Abstract
Background
Among Coronavirus Disease 2019 (COVID-19) manifestations, Olfactory (OD) and Gustatory (GD) Dysfunctions (OGD) have drawn considerable attention, becoming a sort of hallmark of the disease. Many have speculated on the pathogenesis and clinical characteristics of these disturbances; however, no definite answers have been produced on the topic. With this systematic review, we aimed to collect all the available evidence regarding the prevalence of OGD, the timing of their onset and their resolution, their rate of recovery and their role as diagnostic and prognostic tools for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection.
Methods
A systematic review comprising all the observational studies that reported the prevalence and/or the longitudinal trajectories of OGD in COVID-19 patients, as self-reported by patients or measured through objective psychophysical tests.
Results
After the selection process, 155 studies were included, with a total of 70,920 patients and 105,291 not-infected individuals. Prevalence reports were extremely variable across studies, with wide ranges for OD (0%–98%) and GD (0–89%) prevalence. OGD occurred early during the disease course and only rarely preceded other symptoms; out of 30 studies with a follow-up time of at least 20 days, only in 5 studies OGD fully resolved in more than 90% of patients. OGD had low sensitivity and high specificity for SARS-CoV-2 infection; accuracy of OD and GD for infection identification was higher than 80% in 10 out of 33 studies and in 8 out of 22 studies considered, respectively. 28 out of 30 studies that studied the association between OGD and disease severity found how OGD were associated with lower rates of severe pneumonia, hospitalization and mortality.
Conclusions
OGD seem to be highly prevalent in SARS-CoV-2 infection. They occur early, concomitantly with other symptoms and often persist after recovery, in some cases for months; whether a full recovery eventually occurs in all cases is not clear yet. OGD are good predictors of SARS-CoV-2 infection and are associated with a milder disease course.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, Smell, Taste, Anosmia, Ageusia, Chemosensory
Highlights
-
•
Taste and smell disturbances prevalence in COVID-19 is highly variable across studies.
-
•
They appear early in the course of the disease, along with typical COVID-19 symptoms.
-
•
In those who fully recover, they typically resolve within 14 days.
-
•
In a significant proportion of patients, recovery is partial or absent after months.
-
•
They are good infection predictors and are associated with a milder disease course.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); it has been declared a pandemic on March 11, 2020 by the World Health Organization (WHO, 2020). As of January 2021, more than 80 million cases have been reported worldwide, causing over 1,800,000 deaths (John Hopkins Coronavirus Resource Center) as well as significant social and economic suffering.
While approximately half of those infected experience an asymptomatic disease course, the most common clinical manifestations are, in descending order of frequency, fever, cough, headache and sore throat (Lavezzo, 2020). Some patients, typically those of older age and/or suffering from other coexisting clinical conditions, may develop more severe forms of the disease, characterized by worsening of the respiratory dysfunction, acute respiratory distress syndrome, septic shock and, potentially, multi-organ failure (Wu et al., 2020).
In addition to the clinical scenario described above, a variety of other “non-typical” manifestations have been described in patients with COVID-19, including neurological (Mao et al., 2020a), psychiatric and neuropsychiatric (Rogers et al., 2020), cardiovascular (Zheng et al., 2020a), gastrointestinal (Jin et al., 2020), dermatological (Recalcati, 2020) and pediatric (Cruz and Zeichner, 2020) manifestations, which have been postulated to be the result of the trophism of human respiratory coronaviruses for multiple human tissues (Gupta et al., 2020; Schurink et al., 2020).
Among the extrapulmonary manifestations, the perceived high prevalence of olfactory and gustatory dysfunctions (OGD) had great resonance, even in the non-scientific community (Rabin, 2020; Asimov, 2020), eventually becoming a sort of “hallmark” of the disease. Consequently, the presence of OGD in COVID-19 patients has been consistently reported by authors from the early phases of the pandemic.
While a considerable amount of systematic reviews and meta-analyses have already been published on the topic (n = 15) (Hannum et al., 2020; Saniaslaya et al., 2020; Pang et al., 2020; Hajikhani et al., 2020; Ibekwe et al., 2020; Giorli et al., 2020; Von Bartheld et al., 2020a; Chi et al., 2020; Agyeman et al., 2020; Desiato et al., 2020; Rocke et al., 2020; Borsetto et al., 2020; Suratannon et al., 2020; Aziz et al., 2020; Tong et al., 2020), there are several reasons why we feel that our review can add valuable results for the readers.
First, the most updated database search was August 15th (Von Bartheld et al., 2020a); given the high rate of publication on COVID-19 (Putman et al., 2020), we expected that a high number of studies would have been published in the following months. Second, while all the meta-analyses cited above reported the prevalence of OGD, only few explored other aspects of these manifestations. In particular, only one meta-analysis collected data regarding the average duration of OGD (Von Bartheld et al., 2020a) (updated until August 15th - 104 studies included), and only one meta-analysis reported the timing of onset of OGD relative to other COVID-19 symptoms (Chi et al., 2020) (updated until May 8th – 12 studies included); no meta-analysis mentioned if any information on longitudinal trajectories of OGD symptoms was present in the studies included. Regarding the usefulness of OGD as predictors of SARS-CoV-2 infection, only one meta-analysis focused on this aspect, reporting the pooled positive predictive value of OGD in relation to SARS-CoV-2 positivity (Rocke et al., 2020) (updated until April 18th - 12 studies included). Finally, only one study collected data for the estimation of the relationship between the presence of OGD and the severity of the disease, expressed as the need for hospitalization (Giorli et al., 2020) (updated until June 1st – 11 studies included).
In this context, we performed a systematic review of studies focusing on OGD in COVID-19 in order to address the following points:
-
a)
Define OGD prevalence in COVID-19 patients and, if present, in a control group of healthy patients or patients with other upper-respiratory conditions.
-
b)
Define the timing of OGD occurrence, and in particular the timing of onset relative to COVID-19 “typical” symptoms.
-
c)
Define the longitudinal trajectories of OGD in terms of recovery or persistence.
-
d)
Define the potential role of OGD as a diagnostic and predictive tool for SARS-CoV-2 infection.
-
e)
Define the potential role of OGD as a predictor of severity and prognosis of COVID-19.
2. Methods
We structured our systematic review on the basis of the Conducting Systematic Reviews and Meta-Analyses of Observational Studies of Etiology (COSMOS-E) guidelines (Dekkers et al., 2019). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) tool (Liberati et al., 2009) was adopted for reporting.
2.1. Eligibility criteria
The study design of each report was evaluated following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (von Elm et al., 2007). The following design were included: cohort (with or without a control group), case-control and cross-sectional studies. Case series were excluded unless they provided follow-up data regarding OGD. Case reports or studies with sample <10 were not considered for inclusion.
No restrictions were posed regarding the definition of the population of interest, except for the presence of any medical condition associated with OGD (e.g., Parkinson disease).
The only criterion for inclusion was SARS-CoV-2 positivity, defined as SARS-CoV-2 genome detection by real-time reverse transcription polymerase chain reaction (RT-PCR) or antigen detection on nasal, pharyngeal or respiratory sample. We also included studies that defined exposure as positivity to serological testing (irrespective of the assay employed) (Houlihan and Beale, 2020).
Absence of exposure was defined as a negative result on the SARS-CoV-2 testing methods mentioned above. The presence of an unexposed control group was not necessary for a study to be included.
The outcome of interest was the occurrence of olfactory or gustatory disturbances. We did not pose limits regarding the way by which OGD were defined, and we included studies that assessed OGD through review of medical records, face to face or telephone clinical interviews, administration of web- or mobile application-based questionnaires. We also included studies that employed objective testing to assess OGD; no limitations were put regarding the type of psychophysical test adopted.
2.2. Search strategy
To identify potentially relevant records, PubMed, EMBASE and Web of Science databases were searched from December 1st, 2019 to October 8th, 2020. Only studies in English were considered. The search was rerun weekly, and last updated on December 14th.
The following string was used in PubMed and adapted for the other two databases:
"(coronavirus OR sars OR covid) AND (smell OR taste OR olfactory OR gustatory OR odor OR flavor OR anosmia OR hyposmia OR dysosmia OR ageusia OR hypogeusia OR dysgeusia OR chemosensory)."
2.3. Study selection process
Search results were exported into reference manager software Systematic Review Accelerator © and EPPI-Reviewer 4 © for duplication removal. Rayyan QCRI © was used for the screening process.
All the records were screened by title and abstract by two independent authors (AB and GD); on the second level of screening, full text of publications was evaluated by AB and VC; disagreements on study selection were resolved by consensus with the involvement of a third author (AP).
2.4. Data extraction
A preliminary data extraction form was designed by AB; it was then pilot tested on 15 randomly selected studies and fine-tuned accordingly. With the search being rerun on a weekly basis, data from newly included study were updated accordingly.
