Abstract
The Hierarchical Taxonomy of Psychopathology (HiTOP) is an empirical effort to address limitations of traditional mental disorder diagnoses. These include arbitrary boundaries between disorder and normality, disorder co‐occurrence in the modal case, heterogeneity of presentation within disorders, and instability of diagnosis within patients. This paper reviews the evidence on the validity and utility of the disinhibited externalizing and antagonistic externalizing spectra of HiTOP, which together constitute a broad externalizing superspectrum. These spectra are composed of elements subsumed within a variety of mental disorders described in recent DSM nosologies, including most notably substance use disorders and “Cluster B” personality disorders. The externalizing superspectrum ranges from normative levels of impulse control and self‐assertion, to maladaptive disinhibition and antagonism, to extensive polysubstance involvement and personality psychopathology. A rich literature supports the validity of the externalizing superspectrum, and the disinhibited and antagonistic spectra. This evidence encompasses common genetic influences, environmental risk factors, childhood antecedents, cognitive abnormalities, neural alterations, and treatment response. The structure of these validators mirrors the structure of the phenotypic externalizing superspectrum, with some correlates more specific to disinhibited or antagonistic spectra, and others relevant to the entire externalizing superspectrum, underlining the hierarchical structure of the domain. Compared with traditional diagnostic categories, the externalizing superspectrum conceptualization shows improved utility, reliability, explanatory capacity, and clinical applicability. The externalizing superspectrum is one aspect of the general approach to psychopathology offered by HiTOP and can make diagnostic classification more useful in both research and the clinic.
Keywords: HiTOP, externalizing, disinhibition, antagonism, antisocial personality disorder, Cluster B personality disorders, substance use disorders, clinical utility
The Hierarchical Taxonomy of Psychopathology (HiTOP) consortium aims to integrate research on the empirical organization of psychopathology, with the goal of delineating a comprehensive descriptive system1, 2, 3. Taxonomies in frequent use (e.g., the DSM) have notable limitations, such as arbitrary boundaries between psychopathology and normality, diagnostic instability, heterogeneity within disorders, disorder co‐occurrence in the modal case, and inability to conceptualize subthreshold cases. The HiTOP approach mitigates such problems by: a) defining psychopathology in terms of continua ranging from normative to maladaptive; b) delineating continua based on observed covariation among signs, symptoms and syndromes, and c) arranging continua in a hierarchy, ranging from more narrow and specific (e.g., clusters of symptoms) to more broad and general (e.g., spectra of inter‐related diagnostic phenomena).
An approach based on continua or dimensions of human individual differences resolves issues of arbitrary thresholds and diagnostic instability. Thresholds indicating specific clinical options can be described based on evidence, and test‐retest reliability of dimensional psychopathology constructs is notably greater than that of arbitrary diagnostic categories4, 5, 6, 7. No patients are excluded from the system (i.e., individuals with subthreshold or atypical symptoms are all characterized on a set of dimensions), providing a boon to case conceptualization. The HiTOP approach also reduces diagnostic heterogeneity by grouping empirically related symptoms together and arraying them along distinguishable dimensions8, 9, 10, 11. Comorbidity is rendered understandable, because related conditions form elements in psychologically coherent spectrums.
The working HiTOP system currently includes six broad spectrums: internalizing, somatoform, disinhibited externalizing, antagonistic externalizing, thought disorder, and detachment1, 2, 3. These spectrums reflect continuous individual differences in a given domain across the entire population. Broad spectrums, in turn, are combined into larger groupings or superspectra: emotional dysfunction (internalizing and somatoform), externalizing (disinhibited and antagonistic), and psychosis (thought disorder and detachment)12, 13, 14, 15, 16. Above these superspectra, the HiTOP approach also recognizes a general psychopathology factor17, 18.
The working HiTOP system was created by reviewing a considerable body of research, but external validity and utility have been less well documented, because previous reviews of these topics had limited scope. With this in mind, the Utility Workgroup of the HiTOP consortium assembled teams of experts to comprehensively review evidence on the validity and utility of the working HiTOP model. Expert reviews were organized according to the three superspectra. The present paper is the second in this series (the first focused on psychosis 19 and the third will examine emotional dysfunction) and focuses on the externalizing superspectrum.
The externalizing superspectrum encompasses two spectra: disinhibited externalizing and antagonistic externalizing. The disinhibited externalizing spectrum includes tendencies to act on impulse, without consideration for potential consequences. Empirically, disinhibition tends to be accompanied by societally prohibited behaviors that align psychologically with the core of the construct, for example, the use of psychoactive substances to excess 20 and with minimal regard for future consequences. The antagonistic externalizing spectrum includes tendencies to navigate interpersonal situations using antipathy and conflict, and to hurt other people intentionally 21 , with little regard for their rights and feelings.
These spectra encompass both maladaptive traits and more time‐limited symptoms, with the distinction pertaining to the timescale of the phenomena 22 . For example, a series of specific disinhibited behaviors (e.g., a brief period encompassing impulsive purchases and other decisions that reflect immediate reward more than longer‐term consequences) could be driven by a specific life crisis, rather than being generally characteristic of a person. If such behaviors persist across time and circumstances, they become additionally indicative of a disinhibitory trait. Similarly, a specific hostile interaction is an antagonistic phenomenon, while frequent and recurrent hostile interactions are indicative of an antagonistic trait. As described at length throughout this review, disinhibited and antagonistic behaviors tend to co‐occur at notably greater than chance levels, illustrating the phenotypic coherence of the broad externalizing superspectrum 23 .
The goal of this paper is to review the extensive evidence documenting the structural coherence and content of the externalizing superspectrum and the disinhibited and antagonistic spectra, and the utility and validity of these diagnostic constructs.
STRUCTURAL EVIDENCE
Composition of major dimensions
The externalizing superspectrum has long emerged in research on the structure of mental disorders and of maladaptive personality traits. Indeed, studies have revealed that externalizing psychopathology is separate from other superspectra, including internalizing psychopathology in youth24, 25, 26, 27, 28, 29, 30 and both internalizing and thought disorder/psychosis in adults31, 32, 33, 34. Across these bodies of research, clinical diagnoses or dimensional symptom counts of antisocial personality disorder (PD), attention‐deficit/hyperactivity disorder (ADHD), alcohol, cannabis, nicotine, and other substance use disorders (SUDs), and intermittent explosive disorder in adulthood, as well as conduct disorder (CD) and oppositional defiant disorder (ODD) in childhood, clearly reflect a distinct and overarching externalizing superspectrum, as summarized in Table 1 and pictured in Figure 1.
Table 1.
Structural evidence on the externalizing superspectrum and the disinhibited and antagonistic spectra
| Sample size | Sample type | ASPD | AUD | Other SUD | IED | CD | ODD | ADHD | NPD | PPD | HPD | BPD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Externalizing superspectrum | |||||||||||||
| Dunedin Multidisciplinary Health and Development Study (Caspi et al 31 , Krueger et al 35 ) | 1,037 | Community/longitudinal | + | + | + | + | |||||||
| Early Developmental Stages of Psychopathology (Beesdo‐Baum et al 36 , Wittchen et al 37 ) | 3,021 | Community/longitudinal | + | + | + | ||||||||
| NESARC waves 1 and/or 2 (Carragher et al 38 , Eaton et al 39 , Keyes et al 32 , Lahey et al 40 ) | 43,093 & 34,653 | Community/adults | + | + | + | + | – | – | |||||
| Tennessee Twin Study (Lahey et al 27 , Waldman et al 41 ) | 4,050 | Community/children & adolescents | + | + | + | ||||||||
| WMH Surveys (de Jonge et al 42 , Kessler et al 43 ) | 21,229 | Community/ longitudinal | + | + | + | + | + | + | |||||
| Blanco et al 24 | 9,244 | Community/adolescents | + | + | + | + | + | ||||||
| Castellanos‐Ryan et al 25 | 2,232 | Community/adolescents | + | + | + | + | + | ||||||
| Conway et al 44 | 25,002 | University/adults | + | + | + | ||||||||
| Cox et al 45 | 5,877 | Community/adults | + | + | + | ||||||||
| Forbush & Watson 46 | 16,423 | Community/adults | + | + | + | + | + | + | + | ||||
| Forbush et al 47 | 1,434 | Community/longitudinal | + | + | + | + | |||||||
| Gomez et al 26 | 2,099 | Outpatient/youth | + | + | + | ||||||||
| James & Taylor 48 | 1,197 | Community/adults | + | + | + | – | |||||||
| Krueger 49 | 8,098 | Community/adults | + | + | + | ||||||||
| Krueger et al 50 | 1,048 | Community/adolescents | + | + | + | ||||||||
| Martel et al 28 | 2,512 | Community/children | + | + | + | ||||||||
| Martel et al 28 | 8,012 | Community/adults | + | + | + | + | + | ||||||
| Miller et al 51 | 1,325 | Veterans/adults | + | + | + | ||||||||
| Miller et al 52 | 214 | Veterans/adults | + | – | + | + | |||||||
| Olino et al 29 | 541 | Community/children | + | + | |||||||||
| Tuvblad et al 53 | 1,219 | Community/children | + | + | + | ||||||||
| Verona et al 33 | 4,745 | Community/adults | + | + | + | ||||||||
| Verona et al 30 | 223 | Mixed/youth | – | – | + | + | + | ||||||
| Young et al 54 | 668 | Community/adolescents | + | + | + | ||||||||
| Total positive | 11/11 | 16/17 | 17/19 | 2/2 | 15/15 | 10/10 | 14/14 | 0/0 | 0/1 | 0/0 | 2/4 | ||
| Disinhibited spectrum | |||||||||||||
| MIDAS (Forbes et al 13 , Kotov et al 55 ) | 2,900 | Outpatients/adults | + | + | + | + | – | – | – | – | |||
| Norwegian Institute of Public Health Twin Panel (Kendler et al 56 , Røysamb et al 57 ) | 2,794 | Community/adults | + | + | + | + | – | – | – | + | |||
| Conway & Brown 58 | 4,928 | Outpatients/adults | + | + | |||||||||
| Conway et al 59 | 815 | Community/longitudinal | + | + | |||||||||
| Farmer et al 60 | 816 | Community/longitudinal | + | + | + | + | |||||||
| Kim & Eaton 61 | 43,093 | Community/adults | + | + | + | ||||||||
| Slade & Watson 62 | 10,641 | Community/adults | + | + | |||||||||
| Vollebergh et al 63 | 7,076 | Community/adults | + | + | |||||||||
| Wright & Simms 64 | 628 | Outpatients/adults | + | + | + | – | – | – | – | ||||
| Wright et al 34 | 8,841 | Community/adults | + | + | |||||||||
| Total positive | 5/5 | 10/10 | 10/10 | 3/3 | 0/3 | 0/3 | 0/3 | 1/3 | |||||
| Antagonistic spectrum | |||||||||||||
| MIDAS (Forbes et al 13 , Kotov et al 55 ) | 2,900 | Outpatients/adults | + | – | – | + | + | + | + | + | |||
| Norwegian Institute of Public Health Twin Panel (Kendler et al 56 , Røysamb et al 57 ) | 2,794 | Community/adults | – | – | – | – | + | + | + | + | |||
| Farmer et al 60 | 816 | Community/longitudinal | – | – | – | – | + | + | |||||
| Kim & Eaton 61 | 43,093 | Community/adults | + | – | – | ||||||||
| Wright & Simms 64 | 628 | Outpatients/adults | – | – | – | + | + | + | – | ||||
| Total positive | 2/5 | 0/5 | 0/5 | 1/3 | 1/1 | 1/1 | 3/3 | 3/3 | 3/3 | 2/3 | |||
+: indicator included in analysis with meaningful loading (.30 or larger), –: indicator included in analysis but did not load meaningfully. ASPD – antisocial personality disorder, AUD – alcohol use disorder, SUD – substance use disorder, IED – intermittent explosive disorder, CD – conduct disorder, ODD – oppositional defiant disorder, ADHD – attention‐deficit/hyperactivity disorder, NPD – narcissistic personality disorder, PPD – paranoid personality disorder, HPD – histrionic personality disorder, BPD – borderline personality disorder, NESARC – National Epidemiologic Survey on Alcohol and Related Conditions, WMH – World Mental Health, MIDAS – Methods to Improve Diagnostic Assessment and Services.
Figure 1.
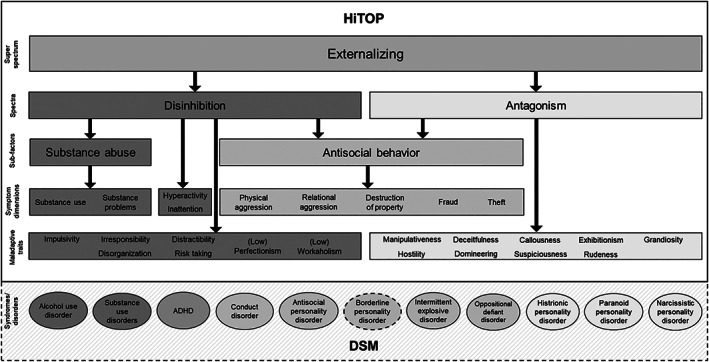
Conceptual model of the externalizing superspectrum. Dashed lines represent provisional inclusion. Specifically, the dashed line surrounding borderline personality disorder represents that this disorder falls under two superspectra (externalizing and internalizing). The dashed line surrounding the diagnoses section indicates that these categorical diagnoses do not belong to the model; they are meant to represent how a dimensional model encapsulates DSM diagnoses. HiTOP – Hierarchical Taxonomy Of Psychopathology, ADHD – attention‐deficit/hyperactivity disorder.
The extant evidence further supports parsing the externalizing superspectrum down into disinhibited and antagonistic externalizing spectra 1 . This bifurcation is more clearly evident in maladaptive trait research and in the adult rather than the child psychopathology literature, and these major domains can be observed in the psychiatric diagnosis literature as well.
As summarized in Table 1, three main observations are evident from this literature. First, the majority of studies identify antisocial PD as an indicator of both disinhibition and antagonism, which supports this disorder as a non‐specific and core indicator of the general externalizing superspectrum. In fact, the criteria for antisocial PD are quite evenly spread across both disinhibited and antagonistic features. Second, alcohol and other SUDs are specific to the disinhibited externalizing spectrum. Third, some DSM PDs (i.e., paranoid, narcissistic and histrionic) appear relatively specific to the antagonistic externalizing spectrum. These findings are also generally consistent with Krueger et al’s multifaceted model of the externalizing spectrum 15 , which considers general externalizing together with more specific liability factors for callous‐aggression (the unique component of antagonism) and substance misuse (the unique component of disinhibited externalizing).
One condition deserving specific consideration is borderline PD, as its relevance to general externalizing, as well as its specificity to antagonism vs. disinhibition, appears dependent on other indicators included in the structural model. In studies in which internalizing psychopathology is also prominently featured, borderline PD tends to load robustly with internalizing and less consistently with externalizing39, 46, 48, 52; moreover, when dimensional traits are considered in addition to psychiatric diagnoses, this PD loads distinctly on internalizing 64 . In other words, the preponderance of research evidence indicates that borderline PD does load with the internalizing spectrum, while its association with externalizing (and even specific placement within antagonism vs. disinhibition)65, 66 is less clear. At this point, borderline PD is therefore best considered an indicator of both internalizing and, to a lesser degree, the general externalizing superspectrum, likely with different components of the disorder being related to these two spectra. As such, borderline PD is only provisionally included in the externalizing superspectrum, as noted in Figure 1.
It is further noteworthy that, while clearly representing antagonistic externalizing in the context of the broader externalizing superspectrum, paranoid and histrionic PD have other influences as well, given their multifaceted nature. For instance, paranoid PD may appear more strongly linked to the psychosis superspectrum when disorders of this type are clearly represented in the set of structural indicators13, 32, 65. Histrionic PD also has direct links (in the negative direction) to the detachment spectrum 64 , which is also supported in the general personality literature67, 68.
Finally, although the externalizing superspectrum is well represented in the youth psychopathology literature, evidence for bifurcation of disinhibition and antagonism prior to adulthood is less clear (see Table 1), likely owing to the lack of clearly defined indicators for making this separation. In contrast with the adult literature, there are no diagnoses or explicit symptom measures of callous‐unemotional traits, narcissism, or paranoia/suspiciousness included in structural modeling studies with children/adolescents, making it virtually impossible for these factors to emerge in the youth literature. Additionally, in young children, substance use is likely to be uncommon. Furthermore, links between personality traits and disorders are less well established in the youth (especially child) literature 69 , making an analysis from this perspective less straightforward. Further research is definitely needed to obtain a clearer picture of psychiatric representations of antagonism in youth, especially beyond what are typically referred to as callous‐unemotional traits 70 .
Role of personality traits
The hierarchical structure of the externalizing superspectrum closely parallels the organization of normal‐range personality traits1, 71. The general externalizing dimension is broadly linked to individual differences in the higher‐order trait factor of constraint vs. disconstraint 72 , which emerges in three‐factor models of normal and abnormal personality72, 73, 74, 75. When additional factors are extracted, this broad constraint vs. disconstraint dimension divides into two more specific components: agreeableness vs. antagonism, and conscientiousness vs. disinhibition73, 75, 76. These two subdimensions, in turn, form the basis for distinguishing antagonistic from disinhibited forms of externalizing.
