsychotics but ziprasidone outperformed placebo, with a small effect size, except for olanzapine and risperidone, which had a large effect size. Small effect sizes emerged regarding response (except for asenapine), and all antipsychotics improved global illness severity. Acceptability was superior vs. placebo for paliperidone, risperidone and olanzapine, without differences for the other antipsychotics. Paliperidone and olanzapine were associated with more discontinuation due to intolerability than placebo, while discontinuation due to inefficacy favored paliperidone, olanzapine, risperidone and ziprasidone (see Table 3).
In head‐to‐head comparisons, risperidone and second‐generation antipsychotics outperformed first‐generation antipsychotics (large effect size), and clozapine outperformed olanzapine on the primary efficacy outcome (large effect size) (see Table 6).
Bipolar disorder
Results for bipolar disorder are shown in Tables 3 and 6.
Regarding bipolar depression, quetiapine was not superior to placebo regarding the primary efficacy outcome, separating only on global illness severity (small effect size). Regarding mania, aripiprazole was more effective than placebo regarding the primary efficacy outcome (large effect size) and response (small effect size), without differences vs. placebo regarding acceptability, while being superior regarding less discontinuations for inefficacy (see Table 3).
Other disorders
Results for tic disorder are shown in Tables 2 and 6. Desipramine and methylphenidate were similar to placebo, but topiramate was superior to haloperidol regarding the primary outcome.
Results for Tourette's disorder are shown in Tables 2 and 7. Antipsychotics (including haloperidol, pimozide, risperidone and ziprasidone) and guanfacine were superior to placebo regarding the primary efficacy outcome (both moderate effect size). No significant difference vs. placebo emerged for methylphenidate (see Table 2). Among psychosocial interventions, behavioral therapy outperformed waiting list or low intensity psychosocial intervention (medium effect size) regarding the primary efficacy outcome (see Table 7).
Results for encopresis are shown in Table 5. No pharmacological intervention was eligible. Behavioral therapy outperformed TAU regarding the primary efficacy outcome and response (small effect size).
Results for developmental coordination disorders are shown in Table 2. In the single meta‐analysis meeting inclusion criteria, skills training had no significant effect vs. waiting list on motor coordination.
Results for PTSD are shown in Table 4. No pharmacological intervention met inclusion criteria. CBT was superior regarding the primary efficacy outcome, response and depressive symptoms vs. waiting list (large effect sizes).
DISCUSSION
Pooling top‐tier evidence from 104 MAs/NMAs of RCTs reporting on the effects of pharmacological, psychosocial and brain stimulation interventions, targeting 20 different outcomes in 15 mental disorders or groups of mental disorders, this umbrella review provides a comprehensive meta‐analytic view of the evidence base regarding the efficacy, acceptability and other relevant outcomes of psychiatric treatments in children and adolescents (see supplementary information for further details).
Considered together with a complementary umbrella review published in this journal 14 , focusing on the detailed evaluation of tolerability and safety of pharmacological interventions, the current review can inform clinicians, youth and their families, as well as other stakeholders, in making evidence‐based decisions regarding the choice and use of pharmacological, psychosocial and brain stimulation interventions in children/adolescents, in monotherapy and in combination. On the basis of these reviews, some evidence‐based recommendation can be made.
For ADHD, amphetamines and methylphenidate are the most effective interventions on a broad set of outcomes. Whilst amphetamines outperform methylphenidate on the primary efficacy outcome, methylphenidate is the medication least different from placebo concerning safety 14 . Some evidence is available regarding behavioral therapy, covering a narrow set of efficacy outcomes, and with small effect sizes compared with those for medications. Importantly, whilst social skills training shows promising results against waiting list, no evidence is available comparing this intervention with placebo. Hence, amphetamines or methylphenidate can be considered the first‐line treatment, augmented with alpha‐2 agonists if needed, and ideally in combination with behavioral therapy as an optimal treatment regimen. Behavioral therapy could be considered if medications are contraindicated.
For autism, aripiprazole and risperidone are the pharmacological treatment options of choice. However, various psychosocial interventions have proven efficacy on a broad set of outcomes, ranging from anxiety (CBT), to irritability, aggressive behavior and functioning (parent‐child interaction therapy), to the primary efficacy outcome and functioning (social skills training, and behavioral therapy with imitative component). These benefits are not only observed vs. waiting list, but also against other active interventions. Given the different outcomes that these treatment modalities target, a variety of therapeutic tools can be considered, according to the patient's and family's resources, needs and choice, as well as the disease course and the presence of environmental stressors.