The following variables were extracted from each study included:
-
1)
Bibliographic information, study design
-
2)
Population and control: number of participants, nationality, type of control group (healthy vs other upper-respiratory viral disease)
-
3)
Exposure: SARS-CoV-2 diagnosis modality, disease severity (outpatient management vs hospitalization)
-
4)
Outcome: OGD assessment modality (subjective and objective), prevalence, timing of onset, longitudinal trajectories
-
5)
Measures of effect: unadjusted and adjusted Odds Ratio (OR) and Relative Risk (RR) for the association between OGD and SARS-CoV-2 infection and COVID-19 severity. Sensitivity (Sn), Specificity (Sp), Positive Predictive Value (PPV), Negative Predictive Value (NPV), Positive Likelihood Ratio (PLR) and Negative Likelihood Ratio (NLR) of OGD for SARS-CoV-2 infection and COVID-19 severity. If not reported by authors of the original study, Sn, Sp, PLR and NLR were calculated from studies that provided data about OGD in both SARS-CoV-2 positive individuals and negative controls.
2.5. Risk of bias assessment
To assess the risk of bias of the studies included, a modified version of the Newcastle-Ottawa Scale was used. The original version of the instrument was adapted for the cross-sectional, cohort and case-control studies included; the three adaptations are made available in the Supplementary Material. A particular focus was put on the representativeness of the sample (i.e., selected on the basis on hospital admission vs general population screening processes), on the ascertainment of the exposure (RT-PCR vs serology) and on the assessment of outcome (i.e., psychophysical testing or validated questionnaires vs anamnestic or chart-based). The maximum score attainable for a study was 9. For the risk of bias evaluation, the following categorization was considered: 0–3 Very High Risk, 4–6 High Risk, 7–9 Low Risk.
3. Results
3.1. Study selection
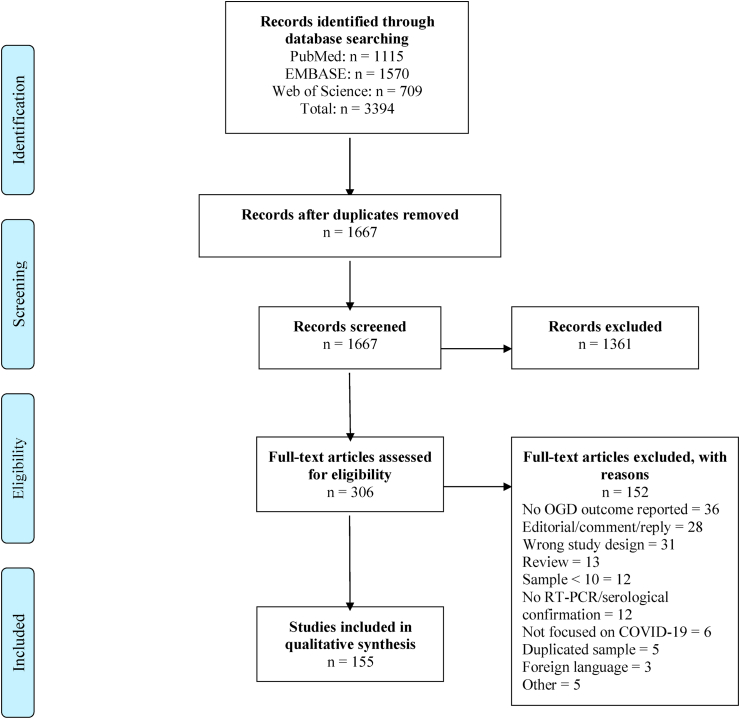
After the selection process, a total of 155 records met the inclusion criteria. A total of 3394 papers were retrieved from databases; after duplication removal, 1667 records remained and were screened through title and abstract. 306 articles were considered for full text examination, and 152 were excluded (Fig. 1).
Fig. 1.
Flow-chart summary of the study selection process (adapted from PRISMA guidelines (Liberati et al., 2009)).
3.2. Study characteristics
The studies characteristics and main findings are summarized below and in Table 1; a detailed report of all the records included is provided in a table format and can be found in the supplementary material section (Supplementary Material - Table 1).
Table 1.
Table summary of the main characteristics of the included studies.
|
DISEASE STATUS |
SARS-CoV-2 + 155 Records 58854 Individuals |
SARS-CoV-258 Records 105291 Individuals |
|
|---|---|---|---|
|
STUDY DESIGN |
Cross Sectional 90 Records 43309 Individuals |
Cohort 64 Records 15386 Individuals |
Case Control 1 Record 159 Individuals |
| COUNTRY (OF STUDY POPULATION) |
Western Countries 111 Records 41902 Individuals |
Asian Countries 13 Records 6671 Individuals |
Rest of the World 31 records 10281 Individuals |
| TESTING METHOD |
RT-PCR 136 Records 53196 Individuals |
Serology 17 Records 5516 Individuals |
Both RT-PCR and Serology 2 Records 142 Individuals |
| DISEASE SEVERITY (87 RECORDS) |
Outpatients (or mild) 20 Records 6036 Individuals |
Inpatients (or from moderate to severe) 37 Records 6207 Individuals |
Both 30 Records 53196 Individuals |
| OLFACTORY DISORDER ASSESSMENT (154 RECORDS) |
SUBJECTIVE 145 Records 57305 Individuals |
OBJECTIVE 21 Records 4316 Individuals |
|
|
Anamnestic, chart-based or non-validated methods 130 Records 47438 Individuals |
Validated questionnaires 15 Records 9867 Individuals |
||
| GUSTATORY DISORDER ASSESSMENT (136 RECORDS) |
SUBJECTIVE 131 Records 53648 Individuals |
OBJECTIVE 8 Records 1550 Individuals |
|
|
Anamnestic, chart-based or non-validated methods 115 Records 43395 Individuals |
Validated questionnaires 16 Records 10253 Individuals |
||
| RISK OF BIAS |
VERY HIGH 11 Records 2104 Individuals |
HIGH 118 Records 47523 Individuals |
LOW 26 Records 9227 Individuals |
When the study design was mislabeled by authors, the design reported was ignored and we labeled the study following the STROBE guidelines (von Elm et al., 2007). In this way, 91 (59%) studies were classified as cross-sectional, and 64 (41%) studies were labeled as cohort studies. Only one study had a case-control design (Joffily et al., 2020).
Since a meta-analysis outlined how OGD prevalence is significantly higher in Caucasians compared to Asian individuals (Von Bartheld et al., 2020b), we included the nationality of the population of the study among the variables collected. In our review, most studies (n = 112) were performed in Europe or North America (n = 41,998 individuals), with only 13 records (n = 6671) collecting data on Asian populations.
A total of 58,854 patients were included; 53,196 were confirmed by RT-PCR (n = 136 studies), while 5516 were defined by antibody detection (n = 17 studies); in two records, both techniques were used (Le Bon et al., 2020a; Merkely, 2020). Given the heterogeneity in the way the studies reported disease severity, when possible, we tried to dichotomize disease severity as the need for hospitalization; otherwise, we labeled disease severity as reported by authors. Disease severity was characterized by 87 papers; 6036 individuals were managed as outpatients and/or were classified as having an asymptomatic or mild form of the disease (n = 20 studies); 6207 were hospitalized and/or were classified as having a moderate to severe disease course (n = 37 studies). Other studies (n = 30, n = 53,196 individuals) considered a variably wide range of disease severity (from asymptomatic to severe, from outpatient course to ICU stay).
A total of 105,291 healthy controls (defined either with RT-PCR or serology) were included (n = 57 studies).
3.3. Subjective OGD assessment tools
A subjective report of OGD presence was collected by most studies (n = 145 for OD, n = 131 for GD); in most cases, clinical information was gathered through retrospective review of medical records, face to face or telephone clinical interviews, administration of web- or mobile application-based questionnaires. However, in a minority of studies OGD presence was assessed through validated questionnaires (n = 15). The Sino-Nasal Outcome Test (SNOT-22) (Hopkins et al., 2009) contains 22 items which evaluates ear-nose-throat symptoms and their impact on quality of life; item 5, which enquires OGD, was used by 4 studies (Mercante et al., 2020), (Ramasamy et al., 2020), (Cocco et al., 2020), (Boscolo-Rizzo et al., 2020). The National Health and Nutrition Examination Survey (NHANES) (Hoffman et al., 2016) contains 8 items which explore subjective and objective OGD; the subjective part was used by 3 records (Lechien et al., 2020a), (Lechien et al., 2020b), (Fantozzi et al., 2020). The Global Consortium for Chemosensory Research (GCCR) offers an online survey (GLOBAL CONSORTIUM FOR CHEMOSENSORY RESEARCH) that employs both binary response and visual analog scales to measure subjective OGD; it was employed by 2 studies (Gerkin et al., 2020), (Parma et al., 2020a). Finally, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) COVID-19 Anosmia Reporting Tool was designed for healthcare providers, and it can be used to submit anonymous information about OGD in COVID-19 patients (COVID-19 Anosmia Reporting Tool); it was employed by 2 studies (Sayin, 2020), (Özçelik Korkmaz et al., 2020).