Antagonistic externalizing has been linked to a variety of specific maladaptive traits that reflect problematic relations with others. It should be noted that some of these traits also show lesser associations with other forms of psychopathology1, 77, 78, 79, 80, 81, 82, 83. The traits that have been most strongly and consistently associated with the antagonistic externalizing spectrum include manipulativeness (i.e., exploiting and taking advantage of others), deceitfulness (i.e., lying and cheating in pursuit of one’s goals), callousness (i.e., being cold‐hearted and lacking empathy), exhibitionism (i.e., engaging in attention‐seeking behaviors), grandiosity (i.e., being arrogant and feeling entitled to special treatment from others), aggression (i.e., engaging in hostile and even violent behavior), rudeness (i.e., being blunt, tactless, and interpersonally insensitive), domineering (i.e., the proneness to be forceful and controlling in relationships), and suspiciousness (i.e., questioning the honesty, fidelity, and motives of others).
Disinhibited externalizing has also been linked to multiple maladaptive traits reflecting disorganization, poor impulse control, and a lack of concern regarding the consequences of one’s behavior1, 77, 78, 80, 81, 82, 83. The specific traits that have been most strongly and consistently associated with the disinhibited externalizing spectrum include impulsivity (i.e., acting spontaneously on the spur of the moment without concern for consequences), irresponsibility (i.e., being undependable and failing to fulfill obligations), distractibility (i.e., problems in attention and difficulties in focusing on tasks), risk taking (i.e., being reckless and engaging in potentially dangerous activities), (low) perfectionism (i.e., having low standards for the completion of work), and (low) workaholism (i.e., being more interested in having fun than in work‐related activities).
These trait correlates, in turn, help to explain the specific types of personality‐related pathology that are subsumed within each spectrum, including both adult1, 67, 84, 85 and youth disorders86, 87, 88, 89, 90, 91, 92, 93. As can be seen in Figure 1, the antagonistic externalizing spectrum subsumes narcissistic, paranoid and histrionic PDs. Disinhibited externalizing includes ADHD, alcohol use disorder, and SUDs. Disorders such as conduct disorder, antisocial PD, intermittent explosive disorder, ODD, and borderline PD contain trait characteristics related to both spectra (e.g., impulsivity and anger/aggression).
VALIDITY EVIDENCE
Behavior genetic evidence
Evidence for a genetically coherent externalizing superspectrum has emerged most strongly from twin studies of constituent disorders and related personality traits in both youth and late adolescent/adult samples.
Specifically, in youth samples, twin studies have shown high heritabilities (h 2 ) and moderate levels of non‐shared environmental influences, but non‐significant shared environmental influences, for ADHD (h 2 =~60‐80%) 94 and ODD (h2=~30‐70%) 93 , as well as for psychopathic traits (such as callous‐unemotionality and narcissism) 95 . These studies have also found moderate heritability (h2=~50%), shared environmental influences, and non‐shared environmental influences for CD96, and moderate heritability for various forms of youth antisocial behavior, including rule breaking and aggression 97 , with its various forms such as reactive, proactive and relational aggression96, 98, 99.
Most importantly, behavior genetic studies have provided evidence for the coherence of the externalizing superspectrum by showing high levels of genetic overlap across ADHD, ODD and CD41, 100, 101, such that the largest contributor to the overlap among these disorders or the covariation among their symptom dimensions is represented by common genetic influences. This is also borne out by studies that have directly estimated the magnitude of genetic influences on an externalizing factor, and have found it to be highly heritable41, 102.
Evidence for the genetic basis of the externalizing superspectrum in youth also includes studies that have demonstrated common genetic influences between these disorders and personality traits such as behavioral disinhibition, neuroticism, and low prosociality54, 103, 104.
Twin studies in late adolescent/adult samples provide considerable evidence for the validity of the externalizing superspectrum54, 103, 104. This evidence comes from studies of PDs, SUDs, and their symptom dimensions and related traits (e.g., antisocial behavior).
The “Cluster B” PDs, when examined individually, exhibit moderate to large heritability estimates 105 . The covariance among these disorders can be accounted for by a genetic common factor, with a second genetic factor accounting for variance in antisocial and borderline PDs 106 . Antisocial PD has also been included as an observed indicator in a highly heritable externalizing factor50, 103. Relatedly, Kendler et al 66 reported evidence for a genetically coherent “Axis I” externalizing factor encompassing antisocial PD as well as CD, alcohol abuse/dependence, and drug abuse/dependence. These authors also found a genetically coherent “Axis II” externalizing factor encompassing dependent, histrionic, narcissistic, obsessive‐compulsive, paranoid and borderline PDs, along with eating disorders.
The DSM‐5 includes an alternative dimensional model of PDs as opposed to the criteria of the categorical diagnostic model. Most relevant to externalizing are the higher‐order domains of antagonism and disinhibition, which are moderately heritable107, 108. In a joint exploratory factor analysis including the DSM‐5 alternative trait model domains, PD symptoms and normal personality domains, three genetic factors emerged: a PD/neuroticism factor, an antagonism/antisocial factor, and a factor reflecting schizoid/detachment 109 .
Twin/family studies compellingly demonstrate that SUDs are genetically influenced, with ~50% of the variance in alcohol use disorders 110 , 50‐60% in problematic cannabis use 111 , ~40‐80% in cocaine use disorders105, 112, 113, 20‐50% in opioid dependence105, 112, and ~60% in nicotine dependence 114 being due to genetic influences. Critically, twin studies indicate that genetic influences are largely shared across SUDs 115 . Further, related psychiatric and behavioral manifestations, such as childhood conduct problems, adult antisocial behavior, behavioral undercontrol and impulsivity 116 , also load on this shared genetic factor, which is highly heritable (~80%)50, 54. A general liability towards externalizing explains the majority of genetic influences for alcohol and other SUDs, including 74‐80% of the genetic influences on alcohol use disorders and 62‐74% of those on other SUDs; it also accounts for 33‐37% of the genetic influences on nicotine dependence.
Molecular genetic evidence
Molecular genetic research also supports an appreciable contribution of genes to individual disorders and traits within the externalizing superspectrum.
Candidate gene studies of ADHD have provided some suggestive evidence of association for genes within the dopamine and serotonin neurotransmitter systems, including the dopamine transporter and D4 and D5 receptor genes (DAT1, DRD4 and DRD5), the serotonin transporter and receptor 1 genes (5HTT and HTR1B), and the synaptosomal‐associated protein 25 gene (SNAP‐25) 117 .
Genome‐wide association studies (GWAS) of various childhood disorders, such as ADHD 118 , CD 119 , and ODD or CD within the context of ADHD 120 , have found evidence for several genome‐wide significant associations and polygenic influences, each with a small effect size, that contribute to the risk for these disorders. In addition, moderate genetic correlations have been found between ADHD and other disorders, such as depression and anorexia nervosa; related traits, such as neuroticism and subjective well‐being (negative); and important life outcomes, including ever having smoked, the number of cigarettes smoked per day, and intelligence and educational attainment (both negative) 120 .
Interestingly, ADHD was not genetically correlated with antisocial behavior in another study, likely due to the relatively small sample size and the heterogeneity of measures of antisocial behavior 121 . In contrast, ODD or CD in the context of ADHD was highly genetically correlated with aggression and antisocial behavior, and its polygenic risk score was more predictive of cognitive functioning, educational outcomes, and having children at a younger age than that for ADHD without ODD or CD 120 . Nonetheless, the maximum variance explained by the polygenic risk score in these outcomes was quite low (0.36%).
In adolescent and adult samples, GWAS of externalizing PDs are still in their infancy, with only borderline and antisocial PDs being investigated to date, using relatively small samples. One molecular genetic study indicated that borderline PD is heritable 122 , but did not test for its genetic association with any other form of externalizing psychopathology. Current GWAS evidence indicates that antisocial behavior is heritable and significantly genetically correlated with CD and neuroticism, but not with schizophrenia, bipolar disorder or ADHD 121 . Furthermore, a study found high genetic correlations of antisocial behavior with lifetime cannabis use and cigarette smoking, but not with alcohol consumption 123 , while another study did not find an association between polygenic risk scores for antisocial PD and either tobacco or alcohol use 124 . A GWAS of antisocial PD 125 reported the most associated gene (ABCB1) to be one involved in immune function and associated with various forms of substance abuse. These studies have also found that many common genetic variants, each with a small effect size, contribute to risk for antisocial behavior. Finally, a large GWAS of normal personality traits did not find that agreeableness has genetic correlations with any externalizing disorders or other forms of psychopathology 126 .
The majority of GWAS on substance use have focused on alcohol‐related phenotypes, including alcohol dependence 127 , alcohol use disorder 128 , number of alcoholic drinks per week 129 , and maximum alcohol intake. Studies of these phenotypes have employed moderately to extremely large sample sizes, thus being well‐powered. One finding which robustly emerged from these GWAS is that genetic influences on alcohol consumption are only moderately correlated with those on alcohol use disorders 130 . Cannabis related GWAS are beginning to reach adequate power131, 132, 133, but still require even larger samples. GWAS on cocaine dependence134, 135 and opioid dependence136, 137, 138 are currently underpowered. It is important to note that, even in large cohorts, polygenic risk scores continue to predict only small proportions of the variance in independent samples (e.g., the polygenic risk score from a GWAS involving ~1 million participants explained only about 2.5% of the variance in alcohol consumption).
Newer multivariate methods such as genomic structural equation modeling (genomic SEM)139, 140 can be used to model the underlying factor structure of genetic correlations from a set of phenotypes of interest using GWAS summary statistics. These methods enable researchers to move beyond a single disorder or behavior in gene identification efforts, and instead focus on identifying genes contributing to the underlying latent factor(s). Genomic SEM is currently being applied in the international Externalizing Consortium, which analyzed genome‐wide data on seven phenotypes related to the externalizing superspectrum from ~1.5 million people and identified nearly 600 significant genetic loci associated with a general liability to externalizing 141 . A polygenic risk score derived from this dataset predicted up to 10% of the variance in general externalizing scores in independent samples, and emerged as significant in both within‐sibling and between‐sibling comparisons. These analyses suggest that focusing gene identification efforts on general externalizing liability, rather than on individual externalizing disorders/behaviors, is a fruitful approach to advancing knowledge of genes contributing to this psychopathological domain.
Environmental risk factors
Decades of observational research have identified a wide range of environmental risk factors for externalizing problems, spanning a variety of social domains. Meta‐analyses document that abuse, neglect, hostile parenting, neighborhood violence, and affiliation with deviant peers all exhibit significant associations with diverse externalizing phenomena142, 143, 144. Longitudinal research in the community confirms that these effects can endure through adolescence and beyond 145 .
Effects of toxic environments are not only robust, but also diffuse. That is, prominent etiological events appear to engender risk for a variety of externalizing mental health conditions and maladaptive personality traits 146 . Indeed, there are essentially no known unique environmental risk factors for any substance use or behavioral disorder.
This observation prompted research on how environmental pathogens relate to composites of externalizing phenotypes. In an epidemiologic sample, various forms of childhood maltreatment predicted individual differences on a latent externalizing dimension constructed from substance use and antisocial behavior disorders 147 . This effect was replicated in a number of cohort studies148, 149. Across studies, the severity of social stress predicted variation in the broad externalizing factor, but not unique components of the specific observed externalizing conditions. This pattern is evident in research on other risk factors that focus on externalizing outcomes which transcend traditional disorder boundaries. Peer victimization, discrimination experiences, and other chronically stressful conditions such as romantic conflict and unemployment, all predicted standing on a latent externalizing spectrum in separate community samples150, 151.
The connection between externalizing problems and environmental stressors over time is almost certainly bidirectional. Research in community samples shows that variation in a latent externalizing factor predicts future rates of both acute life events (e.g., arrest) and ongoing strains (e.g., marital discord)152, 153. These stressful conditions, in turn, presumably set the stage for continued externalizing behavior. This type of person‐environment fit implies a vicious cycle of stress exposure and worsening externalizing problems, akin to the transactional peer selection and socialization effects on externalizing risk in adolescence 145 .
As a whole, longitudinal research has revealed strong connections between a wide range of environmental exposures and the externalizing superspectrum. Much less is known about whether certain environments predispose selectively to disinhibited vs. antagonistic spectra (or any other more homogeneous components) within the superspectrum. The available data at this time suggest that environmental risk is largely non‐specific. More research using genetically informative designs is needed to verify the etiologic roles of putative environmental risk factors by controlling for passive gene‐environment correlation (e.g., parents creating a home environment that is influenced by their heritable characteristics) 154 .
Cognitive and emotional processing abnormalities
Generally speaking, the externalizing superspectrum model helps to organize the literature on cognitive deficits, as reflected in Figure 1. In particular, there is overwhelming evidence that cognitive impairment is prominent in disinhibited forms of externalizing.
Evidence of impaired executive functioning is most substantial for antisocial PD155, 156, 157, 158, 159 and CD160, 161, followed by disinhibitory traits162, 163, 164, 165, 166. Additionally, deficits in sustained attention, inhibitory control, and sluggish cognitive tempo are associated with ADHD162, 167, 168, 169, 170, 171, 172. There is evidence of cognitive deficits in children with ODD, albeit less abundant173, 174, which might be partly explained by high comorbidity with both ADHD and CD175, 176. There is even less evidence of cognitive deficits related to intermittent explosive disorder, which is mostly characterized by impairments in social cognition and emotion regulation177, 178, 179, 180. Impairments in executive functions are extensively reported in individuals with drug and alcohol dependence181, 182, 183, 184, 185, 186, 187, 188.
Under the antagonistic externalizing spectrum, the evidence of cognitive deficits is strong for borderline PD189, 190, whereas findings concerning narcissistic, histrionic and paranoid PDs are mostly derived from symptom, descriptive and trait checklists191, 192.
Antisocial traits are linked with deficits in the ability to regulate emotions and diminished responsiveness to distress in others193, 194, 195, 196. ODD is associated with deficits in empathy, and impaired emotion regulation has been reported in both ODD and intermittent explosive disorder180, 197, 198. There is evidence for emotion dysregulation impairments also in substance dependent individuals199, 200, 201.
Impaired facial affect recognition and emotional regulation deficits are observed in individuals with borderline PD202, 203. The evidence concerning narcissistic and paranoid PDs (respectively, difficulties in emotional empathy and regulation 204 , and hypervigilance and stress reactivity 205 ) has come from symptom, descriptive and trait checklists, rather than behavioral task performance.
Neurophysiological indicators
The best‐established neurophysiological indicator of broad externalizing is reduced amplitude of the visual P300 (P3) event‐related potential (ERP) 206 , a positive‐going ERP that occurs in relation to rare or otherwise salient visual events within an ongoing stimulus series.
Originally thought to be indicative of proneness to alcohol problems 207 , subsequent research showed reduced P3 to be related to various other externalizing conditions as well 208 . Ultimately, it became clear that P3 operates as an indicator of the highly heritable liability for externalizing problems in general209, 210. Like broad externalizing, P3 amplitude is appreciably heritable, and its association with this superspectrum factor reflects additive genetic influences in common between the two211, 212.
Other evidence points to a genetically‐based association between broad externalizing and performance on executive control tasks 213 , and overlap is evident in the relations of P3 amplitude and executive task performance with broad externalizing166, 214. The implication is that reduced P3 reflects a weakness in cognitive control capacity that is associated with heritable risk for externalizing problems in general215, 216, highlighting P3 as a marker of the broad externalizing factor at the superspectrum level of HiTOP.
Another less well‐established candidate indicator of broad externalizing is reduced amplitude of the error‐related negativity (ERN), a negative‐going ERP that is evident following errors in a speeded reaction time task, and is theorized to reflect performance monitoring and error detection processes. Reduced ERN was initially reported for individuals high in impulsive traits217, 218, and later for individuals high in broad externalizing 219 . Further research is needed, though, to evaluate the specificity of the relationship of reduced ERN to broad externalizing, and the neural systems basis of this relationship. In addition, research is needed on the etiologic basis of the association between ERN and externalizing problems, given the limited work of this kind to date 220 .
Studies that have specifically assessed antagonistic externalizing tendencies along with broad externalizing have shown reduced P3 and ERN in relation to the latter, but not to antagonism‐specific variance221, 222. By contrast, high antagonistic externalizing is reliably associated with reduced brain reactivity to fearful face stimuli. Multiple studies have reported reduced amygdala activation to fearful faces in children/adolescents exhibiting antagonistic externalizing tendencies (sometimes termed “callous unemotionality”)223, 224 along with conduct problems, compared to children lacking in antagonistic externalizing. Importantly, this effect has been found to be specific to antagonistic externalizing (callous‐unemotionality) by contrasting groups of children matched for externalizing problems but differing in levels of callous‐unemotionality 210 . Consistent with this, two studies225, 226 reported reduced early‐ERP responses to fearful faces in adults scoring high on a measure of antagonistic externalizing (termed “callousness”); broad externalizing was also assessed in these studies, and effects were shown to be attributable to callousness‐specific variance. This impaired responsiveness to fearful faces may reflect general emotional insensitivity among those high on antagonistic externalizing, or perhaps a more specific deficit in the capacity for empathy or affiliative capacity among these individuals 227 .
Interpretation of the research literature on neurophysiological indicators of problems situated specifically within the disinhibited externalizing spectrum of HiTOP – in particular, substance use problems – is hampered by a failure to differentiate between specific factors versus broad externalizing liability 228 , neglect of the distinction between liability indicators and symptom or “scar” indicators 229 , and the substance‐specific nature of particular indicators 230 . For example, while there is considerable evidence for a distinct role of reward system dysfunction in substance addictions, it remains unclear at this time whether addiction proneness entails heightened or diminished sensitivity to naturally occurring rewards231, 232, 233, due to limitations of existing research. To address these limitations, longitudinal studies are needed that differentiate between neural measures of premorbid liability to externalizing problems in general, as opposed to measures indicative of addiction liability more specifically, or active symptoms or persisting consequences of substance addiction 229 .