For depressive disorders in youth, fluoxetine is the only evidence‐based pharmacological option. All other medications do not improve depression vs. placebo, but placebo effects are considerable. Imipramine, nortriptyline, and likely also venlafaxine should be avoided, given poor acceptability, tolerability and safety. As an alternative to medications, interpersonal therapy is the only psychosocial intervention outperforming placebo. The combination of CBT with fluoxetine also outperformed placebo on the primary efficacy outcome, and was superior to either monotherapy.
For enuresis, imipramine is the most effective pharmacological intervention. It can be combined with oxybutynin to maximize efficacy. However, due to the potential problems with tolerability of this medication in youth, psychosocial interventions should be tried first, including especially alarm behavioral therapy, that is supported by the largest body of evidence. No difference emerges among different types of alarms, and alarm maintains its efficacy after stopping the intervention 86 .
For obsessive‐compulsive disorder, fluoxetine and SSRIs as a class should be considered the first‐line pharmacological treatment. Among psychosocial interventions, CBT and behavioral therapy with exposure and response prevention are effective options. If fluoxetine/SSRIs are ineffective, a switch to psychosocial interventions should be performed, and vice versa 71 .
For anxiety disorders, fluoxetine and fluvoxamine are evidence‐based pharmacological treatment strategies. Among psychosocial interventions, CBT – and in particular group CBT – should be offered as first‐line treatment, likely before medications, given the large effect size and broad beneficial effect even vs. placebo in children and adolescents.
For disruptive behavior/dissocial/conduct disorders, risperidone emerges as the most effective pharmacological agent, but different types of behavioral treatment (including parent training) should be regarded as the first‐line treatment options118, 119.
For anorexia nervosa in children and adolescents, family therapy is the intervention supported by the most significant evidence.
For schizophrenia spectrum disorders, antipsychotic treatment is the cornerstone of treatment. All tested antipsychotics, except for ziprasidone, have broadly similar superior efficacy vs. placebo, with olanzapine and risperidone being the most effective, and lurasidone/aripiprazole a more tolerable treatment option 102 . Ideally, starting with safer medications minimizing the risk of adverse events and maximizing adherence is a recommended strategy 14 .
For bipolar disorder, little meta‐analytic evidence is available overall. For mania, the only positive data are available for aripiprazole, yet lithium is also an evidence‐based treatment based on RCT evidence 120 . For bipolar depression, only quetiapine is superior to placebo, and only on a single outcome, namely global illness severity, but not on the primary symptom outcome. This finding is different from adults 121 , and at least partially due to the larger placebo effects in youth. Our umbrella review did not include lurasidone and olanzapine/fluoxetine combination, as no meta‐analysis has been conducted on them, but these are evidence‐based options to treat bipolar depression in youth based on single RCTs122, 123, which led to their approval by the US Food and Drug Administration for bipolar depression in children and adolescents.
The available evidence presented in this umbrella review is not equally large across individual disorders, and also across monotherapies with pharmacological or psychosocial interventions. Even less meta‐analytic data are available for head‐to‐head studies, within and across treatment modalities, and regarding combination treatments. Furthermore, little meta‐analytic evidence exists on treatment‐resistant youth with a given mental disorder. This is concerning, as early illness onset and disruption of healthy development may portend poorer response and outcomes, requiring information on non‐responding conditions after first‐ and second‐line treatments have been tried.
Among the 104 included meta‐analyses, virtually none reported data on long‐term treatment or relapse prevention. This is problematic, as most of these disorders are chronic and require long‐term treatment.
This umbrella review clearly shows that large effect sizes emerge for psychosocial interventions when they are compared with waiting list or no treatment, where no placebo or expectation of study effect diminishes the treatment effect size. However, when those treatments are compared against psychological placebo or minimally active controls, significant effects either diminish in magnitude or disappear. This finding is relevant for indirect comparisons with pharmacological trials, in which the use of placebo makes the effect size appear smaller. The much greater difficulty of blinding treatment assignment in psychosocial trials is also to be taken into account. The risk of inflated effect sizes due to weak and methodologically flawed comparators (e.g., waiting list, no intervention) is that such interventions might be preferred to other superior treatments, delaying response and remission 121 .
The results from this umbrella review should be considered within its limitations. First, we only considered evidence that was evaluated quantitatively via MAs/NMAs. This approach has excluded data from RCTs that have not (yet) been meta‐analyzed. In particular, Internet‐based psychosocial interventions, whose development has been recent and which may be particularly favored by youth125, 126, have not been sufficiently covered.