3.4. Objective OD assessment tools
OD was evaluated through psychophysical testing in 21 studies. The Sniffin’Sticks' test was the most commonly employed objective test (n = 8 studies) (Sanli et al., 2021), (Lechien et al., 2020c), (Le Bon et al., 2020b), (Otte et al., 2020), (Altin et al., 2020), (Lechien et al., 2020a), (Iannuzzi et al., 2020), (Gözen et al., 2020). Through pen-like odor dispensing devices, it assesses three olfactory functions, i.e. odor threshold, odor discrimination and odor identification (Hummel et al., 1997). The Connecticut Chemosensory Clinical Research Center Test (CCCRC) was employed by 4 studies (Vaira et al., 2020a), (Vaira et al., 2020b), (Vaira et al., 2020c); it consists of a threshold test employing 1-buthanol as the odorant, and an odor identification test with natural items (Cain et al., 1988). The University of Pennsylvania Smell Identification Test (UPSIT) was employed by 3 studies (Moein et al., 2020a, 2020b), (Lima et al., 2020); it utilizes microcapsulated crystals for odor presentation and identification (Doty et al., 1984). Notably, UPSIT and CCCRC has been proved to be highly correlated in their scoring (Cain and Rabin, 1989). Finally, 2 studies (Bhattacharjee et al., 2020), (Li et al., 2020a) used olfactometric techniques; while Li et al. used the Toyota-Takagi (T&T) validated olfactometry, Bhattacharjee et al. ad-hoc designed a ten-channel olfactometer for olfactory detection and matching.
3.5. Objective GD assessment tools
Psychophysical validated evaluation of GD was performed in only 8 records (Vaira et al., 2020d), (Vaira et al., 2020c), (Vaira et al., 2020b), (Vaira et al., 2020a), (Petrocelli et al., 2020), (Hintschich et al., 2020), (Le Bon et al., 2020a), (Altin et al., 2020). In 4 of them (Vaira et al., 2020d), (Vaira et al., 2020c), (Vaira et al., 2020b), (Vaira et al., 2020a), (Petrocelli et al., 2020), a four-item (sweet, salty, sour, bitter) test (Massarelli et al., 2018) was administrated, both in sample of hospitalized patients and a sample of outpatients (self-administered) (Vaira et al., 2020e). “Taste strips”, spoon-shaped filter paper strips impregnated with the four taste qualities (Landis et al., 2009) were used in 2 records (Hintschich et al., 2020), (Le Bon et al., 2020a).
3.6. Prevalence of OGD
Aggregated prevalence (presence of either OD or GD) was given in 56 studies (36%), with a large inter-study variability (range: 1.5% (Pinato et al., 2020) – 91% (Otte et al., 2020)).
Prevalence of OD was reported by 95 studies (62%), with extreme variability between studies (range: 0% (Hauchecorne et al., 2020) – 98% (Moein et al., 2020c)).
Prevalence of GD was reported by 92 studies (59%); the large inter-study variability was confirmed also for this disturbance (range: 0% (Hauchecorne et al., 2020) – 89% (Paderno et al., 2020)).
Considering only studies that performed psychophysical testing for OD (n = 21), the lowest prevalence reported for OD was 19% (Romero-Gameros et al., 2020). In studies reporting both subjective and objective evaluations, those appeared to differ. In some cases, objective evaluation was more sensible to detect OD in those denying such disturbances (Lima et al., 2020), (Gözen et al., 2020). However, quite surprisingly, the opposite held true in some other cases, with subjective reports showing larger prevalence of OD compared to psychophysical testing (Romero-Gameros et al., 2020), (Hintschich et al., 2020), (Lechien et al., 2020a); nonetheless, in Lechien et al. (2020a) objective evaluation was performed only in a small subset of the total sample (93 out of 2013 patients).
Considering only studies that performed psychophysical testing for GD (n = 21), lowest prevalence reported was 20% (Hintschich et al., 2020); in this study, similarly to what reported above for OD, the authors found GD prevalence to be lower when assessed by psychophysical testing compared to subjective reporting (Hintschich et al., 2020).
When comparing prevalence based on SARS-CoV-2 testing, lowest prevalence among studies for OD and GD were 21% (Foster et al., 2020) and 33% (Nouchi et al., 2020), respectively, when considering only SARS-CoV-2 diagnosis through serology (n = 17, 11%). When restricting results to cross-sectional studies, lowest prevalence reported for OD and GD were 5% (Romero-Sánchez et al., 2020), (Mao et al., 2020b) and 6% (Romero-Sánchez et al., 2020), (Mao et al., 2020b), respectively.
Finally, we grouped studies based on nationality of the population, with one group comprising studies carried out in the European Union or United States (n = 111, 71%), and the other including investigations performed in the rest of the world (n = 45, 29%). In the European Union/United States group, prevalence range for OD and GD were 0% (Hauchecorne et al., 2020) - 98% (Moein et al., 2020c) and 080–89% (Paderno et al., 2020), respectively; in the rest of the world group, prevalence range for OD and GD were 1% (Chachkhiani et al., 2020) - 89% (Al-Zaidi and Badr, 2020) and 1 (Chachkhiani et al., 2020) - 87% (Venugopal et al., 2021), respectively.
3.7. OGD onset timing
Data regarding onset of OGD was given by 43 studies (28%). Nearly all the reviewed studies agree in showing that OGD occur early during the course of the disease, being among the first symptoms noted by patients; only 3 papers reported a late presentation of these disturbance, which appeared after all other symptoms in more than 50% of patients (Lechien et al., 2020d), (Lechien et al., 2020a), (Samimi Ardestani et al., 2020). Most of the times OGD occurred concurrently to other COVID-19 symptoms; only in 2 studies OGD preceded other disturbances in more than 50% of patients (Lima et al., 2020), (Gelardi et al., 2020).
3.8. OGD longitudinal course and recovery rate
58 (37%) of studies focused on the longitudinal course and recovery rate of OGD. Across all studies, OGD was generally persistent, and a significant number of patients recovered only partially during the first weeks after resolution of other symptoms.
In particular, 30 (19%) studies obtained follow-up data (either with a prospective or retrospective approach) for at least 20 days after OGD onset; only in 5 OGD persisted in less than 10% of patients that originally developed these symptoms (Lv et al., 2020), (Boscolo-Rizzo et al., 2020), (Barón-Sánchez et al., 2020), (Mishra et al., 2020), (Komagamine and Yabuki, 2020). Of note, among the four studies with the longest follow-up time (i.e. 57.94 ± 1.40 (Otte et al., 2020), 62 (range 25–95) (Li et al., 2020a), 117 (range 41–193) (Stavem et al., 2020) and 125 (45–215) (Petersen et al., 2020) days after symptom onset) recovery rates were 54%, 89%, 88% and 84%, respectively.
In those who recovered, most studies reported that OGD resolved within 2 weeks after onset. In fact, mean or median duration of OGD was more than 14 days in only 3 studies (Sheng et al., 2020), (Garg et al., 2020), (Meini et al., 2020).
3.9. OGD association with SARS-CoV-2 infection risk
Of the 59 studies that enrolled a control group of healthy individuals, 51 studies reported results of statistical test run to evaluate the association between presence of OGD and SARS-CoV-2 infection risk. In all studies, OGD were significantly more prevalent in patients positive to SARS-CoV-2 via RT-PCR or serology (COVID+). 12 studies performed regression analyses adjusted for demographic and clinical variables. OGD were found to be associated with COVID + after adjustment for demographic (Waterfield et al., 2020), (Adorni et al., 2020), (Tudrej et al., 2020), (Venugopal et al., 2021), (Just et al., 2020), (Menni et al., 2020), (Lee et al., 2020), (Ganz-Lord et al., 2020), (Bidkar et al., 2020), clinical (Waterfield et al., 2020), (Adorni et al., 2020), (Tudrej et al., 2020), (Venugopal et al., 2021), (Just et al., 2020), (Lee et al., 2020), (Ganz-Lord et al., 2020) and laboratoristic (Tudrej et al., 2020) variables.
Sn, Sp, PLR and NLR were calculated for 51 studies; data are summarized as their range in Table 2, while the values for each study are reported in Supplementary Tables 3–5. In general, OGD had low sensitivity and high specificity for the diagnosis of SARS-CoV-2. Overall, accuracy of OGD (presence of OD or GD) for SARS-CoV-2 diagnosis was higher than 80% in 13 out of 22 studies, accuracy of OD was higher than 80% in 10 out of 33 studies; finally, accuracy of GD for diagnosis was higher than 80% in 8 out of 22 studies.
Table 2.
Diagnostic performances of presence of olfactory or gustatory dysfunction for the detection of SARS-CoV-2 infection.
OGD: Either Olfactory or Gustatory Dysfunctions (not specified by the studies).
Sensitivity, Specificity and Accuracy data expressed as percentages (%).