Neuroimaging
As with other psychiatric domains, the neuroimaging literature on externalizing has been dominated by case‐control studies of individual disorders, but these are now complemented by growing research taking the transdiagnostic dimensional approach. This work is identifying alterations in a number of circuits involved in social‐emotional processing, aversive learning, emotional regulation, and cognitive control, with varying levels of specificity between antagonism and disinhibition domains, as well as narrower lower‐order constructs that contribute to these domains. We highlight some of the key circuits as a demonstration of the compatibility of neuroimaging data with the HiTOP model of externalizing.
Among the most frequent findings is the observation of reduced amygdala volume, which has been seen in case‐control studies or disorder‐specific symptom measures of psychopathy and antisocial personality 234 , conduct and oppositional problems 174 , borderline personality235, 236, aggression and violence 237 , risk for substance use problems238, 239, and ADHD 240 . While amygdala volume reductions correlate with broad measures of externalizing traits241, 242, they appear most pronounced for callous‐unemotional and antagonistic traits174, 243 as opposed to disinhibition features.
Given the importance of the amygdala in social‐emotional processing, emotional responses to aversive stimuli, and aversive learning 244 , such findings fit with psychological and psychophysiological models emphasizing social‐emotional and fear learning deficits as core features in the etiology of antagonistic spectrum problems245, 246, 247. This having been said, reduced amygdala volume has also been reported for other diagnostic constructs (e.g., post‐traumatic stress disorder)245, 246, 247, such that the specificity of this association would benefit from further study.
Reductions in amygdala volume are paralleled by changes in task‐related activity in disorders with high antagonism characteristics, as repeatedly demonstrated in functional magnetic resonance studies of individuals with antisocial‐psychopathic and borderline personality traits248, 249. Again, the associations appear to most robustly reflect antagonism/callous‐unemotionality rather than disinhibition. For instance, lower task‐relevant activations are seen in the bilateral amygdala among individuals with ODD/CD as compared to ADHD in a number of tasks 174 , and studies using dimensional measures of symptom severity have repeatedly observed reductions in the amygdala response to social‐emotional stimuli in relation to callous‐unemotional traits210, 223, 250.
The amygdala is just one part of a limbic/paralimbic network that has been implicated in different aspects of externalizing251, 252. Neuroimaging studies of psychopathy especially emphasize the orbitofrontal/ventromedial prefrontal cortical (OFC/VMPFC) region 253 , that shares strong structural and functional connectivity with the amygdala 254 . Such an involvement is consistent with the key role of this region in social cognition, including empathy and moral reasoning255, 256, and has helped form the basis of one of the most prominent neural models of psychopathy 253 . Critically, portions of this region have long been associated with the ability to inhibit behavior, with lesions often causing both antisocial behavior and problems with impulsivity and disinhibition 257 . It is thus notable that phenotypic associations with structural and functional features in these circuits extend beyond antagonism or callous‐unemotional traits. Both human and animal models demonstrate the importance of the OFC/VMPFC region to both substance use history and the risk for developing substance use258, 259, 260 as well as behavioral addictions 261 .
Despite indications of overlap that point to involvement beyond antagonism or disinhibition domains, important differences emerge between ventromedial and ventrolateral prefrontal regions, which appear generally consistent with the core cognitive and emotional functions of these regions. Problems with social antagonistic factors are more prominently reflected in ventromedial regions, while alterations in ventrolateral regions (lateral orbital/inferior frontal) are more related to cognitive control (including response inhibition) and executive functions 262 . For instance, deficits in cognitive control show significant associations with task‐related inferior frontal gyrus engagement in both substance dependence and ADHD263, 264.
The dorsal anterior cingulate cortex has been of particular interest in relation to externalizing due to its role in both attention and error monitoring. Alterations in both structure and function have been reported for this area in relation to various externalizing conditions, including ADHD263, 265, 266, psychopathy and violent behavior 252 , disruptive behavior 267 , substance dependence260, 268, 269, and behavioral addictions 261 . These findings are of particular interest given the importance of this region in the generation of the ERN 219 , providing convergent evidence for a core role of this area in cognitive control deficits in externalizing problems as a whole (i.e., at the superspectrum level of the HiTOP system).
In considering the involvement of cortical areas in externalizing psychopathology, it should be noted that some neural correlates may extend quite broadly, even if particular areas play more focal roles in the expression of specific forms of externalizing. For example, the largest meta‐analysis to date of findings for ADHD 265 reported evidence not only for lower surface area in frontal, cingulate and temporal cortical regions, but also lower average effect across the whole cortical area, with the severity of this overall deficit declining from childhood to adolescence and eliminated by adulthood. It will be increasingly important to consider how phenotypic expressions of externalizing are related to, and change with, processes of brain maturation270, 271.
The basal ganglia have been a further focus of interest in the externalizing literature. In particular, dysfunction in mesolimbic and nigrostriatal systems has been repeatedly implicated in reward‐motivational processes relevant to risk for and development of addictions272, 273, 274, and also ADHD265, 275, 276. Differences in the functioning of these systems have been linked to altered processes of reward valuation, discounting behavior and impulsivity that characterize externalizing problems264, 277, 278, 279. Even with respect to antagonistic behaviors, individual differences in the functioning of mesolimbic circuits may dramatically affect the manner in which antagonistic actions are expressed – for example, in the sort of impulsive‐antisocial actions that emerge in these conditions.
In one of the few studies to examine neuroimaging activation in relation to an externalizing factor, while controlling for scores on a general psychopathology factor, fronto‐parietal network hypoactivation during a working memory task was related to increased scores on a “behavioral disturbance” factor, primarily comprising ADHD and CD symptoms 280 . These findings are complemented by recent work reporting relations for the same behavioral disturbance factor with enhanced connectivity within the fronto‐parietal control network, but decreased connectivity within the default mode network 281 . Other dimensional measures of externalizing have similarly been associated with network dysfunction in many of the same regions identified in the foregoing summary of findings282, 283. Consideration of neural networks and their features, as opposed to individual brain regions, almost certainly will prove essential to characterizing the role of neural systems and processes in externalizing problems.
Other biomarkers
Aberrant patterns of DNA methylation have been linked to externalizing psychopathology, including addiction284, 285 and antisocial behaviors286, 287, 288. Epigenetic findings also indicate common downstream biological processes in ODD and ADHD, including dysregulation of long‐term neuronal synaptic plasticity 289 . DNA methylation is thought to represent a molecular pathway through which environmental exposures become translated into phenotypic variation, conferring increased susceptibility to externalizing disorders290, 291. Accordingly, one study identified an epigenetic risk score to broad (tobacco, cannabis and alcohol) substance abuse liability, which mediated the prospective association between prenatal maternal tobacco smoking and adolescent substance use 292 .
An inflammation‐related epigenetic risk score at birth was associated with higher externalizing problems across childhood and adolescence 293 . Elevated levels of pro‐inflammatory markers (e.g., cytokines, C‐reactive protein) in peripheral tissues such as blood have also been reported in externalizing psychopathology294, 295, 296, including ADHD297, 298, 299, antisocial PD 300 , and substance abuse300, 301, 302, 303, although the overall evidence in this respect is mixed.
Meta‐analytic evidence supports lower cortisol levels in patients with ADHD 304 . In general, reduced cortisol is also associated with persistent aggression and other antisocial and disinhibited behaviors in children and adults305, 306, 307. Moreover, blunted cortisol response to stress has been associated with relapse in patients with addiction 308 . Thus, lower cortisol may reflect an impairment in the ability to regulate stress responses that underpins chronic externalizing psychopathology, as well as other forms of psychopathology more broadly 309 .
Low platelet monoamine oxidase B (MAO‐B) enzyme activity, which is a proxy of low central serotonergic functions, has been consistently shown to correlate with impulsive, aggressive and antisocial personality traits and behaviors, including ADHD 304 , alcohol‐related problems, and smoking 310 . The role of MAO‐B in externalizing disorders is thought to be independent of the effects of tobacco smoking on the enzyme 311 . Moreover, there is evidence for low cerebrospinal fluid serotonin metabolite 5‐hydroxyindoleacetic acid (5‐HIAA) levels characterizing alcohol abuse and antisocial behavior, including disinhibited forms of aggression312, 313, although this effect remains debated 314 . Thus, serotonin hypofunction may be a shared biological mechanism underlying disinhibited and antagonistic psychopathology.
Overall, research evidence suggests that conditions within the disinhibited and antagonistic externalizing spectra share common biological signatures. However, conclusions have been constrained by methodological limitations of the existing studies, including small sample sizes, focus on a single disorder, and paucity of longitudinal designs, which are particularly relevant for disentangling biological markers of risk vs. consequences of substance use and/or medication.
Temperamental antecedents
Continuity in the traits that underlie the externalizing superspectrum, beginning in early childhood through adolescence and adulthood, has been documented by research74, 315, 316, 317, 318, 319.
For example, disinhibition is captured by low effortful control in early childhood315, 316, which has been shown to be a robust predictor of subsequent externalizing behaviors320, 321. This literature is paralleled by evidence that low agreeableness and conscientiousness (captured together in low effortful control) together predict externalizing behaviors later in childhood and adolescence315, 320. Negative affectivity has also been found to consistently predict externalizing320, 321, but with low specificity, as it tends to act as a broadband risk for subsequent psychopathology31, 322.
A similar pattern of low effortful control and high negative affectivity has been found to prospectively predict antisocial behavior indicators, including CD, ADHD, ODD and antisocial PD13, 321, 323, 324, 325, 326, 327. By contrast, limited evidence exists for intermittent explosive disorder 328 . A large prospective study (N=4,983) in Australia found that high negative affectivity, low effortful control, and high surgency (extraversion) at age 4‐5 each uniquely predicted the development of ADHD and CD symptoms to age 12‐13 329 . Similarly, a study of two birth cohorts from Norway (N=797) found that high negative affectivity and high surgency predicted increases in ODD symptoms from age 4 to 6 326 . Although not included in traditional models of temperament, callous‐unemotional traits in childhood and adolescence (i.e., low empathy, lack of remorse, and insensitivity to distress of others) also robustly and prospectively predict risk for severe antisocial and related behaviors224, 330.
There is little evidence regarding the childhood antecedents of adult PDs included in the antagonistic spectrum of the HiTOP model (e.g., histrionic, narcissistic and paranoid PD) 317 , while some research has found that negative affectivity316, 331 and low effortful control331, 332 predict borderline PD, mirroring the findings for other externalizing disorders. Finally, SUDs, reflecting disinhibited externalizing in the HiTOP model, are consistently related to low effortful control333, 334, as well as high negative affectivity 334 , with some evidence also pointing to an association with surgency/extraversion (e.g., for cannabis use) 335 .
Overall, the combination of negative affectivity with low effortful control represents a consistent constellation of temperamental traits that acts as an antecedent to the externalizing superspectrum. Disinhibited and antagonistic spectra do not tend to show differential associations with childhood temperament, although there is some evidence that callous unemotionality represents an additional risk factor for severe antisocial behavior.
Illness course
Several authors have described a trajectory of externalizing behaviors that begins with hyperactivity and impulsivity in preschool‐age children, followed by delinquency in middle school, and SUDs and antisocial personality in late adolescence and emerging adulthood336, 337, 338. This pattern of progression of externalizing behaviors suggests a shared etiology, and has led to the suggestion that the so‐called “co‐occurrence” among individual DSM externalizing disorders is largely artifactual, stemming from the split of a unitary construct into multiple diagnoses.
The validity of the externalizing superspectrum is also supported by the high stability over time of externalizing behaviors339, 340, from middle childhood through late adolescence 340 . Olson et al 341 measured externalizing outcomes throughout the school‐age period and at age 17 using a multi‐informant approach. They found that children at risk for externalizing problems later in childhood and at age 17 were perceived as “difficult” and resistant to control as toddlers. Parental perceptions about child behaviors predicted externalizing behavior as early as at 13 months and remained persistent predictors throughout late adolescence.
Antagonistic and disinhibited spectra have not shown substantial evidence of differential patterns of developmental trajectories.
Treatment response
Numerous treatments have proven effective for a broad array of externalizing disorders in children and adolescents, including behavioral/psychosocial342, 343, 344, systems‐ or school‐based345, 346, 347, and psychopharmacological interventions348, 349, 350, 351, while only few treatments have been successfully used for externalizing in adults (for instance, motivational interviewing has long been used to treat SUDs, and treatment effects have been found to last up to two years, with 75% of participants gaining some type of improvement 352 ).
A meta‐analysis of 36 randomized, between‐subjects comparison studies of psychosocial treatment efficacy for externalizing problems in children less than 8 years of age 353 found that general externalizing symptoms showed the largest treatment response, followed by opposition/non‐compliance. Impulsivity/hyperactivity showed the weakest response (although the effect size was still within the “medium” range). These findings suggest that a dimensional approach designed to treat specific components of externalizing may have greater clinical utility than applying individual treatments to individual disorders.
In support of a dimensional approach, Epstein et al 354 carried out a meta‐analysis of 28 studies of psychosocial interventions for childhood externalizing problems. Using random effect variances, they found that dimensional externalizing severity scores accounted for significant additional variance in predicting treatment outcomes.
Furthermore, there appears to be utility for assessing the full range of the externalizing superspectrum in randomized clinical trials designed to treat externalizing psychopathology. For example, in the meta‐analysis by Battagliese et al 355 , the authors stated that they could not examine effects of cognitive‐behavior therapy on certain diagnostic subgroups because no studies measured ADHD symptoms in children with a diagnosis of ODD and only two studies included children affected by CD. Given the high rates of diagnostic co‐occurrence within the externalizing spectrum, assessing and treating the full range of externalizing problems for an individual client may be a parsimonious and effective approach to designing future interventions.
Summary of validity evidence
The validity evidence reviewed herein is summarized in Table 2. This table shows a substantial coherence within the disinhibited and antagonistic spectra, as well as an overlap between them. This supports the validity of a hierarchical conceptualization, involving an overarching externalizing superspectrum with two distinguishable spectra. As shown in the column “Summary of specificity”, most validators (sixteen) are evident for the broad externalizing superspectrum, with some (eight) evident for disinhibition and one for antagonism.
Table 2.
Validators of the disinhibited and antagonistic spectra
| Both spectra | Disinhibited spectrum | Antagonistic spectrum | Summary of specificity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASPD | CD | ODD | IED | BPD | Traits | AUD | SUD | ADHD | Traits | NPD | HPD | PPD | Traits | ||
| Genetics | |||||||||||||||
| Family/twin | ++ | + | + | ++ | + | + | +++ | + | ++ | ++ | ++ | + | B | ||
| Polygenic risk | + | + | + | B | |||||||||||
| GWAS | + | + | + | + | +++ | + | + | ++ | D | ||||||
| Environment | |||||||||||||||
| Neighborhood risk factors | ++ | ++ | B | ||||||||||||
| Peer interactions | ++ | ++ | B | ||||||||||||
| Childhood maltreatment | +++ | +++ | +++ | +++ | +++ | +++ | +++ | B | |||||||
| Cognition/Neurobiology | |||||||||||||||
| Cognitive deficits | +++ | +++ | + | – | ++ | +++ | +++ | +++ | +++ | +++ | – | – | – | B | |
| Emotional processing abnormalities | +++ | +++ | ++ | + | +++ | +++ | ++ | +++ | + | + | + | + | B | ||
| Reduced amygdala volume | ++ | + | + | ++ | + | ++ | + | +++ | B | ||||||
| Involvement of OFC/VMPFC | ++ | +++ | B | ||||||||||||
| Task‐related inferior frontal gyrus engagement | ++ | ++ | D | ||||||||||||
| Aberrations in dorsal anterior cingulate | + | ++ | +++ | + | B | ||||||||||
| Reward system dysfunction | +++ | D | |||||||||||||
| Dysfunction in mesolimbic and nigrostriatal systems | +++ | +++ | ++ | D | |||||||||||
| Reduced amygdala activation to fearful faces | ++ | +++ | A | ||||||||||||
| Blunted P300 | ++ | ++ | B | ||||||||||||
| Blunted error‐related negativity | ++ | D | |||||||||||||
| Biomarkers | |||||||||||||||
| DNA methylation | + | + | B | ||||||||||||
| Elevated pro‐inflammatory markers | + | + | + | D | |||||||||||
| Low cortisol | + | + | + | D | |||||||||||
| Low MAO‐B | + | + | + | + | D | ||||||||||
| Antecedents/Course | |||||||||||||||
| Low effortful control | ++ | ++ | + | ++ | +++ | +++ | ++ | +++ | ++ | B | |||||
| High negative affectivity | + | + | ++ | ++ | +++ | + | ++ | B | |||||||
| Extraversion/surgency | + | + | + | + | B | ||||||||||
| Treatment | |||||||||||||||
| Response to psychosocial interventions | + | + | + | B | |||||||||||
+: some evidence for effect, ++: some replications, +++: repeatedly replicated finding, –: some evidence for reverse effect, – –: some replications for reverse effect, – – –: repeatedly replicated reverse effect, A – linked to antagonism, D – linked to disinhibition, B – linked to both, ASPD – antisocial personality disorder, CD – conduct disorder, ODD – oppositional defiant disorder, IED – intermittent explosive disorder, BPD – borderline personality disorder, AUD – alcohol use disorder, SUD – substance use disorder, ADHD – attention‐deficit/hyperactivity disorder, NPD – narcissistic personality disorder, HPD – histrionic personality disorder, PPD – paranoid personality disorder, GWAS – genome‐wide association studies, OFC/VMPFC – orbitofrontal/ventromedial prefrontal cortex, MAO‐B – monoamine oxidase B.