Second, we focused mainly on efficacy outcomes, while choices need to be made considering both efficacy and tolerability/safety. However, we included all‐cause discontinuation as a global acceptability measure, as well as discontinuation due to intolerability as a core tolerability outcome, because these two events are typically measured and reported across both pharmacological and non‐pharmacological treatment modalities. Detailed tolerability outcomes of pharmacological interventions in youth with mental disorders, that can be used to complement the present work on efficacy, have been recently published in this journal 14 . Such detailed data are not generally reported for psychosocial interventions, which is currently a major unmet need 127 .
Third, as mentioned above, most meta‐analytic evidence concerns the acute and short‐term treatment effects, and much more data are required regarding the efficacy and safety of long‐term and relapse prevention interventions for mental disorders in youth. Fourth, most evidence is available for monotherapy and vs. placebo/no treatment, although combination and augmentation treatments across and within pharmacological and psychosocial treatment modalities are commonly used in clinical practice, in youth as well as in adults 128 . Fifth, although 14 of the 104 included meta‐analyses were NMAs that allow for direct and indirect head‐to‐head comparisons, most data were not derived from direct comparisons of active treatments, limiting the confidence with which comparative treatment choices can be made.
Sixth, since design, population and illness characteristics, as well as choice of control groups and blinding methods influence effect sizes, and these characteristics often differ substantially between pharmacological and non‐pharmacological trials, indirect comparisons of effect sizes across these treatment modalities need to be interpreted with caution. To overcome this limitation, more head‐to‐head comparisons and combination trials need to be conducted both within and across treatment modalities. Finally, we focused on those disorders that are most common and studied in youth, maximizing the chance of finding meta‐analytic evidence, but other mental conditions could also be of interest.
Despite these limitations, inherent in the umbrella review methodology and available RCT data, this study provides the most comprehensive account of the available RCT evidence concerning pharmacological, psychosocial and brain stimulation interventions for the main psychiatric disorders in childhood and adolescents. The large body of literature reviewed here can inform future research aimed at addressing identified gaps, as well as current clinical care and guidelines regarding the choice of interventions for mental health conditions in youth, merging state‐of‐the‐art efficacy and acceptability data with information on tolerability and safety.
ACKNOWLEDGEMENTS
E.G. Ostinelli is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility and the NIHR Oxford Health Biomedical Research Centre (grant BRC‐1215‐20005). Supplementary information on this study is available at https://osf.io/2awu4/.
REFERENCES
- 1. Kessler RC, Berglund P, Demler O et al. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593‐602. [DOI] [PubMed] [Google Scholar]
- 2. Caspi A, Houts RM, Ambler A et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin birth cohort study. JAMA Netw Open 2020;3:e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang PS, Berglund P, Olfson M et al. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:603‐13. [DOI] [PubMed] [Google Scholar]
- 4. GBD 2017 Child and Adolescent Health Collaborators . Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the global burden of diseases, injuries, and risk factors 2017 Study. JAMA Pediatr 2019;173:e190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortese S, Adamo N, Del Giovane C et al. Comparative efficacy and tolerability of medications for attention‐deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2018;5:727‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillay J, Boylan K, Carrey N et al. First‐ and second‐generation antipsychotics in children and young adults: systematic review update. Rockville: US Agency for Healthcare Research and Quality, 2017. [PubMed] [Google Scholar]
- 7. Cipriani A, Zhou X, Del Giovane C et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta‐analysis. Lancet 2016;388:881‐90. [DOI] [PubMed] [Google Scholar]
- 8. Pagsberg AK, Tarp S, Glintborg D et al. Acute antipsychotic treatment of children and adolescents with schizophrenia‐spectrum disorders: a systematic review and network meta‐analysis. J Am Acad Child Adolesc Psychiatry 2017;56:191‐202. [DOI] [PubMed] [Google Scholar]
- 9. Cipriani A, Furukawa TA, Salanti G et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet 2018;391:1357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou X, Teng T, Zhang Y et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta‐analysis. Lancet Psychiatry 2020;7:581‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobson E, Bloch M, Strawn J. Efficacy and tolerability of pharmacotherapy for anxiety disorders. J Clin Psychiatry 2019;80:17r12064. [DOI] [PubMed] [Google Scholar]
- 12. James AC, Reardon T, Soler A et al. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2020;11:CD013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huhn M, Tardy M, Spineli LM et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta‐analyses. JAMA Psychiatry 2014;71:706‐15. [DOI] [PubMed] [Google Scholar]
- 14. Solmi M, Fornaro M, Ostinelli EG et al. Safety of 80 antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta‐review of 78 adverse effects. World Psychiatry 2020;19:214‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed GM, First MB, Kogan CS et al. Innovations and changes in the ICD‐11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 2019;18:3‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Correll CU, Rubio JM, Inczedy‐Farkas G et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta‐analytic evidence. JAMA Psychiatry 2017;74:675‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shea BJ, Grimshaw JM, Wells GA et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borenstein M, Hedges L, Higgins JPT et al. Comprehensive meta‐analysis (Version 2.2.027). www.meta‐analysis.com/.