3.10. OGD association with COVID-19 severity
A total of 30 studies examined the relationship between OGD and severity of COVID-19; 12 did not find any significant association between the two variables (Moein et al., 2020c), (Petrocelli et al., 2020), (Lee et al., 2020; Özçelik Korkmaz et al., 2020; Ninchritz-Becerra et al., 2020; Romero-Gameros et al., 2020; Cocco et al., 2020; Mao et al., 2020b; Petersen et al., 2020; Al-Zaidi and Badr, 2020; Makda et al., 2020; Izquierdo-Domínguez et al., 2020).
In the others, the presence of OD was associated with a milder clinical course, and in particular with decreased risk of developing pneumonia (Klopfenstein et al., 2020), (Castelli et al., 2020), (Romero-Sánchez et al., 2020), (Sanli et al., 2021), lower levels of inflammatory markers (Sanli et al., 2021), decreased need for hospitalization (Salepci et al., 2020; Foster et al., 2020; Izquierdo-Domínguez et al., 2020; Paderno et al., 2020; Yan et al., 2020a; Nouchi et al., 2020; Avcl et al., 2020; Klopfenstein et al., 2020; D'Ascanio et al., 2020; Sisó-Almirall et al., 2020), decreased need for oxygen therapy (Klopfenstein et al., 2020), (Sayin, 2020), decrease need for Intensive Care Unit (ICU) admission (Sisó-Almirall et al., 2020), decrease Acute respiratory Distress Syndrome (ARDS) rates (Foster et al., 2020), decrease need for intubation (Foster et al., 2020), reduced mortality (Sisó-Almirall et al., 2020).
In two records, the presence of persistent OD was associated with increased need for hospitalization (Vaira et al., 2020b), (Vaira et al., 2020c); conversely, another study found that the frequency of smell function recovery was lower among non-hospitalized patients (Foster et al., 2020).
Presence of GD was associated with decreased risk of developing pneumonia ((Romero-Sánchez et al., 2020)) and decrease need for hospitalization (Paderno et al., 2020), (Yan et al., 2020b), (Nouchi et al., 2020), (Sisó-Almirall et al., 2020).
Finally, regarding aggregate OGD prevalence, in a study involving 12,066 patients, ascendant hierarchical clustering was applied to generate four phenotypic clusters, one of which included OD and GD. This cluster was the one associated with the lowest rate of ICU admission and mortality (Rubio-Rivas et al., 2020). Similarly, in another record OGD was associated with lower rates of hospitalization and ICU admission (Chary et al., 2020).
3.11. Risk of bias
Overall, quality of evidence was low for most of the studies included. 11 studies were considered to have a very high risk of bias (n = 2104 individuals), 118 records were classified as having a high risk of bias (n = 47,523 individuals), while only 26 reports were labeled as low risk of bias (n = 9227 individuals).
4. Discussion
The large number of studies included in this review reflects the magnitude of interest that the topic of chemosensory disturbances has attracted among the scientific community. In this review, we focused solely on clinical studies on OGD in SARS-CoV-2 infection; however, numerous other records have also provided some insights on the etiopathological basis of SARS-CoV-2-related OGD, from pre-clinical studies in animal models, to post-mortem histological analyses and neuroimaging findings. A summary of these studies can be found in Box 1.
Box 1.
| UNCOVERING THE ETIOPATHOLOGICAL ROOTS OF SARS-CoV-2 RELATED OLFACTORY DYSFUNCTION: EVIDENCE FROM PRECLINICAL, HISTOPATHOLOGICAL AND IMAGING FINDINGS |
| The etiology of OD dysfunctions in the SARS-CoV-2 infection has been the subject of discussion since the beginning of the pandemic; in this section we try to summarize the most relevant findings on the topic coming from preclinical, histopathological (biopsies, post-mortem findings) and radiological studies on the topic. |
| Of note, we did not find any original study regarding the etiopathogenesis of gustatory dysfunctions in SARS-CoV-2 infection; therefore, all the evidence presented below will focus on OD. |
| Preclinical studies |
| Many have suggested that the high rates of neurological complications seen in COVID-19 (Favas et al., 2020), including OGD, could be the consequence of SARS-CoV-2 neuroinvasive potential (Orrù et al., 2020). |
| All the preclinical studies conducted on murine models agree on the fact that the major target for SARS-CoV-2 infection is the olfactory non-neuronal epithelium, and specifically the sustentacular cells (Bryche et al., 2020; De Melo et al., 2020; Zhang et al., 2020; Rodriguez et al., 2020; Zheng et al., 2020b; Sun et al., 2020), a glial-like cell population that support olfactory sensory neurons in their functions (Vogalis et al., 2005). Sustentacular cells express high levels of the virus entry proteins Angiotensin-Converting Enzyme 2 (ACE2) and Transmembrane Serine Protease 2 (TMPRSS2) (Sun et al., 2020), (Chen et al., 2020); interestingly, these two proteins do not seem to be expressed olfactory sensory neurons (Bilinska et al., 2020). When the virus gets inoculated in murine models, it could be found in the olfactory epithelium as early as two days after administration (Bryche et al., 2020; Zhang et al., 2020; Zheng et al., 2020b) causing severe and acute olfactory dysfunction. Since virus particles were found in only 1% of the olfactory epithelium, it has been suggested that the olfactory dysfunction is mainly caused by down-expression of olfactory receptors as a consequence of inflammatory cytokines release triggered by a strong activation of the innate immune response (Rodriguez et al., 2020). |
| Whether SARS-CoV-2 infects the neuronal cells of the olfactory systems remains unclear. While several studies employing animal models did not find any evidence of SARS-CoV-2 particles in olfactory sensory neurons, olfactory bulb, olfactory tracts and olfactory cortex (Bryche et al., 2020; Rodriguez et al., 2020; Zheng et al., 2020b; Sun et al., 2020), two records reported the presence of the virus in the olfactory sensory neurons (De Melo et al., 2020; Zhang et al., 2020), and one documented infection of the neurons comprising the olfactory bulb (De Melo et al., 2020). Interestingly, since SARS-CoV-2 cellular entry proteins ACE2 and TMPRSS2 have been found to be significantly expressed in olfactory stem cells, with another study showing infection of the immature olfactory sensory neurons (Zhang et al., 2020), it may be suggested that the virus reaches the olfactory neuroepithelium through infection of its immature cells. |
| Histopathological studies on humans |
| Evidence from histopathological findings on human tissues suggest a neuroinvasive potential SARS-CoV-2, with significant microstructural modifications of the olfactory epithelium and the olfactory pathway. |
| Specifically, biopsies taken from patients with COVID-19 related olfactory dysfunction showed the presence of viral particles with concomitant histological alterations of the olfactory epithelium, with a clear inflammatory signature demonstrated by increased levels of tumor-necrosis factor alpha and interleukin-6 (De Melo et al., 2020; Torabi et al., 2020; Vaira et al., 2020f). Of note, histological alterations can still be found several weeks after the acute phase, with biopsies taken from patients with persistent olfactory dysfunction at 3 (Vaira et al., 2020f) and 6 (De Melo et al., 2020) months after initial diagnosis showing evidence of massive olfactory epithelium destruction and persistence of SARS-CoV-2 particles. |
| Results from post-mortem histological analyses confirmed the presence of SARS-CoV-2 in the olfactory epithelium (Meinhardt et al., 2020), with evidence of severe damage of the olfactory nerve (Bulfamante et al., 2020) and inflammatory neuropathy of the olfactory tracts (Kirschenbaum et al., 2020). Moreover, involvement of higher regions of the olfactory pathway such as the olfactory bulb was also reported, with presence of viral genetic material and viral particles (Meinhardt et al., 2020; Morbini et al., 2020) and evidence of inflammatory activity (Morbini et al., 2020) and high degree of astrogliosis and microgliosis (Matschke et al., 2020). |
| Neuroimaging findings in OGD |
| Scoping the literature, we retrieved 20 records in which COVID-19 with OGD were studied with various neuroimaging tools such as Computed Tomography (CT), Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI). |
| Specifically, the olfactory cleft anatomy was studied by 3 records, which reported thickening and obstruction of the olfactory cleft at the CT (Spoldi et al., 2020; Altundag et al., 2020), probably caused by mucosal edema as suggested by MRI T2 hyperintensity (Altundag et al., 2020); this could be seen as a potential explanation of olfactory dysfunction in these patients, since the olfactory cleft represents the entry route of odorant molecules to the olfactory epithelium. Another study, however, reported no evidence of involvement of these olfactory areas (Naeini et al., 2020). |
| 9 MRI studies reported relevant findings regarding olfactory bulb structure in anosmic COVID-19 patients. |
| Olfactory bulb dimensions were often found to be altered. A case report reported an enlargement along with an increase in T2 signal intensity, findings suggestive of edema (Laurendon et al., 2020); conversely, 5 studies reported a decrease in the size of the olfactory bulb (Kandemirli et al., 2020; Chiu et al., 2020; Li et al., 2020b; Tsivgoulis et al., 2020; Liang et al., 2020). Notably, out of these 5 studies, three (Kandemirli et al., 2020; Tsivgoulis et al., 2020; Liang et al., 2020) were performed in patients with persistent (>1 month in duration) anosmia. This finding seems to agree with the notion by which reduced dimension of the olfactory bulb in patients with post-infectious olfactory disorder is associated with longer duration of the chemosensory impairment (Eliezer et al., 2020), (Naeini et al., 2020). |