Notably, cells that are blank in the table indicate a lack of evidence, not the absence of an effect. These may therefore be fruitful areas for future inquiry. Generally speaking, large sample designs where all elements of the externalizing superspectrum are well characterized, along with multiple validators, can improve inferences by helping to address questions of generality and specificity.
Many validators considered here may not be specific to externalizing. For example, pro‐inflammatory biomarkers were characterized as also related to the psychosis superspectrum of HiTOP in our previous paper in this series 19 . These and other factors (e.g., childhood adversity) are likely broadly relevant to psychopathology risk, and not specific to externalizing risk.
Generally speaking, these validity findings dovetail well with the structural perspective on psychopathology taken in the HiTOP consortium. In contemplating the validity of psychopathological concepts, it is no longer sufficient to focus on putative diagnostic categories in isolation. Rather, broad characterization of psychopathological phenomena, along with assessment of specific validators in large samples, can deepen our understanding by revealing the interplay between the structural organization of psychopathology and multiple putative causes and correlates.
UTILITY EVIDENCE
Reliability
Some of the largest studies on the reliability of the diagnosis of mental disorders have come from field trials of the official classification systems, the DSM and ICD. Results of the DSM‐5 field trials documented moderate/good reliability for alcohol use disorder (test‐retest kappa coefficient of 0.40) and questionable reliability for antisocial PD (kappa=0.21) 356 . These estimates are lower than those observed in field trials of DSM‐IV, largely due to the fact that “usual clinical interview approaches” 356 were utilized in the DSM‐5 field trials instead of highly structured diagnostic interviews as in the DSM‐IV field trials 357 . Nevertheless, complementary analysis of DSM‐5 cross‐cutting dimensional measures of externalizing‐related constructs (confined to alcohol, tobacco and illicit drug use) demonstrated higher reliability compared to their categorical counterparts 358 .
Direct comparisons of continuous and categorical measures of psychopathology are rare. In a comprehensive review, Markon et al 4 found that continuous measures of psychopathology were generally more reliable than discrete measures across all psychopathology domains, and that the overall meta‐analytic reliability estimate for the externalizing domain was 0.77.
A growing body of research has examined the reliability of PDs and personality dimensions that fall within the externalizing spectrum. Using the Personality Inventory for DSM‐5 (PID‐5) 359 , a questionnaire specifically developed to operationalize the DSM‐5 dimensional trait model for PDs, a high internal reliability of the disinhibition (McDonald’s omega = 0.80) and antagonism (omega = 0.83) domains was documented 360 .
In a study of the stability of PID‐5 domains over a one‐year period, the externalizing domains of the PID‐5 were relatively stable across a one‐year period in individuals diagnosed with PDs 361 . In a study examining both personality traits and PDs, high levels of stability over a two‐week period (referred to as dependability by the authors) were reported in PID‐5 domains of antagonism (0.86) and disinhibition (0.86) 362 . In addition, the authors provided evidence of clear advantages of dimensional over categorical ratings for PDs traditionally linked to the externalizing domain (e.g., antisocial PD).
Explanatory and prognostic power
Using data from two waves of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) study, a large general population longitudinal investigation, Kim and Eaton 61 demonstrated that an externalizing dimension at Wave 1 predicted Wave 2 mental disorder diagnoses more strongly than individual diagnoses.
Externalizing dimensions have also outperformed diagnoses when explaining variance in suicidality, psychotic‐like experiences and internalizing‐type disorders 363 . Furthermore, the externalizing dimension has been shown to mediate the relations of constructs such as childhood maltreatment with diagnosed externalizing‐type mental disorders (e.g., SUDs) 147 . Similar general vs. disorder‐specific findings are evident when examining constructs such as perceived racial discrimination 151 , stress responsivity 59 , and transmission of externalizing disorders from parents to offspring.
Collectively, this research points to the superiority of the HiTOP conceptualization of externalizing psychopathology in predicting a wide range of disorder validators.
Clinical utility
The utility of integrating the HiTOP model into clinical practice has been recently addressed 364 . Conway et al 44 demonstrated that the HiTOP structure generalizes well to patterns of comorbidity among diagnoses assigned by health practitioners in everyday practice. They further demonstrated that categorical diagnoses did not offer additional incremental validity when predicting suicidality and self‐injury, over and above the identified HiTOP dimensions.
Research on the clinical utility of dimensional versus categorical conceptualizations of externalizing largely comes from the PD field, and draws heavily from studies that examine practitioner ratings of utility. Using case vignettes as well as data obtained from actual patients, these studies evaluate the clinical utility of dimensional and categorical frameworks across various dimensions of utility (e.g., ease of use, utility in communicating with other health professionals, usefulness in formulating a therapeutic intervention, and usefulness in treatment planning). Recently, Bornstein and Natoli 365 summarized this literature and found that dimensional models of PD are rated more positively than categorical models with respect to most areas of clinical utility.
MEASUREMENT
The Externalizing Spectrum Inventory (ESI) is one of the most well‐validated instruments to measure individual facets and global levels of the externalizing superspectrum. The ESI was developed using a bottom‐up process to target 23 unidimensional facets of externalizing and capture the hierarchical structure of broad externalizing (or disinhibition) along with specific factors associated with callousness/aggression and substance abuse 15 .
Independent validation studies have demonstrated that the broad factors of the ESI possess concurrent validity against the Multidimensional Personality Questionnaire (MPQ) 366 , measures of integrity, and a range of DSM‐IV symptoms of externalizing disorders, personality traits, psychopathy, and symptoms of substance dependence219, 367, 368.
Recent efforts have focused on improving the clinical utility of the ESI via the development of data‐driven brief forms and adaptive scales. Patrick et al 16 constructed brief forms of the 23 facets with a total of 160 items (down from 415 items), ranging from 3 to 11 items per facet, which maintained high internal consistency, replicated the structure of the full ESI, and demonstrated similar validity in relation to the MPQ. Additional independent validation has confirmed the favorable psychometric properties of the brief form 369 . More recently, Sunderland et al 370 have demonstrated the feasibility of computerized adaptive versions of the ESI, producing similar scores as the full ESI with acceptable levels of reliability using very few items tailored to each respondent.
Omnibus clinical personality inventories are also available to assess the externalizing spectrum. Primary examples include the Minnesota Multiphasic Personality Inventory‐2‐Restructured form (MMPI‐2‐RF) 371 and the Personality Assessment Inventory (PAI) 372 . Specifically, the MMPI‐2‐RF captures behavioral/externalizing dysfunction at the higher order level, which comprises pervasive dysfunction with under‐controlled or acting‐out behaviors, as well as specific facet measurement (juvenile conduct problems, substance abuse, aggression, and anger proneness), all of which have been shown to directly map onto the same externalizing spectrum model as HiTOP and the ESI373, 374, 375, 376.
The MMPI‐2‐RF Personality Psychopathology Five (PSY‐5‐RF) scales also have trait‐level measures of higher‐order antagonism (aggressiveness) and disinhibition (disconstraint). Furthermore, factor analytic research with the PAI scales has typically revealed three‐ or four‐factor structures, with factors resembling disinhibition and antagonism usually emerging 373 .
There are also several personality measures that map onto, and therefore operationalize, the externalizing superspectrum via dimensional personality traits, including the PID‐5 359 , the NEO Personality Inventory 3 (NEO‐PI‐3) 377 , and the Comprehensive Assessment of Traits Relevant to Personality Disorder (CAT‐PD) 378 . The PID‐5 explicitly measures the antagonism and disinhibition trait domains that emerge from a broader externalizing superspectrum 379 . Similarly, a conjoint analysis of several dimensional personality trait inventories – the PID‐5, CAT‐PD and NEO‐PI‐3 – has provided evidence of a five‐factor solution that bore strong resemblance to the HiTOP model and included factors for antagonism and disinhibition that converged onto a single externalizing dimension in hierarchical analysis 83 .
In child and adolescent populations, a number of measures have been used extensively to assess externalizing and disinhibited behaviors, such as the Child Behavior Checklist (CBCL) 380 , the Strengths and Difficulties Questionnaire (SDQ) 381 , and the Diagnostic Interview Schedule for Children (DISC) 382 , with factor analysis consistently identifying strong coherence between these measures and the broader HiTOP structure383, 384, 385.
Finally, there are numerous scales designed to measure specific facets of externalizing and disinhibited behavior, such as substance use, impulsiveness, and aggression386, 387, 388.
IMPLICATIONS
The HiTOP approach aims to advance our understanding of the natural organization of externalizing psychopathology in at least three major ways.
First, externalizing psychopathology encompasses two spectra, disinhibition and antagonism. These spectra show both similarities and differences, consistent with the fundamentally clarifying idea of disinhibitory and antagonistic aspects of a broader and more general externalizing superspectrum. Nevertheless, to characterize a patient fully, a profile across major psychopathology spectra needs to be considered, as detailed in previous HiTOP publications1, 19, 364.
Second, the HiTOP approach underscores a growing consensus that clinically significant externalizing problems lie on a continuum with normative functioning and maladaptive traits. Developmentally earlier expressions of disinhibitory and antagonistic traits often precede the onset of serious sequalae (e.g., behaviors that are grounds for arrest). In this way, the HiTOP approach melds dimensional and developmental perspectives on psychopathology, as parts of a more integrated approach to understanding both development and broad population‐level variation in socially consequential externalizing tendencies.
Third, the HiTOP approach addresses heterogeneity within externalizing problems by explicating specific trait and symptom dimensions that constitute broader spectra. Figure 1 provides an evidence‐based guide to constituent narrow‐band elements of externalizing. Nevertheless, continued research on the nature of specific sub‐elements of externalizing psychopathology would be welcome, as the field pivots toward basing nosology on evidence, as opposed to diagnosis by tradition and putative authority15, 374.
FUTURE DIRECTIONS
The proposed HiTOP model of the externalizing superspectrum is based on extensive evidence. Nevertheless, intriguing possibilities exist to explore the discrete vs. continuous nature of psychopathology based on data. The HiTOP model is meant to include all empirical psychopathological entities, whether dimensional or categorical in nature. Only dimensions have been established empirically to date. Setting aside the complex political issues implied by this situation (e.g., the way authoritative nosologies tend to recognize committee‐derived categories as opposed to empirically‐derived dimensions), quantitative techniques can adjudicate between more continuous and more discrete accounts of the structure of psychopathology. Further research along these lines can help to continue to place psychiatric nosology on firmer empirical footing3, 19.
Systematic research can also provide a means for linking psychopathological variation with intervention implications in a principled manner. Rather than imposing arbitrary diagnostic thresholds, diagnostic algorithms can link clinical presentations with optimal intervention strategies. Practically speaking, clinical decisions rarely focus on “to treat or not to treat”. Rather, an ordinal set of interventions varying in intensity is typically deployed in response to a corresponding level of clinical need. For example, externalizing problems frequently present as SUDs, because substance dependence creates an acute clinical need. Substance use intervention can range from medically responsible outpatient detoxification, to partial hospitalization, to inpatient services. This rough ordinal scale of intervention is typically tethered to clinical need (e.g., medical complications may require close observation to resolve, and a corresponding inpatient stay). Ultimately, these sorts of treatment options can be tethered to intensity of presentation in a principled way, relying on the types of evidence reviewed herein.
CONCLUSIONS
The HiTOP approach to clinical diagnosis provides an empirically based and hierarchical conceptualization of externalizing disorders that was derived from evidence. The validity evidence reviewed herein is extensive, and the utility of the approach was also reviewed and is readily apparent. Assessment instruments for externalizing constructs already exist, and the HiTOP approach can therefore be readily implemented.
Further research will be beneficial, but the HiTOP model is ready for use by scientists and clinicians interested in basing their approaches on evidence as opposed to putative authority. The HiTOP approach addresses problems of heterogeneity, comorbidity and low reliability, thereby providing valid and reliable descriptions of patients to drive both discovery and intervention.
ACKNOWLEDGEMENTS
R.F. Krueger is partially supported by the US National Institutes of Health (grants no. R01AG053217, U19AG051426). K. Hobbs is supported by the National Science Foundation Graduate Research Fellowship Program (grant no. 00074041). K. Forbush is supported by the US Department of Defense, and the University of Kansas, Research Excellence Initiative. D. Dick is supported by the US National Institutes of Health (grants no. K02AA018755, P50AA022537, R01DA050721). Further information on the HiTOP consortium can be found at http://medicine.stonybrookmedicine.edu/HITOP. D. Watson and R. Kotov are joint senior authors of this paper.
Members of HiTOP Utility Workgroup include, in addition to the authors of this paper, Kamran Afzali, Marina A. Bornovalova, Natacha Carragher, David C. Cicero, Anna R. Docherty, Michael B. First, Eiko I. Fried, Michael N. Hallquist, Katherine Jonas, Kristian E. Markon, Les C. Morey, Stephanie N. Mullins‐Sweatt, Kristin Naragon‐Gainey, Brady Nelson, Thomas M. Olino, Praveetha Patalay, Aaron L. Pincus, Craig Rodriguez‐Seijas, Lauren A. Rutter, Giovanni A. Salum, Alexander J. Shackman, Andrew E. Skodol, Kathryn Tabb, Nicholas Wagner, Ashley L. Watts, Amanda A. Uliaszek, Johannes Zimmermann and Richard E. Zinbarg.