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
- 20. Catalá‐López F, Hutton B, Núñez‐Beltrán A et al. The pharmacological and non‐pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta‐analyses of randomised trials. PLoS One 2017;12:e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luan R, Mu Z, Yue F et al. Efficacy and tolerability of different interventions in children and adolescents with attention deficit hyperactivity disorder. Front Psychiatry 2017;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otasowie J, Castells X, Ehimare UP et al. Tricyclic antidepressants for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2014;19:CD006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Punja S, Shamseer L, Hartling L et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents (Review). Cochrane Database Syst Rev 2016;2:CD009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun C‐K, Tseng P‐T, Wu C‐K et al. Therapeutic effects of methylphenidate for attention‐deficit/hyperactivity disorder in children with borderline intellectual functioning or intellectual disability: a systematic review and meta‐analysis. Sci Rep 2019;9:15908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Battagliese G, Caccetta M, Ines O et al. Behaviour research and therapy cognitive‐behavioral therapy for externalizing disorders: a meta‐analysis of treatment effectiveness. Behav Res Ther 2015;75:60‐71. [DOI] [PubMed] [Google Scholar]
- 26. Faraone SV, Biederman J, Roe C. Comparative efficacy of Adderall and methylphenidate in attention‐deficit/hyperactivity disorder: a meta‐analysis. J Clin Psychopharmacol 2002;22:468‐73. [DOI] [PubMed] [Google Scholar]
- 27. Van Doren J, Arns M, Heinrich H et al. Sustained effects of neurofeedback in ADHD: a systematic review and meta‐analysis. Eur Child Adolesc Psychiatry 2019;28:293‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cortese S, Ferrin M, Brandeis D et al. Cognitive training for attention‐deficit/hyperactivity disorder: meta‐analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry 2015;54:164‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daley D, Van Der Oord S, Ferrin M et al. Behavioral interventions in attention‐deficit/hyperactivity disorder: a meta‐analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry 2014;53:835‐47. [DOI] [PubMed] [Google Scholar]
- 30. Bikic A, Reichow B, McCauley SA et al. Meta‐analysis of organizational skills interventions for children and adolescents with attention‐deficit/hyperactivity disorder. Clin Psychol Rev 2017;52:108‐23. [DOI] [PubMed] [Google Scholar]
- 31. Mulqueen JM, Bartley CA, Bloch MH. Meta‐analysis: parental interventions for preschool ADHD. J Atten Disord 2015;19:118‐24. [DOI] [PubMed] [Google Scholar]
- 32. Cortese S, Ferrin M, Brandeis D et al. Neurofeedback for attention‐deficit/hyperactivity disorder: meta‐analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry 2016;55:444‐55. [DOI] [PubMed] [Google Scholar]
- 33. Bussalb A, Congedo M, Barthélemy Q et al. Clinical and experimental factors influencing the efficacy of neurofeedback in ADHD: a meta‐analysis. Front Psychiatry 2019;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stuhec M, Munda B, Svab V et al. Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta‐analysis with focus on bupropion. J Affect Disord 2015;178:149‐59. [DOI] [PubMed] [Google Scholar]
- 35. Faraone SV, Biederman J. Efficacy of Adderall® for attention‐deficit/hyperactivity disorder: a meta‐analysis. J Atten Disord 2002;6:69‐75. [DOI] [PubMed] [Google Scholar]
- 36. Schachter HM, Pham B, King J et al. How efficacious and safe is short‐acting methylphenidate for the treatment of attention‐deficit disorder in children and adolescents? A meta‐analysis. CMAJ 2001;165:1475‐88. [PMC free article] [PubMed] [Google Scholar]
- 37. Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention‐deficit/hyperactivity disorder: results from a comprehensive meta‐analysis and metaregression. J Am Acad Child Adolesc Psychiatry 2014;53:174‐87. [DOI] [PubMed] [Google Scholar]
- 38. Coghill DR, Seth S, Pedroso S et al. Effects of methylphenidate on cognitive functions in children and adolescents with attention‐deficit/hyperactivity disorder: evidence from a systematic review and a meta‐analysis. Biol Psychiatry 2014;76:603‐15. [DOI] [PubMed] [Google Scholar]
- 39. Storebø OJ, Ramstad E, Krogh HB et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev 2015;11:CD009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bangs ME, Wietecha LA, Wang S et al. Meta‐analysis of suicide‐related behavior or ideation in child, adolescent, and adult patients treated with atomoxetine. J Child Adolesc Psychopharmacol 2014;24:426‐34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hirota T, Schwartz S, Correll C. Alpha‐2 agonists for attention‐deficit/hyperactivity disorder in youth: a systematic review and meta‐analysis of monotherapy and add‐on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 2014;53:153‐73. [DOI] [PubMed] [Google Scholar]