| Alterations in signal intensity within the olfactory bulb were also commonly reported, with diffuse hyperintense foci resembling microhemorrhages (Kandemirli et al., 2020), T2 FLAIR signal abnormalities (Strauss et al., 2020; Chetrit et al., 2020) and injury of the olfactory bulbs demonstrated by pre-contrast and post-contrast fat suppression T1W and STIR images (Aragão et al., 2020). |
| However, it must be outlined that olfactory bulbs hyperintensities in T2-FLAIR are a relative common finding in healthy subjects (Shor et al., 2020); for this reason, inclusion of a control group is warranted for a correct interpretation of these findings. In addition, given the small volumes of olfactory bulbs, high resolution sequences and objective intensity evaluations must be performed in order to avoid misinterpretation of paraphysiological findings. Still, the evidence seems to point out that the olfactory bodies might represent a key target of SARS-CoV-2 infection and a possible neuroimaging marker of olfactory dysfunctions in SARS-Cov-2 infection. |
| Involvement of the olfactory tracts was also reported, with evidence of bilateral T2 FLAIR and fat suppression hyperintensities and DWI abnormalities (Li et al., 2020b; Casez et al., 2020), suggestive of olfactory tract inflammatory neuropathy. |
| Finally, reports of alterations in cortical regions involved in processing of olfactory inputs were also found. Specifically, in two MRI studies on COVID-19 patients with anosmia, alterations of the right gyrus rectus were found in the form of FLAIR hyperintensity (Politi et al., 2020) and hemorrhage (Thu et al., 2020). In addition, a study employing F-FDG brain PET imaging found rectal gyrus metabolism to be greatly reduced on the right side. Moreover, a study reported the presence of FLAIR hyperintensity in the entorhinal cortex of 5 out of 23 patients studied (Kandemirli et al., 2020). |
The main findings of our systematic review can be summarized as following: a) The prevalence of OGD was significant in patients with SARS-CoV-2 infection, with a large variability across studies and a wide range of prevalence estimates, b) OGD appeared early during the disease and, most of the time, concurrently with other COVID-19 typical symptoms, c) In those who fully recover, OGD were generally short-lived, with most of the cases resolving within 14 days. However, in a non-negligible proportion of patients, OGD recovery was partial or absent even after months of follow-up, d) OGD presence was a reliable diagnostic tool for SARS-CoV-2, especially in ruling in SARS-CoV-2 diagnosis, e) OGD were associated with a milder course of disease, a decreased need for hospitalization and lower mortality rates.
4.1. OGD prevalence in SARS-CoV-2 infection
A key point that influences the results of prevalence studies is how outcomes are defined (Radke et al., 2019). In the case of OGD, outcome could be defined as ascertained by subjective testing (clinical interviews, administration of questionnaires, retrospective medical chart screening) or objective testing, carried through psychophysical tests. Intuitively, it could be expected that psychophysical testing is more sensitive in detecting chemosensory dysfunction. However, from the reviewed studies it emerged that psychophysical testing was not always the most sensitive measure, since in some records (Romero-Gameros et al., 2020), (Hintschich et al., 2020), (Lechien et al., 2020a) the prevalence of self-reported OGD was higher than the one derived from objective testing; in other cases, the opposite was true (Lima et al., 2020), (Gözen et al., 2020), with psychophysical testing reporting higher prevalence. Reports showed how absence of self-reported GD has high negative predictive value regarding the presence of GD as determined by objective testing (Soter et al., 2008); in this sense, since high negative predictive value is associated with high sensitivity, this finding suggests how based self-reported OGD could be at least as reliable as objective methods. In fact, self-reported OD severity has been found to correlate fairly well with psychophysical testing OD scores (Seok et al., 2017).
All the studies that employed objective testing used validated psychophysical tests, whose scores could be therefore expected to be highly reliable when compared with each other. However, Marino-Sanchez et al. (Mariño-Sánchez et al., 2020) raised an important point regarding the replicability of tests across different countries. As an example, they refer to the study by Moein et al. (2020c) which found, in an Iranian sample of COVID-19 patients, a prevalence of 98% for OD when measured by UPSIT. Marino-Sanchez et al. suggest how this extremely high prevalence could be influenced by the fact that UPSIT is only validated and commonly used in the US. The validity of this observation seems to be supported by the fact that Moein et al. found a very high prevalence of OD in the healthy controls sample, probably since the UPSIT olfactory stimuli, familiar to the US population, were not recognized by the Iranian sample. The fact that the reliability of OGD testing is highly dependent on whether the test is validated in the country of interest is probably an understated problem across studies on OGD prevalence in SARS-CoV-2 infection, since this potential issue was not addressed in any of the papers included in this review.
Furthermore, it must be noted that OGD are significantly prevalent in the general population, with recent estimates pointing at an OD prevalence of 22% (Desiato et al., 2020) and a GD prevalence of 17% (Liu et al., 2016). Nonetheless, 31% of the studies considered in this review included a control group of SARS-CoV-2 negative patients and run a statistic test to determine whether OGD scores were significantly different in the two groups; in all studies, OGD were more prevalent in the group of SARS-CoV-2 positive individuals.
4.2. OGD timing of onset and longitudinal trajectories
Early anecdotal reports suggested how OGD could represent the first symptom of SARS-CoV-2 infection in the majority of cases (Özçelik Korkmaz et al., 2020). While the reviewed studies confirmed that OGD occur early in the disease course, OGD preceded other COVID-19 symptoms only in a minority of cases.
On the other hand, the notion of high rates of persistence of OGD after the resolution of other COVID-19 symptoms was confirmed by several follow-up studies. The study reporting the longest follow-up time was that by Petersen et al. (2020)., which reported, after a mean of 125 days after OGD onset, the presence of residual OD and GD in 24% and 16% of patients, respectively. At present, whether SARS-CoV-2-related OGD are completely reversible remains an open question; since chronic OGD is associated with disturbances in eating behavior, depression and a general reduction of the quality of life (Croy et al., 2014; Baharvand et al., 2013), future studies following-up patients with residual OGD and reporting their outcomes are warranted.
4.3. OGD as a diagnostic marker for SARS-CoV-2 infection
In about one third of the included studies, a group of both SARS-CoV-2 negative (COVID-) and SARS-CoV-2 positive (COVID-+) were included, thus allowing to test the efficacy of OGD as a diagnostic marker for the infection (Table 2, Supplementary Tables 3–5).
Overall, OGD were proven to have good accuracy in detecting the infection, even more so considering that these were symptoms often self-referred by patients; as a benchmark, clinical symptoms used to diagnose community acquired pneumonia all seem to perform worse than OGD for the diagnosis of SARS-CoV-2 infection (Ebell et al., 2020). Moreover, high specificity and low sensibility values were found consistently across studies, ultimately suggesting that while the absence of OGD is not useful in excluding the possibility of infection, their presence “rule-in” the possibility of SARS-CoV-2 diagnosis, thus representing a strong indication for more definitive testing. In this context, a preprint published by the Global Consortium for Chemosensory Research (GCCR) demonstrated how a continuous rating of current olfactory ability on a 0 to 10 rating scale was the best predictor of SARS-CoV-2 infection in a sample of 4148 COVID+ and 546 COVID-; ratings ≤ 2 were associated with OR ≥ 2, with the presence of anosmia being associated with SARS-CoV-2 infection with an OR of 10 (Gerkin et al., 2020).
4.4. OGD are specific symptoms of SARS-CoV-2 infection
Several studies showed how OGD were associated with SARS-CoV-2 infection independently from demographic, clinical or laboratory variables, ultimately supporting the notion by which OGD are specific symptoms of SARS-CoV-2 infection, and not the product of confounders. One of the most discussed candidates in the list of the potential confounders was the presence of nasal obstruction. Regarding this last point, several of the studies included found nasal blockage to have a significant lower prevalence than OGD in SARS-CoV-2 patients. In particular, two papers from the GCCR (Gerkin et al., 2020; Parma et al., 2020b) showed, through a principal component analysis, how OGD and nasal obstruction were mostly uncorrelated, and thus unlikely to demonstrate a causal relationship with each other.
4.5. OGD as a prognostic maker for SARS-CoV-2 infection
Various studies reported how OGD presence was associated with a generally milder disease characterized by a decreased need for hospitalization, lower rates of severe pneumonia or ARDS, decreased need for oxygen, intubation, and lower mortality rates. Among the hypotheses proposed, the most common was the one suggesting that a strong immunological response in the lymphatic tissue of the nasal mucosa might be associated with higher rates of OGD, but also with a more effective immune response and thus with a lower incidence of complications (Yan et al., 2020c); this hypothesis, however, has never been tested in pre-clinical or clinical settings to date and awaits confirmation.