REFERENCES
- 1. Kotov R, Krueger RF, Watson D et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J Abnorm Psychol 2017;126:454‐77. [DOI] [PubMed] [Google Scholar]
- 2. Kotov R, Krueger RF, Watson D. A paradigm shift in psychiatric classification: the Hierarchical Taxonomy Of Psychopathology (HiTOP). World Psychiatry 2018;17:24‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krueger RF, Kotov R, Watson D et al. Progress in achieving quantitative classification of psychopathology. World Psychiatry 2018;17:282‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychol Bull 2011;137:856‐79. [DOI] [PubMed] [Google Scholar]
- 5. Shankman SA, Funkhouser CJ, Klein DN et al. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM‐5 (SCID). Int J Methods Psychiatr Res 2018;27:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chmielewski M, Clark LA, Bagby RM et al. Method matters: understanding diagnostic reliability in DSM‐IV and DSM‐5. J Abnorm Psychol 2015;124:764‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watson D. Subtypes, specifiers, epicycles, and eccentrics: toward a more parsimonious taxonomy of psychopathology. Clin Psychol Sci Pract 2003;10:233‐8. [Google Scholar]
- 8. Cuesta MJ, Peralta V. Integrating psychopathological dimensions in functional psychoses: a hierarchical approach. Schizophr Res 2001;52:215‐29. [DOI] [PubMed] [Google Scholar]
- 9. Kotov R, Foti D, Li K et al. Validating dimensions of psychosis symptomatology: neural correlates and 20‐year outcomes. J Abnorm Psychol 2016;125:1103‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reininghaus U, Böhnke JR, Chavez‐Baldini U et al. Transdiagnostic dimensions of psychosis in the Bipolar‐Schizophrenia Network on Intermediate Phenotypes (B‐SNIP). World Psychiatry 2019;18:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruggero CJ, Kotov R, Watson D et al. Beyond a single index of mania symptoms: structure and validity of subdimensions. J Affect Disord 2014;161:8‐15. [DOI] [PubMed] [Google Scholar]
- 12. Anderson JL, Sellbom M, Ayearst L et al. Associations between DSM‐5 section III personality traits and the Minnesota Multiphasic Personality Inventory 2‐Restructured Form (MMPI‐2‐RF) scales in a psychiatric patient sample. Psychol Assess 2015;27:801‐15. [DOI] [PubMed] [Google Scholar]
- 13. Forbes MK, Kotov R, Ruggero CJ et al. Delineating the joint hierarchical structure of clinical and personality disorders in an outpatient psychiatric sample. Compr Psychiatry 2017;79:19‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krueger RF, Chentsova‐Dutton YE, Markon KE et al. A cross‐cultural study of the structure of comorbidity among common psychopathological syndromes in the general health care setting. J Abnorm Psychol 2003;112:437‐47. [DOI] [PubMed] [Google Scholar]
- 15. Krueger RF, Markon KE, Patrick CJ et al. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol 2007;116:645‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patrick CJ, Kramer MD, Krueger RF et al. Optimizing efficiency of psychopathology assessment through quantitative modeling: development of a brief form of the Externalizing Spectrum Inventory. Psychol Assess 2013;25:1332‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry 2018;175:831‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lahey BB, Krueger RF, Rathouz PJ et al. A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull 2016;143:142‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotov R, Jonas KG, Carpenter WT et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry 2020;19:151‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mullins‐Sweatt SN, DeShong HL, Lengel GJ et al. Disinhibition as a unifying construct in understanding how personality dispositions undergird psychopathology. J Res Personal 2019;80:55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynam DR, Miller JD. The basic trait of antagonism: an unfortunately underappreciated construct. J Res Personal 2019;81:118‐26. [Google Scholar]
- 22. DeYoung CG, Chmielewski M, Clark LA et al. The distinction between symptoms and traits in the Hierarchical Taxonomy of Psychopathology (HiTOP). Submitted for publication. [DOI] [PubMed]
- 23. Krueger RF, Eaton NR. Transdiagnostic factors of mental disorders. World Psychiatry 2015;14:27‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blanco C, Wall MM, He J‐P et al. The space of common psychiatric disorders in adolescents: comorbidity structure and individual latent liabilities. J Am Acad Child Adolesc Psychiatry 2015;54:45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castellanos‐Ryan N, Struve M, Whelan R et al. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry 2014;171:1310‐9. [DOI] [PubMed] [Google Scholar]
- 26. Gomez R, Stavropoulos V, Vance A et al. Re‐evaluation of the latent structure of common childhood disorders: is there a general psychopathology factor (P‐factor)? Int J Ment Health Addict 2019;17:258‐78. [Google Scholar]
- 27. Lahey BB, Rathouz PJ, Van Hulle C et al. Testing structural models of DSM‐IV symptoms of common forms of child and adolescent psychopathology. J Abnorm Child Psychol 2008;36:187‐206. [DOI] [PubMed] [Google Scholar]
- 28. Martel MM, Pan PM, Hoffmann MS et al. A general psychopathology factor (P factor) in children: structural model analysis and external validation through familial risk and child global executive function. J Abnorm Psychol 2017;126:137‐48. [DOI] [PubMed] [Google Scholar]
- 29. Olino TM, Bufferd SJ, Dougherty LR et al. The development of latent dimensions of psychopathology across early childhood: stability of dimensions and moderators of change. J Abnorm Child Psychol 2018;46:1373‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verona E, Javdani S, Sprague J. Comparing factor structures of adolescent psychopathology. Psychol Assess 2011;23:545‐51. [DOI] [PubMed] [Google Scholar]
- 31. Caspi A, Houts RM, Belsky DW et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci 2014;2:119‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keyes KM, Eaton NR, Krueger RF et al. Thought disorder in the meta‐structure of psychopathology. Psychol Med 2013;43:1673‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verona E, Sachs‐Ericsson N, Joiner TE. Suicide attempts associated with externalizing psychopathology in an epidemiological sample. Am J Psychiatry 2004;161:444‐51. [DOI] [PubMed] [Google Scholar]
- 34. Wright AGC, Krueger RF, Hobbs MJ et al. The structure of psychopathology: toward an expanded quantitative empirical model. J Abnorm Psychol 2013;122:281‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krueger RF, Caspi A, Moffitt TE et al. The structure and stability of common mental disorders (DSM‐III‐R): a longitudinal‐epidemiological study. J Abnorm Psychol 1998;107:216‐27. [DOI] [PubMed] [Google Scholar]
- 36. Beesdo‐Baum K, Höfler M, Gloster AT et al. The structure of common mental disorders: a replication study in a community sample of adolescents and young adults. Int J Methods Psychiatr Res 1998;18:204‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wittchen HU, Beesdo‐Baum K, Gloster AT et al. The structure of mental disorders re‐examined: Is it developmentally stable and robust against additions?. Int J Methods Psychiatr Res 2009;18:189‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carragher N, Krueger RF, Eaton NR et al. ADHD and the externalizing spectrum: direct comparison of categorical, continuous, and hybrid models of liability in a nationally representative sample. Soc Psychiatry Psychiatr Epidemiol 2014;49:1307‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eaton NR, Krueger RF, Keyes KM et al. Borderline personality disorder comorbidity: relationship to the internalizing‐externalizing structure of common mental disorders. Psychol Med 2011;41:1041‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lahey BB, Applegate B, Hakes JK et al. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol 2012;121:971‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waldman ID, Poore HE, van Hulle C et al. External validity of a hierarchical dimensional model of child and adolescent psychopathology: tests using confirmatory factor analyses and multivariate behavior genetic analyses. J Abnorm Psychol 2016;125:1053‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Jonge P, Wardenaar KJ, Lim CC et al. The cross‐national structure of mental disorders: results from the World Mental Health Surveys. Psychol Med 2018;48:2073‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kessler RC, Ormel J, Petukhova M et al. Development of lifetime comorbidity in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry 2011;68:90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Conway CC, Mansolf M, Reise SP. Ecological validity of a quantitative classification system for mental illness in treatment‐seeking adults. Psychol Assess 2019;31:730‐40. [DOI] [PubMed] [Google Scholar]
- 45. Cox BJ, Clara IP, Enns MW. Posttraumatic stress disorder and the structure of common mental disorders. Depress Anxiety 2002;15:168‐71. [DOI] [PubMed] [Google Scholar]
- 46. Forbush KT, Watson D. The structure of common and uncommon mental disorders. Psychol Med 2013;43:97‐108. [DOI] [PubMed] [Google Scholar]
- 47. Forbush KT, South SC, Krueger RF et al. Locating eating pathology within an empirical diagnostic taxonomy: evidence from a community‐based sample. J Abnorm Psychol 2010;119:282‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. James LM, Taylor J. Revisiting the structure of mental disorders: borderline personality disorder and the internalizing/externalizing spectra. Br J Clin Psychol 2008;47:361‐80. [DOI] [PubMed] [Google Scholar]
- 49. Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry 1999;56:921‐6. [DOI] [PubMed] [Google Scholar]
- 50. Krueger RF, Hicks BM, Patrick CJ et al. Etiologic connections among substance dependence, antisocial behavior and personality: modeling the externalizing spectrum. J Abnorm Psychol 2002;111:411‐24. [PubMed] [Google Scholar]
- 51. Miller MW, Fogler JM, Wolf EJ et al. The internalizing and externalizing structure of psychiatric comorbidity in combat veterans. J Trauma Stress 2018;21:58‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller MW, Wolf EJ, Reardon A et al. Personality and the latent structure of PTSD comorbidity. J Anxiety Disord 2012;26:599‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tuvblad C, Zheng M, Raine A et al. A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9‐10 year old boys and girls. J Abnorm Child Psychol 2009;37:153‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Young SE, Stallings MC, Corley RP et al. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet 2000;96:684‐95. [PubMed] [Google Scholar]
- 55. Kotov R, Ruggero CJ, Krueger RF et al. New dimensions in the quantitative classification of mental illness. Arch Gen Psychiatry 2011;68:1003‐11. [DOI] [PubMed] [Google Scholar]
- 56. Kendler KS, Aggen SH, Knudsen GP et al. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM‐IV Axis I and Axis II disorders. Am J Psychiatry 2011;168:29‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Røysamb E, Kendler KS, Tambs K et al. The joint structure of DSM‐IV Axis I and Axis II disorders. J Abnorm Psychol 2011;120:198‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Conway CC, Brown TA. Evaluating dimensional models of psychopathology in outpatients diagnosed with emotional disorders: a cautionary tale. Depress Anxiety 2018;35:898‐902. [DOI] [PubMed] [Google Scholar]
- 59. Conway CC, Starr LR, Espejo EP et al. Stress responsivity and the structure of common mental disorders: transdiagnostic internalizing and externalizing dimensions are associated with contrasting stress appraisal biases. J Abnorm Psychol 2016;125:1079‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farmer RF, Seeley JR, Kosty DB et al. Refinements in the hierarchical structure of externalizing psychiatric disorders: patterns of lifetime liability from mid‐adolescence through early adulthood. J Abnorm Psychol 2009;118:699‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim H, Eaton NR. The hierarchical structure of common mental disorders: connecting multiple levels of comorbidity, bifactor models, and predictive validity. J Abnorm Psychol 2015;124:1064‐78. [DOI] [PubMed] [Google Scholar]
- 62. Slade T, Watson D. The structure of common DSM‐IV and ICD‐10 mental disorders in the Australian general population. Psychol Med 2006;36:1593‐1600. [DOI] [PubMed] [Google Scholar]
- 63. Vollebergh WA, Iedema J, Bijl RV et al. The structure and stability of common mental disorders: the NEMESIS study. Arch Gen Psychiatry 2001;58:597‐603. [DOI] [PubMed] [Google Scholar]
- 64. Wright AGC, Simms LJ. A metastructural model of mental disorders and pathological personality traits. Psychol Med 2015;45:2309‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kotov R. New dimensions in the quantitative classification of mental illness. Arch Gen Psychiatry 2011;68:1003‐11. [DOI] [PubMed] [Google Scholar]
- 66. Kendler KS, Myers JM, Maes HH et al. The relationship between the genetic and environmental influences on common internalizing psychiatric disorders and mental well‐being. Behav Genet 2011;41:641‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Samuel D, Widiger T. A meta‐analytic review of the relationships between the five‐factor model and DSM‐IV‐TR personality disorders: a facet level analysis. Clin Psychol Rev 2008;28:1326‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saulsman LM, Page AC. The five‐factor model and personality disorder empirical literature: a meta‐analytic review. Clin Psychol Rev 2004;23:1055‐85. [DOI] [PubMed] [Google Scholar]
- 69. Shiner RL, Tackett JL. Personality disorders in children and adolescents. In: Mash EJ, Barkley RA (eds). Child psychopathology. New York: Guilford, 2014:848‐96. [Google Scholar]
- 70. Frick PJ, Myers TDW. Conduct disorder and callous‐unemotional traits. In: Lochman JE, Matthys W (eds). The Wiley handbook of disruptive and impulse‐control disorders. Chichester: Wiley‐Blackwell, 2018:37‐54. [Google Scholar]
- 71. Widiger TA, Sellbom M, Chmielewski M et al. Personality in a hierarchical model of psychopathology. Clin Psychol Sci 2019;7:77‐92. [Google Scholar]
- 72. Krueger RF, South SC. Externalizing disorders: cluster 5 of the proposed meta‐structure for DSM‐V and ICD‐11. Psychol Med 2009;39:2061‐70. [DOI] [PubMed] [Google Scholar]
- 73. Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: an integrative hierarchical approach. J Pers Soc Psychol 2005;88:139‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J Pers Soc Psychol 2000;78:122‐35. [DOI] [PubMed] [Google Scholar]
- 75. Watson D, Clark LA, Chmielewski M. Structures of personality and their relevance to psychopathology: II. Further articulation of a comprehensive unified trait structure. J Pers 2008;76:1545‐86. [DOI] [PubMed] [Google Scholar]
- 76. Tackett JL, Slobodskaya HR, Mar RA et al. The hierarchical structure of childhood personality in five countries: continuity from early childhood to early adolescence. J Pers 2012;80:847‐79. [DOI] [PubMed] [Google Scholar]
- 77. Crego C, Widiger TA. Convergent and discriminant validity of alternative measures of maladaptive personality traits. Psychol Assess 2016;28:1561‐75. [DOI] [PubMed] [Google Scholar]
- 78. Somma A, Krueger RF, Markon KE et al. The replicability of the personality inventory for DSM‐5 domain scale factor structure in U.S. and non‐U.S. samples: a quantitative review of the published literature. Psychol Assess 2019;31:861‐77. [DOI] [PubMed] [Google Scholar]
- 79. Tackett JL, Martel MM, Kushner SC. Temperament, externalizing disorders, and attention‐deficit/hyperactivity disorder. In: Zentner M, Shiner RL (eds). Handbook of temperament. New York: Guilford, 2012:562‐80. [Google Scholar]
- 80. Watson D, Clark LA. Personality traits as an organizing framework for personality pathology. Personal Ment Health 2020;14:51‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Watson D, Stasik SM, Ro E et al. Integrating normal and pathological personality: relating the DSM‐5 Trait‐Dimensional Model to general traits of personality. Assessment 2013;20:312‐26. [DOI] [PubMed] [Google Scholar]
- 82. Watters CA, Bagby RM. A meta‐analysis of the five‐factor internal structure of the Personality Inventory for DSM‐5. Psychol Assess 2018;30:1255‐60. [DOI] [PubMed] [Google Scholar]
- 83. Wright AGC, Simms LJ. On the structure of personality disorder traits: conjoint analyses of the CAT‐PD, PID‐5, and NEO‐PI‐3 trait models. Personal Disord Theory Res Treat 2014;5:43‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rojas SL, Widiger TA. Coverage of the DSM‐IV‐TR/DSM‐5 Section II Personality Disorders with the DSM‐5 Dimensional Trait Model. J Personal Disord 2017;31:462‐82. [DOI] [PubMed] [Google Scholar]
- 85. Watters CA, Bagby RM, Sellbom M. Meta‐analysis to derive an empirically based set of personality facet criteria for the alternative DSM‐5 model for personality disorders. Personal Disord Theory Res Treat 2018;10:97‐104. [DOI] [PubMed] [Google Scholar]
- 86. Burke JD, Boylan K, Rowe R et al. Identifying the irritability dimension of ODD: application of a modified bifactor model across five large community samples of children. J Abnorm Psychol 2014;123:841‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Coccaro EF, Lee R, McCloskey MS. Relationship between psychopathy, aggression, anger, impulsivity, and intermittent explosive disorder. Aggress Behav 2014;40:526‐36. [DOI] [PubMed] [Google Scholar]
- 88. Fernandez E, Johnson SL. Anger in psychological disorders: prevalence, presentation, etiology and prognostic implications. Clin Psychol Rev 2016;46:124‐35. [DOI] [PubMed] [Google Scholar]
- 89. Gomez R, Corr PJ. ADHD and personality: a meta‐analytic review. Clin Psychol Rev 2014;34:376‐88. [DOI] [PubMed] [Google Scholar]
- 90. Herzhoff K, Tackett JL. Subfactors of oppositional defiant disorder: converging evidence from structural and latent class analyses. J Child Psychol Psychiatry 2016;57:18‐29. [DOI] [PubMed] [Google Scholar]
- 91. Johnston OG, Cruess DG, Burke JD. Irritability and behavioral symptom dimensions of oppositional defiant disorder in young adults: associations with DSM‐5 pathological personality traits. J Psychopathol Behav Assess 2020;42:424‐35. [Google Scholar]
- 92. Reardon KW, Tackett JL, Lynam D. The personality context of relational aggression: a Five‐Factor Model profile analysis. Personal Disord Theory Res Treat 2017;9:228‐38. [DOI] [PubMed] [Google Scholar]
- 93. Waldman ID, Rowe R, Boylan K et al. External validation of a bifactor model of oppositional defiant disorder. Mol Psychiatry 2021;26:682‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta‐analysis. J Abnorm Psychol 2010;119:1‐17. [DOI] [PubMed] [Google Scholar]
- 95. Ficks CA, Dong L, Waldman ID. Sex differences in the etiology of psychopathic traits in youth. J Abnorm Psychol 2014;123:406‐11. [DOI] [PubMed] [Google Scholar]
- 96. Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta‐analysis of twin and adoption studies. Psychol Bull 2002;128:490‐529. [PubMed] [Google Scholar]
- 97. Burt SA. Are there meaningful etiological differences within antisocial behavior? Results of a meta‐analysis. Clin Psychol Rev 2009;29:163‐78. [DOI] [PubMed] [Google Scholar]
- 98. Tackett JL, Krueger RF, Iacono WG et al. Symptom‐based subfactors of DSM‐defined conduct disorder: evidence for etiologic distinctions. J Abnorm Psychol 2005;114:483‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tackett JL, Waldman ID, Lahey BB. Etiology and measurement of relational aggression: a multi‐informant behavior genetic investigation. J Abnorm Psychol 2009;118:722‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Burt SA, Krueger RF, McGue M et al. Sources of covariation among attention‐deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: the importance of shared environment. J Abnorm Psychol 2001;110:516‐25. [DOI] [PubMed] [Google Scholar]
- 101. Dick DM, Viken RJ, Kaprio J et al. Understanding the covariation among childhood externalizing symptoms: genetic and environmental Influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. J Abnorm Child Psychol 2005;33:219‐29. [DOI] [PubMed] [Google Scholar]
- 102. Cosgrove VE, Rhee SH, Gelhorn HL et al. Structure and etiology of co‐occurring internalizing and externalizing disorders in adolescents. J Abnorm Child Psychol 2011;39:109‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hicks BM, Krueger RF, Iacono WG et al. Family transmission and heritability of externalizing disorders: a twin‐family study. Arch Gen Psychiatry 2004;61:922‐8. [DOI] [PubMed] [Google Scholar]
- 104. Waldman ID, Tackett JL, Van Hulle CA et al. Child and adolescent conduct disorder substantially shares genetic influences with three socioemotional dispositions. J Abnorm Psychol 2011;120:57‐70. [DOI] [PubMed] [Google Scholar]
- 105. Kendler KS, Karkowski LM, Neale MC et al. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population‐based sample of male twins. Arch Gen Psychiatry 2000;57:261‐9. [DOI] [PubMed] [Google Scholar]
- 106. Torgersen S, Czajkowski N, Jacobson K et al. Dimensional representations of DSM‐IV cluster B personality disorders in a population‐based sample of Norwegian twins: a multivariate study. Psychol Med 2008;38:1617‐25. [DOI] [PubMed] [Google Scholar]
- 107. South SC, Krueger RF, Knudsen GP et al. A population based twin study of DSM–5 maladaptive personality domains. Personal Disord Theory Res Treat 2017;8:366‐75. [DOI] [PubMed] [Google Scholar]
- 108. Wright ZE, Pahlen S, Krueger RF. Genetic and environmental influences on Diagnostic and Statistical Manual of Mental Disorders‐Fifth Edition (DSM‐5) maladaptive personality traits and their connections with normative personality traits. J Abnorm Psychol 2017;126:416‐28. [DOI] [PubMed] [Google Scholar]
- 109. Kendler KS, Aggen SH, Gillespie N et al. The structure of genetic and environmental influences on normative personality, abnormal personality traits, and personality disorder symptoms. Psychol Med 2019;49:1392‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta‐analysis of twin and adoption studies. Psychol Med 2015;45:1061‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Verweij KJH, Zietsch BP, Lynskey MT et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta‐analysis of twin studies. Addiction 2010;105:417‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tsuang MT, Bar JL, Harley RM et al. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry 2001;9:267‐79. [PubMed] [Google Scholar]
- 113. van den Bree MBM, Johnson EO, Neale MC et al. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend 1998;52:231‐41. [DOI] [PubMed] [Google Scholar]
- 114. Maes HH, Sullivan PF, Bulik CM et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med 2004;34:1251‐61. [DOI] [PubMed] [Google Scholar]
- 115. Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry 2007;64:1313‐20. [DOI] [PubMed] [Google Scholar]
- 116. Kendler KS, Prescott CA, Myers J et al. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 2003;60:929‐37. [DOI] [PubMed] [Google Scholar]
- 117. Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta‐analytic review. Hum Genet 2009;126:51‐90. [DOI] [PubMed] [Google Scholar]
- 118. Demontis D, Walters RK, Martin J et al. Discovery of the first genome‐wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019;51:63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dick DM, Li T‐K, Edenberg HJ et al. A genome‐wide screen for genes influencing conduct disorder. Mol Psychiatry 2004;9:81‐6. [DOI] [PubMed] [Google Scholar]
- 120. Demontis D, Walters R, Rajagopal VM et al. Identification of risk variants and characterization of the polygenic architecture of disruptive behavior disorders in the context of ADHD. bioRxiv 2019: 791160. [Google Scholar]
- 121. Tielbeek JJ, Johansson A, Polderman TJC et al. Genome‐wide association studies of a broad spectrum of antisocial behavior. JAMA Psychiatry 2017;74:1242‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Witt SH, Streit F, Jungkunz M et al. Genome‐wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. Transl Psychiatry 2017;7:e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tielbeek JJ, Vink JM, Polderman TJC et al. Genetic correlation of antisocial behaviour with alcohol, nicotine, and cannabis use. Drug Alcohol Depend 2018;187:296‐9. [DOI] [PubMed] [Google Scholar]
- 124. Chang L‐H, Whitfield JB, Liu M et al. Associations between polygenic risk for tobacco and alcohol use and liability to tobacco and alcohol use, and psychiatric disorders in an independent sample of 13,999 Australian adults. Drug Alcohol Depend 2019;205:107704. [DOI] [PubMed] [Google Scholar]
- 125. Salvatore JE, Edwards AC, McClintick JN et al. Genome‐wide association data suggest ABCB1 and immune‐related gene sets may be involved in adult antisocial behavior. Transl Psychiatry 2015;5:e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lo M‐T, Hinds DA, Tung JY et al. Genome‐wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet 2017;49:152‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Walters RK, Polimanti R, Johnson EC et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci 2018;21:1656‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kranzler HR, Zhou H, Kember RL et al. Genome‐wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun 2019;10:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu M, Jiang Y, Wedow R et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2019;51:237‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Johnson EC, Sanchez‐Roige S, Acion L et al. Polygenic contributions to alcohol use and alcohol use disorders across population‐based and clinically ascertained samples. Psychol Med (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pasman JA, Verweij KJH, Gerring Z et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nat Neurosci 2018;21:1161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Minică CC, Verweij KJH, van der Most PJ et al. Genome‐wide association meta‐analysis of age at first cannabis use. Addiction 2018;113:2073‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Demontis D, Rajagopal VM, Thorgeirsson TE et al. Genome‐wide association study implicates CHRNA2 in cannabis use disorder. Nat Neurosci 2019;22:1066‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gelernter J, Sherva R, Koesterer R et al. Genome‐wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry 2014;19:717‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cabana‐Domínguez J, Shivalikanjli A, Fernàndez‐Castillo N et al. Genome‐wide association meta‐analysis of cocaine dependence: shared genetics with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2019;94:109667. [DOI] [PubMed] [Google Scholar]
- 136. Cheng Z, Zhou H, Sherva R et al. Genome‐wide association study identifies a regulatory variant of RGMA associated with opioid dependence in European Americans. Biol Psychiatry 2018;84:762‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhou H, Rentsch CT, Cheng Z et al. GWAS including 82,707 subjects identifies functional coding variant in OPRM1 gene associated with opioid use disorder. medRxiv 2019:19007039. [Google Scholar]
- 138. Polimanti R, Walters RK, Johnson EC et al. Leveraging genome‐wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry 2020;25:1673‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Grotzinger AD, Rhemtulla M, de Vlaming R et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav 2019;3:513‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Waldman ID, Poore HE, Luningham JM et al. Testing structural models of psychopathology at the genomic level. World Psychiatry 2020;19:350‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Karlsson Linnér RK, Mallard TT, Barr PB et al. Using the genetic architecture of substance use disorders to aid in gene identification: findings from the Externalizing Consortium. Presented at the NIDA Genetics and Epigenetics Cross‐Cutting Research Team Meeting, Rockville, January 2020.