- 42. Storebø OJ, Andersen ME, Skoog M et al. Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev 2019;6:CD008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fallah MS, Shaikh MR, Neupane B et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta‐analysis. J Child Adolesc Psychopharmacol 2019;29:168‐80. [DOI] [PubMed] [Google Scholar]
- 44. Maneeton N, Maneeton B, Putthisri S et al. Aripiprazole in acute treatment of children and adolescents with autism spectrum disorder: a systematic review and meta‐analysis. Neuropsychiatr Dis Treat 2018;14:3063‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu Q, Li E, Li L et al. Efficacy of interventions based on applied behavior analysis for autism spectrum disorder: a meta‐analysis. Psychiatry Investig 2020;17:432‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oono IP, Honey EJ, McConachie H. Parent‐mediated early intervention for young children with autism spectrum disorders (ASD). BJPsych Adv 2016;22:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parsons L, Cordier R, Munro N et al. A systematic review of pragmatic language interventions for children with autism spectrum disorder. PLoS One 2017;12:e0172242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kreslins A, Robertson AE, Melville C. The effectiveness of psychosocial interventions for anxiety in children and adolescents with autism spectrum disorder: a systematic review and meta‐analysis. Child Adolesc Psychiatry Ment Health 2015;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tarver J, Palmer M, Webb S et al. Child and parent outcomes following parent interventions for child emotional and behavioral problems in autism spectrum disorders: a systematic review and meta‐analysis. Autism 2019;23:1630‐44. [DOI] [PubMed] [Google Scholar]
- 50. Soares EE, Bausback K, Beard CL et al. Social skills training for autism spectrum disorder: a meta‐analysis of in‐person and technological interventions. J Technol Behav Sci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Postorino V, Sharp WG, McCracken CE et al. A systematic review and meta‐analysis of parent training for disruptive behavior in children with autism spectrum disorder. Clin Child Fam Psychol Rev 2017;20:391‐402. [DOI] [PubMed] [Google Scholar]
- 52. Maneeton N, Maneeton B, Putthisri S et al. Risperidone for children and adolescents with autism spectrum disorder: a systematic review. Neuropsychiatr Dis Treat 2018;14:1811‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou MS, Nasir M, Farhat LC et al. Meta‐analysis: pharmacologic treatment of restricted and repetitive behaviors in autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 2020;60:35‐45. [DOI] [PubMed] [Google Scholar]
- 54. Murza KA, Schwartz JB, Hahs‐Vaughn DL et al. Joint attention interventions for children with autism spectrum disorder: a systematic review and meta‐analysis. Int J Lang Commun Disord 2016;51:236‐51. [DOI] [PubMed] [Google Scholar]
- 55. Sturman N, Deckx L, van Driel ML. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Syst Rev 2017;11:CD011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fletcher‐Watson S, Mcconnell F, Manola E et al. Interventions based on the Theory of Mind cognitive model for autism spectrum disorder (ASD) (Review). Cochrane Database Syst Rev Interv 2014;3:CD008785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen D, Raffin M, Canitano R et al. Risperidone or aripiprazole in children and adolescents with autism and/or intellectual disability: a Bayesian meta‐analysis of efficacy and secondary effects. Res Autism Spectr Disord 2013;7:167‐75. [Google Scholar]
- 58. Hirota T, Veenstra‐Vanderweele J, Hollander E et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta‐analysis. J Autism Dev Disord 2014;44:948‐57. [DOI] [PubMed] [Google Scholar]
- 59. D'Alò GL, De Crescenzo F, Amato L et al. Acceptability, equity, and feasibility of using antipsychotics in children and adolescents with autism spectrum disorder: a systematic review. BMC Psychiatry 2020;20:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ospina MB, Seida JK, Clark B et al. Behavioural and developmental interventions for autism spectrum disorder: a clinical systematic review. PLoS One 2008;3:e3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reichow B, Steiner AM, Volkmar F et al. Social skills groups for people aged 6 to 21 with autism spectrum disorders (ASD). Cochrane Database Syst Rev 2012;7:CD008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tachibana Y, Miyazaki C, Ota E et al. A systematic review and meta‐analysis of comprehensive interventions for pre‐school children with autism spectrum disorder (ASD). PLoS One 2017;12:e0186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nevill RE, Lecavalier L, Stratis EA. Meta‐analysis of parent‐mediated interventions for young children with autism spectrum disorder. Autism 2018;22:84‐98. [DOI] [PubMed] [Google Scholar]