5. Limitations
Although the screening process was performed in a rigorous way, the rate of publications on the topic has been unprecedented; moreover, most authors performing clinical studies on SARS-CoV-2 have included, since the beginning of the pandemic, data about prevalence of OGD. Therefore, we could not exclude that some records could have been missed in the selection process.
Studies were highly heterogeneous in their design, with some being performed in hospital wards, others in emergencies department and others being cross-sectional studies on SARS-CoV-2 serology. However, we provided information regarding study design adopted, SARS-CoV-2 testing methodic employed, and the severity of patients studied. In several studies, assessment of OGD presence was carried out retrospectively through phone interviews or the administration of questionnaires weeks or month after the disease onset; in this case, the presence of recall bias could not be ruled out.
Finally, some of the reviewed studies investigated the rates of OGD resolution/recovery following-up the patients. However, while some of these studies clearly stated the attrition rate (number of lost at follow-up), others did not, ultimately limiting the reliability of OGD recovery rates reported.
6. Conclusions
From the reviewed studies it emerged how OGD are prevalent in patients with SARS-CoV-2 infection and how they can provide valuable information for early detection of the disease and for its prognostic stratification. Worrisome rates of OGD persistence are observed in the medium-term, and to date it is not clear whether these disturbances are fully reversible. Quality of the gathered evidence was generally low, with most of the studies having a high risk of bias. Therefore, further studies are warranted on this topic, since OGD may represent one of the long-term complications of the disease.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100268.
Funding sources
This work has been supported by Fondazione Cariplo, grant n° 2020-1366 to PB, within the call to support research into treatment, diagnosis and detection of COVID-19 in partnership with the Lombardy Region and Fondazione Veronesi.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adorni F. Self-reported symptoms of SARS-CoV-2 infection in a nonhospitalized population in Italy: cross-sectional study of the EPICOVID19 web-based survey. JMIR Publ. Health Surveill. 2020;6 doi: 10.2196/21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin. Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zaidi H.M.H., Badr H.M. Incidence and recovery of smell and taste dysfunction in COVID-19 positive patients. Egypt. J. Otolaryngol. 2020;36 [Google Scholar]
- Altin F., Cingi C., Uzun T., Bal C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur. Arch. Oto-Rhino-Laryngol. 2020;277:2775–2781. doi: 10.1007/s00405-020-06155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altundag A. Otolaryngology - Head and Neck Surgery; United States: 2020. Olfactory Cleft Measurements and COVID-19–Related Anosmia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragão M.F.V.V., Leal M.C., Cartaxo Filho O.Q., Fonseca T.M., Valença M.M. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am. J. Neuroradiol. 2020;41:1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimov E. New York Times; 2020. Rediscovering Wine after Covid-19. [Google Scholar]
- Avcl H., Karabulut B., Farasoglu A., Boldaz E., Evman M. Relationship between anosmia and hospitalisation in patients with coronavirus disease 2019: an otolaryngological perspective. J. Laryngol. Otol. 2020;134:710–716. doi: 10.1017/S0022215120001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M. Taste changes (dysgeusia) in COVID-19: a systematic review and meta-analysis. Gastroenterology. 2020;159:1132–1133. doi: 10.1053/j.gastro.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharvand M., Shoalehsaadi N., Barakian R., Jalali Moghaddam E. Taste alteration and impact on quality of life after head and neck radiotherapy. J. Oral Pathol. Med. 2013;42:106–112. doi: 10.1111/j.1600-0714.2012.01200.x. [DOI] [PubMed] [Google Scholar]
- Barón-Sánchez J., Santiago C., Goizueta-San Martín G., Arca R., Fernández R. English Edition. 2020. Smell and Taste Disorders in Spanish Patients with Mild COVID-19. Neurología. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bartheld C.S., Butowt R., Hagen M.M. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem. Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bartheld C.S., Butowt R., Hagen M.M. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem. Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A.S., Joshi S.V., Naik S., Sangle S., Abraham N.M. Quantitative assessment of olfactory dysfunction accurately detects asymptomatic COVID-19 carriers. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3622362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidkar V. Testing olfactory and gustatory dysfunctions among quarantine COVID-19 suspects. Indian J.Otolaryngol. Head Neck Surg. 2020 doi: 10.1007/s12070-020-02210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo ’. Nature. 2020;584(7821):425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- Le Bon S.D. European Archives of Oto-Rhino-Laryngology; 2020. Psychophysical Evaluation of Chemosensory Functions 5 Weeks after Olfactory Loss Due to COVID-19: a Prospective Cohort Study on 72 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon S.D. European Archives of Oto-Rhino-Laryngology; 2020. Psychophysical Evaluation of Chemosensory Functions 5 Weeks after Olfactory Loss Due to COVID-19: a Prospective Cohort Study on 72 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsetto D. Self-reported alteration of sense of smell or taste in patients with COVID-19: a systematic review and meta-analysis on 3563 patients. Rhinology. 2020;58:430–436. doi: 10.4193/Rhin20.185. [DOI] [PubMed] [Google Scholar]
- Boscolo-Rizzo P. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngology - Head and Neck Surgery. 2020;146:1–5. doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryche B. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfamante G. First ultrastructural autoptic findings of sars-cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86:678–679. doi: 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- Cain W.S., Rabin M.D. Comparability of two tests of olfactory functioning. Chem. Senses. 1989;14:479–485. [Google Scholar]
- Cain W.S., Gent J.F., Goodspeed R., Leonard G. Evaluation of olfactory dysfunction in the Connecticut chemosensory clinical research center. Laryngoscope. 1988;98:83–88. doi: 10.1288/00005537-198801000-00017. [DOI] [PubMed] [Google Scholar]
- Casez O. SARS-CoV-2 related encephalitis: MRI pattern of the olfactory tract involvement. Neurology. 2020 doi: 10.1212/wnl.0000000000011150. 10.1212/WNL.0000000000011150. [DOI] [PubMed] [Google Scholar]
- Castelli M. Prevalence and risk factors for lung involvement on low-dose chest CT (LDCT) in a paucisymptomatic population of 247 patients affected by COVID-19. Insight. Imag. 2020;11 doi: 10.1186/s13244-020-00939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachkhiani D. Neurological complications in a predominantly African American sample of COVID-19 predict worse outcomes during hospitalization. Clin. Neurol. Neurosurg. 2020;197 doi: 10.1016/j.clineuro.2020.106173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary E. Prevalence and recovery from olfactory and gustatory dysfunctions in covid-19 infection: a prospective multicenter study. Am. J. Rhinol. Allergy. 2020;34:686–693. doi: 10.1177/1945892420930954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetrit A. Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J. Infect. 2020;81:816–846. doi: 10.1016/j.jinf.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. One-seventh of patients with covid-19 had olfactory and gustatory abnormalities as their initial symptoms: a systematic review and meta-analysis. Life. 2020;10:1–8. doi: 10.3390/life10090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu A. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2020 doi: 10.1007/s00234-020-02554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco A. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. J. Neurol. 2020 doi: 10.1007/s00415-020-10135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I., Nordin S., Hummel T. Olfactory disorders and quality of life-an updated review. Chem. Senses. 2014;39:185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- Cruz A.T., Zeichner S.L. COVID-19 in children: initial characterization of the pediatric disease. Pediatrics. 2020;145:19–21. doi: 10.1542/peds.2020-0834. [DOI] [PubMed] [Google Scholar]
- COVID-19 Anosmia Reporting Tool. https://www.entnet.org/content/reporting-tool-patients-anosmia-related-covid-19
- Dawson P. Loss of taste and smell as distinguishing symptoms of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1101/2020.05.13.20101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers O.M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiato V.M. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am. J. Rhinol. Allergy. 2020 doi: 10.1177/1945892420946254. [DOI] [PubMed] [Google Scholar]
- Dixon B.E. 2020. Symptoms and Symptom Clusters Associated with SARS-CoV-2 Infection in Community-Based Populations: Results from a Statewide Epidemiological Study. medRxiv : the Preprint Server for Health Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R.L., Shaman P., Kimmelman C.P., Dann M.S. University of Pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- D'Ascanio L. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol. Head Neck Surg. 2020 doi: 10.1177/0194599820943530. United States. [DOI] [PubMed] [Google Scholar]
- Ebell M.H., Chupp H., Cai X., Bentivegna M., Kearney M. Accuracy of signs and symptoms for the diagnosis of community-acquired pneumonia: a meta-analysis. Acad. Emerg. Med. 2020;27:541–553. doi: 10.1111/acem.13965. [DOI] [PubMed] [Google Scholar]
- Eliezer M. Loss of smell in COVID-19 patients: MRI data reveals a transient edema of the olfactory clefts. Neurology. 2020 doi: 10.1212/wnl.0000000000010806. 10.1212/WNL.0000000000010806. [DOI] [PubMed] [Google Scholar]