- 142. Cicchetti D, Handley ED. Child maltreatment and the development of substance use and disorder. Neurobiol Stress 2019;10:100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Deater‐Deckard K, Dodge KA, Bates JE et al. Multiple risk factors in the development of externalizing behavior problems: group and individual differences. Dev Psychopathol 1998;10:469‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pinquart M. Associations of parenting dimensions and styles with externalizing problems of children and adolescents: an updated meta‐analysis. Dev Psychol 2017;53:873‐932. [DOI] [PubMed] [Google Scholar]
- 145. Samek DR, Goodman RJ, Erath SA et al. Antisocial peer affiliation and externalizing disorders in the transition from adolescence to young adulthood: selection versus socialization effects. Dev Psychol 2016;52:813‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. McKee L, Colletti C, Rakow A et al. Parenting and child externalizing behaviors: are the associations specific or diffuse? Aggress Violent Behav 2008;13:201‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Keyes KM, Eaton NR, Krueger RF et al. Childhood maltreatment and the structure of common psychiatric disorders. Br J Psychiatry 2012;200:107‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Conway CC, Raposa EB, Hammen C et al. Transdiagnostic pathways from early social stress to psychopathology: a 20‐year prospective study. J Child Psychol Psychiatry 2018;59:855‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vachon DD, Krueger RF, Rogosch FA et al. Assessment of the harmful psychiatric and behavioral effects of different forms of child maltreatment. JAMA Psychiatry 2015;72:1135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Forbes MK, Magson NR, Rapee RM. Evidence that different types of peer victimization have equivalent associations with transdiagnostic psychopathology in adolescence. J Youth Adolesc 2020;49:590‐604. [DOI] [PubMed] [Google Scholar]
- 151. Rodriguez‐Seijas C, Stohl M, Hasin DS et al. Transdiagnostic factors and mediation of the relationship between perceived racial discrimination and mental disorders. JAMA Psychiatry 2015;72:706‐13. [DOI] [PubMed] [Google Scholar]
- 152. Conway CC, Hammen C, Brennan PA. Expanding stress generation theory: test of a transdiagnostic model. J Abnorm Psychol 2012;121:754‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Snyder HR, Young JF, Hankin BL. Chronic stress exposure and generation are related to the p‐factor and externalizing specific psychopathology in youth. J Clin Child Adolesc Psychol 2019;48:306‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ensink JBM, de Moor MHM, Zafarmand MH et al. Maternal environmental risk factors and the development of internalizing and externalizing problems in childhood: the complex role of genetic factors. Am J Med Genet B Neuropsychiatr Genet 2020;183:17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Morgan AB, Lilienfeld SO. A meta‐analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev 2000;20:113‐36. [DOI] [PubMed] [Google Scholar]
- 156. Ogilvie JM, Stewart AL, Chan RCK et al. Neuropsychological measures of executive function and antisocial behavior: a meta‐analysis. Criminology 2011;49:1063‐107. [Google Scholar]
- 157. Pasion R, Fernandes C, Pereira MR et al. Antisocial behaviour and psychopathy: uncovering the externalizing link in the P3 modulation. Neurosci Biobehav Rev 2018;91:170‐86. [DOI] [PubMed] [Google Scholar]
- 158. Bernat EM, Nelson LD, Steele VR et al. Externalizing psychopathology and gain‐loss feedback in a simulated gambling task: dissociable components of brain response revealed by time‐frequency analysis. J Abnorm Psychol 2011;120:352‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Friedman NP, Rhee SH, Ross JM et al. Genetic and environmental relations of executive functions to antisocial personality disorder symptoms and psychopathy. Int J Psychophysiol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Romer D, Betancourt LM, Brodsky NL et al. Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in early adolescence. Dev Sci 2011;14:1119‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lansing AE, Plante WY, Golshan S et al. Emotion regulation mediates the relationship between verbal learning and internalizing, trauma‐related and externalizing symptoms among early‐onset, persistently delinquent adolescents. Learn Individ Differ 2019;70:201‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Becker SP, Leopold DR, Burns GL et al. The internal, external, and diagnostic validity of sluggish cognitive tempo: a meta‐analysis and critical review. J Am Acad Child Adolesc Psychiatry 2016;55:163‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fragkaki I, Cima M, Meesters C. The association between callous‐unemotional traits, externalizing problems, and gender in predicting cognitive and affective morality judgments in adolescence. J Youth Adolesc 2016;45:1917‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. McDonald JB, Bozzay ML, Bresin K et al. Facets of externalizing psychopathology in relation to inhibitory control and error processing. Int J Psychophysiol (in press). [DOI] [PubMed] [Google Scholar]
- 165. Endres MJ, Rickert ME, Bogg T et al. Externalizing psychopathology and behavioral disinhibition: working memory mediates signal discriminability and reinforcement moderates response bias in approach‐avoidance learning. J Abnorm Psychol 2011;120:336‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Brennan GM, Baskin‐Sommers AR. Brain‐behavior relationships in externalizing: P3 amplitude reduction reflects deficient inhibitory control. Behav Brain Res 2018;337:70‐9. [DOI] [PubMed] [Google Scholar]
- 167. Pan XX, Ma HW, Dai XM. Value of integrated visual and auditory continuous performance test in the diagnosis of childhood attention deficit hyperactivity disorder. Chin J Contemp Pediatr 2007;9:210‐2. [PubMed] [Google Scholar]
- 168. Slobodin O, Cassuto H, Berger I. Age‐related changes in distractibility: developmental trajectory of sustained attention in ADHD. J Atten Disord 2018;22:1333‐43. [DOI] [PubMed] [Google Scholar]
- 169. Slobodin O, Blankers M, Kapitány‐Fövény M et al. Differential diagnosis in patients with substance use disorder and/or attention‐deficit/hyperactivity disorder using continuous performance test. Eur Addict Res 2020;26:151‐62. [DOI] [PubMed] [Google Scholar]
- 170. Berger I, Slobodin O, Cassuto H. Usefulness and validity of continuous performance tests in the diagnosis of attention‐deficit hyperactivity disorder children. Arch Clin Neuropsychol 2017;32:81‐93. [DOI] [PubMed] [Google Scholar]
- 171. Zeeuw PD, Aarnoudse‐Moens C, Bijlhout J et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry 2008;47:808‐16. [DOI] [PubMed] [Google Scholar]
- 172. Huang‐Pollock CL, Karalunas SL, Tam H et al. Evaluating vigilance deficits in ADHD: a meta‐analysis of CPT performance. J Abnorm Psychol 2012;121:360‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Xu M, Jiang W, Du Y et al. Executive function features in drug‐naive children with oppositional defiant disorder. Shanghai Arch Psychiatry 2017;29:228‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Noordermeer SDS, Luman M, Oosterlaan J. A systematic review and meta‐analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention‐deficit hyperactivity disorder (ADHD) into account. Neuropsychol Rev 2016;26:44‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Connor DF, Chartier KG, Preen EC et al. Impulsive aggression in attention‐deficit/hyperactivity disorder: symptom severity, co‐morbidity, and attention‐deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol 2010;20:119‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Frick PJ, Nigg JT. Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annu Rev Clin Psychol 2012;8:77‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. McCloskey MS, Phan KL, Angstadt M et al. Amygdala hyperactivation to angry faces in intermittent explosive disorder. J Psychiatr Res 2016;79:34‐41. [DOI] [PubMed] [Google Scholar]
- 178. Coccaro EF, Fanning JR, Keedy SK et al. Social cognition in intermittent explosive disorder and aggression. J Psychiatr Res 2016;83:140‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fahlgren MK, Puhalla AA, Sorgi KM et al. Emotion processing in intermittent explosive disorder. Psychiatry Res 2019;273:544‐50. [DOI] [PubMed] [Google Scholar]
- 180. Fettich KC, McCloskey MS, Look AE et al. Emotion regulation deficits in intermittent explosive disorder. Aggress Behav 2015;41:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology 2012;219:607‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Volkow ND, Li T‐K. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci 2004;5:963‐70. [DOI] [PubMed] [Google Scholar]
- 183. Finn PR, Rickert ME, Miller MA et al. Reduced cognitive ability in alcohol dependence: examining the role of covarying externalizing psychopathology. J Abnorm Psychol 2009;118:100‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kirisci L, Tarter R, Ridenour T et al. Externalizing behavior and emotion dysregulation are indicators of transmissible risk for substance use disorder. Addict Behav 2015;42:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Verdejo‐García A, Pérez‐García M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology 2007;190:517‐30. [DOI] [PubMed] [Google Scholar]
- 186. Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav 2005;81:319‐30. [DOI] [PubMed] [Google Scholar]
- 187. Smith JL, Mattick RP, Jamadar SD et al. Deficits in behavioural inhibition in substance abuse and addiction: a meta‐analysis. Drug Alcohol Depend 2014;145:1‐33. [DOI] [PubMed] [Google Scholar]
- 188. Verdejo‐García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance‐use disorders: review of findings from high‐risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 2008;32:777‐810. [DOI] [PubMed] [Google Scholar]
- 189. Ruocco AC. The neuropsychology of borderline personality disorder: a meta‐analysis and review. Psychiatry Res 2005;137:191‐202. [DOI] [PubMed] [Google Scholar]
- 190. Kalpakci A, Ha C, Sharp C. Differential relations of executive functioning to borderline personality disorder presentations in adolescents. Personal Ment Health 2018;12:93‐106. [DOI] [PubMed] [Google Scholar]
- 191. Coolidge FL, Thede LL, Jang KL. Are personality disorders psychological manifestations of executive function deficits? Bivariate heritability evidence from a twin study. Behav Genet 2004;34:75‐84. [DOI] [PubMed] [Google Scholar]
- 192. Herpertz SC, Bertsch K. The social‐cognitive basis of personality disorders. Curr Opin Psychiatry 2014;27:73‐7. [DOI] [PubMed] [Google Scholar]
- 193. Lovett BJ, Sheffield RA. Affective empathy deficits in aggressive children and adolescents: a critical review. Clin Psychol Rev 2007;27:1‐13. [DOI] [PubMed] [Google Scholar]
- 194. Domes G, Schulze L, Herpertz SC. Emotion recognition in borderline personality disorder – a review of the literature. J Personal Disord 2009;23:6‐19. [DOI] [PubMed] [Google Scholar]
- 195. Frick PJ, White SF. Research Review: The importance of callous‐unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry 2008;49:359‐75. [DOI] [PubMed] [Google Scholar]
- 196. Glenn AL, Johnson AK, Raine A. Antisocial personality disorder: a current review. Curr Psychiatry Rep 2013;15:427. [DOI] [PubMed] [Google Scholar]
- 197. O’Kearney R, Salmon K, Liwag M et al. Emotional abilities in children with oppositional defiant disorder (ODD): impairments in perspective‐taking and understanding mixed emotions are associated with high callous‐unemotional traits. Child Psychiatry Hum Dev 2017;48:346‐57. [DOI] [PubMed] [Google Scholar]
- 198. Jiang W, Li Y, Du Y et al. Emotional regulation and executive function deficits in unmedicated Chinese children with oppositional defiant disorder. Psychiatry Investig 2016;13:277‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Verdejo‐García A, Pérez‐García M, Bechara A. Emotion, decision‐making and substance dependence: a somatic‐marker model of addiction. Curr Neuropharmacol 2006;4:17‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Verdejo‐García A, Rivas‐Pérez C, Vilar‐López R et al. Strategic self‐regulation, decision‐making and emotion processing in poly‐substance abusers in their first year of abstinence. Drug Alcohol Depend 2007;86:139‐46. [DOI] [PubMed] [Google Scholar]
- 201. Wilcox CE, Pommy JM, Adinoff B. Neural circuitry of impaired emotion regulation in substance use disorders. Am J Psychiatry 2016;173:344‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Herpertz SC, Schneider I, Schmahl C et al. Neurobiological mechanisms mediating emotion dysregulation as targets of change in borderline personality disorder. Psychopathology 2018;51:96‐104. [DOI] [PubMed] [Google Scholar]
- 203. Carpenter RW, Trull TJ. Components of emotion dysregulation in borderline personality disorder: a review. Curr Psychiatry Rep 2013;15:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Baskin‐Sommers A, Krusemark E, Ronningstam E. Empathy in narcissistic personality disorder: from clinical and empirical perspectives. Personal Disord 2014;5:323‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lee DS. A longitudinal analysis of executive functions, learning‐related skills, and mathematics achievement. Dissertation, University of California, Irvine, 2017. [Google Scholar]
- 206. Gao Y, Raine A. P3 event‐related potential impairments in antisocial and psychopathic individuals: a meta‐analysis. Biol Psychol 2009;82:199‐210. [DOI] [PubMed] [Google Scholar]
- 207. Begleiter H, Porjesz B, Bihari B et al. Event‐related brain potentials in boys at risk for alcoholism. Science 1984;225:1493‐6. [DOI] [PubMed] [Google Scholar]
- 208. Iacono WG, Carlson SR, Malone SM et al. P3 event‐related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry 2002;59:750‐7. [DOI] [PubMed] [Google Scholar]
- 209. Patrick CJ, Bernat EM, Malone SM et al. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology 2006;43:84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Viding E, Sebastian CL, Dadds MR et al. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous‐unemotional traits. Am J Psychiatry 2012;169:1109‐16. [DOI] [PubMed] [Google Scholar]
- 211. Hicks BM, Bernat E, Malone SM et al. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology 2007;44:98‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Yancey JR, Venables NC, Hicks BM et al. Evidence for a heritable brain basis to deviance‐promoting deficits in self‐control. J Crim Justice 2013;41:309‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Young SE, Friedman NP, Miyake A et al. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol 2009;118:117‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Venables NC, Foell J, Yancey JR et al. Quantifying inhibitory control as externalizing proneness: a cross‐domain model. Clin Psychol Sci 2018;6:561‐80. [Google Scholar]
- 215. Iacono WG, Carlson SR, Taylor J et al. Behavioral disinhibition and the development of substance‐use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol 1999;11:869‐900. [DOI] [PubMed] [Google Scholar]
- 216. Patrick CJ, Foell J, Venables NC et al. Substance use disorders as externalizing outcomes. In: Beauchaine T, Hinshaw S (eds). Oxford handbook of externalizing spectrum. Oxford: Oxford University Press, 2016:38‐60. [Google Scholar]
- 217. Dikman ZV, Allen JJB. Error monitoring during reward and avoidance learning in high‐ and low‐socialized individuals. Psychophysiology 2000;37:43‐54. [PubMed] [Google Scholar]
- 218. Pailing PE, Segalowitz SJ, Dywan J et al. Error negativity and response control. Psychophysiology 2002;39:198‐206. [DOI] [PubMed] [Google Scholar]
- 219. Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error‐related negativity. Psychol Sci 2007;18:326‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Michelini G, Cheung CHM, Kitsune V et al. The etiological structure of cognitive‐neurophysiological impairments in ADHD in adolescence and young adulthood. J Atten Disord 2021;25:91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Pasion R, Cruz AR, Barbosa F. Dissociation of boldness and disinhibition psychopathic traits in ERN modulation. Personal Individ Differ 2016;95:6‐10. [Google Scholar]
- 222. Ribes‐Guardiola P, Poy R, Patrick CJ et al. Electrocortical measures of performance monitoring from go/no‐go and flanker tasks: differential relations with trait dimensions of the triarchic model of psychopathy. Psychophysiology 2020;57:e13573. [DOI] [PubMed] [Google Scholar]
- 223. Marsh AA. Reduced amygdala response to fearful expressions in children and adolescents with callous‐unemotional traits and disruptive behavior disorders. Am J Psychiatry 2008;165:712‐20. [DOI] [PubMed] [Google Scholar]
- 224. Frick PJ, Ray JV, Thornton LC et al. Can callous‐unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol Bull 2013;140:1‐57. [DOI] [PubMed] [Google Scholar]
- 225. Brislin SJ, Patrick CJ. Callousness and affective face processing: clarifying the neural basis of behavioral‐recognition deficits through the use of brain event‐related potentials. Clin Psychol Sci 2019;7:1389‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Brislin SJ, Yancey JR, Perkins ER et al. Callousness and affective face processing in adults: behavioral and brain‐potential indicators. Personal Disord Theory Res Treat 2017;9:122‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Dev Psychopathol 2009;21:913‐38. [DOI] [PubMed] [Google Scholar]
- 228. Vanyukov MM, Tarter RE, Kirillova GP et al. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 2012;123:S3‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Perkins ER, Joyner KJ, Patrick CJ et al. Neurobiology and the Hierarchical Taxonomy of Psychopathology: progress toward ontogenetically informed and clinically useful nosology. Dialogues Clin Neurosci 2020;22:51‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Bartholow BD, Henry EA, Lust SA. Effects of alcohol sensitivity on P3 event‐related potential reactivity to alcohol cues. Psychol Addict Behav 2007;21:555‐63. [DOI] [PubMed] [Google Scholar]
- 231. van Hemel‐Ruiter ME, de Jong PJ, Ostafin BD et al. Reward sensitivity, attentional bias, and executive control in early adolescent alcohol use. Addict Behav 2015;40:84‐90. [DOI] [PubMed] [Google Scholar]
- 232. Blum K, Braverman ER, Holder JM et al. The reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. J Psychoactive Drugs 2000;32:1‐112. [DOI] [PubMed] [Google Scholar]