- 64. Spielmans GI, Gerwig K. The efficacy of antidepressants on overall well‐being and self‐reported depression symptom severity in youth: a meta‐analysis. Psychother Psychosom 2014;83:158‐64. [DOI] [PubMed] [Google Scholar]
- 65. Kato M, Hori H, Inoue T et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta‐analysis. Mol Psychiatry 2020;26:118‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whittington CJ, Kendall T, Fonagy P et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341‐5. [DOI] [PubMed] [Google Scholar]
- 67. Watanabe N, Hunot V, Omori IM et al. Psychotherapy for depression among children and adolescents: a systematic review. Acta Psychiatr Scand 2007;116:84‐95. [DOI] [PubMed] [Google Scholar]
- 68. Cox GR, Callahan P, Churchill R et al. Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents. Cochrane Database Syst Rev 2014;11:CD008324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dubicka B, Elvins R, Roberts C et al. Combined treatment with cognitive‐behavioural therapy in adolescent depression: meta‐analysis. Br J Psychiatry 2010;197:433‐40. [DOI] [PubMed] [Google Scholar]
- 70. Klein JB. Cognitive‐behavioral therapy for adolescent depression: a meta‐analytic investigation of changes in effect‐size estimates. J Am Acad Child Adolesc Psychiatry 2007;46:1403‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Skapinakis P, Caldwell D, Hollingworth W et al. A systematic review of the clinical effectiveness and cost‐effectiveness of pharmacological and psychological interventions for the management of obsessive‐compulsive disorder in children/adolescents and adults. Health Technol Assess 2016;20:1‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maneeton N, Maneeton B, Karawekpanyawong N et al. Fluoxetine in acute treatment of children and adolescents with obsessive‐compulsive disorder: a systematic review and meta‐analysis. Nord J Psychiatry 2020;74:461‐9. [DOI] [PubMed] [Google Scholar]
- 73. McGuire JF, Piacentini J, Lewin AB et al. A meta‐analysis of cognitive behavior therapy and medication for child obsessive‐compulsive disorder: moderators of treatment efficacy, response, and remission. Depress Anxiety 2015;32:580‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Locher C, Koechlin H, Zion SR et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin‐norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta‐analysis. JAMA Psychiatry 2017;74:1011‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Geller D. Which SSRI? A meta‐analysis of pharmacotherapy trials in pediatric obsessive‐compulsive disorder. Am J Psychiatry 2003;160:1919‐28. [DOI] [PubMed] [Google Scholar]
- 76. Uhre CF, Uhre VF, Lønfeldt NN et al. Systematic review and meta‐analysis: cognitive‐behavioral therapy for obsessive‐compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 2020;59:64‐77. [DOI] [PubMed] [Google Scholar]
- 77. Johnco C, McGuire JF, Roper T et al. A meta‐analysis of dropout rates from exposure with response prevention and pharmacological treatment for youth with obsessive compulsive disorder. Depress Anxiety 2020;37:407‐17. [DOI] [PubMed] [Google Scholar]
- 78. Zhou X, Zhang Y, Furukawa TA et al. Different types and acceptability of psychotherapies for acute anxiety disorders in children and adolescents: a network meta‐analysis. JAMA Psychiatry 2019;76:41‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Z, Whiteside SPH, Sim L et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: a systematic review and meta‐analysis. JAMA Pediatr 2017;171:1049‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang H, Zhang Y, Yang L et al. Efficacy and acceptability of psychotherapy for anxious young children a meta‐analysis of randomized controlled trials. J Nerv Ment Dis 2017;205:931‐41. [DOI] [PubMed] [Google Scholar]
- 81. Sigurvinsdóttir AL, Jensínudóttir KB, Baldvinsdóttir KD et al. Effectiveness of cognitive behavioral therapy (CBT) for child and adolescent anxiety disorders across different CBT modalities and comparisons: a systematic review and meta‐analysis. Nord J Psychiatry 2020;74:168‐80. [DOI] [PubMed] [Google Scholar]
- 82. James A, James G, Cowdrey FA et al. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2015;11:CD013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang L, Zhou X, Pu J et al. Efficacy and acceptability of psychological interventions for social anxiety disorder in children and adolescents: a meta‐analysis of randomized controlled trials. Eur Child Adolesc Psychiatry 2019;28:79‐89. [DOI] [PubMed] [Google Scholar]