- von Elm E. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Fantozzi P.J. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2020;41 doi: 10.1016/j.amjoto.2020.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favas T.T. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol. Sci. 2020;41:3437–3470. doi: 10.1007/s10072-020-04801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K.J., Jauregui E., Tajudeen B., Bishehsari F., Mahdavinia M. Smell loss is a prognostic factor for lower severity of coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2020;125:481–483. doi: 10.1016/j.anai.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz-Lord F., Segal K.R., Rinke M. L. Title. Covid-19 symptoms, duration, and prevalence among healthcare workers in the New York metropolitan area. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R. Neurological symptoms as initial manifestation of Covid-19-An observational study. Ann. Indian Acad. Neurol. 2020;23:482–486. doi: 10.4103/aian.AIAN_560_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelardi M., Trecca E., Cassano M., Ciprandi G. Smell and taste dysfunction during the covid-19 outbreak: a preliminary report. Acta Biomed. 2020;91:230–231. doi: 10.23750/abm.v91i2.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkin R.C. Recent smell loss is the best predictor of COVID-19: a preregistered, cross-sectional study. medRxiv. 2020 doi: 10.1101/2020.07.22.20157263. 2020.07.22.20157263. [DOI] [Google Scholar]
- Giorli A. A literature systematic review with meta-analysis of symptoms prevalence in covid-19: the relevance of olfactory symptoms in infection not requiring hospitalization. Curr. Treat. Options Neurol. 2020;22 doi: 10.1007/s11940-020-00641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOBAL CONSORTIUM FOR CHEMOSENSORY RESEARCH https://gcchemosensr.org/projects/
- Gözen E.D. Evaluation of olfactory function with objective tests in COVID-19-positive patients: a cross-sectional study. Ear Nose Throat J. 2020 doi: 10.1177/0145561320975510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Hajikhani B. Olfactory and gustatory dysfunction in COVID-19 patients: a meta-analysis study. Physiol. Rep. 2020;8 doi: 10.14814/phy2.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum M.E. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: a systematic review and meta-analysis. Chem. Senses. 2020 doi: 10.1093/chemse/bjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauchecorne M., Baldini C., Foulon S., Bayle A., Albiges L. Outcome of older cancer patients infected with COVID-19 at gustave roussy cancer center. ESMO Virtual Congress. 2020 doi: 10.1016/annonc/annonc289. [DOI] [Google Scholar]
- Hintschich C.A. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int. For. Allergy Rhinol. 2020;10:1105–1107. doi: 10.1002/alr.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman H.J., Rawal S., Li C.M., Duffy V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2016;17:221–240. doi: 10.1007/s11154-016-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Gillett S., Slack R., Lund V.J., Browne J.P. Psychometric validity of the 22-item sinonasal outcome test. Clin. Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Houlihan C.F., Beale R. The complexities of SARS-CoV-2 serology. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30699-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T., Sekinger B., Wolf S.R., Pauli E., Kobal G. ‘Sniffin’ sticks'. Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Iannuzzi L. Gaining back what is lost: recovering the sense of smell in mild to moderate patients after COVID-19. Chem. Senses. 2020 doi: 10.1093/chemse/bjaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibekwe T.S., Fasunla A.J., Orimadegun A.E. Systematic review and meta-analysis of smell and taste disorders in COVID-19. OTO Open. 2020;4 doi: 10.1177/2473974X20957975. 2473974X2095797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Director- General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020 11. World Health Organization; 2020. [Google Scholar]
- Izquierdo-Domínguez A. Smell and taste dysfunction in covid-19 is associated with younger age in ambulatory settings: a multicenter cross-sectional study. J. Invest. Allergol. Clin. Immunol. 2020;30:346–357. doi: 10.18176/jiaci.0595. [DOI] [PubMed] [Google Scholar]
- Jin X. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020 doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffily L. The close relationship between sudden loss of smell and COVID-19. Braz. J. Otorhinolaryngol. 2020;86:632–638. doi: 10.1016/j.bjorl.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Hopkins Coronavirus Resource Center COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html
- Just J., Puth M.T., Regenold F., Weckbecker K., Bleckwenn M. Risk factors for a positive SARS-CoV-2 PCR in patients with common cold symptoms in a primary care setting – a retrospective analysis based on a joint documentation standard. BMC Fam. Pract. 2020;21 doi: 10.1186/s12875-020-01322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Academic Radiology; 2020. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempker R.R. Clinical Infectious Diseases; 2020. Loss of Smell and Taste Among Healthcare Personnel Screened for Coronavirus 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum D. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396:166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T. New loss of smell and taste: uncommon symptoms in COVID-19 patients in Nord Franche-Comte cluster, France. Int. J. Infect. Dis. 2020;100:117–122. doi: 10.1016/j.ijid.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komagamine J., Yabuki T. Initial symptoms of patients with coronavirus disease 2019 in Japan: a descriptive study. J. General Family Med. 2020 doi: 10.1002/jgf2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis B.N. ‘taste Strips’ - a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009;256:242–248. doi: 10.1007/s00415-009-0088-y. [DOI] [PubMed] [Google Scholar]
- Laurendon T. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology. 2020;95:224–225. doi: 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- Lechien J.R. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19. Ann. Intern. Med. 2020;173:672–675. doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R. Psychophysical olfactory tests and detection of COVID-19 in patients with sudden onset olfactory dysfunction: a prospective study. Ear Nose Throat J. 2020;99:579–583. doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- Lechien J.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Kor. Med. Sci. 2020;35:1–6. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Olfactory dysfunction in recovered coronavirus disease 2019 (COVID-19) patients. Mov. Disord. 2020;35:1100–1101. doi: 10.1002/mds.28172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.W. Anosmia and olfactory tract neuropathy in a case of COVID-19. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.C., Tsai Y.S., Syue L.S., Lee N.Y., Li C.W. Olfactory bulb atrophy in a case of COVID-19 with hyposmia. Acad. Radiol. 2020;27:1649–1650. doi: 10.1016/j.acra.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M.A. Smell dysfunction in COVID-19 patients: more than a yes-no question. J. Neurol. Sci. 2020;418 doi: 10.1016/j.jns.2020.117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Zong G., Doty R.L., Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H. Prevalence and recovery time of olfactory and gustatory dysfunction in hospitalized patients with COVID-19 in Wuhan, China. Int. J. Infect. Dis. 2020;100:507–512. doi: 10.1016/j.ijid.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnavita N., Tripepi G., Di Prinzio R.R. Symptoms in health care workers during the covid-19 epidemic. A cross-sectional survey. Int. J. Environ. Res. Publ. Health. 2020;17:1–15. doi: 10.3390/ijerph17145218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makda A., Kumar S., Kumar A., Kumar V., Rizwan A. The frequency of neurological symptoms in COVID-19 patients at a tertiary care hospital in Pakistan. Cureus. 2020 doi: 10.7759/cureus.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño-Sánchez F., Santamaría-Gadea A., de los Santos G., Alobid I., Mullol J. Psychophysical olfactory testing in COVID-19: is smell function really impaired in nearly all patients? Int. For. Allergy Rhinol. 2020;10:951–952. doi: 10.1002/alr.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarelli O. Sensory recovery of myomucosal flap oral cavity reconstructions. Head Neck. 2018;40:467–474. doi: 10.1002/hed.25000. [DOI] [PubMed] [Google Scholar]
- Matschke J. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J. 2020. Olfactory Transmucosal SARS-CoV-2 Invasion as Port of Central Nervous System Entry in COVID-19 Patients. [DOI] [PubMed] [Google Scholar]
- Meini S., Suardi L.R., Busoni M., Roberts A.T., Fortini A. 2020. Olfactory and Gustatory Dysfunctions in 100 Patients Hospitalized for Covid-19: Sex Differences and Recovery Time in Real-Life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Melo G.D. COVID-19-associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system. bioRxiv. 2020;4 2020.11.18.388819. [Google Scholar]
- Menni C. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020 doi: 10.1101/2020.04.05.20048421. 2020.04.05.20048421. [DOI] [Google Scholar]
- Mercante G. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngology - Head and Neck Surgery. 2020;146:723–728. doi: 10.1001/jamaoto.2020.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkely B. 4th. 2020. Novel Coronavirus Epidemic in the Hungarian Population, a Cross-Sectional Nationwide Survey to Support the Exit Policy in Hungary. ed. 42. GeroScience, pp. 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P., Gowda V., Dixit S., Kaushik M. Prevalence of new onset anosmia in COVID-19 patients: is the trend different between European and Indian population? Indian J.Otolaryngol. Head Neck Surg. 2020;72:484–487. doi: 10.1007/s12070-020-01986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T. Smell dysfunction: a biomarker for COVID-19. Int. For. Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Tabarsi P., Doty R.L. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int. For. Allergy Rhinol. 2020;10:1127–1135. doi: 10.1002/alr.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T. Smell dysfunction: a biomarker for COVID-19. Int. For. Allergy Rhinol. 2020 doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbini P. Ultrastructural evidence of direct viral damage to the olfactory complex in patients testing positive for SARS-COV-2. JAMA Otolaryngology - Head and Neck Surgery. 2020;146:972–973. doi: 10.1001/jamaoto.2020.2366. [DOI] [PubMed] [Google Scholar]