- 233. Joyner KJ, Bowyer CB, Yancey JR et al. Blunted reward sensitivity and trait disinhibition interact to predict substance use problems. Clin Psychol Sci 2019;7:1109‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Weber S, Habel U, Amunts K et al. Structural brain abnormalities in psychopaths – a review. Behav Sci Law 2008;26:7‐28. [DOI] [PubMed] [Google Scholar]
- 235. de‐Almeida CP, Wenzel A, de‐Carvalho CS et al. Amygdalar volume in borderline personality disorder with and without comorbid post‐traumatic stress disorder: a meta‐analysis. CNS Spectr 2012;17:70‐5. [DOI] [PubMed] [Google Scholar]
- 236. Ruocco AC, Amirthavasagam S, Zakzanis KK. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: a meta‐analysis of magnetic resonance imaging studies. Psychiatry Res Neuroimaging 2012;201:245‐52. [DOI] [PubMed] [Google Scholar]
- 237. Pardini DA, Raine A, Erickson K et al. Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biol Psychiatry 2014;75:73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Dager AD, McKay DR, Kent JW et al. Shared genetic factors influence amygdala volumes and risk for alcoholism. Neuropsychopharmacology 2015;40:412‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Hill SY, De Bellis MD, Keshavan MS et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry 2001;49:894‐905. [DOI] [PubMed] [Google Scholar]
- 240. Hoogman M, Bralten J, Hibar DP et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross‐sectional mega‐analysis. Lancet Psychiatry 2017;4:310‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Benegal V, Antony G, Venkatasubramanian G et al. Imaging study: gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol 2007;12:122‐32. [DOI] [PubMed] [Google Scholar]
- 242. Cheetham A, Allen NB, Whittle S et al. Amygdala volume mediates the relationship between externalizing symptoms and daily smoking in adolescence: a prospective study. Psychiatry Res Neuroimaging 2018;276:46‐52. [DOI] [PubMed] [Google Scholar]
- 243. Cardinale EM, O’Connell K, Robertson EL et al. Callous and uncaring traits are associated with reductions in amygdala volume among youths with varying levels of conduct problems. Psychol Med 2019;49:1449‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev 2003;41:88‐123. [DOI] [PubMed] [Google Scholar]
- 245. Karl A, Schaefer M, Malta L et al. A meta‐analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006;30:1004‐31. [DOI] [PubMed] [Google Scholar]
- 246. Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta‐analysis. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:1181‐8. [DOI] [PubMed] [Google Scholar]
- 247. Morey RA, Gold AL, LaBar KS et al. Amygdala volume changes in posttraumatic stress disorder in a large case‐controlled veterans group. Arch Gen Psychiatry 2012;69:1169‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Poeppl TB, Donges MR, Mokros A et al. A view behind the mask of sanity: meta‐analysis of aberrant brain activity in psychopaths. Mol Psychiatry 2019;24:463‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ruocco AC, Amirthavasagam S, Choi‐Kain LW et al. Neural correlates of negative emotionality in borderline personality disorder: an activation‐likelihood‐estimation meta‐analysis. Biol Psychiatry 2013;73:153‐60. [DOI] [PubMed] [Google Scholar]
- 250. Jones AP, Laurens KR, Herba CM et al. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous‐unemotional traits. Am J Psychiatry 2009;166:95‐102. [DOI] [PubMed] [Google Scholar]
- 251. Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res 2006;142:107‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta‐analysis. Psychiatry Res Neuroimaging 2009;174:81‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Blair RJR. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc B Biol Sci 2008;363:2557‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Zald DH, McHugo M, Ray KL et al. Meta‐analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex 2014;24:232‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Boccia M, Dacquino C, Piccardi L et al. Neural foundation of human moral reasoning: an ALE meta‐analysis about the role of personal perspective. Brain Imaging Behav 2017;11:278‐92. [DOI] [PubMed] [Google Scholar]
- 256. Shamay‐Tsoory SG. The neural bases for empathy. Neuroscientist 2011;17:18‐24. [DOI] [PubMed] [Google Scholar]
- 257. Koenigs M, Tranel D. Pseudopsychopathy: a perspective from cognitive neuroscience. In: Zald D, Rauch S (eds). Orbitofrontal cortex. Oxford: Oxford University Press, 2006:597‐619. [Google Scholar]
- 258. Cheetham A, Allen NB, Whittle S et al. Orbitofrontal cortex volume and effortful control as prospective risk factors for substance use disorder in adolescence. Eur Addict Res 2017;23:37‐44. [DOI] [PubMed] [Google Scholar]
- 259. Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry 2008;63:256‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Zilverstand A, Huang AS, Alia‐Klein N et al. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron 2018;98:886‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Qin K, Zhang F, Chen T et al. Shared gray matter alterations in individuals with diverse behavioral addictions: a voxel‐wise meta‐analysis. J Behav Addict 2020;9:44‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Rubia K. “Cool” inferior frontostriatal dysfunction in attention‐deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal‐limbic dysfunction in conduct disorder: a review. Biol Psychiatry 2011;69:e69‐87. [DOI] [PubMed] [Google Scholar]
- 263. Hart H, Radua J, Nakao T et al. Meta‐analysis of functional magnetic resonance imaging studies of inhibition and attention in attention‐deficit/hyperactivity disorder: exploring task‐specific, stimulant medication, and age effects. JAMA Psychiatry 2013;70:185‐98. [DOI] [PubMed] [Google Scholar]
- 264. Morein‐Zamir S, Robbins TW. Fronto‐striatal circuits in response‐inhibition: relevance to addiction. Brain Res 2015;1628:117‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Hoogman M, Muetzel R, Guimaraes JP et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large‐scale clinical and population‐based samples. Am J Psychiatry 2019;176:531‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Norman LJ, Carlisi C, Lukito S et al. Structural and functional brain abnormalities in attention‐deficit/hyperactivity disorder and obsessive‐compulsive disorder: a comparative meta‐analysis. JAMA Psychiatry 2016;73:815‐25. [DOI] [PubMed] [Google Scholar]
- 267. Alegria AA, Radua J, Rubia K. Meta‐analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry 2016;173:1119‐30. [DOI] [PubMed] [Google Scholar]
- 268. Luijten M, Machielsen MWJ, Veltman DJ et al. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci 2014;39:149‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Xiao P, Dai Z, Zhong J et al. Regional gray matter deficits in alcohol dependence: a meta‐analysis of voxel‐based morphometry studies. Drug Alcohol Depend 2015;153:22‐8. [DOI] [PubMed] [Google Scholar]
- 270. Sato JR, Salum GA, Gadelha A et al. Default mode network maturation and psychopathology in children and adolescents. J Child Psychol Psychiatry 2016;57:55‐64. [DOI] [PubMed] [Google Scholar]
- 271. Whittle S, Vijayakumar N, Simmons JG et al. Internalizing and externalizing symptoms are associated with different trajectories of cortical development during late childhood. J Am Acad Child Adolesc Psychiatry 2020;59:177‐85. [DOI] [PubMed] [Google Scholar]
- 272. Tervo‐Clemmens B, Quach A, Calabro FJ et al. Meta‐analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use. NeuroImage 2020;209:116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Volkow ND, Wang G‐J, Fowler JS et al. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 2012;52:321‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Willuhn I, Wanat MJ, Clark JJ et al. Dopamine signaling in the nucleus accumbens of animals self‐administering drugs of abuse. Curr Top Behav Neurosci 2010;3:29‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Ellison‐Wright I, Ellison‐Wright Z, Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta‐analysis. BMC Psychiatry 2008;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Nakao T, Radua J, Rubia K et al. Gray matter volume abnormalities in ADHD: voxel‐based meta‐analysis exploring the effects of age and stimulant medication. Am J Psychiatry 2011;168:1154‐63. [DOI] [PubMed] [Google Scholar]
- 277. Bickel WK, Koffarnus MN, Moody L et al. The behavioral‐ and neuro‐economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology 2014;76:518‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Buckholtz JW, Treadway MT, Cowan RL et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci 2010;13:419‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Plichta MM, Scheres A. Ventral‐striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta‐analytic review of the fMRI literature. Neurosci Biobehav Rev 2014;38:125‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Shanmugan S, Wolf DH, Calkins ME et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry 2016;173:517‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Xia CH, Ma Z, Ciric R et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun 2018;9:3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Abram SV, Wisner KM, Grazioplene RG et al. Functional coherence of insula networks is associated with externalizing behavior. J Abnorm Psychol 2015;124:1079‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Sadeh N, Spielberg JM, Logue MW et al. Linking genes, circuits, and behavior: network connectivity as a novel endophenotype of externalizing. Psychol Med 2019;49:1905‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Cecil CAM, Walton E, Viding E. DNA methylation, substance use and addiction: a systematic review of recent animal and human research from a developmental perspective. Curr Addict Rep 2015;2:331‐46. [Google Scholar]
- 285. Prom‐Wormley EC, Ebejer J, Dick DM et al. The genetic epidemiology of substance use disorder: a review. Drug Alcohol Depend 2017;180:241‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Provençal N, Suderman MJ, Guillemin C et al. Association of childhood chronic physical aggression with a DNA methylation signature in adult human T cells. PLoS One 2014;9:e89839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Provençal N, Booij L, Tremblay RE. The developmental origins of chronic physical aggression: biological pathways triggered by early life adversity. J Exp Biol 2015;218:123‐33. [DOI] [PubMed] [Google Scholar]
- 288. Waltes R, Chiocchetti AG, Freitag CM. The neurobiological basis of human aggression: a review on genetic and epigenetic mechanisms. Am J Med Genet B Neuropsychiatr Genet 2016;171:650‐75. [DOI] [PubMed] [Google Scholar]
- 289. Barker ED, Walton E, Cecil CAM et al. A methylome‐wide association study of trajectories of oppositional defiant behaviors and biological overlap with attention deficit hyperactivity disorder. Child Dev 2018;89:1839‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Knopik VS, Marceau K, Bidwell LC et al. Prenatal substance exposure and offspring development: does DNA methylation play a role? Neurotoxicol Teratol 2019;71:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Barker ED, Walton E, Cecil CAM. Annual research review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. J Child Psychol Psychiatry 2018;59:303‐22. [DOI] [PubMed] [Google Scholar]
- 292. Cecil CAM, Walton E, Smith RG et al. DNA methylation and substance‐use risk: a prospective, genome‐wide study spanning gestation to adolescence. Transl Psychiatry 2016;6:e976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Barker ED, Cecil CAM, Walton E et al. Inflammation‐related epigenetic risk and child and adolescent mental health: a prospective study from pregnancy to middle adolescence. Dev Psychopathol 2018;30:1145‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Slopen N, Kubzansky LD, Koenen KC. Internalizing and externalizing behaviors predict elevated inflammatory markers in childhood. Psychoneuroendocrinology 2013;38:2854‐62. [DOI] [PubMed] [Google Scholar]
- 295. Coccaro EF, Lee R, Coussons‐Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry 2014;71:158‐65. [DOI] [PubMed] [Google Scholar]
- 296. Marsland AL, Prather AA, Petersen KL et al. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun 2008;22:753‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Mitchell RHB, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J Am Acad Child Adolesc Psychiatry 2014;53:274‐96. [DOI] [PubMed] [Google Scholar]
- 298. Anand D, Colpo GD, Zeni G et al. Attention‐deficit/hyperactivity disorder and inflammation: what does current knowledge tell us? A systematic review. Front Psychiatry 2017;8:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Leffa DT, Torres ILS, Rohde LA. A review on the role of inflammation in attention‐deficit/hyperactivity disorder. Neuroimmunomodulation 2018;25:328‐33. [DOI] [PubMed] [Google Scholar]
- 300. Wang T‐Y, Lee S‐Y, Hu M‐C et al. More inflammation but less brain‐derived neurotrophic factor in antisocial personality disorder. Psychoneuroendocrinology 2017;85:42‐8. [DOI] [PubMed] [Google Scholar]
- 301. Ashare RL, Wetherill RR. The intersection of sex differences, tobacco use, and inflammation: implications for psychiatric disorders. Curr Psychiatry Rep 2018;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Nennig SE, Schank JR. The role of NFkB in drug addiction: beyond inflammation. Alcohol Alcohol 2017;52:172‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Harricharan R, Abboussi O, Daniels WMU. Addiction: a dysregulation of satiety and inflammatory processes. In: Calvey T, Daniels WMU (eds). Progress in brain research. Amsterdam: Elsevier, 2017:65‐91. [DOI] [PubMed] [Google Scholar]
- 304. Scassellati C, Bonvicini C, Faraone SV et al. Biomarkers and attention‐deficit/hyperactivity disorder: a systematic review and meta‐analyses. J Am Acad Child Adolesc Psychiatry 2012;51:1003‐1019.e20. [DOI] [PubMed] [Google Scholar]
- 305. Haltigan JD, Roisman GI, Susman EJ et al. Elevated trajectories of externalizing problems are associated with lower awakening cortisol levels in mid adolescence. Dev Psychol 2011;47:472‐8. [DOI] [PubMed] [Google Scholar]
- 306. Loney BR, Butler MA, Lima EN et al. The relation between salivary cortisol, callous‐unemotional traits, and conduct problems in an adolescent non‐referred sample. J Child Psychol Psychiatry 2006;47:30‐6. [DOI] [PubMed] [Google Scholar]
- 307. Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol 2006;59:195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Marceau K, Abel E. Mechanisms of cortisol ‐ substance use development associations: hypothesis generation through gene enrichment analysis. Neurosci Biobehav Rev 2018;92:128‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Zorn JV, Schür RR, Boks MP et al. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta‐analysis. Psychoneuroendocrinology 2017;77:25‐36. [DOI] [PubMed] [Google Scholar]
- 310. Harro J, Oreland L. The role of MAO in personality and drug use. Prog Neuropsychopharmacol Biol Psychiatry 2016;69:101‐11. [DOI] [PubMed] [Google Scholar]
- 311. Kiive E, Fischer K, Harro M et al. Platelet monoamine oxidase activity in association with adolescent inattentive and hyperactive behaviour: a prospective longitudinal study. Personal Individ Differ 2007;43:155‐66. [Google Scholar]
- 312. Moore TM, Scarpa A, Raine A. A meta‐analysis of serotonin metabolite 5‐HIAA and antisocial behavior. Aggress Behav 2002;28:299‐316. [Google Scholar]
- 313. Seo D, Patrick CJ, Kennealy PJ. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav 2008;13:383‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Duke AA, Bègue L, Bell R et al. Revisiting the serotonin‐aggression relation in humans: a meta‐analysis. Psychol Bull 2013;139:1148‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Mervielde I, De Clercq B, De Fruyt F et al. Temperament, personality, and developmental psychopathology as childhood antecedents of personality disorders. J Personal Disord 2005;19:171‐201. [DOI] [PubMed] [Google Scholar]
- 316. Tackett JL, Balsis S, Oltmanns TF et al. A unifying perspective on personality pathology across the life span: developmental considerations for the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. Dev Psychopathol 2009;21:687‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Widiger TA, De Clercq B, De Fruyt F. Childhood antecedents of personality disorder: an alternative perspective. Dev Psychopathol 2009;21:771‐91. [DOI] [PubMed] [Google Scholar]
- 318. Clark LA. Temperament as a unifying basis for personality and psychopathology. J Abnorm Psychol 2005;114:505‐21. [DOI] [PubMed] [Google Scholar]
- 319. Shiner RL. A developmental perspective on personality disorders: lessons from research on normal personality development in childhood and adolescence. J Personal Disord 2005;19:202‐10. [DOI] [PubMed] [Google Scholar]
- 320. Martel MM, Smith TE, Lee CA. Personality development and externalizing psychopathology. In: McAdams DP, Rl Shiner, Tackett JL (eds). Handbook of personality development. New York: Guilford, 2019:534‐50. [Google Scholar]
- 321. Morizot J. The contribution of temperament and personality traits to criminal and antisocial behavior development and desistance. In: Morizot J, Kazemian L (eds). The development of criminal and antisocial behavior: theory, research and practical applications. Cham: Springer, 2015:137‐65. [Google Scholar]
- 322. Lahey BB, Applegate B, Chronis AM et al. Psychometric characteristics of a measure of emotional dispositions developed to test a developmental propensity model of conduct disorder. J Clin Child Adolesc Psychol 2008;37:794‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Caspi A, Henry B, McGee RO et al. Temperamental origins of child and adolescent behavior problems: from age three to age fifteen. Child Dev 1995;66:55‐68. [DOI] [PubMed] [Google Scholar]
- 324. Caspi A. Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. Arch Gen Psychiatry 1996;53:1033‐9. [DOI] [PubMed] [Google Scholar]
- 325. Clercq BD, Fruyt FD. A five‐factor model framework for understanding childhood personality disorder antecedents. J Pers 2012;80:1533‐63. [DOI] [PubMed] [Google Scholar]
- 326. Nielsen IKM. The impact of temperamental dimensions on change in symptoms of oppositional defiant disorder from preschool to first grade. Master Thesis, Norwegian University of Science and Technology, 2014.