- 84. Kreuze LJ, Pijnenborg GHM, de Jonge YB et al. Cognitive‐behavior therapy for children and adolescents with anxiety disorders: a meta‐analysis of secondary outcomes. J Anxiety Disord 2018;60:43‐57. [DOI] [PubMed] [Google Scholar]
- 85. Song P, Huang C, Wang Y et al. Comparison of desmopressin, alarm, desmopressin plus alarm, and desmopressin plus anticholinergic agents in the management of paediatric monosymptomatic nocturnal enuresis: a network meta‐analysis. BJU Int 2019;123:388‐400. [DOI] [PubMed] [Google Scholar]
- 86. Caldwell PHY, Codarini M, Stewart F et al. Alarm interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2020;5:CD002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Caldwell PHY, Sureshkumar P, Wong WCF. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev 2016;1:CD002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Caldwell PHY, Nankivell G, Sureshkumar P. Simple behavioural interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2013;7:CD003637. [DOI] [PubMed] [Google Scholar]
- 89. Buckley BS, Sanders CD, Spineli L et al. Conservative interventions for treating functional daytime urinary incontinence in children. Cochrane Database Syst Rev 2019;9:CD012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Deshpande AV, Caldwell PH, Sureshkumar P. Drugs for nocturnal enuresis in children (other than desmopressin and tricyclics). Cochrane Database Syst Rev 2012;12:CD002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Peng CCH, Yang SSD, Austin PF et al. Systematic review and meta‐analysis of alarm versus desmopressin therapy for pediatric monosymptomatic enuresis. Sci Rep 2018;8:16755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Seida JC, Schouten JR, Mousavi SS et al. First‐ and second‐ generation antipsychotics for children and young adults: comparative effectiveness. Rockville: US Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 93. Loy J, Merry S, Hetrick S et al. Atypical antipsychotic drugs for disruptive behaviour disorders in children and youths. Cochrane Database Syst Rev 2017;8:CD008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pringsheim T, Hirsch L, Gardner D et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention‐deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta‐analysis. Can J Psychiatry 2015;60:52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ipser J, Stein DJ. Systematic review of pharmacotherapy of disruptive behavior disorders in children and adolescents. Psychopharmacology 2007;191:127‐40. [DOI] [PubMed] [Google Scholar]
- 96. McQuire C, Hassiotis A, Harrison B et al. Pharmacological interventions for challenging behaviour in children with intellectual disabilities: a systematic review and meta‐analysis. BMC Psychiatry 2015;15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zeeck A, Herpertz‐Dahlmann B, Friederich HC et al. Psychotherapeutic treatment for anorexia nervosa: a systematic review and network meta‐analysis. Front Psychiatry 2018;9:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Couturier J, Kimber M, Szatmari P. Efficacy of family‐based treatment for adolescents with eating disorders: a systematic review and meta‐analysis. Int J Eat Disord 2013;46:3‐11. [DOI] [PubMed] [Google Scholar]
- 99. Fisher CA, Skocic S, Rutherford KA et al. Family therapy approaches for anorexia nervosa. Cochrane Database Syst Rev 2019;5:CD004780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van den Berg E, Houtzager L, de Vos J et al. Meta‐analysis on the efficacy of psychological treatments for anorexia nervosa. Eur Eat Disord Rev 2019;27:331‐51. [DOI] [PubMed] [Google Scholar]
- 101. Linardon J, Wade TD, de la Piedad Garcia X et al. The efficacy of cognitive‐behavioral therapy for eating disorders: a systematic review and meta‐analysis. J Consult Clin Psychol 2017;85:1080‐94. [DOI] [PubMed] [Google Scholar]
- 102. Krause M, Zhu Y, Huhn M et al. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: a network meta‐analysis. Eur Neuropsychopharmacol 2018;28:659‐74. [DOI] [PubMed] [Google Scholar]
- 103. Arango C, Ng‐Mak D, Finn E et al. Lurasidone compared to other atypical antipsychotic monotherapies for adolescent schizophrenia: a systematic literature review and network meta‐analysis. Eur Child Adolesc Psychiatry 2020;29:1195‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sarkar S, Grover S. Antipsychotics in children and adolescents with schizophrenia: a systematic review and meta‐analysis. Indian J Pharmacol 2013;45:439‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kumar A, Datta SS, Wright SD et al. Atypical antipsychotics for psychosis in adolescents. Cochrane Database Syst Rev 2013;10:CD009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Maneeton B, Putthisri S, Maneeton N et al. Quetiapine monotherapy versus placebo in the treatment of children and adolescents with bipolar depression: a systematic review and meta‐analysis. Neuropsychiatr Dis Treat 2017;13:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Meduri M, Gregoraci G, Baglivo V et al. A meta‐analysis of efficacy and safety of aripiprazole in adult and pediatric bipolar disorder in randomized controlled trials and observational studies. J Affect Disord 2016;191:187‐208. [DOI] [PubMed] [Google Scholar]