- Naeini A.S. Paranasal sinuses computed tomography findings in anosmia of COVID-19. American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2020;41 doi: 10.1016/j.amjoto.2020.102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninchritz-Becerra E. Subjective evaluation of smell and taste dysfunction in patients with mild COVID-19 in Spain. Med. Clínica. 2020 doi: 10.1016/j.medcli.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi A. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur. J. Clin. Microbiol. Infect. Dis. 2020 doi: 10.1007/s10096-020-04056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrù G. Neurological complications of covid-19 and possible neuroinvasion pathways: a systematic review. Int. J. Environ. Res. Publ. Health. 2020;17:1–18. doi: 10.3390/ijerph17186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte M.S., Eckel H.N.C., Poluschkin L., Klussmann J.P., Luers J.C. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol. 2020 doi: 10.1080/00016489.2020.1811999. [DOI] [PubMed] [Google Scholar]
- Özçelik Korkmaz M., Eğilmez O.K., Özçelik M.A., Güven M. European Archives of Oto-Rhino-Laryngology; 2020. Otolaryngological Manifestations of Hospitalised Patients with Confirmed COVID-19 Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paderno A. Otolaryngology - Head and Neck Surgery; United States: 2020. Olfactory and Gustatory Outcomes in COVID-19: A Prospective Evaluation in Nonhospitalized Subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K.W., Chee J., Subramaniam S., Ng C.L. Frequency and clinical utility of olfactory dysfunction in COVID-19: a systematic review and meta-analysis. Curr. Allergy Asthma Rep. 2020;20 doi: 10.1007/s11882-020-00972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses. 2020;45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V. 2020. More than Just Smell - COVID-19 Is Associated with Severe Impairment of Smell, Taste, and Chemesthesis. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.S. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli M. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J. Laryngol. Otol. 2020;134:571–576. doi: 10.1017/S0022215120001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinato D.J. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12:1–13. doi: 10.3390/cancers12071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi L.S., Salsano E., Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurology. 2020;77:1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- Putman M.S., Ruderman E.M., Niforatos J.D. Publication rate and journal review time of COVID-19–related research. Mayo Clin. Proc. 2020;95:2290–2291. doi: 10.1016/j.mayocp.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R.C. The New York Times; 2020. Lost Sense of Smell May Be Peculiar Clue to Coronavirus Infection. [Google Scholar]
- Radke E.G. Development of outcome-specific criteria for study evaluation in systematic reviews of epidemiology studies. Environ. Int. 2019;130 doi: 10.1016/j.envint.2019.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy K., Saniasiaya J., Abdul Gani N. Olfactory and gustatory dysfunctions as a clinical manifestation of coronavirus disease 2019 in a Malaysian tertiary center. Ann. Otol. Rhinol. Laryngol. 2020 doi: 10.1177/0003489420963165. [DOI] [PubMed] [Google Scholar]
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- Rocke J., Hopkins C., Philpott C., Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: a systematic review and meta-analysis. Clin. Otolaryngol. 2020;45:914–922. doi: 10.1111/coa.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S. 2020. Innate Immune Signaling in the Olfactory Epithelium Reduces Odorant Receptor Levels: Modeling Transient Smell Loss in COVID-19 Patients. medRxiv : the Preprint Server for Health Sciences. [DOI] [Google Scholar]
- Rogers J.P. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland L.T., Gurrola J.G., Loftus P.A., Cheung S.W., Chang J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int. For. Allergy Rhinol. 2020;10:832–838. doi: 10.1002/alr.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Gameros C.A. Evaluation of predictive value of olfactory dysfunction, as a screening tool for COVID -19. Laryngoscope Invest. Otolaryngol. 2020 doi: 10.1002/lio2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Rivas M. Predicting clinical outcome with phenotypic clusters in COVID-19 pneumonia: an analysis of 12,066 hospitalized patients from the Spanish registry SEMI-COVID-19. J. Clin. Med. 2020;9:3488. doi: 10.3390/jcm9113488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salepci E. Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur. Arch. Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-06284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi Ardestani S.H. The coronavirus disease 2019: the prevalence, prognosis, and recovery from olfactory dysfunction (OD) Acta Otolaryngol. 2020 doi: 10.1080/00016489.2020.1836397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saniaslaya J., Islam A., Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27492 patients. Laryngoscope. 2020;131(4):865–878. doi: 10.1002/lary.29286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanli D.E.T. Relationship between disease severity and serum IL-6 levels in COVID-19 anosmia. American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2021;42 doi: 10.1016/j.amjoto.2020.102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin İ. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg. 2020;163(3) doi: 10.1177/0194599820931820. 473–479. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurink B. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J., Shim Y.J., Rhee C.S., Kim J.W. Correlation between olfactory severity ratings based on olfactory function test scores and self-reported severity rating of olfactory loss. Acta Otolaryngol. 2017;137:750–754. doi: 10.1080/00016489.2016.1277782. [DOI] [PubMed] [Google Scholar]
- Sheng W.H., Liu W. Da, Wang J.T., Chang S.Y., Chang S.C. Dysosmia and dysgeusia in patients with COVID-19 in northern Taiwan. J. Formos. Med. Assoc. 2020 doi: 10.1016/j.jfma.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor N., Chougar L., Pyatigorskaya N. MR imaging of the olfactory bulbs in patients with COVID-19 and anosmia: how to avoid misinterpretation. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.a6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisó-Almirall A. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PloS One. 2020;15 doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soter A. Accuracy of self-report in detecting taste dysfunction. Laryngoscope. 2008;118:611–617. doi: 10.1097/MLG.0b013e318161e53a. [DOI] [PubMed] [Google Scholar]
- Spoldi C. Isolated olfactory cleft involvement in SARS-CoV-2 infection: prevalence and clinical correlates. Eur. Arch. Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-06165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavem K., Ghanima W., Olsen M.K., Gilboe H.M., Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S.B., Lantos J.E., Heier L.A., Shatzkes D.R., Phillips C.D. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. Am. J. Neuroradiol. 2020;41:1882–1887. doi: 10.3174/ajnr.A6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.H. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133. doi: 10.1016/j.chom.2020.05.020. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratannon N., Dik W.A., Chatchatee P., Hagen P. M. van. COVID-19 in children: heterogeneity within the disease and hypothetical pathogenesis. Asian Pac. J. Allergy Immunol. 2020;38:170–177. doi: 10.12932/AP-170720-0920. [DOI] [PubMed] [Google Scholar]
- Therchilsen J.H. Self-collected versus healthcare Worker-collected swabs in the diagnosis of severe acute respiratory syndrome coronavirus 2. Diagnostics. 2020;10 doi: 10.3390/diagnostics10090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu S.S., Matin N., Levine S.R. Olfactory gyrus intracerebral hemorrhage in a patient with COVID-19 infection. J. Clin. Neurosci. 2020;79:275–276. doi: 10.1016/j.jocn.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- Torabi A. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem. Neurosci. 2020;11:1909–1913. doi: 10.1021/acschemneuro.0c00249. [DOI] [PubMed] [Google Scholar]
- Tostmann A. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, The Netherlands, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivgoulis G. Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: a magnetic resonance imaging study. Eur. J. Neurol. 2020 doi: 10.1111/ene.14537. [DOI] [PubMed] [Google Scholar]
- Tudrej B. 2020. Self-reported Loss of Smell and Taste in SARS-CoV-2 Patients: Primary Care Data to Guide Future Early Detection Strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun Ö. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain. 2020;21 doi: 10.1186/s10194-020-01188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A. Gustatory dysfunction: a highly specific and smell-independent symptom of COVID-19. Indian J.Otolaryngol. Head Neck Surg. 2020 doi: 10.1007/s12070-020-02182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42:1560–1569. doi: 10.1002/hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J. Otolaryngol. Head Neck Surg. 2020;49 doi: 10.1186/s40463-020-00449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J. Laryngol. Otol. 2020;134:703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 2020;42:1570–1576. doi: 10.1002/hed.26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J. Laryngol. Otol. 2020 doi: 10.1017/S0022215120002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena A. Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J. Clin. Med. 2020;9:2780. doi: 10.3390/jcm9092780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal U. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int. J. Infect. Dis. 2021;102:63–69. doi: 10.1016/j.ijid.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Hegg C.C., Lucero M.T. Ionic conductances in sustentacular cells of the mouse olfactory epithelium. J. Physiol. 2005;562:785–799. doi: 10.1113/jphysiol.2004.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield T. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch. Dis. Child. 2020 doi: 10.1136/archdischild-2020-320558. [DOI] [PubMed] [Google Scholar]
- Wu C. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int. For. Allergy Rhinol. 2020 doi: 10.1111/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. For. Allergy Rhinol. 2020;10:806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int. For. Allergy Rhinol. 2020;10:821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.J. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2020 doi: 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.