- 327. Stringaris A, Maughan B, Goodman R. What’s in a disruptive disorder? Temperamental antecedents of oppositional defiant disorder: findings from the Avon Longitudinal Study. J Am Acad Child Adolesc Psychiatry 2010;49:474‐83. [DOI] [PubMed] [Google Scholar]
- 328. Tremblay RE, Galera C, Orri M et al. Developmental trajectories of aggression and other problematic behaviors associated with IED. In: Coccaro EF, McCloskey MS (eds). Intermittent explosive disorder. Cambridge: Academic Press, 2019:3‐15. [Google Scholar]
- 329. Forbes MK, Rapee RM, Camberis A‐L et al. Unique associations between childhood temperament characteristics and subsequent psychopathology symptom trajectories from childhood to early adolescence. J Abnorm Child Psychol 2017;45:1221‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Waller R, Wagner NJ, Barstead MG et al. A meta‐analysis of the associations between callous‐unemotional traits and empathy, prosociality, and guilt. Clin Psychol Rev 2020;75:101809. [DOI] [PubMed] [Google Scholar]
- 331. Chanen AM, Kaess M. Developmental pathways to borderline personality disorder. Curr Psychiatry Rep 2012;14:45‐53. [DOI] [PubMed] [Google Scholar]
- 332. Stepp SD, Whalen DJ, Pedersen SL. The externalizing pathway to borderline personality disorder in youth. In: Sharp C, Tackett JL (eds). Handbook of borderline personality disorder in children and adolescents. New York: Springer, 2014:247‐63. [Google Scholar]
- 333. Ivanov I, Schulz KP, London ED et al. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am J Drug Alcohol Abuse 2008;34:239‐58. [DOI] [PubMed] [Google Scholar]
- 334. Rioux C, Castellanos‐Ryan N, Parent S et al. The interaction between temperament and the family environment in adolescent substance use and externalizing behaviors: support for diathesis‐stress or differential susceptibility? Dev Rev 2016;40:117‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Creemers HE, Verhulst FC, Huizink AC. Temperamental risk factors for adolescent cannabis use: a systematic review of prospective general population studies. Subst Use Misuse 2009;44:1833‐54. [DOI] [PubMed] [Google Scholar]
- 336. Moffitt TE. Life‐course‐persistent and adolescence‐limited antisocial behavior: a developmental taxonomy. Psychol Rev 1993;100:674‐701. [PubMed] [Google Scholar]
- 337. Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annu Rev Psychol 1997;48:371‐410. [DOI] [PubMed] [Google Scholar]
- 338. Kuperman S, Schlosser SS, Kramer JR et al. Risk domains associated with an adolescent alcohol dependence diagnosis. Addiction 2001;96:629‐36. [DOI] [PubMed] [Google Scholar]
- 339. Lynam DR, Charnigo R, Moffitt TE et al. The stability of psychopathy across adolescence. Dev Psychopathol 2009;21:1133‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Wichers M, Gardner C, Maes HH et al. Genetic innovation and stability in externalizing problem behavior across development: a multi‐informant twin study. Behav Genet 2013;43:191‐201. [DOI] [PubMed] [Google Scholar]
- 341. Olson SL, Bates JE, Sandy JM et al. Early developmental precursors of externalizing behavior in middle childhood and adolescence. J Abnorm Child Psychol 2000;28:119‐33. [DOI] [PubMed] [Google Scholar]
- 342. Helmond P, Overbeek G, Brugman D et al. A meta‐analysis on cognitive distortions and externalizing problem behavior: associations, moderators, and treatment effectiveness. Crim Justice Behav 2015;42:245‐62. [Google Scholar]
- 343. Maughan DR, Christiansen E, Jenson WR et al. Behavioral parent training as a treatment for externalizing behaviors and disruptive behavior disorders: a meta‐analysis. Sch Psychol Rev 2005;34:267‐86. [Google Scholar]
- 344. Reyno SM, McGrath PJ. Predictors of parent training efficacy for child externalizing behavior problems – a meta‐analytic review. J Child Psychol Psychiatry 2006;47:99‐111. [DOI] [PubMed] [Google Scholar]
- 345. Barnes TN, Smith SW, Miller MD. School‐based cognitive‐behavioral interventions in the treatment of aggression in the United States: a meta‐analysis. Aggress Violent Behav 2014;19:311‐21. [Google Scholar]
- 346. Kremer KP, Maynard BR, Polanin JR et al. Effects of after‐school programs with at‐risk youth on attendance and externalizing behaviors: a systematic review and meta‐analysis. J Youth Adolesc 2015;44:616‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Mingebach T, Kamp‐Becker I, Christiansen H et al. Meta‐meta‐analysis on the effectiveness of parent‐based interventions for the treatment of child externalizing behavior problems. PLoS One 2018;13:e0202855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Faraone SV, Biederman J, Spencer TJ et al. Comparing the efficacy of medications for ADHD using meta‐analysis. Medscape Gen Med 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 349. Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta‐analysis. Eur Child Adolesc Psychiatry 2010;19:353‐64. [DOI] [PubMed] [Google Scholar]
- 350. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention‐deficit/hyperactivity disorder using meta‐analysis of effect sizes. J Clin Psychiatry 2010;71:754‐63. [DOI] [PubMed] [Google Scholar]
- 351. Ipser J, Stein DJ. Systematic review of pharmacotherapy of disruptive behavior disorders in children and adolescents. Psychopharmacology 2007;191:127‐40. [DOI] [PubMed] [Google Scholar]
- 352. Lundahl BW, Kunz C, Brownell C et al. A meta‐analysis of motivational interviewing: twenty‐five years of empirical studies. Res Soc Work Pract 2010;20:137‐60. [Google Scholar]
- 353. Comer JS, Chow C, Chan PT et al. Psychosocial treatment efficacy for disruptive behavior problems in very young children: a meta‐analytic examination. J Am Acad Child Adolesc Psychiatry 2013;52:26‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Epstein RA, Fonnesbeck C, Potter S et al. Psychosocial interventions for child disruptive behaviors: a meta‐analysis. Pediatrics 2015;136:947‐60. [DOI] [PubMed] [Google Scholar]
- 355. Battagliese G, Caccetta M, Luppino OI et al. Cognitive‐behavioral therapy for externalizing disorders: a meta‐analysis of treatment effectiveness. Behav Res Ther 2015;75:60‐71. [DOI] [PubMed] [Google Scholar]
- 356. Regier DA, Narrow WE, Clarke DE et al. DSM‐5 field trials in the United States and Canada, Part II: Test‐retest reliability of selected categorical diagnoses. Am J Psychiatry 2013;170:59‐70. [DOI] [PubMed] [Google Scholar]
- 357. Cottler L. The DSM‐IV field trial for substance use disorders: major results. Drug Alcohol Depend 1995;38:59‐69. [DOI] [PubMed] [Google Scholar]
- 358. Narrow WE, Clarke DE, Kuramoto SJ et al. DSM‐5 field trials in the United States and Canada, Part III: Development and reliability testing of a cross‐cutting symptom assessment for DSM‐5. Am J Psychiatry 2013;170:71‐82. [DOI] [PubMed] [Google Scholar]
- 359. Krueger RF, Derringer J, Markon KE et al. Initial construction of a maladaptive personality trait model and inventory for DSM‐5. Psychol Med 2012;42:1879‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Quilty LC, Ayearst L, Chmielewski M et al. The psychometric properties of the personality inventory for DSM‐5 in an APA DSM‐5 field trial sample. Assessment 2013;20:362‐9. [DOI] [PubMed] [Google Scholar]
- 361. Wright AGC, Calabrese WR, Rudick MM et al. Stability of the DSM‐5 Section III pathological personality traits and their longitudinal associations with psychosocial functioning in personality disordered individuals. J Abnorm Psychol 2014;124:199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Chmielewski M, Ruggero CJ, Kotov R et al. Comparing the dependability and associations with functioning of the DSM–5 Section III trait model of personality pathology and the DSM‐5 Section II personality disorder model. Personal Disord Theory Res Treat 2017;8:228‐36. [DOI] [PubMed] [Google Scholar]
- 363. Sunderland M, Slade T, Krueger RF. Examining the shared and unique relationships among substance use and mental disorders. Psychol Med 2015;45:1103‐13. [DOI] [PubMed] [Google Scholar]
- 364. Ruggero CJ, Kotov R, Hopwood CJ et al. Integrating the Hierarchical Taxonomy of Psychopathology (HiTOP) into clinical practice. J Consult Clin Psychol 2019;87:1069‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Bornstein RF, Natoli AP. Clinical utility of categorical and dimensional perspectives on personality pathology: a meta‐analytic review. Personal Disord 2019;10:479‐90. [DOI] [PubMed] [Google Scholar]
- 366. Tellegen A, Waller N. Exploring personality through test construction: development of the Multidimensional Personality Questionnaire. In: Boyle GJ, Matthews G, Saklofske DH (eds). The SAGE handbook of personality theory and assessment. Thousand Oaks: Sage, 2008:261‐92. [Google Scholar]
- 367. Blonigen DM, Patrick CJ, Gasperi M et al. Delineating the construct network of the Personnel Reaction Blank: associations with externalizing tendencies and normal personality. Psychol Assess 2010;23:18‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: relations with disinhibitory psychopathology, personality, and psychopathic features. Psychol Assess 2011;24:88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Soe‐Agnie SE, Patrick CJ, Nijman HLI et al. Validation of the full and brief Externalizing Spectrum Inventory in Dutch forensic inpatients. J Forensic Psychiatry Psychol 2016;27:77‐91. [Google Scholar]
- 370. Sunderland M, Slade T, Krueger RF et al. Efficiently measuring dimensions of the externalizing spectrum model: development of the Externalizing Spectrum Inventory‐Computerized Adaptive Test (ESI‐CAT). Psychol Assess 2016;29:868‐80. [DOI] [PubMed] [Google Scholar]
- 371. Ben‐Porath YS, Tellegen A. Minnesota Multiphasic Personality Inventory‐2‐Restructured Form. www.pearsonassessments.com.
- 372. Moray LC. Professional manual for the Personality Assessment Inventory, 2nd ed. Lutz: Psychological Assessment Resources, 2007. [Google Scholar]
- 373. Lee TTC, Sellbom M, Hopwood CJ. Contemporary psychopathology assessment: mapping major personality inventories onto empirical models of psychopathology. In: Bowden SC (ed). Neuropsychological assessment in the age of evidence‐based practice: diagnostic and treatment evaluations. New York: Oxford University Press, 2017:65‐94. [Google Scholar]
- 374. Sellbom M. Elucidating the validity of the externalizing spectrum of psychopathology in correctional, forensic, and community samples. J Abnorm Psychol 2016;125:1027‐38. [DOI] [PubMed] [Google Scholar]
- 375. Sellbom M. Mapping the MMPI‐2‐RF specific problems scales onto extant psychopathology structures. J Pers Assess 2017;99:341‐50. [DOI] [PubMed] [Google Scholar]
- 376. Sellbom M. The MMPI‐2‐Restructured Form (MMPI‐2‐RF): assessment of personality and psychopathology in the twenty‐first century. Annu Rev Clin Psychol 2019;15:149‐77. [DOI] [PubMed] [Google Scholar]
- 377. McCrae RR, Costa PT Jr, Martin TA. The NEO‐PI‐3: a more readable revised NEO personality inventory. J Pers Assess 2005;84:261‐70. [DOI] [PubMed] [Google Scholar]
- 378. Simms LJ, Goldberg LR, Roberts JE et al. Computerized Adaptive Assessment of Personality Disorder: introducing the CAT‐PD project. J Pers Assess 2011;93:380‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Wright AGC, Thomas KM, Hopwood CJ et al. The hierarchical structure of DSM‐5 pathological personality traits. J Abnorm Psychol 2012;121:951‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 2000;21:265‐71. [DOI] [PubMed] [Google Scholar]
- 381. Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Adolesc Psychiatry 2001;40:1337‐45. [DOI] [PubMed] [Google Scholar]
- 382. Shaffer D, Fisher P, Lucas C et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC‐IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 2000;39:28‐38. [DOI] [PubMed] [Google Scholar]
- 383. Carragher N, Teesson M, Sunderland M et al. The structure of adolescent psychopathology: a symptom‐level analysis. Psychol Med 2016;46:981‐94. [DOI] [PubMed] [Google Scholar]
- 384. Lahey BB, Zald DH, Perkins SF et al. Measuring the hierarchical general factor model of psychopathology in young adults. Int J Methods Psychiatr Res 2018;27:e1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. McElroy E, Belsky J, Carragher N et al. Developmental stability of general and specific factors of psychopathology from early childhood to adolescence: dynamic mutualism or p‐differentiation? J Child Psychol Psychiatry 2018;59:667‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Conway KP, Levy J, Vanyukov M et al. Measuring addiction propensity and severity: the need for a new instrument. Drug Alcohol Depend 2010;111:4‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. McKenna H, Treanor C, O’Reilly D et al. Evaluation of the psychometric properties of self‐reported measures of alcohol consumption: a COSMIN systematic review. Subst Abuse Treat Prev Policy 2018;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Suris A, Lind L, Emmett G et al. Measures of aggressive behavior: overview of clinical and research instruments. Aggress Violent Behav 2004;9:165‐227. [Google Scholar]