- 108. Liu HY, Potter MP, Woodworth KY et al. Pharmacologic treatments for pediatric bipolar disorder: a review and meta‐analysis. J Am Acad Child Adolesc Psychiatry 2011;50:749‐62. [DOI] [PubMed] [Google Scholar]
- 109. Jochim J, Rifkin‐Zybutz R, Geddes J et al. Valproate for acute mania. Cochrane Database Syst Rev 2019;10:CD004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bloch MH, Panza KE, Landeros‐Weisenberger A et al. Meta‐analysis: treatment of attention‐deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry 2009;48:884‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yu L, Yan J, Wen F et al. Revisiting the efficacy and tolerability of topiramate for tic disorders: a meta‐analysis. J Child Adolesc Psychopharmacol 2020;30:316‐25. [DOI] [PubMed] [Google Scholar]
- 112. Hollis C, Pennant M, Cuenca J et al. Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis. Health Technol Assess 2016;20:1‐450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zheng W, Li X, XIang Y et al. Aripiprazole for Tourette's syndrome: a systematic review and metaanalysis. Hum Psychopharmacol Clin Exp 2016;31:11‐8. [DOI] [PubMed] [Google Scholar]
- 114. Freeman K, Riley A, Duke D et al. Systematic review (and meta‐analysis) of behavioral interventions for fecal incontinence with constipation. J Pediatr Psychol 2014;39:887‐902. [DOI] [PubMed] [Google Scholar]
- 115. Brazzelli M, Griffiths P, Cody JT et al. Behavioural and cognitive interventions with or without other treatments for the management of faecal incontinence in children. Cochrane Database Syst Rev 2011;12:CD0022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miyahara M, Sl H, Pridham L et al. Task‐oriented interventions for children with developmental co‐ordination disorder. Cochrane Database Syst Rev 2017;7:CD010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gillies D, Taylor F, Gray C et al. Psychological therapies for the treatment of post‐traumatic stress disorder in children and adolescents (Review). Evid Based Child Health 2013;8:1004‐116. [DOI] [PubMed] [Google Scholar]
- 118. Scotto Rosato N, Correll CU, Pappadopulos E et al. Treatment of maladaptive aggression in youth: CERT guidelines II. Treatments and ongoing management. Pediatrics 2012;129:e1577‐86. [DOI] [PubMed] [Google Scholar]
- 119. Knapp P, Chait A, Pappadopulos E et al. Treatment of maladaptive aggression in youth: CERT guidelines I. Engagement, assessment, and management. Pediatrics 2012;129:e1562‐76. [DOI] [PubMed] [Google Scholar]
- 120. Findling RL, Robb A, McNamara NK et al. Lithium in the acute treatment of bipolar i disorder: a double‐blind, placebo‐controlled study. Pediatrics 2015;136:885‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bahji A, Ermacora D, Stephenson C et al. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta‐analysis. J Affect Disord 2020;269:154‐84. [DOI] [PubMed] [Google Scholar]
- 122. DelBello MP, Goldman R, Phillips D et al. Efficacy and safety of lurasidone in children and adolescents with bipolar I depression: a double‐blind, placebo‐controlled study. J Am Acad Child Adolesc Psychiatry 2017;56:1015‐25. [DOI] [PubMed] [Google Scholar]
- 123. Detke HC, DelBello MP, Landry J et al. Olanzapine/fluoxetine combination in children and adolescents with bipolar I depression: a randomized, double‐blind, placebo‐controlled trial. J Am Acad Child Adolesc Psychiatry 2015;54:217‐24. [DOI] [PubMed] [Google Scholar]
- 124. Skarphedinsson G, Hanssen‐Bauer K, Kornør H et al. Standard individual cognitive behaviour therapy for paediatric obsessive‐compulsive disorder: a systematic review of effect estimates across comparisons. Nord J Psychiatry 2015;69:81‐92. [DOI] [PubMed] [Google Scholar]
- 125. Andersson G, Titov N, Dear BF et al. Internet‐delivered psychological treatments: from innovation to implementation. World Psychiatry 2019;18:20‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Linardon J, Cuijpers P, Carlbring P et al. The efficacy of app‐supported smartphone interventions for mental health problems: a meta‐analysis of randomized controlled trials. World Psychiatry 2019;18:325‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cuijpers P. Targets and outcomes of psychotherapies for mental disorders: an overview. World Psychiatry 2019;18:276‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cuijpers P, Noma H, Karyotaki E et al. A network meta‐analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry 2020;19:92‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]


